The Impact of Sleep and Circadian Rhythms in Sports
 Raman K. Malhotra, MD, FAASM
Raman K. Malhotra, MD, FAASM
Professor of Neurology
Washington University in St. Louis


 Raman K. Malhotra, MD, FAASM
Raman K. Malhotra, MD, FAASM
Professor of Neurology
Washington University in St. Louis


of
I have no relevant conflicts of interest to disclose.





“Tom Brady, noted underachiever that he is, aims to be in bed by 8:30 p.m. LeBron James plans his life around nabbing ten hours of shut-eye. Justin Verlander, the Cy Young –winning Houston Astros ace, clocks between ten and 12 hours every night. (And he does it sleeping next to Kate Upton.) In the past few years, the world’s premier athletes have discovered a new performance-enhancing supplement for their training regimens: sleep.”






















(do athletes need more?)

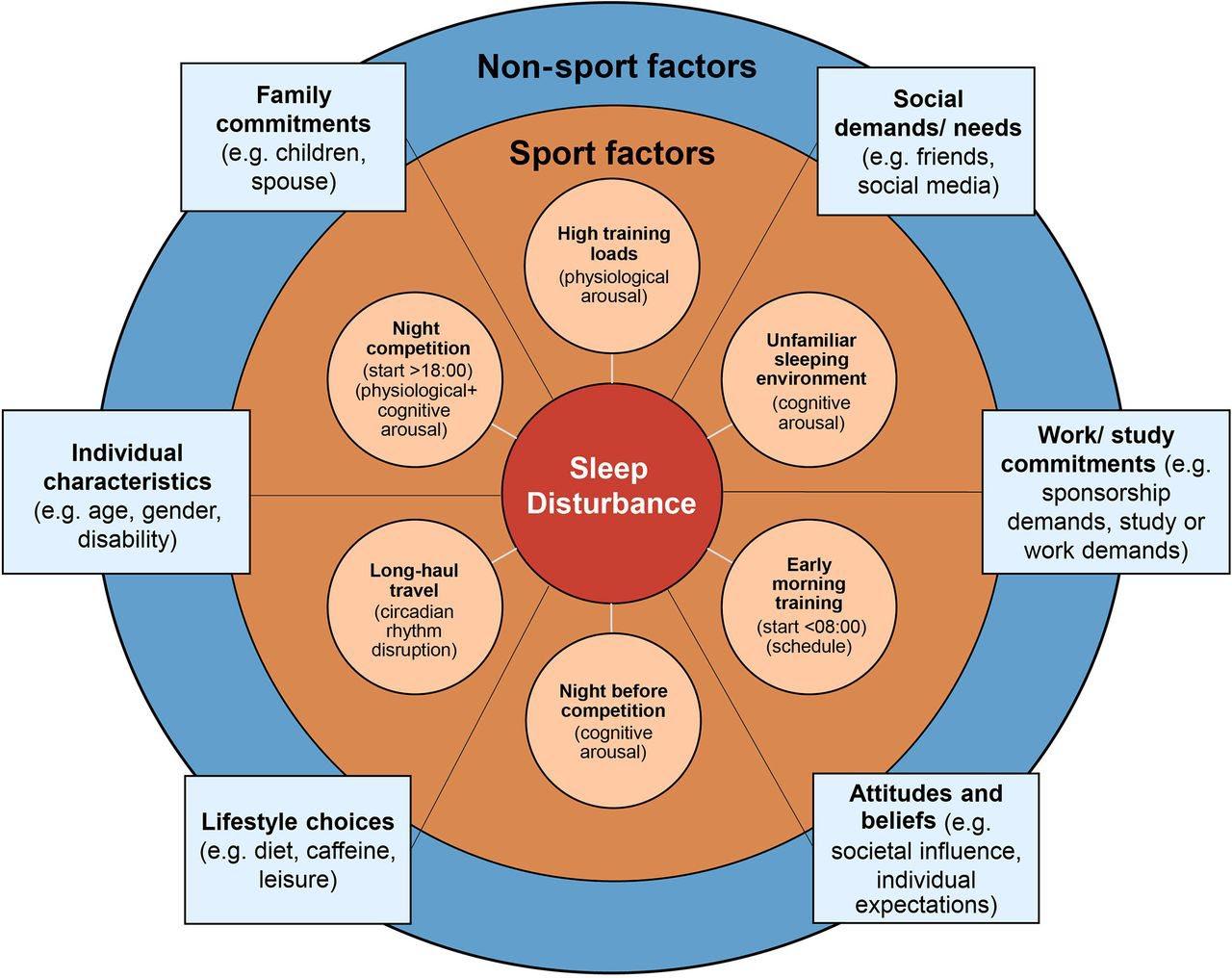
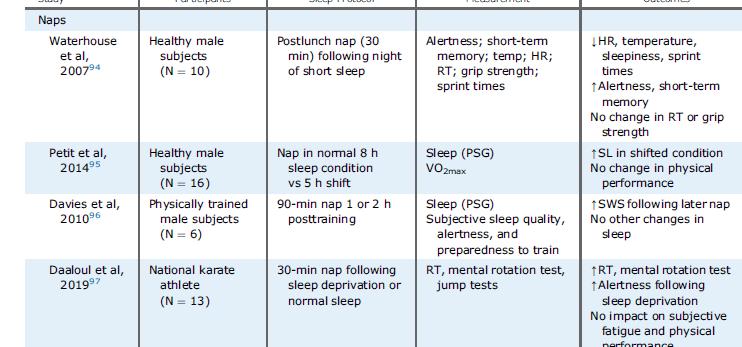
Contributory factors for sleep disturbance in athletes; including sport-specific factors (orange shading) and non-sport factors (blue shading).




Power Speed
Endurance
Running
Breathing

Reaction time
Memory Concentration
Learning Mood Creativity/Adaptability

-Crave food (higher calorie foods)
-Growth hormone, cortisol changes

-Pro-inflammation (impeding recovery)
-Higher physiological demand (fatigue easier) with higher lactate levels, heart rates and respiratory rates noted in the sleep deprived group
Mougin F,et al, Eur J Appl Physiol Occup Physiol, 1991 Charest J, Grandner MA, Sleep Med Clinic, 2020
• Worsened plate discipline1
• Worsened tennis serve accuracy2

• Worsened performance (place in contest)3
1. Kutscher SJ, Song Y, Wang L, et al.. Sleep 2013;36(Abstr Suppl.)
2. Reyner LA, Horne JA. Physiol Behav 2013: 120: 93–96.
3. Juliff LE, Strength Cond Res, 2018

Juliff, Laura E.; Halson, Shona L.; Hebert, Jeffrey J.; Forsyth, Peta L.; Peiffer, Jeremiah J.
The Journal of Strength & Conditioning Research32(1):189-194, January 2018.
Mean sleep characteristics during competition in teams based on final finishing position, with 95% confidence intervals for (A) time in bed, (B) sleep duration, and (C) sleep efficiency. * indicates significant (p ≤ 0.05) difference between team 3 and all other teams. # indicates significant (p ≤ 0.05) difference between both team 1 and 2.

Brauer AA, Athey AB, Ross MJ, Grandner MA. Sleep and Health Among Collegiate Student Athletes.
Chest. 2019 Dec;156(6):1234-1245.

-<7 hours of sleep have a 1.7X higher risk of injury
->8 hours of sleep have a 61% decrease in injury
-No prospective studies demonstrating this
Huang K, Ihm J. Sleep and Injury Risk. Curr Sports Med Rep. 2021 Jun 1;20(6):286-290.

-Insomnia and EDS two or more times a month increased risk of concussion (relative risk was 3.13) in 190 NCAA Athletes

-Concussed patients frequently suffer from insomnia or hypersomnia (up to 50% of concussed patients)
- Poor sleep correlates with worse and prolonged symptoms from concussion
-Pre concussion sleep symptoms can affect assessment of concussion
*Raikes AC, Sleep Med, 2019
-Resistant to effects or short sleepers?
-Overcome decrements with increased effort or competitiveness/adrenaline?

-College athletes get < 7 hours of sleep 3.8 days/week (Turner, RW, 2019)
-39% of athletes self-reported < 7 hours of sleep (Mah CD, 2018)
-PSQI poor sleep quality in 20-50% (Swingbourne, 2016)

-50% of college athletes have an ESS >10 (Mah, 2018)
-91.5% of gymnasts < 8 hours of sleep
- Banking sleep before sleep loss
- SleepExtension has demonstrated improvement in basketball, tennis, track and field, swimming, and other sports in short studies with athletes
(Amal PJ, Med Sci Sports Exerc 2016 )








Brauer AA, Athey AB, Ross MJ, Grandner MA. Sleep and Health Among Collegiate Student Athletes.

Chest. 2019 Dec;156(6):1234-1245.



Most common sleep disorder (up to 24%)

Multifactorial (travel, stress, injuries, poor sleep hygiene)
Night before competition 60% report insomnia (Juliff, 2015)
Treatments similar (CBTI or hypnotics)- avoid use of recreational drugs and etoh to treat

8% of college football players (Dobrosielski, 2016)
27%% in NFL players confirmed with sleep study (14/52 subjects) (George, 2003)
More in lineman and linebackers

• Snoring bothering roommate
• Falling asleep in meetings
• Increased cardiovascular risk
• Prolonged recovery
• Higher perioperative risks
OSAisNOTroutinelyscreenedinthispopulation.
-Many anecdotal cases in the media in a variety of different sports
-One published study showing PAP helped with golf scores
Benton ML, Friedman NS. J Clin Sleep Med. 2013 Dec 15;9(12):1237-42.









Badminton serving (Edwards BJ, 2005)

Tennis serves (Atkinson G, 1998)
Swimming (Deschodt VJ, 2004)
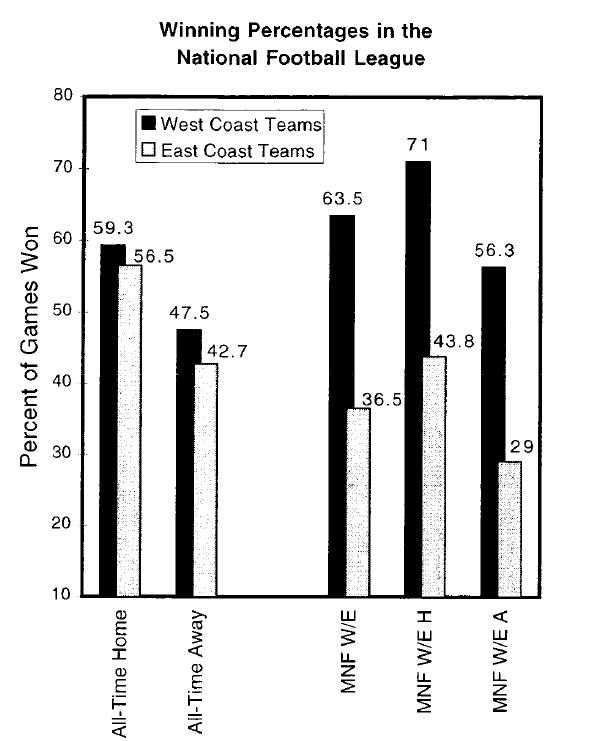
Retrospectively looked at 25 years of MNF games. They start at 9PM EST regardless of location.


West coast (WC) teams would play during their peak (late afternoon) and East coast(EC) teams would play closer to their nadir (late night).
Used the Las Vegas sports spread.
*Smith RS, Guilleminault C, Sleep 1997.
WC teams won 63.5% of the time, EC teams 36.5%

WC teams won by an avg of 14.7 pts/game versus EC teams of 9.0 pts/game
WC teams won 59.3% of their home games, and 71% of their MNF home games. EC teams won 56.5% of home games and only 43.8% MNF home games.
WC teams were winner vs point spread 67.9% of the time on MNF.




(Recht, LD, Schwartz WJ, Nature Oct 1995)
(Winter WC, et al, Int J Sports Physiol Perform, Sep 2009)
Players given MEQ and Assigned as “Morning” or “Evening” Type
Winter WC, Potenziano BJ, Zhang Z, et al. Chronotype as a predictor of performance in major league baseball batters. Sleep 2011;34:A167–8.

Athlete Sleep Behavior Questionnaire
Athlete Sleep Screening Questionnaire
Sleep Logs/Diaries and Wearables





Better tracking/surveys
Use accurate sleep trackers
Better screening for sleep issues
Required sleep education to athletes and coaches
Kroshus E, Wagner J, Wyrick D, et al. Wake up call for collegiate athlete sleep: narrative review and consensus recommendations from the NCAA Interassociation Task Force on Sleep and Wellness

British Journal of Sports Medicine 2019;53:731-736

Change culture about importance of sleep
Better screening by medical teams
More education to athletes, coaches, and other staff
Monitor for accurate trackers and minimize misinformation

-Sleep disorders and insufficient sleep can have a negative impact on athlete performance, health, and academic performance
-Athletes have a high prevalence of insufficient sleep and sleep disorders that are undiagnosed/untreated

-There are minimal studies looking at the effect of treating sleep disorders has on performance (but many anecdotal reports)
-We must increase education/awareness about sleep disorders to this population and their providers to reduce misinformation/myths



(not an extensive review of the topics)
• Restless Leg Syndrome
– Augmentation – Use of opioid medications
• Central sleep apnea
– ASV therapy
– Mask choice
• Non-24 SWD
–
Value of looking at PAP
downloads
– Treatment options
1. I do not have any relationships with any entities producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients, OR
2. I have the following relationships with entities producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients:
Type of Potential Conflict
Details of Potential Conflict
Grant/Research Support
Consultant
Speakers’ Bureaus
Financial support
Other
3. The material presented in this lecture has no relationship with any of these potential conflicts, OR
4. This talk presents material that is related to one or more of these potential conflicts, and the following objective references are provided as support for this lecture:
• 56-year-old woman with history of RLS for over 25 years
– Progressed from legs to involve the torso
– Initially symptoms present in the evenings (9-10 pm), now start at 3 pm, sometimes last all night
– Described sensation as “anxiety, desperate, crawly, perpetual irritation”
– Improve with walking and taking a shower
–
IRLS scale 33 (very severe RLS)
• Prior meds: Requip, Mirapex, Lyrica, tramadol, gabapentin, Neupro patch 12 mg, IV iron
• Current medication:
– Neupro patch 1 mg every 24 hrs (has been tapering dose on her own)
– Gabapentin 300 mg at 7 pm, 600 mg at 9 pm
– Tramadol 50-100 mg at 8 pm
• Associated symptoms
– Feels she is suffering from dopamine withdrawal symptoms (anxiety, depression, prior suicidal ideations)
– Reported snoring
– Feels tired and takes occasional naps 2-3 times a week (ESS 3)
• Sleep schedule
–
BT: 11 pm, LO same, SOL 2 hours (walks and watches TV in the living room if can’t sleep)
FNA: varies –
–
WT: 6-7 am
• PMH: HTN, hypothyroidism
• Meds: buproprion, benazepril, hydralazine, HCTZ, levothyroxine, simvastatin
• Social history
– Retired teacher, now paints at home
– No tobacco or alcohol use, occasional marijuana use, 1 coffee in the mornings
• Family history: brother has RLS
• Physical Exam
– Normal extremity exam
–
Tongue enlarged with scalloping, hard palate is high arched, soft palate is narrow, uvula is normal, tonsils not seen, Friedman class 3
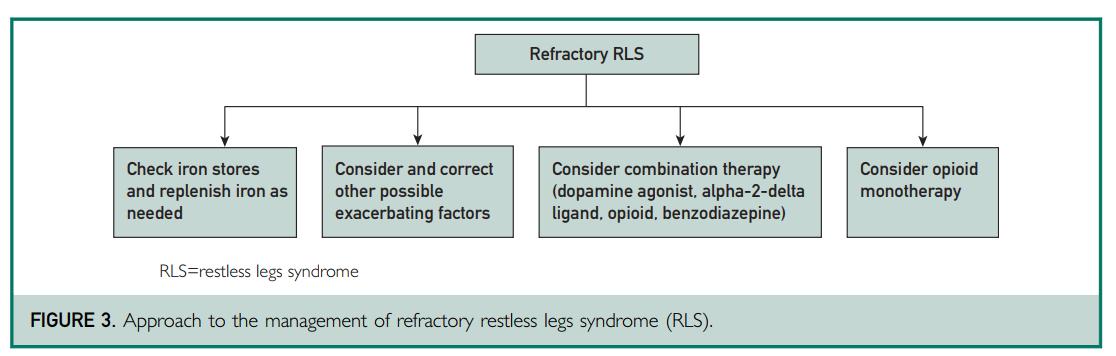
Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless leg syndrome: an updated algorithm. Mayo Clinic Proc. 2021:96(7);1921-1937.


