32 minute read
Catering
from Health Business 20.1
by PSI Media
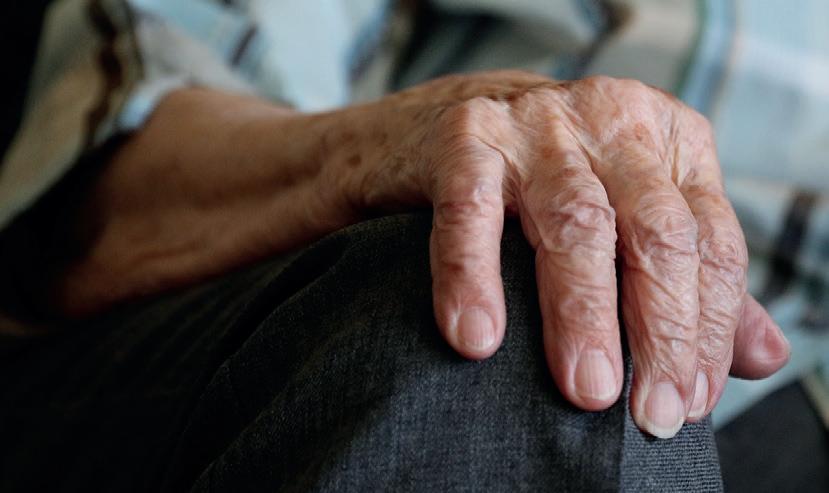
Supporting the elderly to eat and enjoy food together care
Over the last few years, Food for Life has researched intergenerational activity and support for care settings for the elderly. Here, Florence Todd Fordham shares some of the findings
Malnutrition presents a significant health threat to older people and care home residents. In the UK, over one-third of care home residents have been found to be malnourished and require treatment (BAPEN, 2015). This has major impacts on their quality of life, with additional consequences such as social isolation and loneliness. Through the Food for Life Better Care programme we have aimed to positively impact on the lives of older people and care home residents.
What is Food for Life Better Care? Food for Life Better Care was a two year programme to promote good food for older people and included a focus on care homes and intergenerational work in Edinburgh, Calderdale, Kirklees, Leicester, Leicestershire and Rutland. The team used innovative techniques, based on ethnography and codesign, to identify opportunities for change. Food for Life Better Care sought to be both comprehensive – with a whole settings approach to multiple aspects of food – and developmental – to test out and reflect on what worked and what might be enhanced. While each area shared a common overarching framework and approach towards engaging partners, it was anticipated from the outset that the team would adapt the delivery of the intervention to fit local circumstances. The programme has consisted of networking, training, support, development and delivery for a wide range of care homes. This has included creating opportunities to partner with nurseries and schools, some of which are active in the Food for Life schools and Early Years national programmes. Food for Life Served Here, an accreditation from the Soil Association that awards sustainable and healthy catering, was used as a platform for promoting nutrition, hydration and sustainability standards in care homes. Key goals of the Food for Life Better Care programme were to prevent malnutrition and loneliness, to enhance the wellbeing of people in later life, to build capacity within the care sector around food, and to bring communities together through food. FFLSH and sustainability of food provided A key aspect of Food for Life Better Care was supporting better access to nutritious food. Through our work in other settings, such as schools and early years, Food for Life has a wealth of experience in improving the food provision, procurement of ingredients and the overall dining experience. To help caterers make good changes, Food for Life supports food providers to meet Food for Life Served Here criteria. Food for Life Served Here is an independent endorsement, backed by annual inspections, for food providers who are taking steps to improve the food they serve, for climate, nature and health.
The aim of the scheme is to encourage and reward caterers who serve fresh food, source environmentally sustainable and ethical food, make healthy eating easy, and champion local food producers. Food for Life Served Here accreditation is available for all organisations who serve food. The fixed bronze
standards apply to all caterers while silver and gold
are assessed using a
points-based system. Points are achieved at silver and gold for sourcing
environmentally friendly and ethical food, steps taken towards making healthy eating easy and championing local food producers. If you see a Food for Life Served Here logo you know that the majority of food on the menu will be freshly prepared, it will always be free from undesirable trans fats, sweeteners and additives, be cooked by trained chefs, and use ingredients from sustainable and ethical sources. E Therapeutic food activities, dining room experiences and nutrition training activities are greatly beneficial to residents, care home staff and catering teams
Air hugs technology
“Everything you never knew you wanted in a hand dryer”. With ffuuss you will save time on washroom upkeep and reduced janitorial and maintenance cost.
Features
ffuuss has a patented air heating sistem, Preheat, which eliminates the burdens of conventional electric heating and allows a reduction in energy consumption. Everyone can enjoy an optimal drying experience with capacitive sensors. Allows lateral activation of dryer. Suitable for children and people in wheelchairs.

