





• • • • •








Positive Pressure Ventilation Therapy for Improvement of Symptoms and Physiological Measurement in Acute Mountain Sickness : A Systematic Review and Meta Analysis of Randomized Control Studies
Achmad Ivka Raehan, Eisya Akmel Naila, I Nyoman Sebastian Sudiasa, Mohammad Satrio Wicaksono
Faculty of Medicine, Diponegoro University Abstract
Introduction: Acute mountain sickness (AMS) is a high altitude disease characterized by headache, dizziness, fatigue, and gastrointestinal symptoms. AMS leads to hypobaric hypoxia and potentially develops into dangerous conditions such as pulmonary or cerebral edema. Current pharmacological treatments have not been proven to be consistently effective to prevent AMS and shown unpleasant adverse effects. Hence, positive pressure ventilation therapy has shown some promising results in treating AMS. Therefore, this systematic review and meta-analysis is conducted to assess its efficacy.
Objective: The aim of this systematic review and meta-analysis is to evaluate the efficacy of positive pressure ventilation therapy in improving symptoms and physiological measurement in acute mountain sickness.
Method: This systematic review and meta-analysis was reported based on PRISMA. The literature search was conducted on several databases such as PubMed, Cochrane, Science Direct, and Scopus. Results were shown as mean difference (MD) and standard deviation (SD). A fixed-effect model (FEM was used when the included studies were considered homogenous (low variability in studies’ results or variation due to random error), which were indicated by an I2 value less than 40%. Risk of bias was assessed using the cochrane risk-of-bias tool for randomized trials (RoB 2).
Result: Lake Louise Score declining with a significant pooled mean difference (MD) of -1.16 [95%CI: (-1.90) - (-0.41), P = 0.002]. Arterial oxygenation improvement with a significant pooled main difference (MD) of 3.18 [95% CI: (2.19) (4.17), P < 0.00001]. Heart rate measurement without significant effect, (MD) of (-0.1 [95% CI: (-7.26)-6.68, P = 0.96]
Conclusion: This systematic review and meta-analysis showed evidence that positive pressure ventilation therapy is a prospective therapy to improve acute mountain sickness symptoms and arterial oxygenation, but not with heart rate.
Keyword: acute mountain sickness, positive pressure ventilation therapy, arterial oxygen saturation, heart rate.
Positive Pressure Ventilation Therapy for Improvement of Symptoms and Physiological Measurement in Acute Mountain Sickness : A Systematic Review and Meta Analysis of Randomized Control Studies
East Asian Medical Students’ Conference 2022

Authors:
Achmad Ivka Raehan Eisya Akmel Naila
I Nyoman Sebastian Sudiasa Mohammad Satrio Wicaksono
FACULTY OF MEDICINE DIPONEGORO UNIVERSITY 2022
Positive Pressure Ventilation Therapy for Improvement of Symptoms and Physiological Measurement in Acute Mountain Sickness : A Systematic Review and Meta Analysis of Randomized Control Studies
Achmad Ivka Raehan, Eisya Akmel Naila, I Nyoman Sebastian Sudiasa, Mohammad Satrio Wicaksono
Faculty of Medicine, Diponegoro University Abstract
Introduction: Acute mountain sickness (AMS) is a high altitude disease characterized by headache, dizziness, fatigue, and gastrointestinal symptoms. AMS leads to hypobaric hypoxia and potentially develops into dangerous conditions such as pulmonary or cerebral edema. Currentpharmacological treatmentshavenotbeenproventobeconsistently effectivetoprevent AMS and shown unpleasant adverse effects. Hence, positive pressure ventilation therapy has shown some promising results in treating AMS. Therefore, this systematic review and metaanalysis is conducted to assess its efficacy.
Objective: The aim of this systematic review and meta-analysis is to evaluate the efficacy of positive pressure ventilation therapy in improving symptoms and physiological measurement in acute mountain sickness.
Method: This systematic review and meta-analysis was reported based on PRISMA. The literature search was conducted on several databases such as PubMed, Cochrane, Science Direct, and Scopus. Results were shown as mean difference (MD) and standard deviation (SD). A fixed-effect model (FEM was used when the included studies were considered homogenous (low variability in studies’ results or variation due to random error), which were indicated by an I2 value less than 40%. Risk of bias was assessed using the cochrane risk-of-bias tool for randomized trials (RoB 2).
Result: Lake Louise Score declining with a significant pooled mean difference (MD) of -1.16 [95%CI: (-1.90) - (-0.41), P = 0.002]. Arterial oxygenation improvement with a significant pooled main difference (MD) of 3.18 [95% CI: (2.19) (4.17), P < 0.00001]. Heart rate measurement without significant effect, (MD) of (-0.1 [95% CI: (-7.26)-6.68, P = 0.96]
Conclusion: This systematic review and meta-analysis showed evidence that positive pressure ventilation therapy is a prospective therapy to improve acute mountain sickness symptoms and arterial oxygenation, but not with heart rate.
Keyword: acute mountain sickness, positive pressure ventilation therapy, arterial oxygen saturation, heart rate.
Introduction
Acute mountain sickness (AMS) is a common syndrome found in inexperienced travelers after arriving in high-altitude areas, characterized by headache, dizziness, fatigue, and gastrointestinal symptoms such as nausea,vomiting,loss of appetite,dueto lowpartial pressure of oxygen 岷怠峅 Ascent to high altitude, approximately above 2500 m┸ 岷態峅 leads to hypobaric hypoxia, a state in which the inspired oxygen partial pressure (PiO2) is reduced due to low air pressure in the atmosphere, resulting in decreased arterial oxygenation 岷戴峅 Since the severity, onset, and duration of symptoms are influenced by the level of altitude, rate of speed, and personal susceptibility, its prevalence ranges from 15% to 80%%, with an increasing incidence as the altitude increases. The proportion of unacclimatized travelers affected at 3000 m was approximately 75% ┻ 岷替峅 Although AMS is typically not life-threatening, it could potentially develop into dangerous conditions such as pulmonary or cerebral edema┻ 岷泰峅 Consequently, the prevention of these severe conditions is crucial. Pharmacological treatments with acetazolamide, dexamethasone, and analgesic have been used for the prevention of AMS. However, these drugs have not been proven to be consistently effective to prevent the occurrence of AMS┸ 岷泰┸滞峅 and have unpleasant adverse effects such as peripheral and circumoral paresthesias, nausea, and dry mouth ┻ 岷胎峅 Hence, beneficial non-pharmacological treatment was promoted.
The mechanism of AMS is associated with brain response to hypoxia, since the brain has the greatestoxygendemand┻ 岷腿峅 Athigheraltitude,thelevelofcarbondioxideinthebloodincreased as the result of low partial pressure of oxygen. This condition is detected by medullary chemoreceptors, leading to hypoxic ventilatory response (HVR) in which the rate and depth of ventilation increased as the compensation of the reduced partial pressure of oxygen. The failure to intensify theHVR leads to hypoxemiathedevelopmentof AMS┻ 岷苔峅 Altitudehypoxemialeads to both increased sympathetic activity which leads to increased heart rate and endothelial alteration, causing vasogenic edema in the brain, which increases intracranial pressure, causing headache, nausea, vomiting, and weakness. Since the reduced arterial oxygenation plays a significant role in the development of AMS, the ability to reduce the hypoxemic stress, especially in the first hours of exposure, may determine the probability of subsequent altitude illness 岷怠待峅
Improving pulmonary gas exchange is another mechanism linked to improved blood oxygenation and altitude acclimatization, in addition to increased HVR. Pulmonary gas exchange improvement can be gained by respiratory treatment of positive pressure ventilation therapy in which air or a combination of oxygen and other gasses is delivered into the lungs under positive pressure, leading to an increase of gas exchange. Positive expiratory pressure (PEP) has been found as a non-pharmacological respiratory treatment and easy method to treat acute mountain sickness by producing pressure contrary to the pressure produced by airways (expiratory flow resistance), causing an increase in alveolar pressure, leading to increasing of pulmonary gas exchange and arterial oxygen saturation┸ 岷怠怠峅 with the rapid increases in SpO2 ranging between 0-23% have been reported with PEP breathing at high altitude┻ 岷怠態┸怠戴峅
As well as PEP, another pressure-based respiratory therapy named positive end expiratory pressure (PEEP) was also known to be efficient in preventing acute mountain sickness in a hypobaric chamber without any side-effects 岷怠替峅 Utilizing positive expiratory pressure while sleeping enhances ventilation by attracting microatelctatic alveoli and changing breathing pattern, resulting in improvement of oxyhaemoglobin saturation 岷怠腿峅
Although PEP and PEEP were thought to be successful as a treatment to improve conditions at hypobaric chamber and prevent severe complications, it is necessary to assess the efficacy of those interventions in actual field settings at high altitudes. Currently, there was no metaanalysis assessing these pressure-based therapy. Therefore, the current study was conducted to analyze the efficacy of PEP and PEEP as a treatment of AMS symptoms.
Material and Method
This systematic review was conducted based on Preferred Reporting Items for Systematic ReviewsandMeta-Analyses(PRISMA)guidelinethatcan beaccessedthrough(https://prismastatement.org/)
Eligibilitycriteria
Criterias that are considered eligible for this systematic review are: Original research article or research reports using human study with randomized controlled trial design were included in this study. Narrative review, systematic review, meta-analysis, non comparative research, in silico studies, in vitro studies, in vivo studies, technical reports, editor response, scientific posters, study protocol, and conference abstracts were excluded. Unavailable full-text articles, non-english, irrelevant topics were also excluded.
Outcome Measure
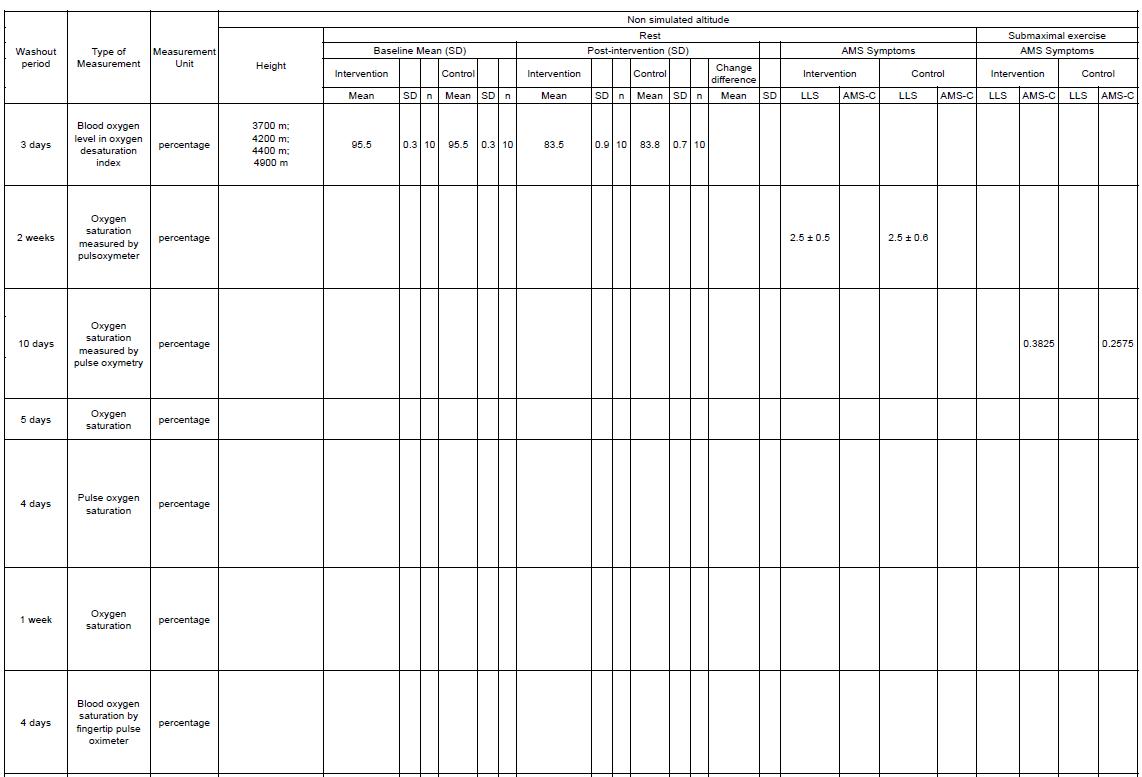
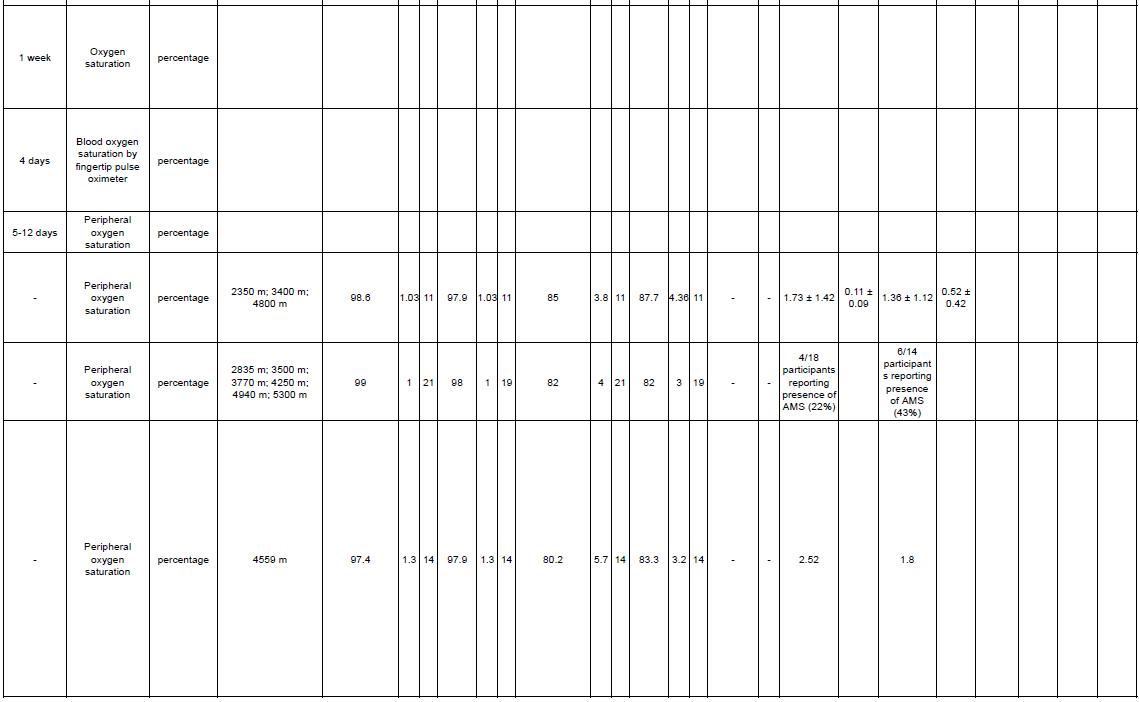
Outcome measures that are assessed in this systematic review are Lake Louis Score (LLS), arterial oxygen saturation, and heart rate. LLS is a robust and practical tool to diagnose and score the severity of acute mountain sickness.
Lake Louise AMS scoring system is divided into three parts: a self-report questionnaire, a clinical assessment, and a functional score. The AMS self-report questionnaire consists of five questions regarding headache, gastrointestinal symptoms, fatigue and/or weakness, dizziness/light-headedness, and functional score each scoring from 0 to 3. The clinical assessment score is given through clinical examination by a physician of three signs: mental status rating from 0 to 4, ataxia rating from 0 to 4 and peripheral oedema rating 0 to 2. After clinicalassessmentshavebeenconducted,theresultscanbeaddedtotheAMSself-reportscore. The funcional score is one optional question regarding functional consequences of recorded signs rating from 0 to 3. The Lake Louise AMS plus the clinical assessment was defined as the sum of the both scoring. Representative point of AMS is when the result of assessment on the self-report questionnaire alone, or in combination with the clinical assessment score as threepoint or greater score┻ 岷態待峅
Physiological measurements suchas arterial oxygensaturation(SpO2)andheartrate(HR)were also assessed in this study. Arterial oxygen saturation (SaO2) is a measure of hemoglobin oxygenation in the arterial compartment of the circulatory system. The value of SaO2 is the same throughout the whole arterial system and directly related to the oxygen supply to organs, and normal values lie between 95% and 100%. Arterial oxygen saturation has been studied to beapredictorofacutemountainsickness┻ 岷態怠峅 HRweremeasuredtorepresent thecardiovascular physiology from participants in the high altitude compared to low altitude.
IndexTest
Studies included were evaluating the LLS AMS Score, arterial oxygen saturation, and heart rate that presented the data with mean difference and standard deviation. Studies that do not present mean data scores before and after intervention were excluded.
Reference Standard
Reference standards are randomized controlled trial studies performed by professionals by evaluating the effect of PEEP, EPAP, and CPAP on LLS score alteration.
Data Sources and Search
This study acquired studies by using searching databases, such as PubMed, Cochrane, Science Direct, and Scopus. Search was conducted from the inception of the database until October 2022. The keywords used were using Boolean operator and mesh. Keywords used in each database can be seen in Table 1. The studies are stored in the authors’ library using mendeley group reference manager.
Table 1. Keyword Used in Literature Searching
Pubmed
Database
Keywords
(((acute mountain sickness[MeSH Major Topic]) OR (altitude sickness[Title/Abstract])) OR (altitude hypoxia[Title/Abstract])) AND (((positive end expiratory pressure[MeSH Major Topic]) OR (continuous positive airway pressure[Title/Abstract])) OR (expiratory positive airway pressure[Title/Abstract]))
Cochrane Library ("positive end-expiratory pressure"):ti,ab,kw OR ("continuous positive airway pressure"):ti,ab,kw OR ("expiratory positive airway pressure"):ti,ab,kw OR (“positive expiratory pressure”):ti,ab,kw AND (acute mountain sickness):ti,ab,kw OR (altitude sickness):ti,ab,kw OR (altitude hypoxia):ti,ab,kw
Science Direct
(“Positive end expiratory pressure” OR “Positive expiratory pressure” OR “Expiratory positive airway pressure” OR “Continuous positive airway pressure”) AND (“Acute
mountain sickness” OR “Altitude sickness” OR “Altitude hypoxia”)
Scopus (“Positive end expiratory pressure” OR “Positive expiratory pressure” OR “Expiratory positive airway pressure” OR “Continuous positive airway pressure”) AND (“Acute mountain sickness” OR “Altitude sickness” OR “Altitude hypoxia”)
Selection process
After searching keywords written in Table 1, studies with non-RCTs were excluded through article type filters of each database. Results from 5 databases were later combined and screened by four independent reviewers (AIR, INSS, IOK, EAN) through title, year of publication, and DOIs for duplicate removal. After duplicate removal, studies were later screened through abstract and full-paper for irrelevance removal. The study selection processes were recorded in the PRISMA flow chart.
Data collection process
Studies after final screening are extracted for the relevant data and recorded in Google Spreadsheet. The recorded datas were: (1) first author, year, (2) country, (3) study design, (4) sample size, (5) gender, (6) mean age, (7) name of intervention, length of intervention, comparison, and (8) outcome that consist of LLS AMS Score, arterial oxygen saturation, and heart rate. All statistical tests for this meta-analysis were conducted using Review Manager (RevMan) v5.4 (Cochrane Collaboration, UK).
Studyriskofbias assessment (Qualitative Synthesis)
Each study included in this study was assessed by four independent reviewers (AIR, INSS, IOK, EAN) according to the Cochrane risk-of-bias tool for randomized trials (RoB 2) which can be accessed in (https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-riskbias-tool-randomized-trials) The discrepancies were later discussed and resolved between reviewers. To maintain the present study’s robustness, we excluded studies that are assessed with a high risk of bias from the meta-analysis.
Quantitative Data Synthesis (Meta-Analysis)
Mean Difference (MD) and Standard Deviation (SD) with the Confidence Interval (CI) of 95% were calculated in this review. A fixed-effect model (FEM was used when the included studies were considered homogenous (low variability in studies’ results or variation due to random error), which were indicated by an I2 value less than 40%. Otherwise, we used a random-effect model (REM). The pooled estimate was presented in a forest plot.
Result and Discussion Studyselection
After conducting literature searching from 4 databases which are PubMed, Cochrane Library, Scopus, and ScienceDirect, 292 studies were generated. Automation tools from each database were used to exclude non-rct studies and resulted in 176 articles being excluded. Afterward, 33 duplicate study articles were removed. Subsequently, authors assessed all of the remaining articles from the title and abstract for irrelevance to the topic, resulting in 75 articles excluded. 7 articles were then retrieved for the full text availability. Lastly, the author assessed eligibility for all the studies and agreed to exclude 3 studies because of an uncontrolled study group and 1 study for an unpresent outcome of interest. This review included 4 studies to be in the systematic review and meta analysis. Our study selection process is presented in the PRISMA diagram flow chart in Figure 1
Figure 1. PRISMA 2020 Flow Diagram.
Studycharacteristics
From 4 studies included in this review , the total participants are 277 participants. Most of the studies (n=3) are conducted in field conditions in nepal and france such as Mount Blanc; the Khumbu region of the Himalayas; and Larkye Pass, Manaslu Circuit in the Nepali Himalaya. the other study are conducted in laboratory condition using hypobaric chamber to simulate the condition in the high altitude.
Riskofbias in studies
The quality of each study were carefully analyzed by using the Cochrane risk-of-bias tool for randomizedtrials (RoB2)l.3studiesshowedhigh riskofbias(Savoreyet al;Jeanet al;Thomas

et al) and one study showed some concern (Grants et. al). The risk of bias were summarized in Figure 2.
Figure 2. Risk of Bias Assessment Result.

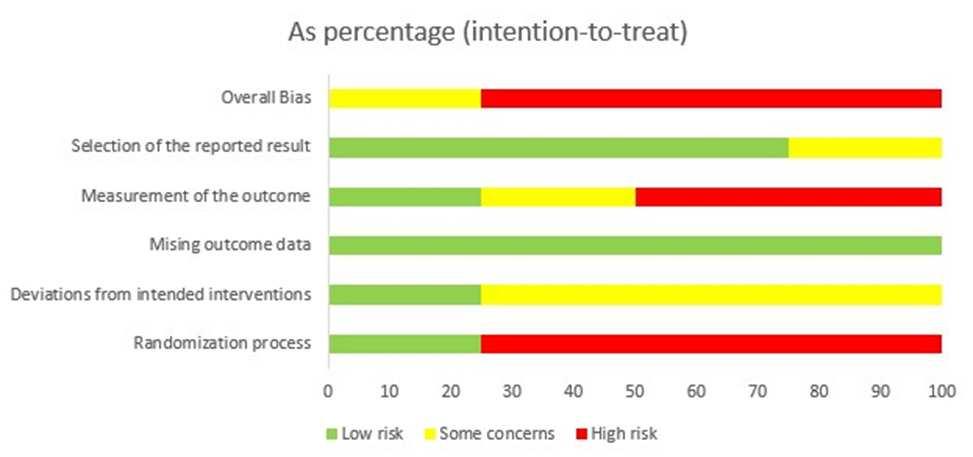
Table 2. Characteristic of Studies
Author, Year Savourey, et al.なひひぱ岷怠替峅 Jean, et al. にどどね岷怠泰峅 Grant S, et al. にどなの岷怠滞峅 Thomas, et al. にどにな岷怠胎峅
Country France France Himalaya, Nepal Nepal
Population Sample size 22 8 223 24
Sex Male Male female and male male Mean Age 27 23 39 41.0 ± 15.6
Intervention Name of intervention 5cm H2O PEEP and to a run without PEEP during an 8-h hypoxic exposure (PB = 589 hPa, 4500 m).
PEEP EPAP face mask with PEP
Length of intervention 8 hour 2 days 5 days 16 to 18 days, measured after 15, 25, 45 minute
Comparison Without PEEP Without PEEP Placebo Sea Level Altitude
Outcome Control t0: 0.09±0.79, t1: 0.45±3.09, t2: 1.18±5.06, t3: 1.54±6.89, t4: 2,22±7.73, t5:4.23±9.70
Departure (Chamonix) :0.38±0.47 Arrival (Cosmiques) :0.75±0.75 Bedtime (Cosmiques):1.63±1.
1.87 2.3 ± 1.7
AMS sympto m (LLS)
Intervention t0: 0.09±0.79, t1: 0.41±2.81, t2: 0.96±4.87, t3: 1.28±6.19, t4: 1.54±6.19, t5: 1.50±6.19
Getting up (Cosmiques) : 2.25±2.38
Summit : 3.63±1.63 Return (Cosmiques) : 2.50±1.38 Return (Chamonix) :0.88±0.66
Departure (Chamonix) :0.00±0.00 Arrival (Cosmiques) :1.13±0.91 Bedtime (Cosmiques):1.13±0.91 Getting up (Cosmiques) :1.13±0.91
Summit : 1.50±0.88 Return (Cosmiques) : 1.76±0.63 Return (Chamonix) :0.75±0.75
1.54 1.7 ± 1.8 p value P<0.01 P<0.01 at Summit; P<0.05 at Return (Cosmiques) p=0.17 P < 0.05
Control t0: 99.14±4.40, t1: 86.23±15,80, t2: 87.55±16.65, t3: 86.70±14.35, t4: 85.09±14.39, t5:
Departure (Chamonix) :81.6±6.1 Arrival (Cosmiques) : 99.6 10.2 Bedtime
78 ± 5 77.7 ± 3.7
SpO2
87.41±18.62 (Cosmiques):105±8.7 Getting up (Cosmiques) : 99.1±3.6 Summit : 103.0 11 Return (Cosmiques) : 102.0 6.2 Return (Chamonix) : 104.0 9.2
Intervention t0: 98.86±4.26, t1: 85.59±21.22, t2: 85.36±19.32, t3: 86.80±,15,00 t4: 86.50±14.30, t5: 87.55±18.76
80 ± 3 86.5 ± 4.6 p value P=0.01 P>0.01 p<0.01 P < 0.05
Heart Rate Control t0: 72.09±43.43, t1: 76.50±45.63, t2: 71.73±45.21, t3: 81.50±35.64, t4: 89.05±41.18, t5:
Departure (Chamonix) :96.0±0.5 Arrival (Cosmiques) :88.0±2.3 Bedtime (Cosmiques):87.0±2.4 Getting up (Cosmiques) :88.0±4.0 Summit : 1.50±0.88 Return (Cosmiques) : 87.0±4.6 Return (Chamonix) :96.0±0.7
Departure (Chamonix) :81.6±6.1 Arrival (Cosmiques) : 99.6±10.2 Bedtime
NA 77.3 ± 14.7
83.36±50.60 (Cosmiques):105±8.7
Getting up (Cosmiques) : 99.1±3.6
Summit : 103.0±11 Return (Cosmiques) : 102.0±6.2 Return (Chamonix) : 104.0±9.2
Intervention t0: 71.64±46.57, t1: 78.77±46.34, t2: 77.91±41.41, t3: 87.50±41.36, t4: 93.36±47.04, t5: 87.73±40.43
Departure (Chamonix) :83.2±9.2
Arrival (Cosmiques) : 99.0±9.5 Bedtime (Cosmiques):103.0±10.3
Getting up (Cosmiques) : 99.0±10.7
Summit : 107.0±12.5 Return (Cosmiques) : 105.0±10.2 Return (Chamonix) : 102.0±6.2
p value P<0.05 at t2,t3. Others NS P>0.01
NA 75.9 ± 13.6
NA P= 0.336
Meta Analysis
Statistical analysis was performed using Review Manager (RevMan) v5.4 (Cochrane Collaboration, UK) . Mean Difference (MD) and Standard Deviation (SD) with the Confidence Interval (CI) of 95% were then calculated in this review The data then processed into pooled standardized mean difference forest plot form. Our study assessed extractable quantitative data and group them into 3 outcomes which include Lake louise Score, Sp02, and Heart rate. The forest plot of the meta-analysis can be seen in Figure 3-5.



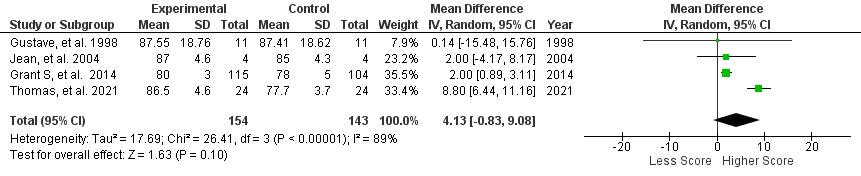
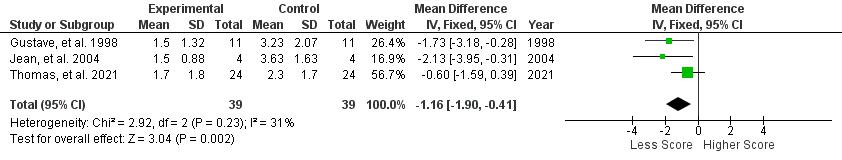
Figure 3. Lake Louise Score
Figure 4. SpO2 (Arterial Oxygen Saturation)
Figure 5. Heart Rate a b
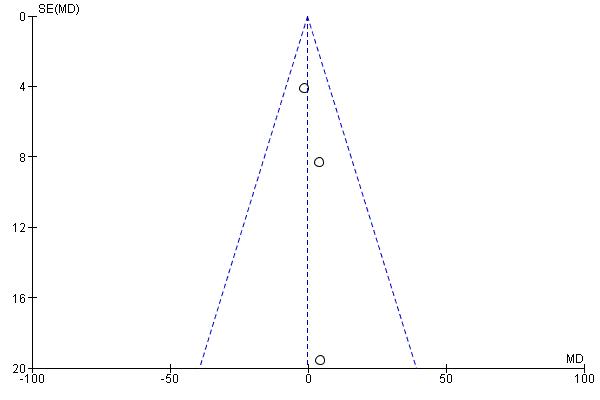
Figure 6. Funnel Plot for Assessing the Level of Publication Bias for Each Outcome: a. LLS; b. SpO2; c. Heart Rate.
Discussion
Positive pressure ventilation therapyinfluence on AMSSeverity
AMS symptoms severity was assessed by Lake Louise Questionnaire, containing 4 symptoms which are headache, nausea/vomiting, fatigue, and dizziness/light-headedness. A headache score of at least one point and a total score of at least three points are required for a positive AMS definition┻ 岷態態峅 Positive pressure ventilation therapy that was conducted in all studies which are positive expiratory pressure (PEP) and positive end expiratory pressure (PEEP) has proven to be successful in reducing Lake Louise Score with a significant pooled mean difference (MD) of -1.16 [95%CI: (-1.90)-(-0.41), P = 0.002]. The reduction of Lake Louise Score, approximately ≤ 3, showed the improvement of individual condition obtained by the positive pressure breathing 岷怠胎峅 Furthermore, all of the studies were found to significantly reduce the score of LLS, with the exception of Grant S, et al. 2015 (p=0.17) which possibly due to wide range demographic variety of the participant. Thus, in general, positive pressure ventilation therapy is effective to relieve severity of AMS symptoms and yet, preventing the complication of AMS.
Oxygen saturation increases due to positive pressure ventilation therapy Ascending to high altitude causes a significant decrease in arterial oxygen saturation that leads to the development of AMS symptoms, and if occurs continuously, resulting in serious complications such as high altitude pulmonary or cerebral edema┻ 岷態戴峅 Positive pressure ventilation therapy has been shown to successfully increase arterial oxygenation during acute hypoxic exposure with a significant pooled main difference (MD) of 3.18 [95%CI : (2.19) (4.17), P < 0.00001]. An increase of oxygen saturation was facilitated by increased pulmonary gasexchangesdueto improvementofintrathoracicpressurethatwasobtainedfromthepositive pressure breathing-therapy┻ 岷怠腿┸態戴峅 Since the low oxygen saturation is highly associated with the worsening of the symptoms┸ 岷態替峅 increased oxygen saturation provides better prognosis of symptoms development.
Positive pressure ventilation therapyeffect towards heart rate
c.
Acute high-altitude hypoxia induces cardiovascular changes through 3 pathways which are cardiac response due to depletion of oxygen delivery, increased pulmonary vascular constriction, and enhancement of sympathetic activity through く-adrenergic stimulation, leading to increasedheart rate 岷にの┸には峅 Asacompensationfordepletion ofoxygenat high altitude, the heart beats faster to increase cardiac output, so that the body could maintain appropriate oxygen delivery to tissues 岷にば峅 Theoretically, increasing oxygen saturation would help to remove the hypoxic condition, thus, the heart could beat at its normal rhythm. However, it has been shown that the positive pressure breathing ventilation does not give a significant effect on heart rate, as shown by pooled MD of -0.19 [95% CI: (-7.26)-6.68, P = 0.96]. This is possibly due to the complex pathway of the cardiovascular system, so that the respiratory intervention does not directly and significantly cause heart rate alteration. The detailed mechanism of this case requires further investigation.
Strength and Limitations
This study is the first systematic review and meta analysis that evaluated the positive pressure ventilation therapy efficacy towards acute mountain sickness. This systematic review assessed different types of positive pressure ventilation therapy effects in reducing acute mountain sickness symptoms on samples that were exposed to hypoxia conditions in mountain hiking. All studies included are randomized controlled trial with significant results in several aspects, such as reducing Lake Louis Score and improving oxygen saturation. Nonetheless this study is not without limitation. The study that was included have a high and moderate risk of bias. The sample size of the included study also were limited in size and conducted in homogeneous places. Therapies used in studies also varied between PEEP and PEP. Positive pressure ventilation therapy is also found to be not significant in reducing heart rate on high altitude induced hypoxia. Other external factors, such as individual detailed backgrounds including ethnicity were not explored and discussed in included studies. Hence, identifying a broader population in mountain settings in the futures studies is necessary. There might be a possibility to miss some important information in studies written in other language than English or Indonesian. Irretrievable full-text is also the limitation of this study.
Conclusion and Recommendation
This systematic review and meta analysis revealed that the use of positive pressure ventilation therapy which includes PEEP and PEP is a prospective therapy in acute mountain sickness. It has been shown that positive pressure ventilation therapy is quantitatively significant in improving AMS symptoms and arterial oxygen saturation. However, it has no significant effect on heart rate. We recommend further randomized control study with a larger sample size to be conducted to observe more about the efficacy of positive pressure ventilation therapy.
Conflict of Interest
All authors declared there are no competing interests in this study.
Reference
1. Prince TS, Thurman J, Huebner K. Acute Mountain Sickness. [Updated 2022 Jul 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430716/.
2. Luks AM, Swenson ER, Bärtsch P. Acute high-altitude sickness. Eur Respir Rev. 2017 Jan 31;26(143):160096. doi: 10.1183/16000617.0096-2016. PMID: 28143879. https://pubmed.ncbi.nlm.nih.gov/28143879/.
3. Rupp T, Saugy JJ, Bourdillon N, Verges S, Millet GP. Positive expiratory pressure improves arterial and cerebral oxygenation in acute normobaric and hypobaric hypoxia. Am J Physiol Regul Integr Comp Physiol. 2019 Nov 1;317(5):R754-R762. doi: 10.1152/ajpregu.00025.2019. Epub 2019 Sep 18. PMID: 31530174. https://pubmed.ncbi.nlm.nih.gov/31530174/.
4. Mairer K, Wille M, Burtscher M. The prevalence of and risk factors for acute mountain sickness in the Eastern and Western Alps. High Alt Med Biol. 2010 Winter;11(4):3438. doi: 10.1089/ham.2010.1039. PMID: 21190503.
5. Taylor AT. High-altitude illnesses: physiology, risk factors, prevention, and treatment. Rambam Maimonides Med J. 2011;2(1):e0022. Published 2011 Jan 31. doi:10.5041/RMMJ.10022. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3678789/
6. LarsonEB, Roach RC,SchoeneRB (1982)Acutemountain sickness andacetazolanide. J Am Med Assoc 248:328-332.
7. Hackett PH, Roach RC. High-altitude illness. New Engl J Med. 2001;345:107 14. doi: 10.1056/NEJM200107123450206.
8. Masuet-Aumatell C, Sánchez-Mascuñano A, Santangelo FA, Ramos SM, RamonTorrell JM. Relationship between Smoking and Acute Mountain Sickness: A MetaAnalysis of Observational Studies. Biomed Res Int. 2017;2017:1409656.
9. Bärtsch P, Swenson ER, Paul A, Julg B, Hohenhause E. Hypoxic ventilatory response, ventilation, gas exchange and fluid balance in acute mountain sickness. High Alt Med Biol. 2002;3:361 76. doi: 10.1089/15270290260512846.
10. Roach RC, Greene ER, Schoene RB, Hackett PH. Arterial oxygen saturation for prediction of acute mountain sickness. Aviat Space Environ Med. 1998 Dec;69(12):1182-5. PMID: 9856544. https://pubmed.ncbi.nlm.nih.gov/9856544/.
11. Acosta P, Santisbon E, Varon J. “The use of positive end-expiratory pressure in mechanical ventilation”. Crit Care Clin 23: 251 261, 2007. doi:10.1016/j.ccc.2006.12.012.
12. Tannheimer M, Tannheimer S, Thomas A, Engelhardt M, Schmidt R. Auto-PEEP in the therapy of AMS in one person at 4,330 m. Sleep Breath. 2009 May;13(2):195-9. doi: 10.1007/s11325-008-0237-z. Epub 2008 Dec 4. PMID: 19052788. https://pubmed.ncbi.nlm.nih.gov/19052788/.
13. Lipman GS, Kanaan NC, Phillips C, Pomeranz D, Cain P, Fontes K, Higbee B, Meyer C, Shaheen M, Wentworth S, Walsh D. Study Looking at End Expiratory Pressure for Altitude Illness Decrease (SLEEP-AID). High Alt Med Biol 16: 154 161, 2015.
14. Savorey G, Caterini R, Launay JC, Guinet A, Besnard Y, Hanniquet AM, et al. Positive end expiratory pressure as a method for preventing acute mountain sickness. Eur J Appl Physiol 1998;77(1 2):32 6.
15. Jean, L. C., Nespoulos, O., Guinet-Lebreton, A., Besnard, Y., & Savourey, G. (2004). Prevention of acute mountain sickness by low positive end-expiratory pressure in field conditions. Scandinavian Journal of Work, Environment and Health, 30(4), 322 326. https://doi.org/10.5271/sjweh.801.
16. Grant. S., Kanaan, N. C., Phillips, C., Pomeranz, D., Cain, P., Fontes, K., Higbee, B., Meyer, C., Shaheen, M., Wentworth, S., & Walsh, D. (2015). Study Looking at End Expiratory Pressure for Altitude Illness Decrease (SLEEP-AID). High Altitude Medicine and Biology, 16(2), 154 161. https://doi.org/10.1089/ham.2014.1110.
17. Thomas, R., Saugy, J. J., Bourdillon, N., Verges, S., & Millet, G. P. (2019). Positive expiratory pressure improves arterial and cerebral oxygenation in acute normobaric and hypobaric hypoxia. American Journal of Physiology - Regulatory Integrative and Comparative Physiology, 317(5), R754 R762. https://doi.org/10.1152/ajpregu.00025.2019.
18. Johnson PL, Popa DA, Prisk GK, Edwards N, Sullivan CE. Non-invasive positive pressure ventilation during sleep at 3800 m: Relationship to acute mountain sickness and sleeping oxyhaemoglobin saturation. Respirology. 2010 Feb;15(2):277-82. doi: 10.1111/j.1440-1843.2009.01678.x. Epub 2009 Dec 27. PMID: 20051046; PMCID: PMC4183457 https://pubmed.ncbi.nlm.nih.gov/20051046/.
19. Potchileev I, Doroshenko M, Mohammed AN. Positive Pressure Ventilation. [Updated 2022 May 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560916/
20. Roach,R.C.,Hackett,P. H.,Oelz,O.,Bärtsch,P.,Luks,A.M.,MacInnis, M.J.,Baillie, J. K., Achatz, E., Albert, E., Zafren, K., Yaron, M., Willmann, G., Wilkes, M., West, J. B., Wang, S. H., Wagner, D. R., Voituron, N., Ulrich, S., Twomey, R., … Andrews, J. S. (2018). The 2018 Lake Louise Acute Mountain Sickness Score. High Altitude Medicine & Biology, 19(1), 4. https://doi.org/10.1089/HAM.2017.0164.
21. Karinen HM, Peltonen JE, Kähönen M, Tikkanen HO. Prediction of acute mountain sickness by monitoring arterial oxygen saturation during ascent. High Alt Med Biol. 2010 Winter;11(4):325-32. doi: 10.1089/ham.2009.1060. PMID: 21190501.
22. Roach RC, Hackett PH, Oelz O, et al. The 2018 Lake Louise Acute Mountain Sickness Score. High Alt Med Biol. 2018;19(1):4-6. doi:10.1089/ham.2017.0164.
23. Nespoulet H, Rupp T, Bachasson D, Tamisier R, Wuyam B, Lévy P, Verges S. Positive expiratory pressure improves oxygenation in healthy subjects exposed to hypoxia. PLoS One. 2013 Dec 23;8(12):e85219. doi: 10.1371/journal.pone.0085219. PMID: 24376872; PMCID: PMC3871630.
24. Oliver SJ, Sanders SJ, Williams CJ, Smith ZA, Lloyd-Davies E, Roberts 576 R, Arthur C, Hardy L, MacDonald JH. Physiological and psychological 577 illness symptoms at high altitude and their relationship with acute mountain 578 sickness: A prospective cohort study. J Travel Med 19: 210 219, 2012.
25. Gonggalanzi, Labasangzhu, Bjertness E, Wu T, Stigum H, Nafstad P. Acute mountain sickness, arterial oxygen saturation and heart rate among Tibetan students who reascend to Lhasa after 7 years at low altitude: a prospective cohort study. BMJ Open. 2017;7(7):e016460. Published 2017 Jul 10. doi:10.1136/bmjopen-2017016460.
26. Bärtsch P, Gibbs JS. Effect of altitude on the heart and the lungs. Circulation. 2007 Nov 6;116(19):2191-202. doi: 10.1161/CIRCULATIONAHA.106.650796. PMID: 17984389.
27. Mallet RT, Burtscher J, Richalet JP, Millet GP, Burtscher M. Impact of High Altitude
on Cardiovascular Health: Current Perspectives. Vasc Health Risk Manag. 2021;17:317-335. Published 2021 Jun 8. doi:10.2147/VHRM.S294121
The Effect of Nitrate Supplementation on Oxygen Saturation Level asAcute Mountain Sickness Prevention:ASystematic Review and Meta-Analysis
Muhammad Rizqi Tri Nafi’an1, Imtiyaz Hafizah Zahra1,DanindraArio Wiryawan1, Qonita Jayanti Wijayatno1
1Faculty of Medicine, Public Health and Nursing, UniversitasGadjah Mada, Indonesia Asian Medical Students’Association Indonesia
Abstract
Introduction:Forthosewhowerenotwell-adaptedtohighaltitude,thereisariskof sufferingfromahigh-altitudeillness(HAI),likeacutemountainsickness(AMS).Theoretically, nitratesupplementationcouldincreasethebody'sresistancetohypoxia.However,theevidenceis stillinconclusive.Therefore,thisstudyaimedtosystematicallyreviewtheeffectofnitrate supplementationonbloodoxygensaturationandAMSsymptoms. Methods:Tworeviewers independentlysearchedpublishedstudiesfromPubMed,Scopus,andCochraneLibrary databasesusingpre-registeredsearchstrategiesfrominceptiontoOctober2022,followinga registeredprotocolonPROSPERO(ID=CRD42022365347).Studyinclusioncriteriawere(1) randomizedcontrolledtrials;(2)studypopulationconsistedofnon-acclimated people/lowlanders;(3)interventionusingdietarynitratesupplementationinformofbeetroot juiceornitratecompound;(4)comparatorusingplaceboorothersupplementationthatdidn’t containnitrate;(5)outcome,whichisoxygensaturation.TheROB2.0toolwasusedtoassess riskofbiasintheincludedstudies.Resultswerenarrativelydescriptivebasedonavotecounting analysis,thenmetaanalysiscomputesusingrandomeffectsmodelswereconducted. Results: A totalof11recordsfrom3randomizedcontrolledtrialstudiesand8randomizedcrossover studieswereincluded.Thevotecountingfavoredthemajorityofstudiesthatstatethatnitrate supplementationcanincreaseoxygensaturationbutisstatisticallyinsignificant.Themeta analysisnon-statisticallysignificantfavorednitratesupplementationdecreasedbloodoxygen levelby–0.7%(95%CI–2.3to0.9)inrestingstate,improvingbloodoxygensaturationby 0.35%(95%CI–1.56to2.27)insubmaximalexercise,andimprovingbloodoxygensaturation by0.73%(95%CI–3.14to4.6)inmaximalexercise. Conclusion: Nitratesupplementation improves oxygen saturation and reduces the risk ofAMS occurrence at high altitude.
Keywords: Highaltitude,nitratesupplementation,beetroot,oxygensaturation,bloodoxygen level
The Effect of Nitrate Supplementation on Oxygen Saturation Level asAcute Mountain Sickness Prevention:ASystematic
Review and Meta-Analysis
Asian Medical Students’Conference 2022
Authors:
Muhammad Rizqi Tri Nafi’an
Imtiyaz Hafizah Zahra DanindraArio Wiryawan Qonita Jayanti Wijayatno
Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Indonesia
Asian Medical Students’ Association Indonesia

