The journey is what you make it. Your thoughts, behaviors, and actions will shape your experience, so make those things work for you and not against you.






The journey is what you make it. Your thoughts, behaviors, and actions will shape your experience, so make those things work for you and not against you.





Sam Salvaggio’s life was turned upside-down when, in 2005, she was diagnosed with relapsing remitting MS. She’s made a name for herself creating content about her experiences that is free of toxic positivity and informed by her advanced education in nutrition and pharmaceutical sciences. Here, she shares some of the tips she’s learned for better living after managing MS for nearly 20 years:
Always keep track of any symptoms, tests, appointments, etc. As the years go on, you’ll find that it’s really helpful to have a record of everything, because things will start to blur together.
Throughout the journey with a chronic illness, you spend a lot of time at the doctor’s office. Healthcare professionals have expertise, but not (usually) the lived experience. Some are great at listening and being respectful, and others have plenty of room for improvement. It can be emotionally and physically draining, but find doctors that really listen, and respect you and your experience. You need people on your team that are in your corner. Always advocate for yourself — it can be exhausting but it’s absolutely critical.
Listen and learn about your condition and your body. Everyone is different, and the more you know about how different variables (e.g., diet, activity, sleep, stress, hydration level) affect your body, the more you can live in a way that helps you. It also allows you to shift your focus from the what-ifs and unknowns to something you can control after diagnosis.
The ups and downs are inevitable. Fighting them is a waste of time and energy. Learn that they will come and go, and that change is the only constant.
The journey is what you make it. Your thoughts, behaviors and actions will shape your experience, so make those things work for you and not against you. Be nice to yourself and don’t beat yourself up. Remember: You have the power.
Chronic illness doesn’t take away your choices, it just changes the choices you have. Accept that and look for what you can do rather than what you can’t.
Of course you don’t want a chronic illness, but that isn’t within your control. What you can do is accept that two things can be true at once: it can be hard, and you can move onward and shift your focus to things you can control instead.
Take anything you hear from others with a grain of salt, especially if they don’t have a chronic illness. If whatever the sentiment is doesn’t sit well with you, then ignore it.
Don’t stop living. Life isn’t over, it’s just different.
Find community. There are so many ways to connect with others who will get what you are going through. You are not alone in this.
Written by Sam SalvaggioLiving with multiple sclerosis (MS) poses daily challenges. But recent advances offer hope and help for people coping with the unpredictability of the disease.
M“S care has been revolutionized by safe and highly effective treatments for the inflammatory components of the disease,” said Mayo Clinic neurologist Dr. William Oliver Tobin. “If caught early enough, we can prevent disability for many patients with MS.”
The first step in preventing disability is early and accurate diagnosis. That can be difficult because the symptoms of MS are similar to symptoms of other autoimmune diseases.
“Mayo Clinic offers a combination of neurological expertise and advanced technology that provides early and accurate diagnosis of MS and other diseases that can mimic it,” said Mayo Clinic neurologist Dr. Dean M. Wingerchuk.


A new diagnostic test for MS is available, thanks to groundbreaking research at Mayo Clinic. The test measures immunoglobulin kappa free light chains rather than oligoclonal bands — the target of current gold-standard testing.
Mayo Clinic researchers were the first to discover that a certain type of T cell (CD8) plays a critical role in causing nerve damage in MS. That discovery might lead to new drugs to slow or stop that nerve damage.
Living with MS is particularly challenging for children and can be especially difficult to diagnose. Mayo Clinic’s Pediatric Multiple Sclerosis Center in Rochester, Minnesota is recognized as a Pediatric Network MS Center by the National Multiple
Sclerosis Society.
Mayo Clinic’s care team includes not only subspecialized neurologists, but also physiatrists, urologists, psychiatrists and psychologists, and neuro-ophthalmologists, as needed.
“With the understanding that each patient with MS has their own journey with the disease, here at Mayo Clinic, our goal is to provide multidisciplinary care that meets patients’ needs,” said Mayo Clinic neurologist Dr. Vanessa V. Marin Collazo.
Written by Mayo Clinic











































Starting in my early 20s, I experienced bouts of intense fatigue. It got so bad that, at the age of 22, I fell asleep at the wheel of my pickup and was hit by an 18-wheeler. Both trucks exploded into flames. By what I call divine intervention, I survived that accident without spending a night in the hospital.
During this time, I worked a high-stress job as an HVAC technician. I loved the action but continuously suffered from extreme fatigue and weakness on the right side of my body.
