EUROPEAN EDITION
MEDICAL PLASTICS news THE NEW POSSIBILITIES OF MEDICAL-GRADE POLYMERS VIRTUAL SUPPLIER AUDITS — THE NEW NORM? TESTS FOR POLYMER COMPATABILITY WITH HARSH DISINFECTANTS
PUMP UP THE V LUME
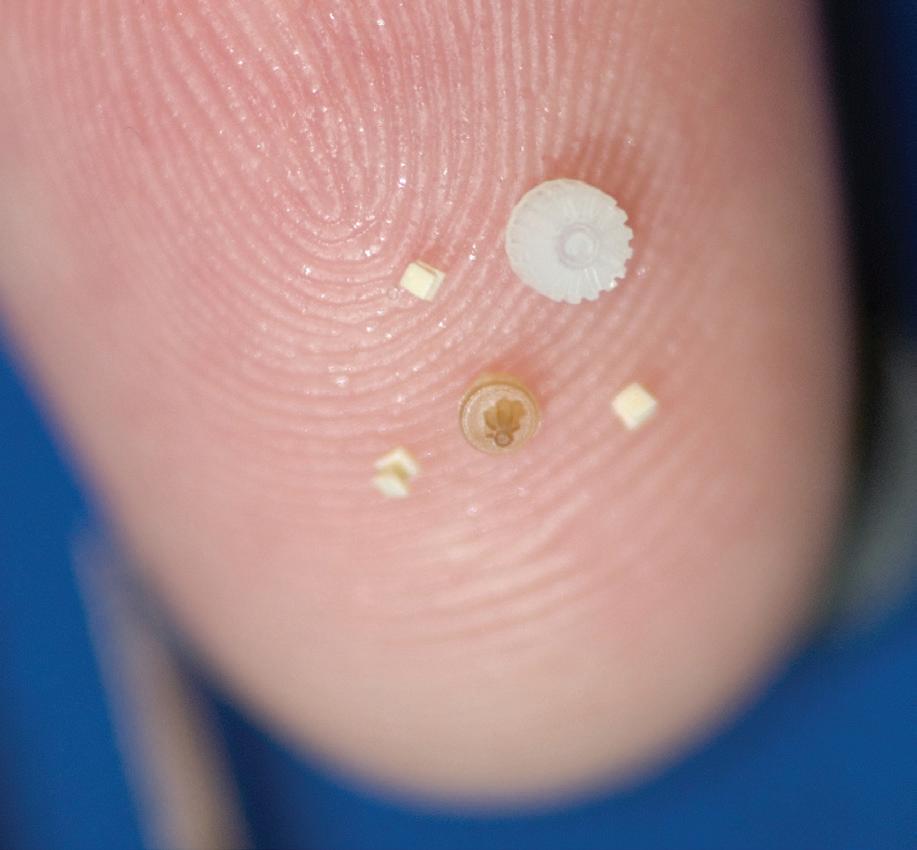
Accumold’s Aaron Johnson focuses on volume manufacture of micro medical components.
ISSUE 60
May - June 2021
WWW.MEDICALPLASTICSNEWS.COM
Aug 10-12 2021 Anaheim, CA Anaheim Convention Center
JOIN North America’s Largest Annual Medtech Event
MDMWest.com/MPN
17959_MDM_W21
REGISTER NOW at
CONTENTS May/June 2021, Issue 60
Regulars 5 Comment Corrine Lawrence on change. Our ability to embrace it has galvanised the medical plastics industry. 6 Digital spy 12 Cover story Accumold’s Aaron Johnson focuses on volume manufacture of micro medical components. 26 06:2021
Features 9 Orthopaedics: Survival instinct — it’s in the bones Benny Hagag, President, MicroPort Orthopaedics on the company’s survival tactics in response to the coronavirus pandemic.
14 Innovation in implants: Thermoplastics: the cool kids of medical 3D printing Implants 3D-printed with medicalgrade polymers offer new possibilities compared with metals such as titanium, says Kumovis. 17 Supply chain: VSAs not VISAs Lloyd R. DeShane, vice president, China operations, Yongshen Mould, outlines how virtual supplier audits look set to become the new normal. 19 Materials: Determining polymer compatibility with harsh healthcare disinfectants — Part I LNP Copolymers’ senior business manager, Nithin Raikar, focuses on the test for compatibility among different materials, such as industry standard PC blends and advanced PC copolymers. 22 Materials: Hydrophilic porous plastics Ken Nzeribe, materials scientist and engineer, Porvair Sciences discusses the advancements in manufacturing hydrophilic porous plastics for healthcare and pharmaceutical industries.
WWW.MEDICALPLASTICSNEWS.COM
3
Recommended by leading blood experts: lipid-resistant and BPA-free CYROLITE®.
When blood is flowing outside of veins, hopefully it’s flowing through CYROLITE®. Our high-performance medical acrylics are chemically resistant to lipids, are BPA- and DEHP-free, and can be reliably sterilized using gamma irradiation, e-beam irradiation, and ethylene oxide. This has impressed both vampires and health-care professionals alike: CYROLITE® meets the requirements of USP Class VI, ISO 10993-1, and REACH. You can learn even more about our blood-friendly acrylic polymers at www.cyrolite.com.
editorial content producer | corrine lawrence corrine.lawrence@rapidnews.com advertising | caroline jackson caroline.jackson@rapidnews.com
Editor’s Comment
vp sales & sales talent | julie balmforth julie.balmforth@rapidnews.com
C O R R I N E L AW R E N C E
head of studio & production | sam hamlyn
#Change
graphic designer | matt clarke junior designer | ellie gaskell publisher | duncan wood Medical Plastics News Europe Print Subscription – Qualifying Criteria UK & Europe – Free US/Canada – £249 ROW – £249 Medical Plastics News NA Print Subscription – Qualifying Criteria US/Canada – Free UK & Europe – £249 ROW – £249 FREE on iOS and Android devices Subscription enquiries to subscriptions@rapidnews.com Medical Plastics News is published by: Rapid Life Sciences Ltd, Carlton House, Sandpiper Way, Chester Business Park, Chester, CH4 9QE T: +44(0)1244 680222 F: +44(0)1244 671074
W
ords. They come, they go, drifting in and out of favour, acquiring their own hashtag if they’re successful enough. Words are barometers of the time, highlighting the highs and lows of life. Change. It’s a common enough word, yet it has re-asserted itself with force during the last 18 months, arriving with the elegance of a crash landing. We’ve probably heard ‘change’ as many times as we’ve heard ‘unprecedented’. But whether we asked for it, like it, or even care about it, change has been thrust upon us. Turning a blind eye to its arrival has delivered a crippling, if not fatal, blow to business longevity. Those companies that have understood and accepted they must change, and quickly, have cheated ruin. Although change can be disconcerting, rudely interrupting the familiar flow of daily life, it is also re-affirming. If we were in any doubt, we have now demonstrated that our collective skills, knowledge, experience, and the occasional leap of faith, can sustain us. We can adapt. We can survive.
© 2021 Rapid Life Sciences Ltd While every attempt has been made to ensure that the information contained within this publication is accurate the publisher accepts no liability for information published in error, or for views expressed. All rights for Medical Plastics News are reserved. Reproduction in whole or in part without prior written permission from the publisher is strictly prohibited.
