

March 2024 Volume 51 | Number 3 www.pharmacos.co.za 50 years R69,95 Incl VAT

The biggest trend in perfumery
March 2024 Volume 51 | Number 3 www.pharmacos.co.za 50 years +
Global
Rotolabel pioneers eco-solutions in labeling How to solve complex API challenges Learn & explore at in-cosmetics
Unisex fragrances
R69,95 Incl VAT

Natural and Organic Ingredients

• Natural & Organic





n


24
8 News
IFF celebrates the art of perfumery
New leadership for Pharmaceutical Task Group
AnnonaSense CLR study results reveal latest data
10 Events
Learn and network at in-cosmetics Global
14 Contract Manufacturing
Unlock new opportunities for your brand with Hersol
The quintessential choice for your cosmetics
18 Perfumery
& Fragrance Ingredients
Well-being fragrance collection by Parfums Plus A revolution in natural ingredient production
IFF’s extraordinary women in perfumery
Fragrance Oils explores the power of fragrance


30

28 Sustainable Packaging
Rotolabel pioneers eco-solutions in labelling HP takes responsibility for sustainable change
Mondi expands EcoWicketBag production
34 Pharma Focus/ Excipients & APIs
Multichem Sourcing delves into the future of liposomes
Solubilising formulation technologies Your excipient choice can make all the difference
Customised excipients and pre-mixes from Nitika
WWW.PHARMACOS.CO.ZA // MARCH 2024 5 Contents March 2024
48 Student Focus
Coschem/P&C Review essay prize winner
51 Association News
Coschem AGM report
Plastics industry responds to SONA
42 images/

Volume 51 | Number 3 www.pharmacos.co.za
Shutterstock.com
How to overcome poor drug solubility with IMCD 50 years
Formulating body mists for the modern consumer

We all desire the fragrances we wear, whether it's the scent of our perfume, body lotion or conditioner, to reflect our personality. In today's world, many consumers seek recognition for their individual preferences and tastes rather than conforming to gender norms, especially when it comes to the fragrances they choose.
The gender-neutral fragrance movement, promoting inclusivity and breaking away from traditional stereotypes, is reshaping the fragrance industry. Perfumers are carefully crafting scents that transcend gender boundaries, allowing anyone to wear them comfortably. Alongside this transformative shift, gourmand fragrances are experiencing a resurgence, featuring edible vanilla and cherry notes, as well as sophisticated savoury options like rice and milk notes. For more insights into these trends, flip to page 16 in this edition of P&C Review
Our pharma focus delves into overcoming complex challenges in formulating with
active pharmaceutical ingredients (APIs). As APIs have become less soluble and more sensitive to temperature and physical stress over the years, the demand for tailored solutions has increased. This edition features various tailored solutions, from liposomes to multifunctional excipients. Explore more on page 34
We're also thrilled to feature Vishay Ramdhav’s essay in our student focus on page 46. Vishay is the deserving winner of this year’s Coschem Cosmetic Science Training/P&C Review Essay Prize. His essay on ‘the ongoing ethical dilemma surrounding animal testing in the cosmetics industry and the push for cruelty-free alternatives’ was selected from all Module 1 students’ essays for this prestigious prize sponsored by P&C Review
Enjoy the read!


The team
EDITORIAL
EDITOR: Abby Vorster
+27 (0)71 359 4519 abby.vorster@newmedia.co.za
LAYOUT & DESIGN: Andipha Nkoloti
COVER: images/ Shutterstock.com
SUB-EDITOR: Gill Abrahams
CONTRIBUTOR: Natalie Gerhardt, Haim Levit, Vishay Ramdhav, C.F. Thomas
ADVERTISING
KEY ACCOUNT MANAGER: Carla Melless
+27 (0)83 260 6060 carla.melless@newmedia.co.za
INTERNATIONAL SALES
Germany/Austria/Switzerland: Eisenacher Medien Erhardt Eisenacher +49 228 249 9860 info@eisenacher-medien.de
Italy: Ngcombroker Giacomo Rotunno +39 370 101 4694 g.rotunno@ngcombroker.com
Taiwan: Ringier Trade Media
Sydney Lai +886 4 2329 7318 sydneylai@ringier.com.hk
CIRCULATION
CIRCULATION MANAGER: Felicity Garbers felicity.garbers@newmedia.co.za
PUBLISHING TEAM
GENERAL MANAGER: Dev Naidoo
GROUP ACCOUNT DIRECTOR B2B: Johann Gerber
PRODUCTION CONTROLLER: Mandy Ackerman
ART DIRECTOR: David Kyslinger
JOHANNESBURG OFFICE
New Media Publishing, Ground floor
272 Pretoria Avenue, Randburg
Tel: +27 (0)11 713 9000
POSTAL ADDRESS
PO Box 784698, Sandton, Johannesburg, 2146
Published by New Media, a division of Media24 (PTY) Ltd
MANAGEMENT TEAM
CEO: Aileen Lamb
COMMERCIAL DIRECTOR: Maria Tiganis
STRATEGY DIRECTOR: Andrew Nunneley
CHIEF FINANCIAL OFFICER: Venette Malone
CEO MEDIA24: Ishmet Davidson
HEAD OFFICE
New Media, a division of Media24 (Pty)
EDITORIAL ADVISORY BOARD







Pharmaceutical & Cosmetic Review is published by New Media 11 times a year and circulates to manufacturers, packers and distributors of pharmaceuticals, health products, cosmetics, detergents, soaps, toiletries and allied products. The journal is an up-to-date source of reference for company directors, factory and production managers, marketing executives, engineers, import agents, buyers and research personnel. While precautions have been taken to ensure the accuracy of its contents and information given to readers, neither the editor, publisher, or its agents can accept responsibility for damages or injury which may arise therefrom. All rights reserved. © Pharmaceutical & Cosmetic Review No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, photocopying, electronic, mechanical or otherwise without the prior written permission of the copyright owners. Pharmaceutical & Cosmetic Review is printed and bound by CTP Printers - Cape Town Copyright: all rights reserved. ISSN 0257-8719 CEO of the Generic and Biosimilar Medicines of Southern Africa
Frittelli
Society of Cosmetic Chemists SA
Vivian
Past-President,
Dr
Consultant, Cosmetic Solutions
P&C Review is affiliated with: CTFA - The Cosmetic, Toiletry & Fragrance Association of South Africa GBM - Generic and Biosimilar Medicines of Southern Africa COSCHEM - The Society of Cosmetic Chemists of South Africa HPA - The Health Products Association of Southern Africa AMA - The Aerosol Manufacturers’ Association of South Africa
Prof
Aubrey Parsons
John Knowlton
Professor Emeritus, Faculty of Health Sciences, Nelson Mandela University Prof N T (Raj) Naidoo
Ltd 8th floor, Media24 Centre, 40 Heerengracht Cape Town, 8001 Tel: +27 21 406 2002 Email: newmedia@newmedia.co.za PO Box 440, Green Point, Cape Town 8051 FROM THE EDITOR Scent without
boundaries
Carla Melless Sales executive +27 (0)83 260 6060 To advertise in www.pharmacos.co.za contact image/ Shutterstock.com 6 MARCH 2024 // WWW.PHARMACOS.CO.ZA






































@Pharmaceutical & Cosmetic Review Sign up to our newsletter Find everything you need for new product development in Formulation Design. BROUGHT TO YOUBY ISSUE 3 IS NOW AVAILABLE ONLINE @Pharmaceutical & Cosmetic Review

IFF celebrates the art of perfumery in new ad campaign
IFF has launched an advertising campaign featuring staple tools of the art of perfumery. The campaign is appearing today in select publications and online.
“IFF perfumers are artists of the invisible, who craft olfactive works of art, connecting with consumers around the world through the scents they create for niche to global brands,” said Sabrya Meflah, IFF president of fine fragrance. “This campaign celebrates their art, which carries a unique emotional power.”
The advertising campaign was shot by renowned luxury, perfume and haute-jewellery photographer, Helmut Stelzenberger, who has worked on ad campaigns for Chanel, Van Cleef, Cartier and others.
“IFF perfumers are artists of the invisible, who craft olfactive works of art ”
The campaign visuals feature the iconic blue-glass bottle – a staple of the IFF brand – held within hands that represent the craftsmanship of perfumery. Meanwhile, the perfumer’s tools – blotters, beakers, pipettes and vials – stand on the scale perfumers use to compound their fragrance formulas. The scale’s digital display shows a date of significance in IFF’s long history: 1889, the year predecessor company Polak & Schwarz was founded, a testimony to the company’s longstanding legacy.
Paboco moves into full-scale production



With the theme ‘Let’s paper up business’, Paboco, the paper bottle company, proudly launches the next gen paper bottle by initiating fullscale production, marking a milestone towards a more sustainable future.
“This is a fantastic opportunity for conscious brands and consumers looking to make a real difference,” says CEO, Tim Silbermann.
The next gen paper bottle signifies Paboco’s dedication to pursuing its vision of fully biobased bottle packaging. With a new state-of-the-art manufacturing site in Slangerup, Denmark, Paboco is gearing up for fullscale production. The aim? To deliver over 20 million paper bottles by the end of 2025.
Built on the idea of less fossil, more natural materials, the next gen paper bottle offers a minimal barrier solution while staying fully recyclable as paper packaging. Paboco empowers brands with design flexibility, allowing them to choose between standard paper bottles or bespoke designs. This versatility allows them to express their identity and connect with consumers on a deeper level.
Initially targeting brands in the beauty and homecare sectors, the next gen paper bottle shows promise across various industries, including premium spirits, food and beverage, vitamins and pills, pet care and more, indicating significant advancements in these areas. This scope offers consumers the opportunity to be part of driving change by choosing FSC-certified fibre-based packaging with less climate and environmental impact.
With ALPLA as the new majority shareholder and a long-trusted partner, Paboco is primed for accelerated growth, positioning itself as a leader in sustainable packaging solutions.
Since 2021, Paboco has been testing its ground-breaking products in partnership with Pioneer Community members: The Absolut Group, Carlsberg Group, The Coca-Cola Company, L'Oréal, and Procter & Gamble.
“After years of innovation and successful collaborations, we are now prepared to launch the Next Gen Paper Bottle on a larger scale,” Silbermann continues. “I welcome more conscious brands that want to join us in changing this industry for good.”
NEWS
8 MARCH 2024 // WWW.PHARMACOS.CO.ZA
The scope of the next gen paper bottle offers consumers an opportunity to take part in driving change by choosing FSC-certified fibre-based packaging with less climate and environmental impact
Pharmaceutical Task Group appoints new leadership
Zwelethu Bashman has been appointed chairman of the Pharmaceutical Task Group (PTG) with Dr Stavros Nicolaou appointed deputy chairman. Bashman is also president of the Innovative Pharmaceutical Association South Africa (IPASA), and managing director of MSD South Africa and sub-Saharan Africa.

“Our goal is to contribute towards an environment that promotes growth and investment in the South African pharmaceutical industry while aspiring to broaden access to medicines for all people living in South Africa,” said Bashman. Stavros Nicolaou has assumed the role of deputy chairman after serving as chairman of the PTG for several years.

PTG was established in 2004 to respond to the Transparent Pricing Regulations. It is an umbrella body that represents four pharmaceutical associations on issues of common interest. Members of these associations are involved in the manufacturing, sale, and distribution of medicines in South Africa and supply more than 80% of market needs.
Four pharmaceutical associations, representing more than 80% of the industry, comprise the membership of the PTG.
“Our goal is to contribute towards an environment that promotes growth and investment in the South African pharmaceutical industry ”

AnnonaSense CLR proven to influence the skin-mind connection

CLR Berlin has revealed new test results for AnnonaSense CLR™ (INI: Annona Cherimola Fruit Extract), showing that it is the first cosmetic active ingredient with real clinical proof for positively influencing the entire skin-brain feedback loop.
The need for stress relief and relaxation is high for consumers. Skin and mind are closely connected. If our skin is in balance, we feel good in our skin in the truest sense of the word.
The way we feel, emotionally, has great impact on virtually all biological processes in the body. When we are stressed, the stress hormone cortisol is produced by the body’s adrenal glands. Cortisol has a highly negative impact on skin health. Unhealthy skin is more sensitive and has a less attractive appearance. When skin is sensitive and unattractive, it affects one’s confidence. This creates a vicious cycle which affects many consumers.
Is it possible for an active ingredient break this vicious cycle and make a real difference – for skin and mind?
To assess whether AnnonaSense CLR can really make a difference for the consumer, a new study was conducted by CLR on a total of 117 volunteers with sensitive skin and/or a stressful life (e.g. healthcare workers).
The volunteers were split into three groups. One group applied a cream containing AnnonaSense CLR; the second group applied the same formulation without AnnonaSense CLR (placebo); and the third group applied the placebo and combined this with a facial massage which is expected to have a large impact on skin and wellbeing.
The study clearly showed that AnnonaSense CLR™ has a strong influence on the entire skin-brain feedback loop. It perceivably improves the look and comfort of skin and it reduces psychological stress and improves quality of sleep. Importantly, AnnonaSense CLR™ reduces the production of cortisol within the body. Lastly, completing the loop, it acts against the negative effects of systemic cortisol of skin by improving skin health.
Available in South Africa from IMCD, AnnonaSense CLR™ is a natural and upcycled ingredient. It is obtained from Annona cherimola, common name Cherimoya, an edible fruit originating from South America.
NEWS
WWW.PHARMACOS.CO.ZA // MARCH 2024 9
A person’s emotional wellbeing can have a significant impact on biological processes in their body (Image: CLR Berlin)
Dr Stavros Nicolaou
Zweli Bashman
image/ Shutterstock.com
Knowledge sharing at the forefront of in-cosmetics Global
As the leading global exhibition dedicated to personal care ingredients, in-cosmetics Global has strengthened its educational programmes for this year’s event taking place in Paris, France from 16 to 18 April.

