














Advanced Engineering. Prototyping. Product Development. Micro-Manufacturing. Learn More at Resonetics.com













Medical equipment requires flawless performance, every time. That’s why so many Bodine products drive everything from complex diagnostic machinery, patient lifts and beds, to medical stirrers and centrifuges. Our gearmotors, motors and controls provide the efficient and reliable power needed for applications when second-best is simply not an option.
Visit bodine-electric.com for more Information
bodine-electric.com • info@bodine-electric.com • 773.478.3515 (USA)


EDITORIAL
Executive Editor Chris Newmarker cnewmarker@wtwhmedia.com @newmarker
Managing Editor
WEB DEV/DIGITAL OPERATIONS









Web Development Manager B. David Miyares dmiyares@wtwhmedia.com @wtwh_webdave
DIGITAL MARKETING
VP, Digital Marketing Virginia Goulding vgoulding@wtwhmedia.com @wtwh_virginia
Jim Hammerand jhammerand@wtwhmedia.com
Senior Editor Danielle Kirsh dkirsh@wtwhmedia.com
Pharma Editor Brian Buntz bbuntz@wtwhmedia.com


Associate Editor Sean Whooley swhooley@wtwhmedia.com @SeanWhooleyWTWH
Editorial DirectorDeviceTalks Tom Salemi tsalemi@wtwhmedia.com
VP Lifesciences Mary Ann Cooke mcooke@wtwhmedia.com 781.710.4659
CREATIVE SERVICES
VP of Creative Services Mark Rook mrook@wtwhmedia.com @wtwh_graphics

Senior Art Director Matthew Claney mclaney@wtwhmedia.com @wtwh_designer

DeviceTalks Tuesdays is a weekly virtual event that brings the insights and energy of our in-person events to your desktop.
Each DeviceTalks Tuesday will kick off with a quick briefing from the editors of MassDevice and Medical Design and Outsourcing. These presentations will give attendees insights on what trends will be moving medtech in the days to come.


Be sure to check back frequently as new live events continue to be added.
WATCH devicetalks.com


Digital Media Manager Patrick Curran pcurran@wtwhmedia.com @wtwhseopatrick


Front End Developer Melissa Annand mannand@wtwhmedia.com
Software Engineer David Bozentka dbozentka@wtwhmedia.com



Web Dev./Digital Production Elise Ondak eondak@wtwhmedia.com
PRODUCTION SERVICES
Customer Service Manager Stephanie Hulett shulett@wtwhmedia.com
Customer Service Rep Tracy Powers tpowers@wtwhmedia.com
Customer Service Rep JoAnn Martin jmartin@wtwhmedia.com
Customer Service Rep Renee Massey-Linston renee@wtwhmedia.com
Customer Service Rep Trinidy Longgood tlonggood@wtwhmedia.com
Digital Marketing Manager Taylor Meade tmeade@wtwhmedia.com @WTWH_Taylor
Digital Marketing Coordinator Matthew Kulkin mkulkin@wtwhmedia.com @WTWH_Matt
Webinar Manager Matt Boblett mboblett@wtwhmedia.com
Webinar Coordinator Halle Sibly hkirsh@wtwhmedia.com

WTWH Media, LLC 1111 Superior Avenue, 26th Floor Cleveland, OH 44114 Ph: 888.543.2447 Fax: 888.543.2447
Senior Graphic Designer Allison Washko awashko@wtwhmedia.com @wtwh_allison
Graphic Designer Mariel Evans mevans@wtwhmedia.com @wtwh_mariel
Director, Audience Development Bruce Sprague bsprague@wtwhmedia.com

Digital Production Manager Reggie Hall rhall@wtwhmedia.com

Digital Production Specialist Nicole Johnson njohnson@wtwhmedia.com


Digital Production / Marketing Designer Samantha King sking@wtwhmedia.com

Marketing Graphic Designer Hannah Bragg hbragg@wtwhmedia.com
EVENTS


Events Manager Jen Osborne josborne@wtwhmedia.com @wtwh_jen
Events Manager Brittany Belko bbelko@wtwhmedia.com
Event Marketing Specialist Olivia Zemanek ozemanek@wtwhmedia.com
Videographer Manager Bradley Voyten bvoyten@wtwhmedia.com @bv10wtwh

Videographer Garrett McCafferty gmccafferty@wtwhmedia.com


Webinar Coordinator Kim Dorsey kdorsey@wtwhmedia.com FINANCE Controller Brian Korsberg bkorsberg@wtwhmedia.com
MEDICAL DESIGN AND OUTSOURCING (ISSN 2164-7135) is published 6 times per year: (January, March, May, July, September, November) by WTWH Media, LLC, 1111 Superior Ave., 26th Floor, Cleveland, OH 44114.
PERIODICALS POSTAGE PAID AT CLEVELAND, OH AND ADDITIONAL MAILING OFFICES.
POSTMASTER: Send address changes to Medical Design & Outsourcing, 1111 Superior Ave., 26th Floor, Cleveland, OH 44114.


Medical devices have never been more complex than they are today.
They’re getting smaller, smarter and safer. They’re more effective, intuitive and sustainable. Medical device companies manufacture some to last longer — and then redesign others for just a single sure-fire use.
Device developers have more options than ever: advanced materials, cutting-edge components and new technologies like 3D printing, artificial intelligence and robotics that lend a helping hand in the operating room and on the production line.


It’s also never been harder to go it alone.
Collaboration is more important than ever, and we at Medical Design & Outsourcing are proud to help with our latest Medical Device Handbook. We’ve tapped our in-house expertise — along with technical professionals and leaders at major medical device OEMs, contract manufacturers, suppliers and partners — to offer actionable information and advice to help medical device designers and engineers break new ground.













In this edition, we’ll once again cover a wide range of topics: components, drug delivery, manufacturing, machining, molding, materials, product design and development, regulatory, reimbursement, software, intellectual property, sterilization services and tubing. All of these articles will also be available online at wtwh.me/ handbook, along with content across all of those categories from previous handbook editions.
To highlight just a few articles, MDO readers of this year’s handbook will:




• Hear Medtronic CEO Geoff Martha, Abbott Medical Devices EVP Lisa Earnhardt and ResMed CEO Mick Farrell discuss how medtech companies can take advantage of healthcare ecosystems;
• Get tips from Boston Scientific for keeping production lines staffed and running despite the global labor shortage;

• Understand the blood clot science behind Johnson & Johnson’s Embotrap stent retriever for thrombectomies to treat ischemic strokes;
• Learn how DermaSensor designed a handheld device for earlier detection of skin cancer;
• Confront and conquer the challenges of developing devices for cardiac ablation;
• Consider the future of artificial intelligence in medical devices with regulatory and reimbursement experts from the industry and FDA.
Outsourcing. Enjoy — and thanks for
American-made Interpower® North American 5-15, 5-20, 6-15, and 6-20 hospital-grade cords, and international hospital-grade cords provide the correct amperages and voltages for vital medical equipment requiring correct amperages and voltages such as portable CT Scanners and X-ray machines, medical-grade treadmills, ECMO machines, and ventilators.


When designing and manufacturing hospital-grade products worldwide, it’s essential to know the medical requirements of the country of export—select hospitals in Australia, Canada, Denmark, Japan, and the United States have proprietary requirements. All Interpower hospital-grade cords are manufactured to the highest standards. Interpower hospital-grade cords come with NEMA hospital-grade plugs bearing the “green dot,” and surpass UL 817 (18.2.4.1) and C22.2 No. 21-14 requirements for hospital-grade power cords and cord sets.
“We test more than the standards require for our own benefit,” Interpower Product Development Manager Ron Barnett said. “We do so because it lends better reliability to our design.”






• World-class customer service
• Value-added services such as lengths, colors, & packaging
• No minimum order or dollar requirements
• Quantity price breaks








































































































































TOR ALDEN leads SteriPack’s usercentered product development group as global VP of design, human factors and development. His expertise focuses on over 30 years of the design, development, research, and human factors of medical, life science and consumer healthcare products. Alden earned his bachelor’s degree in industrial design from Syracuse University and received his Master of Science in management of technology (MOT) from Stevens Institute of Technology.

JOHN BANKS, assembly operations manager at Beacon MedTech Solutions, oversees the manufacturing operations of all products in the Life Sciences portfolio to ensure on-time delivery and quality standards compliant to ISO 13485, ISO 9001 and cGMP. He brings 10-plus years of experience in both technical and leadership roles within the medical device and biopharma industries to help companies accelerate success in the market.














LISA BARTAKOVICS is the senior director of global regulatory affairs and quality at Avery Dennison Medical (ADM), overseeing regulatory affairs including FDA 510(k) submissions of combination products, Class III Design Dossier approval and international product registrations. She is also responsible for the quality system and quality performance, including document control system standardization and bestprocess implementation across ADM's three manufacturing sites in Mentor, Ohio; Turnhout, Belgium; and Longford, Ireland.


DAVID DEVINE is the business development manager, Medical, Branson Welding and Assembly at Emerson. Devine earned a degree in mechanical engineering from the University of Dayton, where he began his career with Emerson as a manufacturing engineering co-op in Sidney, Ohio. He has spent six years with Branson Welding and Assembly, starting as a sales engineer.



DANIEL FRIEDRICHS, Ph.D., leads development engineering efforts at Minnetronix Medical for the commercialization of surgical energy devices including in vivo electroporation systems and other energy-based medical, surgical, and drug-delivery therapies. He is named on more than 35 patents and has worked with an extensive network of clients seeking to commercialize a wide array of technologies.

TONY KAUFMAN is new business ventures lead for the Medical Materials & Technologies Business at 3M and has more than 24 years of medical device design, development and manufacturing experience. Kaufman is Lean Six Sigma Black Belt certified, has utilized finite element analysis modeling in various ways to solve challenges and accelerate product development, and holds a degree in biological systems engineering from the University of Nebraska-Lincoln.
MATT KNUTSON is vice president of manufacturing operations at Donatelle, where he is responsible for production, manufacturing engineering and automation, quality, maintenance and facilities. Knutson has designed, implemented and overseen implementations of both fixed automation and flexible automation systems throughout his career.
RICHARD LANDRY, director of operations at Beacon MedTech Solutions, applies decades of plastic injection molding and contract manufacturing expertise to oversee all manufacturing operations. Working with a cross-functional team, Landry elevates customer programs through particular expertise in engineering and operations management, with an emphasis on quality, training and continuous improvement.
ROBBIE LONG is a senior technical applications engineer with Fictiv and has a long history of working in prototyping and digital manufacturing. Long attended the University of West Georgia and is based in Atlanta.
MATT LOWE, chief product and marketing officer at MasterControl, is a medical device expert with experience in product development and product management at Ortho Development Corp and Bard Access Systems, a subsidiary of BD. Lowe has launched more than a dozen medical devices, has five patents issued, wrote an FDAcleared 510(k) and managed a multisite, multiyear postmarket clinical study for orthopedic devices.
ARJUN LUTHRA is the commercial director at Biointeractions, an R&D company specializing in biomaterial coatings for devices, instruments and implants. The portfolio of multi-action materials confronts chronic challenges such as thrombus formation, infections, device-related complications and other biocompatible challenges.
GAURAV MANCHANDA, medical market development director at Formlabs, launched and leads the medical division for the 3D printing company, where his goal is to advance 3D printing in the healthcare industry by combining clinically validated 3D printing technology, in-house QA/RA expertise, accessible pricing, and a wide range of materials. Manchanda leads 3D printing market development in priority segments including medical devices, pointof-care, orthotics and prosthetics, medical education, and the life sciences.
GREGORY MONTALBANO is the principal of MIDI, a turnkey product development consulting firm specializing in the design and development of medical, biotech, life sciences and home healthcare devices and systems. With 30-plus years of experience, Montalbano has serviced clientele ranging from startups and emerging companies to Fortune 500 organizations representing industry segments from both domestic and international markets.
ZACHARY MURNANE is a senior research and development applications manager for Honeywell’s Spectra business, which produces Spectra MG Bio, an ultra-high molecular weight polyethylene (UHMWPE) fiber. He has more than ten years of experience leading manufacturing and new product development initiatives within Fortune 500 companies, most recently with a focus on expanding the use of UHMWPE within the medical industry.
JAY NOBLE heads Integrated Computer Solutions’ global team of engineers, leveraging his more than 25 years of experience leading global software engineering and professional services teams in the medical, military and industrial spaces. He holds a B.S. in computer science from the University of Massachusetts in Lowell, an M.S. in computer science from Boston University and an MBA in international business from Suffolk University in Boston.
PHILIP REMEDIOS is principal and design director at BlackHägen Design, an R&D consultancy focused on medical device innovation. He has a background in design and engineering and is a named inventor on over 35 patents.
ANDREW (A.J.) TIBBETTS is a shareholder in the Intellectual Property & Technology practice group in Greenberg Traurig's Boston office. Formerly a software engineer, Tibbetts works with organizations large and small, serves on the board of MassMEDIC and guides digital health efforts for MassBio.
FLORIAN SOLZBACHER is the cofounder and chairman of Blackrock Neurotech and professor and chair of the Department of Electrical and Computer Engineering at the University of Utah. A leading authority on chronically implantable sensors for electrophysiological and chemical biomarkers, Solzbacher drives the technological vision for Blackrock with a focus on restorative tech for neurological conditions.
STEPHANIE VAN NESS is associate director of marketing and chief storyteller at Integrated Computer Solutions, writing about user experience design and innovations in technology, from gesture-controlled medical devices and extended reality-powered surgical training simulators to autonomous truck fleets. Her work has appeared widely in medical device and software publications. She holds a journalism degree from Boston University.









COMPONENTS:



How fluorescent signals could allow for deeper sensor implants in the brain; How the Utah Array is advancing BCI science; 5 steps to help medical device makers deal with semiconductor shortages

In vivo electroporation is an engineering solution for drug and gene delivery; How Qnovia aims to use inhaled therapeutics for smoking cessation













Best practices when outsourcing medical injection molding; Two strategies for managing labor shortages at Boston Scientific; Leverage automation to get ahead of supply and demand; 5 questions to ask when transitioning from medtech product development to manufacturing; Affordable 3D-printed medical devices reach commercialization; Three considerations for reshoring your medical device components and assemblies; Is there a better way to weld medical wearable components?




MATERIALS:


How antimicrobial coatings of the future will better prevent infections; Small but mighty medical fiber: Minimally invasive surgery demands new materials; Braiding technology options for innovative biomedical textile structures




PRODUCT DESIGN & DEVELOPMENT: 3 critical starting points for medical device design usability research; Medtronic’s Design for Reliability and Manufacturability after the reorganization; This smart, dissolving pacemaker communicates with sensors on the body for remote patient monitoring; 4 ways to usher in next-gen medical device design; How to center patients when designing distance care medical devices



REGULATORY, REIMBURSEMENT, STANDARDS & IP: Navigating MDR’s newly charted waters; The future for AI in medical devices; IP for healthtech AI; Staying on the compliant side of change control regulations





SOFTWARE: Pushing the boundaries of brain-computer interface software; Meticulous software testing can save the lives of medical device users
STERILIZATION SERVICES: FDA reports sterilization challenge progress as EPA takes aim at EtO emissions


TUBING:

How Medtronic designed the next generation of GI bleeding treatment; Design challenges to overcome when developing cardiac ablation devices


PRODUCT SPOTLIGHT AD INDEX

Medtech leaders discuss how to get the most out of a burgeoning healthcare ecosystem.















DermaSensor’s innovative device could save lives with early skin cancer detection capabilities.


The founder of Johnson & Johnson MedTech’s stroke science research arm explains the science of blood clots and how that influenced the Embotrap’s design.




























CYROLITE® has been working in hospitals and labs for more than 40 years. Thanks to their excellent properties, our high-performance acrylics are perfect for use in a wide range of medical devices. CYROLITE® is highly transparent and easily processed into intricate parts. It can be reliably sterilized using most common methods and is BPA- and DEHP-free. This has impressed both patients and healthcare professionals alike: CYROLITE® meets the requirements of USP Class VI, ISO 10993-1, and REACH. You can find more details at www.cyrolite.com.








































Medical Design & Outsourcing is excited to release the winners of our annual Leadership in Medical Technology program. Since we announced the nominees in our January 2022 issue and online, our user community has voted on what companies they feel best exemplify medical technology leadership in 14 categories. We are happy to celebrate the winners here.




















Founded in 1990, Isometric’s success begins and ends with our people. Isometric is ISO 13485 certified with two adjacent facilities, Class 7 cleanrooms, and over 95 employees.
With unparalleled micro manufacturing capabilities, Isometric provides 100% in-house:






• Micro 3D Printing




• Micro Tooling
• Micro Molding

• Micro Automated Assembly
• CT Scanning
A global micro solutions leader, Isometric serves high precision medical market segments such as diabetes, ENT, ophthalmic, cardiovascular, neurovascular, orthopedic, drug delivery–and more. Isometric’s numerous micro solutions include micro fluidics, molded needles, catheter tips, sensors, transdermal patches, life science disposables, and neurovascular implant devices. Driven by ambition for innovation, Isometric’s team of experts use our internally developed Microns Matter® risk mitigation process to successfully meet tolerances and bring your micro parts to validation faster.





The world trusts Sava’s mechanical cable to power tomorrow’s surgical robots and endoscopic instruments. That’s a fact.
For over 50 years, Carl Stahl Sava Industries has been a staple in the manufacture of world-class surgical robotic, endoscopic device, implantable and wearable mechanical cable and assemblies. Using materials such as tungsten, stainless steel, nitinol, titanium and more, Sava has taken the time and invested greatly in perfecting the ultrafine cables powering the motion systems of the world’s most advanced surgical marvels.

Working alongside the most trusted names in contract manufacturing and surgical instrumentation innovation, Sava has a deep bench of experts with decades of collaborative experience.









Trelleborg Healthcare & Medical helps pharmaceutical and medical device companies improve patient quality of life by partnering in all stages of development, from concept to serial production to create engineered polymer solutions for demanding applications. Trelleborg’s global footprint is backed by experts with 30+ years of industry experience and a portfolio of capabilities, including micromolding, process automation, silicone extrusion, drugeluting solutions, silicone and thermoplastic molding and over-molding, multicomponent molding, device assembly, and silicone dipping and coating. Trelleborg’s Rapid Development Center helps medical device companies navigate and accelerate the development process from concept to market launch.





Rotor Clip is a globally recognized manufacturer of high-quality retaining rings, spiral rings, wave springs and hose clamps. Companies across a broad range of industries rely on Rotor Clip products, value-added services and expertise to build reliable and safe equipment for virtually any application.

Rotor Clip operates in the US, UK, Germany, China, and the Czech Republic - providing worldwide service to its customers. Recognized worldwide, Rotor Clip has received numerous quality awards from major OEMs, automotive manufacturers, and distributors. Rotor Clip also produces its products under IATF 16949, ISO 9001, AS9100, ISO 14001, ISO 13485, and TISAX standards.






Stock Drive Products/Sterling Instrument (SDP/SI) makes it easier for OEMs to get their products to market by designing and delivering precision components and complex drive assemblies while managing costs and inventories. Well known as the one-stop-shop, we provide mechanical and electromechanical components, engineering development, precision manufacturing, and problem-solving customized solutions.

Established in 1950, we are a proven partner to the most recognized names in medical, aerospace, defense, and robotics industries providing innovative solutions, worldclass quality, and engineering support from concept to completion. We look forward to working with you!







Accumold began in a rented garage in 1985. Our primary focus was and still is on very small micro-sized plastic parts and components that other companies can’t produce. The original Micro-Molder® was designed to help manufacture parts with minimal waste and short cycle times for electronic component manufacturing. Although there have been many innovations over the years the basic principals have remained the same and so has our commitment to micro-injection molding technologies.

Today the company now focuses on industries that require fast turn around and complex parts for high tech industries such as Micro Electronics, Micro Optics, and Medical, as well as new emerging markets and technologies.



TRACO Electronic AG , is a Swiss company with headquarters in Baar, Switzerland. TRACO markets its products worldwide under the registered trademark TRACO POWER. As a leading power supply specialist with more than 35 years’ experience we are dedicated to the design and manufacturing of high quality DC/DC and AC/DC power conversion products with an emphasis in medical approved solutions.




The standard product offering in medical grade power solutions includes 15~850 watt AC/DC power supplies in open frame, fully enclosed or encapsulated models and 1~60 watt DC/DC converters with PCB mounting.



With over 100 years of experience, Boker’s has earned the reputation as a stamping leader. They deliver precision stampings in a complete range of sizes: flat blanking and piercings up to 12” x 12” (flat) with thicknesses from .005” to .190” (varies by material) and draws up to 3” deep and 8” in diameter.
With numerous inside diameters, thicknesses and over 2,000 commonly specified and hard-to-find material options— including various types of steel, aluminum, brass, copper, nickel silver, plus non-metallic materials such as PTFE, polyester, fiber and nylon— whatever your requirements, if it can be stamped, Boker’s can turn it into the part you need.



Boker’s also delivers a wide selection of standard and nonstandard washers, spacers and shims. With over 32,000 stock tools, and outside diameters of .080” to 5.140” along with a variety of inside diameters, you have millions of fl at washer possibilities.
All stampings, washers, spacers and shims are available in short, medium and long runs with a 100-piece minimum.

Boker’s also offers 3D printing for prototyping, complete Statistical Process Control (S.P.C.) capability, inhouse tooling, and “Dock-to-Stock” and “Just-in-Time” programs.





Nitto Kohki USA, Inc. designs, develops, engineers, and continuously improves high quality air compressors and vacuum pumps for applications like medical devices, laboratory equipment, applied robotics and environmental sensing equipment.


Nitto Kohki’s unique linear free piston design has one moving part per pump— the piston. The result is a pump with exceptionally reliable performance in tough medical applications. The linear free piston concept enables Nitto Kohki pumps to be smaller and lighter than conventional aerators, allowing designers greater flexibility in system design.









Hobson & Motzer’s world-class facilities offer the most advanced technology in metal transformation and assembly. With more than 100 years of precision manufacturing experience, we have the capabilities and infrastructure to deliver on critical projects. Our commitment to lean and continuous improvement results in superior quality, as well as exceptional supply chain performance and customer satisfaction. Our extensive tool design and engineering expertise drives repeatability and efficient production. We offer precision rapid prototyping and project-based support, from concept to completion. Medical device components include endo-mechanical, electrosurgical, pharmaceutical, ophthalmic blade blanks, custom electrical contacts, and more.




ElectroCraft, Inc. is a global provider of dependable, application-engineered fractional-horsepower motor and motion products. The ElectroCraft Powering Innovation custom manufacturing services cover the following products: AC motors, PMDC motors, brushless DC motors, stepper motors, servo motors, gearboxes, gearmotors, linear actuators, drives, servo drives and integrated motor drives.


Our products are found in thousands of different applications within industrial, commercial and consumer product markets. While ElectroCraft provides a wide array of standard products with many configurable options, we have built our brand on custom OEM solutions that meet the precise performance, cost and quality our customers require.


For OEM Customers who are unsatisfied with having to design around inflexible off-the-shelf products, our technical knowledge and cusomizable product families provide for a design experience which results in motor and motion systems that provide superior reliability and performance at the lowest possible cost.


Chamfr is a female-owned medical device component marketplace founded 5 years ago by 3 co-founders who saw an unmet need in the industry. Engineers needed fast access to components for early stage development but were frustrated by long lead times and lack of accessibility to medical-grade components for early stage development.