My position at MPN puts me in receipt of hundreds of emails a week (I kid you not!), each informing me of how you have survived. Your new products, services, capabilities, initiatives all bear testament to your success. They also share a common thread — your ability to adapt to the world around you, be that working from home, increasing capacity in response to demand, or even diversifying. Change happens faster and better when we work together, as the global response to COVID-19 has proved, and continues to do so. Many of your success stories are the result of collaborations, an appreciation and respect for the other’s expertise. MPN has also recently undergone some change. Our new-look website has been designed to reflect your need for more insight-based content. Please go in and explore. I encourage you to contact me with your thoughts and ideas about how best MPN can serve you, whether that’s through specific content, or helping you to connect with others in the medical plastics industry. Medical plastics — what an incredible industry to be part of. Let’s keep innovating, collaborating, and standing up to #change.
BPA Worldwide Membership ISSN No: 2047 - 4741 (Print) 2047 - 475X (Digital) WWW.MEDICALPLASTICSNEWS.COM
5
DIGITAL SPY
DIGITAL
spy
A
www.idc.uk.com
IDC PURCHASE INVITES A “WEALTH OF PLASTICS MANUFACTURING EXPERIENCE”
I
By acquiring Naiad’s injection moulding facility, IDC expands its manufacturing services for customers. “We can now offer complete product design and production services, as well as manufacturing only solutions for clients who solely need support with production. Naiad has opened the door to a wealth of plastics manufacturing experience, and this, in combination with IDC’s highly skilled team, brings unique capabilities across product design, prototyping, testing and production. This is particularly the case as we see more companies looking to
www.prisymid.com
SOFTWARE COMPANY SEES MEDICAL DEVICE SECTOR PREPARE FOR THE FUTURE
ACQUISITION UPDATE
nternational product design company, Industrial Design Consultancy (IDC), has purchased Naiad Plastics, a UK-based injection moulding company.
LABELLING UPDATE
manufacture in the UK,” said IDC’s MD Stephen Knowles. Naiad will continue to operate from its base in Littlehampton, West Sussex, and will be run by its MD, David Wright. The company has strong environmental policies and works extensively with recycled plastics to support customers in improving the environmental impact of their products. Commenting on what the buyout means for Naiad, the company’s MD, David Wright, said: “This will benefit existing customers with investment in new machinery, a muchimproved working environment and give Naiad the capacity to assist new customers from concept to final product with the design capabilities of IDC.”
fter five significant business wins at the start of the year, PRISYM ID has successfully implemented a further six new regulated labelling software systems as demand from across the medical device and life science sectors increases. The labelling software firm says this performance demonstrates the medical device and life science industries are recovering from the impact of the pandemic and are refocusing on implementing labelling management solutions to help prepare for the future. The company adds that after experiencing the effects of the pandemic on the market, customers are now looking for solutions that
INVESTMENT UPDATE
www.boddingtons.co.uk
Boddingtons invests in new production cell to manufacture syringes
W
inning an order from a leading UK OEM healthcare company for the volume production of insertmoulded syringes has spurred Boddingtons — a medical device manufacturer — into investing in a new production cell. A new Engel 160t electric injection moulding machine together with a 6 axis Kuka robot forms part of the investment. An 8 + 8 mould 180° rotating tool is deployed in the twin shot part of the Engel machine and a total of four mould tools have been made by Boddingtons on behalf of the OEM client. The new production cell will primarily manufacture syringe devices which will be used in applications for ocular healthcare. The main body of the syringe is moulded in medical-grade polypropylene, and a TPE-based plug is then inserted into the
6
will increase their flexibility and allow them to respond more effectively to any change or uncertainty in their business. In particular, many organisations are prioritising speed of implementation as they seek to implement solutions that can help them as they prepare for life post pandemic — as a result, PRISYM ID says that its customers are putting a far greater focus on so-called “time to value” for their technology investments than ever before. Richard Adams, chief executive of the company, said: “The first few months of 2021 have been extremely busy for us as it is clear that the legacy of COVID 19 has focused people’s minds.”
WWW.MEDIC ALPL ASTICSNEWS.COM
syringe tube. Andy Tibbs, Boddingtons’ CEO, said the “complex but satisfying” project will “boost our own manufacturing skill sets”. “Clinical and healthcare need around the world has increased greatly,” noted Tibbs, “and is unlikely to decrease any time soon. We believe that successful medical manufacturers will invest accordingly and will reap the rewards.”
DIGITAL SPY
MOULDING UPDATE
www.hasco.com
HASCO launches new offline tool to assist mould designers
KAREN DRINKWATER, PRESIDENT, BRITISH PLASTICS FEDERATION
for all conventional CAD systems; models of the products can be directly imported and exported; and the assembly environments of the standard mould units can be called up directly, generated and configured with just a few clicks of a button, resulting in significant time savings and making the design of injection moulding tools easier.
3D PRINTING UPDATE
www.stratasys.com
Novel all-in-one medical 3D printer sets new standards
S
tratasys has launched its J5 MediJet 3D printer for healthcare providers and medical device companies that combines multiple applications in one system. With multiple materials and multicolour capabilities, the printer enables users to create highly detailed 3D anatomical models and drilling and cutting guides with approved third party 510k cleared segmentation software. Guides and models are certified as sterilisable
POINT https://www.bpf.co.uk
T
he HASCO SET — the Standard Engineering Tool for mould making — is the company’s newly developed offline software program geared specifically to the needs of mould making designers. Because CAD computers in design — often for security reasons — are not connected with the Internet, the HASCO SET as an offline version offers the optimum solution here. Designers can simply install it and utilise the entire product library comfortably at the CAD workstation. Regular updates guarantee the user access to more than 100,000 quality mould units, current product data and the latest information. The mould-making assistant from the HASCO portal provides further support. With the integrated layout editor, personalised mould structures can be easily configured. Furthermore, interfaces are available
talking
and biocompatible, and the printer is economical and compact enough for small lab spaces. Compared with other 3D printers, the MediJet 3D printer is up to 30% faster, along with a simple workflow that includes automatic build tray arrangement, corrections and support for the latest 3MF file format for simplifying connectivity to third party segmentation and design software. The new printer supports DraftWhite material for affordable single material applications, along with a full array of new flexible, rigid colour, and transparent materials. The multimaterials capabilities support a broad range of medical modelling applications in one office friendly platform, which reduces outsourcing costs or the need for multiple printers.