The Effect of Nitrate Supplementation on Oxygen Saturation Level asAcute Mountain Sickness Prevention:ASystematic Review and Meta-Analysis
Abstract
Introduction:Forthosewhowerenotwell-adaptedtohighaltitude,thereisariskof sufferingfromahigh-altitudeillness(HAI),likeacutemountainsickness(AMS). Theoretically,nitratesupplementationcouldincreasethebody'sresistancetohypoxia. However,theevidenceisstillinconclusive.Therefore,thisstudyaimedtosystematically reviewtheeffectofnitratesupplementationonbloodoxygensaturationandAMSsymptoms. Methods:TworeviewersindependentlysearchedpublishedstudiesfromPubMed,Scopus, andCochraneLibrarydatabasesusingpre-registeredsearchstrategiesfrominceptionto October2022,followingaregisteredprotocolonPROSPERO(ID=CRD42022365347). Studyinclusioncriteriawere(1)randomizedcontrolledtrials;(2)studypopulationconsisted ofnon-acclimatedpeople/lowlanders;(3)interventionusingdietarynitratesupplementation informofbeetrootjuiceornitratecompound;(4)comparatorusingplaceboorother supplementationthatdidn’tcontainnitrate;(5)outcome,whichisoxygensaturation.The ROB2.0toolwasusedtoassessriskofbiasintheincludedstudies.Resultswerenarratively descriptivebasedonavotecountinganalysis,thenmetaanalysiscomputesusingrandom effectsmodelswereconducted. Results: Atotalof11recordsfrom3randomizedcontrolled trialstudiesand8randomizedcrossoverstudieswereincluded.Thevotecountingfavoredthe majorityofstudiesthatstatethatnitratesupplementationcanincreaseoxygensaturationbut isstatisticallyinsignificant.Themetaanalysisnon-statisticallysignificantfavorednitrate supplementationdecreasedbloodoxygenlevelby–0.7%(95%CI–2.3to0.9)inresting state,improvingbloodoxygensaturationby0.35%(95%CI–1.56to2.27)insubmaximal exercise,andimprovingbloodoxygensaturationby0.73%(95%CI–3.14to4.6)inmaximal exercise. Conclusion: Nitratesupplementationimprovesoxygensaturationandreducesthe risk ofAMS occurrence at high altitude.
Keywords: Highaltitude,nitratesupplementation,beetroot,oxygensaturation,bloodoxygen level
Introduction
Highlandsandmountainsoftenbecometouristdestinationsformanypeopleinthe world.Forexample,in2019,1.2milliontouristswerevisitingNepal1.Nepalisoneofthe countrieswithgeographicalconditionsandlandscapesintheformofhighlandsand
1
1
mountains.Nepal'sgeographicalconditionsvaryinaltitude.Thehillyareaisatanaltitudeof 610-4800 m, while the mountainous area is at 4800-88392.
Varioushealthproblemsathighaltitudestartfromlowoxygenlevelsinthebody.For lowlanders,beingathighaltitudesisastressorforthebody3.Wheninanareawithan altitudeof2500mabovesealevel,thereisariskoftouristssufferingfromahigh-altitude illness(HAI)4HAIcanoccurduetohypobarichypoxiaconditionsathighaltitude.HAIis oftencategorizedinto3,namelyacutemountainsickness(AMS),high-altitudepulmonary edema (HAPE), and high-altitude cerebral edema (HACE)5.
TheincidenceofAMSwillincreaseasaltitudeincreases.Atanaltitudeof3000m, AMSincidentscanoccurinapproximately75%ofunacclimatizedtourists6.Somepeople maynotrealizetheyhaveAMS.TheclinicalmanifestationsofAMShaveoftenbeenhidden somanypeopleunderestimateit.Symptomsthatoftenappeararenausea,dizziness, headache,andfatigue7.ThereisascoringmethodcalledtheLake-LouiseScore(LLS)that canbeusedtodiagnoseAMSwithamaximumscoreof12.Scoresof3-5arecategorizedas mild,6-9asmoderate,and10-12assevere5.AMSconditionsthatcontinuetooccurcan trigger the emergence of HAPE and HACE with more severe clinical manifestations.
Theconditionofhypobarichypoxiaisthemainstressorofthebodythatcauses variousclinicaldisordersathighaltitude.Hypoxiacanleadtohypoxemiaandcausecell death3.Forhighlanders,ithappenslessbecausetheyhavebeenacclimatizedandadaptedto low-pressureareas3.Interestingly,severalstudieshaveshownthathighlandershave physiologicaldifferencesinbodychemicalcompositionfromlowlanders,oneofthemis NitricOxide.NOisanitratecompoundthatactsasavasodilator8.Highlandershavehigher amounts of NO compounds than lowlanders9–11.
Thishasattractedtheattentionofresearchersregardingthefunctionofnitratein reducingtheriskofhypoxiaforlowlanderslivinginhighlands.Nitratesarefoundinmany vegetables,especiallybeetroot,spinach,andlettuce.Thenitratecompoundsinthese vegetablesareinanactiveformandcanimmediatelyperformtheirfunctionsinthebody12. Variousstudiesthataimtodeterminethefurtherfunctionofnitratesupplementationuse beetrootasasourceofnitrate.Thehighamountofnitrateanditsactiveformmakebeetroot chosen as the dietary nitrate used in many studies.
Nitratesarethoughttoreducetheriskofhypoxiabyincreasingoxygendeliveryinthe body.Nitratesaresaidtoimprovethefunctionofthemicrocirculation,optimizetheuseof oxygenforATPproduction,andimprovethefunctionoftheendothelialbloodvessels12. However,otherstudieshaveshowntheoppositeresult.Nitratesupplementationincreases
2
2
acutemountainsicknessseverityandsenseofeffortduringhypoxicexercise13.Whilesome studiesshowthatnitratesupplementationdoesnotsignificantlyincreasethebody'sresistance tohypoxiaconditions14.Notonlythat,untilnowtherehasbeennosystematicreviewthat summarizestheeffectivenessofnitratesupplementationinincreasingthebody'sresistanceto hypoxiaconditions.Thus,asystematicreviewshouldbecarriedouttoidentify,evaluate,and summarizeinanunbiasedmannerthefindingofallrelevantindividualstudiesassessingthe effect of nitrate supplementation on low lander’s adaptation to the high altitude.
Methods
AsystematicreviewwasconductedbasedonaregisteredprotocolonPROSPERO (CRD42022365347),whichwasdevelopedinadvanceofthereviewinaccordancewith guidelinesfromtheCochraneHandbookforSystematicReviewandthePreferredReporting Items for Systematic Reviews and Meta-Analyses 2020 Statement.
Inclusion and exclusion criteria
Theauthorsappliedsomeinclusionandexclusioncriteriainmakingthisreview.The inclusioncriteriaforthisstudywere(1)randomizedcontrolledtrials;(2)studypopulation consistedofnon-acclimatedpeople/lowlanders;(3)interventionusingdietarynitrate supplementationinformofbeetrootjuiceornitratecompound;(4)comparatorusingplacebo orothersupplementationthatdidnotcontainnitrate;(5)outcome,whichisoxygen saturation, measured by either blood oxygen levels or peripheral oxygen saturation.
Theexclusioncriteriaforthisstudywere(1)unsuitabletypesofarticles;(2) population consisted of animals; (3) inaccessible studies; (4) study without a control group.
Search method
Theauthorsusedpublishedstudiesfromthreemajordatabases:PubMed,Scopus,and CochraneLibrarydatabasesfrominceptiontoOctober2022.Weappliedthefollowing keywords.AcombinationofMeSHtermsandfreetermsfromkeywords"highaltitude"and ("beetroot"or"nitrate")wereusedtodevelopthesearchstrategy.Thecompletesearch strategyofeachdatabasecanbefoundintheprosperoregistration.Thesearchwaslimitedto studiespublishedinEnglish.Theoutcomeandtypeofstudywereappliedatthescreening stage.
Study selection
3
3
Tworeviewers(M.R.T.N.,Q.J.W.)conductedtwostagesofthescreeningprocess usingtheRayyansoftware15.Beforeconductingthetwostagesscreening,theyremoved duplicatestudiesusingtheRayyan.Forthefirststage,theyscreenedindependentlytitlesand abstractsofallstudiesbycategorizingtheminto"Include","Exclude",and"Maybe".Studies arecategorizedexplicitlyonlyiftheyhavedifferentinterventions,populations,andtypesof studies.Abstractsthatdidnotreportthehealth-researchoutcomesthatwedesiredarenot excludedsincethemajorityofabstractsinresearchdidnotfullyreportalloftheirresearch outcomes.Inthesecondstage,theyscreenedthefulltextofstudiesincludedinthe"Include" and"Maybe"categories.Thethirdreviewer(I.H.Z)facilitatedasajudgetoresolveany disagreements in the first and second stages of the screening process.
Tworeviewers(I.H.Z.,D.A.W.)developedacustomdataextractionform.Outcome dataforallincludedstudieswereindependentlyextractedbythreereviewers(M.R.T.N., I.H.Z., Q.J.W.).Another reviewer (D.A.W.) facilitated a discussion to resolve discrepancies.
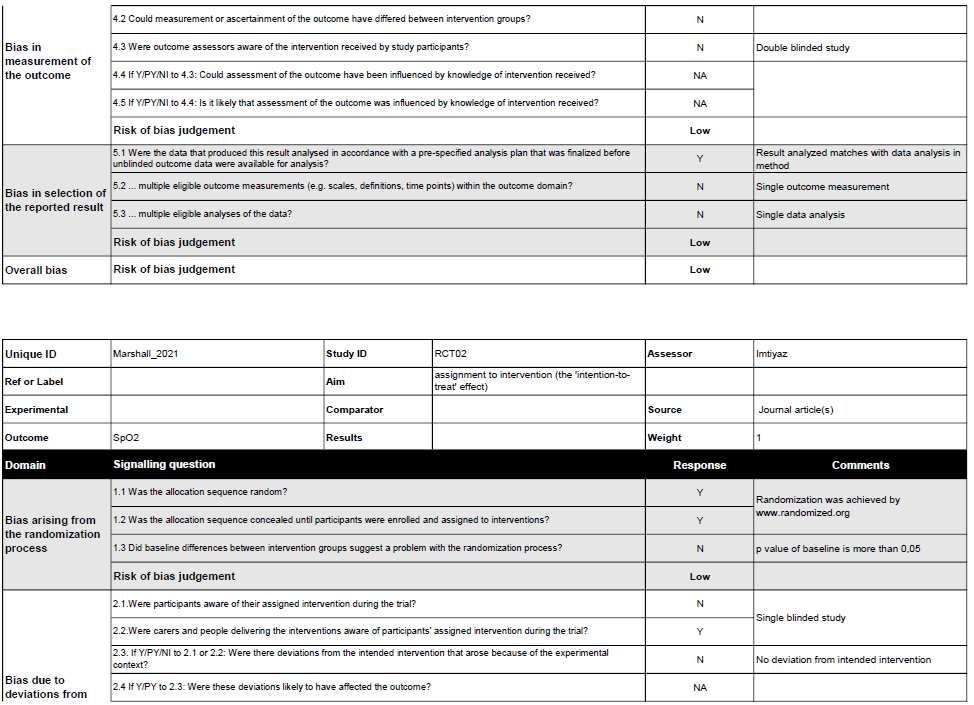
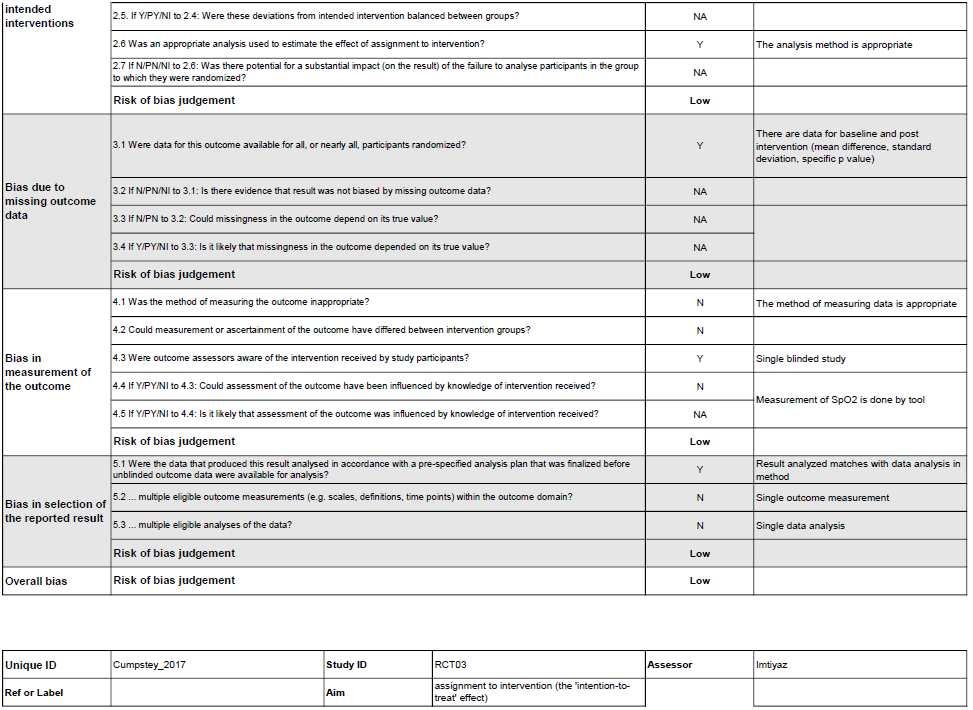
Onereviewer(I.H.Z)assessedtheriskofbiasusingtheCochraneRiskofBias2.0 toolforRandomizedControlledTrialandCrossoverTrial16.CochraneRiskofBias2.0tool forRandomizedControlledTrialconsistoffivedomainsassessment,asfollows:(D1) randomizationprocess,(D2)deviationsfromtheintendedinterventions,(D3)missing outcomedata,(D4)measurementoftheoutcome,(D5)selectionofthereportedresult. CochraneRiskofBias2.0toolforCrossoverTrialconsistofsixdomainsassessment,as follows:(D1)randomizationprocess,(DS)biasarisingfromperiodandcarryovereffects, (D2)deviationsfromtheintendedinterventions,(D3)missingoutcomedata,(D4) measurement of the outcome, (D5) selection of the reported result.
Study outcomes
Themainoutcomeofinterestobservedinthisreviewisoxygensaturation,measured byeitherbloodoxygenlevelsorperipheraloxygensaturation.Otherdatathatrepresent oxygensaturationindirectlyisalsoconsideredasthemainoutcome.Furthermore,thisreview alsoobservesacutemountainsicknesssymptomssuchasheadache,nausea,dizziness, fatigue,andsleepdisturbanceasadditionaloutcomesassessedusingLakeLouisescore (LLS) andAcute Mountain Sickness Cerebral Score (AMS-C).
Synthesis method
4
4
Theauthorswillpresentanarrativesummarythenquantitativeanalysis.We conducteda‘vote-counting’approachasapreliminaryanalysisbycategorizingtheresultsof eachoutcomeintofivecategoriesasfollows:(1)statisticallysignificantpositiveeffects favoringthenitratesupplementationgroup,(2)non-statisticallysignificantpositiveeffects favoringthenitratesupplementationgroup,(3)statisticallysignificantnegativeeffects favoringthenitratesupplementationgroup,(4)non-statisticallysignificantnegativeeffects favoringnitratesupplementationgroup,and(5)notsignificantatall.Theresultsofthevote countingwerebasedonthehighestnumberofvotescountedoneachoutcomemeasureto obtainanindicationoftheeffectivenessofdietarynitratesupplementation.Thiswillaidthe narrativesynthesis(https://doi.org/10.1136/bmj.l6890).Then,meta-analyseswereperformed usingReviewManagerSoftwareversion5.3(RevManv5.3,TheCochraneCollaboration, Oxford,UK)forthelevelofoxygensaturationoutcomesincequantitativedatafromtwoor morestudieswereavailableandappropriate.Arandomeffects’modelusingmeandifference ofthechangefrombaselinewasusedsincetherewasclinicalheterogeneityresultingfrom varietyinthealtitudesandnitratesupplementationdoses.Whentheindividualstudiesdidnot providethestandarddeviation(SD)ofthechangefrombaseline,weobtaineditbyimputing itfromthebaselineSDandposttreatmentSDusingthecoefficientcorrelation.Visual inspectionofforestplotandHigginsI²statisticusedtoassessheterogeneity.Wealso examinedpublicationbiasusingthefunnelplot.Thequalityofevidenceofoursynthesiswas evaluatedusingtheGradingofRecommendations,Assessment,DevelopmentandEvaluation (GRADE) approach.
5
5
Results
Search results
Weidentified544recordsthroughdatabasesearches.Afterconductingtwostagesof screening,weincluded11studiesinthesystematicreview.Amongtheincludedstudies,there werefourrandomizedcontrolledtrialsstudiesandsevenrandomizedcrossovertrialstudies.
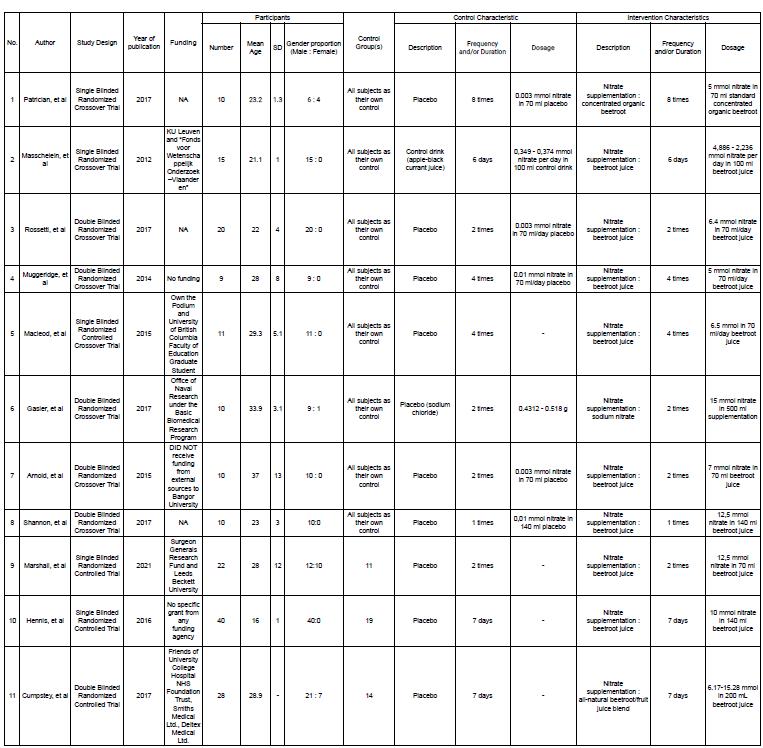
Characteristics of included studies
Afterscreeningandrecheckingtomaintainuniformityandcompatibilitywith inclusionandexclusioncriteria,threerandomizedcontrolledtrialsandeightrandomized crossovertrialstudiesareincluded.Thedetailsofincludedstudiesareshownin Appendix1. Fromtheincludedstudies,threestudiesconductedthetrialintherealsettingofhighaltitude, andthereststudieswereconductedinahypoxiachamber.Allofthestudieswerepublished between2012until2021.Thenumberofparticipantsintheincludedstudiesrangedfrom9to 40.Themeanageoftheparticipantsrangedfrom16yearsto40years.Alloftheincluded studies recruited male subjects and five of those studies recruited female subjects.
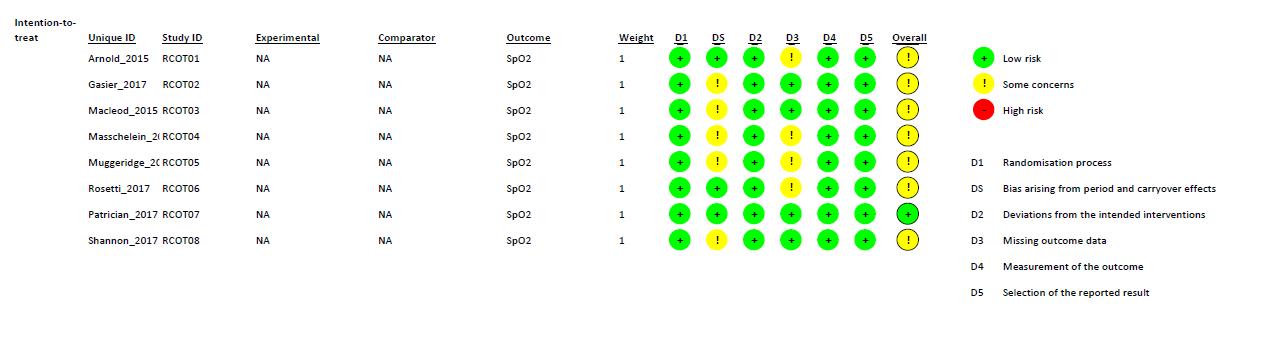
Risk of Bias in individual studies (Qualitative Synthesis)

6
6
Figure 2. Risk of Bias summary using Cochrane Risk of Bias 2.0 tool for Randomized Controlled Trial study.
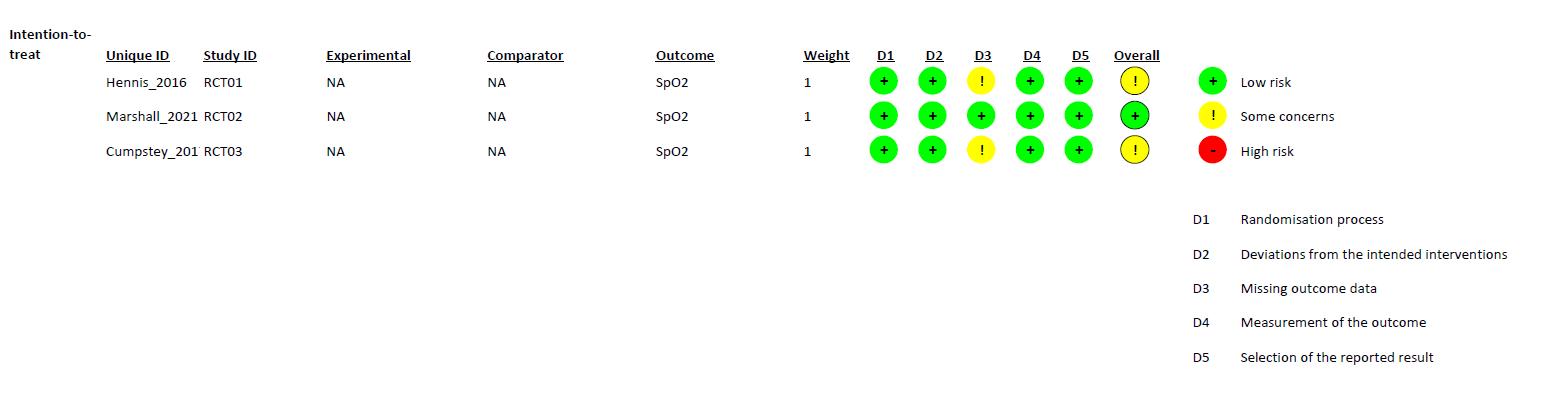
Figure 3. Risk of Bias summary using Cochrane Risk of Bias 2.0 tool for Randomized Crossover Trial study.
WecriticallyassessedthequalityofeachstudywiththeCochraneRiskofBias2.0 toolforRandomizedControlledTrialandRandomizedCrossoverTrial.Thereisonestudy, conductedbyMarshalletal.thathasalowriskofbiasinalldomains.Someofthestudies didnotprovidefurtherinformationregardingthebiasdomains’judgment,whichledto “someconcerns” ofbias.MostofthebiasinRCTsresultedfromthecompletenessof outcomedata.AstudybyHennisetal.andastudybyCumpsteyetal.didn’treportthe baselinedata.ForRCOTsstudies,thereisonestudy,conductedbyPatrician,etal.hasalow riskofbiasinalldomains.TheotherRCOTsstudieshave“someconcerns”ofbias.Most biasesinRCOTsresultedfromDomainS(DS)andDomain3(D3).DSbiasresultedfroman unknownnumberofparticipantsallocatedtoeachgroup,eitherequalornearlyequal.D3bias resultedfromthecompletenessofoutcomedata.StudiesbyArnold,etal.,Masschelein,etal., Muggeridge, et al., and Rosetti, et al. didn’t report the baseline data.
7
7
Vote-counting
Study ID Design Condition
Patrician, 2017








Single Blinded Randomized Crossover Trial Rest
Masschelein, 2012 (a)
Single Blinded Randomized Crossover Trial Rest
Intervention dosage
LLS AMS-C
Oxygen level Acute mountain sickness symptoms improvement Dose Frequency and/or duration vs. Control vs. Control
5 mmol nitrate in 70 ml standard concentrated organic beetroot
4,886 - 2,236 mmol nitrate per day in 100 ml beetroot juice
8 times
6 days Marshall, 2021
Single Blinded Randomized Controlled Trial Rest 12,5 mmol nitrate in 70 ml beetroot juice 2 times Hennis, 2016 (a)
Single Blinded Randomized Controlled Trial Rest 10 mmol nitrate in 140 ml beetroot juice 7 days
8
8
Cumpstey, 2017
Double Blinded Randomized Controlled Trial Rest 6.17-15.28 mmol in 200 mL beetroot juice 7 days
Shannon, 2017 (a)
Double Blinded Randomized Crossover Trial Rest 12,5 mmol nitrate in 140 ml beetroot juice 1 times
Gasier, 2017 (a)







Double Blinded Randomized Crossover Trial Rest 15 mmol nitrate in 500 ml supplementation 2 times
Hennis, 2016 (b)
Single Blinded Randomized Controlled Trial
Submaxima l exercise
10 mmol nitrate in 140 ml beetroot juice 7 days
Masschelein, 2012 (b)
Single Blinded Randomized Crossover Trial
Submaxima l exercise
4,886 - 2,236 mmol nitrate per day in 100 ml beetroot juice
6 days
Shannon, 2017 (b)
12,5 mmol nitrate in 140 ml beetroot juice 1 times
Submaxima l exercise 9
9
Double Blinded Randomized Crossover Trial
Gasier, 2017 (b)







Double Blinded Randomized Crossover Trial
Submaxima l exercise (resistance)
15 mmol nitrate in 500 ml supplementation 2 times
Rossetti, 2017
Double Blinded Randomized Crossover Trial
Submaxima l exercise
6,4 mmol nitrate in 70 ml/day beetroot juice 6 days
Arnold, 2015 (a)
Double Blinded Randomized Crossover Trial
Submaxima l exercise
7 mmol nitrate in 70 ml beetroot juice 2 times
MacLeod, 2015 (a)
Single Blinded Randomized Controlled Crossover Trial
Submaxima l exercise
6,5 mmol in 70 ml/day beetroot juice 4 times
Muggeridge, 2013
Double Blinded Randomized Crossover Trial
Masschelein, 2012 (c)
Submaxima l exercise
5 mmol nitrate in 70 ml/day beetroot juice 4 times
Maximal exercise 4,886 - 2,236 mmol nitrate per 6 days
10
Single Blinded Randomized Crossover Trial 10
MacLeod, 2015 (b)






Single Blinded Randomized Controlled Crossover Trial


Maximal exercise
day in 100 ml beetroot juice
6,5 mmol in 70 ml/day beetroot juice 4 times
12,5 mmol nitrate in 140 ml beetroot juice 1 times Arnold, 2015 (b)
Maximal exercise
Double Blinded Randomized Crossover Trial = increased significantly = decreased significantly = not significant at all = increased but not significant = decreased but not significant
7 mmol nitrate in 70 ml beetroot juice 2 times
Maximal exercise 11
Table 1. Vote-Counting Results Ingeneral,thereisatendencythatnitratesupplementationcanincreaseoxygensaturation,asindicatedbythemajorityofstudies(10/19) (Masschelein,2012;Shannon,2017;Arnold,2015;MacLeod,2015;Muggeridge,2013)showedimprovement,butonly4of themshowed
11
Shannon, 2017 (c)
Double Blinded Randomized Crossover Trial
statisticallysignificantimprovement(Masschelein,2012;Shannon,2017)comparedto1studyarmthatshowedreducedoxygensaturation statisticallysignificant(Marshall,2021)(Table1).Insubmaximalandmaximalexerciseconditions,thistendencyisalsofound.Mostofthe studyarmsunderexerciseconditions,bothsubmaximalandmaximal,showedanincreasedoxygensaturationalthoughnotallofthemwere statisticallysignificant,5/8(Masschelein,2012;Shannon,2017;Arnold,2015;MacLeod,2015;Muggeridge,2013) and3/4(Masschelein,2012; Shannon,2017;Arnold,2015)respectively.However,inrestingconditions,thereisadifferenttendency,inwhichthemajorityofstudies(4/7) showedadecrease(Patrician,2017;Marshall,2021;Cumpstey,2017;Gasier,2017)althoughnotallofthemwerestatisticallysignificantand only 1 study showed a statistically significant decrease (Marshall, 2021).
12
12
Meta analysis
Figure 4. Forest Plot of Oxygen Saturation
TwoRCOTstudiesassessingarterialbloodoxygensaturation(SaO2)andfivestudies (threeRCTandtwoRCOT)assessingperipheralbloodoxygensaturation(SpO2)were includedinthemeta-analyses.Thesesevenstudiesweredifferentiatedintothreestudy comparisonsasfollows,restingstate,submaximalexercise,andmaximalexercise.Inresting state,nitratesupplementationwasfoundtobenotbeneficialbecausebloodoxygensaturation wasdecreasedby–0.7%(95%CI–2.3to0.9).Thereisasmallheterogeneityandthequality ofincludedstudiesdon'tcontributetotheheterogeneitysincesensitivityanalysisexcluding onestudywithhighriskofbias(Patricianetal,2017)don'treducingtheI2anddon’tchange the direction of the effect.
Insubmaximalexercise,nitratesupplementationcouldbebeneficialforimproving bloodoxygensaturationby0.35%(95%CI–1.56to2.27)butthiseffectwasnotstatistically significant.Therewasnosignificantheterogeneityonbloodoxygensaturationoutcomein submaximal exercise.
Inmaximalexercise,nitratesupplementationwasfoundtohaveapotentialbenefits forimprovingbloodoxygensaturationby0.73%(95%CI–3.14to4.6).Unfortunately,the improvementwasnotstatisticallysignificant.Therewasnosignificantheterogeneityon blood oxygen saturation outcome in maximal exercise.

13
13
Overall,regardingbloodoxygensaturation,nitratesupplementationwasfoundtobe notbeneficialbecausebloodoxygensaturationdecreased0.21%(95%CI–1.35to0.93). Therewasnosignificantheterogeneityonbloodoxygensaturationoutcome.Insensitivity analysiseliminatingastudywithhighriskofbias,therewasnosignificanteffecton heterogeneity (I2 still 0%), meaning that the sourceof that low heterogeneity is not a bias.
Publication bias
Ingeneral,thefunnelplotissymmetrical(Figure5).However,thefunnelplotfor submaximalexerciseandmaximalexercisewasasymmetricalindicatingtherewas publication bias.
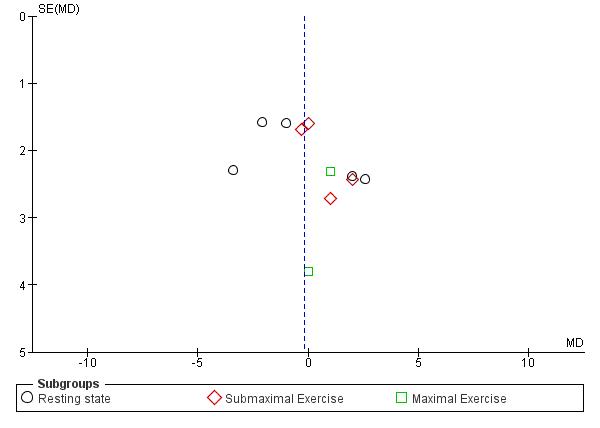
Figure 5. Funnel Plot of Oxygen Saturation
Quality of evidence
OurquantitativesynthesiswasresultedfromRCTsdirectlyassesstheoxygen saturationandhadanarrowCIwhichdidnotcrosstheminimalclinicallyimportant difference(6%increasedofSpO2)forhighaltitudeacclimatization (https://pubmed.ncbi.nlm.nih.gov/21123763).However,therewereseveralindividualstudies havingconcernsofbias.Therefore,thequalityofevidenceismedium.Forthesubmaximal exerciseandmaximalexercise,therewaspublicationbiasdroppingthequalityofevidence into a low quality of evidence.
14
14
Discussion
Inthisreview,wefoundthatthemajorityofstudiesfavorednitratesupplementation asafactorthatcouldincreaseoxygensaturationonhighaltitude.Positiveeffectofnitrate supplementationhasbeenseenonsubmaximalexerciseandmaximalexercisecondition.On theotherhand,inrestingconditionsnitratesupplementationdoesnotshowthesame tendencyastheotherconditions.Inaddition,theclinicalcorrelationofourfindingsisthe nitratesupplementationcanreducetheriskofAMSoccurrence.However,noroftheresults wasstatisticallysignificantandthequalityofevidencewasmedium.Therefore,further studies are still needed to provide stronger evidence.
Thecauseofthosefindingsinbothexerciseconditionscouldbebecausenitrate ingestionhasaneffectonperipheraloxygensaturationthroughimprovingitsefficiency17. Basedonourmetaanalysisdataresult,nitratesupplementationimprovedtheoxygen saturationby0.35%insubmaximalexerciseathighaltitude(95%CI-1.56to2.27,P=0.72). Besidesthat,nitratesupplementationalsoimprovedtheoxygensaturationby0.73%in maximalexerciseathighaltitude(95%CI-3.14to4.6,P=0.71).Thisdataprovedthatthere isapositiveeffectofnitratesupplementationonexerciseathighaltitude.Thenegativeeffect thathasbeenshowninrestconditionstudiescouldbebecausethefrequencyofnitrate supplementation administration is less than other studies that show positive results.
OursystematicreviewhasalreadybeeninaccordancewiththePRISMAguidelines, registeredintoPROSPERO,andfulfillingalmostalloftheAMeaSurementTooltoAssess SystematicReviewsversion2(AMSTAR2)checklist.Thissystematicreviewhaslimitations onaccessingfulldataofsomestudies,butweresolveitbyusingWebPlotDigitizertool https://apps.automeris.io/wpd/isalreadyindicatedtohavehighlevelsofintercoderreliability andvalidity26.Forthefuturestudies,researchersshouldprovidethefulldataoftheir findings so we could lower the risk of bias.
Conclusion
Thisreviewconcludesthatnitratesupplementationmayimproveoxygensaturation andreducetheriskofAMSoccurrenceathighaltitude.Howeverthequalityofevidencewas lowtomedium.Toprovidestrongerevidence,furtherstudiesarestillneededbyproviding full data on their findings to lower the risk of bias.
Funding: This review did not receive external funding.
15
15
Reference
1.DevelopmentandimportanceoftourismforNepal[Internet].[cited2022Oct22]. Available from: https://www.worlddata.info/asia/nepal/tourism.php
2.GeographyofNepal-EmbassyofNepal-Tokyo,Japan[Internet].[cited2022Oct 22].Available from: https://jp.nepalembassy.gov.np/geography-of-nepal/
3.BrownJPR,GrocottMPW.Humansataltitude:physiologyandpathophysiology. ContinuingEducationinAnaesthesiaCriticalCare&Pain[Internet].2013Feb1 [cited2022Oct22];13(1):17–22.Availablefrom: https://academic.oup.com/bjaed/article/13/1/17/281180
4.Altitudesickness-NHS-NHS[Internet].[cited2022Oct22].Availablefrom: https://www.nhs.uk/conditions/altitude-sickness/
5.AkselG,Ç ŞK,ÖzenC.High-altitudeillness:Managementapproach.Turk JEmergMed[Internet].2019Oct1[cited2022Oct22];19(4):121.Availablefrom: /pmc/articles/PMC6819752/
6.PrinceTS,ThurmanJ,HuebnerK.AcuteMountainSickness.StatPearls[Internet]. 2022Jul12[cited2022Oct22];Availablefrom: https://www.ncbi.nlm.nih.gov/books/NBK430716/
7.LeissnerKB,MahmoodFU.Physiologyandpathophysiologyathighaltitude: considerationsfortheanesthesiologist.JAnesth[Internet].2009Nov[cited2022Oct 22];23(4):543–53.Available from: https://pubmed.ncbi.nlm.nih.gov/19921365/
8.AhmadA,DempseySK,DanevaZ,AzamM,LiN,LiPL,etal.RoleofNitricOxide intheCardiovascularandRenalSystems.IntJMolSci[Internet].2018Sep3[cited 2022 Oct 22];19(9).Available from: /pmc/articles/PMC6164974/
9.ErzurumSC,GhoshS,JanochaAJ,XuW,BauerS,BryanNS,etal.Higherblood flowandcirculatingNOproductsoffsethigh-altitudehypoxiaamongTibetans.Proc
16
Conflicts of Interests: The authors declare no conflictof interests.
16
NatlAcadSciUSA[Internet].2007Nov6[cited2022Oct22];104(45):17593–8. Available from: https://pubmed.ncbi.nlm.nih.gov/17971439/
10.BeallCM,LaskowskiD,StrohlKP,SoriaR,VillenaM,VargasE,etal.Pulmonary nitricoxideinmountaindwellers.Nature[Internet].2001Nov22[cited2022Oct 22];414(6862):411–2.Available from: https://pubmed.ncbi.nlm.nih.gov/11719794/
11.HoitBD,DaltonND,ErzurumSC,LaskowskiD,StrohlKP,BeallCM.Nitricoxide andcardiopulmonaryhemodynamicsinTibetanhighlanders.JApplPhysiol(1985) [Internet].2005Nov[cited2022Oct22];99(5):1796–801.Availablefrom: https://pubmed.ncbi.nlm.nih.gov/16024527/
12.DaunceyS.CanDietaryNitrateSupplementsImproveTolerancetoHypoxia? http://dx.doi.org/101177/175114371201300306[Internet].2012Jul1[cited2022Oct 22];13(3):198–204.Availablefrom: https://journals.sagepub.com/doi/abs/10.1177/175114371201300306
13.RossettiGMK,MacDonaldJH,WylieLJ,LittleSJ,NewtonV,WoodB,etal.Dietary nitratesupplementationincreasesacutemountainsicknessseverityandsenseofeffort duringhypoxicexercise.JApplPhysiol(1985)[Internet].2017Oct1[cited2022Oct 22];123(4):983–92.Available from: https://pubmed.ncbi.nlm.nih.gov/28684588/
14.HennisPJ,CumpsteyAF,O’DohertyAF,FernandezBO,Gilbert-KawaiET,Mitchell K,etal.DietaryNitrateSupplementationDoesNotAlterExerciseEfficiencyatHigh Altitude–FurtherResultsFromtheXtremeAlpsStudy.FrontPhysiol.2022Feb 28;13:138.
15.OuzzaniM,HammadyH,FedorowiczZ,ElmagarmidA.Rayyan-awebandmobile appforsystematicreviews.SystRev[Internet].2016Dec5[cited2022Oct 22];5(1):1–10.Availablefrom: https://link.springer.com/articles/10.1186/s13643-016-0384-4
16.RiskofBias2(RoB2)tool|CochraneMethods[Internet].[cited2022Oct22]. Available from: https://methods.cochrane.org/risk-bias-2
17.MasscheleinE,vanThienenR,WangX,vanSchepdaelA,ThomisM,HespelP. Dietarynitrateimprovesmusclebutnotcerebraloxygenationstatusduringexercisein
17
17
hypoxia.JApplPhysiol(1985)[Internet].2012Sep1[cited2022Oct 22];113(5):736–45.Available from: https://pubmed.ncbi.nlm.nih.gov/22773768/
18.ShannonOM,DuckworthL,BarlowMJ,DeightonK,MatuJ,WilliamsEL,etal. EffectsofDietaryNitrateSupplementationonPhysiologicalResponses,Cognitive Function,andExercisePerformanceatModerateandVery-HighSimulatedAltitude. FrontPhysiol[Internet].2017Jun9[cited2022Oct22];8(JUN).Availablefrom: https://pubmed.ncbi.nlm.nih.gov/28649204/
19.ArnoldJT,OliverSJ,Lewis-JonesTM,WylieLJ,MacdonaldJH.Beetrootjuicedoes notenhancealtituderunningperformanceinwell-trainedathletes.ApplPhysiolNutr Metab[Internet].2015Feb10[cited2022Oct22];40(6):590–5.Availablefrom: https://pubmed.ncbi.nlm.nih.gov/25942474/
20.20.MacLeodKE,NugentSF,BarrSI,KoehleMS,SporerBC,MacInnisMJ. AcuteBeetrootjuicesupplementationdoesnotimprovecyclingperformancein normoxiaormoderatehypoxia.IntJSportNutrExercMetab.2015Aug 1;25(4):359–66.
21.MuggeridgeDJ,HoweCCF,SpendiffO,PedlarC,JamesPE,EastonC.Asingledose ofbeetrootjuiceenhancescyclingperformanceinsimulatedaltitude.MedSciSports Exerc. 2014 Jan;46(1):143–50.
22.MarshallAR,RimmerJE,ShahN,ByeK,KippsC,WoodsDR,etal.Marchingtothe Beet:Theeffectofdietarynitratesupplementationonhighaltitudeexercise performanceandadaptationduringamilitarytrekkingexpedition.NitricOxide [Internet].2021Sep1[cited2022Oct22];113–114:70–7.Availablefrom: https://pubmed.ncbi.nlm.nih.gov/34051342/
23.PatricianA,EnganH,LundstenD,GroteL,Vigetun-HaugheyH,SchagatayE.The EffectofDietaryNitrateonNocturnalSleep-DisorderedBreathingandArterial OxygenDesaturationatHighAltitude.HighAltMedBiol[Internet].2018Mar1 [cited2022Oct22];19(1):21–7.Availablefrom: https://pubmed.ncbi.nlm.nih.gov/29211505/
18
18
24.CumpsteyAF,HennisPJ,Gilbert-KawaiET,FernandezBO,PoudevigneM,CobbA, etal.Effectsofdietarynitrateonrespiratoryphysiologyathighaltitude-Results fromtheXtremeAlpsstudy.NitricOxide[Internet].2017Dec1[cited2022Oct 22];71:57–68.Available from: https://pubmed.ncbi.nlm.nih.gov/29042272/
25.GasierHG,ReinholdAR,LoiselleAR,SoutiereSE,FothergillDM.Effectsoforal sodiumnitrateonforearmbloodflow,oxygenationandexerciseperformanceduring acuteexposuretohypobarichypoxia(4300m).NitricOxide[Internet].2017Sep30 [cited2022Oct22];69:1–9.Availablefrom: https://pubmed.ncbi.nlm.nih.gov/28684191/
26.DrevonD,FursaSR,MalcolmAL.IntercoderReliabilityandValidityof WebPlotDigitizerinExtractingGraphedData. http://dx.doi.org/101177/0145445516673998[Internet].2016Oct18[cited2022Oct 22];41(2):323–39.Availablefrom: https://journals.sagepub.com/doi/abs/10.1177/0145445516673998?journalCode=bmo a
19
19
Appendix 1. Extraction Data
20 Appendix
20
21 21


