





• • • • •











EFFICACYANDSAFETYOFSAFINAMIDEVSOPICAPONEASANADJUNCT TOLEVODOPATHERAPYINELDERLYWITHPARKINSON’SDISEASE:A SYSTEMATICREVIEWANDINDIRECTTREATMENTCOMPARISONOF NETWORKMETA-ANALYSIS
FanMaitriAldian,MaxwellSalvadorSuryaAtmaja,MelissaValentinaAriyanto,Visuddho
[CorrespondenceE-mail:fanmaitri35@gmail.com]
Introduction: Parkinson's Disease, a chronic and progressive neurodegenerative disease, becomes a significant population health problem There is an urgent need as an adjunct therapy to levodopa, a gold standard for Parkinson's Disease treatment Safinamide and opicapone may be promising drugs as adjunct therapy of levodopa.
Objective: To compare the efficacy and safety of safinamide and opicaponeasanadjunctto levodopatherapyinPDtreatment.
Method: This systematic review and network meta-analysis of randomized controlled trials were conducted based on the PRISMA NMA Checklist of Items. The outcome of this study was evaluated efficacy (ON-time, OFF-time, UPDRS-III, and PDQ-39) and safety of safinamide and opicapone in Parkinson’s Disease Effect sizes were presented as mean difference for efficacy outcomes and Odds Ratio for safety outcomes with random-effects model and presented using both pairwise and Bayesian network meta-analyses.
Result: Fourteen RCTs were included in this study. The efficacy analyses showed that safinamide (MD = 0 87 h, 95% CrI = 0 31 - 1 48) was superior in increased total ON-time than opicapone (MD = 0.82 h, 95% CrI = 0.18 - 1.47). Safinamide also showed a superior in reducing OFF-time (MD = -1.33 h, 95% CrI = -2.45 - -0.196), UPDRS-III score (MD = -2.8, 95% CrI = -3 92 - -1 66), and PDQ-39 score (MD = -2 05, 95% CrI = -3 47 - -0 702) Safety analysis also showed that safinamide (OR = 1.04, 95% CrI = 0.83 - 1.3) is more applicable for older patients than opicapone (OR = 1.32, 95% CrI = 0.95 - 1.81).
Conclusion: In conclusion, our findings from this indirect treatment comparisonsupportthe use of safinamide in elderly patients with Parkinson's, which effectively inhelpinglevodopa inimprovingON-time,bettertoleratingmotorandnon-motorfeaturesduringtheOFFperiod, andincreasingthequalityoflifewithminimumadverseeffects.
Keywords: Parkinson’s Disease, Safinamide, Opicapone
Pre-ConferenceCompetitionAsianMedicalStudents’Conference2023:Taiwan

EFFICACYANDSAFETYOFSAFINAMIDEVSOPICAPONEASANADJUNCT TOLEVODOPATHERAPYINELDERLYWITHPARKINSON’SDISEASE:A SYSTEMATICREVIEWANDINDIRECTTREATMENTCOMPARISONOF NETWORKMETA-ANALYSIS
Authors:
FanMaitriAldian
MaxwellSalvadorSuryaAtmaja
MelissaValentinaAriyanto
Visuddho
2023
AMSA-UniversitasAirlangga
1
EFFICACYANDSAFETYOFSAFINAMIDEVSOPICAPONEASANADJUNCT TOLEVODOPATHERAPYINELDERLYWITHPARKINSON’SDISEASE:A SYSTEMATICREVIEWANDINDIRECTTREATMENTCOMPARISONOF NETWORKMETA-ANALYSIS
FanMaitriAldian,MaxwellSalvadorSuryaAtmaja,MelissaValentinaAriyanto,Visuddho
[CorrespondenceE-mail:fanmaitri35@gmail.com]
ABSTRACT
Introduction: Parkinson's Disease, a chronic and progressive neurodegenerative disease, becomes a significant population health problem. There is an urgent need as an adjunct therapy to levodopa, a gold standard for Parkinson's Disease treatment. Safinamide and opicapone may be promising drugs as adjunct therapy of levodopa
Objective: To compare the efficacy and safety of safinamide and opicaponeasanadjunctto levodopatherapyinPDtreatment.
Method: This systematic review and network meta-analysis of randomized controlled trials were conducted based on the PRISMA NMA Checklist of Items The outcome of this study was evaluated efficacy (ON-time, OFF-time, UPDRS-III, and PDQ-39) and safety of safinamide and opicapone in Parkinson’s Disease Effect sizes were presented as mean difference for efficacy outcomes and Odds Ratio for safety outcomes with random-effects model and presented using both pairwise and Bayesian network meta-analyses.
Result: Fourteen RCTs were included in this study. The efficacy analyses showed that safinamide (MD = 0 87 h, 95% CrI = 0 31 - 1 48) was superior in increased total ON-time than opicapone (MD = 0.82 h, 95% CrI = 0.18 - 1.47). Safinamide also showed a superior in reducing OFF-time (MD = -1.33 h, 95% CrI = -2.45 - -0.196), UPDRS-III score (MD = -2.8, 95% CrI = -3 92 - -1 66), and PDQ-39 score (MD = -2 05, 95% CrI = -3 47 - -0 702) Safety analysis also showed that safinamide (OR = 1.04, 95% CrI = 0.83 - 1.3) is more applicable for older patients than opicapone (OR = 1.32, 95% CrI = 0.95 - 1.81).
Conclusion: In conclusion, our findings from this indirect treatment comparisonsupportthe use of safinamide in elderly patients with Parkinson's, which effectively inhelpinglevodopa inimprovingON-time,bettertoleratingmotorandnon-motorfeaturesduringtheOFFperiod, andincreasingthequalityoflifewithminimumadverseeffects.
Keywords: Parkinson’s Disease, Safinamide, Opicapone
1. INTRODUCTION
As the global population ages, Parkinson's Disease (PD) becomes the most prevalent neurodegenerative movement disorder 1 PD is a chronic and progressive neurodegenerative disease that is devastating to both patients and caregivers due to its motor and non-motor symptoms.2 Striataldopaminergicneuronslossinsubstantianigra,whichresultsinadecrease of the neurotransmitter dopamine in the synaptic cleft, leads to the motor symptoms of PD,
2
which include resting tremor, bradykinesia, muscular rigidity, and postural reflex disturbance.3 Non-motor symptoms of PD, such as major neuropsychiatric symptoms, autonomous disorders, sleep disorders, and sensory disorders, are also frequent and debilitating to the patient.4,5 These symptoms cause misery to the patient, especially in the advancedstagesofPDpatients,asPDappearstobethemostcommoncauseof Parkinsonism term.6,7
WHO estimates that 8.5 million people were living with PD in 2019; its prevalence has doubled in 25 years, making it the most increasing neurological disorder among others.8 Aging is linear to PD's prevalence; while the general population's prevalence of PD is as much as 0.3%, it occurs in 1-2% of people over 60 years ofage,whichraisedto3.5%inthe group of 85-89 years of age.9 Surprisingly, younger people are not free from PD. 41 per 100,000 people aged 40 to 49 live with PD.2 An estimated 876,665 people live with PD in Indonesia.10 PD contributes to about 1,064,753 deaths from 1994 to 2019.Themortalityrate isincreasingalmostfourfold,from1.76per100,000populationin1994,to5.67in2019.8,11
PD may not be a "direct killer" disease, but it affects almost all aspects of the patient's life, such as increasing fall risk three times and fracture risk 2.24 fold higher than healthy people 12,13 PD impact in many aspects of life is also threatening, a major economic burden, with its incremental costs rising almost threefold, from1-year,asmuchas$9,625to$27,466 in 5-year PD patients.14 In 2017, it was estimated that PD had given a burden in economic aspect as much as $51.9 billion in the United States only, including direct medical costs of $25.4 billion, indirect costs of $14.2 billion, non-medical costs of $7.5 billion, and $4.8 billion due to disability; it is estimated that this number will surpass $79 billion by 2037.15 Other than aging, it turns out that family history, dyspepsia, and environmental factors such as persistent disclosure of pesticides, oils, metal, and general anesthesia were becoming the riskfactorsforPD.16
The main objective of PD therapy is to increase the postsynaptic dopamine receptor stimulation; it is basedondopaminereplacementtomanagemotorandnon-motorsymptoms, trying to avoid motor complications.17 Levodopa (L-dopa),firstsynthesizedin1911,remains a gold standard in PD treatment since its approval by FDA (Food and Drug Administration) in 1970.17,18 Nevertheless, due to loss of tonic dopaminergic regulation, changes in dopaminergic synaptic plasticity, the relatively short levodopa half-life,andthedevelopment of the wearing-off effect, levodopa's positive motor response turns out to be shortened over time; its long-term therapy is correlated with the development of motor and non-motor fluctuations and dyskinesias.19,20 During "On Episodes", their levodopa is working well, and their PD symptoms are improved, but some patients often experience "OFF-time", a flare of PD symptoms despite their standard levodopa therapy; therefore, the adjunct therapy is an urgentneedforlevodopainthetreatmentofPD.21
Acknowledging those mechanisms, some other therapy, such as dopamine agonists,MAO-B inhibitors, and COMT inhibitors, are added to optimize levodopa.22 Monoamine oxidase-B (MAO-B) and catechol-O-methyltransferase (COMT) are known to be involved in the dopamine inactivation pathway; thus, these two enzymes correlate with the dopamine bioavailability inthecentralnervoussystem.23,24 FDAapprovedopicapone,athird-generation
3
COMT inhibitor, in April 2020 for treatingend-of-motormotorfluctuationinadultswithPD as an adjunct therapy 25 Before that, in March 2017, FDA approved safinamide, an MAO-B inhibitor, as an adjunct therapy to levodopa for the patient experiencing "off" episodes.26 However, to date, no meta-analysis has been made comparing the efficacy of opicapone and safinamide as an adjunct therapy for PD treatment. Therefore, in this study, we aim to compare the efficacy and safety of opicapone and safinamide as an adjunct to levodopa therapyinPDtreatment.
2. METHODS
2.1. StudyDesign
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) NMA Checklist of Items (Appendix Table 8)27 and guided by the Cochrane Handbook for SystematicReviews forSystematicReviewsofInterventions.28
2.2. SearchStrategies
A computerized data search of the relevant studies was conducted on Pubmed, Cochrane, ProQuest, ScienceDirect, Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO, and Web of Science up to March 17, 2023. The following main keywords were initially established: “Parkinson’s Disease”, “Safinamide”,and“Opicapone”. We then added several Medical Subject Headings (MeSH) and other free text terms to construct the database-specific search terms. The full search terms for each database are provided in the Appendix Table 1 No publication date andlanguagerestrictionsweresetin allsearches.
2.3. SelectionofStudies
Search results for each database were collected and managed using Google Sheets (https://docs.google.com/spreadsheets/) (Google LLC, Mountain View, CA, USA). After deduplication, the remaining articles were screened based on title and abstract. All articles included in the next screening step were retrieved. After that, we retrieved studies with available and published full-texts and thoroughly assessed them according to the pre-specified eligibility criteria. The reasons for excluding each article from each screening step were reported asappropriateinthespreadsheet.Theliteraturesearchesandoverallstudy selection process were completed by all investigators (FMA, MSSA, and MVA). Any disagreementsareresolvedthroughfourthinvestigators(VV).
2.4. EligibilityCriteria
We used the Population, Index Test, Comparator, Outcome (PICO) framework29 (Table 1) designed for systematic reviews as the basis for formulating and establishing the eligibility criteria. To be included in the systematic review and meta- analysis, studies had to meet the following criteria: (1) the study population consisted of elderly patient with PD receiving levodopa; (2) include at least one adjuvant drug for the treatment of PD, including, safinamide or opicapone; (3) used placebo as control therapy; (4) evaluated efficacy (ON-time, OFF-time, Unified Parkinson’sDiseaseRatingScale(UPDRS)partIIIscores,and
4
Parkinson’s Disease Questionnaire (PDQ-39)) and safety (number of patients with at least had1adverseeffect);(5)employedRandomizedControlledTrial(RCT)design.Studieswere excluded if: (1) the title or abstract was irrelevant; (2) the full-text was irretrievable; (3) the studywasareviewarticle,casereport,caseseries,orconferenceabstract.
Table1.PICOFramework
Definition
Population
ElderlywithParkinson’sDiseaseReceivingLevodopa
Intervention SafinamideorOpicapone
Comparison Placebo
Outcome Efficacy (ON-time, OFF-time, UPDRS-III, and PDQ-39); Safety (numberofpatientswithatleasthad1adverseeffect)
Abbreviations: PICO, Population, Intervention, Comparison, and Outcome; UPDRS, Unified Parkinson’s Disease Rating Scale; PDQ-39, 39-item Parkinson’s Disease Questionnaire.
2.5. DataExtraction
Three investigators (FMA, MSSA, and MVA) extracted data from eachincludedstudyusing a pre-specified checklist developed and tabulated within thespreadsheetbyFMA.Afterthat, VV checked the collected data for their eligibility and any disagreements were promptly resolved. The data extracted include the name of the first author and year of publication, study location (continent), characteristic of population, diagnostic criteria, age, duration of PD (years), duration of treatment with levodopa (years), sample size, adherence rate,gender (% of females), Hoehn & Yahr stage(ON-phase,OFF-phase),dailyonepisode(hours),daily OFF-time (hours), period of treatments (weeks), frequency of drug administration, type of analysis (intention to treat [ITT] or per-protocol [PP]), trial arm (placebo, safinamide, or opicapone), trial size (analyze), efficacy outcome (ON-time, ON-time with troublesome dyskinesia, ON-time without dyskinesia,OFF-time,UPDRStotal,UPDRS-I,UPDRS-II(ON phase and OFFphase),UPDRS-III,UPDRS-IV,andPDQ-39),andsafety(numberofpatients with at least had 1 adverse effect). The extracted characteristics and outcomes of each includedstudywerethenpresentedqualitativelyinatabularformat.
2.6. AssessedOutcomes
The primary outcome of this study was efficacy (ON-time, OFF-time, UPDRS-III, and PDQ-39). ON-time is when levodopa is working well and the symptoms are controlled while OFF-time is when levodopa is no longer working well and returns to or worsening of parkinsonian features 30,31 The increasing of ON-time and decreasing of OFF-time showed the reduction of Parkinsonian features, such as bradykinesia and rigidity.1 UPDRS-III is the most widely applied rating instrument for PD, especially contributing in motor function subscales 32 It contains 27 items, with each item scored on a 5-point scale (from 0 to 4) The total score of part III may range from 0 (no disability) to 108 (total disability) and it is rated by the Investigator.33 Decreasing UPDRS-III score indicates that there is an improvement of motor function ability, such as speech, facial expression, hand movements, leg ability, and
Componentsof PICO
5
postural stability.34 PDQ-39 is a 39-item self-report questionnaire35 and measures the health status and quality of life in PD patients over the last month It assesses how often people with Parkinson's experience difficulties across 8 dimensions of daily living including relationships, social situations and communication. It also evaluates the impact of Parkinson's on specific dimensions of functioning and wellbeing 36 Decreasing PDQ-39 scores showed improving health-related quality of life (HRQoL) on PD patients.37 While the secondary outcome is safety. Assessed safety outcomes were the number of patients with at least one adverse effect during the treatment
2.7. QualityAssessmentofIndividualStudies
Two reviewers (MSSA and MVA) independently conducted a methodological quality assessment to evaluate the risk of bias of each eligible study using the Cochrane Collaboration’s RiskofBias2(RoB2)tool.38 Disagreementsofjudgementswereresolvedby a group discussion involving a third reviewer (FMA). The RoB 2isarevisedtoolconsisting of five bias domains explicitly designed to consider the risk of bias of randomized trials arising from: (1) the randomization process; (2) deviations from intended interventions; (3) missing outcome data; (4) the measurement of the outcome; and (5) the selection of the reported result. The risk of bias on each domain was rated as low risk, high risk, or some concerns (unclear) to the algorithms that incorporated several domain-specific signaling questions. Judgment levels from all domains were later deducedasanoverallriskofbiasfor each study A study is consideredlowriskofbiasifalldomainsshowlowrisk.Ifatleastone domain was rated as unclear, studies were judged as having some concerns. Studies were judged to be athighriskofbiasifatleastonedomainpresentsahighriskorthereweresome concerns in multiple domains that could significantly lower the confidence in the study results.
2.8. StatisticalAnalysis
2.8.1. NetworkMeta-Analysis(NMA)
The synthesized outcomes (effect sizes) included mean difference (MD) among continuous outcomes (change in ON-time, OFF-time, UPDRS-III score, and PDQ-39 score), and Odds Ratio (OR) among dichotomous outcomes (number of patients at least had adverse effects). We calculated missing SD from p-values, t-values, and SE or imputed them withavalidated method according to recommendations from the Cochrane Handbook chapter 7.7.3.2.28 The effect sizeswereestimatedusingbothpairwise39,andBayesiannetworkmeta-analyses40 were performed using MetaInsight version 4.0.41 The random-effects model was used to allow for inevitable heterogeneity42 and assumed exchangeability between treatments, ie., direct and indirect evidence concurs.43 The consistency between direct and indirect evidence was not applicable due to the absence of direct evidence. The rating of heterogeneity was assessed based on CINeMA44-46 by comparing the 95% confidence interval (CI) with the 95% prediction interval and the clinically meaningful threshold. We also assessed statistical heterogeneity in each pairwise and network meta-analysis comparison using τ² and I2 statistics. Surface under the cumulative ranking curve (SUCRA) valueswereestimatedasan additional measure to reflect ranking of each treatment in the network.47 SUCRA values are
6
expressed as a percentage (0-100%) and represent the relative probability of an intervention being among the best options in the network. The higher the value,thehighertherankingof the treatment in the network. We present the results of the SUCRA values with Litmus Rank-O-GramandRadialSUCRAplot.48
We evaluated the transitivity assumption by comparing the distribution of potential effect modifiers, such as sample size, duration of study, mean age, etc, across studies grouped by comparison. We assessed individual studies with the Cochrane risk of bias tool.38 Finally, to assess the credibility of the finding of each network meta-analysis, the findings were incorporated into the Confidence inNetworkMeta-Analysis(CINeMA)application,whichis anadaptationoftheGradingofRecommendationsAssessment,Development,andEvaluation (GRADE)approach.44-46
2.8.2. Subgroup,SensitivityAnalysis,andMeta-RegressionforNMA
To explore the potential impact of differences in patient characteristics across studies on findings observed in the analysis and to assess comparative efficacy in clinically relevant subpopulations, subgroup analyses were performed for the NMA. Subgroups were formed based on one set of variables for which there was sufficient comparative data: (1) Study location (Europe and Non-Europe).Furthersubgroups,suchasdifferentstagingofdiagnostic criteria between studies, were explored but were not possible, owing to a lack of studies in some comparisons. Sensitivity analyses for all outcomes were conducted in one way by excluding moderate and high-risk of bias studies. After the analysis, the significance of the meta-analysisresultswasre-evaluated.
We determined whether the distribution differences of potential effect modifiers were large enough to threaten the validity oftheanalysisbycomparingthedistributionofthesepossible effect modifiers across treatments included in the network meta-analysis by assessing their actual impact on the treatment effect through meta-regression analyses.49 A meta-regression analysis was performed to determinetherelationshipofpotentiallyconfoundingfactors(year of publication, sample size, mean age, proportionoffemales,durationofPD,adherencerate, baseline H&Y score, baseline ON-time duration, baseline OFF-time duration, and period of intervention)tomagnitudeoftheeffectonalloutcomes.
3. RESULTS
3.1. OverviewofStudySelection
A PRISMA flowchart of the overall study selection process is depicted in Figure 1. Initial searches in the seven databases resulted in a total of 2970 hits. 1888 records were then removed automatically by automation tools. We identified duplicates, and 348 records were subsequently removed. Of the remaining 734 records, 477 and 92 were excluded based on their title and abstract, respectively. A total of 76 reports identified as trial registers, conference abstracts, or with no access to full texts were not retrieved. Afterward, we thoroughly assessed the remaining 89 full texts for eligibility, and 75 were further excluded due to their type and method of interventions (n =15),reportedandevaluatedoutcomes(n = 19), and studydesign(n =41).Accordingly,theoverallscreeningprocessledtotheinclusion of14RCTs50-63 inthissystematicreviewandnetworkmeta-analysis.
7
3.2. CharacteristicsandOutcomesofIncludedStudies
The characteristics of the included studies are summarized in Appendix Table 2 The total sample accumulated from 14 RCTs was 5683elderlypatients(meanage:57.4to69.5years), from which 2186 received safinamide, 1477 opicapone, and 2020 werecontrolsamples.The study sample sizes were variable, ranging from 30 to 783. The totalnumberoffemalesfrom all studies was 2388, which accounted for 42.01% of the entire studypopulation.Allstudies use the Hoehn and Yahr scale to measure Parkinson's symptoms progress and the level of disability. Of 14 studies, sixwerelocatedinEurope,andtherestwereinAmerica(n =4)and Asia(n =4).
The mean duration of PDinthepopulationsamplesrangedfrom2.4to8.3years,wheremost of the studies have the mean daily ON-time for 9 to 10 hours. Most studies carried out interventions for 24 weeks (n = 5), ranging from 13 to 78 weeks. All the studies provided once-daily treatment and reported outcomes using ITT analyses. All studies were 2-arm studies;allhadplaceboarms,9hadsafinamidearms,and5hadopicaponearms.
Figure 2 shows the established networks for comparison. Each node represents a treatment. Connections between nodes denote direct comparisons. The node size and thickness of connectionsvaryaccordingtothenumberofstudiesinvolvedinacomparison.
3.3. QualityAssessmentofIncludedStudies
The result of the domain-specific quality assessment is provided in Figure 3, while each study's detailed risk of bias evaluation is summarized in Figure 4 According to Cochrane's RoB 2 tool, twelve studies were rated as low risk in all domains, thus having a low overall riskofbias.TwoRCTswereratedashavingsomeconcerns,withunclearriskin1domain.
8
CINAHL,CumulativeIndextoNursingandAlliedHealthLiterature;CENTRAL,CochraneCentralRegister ofControlledTrials;PRISMA,PreferredReportingItemsforSystematicReviewsandMeta-Analyses
3.4. NetworkMeta-AnalysisResults

3.4.1. NetworkMeta-Analysis
We performed pairwise (Appendix Figure 1) and Bayesian (Figure 5) network meta-analyses to explore the differences between safinamide and opicapone used to treat patients with PD. In terms of efficacy, safinamide (MD = 0.87 h,95%CrI=0.31-1.48)and opicapone (MD = 0.82 h, 95% CrI=0.18-1.47)increasedpatients’totalON-timecompared to placebo. Safinamide (MD = -1.33 h, 95%CrI=-2.45--0.196)reducedthetotalOFF-time while opicapone (MD = 0.121, 95% CrI = -1.11 - 1.58) increased the total OFF-time compared to placebo. We also found similar results in the PDQ-39 score, where safinamide (MD = -2.05, 95% CrI=-3.47--0.702)reducedandopicapone(MD =0.22,95%CrI=-1.34 - 1.81) increased the PDQ-39 score. For the UPDRS-III score, both safinamide (MD = -2.8, 95% CrI = -3.92 - -1.66) and opicapone (MD = -0.786, 95% CrI = -2.73 - 1.19) reducedthe UPDRS-IIIscore(Table2).
Figure1.PRISMAFlowchartoftheStudySelectionProcess
9
Figure2.NetworkofEligibleComparisonsforEfficacyandSafetyOutcomes
Thewidthofthelinesandsizeofnodesareproportionaltothenumberoftrialscomparingeachpairof treatments.UPDRS-III,TheUnifiedParkinson’sDiseaseRatingScalePart3;PDQ-39,39-itemParkinson’s DiseaseQuestionnaire

10
Regarding safety (Table 3), patients who got safinamide (OR = 1.04, 95% CrI = 0.83 - 1.3) and opicapone (OR = 1.32, 95% CrI = 0.95 - 1.81) have a greater chance of experiencing at leastoneadverseeffectduringthetreatments.
3.4.2. SensitivityAnalysis

A sensitivity analysis was performed to derive conclusions about whether the effect of excluding the moderate and high risk of bias studies by Borgohain et al., 201356 and Borgohain et al., 201458 showed similar results for efficacy and safety outcomes. The efficacy outcomes, change in ON-time, OFF-time, and UPDRS-III scores showed identical results to the main analysis. However, we could not do the sensitivity analysis for changein the PDQ-39 score because all studies have a low risk of bias. The results were robust for safety outcomes where safinamide (OR = 1.13, 95% CrI = 0.85-1.49)andopicapone(OR =
 Figure3.Domain-SpecificResultsofQualityAssessmentofIncludedStudiesusingtheRiskofBias2tool
Figure4.DetailedQualityAssessmentSummaryofIncludedStudiesusingtheRiskofBias2tool
Figure3.Domain-SpecificResultsofQualityAssessmentofIncludedStudiesusingtheRiskofBias2tool
Figure4.DetailedQualityAssessmentSummaryofIncludedStudiesusingtheRiskofBias2tool
11
1.33, 95% CrI = 0.95 - 1.83) have similar results to the primary analysis. The effectsizesof Bayesiannetworkmeta-analysisareshowninAppendixFigure2.
Table2.NetworkMeta-AnalysisforEfficacyOutcomes
Thecomparisonsshouldbereadfromlefttoright Forefficacy,dataareinmeandifference(95%CI),anddata above0favorforchangeinON-time,andviceversafortheothers
Table3.NetworkMeta-AnalysisforSafetyOutcomes
Comparisonsshouldbereadfromlefttoright Comparisonsupandbelowthetreatmentareinverse Forsafety, dataareoddsratio(95%CI),anddatabelow1favorthecolumn-definingtreatment.


12
anRandomEffectConsistencyModelofEfficacyand einOFF-time.(C)ChangeofUPDRS-IIIscore.(D) patientswhoatleasthadadverseeffects.
gScalePart3;PDQ-39,39-itemParkinson’sDisease nnaire
3.4.3. SubgroupAnalysis
Subgroup analysis for studies conducted in Europe or outside Europe are reported with a random-effects model. For both subgroups, there were no differences in the changes in UPDRS-III scores (Appendix Table 3). However, we found substantial differences in the ON-time, OFF-time, and PDQ-39 score changes, and number of patients who at least had adverseeffects.
3.4.4. Meta-RegressionAnalysis
Results of meta-regression analyses are presented in Appendix Table 4. We found no significant differences in most of the covariates in all outcomes. However, meta-regression analysis showed that the duration of PD had a significantdifferenceinUPDRS-IIIoutcomes (p = 0.01). We also found that publication year (p < 0.01) and length of intervention (p = 0.04) also had significant differences in PDQ-39 scores. Meta-regression analysis also showed that mean age significantly differs in the numberofpatientswhohadadverseeffects (p = 0.01). However, wewereunabletoperformameta-regressionregardingtheeffectofthe H&Y score and baseline ON-time duration on the UPDRS-III score due to the insufficient numberofstudies.





3.4.5. RankProbability
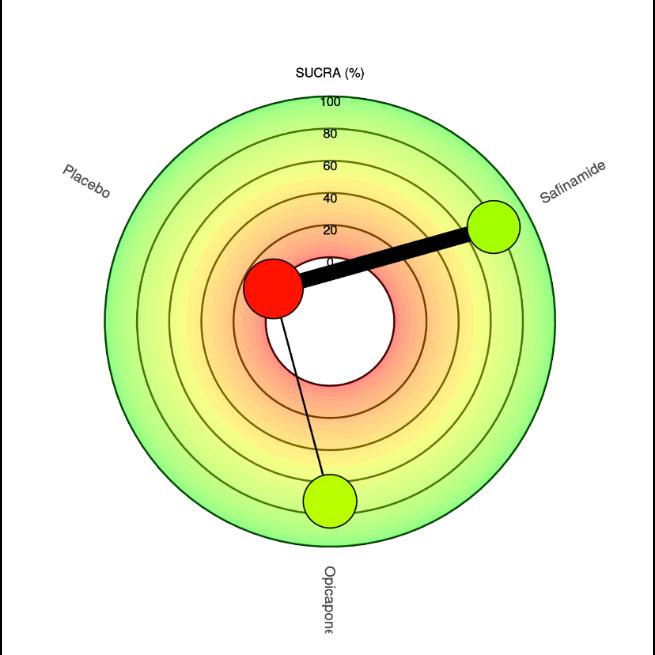
Figure 6 Shows a ranking chart and radial SUCRA plot of the probability of each target strategy ranked in terms of efficiency According to the SUCRA values and rankprobability of the efficacy between safinamide and opicapone, safinamide (SUCRA, 77.5) ranked the highest in extending the ON-time, followed by opicapone (SUCRA, 71.8) and placebo
13
(SUCRA, 0.6). Further, safinamide (SUCRA, 97.4) ranked the highest in reducing total OFF-time, followed by placebo (SUCRA, 30) and opicapone (SUCRA, 22.4). In addition, safinamide (SUCRA, 98.1) ranked the highest in reducing UPDRS-III score, followed by opicapone (SUCRA, 43.5) and placebo (SUCRA, 8.2). Safinamide (SUCRA, 98.8) also ranked the highest in reducing PDQ-39 score, followed by placebo (SUCRA, 31.7) and opicapone (SUCRA, 19.4). Lastly, placebo (SUCRA, 80.2) ranked the least treatment to make adverse effects, followed by safinamide (SUCRA, 62) and opicapone (SUCRA, 7.7).



WealsosummarizedAll SUCRAvaluesinLitmusRank-O-GraminAppendixFigure3.
 A. Changeinon-time
A. Changeinon-time
Treatment Rank1 Rank2 Rank3 SUCRA Treatment Rank1 Rank2 Rank3 SUCRA Opicapone 045 054 001 71.80 Opicapone 0 03 0 38 0 58 22 48 Placebo 000 001 099 0.68 Placebo 0 01 0 58 0 41 30 06 Safinamide 055 044 000 77.53* Safinamide 0 96 0 04 0 01 97 46*
B. Changeinoff-time
C. ChangeofUPDRS-IIIscore
Treatment Rank1 Rank2 Rank3 SUCRA Treatment Rank1 Rank2 Rank3 SUCRA Opicapone 0 04 0 80 0 16 43.57 Opicapone 0 02 0 36 0 63 19.47 Placebo 0 00 0 16 0 84 8 25 Placebo 0 00 0 63 0 37 31 68 Safinamide 0 96 0 04 0 00 98.18* Safinamide 0 98 0 02 0 00 98.86* 14
D. ChangeofPDQ-39score
HigherSUCRA(SurfaceUndertheCumulativeRankingCurve)valuesindicatebettertreatments;sizeofnodes representnumberofparticipantsandthethicknessoflinesindicatenumberoftrialsconducted.*meansthe treatmentwiththehighestSUCRAvalueinaspecificoutcome
3.5. QualityofEvidence(GRADE),Heterogeneity,andPublicationBias

The certainty of the evidence was low to high (Appendix Table 5). We found there are no concerns within-study bias and indirectness in all comparisons as we can see in Appendix Table 6 & Appendix Table 7. However, we still found some major concernsinimprecision andheterogeneitythatsummarizedin AppendixTable5
4. DISCUSSIONS
4.1. MainFindings
We found that safinamide is superior in increasing patients’ total ON-time compared to opicapone. Patients with safinamide also had a better reduction on the total OFF-time compared to patients with opicapone. These findings indicate that safinamide is more effective in reducing parkinsonian features such as depression, apathy and an unwillingness toparticipateinsocialactivities.31 Thesymptomscontrolmayberelatedtolevelsoflevodopa which are maintained by these drugs. The difference in the pharmacokinetic profile of safinamide and opicapone may be responsible for these findings. Thehalflifeeliminationof safinamide is 20 to 26 hours while opicapone is 1 to 2 hours.64,65 The prolonged levodopa effect can be responsible for better results for safinamide.66 However, despite the short half-life of opicapone, the observed half-life of opicapone-induced COMT inhibition in human redbloodcellswas61.6±37.6hours.67 Thisisthemainreasonthattheopicaponecan beusedoncedailyforadjuvanttreatment.
E.
Treatment Rank1 Rank2 Rank3 SUCRA Opicapone 004 008 088 7.70 Placebo 062 036 002 8028* Safinamide 034 055 010 62.02
Numberofpatientswhoatleasthadadverseeffects
Figure6.SUCRAValueandRadialSUCRAplot
15
Schapira AH et al.52 and Bette S et al.26 reported that safinamide is most effective as an adjuvant to levodopa in patients with PD to improve on time and reduce wearing off.26,52 Beside its potent inhibition of MAO-B, it also reduce presynaptic abnormal glutamate release, modulating the glutamatergic stimulation with consequent stabilization of moodand pain relief.68,69 Thus, safinamide may help PD patients better tolerate motor and non-motor features during the OFF period.33 This could be the explanation why it is possible to reduce theOFFtimeandimprovetheONtimewithorwithouttroublesomedyskinesias.
We also found that safinamide can decrease UPDRS-III and PDQ-39 values more than opicapone. From these findings, we can conclude that safinamide is more effective in reducing motor fluctuationsand reducing the frequency of Parkinson's patients experiencing difficulties in daily activities, suchasrelationships,socialsituationsandcommunications.26,33 This may be explained by theassociationofUPDRS-IIIandPDQ-39withbetteron-timeand off-time in safinamide.70 Our studies are comparable to thepreviousstudiesthatassessedthe efficacy of safinamide and opicapone in UPDRS-III and PDQ-39 outcomes.71,72 A previous study by Tsuboi et al.73 also showed that safinamidecouldsignificantlyimprovethePDQ-39 domain after 2 years.73 At the same time, opicapone showed consistent results over 52 weeks.74
Our findings also showed the higher number of patients who at least had adverse effects in patients with opicapone than safinamide. However, this result must be concluded with consideration of the tolerability of both drugs. The use of safinamide must be adjusted for patients with hepatic impairment and cannot be used concomitant with methylphenidate, amphetamine, and their derivatives, or dextromethorphan.65 76% of safinamide dose is cleared from renal, even its pharmacokinetic was not affected by impaired renal function based on existing evidence.65 Opicaponedemonstratedthelowestpotentialforcytotoxicityin comparison with other COMT inhibitors.75 Its use may be avoided in patients with catecholaminesecretingneoplasms.64
These findings also show an increase in treatment-related adverse events (trAEs) in elderly patients of both safinamide and opicapone. Our findings align with other studies; it is reasonable becausethereisachangeinthemetabolicandpharmacokineticprofileofthedrug associated with aging, making trAEs more frequent in the elderly 76 However, trAEs in the elderly treatment using opicapone are found to be more significant than safinamide; a study by Azevedo Kauppila et al.77 reveals that there are greater trAEs in the treatment using opicapone for elderly patients, which causes a larger number of discontinuation of the treatment. This finding concludes that safinamide ismoreapplicableforolderpatientsdueto its age-related safety.78 A study by Lo Monaco etal.79 showsthatsafinamideissafe,evenfor olderpatients,increasingelderlypatients'compliance.
4.2. AccessibilityofSafinamideandOpicapone
Indonesia's National Agency of Drug and Food Control (Badan Pengawas Obat dan Makanan) has given marketing authorization for safinamide and opicapone as adjunctive therapy in PD.80 However, safinamide turns out to be slightly better in market distribution. Since its approval in Europe and The United States in 2015 and 2017, respectively, it was already available in vast amounts in 12 countries in 2017.81,82 The use of safinamide as an
16
adjunct therapy for levodopa in PD treatment has also been proven in Asia, such as Japan, with its approval in 2020, and China, which has started its trial since August 2019.83,84 This indirectcomparisonmeansthatsafinamideismoreaccessiblethanopicapone.
4.3. Cost-EffectivenessofSafinamideandOpicapone
Since levodopa is the gold standard for treating PD, current studies have shown a decline in the brain to levodopa efficacy over time. Safinamide and opicapone have succeeded in showing their respective advantages, which we have analyzed in this indirect treatment comparison. However,westillneedtoconsiderthecost-effectivefactorforsociety,wherewe can measurethebalancebetweenthecostandtheeffectivenessprovided.Fromtheresultswe got in all the outcomes, we can see that safinamide is superior to opicapone. For standard treatment, safinamide is given once daily at a dose of 50 mgandincreasedto100mgafter2 weeks. For a 50 mg tablet, safinamide is estimated at around 6.9 USD, with the Average annual drug cost of 2,520 USD.85 Opicaponeisalsogivenoncedailyatadoseof50mg.The average price of 50 mg tablets of opicapone is 23.85 USD, and theaverageannualdrugcost is 8705.25 USD.86 Based on these findings, the safinamide cost is more effective than opicapone.
4.4. StrengthandLimitations
To the best of our knowledge, this is the first comprehensive systematic review and indirect treatment comparison that compared the effectiveness and safety of safinamide and opicapone on Parkinson's patients. We synthesized evidence from available RCTs, where these study designs are regarded as the most suitable and recommended way to evaluate the efficacy of interventions.87 Furthermore, the total number of samples included in most analyses was relatively sufficient, supported by the wide range of areas covered in Europe, Asia, and America, which may strengthen the generalizability of the study's conclusion. In additiontothelong-termeffects,werevealedthatsafinamideandopicaponearestilleffective after treatments for 2 years. Subgroup and meta-regression analyses were also conducted to searchforpotentialvariablesaffectingthepooledresults.
Although we have made everyefforttoprovidethebestpossiblequalityofthestudy,wealso acknowledged that there are still some limitations. First, we couldn't do subgroup and meta-regression analyses in some covariates due to the lack of studies. Second, we also couldn'tconductconsistencybetweendirectandindirectevidencethatwasnotapplicabledue to the absence of direct evidence. Lastly, we still found some major concerns inimprecision and heterogeneity evaluation, making a downgrade of confidence rating. However, we have already conducted subgroup and meta-regression meta-analyses that revealed most of the concernsarecausedbythedifferenceinlengthofinterventionsandmeanageacrossstudies.
5. CONCLUSION
In conclusion, our findings from this indirect treatment comparison support the use of safinamide in elderly patients with Parkinson's, which effectively in helping levodopa in improving ON-time, better tolerating motor and non-motor features during the OFF period, and increasing the quality of life with minimum adverse effects. Expectedly, the longer duration of treatment using safinamide could increase the therapeutic effects. However
17
considerations regarding the clinical condition of the drug such as side effects and contraindications need to be taken into account before deciding on a therapeutic option. We hope that our results can be beneficial for constructing future treatment guidelines for Parkinson'sDisease.
6. ACKNOWLEDGEMENTS
Wewanttothankstudiesthatareincludedinthisindirecttreatmentcomparison.
7. CONFLICTOFINTEREST
Theauthorsdeclarenoconflictofinterest.
18
8. RERERENCES
1. Balestrino R, Schapira AHV. Parkinson disease. European Journal of Neurology 2019;27:27–42 doi:10 1111/ene 14108
2 Pringsheim T, Jette N, Frolkis A, Steeves TDL The prevalence of parkinson's disease: A systematic review and meta-analysis. Movement Disorders 2014;29:1583–90. doi:10.1002/mds.25945.
3 Marino BLB, de Souza LR, Sousa KPA, Ferreira JV, Padilha EC, da Silva CHTP, et al. Parkinson’s disease: A review from pathophysiology to treatment. Mini-Reviews in Medicinal Chemistry 2020;20:754–67. doi:10.2174/1389557519666191104110908.
4 Kim H-S, Cheon S-M, Seo J-W, Ryu H-J, Park K-W, Kim JW Nonmotor symptoms more closely related to parkinson's disease: Comparison with normal elderly Journal of the Neurological Sciences 2013;324:70–3. doi:10.1016/j.jns.2012.10.004.
5. Pfeiffer RF. Non-motor symptoms in parkinson's disease. Parkinsonism & Related Disorders 2016;22 doi:10 1016/j parkreldis 2015 09 004
6. Mirpour S, Turkbey EB, Marashdeh W, El Khouli R, Subramaniam RM. Impact of DAT-SPECT on management of patients suspected of parkinsonism. Clinical Nuclear Medicine 2018;43:710–4 doi:10 1097/rlu 0000000000002240
7 Lill CM, Klein C Epidemiologie und ursachen der parkinson-erkrankung Der Nervenarzt 2017;88:345–55. doi:10.1007/s00115-017-0288-0.
8. WHO. Parkinson disease. World Health Organization 2022. https://www who int/news-room/fact-sheets/detail/parkinson-disease#:~:text=Overvie w-,Parkinson%20disease%20(PD)%20is%20a%20degenerative%20condition%20of% 20the%20brain,pain%20and%20other%20sensory%20disturbances).
9 Rizek P, Kumar N, Jog MS An update on the diagnosis and treatment of parkinson disease. Canadian Medical Association Journal 2016;188:1157–65. doi:10.1503/cmaj.151179.
10. Kassandra F. Hubungan Antara Penggunaan Obat Anti Hipertensi dan Penyakit Parkinson di Rumah Sakit Bethesda Yogyakarta; 2017
11. Lampropoulos IC, Malli F, Sinani O, Gourgoulianis KI, Xiromerisiou G. Worldwide trends in mortality related to parkinson's disease in the period of 1994–2019: Analysis of vital registration data from the Who Mortality Database Frontiers in Neurology 2022;13. doi:10.3389/fneur.2022.956440.
12. Farombi TH, Owolabi MO, Ogunniyi A. Falls and their associated risks in parkinson's disease patients in Nigeria Journal of Movement Disorders 2016;9:160–5 doi:10 14802/jmd 16011
13. Schini M, Bhatia P, Shreef H, Johansson H, Harvey NC, Lorentzon M, et al. Increased fracture risk in parkinson's disease – an exploration of mechanisms and consequences
19
for fracture prediction with frax. Bone 2023;168:116651.
doi:10 1016/j bone 2022 116651
14 Albarmawi H, Zhou S, Shulman LM, Gandhi AB, Johnson A, Myers DE, et al The economic burden of parkinson disease among Medicare beneficiaries. Journal of Managed Care & Specialty Pharmacy 2022;28:405–14.
doi:10 18553/jmcp 2022 28 4 405
15. Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, et al. Current and projected future economic burden of parkinson’s disease in the U.S. Npj Parkinson's Disease 2020;6 doi:10 1038/s41531-020-0117-1
16 Belvisi D, Pellicciari R, Fabbrini A, Costanzo M, Pietracupa S, De Lucia M, et al Risk factors of parkinson disease. Neurology 2020;95.
doi:10 1212/wnl 0000000000010813
17 LeWitt PA Levodopa therapy for parkinson's disease: Pharmacokinetics and pharmacodynamics. Movement Disorders 2014;30:64–72. doi:10.1002/mds.26082.
18. Tambasco N, Romoli M, Calabresi P. Levodopa in parkinson’s disease: Current status and future developments Current Neuropharmacology 2018;16:1239–52
doi:10.2174/1570159x15666170510143821.
19. Charvin D, Medori R, Hauser RA, Rascol O. Therapeutic strategies for parkinson disease: Beyond dopaminergic drugs. Nature Reviews Drug Discovery 2018;17:804–22 doi:10 1038/nrd 2018 136
20. Salat D, Tolosa E. Levodopa in the treatment of parkinson's disease: Current status and new developments. Journal of Parkinson's Disease 2013;3:255–69.
doi:10 3233/jpd-130186
21 Hauser RA, LeWitt PA, Comella CL On demand therapy for parkinson’s disease patients: Opportunities and choices. Postgraduate Medicine 2021;133:721–7.
doi:10 1080/00325481 2021 1936087
22 Fox SH Non-dopaminergic treatments for motor control in parkinson’s disease Drugs 2013;73:1405–15. doi:10.1007/s40265-013-0105-4.
23. Sampaio TF, dos Santos EU, de Lima GD, dos Anjos RS, da Silva RC, Asano AG, et al MAO-bandcomtgenetic variations associated with levodopa treatment response in patients with parkinson's disease. The Journal of Clinical Pharmacology 2018;58:920–6. doi:10.1002/jcph.1096.
24 Pahwa R, Lyons KE Treatment of early parkinson's disease Current Opinion in Neurology 2014;27:442–9 doi:10 1097/wco 0000000000000113
25. Greenwood J, Pham H, Rey J. Opicapone: A third generation COMT inhibitor. Clinical Parkinsonism & Related Disorders 2021;4:100083.
doi:10 1016/j prdoa 2020 100083
20
26. Bette S, Shpiner DS, Singer C, Moore H. Safinamide in the management of patients with parkinson’s disease not stabilized on levodopa: A review of the current clinical evidence. Therapeutics and Clinical Risk Management 2018;Volume 14:1737–45. doi:10.2147/tcrm.s139545
27. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations Ann Intern Med 2015;162(11):777-784.
28. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6 3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
29 Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Services Research. 2014;14(1).
30. Europe P. Wearing off and motor fluctuations [Internet]. Wearing off and motor fluctuations | Parkinson's Europe Available from: https://www parkinsonseurope org/
31. Olanow CW, Poewe W, Rascol O, Stocchi F. on‐demand therapy for off episodes in parkinson's disease. Movement Disorders. 2021;36(10):2244–53.
32. Sánchez-Ferro Á, Matarazzo M, Martínez-Martín P, Martínez-Ávila JC, Gómez de la Cámara A, Giancardo L, et al Minimal clinically important difference for UPDRS-III in daily practice. Movement Disorders Clinical Practice. 2018;5(4):448–50.
33. Cattaneo C, Jost WH, Bonizzoni E. Long-term efficacy of safinamide on symptoms severity and quality of life in fluctuating parkinson’s disease patients Journal of Parkinson's Disease. 2020;10(1):89–97
34. Unified Parkinson Disease Rating Scale (UPDRS) [Internet]. Theracycle Physical Therapy Exercise Bike and Rehabilitation Equipment Available from: https://www theracycle com/
35. Parkinson's disease questionnaire - 39 [Internet]. Shirley Ryan AbilityLab. Available from: https://www.sralab.org/
36 The parkinson's disease questionnaire (PDQ-39) [Internet] Parkinson's UK 2020 Available from: https://www.parkinsons.org.uk/.
37. Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ, Kurtis MM. Impact of pharmacotherapy on quality of life in patients with parkinson’s disease CNS Drugs 2015;29(5):397–413
21
38. Sterne JAC, SavovićJ, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A revised tool forassessing risk of bias in randomised trials BMJ 2019; 366: 1–8 https://doi.org/10.1136/bmj.l4898PMID: 31462531.
39. Rücker G, Schwarzer G, Krahn U, König J netmeta: Network Meta-Analysis using Frequentist Methods (R package version 0.9-5). https://CRANR-projectorg/ package=netmeta 2017
40. Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials Med Decis Mak 2013;33:607–17
41 Owen, RK, Bradbury, N, Xin, Y, Cooper, N, Sutton, A MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta Res Syn Meth 2019; 10: 569-581
42 Higgins JPT, Jackson D, Barrett JK, et al Consistency and inconsistency in network meta-analysis: concepts and models for multiarm studies. Res Synth Methods. 2012;3:98–110.
43 van Valkenhoef G, Tervonen T, de Brock B, et al Algorithmic parameterization of mixed treatment comparisons. Stat Comput. 2012;22:1099–111.
44. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M & Salanti G. CINeMA: An approach for assessing confidence in the results of a network meta-analysis PLOS Medicine 2020 17 1-19
45. Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M & Salanti G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis Campbell Systematic Reviews 2020 16 e1080
46. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis PLoS One 2014;9:e99682
47 Nevill CR, Cooper NJ, Sutton AJ, A multifaceted graphical display, including treatment ranking, was developed to aid interpretation of network meta-analysis, Journal of Clinical Epidemiology (2023)
48 Daly CH, Neupane B, Beyene J, et al Empirical evaluation of SUCRA-based treatment ranks in network Meta-analysis: quantifying robustness using cohen’s kappa. BMJ Open. 2019;9(9):e024625.
49 Cipriani A, Higgins JP, Geddes JR, Salanti G Conceptual and technical challenges in network meta-analysis Ann Intern Med 2013;159:130–7
50. Kulisevsky J, Martínez-Horta S, Campolongo A, Pascual-Sedano B, Marín-Lahoz J, Bejr-kasem H, et al. A randomized clinical trial to evaluate the effects of safinamide on apathetic non-demented patients with parkinson's disease Frontiers in Neurology 2022;13.
22
51. Stocchi F, Borgohain R, Onofrj M, Schapira AHV, Bhatt M, Lucini V, et al. A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early parkinson's disease patients. Movement Disorders. 2011;27(1):106–12.
52. Schapira AH, Fox SH, Hauser RA, Jankovic J, Jost WH, Kenney C, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with parkinson disease and motor fluctuations JAMA Neurology 2017;74(2):216
53. Nomoto M, Ishida T, Koebis M, Kamei T, Suzuki I, Hattori N, et al. Characteristics of wearing-off and motor symptoms improved by Safinamide adjunct therapy in patients with parkinson's disease: A post hoc analysis of a Japanese phase 2/3 study Journal of the Neurological Sciences. 2022;434:120083.
54. Hattori N, Tsuboi Y, Yamamoto A, Sasagawa Y, Nomoto M. Efficacy and safety of safinamide as an add-on therapy to L-DOPA for patients with parkinson's disease: A randomized, double-blind, placebo-controlled, phase II/III study Parkinsonism & Related Disorders. 2020;75:17–23.
55. Schapira AH, Stocchi F, Borgohain R, Onofrj M, Bhatt M, Lorenzana P, et al. Long-term efficacy and safety of safinamide as add-on therapy in early parkinson's disease. European Journal of Neurology. 2012;20(2):271–80.
56. Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt M, Chirilineau D, et al. Randomized trial of Safinamide add‐on to levodopa in parkinson's disease with motor fluctuations. Movement Disorders. 2013;29(2):229–37.
57. Wei Q, Tan Y, Xu P, Tao E, Lu Z, Pan X, et al. The Xindi Study: A randomized phase III clinical trial evaluating the efficacy and safety of safinamide as add-on therapy to levodopa in Chinese patients with parkinson’s disease with motor fluctuations CNS Drugs. 2022;36(11):1217–27.
58. Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt MH, Chirilineau D, et al. Two‐year, randomized, controlled study of Safinamide as add‐on to levodopa in mid to late parkinson's disease. Movement Disorders. 2014;29(10):1273–80.
59. Ferreira JJ, Rocha J-F, Falcão A, Santos A, Pinto R, Nunes T, et al. Effect of opicapone on levodopa pharmacokinetics, catechol-o-methyltransferase activity and motor fluctuations in patients with parkinson's disease European Journal of Neurology. 2015;22(5).
60. Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares‐da‐Silva P. Long‐term efficacy of opicapone in fluctuating parkinson's disease patients: A pooled analysis of data from two phase 3 clinical trials and their open‐label extensions. European Journal of Neurology. 2019;26(7):953–60.
61 Lees AJ, Ferreira J, Rascol O, Poewe W, Rocha J-F, McCrory M, et al Opicapone as adjunct to levodopa therapy in patients with parkinson disease and motor fluctuations. JAMA Neurology. 2017;74(2):197.
23
62. Ferreira JJ, Lees A, Rocha J-F, Poewe W, Rascol O, Soares-da-Silva P. Opicapone as an adjunct to levodopa in patients with parkinson's disease and end-of-dose motor fluctuations: A randomised, double-blind, controlled trial. The Lancet Neurology. 2016;15(2):154–65.
63. Takeda A, Takahashi R, Tsuboi Y, Nomoto M, Maeda T, Nishimura A, et al. Randomized, controlled study of Opicapone in Japanese parkinson's patients with motor fluctuations. Movement Disorders. 2020;36(2):415–23.
64. Full prescribing information: Contents* - food and drug administration [Internet]. Available from:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212489s000lbl.pdf.
65. Reference ID: 4072731 - food and drug administration [Internet]. Available from: https://www accessdata fda gov/drugsatfda_docs/label/2017/207145lbl.pdf
66 Kaakkola S Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson's disease. Drugs. 2000 Jun;59(6):1233-50. doi: 10.2165/00003495-200059060-00004.
67 Almeida L, Rocha JF, Falcao A, Palma PN, Loureiro AI, Pinto R, Bonifacio MJ, Wright LC, Nunes T, Soares-da-Silva P: Pharmacokinetics, pharmacodynamics and tolerability of opicapone, a novel catechol-O-methyltransferase inhibitor, in healthy subjects: prediction of slow enzyme-inhibitor complex dissociation of a short-living and very long-acting inhibitor. Clin Pharmacokinet. 2013 Feb;52(2):139-51. doi: 10.1007/s40262-012-0024-7.
68. Mancini F, Di Fonzo A, Lazzeri G, Borellini L, Silani V, Lacerenza M, et al. Real life evaluation of safinamide effectiveness in parkinson’s disease Neurological Sciences 2018;39(4):733–9.
69. Cattaneo C, Müller T, Bonizzoni E, Lazzeri G, Kottakis I, Keywood C. Long-term effects of safinamide on mood fluctuations in parkinson’s disease Journal of Parkinson's Disease. 2017;7(4):629–34.
70 Antonini A, Marano P, Gusmaroli G, Modugno N, Pacchetti C, Sensi M, Melzi G, Bergmann L, Zibetti M, Lopiano L. Long-term effectiveness of levodopa-carbidopa intestinal gel on motor and non-motor symptoms in advanced Parkinson's disease: results of the Italian GLORIA patient population. Neurol Sci. 2020 Oct;41(10):2929-2937.doi:10.1007/s10072-020-04401-w.Epub2020Apr28.
71. Żegleń M, Śladowska K, Kawalec P, Brzostek T. Opicapone as an add-on to levodopa for reducing end-of-dose motor fluctuations in parkinson’s disease: A systematic review and meta-analysis Journal of Comparative Effectiveness Research 2022;11(12):889–904.
24
72. Giossi R, Carrara F, Mazzari M, Lo Re F, Senatore M, Schicchi A, et al. Overall efficacy and safety of safinamide in parkinson’s disease: A systematic review and a meta-analysis. Clinical Drug Investigation. 2021;41(4):321–39.
73. Tsuboi Y, Hattori N, Yamamoto A, Sasagawa Y, Nomoto M. Long-term safety and efficacy of safinamide as add-on therapy in levodopa-treated Japanese patients with parkinson's disease with wearing-off: Results of an open-label study Journal of the Neurological Sciences. 2020;416:117012.
74. Takeda A, Takahashi R, Tsuboi Y, Nomoto M, Maeda T, Nishimura A, et al. Long-term safety and efficacy of opicapone in Japanese parkinson’s patients with motor fluctuations. Journal of Neural Transmission. 2021;128(3):337–44.
75. Svetel M, Tomić A, Kresojević N, Kostić V. Pharmacokinetic drug evaluation of opicapone for the treatment of Parkinson's disease. ExpertOpinDrugMetabToxicol. 2018 Mar;14(3):353-360. doi: 10.1080/17425255.2018.1430138. Epub 2018 Jan 24. PMID:29345156.
76. Ross, MSc candidate SB, Wu PE, Atique, MD candidate A, Papillon‐Ferland L, Tamblyn R, Lee TC, et al. Adverse drug events in older adults: Review of Adjudication Methods in deprescribing studies Journal of the American Geriatrics Society 2020;68:1594–602. doi:10.1111/jgs.16382.
77. Azevedo Kauppila L, Pimenta Silva D, Ferreira JJ. Clinical utility of opicapone in the management of sparkinson's disease: A short review on emerging data and place in therapy Degenerative Neurological and Neuromuscular Disease 2021;Volume 11:29–40. doi:10.2147/dnnd.s256722.
78. Rinaldi D, Bianchini E, Sforza M, Alborghetti M, Galli S, Salvetti M, et al. The tolerability, safety and efficacy of safinamide in elderly parkinson's disease patients: A retrospective study. Aging Clinical and Experimental Research 2020;33:1689–92. doi:10.1007/s40520-020-01648-3.
79 Lo Monaco MR, Petracca M, Vetrano DL, Di Stasio E, Fusco D, Ricciardi D, et al Safinamide as an adjunct therapy in older patients with parkinson's disease: A retrospective study. Aging Clinical and Experimental Research 2020;32:1369–73. doi:10 1007/s40520-020-01469-4
80 Indonesia KEPUTUSAN KEPALA BADAN PENGAWAS OBAT DAN MAKANAN
Nomor 246 Tahun 2022 tentang DAFTAR BAHAN OBAT DAN MAKANAN YANG
DIBATASI PEMASUKANNYA KE DALAM WILAYAH INDONESIA DAN
BAHAN OBAT DAN MAKANAN BERUPA BAHAN OBAT TRADISIONAL, BAHAN OBAT KUASI, BAHAN KOSMETIKA, DAN BAHAN PANGAN YANG
DIMASUKKAN KE DALAM WILAYAH INDONESIA UNTUK KEPERLUAN
INDUSTRI KECIL DAN INDUSTRI MENENGAH Sekretariat Negara Jakarta
81. Amanda. Xadago (safinamide) is now available in the USA for parkinson's disease patients. NeuroNews International 2017.
25
https://neuronewsinternational.com/xadago-safinamide-now-available-in-the-usa-for-p arkinsons-disease-patients/
82 Deeks ED Safinamide: First global approval Drugs 2015;75:705–11 doi:10.1007/s40265-015-0389-7.
83. Koebisu M, Ishida T. Safinamide mesilate (equfina® tablets 50 mg): Preclinical and clinical pharmacodynamics, efficacy, and safety Folia Pharmacologica Japonica 2020;155:269–76. doi:10.1254/fpj.20012.
84. AH S, R B, D SG, YH H, HA B, N H, et al. Safinamide. ALZFORUM 2021. https://www alzforum org/therapeutics/safinamide (accessed April 3, 2023)
85 Lowin J, Bergman A, Ray Chaudhuri K, Findley LJ, Roeder C, Schifflers M, et al A cost-effectiveness analysis of levodopa/carbidopa intestinal gel compared to standard care in late stage parkinson’s disease in the UK. Journal of Medical Economics. 2011;14(5):584–93
86. Ongentys prices, coupons, Copay & Patient Assistance [Internet]. Drugs.com. Available from: https://www.drugs.com/price-guide/ongentys
87. Kabisch M, Ruckes C, Seibert-Grafe M, Blettner M. Randomized Controlled Trials. Deutsches Ärzteblatt international 2011;
26
Database
PubMed
Cochrane
APPENDIX
AppendixTable1.SearchStrategies
SearchTerms
#1 "parkinsondisease"[MeSHTerms]
#2 "idiopathic parkinson s disease"[Title/Abstract] OR "lewy body parkinson s disease"[Title/Abstract] OR "primary parkinsonism"[Title/Abstract] OR "paralysisagitans"[Title/Abstract]OR"parkinsonsdisease"[Title/Abstract]
#3 #1OR#2
#4 "safinamide"[SupplementaryConcept]
#5
(("safinamide"[Supplementary Concept] OR "safinamide"[All Fields]) AND "methanesulfonate"[Title/Abstract]) OR "Xadago"[Title/Abstract] OR "FCE-28073"[Title/Abstract] OR "PNU-151774E"[Title/Abstract] OR "FCE-26743"[Title/Abstract] OR ("fbap"[All Fields] AND "methanesulfonate"[Title/Abstract]) OR (("2"[All Fields]AND(("4"[AllFields] AND "3-fluorobenzyloxy"[All Fields]) AND "benzylamino"[All Fields]))AND "propionamide"[Title/Abstract])
#6 #4OR#5
#7 "opicapone"[SupplementaryConcept]
#8 "catecholomethyltransferaseinhibitors"[Title/Abstract]
#9 #7OR#8
#1 0 #3AND(#6OR#9)
#1 1 (#10 ) AND (("random*"[Title/Abstract] OR "randomized controlled trials"[Title/Abstract] OR "trial*"[Title/Abstract] OR "RCT"[Title/Abstract]))
#1 MeSHdescriptor:[ParkinsonDisease]explodealltrees
#2 (("opicapone"OR"CatecholO-MethyltransferaseInhibitors")):ti,ab,kw
#3 (safinamide OR safinamide methanesulfonate OR Xadago OR FCE-28073 OR PNU-151774EORFCE-26743ORfbapmethanesulfonate):ti,ab,kw
#4 #2OR#3
#5 #1AND#4
#6 #5 AND ("trial*" OR "random*" OR "RCT" OR "Randomized Controlled Trial")
#1 "Parkinson"OR"ParkinsonDisease"
ProQuest
#2 noft("opicapone"OR"CatecholO-MethyltransferaseInhibitors")
#3 noft("safinamide" OR "safinamide methanesulfonate" OR "Xadago" OR
27
"FCE-28073" OR "PNU-151774E" OR "FCE-26743" OR "fbap methanesulfonate"OR"2-(4-(3-fluorobenzyloxy)benzylamino)propionamide")
#4 "random*"OR"randomizedcontrolledtrials"OR"trial*"OR"RCT"
#5 #1AND(#2OR#3)AND#4
ScienceDirect ("parkinson") AND (("opicapone"OR"CatecholO-MethyltransferaseInhibitors")OR ("safinamide" OR "Xadago")) AND("random"OR"randomizedcontrolledtrials"OR "trial"OR"RCT")
#1 TITLE-ABS-KEY("parkinson"OR"parkinsondisease")
#2 TITLE-ABS-KEY("opicapone"OR"CatecholO-MethyltransferaseInhibitors")
Scopus
#3 TITLE-ABS-KEY ("safinamide" OR "safinamide methanesulfonate" OR "Xadago" OR "FCE-28073" OR "PNU-151774E" OR "FCE-26743" OR "fbap methanesulfonate"OR"2-(4-(3-fluorobenzyloxy)benzylamino)propionamide")
#4 TITLE-ABS-KEY ("random*" OR "randomized controlled trials" OR "trial*" OR"RCT")
#5 #1AND(#2OR#3)AND#4
#1 (MH "Parkinson Disease") OR "Idiopathic Parkinson's Disease" OR "Lewy BodyParkinson'sDisease"OR"PrimaryParkinsonism"
#2 (MH"opicapone")OR"CatecholO-MethyltransferaseInhibitors"
CINAHLvia EBSCO
#3 (MH "safinamide") OR "safinamide methanesulfonate" OR "Xadago" OR "FCE-28073" OR "PNU-151774E" OR "FCE-26743" OR "fbap methanesulfonate"OR"2-(4-(3-fluorobenzyloxy)benzylamino)propionamide"
#4 #2OR#3
#5 #1AND#4
#6 (MH"RCT")OR"random*"OR"trial*"OR"RandomizedControlledTrial"
#7 #5AND#6
#1 ALL=("parkinson"OR"parkinsondisease")
#2 ALL=("opicapone"OR"CatecholO-MethyltransferaseInhibitors")
Webof Science
#3
ALL=("safinamide" OR "safinamide methanesulfonate" OR "Xadago" OR "FCE-28073" OR "PNU-151774E" OR "FCE-26743" OR "fbap methanesulfonate"OR"2-(4-(3-fluorobenzyloxy)benzylamino)propionamide")
#4 ALL=("random*"OR"randomizedcontrolledtrials"OR"trial*"OR"RCT")
#5 #1AND(#2OR#3)AND#4
Abbreviations:CINAHL,CumulativeIndextoNursingandAlliedHealthLiterature
28
,HoehnandYahrScale;F,Female;SD,Standarddeviation;ITT,Intention-to-treatanalysis;N/A,Notavailable
First Author, Year Study Location Diagnostic Criteria SampleSize Age (Years) %F Durationof Parkinson’sa (Years) H&Ystage a DailyOnEpisode (hours)a DailyOffEpisode (hours)a Periodof Treatments (weeks) Frequency Typeof Analyses Adherence Rate(%) TrialArms Arm1 Arm2 Intervention Control Intervention Control Intervention Control Intervention Control Intervention Control Kulisevsky etal.,2022 Europe H&YStageI-III 15 15 69.5± 98 50 4.81±3.42 3.98± 305 2.1±0.3 2.1± 05 N/A N/A N/A N/A 24 Oncedaily ITT 86.7 Placebo Safinamide Stocchiet al.,2011 America H&YStageI-III 179 90 574± 113 37 2.47±1.38 241± 12 N/A N/A N/A N/A N/A N/A 24 Oncedaily ITT 84.4 Placebo Safinamide Schapira etal.,2017 America H&YstageI-IV 274 275 619± 8.9 376 N/A N/A N/A N/A 930±241 906± 2.50 534±197 538± 2.01 24 Oncedaily ITT 894 Placebo Safinamide Nomotoet al.,2022 Asia H&YStageII-IV 259 136 6807± 859 5486 622±743 795± 51 237±062 233± 059 970±296 1041± 269 635±299 566± 256 24 Oncedaily ITT 1000 Placebo Safinamide Hattoriet al.,2020 Asia H&YStageII-IV 265 141 68.06± 859 5494 838±479 7.97± 51 237±062 2.33± 059 969±296 10.41± 269 635±299 5.66± 256 24 Oncedaily ITT 977 Placebo Safinamide Schapira etal.,2012 America H&YStageI-III 149 78 577± 92 63.4 N/A N/A N/A N/A N/A N/A N/A N/A 49.5 Oncedaily ITT 83.9 Placebo Safinamide Borgohain etal.,2013 Europe H&YstageI-IV 447 222 5987± 9.41 2825 805±39 83± 3.8 28±06 28± 0.7 944±2252 930± 2.155 52±212 53± 2.06 78 Oncedaily ITT 667 Placebo Safinamide Weietal, 2022 Asia H&YstageI-IV 151 154 616± 929 4197 83±49 82± 48 N/A N/A 101±28 103± 31 59±28 56± 31 16 Oncedaily ITT 907 Placebo Safinamide Borgohain etal.,2014 Europe H&YstageI-IV 447 222 59.87± 941 2825 805±39 8.3± 38 28±06 2.8± 07 944±2252 9.30± 2155 52±212 5.3± 206 78 Oncedaily ITT 667 Placebo Safinamide Ferreiraet al,2015 Europe H&YStageI-III 30 10 6752± 904 50 6.3±6.14 85± 74 2.77±0.41 29± 02 N/A N/A 6.75±3.09 710± 300 13 Oncedaily ITT 86.7 Placebo Opicapone Ferreiraet al.,2019 Europe H&YstageI-III 518 265 635± 8.9 4112 77±43 78± 3.9 N/A N/A N/A N/A 639±215 61± 2.1 52 Oncedaily ITT 6892 Placebo Opicapone Leesetal, 2017 America H&YstageI-III 283 144 6325± 875 4054 83±44 77± 37 23±06 24± 06 93±22 96± 24 625±219 61± 23 52 Oncedaily ITT 8693 Placebo Opicapone Ferreiraet al.,2016 Europe H&YStageI-III 356 121 61.42± 1008 4172 724±383 7.7± 42 24±047 2.4± 05 966±224 10.0± 20 661±18 6.2± 199 15 Oncedaily ITT 913 Placebo Opicapone Takedaet al.,2020 Asia H&YStageI-III 290 147 6794± 851 60.18 7.65±4.42 75± 38 N/A N/A 10.45±2.50 99± 27 5.95±2.30 63± 26 15 Oncedaily ITT 99.31 Placebo Opicapone aDataarepresentedinmean±SD Abbreviations:H&Y
29
AppendixTable2.CharacteristicsoftheIncludedStudies
AppendixFigure1.ForestPlotofPairwiseMeta-AnalysesforEfficacyandSafetyOutcomes
(A) Change in ON-time (B) Change in OFF-time (C) Change of UPDRS-III score (D) Change of PDQ-39 score (E) Number of patients who at least had adverse effectsUPDRS-III, The Unified Parkinson’s Disease RatingScalePart3;PDQ-39,39-itemParkinson’sDiseaseQuestionnaire.





A B C D E
30
AppendixFigure2.SensitivityAnalysisForestPlotofRelativeEffectsfromBayesianRandomEffect ConsistencyModelofEfficacyandSafetyOutcomes(A)ChangeinON-time.(B)ChangeinOFF-time.(C) ChangeofUPDRS-IIIscore.(D)Numberofpatientswhoatleasthadadverseeffects.





B D
A B 31
UPDRS-III, The Unified Parkinson’s Disease Rating Scale Part 3; PDQ-39, The Parkinson’s Disease Questionnaire
Number of Patients Who Experienced At Least One Adverse Effects






AppendixTable3.SubgroupAnalysesNetworkMeta-AnalysisResultsforEfficacyandSafetyOutcomes(A) EfficacyoutcomesinEuropestudies.(B)Efficacyoutcomesinnon-Europestudies.(C)Safetyoutcomesin Europestudies.(D)Safetyoutcomesinnon-Europestudies.
UPDRS-III, The Unified Parkinson’s Disease Rating Scale Part 3; PDQ-39, The Parkinson’s Disease Questionnaire


C D Change in ON-time
in OFF-time
UPDRS-III
PDQ-39
Change
Change of
score Change of
score
32
AppendixTable4.SummaryofMeta-RegressionAnalysesinAllOutcomesinNetworkMeta-Analysis
Outcome Measure Covariate Intervention Number of Studies Z p-value p-value differences ON-time YearofPublication Safinamide 5 -299 000 082 Opicapone 4 022 083 SampleSize Safinamide 5 2.18 0.03 078 Opicapone 4 -024 081 MeanAge Safinamide 5 -304 000 0.71 Opicapone 4 0.37 0.71 Female Safinamide 5 -453 000 075 Opicapone 4 032 075 DurationofParkinson Safinamide 4 071 048 041 Opicapone 4 013 089 Adherence Safinamide 5 -4.75 0.00 0.81 Opicapone 4 024 081 H&YScore Safinamide 3 474 000 078 Opicapone 2 -0.28 0.78 ON-time Baseline Duration Safinamide 5 -118 024 069 Opicapone 3 009 093 OFF-time Baseline Duration Safinamide 5 -2.74 0.01 0.68 Opicapone 4 -041 069 PeriodofIntervention Safinamide 5 332 000 095 Opicapone 4 -0.06 0.96 OFF-time YearofPublication Safinamide 4 032 075 012 Opicapone 3 017 087 SampleSize Safinamide 4 -0.15 0.88 047 Opicapone 3 083 041 MeanAge Safinamide 4 033 074 0.09 Opicapone 3 -0.22 0.83 Female Safinamide 4 039 070 026 Opicapone 3 -006 096 DurationofParkinson Safinamide 3 018 085 008 Opicapone 3 028 078 Adherence Safinamide 4 1.00 0.32 0.48 Opicapone 3 026 079 H&YScore Safinamide 2 -085 039 020 Opicapone 2 -0.54 0.59 ON-time Baseline Duration Safinamide 4 -044 066 028 Opicapone 2 -008 094 OFF-time Baseline Duration Safinamide 4 0.03 0.98 0.37 Opicapone 3 -051 061 PeriodofIntervention Safinamide 4 -114 025 015 Opicapone 3 0.83 0.41 UPDRS-II Iscore YearofPublication Safinamide 8 329 000 060 Opicapone 2 053 060 SampleSize Safinamide 8 -0.74 0.46 032 Opicapone 2 -028 078 MeanAge Safinamide 8 485 000 0.59 Opicapone 2 0.54 0.59 Female Safinamide 8 164 010 043 33
Opicapone 2 062 053 DurationofParkinson Safinamide 6 055 058 0.02* Opicapone 2 -0.53 0.60 Adherence Safinamide 8 244 002 052 Opicapone 2 062 053 H&YScore Safinamide 4 -418 000 Insufficient observations Opicapone - -ON-time Baseline Duration Safinamide 5 2.85 0.00 insufficient observations Opicapone - -OFF-time Baseline Duration Safinamide 5 475 000 061 Opicapone 2 -0.51 0.61 PeriodofIntervention Safinamide 8 -127 021 033 Opicapone 2 -062 053 PDQ-39 score YearofPublication Safinamide 5 -0.17 0.86 000* Opicapone 3 -017 086 SampleSize Safinamide 5 -017 086 060 Opicapone 3 1.12 0.26 MeanAge Safinamide 5 -129 020 064 Opicapone 3 -021 084 Female Safinamide 5 -1.21 0.23 068 Opicapone 3 012 091 DurationofParkinson Safinamide 4 019 085 0.57 Opicapone 3 -1.09 0.28 Adherence Safinamide 5 -130 019 047 Opicapone 3 044 066 H&YScore Safinamide 3 -049 063 017 Opicapone 2 075 046 ON-time Baseline Duration Safinamide 4 -0.31 0.76 0.24 Opicapone 3 064 052 OFF-time Baseline Duration Safinamide 4 -093 035 006 Opicapone 3 0.81 0.42 PeriodofIntervention Safinamide 5 -110 027 0.04* Opicapone 3 -101 031 Number ofpatients whoat leasthad adverse effects YearofPublication Safinamide 8 -1.93 0.05 0.48 Opicapone 3 -062 053 SampleSize Safinamide 8 166 010 046 Opicapone 3 0.59 0.56 MeanAge Safinamide 8 -066 051 0.02* Opicapone 3 -063 053 Female Safinamide 8 -0.85 0.40 023 Opicapone 3 -048 063 DurationofParkinson Safinamide 6 -019 085 0.12 Opicapone 3 -0.35 0.73 Adherence Safinamide 8 -127 020 056 Opicapone 3 -030 077 H&YScore Safinamide 4 089 037 026 Opicapone 2 047 064 ON-time Baseline Duration Safinamide 5 -1.95 0.05 0.82 Opicapone 3 -016 087 OFF-time Baseline Duration Safinamide 5 -137 017 029 Opicapone 3 0.74 0.46 34
* p <0.05
H&Y, Hoehn and Yahr Scale; UPDRS-III, The Unified Parkinson’s Disease Rating Scale Part 3; PDQ-39,39-itemParkinson’sDiseaseQuestionnaire





O-GramforEfficacyandSafetyOutcomes
Higher SUCRA (Surface Under the Cumulative Ranking Curve) values and cumulative ranking curves nearer thetopleftindicatebetterperformance
PeriodofIntervention Safinamide 8 149 014 069 Opicapone 3 -022 083
35
A. MeanDifferenceofChangefrombaselineinTotalOn-Time
B. MeanDifferenceofChangefrombaselineinTotalOff-Time
C. MeanDifferenceofChangefrombaselineinUPDRS-III
D. MeanDifferenceofChangefrombaselineinPDQ-39
E. OddsRatioofNumberofSampleSizeWhoExperiencedAtLeastOneAdverseEffects
AppendixTable5.GRADEAssessmentofNetworkEstimatesandHeterogeneity:RiskofBiasperPairwise TreatmentforEachOutcome
Judgments for the six domains across all evaluated treatment effects are reported, except inconherence. The default summary judgment is “High” confidence; downgrading by one, two, or three levels will lead to a confidence rating of “Moderate,” “Low,” or “Very low”




respectively We manually assign an overall level of confidence to each relative effect GRADE, Grading of Recommendations Assessment, Development andEvaluation

36
A. ChangeinON-time
B. ChangeinOFF-time
C ChangeofUPDRS-IIIscore
D ChangeofPDQ-39score
E Numberofpatientswhoatleasthadadverseeffects
AppendixTable6 RiskofbiasContributionsWithin-StudyBias
Each bar corresponds to an estimate of relative effect. Each bar also represents areorderingofacolumnofthe per‐study contribution matrix, where studies with low, moderate, and high risk of bias have been grouped together and colored accordingly Each study is represented by a colored area with white borders and is proportionaltoitscontribution





37
A. ChangeinON-time
B. ChangeinOFF-time
C ChangeofUPDRS-IIIscore
D ChangeofPDQ-39score
E Numberofpatientswhoatleasthadadverseeffects
AppendixTable7.IndirectnessContributionsinGRADEReports
Each bar corresponds to an estimate of relative effect Each bar also represents areorderingofacolumnofthe per‐study contribution matrix, where studies with low, moderate, and high indirectness have been grouped together and colored accordingly Each study is represented by a colored area with white borders and is proportional to its contribution. GRADE, Grading of Recommendations Assessment, Development andEvaluation




38
AppendixTable8.PRISMANMAChecklistofItemstoIncludeWhenReportingASystematicReview
Methods:datasources;studyeligibilitycriteria, participants,andinterventions;studyappraisal;and synthesis methods, such as network meta-analysis.
Results:numberofstudiesandparticipantsidentified; summaryestimateswithcorresponding confidence/credibleintervals; treatment rankings may also be discussed. Authors may choose to summarize pairwise comparisons against a chosen treatment included in their analyses for brevity.
InvolvingaNetworkMeta-analysis Section/Topic Item # ChecklistItem Reportedon Page# TITLE Title 1 Identifythereportasasystematicreview incorporating a network meta-analysis (or related form of meta-analysis) 1 ABSTRACT Structured summary 2
Provideastructuredsummaryincluding,asapplicable: Background:mainobjectives
Other:primarysourceoffunding;systematicreview
1 INTRODUCTION Rationale 3 Describetherationaleforthereviewinthecontextofwhat isalreadyknown, including mention of why a network meta-analysis has been conducted 1-3 Objectives 4 Provideanexplicitstatementofquestionsbeingaddressed, withreferencetoparticipants,interventions,comparisons, outcomes,andstudydesign(PICOS) 3 METHODS Protocoland registration 5 Indicatewhetherareviewprotocolexistsandifandwhere itcanbeaccessed(e.g.,Webaddress);and,ifavailable, provideregistrationinformation,includingregistration number N.A Eligibilitycriteria 6 Specifystudycharacteristics(eg,PICOS,lengthof
been
the
(with justification) 3-4 Information sources 7 Describeallinformationsources(eg,databaseswithdates ofcoverage,contactwithstudyauthorstoidentify additionalstudies)inthesearchanddatelastsearched 3 Search 8 Presentfullelectronicsearchstrategyforatleastone database,includinganylimitsused,suchthatitcouldbe repeated 39
Discussion/Conclusions:limitations;conclusionsand implicationsoffindings.
registrationnumberwithregistryname.
follow-up)andreportcharacteristics(eg,years considered,language,publicationstatus)usedascriteria foreligibility,givingrationale Clearly describe eligible treatments included in the treatment network, and note whether any have
clustered or merged into
same node
Studyselection 9 Statetheprocessforselectingstudies(ie,screening, eligibility,includedinsystematicreview,and,ifapplicable, includedinthemeta-analysis) 3 Datacollection process 10 Describemethodofdataextractionfromreports(eg, pilotedforms,independently,induplicate)andany processesforobtainingandconfirmingdatafrom investigators. 4 Dataitems 11 Listanddefineallvariablesforwhichdataweresought (e.g.,PICOS,fundingsources)andanyassumptionsand simplificationsmade. 4 Geometryofthe network S1 Describemethodsusedtoexplorethegeometryofthe treatmentnetworkunderstudyandpotentialbiasesrelated toit.Thisshouldincludehowtheevidencebasehasbeen graphicallysummarizedforpresentation,andwhat characteristicswerecompiledandusedtodescribethe evidencebasetoreaders. 5-6 Riskofbias withinindividual studies 12 Describemethodsusedforassessingriskofbiasof individualstudies(includingspecificationofwhetherthis wasdoneatthestudyoroutcomelevel),andhowthis informationistobeusedinanydatasynthesis. 5 Summary measures 13 Statetheprincipalsummarymeasures(e.g.,riskratio, differenceinmeans). Also describe the use of additional summary measures assessed, such as treatment rankings and surface under the cumulative ranking curve (SUCRA) values, as well as modified approaches used to present summary findings from meta-analyses 5-6 Plannedmethods ofanalysis 14 Describethemethodsofhandlingdataandcombining resultsofstudiesforeachnetworkmeta-analysis This shouldinclude,butnotbelimitedto:
of multi-arm trials;
of variance structure;
Selection of prior distributions in Bayesian analyses; and
Assessment of model fit 5-6 Assessmentof Inconsistency S2 Describethestatisticalmethodsusedtoevaluatethe agreementofdirectandindirectevidenceinthetreatment network(s)studied Describeeffortstakentoaddressits presencewhenfound 5 Riskofbias acrossstudies 15 Specifyanyassessmentofriskofbiasthatmayaffectthe cumulativeevidence(eg,publicationbias,selective reportingwithinstudies). 6 Additional analyses 16 Describemethodsofadditionalanalysesifdone,indicating whichwerepre-specified.Thismayinclude,butnotbe limitedto,thefollowing:
Sensitivityorsubgroupanalyses;
Meta-regressionanalyses;
Alternative formulations of the treatment network; and
Use of alternative prior distributions for Bayesian analyses (if applicable) 6 40
● Handling
● Selection
●
●
●
●
●
●
RESULTS†
confidence/credibleintervals In larger networks, authors may focus on comparisons versus a particular comparator (e g placebo or standard care), with full findings presented in an appendix League tables and forest plots may be considered to summarize pairwise comparisons If additionalsummarymeasureswereexplored(suchas treatmentrankings),theseshouldalsobepresented
Describeresultsfrominvestigationsofinconsistency This mayincludesuchinformationasmeasuresofmodelfitto compareconsistencyandinconsistencymodels, P values fromstatisticaltests,orsummaryofinconsistency estimatesfromdifferentpartsofthetreatmentnetwork.
Presentresultsofanyassessmentofriskofbiasacross studiesfortheevidencebasebeingstudied.
Giveresultsofadditionalanalyses,ifdone(eg,sensitivity orsubgroupanalyses,meta-regressionanalyses, alternative network geometries studied, alternative choice of prior distributions for Bayesian analyses, andsoforth)
14,Appendix Table6
Summarizethemainfindings,includingthestrengthof evidenceforeachmainoutcome;considertheirrelevance tokeygroups(e.g.,healthcareproviders,users,and policy-makers).
Discusslimitationsatstudyandoutcomelevel(e.g.,riskof bias),andatreviewlevel(e.g.,incompleteretrievalof identifiedresearch,reportingbias). Comment on the
16-17
Studyselection 17 Givenumbersofstudiesscreened,assessedforeligibility, andincludedinthereview,withreasonsforexclusionsat eachstage,ideallywithaflowdiagram 6-7 Presentationof network structure S3 Provideanetworkgraphoftheincludedstudiestoenable visualizationofthegeometryofthetreatmentnetwork 7, Figure2 Summaryof network geometry S4 Provideabriefoverviewofcharacteristicsofthetreatment network Thismayincludecommentaryontheabundance oftrialsandrandomizedpatientsforthedifferent interventionsandpairwisecomparisonsinthenetwork, gapsofevidenceinthetreatmentnetwork,andpotential biasesreflectedbythenetworkstructure 7 Study characteristics 18 Foreachstudy,presentcharacteristicsforwhichdatawere extracted(eg,studysize,PICOS,follow-upperiod)and providethecitations 7,Appendix Table2 Riskofbias withinstudies 19 Presentdataonriskofbiasofeachstudyand,ifavailable, anyoutcomelevelassessment 7,Figure3, Figure4 Resultsof individualstudies 20 Foralloutcomesconsidered(benefitsorharms),present,
interventiongroup,and2)effectestimatesandconfidence intervals Modified approaches may be needed to deal with information from larger networks 7,Appendix Table2 Synthesisof results 21
8-10,Figure 5,Appendix Figure1 Explorationfor inconsistency S5
foreachstudy:1)simplesummarydataforeach
Presentresultsofeachmeta-analysisdone,including
Not applicable Riskofbias acrossstudies 22
Resultsof additional analyses 23
Summaryof evidence 24
10-14, Appendix Figure2&3, Appendix Table3;4;5;7
DISCUSSION
15-16 Limitations 25
41
validity of the assumptions, such as transitivity and consistency Comment on any concerns regarding network geometry (e g , avoidance of certain comparisons)
Describesourcesoffundingforthesystematicreviewand othersupport(e.g.,supplyofdata);roleoffundersforthe systematicreview.Thisshouldalsoincludeinformation regardingwhetherfundinghasbeenreceivedfrom manufacturersoftreatmentsinthenetworkand/orwhether someoftheauthorsarecontentexpertswithprofessional conflictsofinterestthatcouldaffectuseoftreatmentsin thenetwork
PICOS=population,intervention,comparators,outcomes,studydesign.
*TextinitalicsindicateSwordingspecifictoreportingofnetworkmeta-analysesthathasbeenadded toguidancefromthePRISMAstatement
†Authorsmaywishtoplanforuseofappendicestopresentallrelevantinformationinfulldetailfor itemsinthissection
Conclusions 26 Provideageneralinterpretationoftheresultsinthecontext ofotherevidence,andimplicationsforfutureresearch 17
FUNDING Funding 27
N.A
42
ANovelMonoclonalManagementofGeriatricOsteoarthritis:NetworkMeta-Analysis ofComparativeEfficacyandSafetyofTanezumabagainstOralNSAIDs
DerrenD.C.H.Rampengan1,JoshNathanielJowono2 ,ArnoldKeane2 , JuanA.J.M.NuraLele3
AMSA-UniversitasSamRatulangi1,AMSA-UniversitasIndonesia2 , AMSA-UniversitasKristenIndonesia3
Abstract
Introduction: Osteoarthritis (OA) affects 250 million people globally, causing pain, disability, and reduced quality of life. Non-steroidal anti-inflammatory drugs (NSAIDs) are the first-line therapy, but their use in the elderly is limited. Tanezumab, a promising nerve growth factor (NGF) antagonist, has emerged as a potential alternative treatment. However, currentmeta-analyseslackspecificitytoTanezumabandnocomparisontoNSAIDs.
Objective: This study aims to compare the efficacy and safety of Tanezumab with oral NSAIDsingeriatricosteoarthritistreatment.
Method: This study was conducted using the Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA). We systematically searched PubMed, ScienceDirect, and Manual Research through Google Scholar up until 4 April 2023. The selected papers were thoroughly screened and evaluated for potential bias using Cochrane Risk of Bias 2.0. Random-effects meta-analysis was performed to combine effect estimates, using Review Manager5.4.
Result and Discussion: Twelve studies culminating in total of 7031 patients were included and screened with low risk of bias. Tanezumab with 5 and 10 mg doses shows significant effects on decreasing pain [WOMAC Pain; SMD -1.09; 95% CI -2.02 to -0.15; p = 0.002], increasing physical function [WOMAC Physical Function; SMD -5.47; 95% CI -8.71 to -2.24; p = 0.0009],andincreasinggeneralhealth[PGA;SMD-3.83;95%CI-6.94to-0.72;p = 0.02]. Therefore, tanezumab is shown to be efficacious and safe with less adverse effects. This effect is shown because of the novel pain reduction and relation to joints NGF while havingalongeractiveduration.
Conclusion: Tanezumab shows more effective effect on reducing pain and improving function compared to NSAIDs. It shows less adverse effects in comparison toNSAIDs,thus recommendedtobeusedforpatientswithinadequatereactiontotherapyorcomorbidities.
Keywords: Tanezumbab, Osteoarthritis, Geriatric, Oral NSAIDs
ANovelMonoclonalManagementofGeriatricOsteoarthritis:NetworkMeta-Analysis ofComparativeEfficacyandSafetyofTanezumabagainstOralNSAIDs
AsianMedicalStudents’Conference2023
Authors:
DerrenDavidChristianHomentaRampengan1
JoshNathanielJowono2
ArnoldKeane2
JuanAlessandroJeremisMaruliNuraLele3
1 FacultyofMedicine,UniversitasSamRatulangi
2 FacultyofMedicine,UniversitasIndonesia
3 FacultyofMedicine,UniversitasKristenIndonesia

ANovelMonoclonalManagementofGeriatricOsteoarthritis:NetworkMeta-Analysis ofComparativeEfficacyandSafetyofTanezumabagainstOralNSAIDs
INTRODUCTION
Osteoarthritis (OA) affects approximately 250 million people worldwide and is a significant cause of pain and disability among older adults.1 Osteoarthritis causes a limited range of jointsandpain,whichcausesdamagetopatients'dailyactivities.Asaresult,thereis a significant reduction in their quality oflife(QoL).TodateNon-steroidalanti-inflammatory drugs (NSAIDs) are recommended as first-line therapy, but also havesubstantiallimitations.
NSAIDs in the elderly should be prescribed with caution because of comorbidities and polypharmacy, and by the drug's benefit/risk balance, which all together influence cardiovascular(CV),gastrointestinal(GI)andrenal(AE)adverseevents.1,2
Recently, understanding of biotherapeutic approaches has been the reason for studies of cytokine activity in the pathogenesis of OA.2 There were three main types of cytokine blockers used in OA, targeting the nerve growth factor (NGF), interleukin-1 (IL-1), and the tumor necrosis factor-α (TNF-α), respectively 3 Among the three targets,nervegrowthfactor (NGF) are the most promising candidates.4 The anti-NGF mechanism can normalize the expression of nociceptive neuromodulators elicited by inflammation. As a novel NGF antagonist, Tanezumab is a humanized monoclonal IgG2 antibody that blocks NGF from activating trkA receptors on nociceptive neurons. 5 TanezumabwasdevelopedtotargetNGF, binding both circulating and local tissue NGF and preventing interaction with the tropomyosin-relatedkinase-Aandp75receptors.6
So far, some meta-analyses have assessed theefficacyofNGFantagonistsbutarenot specific to Tanezumab.3 In addition, no complementary analysis of different doses was performed in other meta-analyses about Tanezumab for OA.7 Instead, we found a lack of evidence of a dose-response relationship to guide clinical application.8 Furthermore, the meta-network analysis was performed but only compared Tanezumab with placebo and did notconfirmthecomparisonwithNSAIDs.9
To our knowledge, no meta-analyses directly compare NSAIDs and Tanezumab at various therapeutic doses. In order to demonstrate the clinical effect of an effective dose of Tanezumab for OA patients, we conducted a recent meta-analysis over the last five years to assess the safety and efficacy of Tanezumab (2.5 mg, 5 mg, 10 mg) as monotherapy in the treatment of hip or knee compared with NSAIDs. We performed a network meta-analysis to investigate Tanezumab's clinical efficacy and safety in guiding clinicians to make the best practicedecisiontorecommendgivingTanezumabtoosteoarthritispatients.
METHOD
This systematic review and network meta-analysis were conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines on NetworkMeta-Analyses.
Search Strategy
Literature search was performed across multiple databases including PubMed, Cochrane Library, EMbase, and Clinical Trials. For studies comparing Tanezumab to NSAIDS for osteoarthritis treatment up to April 4th, 2023 with the following keywords: “tanezumab” AND (“NSAID” OR“OralNSAID”)AND“osteoarthritis”.Allofthetermswereinlinewith the MeSH (Medical SubjectHeadings)Browser.Thedetailedkeywordsforeachdatabaseare attached in Appendix 1. Suitable advanced search techniques were applied whenever appropriate.TheplannedprocedureisillustratedinFig1.
Study Eligibility Criteria
Before conducting the literature search, the inclusion and exclusion criteria was determined to ensure that the data was specific and relevant as well applied to the PICOS framework (patient/problem, intervention, comparison/control, outcome). The inclusion criteria include (1) clinical trial studies using RCT, (2) studies published within the last 10 years, (3) population: geriatric patients aged ≥ 60 years old with osteoarthritis, (4) peer-reviewed journals, (5) intervention: tanezumab, and (6) study outcomes measured by WOMAC Pain, WOMAC Physical Function, and/or PGA. The exclusion criteria, however, are (1) irretrievable full-text articles, (2) incompatible language, (3) non-human clinical trials, and (4) incomplete outcome reporting. Three independent reviewers selected the titles and
 Figure1.Diagramflowofliteraturesearchstrategy
Figure1.Diagramflowofliteraturesearchstrategy
abstracts of papers based on criteria regarding their accessibility (DDCHR, JNJ, AK). Any disputeswerediscussedbythefourthauthortoreachaconsensus(JAJMN).
Data Extraction
We used a predetermined outcome sheet in tabular form to extract the included studies. It contained the following information: (1) author and year of publication; (2) study characteristic, including the location and duration; (3) study population, including sample size and sample age mean +/- SD); (4) intervention and control, including type, dose, and adverse events in highlighted findings; and (5) study outcomes, including the baseline and post-treatment score of WOMAC Pain, WOMAC Physical Function and/or PGA. Two reviewers (JAJMN and JNJ) qualitatively evaluate the studies, while another author (DDCHR)double-checkedtheretrieveddatawhileconductingstatisticalanalysis.
Quality Assessment
The risk for bias in the final papers thatwereanalyzedwasevaluatedusingtheRevisedTool RoB 2.0, which consists of five domains for studies. The results will thenberecordedinthe domain file bias (.xlsx) then posted to the ROBVIS website for display. Four reviewers separately assessed the quality, and any disagreements settled by consensus between the reviewers.InFigure2,anevaluationofstudyqualityisshown.
Statistical Analysis
Statistical analysis was carried out utilizing Review Manager 5.4.1. (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen). Studies were used to extract mean differences (MD), standard deviations (SD), and p-values for both intervention versus control's pre and post treatment. We then used pooled fixed effect models to interpret impacts.ThemainfindingthatguidedourstatisticalanalysiswastheMDandSDforchanges from the baseline using tanezumab as monoclonal treatment for geriatric osteoarthritis, as demonstrated by lower WOMAC Pain, WOMAC Physical Function and/or Patient’s Global Assessment in each tool's appropriate range. Standardized Mean difference (SMD) with a 95% confidence interval and its respective p-value was used to determine the efficacy and safety of tanezumab which will be presented in forest plots. We used inverse variance, the DerSimonian-Laird random effects model as proposed by Riley et al., since we considered that heterogeneity outside the study could also be discovered from studies.10 Heterogeneity was further evaluated using I2 statistics based on Cochrane threshold, with cut-off limits of 0%, 25%, 50%, and 75% as insignificant, low, moderate, and high heterogeneity, respectively
RESULTS
Study selection
Quality Assessment
The Risk of Bias tools for a randomized control trials used in assessing the quality of the twelve trials shows that, only studies Ekman et al., (1018), Karsdal et al., 2019,
NCT02528188, 2022 and NCT02709486, 2022 reported high risk of bias under selective bias, detection bias, incomplete Outcome data and selective reporting. Regardless of the reported high risk, all trials show high quality and are deemed fit to be included in the meta-analysis.

