

Welcome from EUS-AAEM
The Emergency Ultrasound Section of the American Academy of Emergency Medicine (EUS-AAEM) was founded to foster the professional development of its members and to educate them regarding point-of-care ultrasound. This group will serve as a venue for collaboration among medical students, residents and practitioners who are interested in point-of-care ultrasound. The purpose of our group is to augment the knowledge and expertise of all emergency medicine specialists and to advocate for patient safety and quality care by endorsing bedside ultrasound. Membership is not limited to fellowship trained physicians. All emergency medicine practitioners passionate about ultrasound are welcome to join and participate.
We are proud to publish our e-newsletter with original contributions from many of our members. We encourage all members to submit for future editions. Topics include but are not limited to educational, community focus, interesting cases, resident and student section, and adventures abroad.
For more information, visit us at: www.aaem.org/get-involved/sections/eus/
President’s Message
This past year has been another great year for the Emergency Ultrasound Section as we continue to engage with our membership and promote high-quality ultrasound education to Emergency Medicine physicians regardless of practice setting.
This year has brought changes to the Unmute Your Probe webinar series The current season highlights ultrasound program management topics for the first half of the year and Advanced Emergency Medicine Ultrasonography (AEMUS) Focused Practice Designation (FPD) Exam Review starting in September for those preparing to sit for the FPD in March 2024. Prior Beginner and Advanced topics remain available for viewing through the EUS-AAEM site.

Our Speakers Bureau is connecting interested medical student groups with virtual lectures to advance ultrasound knowledge for the next generation of physicians. The POCUS Report continues to put out quality content twice yearly. We have also begun offering Regional Hands-On Ultrasound Courses aimed at providing customizable ultrasound education for emergency practitioners at sites local to those looking to advance their point of care ultrasound skill.
We are thrilled at the offerings available from EUS at this year’s Scientific Assembly, including a medical student focused case challenge in collaboration with RSA and the Simulation Interest Group and our first annual Poster Competition in addition to multiple workshops and talks of varying lengths.
The Section now has representatives on multiple national boards and workgroups, including recent additions to the ACEP Clinical Ultrasound Accreditation Program board and the AEMUS Scholarly Activity Workgroup. National presence such as this allows EUS-AAEM to continue to advocate for emergency physicians and the role of point of care ultrasound in providing high quality patient care.
It has been a privilege to serve as Chair of the Section, and I look forward to my next role as Immediate Past Chair when Dr Neha Bhatnagar assumes leadership in New Orleans at Scientific Assembly

Opportunities to Get Involved
Help Wanted: Speakers
The Emergency Ultrasound Section is looking for interested residents, fellows and faculty to speak with medical school Emergency Medicine Interest Groups and Ultrasound Interest Groups about the use of point of care ultrasound in the Emergency Department.

The section will provide a list of suggested topics to the Interest Groups, as well as providing you with several slide sets to use if you choose as a basis for lectures. This is a great opportunity for anyone interested in sparking interest in the next generation of Emergency Physicians in the use of point of care ultrasound in the Emergency Department! Interested physicians may sign up here.
An Opportunity for EMIGs
The American Academy of Emergency Medicine Emergency Ultrasound Section has an exciting new opportunity focused on providing emergency ultrasound education to groups like yours. The section has compiled a group of point of care ultrasound enthusiasts who are making themselves available to give virtual talks on a variety of emergency ultrasound topics. This is a great chance to get directed education on an exciting and ever expanding diagnostic topic directed to your group's specific interest. If you think your group would be interested in taking advantage of this opportunity, please sign up through the sign-up link below
Please designate your interest on this worksheet
Education Section
Alternate Ultrasound Medium as a Method of Training Ocular Ultrasound
CPTKaegan
Williams PA-C, CPT Seth Brown PA-C, Melissa Myers, MD FAAEMOcular ultrasound (US) is frequently used in the Emergency Department evaluation of patients presenting with ocular complaints.1,2 Performing ocular US requires the placement of gel on the eyelid, with or without the presence of a barrier such as a Tegaderm™. The absence of a barrier may cause increased patient discomfort but using a barrier has been shown to decrease image quality 3,4 This requires the person obtaining images to choose between patient comfort and high quality images. Alternative methods to create an acoustic window without patient discomfort would assist with gathering high quality images.
One potential alternative is a non-toxic noise putty (Image 1) which can be used as a gel alternative in austere environments and may be expanded for use in a training setting.5 In a training setting, residents and other trainees may be performing repeated scans on each other The scanning process should be as easy as possible to encourage trainees to scan as much as possible.
Image 1: Flarp! Noise Putty
We used a noise putty as a medium for ocular US when practicing Point of Care US with five Emergency Medicine (EM) residents, two EM PA fellows, and two non-medical personnel. The noise putty was not shared between participants. While using this on peers during US training, all nine of the participants noted the noise putty to be more comfortable. The novice US operators noted that the noise putty increased the

