Annual Supplement to Gastroenterology & Endoscopy

Crypt Dysplasia in Barrett’s Esophagus: An Update Nonalcoholic Steatohepatitis: Identifying High-Risk Patients
Nonalcoholic Steatohepatitis: Treatment Options for High-Risk Patients Treatment of Nonachalasic Esophageal Motor Disorders

Improving Bowel Preparation For Inpatient Colonoscopy: A Proactive Approach Comparison of 2021 IDSA and ACG Recommendations for Treatment of C. difficile Infection

SPOTLIGHT SECTIONS: Gastroesophageal Reflux Disease


Inflammatory Bowel Disease
Scope Reprocessing and Infection Control GI Nutrition Emerging AI Applications In Gastroenterology


2022

gastroendonews.com
News •
Elevating the Standard
Visit medical.olympusamerica.com/getready








Pending 510(k); not available for sale in the United States. Olympus may not take orders that might result in contracts for the sale of the device until clearance is achieved.















9 Crypt Dysplasia in Barrett’s Esophagus: An Update 15 Nonalcoholic Steatohepatitis: Identifying High-Risk Patients 23 Nonalcoholic Steatohepatitis: Treatment Options for High-Risk Patients 43 Treatment of Nonachalasic Esophageal Motor Disorders 53 Improving Bowel Preparation for Inpatient Colonoscopy: A Proactive Approach 89 Comparison of 2021 IDSA and ACG Recommendations for Treatment of C. difficile Infection SPOTLIGHT SECTIONS: 35 Gastroesophageal Reflux Disease 63 Inflammatory Bowel Disease 75 Scope Reprocessing and Infection Control 82 GI Nutrition 93 Emerging AI Applications in Gastroenterology gastroendonews.com Annual Supplement to Gastroenterology & Endoscopy News • 2022 GASTROENDONEWS.COM4

















































































GASTROENTEROLOGIST† RECOMMENDED Brands for Digestive Health FREQUENT HEARTBURN Recommend PRILOSEC OTC® For 24 Hour Relief With 1 Pill Each Morning^ * THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE, OR PREVENT ANY DISEASE. † Based on ProVoiceTM Surveys 2022. Prilosec OTC®: Frequent Heartburn Medicine / OTC Acid Reducer Category. Metamucil®: OTC Therapeutic Fiber category. Align®: Probiotic Category. ^ It’s possible while taking Prilosec OTC. Use as directed for 14 days to treat frequent heartburn. May take 1-4 days for full effect. DAILY FIBER SUPPLEMENT Recommend METAMUCIL® To Help With Occasional Constipation*. DAILY PROBIOTIC SUPPLEMENT Recommend ALIGN® to help with occasional:* · Abdominal discomfort · Gas · Bloating PSYLLIUM FIBER SUPPLEMENT ®
EDITORIAL ADVISORY BOARD
ANDREW ALBERT, MD, MPH Chicago, Illinois
MANOOP S. BHUTANI, MD Houston, Texas
BROOKS D. CASH, MD Houston, Texas
ALINE CHARABATY, MD, AGAF Washington, D.C.
AUSTIN CHIANG, MD, MPH Philadelphia, Pennsylvania
ALAN F. CUTLER, MD Farmington Hills, Michigan
SARAH ENSLIN, PA-C Rochester, New York
RONNIE FASS, MD, MACG Cleveland, Ohio
HARISH K. GAGNEJA, MD Austin, Texas
FRANK G. GRESS, MD New York, New York
VIVEK KAUL, MD, FACG, FASGE, AGAF Rochester, New York
GARY R. LICHTENSTEIN, MD Philadelphia, Pennsylvania
JENIFER R. LIGHTDALE, MD, MPH Worcester, Massachusetts
DANA J. LUKIN, MD, PH D, FACG New York, New York
PETER R. MCNALLY, DO Fort Carson, Colorado
KLAUS MERGENER, MD, PH D, MBA Tacoma, Washington
SATISH RAO, MD,PHD Augusta, Georgia
JOEL E. RICHTER, MD Tampa, Florida
DAVID ROBBINS, MD New York, New York
ELLEN J. SCHERL, MD New York, New York
PRATEEK SHARMA, MD Kansas City, Kansas
JEROME H. SIEGEL, MD New York, New York
ASHWANI K. SINGAL, MD, MS Sioux Falls, South Dakota
EDITORIAL STAFF
SARAH TILYOU Managing Editor smtilyou@mcmahonmed.com
MEAGHAN LEE CALLAGHAN Associate Editor lgray@mcmahonmed.com
LANDON GRAY Assistant Editor mcallaghan@mcmahonmed.com
JAMES PRUDDEN Vice President, Editorial
DAVID BRONSTEIN DONALD M. PIZZI Editorial Directors
ELIZABETH ZHONG KRISTIN JANNACONE Senior Copy Editors SALES STAFF
MATTHEW SPOTO Publication Director mspoto@mcmahonmed.com
DON POPOWSKI Account Manager dpopowski@mcmahonmed.com
CRAIG WILSON Sales Associate, Classified Advertising cwilson@mcmahonmed.com
JOSEPH MALICHIO Vice President, Medical Education jmalichio@mcmahonmed.com
ART AND PRODUCTION
MICHELE MCMAHON VELLE Creative Director
JEANETTE MOONEY Senior Art Director
JAMES O’NEILL Senior Systems Manager
RON REDFERN Production Manager
ROB SINCLAIR Circulation Manager
MCMAHON PUBLISHING
VAN VELLE President, Partner MATTHEW MCMAHON General Manager, Partner
LAUREN SMITH MICHAEL P. MCMAHON MICHELE MCMAHON VELLE Partners
RAY AND ROSANNE MCMAHON Co-founders
DISCLAIMER—The reviews in this issue are designed to be a summary of information, and they represent the opinions of the authors. Although detailed, the reviews are not exhaustive. Readers are strongly urged to consult any relevant primary literature, the complete prescribing information available in the package insert of each drug, and the appropriate clinical protocols. No liability will be assumed for the use of these reviews, and the absence of typographical errors is not guaranteed. Copyright © 2022 McMahon Publishing Group, 545 West 45th Street, 8th Floor, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form.
GASTROENDONEWS.COM6









Diagnose foodborne illness in minutes, not days Campylobacter results in 15 minutes with Sofia 2 Accurate, objective and automated results in 15 minutes for Campylobacter with Sofia 2 fluorescent immunoassay technology. Detects four of the most prevalent Campylobacter species including C. jejuni, C. coli, C. lari and C. upsaliensis with excellent performance compared to culture. Sofia 2 Campylobacter FIA • Simple, flexible workflow with dual mode testing • Various sample types including fresh, frozen, Cary Blair or C&S • LIS Connectivity • Room Temperature Storage For more information contact Quidel Inside Sales at 858.431.5814 Enhanced diagnostics featuring data analytics and surveillance AD20352100EN00 (02/22)






truFreeze RFA-F RFA-C Pre ProcedureImmediately Post Procedure48 Hours Post Procedure 0 1 2 3 4 5 6 1. Solomon, SS. et. al. Liquid Nitrogen Spray Cryotherapy Is Associated With Less Postprocedural Pain Than Radiofrequency Ablation in Barrett’s Esophagus: A Multicenter Prospective Study. Journal Clinical Gastroenterology. J Clin Gastroenterol. 2019 Feb;53(2):e84-e90. © 2022 STERIS Corporation. All rights reserved. All company and product names are trademarks of STERIS Corporation, its affiliates or related companies, unless otherwise noted. Offer your Barrett’s Esophagus patients an ablation option that is proven to be less painful1... Pain intensity scores and the presence of dysphagia assessed for LNC vs. RFA1 For more information, visit www.steris.com/trufreeze. A recent multicenter prospective study shows patients undergoing Radiofrequency Ablation (RFA) had at least 5 times greater odds of experiencing pain compared with the Liquid Nitrogen Cryotherapy (LNC) group.1 Clinically proven to reduce post-procedure pain when compared to other modalities of ablation for Barrett’s esophagus and cancer. Spray Cryotherapy System
Crypt Dysplasia In Barrett’s Esophagus: An Update



 ZOE LAWRENCE, MD SETH A. GROSS, MD
NYU Langone Health New York, New York
ZOE LAWRENCE, MD SETH A. GROSS, MD
NYU Langone Health New York, New York
Endoscopic
surveillance of Barrett’s esophagus can help diagnose cellular changes and prevent progression to esophageal adenocarcinoma.




Given that surveillance intervals are determined by the degree of dysplasia, an understanding of the significance of the findings is crucial.

GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 9 PRINTER-FRIENDLY VERSION AVAILABLE AT GASTROENDONEWS.COM
Barrett’s esophagus (BE) is defined as the presence of columnar mucosa with intestinal metaplasia of at least 1 cm within the esophagus.1 Up to 7% of patients with gastroesophageal reflux disease have histologically confirmed BE, with prevalence highest in South America, followed by North America, and lowest in Asia.2 As a precursor to esophageal adenocarcinoma (EAC), BE represents a premalignant state.3 The pathway from a healthy esophagus to cancer includes progression from erosive esophagitis to nondysplastic BE, then to lowgrade dysplasia (LGD), high-grade dysplasia (HGD), adenocarcinoma in situ, and culminates with invasive adenocarcinoma (Figures 1-3).4 As the seventh leading cause of cancer-related death among men in the United States, esophageal cancer is the subject of much research, and the early detection of changes along this dysplasia-to-carcinoma sequence can be lifesaving.5
In 2021, there were an estimated 19,260 new cases of esophageal cancer diagnosed in the United States and approximately 15,530 esophageal cancer deaths.5 Surveillance of BE can help diagnose cellular changes and prevent progression to EAC, but guidelines on endoscopic surveillance intervals are dictated by the degree
of dysplasia identified.1 Thus, an understanding of the significance of the findings is required.
Under the microscope, dysplasia is characterized by architectural and cytological abnormalities. When dysplastic changes are seen in the crypt bases with preserved surface maturation, pathology will be labeled as basal crypt dysplasia or indefinite for dysplasia (IND).6,7 Significant research has gone into understanding the importance of crypt dysplasia, and we discussed the contributions to the literature on crypt dysplasia in a previous review ( Gastroenterology & Endoscopy News 2019;72[7]:1-4). In this current review, we discuss advances that have been made in the past 2 years that clarify the implication of these findings.
Inflammation Versus Dysplasia
The cellular abnormalities that define crypt dysplasia can sometimes be attributed to inflammation or technical issues with specimen handling. Crush artifact and heat damage, such as the damage sustained from cautery, can lead to lack of surface epithelium or damage to the surface and inability to accurately assess the surface maturation.8,9 Similarly, crypt dysplasia or IND will often be used when there is a background of inflammation. However, unlike true dysplasia, inflammation, granulation tissue, and mucosal erosion do not cause DNA abnormality or aneuploidy. Therefore, it has been proposed that DNA content abnormality can be used for the risk stratification when IND is diagnosed. Choi et al found DNA content abnormality in 21 patients with biopsy consistent with IND, 90.5% had normal DNA, and only 1 of those patients developed HGD. The other 9.5% had abnormal DNA and developed HGD or EAC within 2 years. The research demonstrates that evaluation for DNA abnormality can differentiate true crypt dysplasia from the cellular abnormalities caused by inflammation or technical issues.8
In addition to aneuploidy alone, mutational load, or the measure of genetic aberration and instability, can be used to risk-stratify patients with IND and differentiate between those with inflammation and those with dysplasia. In a retrospective study of 28 patients with baseline IND, 88% of those who progressed to LGD or HGD had a mutational load of at least 0.5. The sensitivity and specificity for identifying patients who would progress to HGD were 100% and 85%, respectively, when using a baseline mutational load of at least 1.5. Based on this research, mutational load also can help risk-stratify patients with IND.10
More specific investigation into the cellular abnormalities that may be present in dysplasia has included assessing the presence of abnormal tumor suppressor gene p53 via immunohistochemistry among patients with BE. Redston et al found that 90% of IND patients who progressed to advanced disease had abnormal p53 compared with just 15.4% of nonprogressors.11 Patients with abnormal p53 within IND samples progressed with similar rates to patients with LGD, whereas patients with IND and normal p53 had much lower progression

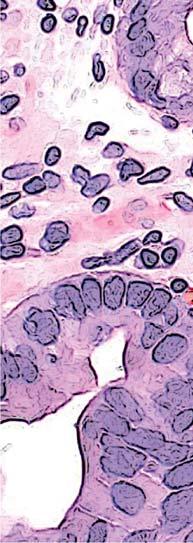
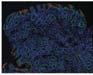
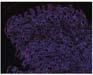
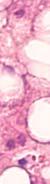
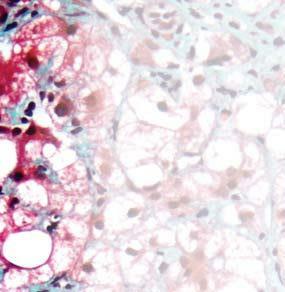
Figure 1. Basal crypt dysplasia. The surface is mature; however, deep glands have increased mitotic activity, cribriform architecture, and luminal debris.
Image courtesy of Yvelisse Suarez, MD.
GASTROENDONEWS.COM10
Image courtesy of Yvelisse Suarez, MD.
rates. Since abnormal p53, like high mutational load and abnormal DNA, would not be expected to be seen in nondysplastic inflamed or damaged tissue, its presence may help differentiate between those samples with IND that are on the pathway to progression versus those that are simply inflamed.
Updates to the Literature
Most of the recent literature on crypt dysplasia in BE consists of retrospective studies. One exception is a multicenter prospective cohort study from Philips et al assessing the risk for neoplasia among patients with IND.9 They found that 24% of patients with IND developed dysplasia. In this study, length of the BE segment was the only significant risk factor for progression to neoplasia. Similarly, Shaheen et al found a correlation between BE segment length and rate of progression from crypt dysplasia to HGD or EAC.12 In this study, of 128 patients with crypt dysplasia, 3 progressed to HGD or EAC, with a progression rate of 1.42% per patient-year. Henn et al found an annual progression rate of 0.98 cases per 100 patient-years from IND to HGD or EAC among a retrospective cohort of 107 patients with IND.13 However, all cases of HGD or EAC were discovered within 1 year of initial IND diagnosis, reinforcing


the need for surveillance EGD within 6 months of IND diagnosis. Among this cohort, 18.7% of patients developed LGD within a median follow-up of 2.39 years. Of note, patients with persistent IND on repeat endoscopy had a higher rate of progression, with 36% developing LGD.
One of the main challenges with understanding IND is determining whether it has a place along the metaplasia-dysplasia-carcinoma sequence. One retrospective single-center Barrett’s registry showed that the rate of progression to HGB and EAC was higher among patients with IND than among those with nondysplastic BE, and was lower in patients with IND than those with LGD.14 This pattern suggests that IND may fall along this spectrum and perhaps represents a midway point between nondysplastic BE and LGD.
To date, there is only 1 systematic review and meta-analysis evaluating the risk for progression in BE-IND. Pooling data from 8 studies with 1,441 patients, Krishnamoorthi et al found an incidence of HGD and/ or EAC of 1.5 per 100 patient-years.15 This rate is similar to the rate of progression in LGD, and the authors point out that, given the similar rate of progression as LGD, there may be a role for endoscopic therapy for IND in the future.
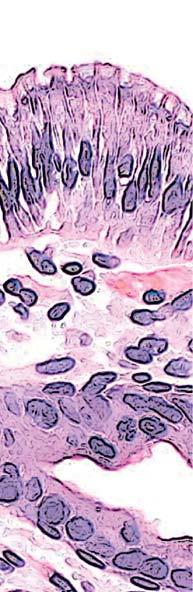
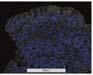
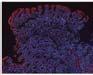
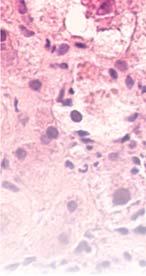
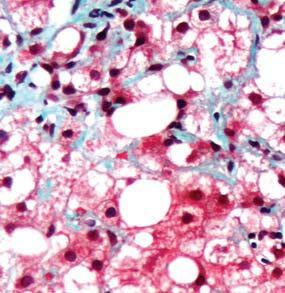
Figure 2. Low-grade dysplasia. Hyperchromatic nuclei extend to the surface, with maintenance of polarity.
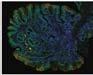
Figure 3. High-grade dysplasia. Crowded irregular glands with hyperchromatic nuclei extending to the surface, with loss of polarity.
Image courtesy of Yvelisse Suarez, MD.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 11
Guidelines Recommendations
The American College of Gastroenterology guidelines for BE recommend that when pathology is indefinite for dysplasia, the patient should be started on twice-daily proton pump inhibitors and undergo repeat esophagogastroduodenoscopy (EGD) within 6 months.1 This recommendation has been modified from the 2015 recommendation to repeat EGD within 3 to 6 months.16 When IND is identified on the surveillance EGD within 6 months, repeat endoscopy should be performed annually.1
In comparison, patients with nondysplastic BE can wait 3 to 5 years for their next EGD, depending on the length of the segment of BE.1 This shorter interval means that patients with crypt dysplasia will have more surveillance endoscopies than those without dysplasia; although endoscopy is a safe procedure, it is not without risks, including the risks of anesthesia. Understanding of the significance of crypt dysplasia,
References
1. Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and management of Barrett’s esophagus: an updated ACG guideline. Am J Gastroenterol. 2022;117(4):559-587.
2. Eusebi LH, Cirota GG, Zagari RM, et al. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70(3):456-463.
3. Coron E, Robaszkiewicz M, Chatelain D, et al. Advanced precancerous lesions in the lower oesophageal mucosa: high-grade dysplasia and intramucosal carcinoma in Barrett’s oesophagus. Best Pract Res Clin Gastroenterol 2013;27(2):187-204.
4. Coleman HG, Xie SH, Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterology 2018;154(2):390-405.
5. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33.
6. Coco DP, Goldblum JR, Hornick JL, et al. Interobserver variability in the diagnosis of crypt dysplasia in Barrett esophagus. Am J Surg Pathol. 2011;35(1):45-54.
7. Lomo LC, Blount PL, Sanchez CA, et al. Crypt dysplasia with surface maturation: a clinical, pathologic, and molecular study of a Barrett’s esophagus cohort. Am J Surg Pathol 2006;30(4):423-435.
8. Choi WT, Tsai JH, Rabinovitch PS, et al. Diagnosis and risk stratification of Barrett’s dysplasia by flow cytometric DNA analysis of paraffin-embedded tissue. Gut. 2018;67(7): 1229-1238.
9. Phillips R, Januszewicz W, Pilonis ND, et al. The risk of neoplasia in patients with Barrett’s esophagus indefinite for dysplasia: a multicenter cohort study. Gastrointest Endosc 2021;94(2):263-270.e2.
therefore, has downstream effects on patient safety and healthcare costs.15
The American Society for Gastrointestinal Endoscopy guideline for screening and surveillance of BE does not contain specific guidance regarding interpretation or management of crypt dysplasia, but it notes that patients with crypt dysplasia may be at a higher risk for progression and points out that further research is needed to clarify the natural history of crypt dysplasia.17
Despite additional research over the past several years, the role that crypt dysplasia plays in the metaplasia-dysplasia-carcinoma sequence from BE to EAC remains ambiguous. It is clear that crypt dysplasia carries with it a risk for progression to HGD and EAC, and strides have been made toward understanding the nuances of this complex diagnosis. Further investigation and high-quality, prospective research on the significance of IND is necessary to elucidate the importance and management of this finding.
10. Trindade AJ, McKinley MJ, Alshelleh M, et al. Mutational load may predict risk of progression in patients with Barrett’s oesophagus and indefinite for dysplasia: a pilot study. BMJ Open Gastroenterol. 2019;6(1):e000268.
11. Redston M, Noffsinger A, Kim A, et al. Abnormal TP53 predicts risk of progression in patients with Barrett’s esophagus regardless of a diagnosis of dysplasia. Gastroenterology. 2022;162(2):468-481.
12. Shaheen NJ, Smith MS, Odze RD. Progression of Barrett’s esophagus, crypt dysplasia, and low-grade dysplasia diagnosed by wide-area transepithelial sampling with 3-dimensional computer-assisted analysis: a retrospective analysis. Gastrointest Endosc. 2022;95(3):410-418.e1.
13. Henn AJ, Song KY, Gravely AA, et al. Persistent indefinite for dysplasia in Barrett’s esophagus is a risk factor for dysplastic progression to low-grade dysplasia. Dis Esophagus. 2020;33(9):doaa015.
14. O’Byrne LM, Witherspoon J, Verhage RJJ, et al. Barrett’s Registry Collaboration of academic centers in Ireland reveals high progression rate of low-grade dysplasia and low risk from nondysplastic Barrett’s esophagus: report of the RIBBON network. Dis Esophagus. 2020;33(10):doaa009.
15. Krishnamoorthi R, Mohan BP, Jayaraj M, et al. Risk of progression in Barrett’s esophagus indefinite for dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2020;91(1):3-10.e3.
16. Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111(1):30-50; quiz 51.
17. Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc. 2019;90(3):335-359.e2.
The authors reported no relevant financial disclosures. Copyright © 2022 McMahon Publishing, 545 West 45th Street, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form. GASTROENDONEWS.COM12
Which Barrett’s esophagus patient is likely to progress to high grade dysplasia or esophageal cancer?
Two non-dysplastic patients nearly identical by pathology and clinical risk factors





NDBE 4cm
Hiatal Hernia No Lesions
NDBE 3cm
Hiatal Hernia
Lesions
TissueCypher Score / Risk Class 3.6 / Low Risk












































Five-year Progression Risk 2.1% Outcome Progression-free for 6.7 years

The FIRST and ONLY precision medicine test that:
•Predicts future development of esophageal cancer in patients with Barrett’s esophagus (BE)
TissueCypher Score / Risk Class 9.6 / High Risk Five-year Progression Risk 21.4% Outcome Progressed to HGD in 2.7 years following baseline
•Is an INDEPENDENT risk predictor from tissue histology and other clinical risk factors
•Transforms patient management by enabling upstaging or downstaging based on individual patient risk
























Know your patient’s individual risk of progression to esophageal cancer
LEARN MORE AT TISSUECYPHER.COM

© 2022 Castle Biosciences. TissueCypher Barrett’s Esophagus Assay is a trademark of Castle Biosciences Inc.TC-015v3 – 042122
No






























































































































































































































































































































































































VELACURTRANSIENT ELASTOGRAPHY 2X MORE DEPTH 30X MORE VOLUME Volume SampledMeasurement Depth 15 12 9 6 3 0 100 cm3 80 cm3 60 cm3 40 cm3 3 cm3 0 cm3
Nonalcoholic Steatohepatitis: Identifying High-Risk Patients



ASHWANI K. SINGAL, MD, MS, FACG, FAASLD, AGAF
Professor of Medicine Department of Medicine University of South Dakota
Sanford School of Medicine
Transplant Hepatologist
Avera McKennan University Hospital Chief, Clinical Research Avera Transplant Institute Sioux Falls, South Dakota
Nonalcoholic
fatty liver disease (NAFLD) is characterized by excessive fat deposition in the liver in the absence of other causes of liver disease, including harmful consumption of alcohol.1 The disease spectrum ranges from nonalcoholic fatty liver or hepatic steatosis to nonalcoholic steatohepatitis (NASH)—characterized by hepatic inflammation and steatosis with or without fibrosis—to cirrhosis and hepatocellular carcinoma (HCC).2-4

 Fibrotic liver Formation of scar tissue within the liver
Cirrhotic liver Scarred tissue replaces healthy tissue in the liver
Liver cancer Formation of malignant tumor in the liver
Liver Steatosis Enlarged liver due to fat deposits
Fibrotic liver Formation of scar tissue within the liver
Cirrhotic liver Scarred tissue replaces healthy tissue in the liver
Liver cancer Formation of malignant tumor in the liver
Liver Steatosis Enlarged liver due to fat deposits
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 15
NASH is the second-leading indication for liver transplantation in the United States5 and likely will become the main indication in the future.6 It also is the fastest-growing reason for simultaneous liver–kidney transplantation, acute-on-chronic liver failure–related hospitalization, and HCC.7-9 NAFLD also is the most common long-term complication in liver transplant recipients.10
This review, part 1 of a 2-part series, focuses on NASH, given its clinical significance and greater effects on patient outcomes, discussing how to identify patients who are at risk for advanced fibrosis. Part 2 discusses ongoing clinical trials with new therapeutic targets (See page 23).
Targeting High-Risk Patients
Patients with NAFLD usually are asymptomatic.11 The most common clinical presentations include incidental elevations in aminotransferases, steatosis on imaging, or a new diagnosis of cirrhosis.12 About 15% to 20% of NAFLD patients who develop NASH are at risk for progression to cirrhosis and liver-related mortality.13 Hence, identification of these patients is crucial so they can be targeted for close follow-up and treatment.
Clinical predictors of NASH are advancing age,
adiposity, and type 2 diabetes mellitus (T2DM).11 Aminotransferases may be normal in
obesity, visceral
Table. Pooled Performance of Serologic- and Radiological-Based Noninvasive Assessment of Fibrosis for NAFLD Test Significant fibrosis (F2-F4) Advanced fibrosis (F3-F4) Cirrhosis (F4) Cutoff valueAUC Sensitivity/ specificity, % PPV/ NPV, % Cutoff valueAUC Sensitivity/ specificity, %PPV/NPV Cutoff valueAUC Sensitivity/ specificity, % PPV/ NPV Serologic biomarkers APRI 0.43-1.50.759/7768/–0.540.98 0.7569/7361/900.54-2.00.75 56/8438/–FIB-4 0.373.25 0.7564/7073/–1.241.45 0.8 78/7140/851.92-2.480.8576/8239/–NFS –1.10.7266/8382/––1.4550.7873/7450/92–0.0140.8380/8143/–BARD 20.8144/7050/6520.8875/6238/8930.8352/8425/ 94 HFS 0.470.8574/9776/92 ELF 0.570.9 45/9585/–1.6450.9330/–87/–NANANANA MLA 0.90.9970.989 Radiological biomarkers TE 6.7-7.00.8274/5963/857.6-8.00.8689/7743/9610.3-11.30.9483/8647/ 98 ARFI/ pSWE 0.8474/900.9592/910.9792/91 2D-SWE 2.7-9.40.8990/9288/933.010.6 0.9190/9288/933.360.92100/8655/ 100 MRE 3.4-3.620.8873/9183/86 3.6-4.80.9386/9171/93 4.2-6.70.9287/9353/ 99 MEFIB 0.90 APRI, AST–to-platelet ratio index; ARFI, acoustic radiation force imaging; AUC, area under the curve; ELF, enhanced liver fibrosis; FIB-4, Fibrosis-4; MEFIB, MRE+FIB-4 combination; MLA, machine learning algorithm; MRE, magnetic resonance elastography; NFS, NAFLD fibrosis score; NPV, negative predictive value; PPV, positive predictive value; SWE, shear wave elastography; TE, transient elastography. Modified from reference 53. GASTROENDONEWS.COM16
more than half of patients with NASH and elevated in NAFLD patients without NASH.14
Liver Biopsy
Liver biopsy is the gold standard for accurate diagnosis of NASH. A NAFLD activity score (NAS) is calculated based on liver histology, with evaluation of steatosis grade (0-3), acinar inflammation (0-3), and cytologic ballooning (0-2).15 A NAS of less than 3 excludes, and 5 or higher confirms, a diagnosis of NASH.15 Liver biopsy also allows assessment of fibrosis stage, the main determinant of outcomes in NAFLD.16 The METAVIR score is one method often used to score fibrosis stage on liver biopsy, with stage F0 correlating with an absence of fibrosis and stage F4 with cirrhosis.17 The Ishak score is another method, using fibrosis stages F0 to F6, with stage F5 equivalent to early cirrhosis and stage F6 indicating established cirrhosis.18
Noninvasive Assessment of Liver Disease
Liver biopsy has been considered the standard to stratify the disease spectrum in patients with NAFLD.13,19 However, biopsy is associated with potential complications: Pain after biopsy is almost universal, bleeding occurs in approximately 1 in 500 liver biopsies, and death occurs in 1 in 10,000.20 In addition, liver biopsy is not an ideal reference and is limited by sampling error, with inaccurate fibrosis staging occurring in up to 33% of cases and interobserver disagreement in up to 30% of cases.21 Liver biopsy also is limited in research studies and clinical trials, with a requirement of at least a 15-mm length of liver tissue with at least 10 portal tracts, a standard not observed frequently in clinical practice.21
Over the past couple of decades, several blood-based and imaging-based biomarkers have been developed for noninvasive assessment of liver disease (NIALD) (Table).
Blood-Based Biomarkers
Blood-based biomarkers to assess fibrosis in NAFLD can be categorized into indirect biomarkers (using patient demographics, standard-of-care laboratory values for liver chemistry, and platelet counts) and direct biomarkers (using specific laboratory tests measuring extracellular matrix deposition and turnover).22
NAFLD Fibrosis Score
This measure incorporates age, the presence of hyperglycemia, body mass index (BMI), platelet count, serum albumin level, and aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio. In the pivotal study, NAFLD Fibrosis Score (NFS) of 1.455 or less had an area under the curve (AUC) of 0.88 (0.85-0.92), with a negative predictive value of 93% to exclude F3 to F4 fibrosis.23 As with many other NIALD biomarkers considering platelet count (see below), NFS is not accurate in patients who have undergone transjugular intrahepatic portosystemic shunt placement or are asplenic.24 Similarly, NFS and other tests, including AST and ALT tests, lose accuracy when they are calculated
during acute-on-chronic liver injury because aminotransferases can be elevated acutely.
Fibrosis-4
This test uses age, AST and ALT levels, and platelet count. In a pivotal study of 832 biopsied patients with HIV and hepatitis C virus (HCV) coinfection, a cutoff of greater than 3.25 had a positive predictive value of 68% and specificity of 97% in the diagnosis of advanced fibrosis.25 Based on the lower and upper cutoffs of 1.30 and 3.25, respectively, Fibrosis-4 (FIB-4) allowed avoidance of 71% of liver biopsies. The test also was found to be useful in diagnosing advanced fibrosis and cirrhosis in NAFLD patients, with an AUC of 0.8 and 0.85 at cutoff values of 1.24 to 1.45 and 1.92 to 2.48, respectively. However, FIB-4 is less specific in patients older than 60 years of age.26
AST-to-Platelet Ratio Index
This index was developed in patients with HCV, with an AUC of 0.83 (95% CI, 0.78-0.88) and 0.90 (95% CI, 0.86-0.94) for predicting significant fibrosis and cirrhosis, respectively.27 The utility of the AST-to-Platelet Ratio Index (APRI) in NAFLD patients was validated with an AUC of 0.77 and 0.91 at cutoff scores of 0.43 to 1.5 and 0.54 to 2.0, respectively.24
BARD Score
This score is the weighted sum of 3 variables (BMI ≥ 28 kg/m 2 =1 point, AST/ALT ratio ≥ 0.8=2 points, diabetes=1 point). In a pivotal study of 827 patients with NAFLD, a score of 2 to 4 had a 17-fold odds of indicating the presence of advanced fibrosis, with 96% accuracy in excluding this condition.28
Hepamet Fibrosis Score
This score consists of age, sex, homeostatic model assessment score, diabetes mellitus status, AST, serum albumin, and platelet count. The Hepamet Fibrosis Score has a better accuracy than FIB-4 and NFS and does not require adjustment for patient’s age.29
FibroTest (FibroSURE in United States)
This test uses total bilirubin, gamma-glutamyl transferase (GGT), alpha2-macroglobulin, apolipoprotein A1, and haptoglobin, corrected for age and sex. In an initial NAFLD validation study, FibroTest showed an AUC of 0.88 (95% CI, 0.82-0.92) in predicting F3 to F4 fibrosis, proposing cutoff values of 0.3 and 0.7 to maximize negative predictive value (90%) and positive predictive value (73%), respectively.30 Conditions different from NAFLD affecting bilirubin (eg, Gilbert’s syndrome), haptoglobin (eg, hemolysis), alpha2-macroglobulin (eg, inflammatory condition), or GGT (eg, alcohol ingestion) may result in an inaccurate estimation of fibrosis.21
Enhanced Liver Fibrosis Score
This measure, which uses matrix metalloproteinase 1 (tissue inhibitor of matrix metalloproteinase 1),
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 17
hyaluronic acid, and amino terminal peptide of pro-collagen III, shows an AUC of 0.90 for distinguishing F3 to F4 fibrosis.31 Caution is advised when interpreting enhanced liver fibrosis (ELF) score results in patients with other diseases associated with increased collagen turnover (eg, interstitial lung disease).31
A meta-analysis comparing serologic markers in the assessment of fibrosis in more than 13,000 patients showed NFS and FIB-4 to be the most accurate, with a pooled AUC of 0.84 and negative predictive value of greater than 90% for the diagnosis of advanced fibrosis.32 One of the limitations of these blood-based biomarkers is that approximately 20% to 30% of patients can fall into an indeterminate category.24

Machine Learning Algorithm
This test consists of body mass index, Pro C3, type IV collagen, and AST-to-GGT ratio.33


Imaging-Based Biomarkers
Although liver biopsy often is needed, imagingbased NIALD can be used to improve accuracy and reduce the need for biopsy.24 In general, imaging techniques are more accurate than serum biomarkers,34 but many of them are not widely available.
Transient Elastography or Vibration-Controlled Transient Elastography





