ANNUAL REPORT 20 21
engineering.pitt.edu
facebook.com/pittengineering
twitter.com/pittengineering
youtube.com/pittengineering
The information printed in this document was accurate to the best of our knowledge at the time of printing and is subject to change at any time at the University’s sole discretion. The University of Pittsburgh is an affirmative action, equal opportunity institution. 09/23 Contents Department of Bioengineering 4 Department of Chemical and Petroleum Engineering 6 Department of Civil and Environmental Engineering 8 Department of Electrical and Computer Engineering 10 Department of Industrial Engineering 12 Department of Mechanical Engineering and Materials Science 14 Office of Diversity 16 Sustainability at Pitt 18 University of Pittsburgh Swanson School of Engineering Annual Report 2021 Executive Editor Paul Kovach, Director, Marketing and Communications Design . . . . . . . . . . . . . . . . . . . Leslie Karon-Oswalt, Senior Graphic Designer Writer Maggie Pavlick, Senior Communications Writer Writer . . . . . . . . . . . . . . . . . . . . Leah Russell, Content Manager/Editor
Placebo-Inspired Project Wins $12 Million Grant to Help Parkinson’s Patients

A University of Pittsburgh-led neurobiological expedition to explore a little-known brain region could show the way to new therapies for Parkinson’s disease. The inter-institutional team, funded by a $12 million, 3-year grant from the Aligning Science Across Parkinson’s (ASAP) initiative, will investigate brain circuits they suspect are involved in a placebo effect occasionally seen in the movement disorder.
Patients with Parkinson’s will sometimes show paradoxical kinesia, or the remarkable return of apparently normal motor function under special circumstances, like riding a bike or having the ability to quickly respond to a fire alarm. Principal Investigator
Peter L. Strick, chair of the Department of Neurobiology and scientific director of Pitt’s Brain Institute, and his collaborators,
including Bioengineering Assistant Professor Helen Schwerdt, believe they may know why this happens.
Parkinson’s disease occurs due to the death of nerve cells in a small brain area called the substantia nigra, part of the collections of nerve cells at the base of the brain known as the basal ganglia that interacts with the cerebral cortex through two prominent networks. One network forms a closed-loop neuronal circuit: a center within the basal ganglia sends outputs to a region in the cortex, which then sends input back to it. This circuit is damaged in Parkinson’s, and motor symptoms of the disease are treated with implanted electrodes, known as deep brain stimulation, at a node within this circuit.
4
Department of Bioengineering
The second network forms an open-loop circuit that receives input from brain sites such as the amygdala that are largely unaffected by Parkinson’s. The Pitt team suspects that the open-loop circuit provides the pathway for paradoxical kinesia and the placebo effect – its activation allows signals to reach the cerebral cortex and bypass the disease-affected closed-loop circuit.
“For some patients, placebos can be surprisingly effective in treating the movement disorders associated with the disease,” said Strick. “We think there is brain circuitry that makes this possible, so we plan to define it and explore its potential impact on Parkinson’s.”
Schwerdt will examine dynamic aspects of dopamine release in these animals, and William R. Stauffer, an assistant professor
of neurobiology, will use single-cell tools to characterize cell-type-specific gene expression and regulation in the two circuits before and after dopamine depletion.
“Implanted sensors degrade after a couple of hours, so much of our previous understanding of dopamine is in that very short time frame,” said Schwerdt. “Neurodegeneration is a long-term process over years or decades, so to understand and effectively treat neurodegenerative disorders, we need a more robust device.”
Before coming to Pitt, Schwerdt developed tools that dramatically extend the lifetime of these implanted sensors. With her improved device, she has successfully tracked dopamine in both rodent and primate species for more than a year.
5
Engineering Catalysts that Turn Seawater into Fuel
What if aircraft carriers could rely on the most abundant of local resources –seawater – to fuel the planes on board?

Thanks to seawater-to-fuel technology that has been in development for several years, scientists are able to use the onboard nuclear reactor and harness the carbon dioxide and hydrogen from seawater to create a liquid fuel that can power a jet engine. The technology would allow aircraft carriers to remain in continuous operation and avoid relying on tanker ships to replenish their fuel.
However, designing catalysts that can effectively create jet fuel from these common compounds is a difficult and costly process.
