Link: http://www.ferdinand-engelbeen.be/klimaat/co2_origin.html
Please see link above for source text.
ORIGIN OF THE RECENT CO2 INCREASE IN THE ATMOSPHERE
Ferdinand Engelbeen
September 16, 2007, updated October 11, 2022
In climate skeptics circles, there is rather much confusion about historical/present CO2 measurements. This is in part based on the fact that rather accurate historical direct measurements of CO2 in the atmosphere by chemical methods show much higher values in certain periods of time (especially around 1942), than the around 280 ppmv which is measured in Antarctic ice cores.
280 +/- 10 ppmv is assumed to be the pre-industrial amount of CO2 in the atmosphere during the current interglacial (the Holocene) by the scientific community. This is quite important, as if there were (much) higher levels of CO2 in the recent past, that may indicate that current CO2 levels are not from the use of fossil fuels, but a natural fluctuation and hence its influence on temperature is subject to (huge) natural fluctuations too and the current warmer climate is not caused by the use of fossil fuels.
To be sure about my skepticism: I like to see and examine the arguments of both sides of the fence, and I make up my own mind, based on these arguments. I am pretty sure that current climate models underestimate the role of the sun and other natural variations like ocean oscillations on climate and overestimate the role of greenhouse gases and aerosols. But I am as sure that the increase of CO2 in the atmosphere since the start of the industrial revolution is mainly from the use of fossil fuels.
There are several reasons why the hypothesis of large non-human CO2 variations in recent history is wrong (see my comment on the late Ernst Beck's compilation of historical measurements) and that most of the recent increase in CO2 in the atmosphere indeed is mainly man-made, but that needs a step-by-step explanation. Follow the steps:
1. Evidence of human influence on the increase of CO2 in the atmosphere.
1. The mass balance
2. The process characteristics
3. The 13C/12C ratio
4. The 14C/12C ratio
5. The oxygen use
6. The Ocean's pH and pCO2
7. The processes involved
2. Conclusion
3. Extra: how much human CO2 is in the atmosphere?
4. References
1. Evidence of human influence on the increase of CO2 in the atmosphere. 1.1. The mass balance
The amount of CO2 emitted by humans nowadays is about 9 PgC/yr (CO2 counted as carbon). The increase in the atmosphere is about 5 PgC/yr. That implies that there is little to no increase in the atmosphere due to other causes, or the amount in the atmosphere in the case of a positive natural unbalance should be higher than the emissions, not lower. No matter how large the natural inputs and outputs were, the net balance shows that the natural outputs every year near always were larger than the natural inputs. To show this over the past 60 years:
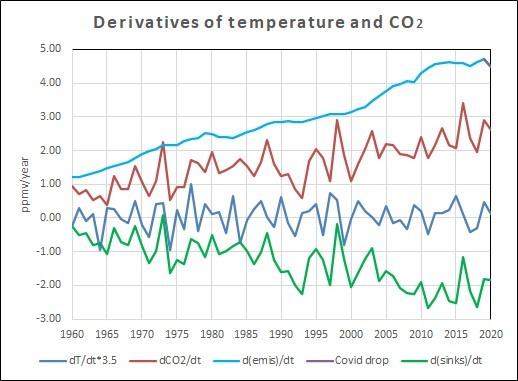
The graph shows the increase of CO2 emissions from fossil fuel burning and cement manufacturing (light blue), The small drop due to the Covid pandemic (purple) and the increase measured in the atmosphere (red).
The CO2 sinks in vegetation and oceans (green) is the difference between human emissions and the increase in the atmosphere. The temperature variability is enhanced with a factor 3.5 to show the same amplitude as for the CO2 variability.
Everything is expressed in ppmv/year (1 ppmv = 2.13 PgC = 2.13 GtC)
The graph is based on calculations of emissions, sampled from national inventories of fuel use and cement manufacturing (taxes!) and fuel burning efficiency [1]. In the best case, these are accurate, in the worst case, the emissions are underestimated, due to the human nature to avoid taxes and for political reasons (as probably is the case for China). That doesn't make any difference for the balance, as that only implies that the net natural sinks have to work harder to give the observed increase in the atmosphere... Inventories of CO2 in the atmosphere are based on very accurate measurements of CO2 at Mauna Loa [2] and several other "background"
stations. The difference between human CO2 emissions (expressed in PgC or GtC carbon per year - PgC/yr or GtC/yr) and CO2 increase in the atmosphere is what the oceans and/or vegetation extra absorb each year (other sinks are much smaller).
Interesting items in that graph:
- Nature was a net sink in all years except for a few borderline El Niño years.
- The trend of the CO2 increase in the atmosphere is around 50% of human emissions.
- That proves that nature is a sink for the other 50%, not the cause of the increase.
- The temperature derivative shows no trend, thus is not the cause of the exponential increase in the atmosphere.
- Temperature variability certainly is the cause of the variability in natural net sink capacity for CO2.