Demography and clinical Characteristics of the included studies
Out of the 12 included trials, 4 trials are obtained from the clinical trial with registration number NCT00809358, 2021, NCT02528188, 2022, NCT02709486, 2022 and NCT02697773, 2022. All studies are publications between 2017 and 2023 published in USA.A total of 22875 patients was included, out of which 6070 patients was treated with Tanezumab 5mg, 5047 patients was treated with 5047, 1251 patients with Tanezumab 10mg
 Fig2.RiskofBiasassessmentoftheincludedstudies
Fig2.RiskofBiasassessmentoftheincludedstudies
and 5258 patients with NSAID while 1445 patients was treated with placebo. The average ageofpatientswas60.5.AllpatientshadKneeorHiporBothOsteoarthritisproblems.
In Table 2: Adverse event; any AE and serious AE was reported in half of the included studies.Otheradverseeventsubjectintheinterventiongrouparefallandnasopharyngitis.
Outcome Measures
The primary outcome measures are Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) that measure Pain, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) which measure Physical Function rating scale andpatient’sglobalassessmentofOA(PGA)-OAScore
Table1:BaselineandClinicalCharacteristicofIncludedStudies
Author FirstName Demography ClinicalCharacteristics IndexJoint,n (%) Design Country Age M(SD) Control Female(%) Condition Interventi on Patients Outcome Periodof FollowUp Knee Hip Ekman et al.,(1015) RCT USA 61.4± 10.0 Placebo TZB5g122(59.2), 10g128(61.5), Naproxen129(62.6) KneeOA TZB(5g& 10g), Naproxen 832 WOMACP, WOMAC PF,PGA 18Weeks 206 (100) 0 Ekman et al.,(1018) RCT USA 60.3± 10.5 Placebo TZB5g134(63.5), 10g128(61.2), Naproxen136(64.5) KneeOA TZB(5g& 10g), Naproxen 849 WOMACP, WOMAC PF,PGA 18Weeks 172 (81.5) 39(18.5) Hochberg et al.,2021 RCT India 60.3(9.5) Placebo NSAIDBID TZB2.5g637(63.6), 5g654(65.5),NSAID 662(66.5) Kneeor HipOA Oral NSAID, TZB2.5g 2,996 WOMACP, WOMAC PF,PGA 16Weeks 144 (14.5) 852(85.5) Karsdal et al.,2019 RCT USA 61.3(7.69) Controlled release oxycodone orPlacebo NSAIDUser22 (84.6%) Moderateto-Severe Kneeor HipOA TZB, TZB+NSA IDs, NSAID 56 WOMACP 35.5(20.4) NR NR NCT02697 773,2022 RCT USA 61.2(9.0) Placebo TZB5mg151(64.8), TZB2.5g145(62.8) Kneeand HipOA TZB 3021 WOMACP, WOMAC PF, WOMACS 8–24weeks 198 (85.0) 35(15.0) NCT02709 486,2022 RCT USA 65.2(10.2) Placebo TZB5mg91(31), TZB2.5g85(30) Kneeand HipOA TZB 2700 WOMACP, WOMAC PF, WOMACS 8–24weeks 236 (83.1) 48(16.9)
TZB = Tanezumab; NR = Not Reported; OA = Osteoarthritis; WOMAC P = WOMAC Pain, WOMAC PF= WOMAC Physical Function; PGA = Patient’s Global Assessment
NCT02528 188,2022 RCT USA 60.3(9.5) NR TZB5mg654(65.5), TZB2.5g637(63.6), NSAID662(66.5) Kneeand HipOA TZB, NSAIDs 3031 WOMACP, WOMAC PF, WOMACS 8–24weeks 852 (85.5) 144(14.5) Brown et al.,2022 RCT USA NR NSAID NR KneeOA TZB 3234 NR 24Weeks 3234 NR Mease2021 RCT NR 62.4(9.3) NR 85%Women Moderateto-Severe Kneeor HipOA TZB,Oral NSAID 40 AUSCANP, AUSCANF, VASGlobal Assessment 16weeks NR NR Schnitzer et al.,2017 RCT USA 61.3(10.0) Oral naproxen 500mg TZBmg392(72.5), TZB10mg392 (72.3),Placebo +NSAID388(72.0) Kneeand HipOA TZB(5g& 10g) 2700 WOMACP, WOMAC PF,PGA 17weeks 452 (83.4) 90(16.6) Schnitzer et al.,2019 RCT USA 61.2 (32-83) Placebo TZB2.5mg145 (62.8),TZB5mg151 (64.8) Kneeor HipOA TZB 696 WOMACP, WOMAC PF,PGA 8weeks 198 (85.0) 35(15.0) NCT00809 358,2021 RCT USA 18-44yrs 44%, 45-64yrs 57%,> 65yrs 38.6% Naproxen 500mg 70.50% Kneeor HipOA TZB 2720 WOMACP, WOMAC PF,PGA 9weeks NR NR
Table2:AdverseEffectAriseFromtheIncludedStudies
AuthorFirst Name AE AdverseEffect/Complication Tanezumabdosen(%) Controln(%) 5g 2.5g 10g NSAID Placebo Ekmanetal.,(A) 1015 Anyadverseevent 107 (51.9) NR 122 (58.7) 104 (50.5) 99 (47.6) AnyseriousAE 7(3.4) NR 6(2.9) 5(2.4) 8(3.8) Nasopharyngitis 2(1.0) NR 6(2.9) 8(3.9) 4(1.9) Fall 5(2.4) NR 2(1) 2(1) 8(3.8) Ekman etal.,(B) 1018 Anyadverseevent 101 (47.9) NR 110 (52.1) 110 (52.1) 85 (40.7) AnyseriousAE 3(1.4) NR 4(1.9) 9(4.3) 4(1.9) Nasopharyngitis 8(3.8) NR 4(1.9) 5(2.4) 2(1) Fall 6(2.8) NR 4(1.9) 5(2.4) 5(2.4) Hochberg et al., 2021 Anyadverseevent 670 (67.1) 629 (62.8) NR 601 (60.3) NR AnyseriousAE 80(8.0) 51 (5.1) NR 46(4.6) NR Nasopharyngitis 67(6.7) 57 (5.7) NR 40(4.0) NR Fall 53(5.3) 65 (6.5) NR 46(4.6) NR Brown et al., 2022 AnyAEofAPS 101 (10.1) 67 (6.7) NR 54(5.4) NR Schnitzer et al., 2014 Anyadverseevent, 405 (74.9) NR 399 (73.6) NR 364 (67.5) Anyseriousadverse event, 44(8.1) NR 46(8.5) NR 43(8.0) Nasopharyngitis 22(4.1) NR 19(3.5) NR 23(4.3)
*NR = Not Reported
Meta-Analysis of Efficacy of TNZ Against NSAID/Placebo

In the meta-analysis of efficacy and safety of tanezumab for Osteoarthritis disease as novel treatment, the results suggest that tanezumab 2.5mg (OR = 0.66, 95% Confidence Interval; 0.51 to 0.86, p – value = 0.002) and tanezumab10mg(OR=0.56,95%ConfidenceInterval; 0.33 to 0.96) significantlycuredOsteoarthritisdiseasecomparedtoNSAIDdrugsorPlacebo. However, it shows no significant effect with tanezumab 5mg (OR = 1.01, 95% Confidence Interval; 0.96 to 1.06, p – value = 0%) shown in Fig 3 Significant and high heterogeneity wasfoundamongstudiesincludedinTanezumab5mgandTanezumab10mg(I2 = 96% and I2 = 97%) respectively. Funnel visualization of the included studies shows a symmetric shape whichimpliesnoevidenceofpublicationbiasinFig4.

Fall 18(3.3) 20 (3.7) NR NR 16(3.0) Schnitzer et al., 2019 PatientswithAEs 143 (61.4) 156 (67.5) NR NR 145 (62.5) Patients with seriousAEs 11(4.7) 7(3.0) NR NR 9(3.9) Nasopharyngitis 17(7.3) 20 (8.7) NR NR 16(6.9) Fall 10(4.3) 13 (5.6) NR NR 9(3.9)
Meta-analysis of Primary Outcome Measure

Efficacy of Tanezumab 2.5mg on the outcomes
The subgroup meta-analysis of the three outcome measures (WOMAC Pain, WOMAC Physical, Function, and patient’s global assessment) suggested Tanezumab 2.5mg has no effect on WOMAC pain improvement (SMD= -0.07, 95% CI; -0.17 to 0.03, p – value = 0.15), improvement in physical function (SMD = -0.04, 95% CI; -0.17 to 0.09, p – value = 0.58) and global cognitive function (SMD = -0.01, 95% CI; -0.08 to 0.06, p – value = 0.97 (Fig 5). Significant and symmetric funnel plot was found with a point outside the funnel which indicates true heterogeneity rather than outlier (Fig 6). The percentage of heterogeneitywasmoderateandsignificant(p<0.05).
Figure 3: Forest Plot Showing the Efficacy of Tanezumab 2.5mg (Fig. 1.1.1), Tanezumab 5mg (Fig. 1.1.2), and Tanezumab 10mg (Fig. 1.1.3) ComparedtoNSAIDdrugs.
Figure 4: Funnel Plot showing Studies Included in the Efficacy of Tanezumab (2.5mg, 5mg and 10mg)againstNSAIDdrugs
Fig. 5: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)ofTanezumab2.5mgvsNSAID/Placebo
Efficacy of Tanezumab5mgontheoutcomes
Tanezumab 5mg indicate significant improvement inWOMACpain(WOMACpain;SMD= -0.72, 95% CI; -1.20 to -0.25, p – value = 0.003), improvement in Physical cognitive function (SMD = -2.33, 95% CI; -3.33 to -1.34, p – value < 0.00001), and global scores (PGA-OA; SMD = -0.60, 95% CI; -1.03 to -0.17, p – value = 0.006) in comparison with NSAID drugs or Placebo Fig 7. High and significant heterogeneity was found among the studies included in all analysis (I2 = 99%, 100% and 99%) respectively Visualization of the studies included in each group suggest a symmetric shape with a point outside the funnel which indicates true heterogeneity rather than outlier, thus, indicating no evidence of publicationbiasFig.8.
 Fig. 6: Funnel plot showing Studies Included in all outcome measure of Tanezumab 2.5mg VsNSAID
Fig. 6: Funnel plot showing Studies Included in all outcome measure of Tanezumab 2.5mg VsNSAID
Efficacy of Tanezumab 10mg on the outcomes
Tanezumab 10mg against NSAID or placebo drugs shows a significant improvement in WOMAC pain score (WOMAC pain; SMD = =1.09, 95% CI; -2.02 to -0.15, p – value = 0.002), Physical cognitive function (SMD = -5.47, 95% CI; -8.71 to -2.23, p – value =

 Fig. 7: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)forTanezumab5mgVsNSAID/Placebo
Fig. 8: Funnel plot showing Studies Included in all outcomemeasureofTanezumab5mgVs NSAID
Fig. 7: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)forTanezumab5mgVsNSAID/Placebo
Fig. 8: Funnel plot showing Studies Included in all outcomemeasureofTanezumab5mgVs NSAID
0.0009),andglobalscores(PGA-OA;SMD=-3.83,95%CI;-6.94to-0.72,p–value=0.02) in comparison with NSAID drugs or Placebo Fig 9. High and significant heterogeneity was found among the studies included in all analysis (I2 = 99%, 100% and 100%) respectively. Visualization of the studies included in each group suggest a symmetric shape with a point outside the funnel which indicates true heterogeneity rather than outlier which shows no evidenceofpublicationbiasFig.10.

 Fig. 9: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)forTanezumab10mgVsNSAID/Placebo
Fig. 9: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)forTanezumab10mgVsNSAID/Placebo
NETWORKMETA-ANALYSISOFTHETHREEDOSEOFTANEZUMABDRUGS
Before estimating the forest network meta-analysis, the net graph depicts the interaction effect between the three doses of the intervention against NSAID drugs (Fig. 11.) The networkforestplotofNSAIDagainstallinterventionconfirmedtheefficacyofTanezumabat all doses compared to the control group (all diamond points fall in the intervention groups)

Fig 13 However, when the treatment was treated with both NSAID and Placebo, theresults showednosignificanteffect.(Fig12).
 Fig. 10: Funnel plot showing Studies Included in all outcome measure of Tanezumab 10mg VsNSAIDorPlacebo
Fig. 10: Funnel plot showing Studies Included in all outcome measure of Tanezumab 10mg VsNSAIDorPlacebo
“*”MeansSignificant

Fig13: Intervention Comparison value EfficacyofTanezumab Tanezumab2.5mgVsNSAID 0% 0.96to1.06 0.65 Tanezumab5mgVsNSAID 96% 0.51to0.86 0.002* Tanezumab10mgVsNSAID 97% 0.33to0.96 0.03* PrimaryOutcomeMeasureforTanezumab2.5mg WOMACPainscore 71% -0.17to0.03 0.15 WOMACPhysicalFunctionscore 86% -0.17to0.09 0.58 PGA-OA 50% -0.08to0.06 0.79 Tanezumab5mg WOMACPainscore 99% 1.20to-0.25 0.003* WOMACPhysicalFunctionscore 100% -3.33to-1.34, <0.00001* PGA-OA 99% 0.006* Tanezumab10mg WOMACPainscore 99% -2.02to-0.15 0.02* WOMACPhysicalFunctionscore 100% -8.71to-2.23 0.0009* PGA-OA 100% -6.94to-0.72 0.02*
DISCUSSION
Tanezumab as a novel drug from pain therapywithhighaffinityandspecificity(Hefti,2006) is shown to be efficacious to reducepaininosteoarthritisandincreasejointsafety.Inclinical trials Tanezumab (2.5mg, 5mg and 10mg) has been investigated to reduced pain and cognitive function in elderly patients of over 60 years with knee or hip osteoarthritis, but findings of systematics review and meta-analysis to confirmed the efficacy of Tanezumab dose remained controversial. The present research has systematically reviewed 12 trials on the efficacy of Tanezumab against NSAID or placebo drugs and performed a meta-analysis on the reported outcome. Our results have shown that Tanezumab 5mg and 10mg significantly affect elderly patients with knee or hiposteoarthritiscomparedwiththeNSAID or placebo drugs, but Tanezumab 2.5mg showed no significant effect. Though patients already treated with NSAID and show no response gained additional benefit when treated with Tanezumab. In the meta-analysis of the three reported primary outcomes, our findings confirmed that Tanezumab 5mg and 10mg again, reduced pain, improved cognitive function and global scores. The findings are consistent with the previous meta-analysis (Cao et al., 2020 & Fan et al., 2020). Though, the study Cao et al., 2020 performed a network meta-analysisbutresultsarestillconsistentwithourresearch.
Tanezumab as a humanized nerve growth factor (NGF)-neutralizing monoclonal IgG2 antibody works by working against the interaction of NGF to the TrkA receptor on nociceptive neurons. While affecting neurons, NGF are found elevated in synovial fluid and affect joints in inflammationandosteoarthritis.IncontrasttoopioidandNSAIDs,tanezumab allows novel mechanisms in pain relief. This allows tanezumab to have less adverse effects than current treatment, such as cardiovascular, gastrointestinal bleeding, respiratory problems, or addiction.11,14,20 Furthermore, tanezumab ability to work with NGF allows sensitization and reduces NSAIDs current side effects that are gastrointestinal disturbance, cardiovascular change, and hypersensitivity reactions. In contrast, tanezumab may cause a more significant number of nasopharyngitis and peripheral sensitization on higher dose treatment.CurrentstudiesalsoshowthattanezumabcanbeusedinconjunctionwithNSAIDs or replacement to non-pharmacological treatment. While current treatment for osteoarthritis still needs rigorous maintenance, tanezumab enables a more planned administration, only once every 8-12 weeks. This has shown a more easy intervention while havingalongsafety surveillance.11,13,15, With a consistent administration, patients WOMAC score reduced significantlyandjointsafetywasimproved.
Although it strengths, tanezumab treatment currently is not used well in clinical settings because of its high cost. Current innovations,suchassubcutaneousinjectionarebeingmade, but notwidelyavailableyettomostpatients.Otherthanthat,itsuseofinjectionsmaychange patients' treatment in comparison to oral treatment of NSAIDs. Besides, tanezumab use in rapid progressive osteoarthritis is also limited. Its effect on joint safetyisrelatedtoaffecting cartilage repair and bone formation, while its mechanism in correlation to rapid progressive osteoarthritisstillremainsunclear 11,13-15,16,20
Strength and Limitations
Within this network meta-analysis and search, there are strengths and limitations concluded. From our knowledge, this is the first network meta-analysis of tanezumab comparison to NSAID as current treatment on geriatric osteoarthritis patients. Results of the network analysis show a more detailed and significant efficacyoftanezumabinmultipledoses.Other than that, the amount of studies with recommended practiced methods areusedtoensurethe randomized controlled trial and research in the field. The risk of biasassessmentresultsalso shows a low inclination. There are some limitations that should be considered while promising results. The studies show a high level of heterogeneity because of the variety in follow up timeandlackoflongtermeffectmonitoring.Thereisalsolimitedaccesstostudies outsideEnglishstudiesandfulltextstudies.
CONCLUSIONANDRECOMMENDATIONS
Inconclusion,tanezumabisfoundeffectivetotreatpaininosteoarthritisshownbysignificant reduction in WOMAC pain scale, increasedphysicalactivityinWOMACPhysicalFunction, and better overall health shown by Patients’ Global Assessment. In comparison to NSAIDs, tanezumab in 5 and 10 mg doses has beenfoundtobebetterwhilehavingfewersideeffects. Thus, further research of tanezumab cost-effectiveness and long term effects will be beneficial to practice in clinical settings. Furthermore, inclusion of tanezumab in osteoarthritis guidelines should be considered to relieve moderate-to-severe pain with inadequate relief from other interventions and patients with comorbidities that limits the use ofNSAIDs,suchasgastrointestinalchange,cardiovasculardisease,andrespiratorychange.
ACKNOWLEDGMENTSANDCONFLICTOFINTERESTS
Theauthorshavenoconflictofinteresttodeclare.
REFERENCES
1. HunterD,Bierma-ZeinstraS.Osteoarthritis.Lancet.2019;393(10182):1745-1759
2. Meng, Fanqiang, et al. "Efficacy and safety of biologic agents for the treatment of osteoarthritis: A meta-analysis of randomized placebo-controlled trials." Therapeutic AdvancesinMusculoskeletalDisease14(2022):1759720X221080377.
3. Lin CL, Heron P, Hamann SR, et al. Functional distinction between NGF-mediated plasticity and regeneration of nociceptive axons within the spinal cord.Neuroscience 2014;272:76–87.
4. Denk, Franziska, David L. Bennett, and Stephen B. McMahon. "Nervegrowthfactor andpainmechanisms."Annualreviewofneuroscience40(2017):307-325.
5. Nair AS. Tanezumab: Finally a Monoclonal AntibodyforPainRelief.IndianJPalliat Care. 2018 Jul-Sep;24(3):384-385. doi: 10.4103/IJPC.IJPC_208_17. PMID: 30111960;PMCID:PMC6069623.
6. Gao, Yijie, et al. "Efficacy and Safety of Anti–Nerve Growth Factor Antibody Therapy for Hip and Knee Osteoarthritis: A Meta-analysis." Orthopaedic Journal of SportsMedicine10.4(2022):23259671221088590.
7. Kan, S. L., Li, Y., Ning, G. Z., Yuan, Z. F., Chen, L. X., Bi, M. C., et al. (2016). Tanezumab for Patients with Osteoarthritis of the Knee: AMeta-Analysis.PLoSOne 11(6),e0157105.doi:10.1371/journal.pone.0157105
8. Kan, S. L., Li, Y., Ning, G. Z., Yuan, Z. F., Chen, L. X., Bi, M. C., et al. (2016). Tanezumab for Patients with Osteoarthritis of the Knee: AMeta-Analysis.PLoSOne 11(6),e0157105.doi:10.1371/journal.pone.0157105
9. Hu, Rui, et al. Clinicaloutcomesoftanezumabwithdifferentdosagesforpatientwith osteoarthritis:networkmeta-analysis.FrontiersinPharmacology12(2021):614753.
10.Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ [Internet]. 2019 Jan 30 [cited 2022 Oct 11];364. Available from: https://www.bmj.com/content/364/bmj.k4597
11. Hochberg MC, Carrino JA, Schnitzer TJ, Guermazi A, Walsh DA, White A, et al. Long‐term safety and efficacy of subcutaneous tanezumab versus nonsteroidal antiinflammatory drugs for hip or knee osteoarthritis: a randomized trial. Arthritis Rheumatol [Internet]. 2021Jun7[cited2023Apr13];73(7):1167–77.Availablefrom: https://onlinelibrary.wiley.com/doi/10.1002/art.41674
12.Neogi T, Hunter DJ, Churchill M, Shirinsky I, WhiteA,GuermaziA,etal.Observed efficacy and clinically important improvements in participants with osteoarthritis treated with subcutaneous tanezumab: results from a 56-week randomized NSAID-controlled study. Arthritis Res Ther [Internet]. 2022 Mar 29 [cited 2023 Apr 4];24(1):78.
Available from:
https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-022-02759-0
13.Brown MT, Sandroni P, Low PA, Gorson KC, Hunter DJ, Pixton GC, et al. Neurological safety of subcutaneous tanezumab versus NSAID in patients with osteoarthritis. J Neurol Sci [Internet]. 2022 Mar [cited 2023 Apr 4];434:120184.
Availablefrom:https://linkinghub.elsevier.com/retrieve/pii/S0022-510X(22)00046-6
Available from:
14.Schnitzer TJ, Bonfanti G, Atkinson J, Donevan S, Viktrup L, Barroso J, et al. Characterizing 16-week responder profiles using group-based trajectory modeling in over 4300 clinical trial participants receiving pharmaceutical treatment for moderate to severe osteoarthritis. AdvTher [Internet]. 2022 Aug 12 [cited 2023 Apr 4];39(10):4742–56.
https://link.springer.com/article/10.1007/s12325-022-02290-3
15.Ekman EF, Gimbel JS, Bello AE, Smith MD, Keller DS, Annis KM, et al. Efficacy andsafetyofintravenoustanezumabforthesymptomatictreatmentofosteoarthritis:2 randomized controlled trials versus naproxen. J Rheum [Internet]. 2014 Oct 1 [cited 2023 Apr 4];41(11):2249–59. Available from:
https://www.jrheum.org/content/41/11/2249
16.Schnitzer TJ,EkmanEF,SpieringsELH,GreenbergHS,SmithMD,BrownMT,etal. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis [Internet]. 2014 Mar 13 [cited 2023 Apr 4];74(6):1202–11. Available from:https://ard.bmj.com/content/74/6/1202
17.Mease P, Kuritzky L, Wright WL, Mallick-Searle T, Fountaine R, Yang R, et al. Efficacy and safety of tanezumab, NSAIDs, and placebo in patientswithmoderateto severe hip or knee osteoarthritis and a history of depression, anxiety, or insomnia: post-hoc analysis of phase 3 trials. CurrMedResOpin[Internet].2022Aug28[cited 2023 Apr 4];38(11):1909–22. Available from: https://www.tandfonline.com/doi/abs/10.1080/03007995.2022.2113689?journalCode= icmo20
18.Stanos SP, Chang WJ, Hultman C, Sadrarhami M, Yamabe T, Park P. AB0859 improvements in physical function in patients with osteoarthritis receiving subcutaneoustanezumabin3randomizedcontrolledtrials.AnnRheumDis[Internet].
2020 Jun [cited 2023 Apr 13];79(Suppl 1):1736.3-1737. Available from: https://ard.bmj.com/content/79/Suppl_1/1736.3
19.Hunter DJ, Schnitzer TJ, Hall J, Semel D, Davignon I, Cappelleri JC, et al. Time to first and sustained improvement in WOMAC domains among patients with osteoarthritis receiving tanezumab. Osteoarthr [Internet]. 2022 Sep [cited 2023 Apr 4];4(3):100294.
Available from:
https://linkinghub.elsevier.com/retrieve/pii/S2665913122000620
20.Schnitzer TJ, Easton R, Pang S, Levinson DJ, Pixton G, Viktrup L, et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee. JAMA [Internet].
2019 Jul 2 [cited 2023 Apr 4];322(1):37. Available from: https://jamanetwork.com/journals/jama/fullarticle/2737173
21.Clinicaltrials.gov. Identifier: NCT00809354-Long-termanalgesicefficacyandsafety of tanezumab alone or in combination with non-steroidal anti-inflammatory drugs (NSAIDs) versus NSAIDs alone in patients with osteoarthritis of the knee or hip. National Library of Medicine: Bethesda (MD); 2021 Jun 24 [cited 2023 Apr 4].
Availablefrom:https://clinicaltrials.gov/ct2/show/NCT00809354
22.Clinicaltrials.gov. Identifier: NCT02528188 - Long term safety and efficacy study of tanezumab in subjects with osteoarthritis of the hip or knee. National Library of Medicine: Bethesda (MD); 2020 Jan 10 [cited 2023 Apr 4]. Available from: https://clinicaltrials.gov/ct2/show/NCT02528188
Database Keywords Hits PubMed [(“tanezumab”)AND(“NSAID”OR“OralNSAID”)AND (“osteoarthritis”)] 54 ScienceDirect ('tanezumab'/expORtanezumab)AND('nsaid'/expOR nsaidOR'oralnsaid'OR(oralAND('nsaid'/expORnsaid))) AND('osteoarthritis.'/expORosteoarthritis.) 12 Manual [(“tanezumab”)AND(“NSAID”OR“OralNSAID”)AND (“osteoarthritis”)] 14
APPENDIXES Appendix1.SearchStrategies
Glucagon-Like-Peptide-1-Receptor-Agonist VS Sodium-Glucose-Cotransporter-2Inhibitors to Its Glycemic Control and Cardio-Renal Outcomes Among Elderly with Type-2 Diabetes Mellitus: Systematic Review and Meta-Analysis
Ni Putu Desya Chandraka Dewi
1 ,
Tsabita Nafisa
Muhammad Syifaul Afnan1 , Sekar Arum Srigati1
1 ,
1Faculty of Medicine University of Jember, Jember, Indonesia
Correspond email: desyachandraka25@gmail.com
ABSTRACT
Introduction: Type-2 diabetes mellitus (T2DM) is the most common systemic disease among theelderly with nearly253millionexpectedpatients in 2045with cardiovasculardisease(CVD) and diabetic kidney disease (DKD) as its most common complications. Sodium-Glucose Cotransporter-2 inhibitor (SGLT2i) and Glucagon Like Peptide-1 Receptor Agonist (GLP1RA) has emerged as the alternative therapy with the possibility to prevent any CVD and kidney diseases progression among T2DM patients, moreover to the elderly. which need to be investigated further.
Objective: To investigate and compare the glycemic control and cardiorenal protection effects of SGLT2i and GLP-1RA in elderly patients with T2DM.
Method: A literature search was conducted through multiple electronic databases. Risk of biases were assessed using RoB 2 tool. Mean and Standard Deviation (SD) with confidence interval (CI) of 95% were used to determine the association between SGLT2i and GLP-1RA in all outcomes.
Result:EightRCTs,yielding7.897participantsin totalareincluded.Thecurrentstudyshowed significant result in GLP-1RA (pooled MD=-0.84, 95% CI (-1.34,0.34), p=0.0009, I²=97% vs pooled MD=-0.52, 95% CI (-1.16, 0.13), p=0.12, I²=96%) compared to SGLT2i as the primary outcome. In the secondary outcome, SGLT2i showed better results in systolic blood pressure, diastolicbloodpressure, andbodyweight,meanwhileGLP-1RAshowedbetterresultsin eGFR. We also reported MACE and adverse events across the study.
Conclusion: This study provides valuable evidence that SGLT2i and GLP-1RA showed a potential effect as glycemic control and cardiorenal protection. However, SGLT2i indeed showed a more significant effect in multiple outcomes in elderly with T2DM. We recommend future study with a more homogenous comorbidity.
Keywords: cardiorenal complications, glucagon-like peptide-1 receptor agonist, glycemic control, sodium-glucose cotransporter 2 inhibitors, type 2 diabetes mellitus
Glucagon-Like-Peptide-1-Receptor-Agonist VS Sodium-Glucose-Cotransporter-2Inhibitors to Its Glycemic Control and Cardio-Renal Outcomes Among Elderly with Type-2 Diabetes Mellitus: Systematic Review and Meta-Analysis
Pre-Conference Competition Asian Medical Students’ Conference
PCC AMSC 2023
Geriatrics: Care for the Future
Ni Putu Desya Chandraka Dewi
Tsabita Nafisa
Muhammad Syifaul Afnan
Sekar Arum Srigati
Faculty of Medicine
University of Jember

Jember
Indonesia
Glucagon-Like-Peptide-1-Receptor-Agonist VS Sodium-Glucose-Cotransporter-2Inhibitors to Its Glycemic Control and Cardio-Renal Outcomes Among Elderly with Type-2 Diabetes Mellitus: Systematic Review and Meta-Analysis
Ni Putu Desya Chandraka Dewi
1 ,
Tsabita Nafisa
Muhammad Syifaul Afnan1 , Sekar Arum Srigati1
1 ,
1Faculty of Medicine University of Jember, Jember, Indonesia
Correspond email: desyachandraka25@gmail.com
ABSTRACT
Introduction: Type-2 diabetes mellitus (T2DM) is the most common systemic disease among theelderly with nearly253millionexpectedpatients in 2045with cardiovasculardisease(CVD) and diabetic kidney disease (DKD) as its most common complications. Sodium-Glucose Cotransporter-2 inhibitor (SGLT2i) and Glucagon Like Peptide-1 Receptor Agonist (GLP1RA) has emerged as the alternative therapy with the possibility to prevent any CVD and kidney diseases progression among T2DM patients, moreover to the elderly. which need to be investigated further.
Objective: To investigate and compare the glycemic control and cardiorenal protection effects of SGLT2i and GLP-1RA in elderly patients with T2DM.
Method: A literature search was conducted through multiple electronic databases. Risk of biases were assessed using RoB 2 tool. Mean and Standard Deviation (SD) with confidence interval (CI) of 95% were used to determine the association between SGLT2i and GLP-1RA in all outcomes.
Result:EightRCTs,yielding7.897participantsin totalareincluded.Thecurrentstudyshowed significant result in GLP-1RA (pooled MD=-0.84, 95% CI (-1.34,0.34), p=0.0009, I²=97% vs pooled MD=-0.52, 95% CI (-1.16, 0.13), p=0.12, I²=96%) compared to SGLT2i as the primary outcome. In the secondary outcome, SGLT2i showed better results in systolic blood pressure, diastolicbloodpressure, andbodyweight,meanwhileGLP-1RAshowedbetterresultsin eGFR. We also reported MACE and adverse events across the study.
Conclusion: This study provides valuable evidence that SGLT2i and GLP-1RA showed a potential effect as glycemic control and cardiorenal protection. However, SGLT2i indeed showed a more significant effect in multiple outcomes in elderly with T2DM. We recommend future study with a more homogenous comorbidity.
Keywords: cardiorenal complications, glucagon-like peptide-1 receptor agonist, glycemic control, sodium-glucose cotransporter 2 inhibitors, type 2 diabetes mellitus
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is the most common systemic disease among the elderly with nearly 253 million expected patients in 2045.[1,2] Hence, proper treatment and glycemic control in the elderly are crucial to prevent poor outcomes and complications. [3,4] Among all the complications, cardiovascular disease (CVD) and diabetic kidney disease (DKD) are the most common complications in the elderly.[4,5]Poor glycemic control and overtreatment could lead to worsening conditions and adverse events such as hypoglycemia which would cause major adverse cardiovascular events (MACE).[6,12]
Up to 2023, T2DM has several drugs to choose from for its therapy, with metformin as its first-line therapy. But the use of metformin has restrictions to be used for diabetes, with the estimated glomerular filtration rate (eGFR) <45 mL/min/1,73 m2 .This restriction is due to the possibility of lactic acidosis and also to prevent the worsening of renal condition, which eGFR can measure.[7,9] Furthermore, Sodium-Glucose Cotransporter-2 inhibitor (SGLT2i) and Glucagon Like Peptide-1 Receptor Agonist (GLP-1RA) has emerged as alternative therapy withthepossibilitytopreventanyCVDandkidneydiseasesprogressionamongT2DMpatients, moreover to the elderly [9,10] SGLT2i mediates hyperglycemia by preventing glucose reabsorption.[11] Aside, these two drugs hold the potential for cardiorenal protection effects. The cardioprotection of SGLT2i is by reducing cardiac preload, blood pressure, and body weight by reducing albuminuria and hypoxic stress and restoring tubuloglomerular feedback as its renoprotection.[5] Meanwhile, GLP1-RA works by lowering postprandial (PP) glycemia, slowing gastric emptying, inducing weight loss, ameliorating atherosclerosis formation and high blood pressure, and halting the progression of heart failure, myocardial infarction, and cardiomyopathy. [6]
In recent years, several studies showed the beneficial effects of SGLT2iand GLP-1RA on glycemic and cardiorenal outcomes.[18, 19] The previous meta-analysis[29] only measured the cardiovascular effects using 3-point of MACE in both drugs but not in its glycemic control and renoprotection ability
Thus, this study aimed to provide the most recent evidence comparing SGLT2i and GLP-1RAin glycemiccontrol andcardiorenal protection effects in elderly patients with T2DM based on the change score of baselines to know the impact precisely
MATERIAL AND METHODS Study Methodology
We adhered this study to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines. And the research protocol is already registered to International Prospective Register of Systematic Review (PROSPERO) (https://www.crd.york.ac.uk/prospero/)
Eligibility Criteria
The following criteria were considered for this studies’ eligibility: population of the studies, type of intervention, outcomes, type of studies, and reference standards.
Population of the studies
All elderly patients (more than 60 years old in age) with T2DM were included in this study. There was no limitation for comorbidity and diabetes duration.
Type of intervention
All interventions included SGLT2i and/or GLP-1RA with placebo as its control comparison no limitation about the SGLT2i and GLP-1RA type.
Outcomes
The outcome of the studies should mention Hemoglobin A1c (HbA1c) as its primary outcome and eGFR, systolic blood pressure (SBP), diastolic blood pressure (DBP), and body weight as its secondary outcome. The outcome measurement should mention the change score of the baseline study or can be extracted to the change score value in mean (standard deviation) data.
Type of studies
Original research articles in randomizedcontrolledstudy type,full-textavailability, and written in Bahasa and English were included. Narrative review, systematic review, metaanalysis, non-comparative research, technical reports, editor response, scientific poster, study protocols, conference abstracts, and other articles irrelevant to the topics were excluded.
Reference Standard
The reference standard was experimental laboratory research performed by qualified professionals by evaluating the effect of SGLT2is and/or GLP-1RA treatment among elderly patients with T2DM.
Data Sources and Search
A literature search process was carried out with multiple electronic databases, such as Cochrane Library, Google Scholar, PubMed, and SpringerLink. The literature search of the database was conducted from inception until 30 March 2023. The keywords were described usingBooleanoperators. Allthestudiesfromthesedatabaseswerestoredin theauthors’library on Rayyan.ai.
Studies Selection
After removing duplicated articles, retrieved articles were screened based on the titles and abstracts by four independent reviewers (TN, NPDCD, MSA, SAS). Potentially eligible full-text articles were thoroughly assessed in Rayyan.ai using the eligibility criteria described above. Any emerging discrepancies were resolved by consensus among the review team. The study selection process was recorded in the PRISMA flow chart.
Data Extraction and Analysis
Selected studies were extracted with Microsoft Excel 2019 (Microsoft Corporation, USA). The following data were recorded: first author, year, setting, study design, age, intervention type, population characteristic, sample size, dosage, assessment period, HbA1c, eGFR, SBP, DPB, Body weight, MACE (nonfatal stroke, nonfatal myocardial infarction, CVD death) and adverse events. All statistical tests for this meta-analysis were conducted using Review Manager v5.4 (Cochrane Collaboration, UK).
Risk Of Bias in Individual Studies (Qualitative Synthesis)
The quality of each study included in this study was assessed by two independent reviewers (NPDCD and MSA) according to the Cochrane Risk of Bias (RoB) tool, mainly the RoB 2 tool (revised tools for risk of bias in randomized trials). Any disagreement is resolved by consensus between reviews.
Quantitative Data Synthesis (Meta-Analysis)
This meta-analysis calculated Mean and Standard Deviation (SD) with the confidence interval (CI) of 95%. We used the heterogeneity level to determine the effect size, either a fixed-effect model (FEM) or random effect model (REM). We used REM when the included studies were considered heterogeneous, indicated by an I2 > 50%. Otherwise, we used FEM when the included studies indicate I2 < 50%. The pooled estimate was presented in our forest plot.
RESULT
Study selection
Our search yielded 600 articles from four different databases as described in Appendix 2 below. Sixty-four duplicates from those databases were removed. Then, the authors read the title and abstract of the 536 remaining articles for preliminary screening. The articles that did not fulfill our eligibility criteria were excluded. The full text was retrieved from 139 articles and 397 studies were excluded because those articles do not clearly explain the effect of either SGLT2i or GLP-1RA supplementation. Finally, 8 full-text articles were included in both qualitativeandquantitativeanalysis. Ourstudy selectionprocess was presentedin thePRISMA diagram in Appendix 3
Study Characteristics and Results of Individual Studies
The summary of our included studies is displayed in Appendix 13. The subject of these studies was elders with age above 60 years old with T2DM receiving either SGLT2i or GLP1RA with placebo as its control treatment. We focused on HbA1c, eGFR, SBP, DBP, body weight, MACE, and AE as our outcome to indicate the effect of SGLT2i or GLP-1RA Out of the eight studies, one study of the GLP-1RA did not provide the standard deviation.
Risk of Bias in Individual Studies (Qualitative Synthesis)
We critically assessed the quality of each study with the RoB 2 tool. Four studies had low risk of bias, four studies lead to concern, and only one lead to high risk of bias. Studies by Allegreti et al. 2019 and Husain et al.,2019 create concern due to incomplete report of outcome data. While studies by Gerstein et al., 2021 creates concern due to no information in result selected from multiple outcome measurements and in study by Ott et al., 2017 there is no information in the accordance of prespecified analysis plan. High risk of bias in study by Seino et al., 2014 is due to overall bias in selection of the reported result. The summary of bias analysis is presented in Appendix 4.
Meta-Analysis
We compared the pooled effect of SGLT2i and GLP-1RA treatment in elderly with T2DM to thechangescoreofHbA1cto analyzeglycemiccontrol. Theeffectiveness ofSGLT2i and GLP-1RA compared to placebo is displayed in Figure 1 and Figure 2. These results indicated that both treatments indeed had potential ability in which the change score showed higher reduction of HbA1c. But, GLP-1RA showed a higher reduction and significant result. (pooled MD=-0.84, 95% CI(-1.34, -0.34), p=0.0009, I²= 97%) in HbA1c than SGLT2i (pooled MD=-0.52, 95% CI (-1.16, 0.13), p=0.12, I²= 96%). However, these results are still unsignificant across studies due to high heterogeneity
Other analysis in our secondary outcome consists of eGFR, SBP, DBP, and BW. In eGFR, SGLT2i showed a probable of increasing the progression of kidney disease by lowering the eGFR (pooled MD=-1.93, 95% CI (-9.26, 5.39), p=0.60, I²= 81%), even though the result showed an unsignificant effect with high heterogeneity. Contrary to SGLT2i, meta-analysis of GLP-1RA showed a potentially good effect to lowering the kidney disease progression by increasing the eGFR (pooled MD=0.68, 95% CI (0.11, 1.26), p=0.02, I²= 40%). The GLP-1RA was also supported by significant p-value and homogeneity in its data across studies. The results are displayed in Appendix 5 and Appendix 6
In SBP, SGLT2i showed a better result in lowering the SBP (pooled MD=-3.61, 95% CI (-4.83, -2.38), p < 0.00001, I² = 0%) with a significant and homogenate result compared to GLP-1RA who has significant yet heterogenic result (pooled MD = -2.16, 95% CI (-3.64,0.68), p = 0.004, I² = 76%). The results are displayed in Appendix 7 and Appendix 8. Next, we also compared the DBP outcome between SGLT2i and GLP-1RA against placebo. SGLT2i showed a significant and homogenate result in lowering the DBP among elderly with T2DM (pooled MD = -1.88, 95% CI (-1.88, -0.31), p = 0.006, I² = 0%). But, the different results showed by GLP-1RA analysis in which also showed a significant and homogenate result to placebo instead of SGLT2i treatment (pooled MD = 0.85, 95% CI (0.26, 1.44), p = 0.005, I² = 0%). These SBP and DBP results indicate a comparison between these two drugs in favoring the effect towards the CVD risk factors among elderly with T2DM. The results are displayed in Appendix 9 and Appendix 10.
We also analyze the potential effects of both SGLT2i and GLP-1RA in lowering body weight in the elderly with T2DM. The body weight reduction showed a promising result in SGLT2i (pooled MD=-3.17, 95% CI (-3.51, -2.82), p<0.00001, I²= 0%) compared to GLP1RA (pooled MD=-1.26, 95% CI (-2.85, 0.33), p=0.12, I²= 94%) due to its significance and homogeneity in reducing the body weight. These results are displayed in Appendix 11 and Appendix 12.

 Figure 1. Forest plot meta-analysis of the effect of SGLT2i treatment on elderly patients with T2DM seen from the change score of HbA1c
Figure 2. Forrest plot meta-analysis of the effect of GLP-1RA treatment on elderly patients with T2DM seen from the change score of HbA1c
Figure 1. Forest plot meta-analysis of the effect of SGLT2i treatment on elderly patients with T2DM seen from the change score of HbA1c
Figure 2. Forrest plot meta-analysis of the effect of GLP-1RA treatment on elderly patients with T2DM seen from the change score of HbA1c
DISCUSSION
We have been looking into how impactful SGLT2i and GLP-1RA are, and which one showed a better outcome toward glycemic control and cardiorenal protection. To examine the effect of both treatments, our study used the change score of the baseline and final follow-up. In this case, the results will be more powerful than comparing the raw data as it removes some variability among the analysis. We conducted analysis of HbA1c as primary outcome along with SBP, DBP, and BW as our secondary outcome.
Studies have shown that HbA1c is suited to assess long-term glycemic control as it has a time of up to 8-12 weeks of correlation with blood glucose levels.[30] Our meta-analysis suggested better effects in GLP-1RA to HbA1c reduction. We analyzed the results using two forest plots as can be seen in Figure 1 and Figure 2. SGLT2i and GLP-1RA showed high heterogeneity (I²= 96% and I²= 97%, respectively) suggesting that it tends to have high inconsistency data across included studies. Therefore, GLP-1RA might be the better option as a T2DM alternative drug of choice for the elderly. Moreover, among SGLT2i, four out of five studies shows a decrease level in HbA1c.[18,20,21,25] Meanwhile, all the included study using GLP-1RA decreased the HbA1c which provide the reason of better outcomes than SGLT2i. [22,24,26] Study by Lundin et al., 2022 is the only SGLT2i study that showed a positive change score to its final follow up which might be caused by the less efficient effects of SGLT2i in newly diagnosed patients with dysglycaemia which was the population of the study.[19]
As DKD is one of the complications of T2DM, we aimed to assess the kidney disease progression in the patients using eGFR which more practical than others.[7,31] In this current study, only two studies of SGLT2i and GLP-1RAmentioned the eGFR data. Our meta-analysis of those studies against placebo showed a low heterogeneity and significant result in GLP-1RA compared to SGLT2i data (I²=40%, p=0.02vs I²= 81%,p=0.60).These results showed abetter favorable effect of GLP-1RA on lowering kidney disease progression by increasing the eGFR. It might be happened due to the physiological effect between glomerular filtration rate activity with the decreasing level of systemic blood pressure which is one of effects from GLP1RA [24,27] High heterogeneity in SGLT2i treatment might be happen due to difference in dosage and intervention period
Cardiovascular risk among elderly can be seen by blood pressure as its independent risk factor.[32] Hence, in our study we compared both its systolic and diastolic blood pressure independently. The blood pressure analysis has shown a better outcome in SGLT2i compared to GLP-1RA with homogeneity and significant data (I²=0%, p<0.00001 vs I²=76%, p=0.004 for SBP and I² = 0%, p=0.006 vs I² = 0%, p=0.005, respectively). The heterogeneity in GLP1RA resulted probably by the limitation of included study and gap number of participants. But, in the lowering DBP analysis, the result showed significant favorable effect toward placebo instead of GLP-1RA. These results can be a consideration on managing hypertension in the elderly with T2DM who undergo the antidiabetic treatment.
Weight loss is known as a recommended way to overweight and obese patients with T2DM to lowering the complications and improving hyperglycemia.[36] Appropriate interventions to maintain a stable weight were also recommended to positively influence health outcomes.[37,38] Glucose lowering drugs, such as SGLT2i and GLP-1RA are reported to have
effect on weight by inducing weight gain or weight loss among patients with T2DM.[39] In this study, SGLT2i hold a better outcomes compared GLP-1RA as both treatments being compared to placebo (I²= 0%, p<0.00001 vs I²= 94%, p=0.12). The favorable effects of this drug on lowering body weight might be due to caloric wasting and proximal tubulardiuretic effects that result from increased urinary glucose excretion. However, GLP-1RA still gives its weight loss effect as it is known to, here, it most likely due to the unfavorable and high heterogeneity data of Seino et al., 2014. [26]
In addition, concern about CVD which is one of the leading causes of death in T2DM patients.[35] Therefore, we evaluate Major Adverse Cardiovascular Events (MACE) including nonfatal stroke,nonfatal myocardial infarction (MI),and CVD death in both SGLT2i and GLP1RA treatments In SGLT2i analysis, only study by Allegreti et al., 2019 reported MACE in their outcome. Two MACE is reported out of 157 total population that supplemented with SGLT2iandnoneintheplacebo arm.Meanwhile twostudiesreportedMACEintheiroutcome. Study by Gerstein et al., 2021 reported total of 189 MACE out of 2171 total population that had SGLT2i. The 189 events consist of 41 nonfatal stroke, 85 nonfatal MI, and 75 CVD death. Compared to placebo, the total MACE reported was 125 consist of 25 nonfatal stroke, 53 nonfatal MI, and 50 CVD death out of 1359 total population in placebo arm. These results conclude that GLP-1RA lowers MACE by 27% [24] Study by Husain et al., 2019 reported a total MACE of 64 out of 1591 total population with GLP-1RA supplementation. This event is consisting of 12 nonfatal stroke, 37 nonfatal MI, and 15 CVD death. The total number is lower in GLP-1RA compared to total MACE of 77 out of 1592 population in placebo arm.[22] Moreover, all three included studies with GLP-1RA stated gastrointestinal adverse events, including vomiting, nausea, and diarrhea.[22,24,26] Meanwhile inSGLT2i supplementation, most of the adverse events are hypoglycemia and infection, especially Genito-urinary tract infection [18,20,21] SGLT2i might cause hypoglycemia by reducing renal proximal tubular reabsorption of glucose, thereby inducing glucosuria [40] However, those adverse events might be associated with other factors aside from the treatment, such as comorbidities, duration of the illness, and others.
Despite the heterogeneity, our results suggest that both SGLT2i and GLP-1RA to elderly with T2DM have promising effects in glycemic controls and cardiorenal protection. It depends on thepatients' conditions, comorbid, and other considerations to usewhetherSGLT2i or GLP-1RA as monotherapy or on combination with other antidiabetic drugs. Furthermore, we hope there will be an update and a large-scale study about the potential effects with a more homogenous comorbidity to assess the cardiorenal protection effect of SGLT2i and GLP-1RA based on their dosage and duration. Furthermore, we hope there will be an update and a largescale study about the potential effects with a more homogenous comorbidity to assess the cardiorenal protection effect of SGLT2i and GLP-1RA based on their dosage and duration.
CONCLUSION
This systematic review and meta-analysis provide valuable evidence that SGLT2i and GLP-1RA showed a potential effect as glycemic control and cardiorenal protection. However, SGLT2i indeed showed a more significant effect in multiple outcomes in elderly with T2DM. We recommend future study with a more homogenous comorbidity.
ACKNOWLEDGEMENTS AND CONFLICT OF INTEREST
Thanks to our seniors and family for fully supporting this systematic review and metaanalysis. The authors declare no conflict of interest.
REFERENCES
1. NCDRiskFactorCollaboration(NCD-RisC).Worldwidetrendsindiabetessince1980: a pooled analysis of 751 population-based studies with 4·4 million participants. The Lancet. 2016 Apr;387(10027):1513–30.
2. Chentli F, Azzoug S, Mahgoun S. Diabetes mellitus in elderly. Indian Journal of Endocrinology and Metabolism [Internet]. 2015;19(6):744. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4673801/
3. Abdelhafiz AH, Rodríguez-Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in Older People - A Less Well Recognized Risk Factor for Frailty. Aging and Disease [Internet]. 2015;6(2):156. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4365959/
4. Raghavan S, Vassy JL, Ho Y, Song RJ, Gagnon DR, Cho K, et al. Diabetes Mellitus–RelatedAll‐CauseandCardiovascularMortalityinaNationalCohortofAdults.Journal of the American Heart Association [Internet]. 2019 Feb 19;8(4). Available from: https://dx.doi.org/10.1161%2FJAHA.118.011295
5. Kalra S, Aydin H, Sahay M, Ghosh S, Ruder S, Tiwaskar M, et al. Cardiorenal Syndrome in Type 2 Diabetes Mellitus – Rational Use of Sodium–glucose Cotransporter-2 Inhibitors. European Endocrinology [Internet]. 2020 Oct 1;16(2):113
21. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7572171/
6. Ma X, Liu Z, Ilyas I, Little PJ, Kamato D, Sahebka A, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. International Journal of Biological Sciences [Internet]. 2021 May 11;17(8):2050–68. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8193264/
7. Christofides EA, Desai N. Optimal Early Diagnosis and Monitoring of Diabetic Kidney Disease in Type 2 Diabetes Mellitus: Addressing the Barriers to Albuminuria Testing. Journal of Primary Care & Community Health. 2021 Jan;12:215013272110036.
8. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovascular Diabetology [Internet]. 2018 Jun 8;17(1). Available from: https://cardiab.biomedcentral.com/articles/10.1186/s12933-018-07286
9. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes 2023 Diabetes Care. 2022 Dec 12;46(Supplement_1):S191–202.
10. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Erratum. 10. Cardiovascular disease and risk management: Standards of Care in Diabetes 2023. Diabetes Care 2023;46(Suppl. 1):S158–S190. Diabetes Care. 2023 Jan 26;
–
11. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nature Reviews Endocrinology. 2021 Jun 25;17.
12. Sharma A, Pagidipati NJ, Califf RM, McGuire DK, Green JB, Demets D, et al. Impact of Regulatory Guidance on Evaluating Cardiovascular Risk of New Glucose-Lowering Therapies to Treat Type 2 Diabetes Mellitus. Circulation. 2020 Mar 10;141(10):843
62.
13. Cornell S. A review of GLP-1 receptor agonists in type 2 diabetes: A focus on the mechanism ofactionofonce-weekly agents. J ClinPharmTher.2020;45 Suppl 1(Suppl 1):17-27. doi:10.1111/jcpt.13230
14. Fonseca-Correa JI, Correa-Rotter R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front Med (Lausanne). 2021 Dec 20;8:777861. doi: 10.3389/fmed.2021.777861. PMID: 34988095; PMCID: PMC8720766.
15. Joshi,S.S.,Singh,T.,Newby,D.E.,&Singh,J.(2021).Sodium-glucoseco-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart, 107(13), 1032–1038. doi:10.1136/heartjnl-2020-318060
16. Nelinson, Donald S., Sosa, Jose M. and Chilton, Robert J.. "SGLT2 inhibitors: a narrative review of efficacy and safety" Journal of Osteopathic Medicine, vol. 121, no. 2, 2021, pp. 229-239. https://doi.org/10.1515/jom-2020-0153
17. Eberly LA, Yang L, Essien UR, et al. Racial, Ethnic, and Socioeconomic Inequities in Glucagon-Like Peptide-1 Receptor Agonist Use Among Patients With Diabetes in the US. JAMA Health Forum. 2021;2(12):e214182. doi:10.1001/jamahealthforum.2021.4182
18. Rau M, ThieleK,HartmannNUK,SchuhA,AltiokE,MöllmannJ, et al. Empagliflozin does not change cardiac index nor systemic vascular resistance but rapidly improves left ventricular filling pressure in patients with type 2 diabetes: a randomized controlled study. Cardiovascular Diabetology [Internet]. 2021 Jan 7 [cited 2021 Mar 17];20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7791833/
19. Lundin M, Ferrannini G, Mellbin L, Johansson I, Norhammar A, Näsman P, et al. SOdium-glucose CO-transporter inhibition in patients with newly detected Glucose Abnormalities and a recent Myocardial Infarction (SOCOGAMI). Diabetes Research and Clinical Practice. 2022 Nov;193:110141.
20. Allegretti AS, Zhang W, Zhou W, Thurber TK, Rigby SP, Bowman-Stroud C, et al. Safety and Effectiveness of Bexagliflozin in Patients With Type 2 Diabetes Mellitus and Stage 3a/3b CKD. American Journal of Kidney Diseases. 2019 Sep;74(3):328
37.
21. Fadini GP, Bonora BM, Zatti G, Vitturi N, Iori E, Marescotti MC, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovascular Diabetology [Internet]. 2017 Apr 4 [cited 2021 May 17];16(1):42.
Available from: https://pubmed.ncbi.nlm.nih.gov/28376855/
22. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. New England Journal of Medicine. 2019 Aug 29;381(9):841–51.
23. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects ofdapagliflozin ondevelopment andprogressionofkidneydiseasein patients with type
–
–
2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. The Lancet Diabetes & Endocrinology. 2019 Aug;7(8):606–17.
24. Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. New England Journal of Medicine. 2021 Sep 2;385(10):896–907.
25. Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, Bramlage P, et al. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovascular Diabetology. 2017 Feb 23;16(1).
26. Seino Y, Takami A, Boka G, Niemoeller E, Raccah D. Pharmacodynamics of the glucagon-like peptide-1 receptor agonist lixisenatide in Japanese and Caucasian patients with type 2 diabetes mellitus poorly controlled on sulphonylureas with/without metformin. Diabetes, Obesity and Metabolism. 2014 Mar 12;16(8):739–47.
27. Lundgren, J.R., Færch, K., Witte, D.R. et al. Greater glucagon-like peptide-1 responses to oral glucose are associated with lower central and peripheral blood pressures. Cardiovasc Diabetol 18, 130 (2019). https://doi.org/10.1186/s12933-019-0937-7
28. Eriksen, B.O., Stefansson, V.T.N., Jenssen, T.G. et al. Blood pressure and age-related GFR decline in the general population. BMC Nephrol 18, 77 (2017).
https://doi.org/10.1186/s12882-017-0496-7
29. Karagiannis T, Tsapas A, Athanasiadou E, Avgerinos I, Liakos A, Matthews DR, Bekiari E. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2021 Apr 1;174:108737.
30. Copur S, Onal EM, Afsar B, et al. Diabetes mellitus in chronic kidney disease: Biomarkers beyond HbA1c to estimate glycemic control and diabetes-dependent morbidity and mortality. J Diabetes Complications. 2020;34(11):107707. doi:10.1016/j.jdiacomp.2020.107707
31. Tarwater K. Estimated glomerular filtration rate explained. Mo Med. 2011;108(1):2932.
32. Fuchs FD, Whelton PK. High Blood Pressure and Cardiovascular Disease. Hypertension [Internet]. 2020 Feb;75(2):285–92. Available from:
https://www.ahajournals.org/doi/full/10.1161/HYPERTENSIONAHA.119.14240
33. Denardo SJ, Anderson RD, Pepine CJ. Blood Pressure Targets After High-Risk Myocardial Infarction. Hypertension. 2008 Jan;51(1):26–7.
34. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, et al. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events. Journal of the American College of Cardiology. 2016 Oct;68(16):1713–22.
35. Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105-113. doi:10.1016/S2213-8587(17)30412-6
36. Brown R, Kuk J. Consequences of obesity and weight loss: a devil’s advocate position. Obes Rev. 2015;16(1):77–87.
37. Nam GE, Kim W, Han K, Lee C-W, Kwon Y, Han B, Park S, Park J-H, Kim Y-H, Kim D-H. Body weight variability and the risk of cardiovascular outcomes and mortality in patients with type 2 diabetes: a nationwide cohort study. Diabetes Care. 2020;43(9):2234–41.
38. Lee H-J, Choi E-K, Han K-D, Kim DH, Lee E, Lee S-R, Oh S, Lip GY. High variability in bodyweight is associated with an increased risk of atrial fbrillation in patients with type 2 diabetes mellitus: a nationwide cohort study. Cardiovasc Diabetol. 2020;19(1):1–10.
39. Hurren KM, Dunham MW. Understanding the impact of commonly utilized, noninsulin, glucose-lowering drugs on body weight in patients with type 2 diabetes. Expert Opin Pharmacother. 2018;19(10):1087–95
40. Scheen AJ. Pharmacodynamics, efficacy and safety of sodiumglucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75(1): 33-59.
APPENDIX
Population Elderly >60 years old with T2DM Intervention sodium glucose cotransporter 2 inhibitors (SGLT2i) or glucagon like peptide 1 receptor agonist (GLP-1RA)
Comparation/Control Placebo
Outcome Change score of HbA1c, eGFR, SBP, DBP, and BW Study Design Randomized controlled trials
Database Keywords Articles
PubMed
(("Aged"[MeSH Terms] OR "elder"[Title/Abstract] OR "older"[Title/Abstract] OR "elder*"[Title/Abstract]) AND ("diabetes mellitus, type 2"[MeSH Terms] OR "Diabetes Mellitus"[Title/Abstract] OR "type 2 diabetes mellitus"[Title/Abstract]) AND ("sodium glucose cotransporter 2 inhibitors"[Title/Abstract] OR "SGLT2i"[Title/Abstract] OR "SGLT2 inhibitors"[Title/Abstract])) AND (randomizedcontrolledtrial[Filter])
(("Aged"[MeSH Terms] OR "elder"[Title/Abstract] OR "older"[Title/Abstract] OR "elder*"[Title/Abstract]) AND ("diabetes mellitus, type 2"[MeSH Terms] OR "Diabetes Mellitus"[Title/Abstract] OR "type 2 diabetes mellitus"[Title/Abstract]) AND ("glucagon like peptide 1 receptor agonist"[Title/Abstract] OR "GLP1RA"[Title/Abstract])) AND (randomizedcontrolledtrial[Filter])
107
91
Google Scholar
SpringerLink
("elder*" OR "older*") AND ("Type 2 Diabetes Mellitus" OR "Diabetes Mellitus") AND ("sodium glucose cotransporter 2 inhibitors" OR "SGLT2 inhibitors") AND (“randomized controlled trial” OR “randomised controlled trial”)
("elder*" OR "older*") AND ("Type 2 Diabetes Mellitus" OR "Diabetes Mellitus") AND ("Glucagon Like Peptide 1" OR “GLP 1” OR "Glucagon-Like Peptide 1" OR “GLP-1”) AND (“randomized controlled trial” OR “randomised controlled trial”)
("Aged" OR "elder*" OR "older*") AND ("diabetes mellitus, type 2" OR "Diabetes Mellitus" OR "type 2 diabetes mellitus") AND ("sodium glucose cotransporter 2 inhibitors" OR "SGLT2i" OR "SGLT2 inhibitors") AND ("Glucagon-Like Peptide 1 Receptor Agonist" OR "GLP-1RA" OR "Glucagon Like Peptide 1 Receptor Agonist")
98
200
98
Appendix 1. PICOS Framework
Appendix 2. Database searching process result
Cochrane
(("Aged"[MeSH Terms] OR "elder"[Title/Abstract] OR "older"[Title/Abstract] OR "elder*"[Title/Abstract]) AND ("diabetes mellitus, type 2"[MeSH Terms] OR "Diabetes Mellitus"[Title/Abstract] OR "type 2 diabetes mellitus"[Title/Abstract]) AND ("sodium glucose cotransporter 2 inhibitors"[Title/Abstract] OR "SGLT2i"[Title/Abstract] OR "SGLT2 inhibitors"[Title/Abstract])) AND ("glucagon like peptide 1 receptor agonist"[Title/Abstract] OR "GLP1RA"[Title/Abstract])) AND (“randomized controlled trial” OR “randomised controlled trial”)

9
Appendix 3. PRISMA Flow Chart
Appendix 4. Study Quality Assessment Based on Cochrane RoB 2.0 Tool


Appendix 5. Forrest plot meta-analysis of the effect of SGLT2i treatment on elderly patients with T2DM seen from the change score of eGFR
Appendix 6. Forrest plot meta-analysis of the effect of GLP-1RA treatment on elderly patients with T2DM seen from the change score of eGFR


Appendix 7. Forrest plot meta-analysis of the effect of SGLT2i treatment on elderly patients with T2DM seen from the change score of SBP
Appendix 8. Forrest plot meta-analysis of the effect of GLP-1RA treatment on elderly patients with T2DM seen from the change score of SBP


Appendix 9. Forrest plot meta-analysis of the effect of SGLT2i treatment on elderly patients with T2DM seen from the change score of DBP
Appendix 10. Forrest plot meta-analysis of the effect of GLP-1RA treatment on elderly patients with T2DM seen from the change score of DBP


Appendix 11. Forrest plot meta-analysis of the effect of SGLT2i treatment on elderly patients with T2DM seen from the change score of BW

Appendix 12. Forrest plot meta-analysis of the effect of GLP-1RA treatment on elderly patients with T2DM seen from the change score of BW

Setting United States, Spain, France, and Japan
Study design
a phase 3, multicenter, multinationa l, randomized, doubleblind, placebocontrolled, parallelgroup trial
randomized, placebocontrolled, phase IV clinical trial
randomized, placebocontrolled trial
event-driven, randomized, double-blind, placebocontrolled trial
randomized, double blind, placebocontrolled, investigatorinitiated trial
prospective, randomised, double-blind, placebocontrolled, cross-over phase IIIb trial
single center, prospective, placebocontrolled, double blind, randomized, 2-arm parallel, interventional and exploratory pilot study
international, multicentre, randomized, double-blind, placebocontrolled, combined single-dose and repeated dose-escalation, parallel-group
** data is reported in Median (IQR)
BD: Twice daily; QD:once daily; T2DM: Type 2 Diabetes Melitus; CKD: chronic kidney disease; CVD: Cardiovascular disease; MI; myocard infark; NR: not reported; INT: Intervention; PL:Placebo; SD: Standard deviation
Appendix 13. Qualitative summary of literature study about SGLT2i and GLP-1RA on glycemic control and cardiorenal outcome in T2DM patient
Study, year Allegreti et al., 2019[20] Fadini et al., 2017[21]
Gerstein et al., 2021[24] Husain et al., 2019[22]
Lundin et al., 2022[19] Ott et al., 2017[25]
Rau et al., 2021[18]
Seino et al., 2014 (1)[26]
Seino et al., 2014 (2)[26]
Italy Canada United States Sweden Germany Germany Japan
Age(mean ± SD) - INT - PL 69.3 ± 8.36 69.9 ± 8.29 66.3 ± 1.8 61.0 ± 1.8 64.6 ± 8.2 64.4 ± 8.3 66 ± 7 66±7 67 ± 8 68 ± 8 60.3 ± 7.6 60.3 ± 7.6 61.2 ± 7.9 62.8 ± 5.4 62.0** QD / 64.0** BD 62.5** Population Characteristic T2DM with 3a/3b CKD T2DM T2DM and CVD or current kidney disease T2DM with CVD or CKD T2DM with MI or unstable angina T2DM T2DM T2DM Total Population 312 31 4076 3183 42 62 42 73 76 Population (INT/PL) 157/155 15/16 2717/1359 1591/1592 20/22 31/31 20/22 34/39 37/39
BD: Twice daily; QD: once daily; HbA1c: hemoglobin A1C; eGFR: estimated glomerular filtration rate; NR: not reported; INT: Intervention; PL: Placebo; SD: Standard deviation
Study, year Allegreti et al., 2019[20] Fadini et al., 2017[21] Gerstein et al., 2021[24] Husain et al., 2019[22] Lundin et al., 2022[19] Ott et al., 2017[25] Rau et al., 2021[18] Seino et al., 2014 (1)[26] Seino et al., 2014 (2)[26]
Bexagliflozi n Dapagliflozin Efpeglenatide Semaglutide Empagliflozin Dapagliflozin Empaglifozin Lixisenatide QD Lixisenatide BD Dosage 20 mg 10 mg 2 mg (first 4 week), 4 mg (second 4 week) or 6 mg 14 mg 25 mg 10 mg 10 mg 10 ug 10 ug Assessment period week 6,12, 24 week 12 week 12, 24 week 83 week 28 week 6 day-1, day-2, week-12 week 6 HbA1 c (mean ± SD) INT - Baseline - Final - Mean Change 8.01 ± 0.786 NR -0.61 ± 1.59 8.2 ± 0.2 7.3 ± 0.4 −0.9 ± 0.2 8.90±1.46 NR -1.42 ± 1.04 8.2 ± 1.6 NR -1 42 ± 6 NR 0.8 ± 5.6 6.62 ± 0.7 NR −0.05 ± 0.3 7.5±0.9 7.1 ± 0.7 -0.4 ± 0.5 8.17 ± 0.75 NR −0.94 ±0.41 8.49 ± 0.85 NR −1.13 ± 0.43 PL - Baseline - Final - Mean Change 7.95 ± 0.812 NR -0.24 ± 0.64 8.2 ± 0.2 8.6 ± 0.4 0.4 ± 0.2 8.94±1.52 NR -0.17 ± 0.81 8.2 ± 1.6 NR -0,3 43 ± 9 NR 0.7 ± 4.2 6.79 ± 0.8 0.12 ± 0.5 7.9 ± 1.3 7.8 ± 1.5 -0.1 ± 0.9 8.38 ± 0.75 NR −0.41 ±0.43 8.38 ± 0.75 NR -0.41 ± 0.43 eGFR (mean ± SD) INT - Baseline - Final - Mean Change 45.44 ± 8.56 NR NR 89.3 ± 4.4 NR NR 72.2 ± 21.9 NR -2.37 ± 10.2 74 ± 21 NR 0.98 ± 16.6 68 ± 13 NR 0.0 ± 5.3 NR NR NR 77 ± 21 68 ± 20 -9 ± 7.8 NR NR NR NR NR NR PL - Baseline - Final - Mean Change 44.78 ± 8.08 NR NR 92.5 ± 4.4 NR NR 72.9 ± 23.3 NR -3.26 ± 9.9 74 ± 21 NR 0.98 ± 17.6 73 ± 14 NR − 1.5 ± 5.6 NR NR NR 88 ± 16 85 ± 16 -3 ± 10 NR NR NR NR NR NR
Supplementation
SBP: systolic blood pressure; DBP: diastolic blood pressure; BW: body weight; NR: not reported; INT: Intervention; PL: Placebo; SD: Standard deviation
Study, year Allegreti et al., 2019[20] Fadini et al., 2017[21] Gerstein et al., 2021[24] Husain et al., 2019[22] Lundin et al., 2022[19] Ott et al., 2017[25] Rau et al., 2021[18] Seino et al., 2014 (1)[26] Seino et al., 2014 (2)[26] SBP (mean ± SD) INT - Baseline - Final - Mean Change 135.9 ± 14.25 NR -3,8 NR NR -4.7 ± 1.3 135.1 ± 15.5 NR -2.56 ± 11.9 135 ± 18 NR –5 ± 18 130 ± 16 NR 1.3 ± 14.1 130 ± 14 126 ± 11 −3 ± 10 135±16 128±15 -7 ± 9.8 NR NR NR NR NR NR PL - Baseline - Final - Mean Change 137.6 ± 14.75 NR NR NR NR -1.0 ± 2.3 134.4±15.6 NR -1.08 ± 11.8 136±18 NR –2 ± 18 131 ± 16 NR 6.5 ± 11.8 130 ± 14 129 ± 11 −1 ± 10 136±18 132±20 -4 ± 12.2 NR NR NR NR NR NR DBP (mean ± SD) INT - Baseline - Final - Mean Change NR NR NR NR NR -1.3 ± 0.6 76.8±9.7 NR 0.47 ± 24.5 76±10 NR –1 ± 11 79 ± 10 NR 1.0 ± 6.6 79 ± 9 75 ± 8 −2 ± 6 82±13 79±11 -3 ± 7.8 NR NR NR NR NR NR PL - Baseline - Final - Mean Change NR NR NR NR NR -0.4 ± 1.6 76.6±9.8 NR -0.11 ± 7.0 76±10 NR –2 ± 10 80 ± 10 NR 3.0 ± 7.8 79 ± 9 77 ± 7 0 ± 6 81±14 82±11 1 ± 8.4 NR NR NR NR NR NR BW (mean ± SD) INT - Baseline - Final - Mean Change 82.90 ± 20.51 NR -1,61 NR NR −3.1 ± 0.5 NR NR -3.21 ± 5.2 91.0±21.4 NR –4.2 NR NR − 1.6 ± 2.4 87.4 ± 13 NR NR 95.8±17.3 96.0±19.6 0.2±12.5 72.51 ± 17.37 -0.95 ± 1.9 79.06 ± 16.89 NR -0.85 ± 1.9 PL - Baseline - Final - Mean Change 82.59 ± 21.12 NR NR NR NR 0.1 ± 0.5 NR NR -0.62 ± 4.8 90.8±21.0 NR –0.8 NR NR 1.0 ± 3.4 87.4 ± 13 NR NR 94.0±14.0 93.7±14.3 NR 76.29 ± 15.78 -0.36 ± 1.9 76.29 ± 15.78 NR -0.36 ± 1.9
Conclusion
Apparent treatment effect in decreasing HbA1c (p=0.001)
Dapaglifozin supplementation significantly improve HbA1c (p = 0.004), SBP (P = 0.035), and BW (P = 0.0001) compared to placebo Yet DBP results shows not reach significant (P = 0.317)
All results shows improvement in Hba1c 95% CI, -1.24 (-1.32,1.17); eGFR 95% CI, 0.89 (0.27, 1.51); SBP (95% CI, 0.8 to 2.2; DBP 95% CI, 0.2 to 1.0; and BW (95% CI, 2.3 to 2.9).
And MACE incident shows Hazard ratio, 0.73 (95% CI, 0.58–0.92) P=0.007
Overall result shows improvement in each outcome, including HbA1c, eGFR, SBP, DBP, and BW. However, cardiovascular risk of SGLT2i is not inferior to placebo (P<0.001 for non-inferiority)
Supplementation of empaglifozin doesn’t shows the predicted effect HbA1c P= 0.944; eGFR P=0.381 SBP= 0.193 DBP= 0.365 but shows significant result in BW P= 0.007
dapagliflozin treatment resulted in numerous beneficial effects Hba1c P< 0.01 SBP P=0.084 DBP P=0.951
Empaglifozin did not shows significant results: eGFR P=0.108; HbA1c P=0.595; SBP P=0.318 ; DBP P=0.197; BW P =0.059
lixisenatide shows significant effect in all outcome p < 0.0001 for both
CVD: Cardiovascular disease; MI; myocardial infract; MACE: Major adverse cardiovascular event; AE: Adverse event; HbA1c: hemoglobin
A1C; eGFR: estimated glomerular filtration rate SBP: systolic blood pressure; DBP: diastolic blood pressure; BW: body weight; NR: not reported; INT: Intervention; PL: Placebo; n: events; N: tota
Study, year Allegreti et al., 2019[20] Fadini et al., 2017[21] Gerstein
al., 2021[24] Husain
2019[22] Lundin
al., 2022[19] Ott
al., 2017[25] Rau
2021[18] Seino
al., 2014 (1)[26] Seino
2014 (2)[26] CVD effect (n/N) Nonfatal Stroke - INT - PL NR NR NR NR 41/2717 25/1359 12/1591 16/1592 NR NR NR NR NR NR NR NR NR NR Nonfatal MI - INT - PL NR NR NR NR 85/2717 53/1359 37/1591 31/1592 NR NR NR NR NR NR NR NR NR NR CVD Death - INT - PL NR NR NR NR 75/2717 50/1359 15/1591 30/1592 NR NR NR NR NR NR NR NR NR NR MACE - INT - PL 2/157 0/155 NR NR 189/2717 125/1359 64/1591 77/1592 NR NR NR NR NR NR NR NR NR NR AE (n/N) - INT - PL 109/157 105/155 NR NR NR NR 301/1591 358/1592 NR NR NR NR NR NR 35/39 29/40 31/41 29/40
et
et al.,
et
et
et al.,
et
et al.,

Appendix 14. PROSPERO Registration




Appendix 15 Cochranes’ RoB-2 Tools




Acceptance, Safety, and Impact on Quality of Life of Exergame for Elderly Patients with Neurodegenerative Diseases: A Systematic Review and Meta-Analysis
Ghina Tsurayya, Teuku Fais Duta, Muhammad Alif Naufal, Meulu Alina Faculty of Medicine, University of Syiah Kuala, Aceh, Indonesia
ABSTRACT
Introduction : Conventional rehabilitation is still considered inefficient and provides a long recovery period for neurodegenerative disease, especially for elderly. Exergame is a technology that provides physical and cognitive training simultantly by combining exercise and gaming activities that seems promising by their effectiveness. Unfortunately, the impacts of exergame on Quality of Life have not been extensively evaluated and summarized in a frame of a comprehensive review.
Objectives : Evaluate the efficacy of exergame in improving the Quality of Life among elderly patients with neurodegenrative diseases. Safety of exergame and the patients’ acceptance toward this technology were also evaluated.
Methods : This study adapted adapted Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline. We systematically research Pubmed, Embase and Scopus for relevant review up to 16 Maret 2023. Thereafter, the included studies were assessed using Physiotherapy Evidence Database (PEDro) Risk of Bias tool version 2.0. Meta-analysis using a random effect was carried out on outcomes reported at least by two studies to calculate the standard mean difference (SMD) and its 95% confidence interval (CI). The difference of influence between exergame and conventional therapy was judged based on Z- and p-values. Heterogeneity was determined by I2 score.
Results : As many as 15 RCTs were included (n=466 participants) published in 2013–2023. All included studies passed the PEDro scale (mean score=8.3). According to Cochrane’s Risk of Bias tool version 2.0, 9 studies were marked as ‘high quality’, 5 – having ‘some concerns’, and 1
‘high risk’. Meta-analysis was performed on Euroqol-5D (n= 2, SMD=0.27, 95%CI: -0.79–0.24, p= 0.30) and PDQ-39 (n= 9, SMD= -0.08, 95%CI: -0.31–0.16, p= 0.53) for Parkinson’s diseases and AD-QoL (n= 2, SMD= -0.21, 95%CI: -0.33–0.76, p= 0.44) for Alzheimer’s disease. All results suggest that a neutral results, which means that exergame is not more or less efficacious when compared with conventional rehabilitation (p>0.05). The included studies reported no adverse events when using exergame, where patients experienced reduced fatigue and fear of falling. High acceptance of exergame was attributed to high level of adherence (80–97%), low attrition rate and the natures of exergame for being enjoyable, easy-to-use, and motivational.
Conclusion : Exergame has similar efficacy with conventional rehabilitation when used to treat elderly patients with neurodegenerative disease. Further, exergame was shown to have high safety and acceptance levels among these populations. We recommend improvement in the game play design to increase the acceptance level and the game console to reduce the spatial requirement. Future studies with higher number of participants are required to obtain conclusive results on the efficacy of exergame.
Keywords : Exergame; Quality of Life; Neurodegenerative; Elderly
–
Acceptance, Safety, and Impact on Quality of Life of Exergame for Elderly Patients with Neurodegenerative Diseases: A Systematic Review and Meta-Analysis
AUTHOR :
Ghina Tsurayya
Teuku Fais Duta

