How the sun warms the Earth, and how the Earth cools
Terigi Ciccone
March 16, 2024.
Abstract. Dozens of natural forces and cycles dominate the Earth’s weather, climate, and climate change, some acting independently and others in complex interactions among and between these forces and cycles. Climate and climate change science is highly complex, involving dozens of scientific disciplines, and is poorly understood. The science of climate and climate change is far from settled.
Introduction. Here, we discuss some significant drivers of climate change, provide an overview of overlooked ones, and challenge some popularly accepted ones. We start by analyzing the science, examining the historical record and geologic evidence, and discussing intriguing possibilities and their possible roles and contributions. Here is a grouping of the significant drivers of climate change:
Exo-Earth. This grouping includes astronomical, orbital-planetary, galactic, and solar forces and cycles that account for around 90% or more of the total climate changes occurring on Earth. Humans have zero capability to control any of them.
Endo-Earth. This category focuses on what occurs on Earth, divided into two categories: 1) Gravity, as it continuously pumps up the atmospheric pressure and resultant temperatures, thereby establishing their respective lapse rates. 2) Plate climatology is powered by the Earth’s molten liquid metal outer core, swirling around and pushing continents, building new islands, and recycling the Earth's crust with its interior. We see its effects with tsunamis, volcanic eruptions, earthquakes, gas vents, El Ninos, warming oceans, atmospheric rivers, etc. These account for maybe 8% of climate change. Here, humans also have zero impact.
Bio-Earth. The remaining 1 to 2% is caused by the earth's biosphere, which includes animal and plant respiration and bio-decomposition, land use changes, albedo, human heat engines, and CO2/GHG emissions, etc., of which the humancaused CO2/GHG activities may account for about 0.01% of this last 1 to 2%.
We will use the NASA Earth Energy Budget (EEB) to guide us in accounting for the energy the sun provides. NASA uses this EEB to tell us how the sun heats the Earth and summarizes it below in Figure-1. First, however, this figure needs to be explained to non-scientific persons. Secondly, it needs to provide more information on where these
numbers come from, their meaning, and the science behind them. This article will give simplified answers to these critical questions. So, we start by explaining what radiation is, what it does, and how it works to warm up the Earth’s atmosphere, waters, and lands. But equally important, we answer the never-addressed question: how does the Earth cool to maintain the stable climate we enjoy?

What is radiation? The sun sends its energy to Earth, not as heat but as radiation, and more about this important point later. There are three fundamental components in NASA Earth's Energy Budget presented in Figure 1. First, the information inside the two light blue ovals is actual and repeated measurements recorded by sophisticated satellite-based instruments. These are the only verifiable numbers on this entire diagram. Second, sophisticated instruments from space also measure the information in the dark-thick blue circle. Still, this value could be more trustworthy; we will get into this later. Lastly, the information inside the bright freehand red collective is assumptions, estimates, and guesses. The information inside this red collective is the essence of the much-debated global warming caused by all the greenhouse gases (GHG), including the much-debated CO2 gas and the greenhouse effect (GHE).
In this article, the term “surface” includes all lands, waters, plants, animals, and all physical objects. Note that the numbers inside this red collective have not been verified by laboratory test data [i] but are estimates based on theoretical assumptions and modeling. Third, all the numbers are energy densities expressed in watts per square meter (W), which estimates the energy amounts as a global average. This global average temperature in substance assumes that the earth is flat and receives the same energy from the poles and the equator. Some of these average numbers have no meaning in our physical world, like saying the average USA family has 2.18 children. Also, the climate change models are based on the sun shining 24 hours/day equally at the poles and the equators and everything in the middle. But, practically, all weather and climate changes occur at the local/regional levels and conditions. They will vary significantly depending on specific locations, geographical features, latitudes, altitudes, geologic conditions, etc.
What is an electromagnetic wave? Solar radiant energy travels from the sun to Earth through electromagnetic waves (EMW). The surface of the sun is scorching, about 10,000 degrees Fahrenheit. But this heat never reaches the earth because heat energy can’t travel in the vacuum of space. The only way the sun can send its energy to Earth is by radiation in the form of electromagnetic waves (EMW). The most significant parts of an EMW are shown in Figure 2: the wavelength, amplitude, and direction of propagation. Frequency is the number of these wavelengths passing per second along the propagation path. We also see a conceptual representation of the electric field perpendicular to a magnetic field. So, the

visible light from the sun is one example of many types of frequencies that reach the Earth.
As we learned in high school science, light sometimes acts as a wave, and other times it acts as a particle. For convenience, we call a specific amount (quanta) of this radiant energy a “photon.” However, people are still determining what this wave or photon looks like, and more about this vital subject will be discussed later. But we know this wave/photon propagates in a vacuum, with no interactions or interference with other photons, no increase or decrease in energy state, and with zero heat. Only when this wave/photon hits, meaning it is absorbed by a physical body, like a brick, water, or a gas molecule, will energy be absorbed by the object, which then warms the object up.
Figure-3 shows that this EMW has a near-infinite number of frequencies, spanning more than 14 orders of magnitude (1014), and it’s called the “spectrum.” The spectrum is measured in wavelengths from meters (m) to the tiniest, measured in micrometers (mm) or even nanometers (nm). One mm = micrometer = (1/1,000,000 of a meter), and one nm = nanometer = (1/1,000,000,000) of a meter.

