




◼ New treatments for alopecia areata, vitiligo expected to benefit many patients see page 4
by LOUISE GAGNON, Correspondent, The ChronicleNew research from the Mayo Clinic suggests patients with lower-limb lymphedema have twice the risk of developing skin cancer than patients without lymphedema, according to findings recently published in Mayo Clinic Proceedings Nov. 2023; 98(11):1653-1659.
 by LOUISE GAGNON, Correspondent, The Chronicle
by LOUISE GAGNON, Correspondent, The Chronicle
With the increasing diversity of Canada’s population, especially in urban centres, dermatology care specifically provided for skin of colour patients is addressing an unmet need. Toronto is a city rich in racial diversity with neighbourhoods such as Scarborough being home to many residents from cultural communities of colour. And Canada’s capital Ottawa is growing in diversity: according to 2021
census data, nearly one-third of Ottawa’s population is comprised of people who identify as visible minorities or racialized, a figure that was 26% in 2016.
 Dr. Reetesh Bose
Dr. Reetesh Bose
n Maintain a high index of suspicion when skin eruptions observed clinically
by LOUISE GAGNON, Correspondent, The ChronicleOne of the important factors to differentiate when faced with viral exanthems is whether the presentation is stemming from a herpetic or coxsackie infection, according to Dr. Catherine McCuaig, a pediatric dermatologist at St. Justine Hospital and a professor at the University of Montreal in Montreal. Speaking at the annual Pedi-
Clinics dedicated to skin of colour patients serve multiple purposes including managing a variety of patient conditions, providing an opportunity for medical students and residents to learn Please
Pediatrics page 18→
about various presentations of skin and hair conditions in patients with skin of colour, and generating interest in therapeutics for skin and hair conditions for which there may be few, if any, treatments, according to dermatologists serving racially diverse communities in Canada.
“It [a dermatology clinic serving patients with skin of colour] is just a fantastic way to really address skin problems in an underserved population and address wait times in an effective way while also teaching residents and learners and contributing to research,” said Ot-
The data stem from a retrospective investigation of 4,437 patients who had lower extremity lymphedema and 4,437 matched controls without lower extremity lymphedema at the Mayo Clinic in Rochester, Minn. from 2000 to 2020.
Dr. Afsaneh Alavi, a dermatologist at Mayo Clinic in Rochester and the study’s senior author, and colleagues looked at the time to development of the first skin cancer for each cohort and calculated the time to development of the first skin cancer for the lymphedema cohort and the non-lymphedema cohort.
Investigators reviewed electronic health records of every pa-




The Soluver products for wart treatment are formulated in a unique acrylic delivery system which eliminates the need for bandage covering while keeping occlusion.
Soluver Original minimizes irritation to the healthy skin surrounding the wart, locks in moisture with a durable, watertight covering and allows for superior penetration of salicylic acid.
Soluver Plus, with its higher percentage of salicylic acid, is ideally suited for resistant common or plantar warts.
Soluver Original is available in a 14 mL size while Soluver Plus is available in a 10 mL size; both include a brush applicator.
AVAILABLE AT SHOPPERS DRUG MART AND MOST DRUG STORES.
Clinics for SoC patients reach underserved communities
Increasing diversity in Canada’s population has led to the establishment of dermatologic clinics specifically for patients with skin of colour . . . . . . . . . . . . . . . . . . . . . . 1
More choices for challenging dermatologic conditions in 2024 Canadian dermatologists discuss new treatments expected to be available in 2024, including new approvals for hard-to-treat conditions such as alopecia areata 4
Vender on Psoriasis: Izokibep for the treatment of moderate-to-severe plaque psoriasis
Also, upadacitinib, a reversible JAK inhibitor, has shown efficacy and safety in patients with psoriatic arthritis who have had an inadequate response to other treatments, including biologic disease-modifying antirheumatic drugs. 8
In this issue’s postgraduate educational supplement, investigators performed a meta-analysis to assess the association between diabetes mellitus, hypertension, and psoriasis. A review of studies that met the inclusion and exclusion criteria found an association between psoriasis and diabetes and hypertension. . . 21
According to a recent study, guselkumab has shown significant improvements in skin clearance, scalp itch, and patient-reported health-related quality of life in skin of colour patients with moderate-tosevere scalp psoriasis.
Data from the study were presented at the Maui Derm Hawaii 2024 conference.
In the Phase 3B VISIBLE study, researchers evaluated data from 108 patients with scalp psoriasis, comparing treatment outcomes on guselkumab to placebo.
By week 16, patients treated with guselkumab reported greater improvements in the Psoriasis

February 2024 • Vol. 30 No. 1
Published eight times per year by the proprietor, Chronicle Information Resources Ltd., with offices at 1460 The Queensway, Suite 212, Etobicoke, Ont. M8Z 1S4 Canada. Telephone: (416) 916-2476; Facs. (416) 352-6199.
E-mail: health@chronicle.org
ISSN No. 1209-0581
Contents © Chronicle Information Resources Ltd., 2024 except where noted. All rights reserved worldwide.
The Publisher prohibits repro-
duction in any form, including print, broadcast, and electronic, without written permissions.
Printed in Canada. The Chronicle of Skin & Allergy is a Canadian publication. The Publisher certifies that advertising placed in this publication meets Revenue Canada requirements for tax deductibility.
Subscriptions: $85.60 per year in Canada, $129.95 per year in all other countries. Single copies: $10.00 per issue (plus 13% HST).
“There is a great unmet need for treatment for alopecia areata.”
Symptoms and Signs Diary and Dermatology Life Quality Index across all skin tones.
Patients also saw improvements in rates of complete scalp clearance, as well as milder effects of skin discoloration according to health-related quality of life data .
“These findings from VISIBLE are key to broadening our understanding of scalp psoriasis, and the roles [guselkumab] can have in treating all patients with this disease,” said Dr. Andrew Alexis, Lead Investigator and Vice Chair for Diversity and Inclusion at Weill Cornell Medicine in New York, at the Maui Derm Hawaii conference.
Medical Editor
Canada Post Canadian Publications Mail Sales Product Agreement Number 40016917. Please forward all correspondence on circulation matters to: The Chronicle of Skin & Allergy, 1460 The Queensway, Suite 212, Etobicoke, Ont. M8Z 1S4
Ideas in the Service of Medicinesm Affiliated journals of the Chronicle Companies include The Chronicle of Cosmetic Medicine + Surgery, Women in Dermatology, Pediatric Chronicle, and Linacre’s Books/ Les Editions Linacre


Dr. Charles Lynde, Director of the Lynde Institute for Dermatology, Markham, Ont.

In this issue of THE CHRONICLE OF SKIN & ALLERGY, I’d like to give a big shout out to Dr. Brittany Waller, Assistant Professor of Dermatology at the University of Saskatchewan and a dermatologist with Origins Dermatology in Regina. She certainly nailed it when it comes to the Therapeutic Update (see page 4) and I quote “the dermatology therapeutic landscape is exploding ... We are constantly seeing favourable clinical trial data relating to novel targeted therapies for a variety of skin conditions, many of which have previously been resistant to conventional treatments.” I must add that many of these conditions have had no effective or approved therapies.
This therapeutic onslaught is led by our new best friend ‘JAK Inhibitor’ which is showing efficacy not only in severe atopic eczema but also in vitiligo and alopecia areata. As you know there are multiple JAKs available both oral and topical, either on the market or soon to be approved. Another excit-
Please turn to Message page 10→
Wayne Gulliver, MD, FRCPC Editor, Cosmetic Dermatology
John P. Arlette, MD, FRCPC
Benjamin Barankin, MD, FRCPC
Marc Bourcier, MD, FRCPC
Eric Goldstein, MD, FRCPC
Peter Hull, MD, FRCPC
Richard Langley, MD, FRCPC
Danielle Marcoux, MD, FRCPC
R.A.W. Miller, MD, FRCPC
Sheldon V. Pollack, MD, FRCPC
H. Eileen Murray, MD, FRCPC
Kim Papp, MD, FRCPC
Yves Poulin, MD, FRCPC
Melanie D. Pratt, MD, FRCPC
Denis Sasseville, MD, FRCPC
Jerry Tan, MD, FRCPC
Ronald B. Vender, MD, FRCPC
Founding Editor Colin A. Ramsay, MD, FRCPC (1936-2003)
Publisher Mitchell Shannon
Editorial Director R. Allan Ryan
Senior Editor John Evans
Assistant Editor Jeremy Visser
Joyce Pitter-Hinds
Comptroller Rose Arciero
n R READER SERVICE: To change your address, or for questions about your receipt of the journal, send an e-mail to health@chronicle.org with subject line “Circulation,” or call during business hours at 416.916.CHROn (2476), or toll-free at 866.63.CHRON (24766).
n L LETTERS: We welcome your correspondence by mail, fax (416.352.6199), or e-mail. Kindly use the co-ordinates listed above.
n A ADVERTISING: For current rates and data, please contact the publisher.
n R REPRINTS: The content of this journal is copyrighted. Please contact Mitchell Shannon for reprint information.

Approvals of therapies for hard-to-treat conditions such as alopecia areata are encouraging news for Canadian dermatologists in 2024.
“The dermatology therapeutic landscape is exploding.” declared Dr. Brittany Waller, Assistant Professor of Dermatology at the University of Saskatchewan and a dermatologist with Origins Dermatology in Regina.
“We are constantly seeing favourable clinical trial data relating to novel targeted therapies for a variety of skin conditions, many of which have previously been resistant to conventional treatments.”
The Health Canada approval of baricitinib, an oral Janus Kinase (JAK) inhibitor, at the end of Jan. 2024 for the treatment of alopecia areata is a major stride, according to Dr. Waller. “It [alopecia areata] is a condition that can have a significant psychosocial impact on the patient.”
Dr. Charles Lynde, Director of the Lynde Institute for Dermatology in Markham, Ont. agreed that the availability of baricitinib, which closely followed the Health Canada approval of ritlecitinib in Dec., 2023, will help to fill a void in therapeutics for alopecia areata.
“There is a great unmet need for treatment for alopecia areata,” said Dr. Lynde, noting the approvals of ritlecitinib and baricitinib. “We have had methotrexate and other immunosuppressants that have been used, but have not had very good success with them.”
Dr. Gurbir Dhadwal, a dermatologist




at Guildford Dermatology in Surrey, B.C., and at St. Paul’s Hospital in Vancouver, shared similar sentiments with regard to emerging therapies for alopecia areata. “It’s exciting to have these JAK inhibitors [baricitinib and ritlecitinib] available for alopecia,” said Dr. Dhadwal.

