




With regulators choosing not to take ‘visible’ action when they discover illegal activity, choosing not to share information with other relevant regulators and not training dentists to understand their legal obligations - is this acceptable to dental patients and legal manufacturers? This long read will attempt to explain what has happened so far and consider why?
This issue: Special feature on “Protecting illegal dentistry and manufacture”. See pages 25-32
l Welcome to your February Edition of The Dental Technician Magazine. Last month we had some fantastic articles and this month we have pulled some more great pieces in, with the introduction to some new writers and our first editorial article from Daniel Shaw, our latest editorial board member.
In this months edition we have included a feature on ‘Illegal Manufacturing’ and the current stance of our regulators. This is a very important article; hence it is a very lengthy read. The article has been put together over several years of investigative journalism by many colleagues including the late and great Larry Browne (previous editor of The Dental Technician magazine). The aim of the article is to give readers enough background information to make their own opinions on the current stance our regulators have on illegal manufacturing in Dentistry. It does leave us still asking lots of questions, questions that the regulators have either refused to answer or have only partially answered in previous correspondence. I would like to run a follow up to this article by asking readers to write to me with their thoughts on the article. I look forward to hearing from you.
I am very positive about the year ahead of us, this sentiment is echoed by Ashley Byrne one of our regular authors from the Editorial Board. He suggests that despite the global ‘doom and gloom’, the dental industry and particularly dental laboratories could have their best year yet. It’s a really positive and inspiring read, enjoy it.

I would like to wish you well for February, I do hope January was a great start to the year for you all. The lighter nights are coming, spring will be upon us before we know it. Have a great month and see you all again in March.
Matt Everatt I Editor











lThere is one thing I want to wish you for this New Year: to accomplish everything you do, driven by true passion. The same mad passion which you feel under your skin every time you do something you deeply enjoy and pushes you to get up every day early in the morning, despite all difficulties, challenges and failures. We all have goals, but only with true commitment we will achieve them. Those who succeeded have tried hard, and even if they have failed thousands of times, they still got back up without looking for excuses.
From architecture to engineering, from gastronomy to cinema, behind the most incredible innovations there are common people driven by great determination who, with patience and enthusiasm, had the courage to dare and look beyond




time. The love for their art created the inventions and masterpieces we can still appreciate today. No matter how far or complex our goal may be, the important thing is that it’s clearly visible in front of our eyes, inspiring our daily actions and choices. Inspiration is not the prerogative of artists but a strong will to pursue one’s job with passion and imagination, aspiring every day to new accomplishments.
As the greats of our history made it, you will make it too. Always keep your passion for your art alive, and your determination will reward you.
Go, and get your goals accomplished!














A VALUABLE COMMUNICATION TOOL BETWEEN DENTIST, DENTAL TECHNICIAN AND PATIENT




A young patient requested an aesthetic restoration for his anterior teeth (13 – 23). In order to ensure the best possible care, a mock-up was realised:













- Digital impression of the patient’s oral situation with the Detection Eye intraoral scanner and model creation in the Zirkonzahn.Modifier software; printing of 3D models with the P4000 Printer





- Design of the mock-up for the upper jaw in Zirkonzahn.Modifier and subsequent milling


- Verifi cation of the fi nal mock-up in the PS1 articulator: plaster-free articulation of the models by means of the JawAligner system
AVAILABLE WITH Ø 95 mm e 98 mm MEET US! DIGITAL & IMPLANT DENTISTRY SHOW, 25/02/2023, STAND A4 IDS, 14 – 18/03/2023, STAND D-020, HALL 01.2















The countdown is officially on for Dental Technology Showcase (DTS) 2023 which returns to the NEC Birmingham on 12-13 May 2023. Registration is completely free for dental labs and is now live on the website at the-dts.co.uk

SECURE YOUR PLACE TODAY TO MAKE SURE YOU DON’T MISS:
• 40+ hours of content, dedicated to the dental lab community
• 50+ highly respected and renowned speakers from around the globe
• 80+ exhibitors, once again tailored for dental technicians and lab owners
• 1,500+ visitors to connect and network with
A wide range of topics will be covered in the 3 theatres at the show, offering a wealth of technical, personal and business advice. Delegates have the opportunity to get involved with industry conversations, share their own experiences and gain up to 12 hours of enhanced CPD at the same time.
Registration is quick and easy – simply complete the short form on the website and your confirmation will be emailed to you.

The The Peer Assessed Rating (PAR) index is the most widely accepted system for analysing a patient’s orthodontic movement and how much they have progressed.
The index is split into the following five categories:
1. upper and lower anterior segments
2. left and right buccal occlusion
3. overjet
4. overbite/open bite

5. centreline
Within each of these categories, the person scoring must attribute scores to various aspects of the patient’s dentition. Ideal positioning and alignment would result in a score of zero; although this is almost never achieved. A score of 2 is often the best possible outcome. Crowding, spacing, poor interdigitation of upper and lower teeth, overjet, crossbite, overbite, open bite, and misaligned centrelines could all result in a higher score. Some of
these areas’ scores are weighted, meaning they have a far greater effect on the end result. For example overjet and anterior crossbites are weighted by a factor of 6, meaning if a patient scores 2 in the overjet section then 12 points are added to their final weighted score.
Orthodontists and dentists are required to submit 20 cases plus 10% of their remaining caseload per year for PAR scoring. This job is often either assigned to a qualified nurse that has undertaken the PAR scoring course conducted by Professor Stephen Richmond or sent to a dental lab where a technician who has done the same course will PAR score the model sets.
As I am sure the reader of this article has experienced before, dental labs are a fast paced environment in which many appliances are often being shipped each day to be fitted in the practice the following day. PAR scoring on the other hand will never have a fit date; it has a deadline set by the NHS. I have come
to the conclusion that this is the reason PAR scoring is often neglected, especially in larger labs in which hundreds of appliances are being manufactured and shipped each day.
Having worked in two of the largest labs in the country, I understand how easy it is for the lab to fall slightly behind, how work then gets prioritised and why PAR scoring is rarely at the top of anyone's priorities as a result. However I do empathise with the orthodontists and dentists that send batches of models to be scored with plenty of time only to then have to call the lab a day before the deadline set and enforced by the NHS.
I started my company, ExpressPARScore, specifically to solve this problem in our dental industry. So, if you have PAR scoring orders that are a burden to your lab get in touch.
E: gabriel@expressPARScore.co.uk T: 07732 333 715

High detailed 3D printed models with Cosmos Dental Model. Available in plaster colour to guarantee high aesthetic results, Cosmos Dental Model is indicated to print prosthodontics dental models allowing high accuracy work



Universal Resin for Dental Model,Beige,DLP,1L

Con dence on implant & prosthetics checking. Cosmos Gingiva mask resin is speci c to print arti cial gingiva models due to the proper colour and exible aspect, which can be combined with model material.
- 500mL bottle


Universal Resin for Gingiva mask,DLP,500mL
Easiness in casting process. Casting material for cropings, crowns, bridges and general frameworks. This 3D printing resin can go directly to the foundry. Easy to use due to the formulationdesigned to leave no residue after casting.
For more information, contact your local territory manager www.straumanngroup-uk.co/team-directory

Sometimes it’s hard to be positive when our days are filled with nothing but negative news. Rising costs, recession looming, and wars continuing to be fought around the world. Whilst influencing our lives in various ways, they are all outside of our circle of control.
So, what about the dental technician and the dental market? We see a lot of doom and gloom here with NHS practices on the downturn, and stories of labs that feed that sector having a tough time. However, in all crises, it’s essential that we do all we can to innovate and adapt to an ever-changing market.


I know there are some who will see me as preaching positivity from my ivory tower at Byrne’s but before you judge me, let me be straight that I’ve had my own challenges over the year. 2022 was a difficult one (people, process, software, time, you name it), topped off by a closing final quarter of really poor sales. Trust me, I’m speaking from experience of tough times this past year.
2023 however, is set to be quite the year of opportunity - in my opinion. I believe it is time for us, as Dental Technicians and labs, to really stand tall and show what we are capable of as an industry. For decades we have been subservient to the broader dental industry, and yet we are vital to the success of modern dental treatment. As lab numbers and technicians continue to decline, the demand for our services increases annually. Global dental technology sales are estimated to be a record high of over £33 Billion in 2024, and many marketing papers quote sales growths of between 4-6% per annum in the UK alone. With a market growth beating nearly every other aspect of manufacturing and being well above most economic growth percentages, dental technology has an awful lot to be positive about.
When any industry identifies as an opportunity for serious money to be made, large groups and corporates start to raise huge funds to invest. We are seeing exactly this happening with the growth of large chains of labs, in parallel with clinical corporate groups acquiring their own labs to supply their practices. We do not need to fear the large groups because there is more than enough work for all of us, providing we adapt and move with the modern market.
Digitisation is key to this growth and if your lab is not offering any digital services, you will soon be left behind. In my lab, 100% of my crown and bridge and implant work has at least one digital stage. In prosthetics, all my trays, splints, guides, temporary dentures, and some definitive dentures, are made digitally, 3D-printed or milled, and they are looking as good as hand-made. This isn’t ‘de-skilling’ anyone, quite the opposite. This is up-skilling, and the smiles and buzz generated amongst the team from learning and challenging these new methods, are testament to the power of digital.
Intra oral scanning (IOS) has really scaled up post-pandemic and in our lab, 70% of all impressions are now IOS and that is rising every month. Needless to say, labs with no digital offering are potentially missing out on work. 2023 is the year where we all really need to help each other in the growth and implementation of digital workflow. Dentists are leaning on technicians more and more for material knowledge, IOS knowledge, and the whole digital workflow. Some recent evidence showed that dentists consider dental technicians to be the most trusted of all dental professionals and suppliers, which
puts us in a very strong position. Technicians are honest and all decisions we make are ultimately in the patients’ interest, so this report came as no surprise to me. Providing we keep up to date on materials and we share what works and what doesn’t with other labs, we can be a force to be reckoned with as a knowledge base in the dental industry.
As patients’ demands continue to rise, the pressure on the clinician and clinical teams is increasing. The majority of patient’s have little interest in the type of implant used, or the method of bonding the teeth, but they do really care about the false teeth we manufacture for them. As digital enables expanding treatment options, it is our responsibility to ensure the clinical team is aware of this and advise accordingly. Dentists and the clinical team have never been busier so keeping up with what we do is near impossible. We are also seeing a decline in chairside restorative work like milling, as clinicians simply do not have the time or resources to finish to the standard that we can.
There is an enormous amount to be positive about in 2023, and I genuinely feel there has never been a better time than this exact moment to be a dental technician. We have growth opportunity, our industry is talking to each other and helping each other to grow. We have finally earned the respect we deserve for our extensive knowledge of restorative dentistry, and this will only grow as we become more digital. It’s shaping up to be quite the year as we Dental Technicians take centre stage. I don’t think I’ve ever been this excited to be a technician and lab owner, as I am right now. Let’s rock 2023 #dentaltechniciansarerockstars