At Chamfr, we are not a single company trying to push our products. Instead, we give medical device engineers access to a wide range of in-stock components and technologies for prototyping to spur the development of innovative medical devices. This allows design engineers to launch their products faster by being able to iterate without long lead times and high-cost minimum orders. With 3000+ components, from over 30 different suppliers, all in-stock and ready to ship, Chamfr is kind of like an Amazon for medical device components.

One of the biggest advantages of buying on Chamfr is transparency. You see who you are buying from so when it’s time to source long term supply, you know exactly where to go and can be confident that you have medical-grade supplier and traceability for your DHF.






COMSOL is a global provider of simulation software for product design and research to technical enterprises, research labs, and universities. Its COMSOL Multiphysics® product is an integrated software environment for creating physics-based models and simulation applications. A particular strength is its ability to account for coupled or multiphysics phenomena. Add-on products expand the simulation platform for electromagnetics, structural, acoustics, fluid flow, heat transfer, and chemical applications. Interfacing tools enable the integration of COMSOL Multiphysics® simulations with all major technical computing and CAD tools on the CAE market. Simulation experts rely on COMSOL Compiler™ and COMSOL Server™ to deploy applications to their design teams, manufacturing departments, test laboratories, and customers throughout the world.













 Kirsh Senior Editor
Kirsh Senior Editor
Fluorescent sensors are typically used to label and image a variety of molecules to give a unique glimpse inside living cells. However, the method has been limited to cells grown in a lab dish or in tissues closer to the surface of the body because the signal from the sensors are lost when implanted too deeply in the body.
A team of Massachusetts Institute of Technology engineers have developed a photonic technique that dramatically improved the fluorescent signal. The researchers showed that sensors could be implanted as deep as 5.5 cm in the tissue and still provide a strong signal. Improved signaling could help fluorescent sensors to track specific molecules inside the brain or other tissues deep within the body for medical diagnosis or monitoring drug effects.
“If you have a fluorescent sensor that can probe biochemical information in cell culture, or in thin tissue layers, this technology allows you to translate all of those fluorescent dyes and probes into thick tissue,” said the lead author on the study, Volodymyr Koman.
Wavelength-induced frequency filtering

Traditionally, scientists use different kinds of fluorescent sensors — including quantum dots, carbon nanotubes and fluorescent proteins — to label molecules inside the cells. The fluorescence of the sensors can be seen by shining a laser light on them. However, the method does not work in thick, dense tissue or deep within tissue because tissue emits some fluorescent light called autofluorescence, which makes implant signals weak.
“All tissues autofluoresce, and this becomes a limiting factor,” Koman said. “As the signal from the sensor becomes weaker and weaker, it becomes overtaken by the tissue autofluorescence.”
The MIT researchers modulated the frequency of the fluorescent light emitted by the sensor so it could become more easily distinguishable from tissue autofluorescence. Called wavelength-induced frequency filtering (WIFF), the method uses three lasers to create a laser beam with an oscillating wavelength. (continued on page 32)

MIT engineers have developed a photonic technique for fluorescent sensors that could improve sensor signals deep in the body.Image courtesy of the Massachusetts Institute of Technology Danielle





















Oscillating beams are shined on the sensors and cause fluorescence emitted by the sensor to double its frequency, according to the researchers. The signal can then be easily picked out from the background’s autofluorescence. The researchers reported they enhanced the sensor signal-tonoise ratio more than 50-fold.



its usability, the team focused on glioblastoma. Patients with this aggressive form of brain cancer typically undergo surgery to remove as much as the tumor as possible and then receive chemotherapy to eliminate the remaining cancer cells.

“We are working on technology to make small sensors that could be implanted near the tumor itself, which can give an indication of how much drug is arriving at the tumor and whether it’s being metabolized,” said Michael Strano, senior author of the study and the Carbon P. Dubbs professor of chemical engineering at MIT. “You could place a sensor near the tumor and verify from outside the body the efficacy of the drug in the actual tumor environment.”


The researchers suggest the method could be used to monitor the effectiveness of chemotherapy drugs. To demonstrate
When the cancer drug temozolomide enters the body, it is broken down into smaller compounds, and the MIT team designed a sensor to detect the compound known as AIC. They found that they could place the implant as deep as 5.5 cm within an animal brain and could read the signal from the sensor even through the animal’s skull.
The researchers suggest the sensors could also be used to detect molecular signatures of tumor cell death. The WIFF method could be used to enhance the signal from other types of sensors, including carbon-nanotube-based sensors that detect hydrogen peroxide, riboflavin and ascorbic acid.
“The technique works at any wavelength, and it can be used for any fluorescent sensor,” Strano said. “Because you have so much more signal now, you can implant a sensor at depths into tissue that were not possible before.”



The MIT team hopes to continue the research using a tunable laser to create the signal and improve the technique even further. They are also working on sensors that are biologically resorbable and do not need to be surgically removed.
The research was funded by the Koch Institute for Integrative Cancer Research and Dana-Farber/Harvard Cancer Center Bridge Project with additional funding from the Swiss National Science Foundation, the Japan Society for the Promotion of Science, the King Abdullah University of Science and Technology, the Zuckerman STEM Leadership Program, the Israeli Science Foundation and the Arnold and Mabel Beckman Foundation.
The study was published in the journal Nature Nanotechnology.





































This gold-standard brain-computer interface technology is creating a platform for better patient outcomes.
Brain-computer interface (BCI) science has seen exciting advances and heightened public attention in recent years, and for good reason. The promise of BCI for individuals with paralysis is monumental: To intuitively restore communication, control external devices, and provide the independence that many able-bodied people take for granted.
Sitting at the core of today’s BCI innovation is a tiny device that has quietly powered advances in the field for decades: the Utah Array.
Studied in humans since 2004, this bundle of microelectrodes, also known as the NeuroPort Array, remains the only FDAcleared high-channel microelectrode platform that can record signals from individual neurons and that is used in BCI applications for movement disorders. In clinical studies, it has been successfully surgically implanted in the brain tissue of patients with paralysis
for up to eight years (and counting) to record movement intentions and restore physical sensations, resulting in life-changing applications for the patients.
Because of its extensive safety and efficacy profile and the quality of neural data it gathers, the Utah Array is often considered the gold standard of BCI technology. But it’s more than just a useful research tool — the Utah Array is driving BCI innovation and unlocking practical, clinical solutions for patients with paralysis.

What is the Utah Array?


The Utah Array is a high-channel count microelectrode array invented by Richard Normann at the University of Utah. Initially intended for applications in visual prosthesis, over time it became widely adopted by the neuroengineering community as the gold standard tool for basic and applied research.

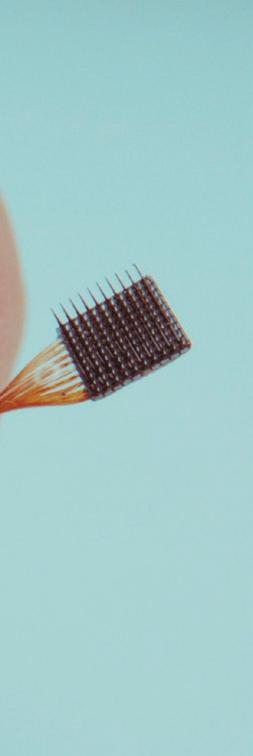

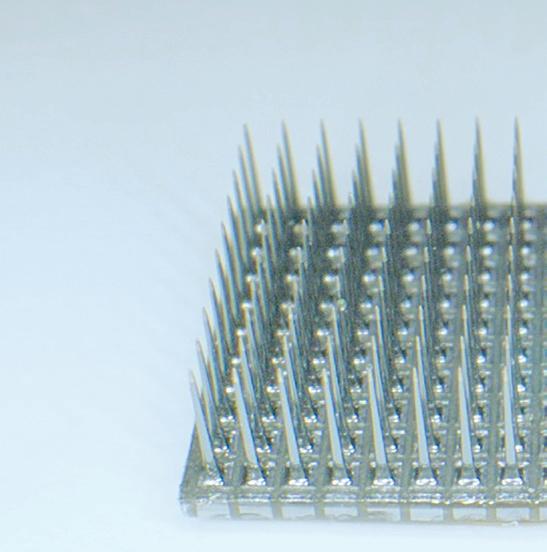

The array gathers neural signals from up to 100 channels per device, and it’s the only BCI implant with documented long-term stability and safety in humans. It has been studied in dozens of patients for a total of more than 30,000 days, demonstrating groundbreaking performance.

The Utah Array has earned commercial FDA clearance for monitoring electrical activity in the brain for 30
days or less, and Blackrock is pursuing clearance for long-term implantation, which will enable commercialization of the BCI platform as a clinical therapy. In studies where Investigational Device Exemptions have allowed implants to remain in place chronically, the Utah Array has been shown to successfully restore function in patients with paralysis and ALS for up to eight years so far. Research is underway to examine how the Utah Array could potentially treat chronic pain and other neurological conditions.
A modified version is used in efforts to restore hearing. Ultimately, the Utah Array is best thought of not as a single device but a family of devices and form factors and as the core of a larger platform and workflow.
Blackrock is actively working with partners to design processes that result in optimal outcomes post-operation (such as avoiding tissue damage or immune response to the implant) and ensure long-term patient safety and higher quality of life.
(continued on page 36)
Neurotech uses its Utah Array in its brain-computer interface to sense brain signals.




















































































































































































(continued from page 34)

Because the Utah Array has been proven to work safely and effectively for years on end, the Blackrock team has turned its attention to making its BCI commercially available as a medical device, bringing the technology out of the lab and into patient homes.
The Utah Array has a few key performance features:




• 100 active electrode channels per implant; can be customized up to 1,024 channels








• Typical configurations include four to six devices (400-600 electrodes)










• Ability to reliably pick up signals from both individual neurons and a summation of high-resolution signals


• Variety of connector types for acute or chronic recording






• Electrode site metal options: iridium oxide or platinum

• Standard electrode lengths: 0.5-1.5 mm (research) or 1.0-1.5 mm (clinical)

The Utah Array’s largest advantage over other BCI electrodes currently in development is its longevity and safety. No other neural implant used in BCI applications has a comparable safety and efficacy profile demonstrated over years of clinical research. In terms of safety, the Utah Array has had no FDA-reported serious adverse events related to the implant since it was first implanted in humans in 2004.
An engineering challenge that Blackrock and other BCI companies face is how to increase channel count and/or develop other higher-density arrays. At this point in time, the Utah Array remains the highest density recording electrode long-term tested in humans. In animal research, no higher channel count array has chronically demonstrated the ability to work reliably in cortex with comparable lifetimes.
In addition, there is a risk-reward tradeoff with higher-density electrodes. Electrode count should always be driven by application needs, which in turn are
determined by customer needs and physiology. Many neuroprosthetics and communication tool applications that interface with motor intent perform well with hundreds-to-one-thousand electrode channels. More channels may cause additional injury to tissue that is harder to heal without adding significant performance benefits. A higher concentration of electrodes alone may not automatically lead to better-performing applications.
Furthermore, the electrode array is only one key component in an entire system and value chain. A keen understanding of the use case, clinical and caretaker workflows, surgical procedures, electronics, firmware and software design and user interfaces — combined with advanced algorithms and machine learning approaches — enables the performance that users experience in the field. That allows them to translate thought-to-text with speeds approaching able-bodied people (150 characters per minute) and to control a computer or mobility devices, allowing patients to truly regain independence and function.
Some use cases do require higher channel count, such as applications for memory and vision. For these applications, Blackrock has developed novel electrodes with 10,000 channels and beyond.
While Blackrock engineers are developing higher-density electrodes that optimize the risk-reward tradeoff, the near-term opportunity of the Utah Array is its application as a medical device. In its current form, the Utah Array powers a BCI platform that, once available in a commercial medical device, will change the way paralysis and neurological disorders are treated.
The Utah Array is the tiny component that is already fulfilling this life-changing promise. And it’s just the beginning.
Today’s product designs for medical equipment require smaller and smaller components that are extremely durable and meet rigorous tolerance specs. Minimally invasive components deliver huge patient care advantages by shrinking the size of devices ranging from minimally invasive biopsy cutters to catheters. As advancements in minimally invasive surgery continue, biomedical engineers will continue to demand reliable, high-precision components with ever-decreasing physical dimensions and complex design structures.
Metal alloy components continue to be called upon to meet these rapidly evolving needs, but traditional manufacturing techniques struggle with shrinking dimensions and intricate structures. Using machine tools to fabricate small components is expensive, difficult and time consuming. Even more significant are the issues machine tools have in producing large numbers of small parts with consistent tolerance quality.
Micro-metal injection molding (MicroMIM) is the solution to these issues. It delivers the highest manufacturing quality while meeting specifications for extremely small components with complex geometries. MicroMIM also creates production efficiencies, yielding highly consistent production runs and a lower cost per part.
MicroMIM technology was developed to meet requirements for metal components with extremely small size specifications. While the average mass of a conventional MIM component is 15-20 grams, MicroMIM is defined as the molding of components that are less than 1 gram or have critical features that are less than 100 microns. Some current MicroMIM parts are as small as a few tenths of a gram.
At this micro scale, tight tolerances and complex geometries are almost impossible to achieve in a production environment with standard machining technology. In addition to variability in quality, the machining cycle times are long and the costs are high, both for unique tooling and metal alloy scrap.
MicroMIM is currently being used to facilitate the production of extremely small, complex-shaped metal components with outstanding mechanical properties. When implemented with well-designed molds, material choices, and mold flow characteristics, this manufacturing method creates production efficiencies and results in little wasted material. It offers a huge leap in component design freedom by eliminating the inherent restrictions of a machining approach and ultimately yields highly consistent production runs with a lower cost per part.
Additionally, MicroMIM produces microscale medical device components with much greater feature definition, smoother surfaces and superior quality than can be achieved using machining tools. A further benefit is the elimination of weld lines that impact product quality. These advantages enable lifescience OEMs to implement advanced engineering concepts and achieve consistently high levels of product quality.
Today’s MicroMIM medical equipment applications include:
• Robotic surgical micro components
• Implantables
• Catheter components and sacrificial cores for stent production
• Staples and stapling anvils
• Laparoscopic and suturing jaws
• Minimally invasive biopsy cutters and graspers
• Dental surgery
• Drug delivery systems
Advances in minimally invasive surgery will inevitably continue, with tremendous benefits for patient care. Smaller and smaller components will be required, challenging both design and production teams. You can position your teams ahead of the curve by adding MicroMIM capabilities to your portfolio.

In addition to expanding design engineering freedom, MicroMIM delivers major business advantages. Because it is a highly repeatable process, with consistent results, production runs can ramp up quickly to support critical new product introductions. This is in stark contrast to machine tool production, where each individual component has a statistically significant likelihood of unacceptable dimension variations. The batch production of microcomponents also facilitates assembly flexibility for the final devices where they are used.
Cost savings with MicroMIM give additional support to profitability. Traditional manufacturing requires high up-front investments in tooling and fixturing to produce parts with tight tolerances. MicroMIM is a cost effective alternative, with lower set-up costs as well as a lower marginal cost-per-piece during production. MicroMIM’s reliable product quality during production runs also drastically reduces manufacturing scrap, driving further overall cost reductions.
As stated, further developments in medical device technology are coming and quickly. Incorporating MicroMIM into sustainable supply portfolios now can give life science OEMs a leg up when the next cutting edge designs hit the market. Read our MIM design guide to learn more about the MIM process and to determine if your components are a good fit for this technology.
Nick Eidem is the director of business development for Advanced Powder Products, one of the country's leading Metal Injection Molding and 3D Metal Printing companies. Nick has over 10 years of experience in manufacturing sales. To learn more go to https://advancedpowderproducts.com/.

Medical device manufacturers are increasingly pessimistic about the supply of semiconductors, according to a Deloitte survey of the industry.
Some said they’ve slowed down or halted manufacturing operations after depleting their semiconductor inventories, and nearly 80% of survey respondents reporting extended lead times, with some stretching more than a year.
“More than 75% of our most-recent survey respondents said that their customers have turned to alternative types of treatment for their patients,” Deloitte’s Stephen Bradley and Bill Murray wrote in a recent report. “As a result, some hospitals and health systems are looking into alternate products, new usage strategies or treatment options.”
The Advanced Medical Technology Association (AdvaMed), which continues to push for the medical device industry to be prioritized for chip supplies, commissioned the survey to follow up on a similar survey conducted in July 2021. At that time, medical device companies said it was getting harder to obtain semiconductors, but many said they had enough inventory on hand to manage and that they had figured out temporary solutions.
A lot has changed since then. Russia’s invasion of Ukraine, for example, jeopardized Ukraine’s neon gas supplies, which are used in lasers that build the microscopic circuits in semiconductors. And Russia itself is one of the top
exporters of the palladium used in chips. Chips — once plentiful and cheap — are also now more expensive to move due to higher fuel costs and decreased shipping capacity, and some chip buyers are hoarding to protect against continued supply chain issues, Deloitte noted.
With some medical device manufacturers expecting the shortage to continue into 2023, Deloitte offered fi ve steps medical device companies should consider:
Deloitte’s surveys found 13% of respondents did not have a chip inventory before the pandemic, but more than 70% now say they have recently increased semiconductor inventory levels.
About half of all medical devices have at least one semiconductor, but the medical device industry’s share of global semiconductor purchases is only 1%.

Alternate suppliers can be even more effective if medical device manufacturers “build speed and flexibility into component substitutions — through planning, manufacturing, and regulatory processes” that allow for a supplier switch when needed, the Deloitte report’s authors wrote.

“More than half of our most recent survey respondents said they previously relied on a single source for 75% of their chip supply,” Bradley and Murray wrote. “All of them are now pursuing alternative sources.”
Nearly a third of survey respondents said they’ve contacted brokers, which can serve as an alternate source while protecting against counterfeit products. Deloitte said the challenge of avoiding counterfeit chips has grown for medtech firms since last year’s survey.
“Many companies are revalidating components to increase sourcing options even though the process can be cumbersome,” they said.
ResMed, in one example of agility, started shipping a new device without some of the chip-enabled features to meet immediate demand.
Deloitte said the follow-up survey found most companies have increased their visibility into multiple supply chain tiers. That helps companies spot trouble sooner and gives them more time to act.
“Digitization of the supply chain can provide visibility from the suppliers all the way to the customer and help enable a quicker response,” Bradley and Murray wrote. “Advanced analytics could enhance the ability to be more proactive in every step of the supply chain.”
With the global chip shortage expected to continue into 2023, device developers and manufacturers are taking action to keep the lines moving.
"More than 75 % of our mostrecent survey respondents said that their customers have turned to alternative types of treatment for their patients."












Reversible electroporation is the use of an electric fi eld to open pores in a cell wall, allowing transport of drugs, DNA or other “cargo” into a cell — in particular, allowing delivery of chemically large molecules and transport of DNA vaccines and customized gene therapies.

In the 40 years since its invention, electroporation has become a mainstay technique in biological sciences. Only recently, however, has wide interest taken hold when it comes to in vivo (human clinical) electroporation. Compared to other delivery vectors and carriers, electroporation stands apart as a particularly elegant engineering solution to a biologic problem, offering safe, convenient and efficacious delivery of a wide array of cargos.

Like any new technology, adoption has been slow. Companies that adapt to this new paradigm will be at the forefront of realizing the profound benefits of this technology.
In fact, electroporation-enabled gene therapies and nucleic acid vaccines are leading contenders to be paradigmshifting technologies.
Electroporation involves using an external electrical device to apply a prescribed electric fi eld to tissue. This transiently opens pores in cell walls, allowing transport of drugs, DNA, and gene therapies.

Photo courtesy of Minnetronix Medical
COVID-19 clearly demonstrated the need for quick-to-develop vaccines that can be transported without cold-chain storage. Electroporation-delivery of such a DNA vaccine is drawing wide attention and robust funding. Companies working in this space have raised significant capital, and hundreds of clinical trials are ongoing.
Diseases for which there is ongoing research into the use of electroporationdelivered therapies include a wide range of neoplasms, several viral infectious diseases and even some degenerative diseases. A sampling of these conditions includes advanced melanoma; HIV; Lassa fever; wet macular degeneration; human papillomavirus-caused cervical, head, and neck cancers; prostate cancer; certain breast cancers; and more.

With such a robust pipeline of diseasespecific agents targeting multiple types of cancers, infectious diseases, and even degenerative diseases, it is easy to see the profound health (and economic) impact this technology is bound to have.
(continued on page 42)
Why the new buzz about an old technique? Harness the potential of an existing technology for modern medicine.Minnetronix Medical

(continued from page 40)


Using electroporation as a delivery vector is quite simple. As an example, there are currently ongoing trials where a drug is administered intramuscularly with a standard hypodermic needle and syringe, then an electroporation application is performed, which causes the cells to form temporary pores, dramatically increasing the volume of that drug which ends up inside the cells.
In this case, the electroporation application involves using a separate device (that looks something like an electric toothbrush) that has pairs of needles that puncture the skin near the injection site and apply a series of electrical pulses. The process only takes a few seconds. Depending on the specifics, it can create a mild buzzing sensation for the patient but is otherwise benign.
In other devices, the injection needle is also used as part of the electroporation circuit, which makes for a more complicated delivery device but removes variability in administration.

Today’s most common in vivo electroporation applications use needle electrodes for intra-tumoral or intramuscular administration of electroporation pulses. However, research shows the ability to use “paddle” type electrodes (which don’t even need to pierce the skin) or electrodes on the surface of, for example, a flexible catheter for intraluminal or surgical applications. As electroporation matures as a delivery technology, it is likely we will see even more innovation in this space.
Developing an in vivo electroporation device requires consideration of both biologic and electro-mechanical factors. Clinical efficacy of electroporation relies on achieving specific electric field strengths, which can be influenced by varying impedance of tissue or sample. Specialized measurement equipment and protocols improve accuracy and performance, which is why an experienced design partner is invaluable. Additionally, like any medical device, such a system must be designed to be safe, effective and compliant to regulations.


Led by a former tobacco company executive, Qnovia looks to offer nicotine replacement therapy through inhaled drug delivery.
Brian Quigley spent just over 16 years at tobacco giant Altria. During his time there, he ran two U.S. business units, including the

smokeless tobacco business.
In July 2020, he joined founder and then-CEO at Qnovia (then Respira Technologies) Mario Danek as chief operating offi cer. Now CEO, with Danek as chief technology offi cer, Quigley leads the company as it looks to bring a prescription smoking cessation therapy to market.
































Quigley acknowledged the potential applications Qnovia could pursue with its inhaled therapeutic technology. For now, though, the focus is firmly on pursuing FDA approval for what would be the first inhalable prescription smoking
cessation therapy. first an
“I joined Qnovia because of what I learned when I first met Mario Danek,” Quigley said in an interview. “He explained this innovation he created. He’s taken proven medical device technology and been able to imbue it with form factors for better compliance, better adoption and
better patient outcomes.”
Qnovia’s RespiRx drug delivery platform leverages a vibrating mesh nebulizer. The aerosol technology has been used to deliver therapeutics for decades, Quigley said.
However, he noted that they “really suffer” from a range of challenges. For instance, the devices are gravity-fed. This means the piece that has to pour the drug product comes into contact with the mesh itself. He called them “clunky” as they require cleaning. According to Quigley, they can

contribute to poor adherence due to the need to clean the devices.

Qnovia took the approach of miniaturizing the technology and making it work in an orientation-agnostic fashion. This helps it to deliver therapeutics in a patient-friendly way, he said.