Figure-3 shows that the visible light range is narrow, with a band of only about 400 to 700 (nm), and the ultraviolet UV range is significantly wider than the visible light. The infrared red IR portion is the widest and carries the weakest energies. The radiant energy of a single photon is measured by the Plank-Einstein formula E=hv, where E is the energy, h is the plank constant, and v is the frequency. This energy is totally different from the Einstein-Newton energy of the material world as calculated by E=MC2 . Note that there is no temperature component in either energy equation. We use chemistry, thermodynamics, and mathematics to extract temperature values from these energies when they interact with the universe's physical matter.[ii]
In Figure-3a, [iii] we also note that frequencies have a vast energy range and that energy depends only on the frequencies. Look at the red line, which shows the span of the
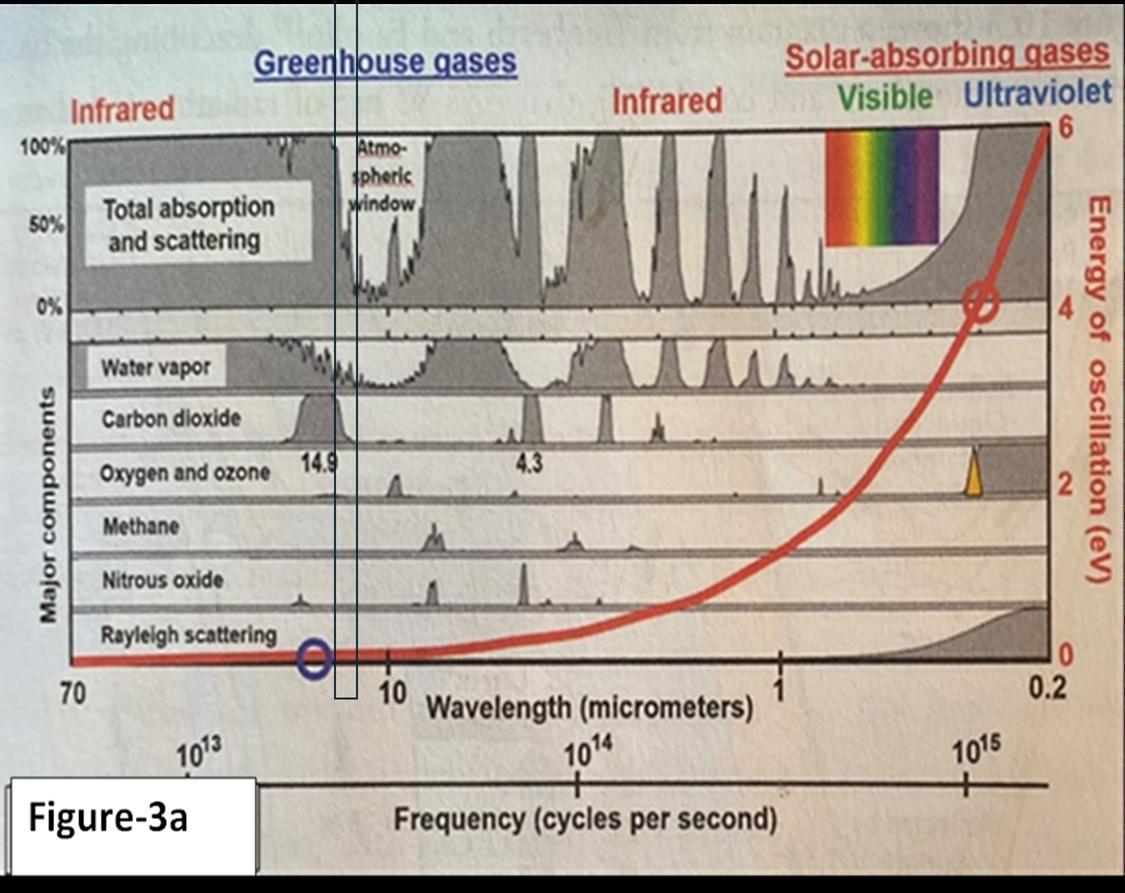
electron energies from the weak IR to the powerful UV rays. Here, we see that CO2, at its most abundant wavelength, is at a range of 14 to 16 mm, and a central peak at 14.9 mm (blue circle) carries an energy of about 0.083 electron Volts (eV). However, a powerful UV photon (red circle), absorbable by ozone and oxygen, will have almost 50 times more electron volts of energy. Note also the slight overlap with the atmospheric window.
These radiant energies are not additive; see Figure-3b [iv]. The white light that enters the prism does not carry the sum of the energies of its constituent colors/frequencies. It’s not that the total energy of 11.8 eV is wrong. It’s that the summing of these energies is nonsensical and non-existent. We cannot add the energy listed for each frequency to the energy of the other frequencies. It’s the same with temperatures; we cannot take the temperatures of 10 bricks and add them together to get a higher temperature. This is a crucial scientific point in refuting the UN IPCC computer algorithms that use the
energy additive concept they refer to as “integration across the wavelength spectrum” to forecast alarming global temperature increases. Note that Figure-3 shows the wavelength of EMW, and shows the frequencies and wavelengths of the EMWs grouped into three major categories: Infrared, visible, and ultraviolet.

Note also that each frequency is associated with a color. In the visible light range, we see reds at the longer/weaker 700 mm wavelengths and violet at the slightly more powerful, shorter wavelengths of 400 nm range. The visible light range will also reflect and scatter in the atmosphere, allowing us to see the objects warming. As a photon travels in a vacuum, the amplitude of the vibrations or the color brightness will diminish over distance. But its Energy level will not, even to the end of the universe. Lastly, a photon’s energy only decreases/goes to zero once the photon interacts with and is absorbed by the matter of the physical world. There, it is stored as chemical energy by increasing the molecular bond’s amplitude of vibrations.
In summary:
1. Ultraviolet radiation (UV) is the most powerful and carries the most energy per photon. These invisible rays have the highest frequencies and the shortest wavelengths. This family includes the most powerful and deadly ones, like Gamma rays, DNA-damaging X-rays, and several other categories of tissue-damaging UV rays.
2. Visible light occupies a narrow band of frequencies; each photon carries a medium energy level.
3. Infrared radiation (IR) is not visible and makes up the widest band of longwave and the lowest energy photons.
More on photons. People need to understand what a photon is and isn’t, how it works, and how it travels. First, photons are an electromagnetic disturbance, sometimes shown as a dot or circle on a diagram. But there are also many other images used. The vital point is that photons have no mass, do not occupy space, and have no shape or charge. Some consider them as electrons without an electrical charge. But if we know its frequency and amplitude and the direction of propagation, we can extract much information. For example, in Figure-2, we see only a conceptual representation using the common understanding of physical waves, like a disturbance traveling through the water's surface. But we have yet to learn what these two perpendicular
waves look like on the sub-atomic scale.[v] Paraphrasing Nobel laureate Richard Feynman, we have no picture in our mind of what this energy wave looks like.
Solar energy arrives towards Earth in the above broad spectrum of frequencies. First, the high-energy UV frequencies are nearly 100% absorbed by the atmosphere and warm it. Almost 100% of the visible light and the IR photons bore through the atmosphere and warm the surface. The last and critical point is that after the atmosphere and surface have absorbed these radiant energies, this absorbed energy can only exit from the earth through Longwave Infrared Radiation (LWIR). How this transformation of energy happens and how it exits into space will be discussed throughout the article.

