18 minute read
Glossary
from What you need to know about epizootic ulcerative syndrome (EUS) – An extension brochure for Africa
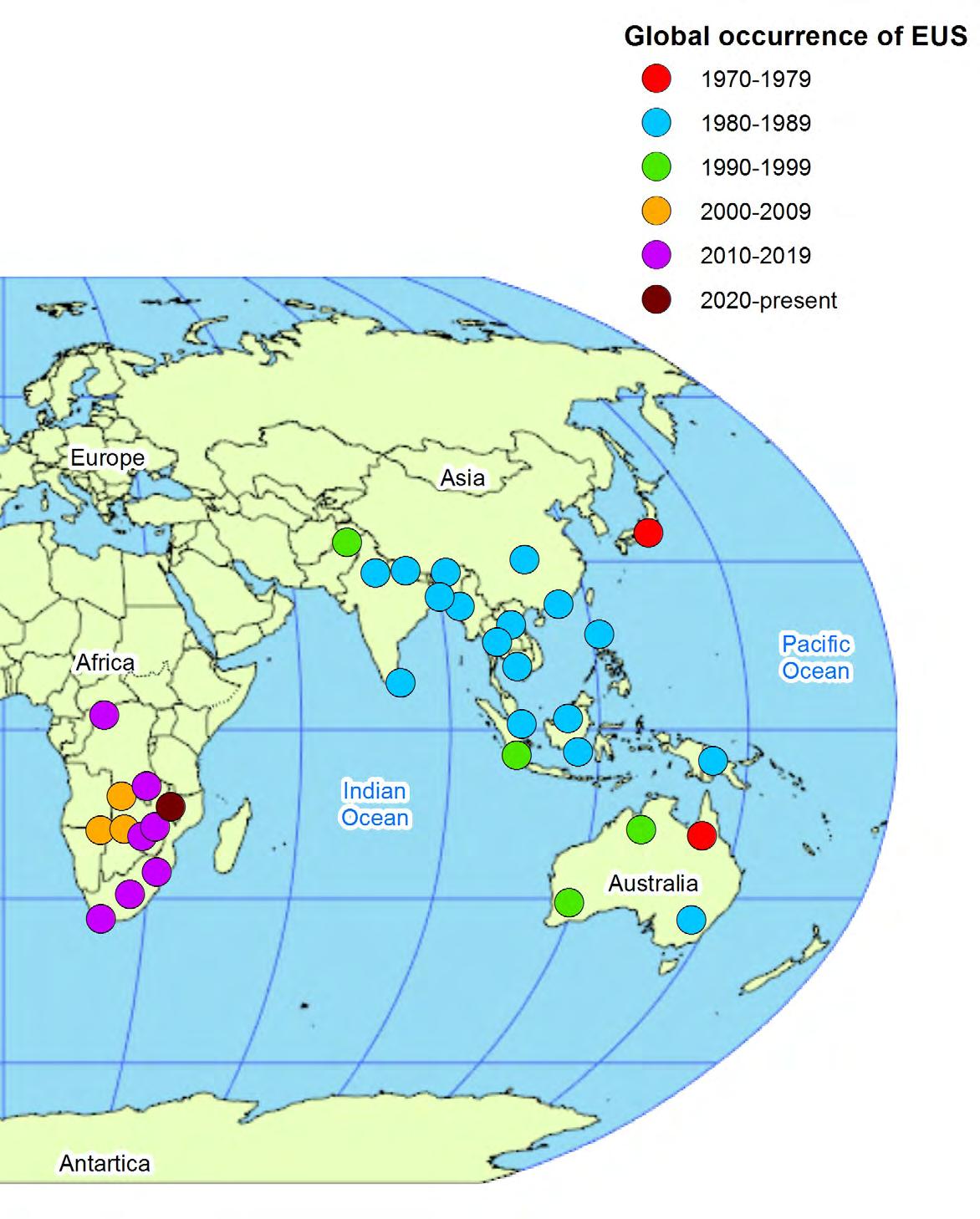
Cambodia (1983 or 1984); Viet Nam (1983?); China (1982?; 1987–1988?; 1989?); China, Hong Kong SAR (1988?); Philippines (1985); Sri Lanka (1987); Bangladesh (1988); India (1988); Bhutan and Nepal (1989); Pakistan (1996); United States of America (North Carolina, Florida and Connecticut – 1984); Botswana (2006?; 2007; 2010; 2020); Namibia (2006?; 2007); Zambia (2007?; 2008; 2014); Canada (2010); Zimbabwe (2016); South Africa (2011; 2014; 2015; 2016; 2017); Democratic Republic of Congo (2015); Malawi (2020).
