The Future of Immunisation and Vaccines
Strengthening
 Aurélia Nguyen, Chief Programme Officer, Gavi, the Vaccine Alliance
Aurélia Nguyen, Chief Programme Officer, Gavi, the Vaccine Alliance
 Andrea Ammon, Director, ECDC
Andrea Ammon, Director, ECDC


Strengthening
 Aurélia Nguyen, Chief Programme Officer, Gavi, the Vaccine Alliance
Aurélia Nguyen, Chief Programme Officer, Gavi, the Vaccine Alliance
 Andrea Ammon, Director, ECDC
Andrea Ammon, Director, ECDC

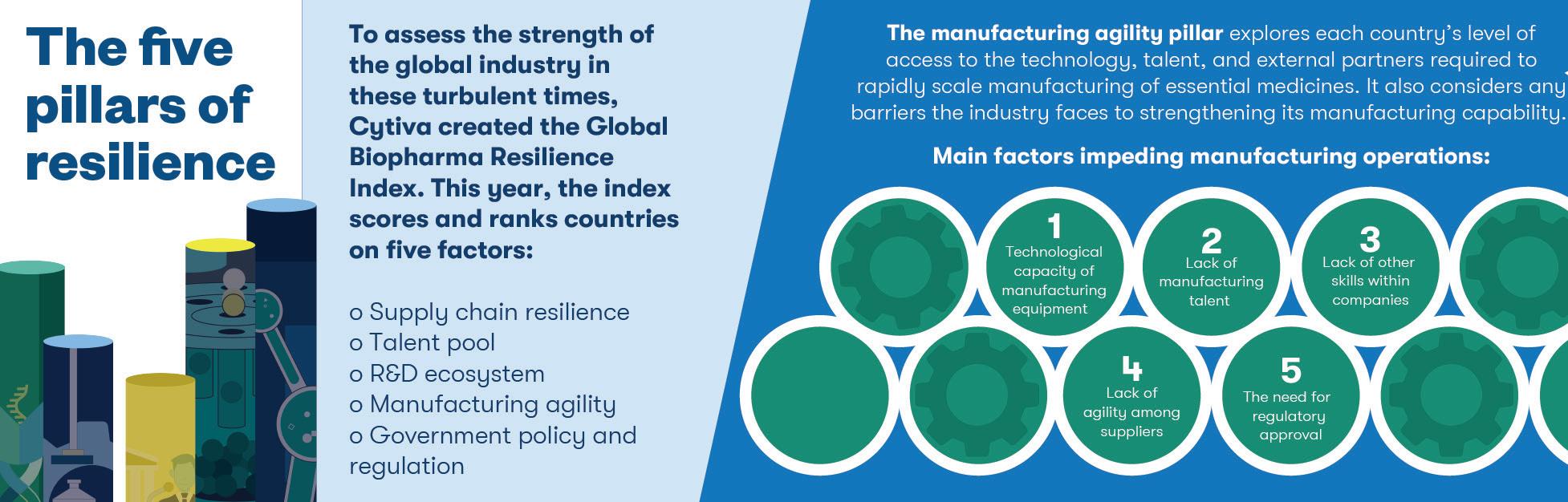
Recent years have highlighted, with sobering clarity, the devastating impact public health emergencies can have on our lives and livelihoods. And with the potential that a new, previously unknown pathogen, often referred to as ‘Disease X,’ could emerge at any time — or that a known pathogen could re-emerge — preparation is essential to minimise the impact on health and society.
At UKHSA, we are focused on readying the UK against all eventualities, and vaccination is core to our strategy. Even amid responding to the global pandemic, we have been keeping one eye on the horizon, building the UK’s resilience against future threats to mitigate their impact. That means ensuring the UK has early access to effective vaccines. Our work with industry and academia is central to that aim, particularly two landmark initiatives: UKHSA’s Vaccine Development and Evaluation Centre (VDEC) and the ModernaUK Strategic Partnership. Both strive to push the boundaries of vaccine research and development for pandemic preparedness, promoting new ways of working with industry that will help us respond to health threats more effectively than ever before.
The COVID-19 pandemic demonstrated that closer collaboration between government,
industry and academia can lead to greater innovation, faster breakthroughs and better health outcomes — and these two initiatives are excellent examples of driving that collaboration forward.
VDEC grew from the legacy of the COVID-19 pandemic and officially launched this summer. It brings together almost 300 scientists working in over 2,800 square metres of laboratory space with highly specialised capabilities at Porton Down to deliver worldleading science.
Its sole aim is to target the deadliest pathogens and those with pandemic potential by working with industry and academic institutions to help find, develop and evaluate new vaccines and treatments where none exist — or improve those that do. Its work includes rapid assessment of vaccines against new variants to ensure that our vaccination programmes are effective.

VDEC scientists have already discovered the possibility of developing a world-first vaccine against Crimean-Congo haemorrhagic fever — a virus spread by the bite of an infected tick that is fatal in about 30% of cases. The vaccine is in stage one clinical trials and, if successful, would be a global breakthrough.
We are working with several vaccine manufacturers to ensure we have future access to effective vaccines as and when we need them. The most recent development in that area is the UK’s 10-year partnership
with Moderna, announced in 2022. The agreement secures future access to up to 250 million lifesaving vaccines per year, catering to demand, as well as funding research into new vaccines combating various threats.

Under the partnership, Moderna is building new mRNA vaccine research and development and manufacturing facilities in the UK and has committed substantial funding to UK-based R&D activities over a 10-year period. The centre will also have the potential to develop vaccines targeting a range of illnesses, such as flu, cancer and rare diseases.
It will significantly boost our ability to respond to future pandemics and, alongside VDEC, support the global 100 Days Mission, launched in 2021 under the UK G7 presidency, with the ambitious aim of deploying an effective vaccine within 100 days of identifying a new pandemic threat.

