Problems with Boltzmann constant and CO2 as source of global warming
 Terigi Ciccone
Terigi Ciccone
May 7, 2023
[1] Quote: “At the assumed average temperature of the earth (15°C, 59°F), it's 398.2 W/m2 . Source http://nov79.com/gbwm/sbc.html, The Stefan-Boltzmann Constant is in Error. It shows about 40 times too much radiation at normal temperatures. (NASA Charts)”.
But I have a problem with the Boltzmann constant and other matters involving CO21 as a source of global warming.
The earth is not a blackbody absorber/emitter. As I previously said, I don't stand by the number 40 that I cited in the above endnote. But here are reasons why I continue to vigorously challenge the value of the Boltzmann constant relative to NASA’s Earth Energy Budget (EEB). The discussion centers on what is the “surface” of the earth and how it compares to an ideal blackbody. I argue that the Earth is not a blackbody absorber/radiator. Pretending that it is, gives us uninspiring results. Precisely, the EEB pretends that there is a “surface on the earth,” about 6 feet above the ground/sea level. Then asserts that this “surface” is in thermal equilibrium and has a uniform temperature of 15 C. Those are absurd assumptions. There is no such surface, and assuming that there is, and further assuming it is in thermal equilibrium, leads to questionable conclusions, as discussed below. I want to be clear that I don’t challenge the validity of the S-B equation or the relationship between radiative energy and temperature. I question that the “surface” defined by NASA in the EEB doesn’t exist and is never in thermal equilibrium.
How the atmosphere and a blackbody absorb and emit radiant energy. In the world of EMI, radiation, which we conveniently refer to as photons for this discussion[2], is absorbed and emitted, as shown in the following example. In this thought experiment, we’ll use an iron stove in the house in thermal equilibrium with its environment. The room is dark, and there is no fire in the stove. This stove is now an ideal blackbody absorber/radiator for EMI, and Mr. Boltzmann is happy. Now I use a remote arm and light a match. It will immediately release photons in all spherical propagation directions. The frequency (Energy) of each photon depends only on the temperature of a microscopic pinpoint on the surface of the match head. Meanwhile, another gazillion photons will be similarly emitted in that split second. Each will have its own frequency (Fn) and its own energy (En), and its own angle of propagation (Dn) towards the stove. Each photon is its own master. They do not collide with other photons, they
1 Throughout this discussion, Co2 represents all greenhouse gasses, including the water vapor that accounts for about 95% of all absorbed LWIR radiation. See the detailed article by Br. S Fred Singer; https://climatecite.com/water-vaporrules-the-greenhouse-system/#:~:text=Water%20vapor%2C%20responsible%20for%2095,This%20is%20insignificant!
2[] First, I rely on the notion expressed by Richard Feynman that energy does not come in “little blobs of definite amounts,” from which I get a few important corollaries. a) radiant Energy equals a constant times the frequency (E=cF), implying that radiant energy is not additive or interferes with other frequencies. b) Radiant energy exists only as potential energy until it interacts with the matter of the physical universe. Then we can microwave a chicken or tune in to NPR radio at FM 89.1 MHz. c) A blackbody emits energy that depends only on the temperature of an infinitesimally small point of a blackbody and does so over a range of frequencies. d) Radiant energy can only flow from matter that’s at a higher energy state to a lower energy state body.
don’t integrate, and molecule absorbs resonance, see Figure-0. molecular bond.
Richard Feynman
average Energy of Prior to the match stove’s surface is molecular bond vibrates frequencies. So, when the stove’s surface, will absorb some dependent on the absorption is done forks. Each bond from this EMI wave
a) the frequencies
b) the receiving
c) not all energy will be absorbed by the stove as some will reflect, some will scatter, some will diffuse, etc. The total energy, absorbed and unabsorbed, will be conserved.
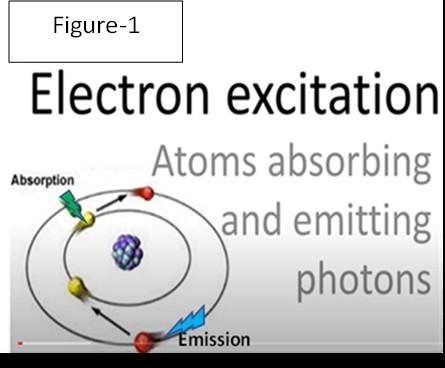
If the arriving EMI energy is insufficient to kick the bonding electron to its next higher energy level, there will be no absorption. See the diagram in Figure-1 where a green photon is absorbed, and the yellow electron jumps to the energized red energy state.
There is no in-betweener.
Let’s look at Figure-2 and see what has happened up to this point. Here we see the length of the molecular bond in its pre-absorption/relaxed state, represented by the black double arrow, called the amplitude of oscillation, as shown in Figure-1.