Bi-lateral air curtains prevent water splashing out of the drying cavity, while residual water from hands is collected in a water tank or plugged directly into the drain.

The hand dryers contain a highquality H13 HEPA filter with a 99.97% efficiency to guarantee hand drying with clean air. ffuuss is the most hygienic hand dryer available today, with Biomaster, an active ingredient with silver ion technology that inhibits bacterial growth.



The air outlets have been designed to cover the entire hand surface. Also, by combining different hole sizes, noise is minimised during operation.

F u l l h a n d c o v e r a g e 108 air outlets
Low energy hand dryer consumption at 1100w. The Hand Dryers will pay for themselves within 12 months compared to paper towels.

What did we find? The evaluation of Food for Life Better Care was led by the University of the West of England, collaborating widely and building on related research to ensure relevance across the UK.
Through the evaluation we were ultimately testing a hypothesis: if older people have better access to nutritious food, therapeutic food activities and shared mealtimes across care homes, community care services and hospitals, will they be less susceptible to malnutrition and loneliness and enjoy enhanced health and well-being.
The programme introduced individuals and organisations to the methods and benefits of co-design and whole settings approaches. Greater collaboration helped move beyond the fragmented and isolating working practices that often feature in adult social care. The programme therefore illustrated an approach that holds the prospect of being transferable and scaleable. Overall, the Food for Life Better Care activities were linked to promising evidence of benefits for care home residents in terms of positive social interactions, mood and mental well-being, improved diet and enjoyment of meals and eating.
Case study In November 2017, the Food for Life Better Care team collaborated with staff and residents at Summerfield House Care and Nursing Home, a large privatelyowned care home in Calderdale, to test a whole settings approach to food using a co-design test-and-learn approach.
Over an 18-month period, the Food for Life Better Care team collaborated with staff and residents at Summerfield House Care and Nursing Home to focus on food and foodrelated activities as a social experience and a bridge to the surrounding community. Food for Life Better Care sought a comprehensive approach, in that the programme was informed by a whole settings approach to consider multiple aspects of food. The
team adopted a ‘test and learn’ approach to the programme. This involved consulting with residents, care home staff and others on types of activities to run, testing them out, and reflecting upon the learning. There were several benefits of the programme to residents, staff, residents’ relatives, schools and the care home itself. The analysis led by the University of West England showed that residents derived social, affective, nutritional and general wellbeing benefits from the programme. Staff who engaged in the programme were noted to feel valued, supported and developed a higher sense of morale in the care home. Communication between staff and residents were observed to have improved during the implementation of the programme. There were testimonies from staff about residents’ relatives’ satisfaction of care due to residents’ exposure and engagement in the Food for Life Better Care activities.
The activities had a positive impact on school pupils who visited Summerfield House Care and Nursing Home to undertake intergenerational growing, gardening and other food-related activities with residents. There were observed and reported increase in empathy for older people; counterstereotypical behaviour towards older people; and development of friendships with residents through the intergenerational work. The quality of food was noted to improve during implementation of the Food for Life Better Care programme in Summerfield House Care and Nursing Home and management had realised savings on food cost and reduction of plate waste.
The future of Food for Life Better Care Because of the experience of successfully delivering Food for Life Better Care, we have learned that therapeutic food activities, dining room experiences and nutrition training activities are greatly beneficial to residents, care home staff and catering teams. This experience and the expertise we have built around improving the health and well-being of older people through food related activities, means we know how to support care homes to develop an outstanding food culture and service. We can offer tailored support to you if you’re a local authority, care home company, caterer. We can provide help by delivering consultancy, staff training and activities for nutrition and dining experience, and for our Food for Life Served Here accreditation scheme. L
If you are interested in developing a Food for Life Better Care programme where you work, please get in touch with Florence Todd Fordham on ftoddfordham@soilassociation.org and we would be happy to build a programme that would best support your needs.
The new name from the laptop experts
Change has come. We have evolved. We bring over 30 years of knowledge and expertise with us. With quality, reliability, security and innovation at our core we can now evolve even faster.