Absorption and emission. For a gas molecule, like CO2, N2, or O2, to absorb a photon and increase its energy state, it must have a frequency equal to (resonant) that of each specific photon. When the frequencies are not resonant, the photon is not absorbed, there is no energy exchanged, and there is no heating. Instead, the photon continues its journey until something else happens somewhere else. For example, in Figure-3c, we see the absorption, by resonance, of an incoming green photon. The increased EMW energy kicks the red electron to a higher energy state, pushing the electron farther from the nucleus. As described below, this increased EMW energy is stored in the atomic and molecular bonds that hold atoms together to form molecules. This results in increased amplitudes of vibrations between these atoms. Then, within the blink of an eye, the molecule emits a new photon (shown blue), and the electron returns to its lower energy state.

NASA provides a graphic representation of this absorption and emission process, as seen in the simplified image Figure-3d. The image shows that the CO2 molecule appears to be absorbing/emitting two photons simultaneously. But this is not the case. An energized molecule can’t absorb a second photon until it emits the first absorbed photon. But, perhaps NASA is attempting to show that the CO2 molecule absorbs an array of frequencies across all its degrees of freedom. As shown in
Figure 3d1, this CO2 absorbable photon has a central peak at just under 15 mm, but its spectrum goes from over 13 to just over 17 mm. But remember that the time for this absorption process to the emission of a new photon occurs within about one billionth of a second, and no heat is generated. See endnote [vi] for details. Understanding these concepts is crucial to understanding how radiation warming occurs or doesn’t occur, as later discussed in greater detail.

More on solar irradiance. As seen in Figure-1, NASA tells us that the total radiant energy arriving from the Sun to Earth is called TSI (Total Solar Irradiance). NASA measures this energy in Watts per square meter per second (W) at the top of the earth's atmosphere. It arrives towards the earth in all frequencies, from the very high energy UV rays to the medium energy Visible Light and ending with the low energy IR radiation. As seen in Figure-3, the longwave/low-frequency Infrared radiation is the weakest. As a general guideline, visible light makes up almost half of the total TSI energy that warms the surface, less by IR and least by UV. Each photon of Visible Light is about 10 times more potent than IR, and UV is about 100 times more powerful than IR. The NASA Earth Energy Budget uses 340.4 W of TSI as the total incoming solar energy. It then estimates that 99.9 W, about 30%, is immediately reflected into space by the air and clouds and from the surface by snow, water, trees, children playing with mirrors, etc.
Almost 100% of the powerful UV rays are absorbed by the atmosphere, shown in Figure-1 as 77.1 W. On the other hand, nearly 100% of the Visible Light and Infrared Radiation of 163.3 W goes through the atmosphere and is absorbed by the earth's surface. Plants also use a tiny amount (0.6 W) for photosynthesis and later store it as potential or chemical energy in plant material. Let us summarize: the energy of 239.9 W (340.4 - 99.9 - 0.6) must be radiated by longwave IR radiation to space to keep the earth in solar thermal balance. Figure-1 matches the 239.9 W of outgoing LWIR radiation in the light blue oval, and the earth appears in thermal equilibrium.
How is the atmosphere warmed?
The atmosphere is warmed by incoming solar radiation, but it is also warmed by heat coming off the surface through conduction, convection, and latent heat of evaporation. Some other heat sources also warm the surface in unknown, questionable amounts, including geologic and volcanic heating, animal respiration, bio-decomposition, and some amounts of Longwave IR radiation(LWIR) from the surface, which reputedly also warms the atmosphere.
Atmospheric warming by incoming solar radiation. On average, about 99% of the atmosphere comprises Nitrogen, Oxygen, and argon. CO2 and all other gases make up less than 1%. The much-maligned CO2 is a trace gas that makes up about 0.042% of the atmosphere. All greenhouse gases combined total about 0.05%, a tiny fraction of the atmosphere. All gasses are well mixed in the atmosphere from sea level to the top of the stratosphere. But water vapor, the gaseous form of water, is the most abundant greenhouse gas on earth. Water vapor is generally limited to the troposphere, a region from sea level to about 7 miles/10 Km up. Water vapor in the atmosphere is what a wild card is in poker. On average, global water vapor is less than 0.3% of the atmosphere, but in tropical regions, it can be more than 4.0% and decreases with latitudes, approaching nearly 0.0% in the polar areas. On average, nature-produced water vapor accounts for about 95% of the so-called greenhouse effect, total C02 about 3.5%, and human-made CO2 less than 0.2% [vii] of the greenhouse effect. As used in this paper, the greenhouse effect is the absorption of a longwave IR (LWIR) photon and the subsequent emission of a photon and has nothing to do with warming or cooling, as detailed below.
Nearly 100% of the very powerful incoming solar UV rays are absorbed by nitrogen (78% of the atmosphere), oxygen (21%), and all other trace gases in the atmosphere, about 1%. These atmospheric gases are warmed by incoming sunlight in two ways: photodissociation of all the gases and oxygen and ozone also by photoionization. In Figure 4a, we see the images of the molecules of the two primary gases, nitrogen and oxygen. Each molecule has two atoms and a covalent electron bond that holds the two atoms together like rubber bands that constantly vibrate. When these molecules absorb a radiant energy photon, the additional energy is stored in these molecular bonds, making the bonds vibrate with greater amplitudes of oscillations. Figure-4b shows a diagram of the CO2 molecule and its molecular bonds, and since it has three atoms, it has more vibrational modes, called “degrees of freedom,” than the two-atom molecules.