Our strategy is about building capacity and flexibility into the system for pandemic preparedness. It is nearly impossible to predict exactly what the next major public health threat will be; but, by laying the foundations ahead of time, we can ensure our response will be quick and effective — saving lives and minimising the impact on society. We have made a good start, and UKHSA continues working to ensure the UK leads the way.
Strategic Account Director: Benedetta Marchesi benedetta.marchesi@mediaplanet.com Managing Director: Alex Williams Country Manager UK: Ellie McGregor | Head of Print & Design: Thomas Kent Designer: Aimee Rayment Content Editor: Angelica Hackett O’Toole | Head of Digital Operations: Harvey O’Donnell Paid Media Strategist: Jonni Asfaha Social & Web Editor: Henry Phillips Digital Assistant: Carolina Galbraith Duarte | All images supplied by Gettyimages, unless otherwise specified
The world’s population is facing a rising tide of threats against health as factors like climate change and globalisation alter the spread of infectious diseases.Richard Stratford Ph.D CEO & Co-Founder, NEC OncoImmunity


The fight against infectious diseases takes a significant leap forward with a cutting-edge AI platform that can design universal vaccines.
New developments within artificial intelligence are unlocking the potential for creating broadly protective universal vaccines against infectious diseases. NEC OncoImmunity’s (NOI) technology can identify cross-reactive immune targets covering numerous strains of a disease, cut development timelines, and make vaccines cheaper for more equitable global distribution.
What is a universal vaccine?
While current vaccines confer immunity to one or several strains of a disease, universal vaccines are designed to teach the immune system to defend against all versions of a pathogen. Significantly, that may even cover versions that do not yet exist.
Vaccines targeting all variants
WRITTEN BY Mark NichollsBiotech company NOI’s AI platform, with proprietary machine-learning-based software, was initially designed for personalised cancer immunotherapy and is now also repurposed to identify optimal antigen/epitope targets for infectious disease vaccine development. Co-founded as OncoImmunity by CEO Dr Richard Stratford and CSO Dr Trevor Clancy in 2014, the company has been a part of NEC group since 2019 and, more recently, NEC Bio B.V.
The company is already developing a portfolio of vaccine designs, which can be further developed into commercial vaccines, and is seeking meaningful
partnerships with established players in the infectious diseases field. Dr Clancy explains that the ability to design universal vaccines has been made possible by the advent of next-generation sequencing (NGS) data, which can rapidly sequence pathogens and genomes, and the evolution of AI algorithms.
Faster infectious disease vaccine production
Dr Stratford says the process of target discovery, previously conducted in wet labs, is now done in silico, with the timelines reduced from years to weeks. Following the pandemic, a global target was set to develop vaccines within a 100-day timeframe to protect against current and emerging threats. “We can already develop personalised cancer vaccines, within a short timeframe; the trick now is to translate that into the infectious disease field,” he adds.
Enabled by AI and partnerships
The AI platform could tackle diseases that have proven refractory to developing vaccines, explains Dr Stratford. “It also speeds up the process and makes it cheaper to develop vaccines,” he adds. Currently, Dr Stratford is looking to build partnerships to progress NOI’s portfolio into clinical development.


A major biotechnology company is helping to ensure the UK is ‘pandemic-prepared’ through the construction of a manufacturing and innovation centre. It aims to develop and produce mRNA vaccines for various known and unknown respiratory diseases.
One of the major learnings of the COVID-19 pandemic was that the world was ill-prepared. As a result, the G7, led by the UK Government, developed the ‘100-Day Mission to Respond to Future Pandemic Threats.’
Central to the mission is a 10-year agreement between the UK Government and Moderna, a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines. Construction of the Moderna Innovation and Technology Centre (MITC) at the Harwell Science Campus in Oxfordshire is underway. It encompasses research, development, clinical trials and a clinical biomarker lab to provide the UK with access to mRNA vaccines for respiratory diseases in the event of another pandemic as well during endemic times.
Timely pandemic response
The company’s UK General Manager Darius Hughes says the centre will ensure the country is ‘pandemicprepared’ by providing the UK Government with direct access to pandemic response capabilities. He explains that the centre will possibly enable the production of mRNA vaccines within 100 days for the nation.
also gives the Government the ability to upscale manufacturing in a very short period — because of the flexibility of mRNA — if we have another pandemic.”
Covering a range of diseases
mRNA is a code or message that the body produces to make a protein. They prompt the body to produce antibodies, which help fight disease. Once its job is done, the messenger is quickly broken down and leaves the body.
As well as COVID-19 vaccines, the Moderna mRNA platform manufactures those ‘messages’ for other viruses and bacteria. “The mRNA platform has the ability to be effective in producing vaccines and medicines across a wide range of therapy areas,” adds Hughes.
UK-MRNA-2300080
Date of preparation: September 2023
“That gives us the ability to onshore mRNA vaccine development for the UK public,” says Hughes. “It
The centre aims to provide a manufacturing hub for ‘endemic and pandemic times’ but also make the UK “one of the leading countries in the world for mRNA technology,” explains Hughes. Moreover, the centre will support local job creation — and skills development across the country — as it helps prepare the UK to respond to respiratory disease threats and future pandemics.
Maternal immunization is a crucial health intervention because it can help protect newborn infants — whose immune systems are not yet sufficiently developed — from day one.
Iona Munjal, MD Executive Director, Vaccine Research & Development, Pfizer WRITTEN BY Tony GreenwayIona Munjal is a doctor who has experienced infant illness firsthand. She’s also a mother. Both roles inform the work she does as Executive Director, Vaccine Research and Development at Pfizer. She is passionately committed to researching and developing breakthrough maternal vaccines that have the potential to protect and make a life-changing difference in the health of newborn babies.
Babies may benefit from maternal immunization

