Official Publication of SDCMS OCTOBER 2022 THE GERM ISSUE Monkeypox 2022 in San Diego WHAT DO I NEED TO KNOW? WHAT DO I NEED TO KNOW?







Thank You to our Sponsors
Stony Anderson
Maria Carriedo Liliana
Osorio
Eimaneh Mostafian Jim Schultz Kosala Samarasinghe
Editor: James Santiago Grisolia, MD
Editorial Board: James Santiago Grisolia, MD; David E.J. Bazzo, MD; Robert E. Peters, MD, PhD; William T-C Tseng, MD
Marketing & Production Manager: Jennifer Rohr
Art Director: Lisa Williams
Copy Editor: Adam Elder
OFFICERS
President: Sergio R. Flores, MD
President–Elect: Toluwalase (Lase) A. Ajayi, MD
Secretary: Nicholas (Dr. Nick) J. Yphantides, MD, MPH
Immediate Past President: Holly B. Yang, MD, MSHPEd, HMDC, FACP, FAAHPM
GEOGRAPHIC DIRECTORS
East County #1: Catherine A. Uchino, MD
East County #2: Rakesh R. Patel, MD
Hillcrest #1: Kyle P. Edmonds, MD
Hillcrest #2: Steve H. Koh, MD (Board Representative to the Executive Committee)
Kearny Mesa #1: Anthony E. Magit, MD, MPH
Kearny Mesa #2: Alexander K. Quick, MD
La Jolla #1: Preeti S. Mehta, MD (Board Representative to the Executive Committee)
La Jolla #2: David E.J. Bazzo, MD, FAAFP
La Jolla #3: Sonia L. Ramamoorthy, MD, FACS, FASCRS
North County #1: Arlene J. Morales, MD
North County #2: Christopher M. Bergeron, MD, FACS
North County #3: Nina Chaya, MD
South Bay #1: Paul J. Manos, DO
South Bay #2: Maria T. Carriedo-Ceniceros, MD
AT–LARGE DIRECTORS
#1: Thomas J. Savides, MD
#2:
C. Motadel, MD,
(Reno) D. Tiangco,
R. Sonneborn,
M. Wilson,
E. Steinberg,
Postlethwaite,
VOTING DIRECTORS
Medical Student: Jimmy Yu
Nicole L. Herrick, MD
Young Physician: Brian J. Rebolledo, MD
Mitsuo Tomita, MD
OFFICERS AND TRUSTEES
Robert E. Wailes, MD
William T–C Tseng, MD, MPH
Sergio R. Flores, MD
Timothy Murphy, MD
DELEGATES AND ALTERNATE DELEGATES
District I: Mihir Y. Parikh, MD
District I Alternate: William T–C Tseng, MD, MPH
At–Large: Albert Ray, MD
At–Large: Robert E. Hertzka, MD
At–Large: Theodore M. Mazer, MD
At–Large:
Opinions expressed by authors are their own and not necessarily those of San Diego Physician or SDCMS. San Diego Physician reserves the right to edit all contributions for clarity and length as well as to reject any material submitted. Not responsible for unsolicited manuscripts. Advertising rates and information sent upon request. Acceptance of advertising in San Diego Physician in no way constitutes approval or endorsement by SDCMS of products or services advertised. San Diego Physician and SDCMS reserve the right to reject any advertising. Address all editorial communications to Editor@SDCMS.org. All advertising inquiries can be sent to DPebdani@SDCMS.org.
San Diego Physician is published monthly on the first of the month. Subscription rates are $35.00 per year. For subscriptions, email Editor@SDCMS.org. [San Diego County Medical Society (SDCMS) Printed in the U.S.A.]
John D. Malone, MD, MPH, FIDSA, FACP, FACPE; Aileen M. Marty, MD, FCAP; Christian K. Bey, MPH, W-EMT; and Kristi L. Koenig, MD, FACEP, FIFEM, FAEMS
Michael Butera, MD, FIDSA
Ni-Cheng Liang,
Frank Edward Myers III, MA, CIC, FAPIC
Adama Dyoniziak
By Kim Delahanty, BSN, PHN, MBA/HCM, CIC, FAPIC
Kelly
MPH #3: Irineo
MD #4: Miranda
MD #5: Stephen R. Hayden, MD (Delegation Chair) #6: Marcella (Marci)
MD #7: Karl
MD, FAAFP #8: Alejandra
MD ADDITIONAL
Resident:
Retired Physician:
CMA
AMA
Kyle P. Edmonds, MD At–Large: Holly B. Yang, MD, MSHPEd, HMDC, FACP, FAAHPM At–Large: David E.J. Bazzo, MD, FAAFP At–Large: Sergio R. Flores, MD At–Large Alternate: Bing Pao, MD CMA DELEGATES District I: Karrar H. Ali, DO, MPH District I: Steven L.W. Chen, MD, FACS, MBA District I: Franklin M. Martin, MD, FACS District I: Vimal I. Nanavati, MD, FACC, FSCAI District I: Peter O. Raudaskoski, MD District I: Kosala Samarasinghe, MD District I: James H. Schultz, MD, MBA, FAAFP, FAWM, DiMM District I: Mark W. Sornson, MD District I: Wynnshang (Wayne) C. Sun, MD District I: Patrick A. Tellez, MD, MHSA, MPH RFS: Rachel Buehler Van Hollebeke, MD Contents OCTOBER VOLUME 109, NUMBER 8 Departments 2 Briefly Noted: Legislative Issues • Public Health 18 Healers Need Healing, Too By
MD 20 In Good Hands By
21 Classifieds SANDIEGOPHYSICIAN.ORG 1 Features 3 What Do I Need to Know? Monkeypox 2022 in San Diego By
8 Biomedical HIV Prevention Strategies and Getting to Zero, 2022 Updates By
13 New Endoscope Reprocessing Guidelines Attempt to Lower the Infection Risk By
16 Infection Prevention and Control in Low- and-ResourcePoor Countries
State Legislature Passes CMASupported Bill to Provide More eRx Flexibility for LowVolume Prescribers
A CALIFORNIA MEDICAL ASSOCIATION (CMA)-supported bill to provide exemptions to e-prescribing requirements for low-volume prescribers has passed the state legislature.

The bill — Assembly Bill 852 — will also provide exemptions for practices affected by federal, state, or local emergencies or disasters. The bill passed the State Senate by a vote of 32 to 2 and is now on Governor Newsom’s desk.
“AB 852 will give physicians more flexibil ity in complying with California’s prescrib ing mandate,” says CMA President Robert E. Wailes, MD. “By allowing exceptions to e-prescribing requirements for healthcare practitioners who meet certain criteria, this bill will ensure that patients are able to get the medications they need without delay.”
Since California’s e-prescribing mandate took effect in January of this year, it has proven to be a costly burden for low-volume prescrib ers. E-prescribing software often comes with a monthly subscription cost, and for providers who write only a handful of prescriptions a year, the software can cost upward of $20 per prescription. This bill will align California law with federal regulations, and ease this burden at a time when physicians are still reeling from the effects of the COVID-19 pandemic.
AB 852 will provide e-prescribing exemp tions for providers who:
• Issue 100 prescriptions or less in a year
• Are practicing in areas affected by natural disasters, officially declared disaster, or an emergency zone
• Are granted a waiver based on other extraor dinary circumstances.
CMA Updates COVID Vaccine Billing Resources to Include New Bivalent Boosters
IN A CRITICAL NEXT STEP FORWARD in our country’s COVID-19 vaccination program, the U.S. Centers for Disease Control and Preven tion (CDC) and U.S. Food and Drug Administra tion (FDA) last week approved the use of updated COVID-19 boosters from Pfizer-BioNTech for people ages 12 years and older and from Moderna for people ages 18 years and older.
The updated boosters are adapted for the BA.4 and BA.5 Omicron subvariants and the original coronavirus strain in a single dose, helping to restore protection that has waned since previous vaccination by target ing variants that are more transmissible and immune-evading.
In the coming weeks, CDC also expects to recommend updated COVID-19 boosters for other pediatric groups.