• Rates of augmentation for pramipexole and ropinirole are 40-70% over 10-year period
• Risk of augmentation is dose-dependent
• Increased risk with iron deficiency

• RLS unresponsive to monotherapy with tolerable doses of first-line agents due to reduction in efficacy, augmentation, or adverse effects
• May consider one-time increase in dopamine agonist and splitting the dosing
• Recommendation is to discontinue dopamine agonist and substitute a drug from a different class
– Gradually taper the dopamine agonist (pramipexole 0.25 mg and ropinirole 0.5 mg every 3 days or slower)
– Allow for a drug-free holiday, or introduce the new agent with an overlap period to reduce severe symptoms and insomnia Silber, 2021
• Continue with Neupro patch 1 mg for now
• Increase gabapentin to 600 mg at 7 pm and 600 mg at 9 pm
• Repeat iron labs
– Ferritin 246, iron saturation 34
• Sleep study to evaluate for OSA
– HSAT showed moderate OSA, RI 16.2, 3 min <88%
• RLS symptoms most bothersome at 10 pm to 1-3 am
– Continue with Neupro patch 1 mg
– Continue gabapentin to 600 mg at 7 pm and 600 mg at 9 pm
– Discussion to start opioid therapy
• Tramadol not working well, experienced GI symptoms with codeine in the past, prefers a short-acting opioid
• Oxycodone 5 mg at 9 pm started
– MAPS reviewed, UDS obtained, Opioid Risk tool 1, Start Talking Form signed, side effects and safety issues reviewed
–
Encouraged to sign up for the RLS opioid registry
• Iron studies normal
• Auto CPAP 4-15 cm water ordered
•
≤ 3: low risk for abuse

•
4-7: moderate risk
•
≥8: high risk
• Assess for risk of abuse (family and personal history of abuse of other substances)
• Consider urine drug screen
• Review and sign Start Talking Form in Michigan
• Query the Michigan Automated Prescription System in Michigan

• Using auto CPAP 4-15 cm water consistently
• RLS is much improved (IRLS 1)
– Increased oxycodone to 5 mg at 7:30 pm and 5 mg at 9:45 pm
–
Tapered off gabapentin on her own
– Taking Neupro patch 1 mg
• Mood improving
• Using auto CPAP 4-15 cm water consistently but less comfortable with it
• RLS is doing alright (IRLS 8)
– Taking oxycodone to 5 mg at 6 pm and at 8 pm
–
Decreased Neupro patch to 0.5 mg
• Insomnia – BT 11 pm, SOL is short – Wakes up at 3-6 am, no ruminating thoughts, no RLS
– Referred for CBT-I
• Stopped using CPAP and returned it
• Insomnia
– Completed 3 CBT-I sessions
– BT 11:30 pm to MN, SOL is short, WASO improved to 20-30 min, WT 6-6:30 am
• RLS is doing worse (IRLS 33)
– Increased oxycodone to 10 mg at 6 pm and at 8 pm –
Weaned off Neupro patch
– Using an iron patch (but iron studies at goal)
• Given worsened RLS, discussion to change to a long-acting opioid, patient is reluctant
• Medication is not "working as well as it did a year ago, but I'm dealing with it".
• BT 11 pm, SOL 30 min, WT 6 am
• Oxycodone 5 mg at 4:30 pm, 5 mg at 6 pm, 10 mg at 10 pm
• RLS most bothersome at 4 pm
• no mood concerns, no constipation, no eds
• ->iron repeated (ferritin 232, iron saturation 30)
• ->decision to start methadone
–
EKG normal at baseline, repeat in 2 weeks
–
UDS ordered –
Start with methadone 2.5 mg once a day and decrease oxycodone to 2-3 pills a day, after 5 days may increase to methadone 5 mg and decrease oxycodone to 1-2 pills a day
• Nearly monthly electronic communication regarding tolerance and symptoms
• Increased methadone to 5 mg at 7 pm and 2.5 mg at 9 pm
• Completely weaned off Neupro patch
• Bedtime 11 pm and wake time 6 am
• Occasional night-time awakenings
• IRLS 6
• Repeat EKG was obtained once on a steady dose
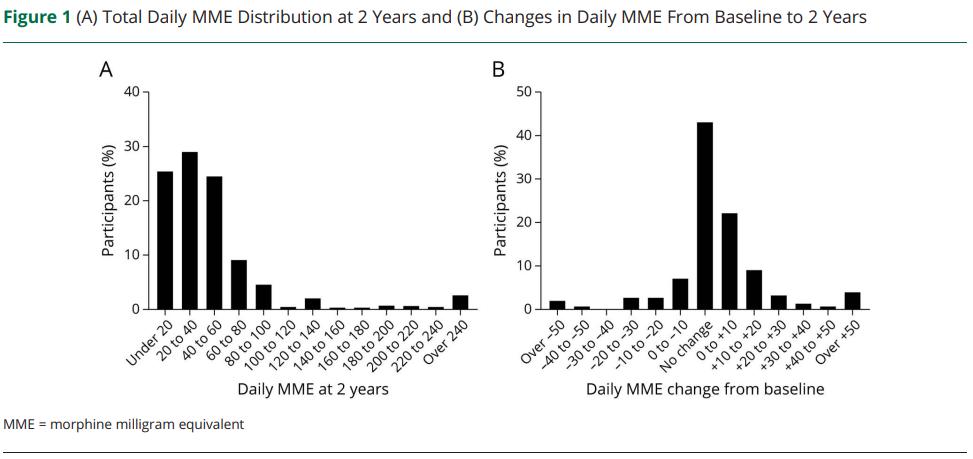
• Leiwah and Winkelman (2022) looked at 63 patients with augmented RLS on DA, followed for 5-59 months
– A2D and/or opioids were added to the existing DA, which were then slowly tapered to elimination if possible
• Iron was replaced to target ferritin >75 µg/L and iron saturation >20%
• 78% were classified as responders
• Average daily dose of A2D: 1244 mg
–
39% discontinued DA agonist with A2D alone
• When opioids were used, mean MME was 36 mg (methadone 9 mg, oxycodone 24 mg)
–
42% discontinued DA agonist with use of opioid
• 448 participants registered in the registry


• Think about augmentation before and during therapy with dopamine agonists
• Alpha 2 delta ligands are preferred first-line agents for RLS
• When augmentation happens, taper off dopamine agonists and transition to alpha 2 delta ligands if possible
• Opioid therapy is generally safe, with low risk for dose-escalation and abuse
• 72-year-old male with history of Interstitial Pulmonary Fibrosis, s/p bilateral lung transplant 2 years prior, as well as diastolic CHF
• HSAT showed severe OSA with RI of 41 (CAI 16.7) (done during COVID-19 pandemic)
• Titration recommended CPAP 9 cm water on room air with close follow up due to persistence of central events (residual AHI 29.3 with all events being central apneas and central hypopneas)
• Echo from 1 year ago - EF 63%, Pulmonary Artery Pressure 33 mmHg
• Using CPAP 9 cm water with a nasal mask
• Average use 6 hours 29 minutes, 87% nights 4+ hrs
• AHI: 33.8 (CAI 10, OAI 2.8)
• Normal leak rates
• Reports feeling more tired with CPAP, denies mouth-venting
• An attended re-titration was recommended, he ademantly declined − Pressure was increased to CPAP 11 cm water
• Download showed residual AHI elevated at 37 with most events being central
• He again was not willing to proceed with an attended titration
• ASV with empiric settings was ordered
• Repeat echocardiogram showed EF of 61%
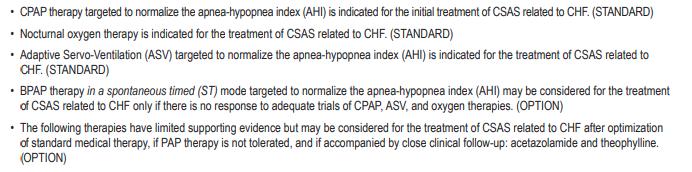
Recommendations for the 2012 AASM Guideline:
“The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”, 2016

• Population: 1325 patients with EF ≤45% and central sleep apnea (AHI ≥15 and >50% CA’s)
• Intervention: guideline-based medical treatment or medical treatment + ASV (attended titration)
– Follow up of 0-80 months, median 31 months
– ASV group had median residual AHI of 6.6 on ASV, 60% compliant (average of ≥ 3 hrs of use)
• Results:
– No difference in primary endpoint (death from any cause, life-saving CV intervention, unplanned hospitalization for worsening CHF)
• 54.1% in control, 50.8% in ASV group
– ASV group had higher rate of secondary endpoints
• All-cause mortality: 29.3% in control, 34.8% in ASV group
• CV mortality: 24% in control, 29.9% in ASV group
• No difference in hospitalization for CHF or any cause, heart transplantation, defibrillator implantation, resuscitation appropriate shock
• No difference in quality of life, 6-minute walk distance or symptoms, although ESS improved in ASV group
• Proposed mechanisms for detrimental effects of ASV
– CSA may be a compensatory mechanism (resting of respiratory muscles, attenuation of sympathetic system activity, avoidance of hypercapnic acidosis, hyperventilation-related increase in end-expiratory lung volume)
– Application of PAP may impair cardiac output and stroke volume
• Goal is to even out breathing over several breaths
– EPAP adjusts to control obstructive events
– Pressure support adjusts to control central events and periodic breathing

– Provider sets EPAP (or auto EPAP), min and max pressure support, and max pressure
– Device manipulates the pressure support (difference between IPAP and EPAP) and back-up rate

• Sleeping better with ASV compared to CPAP, nocturia is improved from 3 times a night to 1 time a night
• Reporting nasal drainage and congestion, using nasal saline but not regularly
• Using a nasal mask, does not think he mouth-vents
• Using ASV average of 7 hrs 29 min, 83% nights used 4+ hrs
• Residual AHI elevated at 22.7
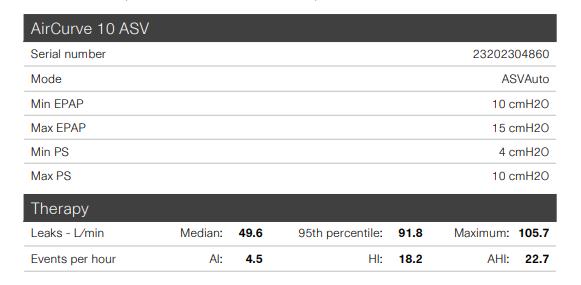

• Elevated AHI felt to be partly due to leak and mouth-venting
– A full-face mask was fitted
– Improving nasal congestion was discussed, declined prescription nasal sprays and was asked to use nasal saline twice a day
– EPAP min was decreased from 10 to 9 cm water
• Reports to be doing well, is sleeping better
• Still has some leaking from the mask but tolerates the full-face mask
• Using ASV every night for average of 7 hrs 41 min
• Residual AHI improved at 1.3


• Consider use of empiric settings for PAP therapy when an attended titration is not feasible, or patient is not willing to proceed
• ASV therapy is contraindicated in patients with CSA and CHF with reduced EF (≤45%) due to increased mortality rate
• Monitor mask leak and mask type
CC: 25-year-old male, difficulty falling and staying asleep
• Estimated BT between midnight and 3 am, SOL up to 3 hours, several night-time awakenings, WT of around 10 am. Naps 2 to 3 times a week, at random times of the day.
• Spends his days in the basement playing video games and watching television, does not go outside the house much
• Loud snoring, witnessed apneas, non-refreshing sleep and excessive daytime sleepiness (ESS 7)
PMH: Asperger’s disease, ADHD
Medications: sertraline
PE: overweight male with BMI of 32 and a crowded oral airway with a Friedman class 3
• Asked to keep sleep logs (actigraphy was not readily available)
• PSG: SE 53%, SOL 180 minutes (LO 10:10 pm), severe OSA (AHI 43) with excessive central apneas (CAI 10.3)
• Titration: CPAP 8 cm water effective
• Using CPAP every night
• More energy and more motivation
• Estimated BT 10 pm, SOL 20 minutes, WT 7-11 am, no naps
• CPAP download: CPAP use of 100%, average use of 9 hours 15 minutes, residual AHI not available
• He was not able to keep sleep logs
• Detailed download…
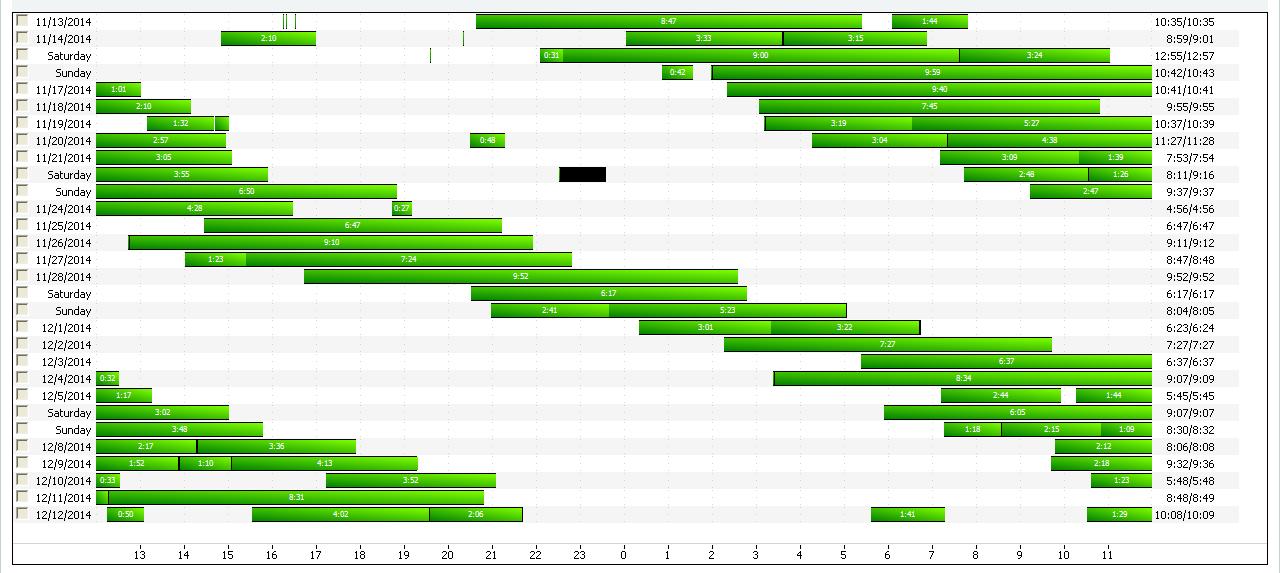