The device features a rechargeable base unit, plus an interface for patients to track their dosing. They can follow their use regimen and capture data through the device. The device also has a prefilled drug product-containing cartridge. Quigley said the patient can stick this into the end of the device to start therapy, then replace it when needed as they move through therapy.
“It has a very familiar patient-friendly format for on-the-go treatment to deliver the drug wherever you need it and to do it effectively,” Quigley said.
Based on each indication Qnovia treats with its technology, each user would have a different use regimen, Quigley said.
Each user received a prescribed target dose based on the therapy, the treatment and the outcome, specifi cally for inhalable nicotine replacement therapy. That particular treatment has a 12-week use regimen. Quigley described it as a traditional “stepdown” model, where the patient fi rst puts down the cigarettes. Then they begin using the device, which helps them reduce nicotine use over 12 weeks before they stop using it altogether.
In the case of smoking cessation therapy, Qnovia has a precisely timed and delivered dose prescribed to the patient based on the number of cigarettes they used to smoke in a day. Each therapy has a specific number of activations to deliver the drug to the body to alleviate withdrawal symptoms. >>
“That kind of algorithm of the dosing, the dose control and the delivery is a function of the indication,” Quigley said. “That would be very different for any future indication. If we were working on asthma or COPD, for example, the device itself can be programmed to deliver that drug differently based on the patient needs.”

Quigley said Qnovia’s technology maintains broad potential in terms of use. That includes effective drugs already approved for delivery via a vibrating mesh nebulizer.










Each of those drugs represents billion-dollar opportunities, he says. However, patients currently have to use large, clunky or at-home nebulizers to dispense the drug, which is where Qnovia could come into play.
Additionally, Quigley pointed to the world of drug development as an opportunity. He said inhalation represents an effective way to deliver compounds to the body, particularly for respiratory disease.
Finally, he pointed to companies looking at drugs delivered in different ways. Those can be delivered with less patient friction but the same efficacy and route of inhalation.
“There’s still a lot of work to be done,” said Quigley. “That’s why we’re focusing first on the smoking cessation therapy indication. As we continue the development, we can identify and build up a pipeline of the other potential drugs we could be delivering or drugs and development that we can partner with other companies to deliver.”
Qnovia took a large step forward in September by raising a $17 million Series A funding round. Its plans include investigational new drug submission to the FDA next year, followed by Phase 1 clinical trials.
According to Quigley, the company has the capital to get through Phase 1 trials and to explore application programming interfaces (APIs) and
expansion opportunities.
“Getting through that Phase 1 human clinical trial is our next major milestone, and we’re funded to do that for sure,” said Quigley. “From there, our fate we would begin with Phase 2 studies in the back half of next year, and then move through Phase 3 in the new drug application.”
As for the future indications, Quigley said it’s hard to predict when public communications will come for those. The company’s focus remains on advancing its smoking cessation treatment.
“We’re really excited to have the support of the investors we have,” Quigley said. “We think that this technology to deliver health therapeutics has big potential. Advancing our smoking cessation application first and foremost is just a massive opportunity to improve the lives of millions of people.
“We’re excited to advance that and to continue to identify and bring to market additional indications that can help improve outcomes.”
CGI Motion standard products are designed with customization in mind. Our team of experts will work with you on selecting the optimal base product and craft a unique solution to help di erentiate your product or application. So when you think customization, think standard CGI assemblies.















Connect with us today to explore what CGI Motion can do for you.






TransMed7’s SpeedBird biopsy devices are made with injection molding, among other manufacturing processes. Photo courtesy of Fictiv




Part design, material selection, tooling and quality assurance are the keys to success.
Injection molding is known for producing high volumes of tight-tolerance parts. What medical designers may not realize, however, is that some contract manufacturers can also cost-effectively prototype functional samples for testing and evaluation. Whether it’s for singleuse devices, repeated-use devices or durable medical equipment, plastic injection molding is a versatile process that can help you bring products to market faster.

Like any manufacturing process, there are best practices for injection molding. They fall into four major areas: part design, material selection, tooling and quality assurance.
By considering what works well and working closely with an experienced manufacturer, you can avoid common mistakes that result in added costs and delays. The following sections explain what medical designers need to consider when outsourcing an injection molding project.



Design for manufacturability (DFM) is the process of designing parts so they are easy to manufacture. Parts with looser tolerances have larger part-topart dimensional variations and are usually easier and less expensive to make. However, most medical applications require tighter tolerances that those used with commercial products. Therefore, during the part design process, it’s important to work with your manufacturing partner and add the right type of commercial or precision tolerances to your drawings.
There isn’t just one type of injection molding tolerance, and omitting drawing details can result in parts that don’t fit correctly or cost too much to produce. In addition to dimensional tolerances, consider whether you need to specify tolerances for straightness/flatness, hole diameter, blind hole depth and concentricity/ovality. (continued on page 48)


(continued from page 46)
With medical assemblies, work with your manufacturing partner to determine how all the parts fit together in what’s known as a tolerance stack-up.

Tolerances vary by material, so don’t just evaluate plastics based on properties and pricing. Choices range broadly from commodity plastics to engineering resins, but these materials all have something important in common. Unlike 3D printing, injection molding can produce parts with exact end-use properties. If you’re designing pilot prototypes, recognize that you have the fl exibility to use the same material as in production. If you need a plastic that conforms to a specifi c standard, consider asking for a certifi cate of assurance (COA) to ensure that the injection molding material — not just its individual ingredients — complies.
Manufacturers mostly create injection molds out of aluminum or steel. Aluminum tooling costs less but can’t match steel tooling’s support for high volumes and precision. Although the cost of a steel mold may take longer to amortize, steel is cost-efficient across a high volume of parts. For example, if a $10,000 steel mold for a single-use medical product is amortized across 100,000 parts, the tooling cost is just 10 cents per part.
Steel tooling can also be the right choice for prototypes and lower volumes, depending on your injection molder’s capabilities. With a master die unit and frame that includes sprues and runners, leader pins, water lines and ejector pins, you only pay for the mold cavity and the core details. Family molds that contain more than one cavity can also reduce tooling costs by having multiple different designs inside the same mold.




With medical injection molding, it’s not

enough to produce good parts most of the time and then have the QA department catch any defects. In addition to tight tolerances, medical parts need a high degree of accuracy. DFM, T1 samples and post-production testing and inspection are important, but process control is essential for variables such as temperatures, flow rates and pressures. So along with the right equipment, your medical injection molder needs to be able to identify critical-toquality (CTQ) attributes.
For disposables, repeated-use medical devices and durable medical equipment, injection molding can help you bring products to market faster after alpha and beta prototyping are complete. Injection molding is known for supporting high-volume production, but cost-effective pilot prototyping is also possible. Injection molders have different capabilities, so consider making careful vendor selection an additional best practice for your next project.





















Collaborative robotics and Spanish-speaking shifts are helping Boston Scientific deal with a global labor shortage.

Boston Scientific executives said two labor-shortage strategies are paying off in a big way for the medical device maker, which has around 41,000 employees across the globe.


Collaborative robotics and Spanishspeaking shifts are helping Boston Scientific deal with labor shortages in the U.S. and elsewhere.
“It’s hit us everywhere. Part of that was just general wage inflation. Although we don’t like the cost of that per se, that’s relatively easy to deal with, right? You give people raises and at least the supply continuity problem goes away,” Global Operations EVP Brad Sorenson said in a recent interview with our DeviceTalks Weekly podcast.

“The bigger issue is, however, that if we used to want to hire 200 people because we had capacity growth that we needed, we’d just put an ad in the paper and do an open house and we could hire them. That’s not the case anymore,” he continued. “Frankly, just getting enough people to work has been difficult. So we’ve undertaken a very aggressive path to try to remove the human labor dependency for things that machines can do.”













As an example, Sorenson said about 30% of Boston Scientifi c employees “look through microscopes every day and make a go/no go decision” for product quality.


“Automated machine learning has really moved the ball on a machine’s ability to do that. And quite frankly, that’s probably not the most rewarding work that our workforce is doing,” Sorenson said. “We’ve started to put in more automation in those type of places to augment our direct labor capability and in parallel undertaken a dramatic change management approach through a program we call Grow, which is really around upskilling our direct labor, creating a better career path in places that have more value add.”


He reported “really good progress” through automated visualization and cobotics, or collaborative robots that work with product technicians or engineers on the line. Such technology could augment around a third of the Boston Scientific labor force, Sorenson said. And it doesn’t mean automation will lead to layoffs. (continued on page 51)
The medical device industry obviously isn’t immune to the challenges posed by a shaky supply chain. But OEM leaders like Boston Scientific need to work doubly hard to ensure they have the supplies needed to provide devices for patient care. devicetalks.com/podcast/


(continued from page 49)
“We get the team on the bus with us,” Sorenson said. “There’s no pushback — ‘Hey robots are trying to take my job away.’ It’s, ‘I get it, you’re going to try to do the part of my job that I don’t particularly love doing every day and give me stuff that’s way more interesting and exciting so I can grow.’ So we think it’s pretty dramatic, and in some parts of the organization, we’ve made big leaps forward. We’ve been able to essentially hold labor flat while we’ve been seeing 15% to 20% increase in output.”

Another labor supply solution came from an employee at a plant in the Midwest who suggested hiring workers who only speak Spanish, said Supply Chain VP Paudie O’Connor.

The facility already had Spanish-speaking employees from Costa Rica who had transferred. After translating documentation into Spanish, the plan worked.

“We had a couple of shifts up and running in no time,” O’Connor said. “Word of mouth in that community really helped. That is growing to be like a standard stable in that organization. About probably 25% of the workforce are only-Spanish speaking. ... It’s just phenomenal to see how it’s come together and each different community learning off each other. We’re giving Spanish classes to supervisors and engineers and anyone that wants to learn.”
With nearly $11.89 billion in annual revenue, Boston Scientific is the 12th largest medical device company in the world, according to our 2022 Medtech Big 100 ranking.

Medical device companies are competing for workers in one of the tightest-ever job markets. Meanwhile, there is pent-up demand for medical products worldwide due to increasing need along with pandemic-fueled supply chain problems.
There is a way medical device companies can overcome labor challenges and fulfill orders to take advantage of market demand. Highvolume production facilities have long relied on help from multimillion-dollar automated assembly lines and robotics. For decades, those systems were too expensive or impractical for medium- and low-production runs.

But robotic technology has advanced and expanded to the point where flexible automation makes sense for lower volumes and to bolster workforces.
Advances in robotics are helping manufacturers improve quality, fill labor shortages and promote personnel.

Many have feared advances in manufacturing would take away jobs and push more costly and fallible human workers out. However, automation systems and humans are both needed to operate manufacturing facilities efficiently, consistently and safely. Not everything can or should be fully automated, and not everything can or should be done by humans.

Flexible automation allows manufacturers to move workers off menial, repetitive tasks, and retrain them for higher-level roles that require cognitive agility and decision-making. Rather than “stealing” jobs from humans, automation and robotics can promote us.
Recently, one medical manufacturer with a product family produced through a labor-intensive, manual assembly and
insert molding process invested in new molding equipment. The team deployed an automation cell with a cobot (collaborative robot) to perform many repetitive tasks previously completed by a human operator. The switch resulted in consistent cycle times — which is key in injection molding — and a consistent level of quality with automatic separation of non-conforming parts. It also met the customer’s pricing target by reducing costs over prior models. In addition, the manufacturer’s investment helped alleviate the labor shortages it faced and enabled them to train plant floor employees for more complex tasks needed in higher-level positions.
Maximizing and optimizing resources There are generally two types of robots used for medical device production: industrial robots and cobots. Medtech

manufacturers often deploy industrial robots in automation cells. They require hard guarding and/or light curtains to prevent injuries.
Cobots can be programmed to learn multiple tasks, and with built-in controls can safely work alongside human colleagues in production. Generally, manufacturers use cobots to perform repetitive tasks that may need to be moved between jobs or work centers. Setup is quick and easy. With proper risk assessment, they do not require hard guarding. Cobots often are deployed for loading/unloading inserts into a thermoplastic or liquid silicone molding process, and automated inspection with machine vision systems or pad printing.
Cobots have revolutionized manufacturing automation costeffectiveness. Cobots are far less expensive than a full-fledged robot and easy to program. As a result, they take over menial jobs while improving precision in tedious tasks and increasing the overall safety of personnel and equipment. Before cobots came into their own, manufacturers spent much time and money designing and implementing safety systems to protect personnel and equipment from hazards. Now cobots can perform many of these tasks.
Once a cobot is programmed, assessed/tested, approved and deployed, it’s ready to handle whatever task it’s assigned. It’s possible to reassign a cobot as needed.
Deploying robots compliantly and cost-effectively Companies need to ensure internal staff or contact manufacturing partners have the knowledge and expertise to deploy and maintain their automation systems compliantly. In highly-regulated markets like the medical device industry, there are numerous, often complex regulations and international standards for automated systems in manufacturing applications. Therefore, proper automation system installation and operational and performance qualification, including process validation, are musts.
Choosing when, where and how to deploy robots requires strategic planning. The goal is to maximize operations and productivity and optimize personnel. Manufacturers should keenly focus on putting employees into roles that require cognitive agility and real-time decision-making. These roles generally are more enriching, which can
help recruiting and retention.
Take inspections, for example. Those that are straightforward, such as measuring a known feature or confirming the presence or absence of a component, can be done efficiently and effectively by robots. But that’s repetitive and often tedious work for humans. However, an inspection process to determine whether foreign material is inside a sealed package can be difficult and nuanced. All the surfaces of the part need to be assessed in all directions. A human can hold the package and tip it every which way, and put it under a microscope if they think they see something. A human has the ability to infinitely manipulate the product being inspected, whereas automation would typically follow a sequence and programming. It’s possible to achieve quality and consistency through thoughtful and deliberate deployment of automation with vision systems.

Humans are needed to design, maintain and oversee operations. This is where the labor shortages are critical and
intensely competitive. Manufacturers need technicians who are well-versed in setting up and adjusting systems and engineers with the deep expertise and knowledge to develop, qualify, troubleshoot and oversee the systems. They also need skilled personnel on the production and assembly lines who can operate and work alongside machines. All of these roles now require a higher level of training and education than the tasks that machines can now perform. Medical device manufacturers can automate many critical tasks, enabling the exact operation to be done in the same manner at the same time. Compared to a multi-shift operation with a variety of skilled human operators, they could be doing the same task, but slightly differently or vastly differently. A robot will do what it is programmed to do repeatedly and consistently. It won’t get fatigued, distracted or bored. The endgame is improved productivity to meet supply and demand, reduced risk and better allocation of resources for all involved.


Choosing a contract design or manufacturing partner begins a long, two-way relationship.
Vascular devices may need contract manufacturing services such as clean room injection molding, additive manufacturing, plasma treatment and coating or automated assembly. Photo courtesy of SteriPack




No matter what type of medical product you are designing, at one point you will be faced with deciding which design house or contract manufacturer is right for you.
OEMs through highly funded startups all face the same issues when preparing to transition from design to manufacturing.
The design-through-manufacturing cycle is getting quicker. On average, the typical product development launch is two years from concept to regulatory approval. This two-year period may initially seem like a long duration, and you might not feel an urgent need to engage a medical solution partner, letting the selection decision wait until the development cycle matures. However, time after time, we have seen a tendency to rush the development process to get to a prototype without engaging molders or manufacturers, leading to disappointment in aesthetics, assembly methods and cost.
Proper and early due diligence is especially important if you are a startup. Your organization might not yet have approved suppliers, the discipline to review past vendors, or even have experienced staff on hand. Startups are lean and fast-moving by nature. Once the startup receives its Series A or B
funding, the plan is to get to market as fast as possible. Does the company have an experienced program manager on the team that can recognize the ideal solution providers, or do you risk the pitfalls of jumping in too soon with a supplier that does not fit? Likewise, the suppliers you may target could be apprehensive about taking on the project based on volumes, investment cost and strategy to market.




There are as many medtech, diagnostic, pharma and consumer health product developers as there are specialty contract manufacturers. So let's look at the potential questions medtech designers and managers should ask when considering medical solution providers.



1. Is my contract manufacturer the proper size or scalable?
There is always a tendency for a company to overreach its growth goals. This idea relies on the assumption that a supplier with a global presence and high-volume capabilities will be safe as your production volumes grow. Even the most prominent companies fall into this trap.
It is ideal to fi nd a global contract manufacturer that has reach for when your product grows, but acts with caution regarding initial volume and cost
HS Design, a SteriPack Company
targets. Gauging a supplier's hunger for your business is paramount. Sometimes a smaller contract manufacturer will not only be more aggressive in timelines but also jump through problems due to gaining that larger OEM. Ask the contract manufacturer to tell you what their units-per-year production sweet spot is and about their ability to scale up and down.
2. What are the specific capabilities my product needs?
The thought that all contract manufacturers are the same couldn't be further from the truth. >>
Depending on the product, it could include micro molding, assembly, complex fluidics, packaging, sterilization, clean room molding — the list goes on. Finding examples of what the supplier is currently doing is always a good first step. Make sure your supplier has a process in place for everything. Do they offer turnkey or consignment preferences? If final packaging is required, will instructionsfor-use (IFUs) need to be provided? What additional services do they offer? Understanding the manufacturing partner's safety, sustainability, supply chain and accessibility is essential.


3. Are regulatory and QMS systems in place?
In today's highly competitive and regulated market, chances are you will have a medical solution partner meeting all the criteria on paper. However, how their QMS system is integrated and provides tracking can significantly differ. Look for a provider that can point to their quality and regulatory manager and have them discuss their certificates, traceability and capabilities for carrying inventory, quarantine and shipping. Are they GMP, ISO 13485 or FDA registered? Are their procedures regularly critiqued through internal notified bodies and customer audits? Strict quality management ensures consistent delivery and instills confidence in customers.
4. Does the solution provider offer holistic design-to-manufacturing services?


Even though you may have spent considerable time selecting your design firm, remember that some design firms are more manufacturing-focused than others. Integrating a design firm into a contract manufacturer will reduce the need for rework. Design and manufacturing processes differ, and ensuring the manufacturer is in meetings early will prevent rework. Do they have in-house molders, or do they rely on outsourced services? What is
the knowledge of medical resins and prototype tooling?
5. Does your current product development partner have a track record with the contract manufacturer?
Most ISO 13485 medical-focused product development fi rms have vetted quality processes, but not all are usercentered or manufacturing-focused. If the fi rm lacks experience in molding and manufacturing, it may not have existing relationships with contract manufacturers. Early integration of the contract manufacturer with the product development fi rm is critical to make the experience as holistic as possible. Ideally, the medical solution provider can show successful case studies of projects transitioning from design fi rms to production.
The devil is in the details. Trust your instinct, but base it on facts. If possible, visually inspect the plant before selecting it to gauge the supplier's quality from the looks of the tooling, molding and assembly fl oors — and even the front lobby. Resist the urge to select based on recommendations and slick presentations. Once you choose your medical solution provider, be prepared for a long-lasting, trusting relationship. The ability to move your product to another supplier once in production is a daunting process and will test the relationship between an OEM and the contract manufacturing partner. In addition, all the regulatory work, design history, tooling and material knowledge will need to be transferred, leading to another round of testing and validation.










That’s what differentiates Freudenberg from the other CMO’s. We invest annually in R&D to deliver innovations like conductive silicone, inline ID measurement for silicone extrusion, twisted lumen technology, and turnkey product solutions that simplify your supply chain and get products to market faster. Learn more about Innovation at Freudenberg. freudenbergmedical.com/innovatio n




3D printer accessibility and affordability are enabling small device firms to develop and market a wave of personalized devices.
Healthcare is becoming more efficient, and patients are beginning to expect a personalized approach.
3D printing isn’t a newly minted manufacturing technology, yet it’s reached an inflection point for bringing change in healthcare and dental applications.
Traditionally, 3D printing was prohibitively expensive and only available to the largest, best-resourced medical centers and device manufacturers. But these days, 3D printers have become more affordable and accessible. As a result, additive manufacturing is surging in healthcare as medical providers and device manufacturers tap into the ability to safely produce novel, patient-specific, biocompatible and sterilizable parts.
As 3D printing becomes the method
of choice for a growing number of medical devices, implants, surgical guides, orthotics, prosthetics, and other medical applications, medical device firms and manufacturers must consider the technology for the commercialization of end-use parts.




The healthcare sector has begun to embrace 3D printing, a trend expected to grow as providers and manufacturers implement personalized care, create new medical devices and improve patient education. Also, there is the development of technology to improve part production, deliver new materials and reduce production time.
3D printing manufacturers and healthcare leaders are teaming up to further advance additive manufacturing.

Major group purchasing organization (GPO) Vizient listed a 3D printing company in its catalog for the first time, and other partnerships are accelerating the delivery of life-changing medical devices while maintaining regulatory compliance.
This support from the industry dovetails with advancements enabling new applications and faster workflows, which will further advance 3D printing toward commercialization in healthcare. Beyond 3D printing hardware and software, materials are also a critical component to advancing 3D printing commercialization in the space.
Healthcare providers, manufacturers, and facilities require biocompatible, sterilizable materials such as BioMed Clear, BioMed White and BioMed Black Resins.
(continued on page 60)





(continued from page 58)
These materials are made in an ISO 13485 certified, FDA-registered facility and are designed for applications requiring contact with skin, blood, tissue, dentin, mucosal membrane or gas pathways in healthcare. They’re also compatible with common solvent disinfection and sterilization methods. Designed with patient safety in mind, these SLA materials — and biocompatible nylon materials for an SLS printer — are critical for creating medical devices, surgical instruments, surgical guides, and new innovations in ground-breaking healthcare research.
Designing 3D printing technology with healthcare commercialization in mind will enable the industry to reach the end goal of improving patient care sooner. Small and large medical manufacturers have started creating medical devices, dentures, implants, prosthetics and more using 3D printing.


Small firms already commercializing devices

Small firms are commercializing 3D-printed medical devices — including surgical instruments, inhalers, smart prosthetic hands, metabolic analyzer masks and more — and can show the path forward. Companies using 3D printing for manufacturing and prototyping can develop new innovative treatments and devices that are customized to patients while remaining affordable.
Medical device company Coalesce Product Development (recently acquired by Novartis) is using 3D printing to develop novel, affordable drug delivery devices such as inhalers and injectors for generic inhalation products that offer significantly better value than brand name alternatives that can cost over $380 per month.
Another company, Restor3d, is bringing the benefits of 3D printing to surgery. Stainless steel instruments traditionally go into more than 132,000 anterior cervical discectomy and fusion procedures performed per year in the U.S. But these instruments had high upfront and ongoing inventory costs and often presented complications in the surgical workflow. Restor3d uses the design freedom and constant improvement enabled by 3D printing to change how spine surgeons operate and improve surgical care delivery. The company’s first 3D-printed, procedure-specific polymer instruments for foot and ankle and cervical spine implants were made from

a combination of metal and 3D-printed polymer parts, benefitting hospitals with reduced sterilization and inventory costs.
The medical device industry has also begun to see applications of 3D-printed parts outside of the human body. Mychael Overstreet — a veteran, firefighter and paramedic — used 3D printing to create Tension Square, a portable device that holds a needle decompression catheter securely in place while preventing damaging kinking, folding or dislodgement and preventable deaths in the field due to pneumothorax or collapsed lungs. With no formal engineering experience,
Overstreet relied on 3D printing to test and finalize his design, enabling him to try a mix of materials to achieve a long-lasting, accurate, lightweight, skinsafe and durable design that is being commercialized with SLS 3D printers.
These companies and innovators have successfully commercialized 3D-printed enduse parts, as the technology stack is now more accessible than ever. Collaboration within the industry will enable medical device firms to learn from these early examples to chart their own path to commercialization and improve patient outcomes, reduce costs and improve efficiency.
(continued on page 62)

(continued from page 60) Designing medical devices with 3D printing Utilizing 3D printing to create commercialized healthcare devices has become an attainable goal, but it’s not yet the norm. Companies can approach 3D printing design and production to deliver precision healthcare across point-of-care facilities and create new medical devices by:








































• Understanding biocompatibility and sterilization requirements of the intended device and reviewing relevant documentation from manufacturers.























































• Becoming familiar with quality and regulatory requirements and best practices for leveraging additive manufacturing while maintaining compliance.


