3D PRINTING
LUKAS PAWELCZYK, HEAD OF FREEFORMER SALES, ARBURG, EXPLAINS HOW THE APF PROCESS WITH THE FREEFORMER IS PARTICULARLY SUITABLE FOR AM IN MEDICAL TECHNOLOGY.
Making the impossible possible
T
he medical plastics market was one of the first to make extensive use of additive manufacturing (AM) technology, creating complex and often custom-designed components and devices in relatively small numbers. This trend has accelerated as the imagination of medical designers and new 3D printing equipment have made the previously impossible possible. Now, a different type of system is extending those boundaries even further by allowing the use of the same plastic granules used in injection moulding, including biocompatible, resorbable, sterilisable, and FDA-approved original materials. Developed and built by Arburg, the German manufacturer of precision injection-moulding machines, the freeformer machine, with its Arburg Plastics Freeforming (APF) process, facilitates sophisticated medical applications that cannot be achieved with any other process.
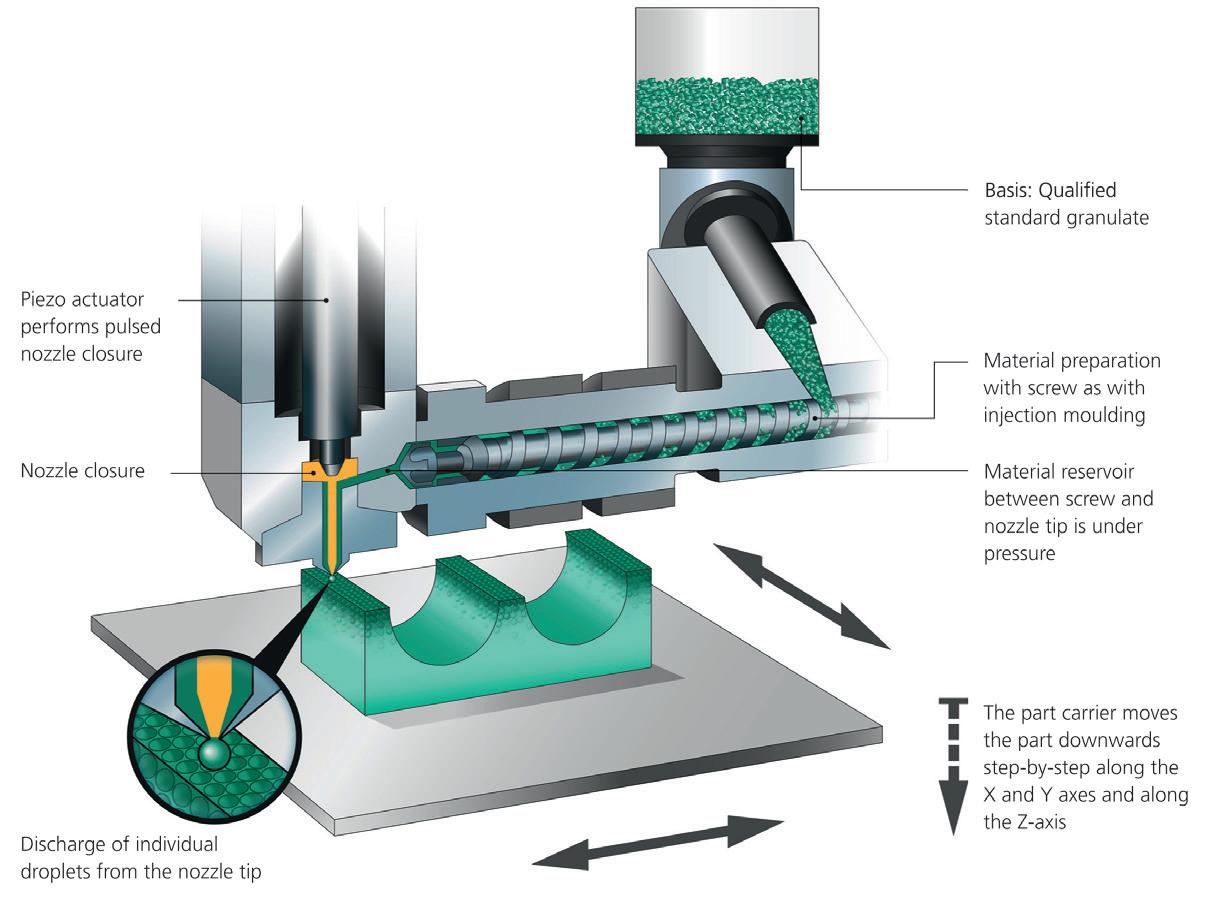
Figure 1: The APF process is based on plastic granules. The qualified original material is melted in an injection unit and discharged via a nozzle in the form of droplets.
‘OPEN SYSTEM’ IS THE KEY Similar to injection moulding, the freeformer operates by melting conventional plastic granules via a heated plasticising cylinder. A high-frequency pulsing rigid nozzle then discharges tiny droplets of the liquid plastic melt (Figure 1). The part carrier, which can be moved along three axes, enables each individual droplet to be set down precisely. The applied droplet bonds with the existing surrounding material so that, layer-bylayer, three-dimensional components with a high mechanical strength are produced. Part production starts from 3D CAD data in basic stereolithography STL format. Unlike conventional filamentfed AM systems, freeformers work using a wide variety of qualified standard plastics granulates. In addition, users can process their own custom-compounded materials with this ‘open system’ and optimise the droplet size and process themselves. Alternatively, they can access Arburg’s material database and select certified plastic granules, such as ABS (acrylonitrile butadiene styrene), amorphous PA (polyamide) and PC (polycarbonate), elastomeric TPU (thermoplastic polyurethane) and semi-crystalline PP (polypropylene), PLLA (poly-L-lactic acid) and other special and certified original materials including biocompatible, absorbable,
WWW.MEDICALPLASTICSNEWS.COM
23