My name is Alexander Brittain, I am a 32 year specialist Orthodontic Technician with a lengthy career in the dental field. Starting out as a dental nurse and covering most areas of GDP I found the need to find something better suited to my abilities, this led me to an introduction with Daniel Shaw at Chesterfield Royal hospital, which opened my eyes to the interesting world of orthodontic technicians. From that moment I knew this was what I wanted to do with my career. After training and a maternity cover job with Daniel Shaw, I joined the fantastic team at UHDB where their department opened my eyes into the world of 3D. This has led me to my current post with ULHT at the Lincoln county hospital, where I have since been working and pursuing bringing our lab into the exciting world of 3D.
Throughout my career I have found several things that I cannot work without and here are my 5:
By far the most important point for me is the team I work with I have been very fortunate since starting out as a technician to have always worked within a fantastic team. This has so many benefits: from helping each other when snowed under and teaching each other tips and tricks. But this goes much further into being able to enjoy your time at work. Being able to have a laugh and joke with colleagues definitely helps your overall mental health and allows you to be a better team.
My second point is my Ash 5 This tool has proven itself to be one of my most valuable assets in the lab. From waxing out, blocking out and carving, it really has been one of my most used tools. But for whatever reason it’s always the instrument I can never find in my drawer when I am looking for it, usually when I’ve
only got one hand free and I’m trying to wax something in place. But that’s on me for not getting it out before I need it.
3. My third point is my mug
This may sound like a not so valuable item, but for me it really is. Having a good cup of coffee can really sort out most problems you may come across, whether work related or not, having a coffee with colleagues usually sets everything straight. This could be an appliance that’s causing a headache or something you have been working on so long you are going cross eyed. Having that 5 minutes away from it can really make all the difference.
4. My fourth point is finding a good hand cream

This may seem unnecessary for most people, but when your hands spend half the day covered in plaster, in and out of hot and cold water frequently and in gloves, my hands get so dry and cracked. This is not ideal when we rely on our hands so much. So for me finding that hand cream that works the best and doesn’t leave them greasy has made my hands so much more comfortable throughout the day. My current go to is the hemp hand cream.
5. My final point is technically more than one item, but having such a broad selection of burrs
As a student I bought a second hand box of tools from a technician on eBay, and the amount of random burrs have really been a God-send throughout my career. Through trial and error I have tried them all, some fantastic and some completely pointless. But through that I have a really good arsenal of burrs for every situation. This has certainly made my trimming and polishing life so much easier.


Edent-Upcera is the UK's official sole distributor for Upcera’s products and technology. Headed up by Richard Breach, a Master Ceramist for over 30 years. His roles as UK Technical Logistics Manager is supported by Jason Blackman, UK Clinical Sales Manager.



COME AND SEE US AT: Digital & Implant Dentistry Show 2023 Novotel Hotel London West, Hammersmith, London I Saturday 25th February 2023 I 9:00-16:00 I Booth: D9
The North Of England Dentistry Show Manchester Central Convention Complex I Saturday 11th March 2023 I 09:00 -16:00 Booth: TBC

company.
n Edent-Upcera offer solutions to labs and technicians who are either looking to outsource digital cases, or are new to digital dentistry as well as supplying established labs with a wide range of first class products from scanners to zirconia and metal milling machines, zirconia and PMMA milling discs.


If you are new to Digital work flows and would like to discuss how to start production in your company please talk to us as we have many different solutions all taylor-made to suit your business profile and meet your client’s needs.
Our aim is to get more UK Dental labs offering digital dental solutions for the dental customers in the hope to reduce the amount of profitable cases being outsourced overseas.
We offer outstanding performance and affordability backed up by impeccable customer service and after sales care.


Here at Edent-Upcera we can also offer free training and installation on all digital products, we have a purpose built demonstration centre designed to show technicians how to get their laboratories using the latest digital technology EdentUpcera has to offer.
Edent-Upcera is leading the way for digital solutions for laboratories and surgeries alike.

lDental Technology Showcase (DTS) is returning to NEC Birmingham and is set to be better than ever before!


It has never been more important for the industry to come together, not just to celebrate its success and resilience, but to problem-solve the common challenges.
As a key part of the dental team, technicians are at the forefront of change, with more digital technology being used to elevate outcomes and make workflows more efficient.
DTS has always been for everyone, whatever your role. Being co-located with the British Dental Conference & Dentistry Show means there is a unique opportunity to see what dentists are doing, and the issues that are concerning them too.

With a trade exhibition featuring renowned brands and pioneering new startups, don’t miss your chance to attend.
Register your interest today.

lA great dental laboratory also needs to be a well-run business that people want to work for and with.
If business skills aren’t your area of expertise, Dental Technology Showcase (DTS) 2023 can help you create a more efficient, profitable and happy workplace.
You will learn about issues as diverse as recruitment and retention, the latest equipment that can shorten turnaround times and improve results, and how to
protect the mental wellbeing of your team.
As always, there will be a busy trade exhibition too, where you can talk with suppliers and manufacturers and maybe get a great deal or two. Life in a lab is so busy, and rarely is there this opportunity to connect with so many people who share the same challenges, in one place.
Register your interest today and save the date for DTS 2023, to grow a thriving business this year and beyond.
DTS 2023 will be held on Friday 12 and Saturday 13 May, NEC Birmingham, co-located with the British Dental Conference & Dentistry Show. For more information, visit the-dts.co.uk or email dts@closerstillmedia.com










According to the World Health Organization (WHO), 1 in 6 people worldwide will be aged 60 years and over by 2030. An aging population means that, for many of us, we can spend more time with loved ones, but it also means that the healthcare sector is facing increased pressures to meet demands and improve patient care. This is especially true within dentistry, as a patient’s dental needs could become more complex as they age.
Nowadays, there is more variety of treatment options for patients who are suffering from tooth loss as a result of aging, from removeable to fixed solutions. As a busy technician, you will likely be fabricating prostheses for your clients’ older patients, many of whom may have specific needs and requirements that mean you must tailor your approach to each case. It is important, both for your clients and your business, to employ high-quality materials so your clients’ patients can enjoy a beautiful and functional dentition.
Tooth loss can be a devastating occurrence for any age group, but for the older generations the attitude seems to be that it is just a part of ‘getting older’. As you know, edentulism not only damages self-confidence, but can actually put the individual at risk of further health issues. For instance, evidence suggests that edentulous patients may lack certain nutrients and this puts them at risk of various health disorders, with one study finding that the participants with a higher number of teeth were better nourished when compared to those who had less. Oral health in older individuals may also begin to decline due to a range of interlinking factors. A study of 353 adults, with a mean age of 74.9, noted a higher prevalence and severity of root caries. This appears to be an issue for older adults worldwide.
There are many reasons as to why a number of older patients have poorer oral health. Researchers have suggested that age-related salivary changes, a poor diet and gingival recession are the main culprits. The occurrence of xerostomia, as a result of polypharmacy, is also thought to affect oral health in older individuals. Oral health in the UK has certainly improved in recent years, and many older adults are retaining their natural dentition for longer. However, it is thought that 6% of British adults have no natural teeth, which means that many treatment options have been designed and improved to restore aesthetics and function.
Dental technicians are able to satisfy far more demands than ever before, thanks to the impressive strides taken in enhancing dental tools, materials and modalities. Dental implants are growing as a popular restorative choice, providing enhanced stability, aesthetics and function. Research has supported the efficacy of dental implants, demonstrating that older adults can enjoy good oral health with this treatment option.
Dentures have not always had the best reputation. Parables have circulated about how these solutions change the appearance of the smile and how they make mastication and speaking challenging. As you well know, a well-made denture will not only be comfortable, but will look discreet and natural. For many patients, the flexibility afforded by dentures still make them a popular modality. They are also considered to be easier to maintain and are more economical than dental implants. As such, you will likely still see many orders for dentures in your day-today work.
Regardless of whether you have been depending on the same product for years, or are seeking something new, it is always worth reassessing what you use in your lab to improve the work you create.
When it comes to denture acrylics, you need a material that will provide you with durability and strength, as well as one that is easy to handle. Kemdent is delighted to offer dental technicians their Acron Hi – High Impact Denture Base

Acrylic, a remarkable acrylic ideal for high-quality, long-lasting dentures. This solution produces a malleable and easily packable ‘dough’, a particular advantage if you are using an injection moulding protocol. It is easy to trim and, thanks to its unique Multi-Matrix Technology, your dentures will be resistant to fractures and breakages. Give your clients and their patients prostheses that last, and consider adding the Kemdent Acron Hi – High Impact Denture Base Acrylic into your dental repertoire.

