Soundings
President’s Message

Welcome to the Spring 2024 issue of Soundings!
As we move into the spring and summer, planning for the annual PAO-HNS annual scientific meeting at the Hotel Hershey is well underway. This year’s agenda will focus on bridging the gap and foster collaboration between academic and
private practice otolaryngologists in the state under the guidance of Sandra Stinnett MD, UPMC, and Robert Brody MD, U Penn. I appreciate the diligence and hard work byof the planning committee, who has arranged for an exciting lineup of talks spanning the spectrum of otolaryngology and including a business practice session. We are excited to welcome Doug Backous, MD, President of the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) and its Foundation, as keynote speaker and Mary Mitskavich, MD, founder and managing partner of Coastal Ear, Nose and Throat, LLC, as the featured speaker for the Women in Otolaryngology event. Also new this year, the meeting will include a private practice speed mentoring session and will be bringing back the family simulation event which is an interactive experience for children and families.
We look forward to seeing you in Hershey in June, register for the meeting now at www.otopa.org.


One of the beautiful things about the PAO-HNS is the ability to engage and collaborate with otolaryngologists in all practice settings throughout the state. As you read through this issue of Soundings, you will may notice some changes in the design. Similar changes are underway with our website. We would like to incorporate features on otolaryngologists in the state who are making an impact locally, nationally, and globally in either clinical practice advancement, research, training or community service/outreach. If you would like to be featured or know of a colleague making an impact in one of these areas, please send us a photo and brief description to include in future issues and on our website. We look forward to hearing from you!
Best regards, Jessyka G. Lighthall, MD, FACS

THE INTEREST OF OUR MEMBERS
THEIR PATIENTS SPRING 2024
PUBLISHED IN
AND
Jessyka G. Lighthall, MD, FACS PAO-HNS President


President Jessyka G. Lighthall, MD, FACS Penn State Health Milton S. Hershey Medical Center Otolaryngology—Head & Neck Surgery
President-Elect Colin T. Huntley,
University—Otolaryngology
Secretary-Treasurer Andrew McCall, MD, FACS University of Pittsburgh Department of Otolaryngology
Administrative Office 400 Winding Creek Blvd. Mechanicsburg, PA 17050-1885 833-770-1544
855-918-3611 (fax)
Soundings accepts classified advertisements; however, there is no guarantee that they will be published. All submissions are subject to review. The advertisement should be of interest/ pertain to otolaryngologists, their practice, and health care in Pennsylvania. Submissions that are self-promotional or commercial in nature will not be accepted. Publication of advertising does not imply endorsement of the products advertised or the statements contained in such advertising by Soundings or the PAO-HNS. The opinions expressed in this newsletter do not necessarily reflect the opinion of PAO-HNS.
MD Jefferson
Head
& Neck Surgery
Visit our website at www.otopa.org 2 SOUNDINGS | Spring 2024 Contents | Spring 2024 1 President’s Message 3 The Face of Semaglutide 6 Spring BOG Update 8 Ergonomics In Otolaryngology 10 Legislative Update 11 TT Placement in ASD Kids 13 Does Obesity Contribute to Increased Operative Time 15 Annual Meeting 16 Member Benefits
The Face of Semaglutide: What the Facial Plastic Surgeon Needs to Know
David Goldrich, MD
Department of Otolaryngology-Head & Neck Surgery, Penn State Milton S. Hershey Medical Center, Hershey Pennsylvania, USA
Bao Sciscent, BS Pennsylvania State University, College of Medicine, Hershey, Pennsylvania, USA
Hanel Eberly, BS Pennsylvania State University, College of Medicine, Hershey, Pennsylvania, USA
Scott Walen, MD FRCS Department of Otolaryngology-Head & Neck Surgery, Penn State Milton S. Hershey Medical Center, Hershey Pennsylvania, USA
Corresponding Author: Scott Walen, MD, FRCS
Introduction
Initially developed for glycemic control in patients with type 2 diabetes (T2DM), Semaglutide (sold under brand name Ozempic/Wegovy) has been creating headlines for its significant effects on weight loss and obesity. With many patients seeking adjunct treatment for weight loss, Semaglutide has become a popular and efficacious option toward weight reduction. One 2021 study demonstrated nearly 15% mean body weight reduction in overweight/obese patients without T2DM taking Semaglutide as compared to a 2.4% reduction among patients taking placebo, a greater effect than many approved weight loss drugs.1 Given advantageous effects as well as multiple administration options including an oral or once-weekly injection formulation, hundreds of thousands of patients have been proscribed Semaglutide, with largely positive results for weight loss as well as emerging data of impact upon associated metabolic and cardiovascular comorbidities such
as systolic blood pressure, hyperlipidemia and stroke.1
As originally conceived toward treatment of T2DM, Semaglutide falls within glucagon-like peptide 1 (GLP-1) receptor agonist class of medications (See Table 1). GLP-1 receptor agonists act upon pancreatic alpha and beta cell receptors and within peripheral tissues to increase glucose-dependent insulin secretion, inhibit glucagon secretion, increase sensation of satiety via hypothalamic stimulation, and decrease gastric emptying (See Figures 1 & 2).2 For diabetic purposes, Semaglutide can be used in addition to metformin or basal insulin when a greater HbA1c reduction is required, or as monotherapy when intolerant of metformin.3 Compared to similar GLP-1 medications such as liraglutide, Semaglutide has been associated with greater dose-dependent
decrease in plasma glucose levels and body weight.4 Similarly, Semaglutide has shown significantly greater HbA1c reduction compared to dulaglutide, exenatide, liraglutide and lixisenatide.5 While generally well-tolerated, adverse side effects can occur in dose-dependent manner and commonly include mild to moderate gastrointestinal side effects such as nausea, constipation, diarrhea and vomiting. Rarer side effects include hepatic disorders, pancreatitis, injection site reactions, and hypoglycemia (See Table 2).1,3 Though no clear association has been made, there has also been reported risk of medullary thyroid carcinoma that endocrinologists and otolaryngologists should be aware of.6
“Ozempic
Face” Semaglutide’s primary mechanism of action for weight loss has been
Continued on page 4

3 SOUNDINGS | Spring 2024 TABLE 1. GLP-1 Receptor agonists Drug (Brand names) Year Approved Administration Dosing Frequency Exenatide (Byettta) 2005 Subcutaneous Twice Daily Liragultide (Victoza) 2010 Subcutaneous Once Daily Exenatide (Bydureon) 2012 Subcutaneous Once Weekly Dulaglutide (Trulicity) 2014 Subcutaneous Once Weekly Lixisenatide (Adlyxin) 2016 Subcutaneous Once Daily Semaglutide (Ozempic) 2017 Subcutaneous Once Weekly Semaglutide (Rybelsus) 2019 By Mouth Once Daily Semaglutide (Wegovy) 2021 Subcutaneous Once Weekly Table 2. Reported Adverse Effects of Semaglutide Adverse Side Effects Reported Frequencies of Side Effects Constipation 11.6%-38.9% Diarrhea 8.6%-34.9% Nausea 7.3%-61.1% Abdominal Pain 6.5%-10% Nasopharyngitis 5.7%-21.5% Psychiatric disorders 5.6%-17.1% Back Pain 5.2%-9.9% Allergic Reactions 4.9%-15.1% Headache 3.4%-15.9% Retinopathy 3%-7.1% Vomiting 2.8%-30.3% Hepatic disorders 1.6%-2.4% Gall-bladder related disorders 0.8%-2.8% Injection site reactions 0%-6.6% Hypoglycemia 0%-2.6% Acute renal failure 0%-0.8% Acute pancreatitis 0%-0.2%
of Semaglutide
Figure 1. Mechanism
The Face of Semaglutide: What the Facial Plastic Surgeon Needs to Know