standoff distance and optimized the image in the middle third of the screen. When comparing the images to those obtained with US gel (Image 2a) versus those obtained with the noise putty (Image 2b) there was no apparent degradation of the quality of the image. It was noted by the participants who were receiving the scan that the noise putty held its form more easily than standard US gel and did decrease the amount of perceived pressure applied to the participants’ eye. All comments were made verbally and without any written questionnaire or stratification tool like the Likert scale. This is consistent with previous research on the use of FLARP as an US gel alternative.5 In this initial test of use, FLARP shows potential promise for use in the training environment. We recommend a future prospective study on FLARP as an US gel alternative.
References
1 Kilker BA, Holst JM, Hoffmann B Bedside ocular ultrasound in the emergency department European Journal of Emergency Medicine 2014;21(4):246-253
2. Gharahbaghian L, Anderson KL, Lobo V, et al.. Point-of-care ultrasound in austere environments: a complete review of its utilization, pitfalls, and technique for common applications in austere settings Emergency Medicine Clinics 2017;35(2):409-441
3. Roth KR, Gafni-Pappas G. Unique method of ocular ultrasound using transparent dressings. The Journal of Emergency Medicine 2011;40(6):658-660

4 Marks A, Patel D, Chottiner M, et al Covered or uncovered: A randomized control trial of Tegaderm versus no Tegaderm for ocular ultrasound. The American Journal of Emergency Medicine. 2022;61:87-89.
5 Ersando JCA, Fetterolf BM, Draper HR, et al Military Special Operations and Prolonged Field Care Manual of Austere and Prehospital Ultrasound 2021:23-26
Image 2: Comparison of US Gel (A) to images obtained with Noise Putty (B)Ultrasound Saves
Right Ventricular Strain from Saddle Pulmonary Embolism
Cayla Fappiano, MD and Andrew Kuschnerait, MDCase Presentation
A 42-year-old female with a past medical history of asthma, remote Roux-en-Y gastric bypass, and right medial meniscal repair one month prior presented to the emergency department with two days of dyspnea, chest pressure, and palpitations. She had mild right lower extremity pain and swelling since her meniscal repair and had been wearing a knee immobilizer The patient had no prior history of DVT, PE, or malignancy She took oral contraceptives and received monthly testosterone injections. She denied fevers/chills, cough, hemoptysis, and orthopnea.
Initial vitals were T 37.4C, P 124, BP 106/70, RR 24, SpO2 88% on RA. On exam, the patient appeared to be in respiratory distress. She was only able to speak a few words at a time. She had non-pitting edema of the right lower extremity with no overlying erythema. Her lungs were clear to auscultation bilaterally

The patient was placed on 2L NC, and her SpO2 improved to the low 90s. Labs revealed an elevated BNP of 6818, negative troponin, leukocytosis, and a negative COVID test. A VBG demonstrated respiratory alkalosis with pH 7.51 and pCO2 26.4. Chest x-ray revealed no acute cardiopulmonary abnormalities.
A bedside cardiac echo was performed by the emergency medicine physician and revealed right ventricular overload. Expedited computed tomography pulmonary angiogram (CTPA) demonstrated saddle pulmonary embolism (PE) extending through the main pulmonary arteries with bilateral panlobular involvement and evidence of right heart strain. Radiology provided an immediate wet read of the CTPA. Heparin was already prepared at the bedside when the patient returned from CT


