


WE AIM TO END MELANOMA
? w hile im proving t he lives of t hose it affect s.




? w hile im proving t he lives of t hose it affect s.

We direct, manage and fund COLLABORATIVE RESEARCH INITIATIVES, on a national and global scale. Our initiatives are innovative. We ask the world?s leading melanoma researchers what they need? what?s missing in the fight? and listen when they respond.
We speak on behalf of melanoma patients and families through our work in LEGISLATION, POLICY, AND ADVOCACY. The invited presence of our staff on numerous boards and committees that directly affect patients is one indication of our leadership in the world of melanoma.
The breadth, depth, and number of our EDUCATION AND SUPPORT resources for the melanoma community are second to none. Whether you?re a patient, survivor, caregiver, family member, healthcare provider, or researcher, we have so much to offer.
L E T T E R F R O M T H E P R E S I D E N T
2023 was a special and sobering year for the AIM at Melanoma Foundation. It was the 20-year ann versary of the death of my s ster, Charlie Gui d.
On November 24, 2003, Charlie d ed of Stage IVmelanoma. In the months before her death, she told my mother, Valerie Gu d, about her wish that other families wou d not have to go through what my family went through? that her death not be n vain. It was in those conversations that the idea for AIM at Me anoma was born, and the Foundation was officially established a few months ater.
In the 20 years since Charlie?s death, AIM has steadfastly remained true to ts mission: to support me anoma research; to educate the public and hea thcare providers; and to advocate on beha f of the melanoma community.
In 2024, AIM will ce ebrate our 20-year milestone a year long. But for this letter in this 2023 Annual Report, it?s important to remember Charlie and her wish. She wou d be proud of what AIM has accomp shed.
Th s annual report describes three notab e successes in 2023 for AIM at Melanoma? one from each of the areas of our miss on. Thank you: You are the reason for our successes, and we are grateful for your support.
Sam Gu d, President

AIM at Melanom a has so m any accom plishm ent s t o celebrat e
"We?renot just collectingtumor specimens. For everypatient in thebank, we arecollectingblood samples, and weareenteringthepatient?sentiremedical record into our database in a de-identified manner. Wearealso askingeach participant to take a comprehensivesurveyaskingeverythingfrom their eye color to their tanninghabitsto their familyhistoryof cancer. Thisdata? along with theblood and tissue? will beinvaluablefor research. I can? t wait to explorethetissue, blood, and data that wehave."
Dr. Jeffrey Wayne, MD, Professor of Surgery and Dermatology, Ch ef, Division of Surgical Oncology, Northwestern University

THEINTERNATIONALMELANOMATISSUEBANKCONSORTIUM(IMTBC)
At t he end of 2023, w e had collect ed 196 fresh frozen
prim ary t issues
Tissue collection ramped up in 2023 and discussions of the first joint research project began.

AIM is helping t o set up clinical t rials for pat ient s w it h m elanom a in count ries out side t he U.S.
All of society benefits when treatment clinical trials are run, because they answer important questions about whether new treatments are safe and whether they work. But in Europe, let alone in the Middle East and Africa, therapeutic clinical trials are not as common as in the U.S. This situation is due to the fact that so many research institutions are located here, and some of the largest pharmaceutical companies are based in the U.S. Additionally, the U.S. offers one of the most profitable drug markets in the world because the prices are largely unregulated by the U.S. government. However, pharmaceutical companies need to accrue enough patients to run their trials, so running trials beyond the U.S. is necessary. And if there are researchers and patients willing and able to participate, it?s better for everyone.
Treatment clinical trials can also benefit the country in which the trial is located. In order to run a clinical trial for an agent that could receive approval through the U.S. FDA,certain conditions must be met, one of which includes offering a tangible benefit to the population undergoing the ?burden?of the research. In undeveloped countries, it is often not allowed to burden the population with a clinical trial if the approval would only benefit people in developed countries that can afford the approved drug. To get around this, companiesagree to provide that agent to the undeveloped country that participated in the clinical trial which led to a successful approval.
Clinical trials can be good for individual patients for several reasons. In some countries, the standard of care lags behind the U.S., and treatment can be limited. A clinical trial may offer the newest treatment, so it could present a treatment benefit for patients with melanoma in those countries. Additionally, clinical trials offer close monitoring of patients and often cover the costs of the investigational agent and necessary tests to determine its safety and efficacy. Therefore, a clinical trial has the potential to offer patients better care than they may otherwise receive at little or no cost. This benefit is particularly clear if there is no other treatment option available. Finally, if there is already a standard of care, clinical trials for cancer usually add the investigational agent onto the standard treatment, giving the patient much more care than they otherwise would receive.
AIM has been working with a physician-researcher outside of the U.S. who sees a lot of a certain subtype of melanoma. Currently, there are very few optionsto treat this specific type of melanoma. In order to find a cure, more research is needed,which includes expanding the diversity of the pool for patients with this type of melanoma. The numbers can be very small if only one country isused for accrual, especially if the cancer israre in that region. This situation reduces the subject pool,the trial size, and the data collection. It may also delay development of a drug if patient accrual cannot be met.
Therefore, partnering with clinicians in other regions of the world providesa larger patient pool and thus, more information about treating it with a test agent.
We introduced this doctor to a pharmaceutical company that is interested in running a trial in this country. In 2023, the physician-researcher and the company began working on the epidemiological study in order to get approval to run the trial. Our fingers are crossed that the trial is approved!
Significant increase in new and com prehensive
cont ent on our w ebsit e, w hich t ranslat ed t o increased view ership

