Standard Precautions for Infection Prevention & Control including Transmission Based Precautions (TBP’s) and Isolation
precautions.
Version: V6
Ratified by: Quality and Safety Committee
Date ratified: 19/07/2023
Job Title of author: Specialist Infection Prevention nurse
Reviewed by Committee or Expert Group Infection Prevention Group
Equality Impact Assessed by: Specialist Infection Prevention Nurse
Related procedural documents
IPPOL03 Infection Prevention & Control Hand Hygiene Policy
IPPOL09 Decontamination of Medical Equipment
IPPOL14 Management of Blood Borne Viruses Policy
IPPOL16 Management and Control of an Outbreak of Infection
IPPOL18 – Management & Safety of Sharps
Review date: 19/07/2026
It is the responsibility of users to ensure that you are using the most up to date document template – i.e. obtained via the intranet.
In developing/reviewing this policy Provide Community has had regard to the principles of the NHS Constitution.
Version Control Sheet
Version Date
Author Status Comment
V1 June2012 Head of Infection Prevention & control Ratified Replaces section3of Infection Control Guidelines. Short review date, because of new sharps safety legislation –May2013
V2 February2013 Head of Infection Prevention & control Ratified Review
V2.1 September 2013 Quality & Safety Administrator No change to reviewdate. Updatedinline with Organisation name change andrestructure.
V3 November 2014 Head of Infection Prevention& control Ratified Review due to EPIC 3 new Guidance
V4 November 2016 Head of Infection Prevention& control 2-yearreview
V5 June2022 Lead for Infection Prevention & control Updatedinline with New National Infection Preventionand ControlManual England June 2022 and includes Transmission based precautions TBP’s
V6 May2023 Updatedinline with National infection prevention and control for England April 2023
1. Introduction
Standard Infection Control Precautions (SICPs) are basic infection prevention and control measures necessary to reduce the risk of transmission of infectious agent from both recognised and unrecognised sources of infection.
These sources of (potential) infection include blood and other body fluids, secretions or excretions (excluding sweat), non-intact skin ormucous membranes, any equipment or items in the care environment that could become contaminated, including the care environment itself if not cleaned and maintained.
To be effective in protecting against infection SICPs must be applied continuously by all staff. The application of SICPs during care delivery must take account of the following risks:
• The risk to and from the individual for whom care is being provided
• The risk of the task to be undertaken
• The level of interaction
• The anticipated level of exposure to blood and or other body fluids.
Doing so allows staff to safely apply each of the 10 SICPs by ensuring effective infection prevention and control is maintained.
2. Purpose
The purpose of this policy is to outline the measures in place to prevent the transmission of Healthcare Associated Infections (HCAIs) and other micro-organisms to both patients and staff. This policy should be read in conjunction with other Infection Control policies.
The policy outlines all the necessary personal protective equipment (PPE) to be worn by healthcare staff.
3. Definitions
SICPs
Standard Infection Control Precautions (SICPs) are infection prevention and control measures necessary to reduce the risk of transmission of micro-organisms from both known and unknown sources of infection.
PPE
Personal Protective Equipment is intended to protect both patients and Healthcare Workers from the risk of cross infection. All Healthcare Workers must be trained in the appropriate use of PPE.
Alcohol Based Hand Rubs (ABHRs)
Used for quick decontamination of hands if hands are not physically soiled
Transmission-based Precautions (TBPs)
TBPs are applied when SICPs alone are insufficient to prevent transmission of a microorganism. TBPs are categorised by the route of transmission of the infectious agent.
Donning & Doffing
A sequence for putting on (donning) and taking off (doffing) Personal Protective Equipment to reduce the risk of contamination during the procedure.
5 Moments of hand Hygiene
World Health Organisation initiative showing the 5 most important moments when you must wash your hands
4. Duties
Chief Executive
The Chief Executive has overall responsibility for ensuring staff working for Provide have the necessary management systems in place to enable the effective implementation of this policy and has overall responsibility for the health and safety of staff, patients and visitors.
Director of Infection Prevention and Control (DIPC) and Executive Clinical & Operations Director
The DIPC will have operational responsibility for the effective implementation of this policy. The Executive Clinical & Operations Director has strategic responsibility for ensuring systems are in place to facilitate nursing staff awareness of this policy and appropriate support is given to enable staff in delivering practice as outlined in this policy.
Assistant Directors (ADs) of Services
All ADs have responsibility for ensuring systems are in place to facilitate awareness by all clinical staff of this policy and give appropriate support to enable staff in the delivery of the practice outlined in this policy.
Infection Prevention Team
The infection prevention team are responsible for ensuring all staff are made aware of this policy and advising staff on all infection prevention and control issues
Head of Estates & Facilities
The Head of Estates & Facilities provides the professional leadership for cleaning services and is responsible for providing the operational cleaning framework within Soft FM services.
All Staff
All staff must comply with this policy and act responsibly, liaising with the infection prevention team in a timely manner for advice and support. All staff have a responsibility to ensure that infection prevention is embedded into their everyday practice and is always applied consistently.
5. Consultation and Communication
This policy will be ratified by the Infection Prevention Group and the Infection Prevention Team. Once ratified the policy will be uploaded onto the Provide Platform under MyCompliance.
6. Monitoring
This policy will be monitored every 3 years as per Provide Company policy by the Infection Prevention Team. This policy will be updated with any new significant/urgent national or local evidence-based guidance prior to this policy’s review date if required.
All staff should have training, and a knowledge and understanding of standard Infection Control Precautions relating to their workplace by attending Provide induction, completing local induction, via the green card workbook, risk assessment for specific purposes and mandatory training days. A record of training is maintained by the individual ward/departmental manager, via electronic staff records using e-learning, ESR.
7. Process Standard Precautions (SICPs) including Enhanced Precautions
SICPs apply to the care of all patients regardless of diagnosis or presumed infection status, where there is possible contact with blood and other body fluids, secretions or excretions (excluding sweat), non-intact skin or mucous membranes, and any equipment or items in the care environment that could have become contaminated
The application of SICPs during care delivery is determined by a risk assessment, which assesses the risk from and to a patient and includes any risk from the task being undertaken, the level of interaction and the anticipated level of exposure to blood and any other body fluids, including exposure to chemicals.
SICPs must therefore be used by all staff, in all care settings, at all times and for all patients/individuals, whether their infection status is known or not This ensures the safety of all patients, staff, visitors and relatives.
THE ELEMENTS OF SCIPs are as follows:
1. Patient placement and assessment for infection risk (screening/triaging/testing)
2. Hand hygiene
3. Respiratory and cough hygiene
4. Personal protective equipment (PPE)
5. Safe management of the care environment
6. Safe management of care equipment (decontamination of equipment)
7. Safe management of healthcare linen
8. Safe management of blood and body fluids (spillage management)
9. Safe disposal of waste (including sharps)
10. Occupational safety: Safe handling of sharps – prevention and exposure management
Enhanced precautions include transmission-based precautions (see section 22)
8. Patient Placement
Patients must be assessed for their risk of infection on arrival at the care area (if possible, prior to accepting a patient). This assessment should influence the patient’s placement taking into account the patient’s clinical/care need(s). The assessment should be continuously reviewed throughout the patient’s stay
Patients who may present a particular cross-infection risk should be isolated on arrival and appropriate clinical samples and screening undertaken as per national and local protocols to establish the cause. This includes but is not limited to patients with symptoms such as:
• Loose stools or diarrhoea
• Vomiting
• Fever
• Unexplained rash
• Respiratory symptoms
As well as:
• Those known or suspected to have been previously positive with a Multi-drug Resistant Organism (MDRO) e.g., MRSA, CPE
• Those with a known epidemiological link to a carrier of CPE
• Those who have been an inpatient in any hospital in the UK or abroad in recent months (usually within the past 12 months).
Following relevant screening, triaging, and testing, patients should be regularly reviewed for respiratory symptoms throughout their hospital stay and should managed in line with local policies if they develop respiratory symptoms during their admission.