 Figure 2: Right ventricular overload and apparent “D” sign in parasternal short view on bedside cardiac echo in the setting of saddle PE
Figure 3: Right ventricular overload and McConnell’s sign in apical four view on bedside cardiac echo in the setting of saddle PE
Figure 2: Right ventricular overload and apparent “D” sign in parasternal short view on bedside cardiac echo in the setting of saddle PE
Figure 3: Right ventricular overload and McConnell’s sign in apical four view on bedside cardiac echo in the setting of saddle PE
Discussion
Pulmonary embolism is a clinical emergency that may present with shortness of breath, chest pain, fatigue, and other vague complaints. Signs of right heart failure like jugular venous distention and peripheral edema may eventually develop. Tachycardia and hypoxia are common vital sign abnormalities. Wells Criteria for PE may help risk stratify patients to help guide diagnostic testing, and CTPA is the gold standard for diagnosis. Bedside POCUS currently exists in a middle ground – it may be helpful to guide testing and treatment, but more research is needed to determine how POCUS might be used most effectively in the emergency department to identify PE.
PE obstructs pulmonary vasculature, leading to increased pulmonary vascular resistance. This leads to right ventricular (RV) pressure overload which can be seen on bedside ultrasound as RV dilation and carries an estimated sensitivity of 50% overall increasing with clot size. In the same study, RV dilation occurred in all cases of saddle embolus.1 The IVC will be expanded secondary to restricted venous return to the right heart. Direct signs on bedside POCUS like thrombus in the right heart and pulmonary artery are obviously of high specificity but have a low detection rate.2 Indirect signs of right heart strain, though more easily detected, are non-specific. Right heart strain can also be caused by pulmonary hypertension, left-sided heart failure, ARDS, severe tricuspid regurgitation, and volume overload.
The “D sign” occurs when right ventricular overload causes the septum to shift towards the left side of the heart, creating a “D” shape on a parasternal short view The D-sign has an estimated sensitivity of 27% overall; however, just like RV overload the sensitivity likely increases for larger pulmonary emboli.1
McConnell’s sign - right ventricular free wall akinesis with sparing of the apex has similar sensitivities in the 20-30% range; however, specificity estimates range from 20% to 100%.1 Recent studies have pointed out that RV ischemia without PE should remain in the differential when McConnell’s sign is seen, which may lower specificity 1 When found, McConnell’s sign can strongly suggest PE in the right clinical scenario, but RV infarct and pulmonary hypertension can also produce this finding. The diagnostic accuracy of POCUS for PE has been assessed in pulmonary critical care physicians, and case reports exist of diagnosis of PE on POCUS before CTPA in the ED, but more studies are needed to determine the true sensitivity and specificity in combination with other decision-making tools and lab studies.2-5
Our patient had right ventricular strain in the setting of saddle pulmonary embolism as seen through “D sign,” McConnell’s sign, RV overload and IVC fixation and dilation on POCUS. She was high-risk by Wells criteria and had an elevated d-dimer She was subsequently admitted to the MICU on a heparin drip and underwent mechanical thrombectomy by interventional radiology Ultrasound of the lower extremities later demonstrated a large non-occlusive thrombus in the right popliteal vein. Bedside POCUS in the setting of our high clinical suspicion led to expedited treatment and disposition for this patient.
Conclusion
Bedside POCUS can support the diagnosis of pulmonary embolism, a clinical emergency requiring rapid recognition. Bedside POCUS may be especially helpful in settings where CT may not be readily available or a patient is not stable enough to go to the scanner. More studies should be performed to determine the utility of bedside POCUS for diagnosis in combination with other clinical decision-making tools or lab tests, and the ability of emergency medicine providers to identify these signs.
References
1 Rafie N, Foley DA, Ripoll JG, et al McConnell's Sign Is Not Always Pulmonary Embolism: The Importance of Right Ventricular Ischemia JACC Case Rep 2022 Jul 6;4(13):802-807
2 Dresden S, Mitchell P, Rahimi L, et al Right ventricular dilatation on bedside echocardiography performed by emergency physicians aids in the diagnosis of pulmonary embolism Ann Emerg Med 2014;63:16–24
3 Zhu R, Ma X Clinical Value of Ultrasonography in Diagnosis of Pulmonary Embolism in Critically Ill Patients J Transl Int Med 2017 Dec 29; 5(4): 200–204 doi: 10 1515/jtim-2017-0034
4 Secko M, Legome E, Rinnert S Saddle embolism diagnosed by point-of-care transthoracic echocardiography before computed tomography angiogram of the chest Am J Emerg Med 2016;34:2467
5 Filopei J, Acquah S, Bondarsky E, et al Diagnostic Accuracy of Point-of-Care Ultrasound Performed by Pulmonary Critical Care Physicians for Right Ventricle Assessment in Patients With Acute Pulmonary Embolism Crit Care Med 2017 Dec; 45(12):2040-2045 doi: 10 1097/CCM 0000000000002723
6 Mansencal N, Vieillard-Baron A, Beauchet A, et al Triage patients with suspected pulmonary embolism in the emergency department using a portable ultrasound device Echocardiography 2008 May;25(5):451-6 doi: 10 1111/j 1540-8175 2007 00623 x PMID: 18452470
Fellows Section
Acute versus chronic right-sided heart failure: Point-of-care ultrasound findings
Rebecca Theophanous, MD MHSc FAAEM
Case Presentation
A 72-year-old-female arrives to the emergency department via ambulance with shortness of breath and cough for several days. She has an increased oxygen requirement and is in acute respiratory distress with oxygen saturations 80% on room air requiring six liters nasal cannula. She has known severe interstitial lung disease and has been non-ambulatory for the past few days from increased generalized weakness. While performing a bedside point-of-care cardiac ultrasound, you note a dilated right atrium and right ventricle on an apical four-chamber view and a flattened septum on a parasternal short axis view. While waiting for chest CT scan, how can you help determine whether her symptoms are from acute or chronic right-sided heart failure to precede with the next treatment steps?
Although similarities exist in point-of-care cardiac ultrasound for acute versus chronic right heart failure, several distinct point-of-care ultrasound findings can help determine which is the cause of your patient’s symptoms.