What are you particularly passionate about in the plastics industry? The need to attract and nurture the best talent. We have the potential to be a powerhouse of change in the sustainability agenda, particularly in the quest to reduce carbon emissions. We need well educated and trained people with creativity to keep us at the cutting edge of innovation. What are the main issues of your medical sector members? The shortage in raw material supply is holding us back from exploiting the bounce back from COVID-19. Meeting increased demand is a challenge. Adjusting to the needs of a new regulatory regime post Brexit will also focus minds, as will how the medical sector can contribute to the ‘green industrial revolution’ promoted by the government. Does the BPF work with or get inspiration from other similar organisations in Europe? Plastics is an international industry and BPF is a leading member of many European organisations such as European Plastics Converters (EuPC) and it works closely with PlasticsEurope. The BPF is also a key member of the global organisation for plastics industry associations, CIPAD. Sharing common positions on key issues is crucial in consistent communications at national, European or global levels. What issues are going to keep you busy for the next 12–18 months? The raw material supply issue is unresolved, and there are still problems connected with Brexit where clarification is required such as CE marking and the new UK REACH regime. Further developing the education and skills base of the industry is a priority to enable our industry to reach its potential. Meeting the government’s Net Zero Carbon Emissions target and developing the UK’s plastics recycling culture further is a key part of the programme, as is positioning the industry to take advantage of export opportunities that could open up in the wake of trade deals being sealed. 7
You expect precision. We deliver. contract manufacturing injection moulding medical devices Our manufacturing includes cleanroom environments, automation, and assembly services, delivering total value solutions that reduce investment and improve cost.
www.carclo-ctp.co.uk +44 208 685 0500 sales@carclo-usa.com United States
• United Kingdom
• Czech Republic • India
• China
ORTHOPAEDICS
THE CORONAVIRUS PANDEMIC HAS FORCED COMPANIES OPERATING WITHIN THE MEDICAL INDUSTRY TO ADAPT QUICKLY, BE IT IN RESPONSE TO DEMAND OR TO DEVISE NEW MODES OF WORKING. ORGANISATIONS UNABLE TO DO SO, HAVE FARED BADLY. BENNY HAGAG, PRESIDENT, MICROPORT ORTHOPAEDICS, TALKS TO MPN ABOUT ITS SURVIVAL TACTICS.
SURVIVAL INSTINCT —
ITS IN THE BONES PLEASE TELL A LITTLE ABOUT MICROPORT ORTHOPEDICS AND ITS FOCUS. MicroPort Orthopedics is a global producer of orthopaedic hip and knee technology, with headquarters in the Greater Memphis area of the US. It is the subsidiary company of MicroPort Scientific, a global medical device company providing innovative services and products to surgeons and patients worldwide, with a focus on total orthopaedic joint care. In 2014, the parent company expanded its global portfolio by acquiring Wright Medical’s total orthopaedic joint business. MicroPort directs its full attention on developing innovative and differentiated technologies that are customised to meet each customer’s needs, in addition to maintaining our current fundamental portfolio. WHAT NEW TECHNOLOGIES AND STRATEGIC CHANGES HAVE BECOME PERMANENT FIXTURES FOR HOW MICROPORT ORTHOPEDICS WILL OPERATE POST-COVID? COVID-19 deeply affected the medical device industry. It hit us unexpectedly and forced many businesses to shift from a culture of working face-to-face with customers to one where the entire organisation went fully remote. Although this initially made it difficult to engage with customers, it did, however, allow us to develop creative solutions and approaches to address this challenge. We focused on developing compelling and informative video and email communications with our employees. It was important to provide critical organization detail to our team during an uncertain time.
We quickly pivoted to develop new tactics, new sales capabilities and new management techniques. These tools hadn’t been available previously, or at least did not exist at the scale required during the pandemic. MicroPort Orthopedics’ ability to sustain the business throughout COVID-19’s first wave is directly attributed to our ability to maintain unity with all of our employees around the world, as well as the support received from our parent company. The decision by MicroPort Scientific to financially invest in our division to sustain the company and minimize impact allowed our global organisation to direct its full attention to developing necessary management and communication skills to enable our business to thrive in this adaptive environment. We monitored the situation daily, identified practical opportunities of growth, and evolved
9
ORTHOPAEDICS
I believe every business had to assess its capabilities and resources, as well as their strategy for future initiatives into an agile organisation where key initiatives were adopted swiftly across the entire business. Our executive leadership provided key insights, support; they even drove these initiatives. I fully expect the business to maintain a similar strategy, drive and flexibility after COVID-19. HOW HAS COVID-19 AFFECTED MANUFACTURERS OF ORTHOPAEDICS? It was difficult for all orthopaedic manufacturers to transition and adjust business operations throughout COVID-19. Unlike many manufacturers, MicroPort had the unique advantage of receiving full support from a significant parent company throughout the period. I believe every business had to assess its capabilities and resources, as well as their strategy for future initiatives. Location also mattered. MicroPort is lucky to have offices in more than 80 countries, with strategies already in development to address the differences between markets. It was easier to adjust our global strategy when each office has the ability to react locally, and fine-tune based on how COVID-19 was trending in specific areas. In addition, with different timing and impact level of COVID waves around the globe, we were able to balance the impact curve and sustain relatively stable, although affected, business. WHAT INNOVATIVE TECHNOLOGY HAS THE PANDEMIC EXPEDITED? Since I joined MicroPort Orthopedics more than a year ago, the company has been developing a global road map initiative that focuses on capturing the larger picture. The key focus areas are who we are today, and where we need to be as a global player during the next 5–10 years, and beyond. The pandemic unexpectedly allowed us to direct greater efforts into this. You will see a greater focus towards developing our technologies that deliver an optimised experience across a range of services, for both patients and surgeons through our medical education services, as well as our services. There is a shift towards a more digital healthcare space. MicroPort Orthopedic is leaning into this trend by providing our surgeon customers a way to digitally connect, seek out patients who are in research mode for total joint replacement, get them into a surgeon’s office for a consultation and at that point, our additional technologies kickstart the Episode of Care for our patients.
and engagement, and we continue by providing efficiencies in the operating room, post-operative pain management, recovery monitoring, and the ability to capture the patient reported outcomes. Our approach to the Episode of Care is unique in its simple yet effective ability to drive outpatient total joint replacement success. THE FUTURE OF ORTHOPAEDIC MANUFACTURING IS RAPIDLY CHANGING. WHAT CHANGES CAN WE EXPECT TO SEE DURING THE NEXT 5 YEARS? Many would answer that robotics is the way of the future. They’ve become significantly popular, and I expect that trend to rise. But this robotic race will only lead to increasingly higher costs for hospitals and business partners; eventually, this technology will have to evolve and change to drive cost and efficiency improvements, possibly even within the span of 5 years from now. That is why MicroPort chooses to instead focus efforts on prioritising new technological alternatives that will not only require significantly less investment by hospitals, but will also minimise the cost burden per case, all while delivering the same, if not better, clinical outcomes as expected from robotics. MicroPort has the strategy and foresight to look beyond the next 5–10 years. The future of medicine will continue to rapidly change as more and more companies begin to invest in discovering how they can also produce differentiated technologies that set them apart from the field.
HOW HAVE ORTHOPAEDIC MANUFACTURERS MADE STRATEGIC CHANGES TO THEIR OPERATIONS IN RESPONSE TO THE PANDEMIC? A typical orthopaedic manufacturer builds programs and products to address the masses with a “one-size fits all” solution. I believe the pandemic’s impact solidified this strategy for many, just to effectively sustain business operations. If a company did not already have the system in place that lends itself to producing customised solutions on a global scale, then the pandemic has not helped. WHAT IS MICROPORT’S ORTHOPEDICS’ EPISODE OF CARE MODEL AND WHAT’S UNIQUE ABOUT IT? MicroPort’s Episode of Care approach enhances operational efficiency and patient satisfaction throughout the 90 days both preand post-surgery. Unlike many orthopaedic companies, we provide a personalised suite of solutions to help surgeons and hospital partners find patients, keep patients and achieve effective and profitable outcomes in the Ambulatory Surgery Centers setting by focusing on what matters most: patient satisfaction and outcomes, delivering on a simple, efficient Episode of Care experience. We begin from pre-operative patient education
WWW.MEDICALPLASTICSNEWS.COM
11
COVER STORY
PUMP UP THE V LUME M
icromoulding typically requires the achievement of truly exacting and sometimes almost impossibly tight tolerances. In many instances the demand is for tiny parts or slightly larger parts with submicron feature sizes. When micron tolerances matter, the customer and micromoulding provider must enter into a close partnership in product development, and it becomes hugely important that the micromoulder owns, manages, develops, and innovates in every aspect of the supply chain. The “get it right the first time” headline over every activity plays to the vital importance of vertical integration, which enhances the quality, compliance, and conformance to design intent. Micromoulders need to understand the critical to quality (CTQ) characteristics to manufacture parts successfully; these characteristics — including moulding, assembly, and packaging — are important to the functionality of the product with regard to end-user experience. In basic terms, the longer the value stream, the more disconnected the value stream, and the more variables can be introduced causing customer issues. Vertical integration supports a shorter value stream reducing silos and suboptimised processes.