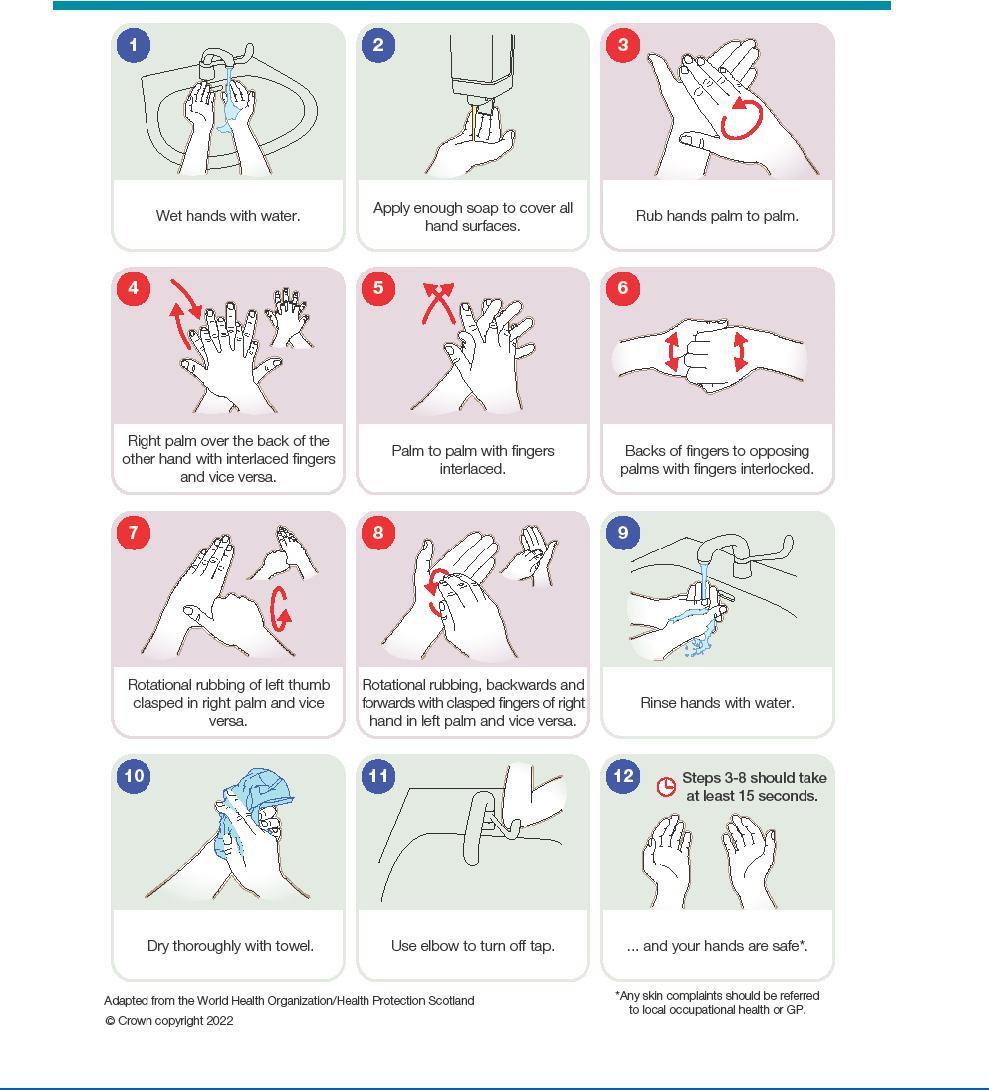
9. Hand Hygiene
Hand Hygiene
Hand hygiene is considered one of the most important ways to reduce the transmission of infectious micro-organisms.
Healthcare Workers must ensure that their hands can be decontaminated effectively by:
• Removing all wrist jewellery and stoned rings when in the clinical area (in community this is when providing direct patient care)
• Ensuring they are ‘bare below elbows’ for all direct patient care and in all clinical areas
• Ensuring that nails are short, clean, and free from false nails, gels or varnish, including clear varnish
• Covering any minor wounds or skin lesions with a waterproof dressing. Staff with large wounds, infected cuts, eczema, dermatitis, or any other skin condition must seek advice from the Occupational Health department.
To perform hand hygiene:
• Wash hands with non-antimicrobial (plain) liquid soap and water if:
o Hands are visibly soiled or dirty
o Caring for patients with vomiting or diarrhoeal illnesses
o Caring for a patient with a suspected or known gastrointestinal infection e.g. norovirus or a spore-forming organism such as Clostridioides difficile.
In all other circumstances, use alcohol-based handrubs (ABHRs) for routine hand hygiene during care.
Alcohol Based Hand Rubs (ABHRs) must be available for staff as near to the point of care as possible. Where this is not practical, such as in the community, personal ABHR dispensers supplied by Provide must be used.
If running water is unavailable, or hand hygiene facilities are lacking (or not considered fit for purpose) then staff may use handwipes followed by ABHR and should wash their hands at the first opportunity.
Hands must be decontaminated in all the following circumstances:
5 Moments of Hand Hygiene
• Before touching a patient.
• After touching a patient
• Before clean/aseptic procedures (alcohol gel should be applied after washing with soap and water to disinfect hands before aseptic procedure is carried out).
• After body fluid exposure risk.
• After touching a patient’s immediate surroundings.
• Immediately after removal of gloves.
Some additional examples of hand hygiene moments include:
• Hands must be cleaned before handling medication
• Before preparing food
• Before donning (putting on) and after doffing (taking off) PPE.
All Healthcare Workers must have induction/training in correct hand washing technique and alcohol gel use
10.Staff Hand Care
Skin Care
• Cuts and abrasions in any area, if on exposed skin, should be covered with a dressing that is waterproof, breathable and is an effective viral and bacterial barrier
• Alcohol based hand rubs used for hand hygiene should contain emollients in their formulation
• Warm/tepid water should be used to reduce the risk of dermatitis; hot water should be avoided
• Hands should be patted dry thoroughly after hand washing, using disposable paper towels; avoid rubbing, which may lead to skin irritation/damage
• Organisational-provided emollient hand cream should be applied regularly to protect skin from the effects of regular hand decontamination
• Refillable dispensers or communal tubs of hand cream must not be used in the care setting.
• Staff with skin problems or reactions to soap or alcohol-based hand rubs used in their area should seek advice from Occupational Health or their General Practitioner (GP) and inform their manager.
11.Respiratory and Cough Hygiene
Respiratory and cough hygiene is designed to minimise the risk of cross-transmission of respiratory illness (pathogens):
• Cover the nose and mouth with a disposable tissue when sneezing, coughing, wiping, and blowing the nose. If a disposable tissue is not available, use your elbow to cover the nose and mouth when coughing or sneezing.
• Dispose of used tissues promptly into a waste bin.
• Wash hands with liquid soap and warm water after coughing, sneezing, using tissues, or after contact with respiratory secretions or objects contaminated by these secretions
• Where there is no running water available, or hand hygiene facilities are lacking, staff may use hand wipes followed by alcohol hand rub and should wash their hands at the first available opportunity.
• Keep contaminated hands away from the eyes nose and mouth.
Staff should promote respiratory and cough hygiene helping those (e.g., elderly, children) who need assistance with this by providing patients with tissues, plastic bags for used tissues and hand hygiene facilities/hand wipes as necessary
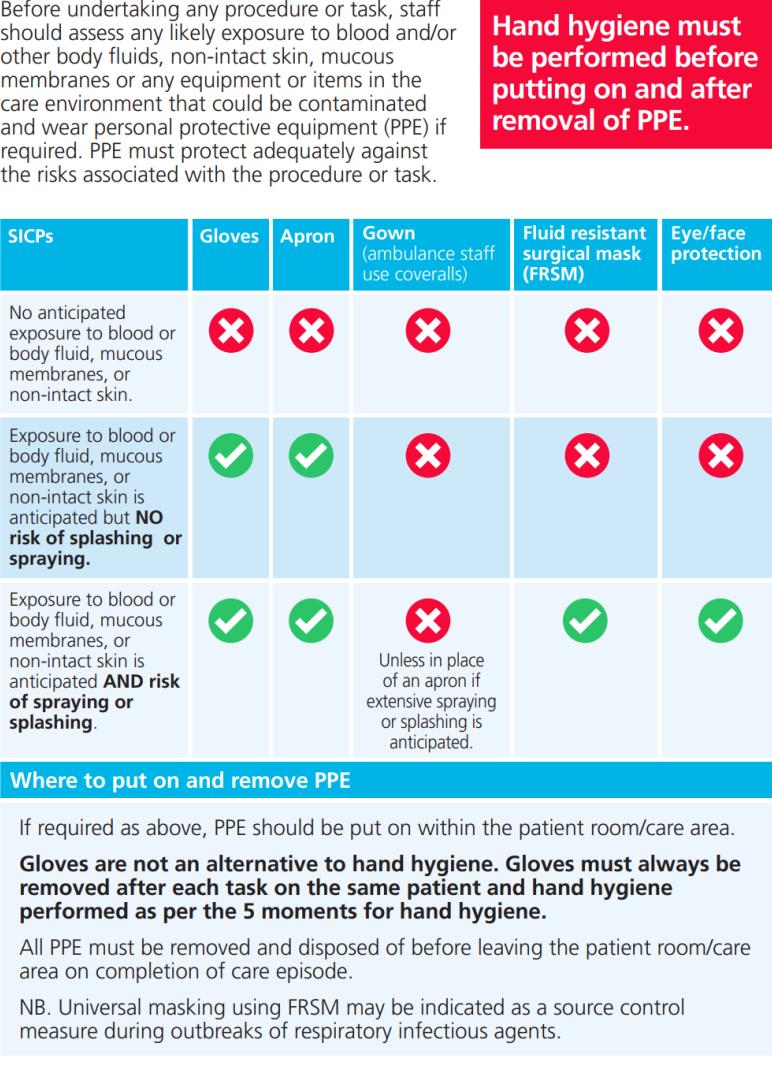
12.Personal Protective Equipment (PPE)
PPE is intended to protect both patient and Healthcare Worker from the risk of crossinfection. All Healthcare Workers and Facilities staff must be trained in the appropriate use of PPE and how to risk assess their individual PPE requirements. PPE includes aprons/gowns, gloves, eye and face protection and some specialised items.