Various opportunities to explore cosmetic science, innovation and market intelligence in the personal care industry are set to be presented this year. With the highly popular Marketing Trends presentations and sessions, the newly expanded Sustainability Zone, must-attend Technical Seminars, and the interactive Formulation Lab®, the 2024 edition will provide invaluable information to those looking to uncover the latest industry trends and opportunities in product development.
MARKETING TRENDS THEATRE
Opening day one of the Marketing Trends programme, Povilas Sugintas, senior consultant – beauty and fashion at Euromonitor International, will lead a session on European Beauty and Personal Care market trends 2024+. This talk will delve into evidence-based insights into the psyche of beauty consumers, exploring consumer trend analysis across the EU, and emphasising critical market areas experiencing growth and presenting opportunities, as well as bottlenecks.
As the focus on natural and organic ingredients continues to grow, Nikola Matic, vice president at chemicals, market research at Kline & Co. will highlight some of the defining aspects driving demand for personal care ingredients in the presentation 2024 and beyond: Where is the personal care ingredients market at, and where is it headed?
Day two of the Marketing Trends Theatre will host a highly interactive and thought-
provoking panel discussion on Sciencebacked beauty vs misinformation . Barbara Green, senior director, Kenvue; Seongmin (Mike) Sohn, CEO & representative consultant, REACH24H Consulting Group, Korea; Michele Superchi, vice president of BEAUTYSTREAMS; Nikola Matic, vice president of chemicals, market research, Kline & Co.; Povilas Sugintas, senior consultant – beauty and fashion of Euromonitor International; Mojgan Moddaresi, managing director (Dr M)™ PhD, PharmD, FRSB, CBiol, MRSB of Personal Care Regulatory; and Belinda Carli, B Nat Therapies; Dip Cos Sci, director at Institute of Personal Care Science, will discuss the challenges of debunking these claims, bridging the gap between R&D and marketing, and leveraging technology to further drive evidence-based skincare to consumers.
For attendees looking to learn about the latest regulatory changes in the Chinese beauty market, April Guo, personal and home care division, general manager at CIRS-REACH Group will lead a session on Chinese regulatory updates , while Amarjit Sahota, founder of Ecovia Intelligence, will guide visitors on Sustainability Schemes & Ethical Labels: Trends and Outlook in the cosmetics industry.
SUSTAINABILITY ZONE AND FORUM
In collaboration with The Green Chemist Consultancy and Ecovia Intelligence, the expanded Sustainability Zone is devoted to inspiring, providing information and
identifying opportunities for brands to minimise their impact on the environment. The Zone encompasses several features, including the Sustainability Display, and Theatre – both sponsored by AAK – the Sustainability Pavilion, powered by Farmforce, and the brand-new Sustainability Zone Forum.
“It is incredibly exciting to see this trade show deliver such a generous range of insightful presentations and hands-on lab sessions led by industry experts”
The introduction of the Sustainability Zone Forum, a one-day programme hosted in the Pegase Theatre, will explore the eco-evolution of the cosmetics industry. Presentations will take place from spokespeople at Syensqo, Expressions Parfumées, OLVEA, RSPO, and more. Here, speakers will look at the industry’s efforts towards more sustainable business structures and offers advice for business owners looking to implement greener practices.
Notably, Emilija Balsyte, fashion and beauty analyst, Euromonitor International, will deliver the highly anticipated results of the research company’s recent report during Euromonitor's sustainability report ; Paul Jenkins, founder and managing director at
10 MARCH 2024 // WWW.PHARMACOS.CO.ZA
EVENTS/IN-COSMETICS GLOBAL image/ Shutterstock.com
ThePackHub, will navigate the cosmetics packaging landscape in Embarking on a journey of innovation: Navigating the future of sustainable cosmetics packaging ; and Jolene Maloney, marketing manager - sustainability for consumer care at Croda will speak on Reducing the carbon footprint of beauty
THE FORMULATION LAB
Poised as a vanguard of innovation and expertise, the much-anticipated Formulation Lab® is proudly sponsored by Brenntag and partnered with Personal Care Magazine. The two purpose-built fully equipped laboratories located on the show floor will offer up to 27 interactive, hands-on experiences in formulation techniques and practices, managed and presented by the event’s technical advisor, Lorna Radford, who is an award-winning cosmetic scientist, founder of Enkos Developments, and Member of the Society of Cosmetics Scientists (SCS) education committee.
Leading a masterclass on Dive into a new skin cleansing era with the brand-new sustainable rheology modifier from LLS beauty will be Sophie Crabeil, EMEA technical service at Lubrizol Life Science. Visitors will discover how to formulate a magic gold sparkling body wash with a honey-like flow, remarkable clarity, excellent suspension, and ideal viscosity at low pH.
With over 30 years of experience working for Clariant in consumer care, Ute Back, technical application manager, personal & home care at Clariant, will introduce attendees to how naturally impactful
TECHNICAL SEMINARS
Visitors to in-cosmetics Global will be able to explore cuttingedge personal care technologies in more than 90 Technical Seminar’s across the three-day event, presented in collaboration with Cosmetics Business.
Must-attend sessions include:
• Anti-ageing: Top to bottom by Joonseok Cha, research director at The Garden of Naturalsolution
• Multifunctionals for all shades of beauty by Sabrina Mizael, senior global product manager at Symrise.


ingredients can be applied to industry trends like skin cycling with their desirable efficacy and sensory elements, during the session Formulate to recalibrate: Unveil your post-show glow
On the final day of the show, Morgane Le Meur, associate technical service & development specialist at Dow, will guide formulators towards creating a gentle and moisturising night cream tailored for sensitive, dry-prone skin during the session Unlocking the secrets of sensitive skincare: Rediscovering silicone ingredients
NETWORK AND LEARN AS ONE
Speaking on the comprehensive schedule of events, Roziani Zulkifli, event director of in-cosmetics Global, said: “It is incredibly exciting to see this trade show deliver such a generous range of insightful presentations and hands-on lab sessions led by industry



experts. Seeing our visitors delve into the latest topics impacting the industry and formulating their own products is a huge part of the show and what distinguishes in-cosmetics Global as the leading industry event.
“The event provides the perfect opportunity for interaction, and we warmly welcome collaboration for the industry to discover, experience, learn and celebrate as one. We’re looking forward to seeing the Marketing Trends, Technical Seminars, Sustainability Zone and Formulation Lab and more come together this April to give our attendees the best experience possible.” in-cosmetics Global, presented by headline sponsor KSM, returns to Porte de Versailles, Paris, France from 16 to 18 April. For more information and to register to attend, visit www.in-cosmetics.com/global . •
WWW.PHARMACOS.CO.ZA // MARCH 2024 11 EVENTS/IN-COSMETICS GLOBAL
What’s on in 2024


2024 SUBSCRIPTION FORM
Please complete in block letters, select your subscription option, and returnthisform,alongwithyourpaymentto:
NEW MEDIA, a division of Media24 (Pty) Ltd, PO Box 440, Green Point, Cape Town 8051 Email: felicity.garbers@newmedia.co.za
SUBSCRIPTION OPTIONS (please tick)1 YEAR2 YEAR
Food Review R588 R905
Pharmaceutical
Food Review +
Full Name: ...........................................................................................
Designation: ........................................................................................
Company: ...........................................................................................
Postal Address:...................................................................................
.................................................................Code: .................................
Country: ..............................................................................................
Tel: ( ).......................................Fax: ( ) ..................................
E-mail: .................................................................................................
Main activity of company:
Approximate number of employees: ..................................................
VAT No: ...............................................................................................
❑ I would like to receive the newsletters and feature announcements via email and may be added to the mailing lists.
Signature:............................................................................................
Please select your preferred method of payment:
❑ Direct Deposit (Complete and email this form to: felicity.garbers@newmedia.co.za
Payee:
Bank:
Acc No:

New Media, a division of Media24 (Pty) Ltd Nedbank Seapoint 1069321540
Branch Code: 10-69-09-00
❑ Credit Card (Mastercard & Visa only)
Name of card: .............................................................................
Expiry Date: ................................................................................
Card Number: .............................................................................
CVC Number...............................................................................
Date: ...........................................................................................
Signature:....................................................................................
❑ TAX INVOICE REQUIRED
Note: The above prices are applicable to South Africa only. International rates available on request.
April
Natural & Organic Products Europe



14 to 15 April
London, UK
www.naturalproducts.co.uk
in-cosmetics Global
16 to 18 April
Barcelona, Spain
www.in-cosmetics.com/global
CPHI Japan
17 to 19 April
Tokyo, Japan
www.cphi.com/japan
Pharma West Africa
8 to 10 May
Accra, Ghana
www.pharma-westafrica.com
Vitafoods Europe
14 to 16 May
Geneva, Switzerland
www.vitafoods.eu.com
Coschem JHB Supplier Day 15 May
Joburg, South Africa
www.coschem.co.za
May June
Coschem KZN Supplier Day 12 June
KZN, South Africa
www.coschem.co.za
Chemspec Europe
19 to 24 June
Düsseldorf, Germany
www.chemspeceurope.com
Cosmetic Review
Pharmaceutical Cosmetic Review R588 R1066 R1620 R905
DIARY
12 MARCH 2024 // WWW.PHARMACOS.CO.ZA

printers and converters of shrink sleeves & flexible packaging


Through an innovative and out of the box approach to our clients’ needs, we are not only willing, but also able to provide practical and efficient cost-saving solutions to most printing and packaging challenges. A pioneering narrow web flexographic printer, ISW specializes in, while not confined to, the manufacturing of highly decorative and complex shrink sleeves, self-adhesive and wraparound labels, and various forms of flexible packaging. By working closely with local and international suppliers, and utilising specially developed in-house processes a multitude of innovative finishes including the likes of thermochromic, glow in the dark, domed, holographic and high lustre metallics along with specialised multi-perforation solutions for promotional items are achieved.




www.iswshrink.co.za
16 Edendale Rd West
Eastleigh, Edenvale.
Tel: (011) 609 1488
Fax: 086 515 7409
Email: sales@iswshrink.co.za




With the manufacturing plant consisting of 10-colour and eight-colour MPS EPW560’s and a full complement of the latest offerings in finishing equipment for the conversion and inspection of our products, ISW maintains the highest level of quality and is one of only a handful of narrow web printers capable of producing wide lay flat shrink sleeves, achieving a L/F of 276mm from a printed web width of 575mm. By offering assistance and advice with every step from concept to design, production and application; we are there to ensure our clients achieve their desired vision efficiently, affordably and consistently.


































































Reg 4870259860
2019/279539/07 VAT
Unlock new opportunities for your brand with Hersol
In the evolving landscape of complementary medicines, finding a contract manufacturer that aligns with your brand’s vision, values and quality standards is paramount. Amidst the plethora of options, Hersol brings a wealth of knowledge and unrivalled expertise to stand out as the ultimate partner in nutraceutical manufacturing.

Consisting of a team of seasoned professionals who are well-versed in the latest trends, regulations and advancements in the field, Hersol Manufacturing Laboratories (Hersol) understands that one size does not fit all.
That is why the business offers customisable solutions tailored to meet your unique specifications and market demands. Whether you are looking to develop a new product from scratch, optimise an existing formulation for better efficacy and consumer appeal or scaleup production, the contract manufacturer’s dedicated departments in research and development, regulatory affairs and quality assurance work closely with you to turn your vision into a reality.
Hersol views its clients as partners and prioritises collaboration in every step of the process to understand their goals, address any concerns and exceed their expectations.
“With responsive communication and a customer-centric approach, we ensure a seamless and hassle-free experience from start to finish. Transparency and integrity are the cornerstones of our business philosophy. We believe in fostering open and honest communication with our clients through every step of the process, from pricing and timelines to raw material sourcing and regulatory compliance,” says Aadila Razak, regulatory affairs manager and NPD pharmacist at Hersol.


SUPREME QUALITY AND EFFICIENCY
Equipped with state-of-the-art facilities, Hersol uses the latest technologies and manufacturing processes to ensure unparalleled quality and efficiency. From raw material sourcing to formulation development and packaging, every step of the manufacturing process is meticulously executed without compromising on quality.
“Licensed by the South African Health Products Regulatory Authority, we adhere to stringent quality control measures throughout our sourcing and manufacturing processes to uphold high standards of authenticity, quality and compliance. This occurs before any raw materials enter our manufacturing facility and any finished product leaves the facility,” she adds.
Hersol’s pilot scale production facilities are equipped with the same state-of-the-art equipment as its full-scale operations, ensuring consistency and accuracy throughout the development process.
Razak comments: “By offering our clients the option of trial batches, we can identify potential issues or optimisations early on, saving time and resources in the long run.”
AN ASSORTMENT OF DOSAGE FORMS
At Hersol Manufacturing Laboratories, versatility is key.
“We understand that different nutraceutical products require different dosage forms to cater to diverse consumer preferences and needs,” Razak explains, adding that this is why the
business specialises in manufacturing a wide range of dosage forms. Whether it’s traditional gelatine capsules or vegetarian capsules, Hersol has the expertise to produce capsules in various sizes, colours and formulations. From standard compressed tablets to chewable and dual layer tablets, the company can manufacture tablets in different sizes, shapes and colours to suit your requirements.
With its state-of-the-art blending and powder filling capabilities, the manufacturer can produce custom powder blends and singleserve sachets for convenient consumption and accurate dosing.
“From tinctures and syrups to emulsions and suspensions, we have the equipment and expertise to manufacture liquid formulations with accurate dosing and consistent quality. In additional to oral dosage forms, we also produce topical products such as creams, lotions and gels,” she adds.
No matter the dosage form, Hersol ensures strict adherence to quality standards and regulatory requirements to deliver safe, effective and market-ready complementary health products. With its diverse manufacturing capabilities, clients are empowered to explore new product formats and expand their product offerings to meet evolving market needs.
EXPERIENCE THE DIFFERENCE
With Hersol Manufacturing Laboratories as your contract manufacturer, you can rest assured that every aspect of your product’s development and production is in capable hands.
“Our unmatched expertise, cutting-edge facilities, unwavering commitment to quality and dedication to customer satisfaction are among the reasons you should choose us as your contract manufacturer,” Razak says. “With us you will experience the difference first-hand while you unlock endless possibilities for innovation and growth in complementary medicines.” •
CONTRACT MANUFACTURING
Hersol Manufacturing Labs –hersol.co.za
14 MARCH 2024 // WWW.PHARMACOS.CO.ZA
Aadila Razak, regulatory affairs manager and NPD pharmacist, and Kevin Duraan, factory co-ordinator
Tumelo Langa, production manager, and Theresa Quinlan, quality assurance manager at Hersol
image/ Shutterstock.com

Discover the power of partnership with Hersol Manufacturing Labs!
At Hersol, we put people first. Our commitment to our clients goes beyond mere production – we’re dedicated to transforming your vision into reality. With our unparalleled expertise in formulation development, meticulous raw material sourcing, and cutting-edge pilot scale production capabilities, Hersol empowers you to bring your innovative concepts to life.
Why choose Hersol as your contract manufacturer?
Because we understand the importance of trust and reliability in every step of the journey. From concept to creation, we ensure that every aspect of product development and production is handled with precision and care.

44 Years of excellence
With our unmatched expertise, cutting-edge facilities, unwavering commitment to quality and dedication to customer satisfaction, choose us as your contract manufacturer, unlock endless possibilities for innovation and growth in the complementary medicines industry and experience the diff erence first-hand.
ACCREDITED BODIES:
Hersol Manufacturing Laboratories is registered with reputable organizations:
• Sahpra Manufacturing licence
• South African Health Products Regulatory Authority
• South African Department of Health
• South African Pharmacy Council
• Health Product Association (HPA)
• Self-Care Association of South Africa

Laurence Solomons CEO
Laurence@Hersol.co.za

• FDA Food Facility
• S.A.N.H.A (South African National Halaal Authority), NIHT (National Independent Halaal Trust) to offer Halaal certified products.
• Montreal Kosher Authorities (Global Kosher Certification) to offer Kosher certified products.