22 22

23 23

24 24

25 RCT 25
26 26
27 27
28 RCOT 28
Acetazolamide Effect on Heart Rate as Prophylaxis of Altitude Sickness: A Meta Analysis of Randomized Controlled Trials
Siti Zahra Arfiani1, Siti Faizatul Aliyah1, Sekar Arum Srigati1, Muhammad Syifaul Afnan1
1Faculty of Medicine University of Jember, Jember, Indonesia
Abstract
Introduction: Altitude sickness is a term used to describe a number of acute syndromes that may occur in unacclimatized individuals at high altitude. High altitude exposure can lead to changes in heart beats which may be linked to AMS development. Acetazolamide is commonly used to prevent and treat AMS in which need to be investigated regarding its effect with the increase of heart rate (HR) in high altitude.
Objective: This systematic review and meta-analysis is aimed to investigate the effects of acetazolamide prophylaxis on HR to prevent altitude sickness.
Materials and Methods: This study was reported based on criteria from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A literature search was conducted with multiple electronic databases. Risk of biases were assessed for each study using the RoB 2 tool. Mean and Standard Deviation (SD) with the confidence interval (CI) of 95% were used to determine the association between acetazolamide and the change of HR in high altitude. Fixedand Random Effect Model wasused based on heterogeneity level and p value <0.05 was considered statistically significant.
Results: The current study showed that the effects of 500 mg dose acetazolamide were not significant (pooled MD= 0.22, 95% CI (-0.19 0.64), p=0.04, I2=58%).
While the administration of 250 mg dose acetazolamide also not significant (pooled MD= -0.15 95% CI (-1.11-0.81), p= 0.76, I²=71%).
Conclusion: This study provides valuable evidence suggesting that acetazolamide is not significant in HR alteration prior to altitude sickness.
Keywords: Altitude sickness, acetazolamide, heart rate, systematic review, metaanalysis
Acetazolamide Effect on Heart Rate as Prophylaxis of Altitude Sickness: A Meta Analysis of Randomized Controlled Trials
Pre-Conference Competition East Asian Medical Students’ Conference 2023
Authors:
Siti Zahra Arfiani
Siti Faizatul Aliyah
Sekar Arum Srigati
Muhammad Syifaul Afnan
FACULTY OF MEDICINE
UNIVERSITY OF JEMBER 2021

Acetazolamide Effect on Heart Rate as Prophylaxis of Altitude Sickness: A Meta Analysis of Randomized Controlled Trials Siti Zahra Arfiani1, Siti Faizatul Aliyah1, Sekar Arum Srigati1, Muhammad Syifaul Afnan1
1Faculty of Medicine University of Jember, Jember, Indonesia
INTRODUCTION
In the past five years, more than 100 million people have traveled to high altitude for work or leisure activities and they are likely to develop symptoms of high-altitude illness (HAI). Altitude sickness is a term used to describe a number of acute syndromes that may occur in unacclimatized individuals at high altitude(1). It is characterized by the development of some or all symptoms of headache, weakness, fatigue, listlessness, nausea, insomnia, and suppressed appetite. Altitude sickness can cause several pathological presentations, including High Altitude Pulmonary Edema (HAPE) and High Altitude Cerebral Edema (HACE). Those are both life-threatening emergencies requiring immediate treatment, while Acute Mountain Sickness (AMS) can be prevented or managed with oral medication. Although, AMS is usually self-limiting, the symptoms may affect well-being as well as motor and cognitive function and in some cases AMS can progress to potentially fatal conditions such as HAPE and HACE(2).
The effects of elevatedaltitude on the human bodyare numerous. The major cardiovascular-related effects associated with elevated altitudes are decreased oxygen delivery to tissues, increased pulmonary vasoconstriction, and increased sympatheticnervousoutflow(3).Of all thecardiovascular-relatedeffectsofexposure to altitude, the heart rate is one of the most well recognized physiological responses under hypobaric hypoxia. High altitude exposure can lead to changes in heart rate variability (HRV) which may be linked to AMS development(4). The research of how elevated altitude exposure affects heart rate will be very useful because it will improve our understanding of how the human body adapts to high altitude. As the number of wilderness travelers increase, the physicians are expected to provide prophylaxis or self-treatment to prevent the occurrence of altitude sickness.
Acetazolamide is a medication commonly used to prevent and/or treat AMS during rapid ascent to high altitude(5). Acetazolamide works by blocking carbonic anhydrase and then acidifying the blood and reducing the respiratory alkalosis associated with high altitude, thus increasing respiration and arterial oxygenation and speeding acclimatization(6). In spite of the wide-range research on the use of acetazolamide to prevent altitude sickness, a systematic review and meta-analysis that specifically investigates the effect of acetazolamide prophylaxis on heart rate has never been conducted. This study is aimed to investigate the effects of acetazolamide prophylaxis on heart rate to prevent altitude sickness. Furthermore, the result of this study could complement research and help both physician and policy maker to establish the guideline for preventing altitude sickness.
MATERIAL AND METHODS Study Methodology
We adhered this study to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines.
Eligibility Criteria
The following criteria were considered for this studies’ eligibility: type of study, samples, outcomes, index test, and reference standards.
Type of studies
Original research articles conducted using acetazolamide to sample in altitude settings with full-text availability, and written in English were included. Narrative review, systematic review, meta-analysis, non-comparative research, technical reports, editor response, scientific poster, study protocols, conference abstracts, and other articles irrelevant to the topics were excluded.
Samples
Healthy participants treated with acetazolamide in altitude settings were included in this study. There was no limitation for the altitude level and dosage for the acetazolamide.
Outcomes
The outcome in this study is the assessment of participants’ heart beat measured in the altitude treated by acetazolamide and placebo in various dosage.
Index test
Studies evaluating the heart beats of healthy patients in altitude with acetazolamide and placebo treatment were included. Studies without heart beats measurement post-acetazolamide and placebo mean and standard deviation in 500 mg and 250 mg dosage were included only in qualitative analysis.
Reference Standard
The reference standard was experimental laboratory research performed by qualified professionals by evaluating the effect of acetazolamide treatment on heart beats alteration in altitude settings.
Data Sources and Search
A literature search process was carried outwith multiple electronic databases, such as PubMed, ScienceDirect, Cochrane Library, SpringerLink, and Google Scholar. The literature search of the database was conducted until October 2022. The keywordsused in electronic databaseswere describedusingBooleanoperators. All the studies from these databases were stored in the authors’ library in Rayyan.ai.
Study Selection
After the removal of duplicated articles, retrieved articles were screened based on the titles and abstracts by two independent reviewers (SFA and MSA). Potentially eligible full-text articles were thoroughly assessed using the eligibility criteria described above. Any emerging discrepancies were resolved by consensus among the review team. The study selection process was recorded in the PRISMA flow chart.
Data Extraction and Analysis
Selected studies were extracted with Microsoft Excel 2016 (Microsoft Corporation, USA) and Rayyan.ai. The following data were recorded: first author, year, setting, study design, treatment type, assessment period, sample size, heart rate (acetazolamide, placebo), p-value, heart rate assessment, way to altitude, and altitude. All statistical test for this meta-analysis was conducted using Review Manager (RevMan) v5.4 (Cochrane Collaboration, UK).
Risk Of Bias in Individual Studies (Qualitative Synthesis)
The quality of each study included in this study was assessed by two independent reviewers (SFA and MSA) according to the Cochrane Risk of Bias (RoB) tool.
Quantitative Data Synthesis (Meta-Analysis)
Mean and Standard Deviation(SD) withthe confidence interval (CI) of95% were calculated in this meta-analysis. We used the heterogeneity level to determine the effect size, either a fixed-effect model (FEM) or random effect model (REM). REMwasusedwhen the includedstudieswere consideredheterogenous(quite high variability in studies results or variation due to random error), indicated by an I2 value more than50%. Otherwise,we usedFEM.The pooledestimate waspresented in our forest plot.
RESULT Study selection
Our search yielded 200 articles from five databases as described in Table 1 below. From hand searching we found 24 articles that matched with our topics. Twentythree duplicatesfrom those databaseswere removed.Then,the authorsread the titles and abstracts of the remaining 201 articles for preliminary screening. The articles that did not fulfill our eligibility criteria were excluded. Full-texts were retrieved for 29 articles and 172studies were excluded because those articles do not clearly explain the comparison between placebo as control and acetazolamide intervention in HR effect. Full-texts were retrieved again for 11 studies and 18 studies were excluded because the studies do not explain clearly the effect of HR after acetazolamide consumption in high altitude conditions. Full-text 10 articles were included in qualitative systematic reviews. Finally, eight studies were included for quantitative analysis. Our study selection process was presented in the PRISMA diagram on Figure 1.
Table 1. Database Searching Process Result
Database Keyword
Google Scholar
("acetazolamide"[MeSH Terms] OR "acetazolamide" OR "acetazolamid") AND ("Altitude Sickness"[Mesh] OR “altitude sickness” OR “Acute Mountain Sickness” OR AMS OR “altitude sickness” OR “high altitude” OR “Mountain Sickness”) AND (“Heart rate”[MesH Terms] OR “Heart rate” OR HR)
ScienceDirect
("acetazolamide"[MeSH Terms] OR "acetazolamide" OR "acetazolamid") AND ("Altitude Sickness"[Mesh] OR "altitude sickness" OR "Acute Mountain Sickness" OR "Mountain Sickness") AND ("Heart rate" OR "HR")
Pubmed
("acetazolamide"[MeSH Terms] OR "acetazolamide" OR "acetazolamid") AND ("Altitude Sickness"[Mesh] OR “altitude sickness” OR “Acute Mountain Sickness” OR AMS OR “altitude sickness” OR “high altitude” OR “Mountain Sickness”) AND (“Heart rate”[MesH Terms] OR “Heart rate” OR HR)
SpringerLink
("acetazolamide" OR "acetazolamid") AND ("Altitude Sickness" OR “Acute Mountain Sickness” OR AMS OR “altitude sickness” OR “high altitude” OR “Mountain Sickness”) AND (“Heart rate” OR “Heart rate” OR HR)
Cochrane Library
("acetazolamide" OR "acetazolamid") AND ("Altitude Sickness" OR “Acute Mountain Sickness” OR AMS OR “altitude sickness” OR “high altitude” OR “Mountain Sickness”) AND (“Heart rate” OR “Heart rate” OR HR)
Study characteristics and results of individual studies
The summary of our included studies was displayed in Table 2. The subject of these studies was healthy participants in various altitude level and receiving acetazolamide treatment with placebo as its control treatment. We focused on the heart rate outcome to indicate the effect of acetazolamide as prophylaxis medicine for AMS. Out of the ten studies included in the qualitative synthesis, eight studies did not use 500 and 250 mg dose as if they only provide one article and not eligible to be included in meta-analysis.
Identification Screening
Identification of studies via databases and registers
Records identified from*: Pubmed (n = 11)
ScienDirect (n = 48) Cochrane Library (n = 16) Google Scholar (n = 80) SpringerLink (n = 44) Hand searching (n = 25) (n= 224)
Records screened (n = 201)
Reports sought for retrieval (n = 29)
Records removed before screening: Duplicate records removed (n = 23)
Included
Reports assessed for eligibility (n = 11)
Records excluded** (n = 172)
Reports not retrieved (n = 18)
Reports excluded: No full text (n = 1)
Studies included in systematic review (n = 10)
Reports excluded: No specific dosage (n = 2)
Studies included in meta-analysis (n = 8)
Figure 1. PRISMA 2020 Flow Diagram











Risk of Bias in Individual Studies (Qualitative Synthesis)
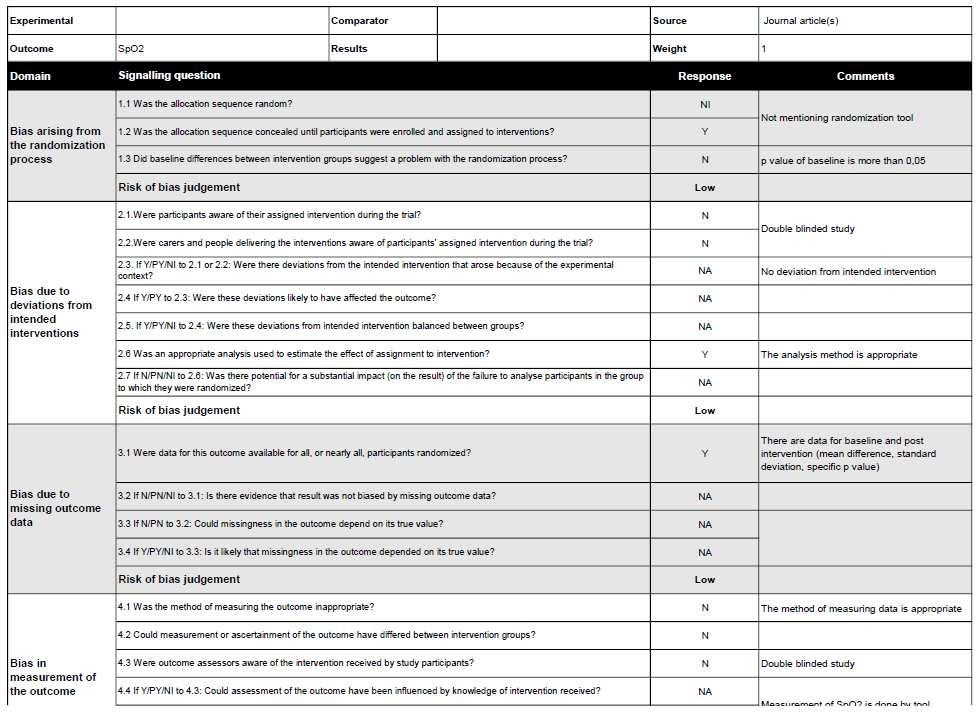
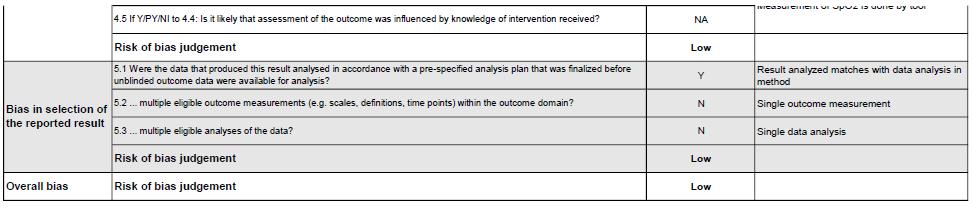
We critically assessed the quality of each study with the RoB 2 tool (revised toolsfor riskof biasin randomized trials).Most of the studies,leadingtoclear (low) risk of bias. Three studies had moderate risk of bias. The study conducted by Bradbury et al. did not mention that a random element was used in generating the allocation sequence and not all randomized participants include an analysis of the intention to treat effect. In other, the study conducted by Bradwell et al (7) mentions that the participants were aware of their assigned intervention during trial. The Study conducted by Ke et al. have some concern for judgment of risk of bias in selection of the reported result. The summary of bias analysis was provided in Figure 2.
Figure 2. Risk of Bias (RoB) 2 tool

Table 2. Qualitative summary of literature study about acetazolamide effect on heart rate Study, Year Basnyat, et al., 2008(8) Bradbury, et al., 2019(9) Bradwell, et al., 1986(7) Faoro, et al., 2007(10) Faull, et al., 2015(11)
Setting Nepal Massachusetts Nepal Huayna Potosi Refugio Guide del Cervino
Study Design two-armed, doubleblind, randomized, placebo-controlled trial
randomized controlled trial double-blind randomized trial double-blind, randomized, placebo-controlled trial
double blind, randomized, placebo-controlled study
Treatment Time 36 hours to 96 hours 1 day before 1 day before 1 day before 3 days before
Assessment Period 4 days 30 hours 18 days 24 hours 22 hours Sample size (ACZ/PL) 187/177 10/10 11/10 NR/NR 10/10 Dosage 250 mg, bid 250 mg, bid 250 mg, bid 750 mg 250 mg, bid HR (mean ± SD) Acetazolamide Placebo 82.6 ± 12.0 82.5 ± 12.0 183 ± 12 177 ± 14 151 ± 4 143 ± 5 67 ± 11 63 + 11 72 ± 23 78 ± 23 p-value 0.91 0.45 NR NR 0.60 HR Assessment pulse oximeter pulse oximeter Bosmat II automatic recorder three-lead ECG pulse oximeter
Way to Altitude walk + flight altitude chamber walk NR cable car Altitude (m) 5000 3500 4846 4700 3459 ACZ: acetazolamide; PL: placebo; NR: not reported; ECG: electrocardiogram; SD: standard deviation
Cont.
Study, Year Ke, et al., 2013(12) Lipman, et al., 2017(13) Moraga, et al., 2007(14) Parati, et al., 2013(15) Rodway, et al., 2013(16)
Setting Lhasa, China White Mountains, California Ollague town, Northern Chile Capanna Regina Margherita, Monte Rosa
Study Design prospective, double-blind, randomized, placebo-controlled trial
prospective, double-blind, randomized, placebo-controlled trial
randomized controlled trial randomized, double blind, parallel group, placebocontrolled study
Mount Everest Base Camp
randomized, double blind, parallel group, placebocontrolled study
Treatment Time 3 days before 4 hours before 24 hours before 2 days before 30 minutes before Assessment Period 4 days 1 day 4 days 4 days 494 minutes
Sample size (ACZ/PL) 9/9 35/35 12/12 19/20 4/4
Dosage 125 mg, bid 125 mg, bid 250 mg, bid 250 mg, bid 125 mg, bid HR (mean ± SD) Acetazolamide Placebo 85 ± 11.05 79 ± 14.96 80.2 ± 13.6 88.3 ± 14.8 94 ± 14 90 ± 15 77.4 ± 42.2 84.1 ± 43.3 66.2 ± 4.78 75.0 ± 13.32 p-value < 0.05 0.02 NR < 0.05 NR
HR Assessment three-lead ECG pulse oximeter pulse oximeter oscillometric device three-lead ECG Way to Altitude commercial airplane walk + drive car cable car + walk walk
Altitude (m) 3658 3810 3696 4559 5300
ACZ: acetazolamide; PL: placebo; NR: not reported; ECG: electrocardiogram; SD: standard deviation
Meta-analysis
We compared the pooled effect size of acetazolamide on heart rate in high altitude. We used the last heart rate assessment for each study comparing the acetazolamide and placebo group. Moderate-high pooled effects size were presented on the forest plot in Figure 3 and Figure 4.
Figure 3. Forest plot meta-analysis of the effect of acetazolamide 250 mg dose on high rate alteration in high altitude
Figure 4. Forest plot meta-analysis of the effect of acetazolamide 500 mg dose on high rate alteration in high altitude
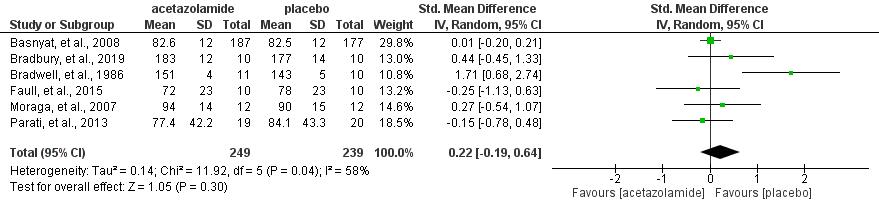

These results indicated that acetazolamide were not significant to heart rate alteration in high altitude both in 250 mg dose (pooled MD= -0.15 95% CI (-1.110.81), p= 0.76, I²=71%) and 500 mg dose acetazolamide (pooled MD= 0.22, 95% CI (-0.19 0.64), p=0.04, I2=58%).
DISCUSSION
We have been looking into how acetazolamide affects the heart rate of the population who are exposed to high altitude. Several reports have shown that administrationof acetazolamide beforehighaltitudeexposure canincreasethe heart rate which may be linked to AMS development (17). Our meta-analysis suggested an unsignificant effect to HR. We analyzed the result using two forest plots as can be seen in Figure 3 and Figure 4. At 500 mg dose of acetazolamide, the intermediate
heterogeneity (I²= 58%) suggested that it tends to have less effect on increasing heart rate when compared to placebo (pooled MD= 0.22, 95% CI (-0.19 0.64), p=0.04, I2=58%) rather than 250 mg. Therefore, administration of acetazolamide at 500 mg can reduce symptoms that may be linked to the development of AMS and safe for patients with acute heart failure(18). Our meta-analysis has also shown that 250 mg acetazolamide tends to increase heart rate when compared to placebo (pooled MD= -0.15 95% CI (-1.11-0.81), p= 0.76, I²=71%). This finding has the opposite effect from 500 mg dose.
Although, our study showed an unsignificant result and has kind of intermediate-high heterogeneity, we still suggest the beneficial effect of acetazolamide as the prophylaxis of AMS. However, the intermediate-high heterogeneity resulted by the small number of participants, notably in the 250 mg dose who hasgap number of participants(12,13).The homogeneity of includedstudies sample characteristic and mechanisms on how the acetazolamide and placebo administered to the sample are unclear.
Despite the limitations of this study, our results suggest that administration of acetazolamide prior to high-altitude exposure is unsignificant to the increase of heart rate. Furthermore, we hope there will be an update and an international largescale study about the administration of acetazolamide prophylaxis for preventing high altitude sickness.
CONCLUSION
This systematic review and meta-analysis provide valuable evidence that acetazolamide as AMS prophylaxis did not show significant effect on heart rate alteration for people exposed to high altitude. We recommend further research which focused to evaluate the effect of acetazolamide on heart rate in the altitude settings.
REFERENCES
1. Winter C, Bjorkman T, Miller S, Nichols P, Cardinal J, O’Rourke P, et al. Acute Mountain Sickness Following Incremental Trekking to High Altitude: Correlation With Plasma Vascular Endothelial Growth Factor Levels andthe Possible Effects of Dexamethasone and Acclimatization Following Re exposure. Front Physiol. 2021 Oct 21;12.
2. Taylor A. High-altitude illnesses: Physiology, risk factors, prevention, and treatment. Rambam Maimonides Med J. 2011 Jan 21;2(1).
3. Parati G, Agostoni P, Basnyat B, Bilo G, Brugger H, Coca A, et al. Clinical recommendations for high altitude exposure of individuals with pre-existing cardiovascular conditions: A joint statement by the european society of cardiology, the council on hypertension of the european society of cardiology, the european society of hypertension, the international society of mountainmedicine, the Italian society of hypertension and the Italian society of mountain medicine. Vol. 39, European Heart Journal. Oxford University Press; 2018. p. 1546 54.
4. Malcolm J, Frcpc AM, Fitchett DH, Howlett JG, Lonn EM, Tardif JC. Resting heart rate: A modifiable prognostic indicator of cardiovascular risk and outcomes? IS HEART RATE A TRUE CARDIOVASCULAR RISK FACTOR? Vol. 24, Can J Cardiol. 2008.
5. Toussaint CM, Kenefick RW, Petrassi FA, Muza SR, Charkoudian N. Altitude, Acute Mountain Sickness, and Acetazolamide: Recommendations for Rapid Ascent. Vol. 22, High Altitude Medicine and Biology. Mary Ann Liebert Inc.; 2021. p. 5 13.
6. Millichap JG, Millichap JJ. Mechanism of action of acetazolamide and idiopathic intracranial hypertension. Vol. 6, Frontiers in Neurology. Frontiers Research Foundation; 2015.
7. Horton R, Zipser R, Fichman M, Terragno NA, Malik KU, Nasjletti A, et al. Interaction between oxprenolol and indomethacin on blood pressure in essential hypertensive patients. Vol. 18, Adv Prostagl Thrombox Res. 1980.
8. Basnyat B, Hargrove J, Holck PS, Srivastav S, Alekh K, Ghimire L v., et al.
Acetazolamide fails to decrease pulmonary artery pressure at high altitude in partially acclimatized humans. High Alt Med Biol. 2008 Sep 1;9(3):209 16.
9. Bradbury KE, Yurkevicius BR, Mitchell KM, Coffman KE, Salgado RM, Fulco CS, et al. Downloaded from www.physiology.org/journal/jappl at Western Sydney Univ [Internet]. 2019. Available from: www.physiology.org/journal/jappl
10. Faoro V, Huez S, Giltaire S, Pavelescu A, van Osta A, Moraine JJ, et al. Effects of acetazolamide on aerobic exercise capacity and pulmonary hemodynamics at high altitudes. J Appl Physiol [Internet]. 2007;103:1161
5. Available from: www.physiology.org/journal/jappl
11. Faull OK, Robertson J, Thomas O, Bradwell AR, Antoniades CA, Pattinson KTS. The effect of acetazolamide on saccadic latency at 3459 meters. Wilderness Environ Med. 2015 Mar 1;26(1):72 7.
12. Ke T, Wang J, Swenson ER, Zhang X, Hu Y, Chen Y, et al. Effect of acetazolamide andgingko biloba on the human pulmonary vascular response to an acute altitude ascent. High Alt Med Biol. 2013 Jun 1;14(2):162 7.
13. Lipman GS, Pomeranz D, Burns P, Phillips C, Cheffers M, Evans K, et al. Budesonide Versus Acetazolamide for Prevention of Acute Mountain Sickness. American Journal of Medicine. 2018 Feb 1;131(2):200.e9200.e16.
14. Moraga FA, Flores A, Serra J, Esnaola C, Barriento C. Ginkgo biloba decreases acute mountain sickness in people ascending to high altitude at Ollagüe (3696 m) in Northern Chile. Wilderness Environ Med. 2007;18(4):251 7.
15. Parati G, Revera M, Giuliano A, Faini A, Bilo G, Gregorini F, et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J. 2013 Mar;34(10):759 66.
16. Rodway GW, Edsell ME, Wong B, Windsor JS. Improving sleep at altitude: A comparison of therapies. Wilderness Environ Med. 2011 Dec;22(4):316 20.
17. Lichtblau M, Berlier C, Saxer S, Carta AF, Mayer L, Groth A, et al. Acute Hemodynamic Effect of Acetazolamide in Patients With Pulmonary Hypertension Whilst Breathing Normoxic and Hypoxic Gas: A Randomized Cross-Over Trial. Front Med (Lausanne). 2021 Jul 22;8.
18. Lim GB. Acetazolamide improves decongestion in acute heart failure. Nat Rev Cardiol [Internet]. 2022;19(11):720. Available from: https://doi.org/10.1038/s41569-022-00784-9
Climbing is a sport that is favored by people who want to feel the thrill and have great courage. In 2017, an estimated 7.1 million people in the United States participated in rock climbing, which has risen markedly from 4.3 million in 2010. The sport was once only popular among outdoor enthusiasts, adventure enthusiasts, and elite competitive athletes who began to build the first indoor rock climbing gyms in the 1980s. In recent years, an explosion of indoor climbing gyms has occurred, with a 10% increase in the number of climbing gyms in 2015 alone. This increased ease of access has led to a new wave of recreationists seeking an alternative form of exercise, causing the sport to flourish. Growth and exposure are only expected to increase with rock climbing’s Olympic debut at the 2020 Summer Olympics in Tokyo, Japan. Behind the increasing popularity of this extreme sport, there are many possibilities of injury and even death that will lurk the athletes. In a study from Colorado, rock climbing rescues accounted for approximately 20% of mountain and wilderness rescue victims. Climbing accidents usually affect a young and healthy population. When assessing climbing-related damages, overuse (overstrain) injuries and acute injuries or accidents should be made. Overuse injuries are generally less severe and can be avoided with informed training in many cases [5]. Acute injuries, on the other hand, can range from minor to life-threatening, involve a single body part or multiple body regions, and usually cannot be avoided by training. Orthopaedic Rock Climbing Injuries are uniquely varied. No anatomic location is spared, and all locations are grouped into three mechanisms. Nineteen percent to 33% of injuries are from chronic overuse, 28% are acute atraumatic from supraphysiologic loading, and 10% to39%are acute traumatic as a result of a fall from height or rockfall.3 5 Reassuringly, only 0.6% of injuries have been reported because of equipment failure.4 Of 975 injuries, 37.6% were evaluated primarily or referred to an orthopedic surgeon n43%of those underwent surgical intervention. Head trauma can be present in the multipleinjured rock climber undergoing damage control orthopedics. Head injury accounts for 1.6% to 3.6% of all rock climbing-associated injuriesandforupto17%ofinjuries in climbers requiring search and rescue. Rockfalls and falls from height are two mechanisms by which climbers can sustain a debilitating or fatal head injury.
Full-body harness designs have been shown to prevent “head down” falls but are rarely used. Despite these risks, helmet use among climbers is staggeringly low. 86.9% of climbers report never using a helmet. The current helmetless culture within the rock climbing community is unique compared with other high-adventure sports. Surprisingly, helmet use is not required in competition by any national or international governing body. Even more concerning is that 27.6% of climbers report climbing under the influence of drugs or alcohol. Take time to counsel patients on helmets and drug and alcohol use to limit avoidable and potentially lethal head injuries.
Prevention and First Aid of Mountaineering’s Accident
East Asian Medical Students’ Conference 2022
Authors: Wan Nafisa Azzahra Ariqoh Sajidah Hanjani Rika Fitri Halimatussa’adah Valesky Goldera Agriphina
AMSA-Universitas Batam

Climbing is one sport that is much liked by people who want to feel the thrill and have great courage. In 2017, an estimated 7.1 million people in the United States participated in rock climbing, which has risen markedly from 4.3 million in 2010. The sport was once only popular among outdoor enthusiasts, adventure junkies, and elite competition athletes who began to build the first indoor rock climbing gyms in the 1980s. In recent years, an explosion of indoor climbing gyms has occurred with a 10% increase in the number of climbing gyms in 2015 alone. This increased ease of access has led to a new wave of recreationalists seeking an alternative form of exercise causing the sport to flourish. Growth and exposure is only expected to increase with rock climbing’s Olympic debut at the 2020 Summer Olympics in Tokyo, Japan. Behind the increasing popularity of this extreme sport, there are many possibilities of injury and even death that will lurk the athletes. In a study from Colorado, rock climbing rescues accounted for approximately 20% of mountain and wilderness rescue victims. Climbing accidents usually affect a young and healthy population.
When assessing climbing-related injuries, a distinction between overuse (overstrain) injuries and acute injuries or accidents should be made. Overuse injuries are generally less severe and can be avoided with informed training in many cases [5]. Acute injuries, on the other hand, can range from minor to life threatening, involve a single body part or multiple body regions and usually cannot be avoided by training. Orthopaedicrockclimbinginjuries are uniquely varied. No anatomic locationisspared and all locations are grouped into three mechanisms. Nineteen percent to 33% of injuries are from chronic overuse, 28% are acute atraumatic from supraphysiologic loading, and 10% to39%areacutetraumatic asa result from a fall from height or rockfall.3 5 Reassuringly, only 0.6% of injuries have been reported because of equipment failure.4 Of 975 injuries, 37.6% were evaluated primarily or referred to an orthopaedic surgeonand43%ofthoseunderwent surgical intervention. head trauma can be present in the multiple-injured rock climber undergoing damage control orthopaedics. Head injury accounts for 1.6% to 3.6% of all rock climbing-associated injuriesandforupto17%ofinjuries in climbers requiring search and rescue. Rockfall and falls from height are two mechanisms by which climbers can sustain debilitating or fatal head injury. Full body harness designs have been shown to prevent “head down” falls but are rarely used. Despite these risks, helmet use among climbers is staggeringly low. 86.9% of climbers report never using a helmet.The current helmetless culture within the rock climbing community is unique compared with other high adventure sports. Surprisingly, helmet use is not required in competition by any national or international governing body. Even more concerning is that 27.6% of climbers reportclimbingundertheinfluenceof drugs or alcohol. Take time to counsel patients on helmet and drug and alcohol use to limitavoidable,andpotentiallylethal, head injury.
METHODS AND MATERIAL Information Source and Search Strategy
The search strategy for this systematic review was based on the Prefered Reporting Items for Systematic Reviews and Meta-Analyses. Electronic database consisting of PubMed, were screening by four reviewers up to 10th Oktober 2022. suitable advanced search techniques were applied whenever appropiate. the literature search was limited by the languange, as the authors were only compatible with english or bahasa indonesia language
Inclusion & Sources and Search Strategy
We are applied the following criteria, review literature found 4 journals related to leadership style and job satisfaction, 1 journal with quantitatif descriptive method, 2 journal with descriptive method, 1 journal with survey descriptive method. The entire journal from 2017-2020.
Data Extraction
INTRODUCTION
we have defined the following datasheets to extract: (1) outhor and year of publication (2) study characteristic, including Bicyle types, Bike fit, include Sudle Height, Suddle Setback, and the Foot- Shoe Pedal Interface. (3) Study injury include Patterns of Injury, Head Face and Neck Injuries, Acute Spine Injuries and Chest and Abdominal trauma. (4) Skin and Soft Injuries include Traumatic and Friction Injuries.