In 2023, our website witnessed a remarkable surge in traffic, achieving an all-time high of 839,313 views. Remarkably, 84%of 839,313 were returning v s tors.
We?re thril ed, because all who come to our website are seek ng and finding nformation about prevention, ear y detection, treatment, or other topics specific to the r needs.
We add new and comprehens ve content frequently, and new content s often a deep dive in the treatment landscape. New and widely v ewed items in 2023 nc uded:
- Notesfrom theLab: mu t ple articles that exp ain the latest treatment ideas and how they work
- All of our In Plain Englisharticles that were captured in a s ngle magazine on our webs te
- TheFutureofMelanoma Treatment in 2024, a webinar hosted by AIM and Anthony J. O szanski, MD, RPh, and Jeffrey M.Farma, MD, FACS, Co-Directors of Fox Chase Cancer Center?sMe anoma and Skin Cancer Program, in December 2023
- Our StageII Treatment Optionsbooklet
- New website sect ons on ocu ar melanoma and cl n cal trials,along with interviews and v deosthat support thiscontent

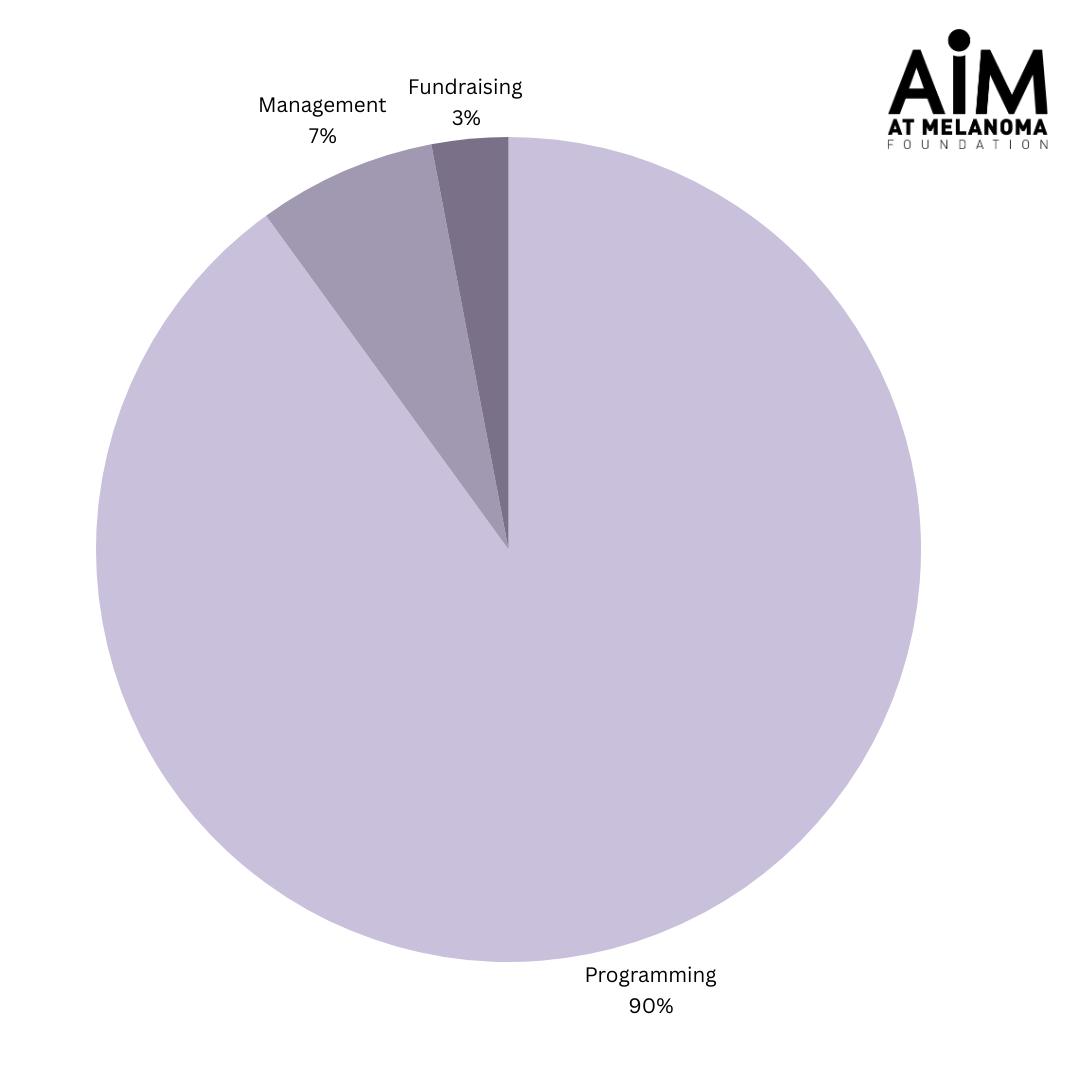
AIM at Melanoma?s goal is to end this disease in our lifetime while improving the lives of those it affects, and we are able to pursue this goal because of our supporters?generosity. We are committed to sound financial practices, transparency, and accountability, all of which are critical to attracting and keeping the generous funding that allows us to fulfill our mission. Thank you for your support and your t rust .
What w ill w e announce as accom plishm ent s in t he next annual report ?
- The Melanoma Tissue Bank Consortium nearing 250 tissues - AIM?s role on panels that receive Department of Defense funding to study melanoma - New content on the AIM at Melanoma website about melanoma in skin of color
Head over to AIMatMelanoma.org for more!
In addition to our generous donors, thank you to our Board of Directors and our Advisory Board. Together, we can #ENDMELANOMA.