And with our technology by your side, so can you.
Discover different at: uk.dynabook.com

Transforming mobile working in healthcare
Nick Offin, head of Sales, Marketing and Operations at dynabook UK, explores the challenges and opportunities presented by mobile working in 2019, and how the latest solutions – from mobile zero clients to wearables – can help healthcare organisations ensure security, productivity and mobility are achieved in equal measure Advertisment Feature
Technology is continually transforming the way people work across a diverse range of industries, with the number of mobile workers predicted to climb from 1.45 billion in 2016 to 1.87 billion by 2022 – accounting for 42.5 per cent of the global workforce. Healthcare is no exception to this, with the global healthcare mobility solutions market predicted to experience an annual growth rate of 28.3 per cent by 2022.
The majority of organisations today already have a mobility strategy in place given the widespread nature of mobile working, and the numerous advantages it can bring. According to an EY study, over 50 per cent of companies have deployed a strategy whereby mobile working is implemented every day. Yet the fast-paced nature of technological innovation, coupled with rapid data explosion driven by the Internet of Things (IoT), mean that such strategies constantly need to be reviewed and refreshed to meet the latest demands.
Unrelenting threats to security in healthcare Remote working and the ever-multiplying swathes of data which are so integral to operations today create increasing opportunity for cyber criminals to strike. The average annual cost of such attacks on healthcare organisations is $12.87 million (£9.26 million) – the fifth most expensive of all industries, given the immense value

attached to sensitive patient data. The more data available to mine, and the more entry points there are providing access to the network, the bigger the risk. Proof of this within an increasingly data-centric healthcare sector is the 211 per cent increase in disclosed security incidents in 2017 when compared with the previous year, according to McAfee.
The edge and data proliferation While security may be the most pressing IT and mobility-related concern for healthcare organisations right now, ensuring efficient and productive working while on the move is also becoming increasingly important. 5G is set to instigate a further boom in the IoT, meaning this ongoing data rush of unprecedented levels is certain to continue and healthcare must be primed to take advantage of this. In order to relieve the strain this data will place on cloud services, a growing number of organisations are integrating an edge-focused element to their mobility infrastructure. Organisations can provide an enhanced quality of service by processing data at the edge of the network, thus reducing the likelihood of data overloads while also helping mobile workers such as GPs to meet compliance regulations, for example by recording consultation notes in a timely manner.


