
7 minute read
Conversion Processes of Carbon Dioxide (CO2) Into Useful Chemicals
CONVERSION PROCESSES OF CARBON DIOXIDE INTO USEFUL CHEMICALS Elizabeth Osadare, Shanelle Bryant, Ariel Taylor, and Emmanuel Dada (Faculty Advisor) Department Of Chemical Engineering, Prairie View A&M University, Prairie View, TX 77446
Abstract
Carbon dioxide (CO 2 ) is the key contributor to greenhouse effect and one of the leading detrimental gaseous compounds that our planet faces. This project focuses mainly on the conversion processes of CO 2 into useful chemicals like urea, ethanol and electro fuels. Technologies involved in combustion processes and how captured CO 2 serves as feedstock for the production of useful chemicals [2] are discussed. The conversion processes are sustainable route of utilization of CO 2 leading to reduction of negative global impact of CO 2 on the planet. [2]. Aspect requiring further research interest is high selectivity for reaction via catalyst needed for higher conversion rate of carbon dioxide into desired chemical products. An extensive research on the conversion processes particularly in the pilot scale area is recommended.
Background
An increase in the CO 2 emissions between 1990 and 2017 correspond to increase in energy usage due to economy growth and industrialization. Though there are many natural sources of CO 2 emission, but the largest source of it is from human-related activities amongst which fossil fuel combustion process is the major contributor. In 2017, CO 2 gas emission was approximately 82% of all the greenhouse gases (GHG) released through human activities in the US alone as described in the Figure 1 below. In general, human activities give rise to the global CO 2 concentrations in the atmosphere thereby altered the earth’s carbon cycle and result in climate change and global warming. [5]
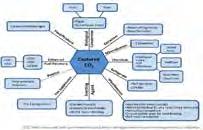
This concept describes commercializatio n potentials and various ways for utilizing the captured CO2 as chemical feedstock [10 ]
This concept describes the hypothetical hydrogenation of captured CO 2 and the use as a feedstock for the synthesis of synthetic liquid fuels.
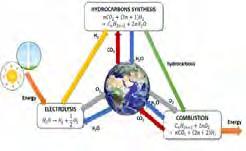


long chain hydrocarbons (HC) [1]
Figure 1. (a): Global greenhouse gas emissions by economic sectors.[6] (b): U.S. Greenhouse gas emission in 2018.[7]
Aim
This research focuses on the conversion processes of captured CO 2 as
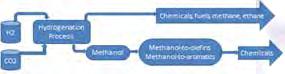
a direct, indirect or co-reactant feedstock for the production of urea, methanol, dimethyl ether (DME) and synthetic liquid fuels amongst many useful chemical products.
Acknowledgements
Office of Undergraduate Research (OUR and Undergraduate Medical
Introduction
Carbon dioxide (CO2) is the key contributor to the greenhouse effect and one of the leading detrimental gaseous compounds that our planet faces. An increase in the CO2 emissions between 1990 and 2017 corresponds to an increase in energy usage due to economic growth and industrialization. Though there are many natural sources of CO2 emission, the largest source is from human-related activities, among which the fossil fuel combustion process is the major contributor.[1] In 2017, CO2 gas emission was approximately 82% of all the greenhouse gases (GHG) released through human activities in the US alone.[6,7] In general, human activities give rise to global CO2 concentrations in the atmosphere, thereby altered the earth’s carbon cycle and result in climate change and global warming. [5]. This project focuses mainly on the conversion processes of CO2 into useful chemicals like Urea, dimethyl ether (DME), methanol, and liquid fuels. The conversion processes are the route to the substantial utilization of CO2, leading to a reduction of the negative global impact of CO2 on the planet. [1], [2].
One of the critical technical issues in the 21st century is the quest for reducing the rising concentration of CO2 in the atmosphere. The current major technological breakthrough to this global concern is the concepts of CO2 capturing, storing, and utilizing the captured CO2 directly or indirectly to generate useful chemical products. [1-4],[8],[10]. Effective CO2 utilization and commercialization potentials are seriously gaining attention in chemical industries as the total annual utilization of CO2 hits 120 million tons [1]. CO2 is a non-toxic, very reactive, and renewable gas [3]. It is thermodynamically stable, and so its application as co-reactant feedstock for the synthesis of useful chemical products, including liquid fuels (long-chain hydrocarbons), is believed to be of help in solving the global concern of CO2 emission and greenhouse effect [2] [3] [4]. Successful implementations of these concepts will extensively reduce the global dependence on fossil fuels for energy, thereby mitigates the undesirable impact of the CO2. as a greenhouse gas. [1],[10]
Aim
To focus on the conversion processes of utilizing captured CO2 as a feedstock directly or indirectly or co-reactant raw materials to produce Urea, methanol, dimethyl ether (DME), and synthetic fuels amongst many useful chemical products.
Methodology
The method under consideration in this research is the hydrogenation process of captured CO2 and using it as a feedstock or co-reactant raw material for chemical synthesis. The focus is to synthesis urea, methanol, dimethyl ether, and liquid fuels.