ENGAGEMENT WITH A MICROMOULDER FOR A MEDICAL DEVICE PRODUCT DEVELOPMENT PROCESS WILL ULTIMATELY RESULT IN THE NEED TO MANUFACTURE PARTS, OFTEN AT VERY HIGH VOLUMES. MICROMOULDERS NEED TO EXCEL AT EVERY STAGE OF PRODUCT DEVELOPMENT, BUT, AS AARON JOHNSON, VP OF MARKETING AND CUSTOMER STRATEGY, ACCUMOLD, HIGHLIGHTS, THERE ARE PARTICULAR CONTINGENCIES THAT SURROUND VOLUME MANUFACTURING THAT MAKES SUPPLIER SELECTION ESPECIALLY IMPORTANT.
VOLUME MANUFACTURING Variability is the enemy of high-volume micromanufacturing, and so focus needs to be maintained on implementing process controls that drive repeatability all the way from cutting micro tool steel to measurement and validation methods. Typically, medical device OEMs want the highest quality products at the lowest possible cost. Product quality requirements are driven by the Voice of the Customer (VOC), which enables good micromoulders to identify critical medical product characteristics which need to be verified during production. Cost, however, is more than just the price charged to mould a part. It also includes the time it takes to get a product to market, whether defects make it to market, and the levels of supply consistency. Micromoulders should strive to supply the highest quality products, through validated processes, with short timelines to market. Realistically, this can only really be accomplished through vertically integrated processes and utilising process validation. When short-listing potential micromoulding suppliers, from a production perspective there are some key questions that medical device OEMs need to ask to ensure the micromoulder is equipped to achieve often exacting objectives. First, do you design, build, and maintain your moulds? Second, what measurement capabilities do you have on site? Third, what are your methods of validation (DOE, IQ, OQ and PQ)? Finally, what resins do you have experience with in production? The successful manufacture of micromoulded medical products is almost entirely down to the VOC. But it is important to realise that the end user isn’t the only customer — there are customers throughout the entire value stream. The shorter and more centrally located the value stream the quicker concerns can be raised and resolved. ASSEMBLY When dealing with miniaturised plastic medical parts and components, the assembly stage of the product development process must be discussed and considered early in the design cycle, demanding a collaborative and pragmatic relationship between medical device OEM and micromoulder. When dealing with microscale parts and components, the cost of manual assembly is prohibitive and often requires levels of precision when dealing
12
W W W. M E D I C A L P L A S T I C S N E W S . C O M
COVER STORY
with submicron tolerances that are impossible to achieve. Automated assembly is, therefore, a must in most micromoulding scenarios, requiring that medical device OEMs select a micromoulding partner that is able to understand the methodology of micro assembly and achieve the extreme positional accuracy required.
The demand is for a laser-like focus on design for micromanufacturing (DfMM), which crucially influences the success of every part of the overall product development process, including assembly. The importance of considering assembly at the design stage of a micromoulding product development process is huge, and is an important factor for any project. Micromoulding just adds more variables that need to be controlled than a standard-sized product for which there are many different manufacturing solution partners in the marketplace. Micromoulded parts can be difficult to feed, inspect, and manipulate without causing damage. Capturing a micromoulded part as it comes out of the mould is often difficult, as is orienting a micro part after moulding. Capturing a part at mould ejection and immediately assembling is often the only route to efficient assembly with low waste. All such considerations should be bottomed-out at the design stage to keep costs under control so a medical device OEM can maintain margins and provide a product to the marketplace at an acceptable price.
Variability is the enemy of highvolume micromanufacturing, and so focus needs to be maintained on implementing process controls that drive repeatability …
SUMMARY Before embarking on a micromoulding project, medical device OEMs should focus on the mould design, mould build, and quality measuring capabilities of the vendor they are engaging to mould their parts. If moulders lack the ability to design, build, and measure high quality moulds to extremely tight tolerances they will struggle to produce consistently high quality parts to tight tolerances at high volume. Producing products to specifications that only allow microns of variations to a specification is much more challenging than producing a product with significantly larger variation allowances. A poorly designed and built tool can eat up your entire variation before you even mould a part, essentially killing the project. So, when micron tolerances matter, informed supplier selection is the absolute key to high volume manufacturing success. 13
INNOVATION IN IMPLANTS
Thermoplastics: the cool kids of medical 3D printing METALS SUCH AS TITANIUM SEEMED TO BE THE LAST WORD IN MEDICAL MANUFACTURING. NOW, IMPLANTS 3D-PRINTED WITH MEDICAL-GRADE POLYMERS OFFER NEW POSSIBILITIES, SAYS KUMOVIS.
T
he discussion about medical 3D printing with polymers has long since grown beyond surgical models and rapid prototyping. Medical R&D activities indicate that longterm applications such as implants, which previously relied on metals and conventional production methods, are likely to be challenged by 3D printed polymer implants in the coming years.1 It has taken years of trials, testing and constant process development to fuse 3D printing technologies and thermoplastics into solutions that open up new opportunities for both medical device companies and hospitals. Possible applications range from trial implants and instruments to short- and long-term implants. The safety and performance requirements of the manufacturing process and 3D printed products vary depending on the local regulatory peculiarities and fields of application. ADDED-VALUE MEDICAL DEVICES Instruments and trial implants, for example, come into contact with patients’ blood during surgery; this, however, is just a temporary contact. And whereas the risks of applications such as these are low compared with implants intended to be permanent, their potential in terms of costs, functionality and ergonomics is all the higher.
Permanent polymer implants also have great potential. These products must meet the industry’s requirements for mechanical properties and those for biocompatibility, including considerations of the long-term interaction with the surrounding tissue. There are already first projects to 3D printing interbody fusion cages from polyetheretherketone (PEEK) or polyetherketoneketone (PEKK), including solutions that help cells adhere to them (Figure 1). HOW TO IMPROVE OSSEOINTEGRATIVE BEHAVIOUR Medical device companies have sound reasons to use metals such as titanium to manufacture implants and instruments. Biocompatibility and mechanical properties have values that meet the high demands outlined above. Titanium, moreover, shows good osseointegrative behaviour; there are, however, polymer alternatives. Meeting the osseointegration challenges of polymer implants requires new developments. First, polymers modified for osteoconductive properties are on the rise.² Second, the 3D printing process combined with post processing can positively affect bone ingrowth. With its 3D printed macrostructure, the Kumovis Improved Implant Interface allows for enlarging the surface of medical devices, thus supporting bone ingrowth. At the same time, this technology coupled with post-processing enables nutrients to travel through capillary effects using a selective microstructure. Coating technology enables adding hydrophilic surfaces to polymer implants. To supply cells — both on the surface and inside the implant — with nutrients, the adaptation of porosity through 3D printing plays a major role. Moreover, applications pointing to tissue engineering could be feasible if new resorbable polymers and the associated processes prove themselves in testing.