Review Data Analysys
The main result which we use in the data analysis was are the potential risks for injury and overuse injury, proper use of bicycles and use of protective equipment and clinical care of these athletes. proper stabilization and transportation to medical facilities, and first aid provided to people with injuries
RESULT AND DISCUSSION
The article shows the anatomic location of theinjuries. It is often found that skeletons are the most common ones to get injured. Following this, safety and protection must be spotted around the sports area to prevent the athletes from getting in danger. The article said that most athletes do not use safety and sadly do the sport under alcohol or drug use. The government should become aware of the casualties of the youngsters.
For injury prevention, the athletes must be equipped with the safety that connects with the sport. Besides equipment, it is also crucial for the athletes or people in charge around the sports field to know about first aid and provide several medicines in case of injure
CONCLUSION AND RECOMMENDATION
Conclusion
This Systematic Reviews and Meta-Analyses shows that mountain biking is an increasingly popular sport with athletes often presenting to an orthopaedic surgeon with injury. Appropriate bike fit and use of
protective equipment, such as a helmet, can decrease the risks of some of these injuries. Clinicians taking care of these athletes should be familiar with common injuries. Proper stabilization and transportation to a medical facility is crucial for the management of cyclists with life-threatening injuries.
Recommendation
Further studies with a longer follow up periodand larger sample size are needed to reduce bias and improve the accuracy of measuring the relationship between groups.
REFERENCES
Ansari, M., Nourian, R., & Khodaee, M. (2017). Mountain Biking Injuries. Currentsportsmedicinereports, 16(6), 404 412. https://doi.org/10.1249/JSR.0000000000000429
Tinoco, J., Sassone, L. M., Stevens, R. H., Martins, D. D., Grangeiro Neto, J. A., & Tinoco, E. (2021). Mouthguard use and attitudes regarding dental trauma among elite cross-country mountain biking and field hockey athletes. Dental traumatology : official publication of International Association for Dental Traumatology, 37(2), 307 313. https://doi.org/10.1111/edt.12636
Cole, K. P., Uhl, R. L., & Rosenbaum, A. J. (2020). Comprehensive Review of Rock Climbing Injuries. The Journal of the American Academy of Orthopaedic Surgeons, 28(12), e501 e509. https://doi.org/10.5435/JAAOS-D-19-00575
Int J Environ Res Public Health. 2020 Jan; 17(1): 203. Published online 2019 Dec 27. doi: 10.3390/ijerph17010203
Effective Dosing of Prophylactic Acetazolamide for Acute Mountain Sickness Incidence and Symptoms: A Systematic Review and Meta-analysis of RCTs
Shakira Amirah1, Sydney Tjandra1, Muhammad Candrika Agyawisnu Yuwono1, Najma Ali1
1AMSA-Universitas Indonesia
ABSTRACT
Introduction: Acute Mountain Sickness occurs as a response to body's acclimatization at high altitudes with 75% occurrence on 3,000 m. Existing dose recommendations for AMS prevention range from 125 mg per 12 h to 750 mg a day. However, the incidence of AMS symptoms based on dose and oxygen saturation remains unexplored.
Objective: Determine the efficacy of acetazolamide for AMS incidence based on dosage, subject, percentage of men, consumption time, and incidence of other symptoms based on acetazolamide’s dose.
Methods: Systematic search through PubMed, Scopus, Cochrane, Wiley, and ProQuest, were done until 17th September 2022. Critical appraisal of included studies with Cochrane Risk of Bias 2.0. We analyzed pooled Odd Ratio (OR) and its p-value using random effects model.
Results and Discussion: Twenty-eight RCTs, yielding 1453 participants in intervention group and 1198 participants are included. Incidence of AMS was lower in acetazolamide group (OR=0.39 [95%CI:0.32-0.47],p<0.00001; I2=0%), subgroup analysis done based on subjects, percentage of man, and consumption time. Meta-analysis incidence of other symptomps done through dosage based subgroup analysis, namely headache (OR=0.46 [95%CI:0.36-0.59],p<0.00001), severe headache (OR=0.19 [95%CI:0.08-0.42],p<0.00001; I2=0%), numbness finger (OR=12.37 [95%CI:6.79-22.56],p<0.00001), numbness face and lips (OR=2.68 [95%CI:0.89-8.03] ,p<0.00001; I2=0%), and visual disturbance (OR=0.66 [95%CI:0.17-2.59], p=0.08).
Conclusion:This meta-analysis found that acetazolamide doses of 125-375 mg produced significant decreasing ofAMS incidence.Doses of250 mgand375 mgsignificantly reduced incidence of headache compared to 125 mg. We recommend factors such as history of AMS and combination with other drugs or gingko biloba to be considered in future studies.
Keywords: Acute mountain sickness, Acetazolamide, Dosage, Meta-analysis, Prophylactic
Effective Dosing of Prophylactic Acetazolamide for Acute Mountain Sickness Incidence and Symptoms: A Systematic Review and Meta-analysis of RCTs
Shakira Amirah1, Sydney Tjandra1, Muhammad Candrika Agyawisnu Yuwono1, Najma Ali1 1AMSA-Universitas Indonesia
ABSTRACT
Introduction: Acute Mountain Sickness occurs as a response to body's acclimatization at high altitudes with 75%occurrenceon3,000 m. Existing doserecommendations forAMS prevention range from 125 mg per 12 h to 750 mg a day. However, the incidence of AMS symptoms based on dose and oxygen saturation remains unexplored.
Objective: Determine the efficacy of acetazolamide for AMS incidence based on dosage, subject, percentage of men, consumption time, and incidence of other symptoms based on acetazolamide’s dose.
Methods: Systematic search through PubMed, Scopus, Cochrane, Wiley, and ProQuest, were done until 17th September 2022. Critical appraisal of included studies with Cochrane Risk of Bias 2.0. We analyzed pooled Odd Ratio (OR) and its p-value using random effects model.
Results and Discussion: Twenty-eight RCTs, yielding 1453 participants in intervention group and 1198 participants are included. Incidence of AMS was lower in acetazolamide group (OR=0.39 [95%CI:0.32-0.47],p<0.00001; I2=0%), subgroup analysis done based on subjects, percentage of man, and consumption time. Meta-analysis incidence of other symptomps done through dosage based subgroup analysis, namely headache (OR=0.46 [95%CI:0.360.59],p<0.00001), severe headache (OR=0.19 [95%CI:0.08-0.42],p<0.00001; I2=0%), numbness finger (OR=12.37 [95%CI:6.79-22.56],p<0.00001), numbness face and lips (OR=2.68 [95%CI:0.89-8.03] ,p<0.00001; I2=0%), and visual disturbance (OR=0.66 [95%CI:0.17-2.59], p=0.08).
Conclusion:This meta-analysis found that acetazolamide doses of 125-375 mg produced significant decreasing of AMS incidence. Doses of 250 mg and 375 mg significantly reduced incidence of headache compared to 125 mg. We recommend factors such as history of AMS and combination with other drugs or gingko biloba to be considered in future studies.
Keywords: Acute mountain sickness, Acetazolamide, Dosage, Meta-analysis, Prophylactic
INTRODUCTION
Acute mountain sickness (AMS) is physical distress due to the body's difficulty adjusting to low oxygen pressure at high altitudes.1 The higher the altitude, the lower the oxygen saturation and the higher the incidence of AMS. The high-altitude environment is at an altitude of 1500 meters;generally, travelers,skiers, hikers, andmountainclimbers can experience AMS at altitudes ranging from 2400 meters.2,3 AMS occurrence on 3,000 m reaches 75%, with symptoms ranging from headache, nausea, and shortness of breath, to inability to exercise.1,2 Most cases of AMS are mild, but if left untreated, they can be life-threatening. Most symptoms disappear after 18-36 hours, whilst less than 1% develop to life-threatening high altitude cerebral edema if untreated.1 This situation calls for medication with low adverse effects, such as AMS prophylaxis.
Acetazolamide is the drug of choice that has long been used for AMS. Compared to other alternative drugs, acetazolamide is deemed to have fewer side effects. Acetazolamide, a carbonic anhydrase, works byhastening altitudeacclimatizationby mimicking natural acclimatizationmore rapidly. It causes increased renal excretion and mild diuresis, which causes metabolic acidosis that increases oxygenation or respiratory rate.4,5 The drug has three common side effects: diuresis, paresthesias, and taste disorders. It is reported that the occurrence of side effects reaches 80-100%. Speculation arises that this side effect is related to plasma drug levels and metabolic acidosis caused by consuming acetazolamide.6
Acetazolamide has been shown to be effective at doses of 125 mg twice daily to 375 mg.7 Based on existing recommendations, the doses for AMS prevention range from 125 mg per 12 h to 750 mg a day, starting 24 hours prior to ascending in elevation. Previous meta-analyses have also described the effective dose of acetazolamide for AMS and ascent profiles.7 However, the incidence of AMS symptoms based on dose and oxygen saturation remains unexplored. Therefore, this study aims to determine the efficacy of acetazolamide on the incidence of AMS based on dosage, subject, percentage of men, and start the time-consuming acetazolamide as well as the incidence of other symptoms divided by the dose of acetazolamide.
1.
2. METHODS AND MATERIALS
2.1 Study Design
This systematic review and meta-analysis was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions 6.2 and reported with regards to the PRISMA checklist.8
2.2 Search strategy
We conducted a comprehensive literature search on PubMed, Scopus, Cochrane, Wiley Library, and ProQuest using the PRISMA framework. We collected data on 10 October 2022, using the keywords “Acetazolamide” OR “Diamox” AND “Acute mountain sickness” OR “AMS” OR “High altitude sickness”. All the terms matched the medical subject headings (MeSH) Browser. The keywords used in the pursuit were customized according to the database used, as shown in Appendix 1. Suitable advanced search techniques were applied whenever appropriate. The planned procedure is illustrated in Figure 1.
Figure 1. Diagram flow of literature search strategy
2.3 Study eligibility criteria
The inclusion criteria applied to the PICOS framework (patient/problem, intervention/exposure, comparison/control, outcome) and included (1) population: people who will go to high altitude place; (2) intervention: acetazolamide in various doses; (3) outcomes: incidence of AMS (primary) and incidence of other symptoms (secondary); (4) pre-treatment or other care as the control; and (5) study: RCTs;. In the meanwhile, the exclusion criteria for this were as follows: (1) incomplete studies at the time of retrieval; (2) studies having unretrievable full-text articles; and (3) studies published in languages other than English as the international language. Additionally, the Mendeley software was used to eliminate duplicates. Three independent reviewers selected the titles and abstracts of papers based on criteria regarding their
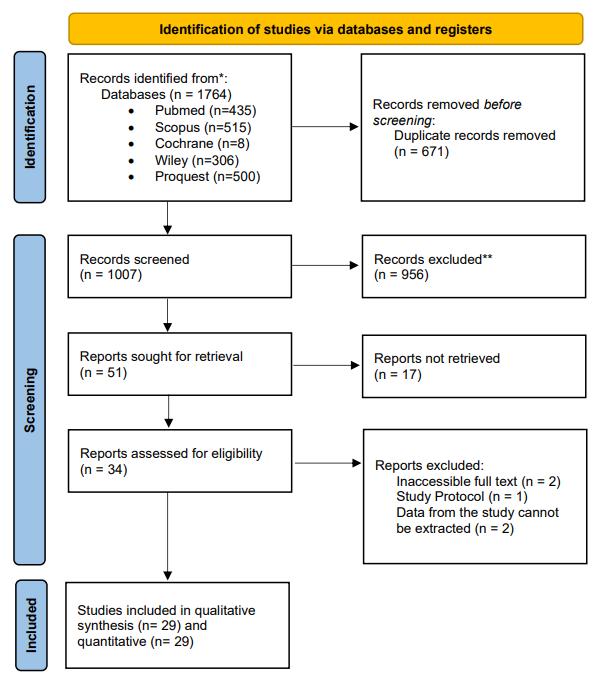
accessibility (SA, ST, NA). Any disputes were discussed to fourth author to reach a consensus (MCAY).
2.4 Data extraction
We extracted the included studies using a predetermined outcome sheet in tabular form, which consisted of (1) author and year of publication; (2) study characteristics, including the study location; (3) study population, including sample size, type of subjects, and percentage of male; (4) Intervention and control, including the name of the intervention, dosage, start time, evaluation time, AMS scale, definition of AMS, mode of ascent, start altitude, and end altitude; and (5) study outcomes, including the incidence of AMS, incidence of heart racing, incidence of headache and severe headache, incidence of numbness, incidence of visual disturbance, and start-final O2 saturation . Study characteristics were assessed qualitatively by two reviewers (ST and NA), and another author (SA) rechecked the accuracy of the extracted data while performing statistical analysis.
2.5 Quality assessment and Publication Bias
The Revised Tool for Risk of Bias in Randomized Trials (RoB 2.0), which consists of five areas for initiative studies, was used to assess the potential for bias in the final studies that were included in the analysis. The author will conduct an analysis of the potential for bias using the formula that was developed by the Cochrane Collaboration. Following that, the findings will be entered into the domain file bias (.xlsx). Following this step, the file will be uploaded to the ROBVISwebsiteinordertofacilitateaccuratevisualizationofthefinalresults.Qualityassessment was performed by four reviewers independently and discrepancies were discussed and resolved by agreement between reviewers. Assessment of the quality of studies are depicted in Appendix 2
2.6 Quantitative data analysis
Statistical analysis was performed using Review Manager ver. 5.4 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen). Mean differences and standard deviation with 95% confidence interval (CI) and p-value were extracted from studies for both pre-post intervention and intervention versus control post-treatment. We then interpreted pooled effects using random effect models. The main result which we use in the statistical analysis was the mean
difference between pre and post treatment using CBT for patients with depression during COVID19 pandemic era, shown by lower depression score in its respective tool used, and also mean difference betweenCBT andcontrol grouppatients. Meandifference(MD) with a95% confidence interval and its respective p-value was used to determine the efficacy of CBT towards depression patients during COVID-19 pandemic era which will be presented in forest plot. We used inverse variance, DerSimonian-Laird random effects model as proposed by Riley et al.9, since we considered that heterogeneity outside the study could also be discovered from studies Heterogeneity was further evaluated using I2 statistics based on Cochrane threshold, with cut-off limits of 0%, 25%, 50%, and 75% as insignificant, low, moderate, and high heterogeneity, respectively.10 Additionally, we also performed sensitivity analysis following the Duval and Tweedie’s trim-and-fill method to identify any outlier study if high heterogeneity was detected.
3. RESULTS
3.1 Search Results and Study characteristics
Quantitativestudieswereconductedon28RCTs,yielding1453participantsinintervention group and 1198 participants in control group who were treated with acetazolamide and placebo respectively.11 38 Qualitative analyses were conducted all RCTs to get primary and secondary outcome. The research was conducted in many countries (Bolivia, California, China, Chile, Colorado, Italy, Pakistan, Tanzania, and Nepal) and released from 1976 to 2019 with mode of ascend including climb and transport. The type of the participants are trekkers, volunteer, and hiker, and they were further randomized into an intervention group, a control group, or several intervention groups for follow-up. Interventions consisted of variant dose of acetazolamide. Outcomes were measured using AMS scale, namely LLQ, AMS, and ESQ. Detailed characteristic of included studies are listed in Appendix 3 Appendix 3 shown that the demographic characteristics are varied which is characterized by the distribution of study locations in almost all continents.
3.2 Study Outcomes - Meta-analysis and subgroup analysis regarding effectivity of acetazolamide vs placebo group in preventing AMS incidences
A summary of study outcomes regarding effectivity of acetozolamide vs placebo is outlined Appendix 4 and Table 1.
Table 1. Summary of meta-analysis outcomes regarding effectivity of acetozelamide in reducing AMS incidence
OR (95% CI) p I2
Incidence of AMS 0.39 (0.32-0.47) p<0.00001 0%
Subgroup analysis based on dosage
Subgroup differences: p=0.11
Dosage 125 mg 0.47 (0.31-0.69) p=0.0001 13%
Dosage 250 mg 0.39 (0.30-0.49) p<0.00001 0%
Dosage 375 mg 0.20 (0.11-0.39) p<0.00001 0%
Subgroup analysis based on subject
Subgroup differences: p=0.33
Trekkers 0.43 (031-0.58) p<0.00001 25%
Volunteers 0.32 (0.22-0.48) p<0.00001 0% Lowlanders 0.21 (0.08-0.52) p=0.0009 0%
Others 0.45 (0.30-0.69) p=0.0002 0%
Subgroup analysis based on percentage men participants
Subgroup differences: p=0.18
>50% male 0.38 (0.31-0.47) p<0.00001 0%
<50% male 0.23 (0.12-0.46) p<0.0001 0%
Subgroup analysis based on start time consuming acetazolamide
3 days before ascent
Subgroup differences: p=0.09
0.18 (0.09-0.38) p<0.00001 0%
2 days before ascent 0.31 (0.19-0.53) p<0.0001 0%
1 days before ascent 0.25 (0.13-0.47) p<0.0001 0%
Day 1 of ascent 0.42 (0.33-0.54) p<0.00001 0%
3.2.1 Effectivity of Acetazolamide in reducing the incidence of AMS
The effectiveness of using acetazolamide compared to placebo in reducing the incidence of AMS is shown in Figure 2. Our quantitative analysis demonstrated that acetazolamide is significantly reduce AMS incident with no heterogeneity found, yielding a pooled odds ratio (OR) value of 0.39 (p<0.00001; 95% CI: 0.32-0.47; I2=0).
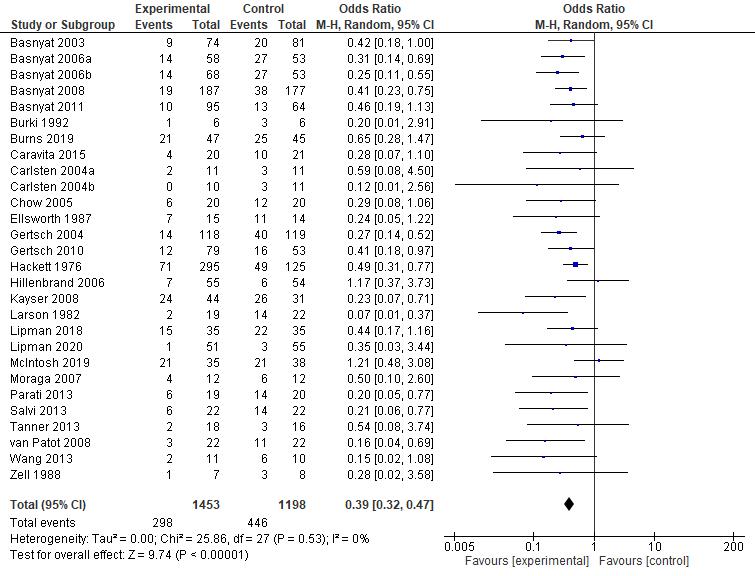
Figure 2. Forest plot showing effectivity of acetazolamide vs control in reducing incidence of AMS
3.2.2 Subgroup analysis based on dose to reduce the incidence of AMS
Furthermore, we also done the subgroup analysis based on acetazolamide dosage on incidence of AMS (Figure 3). Subgroup analysis shown no significant differences (p=0.11) in incidence of AMS based on dosage. All dose variants, both 125 mg, 250 mg, and 375 mg gave significant results with p=0.0001, p<0.00001, and p<0.00001, respectively. Low heterogeneity
was found at a dose of 125 mg with (I2=13%). Heterogeneity was not found at the 250 mg and 375 mg doses, respectively. From the subgroup analysis it was found that acetazolamide at a dose of 375 mg provided the greatest effectiveness in reducing the incidence of AMS with an odd ratio 0.20 (95% CI: 0.32 - 0.47).
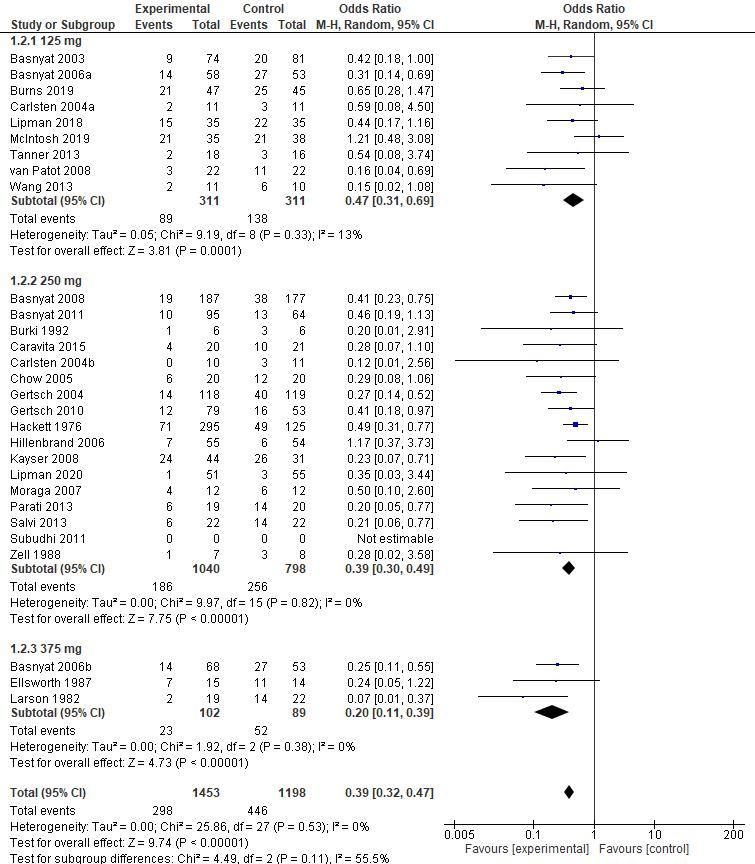
Figure 3. Forest plot subgroup analysis dose of acetazolamide vs control in reducing incidence of AMS
3.2.3 Subgroup analysis based on type of subjects to reduce the incidence of AMS
In the studies that we included in the meta-analysis, we found various types of subjects used as participants, such as volunteers, trekkers, lowlanders, and others. The initial meta-analysis was also done to check whether there were differences in the results that affected the incidence of AMS as seen in Figure 4. As a result, no subgroup differences were found (p=0.33), which means that the different types of participants did not significantly affect the outcome of AMS incidence.
Figure 4. Forest plot of subgroup analysis based on type of subjects
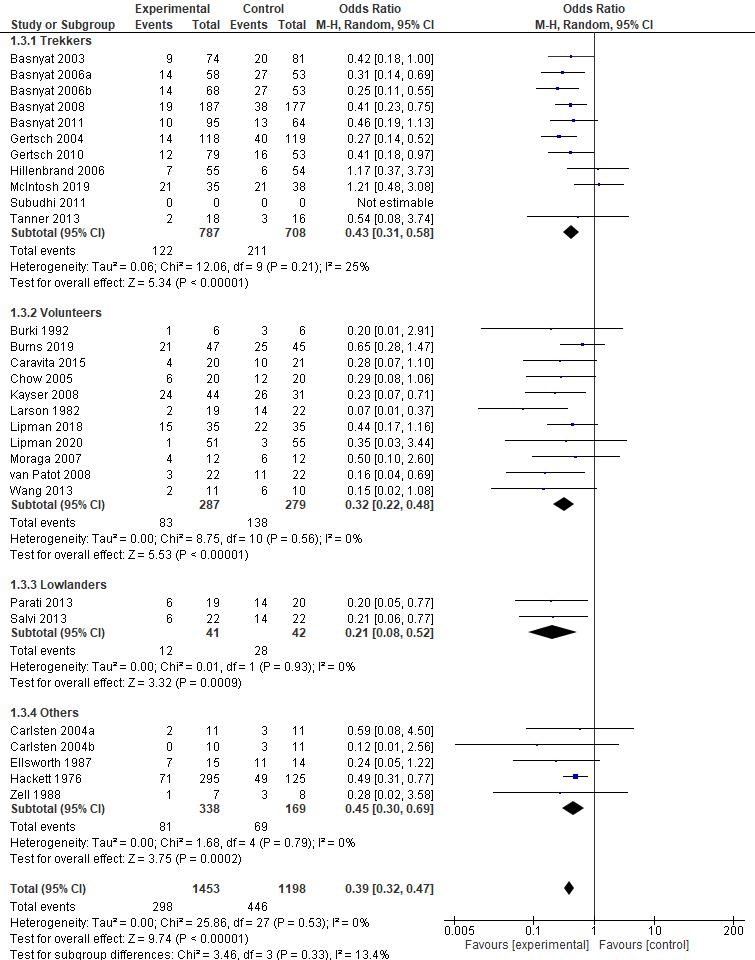
3.2.4
Subgroup analysis based on percentage of men participants
Subgroup analysis was also carried out to check whether it is possible that the difference in the percentage of the number of men as participants affects the effectiveness of acetazolamide in reducing the incidence of AMS as can be seen in Figure 5. The result, there is no significant difference between the subgroups (p=0.18), which means the percentage of the number of men did not affect the results of the effectiveness of acetazolamide.
Figure 5. Subgroup analysis based on percentage of men
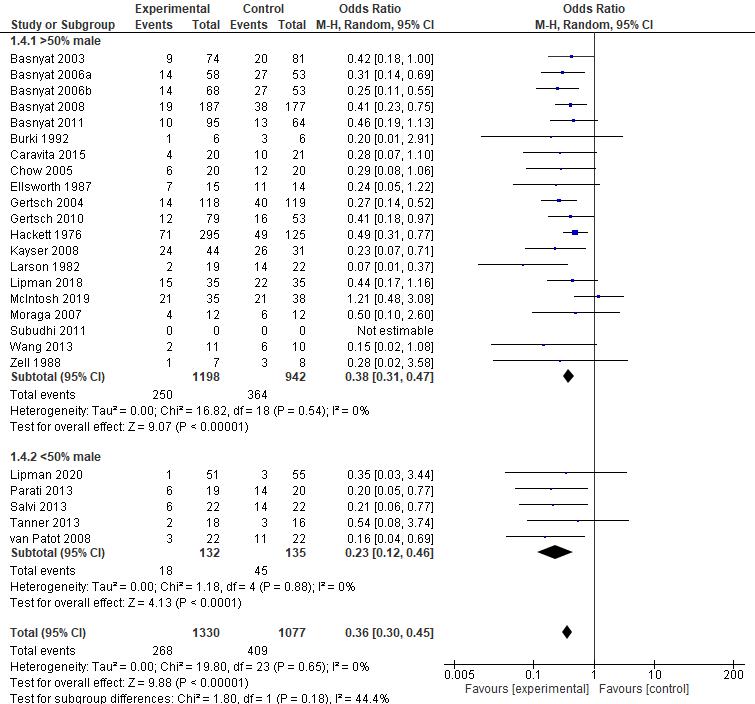
3.2.5 Subgroup analysis based on time consuming acetazolamide
From the various characteristic studies that you found, we also realized that there was a difference in the time to start taking acetazolamide. We performed a subgroup analysis based on the time difference in acetazolamide consumption as shown in Figure 6, the result was that there was no significant difference between subgroups (p=0.09) with heterogeneity not found in any subgroups (I2=0). However, the best time to start taking acetozelamide based on this meta-analysis was 3 days before the ascent with a lowest OR with value of 0.18 (95% CI: 0.09 - 0.38; p<0.00001; I2=0).
Figure 6. Forest plot subgroup analysis based on time consuming acetazolamide vs control in the incidence of AMS
3.3 Study Outcomes - Meta-analysis and subgroup analysis regarding incidence of acetazolamide vs placebo grup
A summary of study outcomes regarding adverse effect is outlined in Table 2
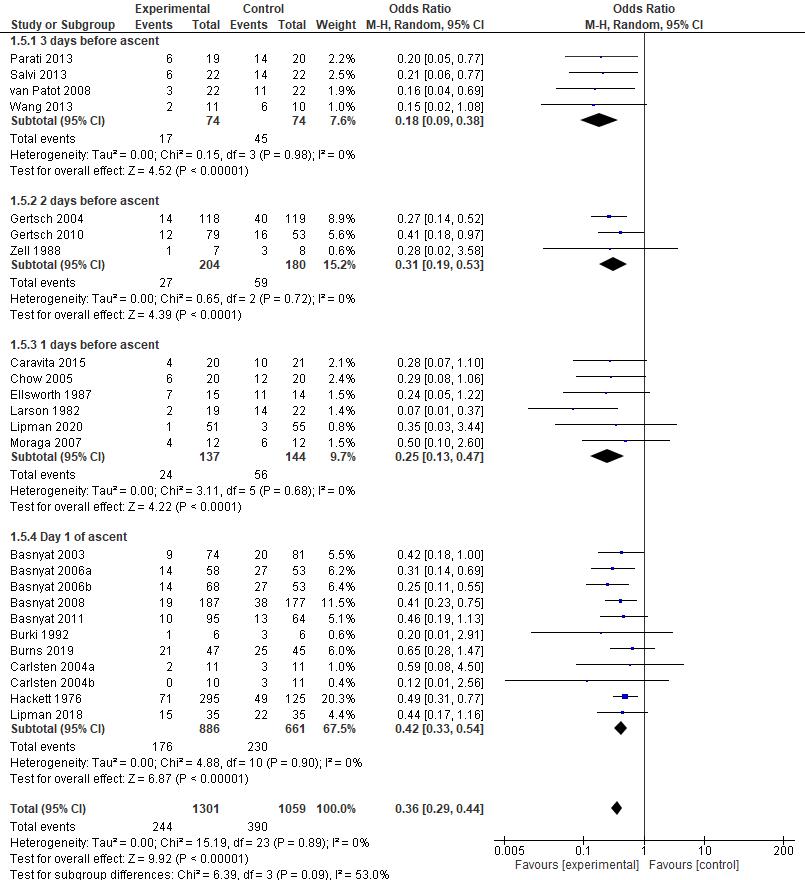
Table 2. Summary of meta-analysis outcome regarding incidence
OR (95% CI) p I2
Incidence of Headache 0.46 (0.36-0.59) <0.00001 85%
Subgroup analysis incidence of headache based on dosage Subgroup differences: p<0.00001
125 mg 1.61 (0.99-2.60) 0.05 97% 250 mg 0.32 (0.21-0.47) <0.00001 63% 375 mg 0.15 (0.08-0.29) <0.00001 0%
Incidence of Severe Headache 0.19 (0.08-0.42) <0.0001 0%
Incidence of Numbness Finger 12.37(6.79-22.56) <0.00001 77%
Incidence of Numbness Face and Lips 2.68 (0.89-8.03) 0.08 0% Incidence of Visual Disturbance 0.66 (0.17-2.59) 0.55 42%
3.3.1 Meta-analysis and sensitivity analysis incidence of headache using acetazolamide vs placebo
The incidence of headache was also reported in 11 studies. We also performed a metaanalysis to assess the incidence of headache between the intervention group (acetazolamide) and the control group (Figure 7). The result was significant with p<0.00001 and OR value: 0.46 (95% CI:0.36-0.59; I2=85%). Ingeneral, it canbe concludedthatacetazolamidecanreducethe incidence of headache. Although high heterogeneity has been found, with an I2 value of 85%, our sensitivity analysis identified Basnyat, et al's study as an outlier, and with its removal the heterogeneity has decreased to much lower value of 46% (Figure 8). When this study is removed, the pooled OR becomes 0.27 (p<0.00001; 95%CI: 0.20-0.36). This might be due to evaluation time that start in the evening (at rest time), where the acetazolamide medicine itself is taken on day 1 ascent.
Figure 7. Forest plot acetazolamide vs control in the incidence of headache
Figure 8. Sensitivity analysis acetazolamide vs control in the incidence of headache
3.3.2 Subgroup analysis incidence of headache based on acetazolamide dose
We also performed a dose-based subgroup meta-analysis on the incidence of headache (Figure 9). The results of our meta-analysis showed significant differences between subgroups with p<0.00001. These results indicate that a dose of 375 mg is the most effective dose in suppressing theincidence ofheadache with OR value0.15 (95%CI: 0.37-0.62; p<0.0001; I2 =0%). However, the 250 mg dose also showed significant results with OR value 0.32 (95% CI: 0.21-0.47; p<0.0001) with medium heterogeneity was found (I2=63%)
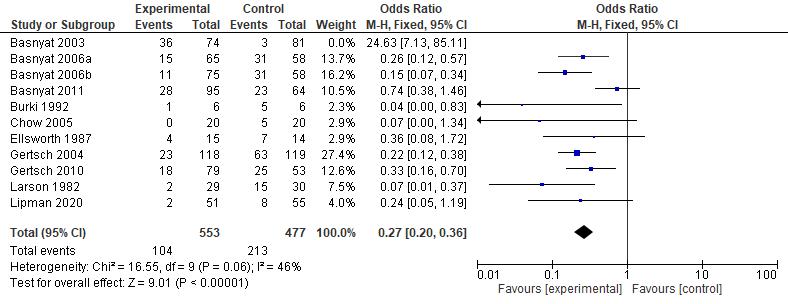
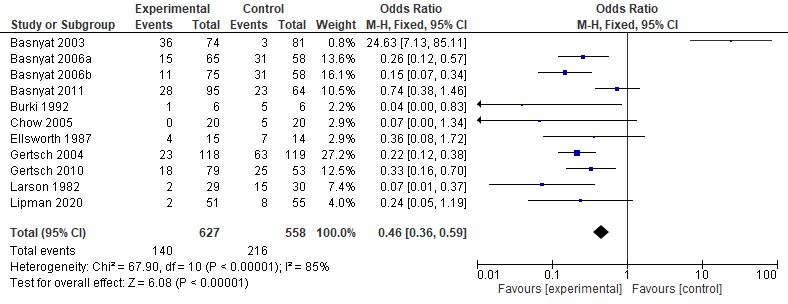
Figure 9. Subgroup analysis based on dose of acetazolamide vs control in the incidence of headache

3.3.3 Meta-analysis incidence of severe headache using acetazolamide vs placebo
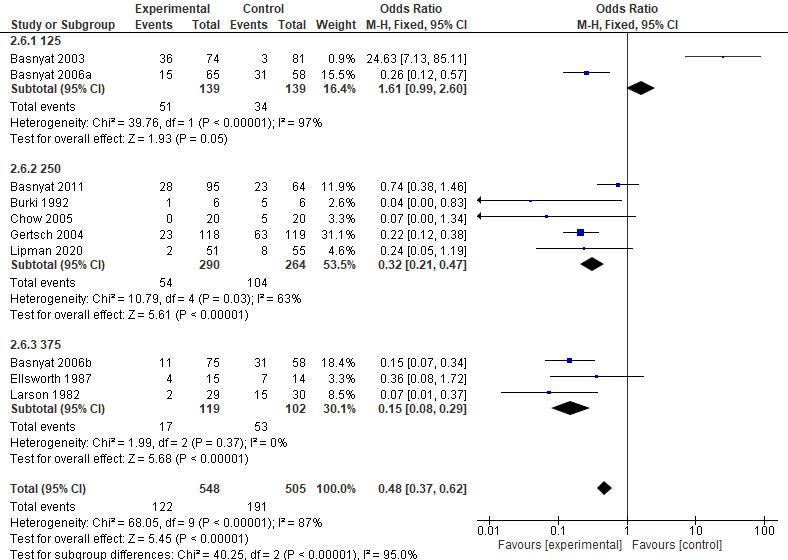
Three of the included studies also reported the incidence of severe headache (Figure 10). Ourmeta-analysisfoundthatacetazolamidesignificantlyreducedtheincidenceofsevereheadache with a pooled OR value 0.19 (95% CI: 0.08-0.42; p<0.00001; I2=0).
Figure 10. Forest plot acetazolamide vs control in the incidence of severe headache
3.3.4
Meta-analysis incidence of visual disturbance using acetazolamide vs placebo
Regarding visual disturbance, our meta-analysis found that acetazolamide reduced the incidence of visual disturbance with a pooled OR value 0.66 (95% CI: 0.17-2.42; [=0.55; I2=42%) (Figure 11). The high heterogeneity and insignificant results may be due to the limited number of studies assessing the effect of acetazolamide on the incidence of visual disturbances.
Figure 11. Forest plot acetazolamide vs control in the incidence of visual disturbance
3.3.5 Meta-analysis incidence of numbness finger using acetazolamide vs placebo

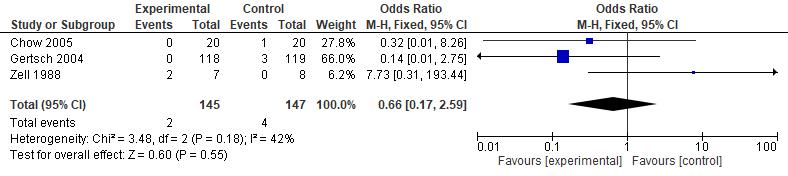
Meta-analysis was also done to assess the effectiveness of acetazolamide in reducing numbness in finger (Figure 12). Our meta-analysis found that acetazolamide significantly increased the incidence of numbness finger with a pooled OR value 12.37 (95% CI: 6.79-22.56; p<0.00001; I2=77. The high OR indicates the side effect of acetazolamide compared to the control which causes numbness in the finger.
Figure 12. Forest plot acetazolamide vs control in the incidence of numbness finger
3.3.6 Meta-analysis incidence of numbness face and lips using acetazolamide vs placebo
Meta-analysis was also done to assess the effectiveness of acetazolamide in reducing numbness in finger (Figure 13). Our meta-analysis found that acetazolamide was not significantly
increase the incidence of numbness in the face and lips with a pooled OR value 2.68 (95% CI: 0.89-8.03; p<0.00001; I2=0).
Figure 13. Forest plot acetazolamide vs control in the incidence of numbness face and lips
3.4 Study Outcomes - Meta-analysis and sensitivity analysis regarding O2 saturation of acetazolamide vs placebo grup
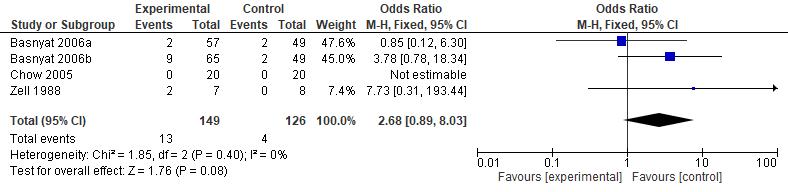
Intervention vs control O2 saturation was also reportedin 10studies (Figure 14).Theresult was significant with p<0.00001 and OR value: 3.14 (95% CI: 1.79-4.50; I2=95%). In general, it can beconcludedthat acetazolamidecanincreaseO2 saturationin theintervention groupcompared to the control group. Although high heterogeneity has been found, with an I2 value of 95%, our sensitivity analysis identified Bradwell, et al's study as an outlier, and with its removal the heterogeneity has decreased to much lower value of 66% (Figure 15)
Figure 14. Forest plot acetazolamide vs control regarding O2 saturation

Figure 15. Sensitivity analysis acetazolamide vs control regarding O2 saturation
4. DISCUSSION
4.1 Current Use of Acetazolamide