• Reviewing 3D printing resources and evaluating various print engines (e.g. FDM, SLS, SLA) and materials with requirements for part performance and manufacturing system compliance in mind.


Moving production back to the United States requires strategic and complex navigation.
Validation is a critical process to keep patients and end users safe. Proper documentation of tooling maintenance and repairs, adjustments to process and part design will help facilitate a successful and efficient validation process.
Reshoring is a topic of conversation with almost every biopharma, life sciences and medical company currently outsourcing production of thermoplastic or silicone parts, products and assemblies to overseas molders.
Just look at the numbers. The Reshoring Initiative released data showing that reshoring and foreign direct investments could drive an additional 350,000 U.S jobs in 2022, a record high.
The reasons for this growth are numerous. Companies may be experiencing issues with quality from current suppliers, capacity issues against fluctuating demand or timeline delays due to supply chain complications. No matter the reason, today’s medical device, equipment and product manufacturers can reap a number of benefits when transferring tools to a


domestic contract manufacturing partner. Still, the decision to reshore is strategic and complex, with many issues to balance as you maneuver the process.
In deciding to move production back to the United States, one of your primary goals should be to ensure a smooth transition. Whether you’re transferring your work to a contract development and manufacturing organization (CDMO) or managing the transition to a captive facility, explore these three considerations to successfully navigate your reshoring initiative.
1: Your existing tools can’t always be salvaged. An early consideration of the reshoring process should be the state of your tooling. Ideally, you would be able to seamlessly collaborate with your >>
overseas partner to ship tooling back to the U.S., make minor repairs, and start injection molding parts. However, in reality it’s often more complex.

For legacy parts, tooling has likely been repaired and modified over the years to ensure volumes continue to be met. However, during the reshoring process, some companies may find that they haven’t been maintained well. Others may find a lack of maintenance or repair documentation, or missing part drawings, 3D files or other design mechanics. In both situations, your program may require entirely new tooling to ensure compliance or produce the quality and volumes you need.
As you begin your reshoring initiative, managing expectations about the state of your tooling will help you identify the right steps for your team while building a strong foundation for ongoing production.

2: Preparation for the validation process can expose potential risks.
As a medical device, product or equipment manufacturer, you have likely experienced the critical FDA validation process to get your product to market while keeping patients and end users safe. As with any move to a new supplier, ensure you prepare the documentation and data you need to ensure a smooth process, no matter who will mold or assemble your parts.

If transferring production to a contract development and manufacturing organization, that team will be critical in supporting you through the process, identifying the potential downfalls through the validation process, especially if your tooling evaluation exposes potential risks.
Quality management teams will use part drawings, tool designs, specs and more to uncover areas in need of attention before validation. Whether the tool needs to be modified to meet design documentation, new documentation is needed, or entirely new tools must be built, a CDMO can ensure ongoing quality against your exacting standards.
Whether your tools are in excellent shape or need work, engaging your team to prepare for validation will help accelerate your timeline and drive efficiency throughout the process.
3: You may need to say goodbye to part of your current supply chain. A known advantage of reshoring is the ability to gain greater control over your
supply chain and improve collaboration with companies closer to home. The move to a domestic supplier for ongoing production of your thermoplastic and LSR components and assemblies may also require reconstruction of your supply chain, both from a geographic and a risk perspective.
Your bill of materials (BOM) — the raw materials, sub-assemblies, subcomponents, parts and quantities needed to manufacture an end product — provides an excellent blueprint for this reconstruction. When moving part production closer to your facility, it just isn’t feasible or logical to maintain your same partners overseas. Whether you choose a vertically integrated CDMO
The decision to reshore production can factor in many other things: financial benefits, brand value, customer loyalty and more. Whatever your reshoring decision, now is the time to ensure you have a smart, stable supply chain to help you accomplish your short- and long-term goals.
If you are a captive molder seeking partners for materials, sub-components and assemblies, or you are seeking a contract manufacturing organization to manage your programs, take steps today to establish your goals, evaluate the performance of your supply chain and guarantee lasting success.
who can manage more of the BOM or choose to manage those relationships individually, reconstructing your value chain can drive sustainable program success long-term with thoughtful and careful vetting of your partners.
Though a total shake-up may seem unnecessary, the move may also help you gain more control amid rising raw material costs, freight and transportation costs, wages, energy costs and more, now and in the future. A 2020 McKinsey Global Report found that companies can now expect supply chain disruptions lasting for at least one month every 3.7 years. With trusted suppliers closer to home, you can help lessen the long-term effects of these recurring disruptions, like issues with quality, on-time delivery and ultimately, your brand reputation.
Reshoring programs can take many shapes, from one-tool transfers to full equipment transfer. No matter the scope of your reshoring initiative, a strong supply chain and engaged team are critical to ensuring success at every stage of the process.


ISO 13485 Certified beaconconverters.com
Serving Healthcare Industries:
Medical Device Pharmaceutical
Biologics
Diagnostics
Animal Health
All products are made to order with a custom fit, naturally reducing the amount of material used.
We are committed to Quality standards set forth by the industry and contribute significant resources to consensus standards group like AMI and ASTM to develop and improve them.
Since 1947, and through every chapter, we have remained committed to providing custom solutions that best serve our customers and the needs of the patients we ultimately serve.
Supply chain challenges have impacted our industry in unprecedented ways. We are here to support you and work through the challenges together.
If you need a responsive, knowledgeable partner, reach out to us at Beacon Converters.
Syringe filters contain thin membranes that, if damaged during assembly, would render them useless.


Emerson officials think their PulseStaking offering provides a new
option for welding the small, delicate structures found in filtration parts.
Technology and market demand are pushing designers and manufacturers to create medical devices that are ever smaller and more compact. The miniaturization trend is especially true of wearable devices used for drug delivery and patient monitoring. Assembling these plastic components, especially those with tiny filters that are frequently used in wearable devices, presents special challenges.
There are plenty of options for joining plastics, including ultrasonic

welding, laser welding and staking and swaging processes that use ultrasonics or thermal technology. However, increasing miniaturization means that the parts to be assembled can be quite fragile and require the utmost care to prevent damage during welding or staking.

The filter media used in medical applications — typically made of polymers such as nonwoven polypropylene (PP) or polyethylene terephthalate (PET) — is usually sealed in a plastic frame or housing. While a larger device might accommodate
filters that are an inch or more in diameter and 0.010 in. or more thick, the structures in wearables might be only 0.1–0.25 in. diameter and 0.005 in. or less thick. Most manufacturers would use ultrasonic welding if they could because it is fast, controllable and economical. However, the vibration it introduces may, in some cases, damage thin or fragile filter membranes. Even the equivalent of a pinhole would render the filters worthless.
To avoid damage, manufacturers of miniature filters and similar products seek
alternatives to ultrasonics and increasingly considering thermal processes. It’s possible to design thermal tooling to apply heat and pressure around the entire circumference of a filter, bonding it to its housing in one step. Since thermal sealing is a nonvibratory process, it eliminates the risk of creating pinholes in the filter. The result is a high-quality seal.
Traditional steady state thermal sealing works OK in this regard. A manufacturer can produce thermal seals relatively quickly and at low cost, without the need for labor-intensive mechanical fastening, expensive adhesive fastening processes or the vibration of ultrasonics. But the process has its pitfalls in terms of cycle-to-cycle repeatability and process control. As designs evolve and more delicate components get assembled into the newest devices, manufacturers have found that thermal staking has some technical and control limitations.
A newer approach from Emerson, called PulseStaking, addresses many of these concerns. PulseStaking technology has been demonstrated to perform as well as or better than existing steady state thermal processes, and it is readily applicable for even the most delicate filter applications. It can work with multiple, closely spaced features on geometrically complex parts, including those with otherwise tricky angles and planes, and can create bonds on a wider range of plastics than traditional heat staking.
The PulseStaking process uses and applies heat much more selectively. Unlike steady state thermal tools that continuously radiate high heat in all directions before, during and after a seal, PulseStaking tips heat and cool instantly — and only when needed — to apply heat in a highly controlled and localized manner. Each tip combines an electrical heating element with a compressed-air cooling system.
This sequence of operations is much more controllable than that of a conventional thermal unit. In steady state thermal sealing, the tool is always energized, wasting heat energy and creating a larger carbon footprint. Beyond that, the staking process is never truly at a steady state. Each cycle draws heat out of the tool, which then needs to be restored before the next cycle. If sufficient reheating time is not built into the process, the weld temperature can vary, and one or two
degrees can mean the difference between a good part and scrap.
In PulseStaking, on the other hand, tips go through a cycle of multiple heating, cooling, and pause or “dwell” intervals to prevent overheating and precisely manage tip and seal temperature until each seal is completed. Thus, cycle consistency does not rely on the temperature of the tool at the start of the cycle.


The volumes associated with most medical devices, including wearables, are often in the hundreds of millions. Consequently, manufacturing lines are likely to be highly automated. Medical applications — like sealing filter media into a housing for a wearable medical device — typically involve complex manufacturing processes. After the filter seal, it is likely that there will be downstream testing, such as a machine-vision quality check. Ultimately, there will be additional processes to install the filter element into the larger component. Therefore, cycle consistency and temperature control become critical to the efficiency and repeatability of any

high-speed multistep process. Variability in the sealing process will result in increased scrap and risk of a defective product, in addition to increased cycle times.
Automation also can require customizing the PulseStaking process to a specific application. Heating tips are available in many standard and custom shapes, and can be operated singly or — if required by production and cycle times — densely grouped into larger tools that can perform multiple operations at once. In addition, the localized heating characteristics of each tip, as noted above, enable sealing operations on complex or angled surfaces, in close proximity to heat-sensitive components such as printed circuit boards, and can even reach into deep cavities or other difficult-toaccess areas without the risk of unintended radiant heating, even if tooling or tips pass very close to nontarget surfaces or heatsensitive components.

As medical devices continue to evolve, becoming more complex and more compact, joining technologies that can accommodate tiny, thin and delicate components like filter elements become ever more important.
Equipped for both heating and cooling, PulseStaking tips precisely manage tip and seal temperature until each seal is completed. Illustration courtesy of Emerson


n the past century, modern medicine has broken countless barriers toward a safer, more effective healthcare protocol. One of the most important discoveries has been antibiotics. Unfortunately, the widespread use of antibiotics has led to growing antibiotic resistance. According to the European Centre for Disease Prevention and Control, more than 4 million people acquire a healthcare-associated infection (HCAI) each year, resulting in 37,000 deaths. Combatting HCAIs is a significant problem for the healthcare sector globally. HCAIs are the sixth leading cause of death in Western countries. However, treating these infections with antibiotics is lowering the effectiveness of the treatments and the public trust in medicine. Researchers have pinpointed
the cause of most of these infections: bacteria-infested surfaces inside of hospitals. There is an identified need to protect surfaces from germs and microbes by using antimicrobial coatings. An antimicrobial coating is an application of a chemical agent on a surface that can stop the growth of disease-causing microorganisms. Apart from increasing the surface’s durability, appearance and corrosion resistance, these coatings also protect from harmful disease-causing microbes. Coat medical equipment with an antimicrobial solution, and it can become up to 99.999% sterile, significantly reducing the risk of infection for the patients. Antimicrobial coatings stick to the surface they are applied on and remain effective for a period, defining them as one of the best options to fight bacteria in a medical environment.


State-of-the-art antimicrobial coatings and their uses



Everything is susceptible to microbes: surfaces, healthcare devices, equipment, walls, textiles. The list is almost endless. From these surfaces, microbes find their way to humans. Unfortunately, strict hygiene regimes and existing disinfectants have limited efficacy and require a considerable level of maintenance to reduce the risk of infection for prolonged time periods.

efficacy
New developments are bringing to light state-of-the-art antimicrobial coatings that offer a new way to combat infections more effectively, efficiently and for longer periods of time. These coatings are proven to provide monoclonal protection, killing a broad spectrum of gram-positive and gram-negative bacteria and enveloped and non-enveloped viruses, including E.coli, MRSA, influenza, vaccinae, adenovirus, norovirus and SARS-CoV-2.
(continued on page 70)

























(continued from page 68)
Our TridAnt antimicrobial coating, for example, is suitable for skin protection and most other surfaces including woven and non-woven fabrics, such as metals (nitinol) as well as polymers (polycarbonate and polyurethanes).







The new antimicrobial technology is non-leaching and, therefore, completely safe to use in all environments and even for implants inside the human body including Class III medical devices. Its active components target microbes (prokaryotic cells) and have reduced risk to human cells, unlike previous technologies. For the first time, medical device technology can kill enveloped and non-enveloped viruses, grampositive and gram-negative bacteria. In addition, it can prevent the formation of biofilms for long-periods of time of up to 365 days (as well as safe enough to protect skin for up to 48 hours) without any noticeable reduction in efficacy over time. As a result, antimicrobial-coated
medical devices are protected with a highly effective and non-leaching shield for the device’s entire lifetime.

























What’s needed to advance the technology to make that future a reality One of the main factors stunting the widespread use of antimicrobial coatings are current regulations. Under today’s European Union (EU) regulations, for example, the coatings are considered medicines and are therefore tested by the European Medicines Agency (EMA) following the same tests and approval processes for drugs. As a result, regulators want to make sure that any positive effects an antimicrobial coating has are systematic, able to be replicated across the board in different settings and for different patients.
In the U.S., the use of antimicrobial coatings is more widespread than in the EU. The FDA allows the use of chlorhexidine and silver-based coatings, regardless of them leaching and
reducing their effectivity overtime. In the EU, however, this is considered a risk — and regulated.
Thorough testing and aiming for perfection in all medical-adjacent products are especially important not only for patient care, but also to bring about a continued cycle of technology innovation. This current testing method helps hospitals, patients and doctors to be certain that antimicrobial coatings are effective against HCAIs. However, this process can be very long — at times up to years — for technologies ultimately to earn accreditation, which slows down progress. Regulations need to be thorough and effective, but they also must move forward to assist with innovation.
Promising and potential uses in the future
There now is the prospect that biocompatible technology can enhance the function of medical devices by eliminating existing microbes and also prevent the formation of new colonies consistently over extensive periods of time. It represents a paradigm shift in the prevention and treatment of surgical infections.
The future will bring further developments in material sterilization, bacterial reduction and viral prevention. As a result, antimicrobial coatings will cement themselves as the golden standard of excellence in healthcare, used in all things that go inside the patient and around open wounds. Additionally, they will help prolong the active lifecycle of medical devices, which has a significant economic, health and social care impact.
As time goes on, the focus in the healthcare sector will switch from infection reaction to infection prevention. Once science understands how to prevent infections, we will also be able to stop pandemics and lockdowns and raise the public’s confi dence in antibiotics and vaccines.
Honeywell’s Spectra MG Bio medical fiber
Photo courtesy of Honeywell
The first minimally invasive surgery (MIS) — an endoscopy — dates back hundreds of years. In orthopedics, it has a history of a century or more. Uptake has accelerated more recently, and has grown fast across a wide range of surgeries in the last few decades. One published analysis of MIS described the expansion as “exponential since the introduction of laparoscopic surgery in the late 1980s.”
MIS is the new standard. In orthopedics, MIS is now widely used for arthroscopies and even full joint replacement for not just knees, but hips, wrists, shoulders and elbows.
From gastroenterology to cardiothoracic and heart surgery, pediatric to urogynecology, MIS has surged.
According to one estimate, the market value of MIS surpassed $60 billion
as of 2020. By 2030 it is estimated to increase more than a third to $94.4 billion, a compound annual growth rate of 4.7%.
MIS is in a period of accelerating growth. That’s not surprising: the benefits are well recognized by medical professionals and the public alike. Smaller incisions mean less pain, fewer complications, less scarring, and shorter hospital stays and recoveries. They also mean a lower total cost of care.

Given a choice, few would want a larger wound requiring more recovery time, but realizing the benefits of MIS depends on a successful surgery. In part, we need high-quality medical devices produced with materials designed to maximize the benefits of MIS procedures.
The rise of MIS poses challenges for medical device companies. Smaller incisions require more precise instruments and smaller medical devices to facilitate surgery. At the same time, requirements for high strength with low stretch, superior flex and bending fatigue performance remain necessary. This applies to Class II devices like catheters and Class III devices such as part of a heart valve. In orthopedics, shrinking incisions do not diminish the need for strong materials.
As with traditional surgery, the qualities and properties of the medical device can have a significant impact. One striking example is the rising use of colored sutures, another trend we’ve seen recently. Colored fibers in sutures can create patterned combinations to aid visibility and suture management in more complex surgeries. That can be particularly valuable when working within the confines of the smaller incisions
of MIS, helping promote faster, more effective and potentially safer surgery.
Medical devices are critically important, and the history of suture materials is even longer than that of MIS. It spans from silk and catgut used over three and half millennia ago right up to the present day, to the synthetic materials of the last half-century.
Some forms of ultra-high molecular weight polyethylene (UHMWPE) fiber can be 15 times stronger than steel and three times stronger than polyester while still being ultra-lightweight. This enables strong sutures with a small diameter/footprint. It also provides superior resistance to chemicals, fatigue and abrasion compared to conventional polyethylene. Look for medical-grade UHMWPE with ISO 13485 certification, the highest quality management standard for the medical device industry.
UHMWPE fiber is available in hues of color for high visibility. That visibility — along with UHMWPE fiber’s high strength, light weight, biocompatibility and non-absorbability — makes this material ideal for use in orthopedic sutures or other medical applications where stark visual contrast can aid in making the procedure more effective.
Such developments don’t just support the increasing preference for and prevalence of MIS. Lighter, stronger, high-performance materials will also be critical to supporting the emerging trends in corrective surgeries, from robotics that reduce procedure durations to smart implants that flag dangerous bacterial presence. UHMWPE will play a crucial role in all of these.

High-strength, lightweight medical fiber helps make surgical procedures more effective.

Biomedical textiles have the potential to enable lower-profile medical devices, less invasive surgical procedures and greater overall flexibility and biocompatibility across a variety of medical applications. But unlocking the full potential that


textiles can offer starts at the earliest stages of the product design and development process. The right decisions must be made on how to create an implantable textile component that’s optimally fit-for-purpose.


Braiding raw material (i.e., fiber) is one of the most prevalent methods of creating biomedical textiles structures, but within this category, there are still technology decisions that must be made. Medical
device OEMs — guided by their textile engineering partner — should assess whether high-density braiding, low-density braiding or 3D braiding is the best option for the product they aim to create.

The braiding process involves yarn prep, twisting and plying. After yarn preparation, it’s possible to make braids by winding bobbins, braiding and rewinding. These bobbins are loaded into the braider and will all contribute to creating a braid. Half of the bobbins will travel clockwise, and the other counterclockwise, interlacing in and out between each other.
An effective tensioning system
Making informed choices between high-density, lowdensity and 3D braiding offers greater design flexibility for implantable textile components that are fit-for-purpose.High-density textile braiding Photo courtesy of Cortland Biomedical
A perfectly sealed noble metal surface is a necessity for systems used to identify pathogens.
A perfectly sealed noble metal surface is a necessity for systems used to identify pathogens.
A perfectly sealed noble metal surface is a necessity for systems used to identify pathogens.












Traditional surfaces for diagnostic devices were built over nickel and similar metals. These interfered with some analytical techniques, however. One answer was a noble metal, gold.
Traditional surfaces for diagnostic devices were built over nickel and similar metals. These interfered with some analytical techniques, however. One answer was a noble metal, gold.


Traditional surfaces for diagnostic devices were built over nickel and similar metals. These interfered with some analytical techniques, however. One answer was a noble metal, gold.
Gold is widely used in biomedical diagnostics, particularly in the sensors and circuit boards that are the heart of all electronics. Gold is, however, inherently porous. As a result, it is imperfect at best for advanced spectroscopy and other emerging techniques.
Gold is widely used in biomedical diagnostics, particularly in the sensors and circuit boards that are the heart of all electronics. Gold is, however, inherently porous. As a result, it is imperfect at best for advanced spectroscopy and other emerging techniques.
Gold is widely used in biomedical diagnostics, particularly in the sensors and circuit boards that are the heart of all electronics. Gold is, however, inherently porous. As a result, it is imperfect at best for advanced spectroscopy and other emerging techniques.

To achieve zero porosity, zero contamination and a sealed, contaminant-free surface, a metallic layer stack comprised of palladium with fine-grained gold overplate has proved optimum.
To achieve zero porosity, zero contamination and a sealed, contaminant-free surface, a metallic layer stack comprised of palladium with fine-grained gold overplate has proved optimum.

To achieve zero porosity, zero contamination and a sealed, contaminant-free surface, a metallic layer stack comprised of palladium with fine-grained gold overplate has proved optimum.
There are two processes, both developed and patented by Uyemura, that are exquisitely suited to spectroscopic and other high purity surface requirements. The processes are economical, compatible with existing equipment and do not require elaborate lab analysis.
There are two processes, both developed and patented by Uyemura, that are exquisitely suited to spectroscopic and other high purity surface requirements. The processes are economical, compatible with existing equipment and do not require elaborate lab analysis.
There are two processes, both developed and patented by Uyemura, that are exquisitely suited to spectroscopic and other high purity surface requirements. The processes are economical, compatible with existing equipment and do not require elaborate lab analysis.



Headquarters: Ontario, CA • ph: (909) 466-5635 • (800) 969-4842 • www.uyemura.com Tech Center: Southington, CT • ph: (860) 793-4011 • (800) 243-3564 • rdepoto@uyemura.com
Headquarters: Ontario, CA • ph: (909) 466-5635 • (800) 969-4842 • www.uyemura.com Tech Center: Southington, CT • ph: (860) 793-4011 • (800) 243-3564 • rdepoto@uyemura.com
Headquarters: Ontario, CA • ph: (909) 466-5635 • (800) 969-4842 • www.uyemura.com Tech Center: Southington, CT • ph: (860) 793-4011 • (800) 243-3564 • rdepoto@uyemura.com
Medigold was developed for medical diagnostics and related applications requiring an ultra-high purity surface.
Medigold was developed for medical diagnostics and related applications requiring an ultra-high purity surface.
(continued from page 72)
Using individual tension controls, axial braid ends can be highly customized (for example, loose or tight). It’s possible to apply different tensions to different braid ends to create a textured surface, which is well-suited for promoting tissue ingrowth.
High-density braids are ideal in scenarios that necessitate design flexibility and custom geometries. These braids can either be bulky or very thin and hollow. Overlaps of the material create a hollow, tubular braid. This braid can either be formed over a mandrel, or independent of any structural aid, depending on the application. Each machine can offer continuous or discrete units. By customizing a variety of bobbins for highdensity braiders, the individual braid can be of different materials, colors and sizes. The formation of the braid can be over almost any item or shape, and one can design the braid wall to be thick or thin.
For more complex solutions, a two-part braid structure may be required. Working with what is essentially a braider inside of a braider, the outer and inner ring can run together at the same time, forming one unique braid on top of the other (a core and jacket). Having an outer ring/jacket can allow the incorporation of qualities that may be totally opposite from the core braid. This is a useful capability for applications where the product needs to hit a certain strength target with distinctive outer surface qualities. Programmable picks per inch (PPI), referring to the braid density, can be adjusted to influence the mechanical properties and surface texture of any part of the braid.
High-density braiding might be the best choice if an OEM is looking for a delivery component or sleeve for other device components with a determined diameter. These braids can combine high coverage with flexibility. To achieve a fixed diameter, they can be braided over a rigid object with an established diameter and then heat set. High-density braids are also a good choice for an open, continuous structure, and can even be manufactured exceptionally “open” to promote cell growth.
Is low-density braiding right for your surgical application?