The American Medical Association (AMA) has announced an editorial update to Current Procedural Terminology (CPT), that includes eight new codes for the bivalent COVID-19 vaccine booster doses from Moderna and Pfizer-BioNTech.
The new product and administrate codes assigned to each bivalent COVID-19 vaccine booster are now included in the CMA CO VID-19 Vaccine Toolkit for Physician Practices and CMA COVID-19 Vaccine Reimbursement Quick Guide.
To help ensure accurate coding and report ing of COVID-19 vaccines and immunization services, AMA also offers a vaccine code finder resource to help identify the appropriate CPT code combination for the type and dose of CO VID-19 vaccine provided to each patient.
2 OCTOBER 2022 BRIEFLY NOTED LEGISLATIVE ISSUES
PUBLIC HEALTH
Monkeypox 2022 in San Diego
WHAT DO I NEED TO KNOW?
BY JOHN D. MALONE, MD, MPH, FIDSA, FACP, FACPE; AILEEN M. MARTY, MD, FCAP; CHRISTIAN K. BEŸ, MPH, W-EMT; AND KRISTI L. KOENIG, MD, FACEP, FIFEM, FAEMS
Introduction
On July 23, the World Health Organiza tion (WHO) declared the global mon keypox (mpox) outbreak a Public Health Emergency of International Concern. A California State Emergency Proclama tion, San Diego County Local Health Emergency Declaration, and Federal Public Health Emergency Declaration followed on Aug. 1, 2, and 4, respectively. Mpox was first described in Asian mon keys imported to Copenhagen in 1958.1 Until recently, mpox was a zoonosis with limited human-to-human transmission, most likely harbored in nature by wild rodents. 2 In 2003, rodents imported from
Ghana to the United States were housed next to prairie dogs, which became infected. The prairie dogs subsequently produced 47 confirmed or probable hu man mpox cases. 3,4
Monkeypox virus is a species of the Orthopoxvirus genus and a member of the Poxviridae family, a group of large, brick- or oval-shaped, complex, doublestranded DNA viruses that replicate in the cytoplasm of the host cell. Other Orthopoxviruses capable of infecting humans include the vaccinia virus, cow pox virus, and smallpox virus (variola, declared eradicated in 1980).
This article will review mpox epi
demiology, transmission properties, clinical presentations, risk reduction (counseling/vaccines), treatments, con siderations for special populations, and reporting requirements. Key concepts are summarized in Figure 1.
Epidemiology
As evidenced by rising case numbers and detection of viral particles in wastewater, human-to-human transmission of the monkeypox virus is ongoing in San Diego County. 5 Laboratory-confirmed human mpox case numbers and demographics as of Sept. 3 are displayed in Figure 2.
While initial mpox patients were pre dominantly men who have sex with men (MSM), the virus is transmissible to any individual regardless of age, gender, sex, race, or sexual orientation. Social group stigmatization can lead to adverse health outcomes for historically marginalized populations.
At-risk community outreach and education are important public health interventions to reduce disease spread. The San Diego County Health and Hu man Services Agency website contains educational brochures for the public and resources for health professionals.6,7 Additional information for healthcare professionals is available on the Centers for Disease Control and Prevention (CDC) website.8
Transmission/Infection Control
Monkeypox virus, like all Orthopoxvi ruses, is stable in the environment for days to months.9 The virus can survive in scabs for months to years and resists des iccation in hot and cold environments.10 Monkeypox viral particles can enter broken skin, the respiratory tract, and mucosal surfaces (e.g., mouth, nose, eyes, vagina, and anus). Close physical contact, especially sexual contact, is a major mode of spread, but transmission can hap pen by inhalation of large droplets and possibly by aerosols.11,12 To mitigate these risks, healthcare practitioners should counsel their patients on risk-reduction behavior.13,14
In contrast to SARS-CoV-2, the concen tration of monkeypox virus in aerosols is usually negligible. However, since the shaking of infected linens can aerosolize
SANDIEGOPHYSICIAN.ORG 3 INFECTIOUS DISEASES
high concentrations of monkeypox virus, these actions can produce infection. For example, in 2018, nosocomial contact transmission via aerosols was document ed in a healthcare worker in England who contacted contaminated bed linens from a Nigerian patient.15
The threat of transmission from an asymptomatic infected person is unclear, but some evidence suggests this is pos sible. In a study of two hundred asymp tomatic MSM undergoing screening for Neisseria gonorrhoeae and Chlamydia trachomatis, 13 (6.5%) had PCR swabs positive for mpox. Two of the 13 later developed symptoms of mpox.16 A similar study of 706 MSM confirmed asymptom atic infection in 3%. The viral load of one patient was significantly higher when he was presymptomatic compared to after he became symptomatic.17
The monkeypox virus has been de tected on multiple contact surfaces, e.g., light switches, door handles, bathroom surfaces, towels, and an outside ban ister. 18 Although it is unclear whether transmission occurs from contact with these surfaces,19 attention to disinfection with approved cleaning agents such as bleach-based or quaternary ammonia compounds is encouraged. 20 Alcoholbased hand sanitizer easily inactivates the virus; frequent hand sanitization with soap and water is encouraged. Careful attention to cleaning protocols20 and han dling of bedsheets is critical. Wash linens and towels in hot water with detergent. In addition, as mpox may be transmissible to pets, 21 animals should ideally be removed from the home to reside with a trusted in dividual until persons living in the home are no longer infectious. 22
Suspected mpox cases should isolate at home pending a laboratory diagnosis. 23 If a person tests positive, isolation should be continued until all lesions have fully healed, leaving a fresh layer of skin. How ever, per CDPH guidelines, home isolation can be discontinued sooner with precau tions. 24
Clinical Presentation/Collection of Laboratory Specimens
The incubation period of mpox is 5–21 days (usually between 6–13 days), with a mean incubation time of 8.5 days. Various clinical symptoms are possible; hence, a
Figure 1. Monkeypox 2022 Key Concepts
Transmission
Affected Populations
Monkeypox is an Orthopoxvirus primarily transmitted through close, skin-to-skin contact. Large droplets, and potentially aerosols from sheets and bedding of infected individuals, can also spread infection; however, this route is much less likely. While transmission during sex is frequent, monkeypox is not considered a sexually transmitted infection (STI). However, co-infections with STIs, e.g., gonor rhea, chlamydia, and syphilis, are common.
While monkeypox 2022 primarily affects men who have sex with men (MSM), spread to other populations is likely. Cases have been reported in women and children.
Infection
Infection can last 21 days or longer. Individuals are no longer infectious and can stop isolation when scabs desquamate from the skin surface and new skin has formed. Lesions can present as small papules and progress to pseudo-pustules. Unlike the classical presentation of monkeypox, lesions are frequently in different stages in the same body area. Constitutional symptoms of myalgia, fever, fatigue, and possibly lymphadenopathy are present prior to the rash in about 60% of cases. These symptoms may manifest concurrent with or after skin lesions ap pear. In addition to co-infection with other STIs, co-infections with SARS-CoV-2, varicella, or other viral infections have been reported.
Sample Collection and Precautions
Individuals with suspect lesions, especially those with a linked history of close, skin-to-skin contact, should have samples collected by vigorously rubbing lesions with a swab. Samples should be submitted to a laboratory for polymerase chain reaction (PCR) testing. Healthcare providers should don full PPE (N95 respirator/ equivalent or higher, gowns, gloves, goggles/face shield, and shoe covers) when evaluating suspect patients or collecting samples. Housekeeping staff should don full PPE and avoid shaking bed linens. Infection control measures must be care fully followed during their removal from patient treatment areas.
Prevention: Vaccination & Behavior Risk Reduction
Vaccination with the Jynneos smallpox/monkeypox vaccine within 3-4 days of exposure can prevent infection. Vaccination within 2 weeks of exposure may at tenuate infection. Due to supply shortages, as of mid-August 2022, the vaccine is primarily reserved for patients identified as high-risk. The Jynneos vaccine was originally FDA-approved for 0.5 ml subcutaneous injection with a repeat booster dose at 28 days. The FDA has issued an Emergency Use Authorization (EUA) for this vaccine to be administered as a 0.1 ml intradermal injection, thereby increas ing the available dose fivefold. The FDA EUA also authorizes injections to persons under 18 years of age, but only via the subcutaneous route. Clinicians should counsel their patients on behavior risk reduction.
Medications and Treatment
The deep-seated skin lesions (pseudo-pustules) can be significantly painful. Graduated pain control regimens should be considered along with other agents such as stool softeners for rectal area lesions. Tecovirimat (TPOXX) is an antiviral medication indicated for high-risk patients and severe cases. As of August 2022, TPOXX is under Expanded Access-Investigational New Drug (EA-IND) protocol requiring patient consent and additional forms.
strong suspicion based on an epidemio logic history is critical. In about 60% of individuals, viremia produces a prodrome that may include fever, chills, myalgias, back pain, and lymphadenopathy. Un commonly, mpox can produce severe and life-threatening conditions: cellulitis, ret ropharyngeal abscesses, deep-necrotizing infections, severe pneumonia, corneal infection/blindness, vomiting and diar rhea producing dehydration/electrolyte imbalances, severe adenopathy causing respiratory compromise, septic shock,
encephalitis, and death. 25
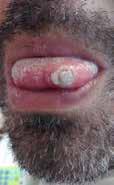
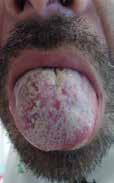
While mpox 2022 presentations may vary, the classic rash lesions progress through stages: macular (1–2 d), papular (1–2 d), vesicular (1–2 d), pseudo-pustular (5 d), and scabbing (7–14 d), followed by new skin formation (Figure 3). [10] Lesions can present at different stages in the same individual. Patients in the 2022 mpox outbreak may have only a single lesion. [26,32,33] Lesions can present in multiple locations, including the throat and anogenital areas (Figure 4). A full skin
4 OCTOBER 2022
INFECTIOUS DISEASES
Male
Median Age
Race/Ethnicity
Hispanic or Latino: 116 (45.7%)
White: 110 (43.3%)
Black or African American: 22 (8.7%)
Asian: (2.4%) Unknown: 51
Orientation
Gay, Lesbian, or Same-GenderLoving: 210 (85.4%)
Bisexual: 19 (7.7%)
Heterosexual or Straight: 13 (5.3%)
Declined to Answer: 4 (1.6%)
Unknown or Missing: 67
Region
Central: 173 (56%)
North Central: 52 (16.8%)
South: 27 (8.7%)
North Coastal: 27 (8.7%)
East: 15 (4.9%)
North Inland: 15 (4.9%)
Unknown or Missing: 4
examination, especially involving the anogenital area, is necessary.
Orthopoxvirus causes “deep-seated and fixed” lesions27 arising from the deep dermis. O. variola tends to grow best at cooler body locations helping to explain why lesions are often more common on the periphery (e.g., hands, feet, and face).28 Lesions of O. variola have an affinity for se baceous glands, which are common on the face. Lesions involving sebaceous glands are more likely to cause scars, which can be severe (Figure 3).29 The differential diag nosis for mpox includes varicella-zoster; genital herpes; syphilis; molluscum con tagiosum; Behçet’s disease; staphylococcal infections; tickborne eschars; and hand, foot, and mouth disease.
Infection with the monkeypox virus does not rule out co-infection with other
diseases, notably sexually transmit ted infections (STIs) that can present similarly, such as gonorrhea, chlamydia, and syphilis. Healthcare professionals should obtain comprehensive patient histories that detail important risk factors and liberally test any individual present ing with suspicious lesions. With nearly 10% of suspected or confirmed cases identifying as heterosexual and bisexual, clinicians should obtain a detailed history from women presenting with suspicious lesions to assess for risk factors.
After donning full personal protective equipment (N95 respirator/equivalent or higher, gowns, gloves, goggles/face shield, and shoe covers), collect specimens by vigorously swabbing lesions after provid ing pain control. [11] Lesions do not need to be unroofed. Several major commer cial testing laboratories are available to process samples. PCR lesion testing is also available at San Diego County Sexually Transmitted Disease clinics. 31
Vaccines
As mpox is closely related to smallpox, options for vaccinations and therapeutics are similar. The currently recommended vaccine for prevention and post-exposure prophylaxis is Jynneos, a third-generation vaccine containing a live, non-replicating virus called modified vaccinia Ankara. As Jynneos, a two-dose vaccine, is in short supply, a second-generation, single-dose vaccine, ACAM2000, is available for mpox under EA-IND; however, this live, replicating virus is contraindicated for some populations who may experience significant side effects.26,32,33,34 One study published in 1988 calculated that vac cination with first- or second-generation vaccines for smallpox imparted approxi mately 85% protection against mpox. 35 There are no human clinical trials on the efficacy of third-generation vaccines, such as Jynneos. The effectiveness of Jynneos is based on animal studies showing when ad ministered as pre-exposure prophylaxis, the vaccine can prevent severe illness and death from aerosolized mpox, though not as effectively as ACAM2000. 36,37 Regarding post-exposure prophylaxis, the relatively long mean incubation period for mpox (8.5 days) allows contact tracers to identify eligible persons with high-risk exposures. Vaccination within four days of exposure
is thought to be most protective against infection. Animal studies support vac cination up to two weeks post-exposure in asymptomatic persons to reduce infection severity. Importantly, the vaccine is not useful for treatment.
The initial supply of the Jynneos vaccine was extremely limited, partly due to a single overseas manufacturer in Denmark, Bavarian Nordic. While the ACAM2000 vaccine supply is greater, it is not expected to be widely used for mpox. ACAM2000 has a significant risk profile, including myopericarditis, potentially fatal dissemination in immunocompro mised individuals, inadvertent spread to partners, and self-inoculation. Both vac cines stimulate the cellular and humoral immune systems by initiating the dermal immune response. The Jynneos vaccine is safe and immunogenic in HIV-positive adults, 38 people with eczema/atopic dermatitis, 39 immunocompromise, and possibly pregnant women.40
Prior vaccination against smallpox may protect from mpox; however, if the last smallpox vaccination was greater than three years ago, high-risk individuals should receive the Jynneos vaccine. Popu lations who may have received recent smallpox vaccination include military service members, some public health workers, and certain laboratory workers.41
The Jynneos vaccine was originally approved by the FDA as a subcutaneous dose of 0.5 ml (1 vial) with a second dose administered at 28 days. To increase vaccine supply for initial doses, most local health jurisdictions have adopted an FDA-designated delay in second dose administration until more vaccine becomes available. Based on very limited studies,42,43,44 on Aug. 9, the FDA issued an EUA permitting intradermal (ID) admin istration of 0.1 ml of Jynneos vaccine on the volar aspect of the forearm for persons ≥18 years, thereby increasing the available supply fivefold. Jynneos vaccine admin istered ID results in more erythema and induration than subcutaneous adminis tration.45 ID administration is contrain dicated in patients with a propensity for keloid formation.46
Treatment
Antiviral medications originally devel oped for treating smallpox and other
SANDIEGOPHYSICIAN.ORG 5
Figure 2. Monkeypox 2022 County of San Diego Demographics as of 9/3/2022) 313 0
Sexual
HHSA
100% 35 Range: 20-65 Confirmed & Probable Cases 10 Hospitalizations Deaths
DNA viruses are being used in patients with mpox at risk for developing severe illness or presenting with severe pain.47 The most commonly used antiviral treat ment is tecovirimat (TPOXX). The treat ment blocks the formation of enveloped viruses, thereby reducing mpox trans mission and clinical disease. TPOXX is under FDA emergency authorization, requiring patient informed consent, preand post-therapy reporting, and a signed 1572 investigator form. These require ments may be simplified if the type of emergency authorization changes. At the time of this writing, the medication is only available from the U.S. Strategic National Stockpile through State and Lo cal Health Jurisdictions.48
For pediatric and adult patients weighing 40 kg to less than 120 kg, the dosing regimen is 600 mg of TPOXX (three 200-mg pills) every 12 hours for 14 days with a fatty meal. For those greater than 120 kg, the dosing regimen is 600 mg of TPOXX every eight hours. For full prescribing information, visit the FDA website.49
Pain management is paramount for mpox patients — some have required hospitalization for intractable pain. In addition to usual analgesic treatments, encouraging results with rapid improve ment over several days with TPOXX are being reported, especially in severe cases of painful oral, genital, anal lesions, and those with potential facial scarring. The timely healing of lesions decreases the potential for transmission and shortens isolation times. For milder cases, ibupro fen, acetaminophen, topical anesthetics, and stool softeners may be adequate.
Special Populations
Certain populations are at higher risk of severe disease with mpox infection. These include children under 8 years, the elderly, pregnant women, persons with skin conditions such as atopic dermatitis or eczema, and people with immunocom promising conditions, including uncon trolled HIV. 50
Children are most likely infected through close contact with an adult parent/caregiver. Post-exposure vaccine prophylaxis can be considered in asymp tomatic exposed children. Along with skin lesions, younger children are prone to encephalitis and have the potential for pharyngeal and respiratory bacterial co-infections. More common childhood infections, such as hand, foot, and mouth disease, can present with lesions resem bling mpox.
Reporting Requirements
Mpox is a reportable disease. All laborato ry-positive cases must be reported within one workday to the Epidemiology and Immunization Services Branch, County of San Diego Health and Human Services Agency (HHSA) using a Confidential Mor bidity Report 51 faxed to (858) 715-6458 or sent by secure e-mail to epi-cdreporting. hhsa@sdcounty.ca.gov. Timely reporting is essential for contact tracing and Jyn neos vaccination of close contacts.