3D printing trial implants with polymers such as polyphenylsulfone (PPSU) can save medical device companies up to
14
50% on manufacturing compared with milled titanium. Furthermore, a PPSU variant with X-ray contrast agent makes the trial implant visible in the X-ray image during the operation. Polymer trial implants are also lighter than their metal counterparts. And individual colour coding of the trial implant makes visible, at a glance, its size and application area.
WHICH POLYMERS TO USE FOR MEDICAL 3D PRINTING Besides using metals, healthcare professionals opt for high-performance polymers for 3D printing. Important medical-grade thermoplastics include the following: • PEEK: a high-performance polymer that has been used in medical
W W W. M E D I C A L P L A S T I C S N E W S . C O M
INNOVATION IN IMPLANTS
applications for years. With the Kumovis R1 3D printer, it is possible to process different types of PEEK filaments: grades for long-term applications such as cranial implants, and grades for short-term and temporary applications such as surgical instruments. • PEKK: a high performance polymer in which, compared with PEEK, one of the ether linkages is replaced by a stiffer ketone group. The resulting PEKK is a newer medical-grade material available for 3D printing. In its amorphous state, it offers high ductility and mechanical strength. • A group of medical materials comprising semicrystalline and amorphous biodegradable polymers with degradation times of less than 6 months to more than 3 years. It includes polylactic acid (PLLA), polylactic co glycolic acid (PLGA), polycaprolactone (PCL) and polydioxanone (PDO). These are already available in filament form for 3D printing in medicine. • Polyphenylsulfone (PPSU): an amorphous polymer that offers, among other things, high impact strength. Similar to PEEK, PPSU is available in different grades for long-term applications (for example, implantable medical devices) and for short-term applications. For 3D printing medical instruments, a small number of PPSU types is available: material in different colours, as well as PPSU filled with X-ray contrast agent to make it visible during medical imaging processes. There are not many options available for medical-grade PPSU. In principle, however, it is possible to add a variety of additives to PPSU. Materials for medical applications must be able to withstand sterilisation. The aforementioned polymers fulfil this requirement. And the field of application will determine the most suitable sterilisation process. When using PPSU to manufacture reusable medical instruments, for example, requirements for reprocessing must be fulfilled. This includes the material’s ability to withstand multiple cycles of hot steam sterilisation. HEAT IS KEY WHEN 3D PRINTING WITH POLYMERS Implant manufacturers face the significant challenge
Figure 1: 3D-printed PPSU trial implants for spinal fusion surgery.
of temperature management when 3D printing with medical-grade thermoplastics. To use high performance polymers, they must have a fully controllable build chamber heating up to 250 °C at any given point to prevent warping on build parts. Moreover, the homogenous heating at high temperatures allows for improving layer adhesion. The Kumovis R1 laminar airflow, for example, enables the required homogenous temperature distribution inside the build chamber. Reducing temperature gradients within the build part is key for good mechanical properties and reproducibility. Patent pending Kumovis technology enables medical device manufacturers to cool certain areas while 3D printing, to increase surface quality and dimensional accuracy. In-house testing at Kumovis, including tensile, flexure, impact and compression tests, now yield values that meet or exceed acceptance criteria for medical applications such as interbody fusion cages or cranial implants. Mechanical properties of parts (for example, tensile strength) 3D printed with the Kumovis R1 can be compared to those from injection moulding and milling. Compared with implants made with titanium or metal alloys, mechanical evaluation of 3D printed polymer implants shows these to be particularly advantageous. Using polymers can reduce differences in elasticity between bone and implant, whereby stress shielding effects and relative movements between bone and implant decrease; thus, optimised implant design, material choice and process parameters can avoid implant loosening. PARTNERSHIPS TO IMPROVE PATIENT CARE 3D printing has outgrown its baby shoes and is ready for series production. Furthermore, the conditions for innovation have rarely been better for manufacturers than they are now, despite new challenges such as the European Union Medical Device Regulation and the rising demand for personalised medicine. New processes and materials enable manufacturers to offer functionalised and individualised implants, as well as cost-efficient solutions for the series production of trials and instruments. Above all, it is important to implement, and realise the advantages of, additive manufacturing and medical-grade thermoplastics during the design phase of each implant. By doing so, healthcare and 3D printing experts can co create economically feasible solutions to a variety of medical challenges, and consequently enable better patient care. References 1. http://documents.epo.org/projects/babylon/eponet.nsf/0/ C2F0871212671851C125859F0040BCCA/$FILE/additive_manufacturing_ study_en.pdf 2. https://medical.evonik.com/en/peek-biomaterial-implants/vestakeep-fusionosteoconductive-peek
WWW.MEDICALPLASTICSNEWS.COM
15
Vyon® for Porous Plastics Solutions Precisely engineered components designed and manufactured for medical and life science applications. Filtration
Media Support
Separation
Wicking
Diffusion
Absorption
Venting
It’s the little things. Vyon®, is the leading brand of porous plastic material found at the heart of innovative product solutions. As small as it can be, Vyon® can be precisely engineered to tight tolerances and has the versatility to be manufactured into a wide range of shapes and sizes to ensure you get the perfect fit for your product or application. Learn more about Vyon® at:
Drop our team a message at:
www.vyonporousplastics.com
enquiries@porvairsciences.com
SUPPLY CHAIN
WHEN SUCH EVENTS AS THE CORONAVIRUS PANDEMIC RESULT IN GLOBAL TRAVEL RESTRICTIONS, COMPANIES NEED AN ALTERNATIVE TO IN PERSON SUPPLIER APPROVAL AUDITS. LLOYD R. DESHANE, VICE PRESIDENT, CHINA OPERATIONS, YONGSHEN MOULD, OUTLINES HOW VIRTUAL SUPPLIER AUDITS NOT ONLY RESOLVE THE IMMEDIATE SITUATION, THEY ALSO LOOK TO BECOME THE NEW NORMAL.