Low-density braiding is a good choice for tethers, cords or pull wires, which are frequently used in delivery systems for minimally invasive surgeries, transcatheter procedures and robotics. If a more advanced braid is needed, textile engineers could incorporate suture tipping and/or fabrication into the process.

Low-density braiding is also ideal for creating custom sutures and soft tissue assemblies, as these braids can fit around small radii and tight bends, whereas metal and wire might fatigue. Low-density braids don’t transfer torque, so one end can be twisted while the other remains stable.
When to choose 3D braiding 3D braiding is a good choice if an OEM is looking for a braid that can be tied around a member and then anchored to a point such as an artificial ligament.
Like normal braiding, 3D and branch braiding using a variation braider involves bobbins on a carrier going around a designated path, and strands coming together in a helical pattern. The ability of the carrier path to change allows this machine to create custom braid structures that can bifurcate, trifurcate, continuing up to octfurcate.
When designing one of these custom-built structures, the first step is to use software to model the actual braid path which will output the product that the OEM is looking for. This allows for an unlimited number of design options and possibilities. 3D braiding allows for considerable customization and furcation to suit the OEM’s needs and make a structure that is completely unique to them.








The future of healthcare lies with the development of devices with designs centered on the safety, needs, desires and challenges of their users.
courtesy of MIDI

 Gregory Montalbano MIDI Medical Product Development
Gregory Montalbano MIDI Medical Product Development


Designing a medical device is not a lighthearted or straightforward task. Every design choice during the development process can influence how the device is used, whether it is effective and if it has the potential to cause its user or intended patient harm.


The vast majority of medical device failures stem from use errors. While this may lead some to believe that the blame lies on the user, the fact is that use errors are frequently the result of unintuitive device design choices.
With this in mind, the FDA and its supporting standards organizations continue to advance effective regulations, standards and guidelines. Developers must embrace proper practices and methods relative to usability research and human factors methods when designing medical devices. But despite their absolute necessity, the sheer number of regulatory and standards documents — and the intricacies of usability research
and human factors standards — can overwhelm medical device developers.
Where do you even begin? Start your medical devices development journey by identifying, studying and understanding the most fundamental and essential components of usability research and human factors engineering for the device in question. These three primary, baseline components are the device users, use environment and user interfaces.
The FDA defi nes device users as “the intended user group of a medical device” who “should be able to use the device without making errors that could compromise the medical care, patient or user safety.” Device users are the fi rst consideration developers must identify and address. A medical device’s user groups can be highly variable depending on the device type. Often, they extend beyond the patient.
(continued on page 78)
Medical device usability research and human factors engineering starts with device users, the use environment and user interfaces.Photo
(continued from page 76)
When evaluating a user group’s ability to operate a device, human factors engineers must observe and document a wide range of unique criteria. They need to consider users’ physical characteristics, such as size, strength, stamina, dexterity, flexibility and coordination, as well as sensory abilities and cognitive abilities such as memory. Other usability factors to be considered for the device/user relationship include the proposed device’s method of communicating information concerning the patient’s status, the disorder the device will be used for, and any patient comorbidities.
Use environment for a medical device is the second essential baseline component for medical device usability research professionals to identify and study. Environmental conditions can significantly impact how a device should be designed and used. Of equal importance is to keep in mind that there can be a wide range of environmental settings in which care may be provided. While some devices are intended for clinical environments, others find use in patients’ homes and other non-clinical environments. Additionally, medical devices have been designed for use within the most challenging uncontrolled environments, including moving vehicles.
You must consider a wide variety of use environment conditions when establishing a complete picture for a medical device’s design. For example, environmental lighting can make it difficult to read displays or identify controls. High ambient noise levels may prevent the user from hearing important device feedback or cause a user to mistake one alert for another. If an environment is heavily cluttered with equipment, a device’s maneuverability can be compromised, and users may become distracted.

User interface for a medical device is the third — though no less critical — essential baseline component. When applying usability research to medical devices, the FDA defines the user interface as including “all points of interaction between the product and the user, including elements such as displays, controls, packaging product labels and instructions for use.”
Software as a medical device (SaMD), graphical user interfaces (GUIs) and the Internet of Things (IoT) have become more common. Therefore the scope of usability research must support emerging digital disciplines together with the physical device design and controls. Development professionals must consider that a medical device’s user interface could be used at any time during setup activities like unpackaging and calibration, during device operation and while performing maintenance.
The natural manifestation of usability research, observations and data collection during the early development stages should reveal the opportunity zones for a medical device’s user interface design. User interface discoveries yield appropriate information delivery systems as well as device size and shape, particularly in handhelds, wearables and disposables. A well-designed user interface will also inform the cognitive logic workfl ow of the overall user-system interaction. This includes the how, when and why for a medical device to provide information and feedback to the user.

It can also make sense to identify hardware components intended for device operation like switches, buttons and knobs — and any accessories or components connected to the device or patient. Device user interface considerations should also include any components that connect, reposition, confi gure or manipulate the device. Lastly, usability information collected should also inform the design of device packaging, labeling, operating instructions and training manuals.



With healthcare moving forward with the combined forces of patients, healthcare professionals, legislation, technology and marketplace influences, it is clear that we are at the precipice of monumental shifts in healthcare and its delivery.
As these shifts occur, we must support user and patient safety as a top priority through effective human factors engineering and usability development methods. The future of healthcare truly lies with the development of devices with designs centered on the safety, needs, desires and challenges of their users.
Medtronic’s DRM program is meant to identify and mitigate risks starting early in development.

"Play small and play big” was one of CEO Geoff Martha’s mantras as he and his team reorganized Medtronic, the biggest corporation in the medtech industry.
The multi-year effort decentralized Medtronic’s three big groups — cardiology, neuroscience and medical surgical — into 20 narrowly focused operating units (OUs).
“The OUs can maintain focus and accountability, execute faster and make quicker decisions, while retaining the advantages of size and scale. … Play small and play big,” Martha said in an October 2020 communique.
Fast forward to today. Mike Hess, VP of corporate technology and innovation, calls Medtronic’s Design for Reliability and Manufacturability (DRM) program a “great example of the play big, play small mindset.”
coaches, part of their project teams.”
Those coaches work with engineers as they advance their individual projects and interact with the corporate DRM team to share information that could be helpful across the wider organization.
Hess describes DRM as “a collection of good engineering practices that when used comprehensively on a project, we believe leads to better quality and better outcomes and better performance, basically, for customers and patients.”
It starts at the very beginning of product development and continues through heavy development, manufacturing transfer and post-transfer.
“The DRM team in corporate, while it’s not a large team by numbers, is a play big activity,” the 32-year Medtronic veteran said in an interview. “We are the ones who are defining the program and the metrics and kind of rolling out all the programming to the whole corporation from the center with some efficiency and some kind of consistency. And then on the play small side, all the operating units have dedicated, embedded resources that work for them [as] their
“Early on, do you understand who your customer is and how they’re gonna use the product and what the product will be exposed to?” Hess said. “And then much later, we talk about manufacturing and transferring it to a factory and do we understand how it’s gonna perform and what equipment we’re going to need, or what techniques or technologies are involved? And do we have all that well characterized and tested? And so all these steps on the process from the idea to getting it to market there’s different questions, different activities you do at various points along the way that are all part of the DRM framework.”
The idea is to increase the odds that the team builds the right product, Hess said, while identifying and addressing technical risks early on, such as NUDs, an acronym that stands for new, unique or difficult technologies.
“We bring in experts from across the company, other businesses, other project teams that have familiarity with these technologies to help give the project team some fresh eyes and some objective assessments. >>
"The DRM team in corporate, while it's not a large team by numbers, is a play big activity. We are the ones who are defining the program and the metrics."
And from all that work, then we put together plans that figure out those risks early … as opposed to letting them percolate through the project, then come back and surprise us later,” Hess said.
Changes in Medtronic’s DRM program
The reorganization is hardly the only change for DRM at Medtronic over the past decades.

“One thing that’s changed which is related to DRM is that the problems that we’re solving have just gotten much more complicated and much more diverse. … So the demands we’re putting on our project teams, as far as what they’re building, have gone up quite a lot because now we’re building on almost every business. We’re building complex systems with multiple pieces that ought to fit and work together, sometimes for quite a long time.”

“A long time ago, we were building much simpler systems that had
a lot less to interact with and a lot less could go wrong,” he continued. “The stakes are higher as we build more complicated systems to tackle bigger healthcare challenges.”


One of the biggest changes for Medtronic’s DRM team is the proliferation of software in medical devices.
“We’ve actually created kind of special DRM frameworks, if you will, for software because it’s different than a lot of our mechanical testing,” Hess said. … “In software, it’s better just to be testing your software constantly as you’re going. We don’t want them to be constantly testing heart valves as they go, because that would be making them all the time and making tiny changes to them.”
He expects continued adjustments to the DRM program to keep up with the growth of AI, machine learning, and algorithms powered by previously unthinkable volumes of data.







 Danielle Kirsh Senior Editor
Danielle Kirsh Senior Editor


Northwestern University researchers said their device could help babies born with heart defects and other patients who need temporary pacing.
Researchers at Northwestern University have developed a dissolving pacemaker with sensors to monitor physiological functions.
The team designed a transient pacemaker — one of the world’s first — last year. The fully implantable, wireless device dissolves in the body after it is no longer needed. The new smart pacemaker features a coordinated network of soft, flexible, wireless, wearable sensors and control units outside of the body that can communicate with each other.
“This marks the first time we have paired soft, wearable electronics with transient electronic platforms,” John Rogers, one of the lead researchers on the project, said in a news release. “This approach could change the way patients receive care providing multimodal, closed-
loop control over essential physiological processes — through a wireless network of sensors and stimulators that operates in a manner inspired by the complex, biological feedback loops that control behaviors in living organisms.”
What makes this device so smart? Algorithms in the smart pacemaker analyze physiological functions, including body temperature, oxygen levels, respiration, muscle tone, physical activity and heart electrical activity, to autonomously detect abnormal cardiac rhythms. The pacemaker can then decide when to pace the heart and at what rate.
All information from the smart pacemaker can be sent to a smartphone or tablet for physicians to remotely monitor patients.

The pacemaker system is designed to eliminate the need for external hardware,

including wires or leads, as it harvests energy from a small, wireless device that softly adheres to a patient’s chest.

The researchers suggest the device could be used in patients who need temporary pacing after cardiac surgery or those who are waiting for a permanent pacemaker.
“For temporary cardiac pacing, the system untethers patients from monitoring and stimulation apparatuses that keep them confined to a hospital setting. Instead, patients could recover in the comfort of their own homes while maintaining the peace of mind that comes with being remotely monitored by their physicians,” said Rogers, who is also a professor of materials science and engineering, biomedical engineering and neurological surgery at Northwestern’s McCormick School of Engineering and Northwestern University Feinberg School
of Medicine. “This also would reduce the cost of health care and free up hospital beds for other patients.”
Designing the dissolvable pacemaker Rogers and the research team designed the pacemaker using four different skin-interfaced devices coordinated with their existing bioresorbable, leadless pacemaker. The soft, flexible device is mounted on the skin and can be gently peeled off after use. The pacemaker naturally dissolves in the body when it is no longer needed.
The system has a body-area network that consists of a battery-free, transient, bioresorbable pacemaker; a cardiac module that sits on the chest to provide power to and control stimulation parameters; a hemodynamics module that sits on the forehead to sense pulse oximetry, tissue oxygenation and vascular tone; a respiratory module at the base of the throat to monitor coughing and respiratory activity; and a multi-haptic feedback module that vibrates and pulses to communicate with a patient.


“We wanted to demonstrate that it’s possible to deploy multiple different types of devices, each performing essential functions in a wirelessly coordinated manner across the body,” Rogers said. “Some are sensing. Some are delivering power. Some are stimulating. Some are



The future of medical device design is in our hands. Here’s what it’ll take to get there.
It's an exciting and challenging time in medical device history. From tracking vital signs to enabling more informed conversations with health professionals, device users can take control of their health in new and powerful ways.



But the rapid acceleration and advancement of data is only just beginning. What lies ahead is a complex web of questions that one person, one team, one company alone can't solve.
For example, how can we further prioritize user experience from the start of the device's development? How can we keep pushing the bounds of wear time? What have we learned from COVID that we can bring forward?
To unlock continued innovation that improves lives, all of us from across the device development process must come together to brainstorm, problem-solve and break down siloes.
It's a big task. Where do we start? As someone who partners with some of the largest medical device companies, I've learned a lot about where we're struggling and what's holding us back. To propel us into the future, here are four areas to focus on first.
1. Create a nimble supply chain
We talk a lot about what we're innovating: What's the device, what's the existing technology, what do we need to accomplish?
To take devices to the next level, we need to talk more about how we're going to innovate. How can we set the entire development ecosystem up for success?
While that can include teams and internal processes, the supply chain is one area that deeply affects the ability to develop devices. The level of medical device regulation poses unique challenges when trying to scale to meet demand. But it's also a competitive area, and we'd benefit from finding ways to not completely eliminate risk, but to properly manage risks. Increasing supply chain nimbleness is a must.
Other highly regulated markets have found success with rapid prototyping. (continued on page 86)
















(continued on page 84)
It could be a way to work within the confines of medical device regulations and quickly understand what works, what doesn't and what we can do about it. Then adjust and tweak before finalizing designs, materials, manufacturing processes and mor.
Adhesives don't typically keep people up at night (though if they do for you, you're in welcome company). Often relegated to being an afterthought in device design, they're seen as a simple material that holds tactical value — sticking one thing to another thing.
But when they aren't thoughtfully designed-in from the start, they have the potential to cause costly and timeconsuming redesigns. Many devices are complex systems, and adhesives are just one, albeit important, layer. With many competing priorities in a limited timeframe, it can be challenging to give every little thing its rightful attention.
How do we reconcile? We can start by identifying the most-often-deprioritized components and understand their role in the entire system. What do they ultimately enable for the design and the end user? Talk with colleagues, mentors and partners inside of and beyond your function to get their perspectives.
From there, your design has the potential to get stronger, and with stronger designs, we're advancing what's possible today.
A real challenge in medtech is handling cybersecurity for novel and legacy devices. It's not a new challenge, but it's taken on a renewed sense of urgency.
Up to now, more passive forms of security measures like shielding films may have sufficed. However, the dialogue needs to evolve to discuss how we can incorporate active measures into devices on the market today and in the future, and how regulatory bodies can validate and defend.
The funny thing about data is that more isn't always better. Similarly, the latest technology isn't always intuitive. So finding the right combination — incorporating the latest tech to get the best data insights while creating a user-friendly experience — is a mix of art and science.
The level and type of data a device needs to collect should inform whether it can be worn on the wrist or elsewhere on the body. Wrist-worn wearables aren't as secure but work well for fitnessrelated metrics, such as tracking calories burned. On the other hand, body-worn wearable patches can adhere directly to skin, creating an intimate connection to the data source. This form factor may be better for tracking medically sound metrics like glucose levels for continuous glucose monitors.
On top of that, if your ideal end user doesn't understand how to use it from when they apply it to when they remove it, they're not likely to want to use it again or recommend it to others.
As expectations of interacting with data, technology and devices
continue to advance, we get to continue problem-solving along with it, testing, validating and fi nding what works, ultimately leaving our mark on the journey toward a healthier world.
Collaborate for the future of medical devices



Together, we can ignite the next wave of device innovation. We can create a reality in which devices are on the cutting edge, providing the insights people need to take control of their health. As a result, people will feel safe, empowered and confident because of them.
Whether you're involved in designing the devices, manufacturing them or beyond, we all have the power to move together and usher in the next generation. How will you help?

Patient-operated medical devices — such as those used in insulin delivery — require a focus on the usability requirements of the less-experienced end user.
User-centered design is critical when developing new medical devices to support distance care. Medtech companies are now specifically designing devices for patient use outside of clinical settings. Digital technologies and ingenious benefits for providers and payers have enabled the shift. However, it is imperative to focus on benefits to end-users in the design process. Device creators must consider environmental factors and design for elderly, infirm or inexperienced users. It may also include issues such as increased battery life, fall detection and other riskmitigating sensors like innovative applications to
trigger emergency responses.

Smart devices have gained traction with apps that track fitness goals and basic statistics, increasing patient acceptance. This trend simplifies the introduction of devices for more complex diagnostics and therapeutic self-care options. These connected technologies can now provide better, safer and more convenient drug delivery mechanisms for patients to receive their meds.
User-centered design methodologies look for confirmatory patterns to identify
design innovations and issues. Incorporate iterative usability testing to optimize opportunities to provide unmet needs.
User studies enable the designer to identify potential challenges for patient end-users. Consider potential user personalities and their specific needs. Keep in mind demographics (including the size of the user and economic variations), psychographics and cultural differences, environments (the device likely will not be in an aseptic environment), and potential or actual comorbidities. Can users have the discretion to blend the device in with the environment or, if wearable, on their attire?
Device designers also need to identify how the device will be packaged and handled before reaching the user. For example, consider temperature and humidity control, shelf life, long-term storage needs and management of biohazardous materials.
The evolution of distance care
Patient-operated healthcare has become known as “distance care,” including wearable and in-home devices for real-time monitoring, pain management and chronic drug delivery. Many of these devices securely collect and transfer data to healthcare providers. They may also incorporate sophisticated embedded intelligence to simplify operator interactions while delivering vital data to remote databanks for analysis and functional optimization. Such features minimize the operational burden on patients with comorbidities that limit cognitive and physical capabilities.
Miniaturization and voice and gesture controls further enhance patient lifestyles. Wearables and portable devices are also being styled to blend with a patient’s attire and often resemble consumer products. In addition, many devices interact with smartphones to leverage processing, photographic and telemetric technology, reducing the capital cost
of distance care. Modern device designs benefit from low-power electronics and dense battery chemistries that provide hours of continuous operation within smaller, lighter configurations.














With the patient-as-operator, device manufacturers must address significant usability requirements that have not been historically relevant with clinicianoperated devices. The breadth of demographic and psychographic variation among patients is massive compared to highly trained healthcare professionals. As such, these devices present a slew of design challenges to overcome interaction limitations. Eyesight, dexterity, strength, cognitive deterioration, and social/cultural/ anthropomorphic variability — all could complicate operability and use safety.
Design teams must consider the entire work/lifecycle for these devices. Drugdelivery combination products may require environmental and physical controls during transport, and the same is true for specimen samples sent by patients. Packaging and environmental specifi cations — shock, vibration, material leaching and climate control — must be well constructed as part of the design brief and ideally should not complicate the end-user burden.
A common scenario in this category is the administration and management of insulin delivery to diabetic patients. Both chronic and critical, it is necessary for drug delivery to be accurate by volume and timely in delivery to meet shelf-life limitations. Real-time blood glucose measurement is necessary to calculate the optimal insulin bolus. Patients previously drew their own blood to test for sugar levels multiple times a day, which could cause multipleuse errors and hazardous outcomes. Continuous blood glucose monitoring is now a stable and mature technology that substantially reduces the workfl ow burden to the patient when coupled with wearable insulin (and glucagon) delivery pumps (artifi cial pancreas). These semi-automated systems greatly improve therapy and outcomes for millions of patients.
There is also a growing need for these devices to ensure appropriate operational compliance and adherence by the patient to support efficacy, safety and reimbursement requirements. Authentication measures are important to document correct practices and require that devices and recording methods interact reliably.
Is the patient using the device as often as they should and in the right way? User identification may also be necessary and is often a large part of reimbursement. In addition, designers can consider utilizing a smartphone, camera or video recording to ensure correct usability. Finally, therapy monitoring with time, data, bolus quality, and/or anatomical placement is important.
A vaccine delivery device may be required by governing authorities to prove correct administration to the patient on a specific date. One method is to utilize a smartphone app that video
records the process and QR codes for batch recognition with a date-stamp. It could be possible to upload the information autonomously for verification and traceability. This may require the device to provide visual markers or electronic sensing to the app for verification. Tamper-resistant packaging ensures the contents are consistent with the QR code database and that the sample collection has not been compromised during transit.
Advancements in device and sensor technology, telemetry, cybersecurity and data processing are transforming healthcare procedural paradigms. Patients as device operators will grow more familiar with and accepting of this technology, propagating higher levels of device and functional capability.
However, usability requirements for self-administered devices are vastly more
challenging than clinical-use devices and require thorough user-centered design methodologies to maximize safety and efficacy. Reliable authentication techniques will be required as critical drug delivery and sample collection become widely adopted.
With fewer future clinicians to care for an ever-aging population, we as patients need to become more involved in our healthcare. AI coupled with massive online biostatistical data will inform these connected patient-operated devices to make optimized and safe real-time decisions with little end-user involvement in the comfort of our homes and as part of our everyday lives.
DDL has over 30 years of experience navigating complex testing standards and regulations for medical device and pharma products. Our reliable quality, responsive attention, and on-schedule completion for packaging, product and materials testing secure confidence in performance and safety while achieving regulatory compliance.


• Packaging and Materials
• Medical Devices
• Combination Products
• CCIT TestingW OFFERING CCIT TESTING
DDL
is
Partner with us and gain a reliable testing expert at your side! Visit us at DDLtesting.com or call us at 1-800-229-4235
Compliance with the EU's MDR often requires multidisciplinary collaboration between a device maker's operations, R&D, regulatory and quality teams, plus partners across the supply chain.
With the Medical Device Regulation in effect, European Union regulatory compliance requires rigorous attention and cross-functional collaboration.

Are you ready for MDR? That question has weighed on medical device manufacturers ever since the European Union proposed stronger device regulations and requirements.


After MDR became law in May 2017, the industry had four years to transition and comply. It’s been more than a year since MDR’s May 26, 2021, application date, yet there still is uncertainty regarding compliance requirements due to the lack of guidance documents and individual interpretation.
One thing is clear. To successfully comply with MDR, it’s crucial to have: quantitative data; strong quality systems; trusted, transparent supply chains; and a cooperative, collaborative organizational culture.
MDR compliance is required to market and sell medical devices in the EU. It applies to all medical devices and their manufacturers regardless of classification, years in business or time on the market.

No devices or companies are “grandfathered.” In other words, manufacturers cannot automatically qualify for MDR certification, even if their devices meet all requirements of the EU’s previous regulatory framework, the Medical Device Directive (MDD). Whereas the MDD was a set of guidelines, the MDR is codified law. Whether a device is brand new to the EU market or sold there for decades, it must pass the MDR muster. MDR compliance is mandatory for a device to bear the CE mark, confi rming the product meets all European health, safety and environmental protection requirements.
The MDR requires the legal manufacturer of a device to provide an extensive technical dossier about every product it seeks to sell in the EU. For many devices, there are heightened requirements for post-market surveillance and documentation than there were under the MDD.
(continued on page 92)






For example, the manufacturer must maintain an updated Summary of Safety and Clinical Performance (SSCP). For Class III and implantable devices, the manufacturer must provide Periodic Safety Update Reports (PSUR).