Summary
This article reviewed diagnosing and managing suspected mpox patients using vaccines, antivirals, pain medications, and reporting requirements. Prompt reporting of suspected mpox cases to public health is essential for timely contact tracing.

Behavioral modifications and vaccination play critical roles in disease prevention and containment.
Mpox 2022 is an unprecedented global pandemic. As our knowledge of mpox is rapidly evolving, clinicians are encour aged to stay informed. In addition to applying the most current information on identification and management of indi vidual patients, healthcare workers play key roles in prevention, treatment, and containment of mpox.
Disclaimer
The opinions and assertions expressed herein are those of the authors. They do not necessarily reflect the official policy or position of the County of San Diego, Health and Human Services Agency; San Diego County Fire – Public Safety Group; or Florida International University. The authors report no conflicts of interest.
Due to the rapidly evolving nature of this outbreak, information should be considered current only at the time of submission for publication and may evolve as the science develops.
Dr. Malone is an infectious diseases physi cian with the Epidemiology and Immuniza tion Service Branch, County of San Diego. Dr. Marty is a distinguished university pro fessor for infectious disease and outbreak response at Florida International Univer sity. Christian K. Beÿ is at the Emergency Medical Services Office, Public Safety Group of San Diego County Fire, County of San Diego. Dr. Koenig is professor emerita of Emergency Medicine & Public Health, UC Irvine, and the Emergency Medical Services Office, Public Safety Group of San Diego County Fire, County of San Diego.
6 OCTOBER 2022
INFECTIOUS DISEASES
Figure 3. Monkeypox 2022 Facial Lesion Progression over 22 days.
Figure 4. Monkeypox 2022 Patients Seen in San Diego Emergency Departments Papular lesions on the arm and tongue of a patient with monkeypox. Images by: Roneet Lev, MD and Valerie Norton, MD
References
1. von Magnus P, Anderson EK, Petersen KB, BirchAndersen A. A pox-like disease in cynomolgus mon keys. Acta Pathol Microbiol Scand . 1959; 46:156-176. DOI: 10.1111/j.1699-0463.1959.tb00328.x
2. Monkeypox in animals. Centers for Disease Control and Prevention. Updated August 17, 2022. Accessed August 21, 2022. https://www.cdc.gov/poxvirus/mon keypox/veterinarian/monkeypox-in-animals.html
3. Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med . 2004;350(4):342–350. DOI:10.1056/NEJ Moa032299
4. Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773-80. DOI: 10.1086/505880
5. LaFee S, Scripps Research Communications. UC San Diego researchers add monkeypox to wastewater surveillance. UC San Diego Health. Published August 10, 2022. Accessed August 21, 2022. https://health. ucsd.edu/news/releases/Pages/2022-08-10-uc-sandiego-researchers-add-monkeypox-to-wastewatersurveillance.aspx
6. Monkeypox educational materials. County of San Diego Health & Human Services Agency. Updated Au gust 15, 2022. Accessed August 21, 2022. https://www. sandiegocounty.gov/content/sdc/hhsa/programs/ phs/community_epidemiology/dc/human-monkey pox/education.html
7. Monkeypox resources for health professionals. County of San Diego Health & Human Services Agency. n.d. Accessed August 21, 2022.https://www. sandiegocounty.gov/content/sdc/hhsa/programs/ phs/community_epidemiology/dc/human-monkey pox/healthprofessionals.html
8. Monkeypox: Information for healthcare profes sionals. Centers for Disease Control and Prevention. Updated August 19, 2022. Accessed August 21, 2022. https://www.cdc.gov/poxvirus/monkeypox/clini cians/index.html
9. Essbauer S, Meyer H, Porsch-Özçürümez M, Pfeffer M. Long-lasting stability of vaccinia virus (Orthopox virus) in food and environmental samples. Zoonoses Public Health . 2007;54(3-4):118-24. DOI: 10.1111/j.18632378.2007.01035.x
10. Master question list for monkeypox virus (MPXV). US Department of Homeland Security Science and Technology Directorate. Published July 19, 2022. Ac cessed August 21, 2022. https://www.dhs.gov/scienceand-technology/publication/st-master-question-listmonkeypox
11. MacIntyre CR, Das A, Chen X, et al. Evidence of long-distance aerial convection of variola virus and implications for disease control. Viruses. 2019;12(1):33. DOI: 10.3390/v12010033. https://www.mdpi.com/19994915/12/1/33
12. Russo AT, Grosenbach DW, Brasel TL, et al. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in Cyno molgus macaques. J Infect Dis. 2018;218(9):1490–1499. DOI:10.1093/infdis/jiy326
13. Monkeypox: Safer sex, social gatherings, and mon keypox. Centers for Disease Control and Prevention. Updated August 5, 2022. Accessed August 24, 2022. https://www.cdc.gov/poxvirus/monkeypox/sexual health/index.html
14. Spicknall IH, Pollock ED, Clay PA, et al. Modeling the impact of sexual networks in the transmission of monkeypox virus among gay, bisexual, and other men who have sex with men — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022. DOI: 10.15585/mmwr. mm7135e2
15. Monkeypox - United Kingdom of Great Britain and Northern Ireland. World Health Organization. Pub lished July 8, 2021. Accessed August 21, 2022. https:// www.who.int/emergencies/disease-outbreak-news/ item/monkeypox---united-kingdom-of-great-britainand-northern-ireland
16. De Baetselier I, Van Dijck C, Kenyon C, et al. Asymp tomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. medRxiv 2022. DOI: 10.1101/2022.07.04.22277226
17. Ferré VM, Bachelard A, Zaidi M, et al. Detection of monkeypox virus in anorectal swabs from asymp tomatic men who have sex with men in a sexually transmitted infection screening program in Paris, France [published online ahead of print, 2022 Aug 16]. Ann Intern Med. 2022;10.7326/M22-2183. DOI:10.7326/ M22-2183
18. Atkinson B, Burton C, Pottage T, et al. Infection-com petent monkeypox virus contamination identified in domestic settings following an imported case of monkeypox into the UK [published online ahead of print, 2022 Jul 15]. Environ Microbiol. 2022;10.1111/14622920.16129. DOI:10.1111/1462-2920.16129
19. Pfeiffer JA, Collingwood A, Rider LE, et al. High-con tact object and surface contamination in a household of persons with monkeypox virus infection — Utah, June 2022. MMWR Morb Mortal Wkly Rep. 2022. DOI: 10.15585/mmwr.mm7134e1
20. EPA releases list of disinfectants for emerging viral pathogens (evps) including monkeypox. Environ mental Protection Agency. Published May 26, 2022. Accessed August 24, 2022. https://www.epa.gov/ pesticides/epa-releases-list-disinfectants-emergingviral-pathogens-evps-including-monkeypox
21. Seang S, Burrel S, Todesco E, et al. Evidence of humanto-dog transmission of monkeypox virus [published online ahead of print, 2022 Aug 10]. Lancet. 2022;S01406736(22)01487-8. DOI:10.1016/S0140-6736(22)01487-8
22. Monkeypox: Pets in the home. Centers for Disease Control and Prevention. Updated August 17, 2022. Accessed August 22, 2022. https://www.cdc.gov/ poxvirus/monkeypox/specific-settings/pets-inhomes.html
23. Koenig KL, Marty AM, Beÿ CK. Monkeypox 2022: Is there a case for quarantine? Evidence Aid. Last up dated July 15, 2022. Accessed August 21, 2022. https:// evidenceaid.org/monkeypox-2022-is-there-a-casefor-quarantine/
24. Monkeypox home isolation guidance for the general public. State of California Health and Human Services Agency, California Department of Public Health. Published August 18, 2022. Accessed August 21, 2022. https://www.cdph.ca.gov/Programs/CID/DCDC/ Pages/MPX/MPX-Home-Isolation-Guidance-for-theGeneral-Public.aspx
25. Clinical management and infection prevention and control for monkeypox: Interim rapid response guidance, 10 June 2022. World Health Organization. Published June 10, 2022. Accessed August 21, 2022. https://www.who.int/publications/i/item/WHOMPX-Clinical-and-IPC-2022.1
26. Koenig KL, Beÿ CK, Marty AM. Monkeypox 2022 Identify-Isolate-Inform: A 3I tool for frontline clinicians for a zoonosis with escalating human com munity transmission. One Health. 2022;15:100410. DOI. org/10.1016/j.onehlt.2022.100410
27. Pauli G, Blümel J, Burger R, et al. Orthopox viruses: Infections in Humans. Transfus Med Hemother. 2010;37(6):351-364. DOI:10.1159/000322101
28. Orenstein W, Offit PA, Edwards KM, Plotkin SA. Plot kin’s Vaccines. 7th ed. Elsevier, Inc; 2018.
29. Fenner F, Wittek R, Dumbell KR. The Orthopoxvi ruses. Academic Press, Inc; 1989.
30. Monkeypox: Specimen collection. Centers for Disease Control and Prevention. Updated August 19, 2022. Accessed August 21, 2022. https://www.cdc.gov/ poxvirus/monkeypox/clinicians/prep-collectionspecimens.html
31. HHS expanding monkeypox testing capacity to five commercial laboratory companies. Department of Health and Human Services. Published June 22, 2022. Accessed August 24, 2022. https://www.hhs.gov/ about/news/2022/06/22/hhs-expanding-monkeypoxtesting-capacity-five-commercial-laboratory-com panies.html
32. Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries –April-June 2022. N Engl J Med. 2022:1-12. DOI:10.1056/ NEJMoa2207323
33. Sklenovská N, Van Ranst M. Emergence of monkey pox as the most important Orthopoxvirus infection in humans. Front Public Health. 2018;6:241. DOI:10.3389/ fpubh.2018.00241
34. Malone JD. Pre-event smallpox vaccination for healthcare workers revisited--the need for a carefully screened multidisciplinary cadre. Int J Infect Dis. 2007;11(2):93–97. DOI: 10.1016/j.ijid.2006.11.005
35. Fine PE, Ježek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human popula tions. Int J Epidemiol. 1988;17(3):643-50. DOI: 10.1093/ ije/17.3.643
36. Hatch GJ, Graham VA, Bewley KR, et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in Cynomolgus macaques. J Virol. 2013 Jul;87(14):7805-15. DOI: 10.1128/JVI.03481-12
37. Earl PL, Americo JL, Wyatt LS, et al. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunode ficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;15;366(1):84–97. DOI: 10.1016/j.virol.2007.02.041
38. Overton ET, Lawrence SJ, Stapleton JT, et al. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine. 2020;38(11):2600–2607. DOI: 10.1016/j.vac cine.2020.01.058
39. Darsow U, Sbornik M, Rombold S, et al. Long-term safety of replication-defective smallpox vaccine (MVA-BN) in atopic eczema and allergic rhinitis. J Eur Acad Dermatol Venereol. 2016;30(11):1971–1977. DOI: 10.1111/jdv.13797
40. Khalil A, Samara A, O’Brien P, et al. Monkeypox vaccines in pregnancy: Lessons must be learned from COVID-19. Lancet Glob Health. 2022;10(9):e1230–e1231. DOI: 10.1016/S2214-109X(22)00284-4
41. Rao AK, Petersen BW, Whitehill F, et al. Use of JYN NEOS (smallpox and monkeypox vaccine, live, non replicating) for preexposure vaccination of persons at risk for occupational exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Im munization Practices - United States, 2022 [published correction appears in MMWR Morb Mortal Wkly Rep. 2022;71(27):886]. MMWR Morb Mortal Wkly Rep. 2022;71(22):734–742. DOI:10.15585/mmwr.mm7122e1
42. Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intra dermal routes of administration in healthy vaccinianaïve subjects. Vaccine. 2015;33(39):5225–5234. DOI:10.1016/j.vaccine.2015.06.075
43. Earl PL, Americo JL, Wyatt LS, et al. Rapid protec tion in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci U S A. 2008;105(31):10889–10894. DOI:10.1073/ pnas.0804985105
44. Greenberg RN, Hay CM, Stapleton JT, et al. A random ized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modi fied vaccinia Ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11(6):e0157335. DOI:10.1371/journal.pone.0157335
45. Monkeypox: JYNNEOS vaccine. Centers for Disease Control and Prevention. Updated August 10, 2022. Accessed August 21, 2022. https://www.cdc.gov/pox virus/monkeypox/interim-considerations/jynneosvaccine.html
46. Monkeypox: Vaccination administration consider ations for specific populations. Centers for Disease Control and Prevention. Updated August 9, 2022. Accessed August 21, 2022. https://www.cdc.gov/pox virus/monkeypox/interim-considerations/specialpopulations.html
47. Desai AN, Thompson GR, Neumeister SM, et al. Compassionate use of tecovirimat for the treatment of monkeypox infection. JAMA. 2022. Published online ahead of print. DOI: 10.1001/jama.2022.15336
48. Monkeypox: Information for healthcare providers on obtaining and using TPOXX (tecovirimat) for treat ment of monkeypox. Centers for Disease Control and Prevention. Updated August 18, 2022. Accessed August 24, 2022. https://www.cdc.gov/poxvirus/monkeypox/ clinicians/obtaining-tecovirimat.html
49. Highlights of prescribing information: TPOXX (teco virimat). Food and Drug Administration. Revised May 2022. Accessed August 21, 2022. https://www.accessda ta.fda.gov/drugsatfda_docs/label/2022/214518s000lbl. pdf
50. Monkeypox: Clinical Considerations for Treatment and Prophylaxis of Monkeypox Virus Infection in People with HIV. Centers for Disease Control and Prevention. Updated August 17, 2022. Accessed August 21, 2022. https://www.cdc.gov/poxvirus/monkeypox/ clinicians/people-with-HIV.html
51. State of California – Health and Human Services Agency / California Department of Public Health con fidential morbidity report. County of San Diego Health and Human Services Agency. Published July 2020. Accessed August 31, 2022. https://www.sandiego county.gov/content/dam/sdc/hhsa/programs/phs/ documents/CMRa.pdf
SANDIEGOPHYSICIAN.ORG 7
Biomedical HIV Prevention Strategies and Getting to Zero, 2022 Updates
BY MICHAEL BUTERA, MD, FIDSA
IN THE 2019 EDITION OF SAN DIEGO Physician magazine we reviewed the biomedical HIV prevention strategies and getting to Zero initiative. Getting to Zero consists of three primary strate gies to end the HIV pandemic.
Strategy 1: Test
CDC recommended in 2006 that all adults regardless of risk be tested for HIV. In 2013 the U.S. Preventive Services Task Force (USPSTF) recommended that all adults ages 15–65 be HIV tested in healthcare set tings with a Grade A recommendation.