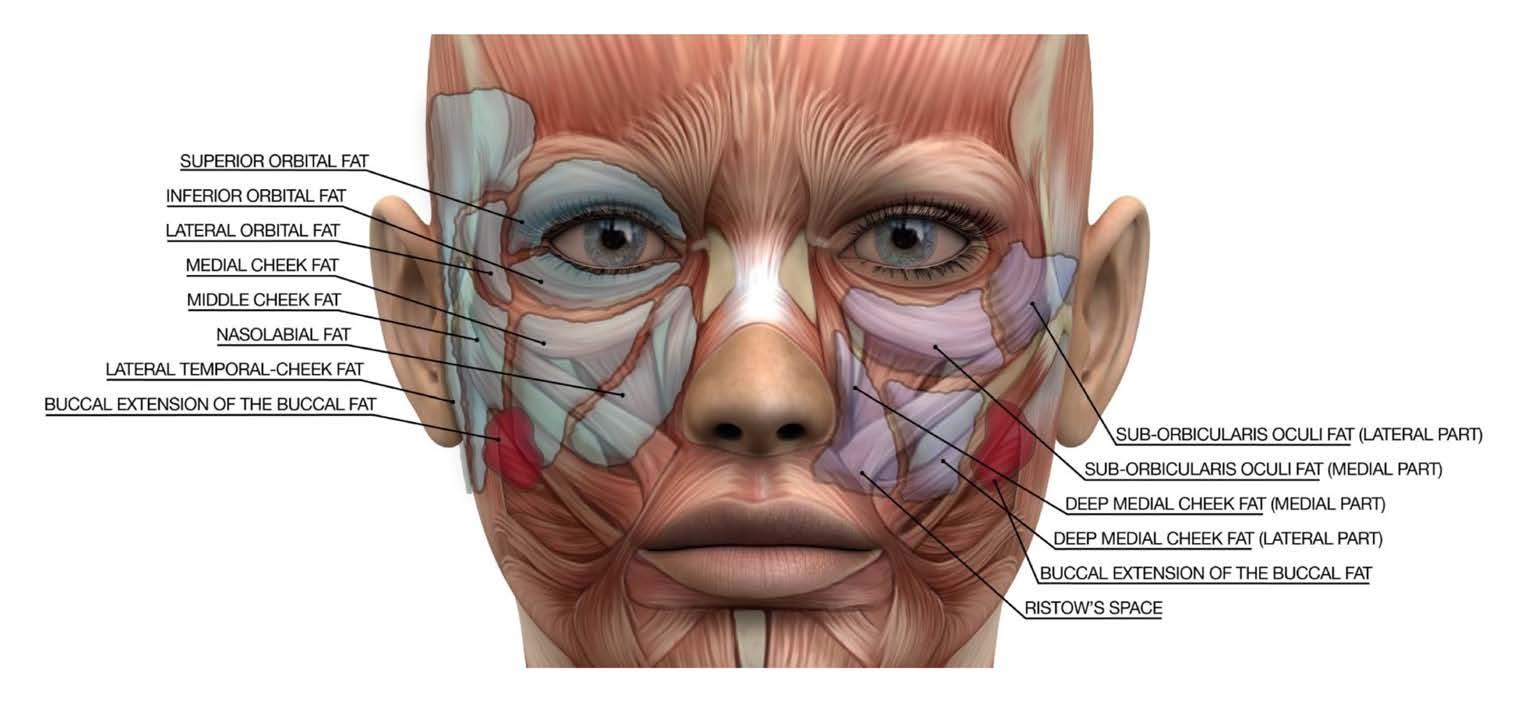
reduction of visceral abdominal fat leading to decreased obesity and waist circumference. However, one emerging concern for the facial plastic and reconstructive surgeon (FPRS) has impact upon facial fat distribution. “Ozempic Face”, a term originally coined by dermatologist Dr. Paul Frank, describes the resulting thinner, gaunt facial appearance that can make patients appear older than their given age. While not a side effect specific to Semaglutide and largely due to effects of rapid systemic weight loss, media popularization has increased awareness of this previously underreported facial impact. Comparable to post-bariatric surgery, patients with rapid weight loss have been shown to appear older than those undergoing mild weight loss. 7 Studies involving 3-D morphologic analysis have demonstrated significant facial volume loss after massive weight loss, and imaging studies have shown correlation between BMI and total cheek fat volume.8 Facial fat has known compartmentalization within superficial dermal and deep subcutaneous layers in the perioral, midface, buccal and deep temporal fat pads (see Figure 3).
Following weight loss impact on these distributions, patients can have excess jowl skin, and volume loss in the midface, nasolabial groove and perioral regions. In similar fashion, normal facial aging shows an evolution of appearance based on fat architecture, where loss of deep medial cheek fat volume can contribute to increased nasolabial fold depth, and deflation of the buccal and superficial lateral temporal fat pads can contribute to droopy appearance of the face.9 Subcutaneous fat loss can also cause
fat loss, but in many studies of Semaglutide, most participants were female and subgroup analyses found greater mean weight reduction among female patients.1,10 Additionally, some patients may be using Semaglutide “off label” and non-diabetic or non-obese patients should be carefully questioned regarding medication use or other medication side effects. For patients suffering from “Ozempic Face”, changing drug regimens can be considered, and discontinuation of Semaglutide can lead to rapid regain
appearance of wrinkles and increased prominence of existing folds. Beyond effects upon facial fat, rapid weight loss can also place strain on the collagen and elastin dermal matrix. Unlike in slower weight loss, the dermal matrix has less ability to adjust to rapid changes or develop compensatory contouring of appearance, leading to less tightened, “saggy” skin.
Treatment Considerations
In evaluation and treatment of this problem, FPRS should be able to recognize higher risk populations and have informed discussions with patients regarding the potential facial effects and best treatment plan for rapid weight loss. In particular, undesirable effects may be more prominent in female patients; not only do women tend to have more facial fat than men and more marked detrimental aging effects with cheek
of weight including facial hollow refilling. However, like the nonspecific systemic patterns of weight loss, distribution of weight regain is unpredictable. Likewise, any consideration of medication cessation should include risk-benefit discussion of likely reversal of beneficial metabolic and cardiovascular effects.
For appropriate patients, several treatments can be used to augment facial fat and target the appearance of an aged face, though the underlying issue of rapid weight loss will not be addressed. Facial fillers can add volume back to the face by filling temporal hollows, cheeks, or jawline. Fat grafting is another option for facial rejuvenation, which involves adipose tissue harvest and facial transfer as autograft. Though a more involved procedure, compared to synthetic fillers, fat
4 SOUNDINGS | Spring 2024
Continued on page 5
Continued from page 3
Figure 2. Effects of Semaglutide on the Body
Figure 3. Facial Fat Compartments
The Face of Semaglutide: What the Facial Plastic Surgeon Needs to
Know
Continued from page 4
grafting can have longer-lasting effects at addressing facial volume. However, while replacing volume, grafts may not have the same mechanical properties as native white adipose tissue. Laser resurfacing, radiofrequency and other modalities can be used to target wrinkles and give skin a more tightened appearance. Other treatment considerations include medication-specific perioperative risk factors, as Semaglutide can be associated with delayed gastric emptying, a potential risk factor for perioperative aspiration if undergoing general anesthesia. Additionally, it can rarely cause systemic complications such as kidney failure or pancreatitis. Likewise, treatment-seeking patients with T2DM or obesity stopping their medication preoperatively may at higher risk of poor glycemic control or perioperative cardiometabolic events.10 Ultimately, understanding the anatomy of facial fat distribution is key to appropriate restoration of facial volume and helping patients achieve their desired appearance. While the role of imaging is unclear at this time, future investigations using CT or magnetic resonance imaging (MRI) of patients treated with Semaglutide may provide more detailed insight into the medication’s effect on facial appearance and post-treatment effects.
With increased Semaglutide usage, an uptick in FPRS referrals for post-treatment facial concerns may occur. Current limitations to the size of the Semaglutidetreated population appear to be medication cost and supply. Due to limited FDA-approved indications, out-of-pocket cost for 30 day supply of Semaglutide is approximately $1,619, significantly more expensive than other weight loss drugs such as Orlistat or Phentermine ($40-$233 per month), and cost-effectiveness of long term therapy may be a consideration in patient use.11 However, with expanding indications, increasing supply, competitor
development, and growing social media awareness, advertising and patient desire for off-label weight loss use of Semaglutide, “Ozempic Face” will likely only become a more prevalent concern.