Paving the way for wearables As edge computing develops, so too will the solutions used to collect and manage this data. Global wearable device shipments will rise to 154 million in the enterprise by 2021, according to ABI Research, as sectors including healthcare recognise how they can utilise such technology to enhance mobile productivity.
Accenture claims that, by 2020, 91 per cent of healthcare solutions providers will include wearables in their IoT offerings to clients. This includes the adoption of long-mooted solutions such as Assisted Reality (AR) smart glasses, which can for example be used by medical workers in the field to record patient information in real-time during an examination. What is clear is that, while most organisations have already embraced mobile working, whether they are executing such a strategy effectively varies from one to the next. Verizon’s Mobile Security Index 2018 found that 35 per cent of healthcare organisations reported data loss or downtime from a mobile device security incident in the past year, while 41 per cent admit to purposefully putting themselves at risk of a security breach for the sake of expediency or business performance.
Building, managing and maintaining a secure and agile IT strategy is more complex now than ever before, from choosing trusted devices to integrating bespoke security solutions at a network level. It is, therefore paramount for CIOs to constantly assess their architecture and integrate the right solutions to ensure security, productivity and mobility in equal measure. L
"IT systems in the NHS are so outdated that some staff need to log into as many as 15 different systems to do their jobs." - Source BBC
With Single Sign-On (SSO), Touch ID, user session roaming, and leveraging AI for ultrafast logins, NHS staff can reduce their login times by 90%.
90% REDUCTION
5 Signi cant Advantages of Parallels RAS
Increase employee and IT productivity Improve security capabilities Combine with multi-factor authentication Reduce password fatigue Streamline the user experience
Parallels ® Remote Application Server (RAS) is an all-in-one virtual
desktop infrastructure (VDI) solution that delivers applications and virtual desktops to any device, anytime, anywhere.
Session roaming allows doctors and nurses to move from one device to another and pick up their sessions where they left off. They can access different applications at the same time and switch effortlessly between tasks and devices while on the go. With SSO, Touch ID, and AI, doctors and nurses experience a smooth login process without having to re-enter their credentials multiple times when using different applications, reducing the number of logins and login times. Parallels RAS enables ultrafast logins by leveraging arti cial intelligence (AI), making applications ready-to-use when you need them. For example, if a doctor begins seeing patients promptly at 9 am, the applications needed will launch at 8.55 am - reducing waiting times.
A simple approach to providing improved patient care
Glen Hodgson, head of Healthcare at GS1 UK, looks at the current state of the eProcurement strategy and asks where the GLN sector is heading over the next few years
Trading beyond borders is often a challenge for suppliers. And that’s not just with reference to supply-chain efficiency and traceability - that can also be extended to order processing, invoicing and payment management too. However, when you compound these seemingly simple tasks with the intricacies of a country’s healthcare framework, an already complicated system becomes even more difficult to navigate.
As a result, the Department of Health and Social Care (DHSC) introduced the NHS eProcurement strategy 1 in 2014 – designed to help improve procurement efficiencies across the NHS, and enable organisations to provide better value and better care for patients.
After all, irrespective of being an NHS trust, or a supplier, distributor or manufacturer, the main aim is to make sure the patient has the right product or device, available in the right place and at the right time, every time. With the eProcurement strategy in place, products can be traced to their point of origin, through to their point of issue/delivery. This enables any defective products to be identified and removed from circulation quickly – a significant patient safety advantage.

Take the example of the Poly Implant Prostheses (PIP) breast implant scandal of 2010 2 . To this day, there are tens of thousands of women still living with these implants a decade after their surgery. Apply this same methodology to anti-counterfeiting and medicines traceability, and there are some clear translatable benefits here too. How does this work? Delivering the aims of the eProcurement strategy relies on two central components to support the transition to e-trading and e-invoicing. Firstly, the use of GS1 standards for the unique identification of products, using Global Trade Item Numbers (GTINs) and of locations, using Global Location Numbers (GLNs). This feeds into the efficient synchronisation of data across a central network – the Global Data Synchronisation Network (GDSN).
Secondly, the use of PEPPOL messaging standards for the electronic exchange of transaction information such as purchase orders, delivery details and the exchange of invoices. This depends on the inputting of accurate product data to the GDSN, which can then be attributed back to any individual organisation on the supplyside using their unique organisation identification number – their legal GLN. For NHS trusts, this information feeds directly into their product catalogues. Once an order is placed, suppliers then send the order directly to the trust’s unique delivery location, also identified using a GLN.
The trust is also identified with a legal GLN, so it’s not mistaken any of the other 153 acute trusts across England. Adding further granularity to the process, the trust can also use a separate GLN to ensure the delivery goes directly to a designated point such as a department, ward or even a specific shelf or patient’s bedside. All GLNs used across the system are held in one central repository – LocationManager 3 . So, any time a shipping location is modified, billing information is updated, or a delivery point is changed, the amendments are available in real time, so suppliers have access to everything they need,
whenever they need it. Without accurate location information in place, products are susceptible to being lost and deliveries are at risk of being E Delivering the aims of the eProcurement strategy relies on two central components to support the transition to e-trading and e-invoicing
Introducing the PURELL ® ES8 Dispensing System