All PPE:
• Should be located close to the point of use (where this does not compromise patient safety).
• Community-based staff inc. domiciliary carers should transport PPE in a clean receptacle (e.g. lidded, plastic box) which should be included in routine cleaning schedules
• Should be stored safely and in a clean, dry area to prevent contamination. This area must have a cleaning schedule and a person(s), with responsibility assigned
• Must be within the expiry date
• Should be single use, unless specified by the manufacturer, or as agreed for extended/sessional use, for example surgical face masks
• Should be changed immediately after each patient and/or after completing a procedure or task (unless sessional use has been agreed and local risk assessment undertaken)
• should be disposed of into the correct waste stream depending on the setting, for example, domestic waste/offensive (non-infectious) or infectious clinical waste
• Should be discarded if damaged or contaminated.
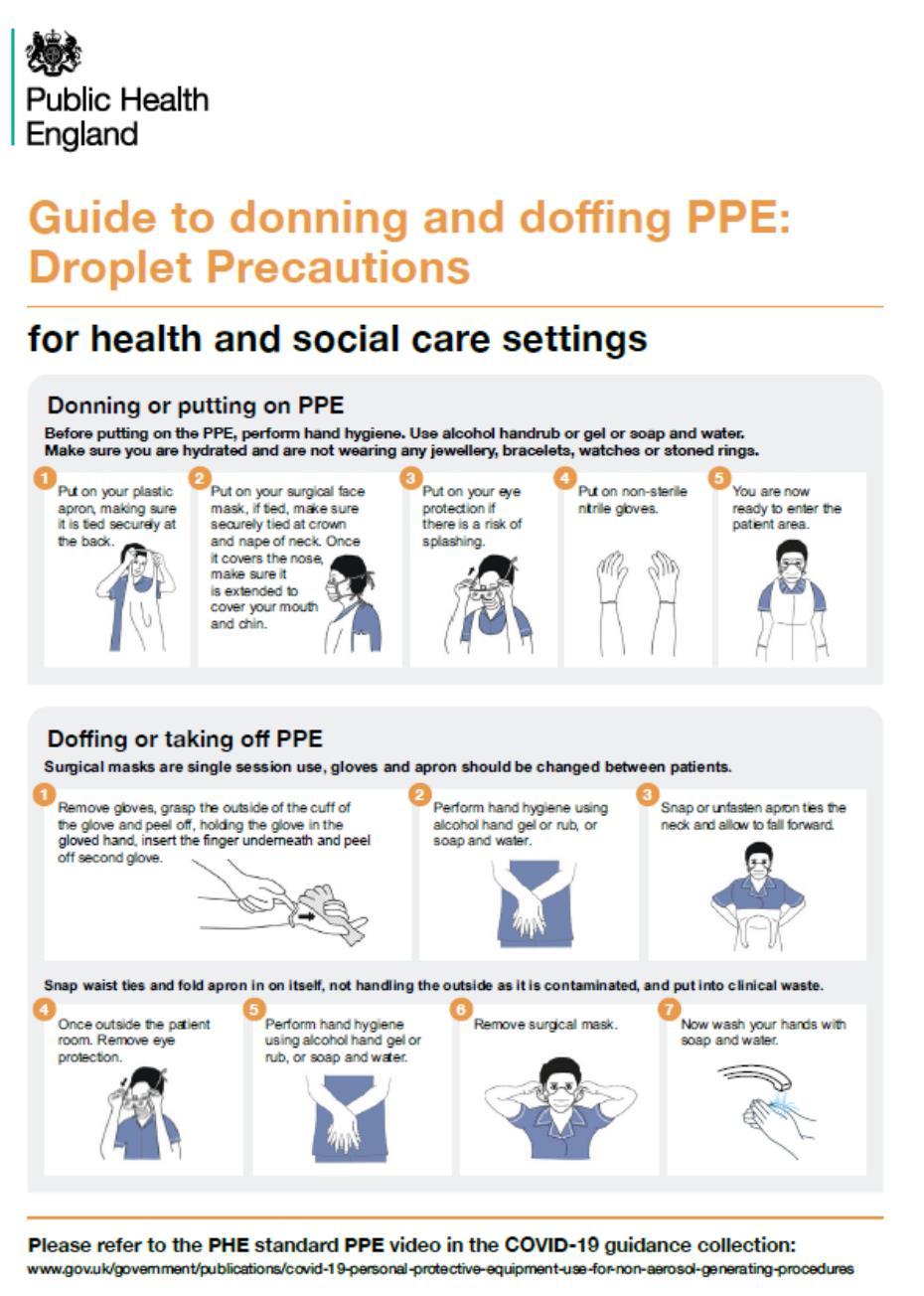
• Should be donned safely (put on) to avoid contamination
• Should be safely doffed (removed) to avoid self-contamination.
Risk Assessment (Please see Appendix 11. PPE Risk Assessment)
The application of SICPs during care delivery is determined by a risk assessment, which should include:
• What is the risk from the patient?
• What is the risk from the task that the Healthcare Worker is about to undertake?
• What is the risk of exposure to blood, body fluids or chemicals?
Gloves:
• Must be single use items
• Nitrile gloves should be worn whenever there is a risk of exposure to body fluids, secretions/excretions, broken skin or mucous membranes
• Must be changed immediately after each task performed
• Must be worn appropriately and removed after use
• Must never be worn inappropriately in situations, such as to go between patients, move around a care area, work at IT workstations
• Must be changed if a perforation or puncture is suspected
• Must be appropriate for use, fit for purpose and well-fitting
• Must conform to current EU legislation (CE marked as medical gloves for single use only)
• Must never be decontaminated with alcohol-based hand rub or soap between use
• Provide operates a latex-free policy. All gloves are latex-free.
Sterile Gloves
Sterile gloves are required for invasive procedures or contact with sterile sites. Please refer to the ANTT policy IPPOL17 – Aseptic Non-Touch Technique Policy (ANTT).
Disposable plastic aprons
Aprons should be:
• worn to protect uniform or clothes when contamination is anticipated/likely
• worn when in direct care contact with a patient or their immediate environment e.g. providing toileting support or changing bed linen
• changed between patients and following completion of a procedure or task
Aprons should not be worn between patients or when moving from location to location unless required for the task underway e.g. taking commode/bed pans for disposal
Full Body Gowns/Fluid Repellent Coveralls
Gowns / coveralls should be:
• worn when there is a risk of extensive splashing of blood and/or body fluids
• worn when a disposable apron provides inadequate cover for the procedure or task being performed (surgical procedures)
• changed between patients/individuals, and immediately after completing a procedure or task
• worn as part of contact precautions with patients with Multi Resistant Organisms/ CPE. See policy IPPOL28
The choice of apron or gown is based on a risk assessment and anticipated level of body fluid exposure. Sessional use of gowns/aprons is not permitted.
Face, Eye and Respiratory Protection
Eye/face protection (including full-face visors):
• Must be worn if blood and/or body fluid contamination to the eyes/face is anticipated/likely, for example, by members of the surgical theatre team and always during Aerosol Generating Procedures (AGPs)
• As part of SICPs a full-face visor may be used to protect the eyes and face from splashing
• Eye or face protection (including full-face visors) must not be impeded by accessories, such as piercings or false eyelashes
• Should not be touched when being worn
• Must cover the full peri-orbital region and wrap around the sides of the face.