I advanced in my career, but my symptoms persisted with no clarity as to the reason behind them. Many doctor visits over the years failed to elucidate the cause of my symptoms. I didn’t think much of it until 2008 brought more than just fatigue.
After being promoted to operations manager, my work-related stress levels increased. Midway through 2008, I began tripping over unseen obstacles. In December, I reached a breaking point — my right arm went limp. I feared I was having a heart attack or a mini-stroke. After ruling out those causes, my doctor advised me to see a neurologist, who conducted a series of tests.
Two weeks later, everything changed: I received the news that I had relapsing multiple sclerosis (RMS). I freaked out. I thought MS

was a death sentence. My neurologist advised me to start therapy immediately. Though overcome with fear, I complied.
My treatment journey was far from easy. I was on an injectable therapy for several years that brought side effects and the lack of motivation to stay compliant. Traveling with the treatment became burdensome as well.
I was determined to explore alternative treatment options. After frank discussions with my neurologist, she made me aware of an oral treatment: MAVENCLAD® (cladribine) tablets. My doctor explained the potential benefits of MAVENCLAD as well as the risk of serious side effects, including the risk of cancer, birth defects, low white blood cell counts, serious infections, and liver problems.
The convenient, short-course dosing schedule of MAVENCLAD particularly interested me. For me, I would only have to take two pills a day for five days, do it again a month later, and my MS treatment would
be done for that year. After repeating this process a year later, that would be it!
After she explained that screening and monitoring would be required before, during, and after treatment, we agreed that MAVENCLAD was the best fit, and I scheduled my first treatment in 2019.
My experience with MAVENCLAD has been positive, affording me the pleasure of waking up and focusing on breakfast rather than having to remember to take a shot. The convenience of the short-course dosing means I no longer have to schedule my life around my treatment.
Thanks to MAVENCLAD, I have redefined the narrative of my life beyond RMS. I’ve finished my treatment courses, haven’t had any relapses, and am able to enjoy traveling, as well as time with loved ones. Today, I try not to make my MS bigger than it is. It’s one part of my life — but with MAVENCLAD, it doesn’t define me.
Written by Ron, Living with MS
MS is one part of my life—but with MAVENCLAD, it doesn’t define me.

Convenient oral dosing*
Well-characterized
No continuous immunosuppression†
$0 co-pay for eligible‡ patients
1:1 patient support options
*Each week of MAVENCLAD treatment is known as a cycle and consists of 1 or 2 MAVENCLAD pills a day for 4 or 5 days (depending on your body weight) in a row. There are 2 MAVENCLAD cycles a year, about a month apart, for 2 years. Your healthcare provider will continue to monitor your health during the 2 yearly treatment courses, as well as between treatment courses, and for at least another 2 years, during which you do not need to take MAVENCLAD.
†Due to it’s dosing schedule, taking MAVENCLAD causes temporary immunosuppression, and allows impacted immune cells to recover after each treatment course.
MAVENCLAD is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, MAVENCLAD is generally used in people who have tried another MS medicine that they could not tolerate or that has not worked well enough.
MAVENCLAD is not recommended for use in people with clinically isolated syndrome (CIS). It is not known if MAVENCLAD is safe and effective in children under 18 years of age and is therefore not recommended.
MAVENCLAD may cause serious side effects, including:
• Risk of cancer (malignancies). You should follow healthcare provider instructions about screening for cancer.
• MAVENCLAD may cause birth defects if used during pregnancy. Women must not be pregnant when they start treatment with MAVENCLAD or become pregnant during MAVENCLAD dosing and within 6 months after the last dose of each yearly treatment course. You should stop treatment with MAVENCLAD and contact your healthcare provider right away if you become pregnant during treatment with MAVENCLAD.
Please see Brief Summary of Important Safety Information on the following pages.
‡Some limitations are required by law. Patients covered by federal or state healthcare programs, including Medicare and Medicaid, are not eligible for assistance. This program is open to residents of the U.S. and Puerto Rico with relapsing forms of multiple sclerosis who are starting MAVENCLAD therapy or presently taking MAVENCLAD.
Read this information carefully before using MAVENCLAD. As this is not all the information about this prescription medicine, talk to your doctor and visit www.mavenclad.com to see the FDA approved labeling and patient Medication Guide.
What is the most important information I should know about MAVENCLAD?
MAVENCLAD can cause serious side effects, including:
• Risk of cancer (malignancies). Treatment with MAVENCLAD may increase your risk of developing cancer. Talk to your healthcare provider about your risk of developing cancer if you receive MAVENCLAD. You should follow your healthcare provider instructions about screening for cancer.
