OUR NEXT EVOLUTION



On the 8 November 2021, VCS Foundation Limited changed its trading name to the Australian Centre for the Prevention of Cervical Cancer (ACPCC); a new era in our evolution as Australia’s leading organisation in cervical cancer prevention.
Ensuring that the ACPCC’s activities and purpose is globally recognised is critical for progressing our collaborations to eliminate cervical cancer as a public health problem in our region.
With a new name, our purpose as a not-for-profit organisation remains unchanged; to eliminate cervical cancer as a public health problem.
ACPCC is here to control cervical cancer for the benefit of all people –we always have been.
THE AUSTRALIAN CENTRE FOR THE PREVENTION OF CERVICAL CANCER

“I think the name aptly describes the significant leadership of the organisation in eliminating cervical cancer as a public health concern in this country, and welcome the announcement of the Australian HPV Reference Laboratory. I also appreciate that these and other key functions of the organisation will continue to support our prevention priorities in Victoria.”
Sincerely,
Maria Bubnic Executive Director, Policy & Programs Public Health Division, Department of Health Victoria“While the name change is significant, reflecting a change from cytology to molecular diagnostics, the work you lead and do, and your collaborations, are and will continue to be the real measures of your success.”
Best wishes
Prof Ian Frazer AC University of Queensland“Our new name ensures that we are recognised as Australia’s leading organisation in cervical cancer prevention. This is an important step to move ahead in the goal to eliminate cervical cancer in our region. We are proud to continue contributing to Australia’s highly successful cervical cancer prevention efforts and to collaborate with partners across the Indo-Pacific region to save thousands more women’s lives.”
Professor Marion Saville, AM Executive Director Australian Centre for the Prevention of Cervical Cancer
Cervical cancer is caused by the Human Papillomavirus, or HPV. It is preventable through HPV vaccination and cervical screening and is one of the most curable cancers if diagnosed early and managed effectively. Yet cervical cancer is the fourth most common cancer among women globally.
In 2020, there was an estimated 604,000 new cases of cervical cancer worldwide and over 342,000 women died from this disease. Cervical cancer is currently the 14th most common cancer in Australia and in 2021, there were over 900 new cases and 237 deaths. These statistics are preventable.
On 17 November 2020, following endorsement at the 73rd World Health Assembly, the World Health Organization (WHO) officially launched the Global Strategy to Accelerate the Elimination of Cervical Cancer
In 2020, there was an estimated 604,000 new cases worldwide and over 342,000 women died from the disease. Nearly 90% of deaths in occured in low- and middle-income countries.
https://gco.iarc.fr/today/data/factsheets/cancers/23Cervix-uteri-fact-sheet.pdf
Elimination is within the reach of all countries. With political will from governments and the commitment and expertise of health and community organisations from all nations, girls and people with a cervix who are born today will live to see a world free of this disease.
Australia is on track to achieve elimination by 2035. We must address inequities in access to culturally safe and inclusive vaccination, screening and treatment services to ensure that we reach the elimination target in Australia, leaving no one behind.
For the first time ever, the world has committed to eliminating a cancer. The goal is to eliminate cervical cancer as a public health problem by reaching an incidence of <4 per 100,000 women in every country within the next 100 years.
To reach elimination, the WHO strategy sets out three targets to be met by every country by 2030:
VACCINATION: 90% of girls vaccinated against HPV by the age of 15 years
SCREENING: 70% participation in twice-lifetime cervical screening with a high precision approach such as HPV testing
In 2021/22, the Australian Government announced the development of a collaborative National Cervical Cancer Elimination Strategy. ACPCC has been contracted to lead the development of the strategy.
This Strategy will inform Australia’s future activities to optimise culturally safe and inclusive vaccination, screening and treatment services in order to close health gaps and eliminate cervical cancer as a public health problem in Australia by 2035.
TREATMENT: 90% complete treatment of pre-invasive lesions and invasive cancer
Cervical cancer is almost entirely preventable. Yet globally, one woman dies of cervical cancer every two minutes.
Announced in November 2021, Australia will be one of the first countries worldwide to offer the game-changing self-collection for cervical screening tests as a choice for all people who are screening under the National Cervical Screening Program.
From 1 July 2022, all women and people with a cervix will have the choice to screen using either a self-collected vaginal sample or a clinician-collected cervical sample. In contrast to other high-income countries, self-collection will be principally offered and provided through primary care.
Self-collection has enormous potential to remove many of the cultural, personal and logistical barriers that discourage many women and people with a cervix from screening. These barriers particularly affect people living in remote areas, Aboriginal and Torres Strait Islanders, culturally and linguistically diverse people, and gender and sexually diverse people.
Over 900 women were diagnosed with cervical cancer in Australia in 2021, with more than 70% of cases occurring in women who have never screened or were not up-todate with screening at the time of diagnosis.
Offering self-collection to all people eligible for screening will be a game changer for the National Cervical Screening Program, helping to address major barriers to participation and under-screening rates.
ACPCC is leading and promoting the increased uptake of HPV self collection, consistent with our 2020-25 Strategic Plan
Offering self-collection to all women and people with a cervix is a 'game changer' for the national cervical screening program.

THREE – SUPPORT COUNTRIES IN THE INDO-PACIFIC REGION TO SCALE UP TO MEET THE 2030 TARGETS IN SUPPORT OF THE WHO STRATEGY TO ELIMINATE
EIGHT – LEVERAGE THE VALUE OF THE CANSCREEN® AND CANVAX® PLATFORMS FOR COST EFFECTIVE SUPPORT OF LOW TO MIDDLE INCOME COUNTRIES (LMICS) AND FOR
ACPCC acknowledges the people and the Elders of the Aboriginal and Torres Strait Islander Nations who are the Traditional Owners of the land and seas of Australia in which we work and live. We pay our respect to Elders past, present and emerging.

To prevent cancer and infectious diseases through excellence in the provision of public health services supporting screening, population-based testing and vaccination.
ACPCC acknowledges the support provided by the Victorian and Commonwealth Governments which has been invaluable in enabling ACPCC to deliver outstanding service to participants in public health programs through its laboratory and registry services.


Australia is a world leader in achieving cervical cancer control in our population and with a new name, the Australian Centre for the Prevention of Cervical Cancer, we are extending our leadership for global elimination.
ACPCC is a not-for-profit organisation established in 1965 to make a positive difference in the lives of Victorian women by reducing the impact of cervical cancer.
With a proud 57-year history of contributing to Australia’s National Cervical Screening Program through laboratory services, registry programs, research and evidence-based policy recommendations, ACPCC is well positioned to leverage its expertise to support the WHO’s Strategy for all countries to end the suffering caused by cervical cancer.
Our unique suite of services are designed to implement, support, monitor and manage population health programs including cancer screening and vaccination. Our multi-disciplinary team leverages deep expertise and an ability to cost effectively support and deliver large scale programs.
ACPCC is a trusted advisor to governments locally and globally, participating in numerous committees that are supporting the shift from cytology to HPV screening and the delivery of HPV vaccination in Australia and around the world.
We are evolving our services and projects as we move towards the target of cervical cancer elimination.
VCS Foundation Ltd, trading as the Australian Centre for the Prevention of Cervical Cancer (ACPCC), is a Company Limited by Guarantee that operates under and complies with the:
• Corporations Act 2001 (Cth)
• Australian Charities and Not-for-profits Commission Act 2012 (Cth)
• Improving Cancer Outcomes Act 2014 (Vic).
VCS Pathology is a specialist laboratory and medical education service committed to gynaecological health including HPV testing, histopathology, cytology and related molecular microbiology, clinical support and advice. We are Australia’s HPV and cervical screening reference laboratory. SARS-CoV-2 testing was introduced in 2020 to support the Victorian Government response during the pandemic.
Our Digital Health team are market leaders in innovative, integrated digital healthcare solutions and services that deliver improved health outcomes. Our core capabilities include population health management platforms and a broad range of IT service management expertise.
With a 25-year track record in building, integrating, deploying and supporting advanced eHealth solutions, our products and services are used by governments and researchers around the world.
Our Population Health team provide a combination of experience in delivering and managing population health services through registry services, epidemiology, research and evaluation, health information management, reporting and statistical analysis which allows us to find the best solutions to improve health outcomes for everyone. This unique skill mix is key to our success and international reputation for high-quality, policy-relevant research focused on preventing cancer and infectious disease. Our team of experts work collaboratively and invest in strategic relationships with clients, government, program partners and stakeholders. We are committed to being strong and effective advocates for population health programs and have extensive experience as advisors and experts in population screening and vaccination.


ACPCC is a key partner of Australia’s National Health and Medical Research Council (NHMRC) funded Centre of Research Excellence (CRE) in Cervical Cancer Control.

The Centre of Research Excellence in Cervical Cancer Control, known as C4, was established in late 2017, to bring together cervical cancer control experts undertaking research and evaluation of HPV vaccination and screening programs. It is funded by the NHMRC.
The work of C4 will ensure the future of cervical cancer control is underpinned by world-class research with the potential to inform substantial reductions in the global impact of cervical cancer. The core group consists of researchers from the Cancer Council NSW, ACPCC, the University of Melbourne and the Kirby Institute with combined expertise in epidemiology, public health, laboratory testing, clinical trial implementation, predictive modelling, and economic evaluation. Our associate investigators bring additional expertise and perspectives from a range of organisations.
For details, please visit the C4 website at www.cervicalcancercontrol.org.au
ACPCC strongly values its working relationships with our partners, which include Government Departments both State and Commonwealth, Cancer Councils, medical colleges, universities, major teaching hospitals, sexual and reproductive health services, primary care and community organisations, and technology and device service providers. ACPCC is a key contributor to state, national and international cervical cancer control policy and initiatives.
ACPCC staff continue to serve in-kind on expert advisory committees and participate in working groups and forums that support both the Commonwealth and Victorian Governments in relation to cancer reporting and prevention, cancer screening and immunisation. See Appendix A for a full list of our Committee involvement in 2020/21.
Through partnerships and collaborations, ACPCC encourages and supports health improvements and health equity. We are proud to be a member of the Global Health Alliance, BioMelbourne Network, The Public Health Association of Australia, Public Pathology Australia and the Union for International Cancer Control.





2021/22 was a year of evolution. We were pleased to announce our new name, the Australian Centre for the Prevention of Cervical Cancer, commencing a new era reflecting our role as Australia’s leading organisation in cervical cancer prevention.
Our new name ensures that we are globally recognised for our purpose in cervical cancer elimination. While the name change is significant and reflects the change from cytology to molecular diagnostics, the work that the ACPCC delivers, our collaborations and impacts will continue to be the real measures of our success.
ACPCC is proud to be supporting the Australian Government to develop a collaborative National Strategy for Cervical Cancer Elimination. The strategy will have a strong focus on addressing current inequities so that Australia can achieve elimination of this preventable disease for everyone. Australia is on track to be potentially the first country in the world to reach the agreed elimination target of 4 cases of cervical cancer per 100,000 women per year.
Australia will be one of the first countries in the world to offer the game-changing self-collection for cervical screening tests as a choice for all people who are eligible for screening. This change was announced in November 2021 and will be implemented from 1 July 2022. It has been made possible by the emergence of evidence that shows self-collected vaginal samples are as accurate as clinician collected cervical samples for the detection of cervical pre-cancer.
By making self-collection universally available it is hoped that there will be greater uptake of self-collection by healthcare professionals and that this will facilitate access to screening for under-screened populations, including but not limited to Aboriginal and Torres Strait Islander people, members of the Lesbian, Gay, Bisexual, Trans and Intersex (LGBTQIA+) community, those from culturally and linguistically diverse communities, people with disability and those living in rural and remote areas of Australia.
Recognising that self-collection would be one of the most important tools available to support Australia’s goal to achieve elimination of cervical cancer by 2035, VCS Pathology was the first laboratory in Australia to be accredited by the National Association of Testing Authorities (NATA) to process self-collected HPV samples. We have invested in new automated technology this year to ensure our readiness for the increases in volumes of selfcollected samples.
Our early adoption of self-collection has positioned the ACPCC to provide expert advice through clinician education and resource materials, to support the expected increase in testing following government’s announcement.
Despite the year being marked by continued COVID-19 lockdowns impacting our people and operations, our staff have worked together to ensure uninterrupted delivery of high-quality testing, expert advice and population health initiatives.
Our Executive Team, Board Directors and staff are excited about this new phase for the organisation, and we look forward to continuing to work with all of our stakeholders into the future.
Our thanks go to fellow Board members for their unwavering leadership and generosity of time; particularly Board Director Dr Christine Selvey who leaves us after 9 years of valuable contribution as an immunisation expert representative. And of course, to all of our employees and stakeholders without whom none of our achievements would be possible.
Mr Tim Humphries Chairman Professor Marion Saville, AM Executive Director
Professor Marion Saville, AM Executive Director
Australia will be one of the first countries in the world to offer the game-changing self-collection for cervical screening tests as a choice for all people who are eligible for screening.

OPERATING RESULT $10,345,325 UP FROM $5,107,489 IN 2020/21
TOTAL EXPENSES $33,328,085 UP FROM $31,392,822 IN 2020/21
TOTAL REVENUE $43,673,410
The support provided by the Victorian and Commonwealth Governments has been invaluable in enabling ACPCC to deliver outstanding service to participants in population-based health programs through its laboratory and registry services.
The positive results for 2021/2022 were in large part a consequence of high volumes of SARS-CoV-2 testing opportunities from the COVID-19 pandemic, not our core cervical screening work. This extraordinary revenue is not expected to continue because, although the pandemic is not over, SARS-CoV-2 PCR tests are not expected to return to the same high volumes, even during future waves of infection. This is a consequence of a change in approach to the pandemic together with the wide uptake of rapid antigen tests.
The ACPCC Strategic Plan 2020-2025 was developed by the ACPCC Board of Directors in consultation with the Executive Team.
Over the five years of the plan, we commit to assisting the Victorian Government, the Commonwealth and countries in the Indo-Pacific region to eliminate cervical cancer as a public health issue.

We continue to support the National Bowel Cancer Screening Program through the delivery of our funded services.
We will build on laboratory service excellence by diversifying the range of VCS Pathology test capabilities and our unique position as the HPV Reference Laboratory.
This Annual Report showcases the activities of ACPCC against the Strategic Plan.
1. Support Victoria’s efforts to eliminate cervical cancer as a public health problem by a target date agreed with the Department of Health, in accordance with the Victorian Cancer Plan 2020-24. Page nos 22-27

2. Support Australia’s efforts to eliminate cervical cancer as a public health problem by 2035. Page nos 28-29
3. Support countries in the Indo-Pacific region to scale up to meet the 2030 targets in support of the WHO strategy to eliminate cervical cancer as a public health problem. Page nos 30-35
4. Lead and promote the increased uptake of self-sampling. Page nos 36-37
5. Support the National Bowel Cancer Screening Program in Victoria. Page nos 38-39
6. Deliver and disseminate the research outcomes of the Compass trial, C4 and other policy relevant research. Page nos 40-43
7. Diversify the range of VCS Pathology laboratory tests by leveraging our existing expertise and capital investment. Page nos 44-51
8. Leverage the value of the canSCREEN and canVAX platforms for cost effective support of Low to Middle Income Countries (LMICs) and for commercially advantageous opportunities. Page nos 52-53
9. Reshape the business model to adapt to our new commercial environment and global opportunities. Page nos 54-55
ACPCC has been engaged by the Commonwealth to develop a National Cervical Cancer Elimination Strategy by early 2023. The overarching target for Australia is to eliminate cervical cancer (<4 cases per 100,000) by 2035.
Cervical Screening Educational Webinars were developed to update health care professionals on changes to the National Cervical Screening Program, including self-collection.
VCS Pathology prepared to scale-up its laboratory services and processes for Universal Self Collection following the Commonwealth’s announcement that all people eligible for a cervical screening test will be able to collect their own sample from 1 July 2022.
canSCREEN® population health management platform was successfully deployed to the Western Highlands province of Papua New Guinea and Vanuatu.
12,368 Victorian Bowel Cancer Screening Participants were successfully contacted to confirm positive results and support follow-up.
VCS Pathology launched its 'Every Kit Counts' campaign for Healthcare Practitioners, including GPs and Nurses, to drive bowel cancer screening participation to 60% and save up to 84,000 lives by 2040.
VCS Pathology reported:
312,878 SARS-CoV-2 tests
96,866 HPV tests (which includes 3,400 Self-collected HPV tests)
40,004 Liquid Based Cytology tests
2,706 Histology cases
5,671 Chlamydia tests
3,305 Gonorrhoea tests
11,316 Compass tests
Total throughput of tests in the laboratory in 2021/22: 472,746
DATA AND REPORTING TOOLS FOR CERVICAL, BOWEL AND BREAST SCREENING PROGRAMS, IN PARTNERSHIP WITH VICTORIAN SCREENING PARTNERS
In May 2019, the Victorian Department of Health endorsed a Victorian Cancer Screening Framework, a new governance and funding model for Victoria’s cervical, breast and bowel screening programs. A three-year strategy and annual activity plan was developed by the Victorian Cancer Screening Steering Committee which comprises members from the Victorian Department of Health, ACPCC, BreastScreen Victoria, Cancer Council Victoria and the Victorian Aboriginal Community Controlled Health Organisation. The Committee works together to reach annual goals and implement the overall strategy.
The Victorian Department of Health and the Victorian Cancer Screening Steering Committee identified the need for a more integrated approach to cancer screening data and surveillance in Victoria to ensure that screening program partners can better utilise combined screening data to deliver screening systems improvements and drive equitable outcomes.
The Victorian Cancer Screening Framework Data Dashboard provides a snapshot of key data from the Bowel, Breast and Cervical Screening Programs to key stakeholders. The Dashboard includes data on invitations, screening numbers, participation, cervical selfcollection, time to colposcopy and time to colonoscopy. Data is grouped and presented in a series of tabs containing tables and charts, stratified by parameters such as time period, Aboriginal and Torres Strait islander status, age group, targeted initiatives, and geographical area.
The latest dashboard submitted to the Steering committee in June 2022 included updates until 31st March 2022 for invitations, screening episodes and targeted areas.
The data captured by our Population Health team ensures that timely, accurate and rich information is available to the Department, cancer screening agencies, health service providers, researchers and other stakeholders for the purposes of:
• Informing cancer screening service planning at the local, regional and state level
• Developing and evaluating initiatives to improve screening program participation, access and performance
• Identifying under-screened groups and monitor their participation rates, and
• Supporting research and evaluation to improve cancer outcomes for all Victorians.
Key objectives of the cancer screening data and surveillance strategy:
• Ensure access to timely and accurate data to support service planning and program delivery across each program’s cancer screening pathway
• Utilise data to establish and monitor baseline participation rates and improvements in screening participation outcomes, and
• Develop consistency and integration across cancer screening datasets.
It is with much regret that in August 2022 we said goodbye to Genevieve Chappell after 14 years of dedicated service leading the Population Health Team at the Australian Centre of Cervical Cancer Prevention.
Genevieve has seen many changes in the organisation, particularly as we transitioned our registry services to the National Cancer Screening Register. She has ably managed the requirements of the Department of Health Victoria and has developed strong relationships with their Screening and Cancer Prevention Team. This rapport leaves us with a solid base to continue our important work with the Department of Health in establishing the Victorian Cancer Screening Data and Monitoring Framework.
Genevieve has been a valued and well-respected presence in the organisation and has steered and supported her staff through many challenges, including the COVID-19 pandemic and associated lockdowns. She will be greatly missed by her immediate colleagues and the Executive Team.