Regular prescription spectacles are not considered to be adequate eye protection and are not classed at PPE.
Fluid Resistant Surgical Face Masks (FRSM Type II and 11R)
Type II masks are worn to provide source control, they protect others from the wearer’s respiratory droplets These do not protect wearer from respiratory droplets. These masks are not fluid repellent and therefore should not be worn for activities where there is a risk of splash of blood, body fluids or hazardous cleaning products, or when caring for an individual that requires transmission-based precautions
Type 11R
Type IIR fluid-repellent surgical masks protect the wearer by providing a fluid repellent barrier between the wearer and the environment. This protects the wearer against blood or body fluid splashes and against the respiratory droplets of others reaching their mouth and nose. These masks also protect others from the wearer’s respiratory droplets known as source control.
Fluid resistant surgical face masks must be:
• Worn if a full-face visor is not available for spraying/splashing.
• Worn with eye protection if spraying of blood, body fluids, secretions, or excretions onto the respiratory mucosa (nose and mouth) is anticipated or likely (type 11R)
• Worn to protect patients from the operator as a source of infection e.g. when performing surgical procedures or inserting a central vascular catheter (CVC) (type 11 or 11R)
• Well-fitting and fit for purpose, and fully cover the mouth and nose (manufacturer’s instructions must be followed to ensure effective fit and protection) Masks must not be touched once put on or allowed to dangle around the neck
• Removed or changed:
o At the end of a procedure/task
o If the mask’s integrity is breached, eg from moisture build-up after extended use or from gross contamination with blood or body fluids
o In accordance with manufacturers’ specific instructions
In some circumstances, face masks may impede communication. Transparent face masks must meet required standards for medical use to ensure protection is maintained.
https://www.gov.uk/government/publications/technical-specifications-for-personalprotective-equipment-ppe/
Please see Transmission Based Precautions (TBPs) for guidance on Respiratory Protective Equipment (RPE) including FFP3 respirator masks and respirator hoods.
13.Headwear and Footwear
Headwear
• Headwear is not routinely required in clinical areas (even if undertaking an AGP) unless part of theatre attire or to prevent contamination of the environment such as in clean rooms
• A local protocol should be in place for theatre headwear and inc. the wearing of a snood to ensure facial hair is also covered e.g. a beard
• Headwear worn for religious reasons (for example, turban, kippot veil, headscarves) is permitted provided patient safety is not compromised. These types of headwear must be washed and/or changed between each shift, or immediately if contaminated, and comply with additional attire in, for example, theatres
Footwear
• Footwear must be non-slip, impervious, clean, and well maintained, and support and cover the entire foot to avoid contamination with blood or other body fluids or potential injury from sharps.
• Footwear such as those described as clogs used in theatre settings only, must ensure the fore foot is covered in full and is not perforated or decorated in design. An ankle strap must be in place for securement to prevent slip and displacement.
• Footwear must be removed before leaving a care area where dedicated footwear is used e.g., theatre. In these circumstances a decontamination schedule should be in place for all footwear and responsibilities assigned
• Dedicated footwear found to be defective should be repaired or replaced before further use
14.Summary of Protective Clothing Table
No direct contact with the patient or their immediate surroundings (bedding etc).
Contact with patient but no contact with wounds and/or potential exposure to blood/ body fluids, but low risk of splashing.
No protective clothing required
Disposable apron and non-sterile disposable gloves required.
Contact with patient (but no contact with wounds). Potential exposure to body fluids anticipated and medium to high risk of splashing to face.
Disposable apron, nonsterile disposable gloves and eye/nose/mouth protection required
Contact with patient’s wound(s) normally sterile sites, but low risk of splashing of blood/ body fluids.
Disposable apron and sterile disposable gloves required.
Contact with patient’s wound(s) and medium to high risk of splashing to face.
Environmental cleaning around patient areas.
Disposable apron, sterile disposable gloves and eye/nose/ mouth protection required.
Disposable apron and colour-coded gloves according to the task.
Click here to enter text.
15.Safe Handling of Sharps
All Healthcare Workers must handle sharps safely to reduce the risk of injury, as exposure to contaminated blood may be associated with Blood Borne Viruses (BBVs). There is a legal requirement to report all sharps injuries and near misses to line managers/employers.
The Health and Safety (Sharp Instruments in Healthcare) Regulations (2013) outline the regulatory requirements which include arrangements for the safe use and disposal of sharps including:
• Sharps must not be passed directly from hand to hand and the handling of sharps should be kept to a minimum.
• Needles must not be bent or broken prior to use or disposal.
• Needles and syringes must not be disassembled by hand prior to disposal.
• Needles must not be re-sheathed.
• The temporary closure mechanism must be deployed on all sharps containers when not in use.
• Temporary closure mechanisms must be deployed after use and when transporting.
• All sharps container lids must fitted securely and correctly.
• All used sharps must be disposed of at point of use.
• The sharps container must not be filled above the black indicator line mark.
• Once filled to the black marker line the lid must be closed securely, and the label completed with name of staff sealing the sharps container and the date of closure.
• All sharps’ containers must conform to UN3291 and BS7320 standards
• Sharps containers must not be placed on the floor but should be located in a safe place between waist and shoulder height (to enable sight of aperture prior to disposal). Alternatively, containers can be attached to a wall/trolley using an appropriate bracket or placed within a correctly fitting sharps tray.
Sharps safety devices
The Sharp Instruments in Healthcare Regulations (2013) aims to reduce and eliminate (where possible) the number of sharps related injuries which occur in healthcare. Its basic guidance states:
• If a sharp instrument is to be used, then a non-sharp alternative is to be sourced and used (where these are available)
• If a non-sharp alternative is not available, then a safety device is to be sourced and used
• If a safety device is not available then all available risk management processes should be employed to minimise risk inc. risk assessment, provision of appropriate sharps bins, sticky mats (for use during surgical procedures), safety procedures and training
16.Safe Management of Care Equipment (and decontamination of equipment)
Care equipment is easily contaminated with blood, secretions, excretions and other body fluids and infectious agents. Consequently, it is easy to transfer infectious agents from communal care equipment during care delivery.
Care equipment is classified as follows:
• Single-use – equipment which is used once on a single patient and then discarded. This must never be reused, even on the same patient. The packaging will carry this symbol:
• Needles and syringes are single-use devices. They should never be used for more than one patient or reused to draw up additional medication.
• Medications from a single-dose vial or intravenous (IV) bag must never be administered to multiple patients.
• Single patient use – equipment which can be re-used on the same patient.
• Reusable invasive equipment – equipment, which is used once then decontaminated, e.g., surgical instruments.
• Reusable non-invasive equipment (often referred to as communal equipment) – reused on more than one patient following decontamination between each use, e.g., commode, patient transfer trolley.