Maternal immunization — vaccinating pregnant women to help protect the health of their babies from their very first breath — is a vital intervention, explains Dr. Munjal. “Infants are most vulnerable to sickness when they’re first born, because they don’t have a developed immune system and may not be able to capably fight off infections,” she says.
“Infant immunization schedules typically begin when a child is two months old. But maternal immunization is a gift that can help protect infants before they are fully immunized.”
Sometimes, nature steps in and equips infants with immunity against certain illnesses while they are still in the womb. Dr. Munjal points out that women who have had infections and have sufficiently high levels of antibodies naturally pass those antibodies (‘the fighting skills, so to speak’) via their placenta to their babies, thereby providing protection from becoming sick with those same infections.
With maternal immunization, modern medicine is simply taking a page out of Mother Nature’s playbook. “At Pfizer, we want to capitalize on the concept of passive protection transference by giving pregnant women vaccines for common infections that their
babies might experience,” notes Dr. Munjal. “It’s a lovely idea for scientific research that piggybacks on a very important piece of evolutionary design and helps to protect the infant from illness from day one.”
Take advantage of existing maternal vaccines
Maternal vaccination is not new, however. The Tdap vaccine (tetanus, diphtheria, and pertussis) is already given to women to protect their newborns against pertussis (whooping cough), an illness that can have lifethreatening complications. Flu and COVID vaccines are also offered to protect the health of the mother. “We’re starting to head into winter and the flu season,” says Dr. Munjal. “So, if a flu vaccine is available and recommended by her doctor, then the vaccine has a dual role of protecting her — which is critical — and subsequently providing antibody protection to her unborn infant.”
At Pfizer, Dr. Munjal and her team have been working on maternal vaccines to help protect infants against respiratory syncytial virus (RSV) and group B streptococcus (GBS). These are two particularly pernicious conditions, she explains. RSV is a contagious lower respiratory tract illness that is both extremely prevalent and potentially life-threatening for young infants. In the United States, approximately 500,000 to 600,000 infants experience lower respiratory tract disease due to RSV each year, and it is a leading cause of hospitalization in children less than one year of age. Meanwhile, group B streptococcus can cause potentially devastating diseases in infants, including sepsis, pneumonia, and meningitis. Pfizer hopes that maternal vaccination
will help protect newborns and young infants from disease. Recently, the U.S. Food and Drug Administration (FDA) approved the company’s RSV vaccine, for the prevention of lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth through six months of age by active immunization of pregnant individuals at 32 through 36 weeks gestational age. The European Commission (EC) also granted marketing authorization for the company’s RSV vaccine for passive protection against LRTD caused by RSV in infants from birth through six months of age following maternal immunization during pregnancy. Additionally, the FDA and EC also approved the vaccine for active immunization of individuals 60 years of age and older for the prevention of LRTD caused by RSV.
Pfizer’s GBS vaccine candidate is currently in a Phase 2 clinical trial, and in July, positive efficacy and safety data from the study were published.

Bringing meaningful change
It’s no exaggeration to say that these immunizations — if they are both successfully developed and approved— could reduce parental and child misery and potentially prevent unnecessary deaths. Frankly, it’s about time that children were protected from such potentially devastating conditions, says Dr. Munjal. “I can’t think of anything more meaningful than bringing change via new vaccines.”
It’s not just pregnant individuals and children in the first world who stand to benefit. In 2022, Pfizer announced a partnership with leaders from Rwanda, Ghana, Malawi, Senegal, Uganda, and the Bill & Melinda Gates Foundation to work towards sustained, equitable access to medicines and vaccines for 1.2 billion people living in 45 lower-income countries. The Bill and Melinda Gates Foundation has also provided support for the development and accessibility of the maternal RSV vaccine and GBS vaccine candidate in lower-income countries with grants totaling nearly US$128 million, including the development of a multidose vial for potential delivery of these vaccines in lower-income countries.
they are, mothers-to-be may be worried about the risks of maternal vaccinations. “We don’t want to negate the fact that shots can cause some discomfort at the site of the vaccination,” says Dr. Munjal. “No one likes getting shots. But what we’ve seen with maternal vaccines, is that reactogenicity — the way vaccines are tolerated — is quite good. So, most of the reactions that pregnant individuals tend to have are mild to moderate.”
During the research and development of vaccines, patient safety is the number one priority.
“For this reason, Pfizer uses a three-tiered system in our clinical trials,” says Dr. Munjal. “First, we as a company, make sure that there are measures in place to record all safety events in the study.
Second, we work with clinical or academic researchers who actively monitor patient safety. Third, all patient data we collect are independently sent to and analyzed by outside groups including regulators, like the FDA in the United States, so they can make their own safety assessment. Patient data are also sent to an independent external data monitoring committee, an advisory group of experts in the field which ensures all safety data is collected and duly analyzed.”
Vaccine development is the very opposite of easy, admits Dr. Munjal. But when the results are successful, ‘gratified’ doesn’t even begin to cover how she and her team feel.
“Clinical studies of maternal and child populations, particularly infants, is a time-consuming and challenging thing to accomplish, but we’re committed to researching and developing innovative maternal vaccines,” she says.
“I’m proud to say that there’s now an option out there that could help reduce the burden of RSV. Then there’s our GBS study. If the vaccine candidate is successfully developed and approved, we could see it changing lives globally. That’s what we’re here for: to develop breakthroughs that change people’s lives.”
Vaccine development can take up to a decade, which doesn’t work when an infectious disease is spreading across the globe. How can manufacturers help to deliver more quickly?
The Coalition for Epidemic Preparedness Innovations (CEPI) is on a mission to explore whether new vaccines could be developed against emerging infectious diseases within just 100 days. With their recent successes and a range of advantages over traditional vaccine platforms, emerging rapid response technologies, like mRNA, are expected to play a significant role in achieving this goal.
Acceleration of vaccine development
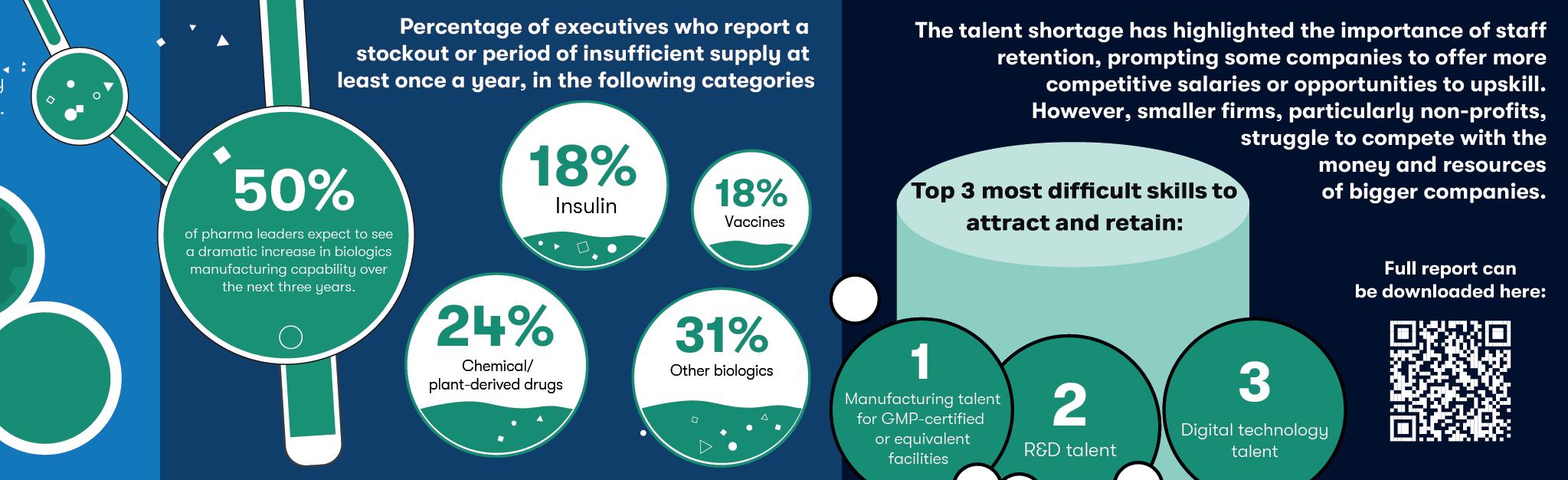
Before COVID-19, Merck held the record for the fastest modern vaccine ever developed. The company began formulating ERVEBO™ in 2014 while the Ebola Virus Disease (EVD) epidemic raged in West Africa. The live attenuated vaccine was authorised by the EMA and FDA five years later in 2019 — half the time than normal.1,2