Figure-5 shows a conceptual model of one of these molecular bond oscillations. The red line shows the path length of each electron. The black arrow shows the bond length in its “relaxed” mode before it absorbs an LWIR photon. It vibrates between the two atoms with a repulsive force as the electron gets closer to the nucleus. And it has an attraction force when it’s farther away. The electron is like a ping-pong ball pulled by two opposing rubber bands. Since this electron has no inertia, these vibrations will last unchanged forever.
But when the molecule absorbs a photon, the increased energy is not converted to heat temperature, nor is the molecule warmed in any way. Instead, the increased energy is stored in the bonds, causing the bond length to stretch to the size of the blue arrow. The increased bond length increases the probability that the atoms of the CO2 molecule bump into an adjacent air molecule with increased kinetic energy, and when it does, the gas temperatures increase. However, the heat generated by these sub-molecular collisions is infinitely small, and the increased heat has never been quantified in scientific laboratory tests. Then, in less than a billionth of a second after the absorption, the CO2 molecule emits a new photon, and the amplitude of the oscillation returns to the resting state of the black double arrow. This increased collision probability during the energized state of the CO2 molecule increases the temperature, which is explained by the kinetic theory of gases, and this CO2 absorption may microscopically warm the atmosphere.
However, see what happens when a nitrogen or oxygen molecule absorbs a powerful UV ray. The green arrow bond length in Figure 5 is reached, and it is above the Energy Threshold, which signifies that the attraction force is broken. This is shown in greater detail in figure-5a, which shows what happens when the molecular bonds explode. There, we see the atoms fly apart with great translational velocities and in random directions. Effectively, this explosion converts photons’ electromagnetic energy to kinetic energy of motion of the two separated atoms. The increased kinetic energy gained by each atom is equal to E V2 (one-half the mass of the atom times its velocity square), and we call this movement "Brownian Motion." These free atoms smash into everything in their trajectories, like molecules, ions, aerosols,

walls, rocks, etc. On impact, these collisions convert a portion of their kinetic energy of motion to the energy of physical heat, which we measure as temperature, and the atmosphere warms up. This is an established fundamental law of thermodynamics called “The Ideal Gas Law.”
Oxygen and ozone also undergo a second type of heating called photoionization. This is when the oxygen molecule gains or loses an electron, converting the molecule into ions. All the accumulated energy is stored in the same electron bonds. When these electron bonds break, the chemical/vibrational energy is converted to kinetic energy, as discussed above. The energy released is half the ion’s mass times its velocity squared, which becomes kinetic energy and subsequently into heat energy through more collisions and more powerful collisions.
An example of this kinetic heating is seen when we pump up bicycle tires. The pumping action squeezes the air molecules closer together. This increases the number of collisions of the air molecules in the tires, and that’s why we feel the tires getting warmer. Then, after some time, the air in the tires and the tire walls cool, and the temperature decreases. This lets the thermal energy step down until it exits into space as LWIR radiation. But gravity never stops and keeps pumping the air molecules downward 24/7/365. The above-described photon absorption/emission process is the same as the tire pressure example, except it occurs at the sub-molecular levels of ions and atoms instead of air molecules. This is the well-established principle embodied in the Ideal Gas Law of thermodynamics. It’s been codified by the formula PV = nRT or restated for temperature measurements as T= PV/nR when temperature is our primary interest. Here, P is pressure, V is volume, n is the number of moles of an ideal gas, R is the ideal gas constant. This thermodynamic model has a corresponding molar equivalent to the Boltzmann constant, where T is the temperature. This is important because it brings gravity as a constant warming source in the atmosphere since it squeezes the air molecules together like the above bicycle tires. Unfortunately, this fundamental principle never seems to enter the discussions centered on the global warming science espoused by the UN IPCC, which is focused only on the “Radiative Forcing” of Greenhouse gases. See endnote [viii] for additional details.
Atmospheric heating by the surface and atmospheric cooling. Our focus starts with the sun-warmed surface shown in the NASA Earth Energy Budget in Figure1 above. It shows that the surface absorbs 163.3 W of solar energy, mostly from visible light and infrared. However, the surface also absorbs some UV light, especially at higher altitudes and during periods of ozone depletion. The absorption process warms the surface, with temperatures warmer than the air above it. We immediately notice this phenomenon when we walk barefoot on a hot, sunny day at the beach or on a black tar parking lot. We may not notice that the surface continuously cools, even while being warmed. This is an important concept. The surface absorbs solar radiation, warms, and continuously radiates LWIR radiation that cools it. The absorption rate is greater
during the warmer daylight hours, and the surface keeps warming. But the cooling process continues 24 hours a day and dominates at night. The other important point is that the hotter the surface is, the faster it radiates the LWIR radiation. However, LWIR radiation is not the only way the surface cools. In fact, it is not even the primary source of cooling. Let’s get into some specifics.
As seen in Figure-1, NASA tells us the surface cools by 18.4 W of heat labeled “Thermals.” This includes the conduction caused by winds as the columns of warm air rise like smoke. This rising column of warm air also moves to the cooler regions of the earth, as shown in Figure-6. This rising stream of warm air also moves to the cooler air and warms it. See Reference note [VIII] for the temperature and pressure lapse rates. In the process, it cools the warm surface as discussed in detail in [ix].
The surface also cools by 86.4 W, called “latent Heat.” This is caused by liquid water changing its physical state and evaporating into water vapor. This is the cooling sensation we feel when we come out of the pool and feel our skin cooling even under a blazing sun. So, a total of 104.8 W (18.4 + 86.4), or about 64% of the 163.3 W absorbed by the surface, goes to warm the atmosphere not by radiation but by this physical heat. Then, as the atmosphere cools, it transforms this 104.8 W of physical heat into LWIR radiation, nearly all of which goes directly into space. The rest of this atmospheric radiation scatters or is reabsorbed/remitted by the atmosphere before finally going to space. No atmospheric cooling radiation from this physical heat warms the surface because the surface is always warmer than the air [IX]. But as Richerd Lindzen reminds us, the scientific process of warming and cooling of the atmosphere and how it radiates to space is poorly understood. So, Based on the arithmetic coming from Figure-1, we are left with an unexplained 58.5 W (163.3-104.80) of energy that needs to leave the surface and go to pace.