This techniquehas the advantages of beingofficebased, reproducible across centers, available as a point-of-care tool, and easy to operate and interpret. The results can be affected by fed state, so patients are advised to fast for at least 3 hours before the test.35 Morbidly obese individuals with a skin-to-liver capsule distance of 25 mm or greater will require an extralarge (XL) probe.
The test is a liver stiffness measurement (LSM) and is reported in kilopascals (kPa). Transient elastography (TE) is 94% to 100% accurate in ruling out F3 to F4 fibrosis at a cutoff of 8 kPa. The sensitivity and specificity are 85% and 82%, respectively, for the diagnosis of advanced fibrosis (stage F3) and 92% and 92%, respectively, for the diagnosis of cirrhosis.36 The AUCs for diagnosis of advanced fibrosis and cirrhosis do not differ with the standard M probe and the XL probe (0.87 vs 0.86 and 0.92 vs 0.94, respectively).37 TE is limited by lack of parenchymal assessment and inaccuracy in patients with ascites, cholestasis, inflammation from acute hepatitis, and heart failure. In addition, accuracy depends on operator experience.35 TE also has a technical failure rate of about 5% in patients who are morbidly obese.38
Figure. Proposed clinical care algorithm for NAFLD. a If MRE is unavailable. FIB-4, Fibrosis-4 score; LSM, liver stiffness measurement; MRE, magnetic resonance elastography.
Patients with
NAFLD (steatosis on imaging and other causes ruled out) FIB-4 LSM ≤8.0 kPa LSM >8.0 kPa MRE Consider liver biopsya FIB-4 <1.3 • Follow-up by primary care physician • Check liver chemistry every 6-12 mo • Lifestyle modifications: diet and exercise FIB-4 ≥1.3 Transient elastography assessment GASTROENDONEWS.COM18
Shear Wave Elastography
This test can be performed using acoustic radiation force imaging (ARFI), also known as point shear wave elastography (pSWE) or 2-dimensional SWE (2DSWE).39 ARFI measures the ultrasound attenuation and velocity of spread of shear waves expressed as meter per second (m/s) and has shown an accuracy of 95% for the diagnosis of significant fibrosis.39,40 Like TE, 2D-SWE measures liver stiffness in kPa, with the added advantage of assessment of hepatic parenchyma, and it has shown an accuracy of 92% for the diagnosis of advanced fibrosis in NAFLD.41 Both ARFI and 2D-SWE are becoming widely available, and despite differences in cutoff values across manufacturers, high operator dependability, and limited experience (eg, few prospective studies), the accuracy is comparable to that of TE, and these methods are good alternatives for patients needing an anatomic ultrasound.42
MR Elastography
This technique involves phase contrast imaging, which measures liver stiffness depending on mechanical wave propagation. With this method, mechanical waves generated in a drum device over the liver are imaged for about 15 seconds in end expiration, and the device automatically provides a color-coded liver stiffness map. MR elastography (MRE) has the unique ability to assess regression and progression during the course of disease or in response to treatment.43 The accuracy of 2D-MRE for diagnosis of advanced fibrosis or cirrhosis, which has been documented in a recent meta-analysis, is independent of BMI, sex, and degree of inflammation.43 Although 3D-MRE is more accurate than the 2D technique, it takes more time, and experience with this approach is limited.42 MRE is more accurate than TE because it is less operator dependent, and it also is more useful for patients who are morbidly obese or have ascites. However, this method is not appropriate for patients with implanted metallic devices, claustrophobia, severe steatosis, or hemochromatosis, and it is further limited by high cost and lack of widespread availability.
Assessment of Steatosis
This review primarily focuses on assessment of high-risk NAFLD patients with fibrosis, but it is worth mentioning noninvasive imaging assessment of steatosis because the standard technique of ultrasonography fails to quantify steatosis, a relevant important assessment, especially in ongoing clinical trials targeting steatosis. 44 In addition, ultrasound assessment can provide false-negative results, leading to diagnosis of steatosis in patients with morbid obesity and/or renal disease.45 In the absence of more widely available and validated methods, the American Association for the Study of Liver Diseases and the European Association for the Study of Liver Diseases recommend ultrasonography to diagnose steatosis.13,19 Techniques for quantifying steatosis are discussed below.
Controlled Attenuation Parameter
This novel ultrasound-based technique to quantify steatosis is incorporated into TE equipment. It is easy to perform, widely available, affordable, accurate, and reproducible. The pivotal study assessing this technique showed AUCs of 0.91, 0.95, and 0.89 for the diagnosis of mild, moderate, and severe steatosis, respectively. In a recent meta-analysis, these respective AUC values have remained at 0.82, 0.86, and 0.88 at cutoffs of 248, 268, and 280 dB/m, respectively.46 However, the tool is limited in patients with morbid or severe obesity or ascites. In a limited evaluation of the XL probe versus M probe, controlled attenuation parameter (CAP) showed no difference in accuracy in quantifying fat.42 Some adjustments in CAP results are needed based on degree of obesity and in patients with NAFLD and diabetes.
MRI Proton Density Fat Fraction
This approach depends on the ability of MRI to separate water and fat tissue signals, using MR-based spectroscopic examination. MRI protein density fat fraction (MRI-PDFF) reliably quantifies hepatic steatosis and is useful for following patients on treatment.47 The technique is superior to CAP for quantifying hepatic fat, with an AUC of 0.99 versus 0.85 for CAP.48 MRI-PDFF has excellent concordance with liver biopsy–based quantification of liver fat.49 MRI-PDFF also has been shown to be a surrogate for histologic improvement, with a 30% or greater relative decrease in hepatic fat being associated with a 2-point reduction in NAFLD activity score, NASH resolution, and improvement in fibrosis.50 However, the technique is limited by lack of widespread availability, high cost, expertise required, and other disadvantages of MRI-based assessment.
Prospective NAFLD Studies Using Noninvasive Assessment
Besides providing cross-sectional assessment of fibrosis stage, noninvasive imaging and serum biomarkers of fibrosis have been shown to predict liver-related outcomes and mortality.51,52
Approach to NIALD for Patients With NAFLD in Routine Practice
Considering the accuracy of NIALD techniques validated against liver biopsy, we proposed a clinical care algorithm (Figure). Of the serum biomarkers, NFS and FIB-4 are favored, having the highest accuracy in excluding advanced fibrosis or cirrhosis.53 Of the imaging-based techniques, TE allows point-of-care clinical decisions and is the favored first-line imaging technique to stratify risk for fibrosis.53 These tests can be used in a simultaneous or sequential fashion. With a simultaneous approach, biopsy can be used for discordant results. With a serial approach, the second test reclassifies patients with indeterminate results on the first test, reducing the need for liver biopsy.54 If a biopsy is not obtained, continued follow-up and
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 19
monitoring are recommended for patients with indeterminate results.
Patient BMI, race, steatosis, and presence of diabetes need to be considered when using the algorithm in routine practice, and these variables affect NIALD. In this regard, CAP values are reduced by 10 dB/m for patients with NAFLD and T2DM. Similarly, CAP values are increased or reduced by 4.4 dB/m for every unit change in BMI above or below 25 kg/m2, respectively.46 As an example, in a patient with NAFLD, BMI of 29.5 kg/m2, and T2DM, a measured CAP value of 320 dB/m (severe steatosis) will translate to an adjusted value of 277 dB/m (moderate steatosis). Similarly, in
References
1. Sayiner M, Koenig A, Henry L, et al. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis 2016;20(2):205-214.
2. Kanwar P, Kowdley KV. The metabolic syndrome and its influence on nonalcoholic steatohepatitis. Clin Liver Dis 2016;20(2):225-243.
3. Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17(5):774.
4. Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology 1995;22(6):1714-1719.
5. Patel V, Sanyal AJ, Sterling R. Clinical presentation and patient evaluation in nonalcoholic fatty liver disease. Clin Liver Dis 2016;20(2):277-292.
6. Benedict M, Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol. 2017;9(16):715.
7. Singal AK, Hasanin M, Kaif M, et al. Nonalcoholic steatohepatitis is the most rapidly growing indication for simultaneous liver kidney transplantation in the United States. Transplantation 2016;100(3):607-612.
8. Axley P, Ahmed Z, Arora S, et al. NASH is the most rapidly growing etiology for acute-on-chronic liver failure-related hospitalization and disease burden in the United States: a population-based study. Liver Transpl. 2019;25(5):695-705.
9. Pocha C, Xie C. Hepatocellular carcinoma in alcoholic and non-alcoholic fatty liver disease—one of a kind or two different enemies? Transl Gastroenterol Hepatol. 2019;4:72.
10. Mikolasevic I, Filipec-Kanizaj T, Mijic M, et al. Nonalcoholic fatty liver disease and liver transplantation—where do we stand? World J Gastroenterol. 2018;24(14):1491-1506.
11. Karim M, Al-Mahtab M, Rahman S, et al. Non-alcoholic fatty liver disease (NAFLD)—a review. Mymensingh Med J 2015;24(4):873-880.
12. Salt W. Nonalcoholic fatty liver disease (NAFLD): a comprehensive review. J Insur Med. 2004;36(1):27-41.
13. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
14. McNair A. Non-alcoholic steatohepatitis (NASH): why biopsy? Gut. 2002;51(6):898; author reply 898-899.
15. Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53(3):810-820.
a patient with NAFLD at a low BMI of 21 kg/m2, a CAP measurement of 235 dB/m (no steatosis) will translate to an adjusted value of 252.5 (mild steatosis).
Summary
The current perspective on identifying high-risk NASH patients is transitioning away from liver biopsy, with the availability of several noninvasive, simple serologic and radiological biomarkers. However, given that the use of these biomarkers for following patients and assessing response to therapeutic intervention needs to be validated in clinical trials, liver biopsy remains a significant tool in patient selection and follow-up.
16. Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643-654.
17. Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47(4):598-607.
18. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696-699.
19. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obes Facts 2016;9(2):65-90.
20. Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology. 2009;49(3):1017-1044.
21. Duarte-Rojo A, Altamirano JT, Feld JJ. Noninvasive markers of fibrosis: key concepts for improving accuracy in daily clinical practice. Ann Hepatol. 2012;11(4):426-439.
22. Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127(6):1704-1713.
23. Younossi ZM, Loomba R, Anstee QM, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68(1):349-360.
24. Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486-1501.
25. Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104-1112.
26. Boursier J, de Ledinghen V, Leroy V, et al. A stepwise-algorithm using an at-a-glance first-line test for the non-invasive diagnosis of advanced liver fibrosis and cirrhosis. J Hepatol 2017;66(6):1158-1165.
27. Wai C-T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-526.
28. Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441-1447.
29. Ampuero J, Pais R, Aller R, et al. Development and validation of hepamet fibrosis scoring system—a simple, noninvasive test to identify patients with nonalcoholic fatty liver disease with advanced fibrosis. Clin Gastroenterol Hepatol. 2020;18(1):216-225.35.
GASTROENDONEWS.COM20
30. Ratziu V, Massard J, Charlotte F, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6(1):6.
31. Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47(2):455-460.
32. Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617-649.
33. Feng G, Zheng KI, Li YY, et al. Machine learning algorithm outperforms fibrosis markers in predicting significant fibrosis in biopsy-confirmed NAFLD. J Hepatobiliary Pancreat Sci. 2021;28(7):593-603.
34. Fedchuk L, Nascimbeni F, Pais R, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209-1222.
35. Vuppalanchi R, Weber R, Russell S, et al. Is fasting necessary for individuals with nonalcoholic fatty liver disease to undergo vibration-controlled transient elastography? Am J Gastroenterol 2019;114(6):995-997.
36. Buzzetti E, Hall A, Ekstedt M, et al. Collagen proportionate area is an independent predictor of long-term outcome in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;49(9):1214-1222.
37. Staufer K, Halilbasic E, Spindelboeck W, et al. Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United European Gastroenterol J. 2019;7(8):1113-1123.
38. Barr RG, Ferraioli G, Palmeri ML, et al. Elastography assessment of liver fibrosis: Society of Radiologists in Ultrasound Consensus Conference statement. Radiology. 2015;276(3):845-861.
39. Kennedy P, Wagner M, Castéra L, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology. 2018;286(3):738-763.
40. Liu H, Fu J, Hong R, et al. Acoustic radiation force impulse elastography for the non-invasive evaluation of hepatic fibrosis in non-alcoholic fatty liver disease patients: a systematic review & meta-analysis. PLoS One. 2015;10(7):e0127782.
41. Herrmann E, de Lédinghen V, Cassinotto C, et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology. 2018;67(1):260-272.
42. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264-1281.e4.
43. Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2016;26(5):1431-1440.
44. Paige JS, Bernstein GS, Heba E, et al. A pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. Am J Roentgenol 2017;208(5):W168-W177.
45. Sasso M, Miette V, Sandrin L, et al. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol 2012;36(1):13-20.
46. Karlas T, Petroff D, Sasso M, et al. Individual patient data metaanalysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022-1030.
47. Ajmera V, Park CC, Caussy C, et al. Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2018;155(2):307-310.e2.
48. Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 2017;152(3):598-607.e2.
49. Caussy C, Reeder SB, Sirlin CB, et al. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68(2):763-772.
50. Tamaki N, Munaganuru N, Jung J, et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease. Gut. 2022;71(5):983-990.
51. Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65(3):570-578.
52. Lombardi R, Petta S, Pisano G, et al. FibroScan identifies patients with nonalcoholic fatty liver disease and cardiovascular damage. Clin Gastroenterol Hepatol. 2020;18(2):517-519.
53. Altamirano J, Qi Q, Choudhry S, et al. Non-invasive diagnosis: non-alcoholic fatty liver disease and alcoholic liver disease. Transl Gastroenterol Hepatol. 2020;5:31.
54. Petta S, Wong V W-S, Camma C, et al. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther 2017;46(6):617-627.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 21
FUTURE OF NASH WITH FIBROSIS
A liver disease with

• Patients with NASH have
• Once a patient develops
consequences – including mortality1-5
disease, cirrhosis, decompensated
carcinoma2,4,6-10
the risk of liver-related mortality


•
- F2 and F3 patients are at ~10-17x
is
risk of mortality compared to F0 patients11

patients with significant fibrosis* as it is a strong
*Fibrosis stage F2 or F3 as defined histologically.12,13
F0=liver fibrosis stage 0; F2=liver fibrosis stage 2; F3=liver fibrosis stage 3; NASH=nonalcoholic steatohepatitis.
References:
of patients with
TA, Yerian L, Lopez R, Zein NN, Feldstein AE. The inflamed liver and atherosclerosis: a link between histologic severity of nonalcoholic fatty liver disease and increased cardiovascular risk. Dig Dis Sci. 2010;55(9):2644-2650. 3. Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):12651273. 4. Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6(7):578-588. 5. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127(1):55-64. 6. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646-650.
7. Zhai M, Liu Z, Long J, et al. The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci Rep. 2021;11(1):5195. 8. Loomba R, Adams L. The 20% rule of NASH progression: the natural history of advanced fibrosis and cirrhosis caused by NASH. Hepatology. 2019;70(6):1885-1888. 9. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188-2195.
10. Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48(7):696-703. 11. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557-1565. 12. Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterol. 2021;1-13. 13. Ratziu V, Bellantani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372-384.
IT’S TIME TO EXPLORE THE
severe
Learn more about the risks starting at F2 and how to identify these patients11 Visit NASHexplored.com/4 today and stay informed ©2022 Madrigal Pharmaceuticals, Inc. All rights reserved. Madrigal Pharmaceuticals, 200 Barr Harbor Drive, Suite 200, West Conshohocken, PA 19428 US-00006 05/22
an increased risk of cardiovascular
liver disease, liver transplant, and hepatocellular
significant fibrosis*,
increases substantially1,11
higher
It
important to identify and actively manage
predictor of liver-related mortality1,11
1. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes
nonalcoholic fatty liver disease. Gastroentrology. 2015;149(2):389-397. 2. Alkhouri N, Tamimi
Nonalcoholic Steatohepatitis: Treatment Options for High-Risk Patients





ASHWANI K. SINGAL, MD, MS, FACG, FAASLD, AGAF
Professor of Medicine, Department of Medicine University of South Dakota Sanford School of Medicine Transplant Hepatologist
Avera McKennan University Hospital Chief of Clinical Research Avera Transplant Institute Sioux Falls, South Dakota
Themanagement of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) involves treatment of liver disease and its complications, control of risk factors, disease stabilization, and prevention of fibrosis progression. Although the only consistently proven treatment is lifestyle modification, development of drug targets to treat NASH has undergone rapid growth over the past several years, with several active clinical trials.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 23
This is part 2 of a 2-part series on NASH. This review discusses ongoing clinical trials of new therapeutic targets for the treatment of NASH. Part 1 discusses how to identify patients with NASH who are at risk for advanced fibrosis (See page 15).
Achieving Treatment Goals
The goals in managing patients with NAFLD include treatment of liver disease and its complications, stabilization of disease, control of risk factors for the disease, and prevention of progression of fibrosis.1 Lifestyle modification with diet, exercise, weight loss, and control of the components of metabolic syndrome is the only consistently proven treatment for NAFLD. The best data on the beneficial effects of weight loss in NAFLD come from paired liver biopsy evaluation in patients undergoing bariatric surgery.2 Weight loss of at least 7% has been deemed necessary for histologic improvement.3 Practice guidelines recommend a goal of at least 7% to 10% weight reduction to improve both fibrosis and inflammation, but a 3% to 5% weight loss has shown success in improving steatosis.1 Given the difficulty in achieving this weight loss goal in the clinical setting, it is recommended that patients with NAFLD be managed by a multidisciplinary team that includes dietitians, physical activity supervisors, and psychologists.1 More frequent clinic visits are associated with greater weight loss in patients with NAFLD.4 Novel approaches, such as text-messaging weight management programs, can help patients achieve meaningful weight loss.5 Restricting calories to 25 to 30 kcal/kg per day to achieve a 10% weight loss over 6 months is desirable; rapid weight loss can worsen NAFLD.6 The Mediterranean diet has been shown to reduce hepatic steatosis and improve insulin sensitivity in nondiabetic patients with NAFLD.7 Combining dietary changes with exercise is more effective than either intervention alone. There is a clear benefit of exercise on hepatic steatosis, even in the absence of weight loss.8 Resistance training may be preferable to aerobic training and is associated with significant improvement in NAFLD parameters.9
Management of diabetes and achievement of optimal glycemic control with a target hemoglobin A1c of less than 7% is desirable. Use of statins to treat dyslipidemias is imperative, with a target low-density lipoprotein cholesterol level of less than 100 mg/dL to decrease cardiovascular mortality.10 Atorvastatin, rosuvastatin, pravastatin, and simvastatin have been assessed in randomized controlled trials and were found to be safe for patients with NAFLD.11 Omega-3 fatty acids and fenofibrate are safe and effective for managing hypertriglyceridemia. Control of blood pressure, with a target below 140/90 mm Hg, is imperative.12 Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are preferred, especially in patients with diabetes because they have been shown to improve insulin sensitivity.1 Heavy use of alcohol is associated with progression of fibrosis and worse prognosis,13 although light to moderate drinking has been shown to have a protective
effect in the development of NAFLD.14 Progression to cirrhosis or hepatocellular carcinoma occurs in only 2.5% of patients with NASH, and usually it is slower than with other chronic liver diseases.15
Many pharmacologic options have been studied, but no FDA-approved drug exists for the management of NAFLD. Vitamin E (800 IU per day) was better than placebo in clinical trials, with a higher proportion of patients showing improved histologic features of NASH (43% vs 19%; P=0.001).16 However, vitamin E did not improve liver fibrosis and did not gain FDA approval for the treatment of NAFLD or NASH. The American Association for the Study of Liver Diseases supports consideration of vitamin E for nondiabetic patients with biopsy-proven NASH.1
Liver transplantation is a definitive treatment for NAFLD patients with cirrhosis and acute-on-chronic liver failure, those with end-stage liver disease, or those with hepatocellular carcinoma. After surgery, these patients often develop an increase in body weight, subsequent insulin resistance, metabolic syndrome, and malignancy.17 Outcomes in patients with NASH after transplant are similar to those for other etiologies, with a lower risk for liver-related mortality.18
Novel Therapeutic Targets
Drug development of targets to treat NASH has undergone rapid growth over the past several years, with more than 100 registered clinical trials currently in different phases. Based on the pathophysiology of NASH, therapeutic targets are categorized into 3 groups related to their anti-inflammatory, antisteatotic, and antifibrotic activity. Several drugs in trials exhibit more than 1 of these 3 activities. Several molecular targets are in later stages of clinical development (Table 1).
Peroxisome Proliferator–Activated Receptors
Peroxisome proliferator–activated receptors (PPARs) (alpha, gamma, and beta/delta) are ligand-activated transcription factors of the nuclear hormone receptor superfamily,19 which regulate metabolism and energy homeostasis. Thiazolidinediones (PPAR-gamma ligands) are effective in patients with diabetes, yielding improvements in steatosis, steatohepatitis, and hepatic fibrosis.16,20 However, these agents are limited by adverse effects, including weight gain, osteopenia, and fluid retention. Elafibranor (Genfit), a liver-targeted dual PPAR-alpha/delta agonist, improved metabolic (including cardiometabolic) features of NASH in a post hoc analysis of a phase 2b trial.21 The phase 3 RESOLVE IT trial evaluating elafibranor did not identify any safety issues but was prematurely terminated due to lack of efficacy (ClinicalTrials.gov Identifier: NCT02704403). Other examples are saroglitazar (Zydus Therapeutics)22 and lanifibranor (Inventia Pharma).23
Thyroid Hormone Receptor-Beta Agonist
Thyroid hormone receptor signaling regulates organogenesis, growth and differentiation, metabolism, and energy homeostasis.24,25 Its beta subunit, expressed in
GASTROENDONEWS.COM24
the liver, regulates metabolic function, and two agonists— MGL-3196 (resmetirom; Madrigal) and VK2809 (Viking)— are being evaluated in NASH. In a phase 2, 36-week trial, MGL-3196 improved steatosis as measured by MRI proton density fat fraction (MRI-PDFF), liver enzymes, and fibrosis biomarkers.25 The greatest effect on NASH occurred in participants with 30% or more body fat. In a phase 2, 16-week trial, VK2809 improved low-density lipoprotein cholesterol levels by more than 50% compared with placebo (8.9%).26 MGL-3196 is moving forward in a phase 3 clinical trial (ClinicalTrials.gov Identifier: NCT03900429), and a phase 2b study is recruiting for VK2809 (ClinicalTrials.gov Identifier: NCT04173065).
Bile Acids and Their Pathways
Bile acids are synthesized from cholesterol in hepatocytes and regulate metabolism, inflammation, and liver fibrosis. Nuclear (farnesoid X receptor [FXR]) and transmembrane (Takeda G protein–coupled receptor clone 5) bile acid receptors have been exploited with the development of agonists of these receptors.27
In the phase 2 FLINT study of NASH patients without cirrhosis, 72 weeks of treatment with obeticholic acid (OCA; Intercept) resulted in improvement in insulin sensitivity, increased fibroblast growth factor (FGF 19) levels, and improved liver histology compared with placebo.28,29 However, OCA was associated with increased low-density and decreased high-density lipoprotein cholesterol levels, as well as severe pruritus requiring drug discontinuation in about 10% of patients.28 Atorvastatin safely helps control these adverse effects.29 These results were replicated in a phase 3 study of NASH patients with stage F2 to F3 fibrosis treated for 18 months with OCA30; 7-year follow-up results are awaited (ClinicalTrials.gov Identifier: NCT02548351). Caution is warranted in patients with cirrhosis due to reported episodes of decompensation in those with primary biliary cholangitis taking higher than recommended doses.
Tropifexor (TXR; Novartis), a non–bile acid agent with high affinity for the FXR, was evaluated in a phase 2 trial (ClinicalTrials.gov Identifier: NCT02855164).31 The interim analysis of this 48-week study showed that TXR decreased liver fat content (by MRI-PDFF) and improved aminotransferases compared with placebo.32 Considering that TXR has an adverse effect profile that is similar to that of OCA, it is suggested that it be used based on body weight (with therapeutic effect greater in patients with a lower body mass index).33 Another non–bile acid FXR agonist, cilofexor (GS-9674; Gilead) resulted in improvements in fat content and aminotransferases in a phase 2, 24-week, placebo-controlled trial in patients with NASH and stage F1 to F3 fibrosis.34 The drug did not result in changes in lipid levels or pruritus especially at a lower dose of 30 mg.
FGF-19, secreted by the intestine and a downstream target of FXR, regulates carbohydrate metabolism and bile acid synthesis, with improved insulin sensitivity.35 In a phase 2 study, the parenteral FGF-19 analog NGM282 (NGM Biopharmaceuticals) given for 12 weeks reduced
liver transaminases, with improvement in all components of NASH and fibrosis on liver histology.36 The drug was safe except for increased serum cholesterol, which was controlled with adjuvant use of rosuvastatin. The molecule has been evaluated in a phase 2b, randomized, placebo-controlled, 24-week study, but the results of this study have not been posted or published yet (ClinicalTrials.gov Identifier: NCT03912532). Many other analogs of FGF-19 and FGF-21 are being investigated as therapeutic targets for NASH (Table 1).
Antagonism of C-C Chemokine Receptor Types 2 and 5
Hepatic inflammation activates monocytes and macrophages and causes upregulation of cytokines and chemokines, which cross-talk with hepatic stellate cells to lay down collagen, resulting in liver fibrosis.37 Cenicriviroc (Allergan), an antagonist of C-C chemokine receptors 2 and 5, was evaluated in a phase 2b clinical trial in patients with NASH and stage F1 to F3 fibrosis. Relative to placebo, cenicriviroc improved liver fibrosis but not NASH at 12 weeks; no improvement was seen at 24 weeks.38 The phase 3 AURORA study recently completed in patients with NASH and stage F2 to F3 fibrosis was terminated for lack of efficacy based on planned interim analysis (ClinicalTrials. gov Identifier: NCT03028740).
Apoptosis Signal–Regulating Kinase 1 Inhibitor
Oxidative stress in hepatocytes activates the apoptosis signal–regulating kinase 1 inhibitor (ASK1), with phosphorylation of p38 mitogen-activated kinase and c-Jun N-terminal kinase, leading to regulation of apoptotic pathways, with hepatic inflammation and myofibroblast activation.39 The ASK1 antagonist selonsertib showed beneficial activity in a phase 2 study in combination with the lysyl oxidase–like 2 inhibitor simtuzumab (Gilead). 40 However, the drug showed no efficacy in a large phase 3 trial41,42 and is not recommended for further evaluation in the landscape of NASH drug development. Caspase inhibition with emricasan (Pfizer) is an attractive option, but 2 studies in F1 to F3 fibrosis and cirrhosis with portal hypertension were negative.43,44 In the first study, emricasan did not meet any of the FDA-approved primary end points of improvement in fibrosis by at least 1 stage without worsening of NASH or NASH resolution without worsening of fibrosis. In the second study, emricasan did not improve hepatic venous pressure gradient in participants with NASH cirrhosis and severe portal hypertension (gradient ≥12 mm Hg).
Acetyl Coenzyme A Carboxylase Inhibition
Lipotoxicity from accumulated fatty acids in NAFLD as a result of de novo lipogenesis and increased influx from peripheral adipose tissue contributes to NASH, with downstream effects of inflammation and fibrosis.45 Acetyl coenzyme A carboxylase (ACC), the rate-limiting step in de novo lipogenesis, with ACC1 in cytoplasm and
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 25
Table 1. Landscape for Compounds Targeted to Treat NAFLD and NASH
DrugTargetIndication Clinical trial namePrimary outcome(s) Current status
FXR agonists
Obeticholic acid (INT-747)1 FXR agonistNASH F2-F3REGENERATE NCT02548351
NASH resolution without worsening of fibrosis; fibrosis improvement without worsening of NASH (on histology)
NASH F4REVERSE NCT03439254 Fibrosis improvement without worsening of NASH (on histology)
EDP-3052 NASH F2-F3NCT04378010Change in ALT; change in liver fat content
MET642NASH and liver fat ≥10% NCT04773964Safety and pharmacokinetics
Tropifexor (TXR, LJN452) Nonsteroidal FXR agonist NASH F1-F3FLIGHT-FXR NCT02855164 Change in transaminases and fat fraction on MRI
Phase 3: Histologic improvement in fibrosis without worsening of NASH (interim analysis)
Phase 3: Active but no data available
Phase 2: Reduction in ALT and liver fat
Phase 2a: Active but no data available
Phase 2: Improved liver chemistry and fat content
Cilofexor (GS-9674)3 NASH F1-F3GS-9674 NCT02854605 Overall safetyPhase 2: Improved liver chemistry, serum bile acids, and liver fat content
PPAR agonists
Elafibranor (GFT-505)4 PPAR-alpha/ delta agonist NASH F1-F3Golden NCT01694849
Resolve-IT NCT02704403
NASH resolution without worsening of fibrosis
Phase 2b: Resolution of NASH without worsening of fibrosis
Phase 3: Terminated; did not meet predefined primary surrogate efficacy end point, no safety issues identified; no effect on resolution of NASH
PioglitazoneT2DM and NASH NCT04501406NASH improvement without worsening of fibrosis
Phase 2b: Recruiting
Saroglitazar5 NAFLD with ALT>50 IU/L EVIDENCES VI NCT03863574 ALT and liver fat contentPhase 2b: Improved ALT, liver fat, and metabolic profile
NASH with fibrosis NCT05011305NASH resolution without worsening of fibrosis
Lanifibranor6 NATIVE NCT03008070 Decrease of ≥2 points in SAF score without worsening of fibrosis
Phase 2: Recruiting
Phase 2b: ≥2 points SAF score improvement higher with 1,200 mg dose vs placebo (55% vs 33%; P=0.007)
ALT, alanine aminotransferase; DGAT2, diacylglycerol O-acyltransferase 2; FASN, fatty acid synthase; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; HVPG, hepatic vein pressure gradient; LDL, low-density lipoprotein; MPC, mitochondrial pyruvate carrier; MELD, Model for End-stage Liver Disease; MRE, magnetic resonance elastography; MRI-PDFF, MRI proton density fat fraction; MRS, magnetic resonance spectroscopy; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptors; SAF, steatosis, activity and fibrosis score; SCD1, stearoyl-CoA desaturase 1; SGLT2, sodium-glucose cotransporter-2 inhibitors; T2DM, type 2 diabetes mellitus; THR, thyroid hormone receptor.
GASTROENDONEWS.COM26
Table 1. Landscape for Compounds Targeted to Treat NAFLD and NASH
DrugTargetIndication Clinical trial namePrimary outcome(s) Current status
Fibroblast GF analogs
Efruxifermin7 Pegylated FGF21
Pegbelfermin (BMS-986036)
NASH with fibrosis NCT03976401Liver fat by MR-PDFFPhase 2a: Improvement in liver fat content (2b ongoing)
NASH F3 FALCON 1 NCT03486899 Improvement in fibrosis without worsening of NASH
NASH CCFALCON 2 NCT03486912 Improvement in NASH without worsening of fibrosis
NASHSafety and liver fat by MR-PDFF
Aldafermin8 FGF19NASH F2-F3ALPINE 2/3 NCT03912532 Improvement in fibrosis without worsening of NASH
NASH CCALPINE 4 NCT04210245 Safety and improvement in ELF score
Phase 2b: Completed but no data available
Phase 2b: Completed but no data available
Phase 2: Recruiting
Phase 2b: Safe but did not meet primary end point
Phase 2b: Active but no data available
GLP analogs
Liraglutide9 GLP-1 analogNASH F0-F4LEAN NCT01237119
Improvement in NASHPhase 2: NASH resolution on histology
Semaglutide10 NASH F2-F3NCT02970942NASH resolution without worsening of fibrosis Phase2b: Higher % of patients with NASH resolution without worsening of fibrosis NASH F2-F3ESSENCE NCT04822181 NASH resolution without worsening of fibrosis; fibrosis improvement without worsening of NASH; time to first liver-related clinical event
Phase 3: Recruiting TirzepatideDual GIP and GLP-1 agonist NASH F2-F3SYNERGY-NASH NCT04166773
NASH resolution without worsening of fibrosis Phase 3: Recruiting Efinopegdutide (MK-6024) NAFLD >10% liver fat content
IONIS DGAT2Rx11 DGAT2 inhibitor Type 2 diabetes and NAFLD
DapagliflozinSGLT2 inhibitor Type 2 diabetes and NASH
NCT04944992Safety; decrease in liver fat content by MR-PDFF
Phase 2: Active but no data available
NCT03334214Safety; liver fat content by MR-PDFF Phase 2: Safe and reduced liver fat content
DEAN NCT03723252 NASH improvement on histology Phase 3: Recruiting
ALT, alanine aminotransferase; DGAT2, diacylglycerol O-acyltransferase 2; FASN, fatty acid synthase; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; HVPG, hepatic vein pressure gradient; LDL, low-density lipoprotein; MPC, mitochondrial pyruvate carrier; MELD, Model for End-stage Liver Disease; MRE, magnetic resonance elastography; MRI-PDFF, MRI proton density fat fraction; MRS, magnetic resonance spectroscopy; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptors; SAF, steatosis, activity and fibrosis score; SCD1, stearoyl-CoA desaturase 1; SGLT2, sodium-glucose cotransporter-2 inhibitors; T2DM, type 2 diabetes mellitus; THR, thyroid hormone receptor.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 27
Table 1. Landscape for Compounds Targeted to Treat NAFLD and NASH
DrugTargetIndication Clinical trial namePrimary outcome(s) Current status
THR agonists
Resmetirom (MGL-3196) Selective THR beta agonist
NASH F1-F3 NCT0291226012
Change from baseline in fat fraction on MRI-PDFF (Histopathology with paired biopsy at 36 wksecondary end point)
NASH F2-F33-MAESTRO NCT03900429 NASH resolution on histology; all-cause mortality and liver-related events
VK2809THR beta agonist NASH F1-F3VOYAGE NCT04173065
Fatty acid synthesis inhibitors
Firsocostat (GS-0976)11 Acetyl coenzyme A carboxylase inhibition
Phase 2: Improved fat fraction and lipid profile, liver chemistry, and NASH histology
Phase 3: Recruiting
Liver fat content and LDLPhase 2: Recruiting
NASH F1-F3NCT02856555Overall safety/adverse events Phase 2: Improved liver fat content, liver chemistry, and serum markers of fibrosis
Aramchol13 SCD1 inhibitorNASH F0-F3ARREST NCT02279524
Change in liver on MRS Phase 2b: Reduced liver fat, improved histology, and liver chemistry
TVB-2640FASN inhibitor NASHFASCINATE-1 NCT03938246 Liver fat contentPhase 2a: Improvement in liver fat content
PXL065MPC inhibitor NCT04321343
Phase 2: Active not recruiting
TesamorelinGHRH analogNAFLDNCT03375788Phase 2: Recruiting
Anti-inflammatory and antioxidant agents
CenicrivirocChemokine receptor 2/5 antagonist
NASH F1-F3CENTAUR NCT0221747514
NASH F2-F3AURORA NCT03028740
Improved NASH without worsening of fibrosis; improvement in fibrosis without NASH worsening
Improvement in fibrosis without worsening of NASH; time to first liver-related event
Phase 2b: No improvement in NASH, but improved fibrosis without worsening of NASH
Phase 3: Terminated early; lack of efficacy on planned interim analysis of Part 1 data
ALT, alanine aminotransferase; DGAT2, diacylglycerol O-acyltransferase 2; FASN, fatty acid synthase; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; HVPG, hepatic vein pressure gradient; LDL, low-density lipoprotein; MPC, mitochondrial pyruvate carrier; MELD, Model for End-stage Liver Disease; MRE, magnetic resonance elastography; MRI-PDFF, MRI proton density fat fraction; MRS, magnetic resonance spectroscopy; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptors; SAF, steatosis, activity and fibrosis score; SCD1, stearoyl-CoA desaturase 1; SGLT2, sodium-glucose cotransporter-2 inhibitors; T2DM, type 2 diabetes mellitus; THR, thyroid hormone receptor.
ACC2 in mitochondria, is an attractive therapeutic target for patients with NASH. The liver-targeted inhibitor of ACC1 and ACC2, GS-0976 (firsocostat, Gilead), reduced de novo lipogenesis after 12 weeks of treatment and showed improvement in steatosis based on MRI-PDFF and markers of liver injury.46 Phase 2 studies using firsocostat in combination with the FXR agonist cilofexor (Gilead) in NASH recently completed and results are awaited (ClinicalTrials.gov Identifier: NCT03449446) (Table 2). Other drugs targeting hepatic fat accumulation
are aramchol (inhibitor of stearoyl-CoA desaturase 1-1),47 TVB-2640 (fatty acid synthase inhibitor),48 and PXL065 (mitochondrial pyruvate carrier inhibitor) (Table 1).
Glucagon-Like Peptide-1 Analogs
Glucagon-like peptide-1 (GLP-1) analogs are approved for treatment of type 2 diabetes mellitus, and have the added advantage of resulting in weight loss and reductions in cardiovascular events.49,50 In a small phase 2 study, a 1.8-mg subcutaneous injection
GASTROENDONEWS.COM28
Table 1. Landscape for Compounds Targeted to Treat NAFLD and NASH
DrugTargetIndication Clinical trial
namePrimary outcome(s) Current status
Anti-inflammatory and antioxidant agents (continued)
Selonsertib (GS-4997)15 Apoptosis signal–regulating kinase 1 inhibitor
NASH F3STELLAR 3 NCT03053050 Fibrosis improvement without worsening of NASH
NASH F4STELLAR 4 NCT03053063 Fibrosis improvement without worsening of NASH
Phase 3: Terminated early due to lack of efficacy based on results of week 48 analysis as prespecified in clinical study protocol; no benefit in fibrosis improvement (STELLAR 3); no benefit in fibrosis improvement, infection, or survival (STELLAR 4)
NASH F2-F33-MAESTRONASH resolution on histology No data available
Vitamin E and pioglitazone16-17 Antioxidant vs PPARalpha/delta agonist
Nondiabetic NASH PIVENS NCT00063622 Improvement in NASH histology Phase 3: Vitamin E better than placebo in improving steatosis and NASH but not fibrosis
Type 2 diabetes and NASH
Vitamin E tocotrienols AntioxidantNASH cirrhosis with MELD score 8-17
Antifibrotics
Belapectin18 Galectin-3 inhibitor NASH cirrhosis (HVPG >6 mm Hg)
NCT01002547Improvement in NASH without worsening of fibrosis
Combination was better in meeting primary end point
NCT02581085Change in MELD scorePhase 2: Recruiting
NCT02462967Reduction in HVPG or fibrosis Phase 2b: No effect on HVPG or fibrosis. However, reduced variceal development in subgroup without esophageal varices at baseline
ALT, alanine aminotransferase; DGAT2, diacylglycerol O-acyltransferase 2; FASN, fatty acid synthase; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; HVPG, hepatic vein pressure gradient; LDL, low-density lipoprotein; MPC, mitochondrial pyruvate carrier; MELD, Model for End-stage Liver Disease; MRE, magnetic resonance elastography; MRI-PDFF, MRI proton density fat fraction; MRS, magnetic resonance spectroscopy; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptors; SAF, steatosis, activity and fibrosis score; SCD1, stearoyl-CoA desaturase 1; SGLT2, sodium-glucose cotransporter-2 inhibitors; T2DM, type 2 diabetes mellitus; THR, thyroid hormone receptor.
of liraglutide for 48 weeks resulted in improvement in all components of NASH without worsening of fibrosis compared with placebo. 51 Semaglutide (Ozempic/Rybelsus, Novo Nordisk), another GLP-1 analog, administered as a 2.4-mg weekly subcutaneous injection for 48 weeks in NASH patients, resulted in NASH resolution without worsening of fibrosis52 A phase 2 combination study with semaglutide and firsocostat is completed and results are awaited (ClinicalTrials.gov Identifier: NCT03987074). Other GLP analogs, such as
tirzepatide and efinopegdutide, are also under investigation (Table 1).
Galectin-3 Inhibitors
High levels of galectin-3 have been found to be associated with NASH and contribute to fibrosis in mice.53 Belapectin (GR-MD-02, Galectin), an inhibitor of galectin-3, was shown to decrease fibrosis in rats and was well tolerated in phase 1 studies.54 However, in a recent phase 2b trial in patients with NASH, cirrhosis,
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 29
and portal hypertension (hepatic venous pressure gradient >6 mm Hg), 1 year of biweekly infusions of belapectin was found to be safe but was not associated with significant reduction in portal hypertension or fibrosis compared with placebo.55 A subgroup analysis of patients without esophageal varices showed that 2 mg/kg of belapectin reduced portal hypertension and development of varices.
The reality that the pathogenesis of NAFLD and NASH stems from multiple complex, simultaneously activated pathways is a major barrier to development of a single molecule that would be effective in all patients. Recognizing this, several combination regimens are being tested for the management of NASH patients (Table 2).
Monitoring Response to Treatment
Currently, there are 2 FDA-approved outcomes followed in most clinical trials: resolution of NASH without worsening of fibrosis and improvement of fibrosis by at least 1 stage without worsening of NASH. In addition,
most clinical trials still rely on paired biopsies to adjudicate these outcomes. However, recognition of NASH with fibrosis as a reversible condition after lifestyle intervention has generated a demand for noninvasive monitoring of changes in fibrosis and/or steatosis in response to pharmacologic intervention. Among the blood-based biomarkers, fibrosis-4 (FIB-4), aspartate aminotransferase-to-platelet ratio index (APRI), and NAFLD fibrosis score (NFS) are the ones more consistently identifying regression or progression of fibrosis in studies using paired biopsies (before and after intervention).
A recent study combining the experience of 2 National Institutes of Health–sponsored studies (N=292) identified an area under the curve of 0.82, 0.81, and 0.80 for APRI, FIB-4, and NFS, respectively, for progression to advanced fibrosis, but none of the tests could detect regression of fibrosis. According to this study, a change in 1 stage of fibrosis (scale, F0-F4) was associated with changes of 0.33 units in APRI, 0.26 in FIB-4, and 0.19 in NFS after a median follow-up of 2.6 years.56
In a similar study of 261 patients without cirrhosis, only
Table 2. Clinical Trials of Combination Therapies for NAFLD and NASH
DrugsTargetIndication
Saroglitazar, Vitamin E
PPAR-gam-
Clinical trial namePrimary outcome(s) Current status
NAFLDNCT04193982Change in NAFL fibrosis score Phase 3: Recruiting
PF-06865571, PF-05221304DGAT2, ACCNAFLDNCT04399538Change in liver fat content Phase 2: Recruiting
MET-409, empaglifozinFXR, SGLT-2NASHNCT04702490Safety and tolerability of MET-409 Phase 2: Active, not yet recruiting
Tropifexor, licoglifozinFXR, SGLT-2NASH F2-F3ELIVATE NCT04065841 Improvement in fibrosis without worsening of NASH; NASH resolution without worsening of fibrosis
Phase 2: Recruiting Semaglutide, cilofexor + firscostat GLP-1, FXR + ACC NASH cirrhosis NCT04971785
Tropifexor, cenicrivirocFXR, chemokine receptor 2/5
NASH F2-3TANDEM NCT03517540 Safety of combination Phase 2: Completed and results awaited
Cilofexor, firscostat + selonsertib FXR, ACC + ASK1 NASH F3-4ATLAS NCT03449446
Adverse effects and safety of combination
ACC, acetyl coenzyme A carboxylase; ASK1, apoptosis signal–regulating kinase 1 inhibitor; DGAT2, diacylglycerol O-acyltransferase 2; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PPAR, peroxisome proliferator–activated receptor; SGLT-2, sodium-glucose cotransporter-2.
ma antioxidant
GASTROENDONEWS.COM30
NFS predicted progression or regression of fibrosis after 1 year. However, a novel index, the Fibrosis Improvement after Lifestyle Interventions (FILI) score—composed of change in hemoglobin A1c, platelet count, and alanine aminotransferase (ALT) normalization—better predicted regressed fibrosis, with a positive predictive value of 94% and negative predictive value of 91%.57
Imaging-based methods also are promising for detecting changes in fibrosis in patients with NAFLD. In patients treated with the apoptosis signaling inhibitor selonsertib for 24 weeks, magnetic resonance elastography (MRE) could not detect changes in fibrosis (n=54), but MRI-PDFF was more sensitive to improvements in steatosis (n=65) compared with those observed in histologic responders.58 Although not validated against paired liver biopsies, MRE/MRI-PDFF or liver stiffness measurement/controlled attenuation parameter (LSM/CAP) improved in response to NASH treatment. In a randomized trial, investigators found a reduction in MRI-PDFF of 30% or greater and a trend toward MRE reduction but no significant changes in LSM/CAP in patients receiving an allosteric inhibitor of acetyl coenzyme A for 12 weeks (n=126).48 A clinical trial evaluating the effectiveness of 24 weeks of dapagliflozin (Farxiga, AstraZeneca) in 57 NAFLD patients with diabetes showed a trend toward improvement in LSM (9.49 to 8.01 kPa; P=NS). However, among 14 patients with baseline LSM of at least 8.0 kPa, there was improvement in LSM, from 14.7 to 11.0 kPa (P=0.0158), and in CAP, from 314 to 290 dB/m.59 Although it is possible to identify regression or progression in staging or grading of NAFLD using noninvasive assessment of liver disease, further validation is needed, including head-to-head comparisons between imagingand blood-based methods. It is possible that the inferior performance of imaging-based methods is due to the short follow-up and absence of an effective and durable intervention in reported studies.
Challenges and Obstacles For Future Therapies
Lack of accurate noninvasive assessment for NASH, short-term clinical trials of 12 to 48 weeks, and
References
1. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
2. Mummadi RR, Kasturi KS, Chennareddygari S, et al. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2008;6(12):1396-1402.
3. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121-129.
4. Dudekula A, Rachakonda V, Shaik B, et al. Weight loss in nonalcoholic fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS One. 2014;9(11):e111808.
spontaneous regression with placebo have complicated clinical trial design, especially with NASH resolution or improvement as an end point. The effectiveness of NASH therapies will be measured by the effects on liver and all-cause mortality, which are driven primarily by the presence of advanced fibrosis and cirrhosis. Given that spontaneous change of 1 stage in fibrosis may take up to 7 years, patient-reported outcomes will be important in the evolving landscape of drug evaluation.60,61
A major obstacle in assessing the effectiveness of NASH therapies has been the high placebo response in clinical trials and unexpected negative results from potential therapies that showed promise in early stages of drug development.62 The placebo response varies depending on the surrogate end point being evaluated, but it likely reflects, in part, the influence of inadvertent lifestyle changes among placebo recipients. In a recent meta-analysis of 39 clinical trials, placebo was associated with improvement of 2 points or more in NAFLD activity score (NAS) in 25% of patients (95% CI, 21%-29%), with similar results in an analysis restricted to patients with no worsening of fibrosis. 63 Similarly, 30% of placebo-treated patients had at least a 1-point improvement in individual components of NAS, and fibrosis stage improved in 21% of placebo-treated patients. It is important to clarify the basis for the high placebo response when designing clinical trials by enriching for patients with more advanced disease using evolving noninvasive surrogates.
In summary, the current climate for drug development for NASH is vibrant and promising. The sustained progress in elucidating the pathogenesis of this disease continues to promote a highly interactive dynamic between basic, translational, and clinical investigators, who are partnering with companies committed to developing new approaches to managing this illness. Hopefully, affected patients and their caregivers soon will be the beneficiaries of this ongoing partnership through improved clinical outcomes, quality of life, and life span.
5. Axley P, Kodali S, Kuo Y-F, et al. Text messaging approach improves weight loss in patients with nonalcoholic fatty liver disease: a randomized study. Liver Int. 2018;38(5):924-931.
6. St. George A, Bauman A, Johnston A, et al. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol. 2009;24(3):399-407.
7. Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67(4):829-846.
8. Keating SE, Adams LA. Exercise in NAFLD: just do it. J Hepatol 2016;65(4):671-673.
9. Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66(1):142-152.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 31
10. Corey KE, Vuppalanchi R. Assessment and management of comorbidities (including cardiovascular disease) in patients with nonalcoholic fatty liver disease. Clin Liver Dis. 2012;1(4):114-116.
11. Pastori D, Polimeni L, Baratta F, et al. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2015;47(1):4-11.
12. Dyson J, Jaques B, Chattopadyhay D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60(1):110-117.
13. Ekstedt M, Franzén LE, Holmqvist M, et al. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44(3):366-374.
14. Weng G, Dunn W. Effect of alcohol consumption on nonalcoholic fatty liver disease. Transl Gastroenterol Hepatol. 2019;4:70.
15. Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643-654.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362(18):1675-1685.
17. Watt KD, Pedersen RA, Kremers WK, et al. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137(6):2010-2017.
18. Singal AK, Guturu P, Hmoud B, et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95(5):755-760.
19. Pu S, Wu X, Yang X, et al. The therapeutic role of xenobiotic nuclear receptors against metabolic syndrome. Curr Drug Metab 2019;20(1):15-22.
20. Mahady SE, Webster AC, Walker S, et al. The role of thiazolidinediones in non-alcoholic steatohepatitis—a systematic review and meta analysis. J Hepatol. 2011;55(6):1383-1390.
21. Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150(5):1147-1159.e5.
22. Gawrieh S, Noureddin M, Loo N, et al. Saroglitazar, a PPAR-alpha/gamma agonist, for treatment of NAFLD: A randomized controlled double-blind phase 2 trial. Hepatology. 2021;74(4):1809-1824.
23. Francque SM, Bedossa P, Ratziu V, et al. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385:1547-1558.
24. Sanyal AJ, Friedman SL, McCullough AJ, et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases–U.S. Food and Drug Administration Joint Workshop. Hepatology 2015;61(4):1392-1405.
25. Harrison SA, Guy CD, Bashir MR, et al. In a placebo controlled 36-week phase 2 trial, treatment with MGL-3196 compared to placebo results in significant reductions of hepatic fat (MR-PDFF), liver enzymes, fibrosis biomarkers, atherogenic lipids and improvement in NASH on serial biopsy. Hepatology 2018;68(suppl 1):abstract 14.
26. Loomba R, Neutel J, Mohseni R, et al. LBP-20-VK2809, a novel liver-directed thyroid receptor beta agonist, significantly reduces liver fat with both low and high doses in patients with non-alcoholic fatty liver disease: a phase 2 randomized placebocontrolled trial. Hepatology. 2018;68:1447A.
27. Keitel V, Kubitz R, Haussinger D. Endocrine and paracrine role of bile acids. World J Gastroenterol. 2008;14(37):5620-5629.
28. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, nonalcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956-965.
29. Pockros PJ, Fuchs M, Freilich B, et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int 2019;39(11):2082-2093.
30. Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184-2196.
31. Tully DC, Rucker PV, Chianelli D, et al. Discovery of tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J Med Chem. 2017;60(24):9960-9973.
32. Sanyal AJ, Lopez P, Lawitz E, et al. Tropifexor (TXR), an FXR agonist for the treatment of NASH—interim results from first two parts of phase 2b study flight-FXR. Hepatology 2018;68(1):1461A-1462A.
33. Sanyal A, Lopez P, Lawitz E, et al. SAT-357-Tropifexor, a farnesoid X receptor agonist for the treatment of non-alcoholic steatohepatitis: interim results based on baseline body mass index from first two parts of phase 2b study FLIGHT-FXR J Hepatol. 2019;70(1):e796-e797.
34. Patel K, Harrison SA, Trotter JF, et al. The non-steroidal FXR agonist GS-9674 leads to significant reductions in hepatic steatosis, serum bile acids, and liver biochemistry in a phase 2, randomized, placebo-controlled trial of patients with NASH. Hepatology. 2018;68(suppl 1):abstract 736.
35. Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol 2014;11(1):55-67.
36. Harrison SA, Rossi SJ, Paredes AH, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71(4):1198-1212.
37. Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64(5):830-841.
38. Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67(5):1754-1767.
39. Brenner C, Galluzzi L, Kepp O, et al. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59(3):583-594.
40. Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2018;67(2):549-559.
41. Gilead announces topline data from phase 3 STELLAR-4 study of selonsertib in compensated cirrhosis (F4) due to nonalcoholic steatohepatitis (NASH). February 11, 2019. Accessed August 16, 2022. www.gilead.com/news-and-press/press-room/ press-releases/2019/2/gilead-announces-topline-data-fromphase-3-stellar4-study-of-selonsertib-in-compensated-cirrhosisf4-due-to-nonalcoholic-steatohepatitis-nash
42. Gilead announces topline data from phase 3 STELLAR-4 study of selonsertib in bridging fibrosis (F3) due to nonalcoholic steatohepatitis (NASH). 2019. April 25, 2019. Accessed August 16, 2022. www.gilead.com/news-and-press/press-room/ press-releases/2019/4/gilead-announces-topline-data-fromphase-3-stellar3-study-of-selonsertib-in-bridging-fibrosis-f3-dueto-nonalcoholic-steatohepatitis-nash
43. Harrison SA, Goodman Z, Jabbar A, et al. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol. 2020;72[5]:816-827.
44. Garcia-Tsao G, Bosch J, Kayali Z, et al. Randomized placebocontrolled trial of emricasan in non-alcoholic steatohepatitis (NASH) cirrhosis with severe portal hypertension. J Hepatol 2020;72(5):885-895.
45. Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115(5):1343-1351.
GASTROENDONEWS.COM32
46. Lawitz EJ, Coste A, Poordad F, et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2018;16(12):1983-1991.e3.
47. Ratziu V, de Guevara L, Safadi R, et al. Aramchol in patients with nonalcholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2b trial. Nat Med. 2021;27(10):1825-1835.
48. Loomba R, Kayali Z, Noureddin M, et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(5):1463-1473.e6.
49. American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(suppl 1):S73-S85.
50. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and metaanalysis. Lancet. 2014;384(9961):2228-2234.
51. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690.
52. Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113-1124.
53. Iacobini C, Menini S, Ricci C, et al. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 2011;54(4):975-983.
54. Jeftic I, Jovicic N, Pantic J, et al. Galectin-2 ablation enhances liver steatosis, but attenuates inflammation and IL-33-dependent fibrosis in obesogenic mouse model of nonalcoholic steatohepatitis. Mol Med. 2015;21(1):453-465.
55. Chalasani N, Abdelmalek MF, Garcia-Tsao G, et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158(5):1334-1345.e5.
56. Siddiqui MS, Yamada G, Vuppalanchi R, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17(9):1877-1885.e5.
57. Vilar-Gomez E, Calzadilla-Bertot L, Friedman SL, et al. Serum biomarkers can predict a change in liver fibrosis 1 year after lifestyle intervention for biopsy-proven NASH. Liver Int 2017;37(12):1887-1896.
58. Jayakumar S, Middleton MS, Lawitz EJ, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70(1):133-141.
59. Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21(2):285-292.
60. FDA. Guidance document. Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment; guidance for instruction. FDA-2018-D-3632. December 2018. Accessed August 16, 2022. www.fda.gov/regulatory-information/ search-fda-guidance-documents/noncirrhotic-nonalcoholic-steatohepatitis-liver-fibrosis-developing-drugs-treatment
61. FDA. Nonalcoholic steatohepatitis: developing drugs for treatment; guidance for industry. Draft guidance. June 2019. Accessed August 16, 2022. www.fda.gov/media/127738/ download
62. Harrison SA, Abdelmalek MF, Caldwell S, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155(4):1140-1153.
63. Han MAT, Altayar O, Hamdeh S, et al. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(4):616-629.e26.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 33