When the COVID-19 pandemic first began, the highly transmissible disease took a matter of months to become a global health emergency at a scale the modern world had never seen. As the virus continued to spread and mutate, all hopes of a way out were pinned on a vaccine.
Single and multi-nation funding opportunities
flooded in. Devex’s global COVID-19 funding dashboard recorded $172 million invested in vaccine research between 1 January 2020 and 27 June 2020.3 With the world watching, the biopharmaceutical community got to work.
Impact of rapid vaccine distribution
Just 326 days after SARS-CoV-2 was first sequenced, the FDA approved Pfizer and BioNTech’s Comirnaty® under Emergency Use Authorization (EUA).4 This turnaround
was not only significantly faster than any previous vaccines, but the formulation also leveraged a vaccine technology that hadn’t been widely used in patients before: mRNA.
On its 100 Days website, CEPI applauds this recordbreaking development timeline. But it also argues that if these 326 days had been reduced to under 100, millions more lives could have been saved. According to CEPI: “Achieving the 100 Days Mission would give the world a fighting chance of containing a future outbreak before it spreads to become a global pandemic.”
UK investment in health innovations
The UK Government is behind the effort to reduce vaccine development timelines. The mission was first put forward during the UK’s G7 Presidency of June 2021, with support from representatives of the life sciences industry. In 2022, global governments pledged a combined $1.5 billion towards the ‘pandemic-busting plan,’ which involves the investigation of rapid response vaccine technologies.5 So, where do we go from here?