Researchers from the University of Pittsburgh and the University of Rochester seek to improve this process in a project
that recently received $300,000 from the Department of Defense Office of Naval Research. The project, led by the University of Rochester’s Marc Porosoff and Pitt’s Giannis Mpourmpakis, will refine a crucial step in the seawater-to-fuel process, making it more energy efficient, safer, and scalable.
The first step of fuel synthesis is converting the carbon dioxide (CO2) extracted from seawater into carbon monoxide (CO). Last summer, the team successfully demonstrated that molybdenum carbide catalysts efficiently and reliably convert CO2 to CO, achieving this critical first step in turning seawater into fuel. The newly funded project will expand on the previous work, seeking to further hydrogenate the carbon monoxide into usable fuels using FischerTropsch synthesis.
“Our goal with this project is to tune hydrocarbon selectivity during the
6
Department of Chemical and Petroleum Engineering
Pitt and University of Rochester Researchers Receive $300K to Develop Catalysts for Naval Seawater-to-Fuel Technology
hydrogenation of a mixture of CO and CO2,” said Porosoff, who is an assistant professor of chemical engineering at the University of Rochester and principal investigator of the project. “To do that, we’ll design, synthesize and test bimetallic, zeolite-based catalysts that selectively hydrogenate CO and create specific compounds, like olefins and heavier hydrocarbons, that can be used as fuels.”
Zeolites – minerals that contain aluminum and silicon – are commonly used as commercial catalysts. The researchers expect that catalysts based on zeolites and bimetallic particles will result in enhanced activity, selectivity and stability in the seawater-to-fuel application. The catalysts offer several other benefits, as well: They avoid reliance on expensive and rare precious metals and are highly tunable, meaning that researchers can control the acid-base properties to stabilize the desired reaction.
Mpourmpakis, associate professor of chemical engineering at Pitt’s Swanson School of Engineering and co-PI on the project, leads the Computer-Aided Nano and Energy Lab (CANELa), which specializes in using theory and computation to investigate
the physicochemical properties of nanomaterials for applications in catalysis, green energy generation and storage, and materials engineering. To test their hypothesis, Porosoff and Mpourmpakis will use computational modeling and machine learning to identify the characteristics of catalysts most likely to achieve their goal: the selective hydrogenation of CO in a mixture of CO and CO2 , limiting unwanted reactions that make less useful compounds like methane.
“This work will combine computational and experimental approaches to hopefully result in significant time, energy and cost savings over conventional experimental approaches,” said Mpourmpakis, who is also the Bicentennial Alumni Faculty Fellow at Pitt. “These control experiments are essential for the design of an integrated, modular system, and enable the implementation of the ‘seawater to fuel’ process in a way that is safe and efficient for the U.S. Navy.”
The two-year project is titled “Selective CO Hydrogenation Over Bimetallic Nanoparticles” and began on April 1st 2021.
7
Mapping PFAS Contamination in Packaged Food

When grabbing a sweet, sticky bun from the grocery store for breakfast, one might rejoice in the fact that it cleanly slides out of the wrapper and onto a plate. While consumers may not think twice about why it is not sticking, researchers are trying to shed light on how this convenient packaging could potentially expose humans to toxic chemicals called PFAS.
Per- and polyfluorinated alkyl substances (PFAS) are a class of man-made chemicals lauded for their nonstick and oil-repellent characteristics. While useful in the food industry, there is evidence that exposure to these persistent chemicals may lead to adverse outcomes in human health.
Supported by the Agriculture and Food Research Initiative (AFRI) of the USDA
National Institute of Food and Agriculture (NIFA), the University of Pittsburgh’s Carla Ng will lead a project that aims to be the first systematic study of the kinds and amount of PFAS that are present in imported and domestic food packaging. She and her collaborators from Indiana University and the USDA – Agricultural Research Service (ARS) will create a database that they hope will help guide better policy around the use of PFAS in the food industry.
“Humans are exposed to PFAS in a variety of ways, but depending on where you live, food is likely your major source,” said Ng, assistant professor of civil and environmental engineering at Pitt’s Swanson School of Engineering. “There are many different types of PFAS, and we don’t have enough information on where they are used,
8
Department of Civil and Environmental Engineering
in what quantities, and whether they’re toxic, so we will use this award to study those details.”
According to the FDA, there are nearly 5,000 different types of PFAS. To add to the complexity of this issue, other countries have adopted different approaches to regulating PFAS and its many varieties.