As the population continues to age, every sphere within healthcare will need to strategise on the most ideal approaches to support older adults and their varying requirements. Every patient deserves to enjoy the pleasures of a functioning dentition that looks and feels natural. Producing work that is of an optimal quality will not only satisfy your clients, but will restore the confidence and manage the oral needs of the nation’s golden agers too.
For more information about the leading solutions available from Kemdent, please visit www.kemdent.co.uk or call 01793 770 256

i World Health Organisation (2021). Ageing and Health [online] World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/ ageing-and-health [Accessed 9 Sep. 2022].
ii Hutton, B., Feine, J. and Morais, J. (2002). Is there an association between edentulism and nutritional state? Journal (Canadian Dental Association), [online] 68(3), pp.182–187. Available at: https:// pubmed.ncbi.nlm.nih.gov/11911815/#:~:text=The%20 evidence%20suggests%20that%20edentulous [Accessed 9 Sep. 2022].
iii Toniazzo, M.P., Amorim, P. de S., Muniz, F.W.M.G. and Weidlich, P. (2018). Relationship of nutritional status and oral health in elderly: Systematic review with meta-analysis. Clinical Nutrition, [online] 37(3), pp.824–830. Available at: https://www.sciencedirect. com/science/article/pii/S026156141730105X [Accessed 9 Sep. 2022].
iv Zhang, J., Leung, K.C.M., Chu, C.H. and Lo, E.C.M. (2019). Risk indicators for root caries in older adults using long-term social care facilities in Hong Kong. Community Dentistry and Oral Epidemiology, [online] 48(1), pp.14–20. Available at: https://onlinelibrary.wiley. com/doi/abs/10.1111/cdoe.12495 [Accessed 9 Sep. 2022].
v Chan, A.K.Y., Tamrakar, M., Jiang, C.M., Lo, E.C.M., Leung, K.C.M. and Chu, C.H. (2021b). A Systematic Review on Caries Status of Older Adults. International Journal of Environmental Research and Public Health, [online] 18(20), p.10662. Available at: https://www. mdpi.com/1660-4601/18/20/10662/htm [Accessed 9 Sep. 2022].
vi Gil-Montoya, J., Ferreira de Mello, A.L., Barrios, R., Gonzalez-Moles, M.A. and Bravo, M. (2015). Oral health in the elderly patient and its impact on general well-being: a nonsystematic review.
Clinical Interventions in Aging , [online] 10, p.461. Available at: https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC4334280/ [Accessed 12 Sep. 2022].
viiThomson, W.M., Ferguson, C.A., Janssens, B.E., Kerse, N.M., Ting, G.S. and Smith, M.B. (2020). Xerostomia and polypharmacy among dependent older New Zealanders: a national survey. Age and Ageing, [online] 50(1), pp.248–251. Available at: https://academic.oup.com/ageing/ article/50/1/248/5874843 [Accessed 12 Sep. 2022]. viiiDentaly.org. (n.d.). UK Dental Facts, Figures and Statistics for Kids and Adults . [online] Available at: https://www.dentaly.org/en/dental-facts-statistics/ [Accessed 9 Sep. 2022].
ixBecker, W., Hujoel, P., Becker, B.E. and Wohrle, P. (2015). Dental Implants in an Aged Population: Evaluation of Periodontal Health, Bone Loss, Implant Survival, and Quality of Life. Clinical Implant Dentistry and Related Research , [online] 18(3), pp.473–479. Available at: https:// onlinelibrary.wiley.com/doi/abs/10.1111/cid.12340 [Accessed 9 Sep. 2022].

 Interview by Matt Everatt
Interview by Matt Everatt
Hi Gareth, thanks for joining me today, it's great to see you are enjoying your new role heading the digital team at DMG. You have always been a great contact for our laboratory over the years and I am certain you are doing a fantastic job in bringing labs and clinics together, leveraging your extensive network and DMG's digital solutions.
In a few sentences, give me a whistlestop tour of your role at DMG?
Gareth Grimes is Key Account and Digital Team Manager at DMG Dental UK. He has worked in the dental industry for more than 15 years, building his network of customers across the UK through the prestigious companies he worked at, including Boutique Whitening, Astek Innovations, and DBG Dental. During the 15 years, he has gained a deep understanding the importance of the running of dental practices and dental labs, which has helped him to build collaborations that are beneficial for all parties. Gareth feels strongly about providing a high level of customer service and support, and prides himself on working with products that are the best in their field.
I had previously done some collaborations with DMG so I was fully aware of the great products they manufacture and had heard about their digital solutions, which were interesting to me. I joined in August last year and my main responsibility was to look after the key accounts within the dental industry but when I looked into the digital side of things, I was hooked. I then inherited the position of Digital Team Manager as well.
DMG seem to have got the right person, I have had first-hand experience of you in your previous role where you have been a conduit between lab and clinic is that something you and DMG are hoping to achieve?
Yes, it is. I have always had the opinion that the key to a successful partnership between dental labs and dental practices is making sure everything runs smoothly and is kept as simple as possible. I believe that the best approach to achieve this is through strong communication and relationships, which is something I have always worked to promote to my customers in previous roles. One of the things that attracted me to DMG was the ethos of relationship building they had taken throughout the design of the digital workflow and software DentaMile. The system promotes networking opportunities for DentaMile users, which is a really exciting benefit for dental labs.
DentaMile software sounds great, I have had a brief look on the DMG website and it seems like there is something for most labs, from the smallest 1-person lab to larger corporates. Can you explain a bit more about it?
DentaMile is our fully encrypted, innovative cloud-based software with a range of user licenses to suit different needs, enabling the dental practice to design the appliance themselves for the lab to print, or just send the scan file for design and print to be carried out by the lab. This means there is the perfect solution for everyone depending on how involved they want to be in the process. From the lab side, this gives great networking opportunities as they can offer licenses to their dental partners and grow their business through printing.
The workflows within DentaMile have been created to make the entire design, fabrication, and processing procedure easier, slicker, and faster - giving users confidence that the appliance will be created without any hold backs.
That sounds great and very innovative, is the software exclusive to DMG?
Yes it is, it has been created in-house. We have a dedicated team in Hamburg at DMG Headquarters who are constantly implementing new ideas to help users get the best from
it. What I love most is that they are open to feedback and really take on comments from our current users in order to improve the software and make it as efficient, practical, and beneficial as possible.
How does it differ to other options?
The DentaMile software is the key differentiator in our workflow. It is fully encrypted making it secure and compliant, and it is also cloud-based so everything can be easily accessed by authorised users wherever you are –allowing design to be carried out away from the lab if required, which is great for home working.
It is open to all intraoral scanning devices and it is fun to use, almost child’s play so to speak – which is great when is comes to dental practices who want to design appliances and then delegate the printing to the lab. Alternatively, it can work the other way around, the dental practice can send the STL file securely over to the lab for design, then it can be sent back and printed chair side for same day dentistry. This is what many patients now want and I think it is where we are heading. DentaMile can help keep labs in the loop with this.
What sort of devices can be designed using the software?
We have smooth and efficient workflows for the production of splints and bleaching trays but with the use of other software alongside it - such as Netfabb - models, implant guides, special trays, gingival masks and casts can also be designed and printed. Over the next 12 months there will be


some big introductions coming, but I can’t say much more on that for now… watch this space!
As you can imagine with my background, I was sold on the bleaching tray workflow. The quality of the print and the softness and clarity of the resin is pretty impressive and the costs associated make it a no brainer!
Are there any automation aspects that speed up the design process?
Our system is not automated but it only requires a very short learning curve, everything else is prompted and fully explained. All the parameters are pre-programmed with our Luxaprint resins and the whole workflow carries RFID-detection of material.
We provide excellent training and ongoing support free of charge, which is carried out by our in-house Technical Support Manager, who is an experienced ex-dental technician.
I guess DMG offer materials and printers to support DentaMile?
We do have our own range of printers, with different capabilities depending on volume but also an entire system including coordinated post-processing. Along with our Luxaprint resins and DentaMile software, an efficient validated workflow is now possible for digital production, providing same day dentistry.
Is there a lab to lab option? Say for cases where a lab may sub out their implant work but do the rest in house?

Of course, the whole idea behind DentaMile is to create a network of printing facilities to support the
whole dental population, obviously there is an option to lock users into labs and keep them hidden from the DentaMile network.
Tell me a little about the start-up costs, do labs need powerful PCs/Macs, is there a need for fast broadband? Because DentaMile is cloud-based it just requires a good Wifi connection or LAN and Google Chrome. Costs are super competitive, the full production license is £1135 + VAT a year and to simply give your customer a license allowing them to securely send cases would be £68 + VAT, which is kept at a low cost to enable labs to add a margin on for their own profit.
So, if I wanted to start up today, what do I need to do and would you help labs seek customers wanting these lab services? Just contact the Cheshire-based head office on 01656 789 401 or email me on gareth@dmg-dental.co.uk so that we can have a conversation about your needs and how our digital solutions can help.
We already have a number of labs using our complete workflow and part of that agreement is to help merge current and new customers on to the DentaMile platform and provide the training. This is something we feel strongly about because we want to make the whole process fast and simple.
Please contact Gareth on 01656 789 401 or gareth@dmg-dental.co.uk to arrange a demo.
















With regulators choosing not to take ‘visible’ action when they discover illegal activity, choosing not to share information with other relevant regulators and not training dentists to understand their legal obligations - is this acceptable to dental patients and legal manufacturers? This long read will attempt to explain what has happened so far and consider why?