On 25 November 2021, ACPCC and Cancer Council Victoria launched the 'Self-Collection Saves Lives' campaign. The campaign, funded by the Victorian Government, was targeted at encouraging healthcare practitioners to offer self-collection for cervical screening to under-screened women and people with a cervix across Victoria. At the time of the campaign, selfcollection was limited to those aged 30 years or over who had never been screened, or who were two or more years overdue.
The campaign will help achieve the Victorian Government’s target of encouraging an additional 10,000 under-screened women and people with a cervix to screen via selfcollection, in line with Victorian Cancer Plan 2020-2024.
Self-collected samples are as accurate as samples collected by a General Practitioner or nurse for the detection of pre-cancerous lesions and cancers. Importantly, our research in Victoria has shown that self-collection is highly acceptable in a range of under-screened groups who otherwise choose not to screen.
Despite self-collection being available since 2018, uptake in Victoria has remained low, with a lack of awareness about self-collection among some health practitioners. It is hoped that the campaign will address this lack of awareness and thereby increase availability to under-screened people in Victoria.
Melbourne-based GP Dr Sally Cockburn, also known as Dr Feelgood, said: “Self-collection is a great option to test for HPV, especially for those who have issues being examined. In particular, certain cultural groups who can’t be examined by a male but don’t have access to a female health professional. Many patients are already eligible and now is a good time to offer it to them.”
Kate Broun, Head of Screening, Early Detection and Immunisation, Cancer Council Victoria, said: “We know that many women and people with a cervix face a range of barriers to accessing screening. Self-collection provides an opportunity to address these barriers. For those who have never screened, research shows that taking part in self-collection even just once, could reduce the risk of developing cervical cancer by around 40 per cent and by even more if they become regular screeners.”
ACPCC offers free clinical education on cervical screening for healthcare professionals. Our Liaison Physicians support healthcare professionals to implement cervical screening clinical guidelines and program policies, and to confidently discuss cervical screening with their patients.
Our Medical Education and Liaison Team have significant experience in health professional education and in clinical practice and are available by phone or email to discuss queries relating to cervical screening and changes in the guidelines.
ACPCC’s clinical education sessions and resources are evidence-based and updated regularly. Cervical screening clinical education is carefully tailored to meet professional and clinical learning objectives and attracts Continuing Professional Development (CPD) Points.
We provide clinical education for:

• General practitioners (GPs)
• Nurse Cervical Screening Providers
• Obstetricians and gynaecologists (O&Gs)
• GP and O&G registrars
• International Medical Graduates
• Aboriginal and Torres Strait Islander Health Workers
• Practice Nurses
• Medical and nursing students. COVID-19 restrictions have resulted in limited face to face contact, however our education sessions can be organised as on-line events, or health practitioners can access our:
Pre-recorded webinars including:
• Cervical Screening: Universal Access to Self-Collection and Optimising the Experience for People with Intellectual Disability

• Meeting the Cancer Screening Needs of the LGBTQIA+ Community.


eLearning modules:
• Cervical Screening, HPV and Self-Collection: Clinical Education Course Developed in partnership with ModMed for GPs and Nurse Cervical Screening Providers.
Online and CPD points are available
| ACRRM: 2.5 Educational Activity hours, 2 performance review hours
| APNA: 4 hours | ACN: 4 hours
• Changes to the cervical screening intermediate risk pathway, developed in partnership with Queensland Health and True Relationships & Reproductive Health
• Breast, bowel and cervical cancer screening, in depth training and education for GPs and Nurses on bowel, breast and cervical screening programs. CPD Points are available
| RACGP: 8-point (4hr) CPD Activity for 2021-23 Triennium.
Work is underway to replace our frequently viewed 'How to take Pap test video' with updated guidelines including self-collection
• Responded to over 800 clinical queries from healthcare professionals
• Provided 24 education sessions, focussing on the changes to the National Cervical Screening Program, reaching a combined audience of approximately 600 healthcare professionals including GPs, nurses, and community health representatives

• Attended 13 GP practice visits (resumed in 2022 following the relaxation of COVID-19 restrictions).
VCS Pathology previously received block funding from the Commonwealth Government for Cervical Screening Tests (CSTs), enabling Nurse Cervical Screening Providers to conduct cervical screening independently. This has been critical in areas and communities across Victoria who face barriers to accessing GPs. Nurse-led cervical screening services have helped reach and engage these underserved communities.
From 1 July 2021, VCS Pathology transitioned to Medicare billing for CSTs, aligning to all other Commonwealth funded laboratories across Australia. Test requests now require a GP or Nurse Practitioner provider number and signature. This means Nurse Cervical Screening Providers can no longer independently provide CSTs and must have a GP or Nurse Practitioner co-sign a CST request.
VCS Pathology conducted a short survey with 65 Victorian Nurse Cervical Screening Providers on the impact of this change. Almost half of all respondents have reported significant impacts to their practice. The main concern raised by nurses was the adverse outcomes for patients –

including continuity with the health service, service accessibility and the valuable relationship built between nurse and patients. Other strong themes emerging from this survey include the loss of autonomy and scope of practice and increased administrative burden across the whole service. Furthermore, a small number of nurses have chosen to stop providing cervical screening completely and had no choice but to give up their aspirations of setting up their own private cervical screening clinic.
The loss of nurse autonomy in providing cervical screening will have a negative impact on Australia reaching the overarching target to eliminate cervical cancer (<4 cases per 100,000) by 2035. ACPCC are advocating to seek innovative models of care, including nurse-led cervical screening and Nurse Colposcopists, to bridge the health inequities currently experienced across vulnerable populations.
The ACPCC is conducting a project with Service Plan funding from the Victorian Department of Health to explore options for a state-wide Nurse Colposcopy Model. The purpose of the project is to increase timely and equitable access to public colposcopy services in Victoria.
The first phase of the project included an environmental scan incorporating a rapid review of the literature, a review of Australian and International models of nurse colposcopy, and key informant interviews.
Following review of the findings of the consultation, if in-principle support for a Nurse-led Colposcopy Model is confirmed, the project will proceed to develop a high-level Nurse Colposcopy model. This will include a plan for implementing a pilot and consideration of sustainability, scalability, risks and barriers. An Advisory Committee will be established to support the project.

A suite of resources has been developed providing updated information on screening pathways, reflecting the recent changes to the National Cervical Screening Program. A comprehensive Cancer Screening Resource Kit for Primary Care is available that includes links to essential resources and websites for:

1. General cancer screening
2. Bowel screening
3. Breast screening
4. Cervical screening
5. Aboriginal and Torres Strait Islander cancer screening
6. Engaging with under-screened Communities
7. Screening during the COVID-19 Pandemic.
These resources are designed to give healthcare providers a clear understanding of the correct screening pathways for their patients and as tools for practitioners to keep up to date with any changes to the screening program. A set of resources to support screening for Aboriginal and Torres Strait Islander people has also been prepared in consultation with the Victorian Aboriginal Community Controlled Health Organisation.
Work is underway to replace our frequently viewed 'How to take Pap test video' with updated guidelines, including self-collection, and will be ready for distribution by November 2022.

The ACPCC’s Population Health research team continues to undertake research and evaluation of Victorian public health activities supporting cervical cancer prevention and eventual elimination. Professor Julia Brotherton has assumed a part time position as Professor of Cancer Prevention Policy and Implementation in the Evaluation and Implementation Unit of the Centre for Health Policy at the Melbourne School of Population and Global Health, University of Melbourne. Collaborative Victorian projects involving the University of Melbourne, and other key partners include:
• Victorian Department of Health funded multi-method evaluation of the North-West Melbourne COVID-19 Response Cancer Screening Project, which aimed to improve participation in selfcollection based cervical screening, bowel screening and hepatitis screening. The pilot project resulted in expansion to a statewide initiative ‘Maximising cancer screening’ (2022-2023) which is again being evaluated by ACPCC and the University of Melbourne team
• Victorian Cancer Agency funded Project Grant ‘Improving the benefits of the renewal of the National Cervical Screening Program for Victorian Aboriginal women.’ This implementation research study is partnering with Victorian Aboriginal Community Controlled Health Organisation and Aboriginal Community Controlled
Health Organisations across Victoria codesigning strategies to support cervical screening and follow-up. A co-designed strategy of offering universal access to self-collection was completed at the Ballarat and District Aboriginal Co-operative as part of this Project.
• Victorian Cancer Agency Cancer Prevention and Screening Research Grant. Piloting a model of universal self-collection for cervical screening in primary care in Victoria: The solution to a decade of declining participation and to longstanding inequity? This study is supporting practices to implement the choice of self-collection to all people eligible for cervical screening and evaluate the impact of the implementation in general practice of universal access on uptake of cervical screening.

The World Health Organization officially launched the Global Strategy to Accelerate the Elimination of Cervical Cancer in November 2020:
• Goal – Eliminate cervical cancer by reaching an incidence of <4 per 100,000 women in every country within the next 100 years.
To reach elimination, the WHO Strategy sets out three targets to be met by every country by 2030:
• VACCINATION – 90% of girls vaccinated against HPV by the age of 15 years
• SCREENING – 70% participation in twice-lifetime cervical screening with a high precision test (such as HPV testing)
• TREATMENT – 90% complete treatment of pre-invasive lesions and invasive cancer.
The adoption of the Strategy at the World Health Assembly was co-led by Australia, leveraging our country’s strong history of global leadership in cervical cancer prevention programs, technology and research innovation.
As a result of Australia's world-leading HPV vaccination and cervical screening programs, innovative clinical trials and high-quality cancer treatment and care, Australia has amongst the lowest rates of cervical cancer incidence and mortality in the world. Based on Australia’s success, the government is playing a leading role in the global commitment to eliminate cervical cancer as a public health problem.
The Australian Government has committed to eliminate cervical cancer in Australia by no later than 2035 and we are on track to achieving this goal.
However, incidence and mortality rates vary across Australia, and certain groups experience lower outcomes.
Whilst modelling suggests that Australia is on track to be the first in the world to reach cervical cancer elimination, addressing these inequities is necessary to achieve elimination for all women and people with a cervix in Australia.
We must address the existing inequities in access to culturally safe and inclusive HPV vaccination, screening, and timely diagnosis and treatment services, to achieve elimination for all women and people with a cervix.
In Australia, cervical cancer inequities exist depending on who you are, where you live and your socioeconomic status.
• Aboriginal and Torres Strait Islanders
• Culturally and linguistically diverse communities
• LGBTQIA+
• People with disabilities
• People who have experienced sexual violence
Where you live
• Remote and very remote areas that have 14.5 and 13.4 new cases per 100,000 women, compared to 10.1 for major cities
• Very remote areas: 3x higher mortality rate than the national average
• People in the lowest SES quintile are 2.75 times more likely to die from cervical cancer than those in the highest SES quintile
The National Cervical Elimination Strategy is being developed in consultation with key stakeholders across the three pillars of cervical cancer elimination and with an overarching equity lens.
We passionately believe that no person, and no groups of people, should be left behind as Australia pursues elimination. Our partnerships with leading Indigenous researchers and communities are about ensuring we play our part in closing the gap in cervical cancer burden for Aboriginal and Torres Strait Islander people.

On the 17 November 2021, the inaugural World Cervical Cancer Elimination Day, the Federal Minister for Health, the Hon Greg Hunt MP, announced that the Australian Centre for the Prevention of Cervical Cancer has been engaged to develop a National Cervical Cancer Elimination Strategy to eliminate this preventable disease by 2035.
The development of a National Cervical Cancer Elimination Strategy will set Australia on the path to meet its commitment to the WHO global strategy.
The Strategy is expected to be completed by April 2023, allowing sufficient time for meaningful consultation with technical experts and priority communities. ACPCC will ensure that under-screened population groups are prioritised and central to the development and endorsement of the Strategy.
The development of the strategy is being overseen by the Expert Advisory Group for the Elimination Response. This governance group, chaired by Professor Karen Canfell from the Daffodil Centre is made up of members who are expert in vaccination, screening and cancer treatment together with leaders of communities known to have poorer access to preventative health and treatment services.
Between May and July 2022, ACPCC undertook consultations to inform the Strategy. We heard from 401 individuals through an online survey, focus groups with priority populations and technical workshops with relevant experts.
An initial draft Strategy has been prepared and, following review by our sub-advisory groups and the Department of Health the draft Strategy will go to public consultation.


Hosted by ACPCC and the NHMRCfunded Centre of Research Excellence in Cervical Cancer Control (C4), PCC2022 will bring together the best and brightest researchers, policymakers and health sector leaders across Australia, New Zealand, the Indo-Pacific region and beyond to discuss how we can work together to achieve a cervical cancer free future for women and girls across our region.
PCC 2022, originally scheduled for March 2022, was delayed due to a spike in COVID-19 cases around the world and was moved to 16-18 November 2022 in anticipation that more people, including overseas speakers, will be able to attend in person at this time.
The PCC2021 conference was a National Finalist in the category Best Charity or Cause-Related Event (Virtual, Live or Hybrid) at the Australian Event Awards.
At the Preventing Cervical Cancer Conference in March 2021, the Minderoo Foundation, together with NHMRC Centre of Research Excellence in Cervical Cancer Control (C4), announced a first-of-its-kind humanitarian and research effort to eliminate cervical cancer in the Western Pacific.


The Western Pacific has among the highest rates of cervical cancer in the world, with an estimated 1,200 deaths in Papua New Guinea (PNG) alone every year. HPV causes almost all cervical cancers worldwide. HPV infection and disease can be prevented by childhood vaccination, whilst screening women for HPV infection in adult life is highly effective in detecting early disease and preventing cervical cancer. The project aligns with the WHO Strategy to eliminate cervical cancer worldwide by the end of the century, through a ‘triple-intervention’ approach, which sets out three simple targets to place all countries on the path toward elimination by 2030: Vaccination, Screening, Treatment.
Within C4, this initiative has been led by Professor Karen Canfell (Daffodil Centre), Professor Marion Saville (ACPCC), Professor Andrew Vallely (Kirby Institute, UNSW and PNG Institute of Medical Research, Goroka) and Professor Deborah Bateson (Daffodil Centre).
The project will put the WHO eliminate cervical cancer concept into practice, leading the world to show how the tripleintervention strategy of HPV vaccination, HPV-based screening and cancer treatment can be introduced into a priority region. It will create a sustainable framework for attracting additional partners and will act as a catalyst for cervical cancer elimination globally.
• The Australian research arm will conduct modelling and analysis to inform the most efficient and effective ways to implement and then expand the initiative.
• The Eliminate Cervical Cancer in the Western Pacific project will be core to a range of active and emerging regional and local partnerships in the Western Pacific region, which will be essential to achieving cervical cancer elimination worldwide.
• A program has been established in PNG and Vanuatu to provide effective and equitable HPV-based cervical screening programs for women aged 30-54 years.
ACPCC's canSCREEN® e-health platform has been optimised for PNG local health care teams, to record and track women's results and follow-up care in remote regions. The canSCREEN® registry went live in PNG on 28 May 2022. The solution includes a mobile application for capturing information whilst offline, and a desktop application integrating to the GeneXpert HPV on-demand PCR test that provides actionable HPV results in approximately one hour. PNG has successfully started uploading patient data and test results into their canSCREEN® registry in the Mt Hagen Well Women’s Clinic. This was the first instance of canSCREEN® supporting the 'screen and treat' model for HPV screening. The PNG team have now screened several hundred patients, with testing continuing to increase as the program expands.
ACPCC is the final stages of rolling out canSCREEN® to Vanuatu, working with our project partners to facilitate implementation.
“Developing a locally appropriate cancer screening registry is crucial in scaling up cervical screening programs in low and middle-income countries such as PNG.