Novel treatments for alopecia areata are highly encouraging, agreed Dr. Ashley O’Toole, a dermatologist with the Skin Centre for Dermatology in Peterborough, Ont. “We were involved in trials with ritlecitinib for alopecia [areata],” she said.
Therapies for vitiligo and HS under study
Since alopecia areata and vitiligo are considered “sibling diseases”, it is


Continued from page 4
promising that there is hope on the horizon for patients with vitiligo, thanks to therapies like ruxolitinib, which may become available in Canada, said Dr. Dhadwal, who is also a clinical instructor at the University of British Columbia.
Some JAK inhibitors may offer benefit in more than one condition. A case in point is ruxolitinib, which is approved for use in atopic dermatitis and in nonsegmental vitiligo in the U.S., noted Dr. O’Toole. “It has more than one use,” she added.
Still another JAK inhibitor that may prove effective in more than one disease state is povorcitinib, which is being investigated as a possible treatment for nonsegmental vitiligo and as a potential HS therapy.
New potential use for roflumilast and new acne topical therapies
The topical therapy roflumilast, which received approval in Canada in 2023 for the management of psoriasis, is anticipated to become available for the management of seborrheic dermatitis, noted Dr. Waller.
“The expected approval of a topical PDE4 inhibitor [roflumilast] foam for seborrheic dermatitis will be a welcome addition to the treatment tool kit for this common, but bothersome, skin condition,” said Dr. Waller. “While this molecule is already showing promise in the treatment of psoriasis, this new [foam] formulation of roflumilast and new indication will also be very welcomed.”
Dr. Monica Li, Clinical Assistant Professor, Department of Dermatology and Skin Science, University of British Columbia, Vancouver, pointed out that the recent approval of roflumilast foam for seborrheic dermatitis in the U.S., which was approved for psoriasis in a cream formulation last year in Canada, may expand treatment options for seborrheic dermatitis.
“We are probably going to see approval [in Canada] at some point this year,” said Dr. Li. “Topical options in general are great because they are targeting affected areas in a very focal sense. For the most part,
if the side effects are localized, we are generally not worried about drug interactions. Topical options are user-friendly for the patient, and also relatively more affordable relative to systemics.”
Similarly, Dr. O’Toole expressed anticipation around the accessibility of roflumilast foam 0.3% for seborrheic dermatitis. “We are looking forward to it [roflumilast foam 0.3%], having seen the results in the clinical trial,” she said.
Canadian dermatologists are embracing a new therapy in the acne arsenal, clascoterone, which was approved for use last year. “I am finding a lot of use [for clascoterone] in people who are not necessarily adolescents,” noted Dr. Lynde. “They are a little older, about 20 to 30. It does not dry the skin and is specifically acting against hormones. It is well tolerated by patients.”
Another emerging acne therapy is a topical gel that features clindamycin phosphate 1.2%, adapalene 0.15%, and benzoyl peroxide 3.1%, which received U.S. FDA approval for patients aged 12 years and up. “It’s an exciting time for topicals,” said Dr. Li, referring to the approval of the triple combination acne treatment.
As there is the possibility of the U.S. FDA prohibiting formaldehyde and formaldehyde-releasing chemicals in haircare products, this potential ban may impact future products, according to Dr. Waller
“If this ban goes through in 2024, it will be interesting to see how many over-the-counter formulations change,” said Dr. Waller. “I also wonder if rates of contact dermatitis will decrease [as a result of the ban].”
Non-psroprietary and brand names of therapies: baricitinib (OLUMIANT, Lilly); ritlecitinib (LITFULO, Pfizer Canada); ruxolitinib (not approved for this indication); povorcitinib (not approved in Canada); roflumilast foam 0.3% (not approved in Canada); clascoterone cream 1% (Winlevi, Sun Pharma Canada); clindamycin phosphate 1.2%, adapalene 0.15%, and benzoyl peroxide 3.1% (not approved in Canada).
Please consult the Product Monograph at https://sunpharma.com/wp-content/ uploads/2023/08/Winlevi_Pm.pdf for important information about:
• Warnings and precautions including, only using PrWINLEVI® (clascoterone) externally; avoiding accidental transfer of WINLEVI® into eyes, lips, mouth, corners of the nose, or other mucous membranes; hypothalamic-pituitary-adrenal axis suppression; local irritation; susceptibility to systemic toxicity in pediatric patients; no available data on the use of WINLEVI® in pregnant women and no studies were conducted to determine the presence of clascoterone or its metabolite in human or animal milk
• Conditions of clinical use, adverse reactions, drug interactions and dosing instructions.
The Product Monograph is also available by calling our medical information department at: 1-844-924-0656
REFERENCE:
Current WINLEVI® Product Monograph, Sun Pharmaceutical Industries Limited.
© 2023 Sun Pharma, or its subsidiaries and affiliates. All rights reserved.
WINLEVI is a registered trademark of Cassiopea, S.p.A., used under exclusive license.
All other trademarks are the property of their respective owners.
INTRODUCING


The FIRST and ONLY TOPICAL anti-androgen indicated in the treatment of acne vulgaris*

INDICATED FOR THE TOPICAL TREATMENT OF ACNE VULGARIS IN PATIENTS 12 YEARS AND OLDER.




This phase II study, known as AFFIRM-35, assessed the dose response, efficacy, and safety of izokibep, an interleukin (IL)-17A inhibitor, in patients with moderate-to-severe plaque psoriasis who had inadequate responses to two or more standard therapies.
Published in the British Journal of Dermatology (doi.org/10.1093/bjd/ljad186), 109 adult patients were enrolled in the study and randomized into five groups: placebo, izokibep 2 mg, 20 mg, 80 mg, or 160 mg, administered every two weeks for 12 weeks. After this initial phase, patients initially receiving placebo were switched to izokibep 80 mg, and dosing intervals for all patients were adjusted based on Psoriasis Area and Severity Index (PASI) scores. The core study was followed by two optional one-year extension periods, resulting in a total study duration of three years.
The primary endpoint was the achievement of a 90% reduction in PASI score (PASI 90) at week 12. Results indicated that PASI 90 response rates increased in a dose-dependent manner, with rates of 0%, 5%, 19%, 71%, and 59% for the placebo, 2 mg, 20 mg, 80 mg, and 160 mg izokibep groups, respectively, at week 12. Rapid dose-dependent improvements were also observed across other efficacy outcomes.
During the placebo-controlled period, adverse events (AEs) in the izokibep groups were generally similar to placebo, except for mild injection site reactions. AEs were predominantly mild to moderate, and the drug was well tolerated.
Izokibep maintained its efficacy at higher dosage levels for up to three years, with no emergence of new safety signals. These findings suggest that izokibep is well-tolerated and effective in treating plaque psoriasis. Higher doses or more frequent dosing may further enhance response rates.
There could never be enough biologics to treat our patients as some come and some disappear depending on economics and adverse events associated with these medications. In this case there is a new interleukin 17 inhibitor called izokibep. This again shows the power of interleukin 17 inhibitors by having an extremely high PASI90 response with this drug. Because this is an early phase study, the dosing has not been finally determined. Although interleukin 23 inhibitors affect the master cytokine, interleukin 23, the end result is always reducing interleukin 17 which these interleukin 17 inhibitors, such as secukinumab, ixekizumab, bimekizumab, and brodalumab directly attack. Phase III studies will determine how izokibep reacts in the larger population.
Psoriatic arthritis (PsA) is a chronic, immune-mediated, inflammatory, musculoskeletal disease characterized by pain and stiffness due to inflammation of the peripheral and axial joints, enthesitis, and dactylitis, often accompanied by skin and nail psoriasis. Despite the availability of various pharmacologic therapies, some patients fail to achieve treatment targets, representing an unmet need. Upadacitinib, a reversible oral Janus kinase (JAK) inhibitor, has shown efficacy and safety in patients with PsA who have had an inadequate response to prior treatments, including biologic disease-modifying anti-rheumatic drugs (bDMARDs).
Published in Clinical and Experimental Rheumatology (2023 Nov; 41(11):2286-2297), the three-year re-
sults from the open-label extension of the randomized controlled phase III SELECT-PsA 2 study demonstrate the long-term safety and efficacy of upadacitinib in patients with PsA and an inadequate response to bDMARDs. In this study, patients were randomized to receive blinded upadacitinib 15 or 30 mg once daily (QD), or placebo for 24 weeks, followed by upadacitinib 15 or 30 mg QD. After 56 weeks, patients could enter an open-label extension where they continued their assigned dose of upadacitinib. The results showed that the efficacy of upadacitinib was maintained up to 152 weeks of treatment, with improvements in efficacy outcomes observed at week 56 sustained through week 152. The study also included a subanalysis of patients with inadequate response to tumor necrosis factor inhibitors (TNFis), and the efficacy outcomes in this subgroup were similar to those reported in the overall population. Upadacitinib was well tolerated throughout the longterm treatment, with no cumulative adverse effects observed through 152 weeks. The long-term safety profile of upadacitinib 15 mg was consistent with its known safety profile across indications, and no new safety signals were identified.
Upadacitinib is an extremely effective and popular medication for the treatment of psoriatic arthritis. The study shows excellent safety, and efficacy, as well as long-term durability. Unfortunately, there is minimal data on improvement of psoriasis of the skin which often precedes psoriatic arthritis. Higher doses may be needed leading to more potential side effects. However, the dose approved for psoriatic arthritis may still give improvement of skin psoriasis. This oral therapy and new mechanism of action can get great relief to patients who suffer from psoriatic arthritis, especially if they have failed other medications. However, it is not necessarily to be used as a stepwise approach, i.e., after failure of other medications, and can often be used first line.
We invite your comments and questions about this feature at www.derm.city. Dr. Ron Vender is a certified dermatologist with more than 30 years of clinical practice experience and over 100 clinical trials in psoriasis. He is founder and director of Venderm Innovations in Psoriasis, a center of excellence for Psoriasis offering a comprehensive management solution for individuals with psoriasis. www.psoriasis.vip

Skinimages.ca is a comprehensive and easily accessible digital database of dermatology and dermatopathology images categorized according to six skin phototypes.

