
6 minute read
NEWS
New FDA Insight Podcast on Emerging Topics
Dr. Anand Shah, FDA’s deputy commissioner for medical and scientifi c aff airs, hosts a new podcast with other FDA leader guests as they provide their insight into issues facing the agency—including the COVID-19 pandemic and other emerging topics. As Dr. Shah explains, “The goal of our podcast is to educate our many stakeholders about the products that we regulate, the issues we face, and the processes that we follow in everyday plain language. We’ll be discussing COVID-19 and non-COVID-19 related topics.” New podcasts appear on Tuesdays and most are under 15 minutes in length. — Keren Sookne
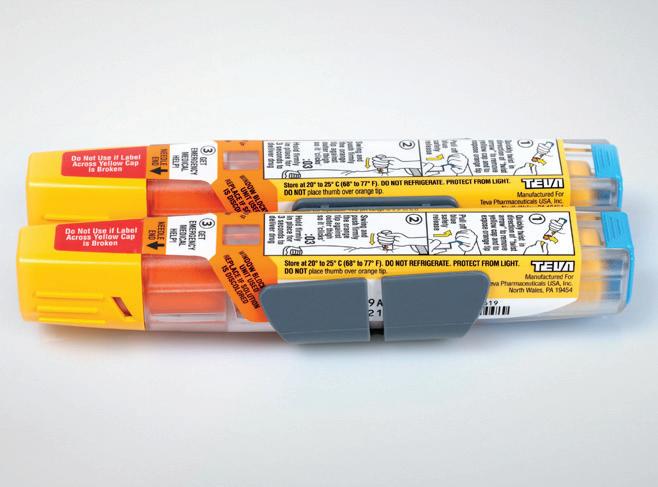
TEVA’s Abrasion-Resistant Adrenaline Injector Label
TEVA off ers adrenaline in a single-dose, prefi lled disposable autoinjector for life-threatening allergies. The company selected an autoinjector label with special protection against abrasion and scratch-resistant reverse printing developed by Schreiner MediPharm. Allowing vital user and product information to remain legible over an extended period of time, the TLMI award-winning label is particularly resistant against chemical/mechanical impacts it may encounter while being carried (e.g. in a purse or backpack). An additional cover for protection is not necessary, which reduces waste and environmental footprint. —Keren Sookne
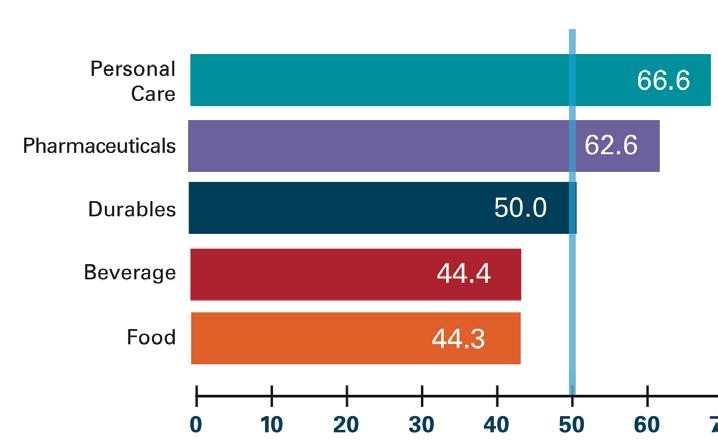

Healthcare Market Equipment Investments Poised for Growth in 2021
According to the May 2020 U.S. Packaging Machinery Purchasing Index report by PMMI Business Intelligence, end users in personal care and pharmaceuticals/medical devices are optimistic about current business conditions driving expansion in 2021, despite COVID-19 shutdowns and oil market surpluses creating a drop in other industries. Respondents in life sciences are reporting some expansion in planned projects, and increased usage of remote access. End users in the personal care industry are outperforming the total market in all metrics, likely due to demand for items such as hand soaps, sanitizers, and other assorted toiletries. Download your FREE copy of the report at hcpgo.to/380. — Kim Overstreet
Proposed Med Device Standard Will Help Validate Cleaning Methods
A proposed ASTM International standard presents methods for detecting and quantifying cleaning markers (analytes) on reusable medical devices.