ACPCC’s canSCREEN e-health platform will be optimised for PNG local health care teams to record and track women’s results and follow-up care.”
ACPCC is leading a project to implement self-collected HPV-based cervical cancer screening in vulnerable populations in urban and rural India. Funded through the GACD and the National Health and Medical Research Council (NHMRC), ACPCC was awarded a $1.3 million grant for a three-year research project implementing cervical cancer screening in hard-to-reach and vulnerable Indian communities.
India is the world’s second most populous nation. The burden of cervical cancer is high, at one-fifth of the total global burden. This equates to 60,000 Indian women dying from cervical cancer every year.
Professor Julia Brotherton is leading the implementation research project with partners in two states of India, Tamil Nadu in Southern India and Mizoram in North-eastern India, along with experts on cancer and disease prevention from the International Agency for Research on Cancer, the Baker Heart and Diabetes Institute and RTI International.
Building on previous collaborative and world-leading research supporting the use of HPV-based screening and treatment for screening, Professor Andrew Vallely from the Kirby Institute at UNSW Sydney is leading a project on HPV testing and treatment for the elimination of cervical cancer in rural and remote Papua New Guinea (PNG). This $1.59 million project is complementary to the Minderoo project and is being conducted in collaboration with ACPCC (investigators Professor Marion Saville and Professor Julia Brotherton), Cancer Council NSW,
Family Planning NSW, and the NHMRC Centre of Research Excellence in Cervical Cancer Control (C4), and incountry partners and stakeholders in PNG. ACPCC is supporting the project with our canSCREEN® registry platform (see page 52), as well as our laboratory, epidemiological and screening expertise. The project will utilise the novel ‘test and treat’ screening model developed by our collaborative research team, comprising HPV testing of self-collected vaginal specimens followed by sameday treatment using a battery operated, portable thermal ablation device. This approach proved highly effective, acceptable and cost-effective in previous field trials.
The team will now establish how best to reach women in rural and remote communities, where cervical pre-cancer and cancer rates are highest, access to health services most constrained, and the most at-risk women in PNG and other high-burden, low-resource countries live.
Professor Marion Saville, Executive Director
The trial leverages the benefits of the canSCREEN® platform, which was successfully implemented in early 2022.
This self-collection research is led by Prof Bev Lawton, Director, Centre for Women’s Health Research Centre, at Victoria University of Wellington, New Zealand.
The Te Tātai Hauora o Hine Centre for Women’s Health Research and Mahitahi Hauora Primary Health Entity has committed to a trial implementing HPV self-collection in Te Tai Tokerau in northern New Zealand. The trial aims to demonstrate that a screening approach founded on the universal offer of HPV self-collection is at least as effective as the current cervical cytology. The study will play a critical part in informing the

transition of New Zealand’s National Cervical Screening Programme from cytology-based screening to HPV based screening, including the option of self-collection.
The trial leverages the benefits of the canSCREEN® platform, which was successfully implemented in early 2022. The canSCREEN® team integrated the data and test results from the laboratory in New Zealand directly into canSCREEN® with automated data population.
The trial has already screened many hundreds of women and is actively using the SMS and email follow-up functionality of canSCREEN® to effectively communicate results and schedule follow-up treatments.
Project ECHO (Extension for Community Healthcare Outcomes) is a movement to share knowledge and amplify local capacity to provide best practice care for people all over the world. We utilise the ECHO model™ to provide peer-to-peer and expert support to multi-sectorial country teams in the Indo-Pacific answering the WHO call to scale up cervical cancer prevention to eliminate cervical cancer as a public health problem.
The SUCCESS (Scale Up for Cervical Cancer Elimination Strategy Success) in the Indo-Pacific ECHO aims to provide ongoing, monthly consultations with practitioners seeking and contributing peer advice and expert input regarding the challenges faced when scaling up to reach the 2030 goals. In establishing the SUCCESS ECHO, we invited and expanded upon the existing community of practice established by the US National Cancer Institute (NCI) in its 2017-18 APEC cervical cancer
prevention ECHO. Using ZOOM, we hold a monthly tele-mentoring session to provide a sustained exchange opportunity with the motto ‘All teach, all learn’. Over 90 minutes, following a short didactic from an expert with discussion, participating country teams, including both WPRO and SEARO WHO region countries, present case studies from their program or scale up projects for feedback and ideas from regional colleagues and international experts. Monthly session themes alternate between the three elimination pillars of screening, treatment and vaccination.

Over 230 colleagues from across the region and beyond are signed up to SUCCESS, with 31 sessions held to date. Participants are from at least 35 countries and include clinicians, researchers, Ministry of Health staff and NGOs.
SUCCESS ECHO is supporting shared learning in relation to elimination and building ongoing networks and collaborations between participants.
ACPCC hosts monthly Screening, Treatment and Vaccination tele-mentoring sessions for Australia and the Indo-Pacific

Project ECHO (Extension for Community Healthcare Outcomes) is a movement to share knowledge and amplify local capacity to provide best practice care for people all over the world.
Despite being a largely preventable cancer, cervical cancer remains the third most common cancer amongst Malaysian women. Only 25% of eligible Malaysian women have ever had a cervical screening test, despite efforts to raise public awareness and provide access to screening in healthcare facilities.
Barriers to a successful screening program include fear, embarrassment, inconvenience, lack of awareness about cervical screening, poor infrastructure, and lack of dedicated resources and staff. To execute its program at scale, ROSE leverages community engagement, upskilling of healthcare professionals, mobilising community volunteers, and collaborating with other strategic community development initiatives and health services, such as breast screening and social welfare.
Program ROSE (Removing Obstacles to Cervical Screening) was established in June 2018 as a collaborative initiative between ACPCC, the University of Malaya and the Malaysian Ministry of Health, to build an innovative cervical screening program to enhance Malaysian women’s personal journeys of maintaining good cervical health. It commenced as a pilot program, targeting 2,000 women across five clinics in Malaysia and has since expanded across the country to screen over 20,000 women.
The Program ROSE service model components of self-sampling, HPV testing and canSCREEN®, together with global partnerships, local expertise and community mobilisation, provides critical learnings and insights into screening models in countries who are striving to eliminate cervical cancer.


The key elements of the ROSE service model are:
1. Self-sampling: women are empowered to take their own sample for screening and have the result sent to their phone via SMS that same day
2. HPV testing: Compared to a Pap smear for cytology testing, PCR-based testing for HPV is a far superior test that can detect all HPV types known to cause cervical cancer
3. Digital Health platform canSCREEN®: to track the progress and management of every woman screened through their lifetime, regardless of where she was screened. Women with a positive HPV test are also provided with a phone number for counselling and organizing their follow-up care.
1 2 3
Featuring selfsampling by women themselves, instead of pelvic examination by healthcare professionals.
As at the 30 June 2022, Program ROSE has screened 20,873 Malaysian women.
Positive (6%)
Negative (94%)
Default for follow up (10%)
Waiting to attend follow up (10%)
Completed follow up (80%)
Using a secure digital e-health platform that empowers women to register and have all their follow up commuicated through their mobile phone.
The ROSE initiative demonstrates how the ACPCC has shared its knowledge and expertise in health, cervical screening and registry expertise with global partners to develop models of care appropriate to the local context.
As part of Cervical Cancer Awareness Week in November 2021, The Hon Greg Hunt announced that all people eligible for a cervical screening test will be able to collect their own sample from 1 July 2022.
Prior to July 2022, self-collection was only available to women aged 30 years or over, who had never screened, or were two or more years overdue.
Australia will be one of the first countries in the world to offer the ‘game-changing’ self-collect option to all participants through its National Cervical Screening Program.
From July 2022, Medicare is funding self-collection of NCSP tests for everyone who is eligible for cervical screening, making it easier to participate, especially for people who screen at low rates.
Whilst self-collection has become a universal option, those who prefer to have a screening sample collected by a doctor can continue to do so. Self-collection allows women to use a simple swab, similar to a COVID-19 nasal swab, to take a screening sample themselves instead of having a traditional cervical screening test completed by a clinician.
Australia is already on track to be the first country in the world to eliminate cervical cancer, but by making self-collection a universal option, we should get there sooner and in a more equitable way.
Self-collection is expected to improve overall screening participation rates, especially in under-screened populations including Aboriginal and Torres Strait Islander women as well as culturally and linguistically diverse women.
The self-collect tests can be accessed through health care providers, including GPs, ensuring these experts continue to play a critical role in supporting patients with cervical screening.
In anticipation of this announcement and to meet the needs of under-screened people prior to this time, VCS Pathology has been receiving self-collected samples since January 2018 and was the first accredited laboratory in Australia to do so. Our Liaison Physicians are well informed and ready to help with any queries relating to the new screening pathways for self-collected samples.
We also released our 'Cervical Screening, HPV and Self Collection' on-line clinical education course reflecting the policy changes around self-collection, accredited by the Royal Australian College of General Practitioners.
From the 1 July 2022, all people eligible for a cervical screening test will be able to collect their own sample, offering women more control and choice.
VCS Pathology has the tools and expertise to guide and educate health care professionals to confidently encourage HPV self-collection in the community.


An important component of the National Bowel Cancer Screening Program (NBCSP) is the follow-up of participants with a positive Faecal Occult Blood Test (FOBT) result. In 2012, ACPCC was contracted by the Victorian Department of Health to operate the Participant Follow Up Function (PFUF) to support Victorian bowel screening participants. The PFUF team have consistently met the targets and expectations of the Department.
The PFUF team assists with the followup of participants who have chosen to participate in the NBCSP and who have had a positive FOBT result. The PFUF team call participants who, according to the National Cancer Screening Register (NCSR), have either not followed up their positive FOBT result with their general practitioner/ healthcare provider or have not acted on their referral and progressed to having a colonoscopy. The PFUF team are routinely contacting the participant, their nominated general practitioners/ healthcare provider, their nominated personal representative and/or their treating specialist.
The purpose of the contact is to encourage follow-up of the result and progression through the screening pathway, and to accurately update the NCSR PFUF Portal with the current status of participants.
Since the commencement of the program, the PFUF team have followed up 87,267 participants, including 13,913 participants in the 2021/22 financial year, successfully reaching 12,368 of these participants to confirm that positive FOBT results were followed up.
On 17 September 2021, VCS Pathology launched its partnership with Cancer Council Victoria 'Every Kit Counts' campaign for Healthcare Practitioners, including GPs and Nurses to drive bowel cancer screening participation to 60% and save up to 84,000 lives by 2040. The initiative was funded by the Victorian Department of Health.
The campaign also targeted healthcare professionals to use the NCSR to identify eligible and under screened patients aged 50-74 years and order the NBCSP test kit together with their patient in an appointment.
On 17 September 2021, VCS Pathology co-launched the 'Every Kit Counts' campaign with Cancer Council Victoria for healthcare practitioners, including GPs and nurses to drive bowel cancer screening participation to 60% and save up to 84,000 lives by 2040.
In November 2021, the Australian Government announced additional funding for the ACPCC to provide operational support for the Compass Trial, jointly led by ACPCC and the Daffodil Centre (a Joint Venture of Cancer Council NSW and The University of Sydney).
Compass is Australia’s largest clinical trial with over 76,000 participants. It will provide crucial data to inform the optimum screening pathway for Australia’s National Cervical Screening Program in the future, with ever greater numbers of people who have received the HPV vaccine ageing into the screening program as they turn 25 years of age.
The roll out of the Compass pilot has already delivered major benefits to the Department and the NCSP by informing the implementation of the renewed screening pathway in December 2017, including laboratory and registry processes. It is expected to inform future changes to the NCSP by confirming that the HPV test is a superior screening method and to assess different ways of deciding which women need further investigations.
This is particularly important to understand in the context of Australia’s HPV Vaccination Program, where vaccinated and unvaccinated cohorts are participating in cervical screening.
Compass recruitment closed on 31 December 2019 and ACPCC has continued to operate the Compass Register to support healthcare providers and the 76,000 trial participants until the conclusion of the Compass Trial.
The Compass Register routinely accepts and records cervical screening test information along with information about related investigations, such as colposcopy, biopsy and treatment in order to provide follow-up services in accordance with the Compass Trial protocols. The services of the Compass Register include the following:
• The Compass Hotline for participants and healthcare providers
• Reminders and communication to women participating in the Compass Trial
• Management and provision of screening histories to healthcare providers and laboratories
• Monitoring and follow up of trial participants
• Provision of reports to healthcare providers to remind them when participants are due for their next Compass screening test, and
• Data processing and reporting.
As women complete their participation in the Trial, the Compass Register manages the exit of participants, with their complete records transferred to the National Cancer Screening Register. ACPCC worked closely with the Australian Department of Health and Telstra Health to establish and implement processes to facilitate the safe exit of participants from Compass to the National Cancer Screening Register. This work was completed in June 2022 with the Compass Register sending communication to all Compass patients to advise them of their safe transition to the National Cancer Screening Register as they exit the trial. We anticipate that the last patient will complete their participation in the Compass trial in 2026.
ACPCC’s work in research extends across the organisation with our expert teams either leading, contributing to or collaborating in studies important to the success of the cervical cancer control and public health, including the impact of the HPV vaccine, selfcollection, acceptability of cervical screening in Australia and the region, Point of Care testing, and COVID-19 testing. Following is a snapshot of our current work, with a full list of published articles for 2021/22 on page 99.
The SCoPE2 Project is a collaboration between the Royal Women’s Hospital Dysplasia Clinic and VCS Pathology to assess the relative clinical accuracy of self-collected vaginal specimens compared with healthcare practitioner collected specimens from the cervix for the detection of HPV and cervical pre-cancer. Samples are tested on a range of clinically validated PCR-based HPV assays.
The SCoPE2 study is an extension of the original SCoPE study undertaken prior to the introduction of self-collection for under-screened individuals in the renewed National Cervical Screening Program. SCoPE2 builds on this by introducing two devices for selfcollection, using either the established collection device (Copan FLOQSwab), or the Rovers Viba-brush. Self-collection devices are still transported dry and are suspended in Copan MSwab media, a non-toxic non-alcohol-based media which may assist in cheaper, easier transport in remote areas of Australia, or in low- or middle-income area in our region.
Recruitment commenced on 4 April 2022 and it is expected that recruitment target of 400 participants will be completed by October 2022.
VCS Pathology and the Victorian Infectious Diseases Reference Laboratory (VIDRL) collaborated to undertake a study to assess the accuracy of self-collected samples for COVID-19 PCR testing. The study was funded by the Victorian Department of Health.
Two different self-collection devices were assessed, the Copan FLOQSwab and the Rhinomed Rhinoswab, against healthcare worker collected specimens. The Hoppers Lane COVID-19 testing site run by IPC Health was used as the site for participant recruitment. Recruitment began in April 2022 and was completed in June 2022. Healthcare worker specimens were tested and reported according to normal practice and then self-collected specimens tested and assessed at VCS Pathology on three different COVID-19 assays (Roche cobas, Hologic Aptima, Seegene SARSCoV-2).
The results from this study demonstrated that self-collected specimens were not inferior to healthcare worker collected specimens. These data are being used as part of an in-house validation which will allow VCS Pathology to give GP clinics the option of offering selfcollection for COVID-19 to their patients.
ACPCC is a collaborator in the PREVENT Project. This implementation research project is investigating the acceptability and implementation of point of care HPV self-collection and same day assessment in rural and remote communities. Led by Aime Powell, University of Notre Dame, this grant-funded project is introducing point-of-care HPV testing which utilises self-collection and same day followup testing in the Kimberly Region of Western Australia.
ACPCC is assisting with the study design, analysis, and project evaluation. We are also supporting HPV testing including provision of the point-ofcare device, training, education and evaluation. The first screening events took place in August 2022.

This trial is implementing universal self-collection, comparing point of care HPV testing with laboratorybased testing in a remote setting to see whether immediate availability of results and appropriate counselling for patients in whom HPV is detected, leads to better colposcopy follow-up rates.
ACPCC is providing training and technical support in relation to HPV-based testing of self-collected specimens using Point of Care (POC) testing equipment. POC testing allows patients to get their screening results within an hour of testing.
The trial had two stages with the first stage completed in early 2022 with nearly 500 participants across the POC and laboratory testing arms. The second stage began in May 2022 with the POC instruments relocated and over 30 POC and over 40 pathology laboratory-based HPV tests already reported.
Professor Julia Brotherton is a Chief Investigator in this Indigenous-led research program that brings together a team of researchers supported by a strong and culturally sensitive governance structure, along with practitioners, consumers, and other key collaborators nationally and internationally. Key projects have included:
‘Screening matters’ which aimed to:

1. describe Indigenous women’s beliefs and attitudes towards participation in cervical screening, and
2. describe Primary Health Care (PHC) providers’ perspectives and approaches to facilitating screening for Indigenous women, and to identify opportunities to maximise participation. Four papers from the study have been published.
The ARC funded ‘Yarning about HPV vaccination’ study has interviewed adolescents, caregivers and school providers about HPV vaccination in 10 schools in Queensland.

ACPCC is a key partner of Australia’s NHMRC funded Centre of Research Excellence (CRE) in Cervical Cancer Control.
The Centre of Research Excellence in Cervical Cancer Control, known as C4, was established in late 2017, to bring together cervical cancer control experts undertaking research and evaluation of HPV vaccination and screening programs. It is funded by the NHMRC. The work of C4 will ensure the future of cervical cancer prevention is underpinned by world-class research that can reduce the global impact of the disease. The core group consists of researchers from the Daffodil Centre, ACPCC, the University of Melbourne and the Kirby Institute with combined expertise in epidemiology, public health, laboratory testing, clinical trial implementation, predictive modelling and economic evaluation. Our associate investigators bring additional expertise and perspectives from a range of organisations. For details of our full team, please visit the C4 website at www.cervicalcancercontrol.org.au
C4 focuses on:
• Evaluating the effectiveness of next generation primary HPV screening and HPV vaccination approach
• Developing better tools for monitoring HPV and for predicting abnormalities
• Assessing the impact of the HPV vaccination program in Australia, and
• Global aspects of cervical cancer control
• Within C4, we run Australia’s largest clinical trial, Compass, which is providing world-first evidence on the interaction between HPV vaccination and screening.
C4 is supporting the WHO strategy for cervical cancer elimination as a public health problem, with contribution of our expertise to multiple areas of research and implementation activity to ensure that this strategy becomes a reality in Australia, our region and beyond. Our key contributions include:
• Modelling work to inform the elimination strategy
• Membership of WHO expert advisory groups
• Working locally with government and key stakeholders to inform strategies for monitoring progress and achieving elimination in Australia
• Working regionally with governments and key stakeholders to inform strategies for scale up to meet the WHO strategy targets, and
• Developing partnerships and grant applications for the undertaking of implementation research to inform scale up for elimination in our region. Within the CRE, ACPCC is actively engaged in Australian policy relevant research at the national level including the following projects:
• STORIES interview study documenting the perceptions of key stakeholders around the implementation of Renewal. This study has involved multiple CRE partners and was led by the late Prof Margaret Kelaher and by Prof Julia Brotherton. ACPCC has played a coordinating role in the study, with data analysis and paper preparation now complete.
• Piloting of a cervical cancer HPV typing system for Australia. This study is piloting receipt and processing of cervical cancer specimens to determine HPV type in a close collaboration between pathology partners in tertiary hospitals, the RWH and ACPCC.
• Comparison of HPV surveillance approaches using routine screening HPV assay data compared with genotyping using Linear Array. This study uses Compass specimens in a collaboration between ACPCC and the Daffodil Centre.
• NHMRC Partnership grant project ‘Identifying and addressing gaps in Australia’s adolescent HPV vaccination program’ led by Kirby Institute and involving health department immunisation partners in NSW, Tasmania and Western Australia and other CRE partners.
• NHMRC funded study 'It’s a gamechanger' using HPV selfcollection to improve equity and participation in Australia's National Cervical Screening Program' led by University of Melbourne and the Daffodil Centre
• NHMRC funded study ‘Embedding community driven models to increase cervical screening via HPV selfcollection to improve cervical cancer outcomes for Aboriginal and Torres Strait Islander people’ led by ANU.