In Figure 1, we also see 40.1 W of radiation going directly into space, which NASA calls the “Atmospheric Window.” This is radiation from the surface that is not resonant (absorbable ) by any atmospheric gases. So, we are left with 18.4 W (58.5 – 40.1 W) of radiation that must move through the atmosphere before it can exit into space. We have only 18.4 W, or about 11.3% (18.4/163.3 W), of the total solar energy absorbed by the surface that must radiate through the greenhouse gases before they can exit into
space. This exposes another deception created by the popular press, media, and NASA unless you read the articles with a skeptical mind. These NASA articles lead the scientifically naïve to think that 100% of all the solar heat from the surface goes through the greenhouse gasses. In reality, however, we see that only about 11.3% does, and of that, 95% is captured by nature-produced water vapor. In reality, total CO2 only captures about 3.62%, and human-made CO2 captures only about 0.12%. See Table 4a below from the paper Dr. S. F. Singer published in the Wall Street Journal. https://climatecite.com/water-vapor-rules-the-greenhouse-system/.

This is a critical question that has no conclusive answer to date. NASA says an atmospheric absorption and amplification process exists that turns this 18.4 W of residual surface heat into 340.3 W, which is labeled in the Earth Energy Budget as “Back Radiation” in Figure-1. Where did the extra energy come from without violating the fundamental laws of thermodynamics? [x]. NASA has formulated complex “Radiative Transfer Equations” that appear to do that. While the mechanics of mathematics appear correct, the scientific basis for mathematics can’t be explained by established laws of physics, chemistry, and thermodynamics. So, let’s get into some of the details of this “Back Radiation” label and the arguments they present. What follows is the central message of this discussion:
Absorption and emission. We are told and know that CO2 and all the GHGs absorb LWIR radiation, which causes the CO2/GHG molecules to warm up. The “warmed” CO2 molecule then emits a comparable LWIR photon back to the surface, warming the surface a second time. Since this absorption/emission process may occur a thousand times before the initial LWIR photon exits into space, it allegedly delays the Earth's cooling rate, and the Earth gets warmer. But this has several misleading bits of information.
First, the absorption of LWIR photons does not increase the temperature of the CO2 molecule, as discussed above. Instead, the increased energy is stored in the CO2/GHG molecules as increased amplitudes of the vibrations of the molecular bonds. However, within about one billionth of a second, after the CO2 molecule absorbs the LWIR photon, it emits a new photon of about the same energy level, and the CO2 returns to its relaxed state. The delay time is meaningless even if we assume 1,000 or 5,000 such absorptions/emissions. Remember, these photons are light, and light travels at 186,000 miles per second. During this brief time between absorption and emission, there is a small probability that the increased amplitudes of vibrations may increase the likelihood that one of the atoms of the CO2 molecule may bump into an adjacent air molecule. However, as explained in [VIII], the energy levels are microscopic, and any resultant heat increase from the energized collisions is unmeasurably slight.
Secondly, less than 50% of the re-emitted photons from the energized CO2 are returned to the surface, and as already explained, they do not warm up the surface. The rest are directed upwards on their journey to space or scattered in the atmosphere until they find their way into space.
Third, those LWIR photons that reach the surface will cool the surface instead of heating it. CO2 photons at their most abundant frequency of 14.9 mm correspond to a surface-emitting temperature of about a minus -84 C. This means they will be cooler than the surface temperature of about 15 C. So, it will have no warming effect and may have a net cooling effect.
Fourth, when the surface absorbs this 14.9 mm photon, it will not re-emit a comparable 14.9 mm photon and be captured again by CO2, as NASA/IPCC argues. Instead, the surface will emit arrays of many photons of many frequencies, about 25% of which will go directly to space through the atmospheric window without further CO2 or GHG impedance.
Fifth, it’s challenging to assume the Boltzmann Constant is correct and averaged to be the same for all surfaces. The UN IPCC defines the surface as about 2 meters above the ground/water, but it is not even a surface. The Stephan-Boltzmann equation still requires the earth to be in thermal equilibrium, which it is not; therefore, the “surface” of the Earth can’t be treated as a black body radiator/absorber.
Lastly, let’s further assume that human burning of fossil fuels is responsible for the increased CO2 and methane, which are responsible for this escalating global warming. We are still left with several incredulous situations on why humans produce CO2/GHGs and are responsible for global warming.
Additional discussions
- The so-called greenhouse effect is nonsensical. Figure-7 shows a schematic of how a real greenhouse works and why it is a false analogy for the CO2 cause of atmospheric warming. Here are the essential flaws. A real-world greenhouse has solar energy (yellow arrow) arriving that goes through the glass and warms everything inside. This real greenhouse has glass walls and a glass roof that prevents latent heat, convection heat, and IR radiation from escaping. See the red arrows reflecting nearly all the energy back inside the greenhouse. The metaphysical CO2 greenhouse has no walls or roof, and the blue arrow shows that all the physical heat and radiation immediately escape to the atmosphere and space.

- CO2 is too diluted to warm the atmosphere [xi]. In Figure8, we see a packet of the atmosphere, an array of 2,499 air molecules, and one molecule of CO2. Alarmists tell us that at about 400 ppm, this one CO2 molecule will heat the other 2,499 air molecules by an alarming amount! That is the equivalent of saying one cup of hot coffee heats the other 2,499 adjacent cups of coffee to an alarming amount, which is non-sensical.