Muhammad Alif Naufal
Meulu Alina PCC
AMSC Asian Medical Students’ Association Indonesia 2023
Acceptance, Safety, and Impact on Quality of Life of Exergame for Elderly Patients with Neurodegenerative Diseases: A Systematic Review and Meta-Analysis
Ghina Tsurayya, Teuku Fais Duta, Muhammad Alif Naufal, Meulu Alina Faculty of Medicine, University of Syiah Kuala, Aceh, Indonesia
ABSTRACT
Introduction : Conventional rehabilitation is still considered inefficient and provides a long recovery period for neurodegenerative disease, especially for elderly. Exergame is a technology that provides physical and cognitive training simultantly by combining exercise and gaming activities that seems promising by their effectiveness. Unfortunately, the impacts of exergame on Quality of Life have not been extensively evaluated and summarized in a frame of a comprehensive review.
Objectives : Evaluate the efficacy of exergame in improving the Quality of Life among elderly patients with neurodegenrative diseases. Safety of exergame and the patients’ acceptance toward this technology were also evaluated.
Methods : This study adapted adapted Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline. We systematically research Pubmed, Embase and Scopus for relevant review up to 16 Maret 2023. Thereafter, the included studies were assessed using Physiotherapy Evidence Database (PEDro) Risk of Bias tool version 2.0. Meta-analysis using a random effect was carried out on outcomes reported at least by two studies to calculate the standard mean difference (SMD) and its 95% confidence interval (CI). The difference of influence between exergame and conventional therapy was judged based on Z- and p-values. Heterogeneity was determined by I2 score.
Results : As many as 15 RCTs were included (n=466 participants) published in 2013–2023. All included studies passed the PEDro scale (mean score=8.3). According to Cochrane’s Risk of Bias tool version 2.0, 9 studies were marked as ‘high quality’, 5 – having ‘some concerns’, and 1
‘high risk’. Meta-analysis was performed on Euroqol-5D (n= 2, SMD=0.27, 95%CI: -0.79–0.24, p= 0.30) and PDQ-39 (n= 9, SMD= -0.08, 95%CI: -0.31–0.16, p= 0.53) for Parkinson’s diseases and AD-QoL (n= 2, SMD= -0.21, 95%CI: -0.33–0.76, p= 0.44) for Alzheimer’s disease. All results suggest that a neutral results, which means that exergame is not more or less efficacious when compared with conventional rehabilitation (p>0.05). The included studies reported no adverse events when using exergame, where patients experienced reduced fatigue and fear of falling. High acceptance of exergame was attributed to high level of adherence (80–97%), low attrition rate and the natures of exergame for being enjoyable, easy-to-use, and motivational.
Conclusion : Exergame has similar efficacy with conventional rehabilitation when used to treat elderly patients with neurodegenerative disease. Further, exergame was shown to have high safety and acceptance levels among these populations. We recommend improvement in the game play design to increase the acceptance level and the game console to reduce the spatial requirement. Future studies with higher number of participants are required to obtain conclusive results on the efficacy of exergame.
Keywords : Exergame; Quality of Life; Neurodegenerative; Elderly
–
Neurodegenerative diseases affect millions of people due to the increasing aging rate of the worldwide population (1). They are characterized by the progressive deposition of specific proteins within district areas of the brain and progressive loss of sensory-motor and cognitive functions (2–4). Out of many different types of neurodegenerative diseases, Alzheimer’s (AD), Parkinson’s (PD), Huntington’s diseases (HD), and multiple sclerosis (MS) are the most prevalent (5).
In general, these diseases have typical symptoms, namely weakness, fatigue, motor impairment, ataxia, dysphagia, urinary complications, sensory loss, depression, cognitive decline, sleep disorders, and so on (6). These conditions could be developed into severe symptoms affecting the daily living of the sufferer’s (7,8). Data suggest that patients with neurodegenerative disease present lower levels of health-related quality of life in emotional, physical, and social terms than that of the general population (6).
Standard rehabilitation consists of conventional physical training (including aerobic exercise or other exercises that aim to improve muscle strength, physical function, balance control, and flexibility) and cognitive training (completing puzzles, painting, drawing, playing dominoes, crosswords, and playing word games). Unfortunately, conventional rehabilitation is still considered inefficient and provides a long recovery period (9) The traditional clinical setting for therapy is often expensive and suffers from poor adherence. Thus, experts have suggested the urgency of improving rehabilitation efficacy through innovation and technology.
Exergame is a technology that provides physical and cognitive training simultantly by combining exercise and gaming activities. Exergame requires its users to engage in physical activities and multiple players interaction, where they can share learning experiences and feel challenged at each difficulty level (10). Dancing, boxing, bating, running, and puzzle solving through quests are among many other fun and exciting activities the patients could experience when using an exergame. Through gamified techniques, exergame allows patients to receive rehabilitation at home. The gamified therapy performed in the clinic can be installed at the patient's home ensuring the continuity of the rehabilitation (11). Moreover, all people across generations are interested in playing games (12).
Further, many games have been designed specifically for healthcare services. Different games are tailored for various disorders according to the requirements of the patients. That is, the games are customized individually according to the patient‘s needs. These exergames are included in the rehabilitation process since they combine the benefits of gaming as well as exercising. The virtual reality games facilitate people to substitute their sedentary screen time with physical activities (13). Unfortunately, the impacts of exergame on Quality of Life have not been extensively evaluated and summarized in a frame of a comprehensive review. Herein, a systematic review and meta-analysis were performed to evaluate the efficacy of exergame in improving the Quality of Life among patients with neurodegenerative diseases. Safety of exergame and the patients’ acceptance toward this technology were also evaluated.
Introduction
2. Materials and Methods
2.1 Search Strategy
The literature search was performed for randomized control trials published from January 2008 (the first year of exergames studies exists) through March 2023 on three major scientific literature databases, which are PubMed, EMBASE, and Scopus. It was conducted using the following keywords: “elderly”, “neurodegenerative”, “Alzheimer’s disease”, “Parkinson’s disease”, “Huntington’s disease”, “Multiple sclerosis”, “exergame”, “quality of life”, “safety”, and “acceptance”.
Database Search Strategy
PubMed (old OR older OR elderly OR aged OR geriatric OR “older people”) AND (exergame OR exergaming OR “virtual reality” OR “virtual world” OR “video game” OR “active video game” OR “human computer interaction” OR “augmented reality” OR “electronic game” OR “active game play” OR “virtual rehabilitation” OR “augmented rehabilitation” OR wii OR kinect OR “balance board" OR “Dance Dance Revolution” OR “digital exercise”) AND (“quality of life” OR “health-related quality of life” OR “life quality” OR HRQOL OR acceptance OR acceptability OR safety) AND (“alzheimer’s disease” OR alzheimer OR “alzheimer’s dementia” OR “parkinson’s disease” OR “parkinson” OR “huntington’s disease” OR “huntington” OR “multiple sclerosis” OR “demyelinating disease” OR neurodegenerative)
EMBASE ( TITLE-ABS-KEY((old* OR elderly OR aged OR geriatric OR "older people"))) AND TITLE-ABS-KEY((exergam* OR "virtual reality" OR "virtual world"OR "video game" OR "active video game" OR "human computer interaction" OR "augmented reality" OR "electronic game" OR "active game play" OR "virtual rehabilitation" OR "augmented rehabilitation" OR wii OR kinect OR "balance board" OR "dance dance revolution") AND TITLE-ABS-KEY(("quality of life" OR "health related quality of life" OR "life quality" OR HRQOL OR Acceptance OR Acceptability OR Safety)) AND TITLE-ABS-KEY((“Alzheimer’s disease” OR alzheimer OR “alzheimer’s dementia” OR “Parkinson’s disease” OR “parkinson” OR “huntington’s disease” OR “huntington” OR “multiple sclerosis” OR “demyelinating disease” OR neurodegenerative))
SCOPUS (old*:ti,ab,kw OR elderly:ti,ab,kw OR aged:ti,ab,kw OR geriatric:ti,ab,kw OR 'older people':ti,ab,kw) AND (exergam*:ti,ab,kw OR 'virtual reality':ti,ab,kw OR 'virtual world':ti,ab,kw OR 'video game':ti,ab,kw OR 'active video game':ti,ab,kw OR 'human computer interaction':ti,ab,kw OR
'augmented reality':ti,ab,kw OR 'electronic game':ti,ab,kw OR 'active game play':ti,ab,kw OR 'virtual rehabilitation':ti,ab,kw OR 'augmented rehabilitation':ti,ab,kw OR wii:ti,ab,kw OR kinect:ti,ab,kw OR 'balance board':ti,ab,kw OR 'dance dance revolutions;ti,ab,kw':ti,ab,kw OR 'digital exercise':ti,ab,kw) AND ('quality of life':ti,ab,kw OR 'health-related quality of life':ti,ab,kw OR 'life quality':ti,ab,kw OR hrqol:ti,ab,kw OR acceptance:ti,ab,kw OR acceptability:ti,ab,kw OR safety:ti,ab,kw) AND ('alzheimer disease':ti,ab,kw OR alzheimer:ti,ab,kw OR 'alzheimer dementia':ti,ab,kw OR 'parkinson disease':ti,ab,kw OR parkinson:ti,ab,kw OR 'huntington disease':ti,ab,kw OR huntington:ti,ab,kw OR 'multiple sclerosis':ti,ab,kw OR 'demyelinating disease':ti,ab,kw OR neurodegenerative:ti,ab,kw) AND [2008-2023]/py
Table 1. Search strategy in Each Database
2.2 Inclusion Criteria
Inclusion criteria that are considerable for eligibility were based on the PICOS framework : Population : Neurodegenerative Patients (Parkinson’s Disease, Alzheimer’s Disease, Huntington’s Disease and Multiple Sclerosis) over the average age of 60 years
Intervention : Exergame-based interventions, consist of augmented reality or virtual reality Control : Received either conventional exercise or no intervention.
Outcome : The primary outcome of this study is Quality of Life, Acceptance, and Safety of using Exergame for the elderly with neurodegenerative disease
Study : Randomized Control Trials (RCTs) testing the effect on QoL, acceptance and safety of exergame on elderly with major neurodegenerative disease over the age of 60 years.
2.3 Exclusion Criteria
The exclusion criteria were as follows : (1) Healthy people as the control group; (2) The subject had other neurodegenerative diseases than inclusion; (3) Irrelevant Outcome; (4) Studies written before 2008; (5) Severe sensory impairments (mainly visual, auditory, color blindness); (6) Published in languages other than English; (7) Review Article, case report, conference proceedings, and abstract, editorials, commentaries; (8) No full access to full-text;
(9) Abstract Only; (10) Severe cardiac disease, uncontrolled diabetes, a history of stroke, traumatic brain injury, a seizure disorder, and other severe systemic diseases.
2.4 Screening and Study Selection
Two authors (G.T & M.A) adapted Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines that can be accessed through (http://www.prisma-
statement.org) into the process of literature screening and selection of this study. The 404 total studies we searched were imported to Zotero 6 (zotero.org) and duplicates removed were performed automatically. Titles and abstracts of studies were screened to identify potential studies that met the inclusion criteria. The full texts of these studies were retrieved and independently assessed for eligibility. The full texts of these studies were independently retrieved and reviewed for eligibility. Thirteen studies were included to be extracted for the assessment of the quality of life, safety, and acceptance. Hand-picked articles that met the criteria were also included in this systematic review. In the end, fifteen studies were concluded and extracted into primary outcomes
2.5 Data Extraction
Study data were also extracted by the same reviewer as the screening. The extracted data included primary outcomes : (1) Quality of life; (2) Safety; (3) Acceptance. We also extracted the relevant study information, including the first author, country/location, age, sample size (male/female), disease duration, stage of disease, interventions, the duration and length of intervention, the comparison group, assessments, and outcome measures. Any ambiguity (e.g. The different selections or judgment of outcome measures) met by these two reviewers was discussed with the help of the third reviewer (T.F.D).
2.6 Outcomes Measures
The primary outcome that was assessed in this systematic review was the impact on quality of life, safety, and acceptance for using exergame as a beneficial exercise method rather than the conventional one. There are many variants for neurodegenerative quality of life. Parkinson’s disease used PDQ-39, EuroQol-5D, EQ-5D VAS, WHO.ADAS 2.0, (WHOQOLOLD), SF-36, and also SF-12. Alzheimer’s disease used AD-QoL. Multiple Sclerosis used MSIS-29. Huntington’s disease used HD-QoL. Safety was assessed by a number of adverse effects such as falls, muscle soreness, excessive fatigue during the follow-up period, reduction of the FSS/Fatigue Severity Scale, and a specially designed questionnaire. Acceptance was assessed using high adherence, reduced anxiety, and a special-design questionnaire for openended and also closed-ended interviews. The outcomes were described and extracted into tables.
2.7 Quality Assessment of Studies
The two reviewers (M.A.N & G.T) separately evaluated the studies in the same timeline, April 2023. The studies that met the inclusion criteria after systematic screening adopted R.O.B 2.0, which recommended a tool to assess the risk of bias in randomized trials included in Cochrane Reviews. 5 domains were assessed to define the risk of bias.
2.8 Data Synthesis and Analysis
Meta-analysis was only performed for outcomes that are reported in at least two studies. Forest plots were generated on the software developed by Cochrane Collaborations – RevMan version 5.4.1. Mean differences and standard deviation with 95% confidence interval (Cl) and p-value were extracted from the study intervention versus control. Meta-analysis using a random effect was carried out on outcomes reported at least by two studies to calculate the
standard mean difference (SMD) and its 95% confidence interval (CI). The difference of influence between exergame and conventional therapy was judged based on Z- and p-values. Heterogeneity was determined by I2 score. P-value <0.05 was considered as statistically significant and I2 >50% highly heterogenous.
3. RESULTS
3.1 Search Result and Study Characteristics
The PRISMA flow diagram for the included studies based on the eligibility criteria in this review has been presented in Figure 1. As many as 15 studies met the inclusion criteria and were included in this review participated by a total number of 466 patients (59% male) ranging from 10 to 25 participants per group. All of the included studies were randomized clinical trials. Among these studies, the average age of participants ranged from 61 to 81 years old. Studies were conducted in Australia, Italy, Brazil, Taiwan, Switzerland, Hungary, and the United States of America. Summary of the characteristics of the included studies along with their outcomes has been presented in Table 2. We included 3 meta-analyses: (1) PDQ-39 Quality of Life of Parkinson’s disease consists of 9 studies, (2) EQ-5D Quality of Life of Parkinson’s disease consists of 2 studies, and (3) AD-QoL of Alzheimer’s Disease that consist of 2 studies.
 Figure 1. PRISMA flow diagram for the screening and selection process of the published studies
Figure 1. PRISMA flow diagram for the screening and selection process of the published studies
Allen, 2017 (14)
Australia Parkinson's Disease n= 19
Age: 67.5 (7.3)
M/F: 12/7
Disease duration, year
(Mean±SD) : 7.9 (3.9)
Hoehn & Yahr : NR
MMSE : 28.8 (1.0)
Italy Parkinson's Disease n= 25
Age: 72 (7)
M/F: 18/7
Disease duration, months
(Mean±SD) : 73.2 (SD
75.5)
Hoehn & Yahr : NR
MMSE : NR
n= 19
Age: 68.4 (8.5)
M/F: 11/8
Disease duration, year
(Mean±SD) : 8.7 (6.1)
Hoehn & Yahr : NR
MMSE : 28.6 (1.1)
n= 26
Age: 70 (10)
M/F: 17/9
Disease duration,months
(Mean±SD) : 73.2 (SD
75.5)
Hoehn & Yahr : NR
MMSE : NR
Received usual care, 3x/week for 12 weeks, while on medication
● Enjoyable ● Easy-to-use
Ribas, 2016 (16)
Brazil Parkinson’s Disease n= 10
Age: 61.70 ± 6.83
M/F: 4/6
Disease duration, year
(Mean±SD) : 6.5 ± 4
Hoehn & Yah r : 1-2,5
MMSE : 27.5 (1.5)
VR was performed 40-minute session, 3x/week, 6 weeks
↑ NR NR 9
Yuan, 2020 (17)
Taiwan Parkinson's Disease n=12
Age: 67.8 ± 5.5
M/F: 2/10
Disease duration, year
(Mean±SD) : NR
Hoehn & Yahr stage: I-III
MMSE : 28.5 ± 1.7
n= 10
Age: 60.20 ± 11.29
M/F: 4/6
Disease duration, year
(Mean±SD) :
7 ± 2.79
Hoehn & Yahr: 1-2,5
MMSE : 27.5 (0,75)
n=12
Age: 66.5 ± 8.8
M/F: 9/3
Disease duration, year
(Mean±SD) : NR
Hoehn & Yahr stage:I-III
Exergame, 30minute session, 2x/week, 12-week period
↔ ● Reduced fatigue ● No adverse effect NR 8
No
exercise ↔ ● No adverse effect
Author
Year (Ref) Country Disease Characteristic Treatment Outcome PEDro Experiment Control Experimental Control QoL Safety Acceptance
,
Perform the exergames 3x/week for 12 weeks, while on medication ↔ No adverse events ● High adherence
9
● Motivational
Pazzaglia , 2020 (15)
Conventional rehabilitation. 40minute session, 3x/week, 6 weeks
Conventional Exercise, 30minute session, 2x/week, 12-week period
Higher confidence for avoiding fall NR 6
IVGB Training for 30 minute training, 6 weeks
●
Santos, 2019 (18) Brazil Parkinson's Disease n=13
Age: 61.7 ± 7.3
M/F: 11/2
Disease duration, year
(Mean±SD) : 7,8 ± 3,7
Hoehn & Yahr stage: I-III
MMSE : NR
MMSE : 26.0 ± 2.6
n=14
Age:64.5 ± 9.8
M/F: 11/3
Disease duration, year
(Mean±SD) : 6,5 ± 2,0
Hoehn & Yahr stage: I-III
MMSE : NR
Nintendo Wii + Conventional Exercise 50 min/day, 2x/weeks for 8 weeks
Conventional exercise, 50 min/day, 2x/weeks for 8 weeks
Jaggi, 2023 (19)
Switzerlan d Parkinson's Disease n=21
Age: 71.89 (9.09)
M/F: 12/7
Disease duration, year
(Mean±SD): 13.37 (2.68)
Hoehn and Yahr grade : IIV
MMSE : 27.79 (1.55)
n=21
Age: 72.86 (10.14)
M/F: 15/6
Disease duration, year (Mean±SD) : 14.24 (3.67)
Hoehn and Yahr grade: I-IV
MMSE : 27.57 (2.71)
Conventional training
Feel safe ● Did not feel anxious or fear of falling ● 97% adherence
7
Yang, 2016 (20)
Taiwan Parkinson's Disease n=10
Age: 72.5 (8.4)
M/F: 7/4
Disease duration, year
(Mean±SD) : 9.4± 3.6
Hoehn & Yahr: II-III
MMSE : 27,5 (4,0)
n=10
Age: 75.4 (6.3)
M/F: 7/5
Disease duration, year
(Mean±SD) :
8.3±4.1
Hoehn & Yahr: II-III
MMSE : 27,2 (2,5)
Conventional
balance, 12 sessions, 2x/week, 6 weeks
Alves, 2018 (21)
Brazil Parkinson's Disease n
- Nintendo WiiTM(n=9)
- Xbox KinectTM(n=9)
Age:
- Nintendo WiiTM = 58.89 (11.16)
- Xbox KinectTM=62.67 (13.81)
M/F:
- Nintendo WiiTM =9/0
- Xbox KinectTM= 8/1
Disease duration, year
(Mean±SD) : NR
n=9
Age: 61.67 (10.74)
M/F: 8/1
Disease duration, year
(Mean±SD) : NR
Hoehn & Yahr: I-III
MMSE : 25.44 (2.29)
No intervention ↑ Nintend o Wii vs Control NR NR 7 ↔ Xbox Kinect vs Control
↑ NR NR 10
Exergame + conventional training, 15 min/session, 5 times/week NR ●
● Low attrition rate
● Easy-to-use
↔ NR NR 9
VR balance training, 12 sessions, 2x/week, 6 weeks
Xbox KinectTM & Nintendo Wii, 2x/weeks,5 weeks, during on medication
Ferraz, 2018 (22)
Hoehn & Yahr: I-III
MMSE :
- Nintendo WiiTM : 27.11 (2.8)
- Xbox KinectTM : 27.44 (2.35)
Brazil Parkinson's Disease n=20
Age: 67 (0.53)
M/F: 10/10
Disease duration, year
(Mean±SD) :6.1±1.3
Hoehn & Yahr stage: II-III
MMSE (median range) : 27.00 (25.00-28.00)
Hungary Parkinson's Disease n=25
Age: 70.0 (4.69)
M/F:12/13
Disease duration, year
(Mean±SD) :7.5±1.76
Hoehn & Yahr stage: II-III
MMSE : NR
n=20
Age: 67.14 (1.87)
M/F: 11/9
Disease duration, year
(Mean±SD) : 6.14±1.33
Hoehn & Yahr stage: II-III
MMSE (median range) : 27.00 (25.00-29.00)
n=25
Age: 70.6 (4.10)
M/F:11/14
Disease duration, year
(Mean±SD) :7.5±2.16
Hoehn & Yahr stage: II-III
MMSE : NR
Functional Training, 50minutes/session, 3x/week for 8
weeks
Exergame use Xbox 360 core system,1 hour/ sessions over 5 weeks , three-5week-long waves ↔ NR NR 10
Pedreira, 2013 (24)
Brazil Parkinson's Disease n=16
Age= 61.1 ± 8.2
M/F: 11/5
Disease duration, year
(Mean±SD) :8.6 ± 4.6
Hoehn & Yahr score: I-III
MMSE : NR
Taiwan Parkinson's Disease n=12
Age : 67.3 ± 7.1
M/F: 6/6
Disease duration, year
(Mean±SD): 7.9 ± 2.7
Hoehn & Yahr Stage : IIII
MMSE : 29.5±0.7
n=15
Age=66.2 ± 8.5
M/F: 11/4
Disease duration, year
(Mean±SD) : 7.3 ± 6.6
Hoehn & Yahr score: I-III
MMSE : NR
n=12
Age= 64.0 ± 3.0
M/F: 5/7
Disease duration, year
(Mean±SD) : 6.4 ± 3.0
Hoehn & Yahr Stage : I-III
MMSE : 29.7±0.6
Exergame using Nintendo Wii, 40 minutes/session, 3 days/week for 4 weeks. ↑ NR NR 7
Physical therapy program, 40 minutes/session, 3 days/week for 4 weeks.
VR-based Wii Fit exercise + treadmill training, 2 sessions/week, 6 weeks ↑ ● No adverse event ● Fall prevention.
Fall-prevention education NR 10
exergames use kinect, 50minutes/session, 3x/week for 8 weeks ↑ NR NR 9
Tollar, 2018 (23)
bicycle ergometer, rode 110-140 m/s, three-5-week-long waves
Liao, 2014 (25)
Fontoura, 2017 (26)
Brazil Parkinson's Disease n=10
Age= 63(7)
M/F: 7/3
Disease duration, year
(Mean±SD) : NR
Hoehn & Yahr Stage : IIII
MMSE : NR
n=10
Age: 62(8)
M/F: 9/1
Disease duration, year
(Mean±SD) : NR
Hoehn & Yahr Stage : I-III
MMSE : NR
VR + Conventional Physiotherapy. 60 minutes/session
2 sessions/week, 5 weeks
60 minutes/sesion
2 sessions/week, 5 weeks
Padala, 2017 (27)
USA Alzheimer' s Disease n= 15
Age: 72.1(5.3)
M/F: 10/5
Disease duration, year
(Mean±SD) : NR
MMSE : 23.3 (2.2)
n=15
Age: 73.9(7.1)
M/F: 13/2
Disease duration, year
(Mean±SD) : NR
MMSE : 22.7 (2,3)
Walking exercise, 30 minutes/session, 5 days/week for 8 ↑ ● No adverse event ● Reduced feer of fall
● 80% adherence rate 7
Padala, 2012 (28)
USA Alzheimer' s Disease n=11
Age=79.3 (9.8)
M/F: 3/8
Disease duration, year
(Mean±SD): NR
MMSE : 22.6 (4.3)
n=11
Age=81.6 (5.2)
M/F: 3/8
Disease duration, year
(Mean±SD): NR
MMSE : 22.0 (4.1)
Table 2. Baseline characteristic and the primary outcome
Wii-fit training, 30 minutes/session, 5 times/week for 8 weeks
Walking exercise 30 minutes daily, 5/w, for 8 weeks
↔ NR NR 7
Conventional physiotherapy,
↑ NR NR 10
Wii-Fit program, 30 minutes/session, 5 days/week for 8 weeks week
Note: ↔, not significant; ↑, significant; MMSE, mini-mental state examination; NR, not reported
3.2 Quality Appraisal
The results from the quality appraisal of the included studies have been presented in Figure 2. As many as 9 studies were found to have passed all Cochrane’s risk of bias criteria, indicating them as high-quality studies (14,15,17–19,22,23,25,26). Five studies were found to have some concerns, where three of them were derived from missing outcome data (16,21,24), while two others were because of deviation from the intended intervention and biased measurement (27,28), respectively. A study was marked with a high risk of bias due to missing outcome data (20).

For PEDro scale-based risk of bias analysis, the results have been presented in Table 2, where the details of this assessment have been presented in APPENDIX A. The mean PEDro score obtained here is 8.3 ranging from 6 to 10, categorized as high quality.

(a) (b)
Figure 2. (a) Traffic Light Plot (b) Summary Plot Risk Risk of Bias 2.0 Tool of the included studies
3.3 Study Outcomes - Meta-Analysis on PDQ-39 on Parkinson’s Disease.
The results of the random effect meta-analysis of the quality of life-based on PDQ-39 have been presented in Figure 3. The effect of exergame therapy on quality of life was assessed in nine studies (14,15,17–19,22,23,25,26) according to PDQ-39 overall mean score. The effect of exergame therapy on quality of life favored the experimental group but was not statistically significant (n= 9, SMD= -0.08, 95%CI: -0.31–0.16, p= 0.53). The I2 of this analysis was abandoned (0%).
3.4 Study Outcomes - Meta-Analysis on Euroqol-5D on Parkinson’s Disease.
The results of the random effect meta-analysis of the quality based on Euroqol-5D only assessed in 2 studies (22,23) and have been presented in Figure 4. The effect of exergame therapy on quality of life favored the experimental group but was not statistically significant (n= 2, SMD= -0.27, 95%CI: -0.79–0.24, p= 0.30). The I2 of this analysis was <50% (35%).


3.5 Study Outcomes - Meta-Analysis on AD-QoL on Alzheimer’s Disease
The results of the random effect meta-analysis of the quality based on the Quality of Life of Alzheimer’s Disease (QoL-AD) were only assessed in 2 studies (27,28) and have been presented in Figure 5. The effect of exergame therapy on quality of life favored the experimental group but was not statistically significant (n= 2, SMD= -0.21, 95%CI: -0.33–0.76, p= 0.44). The I2 of this analysis was abandoned (0%).
Figure 3. The result of exergame in alleviating quality of life in Parkinson’s disease patients using PDQ-39 tools
Figure 4. The result of exergame in alleviating quality of life in parkinson’s disease patients using Euroqol-5D tools
4. DISCUSSION
4.1 General Description of Exergames
4.1.1 General Characteristics of Exergames

Exergame is a new technological invention that has been lately designed for medical purposes. This gaming technology has recently been used to treat patients with PD and other neurologic disorders (24). It combines video-game-based training with augmented virtual reality (VR) and is intended to be engaging and challenging, therefore stimulating motivation and increasing exercise adherence (29) Further advantages of exergaming are the possibility to adapt to the level of exercise difficulty and to provide online visual and/or verbal feedback during the exercise. All these aspects are important to optimize motor learning in individuals with neurologic disorders.
4.1.2 Types of Exergames
Exergames are divided into three types:
A. Augmented Reality (AR)
The type of exergame that combines virtual objects with the real world (environment that is around us). It is used by applying computer-generated virtual information, such as text, images, 3D models, music, video, etc., to the real world. The AR that was reported were Google Glass (30) which contains exercise for stability and balance. One example of these applications is Moving Through Glass (MTG). The MTG application was designed for Google Glass as a portable, round-the-clock extension of the dance classes that are taught through Dance for PD (30).
B. Virtual Reality (VR)
This exergame uses a computer-generated environment with scenes and objects that appear to be real, making the user feel they are immersed in their surroundings. It interacts with simulated objects in that environment as If they were real. This environment is perceived through a device known as a Virtual Reality headset. The VR that are operated for instance Xbox Kinect (26,29,31), Nintendo Wii (16,18,22,24,25,29), regular VR technology (15,32–34), Exergame Dividat Senso (19), Aquasnap (35), IVGB training (17). All VR listed above contains exercises that provide training for stability and balance. The Xbox Kinect requires no
Figure 5. The result of exergame in alleviating quality of life in alzheimer’s disease patients using AD-QoL tools
controller to capture movements and works with infrared sensors. The Nintendo Wii works with a handheld controller and a force platform named Wii Balance Board. The other exergame, Dividat Senso, includes 20 sensors (strain gauges), 5 vibration motors and an LED control. It is connected to a small computer and a large screen in front of the Sensor on which the stimuli of the Dividat exergames are shown. On the other hand, Aquasnap performed an online CT game taking its player as an underwater photographer, exploring the ocean, and completing cognitive tasks by taking pictures of fish. In Yuan (17), the IVGB exercise program consisted of two tasks: a multi-directional step task and a target-directed stepping task.
C. Monitor-based Technology
This type of exergame can allow physicians to better observe the evolution of symptoms over time, in particular with motor fluctuations, In addition, they potentially allow less frequent visits to the expert’s office and facilitate care in rural areas. In this study, the monitor-based technologies that are operated are TelePark (Bendig), and SENSE-PARK (24). Both exercises listed above contain exercises that provide training for stability and balance.
4.2 Effectivity of Exergame in Improving Quality of Life
There are fourteen studies assessing the quality of life, consisting of twelve studies about Parkinson’s disease and two studies about Alzheimer's (14,15,24–28,16–23). Most of Parkinson’s disease studies used Parkinson’s Disease Questionnaire-39 (PDQ-39) and Euroqol-5D (EQ-5D). While the tools for assessing the quality of life in Alzheimer’s patients used Quality of Life in Alzheimer’s Disease (QoL-AD). The tools aforementioned measured the health-related quality of life whereas they had different focuses, domains, and ways to measure and interpret the responses. PDQ-39 was designed to measure the impact of Parkinson’s disease in various aspects that affect the individual’s life involving eight different domains which are mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort. The higher score of PDQ-39 indicates, the lower quality of life (36). Additionally, EQ-5D assesses only five domains consisting of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The higher score of EQ-5D indicates the better quality of life (37). Equally important, QoL-AD evaluates five domains including physical health, mood, functional abilities, social support, and financial resources with a higher score suggesting a better quality of life. Almost all studies have witnessed that exergames have a significant effect on improving the quality of life in Parkinson’s disease patients (15,18,21,22,24–27). Five other studies gave insignificant effects but it improved quality of life by decreasing the score of PDQ-39 and enhancing the score of SF-36 (14,16,17,23,28). Nonetheless, the two studies got higher scores in PDQ-39 than the control group means it’s not effective, probably due to the bias of imbalance baseline (16,24).
In addition, compared to simple observation, our meta-analysis results are in accordance with a study conducted by Elena et. al. (38), although we include two more studies in our analysis. Both studies revealed the results favor exergame-based rehabilitation compared to conventional rehabilitation in order to ameliorate the quality of life in Parkinson’s disease patients. Nevertheless, the effect was not statistically significant (38). Moreover, the results of
our meta-analysis are also in accordance with studies that assessed different neurological diseases (39)
4.3 Safety of Exergame
There are six studies that reported the safety of using an exergame, for instance, fall, muscle soreness, fatigue, and so on (14,16,17,19,25,27). All of the studies revealed that there were no adverse events found when using the exergame as a rehabilitation. There are no adverse events reported within the studies whether using exergame with sitting up, walking, or moving methods. Ribas showed that exergame had a lower value of fatigue in Parkinson’s disease than conventional rehabilitation, and enhanced the confidence level of patients for avoiding falls while intervention (16) Participants also felt a reduction in fear of falling during the intervention, where the console can be set with safety settings (27)
4.4 Acceptance of Exergame
High adherence rate was found in three studies means that they are comfortable doing the task of exergames, (14,19,27), even some of them doing more than the prespecified task as order and low attrition indicates high acceptability for people with a neurodegenerative disorder. Through open-ended and close-ended interviews, it was revealed that participants felt the exergame was easy, safe, well-integrated, enjoyable, easy to learn, and motivated to complete the next task. However, there are some reports of those tired of playing the same game over 12 weeks and did not have space for exergame in their homes (14). Others complained about the complexity, cumbersome, and need for technical support for the intervention (19).
4.6 Strength and Limitation
This present study is the first in reporting the pooled results from studies participated by elderly population. As limitations, the data acquired are limited and cannot be used to infer a conclusion for the whole neurodegenerative disease. For example, there are studies reporting Parkinson’s Disease and Alzheimer’s disease, multiple sclerosis, and Huntington’s Disease but were excluded because the participants > 60 years old. Moreover, the limitation in data is also contributed by the small sample size of the included studies.
5. CONCLUSIONS AND RECOMMENDATIONS
Exergames are not significantly different from conventional rehabilitation in improving the quality of life. However, exergame is still considered a promising modality in rehabilitating patients with neurodegenerative disease attributed to its easy-to-use and entertaining nature Moreover, the exergame was found to have high acceptance and safety for the elderly to use. To ensure maintain the interest of its users, we recommend the game developer improve the visualization, game scenarios, and movement variety. We also highly suggest further research to develop an exergame that has a minimum capacity of spatial, so it does not need a lot of
areas that might burden participants who have a small capacity of space. Investigation with a larger sample size is highly recommended in the future.
6. REFERENCES
1. Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimer’s Dement [Internet]. 2008 Sep;4(5):316–23. Available from:
https://onlinelibrary.wiley.com/doi/10.1016/j.jalz.2008.05.2479
2. Cornblath EJ, Robinson JL, Irwin DJ, Lee EB, Lee VM-Y, Trojanowski JQ, et al. Defining and predicting transdiagnostic categories of neurodegenerative disease. Nat Biomed Eng [Internet]. 2020 Aug 3;4(8):787–800. Available from:
https://www.nature.com/articles/s41551-020-0593-y
3. Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The Diagnostic Challenge of Psychiatric Symptoms in Neurodegenerative Disease. J Clin Psychiatry [Internet]. 2011 Feb 15;72(02):126–33. Available from:
http://article.psychiatrist.com/?ContentType=START&ID=10007287
4. Montero-Odasso M, Pieruccini-Faria F, Bartha R, Black SE, Finger E, Freedman M, et al. Motor Phenotype in Neurodegenerative Disorders: Gait and Balance Platform Study Design Protocol for the Ontario Neurodegenerative Research Initiative (ONDRI). J Alzheimer’s Dis. 2017;59:707–21.
5. Hussain R, Zubair H, Pursell S, Shahab M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci [Internet]. 2018 Sep 15;8(9):177. Available from: http://www.mdpi.com/2076-3425/8/9/177
6. Batista P, Pereira A. Quality of Life in Patients with Neurodegenerative Diseases. J Neurol Neurosci [Internet]. 2016;7(1). Available from:
http://www.jneuro.com/neurology-neuroscience/quality-of-life-in-patients-withneurodegenerative-diseases.php?aid=8682
7. Schneider JS, Sendek S, Yang C. Relationship between Motor Symptoms, Cognition, and Demographic Characteristics in Treated Mild/Moderate Parkinson’s Disease. Bankiewicz K, editor. PLoS One [Internet]. 2015 Apr 23;10(4):e0123231. Available from: https://dx.plos.org/10.1371/journal.pone.0123231
8. Heinzel S, Bernhard FP, Roeben B, Nussbaum S, Heger T, Martus P, et al. Progression markers of motor deficits in Parkinson’s disease: A biannual 4-year prospective study. Mov Disord [Internet]. 2017 Aug;32(8):1254–6. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mds.27062
9. Bondy SC, Campbell A. Aluminum and Neurodegenerative Diseases. In 2017. p. 131–56. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2468748017300085
10. Johnson TM, Ridgers ND, Hulteen RM, Mellecker RR, Barnett LM. Does playing a sports active video game improve young children’s ball skill competence? J Sci Med Sport [Internet]. 2016 May;19(5):432–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1440244015001012
11. Gravenhorst F, Muaremi A, Bardram J, Grünerbl A, Mayora O, Wurzer G, et al. Mobile phones as medical devices in mental disorder treatment: an overview. Pers Ubiquitous Comput [Internet]. 2015 Feb 21;19(2):335–53. Available from: http://link.springer.com/10.1007/s00779-014-0829-5
12. Griffiths MD, Kuss DJ, Demetrovics Z. Social Networking Addiction. In: Behavioral Addictions [Internet]. Elsevier; 2014. p. 119–41. Available from:
https://linkinghub.elsevier.com/retrieve/pii/B9780124077249000069
13. Biddiss E, Irwin J. Active Video Games to Promote Physical Activity in Children and Youth. Arch Pediatr Adolesc Med [Internet]. 2010 Jul 1;164(7). Available from:
http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/archpediatrics.2010.104
14. Allen NE, Song J, Paul SS, Smith S, O’Duffy J, Schmidt M, et al. An interactive videogame for arm and hand exercise in people with Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord [Internet]. 2017 Aug;41:66–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1353802017301773
15. Pazzaglia C, Imbimbo I, Tranchita E, Minganti C, Ricciardi D, Lo Monaco R, et al. Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: a randomised controlled trial. Physiotherapy [Internet]. 2020 Mar;106:36–42. Available from:
https://linkinghub.elsevier.com/retrieve/pii/S0031940618301287
16. Ribas CG, Alves da Silva L, Corrêa MR, Teive HG, Valderramas S. Effectiveness of exergaming in improving functional balance, fatigue and quality of life in Parkinson’s disease: A pilot randomized controlled trial. Parkinsonism Relat Disord [Internet]. 2017 May;38:13–8. Available from:
https://linkinghub.elsevier.com/retrieve/pii/S1353802017300391
17. Yuan R-Y, Chen S-C, Peng C-W, Lin Y-N, Chang Y-T, Lai C-H. Effects of interactive video-game–based exercise on balance in older adults with mild-to-moderate Parkinson’s disease. J Neuroeng Rehabil [Internet]. 2020 Dec 13;17(1):91. Available from: https://jneuroengrehab.biomedcentral.com/articles/10.1186/s12984-020-00725-y
18. Santos P, Machado T, Santos L, Ribeiro N, Melo A. Efficacy of the Nintendo Wii combination with Conventional Exercises in the rehabilitation of individuals with Parkinson’s disease: A randomized clinical trial. NeuroRehabilitation. 2019;45(2):255–63.
19. Jäggi S, Wachter A, Adcock M, de Bruin ED, Möller JC, Marks D, et al. Feasibility and effects of cognitive–motor exergames on fall risk factors in typical and atypical Parkinson’s inpatients: a randomized controlled pilot study. Eur J Med Res. 2023;28(1):30.
20. Yang W-C, Wang H-K, Wu R-M, Lo C-S, Lin K-H. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: A randomized controlled trial. J Formos Med Assoc. 2016;115(9):734–43.
21. Alves MLM, Mesquita BS, Morais WS, Leal JC, Satler CE, dos Santos Mendes FA. Nintendo WiiTM versus Xbox KinectTM for assisting people with Parkinson’s disease. Percept Mot Skills. 2018;125(3):546–65.
22. Ferraz DD, Trippo KV, Duarte GP, Neto MG, Santos KOB, Oliveira Filho J. The effects of functional training, bicycle exercise, and exergaming on walking capacity of elderly patients with Parkinson disease: a pilot randomized controlled single-blinded trial. Arch Phys Med Rehabil. 2018;99(5):826–33.
23. Tollár J, Nagy F, Hortobágyi T. Vastly different exercise programs similarly improve parkinsonian symptoms: a randomized clinical trial. Gerontology. 2019;65(2):120–7.
24. Pedreira G, Prazeres A, Cruz D, Gomes I, Monteiro L, Melo A. Virtual games and quality of life in Parkinson’s disease: A randomised controlled trial. Adv Park Dis. 2013;2013.
25. Liao Y-Y, Yang Y-R, Cheng S-J, Wu Y-R, Fuh J-L, Wang R-Y. Virtual reality–based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2014;29(7):658–67.
26. Fontoura VCB, de Macêdo JGF, da Silva LP, da Silva IB, de Sales M, Coriolano DM. The role of rehabilitation with virtual reality in functional ability and quality of life of individuals with Parkinson’s disease. CEP. 2017;53140:160.
27. Padala KP, Padala PR, Lensing SY, Dennis RA, Bopp MM, Roberson PK, et al. Home-based exercise program improves balance and fear of falling in communitydwelling older adults with mild Alzheimer’s disease: a pilot study. J Alzheimer’s Dis. 2017;59(2):565–74
28. Padala KP, Padala PR, Malloy TR, Geske JA, Dubbert PM, Dennis RA, et al. Wii-fit for improving gait and balance in an assisted living facility: a pilot study. J Aging Res. 2012;2012.
29. Van Beek JJW, Van Wegen EEH, Bohlhalter S, Vanbellingen T. Exergaming-based dexterity training in persons with Parkinson disease: a pilot feasibility study. J Neurol Phys Ther. 2019;43(3):168–74.
30. Tunur T, DeBlois A, Yates-Horton E, Rickford K, Columna LA. Augmented realitybased dance intervention for individuals with Parkinson’s disease: A pilot study. Disabil Health J [Internet]. 2020 Apr;13(2):100848. Available from:
https://linkinghub.elsevier.com/retrieve/pii/S1936657419301608
31. Pompeu JE, Arduini LA, Botelho AR, Fonseca MBF, Pompeu SMAA, Torriani-Pasin C, et al. Feasibility, safety and outcomes of playing Kinect Adventures!TM for people with Parkinson’s disease: a pilot study. Physiotherapy [Internet]. 2014 Jun;100(2):162–8. Available from:
https://linkinghub.elsevier.com/retrieve/pii/S0031940613001223
32. Arlati S, Di Santo SG, Franchini F, Mondellini M, Filiputti B, Luchi M, et al. Acceptance and Usability of Immersive Virtual Reality in Older Adults with Objective and Subjective Cognitive Decline. J Alzheimer’s Dis [Internet]. 2021 Apr 6;80(3):1025–38. Available from:
https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JAD201431
33. Isernia S, Di Tella S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, et al. Effects of an Innovative Telerehabilitation Intervention for People With Parkinson’s Disease on Quality of Life, Motor, and Non-motor Abilities. Front Neurol [Internet]. 2020 Aug 13;11. Available from:
https://www.frontiersin.org/article/10.3389/fneur.2020.00846/full
34. Mrakic-Sposta S, Di Santo SG, Franchini F, Arlati S, Zangiacomi A, Greci L, et al. Effects of Combined Physical and Cognitive Virtual Reality-Based Training on Cognitive Impairment and Oxidative Stress in MCI Patients: A Pilot Study. Front Aging Neurosci [Internet]. 2018 Oct 1;10. Available from:
https://www.frontiersin.org/article/10.3389/fnagi.2018.00282/full
35. van de Weijer SCF, Duits AA, Bloem BR, de Vries NM, Kessels RPC, Köhler S, et al. Feasibility of a Cognitive Training Game in Parkinson’s Disease: The Randomized Parkin’Play Study. Eur Neurol [Internet]. 2020;83(4):426–32. Available from:
https://www.karger.com/Article/FullText/509685
36. Hagell P, Nygren C. The 39 item Parkinson’s disease questionnaire (PDQ-39) revisited: implications for evidence based medicine. J Neurol Neurosurg Psychiatry. 2007;78(11):1191–8.
37. Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. 2014;
38. Elena P, Demetris S, Christina M, Marios P. Differences Between Exergaming Rehabilitation and Conventional Physiotherapy on Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Neurol. 2021;12:683385.
39. Yen H-Y, Chiu H-L. Virtual reality exergames for improving older adults’ cognition and depression: a systematic review and meta-analysis of randomized control trials. J Am Med Dir Assoc. 2021;22(5):995–1002.
APPENDIX A. The Results of PEDro Analysis
Note. 1: present; 0: absence
Criteria:
1: Eligibility criteria were specified, presented as ‘Yes’ or ‘No’
2: Subjects were randomly allocated to groups (in a crossover study, subjects were randomly allocated an order in which treatments were received)
3: Allocation was concealed
4: The groups were similar at baseline regarding the most important prognostic indicators
5: There was blinding of all subjects
6: There was blinding of all therapists who administered the therapy
7: There was blinding of all assessors who measured at least one key outcome
8: Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups
9: All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analyzed by “intention to treat”
10: The results of between-group statistical comparisons are reported for at least one key outcome
11: The study provides both point measures and measures of variability for at least one key outcome
Allen., 2017 Pazzaglia. , 2019 Ribas., 2017 Yuan., 2020 Santos., 2019 Jaggi., 2023 Yang., 2015 Alves., 2018 Ferraz., 2018 Tollar., 2018 Padala., 2017 Padala., 2012 Fountora., 2014 Pedeira. , 2013 Liao. , 2014 I Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 3 1 1 1 1 1 0 1 0 1 1 0 0 1 0 1 4 1 1 0 1 1 1 1 0 1 1 1 1 1 1 1 5 1 1 1 1 1 1 1 1 1 1 0 0 1 0 1 6 1 0 0 0 1 0 1 0 0 1 1 1 1 0 1 7 0 1 1 0 1 0 1 1 1 1 1 1 1 1 1 8 1 1 1 1 1 1 0 1 1 1 1 1 1 1 1 9 1 1 1 1 1 1 1 1 1 1 0 0 1 1 1 10 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 11 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 Total 9 9 8 6 10 7 9 7 9 10 7 7 10 7 10
TheEfficacyofAutologousPlatelet-richPlasmaGelasNovelApproachforElderly
PatientswithDiabeticFootUlcers:AMeta-analysis
KeyzhaAmartyaAdammayanti1,PutuMoradhaKharismaPutri,1 WellyGrivinLayuk1,JuanAlessandro JeremisMaruliNuraLele1
(1) UniversitasKristenIndonesia
[Correspondence E-mail: amartyakeyzha@gmailcom]
Introduction: Diabetic foot ulcers (DFU) are among the most common complications of patients who have diabetes mellitus which is not well controlled. DFUs are still rare in several guidelines on the management of diabetes intheelderly.Autologousplatelet-richgel (APG) as a more recent platelet-derived product has been appliedtotheclinicaltreatmentof diabetic ulcers in this century andhasadvantages,suchaseasy-to-obtainmaterials,simpleto produce,lowcost,andnorejectionreaction.
Objective: This study aim to assess the safety and efficacy of APG as a novel treatment of DFUcomparedwithstandardtreatmentinelderlypatients
Methods: Randomized Controlled Trials were searched by using PubMed, Cochrane, Google Scholar, Wiley, and Plos One. The keyword has been arranged using the Boolean operator including autologous platelet-richgel,diabeticfootulcers,andelderly Thedatawas screened by inclusion and exclusion criteria. The final inclusion study was analyzed and synthesized by tabulation, clusterization, contextual and thematic approach, and assessedfor risk of bias by using ROB 2.0. Meta-analysis was conducted by using Review Manager 5.4 andtheMantelHaenszelmethod.
Results: Twelve RCTs with 873 patients were eligible for the present analysis. Compared with standard care/conventional treatment, APG could significantly improve the healing wound in patients with diabetic foot ulcers (RR=1.40, 95%CI 1.27,1.56; P < 0.00001), shortened the healing time (MD= -11.32, 95% CI -16.87, - 5.76; P< 0.0001), and shortened thelengthofhospitalstay(MD=-20.11,95%CI-38.02,-2.20;P=0.03).
Conclusions: APG treatment can better treat DFU in terms of duration of healing, wound healing,andinfectionthanthestandardtreatment.
Keywords: Autologous platelet-rich plasma gel, diabetic foot ulcers, elderly
TheEfficacyofAutologousPlatelet-richPlasmaGelasNovelApproachforElderly
PatientswithDiabeticFootUlcers:AMeta-analysis
KeyzhaAmartyaAdammayanti
1,PutuMoradhaKharismaPutri,1 WellyGrivinLayuk1,JuanAlessandroJeremis MaruliNuraLele1
(1) UniversitasKristenIndonesia
[Correspondence E-mail: amartyakeyzha@gmail.com]
Introduction:Diabeticfootulcers(DFU)areamongthemostcommoncomplicationsofpatients who have diabetes mellitus which isnotwellcontrolled.DFUsarestillrareinseveralguidelines on the management of diabetes in the elderly Autologous platelet-rich gel (APG) as a more recent platelet-derived product hasbeenappliedtotheclinicaltreatmentofdiabeticulcersinthis centuryandhasadvantages,suchaseasy-to-obtainmaterials,simpletoproduce,lowcost,andno rejectionreaction.
Objective: This study aim to assess the safetyandefficacyofAPGasanoveltreatmentofDFU comparedwithstandardtreatmentinelderlypatients.
Methods: Randomized Controlled Trials were searched by using PubMed, Cochrane, Google Scholar, Wiley, and Plos One. The keyword has been arranged using the Boolean operator including autologous platelet-rich gel,diabeticfootulcers,andelderly Thedatawasscreenedby inclusion and exclusion criteria. The final inclusion study was analyzed and synthesized by tabulation, clusterization, contextual and thematic approach, and assessed for risk of bias by using ROB 2.0. Meta-analysis was conducted by using Review Manager 5.4 and the Mantel Haenszelmethod.
Results: Twelve RCTs with 873 patients were eligible for the present analysis. Compared with standard care/conventional treatment, APG could significantly improve the healing wound in patients with diabetic foot ulcers (RR=1.40, 95%CI 1.27,1.56; P < 0.00001), shortened the healing time (MD= -11.32, 95% CI -16.87, - 5.76; P< 0.0001), and shortened the length of hospitalstay(MD=-20.11,95%CI-38.02,-2.20;P=0.03).
Conclusions: APG treatment can better treat DFU in terms of duration of healing, wound healing,andinfectionthanthestandardtreatment.
Keywords: Autologous platelet-rich plasma gel, diabetic foot ulcers, elderly
TheEfficacyofAutologousPlatelet-richPlasmaGelasNovelApproachforElderly PatientswithDiabeticFootUlcers:AMeta-analysis
AsianMedicalStudentsConference2023

Authors:
KeyzhaAmartyaAdammayanti
PutuMoradhaKharismaPutri
WellyGrivinLayuk
JuanAlessandroJeremisMaruliNuraLele
UniversitasKristenIndonesia
INTRODUCTION
The prevalence of Diabetes Mellitus increases significantly with age.[1,2] Most of the diagnosed cases lie between the fourth and seventh decades of life.[3] According to the most recent data, >326 million people of working age suffer from DMisdifferentfrom122.8million aged over 65 years.[3] These figures are expected to increase to 438.2 million and 253.4 million respectively in the coming decades.[3] DFU are among the most common complications of patients who have diabetes mellitus which is not well controlled. It is usually the result of poor glycemic control, underlying neuropathy, peripheral vascular disease, or poor foot care.[4] Other risk factors include poor vision, gait abnormalities, reduced mobility and medical comorbidities The risk of major amputations increases with age, along with the increased prevalence of these risk factors. Diabetic feet are rarely mentioned in some guidelines for diabetes management in older adults.[5,6] The prevalence of neuropathy, foot deformities, and peripheral arterial disease (PAD) as well as the risk of amputation all increase with age even in nondiabetic patients. The treatment of foot ulcersreliesonpressurerelief(off-loading),wounddebridement,andtreatment of infection and ischemia. It requires an individualized approach considering the patient's co-morbiditiesandfunctionalstatus.[7]
Conventional treatments do not generally work satisfactorily for diabetic refractory ulcers. With the increasing knowledge of the refractory ulcer pathophysiology, alterationsinthe microenvironment, especially deficiency of growth factors and other bioactive substances are considered important causes of poor healing. Biological products or emerging cellular therapies used to makeupthedeficiencyarebelievedtohavesignificantclinicianvaluesandamongthem, platelet-derived products have been used since 1986. Knighton et al conducted the first clinical study demonstrating that locally applying platelet-derived wound healing factors (PDWHFs) promotedthehealingofchronicrefractoryulcers.[8]
APG as a more recent platelet-derived product has been applied to the clinicaltreatment of diabetic ulcers in this century. The first prospective controlled trial on the effectiveness and safety of APG for the treatment of DFU was conducted by Saldalamacchia et al in 2004, providing important evidence on the significance of topical APG application on diabetic cutaneous ulcers.[9] APG is an inexpensive and immunologically safe source of growth factors (GFs). It is believed that APG can accelerate the healing process, so APGhasbeenwidelyused in the field of wound repair.[10,11] As the second generation of platelet-derived preparation, APG has some additional advantages, such as easy-to-obtain materials, simplicity to produce, low cost, and no rejection reaction. A growing bodyofevidencesuggeststhatAPGismoreeffective for chronic wounds than standard care/conventional treatment.[12,13] Interest in the useofAPGis increasing worldwide due to poor outcomes of conventional treatment methods and the increasing frequency of hard-to-heal leg wounds in the aging population. The goal of this research is to evaluate theefficiencyofAPGtreatmentincomparisonwithexistingconventional legulcertreatment.[14]
To our knowledge, there is no meta-analysis that specifically addresses the treatment of APG in the elderly population with DFU. We analyzed the efficacy ofAPGineachofWagner's
classifications as a toolfortreatingdiabeticfootulcerstodemonstratetheclinicaleffectofAPG. We performed a recent meta-analysis over the last ten years to assess the safety and efficacy of APG as a novel treatment of DFU compared with standard treatment. Therefore, we systematically reviewed the clinical efficacy and safety of APG as a breakthrough therapy in elderlypatientswithdiabeticfootulcers.

METHOD
2.1.LiteratureSearch
A systematic review was conducted by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement rules. Randomized controlled trials were searched from medical databases including PubMed, Cochrane, Google Scholar, Wiley, and Plos One. We searched the following keywords that have been arranged by Boolean Operator rules: (autologous platelet-rich gel) AND (diabetic foot ulcer), [Appendix; Database searching]. The search results were downloaded to a personal database, screened, extracted, analyze, and synthesizedbothforqualitativeandquantitativedata.
Fig.1PRISMAflowchartdiagramofthestudy
2.2.EvaluationCriteria
The study was screened by inclusion and exclusion criteria. The inclusion criteria were (1) P= population. Wagner grade 1–4 patients (±50 years of age) presenting with diabetic foot ulcers were included in the study. (2) I = intervention. Eligible studies for inclusion in the meta-analysis hadtouseAPGasatopicalapplication.(3)C=comparison.Studiescomparedthe use of APG (not platelet-derived growth factor [PDGF]) with placebo or other biomaterialsthat were eligible for meta-analysis. (4) O = outcomes. The primary outcomevariableoftheeligible studies was represented by the healing wound. The healing is referred to as completely healed. The secondary outcomes considered were healing time, length hospital stay, surface area reduction and adverse events. The adverse events were defined as advanced infection, formication, or prickling sensation. Infection was defined as the results of microbial culture on the DF wound surfaces being not consistent with the results before APG treatment. The definition of formication was patients describing an itchy feeling of ants crawling ontheulcers, and the prickling sensation was defined as patients describing a feeling of piercing pain on the ulcers. (5) S= study design. Only RCT studies were included. The exclusion criteria were case series, case reports, retrospective studies, animal studies, technical studies, and reviews. Furthermore, a duplicate removal was also performed using EndNote X9 software (Clarivate, Philadelphia, PA, USA). The titles and abstracts of studies were screened according to the criteria of accessibility by three independent reviewers (i.e., JAJMNL, KAA, and PM). Any disagreementswerediscussedtoconsensus.
2.3.DataCollectionandAnalysis
The collecting data in this study followed the PRISMA flow diagram, including the identification of studies in the databases; screening for duplicates,titles,andabstracts;assessing full eligibility text; and extraction and analysis of the included studies. We extracted studies manually on the extraction tabulation. The item data on the extraction were, namely,theauthor, country, design study, characteristic and the number of samples, model, feasibility, and efficacy of autologous platelet-rich gel. We also extracted outcomes including the healing time, healing duration, and any adverse event. Additionally, we analyzed this study using quantitative and qualitativeanalysis.
2.4.QuantitativeAnalysis
Statistical analysis was performed using ReviewManagerver 5.4(TheNordicCochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). The clinical outcome with continuous data (Mean Differences) with (Standard Deviations) and dichotomous data (Risk Ratio), was conductedwitha95%confidenceinterval(CI).Thep-values<0.05wereconsidered significant. The main results used in the statistical analysis were the mean difference between Autologous Platelet-rich Gel and standard care which was shown by decreasing time, surface area reduction and length of hospital stay The risk ratio is also shown for healing wound and
infection. The mean difference and risk ratiowitha95%CIanditsrespectivep-valuewereused to determine the efficacy of APGonalloutcomes,whichwaspresentedinaforestplot.Weused an inversevarianceandDerSimonian–Lairdrandom-effectsmodelasproposedbyRileyetal.,as we considered that aheterogeneityoutsidethestudycouldalsobediscovered.Theheterogeneity was further evaluated using estimated effect (I2)statistics based on theCochranethreshold,with cut-off limitsof0%,25%,50%,and75%asinsignificant,low,moderate,andhighheterogeneity, respectively.[15] According to von Hippel, the I2 is substantial when the number of studies is small. Following Duval and Tweedie, we also performed a sensitivity analysis using trim and fill.[16] The sensitivity test was performed using the Jamovi 2.2.5 software and used when the heterogeneity was high. We also performed a systematic review to analyze and synthesize through a qualitative approach in the form of tabulation, clusterization, thematic analysis, and contextualdescriptionswhichareshowninthecharacteristicstudy.
2.5.RiskofBiasAssessment
Final inclusion studies were evaluated for risk of bias usingtheRevisedToolforRiskof Bias in RandomizedTrials(RoB2.0)consistingoffivedomainsforinitiativestudies.Theauthor will assess the risk of bias according to the algorithm that has been made by Cochrane. The results will then be inputted in the domain file bias (.xlsx). The file willthenbeincludedonthe ROBVISwebsitesothattheresultingdatacanbevisualizedproperly
RESULT
3.1LiteratureSearch
For this meta-analysis, thereweretwelvefinalarticlesthatmettheinclusionoreligibility criteria with a total of 873 participants.ThetypeofinterventionusedisAutologousPlatelet-rich Gel. The participants involved were elderly Based on the search results for articles in primary sources (PubMed, Wiley, Cochrane, Google Scholar,andPlosOne)1.165articleswerequalified inthedatabasesearch.
3.2StudyCharacteristics
The included studies wereconductedfrom2013to2021.Atotalof12RCTswithsample sizes ranging from48to129wereeligibleforthissystematicreview[14,17-27] and873patientswith the intervention group (n = 434) and the control group (n = 439) were enrolled. The age distributions were similar between the APG group and the control group. TheWagnergradesof DF wounds in included studies ranged from 1 to 5. The features of the included studies are summarizedinTable1.
Table1.Characteristicsofincludedstudies
First Author (Year) StudyType Country Age(Year,mean) SampleSize Wagner grade Observation period Intervention Situation IG CG IG CG Alamdari MRetal, 2021[17] Randomised Controlled Trial Iran 5652±714 90 Intervention group(n=43) Controlgroup (n=47) 1-2 6month APG CT GudeW etal, 2019[18] Randomised Controlled Trial New Mexico 669 647 129 Intervention group(n=66) Controlgroup (n=63) 1-5 12weeks Aurix+ UCC UCC RainysD etal 2019[14] Randomised Controlled Trial Lituania 6223 ±1472 6801 ±1489 69 Intervention grup(n=35) Controlgrup (n=34) NR 8weeks APG Receive d identical treatmen tbut without theAPG XieJetal 2019[19] Randomised Controlled Trial China 605 ±827 6110 ±790 48 Intervention group(n=25) Controlgroup (n=23) NR 8weeks APG CT Goda, AA 2018[20] Randomised Controlled Trial Egypt 5688 558 50 Intervention group(n=25) Controlgroup (n=25) NR 12weeks APG PPP Ahmedet al2016[21] Prospective comparative study Egypt 18-80 56 Intervention group(n=28) Controlgroup (n=28) NR 2-12weeks APG Normal saline+ 10% iodin LiWetal (2016)[22] Retrospective Study China 625 ±101 642 ±98 72 Intervention group(n=36) Controlgroup (n=36) NR 12weeks APG+ SC SC LiuGY etal, 2016[23] Randomised Controlled Trial China 54,6±9,6 2 554±8 19 60 Intervention group(n=30) Controlgroup (n=30) 2-3 8weeks APG CT
Abbreviations: IG= intervention group; CG= control group;PRP=platelet-richplasma;PPP=platelet-poorplasma;CT=conventionaltreatment; UCC=usualandcustomarycare;APG=autologousplatelet-richgel;SC=standardcare;TMA=Transmetatarsalamputation;NR=notreport
3.3RiskofBias
Cochrane risk-of-bias tool for randomized controlled studies 2.0 (Cochrane RoB Tool 2.0) was used to assess the risk of bias from seven different aspects. Attrition bias was low, except in two studies. Xie et al studies reported high bias because in the final analyses, some patients in the control group withdrew from the study,[19] and in Serra R et al. all patients were treated after collecting appropriate information on the type of treatment.[27] Reporting bias was low with all included data coming from trials withlowrisks.Anotherbiaswasnotmentionedin allincludedstudies;sotheincludedstudieshadunclearrisks.TheresultsareshowninFig.2
ZhangL etal, 2016[24] Randomised Controlled Trial China 6612±1 111 673±1 053 52 Intervention group(n=25) Controlgroup (n=27) 1-3 8weeks APG+ CT CT LiLetal, 2015[25] Randomised Controlled Trial China 628 ±116 117 Intervention group(n=59) Controlgroup (n=58) 1-3 5weeks APG+S C SC QiKQet al, 2014[26] Randomised Controlled Trial China 6078±8 3 605±8 11 72 Intervention group(n=36) Controlgroup (n=36) 1-3 NR APG CT SerraRet al, 2013[27] Randomised Controlled Trial Italy 675 635 58 Intervention group(n=26) Controlgroup (n=32) NR 6year TMA+ APG TMA

 Fig.2RiskofBias
Fig.3SummaryRiskofBias
Fig.2RiskofBias
Fig.3SummaryRiskofBias
diabetes, especially intheelderly,sowealsoperformedsubgroupanalysesonthehealingwound of diabetic foot ulcers. All trials were included in patients with diabetic foot ulcers. Above all, one trial reported the healing wound of diabetic foot ulcers according to diabetic foot wagner classification. Since no significant heterogeneity was detected among studies (Chi2 =24.14, P= 0.15, I2 = 25%), a fixed-effects model was used for meta-analysis. The result showed that compared with standard/conventional treatment, APG could significantly improve the healing rate in patients with diabetic foot ulcers (RR=1.40, 95%CI 1.27,1.56; P < 0.00001). APG has significant results, especially for grade 1 healing wounds (RR=1.27, 95%CI 1.02,1.58;P=0,03)

thattreatmentusingAPGisrecommendedtocureDFUthanstandardtreatmentusage.


Fig.5FunnelPlotHealingDurationAPG
Heterogeneity was found in the study We did a sensitivity test on the Jamovi 2.2.5 software. A total of k=6 studies were included in the analysis. The observed mean differences ranged from -25.0000 to -5.4600, with the majority of estimates being negative (100%). The estimated average mean difference based on the random-effects model was (hat/mu) =-11.2521 (95% CI: -17.4531 to -5.0510). Therefore, the average outcome differed significantly from zero (z = -3.5564, p=0.0004).AccordingtotheQ-test,thetrueoutcomesappeartobeheterogeneous (Q(5) = 47.4084, p <0.0001, tau = 48.7490, 12 = 91.6996%). A 95% prediction interval for the true outcomes is given by-26.2761to3.7719.Hence,althoughtheaverageoutcomeisestimated to be negative, in some studies the true outcome may in fact be positive. Anexaminationofthe studentized residuals revealed that one study (Alamdari et al 2021) had a value larger than + 2,6383 and may be a potential outlier in the context of this model. According to the Cook's distances, none of the studies could be considered to be overly influential. Neither the rank correlation nor the regression test indicated any funnel plot asymmetry (p = 0.7194 and p = 0.6814,respectively).

P=0.01). However standard care did not significantly cause infection than APG in the fourth week(RR= 0,20, 95%CI 0.02, 1.63; P=0.13), eighth week (RR= 1.00, 95%CI 0.07, 15.21; P=1.00), and on the twelfth week (RR= 0.52, 95%CI 0.19, 1.38; P=0.19) The totalresultsshow that standard care not statistically significant causes more infections compared to APG (RR= 0,41,95%CI0.26,0.64;P=0.41).ThissuggeststhatAPGisbetterthanstandardcare.

A funnel plot is a graphical representation of individual studies' precisionandeffectsize inameta-analysis.Inameta-analysisinvestigatingtheeffectivenessofAPGtreatmentsforDFU. The distribution of studies in the funnel plot appearshomogeneous,itsuggeststhatthereislittle or no publication bias and the studies are similar in terms of theirprecisionandeffectsize.This indicates that the studies are comparable and the results of the meta-analysis are robust. A homogenous funnel plot also suggests that theoverallestimateofthetreatmenteffectislikelyto bereliable.
The results showed that compared to standard/conventional treatment, APG was recommendedforsurfaceareareductionwithMDof-0.79,butnotstatisticallysignificant.
 Fig.7FunnelPlotInfectionAPG
3.7TheEffectofAutologousPlatele-richGelinSurfaceAreaReductionAmongElderly withDiabeticFootUlcerPatients
Fig.8ForestPlotSurfaceAreaReduction
Fig.7FunnelPlotInfectionAPG
3.7TheEffectofAutologousPlatele-richGelinSurfaceAreaReductionAmongElderly withDiabeticFootUlcerPatients
Fig.8ForestPlotSurfaceAreaReduction


The distribution of studies in the funnel plot may appear statistically heterogeneous; we includedonlytwostudiesonlengthofhospitaloutcome.Butinthisstudytherearenooutliers.

DISCUSSION
The number of DFUs among patients aged 45–64 years will similarly increase. More importantly, DFUs in middle-aged working adults cancauseunemployment,disability,andeven death in the prime of life, contributing to the increased family, social, and healthcare burdens.[28,29] The worst and most feared outcome of diabetic foot ulcers is Lower Extremity Amputation (LEA). Diabetes continues to be the leading cause of LEA worldwide. Afootulcer precedes and is responsible for 85% of allLEAinthediabeticpopulation.[30-32] Abouttwo-thirds ofamputationsareperformedinpatientsagedover60yrs.[33]
Therapeutic procedures to manage DFU are fundamentally based on adequate coverage of the wound, early infection treatment, and pressure relief, with a probability of healing being close to 60% in 1 year. Despite treatment, many chronic ulcers fail to heal or persist for months/years and/or recur after healing, requiring additional advanced wound care therapiesfor adequate healing. At 31 months of follow-up, diabetic foot ulcers have around a 40% of recurrence, and 12.3% were not healed at the end of the follow-up period.15–17 Moreover,at3 years there is evidence of a 10% to 20% rate of amputations.[34-37] Nowadays, the use of APG considerasinDFUtreatment.
APG is a concentration ofplateletsinplasmaandcontainsmanyplatelet-releasedgrowth factors. APG, as a secondary platelet-derived agent, is easy to obtain and prepare, and it has become an effective and adjunctive modality for chronic or acute wounds. It mainly includes platelets, leukocytes, fibrin, growth factors, and cytokines, and it has the function of anti-infection[38] and immunomodulatory.[39] APG treatment might shorten the length of hospital
stay, reduce the hospitalization cost to some extent, and not increase the incidence of adverse events.
Oursystematicreviewandmeta-analysisarethefirsttoassesstheefficacyofAPGonthe effects of DFU in elderly. We assessed a meta-analysis of healing duration, healing wound,and infection.OurstudyshowsthattheuseofAPGtreatmentismorerecommendedforthetreatment of DFU in terms of healing duration, healing wound, and infection. The risk of bias was low, except in two studies. Some studies reported high bias because in the final analyses, some patients in the control group withdrew from the study, and in some studies, all patients were treated after collecting appropriate information on the type oftreatment.Reportingbiaswaslow with all included data coming from trials with low risks. Another bias was not mentioned in all includedstudies;sotheincludedstudieshadunclearrisksorsomeconcerns.
According to the results of our study, the healing duration with APG is better than standard care (MD) of -11.32 (95% CI -16.87, - 5.76; P< 0.0001). This is contrary to research conducted by Li Y et al. The results showed that compared with standard care, APG could significantly shorten the healing time ofchronicDFU(P<0.00001).[40] Also,ameta-analysiswas conducted by Ding H et al. (p < 0.001) with wound repair within 4 weeks.[41] while for the healing wound, the results of our study show that APG has a better healing wound in patients with diabetic foot ulcers compared to standard care (RR=140, 95%CI 127,156; P < 000001) A similar studyconductedbyKnightonetal.reportedthattreatmentofpatientswithAPGachieved a 100% wound healing rate at an average of 10.6 weeks.[8] The data also show a similar indication for the results of the wound healing rate by Dingetal.,theaverageintheAPGgroup was 85.8%andrangedfrom68.4%to100%.Relatively,thecontrolgroupwas57.4%andranged from18.2%to75.0%.[41]
For the incidence of infection in DFU patients, TheresultsofourstudystatethatAGPis better for infection prevention than standard care (RR= 0,41, 95%CI 026, 064; P=041) This is similar to the findings of Sun SY et al. all types of antibiotics are not effective for healing wounds while APG combined with negative pressure treatment eliminates bacterial infections and closes wounds.[43] For treatment in the hospital, based on our study results APG treatment might shorten the length of hospital stay, and reducethehospitalizationcosttosomeextentwith diabetic foot ulcers (RR= -20.11, 95% CI -38.02,-2.20;P=0.03).Asimilarstudywasconducted by LiYetal.ThelengthofhospitalstaywassignificantlyshortenedintheAPGgroupcompared tothecontrolgroup.[44]
The causes of diabetic ulcers are unbalanced levels of metalloproteinase (MMP) and MMP inhibitors, exacerbated by the lack of oxygen and nutrients in the wound tissue due to diabetic neuropathy and vasculopathy. Lack of oxygen and nutrition intake will cause epithelial cells to be unable to express healing factors such asPlatelet-derivedGrowthFactor(PDGF)and Vascular Endothelial Growth Factor (VEGF). All of these things cause a decrease inthenormal healing response when a wound occurs.[21,45,46] APG is a biotechnology that is offered as an
additional therapy for diabetic ulcers. APG has been widelyusedinsurgerytoacceleratewound healing. APG contains cytokines, growth factors, chemokines, and a fibrin scaffold which are thought to induce a normal healing response. Alpha granules present inplateletscontaingrowth factors such as PDGF, VEGF, and transforming growth factor(TGFbeta3)whichstimulatecell proliferationanddifferentiation,resultingintheformationofnewcells.Thesegrowthfactorscan also trigger angiogenesis and provide nutrition to ischemic cells.[21,45-47] APG is also thought to act as a defense mechanism at the wound site by providing signals that attract macrophages. In addition,APGalsocontainsleukocytesinsmallnumbers.[48,49]
Currently, the key to diabetic foot ulcer management is education. Pharmacists, nurse practitioners, andprimarycareprovidersmusteducatepatientsaboutthedangersofsmokingand the need for better blood glucose control. In addition, patients with diabetes mellitus need tobe taught about wearing proper shoes, podiatriccare,andcontrollinghyperlipidemia.Notonlythat, diabetes educators and nurses must work together to educate patients and families about preventivemeasurestominimizemorbidityandimproveoutcomes.[50] Besides,studybyKhanet al. implied that telemedicine is a promising intervention to reduce the incidence of amputation amongDFUpatients.(RR:0.73,95%CI:0.54-1.00).[51]
The results of prior studies as well asthoseofourstudy,elucidatetheclinicalbenefitsof APG, mainly in terms of healing wounds, as adjunctive therapy for DFU. Improvements were achieved by this meta-analysis to assess the safety and efficacy of APG as a novel treatment of DFU compared with standard treatment. Therefore, we systematically reviewed the clinical efficacy and safety of APG as a breakthrough therapy in geriatric patients with diabetic foot ulcers. According to our study, APG significantly reduced the duration of healing of DFUs (P< 0.0001).WealsoevaluatedtheefficacyofAPGinincreasingthehealingwoundrates,whichwas not considered in previous works.[14,20] Our findings suggest that APG decreases the time to healingofDFUsregardlessofage(P<0.0001).
This meta-analysis has several limitations. Firstly, all trials haveahighorunclearriskof bias so thetrialsmaybeunderpowered.Secondly,theresultsandfindingsofthisstudymayhave been influenced by confounding factors, such as patient comorbidities, and severity of DFU. Third, the APG dose used by each patient did not have a fixed standard. Future studies should includemorehigh-qualitystudieswithcomprehensivedataandstandardizedAPGdoses.
CONCLUSION
In the current meta-analysis, APG significantly improves diabetic foot ulcer wound healing, shortens healing time, shortens hospital stay, and has antimicrobial effects. APG can also accelerate the conversion of bacterial cultures from positivetonegativeandhasaparticular antibacterial effect. It is safe if it doesnotchangepatients'bloodhematologyorbloodchemistry (albumin) significantly. APG was effective in DF wounds inWagner1andisstillrecommended for Wagner 2 and 3 but is not statistically significant. Furthermore, the use of APG in treating diabeticfootulcersamongelderlypatientsshouldbeconsideredbecauseofitsbenefits.
AcknowledgmentsandConflictofInterest
None. The authors did not receive funding to report. There was no conflict of interest between theauthors.
Reference
1. NCDRiskFactorCollaboration(NCD-RisC).“Worldwidetrendsindiabetessince1980: apooledanalysisof751population-basedstudieswith4.4millionparticipants.” Lancet (London, England). 2016;387(10027):1513-1530.doi:10.1016/S0140-6736(16)00618-8
2. Moradi-Lakeh,Maziaretal.“HighFastingPlasmaGlucose,Diabetes,andItsRisk FactorsintheEasternMediterraneanRegion,1990-2013:FindingsFromtheGlobal BurdenofDiseaseStudy2013.” Diabetes care. 2017;40(1):22-29. doi:10.2337/dc16-1075
3. Ogurtsova,Ketal.“IDFDiabetesAtlas:Globalestimatesfortheprevalenceofdiabetes for2015and2040.” Diabetes research and clinical practice. 2017;128:40-50. doi:10.1016/j.diabres.2017.03.024
4. OliverTI,MutluogluM.DiabeticFootUlcer.[Updated2022Aug8].In:StatPearls [Internet].TreasureIsland(FL):StatPearlsPublishing;2023Jan-
5. Sinclair,Alanetal.“Diabetesmellitusinolderpeople:positionstatementonbehalfof theInternationalAssociationofGerontologyandGeriatrics(IAGG),theEuropean DiabetesWorkingPartyforOlderPeople(EDWPOP),andtheInternationalTaskForce ofExpertsinDiabetes.” Journal of the American Medical Directors Association 2012;13(6):497-502.doi:10.1016/j.jamda.2012.04.012
6. AmericanDiabetesAssociation.“11.OlderAdults: Standards of Medical Care in Diabetes-2018.” Diabetes care. 2018;41(1):S119-S125.doi:10.2337/dc18-S011
7. Pataky,Z,andUVischer “Diabeticfootdiseaseintheelderly.” Diabetes & metabolism. 2007;33(1):S56-65.doi:10.1016/s1262-3636(07)80057-7
8. Knighton,DRetal.“Classificationandtreatmentofchronicnonhealingwounds. Successfultreatmentwithautologousplatelet-derivedwoundhealingfactors(PDWHF).” Annals of surgery, 1986;204(3):322-30.doi:10.1097/00000658-198609000-00011
9. Saldalamacchia, G et al. “A controlled study of the use of autologous plateletgelforthe treatment of diabetic foot ulcers.” Nutrition, metabolism, and cardiovascular diseases: NMCD. 2004;14(6):395-6.doi:10.1016/s0939-4753(04)80029-2
10.Fukawa, Taisuke et al. “Safety and Efficacy of Intra-articular Injection of Platelet-Rich Plasma in Patients With Ankle Osteoarthritis.” Foot & ankle international. 2017;38(6):596-604.doi:10.1177/1071100717700377
11. Suthar, Manish et al. “Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series.” Journal of biomedical science. 2017;24(1) doi:10.1186/s12929-017-0324-1
12.Martinez-Zapata, Maria José et al. “Autologous platelet-rich plasma for treating chronic wounds.” The Cochrane database of systematic reviews 2016;5 doi:10.1002/14651858.CD006899.pub3
13.Fletcher, J. “Differences between acute and chronic wounds and the role of wound bed preparation.” Nursing standard (Royal College of Nursing (Great Britain): 1987) 2008;22(24):62,64-8.doi:10.7748/ns2008.02.22.24.62.c6412
14.Rainys, Domantas et al. “Effectiveness of autologous platelet-rich plasma gel in the treatment of hard-to-heal leg ulcers: a randomised control trial.” Journal of wound care. 2019;28(10):658-667.
15.Higgins, Julian P T et al. “Measuring inconsistency in meta-analyses.” BMJ (Clinical researched.)2003;327(7414):557-60.doi:10.1136/bmj.327.7414.557
16.Duval, S, and R Tweedie. “Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis.” Biometrics 2000;56(2): 455-63. doi:10.1111/j.0006-341x.2000.00455.x
17.MalekpourAlamdari,Nasseretal.“Evaluationoftheefficacyofplatelet-richplasmaon healingofcleandiabeticfootulcers:ArandomizedclinicaltrialinTehran,Iran.” Diabetes & metabolic syndrome. 2021;15(2):621-626.
18.Gude,Warrenetal.“AurixGelIsanEffectiveInterventionforChronicDiabeticFoot Ulcers:APragmaticRandomizedControlledTrial.” Advances in skin & wound care. 2019;32(9):416-426.
19.Xie,Juanetal.“AutologousPlatelet-RichGelfortheTreatmentofDiabeticSinusTract Wounds:AClinicalStudy.” The Journal of surgical research. 2020;247:271-279.
20.Goda,AAetal.Platelet-richplasmaforthetreatmentofdiabeticfootulcer:a randomized,double-blindstudy TheEgyptianJournalofSurgery 2018;37(2):p178-184.
21.Ahmed,Marwaetal.“Platelet-RichPlasmafortheTreatmentofCleanDiabeticFoot Ulcers.” Annals of vascular surgery 2017;38:206-211.
22.Li,Weietal.“Autologousplatelet-richgelinthetreatmentofdiabeticfootulcers:A retrospectivestudy.” Medicine 2022;101(46):e31701.
23.LiuGYDX,SunY,WangMZ,GaoJ,GouJ.Effectofautologousplatelet-richgelonthe treatmentofdiabeticfootulcers.JournalofXi'anJiaotongUniversity(MedicalSciences). 2016;37:4.
24.ZhangLQD,SunYH.Clinicalobservationofautologousplateletrichgelinthe treatmentofdiabeticfootulcers.NingxiaMedJ.2016;38:3.
25.Li,Lanetal.“Autologousplatelet-richgelfortreatmentofdiabeticchronicrefractory cutaneousulcers:Aprospective,randomizedclinicaltrial.” Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2015;23(4):495-505.
26.QiKQCT,PiaoJL,ShangXL.Theapplicationofautologousplatelet-richgelinthe treatmentofdiabeticfootulcers.ChinJDiabetes.2014;22:4.
27.Serra,Raffaeleetal.“Applicationofplatelet-richgeltoenhancehealingof transmetatarsalamputationsindiabeticdysvascularpatients.” International wound journal. 2013;10(5):612-5.doi:10.1111/iwj.12052
28.PanJ,JiaW Early-onsetdiabetes:anepidemicinChina.FrontMed. 2018;12(6):624–633.doi:10.1007/s11684-018-0669-1.
29.KasiyaMM,Mang'andaGD,HeyesS,KachapilaR,KaduyaL,ChilambaJ,etal.The challengeofdiabeticfootcare:ReviewoftheliteratureandexperienceatQueen ElizabethCentralHospitalinBlantyre,Malawi.MalawiMedJ.2017;29(2):218–223.doi: 10.4314/mmj.v29i2.26.
30.PecoraroRE,ReiberGE,BurgessEM.Pathwaystodiabeticlimbamputation.Basisfor prevention.DiabetesCare.1990:13:513-21.
31.BoultonAJ.Thediabeticfoot:fromarttoscience.The18thCamilloGolgilecture. Diabetologia.2004;47:1343-53
32.ReiberGE,PecoraroRE,KoepsellTD.Riskfactorsforamputationinpatientswith diabetesmellitus.Acase-controlstudy.AnnInternMed.1992;117:97-105.
33.Unwin,N.“EpidemiologyoflowerextremityamputationincentresinEurope,North AmericaandEastAsia.” The British journal of surgery.2000;87(3):328-37. doi:10.1046/j.1365-2168.2000.01344.x
34.WinkleyK,StahlD,ChalderT,EdmondsME,IsmailK.Riskfactorsassociatedwith adverseoutcomesinapopulationbasedprospectivecohortstudyofpeoplewiththeirfirst diabeticfootulcer.JDiabetesComplications.2007;21:341–349.
35.MoulikPK,MtongaR,GillGV.Amputationandmortalityinnew-onsetdiabeticfoot ulcersstratifiedbyetiology.DiabetesCare.2003;26(2):491–494.
36.Martinez-ZapataMJ,Martí-CarvajalAJ,SolàI,etal.Autologousplateletrichplasmafor treatingchronicwounds.CochraneDatabaseSystRev 2012;10:Art.No.:CD006899.
37. DubskýM,JirkovskáA,BemR,etal.Riskfactorsforrecurrenceofdiabeticfootulcers: prospectivefollow-upanalysisintheEurodialesubgroup.IntWoundJ. 2013;10(5):555–561
38.MoojenDJ,EvertsPA,SchureRM,etal.Antimicrobialactivityofplatelet-leukocytegel againstStaphylococcusaureus.JOrthopRes.2008;26:404–10.
39.DissA,DohanDM,MouhyiJ,etal.OsteotomesinusfloorelevationusingChoukroun’s platelet-richfibrinasgraftingmaterial:a1-yearprospectivepilotstudywith microthreadedimplants.OralSurgOralMedOralPatholOralRadiolEndod. 2008;105:572–9.
40.LiY,GaoY,GaoY,ChenD,WangC,LiuG,etal.Autologousplatelet-richgeltreatment fordiabeticchroniccutaneousulcers:Ameta-analysisofrandomizedcontrolledtrials.J Diabetes.2019;11(5):359–69.
41.DingH,FuXL,MiaoWW,MaoXC,ZhanMQ,ChenHL.EfficacyofAutologous Platelet-RichGelforDiabeticFootWoundHealing:AMeta-Analysisof15Randomized ControlledTrials.AdvWoundCare.2019;8(5):195–207.
42.Dominijanni,Andreaetal.“Plateletgelinoralandmaxillofacialsurgery:asingle-centre experience.” Blood transfusion = Trasfusione del sangue. 2012;10(2):200-4. doi:10.2450/2012.0059-11
43.SunS,WangC,ChenD,CenS,LvX,WenX,etal.CombatingSuperbugWithout AntibioticonaPostamputationWoundinaPatientwithDiabeticFoot.IntJLowExtrem Wounds.2016;15:74-7.
44.Li,Yanetal.“Autologousplatelet-richgeltreatmentfordiabeticchroniccutaneous ulcers:Ameta-analysisofrandomizedcontrolledtrials.” Journal of diabetes vol.11,5 (2019):359-369.doi:10.1111/1753-0407.12850
45.TranTD-X,LePT-B,VanPhamP.Diabeticfootulcertreatmentbyactivatedplateletrich plasma:aclinicalstudy.BiomedResTher.2014;1(2):8.
46.BabaeiV,AfradiH,GohardaniHZ,NasseriF,AzarafzaM,TeimourianS.Management ofchronicdiabeticfootulcersusingplatelet-richplasma.JWoundCare.2017;26(12): 784–7.
47.KarimiR,AfsharM,SalimianM,SharifA,HidariyanM.TheEffectofPlateletRich PlasmaDressingonHealingDiabeticFootUlcers.NursMidwiferyStud,2016.5(3).
48.LacciK,DardikA.Platelet-RichPlasma:SupportforItsUseinWoundHealing.YaleJ BiolMed.2010;83:1–9.
49.HidajatD,MalikDA,BuditjahjonoS.Platelet-RichPlasmaDalamDermatologi. 2012;39(4):176–85.
50.OliverTI,MutluogluM.DiabeticFootUlcer.[Updated2022Aug8].In:StatPearls [Internet].TreasureIsland(FL):StatPearlsPublishing;2023Jan-.
51.Khan U, Ahmad K, Yadlapalli SS, Haseeb M, Kabir B, Khemani D, Ghulam Moosa P, Khan S. Effectiveness of Telemedicine for the Management of Foot Ulcers in People With Diabetes: A Meta-Analysis. Cureus. 2022 Oct 24;14(10):e30634. doi: 10.7759/cureus.30634.PMID:36439575;PMCID:PMC9683114.
PlosOne (((everything: Autologous Platelet Rich-Gel) OR everything:DiabeticFootUlcer)ANDeverything:"Elderly ")
Appendix Databasesearching Database Keywords Hits PubMed (AutologousPlatelet-RichGel)AND(DiabeticFootUlcer) AND(GeriatricORElderly)Filters:Fulltext,Randomized ControlledTrial,inthelast10years 33 Cochrane AutologousPlateletRich-GelinTitleAbstractKeyword ANDDiabeticFootUlcerinTitleAbstractKeywordAND GeriatricinTitleAbstractKeywordORElderlyinTitle AbstractKeywordinAllText-withPublicationYearfrom 2013to2023,withCochraneLibrarypublicationdate BetweenJan2013andJan2023,inTrials(Wordvariations havebeensearched) 360 Wiley AutologousPlateletRich-Gel"anywhereand"Diabetic FootUlcer"anywhereand"Elderly"anywhere Publication2013-2023,TypePublication:Journal 33 Google Scholar Autologous Platelet Rich-Gel " Diabetic Foot Ulcer " " Elderly" 552
187
Effectiveness and Safety of Specific Monoclonal Antibody Therapy from Agent
Donanemab against Alzheimer's Disease in The Elderly: A Systematic Review and Meta-Analysis of Randomized Control Studies
Aqsa Aufa Syauqi Sadana, Nabila Maitsa Kalyana Ayu Putri, Deby Tiraini Oktavia Silalahi
[Correspondence E-mail: aqsa.syauqi4@gmail.com]
Abstract
Introduction: Alzheimer’s Disease is one of the issues that affects the elderly frequently. A drug called donanemab is being developed to treat Alzheimer’s Disease by acting as an IG1 antibody that is specifically targeted at the N-terminal pyroglutamate Aβ epitope. This is because the buildup of amyloid-β (Aβ) peptides in the brain in the form of amyloid plaques is a critical step in the disease’ pathophysiology and is also a sign of the disease’s early stages, which is indicated to lead to degeneration of brain abilities.
Objective: The aim of this systematic review and meta-analysis is to assess the effectiveness and review the safety of donanemab by reducing amyloid plaque levels as a solution to Alzheimer’s Disease.
Methodology: The authors systematically search through PubMed, Scopus, and ScienceDirect databases. The literature search was conducted based on the PRISMA guideline. Risk of bias was assessed using the cochrane risk-of-bias tool for randomized trials (RoB 2) and statistical data synthesis testing was carried out using meta-analysis. Results were shown as mean difference (MD) and standard deviation (SD).
Result and Discussion: Donanemab was developed to eliminate amyloid plaques that already existed through microglial-mediated clearance. Donanemab’s method of action is supported by the fact that it targets deposited amyloid plaque rather than soluble forms of Aβ, as evidenced by the lack of change in plasma Aβ1-40 and Aβ1-42. In a dose-escalation trial, donanemab was well tolerated up to 10 mg/kg with amyloid deposition was significantly altered (40-50%).
Overall, no clinically significant changes were found in any of the safety assessments.
Conclusion: Seeing its effectiveness and safety, donanemab can reduce amyloid plaque levels.
Donanemab has the potential to treat Alzheimer's disease in the elderly.
Keywords: Donanemab, alzheimer’s disease, elderly, pyroglutamate Amyloid-β (Aβ)
Effectiveness and Safety of Specific Monoclonal Antibody Therapy from Agent
Donanemab against Alzheimer's Disease in The Elderly: A Systematic Review and Meta-Analysis of Randomized Control Studies
Pre-Conference Competition AMSC 2023

Authors:
Aqsa Aufa Syauqi Sadana
Nabila Maitsa Kalyana Ayu Putri
Deby Tiraini Oktavia Silalahi ASIAN
2023
MEDICAL STUDENTS’ ASSOCIATION
Effectiveness and Safety of Specific Monoclonal Antibody Therapy from Agent
Donanemab against Alzheimer's Disease in The Elderly: A Systematic Review and Meta-Analysis of Randomized Control Studies
Aqsa Aufa Syauqi Sadana, Nabila Maitsa Kalyana Ayu Putri, Deby Tiraini Oktavia Silalahi
[Correspondence E-mail: aqsa.syauqi4@gmail.com]
Abstract
Introduction: Alzheimer’s Disease is one of the issues that affects the elderly frequently. A drug called donanemab is being developed to treat Alzheimer’s Disease by acting as an IG1 antibody that is specifically targeted at the N-terminal pyroglutamate Aβ epitope. This is because the buildup of amyloid-β (Aβ) peptides in the brain in the form of amyloid plaques is a critical step in the disease’ pathophysiology and is also a sign of the disease’s early stages, which is indicated to lead to degeneration of brain abilities.
Objective: The aim of this systematic review and meta-analysis is to assess the effectiveness and review the safety of donanemab by reducing amyloid plaque levels as a solution to Alzheimer’s Disease.
Methodology: The authors systematically search through PubMed, Scopus, and ScienceDirect databases. The literature search was conducted based on the PRISMA guideline. Risk of bias was assessed using the cochrane risk-of-bias tool for randomized trials (RoB 2) and statistical data synthesis testing was carried out using meta-analysis. Results were shown as mean difference (MD) and standard deviation (SD).
Result and Discussion: Donanemab was developed to eliminate amyloid plaques that already existed through microglial-mediated clearance. Donanemab’s method of action is supported by the fact that it targets deposited amyloid plaque rather than soluble forms of Aβ, as evidenced by the lack of change in plasma Aβ1-40 and Aβ1-42. In a dose-escalation trial, donanemab was well tolerated up to 10 mg/kg with amyloid deposition was significantly altered (40-50%). Overall, no clinically significant changes were found in any of the safety assessments.
Conclusion: Seeing its effectiveness and safety, donanemab can reduce amyloid plaque levels.
Donanemab has the potential to treat Alzheimer's disease in the elderly.
Keywords: Donanemab, alzheimer’s disease, elderly, pyroglutamate Amyloid-β (Aβ)
Introduction
In the elderly, health problems are closely related to the degeneration process. It is a process that cannot be avoided and it is likely to attack the ability and health of the brain. From the medical aspect, Alzheimer’s Disease is one of the problems in the elderly that often occurs and is no less complicated than other chronic diseases. Alzheimer’s Disease affects memory, behavior, language skills, cognitive skills, and thinking skills that affect a person’s ability to perform daily activities[1]. This degenerative disease occurs because neurons in the brain are damaged and no longer function normally, affecting cognitive function. This makes Alzheimer’s Disease the most common disease among neurodegenerative disorders[1, 2]
Alzheimer’s Disease has become a serious problem that must be faced by many countries, including Indonesia.This is dueto theincreasing prevalenceofdegenerative diseases and increasing life expectancy in many parts of the world. In terms of prevalence, the United States population aged 65 years has a percentage that doubles every 5 years of age. In addition, about 10% of all people over the age of 70 will experience significant memory loss, more than half of which is due to Alzheimer’s Disease[3]. The incidence of Alzheimer’s Disease continues to show an increase, which is strongly related to the age factor[4] .
Currently, there are two types of drugs that have been approved and marketed to treat this disease. These include therapies based on cholinesterase inhibition (donepezil, galantamine, and rivastigmine) and glutamate receptor antagonist therapy (memantine)[5] These treatments are considered ineffective because they only work as symptomatic treatments and have limited effect on Alzheimer’s patients[6] . Given the high and increasing prevalence of Alzheimer’s disease, these drugs are far from meeting the needs of the medical system. In clinicaltrials.gov shows the development of Alzheimer’s drugs over the past 5 years and there are 16 promising treatment candidates that have entered late-stage trials and analyzed their impact on the clinical treatment of Alzheimer's Disease in China[6, 7]. These therapies include disease modification and symptomatic therapy. One of the therapies under development is donanemab which works as a specific monoclonal antibody[8]
Donanemab is a treatment that is being developed in the treatment of Alzheimer’s Disease by working as an IgG1 antibody specifically directed at the N-terminal pyroglutamate Aβ epitope[8]. This mechanism is considered to have good effectiveness in treating alzheimer's disease, considering that the deposition of amyloid-β (Aβ) peptides in the form of amyloid plaques in the brain is very important for pathophysiology and is an early event in Alzheimer’s Disease which is indicated to lead to degeneration of brain abilities[9, 10]. However, this drug is still under development and future research results.
Alzheimer’s Disease through the Amyloid-β (Aβ) Pyroglutamate mechanism has been widely claimed as a successful treatment to improve this condition[10]. However, it is necessary to assess the efficacy of such interventions in actual field settings in the healthcare world. Currently, thereareno in-depth studies ontheeffectiveness, safety, andmeta-analysis assessing specific monoclonal antibody therapy for pyroglutamate Amyloid-β (Aβ) from agent donanemab against Alzheimer’s Disease in the Elderly. Therefore, the current study was conducted to analyze the efficacy and safety of donanemab as a treatment for Alzheimer’s Disease in the elderly.
Material and Method
In this systematic review, the authors conducted a literature search based on the PRISMAguideline.Aninitialscreening process was carriedout using theBooleanmethodwith the “AND” operation to combine keywords in the database, and applied to three databases: PubMed, Scopus, and ScienceDirect.
Eligibility criteria
Criterias that are considered eligible for this systematic review are: Original research article or research reports using human study with randomized controlled trial design were included in this study. Unavailable full-text articles, review, report case, meta-analysis, nonenglish, irrelevant topics were also excluded.
Outcome Measure
The outcome measure assessed in this systematic review was the amyloid plaque reduction rate of donanemab. In Alzheimer’s Disease, donanemab works as an IG1 antibody that is specifically directed at the N-terminal pyroglutamate epitope of Aβ. The deposition of amyloid-β (Aβ) peptides in the form of amyloid plaques in the brain is an early event in Alzheimer’s Disease which is indicated to cause degeneration of brain abilities. Measurement of amyloid plaques is measured by SUVr (Standardized uptake value ratio).
Data Sources and Search
This study conducted a search using the Boolean operator and mesh on each database, the results were 145. Then screening was carried out using inclusion and exclusion criteria based on the title, abstract, and keywords. The keywords used were using Boolean operator and mesh. Keywords used in each database can be seen in Table 1. The studies are stored in the authors’ library using mendeley group reference manager.
Database
Table 1.
Keyword Used in Literature Searching
Keywords
PubMed ((Alzheimer's Diseases[MeSH Major Topic]) OR (Alzheimer Dementia [Title/Abstract])) OR (Senile Dementia [Title/Abstract])) AND (Donanemab[MeSH Major Topic])
Science Direct (“Alzheimer's Diseases” OR “Alzheimer Dementia” OR “Senile Dementia”) AND (“Donanemab”)
Scopus (“Alzheimer's Diseases” OR “Alzheimer Dementia” OR “Senile Dementia”) AND (“Donanemab”)
Index Test and Reference Standard
The study included evaluating the amyloid's reduction plaque levels from donanemab that presented the data with mean difference and standard deviation. The effect of amyloid’s reduction plaque levels from donanemab is assessed from the Randomized Control Trials (RCTs) in the journal obtained as a reference standard.
Study Process
The keywords that have been obtained are written in Table 1. The studies used in this study were Randomized Control Trials (RCTs). Data from on-RCTs were excluded from the databases using article type filters. The 3 databases obtained were then combined into one and filtered by three independent reviewers (AASS, NMKAP, DTOS) through title, year of publication, and DOIs for duplicate removal. Studies were then reviewed and filtered by an abstract study selection process that was recorded in the PRISMA flow chart. Studies after final screening are extracted for the relevant data and recorded in Google Spreadsheet. The recorded datas were: first author, year, country, population, intervention, and outcome that consist of the effect of amyloid’s reduction plaque levels from Donanemab. Each study included in this study was assessed by three independent reviewers according to the Cochrane Risk of Bias 2.0 tool for randomized trials (RoB 2) consisting of 5 domains.
Synthesis Method (Meta-Analysis)
All statistical tests for this meta-analysis were conducted using Review Manager (RevMan) v5.4 (Cochrane Collaboration, UK). Mean Difference (MD) and Standard Deviation (SD) with the Confidence Interval (CI) of 95% were calculated in this review. Random-effect model (REM) was used for study considered heterogeneous (which were indicated by I2 > 40%). Otherwise, we used the Fixed Effect Model (FEM).
Result and Discussion
Study selection
After conducting literature searching from 3 databases which are PubMed, Scopus, and ScienceDirect, 145 studies were generated. Automation tools from each database were used to exclude non-RCT studies and resulted in 64 articles being excluded. Afterward, 23 duplicate study articles were removed. Subsequently, authors assessed all of the remaining articles from the title and abstract for irrelevance to the topic, resulting in 42 articles excluded. 16 articles were then retrieved for the full text availability. Lastly, the author assessed eligibility for all the studies and agreed to exclude 3 studies because of an uncontrolled study group, and 2 studies with no outcome interest were also excluded. This review included 4 studies to be in the systematic review and meta-analysis. Our study selection process is presented in the PRISMA diagram flow chart in Figure 1
 Figure 1. PRISMA 2020 Flow Diagram.
Figure 1. PRISMA 2020 Flow Diagram.
Study characteristics

From 4 studies included in this review, the total participants are 653 participants. There were 2 studies conducted in the United States and Japan. The other 2 were conducted in the United States and Canada. All of the studies (n = 4) were subject and investigator blind, randomized, placebo controlled, and parallel groups. Nevertheless, each of these studies have its own characteristics. There were 2 studies using multiple doses and the rest using TRAILBLAZER-ALZ design.

Risk of bias in studies
The risk of bias was summarized in Figure 2. The quality of each study was carefully analyzed by using the Cochrane risk-of-bias tool for randomized trials (RoB 2) l. There were 2 studies showing high risk of bias (Mintun A, et al. 2021; Shcherbinin S, et al. 2021) and 2 studies showed some concern (Lowe S, et al. 2021; Lowe S, et al. 2021).
Figure 2. Risk of Bias Assessment Result.
Table 2. Characteristic of Studies
Author, Year Lowe S, et al. 2021[11] Mintun A, et al. 2021[12] Shcherbinin S, et al. 2021[13] Lowe S, et al. 2021 [14] Country United States and Japan United States and Canada United States and Canada United States and Japan Population Sex Male and Female Randomized Randomized Men or infertile women Mean Age 21 - 89 years 75 years 60 - 85 years ≥50 years Number of Samples 63 257 272 61 Intervention Name of intervention 10 mg/kg (Q2W) 1400 mg thereafter 1400mg for up to 76 weeks 10 mg/kg (Q2W) Length of intervention 72 weeks 76 weeks 76 weeks 72 weeks Comparison 10 mg/kg 700 mg for the first three doses 700 mg for the first 3 doses 10 mg/kg Outcome Reduction of Amyloid Plaque Levels Interventi on −44.4±14.2 −85.06±3.87 92.8±28.7 -55.8±9.51 Control -0.26±0.12 −67.83±3.16 117.4±33.9 -16.5±11.22 p-value p < 0.0002 p=0.04 P < 0.001 p=0.001
Meta-Analysis
Mean Difference (MD) and Standard Deviation (SD) with the Confidence Interval (CI) of 95% were then calculated in this review. The data then processed into pooled standardized mean difference forest plot form. Our study assessed extractable quantitative data and group them into 1 outcome which is to reduce amyloid deposits in AD . The forest plot of the metaanalysis can be seen in Figure 3 and 5.