Hand hygiene that’s always ready
When you choose new PURELL ES8 hand hygiene dispensers, you’re not only getting trusted hand hygiene products, you’re also getting a revolutionary dispenser design that addresses the two most common service issues – empty dispensers and worn out batteries.
AT-A-GLANCE™ Refill Design We make it easy to monitor product levels with one quick look, saving time and labour, and eliminating complaints about empty dispensers.

Breakthrough Energy-on-the-Refill Technology Each refill comes with a coin cell battery that's integrated into the refill. Touch-free dispensing without the hassle of battery change outs.
For more information:

missed, resulting in unwarranted delays to vital patient care. Without accurate billing information, trusts and suppliers can easily end up with troublesome invoice queries and delayed payments.
All-in-all, this serves to create an efficient procurement system, which is set in place to streamline business transactions across the NHS, improving the purchaseto-pay process for all stakeholders.
Where are we now? Fast-forward to 2020, and the adoption of GLNs continues to play a fundamental role in the current healthcare landscape. Forming part of key mandates and recommendations, the value of GLNs and effective location management has become paramount, with benefits being realised beyond procurement, having demonstrated advantages within clinical settings as well. The use of GLNs underpins a significant part of the NHS Framework Agreement for the Supply of Goods into the NHS 4 , and several suppliers are already on the journey, having barcoded their products, and begun to trade messages via PEPPOL. But more recently though, the Medicines and Healthcare products Regulatory Agency (MHRA) has also recommended the use of GLNs in Field Safety Notifications (FSNs) and Medical Device Alerts (MDAs).
Due to the critical nature of product recalls, it is essential for manufacturers, distributors, suppliers and NHS providers to be able to establish where any remaining inventory is located as quickly as possible, in order to limit potential harm to patients. Therefore, on both of these counts, the use of the legal GLN will feature as a requirement – which in turn supports one of the core aims of the EU device regulations for medical devices (EU MDR) and in-vitro diagnostic medical devices (IVDR).
This particular regulatory update comes into force from May 2020, and will place greater emphasis on the post-market surveillance of
products, including the proactive monitoring of device performance for recertification, annual safety updates for higher-risk class devices, and rapid reporting of safety incidents. Crucially, these changes will impact trusts and wider healthcare organisations that are integral to the supply of products into the NHS.
What next? Over the next few years, the value of GLNs and effective location management will have real potential to drive even more transformative change beyond the realms of efficient procurement and supply chains. There’s no escaping the ‘digital first’ messaging emanating from government, and the drive to go paperless is still at the heart of the agenda, along with the clear focus on interoperability. And that’s not to disregard the overarching sentiment – to improve patient safety across the board: these changes are all steps in the right direction towards achieving a much needed seamless healthcare system. Envisage the bigger picture of being able to track each touchpoint of a patient’s journey from community and primary care through to secondary care anywhere across England – or around the world for that matter. It’s a long road to get to that point, but it’s not as unachievable as it might have appeared to be some decades ago. In reality, there are already some areas of excellence that exist, and trusts and their suppliers across England are making headway. They are beginning to reap the benefits that effective location management has to offer – enabling them to collectively provide better value and, ultimately, a better patient-care experience. L
For more information on GS1 standards and location management, or to start your GLN adoption journey, contact the GS1 UK healthcare team at healthcare@gs1uk.org.
Glen Hodgson is head of Healthcare at GS1 UK. He is charged with supporting the NHS and the healthcare industry to deliver greater efficiency and a more robust approach to patient safety.
With over 20 years of national and international experience, Glen has served at board level in a variety of operational and commercial roles within complex organisational structures inside the pharmaceutical/healthcare arena.
1. https://assets.publishing.service.gov.uk/ government/uploads/system/uploa...
2. https://www.nhs.uk/conditions/pip-implants/
3. https://www.gs1uk.org/our-industries/ healthcare/locationmanager
4. https://assets.publishing.service.gov.uk/ government/uploads/system/uploa...
New report looks at how we can optimise the path to innovation in digital health