Replacing VISAs with VSAs
M
aintaining a strong supply base with the ability to grow the supply chain is an essential requirement for all companies that source products and production in Asia. Pre-COVID supplier approval audits in China/ Asia were fairly standard: supplier quality engineers (SQEs) and program managers would visit plastic tooling and moulding suppliers to audit the quality, capabilities and systems the supplier has in place to understand whether the supplier has the capabilities required to manage programmes for them. They will also check the supplier to confirm they are using certified resins and polymers from approved sources. These detailed audits typically take 6–8 hours of onsite investigation and review with another few hours of follow up if any details require further clarification at a later date. The supplier audit results in a Pass (approved supplier), Fail or Preliminary Approval (based on an agreed timeline in which to correct or improve a specific issue before resubmitting the data to confirm the issue has been addressed and meets the customer’s requirements. The supplier audit includes every aspect of the supplier’s ability to manage not only production but also general business practices (Table I). TRAVEL RESTRICTIONS The global pandemic saw Asia close its doors to visitors in 2020. According to the China Government, travel restrictions are expected to remain in place for all of Asia during 2021, with speculation of opening China in the spring of 2022 once herd immunity is achieved.¹ Even when borders open up, it will be expensive and complicated to travel. Airfares to Asia are at an all-time high and a COVID Vaccine Passport will
be required.²,³ China may still require a 2-week quarantine at the traveller’s expense when entering China. Furthermore, China will only accept a vaccine passport that details a China-produced vaccine; no other countries’ vaccine will be accepted. Travel costs and complications aside, visitors still have the health risks. Many companies do not want to subject their employees to these health risks and the liabilities that come with travel due to new strains of the virus and potential infection. These external factors have (and will continue to do so) forced companies to find a way to perform remote Supplier Audits, which are required for three main reasons: 1. Supplier Requalification — a supplier maintenance procedure, typically performed once a year, to ensure a current supplier is maintaining its quality systems and production requirements. 2. Supplier Production Audit — validation of current production; cost reduction ideas including implementation of automation and other manufacturing improvements; delivery schedules; quality assessment of production; and review of increased or reduced production requirements. 3. New Supplier Qualification — introducing a new supplier into the supply base to replace a supplier that cannot meet current standards or demands, or a new supplier that has a new technology or manufacturing capability/ capacity that is desired by the customer. THE VSA For the reasons stated above the Virtual Supplier Audit (VSA) system was born out of necessity. In preparation for the VSA you must communicate with the Asian supplier in advance to ensure they have the tools needed for the VSA and have made the necessary arrangements (Table II). Although customers prefer to see the information in person, using a hand-held phone/camera comes a close second: it can be used during the factory tour, to facilitate questions, and guide the tour if/when needed. There really is little difference between a VSA and being present at the audit. A follow-up audit can also be arranged if the customer did not see exactly what they wanted or
WWW.MEDICALPLASTICSNEWS.COM
17
SUPPLY CHAIN
needed further validation. On-site supplier validations, however, are not necessarily destined to become things of the past. Many manufacturers still prefer in-person and on-site meetings to gain a personal feel for the teams and management that will produce the product for them. Limited travel has made VSAs essential — a status that looks set to remain certainly for the next 2 years, particularly for those businesses attempting to maintain and grow a strong supply base. Improvements in virtual meeting technologies are making VSAs particularly beneficial as they control the high cost of sending audit staff to Asia, and facilitate real-time reporting of any production issues or the approval process. Once the suppliers have been trained in using VSAs they can apply the same skills and equipment to provide immediate virtual updates on all issues. A significant amount of preparation is required by the customer and the supplier for a smooth VSA. The customer should provide a clear agenda with timings for each topic to be covered and a list of employees required to attend the meeting. The customer should submit the agenda to the supplier at least one week in advance of the VSA affording them enough preparation time, and schedule the time with the staff required for the meeting. A wellplanned VSA will ensure that all the information is ready to present via the share screen and the people needed to answer questions are all present in the meeting. References 1. https://mp.weixin.qq.com/s/ HKAYkNv4S0TxidzKEcdMpw 2. https://www.trip.com/blog/covid-19vaccine-passports/ 3. https://www.straitstimes.com/asia/ east-asia/covid-19-vaccine-travelrules-widen-the-rift-between-chinaand-the-west
18
Table I: An example of a VSA agenda outlined by a customer (Philips Medical) and given to the supplier 3 days prior to the audit. The information requested for review is determined by the customer based on what is important to them. Quality certifications (ISO, TS, etc.)
Design review and approval
HR review, staff growth and training, retention rate
Quality review, equipment, calibrations, software, processes, etc.
Employee safety, human rights and working conditions
Production build schedule reporting and updates
Environment regulations compliance
Inventory controls (incoming and outgoing)
Quotation review
Sampling of tooling, TO and T1
Data release controls, ECN (engineering change notice) process
Final dimensional buyoff (first article approval)
Equipment list
Logistics, shipping, packaging review
Design for manufacturability and analysis review
IP (intellectual property) protection and security of data
Engineering review, capabilities, CADD systems, software, etc.
LEAN manufacturing initiatives
Manufacturing review, equipment, calibrations of equipment, etc. Table II: An example of VSA requirements outlined by a customer (Philips Medical) and given to the supplier 7 days prior to the audit. The customer decides who will attend the meeting and the platform through which it will be conducted (for example, Microsoft Teams). Video camera in the conference room working at high efficiency (no delays accepted). Audio in the conference room working at high efficiency with microphones by each presenter. Cell phone with 5G that can be used for factory tour without delay in audio or video; cell phone also connected to audio/video conferencing platform, such as Teams Meetings. Entire management and factory department leaders available for questions. All documentation for review can be done via share screen. Technical managers or support personnel from other countries tied into the virtual meeting for questions. Allow 6–8 hours for the meeting, with a 30-minute break.
W W W. M E D I C A L P L A S T I C S N E W S . C O M
MATERIALS
Determining polymer compatibility with harsh healthcare disinfectants — Part I WITH THE SPATE OF NEW, HARSH DISINFECTANTS BEING USED IN HEALTHCARE SETTINGS — PRIMARILY IN RESPONSE TO THE COVID-19 PANDEMIC — DEVICE MANUFACTURERS NEED TO CONSIDER NEWER MATERIAL SOLUTIONS WITH IMPROVED CHEMICAL RESISTANCE TO WITHSTAND THE CUMULATIVE EFFECTS OF CLEANING. NITHIN RAIKAR, SENIOR BUSINESS MANAGER, LNP COPOLYMERS FOCUSES ON THE TEST FOR COMPATIBILITY WITH HEALTHCARE DISINFECTANTS AMONG DIFFERENT MATERIALS, SUCH AS INDUSTRY STANDARD PC BLENDS AND ADVANCED PC COPOLYMERS.
A
ccording to the World Health Organization, healthcare-associated infections (HAIs) are the most frequent adverse event in care delivery worldwide. As the COVID-19 pandemic has prompted hospitals and clinics to implement enhanced cleaning protocols, there has been a clear shift in the way plastics are viewed to address infection control challenges. The introduction of new disinfectants has pushed device manufacturers and material suppliers to seek better understanding of the compatibility of new chemical agents on plastic materials. Specifically, device manufacturers need to consider newer material solutions with improved chemical resistance to withstand the cumulative effects of cleaning — the combination of aggressive disinfectants and increased frequency in protocols. Disinfectants such as alcohols, peroxides and quaternary ammonium compounds can cause traditional polymers to become brittle and crack, shortening the lifespan of costly and critical devices. Medical professionals face a dilemma: How can they trust their devices to keep patients safe and function optimally with increased cleaning? To address this challenge, SABIC has developed a new family of materials. The company’s LNP ELCRES CRX polycarbonate (PC) copolymers can provide superior resistance to some of the harsh disinfectants that are the norm in healthcare today.
MATERIAL SELECTION FOR SAFER, LONGER-LASTING MEDICAL EQUIPMENT A primary side effect of repeated cleanings on plastics is polymer embrittlement. When plastics come into contact with chemicals under stress, a phenomenon, known as environmental stress cracking, occurs. In terms of polymer chemistry, exposure to chemicals may result in either physical degradation (stress cracking, crazing, swelling and discoloration) or chemical attack (reaction of chemical with polymer and loss of properties). Amorphous polymers such as acrylonitrile-butadiene-styrene (ABS) and polycarbonate (PC) resins were traditionally used for medical device housings and enclosures. When
W W W. M E D I C A L P L A S T I C S N E W S . C O M
19
MATERIALS
chemical resistance initially became an issue and components made with these materials started to fail from environmental stress cracking, manufacturers began replacing ABS and PC with blends of PC and ABS or semi crystalline polybutylene terephthalate (PBT). Even these incumbents, however, can fall short in chemical resistance, especially in view of additional measures to prevent COVID 19 transmission.