PANEL
GERD Roundtable: Applying Guidelines To Clinical Practice








Part 1, Wading Through the Fog To Optimize Use of PPIs








The American College of Gastroenterology recently released new guidelines on the management of gastroesophageal reflux disease. For our GERD Roundtable series, Gastroenterology & Endoscopy News spoke with a panel of GERD specialists to explore some aspects of the guidelines and discuss how they will be implemented in practice. In Part 1, we discuss optimizing the use of PPIs, and in Part 2, we discuss the management of refractory GERD, patient monitoring and patients who are misdiagnosed.
Gary W. Falk, MD, MS

Division of Gastroenterology University of Pennsylvania Perelman School of Medicine Philadelphia

Ronnie Fass, MD, MACG
Esophageal and Swallowing Center Division of Gastroenterology and Hepatology MetroHealth Medical System Case Western Reserve University Cleveland
Amit Patel, MD, FACG Division of Gastroenterology Duke University School of Medicine Durham VA Medical Center Durham, N.C.

GEN: There have been numerous reports of adverse events associated with proton pump inhibitors. How are these reports influencing provider and patient views on PPIs? How do the new guidelines on GERD address these reports?
Dr. Falk: There is so much that has been written about this topic. There are countless adverse events that have been attributed to PPIs. I think the current guideline synthesizes the information well, emphasizing causation versus association, which most of those studies are. The guidelines committee makes the key point that the putative risks associated with PPIs should not be considered definitive. They also provide some very practical points about the use of PPIs—when they should be used, when they shouldn’t be used, when to try to stop PPI therapy and how to reassure patients.
Michael Vaezi, PhD, MD, MS Center for Swallowing and Esophageal Disorders Division of Gastroenterology Vanderbilt University Nashville, Tenn.


EXPERT ROUNDTABLE GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 35
Dr. Fass: What I’ve seen in my practice is growing concerns among patients and providers alike about the long-term use of PPIs because of the growing number of reports of adverse events. They are not convinced that PPIs are safe in the background of this large number of publications.
It would not be unusual for me to get a referral
of a patient whose primary care provider will ask me to decide about patient’s long-term use of PPIs. They don’t want to be the ones who are leaving the patient long-term on a PPI because of the concerns about adverse side effects. And patients are saying, “Is there something else that you can offer me instead of PPIs?”
Dr. Patel: Studies in the literature have identified several associations that are potentially linked to PPIs— fractures, chronic kidney disease, dementia, even COVID-19. But the problem is that these associations have been primarily observational in nature, so they can’t establish causality between PPIs and these processes. I think the best evidence we have to date is Moayyedi’s large randomized trial ( Gastroenterology 2019;157[3]:682-691.e2).
In this study, older patients who had stable cardiovascular disease or peripheral vascular disease were randomly assigned to pantoprazole 40 mg daily or placebo. They were followed for three years to identify potential PPI side effects, such as pneumonia, enteric infections, fractures, chronic kidney disease, dementia, diabetes, cancer and even all-cause mortality. They found that the only one of these outcomes with event rates that were significantly increased in the pantoprazole groups was enteric infections, and we’re talking about 1.4% versus 1%. None of the other purported side effects were significantly different between the PPI and placebo groups.
I think what this shows us is that we should be up front with patients when we discuss the use of PPIs and their potential adverse effects. We should discuss that the high-quality data like Moayyedi’s show that PPIs don’t really appear to increase the risk significantly for any of these outcomes, except intestinal infections. With that being said, we can’t be 100%, absolutely sure there is no possibility that PPIs might confer a small increase in risk for any of these. However, I think we agree as a GI community that the proven benefits of PPIs outweigh the theoretical risks noted in observational data, for the appropriate indications or clinical settings.
In our current times, another concern that people can have relates to COVID and PPIs. The COVID pandemic started after Moayyedi’s study, but there’s a nice retrospective analysis in press, of a large cohort of U.S. veterans who tested positive for COVID (Gut 2021 Oct 18. doi:10.1136/gutjnl-2021-325701). The authors looked at PPI use and whether it affected COVID-related outcomes, with propensity-score weighting for factors
like age, sex, race, smoking, comorbidities. They found that veterans who used PPIs as outpatients had rates of severe COVID composite outcomes that were similar to the rates in those who had not had any PPI prescriptions in the prior year. I think this data provides further reassurance on PPI safety, particularly in our COVID era.
Dr. Falk: Although PPIs have been associated with many of the adverse events we’ve mentioned, the magnitude of the purported adverse events is very low for all of these associations, and they all fall within a statistical zone of uncertainty. These findings are drawn from observational studies that may be flawed due to to residual confounding and other methodologic problems. The best level 1 evidence from the randomized trial cited previously shows that these adverse events weren’t seen over a period of three to five years. The only thing seen in that study was a small increased risk for increased enteric infection (not Clostridioides difficile). As such, one has to take it all with a grain of salt. I think these adverse events have been overplayed and not placed into appropriate context.
Dr. Vaezi: I would reemphasize that these are all observational studies that only show association, and the causal link is difficult to establish. There are so many confounding elements that potentially could yield the same result. Having said all that, we should do what we were doing before these associations came out. In other words, independent of those, what we need to do is prescribe when indicated and deprescribe when not indicated.
Dr. Falk: What is clearly seen, and people forget about this, is that unrelated to these long-term concerns, patients can have real adverse events that occur with short-term acute use. These include diarrhea, headache and, in a subset of individuals, bloating.
Dr. Fass: There are some unique side effects that have been attributed to specific PPIs, such as the diarrhea mentioned by Gary. But, overall, when we look at this class of drugs, they’re relatively safe. With respect to
GEN: Can you discuss these adverse events and the data behind them more specifically?
EXPERT ROUNDTABLE GASTROENDONEWS.COM36
bone loss, the AGA guidelines specifically mentioned that they don’t feel there should be a recommendation for DEXA scan on an annual basis in patients taking chronic PPI treatment. In addition, we don’t recommend that patients take a vitamin D supplement if they are on chronic PPI treatment.
If patients need PPIs long term, they should take them. The rule is, they should use the lowest dose of PPI that controls their symptoms. I’ve been taking care of many GERD patients over the years, and I haven’t seen many of these side effects during that period of time.
GEN: Given the provider and patient perceptions related to potential adverse events, can you delineate some of the indications for which PPIs are clearly warranted and others that have less evidence for their current use?


Dr. Falk: Amid the fog, there are some areas that are pretty clear from my perspective. For complicated reflux disease, high-grade esophagitis, Barrett’s esophagus, strictures and patients with potential risks for GI bleeding, the role of PPIs is pretty clear cut.
But there are areas where we can do better. For some reason, there’s a common misconception that PPIs should be administered right off the top as twicedaily dosing. That’s clearly not indicated or necessary in most patients. Another area is patients reporting extraesophageal symptoms, where PPIs often are given twice a day for extended periods of time, without benefits. That’s low-hanging fruit, in terms of where PPI dosages can be either stopped or altered.





Dr. Fass: PPIs truly revolutionized the landscape of acid peptic disorders—specifically, in relation to complications of GERD, which many of them we don’t see like we used to see before we started to use PPIs. Some younger gastroenterologists don’t even know what peptic stricture looks like because we don’t see many of them anymore. PPIs have provided very important contributions to the care of patients with GERD.
We have to remember that we have to use them whenever patients need them. That was also the emphasis of the ACG guidelines. Of course, it’s very important to use them in the right patients. I agree with Gary that there
is an overuse of PPIs in situations where it’s not clear whether patients have GERD as the underlying cause of their symptoms. The good example is atypical or extraesophageal manifestations of GERD but also disorders that are unrelated to acid peptic diseases.
Dr. Falk: For the purported extraesophageal manifestations of reflux, the evidence base and support for PPIs is pretty slim, so people with cough, sore throat, globus, etc., who were placed on PPI therapy, if it has not helped—which is often the case—you will want to get those patients off PPIs. For patients with extraesophageal manifestations of reflux without classic symptoms, you really want to figure out a way to get them off PPIs as quickly as possible.


There is a whole gamut of heartburn and acid regurgitation that that goes from complicated reflux disease to functional heartburn. Reflux is not just one disease— there are many phenotypes within the umbrella of GERD. It’s probably not an ideal thing for the primary care doctor to dissect that. That comes more into our area of expertise.
Dr. Fass: I would just add to that about one third of the patients who present with heartburn have some type of functional disorder. In many of them, the PPIs won’t work, and they probably shouldn’t be on PPIs.
EXPERT ROUNDTABLE GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 37
It’s going to be very difficult for the primary care provider to identify them up front, and they will need the help of a specialist to diagnose them. If heartburn patients don’t respond to a PPI twice a day, that may be a clue for a functional or other esophageal disorder.
In addition, patients may take PPIs for dyspepsia-like symptoms. In many cases, when you ask these patients, they say PPI therapy is not working anyway. So, if a patient walks into my office, and they’re on chronic PPI therapy, and they say that it doesn’t work for them, then obviously that patient should not be on a PPI. Some people are taking PPIs long term, not necessarily because it works for them. I’ve also found patients who are unable to come off PPIs, even though they aren’t helping them. Our job is to identify the patients who really need PPIs and for whom they work. For patients who don’t need them or in whom they don’t work, we need to find a different treatment.
Dr. Falk: Another category of patients who are placed on PPIs and then stay on them although they may not need them are people started on PPIs in the hospital for a variety of often unnecessary reasons. This issue recently was addressed by work out of Columbia University (Dig Dis Sci 2022;67[3]:817-825). One of the jobs of the gastroenterologist is to sort out why the patient is on a PPI and if the dose is appropriate or not. Because primary care doctors have such a limited time with patients, we could help both the primary care doctor and the patient by helping to gauge the need for PPIs and the correct dosing to use.
Dr. Patel: Clinical providers—especially GI providers but also primary care providers—should regularly review the indications for PPI use in our patients who take them chronically, and taper and/or de-prescribe aggressively when appropriate. We should absolutely be considering that for patients who don’t have a clear indication for chronic PPI use. There’s an excellent AGA clinical practice update in Gastroenterology from earlier this year that offers practical and valuable guidance for clinical providers on de-prescribing PPIs (2022;162[4]:1334-1342).
Dr. Vaezi: I think it’s not really difficult. It just requires continued education on the side of the experts to our gastroenterology and primary care colleagues to remind them that our criteria have not changed. What happens oftentimes is that we start with an empiric trial, and then we forget to de-escalate. But the recommendations have always been to use empiric therapy as a way of gauging and/or diagnosing the disease, and then if you see that it is, taper, and if you see that it isn’t, taper. These associations with PPIs and other things, faulty at best, have brought this to the forefront of what we should continue to do. That is to educate our providers about what they should do with respect to PPIs.
Polypharmacy is an issue, whether it be with PPIs, or antihypertensives, or you name the medicine. So, I think it’s good for everyone to look at the list, intermittently and deprescribed when possible.
Empiric treatment is recommended for a lot of things. For example, if a patient presents with certain symptoms, say heartburn, regurgitation, and you suspect it’s reflux related, the guidelines say to start with empiric therapy, four to eight weeks and then reassess. We also use it for dyspeptic symptoms. Our primary care colleagues, perhaps inappropriately at times, use it for bloating and burping, which they consider to be more dyspeptic symptoms, and it’s OK to try these medications for a short time. Knowing that burping and abdominal pain are not necessarily symptoms of GERD, the guidelines recommend to try empiric therapy.
But the problem is not the trial as much as it is the de-escalation part. That’s the difficulty. For example, for patients with eosinophilic esophagitis, the recommendation is to try a PPI because we know that up to 50% of patients might respond to it. But, then do you leave them on twice-daily dosing or do you taper it? The answer should be you start tapering at some point. Starting is easy—it’s the tapering part that we have to educate about.
Dr. Patel: We all probably agree that for typical reflux symptoms— heartburn, regurgitation—without any alarm symptoms or red flags, that an empiric PPI trial is a practical or pragmatic approach to these symptoms in clinical practice. It’s efficient, not invasive, generally pretty costeffective for a short-term period, and patients’ symptomatic responses to this “PPI test” can help corroborate our clinical suspicion for GERD in these settings.
In terms of once or twice daily—depending on the indication PPI use can be twice daily—but the ACG GERD guideline recommends once daily before a meal for an eight-week period. Older meta-analytic data suggest that empiric PPI trials in this setting (although these included study trials were one to four weeks in duration, not eight weeks) have reasonable sensitivity for a GERD diagnosis, almost 80%, but limited specificity, 54%, against ambulatory reflux monitoring as the gold standard (Ann Intern Med 2004;140:518-527).
Starting PPIs is easy, and generally indicated in this clinical setting. But it’s incredibly important that providers reassess patients at the end of that empiric trial at eight weeks, evaluate their symptom response with the trial, and then try to get them off the PPI, even if they had a nice response. In that setting, if patients don’t respond or suffer bothersome and/or repeated recurrences of their symptoms when PPIs are stopped, that’s when we start going down our diagnostic pathways laid out in our ACG Esophageal Physiologic Testing and GERD Guidelines, with interventions like esophagogastroduodenoscopy and potentially esophageal physiologic testing.
EXPERT ROUNDTABLE GASTROENDONEWS.COM38
Part 2, Refractory GERD: Diagnosis and Misdiagnosis

GEN: How commonly do you see patients who are refractory to GERD therapy?
Dr. Vaezi: I don’t like the term “refractory reflux.” When I give lectures, I always say, “There’s no such thing as refractory GERD.” What you have are refractory symptoms that you thought were due to GERD. Now we know it isn’t GERD because we treated it, and it didn’t get better. The exception is regurgitation.
The question that we should be asking is what symptom is refractory. For patients with reflux with objective and/or subjective heartburn/regurgitation, surgery and endoscopic therapies, PPIs and H2 receptor antagonists are indicated. Over the decades, we have seen that patients with reflux do well on therapy and generally are not refractory.
However, if a patient comes to me with burping, and I treat them with once a day, then twice a day, then 10 times a day drug therapy, they will still be burping because that’s not GERD. Similarly, if we do fundoplication in this setting, the patient won’t get better.
In contrast, it’s not unusual for a patient with a hiatal hernia who has true GERD (heartburn/regurgitation) to see improvements in their heartburn and regurgitation on a PPI or an H2 blocker that are not sufficient. They continue to have regurgitation because of a
mechanical barrier, the hiatal hernia. That is refractory GERD, because it is truly GERD mechanistically. If it’s anything else, you have to ask: what is GERD? We are in the era of Dr. Google. Patients come to us with the diagnosis at hand. They say, “I have GERD.” They don’t tell us, “I’m burping.” They don’t tell us, “I have pain and dyspepsia.” They say, “I have GERD.” If you take that and run with it, you just ran with the wrong diagnosis.
Dr. Patel: I agree, it’s challenging to study the entity “refractory GERD,” as it is misused by providers and patients alike. If we are talking about patients with symptoms that may stem from GERD, up to half of patients have persistence of symptoms that may or may not be from GERD, despite taking a PPI appropriately.
For patients with proven GERD, supported by objective evidence on endoscopy and/or reflux monitoring, who have symptoms that are refractory to appropriate PPI therapy as we discussed earlier, that we can call refractory GERD symptoms, with unclear associations with ongoing reflux.
So, for this group of patients with refractory GERD