VCS Pathology is Australia’s largest not-for-profit cervical screening laboratory. In 2021/22, VCS Pathology continued to report almost 50% of all cervical screening tests in Victoria. Cervical screening tests are provided free of charge to women and people with a cervix if tests have been taken in accordance with the National Cervical Screening Program guidelines and the patient is Medicare eligible.
VCS Pathology has reported 783,823 HPV Tests since the beginning of the renewed National Cervical Screening Program in December 2017.
The high volumes of cervical screening tests received by VCS Pathology provide us with the capacity to undertake rigorous analysis of trends and quality measures which inform the National Cervical Screening Program. We have shared knowledge and provided critical data to researchers to help ensure that women and people with a cervix in Australia and the Indo-Pacific region have the best, and most appropriate, screening test available to them.
As a not-for-profit laboratory service, healthcare providers can be assured that when choosing VCS Pathology, they are supporting our vital work with under-screened populations in Australia and around the world in the elimination of cervical cancer.
HPV test volumes fluctuate each year in line with cervical screening testing demand under the five-year testing cycle of the renewed National Cervical Screening Program. In line with modelling, the 2021/22 volumes are the lowest forecast in the first five-year cycle, noting that volumes are anticipated to increase in 2022/23.
If HPV is detected in a routine Cervical Screening Test (CST), a reflex Liquid Based Cytology (LBC, ThinPrep or SurePath) is performed on the same specimen.
VCS Pathology has reported 783,823 HPV and 247,305 LBC tests since the beginning of the renewed National Cervical Screening Program in December 2017. This represents almost 50% of all cervical screening tests in Victoria.
VCS Pathology provides a specialist gynaecological histopathology service which has significant benefits for referrers and their patients.
Our quality assurance procedures correlate cervical cytology and histology results to ensure that each case receives the most comprehensive analysis. VCS Pathology reports approximately 50% of CSTs in Victoria. LBC tests taken prior to biopsy are often immediately available for review and correlation.
Histology results are usually available within 2 working days and often within 24 hours (for results sent by electronic transfer). At the end of June 2022, 99.59% of histology cases were reported within 48 hours.
The National Cervical Screening Program changed the threshold for referral to colposcopy on the 1 February 2021. After this date, women with a 12-month follow up HPV (not-16/18) result with LBC prediction negative, pLSIL or LSIL (intermediate risk result) should have a further HPV follow up test in 12 months’ time following their previous HPV test, instead of referral to colposcopy.
This change to the guidelines, along with the forecast cyclic drop in cervical screening numbers in the first fiveyear cycle, has resulted in a fall in the number of histology cases.
Most of the chlamydia and gonorrhoea test requests at VCS Pathology are taken from specimens received for cervical screening. With the decline in cervical screening volumes, the volumes for these two tests have also decreased. In 2021/22, VCS Pathology reported 5,261 chlamydia tests compared with 6,344 tests in the previous year.
VCS Pathology has continued to provide pathology support to COVID-19 screening clinics in Melbourne’s north and western suburbs operated by IPC Health, one of the largest providers of community health services in Victoria.
Since January 2022, VCS Pathology has also assisted the Victorian Rapid Response Team; a collaboration of community health organisations including IPC Health, StarHealth, EACH, DPV and cohealth. The Rapid Response Team are the frontline COVID-19 testing unit, undertaking a wide and varied range of priority testing. This included in-home testing through to testing within 'at-risk' environments such as aged care or correctional facilities.
A major focus of our collaborations has been working with IPC Health to minimise test reporting turnaround times, reducing the disruption to the lives of the Victorian public who wait anxiously for their results.
The laboratory has continued to deliver a rapid turnaround of samples with a combined processing capacity of 2,500 tests SARS-Co-V-2 tests per day across six testing platforms: the Abbott Alinity m, the Cepheid GeneXpert, the Hologic Panther (and Panther Plus), the Roche cobas 6800, the Roche Liat, and the Seegene STARlet.
Since early May 2020, VCS Pathology has processed over 425,000 SARSCoV-2 samples from various screening clinics and drive-through testing sites across metropolitan Melbourne, with 314,197 tests reported in the 2021/22 financial year.
VCS Pathology extends its thanks to all IPC Health staff who have collaborated with us and significantly contributed to the COVID-19 effort in Melbourne’s north and west.

As a result of the reduced incidence of COVID-19 infections in the community, pop-up COVID-19 testing stations have begun winding down. Along with this reduced community accessibility to PCR testing, there is less assurance that Rapid Antigen Testing is always effective, particularly as new variants of the virus surface in the community.
VCS Pathology has worked with the Victorian Department of Health and the Victorian Infectious Diseases Reference laboratory (VIDRL) to validate self-collection as another option for COVID-19 PCR testing for medical staff and patients attending health care clinics. This has the potential to increase the uptake and accessibility of PCR testing for front line healthcare workers.
VCS Pathology has scaled up to meet the anticipated increase in HPV Self Collection volumes with a current capacity to process 1,700 Self Collection samples per day
HPV self-collection volumes have steadily increased in anticipation of the 1 July 2022 change to universal availability as a choice for all screening participants. VCS Pathology has processed close to 10,000 self-collection samples since the testing was first introduced in 2017, with 3,432 of those samples received in 2021/22. To prepare for the expected increased volumes, VCS Pathology has modified laboratory processes for testing, shifting from a manual process of aliquoting specimens from samples to an automated process using the newly installed Copan Universe platform.
VCS Pathology is positioned to meet the increasing demand in volumes with a current capacity to process 1,700 self-collected samples per day.
A huge thanks to you and the VCS Pathology team for assisting our Rapid Response teams with the processing of these priority specimens.
Myself and our Rapid Testing colleagues at Department of Health VIC as well as our testing partners are very appreciative of your willingness to assist during these challenging times.
DanielleSiler, Executive Lead C-19 Rapid Response Collaborative
Health

In late 2021 VCS Pathology further expanded its respiratory testing options to include detection of some bacterial causes of respiratory illness including Bordetella pertussis (‘whooping cough’), and common causes of pneumonia (e.g., Mycoplasma pneumoniae). The addition of this new testing allows VCS Pathology to offer a full respiratory panel which clinicians can request, often when a patient has a respiratory illness but doesn’t test positive for COVID-19.
ACPCC’s laboratory capabilities and turn-around times continued to benefit from our investment in technology and innovation.
VCS Pathology processed significant volumes of SARS-CoV-2 tests in the financial year. A major bottleneck in SARS-CoV-2 test processing, and the turnaround time of reporting in the laboratory, has been pre-analytical processing. Every COVID-19 specimen required manual transfer into a new tube for analysis, as the original tubes couldn’t be processed when containing the nasal swab.
The Copan Universe, purchased by the ACPCC in late 2021, represents the first pre-analytic device designed for transferring COVID-19 specimens at high throughput (~1,000 specimens in 8 hours).

For SARS-CoV-2 testing, the Copan Universe provided immediate benefits to VCS Pathology across resourcing, staff workload, testing capacity, safety and turn-around times.

Furthermore, the Copan Universe introduces the automation of processing of self-collected cervical screening swabs. With the introduction of universal access to self-collection for all screening participants from July 2022, the laboratory is well positioned to handle the increasing self-collected HPV /testing demands.
The BD Onclarity HPV assay is the first clinically validated commercial assay to have a TGA approved manufacturer’s claim for HPV testing of self-collected vaginal swabs. This will allow for faster processing of self-collected specimens. The BD Onclarity will expand the capacity of VCS Pathology to process self-collected vaginal swabs for HPV. It strategically aligns with the recent announcement by the Medical Services Advisory Committee regarding Medicare changes for self-collected HPV tests.
VCS Pathology meets the NATA regulatory requirements for Australian Laboratories reporting under the National Cervical Screening Program. VCS Pathology achieved continued accreditation following a NATA Technical Re-assessment and Level 2 Clinical Governance Assessment in September 2021. Accredited Pathology Laboratory Approval has been extended to February 2025.
In 2020, VCS Pathology was accredited for the extension of the stability claim for self-collected specimens to be tested for HPV as part of the National Cervical Screening Program. This means that VCS Pathology now has accreditation to process self-collected swabs received up to 28 days after patient collection.
VCS Pathology has successfully established eight different nucleic acid amplification testing assays and platforms for the testing of SARS-CoV-2 including Seegene, Abbott, Hologic, Cepheid and Roche. The laboratory can process and report over 1,500 SARS-CoV-2 tests per day and provide electronic reports to both the clinical referrers and the Victorian Department of Health within 24 hours
VCS Pathology is committed to meeting all relevant industry standards including the various requirements of NATA, National Pathology Accreditation Advisory Council (NPAAC), the Royal College of Pathologists Australasia (RCPA) and ACPCC insurance agencies.


The Australian HPV Reference Laboratory was announced in November 2021. Our laboratory team is widely regarded for their leadership, innovation and deep expertise in cervical cancer prevention.

The laboratory works hand-in-hand with quality assurance programs, test manufacturers, other laboratories and researchers within Australia and abroad to enhance and monitor the performance of HPV tests, technology and laboratory procedures to help reduce HPV-related disease.
The Australian HPV Reference Laboratory provides:
• Expertise in the development, evaluation and implementation of HPV assays for use in cervical screening and other paradigms for HPV detection
• Standardised up-to-date technical information, advice, guidance and training of HPV laboratory practices
• Specialist diagnostic and clinical advice for specific clinical cases
• Advice and materials to support quality assurance programs in the Asia Pacific Region
• Sample Archive/Biobank which is used to support the development of technologies and processes to reduce the incidence and impact of HPV-related disease
• Expertise in the development of policy and programs for eliminating HPV-based cervical cancers.
In preparation for the universal availability of self-collection in the National Cervical Screening Program, the Australian HPV Reference Laboratory assisted 15 Australian laboratories with verifying the Roche self-collection protocol through the free supply of a verification panel.
cervical cancer prevention.
Our laboratory team is widely regarded for their leadership, innovation and deep expertise in
The self-collection method validated by Roche involved self-collection with a Copan FLOQSwab and then swirling the swab in a ThinPrep vial at the point of specimen collection, usually at the GP clinic. This new protocol allowed laboratories using Roche HPV tests to undertake testing on self-collected specimens.
There were no commercial options for verification specimens to allow laboratories to confirm that the self-collection protocol on the Roche assays was performing as expected in their laboratory. The Australian HPV Reference Laboratory, utilised quality control material it had assisted in developing alongside MicroBix and Copan, to create a panel of specimens covering the full range of possible HPV results. To date 15 laboratories have returned results for these panels including laboratories in every state and territory (except the Northern Territory) and including five public and ten private laboratories. VCS Pathology assessed the blinded results and returned a report to the relevant laboratory which has assisted in the number of laboratories accredited for self-collection to grow from 3 to 10 as of the end of August 2022.
Since its establishment in 1964, ACPCC has always regarded the provision of a quality service as the most important aspect of its operation. The Executive Director of ACPCC and our staff remain fully committed to the organisation as a Centre of Excellence in cervical screening tests and registry services.
Our quality system comprises the structure, objectives and policies of ACPCC and the description of work practices and procedures that promote a high quality of operation in all aspects of our work. Thus, the quality system forms the basis on which the pathology laboratory and registries operate.
All staff embrace an ethos of quality improvement and a customer focus. We have a broad perspective of our customer base, seeing this as comprising the health practitioners who send us pathology samples for reporting, the community members from whom the samples are taken, the participants recorded on our registries, and our funding providers.
ACPCC is committed to meeting all relevant industry standards, including AS ISO 15189:2012 and the various requirements of NATA, NPAAC, the RCPA and our insurers.
Quality system activities are coordinated by the Director of Cytology and Histology under the guidance of the Executive Director of ACPCC. These activities are supported by the quality management software Q-Pulse, which is designed to support key elements of the Quality System.
Self-collected HPV samples, rapid point of care testing and same day treatment will be crucial tools in providing safe, accessible and cost-effective screening to the many women in the world living in remote communities.
VCS Pathology provides support to laboratories seeking accreditation for HPV Self-Collection.

can SCREEN ® REGISTRY
canSCREEN® is a cancer screening registry platform designed by ACPCC and leveraging over 50 years of registry knowledge to support cervical cancer prevention. The software performs the critical functions of a Cancer Screening Register including:
• Demographic and contact details about screened participants
• Collection of information documenting screening activity
• Information related to all relevant tests and treatments
• Actively follow-up participants
• Monitor and evaluate the performance of the screening program.
canSCREEN® was originally developed by the ACPCC Digital Health team over 8 years ago. It has evolved over time and is regularly updated with the latest technologies (mobile, HL7, GeneXpert integration etc.) and has been developed to be configurable and scalable to meet the diverse range of features and operational requirements, particularly for low-to-middle income countries.
The software:
• Collects and stores all patient and result details in a central database
• Manages patient duplication through automated processes and cues
• Facilitates the follow up process for those apparently overdue for assessment and/ or treatment
• Recalls participants for rescreening at agreed intervals
• Allows data extraction for reporting purposes.
Patient presents for screening
Test is processed on site using instrument such as GeneXpert
Nurse or Clinician enters patient data into canSCREEN, then receives test result directly from GeneXpert or a similar machine

Patient given result within hours of the original cosultation along with any further advice
Patient presents for screening
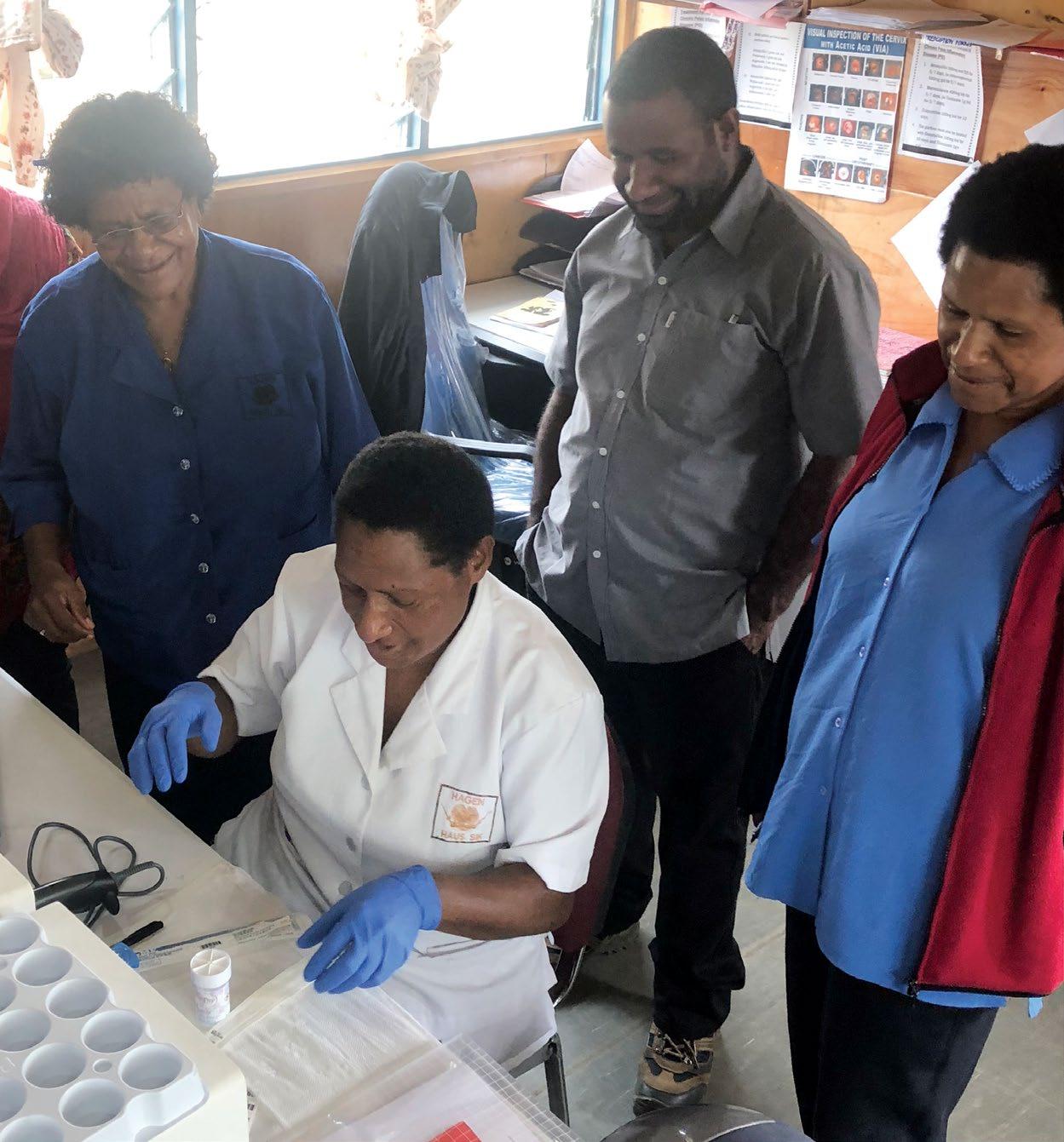
Several canSCREEN® milestones and new functions were achieved for the development of the PNG and Vanuatu applications in the financial year. With the increased demand for its usage and the need to drive down costs and time to deploy, canSCREEN® was streamlined, enabling an 'out of the box' partially configured solution. Some of the new features in canSCREEN® include:
• Integration into GeneXpert Machines
• Development of an off-line mobile app for remote regions
• The change to an evergreen out of the box SAAS solution with pre-configured elements
• Standardised training guide with video enhancements
• Development of software versioning and standards
• Uplift of customer versions to the latest release.
• Centralisation of the app’s location for customers into canscreen.com
• Standardised reporting dashboard and data extracts
• Processes developed for regular version updates and features to customers
• Deployment streamlining.
Results automatically sent to canSCREEN,
Patient can be sent results and follow up info directly