• Previously called Free-Running Type
• Occurs when the hypothalamic circadian pacemaker fails to entrain (synchronize) to the 24-hour day
Circadian rhythms of sleep propensity and alertness drift in and out of synchrony with the 24-hour day
Individuals suffer from periodic nighttime insomnia and daytime somnolence
− Daily delay depends on endogenous circadian period, may range from less than 30 minutes to more than 1 hour
• Suprachiasmatic Nucleus (SCN) in the anterior thalamus is the major circadian pacemaker of the body

• SCN controls the rhythms of sleep-wake propensity, core body temperature, and secretion of various hormones
• SCN contains cells that oscillate
• Period of this rhythm is called tau
• The mean value of tau in humans is 24.2 hrs
• External stimuli (zeitgebers) entrain the SCN to the 24-hour day
• SCN requires daily input from the environment to maintain synchrony to the 24hour day
• Light is the strongest external stimulus to entrain the SCN
• Melanopsin-containing retinal ganglion cells are the major circadian photoreceptors and communicate the presence of light to the SCN via the Retinohypothalamic tract

• Most individuals with N24SWD are totally blind
• Due to non-functional photosensitive retinal ganglion cells resulting in an absence of photoperiod information being transmitted through the retinohypothalamic pathway
• It is estimated to be present in 50% of blind individuals
• Reported in sighted individuals with a history of intellectual disability, autism spectrum disorder, dementia, or delayed sleep-wake disorder with decreased light and social exposure or who have undergone chronotherapy.
− Pathophysiology among sighted individuals is unknown, may be due to excessively long circadian period or inappropriate exposure to light
• Attempts to regulate sleep and wake times may involve the use of alcohol, sedative hypnotics and stimulants
• Depressive symptoms and mood disorders can be common
• The Task Force suggests that clinicians use strategically timed melatonin for the treatment of N24SWD in blind adults
• There is no evidence to support the use of:
− prescribed sleep-wake scheduling
− timed physical activity or exercise
− strategic avoidance of light (as monotherapy)
− use of sleep-promoting medications
− wakefulness-promoting medications
• There is insufficient evidence to support the use of:
− light therapy
− melatonin among sighted patients
− oral vitamin B12
− combination treatments
• CPAP download showed progressive delay in bedtime
• The following were recommended:
1. Prescribed sleep-wake scheduling:
• Scheduled BT of 10 pm, WT 7 am with an alarm clock
• Avoid naps, stay active during the day
2. Light therapy: seek bright light upon awakening and avoid light in the evening
3. Melatonin 1-3 mg at 5 pm
6-month follow up with use of timed light and melatonin (after 4 visits reviewing recommendations)

9-month follow up with timed light and melatonin, states
BT is 1-4 am, WT noon to 3 pm, melatonin 5 mg 1 hour before bedtime

18 month follow up: not able to maintain a regular schedule
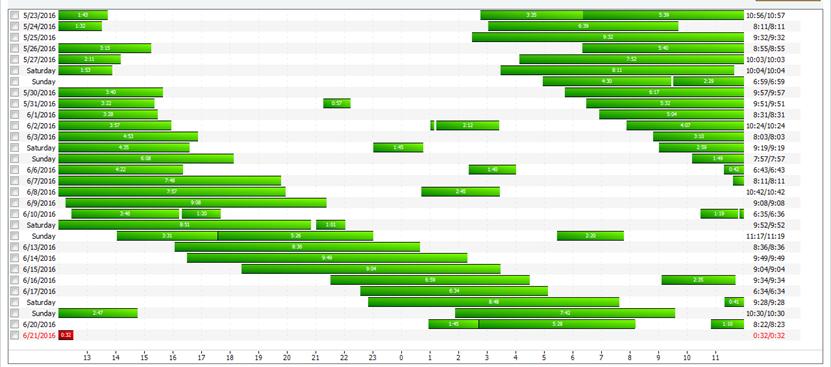
• Not able to maintain a consistent bedtime
• Depends on others for transportation, stays at home on most days
• Still doing well with CPAP
• tasimelteon ordered
− Initially denied, appeal was approved
• FDA approved for treatment of N24SWD in blind patients with N24SWD
• Has been shown to improve sleep onset and maintenance in blind patients with N24SWD
• Selective agonist at melatonin MT1 receptors (associated with sleep induction) and MT2 receptors (associated with circadian rhythm regulation)
• Dosing: 20 mg at night
• Side effects: headache, abnormal dreams, elevated LFTs, URI, UTI
• Monitoring: no routine tests recommended
• Restricted distribution though a centralized pharmacy

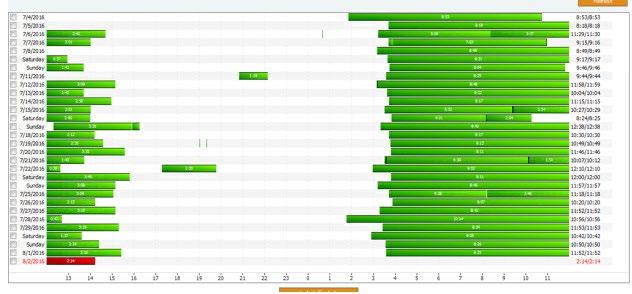
• Continues to take tasimelteon 20 mg at 10 pm
• BT 11 pm, SOL 30-60 minutes, FNA once up to 2 hrs, WT 10-11 am
• Side effects: vivid dreams
• No residual sleepiness
• Has been taking tasimelteon for 7 yrs now
− Desires to have drug-free holidays and stops intermittently with progressive delay in bedtime
• Review all the data available to you (such as raw data from PAP downloads)
• Suspect N24SWD when patients present with intermittent insomnia and excessive sleepiness, alternating with normal periods
− Seen in 50% of blind patients, may also be seen in sighted individuals
• AASM practice parameters: use melatonin in blind individuals
• tasimelteon is FDA approved in blind individuals and seems to be effective
• Laiwah JY, Winkelman JW. How effective are treatment guidelines for augmented RLS? Sleep. 2022:45(7);1-10.
• Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless leg syndrome: an updated algorithm. Mayo Clinic Proc. 2021:96(7);1921-1937.
• Winkelman JW, Wipper B, Zackon J. Long-term safety, dose stability and efficacy of opioids for patients with restless leg syndrome in the national RLS opioid registry. Neurology. 2023: 100(4): e1520-e1528.
• Aurora RN, Bista SR, Casey KR, Chowdhuri S, Kristo DA, Mallea JM, Ramar K, Rowley JA, Zak RS, Heald JL. Updated adaptive servo-ventilation recommendations for the 2012 AASM guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med 2016;12(5):757–761.
• Aurora RN; Chowdhuri S; Ramar K; Bista SR; Casey KR; Lamm CI; Kristo DA; Mallea JM; Rowley JA; Zak RS; Tracy SL. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. SLEEP 2012;35(1):17-40.
• Cowie M, Woehrle H, Wegscheider K. Adaptive servo-ventilation in systolic heart failure. NEJM 2015;373:1095-1105.
• Johnson KG, Johnson DC. Treatment of sleep-disordered breathing with positive airway pressure devices: technology update. Dove Press Journal: Medical Devices: Evidence of Research. 2015;425-437.
• ResMed VPAP Adapt SV and Adaptive Servo-Ventilation, Technology Fact Sheet.
• American Academy of Sleep Medicine. International classification of sleep disorders, 3rd ed, text revision. Darien, IL: American Academy of Sleep Medicine; 2023.
• Berry R. Fundamentals of Sleep Medicine. 2012.
• Johnsa JD; Neville MW. Tasimelteon: a melatonin receptor agonist for non-24-hour sleepwake disorder. Ann Pharmacother 2014;48:1636-1641.
• Morgenthaler TI; Lee-Chiong T; Alessi C; Friedman L; et al. Standards of Practice Committee of the AASM. Practice Parameters for the Clinical Evaluation and Treatment of Circadian Rhythm Sleep Disorders. Sleep 2007;30:1445-1459.
• Skiba V, Drake C. How the CPAP Download Unexpectedly Helped a Young Man with a Sleeping Problem. Journal of Clinical Sleep Medicine. 2015 Sep; 11(9): 1066-1068.
• Xie Y, Tang Q, Chen G. New Insights Into the Circadian Rhythm and Its Related Diseases. Frontiers in Physiology. 2019:10.

 Dr.
Dr.


▪ Establishing a diagnosis of obesity hypoventilation by clinical societies
▪ Inpatient vs outpatient management
▪ Outpatient follow up pitfalls


▪ The individual must fulfill criteria A and B: – A. One or more of the following:
▪ Cor pulmonale
▪ Pulmonary hypertension
▪ Excessive daytime somnolence that is not better explained by other factors
▪ Erythrocytosis
▪ Hypercapnia during wakefulness (PaCO2 > 45 torr)
–

B. Overnight monitoring demonstrates one or both of the following:
▪ An increase in PaCO2 during sleep > 10 torr from awake supine values
▪ Oxygen desaturation during sleep not explained by apnea or hypopnea events.
Sleep-Related Breathing Disorders in Adults: Recommendations for Syndrome Definition and Measurement Techniques in Clinical Research, Sleep, Volume 22, Issue 5, August 1999, Pages 667–689, https://doi.org/10.1093/sleep/22.5.667
– Discussed obesity as a risk factor

– Discussed normal increase in PaCO2 from wake to sleep is 2 to 7 mm Hg
– Hypoventilation = ≥ 10 mmHg
–

Either:
▪ There is an increase in the arterial PaCO2 (or surrogate) to a value > 55 mm Hg for ≥ 10 minutes
▪ There is ≥ 10 mm Hg increase in PaCO2 (or surrogate) during sleep (in comparison to an awake supine value) to a value exceeding 50 mm Hg for ≥ 10 minutes
▪ Exclusion of other causes of alveolar hypoventilation:
– Severe obstructive or restrictive pulmonary disease
– Significant kyphoscoliosis
– Severe hypothyroidism
– Neuromuscular diseases

– Other central hypoventilation syndromes
▪ 10% of patients with OHS have an AHI < 5.
▪ Introduced the idea of “grading” OHS

▪ Agreed with ATS that there is a lack of clarity in the definition
Cabrera Lacalzada C, Diaz-Lobato S. Grading obesity hypoventilation syndrome severity. Eur Respir J 2008; 32: 817–818.
1. Obesity definition: ≥ 30 kg·m−2 (adults) – ATS, ERS, and AASM




▪ Stage zero
– At risk (obesity)
▪ Stages one and two – No daytime hypercapnia

• Addition of sodium bicarbonate
• Stage one = Intermittent sleep hypercapnia and < 27 mmol·L−1 during wake
• Stage two = Intermittent sleep hypercapnia and ⩾27 mmol·L−1 during wake
▪ Stages three and four
– Daytime hypercapnia
▪ Questions raised:

– Are stages one and two completely separate diagnostic entities from stages three and four?
– Does treating intermittent hypercapnia at stages one/two prevent cardiometabolic comorbities?

▪ Reviewed sodium bicarb data predicting awake hypercapnia
– BMI > 40 kg/m2 = patient population with an OHS prevalence of 20 % – < 27 mmol/L has a very high negative predictive value (99.0%; 95% CI, 97.999.6%)
▪ High pretest probability, > 20% (ie BMI > 40)
– Go directly to measuring PaCO2
▪ Low to moderate pretest probability, < 20% ( BMI 30-40)
– If sodium bicarb < 27 mmol/L = no ABG
– If sodium bicarb ≥ 27 mmol/L = consider ABG



3. Inclusion of “sleep disordered breathing” – ATS/ERS
▪ Included – AASM
▪ Does not include


▪ All groups (ATS/ERS/AASM)
– Not primarily due to other condition:
▪ Lung/airway disease
▪ Chest wall disorder
▪ Medication
▪ Neuromuscular disease
▪ Congenital / idiopathic central alveolar ventilation
Alternative causes excluded



1. Outpatient referral for PSG / Inpatient non-acute consult for OSA/Hypercapnia
2. Inpatient acute on chronic hypercapnic respiratory failure


▪ Undiagnosed and not suspected
▪ Diagnosed and unclear etiology
▪ 45 yo, BMI 35
–
ESS = 16
– Snoring, witnessed apneas
– Sodium bicarbonate, 31 mmol/L
– Non-smoker / no pulmonary history

▪ PSG ordered by PCP

– Ok for PSG, add TCO2 monitoring, clinical correlation for ABG
▪ Guidelines: – Obesity √ – Hypercapnia ?
▪ ATS / ERS = use sodium bicarb to guide suspicion for OHS
▪ ERS = if hypercapnia not present, still consider in the future – No alternative explanations √




▪ PSG results

▪ ABG results


– By definitions, yes
–

What if this is just related to severe OSA?
▪ “unloading of carbon dioxide accumulated during longlasting complete or partial obstructive events during sleep”

▪ Non-urgent – Compensated (pH)

– Absence of recurrent hospital admissions
▪ Management strategies: Pickwick study (ATS), 2015
– Life style
– CPAP
– NIV (bilevel with assure volume, 5-6mL/kg actual weight)
Masa JF, Corral J, Alonso ML, Ordax E, Troncoso MF, Gonzalez M, Lopez-Martínez S, Marin JM, Marti S, Díaz-Cambriles T, Chiner E, Aizpuru F, Egea C; Spanish Sleep Network. Efficacy of Different Treatment Alternatives for Obesity Hypoventilation Syndrome. Pickwick Study. Am J Respir Crit Care Med. 2015 Jul 1;192(1):86-95. doi: 10.1164/rccm.201410-1900OC. PMID: 25915102.
▪ Similar improvements:
– ESS score
– Daytime hypercapnia (CPAP – compliance dependent)
– Arterial bicarbonate
– Nocturnal oxygenation

– Sleep quality
▪ Only with NIV:
– FVC
–
FEV1
–
6-MWD
– Reduction in pulmonary arterial pressure (

