
Volume 6 Number 1 October 2024


Volume 6 Number 1 October 2024
An Anglican community inspiring every learner every experience every day
To be a leader in Christian education that is characterised by a global vision that inspires hope
Values
Commitment
Compassion
Courage
Integrity
Respect
Senior Editor
Dr Matthew Hill
Creative Direction
Mrs Susan Layton
Dr Matthew Hill
Research Supervisors
Dr Matthew Hill
Dr Vera Munro-Smith
Dr Katie Terrett

When the New South Wales Education Standards Authority announced a new course “Science Extension” to commence in 2019 we were thrilled that there was an opportunity for a formally asessed capstone experience in Science for our students. From the perspective of the Barker Institute it was an exciting chance to support students doing academic research, alongside other subjects such as History Extension, Music Extension and English Extension 2.
Where many capstone project courses fail is the at final step of the research process – dissemination. Research is not merely the process of conducing an investigation and writing a report, but sharing it with the wider community so that people can learn, critique, have other student researchers at multiple schools build on the projects published. I am so glad to be able to publish this journal each year now celebrating 97 articles each representing genuine contributions to science.

Dr Matthew Hill Director of The Barker Institute

Scientific research is another example of how Barker continues to offer unique experiences for students to shape themselves and shape the world.

The calibre of a school is determined by its students, and again in 2024 our students have made an outstanding contribution to our school community and to our world across various academic and co-curricular domains. This is certainly true for our student researchers in Science.
As a result of a wonderful Science program at Barker, and the expert guidance of three research professionals, another cohort of student Science researchers have played their part in expanding our knowledge of the physical universe. I am thrilled with what can be achieved when space is made in the curriculum for students to follow their passions. This sixth edition of Scientific Research in School, along with the previous five, are testament to what can be accomplished. I am proud of what they have done, and excited for how they may continue to contribute through various academic pursuits.
At every stage we teach our students to question, investigate, and communicate, so it is a joy to see this culminate in academic articles from 13 young researchers.
Science is for everyone. All people in our community – primary school students, secondary school students, teachers, and parents – can enjoy thinking about, and doing Science. This Journal is the capstone, but the journey is a long-term process. In hundreds of science classes each day at Barker, students supported by a superb team of teachers experience constant growth. I regularly watch students of all ages investigate complex topics by designing fair tests, collecting their own data, and reasoning conclusions.
It is a privilege to do Science in a wonderful community, and to share our Science research reports publicly. We invite you to learn, to offer feedback, even to build on these projects in your own contexts.
I am grateful for the work of all Science staff at Barker for their investment in these students, especially the three research supervisors who along with lab staff, supported them through the Science Extension research program.

Mr Phillip Heath AM Head of Barker College
Mrs Virginia Ellis Head of Science

Director, Barker Institute Physics Teacher

and Science Extension Lead

In these 13 academic articles, students wrestle with the extant literature to accurately describe a gap in scientific understanding, before implementing valid methods to produce novel, first-hand results and findings to address this gap. It has been a pleasure to journey with them and wrestle with complex ideas and communication. Together we have endured through complications, celebrated breakthroughs, and explored implications.
We are incredibly proud of them personally, and also the work that they have produced. We look forward to seeing the impact of this work on future research in Science.
PART 1: BIOLOGY AND EARTH &
SCIENCE Effects of Essential Oils extracted from Native Australian Plants and Conventional Antimicrobial Agents against Staphylococcus albus (S.albus)
The Effects of Moisture Content on the Ultimate Tensile Strength of 3D Printed Composites
Graham Kong
Millikan’s Oil Drop in Education; Beneficial or Simply a Waste of Time?
Niraj Sesha
BRAIN DRAIN: Investigating Concussion-Inducing Brain Accelerations from Various Baseball Pitch Speeds
Evan Wang
Investigating the Efficiency and Output Energy of Compressed Air Energy Storage
Thomas Wunderlich
Drag Reduction & Environmental Efficiency in the Superstructure of Maritime Freight Carriers: Harnessing Biomimetic Box Fish Design
Rachel Mathews

In 2024, three diverse projects emerged in the fields of Biology and Earth & Environmental Science.
Tristan focused on expanding his research into antimicrobial agents targeting Staphylococcus albus. With growing global concerns over the increasing resistance of bacteria to commonly used antibiotics, Tristan seized the chance to explore the antimicrobial potential of native Australian plants. This innovative approach could pave the way for new solutions in combating antibiotic resistance.
Courtney’s background in environmental water testing during her school studies fuelled her passion for biology, leading her to conduct a survey of macroinvertebrates in both rural and urban streams. She collected samples from the Cox, Fish, and Duckmaloi Rivers—representing rural environments—as well as the Coups, Waitara, and Hornsby Quarry Creeks in New South Wales. In addition to assessing macroinvertebrate populations, she also evaluated water quality factors across these diverse locations.
During her travels to Lizard Island in Queensland, Eden became deeply interested in the environmental issues posed by microplastics, particularly their impact on marine life. She conducted sampling at several northern beaches in Sydney, including Cabbage Tree (Manly Cove), Manly, Mona Vale, and Whale Beaches. Her findings highlight local concerns that reflect a much larger global problem.
Tristan Lyth Barker College
The global rise in antibiotic resistance necessitates exploring alternative antimicrobial strategies. This research investigated the efficacy of essential oils from native Australian plants (Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, and Australian Sandalwood) compared to conventional agents (hydrogen peroxide, Dettol, bleach, and isopropyl alcohol) against Staphylococcus albus (S. albus). Using the agar disc diffusion method, the zones of inhibition were measured. Dettol had the highest antimicrobial efficacy with a mean zone of inhibition of 52.56 mm, outperforming the other conventional products. Among essential oils, Kunzea oil exhibited the highest efficacy with a mean zone of inhibition of 40.44 mm, making it more effective than Tea Tree oil and comparable to Eucalyptus, Lemon Myrtle, and Sandalwood oils. Additionally, Kunzea oil outperformed conventional antimicrobial agents such as hydrogen peroxide and isopropyl alcohol. Eucalyptus, Lemon Myrtle, and Sandalwood oils also demonstrated strong antimicrobial properties, with Sandalwood oil showing a slightly higher mean inhibition zone compared to Eucalyptus and Lemon Myrtle oils. Statistical analysis using t-tests confirmed significant differences in antimicrobial activity. The findings suggest that essential oils, particularly Kunzea, Eucalyptus, and Sandalwood, have strong antimicrobial properties and could serve as natural alternatives to traditional disinfectants.
The world is facing a global antibiotic resistance crisis, which the World Health Organization (2023) has identified as a critical issue where bacteria develop immunity to the effects of antibiotics, rendering these drugs less effective or entirely ineffective. This growing resistance intensifies the challenges associated with treating bacterial infections and escalates the risk of severe health outcomes, including higher mortality rates. This escalating problem necessitates the exploration of alternative antimicrobial strategies, including the potential of native Australian plants and their essential oils as effective and sustainable solutions. The Australian Group on Antimicrobial Resistance (AGAR) has highlighted the significance of monitoring antibiotic resistance trends through its Australian Staphylococcus aureus (S. aureus) Surveillance Outcome Programme (ASSOP), which reported notable findings in 2021 (Coombs, 2021).
Staphylococcus albus (S. albus) is a bacterium commonly found in the environment and on human skin (Otto, 2009). While it typically exists as a nonpathogenic resident, it can become pathogenic under certain conditions, such as in abnormal lesions or when forming biofilms on medical devices (Lee & Anjum, 2022). This bacterium’s ability to resist
antibiotics, including methicillin, complicates treatment, making prevention and proper hygiene critical (Tuon, 2023). Despite its nonpathogenic status, S. albus’ capability to form biofilms and its antibiotic resistance potential, particularly in hospital settings, underscores its relevance in antimicrobial resistance studies (Otto, 2009). Similarly, Staphylococcus epidermidis (S. epidermidis) , another coagulase-negative staphylococcus with comparable traits, has been recognised for its role in hospitalacquired infections due to its biofilm formation and antibiotic resistance (Lee & Anjum, 2022). S. epidermidis, like S. aureus, is a close relative of S. albus and shares many characteristics, including antibiotic resistance and biofilm formation (Otto, 2009; Pankey & Sabath, 2004).
Conventional antimicrobial agents for treating S. albus infections include hydrogen peroxide, Dettol, bleach, and isopropyl alcohol. Hydrogen peroxide produces free radicals that damage bacterial cell components (McDonnell & Russell, 1999). Dettol contains chloroxylenol, which disrupts microbial cell walls and enzyme function, leading to cell death. Bleach, made of sodium hypochlorite, is a strong oxidiser that kills many microorganisms (McDonnell & Russell, 1999). Isopropyl alcohol denatures proteins and disrupts membranes, causing rapid bactericidal activity (McDonnell & Russell, 1999). Although
effective, antibiotic resistance necessitates alternative antimicrobial strategies. Resistance to these treatments is less common, but their efficacy can be reduced by biofilms or organic matter, emphasising the need for alternatives (McDonnell & Russell, 1999; Otto, 2009).
Essential oils represent a new horizon in combating bacterial antibiotic resistance, offering natural weapons against antibiotic-resistant bacteria responsible for nosocomial infections, as highlighted by Yap et al. (2014) and Iseppi et al. (2021). Extensive research has demonstrated their efficacy against various bacterial strains. For instance, Bachir and Benali (2012) demonstrated significant antimicrobial activity of Eucalyptus oil against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). Carson et al. (2006) reviewed the broad-spectrum antimicrobial properties of tea tree oil, highlighting its efficacy against various pathogens. Van Vuuren et al. (2014) showed promising antimicrobial effects of Kunzea oil, which were similar to those of tea tree oil. Man et al. (2019) confirmed the significant antibacterial properties of lemon myrtle oil. Sadlon and Lamson (2010) discuss the antimicrobial effects of essential oils, including Australian sandalwood, emphasising its broad-spectrum activity against bacteria, viruses, and fungi. Iseppi et al. (2021) evaluated the antimicrobial activity of essential oils in vitro against E. coli and S. aureus, confirming their potent effects. Wińska et al. (2019) discussed the broader implications of essential oils, distinguishing between myth and reality in their antimicrobial properties, while Yuan et al. (2016) compared traditional uses of essential oils with modern applications, reinforcing their relevance in contemporary medicine.
There is considerable research into the properties of oils extracted from various native Australian plants. Cox et al. (2001) determined the antimicrobial actions of tea tree oil, showing its broad-spectrum activity against E.coli, S. aureus, and Candida albicans due to its ability to damage cell membranes. Carson et al. (2006) provided a comprehensive review of tea tree oil’s properties, emphasising its historical and ongoing medicinal use. Astani et al. (2009) compared the antiviral activity of eucalyptus and tea tree oils, highlighting significant reductions in viral infectivity. Research on Kunzea oil, derived from Kunzea ambigua, has shown promising antimicrobial effects. Its active compounds, such as alpha-pinene and 1,8cineole, contribute to its efficacy against a range of pathogens and its utility in treating infections and promoting skin health (Moo 2021) Studies have highlighted Kunzea oil’s potential as a natural alternative to synthetic antimicrobials, further
underscoring the need for continued exploration of essential oils in combating antibiotic-resistant bacteria (Chen 2016).
Comparative studies by Man et al. (2019) investigated the antimicrobial activity of lemon myrtle and other essential oils against a group of human pathogens, showcasing the broad-spectrum efficacy of these natural products. Thielmann et al. (2019) and Sakkas et al. (2018) underscored the potent antimicrobial effects of various essential oils, supporting their potential as alternative treatments. Mani et al. (2023) emphasised the rich chemical diversity and historical use of essential oils in traditional medicine, reinforcing their relevance in the fight against antibiotic resistance. Iseppi et al. (2021) and Wińska et al. (2019) provide evidence supporting the effectiveness of essential oils in comparison to conventional agents.
Native Australian plants were chosen for this study primarily because some of them have not been extensively tested for their antimicrobial properties against Staphylococcus albus (S.albus), especially for Kunzea, lemon myrtle, and Australian sandalwood. This offers a unique opportunity to explore uncharted territories in the field of natural antimicrobial agents. Essential oils from these plants are of particular interest due to their rich chemical diversity and historical use in traditional medicine. Sadgrove (2022) highlights the past and present chemical diversity of Australian essential oils, emphasising their potential in various applications. Additionally, Sadgrove (2013) discusses the role of essential oils in Australian Aboriginal traditional medicine, showcasing their longstanding use and potential benefits. Furthermore, S. albus was chosen for this study because it is safer to use in a school setting, minimising potential health risks while still providing relevant data on antimicrobial efficacy.
Elangovan (2023) reported on the antibacterial properties of Eucalyptus globulus against MethicillinResistant Staphylococcus Aureus (MRSA), further supporting the relevance of native Australian plants in combating antibiotic resistance. Sakkas et al. (2018) examined the antibacterial efficacy of commercially available essential oils against gram-positive pathogens, underscoring the potential of essential oils as effective antimicrobial agents. By investigating native plants like Eucalyptus, tea tree, Kunzea, lemon myrtle, and Australian sandalwood, the study aims to uncover novel antimicrobial agents that could provide alternative solutions to the growing problem of antibiotic resistance. This innovative approach could position Australia at the forefront of natural product
research and development, contributing to global health solutions (Seididamyeh et al., 2023).
In conclusion, the literature review highlights the growing concern of antibiotic resistance and the urgent need for alternative antimicrobial agents. Essential oils from native Australian plants offer a promising solution due to their rich chemical diversity and historical use in traditional medicine. Comparative studies suggest that these essential oils can be effective against antibiotic-resistant bacteria like S. albus, providing a solid foundation for future research and practical applications in combating antimicrobial resistance. The increasing interest in essential oils as alternative antimicrobial agents is further supported by studies comparing their efficacy to conventional treatments. Warnke et al. (2009) demonstrated that while essential oils such as tea tree, eucalyptus, and Kunzea exhibit significant antimicrobial properties, their effectiveness can vary compared to synthetic agents like hydrogen peroxide and chlorhexidine. This underscores the critical importance of continuing research into the potential of essential oils in addressing antibiotic resistance.
How do essential oils extracted from native Australian plants, including Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, and Australian Sandalwood, compare to conventional antimicrobial agents, including hydrogen peroxide, Dettol, bleach, and isopropyl alcohol in inhibiting Staphylococcus albus (S. albus)?
Essential oils extracted from native Australian plants, including Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, and Australian Sandalwood, will exhibit less antimicrobial activity against S. albus compared to conventional antimicrobial agents such as hydrogen peroxide, Dettol, bleach and isopropyl alcohol.
Methodology
Substances
- Essential oils - Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, Australian Sandalwood oils
- Conventional antimicrobial agents - Hydrogen Peroxide, Dettol, Bleach, and Isopropyl Alcohol
- Bacteria - Staphylococcus albus
- 29 nutrient rich agar plates were pre-prepared by the Laborary Teacher’s, according to the standard laboratory protocol.
In conducting research in a school setting, S. albus was chosen over S. aureus due to safety considerations. S. aureus is a known human pathogen with various health risks, including infections and antibiotic resistance. In contrast, S. albus is nonpathogenic, meaning it does not cause diseases in humans, making it safer for educational purposes. A general risk assessment identified minimal risks associated with S. albus, ensuring that with proper safety measures and controlled procedures, it is appropriate for use in a school laboratory setting.
Sterilisation
After closing all windows and doors and turning off fans and air-conditioning to reduce the risk of contamination, the work surfaces were sanitised with antibacterial spray and dried with clean paper towels. The Bunsen burner was lit to ensure a controlled and sterile environment, as well as to sterilise the forceps.
Inoculation and plating
The bottom faces of two plates were labelled “Control 1” and “Control 2.” The remaining 27 agar plates were divided into 9 groups, with 3 plates per group. Each plate in each group was sequentially labelled 1, 2, or 3, with the initial of the essential oil (E for Eucalyptus, TT for Tea Tree, K for Kunzea, LM for Lemon Myrtle, AS for Australian Sandalwood) or conventional antimicrobial agent (HP for Hydrogen Peroxide, D for Dettol, B for Bleach, and IA for Isopropyl Alcohol) being tested and the date of the experiment (e.g., “13/3/24 E 1,” “13/3/24 E 2,” “13/3/24 E 3,” “13/3/24 HP 1,” “13/3/24 HP 2,” “13/3/24 HP 3” for the Eucalyptus and hydrogen peroxide plates).
28 sterile disks were prepared using a sterile hole puncher on sterile filter paper to create equal size disks. Each essential oil (Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, Australian Sandalwood oils) was poured into separate dimples on a sterile spot plate (3-6 mL). Additionally, 10 mL of each conventional antimicrobial agent (Hydrogen Peroxide, Dettol, Bleach, and Isopropyl Alcohol) were poured into individual sterile 50 mL beakers. Three sterile disks were placed into each of the conventional antimicrobial agents using a sterile forceps that was dipped in methylated spirits and then exposed to the Bunsen burner flame for at least 3 seconds to ensure sterility.
3 mL of S. albus broth was pipetted onto every labelled agar plate using a 10 mL disposable pipette (except for “Control 2”). To reduce contamination, the plate lids were opened slightly, with the opening facing the Bunsen burner and away from the experimenter. The lawn plating procedure was used on
every plate with S. albus by using a sterile L-shaped glass lawn spreader and spreading the broth in one direction, then turning the plate 90 degrees and spreading in the same direction. This was repeated until the agar was completely covered with S. albus broth.
One sterile disk from each essential oil (Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, Australian Sandalwood oils) and each conventional antimicrobial agent (Hydrogen Peroxide, Dettol, Bleach, and Isopropyl Alcohol) was placed onto the centre of its corresponding plate using sterile forceps (e.g., a disk soaked in Dettol was placed onto the centre of one of the three plates labelled “Dettol”). Each disk was placed into its assigned plate, with only one disk per plate. The forceps were sterilised using methylated spirits followed by flaming with the Bunsen burner before placing each disk. The remaining one unused sterile disk was placed onto “Control 1.”
The plates were sealed using parafilm and incubated at 30 oC for one week. The zone of inhibition was recorded by measuring the diameter of the clear area surrounding the antimicrobial agent on the agar plate using a metric ruler. Measurements were taken twice: once across the horizontal diameter of the zone of inhibition and once across the vertical diameter (see diagrams 1 and 2). The results were then tabulated. The entire procedure was repeated twice more, making a total of three trials.
Analysis methodology
The vertical and horizontal measurements of the zones of inhibition for both essential oils (Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, and Australian Sandalwood) and conventional agents (hydrogen peroxide, Dettol, bleach, and isopropyl alcohol) were recorded and input into tables. The mean and standard deviation for each essential oil and conventional agent were calculated to understand the central tendency and spread of the data. To compare the means of the essential oils and the conventional antimicrobial agents, t-tests were performed for all possible pairs.
To account for the multiple comparisons and control the overall type I error rate, Bonferroni corrections were applied. This adjustment involved dividing the significance level (alpha = 0.05) by the number of comparisons (26), thus ensuring a more stringent criterion for statistical significance. If the p-value obtained from the t-tests was less than the adjusted alpha value (p=0.00192), the null hypothesis was rejected, indicating a significant difference between the groups. This rigorous approach ensured that the
conclusions drawn from the data were robust and reliable.



