7 minute read
Rare diseases (A Everly, S James, A Waldbieser
Introduction
In the United States, rare diseases are defined as diseases that affect fewer than 1 in 200,000 people. There are thought to be around 7,000 rare diseases known today, but more are being identified. Disease is not limited to a geographic location or cultural association, so rare diseases are a global issue. Despite affecting a large portion of the population, there are still problems surrounding issues such as treatment availability, orphan drug development, and rare disease education and advocacy.
Advertisement
Orphan Drugs
A major issue with rare diseases is pharmaceutical companies are not investing as much research and development in treatments for these diseases.
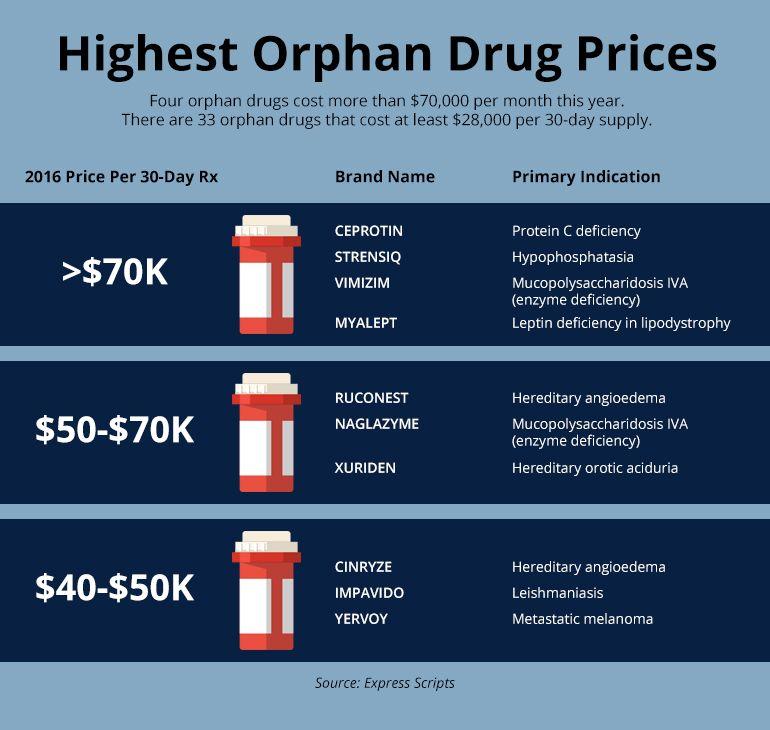
An orphan drug is a drug that is underdeveloped due to limited potential for profitability for pharmaceutical companies. The US passed the Orphan Drug Act to try to encourage the development of these drugs by providing incentives for pharmaceutical companies, such as market exclusivity and reduction of procedural fees. While there have been many successes following this act and many new orphan drugs have entered the market, there are also several concerns. Pharmaceutical companies could create a monopoly since they may be the only company providing a drug. The prices of orphan drugs can be extremely high because of this, as demonstrated by the figure below. In addition, it seems that companies are only focusing on developing treatments for diseases that could be the most profitable for the company. Research for other, lesser-known rare diseases is being neglected. This leads to questions of what should be considered a rare disease. Personal Statement
I believe that legislation needs to be passed to prevent pharmaceutical companies from increasing the price of orphan drugs. Many people are in desperate need of these drugs, but they cannot pay the high prices required for them. The graphic below shows just how expensive orphan drugs can be. I also think that a hierarchy of rare diseases should be established, and research for rare diseases with fewer cases should have more reward and incentive. This would prevent pharmaceutical companies from honing in on a select few diseases to obtain more of a profit. ~ A. Waldbieser
Treatment Accessibility
Treatment accessibility is one of the major issues when it comes to rare diseases. Around the time of the Orphan Drug Act, the FDA and National Institutes of Health federal programs encouraged product development and clinical research for products targeting rare diseases. Ten years after the signing of the US Orphan Drug Act, rare diseases have been a research priority for the European Commission. This created incentives to provide patients suffering from rare diseases with medicinal products which are safe, effective, and produced according to the same quality standards as other products. Since implementation of the ODA, about 1,700 medicinal preparations have been designated as orphan products with about 300 being developed orphan drugs available on the market. The EuOrphan project, which consists of 5 European countries and 13 research institutions, provide a set of web-based services for the dissemination of information on the orphan drugs available worldwide. EuOrphan provides four services: 1) international database containing up-to-date information about orphan medicines including prices and availability throughout Europe, 2) data and statistics on the orphan market at a worldwide level, 3) a consultancy service, and 4) a forum for the recruitment of patients for clinicals trials, organize interest groups, and stimulate new studies.
The affordability of treatments makes it difficult for patients to receive the care that they need. Treatment expenses for rare diseases are typically paid all at once, even though most treatments are received over many years. High costs of treatments put financial burdens on both the patient and payer, and with new treatments continuously being made, the financial burdens are expected to increase even more.
Roland D. The Price Dilemma Over a $16,000 Drug. The Wall Street Journal. https://www.wsj.com/articles/the-price-dilemma-over-a-16-000-drug-1499832421. Published July 12, 2017. Accessed March 31, 2020.