ACPCC is actively evolving its business model in response to our new commercial environment. This has been shaped by many factors including, but not limited to, the move of VCS Pathology from Project Agreement funding to Medicare bulk billing, the expansion of pathology testing to SARSCoV-2 and new collaborative projects in low-to-middle countries in our region
Work is underway to upgrade the existing ACPCC Laboratory Information Management System (LIMS) for VCS Pathology. The existing system was purpose-built and now is now 23 years old, making it difficult to support and to make functional enhancement changes. With data dating back to 1964, this upgrade involves migrating millions of records and will be a significant undertaking and investment for our future. The objectives for a new LIMS are to:
• Improve laboratory efficiency by effectively managing the flow of samples and data
• Provide centralised storage of data required for quality control compliance
• Integrate laboratory instruments and other in-laboratory systems providing a completely digital environment
• Satisfy high throughput demands for laboratory tests by an automated postanalytical phase
• Fully automate billing functions in line with Medicare eligibility requirements
• Assist the laboratory with meeting strict regulatory requirements including tracking and reporting on relevant data.
Key ACPCC staff are actively consulting with potential vendors to find the most suitable application. The work is expected to be completed in the 2022/23 financial year.
Our business change in name from VCS Foundation Ltd to ACPCC has necessitated a complete revision of our website. This has been an opportunity to review the current structure and content of the existing site with the aim to provide a better experience for healthcare professionals and patients using the site.
A consultation phase has been completed and the migration to the new site is expected to be completed by November 2022.
Significant building upgrades and renovations were made to VCS Pathology in the financial year. The upgrade is important for both our continuity of SARS-CoV-2 testing and the general safety of laboratory employees. The improvements have included:
• Upgraded laboratory ventilation system for NATA compliance and staff safety in SARS-CoV-2 testing
• Increased laboratory footprint available for COVID-19 instrumentation
• Dedicated laboratory storage areas
• Work room modifications to allow for social distancing and separation of work areas
• Upgraded work areas for pathologists.
We thank the many vendors and trades persons who supported our laboratory upgrade.
In order to future proof VCS Pathology communications to our customers, ACPCC has partnered with HealthLink to provide secure communications. Since January 2022, ACPCC has been successfully using HealthLink to send COVID-19 results to the Department of Health. The migration of general practitioners and Clinics will be progressed throughout 2022/23.
EXECUTIVE DIRECTOR
Professor
Marion Saville AM
MEDICAL DIRECTOR POPULATION HEALTH
Professor Julia Brotherton


RESEARCH ASSISTANT
Tracey McDermott

Callum Hensman
OPERATIONS DIRECTOR POPULATION HEALTH
Genevieve Chappell
OPERATIONS MANAGER
Tanya O’Farrell
SENIOR HEALTH INFORMATION MANAGER
Karen Peasley
FOLLOW-UP MANAGER
Floriana La Rocca
DIRECTOR
CORPORATE SERVICES
Dr Michelle Critchley

FINANCE MANAGER
Ray Williams
HR ADVISOR
Jessy Warn
DIRECTOR
DIGITAL HEALTH
Michael Lammardo
SENIOR BUSINESS ANALYSTS
Elizabeth Shao
Grit Diessner
Jasvir Kaur
APPLICATIONS DELIVERY MANAGER
Mark Bryant
SENIOR PROJECT MANAGER

John Knox
SENIOR ANALYST PROGRAMMER
Xiaosu Zhou
SYSTEMS ADMINISTRATOR
Simon Chapman
DIRECTOR
MOLECULAR BIOLOGY
Associate Professor

David Hawkes
CLINICAL MICROBIOLOGISTS
Dr Hiu Tat Mark Chan
Dr Linda Chew

Dr Saugata Choudhury
LIAISON PHYSICIANS
Dr Alexis Butler
Dr Wendy Pakes
MOLECULAR SENIOR SCIENTISTS
Ivy Nguyen
Joanne Romano
Marco Ho Ting Keung
DATA INFORMATION MANAGER
Sheree Holt
DIRECTOR CYTOLOGY AND HISTOLOGY
Grace Tan
PATHOLOGISTS
Dr Kristy Dundas

Dr Kais Kasem
Dr Fong Koh
Dr Yi Sun
Dr Karen Talia
CYTOLOGY & HISTOLOGY SUPERVISOR
Diana Stockman
MULTIDISCIPLINARY SENIOR SCIENTISTS
Domenica Giacomantonio
Noni Christou
DIRECTOR
COMMUNICATIONS AND GOVERNMENT RELATIONS
Kate Wilkinson
CLINICAL EDUCATION MANAGER
Hannah Saunders
SENIOR PROJECT OFFICERS
Kim Lee
Lena Elkman
ACPCC has continued to attract and develop a highly skilled workforce. At 30 June 2022, ACPCC employed 134 staff across a range of managerial, professional, technical and operational roles at its Carlton and East Melbourne locations. Our staffing model includes permanent, temporary and casual employees, comprising of 74 full time, 53 part time and 7 casual appointments.
While the pandemic has seen many changes in the ACPCC workforce, the resourcing needs of VCS Pathology have continued to be a major priority for workforce planning. We aim to recruit and retain the best people to deliver on the objectives of our Strategic Plan.
ACPCC is an equal opportunity employer, consistent with our values of Fairness, Integrity, Respect and Excellence. We acknowledge that a diverse and inclusive workforce promotes safety, productivity and wellbeing benefits, and underpins our ability to attract new employees. We have continued to provide compliance training to all employees in the financial year to support an inclusive culture and respectful workplace. This included the delivery of workshops in Equal Opportunity and Anti-Discrimination.
ACPCC values the importance of providing all employees with the same rewards, resources, opportunities for success and outcomes, regardless of gender. We employ, develop and promote our employees based on the strengths of the individual. ACPCC has a long-established history of an inclusive and strong female workforce; a profile which has remained steady over the last decade.
In 2021/22, our workforce consisted of 69% female employees and 31% male employees. Our Executive Management team comprised of 75% female Executives and 25% male Executives, with 63% of ACPCC Board Directors being female and 37% male. ACPCC has continued to report annually to the Workplace Gender Equality Agency. The Agency confirmed the ACPCC’s reporting was compliant for the 2021/22 reporting period.
The 2021/22 year continued the rollout of on-line compliance training to all staff, students and contractors. This followed the need for new COVID-19 safe training options which were accessible to all staff including those working from home. Compliance training modules delivered in the year included Anti-bullying and Anti-Harassment, Discrimination, Cybersecurity, Equal Opportunity, Privacy, Social Media (customised to the ACPCC policy) and Manual Handling. For Executives and members of the Corporate Services team, Whistleblower and Fraud training was also delivered. The financial year saw the ACPCC transition to the Fit2Work platform for national police checks, and the new Employment Hero employee self-service system for tracking of the status of annual confidentiality deeds and other certifications; both providing major benefits to HR team productivity and compliance outcomes.
ACPCC continues to encourage and support the career development of employees across the organisation. The training of our employees is critical to engagement and retention, providing the basis for both succession planning and achieving our strategy. Continuing from the previous year, efforts continued towards upskilling our multidisciplinary VCS Pathology team and introducing flexibility into our workforce across the molecular and cytology work areas. We continued our valued relationship with Swinburne University and the Royal Melbourne Institute of Technology (RMIT) for laboratory and ICT student placements. These placements provide students with invaluable support in building their academic performance, as well as gaining technical and employment skills. The student placement program is evidence of our commitment to continue to provide professional training for future employees and build our capacity to attract industry leaders of the future. The year ahead will see the ACPCC’s transition to Employment Hero for annual performance reviews, replacing the former VESSPA system.
17-19 years (0%)
20-35 years (36%)
36-50 years (29%)
51-65 years (31%)
66+ years (4%)
Executive (7%)
Manager/Supervisors (10%)
Medical Employees (7%)
Professional (12%)
Operations/ Administration (31%)
Scientists/ Laboratory (34%)
ACPCC’s comprehensive health and safety approach directly supports the physical and mental wellbeing of all employees. Health and Safety initiatives conducted throughout the year included custom fittings of N95 masks, COVID-19 safety training, influenza vaccinations, warden training and the promotion of mental health support.
With the introduction of mandatory COVID-19 vaccinations for Victorian healthcare workers in late 2021, significant efforts were made to ensure that 100% of ACPCC staff were compliant with third dose COVID-19 vaccinations within the required timing. Procedures were also put in place to confirm the vaccination status of visitors and contractors entering our premises.
The services of the Employee Assistance Program continued to be used with a total of 14 sessions provided to employees in the financial year. ACPCC staff are regularly encouraged to reach out to this service if they are experiencing mental health difficulties, particularly with the many challenges of the COVID-19 pandemic. All EAP sessions are free to employees, confidential and provided by an external counsellor.
The Lost Time Injury Frequency Rate (LTIFR) rate was 24.87 for the financial year, a decrease from 60.32 in 2020/21. LTIFR is calculated by the number of lost time injuries per million hours worked in the quarter. There were no incidents in the year which resulted in WorkSafe claims.
The Health and Safety Committee continued to meet in accordance with legislative requirements, consistent with our commitment to the health and wellbeing of our workforce and excellence in safety management and best practices. Health and Safety at ACPCC is underpinned by a genuine care for our employees, in alignment with our corporate values of Fairness, Integrity, Respect and Excellence.
The ACPCC Health and Safety Policy was last updated and approved by the Board in May 2022.
ACPCC is not directly subject to the Freedom of Information (FOI) Act 1982. While some of the organisation's government funded activities may be the subject of FOI requests, these requests should be made to the relevant government department for assessment.
ACPCC understands the importance of protecting the privacy and confidentiality of all personal and health information that is held by the organisation. ACPCC collects a range of personal and health information about individuals. ACPCC may collect this information from the individual or from another person dealing with that individual, such as their healthcare practitioner. The type of information that ACPCC collects and the way in which it may use and disclose that information varies according to the services, activities and programs ACPCC provides or undertakes in relation to an individual.
ACPCC has strict privacy and confidentiality practices in place and all staff are required to abide by these.
A Disciplinary Policy and Procedure is in place to ensure staff comply with these practices. All persons who may observe personal and health information held by ACPCC are required to sign a confidentiality statement annually. All personal and health information is stored on the premises of ACPCC or in cloud-based storage. Backup tapes of the information system and some slides are stored in a secure facility off-site. Where services are contracted out, contractors must comply with ACPCC privacy and confidentiality requirements if any personal information is provided to them.
Our Privacy Policy was last updated in 2022 and is available at www.vcs.org.au/ privacy-policy
ACPCC is committed to the highest standards of legal, ethical and moral behaviour. The organisation seeks to maintain an operating environment where legitimate misconduct concerns can be reported without fear of retaliatory actions or retribution, and are managed expeditiously, in confidence and according to internal policy.
ACPCCs Whistleblower Policy and Procedure is compliant with the new whistleblower reforms under Part 9.4AAA of the Corporations Act 2001 (Cth). An updated version of the Policy and Procedure was approved by the Board in August 2021.
Compliance training in whistleblower protection is routinely scheduled for all members of the Executive and Corporate Services teams.

ACPCC (VCS Foundation Ltd) is a registered company limited by guarantee under the Corporations Act 2001 (Vic) and is governed by a Board of up to 11 Non-Executive Directors in accordance with the Constitution.
The Executive Director of the Service is not a member of the Board but acts as Secretary. The Board establishes the organisation’s vision, strategic intent, goals and objectives, employs the Executive Director, identifies and monitors the management of corporate risks, and monitors and assesses the Executive Director and the performance of the organisation.
The Directors present their report on ACPCC ('the Company') for the financial year ended 30 June 2022.
The role of the ACPCC Board of Directors is to:
• Set, approve and monitor the strategic direction of ACPCC
• Take responsibility for the overall performance of the organisation including appointing and managing the performance of the Executive Director, monitoring and working in the best interests of the stakeholders
• Monitor and minimise the risks to ACPCC
• Establish and approve Board policies
• Comply with the Constitution of ACPCC, State and Federal Laws, Directors’ and insurance responsibilities.
The Audit and Finance Committee (a subcommittee of the Board) is responsible for:
• Advising the Board on matters relating to financial strategies and policies, financial performance, viability, sustainability and capital management
• Reviewing the quality of internal financial reporting to the Board
• Ensuring effective governance and financial stewardship to assist directors in discharging their responsibility to exercise due care and diligence in relation to:
- the selection and application of accounting policies in line with accounting standards and legislation
- financial reporting, and
- management and internal control procedures
• Ensuring the effectiveness and independence of external audit function
• Applying appropriate risk management processes contributing to improving the risk management culture in the organisation.
The Quality Assurance Committee (a subcommittee of the Board) is chaired by the Executive Director. It uses statistical analyses to monitor a range of activities including performance targets in the scientific, registries, administration and clerical areas, audits, non-conformance events and document control. Results of the activities are presented at the Quality Assurance Committee meetings and any actions identified are assigned and reported. Detailed reports are presented to the Board on a quarterly basis.
Mr Tim Humphries was elected Chairman at the 2020 AGM and was previously the Chairman of the Audit and Finance Committee. Mr Humphries joined the Board in 2012 as a Director with expertise in Finance, Commerce or Corporate Management. Mr Humphries holds a Bachelor of Commerce from Flinders University, and Master of Business Administration (MBA) from Deakin University. He is a member of the Certified Practising Accountants (CPA) Australia and the Australian institute of Company Directors. Mr Humphries brings a wealth of experience with a career spanning more than 20 years in senior Accounting and Finance roles, and CEO, a position he currently holds. His broad finance experience is complemented with HR, IT, corporate governance, sales and project management skills developed in a wide range of industries including health, aged care, transport and logistics, materials handling, recruitment, and not-for-profit sectors in Australia.
Mr David Wrede was appointed to the Board in May 2010 as the Director with gynaecological expertise and was appointed Vice Chairman in 2017. Mr Wrede studied medicine at Cambridge University and St. Thomas’ Hospital London. His post-graduate training was in General Surgery and Obstetrics and Gynaecology and included two years research into Cervical Cancer and HPV at the St. Mary’s branch of the Ludwig

Institute. Previous appointments in the UK’s National Health Service include Consultant posts with interests in Gynaecological Cancer, Minimal Access Surgery and Colposcopy in Scotland and England. Since moving to Australia, his main clinical focus has been in gynaecological cancer prevention at The Royal Women’s Hospital. He is the clinical lead for the Dysplasia (Colposcopy) services at the Womens' and Box Hill Hospitals. His research profile includes being an investigator on a number of cervical cancer screening projects including COMPASS (led by Prof Marion Saville and Prof Karen Canfell), iPAP (led by A/Prof Dorota Gertig) and EXCISE (co-led with A/Prof Paul Cohen) and he is also an Associate Investigator with the NHMRC Centre of Research Excellence for Cervical Cancer Control. He is a member of the Clinical Guidelines Working Group for the renewed National Cervical Cancer Screening Program and past Secretary of the Management Committee of the Australian Society for Colposcopy & Cervical Pathology. Mr Wrede is an Honorary Consultant to the Familial Centre Clinic on the Parkville Precinct and Honorary Senior Lecturer to the Department of O&G at the University of Melbourne.
Ms Fiona Kelly was appointed to the Board in March 2017 as a Director with expertise in Finance, Commerce and Corporate Management and was appointed Chairman of the Audit and

Finance Committee at the 2020 AGM. Ms Kelly holds a Bachelor of Economics from Monash University and a Master of Business Administration from the University of Melbourne and is a member of Chartered Accountants Australia and New Zealand. Ms Kelly has extensive leadership and management experience from a broad range of senior roles in the professional services and not-forprofit sectors. With a focus on financial leadership, business performance and operational excellence, this experience spans the areas of finance, HR, legal, management of support services (including IT, property management and procurement), project management, technology implementation and organisational development. She is also a Board member of Carey Baptist Grammar School.
Mr Tony Abbenante was appointed to the board in October 2018 as a Director with wide expertise in Information Technology and Communications. He has specialist knowledge and experience in enterprisewide digital health. Mr Abbenante holds a Bachelor of Applied Science in Computer Studies from the University of South Australia and is a fellow of the Australian Institute of Digital Health. He has broad experience in governing national and state-wide programs; this provides a wealth of experience and knowledge to the ACPCC. His career spans more than 27 years in senior digital health roles and he has extensive experience with government. Tony has


deep knowledge and experience in sector delivery, governance and digital health outcomes that deliver valuebased clinical and business outcomes to the health sector.
Dr Jane Collins
Dr Jane Collins was Chairman in 2019/20, a position she also held from 2009-2013. Dr Collins was appointed to the Board in February 2008 to fill the role of a Director with expertise in General Practice. Dr Collins is an experienced General Practitioner, business owner and freelance medical writer. She has a special interest in women’s health as well as the provision and organisation of health care in the wider community. Dr Collins is a co-owner and the Clinical Director of the Clifton Hill Medical Group, an inner urban general practice comprising 10 general practitioners.
Ms Stephanie Reeves
Ms Stephanie Reeves joined the Board in February 2014 as a Director with expertise in Law. She served as Chairman from 2017 to 2019 and was a member of the Audit and Finance Subcommittee of the Board from 2015 - 2020. Ms Reeves has worked as an in-house legal counsel for both small and large ASX Listed companies for many years. She is now Co-Principal of a consultancy business assisting families navigate the aged care sector. She is also a Non-Executive Director of the Royal Automobile Club of Victoria (RACV). Stephanie has previously been a member of the Council of the Royal Melbourne Golf Club, a member of the
Melbourne Cricket Ground Trust, on the Advisory Board of a start-up law firm, Lexvoco, and a Non-Executive Director and Chairman of Crime Stoppers Victoria. Stephanie has a particular interest in corporate governance in both the commercial and not-for-profit sectors.