▪ 2019 task force (conditional):
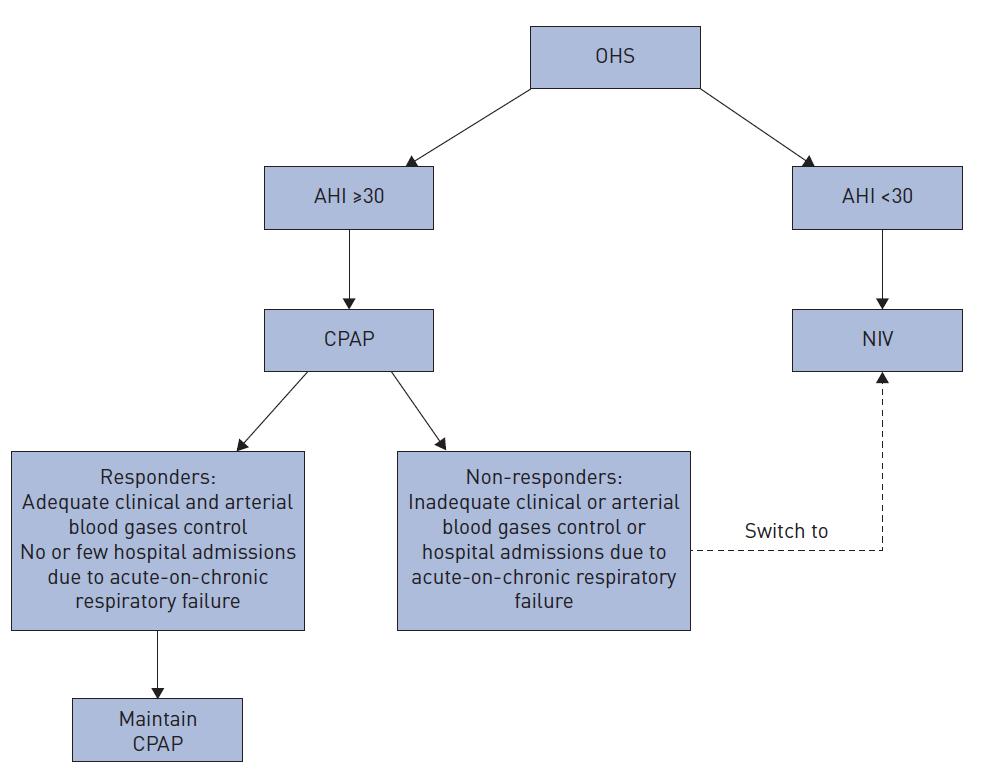
CPAP offered first line for those with AHI > 30 –
–
Weight-loss interventions (25-30 percent of body weight)

▪ ATS and ERS
– If OHS and severe OSA (AHI > 30)

▪ Ok to start with CPAP
▪ ERS – Strongly worded article stating NIV should be started first if AHI < 30
(Outpatient management)
If inadequate treatment with CPAP, transition to bilevel PAP

▪ 45 yo, BMI 42
– Admitted for altered mental status / acute on chronic hypercapnia
– Fourth admission this year
– Non-smoker, no history of asthma

–
History of alcohol dependence, sober x 3 years


–
Obesity √
– Daytime, wakeful hypercapnia √
– Other etiology ruled out ?

▪ Acute hypercapnia presentation in ER –

4 years (Liverpool Hospital in Sydney, Australia, 800,000 pt in ER per year)
– 873 persons included

▪ Hospitalized patients:
– Higher short-term mortality

▪ 11 of 61 patients with OHS admitted to ICU died during the hospitalization
– Misdiagnosed?
▪ 46 patients had been diagnosed with COPD/asthma with subsequent or prior PFTs nondiagnostic of obstructive lung disease
– Coined term “malignant OHS” = BMI > 40 kg/m2, metabolic syndrome, and multiorgan system dysfunction related to obesity
Marik PE, Desai H. Characteristics of Patients With the “Malignant Obesity Hypoventilation Syndrome” Admitted to an ICU. Journal of Intensive Care Medicine. 2013;28(2):124-130.
doi:10.1177/0885066612444261
▪ 50 patients in Sfax, Tunisia over 7 years (2,000 pt admitted to hospital per year)
– CPAP vs bilevel PAP = no difference in readmission rates at 3 months

▪ No prospective RCT data1
▪ Treat underlying triggers (CHF, pneumonia, etc) 1
▪ Observational data = similar short- and long-term outcomes treated with NIV to that of COPD exacerbation requiring NIV2
▪ If decompensated or high risk*, should be in an environment with access to endotracheal intubation1
1. Masa JF, Pépin J-L, Borel J-C, et al. Obesity hypoventilation syndrome. Eur Respir Rev 2019; 28: 180097 [https://doi.org/10.1183/16000617.0097-2018]

*poor outpatient compliance, BMI > 50, or multi-organ failure
2. Carrillo A, Ferrer M, Gonzalez-Diaz G, et al. Noninvasive ventilation in acute hypercapnic respiratory failure caused by obesity hypoventilation syndrome and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 1279–1285
▪ Access to NIV should be available within 1 hour of presentation to ER
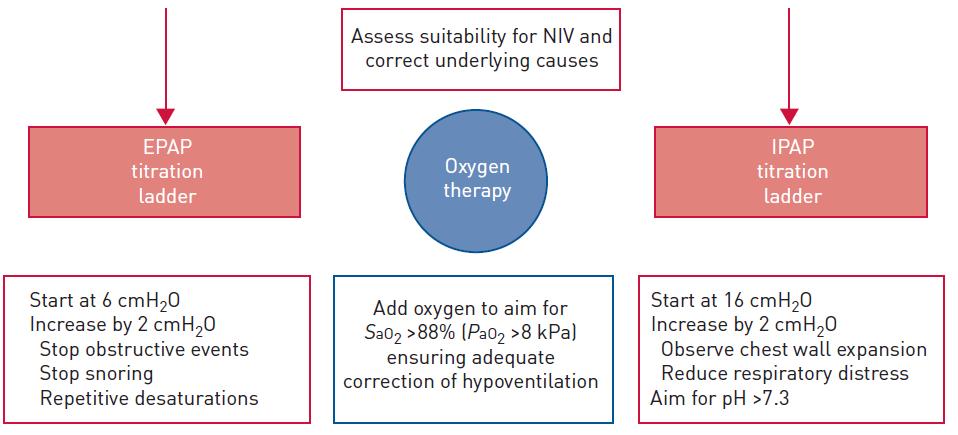
▪ Specifically trained operators (mask fit)
▪ Strategy to titrate
▪ Outpatient follow up
– Re-eval modality if on PAP
– Rapid access to PAP if not using

▪ Reviewed 10 studies
▪ *did not specify the decisionmaking process to discharge with or without
▪ 1162 pts *
– 119 pt discharged without PAP therapy
▪ At 3 months = 20/119 pts had died (16%)
– 1043 pt with PAP therapy

▪ At 3 months = 24/1043 pts had died (2%)
Mokhlesi B, Masa JF, Brozek JL, Gurubhagavatula I, Murphy PB, Piper AJ, Tulaimat A, Afshar M, Balachandran JS, Dweik RA, Grunstein RR, Hart N, Kaw R, Lorenzi-Filho G, Pamidi S, Patel BK, Patil SP, Pépin JL, Soghier I, Tamae Kakazu M, Teodorescu M. Evaluation and Management of Obesity Hypoventilation Syndrome. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2019 Aug 1;200(3):e6-e24. doi: 10.1164/rccm.201905-1071ST. Erratum in: Am J Respir Crit Care Med. 2019 Nov 15;200(10):1326. PMID: 31368798; PMCID: PMC6680300.
▪ Needed information = cost benefit analysis

▪ Noted = Significant effort is required to discharge with PAP
▪ Majority of patients were discharged on NIV (90%) as opposed to CPAP


– Start on NIV prior to discharge (if not available, auto CPAP is preferable to no PAP)
– Continue on NIV (with similar settings) until outpatient work up
– Ideally this should occur within 3 months



▪ No formal recommendations for in-patient management
▪ Sporadic recommendations from JCSM for hypoventilation but not specifically OHS

▪ Bariatric surgery
– OHS in bariatric surgery population (> 60%), compared to general population (0.3 to 0.6%)
– Cleveland, Ohio (7 years)

▪ Used alternative to ABG = PSG-based end-tidal awake CO2 ≥ 45 mmHg or daytime serum bicarb ≥ 27 (looking to include early stages)
Chindamporn P, Wang L, Bena J, et al. Obesity-associated sleep hypoventilation and increased adverse postoperative bariatric surgery outcomes in a large clinical retrospective cohort. J Clin Sleep Med. 2022;18(12):2793–2801
▪ No significant difference:
– Mortality (insufficient power to detect a significant difference)

▪ Increased rate of but not statistically signifcant:
– Re-intubation
– ICU admission
– 30-day re-admission
Chindamporn P, Wang L, Bena J, et al. Obesity-associated sleep hypoventilation and increased adverse postoperative bariatric surgery outcomes in a large clinical retrospective cohort. J Clin Sleep Med. 2022;18(12):2793–2801
▪ No specific recommendations for OHS
▪ Those with OSA and obesity (≥ 35 kg/m2) and intolerant/unaccepting of PAP therapy – Referral to sleep and bariatric surgeons



▪ Vast majority did not assess for OHS or excluded OHS in safety data –
Answer = 2022 study (previously discussed
▪ Suggest interventions that produced sustained weight loss (25-30%)
–
Very low level of certainty
• Lack of data for specific benefits for patients with OHS

• Extrapolation from general obesity data would support









▪ E0470 = – RESPIRATORY ASSIST DEVICE, BI-LEVEL PRESSURE CAPABILITY, WITHOUT BACKUP RATE FEATURE, USED WITH NONINVASIVE INTERFACE
▪ E0471 = – RESPIRATORY ASSIST DEVICE, BI-LEVEL PRESSURE CAPABILITY, WITH BACKUP RATE FEATURE, USED WITH NONINVASIVE INTERFACE


▪ E0470: BOTH –
ABG
▪ Awake, on beneficiary’s prescribed FiO2

▪ > 45 mmHg – Spirometry
▪ FEV1 / FVC ≥ 70%

▪ EITHER:
– ABG PaCO2 during sleep (or immediately upon wakening) on prescribed FiO2, shows PaCO2 worsened ≥ 7 mmHg compared to original result
– Facility based PSG or HST demonstrates:
▪ Oxygen saturation ≤ 88% for ≥ 5 min
▪ Not caused by obstructive events (ie AHI < 5)
▪ Both:
– Currently E0471 is being used

▪ Either:
– ABG:
AND
– Spirometry
▪ FEV1 / FVC ≥ 70%
▪ Awake and on prescribed FiO2
▪ PaCO2 worsens and ≥ 7 mmHg compared to qualifying PaCO2
– Facility based PSG or HST demonstrates:
▪ Oxygen saturation ≤ 88% for ≥ 5 min
▪ Not caused by obstructive events (ie AHI < 5) WHILE using E0470 device

▪ Evaluation period:
–
61-90 days after starting
–
Average of 4 hours per 24 hour period
– Documentation of using and benefiting
▪ Documentation of AHI < 5 in a titration
– Short periods of time on pressures

▪ Affording co-pays
▪ Recalls, availability

▪ Establishing a diagnosis of obesity hypoventilation by clinical societies
▪ Inpatient vs outpatient management
▪ Outpatient follow up pitfalls


No
conflicts of interest to disclose.























At the time of this presentation, Dr. Shelgikar is a board member of the American Academy of Sleep Medicine (AASM) and AASM Foundation. Dr. Shelgikar created this presentation in her personal capacity. The views expressed are her own and do not necessarily represent the views of the AASM or AASM Foundation.
In this presentation, all unattributed images are available under a Creative Commons license.
•39-year-old man diagnosed with narcolepsy
type 1 during adolescence
• Symptomatically well-controlled with
◦ Modafinil 200 mg daily
◦ Methylphenidate 15 mg four times daily
At the end of the visit he asks, “Just out of curiosity, are there any new treatments for narcolepsy?”

Understand
Understand components of the GRADE system used to inform clinical practice guideline development

Identify
Identify treatments noted in 2021 clinical practice guideline

•Adult patients with narcolepsy
•Adult patients with idiopathic hypersomnia
•Adult patients with Kleine-Levin syndrome
•Adult patients with hypersomnia due to medical conditions

• Hypersomnia secondary to alpha-synucleinopathies
• Posttraumatic hypersomnia
• Adult patients with genetic disorders associated with primary central nervous system somnolence
• Adult patients with hypersomnia secondary to brain tumors, infections, or other central nervous system lesions
•Pediatric patients with narcolepsy
How were these clinical practice guidelines developed?

A “transparent framework for developing and presenting summaries of evidence and provides a systematic approach for making clinical practice recommendations.”
Patient population
Adult and pediatric patients diagnosed with NT1 or NT2
Adult and pediatric patients diagnosed with IH
Adult and pediatric patients diagnosed with KLS
Adult and pediatric patients diagnosed with hypersomnia due to a medical disorder, including neurological disorders
Adult and pediatric patients diagnosed with hypersomnia associated with a psychiatric disorder
Intervention
Pharmacological therapy, behavioral therapy, immunotherapy
Pharmacological therapy, behavioral therapy, immunotherapy
Pharmacological therapy, behavioral therapy
Pharmacological therapy, behavioral therapy, immunotherapy
Comparison Placebo, standard therapy, no treatment
Placebo, standard therapy, no treatment
Placebo, standard therapy, no treatment
Placebo, standard therapy, no treatment
Outcome
Excessive daytime sleepiness, cataplexy, disease severity, quality of life, accidents/accident risk, work/school performance/ attendance, fatigue, sleep quality
Excessive daytime sleepiness, disease severity, quality of life, work/school performance/ attendance, cognitive performance, fatigue, sleep inertia
Disease severity, quality of life, work/school performance/ attendance, fatigue, mood
Excessive daytime sleepiness, quality of life, work/school performance/ attendance, difficulty waking in the morning, fatigue
credit: Vishesh Kapur, MD, MPH
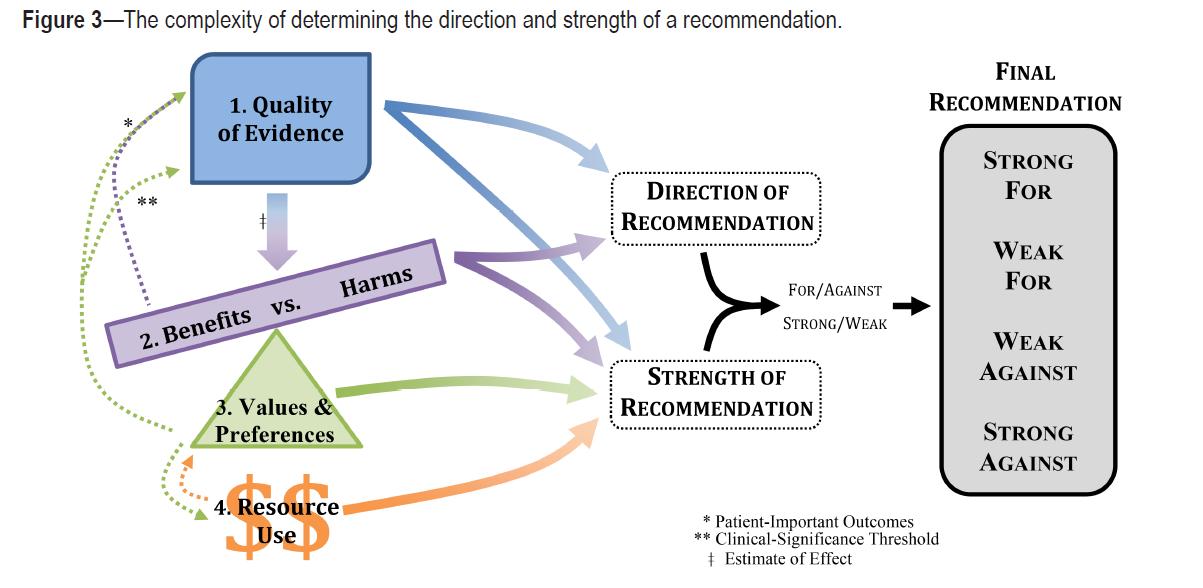
 Slide credit: Vishesh Kapur, MD, MPH Morganthaler et al. , JCSM 2016
Slide credit: Vishesh Kapur, MD, MPH Morganthaler et al. , JCSM 2016
“Based on the clinical expertise of the TF [task force] members and any data published on the topic relevant to patient preferences, the TF determined if patient values and preferences would be generally consistent across the majority of patients, and if patients would use the intervention based on the relative harms and benefits identified.”
•The clinical practice recommendations are based on a systematic review and evaluation of evidence using the GRADE process.
•The recommendations reflect only those interventions for which there was sufficient evidence to make a recommendation.
•The absence of inclusion of certain interventions in this clinical practice guideline should not be interpreted as a statement against their clinical use.
•Interventions for which literature was reviewed but it was determined that insufficient evidence existed to make recommendations are discussed in the systematic review.
•“Insufficient evidence” to determine the effectiveness of a particular intervention does not mean that the intervention does not work, but that evidence is lacking to guide decisionmaking. Additional research is needed to determine the effectiveness of the intervention.

• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates

Recommended for 7 conditions
• 2 with strong for
• 5 with conditional for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Population for which the treatment is recommended
Adult patients with narcolepsy
Adult patients with idiopathic hypersomnia*
Adult patients with hypersomnia secondary to Parkinson’s disease*
Adult patients with hypersomnia secondary to traumatic brain injury*
Adult patients with hypersomnia secondary to myotonic dystrophy*
Adult patients with hypersomnia secondary to multiple sclerosis*
Pediatric patients with narcolepsy
Strength of recommendation
Strong
Strong
Conditional
Conditional
Conditional
Conditional
Conditional
*Indicates off-label use
For which population is modafinil listed as a strong recommendation?

A. Pediatric patients with narcolepsy
B. Adult patients with idiopathic hypersomnia
C. Adult patients with Kleine-Levin Syndrome
D. Adult patients with hypersomnia secondary to multiple sclerosis
• Exact mechanism of action unclear.
• Significantly increases brain dopamine levels via blocking dopamine transporters
• Lower affinity for dopamine receptors compared to amphetamines
• Decreased GABA-mediated neurotransmission via (1) increased turnover of serotonin and (2) enhanced 5-HT2 receptor activity
• An intact central alpha-adrenergic system is required for modafinil's activity
• Hepatic
• Multiple pathways including CYP3A4
• Half-life elimination: Effective half-life = 15 hours
• Time to peak, serum: 2 to 4 hours, may be delayed ~1 hour with food.
• Excretion: Urine (80% as metabolites, <10% as unchanged drug); feces (1%)
• Altered kidney function: In severe, chronic renal failure (CrCl ≤20 mL/minute), exposure to modafinil acid (inactive metabolite) was increased 9-fold.
• Hepatic function impairment:
• In patients with cirrhosis of the liver, clearance is decreased ~60% and steady-state concentrations are doubled.
• Older adults:
• Oral clearance decreased ~20% in patients with a mean age of 63 years of age.
Adults
• Initial dose: 200 mg
• Narcolepsy: Doses up to 400 mg once daily have been well tolerated; no consistent evidence that this dose confers additional benefit
Hepatic impairment dosing
• Mild - moderate hepatic impairment: No adjustments
• Severe hepatic impairment: Reduce dose to ½ that recommended for patients with normal liver function
• IH: May increase dose up to 200 mg twice daily (eg , morning and midday) or 400 mg as a single daily dose in the morning; however, evidence is limited for doses >200 mg/day
Pediatrics
• Children ≥6 years and Adolescents ≤13 years:*
• Patient weight <30 kg: 200 to 340 mg once daily
*From ADHD literature (off-label use)
• Patient weight >30 kg: 300 to 425 mg once daily
• Other dosing recommendations are from adult literature
Tablets (Modafinil Oral)
• 100 mg (per each): $11.19 - $26.40
• 200 mg (per each): $16.93 - $39.94
Tablets (Provigil Oral)
• 100 mg (per each): $56.73
• 200 mg (per each): $85.72
• FDA Schedule IV federally controlled substance because of potential for abuse or dependency.

• Based on animal data, may cause fetal harm. Human data are insufficient to determine risk.
• A 2018 annual report of the ongoing armodafinil/modafinil pregnancy registry in the United States showed a higher rate of major congenital anomalies, and other adverse reactions, in children exposed to the drug in utero.
• May reduce the effectiveness of oral contraception.
Recommended for 2 conditions
• 1 with strong for
• 1 with conditional for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Population for which the treatment is recommended
Adult patients with narcolepsy
Adult patients with idiopathic hypersomnia*
Strength of recommendation
Strong
Conditional
*Indicates off-label use

What EKG finding can pitolisant cause?
A. Sinus tachycardia
B. First-degree atrioventricular block
C. QT prolongation
D. Premature ventricular contractions
• Exact mechanism unclear
• May be mediated through activity as an antagonist/inverse agonist at histamine-3 (H3) receptors
• No appreciable binding to other histamine receptors (H1, H2, H4)
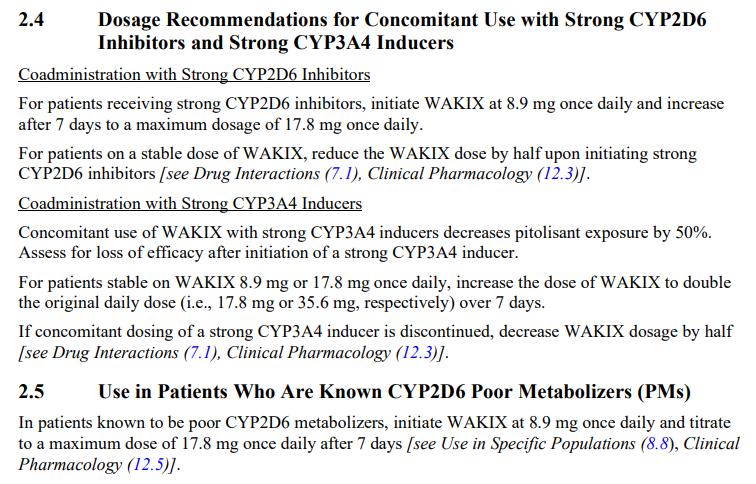
• Metabolized by CYP2D6 and to a lesser extent by CYP3A4 to inactive metabolites
• Half-life elimination: ~20 hours (7.5 to 24.2 hours)
• Time to peak: 3.5 hours (2 to 5 hours)
• Excretion: Urine (~90% , with <2% as unchanged drug); feces (2.3%)

• 4.45 mg
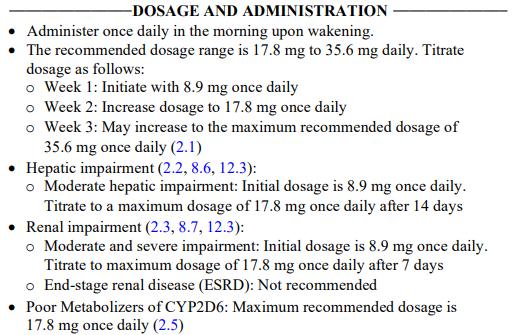
• 17.8 mg
Adjusted dosing
• Hepatic impairment:
• Contraindicated in severe hepatic impairment (Child-Pugh class C).
• Renal impairment:
• Not recommended in patients with end-stage renal disease (eGFR <15 mL/minute/1.73 m2).

Pricing (United States)
Tablets (Wakix Oral)
• 4.45 mg (per each): $145.63
• 17.8 mg (per each): $291.25
CPG
• Based on animal data, may cause fetal harm. Human data are insufficient to determine risk.
• May reduce the effectiveness of oral contraception.

Manufacturer
• “QT Interval Prolongation: Increases in QT interval. Avoid use with drugs that also increase the QT interval and in patients with risk factors for prolonged QT interval. Monitor patients with hepatic or renal impairment for increased QTc.”
Recommended for 4 conditions
• 1 with strong for
• 3 with conditional for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Population for which the treatment is recommended
Adult patients with narcolepsy
Adult patients with idiopathic hypersomnia*
Adult patients with hypersomnia secondary to Parkinson’s disease*
Pediatric patients with narcolepsy
Strength of recommendation
Strong
Conditional
Conditional
Conditional
*Indicates off-label use
Med. 2021;17(9):1881–1893.
What is the maximum nightly dose for sodium oxybate?

A. 6 g
B. 6 mg
C. 9 g
D. 9 mg
• Active moiety of sodium oxybate is gamma-hydroxybutyrate (GHB).
• GHB is a metabolite of gamma aminobutyric acid (GABA)
• Therapeutic effects hypothesized to be mediated by GABA-B receptor activity at
• noradrenergic,
• dopaminergic,
• thalamocortical neurons.
*Be mindful of high sodium content.
• Primarily via the Krebs cycle to form water and carbon dioxide
• Secondarily via beta oxidation
• Significant first-pass effect; no active metabolites; metabolic pathways are saturable.
• Half-life elimination: 30-60 minutes
• Time to peak:
• Extended release: 90 minutes
• Immediate release: 30 to 75 minutes.3.5 hours (2 to 5 hours)
• Excretion: Primarily pulmonary (as carbon dioxide)
• Urine (<5% as unchanged drug); feces (negligible).
ER suspension (Lumryz):
• 4.5 g as a single dose at bedtime. May increase nightly dose by 1.5 g at weekly intervals based on efficacy and tolerability.
• Recommended dosage range: 6 to 9 g once daily at night.
Administration
• Administer on an empty stomach, ≥2 hours after eating.
• Administer while patient is in bed; patient should lie down immediately after dose and remain in bed.
• Maximum dose: 9 g/night.
• Administer within 30 minutes of mixing
IR solution (Xyrem):
• Prepare both doses prior to bedtime
• Initial: 2.25 g at bedtime after the patient is in bed, and 2.25 g administered 2.5 to 4 hours later (4.5 g/night). May increase nightly dose by 1.5 g (0.75 g at bedtime and 0.75 g administered 2.5 to 4 hours later) at weekly intervals based on efficacy and tolerability
• Usual effective dosage range: 6 to 9 g per night.
• Maximum dose: 9 g/night.
Administration
• Administer on an empty stomach (at least 2 hours after eating); administration at similar times each night is preferred.
• Each nightly dose should be administered while patient is sitting up in bed; then patient should lie down and remain in bed; sleep generally occurs within 5 to 15 minutes (generally abruptly without patient feeling drowsy).
• Both doses should be prepared prior to bedtime. An alarm clock may need to be set for the second dose.
• For patients who sleep >8 hours per night the first dose may be administered at bedtime or after an initial period of sleep. After use, rinse containers with water.
IR solution (Xyrem): Dosing is in mg/kg and grams; use precaution. Children ≥7 years and Adolescents
• <20 kg: Limited data available: Initial: 60 to 90 mg/kg/night (total dose) in 2 divided doses; the first dose administered at bedtime after the patient is in bed, then 2.5 to 4 hours later, administer the second dose; titrate every 1 to 2 weeks until efficacy or development of intolerable side effects. Reported maximum daily dose: 180 mg/kg/night.
• 20 to <30 kg: Initial: ≤1 g at bedtime after the patient is in bed, then 2.5 to 4 hours later, administer second dose of ≤1 g. After 1 week, titrate dose based on efficacy and tolerability at weekly intervals; increase dose in ≤0.5 g/dose (≤1 g/night) increments per week; maximum single dose: 3 g/dose; maximum daily dose: 6 g/night.
• 30 to <45 kg: Initial: ≤1.5 g at bedtime after the patient is in bed, then 2.5 to 4 hours later, administer second dose of ≤1.5 g. After 1 week, titrate dose based on efficacy and tolerability at weekly intervals; increase dose in ≤0.5 g/dose (≤1 g/night) increments per week; maximum single dose: 3.75 g/dose; maximum daily dose: 7.5 g/ night.
• ≥45 kg: Initial: ≤2.25 g at bedtime after the patient is in bed, then 2.5 to 4 hours later, administer second dose of ≤2.25 g . After 1 week, titrate dose based on efficacy and tolerability at weekly intervals; increase dose in ≤0.75 g/dose (1.5 g/night) increments per week; maximum single dose: 4.5 g/dose; maximum daily dose: 9 g/night.
Adjusted dosing - adults
• Hepatic impairment:
• ER suspension (Lumryz):
• Do not use as initial therapy in patients with hepatic impairment.
• Patients who have been titrated to a maintenance dose on IR solution may be switched to the ER suspension if the appropriate dosage strength is available.
• IR solution (Xyrem): Reduce initial dose(s) by 50%
• Renal impairment:
• No adjustments.
Adjusted dosing - peds
• Hepatic impairment:
• IR solution (Xyrem):
• Children ≥7 years and Adolescents: Reduce initial total nightly dose by 50% and administer in 2 divided doses.
• Renal impairment:
• No adjustments.
Missed second dose:
• Skip dose and resume usual dosing schedule the next day.
• Do not administer 2 doses at the same time.
Pricing (United States)
Pack (Lumryz Oral)
• 4.5 g (per each): $349.20
• 6 g (per each): $465.60
• 7.5 g (per each): $582.00
• 9 g (per each): $698.40
Solution (Sodium Oxybate Oral)
• 500 mg/mL (per mL): $40.13
Solution (Xyrem Oral)
• 500 mg/mL (per mL): $42.13
• FDA Schedule III federally controlled substance
• Is the sodium salt of gamma hydroxybutyrate (GHB), a Schedule I controlled substance

• FDA black box warning - central nervous system depressant and may cause respiratory depression
• Abuse or misuse of illicit GHB is associated with seizures, respiratory depression, decreased consciousness, coma, and death especially if used in combination with other central nervous system depressants, such as alcohol and sedating medications.
• Based on animal data, may cause fetal harm. Human data are insufficient to determine risk.
Recommended for 1 conditions
• 1 with strong for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Adult patients with narcolepsy
Strong

Solriamfetol is not recommended for use in which clinical context?
A. Severe hepatic impairment
B. End stage renal disease
C. End stage glaucoma
D. Severe restrictive lung disease
• Exact mechanism of action unclear.
• Efficacy may be related to activity as a selective dopamine and norepinephrine reuptake inhibitor (DNRI).
• Unlike traditional stimulants, it does not promote monoamine release.
• Minimal metabolism
• Half-life elimination: ~7.1 hours*
• Time to peak:
• 2 hours (fasting); delayed an additional 1 hour with high-fat meal
• Excretion: Urine (95% as unchanged drug)
*Altered kidney function: Half-life prolonged
• 1.2-fold (eGFR 60 to 89 mL/minute/1.73 m2),
• 1.9-fold (eGFR 30 to 59 mL/minute/1.73 m2), and
• 3.9-fold (eGFR <30 mL/minute/1.73 m2) in patients with renal impairment.
AUC and half-life increased in patients with ESRD. An average of 21% of solriamfetol is removed by hemodialysis.

