
10 minute read
Tissue Adhesive Bleeding and Oozing Practice Summary
Lori Kaczmarek, MSN, RN, VA-BC™ | Vascular Access Clinical Specialist, Adhezion Biomedical, LLC
INTRODUCTION
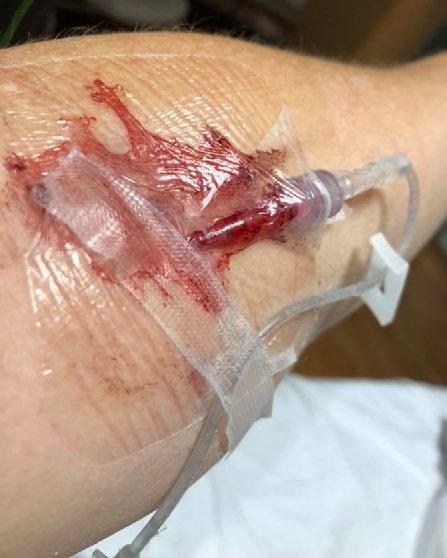
Oozing and bleeding at a vascular access insertion site contributes to the device and/or dressing failure. The device failure may be a result of dislodgement or unintentional removal when the dressing or securements fail. The presence of blood or moisture under the dressing requires unscheduled dressing changes adding to an increased risk of catheter-associated bloodstream infections (CABSI).1-3
A STANDARD OF CARE
A highly purified medical cyanoacrylate or tissue adhesive (TA) was approved by the United States (US) Food and Drug Administration (FDA) for vascular access (SecurePortIV®, Adhezion Biomedical, LLC, Wyomissing, PA USA) in 2017 and since then has seen increased use to improve vascular access outcomes. While mentioned in the 2016 Infusion Nurse Society (INS) Infusion Therapy Standards of Practice,4 TA was still a “consideration” for its benefit with vascular access (VA) and more research was recommended. The development of a unique cyanoacrylate formula, the expanded use, data collection and published reports propelled TA to become one of four recommended securement technologies in the 2021 INS Standards along with additional recommendations for hemostasis and providing a microbial barrier.1,5
BLEEDING AND OOZING
The point of skin penetration for vascular access creates a surgical wound providing a direct route for pathogen entry into the body and an exit site for blood and capillary fluid.6 To control post insertion bleeding, clinicians may resort to using direct manual pressure, use of thrombotic agents, pressure dressings or a combination of interventions. This may lead to frequent or early dressing changes and obscure direct visualization of the insertion point necessary for routine assessment and critical for the prevention of complications.1 7
Use of hemostatic agents or gauze pressure dressings add additional cost, patient, and clinician risk, and require additional time to the care and management of the vascular access device (VAD).2,8,9 Traditional interventions for post insertion bleeding and oozing require additional products or dressings and limit the use or effectiveness of chlorhexidine gluconate (CHG) disks or gel dressings that may be used for microbial protection. Once bleeding and oozing are controlled the patient will require a dressing change (usually within 24 hours) to remove the hemostatic product or soiled gauze and replace with the facility protocol dressing. The manipulation of the VAD and dressing replacement during this process may cause re-bleeding which necessitates yet another cycle of care not to mention additional risk.
HEMOSTATIC EFFECT
Cyanoacrylate tissue adhesive formulas have been used for decades in emergency care and surgical arenas and are known for their ability to provide wound hemostasis.5,10 The innovative cyanoacrylate formula approved for VA provides a potent mechanical hemostatic effect and is
TISSUE ADHESIVE BLEEDING, CONTINUED FROM PREVIOUS PAGE
demonstrated to achieve hemostasis 12-fold faster than thromboplastin.11,12 In addition, the proprietary cyanoacrylate formula exhibits antimicrobial activity against gram positive, gram negative, yeast and fungi commonly known to infect VADs.5,13,14
PRACTICE SURVEY
To better understand TAs role in promoting hemostasis in VA care and management, a practice survey of clinicians was conducted. The survey tool was created using JotForm (JotForm Inc., San Francisco, CA USA). Forty-four (44) clinicians in US hospitals known to routinely use TA for adult (age greater than 18) VA care were invited to participate. The survey was sent electronically to recipients on February 19, 2021. Twenty-one (21) responses were received before the survey closed on March 27, 2021.
Respondent Demographics
The title or position of respondents include Vascular Access Specialist (VAS) (13), Director/Manager (5), Clinical Nurse Specialist (CNS) (2), and Infection Preventionist (IP) (1). Most (9) said they learned of TA by attending a professional conference, while 7 received information or a recommendation from a colleague, and 3 found TA while researching product options. Social media, webinars or podcasts, journal advertisements and local professional network events provided other opportunities to learn about TA.