When the photon is absorbed, as shown in Figure-1, the electron jumps to the red orbit, and the length of the bond vibrations increases
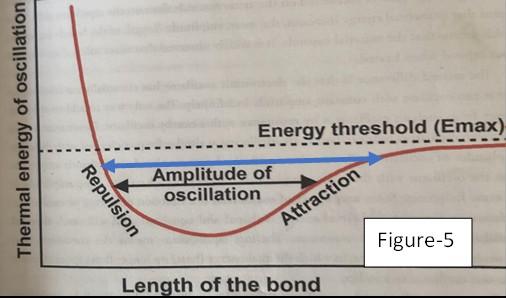
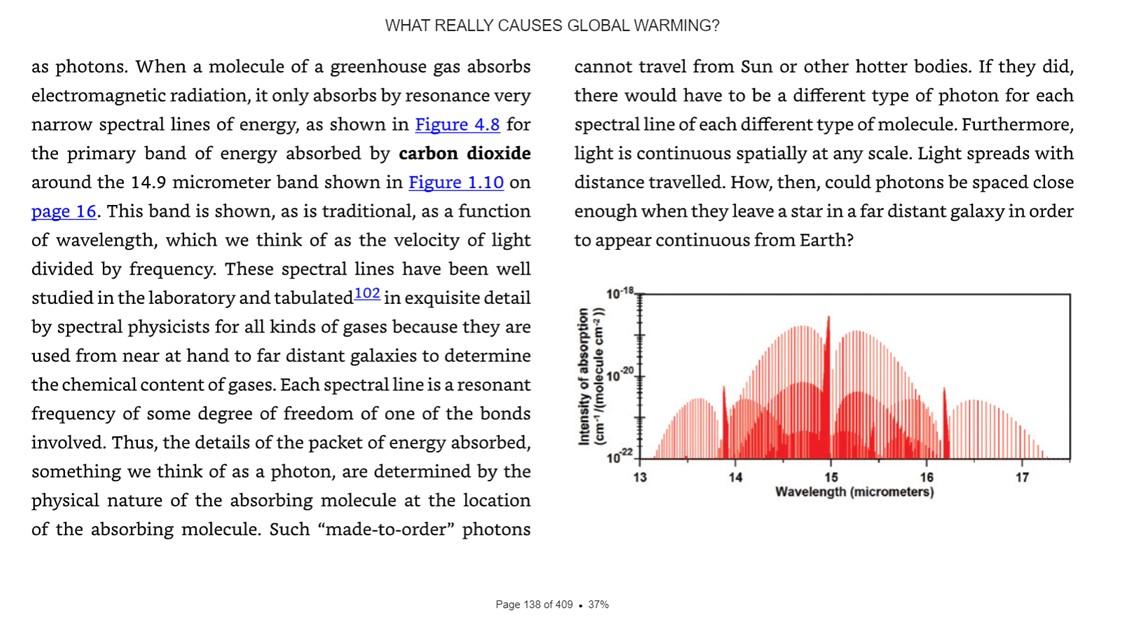
from the black double arrow up to the blue double arrow in Figure-2.3 For example, let’s assume that this is the bond length between the carbon atom and one of the two oxygen atoms in the CO2 molecule. These atoms range out at greater distances in the energized state than in the relaxed state. This increases the probability that this one oxygen atom will collide in the atmosphere with other atoms, molecules, ions, etc.. The increased collisions convert the oxygen atoms’ kinetic energy of motion into heat/thermal energy, and the air warms up an immeasurably small amount. At no point did any increased heat appear due to the absorption of the photon. We only got heat energy because of the increased number and “punch power” of the collisions. The same thing happens on the surface of our blackbody cast iron stove. On the stove, the warmed-up molecules then readily conduct the increased punchheat to warm the rest of the molecules around them, and the entire stove slowly warms a tiny bit. But, within a fraction of a second after the absorption, and independently of whether there were any increased collisions, the energized red electron in Figure-1 will emit a blue photon, and the red electron will fall back into the lower energy yellow orbit. In Figure-2, the same thing happens, and upon the blue photon emission, the blue amplitude arrow falls back down to the black arrow, reducing the chances of increased collisions. Absorption and reemission result in 1 to 10 nanosecond delays, and the time it takes an LWIR photon to escape to space is 0.6 to 30 milliseconds. So, the notion of CO2 trapping LWIR radiation and delaying heat escaping to space is greatly exaggerated.4
Water vapor and clouds do most of this temperature “holding” in the atmosphere. Evaporated water, at 15oC, transports about 60% of the energy from the surface into the atmosphere.5 This energy is released when the water vapor condenses, mostly as clouds. This latent heat release is ABOVE the Greenhouse, and energy is re-radiated from that level by water vapor so CO2 cannot penetrate back to the surface. In addition, thermals, convection, and conduction raise this 60% by an additional 11.5%. So more than 70% of the solar energy absorbed by the surface is the cause of the atmospheric heat/radiation delay and not the CO2 absorption of the LWIR radiation. If we now add the IR radiation passing directly through the atmospheric window, we have almost 90% of all the solar energy absorbed by the surface. Therefore, any additional CO2/greenhouse gas warming is trivial at best.