Table 1a: Average zone of inhibition - essential oils
Table 1b: Average zone of inhibition – conventional antimicrobial agents.
Table 1a and 1b shows the antimicrobial activity of essential oils and conventional antimicrobial agents was evaluated by measuring the zones of inhibition produced by each essential oil (Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, Australian Sandalwood oils) and each conventional antimicrobial agent (Hydrogen Peroxide, Dettol, Bleach, and Isopropyl Alcohol) against S. albus on nutrient agar plates. The diameter
of the clear zones, indicating the extent of bacterial growth inhibition, was recorded in both vertical and horizontal diameters on three different dates: 13th March 2024, 9th May 2024, and 24th May 2024. The averages were calculated for each measurement.

Figure 4 displays the comparison of zones of inhibition for each essential oil (Eucalyptus, Tea Tree, Kunzea, Lemon Myrtle, Australian Sandalwood oils) and each conventional antimicrobial agent (Hydrogen Peroxide, Dettol, Bleach, and Isopropyl Alcohol) against S. albus on nutrient agar plates, ordered from the largest to the smallest zone of inhibition.
Within the essential oil group, Kunzea oil demonstrated the highest average zone of inhibition (40.44 mm), indicating strong antimicrobial activity. This was followed by Sandalwood oil (32.56 mm), Eucalyptus oil (32.44 mm), Lemon Myrtle oil (31.94 mm), and Tea Tree oil (20.83 mm). Among the conventional agents, Dettol showed the highest efficacy with an average inhibition zone of 52.56 mm, whereas Hydrogen Peroxide showed no inhibition. Bleach and Isopropyl Alcohol demonstrated antimicrobial activity with average zones of 29.50 mm and 17.94 mm, respectively. Overall, Dettol outperformed all other substances, while Kunzea oil was the most effective essential oil.
0.00192).
Table 3 shows that significant differences were found in most comparisons between essential oils and conventional antimicrobial agents, with Bonferroni corrections, significant differences (p < 0.00192), applied to control for multiple comparisons. Specifically, the t-tests indicated significant differences in the antimicrobial efficacy for the pairs Eucalyptus vs. Tea Tree, Eucalyptus vs. Kunzea, Tea Tree vs. Kunzea, Tea Tree vs. Lemon Myrtle, Tea Tree vs. Sandalwood, Kunzea vs. Lemon Myrtle, and Kunzea vs. Sandalwood. However, no significant differences were observed for Eucalyptus vs. Lemon Myrtle, Eucalyptus vs. Sandalwood, and Lemon Myrtle vs. Sandalwood when the corrected alpha value was applied. These results underscore the varying effectiveness of different essential oils in inhibiting S. albus, highlighting Kunzea oil as particularly potent among the tested essential oils. The application of Bonferroni corrections ensured the robustness of these findings by accounting for the increased risk of type I errors due to multiple comparisons.
As for the traditional antimicrobial substances, Hydrogen peroxide demonstrated no zone of inhibition, making it significantly less effective compared to Dettol, bleach, and isopropyl alcohol. Dettol showed the highest mean zone of inhibition at 56.67 mm, significantly outperforming both bleach (36.65 mm) and isopropyl alcohol (19.98 mm). After applying the Bonferroni correction, significant differences (p < 0.00192) were observed in all comparisons between conventional agents, including those involving hydrogen peroxide. Although hydrogen peroxide exhibited no zone of inhibition, significant differences were still detected when compared to Dettol, bleach, and isopropyl alcohol. These results emphasise Dettol’s superior antimicrobial efficacy among the tested agents
Table 3 also allows for clear comparison between essential oils and traditional antimicrobial substances. Dettol consistently displayed the highest inhibition zones across all comparisons, indicating strong antibacterial properties. Significant differences were observed when comparing Dettol against Kunzea, Eucalyptus, Sandalwood, and Lemon Myrtle, with inhibition zones ranging from 31.83 mm to 56.67 mm, all of which remained significant after the Bonferroni correction (p < 0.00192). This suggests that Dettol is highly effective in inhibiting bacterial growth compared to these essential oils.
Kunzea exhibited higher inhibition zones than some conventional disinfectants. For example, Kunzea had an inhibition zone of 40.67 mm compared to Bleach’s
29.44 mm, with a significant difference (p < 0.00192). Similarly, when compared to Isopropyl Alcohol, Kunzea showed a significantly higher inhibition zone of 40.67 mm versus 17.95 mm, again with a significant difference (p < 0.00192). These results suggest that Kunzea essential oil possesses strong antibacterial properties, outperforming both Bleach and Isopropyl Alcohol in terms of bacterial inhibition
The results of this study revealed a wide range of antimicrobial activities among the tested essential oils and conventional antimicrobial agents against S. albus. Kunzea oil exhibited the highest average zone of inhibition (40.67 mm), indicating its strong antimicrobial properties. This is likely due to its rich composition of active compounds such as alphapinene and 1,8-cineole, known for their potent antimicrobial effects (Moo, 2021). Eucalyptus oil, with an average zone of inhibition of 32.33 mm, also demonstrated significant antimicrobial activity, consistent with its well-documented efficacy against various bacterial strains (Bachir & Benali, 2012).
In contrast, conventional agents such as hydrogen peroxide and isopropyl alcohol showed lower antimicrobial activity, with average inhibition zones of 0 mm and 17.95 mm, respectively. The ineffectiveness of hydrogen peroxide might be attributed to its rapid decomposition and reduced stability under the experimental conditions (McDonnell & Russell, 1999). The relatively moderate efficacy of isopropyl alcohol can be explained by its mode of action, which primarily involves protein denaturation and membrane disruption, potentially less effective against biofilmforming bacteria like S. albus (McDonnell & Russell, 1999).
Dettol, a well-known disinfectant, demonstrated the highest overall antimicrobial activity with an average inhibition zone of 56.67 mm. This result underscores Dettol’s broad-spectrum antimicrobial efficacy, which is primarily due to its active ingredient, chloroxylenol, known for disrupting microbial cell walls and enzymatic functions (McDonnell & Russell, 1999). The significant antimicrobial activity of essential oils like Kunzea and Eucalyptus, compared to conventional agents, suggests their potential as effective natural alternatives in combating bacterial infections, particularly in the face of rising antibiotic resistance (Yap et al., 2014).
The findings of this study align with previous research that highlights the antimicrobial properties of essential oils. For instance, Carson et al. (2006) reviewed the
broad-spectrum antimicrobial effects of tea tree oil, which, despite showing lower efficacy in this study, has been well-documented for its activity against various pathogens. Similarly, the significant antimicrobial activity of Eucalyptus oil observed in this study is consistent with the findings of Bachir and Benali (2012), who reported its effectiveness against E. coli and S. aureus.
In contrast, the relatively low efficacy of traditional treatments such as hydrogen peroxide and isopropyl alcohol in this study has also been observed in other research. McDonnell and Russell (1999) highlighted the limitations of hydrogen peroxide due to its instability and rapid breakdown, which may account for its lack of antimicrobial activity in this study. The moderate efficacy of isopropyl alcohol aligns with findings that suggest its effectiveness is reduced against biofilm-forming bacteria (McDonnell & Russell, 1999).
Studies like Iseppi et al. (2021) evaluated the antimicrobial activity of essential oils against antibiotic-resistant bacteria and support the significant differences observed between the antimicrobial activities of essential oils and conventional agents in this study. These studies reinforce the potential of essential oils as viable alternatives or complements to traditional antimicrobial agents in clinical and healthcare settings.
The data analysis involved independent samples ttests conducted for each pairwise comparison between essential oils and conventional agents. The original analysis revealed significant differences in antimicrobial activity among the tested substances, with several essential oils showing superior efficacy compared to traditional treatments. However, to account for multiple comparisons and control the overall type I error rate, Bonferroni corrections were applied. This adjustment involved dividing the significance level (alpha = 0.05) by the number of comparisons, resulting in a more stringent criterion for statistical significance (alpha = 0.00192).
The application of Bonferroni corrections ensured that the significant differences observed were robust and not due to chance. For instance, comparisons such as Kunzea vs. Bleach, Eucalyptus vs. Isopropyl Alcohol, and Kunzea vs. Isopropyl Alcohol remained significant even after the Bonferroni correction, underscoring the strong antimicrobial activity of Kunzea oil.
The reliability and validity of the data are supported by the consistent methodologies used across multiple plates and time points. The standard deviations calculated for each group indicate the precision of the measurements, with relatively low variability observed within groups. The use of Bonferroni corrections further enhances the reliability of the findings by reducing the risk of type I errors. The statistically significant differences (p < 0.00192) between treatments, as determined by the t-tests with Bonferroni corrections, confirm the robustness of the findings and suggest that the observed effects are unlikely to be due to chance.
However, the validity of the findings could be influenced by factors such as the variability in the chemical composition of essential oils and the specific strains of S. albus used in the study. Ensuring consistent quality and standardisation of essential oils is crucial for reproducibility and accuracy in future research. Additionally, the use of a single bacterial species limits the generalisability of the results, highlighting the need for further studies involving multiple strains and species to confirm the broader applicability of the findings.
One of the key limitations of this study is the exclusive use of in vitro methods to assess antimicrobial activity. While these methods provide valuable insights into the potential efficacy of essential oils and conventional agents, they do not account for the complexities of in vivo conditions. Factors such as the host immune response, bioavailability, and potential side effects need to be considered in future research to validate the clinical relevance of these findings.
Another limitation is the focus on a single nonpathogenic bacterial species, S. albus. Although this bacterium serves as a useful model for studying antimicrobial efficacy, future research should include pathogenic strains and other clinically relevant bacteria to provide a more comprehensive evaluation of essential oils’ antimicrobial potential. The variability in the chemical composition of essential oils, influenced by factors such as plant source, extraction method, and storage conditions, also poses a challenge to standardisation and reproducibility. Future studies should aim to standardise these variables to ensure consistent and reliable results.
Further implications of this study suggest that essential oils, particularly Kunzea and Eucalyptus oils, could be developed as natural antimicrobial agents to combat antibiotic-resistant bacteria. Their significant
antimicrobial activity highlights their potential role in healthcare settings, especially as alternatives or complements to conventional treatments. However, extensive in vivo studies and clinical trials are necessary to fully explore their therapeutic potential and safety profiles.
Suggested future research directions include exploring the synergistic effects of combining essential oils with conventional antimicrobial agents to enhance their efficacy and reduce the likelihood of resistance development. Additionally, investigating the mechanisms of action of essential oils at the molecular level could provide deeper insights into their antimicrobial properties and guide the development of more effective formulations. The potential use of essential oils in various applications, such as in wound care, sanitisers, and preservation, also warrants further exploration to maximise their benefits in both healthcare and everyday settings.
In this investigation, I explored the antimicrobial efficacy of essential oils extracted from native Australian plants compared to conventional antimicrobial agents against S. albus. The study revealed that Kunzea and Eucalyptus oils, rich in compounds like alpha-pinene and 1,8-cineole, demonstrated significant antimicrobial activity, often surpassing traditional agents like hydrogen peroxide and isopropyl alcohol. Dettol, with its active ingredient chloroxylenol, showed the highest overall effectiveness with a mean inhibition zone of 56.67 mm. Surprisingly, hydrogen peroxide exhibited no antimicrobial activity, which could be attributed to its rapid decomposition and reduced stability under experimental conditions. This unexpected result suggests the need for further investigation into the stability of hydrogen peroxide in similar environments. By applying Bonferroni corrections (alpha = 0.00192), I ensured that the statistical significance of my results was robust and reliable. This research underscores the potential of essential oils, particularly Kunzea and Eucalyptus, as natural alternatives in combating bacterial infections, especially in the context of rising antibiotic resistance. Reflecting on this project, I appreciate the synergy between traditional knowledge and modern scientific approaches, and I am inspired to replicate the experiment with a broader range of bacterial strains and essential oil variations to validate the findings and address any limitations.
My heartfelt thanks and deep appreciation to Ms Kathy Haigh for her unwavering mentorship and for inspiring me to pursue scientific research and this study. Your profound insights, prompt and thoughtprovoking feedback, and encouragement were instrumental in shaping the direction of this study.
Dr Matthew Hill’s technical expertise and Mr Tim Binet’s knowledge in the Biology were crucial in helping me make sense of this experiment, I am immensely grateful. Your prompt feedback and attention to detail in the data analysis reassured my confidence.
Thank you also to Dr Vera Munro-Smith for organising and supervising the experiment. Without your efforts, this research would not have been possible.
Astani, A., Reichling, J. and Schnitzler, P. (2009). Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytotherapy Research, pp.673–679. doi:https://doi.org/10.1002/ptr.2955.
Bachir, R.G. and Benali, M. (2012). Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pacific Journal of Tropical Biomedicine, [online] 2(9), pp.739–742. doi:https://doi.org/10.1016/s22211691(12)60220-2.
Carson, C.F., Hammer, K.A. and Riley, T.V. (2006). Melaleuca alternifolia (Tea Tree) Oil: a Review of Antimicrobial and Other Medicinal Properties. Clinical Microbiology Reviews, [online] 19(1), pp.50–62. doi:https://doi.org/10.1128/cmr.19.1.50-62.2006.
Chen, C.-C., Yan, S.-H., Yen, M.-Y., Wu, P.-F., Liao, W.T., Huang, T.-S., Wen, Z.-H. and David Wang, H.-M. (2016). Investigations of kanuka and manuka essential oils for in vitro treatment of disease and cellular inflammation caused by infectious microorganisms. Journal of Microbiology, Immunology and Infection, [online] 49(1), pp.104–111. doi:https://doi.org/10.1016/j.jmii.2013.12.009. Coombs, G.W., Daley, D.A., Yee, N.W., Shoby, P. and Mowlaboccus, S. (2022). Australian Group on Antimicrobial Resistance (AGAR) Australian Staphylococcus aureus Sepsis Outcome Programme (ASSOP) Annual Report 2020. Communicable Diseases Intelligence, 46. doi:https://doi.org/10.33321/cdi.2022.46.18.