Evidence and Target Areas for Improvement
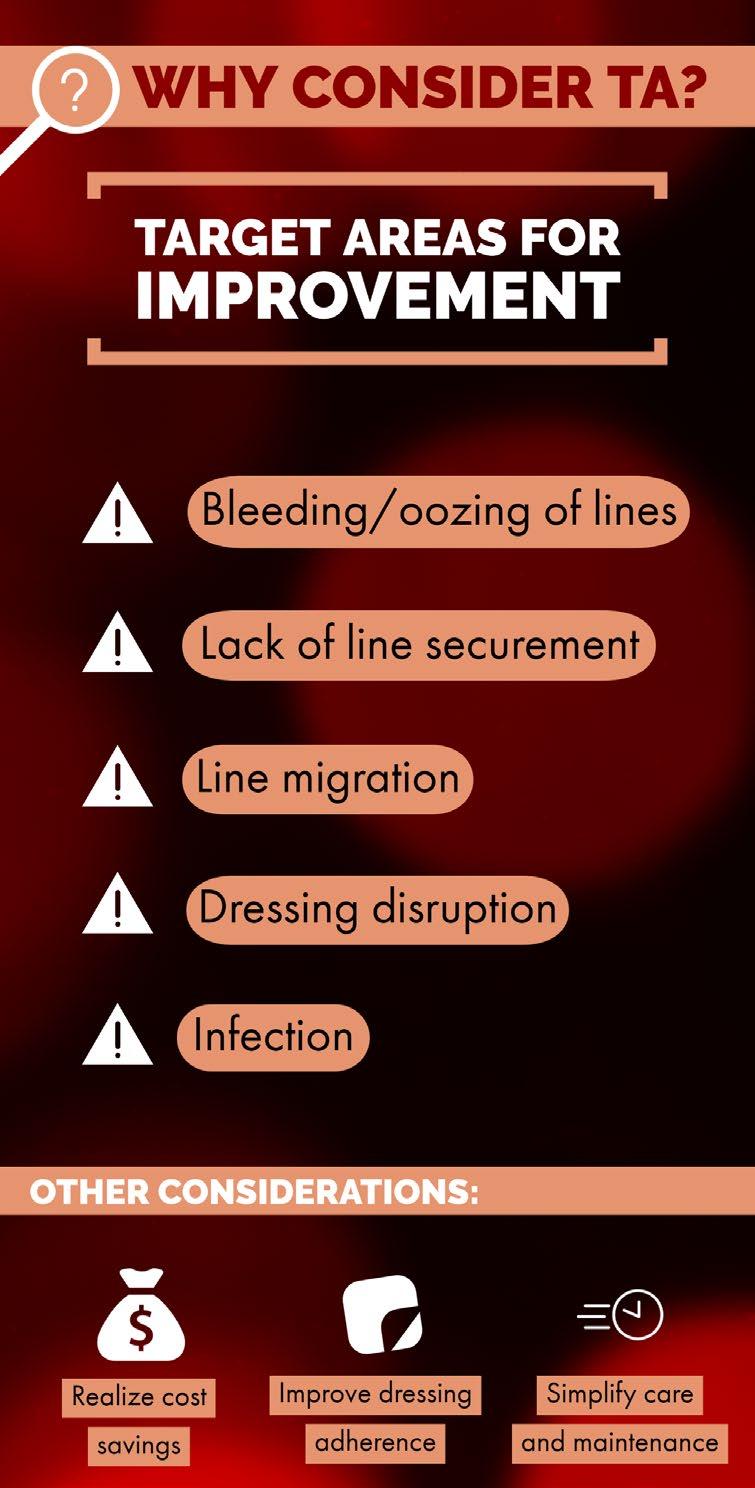
When asked on a 1-5 scale (5 highest) if they found enough evidence to support a trial of TA at their facility, 95% (20) responded 4 or 5. Respondents were asked what prompted them to consider TA. They identified bleeding and oozing of lines (19), desire to improve line securement (13), line migration (10), dressing disruption (10), and infection (9). Others (7) included improving dressing adherence, cost savings, improving both patient and clinician experience/ satisfaction, and simplifying care and maintenance as other reasons they chose to implement TA.