Discussion
Mechanism of Donanemab as Specific Monoclonal Antibody Therapy for Pyroglutamate
Amyloid-β (Aβ)
Donanemab, a biologic that binds to brain-deposited amyloid plaques, is being investigated by Eli Lilly and Company for the treatment of early Alzheimer’s Disease in all genotypes[12]. Donanemab was developed to eliminate amyloid plaques that already existed through microglial-mediated clearance[11]. It specifically targets an N-terminal pyroglutamate
Aβ epitope that is only present in active plaques. Donanemab’s method of action is supported by the fact that it targets deposited amyloid plaque rather than soluble forms of Aβ, as
Figure 3. Reduction of amyloid deposits in AD
Figure 4. Funnel Plot for Assessing the Level of Publication Bias
evidenced by the lack of change in plasma Aβ1-40 and Aβ1-42[11]. It is epitope specific, has no known clinical effects, and does not bind to other Aβ species, neurotransmitters, or their receptors off target. The MOA of donanemab focuses on targeting deposited plaques to remove any amyloid thathas alreadyformed ratherthanjust preventingplaquedeposition orgrowth[15]
Effectiveness and safety of Donanemab in treating Alzheimer's disease in the elderly Lilly undertook TRAILBLAZER-ALZ, a Phase II trial (NCT03367403) in early symptomatic AD to evaluate the safety, tolerability, and efficacy of donanemab[16] Donanemab’s chemical composition has not yet been made public. In a dose-escalation trial, donanemab was well tolerated up to 10 mg/kg. Additionally, amyloid deposition was significantly altered (40-50%) even by 10 mg/kg[11]. The mean terminal elimination half-life was shown to be prolonged by increasing the dose[17]. Therefore, donanemab is considered effective in treating Alzheimer’s Disease, especially in the elderly. However, this drug is still under development and future research results.
The frequency of serious adverse events was similar between the donanemab group and the placebo group. Other safety evaluations, such as electrocardiograms, neurological examinations, safety laboratories, and vital signs, did not reveal any clinically significant changes[12, 18]. Overall, no clinically significant changes were found in any of the safety assessments. It is interesting to note that 90% of study participants generated anti-drug antibodies, but it is unclear what this means for the safety and effectiveness of donanemab[19]
Seeing the effectiveness and temporary safety, donanemab has good potential as a Specific Monoclonal Antibody Therapy for Pyroglutamate Amyloid-β (Aβ) for the treatment of Alzheimer’s Disease in the elderly, although it is still necessary to wait for further developments and research results related to this medicine until its completion which is estimated in 2027.
Strength and Limitations
This review is the first systematic review and meta-analysis to assess the effectiveness and review the safety of donanemab as a solution to Alzheimer’s Disease, especially in the elderly. This systematic review assessed the effect of reducing amyloid plaques levels in patients with Alzheimer’s Disease with the agent donanemab which is developed by Eli Lilly and Company. All studies included Randomized Controlled Trials (RCTs) with significant results in several aspects, such as eliminating amyloid plaques level.
Nonetheless this study is not without limitation. The study that was included has a high and moderate risk of bias. The sample size of the included study also was limited in size.
Through the results of meta-analysis, it was seen that the results were heterogeneous but significant. The limited references in this study is one of the factors. In addition, donanemab is a relatively new Alzheimer’s Disease drug candidate. Unfinished research is also a factor in the lack of reference sources, so more reference sources and further studies are needed on this matter.
Conclusion and Recommendation
This systematic review and meta-analysis revealed that the use of donanemab can reduce amyloid plaque levels in Alzheimer’s patients. Seeing its effectiveness and safety, donanemab can be used as a candidate drug for specific treatment of Alzheimer’s Disease. We recommend further randomized control study with a larger sample size to be conducted to observe more about the efficacy of donanemab as a drug of Alzheimer’s Disease.
Conflict of Interest
All authors declared there are no competing interests in this study.
References
1. Ferrari C, Sorbi S. The complexity of Alzheimer’s disease: an evolving puzzle. Physiol Rev. 2021;101(3):1047–81.
2. Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–39.
3. Head E, Lott IT, Wilcock DM, Lemere CA. Aging in Down syndrome and the development of Alzheimer’s disease neuropathology. Curr Alzhei- mer Res. 2016;13(1):18–29.
4. Arvanitakis Z, Shah CR, Bennett AD. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589–99.
5. Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer’s disease. J Neurochem. 1995;65:1431–44
6. Carlson C, Siemers E, Hake A, et al. Amyloid‐related imaging abnormalities from trials of solanezumab for Alzheimer's disease. Alzheimers Dement 2016;2:75‐85.
7. Panza F, Lozupone M, Seripa D, Imbimbo BP. Amyloid-β immuno-therapy for Alzheimer's disease: is it now a long shot? Ann Neurol. 2019;85:303–15.
8. Irizarry MC, Sims JR, Lowe SL, et al. O4-08-06: Safety, pharmacokinetics (PK), and florbetapir F-18 positron emission tomography (PET) after multiple dose administration of LY3002813, a β-amyloid plaque-specific antibody, in Alzheimer’s disease (AD). Alzheimers Dement 2016;12:P352-P353.
9. Navitsky M, Joshi AD, Kennedy I, et al. Standardization of amyloid quantitation with florbetapir standardized uptake value ratios to the Centiloid scale. Alzheimers Dement 2018;14(12):1565‐1571.
10. Wessels AM, Siemers ER, Yu P, Andersen SW, Holdridge KC, Sims JR, Sundell K, Stern Y, Rentz DM, Dubois B, Jones RW, Cummings J, Aisen PS. A Combined Measure of Cognition and Function for Clinical Trials: The Integrated Alzheimer's Disease Rating Scale (iADRS). J Prev Alzheimers Dis. 2015 Dec 1;2(4):227-241
11. Lowe SL, Willis BA, Hawdon A, Natanegara F, Chua L, Foster J, Shcherbinin S, Ardayfio P, Sims JR. Donanemab (LY3002813) dose-escalation study in Alzheimer's disease.AlzheimersDement (NY).2021 Feb 14;7(1):e12112.doi: 10.1002/trc2.12112. PMID: 33614890; PMCID: PMC7882532.
12. Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, Shcherbinin S, Sparks J, Sims JR, Brys M, Apostolova LG, Salloway SP, Skovronsky DM. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021 May 6;384(18):1691-1704.
13. Shcherbinin S, Evans CD, Lu M, Andersen SW, Pontecorvo MJ, Willis BA, Gueorguieva I, Hauck PM, Brooks DA, Mintun MA, Sims JR. Association of Amyloid Reduction After Donanemab Treatment With Tau Pathology and Clinical Outcomes: The TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022 Oct 1;79(10):1015-1024.
14. Lowe SL, Duggan Evans C, Shcherbinin S, Cheng YJ, Willis BA, Gueorguieva I, Lo AC, Fleisher AS, Dage JL, Ardayfio P, Aguiar G, Ishibai M, Takaichi G, Chua L, Mullins G,Sims JR. Donanemab (LY3002813) Phase 1b Study in Alzheimer's Disease: Rapid and Sustained Reduction of Brain Amyloid Measured by Florbetapir F18 Imaging. J Prev Alzheimers Dis. 2021;8(4):414-424.
15. Bouter Y, Liekefeld H, Pichlo S, Westhoff AC, Fenn L, Bakrania P, Bayer TA. Donanemab detects a minor fraction of amyloid-β plaques in post-mortem brain tissue of patients with Alzheimer's disease and Down syndrome. Acta Neuropathol. 2022 May;143(5):601-603.
16. Busche MA, Hyman BT. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci 2020;23:1183-1193.
17. Pontecorvo MJ, Lu M, Burnham SC, Schade AE, Dage JL, Shcherbinin S, Collins EC, Sims JR, Mintun MA. Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022 Dec 1;79(12):1250-1259.
18. Alexander GC, Emerson S, Kesselheim AS. Evaluation of aducanumab for Alzheimer disease: scientifc evidence and regulatory review involving efcacy, safety, and futility. JAMA. 2021;325(17):1717–8.
19. Fleisher AS, Lowe SL, Liu P, et al. O1-09-01: Significant and sustained florbetapir F18 uptake reduction in patients with symptomatic Alzheimer’s disease with LY3002813, a β-amyloid plaque-specific antibody. Alzheimers Dement 2018;14:P239-P240.
AssessingPainandRecoveryofKneeOsteoarthritisPatientwithPlateletRichPlasma:
ASystematicReviewandMeta-Analysis
RifatRasendriyaRasyid1,DarrenDavidRampengan1,DeboraRiadiAlimSuprapto1,Samuel PartogiNababan1
1AMSA-UniversitasSamRatulangi
ABSTRACT
Introduction: Knee osteoarthritis(OA)isadegenerativejointdiseaseofthekneethatcauses the loss ofarticularcartilageandhigherfrictionduringmobility Olderadultsareatparticular risk for knee OA due to aging. Current standard pharmacological intervention has adverse effects on organ systems, especially considering that a majority of older adults have comorbidities. PRP is an autologousplasmathatcontainsplateletamountshigherthanthatin peripheral blood and has seen growingnumbersofRCTsforuseinkneeOA.However,there isstillalackofconsensusonitseffectivenesscomparedtootherinterventions.
Objective: This meta-analysis and systematic review primarily aimed to investigate the significance of the effectivity of PRP inkneeosteoarthritispatientstowardsVisualAnalogue Scale (VAS), International Knee Documentation Committee Subjective Knee Evaluation Form (IKDC), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)scoresaswellashighlightingitsadverseeffects.
Methods and Materials: This meta-analysis and systematic review were conducted according to the Cochrane Handbook for Systematic Reviews of Interventions 6.3 and were carried out under thePRISMAchecklist.PubMed,CochraneLibrary,EMBASE,Scopus,and ProQuest databases were used. The risk for bias in the included papers was evaluated using the Revised Tool RoB 2.0 and statistical analysis was performed using RevMan version 5.4 softwareforsystematicreviewandmeta-analysis.
Results and Discussion: Eleven RCTs yielding 1884 participants were assessed to evaluate the efficacy of platelet-rich plasma (PRP) for the treatment of knee OA. A reduction in the odds of patientswithkneeOAfrombaselinetoendpointswasobserved(OR=0.48;95%CI; 0.27 to 0.88, p = 0.02). High and significant heterogeneity was found (I2 = 94, p = < 0.00001). PRP indicated a significant and greater reduction in VAS pain undergone by the patients (SMD = -0.75, [95% CI; -1.48, -0.02], I2 = 94%, p = 0.04); While PRP had no significant improvement in IKDC subjective score patients (SMD = 0.86, [95% CI; -0.87, 2.60], I2 = 98%, p = < 0.00001) and indicated better improvement in WOMAC global function (SMD = -1.50, [95% CI; -2.64, -0.35]) andasignificantreductioninWOMACpain (SMD = -1.02, [95% CI; -1.98], -0.06, p = 0.04). Therefore, there is a significant improvement in function and reduction in pain. MildadverseeffectswerefoundinKneeOA patientsafterinjectionsweregiven.
Conclusion: PRP was found to improve the VAS score, WOMAC function score, and WOMAC pain score but PRP did not improve the IKDC score. Only mild adverse effects werefound.FuturestudiesshouldassesstheapplicabilityofPRPinresource-limitedsettings.
Keywords: Knee Osteoarthritis, Platelet-Rich Plasma, Pain and Recovery
AssessingPainandRecoveryofKneeOsteoarthritisPatientwithPlateletRichPlasma:
ASystematicReviewandMeta-Analysis
RifatRasendriyaRasyid1,DarrenDavidRampengan1,DeboraRiadiAlimSuprapto1,Samuel
1
INTRODUCTION
PartogiNababan1
AMSA-UniversitasSamRatulangi
Knee osteoarthritis is a degenerative joint disease of the knee that progressively causes the loss of articular cartilage and higher friction during mobility Multiple factors contribute to the etiology of osteoarthritis with chronic low-degree inflammation caused by proinflammatory mediators due to aging, pro-catabolic mediators, and mechanical stress being key factors. All these factors lead to the structural degeneration of the joint, mainly through the loss of the extracellular matrix. Structural joint degeneration caused by the factors above leads to pain and loss of functionwhicharethepredominantcomplaintofOA. Furthermore,asthediseaseprogresses,painandlossoffunctionalsoprogressivelyworsen.
Older adults are at particular risk for knee osteoarthritis due to aging causing chronic low-degreeinflammation.KneeOAincidenceincreasessharplybetweentheageof50and75 before it starts declining at the age of 80. In Indonesia, considering that the older adult population in 2020 is 283,3% higher than in 1994, assuming that the prevalence of knee osteoarthritisinIndonesiaisrisingisdefinitelynotoutoflogicalthinking.
The first-line treatment approach for knee OA would bepatienteducation,physicalexercise, and weight loss (for overweight or obese individuals). Standardpharmacologicinterventions for knee OA most commonly include oral NSAIDs or topical NSAIDs. However, when it comes to older adults, pharmacological intervention should be used only with the best attention as most pharmacological intervention has adverse effects on organ systems, especially considering that a majority of older adults have comorbidities. Hence, there has been a search for other interventional therapies. Recently, intra-articular injection of hyaluronic acid has also been shown to help knee osteoarthritis by providing lubrication, shock-absorbing properties, and anti-inflammatory properties. However, intra-articular hyaluronic acid injectionisexpensive.Therefore,thereisstillameetingdemandthatisyetto be fulfilled for healthcare professionals treating individuals with knee OA to provide effectivemanagement.
Platelets contain granules that are filled with growth factors, such as platelet-derivedgrowth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF). These growth factors prevent further degradation of the joint as well as potentially recovering structural damage to the joint. However, PRP also contains inflammatory mediators, matrix metalloproteins, and leukocytes that contribute to inflammation and breakdown of the extracellular matrix of the joint which could lead to worsening joint degradation. Despite promising research, encouraging results, and a significantly growing number of reported clinical trials with regard to platelet-rich plasma (PRP) application in knee osteoarthritis (OA), there is still a lack of consensus on its effectiveness compared to
other interventions. Therefore, this meta-analysis andsystematicreviewwasprimarilyaimed to confirm its efficacy and to investigate the significance of all scores that measurepainand global function and effectivity of PRP to assess pain and recovery of knee osteoarthritis patients which is measured by Visual Analogue Scale (VAS), International Knee Documentation Committee Subjective Knee Evaluation Form (IKDC), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). This systematic review also highlights the adverse effects of PRP to assess its feasibility in reducing pain and mobility improvement. Hopefully, thisreviewcouldbepracticallyusedasaguideintheuseofPRPin KneeOAPatients.
METHODSANDMATERIAL
StudyDesign
This Meta-Analysis and Systematic Review were conducted according to the Cochrane Handbook for Systematic Reviews of Interventions 6.3 and were carried out under the PRISMAchecklist.
SearchStrategy
This meta-analysis was performed using the PRISMA framework (Appendix 1). PubMed, Cochrane Library, EMBASE, Scopus, and ProQuest databases were used with the following boolean operator keywords: “Knee Osteoarthritis” AND “platelet rich plasma”. Keywords used were in line with the MeSH (Medical Subject Headings) and were adjusted for each database’ssearchmanager
StudyEligibilityCriteria
Before conducting the literature search, inclusion criteria were applied to the PICOS Framework (Appendix 2) and included (1) Patient: Adult or Elderly with Knee Osteoarthritis; (2) Intervention: Platelet Rich Plasma; (3) Comparison: Standard Care; (4) Outcome: Measured by WOMAC and/or VAS and/or IKDC score; (5) Study: RCT The exclusion criteria, however, are (1) irretrievable full-text articles; (2) non-English literature; (3) non-human clinical trials. The title and abstracts of the paper were reviewed by3(three) independent reviewers (RRR, DRAS, SPN), and any discrepancies were discussed with a fourthreviewer(DDR)toreachaconsensus.
DataExtraction
Data extraction was conducted using a predeterminedoutcomesheetintabularformwiththe following information: (1) author and year of publication; (2) study location; (3) study population (sample size and sample age mean); (4) intervention: name, mechanism, and adverse events; and (5) study outcomes: VAS, WOMAC, IKDC, and KOOS. Three Reviewers (RRR, DRAS, SPN) qualitatively evaluate the studies, while another author (DDR)double-checkedthedatareceivedwhileconductingthestatisticalanalysis.
QualityAssessmentandPublicationBias
TheriskforbiasinthefinalpapersthatwereanalyzedwereevaluatedusingtheRevisedTool RoB2.0,whichconsistsoffivedomainareasforinitiativestudies,andwasusedtoassessthe potentialforbiasinthefinalstudiesthatwereincludedintheanalysis.Theresultswillbe thenrecordedinthedomainfilebias(.xlsx).TheFilewillbethenuploadedtotheROBVIS websiteinordertofacilitateaccuratevisualizationofthefinalresults.Qualityassessment wasperformedbythreereviewersindependentlyanddiscrepancieswerediscussedand resolvedbetweenreviewerstoreachaconsensus.Theevaluationofstudyqualityisshownin Appendix3
QuantitativeDataAnalysis
Statistical Analysis was performed using RevManversion5.4softwareforsystematicreview and meta-analysis. For the purpose of this analysis, the estimated mean and standard deviation reported for each outcome in each study was extracted into a pre-defined Sheet format (.xlsx). Where the study reported the pre-test or baseline estimates and post-test or endpoint estimates, the change in mean and the change in standard deviation were recorded. Where the summary statistics were not recorded but the number of patients treated in each group was recorded, we extracted the number of patients in each groupandthetotalnumber of eligible patients in each study.Acontinuousmeta-analysisofarandomeffectmodelusing standardized mean difference as the effect size was performed for studies that recorded the mean and standard deviation. A binary meta-analysis of a random effect model using odd ratios was performed for a study that recorded the number of patients and total eligible patients. Heterogeneity was measured using (I2) and publication bias was examined using a funnelplot.
RESULTS
SearchResultsandStudyCharacteristics
A total of eleven RCTs were conducted for quantitative analysis, yielding a total of 1884 participants who were assessed using platelet-rich plasma (PRP) as the intervention and conventional therapy as the control. All RCTs were conducted for qualitative analysis. The average mean age is 65.2 years with females having the highest percentage of gender in all trials. All studies used an intra-articular injection of Platelet Rich Plasma as an intervention except Lamo Espinosa et al., (2020) which added bone marrowmesenchymestemcells.The control groups werenotthesameacrossalltrialsbuthyaluronicacid(HA)followedbySham Saline and Bone Marrow cells were the most used control groups. The primary outcome measure was the VAS pain reduction while the secondary outcome measure was the WOMACglobalfunctionandpain.Appendix4includesthestudy’scharacteristics.
StudyOutcome-Meta-analysisoftheEfficacyofPRPintheTreatmentofKneeOA
Eleven trials were identified to evaluate the efficacy of PRP for the treatment of Knee OA. TheresultsshowareductionintheOddsofpatientswithKneeOAaftertheadministrationof PRP from baseline toendpoints(OR=0.48;95%CI;0.27to0.88,p–value=0.02).Highand significant heterogeneity was found (I2 = 94, p < 0.00001) (Appendix 5). The funnel plot
indicates no evidence of publication in all trialsasallpointsareinsidethefunnel(Appendix 6).
StudyOutcome-VASPainScore
Five trials were identifiedintheanalysisoftheVASscorethatmeasurespaininpatientswith OA after the administration of PRP at baseline and endpoint, the results show that PRP indicated a significant and greater reduction in VAS pain undergone by the patients(SMD= -0.75, 95% CI; -1.48, -0.02, p–value = 0.04). Moderately significant heterogeneity was recorded among the included trials (I2 = 94%, p = 0.04) (Appendix 7). [Funnel plot VAS] (Appendix8)
StudyOutcome-IKDCSubjectiveScore
PRP suggested no significant improvement in IKDC subjective score in patients with OA treated with PRP (SMD = 0.86, 95% CI; -0.87, 2.60, p–value = 0.33). High and significant heterogeneity was recorded (I2 = 98%, p–value < 0.00001) (Appendix 9). The Funnel plot showsnoevidenceofpublicationbiasduetoitssymmetry(Appendix10).
StudyOutcome-WOMACglobalFunctionScoreandWOMACpainscore PRP indicated better improvement in WOMAC global function (SMD = -1.50, 95% CI; -2.64, -0.35, p–value = 0.01) compared to the control group. PRP shows a significant reduction in WOMACpain(SMD=-1.02,95%CI;-1.98,-0.06,p–value=0.04).(Appendix 11)
DISCUSSION
Effectivity of Platelet-Rich Plasma in Knee Osteoarthritis
Mechanism of Platelet-Rich Plasma in Knee Osteoarthritis
Knee osteoarthritis is caused by a combination of multiple factors including mainly chronic proinflammatory mediators, pro-catabolic mediators, and mechanical stress which leads to structural degeneration of the joint. PRP is an autologous plasma that contains platelet amounts higher than that in peripheralbloodandcouldhelppreventfurtherdegenerationand even potentially reverse degeneration. Platelets contain granules that are filled with growth factors, such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF). These growth factorsaswellasothersubstances secreted by platelets have been shown to induce endothelial proliferation, induce angiogenesis, inhibit lymphocyte and macrophage proliferation, inhibit apoptosis, and many more mechanisms resulting in potentially recovering properties towards osteoarthritis. However, PRP also contains inflammatory mediators,matrixmetalloproteins,andleukocytes that contribute to inflammation and breakdown of the extracellular matrix of the jointwhich couldleadtoworseningjointdegradation.
Efficacy of Platelet-Rich Plasma in Knee Osteoarthritis
The presentmeta-analysisisthefirstrecentresearchtoinvestigatetheefficacyofPRPandits clinical performance. Our results showed that PRP is abettertherapeuticforthetreatmentof Knee OA. Our findings showed that PRP improved the VAS score, WOMAC function, and WOMAC pain but did not significantly improve the IKDC score. The findings of this study are slightly consistent with a previous meta-analysis that compared the clinical outcomes of Leukocyte – poor PRP and Leukocyte–rich PRP (Kim 2021). The research only included 5 trials in WOMAC function score and found insignificant improvement (P = 0.06), IKDC subjective Score was not significant as similartoourresearch.Also,twotrialswereincluded intheVASscoreandfoundaninsignificantimprovement.
Effectivity of Platelet-Rich Plasma in VAS Score Changes
The Visual Analogue Scale (VAS) is used to measure pain, quality of life, and anxiety. The pain was measured with a line usually 100mm inlength,withanchordescriptorssuchas“no pain” and “worst pain imaginable” asshowninAppendix12.Ameta-analysisonthismatter shows PRP indicated a significant and greater reduction in VAS pain undergone by the patients. One study showed PRP had a great significant reduction on the VAS scale (Lamo Espinosa et al., 2020), while other studies indicate a significant reduction as well but to a lesser extent (Carlos Sainz etal.,2023;Huangetal.,2022;Raessidatetal.,2021;Zhouetal., 2023).Hence,PRPInjectioninKneeOAPatientssignificantlyreducesVASscore.
Effectivity of Platelet-Rich Plasma in IKDC Score Changes
The International Knee Documentation Committee Subjective Knee Form (IKDC) is a patient-reported questionnaire that reports on the patient's perception ofsymptoms,function, and symptom-free sports activity of disorder-affectedknee.Duetoanswersbeingcompletely reliantonthepatient’sperception,thisscoreispurelysubjective.Eachanswertoaquestionis given a score which is then added up to a total score that ranges from 0 to 100, where 100 represents no impairment. A total of 4 studies were included for meta-analysis of the effectiveness of PRP towards IKDC score. PRP was not significant towards the IKDCscore and results favored control. Also, substantial heterogeneity was found. Thus, PRP is not significantinimprovingtheIKDCscore.
Improvement of WOMAC Function and Pain Reduction with Platelet-Rich Plasma
The WOMAC is a 24-item self-reported questionnaire that reports the patient’s pain, stiffness, and function of the joint affected byOA.Pain,stiffness,andfunctionmakeup5,2, and 17 questions out of the total 24 respectively Meta-analysis was performed for the WOMAC function score and pain independently with 8 studies and 7 studies included respectively. PRP was found to be significant in improving both WOMAC function scores and painscores.Althoughlessso,inWOMACpainscores.PRPwassignificantinimproving WOMACfunctionandpainscores.
Adverse Event of Platelet-Rich Plasma in Knee Osteoarthritis
The significance of the intervention resulted in some adverse events and complications as found in someoftheincludedstudiesafterthetreatment;Injectionsitepainwasfoundintwo patients, injection swelling in one patient, Gastrointestinal disorders in one patient, and
Musculoskeletal pain in one patient (Park et al., 2021), and other studies recorded Mild swelling in fourpatients,localpainoccurredin8patientsandSeriousSwellingpainoccurred in three patients after treatment with PRP (Zhou et al., 2023). Post-injection complications occurred in 32% of the patientsrandomizedtothePRPgroup(Raeissadatetal.,2021).Other adverse eventsrecordedarebasedonK-LgradeandAhlbackgrade(Linetal.,2018,Wanget al,2022andCarlosSaizetal.,2023)
StrengthandLimitation
This study included only randomized controlled trials optimizing the study designwhichthe data came from. Furthermore, this study included studies with various control group interventions allowing for comparison to a number ofdifferentinterventions.Thelimitations of this study are that only studies between 2018 and 2023 were included and that high heterogeneitywasfoundinfollow-upperiodsaswellasotherdiseasesreported.
CONCLUSION
This study showed that PRP could be a better treatment option compared to other common interventions. PRP was found to improve the VAS score, WOMAC function score, and WOMAC pain score but PRP did not improve the IKDC score. Also, substantial heterogeneitywasfoundbetweenthestudies.
RECOMMENDATIONS
Further studies with longer follow-up time should be made to assess the long-term effect of PRP in Knee Osteoarthritis. Studies conducted in resource-limited settings are also still lacking, further review assessing the PRP conducted in resource-limited settings is required todetermineitsfeasibility.
CONFLICTOFINTEREST
Thereisnoconflictofinterestinthemakingofthisreview
REFERENCE
1. Mobasheri,A.,&Batt,M.(2016).Anupdateonthepathophysiologyofosteoarthritis. Annals of Physical and Rehabilitation Medicine, 59(5-6), 333–339. doi:10.1016/j.rehab.2016.07.004
2. Mora, J. C., Przkora, R., & Cruz-Almeida, Y (2018). Knee osteoarthritis: pathophysiology and current treatment modalities. Journal of Pain Research, Volume 11,2189–2196.doi:10.2147/jpr.s154002
3. Silverwood, V., Blagojevic-Bucknall, M., Jinks, C., Jordan, J. L., Protheroe, J., & Jordan, K. P. (2015). Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis and Cartilage, 23(4), 507–515.doi:10.1016/j.joca.2014.11.019
4. Higgins J. P. T., Thomas J., Chandler J., Cumpston M.,LiT.,PageM.J.,&WelchV. A. Cochrane Handbook for Systematic ReviewsofInterventionsversion6.3(updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
5. Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. The PRISMA 2020 statement:anupdatedguidelineforreportingsystematic reviews.BMJ2021;372:n71.doi:10.1136/bmj.n71
6. Collins T., Alexander D., & Barkatali B. Platelet-rich plasma: a narrative review EFORTOpenRev.2021Apr1;6(4):225-235.doi:10.1302/2058-5241.6.200017.
7. Michael P. F. J., Chance M., Robert C., Charles P. H., Niall S., Christopher D. M., Brian J. C., John G. K. (2019). The Role of Platelet-Rich Plasma in Cartilage Pathology: An Updated Systematic Review of the Basic Science Evidence, Arthroscopy:TheJournalofArthroscopic&RelatedSurgery,Volume35(3),961-976. https://doi.org/10.1016/j.arthro.2018.10.125.
8. Grevnerts, H. T., Terwee, C. B., & Kvist, J. (2014). The measurement properties of the IKDC-subjective knee form. Knee Surgery, Sports Traumatology, Arthroscopy, 23(12),3698–3706.doi:10.1007/s00167-014-3283-z
9. Gandek, B. (2015). Measurement Properties of the Western Ontario and McMaster Universities Osteoarthritis Index: A Systematic Review. Arthritis Care & Research, 67(2),216–229.doi:10.1002/acr.22415
10.Lamo-Espinosa, J. M., Blanco, J. F., Sánchez, M., Moreno, V., Granero-Moltó, F., Sánchez-Guijo, F., Prósper, F (2020). Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. Journal of Translational Medicine, 18(1). doi:10.1186/s12967-020-02530-6
11. Carlos Saiz, L., Erviti, J., Leache, L. et al. Restoring Study PRGF: a randomized clinical trial on plasma rich in growth factors for knee osteoarthritis. Trials 24, 37 (2023).https://doi.org/10.1186/s13063-022-07049-3
12.Zhou, Y., Li, H., Cao, S., Han, Y., Shao, J., Fu, Q., Wang, B., Wu,J.,Xiang,D.,Liu, Z., Wang, H., Zhu, J., Qian, Q., Yang, X. and Wang, S. (2023), Clinical Efficacy of Intra-Articular Injection with P-PRP Versus that ofL-PRPinTreatingKneeCartilage Lesion: A Randomized Controlled Trial. Orthop Surg, 15: 740-749.
https://doi.org/10.1111/os.13643
13.Wang, Y.-C.; Lee, C.-L.; Chen, Y.-J.; Tien, Y.-C.; Lin, S.-Y.; Chen, C.-H.; Chou, P.P.-H.; Huang, H.-T Comparing the Efficacy of Intra-Articular Single Platelet-Rich Plasma(PRP) versus Novel Crosslinked Hyaluronic Acid for Early-Stage Knee Osteoarthritis: A Prospective, Double-Blind, Randomized Controlled Trial. Medicina 2022, 58,1028.https://doi.org/10.3390/medicina58081028
14.Dulic, O.; Rasovic, P.; Lalic, I.; Kecojevic, V.; Gavrilovic, G.; Abazovic, D.; Maric, D.; Miskulin, M.; Bumbasirevic, M. Bone Marrow Aspirate Concentrate versus Platelet Rich Plasma or Hyaluronic Acid for the Treatment of Knee Osteoarthritis. Medicina 2021, 57,1193.https://doi.org/10.3390/medicina57111193
15.Raeissadat, S. A.,GhaziHosseini,P.,Bahrami,M.H.,SalmanRoghani,R.,Fathi,M., Gharooee Ahangar, A., & Darvish, M. (2021). The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskeletal Disorders, 22(1). doi:10.1186/s12891-021-04017-x
16.Park, Y.-B., Kim, J.-H., Ha, C.-W., & Lee, D.-H. (2021). Clinical Efficacy of Platelet-Rich Plasma Injection and Its Association With Growth Factors in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized Double-Blind Controlled Clinical Trial As Compared With Hyaluronic Acid. The American Journal of Sports Medicine, 49(2), 487–496. doi:10.1177/0363546520986867
17.Kim, J.-H., Park, Y.-B., Ha, C.-W., Roh, Y. J., & Park, J.-G. (2021). Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthopaedic Journal of Sports Medicine, 9(6), 232596712110119. doi:10.1177/23259671211011948
18.Lin, K.-Y., Yang, C.-C., Hsu, C.-J., Yeh, M.-L., &Renn,J.-H.(2019). Intra-articular Injection of Platelet-Rich Plasma Is Superior to Hyaluronic Acid or Saline Solution in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized, Double-Blind, Triple-Parallel, Placebo-Controlled Clinical Trial. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 35(1), 106–117. doi:10.1016/j.arthro.2018.06.035
19.Huang, HY., Hsu, CW., Lin, GC. et al. Comparing efficacy of a single intraarticular injection of platelet-rich plasma (PRP) combined with different hyaluronans forknee osteoarthritis: a randomized-controlled clinical trial. BMC Musculoskelet Disord 23, 954(2022).https://doi.org/10.1186/s12891-022-05906-5
20.Chu J., DuanW.,YuZ.,TaoT.,XuJ.,(2022).Intra-articularinjectionsofplatelet-rich plasma decrease pain and improve functional outcomes than sham saline in patients
with knee osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy 30, 4063–4071(2022)
21.Zaffagnini S., Andriolo L., Boffa A., Poggi A.,CenacchiA(2022).Microfragmented AdiposeTissueVersusPlatelet-RichPlasmafortheTreatmentofKneeOsteoarthritis: A Prospective Randomized Controlled Trial at 2-Year Follow-up.
10.1177/03635465221115821. Epub 2022 Aug 19. Am J Sports Med 2022 Sep;50(11):2881-2892
APPENDIX
Appendix1.LiteratureSearchStrategy
Appendix2 LiteratureReviewPICOSFramework
Population Intervention Comparison Outcome StudyDesign
Geriatricaged 60yearsoldor older
Platelet-rich plasma(PRP)
Conventional therapyas control
VASPainScale, IKDC Subjective Scoreand WOMAC globalfunction andpainscore.
Meta-analysis andSystematic Reviewfrom Randomized ControlledTrial
Appendix3 StudyQualityAssessmentBasedonCochraneRoB2.0*
(*Note:White=UnclearRisk)
Appendix4. CharacteristicsofIncludedStudies

Appendix5.ForestPlotshowingtheefficacyofPRPagainstthecontrolgroupinEleven Trials.



Appendix6.

Appendix7.
 Funnelplot:PRPvsControl
VASscoreForestPlot
Appendix8.Funnelplot;VASPain
Funnelplot:PRPvsControl
VASscoreForestPlot
Appendix8.Funnelplot;VASPain
Appendix9.IKDCScoreForestPlot

Appendix10.Funnelplot:IKDCscore
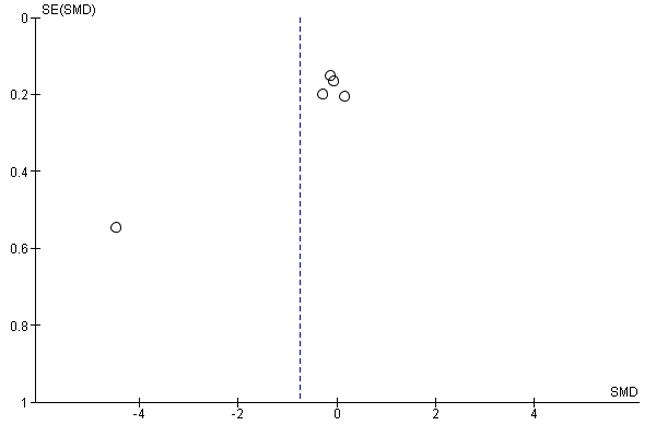

 Appendix11.WOMACFunction(4.1.1),WOMACpain(4.1.2)
Appendix12.VisualAnalogueScale
Appendix11.WOMACFunction(4.1.1),WOMACpain(4.1.2)
Appendix12.VisualAnalogueScale
Ecological Hemodialysis Membrane utilizing Bio-Waste as an Advanced Therapy to Prolong Life Expectancy for Patients with Chronic Kidney Failure
Armadina Fitra Choirunnisa1, Araminta Vania Saraswati1, Davendra Putra Arysatya1, Fadia Qistina Sidqi1
AMSA-UNDIP1
ABSTRACT
Chronic kidney failure is a long-term condition, where the kidney is unable to filtrate blood optimally, leading to a build-up of toxins and fluids in the kidney. Data from Riskesdas (2013) state patients aged >= 75 years occupy the top rank for the chronic kidney failure (CKD) patient group, which is 0.6% higher than other age groups. The most common method of biomedical therapy to overcome chronic kidney failure is hemodialysis. The increasing life expectancy of the world population elicited the increasing numbers of elderly patients starting hemodialysis. An important component of hemodialysis is a semi-permeable membrane able to separate toxic from compounds needed by the body. Chitosan and cellulose acetate are natural polymers that are environmentally friendly and have functional groups to increase the permeability of toxic compounds. Kepok banana peel waste can be processed into cellulose acetate, overcoming the problem of kepok banana peel waste. The purpose of this research is to make a hemodialysis membrane from chitosan and cellulose acetate, isolated from organic waste. This research is a mixed method with quantitative and qualitative approaches. The method used to create this membrane is Non-Solvent Induced phase separation. The Fourier Transform Infrared, Scanning Electron Microscopy, Hydrophilicity, Water Uptake, Porosity, Flux, and Tensile Strength were analyzed and tested. The results show that the type 1 membrane has a higher percentage of porosity and more uniform pores than the control membrane. It has the best ability to pass water with a difference of 1.52%. It also has higher permeability, higher hydrophilicity value, and good tensile strength. It has a chitosan-sodium dodecyl sulfate functional group and has the ability to pass essential compounds. In conclusion, type 1 membrane permeability exceeds the qualities of biopolymer-based membranes and can overcome its physical and chemical weaknesses through some tests that have been done.
Keywords: Cellulose Acetate, Chitosan, Chronic Kidney Failure, Hemodialysis, Membrane
ECOLOGICAL HEMODIALYSIS MEMBRANE UTILIZING BIO-WASTE AS AN ADVANCED THERAPY TO PROLONG LIFE EXPECTANCY FOR PATIENTS WITH CHRONIC KIDNEY FAILURE
Authors:
Armadina Fitra Choirunnisa
Araminta Vania Saraswati
Davendra Putra Arysatya
Fadia Qistina Sidqi
ASIAN MEDICAL STUDENTS’ ASSOCIATION
2023

Ecological Hemodialysis Membrane utilizing Bio-Waste as an Advanced Therapy to Prolong Life Expectancy for Patients with Chronic Kidney Failure
Armadina Fitra Choirunnisa1, Araminta Vania Saraswati1, Davendra Putra Arysatya1, Fadia Qistina Sidqi1
AMSA-UNDIP1
ABSTRACT
Chronic kidney failure is a long-term condition, where the kidney is unable to filtrate blood optimally, leading to a build-up of toxins and fluids in the kidney. Data from Riskesdas (2013) state patients aged >= 75 years occupy the top rank for the chronic kidney failure (CKD) patient group, which is 0.6% higher than other age groups. The most common method of biomedical therapy to overcome chronic kidney failure is hemodialysis. The increasing life expectancy of the world population elicited the increasing numbers of elderly patients starting hemodialysis. An important component of hemodialysis is a semi-permeable membrane able to separate toxic from compounds needed by the body. Chitosan and cellulose acetate are natural polymers that are environmentally friendly and have functional groups to increase the permeability of toxic compounds. Kepok banana peel waste can be processed into cellulose acetate, overcoming the problem of kepok banana peel waste. The purpose of this research is to make a hemodialysis membrane from chitosan and cellulose acetate, isolated from organic waste. This research is a mixed method with quantitative and qualitative approaches. The method used to create this membrane is Non-Solvent Induced phase separation. The Fourier Transform Infrared, Scanning Electron Microscopy, Hydrophilicity, Water Uptake, Porosity, Flux, and Tensile Strength were analyzed and tested. The results show that the type 1 membrane has a higher percentage of porosity and more uniform pores than the control membrane. It has the best ability to pass water with a difference of 1.52%. It also has higher permeability, higher hydrophilicity value, and good tensile strength. It has a chitosan-sodium dodecyl sulfate functional group and has the ability to pass essential compounds. In conclusion, type 1 membrane permeability exceeds the qualities of biopolymer-based membranes and can overcome its physical and chemical weaknesses through some tests that have been done.
Keywords: Cellulose Acetate, Chitosan, Chronic Kidney Failure, Hemodialysis, Membrane
ii
iii TABLE OF CONTENTS ABSTRACT ....................................................................................................................................ii TABLE OF CONTENTS ..............................................................................................................iii CHAPTER I PREFACE ................................................................................................................1 A. Background...........................................................................................................................1 B. Problem Formulation ...........................................................................................................2 C. Research Objectives..............................................................................................................3 D. Research Hypothesis.............................................................................................................3 E. Research Benefits..................................................................................................................4 F. Novelty....................................................................................................................................4 CHAPTER II LITERATURE REVIEW .......................................................................................6 A. Chronic Renal Failure....................................................................................................6 B. Hemodialysis ...................................................................................................................6 C. Membrane .......................................................................................................................7 D. Chitosan...........................................................................................................................7 E. Sodium Dodesil Sulfate ..................................................................................................8 F. Cellulose Acetate on Kepok Banana Peels.......................................................................8 CHAPTER III RESEARCH METHOD........................................................................................9 A. Types of research............................................................................................................9 B. Research variable ...........................................................................................................9 C. Research Tools, Materials, and Samples......................................................................9 D. Time and Place of Research.........................................................................................10 E. Research procedure......................................................................................................10 F. Data Analysis and Testing Techniques...........................................................................11 CHAPTER IV RESULTS AND DISCUSSION..........................................................................14 A. Kepok Banana (Musa acuminatabalbisianacolla) Peel Cellulose Acetate Synthesis 14 B. Effect of the addition of Chitosan-Sodium Dodecyl Sulfate to determine the best dope concentration ..................................................................................................................14 1. Fourier Transform Infrared (FTIR) ................................................................................14 2. Porosity 15 3. Water uptake 16
iv 4. Water flux 17 5. Hydrophilicity 18 C. Effect of the addition of Chitosan-Sodium Dodecyl Sulfate on the final characterization of the best dope membranes ......................................................................19 1. Tensile Strength..............................................................................................................19 2. Scanning Electron Microscopy (SEM)...........................................................................19 3. Fourier Transform Infrared (FTIR) 20 4. Flux Performance 21 CHAPTER V.................................................................................................................................24 CONCLUSIONS AND RECOMMENDATIONS .......................................................................24 REFERENCE...............................................................................................................................26 APPENDIX...................................................................................................................................29
CHAPTER I PREFACE
A. Background
The kidneys acts as a regulator of the balance of body fluids, electrolytes, acid base by filtering blood through the kidneys, selectively reabsorbing water, excreting excess as urine, and removing metabolic waste (urea, creatinine and uric acid) as well as foreign chemicals (Sari, 2012)Based on the Health Research Results Report in 2013, as many as 2 out of 1000 residents or 499,800 Indonesians suffer from kidney failure (Aulia, 2017). Data from Riskesdas (2013) state patients aged >= 75 years occupy the top rank for the chronic kidney failure (CKD) patient group, which is 0.6% higher than other age groups. Progressive and irreversible kidney function disorder where the body's ability fails to maintain metabolism and fluid and electrolyte balance can cause chronic kidney failure which can progress to terminal kidney failure, a condition in which the kidneys lose the ability to maintain body substance so that further treatment is needed in the form of longterm dialysis, or kidney transplantation to prolong life expectancy (Rivandi and Yonata, 2015). The increasing life expectancy of the world population elicited the increasing numbers of elderly patients starting hemodialysis (Nefrol, 2019).
Hemodialysis is a method of biomedical therapy used to replace the role of the kidneys in removing metabolic waste from the body (Rahman et al., 2016). In principle, hemodialysis is based on the toxic diffusion of uremic compounds from the human body into the dialysate solution through a semi-permeable membrane in response to differences in concentration or pressure. Transport through the membrane is achieved due to concentration gradients in both phases. In order to be used for hemodialysis, the membrane must 1) have high permeability, which means that the membrane can transport metabolic substances as quickly as possible, and 2) hemocompatible, or not rejected by the blood (Lusiana et al., 2013).
Most of the semi-permeable membranes used for hemodialysis today are synthetic membranesdue totheir easymanufacturingprocess,thermal stability, mechanical strength, and chemical stability (Wahyuliswari, 2015). Some of these synthetic membranes are Polysulfone and Polyethersulfone. However, the use of synthetic membranes shows that they are not environmentally friendly because they are difficult to decompose in the environment, lack functional groups, are hydrophobic, and have weak interactions with urea and creatinine (Siahaan et al., 2021).
Celluloseacetatemembraneis ahydrophilicmembrane,with ahigh absorption rate, good electrical resistance, limited heat and chemical resistance (Galiano et al., 2018). Kepok banana peel contains high alpha cellulose which has the potential to be used as biomedical materials(Yannasandyetal.,2017).Bananaproduction inIndonesiahasranked 3rd as the largest banana producing country with production figures reaching 7,264,383 tons. This is based on data from the Food and Agriculture Organization (FAO) in 2018 in the form of a ranking table for banana-producing countries in the world (FAO, 2018). One type of banana that develops in Indonesia, among others, is the kepok banana or Musa acuminatabalbisianacolla. A large amount of consumption of kepok bananas is only focused on the flesh, while the skin is immediately discarded. This condition encourages
1
the use of kepok banana peels which are considered by the community as household industrial waste that has not been used optimally.
Kepok banana peel waste can be processed into cellulose acetate, overcoming the problem of kepok banana peel waste. Cellulose acetate is easy to process, a renewable raw material, and has good physical characteristics because it consists of a symmetrical membrane with sufficient size for permeability to water, creatinine, and urea (Wahyuliswari, 2015). With these advantages, cellulose acetate has drawbacks, namely sensitivity to pH in the range of 2-8, low mechanical properties, susceptible to microbes found in nature, and only compatible with some plasticizers (Lestari, 2012). To cover the shortcomings of cellulose acetate, chitosan from crustacean waste is added. Chitosan has hydrophilicity, non-toxic, biological compatibility, and biodegradable properties. However,theresultingmembrane islessporous,proteinadsorption disruptsthepermeation process of creatinine and urea, and its hydrophilicity can still be increased so that it is more optimal (Maiti and Bidinger, 2020). The insufficiency of chitosan and cellulose acetate can be overcome by adding an anionic surfactant sodium dodecyl sulfate, increasing the hydrophilicity, density, and degree of swelling. Moreover, the addition of these additives will allow changes in the form of an increase in the viscosity of the polymer solution and increase in stability with an increase in the number of pores formed.
Chronic kidney failure is associated with the process of various etiologies if not treated. The most common etiology of kidney failure is as follows, glomerulonephritis (25%), diabetes mellitus (23%), hypertension (20%), and polycystic kidney (10%) (Indonesian Renal Registry, 2007-2008). Those who suffer from chronic kidney diseases experience kidney dysfunction which persists for a long time and is constantly recurring. Hemodialysis is one of the possible medical procedures that these patients have to undergo in compensation for the dysfunction of their renal organs. This procedure does not cure the patient; however, it is a form of maintenance to prolong their life expectancy.
Based on these problems and potentials, this research will create a semi-permeable membrane for hemodialysis of chronic kidney failure using chitosan, cellulose acetate, and anionic surfactant sodium dodecyl sulfate. With this research, it is hoped that the semipermeable membrane made from environmentally friendly materials can be used for hemodialysisinpatientswith chronickidneyfailure andprolongtheirlife expectancywhile also offering a better quality of life. This research seeks to provide chronic patients with an increasing life expectancy, which is directly proportional to environmental health.
B. Problem Formulation
Based on the previous background, the problem formulation of this research is as follows:
1. Cellulose acetate can be made from kepok banana peel.
2. The membrane in hemodialysis must have high permeability.
3. The use of semi-permeable membranes in hemodialysis is still synthetic.
4. Environmentally friendly membranes have drawbacks in the form of less pores, protein adsorption that interferes with the permease process, hydrophilicity can be optimized, low mechanical properties.
Based on the previous background, the research questions from this study are as follows:
2
1. How to make cellulose acetate from kepok banana peel?
2. How to make a semi-permeable hemodialysis membrane that has high permeability to urea and is selective to protein using a mixture of anionic surfactant sodium dodecyl with environmentally friendly ingredients chitosan and cellulose acetate?
3. What are the physical and chemical characteristics of the membrane made from sodium dodecyl anionic surfactant additive with environmentally friendly chitosan and cellulose acetate as a hemodialysis membrane?
5. What is the permeability of the membrane made of anionic surfactant additive sodium dodecyl with environmentally friendly chitosan and cellulose acetate in urea and its selectivity to protein?
C. Research Objectives
Based on previous research questions, the objectives of this study are as follows:
1. To find out how to make a cellulose acetate from kepok banana peel.
2. Tofindouthow tomakeasemi-permeablehemodialysismembrane thathashigh permeabilitytoureaandisselectiveto proteinusinga mixtureofsodiumdodecyl anionic surfactant additives with environmentally friendly ingredients chitosan and cellulose acetate.
3. Todeterminethephysical andchemical characteristicsofmembranesmadefrom sodium dodecyl anionic surfactant additives with environmentally friendly materials chitosan and cellulose acetate as hemodialysis membranes.
4. Todetermine thepermeabilityofmembranesmadefromsodiumdodecylanionic surfactant additives with environmentally friendly chitosan and cellulose acetate in urea and their selectivity to protein.
D. Research Hypothesis
The hypothesis in 2023 in this study is:
1. Cellulose acetate can be formed from the synthesis of kepok banana peels which are community waste.
2. The membrane mixed with sodium dodecyl anionic surfactant additive with semi-permeable chitosan and cellulose acetate as an environmentally friendly material has high permeability to urea and is selective to protein.
3. The membrane made of sodium dodecyl anionic surfactant additive with environmentally friendly chitosan and cellulose acetate has good ability to interact or absorb water, and forms a small contact angle.
4. The membrane made of sodium dodecyl anionic surfactant additive with environmentally friendly chitosan and cellulose acetate has good permeability compared to that without the addition of chitosan-sodium dodecyl sulfate in testing for urea and is selective for protein.
3
E. Research Benefits
a. From the research conducted there are benefits that can be drawn, namely:
1. Theoretically
b. Contributing ideas in the health sector, especially in the development of semipermeable membranes with environmentally friendly materials to treat chronic kidney failure.
1. Practically
a. Knowinghowtomakecelluloseacetate fromkepokbananapeels totreat chronic kidney failure.
b. To determine the effect and ability of a semi-permeable membrane formed from mixing sodium dodecyl anionic surfactant additives with environmentally friendly materials chitosan and cellulose acetate to treat chronic kidney failure.
c. Knowing the ability of semi-permeable membranes made from environmentally friendly materials as a solution that can replace synthetic-based membranes in the implementation of hemodialysis.
2. For writers
a. It can provide insight to the reader about chitosan, cellulose acetate, and sodium dodecyl anionic surfactants which can be alternatives in the manufacture of semi-permeable membranes for filtering urea and being selective for proteins.
b. Can improve writing skills, creative thinking, and critical.
F. Novelty
Below are previous studies discussing the use of semi-permeable membranes made from chitosan, cellulose acetate, and sodium dodecyl anionic surfactant to filter urea and be selective on protein. In addition, this sub-chapter will explain the novelty of the research conducted as follows.
Manufacture and Characterization of Chitosan-Cellulose Diacetate-TiO2
Composite Membranes for Detergent Waste Treatment
Effect of Addition of Anionic Surfactant
Sodium Dodecyl Sulfate on Characteristics of Cellulose Acetate Membranes
Study of Creatinine Transport Using Chitosan-Pectin Polyelectrolyte Complex (PEC)
4
No Previous Researcher Year Discussion
Table 1. Previous Researcher
2012
1. Puji Lestari
2. Eka Surya Buana, Dwi Indarti, Asnawati
2014
3. Ni Putu Sri Ayuni, Dwi Siswanta, Adhitasari Suratman
2014
Membrane
Manufacture and Characterization of Cellulose Acetate Nanofiber Membrane Using Electrospinning Technique For Hemodialysis Creatinine
Tripolyphosphate-heparin Modified Chitosan Membrane and Its Application in Urea and Creatinine Permeation
Source: Researchers’ Documents
Table 1. describes previous studies discussing membranes made from chitosan, cellulose acetate, and sodium dodecyl anionic surfactant. The first literature discusses the manufacture, characterization, and the effect of adding TiO2 to the chitosan-cellulose diacetate-TiO2 composite membrane. In the second literature, it is explained about the effect of adding sodium dodecyl sulfate anionic surfactant on the physical properties, chemical properties, and performance of cellulose acetate membranes. Then, the third literature describes the transport of creatinine using chitosan-pectin PEC membranes. Furthermore, the fourth literature discusses the manufacture and characterization of nanofiber membranes made from cellulose acetate by electrospinning technique. The latest literature describes the preparation of alloy membranes from cross-linked chitosan tripolyphosphate (TPP) grafted with heparin and polyvinyl alcohol polyethylene glycol (PVA-PEG). What distinguishes research on semi-permeable membranes made from sodium dodecyl anionic surfactant additives with environmentally friendly materials chitosan and cellulose acetate for filtering urea and selective for protein is the combination of the use of environmentally friendly materials (chitosan and cellulose acetate) with additives (sodium anionic surfactant). dodecyl) forms a semi-permeable membrane to filter urea and is selective for protein in the implementation of hemodialysis in chronic kidney disease.
5
4. Iqlima Ayu Prestisya 2016
5. Retno Ariadi Lusiana, Wahyu Putri Pranotoningtyas 2018
CHAPTER II LITERATURE REVIEW
A. Chronic Renal Failure
The kidney is an essential organ of the body which function as a vital organ for the body. Its specific function is to regulate the volume and chemical composition of the blood. It does it by selectively excreting metabolic waste products and water (Widyastuti et al., 2014). Kidney failure is when the kidney has lost its function in the nephron unit that progresses slowly which cannot be cured. This leads to the body losing its ability to maintain fluid, metabolic function and electrolyte balance, which results in Uremia or a buildup of urea in the blood. This potentially could lead to poisoning to the body’s organs that could lead to more complex health problems (Agustina and Dewi, 2013). Chronic kidney failure is a pathophysiological condition with various etiologies. In 2007-2008 a data from the Indonesian Renal Registry (IRR), shows that the most common etiology of kidney failure are glomerulonephritis (25%), diabetes mellitus (23%), hypertension (20%), and polycystic kidney (10%) (Sudoyo et al., 2006). Chronic kidney patients could also cause more complication to its patients, which includes: a) Anemia, b) Cardiovascular disease, c) Sexual dysfunction,andd)Bonedisease(PrabowoandPranata,2014).Managementofchronickidney failure are focused to treat the primary cause of kidney failure, treat metabolic disorders, minimize comorbid conditions, prevent decline of kidney function optimize growth and development, and treat cardiovascular disease associated with chronic kidney failure.
B. Hemodialysis
Hemodialysis is one of the procedure to replace kidney function that cannot function properly to filtrate blood to create body waste. It uses artificial kidney machine or hemodialysis as its procedure (Ratnawati, 2011). The goal of hemodialysis is to relieve symptoms occurred in the body by controlling uremia, excess of fluid, and electrolyte imbalances in the body of the patients with chronic kidney disease (Rahman et al., 2016). Although it is proven to be a safe procedure, hemodialysis also has its side effects. Such as dizziness, nausea, muscle cramps, infection in the blood vessels, vomiting, heart rhythm abnormalities , digestive disorders and vomiting. To reduce the side effects, intake of foods and sufficient fluid can play a crucial role to create a healthier condition for the patient of chronic kidney failure (Bayhakki, 2012).
Source: (Chan et al., 2013)

6
Figure 1. Hemodialysis
Membrane is a selective semi-permeable layer which lies in between 2 phases. The first phase is the one which contains the incoming components and the acceptor phase which contains particles that can pass through the membrane (Lusiana et al., 2013). The membrane works as a barrier that is very selective to certain components of a fluid and pass other components through the membrane (Mulder, 1991). Hemodialysis main principle is the diffusion of particles through a semipermeable membrane, which means that the membrane plays an important role in hemodialysis (Amiji, 1995). The role of the membrane itself is as an artificial kidney for hemodialysis procedure, which has the goal to separate toxic compounds such as urea and creatinine and compounds that are still needed by the body such as vitamins and proteins (Suwitra, 2006).
Chitosan is considered a reactive biopolymer that can easily undergoes biological degradation to carry out chemical changes. One of its characteristics is that is non toxic both as a coagulant or flocculant and can easily form membranes. Chitosan is a white amorphous solid that is insoluble in mineral acids and alkalis except in certain circumstances. Based on a research, Chitosan can dissolve best in a solution which has 2% acetic acid, 10% citric acid and 10% formic acid. Chitosan cannot dissolve in lactic acid, pyruvic acid, and inorganic acids at a certain pH even if it has been heated and stirred for a long time (Meriatna, 2008). Chitosan acts as a biopolymer that can function as a basic material to form a membrane because it can form thin film membranes and it can also dissolve in dilute acid through a phase of inversion (Lusiana, et al., 2018). Based on the previous research and studies, shows that the manufacture of chitosan-cellulose diacetate composite membranes for detergent waste treatment is in fact possible. Examples such a research by Ni Putu Sri Aryuni in a study of Creatinine Transport Using Chitosan Pectin Polyelectrolyte Complex (PEC) Membranes and Retno Ariadi Lusiana in a study of Tripolyphosphate-heparin Modified Chitosan Membrane and Its Application in Urea and Creatinine Permeation in 2018.

7
C. Membrane
D. Chitosan
Figure 2. Chitosan Structure
Source: (El-banna et al., 2019)
E. Sodium Dodesil Sulfate

Sodium Dodecyl Sulfate is a C12 anionic surfactant that has been developed and prepared which is commonly found in cleaning products because of its optimal solubility of ionic surfactants in the C12 chain that can be very suitable for washing (Lusiana, et al., 2018). In addition, the addition of Sodium Dodecyl Sulfate creates a characteristic of cellulose acetate membrane that is better for hemodialysis.
F. Cellulose Acetate on Kepok Banana Peels
Kepok banana is one of the fruit commodities that are often found in Indonesia. This fruit has various benefits that are good for humans, whether used directly or as processed materials. Kepok banana peel contains cellulose which can be used as raw material for cellulose acetate. Cellulose acetate is one type of polymer that is widely used in industry, one of which is as a polymer in the manufacture of ultrafiltration membranes (Galiano et al., 2018). Cellulose acetate also has an important role in the development of membrane technology such as in the manufacture of reverse osmosis for water purification and desalination membranes because of its porous and dense structure. Cellulose acetatebased membranes have the advantage that they are resistant to acids and bases, and are biodegradable so that they are friendly to the environment (Gaol et al., 2013).

8
Figure 3. Sodium Dodesil Sulfat Source: Researchers’ Documents
Figure 2: Kepok Banana Source: (Badan Litbang Pertanian, 2020)
CHAPTER III RESEARCH METHOD
A. Types of research
The type of approach used in this study is a quantitative and qualitative approach. For the quantitative research, we focuses on emphasizing objective phenomena and is examining it quantitatively. Maximizing the objectivity of this research design was carried out using numbers, data processing, structures and controlled experiments (Hamdi and Bahruddin, 2014). To test the validity and credibility of the data,this study uses a quantitative approach with laboratory study methods. Whereas, the qualitative approach is aimed at understanding the conditions of a context by emphasizing a detailed and in-depth description of the portrait of conditions in a natural context or what actually happened in field conditions (Nugrahani, 2014). In this study, a qualitative approach was carried out by comparing the physical and chemical properties of the various membranes with one another.
B. Research variable
1. Variabel kontrol:
a. Urea
b. Protein
2. Variabel bebas:
a. Surfaktan anionik sodium dodesil (SDS)
b. Kitosan
c. Selulosa asetat from Kepok Banana Peels
3. Variabel terikat:
a. Membran semi-permeabel
5. Variabel Tidak Tetap
a. Komposisi (CA/CS/SDS).
6. Variabel Yang Diteliti
a. Kemampuan membran dalam penyaringan urea dan selektif pada protein untuk hemodialisis.
b. Kemampuan stabilitas termal, kekuatan mekanik, dan ketahanan terhadap bahan kimia.
C. Research Tools, Materials, and Samples
The materials used in this study were cellulose acetate obtained from the synthesis of kepok banana peels, cellulose acetate (Sigma Aldrich) obtained from Hebei Chisure Biotechnology, chitosan (Mw= 40,000 g/mol, DD= 87%) obtained from Biotech Surindo (Indonesia), sodium dodecyl sulfate (merck) obtained from PT Merck Chemicals and Life Sciences, Bovine Serum Albumin (Sigma Aldrich) was obtained from a nitrochemical store, acetic acid, acetone, urea (Mw=60 g/mol), and distilled water were obtained through E-commerce. While the equipment used in this study were glassware, Erlenmeyer with lid, filter paper, electrical tape, watch glass, magnetic stirrer, spatula, pipette, analytical balance, stirrer, measuring cup, petri dish, and digital microscope. The tools needed are obtained from the nearest chemical store and on E-commerce.
9
D. Time and Place of Research
The research began in December 2022 and was completed for 6 months (finished in April 2023) which included literature study on the topic of discussion and observation of the research object. The research plan to be carried out and the activities carried out can be seen in the appendix.
E. Research procedure
1. Literature Study
The literature study was conducted to obtain information relevant to the research problem and used as a theoretical basis in conducting research. The information used is taken from books, journals, research report articles, and internet sites
2. Manufacture of Cs-SDS
Make a homogeneous solution of chitosan and sodium dodecyl sulfate. This was done by dissolving 1.5 g of chitosan in 100 ml of 1% acetic acid. Then make a solution of 3 g of sodium dodecyl with 100 ml of water. Then mix the two solutions until a perfect precipitate forms.
3. Synthesis of Cellulose Acetate from Kepok Banana Peels
The synthesis of cellulose acetate from kepok banana peels was carried out in several stages. The preparation stage is to smooth the clean banana peel then proceed by inserting it into a 17.5% NaOH solution for 24 hours. Materials that are not soluble in solution are the main ingredients, namely alpha-cellulose. The alphacellulose obtained was then separated from the solution using filter paper and washed using warm water at a temperature below 50°C and repeated several times so that the powder obtained reached a neutral condition. After that, it was dried at 40°C and obtained alpha-cellulose solids. Then proceed with the acetylation reaction step by adding acetic anhydride solution with a mass ratio of glacial acetic acid(1:1). Subsequently, the cellulosewasmixedwithglacial acetic anhydridewith a mass ratio of 1:20 which lasted for 6 hours with the reaction temperature maintained at 45°C for cellulose activation. Followed by a neutralization step which aims to dilute glacial acetic acid by adding water to the acetylation product and stirring for 1 hour. The result obtained from the acetylation reaction is a material in the form of a yellowish-white cellulose acetate lump.
4.
Qualitative analysis using the Fourier Transform Infrared (FTIR) was carried out to determine the presence of functional groups in the membrane. The membrane will absorb some of the infrared that enters through the gap. The unabsorbed infrared will then be transferred across the membrane surface.
5.
Making a dope solution using acetone as a solvent from cellulose acetate so that it can become a homogeneous solution with chitosan and sodium dodecyl sulfate. In the manufacture of membranes, the composition of polymer materials and additives will be varied. While cellulose acetate has the same ratio. The variations in the constituent composition are as follows:
10
FTIR Test
Manufacturing of Dope Membranes
Put acetone according to the membrane type composition being processed into a closed Erlenmeyer flask that is equipped with a magnetic stirrer, then place the magnetic stirrer on top of the stirrer. Sequentially add other ingredients slowly and keep stirring for 24 hours. Furthermore, to remove bubbles, the solution is left for 24 hours.
6. Membrane printing using the Non-Solvent Induced phase separation (NIPS) method
The Non-Solvent Induced phase separation process consists of 3 techniques, namely, the immersion precipitation method, air-casting of polymer solution, and precipitation from the vapor phase. Membrane printing is done by pouring dope solution which has been left for 24 hours onto a square glass plate. Then swipe the solutionon the mediasupportorthrough a certain mediaformation. Then immersed in water until solidification occurs.
7. Membrane Testing
The membrane that has been formed from the Evaporation Induced Phase Separation process will then be tested. This research will test semi-permeable membranes that have been formed with Fourier Transform Infrared, Scanning Electron Microscopy, Hydrophilicity, Swelling, Porosity, Testing the membrane resistance at a pH, Flux, and Tensile Strength.
8. Research Result Data Analysis
Data analysis was carried out after obtaining the results of several tests carried out toanswertheobjectivesofthisstudy, namelyrelatedtothemixingeffectand ability of semi-permeable membranes made from sodium dodecyl anionic surfactant additives with environmentally friendly materials chitosan and cellulose acetate on urea and keratinin filtration.
F. Data Analysis and Testing Techniques
The testing and data analysis carried out in this study are as follows:
1. Fourier Transform Infrared (FTIR)
Qualitative analysis using the Fourier Transform Infrared (FTIR) tool is used to determine the presence of functional groups contained in the membrane. The
11
Membran Type CA Aceton Cs-SDS Type 1 18 80 2 Type 2 18 78 4 Type 3 18 76 6 Type 4 18 74 8
Research Documents
Table 2. Membrane Type
Source:
membrane will absorb some of the infrared that enters through the gap. Infrared that is not absorbed will be transferred through the surface of the membrane.
2. Scanning Electron Microscopy (SEM)
Membrane morphology testing can be done by observing the surface of the membrane. This tester will determine the morphology in the form of pore distribution, pore geometry, and porosity on the surface of the membrane with the help of certain detectors. The membrane was placed and attached to the SEM specimen holder using conductive carbon double-sided tape. Then place the membrane by showing the cross section towards the objective lens and the surface of the sample is magnified with a certain magnification.
3. Hydrophilicity
The hydrophilicity of a membrane is calculated from the measurement of its contact angle with the liquid. Through this contact angle will determine how much contact power between the liquid and the membrane.
4. Water uptake
The expansion of the membrane can be measured by measuring the diameter or thickness of the membrane in a dry state. Then soak the membrane in water for 24 hours. The membrane is removed and its diameter or thickness is measured again. The purpose of this test is to estimate the size of a substance that can diffuse into the membrane. The percentage of swelling can be calculated using the following formula:
Water uptake% = ����
Keterangan:
Ww= Weigh when wet
Wd= Weight when dry
5. Porosity
The porosity test was carried out to determine the number of components that can be absorbed by the membrane. Membrane porosity measurements were also carried out to determine the amount of empty space between the membrane materials. Porosity in the membrane is calculated by comparing the volume of free space with the total volume. The calculation by determining the ratio of pore volume to the total volume of the membrane is as follows:
Ρ(%)= ��1 ��0 �������� ����2��
keterangan :
w 1 = membrane wet weight (g)
w 2 = membrane dry weight (g)
ρw = water density (g/cm3)
V T = wet membrane volume (cm3)
ρmd = dry membrane density (g/cm3)
6. Flux
This test will measure the ability of the membrane to pass the volume of water that passes through the membrane per unit surface area of the membrane per unit time.
12
���� ����
The flux test will determine the performance of the membrane as a selective separator layer. The test was carried out 5 times by means of every 15 minutes 2 mL of samples taken from the feed and acceptor phase. Testing on water which will continue to rotate and will produce permeate which will be sampled. Whereas in testing urea and protein, the permeate needs to be complexed first. By taking a sample of 2 mL of permeate, then complexed with picric acid for protein determination and with dimethylamine benzaldehyde for urea analysis. Then disulfjected into UV-Visible spectrophotometer at a wavelength of 486 nm for protein and 430 for urea. These results can be calculated using the formula:
Keterangan:
J V = water flux (L/hr.m2)
V= water volume (L)
A= surface area (m2)
t= time (hour)
7. Tensile Strength
Tensile strength or tensile strength is a test performed as a determinant of the mechanical properties of hollow fiber membranes. This test is carried out with a test tool that will pull the membrane until the membrane breaks. This test is to determine the maximum stress that can be accepted by the membrane.
13
���� = �� �� ��
CHAPTER IV RESULTS AND DISCUSSION
A. Kepok Banana (Musa acuminatabalbisianacolla) Peel Cellulose Acetate Synthesis
The manufacture of cellulose acetate made from kepok banana peels is carried out through 2 stages of work, namely the preparation stage and the acetylation stage. The preparation stage begins with washing 500 grams of kepok banana peel with water until clean and then mashed into a pulp by adding 250 ml of distilled water to facilitate the refining process. After the kepok banana peel is mashed, the resulting pulp will be dark brown. The smooth kepok banana peel was then filtered with filter paper and 17.5% NaOH solution was added and then allowed to stand for 24 hours to obtain alpha-cellulose. The NaOH solution and banana peel puree were then washed with running water until clean and had a normal pH. Furthermore, in the acetylation stage, cellulose is mixed with glacial acetic andrihyde with a mass ratio of 1:5 and continues until the reaction reaches a temperature of 45'C. This process is called the swelling process which aims to activate the cellulose and will have an impact on the surface expansion of the cellulose acetate to the acetylation reaction. Then proceed with the addition of glacial acetic acid in a ratio of 1:1 to acetic andrihyde to replace the acetyl groups in the larger amount of cellulose acetate. In addition, the addition of acetic acid also aims to speed up the reaction. In this process, a yellowish-white lump will appear which is the result of the reaction/cellulose acetate. To prove the synthesis result was successful, functional group analysis was performed using Fourier Transform Infrared (FTIR).
B. Effect of the addition of Chitosan-Sodium Dodecyl Sulfate to determine the best dope concentration
1. Fourier Transform Infrared (FTIR)
Figure 3: Graph of Water Flux Test Results
Source: Researcher Data Processing, 2023
Figure 5. shows the spectrum of FTIR and the characteristic peaks of the functional group of commercial acetate cellulose, the results of cellulose acetate synthesis made from banana skin, and type 4 membranes that are selected membranes. The presence of peaks at wavelength 1731 in the commercial cellulose acetate spectrum indicates the presence of

14
C=O bonds formed. These bonds also appeared in the commercial cellulose acetate spectrum at wavelength 1742 and in the membrane spectrum of type 4 at wavelength 1747. In addition to the similarity of the appearance of C = O bonds in all three spectra, there are similarities in other wave peaks caused by the presence of C-O bonds in all three waves.
2. Porosity
Source:
Porosity testing was carried out to see the effect of the amount of Chitosan Sodium Dodecyl Sulfate on the resulting membrane pores. Porosity is one of the important factors that can affect membrane performance. The resulting pore size affects the performance of the membrane in determining the value of water flux.
Based on the research data, it appears that there are differences in the percentage of porosity in various membranes. The cellulose acetate control membrane had a porosity percentage of 0.428% and the type 4 membrane with a variation of chitosan-sodium dodecyl sulfate 0.8% had the lowest porosity percentage of 0.2%. The highest porosity percentage value was obtained with a variation of chitosan-sodium dodecyl sulfate as much as 0.2%, namely 0.633%. The higher porosity percentage of type 1 membrane than the control membrane is also supported by the SEM data showing that type 1 membrane has more pores than the control membrane. However, the more chitosan-sodium dodecyl sulfate added, the percent porosity will decrease. The presence of membrane pore closure by chitosan-sodium dodecyl sulfate precipitate which was not mixed evenly or homogeneously during the manufacture of dope membranes is thought to be the cause of the decreased percentage of membrane porosity.
15
Figure 7. Porosity Test Results
Researcher Data Processing, 2023
0.428 0.633 0.450 0.387 0.200 0.000 0.100 0.200 0.300 0.400 0.500 0.600 0.700
Kontrol
Tipe 1 Tipe 2 Tipe 3 Tipe 4
POROSITAS
PERSEN POROSITAS TIPE MEMBRAN
3. Water uptake
Table 3. Water uptake test results
Water uptake analysis aims to analyze the ability of the membrane to absorb water. A membrane that has a high water uptake means it has a large ability to interact with water (Lusiana et al., 2019). The water uptake test was applied to all types of membranes, namely control type, type 1, type 2, type 3, and type 4. This test was carried out by weighing before immersion. After that, the membrane was immersed in water for 24 hours. Furthermore, the membrane resulting from immersion is weighed, to compare the weight before immersion. Based on the test results data in table 3., the water uptake results have increased in type 1, type 2, and type 3.
The best test results show that the control type has a value of 2.24, type 1 shows a value of 11.33, type 2 shows a value of 12.85, type 3 shows a value of 12.86, and type 4 shows a value of 1.52. Based on the results of these calculations, with the addition of chitosan-sodium dodecyl sulfate water uptake is significantly higher in type 1, type 2, and type 3. This is due to the fact that chitosan has many functional group structures such as amines and hydroxyl (NH2, and OH), these groups can form intermolecular interactions with water. With the presence of this functional group, it will cause the incoming water to have a direct chemical interaction with the cellulose acetate-chitosan-sodium dodecyl sulfate membrane inside, so that the functional groups on the membrane will attract hydrogen in the water. Hydrogen bond interactions are polar bonds between hydrogen atoms that are bonded to other atoms with large electronegativities and have a lone pair of electrons (Awik et al., 2019). Hydrogen bonding occurs in compounds of hydrogen atoms with atoms that have high electronegativities such as (O, N, and F). Whereas in type 4 there is a decrease in the ability to absorb water caused by an inhomogeneous solution, so that the chitosan in the solution does not completely dissolve.
This is also supported by previous research conducted by Safitri et al. in 2020, which stated that by adding water to interact with N-carboxymethyl chitosan, hydrogen interactions would occur, resulting in an increase in the water uptake value (Safitri et al., 2020). When compared between type 1, type 2, and type 3 which has the highest water uptake value among the others and the difference is not significantly different are type 2 and type 3. Based on this, the water uptake using type 2 is good enough, so you don't have to add chitosan-sodium dodecyl sulfate to the same amount as type 3. However, this must
16
Tipe Wd Ww Ww-Wd Water Uptake(%) Kontrol 0,01795 0,05819 0,04024 2,24178273 Tipe 1 0,00525 0,06474 0,05949 11,3314286 Tipe 2 0,00329 0,04556 0,04227 12,8480243 Tipe 3 0,00283 0,03923 0,0364 12,8621908 Tipe 4 0,0124 0,03124 0,01884 1,51935484
Source: Researcher Data Processing, 2023
be in accordance with the characteristics of other membranes which will be discussed in the following discussion.
Source: Researcher Data Processing, 2023
Source: Researcher Data Processing, 2023
Water flux is carried out to measure the ability of the membrane to pass the volume of water that passes through the membrane per unit surface area of the membrane per unit time. Prior to testing, membranes with a diameter of 4 cm were compacted with distilled water at a pressure of 2 bar for 30 minutes. After that, a water flux test was carried out by measuring the volume of water through the membrane for 5 minutes, 10 minutes, and 15 minutes. In table 4., shows the results of the water flux which has decreased. Overall, based on the tests that have been carried out, the results show that it decreases with the length of time the membrane is used. In the variation of the addition of chitosan-sodium dodecyl sulfate type 1 and type 3, the ability of the membrane to pass water decreased but did not differ significantly between the two. In type 3, it has decreased with a low water passing value. In type 2, after several tests with a pressure of 2 bar, the membrane leaked because the membrane was torn in the middle.
It can be seen from the results of the type 1 water flux test with 2% chitosan-sodium dodecyl sulfate composition, which has a higher value but not significant when compared to types 3 and 4. This shows that the greater the chitosan-sodium dodecyl sulfate content,
17
4. Water flux
Tipe Membran Fluks 5 Menit 10 Menit 15 Menit Tipe 1 4,39 1,06 0,53 Tipe 2 - -Tipe 3 3,87 1,29 0,68 Tipe 4 1,97 0,71 0,39
Table 4. Water flux test results
Figure 8. Results of the Water Flux Test
4.39 1.06 0.53 0 0 0 3.87 1.29 0.68 1.97 0.71 0.39 0 1 2 3 4 5 Menit ke-5 Menit ke-10 Menit ke-15
Tipe 1 Tipe 2 Tipe 3 Tipe 4
Hasil Pengujian Fluks Air
the less the ability to water through the membrane because of the small pores or the amount is few. Based on the statement of Lusiana et al., a membrane with a high flux will also produce a high permeate (Lusiana et al., 2020). The low value of other types of flux can be caused by fouling on the membrane. This can be minimized by washing with humic acid which can significantly reduce the level of fouling. However, it is also necessary to pay attention to the porosity of the membrane (Tan, Chew and Krantz, 2016).
5. Hydrophilicity
Table 5. Hydrophilicity Test Results
Source: Researcher Data Processing, 2023
Hydrophilicity was carried out using a water contact angel dataphysics Germany tool with the sessile drop method. This test is applied to all types or variations of membranes to determine the membrane contact angle. Hydrophilicity is done by dripping water, then the contact angle formed is measured using a water contact angel dataphysics Germany tool. Low water contact angle, shows high hydrophilicity (Putra, 2017). Shown in table 5., the results of the hydrophilicity test which have a small value angle / have a high hydrophilicity value are type 2.
The results of the hydrophilicity test on type 1 membranes produced an average value of 58.3, type 2 had an average value of 52.7, type 3 had an average value of 53.0, type 4 had an average value of 58, 2, and in the control type of 57.7. This shows that the type 2 membrane has more hydrophilic properties than the control type membrane (cellulose acetate). If it is related to the results of porosity and water uptake, the type 2 membrane has a porosity value that is significantly different from type 1 (the best result) and not significantly different from the control membrane. Whereas in the water uptake results, membrane type 2 has a high value compared to other types and control membranes. This is in accordance with the statement of Buana et al. namely the addition of sodium dodecyl sulfate to the cellulose acetate membrane with the right composition can increase the value of hydrophilicity (Buana, Indarti and Asnawati, 2014). The addition of hydrophilic sodium dodecyl sulfate is capable of forming a hydrogen chain between the membrane and H2O (Lusiana et al., 2019).
18
Tipe Membran Rata-rata Hidrophilicity Kontrol 57,7 Tipe 1 58,3 Tipe 2 52,7 Tipe 3 53,0 Tipe 4 58,2
C. Effect of the addition of Chitosan-Sodium Dodecyl Sulfate on the final characterization of the best dope membranes

1. Tensile Strength Table 6. Tensile Strength Test Results
Source: Pengolahan Data Peneliti, 2023
The tensile strength test of the membrane was carried out in the UNIKA integrated laboratory using the Lloyd tool. This test is carried out to determine the maximum stress of the membrane and determine the mechanical properties of the membrane. Based on the tests that have been carried out, the results are shown in table 6, which is 513.91 N/m2 on the control membrane. Meanwhile, for the type 1 membrane, the yield was 931.57 N/m2. The results of this test showed that the addition of chitosan-sodium dodecyl sulfate caused the type 1 membrane to be constantly stronger than the control membrane (cellulose acetate).
This is in accordance with Setiani, et al. in 2013, which stated that the addition of chitosan could increase the tensile ability of the hemodialysis membrane (Setiani, et al. 2013). This increase is caused by chitosan which can form hydrogen bonds between chains, thereby increasing the density of the membrane. In addition, this is also supported by Barkoula et al. that the addition of chitosan can increase the tensile ability of the membrane even though cellulose acetate has brittle properties (Barkoula et al., 2008).
Source: Researcher Data Processing, 2023'
19
Sample Stiffness (N/m2) Young's Modulus (MPa) Extension at Maximum (mm) Tensile Strength (MPa) Membran Kontrol 513,9118 19,27169 1,670483 0,18142033 membran Tipe 1 931,5703 34,9338867 8,33794133 0,64275233
2. Scanning Electron Microscopy (SEM)
Figure 9. SEM Test Results
Morphological characterization and surface images of the membrane were obtained through a Scanning Electron Microscopy (SEM) test with an accelerating voltage of 3.0 kV and a magnification of 5000 times which then obtained data on the pore size of the membrane, as follows: (a) the cellulose acetate control membrane has an identifiable pore size of between 23,103 nm to 59,103 nm and (b) cellulose acetate/chitosan/sodium dodecyl sulfate type 1 membrane with variation of chitosan-sodium dodecyl sulfate 0.2 gram has an identifiable pore size of between 138.103 nm to 16.103nm. The pore size of the type 1 membrane which is larger than the size of the urea particles indicates compatibility with the ability of the membrane to pass urea which is 4.05 Å in size from the membrane pores (Siahaan et al., 2021). Although type 1 membranes have a larger pore size than protein particles which are 140 × 40 × 40 Å, in the cross section of the membrane it appears dense. This makes it difficult for protein particles to pass through the membrane even though they are smaller in size. The characterization of type 1 membranes that have more uniform pores also supports the flux test data which states that the test results on type 1 membranes are better than the control membranes.
Source: Researcher Data Processing, 2023
Figure 10 shows the FTIR spectrum and the characteristic peaks of the functional groups of cellulose acetate, chitosan, chitosan-sodium dodecyl sulfate, and type 1 membrane, the membrane chosen as the best dope. For the spectrum of cellulose acetate, at a wavelength of 1033cm-1 and 1219cm-1 a C-O bond and a C=O bond are formed at a wavelength of 1731cm-1. The wave spectrum of cellulose acetate is also seen in type 1 membranes which show the presence of C-O bonds at a wavelength of 1165cm-1 and C=O bonds at a wavelength of 1743cm-1. In the chitosan spectrum, a C-C bond is formed at a wavelength of 1425cm-1 and a -NH bond at a wavelength of 1645cm-1. The -NH group

