Climate explained by electromagnetic radiation or electromagnetic radiation and thermal aspects ¹
Terigi CicconeOctober 7, 2022
1
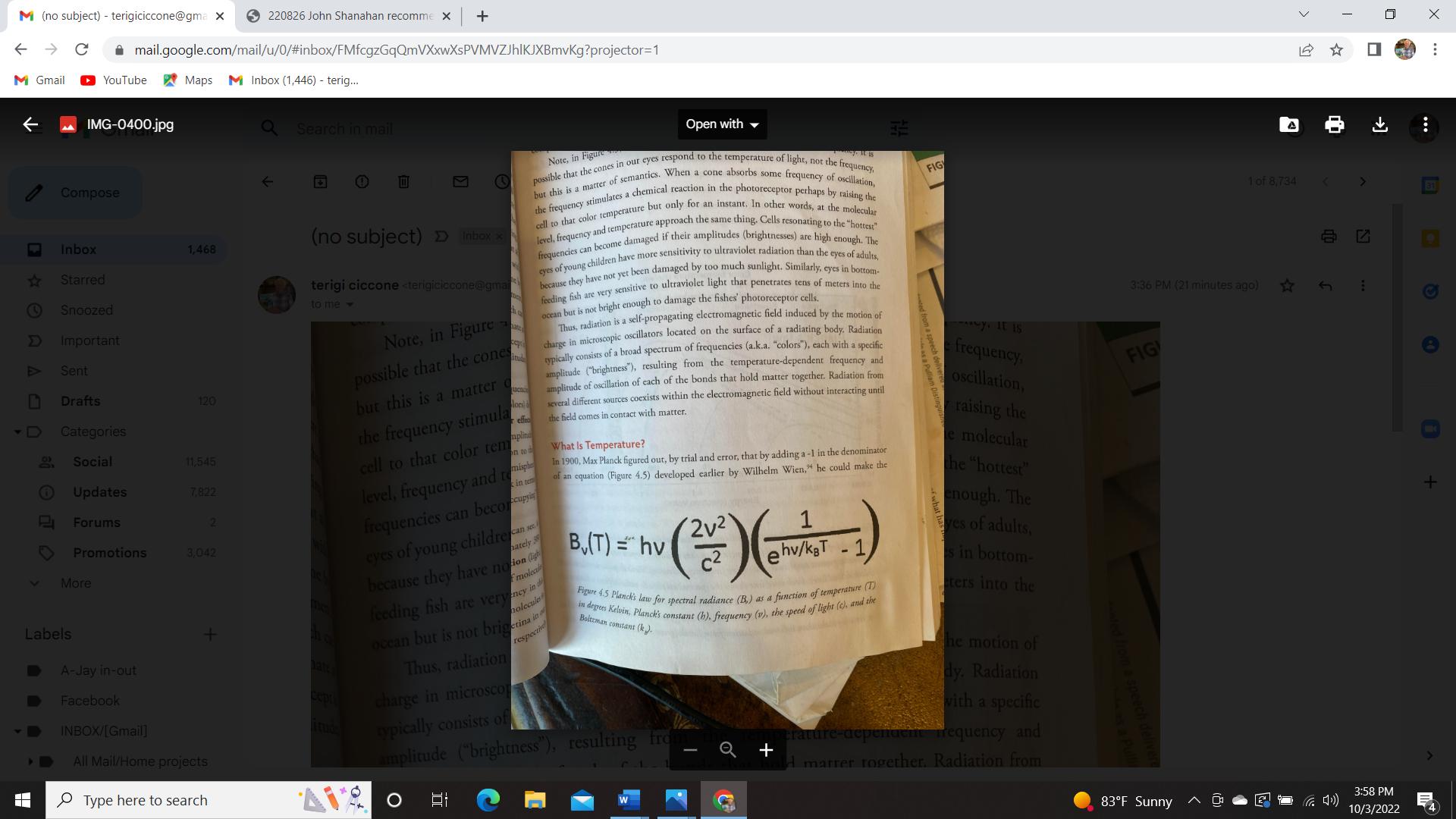
We all know the first law of thermodynamics. It tells us that the total amount of energy in the universe is fixed, leaving out that E=MC2 Thing. However, it applies only to thermally isolated systems. Now the science of climate change is all about how to convert the sun’s electromagnetic/radiant energy to thermal energy that resides only in matter and then vice versa, back to radiant energy. This way, we eliminate the distractions of kinetic and potential energy and focus only on thermal energy, also called chemical energy. Once radiant energy interacts with matter (a brick, elephant, bumble bee, or a molecule or atom), it is stored in the matter as heat. The temperature of a material object is a macroscopic measure of the amount of heat stored in the matter.