Cox, S., Mann, C., Markham, J., Gustafson, J., Warmington, J. and Wyllie, S. (2001). Determining the Antimicrobial Actions of Tea Tree Oil. Molecules, 6(12), pp.87–91. doi:https://doi.org/10.3390/60100087.
Elangovan, S. and Mudgil, P. (2023). Antibacterial Properties of Eucalyptus globulus Essential Oil against MRSA: A Systematic Review. Antibiotics, 12(3), p.474. doi:https://doi.org/10.3390/antibiotics12030474.
McDonnell and Russell. (1999). Antiseptics and Disinfectants: Activity, Action, and Resistance. [online] Clinical microbiology reviews. Available at: https://pubmed.ncbi.nlm.nih.gov/9880479/ [Accessed 1 Jun. 2024].
Iseppi, R., Mariani, M., Condò, C., Sabia, C. and Messi, P. (2021). Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics, 10(4), p.417. doi:https://doi.org/10.3390/antibiotics10040417.
Lee, E. and Anjum, F. (2022). Staphylococcus epidermidis infection. [online] PubMed. Available at: https://www.ncbi.nlm.nih.gov/books/NBK563240/ [Accessed 21 May 2024].
Man, A., Santacroce, L., Jacob, R., Mare, A. and Man, L. (2019). Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens, [online] 8(1), p.15. doi:https://doi.org/10.3390/pathogens8010015.
Mani, J.S., Johnson, J.B., Hosking, H., Ashwath, N., Walsh, K.B., Neilsen, P.M., Broszczak, D.A. and Naiker, M. (2021). Antioxidative and therapeutic potential of selected Australian plants: A review. Journal of Ethnopharmacology, 268, p.113580. doi:https://doi.org/10.1016/j.jep.2020.113580.
Moo, C.-L., Osman, M.A., Yang, S.-K., Yap, W.-S., Ismail, S., Lim, S.-H.-E., Chong, C.-M. and Lai, K.-S. (2021). Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Scientific Reports, [online] 11(1), p.20824. doi:https://doi.org/10.1038/s41598-021-00249-y.
Otto, M. (2009). Staphylococcus epidermidis the ‘accidental’ pathogen. Nature Reviews Microbiology, [online] 7(8), pp.555–567. doi:https://doi.org/10.1038/nrmicro2182.
Pankey, G.A. and Sabath, L.D. (2004). Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram‐Positive Bacterial Infections. Clinical Infectious Diseases, [online] 38(6), pp.864–870. doi:https://doi.org/10.1086/381972.
Sadgrove, N.J. (2022). Purely Australian Essential Oils Past and Present: Chemical Diversity, Authenticity, Bioactivity, and Commercial Value. Diversity, 14(2), p.124. doi:https://doi.org/10.3390/d14020124.
Sadgrove, N.J. and Jones, G.L. (2013). A possible role of partially pyrolysed essential oils in Australian Aboriginal traditional ceremonial and medicinal smoking applications of Eremophila longifolia (R. Br.) F. Muell (Scrophulariaceae). Journal of Ethnopharmacology, 147(3), pp.638–644. doi:https://doi.org/10.1016/j.jep.2013.03.060
Sadlon. and Lamson. (2010). Immune-modifying and Antimicrobial Effects of Eucalyptus Oil and Simple Inhalation Devices. [online] Alternative medicine review : a journal of clinical therapeutic. Available at: https://pubmed.ncbi.nlm.nih.gov/20359267/ [Accessed 6 Jun. 2024].
Sakkas, H., Economou, V., Gousia, P., Bozidis, P., Sakkas, V., Petsios, S., Mpekoulis, G., Ilia, A. and Papadopoulou, C. (2018). Antibacterial Efficacy of Commercially Available Essential Oils Tested Against Drug-Resistant Gram-Positive Pathogens. Applied Sciences, 8(11), p.2201. doi:https://doi.org/10.3390/app8112201.
Seididamyeh, M., Phan, A.D.T., Sivakumar, D., Netzel, M.E., Mereddy, R. and Sultanbawa, Y. (2023). Valorisation of Three Underutilised Native Australian Plants: Phenolic and Organic Acid Profiles and In Vitro Antimicrobial Activity. Foods, 12(3), p.623. doi:https://doi.org/10.3390/foods12030623.
Thielmann, J., Muranyi, P. and Kazman, P. (2019). Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon, [online] 5(6), p.e01860. doi:https://doi.org/10.1016/j.heliyon.2019.e01860.
Tuon, F.F., Suss, P.H., Telles, J.P., Dantas, L.R., Borges, N.H. and Ribeiro, V.S.T. (2023). Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics, 12(1), p.87. doi:https://doi.org/10.3390/antibiotics12010087.
Van Vuuren, S.F., Docrat, Y., Kamatou, G.P.P. and Viljoen, A.M. (2014). Essential oil composition and antimicrobial interactions of understudied tea tree species. South African Journal of Botany, 92(92), pp.7–14. doi:https://doi.org/10.1016/j.sajb.2014.01.005.
Warnke, P.H., Becker, S.T., Podschun, R., Sivananthan, S., Springer, I.N., Russo, P.A.J., Wiltfang, J., Fickenscher, H. and Sherry, E. (2009). The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. Journal of Cranio-Maxillofacial Surgery, 37(7), pp.392–397. doi:https://doi.org/10.1016/j.jcms.2009.03.017.
Wińska, K., Mączka, W., Łyczko, J., Grabarczyk, M., Czubaszek, A. and Szumny, A. (2019). Essential Oils as Antimicrobial Agents Myth or Real Alternative? Molecules, 24(11), p.2130. doi:https://doi.org/10.3390/molecules24112130.
World Health Organization (2023). Antimicrobial Resistance. [online] World Health Organization. Available at: https://www.who.int/news-room/factsheets/detail/antimicrobial-resistance [Accessed 7 Jun. 2024].
Yap, P.S.X., Yiap, B.C., Ping, H.C. and Lim, S.H.E. (2014). Essential Oils, a New Horizon in Combating Bacterial Antibiotic Resistance. The Open Microbiology Journal, [online] 8(1), pp.6–14. doi:https://doi.org/10.2174/1874285801408010006.
Yuan, H., Ma, Q., Ye, L. and Piao, G. (2016). The Traditional Medicine and Modern Medicine from Natural Products. Molecules, [online] 21(5), p.559. doi:https://doi.org/10.3390/molecules21050559.
Courtney Stewart Barker College
Aquatic macroinvertebrates inhabit wetlands, streams, and creeks are essential to nutrient cycles and energy flow within aquatic ecosystems. This study investigates the diversity and density of aquatic macroinvertebrates in rural Vs urban streams. Sampling was conducted in the Cox River, fish river and Duckmaloi River (rural streams) and the coups creek, Waitara creek and Hornsby quarry in NSW Australia. The results of the testing indicated significantly higher diversity and density of aquatic macroinvertebrates in rural streams compared to urban streams. Rural streams exhibited more species-sensitive indicators such as mayfly and stonefly nymphs, while urban streams showed lower diversity and higher presence of pollution-tolerant species like segmented worms and roundworms. These findings underscore the detrimental effects of urbanization on aquatic ecosystems and highlight the importance of conservation efforts to preserve water quality and biodiversity.
Aquatic macroinvertebrates, organisms that inhabit wetlands, streams, and creeks, are integral to aquatic ecosystems. They contribute significantly to nutrient cycling and energy flow, acting as secondary producers and a crucial food source for fish. Their presence and diversity serve as indicators of environmental health, providing measurable responses to factors like temperature, pH, dissolved oxygen, and pollution. This literature review investigates the density and diversity of aquatic macroinvertebrates in rural versus urban areas, emphasizing their role as bioindicators and the impact of environmental stressors on their populations.
Aquatic macroinvertebrates are sensitive to various environmental parameters, including temperature, pH, dissolved oxygen, and pollution levels. Studies have shown that urbanization significantly impacts these organisms due to increased pollution from roads, buildings, and construction sites (Walsh et al., 2005).
Agricultural activities in rural areas, such as vegetation removal, contribute to erosion and runoff, introducing sediments and nutrients that adversely affect macroinvertebrate communities (Allan, 2004).
Seasonal variations also influence the availability of organic matter, affecting the spatial and temporal distribution of macroinvertebrates (Hynes, 1970). Flooding, for instance, facilitates nutrient and organism transfer, promoting biodiversity in these habitats (Junk et al., 1989). Additionally, changes in land use patterns, such as deforestation and urban sprawl, can lead to altered water flow regimes and
increased sedimentation, further impacting macroinvertebrate populations (Poff et al., 1997).
The presence, diversity and abundance of freshwater Macroinvertebrates are widely used as bioindicators due to their sensitivity to environmental changes and their diverse responses to pollution (Rosenberg & Resh, 1993). The presence of certain species indicates healthy water systems, while others signal degradation. For example, the presence of mayfly nymphs, which are sensitive to pollution, often signifies good water quality (Barbour et al., 1999). Conversely, black fly larvae, which thrive in polluted waters, can indicate poor water conditions and pose health risks to livestock (Adler et al., 2004). Stonefly nymphs, another group of sensitive species, are often found in clean, well-oxygenated streams, serving as indicators of high-water quality (Stewart & Stark, 2002).
Land use changes in urban areas affect macroinvertebrate diversity and density. Urban environments typically exhibit lower macroinvertebrate diversity due to pollution and habitat modification and homogenisation. For example, studies in the Hornsby area, a suburban region of Sydney, Australia, reveal the negative impact of urban runoff on aquatic habitats (Walsh et al., 2005).
Rural areas, such as Oberon, dominated by sheep and cattle farming, face challenges from agricultural runoff, which introduces sediments, nutrients, and pesticides into water bodies (Allan, 2004). These inputs can decrease water quality, negatively impacting macroinvertebrate populations. However,
rural streams might retain more natural vegetation and experience less severe chemical pollution compared to urban streams (Hagen et al., 2006). The presence of buffer strips and riparian zones in agricultural landscapes can mitigate some of the negative impacts by filtering runoff and providing habitat for macroinvertebrates (Naiman & Decamps, 1997).
Comparative studies highlight the differences in macroinvertebrate communities between rural and urban streams. Urban streams often exhibit lower species diversity and abundance due to higher levels of pollutants and habitat disturbance (Walsh et al., 2005). In contrast, rural streams, while affected by agricultural runoff, might support more diverse macroinvertebrate populations due to lower levels of urban pollutants and better habitat conditions (Allan, 2004). For instance, a study comparing urban and rural streams in the northeastern United States found that rural streams had significantly higher macroinvertebrate diversity and abundance, attributed to lower levels of impervious surface cover and betterpreserved riparian habitats (Morse et al., 2003).
Hornsby, a suburban area in Sydney, Australia, is subject to frequent monitoring by environmental groups and councils. These studies often report reduced macroinvertebrate diversity in urban creeks due to pollution and habitat alteration (Walsh et al., 2005). Efforts by local councils and environmental organizations aim to improve water quality through stormwater management and habitat restoration projects. Oberon, a rural area with extensive agricultural activities, faces different challenges, primarily from sediment and nutrient runoff. Despite these challenges, rural streams in Oberon might support higher macroinvertebrate diversity due to less chemical pollution and better-preserved riparian zones (Hagen et al., 2006). The implementation of sustainable agricultural practices and the maintenance of natural vegetation buffers are crucial for preserving aquatic biodiversity in these areas.
The density and diversity of aquatic macroinvertebrates vary significantly between rural and urban areas due to differing environmental stressors. Urban streams suffer from pollution and habitat modification, leading to lower macroinvertebrate diversity and abundance. Rural streams, while impacted by agricultural runoff, might retain higher biodiversity. Understanding these differences is crucial for water quality management and conservation efforts, particularly in agricultural and urban planning. Future research should focus on long-term monitoring and the effectiveness of
mitigation strategies in both rural and urban contexts to enhance the health of aquatic ecosystems.
To investigate the diversity and density of aquatic macroinvertebrates in rural areas compared to urban areas.
The diversity and density of aquatic macroinvertebrates will be higher in rural areas compared to urban areas.
1 Prepare Collection Containers: Fill a plastic tray with water from the creek and set it aside. Similarly, fill an ice cube tray with water from the creek and place it next to the plastic tray.
2 Measure Creek Temperature: Immerse a glass thermometer into the creek for 30 seconds. Remove the thermometer and record the water temperature.
3 Determine pH Level:
1. Use the pH testing kit: fill the plastic tube's pH-labelled side with creek water up to the indicated line.
2. Add 5 drops of universal indicator into the water, seal the tube with the red cap, and shake well to mix.
3. Hold the tube up to the light and match the water colour to the pH reader scale. Record the pH level. Dispose of the used water and rinse the tube several times.
4 Assess Salinity:
1. Use a refractometer: place 1-2 drops of creek water onto the tip using a pipette.
2. Close the plastic flap and hold the refractometer up to the light to read the salinity. Record the results and clean the refractometer tip with a cloth.
5 Collect Macroinvertebrates:
1. Position a net in the creek where water flows over small to medium-sized rocks. Place the net downstream of the rocks.
2. Agitate the rocks with your hand to disturb the creek bed, allowing silt, particles, and small rocks to flow into the net. Continue for 30 seconds to 1 minute.
3. Lift the net, ensuring the contents remain inside, and transfer them into the tray of creek
water. Empty the net contents into the tray and set the net aside.
6 Sort and Document Macroinvertebrates:
1. Use forceps to remove macroinvertebrates from the tray water and place them into the ice cube tray, sorting by species, with each section containing one species.
2. Photograph each section of the ice cube tray to document the species present.
Results
Table 1: Macroinvertebrates in various locations
1
3. Record the number of individuals for each species.
7 Return Specimens: After documentation, release the contents of both the ice cube tray and plastic tray back into the creek, ensuring all aquatic macroinvertebrates are returned to their habitat.
Location Cox River Oberon Fish River Oberon Duckmaloi River Oberon Cup’s Creek Normanhurst Waitara Creek Hornsby Quarry Creek
of
Species 1 Mayfly nymph Blackfly larva Non-biting midge larva Whirligig beetle larva Unknown -
Species 2 Non-biting midge larva Whirligig beetle larva Mayfly nymph Round worm Dragonfly nymph -
Species 3 Stonefly nymph Non-biting midge larva CaddisflySegmented worm -
Species 4 Giant water bug Round worm Unknown - Round worm -
Species 5 Pygmy back swimmerBiting midge larva -
Species 6 Round wormSegmented worm -
Species 7 -Whirligig beetle larva
Table 2: Count of species in each location
Discussion
The primary objective of this study was to investigate the diversity and density of aquatic macroinvertebrates in rural versus urban streams. The hypothesis was that rural streams would exhibit higher diversity and density of aquatic macroinvertebrates compared to urban streams. The results of this study supported the hypothesis, revealing significantly greater diversity and density of aquatic macroinvertebrates in rural streams. Only one sample
was taken per stream, so results are specific to one area of the stream.