30 60 90 120 150 180
Advertisement
SWEDEN FINLAND
RUSSIAN FEDERATION
ESTONIA LATVIA
R.F. LITHUANIA POLAND
BELARUS
CZECHIA SLOVAKIA UKRAINE REP. OF MOLDOVAHUNGARY ROMANIA25 AUSTRIA KAZAKHSTAN
MONACO 4ITALY BULGARIA GREECE TURKEY 1 3 6 SAN MARINO HOLY SEE ALBANIA ARMENIA GEORGIA UZBEKISTAN AZERBAIJAN TURKMENISTAN KYRGYZSTAN TAJIKISTAN
MALTA SYRIAN CYPRUS ARAB REP.
LEBANON ISRAEL IRAQ JORDAN
ISLAMIC REP.OF IRAN AFGHANISTAN
Jammu and Kashmir *
LIBYA EGYPT
KUWAIT BAHRAIN SAUDI ARABIA
QATAR UNITED ARAB EMIRATES PAKISTAN
NEPAL BHUTAN
INDIA
BANGLADESH
OMAN MYANMAR MONGOLIA
CHINA
LAO PEOPLE'S DEM. REP. DEM. PEOPLE'S REP. OF KOREA
REPUBLIC OF KOREA JAPAN
CHAD SUDAN ERITREA YEMEN
DJIBOUTI
D'IVOIRE TOGO CAMEROON CENTRAL AFRICAN REP. SOUTH SUDAN ETHIOPIA SOMALIA EQUATORIAL GUINEA UGANDA SAO TOME AND PRINCIPE GABON DEMOCRATIC CONGO KENYA RWANDA REPUBLIC OF BURUNDI ANGOLA THE CONGO UNITED REP. (Cabinda) OF TANZANIA SEYCHELLES SRI LANKA
MALDIVES
Chagos Archipelago (Mauri.)
ANGOLA
ZAMBIA
NAMIBIA ZIMBABWE
BOTSWANA
COMOROS
Agaleda Island
MALAWI
MOZAMBIQUE
Tromelin Island Cargados Carajos Shoals
MADAGASCAR
Réunion (Fr.) Rodriges Island MAURITIUS
ESWATINI THAILAND
VIET NAM CAMBODIA
BRUNEI DARUSSALAM MALAYSIA
SINGAPORE PHILIPPINES
PALAU
I N D O N E S I A
Christmas (Austr.) Cocos (Keeling) Islands (Austr.) TIMOR LESTE
AUSTRALIA
SOUTH AFRICA
LESOTHO
The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Nations.
Final boundary between the Republic of Sudan and the Republic of South Sudan has not yet been determined.
*Dotted line represents approximately the Line of Control in Jammu and Kashmir agreed upon by India and Pakistan. The final status of Jammu and Kashmir has not yet been agreed upon by the parties.
**A dispute exists between the Governments of Argentina and the United Kingdom of Great Britain and Northern Ireland concerning sovereignty over the Falkland Islands (Malvinas).
The initials in parentheses refer to the administering Power
or the Power involved in a special treaty relationship. 60
30
Northern Mariana Islands (U.S.A.)
Guam (U.S.A.)
MARSHALL ISLANDS
FEDERATED STATES OF MICRONESIA
PAPUA NEW GUINEA
NAURU
SOLOMON ISLANDS KIRIBATI
TUVALU
0
Tokelau (N.Z.)