The partitioning between vegetation and ocean as sinks can be calculated from the oxygen balance. That is not of interest for the total balance, as in every year the sum of land+oceans was more sink than source, except for a few borderline El Niño years. But it is interesting anyway to know how much CO2 is absorbed by plants and how much by the (deep) oceans. That was done by Battle ea. and more recently by Bender ea. [3], based on changes of δ13C and oxygen content changes in the atmosphere over the last decade of the previous century and earlier estimates:
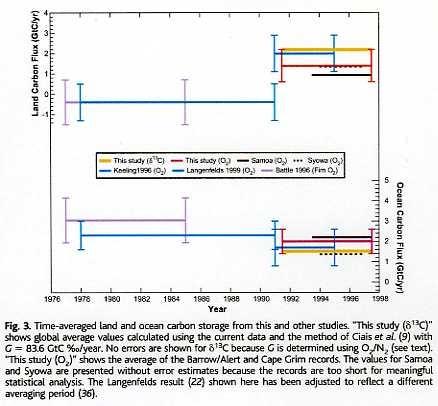
Here the detailed variability of the CO2 sink/source level in 1994-2002 by oceans and vegetation as measured from the O2/N2 balance by Bender ea.:
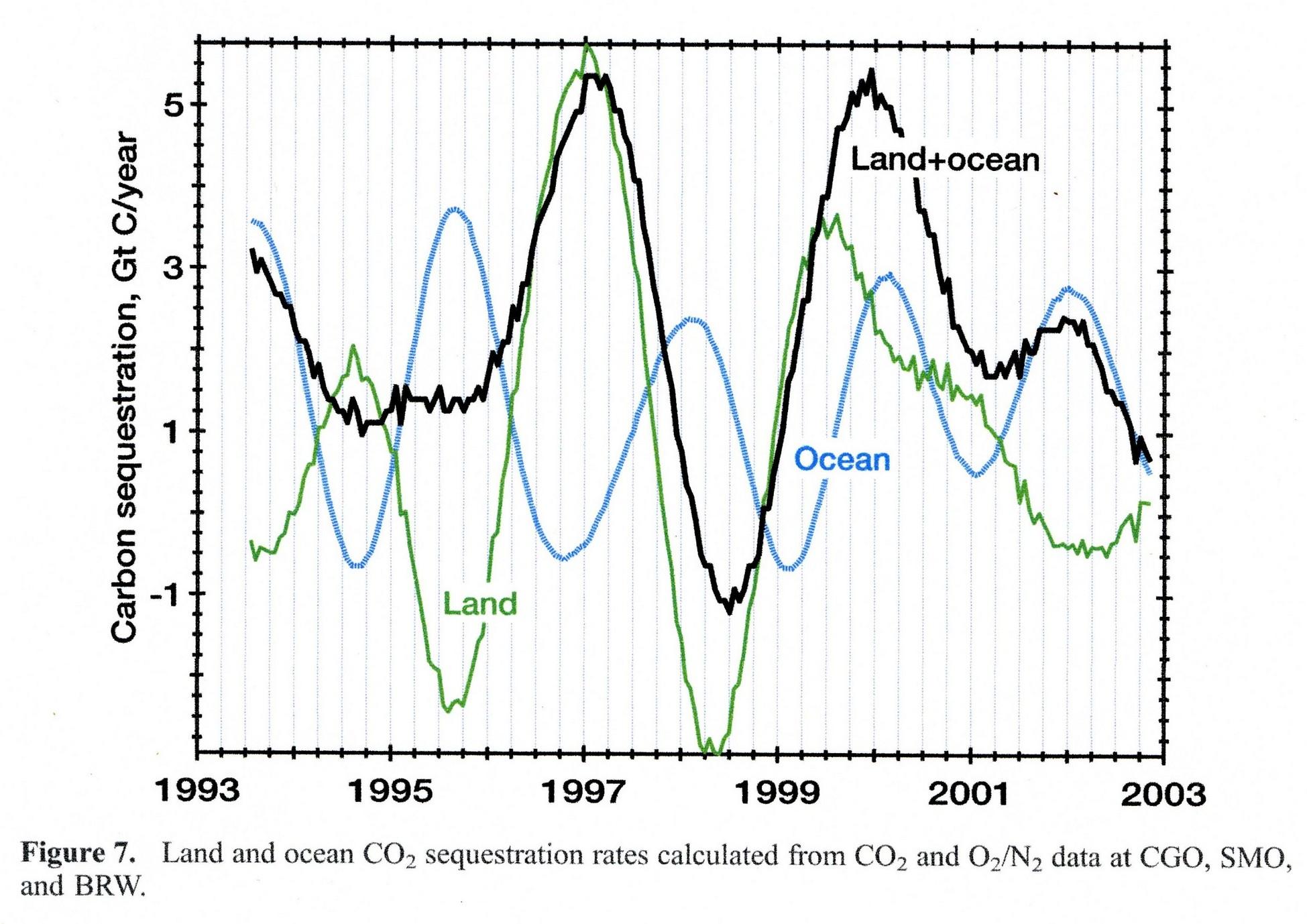
Vegetation by far shows the largest variability (caused by temperature variability), but even so, over the full period, in average it was a net sink for CO2 of around 0.5 ppmv/year (1 PgC/year).
The increase of CO2 in the atmosphere of around 105 ppmv (~225 PgC) since the accurate measurements at the South Pole and Mauna Loa started is near 90% of the increase since the start of the industrial revolution. This is based on measurements at a lot of places where "background" levels of CO2 can be measured (see "where to measure"), with minimum interference of local sinks and sources of CO2. The amount of CO2 in the atmosphere in pre-industrial times is based on ice cores, which are more smoothed: averaged over ~10 years for the high resolution ice cores of Law Dome over the past 150 years to ~560 years over the 800,000 years of Dome C. Nevertheless there are proxies with a better resolution in time, which also point to lower CO2 levels prior to the emissions.
As the first graph shows, in any year of the past over 60 years, the
emissions were larger than the increase in the atmosphere. That means that the total mass balance of all natural variables (temperature, ocean pH, vegetation) which influence CO2 levels, is always towards more sink than source for near every year.
The natural seasonal exchange between vegetation and oceans at one side and the atmosphere at the other side is estimated at about 210 GtC/yr. That is not of interest for what is the mass change over a year, as more CO2 than from the natural releases is absorbed within the same year. The variability in CO2 level after a full seasonal cycle of a year is not more than +/- 1.2 ppmv (+/- 2.5 PgC), mainly caused by temperature changes (El Niño, Pinatubo eruption). Thus the natural variations from year to year are way smaller than human emissions. No matter how high the natural seasonal turnover might be, in near all years over the previous over 60 years, the natural CO2 sinks were larger than the natural CO2 sources... Thus it is near impossible that natural sources were responsible for (a substantial part of) the increase of CO2 in the past over 60 years.
Except that - pure theoretically - a similar, but enormous, increase in natural emissions and sinks that parallels the human emissions may give the same result. Such an enormous (factor 4) increase in natural circulation needs a lot of proof
The increase should mimic the human emissions at exactly the same rate over exactly the same time span. Thus the near quadrupling of human emissions and increase rate in the atmosphere should be paralleled with a near quadrupling of the natural turnover: from an estimated 210 PgC in and out within a year to some 840 PgC in and out.
There is not the slightest indication of such an enormous increase in turnover, which rejects that theory.
The mass balance proves beyond doubt that human emissions are the main cause of the increase of CO2, at least over the past over 60 years. But there are more indications for that...