20
3. Fourier Transform Infrared (FTIR)
Figure 10. FTIR Test Result
4.
that appears in the chitosan spectrum is also formed at a wavelength of 1628 cm-1 in type 1 membranes. This is in accordance with research by (Silverstein, et al., 1981) where the NH functional group is also formed at a wavelength of 1629.7cm-1 . The peak at wavelength 1095 cm-1 in the chitosan-sodium dodecyl sulfate spectrum is associated with the presence of an S-O bond that appears. This bond also appears in the type 1 membrane spectrum atawavelengthof1133cm-1whichindicatesthepresenceof thechitosan-sodium dodecyl sulfate functional group in the membrane dope. The presence of functional groups in the spectrum of chitosan, chitosan-sodium dodecyl sulfate, and cellulose acetate which are also formed in type 1 membranes indicates that type 1 membranes already have 3 combinations of materials used in membrane manufacture.
The performance of the membrane can be determined from the flux results and several accompanying tests. The value of the flux test results shows the flow rate of permeate through the membrane, while the rejection coefficient of the membrane is an illustration of the membrane's ability to hold solute molecules. Prior to testing, the membrane was compacted for 30 minutes so that the flux value obtained was constant. The performance test in this study used urea and protein (Bovine Serum Albumin).
Source: Researcher Data Processing, 2023
Based on the water flux test that was carried out previously, it was found that type 1 membrane has the ability to pass water better than the others. So the control and type 1
21
Flux Performance
A. Urea Flux
Tipe 1 Fluks Permeabilitas (%) 5 Menit 10 Menit 15 Menit Kontrol 5,37 1,94 2,49 15.200 Tipe 1 16,25 3,60 3,05 36.400 Source: Researcher Data Processing, 2023
Table 7. Urea Flux Test Results
Figure 11. Urea Flux Test Results
0 2 4 6 8 10 12 14 16 18 Menit ke-5 Menit ke-10 Menit ke-15 Hasil Pengujian
Urea Kontrol Tipe 1
Fluks
membranes were tested for performance to determine the ability of the membrane to pass the amount of urea through the membrane per unit surface area of the membrane per unit time. Prior to this test, the membrane with a diameter of 4 was compacted using distilled water at a pressure of 2 bar for 30 minutes. After that, replace the aquadest solution with urea and measure the volume of urea that passes through the membrane every 5 minutes for 15 minutes.
Based on the tests that have been carried out, the results of the control membrane have decreased, but urea through the membrane can constantly come out. While type 1 membrane has the ability to pass urea with a higher value than the control membrane. The permeability results also show that type 1 has a higher value, when compared to the control membrane. The test results in table 5 show that more urea passes through the type 1 membrane than the control. In addition, this also shows that type 1 membranes experience additional pores due to the addition of sodium dodecyl sulfate. The size of this pore is able to pass urea with a size of 4.05 Å (Siahaan et al., 2021). This is in accordance with the statement of Buana et al., that the addition of sodium dodecyl sulfate can increase the rejection ability of the membrane (Buana, et al. 2014). The test results also show that if the flux is high, then the permeability of the membrane is high. In other words, type 1 membranes are able to pass urea better than controls.
22
B. Protein Flux
Table 8. Protein Flux Test Results
Tipe Membran Fluks
Source: Researcher Data Processing, 2023
Hasil Pengujian Fluks Protein
Source: Researcher Data Processing, 2023
Apart from using urea to test membrane performance, Bovine Serum Albumin (BSA) is also used as a performance test material with protein. Prior to testing, membranes with a diameter of 4 cm were compacted using distilled water for 30 minutes at a pressure of 2 bar. Furthermore, BSA volume measurements were taken every 5 minutes for 15 minutes.
Based on the tests that have been carried out, the control type obtained greater results than the results of type 1. The results of this test indicate that it is more difficult for the protein to pass through the type 1 membrane or has good performance when compared to the control membrane. Although there is an increase in the number of pores by sodium dodecyl sulfate, many functional groups are formed. The many functional groups that are formed, are able to bind more proteins and make it difficult to pass through the membrane (Bomini et al., 2006). In addition, based on Figure 9., the SEM (cross section) is dense and there are few cavities so that it can hold the protein measuring 140 × 40 × 40 Å from escaping with the urea. Whereas the control membrane has more values than type 1, resulting in wasted protein. The difference in protein flux values between the control membrane and type 1 membrane was quite significant. This performance flux test shows that type 1 membrane can pass urea well, but the membrane remains selective for protein.
23 5 Menit 10 Menit 15 Menit Kontrol 11,03 3,22 2,22 Tipe 1 3 0,59 1,64
Figure 12. Results of Protein Flux Test
0 2 4 6 8 10 12
Menit ke-5
Menit ke-10
Menit ke-15
Tipe Kontrol Tipe 1
CHAPTER V CONCLUSIONS AND RECOMMENDATIONS
A. Conclusion
Based on the research that has been done, it can be concluded that type 1 membrane with the addition of 2% chitosan-sodium dodecyl sulfate to cellulose acetate can be used as a hemodialysis membrane. The synthesis of cellulose acetate was carried out through the stages of preparation, bleching, and acetylation. At the stage of preparation of banana peels that have been softened like porridge, 17.5% NaOH solution is added and allowed to stand for 24 hours. Then do the filtering. Before going through the acetylation process, bleaching was carried out using H2O2 sothat the color ofthecelluloseacetate produced was notdarkbrown. Furthermore, theacetylation process which begins with adding cellulose with glacial acetic anhydride. This activity is called swelling which aims to activate the cellulose and will expand the surface of the cellulose acetate. Then add glacial acetic acid. The cellulose acetate obtained was then tested by Fourier Transform Infrared (FTIR), then it was found that the synthesized cellulose acetate can be used as an environmentally friendly membrane material and reduce organic waste.
To determine how to create the membrane, it was produced by several steps. First, Manufacturing the mixture of Cs-SDS by making a homogeneous solution of chitosan and sodium dodecyl sulfate. This was done by dissolving 1.5 g of chitosan in 100 ml of 1% acetic acid. Then make a solution of 3 g of sodium dodecyl with 100 ml of water. Then mix the two solutions until a perfect precipitate forms. Second, the Synthesis of Cellulose Acetate from Kepok Banana Peels. It was carried out in several stages. The preparation stage is to smooth the clean banana peel. The main ingredient are the undissolvable materials namely alpha-cellulose. The alpha-cellulose obtained was then separated from the solution. After that, it was dried at 40°C and obtained alphacellulose solids. Then proceed with the acetylation reaction step by adding acetic anhydride solution with a mass ratio of glacial acetic acid (1:1). Subsequently, the cellulose was mixed with glacial acetic anhydride for 6 hours with the reaction temperature maintained at 45°C. Followed by a neutralization step. The result obtained from the acetylation reaction is a material in the form of a yellowish-white cellulose acetate lump. Third, Manufacturing of Dope Membranes. Making a dope solution using acetone as a solvent from cellulose acetate so that it can become a homogeneous solution with chitosan and sodium dodecyl sulfate. The composition of polymer materials and additives will be varied, while cellulose acetate has the same ratio. Fourth, Membrane printing using the Non-Solvent Induced phase separation (NIPS) method, Membrane printing is done by pouring dope solution which has been left for 24 hours onto a square glass plate. Then swipe the solution on the media support or through a certain media formation. Then immersed in water until solidification occurs.
The best membrane chemical and physical characteristics/type 1 with a hydrophilicity value of 58.3 and a water uptake test of 11.33%. The results of the hydrophilicity test had a significant difference with the type 1 membrane and not significantly with the control membrane (cellulose acetate). Furthermore, the results of the water uptake test show that there is a difference of 1.53% with the membrane with the highest water uptake value. Based on Scanning Electron Microscopy (SEM) testing, type 1 membranes have pores of uniform size. In the water flux test, type 1 membrane has the highest value among type 2, type 3, and type 4. Furthermore, in the
24
performance flux test, type 1 membrane shows that the membrane can pass urea well, but the membrane remains selective for protein.
The best membrane chemical and physical characteristics/type 1 with a hydrophilicity value of 58.3 and a water uptake test of 11.33%. The results of the hydrophilicity test had a significant difference with the type 1 membrane and not significantly with the control membrane (cellulose acetate). Furthermore, the results of the water uptake test show that there is a difference of 1.53% with the membrane with the highest water uptake value. Based on Scanning Electron Microscopy (SEM) testing, type 1 membranes have pores of uniform size. In the water flux test, type 1 membrane has the highest value among type 2, type 3, and type 4. Furthermore, in the performance flux test, type 1 membrane shows that the membrane can pass urea well, but the membrane remains selective for protein.
B. Suggestion
Based on the research that has been done, suggestions can be put forward as to the need for cooperation between the government, researchers and related institutions to develop further research on membrane mixtures of sodium dodecyl anionic surfactant additives with environmentally friendly materials chitosan and semi-permeable cellulose acetate. This development is expected to be able to find a better formulation by exploring or utilizing environmentally friendly natural resources in Indonesia, so as to create a Golden Indonesia 2045 which is able to rely on domestic technology.
25
REFERENCE
Agustina, K. and Dewi, T. K. (2013) ‘Strategi Coping pada Family Caregiver Pasien Gagal Ginjal Kronis yang menjalani Hemodialisa’, Jurnal Psikologi Klinis dan Kesehatan Mental, 02(03), pp. 7–16. Available at: http://journal.unair.ac.id/strategi-coping-pada-familycaregiver-pasien-gagal-ginjal-kronis-yang-menjalani-hemodialisa-article-8761-media-51category-10.html.
Amiji, A. A. (1995) ‘Permeability and blood compatibility properties of chitosan-poly(ethylene oxide) blend membranes for haemodialysis’, Biomaterials, pp. 593–599.
Aulia (2017) Ginjal Kronis, Kementerian Kesehatan Republik Indonesia. Available at: http://www.p2ptm.kemkes.go.id/ (Accessed: 15 February 2021).
Awik, P. D. et al. (2019) ‘Visualisasi Interaksi Ligan Ibuprofen terhadap Protein Cyclooxygenase secara Komputasi Visualization of the Computational Interaction of Ibuprofen Ligands on Cyclooxygenase Protein Molecular Docking’, Proceeding Biology Education Conference, 16(1), pp. 2–5.
Barkoula, N. M. et al. (2008) ‘Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene’, Polymers and Polymer Composites, 16(2), pp. 101–113. doi: 10.1002/pc.
Bayhakki (2012) Klien Gagal Ginjal Kronik. Jakarta: EGC.
Bonomini, M. et al. (2006) ‘Proteomics characterization of protein adsorption onto hemodialysis membranes’, Journal of Proteome Research, 5(10), pp. 2666–2674. doi: 10.1021/pr060150u.
Buana, E. S., Indarti, D. and Asnawati (2014) ‘Pengaruh Penambahan Surfaktan Anionik Sodium Dodesil ( Effect of Addition Anionic Surfactant Sodium Dodecyl Sulphate OnThe Cellulose Acetate Membrane Characteristics)’, Berkala Sainstek, II(1), pp. 49–53.
Chan, C. T. et al. (2013) ‘Novel techniques and innovation in blood purification: A clinical update from Kidney Disease: Improving Global Outcomes’, Kidney International, 83(3), pp. 359–371. doi: 10.1038/ki.2012.450.
Duolikun, T. et al. (2020) ‘Asymmetric cellulosic membranes: Current and future aspects’, Symmetry, 12(7). doi: 10.3390/sym12071160.
El-banna, F. S. et al. (2019) ‘applied sciences Chitosan as as a a Natural Natural Copolymer Copolymer with with Unique Unique Chitosan Properties for for the the Development Development of of Hydrogels Hydrogels Properties’, Applied Sciences, 9(2193), pp. 1–11.
Gaol, M. R. L. L. et al. (2013) ‘Pembuatan Selulosa Asetat Dari Α -Selulosa Tandan Kosong Kelapa Sawit’, Jurnal Teknik Kimia USU, 2(3), pp. 33–39. doi: 10.32734/jtk.v2i3.1447.
J Bras Nefrol. 2019 Jul-Sep; 41(3): 310–311. Published online 2019 Aug 1. doi: 10.1590/21758239-JBN-2019-0098
Hamdi, A. S. and Bahruddin, E. (2014) Metode Penelitian Kuantitatif Aplikasi dalam pendidikan 1st edn. Edited by A. Anas. Yogyakarta: Deepublish. Available at: books.google.id.
Lestari, P. (2012) PEMBUATAN DAN KARAKTERISASI MEMBRAN KOMPOSIT KITOSAN
26
–
SELULOSA DIASETAT – TiO 2 UNTUK PENGOLAHAN LIMBAH DETERJEN, Skripsi Universitas Airlangga.
Lusiana, R. A. et al. (2013) ‘The influence of PVA.cl.citric acid/chitosan membrane hydrophicility on the transport of creatinine and urea’, Indonesian Journal of Chemistry, 13(3), pp. 262
270. doi: 10.22146/ijc.21286.
Lusiana, R. A. et al. (2019) ‘Synthesis and characterization of composite polyethersulfone (PES) membranes with polyethylene glycol (PEG) and heparin-chitosan (Hep-CS)’, IOP Conference Series: Materials Science and Engineering, 509(1). doi: 10.1088/1757899X/509/1/012123.
Lusiana, R. A. et al. (2020) ‘Permeability improvement of polyethersulfone-polietylene glycol (PEG-PES) flat sheet type membranes by tripolyphosphate-crosslinked chitosan (TPP-CS) coating’, International Journal of Biological Macromolecules, 152, pp. 633–644. doi: 10.1016/j.ijbiomac.2020.02.290.
Lusiana, R. A., Sangkota, V. D. A. and Santosa, S. J. (2018) ‘Chitosan succinate/PVA-PEG Membrane: Preparation, Characterization and Permeation Ability Test on Creatinine’, Jurnal Kimia Sains dan Aplikasi, 21(2), pp. 80–84. doi: 10.14710/jksa.21.2.80-84.
Maiti and Bidinger (2020) ‘PEMBUATAN DAN KARAKTERISASI MEMBRAN PADUAN KITOSAN- POLIETILENGLIKOL6000’, Journal of Chemical Information and Modeling, 14(9), pp. 1689–1699.
Meriatna (2008) Penggunaan Membran Kitosan untuk Menurunkan Kadar Logam Krom (Cr) dan Nikel (Ni) dalam Limbah Cair Industri Pelapisan Logam, Tesis. Universitas Pasca Sarjana.
Mulder, M. (1991) Basic Principles of Membran Technology. 2nd edn. Netherlands: Kluwer Academic Publisher.
Nugrahani, F. (2014) Metode Penelitian Kualitatif: dalam Penelitian Pendidikan Bahasa 1st edn. Surakarta: Cakra Books. Available at: http://digilibfkip.univetbantara.ac.id/.
Nefrol, J Bras. (2019). Geriatric Assesment in Elderly Hemodialysis Patients.
Paristya, W., Arnelli and Cahyono, B. (2013) ‘Formulasi Larutan Detergen Dari Natrium Dodesil Sulfat Dan Sintesis Natrium Dodesilbenzena Sulfonat’, Chem Info, 1(1), pp. 43–50.
Prabowo, E. and Pranata, A. E. (2014) Buku Ajar Asuhan Keperawatan Sistem Perkemihan Yogyakarta: Nuha Medika.
Prestisya, I. A. (2016) PEMBUATAN DAN KARAKTERISASI MEMBRAN NANOFIBER SELULOSA ASETAT DENGAN TEKNIK ELECTROSPINNING SEBAGAI HEMODIALISIS KREATININ, Skripsi. Universitas Airlangga.
Putra, D. I. (2017) Blending Polymer Selulosa Asetat/Polisulfon Terhadap Ketahanan Fouling Protein. Jember.
Rachmanto, B. (2019) Tehnik dan Prosedur Hemodialisa. Available at: rsjdsurakarta.jatengprov.go.id (Accessed: 17 February 2021).
Rahman, M. T. S. A., Kaunang, T. M. D. and Elim, C. (2016) ‘Hubungan antara lama menjalani hemodialisis dengan kualitas hidup pasien yang menjalani hemodialisis di Unit Hemodialisis’, Jurnal e-Clinic (ECL), 4(1), pp. 36
40.
27
–
–
Ratnawati (2011) ‘tingkat kecemasan pasien dengan tindakan hemodialisa di BLUD RSU Dr. M .M Dunda Kabupaten Gorontalo’, Health and Sport, 3(2), pp. 285–362. Available at: http://ejurnal.ung.ac.id/index.php/JHS/article/view/88.
Rivandi, J. and Yonata, A. (2015) ‘Hubungan Diabetes Melitus Dengan Kejadian Gagal Ginjal Kronik’, Medical Jurnal of Lampung University, 4(9), pp. 27–34. Available at: http://juke.kedokteran.unila.ac.id/index.php/majority/article/view/1404/1246
Riskesdas. 2013. Laporan Nasional Badan Penelitian Dan Pengembangan Kesehatan. Departemen Kesehatan. RI.
Safitri, B. et al. (2020) ‘Intermolecular hydrogen bond interactions in N -carboxymethyl chitosan and n H2O: DFT and NBO studies’, AIP Conference Proceedings, 2237(June). doi: 10.1063/5.0005287.
Sari, S. H. (2012) Hubungan Kecepatan Aliran Darah Dializer Dengan Penurunan Kadar Kreatinin Post Hemodialisis Pada Pasien Gagal Ginjal Kronik. Universitas Hasanuddin. Available at: digilib.unhas.ac.id.
Setiani, W., Sudiarti, T. and Rahmidar, L. (2013) ‘Preparasi Dan Karakterisasi Edible Film Dari Poliblend Pati Sukun-Kitosan’, Jurnal Kimia VALENSI, 3(2). doi: 10.15408/jkv.v3i2.506.
Siahaan, P. et al. (2021) ‘The validation of molecular interaction among dimer chitosan with urea and creatinine using density functional theory: In application for hemodyalisis membrane’, International Journal of Biological Macromolecules, 168, pp. 339–349. doi: 10.1016/j.ijbiomac.2020.12.052.
Silverstein, R. M., Bassler, G. C. and Morrill., T. C. (1981) ‘Spectrometric Identification of Organic Compounds’.
Sudoyo, A. W. et al. (2006) Buku Ajar Ilmu Penyakit Dalam. Jakarta: Pusat Penerbitan Departemen Ilmu Penyakit Dalam FKUI.
Suwitra, K. (2006) ‘Penyakit Ginjal Kronik, dalam Sudoyo AW’, Pusat Penerbitan Dapartemen Ilmu Penyakit Dalam FKUI, 1, pp. 581–584.
Syamsu, K. and Kuryani, T. (2014) ‘Cellulose acetate biofilm production from microbial cellulose nata de cassava’, E-Jurnal Agroindustri Indonesia, 3(1), pp. 126–133.
Tan, Y. Z., Chew, J. W. and Krantz, W. B. (2016) ‘Effect of humic-acid fouling on membrane distillation’, Journal of Membrane Science, 504, pp. 263–273. doi: 10.1016/j.memsci.2015.12.051.
Wahyuliswari, R. F. (2015) EFEK VARIASI SUHU BAK KOAGULAN TERHADAP KARAKTERISTIK HOLLOW FIBER DIALISER BERBAHAN PADUAN SELULOSA ASETAT – D-GLUKOSA MONOHIDRAT. Universitas Airlangga. Available at: http://repository.unair.ac.id/27912/.
Widyastuti, R., Butar-Butar, W. and Bebasari, E. (2014) ‘Korelasi Lama Menjalani Hemodialisis Dengan Indeks Massa Tubuh Pasien Gagal Ginjal Kronik di RSUD Arifin Achmad Provinsi Riau Pada Bulan Mei Tahun 2014’, Jurnal Online Mahasiswa Fakultas Kedokteran, 1(2), pp. 1
12.
28
–
APPENDIX




 Appendix 1. Control Membrane, Type 1, Type 2, Type 3, and Type 4
Membrane Condition
Appendix 1. Control Membrane, Type 1, Type 2, Type 3, and Type 4
Membrane Condition
Appendix 2. Hydrophilicity Testing





 Control Membrane (Cellulose Acetate)
Membrane Type 1
Control Membrane (Cellulose Acetate)
Membrane Type 1





 Membrane Type 2
Membrane Type 3
Membrane Type 2
Membrane Type 3


 Membrane Type 4
Membrane Type 4






 Appendix 3. Scanning Electron Microscopy (SEM)
Appendix 3. Scanning Electron Microscopy (SEM)







 Cross section Control Membrane
Cross section Control Membrane







 Scanning Electron Microscopy (SEM) Control Membrane
Scanning Electron Microscopy (SEM) Control Membrane















 Cross section Type 1 Membrane
Cross section Type 1 Membrane



 Scanning Electron Microscopy (SEM) Type 1 Membrane
Scanning Electron Microscopy (SEM) Type 1 Membrane
Comparison of Vitamin D Supplementation Effects on Elderly With and Without Dementia Symptoms: A Systematic Review
Pre-Conference Competition Asian Medical Students’ Conference 2023

Authors:
Vannisa Fadya
Siti Sarah Safira
FACULTY OF MEDICINE
UNIVERSITY OF SYIAH KUALA
Comparison
of Vitamin D Supplementation Effects on Elderly With and Without Dementia Symptoms: A Systematic Review
Vannisa Fadya1, Siti Sarah Safira1
1Faculty of Medicine University of Syiah Kuala
Abstract
Introduction: Dementia is a term for several diseases that affect memory, thinking, and the ability to perform daily activities It can be caused by a number of diseases which over time destroying nerve cells and damaging the brain that leads to deterioration of cognitive function Dementia may also develop because nutritional deficiencies, especially vitamin supplementation A study found that a lack of vitamin D was associated with a substantial increase in the risk of all causes of dementia. Therefore, this systematic review to compare the effects of vitamin D supplementation in elderly with dementia and without dementia.1
Objective: The aim of this systematic review is to compare the effects of vitamin D supplementation in elderly with dementia and without dementia.
Method: This systematic review was reported based on PRISMA. The literature search was conducted on several databases such as PubMed, Science Direct, Wiley, and SpringerLink. Result were shown as mean difference (MD) and standard deviation (SD) or median Risk of bias was assessed using the Cochrane risk of bias tool for randomized trials (RoB 2).
Result: Samples of healthy women receiving vitamin D showed no statistically significant difference over time (p=0.23) and the MMSE scores increased in the control and case group.2 While other 2 studies on samples of elderly with cognitive impairment (MCI and Alzheimer disease) showed an increment of FSIQ score significantly higher in the intervention group than in the placebo group (p<0.001).9,10
Conclusion: This systematic review showed evidence that there are no significant differences in the administration of vitamin D supplements for elderly with normal cognitive abilities, but it has a very important correlation in improving cognitive function for elderly with dementia symptoms.
Keyword: dementia, vitamin D, elderly.
Comparison of Vitamin D Supplementation Effects on Elderly With and Without Dementia Symptoms: A Systematic Review
Pre-Conference Competition Asian Medical Students’ Conference 2023

Authors:
Vannisa Fadya
Siti Sarah Safira
FACULTY OF MEDICINE
UNIVERSITY OF SYIAH KUALA
Comparison of Vitamin D Supplementation Effects on Elderly With and Without Dementia Symptoms: A Systematic Review
Vannisa Fadya1, Siti Sarah Safira1
1Faculty of Medicine University of Syiah Kuala
Abstract
Introduction: Dementia is a term for several diseases that affect memory, thinking, and the ability to perform daily activities. It can be caused by a number of diseases which over time destroying nerve cells and damaging the brain that leads to deterioration of cognitive function. Dementia may also develop because nutritional deficiencies, especially vitamin supplementation A study found that a lack of vitamin D was associated with a substantial increase in the risk of all causes of dementia Therefore, this systematic review to compare the effects of vitamin D supplementation in elderly with dementia and without dementia 1
Objective: The aim of this systematic review is to compare the effects of vitamin D supplementation in elderly with dementia and without dementia.
Method: This systematic review was reported based on PRISMA. The literature search was conducted on several databases such as PubMed, Science Direct, Wiley, and SpringerLink. Result were shown as mean difference (MD) and standard deviation (SD) or median Risk of bias was assessed using the Cochrane risk of bias tool for randomized trials (RoB 2).
Result: Samples of healthy women receiving vitamin D showed no statistically significant difference over time (p=0.23) and the MMSE scores increased in the control and case group.2 While other 2 studies on samples of elderly with cognitive impairment (MCI and Alzheimer disease) showed an increment of FSIQ score significantly higher in the intervention group than in the placebo group (p<0.001).9,10
Conclusion: This systematic review showed evidence that there are no significant differences in the administration of vitamin D supplements for elderly with normal cognitive abilities, but it has a very important correlation in improving cognitive function for elderly with dementia symptoms
Keyword: dementia, vitamin D, elderly.
Introduction
Dementia is a term for several diseases that affect memory, thinking, and the ability to perform daily activities. It can be caused by a number of diseases which over time destroying nerve cells and damaging the brain that leads to deterioration of cognitive function (i.e., the ability to process thought) more than biological aging process. While consciousness is not affected, the cognitive impairment is commonly accompanied, and occasionally preceded, by changes in mood, unstable emotional control, changes in behavior, or motivation and comes in many forms, such as alzheimer, lewy body, frontotemporal, and vascular dementia. Alzheimer disease is the most common form and may contribute to 60–70% of cases. Dementia may also develop after a stroke or in the context of certain infections such as HIV, as a result of harmful use of alcohol, repetitive physical injuries to the brain (known as chronic traumatic encephalopathy) or nutritional deficiencies, especially vitamin supplementation.1
Globally, the prevalence of dementia in 2018 was 50 million, a figure estimated to reach 82 million by 2030 and 152 million by 2050. In May 2017, the WHO recognize dementia as a public health priority, endorsing the Global Action Plan on The Public Health Response to Dementia 2017–2025, which defines a set of actions to promote dementia awareness and research, facilitate the diagnosis, treatment, and support for caregivers and families, and suggest measures to reduce the risk of dementia. One of the supports that can be given is in the form of adequate nutrition which includes supplementary nutrition like vitamins and minerals 1
Vitamin D has a neuroprotective role by promoting amyloid metabolism, clearance in the brain, neuronal and synaptic growth, and neurotransmission.2 Vitamin D obtained from sun exposure, foods, and supplements, is biologically inert and must undergo two hydroxylation process in the body to be activated. The first hydroxylation, which occurs in the liver, converts vitamin D to 25-hydroxyvitamin D [25(OH)D], also known as “calcidiol.” The second hydroxylation occurs primarily in the kidney and forms the physiologically active 1,25dihydroxyvitamin D [1,25(OH)2D], known as “calcitriol”. Vitamin D has other roles in the body, including reduction of inflammation as well as modulation of such processes as cell growth, neuromuscular and immune function, and glucose metabolism.3
An individual can develop vitamin D deficiency when daily intakes are lower than recommended amount over time, limited exposure to sunlight, kidneys inability to convert 25(OH)D into its active form, or inadequate vitamin D absorption in digestive tract. Low vitamin D diets is more common in people with milk allergy or lactose intolerance and those who consume an ovo-vegetarian or vegan diet. Group at risk of vitamin D inadequacy are people with limited sun exposure, people with conditions that limit fat absorption, older adults, and obesity. Older adults are at increased risk of developing vitamin D insufficiency, partly because the skin’s ability to synthesize vitamin D declines with age. In addition, older adults are likely to spend more time than younger people indoors, and they might have inadequate dietary intakes of the vitamin.4
A study found that a lack of vitamin D was associated with a substantial increase in the risk of all causes of dementia But it is still debatable regarding the correlation between vitamin D and dementia. However, vitamin D helps protect nerve tissue from oxidative stress and ensures a balance of calcium levels in the blood which will provide the main signal in cells and ensure that neurons fire properly.4 Certainly, as individuals get older, oxidative stress and aging
of organs and systems can cause cognitive function to not be as good as it was at a young age. However, a study explains that vitamin D can help slow the process. In a meta-analysis of the effects of vitamin D, it was reported a clear link between vitamin D levels and age-related cognitive function.5
This review compares the effect of vitamin D supplementation in elderly with dementia and without dementia. In this context, this study will answer the following questions: i) Does vitamin D affect cognitive function in the elderly with dementia? and ii) Does vitamin D prevent cognitive decline in healthy elderly? Moreover, the key findings of this study acknowledge the recognition of research gaps that need to be analyzed by future researchers.
Material and Method
Study Methodology
This systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Eligibility Criteria
Criteria considered for this reviews’ eligibility are type of study, samples, outcome measures, index test, and reference standards.
Type of study
Original research articles or research reports of randomized controlled trials (RCT) and cohort studies conducted in humans were included. Narrative review, systematic review, metaanalysis, in silico studies, in vitro studies, in vivo studies, scientific posters, study protocol, and conference abstract were excluded. Additionally, unavailable full-text articles, non-english, and irrelevant topics were excluded.
Samples
Elderly with the age above 65 years with and without cognitive impairment or dementia were included in this review. There was no limitation for the type of cognitive impairment.
Outcome measure
The outcome measure after vitamin D supplementation to the elderly is the mini-mental state examination (MMSE). A Mini-Mental State Examination (MMSE) is a set of 11 questions that doctors and other healthcare professionals commonly use to evaluate for possible cognitive impairment (problems with thinking, communication, understanding and memory). A perfect score is 30 points, a score of 24 is the recommended and most frequently used as the cut-point for dementia; a score of 23 or lower indicates dementia. A cut-off score for lesser forms of impairment, e.g., mild cognitive impairment, has not been established or implemented with any consistency 6
Another outcome is the FSIQ (Full Scale IQ), which is a score derived from administration of selected subtests from the Wechsler Intelligence Scales designed to provide a measure of an individual’s overall level of general cognitive and intellectual functioning.7 The highest score
possible is 145, and the lowest score possible is 61; scores between these two extremes represents just one standard deviation from the mean IQ for that group.8
Index Test
Studies included assessing the MMSE score and FSIQ that presented the data with mean difference and standard deviation or median score. Studies that did not elaborate neither mean nor median data were excluded.
Reference Standard
The reference standards are randomized controlled trial studies and cohort studies by evaluating the vitamin D supplementation on MMSE and FSIQ scores’ alteration.
Data Sources and Search
The literature searching process in this review was carried out with multiple electronic databases, such as Pubmed, ScienceDirect, Wiley, and SpringerLink. The search was conducted from the inception of the database until March 2023. The keywords used in the databases were described using Boolean operators that can be seen in Table 1. The studies were stored in the authors’ library using mendeley group reference manager.
Table 1. Keyword Used in Literature Searching
Database
Pubmed
ScienceDirect
Keyword
“Vitamin D” AND “cognitive impairment OR dementia” AND “elderly OR geriatric”
“Vitamin D” AND “cognitive impairment OR dementia” AND “elderly OR geriatric”
Wiley “Vitamin D” AND “cognitive impairment OR dementia” AND “elderly”
SpringerLink
“Vitamin D” AND “cognitive impairment OR dementia” AND “elderly”
Selection Process
The resulted journals of searching through 4 databases were being screened by 2 reviewers (VF and SSS). Studies of RCTs and cohorts were included. Studies before 2018 were excluded through article type filters of each database. After titles and abstracts screening, duplicate articles were removed. Potentially eligible full-text articles were assessed using the eligibility criteria described above. The study selection process was recorded in the PRISMA flow chart.
Data Extraction and Analysis
Selected studies after screening were extracted for relevant data in Microsoft Excel 2016. The following data were recorded: author, year, country, study design, sample, intervention, follow up period, and MMSE or FSIQ outcome.
Risk of Bias in Individual Studies (Qualitative Synthesis)
The quality of each study included in this systematic review was assessed by two independent reviewers (VF and SSS) according to the Cochrane Risk of Bias (RoB) Tool.
Result
Study Selection
Our search yielded 287 articles from 4 databases (Figure 1). Screening through title and abstract by the reviewers left 28 articles. Eight duplicates from databases were excluded. Then, the authors excluded the articles with several reasons, such as incompatible study design (n=6), irrelevant outcome (n=3), incompatible sample criteria (n=2), review articles (n=3), and inaccessible full-text (n=2).
Identification
Records identified from:
Pubmed (n=223)
ScienceDirect (n=10) Wiley (n=14)
SpringerLink (n=40) (n=287)
Title and abstract exclusion (n=259)
Records screened (n=28)
Duplicates excluded (n=8)




Eligibility
Full-text articles assessed for eligibility (n=20)
Studies included in Systematic Review (Qualitative Analysis) (n=4) Included
Full-text articles excluded, with reasons (n=16)
1. Incompatible study design (n=6)
2. Irrelevant outcome (n=3)
3. Incompatible sample criteria (n=2)
4. Review articles (n=3)
5. Inaccessible full-text (n=2)
Figure 1. PRISMA Flow Diagram
Identification of studies via databases
Scre e ning
Study Characteristics and Results of Individual Studies
The subjects of these studies were elderly aged 65 years and older. We focused on the MMSE or FSIQ examination as the outcome for cognitive function measures. We included 4 studies in our review which suits all the eligibility criteria. (Table 2.)
Table 2. Characteristics of Studies
1 Owusu J.E, et al., 20192
Randomiz ed, doubleblind, placebocontrolled clinical trial
America Healthy postmenopa usal African American women aged 65 and older
Half of the women were randomized to receive vitamin D (adjusted to achieve a serum level > 30 ng/mL) with calcium (n=130), and half were randomized to receive placebo with calcium (1,200 mg, n=130)
3 years - - P=0.23 (no statistically significant difference)
2 Jia, Jingya, et.al., 20199
RCT China Patients with AD aged 65 years and older
AD patients (n=210) were randomly divided into intervention (n=105) and control (n=105) groups. Participants received 12-month 800 IU/day of vitamin D or starch granules as placebo. T
RCT China Participants were all native Chinese 12 months FSIQ mean±S D
Subjects (n=183) were randomized to an intervention group (vitamin D 800 IU/day, n = 93) or a placebo group FSIQ mean±SD (104.17±7. 32)
P<0.001 (statistically significant)
No
Country Sample Intervention Follow up period Outcome control case P value
Author; Year Study design
12 months FSIQ mean±S D (82.45±8 .44) FSIQ mean±SD (92.26±7.9 4) P<0.001 (statistically significant)
3 Yang, Tong, et.al., 202010
4. Arosio,
11
speakers aged 65 years and older with MCI.
Cohort study Italy Older adults (64–92 years) in Milan, Italy (n=509)
(the matching starch granules, n = 90), (96.66±9 .76)
Patients met the criteria of mild cognitive impairment (MCI, n=176), 59 were diagnosed with Alzheimer’s Disease (AD), 26 were diagnosed with idiopathic Normal Pressure Hydrocephalus (iNPH), and 133 were identified as mixed dementia (MD) patients. A total of 115 subjects (controls) were diagnosed without cognitive decline.
- MMSE median (27)
MMSE median AD (21), MCI (27), iNPH (21), MD (20)
P<0.001 (statistically significant)
Beatric e, et.al., 2022
Risk of Bias in Individual Studies
We assessed the quality of each study with the Revised Tool for Risk of Bias (RoB 2) tool in RCT studies (Figure 2.). Most of the studies appear to have clear or low risk of bias. Only one study conducted by Jeanette et al did not mention that the assessor did not know which patient is on what treatment during assessment of the outcome.


A B
Figure 2. Risk of Bias Assessment with RoB tool. (A) Risk of Bias Summary. (B) Risk of Bias Graph
DISCUSSION
Vitamin D and cognitive function in the elderly with dementia
Mild cognitive impairment and dementia pose a high risk to the elderly and can interfere with daily activities. The most common cause at this time is progressive Alzheimer's disease. Therefore, it is important to know how to slow down the incident by delaying cognitive decline and determining what prevention can be done, one of which is providing adequate nutrition to the elderly.1
Our findings of 4 studies showed different results on the effect of vitamin D supplementation to prevent or reduce cognitive decline. We found that samples of healthy women receiving vitamin D showed no statistically significant difference over time (p=0.23) and the MMSE scores increased in the control and case group.2 While other 2 studies on samples of elderly with cognitive impairment (MCI and Alzheimer disease) showed an increment of FSIQ score significantly higher in the intervention group than in the placebo group (p<0.001).9,10
In the study that has been assessed, one of them evaluated whether the consumption of vitamin D supplements in individuals with Alzheimer's disease, which is the main cause of dementia, can slow cognitive decline or not. In the research results, it was found that administration of vitamin D was successful in improving cognitive performance by reduction of amyloid beta (Aβ) biomarkers after 12 months and even after 6 months. Accumulation of Aβ protein in the brain is one of the most important causes of Alzheimer Disease and Aβ protein leads to neuronal dysfunction and death, eventually leading to dementia. In addition, there is also clear evidence of improved cognitive function with the FSIQ test after 12 months of vitamin D supplementation.9
Another study also found similar results to previous studies, where supplementation of vitamin D (800 IU/day) for 12 months also increased cognitive function which was evaluated based on the FSIQ test. However, this study states that the administration of vitamin D must be efficient and highly correlated with the transfer of information in the brain.10
Cohort study with the outcome of MMSE score showed that there was a significant relationship between vitamin D in all subjects, regardless of the presence or absence of cognitive impairment. However, this study stated that the levels of vitamin D in Alzheimer's and Mixed Alzheimer's patients had low levels, whereas the vitamin D levels in MCI patients would be higher than normal, indicating that the role of vitamin D would be different for the type of cognitive impairment. They found that MMSE was lower in patients with AD, iNPH, and MD compared to controls and MCI patients (p < 0.001). Regarding vitamin D, both AD and MD patients were characterized by lower levels of vitamin D compared to MCI patients (p = 0.02 and p < 0.001, respectively). Interestingly, MCI showed higher levels than the other groups (p < 0.001 vs. MD, p = 0.02 vs. AD, p = 0.03 vs. iNPH and p = 0.009 vs. controls) 11
Vitamin D as cognitive impairment prevention in healthy elderly
Meanwhile, we found a study with different results that shows there is no relationship between giving vitamin D with cognitive enhancement in people who have not experienced cognitive decline or are said to be normal. In this case, the population taken is AfricanAmerican women who still have good cognitive abilities with the MMSE score for evaluation,
so the results obtained that there is no relationship in maintaining serum 25(OH)D at greater than 30 ng/mL (Endocrine Society guidelines) in terms of cognition. Cognition improved over time in the older African-American, female population, with no difference between women receiving vitamin D and those receiving placebo. The study population was relatively healthy, and vitamin D levels were given higher than the Institute of Medicine's recommendations.2
Strength and Limitation
This is the first systematic review that compares the effect of vitamin D on healthy elderly and elderly with dementia. In addition, we found studies with low risk of bias. However, there are some limitations of this review such as, the dosage of vitamin D given to the intervention group was varied and making it unknown for the optimal dose needed to prevent or reduce cognitive decline and as cognitive functions are declining progressively, a longer period of follow up is needed to investigate the effect of vitamin D supplementation.
CONCLUSION
It concludes that there are no significant differences in the administration of vitamin D supplements for elderly with normal cognitive abilities, but it has a very important correlation in improving cognitive function for elderly with dementia symptoms. Specifically, administration of vitamin D was successful in improving cognitive performance of alzheimer by reduction of amyloid beta (Aβ) biomarkers.
Further studies of randomized controlled trial are needed with a larger sample size and more varied characteristics of elderly to observe vitamin D efficacy as prevention or therapy of dementia.
Conflict of Interest: The authors have no conflicts of interest
REFERENCES
1. Dementia. World Heal Organ [Internet]. 2023; Available from: https://www.who.int/news-room/fact-sheets/detail/dementia
2. Owusu JE, Islam S, Katumuluwa SS, Stolberg AR, Usera GL, Anwarullah AA, et al. Cognition and Vitamin D in Older African-American Women– Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J Am Geriatr Soc. 2019;67(1):81–6.
3. Ross AC, Taylor CL. Dietary Reference Intakes for Calcium and Vitamin D. Inst Med Comm to Rev Diet Ref Intakes Vitam D Calcium [Internet]. 2011; Available from: https://pubmed.ncbi.nlm.nih.gov/21796828/
4. Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PHM, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–8.
5. Farapti F, Fadilla C, Yogiswara N. Effects of vitamin D supplementation on 25(OH)D levels and blood pressure in the elderly: a systematic review and meta-analysis. F1000Research. 2020;9:633.
6. Gluhm S, Goldstein J, Loc K, Colt A, Liew C Van, Corey-Bloom J. Cognitive performance on the mini-mental state examination and the montreal cognitive assessment across the healthy adult lifespan. Cogn Behav Neurol. 2013;26(1):1–5.
7. Lange RT. Full Scale IQ. In: Encyclopedia of Clinical Neuropsychology. New York: Springer; 2011. p. 1103–5.
8. FSIQ Score Chart-Guide. Personality test [Internet]. 2021; Available from: https://personalityanalysistest.com/iq-score/fsiq-score-chart-guide/
9. Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F. Effects of Vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: A randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry. 2019;90(12):1347–52.
10. Yang T, Wang H, Xiong Y, Chen C, Duan K, Jia J, et al. Vitamin D Supplementation Improves Cognitive Function through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J Alzheimer’s Dis. 2020;78(4):1509–18.
11. Arosio B, Rossi PD, Ferri E, Cesari M, Vitale G. Characterization of Vitamin D Status in Older Persons with Cognitive Impairment. Nutrients. 2022;14(6):1–10.
EFFECTIVENESSOFCOMMUNITYBASEDHEALTHCARE(CBHC)FOR ELDERLYPEOPLEININDONESIA:ALITERATUREREVIEW
BryanNaufalAbdullah1,NaufalAfifKurniawan2,RivandyHartono3,Meinisa Sekarningrum4*
1FacultyofMedicine,SebelasMaretUniversity,Surakarta *meinisa.sekarningrum04@gmail.com;+628131-8270-489
ABSTRACT
Introduction:Indonesiaisfacingagrowingelderlypopulationwithover26millionpeople aged60andovernationwide,raisingconcernsaboutprovidingcareandsupportfortheir decliningphysicalandmentalhealth.Whilefamily-basedcareispreferredbyelders,itmay notalwaysbepossibleduetofamilymembers'absence.Community-BasedHealthCare (CBHC)hasemergedasamoreprominentmethodofcaredeliverythatleveragesthepower ofthecommunitytohelpeldersbecomemoreproactiveinmanagingtheirhealthand improvingtheirdailylives.CBHChasthepotentialtoreducemorbidityassociatedwith commonhealthproblemsamongolderadults,andinitiativeslikeposyandulansiaand puskesmassantunlansiacanprovideareferralsystemtohigherhealthfacilitieswhenneeded.
Objective:Tomeasurethecompatibilityofcommunity-basedhealthcareasatreatmentfor geriatricmedicalproblemsandcompareittocommonandcurrenthealthcare.
Method:Thereviewmethodusedinthisliteraturereviewiscarriedoutinelectronic databasessuchasPubMed,NCBI, Badan Pusat Statistik in2020-2021usingkeywords: geriatry, community-based health care, CBHC.
Results:CBHCprovidespersonalizedtreatmentbydoctors,psychologists,andnurses,while alsoallowingforelderinteractionandreducingsocialisolation.Thisapproachcanimprove thequalityoflifeandlifeexpectancyofeldersbymonitoringtheirhealthandpromoting socialinteraction,whichmaypreventmemoryloss.Additionally,Indonesia'seconomic growthhasledtoalackoffreetimeforfamilymemberstoprovidecare.
Conclusion:Inconclusion,CBHCisabetteralternativeasitprovidespersonalizedtreatment byprofessionalsandpromoteselderinteractiontopreventsocialisolationandcognitive decline.CBHCisespeciallynecessaryduetoIndonesia'seconomicgrowth,whichlimits familymembers'freetime.ImplementingCBHCcanimproveelders'qualityoflifeandlife expectancybymonitoringtheirhealthandpromotingsocialinteraction.
Keyword: Geriatric, community-based health care, elderly, social support, CBHC.
EFFECTIVENESSOFCOMMUNITYBASEDHEALTHCARE(CBHC)FOR ELDERLYPEOPLEININDONESIA:ALITERATUREREVIEW
Author:
BryanNaufalAbdullah(G0022052)
NaufalAfifKurniawan(G0022238)
RivandyHartono(G0022188)
MeinisaSekarningrum(G0022128)
FacultyofMedicineUniversitasSebelasMaret
AsianMedicalStudents’AssociationIndonesia
2023

ABSTRACT
Introduction:Indonesiaisfacingagrowingelderlypopulationwithover26millionpeople aged60andovernationwide,raisingconcernsaboutprovidingcareandsupportfortheir decliningphysicalandmentalhealth.Whilefamily-basedcareispreferredbyelders,itmay notalwaysbepossibleduetofamilymembers'absence.Community-BasedHealthCare (CBHC)hasemergedasamoreprominentmethodofcaredeliverythatleveragesthepower ofthecommunitytohelpeldersbecomemoreproactiveinmanagingtheirhealthand improvingtheirdailylives.CBHChasthepotentialtoreducemorbidityassociatedwith commonhealthproblemsamongolderadults,andinitiativeslikeposyandulansiaand puskesmassantunlansiacanprovideareferralsystemtohigherhealthfacilitieswhenneeded.
Objective:Tomeasurethecompatibilityofcommunity-basedhealthcareasatreatmentfor geriatricmedicalproblemsandcompareittocommonandcurrenthealthcare.
Method:Thereviewmethodusedinthisliteraturereviewiscarriedoutinelectronic databasessuchasPubMed,NCBI,BadanPusatStatistikin2020-2021usingkeywords: geriatry,community-basedhealthcare,CBHC.
Results:CBHCprovidespersonalizedtreatmentbydoctors,psychologists,andnurses,while alsoallowingforelderinteractionandreducingsocialisolation.Thisapproachcanimprove thequalityoflifeandlifeexpectancyofeldersbymonitoringtheirhealthandpromoting socialinteraction,whichmaypreventmemoryloss.Additionally,Indonesia'seconomic growthhasledtoalackoffreetimeforfamilymemberstoprovidecare.
Conclusion:Inconclusion,CBHCisabetteralternativeasitprovidespersonalizedtreatment byprofessionalsandpromoteselderinteractiontopreventsocialisolationandcognitive decline.CBHCisespeciallynecessaryduetoIndonesia'seconomicgrowth,whichlimits familymembers'freetime.ImplementingCBHCcanimproveelders'qualityoflifeandlife expectancybymonitoringtheirhealthandpromotingsocialinteraction.
Keyword: Geriatric, community-based health care, elderly, social support, CBHC.
INTRODUCTION
Indonesiaisfacingtheriskofanincreasingnumberofelderlypeople.Statisticsshow thatpeopleaged60yearsoldaremorethan26millionnationwide.9,92%ofthetotal Indonesianpopulationin2020consistsoftheelderly.Thereisagrowingconcernthatthe elderlywillmakeup12,9%ofthetotalIndonesianpopulationin2030.Aselderlyage,they experienceasignificantdeclineintheirdailyactivitiesandmentalcapacity Theirprofile makesitdemandingtosupportandcareforthem.Mostoftheeldersprefertobegivencare bytheirfamily However,studiesshowthatCommunityBasedHealthCareismore prominent.
CommunityBasedHealthCare(CHBC)isaninterventionmethodinservingpatients withtheleveragepowerofthecommunitywhichincludesthehealthcarecommunityandthe societycommunity AfewexamplesofCHBCimplementationinIndonesiaareposyandu lansiaandpuskesmassantunlansia.Theinterventionshouldallowtheelderlytobemore proactiveandimprovetheirdailylives.Nottomention,relievingtheelderlymorbidity.Inthe 55tomorethan75agegroup,healthproblemswiththehighestprevalencearehypertension,
arthritis,stroke,alsomouthandteethproblems.Thesehealthproblemscouldbereferred fromposyandulansiaorpuskesmassantunlansiatoahigherhealthfacility
MATERIALANDMETHOD
ThereviewmethodusedinthisliteraturereviewisthesearchengineNCBI,PubMed,and Badan Pusat Statistik tofindjournalsanddatawithkeywordssuchas: geriatry, community-based health care, elderly, social support, CBHC.
RESULTANDDISCUSSION
Journalpublishedbackin2020revealsthatproblemsfacedbytheIndonesianelderly citizenarealwaysrevolvingaroundtheabsenceofotherhumanbeingswhocouldactastheir companytodosomething.Problemsfacedbytheelderlyincludedifficultiesindoing activitiessuchaseating,bathing,urinating,andlackofentertainmentthatcanbetheir distractionfromtheirhealthproblems.TheseproblemscouldbesolvedbyhavingCBHCas maintreatmentontheelderlypatient.
Per2021,oneinfivecommunity-dwellingeldersinIndonesiaiscategorizedasfrail, meaningtheirabilityindoingdailyactivityandfulfillingdailyneedsaremostlyincapable andtheyneedassistancetobeconsideredfunctional.Thisdependencyandthehighriskof chronicdiseasesespeciallyNCDsurgesthedevelopmentofcommunity-basedcareforthe elders.
RecentpaperpublishedbyEnvironmentalResearchandPublicHealthin2021 discussingthepriorityofimportanceininterventionofchronicdiseaseespeciallydiabetes andhypertensioninKoreaneldersshowedthebeneficialresultinpatientsatisfactionand healthylifestylewhenincludingthecommunitytotakepartingivinghealthcarebyhaving appropriatehealtheducation.Inthisjournal,Koreaistryingtoresolvetheupcomingproblem oftherisingnumberofolderpeopleintheircountry.Withconcernofmanyrisksofchronic diseasetowardselderly,Koreacameupwiththisjournalasapartoftakingastepforward. Indonesiaontheotherhandwillbefacingthesameproblemin1-2decades,thusby learningandadaptingtothissolutionIndonesiamightpreventthispredictedproblemfrom happening.
Anotherpaperpublishedin2020regardingcommunitycareserviceinIndonesia suggestedthatthecommunitytendstovolunteeraslongastheyareprovidedwithclear instructionandotherneededinformationtodotheservices.Thisgivesinformationonthe reliabilityofthecommunitytotakepartatgeriatrichealthcareandopenstheopportunityfor anewalternativehealthcaremethodthatisCBHC.
EffectivenessofCommunityBasedHealthCare
Paperpublishedin2021discussingthelong-termcareforeldersinIndonesiareveals thatactuallyeldersprefertobemedicallytreatedbytheirfamilymemberslikespouses, children,orsomeonetheyknowwell(thepreferenceforcareprovisionarethedaughters). Andyes,family-basedcareiscurrentlythemajoritycaredonetotheelderlypatientandit showsquiteagoodresult.Whatweforgottomentionisthattherearefactorsinwhichthe familymembersofthepatientarenotavailabletogivethecaretothepatientwhoneededit. Thesefactorsincludetheabsenceoffamilymembersduetosomecircumstances.CBHCisin
manywaysbetterthanpersonalcaredonebyeithernursesorfamilymembersbecauseitcan beimplementedtoeachandeveryelderswithoutlimitation.
ByimplementingCBHCtoalleldersinIndonesia,Lifeexpectancyandqualityoflife areexpectedtoincrease.Tomentionafewreason,CBHCareabletoconstantlymonitorthe healthofallelders.Doctors,psychologistaswellasnursesarereinforcedtocareforthe patient.Also,CBHCareabletogathertheelderstocommunicateandinteractwitheach other.Thus,givingtheactualizationtheeldersneedandcreatenewconnectionbetweenthe elders.Thenewbondaremadethrough doingactivitiestheylovetogether Bytime,their brainwillbelievethattheyarehealthyanddecreasethechanceofmemorylossduetothe lackofbrainactivity
AnotherproofofwhyCBHCisthebestgeriatrictreatmentisbecauseofIndonesia's economicgrowth.Yes,Indonesiaiscurrentlyontheirwaytoabettereconomy,whichin achievingabettereconomyneedstheirpeopletobepartofthisgrowthandthusresultingin thelossoffreetimeavailabletoitspeople.Withthisbeingmention,familymembersofthe elderlypeopleinIndonesiatendtofocusmoreontheirworkinordertoearnmoremoneyand thushavingthechancetohaveabetterlife(Indonesianaremostlylivingonthelineof poverty),resultingintheabsenceoftimetheycouldgivetotheirelders,withthatbeing mention,CBHCisinfactthebestwaytotreateldersinIndonesia.
ProsandConsofCommunityBasedHealthCare
Pros
Theadvantageof havingCBHCasthemaintreatmentofelderlyinIndonesiaisthat itmakestheelderstreatedbyprofessionalsthusensuringbetteroutcomes.Having professionalssuchasdoctorsandpsychologiststhatkeeponmonitoringboththeirmedical healthandmentalhealthwillalsomakethefamilyoftheelderslessconcerned.
Cons
ThedisadvantageofhavingCBHCisthemoralreason.Itisamoralsensethat childrenorfamilymembersaretheonewhoisresponsibletotakecareoftheirelders.Having theireldersbetreatedbyCBHCmethodwillinsomewaysmakethefamilyfeelguilty becauseitseemsliketheyare“throwing”theirlovedone.
CONCLUSION
Afterconductingliteraturesearchingfromvariousdatabases,thereviewarticleon long-termcareforeldersinIndonesiahighlightsthatfamily-basedcareisthepreferredand mostcommonmethodofmedicaltreatmentamongelders,withdaughtersbeingthepreferred caregivers.However,therearecircumstanceswherefamilymembersarenotavailableto providecare.CommunityBasedHealthCare(CBHC)isabetteralternativetopersonalcare byfamilymembersornursesasitisaccessibletoalleldersandprovidespersonalized treatmentbydoctors,psychologists,andnurses.CBHCalsoprovidesopportunitiesforelder interaction,reducessocialisolation,andenhancescognitiveactivity,whichmayprevent memoryloss.Furthermore,Indonesia'seconomicgrowthhasledtoalackoffreetimefor familymemberstoprovidecare,makingCBHCthebestoptionforeldercare.Overall, implementingCBHCinIndonesiacanimprovethequalityoflifeandlifeexpectancyof eldersbymonitoringtheirhealth,providingpersonalizedcare,andpromotingsocial
interaction.Withdoctorsandpsychologistsmonitoringboththeirmedicalandmentalhealth, thefamilyoftheelderscanfeellessconcernedabouttheirlovedones'well-being.
RECOMMENDATION
Extendedcomprehensiveinvestigationsareneededtoprovidemuchmorerecent informationregardingthecommunity-basedhealthcareefficiencythroughoutotherviewsand components.ThereareotherpossibilitiestoseekfurtheraroundelderlygrowthinIndonesia alsoitspotentialtochronicdiseasesandNCDs.
REFERENCE
Country Diagnostic Study on Long-Term Care in Indonesia.(2021). https://doi.org/10.22617/TCS210416-2
Sumini,Sukamdi,Pangaribowo,E.H.,Keban,Y.T.,&Darwin,M.(2020).ElderlyCare:A StudyonCommunityCareServicesinSleman,DIY,Indonesia. Journal of Aging Research, 2020 https://doi.org/10.1155/2020/3983290
RahmawatiR,BajorekB.ACommunityHealthWorker-BasedProgramforElderlyPeople WithHypertensioninIndonesia:AQualitativeStudy,2013.PrevChronicDis.2015Oct 15;12:E175.doi:10.5888/pcd12.140530.PMID:26469948;PMCID:PMC4611861.
ByunDH,ChangRS,ParkMB,SonHR,KimCB.PrioritizingCommunity-Based InterventionProgramsforImprovingTreatmentComplianceofPatientswithChronic Diseases:ApplyinganAnalyticHierarchyProcess.IntJEnvironResPublicHealth.2021Jan 8;18(2):455.doi:10.3390/ijerph18020455.PMID:33430108;PMCID:PMC7827405.
Setiati,S.,Soejono,C.H.,Harimurti,K.,Dwimartutie,N.,Aryana,I.G.P.S.,Sunarti,S., Budiningsih,F.,Mulyana,R.,Dwipa,L.,Sudarso,A.,Rensa,R.,Istanti,R.,Azwar,M.K.,& Marsigit,J.(2021).FrailtyandItsAssociatedRiskFactors:FirstPhaseAnalysisof MulticentreIndonesiaLongitudinalAgingStudy. Frontiers in Medicine, 8. https://doi.org/10.3389/fmed.2021.658580
HandollHH,CameronID,MakJC,PanagodaCE,FinneganTP.Multidisciplinary rehabilitationforolderpeoplewithhipfractures.CochraneDatabaseSystRev 2021Nov 12;11(11):CD007125.doi:10.1002/14651858.CD007125.pub3.PMID:34766330;PMCID: PMC8586844.
ZhengZ,LiuW,LuY,SunN,ChuY,ChenH.Theinfluencemechanismofcommunity-built environmentonthehealthofolderadults:fromtheperspectiveoflow-incomegroups.BMC Geriatr.2022Jul16;22(1):590.doi:10.1186/s12877-022-03278-y.PMID:35842581; PMCID:PMC9288733.
OkuyamaK,AbeT,LiX,ToyamaY,SundquistK,NabikaT.NeighborhoodEnvironmental FactorsandPhysicalActivityStatusamongRuralOlderAdultsinJapan.IntJEnvironRes
PublicHealth.2021Feb4;18(4):1450.doi:10.3390/ijerph18041450.PMID:33557194; PMCID:PMC7913898.
THE ASSOCIATION BETWEEN THE USE OF SSRIs AS ANXIETY THERAPY WITH THE RISK OF FALLING IN THE ELDERLY : A SYSTEMATIC REVIEW
Scientific Paper
Authors :
Jenny Khayla Zahirani (G0021098)
Aloysia Flower Lula Utami (G0022017)
Hilmi Amirul Haq (G0022098)
Shafira Nabila Rahadini (G0022199)
Faculty of Medicine Universitas Sebelas Maret
Asian Medical Students’ Association Indonesia

2023
THE ASSOCIATION BETWEEN THE USE OF SSRIs AS ANXIETY THERAPY WITH THE RISK OF FALLING IN THE ELDERLY : A SYSTEMATIC REVIEW
ABSTRACT
A. Introduction
Mental health is getting more attention, the elderly are no exception. One of the antidepressant drugs that are often used by the elderly to treat anxiety is SSRIs. This drug has several side effects for health. Especially for the elderly. This can increase the risks of side effects from SRRIs. Such as inhibit bone formation in osteoblasts and induce bone apoptosis from osteoclasts. So that the use of antidepressant drugs, especially SSRIs, has a high rate of bone loss. This can trigger the potential for falls in the elderly.
B. Objective
This research aim to discuss the association between the use of SSRIs drug type as a therapy for Anxiety and the risk of falling in the elderly with an idea stated in a systematic review.
C. Method
A systematic review was based on two databases, e.g., PubMed, ScienceDirect, using PRISMA. A total of 540 records were obtained in the preliminary search, and finally a total of 15 studies were included.
D. Result & Discussion
A total of 3 studies showed positive results in escitalopram increases risk of fall and/or fracture, while the other 1 studied showed negative results in escitalopram increases risk of fall and/or fracture. But there are studies that prove that escitalopram is more effective than a range of other antidepressants (paroxetine)
There is no study among the 4 articles that showed that paroxetine was associated with fractures causing falls in the elderly. Paroxetine has a lower risk of hip fracture than other SSRIs. But paroxetine has a less favorable tolerability profile than escitalopram.
E. Conclusion
The study concludes that escitalopram has better efficacy for anxiety treatment when compared to paroxetine. However, the side effect of paroxetine is lesser than escitalopram as the use of paroxetine does not give rise to bone mass reduction.
Keyword : SSRIs, falling, elderly
THE ASSOCIATION BETWEEN THE USE OF SSRIs AS ANXIETY THERAPY WITH THE RISK OF FALLING IN THE ELDERLY : A SYSTEMATIC REVIEW
Scientific Paper
Authors :
Jenny Khayla Zahirani (G0021098)
Aloysia Flower Lula Utami (G0022017)
Hilmi Amirul Haq (G0022098)
Shafira Nabila Rahadini (G0022199)
Faculty of Medicine Universitas Sebelas Maret
Asian Medical Students’ Association Indonesia
2023

THE ASSOCIATION BETWEEN THE USE of SSRIs ANXIETY THERAPY WITH THE RISK OF FALLING IN THE ELDERLY : A SYSTEMATIC REVIEW
A. Introduction
Aging is a process that affects humans in biological and psychological aspects. The body changes associated with aging can lead to mental health issues, such as anxiety and depression. Anxiety is shown as feelings of tension, worried thoughts, fear, and dread. This can cause the person with anxiety to experience sweating, feeling restless and tense. Anxiety can get worse over time. Besides anxiety, depression is also a common mental health issue associated with body changes because of aging. Depression can affect a person’s way of feeling, thinking, and handling daily activities. In the worst case, suicide can be the decision made by people who suffer with depression. A study in Hunan Province, China from March to May 2021 with 1173 participants aged 65 years or above shows that anxiety and depression in older adults have the prevalence of 32.74% and 37.34% There are actions needed to follow up the high prevalence of anxiety and depression in older adults.
There are many medications we can use to cure anxiety, and one of them is drugs. There are many subtypes of drugs that we can use. One of them is Selective Serotonin Reuptake Inhibitors (SSRI). Selective serotonin reuptake inhibitors (SSRIs)are a type of medications which are commonly used as prescription for depression therapy. SSRIs are being used in the medical field as the first-line pharmacotherapy to cure depression. Besides, many psychological disorders are being treated with this pharmacotherapy because of its safety, tolerability, and efficacy. Adult and pediatric patients have been approved to use this type of medications. Research in seven European countries (six EU countries and Israel) by Services and Health for Elderly in Long Term care (SHELTER) has been done. The research showed that from 4023 cases residents in the study, 32% of them have been experiencing depressive symptoms. Besides that, antidepressants have been used by nearly half of these people. Each country has a different way of use antidepressant medication usage. From all of the sample, the prevalence is 35.6% (n = 1431). 59.9% of antidepressant users have received selective serotonin reuptake inhibitors (SSRI). The research participant is 60 or years old above those lives in Nursing Homes in those seven countries. The research is conducted during the years 2009 to 2011 (Silvia Giovannini, 2020). For now, Selective Serotonin Reuptake Inhibitors (SSRI) is one of antidepressant drug that has 7 types:
· Citalopram (Celexa)
· Escitalopram (Lexapro)
Fluoxetine (Prozac, Sarafem, Symbyax)
Fluvoxamine (Luvox, Luvox CR)
Paroxetine (Paxil, Pexeva, Paxil CR)
Sertraline (Zoloft)
Vilazodone (Viibryd)
SSRIs are the first-line of medication for anxiety and depression disorders because of their low risk of possible side effects. Citalopram, escitalopram, and sertraline are the types of SSRIswhichareconsideredtobethesafestforolderadults.Thesehavethe lowestpotential for drug-drug in-teractions based on their cyto-chrome P-450 interactions (Bonnie Wiese, 2011)
The therapeutic actions of SSRIs have their basis in upgrading deficient serotonin that researchers postulate is the cause of depression in the monoamine hypothesis. As the name prompts,SSRIsexert action bypreventing thereuptakeofserotonin. Therefore,it increases serotonin activity. SSRIs are a little bit different from other classes of antidepressants. Neurotransmitters are slightly affected by SSRIs. Those neurotransmitters are neurotransmitters such as dopamine or norepinephrine. Compared to TCAs and MAOIs, SSRIs have less side effects. It is because of the fewer effects on adrenergic, cholinergic, and histaminergic receptors.
SSRIs work by inhibiting the serotonin transporter (SERT) at the presynaptic axon terminal. By this action, an increased amount of serotonin (5-hydroxytryptamine or 5HT) remainsin thesynapticcleft.Thisstimulates thepostsynapticreceptorsforamoreextended period. The anxiety and insomnia disorder is relieved by the increasing amount of serotonin.
It has been proven that SSRIs help to relieve anxiety and depression. However, in some cases, the use of SSRIs comes with costs. Data shows that at least one side effect will be experienced by 80% of patients using SSRIs. Other than that, more than one adverse effect will be experienced by over half of patients. Suicidality in patients under the age of 25, anxiety, severe headache, dizziness, weight gain, sexual dysfunction, and gastrointestinal distress are the side effects of the SSRIs use. The risk of serotonin syndrome, as well as SSRI discontinuation syndrome, prolonging the QT interval, and coagulopathy are other side effects.
Based on the explanation above, we can see that currently mental health is getting more attention, and the elderly are no exception. One of the antidepressant drugs that are often used by the elderly to treat anxiety is SSRIs. This drug has several side effects on health. Especially for the elderly who are an age group that is vulnerable to changes in conditions and situations caused by changes in physical, social, and psychological conditions. This can increase the risks of side effects from SRRIs. Such as inhibiting bone formation in osteoblasts and inducing bone apoptosis from osteoclasts. So the use of antidepressant drugs, especially SSRIs, has a high rate of bone loss. This can trigger the potential for falls in the elderly.
This research aims to discuss the association between the use of SSRIs drug type as a therapy for Anxiety and the risk of falling in the elderly with an idea stated in a systematic review.
2. Objectives
This systematic review aims to evaluate the role of SSRIs as the most common medication for Anxiety in causing the increased risk of falling in the elderly. Furthermore, this study also reviews the safest and most effective SSRIs for older adults by comparing the two most effective SSRIs, escitalopram and paroxetine.
3. Method
1. Systematic review based on PRISMA Guidelines
2. 3 Scientific Database: PubMed, NCBI, Science Direct
3. Search terms : (“SSRI” AND “Anxiety” AND “Geriatric” “Falls”)
4. Collect PPE
Inclusion Criteria
a. most effective SSRIs (Escitalopram and Paroxetine)
b. Geriatric
Exclusion Criteria
a. Irrelevant study or outcomes
b. Wrong PICOs component
c. Review, report, editorial article
d. SNRI
e. Publish before 2012 (last 11 years)
4. Result and Discussion Study Selection
Based on the results of a review of 540 articles from 2 data sources (PubMed Center and ScienceDirect). There were 7 articles that met the inclusion criteria. Of the 533 articles that do not meet the requirements. three duplicates were removed from the database, and 371 articles were eliminated by title. Then, 66 articles were eliminated based on abstracts, and 44exclusion criteriawereeliminated,and 49 articles thatwere not included in the inclusion criteria.
 Figure 1.1 Flowchart of study identification and selection based on PRISMA
Figure 1.1 Flowchart of study identification and selection based on PRISMA
Database Keywords Articles Science Direct SSRI AND Anxiety AND Geriatric AND Falls 248 NCBI Pubmed Central SSRI AND Anxiety AND Geriatric AND Falls 292
Table 1.1 Database Searching Process Results
Data Extraction and Analysis
Data extracted from studies found to be eligible were: author’s name, year of publication, study design, adverse effect, prevalence, SSRI increases the risk of fall and/or fracture. The authors examined and summarized the study’s outcomes.
SSRIs inhibit bone formation in osteoblasts and induce bone apoptosis from osteoclasts. So the use of antidepressant drugs, especially SSRIs, has a high rate of bone loss. Selective Serotonin Reuptake Inhibitors (SSRI) were the antidepressant drug that has 7 types that is Citalopram, Escitalopram, Fluoxetine, Fluvoxamine, Paroxetine, Sertraline, Vilazodone. (R. Rizzoli, 2012). Of the 7 types, the most effective are escitalopram and Paroxetine. In general, the results of this systematic review were described in the following tables : studies' characteristics of escitalopram (shown in table 1.), studies' characteristics paroxetine (shown in table 2.)
Table 1. Study Characteristics’ of Escitalopram
Author Journal Year Study Types Adverse Effect Prevalence SSRI increases risk of fall and/or fracture Diniz, B.S. and Reynol ds, C.F. Major 2014 Case–control or cohort studies Osteoporosis, Osteopenia, Bleeding Older people taking 2-4 medicines had a 64% greater risk of hip fracture than older people taking 0-1 medicine (Diniz, 2014) Yes
Marie Anne Gebara 2014 clinical studies significant decreased of the bone resorption marker
Yes
Marie Anne Gebara
2015 Multiple expert panels
- While a shortfall of evidence supporting causation does not indicate that SSRIs do not cause falls (Marie Anne Gebara, 2015)
No
Author Journal Year Study Types Adverse Effect Prevalence SSRI increases risk of fall and/or fracture
Marie Anne Gebara
2016 crosssectional study
Potentially increases fall risk
While some research did not found any change in postural sway with use of paroxetine in older depressed adults, there are foundings which state that sertraline and paroxetine can cause postural sway. (Marie Anne Gebara, 2016)
Yes/Not
Table 2. Study Characteristics’ of Paroxetine
Robyn Tambly n
2020 cohorts
The fracture risk for patients may be reduced by selecting paroxetine, an SSRI with lower risk than citalopram, the SNRI venlafaxine over duloxetine, and the TCA amitriptyline over imipramine or doxepin.
Between 42.9% and 55.6% of research cohorts were 75 years and older, and 29.3% to 45.4% were men.
(Robyn Tamblyn, 2020)
R. Rizzoli 2012 CrossSectional study
Lower BMD (Bone mass Density) causing higher risk for fracture
The same research highlighted differences among the types of antidepressants. Use of the SSRIs citalopram, fluoxetine, and sertraline was associated with a significant dosedependent give rise to the risk of fracture, but the relationship did not achieve significance for paroxetine. (R. Rizzoli, 2012)
No (in Paroxetine case)
No
2014 MetaAnalysis Insufficient data
(unknown)
In a meta-analysis of 117 studies (Cipriani et al., 2009) that reviewed response, mirtazapine, escitalopram, venlafaxine, and sertraline were more efficacious than duloxetine, fluoxetine, fluvoxamine, paroxetine, and reboxetine. In terms of acceptability, escitalopram, sertraline, citalopram, and bupropion were better tolerated than other newgeneration antidepressants. The results of these study indicate that two of the most effective treatments (mirtazapine and venlafaxine) may not be the best for overall acceptability. The foremost clinical indication is that escitalopram and sertraline might be the best option when starting a medication for moderate-to-severe
Insufficien t data
Catheri ne Camero n
(unknown)
depression as they provide the best balance between efficacy and acceptability in anxiety treatment.
Relationship between escitalopram as a therapy for anxiety and falls in the elderly
Based on 3 articles that met the inclusion criteria. We found 2 of 3 articles about escitalopram increasing the risk of falls and/or fractures. Escitalopram has the adverse effect of osteoporosis, osteopenia, bleeding, and significantly decreased bone resorption marker which increases the risk of bone fractures and has the potential to cause falls in elderly humans. Escitalopram was chosen to treat geriatric depression and anxiety in this study. It is a highly selective SSRI, with only few effect on other neurotransmitters and receptors.
Escitalopram does not inhibit its own metabolism and its metabolites have no effect on the metabolismof theparent drug,soits long-termusewillnotcause accumulation inthebody. Escitalopram has less effect on the hepatic cytochrome P450 isoenzyme system than other SSRIs and has very little potential for drug-drug interactions. The oral dose range for escitalopram is 10 – 50 mg/day, and potential side effects include drowsiness and mild gastrointestinalreactions.Thereisevidence that escitalopram ismoreeffectivethanarange of other antidepressants.
Relationship between paroxetine as a therapy for anxiety and falls in the elderly
Based on the 4 articles that met the article inclusion criteria, it was found that 1 source did not have a risk of fall and/or fracture, 2 sources did not show the effect of paroxetine on the risk of falling and there was no data on 1 other source. Paroxetine has an adverse effect that potentially increases fall risk while some studies did not find any change in postural sway with the use of paroxetine in older depressed adults, others have found that sertraline andparoxetine causean increasein postural swaywhichincreasestheriskof bonefractures and has the potential to cause falls in elderly humans.
Escitalopram is more superior than paroxetine, which has a less favorable tolerability profile. Paroxetine is associated with cholinergic muscarinic antagonism and potent inhibition of CYP2D6, and sertraline has moderate drug interaction problems in comparison with escitalopram. Overall, as an allosteric serotonin reuptake inhibitor that is somewhat different from classical SSRIs, escitalopram is the first choice judged by combined efficacy and tolerability, and nonclinical data have offered possible mechanisms
through which escitalopram could be more efficacious, based on its interaction with orthosteric and allosteric binding sites at the serotonin transporter. (Connie Sanchez, 2014)
5. Conclusion and Recommendation
This study was built on the foundation of SSRIs studies that focus on the comparison between escitalopram and paroxetine to find the most effective and suitable drug for anxiety treatment in older adults. The study concludes that escitalopram has better efficacy for anxiety treatment when compared to paroxetine. However, the side effect of paroxetine is lesser than escitalopram as the use of paroxetine does not give rise to bone mass reduction. Thus, paroxetine is more suggested as the treatment option for older adults with anxiety as it is considerably safer than escitalopram. A further study needs to be conducted to support the evidence. The fracture risk for patients may be reduced by selecting paroxetine, an SSRI with lesser risk than citalopram.
6. Acknowledgements and conflict of interest
We would like to thank the authors of previous journals who have inspired us to develop ideas in making this scientific paper.
7. Reference
1. Gebara MA, Shea MLO, Lipsey KL, Teitelbaum SL, Civitelli R, Müller DJ, et al.
Depression, Antidepressants, and Bone Health in Older Adults: A Systematic Review. Journal of the American Geriatrics Society [Internet]. 2014 Jul
15;62(8):1434–41. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4134423/
2. Brännström J, Lövheim H, Gustafson Y, Nordström P. Association Between Antidepressant Drug Use and Hip Fracture in Older People Before and After Treatment Initiation. JAMA Psychiatry. 2019 Feb 1;76(2):172.
3. Tamblyn R, Bates DW, Buckeridge DL, Dixon WG, Girard N, Haas JS, et al.
Multinational Investigation of Fracture Risk with Antidepressant Use by Class,
Drug, and Indication. Journal of the American Geriatrics Society [Internet]. 2020 Jul 1;68(7):1494–503. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7383967/
4. Naharci MI. Psychoactive Drug Use and Falls among Community-Dwelling Turkish Older People. Northern Clinics of Istanbul. 2019;
5. Quach L, Yang FM, Berry SD, Newton E, Jones RN, Burr JA, et al. Depression, Antidepressants, and Falls Among Community-Dwelling Elderly People: The MOBILIZE Boston Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013 Jul 1;68(12):1575–81.
6. Marcum ZA, Perera S, Thorpe JM, Switzer GE, Castle NG, Strotmeyer ES, et al. Antidepressant Use and Recurrent Falls in Community-Dwelling Older Adults. Annals of Pharmacotherapy. 2016 Apr 11;50(7):525–33.
7. Gebara MA, Lipsey KL, Karp JF, Nash MC, Iaboni A, Lenze EJ. Cause or Effect?
Selective Serotonin Reuptake Inhibitors and Falls in Older Adults: A Systematic Review. The American Journal of Geriatric Psychiatry [Internet]. 2015 Oct;23(10):1016–28. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4442757/
8. Ciccone CD. Geriatric Pharmacology. Guccione’s Geriatric Physical Therapy. 2020;102–36.
9. Mulsant BH, Blumberger DM, Ismail Z, Rabheru K, Rapoport MJ. A Systematic Approach to Pharmacotherapy for Geriatric Major Depression. Clinics in Geriatric Medicine. 2014 Aug;30(3):517–34.
Annida Naufal Irvania
Universitas Padjajaran
Abstract:
Parkinson Disease (PD) is caused by loss of pigmented dopaminergic neurons of the substantia nigra pars compacta and the presence of Lewy bodies and Lewy neurites (Hauser et al. 2020). The targeted objective is for high-risk population which related to hereditary. In general, the inheritance patterns of PD are identified by examining the way the disorders are transmitted in the family of the index patient. Such a complex pedigree analysis. The finding states that A total of 28 loci (PARK) in various genes have now been proposed for Parkinson disease (Klein & Westenberger 2012). A familial background plays role at least for 10%. The remaining 90% can be environmental factors, and many more. We can enhance our healthcare insurance and conduct more studies of hereditary about this disease targeted to high-risk populations to reach higher life expectation in Indonesia.
Keyword: Parkinson disease, Hereditary, PARK gene, Studies of hereditary, Healthcare insurance
Is Parkinson’s disease really inevitable due to Hereditary? and Chance to Stop It from Progressing

FACULTY OF MEDICINE UNIVERSITAS PADJAJARAN 2023
Annida Naufal Irvania AMSA-Universitas Padjajaran
Is Parkinson’s disease really inevitable due to Hereditary? and Chance to Stop It from Progressing
Abstract:
Parkinson Disease (PD) is caused by loss of pigmented dopaminergic neurons of the substantia nigra pars compacta and the presence of Lewy bodies and Lewy neurites (Hauser et al. 2020). The targeted objective is for high-risk population which related to hereditary. In general, the inheritance patterns of PD are identified by examining the way the disorders are transmitted in the family of the index patient. Such a complex pedigree analysis The finding states that A total of 28 loci (PARK) in various genes have now been proposed for Parkinson disease (Klein & Westenberger 2012). A familial background plays role at least for 10%. The remaining 90% can be environmental factors, and many more. We can enhance our healthcare insurance and conduct more studies of hereditary about this disease targeted to high-risk populations to reach higher life expectation in Indonesia.
Keyword: Parkinson disease, Hereditary, PARK gene, Studies of hereditary, Healthcare insurance
Introduction:
Parkinson disease (PD) is one of the most common health issue in geriatrics. As the body degenerate by aging process so does the brain. PD is caused by loss of pigmented dopaminergic neurons of the substantia nigra pars compacta and the presence of Lewy bodies and Lewy neurites (Hauser et al. 2020).
Dopamine has major function for our body, it works as neurotransmitter that majorly function in NMJ (Neuromucsular Junction) as signaling molecules toward regio such as muscle. With this degeneration there are several symtoms occurred majorly in body coordination. Further more it can also affect the nonmotoric resulting in physiologic trauma for the patient to distinguish between reality and imaginary in life. According to world wide data PD has affected approximately 1% of every individual older than 60 years and diagnosed too late. Over the past generations, over all the neurological disorder, Parkinson’s disease was the fastest growing (Dorsey, 2016) However we all know that this disease cannot be halted, only can be slowed by appropriate treatments from professional. This condition shall not continue in the future. PD is very tricky because there’s no laboratory biomarkers exist for the condition, it’s purely clinical diagnosis. Evaluation begin with patients experiencing tremor through history and
(Figure 1. Malfunction in neuron for PD (Source: Hauser et al. 2020))
physical examination. The flying rumors about PD’s genetic influence has caused fuss in medical professionals From the positive side, it gives hope to early diagnosis and prevention from such event since PD can’t be cured. From the negative side, if the rumors proved true is it really fate that can’t be changed?
Method:
To prove whether PD is really descendent by genetics many research has been conducted In 1996 findings in mapping mutation of PD patients showed that PD might be hereditary, two years later the linkage of family gene also found but also there’s excluded first gene in a large family, it become clear that this is a complex disorder (Klein & Westenberger 2012). It’s interesting the fact that hustle culture and life habit also play role in the findings. Exposure to severe psychological stress, cardiovascular disease, low socioeconomic status, low education level. This disease that was predominantly happened in developed country with white race prevalence high before the busy-life-habit is now also common around the world make it grows everywhere (Wright Willis et al. 2010) Studies from genetics base in families around the world has been conducted.
If genetic factors are important in a particular disease, concordance in genetically identical monozygotic (MZ) twins will be greater than in dizygotic (DZ) twins, who share only about 50% of genes Early Parkinson disease twin studies generally found low and similar concordance rates for MZ and DZ pairs.so we can state that the developing of Parkinson since birth is almost none (Hauser et al. 2020).
The study continues in families that have spreading PD was examined by genetic nomenclature PARK (to denote their putative link to PD), and numbered in chronological order of their identification (PARK1, PARK2, PARK3, etc.). However there has been difficulties since some of them are non-dividable in later descendant. In general, the inheritance patterns of human disorders are identified by examining the way the disorders are transmitted in the family of the index patient. Such a complex pedigree analysis shown below.
Result:
The finding states that A total of 28 loci in various genes have now been proposed for Parkinson disease (Klein & Westenberger 2012). In Germany confirmed that mutations in the alpha-synuclein gene can cause Parkinson disease. In Five small Greek kindreds were found to have the PD-1 mutation. In one large family in Salerno, Italy, 50 of 592 members had Parkinson disease; linkage analysis incriminated a region in bands 4q21-23, and sequencing revealed an A-for-G substitution at base 209 of the alpha-synuclein gene, termed PD-1 as the main cause too. The finding might have been greater than expected if continuously conducted around the world. For basis data in Indonesia there’s not much to find because the research in this phenomenon is low (Hauser et al. 2020). Although there are many findings, the majority of PD cases are sporadic, i.e., only about 10% of patients report a positive family history (Thomas & Beal 2007).

Discussions:
 (Figure 2. Analytical PD Pedigree (Source: Klein & Westenberger 2012))
(Figure 2. Analytical PD Pedigree (Source: Klein & Westenberger 2012))
With the findings we can state that there is certainty in this occurring disease. A familial background plays role at least for 10%. The remaining 90% can be environmental factors, and many more. From my personal experience dealing with Parkinson relatives is not easy. My grandfather suffers from this disease growing since I was in early High School, which make it 5 years by now. The progressing degeneration comes from nothing and currently paralyze him. He has no memories, can’t do major movements by himself but capable of live everyday life with help. Indonesian health assurance however doesn’t provide treatments and medications for geriatric issue related to physiological and dementia issue like this. The 10% risk is not something inevitable for my family that might have high risk to this disease. Still there are 90% to maximize the lowest potentials if we know about this facts. Since the key point of PD is treatments to slower the progress of degeneration and can’t be healed, this acknowledgment is chance to prevent it What we can do is enhance our healthcare insurance and conduct more studies about this disease targeted to high risk populations. They should know their risk by enough education and start healthy life as habit. Therefore the goals of higher life expectations in healthy old people can increase.
Conclusion:
PD is caused by loss of pigmented dopaminergic neurons of the substantia nigra pars compacta and the presence of Lewy bodies and Lewy neurites (Hauser et al. 2020). This disease is suspected to be hereditary disease. The finding states that A total of 28 loci in various genes have now been proposed for Parkinson disease (Klein & Westenberger 2012) A familial background plays role at least for 10%. The remaining 90% can be environmental factors, and many more. We can enhance our healthcare insurance and conduct more studies about this disease targeted to high-risk populations to reach higher life expectation in Indonesia.
Acknowledgment and Conflict of Interest:
With the following article promoting enhance in healthcare insurance to cover degenerative disease is not a simple thing to do. Indonesia not only sustain one disease or couple disease but many of it. This disease also has vague causes that really need many medical professional assists. Even with full coverage of health insurance such as developed countries there are no guarantee it can be solved as we see white people surveillance the highest from this disease. Conducting research of degenerative disease also take a long time, from us that coming from third country national assist and funding from developed country is needed for some rational reason. However, there are always hope, we can start the action today by maintaining healthy life habit.
References:
Dorsey, E. Ray. 2016. Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology, Volume 17, Issue 11, 939 – 95
Hauser, R. A., Lyons, K. E., McClain, T. A , Pahwa, R. 2020. Parkinson disease. Medscape journal. Accessed April 12, 2023. <https://emedicine.medscape.com/article/1831191overview#a1>.
Klein, C., & Westenberger, A. 2012. Genetics of Parkinson's disease. Cold Spring Harbor perspectives in medicine, 2(1), a008888.
<https://doi.org/10.1101/cshperspect.a008888>.
Thomas, B., Beal, M.F. 2007. Parkinson’s disease. Hum Mol Genet 16 (Spec No 2): R183–R194
Wright Willis, A., Evanoff, B. A., Lian, M., Criswell, S. R., & Racette, B. A. (2010). Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology, 34(3), 143–151. <https://doi.org/10.1159/000275491>
AquaticTherapyforDementiaPatientasAPromisingTreatmentinTheNear Future:ALiteratureReviewStudies
AdamThariqAlJihad,WulanPertiwiTomsio,SalsabilaJoisangadji,NurulFitriMaharani
ABSTRACT
Introduction:
Dementia is a progressive neurodegenerative disorder that affects cognitive functions and activities of daily living.Theburdenofdementiaisincreasingworldwide,andthereisaneedfor effective treatment options. Aquatic therapy is a promising approach for the management of dementia.
Objective: Thisstudyaimedtoevaluatetheeffectivenessofaquatictherapyasatreatmentfordementia.
Methods:
A systematicreviewwasconductedusingdatabasessuchasPubMed,GoogleScholar,Cochrane, Science Direct, Clinical Key, and WHO. The search included articles published between 2013 and 2022. The keywords used were "aquatic therapy," and "dementia,".Studieswereincludedif they evaluated the effects of aquatic therapy on cognitive functions and activitiesofdailyliving indementiapatients.
Results:
The search identified 4 studies that met the inclusion criteria. All the studies showed positive effects of aquatic therapy on cognitive functions and activities of daily living in dementia patients. The studies used various outcome measures, such as the Mini-Mental State Examination, the Montreal Cognitive Assessment, the Barthel Index, and the Timed Up andGo test.Thedurationandfrequencyofaquatictherapyvariedamongthestudies.
Conclusion:
Aquatic therapy is a promising treatment for dementia patients to improve cognitive functions and activities of daily living. The studies showed positive outcomes, and the therapy was well-tolerated by the patients. However, further studies are needed to establish the optimal durationandfrequencyofaquatictherapyandtoinvestigateitslong-termeffects.
Keywords:
Aquatic therapy and dementia.
AquaticTherapyforDementiaPatientasAPromisingTreatmentinThe NearFuture:ALiteratureReviewStudies
INTRODUCTION
Dementia is any disorder where significant decline from one’s previous level of cognition causes interference in occupational, domestic, or social functioning. Generally, dementia should be considered to be an acquired syndrome, with multiple possible causes, rather than a specific disease itself. Every 3 seconds, one person in the world has dementia. The incidence of dementia worldwide isincreasingrapidly,anditisestimatedthat46.8or50 million people are diagnosed withdementiaworldwide.InIndonesia,therewereestimatedto be around 1.2 million people with dementia in 2016, increasing to 2 million in 2030 and 4 millionin2050.
Nowadays, there has not been found a treatment that can cure dementia. The best managementthatcanbedoneislimitedtoadministeringdrugstoreducedementiasymptoms. Common drugs to be given more for Alzheimer's include Acetylcholinesterase inhibitors, Memantine, Risperidone, and Haloperidol. These drugs are useful for short-term treatment, so they are ineffective for long-term treatment, especially with side effects that must be considered.On the other hand, as time passes, Indonesia will face a period when the demographic population is more elderly than productive young people.Thisconditionisone of the important and urgent reasons to find the best and most effective therapy to treat dementia.
By looking at the urgency,wearetryingtofindatherapythatcanhaveagoodimpact and hope to become the first-line therapy for dementia. From the various kinds of literature we obtained, at this time, researchers paid a lot of attention to aquatic therapy for dementia. Aquatic therapy is carried out by patients in water with certain specific criteria. The use of aquatic therapy in managing brain-related rehabilitative issues is exciting and offers new approachestoclinicalrehabilitativecare.
METHODS
The method used is a narrative literature review by analyzing several related references. The author chose 4 journals in the last ten years through PubMed, Google Scholar, Cochrane, Science Direct, Clinical Key, and WHO. Thekeywordsaredementiaand aquatictherapy
RESULTS&DISCUSSIONS
Aquatic immersion and exercise have been studied for their effects on the central nervous system. Research has shown that aquatic immersion and exercise can have positive effects on various cognitive and neurological functions such as balance, gait, and spatial memory These effects are attributed to the unique properties of water, such as buoyancy, hydrostatic pressure, and resistance, which create a low-impact, high-resistanceenvironment that can facilitate neuromuscular adaptations. Additionally, aquatic exercise has been shown
to reduce stress and anxiety, which can also have positive effects on the central nervous system.
One study by Barbosa et al. (2014) investigated the effects of a 12-week aquatic exercise program on cognitive function in older women. The study found that the exercise program resulted in improvements in spatial memory, attention, and executive function, suggesting that aquatic exercise may have neuroprotective effects. Another study by Tari et al. (2019) examined the effects of aquatic exercise on balance in patients with multiple sclerosis. The study found that aquatic exercise resulted in significant improvements in balance, suggesting that it may be a beneficial intervention for individuals withneurological conditions.
Overall, research suggests that aquatic immersion and exercise can have positive effects on the centralnervoussystem,includingimprovementsincognitivefunction,balance, and neurological conditions. These findings highlight the potential of aquatic exercise as a low-impact, high-resistance exercise modality that may be particularly beneficial for individualswithneurologicalconditions.
CONCLUSION
Aquatic immersion and exercise can positively affect the central nervous system, improving cognitive function, balance, and neurological conditions. The properties of water, such as buoyancy and resistance, create a low-impact, high-resistance environment that can facilitate neuromuscular adaptations. Research shows that aquatic exercise can benefit various populations, including older women and patients with multiple sclerosis. Although more research is needed, aquatic exercise has potential as a safe and effective exercise modalityforindividualswithneurologicalconditionsorwhoneedalow-impactoption.
ACKNOWLEDGEMENTS&CONFLICTOFINTEREST
ACKNOWLEDGEMENTS:
This literature review was conducted withthehelpofvariousacademicresourcesand materials. I wouldliketoexpressmygratitudetotheauthorsandresearcherswhoseworkhas contributed to this review I would also like to acknowledge the assistance of my colleagues whoprovidedhelpfulinsightsandfeedbackduringthewritingprocess.
CONFLICTOFINTEREST:
Therearenoconflictsofinteresttodeclareregardingthisliteraturereview.Theauthor has no financial or personal relationships thatcouldinfluencethecontentofthisreview.This review was conducted in an objective and impartial manner, and the findings presented are basedsolelyontheavailableresearchandevidence.
REFERENCES
BarbosaAR,SouzaJA,LealLK,LimaFC,SilvaFA,NogueiraDA.Aquaticexerciseand cognitivefunctioninolderwomen:Arandomizedcontrolledtrial.Archivesofgerontology andgeriatrics.2014;59(3):568-574.doi:10.1016/j.archger.2014.07.008
TariHV,Yeldanİ,ŞahinN,İdimanE,İdimanF.Theeffectsofaquaticexerciseonbalanceof patientswithmultiplesclerosis:Arandomizedcontrolledtrial.MultipleSclerosisandRelated Disorders.2019;27:13-19.doi:10.1016/j.msard.2018.09.024
Gale,S.A.,Acar,D.,&Daffner,K.R.Dementia.TheAmericanjournalofmedicine.2018. 131(10),1161–1169.https://doi.org/10.1016/j.amjmed.2018.01.022
BeckerBE.Aquatictherapyincontemporaryneurorehabilitation:Anupdate.PM&R.2020 Dec;12(12):1251-1259.doi:10.1002/pmrj.12435.
Abstract
NatashaCitaParadhitaKusuma1,DerrenDavidChristianHomentaRampengan2,Nathalia Angelina1,JadeAudreyHomentaRampengan3
FacultyofMedicine,UniversitasTarumanagara1,FacultyofMedicine,UniversitasSam Ratulangi2,FacultyofMedicine,UniversitasAtmaJaya3
Introduction:Nowadays,populationaginghasincreasedthedemandforelderlycareworldwide, therearemorelikelytohaveproblemswithbalancegrowthastheirfacemajorchallenges Balancetrainingbasedonvirtualreality(VR),whichsimulatesanactivitytechnologyfrom balancecontrolandresultsinenhancedstaticanddynamicstability,betoldusedastreatment gap.Ourthisstudyseekstoanswerthequestion,"IsBalanceTrainingonVirtualTechnology canbeanswersforBalancePerformanceintheElderly?"
Methods:ThesystematicreviewliteraturesearchwasconductedaccordingtothePRISMA2020 guidelinesonseveraldatabasesforeligiblestudies2018-2023.Studyqualitywasassessedusing theCochrane’sRiskofBias2Toolforapplicabilityconcernalltheincludedstudieshaslowrisk ofreferencestandardandflowandtimingandmajoritypossesslowriskofpatienceselection.
Results:Totalof651participantsparticipatedwhichtheaverageagewas67years.Eightstudies wasincludedintheassessmentofdiagnosisaccuracyofVRTonbalanceperformance.The findingsthathighsensitivityimpliesthatthetoolVRTaccuratelydiagnosisthebalance performanceinHealthyorderadultswithatrueprobabilityof89%TheresultsonBalance PerformanceshowsthatVRTexhibitsignificantimprovementingaitspeed(GS)Thepooled effectwassignificant(SMD=-0.39,95%CI=-0.76to-0.03,p=0.03),moderateheterogeneity wasfound(I2=57%,p=0.05)andAssessmentoffearoffallthatVRTdidnotdemonstratean inducedsignificantlyimprovement(SMD=0.09,95%CI=-0.15to0.34,p=0.47),withlow heterogeneity(I2=4%).
Conclusion:Theresultsofthisstudydemonstratethepotentialbenefitsofvirtualrealitybalance trainingonbalanceandphysicalperformance,bothofwhichareessentialfactorsinpreventing balanceimpairment.
Keywords:VirtualReality,VRT,BalanceTraining,BalancePerformance

AsianMedicalStudent’sConference2023
: NatashaCitaParadhitaKusuma
DerrenDavidChristianHomentaRampengan
1FacultyofMedicine,UniversitasTarumanagara 2FacultyofMedicine,UniversitasSamRatulangi 3FacultyofMedicine,UniversitasAtmaJaya
TheFutureDiagnosisAccuracyofVirtualBalance TrainingonBalanceandGaitPerformanceinHealthy OlderAdults:AMeta-Analysis
Authors
1
2 NathaliaAngelina1 JadeAudreyHomentaRampengan3
Abstract
NatashaCitaParadhitaKusuma1,DerrenDavidChristianHomentaRampengan2,Nathalia Angelina1,JadeAudreyHomentaRampengan3
FacultyofMedicine,UniversitasTarumanagara1,FacultyofMedicine,UniversitasSam Ratulangi2,FacultyofMedicine,UniversitasAtmaJaya3
Introduction:Nowadays,populationaginghasincreasedthedemandforelderlycareworldwide, therearemorelikelytohaveproblemswithbalancegrowthastheirfacemajorchallenges Balancetrainingbasedonvirtualreality(VR),whichsimulatesanactivitytechnologyfrom balancecontrolandresultsinenhancedstaticanddynamicstability,betoldusedastreatment gap.Ourthisstudyseekstoanswerthequestion,"IsBalanceTrainingonVirtualTechnology canbeanswersforBalancePerformanceintheElderly?"
Methods:ThesystematicreviewliteraturesearchwasconductedaccordingtothePRISMA2020 guidelinesonseveraldatabasesforeligiblestudies2018-2023.Studyqualitywasassessedusing theCochrane’sRiskofBias2Toolforapplicabilityconcernalltheincludedstudieshaslowrisk ofreferencestandardandflowandtimingandmajoritypossesslowriskofpatienceselection.
Results:Totalof651participantsparticipatedwhichtheaverageagewas67years.Eightstudies wasincludedintheassessmentofdiagnosisaccuracyofVRTonbalanceperformance.The findingsthathighsensitivityimpliesthatthetoolVRTaccuratelydiagnosisthebalance performanceinHealthyorderadultswithatrueprobabilityof89%TheresultsonBalance PerformanceshowsthatVRTexhibitsignificantimprovementingaitspeed(GS)Thepooled effectwassignificant(SMD=-0.39,95%CI=-0.76to-0.03,p=0.03),moderateheterogeneity wasfound(I2=57%,p=0.05)andAssessmentoffearoffallthatVRTdidnotdemonstratean inducedsignificantlyimprovement(SMD=0.09,95%CI=-0.15to0.34,p=0.47),withlow heterogeneity(I2=4%).
Conclusion:Theresultsofthisstudydemonstratethepotentialbenefitsofvirtualrealitybalance trainingonbalanceandphysicalperformance,bothofwhichareessentialfactorsinpreventing balanceimpairment.
Keywords:VirtualReality,VRT,BalanceTraining,BalancePerformance
Introduction
Asthepopulationexpand,globallypeopleaged60yearsandolderasknownElderly,thenumber isexpectedmorethanfantastic,from900millionin2015toabout2billionin2050.1Atpresent, thedynamicsoffacechallengingintheElderlymorelikelytohaveproblemswithbalance growthisveryhighastheygrowolderDuetothegrowingageingpopulation,age-related characteristicsinvolveaprogressivelossofvariousphysiologicalfunctionsincludingbalance.2 Basedonthestatistic,proximately28%to45%oftheelderlypopulationhaveamajorbalance problemorfunctionalimmobility.Ascorecaseseachyear,functionalimmobilitycanleadto lowerself-esteemandimpairmentofqualityoflifeforolderadults[forexample,movementis difficult,andfallingisarisk]3andbecamearetheleadingcauseofhospitalizedinjuryinthis population.4
Mostolderadults(79%)havedifficultyimplementingtheirrecommendedbalancetrainingina comprehensivemanner.Withthehighprevalenceofcognitiveimpairmentamongtheelderly,it isimportanttoconsiderwaystoreduceit.Amongpossibleinterventionsisphysicalactivityand lifestylechanges,whichcanmaintainthedailyactivitiesoflifeoftheelderlyandenhancetheir qualityoflife.1Furthermore,variousphysicalactivitiesandmaintaininganactivelifestylecan delayorevenreversethedeclineofcognitivefunctionastheyage.
Boththesensorimotorsystemandtheneuromuscularsystemareinvolvedinmaintainingbalance ItiscommonforelderlypeopletosufferfromimpairedgaitandbalanceastheyageForserve asmaximumtogaittrainingintheelderly,itisimportanttocombineintensivetrainingwith experienceinvariousenvironmentsanddifferentsituations.Theabilitytorecognizeobstacles andnegotiatethemaswellasphysicalabilityarecrucialforsafetobuildbalanceperformto them.3
Accordingthehighprevalenceofbalanceimpairmentarisesfromthecomplexattemptinmaking asuccessfuldiagnosisantrainingfortreatmentThesenumbersincreasethedemandsfor treatment,rehabilitationandsupportservicesoftypesofbalanceimpairment.Asapotential technologytoaddressthetreatmentgapofthiscondition,virtualrealitycanpromotestohelpthe gap.Avirtualrealityreferstoamulti-sensoryinterfacethatincludesreal-timesimulationand interaction.Virtualrealitytrainingcanprovideeducationinastimulatingaimedatimproving motorandcognitivefunctions.Inaddition,itprovidesperformancefeedbacktoaidinlearning
newtrainingstrategies.Interactivevirtualrealitytrainingaremorealluringandengagingthan conventionaltrainingandrehabilitationprograms.Balancetrainingbasedonvirtualreality(VR), whichsimulatesanactivitytechnology,havebeenproposedasanalternativetoconventional balancetrainingtopromotemotoralsocognitivefunctionlearningtogivestrengthenselfefficacyonbalancecontrolleadingtoimprovedstaticanddynamicstability5,6,7
Tothebestofourknowledge,noneofthepreviousreviewsormeta-analyseshaveexaminedthe comparativeeffectsbetweenvarioustypesofexercisesandtheefficacyofmultifacetedbalance interventionswithmorethanonetypeofbalancetrainingforelderlybalanceperformance.The purposeofthisstudyistoconfirmtheeffectofvirtualrealitybalancetrainingonthis population'sbalanceandmobilityWeproposeanexcellentmotion-trackinginterventionthat canmodifyvideospeedinreal-timebasedonmovementtopreventbalanceimpairmentAmore comprehensiveandinclusiveanalysisemployingprecisecodingofexercisetypestargeting functionalaspectsisrequiredtoenhanceprescriptiveguidance.Theresearchquestionwas:
"Whichtypeofbalanceinterventionismosteffectiveforimprovingoverallmeasuresaswellas individualmeasuresandthefuturediagnosticaccuracyofbalancetraininginolderadults?"and
"IsVirtualBalanceTechnologycanbeanswersforBalancePerformanceintheElderly?"
Objective
Thesystematicreviewandmetaanalysisaimstoinvestigateconfirmtheeffectofvirtualreality balancetrainingonthispopulation'sbalanceandmobility.
Methods
Thissystematicreviewandmeta-analysiswereconductedinconformitywiththePreferred ReportingItemsforSystematicReviewsandMeta-Analyses(PRISMA)2020guidelines.The detailedprotocolofthecurrentstudyhasbeenpreviouslyregisteredandpublishedonthe InternationalProspectiveRegisterofSystematicReviews(PROSPERO) (https://www.crd.york.ac.uk/prospero/)withtheregistrationnumberCRD42021287616.8
Eligibilitycriteria
ThePICOformatwasadoptedwhichbasedonthePopulation,Intervention,ComparisonAnd outcome;ThePopulation(P)wasAdultaged60yearsandabove,TheIntervention(I)was virtualrealitybalancetraining,theComparison(C)wasanyothertrainingmethodreportedin thestudies,theoutcome(O)are5STStest,gaitspeed,handgrip,strength,TUG,FRTandDHI. Theeligibilitycriteriaisdividedintoinclusionandexclusion
Inclusioncriteria
SearchLimit:Articlespublishedbetween2018and2023
Studydesign:RCT
ClinicalstudyonHuman
Exclusioncriteria
Studypublishedbefore2018wasexcluded
Animalstudywasremoved
Studywithoutabstractwasexcluded
Table2.PICOframework.
Elderly
(Category: 60years andolder)
Balancetraining-based virtualbalance intervention
Activeorinactivecontrol group
Compareandanalyze balanceperformance control(balanceand functionalmobility)
Qualityoflife
Abbreviations:PICO,Population,Intervention,Comparison,andOutcome;
I C O
P
DatasynthesisandAnalysisTechniques
Themeta-analysiswascarriedoutusingtheRevManreviewversion5.4softwareforsystematic reviewandmeta-analysis.ArandomeffectModelwasadoptedbecauseitwasknownthat patientswererandomizedselectedtogroupThedataextractedtoapre-definedexcelsheetare theestimatedmeanandstandarddeviationoftheoutcomesmeasuredreportedineachofthe studies.Inthecase,whereestimatesarereportedatbaseline,pre-testandattheendpointorat lastmonths,themeanandstandarddeviationdifferenceswastaken.Theheterogeneityamong thestudieswasmeasureusing(I2).95%confidenceintervalwasusedwith5%levelof significance.Thechosenlevelofsignificance(0.05)wouldbecomparedwiththeestimated probabilityvalue.Thedecisionisthat,ifthelevelofsignificance(0.05)islessthattheestimated probabilityvalue,wesaythat,theoutcomeissignificantotherwiseitisnotsignificant.
Studyselection