EXPERT ROUNDTABLE GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 39
symptoms, the question becomes this: Are their symptoms associated with ongoing reflux or not? That’s the group of patients we’re interested in assessing further. These are the kinds of patients who are probably best tested on PPI therapy with combined pH impedance monitoring to help us see if we are dealing with ongoing reflux in these settings, to guide appropriate
therapy. And as Mike pointed out, because PPI therapy only reduces the acidity of reflux episodes without stopping the episodes themselves, antireflux interventions may be appropriate for patients with prominent regurgitation symptoms, particularly in the setting of hiatal hernias.
GEN: How do you approach reflux monitoring for patients?
Dr. Patel: To help guide clinical providers in terms of reflux monitoring modalities and whether to do monitoring on or off PPI, the ACG Esophageal Physiologic Testing guideline and the Lyon consensus recommend that when evaluating patients with typical symptoms but unproven GERD—meaning no advanced erosive esophagitis, confirmed Barrett’s esophagus, reflux-mediated stricture or prior positive reflux monitoring—they should be tested off PPI (Am J Gastroenterol 2020;115:1412-1428; Gut 2018;67[7]:1351-1362). In this setting, catheter-based monitoring and wireless pH monitoring are options. The latter has some advantages, including its longer recording time for patients with infrequent symptoms or day-to-day variation to increase diagnostic yield, as well as less discomfort for patients. In contrast, if we’re evaluating patients with refractory GERD symptoms in the setting of proven GERD, then combined pH impedance testing performed on PPI is most appropriate.
Dr. Vaezi: Early on, impedance pH testing was considered “the best thing since sliced bread” because it could measure acid, nonacid and everything. Then, over time, the discussion became “Well, maybe we don’t need the nonacid part. Should we just perform the acid part? Should we do it on PPIs? Should we do it off?” I started doing a lot of impedance pH testing on-therapy, and I found that the majority of tests were normal. The reason they were normal was because most of the people who have refractory reflux symptoms don’t have reflux as the cause.
The exception is patients who have regurgitation. In fact, the surrogate marker for abnormal impedance is regurgitation as a symptom. So, if a patient already is telling me they regurgitate, I don’t know if I need to do impedance pH testing.
That gets to the question: Am I trying to prove there is reflux or am I trying to prove there isn’t? Often, we do the test to prove there is not reflux. A patient with laryngopharyngeal reflux, a patient with asthma, a patient with heartburn that does not improve with therapy—is it functional? Is it something else? Those I do it off-therapy. I would say 97% of the testing I do is off-therapy.
In the patient Amit was talking about, if you already have heartburn, your heartburn’s better, but regurgitation is not better and you want to test to see what is going on? That’s a good surrogate marker to tell your surgeon, “The patient has reflux, a 4- or 5-cm hernia, and impedance is abnormal, with regurgitation as the symptom.” That might help guide treatment. But overall, I’m starting to do less and less impedance pH testing.
Dr. Patel: The primary metric on reflux monitoring that we are most interested in is acid exposure time— what percentage of time is the pH below 4 at the lower esophageal sensor during the study or recording period. But there’s additional, adjunctive information that we can get from reflux monitoring, such as reflux–symptom association, where we assess for a significant association between the symptoms that patients log during the reflux monitoring period and reflux episodes. There are different mathematical ways to quantitate that relationship. Our data demonstrated that this reflux–symptom association, in addition to the acid exposure time, predicted symptomatic outcomes after antireflux therapy and could be used to help phenotype GERD patients (Clin Gastroenterol Hepatol 2015;13[5]:884-891). Further, we showed that the mean nocturnal baseline impedance from the lower esophagus on pH impedance tracings could also predict reflux burden and symptomatic outcomes (Aliment Pharmacol Ther 2016;44[8]:890-898). With that said, these parameters are more secondary metrics. I agree that acid exposure time is the most important factor. But these adjunctive metrics can be quite helpful in some patients, such as those with borderline acid exposure times, or to increase our confidence in pursuing certain interventions.
Dr. Vaezi: I think the problem with any of the association studies, whether it be with impedance pH or regular pH, is that our patients don’t push the button when they actually have the symptom. We did studies where we listened to patients’ coughing episodes, time-syncing it with when they pushed the button, and we found that 80% to 90% of people did not push the button when they actually coughed. So, using symptom correlation to suggest that this is from reflux is problematic.
EXPERT ROUNDTABLE GASTROENDONEWS.COM40
These tests will make me feel better because I can actually tell the patient, “Oh, well, it’s a symptom association, so you have reflux. Now I’m going to send you to have surgery because your PPI didn’t help you,” or the harder approach, which is, “You don’t have reflux. I don’t know what’s causing your symptoms, but I know it’s not reflux.”
Dr. Patel: I think it is important to remember that we want to treat our patient, not a number. And I think that even with all of our impressive and evolving esophageal diagnostics, there’s a limit to how much granularity is useful clinically. But we have good evidence that some of these other metrics, such as the mean
nocturnal baseline impedance and reflux–symptom association, have potential predictive power and can be helpful when we’re making these important decisions about whether patients are good candidates for reflux interventions. But, most importantly, we have to take the patient, their values and the clinical scenario into account. I do agree with Mike, that if someone has a normal acid exposure time off-PPI, we should not use a positive reflux–symptom association in isolation to refer them for an invasive surgery. Instead, we should look at the whole picture, and these kinds of elements or data can be helpful when we’re engaging in shared decision making with patients about management approaches and options.
GEN: What is leading to poor diagnoses?
Dr. Falk: A common problem that we see in GI and esophageal subspecialty practices is people who have “GERD” despite therapy, or refractory GERD. That is where diagnostic testing comes in as discussed by Drs. Patel and Vaezi. Clinicians need to make sure patients do not get seduced by invasive therapies that may or may not help them. Overall, I think the new ACG guideline ( Am J Gastroenterol 2022;117[1]:27-56) walks through the various tests we have, as well as how best to deploy these tests, and how we should be thinking about invasive options to treat this. Most people with GERD, despite therapy, don’t have reflux as defined by elevated acid exposure time or by mucosal disease.
Dr. Fass: That’s correct. That’s part of the definition that has been changed because there is a difference between refractory GERD and refractory heartburn. In refractory GERD, patients have to have a history of GERD. If that includes documentation of erosive esophagitis in the past, or normal endoscopy patients demonstrate abnormal esophageal acid exposure, they truly have GERD that does not respond to therapy.
When you look at what drives patients’ continuous symptoms, it’s not because their GERD is not under control but something else that affects these patients, such as functional esophageal disorders or other esophageal or gastric disorders.
Then we have functional heartburn and reflux hypersensitivity patients who present with heartburn as the predominant symptom. Heartburn is not specific to reflux, so for many patients with heartburn who don’t respond to PPI therapy, it is likely a non-GERD esophageal disorder.
Dr. Falk: People are still being classified the wrong way, for some reason. This is happening in the primary care and surgical communities. For example, if a barium x-ray describes reflux, then the clinician and the patient think they have reflux despite the problematic performance characteristics of barium radiography as a diagnostic test for GERD. This leads to a chasing-the-tail phenomenon and subsequent problems. I think the GI community knows not to rely on barium studies, but the non-GI community continues to chase abnormal upper GI x-rays.
I think the current guideline also makes reasonable points about medications that don’t work that are still used. When you see patients on multimodality therapy (PPIs, sucralfate, chronic use of H2 blockers at night, prokinetic agents), you usually know that the diagnosis can be in question.
The guidelines include simple, practical points that gastroenterologists can use to simplify the therapeutic and diagnostic approach to these patients.
Dr. Falk reported a financial relationship with Phathom.
Dr. Fass reported financial relationships with AstraZeneca, Celexio, GI Supply, Salix and Takeda.
Dr. Patel reported no relevant disclosures. Dr. Vaezi reported financial relationships with Bayer, Diversatek, Ironwood, ISOThrive, Medtronic, Phathom and Sanofi, as well as consulting in litigations relating to acid-suppressive agent.
Interviews were edited for clarity and brevity.
—Compiled by Sarah Tilyou and Meaghan Lee Callaghan
EXPERT ROUNDTABLE GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 41


































































































Solar™ Go Air Portable Conventional Anorectal Manometry Now Available Solar™ GI High-Resolution Manometry Now Supports Chicago 4.0 Ohmega™ Ambulatory Impedance-pH Recorder Spot® Ex Endoscopic Tattoo EverLift Submucosal Lifting Agent GI Supply is Now Laborie! Scan to Learn More Solar and Ohmega are trademarks of Laborie Medical Techologies Corp. Spot and EverLift are registered trademarks of GI Supply, Inc. © 2022 Laborie Medical Technologies Corp. | All Rights Reserved. laborie.com
Treatment of Nonachalasic Esophageal Motor Disorders


OFER Z. FASS, MD Division of Gastroenterology and Hepatology
Stanford University School of Medicine Redwood City, California
RONNIE FASS, MD, MACG
Esophageal and Swallowing Center Division of Gastroenterology and Hepatology MetroHealth Medical System Case Western Reserve University Cleveland, Ohio
Esophageal
motor disorders encompass a diverse group of diagnoses defined by abnormal peristaltic coordination, vigor, and sphincter function. The list of associated symptoms is broad but commonly includes dysphagia, chest pain, and globus sensation.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 43 PRINTER-FRIENDLY VERSION AVAILABLE AT GASTROENDONEWS.COM
Some patients may be asymptomatic or have symptoms that do not correlate with abnormal findings on manometric testing. When present, symptoms can cause significant distress and decrease patients’ quality of life. In severe cases, esophageal motor disorders can result in weight loss and failure to thrive. Due to limited understanding of the underlying mechanisms of all nonachalasic primary esophageal motor disorders, current treatment has proved challenging, with variable responses to both medical and endoscopic therapies.
The ability to predict response to therapy is limited, and relapses are not uncommon. Esophageal motor disorders related to hypercontractility and impaired esophagogastric outflow generally are more amenable to treatment than those related to hypocontractility. In patients with hypocontractile esophageal motor disorders, therapy is focused on treating potential sequelae, such as gastroesophageal reflux disease (GERD) and dysphagia. Additional research is required to identify effective, long-term treatments and better predictors of response to medical therapy versus endoscopic or surgical intervention. This article covers the underlying evidence and limitations of current medical, endoscopic, and surgical therapies for nonachalasic esophageal motor disorders.
Disorders of Peristalsis: Hypocontractile Disorders
Absent Contractility
Absent contractility is defined as 100% failed peristalsis in the setting of a normal integrated relaxation pressure (IRP) in both the upright and supine positions.1 It could be a primary disorder or due to a systemic
disorder that involves the esophagus, such as scleroderma, mixed connective tissue diseases, amyloidosis, sarcoidosis, and others. Laique et al reported that the prevalence of systemic sclerosis is 63% among patients with absent contractility.2
There is no specific treatment for absent contractility, and smooth muscle function cannot be restored reliably. 3 Furthermore, there is no expectation that peristaltic activity of the esophagus will recover fully. Thus, management is directed primarily toward the sequelae of aperistalsis, most notably GERD. Patients with absent contractility and subsequent GERD may experience refractory symptoms, elevated esophageal acid exposure time, and even erosive esophagitis on upper endoscopy.4 Treatment includes lifestyle changes, dietary modifications, and antisecretory therapy with proton pump inhibitors (PPIs).5,6 In patients presenting with dysphagia as the predominant symptom, esophageal outlet obstruction and achalasia should be ruled out. This is accomplished by using additional or provocative testing, such as rapid drink challenge, solid swallows, timed barium esophagogram (TBE), and endoluminal functional lumen imaging planimetry (FLIP).1,7-11 If esophagogastric junction outlet obstruction (EGJOO) or achalasia is diagnosed, different therapeutic options should be considered.
For patients with dysphagia, nonpharmacologic interventions have been shown to improve symptoms. These interventions are primarily behavioral and include education about a good upright swallowing technique, cutting food into small pieces, lubricating food, and chewing thoroughly before swallowing (Table 1). For patients with reflux symptoms, diaphragmatic breathing, or belly breathing, is another nonpharmacologic intervention that requires the concomitant use of abdominal muscles and diaphragm when breathing. It has been hypothesized that this maneuver helps to bolster the esophagogastric junction (EGJ) barrier and reduce the number of reflux events.12,13 Lastly, Vigneri et al described the use of transcranial brain stimulation to augment esophageal contractions in patients with nonerosive reflux disease and functional heartburn; this procedure may have a role in treating patients with esophageal hypocontractility.14
In patients who do not respond to lifestyle modifications, treatment focuses on reducing GERD with medications including PPIs up to twice daily, histamine-2 receptor antagonists, baclofen, sucralfate, aluminum hydroxide-magnesium trisilicate (Gaviscon, Sanofi), or a combination of a PPI and the other anti-reflux therapies (Table 2). Treatments that target esophageal dysmotility include buspirone, a mixed serotonin 5-hydroxytryptamine 1A receptor (5HT-1A) agonist and dopamine-2 receptor antagonist that has been shown to increase lower esophageal sphincter resting pressure and esophageal contraction amplitudes in patients with scleroderma.15,16 However, in a placebo-controlled, randomized trial, there was
Table 1. Lifestyle Modifications For Patients With Esophageal Motor Disorders Hypocontractile disorders • Avoid carbonated beverages • Decrease bolus consistency • Consume food in upright position • Lubricate fooda • Thoroughly chew food • Swallow effortfully Hypercontractile disorders • Avoid carbonated beverages • Avoid cold drinks • Drink warm water with meals • Take peppermint oil before meals a With fluids, sauces, dressings, or gravy. GASTROENDONEWS.COM44
no difference between buspirone and placebo with respect to improvements in esophageal function metrics or symptom scores.17 Prokinetic agents have demonstrated limited efficacy in treating absent contractility or ineffective motility disorder.7 Prucalopride (Motegrity, Takeda), a 5HT-4 agonist, has been shown to reduce esophageal acid exposure time and accelerate gastric emptying in healthy controls. However, there are no data describing prucalopride’s effect on hypocontractile esophageal disorders.18
Some patients with severe reflux symptoms may require anti-reflux surgery.7 Magnetic sphincter augmentation (Linx procedure) should be avoided in patients with absent contractility because an intact peristaltic contraction is required to propel a food bolus across the ring.19,20 Other anti-reflux surgeries, such as Toupet (270-degree wrap) or Dor fundoplication (180-degree anterior wrap), can be offered instead.
A series of 42 patients with severe hypomotility undergoing Toupet fundoplication revealed significant improvement in heartburn and regurgitation as well as reduced use of PPIs at 2-year follow-up.21 In an earlier study of 26 patients with absent contractility who underwent fundoplication (4 Nissen and 22 Dor), 87% of the patients were eating a normal diet at 2 year follow-up and 93% reported overall improvement on a symptom assessment questionnaire.22 Despite persistent dysmotility on high-resolution manometry (HRM), the long-term incidence of dysphagia was 26.7%, compared with 58.8% preoperatively. Partial fundoplication is preferred to complete Nissen fundoplication, which may cause postoperative dysphagia, especially in patients with absent contractility.23
Ineffective Esophageal Motility
Ineffective motility (IEM) is defined as greater than 70% ineffective swallows (distal contractile integral [DCI], 100-450 mm Hg•s•cm) or greater than 50% failed swallows (DCI, <100 mm Hg•s•cm).1 IEM typically is associated with GERD-related symptoms but also may be observed in patients with chest pain and dysphagia. In addition, IEM has been described in healthy adults and may have no clinical significance if not associated with symptoms. Furthermore, in many patients, IEM does not affect quality of life and does not progress over time.24
Lifestyle changes can significantly improve symptoms in patients with IEM. Behavioral changes for patients with IEM with dysphagia-predominant symptoms include dietary modifications, cutting food into small pieces, chewing thoroughly, and lubricating food (with fluids, sauces, dressings, or gravy). For reflux-predominant symptoms, lifestyle modifications include elevation of the head of the bed, weight loss, and avoidance of late-night meals and trigger foods.
For symptomatic IEM requiring pharmacologic intervention, anti-reflux medications are the cornerstone of management (Table 2). Commonly prescribed medications include PPIs, but other pharmacologic agents,
Table 2. Medical Therapies For Patients With Esophageal Motor Disorders
Hypocontractile disorders
Anti-reflux treatment
• Lifestyle modifications
• H2RAs
• PPIs
• Sucralfate
• Combination of anti-reflux medications
Cholinergic agonists
• Bethanechol
Acetylcholinesterase inhibitors
• Edrophonium
• Pyridostigmine
Dopamine-2 receptor antagonists
• Domperidone
• Metoclopramide
Serotonin receptor agonists
• Buspirone
• Cisapride
• Lintopride
• Mosapride
• Prucalopride (Motegrity, Takeda)
• Sumatriptan
• Tegaserod (Zelnorm, AlfaSigma)
Hypercontractile disorders
Anti-reflux treatment
• Lifestyle modifications
• H2RAs
• PPIs
• Sucralfate
• Combination of anti-reflux medications
Smooth muscle relaxants
• Calcium channel blockers
• Nitrates
• PDE5 inhibitors
Neuromodulators
• SNRIs
• SSRIs
• TCAs
• Trazodone
H2RAs, histamine-2 receptor antagonists; PDE5, phosphodiesterase 5; PPIs, proton pump inhibitors; SNRIs, serotonin–norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 45
such as prokinetic agents and muscarinic agonists, have been studied. As with absent contractility, prokinetic agents demonstrate limited efficacy in improving peristalsis or symptoms. The 5HT-4 agonist mosapride has been shown to induce secondary peristalsis in IEM patients during esophageal rapid air distention, although without improvement in contraction vigor.25 Prucalopride was shown to enhance primary peristalsis and distention-induced secondary peristalsis among 15 patients with IEM.26 Finally, buspirone was not better than placebo in treating dysphagia and IEM.17
Bethanechol, a muscarinic agonist that acts on parasympathetic receptors, is used primarily for treating urinary retention, but it has demonstrated an effect on the smooth muscle of the gastrointestinal tract.27 In a study of 7 patients with severe IEM, bethanechol significantly increased distal esophageal amplitude contractions and improved complete bolus transit time 20 and 40 minutes after administration.28
Patients with reflux symptoms and IEM that are unresponsive, or only partially responsive, to pharmacologic therapy may consider anti-reflux surgery. Before surgery, the multiple rapid swallows (MRS) test should be performed to measure peristaltic reserve.29 A study of 63 patients undergoing anti-reflux surgery and 18 healthy controls demonstrated that the absence of smooth muscle augmentation at the end of an MRS test is associated with late postoperative dysphagia.30 In the study, 11.1% of patients with postoperative dysphagia showed MRS augmentation versus 63.6% of those without postoperative dysphagia and 78.1% of healthy controls.
The degree of hypomotility may affect the choice of anti-reflux surgery, especially if patients demonstrate a high percentage of failed swallows. Magnetic sphincter augmentation is an anti-reflux treatment that may not be optimal for patients with hypomotility, such as IEM, since a forceful peristaltic contraction is required to propel a bolus across the magnetic sphincter.19,20
Disorders of Peristalsis: Hypercontractile Disorders
Distal Esophageal Spasm
Distal esophageal spasm (DES) is defined as greater than 20% premature contractions with normal IRP and DCI. A premature contraction is described on HRM as a distal latency less than 4.5 seconds.1 Typical symptoms include chest pain, dysphagia, and regurgitation.
Like other esophageal motor disorders, DES may be asymptomatic and not clinically significant. DES should correlate with clinical findings of chest pain and dysphagia.31
Treatment of DES is challenging because different phenotypes may exist, with variable responses to therapies. DES can be part of the type III achalasia spectrum or related to opioid treatment.32 Opioids have been linked to shortened distal latency, elevated DCI, and IRP.33-35 For patients with documented DES who
are on chronic opioid therapy, HRM should be repeated with the patient off opioids and symptoms reassessed.
Initial management should constitute lifestyle changes, such as avoidance of carbonated beverages, lubrication of food, and the usage of peppermint oil before meals (Table 1).36 Among patients with DES and reflux disease, it remains unclear whether acid suppression results in a decreased number of premature contractions and improvement in spasm-related symptoms. 32 Regardless, aggressive treatment of GERD may be helpful in a subset of patients with DES.
Other medical therapies with some limited efficacy for DES include smooth muscle relaxants, such as calcium channel blockers (CCBs), and nitrates (Table 2). A case report from 1973 first described the successful use of sublingual nitroglycerin for on-demand treatment of DES with chest pain as the predominant symptom. Sustained symptomatic resolution was achieved with long-acting nitrates.37 A subsequent, small, prospective study demonstrated improvement in symptoms among 5 patients with DES (without reflux disease) after administration of nitroglycerin and long-acting nitrates. Response to therapy was durable, with all 5 patients remaining symptom-free after 6 months to 4 years; 7 patients with reflux-associated spasm derived sporadic benefit from nitroglycerin and longacting nitrates.38
CCBs, particularly nifedipine, were shown to decrease lower esophageal sphincter pressure and reduce prolonged esophageal contractions, but correlation with symptomatic improvement was inconsistent.39 At least 1 study suggested that response to a CCB may take up to 2 weeks and may be transient (about 4 weeks).40
Other medications that have been studied include phosphodiesterase-5 (PDE5) inhibitors and antidepressants. PDE5 inhibitors act as smooth muscle relaxants by slowing the degradation of cGMP by phosphodiesterase.41 Commonly used for the treatment of pulmonary hypertension and erectile dysfunction, PDE5 inhibitors have been shown to reduce lower esophageal sphincter pressure and distal esophageal amplitude contractions for up to 8 hours after administration. Eherer et al administered the PDE5 inhibitor sildenafil to 11 patients with hypercontractile esophageal motility disorders; they found that it improved manometric recordings in 9 of the patients, but only 4 reported an improvement in symptoms and 2 complained of side effects and did not want to continue the study.42 Antidepressants, particularly tricyclic antidepressants (TCAs) such as imipramine, also have been shown to be effective in patients with noncardiac chest pain due to hypercontractile disorders.43 Trazodone also has been shown to improve symptoms of chest pain in patients with hypercontractile esophageal disorders but without any impact on manometric measurements.44
The role of botulinum toxin injections has been investigated with conflicting results. In a double-blind,
GASTROENDONEWS.COM46
randomized controlled study, 22 patients with dysphagia-predominant DES exhibited a significant improvement in symptoms and weight loss after botulinum toxin injection; 50% of patients had improvement at 1-month follow-up compared with 10% of those who received placebo saline injection.45 After 1 year, 30% of patients receiving botulinum toxin injections reported sustained symptom improvement. A subsequent randomized controlled trial of 23 patients with symptomatic hypercontractile esophageal disorders compared botulinum toxin injections with a sham procedure (no injections).46 At 3- and 12-month follow-ups, all patients reported an improvement in symptoms, with no statistical difference between the botulinum toxin and sham groups.
In addition to its role in achalasia, peroral endoscopic myotomy (POEM) has been considered as a therapeutic option for patients with DES (Table 3). Results have been encouraging, although less pronounced than for achalasia. A prospective trial of 30 patients with nonachalasic esophageal motor disorders who underwent POEM, of whom 23 had hypercontractile disorders, revealed clinical response in 80% of patients at 3 months and 63.2% at 6 months.47 This was lower than the response rates observed in patients with achalasia undergoing POEM. A retrospective review demonstrated that among 40 patients with nonachalasic esophageal motor disorders who underwent POEM, 90% reported significant symptom improvement at 6-month follow-up and 82% of patients had sustained improvement at a median 4-year follow-up.48 An international study of 50 patients with nonachalasic esophageal motor disorders provides further evidence of POEM’s efficacy, with clinical success rates of 94.1% for DES, 93.3% for EGJOO, and 75.0% for hypercontractile (jackhammer) esophagus (HE).49 A systematic review of 8 studies that included 179 patients who underwent POEM demonstrated a clinical success rate of 88% for DES compared with 92% for type III achalasia, with no statistically significant difference between the groups.50
Although results for POEM have been encouraging, further studies are needed to identify which clinical characteristics or HRM metrics predict a durable response to POEM. Further prospective studies with sham controls also are needed. In addition, it is uncertain which clinical end points should be used to identify successful response to treatment. The aforementioned studies relied primarily on improvement in the Eckardt score, lower esophageal sphincter pressure, and esophageal emptying time on TBE. Manometric, impedance, and FLIP metrics are lacking.
Although rarely used, laparoscopic myotomy of the distal esophagus has been shown to improve symptoms in patients with spastic esophageal disorders. A study of 20 patients with diffuse esophageal spasm undergoing laparoscopic extended myotomy with anterior fundoplication revealed improved dysphagia and chest pain symptoms at median 50-month
follow-up.51 More studies are needed to determine the value of laparoscopic esophageal myotomy in DES. In addition, the invasive nature of the procedure limits its appeal to patients and physicians alike. However, surgery remains a viable option for patients with refractory symptoms and an inability to tolerate medical therapy.
Hypercontractile Esophagus (Jackhammer)
HE is defined as at least 20% swallows with DCI greater than 8,000 mm Hg•s•cm in the absence of an elevated IRP or mechanical obstruction.1 As with other esophageal motor disorders, the presence of HE on HRM may not be clinically significant. In the absence of typical symptoms (chest pain and dysphagia), therapeutic intervention may not be warranted.52 In addition, lifestyle modifications can be recommended before initiating pharmacologic or invasive therapies.
HE may be secondary to opioid use or in association with GERD.52 These underlying conditions should be addressed with opioid cessation or acid suppression, respectively, before any other therapy is initiated. Despite a high prevalence of GERD among patients with HE, only 6.3% to 20.4% of patients with both HE and GERD report symptomatic improvement with only PPI therapy.53,54
Smooth muscle relaxants, such as CCBs and nitrates, antidepressants, and peppermint oil, have been studied, albeit with limited effect (Table 2). In a retrospective multicenter study of 9 French academic medical centers, Philonenko et al showed that medical therapy (PPIs, CCBs, nitrates, antispasmodics, anxiolytics, and steroids) has poor efficacy and a high relapse rate.55 They found that only 14% of patients responded to medical therapy, 35% with a partial response. A recent meta-analysis of 38 studies underscored the limited efficacy of medical therapies in this setting, finding a response rate of 63% (95% CI, 47%-79%) with various medications (CCBs, nitrates, PPIs, peppermint oil, and antidepressants).56
Table 3. Surgical, Endoscopic Options for Hypercontractile Disorders Endoscopic treatment
• Botulinum toxin injection • Bougie or pneumatic dilation • POEM Surgery • Esophageal myotomy POEM, peroral endoscopic myotomy. GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 47
Endoscopic therapies, including POEM, botulinum toxin injections, and pneumatic dilation, have been used as alternatives or adjuncts to medical therapy (Table 3). The previously mentioned meta-analysis reported an efficacy rate of 79% (95% CI, 74%-85%) for endoscopic therapies, suggesting they are superior to medical treatment.56 A study including 39 patients with HE undergoing endoscopic therapy (27 with botulinum toxin injection, 11 with pneumatic dilation, and 1 with POEM) demonstrated a symptom recurrence rate of 31.2% after botulinum toxin injection and 57.1% after pneumatic dilation.55 The 1 patient treated with POEM remained symptom-free after the intervention.
The role of botulinum toxin injections in treating HE remains to be elucidated. A prospective controlled study of 22 patients with HE compared the efficacy of botulinum toxin injections with placebo saline injections. 45 Patients reported a significant reduction in symptoms after botulinum toxin injection. No significant difference in response was observed for saline injections. In addition, the response to botulinum toxin injections was durable, with 50% of patients reporting sustained improvement in symptoms at 1 month (vs 10% after saline injections) and 30% at 1 year. However, a randomized, placebo-controlled, double-blind study of 33 patients with HE demonstrated no significant difference between botulinum toxin and saline injections.46
POEM has been shown to be the most effective treatment for HE, with a meta-analysis of 38 studies showing an efficacy rate of 82%.56 In the study by Bernardot et al mentioned earlier (30 patients with nonachalasic esophageal motility disorders, 23 with hypercontractile disorders), the response rate to POEM was 80% at 3 months and 63.2% at 6 months.47 Another study assessing the efficacy of POEM in patients with hypercontractile esophageal disorders and predominant
symptoms of chest pain revealed a response rate of 85.7% for up to 15 months after intervention.57
Data are limited on long-term efficacy of POEM. Fiveyear follow-up of a retrospective study reported sustained symptomatic improvement in 6 out of 7 patients with HE who underwent POEM.58
The role of surgery in HE is uncertain due to the absence of prospective randomized controlled trials. Myotomy of the distal esophagus may be considered because of demonstrable efficacy in DES, but no evidence supports its routine use in HE.
Some patients exhibit a spontaneous resolution of symptoms without any intervention. A study including 15 patients with HE reported clinical improvement in 11 patients at 132 weeks, but only 4 received any specific treatment.59 Thus, given the variability in treatment response, it is recommended that patients with HE first be managed conservatively with medical therapy and repeat HRM after 6 to 12 months to assess for manometric durability. Invasive interventions should be considered only in carefully selected patients reporting persistent symptoms despite medical therapy.60
Disorders of EGJ Outflow
Esophagogastric Junction Outflow Obstruction
EGJOO is defined by an elevated median IRP and raised intrabolus pressure with intact peristalsis during HRM.1 An elevated IRP should be noted in both the supine and upright positions to exclude an artifactual increase in IRP.61 Esophagogastroduodenoscopy typically is required for EGJOO diagnosis to rule out secondary/mechanical causes of outflow obstruction.1
EGJOO is a common finding, with a prevalence of 5% to 24% in patients undergoing HRM.59,61-65 Its clinical significance is uncertain and often determined to be an incidental finding. Thus, for EGJOO to be deemed clinically significant, typical symptoms of dysphagia and/or chest pain are required, as well as positive findings during supportive testing, which includes TBE, FLIP, and pharmacologic provocation with amyl nitrite.1
Treatment of EGJOO remains challenging, partly due to its ambiguous clinical significance and unclear natural history.66,67 EGJOO often is an incidental finding during HRM testing that does not have obvious ramifications for patients. In certain cases, EGJOO may represent early achalasia.63,66 However, it is thought that less than 10% of patients with EGJOO progress to full achalasia, with more than 90% never developing a severe esophageal motor disorder.61 Furthermore, 32% to 94% of patients with EGJOO do not require any therapeutic intervention because symptoms resolve spontaneously over time with observation.59,62,64-66
Since the majority of identified cases of EGJOO are incidental and a significant proportion of patients experience spontaneous resolution of symptoms, characterizing the clinical significance of an isolated, elevated IRP is of the utmost importance.1,68,69 Supportive testing not only assists in identifying clinically relevant
Table 4. EGJOO Management Guided by Supportive Testing EGJOO with normal TBE or FLIP EGJOO with abnormal TBE or FLIP • Reassurance • Calcium channel blockers • Proton pump inhibitors • Bougie esophageal dilation • Botulinum toxin injection • Pneumatic esophageal dilation • Surgical myotomy EGJOO, esophagogastric junction outflow obstruction; FLIP, endoluminal functional lumen imaging planimetry; TBE, timed barium esophagogram. Adapted from reference 70. GASTROENDONEWS.COM48
EGJOO but also may predict response to therapy.
Before initiating medical, endoscopic, or surgical intervention, opioid use should be excluded in all patients with EGJOO. Observation and repeat HRM 6 to 12 months after diagnosis is a reasonable approach in patients without severe symptoms.
Experts recommend tailoring the treatment of EGJOO based on the presence or absence of true EGJ obstruction confirmed by supportive testing with TBE or FLIP testing.70 If supportive testing excludes EGJ obstruction, conservative treatments, such as reassurance, PPIs, CCBs, and esophageal dilation, are recommended (Table 4). If EGJ obstruction is confirmed, invasive methods—including botulinum toxin injections, pneumatic dilation, and surgical myotomy—could be considered.
Very few clinical trials can help guide clinicians on how to use medical therapy in EGJOO. As previously described, a significant proportion of patients will experience spontaneous resolution of symptoms without any intervention. The reported symptomatic response rates to PPIs and CCBs are variable. One retrospective study identified 7 patients with dysphagiapredominant EGJOO treated with CCBs, nitroglycerin, muscle relaxants, and antispasmodics. All 7 patients failed to have a significant response to therapy.71 The same study identified 8 EGJOO patients with predominant heartburn and regurgitation symptoms treated with PPIs, of which only 2 (25%) reported sustained improvement in symptoms. Conversely, another study of 37 patients with EGJOO identified 4 who underwent medical treatment, 3 of whom responded to therapy with amitriptyline, hyoscyamine, and PPIs.64 The one patient who failed to respond to medical therapy was treated with a CCB.
Botulinum toxin injections have shown greater efficacy than medical therapy in treating clinically relevant EGJOO, with a sustained response. Among 36 patients with elevated IRP undergoing botulinum injection, 55% experienced symptomatic improvement at 1 year.72 These results endured, with 50% of patients describing symptom improvement at 1.5 years, a response better than that for achalasia. In addition, in a study by van Hoeij et al, all 5 patients treated with botulinum toxin experienced symptomatic improvement after a single injection, although the treatment effect only lasted for 6 to 10 months.66
Bougie and pneumatic dilation have been demonstrated to be effective in treating EGJOO for patients with normal and abnormal TBE, respectively. Among patients with normal esophageal emptying on TBE, bougie dilation to 18 to 20 mm was shown to improve symptoms for up to 1 year after treatment.73 For EGJOO patients with abnormal esophageal emptying on TBE, pneumatic dilation resulted in effective and durable improvement of symptoms. A study of 33 EGJOO patients with abnormal TBE demonstrated 67% improvement in dysphagia symptoms after pneumatic dilation with a 30-mm balloon and up to 79%
improvement with 35-mm balloon dilation. Symptom improvement is durable at 5-year follow-up, and all patients demonstrated improvement in esophageal emptying on TBE.74
Esophageal myotomy, either by POEM or surgery, has been shown to be successful in a small number of case reports. Three patients with EGJOO who underwent POEM reported sustained improvement in symptom severity at 3-month follow-up.71 Similarly, all 4 patients undergoing laparoscopic Heller myotomy reported improvement in symptoms.64 The lack of prospective data and the invasive nature of the procedure has limited its general use in the management of EGJOO. For patients with EGJOO, botulinum toxin injection or pneumatic dilation is recommended before any type of myotomy (either POEM or laparoscopic).70
The role of psychological interventions, such as cognitive behavioral therapy (CBT), requires further exploration as an adjunct to medical or surgical therapy. CBT has been shown shown to improve symptoms and decrease hypervigilance in patients with functional esophageal disorders.75 Whether CBT and other psychotherapeutic interventions have a role in the symptomatic management of esophageal motor disorders has yet to be elucidated.
Conclusion
The treatment of nonachalasic esophageal motor disorders remains a challenge because clinical response is variable and relapse rates are high. Before initiating treatment, an identified esophageal motor disorder should first be deemed clinically relevant. Patients who use opioids should be weaned off these agents, and other medications with a potential effect on esophageal function should be reassessed. An aggressive treatment for GERD should be attempted early in the course of the disease, preferably with a PPI. If symptoms persist despite the aforementioned interventions, then clinicians may consider other medical therapeutic modalities as well.
In hypercontractile esophageal motor disorders, medical therapy should be attempted first. Endoscopic and surgical modalities should be considered in patients who are not responsive to medical therapy or in those with severe symptoms. Patients undergoing endoscopic or surgical intervention should be carefully selected.
For hypocontractile esophageal motor disorders, treatment is focused on controlling GERD. This entails lifestyle modifications, anti-reflux medications, and surgery if indicated. Other medical therapies that may enhance esophageal function could be considered, but the response to these therapies has been inconsistent.
Neuromodulators have a therapeutic role in patients with hypercontractile esophageal motor disorders and may be given alone or in combination with other therapeutic modalities. Psychological intervention should be considered in patients with psychological comorbidities.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 49
1. Yadlapati R, et al. Neurogastroenterol Motil 2021;33(1):e14058.
2. Laique S, et al. J Clin Gastroenterol 2019;53(3):184-190.
3. Smout A, et al. Neurogastroenterol Motil. 2012;24(suppl 1):40-47.
4. Quader F, et al. Am J Gastroenterol 2020;115(12):1981-1988.
5. Triadafilopoulos G, et al. BMJ Open Gastroenterol 2016;3(1):e000126.
6. Gyawali CP, Fass R. Gastroenterology 2018;154(2):302-318.
7. Gyawali CP, et al. Neurogastroenterol Motil 2021;33(8):e14134.
8. Savarino E, et al. Am J Gastroenterol 2020;115(11):1786-1796.
9. Blonski W, et al. Am J Gastroenterol. 2018;113(2):196-203.
10. Wang YT, et al. Clin Gastroenterol Hepatol 2015;13(9):1575-1583.
11. Sweis R, et al. Neurogastroenterol Motil. 2014;26(2):215-228.
12. Pauwels A, et al. Gastroenterology 2018;154(6):S238. Abstract Sa1092.
13. Halland M, et al. Am J Gastroenterol 2021;116(1):86-94.
14. Vigneri S, et al. Clin Neurophysiol 2014;125(9):1840-1846.
15. Karamanolis GP, et al. Arthritis Res Ther. 2016;18(1):195.
16. Karamanolis GP, et al. United European Gastroenterol J. 2015;3(3):266-271.
17. Aggarwal N, et al. Neurogastroenterol Motil. 2018;30(2):e13213.
18. Kessing B, et al. Neurogastroenterol Motil. 2014;26(8):1079-1086.
19. Smith CD, et al. J Am Coll Surg. 2014;218(4):776-781.
20. Asti E, et al. Medicine (Baltimore). 2016;95(30):e4366.
21. Armijo PR, et al. J Gastrointest Surg. 2019;23(1):36-42.
22. Watson DI, et al. Dis Esophagus. 2006;19(2):94-98.
23. Yadlapati R, et al. Am J Gastroenterol. 2018;113(8):1137-1147.
24. Ravi K, et al. Clin Gastroenterol Hepatol. 2015;13(8):1416-1423.
25. Chen C-L, et al. Scand J Gastroenterol 2013;48(12):1363-1370.
26. Lei W-Y, et al. J Gastroenterol Hepatol. 2018;33(3):650-655.
27. Padda IS, et al. Bethanechol. StatPearls [internet]. Last updated November 25, 2021.
28. Agrawal A, et al. J Clin Gastroenterol 2007;41(4):366-370.
29. Marin I, et al. Neurogastroenterol Motil. 2016;28(4):543-553.
30. Shaker A, et al. Am J Gastroenterol. 2013;108(11):1706-1712.
31. Pandolfino JE, et al. Gastroenterology. 2011;141(2):469-475.
32. Roman S, et al. Neurogastroenterol Motil. 2021;33(5):e14119.
33. Ratuapli SK, et al. Am J Gastroenterol. 2015;110(7):979-984.
34. Snyder DL, et al. Am J Gastroenterol. 2019;114(9):1464-1469.
35. Babaei A, et al. Neurogastroenterol Motil. 2019;31(7):e13601.
36. Gasiorowska A, et al. Gastroenterol Hepatol. 2009;5(4):269-279.
37. Orlando RC, et al. N Engl J Med. 1973;289(1):23-25.
38. Swamy N. Gastroenterology. 1977;72(1):23-27.
39. Baunack A, et al. Arzneimittelforschung. 1991;41(6):595-602.
40. Richter JE, et al. Dig Dis Sci. 1984;29(7):649-656.
41. Moreland RB, et al. Life Sci. 1998;62(20):PL309-PL318.
42. Eherer A, et al. Gut. 2002;50(6):758-764.
43. Cannon RO, et al. N Engl J Med 1994;330(20):1411-1417.
44. Clouse RE, et al. Gastroenterology. 1987;92(4):1027-1036.
45. Vanuytsel T, et al. Clin Gastroenterol Hepatol. 2013;11(9):1115-1121.e2.
Mion F, et al. Neurogastroenterol Motil. 2019;31(5):e13587.
47. Bernardot L, et al. Surg Endosc. 2020;34(12):5508-5515.
48. Filicori F, et al. Surg Endosc. 2019;33(5):1632-1639.
49. Khashab MA, et al. Endosc Int Open. 2018;6(08):E1031-E1036.
50. Khan MA, et al. Dig Dis Sci. 2017;62(1):35-44.
51. Leconte M, et al. Br J Surg. 2007;94(9):1113-1118.
52. Chen JW, et al. Neurogastroenterol Motil. 2021;33(6):e14115.
53. Kristo I, et al. Neurogastroenterol Motil. 2018;30(5):e13276.
54. Mallet A-L, et al. Dig Liver Dis. 2016;48(10):1136-1141.
55. Philonenko S, et al. Neurogastroenterol Motil. 2020;32(11):e13918.
56. Wahba G, et al. Neurogastroenterol Motil. 2020;32(11):e13870.
57. Albers D, et al. Z Gastroenterol. 2018;56(11):1337-1342.
58. Nabi Z, et al. J Clin Gastroenterol. 2021;55(7):594-601.
59. Schupack D, et al. Neurogastroenterol Motil. 2017;29(10):1-9.
60. Parker B, et al. Surgery for esophageal motor disorders.
In: Richter JE, Castell DO, Katzka DA, et al, eds. The Esophagus. 6th ed Wiley Online Library; 2021:278-293.
61. Bredenoord AJ, et al. Neurogastroenterol Motil. 2021;33(9):e14193.
62. Pérez-Fernández MT, et al. Neurogastroenterol Motil. 2016;28(1):116-126.
63. Scherer JR, et al. J Gastrointest Surg. 2009;13(12):2219-2225.
64. Lynch K, et al. Dis Esophagus. 2017;30(6):1-6.
65. Song BG, et al. J Neurogastroenterol Motil. 2019;25(1):75-81.
66. van Hoeij FB, et al. Neurogastroenterol Motil. 2015;27(9):1310-1316.
67. Babaei A, et al. Neurogastroenterol Motil. 2019;31(9):e13668.
68. Su H, et al. Neurogastroenterol Motil. 2020;32(11):e13924.
69. Triadafilopoulos G, et al. BMJ Open Gastroenterol. 2018;5(1):e000210.
70. Richter JE, et al. Am J Gastroenterol. 2019;114(4):544-547.
71. Okeke FC, et al. Neurogastroenterol Motil. 2017;29(6):e13061.
72. Porter R, et al. Neurogastroenterol Motil. 2011;23(2):139-144, e27-e28.
73. Clayton S, et al. Gastroenterology. 2017;152(5 suppl 1):S696.
74. Clayton SB, et al. Neurogastroenterol Motil. 2019;31(3):e13522.
75. Kisely SR, et al. Cochrane Database Syst Rev. 2010;20(1):CD004101.
References
46.
GASTROENDONEWS.COM50
BRIEF SUMMARY: Before prescribing, please see Full Prescribing Information and Medication Guide for SUTAB® (sodium sulfate, magnesium sulfate, potassium chloride) tablets for oral use.
INDICATIONS AND USAGE: An osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
DOSAGE AND ADMINISTRATION: Split Dose (2-Day) Regimen: Dose 1 – On the day prior to colonoscopy: A low residue breakfast may be consumed. After breakfast, only clear liquids may be consumed until after the colonoscopy. Early in the evening prior to colonoscopy, open one bottle of 12 tablets. Fill the provided water and drink the entire amount over 15 to 20 minutes. Approximately one hour after
Packaging and tablets not shown actual size.
Dose 2 - Day of colonoscopy: Continue to consume only clear liquids until after the colonoscopy. The morning of colonoscopy (5 to 8 hours prior to the colonoscopy and no sooner than
CONTRAINDICATIONS: Use is contraindicated in the following conditions: gastrointestinal obstruction or ileus, bowel perforation, toxic colitis or toxic megacolon, gastric retention, hypersensitivity to any ingredient in SUTAB. WARNINGS AND PRECAUTIONS: Serious Fluid and Electrolyte Abnormalities: Advise all taking SUTAB, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN). Fluid and electrolyte disturbances can lead to
disturbances or may increase the risk of adverse events of seizure, arrhythmias, and renal impairment; Cardiac arrhythmias: Use caution when prescribing SUTAB for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Consider pre-dose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias; Seizures: Use caution when prescribing SUTAB for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia; Use in Patients with Risk of Renal Injury: Use SUTAB with caution in patients with impaired renal function or patients taking concomitant medications that may affect renal function (such as diuretics, for renal injury. Advise these patients of the importance of adequate hydration with SUTAB and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients; Colonic Mucosal Ulcerations and Ischemic Colitis: Osmotic laxative products may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and SUTAB may increase these risks. Consider the potential for mucosal ulcerations resulting from the Use in Patients with Signifcant Gastrointestinal Disease: If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering SUTAB. Use with caution in patients with severe active ulcerative colitis. Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis, angioedema, dyspnea, rash, pruritis and urticaria have been reported with SUTAB. Inform patients of the signs and symptoms of anaphylaxis and instruct them to seek immediate medical care should signs and symptoms occur. ADVERSE REACTIONS: Most common gastrointestinal adverse reactions are: nausea, abdominal distension, vomiting and upper abdominal pain. Postmarketing Experience: Gastrointestinal: gastric ulceration, gastritis; Hypersensitivity: anaphylaxis, angioedema, dyspnea, rash, pruritus, urticaria. POTENTIAL FOR DRUG ABSORPTION: SUTAB can reduce the absorption of other co-administered drugs. Administer oral medications at least one at least 2 hours before and not less than 6 hours after administration of each dose of SUTAB to avoid chelation with magnesium. Pregnancy: There are no available data on SUTAB use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. No reproduction or developmental studies in animals have been conducted with sodium sulfate, magnesium sulfate, and potassium chloride (SUTAB). Lactation: There are no available data on the presence of SUTAB in human or animal milk, the effects on the breastfed child, or the effects on milk production. Pediatric Use: Safety and effectiveness in pediatric patients has not been established. Geriatric Use: older. No differences in safety or effectiveness of SUTAB were observed between geriatric patients and younger patients. Elderly patients are more abnormalities. STORAGE temperature. Rx only. Manufactured by Braintree Laboratories, Inc. Braintree, MA 02185
See Full Prescribing Information and Medication Guide at SUTAB.com.