1.2. The process characteristics:
Here is a graph of the (global) temperature trend, the cumulative amount of emissions and the increase of CO2 in the atmosphere (1900-2011):
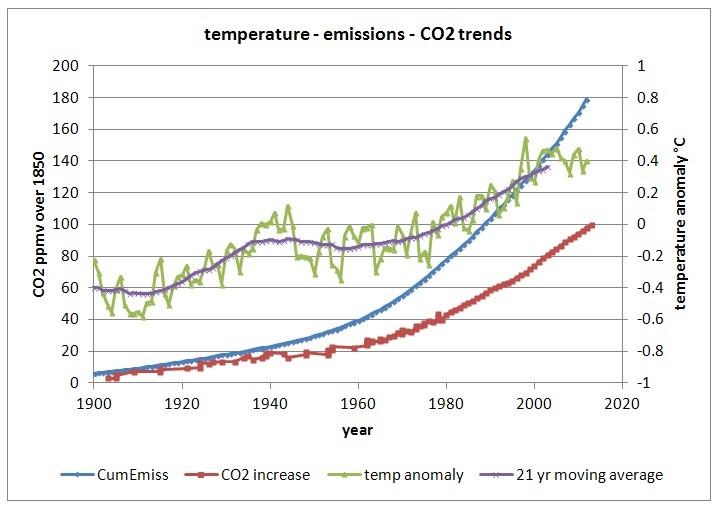
The temperature trend is the yearly averaged global (sea + land) temperature, according to the Hadley Center in the UK [13]. The emissions are from the international inventory data base (DOE, USA, [1]). The CO2 levels in the atmosphere pre-1959 are from ice cores (Law Dome, Siple Dome, [11]) and from 1959 on, the data are taken from measurements at Mauna Loa (Hawaii, [12]). Baseline of the CO2 level is 300 ppmv around 1900 (it was around 285 ppmv in 1850). The 21 year moving average is added, as some have found a good correlation between the 21 year moving average and the CO2 increase. That is right after 1980 (where the correlation was based on), but fails for the whole 1900-current period.
As one can calculate, the correlation between temperature and CO2 levels in the atmosphere is rather weak (corr.: 0.881; R^2: 0.776), and from the detailed T/dCO2 graphs, one can see that a huge change in temperature in a certain year has little influence on CO2 levels, compared to the influence of the temperature change over the whole trend, if temperature was responsible for both the short term variability and the longer term increase:
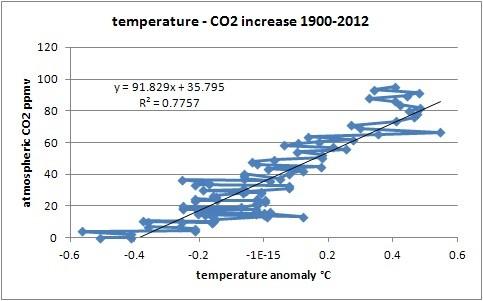
This indicates that temperature is not the cause of the overall increase, but the cause of the variability (+/- 1.2 ppmv) around the increase (currently around 2 ppmv/yr). See more on the detailed process page (see chapter 1.7).
On the other side, the correlation between cumulative emissions and increase in the atmosphere is a near-fit (corr.: 0.999; R^2: 0.998) over the whole period:
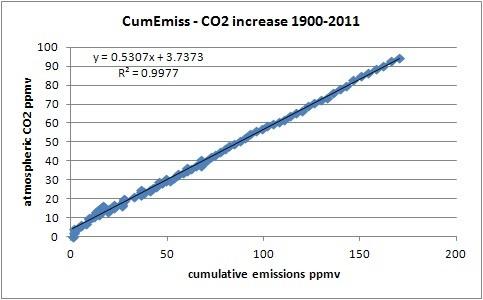
Although one need to be carefull to avoid spurious correlations between accumulated values, in this case accumulation of the emissions is waranted, as for every year in the past 60 years there was a residual mass increase of CO2 in the atmosphere caused by human emissions. Thus human emissions do accumulate in the atmosphere, as mass (that doesn't mean that the original CO2 molecules from fossil fuel use still are all in the atmosphere!). The ratio is 50-55% between increase in the atmosphere and what was emitted. This points to a simple linear first order process, directly related to the partial pressure difference between CO2 in the atmosphere and CO2 in the oceans (and vegetation). The higher the CO2 content in the atmosphere, the higher the push to drive CO2 into the oceans. Currently, the partial pressure difference (pCO2) between the atmosphere and the oceans is about 7 ppmv [4], based on ship's surveys and buoys. The increase of the pCO2 difference causes more and more uptake of CO2 by the oceans (and similarly in vegetation).
Again, this clear relationship points to a direct influence of the emissions on the increase in the atmosphere. There is no known natural process that is able to force CO2 into the atmosphere exactly in the same ratio as is the case here for the emissions. One only need to look at the difference in variability of the temperature curve (which has a limited short-term influence on CO2 levels of 3-4 ppmv/K) with the smoothness of the
emissions curve...
This adds to the weight of the emissions as main cause of the increase in the atmosphere.
1.3. The 13C/12C ratio:
The carbon of CO2 is composed of different isotopes. Most is of the lighter type: 12C (that has 6 protons and 6 neutrons in the kernel), and about 1.1% is heavier: 13C (has 6 protons and 7 neutrons in the kernel). There also is some 14C (6 protons and 8 neutrons in the kernel), which is continuously formed in the upper stratosphere from the collisions of nitrogen with cosmic rays particles. This type of carbon (also formed by above-ground atomic bomb experiments in the 1950's) is radio-active and can be used to determine the age of fossils back to about 60,000 years.
One can measure the 13C/12C ratio and compare it to a standard. In the past, the standard was some type of carbonate rock, called Pee Dee Belemnite (PDB). When the standard rock was exhausted, this was replaced by a zero definition in a Vienna conference, therefore the new standard is called the VPDB (Vienna PDB). Every carbon containing part of any subject can be measured for its 13C/12C ratio. The comparison with the standard is expressed as per mil δ13C: (13C/12C)sampled – (13C/12C)standard δ13C = —————————————————————— x 1,000 (13C/12C)standard
Where the standard is defined as 0.0112372 parts of 13C to 1 part of total carbon. Thus positive values have more 13C, negative values have less 13C. Now, the interesting point is that vegetation growth in general uses by preference 12C, thus if you measure δ13C in vegetation, you will see that it has quite low δ13C values. As almost all fossil fuels were formed from
vegetation (or methanogenic bacteria, with similar preferences), these have low δ13C values too. Most other carbon sources (oceans, carbonate rock wearing, volcanic out gassing,...) have higher δ13C values. For a nice introduction of the isotope cycle in nature, see the e-book of Anton Uriarte Cantolla [5].
This is an interesting feature, as we can determine if CO2 levels in the atmosphere (which is currently below -8 per mil VPDB) were influenced by vegetation decay or fossil fuel burning (both about -24 per mil) towards the negative side or by ocean degassing (0 to +1 per mil in general, but with fractioning at the water-air border) towards the positive side as largest possible sources.
From different CO2 base stations, we do not only have CO2 measurements, but also δ13C measurements. Although only over a period of about 25 years, the trend is clear and indicates an extra source of low δ13C in the atmosphere.