Conclusion
In summary, while GLP-1 receptor agonists like Semaglutide have seen a rise in popularity due to efficacy for weight reduction, it has led to the emerging knowledge of FPRS-specific impact of undesirable facial features. These include sagging skin and facial hollowing causing appearance of premature aging and can lead patients to seek facial rejuvenation procedures. It should be understood that “Ozempic Face” is a misnomer, as the gaunt facial features seen in these patients may be attributable to overall rapid systemic weight loss rather than targeted facial effects of the medication. Nonetheless, with expanding use of Semaglutide and the development of additional similar class medications, the facial plastic surgeon should be familiar with these side effects and appropriate treatment options.
References
1. Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384(11):989-1002.
2. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696-1705.
3. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus oncedaily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366.
4. Nauck MA, Petrie JR, Sesti G, et al. A Phase 2, Randomized, Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog, Semaglutide, Compared With Placebo and Open-Label Liraglutide in Patients With Type 2 Diabetes. Diabetes Care. 2016;39(2):231-241.
5. Chubb B, Gupta P, Gupta J, Nuhoho S, Kallenbach K, Orme M. Once-Daily Oral Semaglutide Versus Injectable GLP-1 RAs in People with Type 2 Diabetes Inadequately Controlled on Basal Insulin: Systematic Review and Network Meta-analysis. Diabetes Ther. 2021;12(5):1325-1339.
6. Smits MM, Van Raalte DH. Safety of Semaglutide. Front Endocrinol (Lausanne). 2021;12:645563.
7. Valente DS, Braga da Silva J, Cora Mottin C, et al. Influence of Massive Weight Loss on the Perception of Facial Age: The Facial Age Perceptions Cohort. Plast Reconstr Surg. 2018;142(4):481e-488e.
8. Peters F, Kroh A, Neumann UP, et al. Morphological changes of the human face after massive weight-loss due to bariatric surgery. J Craniomaxillofac Surg. 2020;48(7):694-699.
9. Rohrich RJ, Pessa JE, Ristow B. The youthful cheek and the deep medial fat compartment. Plast Reconstr Surg. 2008;121(6):2107-2112.
10. Rubino D, Abrahamsson N, Davies M, et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021;325(14):1414-1425.
11. American Diabetes Association Professional Practice C. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S113-S124.
5 SOUNDINGS | Spring 2024
BOG Update Spring 2024
 Karen A. Rizzo, MD, FACS Chair BOG PA Governor/BOG
Karen A. Rizzo, MD, FACS Chair BOG PA Governor/BOG
The Board of Governor’s continues to advocate for legislative and political activity that directly impacts our patients and practices. Negotiations continue with the Medicare physician payment cut relief. In the house the GOP doctors caucus has sent a letter to Senate leadership urging them to mitigate the cuts in their spending package. A bipartisan group of senators also announced the formation of a Medicare payment reform working group to investigate and propose long term reforms to the Medicare physician fee schedule and make necessary updates to the Medicare access and chip reauthorization act (MACRA). Reforming MACRA will be the key long term legislative solution to put an end to the annual fight against Medicare payment cuts.
Recently Dr. James Denneny, our executive vice president and CEO, testified in Maryland against Senate bill 795 which would enact legislation in Maryland detrimental to patient safety granting privileges to audiologists without adequate training to perform them. The bill would allow audiologists to diagnose and treat all diseases of the ear including ordering imaging, performing cultures, and prescribing medications. This bill was first presented
and defeated last year but again has been reintroduced with more sponsors. The Academy is working on a response statement to the Blues, including in Pennsylvania, concerns over denying payment for post-op debridement for FESS patients. Also, comments on certain Blue Shield plans requiring 3 months of medical treatment prior to clearance for septoplasty as inappropriate is in the works.
The inaugural AAO HNS OTO forum was held April 5th to 6th 2024 at the Westin Alexandria. This new event provided a productive forum for leadership discussion and practice management tools highlighting current and future private practice physicians needs in caring for otolaryngologic patients. A focus on networking, benchmarking, innovation, and healthcare trends impacting private practice was discussed by leaders in the field. A BOG advocacy update was also included a presentation from Dr. Mukkamala of the AMA and concluded by the BOG candidate forum, where the president-elect candidates gave an overview of their desire to lead and discuss goals and concerns impacting our specialty and how they would be managed under their leadership.
The BOG will also sponsor a virtual advocacy summit on April 20th giving updates on important legislative and political concerns impacting our specialty,
a panel discussion on pay for ENT call, and interviews of president-elect candidates regarding their goals and concerns.
The following represents the 2024 candidate slate for leadership of the Academy:
1. President-elect: Dr. Eugene Brown and Dr. Lakeisha Henry.
2. At Large Directors (Academic) Dr. Peter Manes and Dr. Lisa Schnayder.
3. At Large Directors (Private Practice) Dr. Mary Mitskavage and Dr. Karen Rizzo.
4. Nominating Committee (Academic) Dr. Heather Edwards, Dr. Margo McKenna, Dr. Stephanie Misono, and Dr. Robert Zitsch, III.
5. Nominating Committee (Private Practice) Dr. Daniel O'Brien, Dr. Annette Pham, Dr. David Yen.
6. Audit Committee: Dr. Art Ambrosio, Dr. Ashutosh Kacker.
I encourage all members of the AAO to vote this May on this excellent slate of candidates.
Karen A. Rizzo, MD FACS PA Governor/BOG Immediate Past Chair BOG
Region 3 Regional Rep Owner/Founder Lancaster Ear, Nose, & Throat, LLC