• MAVENCLAD may cause birth defects if used during pregnancy. Women must not be pregnant when they start treatment with MAVENCLAD or become pregnant during MAVENCLAD dosing and within 6 months after the last dose of each yearly treatment course. Stop your treatment with MAVENCLAD and call your healthcare provider right away if you become pregnant during treatment with MAVENCLAD.
• For women who are able to become pregnant:
Your healthcare provider should order a pregnancy test for you before you begin your first and second yearly treatment course of MAVENCLAD to make sure that you are not pregnant. Your healthcare provider will decide when to do the test.
Women who can become pregnant as well as men with female partners who can become pregnant, should use effective birth control (contraception) on the days on which you take MAVENCLAD and for at least 6 months after the last dose of each yearly treatment course.
Ask your healthcare provider which contraceptive method is right for you.
MAVENCLAD is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include relapsing remitting disease and active secondary progressive disease, in adults. Because of its safety profile, MAVENCLAD is generally used in people who have tried another MS medicine that they could not tolerate or that has not worked well enough.
MAVENCLAD is not recommended for use in people with clinically isolated syndrome (CIS).
It is not known if MAVENCLAD is safe and effective in children under 18 years of age.
Do not take MAVENCLAD if you:
• have cancer (malignancy).
• are pregnant, plan to become pregnant, or are
• a woman of childbearing age or a man able to father a
child and you are not using birth control. See “What is the most important information I should know about MAVENCLAD?”
• are human immunodeficiency virus (HIV) positive.
• have active infections, including tuberculosis (TB), hepatitis B or C.
• are allergic to cladribine.
• are breastfeeding. See “Before you take MAVENCLAD, tell your healthcare provider about all of your medical conditions, including if you:“
Before you take MAVENCLAD, tell your healthcare provider about all of your medical conditions, including if you:
• think you have an infection.
• have heart failure.
• have liver or kidney problems.
• have taken, take, or plan to take medicines that affect your immune system or your blood cells, or other treatments for MS. Certain medicines can increase your risk of getting an infection.
• have had a recent vaccination or are scheduled to receive any vaccinations. You should not receive live or live-attenuated vaccines within the 4 to 6 weeks preceding your treatment with MAVENCLAD. You should not receive these types of vaccines during your treatment with MAVENCLAD and until your healthcare provider tells you that your immune system is no longer weakened.
• have or have had cancer.
• are breastfeeding or plan to breastfeed. It is not known if MAVENCLAD passes into your breast milk. Do not breastfeed on the days on which you take MAVENCLAD, and for 10 days after the last dose. See “Do not take MAVENCLAD if you:”
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
• MAVENCLAD is given as two yearly treatment courses; each course consisting of approximately 2 treatment weeks (also called cycles) about a month apart.
• Handle MAVENCLAD with dry hands and take immediately after opening the blister pack. Take with water and do not chew the tablet. MAVENCLAD can be taken with or without food and should be taken at least 3 hours apart from other oral medicines.
• Wash your hands after handling MAVENCLAD. Limit contact with your skin (especially on your face). Avoid touching your nose, eyes and other parts of the body. If you get MAVENCLAD on your skin or on any surface, wash it right away with water.
• If you miss a dose, take it as soon as you remember on the same day. If the whole day passes before you
• remember, take your missed dose the next day. Do not take 2 doses at the same time. Instead, you will extend the number of days in that treatment week.
Your healthcare provider will continue to monitor your health during the 2 yearly treatment courses, and for at least another 2 years during which you do not need to take MAVENCLAD. It is not known if MAVENCLAD is safe and effective in people who restart MAVENCLAD treatment more than 2 years after completing 2 yearly treatment courses.
What are the possible side effects of MAVENCLAD?
MAVENCLAD can cause serious side effects, including:
• See “What is the most important information I should know about MAVENCLAD?”
• low blood cell counts. Low blood cell counts have happened and can increase your risk of infections during your treatment with MAVENCLAD. Your healthcare provider will do blood tests before, during and after your treatment with MAVENCLAD, as needed.