Strategy 2: Treat
This involved prompt linkage to care, institution and continuity of antiviral therapy, and attaining undetectable viral load. When HIV is suppressed with antiretroviral therapy, the major health
8 OCTOBER 2022
INFECTIOUS DISEASES
benefit is that the progression of HIV disease is effectively halted and the rate of transmission to others eliminated. Undetectable equals untransmittable, and this is true for both sero-discordant heterosexual couples and sero-different MSM couples.
Strategy 3: Prevent Prevention of new HIV transmission by using proven intervention, including preexposure prophylaxis (PrEP), and postexposure prophylaxis (PEP). HIV PrEP involves use of antiretroviral medication in HIV-negative individuals at risk for HIV exposure, and HIV PEP involves treat ment with antiretroviral medication to prevent HIV infection in persons recently potentially exposed via sexual activity, or blood exposure/intravenous drug use.
This review will focus on recent data and studies that have led to changes in recommendations regarding HIV PrEP.
Current Situation
• The U.S. goal includes reducing the
number of new HIV cases 75% by 2025 and 90% by 2030.
• Current estimates are that there are 37.7 million PLHIV in the world, with 1.1 million in the U.S. and still about 35,000 new cases per year in the U.S., and thus not on track to reach 2025 and 2030 targets.
• AIDSVu.org (2022) data shows:
» In 2019 there were 366 new HIV cases in San Diego County (down from 603 in 2009) with 86.6% of these males, 12% African Ameri can, 53.3% Hispanic/Latino, 27.9% Caucasian.
» Decline in new HIV diagnoses in the U.S. from 48,073 in 2008 to 36,720 in 2019.
• 2021 data show 4260 PrEP users in San Diego County, 94 percent men, 64.4 percent between 25 to 54 years of age.
• PrEP use in the USA has also increased from 10,494 in 2012 to 227,000 in 2019, with 92.6% of use in males and 63% in the 25 to 54 age range.
• The PrEP to need ratio of the numbers
of PrEP users to newly diagnosed HIV cases is a way to track unmet need and healthcare disparities. PNR in 2012 0.21 up to 11.64 in 2021, 12.73 among men and only 4.49 among women and no data by ethnic racial group yet available.
The U.S. Public Health Service updated PrEP recommendations for the prevention of HIV infection in the US in 2021. The significant updates are as follows:
• Addition of a recommendation to inform all sexually active adults and adolescents about PrEP.
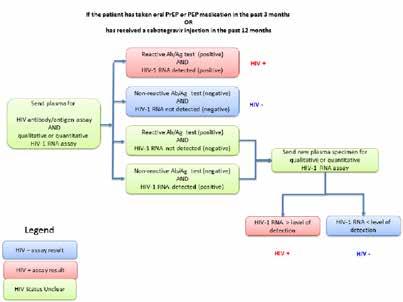
• A new HIV testing algorithm for persons who have taken oral PrEP or PEP in the last three months or inject able cabotegravir PrEP in the past 12 months, which includes HIV ribonucle ic acid (RNA) testing. This was based on observed delays in diagnosis of baseline and incident HIV infections in persons with recent exposure to oral or inject able antiretroviral medication.
• Revision of the recommended
SANDIEGOPHYSICIAN.ORG 9
HOW MUCH ASSURANCE do you have in your malpractice insurance? With yet another of California’s medical liability insurers selling out to Wall Street, there’s an important question to ask. Do you want an insurer with an A rating from AM Best and Fitch Ratings, over $6.5 billion in assets, and a financial award program that’s paid more than $140 million in awards to retiring members? Or do you want an insurer that’s focused on paying its investors? Join us and discover why our 84,000 physician members give us a 90+% satisfaction rating when it comes to exceptional service and unmatched efforts to reward them. Exclusively endorsed by
frequency of assessing estimated creatinine clearance (eCrCl) to every 12 months for persons greater than 50 years of age or with estimated CrCl ≥90 mL/min at PrEP initiation and every six months for all other patients.

• Addition of emtricitabine and tenofo vir alafenamide (F/TAF) as a Food and Drug Administration (FDA) approved option for cis-men who have sex with men (MSM) and transwomen at increased risk for HIV acquisition.
• Recommendations regarding the use of long-acting injectable cabotegravir.
• Guidance for same-day initiation of PrEP and provision of PrEP through telehealth.
• Procedures for off-label use of nondaily oral emtricitabine and tenofovir disoproxil fumarate (F/TDF) for PrEP for MSM. (“On-demand”, “event-driv en,” and “2-1-1” PrEP.)
PrEP Medication Options Have Expanded -Emtricitabine (FTC)/tenofovir diso proxil fumarate (TDF200/300) was the only option for HIV preexposure prophy laxis (PrEP) until 2019. Based on evidence from multiple clinical trials released in 2011–2013, the FDA approved F/TDF for PrEP in persons aged 18 and older and expanded approval for use in adolescents ages 15 and older in 2018 based on a large
clinical trial showing efficacy in this age group. The USPSTF issued a Grade A rec ommendation for PrEP in June 2019.
Studies suggest efficacy starts after seven days for anal sex and after 21 con secutive days for women with vaginal sex possibly related to drug concentrations in rectal vs. vaginal tissues. With consistent use, efficacy up to 96–99% protection for sexual contact risk and 74% for IDU exposures. In the cabotegravir vs. daily oral prep trials, the efficacy of oral Rx was inferior to injectable cabotegravir, likely related to compliance issues.
-Emtricabine/Tenofavir Alafenamide (FTC/TAF 200/25) was FDA approved 10/2019 for daily use PrEP indication in
10 OCTOBER 2022
INFECTIOUS DISEASES
Taken from US Public Health Service PreExposure Prophylaxis for the Prevention of HIV Infection in the United States, 2021 Update. Figure 4a. Clinician Determination of HIV Status for PrEP Provision to Persons without Recent Antiretroviral Prophylaxis Use.
Retrieved
from
https://www.cdc.\gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
sexually active cis-men and trans women (MSM) at risk of HIV acquisition.