For example, PFOA and PFOS have been phased out in the United States, but they are still widely produced in China. While they do not send these specific chemicals to the U.S., there may be residual chemicals that are transferred during production.
“Because of these uncertainties, we want to understand how all the different origins of packaging will impact which PFAS actually wind up in the consumer product,” said Ng. The research team will inspect national supermarket chains and local international food stores to get an idea of the type and geographic origin of food packaging. They will then collect a representative sample of products and analyze the packaging for the presence of PFAS.
“We will use extraction and migration assays to evaluate the packaging,” explained Ng. “Extraction would represent an extreme case where we use harsh chemicals to gather a sample. Alternatively, the migration assays use simulants which represent different types of food – such as fatty, acidic, or salty. It will show, under normal conditions, how much PFAS transferred from packaging to food.”
ARS researcher Yelena Sapozhnikova will contribute to this work by identifying PFAS chemicals migrating from food packaging materials with non-targeted, high-resolution mass spectrometry. Sapozhnikova’s interest in this research is a direct result from her previous work on identification of chemicals from food contact materials.
Once the PFAS structures are identified, they will go to Amina Salamova, associate scientist at IU’s O’Neill School of Public and Environmental Affairs, whose team will quantify how much of each structure is in the sample.
“We’re excited to conduct research that has such big implications for consumer safety,” Salamova said. “This research will help us understand a lot more about a group of chemicals that are widely used but not well understood.”
From there, the analyzed extracts and simulants will go to Pitt to be tested for toxicity. Ng’s lab specializes in molecular modeling that can initially screen the samples before evaluating them in zebrafish for further validation.
The results of the project will reveal whether the chemicals present in the packaging are toxic and if the concentration is high enough to contaminate your food. The researchers hope that this work will inform regulators, provide a risk assessment tool, and potentially reveal hot spots for PFAS exposure in our food system.
9
Snails Carrying the World’s Smallest Computer Help Solve Mass Extinction Survivor Mystery
To understand how one species of tree snail in the South Pacific Society Islands survived when more than 50 others were wiped out, biologists at the University of Michigan needed a tiny sensor that could be fixed to a snail’s shell and track how much sunlight the snail encountered every day.

Inhee Lee, assistant professor of electrical and computer engineering at the University of Pittsburgh Swanson School of Engineering, helped to develop the Michigan Micro Mote (M3), a sensor considered the world’s smallest computer, while at the University of Michigan. Lee was able to adapt the sensor for the biologists’ purposes, helping to solve the mystery of the snails’ survival.
“We were able to get data that nobody had been able to obtain,” said David Blaauw, the Kensall D. Wise Collegiate Professor of Electrical Engineering and Computer Science at the University of Michigan. “And that’s because we had a tiny computing system that was small enough to stick on a snail.”
The M3, considered the world’s smallest complete computer, was announced in 2014 by a team Blaauw co-led. This was its first field application.
The sensor needed to be able to determine whether their white shells gave these snails an evolutionary advantage by tracking light. Since the sensor could already recharge its own batteries with solar cells, Lee realized he could continuously measure the light level by measuring the speed at which the battery was charging.
10
Department of Electrical and Computer Engineering
Inhee Lee Adapts Sensor to Measure the Light Snails Encounter
“It was important to understand what the biologists were thinking and what they needed,” said Lee. “We already had a tiny sensor design, but we needed to change the sensors to detect light. Since the sensor could already recharge its own batteries with solar cells, we can continuously measure the light level by measuring the speed at which the battery is charging.”
After local testing enabled by local Michigan snails, 50 M3s made it to Tahiti in 2017. Bick and Lee joined forces with Trevor Coote, a well-known conservation field biologist and specialist on the French Polynesian snails.
The team glued the sensors directly to the rosy wolf snails, but P. hyalina is a protected species and required an indirect approach. They are nocturnal, typically sleeping during the day while attached underneath leaves. Using magnets, the team placed M3s both on the tops and undersides of leaves harboring the resting P. hyalina. At the end of each day, Lee wirelessly downloaded the data from each of the M3s.
The data revealed a dramatic difference in how much sun reached the habitats of the surviving P. hyalina as opposed to the rosy wolf snail. During the noon hour, the P. hyalina habitat received on average 10 times

more sunlight than the rosy wolf snails. The researchers suspect that the rosy wolf doesn’t venture far enough into the forest edge to catch P. hyalina, even under cover of darkness, because they wouldn’t be able to escape to shade before the sun became too hot.