The MHRA regulates dental device manufacturers and importers. The GDC regulate ‘some’ people who make dental devices if they are GDC registrants and ‘all’ people who fit dental devices to patients, predominantly, but not exclusively dentists. The GDC have a duty to ensure their registrants are complying with the law. The CQC also regulate those who fit dental devices to patients. The GDC and CQC have a role in inspecting dental teaching hospitals.
The Professional Standards Authority (PSA) audit the GDC and CQC (amongst others) but not the MHRA. However, the PSA can only advise (the GDC council has primacy). If the GDC council has decided that GDC staff should take no action if they discover a dentist has fitted illegally manufactured devices to dental patients, the PSA cannot ‘compel’ the GDC to change policy. The PSA can (and does) point out what it sees as breaches of its guidance for good regulation. Something it has not done so far for this issue since the GDC overrode its original attempt to do so.
The Dental Technologists Association (DTA) represents some dental technicians who are GDC registrants and is a GDC stakeholder. The Dental Laboratories Association (DLA) is an organisation that represents some dental device manufacturers. They may be considered to be an MHRA stakeholder.
When questioning the GDC and MHRA and talking with the PSA, The DT gave two examples of illegal activity to both organisations.
A Basic example was of a manufacturer who has had no training and is not registered with the MHRA who makes a simple orthodontic retainer. They leave the plastic edges sharp. When it is fitted to a child the sharp edges cut the child’s gums and they bleed. The manufacturer has received no training; the manufacturer is not registered with the MHRA. No statement of manufacture was made. The DT believes that legally this ‘may’ constitute assault.
A Complex example was of an importer who is not registered with the MHRA but is importing devices with counterfeit dental implants. A replica of a genuine implant, machined to look like the real thing. They do not keep records of this. They are popular because they are considerably cheaper than the real devices made within a legal framework. The MHRA have had a case of illegally imported implants, but will not provide details, and readers may also remember that a DT reader has reported a suspected illegal importer to the MHRA.
While the GDC is meant to have transparent and consistent policies, the MHRA says it is different. The DT has asked the MHRA a number of very focused questions and while the MHRA lumped large numbers of questions together and gave some broad-brush answers avoiding any awkward answers, the way they regulate is clear. The GDC is supposed to be focused on the outcome for each individual dental patient, the MHRA says it only focuses on whole sections of the industry. While the GDC ‘can’ seek to sanction an individual and publish Fitness to Practice (FtP) findings to set an example to others if it chooses, the MHRA does not to publish investigations saying, “we cannot disclose commercially sensitive information where we do not consider it necessary to do so and where the disclosure may not be proportionate to what is sought to be achieved by it. Therefore, we are not able to disclose details of investigations or outcomes unless we are satisfied that the disclosure is necessary and proportionate.”
They go on to say: “We acknowledge that even with the most stringent regulatory processes and oversight, there will always be allegations and incidences of non-compliant medical devices. It would not be possible for MHRA to fully investigate every referral it receives, therefore, we operate a risk-based system to assess allegations and balance the volume of referrals against available resources. This system takes into consideration the potential
hazards associated with the alleged non-compliance and the degree of perceived potential harm as well as the severity and probability that the alleged non-compliance will result in harm. This gives us an overall risk rating for each allegation (‘low risk’, ‘significant risk’, ‘high risk’ and ‘serious risk’).”
The way each regulator works is very different and they are not compatible. There is a regulatory gap, the attempt by the PSA and GDC to close it was stopped. This would appear to be intentional.
If you make a hypothetical figure of illegal manufacture and imports in the UK dental market of say 10%, then the normal role of a regulator would be to try and reduce this to zero. If let’s say, a dental nurse knows the dentist they are working for is fitting devices from an illegal manufacturer and reports it to the GDC, (as the GDC say they should) it should go through the GDC processes and result in a fitness to practice hearing. Just as in the case of counterfeit dental headpieces. It should result in a finding that becomes public and provides moral hazard to the profession and hopefully reduces the 10% figure. The case of illegally imported custom dental implants the MHRA say they have had, would have been the ideal case to work together with the GDC as it did with counterfeit equipment, but the outcome of this case never became published and public. It is clear that the MHRA are not in the business of creating moral hazard for illegal practice in dentistry and the illegal dental device market.
If the GDC were to investigate either of the two example cases at fitness to practice and ask the GDC registrant why they did it, (so far the DT does not believe that the GDC has done this for a dentist or nurse) one may imagine one of two answers. “I did not understand the legal requirements” or “I have not been taught.” In this case it would be a failure of education, a problem which is the GDC’s remit. Or the registrant may say that they “thought as the GDC and MHRA don’t have a policy to take action and give visible sanctions, the law was not being enforced and why bother?”
In the GDC’s response to the DT, they talked of proportionality. If the GDC became aware of the example cases (or something similar) and took no action, would that be ‘proportional’ not to set an example or ‘enabling’ to illegal practice?
“We cannot disclose commercially sensitive information where we do not consider it necessary to do so... ”
Would patients expect for a dentist to fit illegally manufactured devices to them, and the GDC and MHRA not to have clear policy and not to act in the way they did over a dentist’s use of counterfeit handpieces? (see below)
The MHRA and GDC have demonstrated that they can work together against illegal activity in the past with the case of the regulators making an example of a dentist using counterfeit dental drills and equipment he had bought online. The GDC held a Fitness to practice hearing, he was not struck off, but was suspended for 3 months. Dentists and the dental press talked about it for months and there can be few dentists who do not know what will happen if they are tempted to buy fake equipment from online auction sites. Medical regulation in action. Why have the GDC and MHRA decided that they cannot do this for dentists who fit illegally manufactured or imported devices to patients?
UNINSURED?
It is believed that you cannot insure against the consequences of a dental registrant deliberately fitting illegal manufactured or imported devices to a patient, as by doing this the dentist would invalidate their dental indemnity insurance. Having indemnity insurance is a GDC requirement for registration. They can (and do) remove people from the register who do not have it.
The DT has asked the regulators and PSA if a dentist fits an illegally manufactured or imported device to a patient whether they would continue to be insured. None of them have been inclined to comment, with one regulator saying it amounted to giving insurance advice!
The reason why they seem unwilling to give their legal opinion may be that if neither the GDC or MHRA will explain
how they make a regulatory finding and a patient (or their legal advisor) cannot find out if they have been involved in illegal practice, then the concept of illegal dentistry regarding insurance and legal action is not relevant.
First attempt
In 2012 responding to concerns and after discussions with the MHRA, GDC and CQC the PSA (then called the CHRE) released a statement saying; “The GDC has instituted the following checks:
• It will monitor the number of complaints about dental devices, received by its Fitness to Practise Department, and whether, in such cases, patients have been offered a statement of manufacture by the dentist.
• It will also monitor the number of complaints about dental devices, received by the Dental Complaints Service to make sure those cases which raise an issue of fitness to practise are referred to the GDC’s fitness to practise department or the MHRA.
• It will consider, as part of the work being undertaken with the Law Commission to design new legislation, any changes to its powers that may be necessary to fill any regulatory gaps that may be contributing to the dental technicians’ concerns.”
This was published in the DT. Checking back after a year as to how the policy had progressed the then GDC Chief Executive said it had been stopped as being ‘too onerous.’ It is not clear if any actual policy was produced for GDC workers to follow or if the GDC council ordered the changes not to be made. If this guidance from the PSA had been implemented there would not be any doubt that illegal practice would have been stopped.
The second major attempt involved the GDC council itself after a visit from the MHRA to discuss MDR with the GDC. In a letter from a GDC director and cleared for publication and published by the DT in 2016 the GDC director said “there had been an internal project to review our policy in relation to breaches of the MDD, (now MDR) and the regulations of the MHRA. This project has now been completed and all of the below changes implemented. The project reviewed several key areas:
1. Guidance given to caseworkers
2. Awareness of the activities of the MHRA
3. Recording information relating to a breach of MHRA regulations
4. Developing an information sharing agreement
Our internal guidance which is given to caseworkers comprises some 116 pages and is a key document which caseworkers refer to on a regular basis. This document was being reviewed as part of our business as usual activity, and after discussions with the then Head of Casework, the specific sections relating to the MHRA and referrals to the MHRA in light of a breach of the MDD were updated. In addition to this updated guidance, which was circulated to all caseworkers, representatives from the MHRA visited the GDC and provided a training session to our caseworkers on the work of the MHRA, and the regulations surrounding custom made medical devices in particular. The feedback was positive, both from the floor of the meeting where several questions were asked, and from the representatives of the MHRA. Further information relating to the work of the MHRA and custom-made medical devices, was circulated to our caseworkers by email after the training.
During the course of this project, we also reviewed our processes for recording information relating to a breach of the MHRA regulations, to ensure that our processes were as robust as possible, we implemented a new process for recording incidences of MHRA regulation breaches in our cases system. We are also in contact with the MHRA and are in the process of developing a formal Information Sharing agreement, to ensure that an appropriate level of information is being shared between our two organisations so that in situations
“If the GDC became aware of the example cases (or something similar) and took no action, would that be ‘proportional’ not to set an example or ‘enabling’ to illegal practice?”
where there has been a breach of any relevant legislation, public protection can be guaranteed.”
The Director also said: ‘I am not aware of a previous policy of intentionally not requesting a statement of manufacture. During an investigation a Caseworker would regularly request a complete copy of the patient’s records for review. It would naturally follow that a copy of the statement of manufacture of any custom made medical device should be contained within those records. If this were not the case, I would expect a Caseworker to elicit further contact in order to establish why this document was missing.’
Extracts from the GDC caseworker guidance document were also provided: 71.2 Registration with the MHRA A manufacturer of custom-made devices or their authorised representative must register with the competent authority of the member state in which they have registered the business. Registration will include a description of the devices concerned and the business address. This requirement applies to both general medical devices and active implantable medical devices. Any complaint received about a registrant who is manufacturing medical devices as defined in the Medical Devices Directive 93/42/EC and who is not registered with the MHRA should be referred to assessment and a referral made to the MHRA.
Make the statement available to the named patient for whom the device has been manufactured.