Acute right heart failure findings:
a. A dilated right ventricle (RV) can be seen in both acute or chronic right-sided heart failure. The normal RV:LV ratio is 0.6 to 1.0, with a dilated right to left chamber ratio measuring 1:1 or greater being abnormal. This is best visualized in the apical four chamber view.1 (Figure 1)
b. A flattened septum or “D sign” with an enlarged or dilated right ventricular is more suggestive of an acute process such as pulmonary embolism. This can be visualized in a parasternal short view.1 (Figure 2)
c. “McConnell’s sign” or RV apical sparing is also suggestive of an acute process, with decreased movement or hypokinesis of the right ventricular free wall and base, which appears to be being pulled by the normal functioning left ventricular wall. This is visualized in the apical four-chamber view (specificity of 94% and sensitivity of 77% for diagnosing PE).1,2 (Figure 1)
Chronic right heart failure findings:
a. RV hypertrophy is a chronic process that requires time to develop. The RV free wall should be measured in the parasternal long axis or subxiphoid view from inside to outside wall in end diastole. Measurements greater than 5mm are hypertrophied and signify a chronic process.3-5
b. RA enlargement occurs over time in response to elevated RA pressures. The RA size can be compared to the LA in the apical four chamber view and enlargement signifies a chronic process.
c. Tricuspid regurgitation can also lead to RA enlargement and is a more chronic process. A pressure gradient greater than 60mmHg between the RV and RA or tricuspid regurgitation jet with Vmax greater than 3.5m/s on continuous wave doppler (CWD) is suggestive of a chronic process.1,6-7 (Figure 3)