- Figure-9 shows that NASA has another and bigger problem that needs further examination and explanation. The Earth Energy Budget only shows the earth warmed by the sun. But we know that is not the case. The surface has additional sources of heat that NASA ignores or dismisses as insignificant. Relying only on microscopic heating by trace, greenhouse gases as the only other significant source of heat to the earth. Figure-9 shows that the sun delivers only 239.9 W, as discussed above. But the surface is measured to radiate 398.2 W to the atmosphere. That’s more than double the sun’s heat to the surface (163.3 x 2 = 326.6). And yet, the same satellite instruments measure only 239.9 W, exiting from the Earth to space. This begs the question: where does the missing heat of 158.3 W (398.2- 239.9) watts go or come from? Does this not make it evident
Figure-9

that the earth is also substantially warmed by some/all of the heat sources below, which are ignored by the UN IPCC, NASA, and their Earth Energy Budget? This begs the question, is this the missing heat that the UN IPCC attributes to the greenhouse effect? This question remains unanswered to this day. The science of global warming is far from settled.
- The ignored sources of surface heat include:
Animal respiration and digestion, warming their bodies and the air.
Plant/animal respiration, decomposition, and fermentation of organic materials as they rot and decompose. They are also a primary natural source of CO2 and methane.
Geologic heating as their heat is transported from the earth’s outer core to the surface. Mind you, the temperature of this enormous mass of molten metal that makes up about 1/3 of the mass of the earth is about the same as the temperature of the sun's surface. In addition, recent findings also connect the robust El Nino/La Nina warming caused by geologic activities. [xii].
Volcanic eruptions of land and especially submarine basaltic volcanoes can be a significant cause of short-term rapid climate change. [xiii]
Ozone depletion by human-made CFCs. But the basaltic effusive volcanic eruptions release nature-made chlorine and bromine gases that deplete ozone. This ozone depletion allows more of the sun’s UV rays to reach the surface and warm the lands and oceans.
Galactic cosmic rays have been shown to increase or decrease the amount of solar energy that reaches the atmosphere and surface. This is because the sun has several magnetic cycles, the most common being an 11-year-long. During periods of high solar activities, the powerful solar winds reduce the number of galactic cosmic rays entering the earth’s environment. This decreases the volume of clouds, allowing more solar energy to reach the atmosphere and surface by reducing the albedo. Then, when more cosmic rays enter the earth during low solar activity, more cosmic rays enter the atmosphere, increasing cloud cover and cooling the earth. However, NASA only assumes a static albedo of 30%, ignoring the ever-changing cloud cover.
Bottom Line.
Is there an easy-to-understand and explainable resolution to what is happening in the atmosphere and what humanity’s contribution to the alleged global warming is? Unfortunately, the answer is that real climate change scientists do not fully understand the many variables. Perhaps we might have a better answer if NASA used its many $ billions of research dollars to fund scientific research instead of wasting them trying to justify the politically driven human-caused CO2 and methane to support their Anthropogenic cause.
CONCLUSION: THE NASA EXPLANATION OF CLIMATE CHANGE IS FAR FROM “SETTLED SCIENCE,” AND IT IS GROSSLY INSUFFICIENT
Details and references
i[] At the assumed average temperature of the earth (15°C, 59°F, 288 K), the earth’s surface radiates 398.2 W/m2. This measurement is determined using the Boltzmann constant in the measurement instruments. It measures the radiation of the solid/liquid part of the surface and some portion of the air close to the surface. It treats this radiation like the earth’s surface is an ideal black body. But The Earth cannot be treated as an ideal blackbody, nor can the atmosphere. It would seem that these surface readings should have different Boltzmann Constants of X1, X2, X3, etc., for the many different solids, liquids, elements, and atmospheres. Quote: “While there's no such thing as a perfect blackbody, most solid objects are sufficiently close to being a blackbody that they can be treated as one. The sun giving off sunlight, for example, can be treated as a perfect blackbody. On the other hand, the Earth needs to have a correction factor(s). This correction is often taken to be a percentage, i.e., 70% of a perfect blackbody. This means that the radiation emitted still looks like figure 1, but it is 30% less energy, more or less uniformly across all of the wavelengths. The Earth's atmosphere cannot be treated as a blackbody. Please see solar energy to the Earth for a more detailed discussion of this”
https://energyeducation.ca/encyclopedia/Blackbody_radiation#:~:text=On %20the%20other%20hand%2C%20the%20Earth%20needs%20to,Earth%27s %20atmosphere%20cannot%20be%20treated%20as%20a%20blackbody.
In summary.
- The Earth cannot be treated as a blackbody, and it’s often estimated albedo correction of 30% is questionable.
“It is difficult to imagine an object that absorbs and emits all wavelengths with equal probability but not equal magnitude. In other words, the object would be equally capable of giving off any wavelength of light, it just wouldn't tend to because different wavelengths have different energies.”
- Is the Earth a blackbody radiator? “Although a blackbody does not really exist, we will consider the planets and stars (including the earth and the sun) as blackbodies. Even though by definition, they are not perfect blackbodies, for the sake of understanding and simplicity we can apply the characteristics of blackbodies to them.”
https://www.google.com/search?
rlz=1C1JJTC_enUS1018US1018&sxsrf=AJOqlzXMhkb7heLX_erc4SjkP98a3QJRg:1676305832266&q=Is+the+Earth+a+blackbody+radiator %3F&sa=X&ved=2ahUKEwij46rt9ZL9AhVSmmoFHSDVDxgQzmd6BAg WEAU&biw=1536&bih=714&dpr=1.25
The second condition of a black body absorber/emitter is that the surface must be in thermal equilibrium. But this is never the case with the surface of the
earth. First, daylight energy is constantly changing even during daylight hours and at zero at night. This is a fundamental flaw in assuming that the earth has one uniform temperature of about 15 C 24 hours/day from the equator to the poles. Then, the sun is not the only source of heat to the surface. “Earth's internal heat budget is fundamental to the thermal history of the Earth. The flow of heat from Earth's interior to the surface is estimated at 47±2 terawatts (TW)[1] and comes from two main sources in roughly equal amounts: the radiogenic heat produced by the radioactive decay of isotopes in the mantle and crust, and the primordial heat left over from the formation of Earth.” and https://en.wikipedia.org/wiki/Earth%27s_energy_budget. However, this assumption ignores the heat of friction of the molten core as it tries to keep the unbalanced earth dynamically balanced as it spins around its axis; see below for the waterless Earth. The question is how accurate this estimate is as a global average and the heat induced by the enormous and shifting kinetic sources introduced by plate tectonics and orbital forces.