The ESC test has proven to be a useful indicator of expected performance and serves as a screening tool for polymer material candidates
To help maintain the structural integrity of medical devices, SABIC’s Specialties business launched a new product portfolio, LNP ELCRES CRX resins, leveraging a unique new copolymer building block to help meet the needs of this developing market. CRX copolymers are designed to reduce susceptibility to chemical attack and to help minimize crack propagation. In addition to chemical resistance, polymers used in device enclosures and housings need high impact properties to withstand being dropped or resist an external applied force. Additional polymer features include flame retardancy for powered devices and custom colourability to enable styling and aesthetics in part. The retention of impact strength from repeated application of disinfectants is also a key factor for durability over time.
screening tool for polymer material candidates. SABIC follows an established ESC testing procedure (ASTM D543) to screen chemicals and environmental conditions that mimic the part application exposure. To test compatibility with various chemicals, SABIC has used a quantitative ESC test that evaluates retention of tensile properties from 3 to 7 days at 1% strain and at room temperature. These properties correlate to plastic failure modes and often provide insights on resistance to fracture. The test bars are kept saturated with the chemical agent (wrapped in disinfectant wipes) and are bent to a specific strain level (1% in this case) in a test fixture. Constant strain is maintained throughout the test period (Figure 1).
Advanced PC copolymers such as CRX may be a material solution that ticks all the boxes so that hospitals can continue to use aggressive and effective forms of disinfectants to keep patients safe while also keeping medical equipment serviceable for longer periods of time. Device manufacturers may also be less likely to face costly requests to replace devices under warranty.
Note LNP and ELCRES are both Trademarks of SABIC or its subsidiaries or affiliates.
To demonstrate compatibility with a given chemical agent, a material must achieve >90% tensile stress at yield and 80–139% tensile elongation at break, per ASTM D638: Standard Test Method for Tensile Properties of Plastics. With no widely adopted industry standard for chemical resistance testing, SABIC pushed the test conditions (the exposure duration and external stresses) to ensure that testing protocols serve as a highly accelerated version of real life exposure to disinfectant wipes in a healthcare setting. SABIC continues to collaborate with healthcare OEMs who are conducting their own testing to validate the materials in their applications and environment.
Figure 1: SABIC test method.
ENVIRONMENTAL STRESS CRACKING (ESC) TESTING The ultimate laboratory test for a plastic material for use in a finished part would be one that measures the performance over an entire range of temperatures, impact forces, loads, and chemical exposures in its actual end use. Unfortunately, such thorough testing has not yet been fully developed and would be extremely costly. The ESC test has proven to be a useful indicator of expected performance and serves as a
20
W W W. M E D I C A L P L A S T I C S N E W S . C O M
Your UK supplier of choice for bespoke plastic injection moulding and tool manufacture
DESIGN
Whether you are looking to move your existing Plastic Injection Moulding production or sourcing a supplier for a new product, Pentagon will support you at every stage of the process. Delivering a full turnkey solution from one site of UK Manufacture.
DEVELOPMENT MOULDING TOOLING BEYOND THE MOULD
www.pentagonplastics.co.uk websales@pentagonplastics.co.uk Tel: +44 (0) 1403 264 397
MATERIALS — SPONSORED CONTENT
c i l i h p o r s d c i t y s a H us pl o r o p
KEN NZERIBE, MATERIALS SCIENTIST AND ENGINEER, PORVAIR SCIENCES LTD DISCUSSES THE ADVANCEMENTS IN MANUFACTURING HYDROPHILIC POROUS PLASTICS FOR HEALTHCARE AND PHARMACEUTICAL INDUSTRIES.
P
orous plastic polymers are widely used in healthcare and pharmaceutical applications due to its versatile array of mechanical and biochemical properties. Specifically, the composition, structure and surface energy of polymers can be modified and controlled during manufacturing processes to impart hydrophilicity, sometimes referred to as wettability; that is, the ability of a liquid to stay in contact and be absorbed by the porous plastic. During healthcare and pharmaceutical product development, hydrophilicity is a crucial factor in determining the product performance limits, and a challenge that porous plastic manufacturers face is that plastic polymers are naturally hydrophobic; that is, liquids bead up on the surface and do not form strong intermolecular bonds with the material.
22
Figure 1: a) Typical contact angle of hydrophilic and hydrophobic surfaces; b) Schematic representation of a porous structure such as Vyon®. Measuring hydrophilicity requires several factors such as porous volume to accurately measure.
Conversely, hydrophilic materials can attract, absorb, dissolve in, mix with, or be wetted by water; for example, the hydrophilicity of porous plastic materials plays an important role in applications such as liquid absorption, controlled liquid flow and delivery (for example, point-of-care [POC] devices such as eye
W W W. M E D I C A L P L A S T I C S N E W S . C O M
New ShuttlePouch™ Cost-effective, User friendly Specimen Bio-pouch UN3373 compliant secondary packaging solution for specimen vials
ShuttlePouch™
Web: www.UN3373.co.uk | Tel: 023 8048 3000 | Email: sales@alphalabs.co.uk ShuttlePouch June 21.indd 1
04/06/2021 11:46:08
Cleanroom Particle Counters
Portables
Handhelds
Continuous Monitoring Cleanroom Certification • Cleanroom Monitoring PMT (GB) Ltd. | Tel: +44 (0)1684 312950 | email: info@pmtgb.com
MATERIALS — SPONSORED CONTENT
... the composition, structure and surface energy of polymers can be modified ... droppers and nasal sprays) and are thus, at a product level, subject to high levels of regulatory approval (for example, biocompatibility, high bacterial filtration efficiency). Hydrophilicity can be achieved by treatment technologies that render polymeric materials susceptible to fluids by covalently bonding highly reactive polar groups to their molecular chain to create waterattracting surfaces. There are different methods to render porous plastics hydrophilic: •P lasma treatments — this includes plasma cleaning, plasma surface activation, plasma coatings and plasma etching. At very low vacuum, electromagnetic energy is used to evolve highly reactive polar functional groups from gas molecules which are then covalently bonded to the surface of the material. •V acuum deposition process — this includes physical and chemical deposition processes. •D ip-coating — this process involves lowering an article hanging from a support into a liquid coating solution and then pulling it out at a known speed. The coating will stick to the surface of the article as it is drawn up and out of the solution. •S pray-coating — during spray coating, a driver and a nozzle is used to nebulise the coating solution applying it to the surface of the material as a mist. The coating will stick to the surface of the material and render the surface hydrophilic. This is typically a short-term option and is not as robust as plasma treatments or vacuum deposition process.
Figure 2a: Typical hydrophilic porous surface percentage of hydrophilic Vyon®.