Barry Solomons Marketing Director Barry@Hersol.co.za

Kevin Van Wyngaardt Managing Director kevin@hersol.co.za

Riaan Herselman Operations Director Riaan@Hersol.co.za

Tumelo Langa Production Manager Prodman@hersol.co.za
Hersol Manufacturing Laboratories (Pty) Ltd
+27 11 614 6631/2 | email: enquiries@hersol.co.za | web: www.hersol.co.za




tel:
The quintessential choice for your brand
In the vast realm of cosmetic care, finding the perfect contract manufacturer is a task of utmost importance for brand owners. Quintessence Collections, an ISO 22716:2007 certified cosmetic manufacturer, is renowned for its expertise in developing, manufacturing and stability testing products across various categories.

As a second-generation owner-managed business, Quintessence embodies a legacy of excellence, blending traditional values with cutting-edge innovation and standing as a beacon of quality in the industry. With a commitment to customer satisfaction, Quintessence offers a range of services tailored to meet the diverse needs of its customers.
Flexibility is key at this contract manufacturer. The facility specialises in small-batch manufacturing, with minimum batch sizes starting from 5kg. It also has the capabilities to manufacture up to 2 000kg of product per day, ensuring seamless scalability to meet varying demands.
The company’s dedication to quality assurance is evident in its approach to stability testing, which is conducted on manufacturing-sized batches to provide accurate insights into future shelf-life estimations and product durability.
A PASSIONATE BUSINESS
With over 30 years’ industry expertise under its belt, Quintessence nurtures dedicated teams in research and development, manufacturing, and quality control. This wealth of experience enables the company to develop and manufacture products in various formats with
unmatched proficiency, delivering solutions that exceed customer expectations.
Quintessence’s commitment to innovation, excellence and experience translates into tangible value for its customer’s brands, ensuring products that resonate with consumers and stand out in the market.
"Quintessence epitomises the essence of beauty and innovation in contract manufacturing"
The heart of Quintessence lies in its product development. Fuelled by passion and expertise, the company’s R&D team crafts bespoke formulations tailored to meet the unique needs of customers, ensuring products that are not only efficacious but also socially and environmentally responsible. The contract manufacturer meticulously sources raw materials from approved suppliers, both locally and internationally, guaranteeing the highest quality ingredients and uninterrupted supply chains.
AN ECO-FOCUSED PARTNER
With a focus on sustainability, Quintessence upholds stringent quality standards throughout its operations.
From pilot-scale production to filling and packaging, every aspect of the production process is integrated into the company’s quality control system, ensuring precision, consistency and safety in the final product delivered to customers. By prioritising sustainable packaging materials and eco-friendly practices, Quintessence sets the benchmark for sustainability compliance in the industry.
Quintessence epitomises the essence of beauty and innovation in contract manufacturing. With a steadfast dedication to quality, innovation and sustainability, the business emerges as an ideal partner for brands seeking excellence in the beauty and personal care industry.
For inquiries and partnerships, please send an email to info@quint.co.za . •
Quintessence Collections –quintessence.co.za
images/ Shutterstock.com

CONTRACT MANUFACTURING
16 MARCH 2024 // WWW.PHARMACOS.CO.ZA







426 Skilder street, Silvertondale, Pretoria I 0861 LOTION I +27 12 804 6443 I info@quint.co.za www.quintessence.co.za Quintessence Collections, your ideal partner for excellence in contract manufacturing. Creating beauty through innovation, excellence and experience. Contract Manufacturing Product Development Retail Markets

• Thrilling Vibes is vibrant and sparkling. The shimmering pleasure of Sicilian lemons highlights the rosy freshness of geranium, which itself reveals the delicate juicy notes of litchi. This timeless elegant fragrance –designed for both men and women – evolves to a woody and delicately musky trail. Applications include body splash, body wash and hand soap.
• Stylish Vibes is smart and elegant with an enigmatic oriental woody accord made of patchouli and cedarwood built around beautiful contemporary iris notes. This fragrance is ideal for male grooming applications such as shower gel, deodorant and shaving products.
The essence of well-being
Inspired by the flow of positive vibes in fine perfumery, Parfums Plus presents a new fragrance collection designed specifically for cosmetics and toiletries that address ‘well-being’.
• Tropical Vibes is mischievous and cheerful. The playful floral fruity fragrance is built around luminous solar blossoms enhanced with a happy spicy touch of ginger. It evolves to a warm and sensual background composed of patchouli and vanilla, making the fragrance perfect for shower cream, body lotion and body splash applications.
• Sexy Vibes is a stunning oriental accord blended with sparkling tangy notes of red berries and pink grapefruit in top notes. Iconic and daring, this fragrance works well in shower foam, body cream and body splash applications for women.
• Delicious Vibes is a generous floral bouquet of tuberose and orange blossom combined with a luxurious gourmand
accord of vanilla and caramelised popcorn. Suitable applications include body butters and hair mists.
• Addictive Vibes is an enchanting fragrance made of charismatic jasmine and delicious fig milk, blended with crunchy notes of speculoos, all wrapped in a suave and creamy sandalwood background. This unisex fragrance works well in body wash for men or shower cream for women. Fragrances by Parfums Plus are available in South Africa from Cosmetic Technologies. Email costech@global.co.za for more information. •
Parfums Plus – parfumsplus.com

image/
Shutterstock.com
18 MARCH 2024 // WWW.PHARMACOS.CO.ZA
PERFUMERY & FRAGRANCE INGREDIENTS

Wayne Van Wyk www.parfumsplus.com 011 314 0912 costech@global.co.za When creation meets innovation Parfums plus
A revolution in natural ingredient production
Robertet is pursuing its strategy of innovation in natural ingredients with the acquisition of a BioPod. The initiative aims to advance agronomic research in the areas of fragrance, flavours and well-being.
As a world leader in natural raw materials, Robertet is the first industrial player in fragrances and flavours to acquire a BioPod. This is an AI-controlled biofarm developed by Interstellar Lab, which accelerates plant growth and optimises plant molecular composition, while minimising the environmental impact of soil-less cultivation.
Interstellar Lab is a visionary player in biofarming. Its innovation will enable Robertet to accelerate its knowledge of the living, while reducing its environmental footprint for precision agriculture.
The BioPod is a sustainable cultivation tool, which anticipates the challenges of sourcing and the environmental impact of producing natural ingredients. This 11m long, 5m wide and 6m high deployable greenhouse can be installed without a foundation and offers up to 100m2 of highly controlled growing space. The autonomous cocoon is a terrestrial adaptation of a system originally developed for NASA, and operates in a semi-closed circuit, optimising the water cycle and capturing ambient CO 2 . Its transparent membrane captures sunlight and reduces the energy consumption of artificial light.
Equipped with sensors and its own artificial intelligence, BioPod recreates climates autonomously, enabling the production of high-value-added plants and natural ingredients in a sustainable and replicable way. Thanks to cutting-edge technologies and automated control using an algorithmic approach, BioPod maintains ideal conditions for plant growth and molecular composition, while significantly reducing cultivation surface, water and energy consumption.
COMMITTED TO INDUSTRY TRANSFORMATION
This investment comes in addition to the 14 research and creation centres dedicated to exploring the world of natural ingredients to

offer products and solutions to Robertet Group customers, including Claman’s customers in South Africa. Claman is the local agent for Robertet.
Jerome Bruhat, CEO of Robertet, explains: “The arrival of this BioPod in Grasse illustrates our innovation strategy to use cutting-edge technologies to offer our customers the best natural products for a more sustainable industry. We are doing this with a recognised pioneer in bio-farming.”
“BioPod is a sustainable cultivation tool, which anticipates the challenges of sourcing and the environmental impact of producing natural ingredients”
Julien Maubert, director of the raw materials division and the person spearheading the Interstellar project, adds: “We are looking forward to kicking-off our collaboration with Interstellar Lab. We have already engaged in several research programmes to prepare for the future of aromatic plants agronomy, and we are excited to accelerate this research thanks to the remarkable performance of the BioPod. We are determined to continue transforming our industry.”
Barbara Belvisi, founder and CEO of Interstellar Lab, comments: “We are delighted to be bringing BioPods to market with Robertet as our first partner. The leading French, natural ingredients group has unrivalled experience and expertise in cultivating the Earth’s natural resources. We share many common values, such as the preservation of biodiversity. With the BioPod, we are offering a solution to the industry’s ecological transition. We harness technology to benefit the living.” •




20 MARCH 2024 // WWW.PHARMACOS.CO.ZA Claman – www.claman.co.za Robertet Group – www.robertet.com PERFUMERY & FRAGRANCE INGREDIENTS
By investing in a BioPod, Robertet Group is committed to transforming natural ingredient production in a sustainable and ecological way (Images: Interstellar Lab) image/ Shutterstock.com

Extraordinary women in perfumery
IFF recently announced the promotion of three perfumers to vice president perfumers. Patricia Hidalgo, Joa Kim and Birgit Sijbrands are the three women who have attained the vice-president perfumer rank.
Attaining the vice-president perfumer rank at IFF is a feat reserved for those with a unique blend of talent, skill and knowledge, ranging from setting industry milestones to advancing IFF’s technological frontiers, mentoring the next generation of talent and aiding its research and development team in developing patented ingredients.
Valery Claude, vice president, innovation and creation, scent at IFF, comments: “The elevation of these three distinguished women perfumers, who have devoted their lives to the artistry of fragrance, celebrates their extraordinary commitment to perfumery and their role in shaping the future of our craft.”
A RADIANT SPIRIT
Patricia ‘Patty’ Hidalgo – a beacon of creativity and innovation in the fragrance industry with a celebrated career spanning three decades – began her journey at IFF as part of the Fragrance Resources acquisition in 2017. A scholar in biology and chemistry, her technical skills propelled her from a laboratory technician to a towering strength in perfumery creation, where she has since been crafting authentic scents inspired by nature, cultures and wanderlust. Patty’s creations have not only captivated the speciality and prestige markets but have also set new benchmarks for excellence in fragrance creation.
Sabrya Meflah, president of IFF

Fine Fragrance, comments: “Patty is the embodiment of relentless energy, boundless optimism, and a radiant
spirit. Her promotion to VP perfumer is a testament to her artistic flair, mentorship skills and leadership in steering her team toward success.”
A LOVE FOR ART AND PAINTING
Joa Kim’s promotion is a tribute to her more than two decades of dedication to perfumery at the IFF Singapore creative hub. A fine connoisseur of ingredients, creation and the complexity of perfumery delivery systems, Joa’s fervour for perfumery has birthed a plethora of iconic fragrances, which have captivated consumers worldwide. Her portfolio spans an impressive array of categories, from fabric care to beauty and home essentials, catering to both global and regional audiences.
“Joa is the epitome of creative and technical acumen,” says David Ellison, president of IFF Consumer Fragrances. “Her artistry is matched by her technical expertise and exceptional sense of camaraderie and generosity. Joa’s artistic inspiration is deeply intertwined with her love for art and painting, leading to collaborations with the prestigious Van Gogh Museum and the fusion of scent with visual art at the
2022 IFF Singapore Innovation Centre’s inauguration.”
A DEDICATED MENTOR
Birgit Sijbrands’s career is decorated with a remarkable 28-year legacy of fragrance creation. Since her first days at IFF 17 years ago, Sijbrands has been the architect behind a multitude of successful fragrances, fostering robust partnerships with strategic customers across the globe. On par with her achievements, Birgit is admired for her contribution to the diverse expertise within IFF, and her role in mentoring and nurturing the next generation of talent.
“Birgit is the quintessence of dedication, humility and passion,” Ellison adds. “Through her perfumery expertise, she has consistently demonstrated her unwavering commitment to crafting scents that truly awaken the extraordinary senses for a better world.” •

" The elevation of these three distinguished women perfumers celebrates their extraordinary commitment to perfumery "
Patty Hidalgo (top right), Birgit Sijbrands (left), and Joa Kim
PERFUMERY & FRAGRANCE INGREDIENTS 22 MARCH 2024 // WWW.PHARMACOS.CO.ZA IFF – www.iff.com
image/ Shutterstock.com



Flavors & Fragrances

The power of fragrance
Fragrance can affect our emotions and mood. A smell or aroma can create a memory or evoke one, bringing memories back to life. Fragrance can also provide a sense of happiness, reassurance or comfort. Come, and explore the truly transformative benefits of fragrance with Fragrance Oils.
Since the pandemic, the wellness movement continues to be a key trend.
Although this trend has been around for several years, it continues to evolve and has become all-encompassing as consumers look to live a more holistic way of life. For example, having a multi-step hair care routine or applying a luxurious body lotion is recognised as a form of self-care, but to complete the experience and set the right mood, fragrance plays a vital role.
"Fragrance Oils’ team of expert Scentmakers is constantly looking for new and emerging trends"
Fragrance Oils’ team of expert Scentmakers is constantly looking for new and emerging trends. From fine fragrance to colour, fashion and home interiors, we monitor key pillars that shape the market. Our unique global insights allow us to work alongside personal care brands, helping them transform their products with the power of fragrance.
In this article we look at some of the most popular olfactive themes influencing the personal care sector right now. These themes feature in our inspirational Fragrance Collection, bringing the colours, mood and ingredients to life with carefully crafted fragrances that reflect the latest trends.
FRESH AND AIRY
The fresh and airy category embraces the trend for outdoor fragrances that capture the healing and restorative powers of water and green spaces. This is translated through ozonic, marine, and watery green accords, along with comforting, clean cotton notes that offer feelings of freshness and serenity.
BLOOMING AND BRIGHT
A celebration of colour, this bright and youthful theme encompasses vibrant fruits, intoxicating florals and sunny citrus tones. On-trend notes such as juicy peach, ripe mango and luscious orange are blended with solar florals for a touch of luminosity for feelings of radiance and absolute joy.
TEMPTING AND DELIGHTFUL
In times of uncertainty consumers look for familiarity in fragrance and scented products. Recently we’ve seen a resurgence of vanilla for this very reason. The tempting and delightful theme is inspired by mouth-watering gourmand notes, recognising the growing demand for sweet, savoury and salty notes that not only indulge the senses but also convey a feeling of comfort and warmth.
RICH AND LUXURIOUS
Breaking away from familiar fruits and florals, the rich and luxurious trend takes you on an intriguing journey where tantalising spices, warm woods, and rich resins take centre stage.
In fine fragrance, we continue to see powerful, attention-grabbing accords with

leather, oud, amber and patchouli epitomising the desire for rich and luxurious scents – a trend that is now filtering into personal care.
FUNCTIONAL FRAGRANCES ARE KEY
Consumers continue to be more knowledgeable about fragrance, so when it comes to personal care, having the right scent is key. Fragrance, and the feelings and emotions it can evoke, can completely transform a product, by supporting the desired positioning and creating a sensorial experience. With wellness still a key trend across beauty and personal care, we know that functional fragrances which enhance one’s emotional state have never been more important or relevant to consumers of today.
Contact Brenntag SA for more information on the olfactive themes discussed in this article. •
Brenntag SA –www.brenntag.com/en-za Fragrance Oils –www.fragrance-oils.com
images/ Shutterstock.com PERFUMERY & FRAGRANCE INGREDIENTS 24 MARCH 2024 // WWW.PHARMACOS.CO.ZA

Brenntag and Fragrance Oils combine to help you find the perfect scent for your concept.