Wallis and Futune Islands (Fr.) SAMOA
VANUATU FIJI
American Samoa (U.S.A.) Niue (N.Z.)
New Caledonia (Fr.) TONGA
30
NEW ZEALAND
60
A N T A R C T I C A
30 60 90 120 150 Office of Information and Communications Technology Geospatial Information Section 180
Is it safe to eat epizootic ulcerative syndrome (EUS) fish?
The agent causing EUS does not pose any direct human health risk. The deep ulcerations and tissue decay in fish infected with EUS may harbour secondary pathogens which may have health implications to humans consuming such fish.
Thorough cooking may destroy pathogens, such as A. invadans. However, fish tissues compromised by EUS may spoil rapidly and render a product of inferior nutritional value. It is therefore advisable to avoid eating fish showing gross lesions of EUS.
©FAO/D. Huchzermeyer, Rhodes University

Figure 38. It is believed that traditional methods such as smoking used to preserve fish products, as in the case of these fish in the Democratic Republic of Congo (DRC) in 2015, will destroy the agent of epizootic ulcerative syndrome (EUS).
Can epizootic ulcerative syndrome (EUS)-affected fish be treated?
There is no known treatment by which EUS-affected fish can be treated. Control of EUS in natural waters (e.g. rivers) is impossible.
Fish farmers whose farmed fish have been affected with EUS are encouraged to culture, where possible, non-EUS susceptible species or avoid farming susceptible species during the season when EUS is prevalent, i.e. high rainfall period and low temperature season.
A strict ban on the movement of fish from infected waterways or river systems, especially those with lesions of EUS, to other waterways or river systems is recommended; diseased fish should not be moved from one fish farm to another.
Particular care should be taken when moving resistant species, such as Nile tilapia, from hatcheries situated on potentially infected waters. Fish species susceptible to EUS may inhabit the water source of a hatchery and shed infectious spores of Aphanomyces invadans into the water. These can be readily transferred with transport water in which fry and fingerlings are sold.
Properly dried, salted and iced fish have not been reported as potential carriers of EUS, therefore trade of these products can be allowed to continue.

Figure 39. Clarias gariepinus – African sharptooth catfish showing early and advanced lesions of epizootic ulcerative syndrome (EUS), Bangweulu swamps, north Zambia, 2014.
©FAO/D. Huchzermeyer, Rhodes University
Can infection be prevented?
Anumber of important biosecurity measures can minimize or prevent the spread of EUS. These include: ➔ All possible carriers or vectors such as freshly dead fish, birds or terrestrial animals as well as contaminated fishing gears/nets and fish transport containers should be prevented from getting into water bodies or fish ponds. ➔ In outbreaks occurring in small, closed water bodies, disinfection of destocked ponds by liming and improvement of water quality, together with removal of infected fish, are often effective in reducing mortality. ➔ Increasing salinity in holding waters to above 2 parts per thousand may also prevent outbreaks of EUS in aquaculture ponds. ➔ During dry and cold seasons, close observation of wild fish should be made to determine the presence of EUS–diseased fish in neighbouring tanks or canals, in which case, exchange of water should be avoided. ➔ EUS infected fish should never be thrown back into open waters and should be disposed of properly by burying them into the ground or through incineration (burning).

©FAO/D. Huchzermeyer, Rhodes University
Figure 40. Fish that have died as a result of epizootic ulcerative syndrome (EUS), such as this Clarias gariepinus, should be removed from the water. As the fish decays large numbers of Aphanomyces invadans spores can be released into the water, Bangweulu swamps, north Zambia, 2014.
➔ Additional practical aquaculture biosecurity measures should be implemented, including: – improved farm hygiene (e.g. handwashing between tanks, separation of nets/tanks/stocks, regular and correct disinfection procedures, etc.) – optimal husbandry practices – optimal water quality management – proper handling of fish – regular monitoring of fish health – good record keeping (gross and environmental observations and stocking records including movement records of fish in and out of the aquaculture facility, etc.) ➔ Early reporting or notification to concerned authorities of a disease outbreak or suspicion of any abnormal appearance, behaviour or other observations in fish stocks.