For first notification of a complaint relating to a registrant not providing a statement of manufacture for a dental appliance where there are aggravating circumstances OR where this is the second complaint received and the registrant has not rectified the issues previously raised and a reasonable period of time has passed, then the case should be allocated for assessment and a referral made to the MHRA.”
This was an actual piece of the guidance document, with actual policy for GDC workers to follow when they became of aware of illegal activity. It is clear that this policy was quickly stopped or it would be active policy now. The GDC have told the DT that there is no information sharing agreement with the MHRA, so it is clear that processes were stopped.
The GDC at one point also had a patient leaflet about the patient’s right to the Statement of Manufacture saying: “What is a ‘statement of manufacture’? The person who prescribes your treatment (usually your dentist) must offer you a statement of manufacture with full details of the appliance. The statement is like a certificate or warranty and proves that your device has been made to legal standards especially for you. You don’t have to take a copy of the statement, it will be kept on file and you can ask for a copy at any time during the life-time of the appliance.”
It is believed that this patient leaflet was withdrawn.
The DT has asked the MHRA and the GDC who stopped the policy development and why? While both organisations said they ’could’ share data, neither organisation would say why the GDC’s attempt to make a formal information sharing agreement had failed or who had stopped it.
The Kingsholm Group was formed after the previous editor of the DT, carried out an investigation into how dental devices are regulated and threw up some concerning information - a regulatory gap between the MHRA and the GDC. It found no formal GDC education policy for dentists and no clear processes when illegal activity is encountered. The DT passed on concerns (and much of the information in this article) to the DLA, the DTA, the OTA and DAMAS and the Kingsholm group was formed.
At the first meeting the DT editor told the group that The DT would follow the DTA’s advice to its members and report suspected illegal dental device manufacturers advertising on the internet but not appearing to be on the MHRA database to the GDC. The Editor gave the members of the group the names of manufacturers reported as they were advertising on the internet. One reader told the DT editor they had reported a suspected illegal dental device importer to the MHRA. The
editor told the group he was unable to get details of GDC policy in such cases from the GDC executive. There was discussion that dentists are exempt from manufacturing legislation, however another member gave the group a document from the EU Commission explaining in detail why dentists are not exempt from the legislation when they manufacture dental devices. The MHRA have now confirmed this to the DT. The GDC confirmed to the group that they do not require dentists and nurses to receive formal training on the MDR when making and fitting dental devices but it may happen in the future.
The group discussed the issue of whether the legislation was being taught in all dental schools. It also became aware of a consultation on hospital in house exemption. The group failed to form any policy, seemingly because of the views of one group. Because the discussion was held under Chatham House rules, the reasons cannot be reported.
members with Covid. There has been no discussion of these issues and the new information gathered since this point.
The PSA has ‘The standards of good regulation’ and set out the Authority’s expectations about the outcomes that it expects from the GDC (and CQC) and their approach to their work. The standards prioritise the core role of regulators in: Protecting patients and reducing harms. Promoting professional standards and maintaining public confidence in the professions.
All of these three are compatible with the standards required by the MDR. The standards are informed by the PSA’s principles of good regulation which states that regulators should act in a way which is: Proportionate, Consistent, Targeted, Transparent, Accountable and Agile.
5.5 “told us that concerns relating to devices do not routinely appear in fitness to practise or illegal practice cases, or cases brought to the Dental Complaints Service. The GDC said that it has limited evidence of risk in this area , and as such this has not been a focus for the Regulation of Dental Services Programme Board’s Risk and Oversight Group. The GDC has worked with stakeholders including the MHRA, registrants and the dental sector about dental devices.”
There was a vote on whether the group should get more detail on exactly how regulators regulate. The majority agreed to get the information. The group was told that concerns have now been raised with the Professional Standards Authority (PSA) who audit the GDC and the group was told that the issues will be explored during the next GDC audit. The PSA has now audited the concerns.
In a DLA editorial about the Kingsholm group, it told its members about the push for education and that the group would “unite and work together for the common goal of making the climate for UK technology better; l look forward to this continuing and developing.” They also very kindly offered to host the next Kingsholm group meeting but the DT has been told that this has had to be postponed while the DLA helps its
The principals (in bold) should be key to how the GDC deal with illegal devices in dentistry when they become aware of them. Hidden policy is none of these things.
The DT has discussed these concerns with the PSA especially the concern with education and the suspected illegal manufacturers reported to the GDC by the previous Editor of the DT. The focus of the audit when the PSA finally published is surprising. The PSA has told the DT its audit did not obtain a clear account of GDC policy when it becomes aware of its registrants engaged with this form of illegal activity or looking to see if it had been followed. It seems to have focused instead on whether ‘stakeholders’ find the GDC’s policy acceptable. Saying that the GDC:
Clearly this is true. The GDC caseworkers who responded to the DT ‘raising concerns’ to the GDC about the suspected GDC registrants illegally manufacturing and not appearing on the MHRA database told the DT that there would be no more communication on the subject, effectively shutting down the complaints. This was reported to the PSA. This would seem to match the lack of policy to take any action. As stated elsewhere in this article, the GDC had developed a policy expecting caseworkers to gather the Statement of Manufacture (note it is a MHRA legal requirement for a manufacturer to keep a copy of the SoM for 10 years) about any serious case involving a dental device allowing the case handlers to ensure the case did not involve illegal activity and pick up on any other relevant information. Stopping this policy will be responsible for ‘limiting the evidence’. Similarly stopping the GDC project involving the “new process for recording incidences of MHRA regulation breaches in our cases system.” If there is ‘limited evidence’ it cannot be a surprise to anyone, and certainly not the GDC or PSA.
5.6 “The GDC has given us reassurance that it is engaging with stakeholders about this issue, and that it is taking appropriate action when concerns are raised with it about compliance with the legislation. We will however continue to monitor this area.”
The DT has asked the PSA who these ‘stakeholders’ were? The PSA asked the GDC and the GDC said it was the MHRA and the BDA.
It would seem that not recognising the Patients Association, the DTA and the DLA as stakeholders for this issue, is key for this gaslit regulatory gymnastics to work. Is this official GDC council policy?
“A question comes to mind, is the GDC’s policy hidden from the BDA or only the rest of us?.. . ”
So, if the MHRA have no clear policy on working with the GDC and are happy that the GDC doesn’t have any clear policy either, is that a successful audit? Similarly, if a dentist chooses to fit illegally manufactured or imported devices to patients and the GDC take no action, is the organisation that represents dentists happy? This seems to be the implication of what the GDC told the PSA. Are the BDA aware of what the GDC is saying about them? Do they agree? How is any of this consistent with the PSA’s requirement for ‘consistency’ and ‘transparency’? A question comes to mind, is the GDC’s policy hidden from the BDA or only the rest of us?
The DT asked the MHRA some very focused questions on whether manufacturers and dentists in dental teaching hospitals were exempt from complying with the MDR when making and fitting devices to patients in teaching hospitals. The MHRA pointed to an ‘in house exemption’ which some believe may be used by manufacturers and dentists in hospitals not to have to comply with the MDR. If an exemption is claimed by manufacturers and dentists in teaching hospitals and they don’t follow the legal requirements, it would be difficult to argue that the dental hospitals weren’t teaching illegal practice.
The DT asked for more details:
1. Can a dentist in a dental hospital choose not to make the statement of manufacture ‘available’ to their patients and make the patient ‘aware’ of their right to have their statement, offer it to them or give it to them?
2. If manufacturers and dentists in dental hospitals can choose not to implement the MDR, would this be legal for their students not to comply when they leave the dental hospital?
3. If the MHRA and GDC allow dental hospitals to act in a way that would be illegal outside in regard to making and fitting of dental devices, how would the MHRA respond to a suggestion that allowing dental hospitals to claim health institution exemption from the MDR for custom made dental devices conflicts with the GDC’s duty to educate and could be considered to be ‘teaching illegal practice’?
4. Is the MHRA aware of any impediment that would stop manufacturers and dentists in dental teaching hospitals from following the same legal requirements as outside the dental hospital?
5. Does the dental hospital claiming exemption have to notify the MHRA?
The MHRA replied:
“As stated in the regulations, any custom made medical device that is manufactured and placed onto the UK market has a number of requirements that must be met. The manufacturer must be registered with MHRA, or have a UK Responsible Person registered with MHRA on their behalf (if the manufacturer is based outside of the UK). The manufacturer must also inform the patient of the devices statement of manufacture and produce and provide if requested a statement of manufacture to the patient.
If a device is manufactured and used on a patient within the UK then MHRA will expect the previous action to be undertaken and if they are not the manufacturer and/or the UK Responsible Person would be in breach of the Medical Device Regulations 2002 and could face compliance or enforcement action.
MHRA does not require a formal notification or application to manufacture under the in-house exemption in Great Britain and therefore do not hold this information for GB-based institutions applying the exemption.”
“As stated in the regulations, any custom made medical device that is manufactured and placed onto the UK market has a number of requirements that must be met. ”
These answers do not answer all the questions or provide any clarity and guidance. The Dental team should have clear guidance.
The response seems to indicate three contradictory principles:
1. “The manufacturer must be registered with MHRA”;
2. The dental team should be taught by education and example how they should behave when trained;
3. Hospitals may claim an ‘in house exemption’ without notifying the GDC or MHRA.
There is even doubt whether the consultation resulted in any policy. The DT will try and get more information for it’s readers.
During the first Kingsholm group meeting an advanced training manual for dental nurses was produced. Dental nurses can manufacture dental devices under GDC rules and while the manual was extremely comprehensive it contained no guidance on the patient’s right to the Statement of Manufacture or how nurses should make devices within the law.
The GDC has told the DT: “While in education, all dental students and trainees are expected to be appropriately supervised by GDC registrants who are then expected to comply with, and support a student or trainee to comply with, all legislation.”
This would indicate a formal expected educational outcome in dental schools. However, they have also told us there is no formal requirement for dentists and nurses to be taught their legal duty as part of their training syllabus, presumably this is why it can be omitted from a nurses training manual.
The GDC say they require registrants to inform the GDC of any breaches of the law they encounter. Is allowing dental schools to arbitrarily decide not to adopt and teach the legal requirements with a formal expected learning outcome the GDC council’s decision? If it is, they should clearly say why they think this education is not needed.
Understandably the MHRA don’t want to micro manage dentistry, that is the GDC’s role and the GDC say that they don’t want to regulate dental devices as that is the remit of the MHRA. However, it would seem difficult for the GDC to argue that having basic standards, patient protection and information for dental devices is not in the dental patient’s interest or that they don’t have a duty to ensure that their registrants comply with the law. Not regulating, is the cheapest option for both regulators. The GDC have had problems in the past with huge backlogs in patients’ complaints and this may be behind not investigating illegal activity in this area and why policy development was stopped.
Does the MHRA’s point that we “cannot disclose commercially sensitive information where we do not consider it necessary” mean that they hold not causing reputational damage to dentists in higher regard than safe and acceptable dentistry for patients?
Both regulators have said that they ‘could’ work together. So has a decision been made at the highest level that the MHRA and GDC should not work together? A decision perhaps that this aspect of illegal dentistry should go unchallenged and dentists must be given confidence to continue.

With both regulators unwilling to be open, it is very difficult to say. The Health Select Committee of Parliament has the power to get to the truth. If no action is taken when regulators become aware of illegally manufactured devices being fitted to patients, then this regulation is a false assurance for them and for legally practising manufacturers. It is just a waste of time, money and resources in an unregulated market.
Is the current policy designed to make fitting illegally manufactured devices a ‘never event’ or is it designed to give those involved confidence for it to continue? Hopefully this article will have enough information for most readers to make up their mind.
The information in this article has been given to a number of The Dental Technician Editors and editorial board over several years and come from many organisations. Every effort has been made to get the information right, despite its difficult nature. It is very difficult to provide clear information when regulators decline to give a clear and transparent account of their policy.
The DT is planning a future article to examine an actual case where a manufacturer (a dental technician in a dental laboratory) was investigated and faced a misdemeanour charge relating to the MDR at Fitness to Practice for not properly filling out the information given on a patient’s Statement of Manufacture and not putting the patient’s interests first. This clearly proves that the GDC ‘may’ indeed use the legislation in exactly the way it was intended. However, it raises major questions about consistency of GDC policy, and confidence in regulation by this class of GDC registrant. This action may have also caused an increase in the premiums that dental technicians have to pay for their dental indemnity insurance.
“While in education, all dental students and trainees are expected to be appropriately supervised by GDC registrants...”