The success of this project broke new ground from the perspective of the engineers as well as the biologists.
“It’s underappreciated how large a step it is to go from the lab into the field and get meaningful data,” said Blaauw. “It’s essential to achieve success in order to propel the technology forward, but takes a great deal of trust among the collaborators.”
11
Finding the Fountain of Youth for Catalysts
Carbon nanotubes (CNTs) – tiny filaments of carbon, each smaller than one tenthousandth the size of a human hair –have shown transformative potential for electronics, energy storage devices, and high-strength/low-weight composites. The dominant method to synthesize CNTs, called catalytic chemical vapor deposition, uses metal nanoparticles as catalysts that grow CNTs from the bottom up, much like trees growing from seeds in a forest. The high-temperature process, however, changes the nanoparticles over time, meaning the catalysts have a limited lifespan for use.
Industrial Engineering researchers at the University of Pittsburgh have developed a new method to significantly extend the lifetime of nanocatalyst “seeds” for the bottom-up growth of carbon nanotubes.

“An increasing number of chemical conversion technologies depends on oxidesupported metal catalysts, such as iron, nickel, and copper, but catalytic deactivation eventually kicks in, which is undesirable because it greatly limits the yield of the process,” said Mostafa Bedewy, assistant professor of industrial engineering, who led the study. “In the case of carbon nanotube growth from nanoscale catalyst seeds, this progressive deactivation leads to the death of individual nanotubes among a population of billions per square centimeter. What’s interesting in our study is that we leverage rapid temperature changes to delay catalyst deactivation and extend the catalytic lifetime toward growing indefinitely tall CNTs.”
Bedewy, who also holds secondary appointments in the departments of chemical and petroleum engineering,
12
Department of Industrial Engineering
Pitt Engineers Develop a New Method to Extend the Catalytic Lifetime of Nanoparticles
and mechanical engineering and materials science, established the NanoProduct Lab in 2016. Engineers in his research group focus on fabricating high-density arrays of vertically aligned carbon nanotubes “forests.” This is accomplished by catalytic chemical vapor deposition and using surface-bound metal nanoparticles as a catalyst. Bedewy’s research, funded by the National Science Foundation, utilizes a custom-designed multizone reactor for chemical vapor deposition with unique rapid thermal processing capabilities.
The present study builds on previous work by the same group, which showed for the first time the ability to decouple the following three different components of the process from each other using this one-of-a-kind reactor: preparation of catalyst nanoparticles by thin-film dewetting, thermal decomposition of precursor gas, and nucleation and growth of carbon nanotubes.
PhD student Golnaz Tomaraei and former postdoc Jaegeun Lee worked together on this project with assistance from PhD student Moataz Abdulhafez, who are all authors of the paper that appeared in Chemistry of Materials.
“While previous work by other research groups either demonstrated
decoupling of gas-phase reactions from catalytic growth of nanotubes, or demonstrated decoupling of catalyst formation from catalytic growth of nanotubes, our approach is the first to be able to completely decouple these three different processes,” said Lee. “And that is what enables us to rapidly heat the catalyst to a high temperature before growth starts for catalyst formation by reduction and dewetting, before changing temperature back to a lower temperature that is appropriate for growth.”
The team also reveals the underlying mechanisms for the observed boost of catalytic lifetime.
“It turns out from our comprehensive characterization of the metal catalyst and the oxide support layer that the loss of catalyst by subsurface diffusion was suppressed when we implemented the rapid thermal pretreatment step at high temperature, which delayed catalyst deactivation,” said Tomaraei. “We show that thermal treatment at 900°C makes the alumina support layer denser and less porous with higher film crystallinity and Lewis basicity.”
This work is a promising approach for controlling the performance of nanocatalysts by using a unique knob based on dynamic process recipes.
“This outstanding study on thermochemical pretreatment of catalyst for carbon nanotube growth provides valuable insight into some of the fundamental questions in the field and would benefit researchers working on process design and catalyst engineering for chemical vapor deposition of long carbon nanotube arrays,” said Placidus Amama, associate professor and Tim Taylor chair in chemical engineering at Kansas State University, who was not involved in this study.
While the next steps for this work will leverage rapid thermochemical pretreatment for efficient manufacturing of tailored carbon nanotubes, the insights can also be extended to other oxide-supported catalyst systems utilized in other chemical conversion processes in industry.