6 SOUNDINGS | Spring 2024
Pathophysiology of Rhinosinusitis and the Increased Prevalence of Wildfires
Catherine Kwiecien, BA
Alan D. Workman, MD, MTR
Nithin D. Adappa, MD
From wildfires ignited by fallen power lines in Hawaii to those sparked by lightning and drought in eastern Canada, nearly three million acres of land were scorched in 2023 alone.i Human oversight and environmental factors leading to severe heat have been the driving forces fueling the fires resulting in a loss of land, elevating air quality measures, and sending people around the world into hazy, smoke-filled days. With larger and longer burning fires, smoke drifts into densely populated areas, impacting the lives and health of millions of individuals. Studies have shown that generalized smoke can contribute to the generation of chronic rhinosinusitis (CRS), chronic bronchitis, asthma, and chronic obstructive pulmonary disease. ii An increasing prevalence of wildfire smoke (WFS) leads us to hypothesize effects that wildfires specifically will have on respiratory systems, in particular the sinuses, of the greater population as temperatures continue to rise due to climate change.
Wildfire smoke around the world releases particulate matter (PM), carbon monoxide, methane, nitrogen oxides, volatile organic compounds, and even trace metals along with other pollutants into the atmosphere.iii The greatest concern comes from particle pollution, namely the fine particulate matter that measures <2.5 μm in diameter (PM2.5), which is nearly 30-times smaller than a strand of human hair.
When inhaled, PM2.5 can travel deep through the respiratory tract and make its way into the lungs and blood, causing throat, lung, and eye irritation, persistent coughing, phlegm, and labored breathing. Individuals with chronic heart and lung conditions, older adults, and children <5 years of age are most at risk, yet anyone can experience symptoms caused by PM2.5 pollutants.iv Due to
the complex makeup of WFS, it is currently unknown how it may directly impact CRS; however, histological evidence shows elevated type 2 eosinophilic inflammation after exposure to PM2.5 in individuals with worsening symptomology of chronic rhinosinusitis severity.v
Pollutants and PM2.5 exposure cause the sinonasal respiratory epithelium to heighten defenses against airborne pathogens. Through ciliary beating and mucociliary clearance (MCC), it transports mucus from the nasal cavity and into the nasopharynx. When the motility of the cilia is impacted, whether by environmental factors or upper respiratory diseases, the MCC is disrupted as the airway surface liquid (ASL) is contaminated. Recent studies have found the cystic fibrosis transmembrane conductance regulator (CFTR) aids in balancing the homeostasis of the sinuses along with the ASL, and when dehydrated or disrupted, individuals experience viscous mucus due to ciliary disfunction.ii
While initially identified as a gene associated with CF, studies have reviewed how acquired CFTR deficiency can be induced by external factors, namely cigarette smoke, hypoxia, or inflammation; all factors that can also contribute to the pathogenesis of CRS, as well. Because cigarette smoke decreases CFTR gene expression and leads to the destabilization of proteins, there has been a positive correlation with those who are exposed to such smoke and their incidence of CRS. The correlation between WFS and rhinitis is currently in review, however results have been mixed, with many suggesting that exposure to PM2.5 is of greatest concern.
Until we know more about the potential risks associated with WFS inhalation, experts recommend wearing well-fitted N95 masks when the air quality index is elevated, as they filter out 95% of PM2.5 particles.vi Other studies have reviewed strategies to combat chronic sinonasal inflammation via targeting the CFTR once an individual is already managing their disease. In acquired dysfunction cases, such as in CRS, typical treatment can
include CFTR potentiator compounds such as anti-inflammatory medications, antibiotics, and saline irrigations. Novel CFTR corrector therapies include medications that stimulate chloride secretion to increase ASL depth to help loosen thick mucus secretions.ii
The effects of long- and short-term WFS exposure and its correlation with inducing rhinosinusitis are still being studied. As wildfires become more prevalent and exposure to WFS increases in the upcoming years, it is pertinent that the risks and dangers involved, specifically with the smoke, and not only PM2.5, are better understood so the appropriate preventative strategies can be taken.
References
i National Fire News. National Interagency Fire Center. (n.d.). https://www.nifc.gov/fireinformation/nfn
ii Banks, C., Freeman, L., Cho, D. Y., & Woodworth, B. A. (2018). Acquired cystic fibrosis Transmembrane Conductance Regulator Dysfunction. World Journal of Otorhinolaryngology - Head and Neck Surgery, 4(3), 193–199.
iii Noah, T. L., Worden, C. P., Rebuli, M. E., & Jaspers, I. (2023). The effects of wildfire smoke on asthma and allergy. Current Allergy and Asthma Reports, 23(7), 375–387. https://doi. org/10.1007/s11882-023-01090-1 iv Centers for Disease Control and Prevention. (2023, February 16). Particle pollution. Centers for Disease Control and Prevention. https://www. cdc.gov/air/particulate_matter.html
v Patel, T. R., Tajudeen, B. A., Brown, H., Gattuso, P., LoSavio, P., Papagiannopoulos, P., Batra, P. S.,& Mahdavinia, M. (2021). Association of Air Pollutant Exposure and sinonasal histopathology findings in chronic rhinosinusitis. American Journal of Rhinology & Allergy, 35(6), 761–767. https:// doi.org/10.1177/194589242199365
vi Holm, S. M., Miller, M. D., & Balmes, J. R. (2020). Health effects of wildfire smoke in children and public health tools: A narrative review. Journal of Exposure Science & Environmental Epidemiology, 31(1), 1–20. https://doi.org/10.1038/s41370020-00267-4
7 SOUNDINGS | Spring 2024
Ergonomics In Otolaryngology
Nicholas Purdy, DO, FACS Geisinger Health, Danville, PA
Benjamin VanTasel, MS-IV West Virginia School of Osteopathic Medicine
“How can we provide excellent care to the patient if we cannot maintain our own health and safety”1
Physicians must maintain optimal health to provide the best care for their patients. Occupational injuries have long affected surgeons and contribute to chronic pain and early retirement. Worldwide, up to 85% of surgeons experience musculoskeletal (MSK) pain with neck, shoulder, and back pain being most prevalent2 According to OSHA, work related musculoskeletal disorders (MSD) are among the most frequently reported causes of lost or restricted work time.2
Otolaryngologists are particularly at risk for cervical neck strain due to frequent operation in a complex, narrow surgical field. This article will discuss the risk factors, modifications, and preventative measures related to otolaryngologist intraoperative injury. Otolaryngology is a unique surgical specialty that carries a high rate of ergonomic risk. Up to 79% of otolaryngologists report musculoskeletal pain in at least one region.3 Multiple risk factors have been identified regarding otolaryngologic operations. We spend hundreds of hours per year in the operating room performing procedures that require repetitive fine motor movements in ergonomically unfavorable positions. In particular, lengthy open procedures require the surgeon to assume a flexed neck and spine to obtain an adequate view of pertinent anatomy, resulting in greater strain to the cervical and lumbar spine. The use of headlights and loupe magnification increases flexion of the neck and thus places additional stress on the cervical spine. Increased surgeon height is associated with greater risk of musculoskeletal pain, likely related to increased cervical flexion required for surgical field visualization. These factors
lead to increased risk of surgeon morbidity and may be addressed using preventative and intraoperative mitigation techniques. 4,5,6
Many of the aforementioned risk factors can be reduced with implementation of proper biomechanics in the operating room. The ideal posture is upright with an erect spine, retracted and depressed shoulders, elbows outward, and a tucked-in core and buttocks. While not realistic to maintain this posture throughout the entirety of a procedure, surgeons can develop an awareness of body positioning which will lead to greater time spent in an optimal position. In general, the working surface should be at the level of the physician’s elbows, allowing the angle at the elbow to reside between 90-120 degrees.