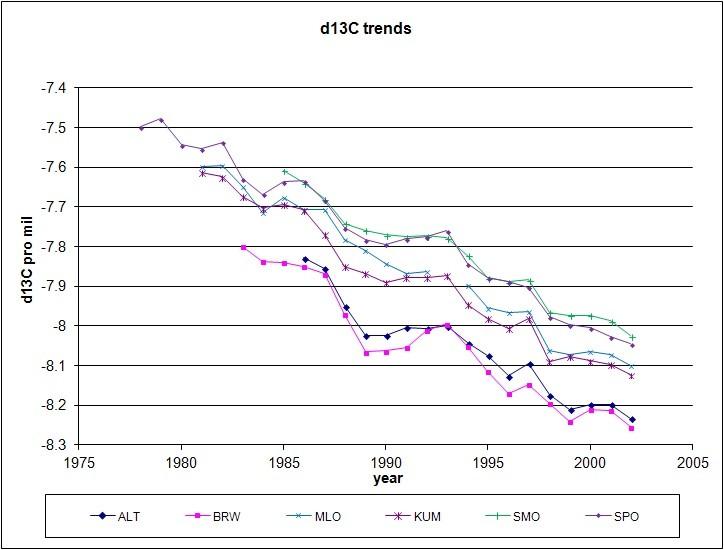
Trends in δ13C from direct measurements of ambient air at 10 base stations.
Data from [6].
ALT=Alert; BRW=Barrow; MLO=Mauna Loa; KUM=Cape Kumukahi; SMO=Samoa; SPO=South Pole.
Again, we see a lag in the trends with altitude and NH/SH border transfer and less variability in the SH. Again, this points to a source in the NH. If that is from vegetation decay (more present in the NH than in the SH) and/or from fossil fuel burning (90% in the NH) is solved in the investigation of Battle ea. [3], where it is shown that there is less oxygen used than can be calculated from fossil fuel burning. Vegetation thus produces O2, by incorporating more CO2 than is formed by decaying vegetation (which uses oxygen). This means that more 12C is incorporated, and thus more 13C is left behind in the atmosphere. Vegetation is thus relative depleting the atmosphere of 12C vs. 13C and thus not the cause of decreasing 13C/12C ratio's.
And we have several other, older measurements of δ13C in the atmosphere: ice cores and firn (not completely closed air bubbles in the snow/ice). These align smooth-less with the recent air measurements. There is a similar line of measurements from coralline sponges and sediments in the upper oceans. Coralline sponges grow in shallow waters and their skeleton is built from CO2 in the upper ocean waters, without altering the 13C/12C ratio in seawater at the time of building. The combination of atmospheric/firn/ice and ocean measurements gives a nice history of δ13C changes over the past 600 years:
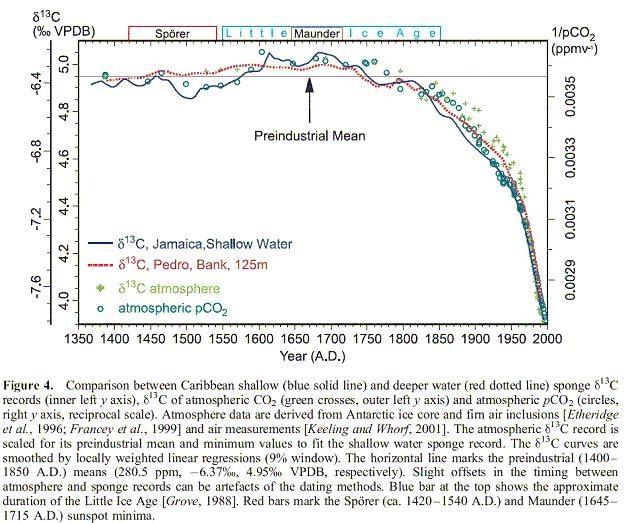
Figure from [7] gives a comparison of upper ocean water and atmospheric δ13C changes.
What we can see, is that the δ13C levels as well as in the atmosphere as in the upper oceans start to decrease from 1850 on, that is at the start of the industrial revolution. In the 400 years before, there is only a small variation, probably caused by the temperature drop in the Little Ice Age. Longer term measurements of the δ13C ratio in CO2 from ice cores show that over the whole Holocene, the variations were not more than +/- 0.2 per mil. Even the change from a glacial to an interglacial period did not give more than 0.2 per mil δ13C change.
Again this is a good indication of the influence of fossil fuel burning...
1.4. The 14C/12C ratio:
14C is a carbon isotope that is made in the atmosphere by the impact of cosmic rays. It is an unstable (radioactive) isotope and breaks down with a half-life time of about 6,000 years. 14C is used for radiocarbon dating of not too old fossils (maximum 60,000 years). The amount of 14C in the atmosphere is variable (depends of the sun's activity), but despite that, it allows to have a reasonable dating method. Until humans started to burn fossil fuels...
The amounts of 14C in the atmosphere and in vegetation is more or less in equilibrium (as is the case for 13C: a slight depletion, due to 12C preference of the biological processes). But about halve of it returns to the atmosphere within a year, by the decay of leaves. Other parts need more time, but a lot is going back into the atmosphere within a few decades. For the oceans, the lag between 14C going into the oceans (at the North Atlantic sink place of the great conveyor belt) and its release around the equator is 500-1500 years, which gives a slight depletion of 14C, together with some very old carbonate going into solution which is completely 14C depleted. In pre-industrial times, there was an equilibrium between cosmogenic 14C production and oceanic depletion.
Fossil fuels at the moment of formation (either wood for coal or plankton for oil) incorporated some 14C, but as these are millions of years old, there is no measurable 14C anymore left. Just as is the case for 13C, the amount of CO2 released from fossil fuel burning diluted the 14C content of the atmosphere. This caused problems for carbon dating from about 1890 on. Therefore a correction table is used to correct samples of after 1890. In the 1950's another human intervention caused trouble for carbon dating: nuclear bomb testing induced a lot of radiation, which nearly doubled the atmospheric 14C content. Since then, the amount is fast reducing, as the oceans replace it with "normal" 14C levels. The half life time is about 14
years.
Again, this adds to the evidence that fossil fuel burning is the main cause of the increase of CO2 in the atmosphere...