Approval based on DISCOVER trial — a manufacturer-sponsored, randomized, double blinded, phase 3 trial of cisgender men who have sex with men (MSM) and transgender women (TGW). Participants had to have a history of recent condom less anal intercourse with >1 partner or bacterial sexually transmitted infection.
2,694 received FTC/TAF vs 2693 FTC/ TDF. 7 new infections occurred in the FTC/TDF group c/w 15 in the FTC/TDF group, most of whom were not taking daily medication consistently based on drug level monitoring. The HIV incidence ratio, 0.47% demonstrated non inferior ity. FTC/TAF is associated with smaller declines in bone density and more favor able renal markers, though the effects on creatinine clearance in both groups were small. Several commentaries published after release criticized non-inclusion of heterosexual women and receptive vagi
nal sex in the study.
Generic F/TAF is not available.

































































Probably no reason to change from Truvada if tolerated — can be considered in persons with creatinine clearance >30 and <50 and those with or at significant risk for osteoporosis.
-Cabotegravir is a long-acting inject able HIV integrase strand transfer inhibi tor that prevents viral DNA insertion into host cell DNA.

In 2020, results from a clinical trial conducted with MSM and transgender women and another conducted with African women reported high efficacy and safety for bimonthly injections of cabote gravir (CAB) for PrEP and this option was included in the U.S. Public Health Center/







Latin America, excluding persons with IDU Hep B and Hep C.
Primary efficacy analysis: 13 in the cabotegravir group (incidence, 0.41 per 100 person-years and 39 in the TDF–FTC group (incidence, 1.22 per 100 person-years). The hazard ratio for incident HIV infection in the cabotegravir group as compared with the TDF–FTC group was 0.34 (95% confidence interval), thus demonstrating a superiority and a 66% reduction in risk of HIV infection compared with TDF/FTC.




HPTN 084-Delany-Moretlwe et al. (2021), studied 3,200 cisgender women at risk for HIV in sub-Saharan Africa. The study found an 88% reduction in HIV

We are a San Diego-based team of experienced and expert healthcare attorneys. We represent doctors in all manner of litigation as well as Medical Board and other government investigations. We advise doctors in contractual business transactions, medical group formation and partnership arrangements, structuring of management services organizations, group mergers and sales, employment contracts and disputes, office leases, and regulatory and compliance matters. David Balfour Litigation, Shareholder dbalfour@buchalter.com Bryn Spradling Business Law, Shareholder bspradling@buchalter.com Hillary Dorne Corporate Transactions hdorne@buchalter.com Carol Salmacia Litigation csalmacia@buchalter.com Li-An Leonard Employment & Leasing lleonard@buchalter.com 655 W. Broadway, Suite 1600, San Diego, CA 92101 | (619) 219-5335 www.buchalter.com
initially given oral cabotegravir for up to 5 weeks to check tolerability before starting injections. None had adverse reactions that precluded them from continuing to the injection phase of the study. Thus, the FDA states that an oral lead-in period is optional.
Dosing of CAB is 600 mg IM monthly for two doses (e.g., day 0 and day 28, then 600 mg IM every two months. Injections should be given in the gluteal muscles.
Once treatment started, both HIV Ag/ Ab and HIV RNA tests should be done every two months because CAB, as well as oral PrEP, can significantly slow sero conversion and detection > 100 days by current HIV Ag/Ab testing and thus delay detection of HIV by Ag/Ab tests. Some subjects who became infected with HIV while on CAB were diagnosed late with potential for development of Integrase inhibitor resistance mutations, which occurred in 1 in 4 of those with unrecog nized HIV at baseline, and 4 of 9 of those with incident HIV during the study.
Adverse effects predominantly con sisted of mild injection site reactions — 81% in HPTN 083, with only 2.4% choosing to discontinue Rx. LISR occurred in 38% in HPTN 084, with no discontinuations.
UGT1A1 inducers decrease CAB plasma concentrations; rifampin, rifapentine, and some anticonvulsants should not be given with CAB, and concurrent use with certain drugs (e.g., rifabutin) requires adjustment of CAB frequency.
CAB is approved for persons age 18 or older and weighing at least 35 kg. There are no restrictions for use in people with kidney disease, osteoporosis, or hepa titis B. Intramuscular (IM) CAB has not been studied in pregnancy, and the FDA recommends a risk-benefit discussion before use in persons who are pregnant or may become pregnant while using IM CAB. Studies in younger age groups are ongoing.
-On Demand PrEP
The 2021 PrEP update and CDC website address the issue of on-demand PrEP.
Molina et al. published the results of a double blinded randomized trial which compared incident new HIV acquisition in MSM individuals engaging in unprotected anal sex comparing ON demand FTC/TDF, two tablets before exposure then daily x
two more days (2:1:1) vs. placebo. There were two new HIV infections among 199 persons in the treatment group vs. 14 new infections in the placebo group with rela tive risk reduction of 86% in the treated group, which compares favorably with daily PREP.
Study included only MSM condomless anal intercourse exposure and there are no studies in other demographics. Some Health Department entities and European Health Agencies are developing guidance for on Demand PrEp, but its use in the USA is not FDA approved and would be an off label.
Dapivirine Vaginal Ring
Prior published studies of Dapivirine vagi nal ring for female controlled HIV preven tion during vaginal sex demonstrated only 33% risk reduction in HIV acquisition c/w placebo and NRTI resistance muta tions emergence on Rx of 18% and thus inferior to currently available and FDA approved PrEP options.
Future Prospects
New agents entering clinical trials in
clude longer acting injectable or implant able antiretroviral medication that may be a game changer with regard to ease and compliance in preventing newly acquired HIV infection.
Nonoccupational Post-Exposure Prophylaxis
Patients not receiving PrEP who seek care within 72 hours after an isolated sexual or injection-related HIV exposure should be evaluated for the potential need for nPEP. If the exposure is isolated (e.g., sexual assault, infrequent condom failure), nPEP should be prescribed, but PrEP or other continued antiretroviral medication is not indicated after completion of the 28day PEP course. Recommendations based on last published guidelines 2016 and covered in our 2019 issue.
Dr. Butera is a fellow of the Infectious Disease Society of America, past presi dent of Infectious Disease Association in California, an epidemiologist at Sharp Coronado Hospital, and co-chair of the San Diego County Medical Society’s GERM committee.
12 OCTOBER 2022
Taken from US Public Health Service PreExposure Prophylaxis for the Prevention of HIV Infection in the United States, 2021 Update. Figure 4b Clinician Determination of HIV Status for PrEP Provision to Persons with Recent or Ongoing Antiretroviral Prophylaxis Use. Retrieved from https://www.cdc.\gov/hiv/ pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf.
INFECTIOUS DISEASES
New Endoscope Reprocessing Guidelines Attempt to Lower the Infection Risk
 BY FRANK EDWARD MYERS III, MA, CIC, FAPIC
BY FRANK EDWARD MYERS III, MA, CIC, FAPIC
TWENTY YEARS AGO, WHEN
someone spoke of the risks of transmission of pathogens in endoscopy, the focus was pri marily on bloodborne pathogens. While large bloodborne pathogen outbreaks have occurred in endoscopy centers [1] these have been related to unsafe injec tion practices such as multi-dosing from single-dose vials and not understanding that due to the properties of blood versus medications, blood will migrate up IV tubing, contaminating the entire IV set. These outbreaks have not been linked
to the scopes themselves. As a result of many such investigations, endoscopy was beginning to be seen as a low-infec tion risk procedure.
This conclusion, however, was quickly dispelled with a number of multi-drugresistant organism outbreaks linked to endoscopy. [2,3] Ultimately, these out breaks were linked to poor reprocessing practices associated with endoscopes. These outbreaks were identified only because they involved bacteria with unique properties that allowed them to be more easily identified.
Transmission
is occurring far more frequently than we have previously been aware. Addition ally, given the growing understanding of gut biome and its role in an individual’s health, even transmission of bacteria with no drug resistance cannot be viewed as inconsequential. Because of these known outbreaks, many profes sional organizations have revised their guidelines around reprocessing endo scopes including the FDA and CDC,[4] Society of Gastroenterology Nurses and Associates (SGNA) , [5] American Society for Gastrointestinal Endoscopy (ASGE), [6] and the Association for the Advance ment of Medical Instrumentation (AAMI), and multiple societies develop ing them together . [7]
While acute care facilities and ac credited facilities must comply with one of these standards, the importance of fol lowing one of these guidelines in physi cian offices rarely visited by government oversight is less obvious. Nevertheless, failure to follow one of these guidelines not only increases the risk to the patients but also the legal liability should a lapse in scope reprocessing occur.
Unfortunately, the guidelines are not always aligned in what tasks should be done to safely protect patients. The AAMI guideline, released this year, has been poorly received by several bodies, which feel that it goes beyond the current state of the science . [8] Some of these critiques are justified while others appear to be based on concerns other than patient safety.
Seven things to examine in a scope reprocessing area:
1. Are the reprocessing staff trained annually on how to reprocess each scope model?
2. Do the reprocessing staff have access to the most current instructions for use (IFU) for each model of endo scope?
3. Are the reprocessing staff insulated from pressures to reprocess scopes more quickly?
4. Does the flow in the reprocessing area move from dirty to clean?
5. Does the scope area have an adequate number of sinks (at least two), equip ment, and adequate lighting?
SANDIEGOPHYSICIAN.ORG 13
INFECTIOUS DISEASES
6. Are endoscopes being reprocessed without the use of AERs?
7. Are scopes reprocessed within 1 hour after use?
So what are the minimum standards agreed upon by all the guidelines? The most important, obvious, and frequently not complied with is following the endoscope reprocessing IFU. Each scope must have its IFU complied. Part of the reason for poor compliance is that enter ing these IFUs into readability programs we find that they are written at the PhD level. Most instructions to easily be complied with must be written at the 5th grade level. Additionally, pressure for faster room and endoscope turnaround often incentivizes speed in reprocessing over patient safety. There is one accepted deviation from following the IFU that is noted in the guidelines. That exception is for conflicting claims. As an illustra
tive fictional example; the clinic owns an Olympus model 1A scope and the IFU says that it must be reprocessed in an Olympus Maxi automatic endoscope reprocessor (AER). If a Sterris 1F AER has a claim that it can reprocess an Olym pus model 1A then the organization can decide which AER it can use as both have proven their claims to the FDA.
The same situation is frequently encountered with brushes. Complying with the IFU doesn’t preclude adding additional steps as long as they do not eliminate other steps in the IFU. An example would be in the IFU it says to use a brush to clean the internal lumen of the endoscope until the brush is vis ibly clean. The physician asks the scope reprocessor how many times they brush the internal lumen to see that it is clean and the reprocessor says, “one time.”
The physician recognizes that when
they brush their teeth they do not do one brush over their teeth. So the facility recommended doing the brushing at least four times and then check if visibly clean. Such a deviation from the IFU is acceptable. The same with adding a pull through (think squeegee) in addition to the brush. What would not be acceptable deviation is changing the concentration of the enzymatic solution used in the sink to be more concentrated than in the IFU for the enzymatic solution, as this increased concentration may be less effective at cleaning or it may cause more deterioration of the endoscope.
The standard of practice is to have persons responsible for endoscope repro cessing attend annual training. Usually these trainings are provided free by the scope manufacturer. The reprocessor should be trained and have competencies on each different scope that they use as outbreaks have occurred assuming that the IFUs for all scopes are similar.