References:1. SUTAB® [package insert]. Braintree, MA. 2 Di Palma JA, Bhandari R, Cleveland M, et al. A safety and subjects undergoing colonoscopy. Am J Gastroenterol
3. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; ACG. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol.
additional information, please call 1-800-874-6756
©2022 Braintree Laboratories, Inc. All rights reserved. 201-289-v4-A
February 2022 For
TABLET CHOICE IN BOWEL PREPARATION
ACHIEVED SUCCESSFUL
* 3 (good) or 4 (excellent) by the blinded endoscopist; scores were assigned following completion of the colonoscopy.



†Patients completed a preference questionnaire following completion of study drug to capture their perceptions of the preparation experience. This questionnaire has not undergone formal validation.

‡Patients were queried for selected gastrointestinal adverse reactions of upper abdominal pain, abdominal distension, nausea, and vomiting following completion of study drug, rating the intensity as mild, moderate, or severe.1,2

ACG=American College of Gastroenterology
MoviPrep is a registered trademark of Velinor AG.



IMPORTANT SAFETY INFORMATION
Packaging and tablets not shown actual size.
SUTAB (24 tablets) are required for a complete preparation for colonoscopy. Twelve (12) tablets are equivalent to one dose. Water must be consumed with each dose of SUTAB and additional water must be consumed after each dose. Complete all SUTAB tablets and required water at least 2 hours before colonoscopy. CONTRAINDICATIONS: Use is contraindicated in the following conditions: gastrointestinal obstruction or ileus, bowel perforation, toxic colitis or toxic megacolon, gastric retention, hypersensitivity to any ingredient in SUTAB. WARNINGS AND PRECAUTIONS: : Encourage adequate hydration, assess concurrent medications and consider laboratory assessments prior to and after each use; Cardiac arrhythmias: Consider pre-dose and post-colonoscopy ECGs in patients at increased risk; Seizures: Use caution in patients with a history of seizures and patients at increased risk of seizures, including medications that lower the seizure threshold; Patients with renal impairment or taking concomitant medications that affect renal function: Use caution, ensure adequate hydration and consider laboratory testing; Colonic mucosal ulcerations


Suspected GI obstruction or perforation: Rule out the diagnosis before administration. Hypersensitivity reactions, including anaphylaxis: Inform patients to seek immediate medical care if symptoms occur. ADVERSE REACTIONS: Most common gastrointestinal adverse reactions are: nausea, abdominal distension, vomiting and upper abdominal pain. DRUG INTERACTIONS: Please see Brief Summary of Prescribing Information on reverse side. See Full Prescribing Information and Medication Guide at SUTAB.com
• NO SODIUM PHOSPHATE1 • SAFE AND EFFECTIVE1,2 • ACG-RECOMMENDED SPLIT-DOSE REGIMEN3 – Two SUTAB doses are required for a complete preparation1 Dose 1 consists of 12 tablets and 16 oz of water Dose 2 consists of 12 tablets and 16 oz of water Each dose is followed by two additional 16 oz of water 92% OF PATIENTS IN TWO PIVOTAL TRIALS
BOWEL CLEANSING WITH SUTAB1,2* 91% OF PATIENTS IN ONE PIVOTAL TRIAL RATED SUTAB AS TOLERABLE TO VERY EASY TO CONSUME2† • 52% of all SUTAB and MoviPrep® patients reported at least one selected gastrointestinal adverse reaction1,2‡ considered severe2‡ 78% OF PATIENTS IN ONE PIVOTAL TRIAL WOULD REQUEST SUTAB AGAIN FOR A FUTURE COLONOSCOPY2†
SUTAB® (sodium sulfate, magnesium sulfate, potassium chloride) tablets for oral use is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults. DOSAGE AND ADMINISTRATION: A low residue breakfast may be consumed. After breakfast, only clear liquids may be consumed until after the colonoscopy. Administration of two doses of
Improving Bowel Preparation For Inpatient Colonoscopy: A Proactive Approach


GEORGE DIMITRIOU, MD, FHM
MOAZ SIAL, MD
PHOENIX FUNG, MD
LEON AVERBUKH, MD
RITA COLE, MBA, BS
JUNGMIN LEE, MD
CARI STEVENS, PHARMD, BCPS
KRISTINA ZAPF, MPH
RYAN BURG, PHARMD
KATIE FARAH, MD, FASGE, CPE
Allegheny Health Network
Allegheny General Hospital Pittsburgh, Pennsylvania
Wexford Hospital Wexford, Pennsylvania
Achieving adequate bowel preparation is challenging but critical to achieve a good-quality colonoscopy in both inpatient and outpatient settings. Inadequate bowel prep is responsible for up to one-third of all incomplete colonoscopies.1,2


In the inpatient setting, inadequate bowel prep can result in delayed medical care, repeat procedures, increased length of stay, and escalating costs. We studied barriers to achieving adequate colonoscopy prep and aimed to implement changes to improve the quality of an inpatient bowel prep regimen in a tertiary care center.
Predictors of Inadequate Bowel Preparation

Hospitalization is a known independent risk factor for poor bowel prep. Reasons for inadequate bowel preparation include 1) the use of a large-volume bowel prep agent that leads to poor patient compliance and an inability to complete prep, 2) communication gaps among physicians, nurses, and patients about the steps involved in completing a bowel prep, and 3) lack of knowledge among patients, providers, and nurses about the importance of bowel prep completion as well as clinician documentation. It is not infrequent to see patients discontinue bowel prep when they feel it is adequate or when they cannot tolerate it due to nausea and/or vomiting. In addition, reporting by nursing staff tends to be subjective.
Documentation of bowel prep intake and stool output often is scant. Procedure cancellations and/or low diagnostic yields due to poor preparation are common.
The presence of predictors of inadequate bowel preparation must be considered when selecting the prep and for determining whether a patient needs special help with their situation.3 Medical conditions associated with inadequate bowel preparation include chronic constipation (and/or use of constipating medications), diabetes mellitus, obesity, cirrhosis, Parkinson’s disease, dementia, and stroke. Polypharmacy, prior colon resection, and a prior history of inadequate colonoscopy prep are additional factors associated with poor prep.4-8 Special interventions may be needed for these individuals to increase the likelihood that they follow instructions and achieve successful completion of prep.

Addressing Barriers to Improve Inpatient Prep
At our tertiary care center, we evaluated barriers to achieving adequate colonoscopy prep and implemented changes to improve the quality of an inpatient

GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 53
bowel regimen. This included creation of a new order set and nursing-driven protocol in the electronic health record (EHR).
Our team conducted an observational prospective study at a single tertiary care teaching institution over a 4-month period to improve bowel preparation, incorporating an assessment tool to allow nurses to better assess bowel prep into the institution’s EHR. First
described by Johnston et al in 2015, the tool—the Nursing Bowel Preparation Assessment Tool (NBPAT)— was developed to minimize repeat colonoscopies and better predict successful bowel preparation.9 The tool has subscores for stool consistency, color, and sediment presence and automatically calculates a total score of 0 to 5.
A high score on the NBPAT (4 or 5) is highly predictive
 Figure 1. Bowel prep assessment flow sheet.
Figure 1. Bowel prep assessment flow sheet.
GASTROENDONEWS.COM54
of adequate bowel prep at the time of colonoscopy (Figure 1).9 Patients with low scores (1 or 2), and potentially those with intermediate scores (3), require additional prep to minimize the risk for an inadequate prep before colonoscopy.
For the study, we evaluated all patients 18 years of age and older who underwent an inpatient colonoscopy, excluding patients with suspected gastrointestinal
obstruction and those receiving preparation via a nasogastric tube at baseline. Polyethylene glycol with electrolyte (PEG-3350) was the 4-liter GI lavage prep used at our medical facilities for bowel cleansing before colonoscopy, with proven safety in a wide range of patients, including those with diabetes, hypertension, cardiac or renal issues, and electrolyte imbalances. Additional prep was given at the discretion of GI fellows.
Table. Education of Prep Protocol (Outline of Order Set Creation)
GI (where applicable) to place the orders only. Order for 4,000 mL once should be omitted from network.
Type in “PEG-3350”
Linked order for two 2,000 mL orders pops up as 2 separate orders (4-L split dose)
Order for AM (morning: 9:00 AM to noon) procedure:
1st dose given at 6:00 PM the night before
2nd dose given at 12:00 AM to be finished by 5:00 AM
Order for PM (afternoon: noon or later) procedure:
1st dose given at 6:00 PM the night before
2nd dose given at 3:00 AM to be finished by 8:00 AM
Diet: Clear liquid
Orders based on: First 2 h (8:00 AM - 10:00 AM) endoscopies; colonoscopies start at 10:00 AM
For high-risk patients (history of bad/inadequate prep, opiates, BMI ≥45, diabetes, cirrhosis, chronic constipation, Parkinson’s, dementia, CVA), order modified prep
Modified Prep: 2 days of clear liquid
Day before procedure: two 2,000 mL orders pop up as 2 separate orders (4-L split dose)
1st dose at evaluation; 2nd dose 4 h later
OR
10 oz magnesium citrate (contraindicated in renal insufficiency and CHF)
Day of procedure: two 2,000 mL orders pop up as 2 separate orders (4-L split dose)
1st dose given at 5 AM
2nd dose given at 9 AM
Nurses will have I/O documentation
Prep intolerance:
6:00 PM – 1st dose given
8:00 PM – patient says can’t drink prep; if nausea present, give antiemetic
10:00 PM – patient still not tolerating or drank less than 50%
• Insert NG tube for rapid preps; push 500 mL every 60 min
• If NG refused, patient continues to try to drink
2nd dose intolerance: give antiemetic or NG tube
Assessment of bowel after prep complete:
AM Procedure I/O at 5:00 AM
PM Procedure I/O at 8:00 AM
Patient READY for procedure if stool is yellow and clear, resembling urine
Patient NOT READY for procedure if stool is dark, murky, brown, dark /light orange, or semi-clear
If not ready, proceed to salvage preparation
Salvage Prep: Additional purgative given after I/O assessment
Give additional rapid prep (2 L) over 3 h
AGH, Allegheny General Hospital; CHF, congestive heart failure; CVA, cerebrovascular accident; I/O, input/output; NG, nasogastric.
EPIC order for split dosing colonoscopy bowel prep (initially for AGH inpatient colonoscopies only)
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 55
Adequate colonoscopy prep at the time of colonoscopy was defined as an intact Boston Bowel Preparation Scale (BBPS) score of greater than or equal to 6, segmental BBPS greater than or equal to 2, and prep quality described as “good,” “excellent,” or “adequate”; all other prep descriptions (<6) were considered inadequate.10,11
We conducted a retrospective cost-based analysis
for repeat inpatient colonoscopies due to incomplete bowel preparation. Data collected for the 6-month (June 2018-December 2018) retrospective analysis included: patient identifiers: medical record number (MRN), date of birth, and gender; exam dates; indication; number of colonoscopy procedures aborted and the reason; prep quality: adequate/inadequate determined from the data points in the Endowriter
 Figure 2. EHR with hard stops implemented.
Figure 2. EHR with hard stops implemented.
GASTROENDONEWS.COM56
Reporting System; whether follow-up colonoscopy was performed or recommended; recommendation for follow-up inpatient or outpatient procedure and timing; and costs associated with hospital stay and colonoscopy.
We also conducted a prospective analysis consisting of 4 phases over a 15-month period, with the following data collected during each phase:
• Demographic data: date, MRN, age, and gender

• Clinical data: indication of colonoscopy
• Prep data: ingested PEG-3350 (mL), reason if unable to finish prep (severe nausea, vomiting, abdominal pain, or bloating), whether or not a nasogastric tube was tried, stool clearance
• Procedure data: BBPS score, adequacy of bowel prep (defined as BBPS score ≥6), rate of abortion of colonoscopy due to poor prep
• Postprocedure data: length of stay, total cost of hospital care
Figure 3. Additional nursing orders.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 57
 Figure 4. EHR protocols for standard, high-risk, and salvage preparations.
Figure 4. EHR protocols for standard, high-risk, and salvage preparations.
GASTROENDONEWS.COM58
Phases in Prospective Analysis
In phase 1, the team collected data before implementation of the intervention to establish the baseline adequacy of bowel preparation for inpatients and the percentage of colonoscopies with inadequate preparations resulting in second-day repeat procedures. No hard stops were built into the EHR at baseline. Then, in intervention 1, investigators educated nurses, fellows, faculty, and patients about the protocol for the prep intake (Table). During this phase, nursing compliance with using and completing the NBPAT was 67%.
During phase 2, intervention 2 was implemented. This included enhancement of prep assessment for consistency, color, and sediment. In addition, we created a targeted order set in the EHR, with hard stops for nursing and physicians. These hard stops triggered action items for providers to intervene if there was evidence of lack of tolerability of bowel preparation. GI fellows were educated about the EHR order set for split-dose bowel preparation (recommended by the American Society for Gastrointestinal Endoscopy3), evaluation of prep adequacy on the day of procedure at 7 AM using a 5-point NBPAT, and same-day additional prep administration for patients with inadequate stool output. Examples of action items included order sets accounting for nausea, vomiting, placement of a nasogastric tube, and salvage prep therapy after assessment of the patient by the GI fellow at 7 AM on the morning of the prep. (Figures 2-4). At this point, nursing compliance with using and completing the NBPAT was 100%.
During phase 3, intervention 3 was implemented. This involved the addition of hard stops for the presence or absence of frank blood and required documentation of prep completion. Nurse workflow for assessment of prep intake was streamlined.
In phase 4, the team conducted a retrospective review of 221 inpatient colonoscopies over a 7-month period to establish baseline colonoscopy prep
adequacy and the frequency of repeat procedures in a tertiary care hospital and prospectively collected data for 7 months after the interventions. Barriers were identified for further intervention. On the day of the procedure, the GI lab status board was updated at 7 AM (Figure 5). The red indication resulted in cancellation of the colonoscopy due to either prep refusal or solid stool output (score of 0 on the NBPAT scale). The yellow indication prompted same-day intervention with salvage prep and postponement of the colonoscopy to the afternoon (NBPAT score of 1-2). The green indication signaled procedure readiness with an NBPAT score of 3 to 5.
Interventions Yielded Benefits
At baseline, 106 of 221 patients (48%) had inadequate prep, with 41 patients (19%) requiring repeat inpatient colonoscopy during the same hospitalization.
After the first intervention (nursing, physician, and patient education), 78 of 215 inpatients (36%) had inadequate prep. Split-dose bowel prep was ordered in both groups, but it was unclear whether the patients followed the regimen correctly. Documentation of prep intake was poor, and partial documentation occurred in 21 patients (10%).
After the second intervention (order set with hard stops and identification of the high-risk population), 51 patients (30%) had inadequate preps.
Assessment of Protocol
At New Community Hospital
The third intervention did not show a significant difference in bowel prep adequacy at our tertiary care hospital. However, in light of the overall improvement observed, we decided to implement this revised protocol with all 3 interventions from the point of opening of a new community hospital to evaluate efficacy.
With the newly built platform in the EHR available at

4
Figure 5. GI procedure status board for bowel preparation adequacy. Figure 6. Cost savings at tertiary care hospital and local community hospital. 60,000 50,000 40,000 30,000 20,000 10,000 0 Tertiary care hospital over 15-month period Community hospital over 11-month period Approximate cost savings compared with baseline pre-intervention cost, $ 46,110 29,203 GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 59
the community hospital’s opening, we found that out of 85 inpatient colonoscopies, 22 (26%) had an inadequate prep over an 11-month period.
Possible reasons for improved bowel prep adequacy at our new community hospital include the presence of a consistent advanced practice provider (APP) in the Division of Gastroenterology, who was well trained in the new EHR protocol and had more time for early morning patient evaluation. In addition, structured nursing training at initial hospital orientation as well as proactive education likely contributed to better process and protocol adherence.
Cost analysis was performed for each patient who had more than 1 inpatient procedure due to inadequate prep. Along with the cost for performing the colonoscopy, it included anesthesia and the cost for a 1-day increase in length of stay. The average cost was estimated to be approximately $1,537 per repeat procedure. With the decrease in repeat inpatient colonoscopies due to inadequate prep during the same hospitalization, the cost savings at our tertiary care hospital was approximated to be $46,110 over the 15-month duration from these simple interventions alone. Assuming roughly half of inpatient colonoscopies would have been inadequate before order set implementation and standardization at the new hospital, the cost savings for the new hospital over an 11-month period was estimated to be $29,203 after 26% of the preps proved to be inadequate (Figure 6).
Protocol Decreases Inadequate Prep With Downstream Benefits
Our evaluation shows that a significant reduction in inadequate bowel prep for colonoscopy can be achieved by educating providers on the split-dose prep order set, early morning bowel prep assessment, and proactive additional same-day “salvage” prep. As a result,
References
1. Marshall JB. High quality bowel preparation - a cornerstone to the effectiveness of colonoscopy as a cancer prevention tool. ASGE Leading Edge. 2014;4(2):1-6.
2. Rex DK. Bowel preparation for colonoscopy: entering an era of increased expectations for efficacy. Clin Gastroenterol Hepatol. 2014;12(3):458-462.
3. Dik VK, Moons LMG, Hüyük M, et al. Predicting inadequate bowel preparation for colonoscopy in participants receiving split-dose bowel preparation: development and validation of a prediction score. Gastrointest Endosc 2015;81(3):665-672.
4. ASGE Technology Committee, Mamula P, Adler DG, et al. Colonoscopy preparation. Gastrointest Endosc 2009;69(7):1201-1209.
5. Ness RM, Manam R, Hoen H, et al. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol 2001;96(6):1797-1802.
6. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc 2010;72(4):686-692.
we revised our current bowel prep order set. However, we found that even though the prep was ordered as a split dose, documentation of intake times and tolerability initially was lacking. Therefore, we implemented an additional intervention to identify high-risk patients and give additional prep as needed.
Inadequate bowel prep can delay appropriate medical care, necessitate repeat procedures, increase length of stay and cost of care, and decrease patient satisfaction. Often, patients do not tolerate the preparation and experience significant side effects such as nausea and vomiting. With the implementation of a simple order set in the EHR for PEG-3350 split dosing as well as education for nurses and patients, we increased compliance and tolerability of bowel preparation, decreased healthcare resource utilization (hospitalization, repeat procedures, and more), and, therefore, improved quality of care and patient experience.
Conclusion
Implementation of a standardized order set as well as related EHR functionality is of paramount importance in any inpatient hospital facility where colonoscopy is performed. In our evaluation, outcome measures included improved nursing compliance, accountability with regard to complete documentation, improved frequency of provider assessment to evaluate for timely tolerability, and identification of high-risk features of poor bowel preparation. These optimizations improve patient experience, increase efficiency, decrease length of stay, and lower cost. Although significant opportunities for improvement related to preparation in colonoscopy exist, through process improvement our study demonstrated tremendous efficacy in the efforts employed to improve quality at lower cost.
7. Hassan C, Fuccio L, Bruno M, et al. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin Gastroenterol Hepatol 2012;10(5):501-506.
8. Calderwood AH, Schroy PC 3rd, Liebermann DA, et al. Boston Bowel Preparation Scale scores provide a standardized definition of adequate for describing bowel cleanliness. Gastrointest Endosc. 2014;80(2):269-276.
9. Borg BB, Gupta NK, Zuckerman GR, et al. Impact of obesity on bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2009;7(6):670-675.
10. Johnston E, Keswan R, Cyrus R, et al. The Nursing Bowel Preparation Assessment Tool (NBPAT) is highly predictive of inpatient bowel preparation adequacy: a prospective pilot study. Gastrointest Endosc. 2015;81(5 suppl):abstract 558.
11. Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25(4):373-384.
The authors reported no relevant financial disclosures.
GASTROENDONEWS.COM60
HARNESS THE POWER OF BOWEL CLEANSING ON DEMAND
Improve visualization, avoid delays, and achieve up to 98% successful procedure rates1

Suboptimal bowel preparation can have a devastating impact on the effectiveness of
The Pure-Vu® EVS System has the potential to dramatically impact patient
improve the efficiency of your facility, and save

Clear
path to success on
with
colonoscopies.
care,
money.
your
demand
Pure-Vu® EVS. Scan to learn more Reference: 1. Neumann H, Latorre M, Zimmermann T, et al. Evaluation of bowel cleansing efficacy in hospitalized patient population using the Pure-Vu system—the REDUCE study. Gastro Endo 2019;89(6):AB509. For more information about the procedure, indications, contraindications, warnings, and precautions, please contact MOTUSGI or consult the complete Instructions for Use (IFU) at www.motusgi.com. Pure-Vu is a registered trademark of Motus GI Holdings, Inc. © 2022 Motus GI Holdings, Inc. MK00051 RevA







References: 1. Hibi T, Ishibashi T, Ikenoue Y, Yoshihara R, Nihei A, Kobayashi T. Ulcerative colitis: disease burden, impact on daily life, an d reluctance to consult medical professionals: results from a Japanese internet survey. Inflamm Intest Dis . 2020;5:27-35. doi:10.1159/000505092 2. Petryszyn PW, Paradowski L. Stool patterns and symptoms of disordered anorectal function in patients with inflammatory bowel diseases. Adv Clin Exp Med. 2018;27(6):813-818. doi:10.17219/acem/68986 3. Gastroenterol Nurs. 2016;39(3): 195-203. doi:10.1097/SGA.0000000000000211 4. J Crohns Colitis. 2016;10(3):315-322. doi:10.1093/ecco-jcc/jjv218 PP-LI-US-0470 09/2021 © Lilly USA, LLC 2021. All rights reserved. ASKABOUTURGENCY.COM ONE SIMPLE QUESTION MAY MAKE A DIFFERENCE.1,2 *Studies were conducted in Japan (n=501) and Poland (n=71). † The study was conducted in Ireland with 247 patients; Crohn’s disease (n=162) and ulcerative colitis (n=85). SOME PATIENTS WITH ULCERATIVE COLITIS ARE TOO EMBARRASSED TO BRING UP BOWEL URGENCY. 1,2* LIKE SARA, WHO CHANGED JOBS BECAUSE OF HER COMMUTE. 1,3,4†
A #MondayNightIBD Conversation
ASUC: A Medical and Surgical Emergency Requiring Comprehensive, Timely Multidisciplinary Care

JOSHUA M. STEINBERG, MD
Director of Inflammatory Bowel Disease
Gastroenterology of the Rockies Denver, Colorado
@joshsteinbergMD
ALINE CHARABATY, MD, AGAF, FACG
Assistant Clinical Director, Division of Gastroenterology
Johns Hopkins School of Medicine Clinical Director, IBD Center
Johns Hopkins–Sibley Memorial Hospital Washington, DC


@DCharabaty
JEAN-PAUL ACHKAR, MD, FACG
Program Director, Gastroenterology Fellowship


Rainin Endowed Chair for IBD Department of Gastroenterology, Hepatology & Nutrition Cleveland Clinic Cleveland, Ohio