Before using any sterile equipment check that:
• The packaging is intact
• There are no obvious signs of packaging contamination
• The expiry date is valid
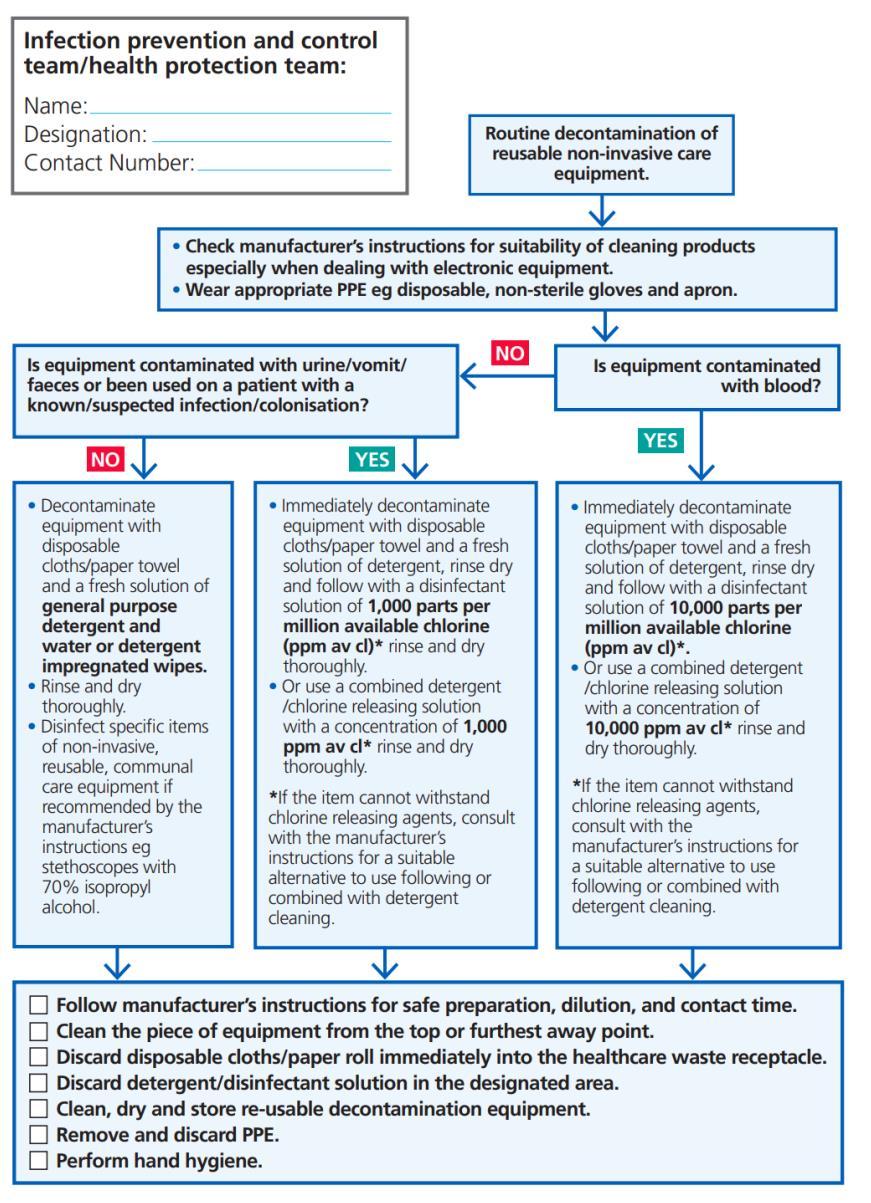
Decontamination of reusable non-invasive care equipment must be undertaken:
• Between each use / between patients
• After contamination with blood and/or body fluid
• At regular, predefined intervals/date as part of an equipment cleaning schedule/protocol
• Before inspection, servicing or being sent for repair. An equipment decontamination status certificate/label will be required if any item of equipment is being sent to a third party, e.g., for inspection, servicing or repair
• Adhere to manufacturers’ guidance for use and decontamination of all care equipment
• Guidance on decontamination may be required prior to procuring, trialling or lending any reusable non-invasive equipment
• Single use equipment must not be re-used
• Used instruments and equipment must be decontaminated in accordance with the manufacturer’s guidelines
• Clean equipment must be labelled using a signed and dated green label (“I am clean” tape or stickers)
• Local cleaning schedules must be in place and completed for all re-usable equipment.
• Audit of decontamination/cleaning must be undertaken to provide evidence and assurance that care equipment is clean and safe.
If providing care in the patient’s home, equipment should be transported safely and decontaminated as above in a designated decontamination room. If equipment is left in situ (at the patient’s home) items should be decontaminated before leaving as part of the episode of care.
17.Safe Management of Care Environment
The care environment:
• Must be visibly clean, free from non-essential items and equipment to facilitate effective cleaning
• Must be well maintained and in a good state of repair with adequate ventilation for the clinical specialty
Routine cleaning
• The environment should be routinely cleaned in accordance with the National Cleaning Standards
• Routine cleaning should be undertaken using a fresh solution of general-purpose neutral detergent in warm water. Water should be changed when dirty or when changing tasks
• The use of detergent wipes is an acceptable alternative for cleaning surfaces/frequently touched sites within the care area
• Routine disinfection of the environment is not recommended with the exception of on sanitary fittings when a 1,000ppm available chlorine solution is recommended
• Staff groups should be aware of their environmental cleaning schedules and be clear on their specific responsibilities
• Cleaning protocols should include responsibility for, frequency of, and method of environmental decontamination.
Guidance on enhanced cleaning / terminal cleaning refer to intranet EFGUI01 Cleaning guideline s for community hospitals and EPOL02 Annual deep cleaning of community hospitals wards.
Safe Management of Linen
Clean linen:
• Should be stored in a clean, designated area, preferably an enclosed cupboard.
• If clean linen is not stored in a cupboard, then the trolley used for storage must be designated for this purpose and be completely covered with an impervious covering that is able to withstand decontamination.
Used linen (previously known as soiled/fouled linen):
• Ensure a laundry receptacle is available as close as possible to the point of use for immediate linen deposit.
• Staff must wear disposable gloves and an apron when handling soiled linen
• Used linen should be placed in an impermeable bag immediately on removal from the bed or before leaving a clinical department
• Used and infectious linen must be stored away from public areas, in a designated, safe, lockable area whilst awaiting collection
• All linen that is deemed unfit for re-use, e.g. torn or heavily contaminated, should be categorised at the point of use, and returned to the laundry for disposal.
Infectious linen:
Infectious linen includes items that have been used by a patient who is known or suspected to be infectious and / or linen that is contaminated with blood and / or other body fluids e.g., faeces.
• Linen in this category must not be sorted but should be placed directly into a pink/red dissolvable bag (alginate), which is then placed inside a white bag and secured before leaving the care area
Do not:
• Shake or sort linen on removal from beds/trolleys.
• Place used linen on the floor or any other surfaces, e.g. a locker/tabletop.
• Re-handle used linen once bagged.
• Overfill laundry receptacles/bags or place inappropriate items in the laundry receptacle, e.g. used equipment/needles, used pads, or catheter bags
Linen used during patient transfer:
• Any linen used during patient transfer e.g., blankets, should be categorised at the point of destination as either used or infectious and managed accordingly.
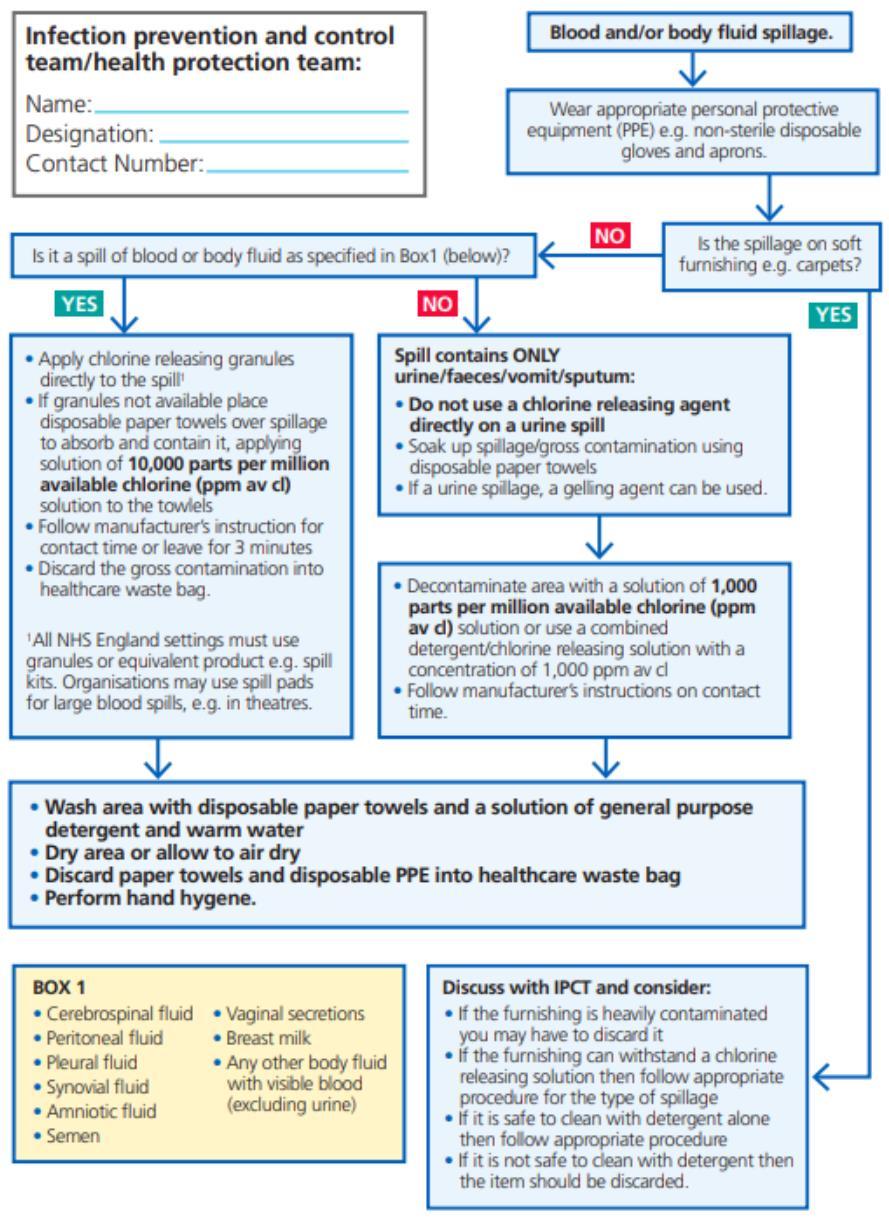
18.Safe Management of Blood and Body Fluid Spillages
All spillages of blood or bodily fluids should be considered as potentially infectious. Spillages must be dealt with quickly and effectively to reduce the risk of exposure and further contamination.