You are invited and encouraged to share your diagnostic expertise by submitting images to skinimages.ca. We accept high quality images, of at least two megabytes. Please use jpg or tif files. This open-access resource aims to create an educational database to raise the standard of care for patients of all skin types, fostering inclusivity and equity in dermatologic education. Your contributions can play a vital role in achieving this goal. If you have questions, please contact skinimages.ca at admin@derm.city

Dr. Yvette Miller-Monthrope is a Toronto based dermatologist and dermatopathologist. She is an assistant professor of medicine at the University of Toronto and the medical director of the pigmentation disorders clinic at Women's College Hospital. She has special interests in diverse skin types, inflammatory dermatoses, curriculum development and clinical-pathological correlation.
SUPPORTED BY



Continued from page 1
tient with a skin cancer diagnosis manually to ensure they excluded cases where the skin cancer, its management, or both were a cause of lymphedema. Cancers that caused secondary lymphedema were excluded, and investigators were diligent about excluding any recurrence or recoding of the same cancer.
Finding highlights need for screening lymphedema patients
Overall, investigators found the lymphedema group had almost two times (1.98) the risk of skin cancer than the non-lymphedema group, a statistically significant difference that highlights lymphedema as a risk factor for skin cancer, according to Dr. Alavi. She noted the study underlines the importance of keeping skin cancer top of mind when dermatologists or other healthcare professionals see patients who have lymphedema of the lower legs.
“We all know that skin cancer is the most common type of cancer worldwide and is increasing in incidence,” said Dr. Alavi in an interview with THE CHRONICLE OF SKIN & ALLERGY. “There needs to be education for everyone who is managing patients with lymphedema, which would include members of a wound care team. In primary care, clinicians need to be aware that lym-

phedema is a risk factor for skin cancer. They need to know not to be afraid to do a biopsy because these are patients at higher risk for cancer. The earlier that we can detect cancer, we have a better outcome.
“What we noticed was not limited to angiosarcoma,” said Dr. Alavi, noting that the risk of angiosarcoma linked to lower extremity lymphedema has been described and is well-established and that squamous cell cancer (SCC), basal cell carcinoma (BCC), and melanoma were all studied and found to be more common in the lymphedema arm than the control arm.
The early detection of skin cancer in a lymphedematous skin is vital as it can allow for less invasive treatment options and decreased complication rates, explained Dr. Alavi.
“If there is lymphedema and skin cancer on top of that, there is poor wound healing because of the lymphedema,” said Dr. Alavi.
Investigators did not find that patient characteristics such as gender or race increased risk of skin cancer in lower extremities. The mean age of patients in both cohorts was 61.6 years.
Findings from subset of patients emphasizes link to skin cancer
Dr. Alavi and her colleagues also identified 24 patients with unilateral lower extremity lymphedema and lower extremity skin cancer. There were 21 cases of skin cancer in 24 lymphedematous legs vs. eight cases in 24 non-lymphedematous (control) legs.
Investigators also found in the subset of 24 patients that the lymphedematous lower extremity was significantly more likely to have one or more skin cancers than the non-lymphedema-
tous. Moreover, the lymphedematous lower extremity was 2.65 times as likely to have skin cancer develop than the non-lymphedematous lower extremity.
Still in the subset of patients, a total of 10 cases of lower extremity BCCs developed on the lymphedematous lower extremity, and one case of BCC developed on the non-lymphedematous lower extremity.
While the study is not prospective in nature, it does represent a substantial number of patients, said Dr. Alavi. “The weakness of our study is the retrospective nature, but I think the strength of our study is its large sample size thanks to our large database,” she said. “Conducting a prospective study would be very difficult in this condition because you would have to follow patients for decades to see if they develop skin cancer.”
Previous clinical experience generated interest in lymphedema
Dr. Alavi recalled that her clinical experience at Women’s College Hospital in Toronto had sparked her interest in the topic of lymphedema and its possible association with skin cancer. While at Women’s College Hospital, Dr. Alavi made an observation about skin cancer being more common in patients with lower extremity lymphedema.
“When I was in Canada and seeing patients at Women’s College Hospital, I saw a few lymphedema patients who had lesions on the leg,” said Dr. Alavi. “When I did biopsy the lesions, they came back as cancer. I remember speaking with our Mohs surgeon and asking about this. He thought it was a very interesting observation. This clinical question, about whether skin cancer is more common in the presence of lymphedema, remained on my mind.”
Continued from page 3
ing molecule is the PD4 inhibitor which has recently been approved for psoriasis with roflumilast soon coming for the treatment of seborrheic dermatitis as well as its present indication of psoriasis. On the acne front the topical clascoterone, which acts at the site of the pilosebaceous unit, has been noted to be well tolerated and effective in our acne patients. New combination therapies are also coming.
In Dr. Catherine McCuaig’s review of viral exanthems she notes that some of the JAK inhibitors may be associated with increased risk of herpes simplex virus infection when used to treat atopic dermatitis (see page 1). She notes this increased risk of herpes simplex virus infection has not been observed with baricitinib. Our attention to the care of skin of colour patients seems to be making a positive impact with clinics established, or soon to be established, in many Canadian communities including Ottawa and the Greater Toronto area. A skin of colour clinic has been in the works here in St. John’s Newfoundland & Labrador for the past few years and we’re hoping
that it too will open its doors to our ever-increasing diverse population.
For those of us who care for patients with lymphedema, Dr. Afsaneh Alavi’s novel and important observation of increased risk of skin cancer in these patients highlights the importance of regular follow up and biopsy in a group that has impaired wound healing (see page 1).
And for those dermatologists who provide the important service of office procedures, the use of inhaled low dose methoxyflurane is now returning to Canada. At Derm Update last fall in Toronto, Drs. Geeta Yadav and Ben Barankin shared some of their positive experiences. As more procedures move from the hospital setting to the clinic setting, dermatologists are in a favourable position to avail themselves of this effective analgesia which supports improved outcomes and patient experience.
As always, THE CHRONICLE team invites and welcomes your comments on this issue, or any other topic in dermatology, at www.derm.city
—Wayne P. Gulliver, MD, FRCPC, Medical EditorContinued from page 1
tawa dermatologist Dr. Reetesh Bose, whose Skin of Colour Dermatology Clinic runs each Friday at the Ottawa Hospital.
Dr. Bose observed the efficacy and cultural sensitivity that a skin of colour dermatology clinic could deliver when he did an elective with dermatologist Dr. Iltefat Hamzavi, who is based at the Henry Ford Health System in Detroit.


“There are a lot of psychosocial and cultural aspects to people’s care that we do not always understand or know or learn about in a dermatology residency,” said Dr. Bose in an interview with THE CHRONICLE OF SKIN & ALLERGY “In certain cultures, you can have individuals who feel that they are less desirable, or see others as less desirable, if they have dark patches on the face or pure pale patches on the face. I have had patients crying in my office because of skin discolouration they have.”
Dr. Morvarid Hessami, head of dermatology at Scarborough Health Network in Toronto, agreed that treating patients with skin of colour is about more than simply recognizing skin pigment but also understanding the cultural context of skin or hair conditions. Depending on the cultural background of the patient, there may be discrimination and stigma attached to having a condition like vitiligo that can make life very distressing for patients with skin of colour, noted Dr. Hessami.
“If someone has vitiligo, they may not have been able to work as a chef in their country of origin or people would not shake their hands because it their condition was thought to be contagious,” said Dr. Hessami.
Clinical use
Can be used with or without medicated topical therapies for atopic dermatitis.
Limitations of use: use in combination with other JAK inhibitors, biologic immunomodulators, or potent immunosuppressants, such as methotrexate and cyclosporine, has not been studied and is not recommended.
Most serious warnings and precautions
Serious infections: patients may be at increased risk for developing serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death; more frequently reported serious infections were predominately viral. If a serious infection develops, interrupt treatment until the infection is controlled. Risks and benefits of treatment should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection. Monitor for signs and symptoms of infection during and after treatment, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Malignancies: lymphoma and other malignancies were observed in patients taking JAK inhibitors to treat inflammatory conditions and were more frequently observed in patients with rheumatoid arthritis (RA) during a clinical trial with another JAK inhibitor versus TNF inhibitors. Thrombosis: including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients taking JAK inhibitors to treat inflammatory conditions. Many of these events were serious; some resulted in death. Consider risks and benefits prior to treating
References: 1. CIBINQO
It is also the due diligence of a dermatologist to explain to their patients with skin of colour that when they have a condition such as psoriasis, they can expect to see dyspigmentation after their psoriasis has been treated.
“Patients [who have had psoriasis] may come in and say that their skin is not clear,” said Dr. Hessami. “It’s a challenge I face every day, to explain to them that the psoriasis has cleared, but that they have post-inflammatory hyperpigmentation that may not easily go away.”
Dr. Marissa Joseph, a pediatrician and dermatologist, stated that her experience is consistent with what Dr. Hessami sees in her clinical practice, pointing out pigmentation disorders can present as primary medical conditions or as secondary to other conditions.
“With common conditions like psoriasis, or atopic dermatitis, or hidradenitis suppurativa, even when patients get better, they are left with profound dyspigmentation and scarring,” said Dr. Joseph, a clinician teacher at the University of Toronto, a dermatologist at Midtown Pediatrics in Toronto, and Medical Director of the Ricky Kanee Schachter (RKS) Dermatology Clinic at Women’s College Hospital in Toronto. “We do not have a lot of dedicated therapeutics or spaces to address that for racialized patients.”
One of the spaces that had addressed profound dyspigmentation and scarring that presented in racialized patients was a skin of colour clinic at Women’s College Hospital in Toronto, which unfortunately ceased operating due to a lack of resources and not because of a lack of need, Dr. Joseph pointed out.
But the growing demand for greater dermatologic care for skin of colour patients, in addition to progress in therapeutics for many conditions that disproportionately affect
Please turn to SoC page 19→
patients who may be at increased risk. In a clinical trial in patients ≥50 years of age with RA, a higher rate of all-cause mortality and thrombosis occurred in patients treated with another JAK inhibitor versus TNF inhibitors. Patients with symptoms of thrombosis should be promptly evaluated and treated appropriately.
Major adverse cardiovascular events (MACE): including non-fatal myocardial infarction, were observed more frequently in patients ≥50 years of age with RA during a clinical trial comparing another JAK inhibitor versus TNF inhibitors.
Other relevant warnings and precautions
•Driving or operating machinery
• Dose-dependent increase in blood lipid parameters, lipid monitoring and management
•Hematological abnormalities
•Use with potent immunosuppressants
•Vaccination
•Monitoring and laboratory tests
•Fertility
•Women of childbearing potential
•Pregnancy and breastfeeding
•Geriatrics
For more information
Consult the Product Monograph at http://pfizer.ca/pm/en/CIBINQO.pdf for important information regarding adverse reactions, drug interactions and dosing, which have not been discussed in this piece. The Product Monograph is also available by calling 1-800-463-6001.
Pfizer Canada ULC. 2. Bieber T, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med 2021;384:1101–12. † Results from a phase 3 randomized, double-blind, placebo-controlled, double-dummy, parallel group, multicentre study
Product Monograph, of CIBINQO in combination with background medicated topical therapies in patients aged ≥18 years with moderate-to-severe atopic dermatitis who had an inadequate response to topical therapy or had received systemic therapy, excluding dupilumab. 2:2:2:1 randomization to CIBINQO 200 mg (n=226), CIBINQO 100 mg (n=238), dupilumab (n=243) or placebo (n=131) for 12 weeks. CIBINQO dose: 200 mg or 100 mg taken orally once daily. Dupilumab dose: 300 mg administered subcutaneously every other week after a loading dose of 600 mg at baseline. Matching placebo was dosed accordingly