Dr Christine Selvey retired from the Board in October 2021 after 9 years of service as the Director with immunisation expertise. Dr Selvey has had responsibility for the implementation of state immunisation programs in Queensland, the Northern Territory and Victoria. She was a member of the National Immunisation Committee (NIC) from 1999-2007 and has been both the NIC and the Communicable Diseases Network Australia (CDNA) representative on the Australian Technical Advisory Group on Immunisation (ATAGI). Dr Selvey has a particular interest in HPV vaccination and was a member of two ATAGI working groups that provided recommendations on the use of HPV vaccines in Australia. With her experience in managing immunisation programs in the two Australian jurisdictions with immunisation registers, and her experience with the Australian Childhood Immunisation Register, Dr Selvey brought a good understanding of the operation of immunisation registers to the Board.
Ms Ange Steele joined the ACPCC Board of Directors in January 2022 as the nurse representative with relevant expertise in preventative health. Ms Steele in a Registered Nurse, and Registered


Midwife and has a Post Graduate Diploma in Adolescent Health and a Master of Public Health. Ms Steele has been a cervical screening nurse for 22 years which includes experience in rural and remote Australia. She is currently the Dysplasia Nurse Coordinator at the Royal Women's Hospital and is the first trained Nurse Colposcopist in Victoria. Ms Steele is also a nurse educator and course coordinator in cervical screening courses for nurses at Family Planning Victoria and is involved in research participation in cervical cancer prevention at The Royal Women’s Hospital.
Ms Genevieve Webb joined the Board in 2019 as a Director with a consumer perspective. Genevieve is a director and consultant in health and human services. She was previously the Director Quality at BreastScreen Victoria, where she led the client centric care and consumer engagement program. Other past roles include CEO of Queen Victoria Women’s Centre, GM Corporate Services at Mind, Executive Director of Relationships Australia (Vic) and Associate Director at KPMG. Genevieve has extensive experience in organisational governance, as Chair of the Audit Committee at the State Revenue Office (Vic) and as a board member of a community health service, a TAFE college and other community organisations. She has qualifications in Psychology, IT and Consumer Engagement and is a Fellow of the Australian Institute of Company Directors.

ACPCC was incorporated under the Corporations Act 2001 on 3 December 2015 and is a company limited by guarantee. If the company is wound up, the constitution states that each member is required to contribute a maximum of $10 each towards meeting any outstanding obligations. At 30 June 2022, the total amount that members of the company are liable to contribute if the company is wound up is $100.
In accordance with the constitution, the person appointed as the Executive Director shall also be the Company Secretary. The Executive Director, Professor Marion Saville AM, has held the position of Company Secretary for the year.
The principal activity of ACPCC during the financial year was to provide public health services. Specifically, this included laboratory and registry services supporting cervical cancer screening and vaccination.
There was a major structural change in the funding profile of ACPCC in 2021/22 with the end of the Commonwealth’s Project Agreement funding for cervical screening at the end of June 2021. The Project Agreement has been critical in sustaining laboratory operations and our staffing model as the ACPCC navigates the cyclical changes in screening volumes and revenue with the renewed National Cervical Screening Program. From 1 July 2021, VCS Pathology became reliant on test volumes, test types and Medicare bulk billing for revenue. The financial result for 2021/22 reports extraordinary income from SARS-CoV-2 testing opportunities during the peak of
the COVID-19 pandemic for the benefit of Victoria as well the ACPCC and is not reflective of our core laboratory services.
The consolidated net result for ACPCC for the financial year ending 30 June 2022 was a surplus of $10.345M after considering depreciation and amortisation. The surplus was well above budget and reflects the collective efforts of the VCS Pathology team in maximising opportunities for COVID-19 testing and the Executive Team in securing new projects and funding, building on our strong relationships with a range of stakeholders.
During the 2021/22 financial year, no Board Director declared a conflict of pecuniary interest in a contract with ACPCC.
During 2021/22 the following Board Members noted their involvement with the Compass Trial Pilot: Mr David Wrede: Principal investigator – Compass Trial Dr Jane Collins: Investigator – Compass Trial
The following meetings were held during 2021/22:
• The Members of the organisation met at the Annual General Meeting 20 November 2021
• The Board of Directors met on eight occasions either in person or via teleconference/Zoom
• The Board’s Audit and Finance Committee met on five occasions, and
• The Board’s Quality Assurance Committee met on 12 occasions for Scientific Quality and four occasions for Operational Quality.
ACPCC records, monitors and reports on enterprise-level risks using the software RiskWare™ which is compliant with ISO 45001:2018 and ISO 31000:2018. The Board of Directors are provided with Quarterly Risk Reports and are immediately notified if a risk is escalated to a High or Extreme rating. The Risk Management Policy and Risk Management Procedure were last updated and approved by the Board in May 2022.
The risk management framework supports the organisation in effectively managing risks by providing a systematic and documented process to identify, mitigate and manage the risks that may impact the achievement of ACPCC’s business and strategic objectives, both positively and negatively. The Executive Director, Business Unit Directors and Managers are responsible for identifying, evaluating and treating Risks in their respective business areas. There were no High/Extreme risks at the end of the 2021/22 reporting period.
I, Marion Saville, certify that ACPCC has appropriate risk management processes in place consistent with the Risk Management Standard AS ISO 31000:2018 and has an internal control system in place that enables the Executive Management team to understand, manage and satisfactorily control risk exposures.
The Audit and Finance Committee verifies this assurance and that the risk profile of the ACPCC has been critically reviewed within the last 12 months.
Professor Marion Saville, AM ACPCC Executive Director Fiona Kelly Chair, ACPCC Audit & Finance CommitteeThe external auditor’s independence declaration for the year ended 30 June 2022 has been received and can be found on page 90 of the financial report. This directors’ report is signed in accordance with a resolution of the Board of Directors.
Director
Timothy Humphries Dated 11/11/2022
The ACPCC’s consolidated net result for the financial year ended 30 June 2022 was an outstanding surplus of $10.345M after accounting for depreciation and amortisation.
This was an extremely positive result compared to budget, reflecting the ACPCC’s efforts in COVID-19 testing for the benefit of Victoria during the peak of the pandemic, and not our core cervical screening work.
In 2021/22, VCS Pathology processed a total of 312,878 COVID-19 specimens from IPC Health screening clinics and drive though test sites across metropolitan Melbourne, demonstrating our continued commitment to the Victorian COVID-19 response. The financial result reported is an exception, understanding that the pandemic is not yet over but the phase of intense PCR testing has ended. It reflects the collective efforts of VCS Pathology and other business units in capturing new
Dr Michelle Critchleyopportunities and continuing service delivery of our funded projects during the many challenges of the pandemic.
The 2021/22 year was a major era of change with the move to Medicare bulk billing by VCS Pathology. This follows the end of Project Agreement funding for cervical screening at 30 June 2021; funding which has provided secure and reliable income for the last 20 years. As forecast, the HPV primary screening volumes reported were in-line with the modelled transition to the five-yearly screening cycle. Approximately 108,000 HPV tests were reported in the financial year compared with just over 122,000 tests in the previous year. The surplus from COVID-19 testing will provide financial resilience in 2022/23 as we move into another low volume HPV year. VCS Pathology continued to maintain its target market share for HPV tests, reporting almost 50% of all cervical screening tests in Victoria. New investment into automated technology has positioned the laboratory to effectively service the volume demands from HPV self-collection from 1 July 2022.
ACPCC continued to deliver the contractual requirements under its Service Plans with the Victorian Government. The organisation was awarded Commonwealth funding to develop Australia’s National Elimination Strategy for Cervical Cancer and continue the important follow-up activities for the Compass Trial. Significant capital investment was made to upgrade VCS Pathology for continuity in SARS-COVID-2 testing and for employee safety. The financial year ahead will see major investment in the future of the laboratory with implementation of a new Laboratory Information Management System.
ACPCC has valued the financial support from both the Commonwealth and Victorian Governments. Government support has enabled ACPCC, as a for purpose, not-for-profit organisation to continue the delivery of service excellence across screening, vaccination and specialist laboratory services. We also acknowledge the support of the Audit and Finance Committee, the Executive and the Corporate Services team in completion of the 2021-22 financial statements.
Ms Fiona Kelly Chairman – Audit & Finance Committee
 Dr Michelle Critchley Director Corporate Services
Dr Michelle Critchley Director Corporate Services
Trading as The Australian Centre for the Prevention of Cervical Cancer
ABN 35 430 554 780
Auditor's Independence Declaration under Section 60 40 of the Australian Charities and Not for profits Commission Act 2012 to the Responsible Persons of VCS Foundation Limited and Controlled Entities
FOR THE YEAR ENDED 30 JUNE 2022
The accompanying notes form part of these financial statements.
The accompanying notes form part of these financial statements.
The accompanying notes form part of these financial statements.
The financial report covers VCS Foundation Limited, a Company registered on 3 December 2015 in Victoria under the Corporations Act 2001 (previously registered as Victorian Cytology Service Inc., an Association incorporated on 3 September 1991 in Victoria under the Associations Incorporation Reform Act, 2012 (Vic)). In accordance with section 601BM of the Corporations Act 2001, this change does not create a new legal entity.
The Company is registered with the Australian Charities and Not for profit Commission (ACNC) and is therefore also required to comply with the Australian Charities and Not for profits Commission Act 2012. The functional and presentation currency of VCS Foundation Limited is Australian dollars.
Comparatives are consistent with prior years, unless otherwise stated.
The financial statements are general purpose financial statements that have been prepared in accordance with the Australian Accounting Standards – Simplified Disclosures and the Australian Charities and Not for profits Commission Act 2012.
The Company is a not for profit entity and therefore applies the additional paragraphs applicable to 'not for profit' organisations under the Australian Accounting Standards ('AAS').
The financial statements, except for the cash flow information, have been prepared on an accruals basis and are based on historical costs modified, where applicable, by the measurement at fair value of selected non current assets, financial assets and financial liabilities.
Significant accounting policies adopted in the preparation of these financial statements are presented below and are consistent with prior reporting periods unless otherwise stated.
Revenue from contracts with customers
The core principle of AASB 15 is that revenue is recognised on a basis that reflects the transfer of promised goods or services to customers at an amount that reflects the consideration the Company expects to receive in exchange for those goods or services. Revenue is recognised by applying a five step model as follows:
1. Identify the contract with the customer
2. Identify the performance obligations
3. Determine the transaction price
4. Allocate the transaction price to the performance obligations
5. Recognise revenue as and when control of the performance obligations is transferred
Generally the timing of the payment for sale of goods and rendering of services corresponds closely to the timing of satisfaction of the performance obligations, however where there is a difference, it will result in the recognition of a receivable, contract asset or contract liability.
None of the revenue streams of the Company have any significant financing terms as there is less than 12 months between receipt of funds and satisfaction of performance obligations.
The revenue recognition policies for the principal revenue streams of the Company are:
Revenue from provision of services is recognised in the accounting period in which the services are rendered. For fixed price contracts, revenue is recognised based on the actual services provided to the end of the reporting period as a proportion of the total services to be provided as the customer receives and uses the benefit simultaneously.
Statement of financial position balances relating to revenue recognition
Contract assets and liabilities
Where the amounts billed to customers are based on the achievement of various milestones established in the contract, the amounts recognised as revenue in a given period do not necessarily coincide with the amounts billed to or certified by the customer.
When a performance obligation is satisfied by transferring a promised good or service to the customer before the customer pays consideration or the before payment is due, the Company presents the contract as a contract asset, unless the Company's rights to that amount of consideration are unconditional, in which case the Company recognises a receivable.
When an amount of consideration is received from a customer prior to the entity transferring a good or service to the customer, the Company presents the contract as a contract liability.
Grant revenue
Government grants are recognised at fair value where there is reasonable assurance that the grant will be received and all grant conditions will be met. Grants relating to expense items are recognised as income over the periods necessary to match the grant to the costs they are compensating.
Grants relating to assets are credited to deferred income at fair value and are credited to income over the expected useful life of the asset on a straight line basis.
Interest income is recognised on a proportional basis taking into account the interest rates applicable to the financial assets, using the effect interest rate method.
Other income
Other income is recognised on an accruals basis when the Company is entitled to it.
At inception of a contract, the Company assesses whether a lease exists i.e. does the contract convey the right to control the use of an identified asset for a period of time in exchange for consideration.
This involves an assessment of whether:
• The contract involves the use of an identified asset – this may be explicitly or implicitly identified within the agreement. If the supplier has a substantive substitution right then there is no identified asset.
• The Company has the right to obtain substantially all of the economic benefits from the use of the asset throughout the period of use.
• The Company has the right to direct the use of the asset i.e. decision making rights in relation to changing how and for what purpose the asset is used.
The non lease components included in the lease agreement have been separated and are recognised as an expense as incurred.
At the lease commencement, the Company recognises a right of use asset and associated lease liability for the lease term. The lease term includes extension periods where the Company believes it is reasonably certain that the option will be exercised. The right of use asset is measured using the cost model where cost on initial recognition comprises of the lease liability, initial direct costs, prepaid lease payments, estimated cost of removal and restoration less any lease incentives received. The right of use asset is depreciated over the lease term on a straight line basis and assessed for impairment in accordance with the impairment of assets accounting policy.
The lease liability is initially measured at the present value of the remaining lease payments at the commencement of the lease. The discount rate is the rate implicit in the lease, however where this cannot be readily determined then the Company's incremental borrowing rate is used.
Subsequent to initial recognition, the lease liability is measured at amortised cost using the effective interest rate method. The lease liability is remeasured whether there is a lease modification, change in estimate of the lease term or index upon which the lease payments are based (e.g. CPI) or a change in the Company's assessment of lease term.
Where the lease liability is remeasured, the right of use asset is adjusted to reflect the remeasurement or is recorded in profit or loss if the carrying amount of the right of use asset has been reduced to zero.
The Company has elected to apply the exceptions to lease accounting for both short term leases (i.e. leases with a term of less than or equal to 12 months) and leases of low value assets. The Company recognises the payments associated with these leases as an expense on a straight line basis over the lease term.
Borrowing costs are recognised as an expense in the period in which they are incurred.
The Company is exempt from income tax under Division 50 of the Income Tax Assessment Act 1997.
Cash and cash equivalents comprise cash on hand, deposits held at call with banks and other short term highly liquid investments with original maturities of three months or less, which are readily convertible to known amounts of cash and are subject to insignificant risk of changes in value.
Bank overdrafts also form part of cash equivalents for the purpose of the statement of cash flows and are presented within current liabilities on the statement of financial position..
Financial instruments are recognised initially on the date that the Company becomes party to the contractual provisions of the instrument.
On initial recognition, all financial instruments are measured at fair value plus transaction costs (except for instruments measured at fair value through profit or loss where transaction costs are expensed as incurred).
All recognised financial assets are subsequently measured in their entirety at either amortised cost or fair value, depending on the classification of the financial assets.
Classification
On initial recognition, the Company classifies its financial assets into the following category, those measured at:
• amortised cost.
Financial assets are not reclassified subsequent to their initial recognition unless the Company changes its business model for managing financial assets.
FOR THE YEAR ENDED 30 JUNE 2022
Assets measured at amortised cost are financial assets where:
• the business model is to hold assets to collect contractual cash flows; and
• the contractual terms give rise on specified dates to cash flows are solely payments of principal and interest on the principal amount outstanding.
The Company's financial assets measured at amortised cost comprise trade and other receivables, cash and cash equivalents and term deposits in the statement of financial position.
Subsequent to initial recognition, these assets are carried at amortised cost using the effective interest rate method less provision for impairment.
Interest income, foreign exchange gains or losses and impairment are recognised in profit or loss. Gain or loss on derecognition is recognised in profit or loss.
Impairment of financial assets is recognised on an expected credit loss (ECL) basis for the following assets:
• financial assets measured at amortised cost.
When determining whether the credit risk of a financial asset has increased significantly since initial recognition and when estimating ECL, the Company considers reasonable and supportable information that is relevant and available without undue cost or effort. This includes both quantitative and qualitative information and analysis based on the Company's historical experience and informed credit assessment and including forward looking information.
The Company uses the presumption that an asset which is more than 30 days past due has seen a significant increase in credit risk.
The Company uses the presumption that a financial asset is in default when:
• the other party is unlikely to pay its credit obligations to the Company in full, without recourse to the Company to actions such as realising security (if any is held); or
• the financial assets is more than 90 days past due.
Credit losses are measured as the present value of the difference between the cash flows due to the Company in accordance with the contract and the cash flows expected to be received. This is applied using a probability weighted approach.
Impairment of trade receivables have been determined using the simplified approach in AASB 9 which uses an estimation of lifetime expected credit losses. The Company has determined the probability of non payment of the receivable
and multiplied this by the amount of the expected loss arising from default.
The amount of the impairment is recorded in a separate allowance account with the loss being recognised in finance expense. Once the receivable is determined to be uncollectable then the gross carrying amount is written off against the associated allowance.
Where the Company renegotiates the terms of trade receivables due from certain customers, the new expected cash flows are discounted at the original effective interest rate and any resulting difference to the carrying value is recognised in profit or loss.
Other financial assets measured at amortised cost
Impairment of other financial assets measured at amortised cost are determined using the expected credit loss model in AASB 9. On initial recognition of the asset, an estimate of the expected credit losses for the next 12 months is recognised. Where the asset has experienced significant increase in credit risk then the lifetime losses are estimated and recognised.
The Company measures all financial liabilities initially at fair value less transaction costs, subsequently financial liabilities are measured at amortised cost using the effective interest rate method.
The financial liabilities of the Company comprise trade and other payables and finance lease liabilities.
Inventories are measured at the lower of cost and net realisable value. Cost of inventory is determined using the first in first out basis and is net of any rebates and discounts received. Net realisable value is estimated using the most reliable evidence available at the reporting date and inventory is written down through an obsolescence provision if necessary.
Each class of property, plant and equipment is carried at cost less, where applicable, any accumulated depreciation and impairment. Assets are capitalised when in excess of $1,000.
Property, plant and equipment, excluding freehold land, is depreciated on a straight line basis over the assets useful life to the Company, commencing when the asset is ready for use. Leased assets and leasehold improvements are amortised over the shorter of either the unexpired period of the lease or their estimated useful life.
The depreciation rates used for each class of depreciable asset are shown below:
These amounts consist predominantly of liabilities for goods and services. Payables are initially recognised at fair value and then subsequently carried at amortised cost and represent liabilities for goods and services provided to the Company prior to the end of the financial year that are unpaid, and arise when the Company becomes obliged to make future payments in respect of purchase of these goods and services. The normal credit terms are usually Net 30 days.
At the end of each annual reporting period, the depreciation method, useful life and residual value of each asset is reviewed. Any revisions are accounted for prospectively as a change in estimate.
Intangible assets represent identifiable non monetary assets without physical substance such as patents, trademarks, and computer software and development costs (where applicable). Intangible assets are initially recognised at cost. Subsequently, intangible assets with finite useful lives are carried at cost less accumulated amortisation and accumulated impairment losses. Costs incurred subsequent to initial acquisition are capitalised when it is expected that future economic benefits will flow to the Company.
Amortisation
Amortisation is recognised in profit or loss on a straight line basis over the estimated useful lives of intangible assets, other than goodwill, from the date that they are available for use. Amortisation methods, useful lives and residual values are reviewed at each reporting date and adjusted if appropriate.
Software and licenses
Software and licenses have a finite life and are carried at cost less any accumulated amortisation and impairment losses. It has an estimated useful life of three years.
At the end of each reporting period, the Company determines whether there is any evidence of impairment for its non financial assets.
Where an indicator exists and regardless for goodwill, indefinite life intangible assets and intangible assets not yet available for use, the recoverable amount of the asset is estimated.
Where assets do not operate independently of other assets, the recoverable amount of the relevant cash generating unit (CGU) is estimated.
The recoverable amount of an asset or CGU is the higher of the fair value less costs of disposal and the value in use. Value in use is the present value of the future cash flows expected to be derived from an asset or cash generating unit. Where the recoverable amount is less than the carrying amount, an impairment loss is recognised in profit or loss. Reversal indicators are considered in subsequent periods for all assets which have suffered an impairment loss, except for goodwill.
Provision is made for the Company’s obligation for short term employee benefits. Short term employee benefits are benefits (other than termination benefits) that are expected to be settled wholly before 12 months after the end of the annual reporting period in which the employees render the related service, including wages and salaries. Short term employee benefits are measured at the (undiscounted) amounts expected to be paid when the obligation is settled. The Company’s obligations for short term employee benefits such as wages and salaries are recognised as a part of current trade and other payables in the statement of financial position.
Provision is made for employees’ long service leave and annual leave entitlements not expected to be settled wholly within 12 months after the end of the annual reporting period in which the employees render the related service. Other long term employee benefits are measured at the present value of the expected future payments to be made to employees. Expected future payments incorporate anticipated future wage and salary levels, durations of service and employee departures and are discounted at rates determined by reference to market yields at the end of the reporting period on corporate bonds that have maturity dates that approximate the terms of the obligations. Upon the remeasurement of obligations for other long term employee benefits, the net change in the obligation is recognised in profit or loss as a part of employee benefits expenses.
The Company’s obligations for long term employee benefits are presented as non current provisions in its statement of financial position, except where the Company does not have an unconditional right to defer settlement for at least 12 months after the end of the reporting period, in which case the obligations are presented as current provisions.
Obligations for contributions to defined contribution superannuation plans are recognised as an employee benefit expense in profit or loss in the periods in which services are provided by employees.
The Company has minimal exposure to liability arising from defined benefit plan liability as highlighted in Note 24. In view of this, the amount is not recognised on the basis that it is immaterial.
FOR THE YEAR ENDED 30 JUNE 2022
Revenue, expenses and assets are recognised net of the amount of goods and services tax (GST), except where the amount of GST incurred is not recoverable from the Australian Taxation Office (ATO).
Receivables and payables are stated inclusive of GST. Cash flows in the statement of cash flows are included on a gross basis and the GST component of cash flows arising from investing and financing activities which is recoverable from, or payable to, the taxation authority are classified as operating cash flows.
The Company has adopted all of the new or amended Accounting Standards and Interpretations issued by the Australian Accounting Standards Board ('AASB') that are mandatory for the current reporting period.
Any new or amended Accounting Standards or Interpretations that are not yet mandatory have not been early adopted.
The adoption of these Accounting Standards and Interpretations did not have any significant impact on the financial performance or position of the Company.
The following Accounting Standards and Interpretations are most relevant to the Company:
Conceptual Framework for Financial Reporting (Conceptual Framework)
The Company has adopted the revised Conceptual Framework from 1 July 2021. The Conceptual Framework contains new definition and recognition criteria as well as new guidance on measurement that affects several Accounting Standards, but it has not had a material impact on the Company's financial statements.
AASB 1060 General Purpose Financial Statements – Simplified Disclosures for For Profit and Not for Profit Tier 2 Entities
The Company has adopted AASB 1060 from 1 July 2021. The standard provides a new Tier 2 reporting framework with simplified disclosures that are based on the requirements of IFRS for SMEs. As a result, there is increased disclosure in these financial statements for key management personnel, related parties, tax and financial instruments.
The AASB has issued new and amended Accounting Standards and Interpretations that have mandatory application dates for future reporting periods. The Directors have decided against early adoption of these Standards, but do not expect the adoption of these standards to have any impact on the reported position or performance of the Company.
The Directors make estimates and judgements during the preparation of these financial statements regarding assumptions about current and future events affecting transactions and balances.
These estimates and judgements are based on the best information available at the time of preparing the financial statements, however as additional information is known then the actual results may differ from the estimates. The significant estimates and judgements made have been described below.
The Company assesses impairment at the end of each reporting period by evaluating conditions specific to the Company that may be indicative of impairment triggers. Recoverable amounts of relevant assets are reassessed using value in use calculations which incorporate various key assumptions.
The receivables at the reporting date have been reviewed to determine whether there is any objective evidence that any of the receivables are impaired. An impairment provision is included for any receivable where the entire balance is not considered collectible. The impairment provision is based on the best information at the reporting date.
Judgement has been exercised in considering the impacts that the Coronavirus (COVID 19) pandemic has had, or may have, on the Company based on known information. There does not currently appear to be either any significant impact upon the financial statements or any significant uncertainties with respect to events or conditions which may impact the Company unfavourably as at the reporting date as a result of the COVID 19 pandemic.
is revenue of $26,712,439 (2021: $8,316,762) which was generated from the testing of specimens for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV 2). This activity commenced in May 2020 and was undertaken throughout the years ended 30 June 2022 and 2021. The extraordinary respiratory activities is also reflected in the increase in consumables expense
The effective interest on short term bank deposits was 0.17% (2021: 0.27%).
(A)
Cash and cash equivalents reported in the statement of cash flows are reconciled to the equivalent items in the statement of financial position as follows:
The carrying value of trade receivables is considered a reasonable approximation of fair value due to the short term nature of the balances.
The maximum exposure to credit risk at the reporting date is the fair value of each class of receivable in the financial statements.
The Company applies the simplified approach to providing for expected credit losses prescribed by AASB 9, which permits the use of the lifetime expected loss provision for all trade receivables. To measure the expected credit losses, trade receivables have been grouped based on shared credit risk characteristics and the days past due. The loss allowance provision as at 30 June 2022 is determined as follows, the expected credit losses incorporate forward looking information.
The Company measures the loss allowance for trade receivables at an amount equal to lifetime expected credit loss (ECL). The ECL on trade receivables are estimated using a provision matrix by reference to past default experience of the debtor and an analysis of the debtor’s current financial position, adjusted for factors that are specific to the debtors, general economic conditions of the industry in which the debtors operate and an assessment of both the current as well as the forecast direction of conditions at the reporting date.
The Company writes off a trade receivable when there is information indicating that the debtor is in severe financial difficulty and there is no realistic prospect of recovery, e.g. when the debtor has been placed under liquidation or has entered into bankruptcy proceedings or when the trade receivables are over 150 days past due, whichever occurs first.
THE COMPANY AS A LESSEE
The Company has leases over a range of assets including land and buildings, and plant and equipment. Information relating to the leases in place and associated balances and transactions are provided below.
Terms and conditions of leases
The Company has entered into the following lease arrangements:
- a premises located in East Melbourne for the term 1 March 2019 to 28 February 2023; and
- plant and equipment, 1 x Cobas 6800 system and 2 x Cobas p 480 v2 instruments, from 1 December 2017 to 30 November 2025.
Neither of the above lease agreements contain options to extend the term of options to purchase the assets on expiry.
MOVEMENT IN CARRYING AMOUNTS
Movement in the carrying amounts for each class of property, plant and equipment between the beginning and the end of the current financial year:
Trade and other payables are unsecured, non-interest bearing and are normally settled within 30 days. The carrying value of trade and other payables is considered a reasonable approximation of fair value due to the short-term nature of the balances.
(A) TRADE AND OTHER PAYABLES CLASSIFIED AS FINANCIAL LIABILITIES AT AMORTISED COST:
Based on past experience, the Company expects the full amount of the annual leave balance to be wholly settled within the next 12 months. Further, these amounts must be classified as current liabilities since the Company does not have an unconditional right to defer settlement of these amounts in the event that employees wish to use their leave entitlements.
(A) DESIGNATED FUNDS RESERVE
The capital funds represent the capital funding received to cover the cost of the upgrade of the VCS/VCCR data base. The amortisation of the upgrade will be allocated against the capital funds over the expected life of the upgrade.
The accumulated surplus represents the funds of the Company that are not designated for particular purposes.
The Company is incorporated under the Australian Charities and Not‑for‑profits Commission Act 2012 and is a Company limited by guarantee. If the Company is wound up, the constitution states that each member is required to contribute a maximum of $10 each towards meeting any outstanding obligations of the Company. At 30 June 2022 the number of members was 8 (2021: 8).
The Company's principal financial instruments comprise of deposits with banks, receivables and payables. The totals for each category of financial instruments, measured in accordance with AASB 9 as detailed in the accounting policies to these financial statements, are as follows:
None of the Company’s financial instruments are recorded at fair value.
The names of persons who were Board members at any time during the year are set out in the Annual Report. There were no transactions that require disclosure for the years ended 30 June 2022 and 30 June 2021.
The Board did not receive any remuneration during the financial years ended 30 June 2022 and 30 June 2021.
Key management personnel remuneration included within employee expenses for the year is shown below:
During the 2021/22 year, nil executives resigned and 1 was appointed. As at 30 June 2022, 8 FTEs were employed as executives (2021: 8). There were no transactions between the Company and the executives during the year.
The Company contributes to a Defined Benefits Scheme ('the Scheme') maintained by Aware Super Fund ('the Fund') and has an ongoing obligation to share in the future experience of the Fund including favourable or unfavourable variations that may arise should the experience of the Fund differ from the assumptions made by the Fund's actuary in estimating the Fund's accrued benefits liability. The trustee of the Scheme has determined that the notional excess of net assets attributable to the staff who are members of the Scheme for the year ended 30 June 2022 totalled $2,906 (2021: $6,084). The Fund's actuary has advised that the contributions will remain unchanged for the current year.
In the opinion of the Directors, the Company did not have any contingencies at 30 June 2022 (30 June 2021: None).
The financial report was authorised for issue on 28 October 2022 by the Directors. No matters or circumstances have arisen since the end of the financial year which significantly affected or may significantly affect the operations of the Company, the results of those operations or the state of affairs of the Company in future financial years.
The registered office and principal place of business of the Company is: VCS Foundation Limited 265 Faraday Street Carlton South VIC 3053
The directors of the Company declare that:
1. The financial statements and notes, as set out on pages 69 to 88, are in accordance with the Australian Charities and Not for profits Commission Act 2012, and:
a. comply with Australian Accounting Standards – Simplified Disclosures; and
b. give a true and fair view of the financial position as at 30 June 2022 and of the performance for the year ended on that date of the Company.
2. In the directors’ opinion, there are reasonable grounds to believe that the entity will be able to pay its debts as and when they become due and payable.
In addition:
1. We certify that VCS Foundation Limited has complied with the terms and conditions of their service agreement with the Commonwealth and Victorian Department(s).
2. We certify that VCS Foundation Limited has used funding received from the Commonwealth and Victorian Department(s) for the year ended 30 June 2022 on the services specified in the service agreement.
This declaration is signed in accordance with subs 60.15(2) of the Australian Charities and Not for profits Commission Regulation 2013.
David Wrede Chairperson Director Tim HumphriesDated this 28th day of October, 2022
We declare that, to the best of our knowledge and belief, there have been no contraventions of any applicable code of professional conduct in relation to the review of the financial report of VCS Foundation Limited for the year ended 30 June 2022.
HLB Mann Judd Nick Walker Chartered Accountants Partner Melbourne 28 October 2022