 Figure 2: Dilated right ventricular with septal flattening or “D sign”
Figure 2: Dilated right ventricular with septal flattening or “D sign”
a. Pulmonary artery systolic pressure (PASP) is also useful in evaluating for chronic right-sided heart failure.(Parasuraman) A PASP value less than 35 mmHg is typically normal, PASP 40-60 mmHg in patients with pulmonary hypertension, and a PASP ≥ 60 mmHg indicating longstanding pulmonary hypertension (PHTN).8 PASP can be estimated using CWD and the modified Bernoulli equation (∆P=4VTR Max2) to calculate the pressure gradient (∆P) across the tricuspid valve. This should be done in the apical four-chamber or parasternal short axis view at the level of the tricuspid valve (TV).6 If no significant pulmonic valve stenosis exists, the RVSP should be similar or equal to the PASP. RVSP is obtained by adding right atrial pressure (RAP) to ∆P. RAP is equivalent to central venous pressure (CVP), [RVSP=4VTR Max2 + CVP]. CVP can be estimated using the subxiphoid IVC view, measuring the diameter and respiratory variability of the IVC. (Table showing CVP estimates below)3
b. The 60/60 sign is another method of determining chronic right-sided heart failure. It requires two values: the tricuspid insufficiency pressure gradient (∆P=4VTR Max2) and the Pulmonic Valve Acceleration Time (PAT), which is the time interval from pulmonic valve opening until maximum blood flow velocity is reached.9 This is measured in a parasternal short axis view at the level of the mitral valve. Tilt the probe towards the base of the heart until the RV outflow tract comes into view, place a pulse wave doppler (PW) gate over the pulmonic valve, toggle to spectral display, and measure the time interval from the start of blood flow to its peak velocity A positive 60/60 sign occurs when the ∆P (= 4VTR Max2) and PAT are BOTH less than 60, which indicates an acute cause of right heart failure (specificity and positive predictive value of 94% and 90%, respectively for acute PE). In contrast, patients with PHTN have greater pressures and longer times, with ∆P and PAT > 60.9 (Figure 4)
Figure 3: Color wave doppler demonstrating tricuspid regurgitationThe patient was stabilized with supplemental oxygen and treated with antibiotics for pneumonia. She was found to have bilateral pulmonary embolisms to the level of the main pulmonary arteries on her CT chest, requiring anticoagulation and interventional pulmonary treatment, with admission to the intensive care unit.
In summary, a flattened septum or “D sign” and RV apical sparing or McConnell’s sign are more common in acute right-sided heart failure, whereas RV hypertrophy >5mm, RA enlargement, an elevated PASP >60mmHg, and a negative 60/60 sign (both ∆P (= 4VTR Max2) and PAT are >60) are more commonly seen in chronic right-sided heart failure patients.
References
1 Bossone E, D'Andrea A, D'Alto M, et al Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013 Jan;26(1):1-14. doi: 10.1016/j.echo.2012.10.009. Epub 2012 Nov 8. PMID: 23140849

2. McConnell MV, Solomon SD, Rayan ME, et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism Am J Cardiol 1996;78:469–73
3. Mikhalkova D, Quader N. Right Ventricular Function and Pulmonary Hemodynamics. In: Quader N, Makan M, Perez JE, The Washington Manual of Echocardiography 2nd edition Lippincott Williams and Wilkins, Wolters Kluwer; 2017, pp 55-71
4 Rudski LG, Wyman WL, Afilalo J, et al Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography J Am Soc Echocardio 2010;23(7):685-713
5 Ho SY, Nihoyannopoulos P Anatomy, echocardiography and normal right ventricular dimensions Heart 2006;62:i2-i13
6 Parasuraman S, Walker S, Loudon BL, et al Assessment of pulmonary artery pressure by echocardiography-a comprehensive review Int J Cardiol Heart Vasc 2016;12:45-51
7 Pellet A, Zeidan A, Avila J Differentiating Acute Versus Chronic Right Heart Failure with Bedside Echocardiography EMRA 2019 https://www emra org/emresident/article/bedside-echo/
8 Bech-Hanssen O, Karason K, Rundqvist B, et al Can pulmonary hypertension and increased pulmonary vascular resistance be ruled in and ruled out by echocardiography J Am Soc Echocardio 2016;26(5):469-78
Figure 4: PAT measurement in parasternal short axis view Patient case resolution9 Kurzyna M, Torbicki A, Pruszczyk P Disturbed right ventricular ejection pattern as a new doppler echocardiographic sign of acute pulmonary embolism. Am J Cardiol. 2002;90(5):507-11.
Use of Point-Of-Care Ultrasound in the Diagnosis of Spontaneous Coronary Artery Dissection Presenting as a Myocardial Infarction in a Young Woman
Deepthi Devireddy, MS-2, Rebecca Loney, MD and Matthew Flannigan, DOIntroduction
TSpontaneous coronary artery dissection (SCAD) is a lesser known cause of myocardial infarction (MI) and acute coronary syndrome in young women. Women diagnosed with SCAD have fewer cardiovascular risk factors that rule in for an MI compared to women with atherosclerosis. Approximately 80% of total SCAD patients are women with a mean age of 43 years. In women aged less than 50 years, studies have shown SCAD to be the underlying cause of MI in 22-43% of cases. Early and accurate diagnosis of SCAD is vital, particularly since SCAD patients are generally young and do not present with many conventional cardiovascular risk factors, which can lead to a delayed or mistaken diagnosis. Coronary angiography, bedside ultrasound, and intravascular ultrasound have been identified as diagnostic tools. The ideal diagnosis and management of SCAD have not been identified through randomized trials, with case reports providing the bulk of information regarding clinical presentation and diagnosis.
Case Description
The patient is a 35 year old female who presents to the Emergency Department following acute onset of left-sided chest pressure with radiation to her left arm. Her pertinent history included an argument with her partner that morning which had caused her severe distress. The patient’s vitals were within normal limits. On exam she was diaphoretic and appeared distressed. Upper abdominal tenderness was noted. An initial EKG showed mild ST elevation in V1-V3 without reciprocal changes (Figure 1). At this point, Takotsubo cardiomyopathy was suspected due to the patient's highly emotional state and non-specific ST segment elevations.
A point-of-care ultrasound (POCUS), performed by an ultrasound-fellowship trained emergency medicine physician showed hypercontractile basilar segments with dyskinesia of lateral and apex segments (Video 1). Based on these ECHO findings, Stat Cardiology consult was requested and a repeat EKG was performed. The results of this second EKG were consistent with STEMI, with ST elevation >2mm in leads V2-V4 and some ST depression in leads II & aVF (Figure 2).