Contact Charis Lewis charis.lewis@brenntag.com brenntag.com
A spritz of affordable luxury – body mists for the modern consumer
As the world grapples with a cost-of-living crisis, consumers are increasingly seeking experiences that are affordable yet indulgent. In the realm of fragrance, this quest for accessible luxury has sparked a notable trend: the rise of hair and body mists. I spoke to Moellhausen perfumer, Anna Chiara Di Trolio, about the dynamics of this burgeoning trend and what inspires her when designing fragrances for the South African market. By
Abby Vorster
Light and carefree, body mists are making a comeback. According to the 2023 Fragrance Trends Report published by Spate (a consumer trend research agency), body mists or sprays are seeing a resurgence in popularity. Online searches for body sprays jumped by 9.8% compared to 2022, and the #bodymist hashtag now has more than 1.4 billion views on TikTok.
"In crafting formulations that balance indulgence with affordability, selecting the right raw materials is key"
Di Trolio, who visited South Africa last year with a delegation from Moellhausen, says that although body mists tend to target younger generations, considering the selling price of these applications and the olfactory direction that generally
distinguishes them, we can see that they will be ever so trendy in 2024.

“With their affordable price point and vibrant scent profiles, these mists are poised to resonate particularly well with the younger demographic in South Africa,” she comments. But what sets body and hair mists apart in meeting the evolving preferences of consumers seeking lighter fragrances?
Di Trolio emphasised the role of affordability and accessibility: "The price plays a key role here. And, consequently, the easy accessibility by everyone."

Creativity and innovation are part of Moellhausen’s DNA and fuel the company’s ambition for fast and continuous development. Drawing inspiration from her travels to South Africa, Di Trolio shared her insights into designing fragrances tailored specifically for this dynamic market.
“I have wonderful memories of my trip to South Africa. The country is so sunny, colourful, and joyful. I try to represent the same mood in my creations when designing new fragrances and fragrance concepts for this market,” she reflects.

Coupled with their fruity top notes and floral heart, these mists offer a compelling olfactory experience that appeals to a wide audience.
SHAPING FRAGRANCES
AROUND LOCAL PREFERENCES
Moellhausen is an Italian Fragrance house with a global footprint. It is active in over 80 countries worldwide on all five continents. The company is an inclusive industrial reference in the field of fragrance compounds, essential oils, aroma chemicals, and innovative and unconventional flavour and fragrance specialties.
Di Trolio’s approach involves infusing fragrances with intensity, vibrancy and a fruity facet, which resonates with local preferences. In crafting formulations that balance indulgence with affordability, Di Trolio says that selecting the right raw materials is key.
“The raw materials need to have a strong and immediate impact, while being able to win over the consumer at the first evaluation,” she explains. “Our formulations aim to deliver long-lasting quality without veering into the realm of generic toiletries.”
CAPTURING JOY IN THE MARKET
As South Africa and beyond embrace the trend of body and hair mists, it is evident that these accessible luxuries are not just a passing fad but a reflection of changing consumer preferences. With fragrances that capture the essence of joy, vibrancy and indulgence, these mists are poised to leave a lasting impression in the hearts and minds of consumers across the country. •
PERFUMERY & FRAGRANCE INGREDIENTS
Moellhausen – www.moellhausen.com
26 MARCH 2024 // WWW.PHARMACOS.CO.ZA
image/ Shutterstock.com
Anna Chiara Di Trolio






Pioneering eco-solutions in labeling
For almost four decades, many of South Africa’s popular and most reputable FMCG producers have relied on Rotolabel as the custodian of the face of their brand – the label. Earning the label of ‘trustworthy’ is thanks to
the supplier’s unwavering consistency, professionalism and its people who are passionate about producing premium quality labels.
Rotolabel is passionate about the environment and focuses on minimising any negative impact of its products. Its label of trust extends to pushing boundaries in the sustainability of its products to ensure a greener future for generations to follow.
The company subscribes to the four Rs in packaging sustainability:
1. Responsible sourcing
2. Reduction of materials
3. Recyclability
4. Increased recycled content.
As the South African leader in sustainability innovation within the label printing space, Rotolabel has introduced some notable initiatives. While self-adhesive labels are a great option for product labelling, recycling of the glassine liner remained a challenge in South Africa.
" Rotolabel is proudly leading sustainability driven change in the South African market"
In partnership with Avery Dennison, in 2018 Rotolabel launched a pilot project in Cape Town for the first ever glassine liner recycling programme in South Africa. Despite many challenges, the business has been able to recycle 86t of glassine liner, which is a significant step in reducing waste to landfill.
Since the successful implementation of the recycling initiative, liners are now being recycled locally with 180t recycled in the last year. This achievement stands as a testament to Rotolabel and Avery Dennison’s bold decision to carry the costs of the initiative, enabling it to come to fruition in South Africa.
FSC CERTIFICATION
Responsible sourcing is one of the cornerstones of sustainability – and while paper can be biodegradable and easily recyclable, it can also be the product of deforestation or poor forestry practices.
The Forest Stewardship Council® (FSC) is an international certification and labelling system that enables people to identify responsibly sourced wood, paper and other forest products. As the original pioneers of forest certification, FSC has over 25 years of experience in setting the gold standard for sustainable forest management.
In today’s world there’s no excuse for paper to not be sourced responsibly, which is why Rotolabel became FSC Chain of Custodycertified (FSC-C119866) back in 2015 – and was the first label printer in South Africa to achieve this.
MATERIAL REDUCTION
One of the critical pillars for a more sustainable future is to reduce packaging. Although pressure-sensitive labels form less than 3% of the overall packaging on a product, reducing the use of resources to produce them can have a significant impact on the environment.
By reducing raw materials, Rotolabel can now offer thinner and lighter topsheets and liners with less grammage, without compromising the label’s performance.
RECYCLED CONTENT
A key driver in the circular economy concept is for raw material suppliers to use recycled content in their virgin products. There is now an array of materials available with up to 100% recycled content – saving resources including water, energy and greenhouse gasses.
RECYCLABLE
It is extremely important to understand which label to apply on what product to ensure compatibility for recycling.
Over the last 18 months, Rotolabel has been collaborating with Avery Dennison to introduce a revolutionary label for PET containers and bottles. CleanFlake™ is a film label with an adhesive that enables true bottle-to-bottle recycling for 100% food-grade rPET. This scientifically designed adhesive ensures that labels get cleanly detached during the recycling process, facilitating the production of food-grade rPET. CleanFlake™ has been approved by the Association of Plastic Recyclers (APR). It has also received formal approvals from key recyclers both in South Africa and abroad.
Rotolabel is proudly leading this sustainability driven change in the South African market, with a 60%+ adoption rate. Despite Rotolabel championing the use of CleanFlake™, many PET packs still use incorrect adhesives, resulting in those being downcycled or ending up in landfills.
Rotolabel is also currently trialling a paperbased wash off material for HDPE and PET containers, which will further help the recycling stream within South Africa. •

Rotolabel – grant@rotolabel.co.za
SUSTAINABLE PACKAGING
28 MARCH 2024 // WWW.PHARMACOS.CO.ZA image/ Shutterstock.com

A proactive approach to sustainability
At HP we have a responsibility towards sustainable change. Our ambition is to make a positive impact for people and communities worldwide. The print and packaging industry is where we are driving significant activities in improving energy efficiency, increasing circularity, reducing waste and empowering customers to reduce the environmental impact of their businesses, writes Haim Levit, SVP and division president, HP Industrial Print.


The print and packing industry is undergoing an unparalleled period of change. Sustainability is a key driver across the entire industry – from consumers expecting new type of prints and packages, to brand owners aiming to reduce, reuse and recycle. It also encompasses our clients – printers and converters –who serve brand owners, as well as all the equipment, substrates and software providers that contribute to the production process.
" Printers and converters play a pivotal role in helping brand owners deliver on their sustainability goals"
It's time to act. At HP we play a critical role at the forefront of innovation bringing groundbreaking technologies and inventive collaborations to market which will transform and decarbonise the print and packaging industry. Three core
pillars shape our approach as we work to deliver on our commitment:
1. Optimising the day-to-day operation
2. Advancing on design circularity
3. Partnering for driving sustainable change together.
EVERYDAY OPTIMISATION
Printers and converters play a pivotal role in helping brand owners deliver on their sustainability goals. Our end-to-end print solutions such as HP Indigo and HP PageWide enable more sustainable production processes. These solutions are equipped with capabilities such as automated digital printing, optimised energy and ink consumption and ink innovations that ultimately reduce waste and empower brands to explore more sustainable substrates.

and flexible packaging alternative to the traditionally wasteful coffee capsules, the company moved its coffee pouches to HP Indigo. Not only did the sustainablefirst packaging and cases deliver on Incapto’s sustainability goals, but they also drove positive change in consumer habits, strengthening its position as a sustainable brand.
To deliver on our shared vision for packaging to be synonymous with sustainability, we collaborated with sustainable packaging provider Rootree to help the business achieve its goal to make 90% of its packaging recyclable and compostable.
Incapto is an example. With the goal to revolutionise the coffee category by delivering a sustainable
Thanks to HP Indigo Electro Ink that can be printed on compostable packaging on the HP Indigo digital press, Rootree was able to make its flexible packaging more sustainable and solidified its position in the sustainable packaging market.

30 MARCH 2024 // WWW.PHARMACOS.CO.ZA SUSTAINABLE PACKAGING
Haim Levit
DESIGN CIRCULARITY
To deliver sustainable solutions we tackle improving efficiency, reducing the need for transportation, and increasing circularity by reducing print media waste. We help our customers on this journey with our sustainability led innovations.
We believe circularity should be weaved into every element of the process from R&D to manufacturing and services. That’s why we prioritise longevity in manufacturing equipment, which is built to last and is more sustainable by design. Through upgrade options and robust maintenance strategies, we ensure a longer equipment lifespan. Incorporating 12% of recycled plastics and metals in our press manufacturing we’re constantly innovating to reduce the environmental impact of our equipment.
With the HP PageWide Advantage 2200 we’re helping customers improve efficiencies. Its 2200 High Efficiency Drying (HED) system reduces up to 60% energy per page. HP xRServices offers instant remote diagnostics and resolution, helping customers to reduce their travel impact. This service typically saves up to 800kg of CO 2 generated by field support engineer’s air travel.
At the end of life of our products or supplies the HP Indigo Take Back programme in the UK recovers presses

" We’re empowering customers to measure and reduce their emissions with tools like the Print Job CO₂ Calculator"
and supplies for remanufacturing and recycling. In the last two years, more than 100 Indigo presses have been recovered and remanufactured as certified pre-owned, reusing more than 90% of the parts and saving more than 60% in CO 2 impacts.
PARTNERING TO DRIVE CHANGE
These days, designing environmentally efficient products and services isn’t enough. We must collaborate with the entire print ecosystem. Through HP’s Amplify Impact pilot programme, we’re extending HP’s Sustainable Impact strategy to our partners to tap into the brand’s extensive knowledge, data, training and resources. The goal is to use these resources to assess and optimise their sustainability performance.
of forests in the fight against climate change. Sadly, nearly half of the world’s forests are under threat. With our Forest Positive by 2030 goal, we’re working to protect and restore forests with every page our customers print, investing in forest conservation and collaborating with environmental organisations. Visit www.hp.com/gben/printers/forest-first-printing.html for more info. •
SEEING IS BELIEVING AT DRUPA 2024

We’re empowering customers to measure and reduce their emissions with tools like the Print Job CO 2 Calculator, which enables the user to assess and actively reduce the carbon footprint of a print job.
Yet, we know that the journey doesn’t end with us and our customers, but with suppliers and third parties throughout the entire supply chain. By leveraging technology and partnering with suppliers, we can consistently reduce CO 2 emissions throughout our supply chain to achieve our 2030 50% reduction goal.
As we look beyond to the natural world, we recognise the importance

Sustainability assets to reduce the impact of print and packaging are tangible, commercially available and already successfully deployed by many of our customers across the globe. At HP we are committed to our responsibility to decarbonising the industry and have the determination to lead and empower print and packaging stakeholders to reduce their environmental impact. We look forward to welcoming you at drupe from 28 May to 7 June, to see first-hand how we’re making this a reality. See you in Düsseldorf!
HP Industrial Print – www.hp.com/gb-en/ industrial-digital-presses.html WWW.PHARMACOS.CO.ZA // MARCH 2024 31 images/ Shutterstock.com
SUSTAINABLE PACKAGING
Mondi expands EcoWicketBag production
Mondi, a global leader in sustainable packaging and paper, is expanding production of its innovative range of paper-based EcoWicketBags. This comes in response to increasing demand for sustainable packaging in the home and personal care (HPC) industry, particularly for products such as nappies and feminine hygiene products.
By expanding the production of EcoWicketBags at its plant in Szada (Hungary), Mondi further leverages the group’s integrated value chain, from in-house paper production to coating and converting.
HELPING CUSTOMERS MEET SUSTAINABILITY GOALS
EcoWicketBags are made from Mondi’s FunctionalBarrier Paper 95/5 – an exceptionally strong kraft paper that can be customised with specific barrier and protective properties to meet diverse product needs. These bags


are available in a variety of sizes for different applications and provide protection from filling to transporting and storage, as well as being ideal for printing customer branding.
" Paper-based packaging has very high recycling rates in Europe"
Paper-based packaging has very high recycling rates in Europe (82%) and Mondi’s EcoWicketBags can support customers in the HPC industry to meet their sustainability goals on their journey towards a circular economy.1 EcoWicketBags are made from a renewable material and designed to be recycled in standard European paper recycling mills according to the 4evergreen guidelines.
CONTRIBUTING TO A BETTER WORLD
EcoWicketBags add to Mondi’s range of recyclable, mono-material polymer WicketBags that follow CEFLEX recycling guidelines. The range also includes WicketBags that use a high amount of post-consumer content in line with customer needs. With various choices available, customers can select the best fit for their product to meet their sustainability goals.