One limitation of this study is the reliance on a single sample per stream, which may not accurately represent the entire stream ecosystem. Streams are dynamic environments with varying microhabitats, such as riffles, pools, and runs, each potentially hosting different communities of macroinvertebrates. Sampling from only one location can lead to an incomplete understanding of the overall diversity and density within the stream. To obtain a more comprehensive picture, multiple samples from different locations and habitats within each stream should be collected. This approach would account for spatial variability and provide a more accurate assessment of the macroinvertebrate communities present in rural and urban streams.
The results from the Cox River, Fish River, and Duckmaloi River in Oberon (rural streams) showed a significantly higher total count and variety of aquatic macroinvertebrates compared to Coup’s creek, Waitara creek and the Hornsby quarry. (urban streams). The rural streams were flowing faster than the urban streams, while both had recent rainfall the rural streams had more movement. While the urban streams where in park like areas and small spaces between houses, the rural streams tested were in paddocks and open spaces.
Specifically, the rural streams had a total of 28, 4, and 31 macroinvertebrates respectively, with multiple species identified including sensitive indicators like mayfly and stonefly nymphs. Conversely, urban streams had much lower counts with only 9, 10, and 0 macroinvertebrates respectively, and included species often associated with poorer water quality, such as segmented worms and roundworms. While one of the rural samples is quite low, when averaging it out there is more abundance in the rural area.
Water temperatures and pH levels can greatly influence the diversity and density of aquatic macroinvertebrates. In this investigation, rural streams exhibited a broader temperature range (19-24 degrees) compared to urban streams (20-21 degrees) with slightly alkaline pH levels (7.7-8.4) in both areas. Higher temperature variability in rural streams likely supports a wider range of species, including sensitive ones like mayfly and stonefly nymphs. Urban streams, despite having similar pH levels, face additional stressors such as pollution and habitat modification, which can disrupt macroinvertebrate communities. The combined stability of temperature and pH in rural
streams, along with less severe pollution, contributes to higher biodiversity.
This difference in macroinvertebrate diversity and density between rural and urban streams is significant as it highlights the impact of human activities on aquatic ecosystems. Rural streams, which are often surrounded by less disturbed natural environments, provide a more stable and suitable habitat for a wider range of macroinvertebrates. On the other hand, urban streams are typically subject to higher levels of pollution, habitat modification, and other stressors that reduce their ability to support diverse and abundant macroinvertebrate communities.
Urban streams like the ones that were tested in the investigation, are more susceptible to various pollutants from road runoff, industrial discharges, and residential areas. Pollutants such as heavy metals, oils, and chemicals from urban runoff can significantly degrade water quality, making it less hospitable for many macroinvertebrate species. Rural streams, while impacted by agricultural runoff, typically face less severe chemical pollution compared to urban streams. Studies have shown that urban runoff often contains a complex mixture of contaminants, including nutrients, heavy metals, pesticides, and organic pollutants, all of which can have deleterious effects on aquatic life (Walsh et al., 2005).
The physical habitat in rural streams is often less altered by human activities compared to urban streams. While they are still altered for purposes such as farming, but they have been changed less than ones in urban areas. These ones have been altered to make way for housing and building structures. Urban streams may suffer from channelization, increased sedimentation, and reduced riparian vegetation, which degrade the habitat quality. Rural streams, especially those with preserved riparian buffers, provide a more diverse and stable habitat, supporting a wider range of macroinvertebrate species. The presence of natural vegetation along stream banks helps to stabilize the soil, reduce erosion, and provide organic matter and shade, which are crucial for maintaining suitable conditions for macroinvertebrates (Allan, 2004).
The rural streams had varying temperatures and pH levels, which can support different types of macroinvertebrates. Although urban and rural streams showed similar pH levels in this study, the overall water quality and habitat structure likely play a more significant role in supporting diverse macroinvertebrate communities in rural areas. Urban streams often experience higher temperatures due to reduced shading and increased surface runoff, which
can stress aquatic organisms and alter community composition (Paul & Meyer, 2001).
While both urban and rural streams can be impacted by nutrient inputs, the sources and types of nutrients can differ. Urban streams are often subject to nutrient pollution from fertilizers, pet waste, and sewage overflows, leading to eutrophication and decreased oxygen levels. Rural streams, although affected by agricultural runoff, may benefit from lower overall nutrient loads and better buffering by riparian vegetation, which helps to maintain water quality (Naiman & Decamps, 1997).
Only six samples were taken, which might not fully represent the variability within each stream or between different streams. Increasing the number of sampling sites and replicates would provide a more comprehensive understanding of macroinvertebrate diversity and density.
The investigation was conducted over a few days in January, which might not capture seasonal variations in macroinvertebrate populations. Macroinvertebrate communities can vary significantly with changes in season, temperature, and flow conditions. Long-term monitoring would help to capture these temporal dynamics and provide a more accurate assessment of stream health.
Some macroinvertebrates could not be identified to the species level, which might have affected the accuracy of the diversity assessment. Advanced identification techniques, such as genetic barcoding, could improve species-level identification and enhance the accuracy of biodiversity assessments.
While temperature and pH were measured, other important parameters such as dissolved oxygen, turbidity, and nutrient levels were not assessed. Including a broader range of water quality parameters would provide a more comprehensive picture of the environmental conditions affecting macroinvertebrate communities.
The findings of this study align with previous research indicating that urban streams generally have lower macroinvertebrate diversity and density compared to rural streams. Studies by Walsh et al. (2005) and Allan (2004) highlight the adverse impacts of urbanization and agricultural activities on aquatic ecosystems. However, rural streams often retain more natural vegetation and have less severe chemical pollution, which supports higher biodiversity.
Research by Walsh et al. (2005) demonstrated that urban streams often suffer from increased pollutant loads, altered flow regimes, and habitat fragmentation, all of which contribute to reduced macroinvertebrate diversity. Similarly, Allan (2004) found that rural streams, although impacted by agricultural activities, tend to have better-preserved riparian zones and lower levels of chemical pollution, supporting more diverse and resilient macroinvertebrate communities.
In contrast, studies in highly urbanized areas, such as those by Paul and Meyer (2001), have shown that urban streams typically exhibit reduced macroinvertebrate diversity due to the combined effects of habitat alteration, pollution, and altered hydrology. These findings underscore the importance of preserving natural habitats and implementing effective pollution control measures to protect aquatic ecosystems.
The results underscore the importance of preserving natural habitats and implementing effective pollution control measures in urban areas to protect aquatic ecosystems. Future research should focus on longterm monitoring to better understand the impacts of seasonal changes and human activities on macroinvertebrate communities. Additionally, investigating the effectiveness of riparian buffer zones and sustainable agricultural practices in mitigating negative impacts on rural streams can provide valuable insights for conservation efforts.
Efforts to conserve and restore riparian buffers in both rural and urban areas can help to mitigate the impacts of pollution and habitat alteration. Riparian buffers play a crucial role in filtering runoff, reducing erosion, and providing habitat for aquatic and terrestrial species.
Implementing stricter pollution control measures in urban areas, such as green infrastructure and stormwater management practices, can help to reduce the influx of contaminants into urban streams. Green infrastructure, including rain gardens, permeable pavements, and constructed wetlands, can help to capture and treat stormwater, reducing pollutant loads and improving water quality.
Promoting sustainable agricultural practices, such as reduced pesticide use, cover cropping, and conservation tillage, can help to minimize the impacts of agricultural runoff on rural streams. The use of vegetative buffer strips and riparian zones can also help to filter out sediments and nutrients before they enter water bodies.
Increasing public awareness about the importance of healthy aquatic ecosystems and engaging communities in conservation efforts can foster greater support for environmental protection measures. Community-based monitoring programs can also provide valuable data and promote stewardship of local water resources.
In conclusion, the study provides evidence that rural streams have higher diversity and density of aquatic macroinvertebrates compared to urban streams, supporting the hypothesis. The findings highlight the significant impact of urbanization on aquatic ecosystems and the need for targeted conservation and management strategies to preserve macroinvertebrate diversity and overall water quality. Addressing the challenges posed by pollution, habitat alteration, and changing land use patterns is crucial for maintaining the health and biodiversity of freshwater ecosystems. Future research should build on these findings by exploring long-term trends and evaluating the effectiveness of various conservation and management interventions in both rural and urban contexts.
Conclusion
The study confirms that rural streams have higher diversity and density of aquatic macroinvertebrates compared to urban streams, supporting the hypothesis. Rural streams, characterized by less disturbed natural environments provide more stable habitats for a wider range of species. Conversely, urban streams suffer from higher levels of pollution and habitat modification, resulting in reduced macroinvertebrate diversity and abundance. These differences emphasize the impact of human activities on aquatic ecosystems. Effective conservation and management strategies, such as implementing sustainable agricultural practices, are crucial for maintaining the health and biodiversity of freshwater ecosystems. Future research should focus on long-term monitoring and the evaluation of mitigation strategies to enhance aquatic ecosystem health in both rural and urban contexts.
I would like to express my gratitude to Dr Vera Munro-Smith faith in me while I worked on this project. As well as her patience and helpful advice throughout the whole process.
References
Adler, P.H., Currie, D.C. & Wood, D.M., 2004. The Black Flies (Simuliidae) of North America. Ithaca, NY: Cornell University Press.
Allan, J.D., 2004. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annual Review of Ecology, Evolution, and Systematics, 35(1), pp.257-284
Barbour, M.T., Gerritsen, J., Snyder, B.D. & Stribling, J.B., 1999. Rapid bioassessment protocols for use in streams and wadeable rivers: Periphyton, benthic macroinvertebrates and fish. 2nd ed. Washington, D.C.: U.S. Environmental Protection Agency, Office of Water
Bonacina, L., Fasano, F., Mezzanotte, V. and Fornaroli, R. (2022). Effects of water temperature on freshwater macroinvertebrates: a systematic review. Biological Reviews, 98(1).
Booth, D.B. & Jackson, C.R., 1997. Urbanization of aquatic systems: Degradation thresholds, stormwater detection, and the limits of mitigation. Journal of the American Water Resources Association, 33(5), pp.1077-1090.
Clarke, A., Mac Nally, R., Bond, N. and Lake, P.S. (2008). Macroinvertebrate diversity in headwater streams: a review. Freshwater Biology, 53(9), pp.1707–1721.
Gerami, M.H., Patimar, R., Negarestan, H., Jafarian, H. and Mortazavi, M.S. (1970). Temporal variability in macroinvertebrates diversity patterns and their relation with environmental factors. Biodiversitas Journal of Biological Diversity, 17(1).
Hagen, E.M., Webster, J.R. & Benfield, E.F., 2006. Are leaf breakdown rates a useful measure of stream integrity along an agricultural landuse gradient? Journal of the North American Benthological Society, 25(2), pp.330-343
Hepp, L.U., Milesi, S.V., Biasi, C. and Restello, R.M. (2010). Effects of agricultural and urban impacts on macroinvertebrates assemblages in streams (Rio Grande do Sul, Brazil). Zoologia (Curitiba), 27(1), pp.106–113.
Hynes, H.B.N., 1970. The Ecology of Running Waters. Toronto: University of Toronto Press.
Junk, W.J., Bayley, P.B. & Sparks, R.E., 1989. The flood pulse concept in river-floodplain systems. In: D.P. Dodge, ed. Proceedings of the International Large River Symposium. Ottawa: Canadian Special Publication of Fisheries and Aquatic Sciences, pp.110-127.
Lundquist, M.J. and Zhu, W. (2019). Aquatic insect diversity in streams across a rural–urban land-use discontinuum. Hydrobiologia, 837(1), pp.15–30.
Moreyra, A.K. and Padovesi-Fonseca, C. (2015). Environmental effects and urban impacts on aquatic macroinvertebrates in a stream of central Brazilian Cerrado. Sustainable Water Resources Management, 1(2), pp.125–136.
Morse, C.C., Huryn, A.D. & Cronan, C., 2003. Impervious surface area as a predictor of the effects of urbanization on stream insect communities in Maine, USA. Environmental Monitoring and Assessment, 89(1), pp.95-127.
Naiman, R.J. & Decamps, H., 1997. The ecology of interfaces: Riparian zones. Annual Review of Ecology and Systematics, 28, pp.621-658.
Paul, M.J. & Meyer, J.L., 2001. Streams in the urban landscape. Annual Review of Ecology and Systematics, 32, pp.333-365.
Poff, N.L., Allan, J.D., Bain, M.B., Karr, J.R., Prestegaard, K.L., Richter, B.D., Sparks, R.E. & Stromberg, J.C., 1997. The natural flow regime. BioScience, 47(11), pp.769-784.
Rosenberg, D.M. & Resh, V.H., 1993. Freshwater Biomonitoring and Benthic Macroinvertebrates. New York: Chapman & Hall.
Schummer, M.L., Eason, K.M., Hodges, T.J., Farley, E.B., Sime, K.R., Coluccy, J.M. and Tozer, D.C. (2021). Response of aquatic macroinvertebrate density and diversity to wetland management and structure in the Montezuma Wetlands Complex, New York. Journal of Great Lakes Research, [online] 47(3), pp.875–883.
Stewart, K.W. & Stark, B.P., 2002. Nymphs of North American stonefly genera (Plecoptera). 2nd ed. Columbus, OH: The Caddis Press.
Vehkaoja, M., Niemi, M. and Väänänen, V.-M. (2020). Effects of urban infrastructure on aquatic invertebrate diversity. Urban Ecosystems
Walsh, C.J., Roy, A.H., Feminella, J.W., Cottingham, P.D., Groffman, P.M. & Morgan, R.P., 2005. The urban stream syndrome: Current knowledge and the search for a cure. Journal of the North American Benthological Society, 24(3), pp.706-723.
Appendix 1: Photos of macroinvertebrate species
Species Photo Non-biting midge larva