Management of Blood and Body Fluid Spillages
• Deal with blood and body fluid spills quickly and effectively. All staff should know who is responsible for spillage management in their work area. In clinical areas this is nursing staff. Domestic cleaning will be required after the initial body fluid spillage has been dealt with. Public and non-clinical areas as the responsibility of cleaning / domestic teams.
• Blood spillage requires disinfection with a chlorine-releasing agent at a concentration of 10,000 ppm. This concentration will kill any blood borne viruses.
• Granules, spillage kits or spill pads are the preferred method of applying chlorinereleasing agents.
Provide service areas are wide and diverse and there are several different types of spill kits available across the organisation. (They should all contain instruction for their use)
• Some kits are enclosed in a hard, ridged box.
• Some kits come in an enclosed plastic packaging.
• These kits may contain the following solutions, tablets or granules: Sodium dichlorocyanurate compound NaDCC (e.g., Presept, Sanichlor, Actichlor, HazTabs, Tristel) or hypochlorite solution.These compounds must be kept in a lockable cupboard to comply with COSHH regulations.
• Absorbent powder (e.g., Vernagel) to soak up the liquid content of the spillage prior to disinfection.
In addition to the spill kit the following equipment may be needed:
• Non-sterile vinyl gloves and nitrile gloves for contact with blood.
• Disposable plastic apron
• Disposable face protection (IF REQUIRED)
• Disposable paper towels
• Orange clinical waste bag
• General-purpose detergent wipes
19.Safe Disposal of Waste (including Sharps)
Provide has a duty tomanage waste in accordance with relevant legislation, minimising risk to health and the environment.
All staff have a responsibility to ensure all waste is disposed of in accordance with the waste management policy.
All waste contaminated with blood or body fluids must be discarded into an orange clinical waste sack, labelled, and sent for incineration according to local policy.
Waste bags must be no more than ¾ full, closed using a ‘swan neck’ method of closure.
Healthcare waste should be secured with a ratchet tag or tape identifying point of origin.
Sharps boxes must:
• Have a temporary closure mechanism, which must be employed when the box is not in use
• Be labelled with date of assembly, point of origin, date of closure and signature.
• Be disposed of when the manufacturer’s fill line is reached.
Store all waste in a designated, safe, lockable area whilst awaiting collection. Collections schedules must be acceptable to the care area and there should be no build-up of waste receptacles.
Waste Streams
Domestic – black bags
• Final disposal to landfill.
Offensive waste aka hygiene waste – yellow/black stripe bags
• Non-infectious waste which consists of soiled dressings, nappies, sanitary towels/tampons, incontinence pads etc.
• Final disposal to deep landfill
Clinical waste aka healthcare waste – orange bags
• Consists of items which are contaminated or likely to be contaminated with blood and/or body fluids
• Waste which unless rendered safe may prove hazardous to any person coming into contact with it
• Treatment methods include incineration, irradiation and other methods to render safe.
20.Occupational Safety: Prevention and Exposure Management
The Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 outline the regulatory requirements for employers and contractors in the healthcare sector in relation as outlined in section 15.
There is a potential risk of transmission of a Blood Borne Virus (BBV) from a significant occupational exposure to a sharp and staff must understand the actions they should take when a significant occupational exposure incident takes place. There is a legal requirement to report all sharps injuries and near misses to line managers/employers and a Datix form must be completed and Medigold Occupational Health provider informed.
A significant occupational exposure/injury is defined as:
• A percutaneous injury, e.g., injuries from needles, instruments, bone fragments, or bites which break the skin
• And/or exposure of broken skin (abrasions, cuts, eczema, etc).
• And/or exposure of mucous membranes including the eyes from splashing of blood or other high risk body fluids.
21.Transmission Based Precautions
Transmission Based Precautions (TBPs) are measures that may be required in addition to Standard Infection Control Precautions (SICPs).
TBPs are applied when SICPs alone are insufficient to prevent transmission of an infectious agent. TBPs are additional infection control precautions required when caring for a patient with a suspected or confirmed infectious agent. TBPs are categorised by the route of transmission of the infectious agent. Some common infectious agents can be transmitted by more than one route.
Clinical judgement and decisions should be made by staff on what additional precautions are required and this will be based on:
• Suspected/known infectious agent
• Severity of the illness caused
• Transmission route(s) of the infectious agent
• Care setting and procedures undertaken.
The application of TBPs may differ depending on the care setting and the known or suspected infectious agent. Application of TBPs should be considered within the framework of the hierarchy of controls.
Types of precautions
Contact Precautions
Used to prevent and control infections that spread via direct contact with the patient or indirectly from the patient’s immediate environment and care equipment.
Contact transmission is the most common route of transmission and consists of two distinct types: direct contact and indirect contact
Direct contact transmission occurs when infectious agents are transmitted directly from an infectious individual to another individual i.e., skin to skin or contact with bodily fluids, such as saliva, faeces, blood, and fluid from open wounds.
Indirect contact transmission occurs when infectious agents are transmitted to an individual via a contaminated environmental surface (e.g. dust or body fluids) or an item of equipment that is contaminated (e.g. has not been decontaminated after use).
Droplet Precautions
Measures used to prevent and control the spread of infections over short distances (at least 3 feet or 1 metre) via droplets from the respiratory tract of one individual directly onto a mucosal surface (mouth/nose) or the conjunctivae (surface of the eye) of another individual. Droplets penetrate the respiratory system to above the alveolar level.
Droplets may be generated from the respiratory tract during coughing, sneezing, talking or singing. These droplets remain in the air for a short period and close proximity is required for transmission.
Airborne Precautions
Measures used to prevent and control spread without necessarily having close patient contact via aerosols from the respiratory tract of one individual onto a mucosal surface (mouth/nose) or conjunctivae of another individual. Aerosols can penetrate the respiratory system to the alveolar level.
Less significant routes of transmission in healthcare inc. food and water borne.
Patient placement / assessment of infection risk
The potential for transmission of infection must be assessed when a patient enters a care area (or prior to arrival). Assessment should be continually reviewed throughout the stay/episode of care. Assessment should influence patient placement decisions in line with clinical/care needs which may need to take priority.
Patients who may present a risk of cross-infection in any setting includes those:
• With diarrhoea, vomiting, an unexplained rash, fever or respiratory symptoms
• Known to have been previously positive with multi-drug resistant organisms (MDROs) e.g. MRSA, CPE
• Who have been an inpatient in any hospital in the UK or abroad or are a known epidemiological link to a carrier of CPE
• To see list of diseases, isolation requirements and respiratory precautions requirements for inpatient settings refer to:
• C1636-national-ipc-manual-for-england-v2.pdf appendix 11
Isolation facilities e.g. single and en suite rooms should be prioritised depending on the known / suspected infectious agent. Patients with an infection spread via the airborne/droplet route may require priority for a single room. Patients with an infection spread via the faecal/oral/contact route e.g. with diarrhoea require a separate toilet / commode but may remain in a bay if single rooms are in short supply.
All patient placement decisions and assessment of infection risk (including isolation requirements) must be clearly documented in the patient records and communicated at handover and on other relevant occasions e.g. when visiting another department.
Multidisciplinary decisions should be made involving the patient’s care team and IPC team. This is of particular importance when determining the use of single rooms.
Source Isolation
• The physical isolation of those service users with suspected or confirmed transmissible infection, usually in a single room, to prevent or reduce the risk of onwards transmission by blocking the route of spread.
• Various levels of isolation are advised for source isolation see appendix 10.