As of early 2023, a vaccine manufacturing facility — the mRNA Innovation and Technology Centre (MITC) — is being established in the UK. The facility will be used to develop and manufacture mRNA vaccines against respiratory viruses. It aims to strengthen the UK’s response capabilities and preparedness for any emergent health risks.
This national mission shows a commitment towards advancing technologies to ensure preparedness for health threats. With continuous effort, vaccine development within 100 days could move from a goal to a lifesaving reality.
Research into the therapeutic use of messenger ribonucleic acid (mRNA) had been ongoing for decades prior to the COVID-19 pandemic. Now, the global community is looking at mRNA technology as the potential answer for fast vaccine delivery.
What makes mRNA vaccines different from traditional formations? Instead of injecting someone with part of a pathogen, mRNA-based vaccines contain nucleic acids that code for a specific protein, or target antigen, related to a virus or disease. When an mRNA vaccine is administered, a patient’s body produces that protein to prompt a desired immune response.
The mechanism and manufacturing of mRNA vaccines offer many advantages to developers, including quick development, high potency, a favourable safety profile and the potential for cost-effective production. This modality could help efficiently control rapidly spreading infectious diseases.
mRNA vaccines can be made more quickly than traditional vaccine platforms because they are produced using an in vitro synthetic process that does not require the time-consuming growth or removal of cells and proteins. Platforms designed to create RNA can also be easily adjusted to target a range of indications or mutated versions of the same virus. This adaptability is what allowed Pfizer and BioNTech to redirect their existing mRNA platform from cancer to COVID in a matter of weeks.
Now that evidence suggests mRNA to be a safe and effective technology, researchers are hoping to use it to accelerate vaccine development for other indications. Unsurprisingly, companies that had success with mRNA during COVID-19 are now looking to expand to new applications. According to GlobalData, 85% of planned mRNA vaccine trials in 2023 are sponsored by industry, up from 34% of mRNA trials initiated in 2021. Moderna and Pfizer hold the two largest mRNA vaccine trials in 2023, for seasonal influenza.6
Arcturus Therapeutics is another company to watch, as they are currently working on an influenza vaccine based on a proprietary self-amplifying mRNA platform.
In August 2022, the Biomedical Advanced Research and Development Authority awarded Arcturus $63.2 million in funding to support this work.7
With focused attention and quick-moving research, exciting developments are expected in the field of mRNA. However, there are still challenges to overcome to fully realise mRNA’s potential.
To start, the stability of mRNA formulations can pose a challenge, as they are prone to degradation by ubiquitous enzymes and require strict temperature control and cold chain logistics. From a safety perspective, there is also a potential for an unwanted immune response and allergic reaction following patient administration.
When it comes to mRNA processing, dedicated consumables and equipment options suited to its characteristics are currently limited.8 An imbalance between supply and demand of plasmid DNA — a key raw material in mRNA production — has additionally become a bottleneck.
Tools to enable a rapid health response
Cytiva offers solutions across the mRNA manufacturing workflow, from plasmid linearisation to aseptic filling. For example, KUBio™ modular ‘box’ environments by Cytiva are designed to support rapid response manufacturing of mRNA in a Biosafety Level 1 (BSL-1) environment.
Installed inside that purpose-built environment, the FlexFactory™ platform can serve as an end-toend manufacturing solution for mRNA drugs. The Figurate™ automation platform provides a central data repository, enabling process analytics, reporting and regulatory compliance.
Achieving CEPI’s ‘100 Days Mission’ will require flexible, configurable and scalable manufacturing solutions. This fast turnaround time will also require a tool provider that is reliable and responsive, delivering high-quality systems with efficiency. When the next epidemic strikes, Cytiva will be ready to support the industry in rising to the challenge of a truly rapid response.
References
2. Gavi. From biodefence to the DRC: How the Ebola vaccine became one of the fastest vaccines to license in history. https://www.gavi.org/vaccineswork/biodefence-drc-how-ebola-vaccine-became-one-fastest-vaccines-license-history;
1. Wolf, J et al. Development of Pandemic Vaccines: ERVEBO Case Study. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7996233/;
3. Devex. Who’s funding the COVID-19 response and what are the priorities? https://www.devex.com/news/interactive-who-s-funding-the-covid-19-response-and-what-are-the-priorities-96833; 4. CEPI. 100 Days. https://100days.cepi.net/100-days/; 5. CEPI. Global community comes together in support of 100 Days Mission and pledges over $1.5 billion for CEPI’s pandemic-busting plan. https://cepi.net/news_cepi/global-community-comes-together-in-support-of-100-days-mission-and-pledgesover-1-5-billion-for-cepis-pandemic-busting-plan/; 6. GlobalData. Clinical Trials Arena. mRNA vaccines: four major clinical trial readouts to watch in 2023.; 7. Yahoo Finance. Arcturus Announces $63.2 Million Award from the U.S. Government to Support Development of Self-amplifying mRNA Vaccine for Rapid Pandemic Influenza Response. https://finance.yahoo.com/news/arcturus-announces-63-2-million-200000755.html?guce_ referrer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvbS8&guce_referrer_sig=AQAAAMcw4zgt1tvCDZ86z2AHBqHHUI8KkLS685B4AGqoxQSpJXb_OUpwYnga1lZdVRd2RGZVGSxrT25Khe01u8rNpfbpg9A7Ld71AzxFHiLEpj3H 2UKr-1zBPthRHjGZG9u3X-yiMK7tjxzqpy0bynIyzE2n7J-yR0lwXKCfQAS-KH1W&guccounter=2; 8. Cytiva. mRNA vaccines: current trends and perspectives. https://www.cytivalifesciences.com/en/us/solutions/cell-therapy/ knowledge-center/resources/current-trends-and-perspectives-in-mrna-vaccines
Nobody can be blamed for not wanting to hear the word ‘pandemic’ ever again. COVID-19, the worst global health emergency in 100 years, claimed the lives of millions of people.
The pandemic wreaked profound economic and psychological distress on many. Yet, just as the COVID-19 years are beginning to slide inelegantly into the rearview mirror of our collective consciousness, we mustn’t let the learnings of our response get lost forever.
It is more or less an evolutionary certainty that we will experience future pandemics and, due to several factors including climate change, mass migration, and continued encroachment by humans into animal habitats, this likelihood is increasing. This means we need to break the cycle of panic-neglect that we have allowed to take root each time we face a global health emergency of this scale.
elderly, or those with underlying health conditions.

Secondly, we need to build a world where vaccines and treatments can be manufactured in every region, rather than have production concentrated in just a few countries, which can then impede equitable access by prioritising their own populations first. These are just two instances where action must be taken now to override the inevitable nationalism and export bans that hampered global efforts to bring COVID-19 under control.
We need to build a world where vaccines and treatments can be manufactured in every region.
What are the lessons from COVID-19 that are invaluable in helping us be better prepared next time? First, we need to ensure contingent at-risk funding is available to allow at-risk lower-income countries to order vaccines or treatments as soon as they come onto the market — just as wealthier countries were able to do. This way, we can collectively be sure that scarce goods are directed first to those who need them, such as health workers, the
Fortunately, progress is being made in both areas. As a co-lead of COVAX, the international initiative for equitable access to COVID-19 vaccines, and as the Chief Programme Officer of the Vaccine Alliance, we are working with our partners, including the African Union, to kickstart development of a thriving African manufacturing ecosystem. Meanwhile, thanks to partnerships with development finance agencies, lines of credit are on hand to provide financing should new vaccines need to be procured.
Aurélia Nguyen Chief Programme Officer, Gavi, The Vaccine Alliance Philippa Whitford
Philippa Whitford