Generally, if your company’s name or brand appears on the product’s packaging, you are considered the legal manufacturer. The MDR compliance bar has proven so high and daunting for some device makers that they have taken their products off the market, even if their devices sold successfully for many years without issue.
Others have asked trusted contractors to serve as the legal manufacturer for their devices. In some cases, those contractors might be better able to supply and maintain the necessary technical documentation.
The MDR-notified bodies — organizations designated by each EU country to assess device MDR conformity — must have permanent access to all product documentation. It’s a major responsibility with serious repercussions for noncompliance.

Keys to a successful transition MDR compliance requires teamwork, resources and experience in navigating regulatory complexities. As the EU said in its MDR compliance guide, “Before continuing with the focus on the product, one needs to consider the company or companies that will be developing, potentially producing and ultimately commercializing this product. The expertise required by developers of medical devices is significant. In addition to the general design and engineering expertise, which is required of any industrial products company, medical device developers also require risk management, quality management, medical and regulatory compliance expertise.” How true it is.
Generating, compiling and submitting MDR compliance documentation requires collaboration between teams in regulatory affairs, R&D, quality and operations. In addition, suppliers to the legal manufacturer often must provide documentation of their
quality systems, raw materials and processes used to make components. EU notified bodies and auditors are honing MDR compliance and monitoring protocols, so manufacturers must be ready to revise filings and supply additional data as requested.

It can be beneficial to work with partners with knowledge of both MDR and FDA regulatory compliance. Some say the pendulum has swung, and EU compliance is now more difficult to achieve than FDA clearance, whereas it used to be the other way around. It takes knowledge and skill to navigate both frameworks, akin to understanding and playing international rugby and U.S. football. They’re similar games with different rules, but everyone’s in it to win.
As in sports, healthcare regulatory compliance takes a team effort from the start, and executing the plays is a job that’s never finished. Rising to the MDR challenge is an ongoing commitment, just as any patient-centered care and safety priorities should be.



Industry experts offer their perspectives on regulatory and reimbursement issues for artificial intelligence in medtech.
Digital health, artificial intelligence (AI), machine learning and more — these concepts continue to generate buzz in the medtech world.
In September, the FDA published guidance on clinical decision support (CDS) software. It helped to clear up what constitutes a medical device and what doesn’t. Early last year, the agency published a predetermined change control plan (PCCP) to help build a regulatory structure for such technology.
These topics and more spurred intriguing commentary on a panel at AdvaMed’s MedTech Conference in Boston last month. The panel featured viewpoints across all angles of the space.
Dr. Yuri Maricich, CMO and head of development at Pear Therapeutics, offered thoughts from the developer of digital therapeutics. Brendan O’Leary, acting director of the Digital Health Center of Excellence at the FDA, provided the regulatory vantage point.
Cybil Roehrenbeck, a partner at Hogan Lovells, offered thoughts from the reimbursement side. Cassie Scherer, senior director of digital health policy and regulatory strategy at Medtronic, provided an industry perspective. Diane Johnson, senior director, strategic regulatory, MD&D at Johnson & Johnson, moderated the panel.
How the medical device industry is approaching AI Scherer explained that the PCCP allows a manufacturer to go to the FDA on a premarket submission with its AI-based offering. You can provide the FDA with your submission, your product and the specific changes you intend to make down the line at the same time.
According to Scherer, this process allows the FDA to put “guardrails” around ensuring safety. It protects the agency and allows the industry side to put out a product with next-generation
versions following in a quick fashion to benefit patients.
Medtronic went through the PCCP process, which Scherer said was difficult. It’s not for everyone, she said, but it “provides a great opportunity.” She called it a “forward-thinking” effort from the FDA.
Despite progress on the regulatory side from her view, Scherer still sees “unique issues” with AI. That includes ensuring that AI remains representative of intended populations and geographies.
Another potential issue she sees is transparency. Doctors aren’t necessarily trained in reading labeling for AI-based products and digital therapeutics. The labeling framework remains robust, too, she said, and she isn’t sure if there is a way to standardize that because of the desire to ensure that different users’ different needs are reflected.
“I think that some uniqueness around AI is really building the patient trust,” said Scherer. “How do we make sure that we give the information in a way that builds that trust and that also is meaningful and actionable?”
Maricich said the FDA’s progressive decisions may help expand even further to allow for more patient-centered opportunities.
The COVID-19 pandemic hammered home that point, he said. We saw the ability of patients to do everything digitally, because “that’s how they do just about everything else in their life” right now.
“The challenge for us as the healthcare delivery sector is for manufacturers, for regulators, for payers to catch up,” Maricich said. “Do what patients are already expecting and integrate this very much into their part of treatment. Part of the engagement areas with patients is making sure that we are thinking about patient populations very broadly.” >>

A trend that Maricich has noticed centers around the need for evidence. Given that different types of organizations and individuals are entering the digital health space — which he thinks is a good thing — there’s more diversity in the people trying to solve problems. However, challenges persist around because people who haven’t been in healthcare suddenly have to solve problems they don’t understand.
Maricich said it’s not enough to say something in healthcare — you must show something, too.
He said developers can’t just believe their assertions and evidence. Real-world evidence with connected devices can provide important insights. Maricich believes that, even with some difficulties in recruiting underserved and minority populations into clinical trials, the different evidence is “most important to make sure we are able to answer the questions most appropriately.”
“I’m excited to see one of the key trends in combining these different types of datasets to answer those appropriate questions,” Maricich said. “But, I’m also excited to answer them much faster.”
Regulating medtech AI: ‘I think international harmonization is key.’ Another intriguing part of the ongoing digital health adoption is that it extends around the world. Johnson broached the question to O’Leary: What’s going on globally related to digital health?
2020. He said a lot of software developers came to the agency claiming they had the solution to solve the testing shortage: software that can diagnose SARS-CoV-2. Better yet, the developers believed their product wouldn’t need regulation because of the CDS draft guidance.

“That was a really scary thing to see, frankly,” O’Leary said. “Because software for diagnosing SARS-CoV-2 is a diagnostic device that FDA regulates.”
The agency wants to make sure it takes a careful approach, O’Leary said. It must be one that doesn’t lead to public health outcomes that the FDA doesn’t want to see. That continues to apply to AI and digital health outside of COVID-19, too.



According to Roehrenbeck, reimbursement for AI services comes much closer to what medical service reimbursement would be. She said that represents a real consideration for businesses pursuing certain codes. Right now, the HHS Office of Civil Rights put out a proposed rule to expand on non-discrimination provisions in the Affordable Care Act.
The rule would include a new section on clinical algorithms. Roehrenbeck says questions remain over where the liability rests for AI.
Cassie SchererO’Leary said he’s seen comments on clinical decision support and final guidance around the subject. That provides an opportunity to improve the agency’s approach to AI and digital health and make it that little bit easier.
The FDA has engaged in multilateral efforts through machine learning practices and guiding principles shared with Health Canada and the Medicines and Healthcare products Regulatory Agency (MHRA). He called it an important step forward and something that offers momentum.
“I think international harmonization is key,” O’Leary said. “You can’t have disjointed approaches to these technologies across jurisdictions.”
Implementation challenges remain from a regulatory perspective, O’Leary said. That never came in clearer than when COVID-19 came around.
O’Leary spent five months directly supporting the FDA’s COVID-19 response in
“It potentially puts the onus on providers, folks who are going to adopt these tools and use them for their patients, to ensure that there is no discriminatory effect based on the use of a clinical algorithm,” said Roehrenbeck. “There are not a lot of guardrails around how this is being proposed.”
As HHS continues its development, Roehrenbeck said she thinks the FDA could provide clear pathways so people can understand these tools.
“Not all AI is great,” she said, adding that one colleague uses the term “glamour AI” to describe the kind of AI that offers information but doesn’t provide any help.
Meanwhile, other AI can completely transform a patient’s experience and save them years of their life.
“As we try to understand what has value and what doesn’t, more standardization and contextualization guardrails would be helpful in that regard,” Roehrenbeck said. “Especially, I think, for clinicians who ultimately are, again, tasked with making sure that these are appropriate for their patients.”



Can you get intellectual property (IP) for artifi cial intelligence (AI)? Absolutely. Should you fi le a patent application? Maybe, but there are alternatives. Should you consider data options? Yes. Is there a standard strategy for AI? Not if you want to see value from your IP.
The healthtech industry is investing heavily in software engineering and data science as it increasingly develops

decision support, medical telemetry, surgical navigation and countless other medical software applications. The software investments naturally raise questions on how best to protect against copycats. It is certainly wise to obtain IP for your AI and software to protect market position or set partnership terms. But while protecting other technologies may be relatively straightforward, protecting AI work can be complex. For companies that leverage IP to advance business interests — rather than getting patents as trophies — a detailed discussion with counsel of the ideas, the competitive market, the business goals and product design is key to obtaining defensible and valued protection for AI.
When establishing IP strategy for a healthtech company or product, it's important to consider AI and software alongside other technologies and products. The work is protectable: Despite recent uncertainty about patentability of software, in both Europe and the U.S., leading data processing cases held healthtech software (e.g., cardiac signal processing) to be patent-eligible.

In addition, the IP is valuable. In summer 2022, after prevailing on validity and infringement of its AI-related patents at trial, a healthtech company asked the U.S. International Trade Commission to block import of Apple Watches. Multiple
examples of successful trade secret enforcements for medical data and software exist. Nowhere is the phrase "data is the new currency" truer than with high-quality, anonymized data sets that healthtech companies gather and leverage in product design. Particularly when a product's hardware matches a prior release or is largely conventional, or runs on someone else's hardware, obtaining IP for the AI, software and data can be crucial for your company.

Against this backdrop of high value and concrete protection, though, companies face challenges. Some AI systems risk labeling as unpatentable mere automations of pre-existing manual processes. Given that many AI techniques leveraged today (including "deep" learning) are decades old, there may be related prior work. For back-end functionality, detecting infringement can be tricky. And in some cases, specific AI techniques are short-lived and soon replaced by improved versions.
These hurdles should not discourage you from protecting AI, but instead serve as helpful guides toward value and away from waste. IP counsel with deep familiarity here will analyze these factors together with product details and the business' objectives. They will build a tailored IP strategy,

With healthtech software investment booming, companies are seeking guidance on when and how to protect that work.Andrew (A.J.) Tibbetts Greenberg Traurig Photo courtesy of Adobe Stock
which may lead away from patents and toward other IP, or trigger in-depth discussions of aligning a patent with value or supplementing with other IP.

Often, the focus may not be on the AI itself. Companies' first thoughts are often of a specific model they trained, but the exact model may be relatively short-lived. There will be retraining and structural improvements. While your team's chosen model performs well, alternatives may be available, and detecting whether a competitor is using your model or another may be difficult. For certain high-value models, trade secrets may be appropriate, but the IP conversation may focus more on other aspects of the product workflow that interact with the AI.
AI outputs typically drive downstream processing, leading to diagnostic output, control system change, a recommendation to a user, etc. That workflow is fruitful ground for AI IP. Though models evolve, AI-driven workflows may persist across product iterations. They may be more visible to customers and drive sales, and it may be possible to detect usage by competitors — both of which can drive patent value. Data rights and licensing for those AI outputs also present high value, particularly in context with corresponding inputs. The input processes may be ripe for IP, such as data curation and preprocessing of raw data into informative features. These input-output contexts may provide important support for patent protection. While diagnostic methods can be difficult to protect, improved analyses that rely on different features or generate different outputs are patentable. It also may be possible to consider trade secret protection where features or processing are not visible. Data rights for access to the input data can be priceless, particularly for training data obtained from partners. Such non-patent IP may be important where the AI is exactly mimicking a pre-existing manual process.
Despite the growth and importance of AI and software in healthtech, there is still uncertainty about protecting investments. Given the technical and legal complexities, defensible and valuable IP protection rests on experienced counsel tailoring the IP strategy through a deep understanding of the product and business.



For over 75 years, MICRO has continually expanded our capabilities in order to provide our customers with the finest in full service Medical Device contract manufacturing.



The latest addition to our global footprint is a newly facilitated 32,000 sq. ft. location in Costa Rica featuring clean room assembly and light manufacturing. This recently added piece of the puzzle will continue to allow us to fulfill our Vision:


Medical device change control regulations have


a lot of boxes to check.
Given the dynamic nature of medical device development, changes are inevitable.
Change control focuses on managing changes throughout a product’s life cycle as a part of current good manufacturing practices (cGMP). Extensive regulations ensure that changes don’t put the safety, reliability and performance of products at risk. Therefore, it’s critical that medical device manufacturers adhere to all of the regulatory change control guidelines.
This article provides four tips for effectively managing change control to remain compliant with cGMP regulations.
are coming
The FDA is close to aligning the current cGMP requirements of its Quality System Regulation (QSR) 21 CFR Part 820 with the International Organization for Standardization’s ISO 13485:2016. Once the ruling is finalized, the regulation will be called the Quality Management System Regulation (QMSR) and the techniques used to inspect medical device companies will change.
One of the primary differences is that QMSR will adopt ISO’s greater emphasis on risk management activities and risk-based decision-making. While the current Part 820 explicitly addresses risk management activities in its section on risk analysis within design validation, the FDA said it expects manufacturers to “integrate risk management activities throughout their QMS and across the total product life cycle.”
Stay in control to remain compliant Change is common with life sciences products, but companies can ensure compliance only when making changes in a controlled manner.
Regardless of who is involved in the process, all discussions, meeting minutes, emails, whiteboard notes and the like associated with the change must be included in the change documentation.
For example, all change processes in production must be documented at the time of performance. Then they are reviewed and approved by the quality control unit and other relevant stakeholders. Having all documents, supporting data and approvals in place during the changes and reviews constitutes a controlled change.

An uncontrolled change refers to modifications made without the review and approval of the quality control unit and other departments affected by the change, which can result in a compliance violation. Uncontrolled changes to any medical device or production processes could impact the quality of a product, jeopardizing the compliance status of the product and the manufacturer.
Data management is essential
Change control procedures should include sufficient supporting data to demonstrate that the revised process or change in materials will result in the product retaining the level of quality that is consistent with the approved specifications.
It is difficult and time-consuming to find and retrieve necessary data to support a change and update the documentation when the data is on paper in a filing
Proper change control is essential to ensure the quality and safety of medical devices. Photo courtesy of MasterControl.
cabinet. And the proposed change can be delayed by an outdated record or one that does not include its revision history.
Changes typically impact various departments and staff. If other quality areas, such as training, CAPA, supplier management and audits, are not integrated and immediately accessible, the organization will experience miscommunication and possibly a change control compliance violation.
Keep regulators in the loop
According to 21 CFR 807, changes may require submitting a new 510(k). The FDA’s “Deciding When to Submit a 510(k) for a Change to an Existing Device” guidance identifies the benchmark for this requirement as when modifications significantly affect the safety or effectiveness of the device.
Effective change control is imperative for companies developing regulated products, and they’re obligated to keep regulators informed of any changes requiring change control procedures. The FDA urges manufacturers to reach out to the agency during the early stages of the change process to eliminate guesswork and duplication of efforts.
When initiating this interaction, ensuring that all data and documentation are up to date, complete and immediately accessible makes this process easier and faster. This gives regulators more confidence in your ability to develop safe and effective products.






Neuroscientist Sumner Norman and AE Studio develop open-source and free tools for the braincomputer interface (BCI) space.
BCI technology has become one of the hottest areas of medtech. Companies are developing a multitude of methods with their own systems that would allow patients to control a computer with their brain. Such technology could enable immobile people to control a mouse cursor, keyboard, mobile device/tablet, wheelchair or prosthetic device by only thinking.


“My goal is to give abilities back to those that have lost them, and eventually, to improve how all of us interact with technology and each other — the ultimate humanmachine interface,” Norman told Medical Design & Outsourcing. “And what’s more human than our brain, the organ that contains our every memory, thought and intention?
Norman’s background includes designing haptic interfaces for teleoperated robotics and exoskeleton robotics for assisting in motor recovery following neurological injuries like stroke. During his Ph.D. studies, he used BCIs to teach patients to create brain states through a process called neurofeedback. He said the method produced strong results but is limited in terms of efficacy and cost/availability.

However, his experiences with BCIs led him to seek ways to build better ones. That includes improvements to hardware, software and protocols, he said. Norman spent the past five years developing ultrasound-based hardware for reading brain signals. This method is now moving into human use, he said.
“At AE studio, we are pushing the boundaries of BCI software,” said Norman. “We develop open-source and free tools to lower the barriers of entry to contribute to neurotechnology and maximally accelerate researchers from all backgrounds. We also work with industry leaders in BCI hardware manufacturing to unlock every bit of performance we can.”

Norman considers BCIs to be “a pretty simple concept at their core.”
They require a method of sensing biophysical effects that take place when brain activity changes. Using an electrode, you may sense the voltage potential created by neurons as they “spike.” Behavior, or attempted behavior like the movement of a person’s hand, must be measured. Machine learning methods then find the correlation between brain patterns and behavior.
(continued on page 102)









(continued from page 100)
“Once this ‘decoder’ is good enough, we can measure the brain state to infer the intended behavior without measuring behavior directly,” said Norman. “We then turn that intended behavior into commands. … Over time, the user learns and adapts to control the BCI more directly.”
BCIs represent “the final frontier” in human-machine interaction, Norman said. With a significant portion of the world already spending large chunks of their lives connected to the internet, it’s now a matter of “waking up to the possibilities.”
Advances in machine learning, cloud architecture and edge computing power mean BCIs are possible. They’re no longer science fiction — they’re just science.
“Much like the internet, they have the potential to completely change the way we live, work and interact,” said Norman. “And, yet, we’re just scratching the surface of what’s possible today. That’s exciting.”
Some big hitters have put their resources behind developing BCI technology.

That includes Elon Musk’s Neuralink and Meta’s Reality Labs. However, a number of companies without the backing of a billionaire like Musk or a Mark Zuckerberg have paved the way themselves.
Testing of Blackrock Neurotech’s technology has been ongoing in human patients for nearly 20 years. In 2021, Blackrock received FDA breakthrough device designation for its MoveAgain BCI system. It provides immobile patients with the ability to control a range of devices by only thinking. Blackrock is one example of companies collaborating with AE to advance BCI technology.
Meanwhile, Synchron develops the catheter-delivered Stentrode brain-computer interface (BCI) implant. The company believes they’re the only BCI company tapping into blood vessels to capture signals from the brain. Synchron has trials for its technology ongoing at multiple locations, with human implants already performed.

Neuralink and Reality Labs “capture headlines, sure, but groups like Paradromics, Synchron and Blackrock Neurotech, are arguably better poised to capture the first generation of BCI users,” said Norman. “While Neuralink is still pushing for their first in-human tests, Blackrock devices have been implanted in nearly 40 humans already, and Synchron is in the thick of their first clinical trial.”
Still, Norman says the first generation of BCI users will be restricted to those with severe forms of neurological injury and disease. That’s because BCI bandwidth is too slow or costs are too high, he said. However, that’s changing.
“The real winners will be those that create the BCI hardware and software that justifies use by people with mild to moderate neurological injury and disease and/or psychiatric and cognitive disorders, and eventually, enables use for all people,” said Norman. “Most groups making strides in building the next generation of BCI are not making headlines — yet.”
What is AE Studio, and how is it contributing to BCI development? Founded in 2016, AE’s goal is to increase human agency with technology. It aims to develop a BCI operating system for maximally increasing human agency. Norman said the company wants to avoid a future in which BCI’s “crowning achievement” is increased consumer spending.
AE helps startups and enterprise clients develop their software with data science and software development consulting. Some revenues are funneled toward developing BCI software, Norman said.
For BCI software, AE builds models that Norman said are “robust, more efficiently calibrated, more easily scaled and more easily deployed.” Data in the BCI field are complex, he said. Experts estimate that approximately 86 billion neurons exist in the brain, with each neuron connected to around 1,000 nearby neurons.
Today’s BCIs interface with “just” hundreds of thousands of neurons, Norman said. However, those neurons can drift in and out of view of an electrode, forcing the BCI out of calibration. He explained that most BCI users must recalibrate every few hours, One of AE’s projects stabilizes decoders over long periods of time for less time calibrating, which includes a tedious exercise of various instructed behaviors.
Norman said the true potential of a platform technology exists in the user’s data. However, one of AE’s areas of focus centers around protecting privacy in this sense.
“At AE, we are building tools today, while user numbers are small, to protect all users as their numbers grow,” said Norman. “As one example, we are currently building a tool that allows us to train sophisticated machine-learning models that benefit from many users while never requiring the data to leave the user’s device. When privacy enables performance, everybody wins.”
The future of BCIs
Norman said the fi rst generation of commercial BCIs will focus on motor intention. An example of a fi rst step would be replacing a computer mouse with a BCI-controlled cursor. Still, questions remain about bringing BCI control to replace something like a keyboard due to diffi culties replicating or improving upon the speed of ten fi ngers on a keyboard.
“Silent speech” is the holy grail in BCI, Norman said, because speech remains the fastest method of communication between humans. There are still plenty of hurdles to clear before silent speech can be integrated into a commercial BCI.
Another class of BCIs relies on the ability to “write” information into the brain instead of reading it out. If BCIs extend there, Norman said, cochlear implants for the deaf represent “likely the most impactful BCI modality” to date. Thus, restoring vision to the blind offers another space into which BCI could move.

Norman explained that, as BCI hardware scales to interact with the brain, so too will their application space.
Studio Chief Neuroscientist Sumner Norman“The coming generation of BCIs will be all-purpose, vastly improving their value proposition,” said Norman. “And if enough people have BCIs, we can test many more approaches that we’ve yet to even imagine.”
Software testing is not optional.
Let me repeat: software testing is not optional!
Software defects are one of the most common reasons for clinical or consumer medical device recalls, and serious issues stemming from software errors abound. A 2020 study by the Australian government identified a rash of problems, such as errors related to resilience and reliability, including software that crashed, froze or functioned only intermittently — or failed to respond at all.
How do software errors present themselves in the real world? Here’s just one example of software gone wrong. In 2016, SoftwareOne’s clinical software — used widely by practitioners in the U.K.’s National Health Service — had been miscalculating patients’ risk of heart attack, errors that had gone undetected since 2009. As a result, at least 300,000 heart patients over that seven-year period were given the wrong drug or medical advice.


The consequences of the software defect were tragic. Many patients who had erroneously been told they were low risk suffered heart attacks or strokes. Many others endured the side effects of heart medication they didn’t actually need.
That’s why every software application must undergo meticulous testing before it is released to users. Testing improves the quality of the product, as well as increases user satisfaction, verifies the system meets requirements, promotes security, identifies preventable errors, and mitigates costs by identifying defects early when they are less expensive to fix.

Yes, testing takes time, but the benefits far outweigh any stress to the schedule.
The primary purpose of software testing is to provide stakeholders with accurate, timely and useful information on the state of the application under test.
Testing can:
• Determine whether requirements have been met, including performance requirements
• Identify areas of weakness (high concentration of defects)
• Help prevent defects, not just find them
• Provide confidence in the system
• Establish the degree of system quality
• Confirm the system is usable, operable, secure and ready to deploy >>
Meticulous software testing can save the lives of medical device users.
Manual testing is the most hands-on type of testing. There are two varieties: White Box testing and Black Box testing.





White Box testing tests the system’s underlying structure, architecture and code to validate input-output flow, which enhances design, usability and security.

Black Box testing approaches testing from an end-user (external) perspective. It involves testing against the specifications and requirements, focusing on what the application is supposed to do.





Though manual testing is employed by every development team at some level, the major drawback is that it is tough to scale in today’s fast-paced development life cycle.