(see figure 1) The physician should stand close to the table, minimizing unnecessary active arm elevation. While standing, the feet should be shoulder width apart with slight flexion at the knees. This slight knee flexion unburdens the spine by activating the gluteus and quadriceps muscles. The physician may also intermittently contract the abdominal and gluteal muscles while standing, encouraging offloading of weight and repositioning of the lumbar spine to neutral. Similarly, cervical muscle strain can be mitigated by decreasing time spent with forward head position as studies have shown that for every inch that the head moves forward, ten pounds of weight are added to the cervical spinal muscle load.1








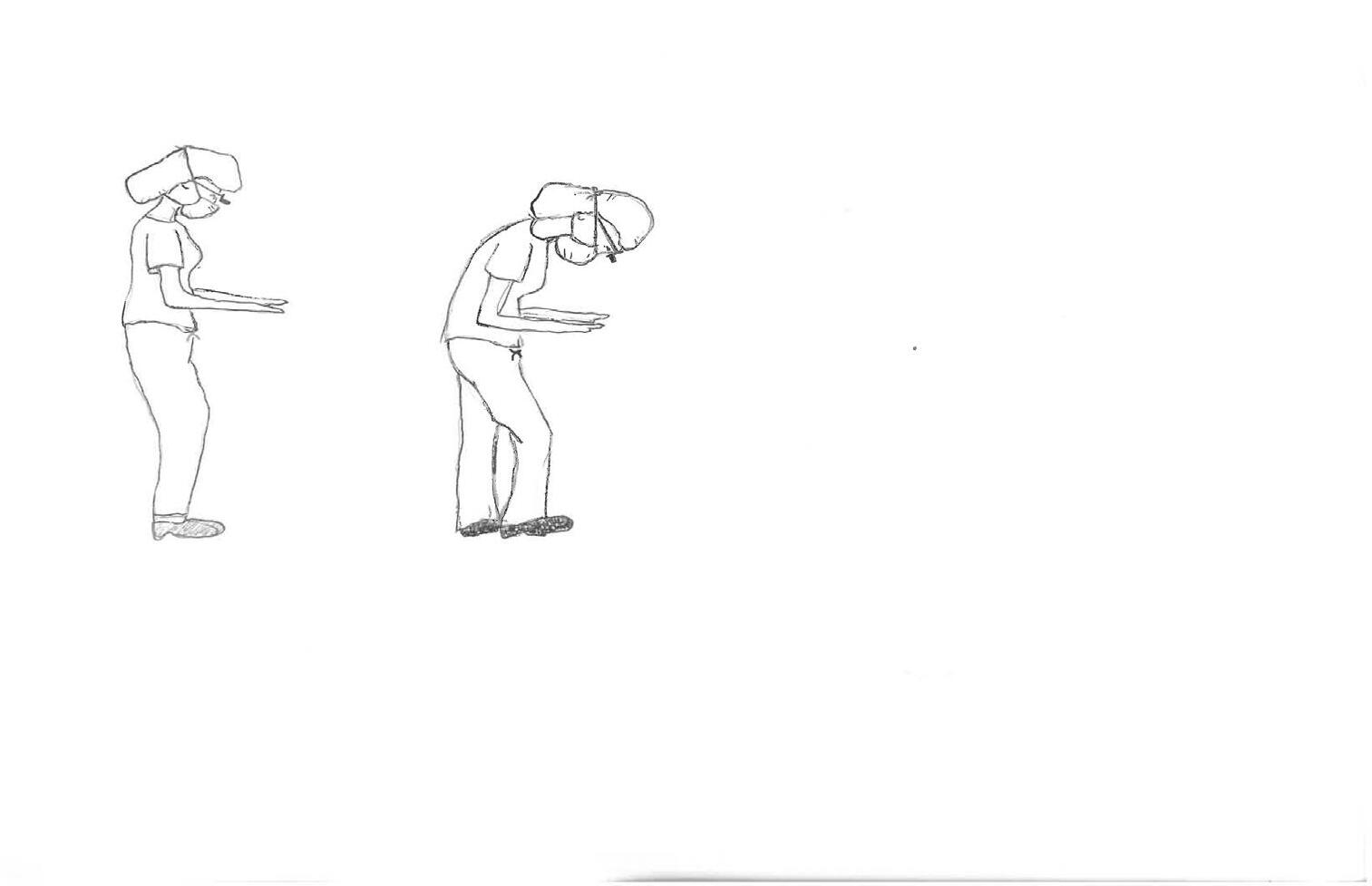

Increased


Acute elbow-arm angle

Rounded low back

Uneven weight distribution
Table too low

A recent article in the cardiothoracic literature by Dairywala, M (et al.) highlights a ‘Surgeon Strength Routine” that surgeons can use to improve their MSK health. It can be performed in 12-15 minutes without equipment and includes elements of stretches, cardiovascular exercise, and strength training. The routine contains 9 different exercises, with detailed instructions. The descriptions are supported by a linked series of brief videos for optimal performance. For example, the first exercise is “Sternocleidomastoid Stretch,” which take 60 seconds to complete, and is illustrated on YouTube. 7
Continued on page 9
8 SOUNDINGS | Spring 2024
Don’t
neck flexion
Do: Arm-elbow angle 90-120 Neutral upright posture Balanced weight distribution Proper table height
Figure 1.
Ergonomics In Otolaryngology
Surgeons often ignore or “push-through” occupational injuries to maintain care for patients and avoid stigmatization. However, the long-term benefit of both prevention and treatment of these workrelated injuries are significant. It has been recognized that intraoperative breaks with stretching exercises significantly reduce pain and improve mental focus in surgeons. (8,9) These ‘microbreaks’ can occur as frequently as every 20–40 minutes or up to every 2 hours. They typically consist of stretching or exercises that target the cervical, thoracic, and lumbar spine as well as the wrists, shoulders, and ankles. Surgeons that include these exercises report improvement in physical performance and reduced pain. (8)
A regular exercise and stretching routine is beneficial in building muscular stability and promoting proper spinal alignment. There is evidence that strength training is preventive of MSK disorders and can improve quality of life for those suffering from MSK disorders. 7 Core stabilization exercises promote alignment and stabilization of the spine. Squatting
exercises promote offloading of upper body weight from the spine and into the pelvic girdle and lower extremities. Stretching the hamstring and hip flexor muscle groups leads to optimal pelvic tilt and decreases anterior and posterior forces on the lumbar spine. Given our long work hours, it can be difficult to find time for strength training. Thus, a recent article in the cardiothoracic literature by Dairywala, MI (et al.) highlights a routine that surgeons can use to improve their MSK health. It can be performed in 1215 minutes without equipment and includes elements of stretches, cardiovascular exercise, and strength training.
The American College of Surgeons’ Surgical Ergonomics Recommendations is a useful resource for surgeons seeking to “address the ergonomic challenges experienced by surgeons and to improve their ergonomic wellbeing”. It contains recommendations on strategies applicable to open, laparoscopic, and robotic surgery. In summary, the first three recommendations focus on improved posture through proper monitor placement, operating table height, and hand and instrument positioning/use. The fourth recommendation focuses on reduction of
The fourth recommendation focuses on reduction of eye strain and MSK fatigue through use of proper lighting and display orientation.
eye strain and MSK fatigue through use of proper lighting and display orientation. The fifth recommendations details strategies to reduce both perioperative and intraoperative stress. We encourage you to read the ACS recommendations and implement them in your practice. 10
Structured educational programs focusing on surgical ergonomics are another valuable method to improve surgeon health. The Duke University general surgery program has laid the groundwork for introducing good ergonomic hygiene by offering resident lead education programs. Their formal program includes loupe fitting education, ergonomic labs, one-on-one observation of the chief residents, and coach training for the chief residents. By focusing on medical students and residents, the goal is to establish healthy ergonomic habits early in their careers.
Conclusion:
Surgeons are at risk for chronic back and other injuries due to operative demands. There are many risk factors such as physician height, length of surgeries, use of accessories like loupes and head lamps, and type of procedure that lead to injury over time. Many of these factors can be mitigated using proper biomechanical positioning, use of operative techniques that require minimal strain to the body, and physical conditioning outside of the operating room. Through increased education and practice of ergonomic principles, we can lessen risks that result in MSK injuries and thus increase surgeons’ quality of life and career longevity, while reducing time off work, and ultimately improve patient care. Continued from page 8

Continued on page 10
9 SOUNDINGS | Spring 2024
Continued from page 9
References:
Ergonomics In Otolaryngology
1. Schlussel AT, Maykel JA. Ergonomics and Musculoskeletal Health of the Surgeon Clin Colon Rectal Surgery. 2019 Nov; 32(6): 424–434
2. www.osha.gov
3. Ryan MT, Montgomery EA, Yang AW, et al. Ergonomics in Otolaryngology: A Systematic Review and Meta-analysis. Laryngoscope, 133:467–475, 2023
4. Walters Z, Chang K, Cervenka B. Ergonomics in Otolaryngologic Surgery: A State of the Art Review. Otolaryngology-Head Neck Surg. 168: 330-338, 2023
5. Stucky CCH, Cromwell KD, Voss RK, et al. Surgeon symptoms, strain, and selections: systematic review and meta-analysis of surgical ergonomics. Ann Med Surg. 2018;27:1-8
6. Vaisbuch Y, Aaron KA, Moore JM, et al. Ergonomic hazards in otolaryngology. Laryngoscope. 2019;129(2):370-376
7. Dairywala M., Gupta S., Salna M. Surgeon Strength: Ergonomics and Strength Training in Cardiothoracic Surgery. Semin Thoracic Surg 34:1220–1229
8. Park AE, Zahiri HR, Hallbeck MS, Augenstein V, Sutton E, Yu D, Lowndes BR, Bingener J. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus. Ann Surg 2017;265(2):340–6
9. Hallbeck MS, Lowndes BR, Bingener J, Abdelrahman AM, Yu D, Bartley A, Park AE. The impact of intraoperative microbreaks with exercises on surgeons: a multicenter cohort study. Appl Ergon 2017;60:334–41
10. Surgical Ergonomics Recommendations Prepared by the American College of Surgeons, Division of Education and Surgical Ergonomics Committee. https://www.facs.org/for-medicalprofessionals/education/programs/surgicalergonomics/recommendations/
11. Ciolac EG, Rodrigues-da-Silva JM: Resistance training as a tool for preventing and treating musculoskeletal disorders. Sports Med 46:1239–1248, 2016
12. Leung K, Segal R, Bernstein J, et al. Surgical ergonomics: Assessment of surgeon posture and impact of training device during otolaryngology procedures. Laryngoscope Investig Otolaryngology. 2022 Oct; 7(5): 1351–1359