The paper, “Boosting Catalytic Lifetime in in Chemical Vapor Deposition of Carbon Nanotubes by Rapid Thermal Pretreatment of Alumina-Supported Metal Nanocatalysts,” (DOI:10.1021/acs. chemmater.0c04692) was coauthored by Jaegeun Lee, Golnaz Najaf Tomaraei, Moataz Abdulhafez, and Mostafa Bedewy.
13
A Microscopic Look At Aneurysm Repair
Hitting a pothole on the road in just the wrong way might create a bulge on the tire, a weakened spot that will almost certainly lead to an eventual flat tire. But what if that tire could immediately begin reknitting its rubber, reinforcing the bulge and preventing it from bursting?
That’s exactly what blood vessels can do after an aneurysm forms, according to new research led by Pitt’s Swanson School of Engineering and in partnership with the Mayo Clinic. Aneurysms are abnormal bulges in artery walls that can form in brain arteries. Ruptured brain aneurysms are fatal in almost 50 percent of cases.
The research, published in Experimental Mechanics, is the first to show that there are two phases of wall restructuring after an aneurysm forms, the first beginning right away to reinforce the weakened points.
“Imagine stretching a rubber tube in a single direction so that it only needs to be reinforced for loads in that direction. However, in an aneurysm, the forces change to be more like those in a spherical balloon, with forces pulling in multiple directions, making it more vulnerable to bursting,” explained Anne Robertson, professor of mechanical engineering and materials science at Pitt, whose lab led the research. “Our study found that blood vessels are capable of adapting after an aneurysm forms. They can restructure their collagen fibers in multiple directions instead of just one, making it better able to handle the new loads without rupturing.”
Researchers have known that blood vessels have the ability to change and restructure over time, but this study represents the first observation of a new, primary phase of restructuring that begins immediately.
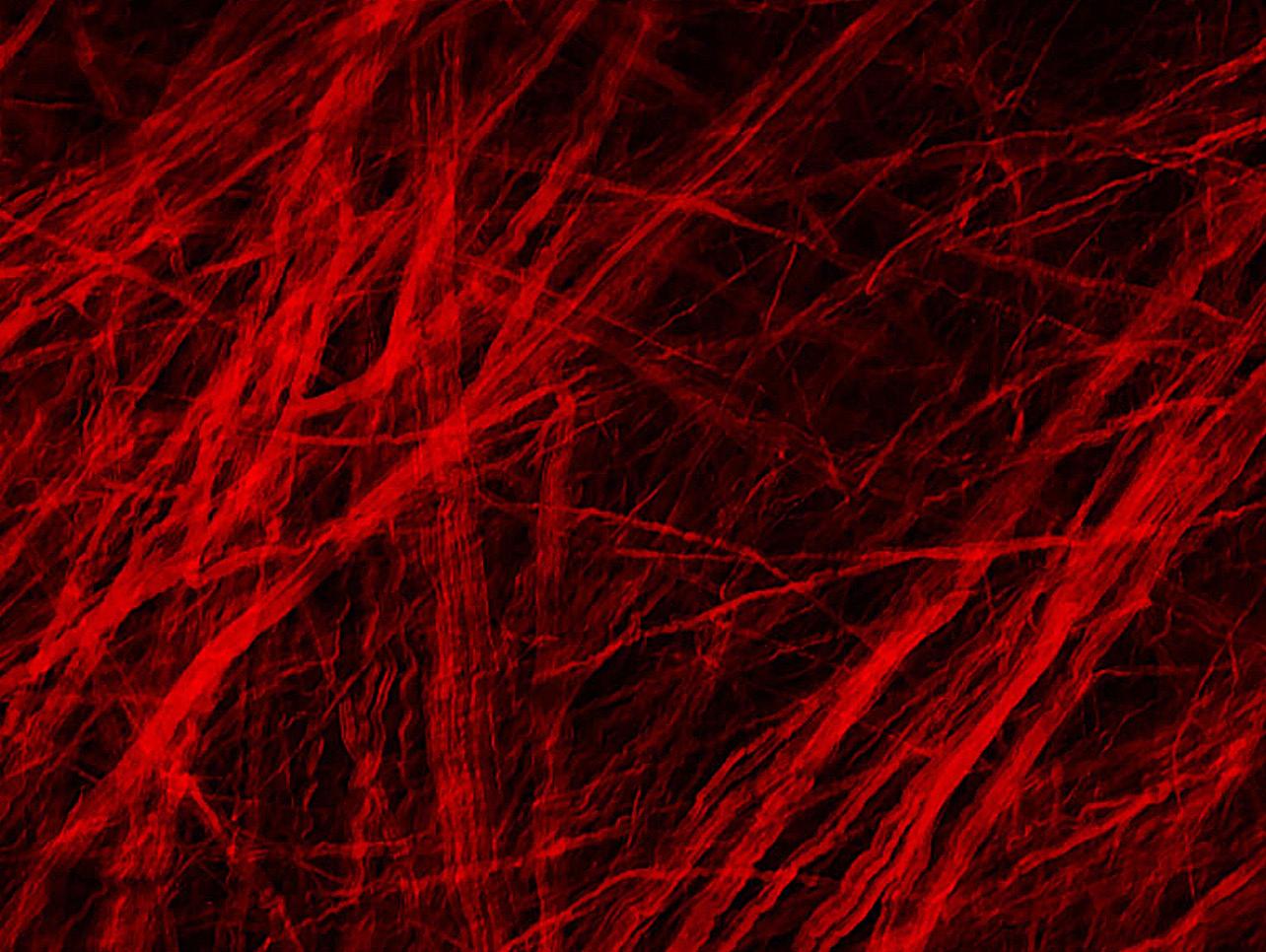
14
Department of Mechanical Engineering and Materials Science
Pitt and Mayo Clinic Discover New, Immediate Phase of Blood Vessel Restructuring After Aneurysm
The researchers used a rabbit model developed by David Kallmes of the Mayo Clinic to observe this restructuring in the brain tissue over time. To see this process up close, the researchers partnered with Simon Watkins at Pitt’s Center for Biologic Imaging, taking advantage of the center’s state-of-the-art multiphoton microscopes to image the architecture of the fibers inside the aneurysm wall.