HYDROPHILIC VYON POROUS PLASTIC Vyon is a porous plastic material manufactured using virgin-grade polyethylene, which is naturally hydrophobic. Vyon is converted from its naturally hydrophobic nature to hydrophilic using a plasma treatment process. The plasma treatment process works in two main steps: 1. C leaning — removal of contamination from the surface 2. Activation — rncreasing the surface energy by the attachment of oxygencontaining molecules HOW IS HYDROPHILICITY MEASURED? Typically, the level of hydrophilicity is quantified by introducing a drop of a liquid of known surface tension onto a solid treated surface and measuring the interfacial contact angle of the liquid to the treated surface. The contact angle, θ, is the angle formed by a liquid at the three phase boundary where the liquid, gas and solid intersect. A surface is considered hydrophilic if the static water contact angle θ on the surface forms a contact angle <90° as shown in Figure 1a. This measurement is done using a goniometer. When dealing with porous surfaces, a different approach is necessary as the interfacial interaction between the liquid and the surface is more complex due to its structure. When liquid is introduced onto a hydrophilic porous surface, the liquid will be pulled or absorbed almost instantly into the porous network of the structure before any contact angle measurements can be taken. An alternative method to quantify hydrophilicity is by assessing the number of hydrophilic pores (hydrophilic porous surface) within the porous structure of the material (Figure 1b). HYDROPHILIC VYON PERFORMANCE Figure 2a shows the typical hydrophilic porous surface of hydrophilic Vyon. The error bar is the standard deviation of 10 measured samples, with a tolerance of ±2%. The small variation observed with hydrophilic Vyon translates to highly reproducible fluid transfer behaviour; that is, the flow rate of liquid through the hydrophilic porous plastic is predictable and consistent.
24
W W W. M E D I C A L P L A S T I C S N E W S . C O M
MATERIALS — SPONSORED CONTENT
as an alternative to stainless steel bed supports as it demonstrates the stiffness and strength required to support the resin bed. In addition, porous plastics are much more costeffective yet deliver similar support and filtration characteristics. In pharmaceutical applications such as controlled drug delivery POC devices, the filtration media within the assembly of the device allows drug delivery, provides a breathable environment to stop the build-up of pressure after dispensing the contents of the POC device, and preserves the integrity of the drug by filtering/ keeping out any air contaminants or bacteria from entering and contaminating the drug in the device reservoir. The filtration media used must have a tightly controlled pore size rating and high hydrophilic porous surface to be able to absorb and deliver the drug immediately when required whist keeping the drug contaminant free. Figure 2b: Liquid (0.5g/l Ponceau.S in deionised water) absorption time of hydrophilic Vyon®.
Figure 2b shows the time it takes for the hydrophilic Vyon to be completely saturated by the liquid dye. The hydrophilic Vyon instantly wets out as soon as it gets in contact with the liquid dye, with a maximum time observed of 2 seconds. This is important because in high throughput applications requiring hydrophilic porous plastics such as process scale chromatography, a quick and consistent rate of liquid transfer through the porous bed support is necessary for immediate flow of the liquids during a preparation procedure while providing support to the powder bed above. If there are blind areas within the structure of the porous bed support due to poor hydrophilicity, the chromatography steps reliant on liquid flow will be affected negatively. APPLICATIONS OF HYDROPHILIC VYON Due to their ubiquitous use and value in modern society and importance in the medical, pharmaceutical and laboratory industries, there is much desire to render plastics hydrophilic for myriad applications. As demonstrated, porous plastics such as Vyon can be efficiently modified to exhibit hydrophilic properties required to increase the wettability of materials for pharmaceutical and healthcare applications. Process-scale chromatography required for drug development, rely strongly on precise flow of liquid through the capture media and bed support. Chromatography bed supports are typically designed from stainless steel (as it is typically hydrophilic) and provide stability and strength to support the resin bed. This stainless steel filter can be expensive and typically requires multiple cleaning cycles. Hydrophilic porous plastics can be used
... Vyon presents a viable solution to meet the technical and regulatory requirements of fast evolving markets
CONCLUSION The properties and characteristics of hydrophilic Vyon allows the rendered material to deliver on key product functionality in pharmaceutical and healthcare applications. Due to the advancements in manufacturing, processing, and functionalisation of porous plastic materials, it presents a viable solution to meet the technical and regulatory requirements of fast evolving markets. For example, with the adoption of automated production, porous filters are robust and rigid enough for fully automated insertion processes (such as, a manufacturing line requiring insertion of the porous plastic into a final component via an automated process), yet also have hydrophilic porous surface properties to fully absorb water and aqueous solutions. Understanding factors such pore size, volume and surface energy are not only important for the rendering process, but also the hydrophilic quality and performance (for example, shelf life and absorption time) achievable with the final product. The versatility of porous plastic materials and ability to tightly control its manufacturing process enables continuous innovation of pharmaceutical and healthcare solutions. Vyon is a registered product.
WWW.MEDICALPLASTICSNEWS.COM
25
MEP develops medical-grade polyacetal resin
1
Mitsubishi EngineeringPlastics (MEP) has developed a new polyacetal resin (POM), the Iupital MA Series.
2
It is available in a range of standard, highflow, high-rigidity, and low-friction grades, enabling flexibility.
3
MAL20 offers improved sliding performance with POM, achieving quiet, smooth movement.
4
The Iupital MA series meets the quality and regulatory requirements for medical materials.
06:2021 START-UP LAUNCHES A TRADING PLATFORM FOR RECYCLED PLASTICS
A
tomler, a Swedish start-up, has developed a platform that aims to streamline trade with post-industrial fractions of PP, PE and PET, thereby increasing the recycling rate of plastic raw materials in Europe. According to Sammy-Sebastian Tawakkoli, who co-founded the company together with Johannes
Schill, the platform makes trading simple, transparent and less risky. Companies can create, for free, their own store on the platform. “Using Atomler not only provides you with a new sales channel, it also helps to increase the recycling rate and thereby improve the reputation of the plastics industry,” said Tawakkoli.
KOKO AND EIRMED FORM MANUFACTURING PARTNERSHIP
K
oKo, a developer of respiratory information systems software and medical devices, and EirMed, a manufacturer of custom designed and engineered medical devices, have formed a manufacturing partnership to streamline the manufacturing of respiratory medical devices deployed globally by medical professionals.
The direct connection between KoKo’s respiratory medical devices and EirMed’s experience and knowledge enables the development and manufacturing of these devices and components. The partnership allows quick optimisation of engineering, manufacturing, assembly, and packaging through rapid cycles.
INDUSTRIAL AND SPECIALTY ADHESIVES MANUFACTURER EXPANDS HQ FACILITY
V
igorous growth has enabled Panacol-Elosol, a manufacturer of industrial and specialty adhesives, to build a larger facility for its corporate headquarters. The new site, which creates double the space for offices, laboratories and production is located in Steinbach near Frankfurt/Main Germany, within the new Steinbach
26
industrial estate “Im Gründchen”, close to the company’s former location.
technology that increases inhouse analytical and testing capabilities.
The new building offers Panacol employees a contemporary work environment with more than 6,000 m² space. The laboratories for R&D, application engineering and quality management are equipped with state-of-the-art
A larger production area with additional new equipment has increased manufacturing capacity and efficiency. The company says the new facilities can be expanded to support further growth in the coming years.
W W W. M E D I C A L P L A S T I C S N E W S . C O M
Unlock the true potential of your machine! Boost your performance with our applications team. You have in-depth knowledge of your product – our ENGEL application engineers know your injection moulding machine inside and out. Let’s team up to reach maximum effi ciency in your production with ENGEL’s new process optimisation service, performance.boost.