2 minute read
Safe endoscopy starts with the SNAP Endoscope Guide
By Dr Dafydd Loughran, Concentric Health CEO
The MediWales award-winning SNAP, Endoscope Guide was designed in response to COVID-19. With the pandemic meaning only 8% of nasendoscopies were able to be carried out, there was a clear demand for a device that enabled safe endoscopy. Alongside endoscope-i, DTR Medical developed a UKmanufactured device that ensures nasendoscopies can be conducted, providing critical diagnosis of Head and Neck Cancer. Why use the SNAP, Endoscope Guide?
A risk to both ENT Clinicians and Speech & Language Therapists is that whilst carrying out nasendoscopy procedures, the healthcare professional is at significantly


higher levels of contracting airborne risks and diseases. The SNAP, Endoscope Guide aims to provide better safety for both the patient and clinician.
“COVID-19 has disrupted many aspects of our home and professional lives. ENT services across the world have been placed into disarray whilst clinicians debated the true meaning of an Aerosol Generating Procedure (AGPs). Like many clinicians, we were concerned to see the huge negative impact that reducing nasendoscopic examinations had on the early diagnosis of cancer, not to mention many of the other commonly presenting ENT conditions. Being both clinicians and innovators, we were able to pool the skill sets within our company, endoscope-i, to conceive, prototype, design, test and manufacture a safe method of performing nasendoscopy through a surgical mask. Safe endoscopy starts with a ‘SNAP’.”
Ajith George, FRCS Chris Coulson, PhD, FRCS Consultant ENT Surgeons
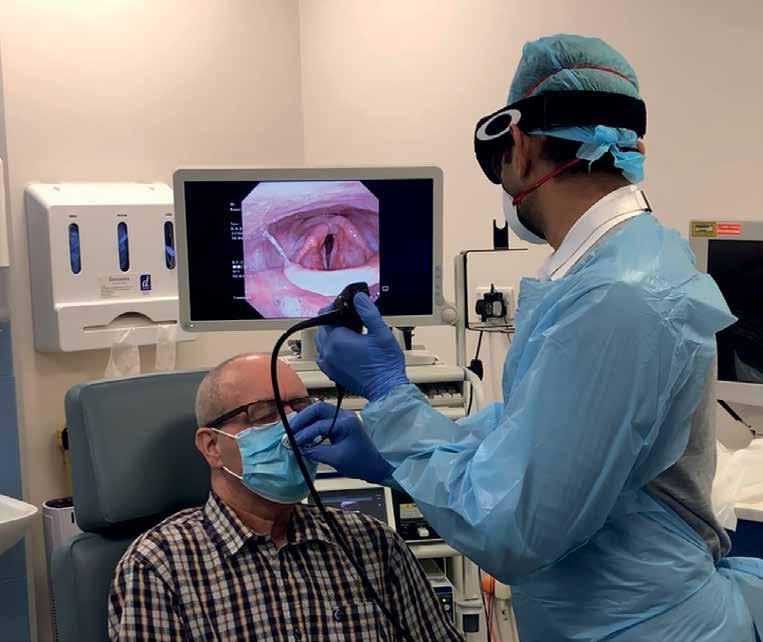
The device ensures a safe passage through a surgical mask, facilitating movement of a scope up to 4mm in diameter, creating an aperture for the endoscope to pass through into the nasal cavity. This means any coughs, splutters or sneezes throughout the procedures are caught in the mask, which is easily doffed after use.
MediWales award-winning
DTR Medical won the ‘Partnership with the NHS’ Award at the 2020 MediWales Innovation Awards. This recognised the company’s collaboration and partnership with Consultant ENT Surgeons Chris Coulson, PhD, FRCS, and Ajith George, FRCS, in the development of this essential product and helping to bring it to market. DTR also ensured a free box was offered to every UK ENT Clinic in the UK, with the opportunity for those who have not yet received one to do so.
In addition to the award, DTR also hosted a webinar alongside MediWales, focused on the importance of continuing nasendoscopy procedures during the coronavirus pandemic.
ENT UK Guidelines
ENT UK recently released their latest guidelines for potential Aerosol-Generated Procedures (AGPs) within the ENT Clinic, with a focus on advice for ENT Surgeons conducting upper-airway endoscopy. The guidelines suggest that patients are advised to wear a mask during nasendoscopy procedures, which is a problem solved using the SNAP. Our device maintains the Medical Device Regulation of the mask and is currently the only regulated and standardised method to do so, recently receiving UK Patent Application Number 2105626.2.
Want to try the SNAP in your Clinic?
The device has been implemented throughout the UK and Ireland from which we are seeing huge success stories where the device is becoming standard practice.
If you are interested in the SNAP and would like to find out more, please contact dtrmarketing@innoviamedical.com