Various classes of drugs have been suggested for high-altitude illnesses, including acute mountain sickness (AMS), namely carbonic anhydrase (CA) inhibitors, steroids, bronchodilators, selective inhibitors of phosphodiesterase type 5 (PDE5), calcium modulators, and non佻steroidal anti佻inflammatory drugs (NSAIDs). Acetazolamide is categorized as a CA inhibitor capable of inhibiting CA in the kidneys, triggering hyperventilation-induced respiratory alkalosis to counter hypoxia, as well as independent pulmonary vasodilation.39
In recent literature, acetazolamide is used as prevention for high-altitude headache as well as AMS in moderate or high-risk ascent traveler profiles, with doses of 125 mg every 12 hours.40, 41 However, trials and reviews showed that effective dosages range from 250 to 750 mg per day.39 Guidelines recommend prophylactic intake to be started a day before the ascent and resumed for two to four days after reaching the target altitude. Contraindications for this drug include patients with a history of anaphylaxis or Stevens-Johnson syndrome from a sulfonamide.41
It is also used as AMS treatment in doses of 250 mg every 12 hours.40 However, for moderate to severe AMS, dexamethasone is preferred as treatment instead of acetazolamide.41 Central sleep apnea, which often happens in altitudes over 2500 meters, is also treated with acetazolamide when daytime activities are interfered by this sleep disorder.40
4.2
Effectivity of Acetazolamide in Acute
4.2.1 Dosage Effectivity
Mountain Sickness Prevention
Historically, a systematic review in 2000 claimed the lowest effective dose for acetazolamide to be 750 mg/day (or 375 mg/dose).42 Our findings are in line with a more recent systematic review and meta-analysis in 2012 refuted this by showing doses of 250 mg and 500 mg daily to be as effective compared to placebo for AMS prevention.43 In our meta-analysis, however, subgroup differences are slightly more apparent (p=0.11) compared to Low et al.’s study (p=0.22)43, with lower heterogeneity values especially for the dose of 750 mg/day (I2=0%). While current guidelines recommend doses of 250 mg/day and recent RCTs emphasize on lowering the dose even further to 125 mg/day30,31, it seems that the evidence for higher doses persist, as the overall effect was strongest for the 500 mg/day subgroup, or 250 mg/dose (Z=7.74) for incidence of AMS. Moreover, incidence of headaches are best responded by higher doses of 500 mg/day or 750 mg/day; outcomes for the 125 mg dose remain borderline ambivalent, favoring controls with an OR of 1.61 (95% CI: 0.99 2.60; p<0.00001; I2 =97%). As paresthesia remained to be a concern in higher doses, our findings signal that the main focus of future studies should probably not to find the lowest-effective dose, but to find a dose that is high enough to optimize effectivity while resulting in acceptable side effects.
4.2.2 Timing of Prophylactic Consumption
Consuming acetazolamide one day prior to altitude ascents is the current gold standard in practical guidelines, but we found prophylactic acetazolamide 3 days before ascent to be the most effective (OR=0.18; 95% CI: 0.09 0.38). While our findings support current recommendations on consuming one day before the ascent or during the day of the ascent to be effective, there is a lack of literature on consuming it two to three days prior to the ascent. This could be the direction for future studies as most studies tested for acetazolamide consumption the day before.
4.2.3
Targeted Patients of Prophylactic Acetazolamide
Although subgroup analysis results proved to remain significant for each subject type, overall effects are lower for lowlanders and other subjects than for trekkers and general volunteers due to the lower number of studies. Subject profiles are worth noting as lowlanders are more susceptible to acute mountain sickness; permanent residents and highlanders usually suffer from
other diseases that are more chronic in nature, such as chronic mountain sickness and pulmonary hypertension.44 Different subgroups also have different physiological responses; recommended acclimatization procedures for prevention differ from high altitude-naive subjects, trekkers, tourists, and other profiles.45 As prophylactic acetazolamide is intended to be a form of prevention, more studies on altitude-specific populations may be relevant in understanding its response variations across groups.
Our meta-analysis also found that sex proportions did not significantly affect the effectiveness of acetazolamide despite the majority of male subjects in almost all studies except five. The subjects of four of the included studies were even 100% male.16,26,32,37 Despite being a minority in trekker and mountaineering demographics, studies, including a meta-analysis, showed that women are more likely to suffer from acute mountain sickness than men46,47, although other studies stated otherwise.48 This phenomenon can be explained by hormones, such as estrogeninduced fluid retention leading to intracranial hypertension in women49, as well as the lack of testosterone which promotes erythropoiesis for better oxygen carrying capacity.50 Hence, the effectivity of acetazolamide will be beneficial for women as a vulnerable group in mountain sicknesses.
4.3 Effect of Acetazolamide Towards Final O2 Saturation
High altitude conditions such as when someone climbs a mountain can lead to oxygen desaturation in the human arterial circulation (hypoxemia) and even end up reducing oxygenation to tissues. These conditions are triggered by low atmospheric pressure in high altitude areas.51 It is also known that hypoxemia is hypothesized to be one of the pathophysiological mechanisms that can underlie the occurrence of AMS. Regarding the pathway, hypoxemia can lead to increased cerebral blood flow (CBF) and intracranial pressure (ICP) which in turn can trigger the activation of the trigeminovascular system and cause AMS symptoms. In addition, hypoxemia can also cause increased vascular permeability. This can lead to vasogenic edema which contributes to the increase in ICP.52 Therefore, through the close relationship between oxygen saturation and AMS, the comparison of oxygen saturation (SaO2) between the use of acetazolamide and placebo is one of the aspects that we highlight in this study.
Previously, it was known that acetazolamide can increase minute ventilation as well as improve arterial pO2 and oxyhemoglobin saturation at high altitude. One of the hypothesized
underlying mechanisms is that renal bicarbonate excretion leads to metabolic acidosis condition, therebyinhibitingtheeffectsofhypoxia-inducedrespiratoryalkalosis.Athighaltitude,hypoxemia will occur due to decreased partial pressure of inspired oxygen. Consequently, minute ventilation will increase so that there is a possibility of respiratory alkalosis occurrence. However, with the administration of acetazolamide, the effect of respiratory alkalosis will be inhibited so that oxygenation will continue to run well.53,54
Based on our meta-analysis results, it is significantly shown that acetazolamide has a more positive effect on oxygen saturation levels. A total of 10 studies included in the meta-analysis showed that the mean oxygen saturation in the acetazola intervention group was higher than in the placebo group. In the study of Basnyat, et al. it is known that there is a change in oxygen saturation of -4.1 ± 4 post-ascent in the subject group given acetazolamide at a dose of 125 mg twice daily. This change of oxygen saturation level was discernibly smaller when compared to the placebo group, which was -5.9 ± 5.3. Furthermore, in this study there was medication noncompliance in theacetazolamidegroup(presenceof19%missedcapsules).Thereisapossibilitythattheoutcome will be better without these missed capsules.11 Another Basnyat, et al., study, compared the performance of two different doses of acetazolamide, 250mg and 750 mg. It was found that the two doses did not show a different final saturation, namely 82.9% and 82.8% respectively (with a change of 8.4%). Through the results of this study, it can be concluded that the lower dose of acetazolamide has the same efficacy as the higher dose of acetazolamide.12
Regarding sex-related differences, the study of Caravita et al. demonstrated that acetazolamide improves oxygenation and decreases periodic breathing at altitude in both sexes without significant differences. Acetazolamide causes a left-ward shift in the CO2 chemoreflex response curve regardless of sex. At high altitude, acetazolamide will trigger a change in CO2 set point to balance hyperventilation-induced hypocapnia and lead to better oxygenation, as well as reducing nocturnal periodic breathing. Therefore, higher sO2 was found in the acetazolamide intervention group.18
A study on the effect of acetazolamide on exercise performance at altitude was conducted by Bradwell, et al. In this study, subjects exercised with 60% of sea-level peak power output after rapid ascent to the Italian alps (16 to 27 hours of altitude exposure). In the acetazolamide group, therewas adecreasein sO2from 91.7%±0.8%to 81.7%±2.4%.Meanwhilein theplacebogroup,
sO2 before exercise was also lower than the acetazolamide group, which was 85.4% ± 0.83% and decreased to 79.2% ± 1.1% after exercise. However, despite showing positive results on the measurement of sO2, the results showed that the administration of acetazolamide reduces the ability to exercise hard. This is based on the perception of exercise difficulty was higher in the acetazolamide group and more subjects in the acetazolamide group were unable to complete the exercise.15
Compared to other drug substances, acetazolamide tends to have more robust and better performance, including the ability to maintain sO2 at high altitudes. In a comparative study with dexamethasone through measurements in the range of 24 hours and 72 hours, the acetazolamide intervention group only had a significant increase from 81.82% in 24 hours to 85.28% in 72 hours, while dexamethasone just had a slight increase from 83.52% in 24 hours to 83.6% in 72 hours.26 Moreover, another comparison study to ginkgo biloba, the acetazolamide intervention group only had a decrease of 1.7 (85.3% to 83.7%), while the ginkgo group experienced a significant decrease of 5.2 (84.8% to 79.5%).22
4.4 Incidence
4.4.1 Headache and Severe Headache
Headacheis oneof the clinical manifestations thatis quiteoftenreportedin variousstudies. Uniquely, there are differences in the effects given by the administration of acetazolamide with different doses on the occurrence of headache. Through our meta-analysis, it is known that 375 mg of acetazolamide is the most effective dose in suppressing the incidence of headache. Besides that, with an OR value of 0.32, acetazolamide at the dose of 250 mg also significantly suppresses the occurrence of headache. It is hypothesized that the pathophysiological mechanism of headache at altitude is a change in cerebral blood flow so that oxygenation will be disturbed and can lead to vasogenic edema as well.55 Hence, coherent towards the study results of changes in oxygen saturation, acetazolamide has the potential to alter the cerebral hemodynamics and suppress the headache caused by high altitude conditions. Overmore, a retrospective study by Celebisoy at al. shows that acetazolamide was effective in reducing the frequency and severity of vertigo and headache attacks in patients with vestibular migraine. It is thought that acetazolamide contributes to this change through perturbation of the CO2 equilibrium and ion channel inhibition. In addition
to reducing the incidence of headache, from a few studies, acetazolamide is also known to significantly reduce the severity of headache.
4.4.3 Numbness (Paresthesia)
The incidence of numbness or paresthesia can be triggered by the administration of acetazolamide. From our meta-analysis, it is known that acetazolamide can significantly cause finger paresthesia with a pooled OR value of 12.37. One of the major results showing a high incidenceofparesthesiaduetoacetazolamideisthestudybyGertschetal. It showsthat85subjects with the acetazolamide intervention experiencing paresthesia (OR value of 0.04, 0.02 to 0.09) and 93 subjects with the combined acetazolamide and ginkgo intervention experiencing paresthesia (OR value of 0.04, 0.02 to 0.08).22 Regarding the comparison between doses, Basnyat et al. study showed that the incidence of paresthesias was higher in the intervention of acetazolamide at a dose of 350 mg compared to a dose of 125 mg (91% vs 71%, respectively). Therefore, To minimize paresthesias occurence on treatment with acetazolamide, 125 mg should be considered as the preferred dosage.12
4.4.3 Vision Disturbance
Study by Gertsch et al. reported that the group of subjects with 250 mg twice daily of acetazolamide intervention had a lower incidence of vision disturbance (blurred vision) compared to the control group.22 The same results were also reported by the study of Chow et al. through the administration of acetazolamide at the same dose.20 Another study of patients with idiopathic intracranial hypertension and mild visual loss showed that acetazolamide improved visual field function by measuring changes in the perimetric mean deviation (PMD). There was a significant change in PMDwith acetazolamideintervention,namely1.43dB (from 3.53 dB at baselineto 2.10 dB). In addition, positive improvement was also observed through other measurement methods, such as papilledema grade, visual function questionnaire (VFQ-25) and 10-item neuro-ophthalmic supplement.56 The action of acetazolamide as a carbonic anhydrase blocker is thought to be related to lowering eye pressure. Despite these few study results showing a low incidence of visual disturbances produced by acetazolamide, it should be emphasized that the limited number of studies may lead to heterogeneity and insignificant results.
4.5 Strengths and Limitations
All studies included in this paper are RCTs, which are considered the gold standard of evidence for interventions. Moreover, heterogeneity was not found in most of our meta-analyses. This, together with the variety of countries in which these studies were conducted, suggests the vast generalizability of our findings. To our knowledge, this study is also the first to date to include the effect of acetazolamide towards oxygen saturation, an essential parameter of efficacy considering acetazolamide’s mechanism of action.
However, several limitations exist, mostly in the diversity of methods. Rate of ascent is one of the main factors towards AMS incidence2, but its value varies between studies; the lack of reporting in this factor also does not allow us to analyze how the efficacy of acetazolamide may shift in different rates of ascent. Secondly, evaluation time on when the Lake Louise Score or similar parameters range from 15 minutes of reaching high altitude21, the morning after arrival12,22,23,32, every morning and evening, up to 96 hours after beginning the drug; this variation may affect AMS incidence, especially with the different definitions of AMS.
5. CONCLUSION AND FURTHER RECCOMENDATION
From this meta-analysis it was found that acetazolamide at doses of 125 mg, 250 mg, or 375 mg produced significant results in reducing the incidence of AMS. This study also found that the doses of 250 mg and 375 mg significantly reduced the incidence of headache compared to 125 mg. In the future, it is necessary to conduct a study taking into account the definition of LLQ (AMS), start altitude and end altitude, as well as similar evaluation times. This aims to determine the optimum time for acetazolamide to work at a certain height. Other factors such as history of AMS also need to be considered in future studies. The combination of acetazolamide with other drugsandgingkobilobaalsoneedstobeconsideredandfurtherstudiesarecarriedoutinthefuture.
ACKNOWLEDGEMENT
The authors have nothing to decleare
CONFLICT OF INTEREST
We declare that we have no competing intention for completing this review.
REFERENCES
1. Hackett PH, Shlim DR. High-altitude travel & altitude illness. Atlanta: CDC; 2020 [cited 2022 Oct 22]. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2020/noninfectious-health-risks/high-altitudetravel-and-altitude-illness
2. Prince TS, Thurman J, Huebner K. Acute mountain sickness. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; [Updated 2022 Jul 12] [cited 2022 Oct 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430716/
3. National Institutes of Health. Acute mountain sickness. Maryland: NIH; c2022 [cited 2022 Oct 22]. Available from: https://medlineplus.gov/ency/article/000133.htm
4. Swenson ER. Carbonic anhydrase inhibitors and high altitude illnesses. Subcell Biochem. 2014;75:361-86. doi: 10.1007/978-94-007-7359-2_18.
5. Farzam K, Abdullah M. Acetazolamide. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; [Updated 2022 Jul 10] [cited 2022 Oct 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532282/
6. Schmickl CN,Owens RL,OrrJE,EdwardsBA,MalhotraA.Sideeffects of acetazolamide: a systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Respir Res. 2020 Apr;7(1):e000557. doi: 10.1136/bmjresp-2020-000557.
7. GaoD,WangY,Zhang R,ZhangY.Efficacyof acetazolamidefortheprophylaxis ofacute mountain sickness: a systematic review, meta-analysis and trial sequential analysis of randomized clinical trials. Am J Med Sci [Internet]. 2021 May [cited 2022 Oct 22];361(5):635-45. Available from: https://www.thoracicmedicine.org/article.asp?issn=18171737;year=2021;volume=16;issue=4;spage=337;epage=346;aulast=Gao doi: 10.1016/j.amjms.2020.12.022.
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. 2021 Mar 29 [cited 2022 Oct 22];372:n71. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc8005924/ doi: 10.1136/bmj.n71
9. Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ [Internet]. 2019 Jan 30 [cited 2022 Oct 11];364. Available from: https://www.bmj.com/content/364/bmj.k4597
10. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ. 2003 Sep;327(7414):557 60.
11. Basnyat B, Gertsch JH, Johnson EW, Castro-Marin F, Inoue Y, Yeh C. Efficacy of lowdose acetazolamide (125 mg BID) for the prophylaxis of acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial. High Alt Med Biol. 2003 Spring;4(1):45-52. doi: 10.1089/152702903321488979.
12. Basnyat B, Gertsch JH, Holck PS, Johnson EW, Luks AM, Donham BP, et al. Acetazolamide 125 mg BD is not significantly different from 375 mg BD in the Prevention of Acute Mountain Sickness: The prophylactic acetazolamide dosage comparison for efficacy (PACE) trial. High Altitude Medicine; Biology. 2006;7(1):17 27.
13. Basnyat B, Hargrove J, Holck PS, Srivastav S, Alekh K, Ghimire LV, et al. Acetazolamide fails to decrease pulmonary artery pressure at high altitude in partially acclimatized humans. High Alt Med Biol. 2008 Fall;9(3):209-16. doi: 10.1089/ham.2007.1073.
14. Basnyat B, Holck PS, Pun M, Halverson S, Szawarski P, Gertsch J, et al. Spironolactone does not prevent acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial by SPACE Trial Group (spironolactone and acetazolamide trial in the prevention of acute mountain sickness group). Wilderness Environ Med. 2011 Mar;22(1):15-22. doi: 10.1016/j.wem.2010.10.009.
15. Bradwell AR, Myers SD, Beazley M, Ashdown K, Harris NG, Bradwell SB, et al. Exercise limitation of acetazolamide at altitude (3459 m). Wilderness Environ Med. 2014 Sep;25(3):272-7. doi: 10.1016/j.wem.2014.04.003.
16. Burki NK, Khan SA, Hameed MA. The effects of acetazolamide on the ventilatory response to high altitude hypoxia. Chest. 1992 Mar;101(3):736-41. doi: 10.1378/chest.101.3.736.
17. Burns P, Lipman GS, Warner K, Jurkiewicz C, Phillips C, Sanders L, et al. Altitude sickness prevention with ibuprofen relative to acetazolamide. Am J Med. 2019 Feb;132(2):247-51. doi: 10.1016/j.amjmed.2018.10.021.
18. Caravita S, Faini A, Lombardi C, Valentini M, Gregorini F, Rossi J, et al. Sex and acetazolamide effects on chemoreflex and periodic breathing during sleep at altitude. Chest. 2015 Jan;147(1):120-131. doi: 10.1378/chest.14-0317.
19. Carlsten C, Swenson ER, Ruoss S. A dose-response study of acetazolamide for acute mountain sickness prophylaxis in vacationing tourists at 12,000 feet (3630 m). High Alt Med Biol. 2004 Spring;5(1):33-9. doi: 10.1089/152702904322963672.
20. Chow T, Browne V, Heileson HL, Wallace D, Anholm J, Green SM. Ginkgo biloba and acetazolamide prophylaxis for acute mountain sickness: a randomized, placebo-controlled trial. Arch Intern Med. 2005 Feb 14;165(3):296-301. doi: 10.1001/archinte.165.3.296.
21. Ellsworth AJ, Larson EB, Strickland D. A randomized trial of dexamethasone and acetazolamide for acute mountain sickness prophylaxis. Am J Med. 1987 Dec;83(6):102430. doi: 10.1016/0002-9343(87)90937-5.
22. Gertsch JH, Basnyat B, Johnson EW, Onopa J, Holck PS. Randomised, double blind, placebo controlled comparison of ginkgo biloba and acetazolamide for prevention of acute mountain sickness among Himalayan trekkers: the prevention of high altitude illness trial (PHAIT). BMJ [Internet]. 2004 Apr 3 [cited 2022 Oct 1];328(7443):797. Available from: https://pubmed.ncbi.nlm.nih.gov/15070635/
23. Gertsch JH, Lipman GS, Holck PS, Merritt A, Mulcahy A, Fisher RS, et al. Prospective, double-blind, randomized, placebo-controlled comparison of acetazolamide versus ibuprofen for prophylaxis against high altitude headache: the Headache Evaluation at Altitude Trial (HEAT). Wilderness Environ Med. 2010 Sep;21(3):236-43. doi: 10.1016/j.wem.2010.06.009.
24. Hackett PH, Rennie D, Levine HD. The incidence, importance, and prophylaxis of acute mountain sickness. Lancet. 1976 Nov 27;2(7996):1149-55. doi: 10.1016/s01406736(76)91677-9.
25. Hillenbrand P, Pahari AK, Soon Y, Subedi D, Bajracharya R, Gurung P, et al. Prevention of acute mountain sickness by acetazolamide in Nepali porters: a double-blind controlled trial. Wilderness Environ Med. 2006 Summer;17(2):87-93. doi: 10.1580/1080-603217.2.87.
26. Hussain MM, Aslam M, Khan Z. Acute mountain sickness score and hypoxemia. J Pak Med Assoc. 2001 May;51(5):173-9.
27. KayserB,Hulsebosch R,BoschF.Low-doseacetylsalicylicacid analog andacetazolamide
for prevention of acute mountain sickness. High Alt Med Biol. 2008 Spring;9(1):15-23. doi: 10.1089/ham.2007.1037. .
28. Larson EB, Roach RC, Schoene RB, Hornbein TF. Acute mountain sickness and acetazolamide. Clinical efficacy and effect on ventilation. JAMA. 1982 Jul 16;248(3):32832.
29. Lipman GS, Pomeranz D, Burns P, Phillips C, Cheffers M, Evans K, et al. Budesonide versus acetazolamide for prevention of acute mountain sickness. Am J Med. 2018 Feb;131(2):200.e9-200.e16. doi: 10.1016/j.amjmed.2017.05.034.
30. Lipman GS, Jurkiewicz C, Burnier A, Marvel J, Phillips C, Lowry C, et al. A randomized controlled trial of the lowest effective dose of acetazolamide for acute mountain sickness prevention. AmJ Med. 2020Dec;133(12):e706-e715.doi: 10.1016/j.amjmed.2020.05.003.
31. McIntosh SE, Hemphill M, McDevitt MC, Gurung TY, Ghale M, Knott JR, et al. Reduced acetazolamide dosing in countering altitude illness: a comparison of 62.5 vs 125 mg (the RADICAL Trial). Wilderness Environ Med. 2019 Mar;30(1):12-21. doi: 10.1016/j.wem.2018.09.002.
32. Moraga FA, Flores A, Serra J, Esnaola C, Barriento C. Ginkgo biloba decreases acute mountain sickness in people ascending to high altitude at Ollagüe (3696 m) in northern Chile. Wilderness Environ Med. 2007 Winter;18(4):251-7. doi: 10.1580/06-WEME-OR 062R2.1.
33. Parati G, Revera M, Giuliano A, Faini A, Bilo G, Gregorini F, et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J. 2013 Mar;34(10):759-66. doi: 10.1093/eurheartj/ehs140.
34. Salvi P, Revera M, Faini A, Giuliano A, Gregorini F, Agostoni P, et al. Changes in subendocardial viability ratio with acute high-altitude exposure and protective role of acetazolamide. Hypertension. 2013 Apr;61(4):793-9. doi: 10.1161/HYPERTENSIONAHA.111.00707.
35. Tanner JB, Tanner SM, Thapa GB, Chang Y, Watson KL, Staunton E, et al. A randomized trial of temazepam versus acetazolamide in high altitude sleep disturbance. High Alt Med Biol. 2013 Sep;14(3):234-9. doi: 10.1089/ham.2013.1023.
36. van Patot MC, Leadbetter G 3rd, Keyes LE, Maakestad KM, Olson S, Hackett PH. Prophylactic low-dose acetazolamide reduces the incidence and severity of acute mountain sickness. High Alt Med Biol. 2008 Winter;9(4):289-93. doi: 10.1089/ham.2008.1029.
37. Wang J, Ke T, Zhang X, Chen Y, Liu M, Chen J, et.al. Effects of acetazolamide on cognitive performance during high-altitude exposure. Neurotoxicol Teratol. 2013 JanFeb;35:28-33. doi: 10.1016/j.ntt.2012.12.003.
38. Zell SC, Goodman PH. Acetazolamide and dexamethasone in the prevention of acute mountain sickness. West J Med [Internet]. 1988 May [cited 2022 Oct 10];148(5):541-5. Available from: https://pubmed.ncbi.nlm.nih.gov/3051673/
39. Estrada VH, Franco DM, Medina RD, Gonzalez Garay AG, Martí-Carvajal AJ, ArevaloRodriguez I. Interventions for preventing high altitude illness: Part 1. Commonly-used classes of drugs. Cochrane Database Syst Rev. 2017 Jun 27;6(6):CD009761. doi: 10.1002/14651858.CD009761.pub2.
40. Luks AM, Hackett PH. Medical conditions and high-altitude travel. N Engl J Med [Internet]. 2022 Jan 27 [cited 2022 Oct 15];386(4):364-73. Available from: https://www.nejm.org/doi/10.1056/NEJMra2104829?url_ver=Z39.88-
2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed doi: 10.1056/NEJMra2104829.
41. Armstrong C. Acute altitude illness: updated prevention and treatment guidelines from the Wilderness Medical Society. Am Fam Physician. 2020 Apr 15;101(8):505-7.
42. Dumont L, Mardirosoff C, Tramèr MR. Efficacy and harm of pharmacological prevention of acute mountain sickness: quantitative systematic review. BMJ. 2000 Jul 29;321(7256):267-72. doi: 10.1136/bmj.321.7256.267.
43. Low EV, Avery AJ, Gupta V, Schedlbauer A, Grocott MP. Identifying the lowest effective dose of acetazolamide for the prophylaxis of acute mountain sickness: systematic review and meta-analysis. BMJ. 2012 Oct 18;345:e6779. doi: 10.1136/bmj.e6779.
44. West JB. High-altitude medicine. Am J Respir Crit Care Med. 2012 Dec 15;186(12):122937. doi: 10.1164/rccm.201207-1323CI.
45. Burtscher M, Hefti U, Hefti JP. High-altitude illnesses: Old stories and new insights into the pathophysiology, treatment and prevention. Sports Med Health Sci. 2021 Apr 16;3(2):59-69. doi: 10.1016/j.smhs.2021.04.001.
46. Hou YP, Wu JL, Tan C, Chen Y, Guo R, Luo YJ. Sex-based differences in the prevalence of acute mountain sickness: a meta-analysis. Mil Med Res. 2019 Dec 9;6(1):38. doi: 10.1186/s40779-019-0228-3.
47. Caravedo MA, Mozo K, Morales ML, Smiley H, Stuart J, Tilley DH, et al. Risk factors for acute mountain sickness in travellers to Cusco, Peru: coca leaves, obesity and sex. J Travel Med. 2022 Aug 20;29(5):taab102. doi: 10.1093/jtm/taab102.
48. Cheng FY, Jeng MJ, Lin YC, Wang SH, Wu SH, Li WC, et al. Incidence and severity of acute mountain sickness and associated symptoms in children trekking on Xue Mountain, Taiwan. PLoS One. 2017 Aug 23;12(8):e0183207. doi: 10.1371/journal.pone.0183207
49. Stachenfeld NS, Silva C, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol (1985). 1999 Sep;87(3):1016-25. doi: 10.1152/jappl.1999.87.3.1016.
50. Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009 Sep;32(8):704-16. doi: 10.1007/BF03345745.
51. Netzer N, Strohl K, Faulhaber M, Gatterer H, Burtscher M. Hypoxia佻related altitude illnesses. Journal of Travel Medicine. 2013;20(4):247 55.
52. Berger MM, Sareban M, Bärtsch P. Acute mountain sickness: Do different time courses point to different pathophysiological mechanisms? Journal of Applied Physiology. 2020;128(4):952 9.
53. Leaf DE, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of Acute Mountain Sickness. Journal of Applied Physiology. 2007;102(4):1313 22.
54. Wang K, Smith ZM, Buxton RB, Swenson ER, Dubowitz DJ. Acetazolamide during acute hypoxia improves tissue oxygenation in the human brain. Journal of Applied Physiology. 2015;119(12):1494 500.
55. RoachRC,HackettPH.Frontiersofhypoxiaresearch:acutemountainsickness.JExpBiol. 2001 Sep;204(Pt 18):3161-70. doi: 10.1242/jeb.204.18.3161. PMID: 11581330.
56. Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA [Internet]. 2014 Apr 23 [cited 2022 Oct 22];311(16):1641 51. Available from:
https://jamanetwork.com/journals/jama/fullarticle/1861803
1. Keywords details in each databases
Databases
PubMed
Scopus
Keywords Hits
(“Acetazolamide" OR "Diamox") AND ("acute mountain sickness" OR "altitude illness" OR "high altitude headache" OR "high altitude")
TITLE-ABS-KEY ( acetazolamide OR diamox ) AND TITLE-ABS-KEY ( "Acute mountain sickness" OR "high-altitude sickness" OR AMS )
435
515
ProQuest (Acetazolamide OR diamox ) AND ( "Acute mountain sickness" OR "high-altitude sickness" OR AMS) 500
Cochrane ( acetazolamide OR diamox) AND ( "Acute mountain sickness" OR "high-altitude sickness" OR AMS) 8
Wiley Library
"(acetazolamide OR diamox)" in Title and "( "Acute mountain sickness" OR "high-altitude sickness" OR AMS)" anywhere
306
Appendix
Appendix 2. Studies quality assessment based on Cochrane RoB 2.0

Appendix 3. Characteristics of Included Studies
Author (Year)
Type of Subjects Location Male (%)
ACZ Dose (mg/bid) and Frequency Control Start Time Evaluatio n Time AMS Scale
Basnyat 2003 Trekkers Mount Everest, Nepal 67.1 125 Placebo Day 1 of ascent
Basnyat 2006 Trekkers Mount Everest, Nepal 64.7 125/375 Placebo Day 1 of ascent
Basnyat 2008 Trekkers Mount Everest, Nepal 62.6 4 250 Placebo Day 1 of ascent
Basnyat 2011 Trekkers Mount Everest, Nepal 66.0 4 250 Placebo Day 1 of ascent
Definition of AMS Mode of Ascent Start Altitud e
End Altitude
At rest in the evening LLQ LLQ ≥ 3; headache + 1 symptoms Climb 4243 4937
The morning after arrival LLQ LLQ ≥ 3; headache + 1 symptoms Climb 3440 4928
36-96 h after beginning of drug LLQ LLQ ≥ 3; headache + 1 symptoms Climb 4250 5,000
36-96 h after beginning of drug LLQ LLQ ≥ 3; headache + 1 symptoms Climb 4300 5,000
Bradwell 2014 Volunteers Refugio Testa Grigia, Italy 70 250 Placebo Day 1 of ascent 16-27 h after HA LLQ Transpor t 1608 3459
Burki 1992 Volunteers Karakorum mountains, Pakistan 100 250 Placebo (Ascorbic Acid) Day 1 of ascent 32-56 h after arrival
Clinical observatio n
Burns 2019 Volunteers White Mountains of California - 125 Ibuprofen Day 1 of ascent 6-18 h post-acent LLQ
Dizziness, nausea/vomiting , headache Transpor t 518 4450
LLQ score ≥ 3 including headache Climb 0 3810
Caravita 2015 Volunteers Capanna Regina Margherita , Italy
51.2 2 250 Placebo 1 day before ascent
Carlsten 2004 Tourists La Paz, Bolivia - 125/250 Placebo (Ascorbic Acid)
Within 2 h of airport arrival
Every morning at HA LLQ LLQ > 4 Transpor t and Climb 122 4559
At 0, 6, 12, and 24 h from initiation of medication
LLQ LLQ ≥ 3 including headache Transpor t 3630 3630
Chow 2005 Volunteers California Barcroft Laboratory 57.5 250 Placebo 1 day before ascent 24 h after HA LLQ LLQ ≥ 3; headache + 1 symptoms Transpor t 1230 3800
Ellsworth 1987 Climbers Mount Rainier, USA 93.3 3 375
Dexamethaso ne 4 mg; Placebo (Lactose)
1 day before ascent
Within 15 min of reaching HA ESQ-III AMS-C > 0.7; AMS-R > 0.6 Transpor t and Climb 13001600 4392
Gertsch 2004 Trekkers Mount Everest, Nepal 70.4 6 250 Placebo 3 or 4 doses before ascent
The morning after arrival LLQ LLQ ≥ 3; headache + 1 symptoms Climb 4358 4928
Gertsch 2010 Trekkers Mount Everest, Nepal 68.5 2 85 thrice Placebo Minimum of 3 doses before ascent
The morning after arrival LLQ LLQ ≥ 3; headache + 1 symptoms Climb 4358 4928
Hackett 1976 Hikers Pheriche, Nepal 71 250 Placebo (Lactose) Day 1 of ascentHackett score AMS ≥ 2 (0 to 5 point scale) Transpor t and Climb 3440 4243
Hillenbran d 2006 Nepali trekking porters
Mount Everest, Nepal - 125 Placebo - - LLQ
Hussain 2001 Volunteers Karakorum range, Pakistan 100 125 (twice daily) Placebo (Multivitamin )
1 day before ascent 24 and 72 h at HA ESQ
LLQ ≥ 3; headache + 1 symptoms Climb 3440 4930
AMS-C > 0.7; AMS-R > 0.6; ESQ Score > 5
Kayser 2008 Volunteers Mount Kilimanjar o, Tanzania 91 250 Placebo -
Daily around 6 PM after at least 2 h of rest after arrival
LLQ
LLQ ≥ 3 including headache
Transpor t and Climb 515 4578
Transpor t and Climb sea level 5896
Larson 1982 Volunteers Mount Rainier, USA 91 375 Placebo 1 day before ascent - GHAQ
Headache of moderate or greater severity, nausea of slight or greater severity, or both
Transpor t and Climb 1300 1600 4394
Lipman 2018 Volunteers White Mountains of California 53.4 125 Placebo; Budenoside Day 1 of ascent
Lipman 2020 Volunteers White Mountains of California
47.1 7 62.5/125 (twice daily) Placebo 1 day before ascent
Evening of ascent and morning after ascent
LLQ
Evening and next morning at HA LLQ
LLQ ≥ 3; headache + 1 symptoms
Transpor t and Climb 1240 3810
LLQ ≥ 3; headache + 1 symptoms
McIntosh 2019 Trekkers
Everest Base Camp, Nepal 64.4 62.5/125 (twice daily) Placebo 1 day before ascent
Every morning and evening LLQ
Transpor t and Climb 1240 3810
LLQ ≥ 3; headache + 1 symptoms Climb 1400
Standard: 5515±33 2 Reduceddose: 5020±42 0
Moraga 2007 Volunteers Ollague, Chile 100 250 Placebo 1 day before ascent
Parati 2013 Lowlanders Capanna Regina Margherita , Italy
Salvi 2013 Lowlanders Capanna Regina Margherita , Italy
48.7 2 250 Placebo 3 days before ascent
The morning after arrival LLQ LLQ ≥ 3 Transpor t sea level 3969
At least 4 h after reaching HA LLQ LLQ ≥ 3 Transpor t and Climb 122 4559
48.7 2 250 Placebo 3 days before ascent - LLQ LLQ ≥ 3 Transpor t and Climb 122 4559
Tanner 2013 Trekkers Manang, Nepal 38.2 4 125 single dose 7.5鳥mg Temazepam Night of enrollmen t
Evening of enrollment and the following day
LLQ LLQ ≥ 3 Climb 760 3540
van Patot 2008 Volunteers Colorado Springs, Colorado 50 125 Placebo (lactulose) 3 days before ascent After 24 h at HA ESQ-III; LLQ
Wang 2013 Volunteers Lhasa, China 100 125 Placebo 3 days before ascent
Zell 1988 Backpackers Sierra Nevada Mountains, California 62.5 250 (twice daily) Placebo 2 days before ascent
Combination of AMS-C score ≥ 0.7 and an LLQ ≥ 3
Transpor t 1600 4394
Daily for around 6 PM LLQ LLQ ≥ 3 Transpor t 402 3561
Every 12 h at altitudes above 3650 ESQ-III ESQ > 0.7 Transpor t and Climb 3650 4050
Appendix 4. Outcome of included studies
Author (Year) Dosage
Basnyat
Incidence of AMS Incidence of Heart Racing Incidence Microturition Incidence of Headache Incidence of Severe Headache Incidence of Numbness, fingers
Incidence of Numbness, face and lips
Incidence of Visual disturbance Final O2 Saturation
Int Con Int Con Int Con Int Con Int Con Int Con Int Con Int Con Int Con
n N n N n N n N n N n N n N n N n N n N n N n N n N n N n N n N Mean SD Mean SD
2003 125 9 74 20 81 36 74 3 81 82.8 4.1 81 5.5
Basnyat 2006a 125 14 58 27 53 34 62 28 53 15 65 31 58 2 57 2 49 2 57 2 49
Basnyat 2006b 375 14 68 27 53 44 73 28 53 11 75 31 58 9 65 2 49 9 65 2 49
Basnyat 2008 250 19 187 38 177 86.45 3.39 85.91 4.08
Basnyat 2011 250 10 95 13 64 28 95 23 64 3 95 7 64 83 0.04 80 0.04
Bradwell 2014 250 91.7 0.08 85.4 0.83
Burki 1992 250 1 6 3 6 1 6 5 6 86.2 1.1 83.4 1.1
Burns 2019 125 21 47 25 45
Caravita
2015 250 4 20 10 21 85.2 3.8 77.1 6.6
Carlsten 2004a 125 2 11 3 11 Carlsten 2004b 250 0 10 3 11
Chow 2005 250 6 20 12 20 4 20 4 20 0 20 5 20 0 20 0 20 0 20 0 20 0 20 1 20
Ellsworth
1987 375 7 15 11 14 4 15 7 14
Gertsch 2004 250 14 118 40 119 23 118 63 119 2 118 16 119 85 118 12 119 0 118 3 119
Gertsch
2010 85 12 79 16 53 18 79 25 53 3 79 7 53 82.6 3.5 81.4 4.3
Hackett 1976 250 71 295 49 125
Hillenbrand
2006 250 7 55 6 54
Hussain 2001 250 85.28 1.19 82.27 2.78
Kayser 2008 250 24 44 26 31
Larson 1982 375 2 19 14 22 8 29 14 30 6 29 0 30 2 29 15 30
Lipman 2018 125 15 35 22 35 88.1 3.8 86.4 5
Lipman 2020 250 1 51 3 55 2 51 8 55
McIntosh 2019 125 21 35 21 38
Moraga 2007 250 4 12 6 12
Parati 2013 250 6 19 14 20
Salvi 2013 250 6 22 14 22 87.5 3.9 82 4.6
Tanner 2013 125 2 18 3 16 82 5 78 8
van Patot 2008 125 3 22 11 22
Wang 2013 125 2 11 6 10
Zell 1988 250 1 7 3 8
Prophylactic Efficacy of Antimalarial Chemoprophylaxis Drugs for Preventing Seasonal
Malaria: A Systematic Review from Randomized Controlled Trials
Muhammad Azka Al atsari1a, Nabilah Puteri Larassaphira1, Lidia Jamal1, Elsa Maydita1
1Faculty of Medicine, Hasanuddin University, Indonesia 1a azka2alatsary@gmail.com
ABSTRACT
Introduction: Malaria infection transmitted to humans through the bite of the female Anopheles. Some modality to prevent malaria has some weakness, as it is shown to be less efficient if used on a large scale and could give rise to human health and environmental problems. Prevention of malaria can be done by using chemoprophylaxis, a medication that uses antimalarial drugs to prevent the infection of malaria disease. In this systematic review, we performed qualitative synthesis analysis regarding chemoprevention as a choice for malaria prevention
Objectives: The aim of this systematic review is to evaluate the efficacy of antimalarial chemoprophylaxis drugs (azithromycin, tafenoquine, and naphthoquinone-azithromycin) to prevent seasonal malaria
Material and Methods: The studies included are randomized controlled trials (RCTs) from 4 databases (PubMed, Science Direct, ProQuest, and Cochrane Library) and selected based on PRISMA2020 guidelines, resulting in 11 studies forqualitative analysis. Risk of bias wasassessed using Review Manager 5.4.1
Results and Discussion: The results showed that azithromycin has a significant impact on a lower prevalence of malaria parasites and number of death (P=0.003). Using naphthoquine-azithromycin also shows significant results in prophylactic efficacy and safety (P<0.001).
Conclusion: The use of medication to prevent malaria showed positive effects in some studies, it can be promisingly applied to countries by considering their resources and choosing which drugs are suitable.
Keywords: efficacy, chemoprevention, malaria
Prophylactic Efficacy of Potentially Antimalarial Chemopreventive Drugs for Preventing Seasonal Malaria: A Systematic Review from Randomized Controlled Trials

Authors:
Muhammad Azka Al atsari
Nabilah Puteri Larassaphira
Lidia Jamal Elsa Maydita
Faculty of Medicine, Hasanuddin University
Asian Medical Students’ Association Universitas Hasanuddin 2022
Introduction
Malaria is a parasitic infectious disease of the genus Plasmodium which is transmitted to humans through the bite of the female Anopheles[1]. It is still a world health problem, especially in malaria endemic areas such as sub-Saharan Africa[2]. Based on data from the latest World malaria report, there were 241 million malaria cases in 2020, an increase of 14 million cases from 2019 and the estimated death from malaria reached 672,000 people in 2020[3] . Because the clinical manifestations of malaria are non-specific, WHO recommends that all patients suspected of malaria undergo parasite-specific laboratory tests to confirm clinical suspicion[3]
Asthisemergingdiseasecontinuesto increase every year,some methodsarepotentially used to prevent malaria, including Insecticide-treated mosquito nets (ITNs), Indoor residual spraying (IRS), and Larval source management (LSM)[4]. However, these sorts of modalities have shown to be less efficient if used on a large scale and could give rise to human health and environmental problems[5]. For example, despite the success of dichlorodiphenyltrichloroethane (DDT) in controlling mosquitoes, it has been banned by several countries due to environmental hazards[4] .
Based on WHO guidelines, prevention of malaria can be done by using chemoprophylaxis, a medication that use antimalarial drugs to prevent the infection of malaria disease[6]. Chemoprophylaxis kills the asexual stages of the plasmodium, which is responsible for the acute clinical phase in malaria. It also acts against all hepatic plasmodium in primary or dormant live phases, making it able to prevent malaria both in acute or in delayed malaria attacks[7]. The example of chemoprophylaxis are azithromycin (AZ) and combination between napthoquine and azithromycin (NPAZ). Prior study also shows that AZ has many advantages compared to other treatment, such as prolonged half-life, better tolerability, better acceptability in young children, and also a good efficacy when administered daily, which is associated with malaria parasitemia reduction[8]. Moreover, prior research also showed that combination between naphtoquinone and azithromycin are safe and their in vivo interaction on Plasmodium parasites were synergetic[9]. Another chemoprophylaxis is tafenoquine. Tafenoquine has a long half-life, enabling weekly dosing for malaria prevention. It has also been proven as an agent to prevent in acute and delayed attacks in malaria[10] .
As to focus on seasonal malaria, chemoprevention is critical to analyze the efficacy of every drug in the targeted populations. Appropriate statistical analysis of safety outcomes can improve the development of anti-malarial drug safety profile. This can be achieved through efficient use of RCTs data by examination, in which can provide a comprehensive drug safety
insight[11]. Therefore, this review aims to identify the efficacy of anti-malarial drugs for malaria prevention in recent randomized clinical trials
Material and Methods Data Collection
Data collections in this study are done by utilizing the methodological guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for more transparent and comprehensive systematic review.
Data Source and Search Strategy
Four reviewers (M.A., N.P., L.J., E.M.) compiled the data in this study using 4 search engines which are PubMed, Science Direct, ProQuest, and Cochrane Library. The keywords used for the search engines were “efficacy” AND “malaria” AND “chemoprevention OR chemoprophylaxis”.
Inclusion Criteria
Included studies are randomized controlled trials (RCTs) consisting of patients who are willing to participate in the research as intervention group, which they are given certain medication to prevent malaria infection, and to compare them with the control group, which are given placebo, for evaluation. This review only includes English literatures, and recent 5years of publication.
Study Selection
Studiesare identifiedusing keywords usedduringthe search.Afterremovingduplicates using Rayyan.ai program, four independent reviewers (M.A., N.P., L.J., E.M.) screened all the retrieved studies based on their title and abstracts. Studies that does not include chemoprevention as a method of intervention, and incidence of malaria infection as an outcome, were excluded. After that, all the full-text studies will be assessed using the inclusion criteria, and consensus would be held among the reviewers should there be any dissents needs to be resolved.
Quality Assessment
The appropriate risk of bias assessment tool according to our study was utilized using Review Manager 5.4.1. This tool measured the risks of bias in selection, performance,
detection, attrition, and outcome reporting of our systematic review qualitatively. Results are then classified into unclear, low risk, or high risk in bias. We evaluated the risk of bias independently and discussed it together to form a summary.
Data Extraction
Data from each study are collected and have been concluded in a table. The following data are extracted from the included studies: 1) first author and publication year; 2) study design; 3) number of participants; 4) type of intervention; 5) intervention and control details; 6) outcomes; and 7) significancy (P-value).
Results
A preliminary search in four electronic databases (PubMed, ProQuest, Science Direct, and Cochrane Library) yielded 1724 articles and 37 duplicate articles were removed. Then, authors read the title and abstracts of the remaining 1687 articles for preliminary screening, resulting in 19 eligible articles for further analysis. However, authors excluded 8 articles due to wrong procedure of intervention and wrong type of intervention. Finally, this systematic review included a total of 11 studies. Search flowchart and selection methods in this systematic review were summarized in Figure 1.
Characteristics of Included Studies
Outcomedatawere availablefrom11studiesusingrandomized controlledtrials(RCTs) with chemoprophylaxis as their method of intervention, placebo as comparison, and prevalence, mortality rate, prophylactic efficacy and safety as outcome. Based on 11 studies using a randomized controlled trial design, it was found that total participants included in this review were 79178 participants (38.340 participants as intervention group and 40.838 participants as control group). Participant included in this review were found to be aged from 1 months to 60 65 years, with intervention durations ranging from 14 days to 3 years.
Figure 1. PRISMA flow chart of study selection
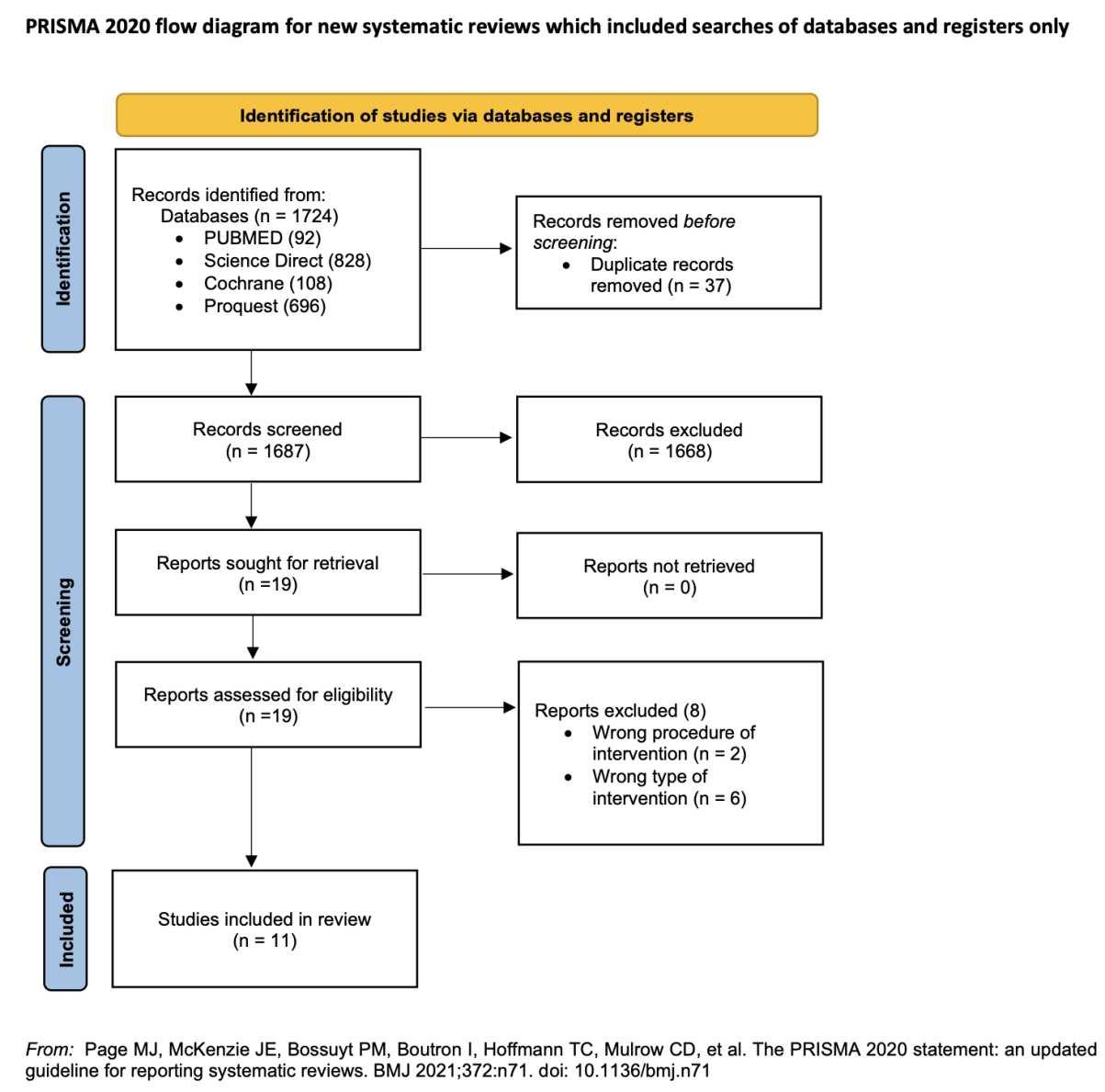
Abbreviations
N/A: Not Applicable
IG: Intervention Group
CG: Control Group
Table 1. Results of Qualitative Analysis of Azithromycin
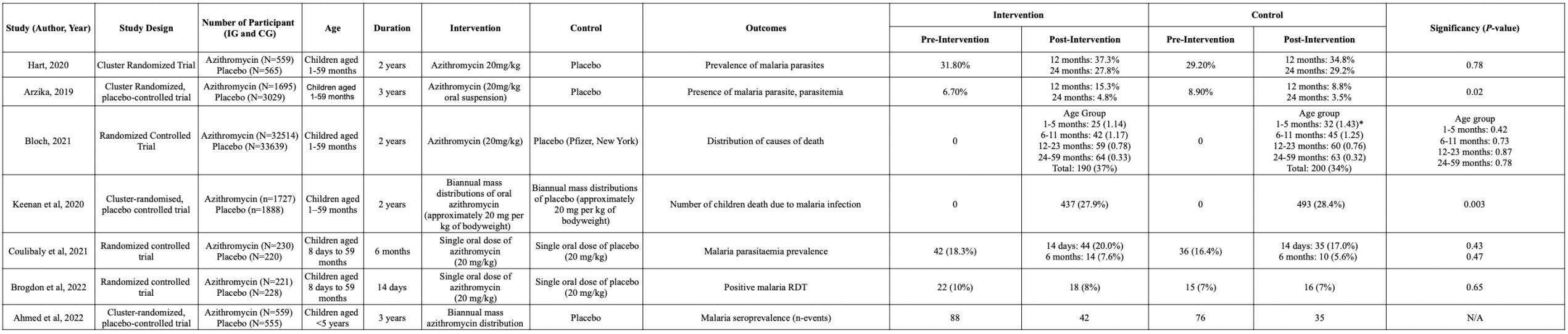
Table 2. Results of Qualitative Analysis of Naphthoquine-Azithromycin
Table 3. Results of Qualitative Analysis of Tafenoquine
Figure 2. Quality assessment of RCTs. (A) Risk of bias graph: review of authors’ judgements regarding the risk of bias item presented as percentage for all included studies. (B) Risk of bias summary: review of authors’ judgements regarding the risk of bias for each included study.