ii[] Quote: “.. the discrepancy between the Boltzmann equation and the experiment may always be blamed upon the experiments. If one does things right, the Boltzmann equation is theoretically completely exact (issues will be discussed below). However, it's pretty hard to measure the distribution functions "directly,” especially if you want the full dependence both on momenta and positions, so one may say that the "weak link" is always blamed on the experimental side (after errors are fixed).” See item 5, of the article”
Accuracy of the Boltzmann equation.”
https://physics.stackexchange.com/questions/2933/accuracy-of-theboltzmann-equation
iii[] The figure From Peter Langdon Ward’s book, What Really Causes Global Warming, fig 10.2.
iv[] This figure is from page 64 of the book by Dr. Peter Langdon Ward titled “What Really Causes Global Warming.” See also a detailed article titled “Radiant Thermal Energy (either heat or radiation) Is Not Additive” Ward2016ThermalEnergy160223.pdf (whyclimatechanges.com)
See the paper
Ward2016ThermalEnergy160223.pdf (whyclimatechanges.com), Quote: “Energy comes in two fundamentally different forms: 1) those generally associated with the net linear displacement or deformation of macroscopic matter, such as kinetic, potential, mechanical, mechanical wave, elastic, and mechanical work energies, and 2) those associated with microscopic oscillations of molecular and atomic bonds that hold matter together, such as thermal, heat, chemical, electric, magnetic, radiant, nuclear, and ionization energies. We study macroscopic energies primarily via classical mechanics. We study microscopic energies primarily via quantum mechanics. Macroscopic energies typically depend on position, distance travelled, velocity, acceleration, mass, or extent of the object and are therefore said to have extensive physical properties. Energy that is extensive “comes in little blobs of a definite amount” that generally can be added together. Kinetic energy, for example, includes mass (m) and velocity (v) in its definition (Ek=½mv2 ). When you double the mass, you double the energy. When you double the velocity, you quadruple the energy. Macroscopic energies are typically additive.
Microscopic energies, on the other hand, involve simultaneous oscillation of all the molecular and atomic bonds that hold matter together. Sometimes, these bonds oscillate at high enough frequencies to be “shaken” apart, causing the matter to melt or become dissociated. Every piece of every atom is involved in these oscillations in some way—the smaller the piece, the higher the frequency of oscillation and the higher the energy holding the bond together. These microscopic oscillatory energies are pervasive throughout matter, do not depend on the size or extent of matter, and are, therefore, described as intensive. Intensive physical properties are typically not additive. Microscopic oscillations have two physical properties—frequency of oscillation and amplitude of oscillation. The mass may affect the frequency of oscillation but is typically not changing and thus does not need to be included in the equation defining oscillatory energy. These atomic oscillators are frictionless, so that length of travel is not a factor affecting energy. The extent or amplitude of oscillation is always changing, but there is no net change except as a very important function of temperature described below. Thus frequency, the rate of change of the length of the bond, has the primary effect on microscopic energy. If energy powers change, higher energy powers a higher rate of change. A higher rate of change for cyclical systems is, by definition, a higher frequency. At atomic scales, energy appears simply to be frequency times an appropriate scaling constant. Think of microscopic energy as the pulse, the heartbeat, of matter that gives rise to temperature and goes to zero at a temperature of absolute zero.”
vi[]
Figure 3d shows how the UN IPCC presents the absorption as a photon bullet and a photon bullet remitted. That is contrary to what Richard Feynman said: “Richard Feynman, one of the best known and most provocative physicists of the 20th century, wrote in his Lectures on Physics: “It is important to realize that in physics today, we have no knowledge of what [radiative] energy is. We do not have a picture that energy comes in little blobs of a definite amount” that can be added together (Feynman et al., 1963) (p. 4-2).” See https://ozonedepletiontheory.info/energy-not-additive/#:~:text=We%20do %20not%20have%20a%20picture%20that%20energy,the%20study%20of %20how%20radiation%20interacts%20with%20matter.
Instead, Feynman said that when a quantum of electromagnetic energy is absorbed and re-emitted by CO2, it is done as an array of frequencies, as shown in Figure-3d1. The re-emitted photons are randomly directed spherically from the CO2 molecule. Almost 50% go upward in the atmosphere and 50% downward towards the surface. So, let’s look at Image-a below.

This is the Earth Energy Budget (EEB) back in 2009, when NASA-NOAA maintained a greater integrity level, and see what they said then about radiation coming from the surface.The complete article is Climate and Earth’s Energy Budget. Here, we now focus only on the portion of the heat that exists from the surface through radiation.
17% of the heat from the surface is released by radiation, of which about 5-6% interacts with the atmosphere, including Greenhouse gases. The other 12% goes directly to space through the Atmospheric window, and the greenhouse gases (CO2) absorb the remaining 5-6% in the atmosphere. Then, instantaneously, a new photon is remitted spherically in random directions, with almost half going up and almost half coming back down and the rest scattered/reflected.
Of the ones that go upward, approximately 30% are emitted at frequencies that overlap with the atmospheric window, and these go directly to space with no further CO2/GHG absorptions. The remaining 70% must compete with other Greenhouse gasses, including the most abundant water vapor, which dominates the troposphere, especially in the first few kilometers. When water vapor absorbs a LWIR photon emitted by a CO2 molecule, it is remitted at the full spectrum of water vapor, not the CO2 spectrum. That means that these Water Vapor-emitted LWIR photons are no longer absorbable by CO2 a second time. This greatly reduces the influence of CO2 activities in the troposphere. So, in the troposphere the chances of an LWIR photon being absorbed a second time by another CO2 molecule are small, and a third time is almost impossible. Above the troposphere, where CO2 quantities dominate over water vapor, only a tiny fraction of the LWIR photons are resonant/absorbable by CO2.
The surface most likely absorbs the LWIR photons that go downward. However, since these CO2 photons are emitted from a CO2 molecule with a radiative temperature of almost -84 C, can't warm the earth that’s radiating its photons at a temperature of 15 C. As discussed above, energy only flows from a higher state to a lower state, never the other way around. So, a more likely scenario is that these LWIR photons might actually cool the surface. At any rate, once absorbed by the surface, the surface will incorporate the CO2 photon and emit its own new photons at the full spectrum radiated by the surface. Essentially, this reduces the impact of the absorbed CO2 photon to zero.
vii[] See the article “Water Vapor Rules the Greenhouse System,” by Dr. S F Singer
http://arizonaenergy.org/WaterEnergy/Water%20Vapor%20Rules%20the %20Greenhouse%20System.htm. In the paper, Dr. Singer concludes by giving an estimated greenhouse effect, as shown in the table below, of the contribution
of greenhouse gases.