Test automation uses automation tools to maintain test data, execute tests and analyze test results without human involvement to improve software quality. It can be used for:


• Unit testing, where a single unit of the application is tested in isolation
• Integration testing, which verifies how the modules communicate and behave together
• Smoke testing, used to evaluate the stability of the system build
• Regression testing, used to determine if recent code changes have affected any existing functionality of the code
Continuous testing is the process of executing automated tests as part of the software delivery process in order to obtain feedback on the risks associated with a software release as rapidly as possible. This approach applies the principles of automated testing in a scaled, continuous manner to achieve the most reliable test coverage for an enterprise.
Verification
Software verification testing is the process in which the software is evaluated in the development phase to determine whether it meets the requirements and design specifications. You are
verifying all the aspects of the software, including unexpected conditions. If an error is found, the development team has an opportunity to correct it before the product reaches the end users. In essence, verification testing determines whether you’re building the product right. Software validation testing, on the other hand, determines whether you’re building the right product. Validation testing evaluates the product or system at the end of the development process to determine whether it meets end-users’ expectations and ensure the system actually meets their requirements.
While it may be tempting to skip or skimp on software testing in an attempt to accelerate feature development and product release, the potential harm to software quality and user satisfaction — even user safety — is just not worth the risk. Careful testing improves consistency, performance and customer satisfaction and is therefore fundamental to the software development process.






A massive verdict in an ethylene oxide lawsuit demonstrates the high stakes of medical device sterilization safety while regulators and industry seek solutions.
The FDA and medtech industry are continuing efforts to make medical device sterilization safer after the EPA identifi ed 23 U.S. facilities where ethylene oxide (EtO) presents a risk to communities.
The FDA said it is similarly concerned about unsafe EtO emissions and highlighted work with medical device manufacturers and sterilizers to reduce EtO usage and develop new
EtO is used on about 20 billion medical devices each year — or about half of all sterile medical devices — and in some cases, it’s the only option. The gas is the most commonly used sterilization method thanks to its ability to permeate packaging at relatively low temperatures and kill bacteria and viruses that could
cause life-threatening infections.
The FDA is seeking safer ways to use EtO and alternative means of sterilization to reduce EtO emissions due to its cancer risk, particularly blood cancers and breast cancer.

“Exposure over the course of a lifetime (24 hours a day for 70 years) to EtO at concentrations expected to be found near some commercial sterilizers can increase a person’s risk of developing cancer,” the EPA warns.
The FDA’s sterilization innovation challenges are making “encouraging progress,” FDA said, with some facilities cutting EtO emissions by an estimated 20% to 35%.


“In general, manufacturers are targeting an ethylene oxide cycle concentration that is 11–66% less than the typical ethylene oxide concentration range,” the FDA said.
Manufacturers and sterilization companies that have worked with the FDA on reduction efforts include Abbott, Andersen Scientific, BD, DMB Apparatebau, Medtronic, Sterigenics, Steris and Taiwan Advanced Sterilization Technologies. (continued on page 108)









(continued from page 106)
Alternative sterilization methods under consideration for certain types of medical devices include vaporized hydrogen peroxide, supercritical carbon dioxide and nitrogen dioxide. The FDA selected NovaSterilis, Noxilizer, Steris and Stryker’s TSO3 from 24 innovation challenge applicants to work with the agency on these efforts.

Some device manufacturers are working with contract sterilizers to validate new or different methods as well as the feasibility for scaling up, FDA said.
Another EtO reduction effort is the FDA’s EtO Sterilization Master File Pilot Program, which lets premarket approval (PMA) holders of high-risk devices change sterilization processes and facilities.
The pilot includes 11 sites and 28 Class III devices (those that “sustain or support
analysts completed risk assessments for American communities near the nation’s approximately 100 commercial sterilizers.
The agency flagged 23 U.S. cities where commercial sterilizers using EtO contribute to an elevated cancer risk for residents of surrounding communities. The EPA said EtO facilities in those cities are contributing to at least one additional cancer case per 10,000 people exposed. Operators of those facilities include device manufacturers such as Medtronic and Edwards Lifesciences.
The Steri-Tech facility in Salinas, Puerto Rico, appears to have the greatest increase in cancer risk of all the facilities at
Sterigenics sterilization facility’s emissions of EtO for a woman’s breast cancer and her son’s non-Hodgkin’s lymphoma.
The woman who won the verdict against Sterigenics, Susan Kamuda, said during the five-week trial that her family had no history of cancer and didn’t know the facility was releasing toxic emissions.
It was the first verdict in hundreds of lawsuits against Sterigenics, which permanently closed the Willowbrook, Illinois facility in 2019 after state officials halted operations due to high emissions of EtO.
The verdict included $38 million in compensatory damages and $325 million in punitive damages. The Cook County jury decided Sterigenics should pay $220 million, parent company Sotera Health should pay $100 million and Griffith Foods — which built the plant — should pay $5 million.

life, are implanted, or present potential unreasonable risk of illness or injury”), the FDA said. Medtech organizations have also expressed interest in the FDA’s new 510(k) master file pilot program.
“While signs of innovation are promising, other methods of sterilization cannot currently replace the use of EtO for many devices,” the FDA said. “To that end, we are equally concerned about the potential impact of shortages of sterilized medical devices that would result from disruptions in commercial sterilizer facility operations. Our supply chain program is ready to work with industry to help prevent and mitigate potential shortages due to reduced supply of certain ethylene oxide sterilized medical devices.”
The FDA’s most recent report of an EtO facility closure was the June 2021 closure of Steril Milano facilities in Italy. The FDA said that the company falsified records for years, leading to voluntary device recalls by at least 10 medtech firms.
New rule coming from the EPA
The EPA is updating the Clean Air Act’s National Emission Standards for Hazardous Air Pollutants (NESHAP) to reflect EtO’s risk and mitigating technologies, with a proposed rule anticipated to be released this year. As part of that effort, EPA scientists and
60 additional cases per 10,000 people.
The EPA risk information is current as of July 27, based on the agency’s latest modeling of EtO emissions from the facilities.
“We are continuing to collect and verify information about these facilities and its emissions,” the EPA said. “If there are updates to EtO emissions or the systems in place at these facilities, there may be resulting updates to the risk information.”
Workers in the facilities and neighbors who live closest are at highest risk, and the CDC says EtO’s dangers may go beyond cancer.
AdvaMed CEO and President Scott Whitaker agreed with the EPA on the need for further research to understand background levels of EtO outdoors, safe levels for health, and ways to make EtO safer while balancing the needs of patients and medical device manufacturers.
“It is critical that the EPA get this right,” Whitaker said. “Too much is at stake, as the FDA notes: this is a ‘vital process for helping to prevent serious infections’ and ‘other methods of sterilization cannot currently replace the use of EtO for many [medical] devices.’”
In September, an Illinois jury awarded $363 million in a lawsuit that blamed a
“We do not believe the jury verdict in this matter reflects the evidence presented in court,” Sterigenics said in a statement to Medical Design & Outsourcing. “Sterigenics is evaluating the verdict and plans to challenge this decision through all appropriate processes, including appeals. We will continue to vigorously defend against allegations about our ethylene oxide operations and emissions. We remain committed to our mission of safeguarding global health. As we have consistently done throughout our history, we will continue to operate in compliance with applicable rules and regulations to ensure the safety of our employees, the communities in which we operate and patients around the world.”
After the verdict, Needham analysts downgraded the stock of Sterigenics competitor Steris. The analysts said they expect attorneys to look for potential lawsuits in communities near EtO facilities, though they believe Steris can manage any EtO legal liabilities due to the company’s size, diversification and balance sheet.
Steris leaders have said their Applied Sterilization Technologies (AST) unit has been cautious on EtO emissions, an assertion the analysts said was supported by the EPA’s data. But they worry the data does not reflect past practices, and there’s a risk that Steris could be found liable for EtO exposure in past decades.
“Since breast and blood cancers are relatively common, we worry that attorneys could have success in finding additional people willing to file more lawsuits,” the analysts wrote.
"While signs of innovation are promising, other methods of sterilization cannot currently replace the use of EtO for many devices."










































It’s a noncontact, nonthermal and nontraumatic hemostatic powder sprayed through a catheter. Here’s how Medtronic created Nexpowder.
There are plenty of ways to treat gastrointestinal (GI) bleeding.
Cauterizing wounds — treating with heat — is one, while clipping the bleed to stop it is another.
However, Medtronic now offers a new way that just might trump them all, said Dr. Austin Chiang, chief medical officer of Medtronic's Gastrointestinal business. The company believes its newly FDA-cleared Nexpowder endoscopic hemostasis system is the future of GI bleeding treatment.

“It’s really innovative,” Chiang said in an interview. “It addresses a lot of the shortcomings of existing technology out there.”
What is Medtronic’s Nexpowder?
Nexpowder is a noncontact, nonthermal and nontraumatic hemostatic powder sprayed through a catheter into the GI tract. It features a proprietary powdercoating technology. Medtronic designed it to minimize catheter clogging and particle scattering without requiring carbon dioxide or air compressors.


Chiang said the spray-on powder differentiates from other offerings on the market because it reacts to moisture, not just blood. It turns into a gel that’s adhesive, sticking to the bleeding area and allowing it to heal. This reaction to moisture also means it can be used in other applications.
“With existing systems out there, sometimes these powders get in the way to clog up the machine and clog up the device or

inhibit your visualization,” Chiang said. “Our device actually is a very targeted type of powder. It’s easy to control and use and it doesn’t clog up the system.”
According to Medtronic, Nexpowder achieves a 94% immediate hemostasis rate. It comes in at two times the adhesive force of other hemostatic sprays, the company said, and it contributes to a mere 3.7% rebleeding rate.

The device has a handle attached to a catheter that is fed down a scope channel. That channel leads down to, for example, an ulcer or bleeding lesion in the stomach.
Chiang then said the person performing the endoscopy directly turns on the spray handle for the device and applies the powder. It delivers the powder in what he described as a motorized fashion, through the catheter onto the target lesion. (continued on page 112)
Sean Whooley Associate EditorMedtronic’s Nexpowder device delivers hemostatic powder through a catheter to stop gastrointestinal bleeding . Photo courtesy of Medtronic

(continued from page 110)
The targeted delivery factor distinguishes Nexpowder from the rest of the market, Chiang said. He said that, historically, a problem has been a “scattered cloud” of powder, rather than direct delivery to the target. The reaction to moisture represents another advantage, he said.

The system features a blue gel to provide confidence that a lesion is fully covered. Additionally, the gel remains blue to simplify follow-up endoscopy.











Altogether, Chiang said the visualization aspect of Medtronic’s technology is another step forward.
“The visualization, because it’s targeted, allows us to actually see what we’re doing,” Chiang explained. ”You can imagine if it’s just a big cloud of powder, you can’t really see through that. It’s actually something that we’ve run into in the past with other systems.”
Adding to the GI portfolio Nexpowder rounds out the Medtronic gastrointestinal portfolio, which features
Nexpowder becomes an adhesive hydrogel upon contact with moisture. Illustrations courtesy of Medtronic



the GI Genius for detecting colorectal polyps through enhanced visualization during colonoscopy.






The portfolio stretches across the stages of GI treatment, Chiang said, with Nexpowder fitting in as something of a final puzzle piece.
“I think [Nexpowder is] important,” Chiang said. “Our vision is really to cover the entire care continuum, and that means detection, resection and also protection. So, if we think about our portfolio, we have GI Genius, which is the kind of fi rst-to-market AI polyp detection device that kind of serves as the detection piece of it. Then, resection is for bigger polyps that may need to be removed. We have tools that are also going to be commercialized very soon that are cleared by the FDA. And then, of course, this is the protection piece of it.




“We want to make sure that we see all of our patients through this entire spectrum.”
Medtronic’s Nexpowder is composed of succinic anhydride and oxidized dextran. Photo courtesy of Medtronic
Innovative solutions engineered to your specific requirements. Working together to create quality medical products that enhance lives.






Acutus Medical’s





AcQBlate Force sensing ablation catheter is one of the cardiac ablation devices on the market today.



Photo courtesy of Acutus Medical
Kirsh Senior EditorCardiac ablation is when a physician intentionally destroys a small amount of tissue in the heart to treat and prevent heart rhythm problems. The procedure creates therapeutic scars in the heart to block the irregular electrical signals that cause an uneven heartbeat.
To perform cardiac ablation, a physician inserts a thin, flexible catheter through veins or arteries into the heart. Sensors on the tip of the catheter detect the electrical signals inside the heart, which allows a cardiologist to pinpoint which area of the heart requires ablation.

Ablation devices mostly use heat (radiofrequency energy), extreme cold (cryoablation) or nonthermal pulsed-field energy to create small scars on the heart and block irregular heart rhythms. Cardiac ablation devices are on the market from prominent medtech players including Medtronic, Boston Scientific, Abbott, Atricure, Acutus Medical and others.
Cardiac electrophysiologist Dr. Steven Mickelsen — who was the founder of pulsedfield ablation device maker Farapulse, now a part of Boston Scientific, and more recently chief translational science officer at Acutus Medical — told Medical Design & Outsourcing about the main challenges in creating cardiac ablation devices.
One half of the challenge in creating cardiac ablation devices is understanding the heart condition that needs treatment. Cardiologists have to determine how deep within the tissue lies the underlying problem, and how they need to produce scar tissue to fix it.
(continued on page 116)
It’s about figuring out how and where to go with the cardiac ablation and then engineering the best catheterbased delivery device.Danielle










(continued from page 114)
For example, a surgeon may create linear scars to isolate sections of the heart electronically for atrial fibrillation ablation, Mickelsen said. The challenge is creating a set of point-by-point scars that collectively all coalesce into a fence around the tissue, or alternatively, a very deep penetration into the ventricular wall.



“Sometimes those understandings of how and where to go and the diagnostic part of it is not well understood when designing these tools. The sciences are evolving as we go,” Mickelsen said.
Once in the highly constrained area of the heart, the device has to be able to transmit mechanical forces from the handle to the tip of the catheter while dealing with the challenge of visualization.

“People think that anytime you’re in the heart, it’s really a heart surgery and that it’s very dangerous and very complex — and in many ways they’re right,” Mickelsen said. “But it’s definitely turned into a simpler problem with technology that’s available in this day and age.”

Once there is a full understanding of the diagnostic need, engineers can begin to make a physical tool that will move within the body. Therein lies the second half of the challenge.

“From an engineering point of view, there are many mechanical challenges of overcoming variances in anatomy and trying to understand the different ways that catheters have to move in order to get the tip of the catheter to the target,” Mickelsen said.

To begin with, the catheter and devices used in cardiac ablation procedures have to be small enough to navigate through small-bore IVs in the leg or via percutaneous access.
The vascular system’s tortuous anatomy presents more obstacles as the catheter makes its way to the heart. To be successful, engineers must understand the different ways that catheters need to move within the body to get the device’s tip to the targeted tissue inside the heart — all while being mechanically driven by a surgeon’s hand outside the patient’s body.

During the procedure, the catheter maneuvers through the heart’s complex structure, where there are several fulcrum points, and goes out through a vein.

 Dr. Steven Mickelsen
Dr. Steven Mickelsen
"From an engineering point of view, there are many mechanical challenges of overcoming variances in anatomy and trying to understand the different ways that catheters have to move in order to get the tip of the catheter to the target."Scripps Memorial Hospital cardiac electrophysiologist
Vice President of the International Ophthalmology Council and the first African to serve as a Trustee Board Member of Ophthalmology, at the American Academy of Ophthalmology.

Extend your Medical business trip to South Africa and unearth the latest Medical trends at the International Conference on Medical Regulation in Johannesburg. Learn more about the medical industry in South Africa, while experiencing our flavourful cuisine, breath-taking sights, and wonderful sense of togetherness. Exceptional for Medical events. Phenomenal for you.
Arrive Focused. Leave Inspired. Find out more at: sancb.southafrica.net















Medtech leaders discuss how to get the most out of a burgeoning healthcare ecosystem.
 SEAN WHOOLEY ASSOCIATE EDITOR
SEAN WHOOLEY ASSOCIATE EDITOR

The word “ecosystem” is used plenty in the tech world. Now it’s coming into the medtech space.







So, what is an ecosystem? How can medtech companies take advantage of one to provide better care and improved patient outcomes? How can they do so with speed in an ever-changing environment? Three major medtech leaders joined McKinsey & Company Senior Partner Rajesh Parekh at AdvaMed’s The MedTech Conference in Boston to try and explain that.
Medtronic Chair and CEO Geoff Martha, Abbott EVP of Medical Devices Lisa Earnhardt and ResMed CEO Mick Farrell offered their views on ecosystems in the space.


What is an ecosystem in healthcare? Earnhardt said an ecosystem brings a variety of stakeholders together to drive value in the space. That amounts to value for the patient and the customer.
A 25-year veteran of the medtech space, Earnhardt said a traditional viewpoint centers around being a medtech company, having a product and delivering that to a hospital or provider. That two-way relationship no longer has to be the norm, though.
“The real opportunity is coming together as an ecosystem, if you will,” said Earnhardt, “coming together as an ecosystem to really drive value with everyone bringing their unique strengths and assets and capabilities to the table.”
Martha said bringing all types of devices and technologies together and making them as integrated as possible facilitates efficient workflow. It also produces better outcomes. >>
“There’s a difference between being connected and integrated,” he said. “It’s really about generating better outcomes, not just for patients but for healthcare workers and systems. We have several different areas we’ll be working on.”
Farrell said ResMed aims to improve upon a term he’s heard a lot — “data lakes” — with “data wells.” Those wells for the respiratory device developer provide a deep set of data on sleep, suffocation, sleep apnea, COPD, neuromuscular disease and respiratory insufficiency.
He said ResMed sees patient data from 33% of their lives, from going to bed to waking up. The other 67% isn’t accounted for. That’s where an ecosystem with cardiovascular outcomes, surgical outcomes, wellness outcomes and more comes in.



“I see an ecosystem as combining ResMed data wells with Medtronic data wells and Abbott data wells,” Farrell said. “And that’s not just across medtech but tech companies, too.”
When posed with the question of how to bring technology from concept to difference-maker, Earnhardt gave the
example of Abbott’s FreeStyle Libre continuous glucose monitor (CGM) for diabetes.
It all revolves around the patient’s need. Abbott recognized that CGM capabilities help those with diabetes. However, limiting technology to just CGM would leave the company “falling short of the potential.” So, it partnered with insulin pumps and coaching platforms to expand the ecosystem.
“It’s bringing the entire ecosystem together to put the patient at the center with their needs,” said Earnhardt. “It’s empowering them with the information they need, when they want it, wherever they are.”
Farrell pointed to a decision to give patients their data for free. Engaging patients — one example he gave was gamification through things like TikTok — creates better outcomes.
“We liberated the data and then provided each person in the ecosystem a way to extract value,” Farrell said. “Then, they selected our products. Physicians prescribed it more. Patients asked their doctors for it more. It created value that went beyond just our space and went into their diabetes care, their cardiovascular care.”
"The real opportunity is coming together as an ecosystem ... to really drive value with everyone bringing their unique strengths and assets and capabilities to the table."Medtronic Chair and
CEO
Geoff Martha
ResMed CEO Mick Farrell
Parekh asked the executives if the growing ecosystem changes the way medtech companies conduct their business. Martha had no doubts about it — “it totally changed the business model.”
For instance, Martha said the move from open surgery in the spine industry is going toward companies with ecosystems and commitment to evolving the space. That’s disrupting the industry, he explained. Similarly, as Farrell also pointed out, the software as a service (SaaS) model adds another wrinkle.
“It’s based on this ecosystem, and we’re not quite there yet in all transparency, but you can see the path it started with market share shifting,” said Martha. “It started with cost takeout and better outcomes. You’re starting to see better outcomes from this and economic outcomes as well. And then the next phase, which we’re just starting to get into, SaaS models and things like that, a true shift to the payment model. I see that coming, but we’re not all the way there yet.”
Farrell said SaaS can be part of the healthcare ecosystem. In fact, he said, ResMed has successfully used it for seven years and recently invested in it further. But it involves utilizing the strengths of others within the ecosystem.
“You can get there, but you don’t invent them all yourself, and it probably won’t come from your biomedical engineers,” said Farrell. “It will probably come from someone outside your ecosystem. “Reach out, partner with them and then find a way to bring it into your value set.”
Martha explained that it’s important to think about “the purpose of what we do.” That’s “innovation with a purpose,” he said.
This involves a need to “play offense” and attract talent to medtech. Historically, he said, the talent he’s talking about gravitated toward “big tech.” To establish this ecosystem, Martha wants to bring those people to medtech.
“It’s shifting to wanting to work for a company that stands for something, that’s contributing to society and in a meaningful way,” Martha said. “Looking in healthcare and medtech, we’re blessed with that. We’re working on
cutting-edge technologies, and our products are getting more data-based, and they’re getting cooler — if you want to put it in that context — by the day. So you’ve got the purpose.”
That “cool” factor represents a driving force behind attracting talent to medtech. Earnhardt joked that she feels medtech has always been cool but “just didn’t take credit for it.”
She sees a “phenomenal opportunity” to recruit based on new perspectives and capabilities. Earnhardt also sees an opportunity to partner in ways that, perhaps, the industry didn’t feel comfortable doing before.
“The beauty is that you’re starting to see more companies really wanting to make an impact here because that’s exactly what we do each and every day,” Earnhardt said. “I’m encouraged by the state of the industry. There’s a lot of complexity to it. One of the challenges we never talked about was sort of that need for speed. How do you balance the need for speed and the environment in which we’re in?
“Maybe that’s a whole other panel.”
"Looking in healthcare and medtech, we're blessed with [purpose]. We're working on cutting-edge technologies, and our products are getting more data-based, and they're getting cooler — if you want to put it in that context — by the day"































































































































Aerospace is not the only industry where reliability and robustness are required. In other sectors too, the new stepper motors of the AM3248 series will bring you one step closer to your goal.
More at: www.faulhaber.com/am3248/en




















































































































































































































 SEAN WHOOLEY ASSOCIATE EDITOR
SEAN WHOOLEY ASSOCIATE EDITOR
DermaSensor's innovative device could save lives with early skin cancer detection capabilities.
ith tens of thousands of deaths each year in the U.S. attributable to skin cancer, 99% of deaths from melanoma — the most deadly skin cancer — are avoidable if detected early, Cody Simmons says.

That’s where DermaSensor comes in. With Simmons as CEO, DermaSensor develops technology that could help primary care providers spot potentially cancerous lesions before forwarding patients to a dermatologist for further evaluation, improving the ability of the healthcare system to catch skin cancers early.
The company won the MedTech Innovator MidStage Companies Pitch Event at DeviceTalks Boston in May and recently raised $10 million as it prepares for a commercial launch in the U.S.
DermaSensor’s technology could become the fi rst device cleared by the FDA to assist primary care physicians in evaluating skin cancer.
“Nearly all of the tens of thousands of skin cancer deaths every year are completely avoidable if they are simply detected early,” Simmons said in an interview. “That’s what’s exciting about the opportunity — and also a bit of the tragedy and frustration in this fi eld — is that pretty much all skin cancers are curable, and pretty much all skin cancers, you can literally see on the surface of the skin. At the end of the day, as [DermaSensor cofounder and Chair] Dr. Maurice Ferre says, it’s really a public health issue.”
Simmons reinforced this in describing how preventing skin cancer deaths is “just a matter of getting patients better access to effective skin cancer checks.” >>
DermaSensor’s device uses a form of optical spectroscopy called elastic scattering spectroscopy (ESS) to take noninvasive samples of tissue, capturing cellular-level information from the skin lesion using hundreds of wavelengths of light in a manner similar to how sonar uses sound.