As before if you wish to submit your ECPD online it will be free of charge. Once our web designers give it the all clear there will be a small charge. This will be less than the CPD submitted by post. This offer is open to our subscribers only.
To go directly to the ECPD page please go to https://dentaltechnician.org.uk/dental-technician-cpd. You will normally have one month from the date you receive your magazine before being able to submit your ECPD either online or by post.
If you have any issues with the ECPD please email us cpd@dentaltechnician.org.uk
0.5HRS VERIFIABLE ECPD
The questions are designed to help dental professionals keep up to date with best practice by reading articles in the present journal covering Clinical, Technical, Business, Personal development and related topics, and checking that this information has been retained and understood.
By completing the Quiz successfully you will have confirmed your ability to understand, retain and reinforce your knowledge related in the chosen articles.
CORRECT ANSWERS FROM THE JANUARY 2023 DT EDITION:
1. Your details
First Name: Last Name: Title: Address: .............................................................................................................................................. ...............................................................................................................................................................
Postcode: Telephone: ..................................... Email: .......................................................GDC No:
2. Your answers. Tick the boxes you consider correct. It may be more than one.
3. Evaluation: Tell us how we are doing with your ECPD Service. All comments welcome. ..................................................................................................................................................... .....................................................................................................................................................
As of April 2016 issue ECPD will carry a charge of £10.00 per month. Or an annual fee of £99.00 if paid in advance.
You can submit your answers in the following ways:
1. Via email: cpd@dentaltechnician.org.uk
2. By post to: The Dental Technician Magazine, PO Box 2279, Pulborough, RH20 9BR
Payment by cheque to: The Dental Technician Magazine Limited. Natwest Sort Code 516135 A/C No 79790852

You are required to answer at least 50% correctly for a pass. If you score below 50% you will need to re-submit your answers. Answers will be published in the next issue of The Dental Technician. Certificates will be issued within 60 days of receipt of correct submission.

This months CPD questions are are focussing on Illegal Manufacturing Feature. Please read the feature article Protecting Illegal Dentistry.
Q1. There are several regulators that govern and regulate the Dental industry, which of these is not a Regulator:
a. HMRC
b. MHRA
c. GDC
d. CQC
Q2. MHRA is an acronym for which agency?
a. Manufacturing Hotels Rulings Agency
b Mental Health Rights Association
c. Medicines and Healthcare products Regulatory Agency
d. Mens Health Rights Agency
Q3. GDC is an acronym for which Dental Regulator?:
a. Governing Dental Corporation
b General Dental Corporation
c. Governance of Dental Companies
d. General Dental Council
Q4. Manufacturers of Medical Devices have a XXXXX obligation to register with the MHRA;
a. Moral
b. Legal
c. Dutiful
d. Sensible
Q5. The XXXXX regulate those who fit dental devices to patients:
a. PSA
b. CQC
c. GDC
d. MHRA
Q6. The PSA who audit the GDC are know as;
a. Patient Safety Advisers
b. Professional Standards Authority
c. Professional Safety Agency
d. Personal Standards Authority
Q7. A previous editor of The Dental Technician passed on concerns (and much of the information in this article) to the DLA, the DTA, the OTA and DAMAS and the XXXXXX group was formed:
a. Kingsholm
b. Chatham House
c. Queensholm
d. Princes Trust
Q8. Which of the following organisations is considered a ‘Stakeholder’ by the GDC?:
a. Dental Teaching Association
b. Dental Technicians Agency
c. Dental Technicians Association
d. Dental Tourism Agency
Q9. Every patient who has a Dental Devices fitted should be offered the SoM, what is this an acronym for?
a. Safety of Mouthguards
b Statement of Monies owed
c. Solution of Milton
d. Statement of Manufacture
Q10. It is a MHRA legal requirement for a manufacturer to keep a copy of the SoM for XXX years:
a. 1
b. 100
c. 10
d. 15 YOU
VIA EMAIL:
cpd@dentaltechnician.org.uk
OR BY POST TO:
The Dental Technician Magazine, PO Box 2279, Pulborough, RH20 9BR.
You are required to answer at least 50% correctly for a pass. If you score below 50% you will need to re-submit your answers. Answers will be published in the next issue of The Dental Technician magazine. Certificates will be issued within 60 days of receipt of correct submission.
PAYMENT BY CHEQUE TO:
The Dental Technician Magazine Limited. NatWest Sort Code 516135 A/C No 79790852
As part of the editorial team, I hope to bring in interesting and innovative aspects of Maxillofacial Technology/Healthcare Science into our magazine.

In this particular case study, I’m giving you a body prosthesis case of a very sensitive nature. A new mother went to her antenatal, mid-term ultrasound (20-week) scan where Spina Bifida was diagnosed within the foetus. This however, wasn’t the main concern at this stage; it was the presence of a sacrococcygeal teratoma tumour, at the base of the coccyx. Mother had 2-week appointments to drain fluid from her womb to avoid premature labour. Yet, at week 35, labour was brought on through a cut at one of the fluid draining appointments.

The baby girl was Born at 11lb via Standard C Section which was necessary due to the size of the tumour. On Day 4 it was tumour removal day and shockingly, it was estimated that half of the birth weight was that of the tumour itself. The young girl continues to have regular check-ups to ensure there are no further complications as her body grows.
In 2019, at the age of 11, the patient was referred to myself for some form of prosthetic camouflage.
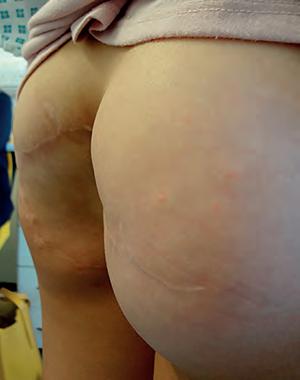
Fig i and ii shows the extent of the scarring and also the lack of projection of the left buttock.

The requirements needed to provide this patient with an adequate prosthesis were relatively simple;
• What does the patient want and what does she want the prosthesis to do for her?
• What are the options available to her?
• How will hygiene be best managed?
In terms of the prosthesis;
• What will the weight of the prosthesis be and can this be reduced?
• What will its size and extension be?
• How will it be attached to the body in the correct location and orientation?
In order to start any form of prosthetic work, it must follow from an accurate impression of
the defect site. I used an old tested impression technique that was taught me by the late Liz Gill where alginate impression material [Schottlander Fidelity 25] was packed onto the surface. Large paper clips were bent and embedded into the alginate prior to setting, to form a key system which allows the plaster backing to accurately support the less stable alginate. Although the prosthesis was intended to sit unilaterally, it was necessary to encapsulate the form of the right buttock to try and match the space lost. (fig iii)
The whole mould was then cast in a type iv stone (Crystacal R [John Winters]), but there needed to be an inhibitor to prevent the backing plaster integrating into the Crystacal working model. For this I sprayed the periphery of the impression backing with MacWax [PolyMed ltd] release agent. (fig iv)
With the same technique of wax separation as with denture manufacture, the mould was soaked for 15 minutes and then dried. A combination of standard modelling wax

and thin casting wax was used to form the try-on prosthesis. Casting wax was used as it is thinner and therefore quicker to adjust in clinic. The try-on was satisfactory (fig v) as it formed adequate coverage and fitted to the surface well. I then performed a second impression, this time with the patient laid on her front. Once this second mould was cast, the wax-up matrix was tried on to it to assess how much the buttock would move.
M511 silicone [Technovent UK] was then colour matched to a natural shade with no great accuracy, as it wouldn’t be a prosthesis that would be visible to anyone. Only the shape and form were required.
One of the crucial aspects of the comfort and usability of this prosthesis was its weight. A prosthesis made purely of silicone would have been extremely heavy and no doubt uncomfortable. Therefore, it was decided to form a silicone sponge matrix that would act as the body with and skin coloured silicone style “glove”.
To achieve this, to two parts of silicone sponge [A-3200-G Technovent] were mixed and packed into the invested wax mould (fig vi). Once cured, it was removed and cut to a smaller shape to allow room for the skin colour silicone within the same mould. Iso-Sep [John Winters] coated the entire surface and was allowed to dry fully, before packing. The skin colour silicone was then added around the sponge to encase it (fig vii). Once packed, the flask was firmly closed, and the silicone allowed to cure with RTV (room temperature vulcanisation) over the weekend. Post cure, any excess was trimmed with scissors and the whole surface was checked and trimmed as necessary to prevent any discomfort.



At the fit appointment it was apparent that the overall projection was inadequate, however it would act as a good starting point to establish whether the patient would be happy wearing a prosthesis in such a sensitive area (fig viii and ix).
I gave the patient a month appointment for her to see how comfortable it was. It wasn’t particularly comfortable as she found the material too hard. She felt that she didn’t want any further prosthesis making for her at this point, therefore I suggested purchasing some padded shapewear underwear and to cut out the filler on the right side of the undergarment so that under clothing, little discrepancy would be noticeable. The patient and her mum were both happy with this suggestion.


The conclusion that stands out in this particular case study is that of providing the patient with exactly what they want.
Important note: for every appointment, the patient’s mother and a nurse chaperone was present alongside me.

Also known as a ‘Scarcity Mindset’ is one that influences behaviours consistent with beliefs that;
l money is something I've never had
l money shouldn’t be spent
l opportunities are limited
l taking any risks at all is dangerous
l success is temporary
l success shouldn’t be celebrated
l accepting the status quo is safest.
You might think, ‘Well it's easy for you with your big lab’. Let me tell you, it’s not been an easy journey. One thing that has been consistent, even through the most difficult times, was my ‘Abundant Mindset’.
As a teenager growing up on one of the roughest and toughest Council estates in the UK, now been ranked in the top 10 worst areas to live, I always believed I could achieve pretty much most things if I set my mind to it. Life in the 80s was tough for our family, but I never once heard my parents moan about the 3 jobs my Dad did, and the late shifts my Mum put in at the sweet factory in Sheffield. They, cracked on, they set a great example, they wanted better things. They didn't moan about what they didn't have, they strived and set out to do things they wanted to do. It was such an encouraging thing to see as a teenager. Certainly one that helped me and set the tone for life with an ‘Abundant Mindset’.
As a Dental Technician, a poverty mindset is almost engrained into our DNA from the outset. I remember my old college lecturer saying to me, “If you want to drive one of those fancy Porsches you'd better retrain as a Dentist, you'll never earn enough as a plaster monkey.” That was it, goal set, at 17 years of age, I told myself I would own an Aston Martin before I was 40! I had 23 years to nail it! I did it, it by the way, aged 39!
That was just the beginning of a professional pathway of bumping into and working alongside some of the most pessimistic people on the planet. It was almost beaten into us when we started our college or university courses. I don't have enough hands with enough digits to
By Matt Everatt FOTA I Author I Editorcount the number of lecturers, colleagues and futures employers that would start conversations with “Why on earth do you want this job?” or “You'll make more money working at Netto.”
True story, I sat in an interview at a very large teaching hospital, being told “You'll struggle to afford to live in London, it's lot more expensive than up North. Will you be able to afford it on a Technicians wage?”I replied "I'll give it a go if you offer me the job thank you very much, when can I start?" I got the job!
It is very easy to get dragged into a poverty mindset by those around you. After all, Jim Rohn - Entrepreneur and author of ‘7 Strategies for Wealth & Happiness’ famously quotes, “You are the average of the 5 people you spend most of your time with.”
It doesn't have to be this way. We can adopt an abundant mindset, first we must identify some of those limiting beliefs we have and consciously flip them. Today, if you spend any time with colleagues, friends or family members, see if you can spot some of those poverty mindset traits.
Here are some examples:
l Belief that you are a victim of others’ decisions and choices. This is a typical trait in our profession, often blaming Dentists for driving prices down, or blaming fellow
technicians and other labs for undercutting prices. “It’s not our fault we have to work long hours to make ends meet, its everyone else’s. It’s beyond my control, if I increase my prices, they’ll just leave and find someone cheaper”. Sound familiar?
l Fear of spending money on non-essentials. I have seen this with so many of my colleagues over the years. Thinking they don't deserve nice things or they ‘really shouldn't buy that’ because they ‘don't need it’, ‘it isn’t essential so I can’t buy it’.
l Constant searching for cheapest alternative, even if its poorer quality.
l Obsessed with getting “deals” and free entry. Spending hours getting voucher codes. I'm not talking about getting a cheeky deal occasionally, we all love a 'free dough balls at Pizza Express' once in a while.
l Belief that you’re lucky when you succeed, incompetent when you fail. Again, I see this so much in our profession. So many people saying they are just lucky, or 'You're just lucky'. Sorry, success isn't luck. Success often comes on the back of lots of previous failures and financial risks, certainly not luck. Look at James Dyson, his 5,126 failures didn’t mean he had failed, they were all just lessons to making his success much better.
l Denying yourself treats or luxury items. This is a big one, I see this all the time. Technicians, looking longingly at the luxury car their customer has just bought, thinking 'it's alright for some, how the other half live', often being in a position themselves to afford such a treat.
l Feelings of guilt when you have more than someone else. You may be embarrassed and have guilty feelings when you buy a new car, or take a really nice holiday after earning a bonus or winning some new business.