1.5 The oxygen use:
To burn fossil fuels, you need oxygen. As for every type of fuel the ratio of oxygen use to fuel use is known, it is possible to calculate the total amount of oxygen which is used by fossil fuel burning. On the other hand, the real amount of oxygen which is used can be measured in the atmosphere. This is quite a challenging problem, as the change in atmospheric O2 from year to year is quite low, compared to the total amount of O2 (a few ppmv in over 200,000 ppmv). Moreover, as good as for CO2 as for oxygen, there is the seasonal to year-by-year influence of vegetation growth and decay. Only since the 1990's, oxygen measurements with sufficient resolution are available. These revealed that there was less oxygen used than was calculated from fossil fuel use. This points to vegetation growth as source of extra O2, thus vegetation is a sink of CO2, at least since 1990. The combination of O2 and δ13C measurements allowed Battle e.a. [3] to calculate how much CO2 was absorbed by vegetation and how much by the oceans. The trends of O2 and CO2 in the period 1990-2000 can be plotted in a nice diagram:
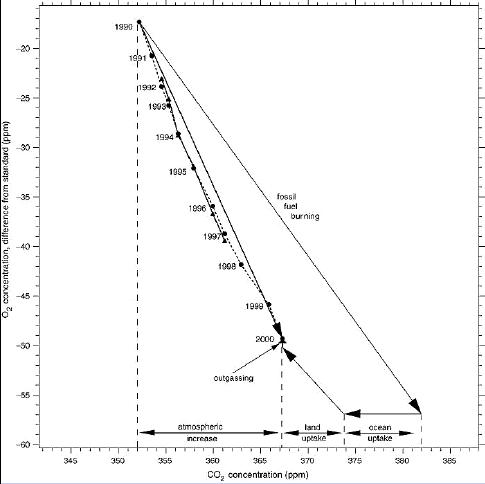
O2-CO2 trends 1990-2000, figure from the IPCC TAR [8]
This doesn't directly prove that all the CO2 increase in the atmosphere is from fossil fuel burning, but as both the oceans and vegetation show a net uptake, and other sources are much slower and/or smaller (rock weathering, volcanic out-gassing,...):
There is only one fast possible source: fossil fuel burning.
1.6 The ocean's pH and pCO2:
If CO2 is increasing in the atmosphere with 50-55% of the accumulated emissions, a part is absorbed by vegetation (see chapter 1.4), another part is
absorbed by the oceans. When CO2 is absorbed by the oceans, this is partially in solution in its original form, but most (99%) of it reacts with available carbonate ions to form bicarbonates. Between 1751 and 1994 the average surface ocean pH is estimated to have decreased from approximately 8.179 to 8.104 (a change of -0.075), based on recent and older oceanic surveys [9].
The ocean's pH can be interpreted the other way out: if for whatever reason the pH is reduced (e.g. by an undersea volcanic event with lots of SO2), this leads to an important increase of pCO2(oceans) and may release a lot of oceanic CO2 into the atmosphere.
That is in contradiction with the observed change in 13C: if oceanic CO2 (from the deep oceans to the surface and further into the atmosphere) was released, this should increase the 13C/12C ratio of both the upper oceans and the atmosphere, while we see the reverse trend happening. Moreover, the release of more CO2 from the upper oceans due to a lower pH would reduce the total amount of carbon (DIC: dissolved inorganic carbon, that is CO2 + bicarbonates + carbonates) in the ocean's surface layer. But we see the reverse trend: DIC is increasing over time [10]. Thus the increase of atmospheric CO2 is going into the oceans, not reverse.
Further, another part of the oceanic survey compares the pCO2 of the atmosphere with that of the oceans at different latitudes in different oceans. This shows that there are huge differences in oceanic pCO2 at different latitudes due to changes in temperature and DIC. This gives a continuous release of CO2 in the tropics (pCO2 of maximum 750 µatm in the upper oceans vs. about 410 µatm for the atmosphere) and a continuous sink of CO2 in the polar oceans, especially in the North-East Atlantic (minimum 150 µatm vs. 410 µatm). That gives a continuous CO2 flux of about 40 PgC/yr between the tropics and the poles through the atmosphere, where the polar waters with extra CO2 sink and return some 1,000 years later near the equator.
The oceans at mid-latitudes are seasonal emitters/absorbers of CO2, depending of the water temperature and sea life (plankton). The average yearly global difference of pCO2(atmosphere) - pCO2(oceans) is about 7
ppmv. That means that in average more CO2 is going from the atmosphere into the oceans than reverse [4]. Moreover, different surveys over time revealed that ocean parts which were net sources of CO2 gradually changed into net absorbers.
Although the ocean pCO2 data are scattered in time and covered area, the trends are clear that the average (increasing) flow of CO2 is from the atmosphere into the oceans and not reverse.
This adds to the overall evidence that human emissions are the main cause of the increase of CO2 in the atmosphere.
1.7 The processes involved:
Temperature and CO2 levels are quite tightly coupled over very long periods, as can be seen in ice cores. There is a quite constant ratio between the temperature proxies ( δD and δ18O) and the CO2 level over the 420,000 years Vostok period, recently confirmed by the 800,000 years record of the Dome C ice core:
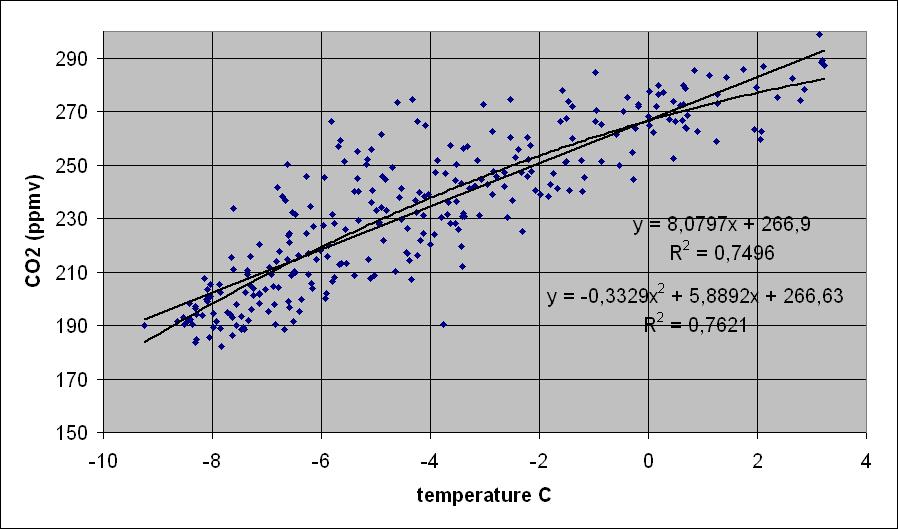
Vostok ice core ratio between CO2 levels and calculated temperature from the δ18O proxy [11].
Most of the deviations are from the CO2 lag after a temperature change which is much larger during a cooling period than during a warming period.