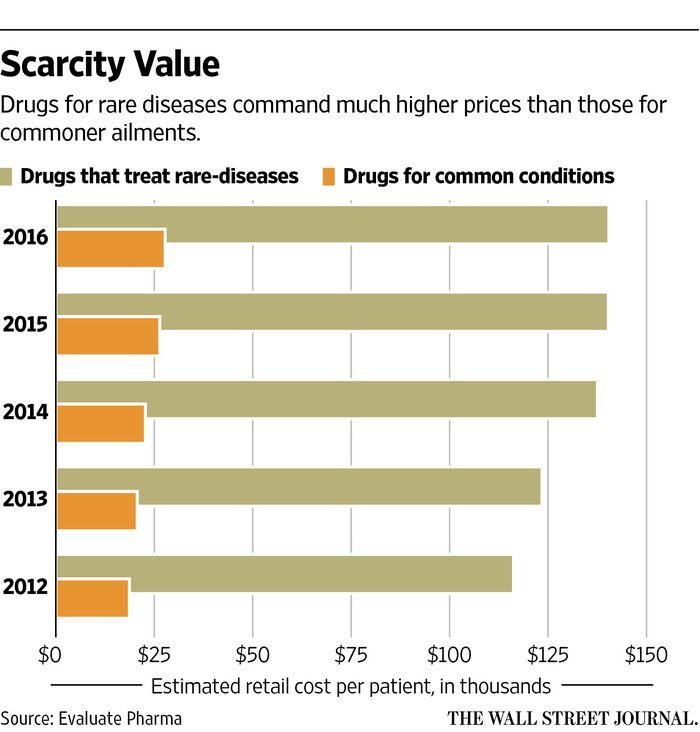
Even with such burdens, pharmaceutical companies have tried to make it easier on patients’ payment plans. Pharmaceutical companies can create value-based contracts that permit payment for therapies over several years. These contracts ensure that the payers are not financially responsible for treatment failures. A bill was also introduced for pharmaceutical and medical device companies to create payment installment deals with insurers linked to the performance of their products. Clinical pathways are usually used to identify the right treatment but there is also opportunity to use pathways to provide guidance on the right payment approach. Another solution would be for manufacturers to partner with financial institutes that provide patients with long term loans for treatments. Other general payment pathways are Medicare, federal reinsurance and subsidies, and reference pricing. However, payment still remains largely unanswered.
Personal Statement
I believe that it is important to provide patients with as much information there is on rare disease treatments to make the process less stressful. When patients have information on how or where to get treatment, it makes it easier for them to take the next step on assessing payment. I understand both sides when it comes to the burdens of patients and payers because patients usually need these treatments to survive but payers also spend a lot of money to make these drugs and have to profit from them. Therefore, finding a balance can help both payers and patients benefit. ~ S. James
Advocacy and Education
Despite being identified as rare diseases, it is estimated by the National Organization for Rare Disorders that around 30 million Americans (as many as 1 in 10) live with a rare disease or disorder. Although there are a significant number of individuals impacted by these conditions, it is a global issue that not much is known about rare diseases or disorders, which is mirrored in the fact that fewer than 10% of the 7,000 known diseases have FDA approved treatments. This emphasizes the unique role that advocacy and education play in informing the general public, as well as policymakers, about the key issues surrounding the topic in order to develop improved conditions for everyone affected by rare diseases and disorders.


NORD. National Organization for Rare Disorders. https://rarediseases.org/. Accessed February 16, 2020.
The National Organization for Rare Disorders (NORD) is one of the key groups that supports education on rare disease issues. They provide resources and information that allow everyone to be involved in some way with rare disease education. One of their biggest projects and campaigns is Rare Disease Day, an international event held on the last day of February that coordinates to raise awareness though numerous activities and projects meant to engage the general public. Each of the 100+ nations that participate aim to target the education of the general public, as well as gain support from policymakers. Various events are held, such as conferences, concerts, family nights, races, and sports events. The purpose is to create equity within the rare disease population as they aim for social inclusion and universal healthcare coverage. They also supply advocacy resources for those wanting to take a more active role, provided through the Rare Action Network (a subset of NORD). Here individuals can connect with others wanting a role in advocacy by finding advocacy issue resources, patient stories, events, and other ways to take action. Groups such as NORD help to drastically minimize and raise awareness for the negative impact that rare diseases and disorders can have on those affected by them, in hopes of one day eliminating them through social inclusion and action.
Personal Statement
I believe the work being done by organizations such as NORD is vital for the wellbeing of the rare disease community. I personally was not aware of how little was known, especially scientifically, about the cause and treatment of these conditions. Through participation in events that NORD and similar groups conduct, significant work can be done if everyone were to participate in some way. I think it would be particularly useful for college students to become involved, for we are a large student body with many voices, and we can help to be the voice of change for an underrepresented group. ~ M. Everly
References 1.) Genetic and Rare Diseases Information Center. U.S. Department of Health and Human Services. https://rarediseases.info.nih.gov/. Accessed February 16, 2020. 2.) J Ethics. 2015;17(8):776-779. doi: 10.1001/journalofethics.2015.17.8.pfor2-1508. Accessed February 13, 2020. 3.) NORD. National Organization for Rare Disorders. https://rarediseases.org/. Accessed February 16, 2020. 4.) Pakizegee, M. and Stefanacci, R. G. Pathways for Paying for Rare Disease Treatments. Journal of Clinical Pathways. 2019;5(1):e1-e3. doi:10.25270/jcp.2019.02.00057. Accessed February 14, 2020. 5.) Rare Action Network. Rare Action Network. https://rareaction.org/. Accessed February 16, 2020. 6.) Stakisaitis D, Spokiene I, Juskevicius J, Valuckas KP, Baiardi P. Access to information supporting availability of medicines for patients suffering from rare diseases looking for possible treatments: the EuOrphan Service. PubMed.gov. https://www.ncbi.nlm.nih.gov/pubmed/17637514. Medicina (Kaunas). 2007;43(6):441–446. Accessed February 16, 2020.