l Fear of being perceived as boastful when you describe achieving a goal. It is very rare to see dental technicians celebrating success, in fact I have seen many people criticise colleagues who have entered or won industry awards for example. More about this one later.

For years I have experienced first hand, a poverty mentality and mindset within our particular sector of Dentistry. It undermines performance and limits success. Let me explain...
l Feeling you haven't got enough reserves or resources. Some of these fears may go back to our childhoods, so it is understandable that we can be worried about not having enough in the bank. Without sounding irresponsible, it is a balance between holding enough back ‘just in case’ and enjoying what you have.
l Believing you could “lose it all” despite your hard work. Perhaps another limiting belief that goes back to our childhoods, particularly if you have seen parents or family members lose jobs or have to close their businesses.
With these examples in mind, consider how others are today. Are the people you spend the most of your time with exhibiting any of these traits? How do those conversations go? Do they make you feel happy, inspired? Remember Jim Rohns’ quote; “You are the average of the 5 people you spend most of your time with.” If you want to feel inspired, encouraged, celebrated, you need to surround yourself with those people in your life that raise you up. This is not to say cut out the others in your life. I have a good friend we all lovingly call ‘Black Cloud’. Every conversation he starts or finishes is doom and gloom, so much so, he has become a bit of a comedy character. If you spent all day with 5 people like him, I’m sure you wouldn’t be in the best place mentally. By the way, I love him dearly, and I love his company when we meet, I see the funny side to his dark perspective on life. Just not everyday!
An ‘Abundance Mindset’, some people say ‘Abundance Mentality’; is one that assures you that you are successful and can replicate that success. You have value and talents in great demand. You can handle most situations that come your way very successfully and in those difficult times, you can bounce back quickly and are resilient.
Simply put, it’s the flipside to those limiting beliefs and trait examples given earlier in this article. When others see problems and fear new things, those with an abundance mindset see solutions, opportunities and relish the new challenges ahead. We could simplify it by using the words Pessimistic and Optimistic, although I think there is a little more to it than simple Optimism and Pessimism.

Here are a few examples of the traits of an Abundance Mindset:
l Considers the opportunities, focusing on areas of growth and improvement. This is a fantastic trait to have. If you have ever had any business coaching, you will have
been encouraged to look at problems as opportunities, to consider difficult situations as ways to improve and grow.
l Leads with confidence and clarity taking responsibility for their own actions. This trait is one that not only leads to having great people on your team and wanting to be part of your team, it inspires others to lead by your example.
l Thinking about the challenges and growth ahead makes them feel empowered and engaged. This is almost polar opposite to how someone with a Poverty Mindset feels and thinks.
l Collaborate well with others by sharing knowledge and success. I see some of the most successful people in our profession sharing their knowledge, ideas and expertise. The process of giving and sharing, ultimately ends in reciprocal help and support from others. Acting as a giver contributes to others wellbeing.
l Think big, embracing risk. This is often where people see those that have achieved great things or success haven’t seen the journey that came before and see them as ‘lucky’ or ‘it’s alright for them’. They haven’t seen the risk and challenges that have gone before the success.
Here are just a few tips that I have pulled together from several authors.
The ability to think big is probably the first step out of your comfort zone of
self-imposed limiting beliefs. Focus your energy on exploring a bigger and better future, think about how the path ahead might look and how you begin the journey to make it possible. If you’re thinking of applying for a new role, offering a new service or how you might take your lab to the next level, think big. Thinking big, sets the direction and the tone of your journey.
Make progress by taking small steps along the way, don’t be afraid to reevaluate your strategies, reconsider your options, adjust and adapt. Remember Dysons 5,126 failures before his success, they all form part of the journey.
Above all, try to think without limits. Try not to be tunnel visioned on one particular thing, try to loosen your focus and create an open minded approach to create an abundance mentality. Choose your words wisely, before you speak, think are you about to deliver something in the scarcity tone or with an abundant tone.
Celebrate yourself and celebrate others too. I have been known to celebrate the opening of a crisp packet! Seriously, enjoy and celebrate good things on a daily basis. If a colleague creates a great piece of work, congratulate them, tell them how great it is. Our Britishness sometimes stifles these kind of celebrations. My American cousins are amazing at celebrating and congratulating each other. Paying compliments and flattering your friends, partner, colleagues and even your competitors will reward you in abundance, seriously give it a try.
It is a choice, you can choose to think Scarcely with a Poverty Mindset or you can choose positivity and go about life with an Abundance Mindset, it really is a choice... So, I task you today to go and think ‘ABUNDANTLY’ without limits and dream BIG!!!
Stephen Taylor will be among the many highly-anticipated speakers at this year’s Dental Technology Showcase (DTS).

Stephen Taylor shares some of the issues he feels dental technicians are facing right now and how he hopes his session in the DTS Lecture Theatre will be of help:
“Even without being aware of the problem, a business culture could make people feel uncomfortable in discussing their mental wellbeing. This can impact on their performance in numerous ways.
“My presentation will highlight some of the signs that people may display. I will explain the need for mental health support within a dental business, and give insight into a framework that can be used to ensure employers can offer a level of support to all members of staff.
“Mental health issues are impacting some people both within a work environment and personal life situations. Currently there is a lot of emphasis on ensuring people are sign-posted to the help they require.”
Reflecting on DTS in general, Steve adds: “Attending DTS allows technicians and employers to update their knowledge, and share experiences with colleagues.”


DTS 2023 will be held on Friday 12 and Saturday 13 May, NEC Birmingham, co-located with the British Dental Conference & Dentistry Show (BDCDS).
For more information, visit the-dts.co.uk or email dts@closerstillmedia.com Register today!
w With Zirkonzahn's new Detection Eye intraoral scanner, the patient's jaw can be easily digitised in less than 60 seconds. The scanner is easy to use and the choice of two different tips (standard and small) makes the impression-taking more comfortable and patient-friendly. The scanner has been designed to be lightweight, compact and ergonomic and the scanning areas do not need to be pre-treated with powder, which simplifies the acquisition process.

Once the data has been captured, it can be easily and quickly loaded into the Model Maker software module, for the design of a model. The created model is then transferred to the new Zirkonzahn. Slicer software, where it is placed on the printing
platform and, if needed, special supports can be generated. The software is conceived for the dental workflow and is supplied with pre-configured settings for a seamless and well-calibrated print process.
The 3D print data generated can be transferred to Zirkonzahn’s P4000 Printer either via USB, LAN or WiFi, and the large print volume (L x W x H: 20 x 12,5 x 20 cm) permits the simultaneous production of, for instance, up to 21 Geller models or 15 dental arches, depending on their structure and dimensions. The P4000 3D printing system works ideally in combination with the Printer Resin Waterbased Beige by Zirkonzahn. This resin is particularly suitable for the

production of dental models and shows low shrinkage values, producing highly stable and reproducible printed objects.The model can be cleaned in an ultrasonic bath and then cured in the L300 Post-Curing Lamp. Subsequently, it can be positioned into the PS1 articulator or ZS1 Mini-Arti without the use of plaster thanks to the new JawAligner PS1 or ZS1 (magnetic spacer plates), in order to check the patient's jaw movements.
For more information: www.zirkonzahn.com

w The ability to produce highly accurate dental products can be enhanced and facilitated with a high quality laboratory micromotor.
The Perfecta range from W&H, available from Blueprint Dental, offers a brushless motor, operating at speeds up to 100,000 rpm, together with an integrated air jet function to instantly blow away chips and debris from the working surface. So, no more reaching for the air gun, saving you time and effort.
With the choice of a foot, table or knee control unit, the technician really can choose which option works best for their own preferred ergonomic set up.
Whatever your choice, the motor speed is easily controllable so you can accurately machine a wide range of materials, including ceramics.
Whichever Perfecta unit fits your needs and budget the best, you know you’re getting a high quality piece of kit which will only enhance your own high levels of craftmanship; all complete with a 2 year warranty for added peace of mind.
Find out more about the W&H Perfecta Laboratory Micromotor visit: www.blueprintdental.co.uk/products/ perfecta-dental-laboratory-control-units or contact Alan Wright on 07904 413211.

w A highly experienced dental technician from Harpenden comments on the Acron High Impact (HI) Acrylic that he has used in the lab for many years now: “The Acron HI from Kemdent is the best of its kind on the market. It packs down really easily, and is simple to handle with plenty of time to work with it. The grinding of the material is very good as well, while the polishing is a dream. Compared to others I have tried, the colour is also great.