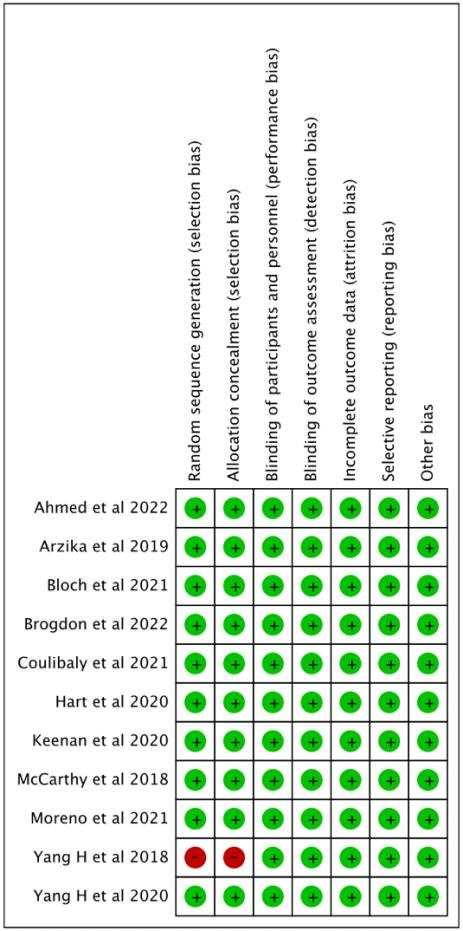
A B
Risk of Bias Assessment
Random allocation sequence generation and allocation concealment clearly described by all studies, except for 1 study. All of the study clearly described if they were blind of participants and personnel and blind outcome assessment. It also didn't deal with outcome assessment, incomplete outcome data and reporting bias. As a summary, all of the studies had a good quality level of methodological quality. The results from Review Manager 5.4.1 for risk of bias assessment were displayed in Figure 2.
Azithromycin Outcome : Prevalence of Malaria Parasites and Number of Death
This review assesses the presence of malaria parasitemia in participants, both in intervention and control groups. This examination becomes the parameter for determining the impact of azithromycin as an intervention on malaria prevention. Unfortunately, study by Hart and Coulibaly reported insignificant changes with the control group, it may caused by individually randomized nature of its trial[12,13]. Nevertheless, it remains to be noted there’s also results shows that prevalence of malaria parasites in azithromycin group was significantly lower compared to placebo group in 2 studi[14,15] .
The other outcome in azithromycin intervention is number of deaths caused by malaria. In untreated cases, malaria could be life-threatening. There were 2 studies reporting the number of death, 1 of them didn’t show significant results[16]. Nevertheless, other study show that using azithromycin could lower the number of death compared to placebo (P=0.003)[17]
NQAZ Outcome : Prophylactic Efficacy and Safety
This review assesses the prophylactic efficacy and safety analysis. The point of this outcome is the drugs that are used are safe (well tolerated) and there’s no serious adverse event that occurred. Although there were only few studies included, but it shows significant result in prophylactic efficacy and safety compared to placebo group (P<0.001)[10,18] .
Tafenoquine Outcome : Adverse Event
As an outcome, adverse events after the intervention is also an important key to assess the safety of drugs. There were no significant results between intervention and control group in 1 study[19]. But, the other study shows that using tafenoquine could lower the number of patients with at least one adverse event occurred compared to placebo[20]
Chemoprevention Features
The strategy for using chemoprevention or chemoprophylaxis is to use a number of medications that are efficacious as antimalarials to prevent, control, and eliminate malaria. However, as the use of number of drugs, the risk of anti-malarial resistance must also be considered[21]. In this review, a qualitative analysis of the efficacy of the use of a number of drugs as malaria chemoprevention has been carried out, including azithromycin, the combination of Naphthoquine-Azithromycin, and tafenoquine. It showed that there were 2 of 7 studies showing positive results in malaria positivity after azithromycin administration, all studies on NQ-AZ and tafenoquine intervention also showed positive and significant results (P<0.05). The analysis related to the details and effectiveness of each drug is described in three subsections below.
Azithromycin
Previous review have reported that Azithromycin has been studied as a potential antimalarial agent, although it exerts slow, this drug activity is potent antimalarial against the apicoplast organelles[22]. Azithromycin is a type of macrolide antibiotic which consists of a 14 , 15-, or 16-membered macrocyclic lactone ring to which one or more deoxy sugars may be attached[23]. Since its discovery, the FDA has approved the use of azithromycin in treating respiratory infections, genitourinary infections , and enteric infections, and others. In this review, the administration of azithromycin as a chemoprevention was carried out according to duration, each study giving it in a different duration, namely biannually and single dose. The dosage is 20 mg/kgBW. To support the qualitative analysis, the use is considered to be safe, as no serious study drug-induced adverse events were reported [12,13,15 17]. Only Brogdon et al, reported a lower likelihood of fever as a side effect than children in the placebo group[24]. These results that are not yet statistically significant allow a more comprehensive and adjusted evaluation of the intervention population group.
Naphthoquine-Azithromycin
Combination therapy of drugs with different mechanisms of action is one of the arts in the treatment of malaria, the efficacy of current chemoprophylaxis is compromised due to drug resistance and non-adherence due to several side effects, an animal model study showed clear evidence of the combination of azithromycin (AZ) and naphthoquine (NQ)[9]. In this review two articles tested the combined use of the two drugs using doses of 400 mg NQ-AZ, 800 mg
Discussion
NQ-AZ, and 400 mg NQ + 400 mg AZ. There was a decrease in the incidence of malaria after 8 weeks of administration in the intervention population. Both regimens were well tolerated with no serious side effects, there was only a slight increase in liver enzymes ALT and AST which returned to normal after the intervention was completed[10,18]
Tafenoquine
Tafenoquine is an 8-aminoquinoline that can inhibit multiple life phases of Plasmodium species, this drug also has a long half-life that allows administration in a single dose[25]. In these 2 studies, unlike the previous drug, no analysis was carried out on the effect of tafenoquine administration on subsequent malaria incidence. In this review, we assessed how tafenoquine administration was effective in adverse event outcomes that varied greatly from those reported, for convenience, using the subject parameter with at least one adverse event. With a loading dose of tafenoquine 200 mg once per week, this drug did not cause serious adverse events (SAEs) during the intervention period and the side effects did not cause participant withdrawal or discontinuation of their participation[19,20] .
Importance of Chemoprevention to Prevent Malaria Incidence
Malaria incidence and deaths worldwide have been reduced dramatically when it rose again in 2020, before the COVID-19 pandemic. Further damage of the recent malaria control will lead to the rise of health, lives, and economics problems of the countries it affected. Chemoprevention strategies for prophylaxis and preventive treatment of malaria can be an effectivetoolsforthe control andeliminationof malaria[21].WorldHealthOrganization(WHO) recent recommendation for malaria incidence is the use of seasonal malaria chemoprevention (SMC) for children under 5 years old, in addition to key interventions for malaria control[26]
A study done by Konate et al, 2020 have shown that SMC has greatly benefitted children under 5 years old by significantly reducing malaria incidence, gametocyte prevalence, and improving their haemoglobin levels after 2 years of routine implementation. This study have reported the reduction of fever prevalence, gametocyte prevalence, and malaria indicator, all during the implementation of SMC[26]
Another study done by Konate et al, 2022, have also found that SMC is also likely feasible for reducing uncomplicated malaria and anemia in older children. The incidence of parasitemia reported in this case was greatly reduced in the intervention group. It has been found that the cumulative incidence of parasitemia in October (which is the height of malaria transmission season) was 60.0% in the control group, and 28.0% in the intervention group. By
the end of this study, they found that the cumulative incidence of parasitemia in the control group was 75.4%, and 40.7% in the intervention group. These suggest that 34.7% of new parasitemia cases were averted[27]. The studies that has been done previously are all in line with our result that SMC could reduce the incidence of malaria.
Advantages and Challenges of Chemoprevention
Malaria chemoprevention is a newly invented tool for the prevention of malaria in areas withseasonal transmission.Previousresearchhasshownthatpopulationswhoreceivedmalaria chemoprophylaxis over an extended period of time had lower levels of malaria antibodies than their counterparts[28]
In Burkina Faso and Mali (Africa), malaria chemoprevention has proven to be effective as an intervention to malaria cases with a high protective efficacy in children. Nevertheless, the season change and logistical reasons might had affect this study. Seasons with low transmission rate produce the most sustainable benefit due to lower risk of infection. This resulted in a reduction of infectious individuals reservoir at the starting period of hightransmission seasons. However, seasons does not affect the malaria prevalence in Malawi which remains high all year rounds. For that reason, it is unclear if there is indeed any optimal time to treat malaria prevalences[28] .
In Australia and USA, the use of tafenoquine as a malaria chemoprophylaxis seems to be safe and effective in preventing malaria cases[19]. Therefore, the high price of tafenoquine among all the chemoprophylaxis drugs has become one of the challenges of mass consumption[29]. Above all the challenges, the use of specific chemoprophylaxis in several countries can be replaced by other types of drugs that are accessible for everyone based on the need.
Limitations
This systematic review was compiled by a relatively limited number of randomized controlled trials in each section of intervention. These will cause insufficient data to measure and to compare. Therefore, the results obtained need to be analyzed comprehensively by including other published articles, considering this to prevent the possibility of patients being incomparable.
Conclusion and Recommendation
Observing the upward trend of malaria infection in the world these decades and its potential to be prevented, chemoprevention serves as a promising solution. As a conclusion, we found that across 11 randomized controlled trials with total of 79,178 participants, the use of medication to prevent malaria showed positive effects in some studies, by reducing the lower number of participants infected with the disease compared with placebo treatments. It can be promisingly applied to countries by considering their resources and choosing which drugs are suitable. The results of this systematic review is expected to be a consideration for further studies and the development of malaria chemoprevention.
References
[1] J. Talapko, I. Škrlec, T. Alebić, M. Jukić, and A. Včev, “Malaria: The past and the present,” Microorganisms, vol. 7, no. 6, 2019, doi: 10.3390/microorganisms7060179.
[2] R. Wang, W. Jing, M. Liu, and J. Liu, “Trends of the Global, Regional, and National Incidence of Measles, Vaccine Coverage, and Risk Factors in 204 Countries From 1990 to 2019,” Front. Med., vol. 8, no. March, pp. 1 12, 2021, doi: 10.3389/fmed.2021.798031.
[3] World malaria report 2019, World malaria report 2019. 2019.
[4] T. A. Tizifa, A. N. Kabaghe, R. S. McCann, H. van den Berg, M. Van Vugt, and K. S. Phiri, “Prevention Efforts for Malaria,” Curr. Trop. Med. Reports, vol. 5, no. 1, pp. 41 50, 2018, doi: 10.1007/s40475-018-0133-y.
[5] A. Blessed K and O. Victor B, “The Challenges of Using Insecticides Treated Nets (ITNs) in Curbing Malaria in Nigeria: A 2000-2018 Systematic Review,” J. Infect. Dis. Epidemiol., vol. 6, no. 4, pp. 1 8, 2020, doi: 10.23937/2474-3658/1510140.
[6] World Health Organization, “WHO Guidelines for malaria - June 2022,” Who, pp. 1 396, 2022.
[7] J. D. Maier et al., “Efficacy and safety of tafenoquine for malaria chemoprophylaxis (1998 2020): A systematic review and meta-analysis,” Travel Med. Infect. Dis., vol. 39, no. September 2020, p. 101908, 2021, doi: 10.1016/j.tmaid.2020.101908.
[8] B. Damle, M. Vourvahis, E. Wang, J. Leaney, and B. Corrigan, “Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19,” Clin. Pharmacol. Ther., vol. 108, no. 2, pp. 201 211, 2020, doi: 10.1002/cpt.1857.
[9] Z. C. Bei, G. F. Li, J. H. Zhao, M. Zhang, X. G. Ji, and J. Y. Wang, “Evaluation of the combination of azithromycin and naphthoquine in animal malaria models,” Antimicrob. Agents Chemother., vol. 64, no. 11, pp. 1 14, 2020, doi: 10.1128/AAC.02307-19.
[10] H. Yang et al., “Efficacy and Safety of a Naphthoquine-Azithromycin Coformulation for Malaria Prophylaxis in Southeast Asia: A Phase 3, Double-blind, Randomized, Placebo-controlled Trial,” Clin. Infect. Dis., vol. 73, no. 7, pp. e2470 e2476, 2021, doi: 10.1093/cid/ciaa1018.
[11] N. Patson et al., “Systematic review of statistical methods for safety data in malaria chemoprevention in pregnancy trials,” Malar. J., vol. 19, no. 1, pp. 1 11, 2020, doi: 10.1186/s12936-020-03190-z.
[12] J. D. Hart et al., “Effects of Biannual Azithromycin Mass Drug Administration on
Malaria in Malawian Children: A Cluster Randomized Trial,” Am. J. Trop. Med. Hyg., vol. 103, no. 3, pp. 1329 1334, 2020, doi: 10.4269/ajtmh.19-0619.
[13] B. Coulibaly et al., “Effect of a single dose of oral azithromycin on malaria parasitaemia in children: a randomized controlled trial,” Malar. J., vol. 20, no. 1, pp. 1 8, 2021, doi: 10.1186/s12936-021-03895-9.
[14] A. M. Arzika et al., “Biannual mass azithromycin distributions and malaria parasitemia in pre-school children in Niger: A cluster-randomized, placebo-controlled trial,” PLoS Med., vol. 16, no. 6, pp. 1 15, 2019, doi: 10.1371/journal.pmed.1002835.
[15] A. M. Arzika et al., “Effect of biannual azithromycin distribution on antibody responses to malaria, bacterial, and protozoan pathogens in Niger,” Nat. Commun., vol. 13, no. 1, p. 976, 2022, doi: 10.1038/s41467-022-28565-5.
[16] E. M. Bloch, Z. Mrango, J. Weaver, B. Munoz, T. M. Lietman, and S. K. West, “Causes of death after biannual azithromycin treatment: A community-level randomized clinical trial,” PLoS One, vol. 16, no. 9 September, pp. 1 12, 2021, doi: 10.1371/journal.pone.0250197.
[17] J. D. Keenan et al., “Cause-specific mortality of children younger than 5 years in communities receiving biannual mass azithromycin treatment in Niger: verbal autopsy results from a cluster-randomised controlled trial,” Lancet Glob. Heal., vol. 8, no. 2, pp. e288 e295, 2020, doi: 10.1016/S2214-109X(19)30540-6.
[18] J. W. Henglin Yang, Y. C. Hui Liu, Xingliang Li, Renhua Nie, Chunfu Li, Hengye Wang, Qinghu Wang, and L. Cuic, “crossm Assess Safety and Prophylactic Efficacy of Naphthoquine- Azithromycin Combination for Malaria Prophylaxis in,” vol. 62, no. 9, pp. 1 9, 2018.
[19] A. Novitt-Moreno et al., “Long-term safety of the tafenoquine antimalarial chemoprophylaxis regimen: A 12-month, randomized, double-blind, placebo-controlled trial,” Travel Med. Infect. Dis., vol. 45, no. October 2021, p. 102211, 2022, doi: 10.1016/j.tmaid.2021.102211.
[20] M. R. James S McCarthy, Bryan Smith, C. D. , Jonathan Berman, Louise Marquart, and and G. D. Leanne West4 Lisa T Read, “Blood schizonticidal activity and safety of tafenoquine when administered as chemoprophylaxis to healthy, non-immune participants followed by blood stage Plasmodium falciparum challenge: A randomized, double- blinded, placebo-controlled Phase 1b study.,” vol. 1, no. 240, 2018.
[21] C. V. Plowe, “Malaria chemoprevention and drug resistance: a review of the literature and policy implications,” Malar. J., vol. 21, no. 1, 2022, doi: 10.1186/s12936-022-
04115-8.
[22] P. J. Rosenthal, “Editorial azithromycin for malaria?,” Am. J. Trop. Med. Hyg., vol. 95, no. 1, pp. 2 4, 2016, doi: 10.4269/ajtmh.16-0332.
[23] A. Firth and P. Prathapan, “Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19 . The COVID-19 resource centre is hosted on Elsevier Connect , the company ’ s public news and information ,” no. January, 2020.
[24] J.Brogdonet al., “Malariapositivityfollowinga singleoral doseof azithromycinamong children in Burkina Faso: a randomized controlled trial,” BMC Infect. Dis., vol. 22, no. 1, pp. 1 9, 2022, doi: 10.1186/s12879-022-07296-4.
[25] K. Y. Lu and E. R. Derbyshire, “Tafenoquine: A Step toward Malaria Elimination,” Physiol. Behav., vol. 176, no. 5, pp. 139 148, 2017, doi: 10.1021/acs.biochem.9b01105.Tafenoquine.
[26] D. Konaté et al., “Effect of routine seasonal malaria chemoprevention on malaria trends in children under 5 years in Dangassa, Mali,” Malar. J., vol. 19, no. 1, pp. 1 6, 2020, doi: 10.1186/s12936-020-03202-y.
[27] D. Konaté et al., “Effectiveness and Community Acceptance of Extending Seasonal Malaria Chemoprevention to Children 5 to 14 Years of Age in Dangassa, Mali,” Am. J. Trop. Med. Hyg., vol. 106, no. 2, pp. 648 654, 2022, doi: 10.4269/ajtmh.21-0046.
[28] G. R. Gore-Langton et al., “Effect of adding azithromycin to the antimalarials used for seasonal malaria chemoprevention on the nutritional status of African children,” Trop. Med. Int. Heal., vol. 25, no. 6, pp. 740 750, 2020, doi: 10.1111/tmi.13390.
[29] N. I. Agudelo Higuita, B. P. White, C. Franco-Paredes, and M. A. McGhee, “An update on prevention of malaria in travelers,” Ther. Adv. Infect. Dis., vol. 8, pp. 1 17, 2021, doi: 10.1177/20499361211040690.
THE EFFECT OF SCORPION VENOM ON THE HUMAN BODY: LITERATURE REVIEW
ABSTRACT
Introduction: term "envenoming" refers to the direct inoculation of venom by a bite or sting. The burden of animal bites and stings is greater in developing nations in Sub-Saharan Africa, South Asia, and Southeast Asia than in developed nations. Indian red scorpion (Mesobuthus tamulus) venom is a severe, sometimes fatal medical issue in tropical and subtropical regions, and it is commonly seen in rural India. Scorpion venom is a potent sodium channel activator that causes severe central and autonomic nervous system indications that, in children especially, might lead in multi-organ failureand death. 30%offatalitiesarethought to berelatedto acutepulmonaryedema. It was found that scorpionsting victims whomreceived bothprazosinand scorpion antivenom had the same increased and decreased incidence and that those who received solely prazosin, and the inclusion of antivenom increased recovery.
Materials and Method: Data was collected from PubMed, Google scholar, Online book and Research gate. With a traditional review design model, this research uses a literature review method. By offering some reliable literature, this study is qualitative research.
Results and Discussion: The parasympathetic nervous system is stimulated briefly and persistently by scorpion venom. Sympathetic signs indicate after the effects of the poison on the sympathetic system, unresponsive to antivenom but reversible with prazosin. Thus, early administration of antivenom in the acetylcholine and prazosin overload stage to counteract the sympathetic and metabolic effects may be synergistic in enhancing recovery. Improvement of parasympathetic stimulation (sweating, salivation, arrhythmias) by antivenom may have contributed to the lower incidence of hypotension in the antivenom plus prazosin group.
Conclusion: Tominimizeanimal bitesandstingstohumans, further isneeded. It'salso meaningful for the general public to be aware of this knowledge so they can be mindful of it when going to explore the wild.
Keyword: envenoming, scorpion, prazosin, inflammation, and handling.
THE EFFECT OF SCORPION VENOM ON THE HUMAN BODY: LITERATURE REVIEW
Author : Nabilah Sillah Nur Rizkal Nina Nur Fazri
Novalia Putri Zilfa Febriyana Putri
Faculty of Medicine University Of Uniba 2022

Introduction
Scorpions are venomous arthropods, members of the class Arachnida and the order of scorpions. This animal is found on all continents except Antarctica, and is known to cause problems in the tropics and subtropics. In particular, scorpions from the Buthidae family were reported to be more venomous than scorpions from other families, although geographic variation may also account for the diversity of scorpion venom (qualitative and quantitative differences) that may affect the severity of scorpion stings. The Indian red scorpion (Mesobuthus Tamulus), with its life-threatening sting, is the most dangerous scorpion species in the world.
Scorpion stings cause varied and complex clinical manifestations, which range from local effects to intense autonomic nervous system responses and systemic inflammatory reactions, similar to those associated withsystemic inflammatoryresponse syndrome and acute sepsis These manifestations often progress to severe cardiac and pulmonary changes that can lead to fatal outcomes, especially in children and the elderly.
Mesobuthus Tamulus, the Indian red scorpion, is the deadliest species of the Buthidae family in India. The venom of the scorpion can delay the closure of neuronal sodium channels, thereby causing "autonomic storms" due to the sudden release of endogenous catecholamines intothecirculation. Autonomic stormsarecharacterized byprolongedsympathetic stimulation. Similar totheparasympathetictransient cardiovascular manifestationsreportedinother species of scorpions. Morbidity and mortality due to scorpion stings include refractory acute pulmonaryedema, cardiogenic shock, and multi-organ failure. There is disagreement about the proper treatment for scorpion stings. It was found that patients withscorpion stings treated with scorpion antivenom and prazosin had the same increased and decreased incidence as patients treated with prazosin alone, and the addition of antivenom accelerated recovery. Despite much scientific experience of severe scorpion envenomation in endemic areas worldwide, standard treatment protocols using drugs and antivenoms are scant.
Material and method
1. Study Design
With a traditional review design model, this research uses a literature review method. By offering some reliable literature, this study is qualitative research.
2. Inclusion Criteria
The inclusion criteria for this study include individuals of every age group living in close proximity to the wild, from very young children to adults.
3. Exclusion Criteria
Those who had never been exposed to the venom of the a scorpion animal were excluded from this study.
4. Instrument
We searched works of literature published in Pubmed, Online book, Google scholar, and Research gate databases to identify literatures that fit our paper topic. Key words thatareusedtosearchtheliteraturesare“scorpion” and“venom”;“scorpion”,“venom” and “interactions”; “scorpion”, “venom” and “inflammation”; “venom” and “handling”.
5. Data analysis In
Initial search results form PubMed, Google Scholar, Online book and Research gate (n=26)
Full-text assessed for eligbility (n=2)
Research not found (n=20)
Full text eligible cross-sectional articles includes in review (n=4)
Results and Discussion: Results
We examined 4 different sources from the many academic journals we studied, each of which included information on how to treat a scorpionsting, the manyspecies ofscorpions and the effects of their venom on the body, etc.
The parasympathetic nervous system is stimulated briefly and persistently by scorpion venom. Clinical symptoms and indicators of excessive acetylcholine point to the existence of
toxins in the blood that can be stopped byantivenom and are moving freely and actively in the body.
Discussion
Based on the information we researched, we discovered that ice should be compacted in regions where scorpions, centipedes, spiders, fire ants, bed bugs, and ticks have stung or bit people. Antitoxins, corticosteroids, and antihistamines can also be administered. If the sting is really painful, medications can be taken orally.
In addition, we discovered that sustained transient parasympathetic stimulation is elicited by scorpion venom. Clinical symptoms and indicators of excessive acetylcholine point to the active and unrestricted circulation of poisons in the blood that can be stopped by antivenom. Ice should be applied to areas that have been stung by scorpions, bit by centipedes, spiders, fire ants, bed bugs, or ticks. Additionally, antihistamines and corticosteroids can be used.
Sympathetic signs indicate after the effects of the poison on the sympathetic system, unresponsive to antivenom but reversible with prazosin. In addition, morbidity and mortality due to envenoming is caused by sympathetic overstimulation and not by parasympathetic stimulation. Thus, earlyadministrationofantivenom intheacetylcholineand prazosinoverload stage to counteract the sympathetic and metabolic effects may be synergistic in enhancing recovery.
On arrival, the absence of sweating and salivation that was present before reporting to the hospital indicated that the toxin had reached the target site and the action was inaccessible with antivenom administration, as in our patient who deteriorated to grade 4. The total dose of prazosin required was significantly higher was lower inthe antivenomplus prazosingroupthan inthe prazosinalone group, indicating that timely administrationofscorpion antivenomresults inrapid neutralizationofcirculating venom, so that theamount ofvenomavailable for neuronal sodium channel activation is low. Improvement of parasympathetic stimulation (sweating, salivation, arrhythmias) by antivenom may have contributed to the lower incidence of hypotension in the antivenom plus prazosin group. Leukocytosis and increased cardiac enzymes promote the release of cytokines such as interleukins.
Evaluation of the clinical grade of the scorpion sting on arrival at the hospital
Grade 1 Severe, excruciating local pain at the sting site radiating along with the appropriate dermatome, mild local edema with sweating at the sting site, without systemic involvement
Grade 2 autonomic storm signs and symptoms are characterized by excess acetylcholine or parasympathetic stimulation (vomiting, profuse sweating of the whole body, ropey saliva, bradycardia, ventricular contractions, hypotension, priapism in men) and sympathetic stimulation (hypertension with blood pressure > 140/90, tachycardia with heart rate > 140/90). 120 per minute, cold extremities, transient systolic murmur).
Grade 3 cold extremities, tachycardia, hypotension or hypertension with pulmonary edema (respiratory rate > 24 per minute, basal lesions or cracks in the lungs).
Grade 4 tachycardia, hypotension with or without edema
Conclusion and Recommendation
As previously stated, evenoming is brought on bythe ability to pierce the bodydirectly with bites or stings. Snake bites and athropods, of which scorpions are one type, have the highest incidence of venomous bites and stings. More than 1.2 million scorpion deaths happen each year, accounting for 3,250 fatal accidents. The venom of scorpions has a variety of toxic complexesand is highlylethal, particularlytochildren. Anger, hypertemia, vomiting, excessive salivation, tremors, and seizures are possible symptoms of scorpion poisoning. The type of scorpion, the poison's chemical makeup, and the victim's physiological response to the poison can all be used to identify additional scorpion poisoning symptoms. The sting of the scorpion should be treated using the following procedure on the area affected.
Recommendation: To minimize animal bites and stings to humans, further is needed. It's also meaningful for the general public to be aware of this knowledge so they can be mindful of it when going to explore the wild.
References:
Buku Ajar Travel Medicine. 2019. Udayana University Press
Reis, Mouzarllem Barros, Karina Furlani Zoccalb , Luiz Gustavo Gardinassic , Lúcia Helena Faccioli. 2019. Scorpion envenomation and inflammation: Beyond neurotoxic effects Petricevich, Vera L. 2019. Scorpion Venom and the InflammatoryResponse.
Trasia, Reqgi First. 2021. PENGOBATAN TERKINI ALERGI, REAKSI TOKSIK, DAN PENYAKIT AKIBAT ARTROPODA. Bagian Parasitologi, Fakultas Kedokteran, Universitas Sultan Ageng Tirtayasa, Kota Serang, Banten, Indonesia.
Bessalem, Sonia Adi, Djelila Hammoudi-Triki, and Fatima Laraba-Djebari. Scorpion Venom Interactions with the Immune System.
Efikasi dan keamanan antivenom kalajengking plus prazosin dibandingkan dengan prazosin untuk sengatan kalajengking berbisa (Mesobuthus tamulus): uji klinis acak label terbuka. Online Journal.
Effects ofAdding Carica papaya Extract in DengueFever Management Compared to just Crystalloid Fluid on Thrombocytopenia:ALiterature Review
Pandhu Pradipta Nararya Pangemanan1, Darmawan SatriaJati1,Gabriella Sachiko Jannesha Sudirman1,Putri Utami Lestari1 1Faculty of Medicine, Padjadjaran University, Indonesia
ABSTRACT
Introduction: Tropicaldiseaseshavebeenaglobalburdenandchallengetoeradicate,oneof themisDengueFever.DengueFeveristransmittedthroughthebiteofaninfectedfemale mosquito, Aedesaegypti or Aedesalbopictus. Themainmanagementiscrystalloidfluid throughoralorIV.Butitisnotstatedthatcrystalloidfluidcanimprovethrombocytopenia. Apparently, Caricapapaya extract can.
Objective: Theliteraturereviewaimstoinvestigateandcomparetheeffectsofadding Caricapapaya extractforthrombocytopeniamanagementwithjustcrystalloidfluidtreatment on Dengue Fever patients.
Method: ThereviewmethodusedinthisliteraturereviewisusingthesearchenginePubMed andGoogleScholar.Weusedthekeywords“dengueANDpapaya”forthemainarticle.The criteriathatwechosearearticlespublishedamaximumoffiveyearsago(2017).Wefound 35articlesfromPubMedand3.660articlesfromGoogleScholar.Wewereabletonarrowit down to 5 main articles used in the results and discussion section.
Results: Twostudiesregardingtheeffectofcrystalloidfluidonthrombocytopeniashowed thatcrystalloidfluiddoesnothaveaneffectonimprovingthrombocytopenia,butworsen thrombocytopenia.Fivestudiesregardingtheeffectof Caricapapaya extractshowedthat Carica papaya leaves can improve platelet count onthrombocytopenia.
Conclusion: CPLEshowspotentialtherapeuticeffectsondenguefeverpatientsthathave thrombocytopenia,includingantithrombocytopeniceffect,immunomodulatoryactivityand antiviralactivity.ThedownsideofCPLEpreparationsisminimalevidence-basedstudyand potentialadverseeffectsoflongtermtreatmentwithCPLEincludinghepatotoxicityand increasedriskofcardiovasculardiseases.CPLEisapotentialcandidateforshortterm treatment of dengue fever.
Keywords: Tropicaldisease,denguefever,thrombocytopenia,crystalloidfluid,carica papaya leaves extract (CPLE)
Effects ofAdding Carica papaya Extract in DengueFever Management Compared to just Crystalloid Fluid on Thrombocytopenia:ALiterature Review
Authors:
Pandhu Pradipta Nararya Pangemanan Darmawan Satria Jati
Gabriella Sachiko Jannesha Sudirman
Putri Utami Lestari Faculty of Medicine Padjadjaran University 2022

INTRODUCTION
Tropical Disease
Tropicaldiseasesarediseasesor infectionsthatareprevalentintropicaland subtropicalregions,suchasIndonesia.Since longago,tropicaldiseaseshavebeena globalburdenandchallengetoeradicate. Therearefiveclassificationsoftropical diseases:foodborne,waterborne,airborne, parasite,anddiseasestransmittedbyinsects, suchasDengueFever[1].DengueFeveris oneofthehealthproblemsandthreatsin mostregionsofIndonesia.Basedonthe MinistryofHealthdata,thereare approximately45.387cumulativecasesin 2022,with432casesleadingtodeath.This numberincreasesmoreduringtherainy season, around September to January[2] . Dengue Fever
DengueFeverisaviralinfection transmittedtohumansthroughthebiteofan infectedfemalemosquito, Aedesaegypti (moreoften)or Aedesalbopictus.Thevirus thatcausedthisdiseaseiscalledDengue Virus(DENV)andhasfourserotypes, rangingfromDENV-1toDENV-4.Infection byanyserotypewillresultinlifelong immunityforthatserotypebutnotother serotypes.Soanindividualcanbeinfected upto4timesbyeachserotype[3].DENVisa partofthe Flavivirus genus,a
single-strandedRNAvirus.Around75%of humansthatareinfectedbyDENVare asymptomatic.However,approximately 0.5%to5%willexperienceseveredengue fever,suchasprogressingtohaemorrhage andshock.Thiscouldleadtodeathinupto 20%ofthecase,whichoftenhappensin children[4] .
Somedenguecasescandevelop flu-likesymptomsthatusuallylast2-7days. AccordingtotheWorldHealthOrganization (WHO),thishappensafteranincubation periodof4-10daysaftertheinfected mosquito’sbite.WHOclassifiesdengueinto 2categories,dengueandseveredengue.Itis classifiedasdenguewhenapatientpresents withhighfever(40°C)andisaccompanied bytwoofthefollowing:severeheadache, muscleandjointpain,rash,painbehindthe eyes,nausea,swollenglands,andvomiting. Itisclassifiedasseveredenguewhena patiententersthecriticalphase(around3-7 daysaftersymptomsappear).Inthefirst 24-48hours,patientspresentwithworsening symptoms,butimprovementinfever (<38°C)[5].Thisisusuallyaccompaniedby warningsignsofseveredengue,suchas abdominalpain,liverenlargement, persistentvomiting,fatigue,risein hematocrit, and thrombocytopenia[4] .
Basedontheguidelinemadebythe MinistryofHealthin2017,themain managementforDengueFeveris rehydrationusingCrystalloidFluidthrough oralorIVwiththeammountof6-7ml/kg/h forDengueFeverwithoutshockand10-20 ml/kg/hforDengueFeverwithshock (DengueShockSyndrome/DSS).Butitis notstatedthatCrystalloidFluidcanimprove thrombocytopenia[6].Apparently,thereisa naturalresourcethatisplentifulinIndonesia whichcanbeusedtoimprove thrombocytopenia,called Caricapapaya extract.Therefore,thisstudyaimedto comparetheeffectofadding Caricapapaya extract as the treatment of Dengue Fever. Carica papaya
Papayaisafruitthatisvery nutritiousbuthasanaffordableprice.Not onlythat,papayaisafruitthatisvery valuableformedicine.Allpartsofpapaya suchasleaves,seeds,fruit,roots,bark,and sapcanbeusedasmedicine.Papayaalso containsasourceofphytochemicalswhich includeantioxidants,vitamins,polyphenols, andflavonoids.Inaddition,papayaalso containsseveralimportantmineralsand enzymessuchaslycopene,papain,and isothyocynate,aswellasseveralproteolytic enzymesthataregoodfortreatinghealth problems[7] .
MATERIALSAND METHODS
Thereviewmethodusedinthis literaturereviewisusingthesearchengine PubMedandGoogleScholar.Weusedthe keywordsdengueANDpapayaforthemain article.Thecriteriathatwechosearearticles publishedamaximumoffiveyearsago (2017).Wefound35articlesfromPubMed and3.660articlesfromGoogleScholar. Afterward,thearticlesareevaluatedtofind articlesthatcorrelatewiththekeywordsand thetopictosupporttheanalysisinthis literaturereview.Wewereabletonarrowit downto5mainarticlesusedintheresults and discussion section..
RESULTSAND DISCUSSION
a.Effects of Crystalloid Fluid on Thrombocytopenia
KryzchandCzempik(2018)dida studyregardingtheeffectofCrystalloid Fluidonplatelets.Thestudyused Plasmalyte(PL)15mL/kgandshowedthat plateletcountsdroppedby21.6%fromthe baseline(BL)(Figure1).Besidesthat,the studyalsoshowedthattherewasadecrease inplateletcomponentofclotstrengthto 83.2% from the baseline (Figure 2)[8]
Meanwhile,anotherstudydoneby Boyd,Brainard,andSmart(2021)showed thatCrystalloidFluid(IV)when administeredinclinicallyrelevantdoses,can
causedose-dependenthypocoagulability, whichmeansCrystalloidFluidcause decrease platelet count[9] .
Frombothofthesestudies,itcanbe concludedthatcrystalloidfluiddoesnot haveaneffectonimproving thrombocytopenia,butworsen thrombocytopenia. b.Effects of Carica papaya extract on Thrombocytopenia Roleof Caricapapaya LeafProductin ImprovingthePlateletCountinPatients with
Dengue Fever
Theuseof Caricapapaya Leaf Extract(CPLE)hasbeenshownto significantlyincreasethenumberof platelets.Thisisrelatedtothecaseof denguefeverinwhichthecaseofdecreased plateletsisamajorconcerninthisdisease. EvenCPLEitselfisanadjuncttherapyfor thrombocytopenia[10].However,further studiesareneededtodeterminetheroleof CPLE in severe cases of dengue fever. CurrentUpdatesontheuseof Carica papaya LeafExtractasaPotentialHerbal Medicine
Theroleof Caricapapaya leavesto controldenguefeverhasbeenproventobe effective.Extractsfrompapayaleavesare proventoincreasethenumberofplateletsin casesofdenguefeverandprevent
complicationsinthrombocytopenia.Thisof coursecanreducetheperiodfrom hospitalizationtothecostofthetreatment itself.Furthermore,papayaleafextractcan beusedasafirstaidtreatmentorallyfor denguefeverpatients.However,theuseor oralconsumptionofpapayaleafextractin thelongtermcancausehepatotoxicitytothe risk of other cardiovascular diseases[11] .
InanotherpilotstudybyDipu etal. (2020),51adultpatientswhotestedpositive onadenguepoint-of-carediagnostictest with severethrombocytopenia(≤30,000/たL) fromatertiarycarehospitalinIndiawere dividedintotwogroups,onegroupwas givenoneCPLE(Caripill)as thrombocytopeniatreatment,whiletheother groupwasgivenplacebo.Thisstudy indicatesthattreatmentwithCPLEinthe managementofseverethrombocytopenia (≤30,000/たL)wasproventobemore effectivecomparedtotheplacebogroup. SafetyandefficacyofCPLEwereindicated by:significantlyincreasedplateletcounts, fewerpatientsinneedofplatelet transfusions,decreasedmediantimeof plateletrecovery,decreasedmeanpercent increaseinTNFgandIFNけlevels,IL-6 meanpercentincrease,fasterclearance kineticsofviralNS1,andwelltolerated characteristic of the CPLE[12] .
Sharma etal. (2019)didan experimentonmaleSpragueDawley(SD) ratsthatwereinfectedwithDENV-2.This experimentshowedthatduring3-7daysof infection,plateletcountstartedtodecrease, then Caricapapaya leafextractwas administeredorally(200mg/kg)totherats. Theresultsshowedthatplateletcountdidn’t decreaseonday4andday7of administration.Plateletcountstartedto increaseonday11andreachnormallevel on day 14[13]
Sarker,Khan,andMohamed(2021) alsodidanexperimentontheeffectof Caricapapaya leafonthrombocytopenia. Theresultsshowedthat Caricapapaya leaveswereabletoimproveplateletcountin denguepatientsandinvivioanimalmodels (rats).Itissuspectedthat Caricapapaya leavesinhibitdestructiveeffectsofDENV onplateletsandincreasetheexpressionof ALOS12genethatisresponsiblefor improving platelet count[14] .
CONCLUSION
CrystalloidfluidandCPLEareboth administeredinthetreatmentofdengue fever.Crystalloidfluidisthemain managementofdenguefeverinIndonesia, whichisameansofrehydrationforpatients withdenguefeverandDSS.However, crystalloidfluiddoesnottreat
thrombocytopeniaandcanevenworsen thrombocytopeniaindenguefeverpatients. CPLEontheotherhand,showspotential therapeuticeffectsondenguefeverpatients thathavethrombocytopenia,includinganti thrombocytopeniceffect, immunomodulatoryactivityandantiviral activity.ThedetrimentofCPLE administration,isthefactthatCPLEisan alternativemedicationthatlacksevaluated evidence,especiallyinformsotherthan caripill.Anotherpotentialadverseeffectof longtermtreatmentwithCPLEincluding hepatotoxicityandincreasedriskof cardiovasculardiseases.Asidefromits adverseeffectinlongtermadministration, CPLEisapotentialcandidateforshortterm treatment of dengue fever.
RECOMMENDATION
Limitedevidence-baseddataisone oftheproblemswithtraditionalmedicine, includingCPLE.Furtherlargerprospective studiesareessentialfordeterminingthe safetyofCPLEusage.Duetotherelevance andcost-effectiveness,weencourageother authors,especiallythosefromdengue endemicregionstostudytheeffectiveness and safeness of CPLE preparations.
REFERENCES
1.Infeksi Tropis [Internet]. INFEKSI TROPIS – Infectious Disease Specialists. [cited 2022Oct22]. Available from: https://www.idspecialists.sg/layanankami/jenis-infeksi/infeksi-tropis/?lan g=ms
2.Rokom. Kasus DBD meningkat, kemenkes Galakkan Gerakan 1 Rumah 1 Jumantik (G1R1J) [Internet]. Sehat Negeriku. 2022 [cited 2022Oct22].Available from: https://sehatnegeriku.kemkes.go.id/b aca/umum/20220615/0240172/kasus -dbd-meningkat-kemenkes-galakkangerakan-1-rumah-1-jumantik-g1r1j/# :~:text=Peningkatan%20kasus%20D BD%20terus%20terjadi,akibat%20D BD%20mencapai%20432%20kasus
3.Darvin Scott Smith MD. Dengue [Internet]. Practice Essentials, Background, Pathophysiology. Medscape; 2021 [cited 2022Oct22]. Available from: https://emedicine.medscape.com/arti cle/215840-overview#a3
4.Dengue fever - statpearls - NCBI bookshelf [Internet]. [cited 2022Oct22].Available from:
https://www.ncbi.nlm.nih.gov/books/ NBK430732/
5.Dengue and severe dengue [Internet]. World Health Organization. World Health Organization; [cited 2022Oct22]. Available from: https://www.who.int/news-room/fact -sheets/detail/dengue-and-severe-den gue
6.Dinas Kesehatan – Kabupaten Pulang Pisau [Internet]. [cited 2022Oct22].Available from: https://www.dinkes.pulangpisaukab. go.id/wp-content/uploads/2020/09/Is i-Buku-DBD-2017.pdf
7.Sarker MM, Khan F, Mohamed IN. Dengue fever: Therapeutic potential of Carica papaya L. leaves. Frontiers in Pharmacology. 2021Apr26;12.
8.Krzych ŁJ, Czempik PF. Effect of fluid resuscitation with balanced solutions on platelets: In vitro simulation of 20% volume substitution. Cardiology Journal. 2017May12;
9.Boyd CJ, Brainard BM, Smart L. Intravenous fluid administration and the coagulation system [Internet]. Frontiers. Frontiers; 1AD [cited 2022Oct22].Available from:
https://www.frontiersin.org/articles/1 0.3389/fvets.2021.662504/full
10.BAV, Doddamane K, Kumar VD. Role of carica Papaya Leaf Product in Improving the Platelet Count in Patients with Dengue Fever [Internet]. Int J Res Med.; 2017 [cited 2022Oct22].Available from: https://ijorim.com/siteadmin/article_i ssue/150156359014%20Kotresh%20 1.pdf.pdf
11.Malabadi RB, Chalannavar RK, R NB, Mulgund G, Meti NT. Www.researchgate.net [Internet]. ResearchGate. International Journal of Research and Scientific Innovation; 2017 [cited 2022Oct22]. Available from: https://www.researchgate.net/publica tion/364577691_First_Report_of_the _Detection_of_DENV_1_Virus_in_ Human_Blood_Plasma_with_Near-I nfrared_Spectroscopy
12.Sathyapalan DT, PadmanabhanA, Moni M, P-Prabhu B, Prasanna P, Balachandran S, et al. Efficacy & safety of Carica papaya leaf extract (CPLE) in severe thrombocytopenia (≤30,000/‒L) inAdult Dengue –Results of a pilot study. PLOS ONE. 2020Feb19;15(2).