Protective Isolation
• Protective isolation is required for patients with a high risk of developing infection because their immune system in compromised.
• Protective isolation is a specialised practice and relies on the use of positive pressure ventilation to protect those at high risk such as neutropenic patients. This type of isolation room is not available within Provide CIC and patients would need to be cared for at another service provider (usually an acute NHS trust).
Cohorting
• When a single room for isolation is unavailable or there are a number of patients with the same infection, then cohorting of a group of patients may be undertaken. This is usually undertaken in a bay where a door can be closed to separate from the rest of the ward. Not all patients with similar symptoms e.g., diarrhoea or respiratory symptoms will have the same aetiology and many patients with the same organism, will have different strains and different patterns of antibiotic resistance. For these reasons, cohorting should only be undertaken following discussion with and approval of the IPT.
Risk assessment of infections & isolation requirements
• Individual policies relating to the specific organism should always be checked to ensure that correct procedures are in place (i.e. Clostridioides difficile policy).
• Any patient suspected or known to be colonised or infected with an organism that may pose a risk to others should be isolated in line with this policy. It is recognised that at times single rooms will need to be prioritised. The following information will help in the risk assessment process by assigning a level of priority for isolation required against specific infections listed in (Appendix 10).
• In the event there is no isolation room available, staff must contact IPT as soon as possible for discussion and/ or escalate to the manager on call and complete a Datix Incident Form
Code • Priority of Isolation Required
• 3
• 2
• 1
• High priority for a single room - discuss with infection prevention team if a single room is not available.
• Single room required - assess patients currently in side rooms to reprioritise if possible. If side room still unavailable, nurse in a bay away from other vulnerable patients (e.g. patients with open wounds or invasive devices, immunocompromised patients etc.). Move to a single room as soon as possible. Discuss further with Infection Prevention Team.
• Separate toilet required – for patients with bacteria or virus spread by the faecal-oral route then a separate toilet should be provided either in a single room or by designating a toilet for separate use (maintaining patient confidentiality ie no name on door)
• 0 Isolation not required OR no longer required
Notification of Infectious Diseases
Some infections are legally notifiable. The aim of notification is to enable public health authorities to undertake surveillance and monitoring of infectious diseases that may have community implications. The responsibility for notification rests with the clinician in charge of the patient’s care.
Notifiable infections may require immediate reporting of an individual case e.g. bacterial meningitis and urgent cases should be discussed initially with local health protection teams. Notifiable infections forms can be downloaded from the internet at:
Notifiable_disease_form.pdf (publishing.service.gov.uk)
Local contact details:
UKHSA East of England Health Protection Team
Office hours contact: 0300-303-8537 option 1.
Out of hours: 01603 481 221 (health care professionals only)
A full list of notifiable disease can be found online here: https://www.gov.uk/guidance/notifiable-diseases-and-causative-organisms-how-toreport
Management of isolation – inpatient unit
• Isolation may be implemented in single rooms or by cohorting infectious patients in the same bay/area. The latter requires separation (of bed space) by at least 3 feet (1 metre) with the door closed. If doors cannot be closed ie fall risk/dementia risk then this should be documented at risk assessment
• Signage should be used to communicate isolation requirements and to prevent the entry of unnecessary visitors and non-essential staff. Patient confidentiality must be maintained at all times
• Infectious patients should be transferred to other departments only if clinically necessary. If the patient has an infection transmitted via the airborne/droplet route then the patient should be encouraged to wear a surgical mask during transfer if this can be tolerated
• Receiving departments and transport staff must be made aware of necessary precautions but confidentiality should be maintained relating to clinical details
Isolation in care settings with or without nursing care
• Residents should remain in their bedroom with the door closed unless for safety reasons (document at time of risk assessment)
• When transferring to another facility, receiving staff should be informed of the infectious status of the resident
• Whilst in isolation, residents should be actively encouraged to remain in the facility for the duration of infectivity and should not visit public places e.g. shops
• Decisions re attendance at day-care facilities need to be made at the time of risk assessment. For example, if the patient has an infection spread by contact e.g. MDRO such as MRSA in a wound, then a clean, dry dressing may enable the resident to continue attending other therapeutic environments.
Outpatient settings
• Patients attending with suspected/known infection / colonisation should be prioritised for assessment / treatment e.g. schedule appointments at the start or end of the clinic session
• Infectious patients should be separated from other patients while awaiting assessment and during care by at least 3 feet (1 metre)
Staff cohorting
During outbreaks of infection, consideration should be given (if staff levels allow) to cohorting staff to care for patient in isolation / cohort areas. This can reduce the risk of transmission between care areas.
Safe management of patient care equipment in an isolation room / cohort area
• Use single use items where possible
• Re-usable items should be dedicated to the isolation room / cohort area and decontaminated prior to use on another patient
• Consider increasing the frequency of decontamination for re-usable noninvasive care equipment when used in isolation/cohort areas
Safe management of the care environment
The care environment must be:
• Visibly clean, free from non-essential items and equipment to facilitate effective cleaning.
• Well maintained, in a good state of repair and with adequate ventilation for the clinical specialty.
Equipment used for environmental decontamination must be either single-use or dedicated to the affected area then decontaminated or discarded following use e.g. cloths, mop-heads etc.
Enhanced cleaning
Inpatient wards (all settings):
• Isolation / cohort rooms/area must be decontaminated at least daily or more frequently on the advice of the IPC team.
• These facilities must be decontaminated using either a combined detergent / disinfectant solution at a dilution of 1,000 parts per million available chlorine (ppm available chlorine (av.cl) OR
• A general-purpose neutral detergent in warm water followed by a solution of 1,000 ppm av.cl.
• Any alternative products MUST be agreed with the IPC team.
• All cleaning products must be managed in accordance with COSHH regulations
• Manufacturers’ guidance and recommended product ‘contact time’ must be followed for all cleaning / disinfection solutions.
Increased frequency of decontamination / cleaning schedules should be incorporated into the environmental cleaning schedules for areas where there may be higher contamination rates eg:
• Toilets / commodes particularly if patients have diarrhoea
• Frequently touched surfaces e.g. door handles, locker tops, over bed tables, bed rails, grab rails etc.
Outpatient / clinic settings
The extent of decontamination between patients will depend on a number of factors including the duration of the consultation / assessment, the patients presenting symptoms and any visible environmental contamination as well as the procedure undertaken e.g. spirometry or AGP on patient with respiratory symptoms. Advice from the IPC team should be sought on a case-by-case basis.
Terminal cleaning / decontamination
Following patient transfer, discharge, or once the patient is no longer considered infectious, remove from the vacated isolation room / cohort area all:
• Healthcare waste and any other disposable items (bagged before removal from the room)
• Bedding / bed screens / curtains – managed as infectious linen (bagged before removal from the room)
• Re-usable non-invasive care equipment (decontaminated in the room prior to removal) refer to appendix 7 of NIPCM
• The room should be decontaminated using products in use during the period of isolation (as above)
• Rooms must be cleaned from highest to lowest points and from least to most contaminated points
Steam Cleaning
Steam cleaning is a dry steam vapour that can instantly clean and dry surfaces without leaving any unhygienic residue. All cleaning staff are trained to operate the machinery.
The Infection Prevention Team will provide guidance as to when it is appropriate to use the steam cleaning process following an infection.
Decontaminating with Hydrogen Peroxide Vapour Technology (HPV)
HPV technology (also known as fogging) can be used as a final decontamination process within a clinical area following certain infections including, but not limited to Clostridioides difficile and other multi-resistant organisms.
The Infection Prevention Team will provide guidance as to when it is appropriate to use the HPV technology and the request will be made from the team directly to the cleaning manager in accordance with the cleaning policy.
Personal protective equipment (PPE): Respiratory Protective Equipment (RPE)
Personal Protective Equipment (PPE) must still be used in accordance with SICPs when using Respiratory Protective Equipment (RPE) as per section 12.
RPE provides additional protection when it is not reasonably practicable to prevent exposure to a substance hazardous to health (as may be the case when caring for a patient with a suspected or known airborne pathogen). In such cases the hazard must be adequately controlled by applying protection measures appropriate to the activity and consistent with the assessment of risk.
When face masks are required:
• Source control
Inpatients with suspected or confirmed respiratory infection should be asked to wear a facemask (FRSM) unless isolated in a single room. FRSM should be worn in multibedded bays, communal areas, e.g., waiting areas for diagnostics, and during transfer if this can be tolerated and is deemed safe for the patient.