@JPAchkarMD
WADE M. BILLINGS, MD
Gastroenterology Fellow
Indiana University School of Medicine Indianapolis, Indiana
@wabillin
WHAT IS @MondayNightIBD?
@MondayNightIBD is an educational Twitter handle created by Aline Charabaty, MD (@DCharabaty), to bring together healthcare professionals to discuss complex IBD clinical case scenarios. On Monday afternoons, an IBD clinician posts a clinical vignette, with a management question linked to a poll listing several reasonable options. A special focus is placed on “gray areas” of IBD management, in which guidelines or randomized controlled trials and solid data are lacking, and on “real-life situations,” in which comorbidities, life events, social history, and other details need to be taken into account in decision making. Clinicians—from gastroenterologists to colorectal surgeons, nutritionists, and psychologists—tweet to discuss their approach to the clinical case, citing peer-reviewed articles and sharing their medical expertise.
This open forum allows for solid scientific discussions and medical education among peers from different areas of the country and the world, at different stages of their careers (trainees, seasoned clinicians, scientists, and academic and private practice clinicians) in a social media setting without the constraints of time and space. Free 1 AMA CME credit is offered with each #MondayNightIBD conversation. The opinions shared do not reflect medical advice.
On Wednesdays, a poll is offered to patients who wish to share their experience on the topic discussed by clinicians earlier in the week. The goal of these polls is to highlight patients’ experiences with IBD and help bring to light their unmet needs.
Acute severe ulcerative colitis is a well-described emergent complication of ulcerative colitis requiring hospitalization and aggressive supportive, medical, and potentially surgical management.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 63 PRINTER-FRIENDLY VERSION AVAILABLE AT GASTROENDONEWS.COM
Truelove and Witts first described and defined acute severe ulcerative colitis (ASUC) in 1955 with the following criteria: at least 6 bowel movements per day associated with rectal bleeding and 1 or more signs of systemic toxicity, including fever, anemia (hemoglobin <10.5 g/dL), tachycardia, or abnormal inflammatory markers (erythrocyte sedimentation rate >30 mm/hour).1 Both the American College of Gastroenterology and American Gastroenterological Association recently published new guidelines involving the diagnosis and management of ASUC,2,3 with updated criteria that include fecal urgency, fecal calprotectin, and an endoscopic score of disease activity (endoscopic Mayo score for UC and UC Endoscopic Index of Severity).3
Initial management of ASUC includes ruling out infectious colitis, particularly Clostridioides difficile and cytomegalovirus (CMV), as well as starting induction therapy with IV corticosteroids, followed by salvage therapies (traditionally infliximab or cyclosporine) and/ or colectomy.
In this article, we review the case of a young patient admitted to the hospital with ASUC who has not responded to IV corticosteroid therapy. This case and subsequent discussion were led on @MondayNightIBD by Jean-Paul Achkar, MD, with informed opinions from healthcare professionals (HCPs) who have a focus in inflammatory bowel disease (IBD), including gastroenterologists, colorectal surgeons, pharmacists, and GI trainees. We review the guidelines and literature on ASUC management, referring to the expert opinions of participants in our discussion and the answers to our #MondayNightIBD poll questions. We also propose an #IBDAlgorithm for the management of patients with ASUC (Figure).
Clinical Case Scenario
A 29-year-old female patient who has a history of UC and is biologic-naive is admitted to the hospital with ASUC. She has not had any meaningful clinical response after 3 days of IV corticosteroid therapy. Stool tesing for C. difficile is negative. The patient’s lab results are notable for an albumin level of 2.9 g/dL and a C-reactive protein (CRP) level of 15 mg/L. The patient undergoes flexible sigmoidoscopy, which reveals a Mayo score of 3 (severe colitis); biopsies do not indicate any signs of CMV colitis.
Our IBD polls asked participants about how they would approach medical (or surgical) management of this very sick patient with ASUC (Tables 1-3; see page 46).
Discussion
ASUC is a true medical emergency that requires multidisciplinary expert and comprehensive care to ensure favorable patient outcomes because short-term colectomy rates have been reported to be as high as 25% to 30%.4
Initial Evaluation
Patients admitted to the hospital with ASUC should have prompt evaluation including bloodwork, with a complete blood count to evaluate for leukocytosis and anemia, and a comprehensive metabolic panel to assess albumin, renal function, and electrolytes in the setting of active disease and fluid loss through frequent stooling. A baseline CRP should be obtained, and CRP should be followed longitudinally throughout the hospitalization for objective assessment of response to medical therapy. A baseline fecal calprotectin can be ordered, but given the prolonged time required to obtain results, it may not be feasible to rely on this assay to guide clinical decision making in real time. Finally, quantiferon gold and hepatitis B serology should be ordered in anticipation of potential infliximab use.
It is imperative that venous thromboembolism (VTE) prophylaxis with low-molecular-weight heparin be initiated, because hospitalized patients with IBD are at risk for developing VTE, with risk being associated with inflammatory burden and reduced mobility.5 Narcotics and anticholinergic agents should be avoided since they can precipitate or mask a toxic megacolon. Total parenteral nutrition should be avoided because of the high risk for catheter infection and upper extremity deep vein thrombosis. Instead, enteral nutrition should be optimized as tolerated. Patients at high risk for urgent surgery should be kept NPO (nothing by mouth). Multidisciplinary care and ongoing communication between the admitting hospitalist, consulting gastroenterologist, colorectal surgeon, nutritionist, and nursing staff are imperative, with close monitoring of the patient for any signs of clinical decompensation throughout the admission.
Ruling Out Infectious Colitis
On hospital admission, appropriate evaluation for infectious colitis is essential because superimposed CMV colitis and C. difficile colitis are commonly seen in these very ill patients.6 More than 50% of IBD patients infected with C. difficile require hospitalization, and 20% of these patients ended up undergoing colectomy.6 Therefore, it is central that stool studies be sent for C. difficile testing. Typically, enzyme-linked immunosorbent assays for toxins A and B or polymerase chain reaction testing are available in most hospitals. If C. difficile is detected, antibiotic therapy should be initiated immediately, with firstline treatment including oral vancomycin or fidaxomicin (Dificid, Merck) but not metronidazole.7
Cytomegalovirus colitis has been shown to affect potentially up to one-third of patients with ASUC who are refractory to corticosteroid therapy; the risk for CMV colitis increases with the presence of deep ulcerations on endoscopy, treatment with corticosteroids, and medically refractory disease.8 Therefore, it is imperative to proceed with flexible sigmoidoscopy for endoscopic evaluation early after admission, not only to assess disease activity and severity but also to assess and biopsy for CMV colitis. Although there has been an ongoing debate in the IBD community about the true pathogenic
GASTROENDONEWS.COM64
nature of CMV in patients with UC, IV antiviral therapy with ganciclovir should be started immediately in patients who have tested positive for CMV and have been refractory to IV corticosteroid or biologic therapy.8
Endoscopic and Radiographic Assessment

Flexible sigmoidoscopy also should be performed in patients admitted to the hospital with ASUC for disease activity assessment. Features suggestive of severe colitis, as per the Mayo score, include ulcerations and spontaneous bleeding with friable colonic mucosa.9 A kidney, ureter, and bladder x-ray or plain abdominal film should be ordered on admission to assess for colonic dilation and to evaluate for features of severe colitis, which include a thickened colonic wall and loss of haustrations. Monitoring for toxic megacolon, an uncommon complication of ASUC with high morbidity and mortality, should be done regularly during the hospital stay.2 In patients with severe abdominal pain and notable distention and tenderness on exam, CT of the abdomen and pelvis should be considered to rule out colonic perforation.10
Salvage Therapy
After initiation of IV corticosteroid therapy (typically the equivalent of 60 mg of IV methylprednisolone daily), patients with ASUC should be monitored closely for clinical response, particularly the frequency of bowel movements, blood in the stool, urgency, and abdominal pain. Also, inflammation should be objectively measured via CRP. A patient with ASUC is deemed refractory to steroid therapy if, by day 3, the number of bowel movements, blood in the stool, and CRP are not decreased by 50% compared with those seen at admission.
In our first IBD poll (Table 1), we asked what HCPs would do for a patient with ASUC refractory to IV corticosteroids, with options including infliximab, cyclosporine, tofacitinib (Xeljanz, Pfizer), or surgery. Traditionally, infliximab and cyclosporine are recommended as socalled salvage therapies in patients with IV corticosteroid–refractory ASUC after 3 days without meaningful clinical/CRP improvement because prolonged steroid use is unlikely to provide additional benefit. An overwhelming majority of the 339 poll responders (88.5%) voted that they would initiate infliximab therapy in this biologic-naive patient with ASUC.
A landmark trial randomly assigned 45 patients who had not responded to IV corticosteroids to receive either a single dose of infliximab (5 mg/kg) or placebo. Colectomy rates were 29% in the infliximab group versus 67% in the placebo group (P=0.017).11 Long-term follow-up data are favorable as well, with one Swedish study showing more than 50% of patients achieving steroid-free remission by 12 months and a 53% colectomy rate at 5 years.12
Alternatively, cyclosporine can be given as a continuous IV infusion (along with IV steroids), starting at 2 mg/kg over 24 hours, with target drug levels in the range of 200 to 400 ng/mL for maximum efficacy, with assessment of response by day 10.13 A landmark trial in
ASUC Management Pearls
• Assess at baseline: blood work, including CBC, CMP, CRP; infectious workup to rule out C. difficile (stool PCR) and CMV (flexible sigmoidoscopy with biopsies within 24-72 h); abdominal imaging with KUB x-ray
• Start IV hydration, enteral nutrition support, and DVT prophylaxis and get colorectal surgery consult
• Initiate IV corticosteroids (40-60 mg IV methylprednisolone daily)

• Assess clinical and CRP response by day 3. If no improvement, consider rescue therapy: infliximab vs cyclosporine, or surgery
• Monitor for complications requiring emergent surgery: perforation, toxic megacolon, and massive gastrointestinal bleeding
ASUC, acute severe ulcerative colitis; CBC, complete blood count; C. difficile,Clostridioides difficile; CMP, comprehensive metabolic panel; CMV, cytomegalovirus; CRP, C-reactive protein; DVT, deep vein thrombosis; KUB, kidney, ureter, and bladder; PCR, polymerase chain reaction.
which 20 patients with ASUC were randomly assigned to receive 4 mg/kg of cyclosporine or placebo demonstrated that 9 of 11 patients had a clinical response at an average of 7 days compared with 0 of 9 patients receiving placebo.14 HCP experience and comfort with cyclosporine are crucial, as are close monitoring of potential adverse effects, including nephrotoxicity, neurotoxicity, and infections. Patients who respond to IV cyclosporine are placed on an oral equivalent dose as well as oral prednisone, and historically have been bridged to maintenance thiopurine therapy. However, more recent data suggest that bridging to biologic therapy, such as ustekinumab (Stelara, Janssen) or vedolizumab (Entyvio, Takeda), has been a successful strategy in maintaining these patients in remission.15,16
Clinical trial data show similar efficacy rates and long-term colectomy-free survival for infliximab and cyclosporine as salvage therapy. Provider and center experience with either therapy should help drive decision making in these cases. Patients with low levels of albumin (<2.3 g/dL) may be considered for cyclosporine therapy, given the risk for infliximab drug loss in the stool. A post hoc analysis of a study comparing infliximab with cyclosporine showed treatment effects favoring infliximab in patients with albumin levels greater than 2.3 g/dL.17,18 Other patients who would benefit from cyclosporine more so than infliximab are those with infliximab failure before admission for ASUC and those at high risk for tuberculosis or hepatitis B reactivation. Alternatively, patients with lower serum cholesterol or magnesium are at greater risk for neurologic adverse events from cyclosporine therapy and should be considered for treatment with infliximab. Successive use of these therapies for ASUC has been shown to be successful in some patients, but it carries a higher risk for
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 65
infections and mortality and should be done only in tertiary care centers.19
In our second IBD poll (Table 2), participants were asked how they would proceed with respect to initial infliximab dosing and how they would manage this patient if she did not respond to the initial dose. Up to 68% of HCPs voted for starting infliximab at 10 -mg/kg, whereas 32% selected 5 mg/kg per day. More than half
ASUC Background
• Life-threatening medical emergency
• Initial presentation in one-third of UC patients
• One-third of patients do not respond to IV steroids
(53%) voted to follow the first 10 mg/kg dose by an early second infliximab dose of 10 mg/kg 5 days later. Dose optimization and accelerated infliximab induction are important concepts to consider in patients with steroid-refractory ASUC and extensive inflammatory burden, deep colonic ulcers, and low albumin. Indeed, these patients are at a higher risk for leaking biologic proteins in the stool and, therefore, not
ASUC Suspected
Truelove and Witts Criteria
≥6 bloody stools per day plus 1 of the following:
• Heart rate >90 beats/min
• HGB <10.5 g/dL
• Temperature >37.8°C
• ESR >30 mm/h or elevated CRP
• In pediatric cases, a PUCAI score >65
ACG UC Severity
≥6 stools per day ± blood with consideration of:
• Urgency
• FCP >150-200 mcg/g
• Mayo 2-3
• HGB <75% normal
• ESR >30 mm/h or elevated CRP
• UCEIS 5-8
Avoid
• NSAIDs
• Opioids
• Anticholinergics
Treatment
Outcomes
Early prediction of steroid nonresponse is a challenge:
• Mayo score of 3 or a UCEIS of ≥7 is predictive of need for surgery
• Low albumin and high CRP are predictive of steroid and infliximab nonresponse and need for surgery
• Infliximab clearance calculation may predict response and need for surgery
• Delay in surgery always raises the risk for postoperative complications
• Shared decision making with early surgical involvement is important
Supportive
• IV fluids, pRBC PRN
• EN as tolerated
• Electrolyte repletion
• DVT prophylaxis
IV Steroids
• 60 mg methylprednisolone per day equivalent
Surgery Consult
• On admission
Triggers
• CDI • CMV
Surgery Indications
• Refractory to medications
• Toxic megacolon
• Perforation
• Severe hematochezia
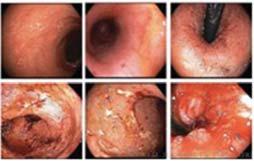
• Multi-organ dysfunction
a Dependent on prior exposures/failures.
ACG, American College of Gastroenterology; ASUC, acute severe ulcerative colitis; CBC, complete blood count; CDI, Clostridioides difficile infection; CMP, comprehensive metabolic panel; CMV, cytomegalovirus; CRP, C-reactive protein; DVT, deep vein thrombosis; EN, enteral nutrition; ESR, erythrocyte sedimentation rate; FCP, fecal calprotectin; HGB, hemoglobin; HBV, hepatitis B; IGRA, interferon-gamma release assays; KUB, kidney, ureter, and bladder; Mayo, endoscopic Mayo score for UC; NSAIDs, nonsteroidal anti-inflammatory drugs; PCR, polymerase chain reaction; PJP, Pneumocystis jiroveci pneumonia; PO, per os/by mouth; pRBC, packed red blood cell transfusion; PRN, pro re nata/as needed; PUCAI, Pediatric Ulcerative Colitis Activity Index; TID, 3 times daily; TPMT, thiopurine methyltransferase; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
Based on references 1-3 and Choy MC, Seah D, Faleck DM, et al. Systematic review and meta-analysis: optimal salvage therapy in acute severe ulcerative colitis. Inflamm Bowel Dis. 2019;25(7):1169-1186.
Figure 1. #IBDAlgorithm for the management of acute severe ulcerative colitis.
Infectious
GASTROENDONEWS.COM66
mounting appropriate therapeutic levels of infliximab to achieve clinical response.20 In a study evaluating the validity of accelerated infliximab dosing, patients who received 3 induction doses within a median period of 24 days had lower rates of colectomy (7%) versus standard dosing (40%), although colectomy rates at 3 months were similar.21 Overall, accelerated infliximab dosing can be considered in selected
patients, but routine use has not been established in ASUC guidelines.
Inpatient
ESR, CRP,
Lipids
Stool
KUB
Rule
CMV
Our third IBD poll (Table 3) asked participants how they would medically manage a patient with ASUC who was taking adalimumab (Humira, AbbVie) therapy before admission with an adequate drug trough level. Votes were almost equally split between starting infliximab therapy (39%) and starting a salvage therapy with a different mechanism of action (32% for tofacitinib and 20% for cyclosporine). In this patient population, current guidelines suggest consideration of cyclosporine as salvage therapy, with bridging to another biologic therapy with an alternative mechanism of action (eg, ustekinumab or vedolizumab), although more robust clinical trials are needed to further elucidate the longterm efficacy of this strategy.
Emerging Therapies in ASUC
Although infliximab and cyclosporine remain the gold-standard salvage therapies for ASUC, emerging data on the use of tofacitinib, an oral small molecule that inhibits the Janus kinase pathway of inflammation, has been presented. In our IBD polls, up to 6% of voters would consider tofacitinib if there is no response to the first 10-mg/kg infliximab dose, and 32% of HCPs reported they would start tofacitinib if the patient developed ASUC while taking adalimumab. Berinstein et al at the University of Michigan performed a retrospective case–control study exploring the efficacy of high-dose tofacitinib in patients who were admitted with ASUC and had previously failed biologic therapy.22 In the study, 40 patients were matched in a 1:3 fashion with controls who only received IV steroids. The investigators found that 10 mg of tofacitinib given orally 3 times daily along with IV corticosteroid therapy was protective against colectomy at 90 days, whereas 10 mg of tofacitinib twice daily was not significantly protective compared with steroids alone. However, rates of further steroid dependence and other complications did not significantly differ between groups. These data are promising and provide another medical therapy for the treatment of ASUC in selected patients, but further prospective studies are needed to validate these findings.
Surgery for ASUC
Only 9% of the voters considered colectomy in ASUC if there was no response to the first 10-mg/kg dose of infliximab. Colorectal surgery consultation is paramount in patients with ASUC and should be done on admission, and definitely by day 3 if there is no response to IV corticosteroids. Indications for urgent surgery in ASUC include toxic megacolon, perforation, uncontrolled severe hematochezia, and multi-organ dysfunction. Certain factors, such as young age, extensive disease and deep ulcers, and C. difficile or CMV infection, increase the risk for colectomy in ASUC and warrant close patient monitoring, decreasing the threshold to proceed with surgery if there is no adequate and timely response to a first salvage therapy. Delaying surgery in ASUC refractory
Lab ResultsImaging •
x-ray • ± CT scan Flexible Sigmoidoscopy With Biopsy • Within 24-72 h • Assess severity •
out
• CBC •
FCP • HBV •
• CDI algorithm •
culture • CMP • IGRA • TPMT Salvage and Alternative Therapy • Infliximab 5-10 mg/kg ± re-dose at 3-5 d • If there is a history of infliximab failure and lower albumin, turn to IV steroids plus: - Cyclosporine A induction with vedolizumab or thiopurine maintenance (PJP prophylaxis while on prednisolone and cyclosporine A and azathioprine) or - Tofacitinib 10 mg TID (small case series) • Surgery • Transition to PO steroids and start maintenance therapya • Lower risk for surgery at follow-up if response to steroids vs need for salvage therapy in ASUC • Vitals • Stool frequency • Blood in stool • Serial exams • CRP • KUB
Admission Monitoring Improvement in ≤3 d? No Yes GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 67
Table 1. IBD Poll 1:
29-year-old admitted with ASUC, not improved after 3 days of IV steroids, C. difficile–negative, albumin 2.9, CPR 15; patient has NOT been on biologics previously; flexible sigmoidoscopy shows Mayo Endoscopy Subscore 3, left-sided colitis; no CMV on biopsies. What would you do next?
Treatment OptionVote, % (N=339)
Start infliximab88.5 Start cyclosporine6.5 Start tofacitinib2.9 Recommend surgery2.1 ASUC, acute severe ulcerative colitis; C. difficile, Clostridioides difficile; CMV, cytomegalovirus; CPR, C-reactive protein
Table 2. IBD Poll 2:
If you decide to start infliximab, what would you use? What would you do if there is no response to the first dose of infliximab?
Treatment OptionVote, % (N=253)
5 mg/kg; repeat in 5 d31.6 10 mg/kg; repeat in 5 d53.4
10 mg/kg; cyclosporine or tofacitinib 6.3 10 mg/kg; surgery8.7
Table 3. IBD Poll 3:
What if the patient has been on adalimumab for 6 months before admission with secondary loss of response despite a good trough level. What would you do next?
Treatment OptionVote, % (N=262)
Start infliximab39.3 Start cyclosporine20.2 Start tofacitinib31.7 Recommend surgery8.8




to medical therapy increases the risk for postoperative complications.23 Colectomy should not be deferred if the patient had received infliximab or cyclosporine because such drug exposure does not increase the risk for postoperative complications. In the acute setting, colectomy is performed, followed by proctectomy and ileal pouch–anal anastomosis at a second stage a few months later, after the patient’s acute condition has stabilized.
In the #PatientExperience poll (Table 4), when patients were asked whether they would choose surgery or further medical therapy to treat complicated IBD, when further medical therapy had a low chance of success, 51% of 180 respondents opted for surgery. Far fewer (17%) would choose further medical intervention, and 32% recognized this would be a difficult decision to make. These results highlight the importance of raising the subject of surgery with the patient early and having a balanced discussion about the risks, benefits, and appropriate timing of surgery, so a shared decision can be reached with the patient’s best interests in mind.
Table 4. #PatientExperience Poll:
If your physician recommends surgery for the treatment of IBD or an IBD complication because the chances of more or different medications helping the disease are low, would you:
Treatment OptionVote, % (N=180)
Proceed with surgery51 Try more medications17 Not sure; tough decision32
Conclusion
ASUC is a true medical and surgical emergency that requires a comprehensive, multidisciplinary, and timely diagnostic and therapeutic approach to optimize patient outcomes. Gastroenterologists’ experience and certain clinical and patient characteristics, such as prior use of anti-TNF, albumin level, and patient preference, should guide the choice of salvage therapy in ASUC refractory to IV steroids. Our -#MondayNightIBD polls revealed that HCPs typically consider salvage therapy with high-dose and accelerated dosing of infliximab. However, the polls also highlight a reluctance to consider colectomy as the next option in patients who are refractory to infliximab, which could lead to an unnecessary delay in surgery and increased morbidity and mortality. Although different medical therapy options are available to gastroenterologists, it is paramount to involve the colorectal surgeon early in patients admitted with ASUC, and not delay surgery when it is indicated.
GASTROENDONEWS.COM68
1. Truelove SC, Witts LJ. Cortisone in ulcerative -colitis; final report on a therapeutic trial. Br Med J. 1955;2(4927):1041-1048.
2. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450-1461.
3. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114(3):384-413.
4. Narula N, Marshall JK, Colombel J-F, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol. 2016;111(4):477-491.
5. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375(9715):657-663.
6. El Ouali S, Achkar JP. Management of hospitalized patients with inflammatory bowel disease and CMV infection or Clostridium difficile infection. In: Feuerstein JD, Cheifetz AS, eds. Management of Inpatient Inflammatory Bowel Disease. Springer; 2022:161-180.
7. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society Of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029-e1044.
8. Sager K, Alam S, Bond A, et al. Review article: cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther 2015;41(8):725-733.
9. Pabla BS, Schwartz DA. Assessing severity of disease in patients with ulcerative colitis. Gastroenterol Clin North Am 2020;49(4):671-688.
10. Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther. 2016;43(4):482-513.
11. Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128(7):1805-1811.
12. Sjöberg M, Magnuson A, Björk J, et al. Infliximab as rescue therapy in hospitalised patients with steroid-refractory acute ulcerative colitis: a long-term follow-up of 211 Swedish patients. Aliment Pharmacol Ther. 2013;38(4):377-387.
13. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94(6):1587-1592.
14. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994;330(26):1841-1845.
15. Shaffer SR, Traboulsi C, Krugliak Cleveland N, et al. Combining cyclosporine with ustekinumab in acute severe ulcerative colitis. ACG Case Rep J. 2021;8(5):e00604.
16. Christensen B, Gibson PR, Micic D, et al. Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol 2019;17(3):486-493.
17. Seagrove AC, Alam MF, Alrubaiy L, et al. Randomised controlled trial. comparison of infliximab and ciclosporin in steroid resistant ulcerative colitis: trial design and protocol (CONSTRUCT). BMJ Open. 2014;4:e005091.
18. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised -controlled trial. Lancet. 2012;380(9857):1909-1915.
19. Steinberg JM, Cohen NA, Rodriguez TG, et al. -Successive use of infliximab and cyclosporine as salvage therapy in acute severe ulcerative colitis [ACG abstract S2442]. Am J -Gastroenterol. 2021;116(suppl):S1033.
20. Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. -Gastroenterology 2015;149(2):350-355.e2.
21. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13(2):330-335.e1.
22. Berinstein JA, Steiner CA, Regal RE, et al. Efficacy of induction therapy with high-intensity tofacitinib in 4 patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2019;17(5):988-990.e1.
23. Randall J, Singh B, Warren BF, et al. Delayed surgery for acute severe colitis is associated with increased risk of postoperative complications. Br J Surg. 2010;97(3):404-409.
References
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 69
EXPERT PICKS
Expert Picks From DDW 2022: IBD
Dana J. Lukin, MD, PhD

Abstract 886. Guselkumab maintenance





‘Longer-term data on the maintenance arm of the VEGA trial will help fully inform safety and efficacy data.’


Abstract 584. IV vedolizumab and chronic pouchitis




‘This is the first randomized controlled trial to demonstrate a benefit with biologic therapy in pouchitis, and there was a clear benefit with vedolizumab over placebo for several clinical and endoscopic end points.’
Thisissue’s installment of “Expert Picks” is from Dana J. Lukin, MD, PhD, the clinical director of translational research at the Jill Roberts Center for Inflammatory Bowel Disease and the director of the advanced fellowship program in IBD at NewYork-Presbyterian/Weill Cornell Medicine, in New York City. As a gastroenterologist with clinical expertise in IBD, Dr. Lukin selected his favorite research from the 2022 Digestive Disease Week centered on the treatment of IBD.
GASTROENDONEWS.COM70
Some immunosuppressive treatments may still have a favorable safety profile for older adults with IBD, especially those without comorbidities, according to this study. Using data from a commercial insurer in the United States, researchers from Massachusetts General Hospital and Harvard University’s T.H. Chan School of Public Health, in Boston, identified patients 60 years of age and older with Crohn’s disease or ulcerative colitis who were newly taking any anti–tumor necrosis factor (TNF) medications, vedolizumab (Entyvio, Takeda) or ustekinumab (Stelara, Janssen). The primary outcome was infection-related hospitalizations, and the secondary outcome was an occurrence of any infection.
The final analysis included 2,369 patients on anti-TNF therapy, 972 on vedolizumab and 352 on ustekinumab. The investigators found a significant relationship between the risk for hospital-related infection and the presence of comorbidities, as defined by the Charlson Comorbidity Index (CCI). Among patients with a CCI
greater than 1, both ustekinumab (hazard ratio [HR], 0.66; 95% CI, 0.46-0.91; P<0.01) and vedolizumab (HR, 0.78; 95% CI, 0.65-0.94; P=0.02) were associated with a significantly lower risk for infection-related hospitalizations compared with anti-TNF therapy. In contrast, there was no difference in risk among patients without significant comorbidities (CCI ≤1).
Dr. Lukin: These data suggest that patient selection is important in determining the course of therapies for older adults with IBD and provide reassurance that TNF antagonist therapy is a viable therapeutic option for patients without significant comorbidities. These results also underscore a favorable safety signal for ustekinumab and vedolizumab among older adults, regardless of comorbidity. Limitations of this study are its retrospective design and use of an insurance claims database, which lacks granular patient and disease activity score detail.
Previous research established the efficacy of switching patients receiving standard doses of intravenous infliximab (Remicade, Janssen) to a subcutaneous version. This study, by French researchers, examined the feasibility of switching patients on an intensified maintenance dose of infliximab to subcutaneous infliximab. The study included 130 IBD patients in clinical remission based on a Clinical Disease Activity Index score of less than 150 or a partial Mayo score of 2 or higher. Regardless of their IV dose, the patients were switched to 120 mg of subcutaneous infliximab every two weeks.
Clinical relapse occurred in 11% of the study patients, including 40% of those previously receiving 10 mg/kg every four weeks (versus 8.6% on 10 mg/kg every eight weeks). A subcutaneous dose increase to 240 mg every two weeks induced clinical remission in 92.3% of patients who relapsed.
Dr. Lukin: This was a European study on switching patients in clinical remission on more than standard doses of IV infliximab to a subcutaneous version. Although the subcutaneous drug is not yet FDA approved, I find this study very interesting. Currently, one of the big drawbacks of infliximab is that it’s an IV medication. It has
already been shown that subcutaneous infliximab works very well compared with the IV dosing among patients on standard maintenance regimens.
This study specifically looked at whether an escalated dose was required in patients who were in -remission but on higher than standard doses of -infliximab. Study inclusion was based on clinical -parameters but not biomarkers or endoscopy, which is a limitation. The investigators analyzed clinical relapse rates according to previous maintenance regimens (10 mg/kg every four, six or eight weeks), as well as response to dose intensification during the followup period. Importantly, only 11% of patients experienced a clinical relapse on the subcutaneous regimen. As expected, patients receiving the highest prior dosing were at greatest risk for relapse, but, fortunately, most were responsive to a doubling of the subcutaneous maintenance dose. These data -suggest that the significant majority of patients in clinical remission on intensified IV infliximab for IBD were successfully transitioned to standard subcutaneous dosing and also demonstrate that a regimen of 240 mg every two weeks is effective for most patients who experience relapse after the transition.
Abstract 455. Comorbidity influences the comparative safety of biologic therapy in older adults with inflammatory bowel diseases (Kochar et al)
Abstract 718. Evolution of clinical and pharmacological parameters after switching from intravenous to subcutaneous infliximab in patients with inflammatory bowel disease: the REMSWITCH study (Buisson et al)
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 71
Abstract 888. Efficacy and safety of guselkumab maintenance therapy in patients with moderately to severely active Crohn’s disease: week 48 analyses from the phase 2 GALAXI 1 study (Panaccione et al)
Abstract 886. Efficacy and safety of combination induction therapy with guselkumab and golimumab in patients with moderately-to-severely active ulcerative colitis: results through week 12 of the phase 2a VEGA study (Feagan et al)
Two major trials using guselkumab (Tremfya, Janssen) were presented at DDW22.
The GALAXI phase 2 trial evaluated the efficacy and safety of guselkumab, a selective interleukin-23 P19 subunit antagonist, in 248 patients with severe and active Crohn’s disease and resistance to conventional therapies. Patients received IV induction and then maintenance dosing over 48 weeks, and ustekinumab was used as an active comparator. At the end of the study, clinical remission ranged from 57.4% to 73.0% across different dose groups and discontinuation rates were low.
The phase 2a VEGA trial evaluated IV guselkumab and the TNF antagonist golimumab (Simponi Aria, -Janssen) separately and in combination as induction therapy in ulcerative colitis. More than 200 patients naive to TNF antagonists and intolerant or refractory to conventional therapy received 200 mg of guselkumab at weeks 0, 4 and 8, 200 mg of golimu-mab at week 0 and 100 mg at weeks 2, 6 and 10, or a combination induction treatment with both medications. The primary end point was clinical response at week 12, and clinical remission at week 12 was a key secondary end point.
Combination induction treatment with guselkumab and golimumab more effectively induced clinical response (combination, 83.1%; guselkumab, 74.6%; golimumab, 61.1%) and clinical remission (36.6%, 21.1% and 22.2%, respectively). When clinical response and clinical remission were assessed using the modified Mayo score, there were significant differences between combination
therapy and golimumab monotherapy but not between combination and guselkumab monotherapy. No safety concerns were identified during the 12-week induction period.
Dr. Lukin: These studies demonstrate promising efficacy data for guselkumab in both Crohn’s disease and ulcerative colitis. The GALAXI trial reported strong data for guselkumab across induction and maintenance periods, with good tolerability and comparable efficacy to ustekinumab. Interestingly, there was no dose-response observed, so optimal dosing of guselkumab may need to be determined.
One significant clinical challenge is to “raise the therapeutic ceiling” of IBD therapies. A potential way to accomplish this is to devise rational combination regimens. The VEGA study demonstrates a feasible strategy for combination therapy including guselkumab and a TNF antagonist in ulcerative colitis. Combination therapy was more effective than golimumab monotherapy in induction of clinical response and other outcomes, without new safety signals. Such data with combination therapies are needed to demonstrate both increased efficacy and continued safety, and this study also will provide rationale to payors that combination strategies can produce favorable results for patients. Longer-term data on the maintenance arm of the VEGA trial will help fully inform safety and efficacy data.
The ELEVATE UC-12 and UC-52 trials evaluated the efficacy and safety of etrasimod (Pfizer), a novel oral selective sphingosine 1-phosphate (S1P) receptor modulator, in ulcerative colitis. Patients with moderate to severe ulcerative colitis (Modified Mayo Score between 4 and 9, centrally read endoscopic score ≥2 and rectal bleeding score ≥1) with previous exposure to at least one treatment were randomly assigned in a 2:1 fashion to receive 2 mg of etrasimod or placebo daily.
Patients with an inadequate response to induction therapy at week 12 could cross over to the 12-week, open-label ELEVATE UC-12 trial. Week 12 responders in ELEVATE UC-52 were continued on therapy until week 52. The primary end point was clinical remission
using Modified Mayo Score at weeks 12 and 52 in ELEVATE-52 and at week 12 in ELEVATE-12. Clinical remission was 27% with etrasimod and 7.4% for placebo at induction (week 12) (P<0.001) and 32.1% and 6.7% at week 52 (P<0.001).
Dr. Lukin: Oral small-molecule therapeutics for IBD are considered highly desirable by patients and clinicians. Etrasimod has demonstrated strong efficacy data in both induction and maintenance, with a clinically meaningful separation from placebo at one year. One advantage of etrasimod is the positioning of this therapy, which does not require prior exposure to a TNF antagonist. This distinguishes it from Janus kinase inhibitors
Abstract 968a. Etrasimod 2 mg once daily as treatment for moderately to severely active ulcerative colitis: results from the phase 3 ELEVATE UC-52 and ELEVATE UC-12 trials (Sandborn et al)
GASTROENDONEWS.COM72
as an oral agent that potentially can be given as firstor second-line therapy. The very low placebo response rate suggests the trial was well designed and that there
is likely a strong treatment effect. Based on these data, etrasimod may become the second S1P receptor modulator approved for ulcerative colitis.
Combination therapy with TNF antagonists and an immune modulator for moderate to severe Crohn’s disease is widely believed to increase therapeutic efficacy and decrease rates of immunogenicity, but it is associated with increased cost, medication burden and potential for adverse effects compared with monotherapy. The duration of combination therapy required for treatment effectiveness and whether deescalation to monotherapy with one of the agents is possible are unknown. Researchers from seven countries studied the effect of withdrawing infliximab or an antimetabolite in patients with sustained remission on combination therapy.
More than 200 patients from 64 centers across Europe, the United Kingdom and Australia were included. All participants had been taking combination therapy for at least eight months and were in steroidfree clinical and endoscopic remission for six months or more. The two co-primary end points were relapse rate and time spent in remission over two years. The relapse rate was 14% for patients who maintained both
therapies, 40% for those who stopped infliximab and 10% for those who stopped the anti-metabolite.
Dr. Lukin: This study assessed withdrawal of medications among patients in remission, a topic of great interest to both clinicians and patients. This is a welldesigned prospective study using objective criteria to assess for relapse and providing an algorithm for retreatment after relapse. This study nicely addresses prior data on withdrawal of either infliximab or azathioprine within a design with three arms, and the results reinforce the importance of continuing the biologic therapy as long-term maintenance and the pitfalls of relying on an immune modulator as monotherapy for this population. These data also suggest the benefit of combination therapy is likely early in the induction and maintenance period, with withdrawal of immune modulators well tolerated once stable remission is achieved. Reassuringly, patients in the immune modulator maintenance arm who experienced relapse responded well to re-treatment with infliximab.
Abstract 584. Vedolizumab intravenous is effective across multiple treatment targets in chronic pouchitis: results of the randomized, double-blind, placebo-controlled EARNEST trial (Travis et al)
Pouchitis is a common clinical presentation among patients undergoing restorative proctocolectomy for ulcerative colitis, with over 50% of patients experiencing at least one episode of acute pouchitis and up to 20% having chronic pouchitis. There are no FDA-approved therapies for chronic antibiotic-dependent pouchitis.
In the phase 4 EARNEST trial, investigators randomly assigned 102 patients with ileal pouch–anal anastomosis for ulcerative colitis and chronic pouch-itis to receive vedolizumab dosed at standard intervals (weeks 0, 2, 6, 14, 22 and 30) or placebo in a 1:1 ratio. All patients received ciprofloxacin for four weeks. The primary outcome was clinical remission as defined by the modified Pouchitis Disease Activity Index (mPDAI).
At week 14, 31.4% of the vedolizumab arm and 9.8% of those receiving placebo acheived remission per mPDAI (P=0.013). In addition, and significant differences favoring vedolizumab were seen in the following
parameters: remission at week 34, response at weeks 14 and 34, full PDAI remission, and reduction in the number of ulcers from baseline. No key differences in adverse events were observed between groups.
Dr. Lukin: This is the first randomized controlled trial to demonstrate a benefit with biologic therapy in pouchitis, and there was a clear benefit with vedolizumab over placebo for several clinical and endoscopic end points. Despite colectomy, chronic pouchitis is likely a manifestation of continued IBD activity in this population; therefore, effective disease-modifying therapies often are needed to allow for symptom control and improvement in the quality of life of patients with pouchitis. These data are important to clearly demonstrate the efficacy of vedolizumab in this population. Additional studies are necessary to investigate the potential benefit of other IBD therapies in patients with chronic pouchitis.
—Written and compiled by Donavyn Coffey
Dr. Lukin reported financial relationships with AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Lilly, Janssen, Palatin, Pfizer and Takeda. He is a member of the Gastroenterology & Endoscopy News editorial board.
Abstract 887. Withdrawal of infliximab or anti-metabolite therapy in Crohn’s disease patients in sustained remission on combination therapy: a randomized unblinded controlled trial (SPARE) (Louis et al)
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 73

















www.driscope.com family of products provides HEPA-filtered air through all internal channels of your endoscope. Convert your existing scope cabinet into an automatic scope drying system. Also available pre-installed in new cabinets. Co (1840 Series Wall Pump pictured) binets. Available in 5, 8, 10 or 16 scope configurations. (23 Series 5 port Manifold pictured) nto Helps you exceed the Multisociety Task Force guidelines! The right choice for instrument channel drying after the AER. Simultaneously process one or two endoscopes. Automatic pressure adjustments from Cystoscope to Colonoscopes. Scope hangers hold up to four scopes at once. Eight side pockets hold disposable connectors or other supplies. Space saving collapsible shelf for additional work area. Available for use with ambient, medical or instrument air. Jet~Cart drying station (Scopes and trays not included) www.dr The A Simultane Automatic Colonosc h sid other su sa (Scopesand trays not no
AAMI Releases New Scope Processing Guidelines

Societies Decline Endorsement

In March 2022, the Association for the Advancement of Medical Instrumentation issued its long-awaited update to its guidance on cleaning and storing flexible and semirigid endoscopes.