 Figure 1: Initial EKG with ST segment elevation indicated by white arrows.
Figure 1: Initial EKG with ST segment elevation indicated by white arrows.
The patient was taken immediately for left heart catheterization, which showed a left main coronary artery dissection extending down the left anterior descending artery. This finding prompted calling cardiothoracic surgery to the catheterization lab, where a diagnosis of cardiogenic shock secondary to SCAD of LAD/left main coronary artery was made. An emergency Coronary Artery Bypass Graft (CABG) surgery was performed. The patient’s left ventricular ejection fraction (LVEF) was severely depressed post-CABG and an intra-aortic balloon pump (IABP) was placed. She was discharged post-op day 12 following weaning off IABP.
Conclusions
Many patients with spontaneous coronary artery dissection may not be diagnosed with appropriate urgency due to low clinical index of suspicion for SCAD. Usage of POCUS and serial EKGs can be beneficial in identifying SCAD in patients presenting with non-traditional risk factors for cardiac events. Bedside POCUS can provide an immediate diagnosis in the right clinical setting, allowing for rapid initiation of treatment. SCAD can cause subtle findings such as ventricular dyskinesia, which can be identified through the use of POCUS. This helps identify patients that would benefit from more urgent coronary angiography, who would not otherwise meet standard criteria for emergent angiography Additional research should be conducted to identify outcome differences in utilizing POCUS in addition to standard cardiac work-up for a subset of patients at “high-risk” for SCAD. More data is also necessary to identify relevant criteria that may help identify this “high-risk” group of patients.
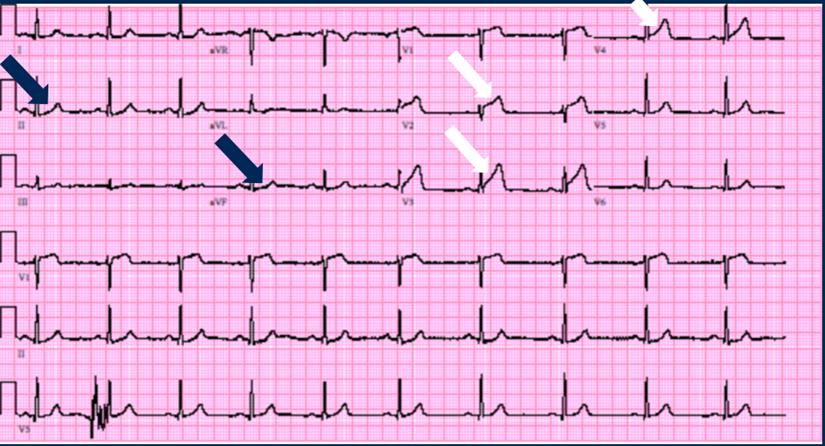 Figure 2: Repeat EKG with ST segment elevation indicated by white arrows and ST segment depression indicated by blue arrows.
Figure 2: Repeat EKG with ST segment elevation indicated by white arrows and ST segment depression indicated by blue arrows.
Out-side the Box US Section
Utilization of Ultrasound to Diagnose Periapical Abscesses in Austere Environments
Charles J. Mears, MD, Eric Sleasman, MS; Andrea Kaelin, MD and Jeremiah Gaddy, MDIntroduction
During the years of 2009-2012 spanning Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF), over 30,000 dental injuries/illnesses were reported, with 2.12% being diagnosed as periapical abscesses (1). Symptoms of periapical abscess are often non-specific, potentially leading to delay in diagnosis. Additionally, in austere environments with minimal resources and diagnostic capabilities, diagnosis becomes even more challenging. This case presents a review of periapical abscesses and how ultrasound (US) can be used as a diagnostic tool in resource limited settings.
Case Report
A 22-year-old United States service member presented to a remote Forward Operating Base (FOB) with a complaint of approximately 14 days of right-sided lip and facial pain and swelling. He was initially evaluated by an enlisted medical provider, diagnosed with sinusitis, and completed a 12 day course of oral amoxicillin. He continued to experience symptoms despite antibiotics and on reexamination was given a dose of intramuscular (IM) ceftriaxone. He was then transitioned to oral clindamycin, and then ertapenem for 7 days IM. Despite this aggressive antibiotic regimen, the patient continued to show no improvement, thus the decision was made to MEDEVAC the patient to a higher echelon of care.
Upon arrival at a Role 3 medical facility he was evaluated by an Emergency Physician (EP) and an enlisted medical provider. Vital signs were unremarkable and the patient was afebrile. Examination was significant for mild tenderness to the right maxilla superior to tooth #7. There was no erythema, warmth, or underlying fluctuance. Intraoral exam was normal. Additionally, he denied a history of dental trauma or poor dentition, prior oral surgeries, neck pain, vision changes or respiratory symptoms.
POCUS (Point of Care Ultrasound) performed by the EP and medic demonstrated an approximate 4 mm fluid collection over the maxilla (Fig. 1). The patient was then referred to dentistry the following day, where panorex imaging was obtained which showed no significant fluid collection, however clinically the patient continued to have swelling. Using ultrasound guided technique, an incision and drainage (I&D) was performed, expressing a significant amount of purulent material. Upon drainage of the abscess, the patient
experienced significant relief and clinically improved over the next few days, returning to duty and allowing the patient to remain in theater.