If I cut up a piece of anything, liquid, air, a rock, each piece will initially have the same temperature, no matter how small the pieces are. This tells me that this energy is stored at the molecular/atomic level. Scientists tell us that atoms/molecules are held together by two opposing electrostatic forces. One attracts the other particles farther away, and the other repels them when they come too close. Together these sub-atomic/molecular particles are in a constant elastic motion of repelling and attracting called molecular bonds, see figure 1. Let’s ignore the degrees of freedom and pretend that all bonds are essentially the same and these cyclic bonds stretch apart and come closer together. They repeat these stretching cycles say, at a frequency of 89.1 MHz. They also oscillate at a constant spectral amplitude of A. If left unimpeded, they will vibrate at this level forever since they have no mass, inertia, frictions, etc., which only exist in the material/physical universe.
Now along comes the sun and bathes these two sample molecules with a huge wave of different frequencies, and one of those frequencies equals the frequency that this nitrogen molecule vibrates at. The nitrogen will absorb and store it at its molecular bonds by making it vibrate with greater amplitude, making the two nitrogen atoms move farther apart and closer together. This increased vibration is thermal energy stored by this molecule. Now, if it’s a powerful UV ray, the vibration amplitude may exceed the net bond strength, the bond will break, and these two
1 This article is a response to a request by John Shanahan for an explanation of climate processes beyond spectra analysis of sunlight in and infrared radiation out. Ordinary people see Energy In = Work Done + Energy Remaining That applies to the food energy we consume the work we do, and how