Stonefly nymph

Giant water bug

Pygmy back swimmer

Round worm

Blackfly larva

Whirligig beetle larva

Mayfly nymph

Caddisfly

Unknown

Biting midge larva

Segmented worm

Unknown

Dragonfly nymph

Eden Cowdery Barker College
Microplastics are fragments of any type of plastic between 1 μm and 5 mm in length. They arise from either the fragmentation of larger plastics or intentionally manufactured microbeads, such as those present in some facial cleansers. They can absorb organi c pollutants and are carriers of organic contaminants into the bodies of aquatic animals, then these aquatic animals act as a pathway for microplastics to enter the human food chain as they act as seafood. This report explores the distribution of microplastics across four beaches along Sydney’s Northern beaches, Cabbage Tree Beach (Manly Cove), Manly Beach, Mona Vale Beach and Whale Beach. 48 samples were collected in total, 4 at each beach in the northern, southern and central areas. Microplastics were sep arated from sand with density separation, followed by sieving, and then viewed under a stereo microscope to determine the number of microplastics present. Macroplastics are plastics greater than 5mm and were also recorded in this experiment. The results re vealed that Manly Beach had the highest number of microplastics present in its sediment, followed by Manly Cove, Whale Beach then Mona Vale Beach. Whale Beach had no macroplastics present whereas, Manly Beach had the largest number of macroplastics present
80 % of all marine pollution is attributed to plastics (Fava, 2022), of which the most common items found are plastic bags, plastic bottles, wrappers, synthetic rope, fishing items, plastic caps/lids and industrial packaging (Morales -Caselles et al., 2021) Microplastics are fragments of any type of plastic between 1 μm and 5 mm in length (Gola et al., 2021), and may be found as fibres, fragments, spheroids, beads, granules, pellets or flakes. Primary microplastics are intentionally manufactured microbeads of different sizes whereas, secondary microplastics arise from the fragmentation of larger plastic objects by mechanical degradation, biodegradation or photodegradation (Mercogliano et al., 2020). Exposure to UV radiation is the most impactful method of fragmentation, resulting in rapid environmental degradation of polymers (Hale et al., 2020; Andrady, 2017). Additionally, microplastics can absorb organic pollutants and are carriers of organic contaminants into terrestrial, marine and freshwater environments (Mamun et al., 2022).
Scientists reported the presence of small plastic particles in the ocean as early as the 1970s, however, research into their distribution and impacts didn’t commence until 2004, with a pioneering study led by marine ecologist Richard Thompson (Rochman, 2018).
The abundance of microplastics increases as fragment size decreases, as does the number of organisms
capable of ingesting them (Hale et al., 2020). Microplastic particles <20 µm can penetrate organs and microplastics <10 µm can penetrate cell membranes (Kannan & Vimalkumar, 2021) The rate of polymer biodegradation also increases as particle size decreases and surface area increases (Hale et al., 2020).
Plastics are composites of long-chain organic polymers. Polymer chains are produced by combining chemical monomers, often derived from fossil fuels, into strands of repeating units (Hale et al., 2020). Thermoplastic polymers, any plastic polymer material that becomes mouldable at an elevated temperature and solidified upon cooling, can be separated into two categories, amorphous or semi-crystalline. Highdensity polyethylene (HDPE) and polypropylene (PP) are popular thermoplastics used in the packaging industry and are classified as semi-crystalline. Polystyrene (PS) and acrylonitrile butadiene styrene (ABS) are classified as amorphous. The arrangement of the molecular chains separates these two classes and how the polymer behaves when heated. A semicrystalline polymer exhibits organised and tightly packed molecular chains (Impact Plastics, 2017). They are used in many different industries and applications as they tend to have high -temperature resistance, can act as dielectrics, and have high durability and a low friction coefficient. Each specific polymer has variations to its uses however, they are generally used for moving and sliding parts like rollers
and rails, as well as durable housings for consumer goods (Team Xometry, 2023). Whereas amorphous plastics have more disorganised polymer chains. The molecules are oriented randomly and are intertwined, causing them to have a range of temperatures at which they will melt. Amorphous polymers typically have better impact resistance but poor wear resistance (Impact Plastics, 2017). Amorphous polymers are used in motor vehicle interiors, keyboards and electrical outlet faceplates. Furthermore, some amorphous polymers are transparent causing them to be used in glass-like applications, particularly those in which resistance to breakage is a priority, such as safety equipment or medical applications (Team Xometry, 2023).
The dramatic increase in plastic production and use, and insufficient waste management have led to its accumulation in the marine environment. Global plastic production increased to 390.7 million tonnes in 2021, from 365.5 million tonnes in 2018 (Plastics Europe, 2022), and plastic production and consumption are predicted to double over the next 10 years (Ocean Conservancy, 2017). 76 % of these manufactured plastics end up as plastic waste, and 72 % of this is emitted into the environment (Dimassi et al., 2022), amounting to approximately 12.2 million tonnes of plastic ending up in the ocean every year (Surfers Against Sewage, 2023). 94 % of the plastic that enters the ocean, ends up on the ocean floor, it is estimated that there is on average 70 kg of plast ic in each square kilometre of seabed (Eunomia, 2016). The Planetary Boundary Threat Framework sets a limit for a global ‘safe operating space’ for humanity (Villarrubia-Gomez, Cornell & Fabres, 2018). It defines precautionary boundaries for several categories, including ocean acidification, climate change, biosphere integrity, etc. They are set at levels to avoid thresholds or shifts in Earth-system functioning that would generate rising risks for the world’s societies. Marine plastic pollution is not considered a planetary boundary threat, however, the irreversibility and global omnipresence of plastic pollution in marine environments means that two of the three essential conditions for a planetary boundary threat are already met (Jahnke et al., 2017).
Microplastics are not only affecting the marine environment, but they are directly affecting marine life. Marine environments closer to urban areas have higher levels of microplastics, and aquatic animals from these areas have shown high numbers of microplastics present in their tissue (Gola et al., 2021). Other water pollutants, such as dyes, heavy metals and other chemicals can easily attach to microplastics, causing them to act as carriers into the bodies of
aquatic animals and these organisms provide an easy pathway for microplastics to enter the human body as these contaminated living organisms act as seafood (Gola et al., 2021). A European study showed that a consumer can ingest up to 11,000 microplastics per year based on shellfish consumption alone (Van Cauwenberghe & Janssen, 2014). One in three fish caught for human consumption contain plastic (Surfers Against Sewage, 2023). Microplastics can be ingested by marine organisms and transferred from one tropic level to the next (Mercogliano et al., 2020). The rate of polymer biodegradation also increases as particle size decreases and surface area increases (Hale et al., 2020). Microplastics were believed to simply pass through the gastrointestinal tract of animals and humans with no effect. However, stud ies have shown that they are sometimes taken up and distributed throughout the circulatory and lymphatic systems and may be stored in the fatty tissues of different organisms. This has shown potential carcinogenic effects, liver dysfunction and endocrine disruption (Lehel & Murphy, 2021). Plastic’s resistance to degradation has caused microplastics to become part of the human food chain. Microplastic exposure to humans is caused by foods of both animal and plant origin. Living organisms can accumulate microplastics in cells and tissues, resulting in threats of chronic biological effects and potential health hazards for humans, including body gastrointestinal disorders, immunity, respiratory problems, cancer, infertility and chromosome alterations (Mamun et a l., 2022). 44 % of all seabird species are known to ingest plastic and 267 species of marine organisms worldwide are known to have been affected by plastic debris (Moore, 2008). Furthermore, it is estimated that by 2050, the amount of plastic in the ocean will outweigh all fish in the sea (Fava, 2022).
How does the distribution of microplastics vary across beaches along Sydney’s Northern Beaches?
The number of microplastics found in sediment along Sydney’s Northern Beaches will be higher at beaches in areas with a higher population density and greater levels of tourism.
48 total samples were collected from four beaches, Cabbage Tree Beach (Manly Cove), Manly Beach, Mona Vale and Whale Beach (Figure 1). 4 samples were collected at each of the Northern, Southern and Central areas of each beach (Figure 2). At each sample
location, the length of the beach from the high tide line to the back of the beach was measured and divided by four, allowing each sample to be taken an equal distance from the other (see Figure 3). The first sample was collected at the high tide line and the subsequent samples were collected equal distances apart travelling away from the high tide line. Each sample container was labelled with the beach, location, sample number, and distance from the high tide line, for example, Manly Beach Central, sample 3, +34 m.



357 grams of sodium chloride (NaCl) was combined with 1 L of water to create a saturated solution. For each sample, 50 grams of sediment was weighed out and placed into a 250 mL beaker. Approximately 50 mL of NaCl solution was poured into the beaker. The sediment was disturbed by stirring the mixture with a spatula allowing any organic and plastic material to float on top of the solution (Figure 4). The floating material and top layer of water were poured through a 5mm sieve into a 250 mL beaker, making sure to not pour through any sediment. The water and material in the beaker were then viewed under a stereo microscope and the microplastics present were identified, counted and recorded (Figure 5).


Table 1: Average number of microplastics and macroplastics found in the north, middle and south of each beach
Manly Beach exhibited the highest average number of microplastics and macroplastics in its sediment. Whereas Mona Vale Beach had the lowest average number of microplastics in its sediment, and Whale Beach had no macroplastics present. Figure 6 displays that Northern Manly Beach had a significantly higher number of microplastics compared to all other sampling locations
This experiment was conducted because of the increasing prevalence of microplastics and more recent studies revealing their potentially harmful effects on humans and animals, such as their potential carcinogenic effects, liver dysfunction and endocrine disruption (Lehel & Murphy, 2021; Wang, Zhao & Xing 2021; Mamun et al., 2022). This experiment aimed to identify the beaches in Sydney’s Northern
Beaches with the highest amount of microplastic pollution. Allowing action to be taken to minimise the pollution and therefore also minimise the risks associated with it.
All results displayed are the average number of microplastics present in a 50 g sample of sediment taken from beaches along Sydney’s Northern Beaches. Table 3 displays that Manly Beach had a significantly higher average number of microplastics in its sediment compared to all other sampled beaches, with 4.4 microplastics per 50 g. Additionally, Table 3 indicates that Manly Beach had the highest number of macroplastics, averaging 0.6 macroplastics per 50 g of sediment. As displayed in Table 6, Manly Beach has a significantly larger number of nearby shops and facilities at the North, South and Middle sample locations. Some of these shops include takeaway food restaurants such as the Salty Rooster, Kazzi Beach Greek Manly, Starbucks, Yo-Chi, Manly Seaside Kebabs, and many more. Many tourists and locals purchase food from these takeaway restaurants to consume on the beach and leave their rubbish behind. Causing significant amounts of plastics to be left in the sediment and undergo degradation and fragmentation over time to become microplastics. Furthermore, Manly Beach is a popular beach for locals and tourists to visit, causing it to receive up to 8 million visitors annually (National Environmental Protection Council, 2012).
Tables 2 and 3 indicate that Mona Vale and Whale Beach exhibited comparable amounts of microplastics in their sediment samples. This similarity might stem from having fewer nearby shops or being less