The Coalition for Epidemic Preparedness Innovations (CEPI) aims to shorten vaccine development to 100 days in any future pandemic, with the ‘100-Day Mission.’
With a significant risk of future pandemics, it is vital we learn from COVID-19. Despite a pandemic being top of the UK Government’s risk register since 2009, the UK was still utterly unprepared, leaving lockdown as the only option to control viral spread until vaccines became available in December 2020.
The speed of development of the COVID-19 vaccines was one of the great achievements of the pandemic, taking just 336 days. Rather than starting from zero, COVID-19 vaccines were developed from ongoing research. Oxford University’s work on Middle East Respiratory Syndrome (MERS), another Covid virus, led to the AstraZeneca vaccine, while BioNTech’s research into mRNA technology led to their vaccine, which was mass produced by Pfizer. The ‘100-Day-Mission’ in a future pandemic requires ongoing government funding of life sciences, including immunological, virological and vaccine research, so viruses with epidemic potential are well understood and candidate vaccines can be developed quickly.
While governments spend vast sums on advanced weapons, which they hope never to use, bioresilience must be included as a key part of defence and security. The OECD estimates that the pandemic cost the global economy over $9 trillion, so avoiding a repeat would be money well spent. The achievement in developing the COVID vaccines was, however, marred by the failure of the international community to provide global vaccine equity.
Despite the rhetoric of spring 2020, of organising a ‘global response to a global crisis’, wealthy countries stockpiled doses while low and middle-income countries struggled to vaccinate even their healthcare workers. Protracted negotiations regarding waiving Intellectual Property (IP) rights or sharing technological procedures meant there was limited vaccine production in the Global South, but manufacturing initiatives in Sub-Saharan Africa and South America should be supported to improve future resilience. A virus with epidemic potential could emerge in any part of the world, so it is vital we remember: ‘No one is safe until everyone is safe’ — but maybe next time we will live up to it.

The ‘100-Day-Mission’ in a future pandemic requires ongoing government funding of life sciences, including immunological, virological and vaccine research.Dr MP Chair of APPG Vaccinations for All and MP for Central Aryshire
 WRITTEN BY Mark Nicholls
WRITTEN BY Mark Nicholls
A new formulation and drying technology for biotherapeutics could solve key challenges in delivery and stability for today’s mNRA platform, unlocking and expanding its potential.
Following its rapid development for Covid-19 vaccines, mRNA has transformed the pharmaceutical industry forever.

Enabling new dosage forms and stability for mRNA
However, mRNA components are very fragile and can only be stored at cryogenic temperatures and administered via injection. A new technology has now been developed that transforms mRNA/ LNP by formulation and drying into easily manageable powder - unlocking a range of new possibilities.
The robust, particle-engineered powder can be easily reconstituted back into solution in the clinic, for injection, or applied for novel dosage forms such as inhalation or transdermal patches.
Smart chemical engineering using mass transfer
CEO Ann Gidner says the process of transforming the pharmaceuticals from liquid into a stable amorphous powder is conducted using smart chemical engineering. After specific formulation, a drying column removes the solvent molecules — typically water — in a rapid, one-step, high-yield and low-cost process with little or no degradation.
Existing drying techniques involve freeze-drying or spray-drying
using heat, both harming the activity of mRNA/LNP. Ziccum’s LaminarPace technology instead uses a mass transfer principle to create more stable, thermostable and user-friendly forms of mRNA and biotherapeutics. “This is a complete game-changer to the industry, this is not possible with any other technology,” insists Gidner.
Overcoming the key challenges to mRNA therapy mRNA enabled efficient COVID-19 vaccination, thanks to using LNP (lipid nanoparticles) as a key delivery system. Via mRNA, a genetic code is delivered into the patient’s body enveloped in LNPs and translated into an active remedy.
Gidner argues that dry formulations would enable mRNA to fulfil its potential. Development of a broad range of treatments — from cancer and flu vaccines to prophylactic vaccines would be possible. “By solving the inherent limitations of mRNA, you would open up completely new treatments and our technology can achieve this — it enables delivery and solves stability.”
LaminarPace has been tested successfully by Ziccum using commercially available mRNA. Now, two leading pharma and biopharma corporations have signed agreements for evaluation studies.
Demetre Daskalakis, MD, MPH Acting Director of CDC’s National Center for Immunization and Respiratory Diseases
Last autumn, amid the COVID-19 pandemic, flu and respiratory syncytial virus (RSV) surged to levels not seen in recent years. The simultaneous circulation of these respiratory viruses took a toll on already fragile healthcare systems.
As we head into another potentially busy autumn and winter season, we must protect people against the most serious effects of these respiratory viruses. This is a top priority for CDC and we find ourselves in the strongest position yet to achieve this, with powerful new tools available to healthcare providers and the public.
For the first time, there are vaccines to protect against these three major respiratory diseases. CDC has also improved our surveillance capabilities, and we are harnessing our detection and prediction tools to help people understand the risk of illness in their communities.
There is much at stake:
• More than 1.1 million Americans have died from COVID-19 since the pandemic began, and while hospitalisations were declining, they have begun to tick up again. With new variants appearing and the colder months approaching, we need to take precautions to avoid a surge.
• This past season, we saw between 27 and 54 million flu illnesses, with up to 650,000 hospitalisations and between 19,000 and 58,000 deaths, according to CDC preliminary estimates.
• RSV is the leading cause of hospitalisation in U.S. infants, causing an estimated 58,000 to 80,000 hospitalisations and 100 to 300 deaths among children ages 5 and under every year. Older adults are also at risk: RSV hospitalises 60,000 to 160,000 adults ages 65 years and older and causes 6,000–10,000 deaths among this population each year.
Earlier this year, we saw breakthrough developments come to fruition in our fight to prevent RSV, with the US Food and Drug Administration approving two immunisations: the first-ever RSV vaccines for older adults and the long-acting monoclonal antibody nirsevimab for infants and some older babies. In midAugust, the FDA approved the first RSV vaccine for use in pregnant people to protect infants against severe RSV after birth.
A new COVID-19 vaccine is expected to be available early this autumn. While we don’t know what’s in store for this autumn and winter, we do know there are safe and effective immunisations, proven treatments and common-sense precautions to help protect ourselves and our loved ones against RSV, flu and COVID-19.
This is a complete game-changer, it is not possible with any other technology.
Malaria, a deadly disease caused by Plasmodium parasites and transmitted by mosquitoes, remains a persistent public health concern. To minimise its threat to pregnant women, malaria clinical trials must also consider them.
The World Health Organization (WHO) estimates that 247 million clinical cases and 619,000 malaria deaths occurred globally in 2021, with the great majority of these in Africa.
Pregnant women in low and middle-income countries (LMICs) where malaria is endemic are particularly vulnerable to its adverse effects. Malaria during pregnancy can lead to complications such as maternal anaemia, preterm birth, low birth weight and even maternal and neonatal mortality. These dire consequences underscore the critical importance of effective preventive measures, including vaccines.
Creating a malaria vaccine tailored for pregnant women offers great potential in reducing the impact of malaria during pregnancy. Such a vaccine could enhance maternal immunity against Plasmodium parasites, lowering infection risks for pregnant women and providing protection for their foetuses, thereby improving maternal and neonatal outcomes.
Historically, pregnant women have been excluded from clinical trials due to concerns about potential harm — resulting in limited data on vaccine efficacy for this group. To address this, concerted efforts are needed to involve pregnant women in clinical trials and consider their distinct requirements in vaccine development.