Piotr Barczykowski, sales director consumer flexibles at Mondi, says: “Our Szada plant in Hungary is well positioned to serve customers across Europe and we are pleased to respond to consumer and customer demands for sustainable solutions with our paper-based EcoWicketBags.” •
REFERENCE:
1. https://www.statista.com/statistics
Mondi – www.mondigroup.com





































































32 MARCH 2024 // WWW.PHARMACOS.CO.ZA Available online: www.thebuyersguide.co.za The directory for manufacturers of food, beverages, pharmaceuticals, cosmetics, toiletries, packaging and the printing industry 2023/2024 Buyer’s
THE TBG 2023.indd 1 2022/10/06 13:16
guide
SUSTAINABLE PACKAGING
are designed for recycling and offer the advanced barrier protection required for diapers and feminine hygiene products.
EcoWicketBags
































Tel 011 608 4944 | Fax 011 608 4948 | Email sales@sensetek.biz Sensetek.indd 1 Tel 011 608 4944 | Fax 011 608 4948 | Email sales@sensetek.biz Supplied by Sensetek Sensetek.indd 1 2018/03/28 7:54 AM Tel 011 608 4944 Fax 011 608 4948 Email sales@sensetek.biz 2017/11/01 12:00 PM Supplied by: Tel 011 608 4944 Fax 011 608 4948 Email sales@sensetek.biz

Gattefossé, your reliable supplier for drug development
As a leading provider of functional lipid excipients and formulation solutions to healthcare industries worldwide, Gattefossé is committed to delivering high quality excipients as well as technical support and inspiring formulations for drug delivery.
ORAL DRUG DELIVERY
Lipid excipients for oral drug delivery include solubility and bioavailability enhancers, lubricants, modified release, taste-masking, API protection and suspending agents. Excipients are used in a variety of processes enabling the formulation of different dosage forms, mainly tablets, granules, hard and soft capsules.
Gattefossé offers a complete portfolio of excipients for oral bioavailability enhancement. This portfolio was recently enhanced with the launch of two new excipients:
1. Labrafac™ MC60, glycerol monocaprylocaprate, an old-new excipient with worldwide precedence of use.
2. Gelucire® 59/14, a new grade in its Gelucire® range.
Compritol® 888 ATO is a lubricant for challenging pharmaceutical tablets, offering extreme inertness and high flexibility in formulation development and production. When used at 10% to 30%, it offers a smart solution to sustain drug release. Compritol® 888 ATO forms an inert matrix from which the drug diffuses slowly over time. It has also been shown to prevent alcohol dose dumping for safer formulations.
Precirol® ATO 5 is ideal for taste-masking and API protection thanks to the formation of a film coating around the drug particle.
TOPICAL DRUG DELIVERY
Lipid excipients for topical drug delivery include solubilisers, emulsifiers and viscosity
modifying agents. Emulsifiers are designed for challenging formulations and deliver excellent texture and sensorial properties. Solubilisers provide skin penetration enhancement and viscosity agents stabilise formulations. These excipients are used in creams, emulgels, lotions, foams, microemulsions, bi-gels, and gels.
"Gattefossé offers a complete portfolio of excipients for oral bioavailability enhancement "
Tefose® 63 is a multi-functional emulsifier used in combination with Labrafil® M 1944 CS, delivering exceptional heat stability to topical emulsions.
Transcutol® P is a safe and effective solvent widely used to deliver drug to and through the skin.
New in the Gattefossé range, Emulfree® Duo is an oil-stabilising agent that enables the preparation of bi-gels, which is an emerging pharmaceutical dosage form.
RECTAL AND VAGINAL DRUG DELIVERY
Lipid excipients for suppository and pessary formulation include hard fat and hard fat with additives. These bases provide excellent physico-chemical stability and optimise
drug delivery for a wide range of APIs and manufacturing equipment.
Alternative dosage forms (creams, gels, foams etc) for rectal or vaginal mucosal delivery can be formulated with Gattefossé’s safe and non-irritant emulsifiers and thickeners.
PRODUCT DEVELOPMENT SUPPORT
With an international network of technical representatives like Carst & Walker in South Africa, and Technical Centres of Excellence in USA, France, India and China, Gattefossé provides bespoke technical and regulatory support to accelerate drug development.
For more information on Gattefossé’s excipients, and to download up-to-date technical and regulatory documentation, brochures and case studies, visit www.gattefosse.com. •


Carst & Walker –
#consumer-specialities
Aurora Pakkies, product manager aurora.pakkies@carst.com

PHARMA FOCUS/SPONSORED CONTENT
carst.com/market-segments/
34 MARCH 2024 // WWW.PHARMACOS.CO.ZA images/ Shutterstock.com

Delivering the goods
Considerable research has gone into developing alternative delivery methods for medicines and nutraceutical products, with liposomes emerging as one of the more promising avenues. In this article, Natalie Gerhardt of Multichem Sourcing explores the technology and its benefits.
In today’s world, we anticipate swift and effortless delivery of our needs and desires. This applies to everything from ecommerce purchases to the medicines and supplements we administer to maintain optimum health.
When it comes to medicines and nutraceutical products, conventional delivery methods may be painful (injections or IVs). There is also the issue of sub-optimal bioavailability.
That is, the active ingredients in the medicine or supplement may become diluted or degraded during interaction with the human body, or their action may be slowed down. In the case of tablets and capsules, excipients, binders, fillers and flow agents can contribute to poor disintegration rates, leading to poor absorption. This can happen even when the active ingredients are delivered at the right dose.
For a formulator, the most pressing challenge is to ensure that the active ingredients move into the bloodstream and reach the cells where they are needed.
THE TECHNOLOGY AT A GLANCE
In a liposome, active ingredients are surrounded by a sphere of phospholipids (the same fat molecules that make up cell walls). This protects the active ingredients and allows them to better survive the digestive process and enter the intestines. Here, the liposome fuses with the cell walls and the active ingredients are released.
Depending on the ingredient, absorption may increase by a factor of up to 20 – a positive result that has

been supported by many scientific studies worldwide. More recently, the liposome delivery method has been used more frequently in natural health applications and nutritional supplements.
In an article entitled Curing The Incurable , author Thomas E Levy MD states that, “a much smaller oral dose
Did you know?
The name liposome is derived from two Greek words: ‘Lipos’ meaning fat and ‘Soma’ meaning body.

of liposome encapsulated vitamin C, (5g to 10g), often results in a clearly superior clinical response than a much larger dose of vitamin C given intravenously (25g to 100g).”
BENEFITS OF LIPOSOMES
The primary advantage of liposomes is that they increase the bioavailability of their ‘payloads’ – that is, of the substances they contain. Enhanced bioavailability has multiple ancillary benefits, including more precise doses, and reduced costs, as smaller quantities are required to achieve the same outcome. This helps avoid
Liposome diagram (Image: LipoCellTechT)
PHARMA FOCUS/EXCIPIENTS & API s 36 MARCH 2024 // WWW.PHARMACOS.CO.ZA
reduced impacts associated with under-delivery, as well as toxicity risks caused by over-delivery.
In a nutraceutical context, liposomes ensure efficient delivery to target cells and enhanced absorption rates of nutrients, with multiple physical health benefits including support for healthy cell structures and maintenance of healthy liver tissue.
Liposomes are biocompatible and biodegradable (the phospholipids used are derived from seed oils eg, sunflower oil). Liposome-enabled delivery allows for adjustable and incremental dosing and avoids the need for IVs and injections.
SOLVING THE CHALLENGES OF LIPOSOMAL DELIVERY
Many studies exist on the efficacy of medicinal liposomal delivery systems, both liquid and dry. Indeed, their efficacy is no longer in question. There are also multiple studies showing that liposomes are an effective delivery system for vitamins and other supplements.
"The primary advantage of liposomes is that they increase the bioavailability of their ‘payloads’"
However, liquid liposomes (whether delivered by injection or orally) are imperfect; they degrade quite quickly and have a short half-life. They are also more likely to break down in the stomach. This is not to say that they are ineffective, but rather that they are less stable. Liquid liposomes tend to demonstrate low solubility and can be prone to leakage of the encapsulated molecules.
Innovative and promising methods developed to address these issues include the use of so-called proliposomes. Work on pharmaceutical applications for these dry liposomes began in the 1980s and soon showed that their stability is far superior
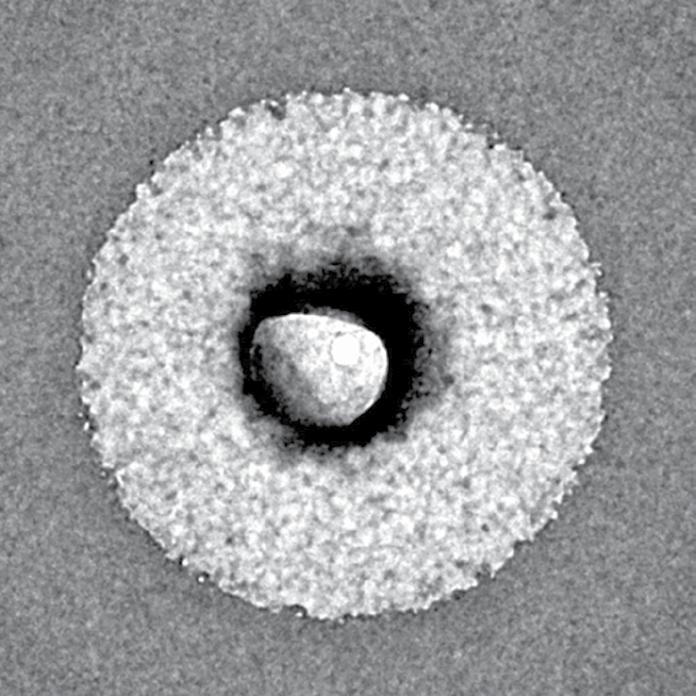
to that of regular liquid liposomes. They are a dry, free-flowing granular material (powder), which immediately forms a liposomal dispersion on contact with water or biological fluids within the body. When the dry powders are hydrated and gently mixed (as occurs in the stomach), the contents rapidly disperse to create a liposomal suspension in the aqueous solution.
ENSURING QUALITY
In today’s world, ingredient fraud is a major issue. Most manufacturers are not able to test the exact purity and stability of the liposomes they purchase. While many suppliers offer these products, only a handful can genuinely produce the stable form.
Multichem Sourcing, a SAHPRA licensed importer, rigorously vets suppliers and can confidently offer a wide range of the high-quality liposomal ingredients.
The company’s current liposome range includes ascorbic acid (vitamin C), glutathione, vitamin B complex, curcumin, NMN, PEA and omega derivatives, among others.
THE FUTURE OF LIPOSOMES
The increased use of liposomes in nutraceuticals has been linked to the growth in the use of pure compounds and complex botanicals – a trend that is aligned with intensified consumer focus on all aspects of wellness.
The future looks bright for liposomes, with new and advanced production methods allowing for smaller and more stable liposomes and consequently, faster absorption rates and increased circulation time within the body. •
ABOUT THE AUTHOR
Natalie Gerhardt (BSc. Hons. in Biochemistry and Microbiology) is the managing director of Multichem Sourcing, a raw materials and chemicals sourcing specialist based in South Africa. The SAHPRA licensed company focuses on sourcing and supplying APIs and raw materials required for API synthesis. Multichem Sourcing supports the pharmaceutical, nutraceutical, cosmetics, agricultural and veterinary industries.


Multichem Sourcing –www.multichem-group.com
Liposome under the microscope (Image: LipoCellTechT)
image/ Shutterstock.com
WWW.PHARMACOS.CO.ZA // MARCH 2024 37
PHARMA FOCUS/EXCIPIENTS & API s
Solubilising formulation technologies based on phospholipids
C.F. Thomas of Lipiod explores the vital role of phospholipids in intravenous formulations for poorly water-soluble drug substances.
All lipids that contain phosphorus are called phospholipids. Phospholipids are surface-active, amphiphilic molecules, which comprise a polar headgroup and a lipophilic tail. They can be found in any cell membrane and are therefore intrinsically biocompatible and biodegradable. The phospholipid molecule comprises a glycerol backbone, which is esterified in positions 1 and 2 with fatty acids and in position 3 with phosphate (see figure 1). The systematic designation of e.g. phosphatidic acid (PA) is 1,2-diacylsn-glycero-3-phosphate (where sn means stereospecific numbering).
Examples of headgroups that are esterified to the phosphate group are choline, ethanolamine, glycerol, inositol and serine. The resulting molecules are called phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), inositol (PI) and phosphatidylserine (PS), respectively. Phosphatidylcholine is also called lecithin. ‘Lecithin’, as described in the USP is, however, not pure phosphatidylcholine but may contain other (phospho)lipids alongside the main component phosphatidylcholine. When no base is esterified to the phosphate group, the phospholipid represents phosphatidic acid (PA). In general, natural phospholipids are neutral (PC, PE) or negatively charged (PG, PS, PI, PA). Positively charged phospholipids rarely exist in nature.
An example of a positively charged phospholipid is lysyl phosphatidylglycerol. Net positively charged, synthetic pHsensitive cationic lipids, which are not necessarily phospholipids, play an important role in the makeup of lipid nano particles (LNPs), suitable for RNA delivery.1
THE ROLE OF PHOSPHOLIPIDS
Phospholipids are used in the formulation of poorly water-soluble drugs for oral and especially intravenous administration. In addition, they play an important
role in the physiological dissolution mechanisms after oral and parenteral administration of poorly water-soluble drugs. For intravenous administration, several formulation technologies based on phospholipids can be applied to solubilise poorly water-soluble drugs.
" Phospholipids are also used as wetting surfactants for suspensions of poorly water-soluble compounds for intramuscular injection"
These are oil-in-water emulsions containing egg phospholipids as emulsifier, mixed micelles comprising the bile salt sodium glycocholate and soybean phosphatidylcholine, and liposomes with phospholipids as the main lipid component of the liposomal membrane. 2,3 In general, poorly water-soluble, lipophilic drugs are associated with or dissolved in the dispersed lipophilic domains of these formulations and prevent precipitation of the drug substances. Upon intravenous administration, the drug substances transition from the dispersed formulation particles to other lipid domains/particles in the blood circulation by means of diffusion and collision mechanisms
eventually reaching the target drug substance receptor. 4 Phospholipids are also used as wetting surfactants for suspensions of poorly water-soluble compounds for intramuscular injection.
OIL-IN-WATER EMULSION
Examples of marketed drug products comprising oil-in-water emulsions are Diazemuls® with diazepam, (Dumex, Denmark), Liple® with prostaglandin E1 (Mitsubishi Pharma Corporation, Japan) and Diprivan® with propofol (AstraZeneca, UK). 2
The lipophilic drug substance is solubilised in the oil phase of the dispersed oil emulsion droplets (see figure 2). A prerequisite for the use of oil-in-water emulsions is a sufficient solubility of the drug substance in the oil phase of these emulsions, which allows administration of a therapeutic dose of the drug substance in an acceptable volume and concentration of the oil.
MIXED MICELLES
Mixed micelles comprising phosphatidylcholine and sodium glycocholate are also used for solubilisation of lipophilic compounds (see figure 3).
The role of the phospholipid component in the mixed micelle is to eliminate the haemolytic properties of the sodium glycocholate. Depending on the stability of the drug, mixed micelles can be used in
Figure 1: Molecular structure of a typical phospholipid, 1-palmitoyl, 2-oleoyl-sn-glycero-3phosphocholine (POPC), also named 1-palmitoyl-2 oleoyl-phosphatidylcholine
38 MARCH 2024 // WWW.PHARMACOS.CO.ZA PHARMA FOCUS/EXCIPIENTS & API s N