much energy we have left. It applies to fuel in the tank of a car or piece of equipment, the work it does, and how much fuel is left in the tank. It applies to the energy to create a hurricane, the destruction and rain it delivers, and the minor storm left over when it no longer is a hurricane. This explanation by retired engineer, Terigi Ciccone, is helpful.
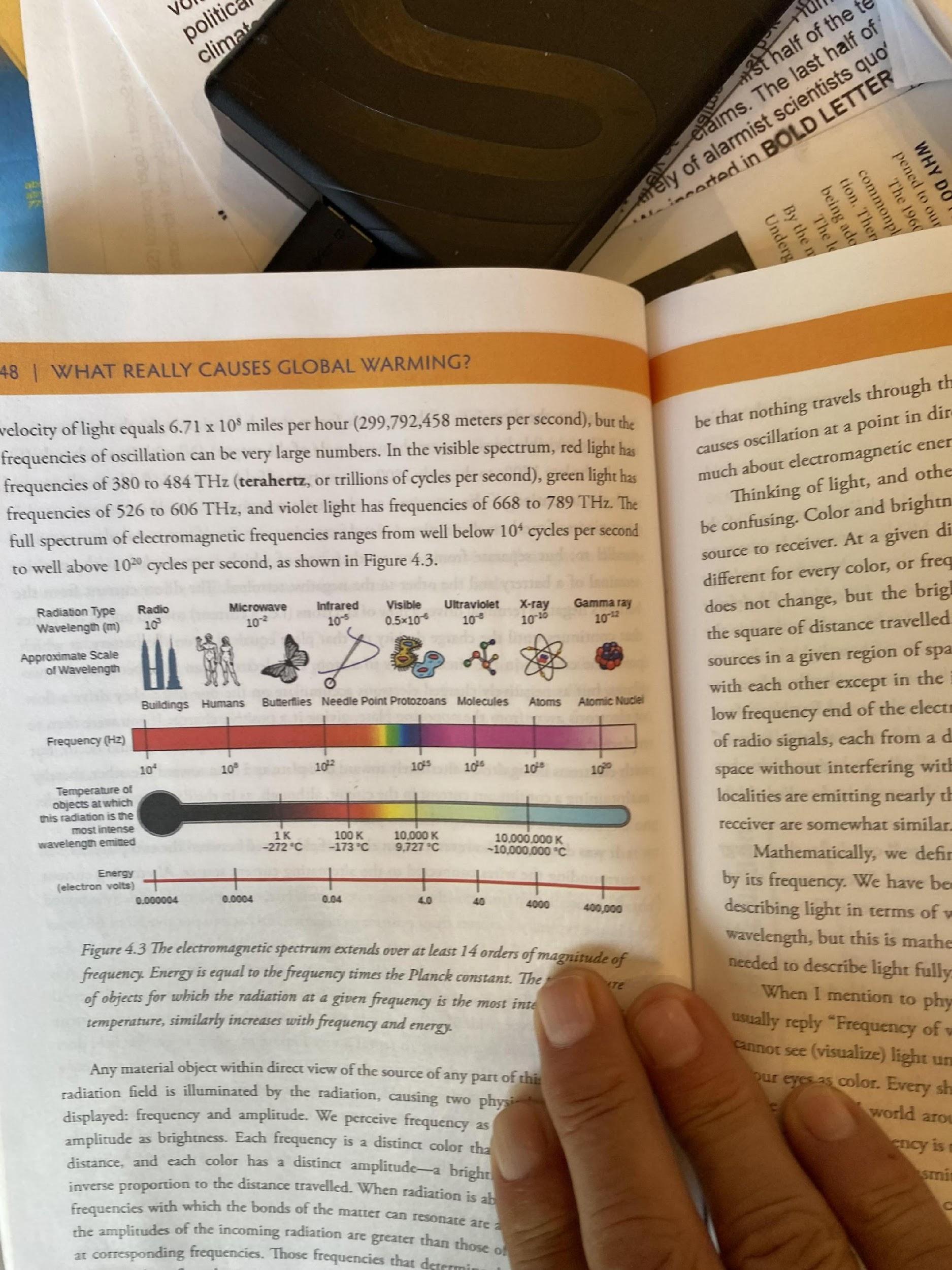
In the below chart, note the enormous frequency/Energy span of the EM spectrum, at least 14 orders of magnitude. For example, the color temperature of a typical house at about 30.6x10 second corresponds to less than -173 C. How is -173 C going to warm anything of consequence on earth?
ADDENDUM 1, NUMBERS HAVE MEANING. terawatts anything on earth. Then 20% is absorbed by and warms the atmosphere. Then as it cools, it exits directly into space, leaving 50% to be absorbed by the surface. So, our focus is on what happens to this 50%?
8% of the solar energy is shown as “convection,” which means that 8% is used to power the breezes, the winds, the hurricanes, the ocean currents, etc. This 8% powers the weather engines. After doing its weather thing, this atmospheric heat cools and radiates directly into space. 19% enters the water cycle. First, as water evaporates to water vapor, it heats the troposphere. Then as it cools by condensation/precipitation, this heat is radiated into space. Lastly, we see that 17% of the solar energy that heated the surface escapes directly to space through what NASA calls an atmospheric window. Meaning this IR radiation does not interact with the air molecules. solar energy arriving on earth that needs to go through the so-called “greenhouse effect, GHE” process before it can escape to space. 2
But it s electrical energy, and not heat. Enough to electrically power the equivalent of 44 USAs all at once.
