3 minute read
Four IOL Delivery Devices to Rule Them All
by Brooke Herron
As surgical techniques evolve, instrumentation and devices must also adapt to meet those needs. In this sphere, one area that has seen remarkable progress is in intraocular lenses (IOLs).
And it’s not just the lenses that have changed — the way they're delivered to the eye has evolved, too. Originally, IOLs were delivered via a reusable syringetype injector, which have become single-use to help ensure sterile conditions. Additionally, and based on surgeon requests, the size and design has progressed to cartridges with parallel end tips, which allow for smaller incisions than previous conical tips; and lateral rails that secure the lens position during butterfly flap closure. Further, based on demand from the lens industry, gliding agents have also been upgraded to sophisticated hydrophilic coatings.
These technologies have been mastered by O&O MDC, which is recognized as one of the three world leaders in this regard, according to Director of International Sales Vasileios Skountis.
Developing delivery systems that reign supreme
The O&O product catalog features IOL delivery devices, all of which are singleuse. Using an innovative cartridge design, each device provides easy lens loading under direct visualization — which reduces preparation time and enables a faster, safer and more efficient surgery. Presenting their court of products...
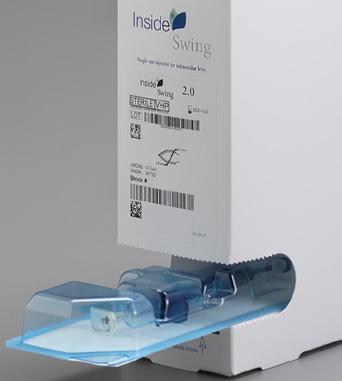
Inside SWING is a hydrophilic and hydrophobic delivery system. It can be used in micro-incision cataract surgery (dependent on the cartridge type) in either traditional or wound-assist surgical techniques.

Why O&O MDC?
A subsidiary of He Vision Group, O&O MDC is an Italian ophthalmic medical device engineering company specializing in IOL manufacturing, delivery systems, polymers for IOLs and packaging accessories. The accumulation of nearly two decades of ophthalmic experience has allowed O&O MDC to extend the company’s core business and expand its range of products, resulting in groundbreaking manufacturing solutions for the production of IOLs and ancillary systems. Further, the company’s specially made, single-use products have attracted customers from around the world.
According to O&O MDC, their innovative technologies are developed using trusted systems, products and expertise — making them the “One and Only” company capable of producing polymers with the qualities required for IOLs (e.g., a thick lens). Plus, their manufacturing experience allows them to make a polymer with both the optical and physical properties for a safe, easy and precise IOL injection — and these injection cartridges remain a major source of company revenue.
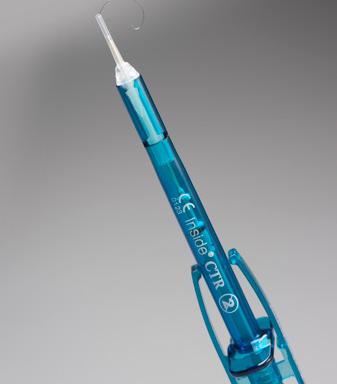
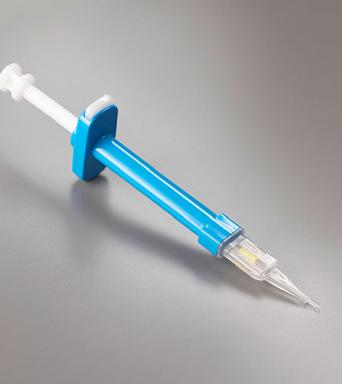
CLOCKWISE: Inside SWING, Inside TWIN, Inside EASY, Inside CTR.
Inside TWIN is a single-use delivery system that enables simple, efficient and consistent IOL loading into the cartridge. In conjunction with the company’s Twin Glide cartridges, the device provides consistency and accuracy of IOL insertion during injection. Plus, the design allows surgeons to choose the preferred method of injection (push or screw). Inside EASY is a hydraulic delivery system that can be used with any O&O butterfly-style cartridge. This ensures accuracy and precision of the IOL’s insertion during delivery, while the cartridge design ensures comfortable lens loading.
The main difference between TWIN, SWING and EASY devices lies in the type of cartridges used – thus, making the loading methods of each injector different. For example, in the TWIN and SWING injectors, the lens is top loaded; meanwhile in the EASY injector, the lens is loaded in a butterfly cartridge.
Inside CTR is a delivery system that was created to simplify and safely implant a capsular tension ring (CTR). This helps stabilize the capsular bag throughout the surgical process. This injector is equipped with a translucent guide, which allows the surgeon to visualize movement of the CTR in the cartridge funnel, right up to the point of insertion. This CTR system ensures centration of the IOL, even with postoperative capsular bag shrinkage; it also replaces the need for a scleral IOL and simplifies potential IOL implantation.