Spring Legislative Update
Philip Dunn
PAO HNS Lobbyist
Governor Shapiro unveiled his Fiscal Year 2024-2025 budget in February. This proposal represents an 8.4% increase ($3.7 billion) over the prior fiscal year. The Pennsylvania General Assembly will now grapple with the details with an eye towards final passage by the end of June.
The budget document shows an 8.5% increase in the Department of Health. Of note are three new initiatives:
1. Ten million for the Long-Term Care Transformation Office
2. One million for Firearm Injury Prevention
3. Four million for Medical Debt Relief
The entire budget tracking spreadsheet can be accessed here: 2024-25 PA Budget
Other Issues
Medical Licensure Compact Act
Although Pennsylvania enacted a medical licensure compact act in 2016, it remains to be one of four states that continues to experience delays in implementation due to language concerns with the FBI over background checks. Once this obstacle is removed, final enactment will take place directly.
Septoplasty
In December 2023, Highmark BC/BS issued a Policy Directive that stated that a twelve week course of either an intranasal
antihistamine or intranasal steroid would be required prior to procedure approval.
In February, Highmark issued a Policy Update that reduced the treatment course from twelve weeks to four weeks before approval would be granted. This policy change is to become effective on May 27, 2024.
PAO, with AAO, is working on letters to Highmark, the Pennsylvania Insurance Commission, and the Senate and House Insurance Committees to indicate opposition to this policy change.
Another septoplasty issue thas arisen lately, Highmark’s refusal to reimburse for debridements after sinus surgery. Highmark claims that this procedure is not just part of post-operative care. This issue will be further flushed out with PAO actively expressing concerns with this issue.
10 SOUNDINGS | Spring 2024
Rates of Tympanostomy Tube Placement In Children with Autism Spectrum Disorder
Flora Yan, MD Arnav Shah, BS Glenn Isaacson, MD
Departments of Otolaryngology—Head & Neck Surgery and Pediatrics, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA
Corresponding Author:
Glenn Isaacson, MD
Tympanostomy tube (TT) placement is one of the most commonly performed pediatric surgical procedures and is indicated for patients with prolonged otitis media with effusion (OME) and documented hearing loss or for recurrent acute otitis media.1 Recent clinical practice guidelines place emphasis on targeting children who may be at increased risk for developmental delays or disorders, such as those with autism spectrum disorder (ASD).1 What this emphasis specifically entails is not strictly defined, however may include a lower threshold for tube placement in children with ASD such as in the setting for a unilateral as opposed to bilateral effusion or with a history of OME without documentation of hearing loss. ASD is one of the most common neurodevelopmental disorders with a prevalence to be 1 out of 44 American children.2,3 It is characterized by social and communicative deficits as well as repetitive and idiosyncratic behaviors.2 These children often experience difficulties with sensory processing and require a unique approach by clinicians when delivering medical care.2 It is our clinical impression that rates of TT placement may be higher in children with ASD. Therefore, we sought to examine the rates of TT placement in children with ASD as opposed to those without.4 We conducted a retrospective analysis using data from the National Health Interview Survey (NHIS) from the 2014 calendar year as this had available survey questions regarding both TT placement and a diagnosis of ASD.
The NHIS is a nationally representative household-based survey study administered by the Centers for Disease Control and Prevention.5 Legal guardians answered the survey questions on behalf of their child. In our study, individuals less than 18 years were included. Children with Trisomy 21, unknown TT status, or unknown ASD diagnosis status were excluded. Statistical analysis consisted of using the chi-square test for univariate analysis of categorical variables as well as a multivariable logistic regression analysis when identifying risk factors for ear tube placement. The student’s T-test was used to compare means when applicable.
In total, 11,730 children were included with 239 (2%) of them having a diagnosis of ASD (Table 1). The overall rate of TT placement in children without
ASD was 8.6% compared to 14.2 % in children with ASD (p=0.002). When controlling pertinent clinical and demographic variables, a diagnosis of ASD conferred a 1.52 increased odds of TT placement (adjusted odds ratio 1.52, 95% confidence interval 1.04-2.22). Interestingly, the age of TT placement for children with ASD compared to those without did not significantly differ (p0.857).
Our study uses a nationally representative sample of children within the United States to compare prevalence of TT placement in children with and without ASD. We found that 14.2% of children with ASD underwent a tympanostomy tube placement compared to 8.6% children without ASD, with a diagnosis of ASD incurring a 1.5 times increased
Table 1: Clinical and Demographic Overview of Included Patients
± 4.7 Gender, (n [%])
(51.0)
(49.0)
Autism, (n [%])
Yes
Race, (n [%])
239 (2.0)
(98.0)
(71.5)
(15.6)
Other / Unknown 1,516 (12.9)
Hispanic, (n [%])
Age at Tympanostomy Tube Placement (n [%])
(28.6)
(71.4)
0 -11 months 239 (22.4) 12-23 months
2-3 years
(21.9)
(26.3) 4-5 years
(11.6) ≥ 6 years 68 (6.7) Unknown
12 (1.2)
Emotional/Behavioral Difficulties, (n [%])
Yes 2,038 (19.7)
(79.3)
Unknown 104 (1.0)
on page 12
Continued
11 SOUNDINGS | Spring 2024
Variables Patients
9.8
Male
Female 5,750
Age (mean ± SD)
5980
No 11,491
White 8,384
Black 1,830
No 8,373
Yes 3,357
216
268
118
No 8,186
Rates of Tympanostomy Tube Placement In Children with Autism Spectrum Disorder
Continued from page 11
odds of TT placement. These findings were similar to previous studies in the literature.6,7 In a caregiver survey to only caregivers of children with ASD, conducted in conjunction with the Simons Simplex Collection - an autism research initiative including 12 data collection sites across North America, Ackerman, Reilly, and Bernier reported at least one TT placement in 15.5% of children with ASD.6 Unfortunately, they were not able to compare this rate to a control group of children without ASD.6 Similarly, in a retrospective case-control study using the TRICARE Management Activity Military Health System (MHS) database of United States uniformed services dependents, Adams et al. demonstrated the prevalence of TT placement in children with ASD to be 9.5% compared to those without ASD at 4.5%, with higher rates of otitis media and complications of otitis media in children with ASD.7 Our study differed in that we were able to compare a cohort of children with ASD to a control group (children without ASD). Additionally, we were able to use a nationally representative sample of children to conduct this study.
Why are rates of tympanostomy tube placement in children with ASD nearly twice as high as those without ASD? It may be that there are increased rates of otologic disease in children with ASD. A retrospective case-control study by Niehus and Lord8 examined 99 children, of whom 75 were subsequently diagnosed with ASD and demonstrated a 2-fold increase in the number of diagnosed otitis episodes in children with ASD (3.35 vs 1.57 per child). Interestingly, they found no differences in rates and age of vaccination or number of pediatrician visits between the two cohorts. Given ASD is a neurodevelopmental disorder and, in of itself, does not have any risk factors for predilection for otologic diseases, we hypothesize that there may be an over diagnosis of ear disease in this population of children who are difficult to examine. Identification of OME or acute otitis media dependent on both otoscopy findings of redness of the eardrum.9 Straining
or crying may produce similar findings of vascular engorgement of the ear canal and tympanic membrane vessels that can lead to false findings on otoscopy.10 This can be apparent in children with ASD as they may struggle with the physical otoscopy exam due to aversion to sensory stimuli. Additionally, diagnosis of OME or acute otitis media depend on tympanometry and audiometric evaluation, which can be challenging to conduct in children with ASD.