Tablet, Oral, as hydrochloride:

• Sunosi: 75 mg [scored]
Contraindication:
Concurrent treatment with a monoamine oxidase inhibitor (MAOI) or use of an MAOI within the preceding 14 days.
• Sunosi: 150 mg
Warnings and precautions:

Pricing (United States)
Tablets (Sunosi Oral)
• 75 mg (per each): $32.92
• 150 mg (per each): $32.92

• FDA Schedule IV federally controlled substance because of potential for abuse or dependency.
• Based on animal data, may cause fetal harm. Human data are insufficient to determine risk.
Recommended for 3 conditions
• 3 with conditional for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Population for which the treatment is recommended
Adult patients with narcolepsy
Adult patients with hypersomnia secondary to dementia with Lewy bodies*
Adult patients with hypersomnia secondary to traumatic brain injury*
Strength of recommendation
Conditional
Conditional
Conditional
*Indicates off-label use
Which side effect has been reported with use of armodafinil?

A. Drug reaction with eosinophilia and system symptoms (DRESS)
B. Eosinophilic esophagitis (EoE)
C. Hypereosinophilic syndrome (HES)
D. Eosinophilic enteritis (EoN)
• Exact mechanism of action unclear.
• R-enantiomer of modafinil
• Binds to the dopamine transporter and inhibits dopamine reuptake, which may result in increased extracellular dopamine levels in the brain
• Does not appear to be a dopamine receptor agonist
• Does not appear to bind to or inhibit the most common receptors or enzymes that are relevant for sleep/wake regulation.
• Hepatic
• Hepatic, multiple pathways, including amine hydrolysis and CYP3A4/5
• Metabolites include R-modafinil acid and modafinil sulfone
• Half-life elimination: ~15 hours
• Time to peak, serum: 2 hours (fasted)
• Excretion: Urine (based on modafinil)
• 80% predominantly as metabolites
• <10% as unchanged drug
Hepatic function impairment
• In patients with severe hepatic impairment, clearance of modafinil was decreased by ~60%
• Steady-state concentration was doubled.
Older adults:
• Systemic exposure of armodafinil was ~15% higher and clearance was ~12% lower in patients > 65 years of age.
Adults
Not recommended in patients with a history of left ventricular hypertrophy or patients with mitral valve prolapse who have developed mitral valve prolapse syndrome with previous CNS stimulant use.*
• Nuvigil: 50 mg, 150 mg, 200 mg, 250 mg
• Generic: 50 mg, 150 mg, 200 mg, 250 mg
Serious and life-threatening rashes including Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.*
Hepatic impairment dosing
• Mild - moderate hepatic impairment: No adjustments
Rare cases of drug reaction with eosinophilia and system symptoms (DRESS), also known as multiorgan hypersensitivity, and cases of angioedema and anaphylactoid reactions have been reported.*
• Severe hepatic impairment: Reduce dose due to decreased clearance and increased steady-state concentration of modafinil in this patient population.
*Also applies to modafinil.
Tablets (Armodafinil Oral)
• 50 mg (per each): $7.26 - $7.27
• 150 mg (per each): $21.86 - $21.89
• 200 mg (per each): $21.89
• 250 mg (per each): $21.86 - $21.89
Tablets (Provigil Oral)
• 50 mg (per each): $13.47
• 150 mg (per each): $40.54
• 200 mg (per each): $40.54
• 250 mg (per each): $40.54

• FDA Schedule IV federally controlled substance because of potential for abuse or dependency.
• Based on animal data, may cause fetal harm. Human data are insufficient to determine risk.
• A 2018 annual report of the ongoing armodafinil/modafinil pregnancy registry in the United States showed a higher rate of major congenital anomalies, and other adverse reactions, in children exposed to the drug in utero.
• May reduce the effectiveness of oral contraception.
Recommended for 1 condition
• 1 with conditional for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Population for which the treatment is recommended
Adult patients with narcolepsy
Strength of recommendation
Conditional
Presumed mechanism of action
• Promotes release of catecholamines (primarily dopamine and norepinephrine) from their storage sites in the presynaptic nerve terminals.
• Blocks reuptake of catecholamines by competitive inhibition.
Metabolism
Dosing
Pricing (United States)
CPG remark
Hepatic to some degree by CYP2D6
5 - 60 mg orally daily in divided doses
$0.47 - $28.13 per unit, depending on preparation
• Black box warning stating high potential for abuse, and prolonged administration may lead to dependence.
• Based on animal data, dextroamphetamine may cause fetal harm. Human data are insufficient to determine risk.
Recommended for 2 conditions
• 2 with conditional for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Population for which the treatment is recommended
Adult patients with narcolepsy
Adult patients with idiopathic hypersomnia*
Strength of recommendation
Conditional
Conditional
*Indicates off-label use
Presumed mechanism of action
• Blocks the reuptake of norepinephrine and dopamine into presynaptic neurons
• Appears to stimulate the cerebral cortex and subcortical structures similar to amphetamines
Metabolism De-esterification by carboxylesterase CES1A1 to alpha-phenyl-piperidine acetic acid (PPAA; ritalinic acid) which has little to no pharmacologic activity.
Dosing 5-60 mg daily, usually in divided doses
Average retail cost $0.17 - $27.62 per unit, depending on preparation
CPG remark
• Black box warning stating that it should be given cautiously to patients with a history of drug dependence or alcoholism.
• Based on animal data, methylphenidate may cause fetal harm. Human data are insufficient to determine risk.
Recommended for 1 conditions
• 1 with conditional for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Population for which the treatment is recommended
Adult patients with idiopathic hypersomnia*
Strength of recommendation
Conditional
*Indicates off-label use
Presumed mechanism of action
• Binds to the 50S ribosomal subunit of the 70S ribosome of susceptible organisms
• Negative allosteric modulators of GABA-A receptors
Metabolism
• Partially hepatic via CYP3A4; converted to 14-OH clarithromycin (active metabolite)
• Undergoes extensive first-pass metabolism
Dosing 500 mg twice daily (with breakfast and lunch)
Average retail cost $1.47 - $8.95 per unit, depending on preparation
CPG remark
• FDA alert advising caution in individuals with heart disease (potential for increased risk of cardiac events and death in people with a history of myocardial infarction or angina)
• Risks associated with antibiotic use (e.g., antibiotic resistance, superinfection) should be weighed when considering the use of clarithromycin for patients with IH.
• Based on animal data, may cause fetal harm. Labeling states clarithromycin should not be used by pregnant women.
Recommended for 1 conditions
• 1 with conditional for
• Modafinil
• Pitolisant
• Sodium oxybate
• Solriamfetol
• Armodafinil
• Dextroamphetamine
• Methylphenidate
• Clarithromycin
• Lithium
(Not included in the CPG)
• Calcium, magnesium, potassium and sodium oxybates
Population for which the treatment is recommended
Adult patients with Kleine-Levin syndrome*
Strength of recommendation
Conditional
*Indicates off-label use
Presumed mechanism of action
• Uncertain in KLS and bipolar disorder
• Increases glutamate clearance (may have neuroprotective effect)
• May influence the reuptake of serotonin and/or norepinephrine, and inhibit second messenger systems involving the phosphatidylinositol cycle.
Metabolism
Not metabolized; excreted in the urine unchanged
Dosing Usually starts at 300 mg given 2 or 3 times a day.
Average retail cost $0.14 - $15.08 per unit, depending on preparation
CPG remark
• Black box warning stating that lithium toxicity is closely related to serum lithium concentrations and can occur at doses close to therapeutic concentrations.
• Accessibility of facilities to conduct prompt and accurate serum lithium determinations should be determined before initiating therapy.
• Based on animal studies, lithium may cause fetal harm. Human studies suggest fetal harm but are insufficient to determine risk.
•Not included in the 2021 AASM clinical practice guideline
•The studies were not published by the time the task force did the final literature review and finalized recommendations
•FDA approved for
• Treatment of cataplexy or excessive daytime sleepiness in patients 7 years and older with narcolepsy
• Adults with idiopathic hypersomnia
Presumed mechanism of action
• Unsure, may be mediated through gamma-aminobutyric acid (GABA)-B actions at the noradrenergic, dopaminergic, and thalamocortical neurons
Metabolism Hepatic; elimination is via Krebs cycle metabolism (byproducts eliminated through expiration)
Dosing
Average retail cost
• 4.5-9 g nightly, given orally in 2 divided doses (hepatic dosing) – narcolepsy
• Once nightly ≤3-6 g per night – idiopathic hypersomnia
• $38.80 for 500 mg/mL (per mL)
Other remarks
• FDA black box warning - central nervous system depressant and may cause respiratory depression
• Abuse or misuse of illicit GHB is associated with seizures, respiratory depression, decreased consciousness, coma, and death especially if used in combination with other central nervous system depressants, such as alcohol and sedating medications.
Manufacturer note
• Contraindicated in combination with sedative hypnotics or alcohol
• Contraindicated in patients with succinic semialdehyde dehydrogenase deficiency.
• Caution patients against hazardous activities for at least 6 hours after taking the medication.
• May impair respiratory drive, especially in patients with compromised respiratory function.
• Increased central apneas and clinically relevant desaturation events have been observed in adult and pediatric patients.
• Parasomnias
• Each mL contains 0.5 g of total salts present as 0.234 g calcium oxybate, 0.096 g magnesium oxybate, 0.13 g potassium oxybate, and 0.04 g sodium oxybate (equivalent to 0.413 g total oxybate)
• Monitor closely for emergence of depressive symptoms if prior history of depressive illness and/or suicide attempt
*Per ml: 40 mg total oxybate in this preparation vs. 91 mg sodium in sodium oxybate preparation

Understand
Understand components of the GRADE system used to inform clinical practice guideline development