GI
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 75 PRINTER-FRIENDLY VERSION AVAILABLE AT GASTROENDONEWS.COM
In a statement announcing the new guidance, AAMI noted that it “has been updated to reflect the consensus of industry, clinicians, and sterilization professionals from around the world.” But a response from seven leading gastroenterology societies, all of which participated in the AAMI Endoscope Reprocessing Working Group’s discussion process for the new guidelines, called that consensus into question.

“Changes to ST91 for flexible and semi-rigid endoscopes create obstacles to implement standards and offer impractical, inappropriate or conflicting guidance,” said a joint statement from the American Association for the Study of Liver Diseases, American College of Gastroenterology, American Gastroenterological Association (AGA), American Society of Colon and Rectal Surgeons, American Society for Gastrointestinal Endoscopy (ASGE), Society of American Gastrointestinal and Endoscopic Surgeons, and Society of Gastroenterology Nurses and Associates, first published online in Gastrointestinal Endoscopy on March 11, 2022 (doi:10.1016/j.gie.2022.02.004).
Recommendations Lack Evidence
“We believe the document fails in three main areas,” explained Lukejohn Day, MD, FASGE, a professor of medicine at the University of California, San Francisco and the chief medical officer at the Zuckerberg San Francisco General Hospital, who served as ASGE’s representative to the AAMI working group for the past three years.
“First, it is not data-driven and does not have a great deal of evidence to support some of the recommendations that are made, which is in contrast to the Multi-Society Task Force reprocessing guidelines” released last year (Gastrointest Endosc 2021;93[1]:11-33. e6), Dr. Day said in an interview.
Dr. Day, who was a lead author of those guidelines,

noted that AAMI did not employ the commonly accepted GRADE (Grading of Recommendations, Assessment, Development and Evaluations) framework, a widely adopted tool for grading the quality of evidence and making practice recommendations.
“GRADE includes a clear scan of the environment for all of the relevant papers, recommendations, and has levels of meaning associated with them, along with the significance of the evidence supporting that recommendation,” said Michael Kochman, MD, AGAF, the director of the University of Pennsylvania’s Center for Endoscopic Innovation, Research and Training, in Philadelphia, and the AGA’s representative to the working group. “Quality is graded along with the importance of the recommendation itself. That was completely lacking in the AAMI process,” Dr. Kochman added.
A second problem area cited by the GI societies was the impracticality of some of the implementation strategies, which Dr. Day said were infeasible and not considered standard best practices.
“One example is the recommendation that end users should bring together the biopsy caps, air and water buttons, and endoscopes to form one collective set that would undergo reprocessing, or if not possible, label each of these devices and accessories in order to track them throughout the reprocessing pathway,” he said. “There is no evidence to suggest that this is a best practice, reduces infection risk or improves overall patient outcomes. Furthermore, it’s also not practical to implement these practices in endoscopy centers. Tracking scopes is definitely done from start to finish, but accessories like buttons and biopsy caps that go through reprocessing are not tagged/labeled as a scope is. There are no data or studies to suggest that implementing this practice would change overall patient outcomes or minimize infectious risks to patients.”
Another problematic recommendation, the multi-society statement said, is the use of cleaning verification tests after each use for all high-risk endoscopes, pointing out that these tests aren’t approved for this use by the FDA and have not demonstrated clear outcome metrics. “The FDA has not made any determination that any of these cleaning verification tests can do anything that is claimed in the AAMI guideline. We shouldn’t make such a recommendation to the end users when the data is not there,” Dr. Day said.
The guidelines also recommend the use of borescopes in endoscope inspection. “Borescopes can detect certain findings. The significance of those findings is not always clear. The manufacturers don’t recommend it, and we have to be careful about implementing techniques that don’t have clear levels of evidence supporting their use, as we also need to be fiscally and practically responsible to society with
‘We cannot endorse the guideline, but it is a published standard, and it can be utilized.’
—Michael Kochman, MD, AGAF University of Pennsylvania Center for Endoscopic Innovation, Research and Training, Philadelphia
GASTROENDONEWS.COM76
solutions that are data proven,” Dr. Kochman said.
Finally, the societies also raised concerns about the transparency of AAMI’s process. “We felt that there were some conflicts of interest, and that many members of the working group represented for-profit industries whose devices could potentially benefit from some of the recommendations made in the guideline,” Dr. Day said.
Lack of Transparency
Those potential conflicts were an issue from the beginning, said Bret Petersen, MD, MASGE, a professor of medicine in the Division of Gastroenterology and Hepatology at Mayo Clinic in Rochester, Minn., who preceded Dr. Day as ASGE’s representative to the working group. “There was not much transparency around the table, nor acknowledgment of conflicts when discussing specific issues. That did improve over time, but not to the degree that most medical practitioners are familiar with, wherein conflicts are clearly disclosed and individuals must often recuse themselves from some discussions based on those conflicts. It’s fair to say that the participation of the physicians’ organizations instilled some ongoing discussion of these issues, which did improve the process. Of course, some would argue that the medical societies are also conflicted, but I hope that our stake in the process is the outcomes for our patients.”
In the press release announcing the new version of ST91, working group co-chair Garland-Rhea Grisby, an endoscope service manager for Kaiser Permanente in Northern California, said sterilization professionals often ask for more stringent requirements. “When they want to introduce change in their facility that can improve safety, they might get pushback from leadership because there are few guidelines out there that say what they should do or how to do it,” he said. “That’s why they need this document to back up and outline what needs to be done.”
AAMI Maintains Process Was Valid
AAMI provided a statement to Gastroenterology & Endoscopy News for this article. “Our standards are developed in accordance with our American National Standards Institute-accredited procedures, ensuring due process, openness and transparency,” wrote Amanda Benedict, the vice president of standards. “The process for ST91:2021 considered and responded to the views of all stakeholders involved in developing the document, and the final standard reflects consensus among experts and stakeholders that the requirements will improve patient safety. Patient safety is our north star, and we collectively stand behind the process that delivers needed change.”
Dr. Kochman stressed that the GI societies are not telling any organization not to follow the AAMI guideline. “We cannot endorse the guideline, but it is a
2022 ANSI/AAMI ST91 Guidelines Overview
The new guidance, ANSI/AAMI ST91:2021 Flexible and Semi-Rigid Endoscope Processing in Health Care Facilities, which replaces the 2015 version, took an unusually long time to develop, with the process originally beginning in 2017. It includes:
• classification for high-risk scopes, such as bronchoscopes and ureteroscopes;
• updated guidance for drying of scopes, as well as proper storage and handling;
• recommendations against manual highlevel disinfection after washing, as opposed to in automated endoscope reprocessors (AERs);
• guidance for testing water in AERs to avoid the final-rinse water recontaminating the endoscopes; and
• guidance for determining the length of storage, or “hang time,” that a scope can withstand before needing to be reprocessed.
AAMI, Association for the Advancement of Medical Instrumentation; ANSI, American National Standards Institute.
published standard, and it can be utilized.”
“There are multiple guidelines related to scope reprocessing. Some institutions follow the multisociety guidelines, some follow AAMI, and others may implement more than one, with their own selected differences between them, which should be explicitly delineated,” Dr. Petersen said. “The requirement for any single institution is to identify which guidelines they intend to follow, and then to demonstrate that they are doing so for their Joint Commission reviews and accreditation.”
—Gina Shaw
Dr. Day reported financial relationships with 3T Biosciences, Boehringer Ingelheim and Pfizer. Dr. Kochman reported financial relationships with Applied Clinical Intelligence, Boston Scientific, Dark Canyon Labs, Medtronic, Novella, Olympus, Pentax and Virgo Systems.
Dr. Petersen reported financial relationships with Ambu, Boston Scientific, Olympus and Pentax.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 77
Reports of Endoscope Contamination Rose Significantly Since 2014, New Analysis Says Enhanced vigilance and more procedures likely contributed to the increase
Adverseevent reports submitted to the FDA, describing the actual or potential contamination of a reprocessed flexible endoscope, have increased significantly over the past seven years, according to a review and analysis.

‘Precleaning starts immediately after each procedure is finished, and manual washing begins within one hour. We’ve been quite pleased with our processes, and I think having qualified techs who are dedicated to each procedure room and making sure there is no lag time have really helped us.’


—Linda Hubbard, RN, Endoscopy Center of Western New York
The report, released in January by Lawrence Muscarella, PhD, the president of LFM Healthcare Solutions and a national authority on the causes and prevention of healthcare-related infections, included more than 10,000 adverse event reports describing contamination of a flexible endoscope submitted to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database between 2014 and 2021. Dr. Muscarella focused on three GI endoscopes (colonoscopes, duodenoscopes and gastroscopes), as well as bronchoscopes, otorhinolaryngologic endoscopes and urologic endoscopes.
GASTROENDONEWS.COM78
Although duodenoscopes have received particular scrutiny for their association with transmission of carbapenem-resistant Enterobacteriaceae (CRE) and other superbugs, the 804% increase in adverse event reports submitted to the FDA for these endoscopes was lower than for any other type of endoscope except bronchoscopes, which increased from 52 in 2014 to 259 in 2021 (or by 398%).
“Although FDA and others in recent years have diligently worked to reduce the contamination and infection risk of reprocessed duodenoscopes and other endoscope types, the findings of this analysis suggest that use of a duodenoscope remains a risk factor for patient exposure to multidrug-resistant organisms including CRE, warranting continued vigilance and care to ensure patient safety,” Dr. Muscarella wrote. “That said, this analysis found that the number of reports submitted to FDA linking a duodenoscope to patient deaths was significantly less in 2021 than just a few years ago.”
The analysis’s recommendations included the suggestion that healthcare providers consider “sterilization of gastroscopes (when feasible), or the use of a singleuse gastroscope (when available and as warranted) to improve patient safety.” Dr. Muscarella recommended performing a risk assessment to help guide this consideration. He also recommended that health experts evaluate whether the issuance of a letter or alert informing healthcare providers of the risk for “reprocessed” gastroscopes transmitting multidrug-resistant bacteria, along with providing evidence-based practices that reduce this risk, is now warranted.
More Procedures and Testing Led to Some of the Reports
In an interview,Dr. Muscarella acknowledged that at least some of these increases in adverse event reports associated with endoscopes could be attributable to more procedures being performed and a higher level of vigilance that began in 2014 after U.S. health officials, for the first time, linked a duodenoscope at a single facility to multiple infections of CRE at a hospital in northern Illinois.
“On the heels of this and other outbreaks, the FDA ordered manufacturers to perform surveillance testing of duodenoscopes, and we’re seeing this type of testing being performed more now on other types of scopes, too. There also may be better compliance with medical device reporting regulations, so an increase in submitted reports does not necessarily translate into an increase in the contamination and infection risk,” he said. “Surveillance testing, in which endoscopes are randomly sampled and cultured after reprocessing without there necessarily being an infection or an outbreak, is more commonly being performed in hospitals today compared to years ago. If we don’t put something under the microscope to examine it, then we may never see its fine details. Now there’s closer examination of what’s going on.”
Potential Factors Impeding Cleaning and Disinfection
Dr. Muscarella described a few factors that he identified in reviewing the MAUDE reports that could interfere with the effectiveness of high-level disinfection and even sterilization. “With duodenoscopes, we learned that the complexity of their design can impede cleaning,” he said. “But another issue has to do with limited staff and financial resources and the problem of continuing to use a damaged endoscope unwittingly, or not maintaining, repairing and servicing instruments according to the manufacturers’ instructions and maintenance schedules.”
His analysis noted that several relatively recent adverse event reports discuss the deterioration of an adhesive, sealant or glue used at the distal end of some flexible endoscope types, causing a gap or crack to form in the adhesive and resulting in the potential for microbial contamination and an increased infection risk due to ineffective reprocessing. “In November of 2021, a duodenoscope model was recalled in part for this ‘adhesive deterioration’ phenomenon, but I found the problem is not unique to that one scope,” Dr. Muscarella said.
“There are stresses occurring at the distal end of the scope, which can be due to a number of factors including mishandling—like banging the endoscope inadvertently on a hard surface. This can cause cracking, chipping and gapping, almost like eating something hard and chipping an acrylic filling on your tooth, and then there’s a gap where bacteria could invade and accumulate. High-level disinfection of endoscopes is effective, provided certain criteria are satisfied, but the process can be challenging to achieve if the endoscope is damaged, a component becomes loose, the endoscope is particularly complex in design, or the endoscope is not sufficiently serviced and maintained.”
Need to Consistently Follow Manufacturers’ Guidelines
Dr. Muscarella’s findings with regard to gastroscopes are “troubling,” said Deepak Agrawal, MD, MPH, an associate professor of gastroenterology and hepatology at Dell Medical School at The University Texas at Austin. “If this is true, we would need to change what we are doing. But there are a few things that remain to be clarified.
‘Before we pursue something like sterilizing scopes or going to disposable scopes, which are cost-prohibitive and environmentally damaging, we should work further to mitigate risk with consistent reprocessing.’
—Deepak Agrawal, MD, MPH Dell Medical School at The University of Texas at Austin
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 79
First, the MAUDE reports do not necessarily correlate contamination with infection, when patients are actually getting sick. Those things are very difficult to objectively prove with case reports, except for well-published case studies where they can trace one [type of] bacteria arising from the scope and see the same bacteria in other patients. In our practice, we do not see this.”
Dr. Agrawal also cited lapses in following manufacturers’ reprocessing guidelines. “It’s clear that there were breaches in protocol for some of these events,” he said. “What would these numbers look like if reprocessing guidelines were consistently followed the way they are written? Before we pursue something like sterilizing scopes or going to disposable scopes, which are cost-prohibitive and environmentally damaging, we should work further to mitigate risk with consistent reprocessing.”
‘High-level disinfection of endoscopes is effective, provided certain criteria are satisfied, but the process can be challenging to achieve if the endoscope is damaged, a component becomes loose, the endoscope is particularly complex in design, or the endoscope is not sufficiently serviced and maintained.’
—Lawrence Muscarella, PhD LFM Healthcare Solutions
Increased Event Reports For All Scope Types
A ll six types of endoscopes had a significant increase in adverse events reported over the study period. Examples of adverse events Dr. Muscarella identified as satisfying his analysis’s inclusion criteria included:
• contamination of the reprocessed endoscope with microorganisms, patient fluids and/or biofilms;
• a documented association between use of a flexible endoscope and patient infection;
• failure to clean or otherwise reprocess the endoscope correctly according to the manufacturers’ instructions; and
• use of a damaged, cracked or otherwise broken endoscope (e.g., a loose component) that could pose an infection risk.
Gastroscopes, including endoscopic ultrasound endoscopes, had the most significant increase in adverse event reports of any of the six endoscope types—from 13 to 1,135 reports—or by approximately 8,630%, when comparing 2014 with 2021.
Adverse event reports associated with colonoscopes also increased substantially, from 23 to 820, or approximately 3,465% when comparing 2014 with 2021.
Harish K. Gagneja, MD, FACG, AGAF, FASGE, a gastroenterologist with Austin Gastroenterology in Texas, agreed. “With gastroscopes and colonoscopes, we know that not much has been reported with regard to passive infections,” he said. “ERCP [endoscopic retrograde cholangiopancreatography] scopes are a different story because of the complex design, but this is an issue that can be addressed with more due diligence in cleaning and disinfection, and adequate storage. You must ensure that your reprocessing staff are adhering to manufacturers’ instructions and that you have proper storage units in your facilities.”
Dr. Muscarella agreed, recommending that GI endoscopy units routinely perform internal audits of staff practices to ensure infection prevention competency and endoscope reprocessing acumen.
Centers that maintain such strict protocols say they have not needed to pursue either sterilization or singleuse endoscopes. “We are purchasing new colonoscopes shortly, and we are not going disposable,” said Linda Hubbard, RN, the director of nursing for the Endoscopy
Center of Western New York. “We log every scope that is reprocessed and regularly review all of those logs, and ... have not had any infections for a number of years. Michelle Velez, our lead endoscopy technician, ensures that all of our reprocessing staff is up to speed on those competencies, and they are reviewed constantly. Precleaning starts immediately after each procedure is finished, and manual washing begins within one hour. We’ve been quite pleased with our processes, and I think having qualified techs who are dedicated to each procedure room and making sure there is no lag time have really helped us.”
—Gina Shaw
Drs. Agrawal and Gagneja and Ms. Hubbard reported no relevant financial disclosures. Dr. Gagneja is a member of the Gastroenterology & Endoscopy News editorial board. Dr. Muscarella reported a financial relationship with Ambu, which sponsored the MAUDE study.
GASTROENDONEWS.COM80





eNe w sle tt er In Your Inbox *Based on data from Kantar Media, May 2020 Articles from the current month’s issue Articles ahead of print Web-exclusive content to receive your free e-Newsletter from the best-read* gastroenterology publication in the country OPT IN Sign up @ GastroEndoNews.com/enews tl
New Practice Update: Role of Nutrition in IBS



“Anywhere between two-thirds and three-fourths [of IBS patients] relate symptoms to eating food,” said lead author of the update, William Chey, MD, a professor of gastroenterology and nutrition at the University of Michigan, in Ann Arbor. Based on the increasing evidence for diet therapy, the AGA saw a need for a clinical practice update, he said.
After reviewing the literature, Dr. Chey and his fellow authors devised best practice updates for gastroenterologists (Gastroenterology 2022;162[6]:1737-1745.e5).

Among them was updated best practice advice related to the increasingly popular low-FODMAP (fermentable oligo-, di- and monosaccharides and polyols) diet. The















‘Around 50% to 70% of patients report improvements in their overall IBS symptoms with low-FODMAP.’
—William Chey, MD University of Michigan, Ann Arbor
As mounting evidence points to dietary interventions as a primary treatment for irritable bowel syndrome, a new clinical practice update from the American Gastroenterological Association outlines the role of nutrition in IBS management.
GASTROENDONEWS.COM82 PRINTER-FRIENDLY VERSION AVAILABLE AT GASTROENDONEWS.COMP PR PRI RI
authors advise that the low-FODMAP diet, which consists of three phases (Table), is “the most evidence-based diet intervention for IBS.”
“Around 50% to 70% of patients report improvements in their overall IBS symptoms with low-FODMAP,” Dr. Chey said. Of the IBS symptoms, “pain and bloating are most likely to get better.”
In a recent survey, Dr. Chey and his colleagues found that gastroenterologists spend five minutes or less talking about diet in interviews with IBS patients (Am J Gastroenterol 2022 Jan 11. doi: 10.14309/ajg.0000000000001602). “It takes more than five minutes to explain the purpose and how to implement the low-FODMAP diet, especially the three different phases,” Dr. Chey said.
Where gastroenterologists struggle to find five minutes to review diet, dietitians can devote 45 minutes to an hour to patients, explaining how the diet works and walking them through the restriction, reintroduction and personalization phases. This is one of the reasons the clinical practice update emphasizes referral to a GI dietitian.
Noting that he has recommended the lowFODMAP diet to patients, including those with IBD/ IBS overlap, Berkeley Limketkai, MD, PhD, the director of clinical research at the UCLA Center for Inflammatory Bowel Diseases, said he usually involves “a dietitian, so [patients] don’t fall at risk of malnutrition and other nutritional deficiencies while pursuing the FODMAP diet.”
Digital Tools, Remote Counseling Can Expand Access
Understandably, not every GI physician has access to a team of dietitians. In that case, “there are a lot of resources available that can help a physician advise patients,” Dr. Chey said. Monash University, where the low-FODMAP diet was developed, has a website that provides extensive information on the diet (monashfodmap.com). It also offers a mobile app that patients can use to navigate food choices.
“The nice thing about the advent of telemedicine and digital resources is that you can access advice outside of your geographic vicinity,” Dr. Limketkai said. “There are quite a number of dietitians [who] do remote nutrition counseling,” he added.
“The habitual diets of the majority of IBS patients are quite abnormal,” Dr. Chey said, “and they are deficient in many macro- and micronutrients.” Patients can benefit tremendously from an appointment with a dietitian even if the end goal isn’t to start a restrictive diet, he said. In addition, the update notes that healthy eating according to the National Institute of Health and Care Excellence Guidelines and increasing soluble fiber both can be efficacious in alleviating IBS symptoms.
Whether by a dietitian or the gastroenterologist, IBS patients on dietary interventions should be screened for disordered eating, eating disorders or malnutrition,
Table. 3 Phases of Low-FODMAP Diet
1. Restriction (≤4-6 weeks)
2. Reintroduction of FODMAP foods
3. Personalization based on results from reintroduction
FODMAP, fermentable oligo-, di- and monosaccharides and polyols.

according to the practice update. Dietary interventions should be attempted for predetermined time periods and abandoned if they are not beneficial, the authors wrote.
Dr. Chey and his co-authors also reported that although observational studies found most IBS patients improve with a gluten-free diet, randomized controlled trials report mixed results. It may be that the patients improve not because of reduced gluten, but because they ingest less sugars that act as FODMAPs to trigger IBS symptoms, Dr. Limketkai said. However, because the low-FODMAP diet is more difficult to execute, a glutenfree diet may be a good place to start for some patients with mild disease, he said. If patients improve, there’s no need to progress to a more intensive diet. If they don’t improve, he said the low-FODMAP diet is a natural next step.
Dietary therapy should be a first-line intervention for IBS patients, according to Dr. Limketkai. That can mean something simple such as adding peppermint oil or increasing soluble fiber, or something more involved, such as meeting with a dietitian or starting one of the diets used to treat IBS.
However, to date, there are no consistently reliable biomarkers for determining which dietary interventions are best suited for specific IBS patients. The practice update authors reviewed studies on immunoglobulin G antibodies, celiac-related genetic factors HLA DQ2/8, sucrase-isomaltase variants, pretreatment fecal microbiome and metabolites, and food sensitivity testing as ways of predicting the appropriate diet. “Unfortunately, none of the biomarkers are foolproof,” Dr. Chey said.
The practice update was intended to be very practical, in terms of content and breadth, for busy clinicians, Dr. Chey said. The bottom line, he added, is that food is a major driver of symptoms for IBS patients, and diet modification should be used as a primary intervention.
Dr. Chey reported financial relationships with AbbVie, Allakos, Alnylam, Arena, Biomerica, Commonwealth Diagnostics International, Comvita, Everlywell, Gemelli, GI on Demand, Ironwood, ISOThrive, ModifyHealth, Nestlé, Phathom, Progenity, QOL Medical, Quest, RedHill, Salix, Urovant and Vibrant. Dr. Limketkai reported no relevant financial disclosures.
—Donavyn Coffey
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 83
VICTORY











THE IS FOR THE MOST STUDIED MULTI-STRAIN PROBIOTIC The Visbiome unique, proprietary formulation is the only 8-strain probiotic recommended in leading guidelines and expert reviews* •Visbiome can reduce symptoms of dysbiosis associated with IBS or UC including bloating, flatulence, and stool frequency •The addition of Visbiome to conventional treatment helps improve clinical response and remission rates LEARN MORE AT VISBIOME.COM FOR THE DIETARY MANAGEMENT OF DYSBIOSIS ASSOCIATED WITH IBS AND ULCERATIVE COLITIS Visbiome is a medical food intended for the dietary management of dysbiosis associated with IBS, UC, antibiotic associated diarrhea, and pouchitis. *Singh et al. Gastroenterology. 2019, Nguyen et al. Cochrane Library. 2019, Forbes et al. Clinical Nutrition. 2019
Teasing Out Malnutrition In IBD Patients






Byhigh school, Macy Stahl had learned how food affected her Crohn’s disease, and she was able to figure out some semblance of a normal diet, even if it was regimented.
However, when she was 16, one by one, the foods she’d learned to depend on left her doubled over in pain. Her disease flares were more severe and more unpredictable than they had been before. Her already slender 100-pound figure was reduced to 60 pounds in just three months, and Ms. Stahl was severely malnourished.

Like Ms. Stahl, many patients with IBD, as much as 85%, are malnourished (Nutrients 2020;12[2]:372). For some people, the onset of malnutrition is fast, and the damage is easily identified, as it was in Ms. Stahl’s case. But for many others, malnutrition is less severe, but it lurks



GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 85
in the background as constant fatigue or weakness due to ongoing vitamin, mineral and protein deficiencies.
A recent study found that malnutrition is going undetected in patients with IBD and that finding it in these patients will require more than questions about body mass index (BMI) and weight loss. Physicians need to be aware of what’s driving malnutrition in their patients, and how and where they can get support.
“In one-third of outpatients, there is still malnutrition,” said Daisy Jonkers, PhD, an associate professor at NUTRIM School of Nutrition and Translational Research in Metabolism at Maastricht University, in the Netherlands. In a study she and her colleagues presented at the 2022 European Crohn´s and Colitis Organisation (ECCO) meeting, a large number of IBD patients had a normal BMI, but when they conducted a closer analysis of fat mass and fat-free mass, they found low muscle mass in patients who appeared normal (abstract P116). “If the patient opposite you has a normal body composition at first glance, you won’t consider malnutrition,” Dr. Jonkers told Gastroenterology & Endoscopy News
“Patients with higher BMIs may go underrecognized as being at risk for malnutrition,” agreed Carolyn Newberry, MD, the director of GI Nutrition at the Innovative Center for Health and Nutrition in Gastroenterology at Weill Cornell Medical Center, in New York City. “The people who are the sickest in a lot of GI diseases, they may be overweight but undernourished,” she added.
In addition, if an IBD patient’s malnutrition is flying under the radar, there’s a chance it’s making their disease worse. “It’s a vicious circle for the disease itself,” Dr. Jonkers said. “Lack of protein and essential nutrition can affect your immune system, leading to worse inflammation and worse disease outcome.” Apart from that, with a low muscle index you will also be very tired, she said.
How to Find Affected Patients
Finding these patients is imperative, but screening for them in a way that’s clinically feasible is the challenge. A DEXA scan is the most accurate, but it is prohibitively expensive and exposes the patient to radiation. “You can’t do that every month or twice a year,” Dr. Jonkers said. She recommended grip strength or a bioimpedance, which require inexpensive tools and can be administered by a nurse.
Berkeley Limketkai, MD, PhD, an IBD specialist and the director of clinical research at the Center for Inflammatory Bowel Diseases at the University of California, Los Angeles, said he understands the motivation to look for sarcopenia, but the added equipment and time needed to check for low muscle mass may not be worth the added sensitivity. However, he does screen every patient using the Malnutrition Screening Tool (MST) and assesses them via physical examination. Kelly Issokson, RD, a dietitian at the Nutrition and Integrative IBD Program at Cedars-Sinai Medical Center, in Los Angeles, uses the same approach. The MST uses two questions:
Have you recently lost weight without trying? Have you been eating poorly because of a decreased appetite?
But such outpatient screening tools for malnutrition are not as well validated as those used for inpatients, according to Dr. Newberry. Dr. Jonkers also noted that these questionnaires are not sufficient to capture every IBD patient with malnutrition, but she added that if providers aren’t screening for malnutrition yet, tools such as the MST could be a place to start.
Diets That Soothe—and Starve
The widespread malnutrition in patients with IBD is tied inextricably to the diets patients use to nurse their disease. There are a dozen or more popular diets commonly used in the IBD community. Most of them are elimination diets that attempt to remove inflammatory, processed foods.
Ms. Issokson said the specific carbohydrate diet has led to improvements in symptoms and inflammation scores for many of her patients. The low-FODMAP (fermentable oligo-, di- and monosaccharides and polyols) diet is common among Dr. Limketkai’s patients. A recent abstract presented at ECCO found the new CDTreat diet, meant to mimic enteral nutrition, significantly improved quality of life in patients with Crohn’s disease (abstract DOP68).
These diets may ease symptoms for some patients, but a separate study presented at ECCO looked at the nutritional composition of seven popular IBD diets used in pediatric patients with IBD and found them lacking (abstract P391). The study included the Crohn’s disease exclusion diet, CD-TREAT, specific carbohydrate diet, IBD anti-inflammatory diet, autoimmune protocol diet, gut and psychology syndrome diet, and low–FODMAP diet.
Based on the dietary needs of 11- and 16-year-old boys and girls, some of the diets were deficient in vitamin D and calcium, some exceeded saturated fat allowance, and some consisted of more than a recommended proportion of calories from fat. The investigators concluded that “it is imperative that clinicians are aware of the risks of inadequate nutrient intake with restrictive diets.”
Since these restrictive diets can put IBD patients at risk, they should be used with extreme caution in patients with or who are at risk for malnutrition, according to Ms. Issokson. “With malnutrition, the priority is getting enough calories, protein and micronutrients for your body to function, heal and recover from the flare. It doesn’t matter how anti-inflammatory the diet is—healing will be delayed in the setting of malnutrition.”
She also cautioned that just because a patient is feeling better, it doesn’t mean inflammation in the gut is improving. Even if they feel that a diet is working, confirmation with further testing is essential. A diet that isn’t nutritionally adequate or improving luminal disease can be more problematic than helpful in the long run.
It’s certainly not necessary or even reasonable for gastroenterologists to know the ins and outs of every therapeutic diet, but it’s important to know if patients are on such a diet and for how long, Dr. Newberry said. This
GASTROENDONEWS.COM86
knowledge can pinpoint patients who need additional nutritional support because they are at risk for becoming malnourished or need help deciding when and how to come off a diet without instigating a flare.
Emerging Risks: Eating Disorders





Sometimes IBD patients create their own diet based on their unique dietary triggers and the demands of their day. But this approach can be problematic because it can foster food fear and aversion. Dr. Limketkai regularly hears of patients who don’t eat because of an event, a meeting, a flight or a doctor appointment.
Because of the rise in malnutrition brought on by disordered eating, Dr. Limketkai said psychologists are increasingly critical members of his team. They can help patients process their food fear or anxiety in the context of their gastrointestinal symptoms, he said.
“We are recognizing a high amount of disordered eating in IBD,” Ms. Issokson said. This is another group of patients who are best off avoiding common restrictive IBD diets. Restrictive diets can reinforce the behaviors that lead to harm and accelerate malnutrition, she said.
Adding to the Care Team
Patients benefit not only from the expertise of dietitians and psychologists, but also from their time and ongoing monitoring, Dr. Limketkai said. Gastroenterologists often don’t have time to walk patients through dietary changes week by week or the nuanced manifestations of food aversion, but these are critical processes in the health of an IBD patient. If providers are trying to decide when to bring in other experts, they should
reference the Crohn’s and Colitis Foundation’s Nutrition Care Pathway.
For GI specialists, Dr. Newberry said, “it’s really about recognizing these patients, not overlooking them. Once malnutrition is identified as a problem, the best next step is to get them hooked in with a nutrition support provider.”
If your institution has a registered dietitian who specializes in IBD, then you’re set. If not, you’re in good company. Dietitians are a critical, but certainly not universal, resource.
If a gastroenterologist is in private practice or doesn’t have access to nutrition specialists at an institution, they should try to find a local dietitian, in either a nearby hospital or private practice. Gastroenterologists should express the patient’s need for dietary counseling and ask them to consider expanding their work with IBD. This is how Ms. Issokson came to specialize in this area. Physicians at Cedars-Sinai expressed a need and she began working to help those patients.
The same is true for psychologists. Psychologists should consider asking a local practitioner to join alongside them and their patients. In an ideal world, they also could connect malnourished IBD patients to an exercise physiologist, Dr. Newberry said.
“The intersection of GI and nutrition is becoming a multidisciplinary approach,” Dr. Limketkai said. Building a team of physician collaborators gives gastroenterologists more time to focus on the disease, while other experts support and advise them through the nutritionrelated symptoms.

—Donavyn Coffey
We are recognizing a high amount of disordered eating in IBD.
—Kelly Issokson, RD, Nutrition and Integrative IBD Program at Cedars-Sinai Medical Center, Los Angeles.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 87
IT RECURS IN UP TO 35% OF CASES WITHIN 8 WEEKS AFTER INITIAL DIAGNOSIS. 2,3













THE CONSEQUENCES OF RECURRENCE ARE SIGNIFICANT, POTENTIALLY DEADLY 2


patients.


References: 1. Centers for Disease Control and Prevention. 2019 Antibiotic Resistance Threats Report: Clostridioides Difficile. https://www.cdc. gov/drugresistancxqe/pdf/threats-report/clostridioides-difficile-508.pdf. Accessed April 8, 2021. 2. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834. 3. Cornely OA, Miller MA, Louie TJ, Crook DW, Gorback SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):s154-s161.