Significantly more CIBINQO patients achieved ≥75% improvement in the EASI score from baseline (defined as EASI-75) at Week 12 vs. placebo1,2†
Rapid and significant itch relief was seen as early as Week 2 vs. placebo as measured by PP-NRS4 (2º endpoint)
Patients achieving a PP-NRS response with ≥4-point improvement from baseline
Significantly more CIBINQO 200 mg patients achieved PP-NRS4 vs. dupilumab as early as Day 4 and remained higher through Week 2
Proportion of PP-NRS4 responders with CIBINQO 100 mg was similar to dupilumab at Week 2 and over time.
CIBINQO is only indicated in patients who have had an inadequate response to other systemic drugs or for whom these treatments are not advisable. Over 50% of patients in these studies did not have prior exposure to systemic therapy.
Effective pain management encourages patients to complete regimens of skin treatments and be willing to experience more aggressive treatment options, leading to better outcomes. Asking patients about their pain experience and having a range of effective analgesia options supports good pain management.
These were messages presented by Drs. Geeta Yadav and Ben Barankin during a presentation at Dermatology Update in Toronto.
Recognizing patients’ pain experience
Patients understand their own experience and tolerance of pain, said Dr. Yadav. founder of FACET Dermatology in Toronto.
She is an expert in both medical and cosmetic dermatology with a medical practice that specializes in atopic dermatitis, psoriasis, and skin cancer.
“I have patients who will not undergo [a specific laser treatment] because they are worried about the amount of pain. So they may not be getting the result that I want to be able to give them for their rosacea, for example, because of their anxiety around the pain.”
Having analgesia options available just makes
sense for many dermatologists, Dr. Yadav said, comparing it to stocking a range of different neuromodulator brands for patients who may prefer one


neuromodulator over another.
“I put a lot of effort in our practice talking to our nurses and our estheticians to make sure that we are hurting our patients as little as possible,” said dermatologist Dr. Ben Barankin, Medical Director and Founder of the Toronto Dermatology Centre where he specializes in medical, surgical, and cosmetic care

of the skin.
“If [patients] have a positive experience, they are more likely to come back regularly, they are more likely to tell their friends, and they are more likely to rate a physician positively online,” he said. “‘If you bruise them, you lose them’ we say with fillers and Botox treatments, and similarly if we cause patients too much pain, we can lose them as well. Patients with optimal pain control are going to take fewer breaks during their procedure as well, and that allows the physician to move faster.”
Dermatologists have many options for analgesia, said Dr. Barankin, from vibration and ice to “talkesthesia,” potent topical anesthetics, and inhaled analgesia.
Different analgesia options are suited for different applications, he noted. Topical anesthetics are difficult to apply to the scalp, for instance. NSAIDs may be unsuitable for procedures involving multiple injections due to increased bruising risk.
Some practices may also choose to not use certain options for practical reasons, Dr. Barankin said. Some inhaled analgesia devices are large rolling units that can compete for limited practice floor space with lasers and other devices. He also noted he had concerns in his practice about flammability if the large, inhaled analgesia devices were used for procedures involving lasers.
Dr. Yadav noted that a practice may not want to have opioids in its armamentarium due to the degree of care involved in the storage, handling, and documentation required for safety compliance.
Another analgesia option that is returning to Canada is the handheld, patient-administered lowdose inhaled methoxyflurane “green whistle” device, Dr. Yadav said. The device is available under the brand name Penthrox, she said.
“Methoxyflurane has been around for a very long time,” Dr. Barankin said. “This is not a new product, it has been used around the world in various medical and surgical specialties, especially in Australia, and in many different age groups.”
“It is a well-known product, a safe product, and a very effective product when used properly,” he said.
Dr. Yadav noted that methoxyflurane is analgesic at low doses and anesthetic at high doses with largely
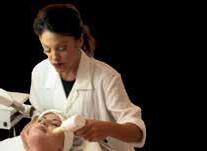
Figure 1: Toronto dermatologist Dr. Geeta Yadav observes as a patient inhales methoxyflurane. The agent is self-administered under supervision of a healthcare practitioner trained in its administration, using the hand-held Penthrox inhaler. Rapid and effective pain relief is seen in about 4 minutes, within 6 to 10 breaths. One unit lasts 30 to 60 minutes. Image courtesy of Dr. Geeta Yadav Dr. Geeta Yadav Dr. Ben Barankin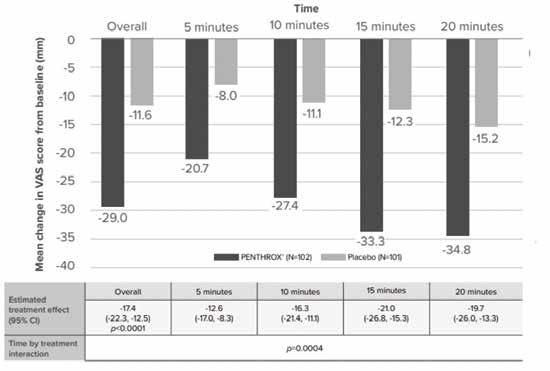
dose-dependent side effects.
“In the Canadian product monograph, it states the use of Penthrox as an anesthetic agent is contraindicated,” said Dr. Yadav. “The role of inhaled methoxyflurane in acute pain management is a rapid onset of analgesia.”
She noted low-dose inhaled methoxyflurane has a well-established safety profile, with no clinically significant effects on vital signs or consciousness levels. “The nephrotoxicity previously associated with methoxyflurane was at a high anesthetic dose and not at the analgesic dose,” Dr. Yadav said. “That is important to highlight for people who might be wondering, as I did before I brought it into my practice, if I need patients to consent separately for this.”
“The official indication is for short-term relief of moderate to severe acute pain associated with trauma or interventional medical procedures in a conscious adult patient,” Dr. Yadav said. She added that it is not indicated for use during pregnancy, there is not currently adequate data to support pediatric use, and the risk of adverse events may be elevated in patients over 65 years of age.
Citing a double-blind study (Adv Ther 2015;

1. Penthrox Patient Monograph

2.Methoxyflurane in Pre-Hospital Settings: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines

3. Delivering the next generation Penthrox 'green whistle'
33:2012–2031), she noted low dose, inhaled methoxyflurane has a rapid onset of action (a decrease of 20.7 mm on a Visual Analogue Scale (VAS) five minutes after administration).
The duration of effect is also short, Dr. Yadav said, noting this is advantageous because it allows patients to return to activities soon after the procedure. Because of this short duration, patients control their own analgesia during the procedure by breathing from the hand-held administration device, she said.
When considering if low dose, inhaled methoxyflurane is a good choice for a given procedure, one consideration is the duration of the procedure, Dr. Barankin said. “If it is under three minutes, there is no real point in getting into using this device and counselling about it. But if we are talking about considering 15-minute plus procedures, for sure, a physician should be thinking about [methoxyflurane].”
As an example, Dr. Barankin mentioned patients undergoing the removal of large numbers of skin tags from their axillae. “They do very well with this device. They’re very happy,” he said. “Yes, we can say the pain of removal is not that bad. But you know, 30, 40, 50 lidocaine injections into the armpits are not pleasant.”
Also related to injections in the armpits, Dr. Barankin said that in general neuromodulator injections for axillary hyperhidrosis may not warrant methoxyflurane analgesia. “It’s a fairly quick procedure. Most people find the pain modest,” he said. However, he has a patient who rated it a six out of 10 on the VAS pain scale. For a patient who has that experience of pain with this procedure, it is worth asking if they would like some form of analgesia, he said. Whether that is a potent topical anesthetic, Tylenol or Advil, or low dose inhaled methoxyflurane.
Neuromodulator injections to treat palmoplantar
hyperhidrosis are a different story, Dr. Barankin said, noting the treatment in this area is “very painful, very unpleasant. We used to do nerve blocks for these patients, and that’s not without its risks. We’ve done ice and medical vibration. That certainly can be helpful, but there are issues with dripping and wetness related to ice, and the potent topical anesthetics help but they're not amazing. So for palmoplantar hyperhidrosis botulinum toxin treatments, we have found [low dose inhaled] methoxyflurane to be a very effective option.”
The value of methoxyflurane analgesia for patients undergoing platelet-rich plasma (PRP) injections also varies by site, Dr. Yadav said.
“Generally, the pain of the facial PRP for antiaging or acne scarring is not so bad. You certainly could consider the device, but we have not necessarily seen a big need. However, PRP for the scalp for androgenic alopecia, that is a lot of needle pokes.”
She noted some patients are fine with the level of pain or are adequately relieved by vibration. “But for PRP for androgenic alopecia, I would say this is a great use of this product.”
Dr. Barankin’s recommendation was the same for Kenalog injections for treating alopecia areata, particularly of the ophiasis type, as well as treatment of syringomas and dermatosis papulosa nigra around the eyes and intralesional injection treatments for keloids.
Low dose, inhaled methoxyflurane analgesia is also an effective option for when delivering energy device treatments over large body areas, Dr. Yadav said. That is especially true for aggressive treatments such as laser resurfacing or hair removal, she said, noting some patients find radiofrequency microneedling significantly painful.
“Some patients do grin and bear it but I would say in these cases physicians definitely should be offering methoxyflurane analgesia and have it available for their patients,” she said.
For some energy-based treatments, such as photodynamic therapy (PDT), there are less painful parameters that can be used, such as daylight PDT, said Dr. Yadav. However, outcomes can be better with IPL or laser-activated PDT. Effective analgesia, such as low dose inhaled methoxyflurane, may help patients achieve better outcomes in these cases by allowing them to tolerate the stronger therapy options, she said.
“People can deal with dental work without dental freezing,” Dr. Barankin said. “But the dentist does not even really ask patients if they would like dental freezing, they just do it. In a sense, [dermatologists] should almost be just doing [low dose, inhaled methoxyflurane analgesia] or the other measures and not be inflicting so much pain.”
Written by John Evans, Senior Editor, THE CHRONICLE OF SKIN & ALLERGY Printed in Canada for Chronicle Information Resources Ltd., 1460 The Queensway, Suite 212, Etobicoke, Ont. M8Z 1S4 Canada. Telephone 416.916.2476; facsimile 416.352.6199; email: health@chronicle.org. Copyright 2024 by Chronicle Information Resources Ltd., except where noted. All rights reserved. Reproduction in any form is expressly prohibited without written permission of the publisher.
Table 1: Low dose, inhaled methoxyflurane significantly reduced mean change in VAS pain intensity score from baseline vs. placebo at the following time points (5, 10, 15 and 20 minutes) assessed ITT population (Paladin data on file).