What can one do in the event of a disease outbreak?
➔ Immediately stop all live fish movements (quarantine). ➔ Dry and disinfect all fishing gear and boats before moving to another water body. ➔ Report immediately a suspected outbreak to concerned authorities (nearest fisheries or veterinary authority) and ask for guidance concerning collection of samples (see page 29). ➔ The abnormal congregation of aquatic birds feeding on fish has been typical of some outbreaks of EUS observed in Botswana and Zambia and may draw attention to presence of large numbers of sick fish. ➔ Taking note of simple observations that deviate from the normal will assist in Level I diagnosis and surveillance and will help to alert to presence of
EUS. Abnormal swimming behavior such as swimming near the surface, swimming with the current, sinking to the bottom, loss of balance, flashing and cork-screw swimming and in non-airbreathing fish gulping at the surface are all indications of sick fish. If any of these observations are noted together with presence of red spots and ulcers/wounds on the fish this will be a strong indication of EUS. ➔ It is important to note the date and time of observed disease outbreaks to determine annual patterns during which fishermen and aquaculturists should be alert for the dangers of EUS. ➔ Further important information to record is the species of fish affected, an estimate of mortality (numbers of fish found dead) and the pattern of mortality (small number of fish dying each day, large number of fish dying at one time, etc.). ➔ Adverse climatic events such as flooding following on drought, sudden cold spells and the presence of disturbed soils that might cause acid leaching into waters should be recorded. ➔ Anticipation of outbreaks and early warning allow containment measures to be instituted timeously. These may include the need for additional disinfection of fishing equipment, discouraging fishermen from moving between EUSaffected and non-affected waters with their fishing gear, avoiding sourcing water for fish farms from water ways that might be contaminated with the zoospores of Aphanomyces invadans during an outbreak of EUS and avoiding moving fingerlings for fish farming from hatcheries on EUS-affected water sources. During an EUS outbreak, a ban on trade in fish across provincial and national borders and between catchments may be instituted by the authorities to reduce the spread of EUS.
Can I collect epizootic ulcerative syndrome (EUS) samples for laboratory examination?
➔ Live samples, if available, are best for laboratory examination. The fish should be packed in double plastic bags, filled with water to one third of their capacity with the remaining 2/3 volume inflated with air/oxygen. Bags should be tightly sealed (with rubber band or tape) and protected from temperature fluctuation. ➔ If live fish that can be transported to the laboratory are not available, freshly dead or moribund/sick fish with clinical lesions can be used. ➔ Using a scalpel or a blade, take samples of skin/muscle sections (<1 cm3), including the edge of the lesion and the surrounding tissue. Parts of internal organs may also be collected by dissecting the whole fish. ➔ Fix the tissue samples immediately in 10 percent formalin (10 ml of formalin in 90 ml of water, preferably phosphate-buffered saline made up in distilled water) in a plastic or bottled container. The amount of formalin should be 10 times the volume of the tissue to be fixed. Tissues should be fixed for at least 24 hours before processing. ➔ After 24 hours, fixed tissues can be wrapped into formalin-moistened tissue paper and placed into small plastic bags to prevent leakage or smell during transport. ➔ Using a scalpel or a blade, collect a second smaller sample of muscle (<0.5 cm3) from the edge of the lesion. ➔ Fix the muscle tissue immediately in 90-100 percent ethanol. ➔ Make sure that samples are properly labelled with the following information: date of sampling, type of tissue samples (e.g. skin, muscle, gills, kidney, other internal organs), collection locality (place of collection), species of fish (weight and length measurements if possible), name of collector, type of fixative used (10 percent formalin, etc.). ➔ Samples can be packed Figure 41. Positions relative to a typical epizootic into a padded envelope or ulcerative syndrome (EUS) lesion in Clarias gariepinus container and sent by mail from which suitable samples can be dissected for fixation if no courier services exist. in (a) 10% formalin - sample should include skin and ➔ Call the laboratory to inform of the kind of samples collected and when they muscle and extend from the ulcer into surrounding healthy appearing tissue, and (b) 90-100% ethanol - the skin is removed and the underlying muscle is sampled adjacent to the edge of the ulcer or below the ulcer), are expected to arrive or to Bangweulu swamps, north Zambia, 2014. be delivered.