A few more observations. First, as the match burns, it has a low temperature, emitting photons at lower frequencies/energy. But as the flame temperature increases, the
3 Figure-1 from the book What Really Causes Global Warming, figure 4.4 by Pater Langdon Ward.
4 Delay Time for Terrestrial InfraRed Radiation to escape Earth’s Atmosphere, https://www.researchgate.net/publication/336915543_Delay_Time_for_Terrestrial_InfraRed_Radiation_to_escape_Earth's_Atmosph ere
5 Laws of Physics Define the Insignificant Warming of Earth by CO2H. Douglas Lightfoot1,* and Gerald Ratzer. See site https://setpublisher.com/pms/index.php/jbas/article/view/2456/2228
energy/frequencies also increase. But, as the flame dies, the energy/frequencies also decrease. The solar energy arriving on earth works the same way. It starts at a zero-baseline temperature, gradually increasing as it works through the morning, reaches a maximum in the early afternoon, decreases thereafter, and returns to the zero baselines at sunset. But unlike the stove, the earth’s other heat sources continue, such as geologic/volcanic heat, animal respiration, atmospheric circulation, fermentation of biological waste, the stored heat in the oceans6 , ozone depletion, etc. When the match burns out, the stove has no other heat sources, so it returns to its baseline ambient temperature. In the house, the furnace or AC dictates the baseline temperature. For the Earth, it’s the same principle but much more complicated, and the process and forces are poorly understood. Other forces maintain/mitigate this baseline temperature on Earth. This includes the heat stored in the oceans, which continues to warm the air and “surface” at night, and morning fog is evidence of its tracks. There is also the warmth from the winds/storms from areas still warmed by the sun, thanks to the Earth’s rotation, tilt, and dozens of other forces. Lastly, and perhaps most important, is the baseline temperature component that’s rarely talked about, the Ideal Gas Law. Yup, the old PV=NrT as gravity keeps accelerating/pushing down the air molecules, squeezing them together, and the unimaginable number of atmospheric gas collisions going on 24/7-365. These collisions have huge linear velocities and huge masses compared to the abovementioned subatomic vibrational collisions.
NASA tells us that there is 33 degrees C of missing heat. That 33 C or 159 W is the difference between what the “surface” radiates to the atmosphere 398 W versus what the sun sends to the Earth and what the Earth radiates to space 239 W (398-239= 159). NASA, why not tell us you don’t know the source/cause components of this missing 33 C/159 W of heat instead of calling it the CO2/greenhouse effect? And why have NASA and the UN IPCC dropped the word/concept of LWIR radiation saturation? Heinz Hug demonstrated CO2 saturation when CO2 was at 357 ppm. More recent precision research by physicists Happer and Wijngaarden determined that the present atmospheric carbon dioxide and water vapor levels are almost completely saturated. In radiation physics, the technical term “saturated” implies that adding more greenhouse gas molecules will not cause more warming.7 In plain language, this means
6 The oceans have a heat capacity (enthalpy) about 1,000 times greater than the oceans. That’s why we have onshore winds at night and offshore winds after sunrise. The oceans are not heated solely by the sun but also by geologic and volcanic heat. The air in the nighttime areas is also kept warm by the natural heat of fermentation, animal respiration, and perspiration. Latent heat from evaporation is still stored in the atmosphere. The Smithsonian Institute tells us there has been a noticeable increase in volcanic activities over the past 150-plus years and continues to this day. The bottom line, the “surface” is nowhere near thermal equilibrium. It flunks the test of Earth being a blackbody radiator. Using one temperature averages over a dynamic, spherical planet, rotating at 1,000 MPH at the equator, with temperatures variable with latitudes, longitudes, is an absurd notion.
7 https://www.heartland.org/news-opinion/news/study-suggests-no-more-co2-warming
that from now on, our emissions from burning fossil fuels could have little or no further impact on global warming.
Recap. When CO2/GHG absorbs an IR photon, the increased energy is stored in the molecular bonds as vibration lengths/amplitudes of vibrations, not heat. It only becomes heat if there are additional collisions of the CO2 atoms with other stuff in the air, and the amount of increased heat is immeasurably small. The energized CO2 molecule will, within a fraction of a second, emit a photon and return to its relaxed state. The emitted photon will have a range of frequencies generally lower than the absorbed photon. This absorption and emission energy step down may continue many times, and each time the emitted photon steps down the energy scale until the photon finally is able to exit the earth, as discussed above.
As far as this engineer understands, what I’ve discussed above is consistent with the laws of physics, chemistry, and thermodynamics.