The healthcare industry is an ever-evolving space and there is a need to consistently reassess the pathways for innovation so as not to hinder new and exciting developments from reaching the market. For this reason, in 2019, EIT Health hosted a series of round tables across Europe, attended by key stakeholders, to examine the areas of the innovation pathway where improvements can be made
In recent years, there has been rapid growth in the field of medical and health technology. Not only has the number of players in this sector increased, but the diversity of products and services has evolved exponentially. While there are clear benefits in this change in dynamic, we must consider the impact this has in terms of how adequately innovators (those developing products and services) are able to navigate the path to market in a field that is highly regulated and often complex and slow to evolve in line with new technologies. This is particularly relevant when we consider that we are seeing more and more solutions being developed by innovators who are not sector specific, and therefore may lack relevant experience and understanding of the specificities of the healthcare market. The regulatory and reimbursement landscapes are also ever-changing, posing new challenges in terms of development, testing, implementation, usability and adoption of new healthcare solutions. As a result, innovators and other stakeholders can face further hurdles in simply keeping abreast of how to access the healthcare market. In light of this environment, the EIT Health Think Tank selected the topic ‘Optimising Innovation Pathways: Future Proofing for Success’ for consideration and debate in its 2019 Round Table Series. The UK Round Table focused on the digital health market as the introduction and evolution of mHealth applications has seen this market grow rapidly over the past few years. According to a study from Deloitte, the UK market rose from £2bn to £2.9bn between 2014 and 2018.
This trend shows the long-established structure of the healthcare industry, where the pharmaceutical sector has traditionally stood at the forefront, is beginning to shift towards digitalisation – opening the path for new entrants and for giants such as Google, Apple and Amazon to diversify into the healthcare space. In fact, only 32 per cent of mHealth developers come from traditional healthcare stakeholders such as hospitals, health insurers and Pharma companies.
The current situation: a focus on today’s innovation pathway The innovation pathway, or route to market for new products and services, is comparable in the UK to the rest of Europe. It is a continuous and modular pathway whereby all parts are interconnected and reliant on each other. Regulatory and reimbursement stages of the pathway were historically developed to support the introductions of more traditional treatments such as pharmaceutical medicines or medical devices. This has led to a pathway that may be considered to favour more traditional innovation, and in need of reform in order to address emerging technologies such as digital health in all its many forms.
This is particularly relevant when assessing the regulatory processes, which is struggling to keep pace with the rapid introduction of new technologies. In the current landscape, where new technological discoveries are constantly being made, there is increasing need for a more agile regulatory framework. Such reform of the regulatory pathway in Europe, and consequently, the UK, has indeed been addressed recently, as evidenced by the European Medicines Agency, with the proposed introduction of new guidance for medical devices and in-vitro diagnostics which are planned to be introduced in

2020. These new guidelines have been developed in response to the increasing pace of innovation and evolution of the types of products and services requiring assessment. Regulatory capacity to assess innovations based on the introduction of these guidelines, however, is expected to be challenging. Currently, regulators have the capacity to evaluate approximately 600 products per year; whereas approximately 6,000 are expected to be impacted by the new MDR. This will create a backlog of solutions both new and already on the market that require review via the new guidance and may interrupt and slow access for patients and citizens. Pathway optimisation is profoundly important for health innovation, as it will in turn ensure that patients and citizens benefit from access to promising solutions as quickly as possible.
Conclusions and recommendations for optimising the path to market The body of evidence collected during the Round Table Series, demonstrated that there are a number of key stages within the innovation pathway where improvements could be made to aid and speed up the route to market for promising innovative solutions. Participants of the UK Round Table Meeting were asked to agree on a set of recommendations that, if implemented, could help to optimise the pathway for digital health solutions. Where possible, they also identified potential stakeholders who would need to be engaged for further discussion.
Bolstering the ideation stage Ideation marks the beginning of an innovation’s life cycle, and if errors are made