ThePRISMAflowchartdisplayedinFigure1explainedthesearchprocessThesearch generatedexplainedinTable1
Figure1.
PRISMAFlowDiagramoftheStudySelectionProcess.
DataExtractionandAnalysis
Thefollowingvariableswasextractedintoapre-definedexcelsheet;Authorfirstname,study design,Modeoftraining,ageofparticipants,BodyMassIndex,interventionusedineachstudy, DurationofTraining,assessmentoffearoffalling,followupperiod,outcomemeasureineach studies,themeanandstandarddeviationoftheoutcomesreportedineachstudies.
("BalanceTraining")OR
("BalanceExercise")OR
("Instabilitytraining")AND ("OlderAdults")OR
("Elderly")OR("Elder")OR
("Older")AND("Virtual Therapy")OR("VR")AND ("Falls") AND ("Performance")AND ("FunctionalMobility")
ScienceDirect (((((((((((BalanceTraining) OR(BalanceExercise))OR (Instabilitytraining))AND
Database Keywords Results MEDLINE
(OlderAdults))OR(Elderly)) OR(*Elder))OR(*Older)) AND(VirtualTherapy))OR (VR))AND(Falls)))AND (Performance) 91 GoogleScholar
Table1.DatabaseSearchingProcessKeywords
(((((((((((BalanceTraining) OR(BalanceExercise))OR (Instabilitytraining))AND
394
(OlderAdults))OR(Elderly)) 234
OR(*Elder))OR(*Older)) AND(VirtualTherapy))OR
ArticlescomprisesofPubMed(n=91),googlescholar(n=394),andsciencedirect(n=234), Proquest(4,171),Hindawi(5),Cochrane(12).Intotal,4,907articlesweregeneratedofwhich 4,184replicatewereexcluded.Theremaining93articleswasscreened.Outofwhich33 irrelevantarticleswereremovedwhenscreenedtheabstract.Then,whenwentthroughthefull text,10articleswereexcluded.From65studiesselectedforfull-textscreening,10studieswere
(Performance) ProQuest "BalanceTraining"AND "OlderAdults"OR"Elderly" AND"VirtualTherapy"OR "VR"AND"Falls"AND "Performance" AND "FunctionalMobility" 4,171 Hindawi
(Instabilitytraining))AND (OlderAdults))OR(Elderly))
AND(VirtualTherapy))OR (VR))AND(Falls)))AND (Performance) 5 Cochrane ElderlyinTitleAbstract KeywordANDBalance TraininginTitleAbstract KeywordANDVirtual TherapyinTitleAbstract KeywordANDFalls 12
(VR))AND(Falls)))AND
(((((((((((BalanceTraining) OR(BalanceExercise))OR
OR(*Elder))OR(*Older))
excludedforthefollowingreasons:10hadfulltextnotavailable,irrelevantoutcome,and incompatiblestudyparticipant.Theremaining14articleswasassessforeligibilityandonly8 articlesmeetstheinclusioncriteria.Finally,studiesincludespublishedbetween2018-2023,were generatedfromhandsearching,fulfilledtheeligibilitycriteriaandwereincludedinthis systematicreview
QualityAssessmentandRiskofBiasinStudy
TheCochraneRiskofBiastoolfordiagnosisofAccuracyissummarizedinFigure2andFigure 3.Allthe8includedarticleswereofgoodquality.Highestpercentageofthestudieshaslowrisk ofpatienceselection,Indextest,referencestandard,flowandtiming.Fortheapplicability concernalltheincludedstudieshaslowriskofreferencestandardandflowandtimingand majoritypossesslowriskofpatienceselection
 Figure2:PercentageSummaryofthequalityassessmentjudgment
Figure2:PercentageSummaryofthequalityassessmentjudgment
Characteristicsofincludedstudies

AsshowninTable2.Thecharacteristicsofthe8randomizedcontrolstudieswaspresented,A Totalof651participantsparticipatedinallstudiesofwhichtheaverageagewas67years MajorityoftheparticipantswereFemaleofabout60%TheaverageBodyMassIndexofthe participantswasaround27kg/m2andthetraininglastedfor60minutesineachgroup.TheMode oftrainingemployedinvarioustrialsare;Virtualrealityorexercise(Phuetal.,2019,Kimetal., 2022,LimaRebelo2021andYousefiBabadietal.,2021),singleanddualtasktraining (Javadpouretal.,2022),ConditionSpecifitandComprehensivebalanceTraining(Mahjur2022), reactivebalancetrainingandcognitivetask(Okubo2019andYuzluetal.,2021).
MajorityofthetrailsusedBalancetraining(Javadpouretal,2022,Okubo2019,Mahjur2022, Yuzluetal.,2021andYousefiBabadietal.,2021),someofthestudiesgroupedtheintervention intotwo,BRUandExercise(Phuetal.,2019),VRTandCBT(YousefiBabadietal.,2021), Singletraininganddualtraining(Javadpouretal.,2022),VRGandMIG(Kimetal.,2022), consecutiveandintegrateddual-tasktraining;CDTTandCDTT(Yuzluetal.,2021).
Figure3:TheQualityassessmentgraphofthe8includedstudies
Themethodusedtoassessedphysicalperformanceandmobilityofhealthyolderadultsare; 5STS,gaitspeed,handgripstrength,FRT,HSandTUG.Thefearoffallingwasassessedusing FallsEfficacyScale(Phuetal.,2019,LimaRebelo2021)andTinettiFallsEfficacyScale(Kim etal.,2022,Okubo2019,andYuzluetal.,2021).
Results
DiagnosisAccuracyofVirtualBalanceTrainingonBalancePerformanceinHealthyOlder Adults
EightstudieswasincludedintheassessmentofdiagnosisaccuracyofVRTonbalance performanceThepooledmeta-analysisofthesensitivityandspecificityreportedinFigure4 Showsthat,thestudiesJavadpouretal,2022,YousefiBabadietal,2021,Yuzluetal,2021and Phuetal.,2019hashighsensitivityvalues88%,80%,97%and97%respectively.However,the percentagesensitivityvaluesofKimetal.,2022,LimaRebelo2021andMahjur2022are moderatelyhigh55%,55%and58%.OnlystudyOkubo2019hassensitivityvalueof42%.The highsensitivityimpliesthatthetoolVRTaccuratelydiagnosisthebalanceperformancein Healthyorderadultswithatrueprobabilityof89%
 Figure4:ThesensitivityandspecificityforestplotofVirtualBalanceTrainingPerformance
Figure4:ThesensitivityandspecificityforestplotofVirtualBalanceTrainingPerformance
Meta-Analysis
EffectsofVirtualBalanceTrainingonBalancePerformanceinHealthyAdults
Baseonthedataextractedfromallstudies,weperformasubgroupmeta-analysestocompare variousVRTtypestocontrolgroupInthemeta-analysisofTUGthatassessedthemobility performanceTheanalysissuggestthat,thevirtualbalancetraining(VRT)significantlyexhibits greaterimprovementinTUG(SMD=-0.31cm,95%CI=-0.55,-0.06,p–value=0.01),low heterogeneitywasfound(I2=4%,p=0.38)Figure5A.Thefunnelplotshowsasymmetric symbolwhichimplieslackofpublicationbiasFigure7A
Forthe5STSandFRT,themeta-analysissuggestthatincomparisontothecontrolgroup,VRT didnotinducesignificantlygreaterimprovementin5STSat5%levelofsignificance(5STS, SMD=028,95%CI=-001to058,p=006)(FRT;SMD=047,95%CI;-125to218,p= 059)ThefunnelplotshowsasymmetricsymbolwhichimplieslackofpublicationbiasFigure 7BFigure7C.
Theresultsofmeta-analysisshowsthatVRTexhibitsignificantimprovementingaitspeed(GS).
Thepooledeffectwassignificant(SMD=-0.39,95%CI=-0.76to-0.03,p=0.03),moderate heterogeneitywasfound(I2=57%,p=005)Thefunnelplotissymmetricwhichimpliesthat thereisnoevidenceofpublicationbiasFigure7D.
Figure5:ResultsofMeta-analysis(A)TimeUpandGo(TUG),(B)5sittostand,(C)Functional ReachTest(FRT),(D)gaitspeed(GS)

Meta-analysisofassessmentoffearoffall
Thefearoffallingwasassessedusingthefearefficacyscale.Fivestudieswasincludedinthe meta-analysis.Theresultsshowsthat,VRTdidnotdemonstrateaninducedsignificantly improvement(SMD=009,95%CI=-015to034,p=047),withlowheterogeneity(I2=4%) Themeta-analysisofhandicapscalealsoshowsnoimprovementinHSbyVRTcomparetothe controlgroup(SMD=-0.10,95%CI=-0.56to0.36,p=0.68)withmoderateheterogeneityI2= 41%.
Wecouldnotperformmeta-analysistoinvestigatetheeffectofVRTonsomestudyoutcome suchasFullertonAdvancedBalancescale,Activities-specificBalanceConfidencescale,Balance sensoryandBodycenterMovementduetosmallnumberofstudy
 Figure6:ResultsofMeta-analysis.(A)Fallefficacyscale(FES),(B)HandicapScale
Figure6:ResultsofMeta-analysis.(A)Fallefficacyscale(FES),(B)HandicapScale
Outcome
Therearecategoriesassessedinthisstudy:(1)Theprimaryoutcome:Compareandanalyze balanceperformancecontrol(balanceandfunctionalmobility)(2)Thesecondaryoutcome: QualityofLifeinelderlytomaintainBalancePerformance;whichusesvirtualtraining frequencyfollowinginterventionconcepts:balancerehabilitationwithconsolesensors,social functioning,durationoftrainingtomaintainoverallqualityoflife.Virtualbalancetraining frequencywasthemostassessedoutcome.Itwasstudiedin7outof8includedstudieshave typesofintervention,whilequalityoflifebasedassessmentoffearoffallingwasonlystudiedin 2outof8studies.Theoutcome;comparingandanalyzingaboutbalancetrainingcontroland
 Figure7:FunnelPlots
Figure7:FunnelPlots
frequencyalsoforqualityoflifeincludedinstudieshaddifferentresults.Onewithimproved qualityoflifeandtheotherwiththesamequalityoflifeamongthosewhoutilizedbalance trainingandthosewhodidnot.Therewasassessmentandcompareaboutbalancetrainingin virtualimprovedsignificantornotsignificantly.Detailsofsummarysignificantlystudyoutcome basedpreandpostinterventionbalancetrainingcanbeseeninAppendix2Detailsofthe studiesincludedinthisreviewaredisplayedinAppendix1
Table2:CharacteristicsofIncludedstudies
First Author Training Modes DesignInterventionAge (Years) (M ±SD) BMI (kg/m2) GenderDuration of Training Noof Particip ant Study Outcome Assessment offearof falling Phuetal., 2019 VirtualReality orExercise RCTBalance Rehabilitation Unit Median 78years Median2767% Female 60mins1955STStest,gait speed, handgrip strengthand TUG FallsEfficacy Scale Yousefi Babadiet al.,2021 VirtualReality Trainingor Conventional balance training RCTKinectand Console Sensors 60-75 years 24.72(1.79)17Males60-min session 36TUG,SLS, FRT Fullerton Advance BalanceScale
Mahjur 2022 Condition Specifitand Comprehensiv ebalance Training RCTNR68.1± 3.6 years NRMale 60% 60-min session 178TUG,ABS, gaitspeed NR Javadpour etal.,2022 STandDT Balance Training RCTSingleTask AndDual Task 67.65± 2.42 67.17± 8.65 Female 16,Male 7 40–60min69Fullerton Advanced Balancescale, TUG,Gait speed, Activitiesspecific Balance Confidence NR Kimetal., 2022 Virtual reality(VR) andmotor imagery training RCTTheGaitview AFA-50 system 75.75(10 .15) 23.82(5.23)5Male& 7Female 30min34BodyCenter Movement Area,Balance Sensorywith eyesopenand alsowitheyes close TinettiFalls Efficacy Scale
(A)IDTT=integrateddual-tasktraining,FRT=Functionalreachtest,CDTT=consecutivedual-tasktraining;
(A)Depression,(B)Anxiety,(C)Stress.
Yuzluet al.,2021 Balance Exerciseand cognitivetasks RCTBergBalance Scale(BBS) 60yrs26.1(2.7)Male5 (17.2), Female 24(82.8) NR58TUG,gait speed TinettiFalls Efficacy Okubo 2019 reactive balance training RCTTrip65-90yrs 26.4 ± 3.9 Female 59% 120min44 Fall TinettiFalls Efficacy Lima Rebelo 2021 Immersive virtualreality RCT 69.25(5. 67) Male 4(20%), Female 16(80%) 50min37 TUG, DGI,FRTand DHI FallsEfficacy Scale International
DISCUSSION
MainFindings
Ourmeta-analysisshowedthathealthyolderpeople'sbalanceperformancewasenhancedby virtualreality(VR)balancingtraining(TUGandGS)Tothebestofourknowledge,this systematicreviewandmeta-analysisisthefirststudytosystematicallyreviewthemostrecent publicationonthediagnosticefficacyofvirtualbalancetrainingonbalanceperformancein healthyolderadults.Therewereeighttop-notchrandomizedcontrolledtrialsincluded.The meta-analysis'sconclusionsshowedthatTUGandGSshowedmorenotableperformancegains thanthecontrolgroup.Ittakesatleast90minutesperday,threeormoredaysperweek,fora totalofabout720minutesperweektoimproveperformance
ThesignificantenhancementofTUGandGSrelativetothecontrolgroupfortargetsteptraining andotherbalancetrainingisconsistentwiththeresultsofapreviousmeta-analysis.(Liu2022 andNerietal.,2017)4,17.Ourmeta-analysisnotonlyinvestigatedtheperformanceofTUGin comparisontomorethanonecontrolgroup,butalsothediagnosticaccuracyofVirtualbalance trainingbydiscoveringhighsensitivityandahighprobabilityoftruepositiveacrossalltrials. Despitethefactthatthetwostudyoutcomes(TUGandGS)arecommonlyusedtoevaluatethe mobilityandequilibriumofhealthyadults,ourresearchdidnotindicateasignificant improvementinGSusingVRTcomparedtothecontrolgroupLiu's2022meta-analysisalso discoveredanon-significantimprovementinGSwhencomparingVRTtoTPTThestudyfrom Noorollaet.alfindingsrevealedthatthemeanofthepost-interventionTUGtestreducedby about2.33sintheinterventiongroup(p<0.001),whilethetwogroupswerenotsignificantly different(p=0.88)andthemeanscoreofFESdecreasedsignificantlyfollowingtheexercises (p<0.001),butdidnotchangeinthecontrolgroup(p=0.217).Sincewehaveprovidedmore evidencetosupportthediagnosticaccuracyoftheVRT-basedintervention,thedegreeof satisfactionandwillingnesstouseitrequiremoreattention;consequently,futureresearchshould devotemoreresourcestothisarea.
Themeta-analysisoftheFRTand5STS,whichassessmobilityandbalance,revealsno significantdifferencebetweentheVRTandthecontrolgroup.Thefindingsarecomparableto Liu'smeta-analysisfrom2022.However,theeffectsofVRTontheperformanceofFRTand 5STSweresignificantlygreaterthanthoseofTRT(Liu,2022),andithasbeendemonstratedthat TPTexhibitsgreaterimprovementsinthevestibularsystemthanVRT(Babadi,2021)16Since ourstudyonlycomparedallcontrolgroups,futureresearchshouldcomparedifferentcontrol groupsandaddmoretrialstothepresentstudy
VirtualReality(VR)forBalanceTrainingasDiagnosisTools
Ithasbeenreportedthatvirtualreality(VR)balancetrainingcanreducefatigue,tension,and depression,aswellasproviderelaxation,whichreflectsthepositiveattitudeofseniorstoward theuseofthisnewtechnology18Ourresultsarecomparabletothoseofpreviousresearchon healthypopulationsingeneral.Inaddition,wefoundthatimprovingstatic/dynamicsteady-state, proactive,andreactivebalance,aswellasperformanceonbalancetestbatteries,hadsignificant effectsinhealthyolderadults.Theseresultsmaybeattributabletothefactthatvirtualbalance trainingisassociatedwithacceptanceskills,whichenhancedthehealthresilienceofbalance performance19 .
Subgroupanalysesrevealedthatthetypeofcontrolconditionsusedtoevaluatethevirtual balancetrainingmayhaveaffectedtheeffectsizeofthepooledeffectonbalanceperformance. Theeffectsizesofstudieswithinertcontrolstendedtobelargerthanthosewithactivecontrols. Ithasbeendemonstratedthattheprovidedactiveinterventions,suchasmanipulatingthesensory inputs(multisensoryexercise)oraddingacognitivefunction(CDTTorFRTexercise),playa significantroleinthetreatmentofindividualsbyassessingfunctionalmobilityandbalance.
Accordingtometa-analyses,aprolongeddurationoftraininginterventionswasassociatedwith improvedbalanceperformanceThisresultwasconfirmedbythesensitivityanalysisresultof Phuetal.,whosestudyhadthelongesttrainingduration(60min-session)andthemost participants(195)8.Theprolongeddurationofeachtrainingcandemonstratethatpoorfalls efficacyandlowconfidenceinbalancecanimpedetheperformanceofdailyactivities,leadingto acycleofdiminishedphysicalcapacity,lossofindependence,andadditionalfalls.Withtheaid
ofadvancingtechnology,balance(physical)therapy'spracticalapplicationshaveexpanded significantly25.Therefore,VR-basedbalancingtrainingmaybeadvantageousasawell-likedand cutting-edgetechnicalmethodbecauseitmaybemoreinteresting.
BarriersandOpportunities
Advancementoftechnologyhasspreadtocommunity-dwellinginelderlyindiscriminately. However,digitalliteracyisnotyetuniformthroughouttheworld'scommunities.Someelderly andelderly’saroundpopulationsmaybeunfamiliarwithadvancedtechnologiessuchas immersivevirtualreality.Thiscouldbemanagedbyincreasingsociety'sawarenessand socializationofproperalsoasanimportantthingstoknowusageproceduresTheresearcherin somestudieshasstatedthatprimarycommunity-dwellingandclinicaltherapiesintheelderly’s aroundplayroleineffortabletomaintaindiagnoseandmanagebalanceperformancefor functionalmobilityandrealizetheimpactofbalanceperformanceonqualityoflife[healthy olderadults].Theelderly'sfamilyandfriendscanassisttheminusingtoolsthatwillmakethe processofvirtualbalancetrainingdiagnosisandmanagementaseffectiveaspossible.Virtual reality(VR)balancetrainingmayplayapivotalroleinbridgingthetreatmentdivideforbalance impairment.
ConclusionandRecommendation
Theeffectsofvirtualbalancetrainingforolderindividualswerepromisingforenhancing functionalposturalbalanceinthispopulationduringrestrictedsituations,suchasage-related immobilityTheresultsofthisstudydemonstratethepotentialbenefitsofvirtualrealitybalance trainingonbalanceandphysicalperformance,bothofwhichareessentialfactorsinpreventing balanceimpairment
Giventhegrowinginterestandpotentialutilityofvirtualbalancetraininginclinicalpractice,we encourageadditionalresearchtoinvestigateadditionalevidenceregardingthemaximumbenefits ofVRbalancetrainingandthepotentialinfluencingfactorsoftreatmentefficacy,alongwitha numberofotherconsiderations.Futurestudiesandresearcherscouldinvestigatethefollowing:
(1)determiningwhethertheobservedbeneficialeffectsaresustainedoverlongerfollow-up periods;(2)evaluatingtheeffectsofVRbalancetrainingforbalanceperformanceinpatients withdifferentcharacteristics;(3)identifyingsubgroupsofseveralfactorsthatcouldnotbe analyzedinthecurrentstudy;and(4)consideringbiasesandqualitycriteriawhenconducting studiesandpublishingresults,asthequalityofthestudieswasnotadequatelyevaluatedLastly, tothebestofourknowledge,thisisthefirstsystematicreviewandmeta-analysisthatexplicitly examinestheeffectsofVRTonthefunctionalmobilityofhealthyolderindividualsutilizing onlyhigh-qualityRCTs.FuturestudiesimplementingVRTwithrigorousdesignswilluseour findingstoinvestigatetheeffectsofVRTonfunctionalmobilityandbalanceinolderadultsand confirmourfindingsandconclusions.
Limitation
Theresearchencounteredsomelimitationswhileinterpretingtheresults.Thefirstlimitationis timelimitswhichwasrestrictedtostudypublishedinthelast5years.Relevantstudymightbe excludedwhichcouldhaveimprovethesignificantoftheresults.Thestudysuggestthat,future researchshouldexpandthesearchlimit.Thesecondlimitationisseverallimitationsneedtobe noted.Thenumberofincludedstudiesisstillrelativelysmall,whichlimitsthescopeofthe analysisLastly,someoftheresultsshowslowheterogeneitywhichimpliesthat,atthetimeof theresearch,morestudiesarelikelytobepublishedwhichmightcausepublicationbias,thus, needmorecautioneventhoughthesensitivityanalysisshowedrobustresults.
Phuetal., 2019
Virtualrealitybalancetraining provideaviablealternative interventionforfallsprevention
Improving balanceand physical performance
Therewaspre-andpostinterventionstudy.Both interventionsshowed significantlybetter improvementthanthenoninterventiongroupinTUG (p<0.001),gaitspeed (p=0.021),limitsof stabilityinposturography assessment(p=0.008), FES-Iscore(p=0.013)and handgripstrength (p=0.021).OnlytheBRU groupimprovedcontrolof staticpostureintheeyes
Virtualrealityasapotentialpractical alternativetoimproveoutcomesofbalance trainingforreductionoffallsriskinolder adults
Appendix1. NoAuthor, year Aim OutcomeSummaryofResults Conclusion 1
Yousefi
Comparisonbetweentheeffects ofvirtualrealitytraining(VRT) andConventionalbalance training(CBT)onthebalance oftheelderly
Comparefrom VirtualReality (VR)Balance Training frequency
closed(p=0.002)andfoam eyesclosed(p=0.006)
tasks
Inbothgroups(VRT, CBT),SLSwithopenand closedeyes,FRT,TUG, andFABSwere significantlyimproved (P˂0.05).Afterthe intervention,changesin bothgroupsweresimilar (P>0.05),whichindicates thatneitherVRTandCBT trainingmethodswere superiortotheother
Virtualrealitytrainingprogramcanbeused asanewtrainingmethodtoimprovethe elderly'sbalanceindailyprogramsof nursinghomes
Investigatetheeffectofhomebasedspecificand comprehensivebalancetraining onbalanceandfunctionalstatus
Qualityoflife (balanceand functional
Theregroupswasshowed moresignificant improvementsthanthe controlgroupinall variablesexceptsectionII
Virtualhome-basedbalancetrainingprovide encouragingdataontheefficacyofhomebasedexerciseprogramsintheelderly population
2
Babadietal., 2021
3. Mahjuret.al, 2022
inolderadults. status) ofBESTest
Comparetheeffectsofsingleversusdual-taskbalance trainingonthegaitsmoothness andbalanceofcommunitydwellingolderadults
Improved significantlyin thetraining groupsfor Qualityoflife
Therewaspre–post assessmentsshowed statisticallysignificant changesinallofthe variables(STandDTHRs, STandDTgaitspeeds, FAB,andABC)inboth theSTandDTbalance traininggroupsstatistically significantdifferencesin themeanchangeofall variablesbetweenthethree groups
ProgressivebalanceexercisesunderbothST andDTconditionsareofpotentialbenefits forimprovinggaitsmoothnessandbalance performanceamongasymptomaticolder adults.
Examinethebalancekeeping effectofavirtualreality(VR) programandmotorimagery training(MIT)andpropose trainingthatcouldimprove
Improve physicalactivity amongolder adults (Qualityoflife)
Therewasasignificant differencebetweenthe MITgroupandCGinthe openeyesbalancescore post-test(d=1.13,95%
VRandMITastrainingmethodstoprevent physicalweaknessinisolatedolderadults
4. Javadpouret al.,2022
5.Kimetal., 2022
physicalactivityamongolder adults
confidenceinterval,0.40–12.33)
Comparetheeffectsof integratedandconsecutive cognitivedual-taskbalance traininginolderadultson balance,fearoffalling,andgait performance
Comparethe virtualreality (VR)balance training frequency
Therewasnodifferencein group-timeinteractionin theBergBalanceScale, TUG-standard,10MWTsingletask,and10MWTdualtasktests.Group-time interactionwasdifferentin theTUG-cognitiveand TinettiFallsEfficacyScale scores.Also,theeffectof timewassignificantly differentinallscales exceptforthe10MWTsingletaskinbothgroups.
Theimpactofintegratedandconsecutive dual-taskbalancetrainingonbalanceand gaitperformanceinolderadultswasnot statisticallysignificantlydifferent
Examinedwhetherreactive balancetraining(exposuresto Analyzereactive balancetraining
Relativetothecontrol group,theintervention
Thereactivebalancetrainingreduced perturbation-inducedfallsby60%indicating
6.Yuzluetal., 2021
7.Okubo2019
2021
slipsandtrips)couldimprove balancerecoveryandreduce perturbation-inducedfalls amongolderadults
frequencygroupexperiencedfewer totalfalls(rateratio [RR]=0.40,95% confidenceinterval [CI]=0.22-0.76),slipfalls (RR=0.33,95%CI=0.120.90)andtripfalls (RR=0.49,95%CI=0.211.12)
improvedbalancerecoveryfromtripsand slipsalsoinfallpreventioninterventions
Comparingeffectsof immersivevirtualreality(IVR) trainingcomparedto conventionalphysiotherapyon balanceandriskoffallsin olderadults
Qualityoflife. Theprimary outcomewas functional balance. Secondary outcomeswere staticbalance, gaitspeed, functionalrange, dizziness
Intheintergroup comparison,therewasno significantdifference.The functionalbalancescorein theexperimentalgroup increasedby3.00(95%CI 1.42to4.57)andinthe controlgroupby3.88 (95%CI2.16to5.59).
Bothgroupsimprovedin assessmentsofsensory
ImmersiveVirtualRealitytrainingprovedto beeffectiveforbalance-relatedoutcomes, althoughitwasnotsuperiortoconventional therapy
8.
LimaRebelo
symptoms,and fearoffalling.
interactionandanterior reach.Onlythe experimentalgroup presentedincreased mobilityandreduced dizziness.Aftertwo months,therewasa maintenanceofgainsin functionalbalanceanda reductionofthegainsin functionalreachforboth groups.
Appendix2.StudyOutcome
Author, Year Assessment Intervention Control Pre Post pValue Pre Post p-Value N MeanNMean NMeanNMean Phuetal., 2019 FallsEfficacy Scale–International73 32(25,41) 51 26(22,33)<0.001 48 32(24,43) 49 34(23,43)0.676 Yousefi Babadiet al.,2021 Fullerton Advance BalanceScaleVirtualReality Training 36 0.72 36 5.29 <0,05
Mahjur et.al, 2022 NR Javadpour etal., 2022 ST=singletask TUG 23 11.93±2.42 23 11.15±2.12 <.001 23 12.78± 1.9823 12.28± 1.70 .147 DT=dualtask TUG 23 12.06±2.31 23 10.96±2.22 Kimet al.,2022 VRGFalls Efficacy 34 6.3±3.3 34 19.7±19.9 34 6.2(3.7to 8.7)34 3.3(5.1 to1.4) Yuzluet al.,2021 TinettiFall EfficacyScale29 31.20(26.20 to36.20)25 18.40(16.40 to20.50) .007 25 12.76 (7.17–18.34) <.001 Timed“Up& Go”cognitive29 18.20(16.6 to19.80)25 14.24(13to 15.40) .022 25 3.96(2.69–5.22) <.001
Okubo et.al, 2019 PPA: Physiological Profile Assessment 19 0.71±1.11 19 0.80±0.920.338 22 0.44± 0.7922 0.50±1.080.773 Lima Rebelo et.al, 2021 DGI 20 3.00 20 0.65 17 3.88 17 1.88 TUG 20 1.71 20 0.29 17 1.22 17 0.54 FRT 20 4.3020 3.19 17 8.64 17 4.47 DHI 20 7.50 20 0.20 17 2.00 17 2.06 FES-I 20 1.80 20 2.00 17 1.41 17 2.18
References
1.Abuseofolderpeople[Internet].WorldHealthOrganization.WorldHealthOrganization; [cited2023Apr9].Availablefrom:https://www.who.int/news-room/fact-sheets/detail/abuse-ofolder-people
2.Mohammed,R.,Shahanawaz,S.,Dangat,P.,Bhatnagar,G.,&Jungade,S.(2021).Balance enhancementinolderadults:Isfunctional-tasktrainingbetterthanresistancetrainingin enhancingbalanceinolderadults?Cureusdoi:107759/cureus19364
3.Kozinc,Ž,Löfler,S.,Hofer,C.,Carraro,U.,&Šarabon,N.(2020).Diagnosticbalancetests forassessingriskoffallsanddistinguishingolderadultfallersandnon-fallers:Asystematic reviewwithmeta-analysis.Diagnostics,10(9),667.doi:10.3390/diagnostics10090667
4.Liu,C.J.,andLatham,N.K.(2022).IsVirtualrealitytrainingmoreeffectivethantraditional physicaltrainingonbalanceandfunctionalmobilityinhealthyolderadults?ASystematic ReviewandMeta-AnalysisCochraneDatabaseSystRev2009:Cd002759doi: 101002/14651858CD002759pub2
5AzhdarM,MirzakhaniN,IraniA,AkbarzadehBaghbanAR,DaryaborA,SangiS,etalThe EffectofBalanceTrainingonCognitiveandOccupationalPerformanceoftheElderly.Journal ofBabolUniversityofMedicalSciences.2022;24(1):41-9
6.LeeK.Virtualrealitygaittrainingtopromotebalanceandgaitamongolderpeople:A randomizedclinicaltrial.Geriatrics.2020;6(1):1.
7Prisma[cited2023Apr9]Availablefrom:http://wwwprismastatementorg/PRISMAStatement/FlowDiagramaspx
8PhuS,VogrinS,AlSaediA,DuqueGBalancetrainingusingvirtualrealityimproves balanceandphysicalperformanceinolderadultsathighriskoffalls.ClinIntervAging.2019 Aug28;14:1567-1577.doi:10.2147/CIA.S220890.PMID:31695345;PMCID:PMC6717859.
9.YousefiBabadiS,DaneshmandiH.Effectsofvirtualrealityversusconventionalbalance trainingonbalanceoftheelderly.ExpGerontol.2021Oct1;153:111498.doi: 10.1016/j.exger.2021.111498.Epub2021Jul24.PMID:34311059.
10.MahjurM,NorastehAA.Effectsofhome-basedspecificandcomprehensivebalance-training programsonbalanceandfunctionalstatusinhealthyolderadults.ExpGerontol.2022Mar; 159:111701.doi:10.1016/j.exger.2022.111701.Epub2022Jan13.PMID:35033547.
11.JavadpourS,SinaeiE,SalehiR,ZahednejadS,MoteallehA.ComparingtheEffectsof Single-TaskversusDual-TaskBalanceTrainingonGaitSmoothnessandFunctionalBalancein Community-DwellingOlderAdults:ARandomizedControlledTrialJAgingPhysAct2022 Apr1;30(2):308-315doi:101123/japa2020-0523Epub2021Aug27PMID:34453027
12.KimSH,ChoSH.BenefitsofVirtualRealityProgramandMotorImageryTrainingon BalanceandFallEfficacyinIsolatedOlderAdults:ARandomizedControlledTrial.Medicina (Kaunas).2022Oct28;58(11):1545.doi:10.3390/medicina58111545.PMID:36363502; PMCID:PMC9692723.
13YuzluV,OguzS,TimurtasE,AykutogluE,PolatMGTheEffectof2DifferentDual-Task BalanceTrainingMethodsonBalanceandGaitinOlderAdults:ARandomizedControlledTrial PhysTher2022Mar1;102(3):pzab298doi:101093/ptj/pzab298PMID:34972869
14.Okubo,Y.,Sturnieks,D.L.,Brodie,M.A.,Duran,L.,&Lord,S.R.(2019).Effectof reactivebalancetraininginvolvingrepeatedslipsandtripsonbalancerecoveryamongolder adults:Ablindedrandomizedcontrolledtrial.TheJournalsofGerontology:Series A.doi:10.1093/gerona/glz021
15.LimaRebêlo,F.,deSouzaSilva,L.F.,Doná,F.,SalesBarreto,A.,&deSouzaSiqueira Quintans,J(2021)Immersivevirtualrealityiseffectiveintherehabilitationofolderadultswith balancedisorders:ArandomizedclinicaltrialExperimentalGerontology,149, 111308.doi:10.1016/j.exger.2021.111308
16.Babadi,S.Y.,andDaneshmandi,H.(2021).Effectsofvirtualrealityversusconventional balancetrainingonbalanceoftheelderly.Exp.Gerontol.153,111498.doi:
10.1016/j.exger.2021.111498
17.Neri,S.G.,Cardoso,J.R.,Cruz,L.,Lima,R.M.,deOliveira,R.J.,Iversen,M.D.,etal. (2017)Dovirtualrealitygamesimprovemobilityskillsandbalancemeasurementsin community-dwellingolderadults?Systematicreviewandmeta-analysisClinRehabil31, 1292–1304doi:101177/0269215517694677
18.KimY,VakulaMN,BoltonDAE,DakinCJ,ThompsonBJ,SlocumTA,TeramotoMand BresselE(2022)WhichExerciseInterventionsCanMostEffectivelyImproveReactiveBalance
inOlderAdults?ASystematicReviewandNetworkMeta-Analysis.Front.AgingNeurosci. 13:764826.doi:10.3389/fnagi.2021.764826
19.SilvadaRochaJ,PotonR,RosaL,daSilvaNL,FarinattiP.Pilatesandimprovementof balanceandpostureinolderadults:Ameta-analysiswithfocusonpotentialmoderators.Health SciencesReview2022;5:100054
20LeeKVirtualrealitygaittrainingtopromotebalanceandgaitamongolderpeople:A randomizedclinicaltrialGeriatrics2020;6(1):1
21.JCPSP.[cited2023Apr9].Availablefrom:https://jcpsp.pk/article-detail/wii-fit-for-balancetraining-in-elderly-a-systematic-review
22.Zahedian-NasabN,JaberiA,ShiraziF,KavousiporS.Effectofvirtualrealityexerciseson balanceandfallinelderlypeoplewithfallrisk:Arandomizedcontrolledtrial.BMCGeriatrics. 2021;21(1)
23TekinF,Cetisli-KorkmazNEffectivenessofatelerehabilitativehomeexerciseprogramon elderadults’physicalperformance,depressionandfearoffallingPerceptualandMotorSkills 2022;129(3):714–730.
24.Balanceexercises[Internet].NHSchoices.NHS;[cited2023Apr8].Availablefrom:
https://www.nhs.uk/live-well/exercise/strength-and-flexibility-exercises/balance-exercises/
25.UrabeY,FukuiK,HaradaK,TashiroT,KomiyaM,MaedaN.Theapplicationofbalance exerciseusingvirtualrealityforrehabilitation.Healthcare.2022;10(4):680.
26ChauPH,KwokYY,ChanMK,KwanKY,WongKL,TangYH,etalFeasibility, acceptability,andefficacyofvirtualrealitytrainingforolderadultsandpeoplewithdisabilities: Single-armpre-poststudy.JournalofMedicalInternetResearch.2021;23(5).
27.Campo-PrietoP,Cancela-CarralJM,Rodríguez-FuentesG.Feasibilityandeffectsofan immersivevirtualrealityExergameprogramonphysicalfunctionsininstitutionalizedolder adults:Arandomizedclinicaltrial.Sensors.2022;22(18):6742.
28.ParkJ-H.Isdual-tasktrainingclinicallybeneficialtoimprovebalanceandexecutivefunction incommunity-dwellingolderadultswithahistoryofFalls?InternationalJournalof EnvironmentalResearchandPublicHealth2022;19(16):10198
29NishchykA,ChenW,PrippAH,BerglandATheeffectofmixedrealitytechnologiesfor fallspreventionamongolderadults:Systematicreviewandmeta-analysis.JMIRAging. 2021;4(2).
30.FarlieMK,RobinsL,HaasR,KeatingJL,MolloyE,HainesTP.Programmefrequency,type, timeanddurationdonotexplaintheeffectsofbalanceexerciseinolderadults:Asystematic reviewwithameta-regressionanalysis.BritishJournalofSportsMedicine.2018;53(16):996–1002.
INTENSIVE VERSUS STANDARD BLOOD PRESSURE TREATMENT FOR ELDERLY WITH HYPERTENSION, WHICH ONE IS MORE COST-EFFECTIVE? A SYSTEMATIC REVIEW
Authors:
Aurielle Annalicia Setiawan

Michelle Gunawan
Ivena Leonita
Muhammad Yusuf
AMSA-UNIVERSITAS BRAWIJAYA
Intensive versus Standard Blood Pressure Treatment for Elderly with Hypertension, Which One is More Cost-Effective? A Systematic Review
Abstract
Introduction: Around 70% of adults ≥65 years have hypertension, and its prevalence and severity both increase with age. However, the optimal target for blood pressure management of hypertension in elderly remains controversial.
Aim: This review aims to evaluate the cost-effectiveness of intensive versus standard blood pressure treatment for hypertensive elderly.
Methods: PRISMA was used to report the entire generating process of this review. Relevant studies were identified from PubMed, Cochrane, ScienceDirect, ResearchGate, and ProQuest, up to the 8th April 2023. Studies were eligible if they compare the cost-effectiveness of intensive and standard blood pressure management in hypertensive elderly, reporting incremental costeffectiveness ratio (ICER) or incremental cost and incremental effectiveness in the measure of quality-adjusted life-year (QALY), published in the last decade. Risk of bias and quality assessment was assessed using CHEERS checklist.
Results: Eight studies were eligible for qualitative analysis. All of the included studies reported QALY and ICER as cost-utility analysis, and most of the studies performed 1-way probabilistic sensitivity analysis (PSA), PSA in combination with 1-way sensitivity analysis, or 2-way sensitivity analysis. All studies reported that intensive blood pressure control was always considered cost-effective compared to the standard blood pressure control in treating the elderly with HT in several high-income or upper-middle-income countries with the majority of SPRINT and STEP trial efficacy assumption basis. Some reported higher cardiovascular events in the standard blood pressure control group compared to the intensive group.
Conclusion: Intensive blood pressure treatment would be cost-effective to introduce in elderly hypertensive populations. These findings could aid the policymakers and provide evidence for the introduction of this strategy.
Keywords: cost-effectiveness, elderly, hypertension, intensive blood pressure treatment
INTENSIVE VERSUS STANDARD BLOOD PRESSURE TREATMENT FOR ELDERLY WITH HYPERTENSION, WHICH ONE IS MORE COST-EFFECTIVE? A SYSTEMATIC REVIEW
Authors:
AurielleAnnaliciaSetiawan
MichelleGunawan
IvenaLeonita
Muhammad Yusuf
AMSA-UNIVERSITASBRAWIJAYA

Intensive versus Standard Blood Pressure Treatment for Elderly with Hypertension, Which One is More Cost-Effective? A Systematic Review
Abstract
Introduction: Around 70% of adults ≥65 years have hypertension, and its prevalence and severity both increase with age. However, the optimal target for blood pressure management of hypertension in elderly remains controversial.
Aim: This review aims to evaluate the cost-effectiveness of intensive versus standard blood pressure treatment for hypertensive elderly.
Methods: PRISMA was used to report the entire generating process of this review. Relevant studies were identified from PubMed, Cochrane, ScienceDirect, ResearchGate, and ProQuest, up to the 8th April 2023. Studies were eligible if they compare the cost-effectiveness of intensive and standard blood pressure management in hypertensive elderly, reporting incremental costeffectiveness ratio (ICER) or incremental cost and incremental effectiveness in the measure of quality-adjusted life-year (QALY), published in the last decade. Risk of bias and quality assessment was assessed using CHEERS checklist.
Results: Eight studies were eligible for qualitative analysis. All of the included studies reported QALY and ICER as cost-utility analysis, and most of the studies performed 1-way probabilistic sensitivity analysis (PSA), PSA in combination with 1-way sensitivity analysis, or 2-way sensitivity analysis. All studies reported that intensive blood pressure control was always considered cost-effective compared to the standard blood pressure control in treating the elderly with HT in several high-income or upper-middle-income countries with the majority of SPRINT and STEP trial efficacy assumption basis. Some reported higher cardiovascular events in the standard blood pressure control group compared to the intensive group.
Conclusion: Intensive blood pressure treatment would be cost-effective to introduce in elderly hypertensive populations. These findings could aid the policymakers and provide evidence for the introduction of this strategy.
Keywords: cost-effectiveness, elderly, hypertension, intensive blood pressure treatment
Hypertension is a worldwide epidemic, which affects at least 1 billion people globally, and is projected to be 1.5 billion by 2025. In Southeast Asia, about one-third of adults have currently been diagnosed with hypertension, and it is estimated that 1.5 million deaths are associated with hypertension annually[1]. It is one of the primary modifiable risk factors for cardiovascular disease and premature death. Around 70% of adults ≥65 years have hypertension, and its prevalence and severity both increase with age[2,3] .
In hypertension management, the optimal target for blood pressure management remains controversial. According to the NHLBI and SPRINT trial, there are two approaches in lowering blood pressure; intensive and standard, in which intensive targets to lower blood pressure to <120 mg/hg systolic, while standard approach targets to lower blood pressure to <140 mg/hg systolic[4] The intensive approach was found to be beneficial compared to the standard approach in reducing CVD events by 25% and all-cause mortality by 27% for patients with high CVD risk. However, it was found in this trial that patients undergoing intensive approach to lower blood pressure required additional medications and physician visits to achieve their target and experienced higher rates of adverse events[5]. Therefore, the cost-effectiveness of this approach compared to the standard approach becomes a question. This systematic review aims to evaluate the costeffectiveness of both of these approaches, taking consideration of its incremental costeffectiveness ratios, willingness to pay, and other parameters.
II. Material and Methods
a. Data extraction and quality assessment
This systematic review was conducted by searching studies comparing the costeffectiveness of intensive and standard blood pressure management. PRISMA was used to report the process of this study. Literature search was conducted through PubMed, Cochrane, ScienceDirect, ResearchGate, and ProQuest, up to the 8th April 2023. The following keywords were used in the search: (“hypertension”) AND (“intensive blood pressure management”) AND (standard blood pressure management”) AND (“cost-effectiveness”).
b. Inclusion criteria
Inclusion criteria were 1) patients who were hypertensive and above the age of 45 years old, 2) studies comparing the cost-effectiveness of intensive and standard blood pressure management, 3) reporting incremental cost-effectiveness ratio or incremental cost and incremental effectiveness in the measure of quality-adjusted life-year (QALY), 4) published between 20132023, and 5) written in English.
c. Exclusion criteria
I. Introduction
Exclusion criteria were 1) studies that do not report incremental cost-effectiveness ratio or incremental cost or incremental effectiveness, 2) studies in which the full text of the article was not found, and 3) papers not qualifying as a research article.
d. Data extraction
All authors extracted the selected studies and evaluated their eligibility. Authors evaluated the journals independently, and disagreements among the authors were discussed and eventually resolved. The following information was extracted: author's name, year of publication, name of trial, perspective, time horizon, type of cost, currency, QALY, GDP per capita, discount rate for cost and utility, sensitivity analysis, and outcomes, which consists of the cost, utility, incremental costs incremental cost-effectiveness ratio, probability of cost-effectiveness, and other additional outcomes.
e. Quality assessment
Quality assessment was independently conducted by four authors, with a consensus criterion. The authors used CHEERS to examine the risk of bias in each study.
III. Results
a. Study Selection
We identified 1652 records in PubMed, Cochrane, ScienceDirect, ResearchGate, and ProQuest. 1632 records were removed prior to screening, leaving 20 for screening titles and abstracts. A total of seven articles was excluded, leaving 13 articles for further full-text review. Three articles could not be retrieved, resulting in ten articles to be assessed further for eligibility. Review of full-text of these articles led to exclusion of two studies, resulting in eight eligible studies for the systematic review. The PRISMA flowchart diagram can be found in Figure 1.
b. Study Characteristics
1. Population
Details of study characteristics are presented in Appendix 1. Studies included were published between 2016 and 2023 and were done in six countries; five of which are high income countries, including the United States (US), United Kingdom (UK), Taiwan, Korea, and Saudi Arabia; and one upper-middle income country which is China[6]. The included studies utilized various studies to determine the input of cardiovascular event risk comparison between the intensive and standard HT treatment, but the most frequently used studies were Systolic Blood Pressure Intervention Trial (SPRINT) trial in five studies and Strategy of Blood Pressure Intervention in older Hypertensive Patients (STEP) trial in three studies. Therefore, the majority of the included cohorts comprised older adult aged older than 50 years .
2. Intervention and Comparator
Three studies defined intensive blood pressure control by using the systolic blood pressure below 120 mmHg[7-9], one study used SBP ranging from 110 to 130 mmHg[10], and one used SBP/DBP below 133/76[11]. As for standard blood pressure control, three defined it by using the SBP below 140 mmHg[6-8], one used SBP/DBP below 140/90[11], and one used SBP ranging from 130 to 150 mmHg[10]. The other studies did not define the blood pressure cut off for intensive and standard blood pressure control.

 Figure 1. Flowchart of study identification and selection based on PRISMA.
Figure 1. Flowchart of study identification and selection based on PRISMA.
3. Outcome
All of the included studies reported a cost-utility analysis (CUA), with quality-adjustedlife-year(QALY)beingthemeasurementofutilityandincrementalcost-effectivenessratio(ICER) as the primary measurement of CUA. The amount of willingness-to-pay (WTP) threshold varies in each study and mostly was based on the gross domestic product (GDP) per capita of each respective country, except for US and Korea that have a country-specific WTP.
4. General characteristics of economic evaluation
Five studies used a time horizon of lifetime[9,10,12-14], while two used a time horizon of 10 years[8,11], and one study used 5, 10, 15 years and lifetime[7]. Most studies used a 3% discount rate for outcome[8-11,14], except for two studies that used 3.5%[10] and one study used 5%[7], and the other two[11,12] did not report the discount rate. Most of the studies used Markov model[7-11,14] except for one study that used microsimulation[13], and one study did not report the modeling approach[12]
5. Sensitivity Analysis
Six studies performed sensitivity analysis to quantify the variations in ICER values using only 1-way probabilistic sensitivity analysis (PSA)[7,8,14], or in combination with 1-way sensitivity analysis[9-11]. Onestudy also used 2-way sensitivity analysis[9]. Themost sensitiveparameters most commonly mentioned in the studies were cost of treatment; risk, utility, and cost of cardiovascular disease such as stroke, acute coronary syndrome, and heart failure; and utility of adverse events and number of physician visit.
6. Perspective
The payer perspective (n = 5)[7,8,12-14] was most frequently used among the 8 studies, followed by healthcare system (n = 2)[9,10], and one study did not report the perspective used in their study[11] .
7. Cost-Effectiveness Results
SummaryofoutcomesispresentedinAppendix2.Allstudiesreportedthatintensiveblood pressure control is cost-effective. Five studies stated that intensive blood pressure control is cost effective[7,9,10,13,14], while one stated that it is very likely to be cost effective[12]. One study also reported that intensive blood pressure control is more cost-effective in the long term[11]. Moise et al. reported that in addition to being cost-effective, intensive blood pressure control is also costsaving, i.e having a greater utility with a lower cost, mainly in male patients[8]
8. Cardiovascular events
Four studies reported cardiovascular events in patients with standard and intensive blood pressure control[8,9,13,14]. The cardiovascular events include cardiovascular deaths, myocardial infarction, heart failure, and stroke. All of them reported higher cardiovascular events in standard blood pressure control group compared to intensive group.
9. Risk of Bias Assessment
Risk of bias was assessed using Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement. The overall risk of bias is low in all studies, although there are some elements that are moderate to high risk. Summary of risk of bias is presented in Appendix 3.
IV. Discussion
This research was the first to conduct a systematic review with the objective of evaluating the cost-effectiveness of both the standard and the intensive blood pressure target in the elderly hypertensivepopulation. Eight cost-effectiveness studies with various qualities wereincluded. The findings showed that intensive blood pressure control was always considered cost-effective compared to thestandard blood pressure control in treating the elderly with hypertension in several high-income or upper-middle-income countries, including USA, UK, Taiwan, Korea, Saudi Arabia, and China, as well as various settings. The majority of the studies based their efficacy assumptions on SPRINT trial and STEP trial. While the quantitative analysis was planned to be performed, none ofthestudies provided theminimum required data, for instance, themeasurement of the dispersion of the ICER, incremental cost, or incremental utility.
Overall, our review found that most of the studies have taken into consideration its ICER, WTP, and other important parameters reflecting the cost-effectiveness of both approaches. Most of the studies have accounted for their local-specific information, such as time horizon, currency, discount rate, and GDP, making the result considered generalized. The CHEERS checklist was used to assess the methodological quality of the included studies. The quality of the studies varies but is still within good limits, however, one study has unclear quality because it is only an abstract. Some of the limitations in the evidence included in this study are the heterogeneity in the population and comparators between studies. Heterogeneity in the population was evident as shown by the different range of population ages in each study. Several studies conducted in Taiwan, China, and Saudi Arabia did not specify the definition of intensive blood pressure target and standard target and several others had different definitions of both targets, causing bias in terms of the intervention given. Clinical characteristics differences were surely evident given the different assumptions used in each study, however, the variability was assessed by sensitivity analysis to show the robustness of the results in each study.
We minimized the effects of publication bias by conducting extensive searches for published and unpublished RCTs, reviewing bibliographies for additional RCTs, and by seeking missing data directly from authors. Additionally, we minimized selection bias by involving two review authors in study selection and data extraction, with arbitration and independent checking by a third review author. We also gathered data for all outcomes that were reported.
This systematic review stands as a mediator regarding the effectiveness and costeffectiveness of intensive blood pressure-lowering strategies to treat elderly hypertensive patients
in the midst of various existing evidence. The results shown in this study are in line with previous studies[15]. The intensive blood pressure lowering strategy is a strategy that is certainly more effective in reducing cardiovascular events bound to the decrease of indirect/additional costs compared to the standard blood pressure lowering strategy. The STEP study showed that intensive BP treatment targeting SBP < 130 mmHg markedly reduced theincidenceofcardiovascular events including acute coronary syndrome, stroke, acute decompensated heart failure, coronary revascularization, atrial fibrillation, or death from cardiovascular causes in older hypertensive patients aged 60-80 years in China[16]. Nevertheless, this method is accompanied by an increase in direct medical costs required for precise blood pressure measurement, drug prescribing, and monitoring, as well as in the educational process[15]. For instance, the mean number of antihypertensive medications administered per patient was 1.9 in the intensive-treatment group and 1.5 in the standard treatment group in the step trial[16]. This review shows that even under such conditions theintensiveblood pressurelowering strategy remains an option with ahigh probability of cost-effectiveness because it increases the utility quantified as QALY and also reduces the indirect medical costs associated with cardiovascular events and adverse events. This review evaluated the cost-effectiveness values from the perspectives of payers and the healthcare system thus confirming the equality of the result between two important parties. This conclusion can only be specified in countries with high to upper-middle income levels, due to the lacking of trials involving lower-middle-income countries.
The application of intensive blood pressure control in elderly patients is supported by this systematicreviewbecauseitiscost-effectiveandreducestheriskofcardiovascularevents.Despite the promising results, implementing intensive blood pressure control in routine clinical practice may be difficult. These difficulties include increased effort for high-quality and frequent blood pressure measurement, an increase in the number of medications prescribed, higher doses, and increased adherence [15]. It is common for patients to be concerned about the amount of medication they must take. This makes it difficult for doctors to educate patients about the importance of taking their medications and how it affects their health. The risk of not taking the medications should be emphasized by the physician. As previously stated, additional research, particularly in low to middle-income countries, is required to understand the potential differences in outcomes in countries with different economic conditions than those included in this systematic review. The definition of standard and intensive blood pressure control also varies between studies. Blood pressure cutoff should be uniformly defined in the future to avoid confusion.
V. Conclusion
Intensive blood pressure treatment would be cost-effective to introduce in elderly hypertensive populations. These findings could aid the policymakers and provide evidence for the introduction of this strategy. Further research with better design could prove the cost-effectiveness of intensive blood pressure treatment, especially in middle-low-income countries.
VI. Acknowledgement and Conflict of Interest
The authors have no acknowledgement and conflict of interest to declare.
References
1. Mohammed Nawi A, Mohammad Z, Jetly K, Abd Razak MA, Ramli NS, Wan Ibadullah WA, Ahmad N. The prevalence and risk factors of hypertension among the urban population in southeast asian countries: a systematic review and meta-analysis. International Journal of Hypertension. 2021 Feb 10;2021:1-4.
2. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nature Reviews Nephrology. 2020 Apr;16(4):223-37.
3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ. Heart disease and stroke statistics--2015 update: a report from the American Heart Association.(2015) Circulation 131: e29-322. Pubmed| Crossref| Others.
4. Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff Jr DC, Johnson KC, Killeen AA, Lewis CE. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clinical trials. 2014 Oct;11(5):532-46.
5. Richman IB, Fairley M, Jørgensen ME, Schuler A, Owens DK, Goldhaber-Fiebert JD. Costeffectiveness of intensive blood pressure management. JAMA cardiology. 2016 Nov 1;1(8):872-9.
6. The World Bank. World Bank Country and Lending Groups [Internet]. [Reviewed 2017. Cited
2023 April 8] Available from: https://datahelpdesk.worldbank.org/k nowledgebase/articles/906519world-bank-country-and-lendinggroups.
7. Lee YS, Lee HY, Kim TH. Costeffectiveness analysis of intensive blood pressure control in Korea. Hypertension Research. 2022 Mar;45(3):507-15.
8. Moise N, Huang C, Rodgers A, Kohli-Lynch CN, Tzong KY, Coxson PG, Bibbins-Domingo K, Goldman L, Moran AE. Comparative costeffectiveness of conservative or intensive blood pressure treatment guidelinesinadultsaged35–74years: the cardiovascular disease policy model. Hypertension. 2016 Jul;68(1):88-96.
9. Richman IB, Fairley M, Jørgensen ME, Schuler A, Owens DK, Goldhaber-Fiebert JD. Costeffectiveness of intensive blood pressure management. JAMA cardiology. 2016 Nov 1;1(8):872-9.
10. Liao CT, Toh HS, Sun L, Yang CT, Hu A, Wei D, Melgarejo J, Zhang ZY. Cost-effectiveness of Intensive vs Standard Blood Pressure Control Among Older Patients With Hypertension. JAMA Network Open. 2023 Feb 1;6(2):e230708-.
11. Xie X, He T, Kang J, Siscovick DS, Li Y, Pagán JA. Cost-effectiveness analysis of intensive hypertension control in China. Preventive Medicine. 2018 Jun 1;111:110-4.
12. Liao C, Toh HS, Wei D, Melgarejo J, Chang W, Zhang Z. AW-NI-5:
COST-EFFECTIVENESS OF INTENSIVE VERSUS STANDARD BLOOD-PRESSURE CONTROL AMONG HYPERTENSIVE PATIENTS IN TAIWAN: A SIMULATION MODELLING STUDY. Journal of Hypertension. 2023 Jan 1;41(Suppl 1):e140-1.
13. Fan J, Zheng W, Liu W, Xu J, Zhou L, Liu S, Bai J, Qi Y, Huang W, Liu K, Cai J. Cost-Effectiveness of Intensive Versus Standard Blood Pressure Treatment in Older Patients With Hypertension in China. Hypertension. 2022 Nov;79(11):2631-41.
14. Almalki Z, Alatawi Y, Alharbi A, Almaklefi B, Alfaiz S, Almohana O, Alsaidan Y, Alanezi A. Costeffectiveness of more intensive blood pressure treatment in patients with high risk of cardiovascular disease in Saudi Arabia: a modelling study of meta-analysis. International Journal of Hypertension. 2019;2019.
15. Derington CG, King JB, Bryant KB, McGee BT, Moran AE, Weintraub WS, Bellows BK, Bress AP. Costeffectiveness and challenges of implementing intensive blood pressure goals and team-based care. Current hypertension reports. 2019 Dec;21:1-2.
16. Zhang WL, Cai J. STEP to blood pressure management of elderly hypertension: evidence from Asia. Hypertension Research. 2022 Apr;45(4):576-82.
Appendix 1. Study characteristics
BP, blood pressure; CNY, Chinese yuan; GDP, gross domestic product; NA, not available; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-years; SBP, systolic blood pressure; SHEP, Systolic Hypertension in the Elderly Program; SPRINT, Systolic Blood Pressure Intervention Trial; STEP, Strategy of Blood Pressure Intervention in older Hypertensive Patients; USD, US Dollar; WTP, willingness-to-pay
Author, year Country Intensive Target BP (mmHg) Standard Target BP (mmHg) Trials Used as Data Source Modelling Approach Perspective Type of Cost Liao et al., 2023 China,US,UK SBP:110-130 SBP:130-150 STEP Markov Healthcare system Direct Liao et al., 2023 Taiwan NA NA SPRINT,STEP NA Payer NA Fan et al., 2022 China NA NA STEP Microsimulation Payer NA Lee et al., 2022 SouthKorea SBP<120 SBP<140 SPRINT Markov Payer NA Almalki et al., 2019 SaudiArabia NA NA Xieetal.,2016 Markov Payer Direct Xie et al., 2018 China <133/76 <140/90 SPRINT Markov NA NA Moise et al., 2016 US SBP<120 SBP<140 SPRINT, SHEP,Lawet al., Markov Payer NA Richman et al., 2016 US SBP<120 SBP<140 SPRINT Markov Healthcare system Directand non-direct
Standard China:$12.362/QALY(1time
UK:$29.940/QALY US:$50.000/QALY
Intensive China:$64 090/QALY(3 timesGDP)
UK:$100.000/QALY US:$44.910/QALY
Author, year Currency, year WTP Threshold GDP Discount Rate Sensitivity Analysis Time Horizon Liao et al., 2023 USD,2022
GDP)
China: $12.362 China:3% US&UK:3.5% 1-waysensitivity analysesandPSA (MonteCarlo) Lifetime Liao et al., 2023 USD,2022 $36.000(1timeGDP) $36.000 NA NA Lifetime Fan et al., 2022 CNY,2020 72.000CNY(1timeGDP) 72.000 CNY NA NA Lifetime Lee et al., 2022 USD,2017 $9.492-32.907/QALY NA 5% 1-wayPSA 5,10,15years andlifetime Almalki et al., 2019 USD,2018 $60.000/QALY $20.796 3% 1-wayPSA (MonteCarlo) Lifetime,30years maximum Xie et al., 2018 CNY,2014 46.491CNY/QALY(1time GDP) 46.491 CNY 3% 1-waysensitivity analysesandPSA (MonteCarlo) 10years Moise et al., 2016 USD,2014 $50.000/QALY NA 3% 1-wayPSA (MonteCarlo) 10years
Appendix 2. Summary of Outcomes
Male:-$1240(95%UI, -$3000–-$200)
Female:-$290(95%UI, -$1400–+$200)
Allhigherinintensive group
Annual QALY gained (vs standard)
Male:40,100(95%UI 23,000-47,000)
Female:14,500(95% UI4,000-28,000)
Allhigherinintensive group
Author, year Standard Intensive Incremental Cost (Mean) Incremental Utility (QALY) (Mean) Cost Utility (QALY) Cost Utility (QALY) Liao et al., 2023 China:$23,599 UK:$70,771 US:$212,300 China:9.29 UK:9.83 US:10.66 China:$26,566 UK:$72,081 US:$219,671 China:9.53 UK:10.11 US:10.95 China:$2,967(95%UI $2,394-$3,694) UK:$1,961(95%UI $1,337-$2,362) US:$7,371(95%UI $5,673-$9,320)
0.24)
0.29)
0.30) Allhigherinintensive group Liao et al., 2023 $20,239–33,414 7.37–15.74 $39,348–22,978 7.60–16.28 Lifetime cost Standard:$20,239–33,414 Intensive:$39,348–22,978 Standard:7.37–15.74 Intensive:7.60–16.28 Fan et al., 2022 NA NA NA NA NA 0.16higherinintensive group Lee et al., 2022 $70,239 16,24 $70,647 16,53 $408 0.29higherinintensive group Almalki et al., 2019 $48,186 10.36 $59,920 10.94 $11,734 0.58higherinintensive group Xie et al., 2018 Byage 65-74:4,713 CNY 75-84:6,523 CNY Byage 65-74:8.21 75-84:7.26 Byage 65-74:5,282 CNY 75-84:6,760 CNY Byage 65-74:8.31 75-84:7.4 Byage 65-74:569CNY 75-84:237CNY Byage 65-74:0.1 75-84:0.14
group Moise et al., 2016 NA NA NA NA
China:0.24(95%UI0.23-
UK:0.28(95%UI0.27-
US:0.29(95%UI0.28-
Allhigherinintensive
Richman et al., 2016 $155,261 9.6 $176,584 10.5 $21,323 0.9higherinintensive group Author, year ICER Cost-effectiveness probability of intensive treatment Conclusion Additional Outcomes Liao et al., 2023 China:$12,362/QALY UK:$7,004/QALY US:$25,417/QALY China:94.3%atWTPof1 timeGDP UK:99.1%at$29,940/QALY US:86.9%at$50,000/QALY China:intensiveiscosteffective UK:intensiveiscosteffective US:intensiveiscosteffective NA Liao et al., 2023 Byage 50-54:$10,989/QALY 55-64:$11,220/QALY 65-74:$11,485/QALY 75+:$11,865/QALY Allprobabilitiesaregreater than99.8%belowtheWTPof $36,000 Intensive:verylikelyto becost-effective NA Fan et al., 2022 $3,018/QALY 82-95%atWTPof1time GDPinChinain2020 Intensive:cost-effective Cardiovascular events Standard:41.28% Intensive:36.88% Lee et al., 2022 $1,373/QALY - Intensive:cost-effective NA Almalki et al., 2019 5years:$44,562/QALY 10years:$30,111/QALY
87.25%atWTPof $60,000/QALY Intensive:cost-effective Clinical outcomes in 30 years (per 10,000 patients), incremental value CVdeaths:-142 strokes:-341 MI:-241
Alllowerinintensivegroup Xie et al., 2018 All:7,876CNY/QALY 53%morecost-effectivethan standardatWTPof1time Intensive:morecosteffectiveinthelongterm NA
20years:$22,425/QALY 30years:$20,358/QALY
HF:-149
Moise et al., 2016
Male,byage
45-54:12,472CNY/QALY
55-64:9,401CNY/QALY
65-74:4,662CNY/QALY
75-84:1,347CNY/QALY
Female,byage
45-54:12,549CNY/QALY
55-64:10,623CNY/QALY
65-74:5,689CNY/QALY
75-84:1,693CNY/QALY
Treated but uncontrolled group
Intensive
Male:$18,900/QALY(cost saving)
Female:$2,000/QALY
Untreated group
Intensive
Male:$30,900/QALY(cost saving)
Female:$20,000/QALY(cost saving)
GDP;60%atWTPof2times GDP
Richman et al., 2016
$23,777/QALY
Male:100%atWTPof $50,000/QALY
Female:82%atWTP $50,000/QALY
Intensive:consistently cost-savinginmen,but notalwaysinwomen
Intensive vs standard
Annual cardiovascular events averted
MeninhighCVDrisk:-29,000 (95%UI-17,000to-47,000)
WomeninhighCVDrisk:14,000(95%UI-5000to23,000)
Annual cardiovascular deaths averted
MeninhighCVDrisk:-11,000 (95%UI-2,000to-18,000)
WomeninhighCVDrisk:4,000(95%UI-2,000to6,000)
Cardiovascular events, standard vs intensive
84%atWTPof $50,000/QALY Intensive:cost-effective
NonfatalMI:3.8%vs3.2%
Nonfatalstroke:2.1%vs2.3%
Heartfailure:7.8%vs2.0%
Death:7.8%vs5.7%
BP, blood pressure; CNY, Chinese yuan; GDP, gross domestic product; ICER, incremental cost-effectiveness ratio; NA, not available; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-years; SBP, systolic blood pressure; SHEP, Systolic Hypertension
in the Elderly Program; SPRINT, Systolic Blood Pressure Intervention Trial; STEP, Strategy of Blood Pressure Intervention in older Hypertensive Patients; USD, US Dollar; WTP, willingness-to-pay
Appendix 3. CHEERS Checklist of the Included Studies
Title
Abstract
Background and objectives
Health economic analysis plan
Study population
Setting and location
Comparators
Perspective
Time horizon
Discount rate
Selection of outcomes
Measurement of outcomes
Valuation of outcomes
Measurement and valuation of resources and costs
Currency, price date, and conversion
Rationale and description of model
Analytics and assumptions
Characterizing heterogeneity
Characterizing distributional effects
Characterizing uncertainty
Liao et al., 2023 Liao et al., 2023 Fan et al., 2022 Lee et al., 2022 Almalki et al., 2019 Moise et al., 2016 Richman et al., 2016
Approach to engagement with patients and others affected by the study Study parameters
Summary of main results
Effect of uncertainty
Effect of engagement with patients and others affected by the study Study findings, limitations, generalizability, and current knowledge
Source of funding
Conflicts of interest
Green: reported; Yellow: unclear; Red: not reported.
ASSESSMENT OF EFFECTIVENESS BETWEEN AMLODIPINE MONOTHERAPY
AND COMBINATION WITH OTHER ANTI-HYPERTENSIVE DRUGS: A COMPREHENSIVE SYSTEMATIC REVIEW AND META-ANALYSIS
Aurelia Bunanta1, Jason Wiliam2, Kristanto Tanjaya3 aurelbunanta@gmail.com1, jasonwiliam159@gmail.com2, krisnto27@gmail.com3
Abstract
Introduction: In 2013, the prevalence of hypertension experienced by geriatric, people who are aged above 60, was 57.6% and it will continue to increase with age. The increase in systolic and diastolicblood pressureis influenced bytwo mainmechanisms, namelyanincreasein total peripheral resistance and an increase in cardiac output Various complications and damage to different organs could appear as a result of long-term hypertension.
Objective: In this study, the most efficient, trustworthy, and secure amlodipine monotherapy and combination therapy for hypertensive geriatrics will be determined.
Methodology: Twenty literature sources were used in the systematic review writing process, while 10 literature sources were used in the quantitative analysis process. End-treatment systolic and diastolic parameters are used to determine drug effectiveness, while night-time systolic is used to measure the risk of heart failure and atherosclerosis. The data utilized is heterological so the calculation uses a random effect model.
Results and Discussions: The best monotherapy treatment option is lacidipine 4-6 mg with the greatest decrease in end-treatment systolic and diastolic (-23.8 ± 1.0; -23.4 ± 0.6).
Combination therapy of amlodipine 5 mg with valsartan 80 mg was the most significant in reducing end-treatment systolic and diastolic (−15.1 ± 1.2; −5.4 ± 1.0). This combination also gave the greatest decrease in nocturnal systolic (−7.2 ± 1.7). Compared to other antihypertensive drugs, amlodipine has the potential to be used as monotherapy or in combination with other therapies for the treatment of hypertension. Adverse effects caused by amlodipine administration (headache, ankle and peripheral edema, dizziness, flushing, palpitations) and/or combination therapy were lower than in the control group.
Conclusions: The best hypertension drugs were lacidipine monotherapy 4-6 mg and combination of amlodipine 5 mg with valsartan 80 mg for systolic and diastolic end treatment.
Keywords: Amlodipine, Hypertension, Night-time Systolic, Therapy
ASSESSMENT OF EFFECTIVENESS BETWEEN AMLODIPINE MONOTHERAPY
AND COMBINATION WITH OTHER ANTI-HYPERTENSIVE DRUGS: A COMPREHENSIVE SYSTEMATIC REVIEW AND META-ANALYSIS
Aurelia Bunanta1, Jason Wiliam2, Kristanto Tanjaya3 aurelbunanta@gmail.com1, jasonwiliam159@gmail.com2, krisnto27@gmail.com3
Abstract
Introduction: In 2013, the prevalence of hypertension experienced by geriatric, people who are aged above 60, was 57.6% and it will continue to increase with age. The increase in systolic and diastolicblood pressureis influenced bytwo mainmechanisms, namelyanincreasein total peripheral resistance and an increase in cardiac output Various complications and damage to different organs could appear as a result of long-term hypertension.
Objective: In this study, the most efficient, trustworthy, and secure amlodipine monotherapy and combination therapy for hypertensive geriatrics will be determined.
Methodology: Twenty literature sources were used in the systematic review writing process, while 10 literature sources were used in the quantitative analysis process. End-treatment systolic and diastolic parameters are used to determine drug effectiveness, while night-time systolic is used to measure the risk of heart failure and atherosclerosis. The data utilized is heterological so the calculation uses a random effect model.
Results and Discussions: The best monotherapy treatment option is lacidipine 4-6 mg with the greatest decrease in end-treatment systolic and diastolic (-23.8 ± 1.0; -23.4 ± 0.6).
Combination therapy of amlodipine 5 mg with valsartan 80 mg was the most significant in reducing end-treatment systolic and diastolic (−15.1 ± 1.2; −5.4 ± 1.0). This combination also gave the greatest decrease in nocturnal systolic (−7.2 ± 1.7). Compared to other antihypertensive drugs, amlodipine has the potential to be used as monotherapy or in combination with other therapies for the treatment of hypertension. Adverse effects caused by amlodipine administration (headache, ankle and peripheral edema, dizziness, flushing, palpitations) and/or combination therapy were lower than in the control group.
Conclusions: The best hypertension drugs were lacidipine monotherapy 4-6 mg and combination of amlodipine 5 mg with valsartan 80 mg for systolic and diastolic end treatment.
Keywords: Amlodipine, Hypertension, Night-time Systolic, Therapy
1
Introduction
A geriatric or old person is someone who is over the age of 60[1]. In 2015, the world's elderly population, over the age of 60 years or more, was 901 million. According to Indonesia Basic Health Research in 2013, hypertension is the most disease experienced by the elderly, with a prevalence of 57.6% [2.3]. The prevalence of hypertension will continue to increase with age (5% at the age of 20-39 years, 26% at the age of 40-59 years, and 59.6% at the age of 60 years and over. Hypertension in the long term will cause various complications and damage to various organs, such as thekidneys,heart, and brain[2].Ofall hypertensivepatients whoreceive treatment, only 10-20% of them achieve blood pressure control targets[3]. On the other hand, the mortality rate due to hypertension reaches 7.5 million, or about 12.8% of the total deaths in the world.[2]
The increase of systolic and diastolic blood pressure is influenced by various things, but there are two main mechanisms, namely an increase in total peripheral resistance and an increase in cardiac output. Anything that causes an increase in one or both of them will cause blood pressure to increase[8]. If not treated immediately, hypertension will cause complications such as coronary heart disease, heart failure, stroke, chronic kidney disease, and retinal damage.[1]
The approach to diagnosing hypertension can be done through anamnesis, determination of cardiovascular risk, physical examination, screening and detection of hypertension, and HMOD (Hypertension-mediated Organ Damage) assessment. Management of hypertension consists of non-pharmacological therapy (lifestyle changes) and pharmacological therapy (drugs). Hypertensive patients need to take life-long antihypertensive drugs, both single and combination drugs to control blood pressure back to normal limits. Several antihypertensive drugs are available in Indonesia, such as ACE (Angiotensin Converting Enzyme)-inhibitor, ARB (Angiotensin Receptor Blocker), diuretic thiazide, CCB (Calcium Channel Blocker), and BB (Beta-blocker)[2]. Until now, the first line treatment for uncomplicated hypertension is using diuretic drugs. However, several studies have reported the incidence of drug resistance resulting in patient failure to control blood pressure, especially at night. In addition, the low patient adherence to taking the drug is also a reason for therapy failure[9]. Amlodipine, another antihypertensive drug that belongs to the dihydropyridine CCB class, has an advantage (half-life of 30-50 hours) that enables it to be consumed once a day.[7]
Each antihypertensive drug has its own advantages and disadvantages. Thiazides can increasefluid secretion throughurineso thattheheart'sburdenis lighter.However,ontheother hand, it can cause dehydration and hypotension, especially in the elderly. Beta-blockers can
2
reduce cardiac pumping capability. However, it often causes bradycardia and heart failure when dosing is inappropriate. ACE and ARB can induce vasodilation which reduces blood pressure, but are often associated with increased creatinine levels and renal impairment. Whereas CCB drugs such as amlodipine can cause coronary and peripheral artery dilatation and are suitable for the elderly, albeit interactions with grapefruit cause severe hypotension.[10] . The main objective of this systematic review and meta-analysis was to determine the best, most stable, and safe monotherapy or combination drugs for people with hypertension. The secondary target of the research is to determine the minimum side effects of the drug, the best dose of amlodipine as monotherapy, and the best administration time of amlodipine.
Methods and Materials
The content in the methods section, including the inclusion, exclusion criteria, and objectives of the research, was agreed upon before the writing process was carried out. The writing of this systematic review was carried out systematically based on the suggested method PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis)[11]. The writing process consisted of several steps, namely 1) determining inclusion, exclusion, and research target criteria, 2) literature search process, 3) screening and selection of literature results, 4) literature quality assessment, 5) data extraction, 6) quantitative analysis, and 7) writing a systematic review conducted by three researchers, namely AB, JW, and KT independently. The study was conducted from 9 February 2023 to 1 April 2023. Literature inclusioncriteriawere1)studiespublishedwithinthelasttenyears,2)studiesusingamlodipine or a combination of amlodipine as a treatment for hypertension, 3) free access articles, and 4) geriatric samples aged >60 years. While the literature exclusion criteria were 1) use of nonamlodipine monotherapy or combination, 2) diagnostic studies with non-human samples, and 3) samples aged <60 years.
A literature search was performed on PubMed, ScienceDirect, and Google Scholar platforms with the keywords: "Hypertension" AND ("Treatment*" OR "Therapy*") AND ("Amlodipine" OR "Combination Amlodipine" OR "Amlodipine Monotherapy") AND (" Systolic" OR "Diastolic" OR “Night Time Systolic ") adapted to the format for each platform. The main target of the study is to analyze the end-treatment systolic, diastolic, and night-time systolic as an assessment to compare the effectiveness of monotherapy and combinations antihypertensive drugs for geriatric patients. The secondary target of the research is to determine the minimum side effects of the drug, the best dose of amlodipine as monotherapy, and the best administration time of amlodipine. The quality analysis process was carried out
3
using RoB 2.0 tool (Risk of Bias) which has been adapted to hypertension therapy studies
[33]
The data extraction process was carried out using the Google Sheet platform by the three researchers independently, and then the results were discussed together. The quantitative analysis process was carried out using the Cochrane RevMan 5.4 application (The Nordic Cochrane Center)[7]. Systematic review writing was carried out using Google Docs and Microsoft Word. Problems that might arise in the course of the writing process were resolved by the three authors through joint discussions.
Results
A. Number of Inclusion Articles
A search on the electronic platform using predetermined keywords resulted in 620 literature sources. At the end of the screening process and literature assessment, 20 literature sources were used in the systematic review writing process, while 10 literature sources were used in the quantitative analysis process[3, 10, 19

B. Qualitative Analysis Results
28, 11, 29
35, 12
18]. Of the 20 literature sources, 10 of them could not be used in quantitative analysis due to inadequate data[13, 33] (Figure 1)
The results of the risk assessment of bias show that the two literature sources[28, 31] have ahigh risk ofbiasdueto thetypeof research being conductedopenlabeled RCTs(Randomized Controlled Trials). Six studies[12, 15, 17, 21, 23, 26] own some concerns bias due to unclear sample
4
–
–
–
Figure 1. PRISMA Flowchart
selection, incomplete data information, as well as the process in which the research was conducted so that there is inadequate information for the first, second, third, fourth, and fifth domain at Cochrane RoB2[33]. Other literature is included in the low risk of bias (Table 1).