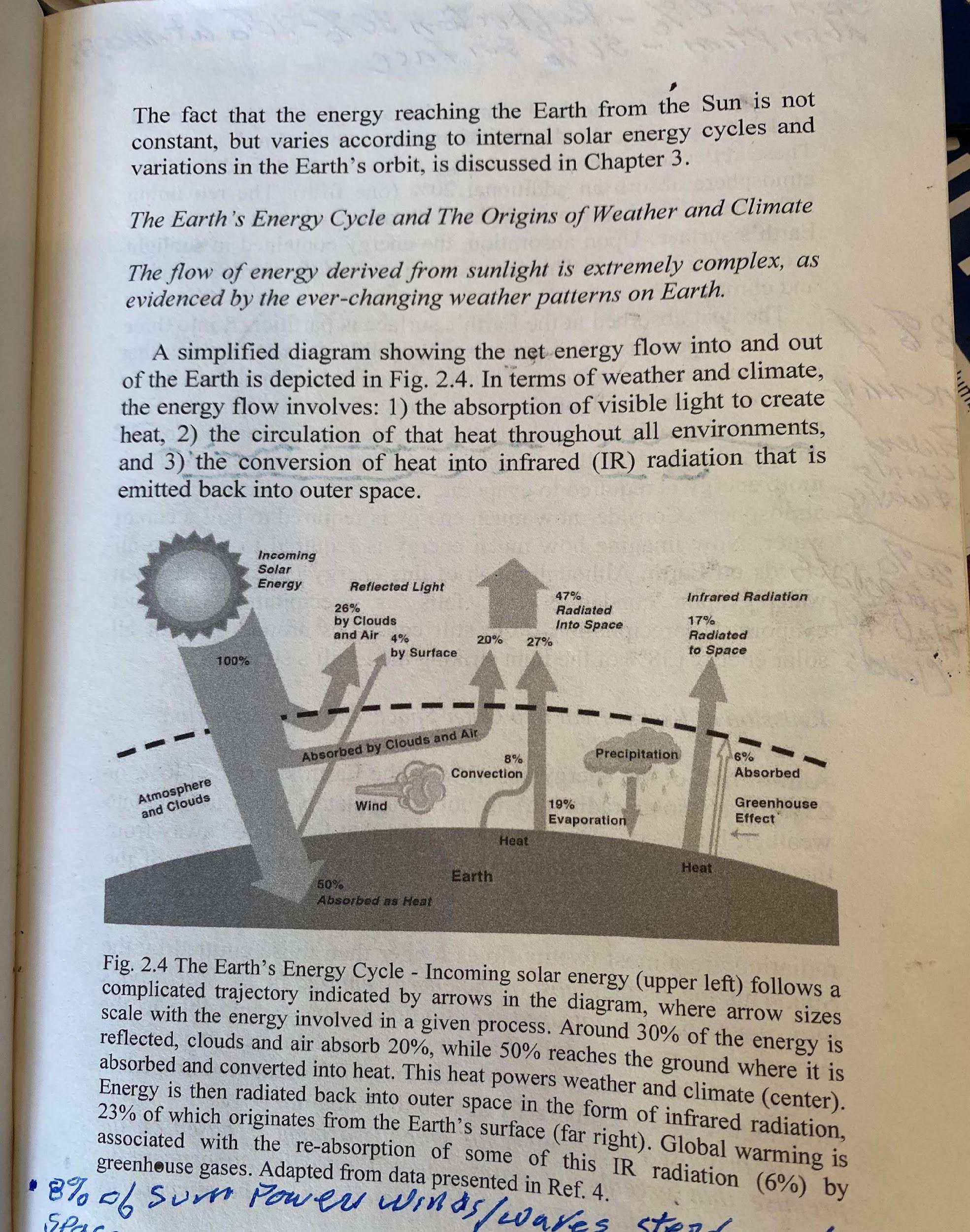
GHGs, as discussed in the main article. So, the question is, how does this 6% solar energy move from the surface through the atmosphere before it exits to space?