Demonstrated lower worst pain overall during bone marrow biopsy vs placebo in adults1
Mean worst pain overall (the highest of two patient-rated pain scores (NRS) at two time points: during aspiration and core biopsy) during bone marrow procedure was significantly lower for PENTHROX® vs placebo (4.9 vs 6.0; p=0.011).1†
The mean worst pain score for PENTHROX® vs placebo during aspiration was 3.3 vs 5.0 ( p<0.001) and during core biopsy was 4.5 vs 5.4 ( p=0.073).1†
Demonstrated safety and tolerability profile1
The most common reactions were related to the CNS system, including dizziness (29.5%), headache (21.5%), and somnolence (5.4%).
Serious dose-related nephrotoxicity has only been associated with methoxyflurane when used at high doses over prolonged periods. The recommended maximum dose for PENTHROX® should not be exceeded.
Please refer to the Product Monograph for complete safety and tolerability profile.
Fictitious patient. May not be representative of all patients.
PENTHROX® is the only analgesic in a hand-held inhaler ‡

PENTHROX® (methoxyflurane) is indicated for short-term relief of moderate to severe acute pain, associated with trauma or interventional medical procedures, in conscious adult patients.

• Fractures2–4

Trauma in Emergency Departments
Interventional
Medical Procedures
Dislocations2–4
L acerations2,3
Contusions2–4
• Foreign body removal3

• Bone marrow biopsies5
Colonoscopies6

CLINICAL USE:
Due to dose limitations of a treatment course of PENTHROX® and the duration of associated pain relief, PENTHROX® is not appropriate for providing relief of break-through pain in chronic pain conditions. PENTHROX® is also not appropriate for relief of repetitive pain.
PENTHROX® is not indicated for use during pregnancy or the peripartum period, including labour.
CONTRAINDICATIONS:
Altered level of consciousness due to any cause including head injury, drugs, or alcohol
Clinically significant renal impairment
History of liver dysfunction after previous methoxyflurane use or other halogenated anesthetics
Hypersensitivity to methoxyflurane or any other halogenated anesthetics
Known or genetically susceptible to malignant hyperthermia or a history of severe adverse reactions in either patient or relatives
Clinically evident hemodynamic instability
Clinically evident respiratory impairment
Use as an anesthetic agent
MOST
Nephrotoxicity: Supratherapeutic doses of methoxyflurane inhalation have been shown to lead to serious, irreversible nephrotoxicity in a dose-related manner. Dosing limitations should be followed meticulously to prevent or limit risk of nephrotoxicity. Consecutive day use of PENTHROX® is not recommended because of nephrotoxic potential. The lowest effective dose should be administered, especially in the elderly or in patients with other known risk factors of renal disease.

Hepatotoxicity: Very rare cases of hepatotoxicity have been reported with methoxyflurane inhalation when used for analgesic purposes. Use with care in patients with underlying hepatic conditions or having risk factors for hepatic dysfunction. PENTHROX® must not be used in patients who have a history of showing signs of liver damage after previous methoxyflurane use or halogenated hydrocarbon anesthesia.
OTHER RELEVANT WARNINGS AND PRECAUTIONS:
Administer with caution in elderly patients with hypotension and bradycardia due to possible reduction in blood pressure
Drug dependence
May influence the ability to drive and operate machinery
Potential CNS effects
Do not administer concomitantly with alcohol ingestion
To reduce occupational exposure to methoxyflurane, the PENTHROX® Inhaler should always be used with the activated carbon chamber to adsorb exhaled methoxyflurane
Drug interactions - inducers of CYP 2E1 and CYP 2A6
Local skin reactions or irritation to the eyes and mucous membranes
Exercise caution if administering to a nursing mother
FOR MORE INFORMATION:
Please consult the Product Monograph at https://health-products. canada.ca/dpd-bdpp/index-eng.jsp for important information relating to adverse reactions, drug interactions, patient counselling, and dosing/disposal information (regarding the total maximum dose for a single administration or over the first day of treatment, in a single 48-hour period and entire treatment course) which have not been discussed in this piece. The Product Monograph is also available by calling us at 1-888-867-7426.
References:
1. PENTHROX Product Monograph, Paladin Labs Inc., April 2022. 2. Borobia AM, Collado SG, Cardona CC, et al. Inhaled Methoxyflurane Provides Greater Analgesia and Faster Onset of Action Versus Standard Analgesia in Patients With Trauma
Pain: InMEDIATE: A Randomized Controlled Trial in Emergency Departments. Annals of Emergency Medicine 2020;75(3):315–28. 3. Coffey F, Wright J, Hartshorn S, et al. STOP!: a randomised, doubleblind, placebo-controlled study of the efficacy and safety of methoxyflurane for the treatment of acute pain. Emerg Med J. 2014;31(8):613–8.
4. Voza A, Ruggiano G, Serra S, et al. Inhaled Methoxyflurane versus Intravenous Morphine for Severe Trauma Pain in the Emergency Setting: Subgroup Analysis of MEDITA, a Multicenter, Randomized, Controlled, Open-Label Trial. JPR 2020;Volume 13:491–502. 5. Spruyt O, Westerman D, Milner A, et al. A randomised, double-blind, placebo-controlled study to assess the safety and efficacy of methoxyflurane for procedural pain of a bone marrow biopsy. BMJ Support Palliat Care. 2014;4(4):342–8. 6. Nguyen NQ, Toscano L, Lawrence M, et al. Patient-controlled analgesia with inhaled methoxyflurane versus conventional endoscopist-provided sedation for colonoscopy: a randomized multicenter trial. Gastrointestinal Endoscopy 2013;78(6):892–901.
† A phase IV, randomized, double-blind, singlecentre, placebo-controlled study to evaluate the efficacy and safety profile of PENTHROX® for the treatment of incident pain in adult patients requiring analgesia associated with a planned bone marrow biopsy (BMB) procedure. 49 patients were randomized to PENTHROX® and 48 patients to placebo. All patients received local anaesthetic as well as study drug.
NRS = numeric rating scale
Continued from
page
atric Dermatology Update organized by the dermatology department at St. Justine Hospital and the University of Montreal held recently in Montreal, Dr. McCuaig noted it can be a challenge to distinguish between eczema coxsackium and eczema herpeticum. A molecular test may have to be conducted to confirm the diagnosis, she said.

Dr. Catherine McCuaig
“To differentiate between coxsackie eczema and herpetic eczema may be difficult,” said Dr. McCuaig. “There are cases where we recommend hospitalization while we wait for PCR [polymerase chain reaction] results. We are also guided by the systemic health of the child.”
One of the recent observations has been that use of the Janus kinase inhibitors upadacitinib and abrocitinib to treat atopic dermatitis has led to increased rates of herpes simplex virus infection, but an increase in herpes simplex virus infection with the use of baracitinib has not been observed, noted Dr. McCuaig.
High index of suspicion needed for morbilliform eruptions
Morbilliform eruptions are common in the pediatric in-patient setting, and several possible diagnoses can be suspected when such eruptions present, according to Dr. McCuaig.

Addison’s disease
T
o differentiate between coxsackie eczema and herpetic eczema may be difficult...There are cases where we recommend hospitalization while we wait for PCR [polymerase chain reaction] results. We are also guided by the systemic health of the child”Clinicians should ask about the travel history when seeing a patient as Chikengunya virus or Dengue virus can result in maculopapular eruptions. “Has the patient travelled to the Caribbean?” asked Dr. McCuaig. “If yes, you need to think of Chikengunya or Dengue” (Dermatol Clin 2022 Apr; 40(2):191 2022).
Other conditions that can be suspected if morbilliform eruptions are present are inflammatory conditions, such as Kawasaki disease, as well as multisystem inflammatory syndrome, with the latter condition having emerged during the Covid-19 pandemic, explained Dr. McCuaig.
With respect to multisystem inflammatory syndrome in children (MIS-C) (which was first identified in the early day of the Covid-19 pandemic), it differs from Kawasaki disease as it tends to affect children at an older age. Kawasaki disease usually appears in children less than five years of age, MIS-C appears typically in children aged five to 13. “It [MIS-C] appears in children, by definition under 21 years of age, and presents with a fever with multi-organ involvement and evidence of very significant inflammation,” said Dr. McCuaig.
Containing the spread of contagious conditions
It’s key to report cases of measles or German measles if that is suspected in a child because of the threat of spread of these conditions, stressed Dr. McCuaig.
“If you suspect it’s measles, you must advise [public health] authorities, isolate the patient, and confirm [the diagnosis] with PCR,” said Dr. McCuaig. “You also need to notify everyone who had been in contact with that patient. If there are others in the waiting room, they need to be notified.”
Vaccinations for measles, rubella or German measles, mumps and chicken pox are supposed to take place when children are aged 12 and 18 months, but Dr. McCuaig noted there were 200,000 deaths worldwide due to measles.
Unfortunately, there are children who are unvaccinated for various reasons, said Dr. McCuaig. She pointed out some children may be from immigrant families and had not been vaccinated at a young age before coming to Quebec, some children may have been immunosuppressed and were not vaccinated at the scheduled time, and some children may have “granola” parents who
Dr. Catherine McCuaig
chose not to have them vaccinated.
With respect to identifying measles, clinicians are taught to check for “the three C’s” in the prodromal phase of the illness including cough, coryza, and conjunctivitis, as well as Koplik spots and fever, Dr. McCuaig said,
With respect to chicken pox, a child should be isolated because the condition is extremely contagious and the incubation period is long, added Dr. McCuaig. “In general, we just observe,” she said, noting treatment consists of oral valacyclovir or intravenous acyclovir in patients who are immunodeficient.
Hand, foot and mouth disease
Hand, foot, and mouth disease typically presents in children younger than five years of age and affects typically affects the sites of the hands, feet, and mouth, but a recent systematic review of 85 studies explored an atypical presentation of hand, foot, and mouth disease with exanthems appearing on sites other than the hands, feet, and mouth (DOI: 10.1111/pde.15461).
The atypical presentation has been associated with coxsackievirus A6 and the most commonly reported morphologies are vesicles (53%), papules (49%), and bullae (36%).
According to Dr. McCuaig, the systematic review highlights the importance of recognizing this atypical presentation of the condition.
Novel presentations linked to SARS CoV-2 infection or monkey pox infection
A novel condition that has been characterized in pediatric patients linked to Covid-19 is GianottiCrosti Syndrome, according to Dr. McCuaig. Maculopapular rashes have been observed typically during the active phase of Covid-19, but the Gianotti-Crosti syndrome associated with Covid-19 seems to present after active infection ( Pediatr Dermatol 2021; 38:629–631).
Chronicle's digital audio, e-newsletters, and web portal keep physicians updated reliably 24/7. Each is worthy of your time. And they're available without charge. Find them all at https://linktr.ee/dermcity

www.derm.city
Since 2015, derm.city has been a leading hub and repository for dermatology information and interactive knowledge-exchange in video, text, images, and audio. It's the official site of The Chronicle of Skin & Allergy, now newly enhanced with added useful features. Thrice weekly updates on the latest findings in dermatology, summarized for quick learning. Visit derm.city at https://derm.city
A dermatology newsletter focused on ethnodermatology care from top physicians in the field, delivered to your inbox every Monday.
Supported by Bausch Health Canada