frequented beaches, resulting in fewer visitors leaving rubbish there. Furthermore, Table 5 shows that the Northern samples at all the beaches had the highest average number of microplastics, with an average of 3 microplastics per 50 g. The South followed with an average of 1.5 microplastics per 50 g, and the Middle had the lowest average of 0.8 microplastics per 50 g. This may be attributed to numerous facilities and shops in the Northern ends of Manly Cove, Manly Beach and Mona Vale or dominant weather an d swell patterns causing waterborne micro and macroplastics to wash up on the Northern ends of beaches. Table 4 indicates that sample 4 had the highest average number of microplastics in its sediment, averaging 3 microplastics per 50 g. Sample 4 was collec ted at the back of the beach, the furthest distance away from the high tide line. Displaying that the back of the beach had the largest presence of microplastics. This could be because, at Manly Cove and Manly Beach, a pathway runs along the back of the beach where many people choose to sit, often leaving their rubbish behind. Ultimately, causing a build-up of litter to materialise at the back of the beach. Or, this could be because of wind and the frictional movement of sediment, caused by people walking along beaches, producing an accumulation of sand and microplastics to form at the back of the beach.
Table 2: Number of microplastics found in each sample
Table 4: Average number of microplastics found in each sample (in terms of distance from high tide line)
Sample
Table 5: Average number of microplastics in the middle, northern and southern areas of beaches
Table 6: Summary of nearby facilities and shops in each sample location
Beach Location Nearby facilities/shops
North
Manly Cove
Public toilets, Manly Waterworks (water park), The Boathouse Kiosk, Manly Art Gallery and Museum, Manly kayak centre, public seating area
Middle Public seating area
South
Public seating area, bus stop, Manly wharf, several restaurants and fast-food stores (The Bavarian Manly Wharf, Hugo’s Manly, Betty’s Burgers, Guzman Y Gomez, Gelatissimo, Max Brenner), Public toilets
North Queenscliff Surf Life Saving Club, the Salty Rooster (Chicken Takeaway Shop), bus stop, public toilets, The Corner at Queensie Cafe
Middle
3: Average number of microplastics found at each beach and the area’s populations
Mona Vale
Whale Beach
South
Playground, North Steyne Life Saving Club, Manly Surf School, Public toilets, restaurants (Kazzi Beach Greek Manly, Tarboosh Lebanese Kitchen Manly), Bus stop
Manly Point Café, Manly Live Saving Club, Little Pearl restaurant, fast food and takeaway shops (Manly Grill, Starbucks, Gelato Messina, Manly Seaside Kebabs, Yogurtland, Yogurberry, Butterboy, Yo-Chi), Hotel Steyne, Public toilets
North Armchair Collective Cafe, The Basin Dining Room, public toilets
Middle Mona Vale Golf Club
South Bus stop, Mona Vale Hospital
North N/A
Middle Whale Beach Surf Life Saving Club
South Public toilets, BBQ’s
Finally, Table 1 and Figure 6 indicate that, overall, Northern Manly Beach had the highest number of microplastics present in its sediment, with an average of 8.8 microplastics per 50 g. This significant difference could be due to Manly Creek connecting to the Northern end of Manly Beach. Manly Creek is intermittently open and closed to the ocean and has poor water quality and high amounts of pollution. With Manly Creek connecting to the Northern end of
Manly Beach, pollution from the creek flows onto the back of the beach.
Table 3 displays the population and population density of Manly, Mona Vale and Whale Beach compared to the average number of microplastics found in their sediment. Manly has a very large population of 44,481 compared to Mona Vale’s population of 11,130 and Whale Beach’s population of 1,977. Manly has a large population density of 3,098 people/km2, compared to Mona Vale’s 2,285 people/km2 and Whale Beach’s 605 people/km2 (Northern Beaches Council, 2023). Ultimately causing larger amounts of pollution in Manly, due to a higher density of people in the area. Similarly, an Argentinian study in 2023 found that areas with the highest levels of tourism presented the largest amounts of microplastics, however, they found the occurrence of microplastics was also influenced by the proximity to the mouth of a river, littoral drift, agricultural land use and irrigation areas (Ronda et al., 2023).
It was expected that Whale Beach and Manly Cove would present the largest numbers of microplastics in their sediment due to prior experiments conducted by Australian organisations in 2022 (AUSMAP, 2022). However, this experiment found that they both had relatively low numbers of microplastics present in their samples. The AUSMAP survey conducted in April 2022, by the Australian organisation Cove Collective, found 2292 microplastics per square metre at South Whale Beach, finding it to be the most polluted beach with microplastics along all of Sydney’s Northern Beaches. However, the results of this experiment showed that South Whale Beach had an average of 1 microplastic per 50 g. Furthermore, another AUSMAP survey conducted in October 2022, by the organisation Living Ocean, found 1572 microplastics per square metre at Manly Cove. The results of this experiment found that Manly Cove had an average of 1.9 microplastics per 50 g of sediment. Both results of this experiment are significantly less than the expected values reported in AUSMAP’s 2022 surveys. Both organisations, Cove Collective and Living Ocean, implemented relatively similar sample collection techniques compared to this experiment, however, they implemented a different separation method. These organis ations used only sieving to separate microplastics from the sediment. They placed the dry samples in a sieve and used water to wash the sand through, leaving only the microplastics in the sieve (Tymoszuk, 2018).
The stereo microscope utilised in this investigation had a magnification of 100 x, allowing particles up to 2 mm to be seen in the field of view. However, this
limits the accuracy of visual sorting of microplastics as they can be any fragment of plastic between 1 μm and 5 mm in length. To improve the accuracy of microplastic identification methods that are not readily available in a school laboratory, such as Micro-Raman spectroscopy or time -of-flight secondary ion mass spectrometry, should be implemented (Dong et al. 2023). Furthermore, the sample size of this experiment should be increased to determine the reliability of the results. By increasing both the number of samples taken at each beach and the number of beaches sampled, the reliability of the investigation can be determined.
The brine solution used for density separation should be amended to improve the accuracy of results. In this experiment, microplastics were separated from sediment using density separation, with a saturated sodium chloride (NaCl) solution, however, Quinn, Murphy & Ewins’ (2016) experiment determined that microplastic recovery increased as the solution density increased. They compared sodium chloride (NaCl), sodium bromide (NaBr), sodium iodide (NaI), and zinc bromide (ZnBr2) brine solutions, discovering that NaI and ZnBr2 had significantly higher rates of microplastic recovery. The implementation of NaI or ZnBr2 brine solutions would have been more appropriate for microplastic extraction and allowed this experiment to collect more accurate results. However, ZnBr2 can cause severe skin burns and eye damage as well as long-term toxic effects on aquatic life, ultimately causing NaI to be the most suitable brine solution for this experiment. Furthermore, to improve the accuracy of results, a larger sample size should be examined. When analysing samples, only 50 g of the sediment samples were examined rather than the whole sample that was collected. Additionally, more samples should be collected at each beach to further improve the accuracy and determine the reliability of the results.
Filtration is another popular and simple method of separation of microplastics from sediment. It involves separating solids based on their size, where the size of the filter paper used determines the size of the solid that is separated (Tirkey & Upadhyay, 2021). This method is similar to sieving as microplastics are separated from sediment based on their size. However, this method is not entirely accurate as the solution may be contaminated with airborne fibres (Nava & Leoni, 2021).
Another approach to the identification and quantification of microplastics involves Fouriertransform infrared (FTIR) spectroscopy (Peez, Janiska & Imhof, 2018). Following the density separation
method for the extraction of microplastics from sediments, visual counting with a stereo microscope was conducted to quantify the microplastics. When counting microplastics under a microscope there could be up to 70 % error, and this increases as particle size decreases (Chen et al., 2020), therefore as FTIR spectroscopy is a more accurate method of identification, it eliminates this error. However, FTIR spectroscopy is very expensive and not easily accessible in schools, making it an unsuitable method of microplastic identification for this experiment. Limiting plastic pollution can reduce the number of microplastics present in the sediment at beaches. Takeaway restaurants and fast-food shops should eliminate plastic consumer packaging to reduce plastic pollution. Many fast-food restaurants such as McDonald’s and Guzman Y Gomez have introduced initiatives to eliminate plastic packaging. For example, Guzman Y Gomez introduced bagasse sugarcane pulp, a compostable and renewable resource with a low carbon footprint for their packaging (BioPak, 2021). Furthermore, the Northern Beaches Clean-Up Crew is a group of volunteers that meet up once a month at one of Sydney’s Northern beaches to pick up visible rubbish. This clean-up crew assists in reducing the number of microplastics present at each beach by removing plastics before they can undergo degradation and fragmentation to become microplastics.
This experiment investigated the varying distribution of microplastics across beaches along Sydney’s Northern Beaches. The results showed that Northern Manly Beach had the highest number of microplastics in its sediment. This was found to be due to various factors such as the high levels of tourism, numerous nearby takeaway and fast-food restaurants and the connection of Manly Creek onto Northern Manly Beach.
The method implemented should be amended and improved to advance further research and the accuracy of results. A Sodium Iodide (NaI) brine solution should be used to separate microplastics from sediment to increase the recovery of microplastics from the sediment. Furthermore, the number of samples collected at each beach should be increased to further determine the reliability and improve the accuracy of the results.
Thank you to Dr Vera Munro-Smith for helping me construct my research project and for constantly giving feedback on it.
Andrady, AL 2011, ‘Microplastics in the Marine Environment’, Marine Pollution Bulletin, vol. 62, no. 8, pp. 1596–1605, viewed 11 May 2024, <https://www.sciencedirect.com/science/article/pii/S00253 26X11003055>.
Andrady, AL 2017, ‘The Plastic in microplastics: A Review’, Marine Pollution Bulletin, vol. 119, no. 1, pp. 12–22, viewed 8 April 2024, <https://www.sciencedirect.com/science/article/pii/S00253 26X1730111X>.
AUSMAP n.d., Hotspot Map, AUSMAP, viewed 11 December 2023, <https://www.ausmap.org/hotspot-map>.
Bardon, J 2024, ‘Australia Considers Mandating Recycled Plastic Packaging as Beaches Drown in Rubbish’, ABC News, 16 June, viewed 17 June 2024, <https://www.abc.net.au/news/2024-06-17/nt-plastic-taxmandating-recycled-plastic-packaging/103978192>.
BioPak n.d., GUZMAN Y GOMEZ: Go Green | BioPak Singapore, www.biopak.com, viewed 17 June 2024, <https://www.biopak.com/sg/resources/guzman-andgomez-go-green>.
Chen, Y, Wen, D, Pei, J, Fei, Y, Ouyang, D, Zhang, H & Luo, Y 2020, ‘Identification and Quantification of Microplastics Using Fourier Transform Infrared Spectroscopy: Current Status and Future Prospects’, Current Opinion in Environmental Science & Health, vol. 18, pp. 14–19.
Clark, JR, Cole, M, Lindeque, PK, Fileman, E, Blackford, J, Lewis, C, Lenton, TM & Galloway, TS 2016, ‘Marine Microplastic debris: a Targeted Plan for Understanding and Quantifying Interactions with Marine Life’, Frontiers in Ecology and the Environment, vol. 14, no. 6, pp. 317–324, viewed 6 April 2024, <https://esajournals.onlinelibrary.wiley.com/doi/abs/10.100 2/fee.1297>.
Clean Up Australia n.d., Manly (Queenscliff) Clean up, Clean Up Australia, viewed 17 June 2024, <https://register.cleanup.org.au/fundraisers/northernbeache scleanupcrew/balmoral-beach-cleanup>.
Cverenkárová, K, Valachovičová, M, Mackuľak, T, Žemlička, L & Bírošová, L 2021, ‘Microplastics in the Food Chain’, Life, vol. 11, no. 12, p. 1349, viewed 12 May 2024, <https://www.mdpi.com/2075-1729/11/12/1349>.
Dimassi, SN, Hahladakis, JN, Yahia, MND, Ahmad, MI, Sayadi, S & Al-Ghouti, MA 2022, ‘Degradationfragmentation of Marine Plastic Waste and Their Environmental implications: a Critical Review’, Arabian Journal of Chemistry, vol. 15, no. 11, p. 104262, viewed 6 April 2024, <https://www.sciencedirect.com/science/article/pii/S18785 35222005780>.
Dong, H, Wang, X, Niu, X, Zeng, J, Zhou, Y, Suona, Z, Yuan, Y & Chen, X 2023, ‘Overview of Analytical Methods for the Determination of microplastics: Current Status and Trends’, TrAC Trends in Analytical Chemistry, vol. 167, p. 117261, viewed 17 June 2024, <https://www.sciencedirect.com/science/article/abs/pii/S01 65993623003485#:~:text=Micro%2DRaman%20spectrom etry%20and%20time>.
Eunomia 2016, Plastics in the Marine Environment –Eunomia Research and Consulting, Eunomia, viewed 8
April 2024, <https://eunomia.eco/reports/plastics-in-themarine-environment/>.
Fava, M 2022, Ocean Plastic Pollution an overview: Data and Statistics, Ocean Literacy Portal, UNESCO, viewed 6 April 2024, <https://oceanliteracy.unesco.org/plasticpollution-ocean/>.
Fendall, LS & Sewell, MA 2009, ‘Contributing to Marine Pollution by Washing Your face: Microplastics in Facial Cleansers’, Marine Pollution Bulletin, vol. 58, no. 8, pp. 1225–1228, viewed 6 April 2024, <https://www.sciencedirect.com/science/article/abs/pii/S00 25326X09001799>.
Garrick, M 2022, ‘“If This Was Bondi, there’d Be outrage”: Beaches Hit by Thousands of Tonnes of Plastic Waste’, ABC News, 22 July, viewed 17 June 2024, <https://www.abc.net.au/news/2022-07-23/plastic-wastegrowing-on-arnhem-land-beaches-indigenousrangers/101254974>.
Gewert, B, Plassmann, MM & MacLeod, M 2015, ‘Pathways for Degradation of Plastic Polymers Floating in the Marine Environment’, Environmental Science: Processes & Impacts, vol. 17, no. 9, pp. 1513–1521, viewed 23 March 2024, <https://pubs.rsc.org/en/content/articlehtml/2015/em/c5em 00207a>.
Gola, D, Kumar Tyagi, P, Arya, A, Chauhan, N, Agarwal, M, Singh, SK & Gola, S 2021, ‘The impact of microplastics on marine environment: A review’, Environmental Nanotechnology, Monitoring & Management, vol. 16, no. 100552, p. 100552.
Hale, RC, Seeley, ME, La Guardia, MJ, Mai, L & Zeng, EY 2020, ‘A Global Perspective on Microplastics’, Journal of Geophysical Research: Oceans , vol. 125, no. 1, viewed 4 December 2023, <https://doi.org/10.1029/2018JC014719>.
Hidalgo-Ruz, V, Gutow, L, Thompson, RC & Thiel, M 2012, ‘Microplastics in the Marine Environment: a Review of the Methods Used for Identification and Quantification’, Environmental Science & Technology, vol. 46, no. 6, pp. 3060–3075, viewed 9 March 2024, <https://pubs.acs.org/doi/10.1021/es2031505>.
Hirai, H, Takada, H, Ogata, Y, Yamashita, R, Mizukawa, K, Saha, M, Kwan, C, Moore, C, Gray, H, Laursen, D, Zettler, ER, Farrington, JW, Reddy, CM, Peacock, EE & Ward, MW 2011, ‘Organic Micropollutants in Marine Plastics Debris from the Open Ocean and Remote and Urban Beaches’, Marine Pollution Bulletin, vol. 62, no. 8, pp. 1683–1692, viewed 6 April 2024, <https://www.sciencedirect.com/science/article/abs/pii/S00 25326X1100316X>.
Impact Plastics 2017, The Difference between Amorphous & Semi-crystalline Polymers, blog.impactplastics.co, viewed 12 May 2024, <https://blog.impactplastics.co/blog/thedifference-between-amorphous-semi-crystallinepolymers>.
Jahnke, A, Arp, HPH, Escher, BI, Gewert, B, Gorokhova, E, Kühnel, D, Ogonowski, M, Potthoff, A, Rummel, C, Schmitt-Jansen, M, Toorman, E & MacLeod, M 2017, ‘Reducing Uncertainty and Confronting Ignorance about the Possible Impacts of Weathering Plastic in the Marine Environment’, Environmental Science & Technology Letters, vol. 4, no. 3, pp. 85–90, viewed 6 April 2024, <https://pubs.acs.org/doi/full/10.1021/acs.estlett.7b00008>.
Kannan, K & Vimalkumar, K 2021, ‘A Review of Human Exposure to Microplastics and Insights into Microplastics as Obesogens’, Frontiers in Endocrinology, vol. 12, viewed 11 May 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8416353/ >.
Kunz, DrA 2020, Microplastic Research in Taiwan, Microplastic Research in Taiwan, viewed 1 February 2024, <https://microplasticresearch.wordpress.com/microplasticin-beach-sediments/>.
Lares, M, Ncibi, MC, Sillanpää, M & Sillanpää, M 2019, ‘Intercomparison Study on Commonly Used Methods to Determine Microplastics in Wastewater and Sludge Samples’, Environmental Science and Pollution Research, vol. 26, no. 12, pp. 12109–12122.
Lehel, J & Murphy, S 2021, ‘Microplastics in the Food chain: Food Safety and Environmental Aspects’, Reviews of Environmental Contamination and Toxicology, vol. 259, pp. 1–49, viewed 12 May 2024, <https://link.springer.com/chapter/10.1007/398_2021_77#c iteas>.
Mamun, AA, Prasetya, TAE, Dewi, IR & Ahmad, M 2023, ‘Microplastics in Human Food chains: Food Becoming a Threat to Health Safety’, Science of the Total Environment, vol. 858, no. Pt 1, p. 159834, viewed 6 April 2024, <https://pubmed.ncbi.nlm.nih.gov/36461575/>.
Mercogliano, R, Avio, CG, Regoli, F, Anastasio, A, Colavita, G & Santonicola, S 2020, ‘Occurrence of Microplastics in Commercial Seafood under the Perspective of the Human Food Chain. A Review’, Journal of Agricultural and Food Chemistry, vol. 68, no. 19, pp. 5296–5301, viewed 6 April 2024, <https://pubs.acs.org/doi/full/10.1021/acs.jafc.0c01209#>.
Microplastic Research Taiwan 2021, Microplastic Extraction from Beach Sediments, YouTube, Microplastic Research Taiwan, viewed 1 February 2024, <https://www.youtube.com/watch?v=W8mLqxpzW0o&t= 1s>.
Molloy, F 2022, You Know you’re Swimming in it: Worst Beaches for Microplastics Revealed, The Lighthouse, viewed 12 February 2024, <https://lighthouse.mq.edu.au/article/january-2022/Youknow-youre-swimming-in-it-worst-beaches-formicroplastics-revealed>.
Moore, CJ 2008, ‘Synthetic Polymers in the Marine environment: a Rapidly increasing, long-term Threat’, Environmental Research, vol. 108, no. 2, pp. 131–139, viewed 4 May 2024, <https://www.sciencedirect.com/science/article/abs/pii/S00 1393510800159X>.
Morales-Caselles, C, Viejo, J, Martí, E, GonzálezFernández, D, Pragnell-Raasch, H, González-Gordillo, JI, Montero, E, Arroyo, GM, Hanke, G, Salvo, VS, Basurko, OC, Mallos, N, Lebreton, L, Echevarría, F, van Emmerik, T, Duarte, CM, Gálvez, JA, van Sebille, E, Galgani, F & García, CM 2021, ‘An Inshore–Offshore Sorting System Revealed from Global Classification of Ocean Litter’, Nature Sustainability, vol. 4, no. 6, pp. 484–493, viewed 11 May 2024, <https://www.nature.com/articles/s41893-02100720-8>.
National Environmental Protection Council 2012, Manly, National Environmental Protection Council, viewed 17 June
2024, <https://www.nepc.gov.au/sites/default/files/202209/117-manly-council.pdf>.
Nava, V & Leoni, B 2021, ‘Comparison of Different Procedures for Separating Microplastics from Sediments’, Water, vol. 13, no. 20, p. 2854, viewed 17 June 2024, <https://www.mdpi.com/20734441/13/20/2854?utm_campaign=releaseissue_waterutm_ medium=emailutm_source=releaseissueutm_term=doilink9 5>.
Northern Beaches Council 2023, About the Profile Areas | Mona Vale | profile.id, profile.id.com.au, viewed 11 May 2024, <https://profile.id.com.au/northernbeaches/about?WebID=370>.
Ocean Conservancy 2017, Plastics in the Ocean, Ocean Conservancy, viewed 8 April 2024, <https://oceanconservancy.org/trash-free-seas/plastics-inthe-ocean/>.
Peez, N, Janiska, M-C & Imhof, W 2018, ‘The First Application of Quantitative 1H NMR Spectroscopy as a Simple and Fast Method of Identification and Quantification of Microplastic Particles (PE, PET, and PS)’, Analytical and Bioanalytical Chemistry, vol. 411, no. 4, pp. 823–833.
Plastics Europe 2022, Plastics - the Facts 2022 • Plastics Europe, Plastics Europe, viewed 8 April 2024, <https://plasticseurope.org/knowledge-hub/plastics-thefacts-2022/>.
Quinn, B, Murphy, F & Ewins, C 2016, ‘Validation of Density Separation for the Rapid Recovery of Microplastics from Sediment’, Analytical Methods, vol. 9, no. 9, pp. 1491–1498, viewed 17 June 2024, <https://pubs.rsc.org/en/content/articlelanding/2017/ay/c6a y02542k/unauth>.
Rochman, CM 2018, ‘Microplastics Research from Sink to Source’, Science, vol. 360, no. 6384, pp. 28–29, viewed 8 April 2024, <http://science.sciencemag.org/content/360/6384/28.full>.
Ronda, AC, Menéndez, MC, Tombesi, N, Álvarez, M, Tomba, JP, Silva, LI & Arias, AH 2023, ‘Microplastic Levels on Sandy beaches: Are the Effects of Tourism and Coastal Recreation Really important?’, Chemosphere, vol. 316, p. 137842, viewed 8 April 2024, <https://www.sciencedirect.com/science/article/pii/S00456 5352300108X>.
Surfers Against Sewage 2023, Plastic pollution: Facts & Figures, Surfers against Sewage, viewed 8 April 2024, <https://www.sas.org.uk/plastic-pollution/plastic-pollutionfacts-figures/>.
Team Xometry 2023, Semi-Crystalline Vs Amorphous Polymers: Which One Is More Durable? , www.xometry.com, viewed 7 April 2024, <https://www.xometry.com/resources/materials/semicrystalline-vs-amorphouspolymers/#:~:text=PP%20(polypropylene)%20is%20a%20 widely>.
Tirkey, A & Upadhyay, LSB 2021, ‘Microplastics: an Overview on separation, Identification and Characterization of Microplastics’, Marine Pollution Bulletin, vol. 170, p. 112604, viewed 17 June 2024, <https://www.sciencedirect.com/science/article/abs/pii/S00 25326X2100638X>.
Tosin, M, Weber, M, Siotto, M, Lott, C & Degli Innocenti, F 2012, ‘Laboratory Test Methods to Determine the
Degradation of Plastics in Marine Environmental Conditions’, Frontiers in Microbiology, vol. 3, viewed 12 May 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3380294/ >.
Tymoszuk, K 2018, First Official AUSMAP Trainers!, AUSMAP, viewed 17 June 2024, <https://www.ausmap.org/post/first-official-ausmaptrainers>.
Van Cauwenberghe, L & Janssen, CR 2014, ‘Microplastics in Bivalves Cultured for Human Consumption’, Environmental Pollution, vol. 193, no. 0269-7491, pp. 65–70, viewed 11 May 2024, <https://www.sciencedirect.com/science/article/pii/S02697 49114002425>.
Villarrubia-Gómez, P, Cornell, SE & Fabres, J 2018, ‘Marine Plastic Pollution as a Planetary Boundary Threat –the Drifting Piece in the Sustainability Puzzle’, Marine Policy, vol. 96, pp. 213–220, viewed 11 December 2023, <https://www.sciencedirect.com/science/article/pii/S03085 97X17305456>.
Wang, C, Zhao, J & Xing, B 2021, ‘Environmental source, fate, and Toxicity of Microplastics’, Journal of Hazardous Materials, vol. 407, p. 124357, viewed 8 April 2024, <https://www.sciencedirect.com/science/article/abs/pii/S03 04389420323475>.