Lastly, increased rates of TT placement in children with ASD may be due to clinicians’ predilection to have a lower threshold of TT placement given existing behavioral and communication problems, especially with clinical practice guidelines, since 2004, advocating for more aggressive treatment of middle ear effusions and hearing loss in children with developmental delays.1,11 It is important to recognize the limitations to our study. First, the NHIS is a survey that was completed by parents on their children’s behalf and accuracy is dependent on the parents’ ability to recall their children’s medical history. There is an inherent selection bias when evaluating the NHIS data as there may be a select population who is more likely to answer a voluntary survey study. Additionally, we used NHIS from 2014, as this was the most recent data set to include by TT and ASD questions. This data is nearly a decade old and may not accurately represent current clinical practice. Overall, using the National Health Interview Survey, a nationally representative survey of children within the United States, the rates of tympanostomy tube placement were significantly higher in children with ASD compared to those without (14.2% vs 8.6%, p = 0.002) regardless of age at tube placement. Future studies may be directed at elucidating the cause for this increased rate as well as to evaluate the long-term hearing and developmental outcomes for children with ASD after TT placement.
References
1 Rosenfeld, Richard M et al. “Clinical Practice Guideline: Tympanostomy Tubes in Children (Update).” Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery vol. 166,1_suppl (2022): S1-S55. doi:10.1177/01945998211065662
2 Hyman, Susan L et al. “Identification, Evaluation, and Management of Children With Autism Spectrum Disorder.” Pediatrics vol. 145,1 (2020): e20193447. doi:10.1542/peds.2019-3447
3 Maenner MJ, Shaw KA, Bakian AV, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill Summ. 2021;70(11):1-16.
4 Yan, Flora et al. “Tympanostomy Tube Placement in Children with Autism Spectrum Disorder.” The Laryngoscope vol. 133,9 (2023): 2407-2412. doi:10.1002/lary.30494
5 The Child & Adolescent Health Measurement Initiative. National Health Interview Survey - Child. https://www.childhealthdata.org/old(pre-july-2018)/learn/NHIS-Child. Published 2018. Accessed March 3rd, 2022.
6 Ackerman, Sean et al. “Tympanostomy tube placement in children with autism.” Journal of developmental and behavioral pediatrics : JDBP vol. 33,3 (2012): 252-8. doi:10.1097/ DBP.0b013e31824b9f57
7 Adams, Daniel J et al. “Otitis Media and Related Complications Among Children with Autism Spectrum Disorders.” Journal of autism and developmental disorders vol. 46,5 (2016): 163642. doi:10.1007/s10803-015-2689-x
8 Niehus, Rebecca, and Catherine Lord. “Early medical history of children with autism spectrum disorders.” Journal of developmental and behavioral pediatrics : JDBP vol. 27,2 Suppl (2006): S120-7. doi:10.1097/00004703200604002-00010
9 Pichichero, M E. “Acute otitis media: Part I. Improving diagnostic accuracy.” American family physician vol. 61,7 (2000): 2051-6.
10 Isaacson, Glenn. “Acute Otitis Media and the Crying Child.” The Pediatric infectious disease journal vol. 35,12 (2016): e399-e400. doi:10.1097/INF.0000000000001335
11 Rosenfeld, Richard M et al. “Clinical practice guideline: Otitis media with effusion.” Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery vol. 130,5 Suppl (2004): S95-118. doi:10.1016/j. otohns.2004.02.002
12 SOUNDINGS | Spring 2024
Does Obesity Contribute to Increased Operative Time in Otolaryngology?
Chihun Jim Han, MD; Colin Huntley, MD, FACS
Thomas Jefferson University Hospital, Department of Otolaryngology
Obesity rates continue to rise both in the United States and globally. No single country has seen a decrease in obesity rates over the past 33 years1. In the United States, the rate of obesity is estimated to be around 41.9%, and every state now has an obesity rate exceeding 20%2. In the state of Pennsylvania, the rate of obesity is 33.4%2. Given this trend and the prevalence of obesity, many otolaryngologists now routinely care for obese patients within their practices. Furthermore, an increasing number of patients undergoing ENT surgery will be obese in the foreseeable future.
Increased body mass index (BMI) and fatty tissue in obese patients may pose greater challenges and complexity during surgeries3. Anecdotally, these anatomical and technical difficulties translate to overall extended operative length. In this article, we summarize the relevant literature exploring the association between obesity and operative time.
Several studies have previously explored the correlation between obesity and operative time in otolaryngologic surgeries. In 2023, Madsen (et al.) published an analysis of seven-year data from the National Surgical Quality Improvement Program (NSQIP) of 5.6 million patients within nine surgical specialties. Their findings demonstrated that operative time was longer in overweight patients than in regular-weight patients (89 mins vs 83 mins). Specifically, the subgroup analysis of otolaryngology patients (N = 152,783) revealed a similar finding, showing obese patients had a slightly increased operative time compared to the non-obese patients (86 min vs. 80 mins, p <0.0001)4 . While this study established a significant relationship between operative time and obesity using a large sample of otolaryngology patients, whether such trends permeate throughout different
otolaryngology procedures was not further investigated. Examining the procedurespecific correlation is crucial, as this allows otolaryngologists to enhance patient safety, improve surgical outcomes, and provide effective counseling to patients regarding the potential risks and benefits that are unique to each procedure.
Two studies have explored the relationship between obesity and operative time during tracheostomy. Cordes (et al.) prospectively analyzed 151 patients undergoing open tracheostomy in a single tertiary care center from 2006 to 2011. They found that mean procedure duration, defined as the time from skin incision until placement of tracheostomy ties, was significantly longer in obese patients than non-obese patients (24.74 ± 9.41 min vs. 46.17 ± 24.6 min, p <0.0001)5. Barrera et investigated the impact of obesity on surgical outcomes of 387 patients undergoing open tracheostomy from 2012 to 2018 in a single tertiary care center. Subgroup analysis of 163 cases that had documented operative time showed that mean operative time was also significantly longer in obese patients than in non-obese patients (54.9 ± 19.3 vs 39.6 ± 16.0 minutes, P = .024)6. Authors of both studies concluded that the increase in operative time likely reflected the technical difficulties of performing tracheostomy in obese patients. They typically exhibited less palpable landmarks, increased submental and upper thoracic tissue, and restricted neck extension, all of which contribute to the technical challenges during the operation and difficulties in achieving optimal surgical field exposure. Furthermore, obese patients are more likely to have comorbid medical conditions, which can result in intraoperative hemodynamic instabilities and heightened anesthetic risks5,6. These factors further compound the complexity of the case.
Interestingly, the association between obesity and prolonged operative time was not demonstrated in other open-neck surgeries. Goshtasbi (et al.) analyzed surgical outcomes of 597 patients undergoing open transcervical Zenker surgery using the NSQIP database. They
found no significant difference in operative time between the obese and non-obese cohorts (107.3 ±69.8 vs. 96.6 ±66.1, p = 0.109)7. Farag (et al.) performed a retrospective analysis of 469 patients who underwent total thyroidectomy, lobectomy, and completion thyroidectomy with or without neck dissection in a single tertiary institution between 2015 and 2017. All surgeries were performed by a single high-volume surgeon. They found no significant difference in operative time (129.6 vs. 125.7, p = 0.52) and total OR time (189 vs. 180, p =0.41) between the obese and non-obese groups. To account for these findings, the authors of the Farag (et al.) study pointed out that the mean BMI of the study cohorts was 30.5 kg/ m². This finding highlighted that the senior surgeon's familiarity with and experience performing these procedures regularly in obese patients may have contributed to the lack of statistical significance8. Another plausible explanation for the lack of statistical significance could be the inherently longer duration of operative time in thyroid surgeries and open Zenker's procedures compared to tracheostomies. These types of surgeries often involve intricate techniques and meticulous dissections, regardless of the patient's obesity status. Therefore, the relatively longer operative times across all patients might have masked any potential differences specifically related to obesity in this study.