Outpatients (including Urgent and emergency care (UEC) and primary care) with respiratory symptoms who present for treatment should be asked to wear a facemask/covering (or offered one on arrival unless placed in a single room) if this can be tolerated and is deemed safe for the patient. Outpatients without respiratory symptoms are not required to wear a facemask unless this is a personal preference.
The request for patients to wear a facemask must never compromise their clinical care, such as when oxygen therapy is required or where it causes distress, e.g., paediatric/mental health settings.
Visitors and individuals accompanying patients to inpatient, outpatient appointments or the emergency department are not required to wear a facemask unless this is a personal preference. 36 |National infection prevention and control manual for England.
If cluster transmission of a respiratory pathogen is known or suspected, consider extending the use of FRSM as source control to health and care staff
RPE i.e., a filtering face piece (FFP) or a respirator hood must be considered when a patient is cared for with a known/suspected infectious agent/disease spread wholly or partly by the airborne route and when undertaking aerosol generating procedures (AGPs) on patients with a known/suspected infectious agent spread wholly or partly by the airborne or droplet route.
The decision to wear an FFP3 respirator/hood should be based on clinical risk assessment e.g. task being undertaken, presenting symptoms, infectious state of the patient, risk of acquisition and the availability of treatment. All staff who are assessed as required to wear an FFP3 mask must be fit tested annually on the specific mask in use.
General principles for RPE include:
• To be worn when caring for patients with a suspected or confirmed infection spread wholly by the airborne route, such as tuberculosis (TB) (during the infectious period), or when required to do so in response to national directives e.g., during specified outbreaks.
• Worn when performing Aerosol Generated Procedures (AGPs) on a patient with a suspected or confirmed infection spread wholly or partly by the droplet or airborne route.
• Should be fit checked (according to the manufacturer’s guidance) every time a respirator is donned to ensure an adequate seal has been achieved. (Test if there is any leakage at the top, sides and bottom of the mask)
• RPE must be fluid-resistant
• Fit testing to be undertaken on all health and care staff who may be required to wear a respirator to ensure they are able to maintain an adequate seal/fit according to the manufacturer’s guidance whilst providing care
• If staff fail fit-testing then an alternative respirator hood should be considered to provide appropriate protections
• Facial hair may interfere with the respirator sealing surface – this should be discussed on a case-by-case basis at the time of fit testing with alternative arrangements made (e.g. respiratory hood) if appropriate
• RPE must be compatible with other facial protection used (protective eyewear) so that this does not interfere with the seal of the respiratory protection
• RPE must be discarded and replaced if breathing becomes difficult, the respirator is damaged or distorted, the respirator becomes obviously contaminated by respiratory secretions or other body fluids, or if a proper face fit cannot be maintained
• RPE must not be touched once put on - if adjustments are needed ensure hand hygiene is undertaken beforehand
• RPE must be removed outside the patient’s room or cohort area and disposed of in an orange clinical bin. (Following correct doffing procedure)
NB: All services must ensure that any new staff that may be required to wear an FFP3 mask to undertake their role receive fit testing as part of their induction.
National Priority Risk Categorisation for fit testing with FFP3
The following risk categorisation is the minimum requirement for staff groups that require FFP3 fit testing:
Level
1 – Preparedness for business as usual
Staff in clinical areas most likely to provide care to patients who present at healthcare facilities with an infectious pathogen spread by the airborne route; and/or undertake aerosol generating procedures.
Level 2 – Preparedness in the event of an emerging threat
Assessment as per local organisations emergency preparedness plans apply. Staff should refer to current policies and operating procedures available on trust intranet.
Aerosol generating procedures
Aerosol generating procedures (AGPs) are medical procedures that can result in the release of aerosols from the respiratory tract. The criteria for an AGP are a high risk of aerosol generation and increased risk of transmission (from patients with a known or suspected respiratory infection).
The list of medica procedures that are considered to be aerosol generating and associated with an increased risk of respiratory transmission is:
• Awake* bronchoscopy (including awake tracheal intubation)
• Awake* ear, nose and throat (ENT) airway procedures that involve respiratory suctioning
• Awake* upper gastro-intestinal endoscopy
• Dental procedures using high speed or high frequency devices e.g. ultrasonic scalers/high speed drills)
• Induction of sputum
• Respiratory suctioning**
• Surgery or post-mortem procedures (like high speed cutting / drilling) like to produce aerosol from the respiratory tract (upper or lower) or sinuses
• Tracheostomy procedures (insertion or removal).
* Awake including conscious sedation (excluding anaesthetised patients with secured airway)
** Available evidence relating to respiratory tract suctioning is associated with ventilation. In line with a precautionary approach, open suctioning of the respiratory tract regardless of association with ventilation has been incorporated into the current (COVID-19) AGP list. It is the consensus view of the UK IPC cell that only open suctioning beyond the oro-pharynx is currently considered an AGP. This means that oral / pharyngeal suctioning is NOT an AGP.
Infection prevention and control when caring for the deceased
The principles of SICPs and TBPs continue to apply while deceased patients remain in the care environment. There remains the potential for ongoing transmission via contact although the risk is usually lower than when the patient was alive.
Staff should advise relatives of the precautions they will need to take whilst viewing and/or having physical contact with their relative. These would be similar to those taken whilst they visited the patient prior to their death.
Undertakers / mortuary staff should be notified of the TBPs in place prior to transfer to the mortuary. In some circumstances a body bag will be required for transportation of the deceased. This will apply to any patient previously being cared for with a category 3 or 4 disease.
Categorisation of Infectious Diseases
The Advisory Committee on Dangerous Pathogens (ACDP) categorise infectious diseases into 1 of 4 categories depending on the significance of the disease and its potential morbidity/mortality. Most health and care associated infections occur as a result of infection with Category 1 or 2 pathogens e.g. MRSA, C. difficile, influenza, scabies, norovirus etc. In most cases, no additional precautions will be required other than to use Standard Infection Control Precautions as used when the person was alive.
Hazard Group 3 pathogens
These organisms can cause significant disease to HCWs and others and other additional precautions need to be taken by mortuary / undertaker staff when handling an infected body. In addition, some procedures, such as embalming may not be appropriate depending on the pathogen.
Infections include:
• Hepatitis B, C, etc;
• HIV & AIDS;
• Mycobacterium tuberculosis (TB)
• Some Salmonella infections e.g. Salmonella typhimurium;
• Spongiform encephalopathy (Creutzfeldt-Jakob disease);
• SARS-CoV-2 (Covid-19);
• Monkeypox virus (MPV)
Hazard Group 4 pathogens
These organisms are extremely hazardous and may cause serious epidemic disease. Most are classified as viral haemorrhagic fevers and will be cared for in a regional secure isolation facility. To see list of diseases, isolation requirements and PPE requirements refer to: C1636-national-ipc-manual-for-england-v2.pdf
22. References
National infection Prevention and Control Manual IPC manual for England version 21July 2022 updated September 2022. C1636-national-ipc-manual-for-england-v2.pdf
Please note where there is a legal duty to implement recommendations the word’ must’ is used
COVID-19: guidance for maintaining services within health and care settings –infection prevention and control recommendations https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infectionprevention-and-control
Infection: Prevention and Control of healthcare –associated infections in primary and community care (2012). NICE Clinical Guidelines 139 www.nice.org.uk/cg139
The Health and Social Care Act (2008, 2015): Code of Practice on the Precautions and Control of Infections and related guidance.
Health and Safety at Work Act 1974, Management of Health and Safety at Work Regulations 1999, Health and Safety Regulations 2002, Control of Substances Hazardous to Health Regulations 2002 and Personal Protective Equipment Regulations 2002.
Loveday et al (2013) EPIC3: National Evidence Based Guidelines for preventing healthcare associated infections in NHS hospitals in England. Journal of Hospital Infection 86S1 S1-70.
Appendix 1: Best practice – How to hand wash, step-by-step images
Appendix 2: Best practice – How to hand rub, step-by-step images
Appendix 3: Best Practice - surgical hand antisepsis using antimicrobial soap
Appendix 4: Best practice – decontamination of reusable noninvasive care equipment
Appendix 5a: Personal protective equipment (PPE) when applying standard infection control precautions (SICPs)
Appendix 5b: Personal protective equipment (PPE) when applying transmission-based precautions (TBPs)
Appendix 6 :Putting on and removing PPE donning and doffing
Appendix 7: Best Practice linen bagging and tagging
Appendix 8: Best Practice -management of blood and body fluid spills
Appendix 9: Best Practice – management of occupational exposure incidents.