Graphic provided by Adhezion Biomedical, LLC
Device Application
In this survey, TA is mostly applied to peripherally inserted central catheters (PICC) (20), midlines or long-dwell peripheral intravenous catheters (PIV) (14), central venous catheters (CVC) (8), PIV (4), and arterial line (AL) (3). The VASs in the survey did not report CVC or AL insertions as routine part of their repertoire which may explain lower TA use on those devices. The FDA approved this TA formula for use on
TISSUE ADHESIVE BLEEDING, CONTINUED FROM PREVIOUS PAGE
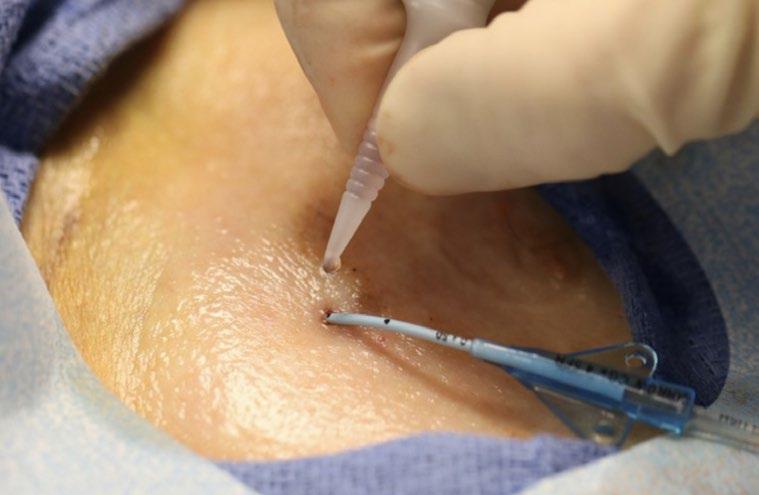
all vascular access devices, it has no age restriction, and it is demonstrated to be safe on polyurethane and silicone catheters. 5,15
During their TA trial, most (86%) aimed to reduce unscheduled dressing changes, reduce post-insertion bleeding (76%), and decrease CLABSI (62%). Other target areas were line migration (52%) and improved dressing adherence (42%). Additional comments included the goal to eliminate a dressing change in the first 24 hours, dissatisfaction with previous securement device, and finally skin integrity issues with current products.
Clinical Impact
• Clinicians were asked how TA impacted their experience with post insertion bleeding and oozing. A summary of responses include: • “Improved a lot. Eliminated frequent dressing changes.” • “We have almost eliminated premature dressing changes and completely removed [hemostatic product] from our process.” • “Decreased dressing change frequency and prevention of accidental migration of catheter during dressing changes.” • “We do not get callback for PICC line oozing.” • “99% of the time we no longer need to place a pressure dressing on patients when they get a
PICC or midline.” • “Once I have placed a PICC line and apply the
SPIV I place the dressing with the reassurance that the insertion site won’t continue to bleed or need frequent dressing changes.”
Coated or Protected Catheter Use
Eight of the 21 respondents replied that they use protected or coated catheters at their facility and shared similar experiences with increased bleeding and oozing relating to their use. One nurse reported that pressure was sometimes held up to 10 minutes to control bleeding. In this patient subset, the clinicians all reported successful or improved hemostasis since the TA was implemented.
Protocol Changes
Asked about any protocol changes made following the implementation of TA, a summary of responses include:
• “Currently we are working to eliminate the [CHG disk] from our practice due to the great success of [TA]” • “No longer use sutures on PICC; still use securement dressing but moving to eliminate this soon.” • “All PICC and midlines are protected by [TA] starting with insertion.” • “Eliminated the first 24-hour dressing change.” • “Use [TA] as the first line tool for post-insertion bleeding.” • “We added an additional layer of protection by implementing [TA] to [extended dwell] PIV, midlines, PICCs and pediatric PIVs.” • “Used on every line.” • “We do not use [hemostatic product] anymore.”
Improvements Realized
Of the 21 survey respondents, 20 reported improvements bleeding/oozing reduction or eliminated, 17 reported decrease in unscheduled dressing changes or early dressing failure, and 16 commented about the clinician satisfaction. Other improvements realized included improved patient/ parent satisfaction, ease of dressing change care and management, and improved insertion site visualization.
TISSUE ADHESIVE BLEEDING, CONTINUED FROM PREVIOUS PAGE

Graphic provided by Adhezion Biomedical, LLC
No Adverse Events
No adverse events or skin issues were reported by the respondents. Fifteen (15) additional comments were added reflecting that they rarely if ever use adhesive remover in this adult population. When used, the facility’s currently stocked adhesive remover [pad] would be allowed to sit over the insertion site for 10-15 seconds to saturate the area where adhesive is present. This action is sufficient to gently wipe and remove glue residue.
Referring to a Colleague
Using a 1-5 scale (5 highest) regarding education and implementation, 95% (20) rated the ease by which they were able to educate caregivers/clinicians to use TA a 4 or 5. The clinicians surveyed were asked what they would tell another colleague who asked if they should consider adding TA to their practice. The responses include: • “Great product, easy application, cost effective.” • “This is a must-have in your toolbox.” • “[TA] is very easy to use and works very well.” • “Take the opportunity to initiate a 14-day trial. Make sure you have pre and post metrics to compare…and the outcomes will speak for themselves.” • “It will definitely benefit the patient.” • “I would highly recommend the use of the TA. I use it on all my PICC line placements to assist with infection prevention and movement of the catheter. I feel I have added another level of protection for my patient.” • “A small amount of the TA applied to the insertion site will go a long way. The need for pressure dressings, thrombolytic patch is not needed as long as you use the TA at the insertion point.” • “Absolutely one of the best products moves we have done.” • “It will change your practice for the better!”