In endoscopic sinus and anterior skull base procedures, varied associations were observed between obesity and operative time. In their analysis of 1996 patients undergoing endoscopic sinonasal surgery, Pai (et al.) found that obesity was associated with prolonged operative time in routine sinus surgeries. However, such association was not found in extended sinus procedures (e.g., CSF leak repair, orbital decompression, transsphenoidal approach to the pituitary tumor). The lower body mass index was associated with Continued on page 14
13 SOUNDINGS | Spring 2024
Does Obesity Contribute to Increased Operative Time in Otolaryngology?
from page 13
prolonged operative time in extended sinus procedures9. On the contrary, Lee (et al.) analyzed the outcomes of 789 undergoing endoscopic pituitary surgery. They showed that obese patients undergoing endoscopic pituitary surgery had significantly longer operative time compared to the non-obese cohort (154.8 ± 79.3 vs. 141.0 ± 73.6, P = .011)10 Anecdotally, large shoulders, short necks, and increased adipose density in the neck and chest in obese patients contribute to technical difficulty during endoscopic sinus procedures. This factor may be amplified in pituitary surgery. During pituitary surgery, the endoscopes and instruments are placed through both nasal cavities by two surgeons standing across the operative table. This situation can make it challenging for both surgeons involved in the procedure to achieve a comfortable and optimal posture, particularly when dealing with a patient of large habitus. In addition, patients undergoing endoscopic nasal surgery typically utilize image guidance hardware. Calibrating such equipment may be more challenging with a larger body habitus, which can contribute to increased operative time during the procedure.
Similarly, conflicting findings regarding the impact of obesity on operative time were observed in otologic surgery. Luryi (et al.) and Gostasbi (et al.) found no significant association between obesity and prolonged operative time in patients undergoing surgery for vestibular schwannomas12. However, Hatch (et al.) reported significant differences in both total operative time (175.4 vs. 158.6; p – 0.0002) and surgical operative time (123.0 vs 113.7, p = 0.03) in obese and non-obese patients undergoing cochlear implant (CI)13. Just as in endoscopic sinonasal surgery, the challenges posed by large shoulders and relatively short necks in obese individuals can escalate technical difficulty, particularly in the context of middle ear and mastoid surgeries
compared to skull base procedures3. This heightened difficulty arises due to the closer proximity of the surgeon's dominant hand to the patient's shoulder, which interferes with precise maneuvers and fine adjustments required for otologic surgery. This correlation is well illustrated in the findings by Lenkeit (et al.), where an increase in body weight was linked to longer operative times only when the patient's shoulder was situated next to the surgeon's dominant hand during the stapes surgeries. In such cases, the operative time increased significantly in overweight patients compared to those with a normal BMI.I (70 minutes vs. 40 minutes, p = 0.019)14
This review highlights the intricate relationship between obesity and operative time across ENT surgeries. As noted by Barrera (et al.), the prolonged operative times in obese patients likely stem from the technical complexities of performing procedures in individuals with larger habitus, particularly for surgeons less familiar with operating on such patients. With the rising prevalence of obesity, further research is needed to explore these associations comprehensively. This will ultimately lead to improved surgical outcomes and enhanced care for obese patients.
References:
1. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 19802013: a systematic analysis for the Global Burden of Disease Study 2013 [published correction appears in Lancet. 2014 Aug 30;384(9945):746]. Lancet. 2014;384(9945):766-781. doi:10.1016/S01406736(14)60460-8
2. Centers for Disease Control and Prevention (CDC). Overweight & Obesity: Adult Obesity Facts https:// www.cdc.gov/obesity/data/adult.html. Accessed March 15, 2024
3. Stevens, Shawn M.; O’Connell, Brendan P.; Meyer, Ted A.. Obesity related complications in surgery. Current Opinion in Otolaryngology & Head and Neck Surgery 23(5):p 341-347, October 2015. | DOI: 10.1097/MOO.0000000000000194
4. Madsen HJ, Gillette RA, Colborn KL, et al. The association between obesity and postoperative outcomes in a broad surgical population: A 7-year American College of Surgeons National Surgical Quality Improvement analysis. Surgery. 2023;173(5):1213-1219. doi:10.1016/j. surg.2023.02.001
5. Cordes SR, Best AR, Hiatt KK. The impact of obesity on adult tracheostomy complication rate. Laryngoscope. 2015;125(1):105-110. doi:10.1002/ lary.24793
6. Barrera SC, Sanford EJ, Ammerman SB, Ferrell JK, Simpson CB, Dominguez LM. Postoperative Complications in Obese Patients After Tracheostomy. OTO Open. 2020;4(3):2473974X20953090. Published 2020 Aug 26. doi:10.1177/2473974X20953090
7. Goshtasbi K, Verma SP. Early Adverse Events Following Transcervical Hypopharyngeal Diverticulum Surgery. Ann Otol Rhinol Laryngol. 2021;130(5):497503. doi:10.1177/0003489420962136
8. Farag M, Ibraheem K, Garstka ME, et al. Thyroid surgery and obesity: Cohort study of surgical outcomes and local specific complications. Am J Surg. 2019;217(1):142-145. doi:10.1016/j. amjsurg.2018.07.038
9. Pai KK, Omiunu A, Vedula S, et al. Impact of Prolonged Operative Time on Complications Following Endoscopic Sinonasal Surgery. Laryngoscope. 2023;133(1):51-58. doi:10.1002/ lary.30057
10. Lee YJ, Wong A, Filimonov A, et al. Impact of Body Mass Index on Perioperative Outcomes of Endoscopic Pituitary Surgery. Am J Rhinol Allergy. 2018;32(5):404-411. doi:10.1177/1945892418787129
11. Goshtasbi K, Abouzari M, Soltanzadeh-Zarandi S, et al. The association of age, body mass index, and frailty with vestibular schwannoma surgical morbidity. Clin Neurol Neurosurg. 2020;197:106192. doi:10.1016/j.clineuro.2020.106192
12. Luryi AL, Babu S, Michaelides EM, Kveton JF, Bojrab DI, Schutt CA. Association Between Body Mass Index and Complications in Acoustic Neuroma Surgery. Otolaryngol Head Neck Surg. 2020;162(4):538-543. doi:10.1177/0194599820906400
13. Hatch JL, Boersma IM, Weir FW, et al. The influence of obesity on operating room time and perioperative complications in cochlear implantation. World J Otorhinolaryngol Head Neck Surg. 2018;3(4):231-234. Published 2018 Jan 19. doi:10.1016/j.wjorl.2017.12.004
14. Lenkeit CP, Fritz CG, Choi JS, et al. Quantifying the effect of shoulder size on operation duration: an analysis of stapes surgery outcomes. J Laryngol Otol. 2024;138(3):258-264. doi:10.1017/ S0022215123000890
14 SOUNDINGS | Spring 2024
Continued

15 SOUNDINGS | Spring 2024

400 Winding Creek Blvd.
Mechanicsburg, PA 17050-1885
BENEFITS OF MEMBERSHIP
Soundings Newsletter
Members receive hard copies of Soundings, the PAO-HNS member newsletter
Legislative Representation
Representation in the state legislature via our own lobbyist
Direct Input with Medicare
Representation on the Novitas Solutions Carrier Advisory Committee (CAC), which has input into local Medicare reimbursement policy
Specialty Events Listings
Members may post their specialty events at no cost
Priority Review for ENT Journals
Priority review for possible publication in ENT Journal, the official journal of the PAO-HNS
National Representation
Representation on the American Academy of Otolaryngology-Head Neck and Neck Surgery's Board of Governors
Discounted Registration forAnnual Science Meeting
Discounted registration to our annual Scientific Meeting featuring CME-approved educational seminars focused on current otolaryngology topics and family-oriented social functions
PRSRT STD US POSTAGE PAID
PA PERMIT NO 922
HARRISBURG










 Karen A. Rizzo, MD, FACS Chair BOG PA Governor/BOG
Karen A. Rizzo, MD, FACS Chair BOG PA Governor/BOG


