at this early phase, the future success of the innovation may be severely hampered. Through the Think Tank activity, it is clear that a number of activities could be optimised to support innovators at this stage with regards to digital health solutions:
Develop a systematic process for needs identification and assessment to provide better guidance on the evaluation of solutions Digital health solutions should consider both the ‘clinical need’ and the ‘system need’. While the underlying need may be similar for the two, the environment and context can be vastly different. Therefore, innovators should be encouraged to place greater emphasis on the broader ‘problem identification and mapping’ and this process should be structured and standardised to offer clarity. HealthTech Connect, provided by NICE, offers a service that presents a centralised repository of granular needs, and EIT Health should consider consulting and promoting this service to ensure innovators are effectively engaging with this resource. Additionally, the needs of the end user (i.e. patient or citizen) should be fundamental to this process, and a cocreation strategy should be at the forefront throughout the innovation pathway.
Map incubators and accelerators available in the UK and promote engagement amongst innovators Many impactful incubators and accelerators are in existence across the UK, however they are often accessed locally and awareness may be low amongst the wider context of the innovation community. EIT Health should consider conducting a mapping exercise of quality health incubators and accelerators in the countries and regions and promoting this as resource to the EIT Health network. Further discussions should also take place with Academic Health Science Networks, the National Institute of Health and Care Excellences’ HealthTech Connect or the NHS innovation accelerator to capitalise on existing resources and add value where necessary.
Improving the development phase Embrace failure as part of the innovation process – be prepared to ‘fail fast’ – and systematise learnings. There is great value in failing within the innovation process – failure allows us to learn and strengthen our ability to innovate. Failing fast also allows for time, cost and resource efficiency. However, culturally, we are still fearful of admitting failure which hampers our ability to learn. EIT Health can help in changing attitudes to failure and promoting positive sharing of learnings within health innovation. EIT Health should consider sharing results and learnings from funded innovation projects that have failed or not continued. Clarify the evidence generation requirements for digital health solutions to demonstrate true value and facilitate conversation between innovators and regulatory and reimbursement bodies. The generation of evidence occurs too late in the pathway; it is needed throughout the process. There should also be clarity around the appropriate body of evidence required for regulatory and reimbursement bodies for digital health solutions as a distinct methodology in contrast to evidential requirements for medicines and medical devices. Innovators should be supported by early access to, and dialogue with, such stakeholders (HTA bodies, commissioners, trust executive teams, etc.) to develop and sustain a strong value proposition.
There are a number of resources in existence in the UK which may help including the NICE MedTech Early Technical Assessment (META). Additionally, open-access databases that can be used for testing, such as from NHS Digital. EIT Health can help to facilitate such a relationship, connecting innovators with decision making authorities to clarify data collection requirements, as well as signposting innovators within the network to existing resources in the UK.
Market entry – navigating the changing regulatory landscape Assess the impact of the new medical device regulation on the access to digital health solutions.
The introduction of the medical device regulation in 2020 is expected to slow access to digital health solutions due to changing guidance as well as regulatory capacity. While estimations have been calculated, it is not clear what the full impact will be on digital health solutions, and guidance is lacking for innovators in approaching the new regulation. ORCHA has developed a briefing on this for Digital Health Apps
Adoption Promote incentives and a value-based approach to the provision of new digital health technologies. The current reimbursement system is heavily focussed on cost, which presents a challenge for digital health solutions which aim to improve more long-term outcomes such as adherence or care quality.
EIT Health should consider assessing the value-based healthcare procurement landscape for digital health solutions in Europe specifically, and discuss potential reform with policy makers and reimbursement bodies. L
Find out more about how EIT Health it is enabling innovation in healthcare throughout Europe and access the full report at www.eithealth.eu/think-tank-topic
Digital transformation in NHS trusts
Over the last 20 years, new technology, and the changes this technology has had on general workplace practices, has slowly eroded the paper-based past