viii[] Air molecules, including CO2, do not sit stationary in the air, waiting to absorb LWIR radiation. Instead, they constantly fly around in random directions, bumping into things like other air molecules. And each time they bump, some of their kinetic energy of motion transforms into the thermal energy of heat. This is part of the natural order that converts kinetic energy into thermal (heat) energy, which warms the air.
Gravity is a source of atmospheric warming as it continuously compresses the air molecules closer together, exponentially increasing the number of collisions by the atmospheric molecules. The kinetic energy of a moving molecule or atom can be calculated by the formula Kv=1/2mv2 and by its Boltzmann temperature equivalent of 1.5kBT. Here, m is the particle’s mass, Kb is the Boltzmann constant, and T is air temperature. Source
https://phys.libretexts.org/Bookshelves/Thermodynamics_and_Statistical_Me chanics/
Supplemental_Modules_(Thermodynamics_and_Statistical_Mechanics)/ Thermodynamics/1.12%3A_Brownian_Motion
We see the result as an atmospheric lapse rate. The actual temperature then is based on the number of collisions that occur in a period and the number of molecules in a volume of air. And as we all know, air density is exponentially related to altitude above sea level. For example, the Earth’s atmosphere extends outward to about 1,000 kilometers. However, most of the atmosphere’s mass (greater than 99 percent) is within the first 40 kilometers. The figure below shows the atmospheric lapse rates of temperature, pressure, and air density and how they decline with increased altitude. Source
https://en.wikipedia.org/wiki/Atmosphere_of_Earth#/media/File:Comparison _US_standard_atmosphere_1962.svg

ix[] Heat can only flow in one direction, from a hotter object (higher energy state) to a colder one (lower energy state), as described by the second law of thermodynamics. See the image below. A hot stove with a surface temperature of 250 F will warm your body with a surface temperature of 80F radiation. Your 80 F body will also radiate longwave IR radiation that cools your body. But, our body’s 80 F radiant energy cannot warm the 250 F stove.

x[] What is the Second Law of Thermodynamics? The second law of thermodynamics states that any spontaneously occurring process will always lead to an escalation in the entropy (S) of the universe. The law explains that an isolated system's entropy (energy) will never decrease over time.
https://www.sciencedirect.com/topics/chemistry/first-law-ofthermodynamics#:~:text=The%20first%20law%20of%20thermodynamics %20states%20that%20energy%20can%20neither,energy%20within%20the %20control%20volume.
The first law of thermodynamics states that energy can neither be created nor destroyed, only altered in form. Energy transfer is associated with mass crossing
the control boundary, external work, or heat transfer across the boundary for any system. These produce a change of stored energy within the control volume. First Law of Thermodynamics - an overview - ScienceDirect.com
xi[] See the article Heat Trapping. Quote: “The free path before an IR photon collides with a CO2 molecule in the atmosphere is 33 meters. CO2 can’t retain thermal energy for more than 100 µs at surface level.”
https://energyeducation.ca/encyclopedia/Thermal_equilibrium.
See also
https://www.researchgate.net/publication/346967791_Delay_Time_for_Terres trial_InfraRed_Radiation_to_escape_Earth's_Atmosphere-2020b and http://nov79.com/gbwm/emit.html.
xii[] CO2/GHG does not power El Ninos induced global warming; instead, the main source of the warming of the Pacific Ocean waters is geologic/tectonic/volcanic heating from submarine eruption. See the article “Why El Niño and La Niña are One Continuous Geological Event”
http://www.plateclimatology.com/why-el-nio-and-la-nia-are-one-continuousgeological-event/ . See also the article “How Geological Heating Refuels El Niño”
http://www.plateclimatology.com/how-geological-heating-refuels-el-nio.
See also article Argo Data Confirms El Niño/La Niña Caused By Underwater Volcanoes. https://www.strata-sphere.com/blog/index.php/archives/18084
See also the video Volcanic Eruptions, a Driver of Natural Climate Variability – ignored by IPCC - Professor Wyss Yim.
https://www.youtube.com/watch?v=OlTlMXR_tSw. The slides used in the video can be downloaded here (PDF) Volcanic eruptions, a driver of climate variability -ignored by IPCC (researchgate.net)
xiii[] See Peter Langdon Ward’s Video. “Geologic evidence for how volcanoes have driven climate change throughout Earth history”
https://www.youtube.com/watch?v=FPH7HPaNHTg&t=1922s
Nature Communications; 13 March 2023. “Bottom marine heatwaves along the continental shelves of North America.”
https://www.nature.com/articles/s41467-023-36567-0
“The Axial Seamount - Nature’s Response To 500 Years of Cooling” (99+) The Axial Seamount - Nature’s Response To 500 Years of Cooling | Jim Le Maistre - Academia.edu
Worldwide volcanic activities growing. Increase in Worldwide Volcanic
Eruptions