The temperature proxies of Vostok and other inland ice cores are measured in ice: the heavier isotopes are increasing in ratio with higher seawater temperatures at the area of water evaporation and (mainly) by the temperatures in the atmosphere where the snow is formed. Anyway, there is a clear relationship between temperature and CO2 levels (some 8 ppmv/K for Antarctic temperatures) , where CO2 lags with 800 +/- 600 years during a deglaciation and several thousands of years during a decrease towards a glaciation.
The same ratio can be seen in the medium resolution (~20 years averaging) Law Dome DSS ice core:
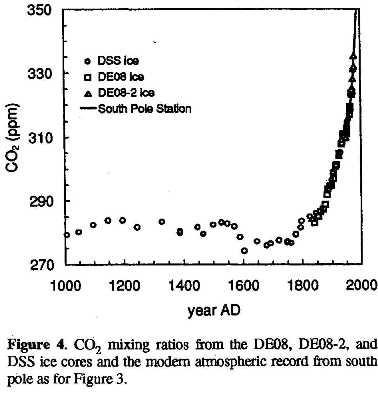
Law Dome ice cores CO2 levels according to Etheridge e.a. 1996 [12].
If we may assume that the temperature difference between the MWP (Medieval Warm Period) and LIA (Little Ice Age) was around 0.8 K (Moberg, Esper and several others) and the drop of CO2 was 6 ppmv (with a lag of ~50 years), then we are again around 8 ppmv/K for the CO2temperature ratio.
On shorter periods there is a direct influence of a temperature rate of change variability on the CO2 rate of change variability:
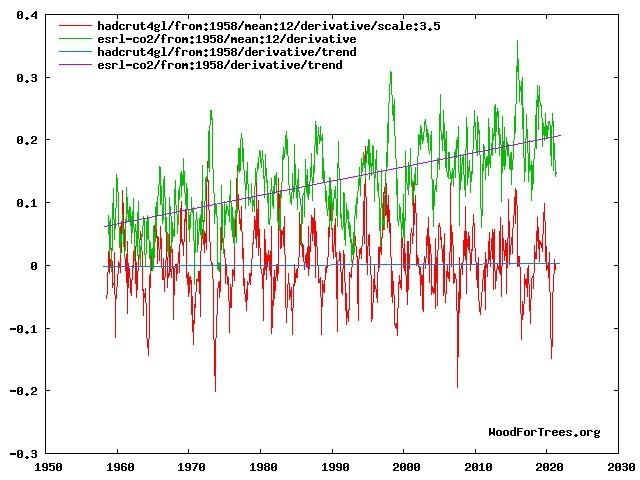
dT/dt versus dCO2/dt plot from the Wood for Trees tool [13]
There is a small (pi/2) lag between temperature changes and CO2 changes. That is a matter of process dynamics: it takes time to increase/decrease the CO2 output from an increase in temperature and back. For dynamic changes with a relative high frequency, it can be mathematically shown that this gives a lag of pi/2 of the frequency. As taking the derivative of both the temperature and the CO2 changes shifts both pi/2 backwards, the same lag of pi/2 still holds for the derivatives.
Further I have used a factor 3.5 for the temperature plot: that makes that the amplitudes of the temperature and CO2 rate of change are similar. A similar fortifying factor can be derived for the global CO2 change and the temperature change over the seasons: a factor 4-5 ppmv/K between CO2 and seasonal temperature variability.
More important than the lag is the fact that there is no trend in the derivative of the temperature, while there is a trend in the CO2 rate of
change. That is because the temperature increased more or less linear, while both the CO2 emissions and the CO2 increase in the atmosphere are slightly quadratic increasing over time.
Some insist that the increase of CO2 in the atmosphere is a direct result of the slight temperature change (0.6°C) over the past 50+ years. That is because if you plot the temperature trend with a factor and an offset with the derivative of CO2, that can give a perfect match in variability timing and trend. But that is a spurious match: the perfect timing is because there is a pi/2 lag of CO2 changes after temperature changes on short term. If you take the derivative of CO2, there is a pi/2 shift in time and thus a perfect timing with the temperature variability. Thus while mathematically possible, there is not the slightest resemblance to a physical process, except if and only if both the short term variability and the longer term trend have the same cause, but that is proven wrong.
Let us see what causes the short term variability. First the seasonal changes:
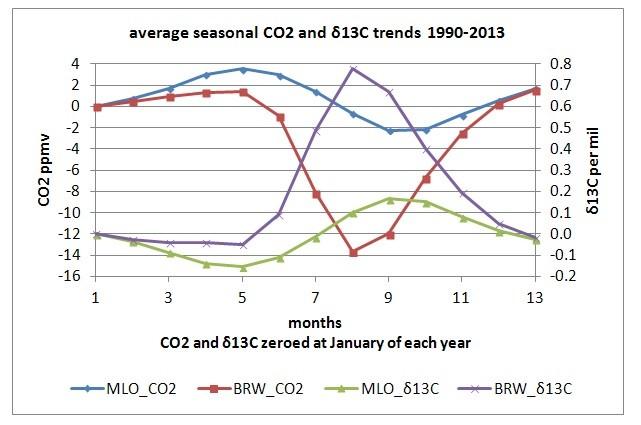
CO2 and δ13C changes over the seasons for Barrow and Mauna Loa, data
from [6]
As one can see, increasing temperatures in spring start to grow leaves in the extra-tropical forests, which reduce CO2 levels and specifically 12CO2 levels, leaving relative more 13CO2 in the atmosphere. That gives an increase in δ13C in spring-summer, while the opposite happens in fall-winter.