Results and Discussion
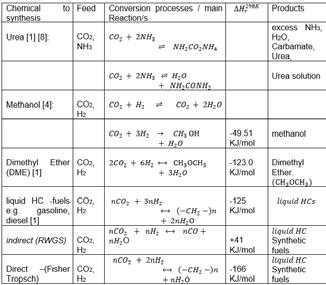
The table below shows the chemical reactions for each process in the conversion of CO2 to some useful chemicals.
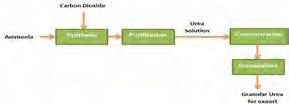
The urea solution is further concentrated to 99.6% w/w molten urea, then granulated for use as fertilizer and other chemical feedstocks [8]. Methanol is further processed to form gasoline and useful chemicals such as formaldehyde (methanal), acetic acid, and formalin, which are organic solvents and raw materials for pharmaceutical products,[1] DME is a low CO2 fuel with good octane number. It can be used as a clean-burning fuel alternative to liquefied petroleum gas (LPG), liquefied natural gas (LNG) and diesel. [11] Finally, the hydrocarbon synthesis Fisher-Tropsch reaction produces long-chain HCs- olefins (C1 to C11) and oligomerize to form gasoline.
Conclusion
There is a significant advancement in the development of a catalyst that provides high selectivity of converting CO2 to useful chemicals and value-added fuels. Finding reveals that Fe-based catalyst is more suitable when compared with Co-based catalyst in converting CO2 to long-chain hydrocarbons to produce synthetic liquid fuels. Adding potassium and copper to the Fe-based catalyst favors the conversion of CO2 and promotes the synthesis of liquid fuels.[1]. When successfully implemented, the concept of power-toliquid fuels using CO2, among other routes, is an important clean energy route. This will extensively reduce the global dependence on fossil fuel, thereby mitigates the undesirable impact of the CO2 as a greenhouse gas. However, the limiting factors affecting CO2 conversion processes include the cost of the energy and the hydrogen [10]. In conclusion, the optimum yield of desirable products is ultimately needed to compensate for the production cost. This is achievable by hydrogenation of CO2 and is subject to the catalyst used for the conversion processes. Therefore there is a need to develop mixed catalysts needed for high selectivity and optimum product yield in the conversion of CO2 to useful chemicals at a reduced cost.
References
[1] C.Panzone, R. Philippe, A. Chappaz, P. Fongarland, and A. Bengaouer, “Powerto-Liquid catalytic CO2 valorization into fuels and chemicals: focus on the FischerTropsch route”, Journal of CO2 Utilization, vol. 38, pp. 314-347, 2020. [2] J.Wu and X. Zhou, “Catalytic conversion of CO2 to value-added fuels: Current status, challenges, and future directions”, Chinese Journal of Catalysis, vol. 37, no. 7, pp. 999-1015, 2016. [3] Y.Qin, G. Niu, X. Wang, D. Luo, and Y. Duan, “Status of CO2 conversion using microwave plasma”, Journal of CO2 Utilization, vol. 28, pp. 283-291, 2018. [4] C.Zhang, K. Jun, G. Kwak, Y. Lee and H. Park, “Efficient utilization of carbon dioxide in a gas-to-methanol process composed of CO2/steam–mixed reforming and methanol synthesis”, Journal of CO2 Utilization, vol. 16, pp. 1-7, 2016. [5] “Putting CO2 to Use – Analysis - IEA”, IEA, 2020. [Online]. Available: http:// www.iea.org/reports/putting-co2-to-use. [6] “Global Greenhouse Gas Emissions Data | US EPA,” US EPA, 2020. [Online]. Available: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data. [7] “Overview of Greenhouse Gases | US EPA,” US EPA, 2020. [Online]. Available: https://www.epa.gov/ghgemissions/overview-greenhouse-gases. [8] “Ammonia and Urea Production,” Nzic.org.nz, 2020. [Online]. Available: https:// nzic.org.nz/app/uploads/2017/10/1A.pdf. [9] “Ministry of Energy and Energy Industries | Urea,” Energy.gov.tt, 2020. [Online]. Available: http://www.energy.gov.tt/our-business/lng-petrochemicals/ petrochemicals/urea/. [10] “ICEF Innovation for Cool Earth Forum”, Icef-forum.org, 2020. [Online]. Available:https://www.icefforum.org/platform/thematic_discussion_topic13_ session2.php. [11] Week, “Francois Bollon - DME: A Sustainable Alternative Fuel for Transportat…”, Slideshare.net, 2020. [Online]. Available: https://www.slideshare.net/EMA_SIEW/ francois-bollon-dme-a-sustainable-alternative-fuel-for-transportation
Shanelle Bryant is a junior majoring in chemical engineering. Ariel Taylor is a senior, majoring in chemical engineering, and Elizabeth Osadare is a graduate student majoring in chemical engineering. Dr. Emmanuel Dada an Assistant Professor of Chemical Engineering with a research interest in carbon dioxide (CO2) utilization; plastic waste, artificial intelligent, process safety, renewable and clean energy.