We mostly send our invoices in PDF form rather than in the post, and we sign our signatures digitally as opposed to printing out documents and putting pen to paper.
But in the NHS, paperwork is still very much alive. From lab results to surgery notes, no sector encounters more paperwork than healthcare. This is still a consistent hindrance to NHS staff, who are required to carry out extensive admin in order to do their jobs.
Looking to a paperless future The integration of smart document capture technology – and the bypassing of excessive admin – is an important step towards delivering a paper-free health service – an objective that many NHS trusts are hoping to achieve by the end of the decade.
More and more trusts are beginning to use scanning platforms to cut down the reliance on paper, but this doesn’t solve the whole problem. We’ve spoken to trusts who have scanned documents into old systems, only for the system to generate one massive file which then needed to be manually searched to pinpoint the information needed. Beyond scanning platforms, the NHS requires intelligent systems – perhaps the product of multiple vendors’ technology – that not only scans a document, but is then able to identify exactly what each document is and store it in a fully-integrated digital platform. In a fit-for-purpose modern-day health system, access to an intelligent, central patient platform is essential to help alleviate some of the administrative strain – one that removes the need for labour-intensive filing and the organising of physical records. By using sophisticated data capture technology, hospital staff can collate and access patient records from a wide variety of sources all in one place, giving them greater visibility into patient history and affording them quick and easy retrieval of information. This technology can change the functionality of the NHS. From the patient’s perspective, clever, integrated digital systems can connect them to care more efficiently and effectively than ever before. And for staff continuously weighed down by administrative tasks, these new technologies will allow them to focus their efforts on the more pressing and personal aspects of care.
Technology in healthcare today At first glance, data capture initiatives may not seem the most glamorous examples of new technology in healthcare today – particularly when compared with robot brain surgeons in Canada, and the developments in 5G and extended reality that are creating new possibilities in simulation training and remote patient treatment.
But the conversion of outdated healthcare systems into modern, streamlined and highly effective ones will underpin the continued success of health services across the world. In the future, further improvements to machine learning and artificial intelligence technologies will radically improve our ability to identify illnesses and diagnose patients. These technologies feed off massive sets of data, and there is an obvious abundance of this in the NHS. But in order to utilise these developments properly, our health services must first

organise the way they store, use and access the extensive and ever-increasing patient information that staff encounter every day.
Ephesoft in the NHS In recent years, a number of NHS trusts across the UK have selected Ephesoft’s technology – together with specialised healthcare technology partners – to transform their working processes. This partner ecosystem can offer complete data capture solutions to NHS trusts that organises, processes and extracts data from a wide variety of clinical documents – from referral notes to discharge summaries and everything in between.
By scanning stickers containing a patient’s date of birth, NHS number and case note number, for example, each record can be clearly identified, ensuring that staff can always access the relevant documents. The technology also has the ability to develop itself over time by learning the layouts of different document types as it comes into contact with them. Given the sheer number of clinical records at play in the NHS, this function ensures that no time is lost when document layouts change – the system learns to recognise the record type and remembers it for next time.
The benefits of an NHS without paper records are numerous and include reduced storage costs; the elimination of lost case notes; eradicating the need for manual entry of patient data; efficient and remote access to extensive patient information all in one place; and digitally secured records protected by rolebased access, safeguarding any sensitive data. But most importantly, the time saved on filing, preparing, auditing and locating patient records – combined with removing the need to ferry documents across wards – will free up the time of hospital staff that can be better spent in patient-facing care. To find out more about how Ephesoft can help increase efficiency in your NHS trust, visit the website below.. L
FURTHER INFORMATION
www.ephesoft.com