Pharma Contact Natalie MacGregor Natalie.MacGregor@brenntag.com brenntag.com Value added solutions with Brenntag South Africa
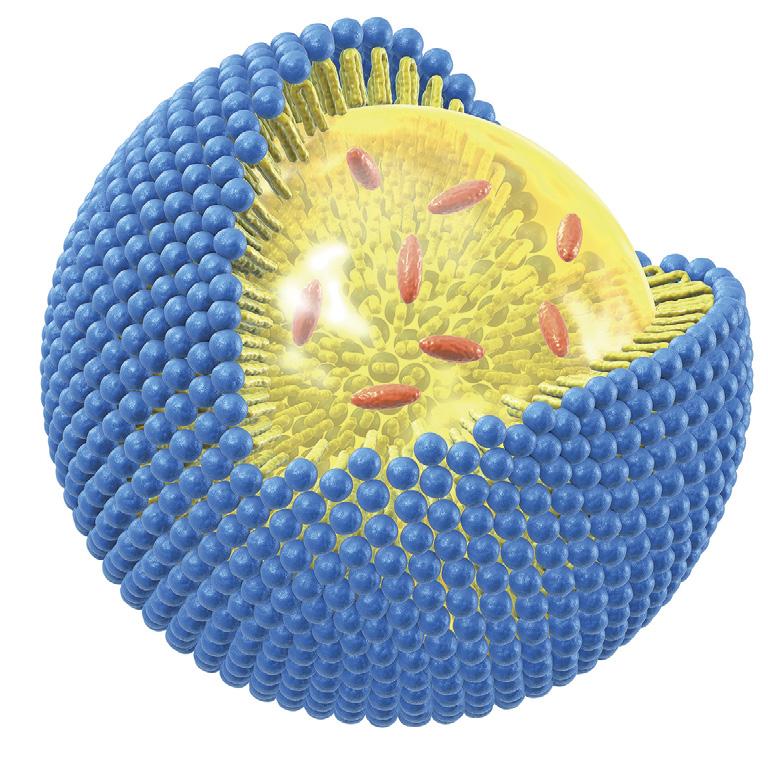

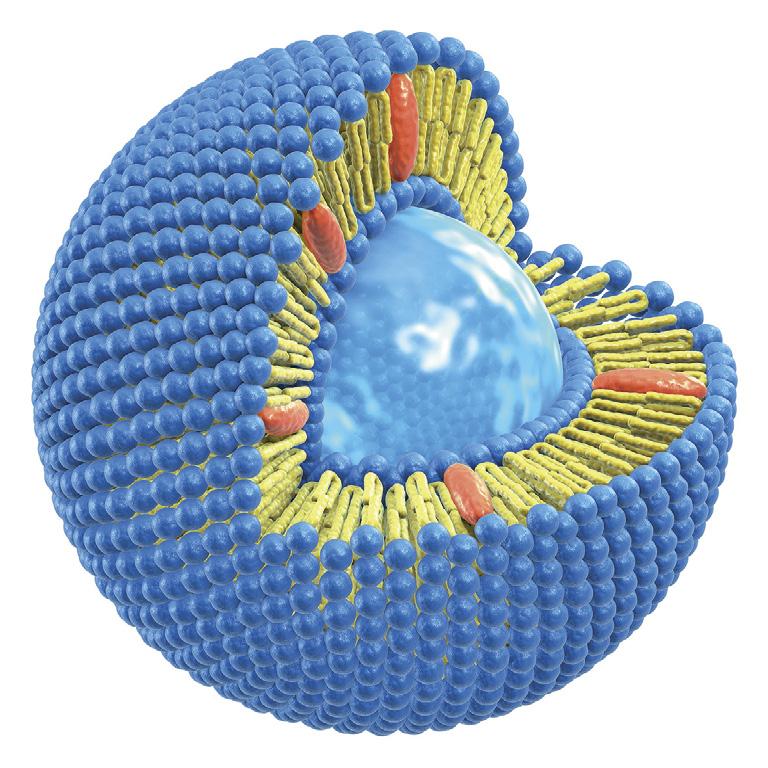
liquid or freeze-dried form. Examples of products are diazepam 5-7 and lipophilic vitamins (Konakion® MM paediatric, Konakion®; and Cernevit®), as well as multivitamins for infusion.
LIPOSOMES
Liposomes are well known for their use in drug targeting but are less known for their potential to solubilise poorly water-soluble compounds for intravenous use. Lipophilic drug substances can be embedded in the liposomal membrane consisting of a double layer of phospholipids (see figure 4).
Examples of poorly water-soluble drug substances that use liposomes as a solubilisation technique are amphotericin B and benzoporphyrin. In Ambisome® comprising amphotericin B, liposomes are used to replace the haemolytic cholate salt in the traditional formulation Fungizone to solubilise amphotericin B. 8 The product Visudyne® (Verteporfin for injection) containing benzoporphyrin solubilised in liposomes is used for treatment of aged-related macular degeneration. 8 In both of examples, the drugs are embedded in the liposomal membrane by means of association with the fatty acid domain of the phospholipid bilayer membrane of the liposomes.
The examples presented in this article of solubilising formulation technologies based on phospholipids underscore the versatility of these excipients. The large-scale availability of natural as well as synthetic phospholipids in the pharmaceutical industry which are accepted by regulatory authorities highlights the use of these excipients in the arsenal of formulation technologies for poorly water-soluble drug substances. 2
Lipiod is represented by Brenntag in South Africa. Contact Brenntag SA for more information. •
REFERENCES:
1. Jeong, M., et al. "Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications". Advanced drug delivery reviews (2023): 114990.
2. Van Hoogevest, P. and A. Wendel. "The use of natural and synthetic phospholipids as pharmaceutical excipients". European journal of lipid science and technology 116.9 (2014): 1088-1107.
3. van Hoogevest, P., et al “Role of phospholipids in the oral and parenteral delivery of poorly water soluble drugs”. J. DRUG DEL. SCI. TEC 21.1 (2011): 5-16.
4. Fahr, A., et al. "Transfer of lipophilic drugs between liposomal membranes and biological interfaces: consequences for drug delivery”. European Journal of Pharmaceutical Sciences 26.3-4 (2005): 251-265.
5. Forrest P. and Galletly D. C. “A double-blind comparative study of three formulations of diazepam in volunteers” Anaesthesia Intensive Care 16 (1988): 158-163.
6. Guentert T. W., et al “Interaction of mixed micelles formed from glycocholic acid and lecithin with the protein binding of various drugs”. British Journal Clinical Pharmacology, 23 (1987): 569-577.
7. Galletly D.C. et al “Diazepam mixed micelle, Comparison with diazepam in propylene glycol and midazolam”. Anaesthesia Intensive Care 13 (1985): 352-354.
8. van Hoogevest, P. et al. "The use of phospholipids to make pharmaceutical form line extensions". European Journal of Lipid science and Technology 123.4 (2021): 2000297.
Brenntag SA – www.brenntag.com/en-za Lipoid – lipoid.com/en/
PHARMA FOCUS/EXCIPIENTS & API s
40 MARCH 2024 // WWW.PHARMACOS.CO.ZA
Figure 2: Oil particle of an oil-in-water emulsion with phospholipid emulsifiers and oil phase containing the lipophilic drug substance
Figure 3: Mixed micelles of soybean phosphatidylcholine and bile slat containing a lipophilic drug substance
Figure 4: A liposome with a lipophilic drug substance embedded in the liposomal membrane

Johannesburg: +27 11 856 4500 Cape Town: +27 21 830 5306 Durban: +27 31 313 3338 Email: info@savannah.co.za www.savannah.co.za From basic ingredients to specialty actives for key application areas: CUTTING EDGE TECHNOLOGY
QUALITY INGREDIENTS FOR WELLNESS INDUSTRIES
| Tablets | Liquids | Syrups | Emulsions | Creams | Pastes
HIGH
Capsules
The right excipient can make all the difference

Multifunctional excipients such as galenIQ™ allow manufacturers to produce traditional and innovative dosage forms of pharmaceuticals and nutraceuticals.
Characterised by their ability to address technical challenges, multifunctional excipients also enhance formulations. Technical challenges in manufacturing are varied – for example easy swallowing and a good taste are priorities for paediatric and

geriatric dosage forms. Ensuring better ease of use, some manufacturers are looking for medications that do not need to be taken with food or water.
" Tablets are still the most widely used drug delivery system"
To simplify dosage regimen, controlled release applications can reduce frequency of administration and extend the duration of the desired effect. Multifunctional excipients such as galenIQ™ can help manufacturers overcome these challenges while contributing to more efficient production processes.
AN EASY PILL TO SWALLOW
Tablets are still the most widely used drug delivery system. However, some patient groups, including the elderly, people with dysphagia, and children may find it difficult to swallow conventional solid dosage forms because of their size and shape. Therefore, multi-particulate drug delivery systems –such as minitablets – have gained favour.
With a diameter of between 1mm and 4mm, minitablets are easy to take, while enabling exact dosing. They can also be coated in an accurate and convenient way and offer a high degree of dispersion in the gastrointestinal tract, reducing the risk of high local drug concentrations.
Manufacturing minitablets requires a filler-binder with excellent flowability. The excipient should also enable the production of high-hardness tablets at low compression forces to avoid damaging the multiple-tip punches used to make them. In addition, good taste and water solubility are important characteristics, especially when it comes to orodispersible minitablets that are tailored for fast drug release. galenIQ™ 721, BENEO’s water-soluble filler-binder, meets all these requirements.
EXCELLENT TASTE AND FUNCTIONALITY COMBINED
galenIQ™ is the pharmaceutical grade of BENEO’s isomalt, a disaccharide alcohol derived from beet sugar, which is the main reason why it has a taste profile similar to sucrose.
This innovative excipient may reduce the bitterness of APIs and could contribute to a pleasant taste and mouthfeel in the final pharmaceutical or nutraceutical product. In addition, galenIQ™ 721 offers
Ensuring a pleasant taste and helping to ensure compliance are key attributes of medicines or vitamins for children (Image: SensorSpot/iStock)
Gummies are a visually appealing dosage format with a pleasant taste (Image: ©Farknot Architect/Shutterstock)
42 MARCH 2024 // WWW.PHARMACOS.CO.ZA PHARMA FOCUS/EXCIPIENTS & API s
MANAGING OILY PLANT EXTRACTS
According to Grand View Research’s global botanical ingredients market report, demand is burgeoning for ingredients such as herbs, leaves, spices, flowers etc. Valued at $164.4bn in 2022 and expected to grow at a CAGR of 6.9% from 2023 to 2030, the industry is experiencing significant growth. Yet plant extracts are often sticky or oily and can be difficult to formulate into a solid format, such as a tablet. galenIQ™ 721 offers a high oil-binding capacity, agglomerate stability as well as flowability. Acting like a sponge, it absorbs the oily plant extract which remains as a dry flowable powder. Its morphology helps to retain the homogeneity of the mixture, making it easier to produce robust tablets with a high content uniformity. Due to the excipient’s water solubility, plant extract tablets disintegrate rapidly without the use of a super-disintegrant. In addition, the excipient reduces the often-unpleasant taste of oily plant extracts, enhancing the palatability of the tablet and promoting patient compliance.

Pharmaceuticals and nutraceuticals formulated with botanical ingredients are increasingly popular
(Image: ©Dima Sobko/Shutterstock) excellent flowability, great compressibility and ensures content uniformity. These functionalities enable high tablet hardness at a low compression force.
BEYOND TABLETS AND CAPSULES
Besides the agglomerated grade for direct compression, BENEO’s product range comprises different versions that serve a broad variety of dosage forms. The filler-binder is available in a wide array of particle size distributions, morphologies and levels of solubility. This makes it a highly flexible excipient that can be used to formulate tablets, chewables, lozenges, effervescents, capsules, powder blends and syrups.
"
Pharmaceutical grade galenIQ™ can act as an excipient for more innovative delivery forms"
With isomalt being an established ingredient in confectionery, the pharmaceutical grade galenIQ™ can act as an excipient for more innovative delivery forms such as medicinal gummies. Compared to capsules or tablets, gummies
are visually appealing and can therefore lead to better patient compliance, especially in children.
Because of its sugar-like taste, galenIQ™ contributes to an appealing taste profile. Regarding processing, when compared to sugar it is much less sensitive to heat and acid and is more stable during the preparation process. galenIQ™ also makes it possible to embed APIs such as vitamins and minerals in the appealing gummy form – whether based on gelatine or plantbased alternatives.
AN EXCIPIENT THAT TICKS MANY BOXES
There is a broad range of excipients on the market, but it can be difficult to find a multifunctional solution that combines several properties, such as excellent flowability, low hygroscopicity
and chemical stability to name a few. In addition, compliance is key – medicines that taste good can considerably increase acceptance by certain patient groups.
Choosing the right filler-binder can enhance the overall taste and mouthfeel of pharmaceutical and nutraceutical products and thus improve compliance. All galenIQ™ grades are physically and chemically stable, non-hygroscopic and enhance the palatability of the final form. galenIQ™ is available in South Africa from Savannah Fine Chemicals. •
BENEO – www.beneo.com/ pharmaceutical-excipients
Savannah Fine Chemicals –www.savannah.co.za
 Minitablets are easy to administer with a diameter ranging from 1mm to 4mm (©funnyangel/Shutterstock)
Minitablets are easy to administer with a diameter ranging from 1mm to 4mm (©funnyangel/Shutterstock)
among consumers
WWW.PHARMACOS.CO.ZA // MARCH 2024 43 PHARMA FOCUS/EXCIPIENTS & API s
A pioneer in excipient manufacturing
In the dynamic landscape of pharmaceuticals, Nitika Pharmaceutical stands out as a leader in excipient manufacturing, contributing significantly to the industry’s global footprint. As a leading Indian pharmaceutical company, Nitika exports its high-quality products to over 90 countries, leveraging a robust network and distribution channels.
At the heart of Nitika’s success is an unwavering commitment to customer satisfaction. The company focuses on delivering topnotch products at a reasonable cost, supported by timely deliveries and dedicated after-sales support. The excipient manufacturer’s dedicated experts, coupled with a proven track record and sophisticated instruments, underscore its commitment to excellence.
STATE-OF-THE-ART FACILITY
Nitika Pharmaceutical recently inaugurated India’s largest manufacturing plant, specialising in customised excipients and pre-mixes. With a yearly installed capacity of 17 000mt and automated cuttingedge technology, this facility marks a significant leap in production capabilities.
MICROCRYSTALLINE CELLULOSE
A flagship product of Nitika, spray dried Tabcell microcrystalline cellulose is available in various grades (101, 102, 112, and 200). Its key features include excellent flowability, free-from black particles, consistent quality (maintained by Nitika MCC 101), superior whiteness index, and outstanding compressibility.
These attributes ensure a seamless tablet manufacturing process and a visually appealing final product.
Did you know?
The versatility of microcrystalline cellulose as an excipient lies in its various applications and the ability to modify its physical parameters. The wide variety of bulk and spraydried grades allows for tailoring microcrystalline cellulose to achieve a desired activity, making it a valuable tool in pharmaceutical formulation development.
MAGNESIUM STEARATE
Nitika’s Tablube, holding the first position in the Asian market and the second globally, is a vegetable-source excipient with USDMF and RSPO certification. Noteworthy features include specific particle size distribution and surface area, high whiteness index (>90%), high batch-to-batch consistency, and reproducible bulk density. Tablube empowers formulators to achieve precision and consistency in their formulations.
SODIUM STEARYL FUMARATE
A specialised tablet lubricant, Novalube sodium stearyl fumarate addresses formulation and manufacturing challenges often encountered with other lubricants. Its advantages include minimal impact on dissolution and disintegration rates, robustness to over-lubrication, high API compatibility, and ease of manufacturing. These benefits make Novalube an ideal choice for tablet formulation, aligning with Nitika’s commitment to innovation and customer satisfaction.