The below table, published by Dr. S Fred Singer in the Wall Street Journal, gives us a convenient summary of how this 6% goes through the GHE process and in the context of what parts are nature-made vs human-made. Dr. Singers did all the calculations considering each GHG's volume and adjusted it by its ability to absorb IR radiation and the energy carried by each IR frequency. Immediately we see that 95% of the GHE is captured by nature-produced water vapor. So, water vapor, and the liquid droplets and ice contained in the atmosphere as clouds, capture 95% of the 6% of outbound IR energy. We note that CO2 captures 3.618% and methane captures 0.36%.
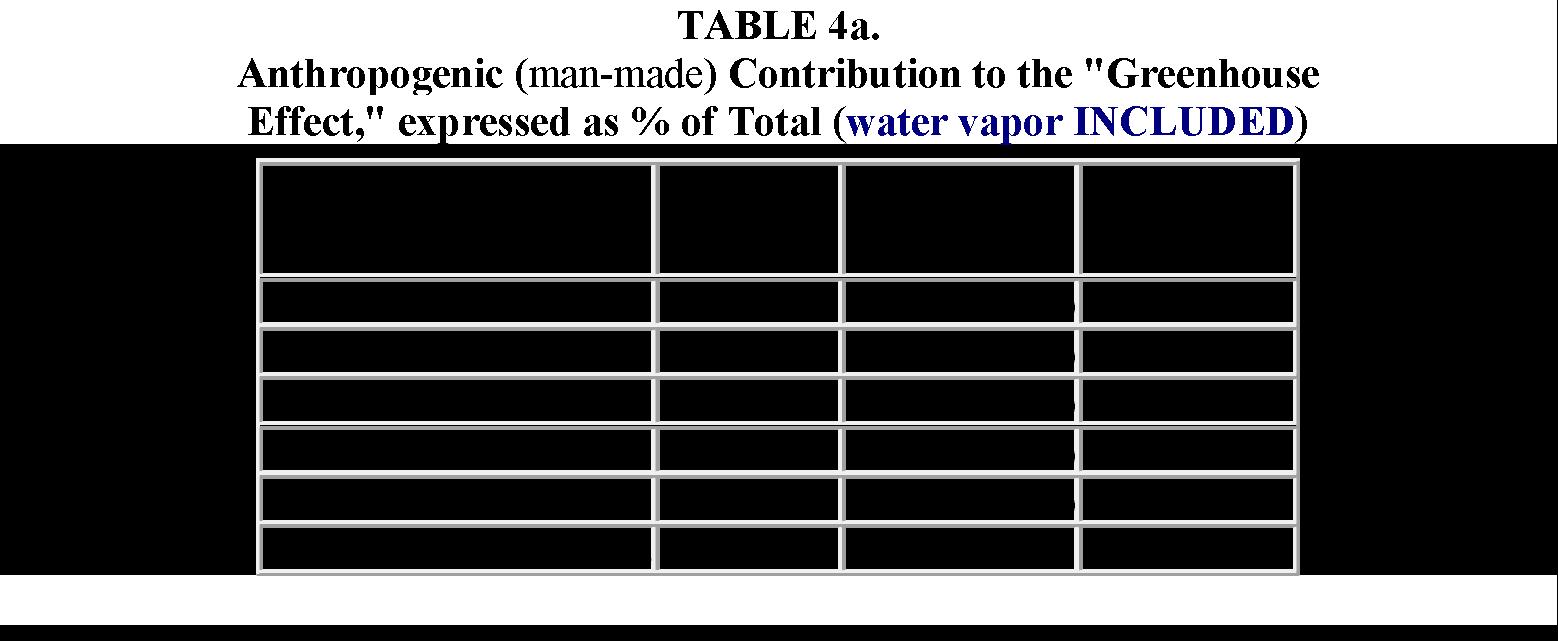
In total, we note that Nature-Made GHGs, including water vapor, capture 99.72% of the 6%, and human activities only capture a miserly 0.28%. The IPCC/NASA, knowing this dominance of Nature-Made water vapor, never mentions water vapor. Instead, IPCC/NASA only considers the other GHGs, and elaborates/embellishes the false dominance of CO2 (to demonize fossil fuels) and the equally false power density of methane (to demonize meat production.)