13.Sharma N, Mishra KP, Chanda S, Bhardwaj V, Tanwar H, Ganju L, et al. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation.Archives of Virology. 2019Feb21;164(4):1095–110.
14.Sarker MM, Khan F, Mohamed IN. Dengue fever: Therapeutic potential of Carica papaya L. leaves. Frontiers in Pharmacology. 2021Apr26;12.
TABLE & FIGURES
Figure1. platelet count (PLT)
Figure2. EffectofPlasmalyte(PL)on platelet-dependent clot strength
Abstract
In low- and middle-income countries, accidents resulting from burn injuries in wilderness activities often cause high mortality and morbidity due to lack of therapeutic options and resources. Current research demonstrates the potential of Aquacel Ag in providing a costeffectiveand rapidhealingprocess forburninjurypatients. Thisliteraturereviewincludesvalid experimental studies drawn from the PubMed, ScienceDirect, EBSCO, Cochrane, ProQuest and Taylor & Francis Journal databases, which met the inclusion criteria. The search method used a boolean operator with literature quoted from 2012 2022. Seven studies with a total 451 of burn injury patients were included in this review. Seven studies with 460 burn patients treated with Aquacel Ag were included in this review. When compared to other regimens, Aquacel Ag has shown to reduce healing time, improve cost-effectiveness, be easier to apply, and increase convenience for the patient with no serious side effects. The effectiveness and safety of Aquacel Ag in burn injury management are all significant, but it is recommended that further research be conducted more in Asian populations, especially Indonesia.
Keywords: Aquacel, Hydrofiber, Dressing, Burn, Burn Injury
EFFECTIVENESS AND SAFETY OF AQUACEL AG IN BURN INJURY MANAGEMENT: A SYSTEMATIC REVIEW AND META-ANALYSIS OF RANDOMIZED CONTROLLED TRIAL
(SUBTHEME : Thermal and Cold Injuries)
Submitted to Enter the Competition Pre-Conference Competition East Asian Medical Students’ Conference (PCC EAMSC 2023: NEPAL)
Team Member :
1. Imke Maria Del Rosario Puling (Brawijaya University)
2. Filzatuz Zahro Ibrahim (Brawijaya University)
3. Ni Made Alvionita Frencia Augustine (Brawijaya University)
4. Sebastian Emmanuel Wilyanto (Brawijaya University)
Effectiveness and Safety of Aquacel Ag in Burn Injury Management: a Systematic Review and Meta-Analysis of Randomized Controlled Trial
Imke Maria Del Rosario Puling1, Filzatuz Zahro Ibrahim1, Ni Made Alvionita Frencia Augustine2 ,Sebastian Emmanuel Wilyanto1
1Second Year Medical Student, Faculty of Medicine, Brawijaya University
2Third Year Medical Student, Faculty of Medicine, Brawijaya University imkepuling@gmail.com
Abstract
In low- and middle-income countries, accidents resulting from burn injuries in wilderness activities often cause high mortality and morbidity due to lack of therapeutic options and resources. Current research demonstrates the potential of Aquacel Ag in providing a costeffectiveandrapidhealingprocess forburninjurypatients. Thisliteraturereviewincludesvalid experimental studies drawn from the PubMed, ScienceDirect, EBSCO, Cochrane, ProQuest and Taylor & Francis Journal databases, which met the inclusion criteria. The search method used a boolean operator with literature quoted from 2012 2022. Seven studies with a total 451 of burn injury patients were included in this review. Seven studies with 460 burn patients treated with Aquacel Ag were included in this review. When compared to other regimens, Aquacel Ag has shown to reduce healing time, improve cost-effectiveness, be easier to apply, and increase convenience for the patient with no serious side effects. The effectiveness and safety of Aquacel Ag in burn injury management are all significant, but it is recommended that further research be conducted more in Asian populations, especially Indonesia.
Keywords: Aquacel, Hydrofiber, Dressing, Burn, Burn Injury
Introduction
Burninjuryisaform ofinjurythatiscaused by heat, cold, electricity, chemicals, radiation, or friction. Burn injuries vary significantly in terms of the tissues affected, severity, and the complications it causes. Patients with burn injury may experience a variety of potentially deadly complications, such as shock, infection, electrolyte imbalances, and respiratory failure, depending on the location affected and the depth of the burn. Aside from physical consequences, burn injuries can cause severe psychological and emotional despair, as well as depression in the quality
of life, due to long-term hospitalization, scarring, and deformity12
Burn injuries, mainly caused by direct contact with hot liquids or hot objects (e.g stoves, coals, lanterns), contributes to 2-8% of wilderness trauma. Higher mortality and morbidity presents in low and middle income countries due to lack of therapeutic options and resources3. Being a leading cause of trauma globally with approximately 265,000 deaths per year, burn injuries still present a high mortality rate even in places with well-equipped burn centres4. Moreover, burn injuries tend to have a relatively long period of treatment
and high frequency of hospital readmissions, hence also adds to the economic burden and patient’s wellbeing5 .
Burn injury dressings mainly aim to promote healing by creating a moist wound environment and providing infection barriers to the wound, whilst reducing pain and possible anxiety experienced by the patient6. Current therapy regimens suggest silver sulfadiazine as the gold standard for burn injuries. However, silver sulfadiazine therapy has several disadvantages. Prolonged use of silver sulfadiazine can potentially cause hypertrophic/atrophic scars, delay in wound healing time, leukopenia in large surface burn injuries, and allergic reactions (including hypersensitivity). Moreover, the application and removal of silver sulfadiazine is painful and induces difficulties in assessing the burn wound6 9 Another standardized treatment is chlorhexidine-impregnated tulle gras dressing, however it is reported to be less effective in terms of adhesive capability, hence frequent painful dressing changes are required during the treatment6. Ideal healing environment may ensure rapid reepithelization and reduce potential of scarring, hence may cut the healing time effectively. Therefore, innovation in burn injury treatments is needed to create a more
effective, safe, and comfortable healing process for burn injury patients.
Aquacel Ag Hydrofiber dressing (Convatec Inc.) is made up of sodium carboxymethylcellulose combined with Hydrofiber has a high absorption capacity and silver ions at a concentration of 1.2%. The Hydrofiber dressing hydrates and afterward expands in response to contact withwoundexudateto createacohesivegel that traps and holds bacteria while conforming to the wound surface. Once hydrated, silver ions are available to provide a bactericidal effect within the dressing matrix and at the wound-dressing interface that protects the wound against infection8,9. According to multiple studies, Aquacel Ag has a number of advantages over other burn treatments, including excellent bacterial control, rapidunimpaired wound healing, costeffectiveness, ease of use, and patient comfort6 9. Thus, it has a high potential in providing a more effective and comfortable healing process for burn injury patients, especially in areas with high burn injury cases low and middle income countries. Based on this explanation, the authors wrote a meta-analysis that aims to determine the pain score and healing time of Aquacel Ag as an innovative effort to treat pain and manage burn injuries.
Method
The meta-analysis was made based on the PRISMA statement guidelines derived from PubMed, Google Scholar, ScienceDirect, EBSCO, Cochrane, and ProQuest databases. The literature search was carried out by the four authors with
keywords using boolean operators, namely: [(Aquacel) OR (Hydrofiber)) AND ((burn) OR (injury) OR (burn injury)]. The literature search flow is structured like the flow diagram in Figure 1.
Figure 1. Flow diagram of literature search Inclusion and exclusion criteria were determined before the literature search so that the results obtained were specific and similar. The inclusion criteria were 1) clinical trial studies with RCT (Randomized-Controlled Trial) design, 2) studies published in the last 10 years, 3) studies written in English, 4) study population consisting of patients diagnosed
with burn injury, 5) peer-reviewed journals, and 6) studies with Aquacel Ag Hydrofiber dressing intervention. The exclusion criteria were 1) access to paid articles, 2) journals not accessible online, and 3) research other than clinical trials in humans. Inclusion criteria of this systematic review refers to the PICO framework in table 1.

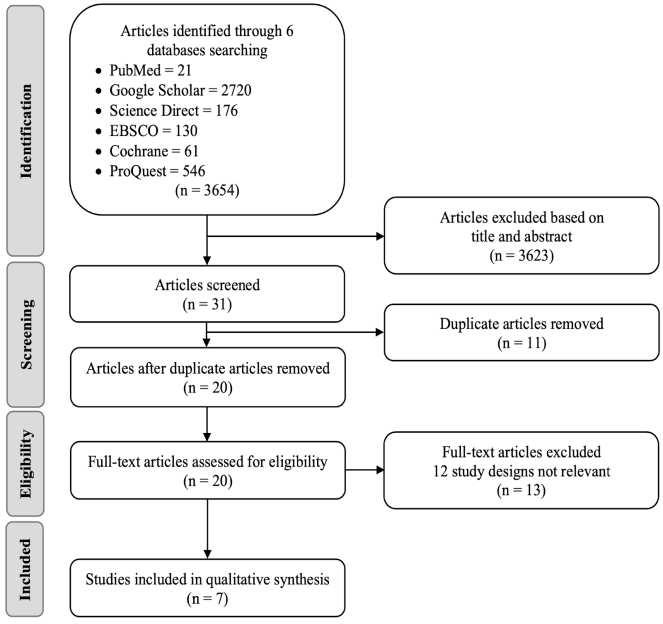
Table 1. PICO Framework
The literature quality assessment was carried out based on the Cochrane RoB Tool 2 guidelines used to assess
Randomized Controlled Trial studies and summarized in figure 210 .
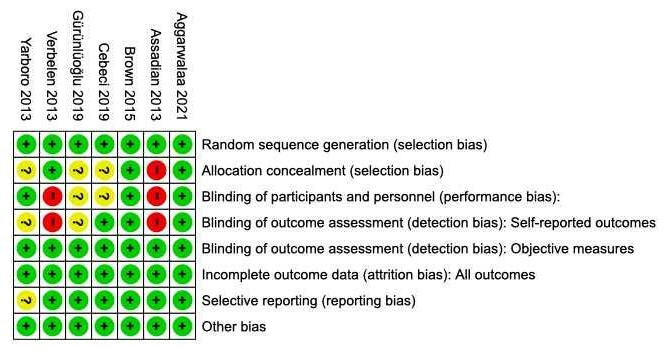
Figure 2. Literature Quality
Meta Analysis
Assessent
Based on Cochrane ROB Tool 2 in Cochrane Review Manager (RevMan 5.4.1)
The meta analysis used in this review is continuous meta-analysis with standard mean different measurement performed by RevMan Cochrane11. Heterogeneity of this meta-analysis was measured with chisquare statistic and heterogeneity rate estimate with I2 statistic, which show the ratio of the percentages of variability in effect estimates due to heterogeneity rather than chance. Meta-analyses were performed for continuous data, inverse variance, fixed effects model and 95% confidence interval (95% CI). General p value <0.0001.
versus Other Interventions and Healing TIme (in Days) Aquacel vs Other Interventions as shown in figure 3 and figure 4. Transforming methacrylate dressing (TMD), ActicoatTM, Silvercontaining hydrofiber dressings (Bactigras), and silver sulfadiazine are included in the other interventions category.
There are 2 groups in general analysis, which are VAS and LASA Score Aquacel
The heterogeneity within groups are relatively low and high heterogeneity is due to various interventions as shown in figure 3. However, it is all supporting the data which claimed the effectiveness of Aquacel Ag, by assessing its pain score and healing time. Therefore, the data were assessed for low heterogeneity within the groups, as
well as good for demonstrating statistical efficacy and reliability.
Figure 3. Forest Plot VAS and LASA Score Aquacel versus Other Interventions


Figure
4. Forest Plot Healing TIme (in Days) Aquacel vs Other Interventions
Effectiveness of Aquacel Ag in Reducing Healing Time
Effectiveness of wound care treatment can be assessed based on its ability to reduce healing time, hence promoting faster healing process. Overall, most studies showed similar results in which concluded that Aquacel Ag reduces healing time significantly.
release silver into the wound for up to 14 days9,12
Burn wounds are at a risk of developing infections8. The sodium carboxymethyl cellulose fibers in the aquacel dressing absorb water and turn into gel when they come into contact with exudate that contain bacteria, resulting in a moist wound environment. It forming a choesive gel that reduces dead spaces as well. Additionally, it prevents infection by continuing to
A Study by Cebecci (2019) conducted in Turkey compared the effectiveness of Aquacel tochlorhexidine-impregnatedtulle gras dressing to patients with seconddegree burns by assessing the patients on days 7, 15, and 22, continued until full epithelialization occurred9. The study concluded that Aquacel significantly limited the healing process compared to the control arm (U = 315.0, P < .05)9. This is based on the data that all burn wounds healed by day 22 in the intervention arm, while the control arm hadn't healed. The intervention arm also presents significantly faster full epithelialization compared to the control arm (10±3 days vs. 13.7±4 days)9
This matter is supported by a previous study by Muangman and Duteille which stated that Aquacel Ag significantly reduced the duration of wound closure compared to Silversulfadiazine9,13
Studies by Yarboro (2013) and Duteille (2012) stated a similar conclusion that Aquacel is more effective than silver sulfadiazine7,13. The study focuses on the dressing changes requirements. In silver sulfadiazine dressings, it is required to change the dressing (10.27 ± 7.46) to achieve 100% re-epithelialization, whilst Aquacel dressings require less changing (4.10 ± 1.38). Hence, it not only shortens the healing time, but also limits the frequency of pain felt by patients when changing dressings7,13
Cost-Effectiveness of Aquacel Ag
Other than that healing time reduction, effectiveness in terms of cost can also be considered as a parameter.
It was shown that Aquacel Ag was more cost-effective than Acticoat™ when considering the frequency of dressing applications and interventions, total dressing surface area (cm2), and total duration spent per patient8. Another study by Cebeci et al. (2019) comparing Aquacel Ag with chlorhexidine-impregnated tulle gras dressings also demonstrated another
economic advantage of Aquacel Ag7 . Although Aquacel Ag is more expensive, patients who used Aquacel Ag had shorter hospitalization, and the daily workload for changing the dressings was dramatically decreasedbecausethe Hydrofiberdressings attached to the wound surface more effectively, necessitating fewer reapplications and therefore reducing expenditures7. In contrast, a study by Aggarwalaa et al. (2021) showed that Mepilex Ag outperformed Aquacel Ag by 53% in terms of cost-effectiveness while also achieving faster re-epithelialization14 However, it is noted in the study that the majority of assessments on costeffectiveness suffered from several shortcomings including inconsistent study methods, merged adult and pediatric populations, and imprecise measurement and reporting of parameters.
Comfortability of Aquacel Ag Usage
Because the nerve endings are still intact, second-degree burns are the most painful. Accordingto several research, thenecessity for frequent dressing changes and the discomfort associated with such changes represent the greatest challenge in caring for patients with second-degree burns. Study conducted by Cebecci show analgesia use and number of dressing changes (only once) were lower with Aquacel dressing9
Pain in burn injury associated with dressing removal, application, and while dressing is in place. Thus, reducing dressing changes was closely related to a decrease in pain scores. Aquacel requires fewer dressing changes which was mentioned before. A study conducted in the UK by Duteille and colleagues stated that patients gave a score of 91% out of 100% or excellent for the convenience of using Aquacel. In addition, the pain score at baseline was 3.43 out of 10, at the end of the study it dropped to 1.15 out of 1013
to acticoat, aquacel is also superior because it requires fewer dressing changes so it is associated with lower pain in patients6 . A study by Cebecci also mentioned that the aquacel arm needed less debridement and analgesic, also lower pain scores, faster epithelialization, wound healing, and burn area reduction more quickly9 .
Other Advantages Proposed by Aquacel Ag
Ease of Use
Another study conducted by Yarboro (2013) also showed a significant comparison between aquacel arm and silver sufadiazine arm in terms of pain7. This study states that the pain score on silver sulfadiazine is 4.70 ± 2.22, while that of aquacel is 2.92 ± 1.12. This study also showed that patients felt more comfortable during insertion and dressing removal on the aquacel arm than silver sulfadizine (7.14 ± 1.50 vs 6.98 ± 1.76, respectively, out of 10)7. Studies conducted in the Netherlands by Verbelen and in Taiwan by Huang also showed that auacel ag was preferred by patients to silver sulfadiazine because it was more comfortable, less painful, and less expensive8,15. Compared
One study, Verbelen (2013), stated that Aquacel Ag application is significantly easiercomparedto otherregimensanalyzed in the studies. The data was measured and based on a 5-point Likert scale filled by the nurses in charge9. In contrast, a study by Aggarwalaa (2021) stated that other interventions (Mepilex Ag and Acticoat) were more favourable16. However Aquacel Ag application was more favourable compared to Biobrane. Both studies did not specify any analysis or reasoning on the issue. Favourable use in Aquacel Ag may result from the contents of the dressing which can attach smoothly, but strongly to wounded skin for approximately 14 days. Further research is needed to analyze this matter. Studies on pain scores, healing time, cost-effectiveness, and ease of use are summarized in table 2.
Minimal Adverse Effect (Safety)
A study by Aggarwalaa (2021) reported that Aquacell disclosed less scars at three and six months post-burn injury compared to other dressings used in the study14 .

Aquacel Ag also presents limited risk of skin maceration. This correlates with the vertical absorption properties that maintains the moisture only at the wounded area14. Based on further reviews, risks associated with all burn wound care are pain, bleeding, and infection. However, all studies did not mention any significant adverse effects directly caused by application of Aquacel Ag in burn injury patients. Hence, it can be concluded that Aquacel Ag can be a safe choice in wound dressing, especially in burn injury.
Conclusion
The effectiveness and safety of Aquacel Ag in burn injury management are all significant. This is based on the analysis and deep studies we’ve done which found the re-epithelization ability provided by the usage of Aquacel Ag dressing. Not only that,thehealingtimeneededisalsoreduced based on full epithelization days for injury
healing. Its effectiveness can also be seen from the cost effectiveness aspect due to reduced healing time so the hospitalisation time is also reduced and the cost needed is cheaper. This is coupled with no need to reapplication the dressing and easy techniques. On the aspect of convertibility, the usage of Aquacel Ag is less painful when it’s placed on and unplaced from the burn injury site so coherently, less dressing changeis needed.Thesafety ofAquacel Ag is also validated due to the minimal effects provided from its usage although some adverse effects occur due to patient’s comorbidities. A limitation of this literature review is that the included studies still included a small sample of Asian populations. Therefore, it is recommended that further research be conducted on the efficacy and safety of the Aquacel Ag in Asian populations, especially in Indonesia.
Conflict of Interest
The authors declare that there were no relevant affiliations, authorships, publications, or financial involvement with any party that could be considered as a potential conflict of interest with the materials reviewed.
References
1. GÜRÜNLÜOĞLU K, DEMIRCAN M, TAŞÇI A, ÜREMİŞ MM. The effects of two different burn dressings on serum oxidative stress indicators in children with partial burn. Am Burn Assoc. 2019;
2. Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Prim [Internet]. 2020;6(1). Available from: http://dx.doi.org/10.1038/s41572020-0145-5
3. Bitter CC, Erickson TB. Management of Burn Injuries in the Wilderness: Lessons from LowResource Settings. Wilderness Environ Med [Internet]. 2016;27(4):519 25. Available from: http://dx.doi.org/10.1016/j.wem.201 6.09.001
4. Tan Chor Lip H, Tan JH, Thomas M, Imran FH, Azmah Tuan Mat TN. Survival analysis and mortality predictors of hospitalized severe burn victims in a Malaysian burns intensive care unit. Burn Trauma. 2019;7:1 7.
5. Latifi NA, Karimi H, Motevalian SA, Momeni M. Economical burden of burn injuries in a developing country. J Burn Care Res. 2017;38(6):E900 5.
6. Brown M, Dalziel SR, Herd E, Johnson K, Wong She R, Shepherd M. A randomized controlled study of silver-based burns dressing in a pediatric emergency department. J Burn Care Res. 2016;37(4):e340 7.
7. Yarboro DD. A comparative study of the dressings silver sulfadiazine and aquacel Ag in the management of superficial partial-thickness burns. Adv Ski Wound Care. 2013;26(6):259 62.
8. Verbelen J, Hoeksema H, Heyneman A, Pirayesh A, Monstrey S. Aquacel® Ag dressing versus ActicoatTM dressing in partial thickness burns: A prospective, randomized, controlled study in 100 patients. Part 1: Burn wound healing. Burns [Internet]. 2014;40(3):416 27. Available from: http://dx.doi.org/10.1016/j.burns.20 13.07.008
9. Cebeci SP, Acaroglu R. Use of silver-containing hydrofiber and chlorhexidine-impregnated tulle gras dressings for second-degree burns. Adv Ski Wound Care. 2019;32(7):1 5.
10. The Cochrane Collaboration. Review Manager, Version 5.4.1. Copenhagen, Denmark: The Nordic Cochrane Centre; 2020.
11. J H, J T, J C, M C, L T, M P, et al.
Cochrane Handbook for Systematic Reviews of Interventions Version. 6.2. London, UK: Cochrane; 2021.
12. Huang SH, Lin CH, Chang KP, Wu SH, Lin SD, Lai CS, et al. Clinical evaluation comparing the efficacy of Aquacel Ag with Vaseline gauze versus 1% silver sulfadiazine cream in toxic epidermal necrolysis. Adv Ski Wound Care. 2014;27(5):210 5.
13. Duteille F, Jeffery SLA. A phase II prospective, non-comparative assessment of a new silver sodium carboxymethylcellulose (AQUACEL® Ag BURN) glove in the management of partial thickness hand burns. Burns [Internet]. 2012;38(7):1041 50. Available from: http://dx.doi.org/10.1016/j.burns.20 12.05.001
14. Shivani Aggarwala, Varun Harish, Sarah Roberts, Megan Brady, Sepher Lajevardi, James Doherty, Mario D’Souza, Peter Haertsch, Peter K M Maitz ACIF. Treatment of partial thickness burns: a prospective, randomised controlled trial comparing BiobraneTM , ActicoatTM, Mepilex® Ag and Aquacel® Ag. J Burn Care Res. 2020;1 10.
15. Swindells S, Andrade-Villanueva
JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, et al. Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV1 Suppression. N Engl J Med. 2020;382(12):1112 23.
16. Aggarwal AK, Shashikanth VS. Platelet-rich plasma prevents blood loss and pain and enhances early functional outcome after total knee arthroplasty鳥: a prospective randomised controlled study. 2014;387 95.
Extraction Data
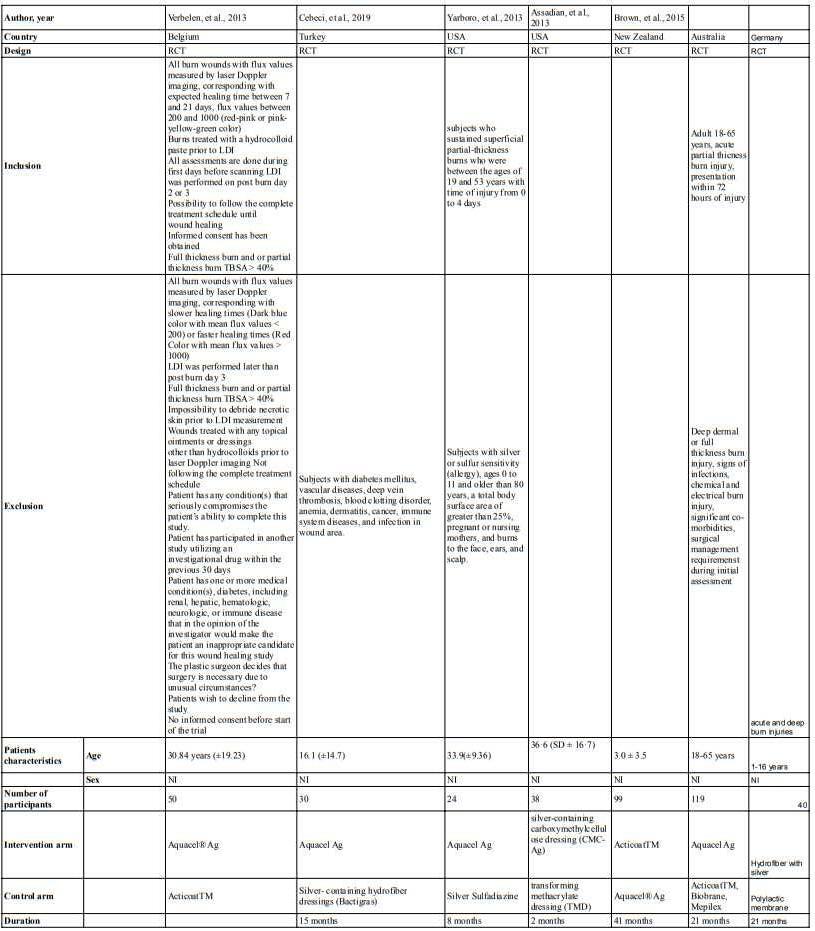
Attachment

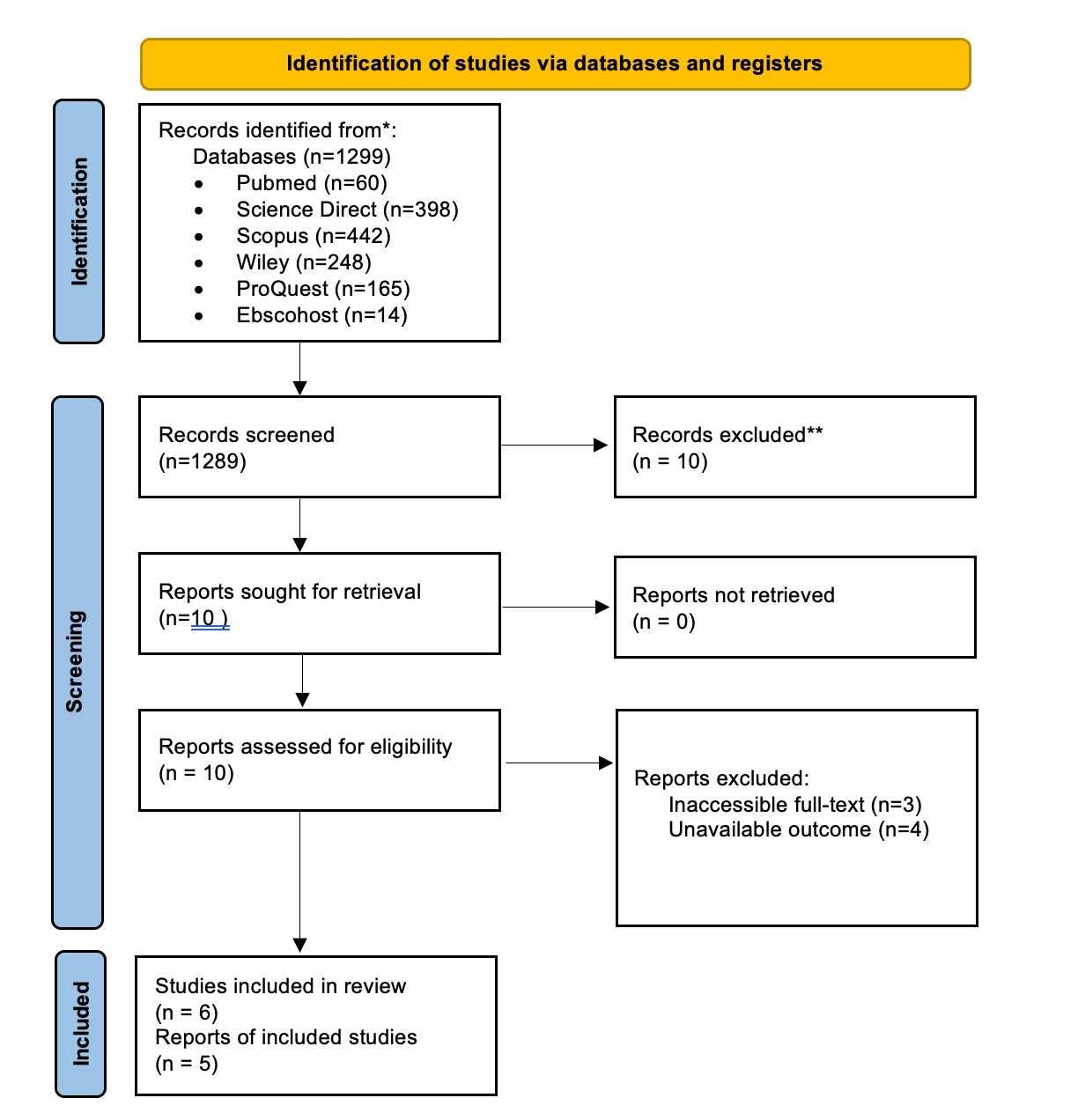

A NOVEL EXPRESSION C-TERMINAL NS1 MAKES DENGUE VIRUSES A PIVOTAL TARGET FOR PROTECTIVE IMMUNITY: A SYSTEMATIC REVIEW AND META-ANALYSIS
Natasha Cita P. K, Derren David C.H. Rampengan, Nabilah Puteri L, Felicia Angelica Gunawan
AMSA-Universitas Tarumanagara, AMSA-Universitas Sam Ratulangi, AMSA Universitas Hassanudin
Introduction: Currently, the major public health challenge is Dengue Virus (DENV) as DENV is one of the causal agents which rapidly spread out in tropical and subtropical regions and become the leading emerging issue in the world with high incidence and severity. The prevalence is estimated to be 3.9 billion people worldwide. Southeast Asia is one of the regions with the highest burden of infection For the dengue virus elicits immune responses against a variety of viral antigens, a recently introduced early diagnostic accuracy marker for detecting dengue virus (DENV) is currently recommended only for seropositive individuals through Nonstructural Protein 1 (NS1).
Objectives: The study aims to evaluate NS1 as an early-stage biomarker for the diagnostic accuracy of dengue and determining the severity of dengue.
Material and Methods: This meta-analysis was conducted using PRISMA framework. The literature search was conducted in MEDLINE, Cochrane, Science Direct, and selecting Randomized controlled trials systematically, with critical appraisal assessed using Cochrane RoB 2. We utilize inverse variance to analyze pooled odds ratio (OR) with associated 95% confidence intervals (CI) and its p-value using sensitivity and subgroup analysis.
Results and Discussion: Fifteen articles were included in the study. In analyzing the efficacy of different parameters of NSI as a screening biomarker in predicting the occurrence of dengue viruses, a total of 8154 patients were reported. The current results showed that the NS1 was very beneficial (sensitivity values were estimated to
be 97% (95% CI 0.93 0.98) and the specificity value was estimated to be 100% (95% CI 98-100)).
Conclusion: This study presents NS1 as an intervention that showed significant results using the ELISA antibodies and RT-PCR screening method with high heterogeneity in the prediction accuracy of dengue severity using the IgM and IgG antibodies method.
Keywords: NS1, Nonstructural Protein 1, Dengue Virus, DENV
A NOVEL EXPRESSION C-TERMINAL NS1 MAKES DENGUE VIRUSES A PIVOTAL TARGET FOR
PROTECTIVE IMMUNITY: A SYSTEMATIC REVIEW AND METAANALYSIS

Scientific Paper
Natasha Cita Paradhita Kusuma
Derren David Christian Homenta Rampengan
Nabilah Puteri Larassaphira
Felicia Angelica Gunawan 2022
EXPRESSION C-TERMINAL NS1 MAKES DENGUE VIRUSES A PIVOTAL TARGET FOR PROTECTIVE IMMUNITY: A SYSTEMATIC REVIEW AND META-ANALYSIS
Natasha Cita Paradhita Kusuma, Derren David Christian Homenta Rampengan, Nabilah Puteri Larassaphira, Felicia Angelica Gunawan
AMSA-Universitas Tarumanagara, AMSA-Universitas Sam Ratulangi, AMSA-Universitas Hassanudin
Introduction
Currently, the dynamics incidence has risen dramatically around the world in recent decades. As populations grow and major public health challenges are prevalent, dengue represents a major threat to half the world's population. More than 80% of infections are mild and asymptomatic despite 100-400 million occurring every year. The prevalence of dengue is estimated to be 3.9 billion people worldwide. Southeast Asia, Americas, and Western Pacific are the region with the highest burden of infection, despite being infected in 129 countries. 1, 2, 14
As an important neglected tropical disease caused by arbovirus is Dengue Virus (DENV), one of the causal agent which rapidly spread out in tropical and sub-tropical regions.7,26 Based on geographic appearance, there are 390 million dengue virus infections each year (95% credible interval 284 528 million), ofwhich 96 million (67 136 million) manifest clinically. Based on that, showed the dengue virus has created an important emerging issue because of its rapid spread, high incidence, and severity.6, 17
DENV is a vector-dependent virus infection caused by four different serotypes of dengue virus (DENV-1, DENV-2, DENV-3, and DENV-4), which is the utmost challenge for vaccine production. 24, 27 . DENV serotype identification is essential for two primary reasons: (i) the presence of more pathogenic DENV serotypes has been associated with severe dengue; and (ii) the presence of multiple DENV serotypes in primary and secondary infections is believed to increase the risk of severe infection. 9 Immunity against a particular serotype develops after primary exposure to it. Secondary infections usually lead to more severe forms ofthe disease. It is possible to suffer from a wide variety of clinical manifestations caused by dengue, starting with an unsightly infection to a potentially fatal disease. 11
Nonstructuralprotein1 (NS1) can be used as anearly diagnostic biomarker.15 Dengue virus elicits immune responses against a variety of viral antigens, including antibodies against dengue non-structural protein 1 (NS1), which are rapidly induced and detected.25 It is present in blood during both primary and secondary infections. Using formulations based on NS1 induces strong immune responses and gives potential immunity against dengue infection. The commonly used NS1 (Non-Structural protein 1) viral antigen testing (either by rapid testing or ELISA) is most sensitive (97%) with specificity varying 100%.
In facing assess the performance of NS1-based detection in the evaluation of dengue. However, to our knowledge, until now there is no systematic review and meta-analysis that covers the detect dengue virus through NS1. Considering that, we conducted this study to demonstrate adequate sensitivity and specificity of NS1-based tests in dengue diagnosis.
OBJECTIVE
The aim of this systematic review and meta-analysis is to screen NS1 as a marker for predicting dengue severity and to determine whether it can be for indicative protection of dengue infection.
MATERIALS AND METHODS
Search Strategies and Data Source
The methodology were in stages, the first stage of the study is the protocol development, followed by the inclusion and exclusion criteria. Then, we performed literature research for studies from databases. The studies were screened by two authors, the screening process covered the abstract, title, full-text screening, data extraction follows, and lastly, a synthesis of the previous findings. This systematic review was conducted using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist to assess NS1 for dengue virus. We searched relevant publications using electronic databases, including MEDLINE, the Cochrane Library, and ScienceDirect. The keywordsused according to the databases are mentioned in Table 1 Thescope of the literature search was based on the inclusion and exclusion criteria. We started by forming two or more search strings to form keywords. The keywords were searched within the title, abstract, and link.
Inclusion criteria
The selection process of the included articles follows the following criteria in order to determine the overall validity of the Meta-analysis; studies were only eligible for the research if they are related to NS1 for dengue virus. Articles published between 2017 and 2022 were included. We include studies written in English language, randomized control trials, clinical studies, prospective and retrospective studies.
Exclusion criteria
Due to the quality of the current research, we exclude studies not writing in English language, we excluded systematics literature review, meta-analysis, and case studies. We excluded studies not relevant to the present research, study with poor methodology and results were excluded.
Study selection and Screening process
After applying the keywords into the above mentioned database, 4,566 publications were generated in total from all database. At the first stage, 3,800 articles were duplicate, review and meta-analysis were excluded. At stage 2, two authors went through the abstracts and title of the studies in stage 1 in order to select studies that were related to our studies, and again, 610 articles were excluded due to lack of information about NSI screening method. After stage 2 and 3, the number of remaining articles were 156, in the next stage, the full text screening were performed on the remaining 156 papers in order to evaluate the methodology and data analysis carried out on each paper which must be in accordance with the scope of the current study, during this process, 115 studies were excluded which left us with 41 studies. Two authors independently cross check the abstract and the title and 26 studies were excluded, leaving us with 15 studies. Any disagreements between authors were resolved by discussion.
Quality assessment
After the screening and selection process mentioned above, the quality of the 15 included studies was assess in order to be sure of the quality of the selected studies. The Risk of bias assessment tools was followed with a particular focus on six domains; the random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting of selected studies and other bias. Three main key
was used to access the studies; high risk with red symbol, low risk with green symbol and unclear risk with white symbol.
Data extraction
A pre-defined sheet was used to gather the following information from the included studies: the First Author’s Name, the geographical location of the author, the total number of patients, the diagnostics method employed, the number of patients confirmed positive, and number of patients confirmed negative, true positive, true negative, false positive and false negative, the clinical outcome. Laboratory outcome and demography data.
Data Analysis Techniques
A meta-analysis of random effect models was used to analyze the different screening methods reported in the studies. The chosen effect size calculated was the odds ratio (OR) with associated 95% confidence intervals (CI). A 5% CI was used and the possibility of publication bias was assessed using a funnel plot of the included studies and effect size against the standard error. The heterogeneity among the studies was measured using the I2 statistic. To analyze the accuracy of the screening methods, the studyemployed the studyfirst to estimate the sensitivity and specificity using the random effect model forest plot in STATA 14.0. Four metrics were used to estimate the accuracy; the positive predictive Value (PPV), negative predictive value (NPV), HSROC, and Confidence Interval.
RESULT AND DISCUSSION
Study Selection
A total of 129 articles were retrieved from the database search. After removing duplicates, the remaining 31 articles were assessed for eligibility as stated in the Methods. During the process of reading through the abstracts and titles, 10 articles were excluded, mainly due to poor results and insufficient data needed for the analysis. Full-text was examined in the remaining 21 articles, and of these, 6 were excluded for the following reasons: 4 articles were animal studies and were not relevant to the present research, and 2 article was in a language other than English. The remaining 15 articles were considered for qualitative evaluation.
Table 1. Database Searching Process Keywords
Database Keywords Results
MEDLINE
(((((NS1) OR (Non-Structural Protein 1)) OR (Viral Nonstructural Proteins)) OR (Dengue Antigen)) AND (Dengue Virus)) OR (DENV)
3,354
The Cochrane Library
(((((NS1) OR (Non-Structural Protein 1)) OR (Viral Nonstructural Proteins)) OR (Dengue Antigen)) AND (Dengue Virus)) OR (DENV)
142
ScienceDirect
(((((NS1) OR (Non-Structural Protein 1)) OR (Viral Nonstructural Proteins)) OR (Dengue Antigen)) AND (Dengue Virus)) OR (DENV)
Database search (Medline = (3354) Cochrane library (n = 142)
Records after duplicates removed (n = 766)
Records Screened (n = 156)
Data assessed for eligibility (n = 41)