Endoscope storage is an area of con troversy. Storage issues can be divided into two categories: (1) how long can a scope be stored before being reprocessed, and (2) how should the scope be stored. In the previous version of the AAMI guideline and continuing through the current version, the AAMI standard began questioning limiting hangtime to a prescribed date. It has been pointed out that reprocessing scopes not contami nated increases wear on the endoscopes and causes micro groves in the interior lumen making it easier for bacteria to adhere to the interior lumen. In the last year the joint commission infection con trol consultant has said in lectures that she encourages institutions to not have any dates for reprocessing.
As to what to store the scopes in is an area where clear divides are developing. First, check the IFU as to storage require ments for the model scope as minimum standard. Some standards suggest HEPA filtered cabinets to demonstrate drying in the cabinet. This benefit has not been demonstrated in scopes with interior channels. The new AAMI standard ap pears to be moving toward the European standard of drying cabinets. Drying cabi nets in that document refers to cabinets that have connections to the ports and
14 OCTOBER 2022
INFECTIOUS DISEASES
blow air through the interior lumens to assist in drying. While many AERs claim that they have the ability to dry the in terior lumens, research suggests that is not the case [9] and therefore the ability of bacteria to reproduce in the presence of water and their inability to live outside the presence of water suggests that these cabinets may lower the risk to patients with lumened or channeled scopes.
One movement is clear. Endoscopes are moving towards being disposable. This movement has been driven by the FDA in part because of growing recogni tion of how difficult it is to clean scopes. The controllers that aren’t high-level disinfected are permanent only the tip in the case of ERCP scopes or in the case of cystoscopes and others the insertion tube is disposable.
After this discussion, the logical ques tion is how does one assure the scopes are cleaned after being reprocessed. The answer there is less than universal. All the approaches described below occur before placing the scope in the AER. The FDA and CDC have described culturing approaches, but these take at least two staff and it means the scopes await the results and are not used beforehand or reprocessed after culturing, either being time intensive. Another approach is to use ATP, a test for the presence of organ isms. This does not differentiate between dead and live bacteria. Other tests exist for carbohydrates and proteins. The issue with these tests (ATP and carbohy drates and proteins) is that they do not differentiate as to how the bacteria are distributed in the scope. This is not an unimportant fact, as AERs are designed to effectively kill bacteria that are not in a large biofilm or bioburden. If the bacteria exist in a large concentrated bioburden, high-level disinfection or sterilization will not be effective. For this reason borescopes (a scope that can visualize the interior lumen to assess the effectiveness of the cleaning) was recom mended for consideration by AAMI. The idea was condemned by other groups, but ironically all guidelines state the need to visually assess for cleanliness before a scope is placed in the AER.
Two other points were mentioned above: One is reprocessing lumened
scopes not using an AER. Literature shows that in cases where people were aware they were being observed, the success rate of following the IFU for all steps was below 2% and other data suggesting such manual reprocessing is unsafe. [10] Lastly, delays in reprocessing may allow items to dry inside and outside the scope, making the device more difficult to clean. Because of this, all IFUs describe what to do when reprocessing is delayed with the caveat this should not be a routine practice.
References:
1 Leary E, Diers D. The silence of the unblown whistle: the Nevada hepatitis C public health crisis. Yale J Biol Med. 2013 Mar;86(1):79-87. Epub 2013 Mar 12. PMID: 23483090; PMCID: PMC3592579.
2 https://www.cdc.gov/hai/outbreaks/ cdcstatement-la-cre.html
3 Epstein L, Hunter JC, Arwady MA, et al. New Delhi Metallo-β-Lactamase–Produc ing Carbapenem-Resistant Escherichia coli Associated With Exposure to Duode noscopes. JAMA. 2014;312(14):1447–1455. doi:10.1001/jama.2014.12720
4 https://www.fda.gov/media/111081/ download
5 https://www.sgna.org/Portals/0/ SGNA%20Standards%20of%20 infection%20prevention%20 in%20reprocessing_FINAL. pdf?ver=2018-11-16-084835-387
6 https://www.asge.org/home/resources/ key-resources/guidelines#newly-pub lished

7 https://www.giejournal.org/article/ S0016-5107(20)34851-3/fulltext
8 https://www.giejournal.org/article/ S0016-5107(22)00098-0/fulltext
9 Ofstead CL, Heymann OL, Quick MR, Eiland JE, Wetzler HP. Residual moisture and waterborne pathogens inside flexible endoscopes: Evidence from a multisite study of endoscope drying effectiveness. Am J Infect Control. 2018 Jun;46(6):689696. doi: 10.1016/j.ajic.2018.03.002. Epub 2018 Mar 30. PMID: 29609854.
10 https://www.infectioncontroltoday. com/view/moving-away-manual-repro cessing-endoscopes
Frank Edward Myers III is director of infection prevention and clinical epidemi ology at UC San Diego Health.
SANDIEGOPHYSICIAN.ORG 15
(858) 569-0300 www.soundoffcomputing.com TRUST A COMMON SENSE APPROACH TO INFORMATION TECHNOLOGY Trust us to be your Technology Business Advisor HARDWARE SOFTWARE NETWORKS EMR IMPLEMENTATION SECURITY SUPPORT MAINTENANCE Endorsed by
Infection Prevention and Control in Low- and Resource-Poor Countries
and healthcare-associated pneumonia (14.8%), including ventilator-associated pneumonia (VAP). [6] Fifty percent of the surgical site infections are also resistant to antimicrobial agents, making treat ment of surgical site infections chal lenging. [2]
The global burden is hard to define due to the inability to acquire reliable, trans parent, robust, standardized, compa rable data. Surveillance data is complex and requires the use of standardized epidemiological definitions, not clini cal diagnostic definitions. Surveillance data collection and analysis expertise, in low- and resource-poor countries and in the United States can be inconsistent, lacking, and subjective. Of note, the pooled HAIs prevalence was significantly higher in high- than in low-quality stud ies (15.5% vs. 8.5%, respectively). SSI is the most surveyed and most frequent type of infection in low- and middle-income countries with incidence rates ranging from 1.2 to 23.6 per 100 surgical proce dures and a pooled incidence of 11.8%. By contrast, SSI rates vary between 1.2% and 5.2% in developed countries. [3]
INFECTION PREVENTION AND
control (IPC) is a key essential component of patient safety regard less of where patients live. Often in low- and resource-poor countries there is little to no focus on infection prevention and control outside of infectious disease outbreaks. There are more medical and non-medical problems prevailing in these countries, making infection pre vention and control a low priority.
In recent years, healthcare-associated infections (HAIs) in low and resource poor countries have been recognized

as a global health problem. The WHO [2] estimates 1 in 10 patients receiving healthcare get an infection — signifi cantly higher in the ICU setting. This is due to high volume and high risk using invasive devices and procedures without following IPC strategies, such as aseptic and sterile technique. The second high est risk is the surgical patient. Surgi cal site infections (SSI) were the most reported and most frequent type of hospital-acquired infection (29.1%), fol lowed by urinary tract infections (UTI) (23.9%), bloodstream infections (19.1%),
In many healthcare facilities, there is a need of IPC knowledgeable medical teams, IP roles/positions in healthcare facilities, access to validated microbiologi cal labs and data, non-expired lab sup plies, functional surveillance programs, continuous supply of antiseptics, safe water supply, personal protective equip ment, essential antibiotics to treat infec tions, appropriate number of healthcare personnel trained in infection control, and appropriate healthcare infrastruc ture and political commitment. [5,6]
Millions of patients are affected by these infections each year, posing a major threat to patients’ safety. They increase morbidity and mortality and may lead to disability, reducing patients’
16 OCTOBER 2022
A Global View from an Infection Preventionist (IP) with Real Life Experiences
BY KIM DELAHANTY, BSN, PHN, MBA/HCM, CIC, FAPIC
INFECTIOUS DISEASES
health-related quality of life [1]. HAIs also led to significant financial losses for health systems. [2] If the main bread win ner of the family, tribe or community has survived a surgical injury due to living in a conflict zone, but has a life threatening or changing infectious event rendering them disabled and not able to work, have we improved patient safety and health care? Medical teams need to provide emergent urgent care in a safe aseptic/ sterile way to avoid this collateral dam age and socioeconomical burden. Treat ing the WHOLE patient not just the HOLE in the patient will benefit the patient, their family and community.
Solid recommendations have been issued by national and international or ganizations, but their application needs to be strengthened and accompanied by performance monitoring both in highincome and low-, middle-, and resourcepoor income countries. [4] HAIs must be treated as a priority patient safety issue within comprehensive approaches to be tackled effectively. One way is to inte grate efforts with the ministry of health,
nongovernmental organizations, and other national and international public health programs. How? Reduce HAIs by assisting with the financial burden of this program, assessment, planning, and implementation of infection prevention and control policies, including timely actions at national, international, and institutional levels.
References:
1. Pittet D, Allegranzi B, Storr J, Bagheri Nejad S, Dziekan G, Leotsakos A, Don aldson L. Infection control as a major World Health Organization priority for developing countries. J Hosp Infec. 2008;68(4):285–92.
2. World Health Organization, Patient Safety. Health care-associated infec tions, Fact Sheet.


3. www.worldbank.org/en/country/mic/ overview.
4. World Health Organization. Report on the burden of endemic healthcare associ ated infection worldwide. 2011. Avail able at: http://apps.who.int/iris/bitstre am/10665/80135/1/9789241501507_ eng.pdf.
5. Infection Control in Limited Resources Countries: Challenges and Priorities Diana Vilar-Compte1 & Adrián Cama cho-Ortiz 2 & Samuel Ponce-de-León. Curr Infect Dis Rep (2017) 19: 20 DOI 10.1007/s11908-017-0572-y.
6. Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. Burden of endemic health-care associated infec tions in developing countries: system atic review and metaanalysis. Lancet 2011;377:228–41.

Kim Delahanty has been an infection preventionist in academic and private hospital systems for over 20 years and is currently the owner of Delahanty Infec tion Prevention Clinical Epidemiology Consulting after working as the adminis trative director of Infection Prevention and Clinical Epidemiology at UC San Diego Health for 16 years. She currently is the IPC advisor/referent for Medicins San Frontieres /Doctors Without Borders for all USA projects and 16 countries.
Vista Community Clinic has outstanding opportunities for Full-Time and Part-Time Physicians. We are looking for dedicated, motivated and enthusiastic team players who want to make a difference in the community.
Vista Community Clinic is a federally qualified, not-for-profit healthcare clinic with more than 800 employees and nine state-ofthe-art clinics treating more than 69,000 patients every year. We provide healthcare throughout the Southern California regions of North San Diego, Orange and Riverside Counties.
Our compensation and benefits program includes: Competitive compensation, sign-on bonus, relocation bonus, health, dental, vision, company-paid life, longterm disability, flexible spending accounts , 403(b) retirement plan, malpractice coverage, NHSC loan repayment eligible organization, CME allowance, and no oncall hours
For more information visit www.vcc.org or email hr@vcc.org EEO

SANDIEGOPHYSICIAN.ORG 17
Join a team that’s been helping to redefine what it means to be a community clinic for 50 years.
PHONE : 800-919-9141 OR 805-641-9141 FAX: 805-641-9143 EMAIL: JNGUYEN@TRACYZWEIG.COM TRACYZWEIG.COM PHYSICIANS NURSE PRACTITIONERS PHYSICIAN ASSISTANTS LOCUM TENENS PERMANENT PLACEMENT
Healers Need Healing, Too
BY NI-CHENG LIANG , MD
THE COVID-19 PANDEMIC HAS REVEALED THE underbelly of how broken the American healthcare system is, in particular for physicians. Healers that seek to heal others have burned out, been morally injured, and are leaving the profession. Sadly, the unfortunate truth to our colleagues leaving is that too often the path to exit ing is suicide.
Dr. N. Douge writes in a Fortune.com article, “My medical education and training did not equip me for the mental and emotional strain medicine imposes on its practitioners today.” Physicians who are from marginalized groups and who are female are at higher risk for being on the receiving end for enduring moral injury.
Leaving medicine and cutting back work hours are viable solutions for some. For others who are unable to, who’s coming to help? Though medical societies, healthcare systems, and undergraduate and postgraduate medical training programs have acknowledged the importance of wellbeing and burnout prevention with the incorporation of well-intentioned well
ness programs across the country, the response has been mixed, and burnout statistics have not changed — or have actually worsened in some subspecialties.