Read the entire Singer article at this site https://climatecite.com/water-vapor-rules-the-greenhouse-system/#:~:text=Water%20vapor%2C%2 0responsible%20for%2095,This%20is%20insignificant!
As detailed above, this GHE, and especially the human-made portion, contributes only a negligible amount of heating of the air and zero-heating of the surface. Regarding the air because of a) the low IR energy levels involved with these photon absorption of IR frequencies. And b) because of the very short time, fractions of a second, between the absorption of these IR photons by the greenhouse gasses and their re-emissions.
ADDENDUM-3, WHAT MAKES WATER VAPOR SUCH A DOMINATING GREENHOUSE GAS? On a molecule-by-molecule basis, water vapor is about two times more potent than methane
and CO2 in absorbing Infrared Radiation3. Water vapor volume also dwarfs all the other GHGs combined. Water vapor resides primarily in the lower troposphere. Tropospheric height varies with latitude. At the equator it’s 11-12 miles high and decreases exponentially as we approach the poles, where it may be only 4-6 miles high. Over the oceans, which cover 71% of the planet’s surface, water vapor accounts for about 3.2% of the air’s molecular concentration. Over lands, water vapor reduces significantly, especially over deserts and high elevations. On average, water vapor accounts for about 2.7% of the air by volume. Said differently, on average, water vapor constitutes 27,000 ppmv (parts per million by volume) compared to CO2 at 420 ppmv. For every CO2 molecule in the air there are 65 molecules of water vapor. Similarly, there are about 14,000 molecules of water vapor for every methane molecule. Combining the water vapor’s 2X absorption and the above concentrations, water vapor absorbs 130-times more heat radiated from the earth than CO2 and 28,000 more than methane in the troposphere.