Studies included in quantitative (metaanalysis/Systematics review) (n = 15)
1,070 Fig 1. Prisma Flowchart
Records excluded= 610 Insufficient
Non relevant = 100
No results = 15
Screenin g Identification Eligibility
Included
Study Characteristics
Fifteen articles were included in the quantitative analysis out of which 1 article was published in 2017, two articles in 2018 and five articles in 2019. Three articles were published in 2020, two articles in 2021 and 2022 respectively. In analyzing the efficacy of difference parameters of NSI as a screening biomarker in predicting the occurrence of dengue viruses a total of 8154 patients were reported with an average age of 21.5. Four screening biomarkers were utilized; IgM, IgG, ELISA and RT-PCR in which IgM; IgG were used in Nine Studies (Cuellar et al, 2020, Jang et al. 2019, Haroon et al. 2018, Sigera et al, 2019, Chia Lai et al. 2022, Rao et al. 2019, Tran et al. 2019, Pan et al. 2021 and Nagar et al. 2020), ELISA was used in Four articles; Lai et al 2019, Wongsawat et al. 2021, Alidjinou et al. 2022 and Gao et al et al. 2018. RT-PCR in two articles; (Santoso et al 2020 and Ahamed et al. 2017),
Table 2. Characteristics of the Included Studies
First Author Year Country Parameter Confirmed Test Age Total Number of Patients
Cuellar et al. 2020 Paraguay IgM; IgG 2 <15 709
Lai et al 2019 Taiwan ELISA 1 NR 146
Jang et al. 2019 Korea NS1; IgM; IgG 3 7.5(1 - 14) 172
Haroon et al. 2018 Pakistan NS1; IgM; IgG 3 1 - 60+ 2000
Gao et al 2018 China ELISA 1 NR 108
Sigera et al. 2019 Sri Lanka IgM; IgG 2 27.5(2040) 122
Chia Lai et al. 2022 Spain IgM; IgG 2 NR 187
Rao et al. 2019 India IgM; IgG 2 24(16 - 34) 1372
Alidjinou et al. 2022 France ELISA 1 26.9(0 - 94) 472
Tran et al. 2019 Vietnam IgG; IgM 2 NR 120
Pan et al. 2021 China IgG; IgM 2 NR 1498
Wongsawat et al. 2021 Thailand ELISA 1 24 (15 - 72) 778
Nagar et al. 2020 India IgM; IgG 2 NR 150
Ahamed et al. 2017 India RT-PCR 1 4.3±6.8 171
Santoso et al. 2020 Indonesia RT-PCR 1 1-25+ 149
Quality Assessment of the Included Studies
Ofthe 15 included studies, 13 were at low risk of bias under the random sequence generation, and 2 were at high risk of bias. For allocation concealment, 13 studies were of low risk, 1 of high risk. And 1 of unclear risk. For blinding of participants and personnel, 14 were of low risk, 1 were of unclear risk, and zero study reported high risk. For blinding of outcome assessment, 13 studies were of low risk and 2 were of unclear risk. For incomplete outcome data, 14 were of low risk, 1 were of high risk, and 0 were of unclear risk. For selective reporting bias, 14 studies were of low risk, 1 were of unclear risk. For other bias, 15 studies were of low risk. The overall percentage of low, unclear and high risk were presented in Figure 2
Figure 2. The overall percentage of the ROB

Figure 3. The Summary Assessment of the ROB.
Meta-Analysis of Prediction Accuracy of dengue severity using the IgM and IgG Antibodies Method.
Nine Studies (Cuellar et al. 2020, Jang et al. 2019, Haroon et al. 2018, Sigera et al. 2019, Chia Lai et al. 2022, Rao et al. 2019, Tran et al. 2019, Pan et al. 2021 and Nagar et al. 2020) reported the screening Results of the IgM and IgG Antibodies method in prediction of dengue severity and efficacy of dengue infection. Table 3. The least sensitivity value was found to be 87% (95% CI 78.6 - 92.5) (Chia Lai et al. 2022) and the highest was found to be 100% (95% CI 0.93 1.00) also the least Specificity value was estimated to be 67% (95% CI 84.7 - 99.5) while the highest value was estimated to be 100% (95% CI 0.99-1.00). The overall meta-analysis results shows that the summarysensitivityvalueswasestimated to be97% (95% CI 0.93 0.98) and thespecificity value was estimated to be 100% (95% CI 98-100) Figure 4. The average PPV was estimated to be 96% while the average NPV was estimated to be 88%. The heterogeneity among the nine studies was analyses Figure 4. For the sensitivity analysis, high heterogeneity was found (I2 = 89.52%, 95% CI = 84.14 95) and for the specificity Analysis, high heterogenetic was detected (I2 = 89.96% 95% CI = 84.79 96).
Table 3. The Screening Test results using IgM and IgG Antibodies as a Biomarker detection of Dengue Virus for individual studies.
Author First Screening Time TP FP FN TN Sensitivity (%) 95% CI
Cuellar et al.
2020
Jang et al. 2019
Haroon et al.
2018
Specificity (%) 95% CI
PPV % NPV%
30 Min 258 1 0 450 1.00(0.93 1.00) 1.00(0.991.00) 99.61 100
25 Min 102 1 7 63 0.94(0.870.97) 0.98(0.921.00) 99.02 90
15 Min 100 2 8 890 0.93(0.860.97) 1.00(0.991.00) 98.03 99.10
Sigera et al. 2019 NR 85 1 3 33 96.6(90.199.7) 0.67(84.799.5) 98.83 91.66
Chia Lai et al. 2022
30 Min 91 5 14 77 0.87(78.692.5) 0.94(86.397.9) 94.79 84.61
Rao et al. 2019 15Min 746 2 45 579 0.94(0.920.96) 1.00(0.991.00) 99.73 92.78
Tran et al. 2019 30Min 66 0 4 50 0.94(0.860.98) 1.00(0.931.00) 100 92.59
Pan et al. 2021 30Min 500 0 2 996 1.00(0.991.00) 1.00(1.001.00) 100 99.79
Nagar et al. 2020 NR 142 1 7 45 0.95(0.010.98) 0.98(0.881.00) 99.30 86.53
NR = Not Reported TP = True Positive, FP = False Positive, FN = False Negative and TN = True Negative
Figure 4. The Forest plots Showing the Sensitivity and specificity of The Screening Results of IgM and IgG Antibodies
IgM& IgGMethod
1
.8 0
.4






.6 .2
1 .8 S.6pecificit.4 y .2 0
Study estimate
HSROC curve 95% prediction region

Summary point 95% confidence region
Figure 5. Hierarchical summarized receiver operating characteristic (HSROC) curves of Novel Expression C-Terminal NS1 based on screening tests for dengue viruses using IgM and IgG Method is 0.97
Meta-Analysis of Prediction Accuracy of dengue severity using the ELISA Antibodies Method.
Four Studies (Lai et al. 2019, Alidjinou et al. 2022, Wongsawat et al. 2021 and Gao et al et al. 2018) reported the screening Results using ELISA Antibodies method in prediction of dengue severityand efficacy ofdengue infection. Table 4. The least sensitivity value was found to be 83% (95% CI 72% 91%) (Lai et al. 2019) and highest was found to be 98% (95% CI 94%-100%) (Alidjinou et al. 2022) also the least specificity value was estimated to be 63% (95% CI 51%73%) while the highest value was estimated to be 100% (95% CI 0.98-1.00). Average PPV estimated fromall studieswas found to be 91% and the Average NPV was found to be 79.9%. The
meta-analysis retuned overall sensitivity of 91% with 95% CI of 76% - 97% while the specificity was found to be 98% (95% CI 0.71-1.00) Figure 6.Despite the small number of included studies, the results were statistically significant (Table 6).
Table 4: The Screening Test results using ELISA Antibodies as a Biomarker detection of Dengue Virus for individual studies.
Author First Screening Time TP FP F N TN Sensitivity (%) 95% CI
Specificity (%) 95% CI
PPV % NPV %
Lai et al. 2019 15 Min 55 30 11 50 0.83(0.72 0.91) 0.63(0.510.73) 64.71 81.97 Alidjinou et al. 2022 30 Min 151 1 3 318 0.98(0.941.00) 1.00(0.981.00) 99.34 99.07 Wongsawa t et al. 2021 30 Min 449 1 78 200 0.85(0.820.88) 1.00(0.971.00) 99.78 71.94
Gao et al et al. 2018 15 Min 108 2 35 71 0.76(0.680.82) 0.97(0.901.00) 98.18 66.98
NR = Not Reported TP = True Positive, FP = False Positive, FN = False Negative and TN = True Negative
Figure 6. The Forest plots Showing the Sensitivity and specificity of The Screening Results of ELISA Antibodies
ELISAMethod
1
.8 0
.4






.6 .2
1 .8 S.6pecificit.4 y .2 0
Study estimate
HSROC curve 95% prediction region
Summary point 95% confidence region
Figure 7. Hierarchical summarized receiver operating characteristic (HSROC) curves of Novel Expression C-Terminal NS1 based on screening tests for dengue viruses using ELISA Method is 0.88.
Meta-Analysis of Prediction Accuracy of dengue severity using the RT-PCR screening Method.
Two Studies (Ahamed et al. 2017 and Santoso et al. 2020) were included in the diagnostics test results ofthe RT-PCR Method in the detection ofdengue virus. In summary, the overall sensitivity of found to be 81% and that of specificity was found to be 94.5%. The PPV was estimated to be 87.5% and NPV was found to be 73.3%. We could not analyze the meta-analysis of the sensitivity and specificity due to small number of included studies which have violated the requirement for meta-analysis. Despite the small number of included studies, the results were statistically significant (Table 5).
Table 5. The Screening Test results using RT-PCR Method as a Biomarker detection of Dengue Virus for individual studies.
NR = Not Reported TP = True Positive, FP = False Positive, FN = False Negative and TN = True Negative
Table 6. Summary Results of The Prediction of Dengue Virus by the Screening Methods
NR = Not Reported TP = True Positive, FP = False Positive, FN = False Negative and TN = True Negative
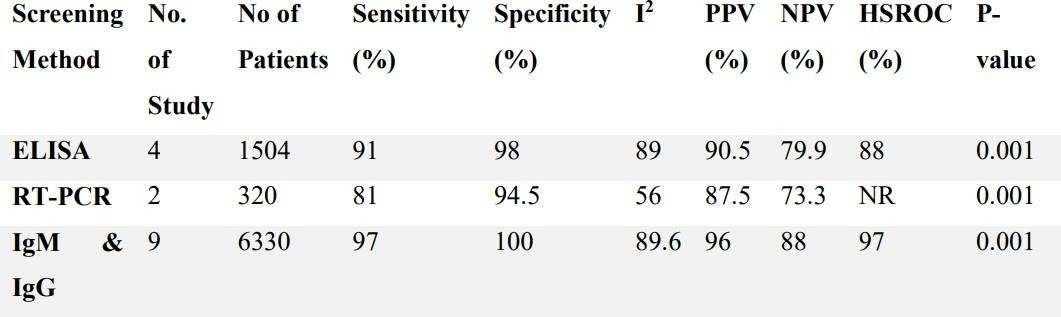

Supplementary Materials (Overall Clinical Outcome) Fever Myalgia Arthralgia Headache NS1+ NS1- NS1+ NS1- NS1+ NS1- NS1+ NS1469(97.9 %) 2228(99.1 %) 207(43.2 %) 107(46.5 %) 160(33.4 %) 104(45.2 %) 261(54.5 %) 153(66.5 %)
Supplementary Materials (Overall Lab Outcome)
Hemoglobin(gr/dl)
Platelets(mm) Hematrocrit(%)
NS1+ NS1- NS1+ NS1- NS1+ NS1138 ± 3.2 144 ± 3.2 847.373 ± 77738.9 879881.4 ± 77875.3 406 ± 6.2 41.8 ± 6.2
13.65 11.35 126 147 39.45 33.8
Limitation
The first limitation is the small number of studies analyzing one of the NSI screening methods. We could not perform the forest plot of the sensitivity and specificity due to the small number of studies. Second. Few studies reported the clinical outcome of the patients, which caused us not to perform any analysis on the clinical outcome, therefore, more studies are needed on NSI - to analyze the clinical outcome. Third. We are limited to studies published between 2017 and 2022, however, relevant studies might be excluded from the research due to the search limit
Conclusion
Observing the urgencyofdengue viruses, this meta-analysis performed that NS1 can be considered as a screening biomarker in predicting the occurrenceof dengue viruses. Although the result shows high heterogeneity in the prediction accuracy of dengue severity using IgM and IgG antibodies method, NS1 as an intervention showed significant results on prediction accuracyusing the ELISA antibodies and RT-PCR screening method.
References
1. World Health Organization, https://www.who.int/en/news-room/factsheets/detail/dengue-and-severe-dengue. Accessed 21 October 2022.
2. SongprakhonP, Thaingtamtanha T, Limjindaporn T, Puttikhunt C, Srisawat C, Luangaram P, et al. Peptides targeting dengue viral nonstructural protein 1 inhibit dengue virus production. Sci Rep. 2020 Jul 31;10(1):12933.
3. Nagar PK, Savargaonkar D, Anvikar AR. Detection of Dengue Virus-Specific IgM and IgG Antibodies through Peptide Sequences of Envelope and NS1 Proteins for Serological Identification. J Immunol Res. 2020 Aug 4;2020:1 8.
4. Martínez-Cuellar C, Lovera D, Galeano F, GattiL, Arbo A. Non-structuralprotein 1 (NS1) of dengue virus detection correlates with severity in primary but not in secondary dengue infection. Journal of Clinical Virology. 2020 Mar;124:104259.
5. Coelho, D. R., Carneiro, P. H., Mendes-Monteiro, L., Conde, J. N., Andrade, I., Cao, T., Allonso, D., White-Dibiasio, M., Kuhn, R. J., & Mohana-Borges, R. (2021). ApoA1 Neutralizes Proinflammatory Effects of Dengue Virus NS1 Protein and Modulates Viral Immune Evasion. Journal of virology, 95(13), e0197420. https://doi.org/10.1128/JVI.01974-20
6. Quirino-Teixeira, A. C., Rozini, S. V., Barbosa-Lima, G., Coelho, D. R., Carneiro, P. H., Mohana-Borges, R., Bozza, P. T., & Hottz, E. D. (2020). Inflammatory signaling in dengue-infected platelets requires translation and secretion of nonstructural protein1. Blood advances, 4(9), 2018 2031. https://doi.org/10.1182/bloodadvances.2019001169
7. Pan YH, Liao MY, Chien YW, Ho TS, Ko HY, Yang CR, et al. Use of seroprevalence to guide dengue vaccination plans for older adults in a dengue non-endemic country. Gubler DJ, editor. PLoS Negl Trop Dis. 2021 Apr 1;15(4):e0009312.
8. Lai SC, Huang YY, Shu PY, Chang SF, Hsieh PS, Wey JJ, et al. Development of an Enzyme-Linked Immunosorbent Assay for Rapid Detection ofDengue Virus (DENV) NS1 and Differentiation of DENV Serotypes during Early Infection. Caliendo AM, editor. J Clin Microbiol. 2019 Jul;57(7):e00221-19.
9. Poltep K, Nakayama EE, Sasaki T, Kurosu T, Takashima Y, Phadungsombat J, et al. Development of a Dengue Virus Serotype-Specific Non-Structural Protein 1 Capture Immunochromatography Method. Sensors. 2021 Nov 24;21(23):7809.
10. Haroon M, Jan H, Faisal S, Ali N, Kamran M, Ullah F. Dengue Outbreak in Peshawar: Clinical Features and Laboratory Markers of Dengue Virus Infection. Journal of Infection and Public Health. 2019 Mar;12(2):258 62.
11. Wongsawat E, Suputtamongkol Y, Assanasaen S, Silpasakorn S, Avirutnan P, Puttikhunt C, et al. Performance ofa New Microfluidic Dengue NS1 Immuno-magnetic Agglutination Assay for the Rapid Diagnosis of Dengue Infection in Adults. The American Journal of Tropical Medicine and Hygiene. 2021 Sep 15;105(3):771 6.
12. Nascimento EJM, George JK, Velasco M, Bonaparte MI, Zheng L, DiazGranados CA, et al. Development of an anti-dengue NS1 IgG ELISA to evaluate exposure to dengue virus. Journal of Virological Methods. 2018 Jul;257:48 57.
13. Lai, S. C., Huang, Y. Y., Wey, J. J., Tsai, M. H., Chen, Y. L., Shu, P. Y., Chang, S. F., Hung, Y. J., Hou, J. N., &Lin, C. C. (2022). Development ofNovelDengueNS1 Multiplex Lateral Flow Immunoassay to Differentiate Serotypes in Serum of Acute Phase Patients and Infected Mosquitoes. Frontiers in immunology, 13, 852452. https://doi.org/10.3389/fimmu.2022.852452
14. Tran, T. V., Nguyen, B. V., Nguyen, T., Tran, T. T., Pham, K. G., Le, Q. B., Do, B. N., Pham, H. N., Nguyen, C. V., Dinh, D., Ha, V. T., Doan, T., & Le, H. Q. (2019). Development of a highly sensitive magneto-enzyme lateral flow immunoassay for dengue NS1 detection. PeerJ, 7, e7779. https://doi.org/10.7717/peerj.7779
15. Alidjinou, E. K., Tardieu, S.,Vrenken, I.,Hober, D., &Gourinat, A. C. (2022). Prospective Evaluation of a Commercial Dengue NS1 Antigen Rapid Diagnostic Test in New Caledonia. Microorganisms, 10(2), 346. https://doi.org/10.3390/microorganisms10020346
16. Espinosa DA, Beatty PR, Reiner GL, Sivick KE, Hix Glickman L, Dubensky TW, et al. Cyclic Dinucleotide Adjuvanted Dengue Virus Nonstructural Protein 1 Induces Protective Antibody and T Cell Responses. JI. 2019 Feb 15;202(4):1153 62.
17. Gao X, Wen Y, Wang J, Hong W, LiC, Zhao L, et al. Delayed and highly specific antibody response to nonstructural protein 1 (NS1) revealed during natural human ZIKV infection by NS1-based capture ELISA. BMC Infect Dis. 2018 Dec;18(1):275.
18. Jang WS, Kwak SY, May WL, Yang DJ, NamJ, Lim CS. Comparative evaluation of three dengue duo rapid test kits to detect NS1, IgM, and IgG associated with acute dengue in children in Myanmar. Wu HC, editor. PLOS ONE. 2019 Mar 13;14(3):e0213451.
19. Sigera PC, Amarasekara R, Rodrigo C, Rajapakse S, Weeratunga P, De Silva NL, et al. Risk prediction for severe disease and better diagnostic accuracy in early dengue infection; the Colombo dengue study. BMC Infect Dis. 2019 Dec;19(1):680.
20. Lai SC, Huang YY, Wey JJ, Tsai MH, Chen YL, Shu PY, et al. Development of Novel Dengue NS1 Multiplex Lateral Flow Immunoassay to Differentiate Serotypes in Serum of Acute Phase Patients and Infected Mosquitoes. Front Immunol. 2022 Mar 4;13:852452.
21. Rao C, Kaur H, Gupta N, Sabeena S, Ambica R, Jain A, et al. Geographical distribution of primary & secondary dengue cases in India 2017: A cross-sectional multicentric study. Indian J Med Res. 2019;149(4):548
22. Ahamed SF, Vivek R, Kotabagi S, Nayak K, Chandele A, Kaja MK, et al. Enhancing the sensitivity of Dengue virus serotype detection by RT-PCR among infected children in India. Journal of Virological Methods. 2017 Jun;244:46 54
23. Santoso MS, Yohan B, Denis D, Hayati RF, Haryanto S, Trianty L, et al. Diagnostic accuracyof 5 different brands ofdengue virus non-structural protein 1 (NS1) antigen rapid diagnostic tests(RDT) inIndonesia. Diagnostic Microbiologyand Infectious Disease. 2020 Oct;98(2):115116.
Effectiveness of Equine-immune F(ab')2 Antivenin on Preventing Complications in Latrodectism: A Systematic Review
Paulus Bergina Tarigan1, Ridel Gabriel Janevandro Lala2, Jessica Keizah Ponto3, Kirey Injili Putri Mantiri4 FacultyofMedicine, SamRatulangi University, Manado, Indonesia
ABSTRACT
Introduction: Lctrodectus venom contains 7 components, one of them is g-Latrotoxin that works as a mediator of toxicity in vertebrates. g-Latrotoxin, a potent neuroexcitatory toxin, in this venom cancauseadepletionofacetylcholinesonthemotoricnerveendsandthereleaseof catecholamines on adrenergic nerve ends as well as other neurotransmitters such as dopamine and norepinephrine within the central nervous system. Characterized of Lactrodectism is localized pain and sweating, symptoms usually come in waves for about 48 to 72 hours, severe cases and with systemic involvement,generalizedpain,sweating,hypertension,andmalaise,persistsfordayscanbefound, fatalities are rare, but still can occur because of other factors such as pulmonary oedema and heart failure. In arecentstudy,therehasbeenfoundanEquine-immuneF(ab`)2antiveninasanantivenin to a latrodectus envenomation, it was an experimental equine F(ab)2 antibody preparation purifies to reduce non-immunized serum components such as albumin.
Objectives: To identify Equine-immune F(ab’)2 antivenin as a recent antivenin on preventing complication throgh result of randomized controlled trial (RCTs)
Methods and Matherials: this systematic review was conducted to English-language studies articles from inception to October 2022 from 3 database which are ScienceDirect, PubMed, and GoogleScholar. We looked for RCTs identify Equine-Immune F(ab’)2 antivenin to preventing Complications.
Results: Among 232 articles screened, two fulfilled our criteria. In these two studies, we found there was no significant ability to reducing pain but can preventing complication. We also found that adverse event no happened in Equine-Immune F(ab’)2 group.
Conclusion: Equine-Immune F(ab')2 antivenin can used as an alternative treatment for patients with latrodectism who have moderate to severe pain. although keep in mind, patients taking this antivenin did not have any adverse events. Cautions use of Equine-Immune F(ab’)2 antivenin should be implemented according to existing guidelines
Keywords: Latrodectus, F(ab')2
Effectiveness of Equine-immune F(ab')2 Antivenin on Preventing Complications in Latrodectism: A Systematic Review Scientific Paper
Authors :
Paulus Bergina Tarigan
Ridel Gabriel Janevandro Lala Jessica Keizah Ponto
Kirey Injili Putri Mantiri
Faculty of Medicine, Sam Ratulangi University, Manado, Indonesia
Asian Medical Student Association-Indonesia
2022

Effectiveness
Of Equine-immune F(ab')2
antivenin On Preventing Complications In Latrodectism: A Systematic Review
Paulus Bergina Tarigan1, Ridel Gabriel Janevandro Lala2, Jessica Keizah Ponto3, Kirey Injili Putri Mantiri4 . FacultyofMedicine, SamRatulangi University, Manado, Indonesia
ABSTRACT
Introduction: Lctrodectus venom contains 7 components, one of them is g-Latrotoxin that works as a mediator of toxicity in vertebrates. g-Latrotoxin, a potent neuroexcitatory toxin, in this venom cancauseadepletionofacetylcholinesonthemotoricnerveendsandthereleaseof catecholamines on adrenergic nerve ends as well as other neurotransmitters such as dopamine and norepinephrine within the central nervous system. Characterized of Lactrodectism is localized pain and sweating, symptoms usually come in waves for about 48 to 72 hours, severe cases and with systemic involvement,generalizedpain,sweating,hypertension,andmalaise,persistsfordayscanbefound, fatalities are rare, but still can occur because of other factors such as pulmonary oedema and heart failure. In arecentstudy,therehasbeenfoundanEquine-immuneF(ab`)2 antiveninasanantivenin to a latrodectus envenomation, it was an experimental equine F(ab)2 antibody preparation purifies to reduce non-immunized serum components such as albumin.
Objectives: To identify Equine-immune F(ab’)2 antivenin as a recent antivenin on preventing complication throgh result of randomized controlled trial (RCTs)
Methods and Matherials: this systematic review was conducted to English-language studies articles from inception to October 2022 from 3 database which are ScienceDirect, PubMed, and GoogleScholar. We looked for RCTs identify Equine-Immune F(ab’)2 antivenin to preventing Complications.
Results: Among 232 articles screened, two fulfilled our criteria. In these two studies, we found there was no significant ability to reducing pain but can preventing complication. We also found that adverse event no happened in Equine-Immune F(ab’)2 group.
Conclusion: Equine-Immune F(ab')2 antivenin can used as an alternative treatment for patients with latrodectism who have moderate to severe pain. although keep in mind, patients taking this antivenin did not have any adverse events. Cautions use of Equine-Immune F(ab’)2 antivenin should be implemented according to existing guidelines
Keywords: Latrodectus, F(ab')2
Introduction
In medical setting especially in the North America, there are two important groups of spiders one of them is widow spiders (Latrodectus). Currently, there are five species of spiders in the Lactrodectus genus: brown widow (Lactrodectus geometricus), red widow (Lactrodectus bishop), black widow (Latrodectus mactans or southern black widow), western black widow (Latrodectus hesperus), and northern black widow (Latrodectus variolus).1 These spiders love to hid in dimly lit and secluded areas such as barns, cabins, outhouses, and stables.2 An epidemiologic data report that was concludedby thepoison control centerin USAsuggestthatin47statesthereare23,409 Latrodectus exposures ranging from year 2000 to 2008, 65% of the exposures reports minor clinicals effect, 33.5% reports mild clinical effect, and 1.4% of the exposures report a major clinical effect, but there are no death cases reported. Another report by the American Association of Poison Control Centers stated that there are 1,015 cases of Latrodectus exposures in year 2018. 3
Lactrodectus spp produces venom as a mechanism to defend themselves if they ever got in a situation where they feel threatened, this venom derived from a mixture of complex components from mostly
proteinsandpeptideswherebothcomponents play an important part in biological mechanism such as paralyzing, limiting a prey or an enemy, and even killing an enemy. Lactrodectus spp doesn’t only stores their venom in their venom glands, they also store it in other body parts such as legs, gut, even eggs and their young. A lactrodectus venom contains 7 active components, and one of them is called g-Latrotoxin that works as a mediator of toxicity in vertebrates.3
This spider venom works by massively releasing neurotransmitters with neurotoxic manifestations and high morbidity. g-Latrotoxin, a potent neuroexcitatory toxin, in this venom can cause a depletion of acetylcholines on the motoric nerve ends and the release of catecholamines on adrenergic nerve ends as well as other neurotransmitters such as dopamine and norepinephrine within the central nervous system, which causes all the symptoms that occurs when a lactrodectus envenomation happens such as musculoskeletal pain, abdominal pain, thoracic pain, hypertension, tachycardia, and diaphoresis.3
Envenomation caused by Latrodectus spp is referred to as lactrodectism, a neurotoxicity but without a significant local injury. A lactrodectus bite would most likely
go unnoticed or feels like a pinprick, the bite site will form a “target lesion” which consists of redness at the central parts, surrounded by a blanching area and an outer halo of redness. There might not be puncture wounds due to spider’s small fang size.1 Lactrodectism is characterized by a localized pain and sweating, symptoms usually come in waves for about 48 to 72 hours, in more severe cases and with systemic involvement, generalized pain, sweating, hypertension, and malaise, that persists for days can be found, but fatalities are a rare scenario, but it still can occur because of other factors such as pulmonary oedema and heart failure.4
The most common manifestation is muscle pain in the abdomen, back, and legs.3 Nausea and restlessness can also be found.5 Manifestations that can be seen on patient’s face is called “facies latrodectismica” which means facial muscle twisting, blepharititis, rhinitis, cheilits, and trismus masseter. Symptoms might also consist of other neurological syndromes that are related to autonomic nervous system such as sweating, saliva production, precordial pressure, anxiety, and mental symptoms contributes to the “pavor mortis” (fear of death) phenomenon.6
Changes in ECG might happen to patients with L. mactans bite, although
myocardium damage and manifestations that are linked to the heart is uncommon. On this rare case, we might find a troponin increase and ECG shift is reported on all studies. Cardiogenic pulmonary oedema could also happen.7
Treatment to lactrodectism usually starts with carefully monitoring the vital signs and intensive caresupport, ifnecessary, followed by the use of analgesia, nonopioid oral pain medications, if the pain is poorly controlled opioids are sometimes indicated, benzodiazepines is also recommended for the painful musclespasms,local woundcare, and tetanus vaccine prophylaxis and when the symptoms become severe an antivenin therapy might be needed and has been demonstrated useful even when there is a 90 hours delay after the exposure. Indication of antivenin administration are hypertension (if uncontrolled), pregnancy, comorbid (e.g., coronary artery disease, chronic obstructive pulmonary disease), intractable pain, respiratory difficulty and priapism. Anaphylaxis and serum sickness may occur as a potential reaction to antivenin. The use of calcium gluconate and methocarbamol do not give out effective results, therefore they are not recommended as a treatment. In place of antivenin, high dose of benzodiazepine and opioid can be used, but a prolonged
disease may occur as a consequence. Classically, antivenin works by binding to latrotoxin and preventing interactions at the presynaptic membrane. An antivenin was introduced around year 1950, this antivenin contains immunoglobulin and other horse serum components that is considered effective, but they might also cause side effects such as allergy and anaphylaxis. In a recent study, there has been found an Equineimmune F(ab’)2 antivenin as an antivenin to a latrodectus envenomation, it was an experimental equine F(ab)2 antibody preparation purifies to reduce nonimmunized serum components such as albumin.8
Matherials and Methods
1. Study registration and Methodology
this systematic review was conducted based on the preferred reporting item for systematic review and meta-analysis (PRISMA) criteria.
2. EligibilityCriteria
Our research includes both randomized clinical trials and clinical trials assessing the efficacy of Equine-immune F(ab')2 antivenin. Review articles,
commentaries, conference abstracts, editorial letters, studies that cannot be accessed through full papers, and studies conducted in languages other than English are not eligible.
This study only looked at the last five years of research.
3. Search strategy
We utilized journals or research published in the Google Scholar database, PubMed, ScienceDirect, and ClinicalTrials from inception to October 2022 to search the data. The inclusion criteria are used in the form of research papers that feature several studies on the efficacy of Equine-immune F(ab')2 antivenin. "Latrodectus" and "F(ab')2" were the keywords used in the search. The search is restricted to Englishlanguage studies published from inception to October 2022.
4. Study selection and data extraction
The studies were gathered from various databases using the keywords mentioned above. The author monitors searches that are similar to engines. After removing titles that are similar or overwriting, the journals or studies gathered are screened based on their titles and abstracts. Then, based on inclusion and
exclusion criteria, all journals and literatures are fully prepared to be used as eligible journals and literatures. We extracted relevant data from eligible studies, including the study's methodological characteristics, study design, study population, and intervention details. The review team worked together on all aspects of study selection and data extraction.
5. Risk of Bias in individual studies
Although statistical analysis is designed based on a priori knowledge, numerous studies throughout this systematic review use assessment instruments such as the visual analog scale (VAS) and numerical rating scale (NRS), including the verbal numerical rating scale (VNRS).9 In clinical research, pain quantification with VAS and NRS has a strong correlation. The NRS used is easier for researchersto manage andis well understood in existing studies.
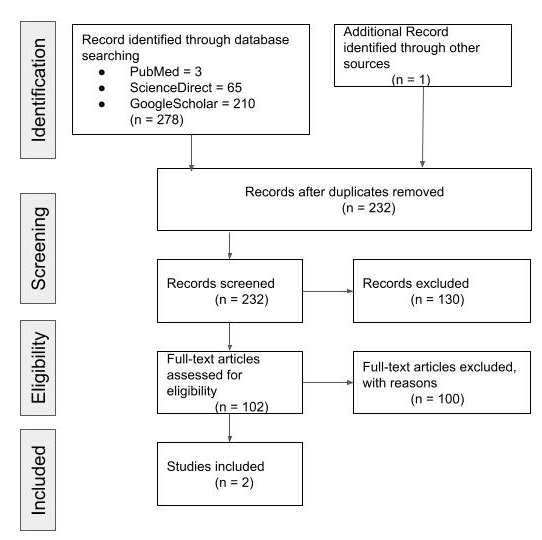
Results
1. Studysearch results
The search for data as literature for this study was obtained through an online search of three databases where 278 studies were identified. After removing the articles with the same title, the remaining 232 articles
were found. The remaining articles are then screened based on inclusion and exclusion criteria. Two articles were selected for review. The summary of our literature search and selection process is illustrated in our PRISMA diagram in Figure 1.
2. Risk of Bias in individual studies
There was significant bias in the selection of baseline characteristics since there was a variation in baseline characteristics that was relatively lower in pain scores in the IM group (VAS score 40 mm) than in pain scores in group IV (VAS score 40 mm).10
Furthermore, most researchers have a high bias because the ratings in these studies differ due to modifications or combinations in the measurement of the score system that
lead to different results. Moreover, evaluating Lactrodectism is complicated since the study did not provide a validated measuring stick and is solely based on previous experience in terms of severity and numerous different autonomic effects, including psychological effects.
High research bias was also discovered in several studies where there were deviations from the intervention protocol, specifically the provision of assistance to some subjects.
3. Characteristic of Included studies
Our study here include subjects aged 10 and up as experimental subjects. Patients who had symptoms suspected to be directly associated to black spider venom in even less than 24 hours, who might have been diagnosed by an observer with the approval of a physician who was not directly involved with the study, and who had moderate to severe pain intensity based on visual analoguescale(VASscore>40mm)werethe main inclusion criteria.Characteristic of studyinterventions
The effectiveness of equine-immune F(ab')2 antivenin in reducing pain symptoms is generally communicated in studies. Each antivenin was given to a different group at a
different dose. Based on all this, the outcome was discovered to reduce treatment failure and pain.11
The caracteristic of included studies and study intervention is summarized in Table 1.
Author N Inclusion Criteria Design Intervention Primary Outcome Secondary Outcome Summary Results
Dart. et al. (2019)10
60 1. aged 10 years and older 2. presenting within 24 hours of symptom onset related to presumed black widow spider envenomation
3. have moderate to severe pain intensity, defined as a score greater than 40 mm on the visual analog scale (VAS)
A multicenter, randomized, double-blind, placebocontroll ed tria
10-mL vial of lyophilized white powder contains sufficient F(ab胡)2
reduced treatment failures which was defined as failure to achieve and maintain clinically significant reduction in pain for 48 hours posttreatment
1. measures of pain intensity differences
2. summed pain intensity difference
1. Treatment failure was more common in the placebo group compared with the black widow spider study medication group
2. The VAS score decreased progressively throughout the ED observation period for both groups
3. Model-estimated mean pain intensity difference increased over time within both the antivenom and placebo groups
4. For the black widow spider study
medication patients and the placebo patients, respectively, pruritus was reported in 62.1% and 54.8%, arthralgia in 24.1% and 19.4%, and rash in 13.8% and 25.8% Dart. et al. (2018)11
60 1. Moderate to severe pain intensity measured using the visual analog scale (VAS score ≥ 40mm) at the start of screening phase (VAS 0)
2. Diagnosis of latrodectism by the Investigator, with concurrence of diagnosis by a
Randomized Clinical Trials Antivenin Latrodectus (Black Widow) Equine Immune F(ab)2
Analatro: 30 mL of lyophilized antivenom, reconstituted in 50 mL saline
Number of Participants With Treatment Failure
1. Number of Participants With at Least 13 mm Reduction in Pain Score at 30 Minutes PostTreatment
2. Drug-related Adverse Events
3. Number of Participants With at Least 13 mm Reduction in
1. For the Antivenin Latrodectus (Black Widow) Equine Immune F(ab)2 groups no Serious Adverse Events detected 0/29 (0.00%) versus placebo group 2/31 (6.45%)
2. the Other (Not Including Serious)
Adverse Events result for Antivenin Latrodectus (Black Widow) Equine Immune F(ab)2 total
physician not directly involved with the study
3. Moderate to severe pain intensity measured using the visual analog scale (VAS score ≥ 40mm) at Baseline (VAS 1)
infused over 10 minutes, up to 2 doses
Pain Score at Any Time Point
4. Drug-related Serious Adverse Events
26/29 (89.66%) and plasebo group total 27/31 (87.10%)
Discussion
The poison of Latrodectus spp. is not a lethal poison for humans, but if the initial treatment is not completely right, a patient will experience a distressing circumstances. Thecondition that is emphasizedin this study is how effective the antivenin Latrodectus Equinen Immune F(ab')2 is as the first treatment for pain symptoms in patients.
Dart et al. evaluated the VAS of symptoms caused by the toxin Lactrodectus spp. as a basic measurement in their study comparing the efficacy of Latrodectus Equinen Immune F(ab')2 with placebo. So even though pain was the reported symptom and previous pain research projects were the primary examination, the assessment was chosen as the primary evaluation. Subjects were randomly assigned to receive Latrodectus Equinen Immune F(ab')2 by intravenous administration for 10 minutes, with each dose consisting of 3 vials of reconstituted antivenom in a total volume of 50 mL of normal saline where this dose is strongly suggested or has been validated in previous research.
The study results findings were contrasted to 6 patients who were unable to meet the assessment criteria because their VAS scores were less than 40 mm and medical management on placebo failed,
namely 24 of 31 subjects who were given antivenin and 15 of 29 subjects who did not.
Benzodiazepines and opiates were given first just to treat temporary pain in previous instances in which individuals had toxic side effects from Latrodectus spp. They were then given antivenom prepared by diluting in 50 mL of normal saline intravenously for 10 minutes; they experienced a reduction in pain and the patient felt better within 30 minutes of management.
Conclusion and recommendation
This systematic review reveals that equine-immune F(ab’)2 antivenin as therapy in patients with latrodectism who do not have a significant ability to reduce pain but has the ability to prevent complications in patients with latrodectism. Equine-immune F(ab')2 showed no adverse event compared to the placebo group. However, it is important to note that treatment using equine-immune F(ab')2 antivenin should keep in mind the patient's condition, used with caution and with the existing guidelines.
Acknowledgements and Conflict of Interest
The Authors have nothing to declare and to conflict of interest.
REFERENCES
1. Orloff K, Zimmerman K. Bites and Stings. Mosby;
2. Erickson TB, Cheema N. Arthropod Envenomation in North America. Vol. 35, Emergency Medicine Clinics of North America. W.B. Saunders; 2017. p. 355 75.
3. Warpinski GP, Ruha AM. North American Envenomation Syndromes. Vol. 40, Emergency Medicine Clinics of North America. W.B. Saunders; 2022. p. 313 26.
4. Piscopo Massari P Scicchitano M Sanasi M De Palo P Caldarola M Liccese G Calculli AF. Acute Myocarditis After Black Widow Spider Bite: A Case Report. Cardiol Ther [Internet]. 9. Available from: https://doi.org/10.6084/m9.figshare.1 2292886.
5. Herness J SMNR. Arthropod Bites and Stings. 2022;
6. Mollie Williams A, Anderson J, Nappe Affiliations TM. Black Widow
Spider Toxicity Continuing Education Activity.
7. Gogos C, Sachpekidis V, Davora F, Bompotis G. Acute myocardial injury
caused by black widow spider (Latrodectus) bite. Vol. 4, European Heart Journal - Case Reports. Oxford University Press; 2020.
8. Paller, Macini. Infestations, Bites, and Stings.
9. Ryan NM, Buckley NA, Graudins A. Treatments for latrodectism a systematic review on their clinical effectiveness. Vol. 9, Toxins. MDPI AG; 2017.
10. Dart RC, Bush SP, Heard K, Arnold TC, Sutter M, Campagne D, et al. The Efficacy of Antivenin Latrodectus (Black Widow) Equine Immune F(ab胡)2 Versus Placebo in the Treatment of Latrodectism: A Randomized, Double-Blind, PlaceboControlled, Clinical Trial. Ann Emerg Med. 2019 Sep 1;74(3):439 49.
11. Dart RC. Black Widow Spider Antivenin for Patients With Systemic Latrodectism (BWSP3). 2018;1.












































































