When Simmons joined as CEO in 2016 to scale their research project into a company, there were not yet any issued patents, no employees and no medical advisors. However, the promising technology, major
The biggest challenge came in the early days when shrinking the 30-pound, microwave-sized desktop prototype into a low-cost, handheld device that can be widely accessible and used by frontline providers. Over time, the spectral data collected during algorithm development and testing has remained consistent due to keeping the same fiber-optic probe configuration, but the rest of the device is now the size of a thick smartphone.
That technology was invented by Irving Bigio, a Boston University professor and scientific advisor to the DermaSensor. Dr. Ferre and DermaSensor Director Christopher Dewey, along with a few other early investors (including Intuitive Surgical founder and major surgical robotics player Dr. Fred Moll) worked to use the technology in a 30-pound desktop spectroscopy system, which represented the early stages of DermaSensor’s founding a decade ago.
clinical unmet need and knowledgeable early investors proved to be a good foundation for Simmons to build upon.






















“It’s been a great journey working with world-class medical device entrepreneurs, executives and investors, and I have learned a tremendous amount from them over the last six years,” he said. “We’re working toward this common goal of solving skin cancer through developing this effective, highly accessible detection tool.”

An advantage held by DermaSensor, according to the CEO, is that the technology isn’t taking a surface-level photo of the mole. The pointed tip of the device is placed on the lesion and five scans of a mole are taken in a “point and click” manner. After that, DermaSensor’s proprietary algorithm provides a result in seconds instead of sending the spectral data to a lab or physician for review.
Simmons also pointed to important efforts from the American Academy of Dermatology related to improving physicians’ training with and assessment of skin color. He said that photo-based technologies used in Europe (none are FDA-cleared for use in the U.S.) also have challenges with darker skin types since most image datasets for algorithm training are on lighter skin types. Because DermaSensor’s technology takes samples of tissue beneath the skin surface at a cellular and sub-cellular level, rather than using visual surface-level photos, literature suggests that skin color has no impact on the device’s performance.
beneath biopsy
“You can do a surgical biopsy of a skin lesion, genetic testing of a skin lesion, or a patient or a primary care provider can use telemedicine by taking a photo of a lesion and send that to a dermatologist for their assessment,” Simmons said. “But, there’s no FDA-cleared product available in the U.S. right now that uses any kind of photo or optical approach to do a point-of-care assessment or test of a lesion and provide that point-of-care result to help inform the doctor. That’s what we’re focused on.”
(continued on page 126)
"It's been a great journey working with worldclass medical device entrepreneurs, executives and investors, and I have learned a tremendous amount from them over the last six years."
READY.
Introducing KNF FP 70, delivering 120 - 850 ml/min while producing up to 29.4 psig (2 barg) pressure under continuous operation. Integrated dampers provide a smooth, gentle flow and innovative 4-point valves ensure reliable self-priming even at very low motor speed.
FP 70 is well-suited for a range of applications including medical technology, inkjet and 3D printing, and analytical instruments. With its introduction, the KNF smooth flow pump series now boasts a flow rate range of 120 ml/min to 12.4 l/min.


(continued from page 124)
Bringing DermaSensor to market DermaSensor followed a unique path to market with its product, which received FDA breakthrough device designation in 2021 and has regulatory authorization in Europe, Australia and New Zealand.




The company has an ongoing commercial pilot effort in Australia, as the regulatory pathway for this type of device was different abroad, with early validation studies proving sufficient as clinical evidence for use there. Simmons said DermaSensor has received valuable insights from Australia, where the commercial team members jokingly refer to themselves as the “company guinea pigs.”

One in five people in the U.S. get skin cancer in their lifetime, compared to two of three in Australia.
“It’s a major public health issue in Australia,” Simmons said. “It’s leaps and bounds higher in Australia than anywhere else in the world.”
Of course, amid the effort to launch abroad, another public health issue emerged: COVID-19.
Still, the persisting issue of undetected skin cancer remains, and the problem of long wait times for dermatologists leaves plenty of room for improvement. Simmons referenced a publication that found that just 15% of Americans reported receiving a full-body skin cancer check in their lifetime.



“With wait times for dermatologists already being months long, just imagine if Americans actually became compliant with getting regular skin cancer checks from dermatologists,” Simmons said. “It would go from an overwhelmed system to being completely unfeasible.”

Putting DermaSensor in the hands of primary care physicians could alleviate those wait times and provide people with potentially life-saving cancer detection.

Understandably, Simmons said, focus shifted to the pandemic. But, ahead of a potential U.S. launch, DermaSensor has recently heard back on the benefits of its technology.

“It’s been challenging for us as it could have been over a year of us selling and marketing our product,” Simmons said. “However, we’ve been able to do some in-person conferences [this spring and summer] and we’ve gotten a lot of great, excited feedback from the physicians that have been using the device on many hundreds of patients.”

With ongoing commercial efforts overseas and a push toward regulatory authorization stateside, DermaSensor’s future looks bright.
“Primary care physicians are not dermatologists. It’s not their expertise. It’s not their specialty. And they’ll be the fi rst ones to tell you that the majority do not consider themselves as experts in skin lesion assessment and care,” Simmons said. “Equipping them with a tool that improves their performance and improves their detection rate can help get high-risk patients to dermatologists as quickly as possible.
“We think our product can have a big impact on a lot of these important outcomes.”
"We've been able to do some in-person conferences [this year] and we've gotten a lot of great, excited feedback from the physicians that have been using the device on many hundreds of patients."DermaSensor
At Canon Virginia, Inc., we’ll help you solve your most daunting challenges while engineering new levels of quality and process improvement. Because when it comes to helping you succeed, doing the impossible is all in a day’s work. Learn more at cvi.canon.com/mfg.


The




































MedTech's stroke science research arm explains the science of blood clots and how that infl uenced the Embotrap's design.









 JIM HAMMERAND MANAGING EDITOR
JIM HAMMERAND MANAGING EDITOR
To catch a clot, you must fi rst understand a clot, and Michael Gilvarry has spent years doing just that.
Gilvarry is the GM of Cerenovus in Galway, Ireland. He founded the Johnson & Johnson MedTech unit’s stroke science research arm and leads the acute ischemic stroke R&D portfolio. That research led to the development and design of the Embotrap stent retriever device for thrombectomies, which helps stroke patients by removing the clots blocking the flow of blood to their brains.
“How the device design is informed by stroke science is something that we feel is really unique about the device,” he said in an interview.
“All of that work we’ve done has led to a very differentiated device design. We’ve demonstrated through testing on the bench and we see in our clinical studies that it’s really led to a device that’s very unique and is yielding very good results and good outcomes for patients.”
founder of Johnson & Johnson A blood clot captured by the Embotrap stent retriever Photo courtesy of Johnson & Johnson MedTechGilvarry spoke with Medical Design & Outsourcing to explain how the Embotrap stent retriever's design, materials and components work together to treat ischemic stroke. The conversation has been lightly edited for space and clarity.


MDO: What’s the typical patient profile for a thrombectomy?
Gilvarry: The patient profile is quite wide-ranging. People often think of stroke as being associated with older people and generally that is the case. About 75% of stroke victims are over the age of 65. But it’s not exclusively older patients. Our devices have been used to treat patients in their twenties and thirties, and unfortunately, it’s common enough depending on the type of comorbidities they might have. The symptoms that somebody develops when they get a stroke are the ones that we’re all familiar with through the public awareness campaigns: the face droop, the loss of power in the arms and [trouble speaking]. That’s caused by a blood clot that normally travels from somewhere else in the body — from the heart, in a large percentage of patients, for example — and that causes a blockage in one of the large vessels in the brain and that triggers the ischemia that leads to the symptoms. The goal of the thrombectomy procedure is to remove the blood clot and restore blood flow to the brain.
MDO: How is the thrombectomy procedure performed with a stent retriever?
Gilvarry: The device is normally accessed to the vascular system through the groin. In some cases, through the radial artery as well, but it’s predominantly through the femoral artery. The devices are tracked up using a series of catheters. There’s access catheters to navigate around the aortic arch up into the carotid artery. And that’s essentially where the basecamp is set up. Then the thrombectomy devices are tracked further into the vessels in the brain. The Embotrap device is deployed across the clot. And to do that, it’s tracked through a microcatheter less than a half millimeter in diameter that crosses the blood clot. The Embotrap device is placed through the microcatheter underneath the clot. And then the microcatheter is unsheathed and the device opens up




inside the blood clot. There’s a wire that goes all the way back outside the body the physician pulls to [withdraw the device,] trapping the blood clot and bringing it back through the access catheter, normally with co-aspiration.
MDO: How many passes does it take to remove the blood clot from the vessel?
Gilvarry: It’s removed ideally in one pass. One pass is something we really focused on in the Embotrap device, to get a very efficient clot removal. It’s been shown that good clinical outcomes are positively correlated with a single-pass procedure.
MDO: How long does a thrombectomy procedure take?
Gilvarry: It’s very fast. We’ve had procedures that can take less than 10 minutes. It’s a metric that’s widely recorded because they say “time is brain” in stroke. The patients are often awake actually during the procedure, and it’s just remarkable to hear the stories of patients recovering on the table. … One of the things a doctor will often do as soon as the procedure is finished is ask the patient to squeeze their hand and they can tell straight away if they’ve got power back in their arm that the signals are very good, that the patient’s going to recover.
MDO: Is thrombectomy a risky procedure?
Gilvarry: Overall, the benefits of this procedure far outweigh the risks. The clinical trials have shown good outcomes increase by 74% compared to tPA clotbusters alone, and the rate of death and disability has been reduced by more than half. There’s a very, very strong risk/ benefit ratio here.
MDO: How does Embotrap do the job differently than its predecessors?
Gilvarry: We really focused on building features into the Embotrap device that address the various properties of blood clots. The open inlet windows, for example, in the Embotrap device are designed to encourage the clot inside the device. People generically call these devices stent retrievers, but the Embotrap device does not behave like a stent. A coronary stent, for example, is designed to open up a vessel and push everything outward. But the Embotrap outer cage is different. >>
It’s designed with these openings to allow the clot inside the device. Then there is an inner channel, which stabilizes the clot during its removal. It’s almost like a stent within the stent. The inner channel is a closed-cell structure cut from a tiny nitinol tube. … And then the device has a closed end to prevent the clot from traveling all the way through the device as it’s being removed.
MDO: What did you learn about blood clots that helped you design this device to capture them?
Gilvarry: What was remarkable even 10 years ago is how little there was published around the properties of blood clots. And it’s really been a journey of discovery around those properties. Thrombectomy wasn’t the standard procedure so there were very few clots to study even 10 years ago. We started doing tests to define the physical properties as you would with any material. One of them is friction. Trying to understand the frictional properties of blood clots, what we found is that changes substantially both with the composition of the blood clot, but also with how it’s manipulated. If the blood clot is compressed, it can increase friction.

of these is quite small. They’re collapsed down into a catheter that’s less than a half millimeter, and it’s the nitinol that allows it to be collapsed down into that very-lowprofile stage in order to be tracked at very, very low forces through the microcatheter up into these vessels. And then once it’s unsheathed, the shape memory properties of nitinol and the superelastic properties help this predefined shape to open up inside the blood clot.
Gilvarry: The tungsten markers are embedded in the struts — very, very small scale, but at each section of the device, there are four markers so when the device opens up inside the vessels or indeed inside the clot, the physician can visualize how the device either conforms to the vessel or how it’s interacting with the clot based on these markers. Depending on the length of the device, there are three to five different segments. That’s really important because what that allows is for the device to maintain its connection with the vessel wall as it’s being retrieved [and] helps engage with the clot all the way through the retrieval process.
That’s one example of many things that we studied and learned and built into the features of the Embotrap device. The device itself has quite large, open inlet windows, and the intent there is to try and encourage the clot to go inside the device so it’s not overly compressing it, to trap it inside the device and then remove it from the vessel.
MDO: How do the materials used in the Embotrap device help with clot removal?
Gilvarry: The device itself is [mostly] made from nitinol, or nickel-titanium alloy. The superelastic and shape memory properties of nitinol make it particularly suited to this device. These vessels are very tortuous. Even compared to coronary arteries, there’s a lot more tortuosity in the pathway to get to the vessels in the brain. So the scale
MDO: How is the Embotrap stent retriever manufactured?
Gilvarry: The business end of the device, the nitinol parts, are made by laser cutting nitinol tube into the pattern that we have designed in the Embotrap. We use very-high-frequency lasers to do that because the scale of these are so small. We’re trying to put very fine patterns into very, very small tubes. The device then is heat-treated to set the shape and electropolished to give it the surface finish we need to be used in interventional procedures. It’s a very intricate process to add the radiopaque markers in the device. And then it’s assembled onto the wire, which is made from coated nitinol material. A lot is assembled, then sterilized with EtO and shipped to the customer. … Some of [the manufacturing] is outsourced and some is in-house.
"We really focused on building features into the Embotrap device that address the various properties of blood clots."
HDPE Die Cut Wallets are commonly used for organizing and protecting heavy or bulky devices as an alternative to Thermoformed Trays. Die Cut Wallets allow devices to be stabilized on the card and the flexible card allows for vertical stacking of components to allow for a small footprint. Wallets are highly customizable and available in a variety of materials, sizes and configurations.
Insert wallets are typically packaged in a flexible peel pouch but can be used to organize within thermoformed trays and kits as well.

Standard materials are natural or white HDPE in various thicknesses. Quick turnaround from conception to production using cost effective tooling in sustainable, recyclable, standard materials allows for meeting short timelines for sterile barrier system validations.

Biocoat is a leading full-service hydrophilic coating provider specializing in supplying lubricious hydrophilic coatings, custom coating services, and coating equipment for medical devices. Biocoat is the only hydrophilic coatings provider to offer industry-leading performance in Thermal and UV light coatings. Biocoat’s service team creates unique solutions that offer high-touch services from your discovery phase through delivery. Biocoat also offers EMERSE, an automated dip coating system designed for medical device companies that require highquality and reliable technology to coat their devices in-house. EMERSE is available for both UV and Thermal curing options. With Biocoat’s full-service offering, our coating experts will be able to find the perfect solution for your project.
Beacon Converters
45 Mayhill Street
Saddle Brook, NJ 07663

beaconconverters.com info@beaconconverters.com
123 Rock Rd
Horsham, PA 19044 215-734-0888
www.biocoat.com


DDL is an independent third party ISO 17025 accredited testing laboratory that provides packaging, medical device and materials testing to the medical device and pharmaceutical industries. We employ a team of engineers, technical and quality experts devoted to helping our customers bring medical device and drug-delivery products to market. Our single source, totally integrated approach enables organizations of all sizes from start-ups to globally recognized corporations maximize product performance, reliability and safety while seamlessly achieving regulatory compliance. We work hard to build strong partnerships with our clients and have an unwavering commitment to assist in getting products to market on time. Please visit www. DDLTesting.com to learn more.
Injection molded optics, engineered for your success.

As a custom optics manufacturer, we can produce custom injection molding for aspheric lenses and free-form lenses and mirrors, as well as Fresnel and diffractive optics. We also have the in-house capability to provide custom designed diamond turning and injection molding for prototypes, thin film optics, reflective coatings, and integrated optical solutions. We support a broad range of consumer, medical, instrument LED lighting, and biomedical and analytical instrument marketplaces.

Our in-house team consists of experts in diamond turning, thin film coating, mold fabrication, and optomechanical assembly. We are ready to work with your technical teams to create highperformance, molded, optical element solutions for your program’s plastic optic needs.
DDL,
(800) 229-4235 https://www.ddltesting.com
408 St. Paul Street 800.252.5335 or Rochester, NY 14605 USA 585.295.0200
www.gsoptics.com
Medical Device Components, a business of Johnson Matthey, provides high-quality precision micromachined components, implant-grade Nitinol parts, and advanced coating surface solutions. Our vast knowledge in specialty metals such as platinum, palladium, and gold, spanning over 200 years, coupled with our expertise in material science, microfabrication techniques, and metal management services helps to accelerate medical device time-to-market. Our products include:
• Platinum alloy marker bands and electrodes
• PGM tube, sheet, rod, and wire
• Nitinol tube, sheet, and foil
• Custom micromachined PGM or Nitinol components via Laser, EDM, or Swiss
Our new Nitinol center of excellence brings low cost country manufacturing capacity, adding to our four global manufacturing facilities. JM-MDC continuously launches sustainable manufacturing capabilities to help stabilize supply chains and maximize product continuity.


Medical Device Components
–
+1-610-648-8000 www.jmmedical.com • medicalinfo@jmusa.com
Manage every part of the security lifecycle and future-proof your prod uct for upcoming Wi-Fi and Bluetooth generations with the Summit System-on-Module (SOM) 8M Plus. The new Summit SOM 8M Plus is Laird Connectivity’s most powerful and feature-rich SOM yet. It lever ages NXP’s I.MX 8M Plus architecture into a powerful platform with:

• Reliable connectivity: Wi-Fi 5 and Bluetooth 5.3
• Machine learning, graphics, video, vision and audio – up to 3 displays
• Secure enclave and secure boot
• Robust software and speed to market
• Global approvals
• Personal support from design to manufacture
With decades of experience developing and producing complex embedded and wireless products, we are the ideal SOM provider to help you bring your application to market smarter and faster. Learn more: www.lairdconnect.com/summit-som-8m-plus
www.medicaldesignandoutsourcing.com
www.lairdconnect.com sales@lairdconnect.com

The sum of Miyano’s physical design is ideal for the rigors of medical manufacturing. High volumes, tough materials, zero defects – all these factors make successful medical manufacturing difficult, but we design machinery with these conditions in mind. Miyano doesn’t just survive long runs in tough medical materials, it thrives with them year after year. Stainless steel, titanium, nickel, and other super alloys are some of our customer’s most common materials for a reason – they know Miyano can handle cutting them for decades. Whether you make products for the orthopedics, cardiovascular, surgical device, or (other specialties etc) sector, Miyano has solutions that will directly improve your manufacturing quality, throughput, and labor ratio per part.
40 Boroline Road # 6 Allendale, NJ 07401 (201)-818-0100 www.Marucit.com
Our business model is designed to adapt to our client’s specific manufacturing and material development needs. We offer the resources, knowledge, and experience necessary to provide rapid research and development and a smooth transition into commercial production.
• 12 ISO Class 7 Cleanroom Suites that are ISO Class 6 capable.


• Independent suite design to facilitate proper line clearance.
• Stringent BioBurden control.
Our team puts the quality and integrity of the device first in every stage of development. TESco has a rigorous and robust quality management system, certified to ISO 13485: 2016 and registered with the FDA.
• Material Selection and Compounding
• Injection Molding & Extrusion
• Packaging and Sterilization Support
• FDA Submission Assistance
978.649.5527 info@tescoassociates.com www.tescoassociates.com

































A perfectly sealed noble metal surface is a necessity for systems used to identify pathogens. Gold is widely used in biomedical diagnostics, particularly in sensors and circuit boards, but is inherently porous. To achieve zero porosity, zero contamination and a sealed, contaminant-free surface, a metallic layer stack comprised of palladium with finegrained gold overplate has proved optimum. Two processes, both developed and patented by Uyemura and available under the tradename Medigold, are exquisitely suited to spectroscopic and other high purity surface requirements. The processes are economical, compatible with existing equipment and do not require elaborate lab analysis.
Tech Center: 860.793.4011 www.uyemura.com/medigold.html
WTWH Media, LLC
1111 Superior Ave., Suite 2600, Cleveland, OH
WTWH Media, LLC
1111 Superior Ave., Suite 2600, Cleveland, OH 44114
Courtney Nagle; WTWH Media, LLC
1111 Superior Ave., Suite 2600, Cleveland, OH 44114
Chris Newmarker; WTWH Media, LLC
1111 Superior Ave., Suite 2600, Cleveland, OH 44114
WTWH Media, LLC
Scott McCafferty
Mike Emich
Marshall Matheson
Bruce A. Sprague (888) 543-2447
Accumold 59
Advanced Powder Products 35, 37 AllMotion 6 Altech Corporation 8, 9 B. Braun BC
Bay Associates Wire Technologies, Inc. 48
Beacon Converters 65, 131 Biocoat 77, 131 Bird Precision 53 BMP Medical 47
Johnson Matthey 75, 133
Keystone Electronics Corp 1 KNF Neuberger 125
Laird Connectivity 33, 133
1111 Superior Ave., Suite 2600, Cleveland, OH 44114
1111 Superior Ave., Suite 2600, Cleveland, OH 44114
1111 Superior Ave., Suite 2600, Cleveland, OH 44114
1111 Superior Ave., Suite 2600, Cleveland, OH 44114
Bodine Electric Company 2 Boker’s, Inc. 36
Canon U.S.A. 107
Canon Virginia, Inc. 127 Carl Stahl Sava Industries 95 CGI Inc. 45
Cirtec Medical 81 Components Corporation 42 Cretex Medical 13 DDL 89, 132
Eagle Stainless Tube 51 ebm-papst 29 Elsner Engineering 91 Faulhaber 122 Filmecc USA, Inc, subsidiary of Asahi Intecc USA. 44 Freudenberg Medical 57
GS Plastic Optics 39, 132 Hobson & Motzer 5 HS Design, Inc. 54 Interpower 7
Life Science Outsourcing 101 Marubeni Citizen-Cincom 61, 134 Master Bond 70 maxon cover/corner, 3, 115 MedTech Strategist 92 MICRO 97 MicroLumen 112, 113
New England Wire & Tubing Technologies 111

Nordson MEDICAL 50
Novotechnik 41
Packworld USA 85
Penn United 105 Pulse Technologies 99 Qosina 80 Resonetics IFC Rohm GmbH 14
SENKO Advanced Components 62
Smalley Steel Ring 31
Solar Atmospheres 88

Solenoid Solutions, Inc. 78

Stock Drive Products/Sterling Instrument 32
Tegra Medical BC
TESco Associates 69, 134
The Mediashop 117 Uyemura 73, 135
0 0 5,030 6,485 10,047 9,706 50.1% 66.8%
Ryan Ashdown rashdown@wtwhmedia.com 216.316.6691
Jami Brownlee jbrownlee@wtwhmedia.com 224.760.1055
Ashley N. Burk 737.615.8452 aburk@wtwhmedia.com
Mary Ann Cooke mcooke@wtwhmedia.com 781.710.4659
Jim Dempsey jdempsey@wtwhmedia.com 216.387.1916
Courtney Nagle cnagle@wtwhmedia.com 440.523.1685
Jim Powers jpowers@wtwhmedia.com 312.925.7793 @jpowers_media
Mike Francesconi mfrancesconi@wtwhmedia.com 630.488.9029
LEADERSHIP TEAM
Scott McCafferty
smccafferty@wtwhmedia.com 310.279.3844
@SMMcCafferty
VP of Sales
Mike Emich memich@wtwhmedia.com 508.446.1823
@wtwh_memich
Brian Toole btoole@wtwhmedia.com 267.290.2386
EVP
Marshall Matheson mmatheson@wtwhmedia.com 805.895.3609
@mmatheson







Why do medical professionals have so much confidence in our devices, sets and kits? It starts with five decades of contract manufacturing. The pharmaceutical industry relies on us to build custom kits with all the devices necessary to dispense and administer your drug. Our product portfolio contains hundreds of standard components plus capabilities including design, regulatory, manufacturing, packaging and sterilization. Think of us as your best, most direct route to market. After all, our devices and capabilities do more than simply meet expectations. They deliver confidence. Always have. Always will.