SUMMARY
Post insertion bleeding and oozing at the vascular access insertion point contributes to negative patient outcomes. Frequent dressing changes expose clinicians to potentially infectious blood and body fluid and add bloodstream infection risk, patient discomfort, and increase cost through use of additional supplies and clinician time.
The use of an innovative highly purified cyanoacrylate TA at the point of catheter insertion provides a potent mechanical hemostatic effect. Additional benefits
include sealing the insertion site and preventing microbial invasion at the skin-catheter tract. TA secures the catheter and can also be applied under the dressing to improve dressing adherence. These added benefits simplify care and maintenance of vascular access devices. Using one product for all VA devices which targets multiple care points (i.e., securement, bleeding and oozing, microbial barrier) can improve clinician competency and improve consistency in use and application.
REFERENCES
1. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th Edition. J Infus Nurs. Jan-Feb 01 2021;44(1S Suppl 1):S1-S224. doi:10.1097/ NAN.0000000000000396
2. Timsit J, Bouadma L, Ruckly S, et al. Dressing disruption is a major risk factor for catheter-related infections. Crit Care Med. Jun 2012;40(6):1707-14. doi:10.1097/CCM.0b013e31824e0d46
3. Association for Vascular Access (AVA). Resource Guide for Vascular Access: Recommended Study Guide for Vascular Access Board Certification. vol 3. 2019:148.
4. Gorski L, Hadaway L, Hagle M, McGodrick M, Orr M, Doellman D. Infusion therapy standards of practice. Journal of Infusion Nursing. 2016;39 (suppl 1):S1-159.
5. Adhezion Biomedical LLC. SecurePortIV: Indication for Use (IFU). SPI-IFU01-1903. June 2019. Accessed April 14, 2021. www.SPIVTraining.com
6. Moureau NL. Vessel Health and Preservation: The Right Approach for Vascular Access. vol 1. Springer International Publishing; 2019.
7. Corley A, Ullman AJ, Mihala G, Ray-Barruel G, Alexandrou E, Rickard CM. Peripheral intravenous catheter dressing and securement practice is associated with site complications and suboptimal dressing integrity: A secondary analysis of 40,637 catheters. Int J Nurs Stud. Dec 2019;100:103409. doi:10.1016/j. ijnurstu.2019.103409
8. Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. Apr 2008;34(4):431-45. doi:10.1111/j.1524-4725.2007.34090.x
9. Moureau N. Impact and Safety Associated with Accidental Dislodgement of Vascular Access Devices: A Survey of Professions, Settings, and Devices. Journal of the Association for Vascular Access. 2018;23(4):203-215. doi:10.1016/j.java.2018.07.002
10. DeAnda A, Jr., Elefteriades JA, Hasaniya NW, Lattouf OM, Lazzara RR. Improving outcomes through the use of surgical sealants for anastomotic sealing during cardiovascular surgery. J Card Surg. May-Jun 2009;24(3):325-33. doi:10.1111/j.15408191.2009.00809.x
11. Guido A, Zhang S, Yang C, Pook L. An innovative cyanoacrylate device developed to improve the current standard of care for intravascular catheter securement. J Vasc Access. Sep 9 2019:1129729819872881. doi:10.1177/1129729819872881
12. Zhang S, Guido AR, Jones RG, Curry BJ, Burke AS, Blaisdell ME. Experimental study on the hemostatic effect of cyanoacrylate intended for catheter securement. J Vasc Access. Jan 2019;20(1):79-86. doi:10.1177/1129729818779702
13. Prince D, Kohan K, Solanki Z, et al. Immobilization and Death of Bacteria by Flora Seal® Microbial Sealant. International Journal of Pharmaceutical Science Invention. 2017;6(6):45-49.
14. Prince D, Solanki Z, Varughese R, Mastej J, Prince D. Antibacterial effect and proposed mechanism of action of a topical surgical adhesive. Am J Infect Control. Jan 2018;46(1):26-29. doi:10.1016/j.ajic.2017.07.008
15. Di Puccio F, Giacomarro D, Mattei L, Pittiruti M, Scoppettuolo G. Experimental study on the chemicophysical interaction between a two-component cyanoacrylate glue and the material of PICCs. J Vasc Access. Jan 2018;19(1):58-62. doi:10.5301/jva.5000816