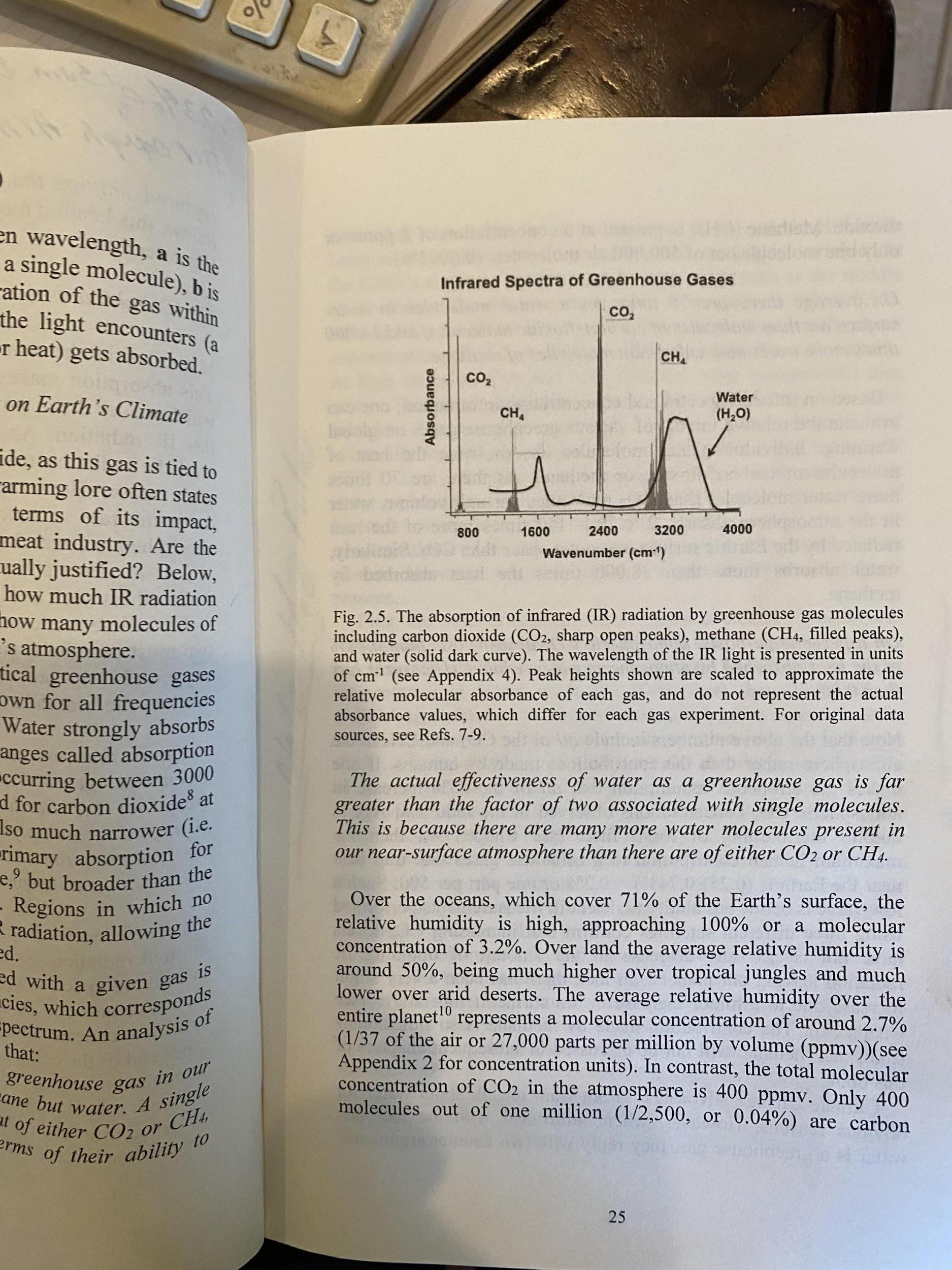
The figure below shows an inconvenient fact never mentioned in the media/press/IPCC. We see that CO2 absorbs primarily in two narrow wavelength bands, with the dominant at about 2,400 cm, or 15 micrometers. -1 However , CO2 must compete with the more dominant and voluminous water vapor molecules at this central wavelength. With methane, it’s even worse, as it must compete with water vapor for available IR across both wavelength bands.
Another inconvenient fact, about 50% of the water vapor molecules are found within an atmospheric height of about 1.2 miles. Above this, it exponentially decreases, and 99% of the atmospheric mass is within the first 20 miles of elevation. This means that water vapor has, or nearly has, already saturated before the IR radiation reaches the uppermost layers of the atmosphere where methane and CO2 do not compete with water vapor.
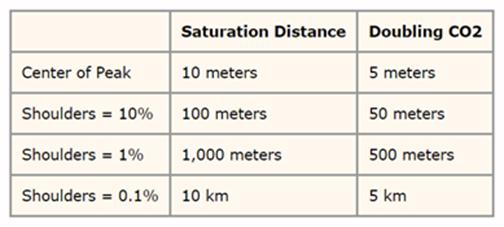
This saturation was tested by the Heinz Hug experiment when the CO2 concentration was 357 ppmv. He found that the central peak of the CO2 main band saturated at the height of only 10 meters above sea level and the extreme wings at the altitude of 10 Kilometers above sea level. He then repeated the experiment by doubling the CO2 to 714 ppmv and found that the saturation height had been cut in half at all elevations. See the table for the measured saturation heights and the diagram below for a visual representation of this CO2 15-um band.
Here are articles by Heinz Hug:
The Climate Catastrophe - A Spectroscopic Artifact?
Measurement of Carbon Dioxide Absorption
A Critical Review of the Hypothesis That Climate Change is Caused by Carbon Dioxide
Hug and Barrett versus IPCC Climate as a result of the earth heat reflection