• serious infections such as:
• life-threatening or fatal infections caused by bacteria, viruses, parasites or fungi
• TB, hepatitis B or C, and shingles (herpes zoster). Fatal cases of TB and hepatitis have happened with cladribine during clinical studies. Tell your healthcare provider right away if you get any symptoms of the following infection related problems or if any of the symptoms get worse, including:
fever
aching painful muscles
headache
feeling of being generally unwell
loss of appetite
burning, tingling, numbness or itchiness of the skin in the affected area
skin blotches, blistered rash, and severe pain
• progressive multifocal leukoencephalopathy (PML). PML is a rare brain infection that usually leads to death or severe disability. Although PML has not been seen in MS patients taking MAVENCLAD, it may happen in people with weakened immune systems. Tell your healthcare provider right away if you have any new or worsening neurologic signs or symptoms. These may include:
weakness on 1 side of your body
loss of coordination in your arms/legs
decreased strength
problems with balance
changes in your vision
changes in your thinking or memory
confusion
changes in your personality
• liver problems. MAVENCLAD may cause liver
problems. Your healthcare provider should do blood tests to check your liver before you start taking MAVENCLAD. Call your healthcare provider right away if you have any of the following symptoms of liver problems:
nausea
vomiting
stomach pain
tiredness
loss of appetite
your skin or the whites of your eyes turn yellow
dark urine
• allergic reactions (hypersensitivities). MAVENCLAD can cause serious allergic reactions. Stop your treatment with MAVENCLAD and go to the closest emergency room for medical help right away if you have any signs or symptoms of allergic reactions. Symptoms of an allergic reaction may include: skin rash, swelling or itching of the face, lips, tongue or throat, or trouble breathing.
• heart failure. MAVENCLAD may cause heart failure, which means your heart may not pump as well as it should. Call your healthcare provider or go to the closest emergency room for medical help right away if you have any signs or symptoms such as shortness of breath, a fast or irregular heartbeat, or unusual swelling in your body. Your healthcare provider may delay or completely stop treatment with MAVENCLAD if you have severe side effects.
The most common side effects of MAVENCLAD include:
• upper respiratory infection
• headache
• low white blood cell counts
These are not all the possible side effects of MAVENCLAD. Call your doctor for medical advice about side effects. You may report suspected adverse reactions to EMD Serono at 1-800-283-8088 ext. 5563 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Distributed by: EMD Serono, Inc., Rockland, MA 02370
MAVENCLAD is a registered trademark of Merck KGaA, Darmstadt, Germany.
For more information, call toll-free 1-877-447-3243 or go to www.mavenclad.com
©2024 EMD Serono, Inc. All rights reserved. US-MAV-02340
Printed in USA 03/2024

As the president of the Board of Governors of the Consortium of Multiple Sclerosis Centers (CMSC) who leads her own MS Center in St. Louis, Dr. Anne H. Cross is one of the world’s foremost thought leaders and researchers in MS. We talked to her about the latest innovations in detecting and treating MS, and the steps patients should take if they start experiencing unexplained neurological symptoms.
 INTERVIEW WITH Anne H. Cross, M.D. Professor of Neurology, Washington University School of Medicine in St. Louis
INTERVIEW WITH Anne H. Cross, M.D. Professor of Neurology, Washington University School of Medicine in St. Louis
What challenges do MS patients currently face when seeking an initial diagnosis for their condition?
When a person develops neurological symptoms that might be from MS, such as visual changes, weakness in one or two limbs, incoordination, or changes in sensation, they first have to make the step to see a doctor (ideally a neurologist). The clinician they see then must consider MS as a possible diagnosis.
It is important, therefore, for a person with new neurologic symptoms to see a doctor and to provide a chronological list of their symptoms. However, sometimes the first symptoms are not typical of MS, or are so mild that the clinician cannot find anything wrong on examination.
How can early diagnosis and intervention impact an MS patient’s treatment and quality of life?
MS is an inflammatory neurological disease that is restricted to the central nervous system. Most people have relapsing MS, which can be relapsing remitting in course, or be progressive but have superimposed relapses or “attacks.”
Relapsing MS is the easiest form of MS to treat. We have a number of therapies that can reduce the number
of relapses, or in some people, stop relapse altogether. For people with relapsing MS, several of these medications have been shown to alter outcomes for the better.
Of course, these medications have both benefits and risks that vary among the available agents. Some of these “disease-modifying therapies” (DMTs) suppress the immune system, and that is of concern. Some may increase risk of developing cancer. But, these DMTs have revolutionized the lives of people with MS because they can delay or stop progressive disability accumulation.
What are some current advancements being made to increase early diagnosis and intervention of MS?