The European Vaccine Initiative (EVI) is currently coordinating several efforts to develop a vaccine for malaria in pregnancy. This includes the testing of advanced malaria vaccine candidates in women of childbearing age. Moreover, two placental malaria (PM) vaccine candidates are also being prepared for clinical trials in Europe and Africa.
Advancing malaria vaccine development aligns with global health goals, improving maternal and child health and reducing child mortality. Prioritising vaccine research, development and distribution is crucial for a healthier future for pregnant women in LMICs.


Despite the well-documented safety and effectiveness of vaccines, EU/EEA countries and nations worldwide are constantly grappling with outbreaks of vaccine-preventable diseases, revealing insufficient vaccination coverage rates.
Undoubtedly, the EU/EEA’s routine immunisation programmes have demonstrated commendable resilience, even during the Covid-19 pandemic. However, as we praise these achievements and the immense dedication they entail, we are also facing persisting disparities in vaccination rates between countries and regions.

In addition, the existence of pockets of population sub-groups in the EU/EEA that did not finish the primary vaccination course for routine immunisation (partially vaccinated) — or are not immunised at all — is very concerning. For example, according to ECDC data, between 2012 and 2021, approximately 2.4 million children in the EU/EEA may not have received three doses of the polio vaccine on time. Despite the European region’s uninterrupted polio-free status of two decades, the continued periodical detection of the virus in other regions, in its wild form or as vaccine-derived strains, serves as stark reminders that the threat lingers. As long as there are non-vaccinated or under-vaccinated population groups and polio is not eradicated globally, the risk of the virus being reintroduced in Europe remains.

It is equally crucial to emphasise the importance of a lifelong
Andrea Ammon Director, ECDCVaccination programmes protect people of all ages, including adolescents, pregnant women, adults, individuals living with chronic diseases and the elderly. People across all age groups should be encouraged to consult their respective national vaccination schedules, talk to their healthcare providers and ensure they adhere to recommended vaccination plans.
Continuous work is essential to identify immunity gaps, especially in hard-to-reach populations, such as refugees, migrants, asylum seekers and other socially or medically vulnerable and underserved population groups.
Additionally, there is a continuous need to understand the drivers of suboptimal vaccine uptake in a given population, to ensure that appropriate interventions are implemented in response. This includes tailored communication strategies aimed at building trust and addressing issues with vaccine acceptance. Healthcare professionals should be empowered to be effective advocates of vaccination. Supporting and strengthening national routine vaccination programmes remains a key priority for ECDC, on the principles of quality, safety and efficacy of vaccines, as well as timely and equitable access.
Artificial intelligence (AI) enables the development of precise treatments targeting health concerns.

Founded in 2008, the clinicalstage biotech Evaxion aims to lead the exploration of AI to design new, superior vaccines and immunotherapies.
Using AI to create targeted vaccines
The biotech has four key validated AI platforms: EDEN, RAVEN, PIONEER and ObsERV. These platforms enable the identification of antigens for use in nextgeneration vaccines.
With AI, the biotech company can find targets more quickly than current approaches. It can also find new targets that cannot be found in other ways. The technologies are based on a machine-learning group of AI models trained on Evaxion’s unique datasets. The models identify therapeutically relevant targets based on predictions as to how they will mount an immune response.
The EDEN platform excels at identifying vaccine targets that can trigger a robust immune response, protecting against bacterial infections. It does this by recognising traits shared by highly protective vaccine candidates. Meanwhile, the RAVEN platform aims to identify vaccine targets against any existing, emerging and mutating viruses.
immunotherapies. These tumour-specific epitopes are selected to trigger a strong T-cell response, training the patient’s immune system to target and kill tumour cells with limited or no impact on healthy cells.
Therapeutic cancer vaccines
Currently, the majority of cancer treatments are based on a ‘onesize-fits-all’ approach. PIONEER is used to identify patient-specific tumour neoantigens efficiently for personalised oncology
The burden of respiratory diseases in the UK has increased approximately threefold compared to pre-pandemic levels as the continued high burden of COVID-19 puts pressure on healthcare systems.