This year, our Chemists have tackled an array of projects from different branches of chemistry and biochemistry.
The constant threat of new and evolving drug resistant pathogens inspired Julian and Patrick to focus on synthesising new drug candidates based on previous research at Barker. Patrick explored the synthesis and purification of a new analogue of pyrimethamine, a once potent antimalarial drug, continuing Max’s work from 2023. As part of a new drug discovery effort, Julian synthesised significant quantities of a new 2-aminothiazole drug analogue in the search for new treatments for the skin infection, mycetoma. This compound is now awaiting testing against the disease-causing pathogen.
Jai and Oliver also paved new territory with investigations in biochemistry and immunology. Jai investigated the effects of temperature on the stability of Vitamin C in oranges, an important investigation in the context of the potent antioxidant properties of this naturally occurring compound. Oliver worked closely with university research scientists to probe the enzymatic digestion of Infliximab, an important monoclonal antibody used to treat autoimmune diseases.
Huw developed his own novel project, inspired by his interest in green solvents and peptide synthesis. Using Cyrene as the solvent, he investigated amide bond formation using PyBop as a coupling agent. This represents a new investigation in this fast-emerging area of research.
Oliver Sam Barker College
Infliximab (IFX) is a monoclonal antibody used to treat autoimmune diseases such as inflammatory bowel disease and rheumatoid arthritis. Patients on IFX can experience adverse events if they develop antibodies to IFX (ATIs) during treatment. Mass spectrometry is a method currently being researched for use in therapeutic drug monitoring of IFX and ATIs. Several studies have been published using trypsin as the enzyme in sample preparation. However, there are no other published studies using alternative proteases. The aim of this study is to develop and optimize a method for the digestion of IFX using Arg-C, an alternative enzyme to trypsin. A generic optimized method for Arg-C digestion of IFX was developed and used to measure IFX concentration in a blank matrix. The range the assay is 1.0-100 µg/ml using 4PL regression curve analysis (r2= 0.98). This method can be used for research into the formation of IFX-ATI complexes. Further work needs to be conducted using this method to calculate concentrations of IFX in more complex matrices such as patient serum.
Infliximab (IFX) is a murine chimeric, monoclonal antibody that interferes with the actions of tumour necrosis factor (TNF). Primarily administered to treat patients with conditions such as psoriasis and psoriatic arthritis, inflammatory bowel diseases, rheumatoid arthritis, and other auto-immune diseases(Winterfield and Menter, 2004). Each molecule of IFX has two binding sites in the variable light chain sequence portion of the antibody and so can bind two molecules of TNF. Treatment with Infliximab can lead to adverse reactions in patients such as immunogenic reactions (rejection of the drug) and patients can develop antibodies to IFX (ATI) which reduce the effectiveness of treatment.
Standard testing for IFX and ATI levels is performed using enzyme-linked immunoassay (ELISA). (Mitrev et al., 2017). While ELISAs have benefits such as they are cheap to run and can be automated, there are significant drawbacks as a method (Bloem et al., 2017, Bendtzen, 2015). Most importantly of interest in this study, is that ELISAs are affected by the presence of IFX and ATIs in serum. This means that IFX and ATIs can measure negative on both assays and so do not give a complete picture of antibody development in patients who reject treatment. Mass spectrometric methods have the potential to overcome these issues as digestion of proteins and identification of signature sequences overcomes drug interference.
Mass Spectrometry assays provide structural information of proteins that increases understanding of their mechanism of action. Previous methods, which include immunoassays, rely on photometric measurement of intact proteins but the proteins need to be active for detection. Mass spectrometers have been developed to combine protein chemistry applications to provide much more information. A paper published in 2004 by Domon and Aebersold, summarised the different types of mass spectrometers, how they work and the advantages and disadvantages of each (Domon and Aebersold, 2004). These machines use bottom-up proteomics, which means they use digested proteins rather than intact. They have high mass accuracy and work best with a targeted approach (meaning you know what sequence you are looking for). Triple quads are comprised of 3 quadropoles, two of which (quadropole 1 and 3) have magnetic fields that select for predicted mass to charge ratio. These consist of 4 cylindrical rods that have an oscillating electrical current that changes the trajectory of the ion in the rod. The middle (2nd quadropole) is a collision chamber containing an inert gas which collides with peptides as they exit the first chamber and fragments them into their product ions. The combination of peptide mass to charge ratio, the product ions created, and the time which they hit the detector allows for accurate identification of a protein. The peak areas of fragments hitting the detector can then be used to quantify a peptide.
Swaney et al’s outlook on the value of the use of multiple proteases within large scale mass spectrometry-based proteomics is a helpful identification of why the use of multiple proteases, or even simply alternate proteases, is useful (Swaney et al., 2010). Throughout their research they discovered that the use of multiple proteases for the enzymatic digestion of more complicated protein mixtures produced far more unique peptides (92 -95) than from a single protease digest (trypsin) with only a 1% false discovery rate. This is in comparison to trypsin which only produces on average 61 peptides per protein (Vandermarliere et al., 2013).
Trypsin is the most commonly used enzyme for digestion of proteins for bottom-up proteomic analysis (Vandermarliere et al., 2013). It specifically cleaves the C-terminus of both Arginine (R) and Lysine (K) (Figure 1) and works efficiently in neutral conditions making it suitable for many downstream applications. The cleavage site works well as it leaves a C-terminal basic residue that ionises well for mass spectrometry analysis. Unfortunately, it has been shown that trypsin does not always cleave at every R and K residue, especially if the next residue is proline. For both untargeted proteomics (identification of an unknown protein) and targeted proteomics (quantitation of known proteins) accurate knowledge of the digestion activity and efficiency is important. Algorithms that can predict how an enzyme will digest an amino acid sequence have been developedthis method is called an in-silico digestion where the sequences of digested proteins can be predicted. Arg-C digests at the C-terminal of arginine residues but not lysine. It also digests more efficiently Arginine-Proline sites compared to Trypsin. Indepth knowledge of the actions of the enzymes are needed for understanding the digestion patterns (Giansanti et al., 2016)

Figure 1: Locations in a typical peptide sequence where enzymes digest the protein. Source: https://www.promega.com.au/products/massspectrometry/proteases-and-surfactants/arg_c_sequencing-grade/?catNum=V1881
Analysis of Infliximab using LC-MS/MS has been studied using various sample preparation techniques which include digestion with Trypsin. In a review written in 2019, the authors discuss the different sample preparation methods for extraction of IFX peptides from serum and the tryptic peptides used for analysis (Willrich, 2019). Methods range from simple extraction from buffer using protein precipitation to immunoaffinity capture which utilises the ability to pull IFX out of patient serum eliminating the risk of a high endogenous IgG background. To date there have been no studies published using other enzymes to digest infliximab.
Several assays have been developed that allow for indirect quantitation of ATIs using immunoassay pre-analytical steps, however this does not overcome the inherent drug sensitivity issues with immunoassay methods (Smeijsters et al., 2023, El Amrani et al., 2019b). Currently ATIs cannot be directly measured using mass spectrometric methods as they differ between patients and no common sequences have been identified. Sequencing of proteins requires digesting proteins using different enzymes to produce fragments based on known digestion points and their fragmentation patterns in a mass spectrometer. Future research work in the immunology laboratory at Liverpool Hospital is to isolate and sequence ATIs. Isolation of ATIs will be done in complex with IFX to remove them from serum using immunoaffinity. A precursor to this work is to optimise the digestion of IFX with a range of enzymes. The aim of this study was to develop and optimise a digestion and mass spectrometry method to digest IFX using Arg-C for use in future research in the immunology laboratory.
Scientific Research Question
Can an optimised protocol for the digestion of IFX using Arg-C enzyme be developed and used to quantify IFX in a blank matrix?
Scientific Hypothesis
An optimised method for the digestion of IFX with Arg-C can be developed and used to quantify IFX in a blank matrix, and be useful for ongoing research in quantifying IFX levels in more complex matrices such as serum.
Methodology
Materials
Infliximab (Remicade ™) was obtained from the pharmacy at Liverpool Hospital. Sequencing grade Arg-C was obtained from Promega. Mass spectrometry grade acetonitrile and isopropanol were obtained from Fisher Biotec. Dithiothritol (DTT), CaCl2, formic acid, NH4HCO3 from Merck Pty Ltd. C18 column was obtained from Phenomenex.
In silico analysis and transition prediction
A Blast search on NCBI protein (Home - ProteinNCBI (nih.gov)) was performed to find the FASTA sequence of IFX was obtained In silico digestion was performed using online predictor, Protein Prospector
(https://prospector.ucsf.edu/prospector/mshome.ht m). Skyline software was used to calculate transition predictions
(https://skyline.ms/project/home/software/Skyline/b egin.view)
Sample preparation
IFX was reconstituted to 10mg/ml using de-ionised water. Working dilutions of samples were prepared by spiking IFX into 50mM Tris (pH 7.6-7.9), 5mM CaCl2, 2mM EDTA (incubation) buffer to concentrations of 100 µg/ml for optimisation of method. A negative control was also included. For quantitation of IFX using Arg-C, samples were diluted to a concentration of 0-100 µg/ml in incubation buffer.
The protocol for digestion was adapted from product information (appendix 1). Arg-C was reconstituted in incubation buffer to a concentration of 20 ng/µl. Samples suspended in incubation buffer and 100 µL was placed into a reaction tube with 10 µL of Arg-C dilution (for a final concentration of 1 in 50 enzyme to protein ratio). Next, 12.2 µL of 10X activation
buffer (50mM Tris (7.6-7.9), 50mM DTT, 2mM EDTA) and samples were incubated for 2 hours @ 37oC in a shaking incubator.
After initial digestion experiment, the methods were optimised for digestion time (hours) and ratio of protein to enzyme. Enzyme ratios tested were 1:20, 1:50, 1:100, 1:200, 1:350 enzyme to protein. Digestion times tested were 1, 2, 4, 6 and 18 hours @ 37oC in a shaking incubator.
Mass spectrometric analysis was performed on a Sciex 6500 triple quad mass spectrometer in positive mode with a Sciex ExionLC, HPLC. The samples were run on a C18 sepharose bead column, 2.1mm X 50 mm. Solvent A was 0.1% formic acid in H2O, Solvent B was 0.1% formic acid/ 25% isopropanol/75% acetonitrile. The initial scan was performed with the gradient previously used for tryptic digestion and was 6 mins long with solvent ratios as follows: 0 mins 95% A/ 5% B, 2.5 mins 85% A/15% B, 4.3 mins 5% A/ 95% B, 4.8 mins 5%A/ 95% B, 4.9 mins 95% A/5% B. This gradient was optimised for optimal separation of peaks. Samples were optimised for collision energy (volts).
Spectra analysis
Initial runs were performed using a targeted, open MRM method and run through Multiquant and Skyline analysis to predict peptide sequences. All predicted transitions with m/z ratios < 1200 (nearing the limit of the detector) were inputted to the Analyst method. Best performing peaks were selected along with transitions that eluted at the same retention time with significant peak areas.
Quantitation of IFX using Arg-C method. Samples of IFX from 0-100 µg/ml in duplicate were prepared using optimised method to create a calibration curve.
In silico digestion
FASTA sequence for Infliximab was obtained from NCBI Protein, after performing a BLAST search Home - Protein - NCBI (nih.gov). The sequence was put into Protein Prospector, a free online program that calculates predicted products of digestion and fragmentation. https://prospector.ucsf.edu/prospector/mshome.htm
Results
In silico digestion
FASTA sequence for Infliximab obtained from a Blast search on NCBI protein.

Figure 2: Schematic structure of infliximab. Created with BioRender.com – Download Scientific Diagram (researchgate.net)
i. Infliximab Fab, Variable light chain region:
>pdb|4G3Y|L Chain L, infliximab Fab L DILLTQSPAILSVSPGERVSFSCRASQF VGSSIHWYQQRTNGSPRLLIKYASES MSGIPSRFSGSGSGTDFTLSINTVESE DIADYYCQQSHSWPFTFGRGEC
ii. Infliximab Fab, Variable Heavy Chain:
>pdb|4G3Y|H Chain H, infliximab Fab H EVKLEESGGGLVQPGGSMKLSCVAS GFIFSNHWMNWVRQSPEKGLEWVAE IRSKSINSATHYAESVKGRFTISRDDS KSAVYLQMTDLRTEDTGVYYCSRNY YGSTYDYWGQGTTLTVSSASTKGPS VFPLAPSSKSTSGGTAALGCLVKDYF PEPVTVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSSSLGTQTYICNVN HKPS TKVDKKVEPKSCDKT
iii. Infliximab Constant region
>pdb|5VH5|A Chain A, Infliximab Fc MKKTAIAIAVALAGFATVAQADVES KSCDKTHTCPPCPAPELLGGPSVFLFP PKPKDTLMISRTPEVTCVVVDVSHED PEVKFNWYVDGVEVHNAKTKPREEQ YNSTYRVVSVLTVLHQDWLNGKEY KCKVSNKALPAPIEKTISKAKGQPREP QVYTLPPSRDELTKNQVSLTCLVKGF YPSDIAVEWESNGQPENNYKTTPPVL DSDGSFFLYSKLTVDKSRWQQGNVF SCSVMHEALHNHYTQKSLSLSPGK
Optimisation of liquid chromatography gradient
Following the initial run using previous mass spectrometry parameters (Appendix 3), four main peaks were identified (Appendix 4). The gradient was then optimised to bring the peaks to the middle of the gradient, reduce the spread of the peaks shorten the gradient overall. A hold period of 0.5 mins was introduced to the start of the run to prevent fronting and tailing of the peaks.

Peptides were analysed using Multiquant software analysis program. Precursors with a minimum of two co-eluting transitions were selected and the highest two transitions (in terms of peak area) were selected for continued analysis. Peak shape was also taken into consideration. In addition, peptide (ASQF) that is produced both with a tryptic and ArgC digestion was included as a positive control. The peptides selected for further analysis of Arg-C are listed in Table 1.
Figure 4: Distinct peaks observed and isolated for retention time SKSINSATHYAESVKGR (a), ASQFVGSSIHWYQQR (b), LLIKYASESMSGIPSR (c), DDSKSAVYLQMTDLR (d), QSPEKGLEWVAEIR (e),
Table 1: Comparison of m/z and retention time of QSPEKGLEWVAEIR (a), DDSKSAVYLQMTDLR (b), LLIKYASESMSGIPSR (c), KSINSATHYAESVKGR (d) peptides compared with their respective location in the IFX antibody
Collision energies are predicted by fragmentation prediction software. Often these need to be varied to improve recovery of selected peptides. Repeat injections of high concentration IFX samples (100ug/ml) were performed. Each injection had varied collision energies selected plus or minus predicted CE voltage and peaks areas were analysed to look at variations in signal. Selected CE voltages are listed in the table below (Table 2).

Figure 5: Peak area comparison of varying collision energies voltages for QSPEKGLEWVAEIR (a), DDSKSAVYLQMTDLR (b), LLIKYASESMSGIPSR (c), KSINSATHYAESVKGR (d) peptides
Table 2: Comparison of m/z and retention time of QSPEKGLEWVAEIR (a), DDSKSAVYLQMTDLR (b), LLIKYASESMSGIPSR (c), KSINSATHYAESVKGR (d) peptides with their respective location in the IFX antibody Peptide sequence Location in IFX sequence
(DDSKS)AVYLQMTDL R Heavy chain FAB amino acid positions
(QSPEK)GLEWVAEIR Heavy chain FAB amino acid positions
(LLIKY)ASESMSGIPSR Light chain FAB
(SKSIN)SATHYAESVK GR Heavy chain FAB amino
Optimisation of ratio of enzyme to protein ratio
We optimised the ratio of enzyme to protein and investigated optimal digestion time. We chose the 2-hour incubation time to first determine the optimal concentration of enzyme. In the most abundant peptide sequences (QSPEK and LLIKY), 1 in 50 performed optimally, whereas the less abundant peptides performed better at lower enzyme to protein ratios (DDSKS 1 in 100- 200, and SKSIN 1 in 350). See graphs below. A significant loss of signal was observed at ratios of greater than 1 in 200 with the exception of SKSIN.

Figure 6: peak area comparison of varying ratios of enzyme to protein, 1 in 50, 1 in 100, 1 in 200 and 1 in 350 at 2 hours digestion time. Transitions were measured for peptides QSPEKGLEWVAEIR (a), DDSKSAVYLQMTDLR (b), LLIKYASESMSGIPSR (c), KSINSATHYAESVKGR (d)
Optimisation of digestion times.
From the previous experiment, variations in digestion times at 37oC (as per the product information) at a ratio of 1 in 200, enzyme to protein. Digestions times tested were 1, 2, 4, 6 and 18 hours, shaking at 37oC.

Figure 7: peak area comparison of varying digestion times (1,2,4, 6 and 18 hours) at 1 in 200 ratio enzyme to protein. Transitions were measured for peptides QSPEKGLEWVAEIR (a), DDSKSAVYLQMTDLR (b), LLIKYASESMSGIPSR (c), KSINSATHYAESVKGR (d)
Quantitation of IFX using Arg-C protocol.
The protocol with the parameters optimised in the results above was then used to develop a calibration curve. These results were then analysed using non-linear (4PL) regression. The DDKS peptide was found to not be dose responsive. The remaining three peptides showed good correlation with r2 scores >0.97. The data was analysed for intra- and inter assay precision (%CV) and accuracy (% recovery)
Results are summarised in table 3.