Such is the realistic pace of change when the culture of medicine has set unrealistic, superhuman expectations of physicians — which has been indoctrinated in the physi cians themselves. COVID-19 painted pictures of health care heroes in capes, further adding to the superhuman strength façade, when physi cians are in fact human. We are heroes who are human
beings. Physicians are humans who want to heal others, often neglecting heal ing ourselves because of the indoctrination.
The cavalry to save physicians is us, and we are exhausted. And yet we need to be part of the change that we want to see. That change can come from within, from unlearning the mantra that many were fed and were expected to abide by: ”Eat, pee, and sleep when you can.” How about we simply recognize our body’s own basic needs in the moment, and honor them? Let’s drop
18 OCTOBER 2022
PHYSICIAN WELLNESS
the cape. We can start changing the culture of medicine by treat ing ourselves and our colleagues like human beings (and not humans who have to keep doing), and having human expecta tions for ourselves, however perfectly imperfect.
For me, the realization that I cannot help others unless I take care of myself first and foremost came early in my career, more than 11 years ago, when I became a cancer patient during pulmonary and critical care fellowship. I was unable to wait for the system to change in order to create a more hospitable environment from which to learn medicine. I turned to the evidence-based modalities of mindfulness, and later on, coach ing, on my own terms. Incorporating the two has helped equip me to respond to the mental and emotional strain of medicine in healthier ways, with space to try to reduce the suffering of colleagues. I’ve empowered myself through mindfulness and coaching to create the space that I need to stay here on this good earth in health. That involved changing myself and the way I practice medicine.
The journey has been a personal one that I’ve gone on to share to try to help reduce suffering. The journey is ongoing and a dynamic process of intention, commitment, and give and take. It’s been an imperfect one. I’ll share some of the ongoing lessons here in hopes that they may help you as they have helped me and the colleagues and trainees I’ve worked with over the last decade:
1. Give yourself permis sion to unlearn that which does not serve. This may be indoctrinated deeply from childhood or medi cal training days such as perfectionism, self-sacrifice, self-deprecation, tendency toward overwork, to name a few. Traumas may surface here, so mental health ser vices and/or coaching may be helpful.
2. Check in with your pillars of health regularly: sleep, healthy nutrition, exercise, stress reduction, social connec tion. Meeting those basic fundamental needs and saying “no” when a task threatens your ability to ad dress those needs helps set you up for success in your wellbeing journey. Consider the intentional inclusion of screen-free time in nature while addressing the pillars of health.
3. Build in moments of mindfulness that work for you. Mindful ness is paying attention on purpose to the present moment without judgement. We know from the breadth of published literature that cultivating mindfulness helps to reduce stress and prevent burnout. However, not everyone has time or wants to cultivate a formal practice, so instead, you can build in purposeful, non judgmental awareness in ways that work for you. Some ideas include noticing the sensation of soap or hand sanitizer gel before a clinical encounter, taking mindful breaths while you are aus cultating a patient’s lungs, going for a mindful walk (around the parking lot like I do) during lunch, actually taking a lunch break, and eating mindfully — using all of your senses to actually see, taste, enjoy your food, even if for a few bites.
4. Ask yourself help ful questions when difficult situations arise. As physicians we are condi tioned to be expert cata strophizers on behalf of our patients. As a result, we can often catastrophize events in our own lives, augment ing stress levels, sometimes unnecessarily. Rather, can we meet difficult situations with curiosity, kindness, and self-compassion?
• Isn’t it interesting that this is happening?
• What is my direct experi ence of this difficulty? Naming body sensations and emotions in the mo ment of difficulty can help disarm the amygdala that has been activated. Feel to heal and name to tame.
• What can I control? What is outside of my control?
• My co-host of the Mindful Healers Podcast, Dr. Jessie Mahoney, recommends asking yourself, “What would love do?” par ticularly for difficulty that arises among loved ones.
• Is this thought actually true? Thoughts influence how we feel, and our thoughts about a circum stance may not be true. Noticing false beliefs with kindness and curiosity can sometimes lessen suffering.
• How might this be hap pening “for me” as op posed to “to me”?
5. When witnessing suf fering, this statement may help: “Everyone is on their own life journey. I am not the cause of this person’s suffering nor is it entirely within my power to make it go away even though I wish I could. Moments like these can be difficult to bear, yet I may still try to help if I can.”
This is from the Mindful Self-Compassion curricu lum.
I realize all of the above are easier said than done. But getting help helps. And perhaps your medical society or healthcare system can help with some of the above, or you can also invest in yourself, choosing from what resonates among the myriad of wellbeing offer ings and mental health sup port. Never forget that you are your own expert when it comes to meeting your needs. Maybe trying one, some, or all of the above can help foster your own person al wellbeing — and, maybe, to then be the change that you would like to see.
So, what is it that you need to help you heal so that you can live in alignment with your values?
How can you continue to heal others without sacrific ing your own wellbeing?
Remember that you are not alone.
Dr. Liang is a pulmonologist with Scripps Health.
SANDIEGOPHYSICIAN.ORG 19
In Good Hands
BY ADAMA DYONIZIAK
WHAT DO A CROONER, A QUARTER back, an actor, a president, a prime min ister, and a comedian have in common with Project Access? Frank Sinatra, John Elway, Paul Newman, Ronald Reagan, Margaret Thatcher, and Bill Murray all had Dupuytren’s contrac ture just like Froilan V., our Project Access patient.
Dr. Lindsey Urband, a Project Access volunteer since 2019, performed hand surgery on Froilan, who had a severe form of this condi tion in both hands. Froilan’s ability to support his family was very limited. When he was able to work, he often experienced pain and soreness in his hands. When planting greenery, his tools would rub against the outside of his fingers due to their curvature. “The skin would peel away from my fingers as I would hit that part of my hand while working. It would start hurting, then blis ter, then I would have to peel the skin from my finger and suffer through it so that I could keep working,” Froilan says. He maintained a positive attitude, and credits his wife and family for keeping him motivated and feeling supported.

Froilan felt comfortable with Dr. Urband, especially because she spoke Span ish. “I am completely appreciative of the doctor,” he says. “She is very kind; she wanted to help me to the maximum effect. I give her thanks for everything she has done. I have seen progress for myself compared to how I was before. I thank her 100%.”
Dr. Urband is very happy that Froilan is making progress. “The best thing about being a physi cian is the ability to make a connection on an individual basis to positively influence a person’s life,” she says. Dr. Urband’s childhood interests in being both a detective and a scientist led her to pursuing a career in medicine. “At the age of 12, I saw an orthopedic surgeon when I tore my ACL,” she explains. “When I expressed interest in medicine, he gave me a VHS tape of surgeries to watch at home, which were fascinating. In college, I majored in Spanish and interpreted on medical missions to Guatemala. It all came to gether during my orthopedic residency: the hand rotation was the best because it was a microcosm of orthopedic surgery.”
Froilan continues his post-surgery reha bilitation with at-home physical therapy to help increase the range of motion in his fingers and to break down the scarring. “If I recuperate from this, I might be able to find work,” he says. His optimism and determination keep him going. He also speaks about having to be patient. “There is no need to start feeling desperate; today there was some progress,” he says. “I don’t want to rush anything because if I hurt myself, I throw away the progress from the surgery.”
Dr. Urband is a hand, upper extremity and microsurgeon with the San Diego Outpatient Surgery Center. Originally from Minne sota, she enjoys hiking, running, cycling, paddle boarding, alpine skiing, golf, salsa dancing, bak ing, and reading. Most people don’t know that she was the 2008 national champion in long track speed skating and climbed Mount Kilimanjaro in 2019. “Being a Project Access volunteer is very rewarding,” she says. “I contribute to my local community by touch ing the lives of those who are less fortunate.”