C. Research Targets
Hypertension as a lifelong disease with a high prevalence and causing severe clinical manifestations requires effective and stable therapy even though it has not shown severe complications because early treatment has been shown to be effective in reducing mortality and morbidity of this disease. However, drug resistance occurs and becomes a barrier to the success of therapies that cause sudden changes in blood pressure and lead to strokes or other heart diseases. The first-line treatment for hypertension used in Indonesia is diuretic drugs which reduce sodium absorption and increase fluid reabsorption. However, there are many other drug options that have the potential to be used as first-line therapy in hypertension, one of which is amlodipine which blocks calcium channels.
1. Results of End-treatment Systolic Analysis
A summary of the ten literature sources can be found in Appendix 1. Five literature sources use amlodipine as monotherapy treatment[12, 14, 20, 23, 29] and five other literature sources use amlodipine as combination[16, 19, 21, 24, 25]. The combined analysis of the systolic value of amlodipine monotherapy showed high results with heterogeneity (Standard Mean Difference
[SMD]: 2.27; 95% CI: 018 - 4.35; p=0,03; I2=99%) (Figure 2). While the combined amlodipine systolic value had a fairly high result with a high heterogeneity value (SMD: 0.89;95% CI:1.56--0.22; p=0,009;I2=97%)(Figure3).Itisknownthatlacidipine4-6gristhemosteffective drug. Nonetheless, lacidipine has the same mode of action as amlodipine, the difference in efficacy is determined by the ability of lacidipine to lower blood pressure in patients with mild
5
Table 1. Cochrane Risk of Bias Tool 2 Result
to moderate hypertension and prevent thickening of the carotid intima-media and atherosclerotic plaques.[12]. While the combination of amlodipine 5 mg with valsartan 80 gr showed the largest end-treatment systolic reduction among the other combinations.
2. Results of End-treatment Diastolic Analysis
A summary of the ten literature sources can be found in Appendix 1. Five literature sources use amlodipine as monotherapy treatment[12, 14, 20, 23, 29] and five other literature sources use amlodipine as combination[16, 19, 21, 24, 25]. The combined analysis of the diastolic value of amlodipine monotherapy has quite high results with high heterogeneity values (SMD: 2.86; 95% CI: 0.27 - 5.45; p=0,03; I2=99%) (Figure 4). Meanwhile, the combined amlodipine diastolic value had a fairly high result with high heterogeneity (SMD: -0.76; 95% CI: -1.34 -0.17; p=0,01; I2= 96%) (Figure 5). It is known that lacidipine 4-6 gr is the most effective drug. While the combination of amlodipine 5 mg with valsartan 80 gr showed the greatest endtreatment diastolic reduction among other combinations. Amlodipine works by decreasing the contraction of vascular smooth muscle. Meanwhile, valsartan works as a vasodilator. When combined, the efficacy of lowering blood pressure will be stronger than monotherapy[19]
3. Results of Night-time Systolic Analysis
A summary of the four literature sources can be found in Appendix 1. The other four literature sources use amlodipine as combination[16, 19, 21, 25]. The combined amlodipine systolic value was quite high with high heterogeneity (SMD: -1.59; 95% CI: -3.08 - -0.11; p=0,04; I2=99%) (Figure 6). The combination of amlodipine 5 mg with valsartan 80 gr showed the largest reduction in systolic at night among other combinations. Night-timesystolic determines the risk of atherosclerosis and heart failure for hypertensive patients[32]. Thus, the greater the systolic reduction at night, the lower the risk of suffering cardiovascular diseases.
4. Amlodipine Monotherapy Dose
Based on Qi Chen's research results[15] in 2017 amlodipine 5 mg was more effective than amlodipine 2.5 mg. It is proved by the decrease in end-treatment systolic (-23.6 ± 0.6;20.6 ± 0.6), end-treatment diastolic (-14.3 ± 0.3; -12.7 ± 0.4), and night-time systolic (- 7.6 ± 0.7; -5.6 ± 0.7). Based on the research results of Mizuno and Fukutomi[27, 28], amlodipine at a dose of 10 mg was able to reduce night-time systolic more than the combination of aliskiren and amlodipine. The safe dose for amlodipine consumption is 5-10 mg daily[15]
6



7
Figure 2. Comparison Effects Between Amlodipine Monotherapy and Other Antihypertensive Drugs on End-treatment Systolic Blood Pressure
Figure 3. Comparison Effects Between Amlodipine Combination and Other Antihypertensive Drugs on End-treatment Systolic Blood Pressure
Figure 4. Comparison Effects Between Amlodipine Monotherapy and Other Antihypertensive Drugs on End-treatment Diastolic Blood Pressure
Discussions
1. Amlodipine Time of Administration
According to Ohishi, et al. (2013)[13], administering the combination of telmisartan plus amlodipine in the morning was able to reduce end-treatment systolic blood pressure more than administeringit at night (-15.3± 11.2; -13.5± 14.5).Meanwhile,thedecreasein diastolicblood pressure did not differ between administration in the morning or at night (-8.0 ± 8.6; -8.0 ± 7.8) respectively. However, administration at night was able to reduce night-time systolic blood pressure more than administration during the day (-8.3 ± 9; -6.5 ± 13.2). Administration of antihypertensive drugs in the morning is not good at reducing systolic blood pressure in the evening because the effect of the drug decreases over time[13]


2. Other Amlodipine Combinations
According to prior research done by Wang, et al. (2017); Mizuno, et al. (2019); and Fukutomi, et al. (2014)[18, 27, 28], results showed that the combination of amlodipine with other
8
Figure 5. Comparison Effects Between Amlodipine Combination and Other Antihypertensive Drugs on End-treatment Diastolic Blood Pressure
Figure 6. Comparison Effects Between Amlodipine Combination and Other Antihypertensive Drugs on Night-time Systolic Blood Pressure
antihypertensive drugs provided greater efficacy in end-treatment systolic and diastolic and night-time systolic than amlodipine monotherapy. According to Wang, et al. (2017); Tsioufis, et al. (2016); Fukutomi, et al. (2014); and Fogari, et al. (2014)[18, 26, 28, 31], the best combination is amlodipine 5 mg with enalapril 20 mg which can reduce end-treatment systolic and diastolic (-23.82 ± 3.9; -10.43 ± 6.36) respectively. Meanwhile, the combination of sacubitril/valsartan plus amlodipine is the most effective combination in reducing night-time systolic (-14.0 ± 1.0)[18]. Enalapril works by blocking ACE which is a vasoconstrictor[26]. Sacubitril/valsartan is first-class angiotensin and neprilysin receptor inhibitors that result in vasodilation[18]. If these drugs are combined with amlodipine, the reduction in blood pressure will be greater.
3. Summary of Side Effects of Antihypertensive Drugs
Based on our search results, out of 20 journals that met the inclusion criteria, 10 RCTs were obtained that reviewed the efficacy and safety aspects of intervention therapy. Broadly speaking, the ten studies suggest that the number of adverse events (AE) was slightly lower in the amlodipine and/or combination therapy group than was in the control group. A study by Wang, et al. (2017) showed that the amlodipine group had significantly lower (p>0.20) side effect rates than the lacidipine group (30.3% vs 26.7%). Side effects that appeared in the amlodipine and lacidipine groups respectively were headache (n=6 vs n=9), ankle and peripheral edema (n=11 vs n=6), dizziness (n=7 vs n=5), flushing (n=5 vs n=5), palpitations (n=3 vs n=4), and other side effects (n=14 vs n=15). Research by Huang, et al. (2019) showed no serious side effects and compared the incidence of AE, namely 40 (15.6%) in the amlodipine group and 42 (16.9%) in the nifedipine-GITS group. The study by Chen, et al. (2017) aimed to observe the best dose of amlodipine administration, the result was that amlodipine 2.5 mg/dl showed a slightly lower AE incidence rate than amlodipine 5 mg/dl (20.0% [n=70] vs 17.7% [n=62]; p=0.50).
Conclusions
The results of this systematic review showed that the best choice of monotherapy drug was lacidipine 4-6 mg with the greatest decrease in systolic and diastolic (-23.8 ± 1.0; -23.4 ± 0.6). Meanwhile, the combination therapy of amlodipine 5 mg with valsartan 80 mg was the most significant in reducing end-treatment systolic and diastolic, as well as night-time systolic (−15.1 ± 1.2; −5.4 ± 1.0; −7.2 ± 1.7). Based on data excluded from the meta-analysis, the combination of amlodipine 5 mg with enalapril 20 mg provided the greatest decrease in endtreatment systolic and diastolic blood pressure (-23.82 ± 3.9; -10.43 ± 6.36) and the
9
combination of amlodipine with sacubitril/valsartan was the best in reducing systolic at night (-14.0 ± 1.0).
The timing of amlodipine administration during the day provides a better result than the night intervention. Conversely, the administration of amlodipine at night lowers night-time systolic better. The incidence of side effects caused by amlodipine (headache, ankle and peripheral edema, dizziness, flushing, palpitations) and/or combination therapy were lower than in the control group.
Acknowledgments and Conflicts of Interest
We thank all contributors for their hard work in creating this paper. We would also like to express our gratitude to dr. Happy Kurnia Permatasari, Ph.D and dr. Edwin Kinesya for the supervision during the conceptualization and writing of this paper. Moreover, our biggest gratitude was given to dr. Eriko Prawestiningtyas, Sp. F as vice dean of student affairs at Brawijaya University, who has provided feedback in the research and writing process of this paper and for the motivation she gave us regarding this research.
References
1. Ministry of Health of the Republic of Indonesia. Clinical practice guide for physicians in primary health care facilities. Jakarta: Ministry of Health of the Republic of Indonesia; 2014.
2. Yulanda G, Lisiswanti R. Management of Primary Hypertension. Majority Journal. 2017 Feb 1;6(1):28-33.
3. Kandarini Y. Pharmacological Management of Hypertension Therapy. Denpasar: Faculty of Medicine, Udayana University. 2016.
4. Ministry of Health of the Republic of Indonesia. Decree of the Minister of Health of the Republic of Indonesia Number HK.01.07/MENKES/4634/2021 concerning National GuidelinesforMedicalServicesfortheTreatment ofAdultHypertension.Jakarta:Ministry of Health of the Republic of Indonesia; 2021.
5. Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, Whelton PK. Hypertension (Primer). Nature Reviews: Disease Primers. 2018;4(1):18014.
6. JAMA. Special Communication 2014 Evidence – Based Guideline For the Management of High Blood Pressure in Adults Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA, 2014, 311(5).507-520.
10
7. Bulsara KG, Cassagnol M. Amlodipine. InStatPearls [Internet] 2022 Jan 24. StatPearls Publishing.
8. Kadir A. Relationship between pathophysiology of hypertension and renal hypertension. Wijaya Kusuma Medical Scientific Journal. 2018 Feb 13;5(1):15-25.
9. Acelajado, M.C., Hughes, Z.H., Oparil, S. and Calhoun, D.A. Treatment of resistant and refractory hypertension. Circulation research, 2019, 124(7), pp.1061-1070.
10. Ministry of Health of the Republic of Indonesia. Technical Guidelines for the Discovery andManagementofHypertension.Jakarta:MinistryofHealthoftheRepublicofIndonesia; 2013.
11. Page M J, McKenzie J E, Bossuyt P M, Boutron I, Hoffmann T C, Mulrow C D et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews BMJ 2021; 372 :n71 doi:10.1136/bmj.n71
12. Wang Y, Li Y, Huo Y, Wang JG. Treatment effect of lacidipine and amlodipine on clinic and ambulatory blood pressure and arterial stiffness in a randomized double-blind trial. Blood Pressure. 2021 Mar 4;30(2):108-17.
13. Ohishi M, Kawai T, Hayashi N, Kitano S, Katsuya T, Nagano M, Hirotani A, Yamamoto K,KamideK,Rakugi H. Effect oftablets with acombinationoftelmisartanandamlodipine on patients with hypertension: the Cotalo study. Hypertension Research. 2013 Jul;36(7):620-6.
14. Huang QF, Sheng CS, Li Y, Dou Y, Zheng MS, Zhu ZM, Wang JG, Amlodipine Morning Blood Pressure Surge Study (ARMORS) Investigators. A randomized controlled trial on the blood pressure–lowering effect of amlodipine and nifedipine‐GITS in sustained hypertension. The Journal of Clinical Hypertension. 2019 May;21(5):648-57.
15. Chen Q, Huang QF, Kang YY, Xu SK, Liu CY, Li Y, Wang JG. Efficacy and tolerability of initial high vs low doses of S‐(‐)‐amlodipine in hypertension. The Journal of Clinical Hypertension. 2017 Oct;19(10):973-82.
16. Poulter NR, Dolan E, Gupta AK, O’Brien E, Whitehouse A, Sever PS. Efficacy and safety of incremental dosing of a new single-pill formulation of perindopril and amlodipine in the management of hypertension. American Journal of Cardiovascular Drugs. 2019 June 1;19:313-23.
17. Eguchi K, Imaizumi Y, Kaihara T, Hoshide S, Kario K. Comparison of valsartan and amlodipine on ambulatory blood pressure variability in hypertensive patients. Clinical and Experimental Hypertension. 2016 Nov 16;38(8):721-4.
11
18. Wang JG, Yukisada K, Sibulo Jr A, Hafeez K, Jia Y, Zhang J. Efficacy and safety of sacubitril/valsartan (LCZ696) add-on to amlodipine in Asian patients with systolic hypertension uncontrolled with amlodipine monotherapy. Journal of Hypertension. 2017 Apr 1;35(4):877-85.
19. Xu SK, Huang QF, Zeng WF, Sheng CS, Li Y, Wang JG. A randomized multicenter study on ambulatory blood pressure and arterial stiffness in patients treated with valsartan/amlodipine or nifedipine GITS. The Journal of Clinical Hypertension. 2019 Feb;21(2):252-61.
20. Thuc Sinh C, Van Minh H, Van Huy T. Effects of lercanidipine versus amlodipine in hypertensive patients with cerebral ischemic stroke. Current Medical Research and Opinion. 2015 Jan 2;31(1):163-70.
21. Merchant N, Rahman ST, Ahmad M, Parrott JM, Johnson J, Ferdinand KC, Khan BV. Changes in biomarkers and 24 hours blood pressure in hypertensive African Americans with the metabolic syndrome: comparison of amlodipine/olmesartan versus hydrochlorothiazide/losartan. Journal of the American Society of Hypertension. 2013 Sep 1;7(5):386-94.
22. Yoshida H, Akasaka H, Saitoh S, Shimamoto K, Miura T. Comparative effects of telmisartan and valsartan as add-on agents for hypertensive patients with morning blood pressure insufficiently controlled by amlodipine monotherapy. Hypertension Research. 2014 Mar;37(3):225-31.
23. Zaman ZA, Kumari V. Comparison of the effects of amlodipine and cilnidipine on blood pressure, heart rate, proteinuria and lipid profile in hypertensive patients. Int J Basic Clin Pharmacol. 2013 Mar;2(2):160-4.
24. Cho EJ, Lee HY, Sung KC, Park S, Sohn IS, Park CG, Choi DJ, Ha JW, Ahn YK, Shin J, Hong SJ. Comparison of 24-hour ambulatory central blood pressure reduction efficacy between fixed amlodipine or up-titrated hydrochlorothiazide plus losartan: the K-central study. American journal of hypertension. 2019 Sep 24;32(10):992-1002.
25. Shin J, Lee HY, Chung WJ, Youn HJ, Cho EJ, Sung KC, Chae SC, Yoo BS, Park CG, Hong SJ, Hong TJ. The association of smoothness index of central blood pressure with ambulatory carotid femoral pulse wave velocity after 20-week treatment with losartan in combination with amlodipine versus hydrochlorothiazide. Journal of hypertension. 2019 Dec;37(12):2490.
26. Tsioufis C, Dimitriadis K, Mantzouranis E, Mani I, Tousoulis D. Differential effects of lercanidipine/enalapril versus amlodipine/enalapril and hydrochlorothiazide/enalapril on
12
target organ damage and sympathetic activation in non-obese essential hypertensive subjects. Current Medical Research and Opinion. 2016 Sep 30;32(sup2):35-41.
27. Mizuno H, Hoshide S, Fukutomi M, Kario K. Differing effects of aliskiren/amlodipine combination and high‐dose amlodipine monotherapy on ambulatory blood pressure and target organ protection. The Journal of Clinical Hypertension. 2016 Jan;18(1):70-8.
28. Fukutomi M, Hoshide S, Mizuno H, Kario K. Differential effects of aliskiren/amlodipine combination and high-dose amlodipine monotherapy on endothelial function in elderly hypertensive patients. American journal of hypertension. 2014 Jan 1;27(1):14-20.
29. Buscemi's, Buscemi C, Borzì AM, Cosentino L, Rosafio G, Randazzo C, Colomba D, Di Raimondo D, Pluchinotta FR, Parrinello G. Metabolic and cardiovascular effects of switching thiazides to amlodipine in hypertensive patients with and without type 2 diabetes (the diuretics and diabetes control study). Metabolic Syndrome and Related Disorders. 2020 Mar 1;18(2):110-8.
30. Shi J, Yuan Y, Deng X, Pan Y, He M, Liu G, Sun D, Wang J, Wang W, Li Y. Metoprolol has a similar therapeutic effect as amlodipine on BP lowering in hypertensive patients with obstructive sleep apnea. Sleep and Breathing. 2019 Mar 14;23:227-33.
31. Fogari R, Derosa G, Zoppi A, Lazzari P, D'Angelo A, Mugellini A. Comparative effect of canrenone or hydrochlorothiazide addition to valsartan/amlodipine combination on urinary albumin excretion in well-controlled type 2 diabetic hypertensive patients with microalbuminuria. Expert opinion on pharmacotherapy. 2014 Mar 1;15(4):453-9.
32. Kario K, Hoshide S, Mizuno H, Kabutoya T, Nishizawa M, Yoshida T, Abe H, Katsuya T, Fujita Y, Okazaki O, Yano Y. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020 Nov 10;142(19):1810-20.
33. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ 2019; 366: l4898
13
1 RCT: Randomized Controlled Trial; GITS: Gastro Intestinal Therapeutic System; HCTZ: Hydrochlorothiazide, CCB: Calcium Channel Blocker; ARB: Angiotensin Receptor Blocker; ACE-I: Angiotensin Converting Enzyme-Inhibitor; NI: not informed

14 Appendix
1
Appendix 1. Study Characteristics
2 RCT: Randomized Controlled Trial; GITS: Gastro Intestinal Therapeutic System; HCTZ: Hydrochlorothiazide, CCB: Calcium Channel Blocker; ARB: Angiotensin Receptor Blocker; ACE-I: Angiotensin Converting Enzyme-Inhibitor; NI: not informed

15 2
ExploringTheImpactofMusicTherapyUseComparedtoStandardofCareOn DepressionInDementiaPatients:ASystematicReview
RevandaAnnisaCahyani(AMSA-Brawijaya),CaesarRamadhanSusanto (AMSA-Brawijaya),KaylaFaraditaAisyah(AMSA-Brawijaya),VincentEnricoAnderson (AMSA-Brawijaya)
ABSTRACT
Introduction: Dementia is defined as an acquired loss of cognition in many cognitive domains,thesymptomsaresevereenoughtointerferewithsocialorprofessionalfunctioning. In recent years, the non-pharmacological interventions are massively being assessed, including activities that use sensory function (listening to music, watching TV, and many others), physical activities, educational, and psychosocial. Thus, this systematic review is made to assess the benefit of music therapyasanalternativesubstitutionofStandardofCare ondepressionindementiapatients.
Objectives: Our purpose in making this systematic review is to notice the exact effects of music therapy implemented to elders diagnosed with dementia which isoverallmeasuredby parameters such as NPI (Neuropsychiatric Inventory), GDS-SF (Geriartric Depression Scale-ShortForm),andCBS-QoL(Cornell-BrownScaleforQualityofLife).
Methods: Inclusion criteria in these studies are randomized controlled trials (RCTs), data collections are generated using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and the data for this study was arranged using 7 databases (ClinicalKey, PubMed, ProQuest, Google Scholar, Wiley, Cochrane Library, and Springer). Risk of bias was assessed by using the Cochranerisk-ofbiastoolforrandomizedtrials(RoB 2).
Results: The results showed that music therapy has significant impacts in plummeting depression on dementia patients as shown by the alterations of quantitative indicators from baseline to post-intervention, which is viewed as an overall surge in qualitative care despite theincreaseordecreaseinquantity,intheparameteroutcomesmeasured.
Conclusion: The integration of musictherapyasanon-pharmacologicalmethodtoraisecare has been one of the patient-centered approaches in geriatric medicine, especially dementia. Overall, the results have shown promising and elevating qualitiesingeriatriclivescompared topatientsgiventheStandardofCare.
Keyword: Music Therapy, Dementia, GDS-SF, Neuropsychiatric Inventory, Depression
Exploring The Impact of Music Therapy Use Compared to Standard of Care on Depression in Dementia Patients: A Systematic Review
UNIVERSITAS BRAWIJAYA
Authors:
Revanda Annisa Cahyani
Caesar Ramadhan Susanto
Kayla Faradita Aisyah
Vincent Enrico Anderson
Faculty of Medicine, Brawijaya University
Asian Medical Students’ Association Universitas Brawijaya
2023

Exploring The Impact of Music Therapy Use Compared to Standard of Care On Depression In Dementia Patients: A Systematic Review
Revanda Annisa Cahyani (AMSA-Brawijaya), Caesar Ramadhan Susanto (AMSA-Brawijaya), Kayla Faradita Aisyah (AMSA-Brawijaya), Vincent Enrico Anderson (AMSA-Brawijaya)
ABSTRACT
Introduction: Dementia is defined as an acquired loss of cognition in many cognitivedomains, the symptoms are severe enough to interfere with social or professional functioning.In recent years,thenon-pharmacologicalinterventions are massivelybeing assessed,includingactivities that use sensory function (listening to music, watching TV, and many others), physical activities, educational, and psychosocial. Thus, this systematic review is made to assess the benefit of music therapy as an alternative substitution of Standard of Care on depression in dementia patients.
Objectives: Our purpose in making this systematic review is to notice the exact effects of music therapy implemented to elders diagnosed with dementia which is overall measured by parameters such as NPI (Neuropsychiatric Inventory), GDS-SF (Geriartric Depression ScaleShort Form), and CBS-QoL (Cornell-Brown Scale for Quality of Life).
Methods: Inclusion criteria in these studies are randomized controlled trials (RCTs), data collections are generated using Preferred Reporting Items for Systematic Reviews and MetaAnalyses (PRISMA), and the data for this study was arranged using 7 databases (ClinicalKey, PubMed, ProQuest, Google Scholar, Wiley, Cochrane Library, and Springer). Risk of bias was assessed by using the Cochrane risk-of bias tool for randomized trials (RoB 2).
Results: The results showed that music therapy has significant impacts in plummeting depression on dementia patients as shown by the alterations of quantitative indicators from baseline to post-intervention, which is viewed as an overall surge in qualitative care despite the increase or decrease in quantity, in the parameter outcomes measured.
Conclusion: The integration of music therapy as a non-pharmacological method to raise care has been one of the patient-centered approaches in geriatric medicine, especially dementia. Overall, the results have shown promising and elevating qualities in geriatric lives compared to patients given the Standard of Care.
Keyword: Music Therapy, Dementia, GDS-SF, Neuropsychiatric Inventory, Depression
INTRODUCTION
Approximately around 8.5% (617 million) of people in the population are elders. Age is the main risk factor for developing dementia, and there are already at least 50 million people worldwidesufferingbecauseofdementia1.Dementiaisdefinedas anacquiredlossofcognition in many cognitive domains, the symptoms are severe enough to interfere with social or professional functioning. There are already many interventions that are being usedto enhance the symptoms of dementia, either pharmacological or non-pharmacological. In recent years, the non-pharmacological interventions are massively being assessed, including activities that use sensory function (listening to music, watching TV, and many others),
physical activities, educational, and psychosocial. The non-pharmacological interventions can be used as a supplemental therapy as they offer economical and feasible interventions that can be done in patients' houses, while also serving a variety of strategies to enhance outcomes for dementia patients, reduce behavioral occurrences, and enhance or maintain quality of life2 .
As stated above, one of many non-pharmacological interventions that are used in many clinical practices for dementia is music therapy. Many authors highlight that music therapy has a positive impact on behavior, mood, and cognition. As a result, music therapy affects brain physiology and can enhance some cognitive functions. Additionally, manyauthors also state that music can enhance the depression in dementia patients, depression is caused by stress inflammatory and then causes neuronal degeneration. Strong neuroplastic changes are stimulated by music instruction. Consequently, music may prevent neuronal degeneration by promoting cerebral plasticity and encouraging the formation of new neural connections in the brain2. However, there are still many studies questioning the significant effect of music therapy on alleviating depression. Because of that, the objective of this systematic review is to objectively analyze the impact of music therapy on alleviating depression and quality of life in dementia patients.
METHOD
Data Collection
In this study, the data collections are generated using the methodological guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), thus the systematic review will be more extensive and clear.
Data Source and Search Strategy
The data for this study was arranged using 7 search engines. The reviewers used ClinicalKey, PubMed, ProQuest, Google Scholar, Wiley, Cochrane Library, and Springer.Multiplekeywordsareutilized,including“Dementia” AND“MusicTherapy” OR “Music Intervention” AND “GDS-SF”. Below is attached the PICOS used as the strategy to write this systematic review.
Population Elderly people with Dementia, including Alzheimer’s Disease, Parkinson’s Disease, and others.
Intervention Music Therapy
Control Standard of Care
Outcome
Declined Depression and Enhanced Quality of Life
Study Design Systematic Review Article
Figure 1. PICOS Framework
Inclusion Criteria
Inclusion criteria in these studies are randomized controlled trials (RCTs) that involve dementia patients who are willing to take part in the study as the intervention
group. The group receive music therapy and are then compared to the control group, who receives standard care such as conventional treatment, to assess theireffectiveness. Only English-language literary works from the past 10 years are reviewed.
Risk of Bias in Studies
The quality of each study was qualitatively analyzed using the Cochranerisk-of bias tool for randomized trials (RoB 2) which includes five domains of study qualities as mentioned in Figure 3a and 3b. Three reviewers assessed the studies independently (RAC, CRS, VEA) and one reviewer (KFA) double-checked the criticalappraisal then plotted it into sheets (.xlsx) before submitted to ROBVIS website and generated the summary plot and the traffic light plot.
RESULTS
The data gathered using 7 electronic databases (ClinicalKey, PubMed, ProQuest, Google Scholar, Wiley, Cochrane Library, and Springer), 2052 articles were eliminated from the 4116 articles, 1012 papers qualified for further study after writers read the titles and abstracts of the remaining 3064 articles for preliminary screening. 20 papers were discarded by the authors because they used the incorrect intervention type and procedure, and double intervention. Finally, a total of 14 studies were included in this systematic review. Figure 2 provides an overview of the systematic review's search flowchart and selection method.
Characteristics of Included Studies
Highlighted findings and outcome of these studies using randomizedcontrolled trials (RCTs) with music therapy as the main intervention, standard of care as control/ comparison group, and geriatric depression scale, neuropsychiatric inventory as the outcome parameters. The participants included in these studies are elderly adults in the age from 70-90 years that are given intervention ranging from 4 weeks to 6 months.
Search Results

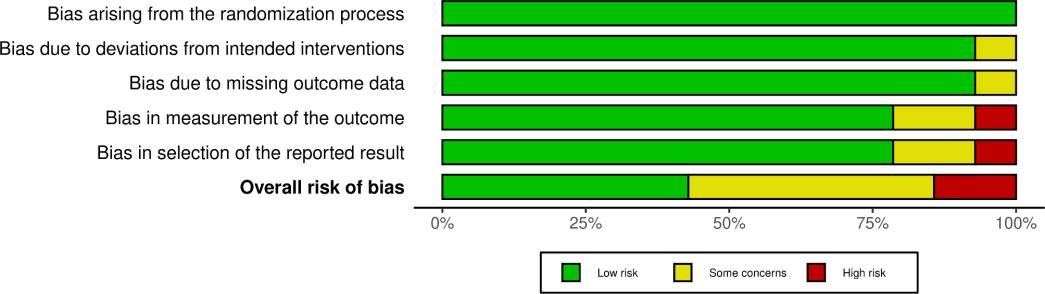 Figure 2. PRISMA Flow chart of study selection
(A)
(B)
Figure 2. PRISMA Flow chart of study selection
(A)
(B)
DISCUSSIONS
Effects of Music Therapy to the Brain

Sound waves are an incredible source of energy, and those waves being able to be perceived by humans involves a complex mechanism. The beginning of this process starts with the sound waves entering the ear, striking the eardrum, and producing vibrations that are further changed into electric signals. These signals will then travel by the sensory nerves to the brainstem. Auditory cortices and many parts of the brain will then be activated after dispersal. The parts of the brain, however, are selectively activated depending on the type of the music.
Over time, musical training has been shown to increase the connectivity of certain brain regions, and can be implemented as a form of therapy. Frequencies are oftenly stimulated and the synaptic connections of that certain brain region will be richer. Alterations in the brain circuitry and connectivity suggest opportunities to activate certain regions to also promote healing especially to patients with dementia, depression, and other neurological conditions. Several areas of the brain that are affected include the corpus callosum, motor cortex, prefrontal cortex, sensory cortex,
Figure 3. Quality Assessment of RCT’s. (A) Risk of Bias domains (B) Risk of Bias Summary
auditory cortex, virtual cortex, nucleus accumbens, amygdala, hippocampus, and cerebellum.
Depression in Dementia
Depression is commonly observed during the clinical course of dementia. It is found that depression can be both a risk factor and a prodrome for cognitive decline and dementia. Depression in elderly may be the first sign of a developing dementia, with emotion and mood is most commonly low but can be irritable, angry, or anxious, disturbed biological rhythms in sleep, appetite and energy are common and patients may be negative, hopeless or even nihilistic3. The impact of depression on dementia may be caused by several mechanisms. As a result of stress chronic inflammatory changes, increase in interleukin-6, C-reactive protein, and tumor necrosis factor-α may be associated with an increase in the risk of dementia and depression.
Music therapy showed a significant effect in reducing depression symptoms in dementia. Listening to preferred music may stimulate the limbic region, paralimbic region, and mesolimbic region which is associated with emotional processing and stimuli. In addition to that, music increases the ability to communicate with others and relate with past pleasant memories that leads to the reduction of anxiety and depressive symptoms4
Outcome Parameters
Geriartric Depression Scale Short Form (GDS-SF)
Depression is a mental disease that frequently affects elderly persons yet goes undiagnosed in this age group. It may be used to depict a sickness, symptoms, or even mood5. Geriatric Depression scale- Short Form (GDS-SF) is a screening tool used to identify depression in older adults by asking 15 questions, used with healthy, medically ill and mild to moderately cognitively impaired adults, easy to administer and score. The questions are satisfaction of life, emptiness, bored, interest activity, good spirits, fear, happiness, doing things at home or outside, problems with memory,feeling helpless, feeling grateful to be alive, full of energy, hopeless situation, and how people react to ourself (better off or no) that deliver as yes or no questions. The studies showed a decrease in the post-intervention GDS score which indicates asignificant effect of music therapy in the reduction of depressive symptoms.
Neuropsychiatric Inventory (NPI)
The Neuropsychiatric Inventory is a comprehensive indicator that can be used for the studies of neuropsychiatric symptoms including dementia. The NPI consists of 10-12 items that are integrated and compiled with questions, subquestions, and ratings of quantitative rates such as frequency and severity. For as long as it has beenexecuted, the NPI has been shown to be valid and reliable. The NPI specifically captures the cost of behavioral symptoms in dementia due to its robust psychometric properties and proliferated versions to respond to specific clinical contingencies.
Cornell-Brown Scale for Quality of Life (CBS-QoL)
In Dementia, QoL is an important cursor used to identify the success of an intervention. It provides a global assessment of quality of life for those who are diagnosed with the abnormality. The scale itself was developed with the thoughts of assessing positive effects, physical and psychological satisfactions, self-esteem, and the relative absence of negative effects and experiences. The Cornell-Brown Scale is assessedafter ajointinterviewbetweenthepatient, caregiver,andtheclinician.Separate interviews without the presence of one side can be held between the patient and the caregiver if necessary to assure reliable and valid collection of data. The interview is conducted in a semi-structured method by assessing the negative pole andthen the positive pole. Both poles after being answered, will be rated respectively withthe score
1/+1 and -2/+2. If neither of the answers belong to each pole, it is scored as a zero. Questions that are forwarded to the patients include topics such as mood related signs and ideational disturbance.
CONCLUSION
Dementia has stayed at the top cause of diseases that are mainly affected by age, thus making it unavoidable for the elders. Older adults also pose greater challenges to organize the optimal care plan or cure, since they are usually equipped with lots of physical and mental morbidities. As time goes by, the integration of novel non-pharmacological methods play a cumbersome yet important role in managing it. Music therapy has been one of the infamous non-pharmacological patient-centered approaches in geriatric medicine, especially dementia. It has been noted that sound waves play a significant role in enhancing and calibrating certain regions of the brain, which brings positive impacts on several functions of the brain itself. The effectiveness of the therapy is measured by indicators such as NPI (Neuropsychiatric Inventory), GDS-SF (Geriartric Depression Scale-Short Form), and CBSQoL (Cornell-Brown Scale for Quality of Life), which acts as the implementation viewed by the triggering of sound waves to the brain. Overall, the results have shownpromising and elevating qualities in geriatric lives compared to patients given the Standard ofCare.
ACKNOWLEDGEMENTS AND CONFLICT OF INTEREST
No competing interests were disclosed by any of the authors for this work.
REFERENCES
1. Ponjoan A, Garre-Olmo J, Blanch J, Fages E, Alves-Cabratosa L, Martí-Lluch R, et al. epidemiology of dementia: Prevalence and incidence estimates using validated electronic health records from primary care. Clinical Epidemiology. 2019; Volume 11:217
28.
2. Moreno-Morales C, Calero R, Moreno-Morales P, Pintado C. Music therapy in the treatment of dementia: A systematic review and meta-analysis. Frontiers in Medicine. 2020;7.
–
–
3. Kitching D. Depression in dementia. Australian Prescriber. 2015;38(6):209–11.
4. Cockerham, W. C. (2016). Sociology of mental disorder. Oxfordshire UK: Taylor & Francis.
5. Baker FA, Lee YEC, Sousa TV, Stretton-Smith PA, Tamplin J, Sveinsdottir V, et al. Clinical effectiveness of music interventions for dementia and depression in elderly care (MIDDEL): Australian cohort of an international pragmatic cluster-randomized controlled trial. Lancet Heal Longev [Internet]. 2022;3(3):e153–65. Available from: http://dx.doi.org/10.1016/S2666-7568(22)00027-7
6. Ridder HMO, Stige B, Qvale LG, Gold C. Individual music therapy for agitation in dementia: An exploratory randomized controlled trial. Aging Ment Health. 2013;17(6):667–78.
7. Ho RTH, Fong TCT, Sing CY, Lee PHT, Leung ABK, Chung KSM, et al. Managing behavioral and psychological symptoms in Chinese elderly with dementia via groupbased music intervention: A cluster randomized controlled trial. Dementia.2019;18(7–8):2785–98.
8. Hsu MH, Flowerdew R, Parker M, Fachner J, Odell-Miller H. Individual musictherapy for managing neuropsychiatric symptoms for people with dementia and their carers: A cluster randomized controlled feasibility study. BMC Geriatr [Internet]. 2015;15(1):1–19. Available from: http://dx.doi.org/10.1186/s12877-015-0082-4
9. Cheung DSK, Lai CKY, Wong FKY, Leung MCP. The effects of the music-withmovement intervention on the cognitive functions of people with moderate dementia: A randomized controlled trial. Aging Ment Heal [Internet]. 2018;22(3):306–15. Available from: http://dx.doi.org/10.1080/13607863.2016.1251571
10. Cockerham, W. C. (2016). Sociology of mental disorder. Oxfordshire UK: Taylor & Francis.
11. Kim H, Engström G, Theorell T, Hallinder H, Emami A. In-home online music-based intervention for stress, coping, and depression among family caregivers of persons with dementia: A pilot study. Geriatr Nurs (Minneap). 2022;46:137–43.
12. Raglio A, Bellandi D, Baiardi P, Gianotti M, Ubezio MC, Zanacchi E, et al. Effect of Active Music Therapy and Individualized Listening to Music on Dementia: A Multicenter Randomized Controlled Trial. J Am Geriatr Soc. 2015;63(8):1534–9.
13. Tz-Han L, Wan-Ru W, Chen IH, Hui-Chuan H. Reminiscence music intervention on cognitive, depressive, and behavioral symptoms in older adults with dementia. Geriatric Nursing. 2023 Jan 1;49:127-32.
14. Chu H, Yang CY, Lin Y, Ou KL, Lee TY, O’Brien AP, et al. The Impact of Group Music Therapy on Depression and Cognition in Elderly Persons With Dementia: A Randomized Controlled Study. Biol Res Nurs. 2014;16(2):209–17.
15. Yu AL, Lo SF, Chen PY, Lu SF. Effects of Group Music Intervention on Depression for Elderly People in Nursing Homes. Int J Environ Res Public Health. 2022;19(15).
16. Cho HK. The effects of music therapy-singing group on quality of life and affect of persons with dementia: A randomized controlled trial. Front Med. 2018;5(OCT):1–13.
17. Ibenthal E, Kehmann M, Backhaus C. Effectiveness of personalized music systems to influence neuropsychiatric symptoms associated with dementia: A quasi-experimental study. Explore [Internet]. 2022;18(3):319–26. Available from: https://doi.org/10.1016/j.explore.2021.03.004
18. Gómez-Gallego M, Gómez-Gallego JC, Gallego-Mellado M, García-García J. Comparative efficacy of active group music intervention versus group music listening in Alzheimer’s disease. International journal of environmental research and public health. 2021 Jul 30;18(15):8067.
APPENDIX
As attached at secluded pages below.
2
1
Effect of Active Music Therapy and Individualized Listening to Music on Dementia: A Multicenter Randomized Controlled Trial
Raglio, A. et al., 2015 Italia Multicenter
Intervention : 31 81.0 ± 7.6 years
Music Therapy and Standard of Care
Listening to Music and Standard of Care 10 Weeks
Control : 32 81.7 ± 7.8 years
4
3
Reminiscence music intervention on cognitive, depressive, and behavioral symptoms in older adults with dementia
Tz-Han, L. et al., 2022 Taiwan single
intervention : 25 82.8 ± 7.8
Control : 20
8-hour reminiscence music therapy
Participate in leisure activates such as listening to music or watching television
4 weeks
Intervention : 18 85 ± 8
The Effects of Music TherapySinging Group on Quality of Life and Affect of Persons With Dementia: A Randomized Controlled Trial
Cho, H. K., 2018 US
Control : 17 (MM-Listening) 87 ± 5 (MMListening)
18 (TV group) 87 ± 5 (TV Group)
Music TherapySinging
Music MedicineListening and TV TV Watching
Managing behavioral and psychological symptoms in Chinese elderly with dementia via groupbased music intervention: A cluster randomized controlled trial
Ho, R. T. H., et al., 2018 China
Intervention : 40 85 ± 7
Music intervention with multisensory components
Standard care 8Weeks
Control : 33 85,7 ± 7
No. Title Author, Year Country Center Population Sample Age Mean ±SD Intervention Control Duration
Single
Single
5
Effectiveness of personalized music systems to influence neuropsychiatric symptoms associated with dementia: A quasiexperimental study
Ibenthal, E. et al., 2022 Germany Multicenter
: 14 88 ± 6 years Personalized music systems
Kim, H., et al., 2022 Sweden
Multicenter
weeks + 4 weeks Follow Up (Without music) Control : 14 80 ± 5 years
Intervention : 24 73.17 ± 11.52
Residents received standard care
: 11 77 ± 5.78
7 Standard/ Usual
care without music intervention
weeks and
Weeks
Control : 28 79.03 ± 7.71 years
Intervention : 40 81.0 ± 7.6 years
minutes active music Therapy Standard care
Weeks
10
No. Title Author, Year Country Center Population Sample Age Mean ±SD Intervention Control Duration
Intervention
8
6 Listening
In-home online music-based intervention for stress, coping, and depression among family caregivers of persons with dementia: A pilot study Standard care 8Weeks Control
music intervention
Group
Effects of Group Music Intervention on Depression for Elderly People in Nursing Homes 5
Yu, A., et al., 2022 Taiwan Multicenter 10
Intervention : 30 80.30 ± 6.88 years
music interevention with usual care
8
Effect of Active Music Therapy and Individualized Listening to Music on Dementia: A Multicenter Randomized Controlled Trial
Raglio., et al., 2015 Italy Multicenter
20
Control : 40 82.4 ± 6.8 years
9
Individual music therapy for agitation in dementia: an exploratory randomized controlled trial
Ridder, H. M. O., et al., 2013 Denmark, Norway Multicenter
Intervention : 21 82.17 ± 8.841 years
Biweekly individual music therapy over a period of six weeks (total 12 sessions)
Standard care 7Weeks
11
The Impact
of
Comparative Efficacy of Active Group Music Intervention versus Group Music Listening in Alzheimer’s Disease
Multicenter
Control: 52
Usual nursing home care (watching television, afternoon tea, and taking walks)
6 weeks
12
Individual music therapy for managing neuropsychiatric symptoms for people with dementia and their carers: a cluster randomised controlled feasibility study
Hsu, M. H., et al., 2015 UK Single
Intervention: AMI: 28
Intervention: AMI: 83.93 ± 8.01
Control: 41 Control: 80.02 ± 5.78
Active Music Intervention consists of welcome song, rythmic exercises, dance exercises, music quiz, and goodbye song.
Standard care (watching nature documentaries without music)
3 months
Intervention:8 84.56 ± 6.64 years
Control:9 82.50 ± 13.04 years
1:1 Active music therapy for a period of 5 months, once a week, live interactive therapy methods of Odell-Miller and Ridder. After each session, two videoclips were presented to the care staff participants in the intervention group unit in order to communicate elements of musictherapy.
Standard care (medical care, personal care, provision of basic needs, and home services)
5 months
Intervention: 54 85.71 ± 6.68 years
TheEffects of the Music-with-
Listened totheir
No. Title Author, Year Country Center Population Sample Age Mean ±SD Intervention Control Duration
Group
Therapy
Control : 21 80.2 ± 8.672 years 10 Persons
Music
on Depression and Cognition in Elderly
With Dementia: A Randomized Controlled Study
Chu, H. et al., 2013 Taiwan
Intervention: 52 82 ± 6 years Music Therapy
Gomez-Gallego, M. et al., 2021 Spain Multicenter
13
movement Intervention on the Cognitive Functions of People with Moderate Dementia: s randomoized controlled trial
Cheung, D. S. K. et al., 2016
Control: 53 85.58 ± 7.46 years
and moved their limbs and trunk, twice a week for six weeks
casually, twice a week for six weeks
Intervention:77 86.0 ± 7.5 years
14
Clinical effectiveness of music interventions for dementia and depression in elederly care (MIDDEL): Australian cohort of an international pragmatic clusterrandomised controlled trial
Baker, F. A., et al., 2022 Australia Multicenter
Control:80 87.20 ± 6.5 years
Table 1. Tables of Studies’ Characteristics
Group Music Therapy twice per week for 3 months, each session is held for 45 minutes and thereafter once per week for 3 months. Standard care
No.
Title
Year
Author,
Country
Center Multicenter Population Sample Age Mean ±SD Intervention
Control Chatted
Duration 12
Hong Kong
preferred music
weeks
6 months
± 8 (MMListening)
± 6 (TV Group)
Agitation : 3.4 ± 3
Aberrant motor : 2.9 ± 3
Irritability : 2.5 ± 3
Dysphoria : 2.2 ± 3
There were no significant differences in behavior between groups. All groups showed improvement over time in behavioral symptoms, depression, and quality of life, as shown by the reduction in NPI global score. But, the effects that observed in the MT group were shown much more significant improvement over time for delusion, anxiety, and disinhibition.
Neuropsychiatric Inventory
± 7 (MMListening)
± 6 (TV Group)
Agitation : 1.4 ± 2
Aberrant motor : 2.2 ± 2
Irritability : 1.8 ± 2
Dysphoria : 1.2 ± 2
The GEE model showed there are no significant differences in stress scores between the two groups. the intervention group showed significant decrease in CSDD score compared to the control group. therefore, reminiscence music program improves depression symptoms.
Clinical outcomes, including demographic characteristics (i. e., age, gender, and educational level), cognition, depression, and behavioral problems, as well as feasibility outcomes, comprising adherence and acceptability.
There were significant effects of a music therapy-singing group on quality of life of persons with dementia (t = 7.02, p = 0.001) and only the singing group significantly increased quality of life between the pre-test and the post-test. The effects of a music medicine-listening group (t = 1.39, p = 0.187) or those of a control-TV group (t = 1.82, p = 0.118) on quality of life were not significant.
Quality of LifeAlzheimer’s Disease
Music intervention showed a significant and moderate effect on agitation and dysphoria and a significant and large effect on aberrant motor behavior. But, BPSD (Agitation, aberrant motor, irritability, and
Author,Year Primary Outcome Highlighted findings Outcome Parameter Baseline PostTreatment Raglio, A. et al., 2015 33.1 ± 16.2 23.7 ± 10.7
33.0 ± 14.2 29.1 ± 17.0 Tz-Han, L. et al., 2022 6.4 ± 2.3 6.0 ± 2.5
4.6 ± 3.0 5.1 ± 3.0 38.7 ± 4 47.2 ± 6
Cho, H. K., 2018
39.2
41.4
45.7
40.7
Ho, R.T. H., et al., 2018
et al., 2018
Agitation : 2.1 ± 3
Aberrant motor : 1.4 ± 2
Irritability : 1.7 ± 2
Dysphoria : 0.5 ± 1
Agitation : 1.8 ± 2
Aberrant motor : 2.9 ± 3
Irritability : 1.9 ± 2
Dysphoria : 0.6 ± 1
There were no significant intervention effects on irritability or subjective mood
irritability,and dysphoria)
There is possible effects were observed for delusions and nighttime disturbances in people with dementia. Caregivers' perceived distress resulting from neuropsychiatric symptoms was not affected. There is an increasing trend of the severity value of apathy was observed, while the severity values of delusions and nighttime disturbances tended to decrease. The trends did not reach statistical significance. Caregivers' perceived distress was not affected.
Neuropsychiatric symptoms and caregivers' distress using the Neuropsychiatric Inventory Questionnaire (NPI-Q)
There were significant withingroup differences in the total scores of PSS-13 in the comparison group at post-test. These findings indicate that while overall stress were maintained from baseline to posttest among caregivers in the intervention group, those in the comparison group reported significantly higher overall stress and lower coping scores at posttest.
Stress level in dementia identify by Perceived Stress Scale 13 (PSS-13)
Ibenthal, E. et al., 2022
-0.025 ± 0.36 Distress : 0.25 ± 0,56 Total : 2.0 ± 0.6
Severity :
Severity : -0.4 ± 0.4 Distress : 1.0 ± 0,8 Total : 1.5 ± 0.5 Kim, H., et al., 2022 10.0 ± 5.65 8.88 ± 6.07
10.82 ± 5.42 11.64 ± 5.09
The time-by-group interaction exhibited a significant difference for depression (GDS-SF) scores for the time, group, and time-bygroup interaction in the music group and control group
status identify by the Geriatric Depression ScaleShort Form (GDSSF)
There were no significant differences in behavior between groups, all groups showed improvement over time in behavioral symptoms, depression, and quality of life, as shown by the reduction
Scale for Quality of Life in Dementia (CBSQoL), Cornell Scale for Depression in Dementia (CSDD), Neuropsychiatric
Frequency of agitation slightly increased during SC, but decreased during MT, difference was not statistically significant (-0.21).Agitation disruptiveness increase during SC (3.26) and decrease during MT (-3.51), difference reached significance (p = 0.027). Quality of Life decreased during SC (-5.88) but increased during MT (10,42), difference was not significant (p = 0.439).
Cohen-Mansfield Agitation Inventory
(CMAI-fr), Cohen-Mansfield Agitation Inventory
(CMAI-di), and Quality
Life (ADRQL)
Depression improvement measured by CSDD (Cornell Scale for Depression)
Yu, A., et al., 2022 8.65 ± 3.38 3.96 ± 1.54 and 2.96 ± 1.29
10.16 ± 2.91 10.39 ± 2.87 and 10.17 ± 2.77 Raglio., et al., 2015 NPI : 33.1 ± 16.2 CSDD : 9 (3–20) CBS-QoL : 5.9 ± 7.2 NPI : 23.7 ± 10.7 CSDD : 6.5 (0–17) CBS-QoL : 4.9 ± 6.9
Depression
NPI : 36.7 ± 19.2 CSDD : 8 (1–29) CBS-QoL : 6.9 ± 9.1 NPI : 28.9 ± 13.3 CSDD : 7 (0–26) CBS-QoL : 4.9 ± 6.9 Ridder, H. M. O., et al., 2013 CMAI-fr : 30.21 ± 12.66 CMAI-di : 15.71 ± 8.15 ADRQL: 334.14 ± 57.36 CMAI-fr : 29.05 ± 15.98 CMAI-di : 15.65 ± 11.8 ADRQL: 333.26 ± 62.57
of
CMAI-fr : 30.98 ± 16.64 CMAI-di : 16.95 ± 13.62 ADRQL: 314.09 ± 85.46 CMAI-fr : 32.12 ± 13.98 CMAI-di : 20.81 ± 14.32 ADRQL: 315.66 ± 76.46 Chu, H. et al., 2013 17.4 ± 10 8.2 ± 7
Frequency
Disruptiveness
Group music therapy reduced depression in persons with dementia. Cognitive function significantly improved slightly at the 6th session, the 12th session, and 1 month after the sessions ended; in particular, short-term recall function improved 15.7 ± 10 13.8 ± 10
GomezGallego, M. et al., 2021
±
NPI-NH: 2.67 ±
0.86 ± 0.43
87.50 ±
NPI-NH: 12.33 ± 11.20
NPI-NH: 3.00 ± 4.38
1.80 ± 0.59
Both groups did not show a significant difference. But, the intervention group showed a larger decrease in the GDS score than the control group. this showed that AMI contributed a larger effect in improving depression syndrome in dementia.
Depression Scale (GDS)
to assess the effect of the interventions on residents’ affective state. Possible scores range from 0 to 15, with higher scores indicating more depressive symptoms.
Symptoms increased in SC while in MT, it is decreased (p = 0.002). Occupational disruptiveness in SC group was found to increase steadily over the course of the prject, and continued to increase during the 2 months follow up. Same goes with MT group with significance p = 0.001. DCM (wellbeing) increased in MT group but decreased in SC group, p = 0.003.
NPI-NH: Symptom score
NPI-NH: Disruptiveness score
DCM: Wellbeing score
DCM-PE: Personal enhancers
Significant changes in these outcomes from baseline to T1 were observed in the MM group; while no changes in any of these variables from baseline to T1 were evident in the SA group. The results indicated that MM is more preferable than other group.
GDS which consists of 15 items of yes/no questions with the total score ranged from 0 to 15, and were used for assessing the depressive symptoms of the participants in this study
Intervention: 6.71 ± 1.60 Intervention: 6.46 ± 1.55
Control: 5.88
1.87 Control: 5.85 ± 1.84
10.78
4.68
DCM-PE:
17.68
±
Hsu, M. H., et al., 2015 NPI-NH: 17.33
DCM:
31.82
DCM:
DCM-PE: 74.90 ±
NPI-NH: 17.57
6.08 NPI-NH: 3.00 ± 3.22 DCM: 1.44 ± 0.49 DCM-PE: 94.45 ± 7.85 NPI-NH: 26.57 ± 7.14 NPI-NH: 10.71 ± 6.02 DCM: 0.61 ± 0.49 DCM-PE: 50.90 ± 43.70 Cheung, D. S. K. et al., 2016 N/A 5.99 ± 3.57
±
N/A 5.98 ± 3.46
Baker, F. A., et al., 2022
MADRS: 77,17.6 ± 8.1
NPI-Q severity: 77, 11.3 ± 7.8
QoL-AD score: 48, 26.9 ± 7.1
EQ-5D-VAS: 76, 58.4 ± 18.4
PCTB scale: 36, 8.8 ± 3.8
MADRS: 80, 16.6 ± 7.0
NPI-Q severity: 80, 8.9 ± 5.5
QoL-AD score: 58, 26.7 ± 7.9
EQ-5D-VAS: 80, 57.3 ± 18.9
PCTB scale: 23, 8 ± 4.7
ADRS: 45, 12.0 ± 8.7
NPI-Q severity: 66, 8.2 ± 6.8
QoL-AD score: 26, 26.1 ± 6.5
EQ-5D-VAS: 66, 59.8 ± 17.8
PCTB scale: 25, 9.9 ± 3.7
MADRS: 50, 13.7 ± 7.8
NPI-Q severity: 50, 7.2 ± 5.2
QoL-AD score: 31, 27.0 ± 7.0
EQ-5D-VAS: 50, 60.7 ± 18.6
PCTB scale: 12, 7.9 ± 4.5
GMT results in lower depressive symptoms, neuropsychiatric symptoms, and perceived burden on staff but higher in one of the three measures of quality of life.
MADRS: MontgomerryAsberg Depression Rating Scale, NPI1: Neuropsychiatric Inventory Questionnaire, QoL-AD: Quality of Life in Alzheimer;s Disease, EQ-5DVAS=EQVisual Analogue Scale, PCTB: Professional Care Team Burden Scale
Table 2. Table of Studies’ Outcomes
The Potency of Global Positioning System (GPS) Based Safety Tracking Devices for Geriatric Patients with Dementia: A Literature Review
Prajnasari Ridwan, Nailah Fairuz Shafiyyah, Iravira Azziaty Evvendi, Nadhirah Sofhi Amanda Faculty of Medicine, Sriwijaya University
[Correspondence E-mail: prajnasarir@gmail.com]
Introduction: Dementia, a worsening sign of cognitive or neuropsychiatric impairment, is growing in the geriatric population, which caused more people to go missing since they were unable to return home and were unaware of their surroundings. The likelihood of getting lost rises as more dementia patients go about retaining mobility and improving health. Monitoring the patient's movement has proven to be one of the most effective ways to prevent a negative outcome.
Objective: This literature review aims to present the potential of new devices for dementia patients to lower the number of missing people, give independence and convenience, and improve search efficiency and search times.
Method: A literature review was conducted through Pubmed and ScienceDirect with keywords: "Dementia AND GPS (Global Positioning System)". There were ten articles used that were relevant and met the criteria.
Results: The use of GPS tracking devices has been tested in several countries showing results almost twice as effective in finding lost dementia patients, and it can reduce the time to find the wayfarers. Patients can live a normal life without burdening the caregiver. Those who initially stayed home all day can now go out and maintain their mental health. However, the range of signals that this GPS device can detect still requires a lot of attention.
Conclusion: The ability to move is essential for geriatric patients to maintain their quality of life. Tracking patients with dementia is beneficial for the patient and the caregiver. Tracking prevents patients from getting lost, and wandering into unsafe areas and reduces the time needed to find them. To provide the same safety procedure in dementia patients, it is better to make GPS safety tracking devices insured. Further research is required to ensure patient comfort while using devices in daily activity.
Keywords: GPS, wanderer, dementia, geriatrics
SCIENTIFIC PAPER COMPETITION
PRE-CONFERENCE COMPETITION ASIAN MEDICAL STUDENT'S CONFERENCE
(PCC AMSC) 2023
THE POTENCY OF GLOBAL POSITIONING SYSTEM (GPS) BASED SAFETY TRACKING DEVICES FOR GERIATRIC PATIENTS WITH DEMENTIA: A LITERATURE REVIEW
Authors:
Prajnasari Ridwan
Nailah Fairuz Shafiyyah

Iravira Azziaty Evvendi
Nadhirah Sofhi Amanda
OF MEDICINE SRIWIJAYA UNIVERSITY
FACULTY
2023
The Potency of Global Positioning System (GPS) Based Safety Tracking Devices for Geriatric Patients with Dementia: A Literature Review
Prajnasari Ridwan, Nailah Fairuz Shafiyyah, Iravira Azziaty Evvendi, Nadhirah Sofhi Amanda Faculty of Medicine, Sriwijaya University
[Correspondence E-mail: prajnasarir@gmail.com]
Introduction: Dementia, a worsening sign of cognitive or neuropsychiatric impairment, is growing in the geriatric population, which caused more people to go missing since they were unable to return home and were unaware of their surroundings. The likelihood of getting lost rises as more dementia patients go about retaining mobility and improving health. Monitoring the patient's movement has proven to be one of the most effective ways to prevent a negative outcome.
Objective: This literature review aims to present the potential of new devices for dementia patients to lower the number of missing people, give independence and convenience, and improve search efficiency and search times.
Method: A literature review was conducted through Pubmed and ScienceDirect with keywords: "Dementia AND GPS (Global Positioning System)". There were ten articles used that were relevant and met the criteria.
Results: The use of GPS tracking devices has been tested in several countries showing results almost twice as effective in finding lost dementia patients, and it can reduce the time to find the wayfarers. Patients can live a normal life without burdening the caregiver. Those who initially stayed home all day can now go out and maintain their mental health. However, the range of signals that this GPS device can detect still requires a lot of attention.
Conclusion: The ability to move is essential for geriatric patients to maintain their quality of life. Tracking patients with dementia is beneficial for the patient and the caregiver. Tracking prevents patients from getting lost, and wandering into unsafe areas and reduces the time needed to find them. To provide the same safety procedure in dementia patients, it is better to make GPS safety tracking devices insured. Further research is required to ensure patient comfort while using devices in daily activity.
Keywords: GPS, wanderer, dementia, geriatrics
INTRODUCTION
Dementia is a syndrome defined as a medical condition where a person’s cognitive capabilities are worsening than a couple of months or years before, resulting in a disturbance of function in daily activities.(1) Some types of cognitive abilities degradation like language, attention, orientation, assessment, and planning, may happen in people with dementia, and the most common is impaired memory. Dementia is therefore not categorized as a single disease but rather as a collection of broad phrases that describe various signs of cognitive or neuropsychiatric impairment.(2) The quality of life for dementia patients is enhanced with nonpharmacologic approaches. It may include physical activity, mental exercises, music therapy, or aromatherapy, which appear tobe effective ways to enhance cognitive function and manage abnormal behavior and moods in dementia patients. Exercising and strolling are essential to support the good life of people with dementia.(3)
There are an estimated 55 million cases of dementia worldwide, and it has been rising. Dementia prevalence rose with age, from 1.0% of those in their 60s and 64s to 26.3% in their 85s and older. In the United Kingdom, around 800.000-850.000 people are diagnosed with dementia, and 5% of them go missing for the first time every year. Some of them even encounter recurring situations.(4) The American Alzheimer’s Association (2011) found that 6 from 10 people with dementia enter society without the presence of a caretaker. Rowe et al. observed that 30% of the missing people with dementia who were reported missing in 325 newspaper articles were later discovered deceased. When compared to those living, the dead were discovered closer to where they were last seen. To discover older people with dementia who are missing more quickly and effectively, a tool is required.
GPS technology can track the patient's mobility by receiving signals from satellites orbiting the Earth, which can then be relayed to a caregiver's device.(5) Patients can easily and safely use this technology by attaching a GPS device, such as a GPS-enabled smartwatch, wristband, or tracker that is hidden in an insole for shoes or slippers. (6)
METHOD
This is a literature review study. The search engine used was Pubmed, and Science Direct, with the keyword used as the main source for this review being “Dementia AND GPS (global positioning system)”, followed by several related keywords like “Dementia AND Geriatric” and “GPS AND Technology”. After selecting and screening, tenth articles were relevant and met the criteria used in the results and discussions part.
RESULTS
Devices equipped with GPS are used to track dementia patients. It comes with a bunch of wearable designs like wristbands, watches, or even attached to clothing.(6) GPS technology helps determine the patient's location by transmitting data to a server that can be accessed by the caregiver using another device.(5) The study to learn the feedback of the devices used by dementia patients uses data about the user experience collected by the caregiver (7) This GPSlocating device is needed in all geriatric patients diagnosed with dementia. Although this review focused in geriatric patients with dementia, this device is also useful for someone with autism or any other condition with cognitive impairment. The use of GPS tracking devices has been tested in several countries showing results almost twice as effective in finding lost
dementia patients, and it can reduce the time it takes to find wayfarers.(8) The caregivers can provide freedom to dementia patients with a sense of calm. Nearly 50% of patients use the device for more than 1 year. Yet, there are still areas for improvement in the device, especially focused on the device's battery life and the tendency not to use the device.(6)
DISCUSSIONS
The working principle of the GPS
Using technology that can track the patient's mobility is one of the solutions that can be used tosolvethe problem.Therefore,GPSor Global PositioningSystemcanbe usedtoidentify a person's location precisely anywhere on Earth’s surface by constantly receiving signals from multiple satellites orbiting the Earth. The device then calculates the distance between each satellite and itself using the time it takes for the signal to travel from the satellite to the device. The signal is relay through mobile phone networks to a caregiver’s computer or mobile device.(6)
Tracking patients with dementia can be an important safety measure for their wellbeing. Tracking devices can help caregivers locate individuals with dementia if they become lost or disoriented, reducing the risk of harm or injury. Locator devices may reduce the time required to find missing individuals with dementia and the costs associated with search and rescue operations. With the assistance of a tracking device, some people with dementia may still be able to safely navigate their surroundings, allowing them to maintain some level of independence.(5) Patients can easily and safely use this technology by attaching a GPS device, such as a GPS-enabled smartwatch, wristband, or tracker that is hidden in an insole for shoes or slippers. In order for the GPS device to receive signals from the GPS satellites, it must be turned on and have a clear view of the sky.(6) The GPS gadget will utilize the signs from the satellites to decide the individual's area and send that data to the following server. The caregivers can see the person's current location on a map on the tracking server from their computer or mobile device.(5) Some GPS devices also offer additional features, such as geofencing, which allows you to set up virtual boundaries and receive alerts when the person or object being tracked enters or exits a designated area.(9)
Safety and effectiveness of implementing mobile tracking devices
Based on several studiesthat have been implementedin several countries (UK, Canada, US, Norway, Netherlands, and Sweden) with the use of three types of tracking devices (ST200 Prime, SmartSole, and TRiLOC), the results show that overall GPS tracking devicesare almost twice as effective in finding lost dementia patients or wayfarers and can work more efficiently over long distances. GPS technology is also beneficial for caregivers because it can reduce the time it takes to find wayfarers.(6)
In fact, the use of mobile tracking is reliable because the caregiver gives the wayfarer more freedom. As many as 60% of caregivers stated that they provide flexibility to wayfarers, and another 30% stated that they could do more activities to do their work due to the use of GPS. The Norwegian study assessed 208 people with dementia; almost more than 50% of patients used mobile tracking for more than one year; 23% used it for up to two years, and 12% used it for more than two years. However, there is a need to pay attention to the cost-
effectiveness of this device and the fast battery drain due to frequent use of the connected GPS.(6)
Opposing influence of tracking device for dementia patient
Tracking Devices for Geriatric Patients with Dementia can help search for dementia so that cases of missing elderly with dementia are reduced, proven effective as in the search for extractions andlost objects in several studies.(8) This portable GPS candetect precise locations, so this system has been launched a lot and facilitates the process of testing and making products. This tool will make it comfortable for the patient's family because they will be easy tofind when they get lost; onthe other hand, patientscanlive normal lives.Those whoinitially stayed at home all day can now go out and maintain their mental health.(10)
However, this tool's manufacture is incompatible with the battery power that can be accommodated, not to mention the social stigma that ensnares it inhumanely.(6) The successful launch of these devices involved a lot of parties in its implementation to regulate the launch of this health product, such as elderly nurses, GPS tracker services, and finished products, to the local government. There are also several obstacles need to be addressed, particularly in patient comfort and device resistance and sensitivity.(10)
CONCLUSION
The ability to move and walk outside is essential for geriatric patients to maintain their quality of life. Tracking patients with dementia can benefit both the patient and the caregiver because it can prevent the patient from getting lost, and the caregivers can quickly locate them and prevent them from wandering into unsafe areas. To provide the same safety procedure in dementia patients, it is better to make GPS safety tracking devices insured. Further long-term research is needed to ensure patient comfort while using devices in daily activity.
ACKNOWLEDGEMENTS AND CONFLICT OF INTEREST
We are grateful for the guidance givenbydr.Ziske Mariske M.Si. Med.,oursupervisor, and Nancy Febriyanti Napitupulu as A-Partner for all the instruction and advice. All writers guarantee that there is no outside influence or potential conflict of interest.
REFERENCE
1. Arvanitakis Z, Bennett DA. What Is Dementia? Vol. 322, JAMA - Journal of the American Medical Association. American Medical Association; 2019. p. 1728.
2. Moon Y, Oh-Park M, Lee J. Geriatric Psychiatric and Cognitive Disorders: Depression, Dementia, and Delirium. In: Geriatric Rehabilitation. Elsevier; 2018. p. 169–80.
3. Greene KS, Clarke CL, Pakes F, Holmes L. People with dementia who go missing: A qualitative study of family caregivers' decision to report incidents to the police. Policing (Oxford). 2019 Jun 1;13(2):241–53.
4. Zhang Y, Xu Y, Nie H, Lei T, Wu Y, Zhang L, et al. Prevalence of dementia and major dementia subtypes in the Chinese populations: A meta-analysis of dementia prevalence surveys, 1980-2010. Vol. 19, Journal of Clinical Neuroscience. 2012. p. 1333–7.
5. Cullen A, Mazhar MKA, Smith MD, Lithander FE, Breasail M, Henderson EJ. Wearable and Portable GPSSolutionsfor MonitoringMobilityinDementia: ASystematic Review. Sensors. 2022 May 1;22(9).
6. GPS Locator Devices for People With Dementia CADTH ISSUES IN EMERGING HEALTH TECHNOLOGIES 2. 2016.
7. Van Kasteren TLM, Englebienne G, Kröse BJA. An activity monitoring system for elderly care using generative and discriminative models. Pers Ubiquitous Comput. 2010 Sep;14(6):489–98.
8. Riyadi S, Bachtiar L, Yunita S. Smart System Pendeteksi Keberadaan Narapidana Di Lapas Kabupaten Kotawaringin Timur. Vol. 6, Generation Journal.
9. Bhavnani SP, Narula J, Sengupta PP. Mobile technology and the digitization of healthcare. Vol. 37, European Heart Journal. Oxford University Press; 2016. p. 1428–38.
10. Prajapati G, Bhimrao B, Sharmila K. Role of Assistive Devices in Wellbeing of Elderly: A Review [Internet]. Available from: https://www.enableireland.ie/sites/default/files/publication/
Partnered-community-based Tango Dance for Improving Balance, Gait, and Modify Disease Severity in Elderly with Parkinson’s Disease: Systematic Review and Meta-analysis of Randomized Controlled Studies
Ilmizab Haq, Aqilla Sakanti Chandrarini, Ragil Kusnandar Miftakhurrozaq, Masrahma
Reinataya
De Rorna
Correspondence E-mail: ilmizab.haq@gmail.com
Introduction: People with Parkinson’ s Disease (PD) will easily be identified by looking at its physical look which can be seen as a slow progression with accumulating disability) Physical and music therapy intervention is believed to help maintain the effectiveness of target balance and complex gait tasks in PD patients, one of the therapeutic intervention that has a highly demand result is through dance. The Tango Dance (TD) has been showing a significance result in improving the balance and complex gait tasks in PD patients) Moreover, community-based TD can also entertain PD patients and improve their mental health so that it maintains their passion to keep on doing the therapy)
Objective: This systematic review and meta-analysis is aimed to evaluate the efficacy of TD intervention given to patients with PD in a community area
Method: This meta-analysis followed the guidelines provided by PRISMA. Study searches were conducted through various electronic databases, such as PubMed, Cochrane Library, Scopus, and Google Scholar. The risk bias assessment of each study was carried out using the Cochrane Risk of Bias (RoB) 2.0. Mean Difference (MD) and Standard Deviation (SD) with the Confidence Interval (CI) of 95% were analyzed using Cochrane Review Manager (RevMan) v5.4.
Results: Tango dance therapy showed to have more effect on lowering Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS-3) when compared to control (pooled MD=-1.32 (95% CI (-2.64,-0.01), p=0.0001, I2=0%)); lowering Timed Up and Go (TUG) (pooled MD=-1.52 (95% CI: -3.08, 0.05), p=0.06, I2=35%)); shorten Freezing of Gait (FOG) time (pooled MD=-0.88 (95% CI: -2.82, 1.07; I2=0%)). Therefore, tango dance therapy can reduce motor symptoms of its disease and also improve balance and gait.
Conclusion: This meta-analysis shows that tango dance is quantitatively significant in improving balance, gait, and functional mobility on people with Parkinson Disease.
Keywords: Balance, Gait, Tango dance, Parkinson’s disease
Partnered-community-based Tango Dance for Improving Balance, Gait, and Modify Disease Severity in Elderly with Parkinson’s Disease: Systematic Review and Meta-analysis of Randomized Controlled Studies
Pre-Conference Competition Asian Medical Students’ Conference
PCC AMSC 2023
Geriatrics: Care for the Future
Ilmizab Haq
Aqilla Sakanti Chandrarini
Ragil Kusnandar Miftakhurrozaq
Masrahma Reinataya De Rorna
Faculty of Medicine
University of Jember

Jember
Indonesia FACULTY
2023
OF MEDICINE JEMBER UNIVERSITY
Introduction
Parkinson’s Disease (PD) is a neurodegenerative disorder caused by a specificdeathofthedopaminergicneuronsintheSubstantiaNigrapars compacta (1) On the year of 2016, PD has approximately affected 6.1 million people worldwide.(2) Around 3–5% of the total PD cases is explained by genetic causes that will be a hereditary disease. People with PD will easily be identified bylooking at its physical look which can be seen as a slow progression with accumulating disability.(3) As a global burden of neurological disorder, PD must be fully pay attention since it has risen the prevalence and incidence of the patient in the last two decades (3–5) Therapeutic intervention is believed to help maintain the effectiveness of target balance and complex gait tasks in PD patients, one of the therapeutic intervention that has a highly demand result is through dance.
The Tango Dance (TD) originated from Argentine has been showing a significance result in improving the balance and complex gait tasks in PD patients (6) TD’s technique requires specific steps that should follow a rhythmic on all direction so it is useful for freezing of gait (7,8) Moreover, TD can also entertain PD patients and improve their mental health so that it maintains their passion to keep on doing the therapy.(9)
TD is included as an example of dance movement therapy that has shown a good connection related with the dopaminergic systems pivotal for establishing and maintaining behaviour. In fact, TD will release a music induced emotion that could increase the reward signals through the tegmental area of dopamine release and be recognized as a pleasurable stimuli (10) Research have proven that TD improves the negative psychological symptoms also modulated serotonin and dopamine concentrations.(11) TD itself can not be separated with the requirements in working of memory, control of attention, and multitasking skill to incorporate new learned dance and previous learned dance elements.(12) Besides, they will also be forced to maintain the rhythm with the music and also to keep the harmonization between one and another while dancing. By doing these, they are starting to learn to concentrate on a specific movement. Another TD specific benefit is that the dancer must not always rely on specific dance movement that they need to memorize, they can just do it with a easy and enjoyable way.(13) The result that hopefully can be obtained including to give a positive results on mood, improvements in balance, improvements in complex gait, improvements in spatial cognition, and also lower frequency of falls (12,14,15)
Even after all the positive impacts that can be obtained from TD, we should not be relying only on TD and wishing that we could improve balance, complex gait, and also spatial cognition for patients with PD. In fact, TD should be started along only as a booster with any other treatments that will also help to maintain improving even for the non-motor manifestations such as cognitive, motoric, and
perceptual abilities of patients with PD.(16–18) Maintaining a regular schedule to do TD for patients with PD will be far more than just an entertainment activity. In consequence, this systematic review and meta analysis aims to evaluate the effectiveness of Tango Dance intervention given to patients with Parkinson’s Disease in a community area of all ages.
Material and Method
The process of creating this systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guideline.
Eligibility criteria
Criteria that are considered for this systematic review and meta analysis eligibility are type of article, study population, intervention, comparator, and outcome measure.
Type of Article
Original research articles using human study with Randomized Controlled Trial (RCT) and written in English were included in this study. Narrative review, systematic review, meta-analysis, non comparative studies, in vitro studies, in vivo studies, technical reports, editor response, scientific poster, study protocol, conference abstracts and other articles that irrelevant with the topic of this systematic review were excluded.
Study Population
People of all ages with Parkinson’s Disease (PD) without any other disease history were included in this study.
Intervention
Intervention of Tango Dance therapy given to the population with Parkison’s Disease in a community area.
Comparator
ComparatorgiventothepopulationwithParkison’sDiseaseinacommunity area with no Tango Dance practice at all and those who were only given Tango Dance lessons without doing any practice after.
Outcome Measure
Outcome measures that are assessed in this systematic review and meta analysis are The Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease RatingScale (MDS-UPDRS-3),The TimedUp and Go (TUG), and Freezing of Gait (FoG).
The Movement Disorder society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) was a new version of the UPDRS that would retain the strengths of the UPDRS scale, but also resolve identified problems and specifically incorporate a number of clinically pertinent Parkinson’s Disease related problems that is not captured in the UPDRS. The content and structure of the MDS-UPDRS are questionnaires for self-evaluation, and a wide body of instructions for raters. The 4 components included in MDS-UPDRS are Part I concerns nonmotor experiences of daily living, Part II concerns motor experiences of daily living, Part III is focused on the motor examination, and Part IV concerns motor complications (19,20)
The Timed Up and Go (TUG) test is a valid, inexpensive, precise, and timeefficient way to evaluate overall functional mobility. The TUG required patients to stand up out of a chair, walk 3 meters, turn around, walk back to the chair, and sit down. Patients were given the following instructions: stand up on the word ‘go,’ walk to the tape, turn around, walk back to the chair, and sit down. The timing of thetest began at theword “go,” andendedwhenthe participant was seated. Patients can only performed the test one time, if a clear error was made, they were asked to repeat the TUG.(21)
Freezing of Gait (FoG)is definedas atemporarysharpfeelingofan absence or marked reduction of proceeding progression of the feet despite the intention to walk. FoG consisted of three patterns that have been distinguished including: 1) trembling in place, 2) shuffling forward, and 3) complete akinesia. These episodes are more likely to occur when initiating walking, turning, and passing through narrowpassages orcertaincircumstancessuchasapproachingadestination.(22) FoG causes falls, reduces quality of life, and likelihood of independent living.(23)
Index Test
Included studies were evaluating the intervention of Tango Dance to Parkinson’s Disease patients of all ages without any other disease intervention in a community area. Studies that do not present a mean difference and standard before and after the intervention were excluded from this meta analysis.
Data Source
The study references that were used in this systematic review and meta analysis were obtained from some databases, such as PubMed, Cochrane Library, Scopus, and Google Scholar. The keywords used in the process of literature search were using mesh and Boolean operators. Keywords that are used in each database can be seen in Table 1 below.
Table 1.
Keyword Used in Literature Searching
Database
Keywords
PubMed ("parkinson disease"[MeSH Terms] OR ("parkinson disease"[Title/Abstract] OR "parkinson s disease"[Title/Abstract] OR "idiopathic parkinson s disease"[Title/Abstract] OR "paralysis agitans"[Title/Abstract]))AND("dancetherapy"[MeSHTerms]OR ("dance therapy"[Title/Abstract] OR "tango dancing"[Text Word] OR "tango dance"[Text Word]))
Cochrane Library (parkinson disease):ti,ab,kw OR (parkinson's disease):ti,ab,kw OR (idiopathic parkinson's disease):ti,ab,kw OR paralysis agitans:ti,ab,kw AND (dance therapy):ti,ab,kw OR tango dancing OR tango dance
Scopus ( TITLE-ABS-KEY ( "parkinson disease" ) OR TITLE-ABS-KEY ( "parkinson's disease" ) OR TITLE-ABS-KEY ( "idiopathic parkinson's disease" ) ) AND ( TITLE-ABS-KEY ( "dance therapy" ) OR ALL ( "tango dance" ) OR ALL ( "tango dancing" ) ) AND ( LIMIT-TO ( DOCTYPE , "ar" ) )
Google Scholar ("parkinson disease" OR "parkinson's disease" OR "idiopathic parkinson's disease") AND ("dance therapy" OR "tango dance" OR "tango dancing") -review
Study Risk of Bias Assesment
Each one of four authors assess all studies that were included in this systematic review and meta analysis according to the Cochrane risk-of-bias toolD for randomized trials. Difference results of assessment were later discussed and resolved between four authors to reduce the chance of bias between each author.
Study Quantitative
Mean Difference and Standard Deviation with the Confidence Interval (CI) of 95% were calculated in this review. We used a Random Effect Model (REM) because the studies results were considered non-homogenous (high variability in studies results), The result of estimation pooling was presented in a forest plot.
Result
Study selection
PubMed, Cochrane Library, Scopus, and Google Scholar databases were systematically searched for relevant English papers; thus 465 articles were generated. Afterward, 122 duplicated articles from mentioned databases were
detected and removed. Authors assessed all of the remaining articles from the title and abstract for irrelevant topic, resulting in 292 articles excluded. 51 articles from databases were retrieved for the full text availability and authors assessed its eligibility. Final screening results into 19 studies excluded under the reason of wrong study design, 8 studies excluded by its wrong population, 6 studies excluded because of wrong comparison, and 11 studies excluded due to its wrong intervention.Therefore,thecurrent reviewincluded5studiestobein thesystematic review and meta analysis. Our study selection as presented in the PRISMA diagram flow chart in Figure 1
 Figure 1. PRISMA 2020 Flow Diagram
Figure 1. PRISMA 2020 Flow Diagram
Study characteristics
From 5 studies included in the systematic review, there are in total 162 people with Parkinson’s Disease (PD) participating in the trials. The frequency of intervention ranges from 10 sessions to 26 sessions for the duration of intervention ranging from 10 weeks until 13 weeks. Majority of the trials (n=4) were conducted in the United States of America (USA). Further details of included studies are shown in Table 2.
2. Summary of the Included Study Results
Author, year Hackney et al, 2009 Duncan et al, 2011 McKee et al, 2013 Romenets et al, 2015 Michels et al, 2018
Table
Washington, USA Washington, USA Atlanta, USA Quebec, Canada Chicago, USA Study design Randomized controlled trial Randomized controlled trial Randomized controlled trial Randomized controlled study Randomized controlled study Population Sample Size 31 52 33 33 13 Mean age (years) Control 66.5±2.8 Interventio n 68.2±1.4 Control 69.0±1.6 Interventio n 69.3±1.9 Control 74.4±6.5 Interventio n 68.3±7.5 Control 64.3±8.1 Interventio n 63.2±9.9 Control Mean 75.50 Interventio n Mean 66.44 Time with PD (years) Control 6.9±1.0 Interventio n 6.9±1.3 Control 7.0±1.0 Interventio n 5.8±1.1 Control 7.0±5.5 Interventio n 7.2±4.9 Control 7.7±4.6 Interventio n 5.5±4.4 NI Interventio n Name of intervention Partnered tango dance with healthy volunteer Communitybased tango dance Communitybased adapted tango dance Partnered argentine tango dance with healthy spouse or friends Communitybased tango dance & talk therapy Frequency 20 sessions 26 sessions 20 90-mins sessions 24 60-mins sessions 10 60-mins sessions Duration of intervention 13 weeks 13 weeks 12 weeks 12 weeks 10 weeks
Place
Risk of bias in studies
We critically assessed the quality of study using the Cochrane revised riskof-bias tool for randomized trials (RoB 2). Four studies showed low risk in overall bias (Duncan et al.,2011; Hackney et al., 2009; Michels et al.,2018; & Romenets et al.,2015) and one study (Mckee et al.,2013) showed some concerns in the first domain prior to the randomisation process. The study conducted by Mckee et al. did not provide any information regarding the randomisation method for the participants.
Comparison No intervention No intervention Lecture Wait-list group Focus Group Discussion without physical intervention Primary Outcome MDSUPDRS-3 Control 32.4±10.72 Interventio n 26.0±9.35 Control 45.6±11.73 Interventio n 39.9±9.18 Control 29.3±7.5 Interventio n 24.0±7.9 Control 26.3±13.5 Interventio n 19.1±10.2 Control 39.00±11.97 Interventio n 23.44±10.61 p-value P=0.002 NI P<0.05 P=0.85 NI Secondary Outcome TUG (seconds) Control 14.4±10.72 Interventio n 10.0±2.99 NI Control 10.5±1.7 Interventio n 10.3±3.2 Control 8.0±2.2 Interventio n 6.1±1.5 Control 14.12±10.09 Interventio n 7.84±1.62 p-value NS NI NS P=0.042 NI Freezing of Gait Control 5.9±10.3 Interventio n 7.5±4.86 Control F=3.33 Interventio n F=4.2 Control 5.7±4.1 Interventio n 4.7±4.2 Control 4.1±4.2 Interventio n 2.7±3.8 NI p-value NS NI NI P=0.026 NI
Study Outcomes in term of MDS-UPDRS-3
There were 5 studies that reported intervention vs control MDS-UPDRS-3 with significant result P=0.002 and MD value: -1.32 (95% CI:-2.64,-0.01, I2=0%) as seen in Figure 3. This quantitative analysis Concluded that tango dance therapy significantly decreased motor symptoms in Parkinson’s Disease using MDSUPDRS-3 scoring with no heterogeneity found (I2=0%).


 Figure 2. Risk of Bias Assessment Result
Figure 3. Study Outcomes in Term of MDS-UPDRS-3
Figure 2. Risk of Bias Assessment Result
Figure 3. Study Outcomes in Term of MDS-UPDRS-3
Study Outcomes in term of TUG
With 4 studies included, the result was not significant effect of tango dance therapy on lowering TUG with p=0.06 and an MD value of -1.52 (95% CI: -3.08, 0.05, I2=35%) compared to the control group are shown in Figure 4. The heterogeneity is low (<50%), hence sensitivity analysis is not needed.
Study Outcomes in term of FoG
Furthermore, we also did the quantitative analysis based on digit FoG. Meta-analysis showed no significant differences (p=0.38) in lowering FoG with neglected heterogeneity (I2=0%). From the meta-analysis it was found that tango dance therapy lowered FoG with MD -0.88 (95% CI: -2.82, 1.07; I2=0%). Figure 5


Discussion
MDS-UPDRS-3 score improvement after tango dance intervention
From all of the studies we obtained, there is a significant decrease of MDSUPDRS-3 score that represents motor symptoms in Parkinson’s Disease with pooled MD: -1.32 (p=0.0001; 95% CI:-2.64,-0.01, I2=0%)
These improvements in MDS-UPDRS-3 scores suggest that participation in theTango program mayhavea disease modifyingeffect. Examination of specific components of the MDS-UPDRS-3 indicates that tango may have a positive influence not only on balance and gait, as might be expected, but also bradykinesia and rigidity. This supports the idea that tango may have a broad impact on motor symptom progression rather than just targeting the gait and balance aspects specifically practiced in the context of dancing.(24)
Figure 4. Study Outcomes in Term of TUG
Figure 5. Study Outcomes in Term of FoG
Effectivity of Tango dance in decreasing Timed Up and Go
The improvement of Timed Up and Go in tango dance group is not significant, with pooled MD : -1.52 (p=0.06; (95% CI: -3.08, 0.05, I2=35%) compared to the control group
From four studies with TUG measurement in it, the study conducted byRomenetset al.weretheonlyonethatshowsignificantimprovementsofbalance during gait in favor of tango in the Timed Up and Go (−1.3 ± 1.6 vs. 0.1 ± 2.3, p = 0.042) and Dual Task TUG score (0.4 ± 0.9 vs. −0.2 ± 0.4, p = 0.012). Other studies that were conducted by Hackney et al., McKee et al., and Michels et al. found nonsignificant improvements of TUG in tango dance group compared to control group.(8,15,25)
Tango dance effect on Freezing of Gait (FOG)
Our meta analysis show that there is significant lowering of FOG in tango dance group compared to control group, with pooled MD -0.88 (95% CI: -2.82, 1.07; I2=0%).
The effects of tango dance on Freezing of Gait may be due in part to the specific nature of the movements performed in tango. For example, tango incorporates movements that are similar to strategies commonly taught to people with FOGby physical therapists. Visual cues, suchas afootto stepover,can relieve FOG. Moreover, tango also involves rhythmic rocking, or alternating shift of center of mass from foot to foot, another strategy commonly used to address freezing (26)
Strength and Limitation
This study only includes RCTs, whereas the previous study only reviewed case study type and observational study type. Sensitivity analysis was used in this study to examine the heterogeneity of included studies. To our knowledge, this study is the first review to account for the comparison of Tango Dance Therapy in people with Parkinson’s Disease with control group without physical treatment. Additionally, The statistically significant results on all assessments and the low heterogeneity of our meta-analysis also provide stronger evidence for this intervention. However, there are some limitations on our review. Firstly, all included studies conducted in one same continent, the American continent. Secondly, outcome measurement between studies also differs, which offers a challenge to the meta-analysis. Lastly, there are many types of Tango Dance that can be used as an intervention.
Conclusion
This systematic review and meta-analysis shows that tango dance is quantitatively significant in improving balance, gait, and functional mobility on
people with Parkinson Disease. All of these improvements were measured via MDS-UPDRS-3, FOG and TUG as our outcome. We recommend further randomized control study with moreoutcomes to provideevidencethat tango dance has an effect on lowering the symptoms of Parkinson Disease, so that the tango dance can become a non-pharmacological therapy which support the outcome of definitive pharmacological therapy. Additionally for future research, we hope there will be research conducted in other countries so that the result is able to represent various races and ethnicities.
Conflict of Interest
No conflict of interest between authors.
Reference
1. Kalia L V., Lang AE. Parkinson’s disease. Lancet [Internet]. 2015;386(9996):896–912.Availablefrom: http://dx.doi.org/10.1016/S01406736(14)61393-3
2. Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–80.
3. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet [Internet]. 2021;397(10291):2284–303. Available from: http://dx.doi.org/10.1016/S0140-6736(21)00218-X
4. Dorsey ER, Sherer T, Okun MS, Bloemd BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis. 2018;8(s1):S3–8.
5. Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, et al. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Heal [Internet]. 2020;5(10):e551–67. Available from: http://dx.doi.org/10.1016/S24682667(20)30190-0
6. Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in parkinson’s disease: A preliminary study. J Neurol Phys Ther. 2007;31(4):173–9.
7. Brichetto G, Pelosin E, Marchese R, Abbruzzese G. Evaluation of physical therapy in parkinsonian patients with freezing of gait: A pilot study. Clin Rehabil. 2006;20(1):31–5.
8. Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: A comparison of Argentine tango and American ballroom. J Rehabil Med. 2009;41(6):475–81.
9. Hackney ME, Earhart GM. Recommendations for implementing tango classes for persons with Parkinson disease. Am J Danc Ther. 2010;32(1):41–52.
10. Menon V, Levitin DJ. The rewards of music listening: Response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28(1):175–84.
11. Jeong YJ, Hong SC, Myeong SL, Park MC, Kim YK, Suh CM. Dance movement therapy improves emotional responses and modulates neurohormones in adolescents with mild depression. Int J Neurosci. 2005;115(12):1711–20.
12. Foster ER, Golden L, Duncan RP, Earhart GM. Community-based argentine tango dance program is associated with increased activity participation among individuals with parkinson’s disease. Arch Phys Med Rehabil
[Internet]. 2013;94(2):240–9. Available from: http://dx.doi.org/10.1016/j.apmr.2012.07.028
13. McNeill LH, Kreuter MW, Subramanian S V. Social Environment and Physical activity: A review of concepts and evidence. Soc Sci Med. 2006;63(4):1011–22.
14. McKinley P, Jacobson A, Leroux A, Bednarczyk V, Rossignol M, Fung J. Effect of a community-based argentine tango dance program on functional balance and confidence in older adults. J Aging Phys Act. 2008;16(4):435
53.
15. McKee KE, Hackney ME. The effects of adapted tango on spatial cognition and disease severity in parkinson’s disease. J Mot Behav. 2013;45(6):519–29.
16. Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, et al. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67(4):492–6.
17. Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated parkinson disease: The norwegian parkwest study. Neurology. 2009;72(13):1121–6.
18. Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol [Internet]. 2010;9(12):1200–13.
Available from: http://dx.doi.org/10.1016/S1474-4422(10)70212-X
19. Ramaker C, Marinus J, Stiggelbout AM, van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Mov Disord. 2002;17(5):867–76.
20. Martinez-Martin P, Rodriguez-Blazquez C, Alvarez-Sanchez M, Arakaki T, Bergareche-Yarza A, Chade A, et al. Expanded and independent validation of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). J Neurol. 2013;260(1):228–36.
21. Bohannon RW. Reference values for the timed up and go test: A descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–8.
22. Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol [Internet]. 2011;10(8):734–44. Available from: http://dx.doi.org/10.1016/S1474-4422(11)70143-0
23. Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116–24.
24. Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil
–
Neural Repair. 2012;26(2):132–43.
25. Duncan RP, Earhart GM, Hackney ME, Earhart GM, McKee KE, Hackney ME, et al. The effects of adapted tango on spatial cognition and disease severity in parkinson’s disease. Complement Ther Med [Internet].
2018;26(6):475–81.
Available from:
https://doi.org/10.1016/j.ctim.2018.07.005
26. Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: A randomized control study. Complement Ther Med [Internet].
2015;23(2):175–84.
Available from:
http://dx.doi.org/10.1016/j.ctim.2015.01.015
Appendix 1. Risk of Bias Analyis






ImprovingVariousMotoricFunctionParametersandAlleviatingPainofPatientswith Parkinson’sDiseaseThroughAquaticTherapy
SydneyTjandra1,NajmaAli1,VindasyaAlmeira1,MuhammadAliZainalAbidin1 1
FacultyofMedicine,UniversitasIndonesia
Introduction: Parkinson's disease (PD) as one of the common geriatric diseases cost 8,5 billion people in 2019 with large gap of DALYs estimated 5.8 billion and lead to 329 thousand deaths. Expansion of PD cases is another crucial issue that could exacerbate with the growing global elderly population. Difficulties of curing the abnormalities of stability in pharmacological treatment leads to the alteration of concerns in improvement of non-pharmacological interventions, such as a rehabilitation program as the most recommended way of treatment for people with PD. Aquatic therapy has proven to inducea neuroprotective system that will decrease the development of neurological disorder effectivelyandimprovebalanceandfunctionalmobility.
Methods: In accordance to the Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA), PubMed, Scopus, Cochrane, andWileyweresearchedforrelevant literature up to 10 April 2023. Included documents were further assessed for risk of bias using the Cochrane Risk of Bias 2.0. Random-effects meta-analysis was done to pool the effectestimateswithReviewManager5.4.
Results and Discussion: Nine randomized studies cumulating a total of 277participantsare included. On balance and gait, aquatic therapy significantly increased Berg Balance Scale scores by 2.64 (p=0.04; 95% CI: 0.11-5.16; I2=44%) and Dynamic Gait Index (p<0.00001; 95% CI: 2.89-6.98; I2=0%). Motoric function based on the Time Get Up and Go Test also improved by a MD of -1.72 (95% CI: -2.85-(-0.58); I2=0%). These results arealsoclinically significant,furthersupportedbypainreduction.
Conclusion: Aquatic therapy significantly improved various motoric parameters and alleviated pain, both statistically and clinically Consideringitscost-effectiveness,feasibility, and acceptability, we recommend aquatic therapy as one of the top non-pharmacological treatmentsincorporatedintoguidelinesforParkinson’sdisease.
Keywords: cognitive function, ai chi, older adults, aquatic therapy
ImprovingVariousMotoricFunctionParametersandAlleviatingPainofPatientswith Parkinson’sDiseaseThroughAquaticTherapy
SydneyTjandra1,NajmaAli1,VindasyaAlmeira1,MuhammadAliZainalAbidin1 1FacultyofMedicine,UniversitasIndonesia
Introduction
Parkinson's disease (PD) is one of the commongeriatricdiseaseshappeningtomorethan8,5 billion people in 2019 with a large gap from DALYs estimations (5.8 billion).The rapid developmentofthisneurologicaldisordercausesslownessofmovement,freezingofgait,and balance impairment as common symptoms, which lead to 329 thousand deaths in the same year.1 According to a cohort study, the estimated number of PD patients over 45 yearsoldis 6,7 million individuals per year, increasing to 9,3 million individuals per year forthoseover 65 years old; higher risk of incidence also increaseswithageovertime.TheexpansionofPD cases in Asia and Europe is another crucial issue that could exacerbate with the growing global elderly population; around 308 thousand new cases were found based on the US Bureaufrom2020to2030.²
6 outof10peoplewithPDtendtoexperiencefallsinmorethan60%ofcasesandfreezingof gait as the primary causes. Additionally, shifting age into greater existence ofolderadultsin Western nations also can worsen the economic burdens with 52 billion dollars as estimated cost.3 The decline of this movement functionality including the reduction in quality of life and also the difficulties of curing the abnormalities of stability in pharmacologicaltreatment is the reason why researchers are still looking intoseveralwaystoincreasetheinnovationof nonpharmacologicaltreatmentinordertoimprovetheconveniencesofgeriatriclife.
Current treatment for Parkinson’s disease entails the consumption of pharmacological treatments such as levodopa.4 However, as this disease is progressive, non-pharmacological interventions such as a rehabilitation programisthemostrecommendedwayoftreatmentfor people with PD. Exercise, physical activity, and physiotherapy are the key points to this program in order to improve balance stability and stimulate neuroprotection which was suggested by some researchers to reduce neurodegeneration.3 Aquatic therapy, a form of water-based physical activity, has become one of the focuses of medical research becauseof this innovative treatment for people with a freezing gait in PD cases offers an effective outcome that also could reduce the fear of falls as a common barrier of treatment. Aquatic therapy has proven to induce a neuroprotective system that willdecreasethedevelopmentof neurological disorder effectively and improve balance and functional mobility.5 Although several recent randomized controlled trials6-8 have been conducted to prove theeffectiveness of this intervention, to our knowledge to date, no review has systematically synthesized its benefits for Parkinson’s disease. Hence, this systematic review aims to assess the effects of aquatictherapytowards.
Methods
Study Design
We conducted a systematic review and meta-analysis based on the Cochrane Handbook for SystematicReviewsofInterventions6.2,reportedinaccordancetothePRISMAchecklist.9

Search strategy
We conducted a comprehensive literature search on PubMed, Scopus, Cochrane, and Wiley Library using the PRISMA framework. We collected data on 10 April 2023, using the keywords (water training OR water exercise OR aquatic therapy) AND (older adult OR geriatr*) AND (functionality OR mobility), as well as their respective medical subject headings (MeSH) terms. The keywords used in the pursuitwerecustomizedaccordingtothe database used, as shown in Appendix 1 Suitable advanced search techniques were applied wheneverappropriate.TheplannedprocedureisillustratedinFigure1
Figure1.Diagramflowofliteraturesearchstrategy
Study eligibility criteria
In accordance to the PICOS framework (patient/problem, intervention/exposure, comparison/control, outcome), the inclusion criteria included (1) population: elderly with Parkinson’s disease; (2) intervention: aquatic therapy; (3) outcomes: functionality and mobility parameters;(4)usualcareorland-basedtherapyasthecontrol;and(5)studydesign: randomized-controlled trials (RCTs). In the meanwhile,theexclusioncriteriaforthiswereas follows:
(1) outcomes in qualitative measures; (2) incomplete studiesatthetimeofretrieval;
(2) irretrievable full-text articles;and(3)studiespublishedinlanguagesotherthanEnglishas the international language. Additionally, the Mendeley software was used to eliminate duplicates. Three independent reviewers selected the titles and abstracts of papers based on criteria regarding their accessibility(ST,NA,VA).Anydisputeswerediscussedbythefourth authortoreachaconsensus(MAZA).
Data extraction
In tabular form, we extracted the following predetermined outcomes into an outcome sheet:
(1) author and year of publication; (2) study study location; (3) study population, including characteristics, sample size and gender distribution, as wellasage;(4)controltreatment,and
(5) intervention, including the types, frequency, and follow-up interval. Studycharacteristics were assessed qualitatively by two reviewers (VA and MAZA) while two other authors (ST andNA)recheckedtheaccuracyoftheextracteddatawhileperformingstatisticalanalysis.
Quality assessment and Publication Bias
The Revised Tool for Risk of Bias in Randomized Trials (RoB 2.0), which consists of five areas for initiative studies, was used to assess the potential for bias in the final studies that were included in the analysis. Using the formula that was developed by the Cochrane Collaboration, authors conducted the bias assessment; findings were enteredintothedomain file bias spreadsheet (.xlsx). Following this step, the file was uploaded to the ROBVIS website to facilitate the accurate visualization of the final results. Four reviewers independently assessed the quality of studies; discrepancies were discussed and resolved by consensus.QualityassessmentofthestudiesaredepictedinAppendix2.
Quantitative data analysis
Statistical analysis was performed using Review Manager ver 5.4 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen). The main resultweusedinthestatistical analysis was the mean differencebetweenpreandpost-interventionusingaquatictherapyfor older adults, shown by assessment scores of each respective scale, as well as the mean differences between intervention and control group patients. Mean difference (MD) with a 95% confidence interval and its respective p-value was used to determine the efficacy of aquatic therapy, presented in forest plots. We used inverse variance, the DerSimonian-Laird random-effects model as proposed by Riley et al.,10 since we considered that heterogeneity outside the study could also be discovered fromstudies.Heterogeneitywasfurtherevaluated using I2 statistics based on Cochrane threshold, with cut-off limits of 0%, 25%, 50%, and 75% as insignificant, low, moderate, and high heterogeneity, respectively.9 Additionally, we
also performed sensitivity analysis following Duval and Tweedie’s trim-and-fill method to identifyanyoutlierstudyifhighheterogeneitywasdetected.
Results
Search Results and Study Characteristics
Quantitative and qualitative analyses were conducted on 9 included RCTs,2,5-8,11-14 yielding 277 participantsintheinterventiongroupandcontrolgroupwhoweretreatedwithoutaquatic therapy Trials were conducted in various countries (Ireland, Spain,Turkey,Italy,Brazil,and China) and published from2011to2018withwater-basedinterventionsinvariousnames(Ai Chi, in-water training, etc.). Subjectswerefurtherrandomizedintoaninterventiongroupora controlgroupwithstandardofcareorland-basedtherapy.Detailedcharacteristicsofincluded studiesarelistedinAppendix3.
Study Outcomes in terms of BBS (BergBalanceScale)
The effectiveness of using aquatic therapy compared to control in increasing BBS scores is shown in Figure 2 Our meta-analysis demonstrated that aquatic therapy significantly increased BBS scores with a low heterogeneity of 44%, yielding a mean difference value of 2.64(p=0.04;95%CI:0.11-5.16;I2=44%).
Meanwhile, BBS scores were also increased post-intervention compared to pre-intervention with 33% heterogeneity and a mean difference of 6.67 (p<0.00001; 95% CI: 4.65-8.69; I2=33%),asshowninFigure3


Study Outcomes in terms of DGI (Dynamic Gait Index)
The effectiveness of using aquatic therapy compared to control in increasing DGI scores is shown in Figure 4 Our meta-analysis demonstrated that aquatic therapy significantly
Figure2.ForestplotshowingeffectivityofaquatictherapyinincreasingBBSscores betweengroups
Figure3.Forestplotshowingeffectivityofaquatictherapyinincreasingpost-intervention BBSscorescomparedtopre-intervention
increased DGI scores with neglected heterogeneity, yielding an increased mean of 4.93 (p<0.00001;95%CI:2.89-6.98;I2=0%).

Meanwhile, DGI scores were also increased post-intervention compared to pre-intervention with no heterogeneity found and a mean difference of 4.98 (p<0.00001; 95% CI: 3.49-6.47; I2=0%),asshowninFigure5


Study Outcomes in terms of UPDRS (Unified Parkinson's Disease Rating Scale) III
The effects of using aquatic therapy towards UPDRS III scores are shown in Figure 6. Our quantitative analysis demonstrated that aquatic therapy slightly reduced UPDRS III scores with no heterogeneity found, yielding a mean difference value of -0.89 (p=0.33; 95% CI: (-2.68)-(0.90);I2=0%)whichisnotstatisticallysignificant.
Meanwhile, UPDRS III scores were significantly decreased post-intervention compared to pre-intervention with no heterogeneity found and a mean difference of -4.35 (p<0.00001; 95%CI:(-6.19)-(-2.51);I2=0%),asshowninFigure7.
Figure4.ForestplotshowingeffectivityofaquatictherapyinincreasingDGIscoresbetween groups
Figure5.Forestplotshowingeffectivityofaquatictherapyinincreasingpost-intervention DGIscorescomparedtopre-intervention
Figure6.ForestplotshowingtheeffectofaquatictherapytowardsUPDRSIIIscores betweengroups
Study Outcomes in terms of UPDRS Total
The equivocal effects ofaquatictherapytowardsUPDRSTotalscoresareshowninFigure8. Through our meta-analysis, we found thataquatictherapyyieldedameandifferencevalueof 0.44(p=0.99;95%CI:(-7.08)-(7.95);I2=0%)comparedtoitsrespectivecontrolgroup.
Meanwhile, UPDRS Total scores were significantly decreasedpost-interventioncomparedto pre-intervention with no heterogeneity found and a mean difference of -8.71 (p=0.03; 95% CI:(-16.77)-(-0.65);I2=0%),asshowninFigure9.