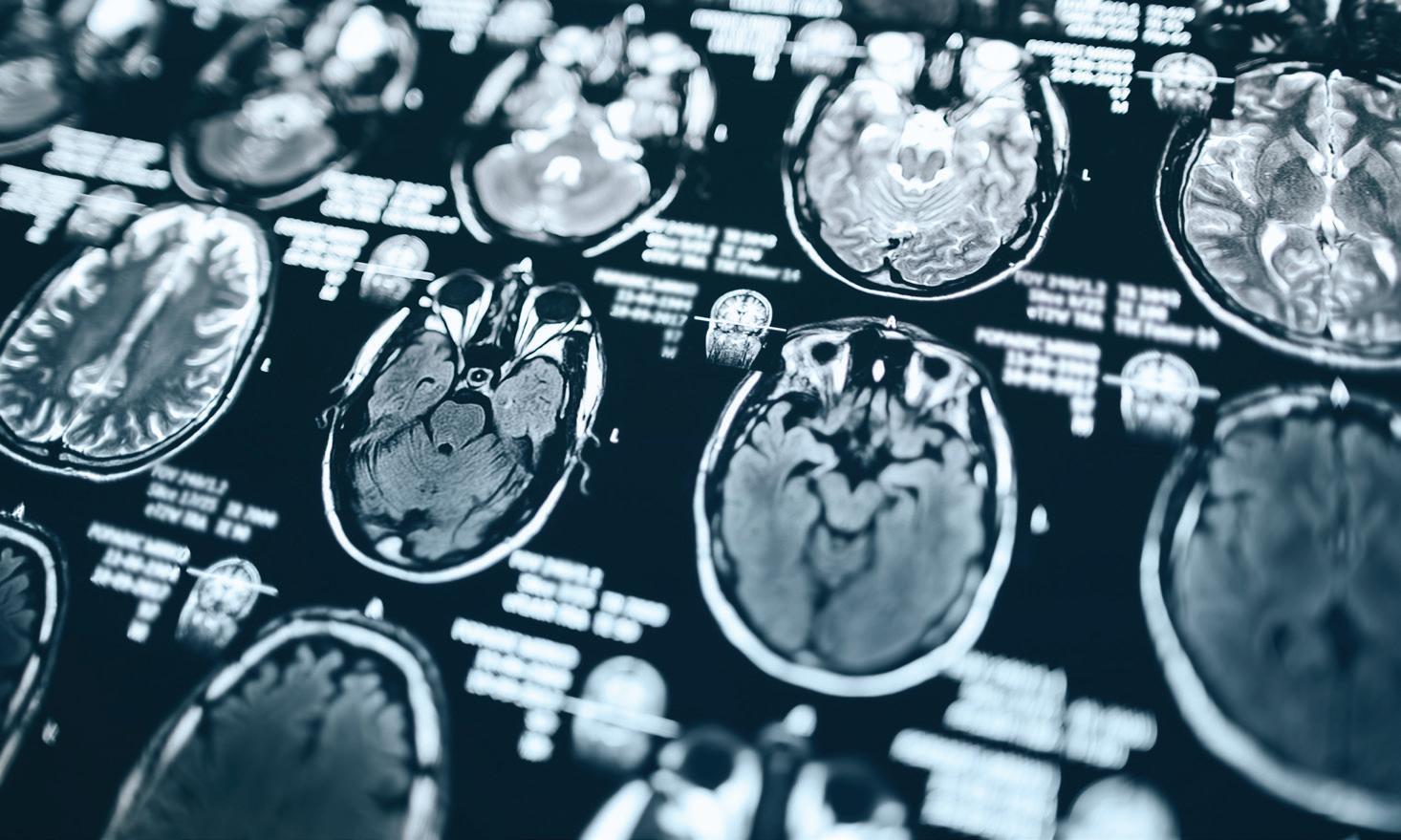
For early diagnosis, one new diagnostic test relates to an imaging finding that is rare in diseases other than MS.
With special MRI sequences (or protocols), brains of people being evaluated for MS can be examined for central veins in lesions. These central veins are typical of MS white matter lesions, and can be seen now on MRI by combining T2-weighted images together with susceptibility weighted imaging. If more than half of white matter lesions have a “central vein sign,” this greatly supports the diagnosis of MS.
As far as efforts at early intervention, accumulating evidence suggests it makes a positive difference. If the MS patient initiates and remains on one of the several DMTs now available, it can have beneficial long-term effects.
What are some recent developments or emerging therapies for the treatment of MS? MS is mediated by abnormal immune system responses. Successful recent developments include the FDA approval of three B lymphocyte (B cell) depleting agents. These are all monoclonal antibody therapies that kill B cells in the blood. Amazingly, these agents can reduce MS relapses by about 90% or more in most people.
Several new oral medications, called Bruton’s tyrosine kinase inhibitors, are being studied as well. These inhibit B cells and other immune system cells called myeloid cells.
Progressive MS, which involves more neurodegeneration than relapsing MS, has been difficult to effectively treat. A number of studies are ongoing now, mostly in early stages, to target the neurodegenerative elements of MS. It is perhaps important to know that relapses and neurodegenerative aspects of MS often coexist in the same person.