A biweekly newsletter on clinical and social issues affecting female patients and physicians, delivered straight to your inbox.
Supported by Galderma Canada
Dr. Geeta Yadav and expert dermatologists discuss clinical developments, news, trends and myths in acne treatment and research. First season of three episodes now available for listening and downloading.
Supported by Cipher Pharmaceuticals

a skin spectrum podcast

Dr. Neil Shear, dermatologist and Professor Emeritus at the University of Toronto, speaks with many guests from the world of dermatology, including Dr. Boluwaji Ogunyemi, Dr. Marc Bourcier, and Dr. Rachel Asiniwasis, to hear their stories and insights.
Dr. Ron Vender delves into recent psoriasis studies and comments articles about the efficacy of PASI scores, intermittent fasting for psoriasis, and biologic fatigue.
Supported by Sun Pharma Canada

Chronicle Podcast System • www.chronicle.ca "Ideas in the Service of Medicine"


Have a comment about the articles in this issue?
Share your knowledge and opinions with peers worldwide.

derm.city
where dermatology lives

Hyder Mirghani,1 Abdulaziz Altemani,2 Ethar Alsaedi,3 Rahaf Aldawish,4 Mohammed Alharbi,5 Reema Alzahrani,6 Saleh Alatawi,7 Sarah Altemani,7 and Ahmed H. Alanazi8
1Internal Medicine Department, Faculty of Medicine, University of Tabuk, Tabuk, SAU 2Dermatology Department, King Fahad Specialist Hospital, Ministry of Health, Tabuk, SAU 3Dermatology Department, King Faisal Hospital, Ministry of Health, Makkah, SAU
4Dermatology Department, College of Medicine, Sulaiman Alrajhi University, Qassim, SAU 5Family Medicine Department, Waerh Primary Healthcare Center, Ministry of Health, Madinah, SAU
6Dermatology Department, Faculty of Medicine, King Abdulaziz University, Jeddah, SAU 7Dermatology Department, Faculty of Medicine, University of Tabuk, Tabuk, SAU 8Internal Medicine Department, King Salman Armed Forces Hospital, Ministry of Defense, Tabuk, SAU
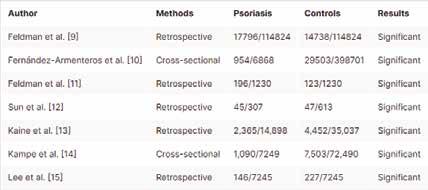
Psoriasis is a systemic disease affecting various organs; however, it is usually thought of as a skin disease. A multidisciplinary approach is needed for better outcomes. The current meta-analysis assessed the association between diabetes mellitus, high blood pressure, and psoriasis. We searched four databases, including Cochrane Library, PubMed, MEDLINE, and Google Scholar, for relevant articles using the following keywords: psoriasis, hypertension, high blood pressure, cardiovascular risk factors, and diabetes mellitus. The author’s name, year, and country of publication, diabetes, and hypertension among patients with psoriasis and control subjects were collected and entered into a Microsoft Excel sheet. Out of 1,209 articles retrieved, 903 articles remained after duplication removal. From the 82 full texts screened, only seven studies fulfilled the inclusion and exclusion criteria. Psoriasis was associated with diabetes and hypertension: odds ratio 1.38, 95% CI 1.171.64; p-value 0.0002, chi-square 224.93, and odds ratio 1.60, 95% CI 1.41-1.81, p-value 0.00001, chi-square 226.59, respectively. Substantial heterogeneity was observed (I2 for heterogeneity, 97%, p<0.001). A broad approach is needed to address the associated comorbidities and select the appropriate therapeutic approach. Randomized controlled trials investigating the best drugs for the treatment of psoriasis and its associated cardiovascular risk factors are needed.
Psoriasis is a chronic inflammatory disease with a strong genetic predisposition; the prevalence varies according to the location, with the highest prevalence observed in Scandinavian regions (11%) and the lowest in some African and Asian countries.1 There are many clinical subtypes (plaque psoriasis, guttate psoriasis, inverse psoriasis, pustular psoriasis, erythrodermic psoriasis, nail psoriasis, and scalp psoriasis) with plaque psoriasis (psoriasis vulgaris) being the most common. Other variants include pustular psoriasis, which can be localized or generalized, and inverse psoriasis involving skin folds.2 Psoriasis affects the skin, joints, oral cavity, scalp, and nails. In addition, it is associated with various disorders, including psychiatric disease, cardiometabolic issues,
and streptococcal infections. Other factors include smoking, alcohol consumption, and obesity.3 Psoriasis is considered an immune-mediated systemic disease and is associated with various comorbidities, significantly impacting patients, communities, and healthcare systems.4 Genetic predisposition and environmental triggers lead to a chronic proinflammatory process, releasing interleukins, tumor necrosis factor, and interferons. These cytokines initiate a vicious cycle of chronic inflammation and keratinocyte hyperproliferation.5 Targeting the inflammatory pathways and cytokines contributing to the disease pathogenesis can revolutionize psoriasis treatment.6 Importantly, psoriasis shares its pathogenesis with various comorbidities, including diabetes, myocardial infarction, metabolic-associated fatty liver disease, dyslipidemia, and hypertension.7-8 How-

ever, there is a lack of addressing these dangerous, associated comorbidities.
Psoriasis has been associated with an increased risk of developing other health conditions, including hypertension (HT) and diabetes mellitus (DM). Chronic inflammation in psoriasis can contribute to the development of these conditions. Studies have shown that individuals with psoriasis are at a higher risk of developing hypertension.4 The chronic inflammation associated with psoriasis may contribute to endothelial dysfunction and arterial stiffness, which are risk factors for hypertension. Psoriasis has also been linked to an increased risk of developing type 2 diabetes mellitus. It can also lead to insulin resistance, a key factor in the development of type 2 diabetes.8
It is evident that the association between psoriasis, diabetes mellitus, and hypertension presents a significant challenge in the field of healthcare. The complex interplay of these conditions requires a comprehensive approach to diagnosis and management. Further-
more, the potential impact on patient outcomes and quality of life necessitates further research and collaboration among healthcare professionals. Addressing this challenge will require a multidisciplinary approach and a deeper understanding of the underlying mechanisms linking these conditions. Therefore, this meta-analysis aimed to assess the relationship between psoriasis, diabetes, hyperlipidemia, hypertension, and obesity.
REVIEW
Materials and methods
Eligibility criteria according to PICOS (Population, Intervention, Comparison, Outcome, Study)
This meta-analysis included retrospective and prospective cohorts, crosssectional, and case-control studies, evaluating the association between psoriasis, diabetes, and hypertension. Case reports, case series, and studies on animals were not included.
Outcome measures
The association of psoriasis with metabolic syndrome.
Literature search and data extraction
Two reviewers searched three databases (PubMed MEDLINE, the first 100 articles in Google Scholar, and Cochrane Library) from inception up to May 2023. The keywords psoriasis, hypertension, high blood pressure, and diabetes mellitus were used. In addition, the references of the included studies were searched for relevant articles. We identified 1,209 studies and 903 studies after the removal of duplication. From them, 82 full texts were screened and only seven studies were included in the final meta-analysis. A datasheet was used to extract the author's name, year, and