DIVERSE RANGE AND REGULATORY COMPLIANCE
Nitika Pharmaceutical also offers a range of antacids, actives and mineral salts, ensuring compliance with pharmacopeial standards. The company provides comprehensive support for regulatory requirements, reflecting its vision of ensuring a ‘happy customer’ through the delivery of topquality pharmaceutical products.
Nitika Pharmaceutical's journey is marked by innovation, dedication and a relentless pursuit of excellence. These and other Nitika pharmaceutical excipients are available in South Africa from CJP Chemicals. •
CJP Chemicals – www.cjpchemicals.co.za Nitika Pharmaceutical –nitikapharma.com
Microcrystalline cellulose
44 MARCH 2024 // WWW.PHARMACOS.CO.ZA PHARMA FOCUS/EXCIPIENTS & API s
Magnesium stearate
OUR PRODUCTS




NITIKA provides a wide range of high quality pharmaceutical excipients across the spectrum of the pharmaceutical industry worldwide.
NITIKA is one of the largest exporter of excipients to a diverse number of markets in South Asia, Middle East, US.









We have the fully formulated and quality products to assist all pharmaceutical industry in the development of high quality products.


































www.nitikapharma.com

Liza Viviers Tel: 011-494-6700 • Email: lizav@cjpchemicals.co.za • Web: www.cjpchemicals.co.za
Solubility enhancement strategies
For decades, poor drug solubility has remained one of the biggest hurdles in pharma product development. About 40% of marketed drugs belong to class II and IV and about 80% of pipeline drugs exhibit low solubility.1 Low drug solubility and permeability negatively affect bioavailability, dosing frequency, drug effectiveness, and ultimately patient compliance. IMCD explores how to break through one of drug development’s toughest barriers.
TThere are numerous approaches available tackle the challenge of poor drug solubility. Some of the most common solutions can be grouped into the following categories:
• Physio-chemical modification of the API – salt formation, prodrug and particle size reduction (micronisation, nanomilling)
• Use of dispersion technologies like hot-melt extrusion or spray drying
• Complexation, co-crystals
• Lipid-based approach, self-emulsifying systems, SMEEDS, or SEEDS2
• Nanotechnology using micelles, vesicles, liquid crystals, dendrimers and nano-capsules
• Use of surfactants, solubilisers, cosolvents and amorphous solid dispersions (ASDs). Some technologies offer an easier approach to overcome the challenge, whereas others are more complicated. The main task for formulators is to find the right solution for their drug and application.
Excipient-based approaches are an attractive option. Classical solubilisers like glycol-based surfactants are usually top contenders as they offer versatility for both oral and parenteral applications. Some orally applied polymers like polyoxyethylene and poloxamers are troublesome as they have low melting temperatures. Other
commonly used polymers, like povidone and copovidone, have limited solubilisation capacity for numerous actives. 3
AN ALL-ROUND SOLUTION
In recent years, ASDs have emerged as a preferred method to increase the aqueous solubility of drugs. ASDs fundamentally consist of an amorphous active ingredient stabilised by a polymer. Amorphous compounds are those that lack a uniform structure or order, making them more readily available to the body. They are however unstable, which is why a stabilising polymer is needed to offer solubility enhancement. 4
Some of the most common excipients used in ASD are polyvinylpyrrolidone (PVP), vinylpyrrolidone/vinyl acetate copolymers (PVP/VA), hydroxypropyl methylcellulose (HPMC), hydroxypropyl methylcellulose acetate succinate (HPMC-AS), and polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol. The latter is a novel tri-block polymeric solubiliser, which has amphiphilic properties. It has excellent solubilising efficiency for hydrophobic drugs along with an inherent property to provide sustained release. Having hydrophilic and non-ionic properties, its solubility does not change throughout the gastro-intestinal tract (GIT). Moreover,
it has a slightly surface-active property, which helps to maintain the super saturation position form for poorly soluble drugs in the GIT. Therefore, this polymer is increasingly becoming sought after for solid dispersions.
OPEN TO INNOVATION
Excipients have a key role to play in maximising the bioavailability of poorly soluble actives. Yet there is no ‘one-sizefits-all’ solution capable of solving each formulation challenge. Every formulator needs to evaluate the available options and choose the right one for their drug. Today, the pharma industry has some interesting challenges that need modern and innovative solutions. Luckily, excipient manufacturers have plenty of ground-breaking and novel excipients to tackle these issues.
Accepting and employing novel approaches can potentially provide intellectual property advantages and positively affect cost and complexity in the long run. IMCD can assist with its wide range of highly effective solubilisation excipients. The distributor partners with leaders in optimising the bioavailability and solubility of challenging APIs. IMCD’s solutions enable effective solubilisation and bioavailability in various dosage forms – from solid dispersions to lipid-based drug delivery systems and soft gels. •
REFERENCES:

1. https://drug-dev.com/special-feature-excipientsenhancing-the-new-poorly-soluble-apis/
2. Singh, Bhupinder & Bandyopadhyay, Shantanu & Kapil, Rishi & Singh, Ramandeep & katare, Om. (2009). Self-Emulsifying Drug Delivery Systems (SEDDS): Formulation Development, Characterization, and Applications. Critical reviews in therapeutic drug carrier systems. 26. 427-521.
3. Wessel M, Reynolds T, Konagurthu S, Crew M. Solubilization technology- How to Choose the Right Solubilization Technology for your API, Drug development and delivery, 2015.
4. Hardung, Djuric, Alic - Combining HME and solubilization Soluplus, the solid solution, Drug Delivery Technology 10(3):20-27 2010
IMCD South Africa – www.imcdsa.co.za IMCD’s solutions enable effective solubilisation and bioavailability in various dosage forms
PHARMA FOCUS/EXCIPIENTS & API s
46 MARCH 2024 // WWW.PHARMACOS.CO.ZA

Looking to develop innovative products for a healthier world?
IMCD Pharmaceuticals
Whether you are looking to improve an existing formulation, or innovate to find a new solution, our technical experts are here to help. With a global network of six labs, we assist you at every stage of your product development, providing unparalleled solutions with the future always front of mind.
We are your end-to-end partner in the following markets:
• Active Pharmaceutical Ingredients
• Excipients
• Nutraceuticals
• Regulated Synthesis
• Biopharma
• Agrochemicals
Let’s collaborate and advance ideas for a healthy future. Contact us today!
IMCD South Africa
275 Oak Ave
Randburg
W: www.imcdsa.co.za

Imagine a world where beauty thrives without causing harm
The winner of this year’s Coschem cosmetic science training/P&C Review essay prize is Vishay Ramdhav. To acknowledge Vishay’s achievement, his essay on the topic “the ongoing ethical dilemma surrounding animal testing in the cosmetics industry and the push for cruelty-free alternatives”, is published in this student focus. Vishay has also received a prize sponsored by P&C Review.
According to Mahatma Gandhi, “A nation's greatness and moral progress can be judged by the way its animals are treated”.
In the vast realm of beauty and self-care, a swirling ethical dilemma persists, casting a shadow over the cosmetics industry. Animal experimentation, a contentious practice that has long sparked debate and deep inquiries about our moral responsibility to the creatures who inhabit our world, is the topic at hand.1
With the quest for cruelty-free alternatives gaining momentum, this essay explores the ongoing ethical conundrum surrounding animal testing in the cosmetics industry, the relentless drive for a change towards a kinder, more compassionate approach, and the growing momentum for cruelty-free alternatives.
Store shelves are decorated with colourful displays of lipstick, dazzling eyeshadow palettes and velvety foundations. These products can improve our looks, raise our
confidence, and give us a short sight of our own beauty.2 Yet beneath the beauty a darker reality exists, one in which animals suffer for aesthetic research. Animal testing has been used by the cosmetics industry for many years to guarantee the efficacy and safety of its products. The justifications provided for these actions include the pursuit of human welfare, arguing that animal testing is necessary to protect consumers from potential harm. Regulatory agencies, guided by these principles, have mandated these tests as a prerequisite for cosmetic product approvals.
The cosmetic industry has embarked on a journey of contemplation and discovery, paving the way for a more ethical and compassionate industry.
A CLOSER LOOK AT THE SPECIFICS
For many people, cosmetics are a part of everyday life, from shampoo and toothpaste to a full face of makeup.3 These products make us

covered in Karyn Siegel-Maier's piece Guilt free Beauty 3 The author claims that Erasistratus of Alexandria documented the mutilation of living animals to examine the body as early as the third century B.C.
" The concept of cruelly injuring animals for human gain has been around for far too long"
The concept of cruelly injuring animals for human gain has been around for far too long. The preferred testing animal is a rodent. They can be purchased from breeders or intentionally bred by scientists.
It might be challenging to determine how much discomfort an animal is experiencing.4
Animals are forced to consume drugs, inhale chemicals, have chemicals applied to their skin, or injected into them during these examinations. After more observation and testing, the species are frequently killed so scientists can collect data.
Genetic alteration is also a typical animal experiment. For instance, specified traits are being removed or inserted into the DNA of mice or other species as they are reproduced. These characteristics are recognised to play a key role in human illnesses. Far from being harmless, many animals perish while they are still infants because the foreign features they are given are too extreme in comparison to their own DNA.5
THE CONTROVERSY CONTINUES
Animal testing in the cosmetics industry has always been a controversial topic. While some believe it plays a vital role in the development
48 MARCH 2024 // WWW.PHARMACOS.CO.ZA STUDENT FOCUS


and safety of cosmetics, it seriously violates the survival rights of experimental animals.
Utilitarianism and Kantian theories (deontological ethics) are both universal moral theories and rely on the use of reason.6 Utilitarianism advocates the pursuit of maximum happiness. Happiness involves not only those involved in the act but also everyone affected by it. Animals experimented on in the cosmetics industry suffered great pain and sadness, which means that the cosmetics industry goes against the morality of utilitarianism. Therefore, animal experiments conducted by the cosmetics industry are unethical.
Kantian is very different from the utilitarianism theory in that Kantian believes that whatever the outcome, at least some actions are right or wrong. In this case, animal testing is justified in the cosmetics industry, mainly because it helps protect consumers. From a Kantian point of view, animal testing in the cosmetics industry is both moral and beneficial.7
Yet there is a glimmer of hope with industry facing a paradigm shift set about by regulatory agencies because of accumulating evidence and public pressure. 8 Additionally, consumers themselves have emerged as powerful catalysts for change. Alternative testing techniques now offer accurate, dependable and cruelty-free options thanks to scientific developments. The use of in vitro testing, recreated human skin models, and computer simulations have the potential to replace animal testing while still verifying the efficacy and safety of cosmetics. Advancements in scientific methods and tools have challenged this notion. In vitro techniques, such as testing on human cell cultures, have shown promising results and are considered more reliable than
animal testing. Human tissue samples and human volunteers also play a crucial role in safety testing. In advanced stages of the testing process, human volunteers can replace animals in specific studies. For instance, skin sensitivity testing of cosmetics often relies on work conducted with human volunteers. This approach has become commonplace.9
CONSUMERS AND REGULATORS DRIVE CHANGE
The ethical implications of animal testing are becoming more widely understood, and there is a growing market for cruelty-free goods, which is encouraging brands to review their procedures.10 According to market trends, ethical consumers are voting with their money, promoting the growth of cruelty-free brands and putting pressure on businesses to completely stop using animals in research. We must face the difficulties that lie ahead as we negotiate the complex terrain of the moral conundrum surrounding animal testing. The constraints of science and technology, the requirement for worldwide regulatory harmonisation, and the importance of activism and education all influence the direction of this on-going discussion.11 Nevertheless, despite these obstacles, there is a definite impetus for change and the vision of a cruelty-free cosmetics industry beckons us towards a more compassionate future.12
The European Union banned animal testing for cosmetic purposes in 2013, while countries like India, Israel, and Norway have implemented similar measures.13 These regulatory shifts signal the landscape is changing.
Worldwide, companies have made significant progress in reducing or eliminating animal testing, thanks to funding research for alternative methods and the safety
of commonly used cosmetic ingredients. However, challenges remain in some parts of the world. China, a crucial market for cosmetics, requires animal testing for all products sold within its borders. This means that companies with cruelty-free policies in Europe or the US must submit their products for animal testing in Chinese laboratories if they want to do business there.14
"Companies have made significant progress in reducing or eliminating animal testing"
Raising consumer awareness and advocating for transparency in company practices are essential for promoting change. Engaging in dialogue with companies operating in countries that still allow animal testing can also be helpful. Although progress is being made, achieving a completely cruelty-free cosmetics industry requires continued scientific advancements and advocacy efforts.
Recently, more than 30 countries and governments across the world have pushed the need for innovation within the cosmetics industry by passing through restrictions and bans on animal testing in the production of cosmetics.15
AN ENVIRONMENTAL DILEMMA
Research within the field of cosmetics shows that natural cosmetics have become a major trend, due to an increased concern for health and the environment.16,17
In a survey on the purchasing habits of 15 000 women when it comes to cruelty-free cosmetics, 36% answered that they only buy from beauty brands that are cruelty-free.18
Consumers concerned about animal cruelty also use the term ‘vegan’ for ethical products. Veganism can be defined as ‘a philosophy and way of living which seeks to exclude – as far as is possible and practicable – all forms of exploitation of, and cruelty to, animals for food, clothing or any other purpose’.19
However, the topic of veganism and crueltyfree often evokes strong and emotional reactions and are more sensitive compared to green and organic, which are topics that have been more frequently studied previously.20
The pressure for cosmetic companies to invest in alternative methods for animal testing is being altered by many different actors such as policymakers, industry professionals, and most importantly individual consumers.21 Additionally, acknowledgment images/
STUDENT FOCUS WWW.PHARMACOS.CO.ZA // MARCH 2024 49
Shutterstock.com