Froilan believes that “you have to give love to receive love. The doctor, the staff, the Project Ac cess team … they are all giving love and will receive it back.”
Since 2008, Project Access has facilitated $24 million in care for 7,500-plus uninsured patients by providing free consultations and surgeries.
Project Access patients are in good hands with our volunteer physicians. To join, visit www.champi onsforhealth.org/volunteer.
Adama Dyoniziak is executive director of Champions for Health.
CHAMPIONS FOR HEALTH 20 OCTOBER 2022
CLASSIFIEDS
VOLUNTEER OPPORTUNITIES
CHAMPIONS FOR HEALTH - PROJECT ACCESS SAN DIEGO: Volunteer physicians are needed in the following specialties: endo crinology, rheumatology, vascular surgery, ENT or head and neck, general surgery, GI, and gynecology. These specialists are needed in all regions of San Diego County to provide short term pro bono spe cialty care to adults ages 26-49 who are uninsured and not eligible for Medi-Cal. Volunteering is customized to fit your regular schedule in your office. Champions for Health is the foundation of the San Diego County Medical Society. Join hundreds of colleagues in this endeavor: Contact Evelyn.penaloza@championsfh.org or at 858-300-2779.
CHAMPIONS FOR HEALTH PROJECT ACCESS: Volunteer physi cians are needed for the following specialties: endocrinology, ENT or head and neck, general surgery, GI, gynecology, neurology, ophthal mology, orthopedics, pulmonology, rheumatology, and urology. We are seeking these specialists throughout all regions of San Diego to support those that are uninsured and not eligible for Medi-Cal receive short term specialty care. Commitment can vary by practice. The mission of the Champions for Health’s Project Access is to improve community health, access to care for all, and wellness for patients and physicians through engaged volunteerism. Will you be a health CHAMPION today? For more information, contact Andrew Gonzalez at (858) 300-2787 or at Andrew.Gonzalez@ChampionsFH.org, or visit www.ChampionsforHealth.org.
PHYSICIAN OPPORTUNITIES
PRIMARY CARE PHYSICIAN: Imperial Valley Family Care Medical Group is looking for Board Certified/Board Eligible Primary Care Physician for their clinics in Brawley & El Centro CA. Salaried/full time position. Please fax CV/salary requirements to Human Resources (760) 355-7731. For details about this and other jobs please go to www.ivfcmg.com
INTERNAL MEDICINE PHYSICIAN: Healthcare Medical Group of La Mesa located at 7339 El Cajon Blvd is looking for a caring, compas sionate, and competent physician for providing primary care services. We require well–organized and detail–oriented with excellent written and oral communication skills, and excellent interpersonal skills to provide high–quality care to our patients. We provide a competi tive salary, paid time off, Health insurance, 401K benefits, etc. We provide plenty of opportunities to refine your clinical competency. Our CEO Dr. Venu Prabaker who has 30 years of teaching experience as a faculty at multiple universities Including Stanford, UCSD, USC, Midwestern, Western, Samuel Merritt, Mayo, etc. — will be providing teaching rounds once a week. You will also get plenty of opportunities to attend other clinical lectures at many of the 4–to–5–star restau rants in San Diego. We also have once a week– one–hour meeting for all the staff for team building and to create a “family atmosphere” to improve productivity and thereby create a win–win situation for all. Visit us at caremd.us.
ASSISTANT, ASSOCIATE OR FULL PROFESSOR (HS CLIN, CLIN X, ADJUNCT, IN-RESIDENCE) MED-GASTROENTEROLOGY: Faculty Position in Gastroenterology. The Department of Medicine at University of California, San Diego, Department of Medicine (http:// med.ucsd.edu/) is committed to academic excellence and diversity within the faculty, staff, and student body and is actively recruiting faculty with an interest in academia in the Division of Gastroen terology. Clinical and teaching responsibilities will include general gastroenterology. The appropriate series and appointment at the Assistant, Associate or Full Professor level will be based on the can didate’s qualifications and experience. Salary is commensurate with qualifications and based on the University of California pay scales. In-Residence appointments may require candidates to be self-funded. For more information: https://apol-recruit.ucsd.edu/JPF03179 For help contact: klsantos@health.ucsd.edu
CARDIOLOGIST POSITION AVAILABLE: Cardiology office in San Marcos seeking part-time cardiologist. Please send resume to alberto chaviramd@yahoo.com.
DERMATOLOGIST NEEDED: Premier dermatology practice in La Jolla seeking a part-time BC or BE dermatologist to join our team. Busy practice with significant opportunity for a motivated, entrepre neurial physician. Work with three energetic dermatologists and a highly trained staff in a positive work environment. We care about our patients and treat our staff like family. Opportunity to do medical/ surgical and cosmetic dermatology in an updated medical office with state-of-the art tools and instruments. Incentive plan will be a per centage based on production. If you are interested in finding out more information, please forward your C.V. to jmaas12@hotmail.com.
RADY CHILDREN’S HOSPITAL PEDIATRICIAN POSITIONS: Rady Children’s Hospital of San Diego is seeking board-certified/eligible pe diatricians or family practice physicians to join the Division of Emer
gency Medicine in the Department of Urgent Care (UC). Candidate will work at any of our six UC sites in San Diego and Riverside Counties. The position can be any amount of FTE (full-time equivalent) equal to or above 0.51 FTE. Must have an MD/DO or equivalent and must be board certified/eligible, have a California medical license or equiva lent, PALS certification, and have a current DEA license. Contact Dr. Langley glangley@rchsd.org and Dr. Mishra smishra@rchsd.org.
PER DIEM OBGYN LABORIST POSITION AVAILABLE: IGO Medical Group is seeking a per diem laborist to cover Labor and Delivery and emergency calls at Scripps Memorial Hospital in La Jolla. 70 deliver ies/month. 24-hour shifts preferred but negotiable. Please send inquiries by email to IGO@IGOMED.com.
MEDICAL CONSULTANT – SAN DIEGO COUNTY: The County of San Diego, Health and Human Services Agency’s Public Health Services is looking for a Board Certified Family Practice or Internal Medicine physician for the Epidemiology and Communicable Disease Division. Under general direction, incumbents perform a variety of duties nec essary for the identification, diagnosis, and control of communicable diseases within the population. This position works closely with the medical and laboratory community, institutional settings, or hospital control practitioners. Learn more here: https://www.governmentjobs. com/careers/sdcounty?keywords=21416207
KAISER PERMANENTE SAN DIEGO - PER DIEM PHYSIATRIST: Southern California Permanente Medical Group is an organization with strong values, which provides our physicians with the resources and support systems to ensure they can focus on practicing medicine, con necting with one another, and providing the best possible care to their patients. For consideration or to apply, visit https://scpmgphysicianca reers.com/specialty/physical-medicine-rehabilitation. For questions or additional information, please contact Michelle Johnson at 866-5031860 or Michelle.S1.Johnson@kp.org. We are an AAP/EEO employer.
PRIMARY CARE PHYSICIAN POSITION: San Diego Family Care is seeking a Primary Care Physician (MD/DO) at its Linda Vista location to provide direct outpatient care for acute and chronic conditions to a diverse adult population. San Diego Family Care is a federally quali fied, culturally competent and affordable health center in San Diego, CA. Job duties include providing complete, high quality primary care, and participation in supporting quality assurance programs. Benefits include flexible schedules, no call requirements, a robust benefits package, and competitive salary. If interested, please email CV to sdfcinfo@sdfamilycare.org or call us at (858) 810- 8700.
FAMILY MEDICINE OR INTERNAL MEDICINE PHYSICIAN: TrueC are is more than just a place to work; it feels like home. Sound like a fit? We’d love to hear from you! Visit our website at www.truecare.org. Under the direction of the Chief Medical Officer and the Lead Physician, ensure the provision of effective quality medical service to the patients of the Health center. The physician is responsible for assuring clinical procedures are continually and systematically followed, patient flow is enhanced, and customer service is extended to all patients at all times.
PUBLIC HEALTH LABORATORY DIRECTOR: The County of San Diego is seeking a dynamic leader with a passion for building healthy communities. This is a unique opportunity for a qualified individual to work for a Level 3 Public Health Laboratory. The Public Health Services department, part of the County’s Health and Human Services Agency, is a local health department nationally accredited by the Public Health Accreditation Board and first of the urban health departments to be accredited. Public Health Laboratory Director-21226701UPH.
NEIGHBORHOOD HEALTHCARE MD, FAMILY PRACTICE AND INTERNISTS/HOSPITALISTS: Physicians wanted, beautiful Riverside County and San Diego County. High-quality family practice for a private-nonprofit outpatient clinic serving the communities of Riverside County and San Diego County. Work full-time schedule and receive paid family medical benefits. Malpractice coverage provided. Be part of a dynamic team voted ‘San Diego Top Docs’ by their peers. Please click the link to be directed to our website to learn more about our organization and view our careers page at www.Nhcare.org.
PHYSICIAN WANTED: Samahan Health Centers is seeking a physician for their federally qualified community health centers that emerged over forty years ago. The agency serves low-income families and individuals in the County of San Diego in two (2) strategic areas with a high-density population of Filipinos/Asian and other lowincome, uninsured individuals — National City (Southern San Diego County) and Mira Mesa (North Central San Diego). The physician will report to the Medical Director and provide the full scope of primary care services, including but not limited to diagnosis, treatment, coor dination of care, preventive care and health maintenance to patients. For more information and to apply, please contact Clara Rubio at (844) 200-2426 EXT 1046 or at crubio@samahanhealth.org.
PRACTICE FOR SALE
OTOLARYNGOLOGY HEAD & NECK SURGERY SOLO PRACTICE FOR SALE: Otolaryngology Head & Neck Surgery solo practice located in the Ximed building on the Scripps Memorial Hospital La Jolla campus is for sale. The office is approximately 3,000 SF with 1 or 2 Physician Offices. It has 4 fully equipped exam rooms, an audio room, one procedure room, one conference room, one office manager room as well as in-house billing section, staff room and a bathroom. There is ample parking for staff and patients with close access to radiology and laboratory facilities. For further information, please contact Christine Van Such at (858) 354-1895 or email: mahdavim3@gmail.com.
OFFICE SPACE / REAL ESTATE AVAILABLE
HILLCREST OFFICE TO SUBLEASE OR SHARE: Beautifully ap pointed office in Hillcrest next to Mercy Hospital to sublease or share. Office is approximately 1500 sq feet and has AAAASF certified oper ating room to share or use as needed.Currently occupied by plastic surgery, the office is ideal for Dermatology, Gynecology, Podiatry, or other specialty. Multiple days per week full use of office available as needed.Please contact amez.cookie@gmail.com or at 858-300-2779.
SUBLEASE AVAILABLE: Sublease available in Del Mar off 5 freeway. Share rent. 2100 sq ft office in professional building. Utilities included. Great opportunity in a very desirable area. 858-342-3104.
CHULA VISTA MEDICAL OFFICE: Ready with 8 patient rooms, 2,000 sf, excellent parking ratios, Lease $4000/mo. No need to spend a penny. Call Dr. Vin, 619-405-6307 vsnnk@yahoo.com.
OFFICE SPACE AVAILABLE – BANKERS HILL: Approximately 500 sq foot suite available to lease, includes private bathroom. Located at beautiful Bankers Hill. For more details, please call Claudia at (619) 501-4758.
OFFICE AVAILABLE IN MISSION HILLS, UPTOWN SAN DIEGO: Close to Scripps Mercy and UCSD Hillcrest. Comfortable Arts and Crafts style home in upscale Mission Hills neighborhood. Converted and in use as medical/surgical office. Good for 1-2 practitioners with large waiting and reception area. 3 examination rooms, 2 physician offices and a small kitchen area. 1700 sq. ft. Available for full occupancy in March 2022. Contact by Dr. Balourdas at greg@thehanddoctor.com.
OFFICE SPACE IN EL CENTRO, CA TO SHARE: Office in El Centro in excellent location, close to El Centro Regional Medical Centre Hos pital is seeking doctors of any specialty to share the office space. The office is fully furnished. It consists of 8 exam rooms, nurse station, Dr. office, conference room, kitchenette and beautiful reception. If you are interested or need more information, please contact Katia at (760) 427-3328 or email at Feminacareo@gmail.com.
SUBLEASE AVAILABLE IN DEL MAR OFF 5 FREEWAY. Share rent. 2100 sq ft office in professional building. Utilities included. Great opportunity in a very desirable area. 858-342-3104
OFFICE SPACE / REAL ESTATE WANTED
MEDICAL OFFICE SPACE WANTED IN HILLCREST/BANKERS
HILL AREA: Mercy Physicians Medical Group (MPMG) specialist is looking for office space near Scripps Mercy Hospital. Open to lease or share office space, full time needed. Please respond to rjvallonedpm@ sbcglobal.net or (858) 945-0903.
NON-PHYSICIAN POSITIONS AVAILABLE
OFFICE MANAGER: 1. Hiring, Training, Managing staff on proce dures/policies. Monitors continuing compliance and office statistics. Oversee stocking/maintenance of supplies, retail. Equipment/ facili ties management. Daily bookkeeping, collections.2. Ensure smooth/ efficient patient flow with increasing production/collections.3. Create a friendly environment where patients expectations are exceeded, where staff can work together as a team. 4. Ensure staff working at maximum productivity/efficiency. Salary: 60-70K depending on experience/qualifications. Benefits: health care reimbursement, pto, retirement, employee discount, bonuses, commission.Contact: info@ manageyourage.com
ASSISTANT PUBLIC HEALTH LAB DIRECTOR: The County of San Diego is currently accepting applications for Assistant Public Health Lab Director. The future incumbent for Assistant Public Health Lab Director will assist in managing public health laboratory personnel who perform laboratory activities for the purpose of identifying, controlling, and preventing disease in the community, as well as assist with the development and implementation of policy and procedures relating to the control and prevention of disease and other health threats. Please visit the County of San Diego website for more infor mation and to apply online.
SANDIEGOPHYSICIAN.ORG 21
Celebrating Independent Physicians
(CAP), we celebrate you—the independent and solo practitioner who keeps healthcare personal. We are here to support you with exceptional
At the Cooperative of American
malpractice coverage supplemented by a host of outstanding

management and practice

so you can stay focused on what’s important— patient care.
For over 40 years,
has
San Diego County Medical Society 8690 Aero Drive, Suite 115-220 San Diego, CA 92123 [ Return Service Requested ] $5.95 | www.SanDiegoPhysician.org PRSRT STD U.S. POSTAGE PAID DENVER, CO PERMIT NO. 5377
Physicians
medical
risk
management services,
CAP
delivered financially secure medical malpractice coverage options and practice solutions to help California physicians realize professional and personal success. Find out what makes CAP different. CAPphysicians.com 800-356-5672 MD@CAPphysicians.com Medical professional liability coverage is provided to CAP members by the Mutual Protection Trust (MPT), an unincorporated interindemnity arrangement organized under Section 1280.7 of the California Insurance Code. Scan the QR code to see how much you can save on your medical malpractice coverage More than 12,500 California physicians rely on CAP to protect their practices every day.






















































 BY FRANK EDWARD MYERS III, MA, CIC, FAPIC
BY FRANK EDWARD MYERS III, MA, CIC, FAPIC