Identify
Identify treatments noted in 2021 clinical practice guideline


of Michigan
No disclosures
• All oxybates all the time
• Bring on the babies!
• Normal sleep
• Narcolepsy
• Drugs
• Sleep deprivation
• Diagnostics
• Let's be legal!
• What is the mechanism of action of sodium oxybate?
• What was the initial purpose behind the development of sodium oxybate?
• How does the body metabolize sodium oxybate?
• What is the mechanism of once-nightly sodium oxybate (Lumryz)?
• What is the primary distinction between the two available twice nightly dosed oxybate medications?
• What is the sodium content in sodium oxybate?
• How does the presence of food affect the absorption of (sodium) oxybate?
• Which medications used to treat hypersomnia are known to potentially interfere with the effectiveness of oral contraception pills (OCPs)?
• What mechanisms can lead to a decrease in the effectiveness of OCPs?
• What is the pregnancy classification for armodafinil and modafinil?
• What occurs with CO2 levels as you transition into sleep?
• Slow wave sleep is associated with the secretion of which hormone?
• Sleep onset is linked to the secretion of which hormone?
• What happens to the autonomic nervous system as you progress from N1 to N4 sleep stages?
• Bonus: What happens during REM sleep?
• What is the typical pattern of gastric acid secretion?
• Which cells are responsible for secreting ghrelin and leptin?
• Which HLA genotype is linked to narcolepsy?
• What are the MSLT criteria for sleep latency in narcolepsy?
• What is the mechanism underlying the dog model of narcolepsy?
• What are the diagnostic criteria for hypocretin levels in cerebrospinal fluid (CSF) used to diagnose narcolepsy?
• What other concurrent sleep disorders can be observed in individuals with narcolepsy?
• What are the diagnostic criteria for hypersomnia attributed to a medical disorder?
• What are the two effects that β-blockers have on sleep?
• What is the mechanism of the GABA A alpha receptor type?
• How does caffeine function as a stimulant?
• Which medication used for hypersomnia can potentially cause QT prolongation?
• What is the mechanism of action of Pitolisant (Wakix)?
• What is the mechanism of action of Solriamfetol (Sunosi)?
• Which aspect of sleep rebounds after sleep deprivation?
• What are the metabolic changes associated with sleep deprivation?
• Is there an interaction between acute sleep deprivation and vaccination effectiveness?
• How does the EEG (Electroencephalogram) change with prolonged sleep deprivation?
• What are the criteria for ending a Maintenance of Wakefulness Test (MWT)?
• What are the criteria for ending a Multiple Sleep Latency Test (MSLT)?
• In a Maintenance of Wakefulness Test (MWT) the technician encourages the patient to what?
Brunner, D. P., D. J. Dijk, and A. A. Borbely. 1993. 'Repeated Partial Sleep-Deprivation Progressively Changes the Eeg during Sleep and Wakefulness', Sleep, 16: 100-13.
Chen, Cuiping, Jack Jenkins, Katie Zomorodi, and Roman Skowronski. 2021. 'Pharmacokinetics, bioavailability, and bioequivalence of lower-sodium oxybate in healthy participants in two open-label, randomized, crossover studies', Clinical and Translational Science, 14: 2278-87.
Dzaja, A., M. A. Dalal, H. Himmerich, M. Uhr, T. Pollmacher, and A. Schuld. 2004. 'Sleep enhances nocturnal plasma ghrelin levels in healthy subjects', American Journal of Physiology: Endocrinology and Metabolism, 286: E963-7.
Gottesmann, Claude. 2002. 'GABA mechanisms and sleep', Neuroscience, 111: 231-39.
Kaplan, Sigal, Debra L. Braverman, Ilana Frishman, and Netta Bartov. 2021. 'Pregnancy and Fetal Outcomes Following Exposure to Modafinil and Armodafinil During Pregnancy', JAMA Internal Medicine, 181: 275-77.
Krahn, Lois E., Donna L. Arand, Alon Y. Avidan, David G. Davila, William A. DeBassio, Chad M. Ruoff, and Christopher G. Harro d. 2021. 'Recommended protocols for the Multiple Sleep Latency Test and Maintenance of Wakefulness Test in adults: guidance from the American Academy of Sleep Medicine', Journal of Clinical Sleep Medicine, 17: 2489-98.
Lange, T., B. Perras, H. L. Fehm, and J. Born. 2003. 'Sleep enhances the human antibody response to hepatitis A vaccination', Psychosomatic Medicine, 65: 831-5.
Lin, L., J. Faraco, R. Li, H. Kadotani, W. Rogers, X. Lin, X. Qiu, P. J. de Jong, S. Nishino, and E. Mignot. 1999. 'The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene', Cell, 98: 365-76.
Mignot, E. J. 2012. 'A practical guide to the therapy of narcolepsy and hypersomnia syndromes', Neurotherapeutics, 9: 739-52.
Qureshi, Asher, and Teofilo Lee-Chiong. 2004. 'Medications and their effects on sleep', Medical Clinics, 88: 751-66.
Seiden, David, Charles Tyler, and Jordan Dubow. 2021. 'Pharmacokinetics of FT218, a Once-Nightly Sodium Oxybate Formulation in Healthy Adults', Clinical Therapeutics, 43: 672.e172.e14.
Spiegel, K., E. Tasali, P. Penev, and E. Van Cauter. 2004. 'Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite', Annals of Internal Medicine, 141: 846-50.
Thorpy, Michael J. 2020. 'Recently Approved and Upcoming Treatments for Narcolepsy', CNS Drugs, 34: 9-27.
Thorpy, Michael J., and Richard K. Bogan. 2020. 'Update on the pharmacologic management of narcolepsy: mechanisms of action and clinical implications', Sleep Medicine, 68: 97109.
• No conflict of interest
• Nothing to Disclose
• Sleep apnea Prevalence: 15-30% in males, 10-15% in females.
• Risk factors: Older age, Male sex, Obesity, Craniofacial structural abnormalities, Smoking, and family history of OSA
• Prevalence increases with other comorbid conditions such as atrial fibrillation, hypertension, obesity hypoventilation syndrome, Pulmonary hypertension, Congestive heart failure, stroke, pregnancy, chronic lung disease, and End-stage renal disease.
• Conventional therapy modality –Positive Airway Pressure (PAP) therapy
• CPAP Adherence:
• Recommended to use 4 hr or more per night. Studies have shown improvement in daytime sleepiness, quality of life, blood pressure control, performance in neurobehavioral tests, and decreased automobile accidents.
• Approximately 50% of the patients show non-adherence
• Factors for Non-Adherence: moderate to severe AHI, Poor motivation, Problems in use within 2 weeks of CPAP, Younger age, Claustrophobia, Nasal congestion, poor sleep, non-supportive bed partner,
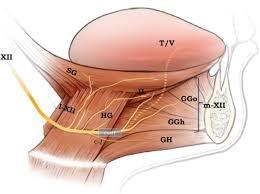
• History of hypoglossal nerve stimulation therapy
• Schwartz et al studied the upper airway collapsibility in a cat model by stimulating the hypoglossal nerve with different frequencies and analyzed pressure-volume relationships.
• Miki et al stimulated the genioglossal muscle in an anesthetized dog
• Bailey et al and Yoo et al studied selective stimulation of the tongue protrudors branch ( medial branch) and whole hypoglossal nerve truck.
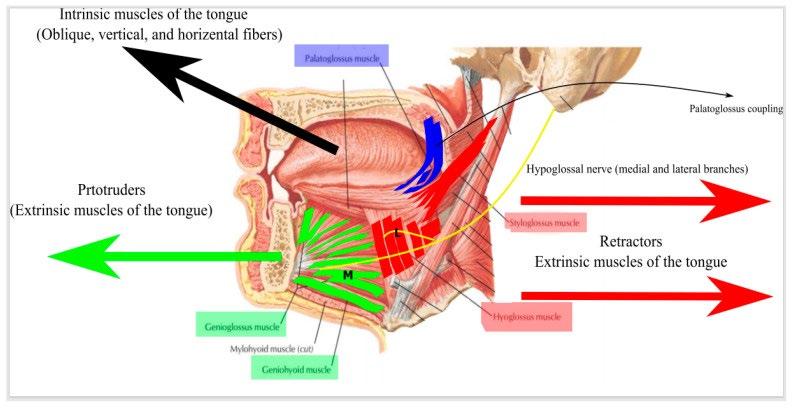
• Hypoglossal nerve stimulation (HGNS)
• Upper airway patency is controlled by dilator muscles. The most important muscle is the genioglossus.
• 3 HGNS devices were tested in clinical trials.
• Apnex Medical Inc
• ImThera aura 6000 – Still Phase III trial
• Inspire device- FDA approved in 2014
• Human studies:
• Schwartz et all – selective intramuscular stimulation of the tongue muscles. 9 patients with severe AHI more than 60/hr, noted an increase in maximum inspiratory airflow (VI Max) with genioglossal stimulation 288ml/s to 501ml/s, no associated arousal with EEG monitoring, and a significant decrease in AHI 69 to 9 /hr.
• Oliven et al. 7 patient with OSA with AHI 5/hr,
Surface stimulation of GG with sublingual electrode- no change
Stimulation of HG muscle resulted in airway collapse
Co-activation of both resulted in the opening of the airway and an increase in the oropharyngeal area.
When these patients were under anesthesia,
stimulation of GG with fine-wire electrodes intramuscular stimulation resulted in an increase in the airway opening
HG stimulation showed a collapse of the airway
Coactivation of these muscles showed an increase in the airway opening but no significant difference from the GG activation alone.
• A few landmark studies prior to STAR trial
• Apnex Medical Inc studies:
• Eastwood et al: 21 patients, decreased AHI to 19 from 43/hr at 6 months compared with baseline. Improved quality of life, ESS score. At 6 months, implant use was 89% of the night, average use of 5.8 hr per night.
• Kezirian et al: 31 patients with moderate to severe OSA. PSG follow-up at 6 and 12 months. Decrease AHI 45 to 21/hr at 6 months and persist at 12 months.
• Noted that BMI>35kg/m2 did not respond favorably.
• ImThera Medical Inc.:
• Mwenge et al. Targeted unilateral hypoglossal cyclical nerve stimulation, 13 patients with moderate to severe OSA,
• PSG done at 3 months and 12 months
• Decrease in AHI 45.2 to 21.7/hr at 3 months and 21/hr at 12 months.
• Improved ODI, and decreased arousal index
• Fatigue improved in both 3 and 6 months
• ESS improved at 3 months and some improvement in 12 months
• Inspire Medical System:
• Van de Heyning et al: 2-part phase II clinical trial
• 22 patients enrolled, hypoglossal nerve trunck stimulation, AHI did not change at 6 months (56.1/hr vs 51.1/hr post-implant)
• Analysis showed that 3 factors showed poor predictors of therapy
• AHI >50/hr
• BMI > 32kg/m2
• Complete concentric collapse (CCC) at the soft palate on DISE
• 2nd part of the study: 9 patients with the above criteria (AHI <50/hr, BMI <32kg/ m2, and no CCC on DISE), medial branch of the hypoglossal nerve stimulation.
• At 6 months, post-implant AHI significant decrease from 38.9 to 10 /hr
• Criteria:
• PSG – AHI 20-50 /hr
• Central and mixed apneas index <25% of AHI
• No anatomical abnormalities such as enlarged tonsils
• No concentric collapse with Drug-induced sleep endoscopy
• PSG- 1 month after implant, 2 months after activation, at 6and 12-month visits
• Sleep questionnaires- ESS, FOSQ at each follow-up visit.
• Primary outcome measures: 1) AHI 4% decrease in oxygen desaturation and a 30% reduction in airflow, 2) ODI with a 4% decrease in oxygen desaturation
• Secondary outcome: Change in ESS, FOSQ
• Randomized therapy withdrawal trial
• 126 patients
• AHI 15-65/hr
• Primary outcome: decrease AHI < 20/hr, and reduction of AHI by at least 50% from baseline
• Secondary outcome: Quality of life measure changes ESS, and FOSQ
• No control group, patient is served as own control group
• Follow up baseline, 2, 6 and 12 months
• PSG at all follow-up visits
• 124 patients completed 12 months of follow up
• At 12 months 46 patients had therapy off study for 1 week to study the result is related to HGNS.



• 18 months:
• 123 patients
• Median AHI 9.7/hr, 67 % reduction
• Median ODI 8.6/hr, 67% reduction
• Adherence 84%
• Stimulation parameters remained the same
• 36 months:
• 116 patients
• 94 volunteered for PSG
• Mean AHI 11.5/hr
• Mean ODI 9.1/hr
 B Tucker Woodson 1, Kingman P Strohl 2, Ryan J Soose 3, M Boyd Gillespie 4, Joachim T Maurer 5, Nico de Vries 6 7, Tapan A Padhya 8, M Safwan Badr 9, Ho-Sheng Lin 10, Olivier M Vanderveken 7, Sam Mickelson 11, Patrick J Strollo Jr 12, Otolaryngol Head Neck Surgery, 2018 Jul;159(1):194-202.
B Tucker Woodson 1, Kingman P Strohl 2, Ryan J Soose 3, M Boyd Gillespie 4, Joachim T Maurer 5, Nico de Vries 6 7, Tapan A Padhya 8, M Safwan Badr 9, Ho-Sheng Lin 10, Olivier M Vanderveken 7, Sam Mickelson 11, Patrick J Strollo Jr 12, Otolaryngol Head Neck Surgery, 2018 Jul;159(1):194-202.
Hypoglossal nerve stimulation therapy 5-year outcome
• Participants are from STAR trial cohorts that finished 5-year visits.
• 126 had implants under the STAR trial
• Followed 12, 24, 36, 48 and 60 months
• Criteria Same: Self-reported outcomes regarding health status, adverse events, snoring, BMI, subjective sleepiness, and sleep-related quality of life questionnaires from ESS, FOSQ
• PSG -2007 AASM scoring criteria- hypopneas- 30% airflow reduction and 4% decrease in oxygenation, sleep stages, and arousals.
• PSGs performed at 6 months, 12 months under the protocol, and voluntarily at 3 and 5 years.

 B Tucker Woodson 1, Kingman P Strohl 2, Ryan J Soose 3, M Boyd Gillespie 4, Joachim T Maurer 5, Nico de Vries 6 7, Tapan A Padhya 8, M Safwan Badr 9, Ho-Sheng Lin 10, Olivier M Vanderveken 7, Sam Mickelson 11, Patrick J Strollo Jr 12, Otolaryngol Head Neck Surgery, 2018 Jul;159(1):194-202.
B Tucker Woodson 1, Kingman P Strohl 2, Ryan J Soose 3, M Boyd Gillespie 4, Joachim T Maurer 5, Nico de Vries 6 7, Tapan A Padhya 8, M Safwan Badr 9, Ho-Sheng Lin 10, Olivier M Vanderveken 7, Sam Mickelson 11, Patrick J Strollo Jr 12, Otolaryngol Head Neck Surgery, 2018 Jul;159(1):194-202.
• Another trial, a post-market study, in Germany by Steffen et al at ENT centers
• 60 patients ( 15 failed MAD, 14 failed surgery)
• Study design similar to STAR trial except AHI15-65/hr and BMI 35kg/m2, follow up 12 months
• AHI reduction 28.6 to 9.5 /hr at 12 months
• Response rate 73%
• Average use 5.6hr per night.
• Long-term follow-up , at 2 years: 41 returned for follow-up, and at 3 years: 38 returned for follow-up
• About 76% at 2 years and 68% at 3 years, AHI below 15/h.
• The median AHI was reduced from 28.6/h to 9.0/h at 2 years and 10.0/h at 3 years
• The Median ODI decreased from 27.0 to 6.3/h at 2 years, and 8.3/h at 3 years
• Usage was stable at approximately 45 hours per week at 2 and 3 years.
• Serious device-related adverse events were rare, with two-device explantation between 12 to 36 months postoperatively.
• Long-term follow-up study at 6 months using Im Thera aura 6000 system by Freidman et al:
• 46 patients received implant • AHI reduction 35.7 to 8.5 /hr • ODI reduction 32.6 to 7.9/hr
• Arousal index reduction 43.9 to 20.4/hr
• ESS reduction 13 to 8.4
• Genio system (Nyxoah SA, Belgium)
• Bilateral hypoglossal nerve stimulation with a paddle close to the distal end of the medial branch to stimulate GG muscle alone
• No batteries
• External activation unit to be placed under the chin for activation
• Cyclical stimulation, so there is a pause between breathing cycles.
• Not synchronized with respiration, no sensing electrode
• Side effect: irritation with an adhesive patch, tongue abrasion, twitching of tongue muscle.
• Mean reduction of AHI from 24 to 13/hr and ODI reduction from 19 to 10/hr at 6 months, ESS reduction from 11 to 8, snore reduction from 96% to 35%.

• Multicentered perspective observational registry aimed to collect 5000 patients
• Data collected from US and Europe
• As of July 2022, 3,988 subjects enrolled in the registry. 57 patients were with AHI >65, and 279 patients were >32. AHI, ESS, Therapy Usage, CGI (Clinical Global Impression), and/or Patient Satisfaction data.




Summary
Hypoglossal nerve stimulation is an effective treatment for patients intolerant to PAP therapy
• More than 50% reduction in AHI and remained stable over several months
• A significant improvement is noted in daytime sleepiness and fatigue with HGNS therapy
• Favorable adherence compared to PAP therapy.
• Minimal adverse effects
AHI reduction: 29.3 to 9/hr ( 68% reduction, p<0.001)
ODI reduction 25.4 to 7.4/hr ( 70 % reduction , p <0.001)
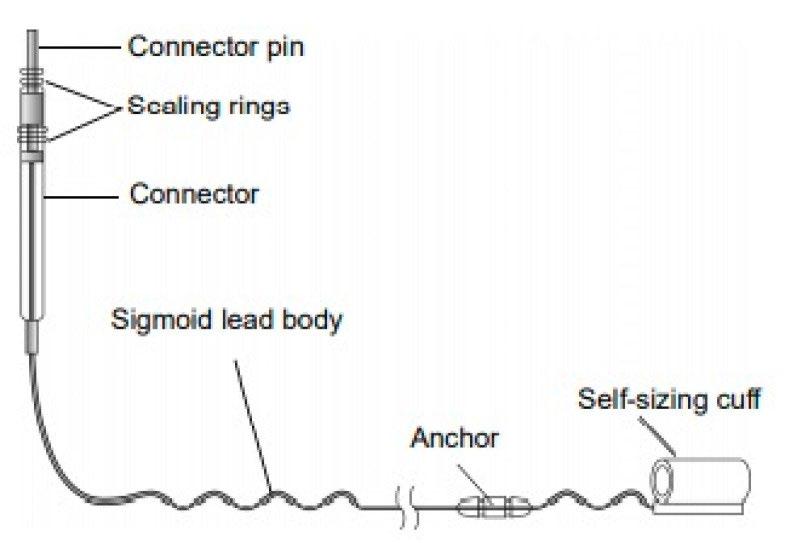