The goal of the proposed standard is to help medical device manufacturers, testing laboratories, and regulatory bodies identify the appropriate method(s) for evaluating whether a medical device can be adequately cleaned.
ASTM’s committee on medical and surgical materials and devices (F04) is developing the proposed standard. ASTM welcomes participation in the development of its standards. Become a member at www.astm.org/JOIN. — Keren Sookne
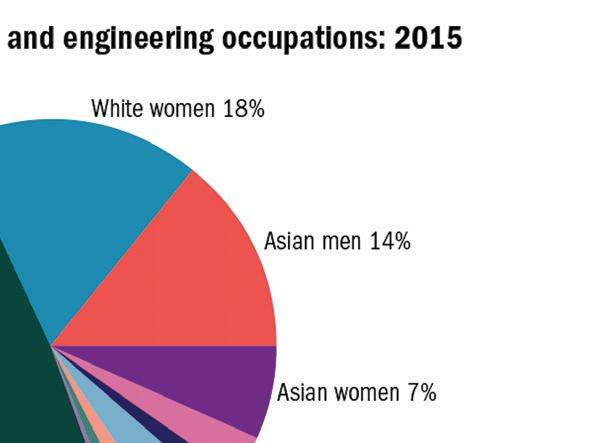
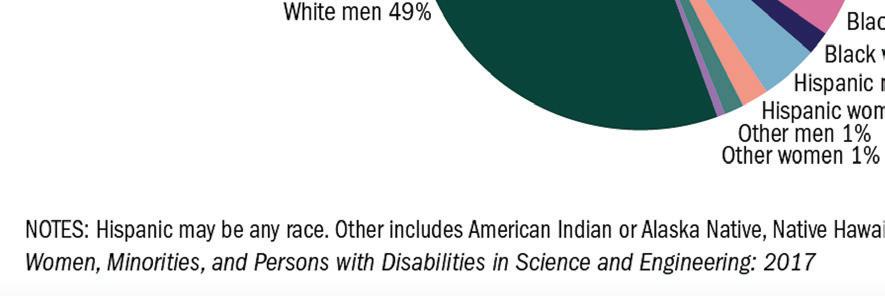
Viral Hashtag Highlights Disparities in Academia, Healthcare
It's critical to hear the experiences of Black scientists and academics as one facet of workplace and societal equality eff orts (to be clear, there are many facets). To view tweets with personal stories, check out Twitter for #BlackInTheIvory, which refers to the “ivory tower” of academia.