Study Outcomes in terms of TGUG
Furthermore, we also did the meta-analysis based on Time Get Up and Go (TGUG) Test (Figure 4), showing significant reductions (p=0.003) in decreasing TGUG scores with neglected heterogeneity (I2=0%) with a mean difference of -1.72 (95% CI: -2.85-(-0.58); I2=0) compared to the control group, as shown in Figure 10 From the meta-analysis, itwas found that aquatic therapy reduced TGUG score compared to pre-intervention with a MDof 3.02(95%CI:-4.06-(-1.97);I2=0),asshowninFigure11.
Figure7.ForestplotshowingtheeffectofaquatictherapytowardsUPDRSIIIscores comparedtopre-intervention
Figure8.ForestplotshowingtheeffectofaquatictherapytowardsUPDRSTotalscores betweengroups
Figure9.Forestplotshowingtheeffectofaquatictherapytowardspost-interventionUPDRS Totalscorescomparedtopre-intervention
Study Outcomes in terms of VAS (Visual Analog Scale)
The effectiveness of using aquatic therapy compared to control in reducing VAS scores is shown in Figure12 Ourquantitativeanalysisdemonstratedthataquatictherapysignificantly reduced VAS scores with no heterogeneity found, yielding a mean difference value of -1.20 (p=0.0001;95%CI:(-1.81)-(-0.59);I2=0%).
Meanwhile, VAS scores were also reduced post-intervention compared to pre-intervention with 0% heterogeneity and a mean difference of -1.40 (p=0.0004; 95% CI: (-2.18)-(-0.62); I2=0%),asshowninFigure13.



 Figure10.ForestplotshowingeffectivityofaquatictherapyinreducingTGUGscores betweengroups
Figure11.Forestplotshowingeffectivityofaquatictherapyinreducingpost-intervention TGUGscorescomparedtopre-intervention
Figure12.ForestplotshowingeffectivityofaquatictherapyinreducingVASscoresbetween groups
Figure13.Forestplotshowingeffectivityofaquatictherapyinreducingpost-intervention VASscorescomparedtopre-intervention
Figure10.ForestplotshowingeffectivityofaquatictherapyinreducingTGUGscores betweengroups
Figure11.Forestplotshowingeffectivityofaquatictherapyinreducingpost-intervention TGUGscorescomparedtopre-intervention
Figure12.ForestplotshowingeffectivityofaquatictherapyinreducingVASscoresbetween groups
Figure13.Forestplotshowingeffectivityofaquatictherapyinreducingpost-intervention VASscorescomparedtopre-intervention
Discussions
Aquatic Therapy Interventions
Although aquatic therapyislessconsideredforPDtherapy,itseffectstoimprovestabilityare quite positive. A hydrotherapy pool environment can help PD patients feel less afraid of falling; keepingthewarmtemperatureofthepoolcouldgiveasenseofrelaxationandhelpin reducing muscle tone, which would be beneficial to increase the progress and lower the freezing of gait in PD patients.5 Previous studies comparing water-based and land-based therapy took 45 minutes of exercise for twice-weekly sessions in 4 weeks that assess the participants progression by stability of posture, body movement, and mobility of trunk in both have same step of therapy activities.1 Frequencies also vary from twice weekly in most studies to four times in five days7 , or even five times a week8; durations range from 3013 to 607,8 minutes per session. Meanwhile, follow-up intervals range from 17 days12 to 6 months.7,8,13
A comparable method of water-based therapy is called Ai chi, another form of tai chi that provides expansive mobility and concentrates on deep-breathing therapy that tends to enhance dynamic balance more effectively than land-based therapy. With 16 steps of movement variations and intervention of hydrostatic pressure and water buoyancy, ai chi could be concluded as a program that is firmly believed to have a positive impact on motor function. According to European guidelines, the continuation of the therapy program is a priority and the water buoyancy concept of reducing gravity and improvement of geriatric conveniencealongthetherapycanbeanotheradvantageofthiswater-basedtherapy.5
There is also study by Silva et al. that focuses on midbrain dysfunction whichleadingtothe loss of dopaminergic neuron function. This neuronregulatescontrolofmotoricactivitiesand that usually needs to coordinate with cognitive function as usually appears in our daily activities. Using this concept, dual task is defined astheperformanceofneuronalfunctionin combination with physical activities therapy that must be combated on PD patients, since performing both physical and brain activities at the same time may result in the loss of motoric control and reduce automaticity of brain-motor function. Focusing more on body movementpracticesaidintheactivationoftheintactportionofthepremotorcortex,resulting inanincreaseofnormalmovementandhighbalancequality 6
Effectiveness of Aquatic Therapy in Improving Motoric Function
Aquatic therapy can be beneficial for improving motoric function, as the buoyancy and resistance of water provide a low-impact environment for exercising and strengthening muscles. This can be particularly beneficial for individuals with conditions that limit their ability to exercise on land, such as elderly with joint pain or neurological disorders.15 This meta-analysisassessestheeffectspecificallyonseveralmotoricfunction:
1. BBS
The results of the study indicate a positive impact of aquatic therapy intervention on the population's BBS score, surpassing both other groups and pre-intervention scores. From the pre-intervention’sBBSscorewecouldinterpretthatseveralstudies'populationsareinthe category of needing assistance. The initial BBS scores suggest that several participants required assistance, with scores ranging between 21 to 40.16 However, after undergoing the intervention, the study conducted by Zhu Zhizong, et al was the only one to register a score of 39. Study by Zivi Ilaria, et al shows themostsignificantincreasingscorefrom36to51in its post-intervention BBS score. This meta analysis show an almost clinically significant score of 2.64, with significant BBS was 3 points.17 In comparison to the control group, the mean BBS score difference ranged from 0.6 (palamara grazi) to 10.0 (zivi ilaria). Furthermore, along with the increasing BBS score, study shows balance impacts self-confidence related to preventing falls, decreased anxiety regarding falling, increasing walkingpace,andimprovedphysicalcapabilities.18
2. DGI
Studies bySilvaAVandIsraelVLandZiviIlaria,etalbothshowsignificantimprovementof DGI score after intervention and compared to other groups. Study show a clinically significant result of bigger than its minimum being 0.6 with subject of score under 22.19 Study shows that the clinically significant DGI score prudently establishes at more than 7 points,howeverminimumdetectablechangeis2.9DGIpointsshowingthatitdoesaffectgait function.20
3. UPDRSIII
In this studies, both pre- and post-intervention assessments using the UPDRS III showed a decrease in motoric symptoms. The post-intervention scoreshowedasignificantreductionin motoric symptoms compared to the pre-intervention score. This is a positive outcome that suggests the intervention has had a beneficial effect on the individual's motoric symptoms. The minimal decreasing number of UPDRSIIIclinicallysignificantat3.25points,thisstudy showsasignificanceof-4.35.21
4. UPDRSTotal
While the studies conducted by Cruzl and Vivas Jamile, et al, did not show statistical significance in terms of the UPDRS Total number, the mean difference of 8.71 suggests a noteworthy decrease in motoric symptoms. Although the mean difference did not meet the threshold for clinical significance, it was close enough to 8.97 to be considered almost clinicallysignificant.22
5. TGUG
According tothestudies,postaquaticinterventionhasresultedinasignificantdecreaseinthe time taken for TGUG test that evaluate overall functional mobility.Theresultsofthestudies indicate that most of the study population did not fall within the normal time rangeofunder 10 seconds, with only a few studies reporting normal times. A reductionintimegreaterthan 0.8 seconds isconsideredclinicallysignificant,studiesshowedasignificantreductionof3.02 seconds after the aquatic intervention. These findings suggest that aquatic intervention may
be an effective intervention for improving functional mobility in individuals with mobility impairments.23
Aquatic Therapy for Pain Alleviation
Both studies by Cruz et al. included VAS as one of the outcomes, together with a mean reduction of around 12–14 mm of VAS according to our meta-analysis.3,5 Old studies would consider this clinically significant as it is over 9 mm;24 althoughstandardsmaybehigherfor patients with severe pain (VAS scores of 6 to 7), a more recent publication considers a clinically significant change to be 13 mm for patients with VAS scores of 3 to 4.25 Hence, aquatictherapysignificantlyalleviatespain,bothstatisticallyandsignificantly
Despite the lack of it being discussed, pain alleviation is crucial for fall prevention in Parkinson’s disease as it interferes with the cognitive processes through distraction.3 Pain significantly increased the odds of falling, according to meta-analyses.26 and case reports.27 With lower pain scores, patients have less distractors, eventually leading to better balance, bettergait,andlessofthedepressivesymptomsoftencomingwithPD.5,28
Advantages of Aquatic Therapy
Exercise therapy can benefit from the helpful and water-resistant properties of water, where the density, temperature, and hydrostatic pressure of water aid in movement. The buoyant force of water can alleviate joint pain and reduce weight-bearing load on the body
Additionally, the density of water forces the body upwards, high water temperature can stimulatemusclestretching,andhydrostaticpressurecanreduceedemadevelopment.
29,30
The study conducted by Sizoo et al. revealed that aquatic exercise is highly enjoyable, with four participants experiencing high levels of enjoyment and five participants experiencing complete enjoyment. Additionally, theuseofaquaticexercisehelpspatientswithPDtoboost their confidence and self-efficacy Furthermore, peer support has a positive impact on patients,enablingthemtoimprovetheirsupport-relatedoutcomes.
29,30
Aquatic therapy is also a cost-effective rehabilitation method, as evidenced by a study conducted by Teng et al. The research revealed that aquatic therapy was associated with an ICER (incremental cost-effectiveness ratio) of SGD 27,471 per QALY (quality-adjusted life-year) gained.Therefore,aquatictherapyisanaffordableoptionforrehabilitation.31
Strengths and Limitations
As this meta-analysis serves as intervention evidence, a strength would be its exclusivity towards selecting randomized controlled trials, the gold standard of its design. We also believe that this is the firstsystematicreviewandmeta-analysisassessingvariousparameters of aquatic therapy benefits towards Parkinson’s disease. The insignificant or low heterogeneity on all meta-analyses also provide stronger evidence for this intervention. We analyzed not only whether the effects are quantitatively statistically significant, but qualitatively clinically significant. Furthermore, included studies were done in diverse countries, suggesting higher generalizability of our findings. However, the variety of
frequencies have not been served with a subgroup meta-analysis. The lack of consensus on the best outcome measures also lead to differing outcome measures, a challenge to the meta-analysis.LanguageofincludedstudieswasalsolimitedtoEnglish.
Conclusion
Aquatic therapy significantly improved various motoric parameters and alleviated pain,both statistically and clinically Balance (BBS), gait (DGI), time to get up and go (TGUG), and pain (VAS)scaleswereimprovedstatisticallyandclinically However,UPDRSscorechanges were not as prominent. As it reduces gravityandthefearoffallsbydesign,thisintervention, together with its enjoyment and cost-effectiveness, offers a promising advantage for elderly patients. Further studies with standardized outcomes, as well as studies on implementation, will be beneficial to the field. We recommend aquatic therapy as one of the top non-pharmacologicaltreatmentsincorporatedintoguidelinesforParkinson’sdisease.
AcknowledgementsandConflictofInterest
Theauthorsdeclarethattheyhavenoconflictsofinterestinthiswork.
References
1. Parkinson disease [Internet]. World Health Organization. World HealthOrganization; [cited 2023 Apr 13]. Available from:
https://www.who.int/news-room/fact-sheets/detail/parkinson-disease#:~:text=The%20 prevalence%20of%20PD%20has,of%20over%20100%25%20since%202000
2. Carroll LM, Volpe D, Morris ME, Saunders J,CliffordAM.Aquaticexercisetherapy for people with Parkinson disease: a randomized controlled trial. Arch Phys Med Rehabil [Internet]. 2017 Apr [cited 2023 Apr 11];98(4):631-8. Available from:
https://linkinghub.elsevier.com/retrieve/pii/S0003-9993(17)30002-3 doi: 10.1016/j.apmr.2016.12.006.
3. Pérez de la Cruz S. Effectiveness of aquatic therapy for the control of pain and increased functionality in people withParkinson'sdisease:arandomizedclinicaltrial. Eur J Phys Rehabil Med [Internet]. 2017 Dec [cited 2023 Apr 11];53(6):825-32. doi: 10.
4. Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: A Review. JAMA [Internet]. 2020 Feb 11 [cited 2023 Apr 11];323(6):548-60. Available from: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2019.22360 doi: 10.1001/jama.2019.22360.23736/S1973-9087.17.04647-0.
5. Pérez-de la Cruz S. A bicentric controlled study on the effects of aquatic Ai Chi in Parkinson disease. Complement Ther Med [Internet]. 2018 Feb [cited 2023 Apr 11];36:147-53.doi:10.1016/j.ctim.2017.12.001.
6. Silva AZ, Israel VL. Effects of dual-task aquatic exercises on functional mobility, balance and gait of individuals with Parkinson's disease: A randomized clinical trial
with a 3-month follow-up. Complement Ther Med. 2019 Feb;42:119-124. doi: 10.1016/j.ctim.2018.10.023.
7. Palamara G, Gotti F, Maestri R, Bera R, Gargantini R, Bossio F, et al. Land plus aquatic therapy versus land-based rehabilitation alone for the treatment of balance dysfunction in parkinson disease: a randomized controlled study with 6-month follow-Up. Arch Phys Med Rehabil. 2017 Jun;98(6):1077-85. doi: 10.1016/j.apmr.2017.01.025.
8. Kurt EE, Büyükturan B, Büyükturan Ö, Erdem HR, Tuncay F Effects of Ai Chi on balance, quality of life, functional mobility, and motor impairment in patients with Parkinson's disease. Disabil Rehabil. 2018 Apr;40(7):791-7. doi: 10.1080/09638288.2016.1276972.
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline forreportingsystematicreviews. BMJ [Internet]. 2021 Mar 29 [cited 2022 Oct 22];372:n71. Available from:
http://www.ncbi.nlm.nih.gov/pmc/articles/pmc8005924/doi:10.1136/bmj.n71
10.Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ [Internet]. 2019 Jan 30 [cited 2022 Oct 11];364. Available from: https://www.bmj.com/content/364/bmj.k4597
11. Carroll LM, Volpe D, Morris ME, Saunders J,CliffordAM.Aquaticexercisetherapy for people with Parkinson disease: a randomized controlled trial. Arch Phys Med Rehabil [Internet]. 2017 Apr [cited 2023 Apr 11];98(4):631-8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003-9993(17)30002-3 doi: 10.1016/j.apmr.2016.12.006.
12.Vivas J, Arias P, Cudeiro J. Aquatic therapy versus conventional land-based therapy for Parkinson's disease: an open-label pilot study. Arch Phys Med Rehabil. 2011 Aug;92(8):1202-10.doi:10.1016/j.apmr.2011.03.017.
13.Zhu Z, Yin M, Cui L, Zhang Y, Hou W, Li Y, Zhao H. Aquatic obstacle training improves freezing of gait in Parkinson's disease patients: a randomized controlled trial.ClinRehabil.2018Jan;32(1):29-36.doi:10.1177/0269215517715763.
14.Zivi I, Maffia S, Ferrari V, Zarucchi A, MolatoreK,MaestriR,etal.Effectivenessof aquatic versus land physiotherapy in the treatment of peripheral neuropathies: a randomized controlled trial. Clin Rehabil. 2018 May;32(5):663-70. doi: 10.1177/0269215517746716.
15.Shariat A, Ghayour Najafabadi M, Ghannadi S, Nakhostin-Ansari A, HakakzadehA, Shaw BS, Ingle L,ClelandJA.Effectsofaquatictherapyonbalanceinolderadults:a systematic review and meta-analysis. Eur Geriatr Med. 2022Apr;13(2):381-393.doi: 10.1007/s41999-021-00577-2.
16.Cleveland Clinic. Berg balance scale [Internet]. Cleveland Clinic; [cited 2023 Apr 13]. Available from: https://my.clevelandclinic.org/health/diagnostics/22090-berg-balance-scale
17.Gervasoni E, Jonsdottir J, Montesano A, Cattaneo D. Minimal Clinically Important Difference of Berg Balance Scale in People With Multiple Sclerosis. ArchPhysMed Rehabil.2017Feb;98(2):337-340.e2.doi:10.1016/j.apmr.2016.09.128.
18.Halvarsson A, Dohrn IM, Stähle A. Taking balance training for older adults onestep further: the rationale for and a description of a proven balance training programme. Clin Rehabil (Internet]. 2015 May [cited 2023 Apr11];29(5):417-25.Availablefrom: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4419050/ doi: 10.1177/0269215514546770.
19.Corrini C, Torchio A, Anastasi D, Parelli R, Meotti M, Spedicato A, et al. Minimal clinically important difference of modified dynamic gait index in people with neurological disorders. Gait Posture. 2021 Oct;90:210-214. doi:10.1016/j.gaitpost.2021.08.024.
20.Romero S, Bihop M, Light K. Minimumdetetctablechangeodthebergbalancescale and dynamic gait index in older person at riskforfalling.GeriatricPhysicalTherapy. 2011.doi:10.1519/JPT.0b013e3182048006
21.Horváth K, Aschermann Z, Ács P, Deli G, Janszky J,KomolyS,BalázsÉ,TakácsK, Karádi K, Kovács N. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism Relat Disord. 2015 Dec;21(12):1421-6.doi:10.1016/j.parkreldis.2015.10.006.
22.Makkos A, Kovacs M, Pinter D, Janszky J, Kovacs N. Minimal clinically important difference for the historic parts of the unified dyskinesia rating scale. Parkinsonism andRelatedDisorders.doi:10.1016/j.parkreldis.2018.08.018
23.Gautschi OP, Stienen MN, Corniola MV, JoswigH,SchallerK,HildebrandtG,Smoll NR. Assessment of the minimum clinically important difference in the timed up and go test after sirgery for lumbar degenerative disc disease. Neurosurgery 2017 Mar 1;80(3):380-385.doi:10.1227/NEU.0000000000001320.
24.Kelly AM Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or causeofpain?AcadEmergMed[Internet].1998Nov[cited 2023Apr11];5(11):1086-90.doi:10.1111/j.1553-2712.1998.tb02667.x.
25.Sadovsky R. Clinically important changes in pain severity on VAS. Am Fam Physician [Internet]. 2002 [cited 2023 Apr 11];65(9):1916-21. Available from: https://www.aafp.org/pubs/afp/issues/2002/0501/p1917.html
26.Stubbs B, Binnekade T, Eggermont L, Sepehry AA, Patchay S, SchofieldP.Painand the risk for falls in community-dwelling older adults: systematic review and meta-analysis. Arch Phys Med Rehabil [Internet]. 2014 Jan [cited 2023 Apr 12];95(1):175-187.e9. Available from: https://core.ac.uk/reader/17345794?utm_source=linkout doi: 10.1016/j.apmr.2013.08.241.
27.Tinetti ME, Kumar C. The patient who falls: "It's always a trade-off". JAMA [Internet]. 2010 Jan 20 [cited 2023 Apr 12];303(3):258-66. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3740370/ doi: 10.1001/jama.2009.2024.
28.Cholewa J,Boczarska-JedynakM,OpalaG.Influenceofphysiotherapyonseverityof motor symptoms and quality of life in patients with Parkinson disease. Neurol NeurochirPol[Internet].2013May-Jun[cited2023Apr11];47(3):256-62.
29.Sizoo SJM, Akkerman M, Trommel N, Esser JJPH, Veen-van der Velden M, Oen IMMH, et al. Feasibility and acceptabilityofaquaticexercisetherapyinburnpatients
A pilot study. Burns Open [Internet]. 2021;5(1):10–20. Available from: https://www.sciencedirect.com/science/article/pii/S2468912220300511
30.Terrens AF, Soh SE, Morgan P The safety and feasibility of a Halliwick style of aquatic physiotherapy for falls and balance dysfunction in people with Parkinson's Disease: A single blind pilot trial. PLoS One. 2020 Jul 30;15(7):e0236391. doi: 10.1371/journal.pone.0236391.PMID:32730325;PMCID:PMC7392279.
31.Teng M, Zhou HJ, Lin L, Lim PH, Yeo D, Goh S, et al. Cost-effectiveness of hydrotherapy versus land-based therapy in patients with musculoskeletal disorders in Singapore. J Public Health (Oxf) [Internet]. 2019;41(2):391–8. Available from: http://dx.doi.org/10.1093/pubmed/fdy044
–

Appendix Appendix1.Keywordsdetailsineachdatabases Database Keywords Hits PubMed (watertrainingORwaterexerciseORaquatictherapy)AND(older adultORelderlyORgeriatr*)AND(functionalityORmobility) 242 Scopus TITLE-ABS-KEY(“watertraining”OR“waterexercise”OR “aquatictherapy”)ANDTITLE-ABS-KEY("olderadult" OR “elderly”OR“geriatr*”)AND(“functionality”OR“mobility”) 568 Cochrane ("visualtherapy"OR"Arttherapy"ORcreative)AND("older adult"OReldery)AND(cognitive) 13 Wiley ("visualtherapy"OR"Arttherapy"ORcreative)AND("older adult"OReldery)AND(cognitive) 165 Appendix2.
StudiesqualityassessmentbasedonCochraneRoB2.0
Appendix3.Characteristicsofincludedstudies


ScreeningPerformanceofSARC-F+EBMasaNovel AlternativeScreeningToolforSarcopeniaComparedto SARC-F:ASystematicReviewandMeta-Analysis
Michael Antonio Boentoro1 , Karen Graciana Setiawan1 , Btari Larasati1 , Emir Gibraltar Faisal1
1FacultyofMedicine,UniversitasIndonesia
ABSTRACT
INTRODUCTION: Sarcopenia is a condition, where skeletal muscles gradually lose mass and strength. Early detection and proper diagnosis of sarcopenia is crucial as it is one ofthe primary health concerns among older individuals. Screening tools, like SARC-F and SARC-F+EBM, are a more practical and affordable alternative for identifying individuals at riskofsarcopenia.
OBJECTIVE: The objective of this study is to provide the result about the performance of SARC-F+EBM as a screening tool for Sarcopenia compared to SARC-F. We evaluate the sensitivity,specificity,andAUCofSARC-F+EBM.
METHOD: Seven databases were sought and obtained papers that screened based on specific eligibility criteria. The data were extracted using a two-by-two table sample, which included TP, FP, TN, and FN, and analyzed using an alternative approach for two-way contingency table analysis, which served as the predictive value. We assessed the quality usingQUADAS-2andstatisticalanalysissoftware.
RESULTS AND DISCUSSIONS: The pooled sensitivities of SARC-F+EBM are substantially greater than SARC-F, with sensitivity of 68.2% (95% CI 55.6-97.1%) and 37.0% (95% CI 26.0-49.1%), respectively. The addition of sarcopenia factors, such as age and body mass index, boost SARC-F's sensitivity. The pooled specificities of SARC-F and SARC-F+EBM are similar, at 71.4%(95%CI68.6-74.0%)and70.9%(95%CI68.2-73.6%), respectively Both SARC-F+EBM and SARC-F are relatively good at ruling out people without sarcopenia. The AUC of SARC-F is 0.247, while the AUC of SARC-F+EBM is 0.7742. The higher AUC value of SARC-F+EBM shows a better diagnostic performance comparedtoSARC-F.
CONCLUSION: This meta-analysis demonstrates that SARC-F+EBM is a superior sarcopenia screening method to SARC-F. It is shown by the significantly higher sensitivity andhigherAUCthanSARC-F.
KEYWORDS: SARC-F+EBM, Sarcopenia, Geriatric, Screening tool
ScreeningPerformanceofSARC-F+EBMasaNovel AlternativeScreeningToolforSarcopeniaComparedto SARC-F:ASystematicReviewandMeta-Analysis
Authors:
MichaelAntonioBoentoro
KarenGracianaSetiawan

BtariLarasati
EmirGibraltarFaisal
AMSA-UNIVERSITASINDONESIA
2023
Sarcopenia is a condition where skeletal muscles gradually lose mass and strength, and is a result of a combination of factors.1 Typically, this condition is associated with aging, where there is an imbalance between muscle protein anabolic and catabolic pathways, causing an overall loss of skeletal muscle. Although the average age of individuals diagnosed with sarcopenia is around70yearsforbothmalesandfemales,thedeclineinmusclemassstartsat 40 years of age.Numerousstudieshavebeenconducted,involving263intotal,thatshowthe prevalence of sarcopenia ranging from 0.2% to 86.5% depending on the diagnostic criteria used. Additionally, the prevalenceofsarcopeniavariesbysetting,withnursinghomeshaving aprevalenceof41%,whichisfourtimeshigherthancommunitydwellingindividuals.2,3,4,5
The adverseeffectsthatsarcopeniahasonindividuals'health,bothintheshortandlongterm, contribute totheoverallburdenofthedisease.4 Thosewithsarcopeniaareatanincreasedrisk of falls and fractures,andhavedifficultyperformingdailyactivities.Additionally,sarcopenia is associated with cardiac disease, respiratory disease, cognitive impairment, mobility disorders, lower quality of life, loss of independence, and higher likelihood of needing long-term care placement. The presence of sarcopenia also increases the risk of hospitalization and raises the cost of care during hospitalization. A study found that older adults with sarcopenia on admission were more than 5 times more likely to incur higher hospital costs than those without sarcopenia.1 Overall, sarcopenia is a complex disease that progressivelyworsensandhasahigherburdenofmorbidityandmortality.3
Early detection and proper diagnosisofsarcopeniaiscrucialasitisoneoftheprimaryhealth concerns among older individuals.2,6 According to the 2019 guidelines by the European Working Group onSarcopeniainOlderPeople(EWGSOP2),sarcopeniacanbediagnosedby evaluatingmusclemassandfunction.However,someofthemethodsusedfordiagnosis,such as CT, MRI, and DXA, are complex, time-consuming, expensive, and involve radiation. Therefore,usingthesemethodsforroutineassessmentofsarcopeniaintheelderlymaynotbe feasible. Screening tools are a more practical and cost-effective alternative for identifying individualsatriskofsarcopenia.7
The SARC-F questionnaire is the most current and widelyusedsarcopeniascreeningsurvey. Five areas are evaluated by the SARC-F questionnaire: strength, assistance with walking, rising from a chair, climbing stairs, andfalls.SarcopeniaischaracterizedbyaSARC-Fscore of 4. Although SARC F is a practical and simple test, it predicts sarcopenia with a verylow sensitivity and a very high specificity Itssensitivity,whichrangesbetween3.8%and33.3%, is a significant drawback because many sarcopenic patients may be missed. Additionally, a study reveals that the SARC-F's use is quite constrained and is only appropriate for usage with elderly patients inhospitalsornursinghomes.Thus,duetoitslowsensitivity,additional screeningmethodsarenowbeingresearched.6,8,9
According to a study conducted by Kurita et al in 2019, the addition of"EBM"(Elderlyand BMI) to the SARC-F screening tool resulted in an overall improvement in diagnostic
1. INTRODUCTION
accuracy and sensitivity for identifying sarcopenia.10 SARC-F+EBM takes into account the patient's age (75 years or older) and BMI (less than 21 kg/m2). Compared to the original SARC-F,theSARC-F+EBMhasahighersensitivityof55%andspecificityof70.7-71.6%.A score of 12 or more on the SARC-F+EBM indicates a positive screening for sarcopenia.11,12 However, there is limited research comparing the accuracy of SARC-F+EBM toSARC-Fas a screening tool for sarcopenia. Therefore, given the high disease burden of sarcopenia, this meta-analysis aims to investigate whether SARC-F+EBM is a more effective screening tool forsarcopeniacomparedtoSARC-F
2. MATERIALS&METHODS
Literature search
PubMed (Medline), Cochrane, Science Direct, EBSCOHost, ProQuest, Wiley, and SpringerLink databases were searched from inception to April 2023. To avoidsearch omissions, we includedtermswithwidermeaningssuchas“Elderly”OR“Geriatrics” to specify the references. The list of references of articles was also reviewed for any additional papers. The literature search was independently conducted by four reviewers(M.A,B.L,K.G,E.G),whohadreceivedsystematictraining.
Eligibilitycriteria
The inclusion criteria were as follows: (І) participants were 60 years old and above; (II) study involved the screening accuracy of SARC-F+EBM for sarcopenia and a two-by-two table, including true positives (TP), false positives (FP), true negatives (TN) and false negatives (FN), could be created; (III) the diagnostic criteria for sarcopenia was EWGSOP or EWGSOP2 or AWGS or FNIH or IWGS; (IV) the languages were limited to English.Theexclusioncriteriawereasfollows:(І)meeting proceedings, letters, reviews, commentary, editorials and grey literature; (II) insufficient and inaccurate data after contacting original authors; (III) duplicate literaturesoftheincludedtrialsorthefulltextwasnotavailable.
Literatureselection
After duplicate studies were removed, two reviewers (M.A, B.L, K.G, E.G) independently assessed the eligible studies according to the inclusion and exclusion criteria via screening titles and abstracts. Full-texts were retrieved when both reviewers agreed that studies were relevant or when consensus was notreached.Any disagreements were resolved by discussion, when consensus was not reached, athird reviewer(E.G)madethefinaldecision.
Dataextraction
Data were extracted from the included studies by two independent reviewers (M.A, B.L, K.G, E.G) using a structured data extraction form: authors, year, country, population, sample size, age, gender, cut-off value of SARC-F+EBM, diagnostic
criteria for sarcopenia, prevalence of sarcopenia in the sample and a two-by-two tables, including TP, FP, TN and FN.13 The statement notes that the provided data is incomplete. As a result, an alternative approach to estimating the two-by-two results is to use predictive data using 2-way contingency table analysis. This method computes various statistics from a 2-by-2 table. It will calculate the Yates-corrected chi-square, the Mantel-Haenszel chi-square, the Fisher Exact Test, and other indices relevant to various special kinds of 2-by-2 tables. This approach involves utilizing statistical models and machine learning techniques to estimate the missing data. The predictive models can be trained on a subset of the available data and then used to estimate the missing values. However, it is important to keep in mind that the accuracy of predicted data depends on both the quality of the training data and the chosen predictive model. Therefore, it is essential to select an appropriate modeland validatetheresultsbeforeusingpredicteddatatomakedecisions.13
Qualityassessment
As recommended by the PRISMA-DTA group, three reviewers (M.A, B.L, K.G) independently assessed the included studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2)14 from four domains comprising 3items: Patient Selection, Index Test, Reference Standard and FlowandTiming.Allincluded studies were assessed for risk ofbiasineachdomainandforapplicabilityconcernsin the first three domains, and each domain is judged as “high”, “low” or “unclear”. Disagreements would have been resolved by consensus or referral to a third author (E.G).
Statisticalanalysis
The screening accuracy of SARC-F+EBM for sarcopenia in the elderly was the primaryoutcomeforourmeta-analysis.Differentdiagnosticcriteriayieldvariabletest accuracy results of SARC-F, thus statistics analyses were performed for each diagnostic criterion. Heterogeneity among studies was tested using Cochrane’s Q statistic. The degree of heterogeneity was assessed using the I2 statistic, with I2 values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively The threshold effect was analyzed by visual assessment of the coupled forest plots of sensitivity and specificity. The Spearman correlation coefficient between the sensitivity and false-positive rates greater than 0.6 was assessed to indicateaconsiderablethresholdeffect.
The summary estimates of sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and their 95% confidence interval (CI) were calculated using hierarchical logistic regression modeling including bivariate modeling and hierarchical summary receiver operating characteristic (HSROC) modeling. For graphical presentation of the results, HSROC curveswith95%CIandpredictionregionwereplotted.
All statistical analyses were performed using the Revman 5.3 software (Cochrane Collaboration,Oxford,UK)andMeta-DiSc1.4software(UniversidadComplutense).
Databases Keywords Hit Pubmed "SARC"[AllFields]AND"F"[AllFields]AND("evidbased med"[Journal]OR"ebm"[AllFields])AND ("sarcopenia"[MeSHTerms]OR"sarcopenia"[AllFields]OR "sarcopenias"[AllFields])AND("geriatric"[AllFields]OR "geriatrics"[MeSHTerms]OR"geriatrics"[AllFields]OR ("aged"[MeSHTerms]OR"aged"[AllFields]OR "elderly"[AllFields]OR"elderlies"[AllFields]OR"elderly s"[AllFields]OR"elderlys"[AllFields])OR("aged"[MeSH Terms]OR"aged"[AllFields]OR("older"[AllFields]AND "adults"[AllFields])OR"olderadults"[AllFields]))
Table1.Keywordssearchedineachdatabases
3. RESULTSANDDISCUSSION
Search results and study characteristics
3 Cochrane Library SARCFEBMANDSarcopeniaANDElderlyORGeriatric
ScienceDirect SARCFEBMANDSarcopeniaAND ElderlyOROlder AdultORGeriatric 0 EBSCOHOST SARCFEBMANDSarcopeniaAND ElderlyOROlder AdultORGeriatric 1 SpringerLink SARCFEBMANDSarcopeniaAND ElderlyOROlder Adult 1 Wiley SARCFEBMANDSarcopeniaAND ElderlyOROlder Adult 0 EMBASE SARCFEBMANDSarcopeniaAND ElderlyOROlder Adult 1 ProQuest SARCFEBMANDSarcopeniaAND ElderlyOROlder Adult 2
0
From a total of 8 records identified following a detailed search, 3 studies with 1167 participants on SARC-F+EBM and 1125 participants on SARC-F were finally included (Figure5).ThecharacteristicsoftheincludedstudiesaresummarizedinTable2andTable3.
The quality assessments of the included studies are summarized in Appendix 2. Of these studies, the patient selection and flow and timing were low risk of bias, whereas reference standards weregenerallyassociatedwithunclearriskofbias.Andthemaincauseofhighrisk of bias in the index test domain was mainly to be thresholds not pre-specified. On the other hand, in terms of applicability concerns, although high applicability concerns (use of an adapted questionnaire rather than the validated SARC-F+EBM questionnaire) arose in the indextestdomain,overall,theapplicabilityconcernswerelow
The results of the evaluation of the quality of the studies included using QUADAS-2 are shown in Appendix 2 In the majority of the studiesincludedinourstudy,theindextestand reference standard were unclear risks of bias, whereas patient selection and flow and timing weregenerallyassociatedwithalowriskofbias.
Diagnostic performance of SARC-F+EBM
Using EWGSOP2 and AWGS as diagnostic criteria, the sensitivity of the individual studies was 77.8% (60.8-89.9%), 55.0 (31.5–76.9), 60.0% (35.7-4.55), respectively and the specificity of the individual studies was 69.6% (66.5-72.5%), 70.7 (60.7–79.4), 86.1% (76.8-92.0).WhileothercharacteristicsofthestudiessuchasSARC-FlistedintheTable3
Authors, Year Studyperiod SampleSize Prevalence KuritaN,etal (2019) August2016-February 2019 959 36/959 Krzymińska-Si emaszkoR,et al(2020) March-November2019 115 16/115 HaxVanessa, etal(2021) March-December2019 94 15/94
Table2.TableCharacteristics
Quality assesment
Authors,Year Sensitivity(95%CI) Specificity(95%CI) KuritaN,etal(2019) SARC-F+EBM=77.8% (60.8-89.9%)
SARC-F+EBM=69.6%
SARC-F=41.7%
(66.5-72.5%) SARC-F=68.5%
Krzymińska-SiemaszkoR,et al(2020)


HaxVanessa,etal(2021)
(25.5-59.2%) (65.4-71.5%)
SARC-F:37.5(15.2–64.6)
SARC-F+EBM:55.0 (31.5–76.9)
SARC-F=40.0%(19.8-64.2)
SARC-F+EBM=60.0% (35.7-4.55)
SARC-F:85.9(77.4–92.1)
SARC-F+EBM:70.7 (60.7–79.4)
SARC-F=81.0%(71.0-88.1)
SARC-F+EBM=86.1% (76.8-92.0)
SARC-F+EBM results
a. Pooled results of SARC-F+EBM on Specificity and Sensitivity
Analyses were performed to assess the result of SARC-F+EBM. The pooled results from 3 studies conducted with various diagnostic tools such as EWGSOP and AWGS with a summary sensitivity of 0.68 (95% CI; 0.56-0.79; I2=43%) and specificity 0.71 (95% CI; 0.68-0.74;I2=81.6%
b. SROC of SARC-F+EBM
Table3.TableCharacteristics
Figure1.PooledresultsofSARC-F+EBMonSpecificityandSensitivity
SARC-F results
a. Pooled results of SARC-F on Specificity and Sensitivity



Figure2.SROCSARC-F+EBM
Figure3.PooledresultsofSARC-FonSpecificityandSensitivity
Analyses were performed to assess the result of SARC-F. The pooled results from 3 studies conducted with various diagnostic tools such as EWGSOP and AWGS with a summary sensitivity of 0.37 (95% CI; 0.26-0.49; I2=43%) and specificity 0.71 (95% CI; 0.69-0.74; I2=91.9%
b. SROC results of SARC-F
Figure 2 displays the SROC curve with AWGS andEWGSOPasthereferencestandard.The ranges of SARC-F sensitivity and specificity in the studies with EWGSOP as the reference standard were 0.07 to 0.27 and 0.80 to 0.97, respectively (solid circle in Figure 2). The summary ROCcurverepresentstherelationshipbetweensensitivityandspecificityacrossthe includedstudieswitha95%confidenceellipseanda95%predictionellipse.
 Figure4.SROCSARC-F
Figure4.SROCSARC-F
Furthermore, the three studies used in writing (cross sectional) were analyzed quantitatively and qualitatively. The journals included in this systematic review span the years 2019-2021. In addition, the study's assessment of bias showed a bias that varied from lowtomoderately high,butmoststudiesshowedamoderatebiasvalue(Appendix2).
3.1DifferencesbetweenSARC-F+EBMandSARC-FScreeningTool
Sarcopenia is defined as the fast loss of skeletal muscle mass, muscle strength, and physical performance caused by old age or chronic disease. Furthermore, it can have negative consequences such as falls, fractures, functional incapacity, increased hospital admission rates, decreased quality of life, and even mortality 15 Screening may allow for the early
 Figure5.PreferredReportingItemsforSystematicReviewsandMeta-analyses(PRISMA) DiagramFlowchart
Figure5.PreferredReportingItemsforSystematicReviewsandMeta-analyses(PRISMA) DiagramFlowchart
detection of sarcopenia and the prevention of major health implications. The followingtools were used to assess the risk of sarcopenia in each subject studied: SARC-F and SARC-F+EBM.12
The SARC-F questionnaire was the first diagnostic tool, and it is now the screening tool suggested by EWGSOP2 and the International Conference on Sarcopenia and Frailty Research (ICSFR).7 The standard SARC-F includes five items that assess strength, walking assistance, ability to rise from a chair, climb stairs, and self-reported number of falls in the previous year (each item is scored between 0 and 2). The score runs from0to10,scoreof4 orhigherpredictedsarcopeniaandpooroutcomes.16
Three years later, in 2019, Kurita et al expanded the SARC-F questionnaire to include two routine sarcopenia predictors: older age and low BMI. SARC-F+EBM (where E stands for Elderly persons and BM is for Body Mass Index) is a modified versionofthequestionnaire. SARC-F+EBM investigates seven domains.ThefirstfiveitemsarethesameasinSARC-F 12 The sixth factor is age. Patients below 75 years old received 0 points, while those ≥75 received 10 points.16 Aging is a risk factor for sarcopenia because the aging-related denervation/reinnervation processhasamajorimpactonquantitativechangesinmuscle,such as muscle fiber loss and atrophy, resulting in overall muscle wasting and loss of muscle function as people get older 17 Finally, the seventh item is BMI, where patients who are not underweight (>21 kg/m2) earned zero points, and patientswhoareunderweight(≤21kg/m2) scored ten points. The scale runs from 0 to 30. A total score of ≥12 indicates positive sarcopenia screening.16 The inclusion of low BMI (≤21 kg/m2) in the questionnaire is particularly pertinent, as it iswidelyknownthatelderlypeoplewithlowBMIaremoreprone to sarcopenia than patients with higher BMI values. Furthermore, a lowBMI(≤21kg/m2)is connected with being underweight and undernourished, both of which increase the risk of sarcopenia. Vandewounde et al., who proposed the notion of Malnutrition-Sarcopenia Syndromein2012,explicitlydescribedsuchalink.12
3.2SensitivityDifferencesbetweenSARC-F+EBMandSARC-F
Sensitivityisthepercentageoftruepositivetestsamongallpatientswithacondition.Inother words,itistheabilityofatesttoproduceapositiveresultforadiseasedindividual.18
In this meta-analysis study, the pooled sensitivities of SARC-F+EBM are substantially greater than those of SARC-F, with sensitivity of 68.2% (95% confidence interval [CI] 55.6-97.1%) and 37.0% (95% CI 26.0-49.1%), respectively. The low sensitivity of SARC-F means that a high proportion of subjects with sarcopenia are overlooked if diagnosed using this questionnaire.12 Meaning, despite the fact that SARC-F is simple toperform,affordable, and has been validated in several populations, its low sensitivity16 makes it nonoptimal for use as a screening toolforsarcopeniainolderpersons.Meanwhile,ithasbeensuggestedthat the addition of sarcopenia factors such as age and low body mass index could boost the SARC-F'ssensitivity 19
Our findings are consistent with previous studies that indicate great sensitivity in SARC-F+EBM and low sensitivity in SARC-F In a study of 959 hospitalized Japanese patients with musculoskeletal disorders, they discovered that SARC-F+EBM was more sensitive than SARC-F (77.8% vs 41.7%, respectively).12 A systematic review and meta-analysis of 21,855 participants found that SARC-F had low to moderate sensitivity (28.9%-55.3%).19
3.3 SpecificityDifferencesbetweenSARC-F+EBMandSARC-F
The percentage of true negatives among every subject who does not have the condition or disease is referred to as specificity.Itisthetest'sorinstrument'scapacitytoproducenegative results or a normal range for subjectswhodonothavetheconditionordisease.Specificityis counted by dividing the number of truenegativesbythetotalofbothtruenegativesandfalse positives,thenmultipliedby100%.18
In this meta-analysis study, the pooled specificities of SARC-F+EBM and SARC-F are similar, with the specificity of SARC-F screening tool being slightly higher than SARC-F+EBM. SARC-F has a pooled specificity of 71.4%, with a 95% CI at 68.6-74.0%. Meanwhile, SARC-F+EBM has a pooled specificityof70.9%,witha95%CIat68.2-73.6%. These show that both the novel screening tool SARC-F+EBM and themorecommonlyused SARC-F screening tool are relatively good at ruling out people without sarcopenia. Both detecttruenegativesatapproximately71%,whileonlygivingfalsepositivesataround29%.
The pooled specificity of SARC-F in this study is in accordance with previous studies. A cross-sectional study in 2022 involving 689 subjects shows thatthespecificityofSARC-Fis ranging from 63.1% to 67.3%.15 Another study in 2021 involving 73 subjects shows thatthe specificity of SARC-F is 85.7%, with a 95%CIat73.8-93.6%.20 Toadd,asystematicreview and meta-analysis focusing on SARC-F, which involves 21,855 subjects, the specificity is moderate to high, ranging from 68.9-88.9%.19 Therefore if a patient's SARC-F screening results show non-sarcopenic (negative), sarcopenia can be ruled out without further testing, suchasdual-energyX-rayabsorptiometry(DEXA),whichrequiresx-rayexposure.12
On the other hand, according to the study by Hax et al in 2021, the specificity of SARC-F+EBM is 86.1%, with a 95% CI at 76.8-92.0%.16 However, other two studies by KuritaetalandKrzymińska-SiemaszkoetalshowthatthespecificitiesofSARC-F+EBMare 68.5%,rangingfrom65.4-71.5%,and70.7%,rangingfrom60.7–79.4%,respectively 10,12
3.4 AreaUndertheCurveDifferencesbetweenSARC-F+EBMandSARC-F
The receiver operating characteristic (ROC) curve is used to evaluate a test's overall diagnostic performance and to compare the results of two or more diagnostic tests. For all cut-off values measured from test results, the ROC curve connects the coordinate points by utilizing specificity as the x-axis and sensitivity as the y-axis. The area under the curve (AUC) is a popular way to assess theprecisionofdiagnostictests.TheclosertheROCcurve is to the upper left corner of the graph, the higher the test's accuracy because the sensitivity
and specificity are both one in the upper left corner. As a result, the AUC of the ideal ROC curve is 1. AUCmustbeabove0.5forthetesttobeconsideredmeaningfulasanAUCof0.5 is predictingthepresenceorabsenceofadiseasethroughanaccidentalmethod,liketossinga coin. It is considered fair if 0.7 ≤ AUC<0.8,goodif0.8≤AUC<0.9,andexcellentif0.9≤ AUC.21
This study shows that the AUC of SARC-F is only 0.247. This value is less than 0.5,which can be considered not meaningful. On the other hand, theAUCofSARC-F+EBMis0.7742, which is much higherthantheAUCofSARC-F.Thisvalueisconsideredfairasitisbetween 0.7 and 0.8. A higher AUC value shows a better diagnostic performance.21 This shows that SARC-F+EBMisabetterscreeningtoolcomparedtoSARC-F
This higher AUC value of SARC-F+EBM compared to SARC-F can be seen in all 3 of the included studies. The AUC values of SARC-F+EBM in Kuritaetal,Krzymińska-Siemaszko et al, and Hax et al are 0.824 (ranging from 0.762 to 0.886), 0.714 (ranging from 0.583 to 0.844), and 0.832 (ranging from 0.713 to 0.952), respectively. Meanwhile, all the AUC values of SARC-F in Kurita et al, Krzymińska-Siemaszko et al, and Hax et al are lower, at 0.557 (ranging from 0.452 to 0.662), 0.693 (ranging from 0.552 to 0.834), 0.588 (ranging from0.420to0.756),respectively.10,12,16
3.5StrengthsandLimitationsofTheStudy
Thisisthefirstsystematicreviewandmeta-analysisthat,toourknowledge,hasevaluatedthe performance of SARC-F+EBM in comparison with SARC-F as screening tools for sarcopenia. This meta-analysis also includes recent studies in the last 5 years. However,itis important to note several limitations of this study Firstly, the number of available studies is relatively limited, which may impactthegeneralizabilityofthefindings.Secondly,theuseof 2-way contingency table analysis as a predictive value for 2-by-2 tables may not be entirely accurate due to incomplete data provided by the author. Nevertheless, this study provides valuableinsightsintotheeffectivenessofSARC-F+EBMasascreeningtoolforsarcopenia.
4. ConclusionandRecommendations
In conclusion, this meta-analysis shows that SARC-F+EBM is a better screening tool for sarcopenia, compared to SARC-F. It has a significantly higher sensitivity and higher AUC than SARC-F. As a result, SARC-F+EBM should be considered for routine screening standardsforsarcopeniainthegeriatricpopulation.
However, further primaryresearchandstudies,especiallythoseinvolvingbiggerpopulations, are needed to validate our results about the diagnostic performance of SARC-F+EBM for sarcopenia.
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing.2018Sep24;48(1):16–31.
2. Papadopoulou SK. Sarcopenia: A Contemporary Health Problem among OlderAdult Populations.Nutrients.2020May1;12(5):1293.
3. Petermann‐Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta‐analysis.JournalofCachexia,SarcopeniaandMuscle.2021Nov23;
4. Cruz-JentoftAJ,SayerAA.Sarcopenia.TheLancet.2019Jun;393(10191):2636–46.
5. Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz‐Jentoft AJ, Dent E, et al. Sarcopenia: A Time for Action. An SCWD Position Paper Journal of Cachexia, SarcopeniaandMuscle.2019Sep15;10(5):956–61.
6. Ackermans LLGC, Rabou J, BasraiM,SchweinlinA,BischoffSC,CussenotO,etal. Screening, diagnosis and monitoring of sarcopenia: When tousewhichtool?Clinical NutritionESPEN.2022Apr;48:36–44.
7. Lu JL ., Ding LY ., Xu Q, Zhu S, Xu XY ., Hua HX ., et al. Screening Accuracy of SARC-F for Sarcopenia in the Elderly: A Diagnostic Meta-Analysis. The journal of nutrition,health&aging.2020Sep25;25(2):172–82.
8. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group forSarcopenia:2019ConsensusUpdateonSarcopeniaDiagnosisand Treatment. Journal of the American Medical Directors Association. 2020 Mar;21(3):300-307.e2.
9. Kera T, Kawai H, Hirano H, Kojima M, Watanabe Y,MotokawaK,etal.Limitations of SARC-F in the diagnosis of sarcopenia in community-dwelling older adults. ArchivesofGerontologyandGeriatrics.2020Mar;87:103959.
10.Kurita N, Wakita T, Kamitani T, Wada O, Mizuno K. SARC-F Validation and SARC-F+EBM Derivation in Musculoskeletal Disease: The SPSS-OK Study. The journalofnutrition,health&aging.2019Jun24;23(8):732–8.
11. Nishikawa H, Asai A, Fukunishi S, Takeuchi T, Goto M, Ogura T, et al. Screening ToolsforSarcopenia.InVivo.2021;35(6):3001–9.
12.Krzymińska-Siemaszko R, Deskur-Śmielecka E, Kaluźniak-Szymanowska A, Lewandowicz M, Wieczorowska-Tobis K. Comparison of Diagnostic Performanceof SARC-F and Its Two Modified Versions (SARC-CalF and SARC-F+EBM) in Community-Dwelling Older Adults from Poland. Clinical Interventions in Aging. 2020Apr;Volume15:583–94.
13.JavaStat 2-way Contingency Table Analysis [Internet]. statpages.info. [cited 2023 Apr13].Availablefrom:https://statpages.info/ctab2x2.html
14.14.Whiting PF. QUADAS-2: A Revised Tool for the Quality Assessment of DiagnosticAccuracyStudies.AnnalsofInternalMedicine.2011Oct18;155(8):529.
15.Xu Z, Zhang P, Chen Y, Jiang J, Zhou Z, Zhu H. Comparing SARC-CalF With SARC-F for ScreeningSarcopeniainAdultsWithType2DiabetesMellitus.Frontiers inNutrition.2022Mar31;9.
16.Hax V, do Espírito Santo RC, dos SantosLP,FarinonM,deOliveiraMS,TrêsGL,et al. Practical screening tools for sarcopenia in patients with systemic sclerosis. Mota JF,editor PLOSONE.2021Jan22;16(1):e0245683.
17.Larsson L, Degens H, Li M, Salviati L, Lee Y il, Thompson W, et al. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiological Reviews. 2019 Jan 1;99(1):427–511.
18.Trevethan R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, andPitfallsinResearchandPractice.FrontiersinPublicHealth.2017Nov20;5(307).
19.Voelker SN, Michalopoulos N, Maier AB, Reijnierse EM. ReliabilityandConcurrent Validity of the SARC-F and Its Modified Versions: A Systematic Review and Meta-Analysis. Journal of the American Medical Directors Association. 2021 Sep 1;22(9):1864-1876.e16.
20.Piotrowicz K, Głuszewska A, Czesak J, et al. SARC-F as a case-finding tool for sarcopenia according to the EWGSOP2. National validation and comparison with otherdiagnosticstandards.AgingClinExpRes.2021;33:1821–1829.
21.Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians.KoreanJournalofAnesthesiology.2022Feb1;75(1):25–36.
Appendix
Authors,Year
KuritaN,etal(2019)
Krzymińska-SiemaszkoR, etal(2020)
HaxVanessa,etal(2021)
Appendix1.OutcomeTable
Outcome TruePositive TrueNegative FalsePositive FalseNegative
Appendix3.StudybiasassessmentinDiagnosisusingQualityAssessmentofDiagnosticAccuracyStudies-2(QUADAS)
SARC-F+EBM=28 SARC-F=15.01 SARC-F+EBM=642.41 SARC-F=632.255 SARC-F+EBM=280.59 SARC-F=290.745 SARC-F+EBM=8 SARC-F=20.99
SARC-F=6 SARC-F+EBM=70 SARC-F=85.04 SARC-F+EBM=29 SARC-F=13.96 SARC-F+EBM=7.2 SARC-F=10
SARC-F+EBM=8.8
SARC-F+EBM=68.02 SARC-F=64 SARC-F+EBM=10.98 SARC-F=15 SARC-F+EBM=6 SARC-F=9
SARC-F+EBM=9 SARC-F=6

Hormone Replacement Therapy as Treatment for Hypertension Postmenopausal Woman: A Systematic Review
Denassta Ruhala Sumarnaputri, Sabrina Intan Puspitasari, Almawaddah Warahmatan Lillalamin, Marshell Dimas Aditya
Faculty of Medicine Islamic University of Maulana Malik Ibrahim Malang, Malang, Indonesia
Abstract
Introduction: This decade, hypertension has become an important global health issue as it is a common risk factor for cardiovascular disease and affects almost all elderly people with the prevalence rate of hypertension increasing by 5.2% worldwide. One of the blood pressurelowering interventions that can be used in postmenopausal women is to use replacement hormone therapy, but many are still opposed due to the adverse effects it has on postmenopausal women patients.
Objective:ThisstudywasconductedtodeterminethepotentialofHormoneReplacementTherapy in reducing blood pressure in postmenopausal women.
Method: This systematic review was reported based on criteria from Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA). A literature search was conducted with multiple electronic databases, such as PubMed, ScienceDirect, Scopus, and Web of Science. The search was conducted from the inception of the database until April 2023. We looked up RCTs comparing treatment with hormone replacement therapy and without hormone replacement therapy or placebo.
Results: Among 958 studies screened, six fulfilled our criteria. In these six studies, we found that hormone replacement therapy has great potential for treating hypertension. But on the other hand, this hormone therapy is not recommended for patients who have heart attacks and strokes. It may cause thromboembolism. And then, The use of HRT such as estrogen in combination with progesterone rather than estrogen alone can lead to uterine cancer risk.
Conclusion: This systematic review provides evidence suggesting the great effectiveness of hormone replacement as a treatment for hypertension.
Keyword
Postmenopausal, Hypertension, Hormone Replacement Therapy
Hormone Replacement Therapy as Treatment for Hypertensive Postmenopausal Woman: A Systematic Review
Asian Medical Student’s Conference 2023

Authors:
Denassta Ruhala Sumarnaputri
Sabrina Intan Puspitasari
Almawaddah Warahmatan Lillalamin
Marshell DimasAditya
FACULTY OF MEDICINE ISLAMIC UNIVERSITY OF MAULANAMALIK IBRAHIM MALANG 2023
Hormone Replacement Therapy as Treatment for Hypertensive Postmenopausal Woman: A Systematic Review
Denassta Ruhala Sumarnaputri, Sabrina Intan Puspitasari,Almawaddah Warahmatan Lillalamin, Marshell DimasAditya Faculty Of Medicine, Islamic University of Maulana Malik Ibrahim Malang, Indonesia
INTRODUCTION
Hypertension is described as a systolic blood pressure of more than 140 mmHg or a diastolic blood pressure of more than 90 mmHg. Hypertension is a global health problem that is a common risk factor for cardiovascular disease and affects almost all adults aged 60 years or older. Various mechanisms involved in the maintenance of normal blood pressure such as the sympathetic nervous system, renin-angiotensin-aldosterone system, endothelial function, and water retention and can develop the disease. In the last decade, the prevalence rate of hypertension increased by 5.2% worldwide. Hypertension is estimated to occur in 1.4 billion people worldwide who have high blood pressure and only 14% have it under control. Findings from Indonesia's national survey show that more women are diagnosed with hypertension than men. The survey confirmed that 50.1% of women had hypertension and 33.7% of men had hypertension. Among those diagnosed, only about 11.5% received treatment and only 14.3% had their blood pressure controlled to less than 140/90 mmHg. Men and women have different patterns of hypertension. Hypertension is a significant heterogeneity related to gender. Young women are protected from developing hypertension by endogenous estrogen. As they age, they become more likely to develop hypertension and associated cardiovascular outcomes. Women also have unique forms of hypertension associated with pregnancy and menopause.
Menopause is defined as the last menstrual period resulting from the cessation of ovarian activity. However,menstrual cycleand endocrine changes are Observableevenbeforemenopause, with an average of 12-24 months, irregular cycles, and elevated FSH levels. After menopause, there is an increase in systolic blood pressure thought to be secondary to the withdrawal of the vasodilator effects of endogenous estrogen, increased arterial stiffness and salt sensitivity, reduced endothelial nitric oxide production, and increased angiotensin II receptor expression. The increase in systolic blood pressure and pulse pressure in pre-and post-menopausal women is greater than that of their male peers. In addition, diastolic blood pressure was similar in both sexes.
Therefore, the prevention and treatment of clinical disorders associated with menopause have become progressives become a public health problem and have been for the past decade in general, for most postmenopausal women Prolonged hormone replacement therapy has clear benefits (HRT) estrogen and progestin. In fact, most of the disturbance may arise directly or indirectly from this dramatic drop in postmenopausal gonadal steroid levels. However, HRT for postmenopausal women is difficult. Considered as physiological hormone replacement therapy (Except for premature ovarian failure as a result of surgery, vascular, infectious, autoimmune,
toxic, or iatrogenic pathological conditions). In fact, natural menopause is not a pathological condition, but a normal part of the aging process.
Various interventions have been tried to address the state of hypertension in elderly women. Current hypertension medications are less effective and have many contraindications for menopausal women. In addition, studies have found that long-term hormone replacement therapy is effective in reducing blood pressure significantly. On the other hand, hormone replacement therapy has adverse effects that are quite concerning based on previous research studies.
Combination treatment (estradiol-progesterone) effectively relieves menopausal complaints and makes cycle control better and makes bleeding patterns and mood disturbances more influenced by E2-DRSP. HRT with anti-mineralocorticoid effects could offer a novel potential mechanism for reducing cardiovascular endpoints in postmenopausal women. The combination of HRT can cause a decrease in systolic and diastolic, in contrast to the group that was given amlodipine only which only had a systolic decrease effect at night. But, the negative effect of HRT, DRSP has anti mineralocorticoid effect risk of osteoporosis.
In this systematic review, we determine to further review the effect of Hormone Replacement Therapy in reducing blood pressure in postmenopausal women by comparing the inclusion of existing studies.
MATERIAL AND METHOD
Study Methodology
We preferred used to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines
Eligibility Criteria
The following criteria were considered for study eligibility: type of study, samples, outcome measure, index test, and reference standards.
Type of Studies
The original research articles using Postmenopausal Women were included. Narrative review, systematic review, meta-analysis, and non-comparative research are excluded. Articles without full-text availability, non-English, and irrelevant topics were also excluded. Articles that did not use a Hormone Replacement Intervention Method, no outcome intervention, no RCT, no mean and SD values, and did not the last 15 years of research are also excluded.
Samples
Post-menopausal Women treated with Hormone Replacement Therapy were included in this systematic review. There was no limitation for the type of Hormone Replacement Therapy specificity.
Index test
Studies evaluating Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) and the use of hormone replacement therapy as an intervention were included.
Outcome measure
The outcome measures SBP and DBP are blood pressure measurements.
Reference standard
The reference standard was an article evaluating SBP and DBP before-after the use of hormone replacement therapy.
Data source and search
A literature search process was carried out with multiple electronic databases, such as PubMed, ScienceDirect, Scopus, and Web of Science. The search was conducted from the inception of the database until April 2023. The keywords used in electronic databases were described using Boolean operators. All the studies from these databases were stored in the author’s library in a spreadsheet.
Studies selection
After duplicate removal, retrieved articles were screened based on their titles and abstracts by four independent reviewers (DRS, AWL, SIP, and MDA). Potentially eligible full-text articles were thoroughly assessed using theeligibility criteriadescribedabove.The study selectionprocess was recorded in the PRISMA flow chart.
Data Extraction and Analysis
Selected studies were extracted with a Spreadsheet. The following data were recorded: Author, year, DOI, database, trial identifier, study location, characteristic population, diagnostic criteria, age at baseline, sample size, participants who dropped out in the intervention group, adherencerate,periodoftreatments,frequency,ITT/PPAS,systolicbloodpressure,diastolicblood pressure, and adverse effect.
Risk of Bias in Individual Studies
The quality of each study included in this systematic review was assessed by 4 independent reviewers (DRS, AWL, SIP, and MDA) according to the Cochrane risk-of-bias tool for randomized trials (RoB 2) and synthesized our summary using The Cochrane Review Manager (RevMan) 5.4. To maintain the present study’s robustness, we excluded studies with a high risk of bias from the systematic review.
Risk of bias across studies (Publication bias)
When all 6 studies are ready to use for systematic review, the presence of publication bias wouldbeevaluatedbygeneratingtheCochranerisk-of-biastoolforrandomizedtrials(RoB2). An asymmetrical shape indicates the presence of publication bias potential, whereas a symmetrical shape indicates the absence of publication bias.
RESULT
Study Selection
Our search yielded 1071 articles from 4 databases as described in Table 1 below. From hand searching, we found 31 articles that matched our topics. One hundred thirty-one duplicates from thosedatabases wereremoved. Then,the authorsread thetitles andabstracts ofthe remaining 774 articles for preliminary screening. The authors excluded the articles that did not fulfill our eligibility criteria. Full texts were retrieved again for 57 studies, and 27 studies were excluded because the studies don’t explain clearly the evaluation of Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) and the use of hormone replacement therapy as an intervention. Full-texts 6 studies were included in systematic reviews (fifty-four studies from database selection). Our study selection process was presented in the PRISMA diagram in Figure 1
Figure 1. Database Searching Process Results
Database
Keywords
PubMed (“Hypertension” OR “Blood Pressure”) AND (“Menopause” OR “Menopausal” OR “Postmenopause” OR “Postmenopausal”) AND (“Hormone therapy” OR “Hormone therapies” OR “Hormone Replacement”) AND (“RCT” OR “Trial*” OR “Random*”)
ScienceDirect (“Hypertension” OR “Blood Pressure”) AND (“Menopause” OR “Postmenopause”) AND (“Hormone therapy” OR “Hormone Replacement”) AND ("RCT" OR "Trial" OR "Random")
Scopus (“Hypertension” OR “Blood Pressure”) AND (“Menopause” OR “Menopausal”OR “Postmenopause”OR“Postmenopausal”)AND(“Hormone Therapy” OR “Hormone Therapies” OR “Hormone Replacement”) AND (“RCT” OR “Trial*” OR “Random*”)
Web of Science (“Hypertension” OR “Blood Pressure”) AND (“Menopause” OR “Menopausal” OR “Postmenopause” OR “Postmenopausal”) AND (“Hormone therapy” OR “Hormone therapies” OR “Hormone Replacement”) AND (“RCT” OR “Trial*” OR “Random*”)
 Table 1. Prisma Flowchart
Table 1. Prisma Flowchart
Study characteristics and results of individual studies
The full details of each study were displayed in Table 2. The subject of these studies was Hormone Replacement Therapy as Treatment for Hypertensive Postmenopausal Women. We focused on the SBP and DBP blood pressure scales to indicate the effectiveness of HRT. Out of the 7 studies included in the data extraction, 45 studies did not provide a randomized control trial. We could not extract the SBP/DBP score as the mean and SD data were unavailable in both groups (experimental and in the control group).
Aim
Table 2. Characteristic of Included Studies
To assess atherosclerosis progression and CVD risk factors after MHT initiated in early menopause.
To describe the effects of lowdose hormonal replacement therapy (HRT) on quality of life, metabolic parameters, and blood pressure in postmenopausal women.
To determine whether irbesartan, an angiotensin receptor blocker, has a greater effect on the vascular function when combined with estradiol, compared with irbesartan alone, in hypertensive postmenopausal women.
Gambacciani
Mirza
Study Harman et al., 2014
et al., 2011
et al., 2008 Country Olympia, Washington Rome, Italy London, UK Year 2014 2011 2008
Trial Identifier N/A N/A N/A
Characteristic population Postmenopausal Women 42-58 years Postmenopausal Women 45-55 years Postmenopausal Woman 45-70 years Diagnostic criteria Blood Pressure Measurement Blood Pressure Measurement Blood Pressure Measurement Age at Baseline (mean + SD) Experimental (Mean) 52.5 52.7 58 Experimental (SD) 2.5 0.5 5 Control (Mean) 52.8 53.8 58 Control (SD) 2.6 0.4 5 Sample Size Intervention (Pre or Baseline) 222 35 9 Intervention (Post) 178 32 9 Control (Pre or Baseline) 275 35 13 Control (Post) 217 20 13
Participants Dropped-Out in Intervention Group 44 3 0 Adherence Rate (%) 80.2 91.4 100 Periode of treatment Experiment 3 Month 3 Month 12 Control 3 Month 3 Month 12 Frequency Daily Daily Daily ITT/PPAS ITT PPAS ITT Systolic Blood Pressure Control Before 119.8 + 4.4 124.6 + 1.7 152 + 18 Control After N/A 125.8 + 1.9 151 + 20 Intervention Before 117.4 + 15.6 125.9 + 1.8 158 + 14 Intervention After N/A 121.8 + 1.4 141 + 23 Diastolic Blood Pressure Control Before 75.4 + 9.5 73.5 + 2.1 91 + 5 Control After 75.3 + 8.3 73.5 + 1.0 89 + 10 Intervention Before 74.1 + 9.7 73.1 + 1.2 97 + 6 Intervention After 75.0 + 9.2 73.4 + 1.0 87 + 14 Adverse Affect improving some markers of CVD risk. N/A N/A
Aim
To examined the combined effects of estrogen and the opioid antagonist, naltrexone, on blood pressure responses to psychological stress in postmeno-pausal women.
To investigate the effects of 24-month long-term trans-dermal hormone replacement therapy (HRT) on the circulating levels of components of the renin–angiotensin–aldosterone system (RAAS) and bradykinin, blood pressure (BP) and lipid profile in normotensive postmenopausal women (PMW).
to explore the impacts of micronized progesterone (MP4) combined with percutaneous estradiol gel (PEG) on hemodynamics in postmenopausal Korean women with grade 1 hypertension.
AJ et al.,2014 Ichikawa et al., 2008 Yoon et al., 2021
Tokyo,
2014 2008 2021
Identifier N/A N/A N/A
Study Allen
Country USA
Asia Korea,Asia Year
Trial
Characteristic population Postmenopausal Woman 41-69 years Postmenopausal Woman 41-69 years Postmenopausal Women 49-75 years Diagnostic criteria Blood Pressure Measurement Blood Pressure Measurement Blood Pressure Measurement Age at Baseline (mean + SD) Experimental (Mean) 57.52 56.3 57.5 Experimental (SD) 7.05 9.1 4.4 Control (Mean) 58.92 57.2 56.6 Control (SD) 4.8 6 3.8 Sample Size Intervention (Pre or Baseline) 27 12 17 Intervention (Post) 27 10 17 Control (Pre or Baseline) 15 10 16 Control (Post) 15 6 16
Participants Dropped-Out in Intervention Group 0 2 0 Adherence Rate (%) 100 83,3 100 Periode of treatment Experiment 12 24 month 12 weeks Control 12 24 month 12 weeks Frequency Daily 12 month daily ITT/PPAS ITT PPAS ITT Systolic Blood Pressure Control Before 71.79 + 5.36 121.4 + 12.5 147.9 ± 14.3 Control After 69.12 + 7.08 121.2 + 14.6 146.9 ± 11.0 Intervention Before 131.40 + 16.25 119.5 + 13.2 150.0 ± 11.6 Intervention After 120.75 + 18.73 111.5 + 12.7 148.5 ± 11.6 Diastolic Blood Pressure Control Before 125.67 + 17.34 74.2 + 5.8 92.8 ± 9.4 Control After 121.98 + 17.00 74.6 + 9.4 91.0 ± 10.6 Intervention Before 71.79 + 5.36 75.7 + 8.0 94.5 ± 10.4 Intervention After 69.12 + 7.08 67.5 + 5.9 93.3 ± 10.1 Adverse Affect 71.37 + 10.24 Oral HRT significantly increases the risk of coronary heart disease and stroke in PMW without established coronary disease. 92.8 ± 9.4
Risk of Bias in Individual Studies
We did a critical assessment of the six randomized control trial studies included in our study using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) and synthesized our summary using The Cochrane Review Manager (RevMan) 5.4. The risk of bias in individual studies is summarized in the figure based on the author’s judgements. An unclear risk of reporting bias is present in the study by Harman et al. (2014), and Yoon et al. (2021) there is little risk of bias. In a study by Allen et al. (2014), Ichikawa et al. (2008), and Gambacciani et al. (2011) there is an unknown risk of wear strain.
Risk of bias summary: review authors' judgments about each risk of bias item for each included study

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Selection Bias. Selection bias in the context of random sequence generation is low risk in all six studies included in this systematic review. A study by Allen et al. (2014), Gambacciani et al. (2011), Harman et al. (2014), Ichikawa et al. (2008), Mirza et al. (2008), Yoon et al. (2021). All included double-blind, randomized controlled trials. All study assignments were blinded and all study baselines were balanced.
Performance Bias. In regard to the blinding of participants and personnel in performance bias for intention to treat study, all six studies were double-blinded and used appropriate analysis to estimate the effects of interventions, and therefore all studies included a low risk of bias in terms of performance.
Detection Bias. Detection bias regarding the blinding of outcome assessment was low risk in all studies included in this systematic review, as the methods used to measure the outcomes were appropriate and not different between the intervention groups. The outcome assessors were not aware of the intervention received by study participants.
Attrition Bias. Attrition bias in terms of the presence of incomplete outcome data is low risk in the studies were done by Gambacciani et al. (2011), Harman et al. (2014), Mirza et al. (2008), and Yoon et al. (2021). This is because the studies had complete, or nearly complete, outcome data from randomized participants. However, the study has some concerns about its risk of attrition due to its incomplete outcome data and the absence of a clear explanation as to why its primary outcomes were not measured in all randomized participants.
DISCUSSION
Blood Pressure
Blood pressure is affected by cardiac output x peripheral resistance. Where cardiac output is affected by stroke volume and heart rate. Stroke volume is influenced by cardiac muscle which is indirectly affected by venus return atrial pressure and diastolic ventricular volume which is influenced by blood flow from the vascular either out or in of the kidney. Heart rate is influenced by the conduction of the SA node where the SA node is influenced by sympathetic nerves to the heart, this nerve is influenced by the the vasomotor nerve in the brain. Total peripheral resistance is affected by atrial smooth muscle, blood viscosity, and other controls. Atrial smooth muscle is influenced by neuro and hormonal control, also influenced by local control. Blood viscosity is affectedby hematocrit. So,blood pressurecontrol involves theorgansystems ofthekidneys,heart, blood vessels, and also brain.
Mechanism HRT in Hypertension
Hormone therapy (HT) consists of estrogen alone (estrogen-only HT) or estrogen combined with a progestogen (combined HT). It is available in a variety of formulations and doses that can be taken orally, vaginally, or intranasally, or as an implant, skin patch, cream, or gel. Clinical eLects vary according to the type of HT and its duration of use. Hormone therapy (HT) has been utilized for over 50 years for the treatment of women with hot flashes and other menopausalsymptoms.Mostendocrineglandsuseonlystructurallyidenticalsyntheticcompounds for hormone replacement to natural human hormones. HRT with a combination of estrogen and progesterone may be accompanied by female hypotension hypertension. Although transdermal administration of 17-β-estradiol did not significantly reduce office blood pressure in a postmenopausal hypertensive woman, daily blood pressure increased in her first 24 hours compared with placebo. dropped sharply by 3 mmHg. The evidence suggests that a woman with hypertension can be safely prescribed HRT, but careful monitoring is required. HRT use for hypertensive women is limited through a perceived fear of the hypertensive effects caused by suspected similarities between HRT and oral contraceptives drug. The evidence so far suggests at least a neutral case with no mild antihypertensive effect of HRT on blood print. Mechanisms
Underlying Blood pressure modifications by HRT, particularly its effects on the renin-angiotensin system, remain controversial. Available data suggest HRT may be safe, It is prescribed to women with hypertension under close monitoring.
Conjugated estrogens alone were not associated with significant changes in SBP or DBP. The combination of conjugated estrogen and progestin did not change SBP, but slightly decreased mean DBP. Using the estrogen patch slightly decreased DBP and increased drug intake. Cardiovascular risk factors and vascular events in women change dramatically after menopause. HRT use does not have a significant adverse effect on blood pressure in menopausal hypertensive women who require it.
Cost-Effectiveness Of Hormone Replacement Therapy
Weevaluatedthecost-effectiveness oftreating hypertension.Target BP witha75%control rate and was projected to prevent approximately 22,348.66 total productive life-year losses annually. This could be explained by the increasing trend of hypertension and inadequate BP control. A cost-effectiveness analysis conducted to evaluate the cost-effectiveness of prevention and treatment of cardiovascular disease in Ethiopia showed that combination drug treatment for individuals having >35% absolute risk of a CVD. The average cost-effectiveness of treating hypertension was 800.23 USD/DALYaverted.A WHO-CHOICE analysis ofthecosteffectiveness of population-level and individual-level interventions to combat non-communicable disease in Eastern sub-Saharan Africa Asia showed that treatment options for cardiovascular disease (CVD) are highly cost-effective (ICER < 100 in.$ per DALY averted). Hypertension among adults aged 40–64 years was very cost-effective with an ICER of 625.27 USD per DALY averted. Treating hypertensive adults aged over 64 years was more cost-effective for men than women with ICER of, 503.34 USD per DALY averted 906.63 USD per DALY averted respectively. This could be due to the high risk of CVD risk factors like smoking, and alcohol consumption in men than in women. Because at a younger age, coronary heart disease (CHD) incidence and mortality. This is supported by evidence from a study conducted to evaluate the cost-effectiveness of hypertension therapy according to 2014 guidelines in the USA showed that treatment of stage 1 hypertension was intermediate or low cost-effective for women between the ages of 45 and 84 years. Treating hypertensive adults aged 45–84 years with diabetes and CKD is very cost-effective for men and cost-effective for women. This could be explained by variation in risk of cardiovascular and cerebrovascular complications, and life expectancy in men and women. Treating hypertension in patients without diabetes and or CKD was cost-effective for both men and women aged 45–84 years with ICER of 1,101.59 USD per DALYs averted in men and 1,034.52 USD per DALY averted in women.
ABOUT THIS SYSTEMATIC REVIEW
Randomized clinical trials and observational data provide evidence that estrogencontaining MHT may decrease coronary heart disease and mortality in women younger than 60 years of age and within 10 years of menopause. Thus, MHT can be safely offered to symptomatic younger menopausal women (Sood et al., 2014). There was a small but statistically significant increase in SBP in both CEE-alone and CEE + MPA arms compared with placebo during both the intervention and cumulative follow-up phases among postmenopausal women with hypertension at baseline (Jiang et al., 2022).. Although transdermally administered, 17-b oestradiol in hypertensive postmenopausal women did not significantly lower office blood pressure, but daytime blood pressure was acutely reduced by 3 mmHg during the first 24 h when compared to placebo (Felmeden, 2000). Older women who are distant from the onset of menopause, have established atherosclerosis, and are given standard dose oral MHT are at increased risk for coronary heart events. (Sood et al., 2014). HRT can be used to manage menopausal symptoms in
the short term, but HRT is not recommended for patients with a history of heart attack/stroke, as HRT may increase the risk of blood clots. And it should be noted that women with a history of CAD are advised to choose other therapies besides HRT. (Cho, 2019).
In this systematic review, one of the journal about comparing oral conjugated equine estrogen (o-CEE) with placebo, it was suggested that hormone replacement therapy may improve some symptoms of CVD risk (Harman, 2014). In another study, the administration of E2+DRSP improved vasomotor symptoms and general aspects of quality of life and may positively influence cardiovascular risk factors and control high-normal blood pressure. (Gambacciani, 2011). HRT is recommended to be used in combination with other medications. The use of Estrogen+Progesterone can have a positive effect on the user, even lowering blood pressure, compared to using just one such as Estradiol alone. This may lead to side effects. Then irbesartan and estradiol, when used in combination, may lead to a greater reduction in blood pressure in postmenopausal hypertensive women. This effect may be mediated through increased vasodilation and lower aldosterone levels (Mirza, 2008). Administration of HRT in combination with opioid blockade does not necessarily change the effect of naltrexone on cardiovascular function. Combined effects of HRT and opioid blockade showed a significant interaction between HRT and opioid blockade for systolic blood pressure response to stress. Cellwise comparisons indicate that the largest stress-induced increase in systolic blood pressure was observed in women taking estrogen with intact opioid receptors. Opioid blockade eliminated the effect of estrogen on SBP reactivity, suggesting a moderate, opioid-mediated excitatory effect of estrogen replacement on SBP responses to stress (Ichikawa, 2008). MP4 might not adversely influence the estrogen effect on ambulatory BP and arterial stiffness. MP4 is also considered safer than MPA in terms of CVD, venous thrombosis, and breast cancer. Accordingly, PEG/MP4 may be a favorable regimen of MHT for postmenopausal women with hypertension. When combined, favorable impacts of active treatments on central aortic BPs and arterial stiffness became evident, likely due to enhanced statistical power. Despite a paucity of literature, MP4 might have independent action on BP, too. MP4 shows anti mineralocorticoid effects and lowers the pressure response to angiotensin II. In addition, MP4 increases nitric oxide production, suppresses vasoconstriction by the modulation of calcium channels, and decreases vascular resistance (Yoon, 2021)
LIMITATION
We are limited here in reviewing the article because we do not know if any of the drugs have side effects when combined with HRT.
CONCLUSION
This systematic review revealed that Various interventions have been tried to address the stateofhypertensioninelderlywomen.TheuseofHRTisrecommendedto beusedincombination with other drugs. The use of Estrogen + Progesterone can have a positive effect on its users, it can even lower blood pressure, compared to using only one such as Estradiol alone. This can actually trigger cardiovascular risk factors. It can be concluded that when HRT is used in combination, it can cause a greater reduction in blood pressure in postmenopausal hypertensive women. This effect may be mediated through increasing vasodilation and decreasing aldosterone levels. HRT can be used to treat menopausal symptoms in the short term, but it is not recommended to use HRT in patients who have a history of heart attack/stroke, because HRT can increase the risk of blood clots. And it should be noted that women with a history of CAD are advised to choose a therapy other than HRT.
Acknowledgements and Conflict of Interest
we have nothing to declare and no conflict of interest.
REFERENCES
Allen AJ, McCubbin JA, Loveless JP, Helfer SG. Effects of estrogen and opioid blockade on blood pressure reactivity to stress in Postmenopausal women. Journal of Behavioral Medicine. 2012;37(1):94–101.
Cho L. Estrogen & The Heart: Risks, benefits & side effects [Internet]. Cleveland Clinic. 2019 [cited 2023Apr15]. Available from: https://my.clevelandclinic.org/health/articles/16979-estrogen hormones#resources
Felmeden DC. Hormone replacement therapy and hypertension. Blood Pressure. 2000;9(5):246–9.
Gambacciani M, Rosano G, Cappagli B, Pepe A, Vitale C, Genazzani AR. Clinical and metabolic effects of drospirenone–estradiol in menopausal women: A prospective study. Climacteric. 2011;14(1):18–24.
Harman SM, Black DM,Naftolin F,Brinton EA,BudoffMJ, CedarsMI,et al. Arterialimaging outcomes and cardiovascular risk factors in recently menopausal women. Annals of Internal Medicine. 2014;161(4):249.
Ichikawa A, Sumino H, Ogawa T, Ichikawa S, Nitta K. Effects of long-term transdermal hormone replacement therapy on the renin-angiotensin- aldosterone system, plasma bradykinin levels and blood pressure in normotensive postmenopausal women. Geriatrics & Gerontology International. 2008;8(4):259–64.
Jiang X, Aragaki AK, Nudy M, Manson JAE, Shadyab AH, Wild RA, et al. The Association of Hormone Therapy with blood pressure control in postmenopausal women with
hypertension: A secondary analysis of the Women's Health Initiative Clinical Trials. Menopause. 2022;30(1):28–36.
Junge W, El-Samalouti V, Gerlinger C, Schaefers M. Effects of menopausal hormone therapy on hemostatic parameters, blood pressure, and body weight: Open-label comparison of randomized treatment with estradiol plus drospirenone versus estradiol plus norethisterone acetate. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2009;147(2):195–200.
Manhem K,Ghanoum B, Johansson M, Milsom I, GustafssonH. Influence ofchronichormone replacement therapy on left ventricular mass and serum-ace activity. Blood Pressure. 2010;19(5):295–300.
Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and Postmenopausal Women. Cochrane Database of Systematic Reviews. 2017;2017(1).
Mirza FS, Ong P, Collins P, Okamura K, Gerhard-Herman M, Williams GH, et al. Effects of estradiol and the angiotensin II receptor blocker irbesartan on vascular function in Postmenopausal women. Menopause. 2008;15(1):44–50.
Sood R, Faubion S, Kuhle C, Thielen J, Shuster L. Prescribing menopausal hormone therapy: An evidence-based approach. International Journal of Women's Health. 2014;:47.
Szekacs B, Vajo Z, Acs N, Hada P, Csuzi L, Bezeredi J, et al. Hormone replacement therapy reducesmean24-hourbloodpressureanditsvariabilityinpostmenopausalwomenwith treated hypertension. Menopause. 2000;7(1):31–5.
Tosteson ANA, Weinstein MC. 12 cost-effectiveness of hormone replacement therapy after the Menopause. Baillière's Clinical Obstetrics and Gynaecology. 1991;5(4):943–59.
Yoon B-K, Sung J, Song Y-M, Kim S-M, Son K-A, Yoo JH, et al. Effects of menopausal hormonetherapyon ambulatoryblood pressureandarterial stiffness in postmenopausal Korean women with grade 1 hypertension: A randomized, placebo-controlled trial. Clinical Hypertension. 2021;27(1).























 Figure3.Domain-SpecificResultsofQualityAssessmentofIncludedStudiesusingtheRiskofBias2tool
Figure4.DetailedQualityAssessmentSummaryofIncludedStudiesusingtheRiskofBias2tool
Figure3.Domain-SpecificResultsofQualityAssessmentofIncludedStudiesusingtheRiskofBias2tool
Figure4.DetailedQualityAssessmentSummaryofIncludedStudiesusingtheRiskofBias2tool










 A. Changeinon-time
A. Changeinon-time





































 Figure1.Diagramflowofliteraturesearchstrategy
Figure1.Diagramflowofliteraturesearchstrategy

 Fig2.RiskofBiasassessmentoftheincludedstudies
Fig2.RiskofBiasassessmentoftheincludedstudies



 Fig. 6: Funnel plot showing Studies Included in all outcome measure of Tanezumab 2.5mg VsNSAID
Fig. 6: Funnel plot showing Studies Included in all outcome measure of Tanezumab 2.5mg VsNSAID

 Fig. 7: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)forTanezumab5mgVsNSAID/Placebo
Fig. 8: Funnel plot showing Studies Included in all outcomemeasureofTanezumab5mgVs NSAID
Fig. 7: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)forTanezumab5mgVsNSAID/Placebo
Fig. 8: Funnel plot showing Studies Included in all outcomemeasureofTanezumab5mgVs NSAID

 Fig. 9: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)forTanezumab10mgVsNSAID/Placebo
Fig. 9: Forest Plot of Primary Outcome Measure (WOMAC Pain; 2.1.1, WOMAC Physical Function;2.1.2andPGA;2.1.3)forTanezumab10mgVsNSAID/Placebo

 Fig. 10: Funnel plot showing Studies Included in all outcome measure of Tanezumab 10mg VsNSAIDorPlacebo
Fig. 10: Funnel plot showing Studies Included in all outcome measure of Tanezumab 10mg VsNSAIDorPlacebo



 Figure 1. Forest plot meta-analysis of the effect of SGLT2i treatment on elderly patients with T2DM seen from the change score of HbA1c
Figure 2. Forrest plot meta-analysis of the effect of GLP-1RA treatment on elderly patients with T2DM seen from the change score of HbA1c
Figure 1. Forest plot meta-analysis of the effect of SGLT2i treatment on elderly patients with T2DM seen from the change score of HbA1c
Figure 2. Forrest plot meta-analysis of the effect of GLP-1RA treatment on elderly patients with T2DM seen from the change score of HbA1c





















 Figure 1. PRISMA flow diagram for the screening and selection process of the published studies
Figure 1. PRISMA flow diagram for the screening and selection process of the published studies







 Fig.2RiskofBias
Fig.3SummaryRiskofBias
Fig.2RiskofBias
Fig.3SummaryRiskofBias






 Fig.7FunnelPlotInfectionAPG
3.7TheEffectofAutologousPlatele-richGelinSurfaceAreaReductionAmongElderly withDiabeticFootUlcerPatients
Fig.8ForestPlotSurfaceAreaReduction
Fig.7FunnelPlotInfectionAPG
3.7TheEffectofAutologousPlatele-richGelinSurfaceAreaReductionAmongElderly withDiabeticFootUlcerPatients
Fig.8ForestPlotSurfaceAreaReduction




 Figure 1. PRISMA 2020 Flow Diagram.
Figure 1. PRISMA 2020 Flow Diagram.










 Funnelplot:PRPvsControl
VASscoreForestPlot
Appendix8.Funnelplot;VASPain
Funnelplot:PRPvsControl
VASscoreForestPlot
Appendix8.Funnelplot;VASPain



 Appendix11.WOMACFunction(4.1.1),WOMACpain(4.1.2)
Appendix12.VisualAnalogueScale
Appendix11.WOMACFunction(4.1.1),WOMACpain(4.1.2)
Appendix12.VisualAnalogueScale












 Appendix 1. Control Membrane, Type 1, Type 2, Type 3, and Type 4
Membrane Condition
Appendix 1. Control Membrane, Type 1, Type 2, Type 3, and Type 4
Membrane Condition





 Control Membrane (Cellulose Acetate)
Membrane Type 1
Control Membrane (Cellulose Acetate)
Membrane Type 1





 Membrane Type 2
Membrane Type 3
Membrane Type 2
Membrane Type 3







 Appendix 3. Scanning Electron Microscopy (SEM)
Appendix 3. Scanning Electron Microscopy (SEM)







 Cross section Control Membrane
Cross section Control Membrane







 Scanning Electron Microscopy (SEM) Control Membrane
Scanning Electron Microscopy (SEM) Control Membrane















 Cross section Type 1 Membrane
Cross section Type 1 Membrane



 Scanning Electron Microscopy (SEM) Type 1 Membrane
Scanning Electron Microscopy (SEM) Type 1 Membrane









 Figure 1.1 Flowchart of study identification and selection based on PRISMA
Figure 1.1 Flowchart of study identification and selection based on PRISMA



 (Figure 2. Analytical PD Pedigree (Source: Klein & Westenberger 2012))
(Figure 2. Analytical PD Pedigree (Source: Klein & Westenberger 2012))


 Figure2:PercentageSummaryofthequalityassessmentjudgment
Figure2:PercentageSummaryofthequalityassessmentjudgment

 Figure4:ThesensitivityandspecificityforestplotofVirtualBalanceTrainingPerformance
Figure4:ThesensitivityandspecificityforestplotofVirtualBalanceTrainingPerformance

 Figure6:ResultsofMeta-analysis.(A)Fallefficacyscale(FES),(B)HandicapScale
Figure6:ResultsofMeta-analysis.(A)Fallefficacyscale(FES),(B)HandicapScale
 Figure7:FunnelPlots
Figure7:FunnelPlots


 Figure 1. Flowchart of study identification and selection based on PRISMA.
Figure 1. Flowchart of study identification and selection based on PRISMA.











 Figure 2. PRISMA Flow chart of study selection
(A)
(B)
Figure 2. PRISMA Flow chart of study selection
(A)
(B)



 Figure 1. PRISMA 2020 Flow Diagram
Figure 1. PRISMA 2020 Flow Diagram


 Figure 2. Risk of Bias Assessment Result
Figure 3. Study Outcomes in Term of MDS-UPDRS-3
Figure 2. Risk of Bias Assessment Result
Figure 3. Study Outcomes in Term of MDS-UPDRS-3




















 Figure10.ForestplotshowingeffectivityofaquatictherapyinreducingTGUGscores betweengroups
Figure11.Forestplotshowingeffectivityofaquatictherapyinreducingpost-intervention TGUGscorescomparedtopre-intervention
Figure12.ForestplotshowingeffectivityofaquatictherapyinreducingVASscoresbetween groups
Figure13.Forestplotshowingeffectivityofaquatictherapyinreducingpost-intervention VASscorescomparedtopre-intervention
Figure10.ForestplotshowingeffectivityofaquatictherapyinreducingTGUGscores betweengroups
Figure11.Forestplotshowingeffectivityofaquatictherapyinreducingpost-intervention TGUGscorescomparedtopre-intervention
Figure12.ForestplotshowingeffectivityofaquatictherapyinreducingVASscoresbetween groups
Figure13.Forestplotshowingeffectivityofaquatictherapyinreducingpost-intervention VASscorescomparedtopre-intervention









 Figure4.SROCSARC-F
Figure4.SROCSARC-F
 Figure5.PreferredReportingItemsforSystematicReviewsandMeta-analyses(PRISMA) DiagramFlowchart
Figure5.PreferredReportingItemsforSystematicReviewsandMeta-analyses(PRISMA) DiagramFlowchart


 Table 1. Prisma Flowchart
Table 1. Prisma Flowchart