of the implications of animal-testing on the environment is imperative. Every year millions of animal carcasses used in research laboratories are discarded and contaminated with toxic and hazardous chemicals. This waste of animal bodies and tissue has the most obvious impact on the environment. 22
While alternative testing methods show promise, they are not without limitations. Some argue that these methods may not fully replicate the complex interactions and physiological responses of living organisms. Further research and development are needed to refine and validate these alternative approaches.
Achieving global harmonisation in regulations and standards for cruelty-free testing poses a significant challenge. Varying regulations across countries can create barriers to the adoption of alternative testing methods and hinder the progress towards a cruelty-free cosmetics industry. 23
Promoting awareness and education about the ethical implications of animal testing is crucial to effecting meaningful change. Increased advocacy efforts by animal welfare organisations, scientific communities, and consumers can drive further momentum for cruelty-free alternatives.
While challenges and limitations persist, it is evident that the cosmetics industry is moving towards a future where ethical considerations are paramount. By embracing cruelty-free alternatives, the industry can uphold its commitment to both human safety and animal welfare, creating a more sustainable and conscientious approach to beauty.24
WHERE BEAUTY AND KINDNESS MERGE
If humans had made the decision a century ago to cease using animals in scientific and medical research, the world we live in today would be very different. Many of us owe our existence to the fact that we or our parents did not succumb to diseases that have been controlled through knowledge gained from animal research. The understanding of genetics, insights into brain functions, and the ability to comprehend new diseases like
HIV/Aids would not have been possible without the biological information obtained from such research. Even the animals we keep as pets or raise for food would lead shorter, less healthy lives, as many vaccines and treatments that are now essential in veterinary medicine would never have been developed.25
By looking into common tests, the animals after the experiments, and the initial results gained, one can say that changes should be made so that animals going through medical experiments and/or product testing experience the least amount of suffering as possible, or completely stop the practice.
"Animals can’t be fully relied on for testing because they don’t get a significant number of human ailments"
The Ethics of Research Involving Animals addresses the intricate and multifaceted ethical considerations surrounding the use of animals in scientific research. The book explores various perspectives, ranging from the necessity of animal research to the ethical responsibilities of researchers, and the implications for animal welfare. One of the central themes of the book is the crucial role that animal research has played in advancing scientific knowledge and contributing to human and animal health.
Throughout its exploration of the ethics of research involving animals, the book emphasises the complexity and nuance of the subject. It recognises the necessity of scientific advancement, the responsibility to minimise animal suffering, and the ongoing need for ethical considerations. The author encourages ongoing dialogue and engagement among scientists, ethicists, policymakers, and the public to continue refining and improving the ethical framework surrounding animal research. 26
In the end, most animal testing has a negative impact on human wellness and makes flawed contributions
to medical advancements. Whether they are mice or monkeys, illnesses that are artificially induced in lab animals are not the same as those that naturally occur in people. Furthermore, it becomes increasingly unlikely that experiments on creatures would produce results that can be accurately translated and applied to the human condition in a substantial way because creature species naturally differ from one another from different angles.27
Crucially, the power of consumers cannot be underestimated. Their increasing demand for cruelty-free products continues to shape the market, compelling cosmetic companies to adopt ethical practices and phase out animal testing. Consumer awareness, education and advocacy play a pivotal role in driving change and fostering a more compassionate cosmetics industry. While challenges and limitations persist, the trajectory is clear. The cosmetics industry is evolving, propelled by ethical imperatives and scientific progress. By embracing cruelty-free alternatives, the industry not only upholds its commitment to human safety but also demonstrates a profound respect for the lives of the animals that inhabit our world. Animals can’t be fully relied on for testing because they don’t get a significant number of human ailments.
It is imperative to acknowledge that the journey towards a cruelty-free cosmetics industry is ongoing. Global harmonisation of regulations, continued scientific advancements and sustained consumer advocacy remain crucial. The transformation we seek requires collective effort, commitment, and a shared vision of a world where beauty thrives without causing harm. In the end, the ethical dilemma surrounding animal testing in the cosmetics industry urges us to confront our values, our responsibilities, and our capacity for change. It calls for a shared determination to forge a future where compassion and progress walk hand in hand – a future where beauty and kindness are inextricably entwined.
* References available on request
ABOUT THE AUTHOR
Vishay Ramdhav is the R&D technical manager at Sachet Manufacturers. He is an astute, diligent, motivated and enthusiastic chemical engineer who has recently been associated with the Society of Cosmetic Chemists South Africa as a professional scientist. Vishay is currently pursuing an advanced diploma in Cosmetic Science, covering a wide range of disciplines in this developing industry as it becomes more technologically advanced and focused.


STUDENT FOCUS 50 MARCH 2024 // WWW.PHARMACOS.CO.ZA
image/ Shutterstock.com

A year of achievements and exciting opportunities
Reflecting on the past year, it’s evident the collective efforts of Coschem members have yielded remarkable and inspiring results. This was highlighted at the Society’s AGM on 8 February which was hosted as a virtual event.
The Society of Cosmetic Chemists (Coschem’s) outgoing president, Jacques Strydom expressed his heartfelt gratitude to members, committee members and the council for their invaluable contributions and unwavering support throughout 2023. Under his leadership, the society has seen significant growth with membership numbers surpassing 500 – a target Jacques aimed to achieve. Coschem’s flourishing state is also attributed to the unwavering support of its corporate sponsors, who were duly thanked for their commitment.
As part of the constitutional requirements, several committee members, including Liesl Keulder, Charis Lewis and Sapphirah Phala stepped down from council this year, making way for new members to join and contribute fresh perspectives to the society’s governance. Jacques also handed over the role of president to Johrinda Nel.
STRONG FISCAL HEALTH
The 2023 annual report and financial statements, showcasing Coschem’s fiscal health and efficiency, are for download on the Society’s website.
Ettiene Retief of FTR Accounting Services provided an overview of the annual financial statements, highlighting Coschem’s robust financial position. Through prudent financial management and dedicated efforts, Coschem has not only met its financial targets but also generated a surplus. This surplus presents an opportunity to advance the Society’s mission and support members in meaningful ways, with funds allocated towards hosting the IFSCC 2029 Congress.
A PROMISING YEAR AHEAD
In her closing remarks as incoming president, Johrinda expressed gratitude to Jacques for his dynamic leadership and outlined her vision to focus on connections in 2024. She emphasised plans to provide guidance and support to regional chapters, fostering a sense of unity and collaboration among members.
As Coschem looks ahead to the future, the stage is set for a year of growth, success and vast possibilities. With a strong foundation and the unwavering dedication of its members, the Society is poised to continue making strides in the field of cosmetic science. •
Coschem – www.coschem.co.za
2024 COUNCIL MEMBERS AND OFFICE BEARERS




Did you know?
South Africa secured the hosting rights for the prestigious IFSCC 2029 Congress. This exciting news is a testament to Coschem’s growing influence and reputation on the global stage.










ASSOCIATION NEWS
WWW.PHARMACOS.CO.ZA // MARCH 2024 51
Marcel van Rooyen, vice president and chair social committee
Wayne van Wyk, co-opted scientific committee
Lee-Ann Raaff, new council member
Anton Weber, Cape Town chapter chair
Kelleen David, new council member
Jacques Strydom, immediate past president
Stephanie Gompel-Ratsiane, co-chair education committee
Ivor Zwane, honorary secretary
Kudzai Kahle Gwazira, education officer
Yurita Boodhram, chair PR committee
Beverley Claire Gardner, coopted scientific committee
Erica de Kock, honorary treasurer
Johrinda Nel, president and chair membership committee
Ann-Tarlia Moodley, new council member (chair KZN chapter)


Plastics industry responds to SONA
Plastics SA has issued a response to President Cyril Ramaphosa’s State of the Nation
Address (SONA) delivered on 8 February, stating that the impact of many of the obstacles he discussed are profoundly felt by the plastics industry.
It was stated that government has taken certain steps to address the youth unemployment challenge in the country. Despite this, our unemployment rate is the highest it has ever been.
In response, Plastics SA executive director, Anton Hanekom comments: “The plastics industry was identified as a priority sector for the South African economy as it provides employment to 60 000 people. Yet the recent economic downturn, energy crisis and labour challenges are forcing many plastics manufacturers and recyclers to downscale their operations. Immediate and drastic measures are needed to protect and restore the entire manufacturing sector, but especially to revitalise the competitiveness of the plastics sector.”
He also urged government to extend the RAF fuel rebate offered to food manufacturers on diesel for generators to include plastic packaging manufacturers.
"It is important to recognise the crucial role plastics play in the future of South Africa"
The industry continues to provide cutting edge, hands-on training in the latest manufacturing and leadership techniques that not only address our unemployment crisis, but also help to stimulate economic growth and ensure competitiveness.
IMPROVING INFRASTRUCTURE
Plastics play a crucial role in modern infrastructure, offering durability, versatility and cost-effectiveness in various construction applications.
“HDPE and PVC pipes are commonly used for water distribution and sewage systems
due to their corrosion resistance and longevity. Plastics such as polystyrene and polyurethane are employed in insulation, enhancing energy efficiency in buildings. The lightweight nature of plastics facilitates easier transportation and installation, reducing labour costs and environmental impact,” Hanekom added.

The plastics industry eagerly awaits the commencement of infrastructure projects highlighted during SONA to provide products that will contribute to cost effectiveness, durability and a low carbon footprint.
PROTECTING THE ENVIRONMENT
Finding solutions to South Africa’s pollution problem and protecting the environment are key focus areas for governments and plastics industries worldwide. Hanekom reiterated that plastics have an important role to play in combatting climate change.
“Numerous studies and life cycle analyses have proven that when plastics are collected and recycled as part of a circular economy, they have a smaller environmental footprint compared to other packaging materials. They can be re-used many times over,” he explained.
Plastics SA is relentless in its efforts to promote and educate end-users on recycling, supporting the industry with the development of end-markets and working with local and national government to improve waste management and collection systems countrywide.
DEALING WITH CLIMATE CHANGE
Government has had to confront the effects of climate change, and in this year’s address the President highlighted plans to invest in green
energy. He invited the private sector to participate in the expected boom that will be generated by green hydrogen energy projects.
“Hydrogen is viewed as a promising alternative to fossil fuel, but the methods used to make it either generate too much carbon dioxide or are too expensive. It is exciting to note, however, that researchers have recently found a way to harvest hydrogen from plastic waste using a low-emissions method, which could more than pay for itself and could provide a win-win solution for us,” Hanekom said.
OTHER GROWTH SECTORS
There were other areas of focus and investment singled out during SONA where plastics have an important role to play.
“Whether it is stabilising the country’s energy supply, fixing logistical problems or boosting electric vehicle manufacturing, plastics are key in every area.”
While concerns about plastic pollution are valid, it is important to recognise the crucial role plastics play in the future of South Africa. With proper waste management and recycling initiatives, these issues can be effectively mitigated, allowing for the continued utilisation of plastics in various sectors.
The industry’s willingness to cooperate and support national objectives underscores the potential for positive change. However, it is essential for political will and decisive action to align with these efforts to steer the country and its economy towards growth and prosperity. Addressing plastic pollution while harnessing the benefits of plastics responsibly will see South Africa forge a sustainable path for generations to come. •
ASSOCIATION NEWS Plastics SA – www.plasticsinfo.co.za
PRESS RELEASE FOR IMMEDIATE RELEASE PLASTICS INDUSTRY RELEASES LATEST RECYCLING FIGURES Johannesburg, 22 November 2021 Plastics SA, the umbrella body representing the local plastics industry, has just released the official plastic recycling statistics for the year ending 31 December 2020. Each year, data is collected from plastics recyclers around the country by Plastix 911 on behalf of Plastics SA. PETCO provides figures from their listed PET recyclers, whilst raw material suppliers Sasol and Safripol provide input on the production and domestic demand of plastics raw materials. P astic ndustry market sectors: South Africa’s plastics industry is dominated by the packaging sector (which accounts for roughly 52 % of the local market), followed by building & construction (13 %), agriculture (9 %), automotive and transport applications (7 %) Due to increased awareness of hygiene caused by the outbreak of the Covid-19 pandemic, the demand for flexible packaging increased by 2 % in 2020 Demand for rigid packaging (linked to on-the-go meals, PET beverage bottles and take-away containers) shrunk, although packaging used for domestic- and personal care increased due to the greater emphasis on cleaning and the increased demand for hand sanitisers. Packaging sheeting was also used to manufacture face shields locally. Consumpt on of virgin and recycled plast cs n SA South Africa, like most countries around the world, witnessed a decline in collection and recycling rates during 2020, compared to pre-COVID-19 rates. In addition, many recyclers were unable to operate at full capacity for several months during the past year due to social distancing norms Other factors that adversely affected the plastic recycling activities include ongoing loadshedding, water shortages and high labour costs which forced many operations to scale down, or even close their doors permanently. South Africa converted 1 739 480 tons of polymer into plastics products during 2020 a decrease of 5.6 % from 2019. This is the total amount of locally produced polymers, imported polymers and recycled polymers sold to local convertors in South Africa, and excludes polymers exported, virgin and recycled. Locally recycled polymer represent ed 17 % of the total domestic consumption, a drop from 18.3 % in the previous year. Per capita consumption for locally converted plastics (virgin and recycled) decreased to 29 kg/person (down from 31 kg/person recorded in 2019). Per capita consumption for virgin material only, dropped from 26 kg to 24 kg. Virgin consumption has increased by 11 % since 2011, whilst recycled tonnages, locally converted, increased by 35 % over the same 10 year period. P astic recyc ing n SA South Africa recorded an nput recycl ng rate of 43.2 % during 2020. 461 500 tons of plastic waste were collected for recycling, of which 312 600 tons were successfully recycled back into raw materials 296 500 tons of recyclate were used to produce new products while 97 260 tons of recyclate were used to produce new packaging. more 2/…
52 MARCH 2024 // WWW.PHARMACOS.CO.ZA
image/ Shutterstock.com
Plastics SA executive director, Anton Hanekom
Shining a light on the pharmaceutical and cosmetic industry in Africa
Connect with us online and reach more buyers via P&C Review’s social media communities.
You can also register as a user on www.pharmacos.co.za to gain free and instant access to every digital edition of the magazine, which you can download in PDF format and save.
Subscribe to receive the P&C Review newsletter for industry news and other relevant and useful content delivered in your inbox.

1

1








2











2









095+ E-mail subs
Print and digital subscriptions
000+ (Jan-August 2022) Page views per month
925
76
LinkedIn
560+
followers
500+ Facebook followers



Tel: (010)745-7097 / (011)624-3493 Email: admin@mocopack.co.za | sales@mocopack.co.za www.mocopack.co.za 5 Sandvale Road, Isandovale, Edenvale, 1609 Frosting & Printing available We HAVE MOVED






























































































































































































































































































































 Minitablets are easy to administer with a diameter ranging from 1mm to 4mm (©funnyangel/Shutterstock)
Minitablets are easy to administer with a diameter ranging from 1mm to 4mm (©funnyangel/Shutterstock)

































































