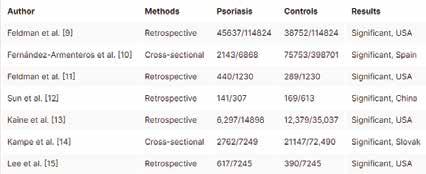

country of publication, diabetes, and hypertension among patients with psoriasis and control subjects.
The Newcastle Ottawa Scale was used to assess the quality of the included studies.
The RevMan system (The Cochrane Collaboration, revman.cochrane.org) was used for data analysis and the DerSimonian and Laird method was applied.
Seven studies were pooled, five retrospective and two cross-sectional studies. Dichotomous data were entered manually and random effects were applied due to the substantial heterogeneity. A p-value of <0.05 was considered significant.
The total number of the articles retrieved was 1,209; 903 remained after the removal of duplication, of them, 82 full texts were screened. Only seven studies fulfilled the inclusion and exclusion criteria. All the included studies were of good quality according to the Newcastle Ottawa Scale. Five of the in-
cluded studies were retrospective and two were cross-sectional (Figure 1).
Four of the studies were published in the USA, two in Europe, and one in Asia. The age of the participants ranged from 42 to 55 years, 44.7% to 55.6% were women, and patients with psoriasis had more comorbidities (Tables 1 to 4).
In the present meta-analysis, seven studies were included (773,761 patients and 79,184 events), five studies were retrospective and two were cross-sectional. Diabetes was common among patients with psoriasis compared to control subjects; odds ratio: 1.38, 95% CI: 1.17-1.64, p-value=0.0002, chisquare=224.93; and standard difference=6. However, substantial heterogeneity was found, I2 For heterogeneity, 97%, and p-value<0.001; therefore, the random effect was applied (Figure 2).
Hypertension was more common among patients with psoriasis compared to their counterparts; odds ratio 1.60, 95% CI: 1.41-1.81, pvalue=0.00001, chi-square=226.59, and standard difference=6. However, substantial heterogeneity was found, I2 for
heterogeneity, 97%, and p-value <0.001. The random effect was used due to the substantial heterogeneity (Figure 3).
First and foremost, numerous epidemiological studies have consistently demonstrated a strong association between psoriasis and diabetes mellitus. A systematic review and meta-analysis published in the Journal of the American Academy of Dermatology in 2012 found that individuals with psoriasis had a significantly increased risk of developing diabetes compared to those without the skin condition.8
In addition to the link between psoriasis and diabetes, a growing body of evidence has also implicated hypertension as a comorbidity of psoriasis. A systematic review and meta-analysis found that individuals with psoriasis had a significantly higher prevalence of hypertension compared to those without the skin condition. This association persisted across different age groups and was independent of traditional cardiovascular risk factors. Moreover, a large-scale cohort study conducted in the United States demonstrated that the risk of developing hypertension was significantly elevated in patients with severe psoriasis, further underscoring the need for comprehensive cardiovascular risk assessment in this population.16 A few other meta-analyses assessed the relationship between psoriasis and hypertension and found an association between mild and severe psoriasis and high blood pressure, confirming the findings of another metaanalysis.16-17 Plausible explanations might include stress, anxiety, depression, psoriasis drugs, and a sedentary lifestyle among patients with psoriasis.18 In addition, psoriasis is a systemic inflammatory disorder associated with other comorbidities, including obesity and diabetes, which might increase the risk of high blood pressure.19 The previous study was limited by many confounders that might affect their conclusion, emphasizing the importance of adjusting for confounders to avoid the risk of bias and heterogeneity.20 Furthermore, the relationship between diabetes mellitus and hypertension has been extensively studied, with evidence suggesting a bidirectional association between the
two conditions. A systematic review and meta-analysis published in Diabetes Care in 2011 found that individuals with diabetes had a significantly higher risk of developing hypertension compared to those without diabetes. Conversely, individuals with hypertension were found to have a significantly increased risk of developing diabetes. These findings underscore the intricate interplay between diabetes and hypertension and the need for integrated management strategies to mitigate the risk of cardiovascular complications in affected individuals.20
Screening for hypertension among patients with psoriasis is vital to avoid serious complications, including myocardial infarction, stroke, and renal complications.21 In the present study, we found that patients with psoriasis were more likely to have diabetes compared to their counterparts without the disease, which is in line with another systematic review and meta-analysis,22 which included five studies and observed similar findings.
Systemic inflammation and adipocytokine dysregulation increase insulin resistance and the progression to type 2 diabetes.4 Importantly, etanercept therapy was found to reduce fasting insulin and improve insulin sensitivity,23 an effect that was not observed when using PUVA (psoralen plus ultraviolet-A) therapy.4 These findings imply that biological therapy and inflammation prevention are real paradigm shifts in optimizing the treatment of patients with psoriasis to improve outcomes.
Another meta-analysis found shared four genetic loci between diabetes and psoriasis, suggesting a causal relationship.24 Low levels of adiponectin and high levels of fetuin-A were observed among patients with psoriasis,25 with high levels of fetuin-A suggested to increase insulin resistance and keratinocyte proliferation, enhancing skin inflammation and the development of metabolic syndrome.26 Interestingly, genetically predicted glycated hemoglobin (HbA1c) increases the risk of psoriatic arthritis, and genetic liability to both psoriasis and psoriatic arthritis increases the risk of cardiometabolic disease, including coronary artery disease.27 Screening for cardiovascular and metabolic disorders among patients with psoriasis is important for timely management to reduce mortality and improve the quality of life.28
The association of diabetes mellitus and psoriasis has important therapeutic implications. A lower level of low-density lipoproteins is recommended, as most statins might increase the incidence of newly diagnosed diabetes. In addition, psoriasis is associated with dyslipidemia. Therefore, choosing a lipid-lowering drug with a low risk of diabetes (including pravastatin and pitavastatin) is important.29
Table 2: Basic characteristics of patients with psoriasis and control subjects (data are presented as percentages and mean±SD) Table 3: Hypertension among patients with psoriasis and control subjects (data are presented as percentages. Table 4: Quality assessment of the included studies (quality was calculated from the maximum score of 9).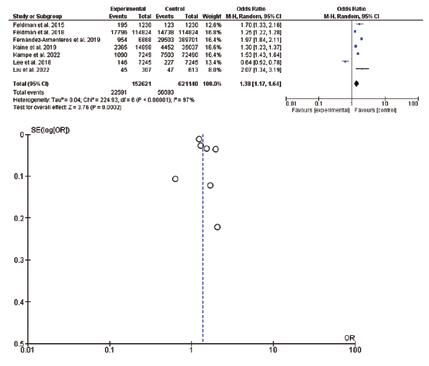

The limitations of this study were the small number of included studies, the observational nature of the studies, and the significant heterogeneity observed.
There is clear evidence of an association between psoriasis, hypertension, and diabetes mellitus, with psoriasis often preceding the development of these comorbid conditions. Patients with psoriasis have a higher chance of developing diabetes mellitus; in addition, hypertension was more common among patients with psoriasis compared to their counterparts without the disease. The substantial heterogeneity observed limited the current conclusion.
Mohammed Alharbi, Reema Alzahrani, Saleh Alatawi , Sarah Altemani
Critical review of the manuscript for important intellectual content: Abdulaziz Altemani, Ahmed H. Alanazi, Hyder Mirghani
Supervision: Abdulaziz Altemani, Hyder Mirghani
Drafting of the manuscript: Ethar Alsaedi, Rahaf Aldawish, Mohammed Alharbi, Reema Alzahrani, Saleh Alatawi , Sarah Altemani
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
The authors would like to acknowledge the Saudi Digital library for accessing the data.
1. Rendon A, Schäkel K: Psoriasis pathogenesis and treatment. Int J Mol Sci 2019; 20:1475.
2. Boehncke WH, Schön MP: Psoriasis. Lancet 2015; 5:983-994.
3. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN: Psoriasis. Lancet 2021; 3:1301-1305.
4. Korman NJ: Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol 2020; 182:840-848.
5. Swindell WR, Johnston A, Xing X, Voorhees JJ, Elder JT, Gudjonsson JE: Modulation of epidermal transcription circuits in psoriasis: new links between inflammation and hyperproliferation. PLoS One 2013; 8:e79253.

patients with comorbid psoriatic arthritis. Arthritis Care Res (Hoboken) 2015; 67:708717.
12. Sun X, Zhao H, Wang R, et al: Psoriasis complicated with metabolic disorder is associated with traditional Chinese medicine syndrome types: a hospitalbased retrospective case-control study. Curr Med Res Opin 2023; 39:19-25.
13. Kaine J, Song X, Kim G, Hur P, Palmer JB: Higher incidence rates of comorbidities in patients with psoriatic arthritis compared with the general population using U.S. administrative claims data. J Manag Care Spec Pharm 2019; 25:122-132.
14. Kampe T, Dorko E, Rimárová K, et al: Prevalence of cardiovascular risk factors in patients with psoriasis. Cent Eur J Public Health 2022; 30:S05-10.
15. Lee S, Xie L, Wang Y, Vaidya N, Baser O: Comorbidity and economic burden among moderate-to-severe psoriasis and/or psoriatic arthritis patients in the US Department of Defense population. J Med Econ 2018; 21:564-570.
16. Duan X, Liu J, Mu Y, et al: A systematic review and meta-analysis of the association between psoriasis and hypertension with adjustment for covariates. Medicine (Baltimore) 2020; 99:e19303.
17. Armstrong AW, Harskamp CT, Armstrong EJ: The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens 2013; 31:433-42; discussion 442-443.
18. Qureshi AA, Choi HK, Setty AR, Curhan GC: Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol 2009; 145:379382.
19. Kaushik SB, Lebwohl MG: Psoriasis: which therapy for which patient. Psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol 2019; 80:27-40.
20. Voils CI, Crandell JL, Chang Y, Leeman J, Sandelowski M: Combining adjusted and unadjusted findings in mixed research synthesis. J Eval Clin Pract 2011; 17:429-434.
21. Parati G, Piepoli MF: Editorial comments: focus issue arterial hypertension and the 2021 ESC guidelines on cardiovascular prevention. Eur J Prev Cardiol 2022; 29:1-4.
22. Armstrong AW, Harskamp CT, Armstrong EJ: Psoriasis and the risk of diabetes mellitus: a systematic review and metaanalysis. JAMA Dermatol 2013; 149:84-91.
23. Campanati A, Ganzetti G, Di Sario A, et al: The effect of etanercept on hepatic fibrosis risk in patients with non-alcoholic fatty liver disease, metabolic syndrome, and psoriasis. J Gastroenterol 2013; 48:839846.
Individuals with psoriasis should be aware of these potential risks and work with their healthcare providers to monitor and manage their overall health. Randomized controlled trials are needed to assess the best therapeutic approach for psoriasis and its associated cardiovascular risk factors.
All authors have reviewed the final version to be published and agreed to be accountable for all aspects of the work.
Concept and design: Abdulaziz Altemani, Sarah Altemani , Hyder Mirghani
Acquisition, analysis, or interpretation of data: Abdulaziz Altemani, Ahmed H. Alanazi, Ethar Alsaedi, Rahaf Aldawish,
6. Baliwag J, Barnes DH, Johnston A: Cytokines in psoriasis. Cytokine 2015; 73:342350.
7. Puig L: Cardiometabolic comorbidities in psoriasis and psoriatic arthritis. Int J Mol Sci 2017; 19:58.
8. Wu JJ, Kavanaugh A, Lebwohl MG, Gniadecki R, Merola JF: Psoriasis and metabolic syndrome: implications for the management and treatment of psoriasis. J Eur Acad Dermatol Venereol 2022; 36:797806.
9. Feldman SR, Hur P, Zhao Y, Tian H, Wei Z, Wang X, Herrera V: Incidence rates of comorbidities among patients with psoriasis in the United States. Dermatol Online J 2018; 15:13030.
10. Fernández-Armenteros JM, Gómez-Arbonés X, Buti-Soler M, et al: Psoriasis, metabolic syndrome and cardiovascular risk factors. A population-based study. J Eur Acad Dermatol Venereol 2019; 33:128-135.
11. Feldman SR, Zhao Y, Shi L, Tran MH, Lu J: Economic and comorbidity burden among moderate-to-severe psoriasis
24. Patrick MT, Stuart PE, Zhang H, et al: Causal relationship and shared genetic loci between psoriasis and type 2 diabetes through trans-disease meta-analysis. J Invest Dermatol 2021; 141:1493-1502.
25. Wolk K, Sabat R: Adipokines in psoriasis: an important link between skin inflammation and metabolic alterations. Rev Endocr Metab Disord 2016; 17:305-317.
26. Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Häring HU, Stefan N: Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One 2008; 3:e1765.
27. Zhao SS, Bellou E, Verstappen SM, et al: Association between psoriatic disease and lifestyle factors and comorbidities: cross-sectional analysis and Mendelian randomization. Rheumatology (Oxford) 2023; 62:1272-1285.
28. Gupta S, Syrimi Z, Hughes DM, Zhao SS: Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int 2021; 41:275-284.
29. Carmena R, Betteridge DJ: Diabetogenic action of statins: mechanisms. Curr Atheroscler Rep 2019; 21:23.
Figure 2: Diabetes mellitus among patients with psoriasis and control subjects Feldman, et al;9 Fernández-Armenteros, et al;10 Feldman, et al;11 Sun, et al;12 Kaine, et al;13 Kampe, et al;14 Lee, et al15 Figure 3: Hypertension among patients with psoriasis and control subjects Feldman, et al;9 Fernández-Armenteros, et al;10 Feldman, et al;11 Sun, et al;12 Kaine, et al;13 Kampe, et al;14 Lee, et al15Continued from page 11
racialized patients, is giving rise to a heightened desire and need to revive the skin of colour dermatology clinic that had operated at Women’s College Hospital, said Dr. Joseph.
“With the renewed interest in diversity and representation, there has been a lot of discussion around
recreating a space like that again,” said Dr. Joseph, noting fellow dermatologist Dr. Yvette Miller-Monthrope, an Assistant Professor of Medicine at the University of Toronto and a dermatopathologist, is slated to lead the rebirth of the skin of colour clinic at Women’s College Hospital. “We are in the process of trying to obtain







funding. We are actively recruiting donations of support through the Women’s College Hospital Foundation to assist in this regard.”
The rebirth of this clinic would also help address hair conditions that affect racialized patients, such as central centrifugal cicatricial alopecia, a scarring alopecia that occurs almost exclu-

sively in Black women. One of the possible reasons for decreased access to dermatologic care among skin of colour patients is that concerns of these patients may not be regarded as top priorities when a condition like melanoma, which mainly affects individuals with lighter skin, is viewed as more pressing and urgent