We have audited the financial report of VCS Foundation Limited (“the Foundation”) which comprises the statement of financial position as at 30 June 2022, the statement of profit or loss and other comprehensive income, the statement of changes in equity and the statement of cash flows for the year then ended, and notes to the financial statements, including a summary of significant accounting policies, and the directors’ declaration.
In our opinion, the accompanying financial report of the Foundation has been prepared in accordance with Division 60 of the Australian Charities and Not for profits Commission Act 2012, including:
In our opinion, the accompanying financial report of the Entity is in accordance with Division 60 of the Australian Charities and Not for profits Commission Act 2012 including:
(a) giving a true and fair view of the Foundation’s financial position as at 30 June 2022 and of its financial performance for the year then ended.
(b) complying with Australian Accounting Standards and Division 60 of the Australian Charities and Not for profits Commission Regulation 2013.


We conducted our audit in accordance with Australian Auditing Standards. Our responsibilities under those standards are further described in the Auditor’s Responsibilities for the Audit of the Financial Report section of our report. We are independent of the Foundation in accordance with the auditor independence requirements of the Accounting Professional and Ethical Standards Board’s APES 110 Code of Ethics for Professional Accountants (“the Code”) that are relevant to our audit of the financial report in Australia. We have also fulfilled our other ethical responsibilities in accordance with the Code.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion.
Management is responsible for the preparation of the financial report that gives a true and fair view in accordance with the Australian Accounting Standards and the Australian Charities and Not for profits Commission Act 2012 and for such internal control as management determines is necessary to enable the preparation of the financial report that is free from material misstatement, whether due to fraud or error.
In preparing the financial report, management is responsible for assessing the Foundation’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless management either intend to liquidate the Foundation or to cease operations, or have no realistic alternative but to do so.
The directors are responsible for overseeing the Foundation’s financial reporting process.
Our objectives are to obtain reasonable assurance about whether the financial report as a whole is free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with Australian Auditing Standards will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of this financial report.
As part of an audit in accordance with the Australian Auditing Standards, we exercise professional judgement and maintain professional scepticism throughout the audit. We also:
• Identify and assess the risks of material misstatement of the financial report, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control.
• Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Foundation’s internal control.
• Evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by the management.
• Conclude on the appropriateness of the management’s use of the going concern basis of accounting and, based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the Foundation’s ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our auditor’s report to the related disclosures in the financial report or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our auditor’s report. However, future events or conditions may cause the Foundation to cease to continue as a going concern.
• Evaluate the overall presentation, structure and content of the financial report, including the disclosures, and whether the financial report represents the underlying transactions and events in a manner that achieves fair presentation.
• We communicate with the directors regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit.
We also provide the directors with a statement that we have complied with relevant ethical requirements regarding independence, and to communicate with them all relationships and other matters that may reasonably be thought to bear on our independence, and where applicable, related safeguards.
HLB Mann Judd Nick Walker Chartered Accountants Partner Melbourne 28 October 2022