“I would recommend the Acron HI to colleagues and have done so several times. Everything about it is ideal – the colour, the way it packs and it comes out really clean when you follow the stages as per the manufacturer’s instructions.”
For more information about the leading solutions available from Kemdent, please visit www.kemdent.co.uk or call 01793 770 256.

w Kemdent is proud to have manufactured one of the first eco modelling wax ranges, demonstrating their dedication to protecting the environment.
Included in the range is the Anutex Eco modelling wax. Thanks to new manufacturing techniques, this innovative material has been made using 30% less energy than previous materials. However, the quality of the wax has not been sacrificed in the process.
The Anutex Eco modelling wax still provides an exceptional performance, as

w If you’re looking to invest in products that simplify your workflow and produce beautiful results for your clients, look no further than Kemdent.
Since 1922, Kemdent has been supporting dentistry with reliable, highquality solutions. Included in their portfolio is the Acron Hi – High Impact Denture Base Acrylic. Gareth, a dental technician from J T Hemming Dental Laboratory, shares his experience of using the product: “I’ve been working at the laboratory for 13 years and we’ve always used the Acron Hi –High Impact Denture Base Acrylic.
“It’s very robust, is easy to handle and has a really good finish, and the colour is preferred by our clients.
“The working time is great, as you get plenty of time to pack other jobs and the product doesn’t go off.”
it softens without becoming flaky, does not stick to fingers and will not irritate the soft tissues in the mouth.
Additionally, the Anutex Eco does not distort in the mouth, trims easily and leaves a smooth, glossy finish after flaming.
Kemdent continues to show their dedication to busy dental professionals with their range of high-grade, reliable dental products and materials.
To discover more, get in touch today.
770 256.

w Simeda customised prosthetic solutions from Anthogyr, a Straumann Group brand, offers quality prostheses for various restorative cases.
Anthogyr offers precision customised restorations for dental implants and multiplatform solutions (MPS). There is over a decade of manufacturing expertise, and a wide variety of materials available including titanium, cobalt-chromium and zirconia to deliver excellent results every time.
The Simeda customised prosthetic solutions from Anthogyr provide customised abutments and prosthetics for implant bridges, suprastructures and bars.

Consider Anthogyr for quality, customised prosthetic restorations for your restorative cases.
For more information on Simeda customised prosthetic solutions from Anthogyr, please visit www.straumann.com/anthogyr


w Createch Medical, part of the Straumann Group, provides a comprehensive directto-lab milling service, to help you provide excellent outcomes in every case. Createch Medical gives you access to advanced technology, allowing you to maximise your design creativity – finding ideal, custom solutions in all cases.
There is a wide range of solutions available when you choose Createch Medical, compatible with over 600 implant systems. The team offers a range of services including the measurement, design and milling of implant supported prostheses and frameworks.
Choose the Createch Medical directto-lab milling service for unbeatable customisation, including in complex cases.
REGISTER FOR YOUR ACCOUNT WITH CREATECH MEDICAL AT http://www.createchmedical.com/en/file-sending/login-registro/ FOR MORE INFORMATION ON THE CREATECH MEDICAL DIRECT-TO-LAB MILLING SERVICE FROM THE STRAUMANN GROUP, VISIT https://www.straumann.com/gb/en/dental-professionals/products-and-solutions/implant-borne-prosthetics/products/createchmedical.html
This integration will see the company offering its products and services in the UK on top of Spain, France, Portugal, the Netherlands, Belgium, Sweden, Denmark and Norway through 74 laboratories and more than 1,650 employees. Corus plans to further develop in the UK market integrating more laboratories.
l OXFORD, 1 February 2023 Corus, the leading digital dental laboratory group in Europe, has integrated Byrnes Dental Laboratory the UK reference in this field. With this addition, Corus enters in a new market to further consolidate Corus European footprint and leadership serving high-end digital prosthesis and orthodontics to advanced dental clinics in nine European countries.
The Byrnes Dental Laboratory was founded in 2006 by Tony and Ashley Byrne. With the leadership of Ashley Byrne since 2009 the company has not only grown successfully but also has become a reference in digital dental prosthesis in the UK and abroad. The lab, located in the Oxford area, delivers high-quality innovative products and services to advanced dental clinics spread amongst England, Scotland, and Ireland. Byrnes’ differential position in the market has been based on a highly innovative and skilled team of 47 people working in a state-of-theart facility.
Now, as a shareholder of Corus, Ashley Byrne will maintain his responsibilities and leadership within Byrnes dental, in addition to contributing to the development strategy in the United Kingdom and the continuous product and services innovation within the company.

This is a first step for Corus in the United Kingdom with further expansion planned
in England, Scotland, Wales and Ireland. The company seeks to integrate reputed and advanced dental laboratories lead by local entrepreneurs, who then become shareholders, maintaining the highest level of service nationally to their existing clients. Corus works as a fully integrated digital platform, where dentists can interact with the laboratory and patient whilst ensuring the traceability of the prescription and delivering efficient and high-quality products and services to meet the patient expectations. The Corus network offers a global solution to dentists based on an innovative and differentiated portfolio of protocols of work, products, services, training and education while maintaining a national and personal environment, thus ensuring a better patient dental care and experience. With 74 laboratories and over 1,650 employees the group serves over 13,000 dental practices and more than 1.5 million patients spread across the United Kingdom, Spain, France, Portugal, the Netherlands, Belgium, Sweden, Denmark and Norway.
lByrnes dental laboratory is starting a new chapter. We are the first UK lab joining an exceptional and disruptive group of 73 high end dental laboratories based in Spain, Portugal, France, Belgium, Netherlands, Sweden, Norway, and Denmark.
This group of quality laboratories, called Corus, is a totally unique offering to the UK market, bringing the concept of shared knowledge and innovation, complimented by game-changing lab software and clinical connection.
These labs are headed up by experienced lab owners with an
Nicolas Bonnard, co-CEO at Corus, said: "Byrnes dental lab has an incredible team. We share values and vision. Driven by innovation and high-tech state-of-the-art manufacturing processes and services, they have been leading digitalization of modern odontology in the UK.”
Jeroen Van Asten, co-CEO at Corus, added: "This move will enhance our skills, consolidate our position as leaders in Europe, and allure other entrepreneurs willing to write together a new chapter in advanced digital dentistry for benefit of dentists and the patients.”
Ashley Byrne, CEO The Byrnes Dental Laboratory, stated: “Everyone at Byrnes Dental is incredibly excited to join the Corus family, working alongside many of Europe’s leading dental labs. This will enhance and improve our offering to new levels of high-end technical dentistry for our clients and patients.”
Steven&Bolton and BDO have advised Corus on the transaction. For Byrnes Dental Lab, the advisors were Herrington Carmichael and DSA Prospect.
Corus is the leading European company in cutting-edge dental prostheses and orthodontics.
Founded in 2015, Corus is immersed in the paradigm shift towards a new dentistry, 100% digitalised, personalised and with added value for the dentist and the patients.
Since its creation, Corus has integrated more than 80 dental prosthesis laboratories distributed between Spain, France, Portugal, Netherlands, Belgium, Sweden, Norway and Denmark and with a team of more than 1,700 professionals, offering to dentists differentiating and exclusive protocols, products, services, training and education.
entrepreneurial spirit and the shared vision of digitising the industry, dynamic innovation, and excellence in patient experience. Byrnes is proud to be the UK flag ship and will be seeking like-minded lab owners and teams with a similar vision of improving the industry through disruption.

support, I am now a proud and qualified GDC registered technician with a Dental Technology qualification
You started with an entirely analogue process of models and dentures, now your day is more digital than analogue. How did you find that transition?
I found it a little scary initially, but in all honesty, it was not as bad as I thought. I found it very helpful to have had the analogue knowledge of hands-on experience to take full advantage of what digital dentistry could offer. Being able to go back and retrieve a digital file so that I can improve the case has been vital to learn from my mistakes and move forward.
Tanya’s inspirational story: from a mature student and technophobe to a registered technician and digital queen!
Before becoming a dental technician, what did you do?
I was a teaching assistant for 10 years in a special needs facility within a mainstream school. I worked one-to-one with children, helping them to access mainstream education.
What age did you decide to become a dental technician and why?
43!! I was a single parent of two grown up children, so I felt I needed to up my game and I would need to earn more to be able to run my life without external assistance. Byrnes dental lab gave me the opportunity to do this late in life.
Once I started, Ashley encouraged me to enrol in day release at Lambeth College. Whilst I was concerned about this and how going back to education would be at my age, through the teams’
How much computer knowledge did you have when you started digital? Basic knowledge. I could navigate my way around okay, working in a school and studying for three years to be a dental technician helped with this. I was certainly no expert on a computer. Being methodical has definitely helped, organisation is key to a good digital work flow. Pushing myself at college for distinctions gave me a drive to learn more. Despite my basic knowledge, that drive has really accelerated my digital dentistry skills.
What digital knowledge do you have now, and what do you do at your lab?
I can use both exocad and 3Shape, and can make special trays, occlusal splints, base plates, and verification jigs. In addition, I am also now regularly producing partial and full temporary dentures. I’m trained in multiple printers including Carbon, Asiga, and NextDent. My day is pretty varied doing CAD, printing, and finishing.
What do you enjoy most about digital over analogue?
Obviously, it’s cleaner, less wasteful, and
better for the environment by the removal of gypsum and waste materials in general. We even have a non-IPA cleaning solution at the lab which adds to the environmental benefit. I am keen to push myself, learn new things, and be a better dental technician. Digital is a great learning tool as I can make mistakes, delete them, and redo the work easily and with zero waste.
For someone who had very little computer knowledge when you started digital dentistry, what challenges did you have and how did you get over them?
I would get frustrated that I didn’t know enough but starting when I did, I think for me, was perfect timing. I still need to learn more, and self-learning is really important. Try the software out, ask questions on forums, look at YouTube when stuck, and generally searching the inter-net has been really helpful for me.
Any hints and tips for the mature generation that may be fearing the change to digital?
Don’t be fearful. What have you got to lose if you try this new way of dental technology? You will feel worried at first, but you CAN do this! The sense of achievement when you do master it is fantastic. Being a part of the future and futureproofing yourself is really important in this industry.
Any final words on digital dentistry? Being an older person (now 49) and late to the industry, has allowed me to see that my brain can be challenged, active, alive, and curious.
If I hadn’t jumped in and pushed myself, or allowed myself to be guided when lacking confidence, I wouldn’t appreciate or know what I really can achieve. Coming into it with no experience or background in dental technology was hard, but also a blessing because I came into it without bias.
Finally, the support of patient team members and the open mindedness of Ashley and Alison has been absolutely fundamental in my learning. Without that I wouldn’t be enjoying what IS the future of dental technology. How exciting is that!
[ Pushing myself at college for distinctions gave me a drive to learn more.]