“We found that the first phase of restructuring involves laying down an entirely new layer of collagen fibers in two directions to better handle the new load, while the second phase involves remodeling existing layers so their fibers lie in two directions,” explained Chao Sang, who was a primary investigator on this research as part of his doctoral dissertation in Pitt’s Department of Mechanical Engineering and Materials Science.
“The long-term restructuring is akin to a scar forming after a cut has healed, while this first phase that we observed can be thought of as having a role similar to clotting immediately after the cut – the body’s first response to protect itself,” added Robertson, who has a secondary appointment in the Pitt’s Department of Bioengineering. “Now that we know about this first phase, we can begin to investigate how to promote it in patients with aneurysms, and how factors like age and preexisting conditions affect this ability and may place a patient at higher risk for aneurysm rupture.”
The investigative team includes Robertson and graduate students Chao Sang and Michael Durka from Pitt, Simon Watkins from the Center for Biologic Imaging, and David Kallmes, Ramanathan Kadirvel, Yong Hong Ding, and Daying Dai from the Mayo Clinic’s Department of Radiology.
15
Sussan Olaore Receives Chancellor’s Award for Commitment to Equity, Diversity and Inclusion
In recognition of her commitment to diversity, equity and inclusion, Sussan Yetunde Olaore was selected as one of nine recipients of the University of Pittsburgh’s 2021 Chancellor’s Award for Staff.
Olaore has been the Pitt STRIVE program coordinator since she joined the Swanson School of Engineering in 2016. She manages day-to-day operations, helping underrepresented minorities (URMs) successfully transition into and complete doctoral engineering programs at Pitt.
The success of Pitt STRIVE was recognized by the University in 2019 when it received the University Prize for Strategic, Inclusive and Diverse Excellence (UPSIDE) Award by the Office of Diversity and Inclusion.

“We received this grant to help underrepresented students pursue PhDs, and it is really fulfilling to see these students
thrive in the program and find jobs in industry or academia,” Olaore said.
The program also aims to cultivate a more diverse professoriate in U.S. colleges and universities, where URMs take up a small fraction of the professoriate compared to student populations. For Olaore, contributing to this mission has been a source of pride.
“I’m thrilled that two of our alumni have become professors in the area,” she said. “Katrina Knight is now an assistant professor in Pitt’s Department of Bioengineering, and Sossena Wood, a Presidential Post-Doctoral Fellow in Biomedical Engineering at Carnegie Mellon University, will be appointed assistant professor in the next academic year.