Method
Probe
L38 Linear Array Probe
Technique
Orient the probe in a sagittal plane on the surface of the face. Depending on the location of pain and swelling, start the scan at the midline of the face and work anterior to posterior For suspected maxillary abscess, work from the midline across the maxilla towards the tragus of the ear For mandibular abscess scan midline towards the ear lobule. Document depth and width of the abscess and approximate tooth number for localized incision and drainage.
Conclusion
Although the military takes precautions to limit dental emergencies through dental readiness, dental emergencies in conflict zones are still prevalent. As shown through the case presented above, there is a shortfall in the capability of outstations with limited medical resources to diagnose periapical abscesses, or potentially other dental diseases in the field. This shortfall led to the misdiagnosis of a patient, a deficit to the manpower of the force, and undue risk possibly to MEDEVAC platforms. Although this patient was effectively managed in-country and was MEDEVAC’ed once to a higher echelon of care, there is a distinct possibility that it could have been handled more effectively with the capability of ultrasound.
Figure 1: Service members POCUS demonstrating 4 mm periapical abscess (arrows).Ultrasound training and implementation to enlisted medic level health care providers could be a force enhancer moving forward. With ultrasound's ability to help identify dental diseases and the other capabilities, such as eFAST exams, this could be a low cost addition to the medics’ repertoire of skills.
References
1 Simecek, J W , Colthirst, P, Wojcik, B E , Eikenberg, S , Guerrero, A C , Fedorowicz, A , Szeszel-Fedorowicz, W , & DeNicolo, P (2014) The incidence of dental disease non battle injuries in deployed U S Army personnel Military Medicine, 179(6), 666–673. https://doi.org/10.7205/milmed-d-13-00511