Now what causes the year by year variability in the rate of change:
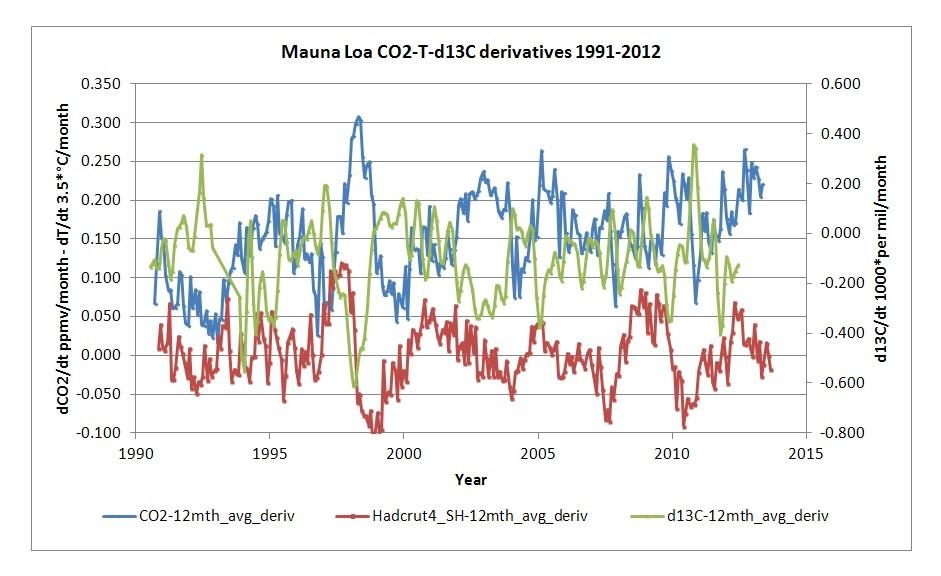
Temperature, CO2 and δ13C rate of changes over time. Data from [6] and [13]
As one can see: the year by year variability again is caused by temperature, but the CO2 result is opposite to the seasonal changes: an increase of the temperature rate of change gives an increase in CO2 rate of change. But again it is vegetation which makes the difference: the δ13C rate of change is opposite to the CO2 rate of change. In this case it is thought that vegetation in the tropical forests is suffering from elevated temperatures (and changed rain patterns/droughts) during El Niño's.
But even as vegetation is responsible for most of the short term variability, it is not responsible for the trend in CO2: vegetation is a proven sink for CO2, as can be calculated from the oxygen balance (see chapter 1.5). The earth is greening...
A more detailed process analyses is made at the CO2 variability page.
Thus while temperature changes are responsible for the short term and pre-industrial (very) long term variability, temperature is not responsible for the current increase of CO2 in the atmosphere.
2. Conclusion
From the available evidence it is quite clear that human emissions are the main cause of the increase of CO2 in the atmosphere. There is a small influence of temperature on this increase, as warmer oceans emit some CO2 (but warmer land absorbs more CO2 in vegetation!). The influence of temperature is limited: based on the variability of the CO2 increase around the trend, the short-term (seasons to 2-3 years) ratio is 3-5 ppmv/K (based on the seasonal and opposite temperature related 1992 Pinatubo and 1998 El Niño events). The very long term influence of temperature on CO2 levels (Vostok ice core) is about 8 ppmv/K for Antarctic temperatures. For global temperatures that makes about 16 ppmv/K as Antarctic temperatures change twice as fast as global temperatures. Thus at maximum, the influence of temperature on the current increase since the LIA is 0.8 ºC x 16 ppmv/K = 13 ppmv of the over 120 ppmv increase since the start of the industrial revolution.
That nicely coincides with Henry's law, which gives an in/decrease of 12-16 ppmv/K for the current average ocean surface temperature when in dynamic equilibrium with the atmosphere.
There are only two fast main sources of CO2 to the atmosphere, besides the burning of fossil fuels: oceans and vegetation. Vegetation is not a source of CO2, as the oxygen deficiency (see chapter 1.5) showed. Neither are the
oceans, as the δ13C trend (see chapter 1.3) and the pCO2/pH trends (see chapter 1.6) show. This is more than sufficient to be sure that human emissions are the cause of most of the increase of CO2 in the atmosphere over the past 1.7 century.
Thus we may conclude:
All observed evidence from measurements all over the earth show with overwhelming evidence that humans are causing the bulk of the recent increase of CO2 into the atmosphere.
But...
That humans are the cause of the recent increase of CO2 doesn't tell anything about the influence of increased CO2 on temperature!
3. Extra: how much human CO2 is in the atmosphere?
A lot of people is confused about this point: Only about 10 percent of the atmosphere is currently from human origin, while there is a 35% increase in CO2 amount. That is because every year about 210 PgC of CO2 (about 25% of the total CO2 content) is exchanged between the atmosphere and oceans/vegetation. That means that every single CO2 molecule from human or natural origin has a 25% chance per year to be incorporated in vegetation or dissolved into the oceans. This makes that the half life time (the "residence" time) of human CO2 in the atmosphere is only about 4 years. This was confirmed by the fate of 14C, increased due to atomic bomb testing, after the tests stopped. Thus if humans emit 8 PgC in a given year, next year some 6 PgC is still of human origin, the rest was exchanged with CO2 from the oceans and vegetation. The second year, that still is 4.5 PgC, then 3.4 PgC, etc... This is not accurate, as some of the "human" CO2 comes back next year(s), especially from vegetation, as much of vegetation is oneyear old leaves, which rotting returns a high part of CO2 incorporated in previous years. This is less the case for the oceans, where more of the
absorbed CO2 disappears into the deep oceans, where it isn't directly traceable anymore. There are techniques to follow human CO2 even there, where they use other recent human-made gases like CFC's and the extra 14CO2 spike from the atomic bomb tests 1945-1960 to track the past emissions. Anyway the "half life", that is the time period in which half of the human induced individual CO2 molecules disappears, is around 4 years.
Over longer periods, humans continue to emit (currently about 9 PgC/year) CO2. The accumulation over the last years thus is 9 + 6.8 + 5.1 + 3.8 + 2.8 +... or about 83 PgC from the emissions over the past 170 years, including what returned from vegetation and the ocean surface, based on the observed 13C/12C ratio. That is only 10% of the current atmosphere... Some conclude from this that humans are only responsible for 10% of the CO2 increase and thus, as far as that influences temperature, also only for 10% of the temperature increase. But that is a wrong assumption...
The previous paragraphs are about how much human induced CO2 still is in the atmosphere. That is about the origin and fate of individual CO2 molecules, which atmospheric lifetime is governed by the seasonal turnover (back and forth flows) of about 210 PgC in/out the atmosphere from/to oceans and vegetation, and has nothing to do with the fate of the extra amount of CO2 (as mass) that humans emit, neither with the increase of total amount of CO2 in the atmosphere as result of that. The latter is governed by the net amounts which year by year are incorporated into oceans and vegetation. That is only 1-7 GtC/year (variable due to temperature variability) or in average 50-55% of the emissions. The half life time of this extra CO2 (as mass) is much longer than the half life time of an individual CO2 molecule: around 35 years [14]. Thus if we should stop all CO2 emissions today, then the increase of 120 ppmv since the start of the industrial revolution would be reduced to 60 ppmv after some 35 years, further to 30 ppmv after 70 years and 15 ppmv after 105 years...