©FAO/D. Huchzermeyer, Rhodes University
References
Andrew, T.G., Huchzermeyer, K.D.A., Mbeha, B.C. & Nengu, S.M. 2008. Epizootic ulcerative syndrome affecting fish in the Zambezi River system in southern Africa, Veterinary Record 163: 629–632. (also available at http://dx.doi.org/10.1136/vr.163.21.629,PMid:19029110).
Baldock, F.C., Blazer, V., Callinan, R., Hatai, K., Karunasagar, I. Mohan, C.V. & Bondad-Reantaso,
M.G. 2005. Outcomes of a short expert consultation on epizootic ulcerative syndrome (EUS): re-examination of causal factors, case definition and nomenclature. In P. Walker, R. Lester and M.G. Bondad-Reantaso (eds). Diseases in Asian Aquaculture V, pp. 555–585. Fish Health
Section, Asian Fisheries Society, Manila.
Blazer, V., Bondad-Reantaso, M.G., Callinan, R.B., Chinabut, S., Hatai, K., Lilley, J.H. & Mohan, C.V.
2005. Aphanomyces invadans (A. piscicida): A Serious Pathogen of Estuarine and Freshwater
Fishes, pp. 24–41. In Cipriano, R.C., Shchelkunov, I.S. and Faisal, M. (editors). Health and
Diseases of Aquatic Organisms: Bilateral Perspectives. Proceedings of the Second Bilateral
Conference Between Russia and the United States. 21–18 September 2003. Sheperdstown,
West Virginia. Michigan State University, East Lansing, Michigan. Bondad-Reantaso, M.G., McGladdery, S., East, I. & Subasinghe, R.P. (eds). 2001. Asia diagnostic guide to aquatic animal diseases. FAO Fisheries Technical Paper No. 402. Supplement 2. Rome,
FAO. 240p. Choongo, K., Hang’ombe, B., Samui, K.L., Syachaba, M., Phiri, H. & Maguswi, C. 2009. Environmental and climatic factors associated with epizootic ulcerative syndrome (EUS) in fish from the
Zambezi floodplains, Zambia. Bulletin of Environmental Contamination and Toxicology, 83: 474–478. (also available at http://dx.doi.org/10.1007/s00128-009-9799-0, PMid:19565173). FAO. 2009. Report of the international emergency disease investigation task force on a serious fish disease in Southern Africa, 18-26 May 2007. Rome, Italy. (also available at http://www.fao.org/ docrep/012/i0778e00.htm). FAO. 2017. Report of the International Emergency Fish Disease Investigation Mission on a
Suspected Outbreak of Epizootic Ulcerative Syndrome (EUS) in the Democratic Republic of the Congo, 13–19 March 2015. Rome, Italy. (also available at http://www.fao.org/3/a-i6596e.pdf). Hatai, K., Nakamura, K., An Rha, S., Yuasa, K. & Wada, S. 1994. Aphanomyces infection in dwarf gourami (Colisa lalia). Fish Pathology., 29, 95–99 Huchzermeyer, K.D.A. & Van der Waal, B.C.W. 2012. Epizootic ulcerative syndrome: Exotic fish disease threatens Africa’s aquatic ecosystems. Journal of the South African Veterinary
Association 83(1), Art. #204, 6 pages. (also available at http://dx.doi.org/10.4102/jsava.v83i1.204). Huchzermeyer, C.F., Huchzermeyer, K.D.A., Christison, K.W, Macey, B.M, Colly, P.A., Hangombe,
B.M. & Songe M.M. 2017. First-record of epizootic ulcerative syndrome from the Upper Congo catchment: an outbreak in the Bangweulu swamps, Zambia. Journal of Fish Diseases. 41(1): 87-94, DOI:10.1111/jfd.12680 Kaphuka, B., Njunga, G. R., Kamwendo, G. & Chirwa, B. B. 2020. Active surveillance of infection caused by Aphanomyces invadans in Malawi. International Journal of Fisheries and Aquaculture (12) 1-5 (also available at http://doi: 10.5897/IJFA2019.0728). Lilley, J.H., Callinan, R.B., Chinabut, S., Kanchanakhan, S., MacRae, I.H. & Phillips, M.J. 1998. EUS