ACPCC Australian Centre for the Prevention of Cervical Cancer
ATAGI Australian Technical Advisory Group on Immunisation
CPD Continuing Professional Development
CRE Centre of Research Excellence
CST Cervical Screening Test
Cth Commonwealth
ECHO Extension For Community Healthcare Outcomes
FOBT Faecal Occult Blood Test
FTE Full time equivalent
GP General Practitioner
HPV Human Papillomavirus
HR Human Resources
ICT Information Communication Technology
ISO International Standards
LBC Liquid Based Cytology
LTIFR Lost time injury Frequency Rate
NATA National Association of Testing Authorities, Australia
NBCSP National Bowel Cancer Screening Program
NCSP National Cervical Screening Program
NCSR National Cancer Screening Register
NHMRC National Health and Medical Research Council
NPAAC National Pathology Accreditation Advisory Council
PAIVnG Providing Access to Immunisation for Vulnerable Groups
PCC Preventing Cervical Cancer Conference
PFUF Participant Follow Up Function, (National Bowel Cancer Screening Program)
RACGP Royal Australian College of General Practitioners
RCPA Royal College of Pathologists Australasia
TAT Turn Around Time
VCS VCS Foundation Limited
WHO World Health Organization
GIAHC Advisory Board -partnership opportunities in the Indo-Pacific region for CCE- Global Initiative Against HPV and Cervical Cancer (GIAHC)
International Taskforce on Cervical Cancer Elimination in The Commonwealth. March 2021
International Papillomavirus Society Policy Committee
Non-trials workgroup, One dose HPV vaccine consortium (PI PATH, Funding Bill and Melinda Gates Foundation)Member and external advisor
WHO Director-General’s Expert Advisory Group on the Elimination of Cervical Cancer
Rapporteur and technical writer, Strategic Advisory Group of Experts HPV Working Party 2021-2022, World Health Organization, Geneva
Data Monitoring and Safety Board, ADDVAC study (Chair)
Prof Marion Saville
GIAHC is one of the founding members of the Cervical Cancer Action for Elimination (CCAE), a network of organizations working together to accelerate global progress towards a world free from cervical cancer
Prof Marion Saville
Australian-led resolution by member states of the World Health Organization (WHO): Cervical Cancer Prevention and Control: Accelerating the Elimination of Cervical Cancer as a Public Health Problem. Australia stands ready to work with the international community to take these commitments forward
Prof Julia Brotherton Engagement with lead policy makers in HPV and cervical screening
Prof Julia Brotherton Close ongoing connections with international HPV immunisation experts relating to ongoing research and evaluation of HPV vaccine impact
Prof Julia Brotherton Direct engagement with lead international group strategy setting for global cervical cancer elimination
Prof Julia Brotherton Supporting the work of the global expert committee providing advice on use of HPV vaccines
Prof Julia Brotherton Study assessing The Population-Level Impact of Adding Male Single Dose HPV Vaccination to Female HPV Vaccination in Tanzania: A Cluster-Randomised Trial. Study Sponsor London School of Hygiene & Tropical Medicine (CIA D Watson Jones)
Invited Council Member, HPV Vaccine Council, CHIC
Member of the IPVC 2021 Public Health Workshop Committee
International Agency for Research on Cancer (IARC) Working Group for Handbook 18 on Cervical Screening 2020/2021
Prof Julia Brotherton
The Council is a global forum of experts intended to foster efficient translation of implementation research to guide practice and more equitable access to immunization and is part of the Bill and Melinda Gates Foundation funded Coalition to Strengthen the HPV Immunization Community (CHIC) project
Prof Julia Brotherton Supporting public health stream of the leading international HPV conference
Prof Julia Brotherton IARC prepares and distributes authoritative information on the causes and prevention of cancer throughout the world
Member of the International Collaboration on Cancer Reporting (ICCR) Expert Committee working on the 4th edition cervix cancer dataset
Host of the ISGyP LiVE team’s monthly online Journal Club
Member of Working Group 1 (cancer audit) of the CervScreen Initiative, a collaboration between the International Agency for Research on Cancer (IARC), Department of Health (DoH) and Health Service Executive (HSE) of Ireland
NATIONAL
Australian Government Department of Health: National Cervical Screening Program Quality and Safety Committee
Australian Government Department of Health: National Cervical Screening Program’s Self-collection Implementation Committee
Australian Government Department of Health: National Cervical Screening Program 'Clinical Expert Panel'
Australian Government Department of Health: National Cervical Screening Program 'Self-Collection Expert Advisory Group'
National Pathology Accreditation Advisory Council (NPAAC) 'Standards Cervical Screening Drafting Committee'
Dr Karen Talia Information obtained through membership of this Committee is communicated to VCS Pathologists, ensuring that our reporting of cervical biopsies and excision specimens is in line with world's best practice. This is reflected in our recent updating of the histology reporting codes
Dr Karen Talia Mentor trainees and early career pathologists (including VCS Pathologists) in critically evaluating and presenting journal articles
Prof Julia Brotherton This initiative aims to create an improved implementation framework for audit, communication transparency, legal and ethical environment in cervical cancer screening
Prof Marion Saville The QSMC will provide advice on the safety and quality of the NCSP
Prof Marion Saville Supports self-collection policy development and adoption in never and under screened Australian women
Prof Marion Saville
Provision of clinical expertise to the Federal Government as required
Prof Marion Saville Supports self-collection policy development and adoption in never and under screened Australian women
Prof Marion Saville Directly supports Australian laboratory standards for pathology laboratories reporting cervical screening tests.
National Quality Safety Monitoring Committee
Prof Marion Saville
RCPA QAP Cytopathology Advisory Committee
RCPA QAP Molecular Infectious Diseases
National Cervical Screening Program Data Dictionary Working Group
Provision of clinical expertise to the Federal Government as required.
A/Prof David Hawkes Peak committee governing the NCSP quality framework. Includes best practices for data management and reporting Horizon Scanning Working Group, a subcommittee of the NCSP Quality and safety monitoring group
Prof Marion Saville Directly assists the performance monitoring of laboratories reporting services under the NCSP. ACPCC provides feedback and support as committee members. This extends to facilitating a 'dummy run' with the RCPA QAP to test data entry processes from a lab perspective using ‘Gotomeeting’
A/Prof David Hawkes Technical advice supporting the quality and testing framework for molecular microbiology testing.
Prof Marion Saville
Genevieve Chappell
Karen Peasley
Assist the Australian Institute of Health and Welfare (AIHW) in the review of the National Cervical Screening Program (NCSP) Data Dictionary Version 1.0 in light of changes to clinical management guidelines, the program or data. This will include the review of data items, performance indicator specifications and coding sheets
RCPA Microbiology Examiner
Dr Hui Tat Mark Chan
Contributing to ensure Clinical microbiologists in training meet adequate standards to practise as independent professionals
NATIONAL
National Cancer Screening Register Cervical Reporting User Forum
Member
Karen Peasley Peak committee governing the NCSP quality framework. Includes best practices for data management and reporting.
Kate Wilkinson This is a working group comprised of communications staff from Public Pathology Australia member organisations. The purpose is to collaborate on communications campaigns and strategies to increase community and government awareness of the value of public pathology services in supporting Australian’s health STATE
Public Pathology Australia Communications Team
Cancer Council NSW, Scientific Advisory Committee for cervical stream of ‘Pathways to a Cancerfree future’
Cancer Council Victoria – Selfcollection Roll Out Committee
Victorian Department of Health Victorian Cancer Screening Steering Committee
Victorian Cancer Screening Data and Surveillance Working Group (auspiced by Department of Health Vic)
Prof Marion Saville Prof Julia Brotherton
Assists in setting future direction of the National Cervical Screening Program.
Prof Marion Saville
Prof Marion Saville
Benefits to National Cervical Screening Program in self-collection and promoting participation in under-never screened women. Assists NCSP implementation and achievement of outcomes.
The committee provides guidance to support Victoria’s delivery of and investment in cancer screening programs and facilitates collaboration across screening programs to maximise efficiencies and reduce duplication. ACPCC provides expertise to support strategic screening priorities including participation and recruitment, data and reporting, research and evaluation and health workforce education
Genevieve Chappell Chaired by VCS Foundation, this group is responsible for developing and implementing the Cancer Screening Data and Surveillance Strategy 2019-2022. The strategy aims to improve surveillance of the effectiveness and outcomes of the breast, bowel and cervical screening programs in Victoria through an integrated and consistent approach to data collection, use and reporting. Cervical screening data produced will inform Victorian initiatives to improve under and never-screened women’s participation, and support cervical screening research and education activities
Department of Health Vic Evaluation and Research Working Group, Victorian Cancer Screening Framework
Prof Julia Brotherton
Chaired by VCS Foundation, this group is responsible for developing and implementing the Cancer Screening Primary Care and Workforce Strategy 2019-2022. The Strategy will support an integrated, evidence-based cancer screening education and training approach and supporting systems, resources, tools and communications for the cancer screening workforce to improve adherence to clinical guidelines, increase health care practitioner endorsement of screening, and increase participation of underscreened populations
Victorian Cancer Screening Primary Care and Workforce Working Group (auspiced by Department of Health Vic)
Victorian Cancer Screening Participation & Recruitment Working Group (auspiced by Department of Health Vic)
Prof Marion Saville Kate Wilkinson Kim Lee
Supporting the work of the global expert committee providing advice on use of HPV vaccines
Kate Wilkinson Chaired by Cancer Council Victoria, this group is responsible for developing and implementing the Cancer Screening Participation & Recruitment Strategy 2019-2022. The Strategy will support community engagement and awareness activities designed to boost participation across all three cancer screening programs
Medical Laboratory Quality Network Committee
Department of Health Vic: Steering Committee, Cancer Council Victoria’s Eliminate Cervical Cancer Fund
Victorian Government COVID-19 Laboratory Liaison Group
Breast Screen Victoria Data Hub Project Board Member
ACPCC EMPLOYEE PURPOSE/CONTRIBUTION
A/Prof David Hawkes Victorian peer organisation for the development and application of quality frameworks within public laboratories
Prof Marion Saville Prof Julia Brotherton
Providing expertise to progress Victorian Government’s commitment to eliminate cervical cancer. The four-year plan tabled aligns with Cancer Council Victoria’s objective to eliminate cervical cancer by 2030 – possibly becoming the first jurisdiction in Australia to do so

A/Prof David Hawkes Providing information relating to experiences for VCS Pathology during the COVID-19 pandemic. This committee is also a source of information on current and future changes to the state-based pandemic processes
Genevieve Chappell Group responsible for overseeing work to facilitate the transfer of BreastScreen Victoria (BSV) data to ACPCC for data and reporting purposes under the Victorian Cancer Screening Framework. The project will establish a data hub to enable ongoing transfer of BSV datasets to ACPCC and other external reporting agencies
HPV Working Group, Positive Life, NSW Prof Julia Brotherton
Australian Society of Cytology Committee, Victorian Branch
Grace Tan
Networking with advocates and experts in the HIV and sexual health community and providing technical advice in relation to the spectrum of HPV related diseases, screening and vaccination
Organise and deliver the scientific program for the local ASC branch
The Daffodil Centre
Cancer Council Victoria
The Department of Health Victoria
Clinicians and Nurse Cervical Screening Providers
VACCHO
Royal Women’s Hospital
Public Pathology Australia
Compass Trial, Research, WHO Strategy to Eliminate Cervical Cancer
Supporting the National Cervical Screening Program in Victoria
Victorian Cancer Screening Framework
Supporting the National Cervical Screening Program in Victoria
Supporting the National Cervical Screening Program in Victoria
ACPCC Tenancy (Carlton) and Research
National collaborative laboratory relationships
University of Melbourne Research
University of Sydney Research
Kirby Institute – University of New South Wales Research
National Centre for Immunisation Research and Surveillance (NCIRS) Research
Menzies School of Health Research Research
Australian National University Research
University of Malaya Program ROSE
Jean Hailes
The National Endometriosis Clinical and Scientific Trials Registry
Public Health Association of Australia Member
Global Health Alliance Member
BioMelbourne Network Member
Union for International Cancer Control, UICC Member
Prince of Wales Hospital, NSW
Royal Brisbane Hospital, Brisbane, QLD
Mercy Hospital for Women/Austin Pathology, VIC
Westmead Hospital, NSW
Royal Prince Alfred Hospital and Chris O’Brien Lifehouse, NSW
Royal Women’s Hospital, VIC
Pathology North, NSW
Monash University, VIC
Australian Cervical Cancer Typing Study
Australian Cervical Cancer Typing Study
Australian Cervical Cancer Typing Study
Australian Cervical Cancer Typing Study
Australian Cervical Cancer Typing Study
Australian Cervical Cancer Typing Study
Australian Cervical Cancer Typing Study
Research relating to Multiple Sclerosis treatments and cervical HSIL/cancer risk
Notre Dame University, WA Research
Burnet Institute Research
International Agency for Research on Cancer
Baker Heart and Diabetes Institute
Christian Medical College Vellore, India
Zoram Medical College, Mizoram, India
RTI International
IPC Health
Global Alliance for Chronic Diseases project
Global Alliance for Chronic Diseases project
Global Alliance for Chronic Diseases project
Global Alliance for Chronic Diseases project
Global Alliance for Chronic Diseases project
Global Alliance for Chronic Diseases project
Brotherton JML member of authoring IARC Working Group on the Evaluation of Cancer-Preventive Interventions
Acuti Martellucci C, Morettini M, Brotherton J, Canfell K, Manzoli L, Flacco ME, Palmer M, Giorgi Rossi P, Martellucci M, Giacomini G, D'Errico MM, Pasqualini F
IARC Handbook of Cancer Prevention
Cancer Epidemiology Biomarkers and Prevention
Brotherton JML, Hawkes D, Saville M. Medical Journal of Australia
Brotherton JML, Hendry A, Dey A, Hull B, Beard F. Australia and New Zealand Journal of Public Health
Chadwick V, Bennett KF, McCaffery KJ, Brotherton JML, Dodd RH.
Creagh NS, Zammit C, Brotherton JM, Saville M, McDermott T, Nightingale C, Kelaher M.
Griffin-Mathieu G, Haward B, Tatar O, Zhu P, Perez S, Shapiro GK, McBride E, Thompson EL, Smith LW, Lofters AK, Daley EM, Guichon JR, Waller J, Steben M, Decker KM, Mayrand M-H, Brotherton JML, Ogilvie GS, Zimet GD, Norris T, Rosberger Z.
Guerra G, Kong J, Millen R, Read M, Liu D, Roth S, Sampurno S, Sia J, Bernardi M-P, Chittleborough T, Behrenbruch C, Xu J, Haynes N, Yu J, Lupat R, Hawkes D, Di Costanzo N, Tothill R, Mitchell C, Ngan S, Heriot A, Ramsay R, and Phillips W.
Chan HT, Keung MHT, Nguyen I, Ip EO, Chew SL, Siler D, Saville M, and Hawkes D.
Pujaria R, Newman M, Talia KL, Pendlebury A, Hawkes D, IrelandJenkin K, McCluggage WG.
PsychoOncology
Womens Health (London)
IARC (2022). Cervical cancer screening.
Impact of a human papillomavirus vaccination programme within organized cervical cancer screening: cohort study.
Reasons for rejection of self-collected samples for cervical screening
National HPV vaccination coverage: two-dose schedule estimates show slightly improved completion and historical estimates lower than from the HPV Register.
Psychosocial impact of testing HPV positive in Australia’s HPV-based cervical screening program: a crosssectional survey.
The experience of under-screened and never-screened participants using clinician-supported selfcollection cervical screening within the Australian National Cervical Screening Program
IARC Handb Cancer Prev. 18:1–456. Available from: https://publications.iarc. fr/604
Ca Epi Biomark Prev. 2022; doi:10.1158/1055-9965.EPI21-0895
Med J Aust. 2022;216(4):214. https://doi.org/10.5694/ mja2.51412
Aust NZ J Public Health 2022; Online; doi: 10.1111/1753-6405.13233
Psycho-oncology. 2022 Feb 6. doi: 10.1002/pon.5897.
Womens Health (Lond). 2022;18:17455065221075905. doi:10.1177/17455065221075905
JMIR Research Protocols
Ensuring a successful transition from Pap to HPV-based primary screening in Canada: a study protocol to investigate the psychosocial correlates of women’s screening intentions.
Cell Death Disease Molecular and Genomic Characterisation of a Panel of Human Anal Cancer Cell Lines.
Journal of Clinical Virology Plus
Acta Cytologica
Exploring beyond the limit: how comparative stochastic performance affects retesting outcomes in six commercial SARS CoV-2 nucleic acid amplification tests.
Seborrheic keratosis-like lesion of the cervix: first report of the cytological features of a low risk HPV 42 associated lesion.
JMIR Res Protoc 2022;11(6):e38917. doi: 10.2196/38917
Cell Death Dis . 2021 18;12(11):959. doi: 10.1038/ s41419-021-04141-5
J Clin Virol Plus. 2022 ;2(3):100079. doi: 10.1016/j. jcvp.2022.100079. Epub 2022 Apr 30
Acta Cytologica 2021;65:448–452 https://doi. org/10.1159/000517479
Sultana F, Gertig DM, English D, Simpson JA, Drennan KT, Wrede CDH, Mullins RM, Heley S, Saville M, Brotherton JML.
Journal of Medical Screening
Talia KL, Ganesan R. Surgical Pathology Clinics
Talia KL, McCluggage WG.
International Journal of Gynecological Pathology
Talia KL, Rahimi S, Hawkes D, McCluggage WG .
Thompson EF, Hoang L, Höhn AK, Palicelli A, Talia KL, Tchrakian N, Senz J, Rusike R, Jordan S, Jamieson
A, Huvila J, McAlpine JN, Gilks CB, Höckel M, Singh N, Horn LC.
Vujovich-Dunn C, Wand H, Brotherton
JML, Gidding H, Sisnowski J, Lorch
R, Veitch M, Sheppeard V, Effler P, Skinner SR, Venn A, Davies C, Hocking
J, Whop LJ, Leask J, Canfell K, Sanci
L, Smith M, Kang M, Temple-Smith M, Kidd M, Burns S, Selvey L, Meijer D, Ennis S, Thomson CA, Lane N, Kaldor
J, Guy R.
Whop L, Butler T, Lee N, Cunningham
J, Garvey G, Anderson K, Condon J, Tong A, Moore S, Maher C, Mein J, Warren E, Brotherton J.
Wirtz C, Mohamed Y, Engel D, Sidibe
A, Holloway M, Bloem P, Kumar S, Brotherton J, Reis V, Morgan C.
Yeasmeen T, Kelaher M, Brotherton JML.
International Journal of Gynecological Pathology
International Journal of Gynecological Cancer
HPV self-sampling and follow-up over two rounds of cervical screening in Australia – the iPap trial.
Neuroendocrine neoplasia of the female genital tract.
Microscopic sertoliform sex cord proliferations: a rare incidental finding associated with endometriosis and ovarian epithelial neoplasia.
HPV42-associated seborrheic keratosis-like lesion of the cervix: first reported case with high-grade morphology.
Molecular subclassification of vulvar squamous cell carcinoma: Reproducibility and prognostic significance of a novel surgical technique.
BMC Public Health Measuring school level attributable risk to support school-based HPV vaccination programs.
J Med Screening. 2022;29(3):185-193. doi:10.1177/09691413221080635. Published online 22/3/22.
Surg Pathol Clin. 2022 ;15(2):407-420. doi: 10.1016/j.path.2022.02.012.
Int J Gynecol Pathol. 2022 Mar 14. doi: 10.1097/ PGP.0000000000000873. Epub ahead of print. PMID: 35283445
Int J of Gynecol Pathol: November 12, 2021 - Volume - Issue -doi: 10.1097/ PGP.0000000000000835
Int J Gynecol Cancer 2022 28;ijgc-2021-003251. doi: 10.1136/ijgc-2021-003251.
BMC Public Health. 2022; 22:822 https://doi.org/10.1186/ s12889-022-13088-
Australia and New Zealand Journal of Public Health
Aboriginal and Torres Strait Islander women’s views of cervical screening by self-collection: a qualitative study.
Aust NZ J Public Health. 2022; 2022;46(2):161-169. doi: 10.1111/1753-6405.13201.
Vaccine. Integrating HPV vaccination programs with enhanced cervical cancer screening and treatment, a systematic review.
Ethnicity and Health
Understanding the types of racism and its effect on mental health among Muslim women in Victoria
Vaccine. 2022;40 Suppl 1:A116-A123 https:// doi.org/10.1016/j. vaccine.2021.11.013
Ethn Health. 2022 :1-17. doi: 10.1080/13557858.2022.2027882.
Australian Centre for the Prevention of Cervical Cancer
ANNUAL REPORT 2021/22
Australian Centre for the Prevention of Cervical Cancer
PO Box 178, Carlton South VIC 3053
265 Faraday Street, Carlton VIC 3053
Telephone: (03) 9250 0300
Website: www.acpcc.org.au
© Australian Centre for the Prevention of Cervical Cancer
ACN: 609 597 408
Copies of this report are available online: www.acpcc.org.au
Printed copies can be ordered from:
Australian Centre for the Prevention of Cervical Cancer
Telephone: (03) 9250 0322
Email: directorate@acpcc.org.au
ISSN 2202-4441
VCS Foundation acknowledges the support of the Victorian Government