©2021 Ferring B.V. US-MBIO-2100122








Now is the time to learn how Ferring is shedding light on the link between disease and disruptions in the gut microbiome, exploring the potential for repopulating its diversity and restoring hope to
THE CDC ACKNOWLEDGES C. DIFFICILE INFECTION AS A MAJOR AND URGENT THREAT 1 A VICIOUS CYCLE WITH SIGNIFICANT BURDEN WHAT COULD BE THE CONSEQUENCES OF RECURRENT C. DIFFICILE INFECTION? Learn why it requires aggressive action LEARN MORE NOW
Comparison of 2021 IDSA and ACG Recommendations for Treatment of C. difficile Infection
MARK H. WILCOX, MD Consultant/Head of Microbiology Research & Development Infection Lead
NIHR Leeds Diagnostic Technologies Medical Technology and In Vitro Diagnostics Co-operative Leeds Teaching Hospitals Professor of Medical Microbiology






Sir Edward Brotherton Chair of Bacteriology University of Leeds National Cinical Director
Antimicrobial Resistance & Infection Prevention and Control NHS England United Kingdom




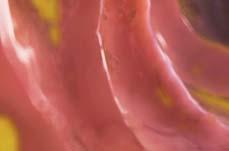


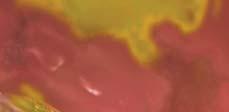
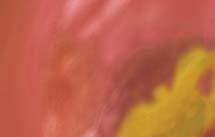
In 2021, the Infectious Diseases Society of America (IDSA) and the American College of Gastroenterology (ACG) each updated its own CDI management guideline,1,2 from previous versions published in 2018 (but confusingly noted as a “2017 update”) and 2013, respectively.3,4 Both guidelines used the GRADE criteria to determine the strength of evidence underpinning the recommendations. While the ACG 2021 update is a full series of recommendations covering prevention, diagnosis, treatment, prevention of recurrence, and special populations, the IDSA 2021 update is more focused, given the shorter gap since the previous version. Hence, the IDSA 2021 guideline addresses 3 specific questions (Table 1).1 This review compares and contrasts how the ACG and IDSA cover these 3 key practice points for the treatment of CDI.

Treatment of Initial Episode of CDI


The IDSA 2021 guideline recommends fidaxomicin (Dificid, Merck) (200 mg orally given twice daily for 10 days) instead of vancomycin (125 mg orally given 4 times daily for 10 days) to treat an initial episode of CDI (conditional recommendation, moderate certainty of evidence), which is a shift from its previous position.1,3 Furthermore, this recommendation “places a high value in the beneficial effects and safety of fidaxomicin, but its implementation depends upon available resources. Vancomycin remains an acceptable alternative.” 1 Continuing its stance from 2018, 3 the IDSA guideline prefers both fidaxomicin and vancomycin to metronidazole, commenting that metronidazole (500 mg orally 3 times daily for 10-14 days) is an alternative for nonsevere CDI, if the former agents are unavailable.1

Clostridioides difficile infection is difficult to diagnose, prevent, and treat. In addition, minimizing the risk for recurrent infection remains a significant challenge.
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 89
Table 1. 3 Questions Addressed
In 2021 IDSA CDI Guideline
1. In patients with an initial CDI episode, should fidaxomicin be used rather than vancomycin?
2.In patients with recurrent CDI episode(s), should fidaxomicin be used rather than vancomycin?
3.In patients with a CDI episode, should bezlotoxumab be used as a cointervention along with standard-of-care antibiotics rather than standard-of-care antibiotics alone?
CDI, Clostridioides difficile infection; IDSA, Infectious Diseases Society of America.
The IDSA recommendation in favor of fidaxomicin was based on a pooled analysis of 4 studies,5-8 including 2 trials 7,8 that were not considered in the 2018 version. 3 The Guery et al clinical trial is considered below in more detail.7 The 2021 IDSA guideline panel acknowledged the relatively high acquisition cost of fidaxomicin, but noted that cost-effectiveness analyses probably support its use due to superior sustained clinical response rates compared with vancomycin 4 weeks after the end of therapy (risk ratio, 1.16; 95% CI, 1.09-1.24).1 However, the panel noted that uncertainty remains about cost-effectiveness estimates. Also, the IDSA guideline notes there is a chance that implementing this recommendation “probably reduces equity due to variation in medical insurance coverage.”1
The 2021 ACG guideline is more circumspect in its recommendations for the treatment of primary CDI, with no stated preference for vancomycin or fidaxomicin. It even states that metronidazole remains a reasonable choice, which is somewhat strange given that the panel reviewed the same evidence demonstrating inferiority of metronidazole.2 Instead, this guideline only recommends avoiding metronidazole in severe CDI.2 The key randomized controlled trials (RCTs) that compared the efficacy of vancomycin and metronidazole found that the latter was inferior regardless of CDI severity.9 The ACG guideline is similarly cautious regarding the comparative cost-effectiveness of vancomycin and fidaxomicin: “Although vancomycin is less expensive, lower recurrence rates of fidaxomicin imply overall similar cost-effectiveness for both agents.”2
Therefore, regarding question 1 (and 2) in Table 1, the IDSA and ACG 2021 guidelines agree and disagree, which appears to reflect a different interpretation of the available evidence. On this point, there is a puzzling anomaly; the IDSA 2021 guideline 1 does not consider a notable, high-impact 2018 network metaanalysis of studies examining the treatment of nonrecurrent CDI.10 The ACG 2021 guideline discusses
this meta-analysis, but states: “In a network metaanalysis comparing 13 agents across 24 trials comprising 5,361 patients, vancomycin was rated the best option for achieving primary cure of severe infection, although fidaxomicin had higher sustained cure (ie, fewer recurrences).”2 Actually, the stated conclusion of this meta-analysis was that “among the treatments for non-multiply recurrent infections by C. difficile , the highest quality evidence indicates that fidaxomicin provides a sustained symptomatic cure most frequently. Fidaxomicin is a better treatment option than vancomycin for all patients except those with severe infections with C. difficile and could be considered as a first-line therapy. Metronidazole should not be recommended for treatment of C. difficile.”10
The higher sustained cure associated with fidaxomicin may be especially beneficial in patients at greater risk for recurrence of CDI,11,12 including those with the following reisk factors: age at least 65 years; previous hospitalization, severe CDI, concurrent antibiotics and hypervirulent ribotype (027/078/244) infections.13,14 Given that the high acquisition cost of fidaxomicin remains a potential barrier to its widespread use, further cost-effectiveness analyses are needed that measure the total (patient and insurance) cost savings from reduced CDI recurrences to determine how much the greater initial drug price is offset. Of note, the nonfinancial benefits of reducing CDI recurrences need to be quantified, considering the considerable impacts on patients’ quality of life and families.15-17
Treatment of Recurrent CDI
The ACG 2021 guideline states: “We suggest tapering/pulsed-dose vancomycin for patients experiencing a first recurrence after an initial course of fidaxomicin, vancomycin, or metronidazole (strong recommendation, very low quality of evidence).” In addition, “we recommend fidaxomicin for patients experiencing a first recurrence after an initial course of vancomycin or metronidazole (conditional recommendation, moderate quality of evidence).”2 The recommendation in favor of tapering/pulsed-dose vancomycin, which comes before the one recommending fidaxomicin, is puzzling given the very modest evidence base, including no RCTs, and noting the relatively high cost of a 4- to 6-week course of oral vancomycin. Conversely, the IDSA 2021 guideline states: “The panel suggests the use of fidaxomicin as the preferred therapy for patients with recurrent CDI episode(s) to improve sustained response after therapy. More well-designed RCTs for patients with recurrent CDI, particularly multiply recurrent CDIs, are needed to improve the strength of recommendations. In particular, studies with more appropriate controls for extended-pulsed fidaxomicin should help clarify the role of this dosing strategy for patients with recurrent CDI both in terms of efficacy and quality of life.”1
The latter point follows on from a phase 3b clinical trial in adults older than 60 years of age, 80%
GASTROENDONEWS.COM90
Table 2. Effectiveness of Bezlotoxumab With SOC Antibiotics, According to Risk Factors for CDI Recurrence
CDI recurrence risk factor
Number needed to treat to prevent a CDI recurrence
Patients aged ≥65 y 3.8
Patients who are immunocompromised 4.7
Patients with severe CDI on presentation 5.1
Patients aged ≥65 y and ≥1 previous episode in prior 6 mo2.5
Patients who are immunocompromised and ≥1 previous episode in prior 6 mo3
Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo2.5
CDI, Clostridioides difficile infection; SOC, standard of care.
of whom had primary CDI, that compared vancomycin with an extended-dosing fidaxomicin regimen.7
The extended-pulsed regimen comprised the same total dosage as in a standard fidaxomicin course of 200 mg twice daily for 10 days, but was 200 mg of fidaxomicin twice daily for 5 days followed by the same dose (200 mg) given once daily on alternate days until day 25. This longer period of fidaxomicin dosing had been shown in a clinically reflective gut model to be associated with less risk for CDI recurrence, likely due to the extended period during which C. difficile inhibitory levels of antibiotic are present in the large intestine.18 The clinical trial found a very low and significantly reduced rate of CDI recurrence in patients receiving the extended-pulsed regimen (4% vs 17% at day 30 after the end of treatment; P<0.001).7 However, the open study design and absence of comparator groups (extended-pulsed vancomycin and standarddosage fidaxomicin) limit the full interpretation of the study results. Nevertheless, this is further evidence of the reduced risk of CDI recurrence (increased chance of sustained cure) in fidaxomicin versus vancomycin recipients.
Treatment of CDI With Bezlotoxumab And Standard of Care Antibiotics
Bezlotoxumab (Zinplava, Merck) is a monoclonal antibody against C. difficile toxin B given as an IV infusion at any point during the first 10 days of standard-ofcare (SOC) antibiotic therapy for CDI.19 Bezlotoxumab was the first agent to be licensed for the prevention of CDI recurrence. The ACG 2021 guideline states: “We suggest bezlotoxumab be considered for prevention of CDI recurrence in patients who are at high risk of recurrence (conditional recommendation, moderate quality of evidence).”2 The IDSA 2021 guideline is similar: “For patients with a recurrent CDI episode within the last 6 months, we suggest using bezlotoxumab as a cointervention along with SOC antibiotics rather than SOC antibiotics alone (conditional recommendation,
very low certainty of evidence).”1
More than three-fourths of patients recruited to the 2 MODIFY phase 3 trials of bezlotoxumab had 1 or more risk factors for poor CDI outcome, including recurrent infection (Table 2).19-21 Analysis of the treatment outcomes for those with risk factors showed that the benefits of bezlotoxumab were greatest for patients 65 years of age or older, those experiencing a recurrent episode of CDI, immunocompromised patients, and severe CDI.20 More than 30% of patients with any 1 of the risk factors who received placebo (plus SOC antibiotics) had recurrent CDI, compared with about 21% of those without a risk factor. Also, the risk for recurrent CDI was higher for those with more risk factors. Those with 1 risk factor had an increased risk for recurrent CDI of 31.3%, while those with 3 or more risk factors had an increased risk of 46.1%. Conversely, recurrent CDI was not reduced significantly in bezlotoxumab recipients who had none of these predefined risk factors for recurrence. Of note, recurrent CDI, use of fecal microbiota transplants (FMTs), and CDI-associated 30-day readmissions were all reduced in bezlotoxumab recipients who had risk factors for recurrence.20
Both guideline panels reviewed the costeffectiveness data for the use of bezlotoxumab and broadly agreed that such analyses favor the addition of bezlotoxumab to SOC antibiotics in patients with a high risk for recurrence, particularly those with a recurrent CDI episode within the last 6 months.1,2 The number needed to treat (NNT) with bezlotoxumab to prevent a case of recurrent CDI is low for patients with risk factors for recurrence, especially for those who have had a recurrent CDI in the previous 6 months (NNT=2.5) (Table 2).21 However, it was noted that the high acquisition cost of bezlotoxumab means that “implementing this recommendation also probably reduces equity due to variation in medical insurance coverage.”1
For patients with multiple CDI recurrences, both
GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 91
guidelines consider a role for FMT. While the use of FMT is beyond the scope of this review, safety concerns remain regarding the potential for inadvertent transmission of pathogens via donor fecal samples.22-24 Such concerns have been extended to SARS-CoV-225 and, most recently, adenoviruses that are associated with severe hepatitis.26 Although the newly recognized
References
1. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029-e1044.
2. Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116(6):1124-1147. Erratum in: Am J Gastroenterol. 2022;117(2):358.
3. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
4. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
5. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
6. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomized controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
7. Guery B, Menichetti F, Anttila VJ, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomized, controlled, openlabel, phase 3b/4 trial. Lancet Infect Dis. 2018;18(3):296-307.
8. Mikamo H, Tateda K, Yanagihara K, et al. Efficacy and safety of fidaxomicin for the treatment of Clostridioides (Clostridium) difficile infection in a randomized, double-blind, comparative phase III study in Japan. J Infect Chemother 2018;24(9):744-752.
9. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014;59(3):345-354.
10. Beinortas T, Burr NE, Wilcox MH, et al. Comparative efficacy of treatments for Clostridium difficile infection: a systematic review and network meta-analysis. Lancet Infect Dis 2018;18(9):1035-1044.
11. Madoff SE, Urquiaga M, Alonso CD, et al. Prevention of recurrent Clostridioides difficile infection: a systematic review of randomized controlled trials. Anaerobe. 2020;61:102098.
12. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(suppl 2):S93-S103.
13. Wilcox MH. Progress with a difficult infection. Lancet Infect Dis 2012;12(4):256-257.
severe hepatitis cases have occurred primarily in children, the epidemiology and transmission of potential viral cause(s) are poorly characterized. The need for extensive screening/testing of donors/samples adds to the costs of FMT. It is probable that the regulatory position on FMT will change as defined microbiomebased products become available.
14. Davies K, Lawrence J, Berry C, et al. Risk factors for primary Clostridium difficile infection; results from the observational study of risk factors for Clostridium difficle infection in hospitalized patients with infective diarrhea (ORCHID). Front Public Health. 2020;17(8):293.
15. Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality of life questionnaire. J Clin Gastroenterol. 2016;50:631-637.
16. Wilcox MH, Ahir H, Coia JE, et al. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother. 2017;72(9):2647-2656.
17. Barbut F, Galperine T, Vanhems P, et al. Quality of life and utility decrement associated with Clostridium difficile infection in a French hospital setting. Health Qual Life Outcomes. 2019;17(1):6.
18. Chilton CH, Crowther GS, Todhunter SL, et al. Efficacy of alternative fidaxomicin dosing regimens for treatment of simulated Clostridium difficile infection in an in vitro human gut model. J Antimicrob Chemother. 2015;70(9):2598-2607.
19. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017;376(4):305-317.
20. Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis. 2018;67(5):649-656.
21. Prabhu VS, Dubberke ER, Dorr MB, et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis 2018;66(3):355-362.
22. DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381:2043-2050.
23. FDA. Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to transmission of multi-drug resistant organisms. 2019. Accessed May 10, 2022. https://bit.ly/3gKXZEDidse
24. FDA. Update to March 12, 2020 safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse events likely due to transmission of pathogenic organisms. 2020. Accessed May 10, 2022. https://bit.ly/3yx1REnIDSE
25. FDA. Safety alert regarding use of fecal microbiota for transplantation and additional safety protections pertaining to SARS-CoV-2 and COVID-19. 2020. Accessed May 10, 2022. https://bit. ly/2U0DUAbIDSE
26. European Centers for Disease Control. Increase in severe acute hepatitis cases of unknown aetiology in children. April 28, 2022. Accessed May 10, 2022. https://bit.ly/3stbOitIDSE
GASTROENDONEWS.COM92
EXPERT PICKS
AI and Machine Learning In the Management Of Liver Disease


 ASHWANI K. SINGAL, MD, MS
ASHWANI K. SINGAL, MD, MS
Oral abstract 74.



‘The researchers may be filling a need with this ML model, which has both a high accuracy rating, at 89%, and simplicity.’


Poster 356. ‘This is an important study that derives a ML-based model with common clinical and laboratory data routinely assessed in the standard of care of patients with alcohol-associated hepatitis.’

In this edition of “Expert Picks,” transplant hepatologist Ashwani K. Singal, MD, MS, a professor of medicine at the University of South Dakota Sanford School of Medicine and the chief of clinical research at Avera Health’s liver disease program, in Sioux Falls, S.D., highlights a selection of abstracts presented at the 2021 Liver Meeting on artificial intelligence and machine learning in the management of patients with liver disease.

GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 93
HEPATOLOGY IN FOCUS
In this study, investigators developed machine learning (ML) models to detect fibrotic nonalcoholic steatohepatitis (NASH) and compared the models with traditional noninvasive tests. To build the models, they used data from 1,016 patients with histologically confirmed nonalcoholic fatty liver disease (NAFLD), considering demographic and clinical variables, along with medications and laboratory tests within three months of liver biopsy. All liver biopsies were scored per the NASH Clinical Research Network criteria. NASH with significant activity was defined as steatohepatitis with an NAFLD activity score of at least 4 and a fibrosis stage rated 2 or higher.
The researchers divided the patient cohort into training (80%) and validation sets (20%), evaluating concordance-statistic (c-statistic) or area under receiver operating curve (AUROC) values on the validation set. ML models were constructed using seven parameters: patient age, aspartate transaminase level, alanine transaminase level, presence of diabetes mellitus, albumin level, platelet count and hematocrit ratio. The best performing model achieved an AUROC of 0.95 (95% CI, 0.91-0.97) and an accuracy of 89.2%. This model outperformed both the Fibrosis-4 index (AUROC, 0.72; 95% CI, 0.68-0.75) and NAFLD fibrosis score (AUROC, 0.55; 95% CI, 0.51-0.59) in predicting NASH.
Dr. Singal: As we know that fibrosis stage is the most important determinant driving liver and nonliver (cardiac and renal) outcomes in NAFLD patients, fibrosis risk determination is crucial when encountering a patient with NAFLD. Because liver biopsy is invasive and not liked by patients and providers alike, there are calls for more tests that are both noninvasive and accurate. Although some noninvasive tests exist, such as serum and radiological marker tests, more accurate and simple biomarker tests are needed. In this regard, the researchers may be filling a need with this ML model, which has both a high accuracy rating, at 89%, and simplicity, using the demographic and laboratory data already routinely collected in the care of NAFLD patients.
ML is emerging as an important tool to derive models for diagnosis and prognosis of any disease using clinical information, laboratory data, or more sophisticated information such as findings from radiology or histology evaluation. Furthermore, a lot of advanced research is being performed using various “-omics” approaches, such as proteomics, lipidomics, transcriptomics and even gene expression studies. At the 2021 Liver Meeting, several abstracts were presented using the ML and AI approaches (including abstracts 294, 356 and 1591, discussed here) aiming to diagnose and/ or predict disease outcomes.
Short-term survival in alcohol-associated hepatitis is predicted using static models with admission data only or a dynamic model that includes data on day 7 (known as the Lille score).
In this study, Dunn et al (including Dr. Singal) derived and validated a LASSO ML algorithm that predicted 90-day survival in alcohol-associated hepatitis. LASSO is a commonly used ML algorithm that can help minimize noncontributing predictors and works without overfitting, a condition in which algorithms work well in the data set but fail when additional external data are added. This algorithm used admission and day 7 simple and composite variables.
In this retrospective study, the researchers used data from 900 patients with severe alcohol-associated hepatitis from four medical centers. To develop the final LASSO score, they collected 23 variables, including blood urea nitrogen level, glomerular filtration rate, neutrophil-tolymphocyte ratio, albumin level, discriminant function score, and ABIC score, at admission or at day 7.
The model was found to be statistically superior
(LASSO c-statistic, 0.80; 95% CI, 0.78-0.81) to the conventional static (Model for End-stage Liver Disease [MELD] score c-statistic, 0.72; 95% CI, 0.69-0.76) and dynamic models (Lille score c-statistic, 0.74; 95% CI, 0.71-0.77). An online calculator is currently under development for public use.
Dr. Singal: This is an important study that derives a MLbased model with common clinical and laboratory data routinely assessed in the standard of care of patients with alcohol-associated hepatitis. This model is more accurate than static MELD scores and dynamic Lille scores because it was derived using the LASSO model, a specific regression model that factors in dynamic change of variables over time, reducing bias and overfitting of the model. When available for public use with an online calculator, it would help clinicians more confidently and accurately choose an appropriate care pathway, such as liver transplantation or palliative care, especially in patients who are ineligible for or unresponsive to corticosteroid treatment.
Oral Abstract 74. Machine learning model outperforms noninvasive tests to detect fibrotic nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease (Aggarwal et al)
Poster 356. Machine learning improves 90 days survival prediction compared to conventional static and dynamic models (Dunn et al)
HEPATOLOGY IN FOCUS GASTROENDONEWS.COM94
Poster 294. A new serological collagen model predicts risk of liver-related events in early alcohol-related liver disease with high precision in derivation and validation cohort (Johansen et al)
The individual risk for developing symptomatic alcohol-related liver disease is highly variable, causing a need for better patient risk stratification tools, especially in those with early disease.
In this prospective study of 462 patients with biopsyproven, early alcohol-related liver disease (ALD) (222 in the derivation cohort seen in secondary care and 240 in the validation cohort seen in primary care), the researchers created and validated a collagen model using PRO-C3 (type III collagen formation), platelet count, and aspartate transaminase and alanine transaminase levels. The new model stratified two risk levels at a cutoff score of 0.35, with 11.6-fold odds of a liver-related event on follow-up among early ALD patients with high scores (95% CI, 6.5-20.8; P<0.001). At both risk score levels (above and below 0.35), the new model was more accurate (c-statistic, 0.842) than other noninvasive test models such as the Fibrosis-4 index (c-statistic, 0.78), PRO-C3 level (c-statistic, 0.79), and ADAPT score (a PRO-CE–based score to diagnose advanced fibrosis
in patients with NAFLD) (c-statistic, 0.81). The authors concluded that the new collagen model is more accurate and can be used for risk stratification in primary and secondary care practices.
Dr. Singal: We use noninvasive serum markers (Fibrosis-4 index, enhanced liver fibrosis test [FibroSure, eviCore]), and radiological markers (liver elastography or magnetic resonance elastography) in a sequential manner to determine fibrosis risk in patients with early ALD. There are no reliable, accurate, noninvasive models that can predict liver-related events among early ALD patients. Because of this gap, the collagen score derived and validated in this prospective cohort study on early ALD patients recruited from primary care provides important, novel information with an accurate prediction of developing a liver-related event. External validation—especially in multicenter, international cohorts—is needed before recommending a check of PRO-C3 in the routine management of ALD patients.
This study examined whether an ML algorithm can accurately predict hepatic venous pressure gradient (HVPG) and the presence of varices using information from liver histology. The investigators performed a post hoc analysis on 143 patients with NASH cirrhosis recruited in the belapectin (Galectin) phase 2a trial (ClinicalTrials.gov Identifier: NCT02462967) with available liver histology and HVPG data.
They found that HVPG was accurately derived with an ML algorithm using information from 457 histologic variables (252 related to septa, 21 related to nodules and 184 related to fibrosis). Their test, called the SNOF (Septa NOdule Fibrosis) score, predicted clinically significant portal hypertension (CSPH) (both HVPG ≥ 10 and ≥ 12 mm Hg) well, with an area under the curve (AUC) of 0.62 and 0.64, respectively. The researchers said the use of ML histologic scores may increase the accuracy of efficacy end points in NASH cirrhosis trials.
Dr. Singal: Identifying CSPH is important in clinical practice because it is a surrogate for clinical outcomes such as the development of varices, decompensation and patient survival. Measurement of the portal pressure gradient and HVPG is invasive and involves cannulation of the internal jugular vein to access the hepatic veins. Furthermore, the technique requires expertise and is available at only a few centers. By using histologic information, the ML-based SNOF score could be important, since it provides accurate information on CSPH without invasive methods that require special training. If a liver biopsy is obtained for the clinical care of NASH patients, this score—apart from gauging prognosis—also could be used to recommend nonselective beta-blockers, which recently were shown to reduce the risk for liver-related events and hepatic decompensation in patients with CSPH (JHEP Rep 2020;2[1]:100063). Furthermore, clinicians can avoid performing invasive HVPG measurements or other tests, such as splenic or liver elastographic measurements, to diagnose CSPH.
—Compiled and written by Kate O’Rourke
Poster 1591. Development of machine learning histological scores that correlated with portal pressures and development of varices in NASH patients with cirrhosis (Noureddin et al)
HEPATOLOGY IN FOCUS GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 95
Meta-analysis Shows AI Approaches Top Most Interventions For Improving ADR
Higheradenoma detection rates are achieved using artificial intelligence devices than with many other adjuncts to colonoscopy, according to a meta-analysis that ranked the approaches.

“The conclusion of our network metaanalysis is that AI definitely improves adenoma detection rate compared to the majority of endoscopic interventions and should be encouraged for widespread use in clinical settings,” said lead investigator Muhammad Aziz, MD, a gastroenterology fellow at the University of Toledo, in Ohio.
Recent randomized trials and metaanalyses showed that ADRs can be improved with the use of AI over standard colonoscopy (World J Gastroenterol



2021;27[29]:4802-4817). But because the previous studies compared AI with highdefinition colonoscopy and not adjunct endoscopic modalities, Dr. Aziz and his co-investigators conducted a systematic review and network meta-analysis of randomized trials to assess the effectiveness of AI versus other interventions aimed at increasing ADR. They calculated relative improvements in ADRs and determined whether AI was superior to another intervention (but not multiple interventions used together).

GASTROENDONEWS.COM96
Data Set
The study, which Dr. Aziz presented at the 2022 Digestive Disease Week (abstract 569), identified 81 randomized controlled trials involving 47,107 patients undergoing colonoscopy for screening or surveillance (61%) and/or diagnosis (39%). The primary outcome was ADR, and they also measured polyp detection rates (PDRs).
The investigators compared AI with 20 discrete interventions falling within these categories: high-definition colonoscopy, distal attachments, dye-based chromoendoscopy, virtual chromoendoscopy (narrow band imaging, blue laser imaging, i-Scan [Pentax Medical], autofluorescence imaging, linked color imaging, flexible spectral imaging color enhancement), full-spectrum endoscopy (FUSE; EndoChoice), water exchange and water immersion.
AI Comes Out on Top For ADR and PDR
Compared with high-definition colonoscopy, AI had the highest relative increase in ADR (relative risk [RR], 1.46). The next most effective modalities were G-EYE (Smart Medical; RR, 1.32), blue laser imaging (RR, 1.27),
Table. ADR With AI Versus Other Endoscopic Interventions
Endoscopic intervention Relative
Autofluorescence
second
EndoRing (Jordco)1.351.13-1.60
spectrum endoscopy
second-generation narrow band imaging (RR, 1.27), and i-Scan with surface and contrast enhancement (RR, 1.26).
Another finding was the extent to which AI was superior to the other interventions in improving ADR (Table). The only interventions AI did not show a statistically significant advantage over “were blue laser imaging, second-generation narrow band imaging and G-EYE,” Dr. Aziz reported.
“In P-score ranking for ADR, AI was superior—0.96— almost perfect for ADR, compared to high-definition colonoscopy, which was only 0.10,” he said.
For improving PDR, again AI ranked highest (RR, 1.44). The next best modalities were i-Scan with surface and contrast enhancement, blue laser imaging, G-EYE and second-generation narrow band imaging (RR, 1.23-1.28). Compared with other interventions in improving PDR, AI proved superior to 13 approaches. In P-score ranking, AI scored 0.98, while the lowest in this case was FUSE, at 0.04, Dr. Aziz reported.
“Of course, our study is not without limitations, first and foremost that there were no direct comparative studies of AI versus other interventions. All the evidence we are presenting is indirect,” Dr. Aziz acknowledged. “But its strength is that it’s based on a high number of robust-quality randomized controlled studies with large patient populations, and with low heterogeneity for the primary outcome.”
Dr. Aziz said to better understand the magnitude to which AI may improve ADR, researchers should compare it directly with other modalities in randomized controlled trials.
Combined Techniques Predicted for the Future
Jeremy Glissen Brown, MD, MSc, an assistant professor of medicine at Duke University, in Durham, N.C., called the systematic review and network metaanalysis, which pooled data from 81 randomized trials, “an impressive study.”
“CADe [computer-aided detection] aims to address the problem of missed polyps that technically may appear in the visual field. Other adjunctive technology that highlights polyps via dyes (chromoendoscopy) or better image quality (high-definition endoscopes), or through alteration of the image output (e.g., linked color imaging or narrow band imaging) may operate in a similar fashion. It is encouraging that CADe performs favorably compared to many of these modalities, which in many cases are now standard of care,” he said.
Dr. Glissen Brown urged caution, however, in interpreting comparisons between CADe and Endocuff (Olympus) or FUSE. “CADe may be seen as complementary to many of these modalities,” he said. “Devices
as Endocuff seek to decrease missed adenomas
increasing the amount of mucosa exposed. In the
we will likely see combined techniques here.”
—Caroline Helwick
Drs. Aziz and Glissen Brown reported no relevant financial disclosures.
Large
such
by
future,
risk 95% CI
imaging 1.361.08-1.72 Dye-based chromoendoscopy 1.261.07-1.48 Endocuff: first generation (Olympus) 1.251.08-1.44 Endocuff:
generation (Olympus) 1.271.10-1.46
Flexible spectral imaging color enhancement 1.301.11-1.53 Full
(EndoChoice) 1.441.22-1.70 High-definition colonoscopy 1.461.31-1.62 i-Scan (Pentax Medical)1.251.01-1.55 Linked color imaging1.251.09-1.45 Narrow band imaging1.371.21-1.56 Water exchange1.271.08-1.49 Water immersion1.521.21-1.90 GASTROENTEROLOGY & ENDOSCOPY NEWS SPECIAL EDITION • OCTOBER 2022 97
Demystifying AI and Computer Vision
A Q&A With Jason Samarasena, MD, MBA, AGAF, FACG


Artificialintelligence was founded as an academic discipline in 1956, but only in the last decade have its specific applications in medicine started to become mainstream.
Some researchers have begun to prefer the term “augmented intelligence” because these applications augment or assist human intelligence instead of replacing it entirely. Publications and presentations related to applications of AI and computer vision in gastroenterology have increased exponentially over the past several years. For this month’s column, I spoke with Jason Samarasena, MD, MBA, an interventional gastroenterologist and the associate chief of the Division of GI Hepatology at the University of California, Irvine’s Digestive Health Institute, about demystifying AI and computer vision for gastroenterologists. The following is an excerpt from the discussion.
falls short during training programs, either because of lack of ample case experience with neoplastic lesions or because visual classification systems are too vague or overly complicated. Whatever the reason, there remains significant room to improve highquality examinations such as for Barrett’s esophagus. Computer-aided detection (CADe) algorithms help by red-flagging areas of suspicion for neoplasia, and computeraided diagnosis (CADx) algorithms specialize in characterizing the lesion of interest—for example, an esophageal nodule being examined could be highgrade dysplasia or nondysplastic Barrett’s esophagus.
Dr. Atreja: Is computer vision ready for general use?
Dr. Samarasena: It is because of our most coveted tool, endoscopy. The stream of still images shot by a fast-clicking camera during endoscopy is composed of pixels representing magnitudes of brightness and color that are data points that a computer can use to find patterns. These still frames and patterns are ripe for computer vision, which leans on AI to augment our perception and interpretation of things we perceive visually.
Dr. Samarasena: Both CADx and CADe AI algorithms require training on thousands of images in a training set and then testing on a completely unique validation set to evaluate the accuracy and performance of the algorithm. Finally, it requires validation among diverse populations and regular refinement. Fortunately, real-time processing speeds are now possible with off-the-shelf computing hardware. We have an FDA approval already in this space (GI Genius, Medtronic) and hope to see many more applications become a reality for us in the next few years across various diseases and procedures. In August, the American Society for Gastrointestinal Endoscopy held its 4th Global Gastroenterology & Artificial Intelligence Summit, and this month, the American Gastroenterological Association is convening a special workshop focused on the Future of AI in Gastroenterology to facilitate the growth of a community of researchers, clinicians and industry liaisons dedicated to studying AI in GI and bringing it to clinical settings.
Dr. Samarasena: Real-time interpretation of the visual matter displayed on the screen is a key skill for endoscopists but is also a challenging one that often
Suggested Reading

Abadir AP, Ali MF, Karnes W, et al. Artificial intelligence in gastrointestinal endoscopy. Clin Endosc. 2020;53(2):132-141.

Editor’s note: Contact Dr. Atreja (aatreja@ucdavis.edu) with suggestions for specific themes and research to cover.

Dr. Atreja: Why is gastroenterology especially primed for use of computer vision?
Dr. Atreja: How can computer vision algorithms help endoscopists?
DIGITAL GI CORNER
ASHISH ATREJA, MD, MPH, FACP, AGAF Chief Information Officer Chief Digital Health Officer Professor of Gastroenterology UC Davis Health Sacramento
JASON SAMARASENA, MD, MBA, AGAF, FACG
GASTROENDONEWS.COM98


























Complete archives Web-only content Multimedia And more …






































www.ovesco.com one & done OTSC® – superior outcome in hemostasis ® therapy demonstrate 96.0% vs. 71.4% (p=0.017) 91.7% vs. 73.1% (p=0.019) 96.8% vs. 86.5% (p=0.007) 3 1 Jensen DM, Kovacs T, Ghassemi KA, Kaneshiro M, Gornbein J. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2315-2323 2 Meier B, Wannhoff A, Denzer U, Stathopoulos P, Schumacher B, Albers D, Hoffmeister A, Feisthammel J, Walter B, Meining A, Wedi E, Zachäus M, Pickartz T, Küllmer A, Schmidt A, Caca K. Gut. 2022 Jul;71(7):1251-1258. 3 Lau J, Tan CH, Sun X, Song H, Li L, Li R, Li P, Feng J, Wang B, Leung WK, Hartley I, Moss AC, Suen BY, Yu Y, Chan FK. Over-the-scope clips versus standard endoscopic treatment. UEG Week Virtual (October 3-5) 2021 OTSC® is a registered brand of Ovesco Endoscopy AG.




































































 ZOE LAWRENCE, MD SETH A. GROSS, MD
NYU Langone Health New York, New York
ZOE LAWRENCE, MD SETH A. GROSS, MD
NYU Langone Health New York, New York




























 Fibrotic liver Formation of scar tissue within the liver
Cirrhotic liver Scarred tissue replaces healthy tissue in the liver
Liver cancer Formation of malignant tumor in the liver
Liver Steatosis Enlarged liver due to fat deposits
Fibrotic liver Formation of scar tissue within the liver
Cirrhotic liver Scarred tissue replaces healthy tissue in the liver
Liver cancer Formation of malignant tumor in the liver
Liver Steatosis Enlarged liver due to fat deposits










































































 Figure 1. Bowel prep assessment flow sheet.
Figure 1. Bowel prep assessment flow sheet.
 Figure 2. EHR with hard stops implemented.
Figure 2. EHR with hard stops implemented.

 Figure 4. EHR protocols for standard, high-risk, and salvage preparations.
Figure 4. EHR protocols for standard, high-risk, and salvage preparations.























































































































































 ASHWANI K. SINGAL, MD, MS
ASHWANI K. SINGAL, MD, MS






























