Technical Handbook. AAHRI, Bangkok. 88pp. OIE. 2019. Manual of Diagnostic Tests for Aquatic Animals. World Organization for Animal Health,
Paris. Malherbe, W., Christison, K.W., Wepener, V. & Smit, N.J. 2019. Epizootic ulcerative syndrome –
First report of evidence from South Africa’s largest and premier conservation area, the Kruger
National Park IJP: Parasites and Wildlife (10) 207-210 (also available at https://doi.org/10.1016/j. ijppaw.2019.08.007) McHugh, K.J., Christison, K.W., Weyl, O.L.F. & Smit, N.J. 2014. Histological confirmation of epizootic ulcerative syndrome in two cyprinid species from Lake Liambezi, Zambezi Region,
Namibia. African Zoology 49(2), 311–316. Nsonga, A., Mfitilodze, W., Samui, K.L. & Sikawa, D. 2012. Epidemiology of epizootic ulcerative syndrome in the Zambezi River system. A case study for Zambia.Human and Veterinary
Medicine. International Journal of the Bioflux Society, 5(1):1-8. Sibanda, S., Pfukenyi, D. M., Barson, M., Hang’ombe, B. & Matope, G. 2018. Emergence of infection with Aphanomyces invadans in fish in some main aquatic ecosystems in Zimbabwe: A threat to national fisheries production. Transboundary and Emerging Diseases: 1–9.DOI: 10.1111/ tbed.12922
Songe, M.M., Hang’ombe, M.B., Phiri, H., Mwase, M., Choongo, K., Van der Waal, B., Kanchanakhan,
S., Reantaso, M. B. & Subasinghe, R. P. 2012. Field observations of fish species susceptible to epizootic ulcerative syndrome in the Zambezi River basin in Sesheke District of Zambia.
Tropical Animal Health and Production, 44:179–183 DOI 10.1007/s11250-011-9906-1
Glossary
Disease
Any deviation from or interruption of the normal structure or function of any part, organ, or system (or combination thereof) of the body that is manifested by a characteristic set of symptoms and signs and whose aetiology, pathology and prognosis may be known or unknown.
Epidemiology Science concerned with the study of the factors determining and influencing the frequency and distribution of disease or other health related events and their causes in a defined population for the purpose of establishing programmes to prevent and control their development and spread.
Epizootic Affecting many animals at the same time; widely diffused and rapidly spreading disease (syn. Epidemic – used for human disease).
Epizootiology The study of factors influencing infection by a pathogenic agent.
Fungi
Heterotrophic organisms possessing a chitinous* wall, with the majority of fungal species growing as multicellular filaments called hyphae forming a mycelium. Fungi are more closely related to animals than plants, yet the discipline of biology dedicated to the study of fungi, known as mycology, often falls under a branch of botany.
* Note that in contrast to the true fungi, the oomycetes, of which Aphanomyces is a member, have a cellulose cell wall.
Granulomas Any small nodular delimited aggregation of inflammatory cells, or modified macrophages resembling epithelial cells (epithelioid cells) formed by the body to ward off an infection or foreign substance.