• Criteria:
• Age 18 and older
• PAP intolerant
• AHI 15-60/hr ( currently FDA approval extended to 100)
• Less than 25% of central apneas and mixed apnea index
• BMI
• BCBS and other commercials:32
• Medicare: 35
• FDA approved: 40
• DISE showing NO Concentric collapse
Inspire device Procedure Implant:
2 incisions: 1) right upper neck 5 cm long between the mandible and hyoid bone 2) infraclavicular area for pulse generator and sensing lead placement
• Predictor of hypoglossal nerve stimulation
• BMI
Anatomical position of the oral cavity lower Mallampati scales II/ III and Friedman’s tongue position II/III
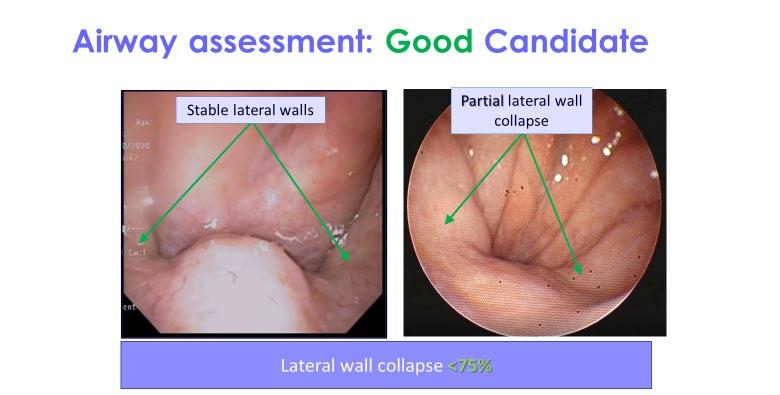
• In Down syndrome FDA approved, 13 to 18 yrs of age AHI 10-50/hr
• OSA is prevalence up to 66% in Childhood patients with Down syndrome
• Airway hypotonia is the main cause of obstruction.
• Current therapies: Adenotonsillectomy and PAP therapy,
• 73% ineffective in Adenotonsillectomy
• 46% adherence to PAP therapy
• Clinical trial 20 patients including case series and case reports - 87.6% reduction in AHI, AHI reduction 24.2 to 3/hr at 2 months, 9.2 hrs use per night.
• Criteria: BMI >95th percentile for age and gender, child should be able to communicate discomfort, age 10 years or more.
• History
• In 1783 German physician Christoph Wilhelm Hufeland developed the idea of stimulation of the Phrenic nerve.
• 1855 French Neurologist Duchenne de Boulogne
• 1856 Hugo Wilhelm von Ziemssen first performed the diaphragm pacing on a 27 yr. old girl with respiratory paralysis due to fumes.
• In 1948 Sarnoff and his group did experiment on a 5 year old patient who had respiratory paralysis after cerebral aneurysm rupture, lasted for 53 hrs.
• The concept of adjusting amplitude and rate of stimulation by John Judson and William Glen in 1968, developed a commercial device in 1970, and FDA approval in 1987.
• In the 1990s, long-term phrenic nerve stimulation for certain diseases was expanded.
• In 2006 Respicardia Incorporation developed a device “Remede” that can be implanted transvenously. Respicardia was acquired by Zoll in 2021
• Received FDA approval for this device on October 2017. CMS approval in 2018
• Indication
• Medicare indication describes as “the implantation of a phrenic nerve stimulator is covered for selected patients with partial or complete respiratory insufficiency” and “ effective only with intact Phrenic nerve”
• Patients with spinal cord injury
• Central sleep apnea (CA)- Congestive heart failure, Atrial fibrillation, Stroke, Idiopathic central apnea, opioid use
• Congenital central hypoventilation
• Diaphragm paralysis


• Therapy
• Starts automatically during sleep hours- to be programmed
• Can change the stimulation amplitude with position
• Starts with low stimulation until synchronized breathing and device.
• Increase stimulation with rate and depth of breathing to overcome central sleep apnea
A novel therapeutic approach for central sleep apnea: Phrenic nerve stimulation by the remedē® System, Susan Joseph a, Maria Rosa Costanzo b

• Remede Pivotal trial
• Primary endpoint: >50% reduction in the AHI at 6 months, free of adverse event with the device in treated patients at 12 months
• Secondary endpoint: Analyze CAI, AHI, ODI, Arousal index, % REM sleep, global assessment, Epworth sleepiness scale.
• Inclusion criteria:
• CA confirmed by PSG within 40 days of implant, AHI>20/hr, CAI>50 % of all apneas, and at least 30 central apnea events, Obstructive apnea index <20% of the AHI and medically stable without medication change or hospitalization or illness in the past 30 days of the implant.
• Exclusion criteria:
• Evidence of phrenic nerve palsy, CA related to pain medication, stroke within the past one-year, poor pulmonary function, baseline oxygen saturation at rest while awake in room air <92%, life expectancy, and transplant or LVAD less than 12 months, any major cardiac issues, MI or interventions within past 3 months and enrollment in another study that might interfere with phrenic nerve stimulation.
Baseline characteristics of the intention-to-treat population
 Maria Rosa Costanzo, Piotr Ponikowski, Shahrokh Javaheri, Ralph Augostini, Lee Goldberg, Richard Holcomb, Andrew Kao, Rami N Khayat, Olaf Oldenburg, Christoph Stellbrink, William T Abraham, for the remedé System Pivotal Trial Study Group* Lancet 2016; 388: 974–82
Maria Rosa Costanzo, Piotr Ponikowski, Shahrokh Javaheri, Ralph Augostini, Lee Goldberg, Richard Holcomb, Andrew Kao, Rami N Khayat, Olaf Oldenburg, Christoph Stellbrink, William T Abraham, for the remedé System Pivotal Trial Study Group* Lancet 2016; 388: 974–82
 Maria Rosa Costanzo, Piotr Ponikowski, Shahrokh Javaheri, Ralph Augostini, Lee Goldberg, Richard Holcomb, Andrew Kao, Rami N Khayat, Olaf Oldenburg, Christoph Stellbrink, William T Abraham, for the remedé System Pivotal Trial Study Group* Lancet 2016; 388: 974–82
Maria Rosa Costanzo, Piotr Ponikowski, Shahrokh Javaheri, Ralph Augostini, Lee Goldberg, Richard Holcomb, Andrew Kao, Rami N Khayat, Olaf Oldenburg, Christoph Stellbrink, William T Abraham, for the remedé System Pivotal Trial Study Group* Lancet 2016; 388: 974–82
Maria Rosa Costanzo, Piotr Ponikowski, Shahrokh Javaheri, Ralph Augostini, Lee Goldberg, Richard Holcomb, Andrew Kao, Rami N Khayat, Olaf Oldenburg, Christoph Stellbrink, William T Abraham, for the remedé System Pivotal Trial Study Group* Lancet 2016; 388: 974–82
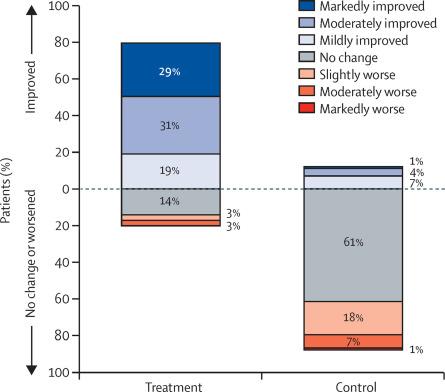

Oldenburg,
2016; 388: 974–82
Maria Rosa Costanzo, Piotr Ponikowski, Shahrokh Javaheri, Ralph Augostini, Lee Goldberg, Richard Holcomb, Andrew Kao, Rami N Khayat, Olaf Christoph Stellbrink, William T Abraham, for the remedé System Pivotal Trial Study Group* Lancet• 5-year safety and efficacy data: Phrenic nerve stimulation for Central sleep apnea.
• Patients in the Pivotal trial got enrolled in the Post Approval Study (PAS) after the FDA approval
• Control group from the pivotal trial activated the device after a 6-month visit to the primary end-point assessment.
• Out of 151 enrolled in the Pivotal trial only 53 enrolled in PAS
• Overnight in-lab attended Polysomnogram performed at 1, 2 and 5 years and Home sleep apnea test at 3 years.
• AHI, CAI, OAI, hypopnea index, ODI 4, % sleep time at different sleep stages, and arousal index.

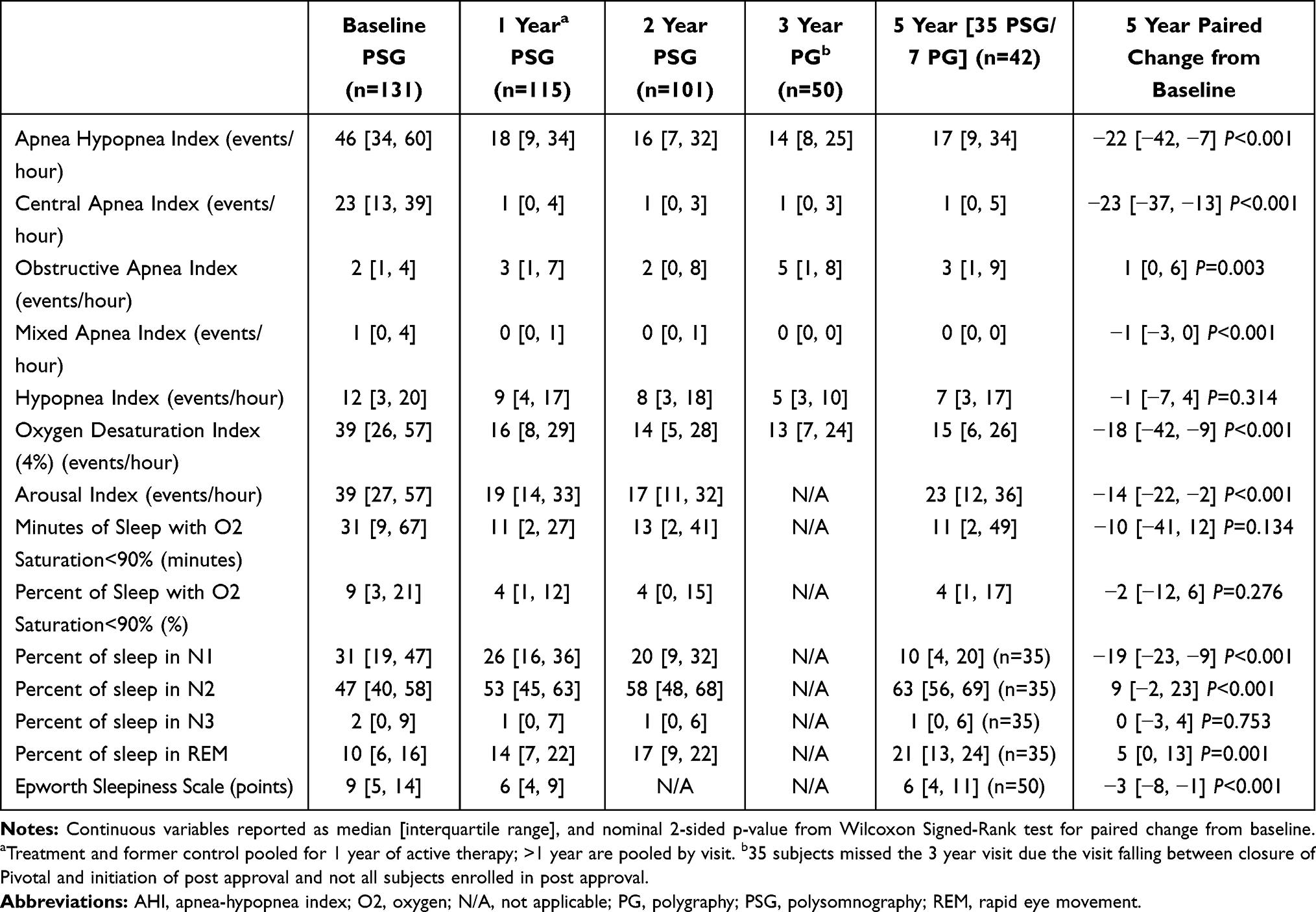
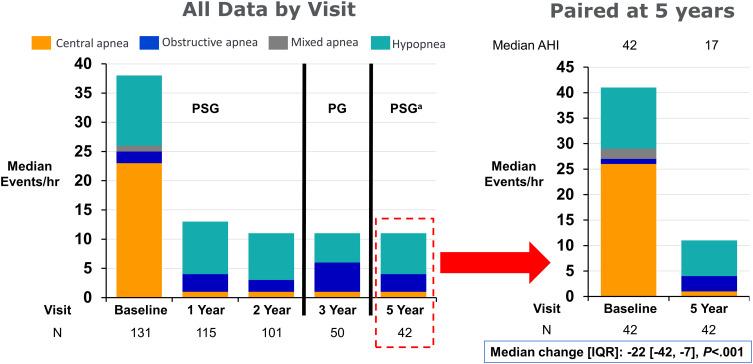
• Conclusion: 5 year follow up
• Transvenous Phrenic nerve stimulation for central sleep apnea is effective in decreasing the CA and the effect continues even at 5 years.
• AHI baseline to 5 year- 43 to 17/hr, CAI 23 to 1/hr . Obstructive and mixed apnea index remained unchanged. Subgroup analysis of Heart failure and Non-heart failure groups showed the same improvement.
• Sleep stage percentage N1 decreased, N2 and REM increased at 5 years compared to the baseline.
• Daytime sleepiness improved by at least > 3 points reduction at 5 years compared to the baseline
• Survival rate in the heart failure group was 78% and heart failure hospitalization decreased to 55%
• The Hypoglossal Nerve Stimulation as a Novel Therapy for Treating Obstructive Sleep Apnea— A Literature Review, Mashaqi S, Patel S, Parthasarathy S et al., Int.J. Environ. Res. Public Health, 2021, 18, 1642
• Patrick J. Strollo, Jr., M.D., Ryan J. Soose, M.D., Joachim T. Maurer, M.D.., et al., for the STAR Trial Group*N Engl J Med 2014; 370:139-149
• Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnea: The STAR Trial B Tucker Woodson 1, Ryan J Soose 2, M Boyd Gillespie 3, Kingman P Strohl 4, Joachim T Maurer 5, Nico de Vries 6, David L Steward 7, Jonathan Z Baskin 4, M Safwan Badr 8, Ho-sheng Lin 8, Tapan A Padhya 9, Sam Mickelson 10, W McDowell Anderson 9, Olivier M Vanderveken 11, Patrick J Strollo Jr 2; STAR Trial Investigators, Otolaryngol Head Neck Surg. 2016 Jan;154(1):181-8.
• Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes, B Tucker Woodson 1, Kingman P Strohl 2, Ryan J Soose 3, M Boyd Gillespie 4, Joachim T Maurer 5, Nico de Vries 6 7, Tapan A Padhya 8, M Safwan Badr 9, Ho-Sheng Lin 10, Olivier M Vanderveken 7, Sam Mickelson 11, Patrick J Strollo Jr 12 , Otolaryngol Head Neck Surg, 2018 Jul;159(1):194-202.
• Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study, Steffen A, Sommer J, Hofauer B et al., Laryngoscope, 2018 Feb;128(2):509-515.
• Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea, Armin Steffen 1, Ulrich J Sommer 2, Joachim T Maurer 3, Nils Abrams 4, Benedikt Hofauer 5, Clemens Heiser 6 Sleep Breath, 2020 Sep;24(3):979-984.
• Targeted hypoglossal nerve stimulation for the treatment of obstructive sleep apnea: Six-month results. Friedman M, Jacobowitz O, Hwang MS, Bergler W, Fietze I, Rombaux P, Mwenge GB, Yalamanchali S, Campana J, Maurer JT.Laryngoscope. 2016 Nov;126(11):2618-2623
• Maria Rosa Costanzo, Piotr Ponikowski, Shahrokh Javaheri, Ralph Augostini, Lee Goldberg, Richard Holcomb, Andrew Kao, Rami N Khayat, Olaf Oldenburg, Christoph Stellbrink, William T Abraham, for the remedé System Pivotal Trial Study Group* Lancet 2016; 388: 974–82
• Costanzo MR, Javaheri S, Ponikowski P, Oldenburg O, Augostini R, Goldberg LR, Stellbrink C, Fox H, Schwartz AR, Gupta S, McKane S, Meyer TE, Abraham WT; remedē®System Pivotal Trial Study Group.Nat Sci Sleep. 2021 Apr 29;13:515-526. doi: 10.2147/NSS.S300713. eCollection 2021.
• A novel therapeutic approach for central sleep apnea: Phrenic nerve stimulation by the remedē® System, Susan Joseph a, Maria Rosa Costanzo b International Journal of Cardiology, Volume 206, Supplement, March 2016, Pages S28-S34