
5 minute read
Genetics in The War Against Viruses
Genetics in the War
Against Viruses
Advertisement
Aayush Purohit
Image courtesy of Creative Commons.
Viral infections pose immense threats to our health due to their resilient and lethal nature. As antibiotic treatments are impossible, finding ways to slow down their attacks is essential. For example, a group of viruses known as arenaviruses has been seen to have a devastating effect on populations. Those diagnosed with an arenavirus infection face a high mortality rate of 50% in most cases. 1 The work of Dr. Jason Whitmire, an associate professor in the Genetics and Microbiology & Immunology departments of the UNC School of Medicine, focuses on arenavirus infections. Dr. Whitmire’s lab investigates viral infections in order to try and analyze the resulting immune responses that they can provoke, as well as the mechanisms behind these responses, with one particular project of his involving experimentation with mice as a model for human pathology and examining genetics as it relates to regulating the impact and mechanisms of disease. Looking at the details of these systems can reveal answers to questions that plague patient healthcare everywhere. The Whitmire lab seeks to model arenaviruses in particular. Arenaviruses, which are pathogens that can lead to severe hemorrhagic disease, a rapid loss in blood with potentially fatal outcomes. In order to accomplish the task, they infected mice with a virus, lymphocytic choriomeningitis virus (LCMV). The viral infection causes symptoms such as weight loss, elevated cytokines, thrombocytopenia, and lung edema in mice, which are characteristics that resemble those found in humans suffering from an arenavirus infection. Conducting genetic studies using real diseases is easier and simpler to conduct in mice, and their results have the capability to be applicable to humans. Identifying a specific location, or locus, in the genetic makeup of the mice is a difficult task, but one that can show the region responsible for influencing the seriousness and impact of the disease. 1 For their experimentation, the researchers had to do a couple of different things in order to put together the different pieces of the puzzle. They had to monitor the health, particularly that of the immune system, of the mice as the disease progressed through their system. Doing so meant that they looked at different symptoms and effects within the mice. The researchers proceeded to conduct genetic tests to sequence the genes of these infected mice. Analyzing the genetic information they acquired enabled them to see which regions and genes had been changed or altered. They could then highlight the specific regions possibly responsible for changing the immune response “A specific region in the mice’s DNA called the PL allele was observed to affect various immune system features...”
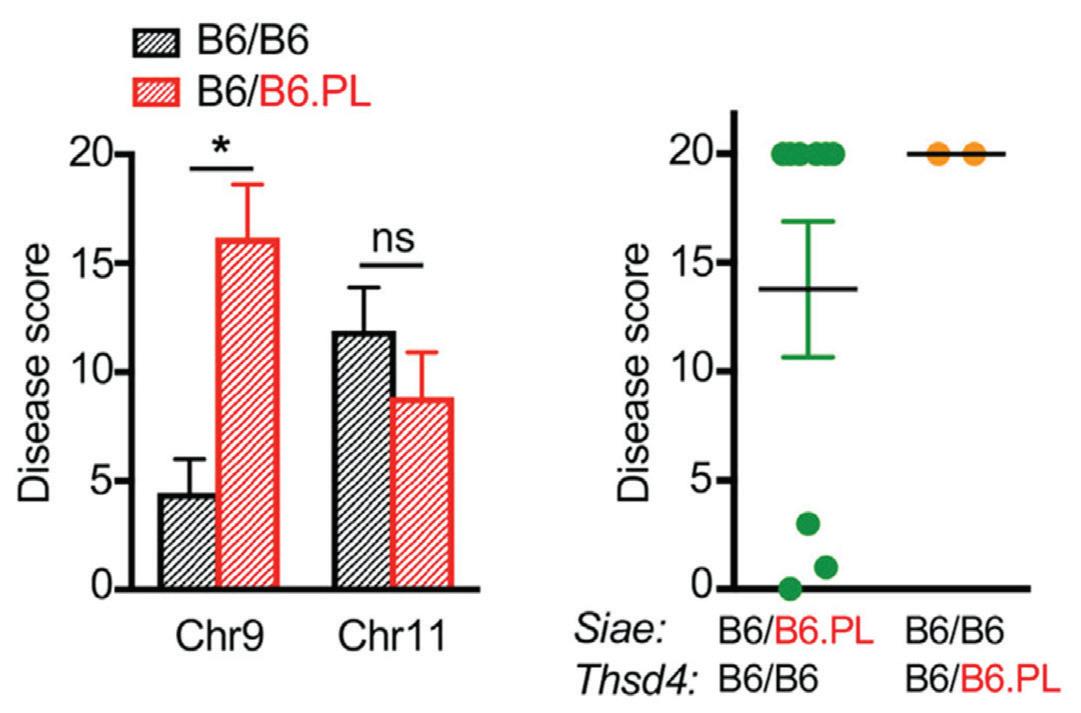
of the mice and the overall outcome of the viral infection. Finally, through these genetic tests that looked at the sequence of DNA and comparisons of these results with the phenotypes (symptoms and characteristics which could be physically observed in the mice), the researchers were able to develop a few major conclusions. An initial result that stood out to the researchers was that the mice developed lethal hemorrhagic disease through their exposure in the lab to the LCMV infection, confirming their hypothesis. Moreover, a specific region in the mice’s DNA, the PL allele was observed to affect various immune system features by such actions as enhancing T cell response (contributes to the development of the disease), thrombocytopenia (deficiency of platelets which increases tissue bleeding), and lung edema (presence of excess fluid), all of which diminish host health. The PL allele was also found to specifically affect those genes that regulate immune system mechanisms from the chromosome 9 region, meaning that it could potentially speed up or slow down the rate at which the host would be able to fight off an attack from disease. 1 Identifying this allele was a very important discovery because the allele can exist and be expressed in many different locations, but the researchers were able to use genotyping to find the location related to the outcome of the viral infection. Dr. Whitmire plans to continue with this work by continuing to map different mutations in genes that can be associate with changes to the immune system, and thereby complete the bigger picture of how viruses can specifically target and weaken hosts. Ultimately, Dr. Whitmire’s research provides an interesting lens to see that even minor minute genetic differences in a host can extraordinarily change their health outcome. The analysis of the genetic makeup of mice has great potential in answering further questions concerning how genes and their differences can change the life course that viruses have and improve the immune response to such dangerous threats. Immunology is becoming an increasingly studied field of science because of its major applications and implications in a wide variety of areas related to improving human health and finding solutions to dealing with pathogens. Dr. Whitmire has done a lot of great work in his field, but still has plenty more to do, “the research is on the right path, but the important thing is to stick with it and to continue building on the foundation of knowledge and insight that has already been created.” 2 If researchers are able to look at different studies in different fields and incorporate some of those ideas and concepts in their own studies, developing novel therapeutic solutions to combat viral threats will be easier than ever before.
References
1. Misumi I, Cook KD, Mitchell JE, Lund MM, Vick SC, Lee RH, Uchimura T, Bergmeier W, Mieczkowski P, de Villena FP-M, Ting JPY, Whitmire JK. (2019). Identification of a locus in mice that regulates the collateral damage and lethality of virus infection. Cell Rep. 27(5): 1387-1396. Doi: 10.1016/j. celrep.2019.04.004 2. Interview with Dr. Jason Whitmire, February 11, 2020.