Camp Liberté was created by a group of dermatologists dedicated to offering children with moderate to severe skin conditions an opportunity to enjoy a summer camp experience. With locations in eastern and western Canada, Camp Liberté hosts more than 40 children per summer at no cost to parents, thanks to the support of generous donors.
Our camps are fully equipped with volunteer dermatologists, residents and nurses to care for children with a wide range of skin conditions, including atopic dermatitis, epidermolysis bullosa, and alopecia areata.
A gift to Camp Liberté provides Canadian children with skin conditions an opportunity to grow in confidence and self-esteem through a multi-cultural outdoor camping experience in a fun, safe, bilingual, environment.



Le Camp Liberté a été créé par un groupe de dermatologues déterminés à offrir à des enfants qui ont des problèmes de peau variant de modérés à graves la possibilité de vivre l’expérience d’un camp d’été. Avec ses emplacements dans l’est et l’ouest du Canada, le Camp Liberté accueille plus de 40 enfants par été sans qu’il en coûte quoi que ce soit aux parents, grâce à l’appui de généreux donateurs. Nos camps bénéficient des services complets de dermatologues, de médecins résidents et d’infirmières bénévoles qui s’occupent d’enfants aux prises avec un vaste éventail de problèmes de peau, y compris la dermatite atopique, l’épidermolyse bulleuse et la pelade.
Un don au Camp Liberté permet à des enfants canadiens qui ont des problèmes de peau d’accroître leur confiance en soi et leur estime de soi en vivant une expérience multiculturelle de camping en plein air offerte dans un environnement bilingue, sécuritaire et amusant.




because it can carry a significant mortality risk, pointed out Dr. Bose.
“Patients who have darker skin can have a really hard time trying to see a dermatologist because skin cancer [which is more common in Caucasian patients] is on the rise,” explained Dr. Bose. “In Ottawa, wait times [to see a dermatologist] are very, very long across the whole city.”
Given the prolonged wait times for dermatologist care, Dr. Bose views the Skin of Colour Dermatology Clinic at the Ottawa Hospital as an avenue to expedite treatment for racialized patients.
“I think having the Skin of Colour Dermatology Clinic is a way to address some of the health inequities that are present [in the health system],” said Dr. Bose.




Chronicle Companies is pleased to support Camp Liberté with monetary and inkind donations through Sandi’s Fund, established to honour our late friend and colleague, Sandra Gail Leckie, RN. Sandi was a nurse, pharmaceutical industry executive and health educator who had a life long affinity for children and children’s charities.
Chronicle Companies contributes prots from the annual National Pharmaceutical Congress (www.pharmacongress.info) to Sandi’s Fund for Camp Liberté, and has partnered with Camp Liberté to provide communications assistance for this valuable philanthropic undertaking.



This randomized, controlled trial studied the efficacy of platelet-rich plasma (PRP) versus 5% topical minoxidil for the treatment of androgenetic alopecia (AGA). Researchers randomly divided 70 AGA patients aged between 18 and 60 years of either gender into two groups. Group A was given 5% topical minoxidil and Group B was given PRP. Both groups were followed up over a period of six months, and the final analysis was done with the help of global photography, hair pull test, and patient satisfaction score. At the end of the sixth month, the investigators found 27 patients (77%) in Group A had a negative hair pull test as compared to only 14 (40%) in Group B (p=0.001). In Group A, 32 patients (91.4%) reported improvement in hair scalp from baseline. In Group B, 26 patients (74.3%) reported improvement from baseline (p=1.00). PRP was effective in 26 patients (74.5%) and 5% topical minoxidil in 15 patients (43.7%) (p=0.007). The authors conclude PRP therapy can be a useful alternative to topical minoxidil in the treatment of AGA.
G Afzal, N Ahmed, F Zahoor, T Malik, and O Farooq: Efficacy of platelet-rich plasma versus 5% topical minoxidil for the treatment of androgenetic alopecia, in Journal of the College of Physicians and Surgeons—Pakistan (Jan. 2024; 34(1):11-15).
The objective of the study was to assess the impact of oral isotretinoin on ovarian functions of acne patients who have polycystic ovarian syndrome (PCOS). Forty women with a clinical diagnosis of acne as well as PCOS participated in this prospective clinical trial. Participants were given oral doses of isotretinoin ranging from 0.5 to 1 mg/kg, for a total of 120 to 150 mg/kg. Investigators obtained blood samples on the second to fifth days of each patient’s menstrual cycles to establish baseline values of hormone levels. They also evaluated global acne grading system (GAGS) scores, follicle count, and bilateral ovarian volumes both before and after isotretinoin treatment. There were significant reductions in global acne score and ovarian volume from pre-treatment to post-treatment, and researchers observed a significant reduction in free testosterone level and hirsutism scores after treatment.
HI Elnagar, OA Hashem, HO Aboelwafa, E Elhelw, and ML Elsaie: The impact of oral isotretinoin on ovarian functions of acne patients complaining of polycystic ovarian syndrome: a prospective study, in Journal of Ovarian Research (Jan. 20, 2024; 17(1):21).
What are these lesions?
A . Psoriasis
B B. Basal cell carcinoma
C C. Seborrheic dermatitis
D Pemphigus foliaceus
THE EDITORS invite your participation in this regular feature of the journal. Please send all images and correspondence to:
Medical Editor,
The Chronicle of Skin & Allergy
1460 The Queensway, Suite 212, Etobicoke, Ont. M8Z 1S4
Telephone: (416) 916-2476
E-mail: health@chronicle.org
Dermatology news your patients may be reading
In a USA Today article (Jan. 22, 2024), Sarah Ferguson, Duchess of York, revealed she is undergoing treatment for skin cancer. The melanoma was discovered when several moles were removed while she was undergoing reconstructive surgery following a mastectomy. She was diagnosed with breast cancer six months previously. Dr. Barry D.Goldman, a clinical instructor at Cornell New York-Presbyterian Hospital, said that 80 to 90% of all skin cancers are on the face and neck, so the article emphasized the importance of sunscreen, recommending daily application to the entire face and uncovered areas, using at least SPF 30. The American Academy of Dermatology also recommends applying one shot glass of sunscreen to cover the entire body in addition to wearing sunglasses, seeking shade, wearing protective clothing, and seeing a dermatologist for any suspicious skin changes.
CBC News reports (Jan. 21, 2024) that the trend of young children shopping at Sephora for expensive skincare and beauty products is sparking debate among parents, dermatologists, retailers, and social media users. Referred to as “Sephora kids,” these children and tweens emulate influencers by posting videos of their hauls and skincare routines online. While some view this trend as harmless, others express concern about using adult products such as retinol on pediatric skin. According to Dr. Ashleigh Vallée, a general practitioner in Coal Harbour, B.C., children tend to have thinner, more sensitive skin and should stick to products marketed for sensitive or hypoallergenic skin. Despite criticisms, popular brands like Drunk Elephant defend their products’ suitability for all skin types while emphasizing certain products have some ingredients that are not intended for children.
An article on Health Hive (Jan. 24, 2024) looked into the shifting demographics of beauty retailers like Ulta and Sephora, noting a trend toward a younger clientele. This transformation is attributed to the rising popularity of skincare among Generation Alpha (born from 2010 to 2024). Dr. Nananamibia Duffy, a dermatologist based in Rochester, N.Y., urges tweens and teens to avoid products with active ingredients meant for mature skin. She recommends a simple three-step skincare routine for eight to 12-year-olds: cleanse with gentle bar soaps, moisturize with lotions or creams suitable for their skin type, and prioritize sun protection with moisturizers containing SPF 15-30. Dr. Duffy emphasizes the importance of sunscreen for all ages and highlights the misconception that a sunburn offers protection against future sun damage, mentioning the irreversible effects of sun damage and the need for preventive measures.
PREFER TOPICAL CORTICOSTEROIDS FOR CHILDREN WITH ALOPECIA
An article from Managed Healthcare Executive (Jan. 24, 2024) highlights a survey analysis published in the Journal of the American Academy of Dermatology, outlining the treatment preferences of pediatric dermatologists for pediatric alopecia areata (PAA). Researchers examined responses from 53 dermatologists treating PAA. According to the publication, pediatric fellowship-trained dermatologists predominantly prescribed class 1 corticosteroids for patients aged eight years or younger, regardless of scalp involvement, while non-fellowship-trained dermatologists preferred class 2 corticosteroids for patients with less than 25% scalp involvement. The findings highlight the need for additional studies on drug safety and efficacy, particularly regarding Janus kinase inhibitors, in PAA treatment.
Photo courtesy of Wikimedia Commons rroC tce rewsnaDeveloped with the help of Canadian Dermatologists specifically for psoriasis of the scalp, therapeutic shampoo contains the highest concentration of active ingredients to ensure results.
















4% salicylic acid to loosen scale







10% crude coal tar solution to slow cellular turnover and to provide anti-itch
Conditioning base formulation leaves hair soft & manageable



Available at Shoppers Drug Mart or through your local pharmacist.





The first
only PDE4i indicated in the topical treatment of plaque psoriasis*

ZORYVE is indicated for topical treatment of plaque psoriasis, including treatment of psoriasis in the intertriginous areas, in patients 12 years of age and older.
Please consult the Product Monograph at http://arcutis.ca/zoryvepm-hcp for contraindications, warnings, precautions, adverse reactions, interactions, dosing, and conditions, of clinical use. The Product Monograph is also available by calling us at 1-844-692-6729.
PDE4i: phosphodiesterase-4 inhibitor
*Comparative clinical significance has not been established. †Subject to restrictions. For program terms and conditions go to www.zoryveassist.ca and click Terms and Conditions.
Reference:
1. ZORYVE Product Monograph. Arcutis Biotherapeutics, Inc. April 27, 2023.
The