Not all cancer patients will benefit from personalised neoantigen immunotherapy. Recent findings identify endogenous retroviruses (ERVs) as a potential additional source of cancer antigens to be used in personalised cancer immunotherapies, leading to the development of the cancer AI platform ObsERV. It enables a new treatment paradigm in cancer, broadening the application of personalised cancer immunotherapy to support more patients in need, and it is unique to Evaxion. This platform exemplifies the ongoing, iterative biologydriven AI platform development, as evidenced by the pipeline of differentiated vaccines for infectious disease and cancer.
New Airfinity modelling estimates the rollout of next-generation vaccines could reduce the number of hospitalisations by 39%. It also estimates that total hospitalisations will reach 200,000 this winter season — up from 60,000 in 2018/19.

The rollout of new, highly efficacious next-generation vaccines, including combination and universal vaccines, could reduce hospitalisations by 80,000. New vaccines could start to make an impact from the 2024/25 season. The most advanced vaccines in the pipeline are Pfizer/BioNTech’s
mRNA BNT-161 and Moderna’s mRNA-1010, both of which will reach primary completion by March 2024.
Encouraging vaccine uptake
Dr Louise Blair, Senior Director of Analysis and Insights at Airfinity, says: “Next-generation vaccines could help reverse falling COVID-19 uptake rates. Across nearly all G7 nations, uptake of COVID-19 jabs has declined sharply since they were rolled out. Conversely, influenza vaccine uptake remains stable and, on average, is higher than COVID-19 uptake. New vaccines could change this and help reduce pressure on hospitals.”
The majority of cancer treatments are based on a ‘one-size-fits-all’ approach.Angela R. Branche, MD, FIDSA Associate Professor of Medicine, University of Rochester


Infectious diseases (ID) professionals are uniquely positioned to educate the public on the positive impact of vaccination and help protect people in the community against preventable infectious diseases.
Despite the efficacy of the Covid-19 vaccines in preventing severe illness and death, uptake rates are declining. This includes vulnerable groups, such as older adults and pregnant people. There are lower rates of children entering school with all state-required routine vaccines for the two years following the onset of the pandemic, leaving approximately 250,000 children unprotected against measles — a highly contagious virus.
As an infectious diseases physician, I’m deeply concerned about these declining rates because I know how crucial vaccination is to prevent hospitalisation, reduce the risk of developing debilitating longCovid syndromes and save lives.
Furthermore, marginalised groups, especially indigenous/Native Americans and Black and Hispanic communities, face disparities that have led to an understandable distrust of healthcare.
Infectious disease (ID) doctors can build trust and confidence in healthcare, though almost 80% of US counties are without a single ID-trained clinician. The impact of this deficiency was felt most acutely as clinicians raced to
With a vaccine pipeline that can tackle today’s and tomorrow’s challenges, the life-saving potential of vaccines is more far-reaching than we know.
Laghnimi-Hahn Associateimprove Covid-19 vaccine uptake and curb the staggering morbidity and mortality straining our healthcare systems.
Because ID is a diverse field of medicine, we are trusted voices to many who struggle to understand or accept federal health care policies. ID physicians are also trained to convert complex biological processes into accessible health information.
In the conduct of clinical trials of Covid-19 vaccines, we developed honest language around the science and safety of vaccines. Even with resources that are lacking in many other communities, the collective effort to inform, educate, build trust and, ultimately, vaccinate our local community was overwhelming. Many other communities are not as fortunate. I fully support federal healthcare agencies, like the National Institutes of Health and the Centers for Disease Control and Prevention, which fund research and develop policies that safeguard the health of our communities. However, trust is built at the local practice level; when it comes to vaccines, infectious diseases physicians are essential to those efforts.
From routine childhood immunisation to seasonal flu or COVID-19, vaccines are important for our health. In fact, 4 million deaths are prevented each year from childhood vaccination alone. Within the next seven years, it is estimated that another 50 million deaths could be prevented, and that’s the tip of the iceberg when it comes to the future of vaccines.
By 2030, the number of people aged 60 years and over is expected to increase by more than one-third, rising to 1.4 billion — and 2.1 billion by 2050. New and improved vaccines can protect this growing demographic against severe and life-threatening illnesses caused by infections such as influenza, pneumococcal, herpes zoster and respiratory syncytial virus (RSV). They can also have a positive socioeconomic and cost-saving impact and reduce pressures on health systems.
In some cases, we can aspire to eliminate certain illnesses. For instance, Human papillomavirus (HPV) vaccines can help prevent up to 90% of cervical cancer cases. With the widespread use of these vaccines, a world in which girls and women do not face the threat of cervical cancer is possible.
Vaccines can protect us against future health emergencies, whether Ebola or the next influenza pandemic. A life-course approach to vaccination for all ages helps control vaccine-preventable diseases, reduce hospitalisations and build herd immunity. Investment in prevention funding, including in infrastructure and extension of immunisation services, helps us build resilience for future outbreaks.
Against the rising threat of antimicrobial resistance (AMR), vaccines give us hope. By 2050, it is estimated that drug-resistant infections could cause as many as 10 million deaths annually. New and existing vaccines can help prevent half a million AMR-related deaths annually by preventing infections in the first place; reducing the use, misuse or overuse of antibiotics and slowing the spread of ‘superbugs.’
Warmer global temperatures and increased rainfall due to the climate crisis also put more people at risk of mosquito-borne diseases like Dengue or Zika. Vaccines in development hold the promise of protecting more people. In the future, we will face several known and unknown viruses. Vaccines are an important line of defence — and, equally, a source of hope — for healthier lives worldwide.
In the conduct of clinical trials of Covid-19 vaccines, we developed honest language around the science and safety of vaccines.