In a recent Nature article, Nidhi Subbaraman spoke to the viral hashtag’s founders, Dr. Shardé Davis and Joy Melody Woods—assistant professor at UCONN and PhD student at the University of Texas at Austin, respectively—about how the hashtag began, the fear of repercussions, and more. Note that the stories shared are not an exhaustive list. — Keren Sookne
Study Evaluates Benefi ts of Atmosphere Control in Blisters
FreeThink Technologies, Inc., Aptar CSP Technologies, and PCI Pharma Services collaborated on a research study comparing the effi cacy of Activ-Blister packaging confi gurations with cold-form foil in maintaining the stability of a model tableted drug product. Activ-Blister technology with molecular sieve (as well as Activ-Blister technology with silica gel) was found to be more protective than cold-form foil or thermoform blister alone. The advantage over cold-form foil was particularly pronounced when the drug product had a high initial water content. The ASAPprime program was found to be eff ective at modeling packaging confi gurations incorporating molecular sieve and silica gel versions of Activ-Blister. For more: hcpgo.to/379. — Keren Sookne

Companies Collaborate to Bring Circular Materials to the Biomedical Industry
Westfall Technik is announcing a new partnership with Polycarbin—a software enabled biomedical plastic recycling company that has developed a method for diverting single-use scientifi c plastics from landfi lls and incinerators, and recycling it back into the hands of scientists and clinicians as circular economy products. Using a system that leverages low-cost, frontend segregation and a waste analytics platform, Polycarbin is positioned to capture and repurpose valuable scientifi c plastics. Polycarbin’s services aim to provide more cost-eff ective and environmentally responsible waste management solutions to research labs, biopharma, and clinical labs while closing the loop on the biomedical plastic life cycle. — Keren Sookne
Med Devices and the FDA: Extended Deadline, Worker Safety
The FDA issued an immediately-in-eff ect guidance on Jul. 1, 2020 on its policy regarding compliance dates for class I and unclassifi ed devices that are not implantable, life-supporting, or life-sustaining. The guidance cited coronavirus as one reason for the extension, noting that “for those labelers that have not already implemented UDI requirements for class I and unclassifi ed devices, preparing to implement UDI requirements while addressing the challenges related to Coronavirus Disease 2019 (COVID-19) could be very diffi cult and could divert resources from COVID-19 response eff orts.” Additionally, CDRH recognized the need for reducing the risk of COVID-19 exposure among medical device manufacturing personnel, issuing 10 recommendations for worker safety in device production environments. — Keren Sookne
Custom Medical Thermoformed Packaging and Device Solutions
TEQ is dedicated to being a leader of quality thermoform packaging and devices. Through continuous innovation and global locations, TEQ is uniquely positioned to provide customers total packaging solutions, now with the ability to offer medical heat sealing machines and tooling.


Medical Device and Pharmaceutical Packaging
TEQ produces only the highest quality medical, and pharmaceutical packaging that meets the most exacting quality control standards. Medical Heat Sealing Machines and Tooling
Sonoco Alloyd is the leading provider of medical heat sealing machines that improve operating efficiencies and reduce costs through ergonomically enhanced designs.
Thermoformed Medical Instrument Covers
From ear thermometer probe covers to medical light handle covers, TEQ can manufacture the most complex thermoformed medical devices.
“The big picture? Packaging isn't seperate from society, it's a reflection of it. As such, the industry is constantly struggling to reinvent itself to match the society it serves. And that's not going to stop anytime soon. ” -MATT REYNOLDS, EDITOR AT PACKAGING WORLD
“Another key [UDI] benefit, because we're calling that product by the same name across the healthcare supply chain… rich and robust data that we can access around supply consumption and utilization rates. One of the buzz terms we've heard during the COVID-19 crisis was, 'What's my burn rate? How fast am I going through gowns, masks, gloves?' ” – MIKE SCHILLER, SENIOR DIRECTOR AT AHRMM
“As reported in the peer-reviewed article, Importance of Climate Risks for Institutional The Investors, researchers found in a survey of 439 investment professionals that only 7% of
institutional investors said they had done
nothing to manage climate risks in the last five years. ” -REUSABLE PACKAGING ASSN.’S INNER LOOP BLOG (JUN. 8)
Jun. 25, 2020
THE DATE that the WHO marked as the end of the 2018 Ebola outbreak in the Democratic Republic of the Congo (DRC), noting that vigilance against flare-ups must continue.
52%
THE PERCENTAGE of CPGs that agree that consumers need to be better educated to understand sustainability and what it really means for packaging design and costs. Source: PMMI’s “Packaging Sustainability: A Changing Landscape”
$301 MILLION
THE PROJECTED GROWTH of the medical device security solutions market between 2020 and 2024.
Source: Technavio
23%
THE REDUCTION in emergency department (ED) visits for heart attack in the 10 weeks following declaration of the COVID-19 national emergency. Per the CDC, stroke and hyperglycemic crisis visits declined as well.