“I also helped recruit David Jordan, who defended his dissertation in the spring and is now a postdoctoral researcher at the University of Arizona.”
16
Office of Diversity
In addition to academic support, the Pitt STRIVE program and its administration have also provided personal support to URM students. They created the Pitt STRIVE Graduate Community that fosters opportunities and allows students to socialize and work in multicultural groups. They also established an annual MentorMentee Retreat which provides a platform for participants to interact in an informal setting, breaking down barriers and protocols between mentors and mentees. Throughout the pandemic, when in-person events were canceled, Olaore organized virtual hangouts to provide a safe social space and nurture collegiality.
“It’s very simple. The STRIVE program would not have been nearly as successful without Sussan,” said Steven Abramowitch, associate professor of bioengineering at Pitt. “She is selfless and extremely dedicated to our students.”

17
Covestro LLC and University of Pittsburgh Collaborate to Establish Groundbreaking Circular Economy Program
A new collaboration between the University of Pittsburgh and Covestro LLC takes aim at decreasing global waste and its impact on the environment and climate. The Covestro Circular Economy Program will enable students at the University of Pittsburgh to become experts in circular economy principles, informed by Covestro’s successes in this area, and ultimately create circular, sustainable products and service solutions.
Pitt’s Mascaro Center for Sustainable Innovation (MCSI) and Swanson School of Engineering will house the new Covestro Circular Economy Program at the University of Pittsburgh.
“The current linear consumption economy of ‘Take, Make, Waste’ is wholly unsuited for exponential global growth, especially as third-world economies evolve,” says Eric Beckman, Distinguished Service Professor and MCSI Director Emeritus and Chief of Innovation and Translation. “Principles of
the circular economy, however, improve efficiency and eliminate waste by designing sustainability into a product, from its base materials and construction to packaging, delivery, and life expectancy.”
The Covestro Circular Economy Program represents the first graduate-level circular design academic program in the U.S. to specifically address the challenge of global waste and material use. The program aims to create opportunities for the research, education, and innovative advancement of circular economy principles that begin with academia and fuel real-world solutions designed to save the planet.
The initial funding will help to establish a transdisciplinary academic, research, innovation and cooperative employment initiative to prepare students with circular economy training and expertise to carry into academia, industry, government and NGOs. Pitt and Covestro are also seeking to
18
Sustainability at Pitt
collaborate with corporations, foundations, and governments to expand the program’s reach and potential.
“Circular design involves a paradigm shift in thinking for everyone, from individuals to corporations to societies,” says Richard Skorpenske, head of Sustainability and Public Affairs at Covestro LLC. “As a leader in driving toward a circular economy, we see the Covestro Circular Economy Program as an important multiplier to build a robust foundation of circularity-focused thinkers, and we are proud to launch it alongside Pitt as founding partner.”
The Covestro Circular Economy Program is solving for a gap that currently exists within the academic arena. Professional training in the relevant sciences has not included holistic training in circular approaches. While the private sector embeds circular design principles into its innovation approach, academia has yet to integrate design principles in advanced degree programs. By establishing the program, Covestro and the University of Pittsburgh are providing a dedicated academic setting for passionate students and professionals to innovate new approaches to materials, design, and planning.
Through the Covestro Circular Economy Program, Covestro and Pitt plan to create new fundamental science that supports the assembly of new tools to aid circular design. The Program will enable graduate students at Pitt to become experts in circular economy principles, informed by Covestro’s advances in this area, and ultimately create circular, sustainable products and service solutions. The first cohort of graduate students will be recruited for fall 2022.
“I am very excited to apply the fundamental research we’ve developed in circular economy and expand it to create a holistic program with potential global impact,” says Melissa Bilec, William Kepler Whiteford Professor and MCSI co-director. “I believe that, like Covestro, other organizations will see the benefit of engaging in such a program that trains scholar-workers in the many possibilities that circular economy presents. Most especially, the transdisciplinary nature of our program will be its most distinct attribute. As the benefits of the circular economy grow across the globe, the necessity for it to transcend traditional STEM fields will become apparent. This opens our program to anyone with a true passion for sustainability and global impact.”
19
Swanson School of Engineering 104 Benedum Hall 3700 O’Hara Street Pittsburgh, PA 15261 engineering.pitt.edu