Granulomatosis Any condition characterized by the formation of multiple granulomas. Heterokonts Or stramenopiles are a major line of eukaryotes presently containing about 10 500 known species; includes the group oomycetes to which Aphanomyces invadans belongs. As opposed to higher fungi, the oomycetes have a cell wall composed of cellulose.
Infection
Invasion and multiplication of an infectious organism within host tissues. May be clinically benign (cf subclinical or carrier) or result in cell or tissue damage. The infection may remain localized, subclinical and temporary if the host defensive mechanisms are effective or it may spread to form an acute, subacute or chronic clinical infection (disease).
Lesion Any pathological or traumatic change in tissue form or function. Mycology The study of fungi (Mycota).
Mycosis
Any disease resulting from infection by a fungus.
Glossary
Oomycetes A group of filamentous, unicellular heterokonts or stramenopiles physically resembling fungi; they are microscopic, absorptive organisms that reproduce both sexually and asexually and are composed of mycelia. The genus Aphanomyces is known to produce only by asexual means.
Outbreak The sudden onset of disease in epizootic proportions.
Pathogen An infectious agent capable of causing disease.
Predispose To make susceptible to a disease that may be activated by certain conditions, as by stress. Sporangium (Mycology) hyphal swelling which contains motile or non-motile zoospores; release is via a pore or breakdown of the sporangial wall (syn. zoosporangium).
Spore
Infective stage of an organism that is usually protected from the environment by one or more protective membranes (syn. zoospores).
Sporogenesis Formation of or reproduction by spores; sporulation.
Stress The sum of biological reactions to any adverse stimuli (physical, internal or external) that disturb the organism’s optimum operating status. Susceptible An organism which has no immunity or resistance to infection by another organism. Syndrome An assembly of clinical signs which when manifest together are indicative of a distinct disease or abnormality (syn. pathognomic/ pathognomonic).
Ulcer
Excavation of the surface of an organ or tissue, involving sloughing of necrotic inflammatory tissue.

Figure 42. Use of a sturdy scoop net is ideal for sampling small sick fish from the shallows along a river’s edge during an epizootic ulcerative syndrome (EUS) outbreak, Chobe River, Botswana, 2007. Figure 43. Typical catch of small fish species in a gill net left over night during surveillance for epizootic ulcerative syndrome (EUS)-affected fish, Chobe River, Botswana, 2007.



Figure 44. Shallow vegetated areas of wetlands often harbour sick fish during an epizootic ulcerative syndrome (EUS) outbreak. Scoop nets are ideal for sampling such areas, Bangweulu swamps, north Zambia, 2014. Figure 45. Bemba speaking fishermen with a seasonal catch of small flood plain fish being preserved by drying in the sun (traded as kasepa). During epizootic ulcerative syndrome (EUS) outbreaks, infected fish can readily be observed on drying racks, Bangweulu swamps, north Zambia, 2014.


Figure 46. Inspecting live air-breathing fish for signs of epizootic ulcerative syndrome (EUS) at a fish market, Equateur Province, Democratic Republic of Congo (DRC), 2015. Figure 47. Fishermen searching for epizootic ulcerative syndrome (EUS)-affected fish from a pirogue during an EUS investigation, Equateur Province, Democratic Republic of Congo (DRC), 2015.


Figure 48. Searching for fish showing signs of epizootic ulcerative syndrome (EUS) in the shallows along a river bank. Note the red-brown colour of the water associated with low pH typical of waters draining from tropical rain forests, Equateur Province, Democratic Republic of Congo (DRC), 2015. Figure 49. Rivers draining tropical rain forests in Africa often have a low pH of 4.5 favouring outbreaks of epizootic ulcerative syndrome (EUS), Equateur Province, Democratic Republic of Congo (DRC), 2015.




Fisheries - Natural Resources and Sustainable Production E-mail: Melba.Reantaso@fao.org www.fao.org/fishery
Food and Agriculture Organization of the United Nations Viale delle Terme di Caracalla 00153 Rome, Italy