The IPCC comes with much longer half life times, according to the Bern model. This is a combination of relative fast (upper oceans), slower (deep oceans and more permanent storage in the biosphere) and very slow (rock
weathering) sinks for the extra CO2. They assume that the first, relative fast, sinks of CO2 will reduce in capacity over the years. That is only true for the ocean surface layer, which follows the atmosphere quite rapidly (1-3 years), but is saturated at 10% of the change in the atmosphere, due to the buffer/Revelle factor. Some media talk about hundreds to thousands of years that the extra CO2 will reside in the atmosphere. That is true for the last part of the curve, as the smaller amounts of CO2 are getting slower and slower into the sinks. But the bulk (85 %) of the extra CO2 will disappear within 105 years as there is no sign of a slowdown of the sink capacity of the deep oceans and vegetation.
From several discussions, I know that it is quite difficult to understand the two different mechanisms which govern the fate of human CO2 in the atmosphere: the fate of individual molecules, governed by exchange rates ("turnover") and the fate of an increase in total CO2, governed by absorption rates (sink capacity). Here I try to give an example of how to see the difference:
Let us say that you start the day in your shop with € 1000.00 in your cash register, all euro's are in 1 euro pieces, all stamped in France. During the day, you have about € 200.00 expenses from goods delivery and you receive € 192.00 back from sales. At the end of the day, you have € 992.00 in your cash register, not only with French euro's anymore, but part of them are now stamped in Germany, Belgium, Spain,... Next day, you add some € 16.00 from your own personal money, only euro's stamped in The Netherlands, to the cash register to start a fresh day with € 1008.00. During that day the same happens as in the previous day: € 200.00 expenses, € 192.00 income. Thus the day ends with € 1000.00 in your cash register, with now an increase of Netherlands euro's (but less than what you have added). Next day, you add € 16.00, again in Netherlands euro's and end the day with € 1008.00. You can repeat that for a few weeks, until you run out of personal money... Over several weeks, you will see that the number of euro's from The Netherlands slowly increases in ratio, but that the
increase of the total amount in the cash register is only 50% of what you add on a daily base. That means that you have a problem: your expenses are larger than your income. That also means that despite the huge daily exchanges (which result in a rapid reduction of Netherlands euro's), that has no influence at all on the total amount of money you have at the end of the day, only what you have added yourself and the (negative) difference of the total balance counts. In this case there is no (net) addition of money from your daily bussiness, only a daily loss.
The difference between the two half life times of CO2 is comparable to at one side the fate of the number of Netherlands euro's in the cash register at the end of each day (which depends of the amounts which were added and exchanged that day and the composition of the exchanges), while on the other side, the second half time only depends of the total sum of euro's that is added and what rests from all transactions at the end of the day. That is independent of the height of each individual transaction or the number of transactions, or the composition of the transactions: the total loss/gain at the end of the day is what you have earned or lost that day... In this case, there is a continuous loss of CO2 (as quantity!) added by humans, which means that all natural flows of CO2 in/out the atmosphere together, over a full year, gives zero net addition to the atmosphere: nature acts as a sink for human CO2...
As shown in chapter 1, there is little doubt that humans are fully responsible for most of the increase of CO2 in the past (at least over halve) century, that means that - as far as there is an influence of CO2 on temperature - that humans may be responsible for (a part of) the temperature increase. How much, that is an entirely different question, as that mainly depends of the (positive and negative) feedback's that follows any increase of temperature...
4. References
[1] Carbon dioxide emissions inventory from the US Department of Energy (DOE):
http://www.eia.doe.gov/pub/international/iealf/tableh1co2.xls updated for recent years at: http://www.eia.gov/cfapps/ipdbproject/IEDIndex3.cfm? tid=90&pid=44&aid=8
[2] Yearly averaged carbon dioxide concentrations at Mauna Loa Observatory, Hawaii, 1958 to last full year, NOAA: ftp://ftp.cmdl.noaa.gov/ccg/co2/trends/co2_annmean_mlo.txt
[3] Global Carbon Sinks and Their Variability Inferred from Atmospheric O2 and d13C, Battle ea., Science, Vol. 287 31 March 2000. http://www.sciencemag.org/cgi/reprint/287/5462/2467.pdf can be viewed with a free subscription at Science. More up-to-date (up to 2002) by Bender e.a.: http://www.bowdoin.edu/~mbattle/papers_posters_and_talks/ BenderGBC2005.pdf
[4] Uptake and Storage of Carbon Dioxide in the Ocean: The Global CO2 Survey, Feely e.a. on the NOAA pages: http://www.pmel.noaa.gov/pubs/outstand/feel2331/exchange.shtml
[5] The 13C cycle in nature, Anton Uriarte Cantolla. Some of the figures and text are from the book "Earth's Climate History" (e-book). More info about stable isotopes in nature, food and humans at: http://www.physics.utoronto.ca/students/undergraduate-courses/coursehomepages/jpa305h1-310h1/stableisotopes.pdf
[6] Atmospheric Carbon Dioxide and Carbon Isotope Records at CDIAC: http://cdiac.ornl.gov/trends/co2/contents.htm
More data at the "carbon tracker": http://www.esrl.noaa.gov/gmd/dv/iadv/
[7] Evidence for preindustrial variations in the marine surface water
carbonate system from coralline sponges, Böhm ea., GEOCHEMISTRY GEOPHYSICS GEOSYSTEMS, 2002. http://onlinelibrary.wiley.com/doi/10.1029/2001GC000264/full
[8] The Carbon Cycle and Atmospheric Carbon Dioxide, IPCC TAR, Chapter 3, page 206, fig. 3.4 http://www.grida.no/climate/IPCC_tar/wg1/pdf/TAR-03.PDF
[9] The acidification of the oceans, according to Wikipedia http://en.wikipedia.org/wiki/Ocean_acidification#cite_note-key04-1 Based on investigations done by Key e.a. and Orr e.a.
[10] Trends of ocean pH, DIC, pCO2,... data at Bermuda in fig. 5 of Battle e.a.
[11] Ice core data from NOAA: http://www.ncdc.noaa.gov/paleo/icecore/current.html
[12] Etheridge e.a., GRL 1996, Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn: http://www.agu.org/pubs/crossref/1996/95JD03410.shtml
[13] Wood for Trees plot tool for different temperature series vs. CO2 data, http://www.woodfortrees.org/plot/
[14] Calculations by Peter Dietze at the web site of the late John Daly: http://www.john-daly.com/carbon.htm
On the net: 16 September, 2007. Transferred from the CO2 measurements page to this page: 30 June 2014. Last updated: 11 October 2022.
To the family home page
To the climate change page
To the CO2 measurements page
To the co2 variability page
ferdinand.engelbeen@telenet.be