Figure 8: Graphs of measured peak area on samples with increasing spiked IFX (0-100 µg/ml) to generate a quantification curve. Non-linear regression analysis was performed on peptides QSPEK (A), LLIYK (C) and SKSIN (D)
Table 3: R2 scores calculated using 4PL non-linear regression analysis. Results were also analysed for precision across the range 1.0-100 ug/ml (CV %), signal to noise ratio of peak areas at 1 ug/ml, limit of detection (LOD) defined as the lowest tested concentration with a S/N ratio of < 10:1, upper and lower limit of quantification (ULOQ and LLOQ respectively) defined as the highest and lowest concentration with CV<20% and recovery rate (% of spiked concentration) Peptide DDSKS (B) was not dose responsive across the concentration range
Only SKSIN demonstrated acceptable CVs for the whole concentration range tested. As SKSIN was the best performing peptide, recovery rate was calculated by using spiked samples and interpolated from the 4PL calibration curve
In this study, a new method for quantification of Infliximab in a blank matrix using Arg-C for the protein digestion was successfully developed Previous studies have demonstrated effective digestion of IFX using trypsin (El Amrani et al., 2016, El Amrani et al., 2019a). Some studies have also shown that a mix of proteases can produce cleaner chromatograms and allow for detection at lower levels of protein (Giansanti et al., 2016). Unexpectedly the peptide, SKSIN (810.41++ transition), the least abundant peptide detected was chosen as the best performing peptide for this application. The qualitative peptides chosen (for confirmation of protein identity) were SKSIN (746.42+) and QSPEK (713.89++).
To develop the protocol, stepwise analysis was performed which involved repeatedly adapting conditions of the experiment to improve the abundance of peptide detection via calculation of peak area on the chromatogram. The immunology laboratory at Liverpool Hospital has an established digestion method and mass spectrometry parameters for quantification of IFX in blank matrix using trypsin (unpublished results). In the initial run, the published product information of Arg-C digestion of proteins, combined with MS conditions for previous trypsin digestions were used. In-silico digestion data was used to predict the expected transitions (Figure 2 and Appendix 2) with open MRM windows to detect any peaks which matched these predict transitions and fragmentation patterns. This revealed 4 main peaks detectable above background. Repeated injections were made with different programmed gradients of organic solvent through the HPLC column to bring these peaks into more suitable positions for analysis (mid-range during the running time of a cycle). As samples were diluted in 5% solvent B, a hold period 30 seconds (0.5mins) of 5% Solvent B (25% Isopropanol, 75% Acetonitrile, 0.1% Formic Acid) was used. This enables the proteins to group together and form a band at the front of the column and avoids the spreading of peaks in the chromatogram. Following on from this the % Solvent B was increased from 5-15% more quickly than the initial gradient conditions (over 2 mins instead of 2.5 mins). This brought forward later eluting peaks (Figure 3). To further optimise the protocol, variations in CE were performed by repeated injections at different collision voltages (varied from the predicted voltage) by plus or minus 1 volt in a stepwise fashion.
This analysis of mass spectrometry and chromatographic parameters was not exhaustive.
Improvements in the chromatography could be made through further shortening the run time by increasing the rate at which organic solvent increases during the time (sharpening the gradient). Another factor that has been shown to increase abundance of detection is increasing or decreasing the temperature in the column oven and changing the solvent make up. These would be useful to pursue if switching to an alternate matrix (like serum) which may increase background. In addition, fragmentation efficiency can be improved by changing the psi of the nebuliser gas which is the gas that protenates the nanoparticles of protein as it enters the first quadropole. Variation in this can change the time of flight through each quadropole. Again, this can improve detection as optimised changes in the chromatography column and quadropoles can help package the product ions together to hit the detector directly causing sharper and taller peaks and increasing peak area.
Following on from optimisation of the chromatographic and mass spectrometry parameters, optimisation of digestion was performed by varying digestion time and ratio of enzyme to protein. For routine use in a clinical laboratory the least amount of enzyme in the shortest amount of time provides the best option from a cost and turnover perspective. The product information provided suggested protein to enzyme ratios and time indications and so the first run was performed with a 1:20- 1:350 enzyme: protein ratio with a 2hour digestion. Following selection of the enzyme to protein ratio of 1:200, the time of digestion analysis was performed. The digestion time of 6 hours was selected. All digestions were performed at 37oC. The digestions were not optimised for temperature. A time trial at 1:350 for the SKSIN peptide could be a valuable addition to results as the yield appears to continue to increase at this ratio, unlike other peptides (Figure 7).
In this experiment SKSIN was selected as the primary peptide for analysis during the development of this methodology. Replicates of IFX were analysed in a concentration of 0.5-100.00 to develop a calibration curve. On analysis, DDKS was discounted as a non-linear regression analysis could not be performed as it was not dose responsive across the concentration range tested. This result can be due to the conformation of antibodies, meaning that the enzyme cannot digest some sites as efficiently due to structure of the protein. This could be overcome by trying an alternate denaturing agent (such as TCEP) or first incubating the sample at 60 oC to unfold the protein before digestion (van der
Gugten et al., 2019, Suttapitugsakul et al., 2017)
The other three peptides were dose responsive and when analysed with 4PL non-linear regression analysis with an r2 >0.97. In addition, the S/N ratio was > 10:1 across all peptides tested.
For clinical tests, an intra- (within) and inter(between) assay precision (% CV) <20 % is required for a method to be considered for adoption into routine analysis (Mitrev et al., 2017). The precision of a test is the expected variability of repeated measures. In other words, in clinical terms, it is the expected variability between results of the same sample should it be tested again. In immunoassays, this is <20%. The ULOQ and LLOQ (which form the range of any given test) are determined by the highest and lowest values that have CVs of <20%.
Only SKSIN met the criteria for both intra- and inter assay CV of <20% across the full range tested giving the test a range from 1.0-100 ug/ml. In addition, recovery rate as a measure of accuracy should be between 85-115%. This was tested in our selected peptide, at 98% for the 810.41++ transition. For measurement of IFX in clinical settings, 2.0 ug/ml is an important level as it is traditionally the level where automatic testing for ATIs is performed (Mitrev et al., 2017). This is due to the limits of immunoassay, as ATIs cannot be detected in drug levels higher that 2.0 ug/ml (Lee et al., 2016, West et al., 2021). Low precision and high accuracy are vital at this level. Therefore, even though SKSIN 810.41+ transition had the lowest abundance of the all the peptides selected, the high precision and accuracy, and broadest range for testing meant it was the best peptide to use in the quantification method.
Further research is required to increase the abundance of the SKSIN peptide as discussed above. Due to the high signal to noise ratio, it is worthwhile trying to expand the test range to test at lower levels and so further work could be conducted at lower concentrations. This would include conducting further research to try and improve the %CV of the QSPEK peptide. A first step would be to repeat more at the critical level of 2.0 ug/ml and try the digestion at a lower enzyme to protein ratio (such as 1:50) to improve digestion. It should be noted, that the method developed is a generic method and may need to be adapted to more complex matrices such as serum or plasma. As the introduction of a more complex matrix will make the chromatography less clean and increase the background, it is important to produce the best data on a blank matrix for best performance.
The development of this method has implications for further research into IFX level testing and sequencing of ATIs. This research is currently being performed in the immunology laboratory at Liverpool Hospital. As the sequence of IFX is known, the method was optimised by looking for known sequences. This method (without the QQQ component), however, can be used for an untargeted approach to ‘build’ and amino acid sequence from scratch by using it in concert with other enzymes. By using an untargeted method (ie scanning across the range and using analysis software designed for high resolution MS data such as MaxQuant) in a highresolution machine such as an orbitrap, amino acid sequences can be predicted (Hughes et al., 2010, Steen and Mann, 2004). It is the aim to use this protocol in combination with trypsin and other enzyme digestions to study IFX in complex with ATIs in serum and build sequences of the ATIs using high resolution mass spectrometry.
In this study a generic protocol for the digestion of IFX in a blank matrix using Arg-C as an alternative protease was successfully developed. The protocol was optimised both for digestion and mass spectrometry variables. This has not been published before. This method has precision and accuracy within required guidelines for clinical assays for measuring IFX levels in patient samples. It is quantitative across a range of 1.0-100 µg/ml which extends the range compared to traditional methods such as ELISA. The advantages of this method are that it is comprised of a few simple steps, uses a small amount of protease (1:200) and has a short digestion time (6 hours) making it cost effective and timely for use in a clinical laboratory. The data suggests this range could be expanded further, especially at the lower end of the quantitative range. Further work is required to adapt this method to alternative matrices such as serum or plasma for more complex analysis. This includes optimising additional parameters to increase the abundance of the peptide chosen for quantitative analysis. The principles applied in this project can be used to develop protocols for other enzymes for use in the research at the immunology laboratory at Liverpool Hospital. The development of this protocol is important to ongoing research work into better understanding the development of ATIs in patients experiencing loss of response to IFX treatment.
Various appendices to this report are available by contacting the researcher or publisher.
I would like to express my deepest gratitude to the following for their participation and aid in the development of my research report:
Mrs Melissa Sam for supervision, assistance in project design and technical training. Dr Catherine Toong, clinical director of immunopathology, for supervision and assistance in project design. Mr Chris Hodgkins for assistance in training on LCMS. Science extension teachers, extended to Dr Katie Terrett in particular for assistance in project design, setup, writing and publication of research report. NSW Health Pathology and The Ingham Institute of Applied Medical Research for providing laboratory space and reagents required.
Bendtzen, K. 2015. Immunogenicity of Anti-TNF-α Biotherapies: II. Clinical Relevance of Methods Used for Anti-Drug Antibody Detection. Front Immunol, 6, 109.
Bloem, K., Hernández-Breijo, B., Martínez-Feito, A. & Rispens, T. 2017. Immunogenicity of Therapeutic Antibodies: Monitoring Antidrug Antibodies in a Clinical Context. Ther Drug Monit, 39, 327-332.
Domon, B. & Aebersold, R. 2006. Mass spectrometry and protein analysis. Science, 312, 212-7.
El Amrani, M., Bosman, S. M., Egas, A. C., Hack, C. E., Huitema, A. D. R. & Van Maarseveen, E. M. 2019a. Simultaneous Quantification of Free Adalimumab and Infliximab in Human Plasma Using a Target-Based Sample Purification and Liquid Chromatography-Tandem Mass Spectrometry. Ther Drug Monit, 41, 640-647.
El Amrani, M., Göbel, C., Egas, A. C., Nierkens, S., Hack, C. E., Huitema, A. D. R. & Van Maarseveen, E. M. 2019b. Quantification of neutralizing anti-drug antibodies and their neutralizing capacity using competitive displacement and tandem mass spectrometry: Infliximab as proof of principle. J Transl Autoimmun, 1, 100004.
El Amrani, M., Van Den Broek, M. P., Göbel, C. & Van Maarseveen, E. M. 2016. Quantification of active infliximab in human serum with liquid chromatographytandem mass spectrometry using a tumor necrosis factor alpha -based pre-analytical sample purification and a stable isotopic labeled infliximab bio-similar as internal standard: A target-based, sensitive and cost-effective method. J Chromatogr A, 1454, 42-8.
Giansanti, P., Tsiatsiani, L., Low, T. Y. & Heck, A. J. 2016. Six alternative proteases for mass spectrometrybased proteomics beyond trypsin. Nat Protoc, 11, 9931006.
Hughes, C., Ma, B. & Lajoie, G. A. 2010. De novo sequencing methods in proteomics. Methods Mol Biol, 604, 105-21.
Lee, M. W., Connor, S., Ng, W. & ToonG, C. M. 2016. Comparison of infliximab drug measurement across three commercially available ELISA kits. Pathology, 48, 60812.
Mitrev, N., Vande Casteele, N., Seow, C. H., Andrews, J. M., Connor, S. J., Moore, G. T., Barclay, M., Begun, J., Bryant, R., Chan, W., Corte, C., Ghaly, S., Lemberg, D. A., Kariyawasam, V., Lewindon, P., Martin, J., Mountifield, R., Radford-Smith, G., Slobodian, P., Sparrow, M., Toong, C., Van Langenberg, D., Ward, M. G. & Leong, R. W. 2017. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther, 46, 1037-1053.
Smeijsters, E. H., Van Der Elst, K. C. M., Visch, A., Göbel, C., Loeff, F. C., Rispens, T., Huitema, A. D. R., Van Luin, M. & El Amrani, M. 2023. Optimization of a Quantitative Anti-Drug Antibodies against Infliximab Assay with the Liquid Chromatography-Tandem Mass Spectrometry: A Method Validation Study and Future Perspectives. Pharmaceutics, 15.
Steen, H. & Mann, M. 2004. The ABC's (and XYZ's) of peptide sequencing. Nat Rev Mol Cell Biol, 5, 699-711.
Suttapitugsakul, S., Xiao, H., Smeekens, J. & Wu, R. 2017. Evaluation and optimization of reduction and alkylation methods to maximize peptide identification with MS-based proteomics. Mol Biosyst, 13, 2574-2582.
Swaney, D. L., Wenger, C. D. & Coon, J. J. 2010. Value of using multiple proteases for large-scale mass spectrometry-based proteomics. J Proteome Res, 9, 13239.
Van Der Gugten, J.G., Bressler, B. & Demarco, M.L. 2019. An automated mass spectrometric blood test for therapeutic drug monitoring of infliximab. Clin Mass Spectrom, 12, 16-22.
Vandermarliere, E., Mueller, M. & Martens, L. 2013. Getting intimate with trypsin, the leading protease in proteomics. Mass Spectrom Rev, 32, 453-65.
West, T. A., Sam, M. & Toong, C. 2021. Comparison of three commercially available ELISA assays for antiinfliximab antibodies. Pathology, 53, 508-514.
Willrich, M. A. V. 2019. Analysis of Tryptic Peptides from Therapeutic Monoclonal Antibodies Using LCMS/MS. Methods Mol Biol, 1872, 85-99.
Winterfield, L. S. & Menter, A. 2004. Infliximab. Dermatol Ther, 17, 409-26.
Julian Wu
Barker College
Mycetoma is a chronic neglected tropical disease, causing severe subcutaneous tissue infection which spreads to affect skin, deep tissue, and bone. Treatment of the disease is expensive and ineffective, with success rates below 30%, highlighting the need for a more effective treatment. This report describes the synthesis of a novel trifluoromethyl substituted 2-aminothiazole analogue of a lead mycetoma drug candidate. The synthesis of the trifluoromethyl analogue was performed in a high school laboratory, with a 65% overall yield producing 6.558g of the desired product. This is a significant increase in yield compared to previous studies using similar methodology to synthesise the same analogue and will thus allow biological testing to help verify the in vitro efficacy of the analogue. Due to time constraints, however, the compound could not be tested, but biological testing is planned for the immediate future.
Mycetoma is a chronic granulomatous infection of subcutaneous tissue that spreads to affect skin, deep tissue, and bone. Symptoms are characterised by subcutaneous mass, multiple sinuses, and discharge containing grains. This results in a general loss of function in the infected area, and in extreme cases, fatal deformity (WHO, 2022). Mycetoma was officially recognised as a neglected tropical disease (NTD) at the World Health Assembly in 2016, mostly affecting males aged 15-30 in developing countries. In a comprehensive literature review by Emery & Denning (2020), Sudan, Mexico, and India are reported to have the highest number of cases, with 10608, 4155, and 1116 affected individuals respectively reported in the literature. The disease had a reported total of 19494 cases globally between 1876 to 2019 (Figure 1) (Emery & Denning, 2020).

Source: Emery & Denning (2020)
Mycetoma can be caused by either bacteria or fungi, and is thus etiologically classified as either Actinomycetoma (caused by bacteria) or Eumycetoma (caused by fungi). Actinomycetoma has treatment success rates varying from 60% to 90% (Ameen & Arenas, 2009). It is usually treated using a combination of antibiotics such as cotrimoxazole (Figure 2), which is a mixture of predominately sulfamethoxazole, trimethoprim, dapsone, and streptomycin (Vineet Relhan et al., 2017).

Contrarily, Eumycetoma, with over 40 species of causative fungi, the most common being Madurella mycetomatis, still lacks an effective treatment. Currently, treatment involves prolonged antifungal therapy in addition to surgery, with a cure rate of only 25% to 30% using Itraconazole, the most commonly used antifungal agent. In most cases, use of Itraconazole is followed by Terbinafine after failure of the initial treatment path (Chandler, Bonifaz & Wendy, 2023). Patients often only seek treatment with
advanced symptoms, which leads to higher failure rate due to fibrosis of tissue because of the infection. Furthermore, such treatments have high cost and are thus unavailable in endemic areas. These treatments often still have side effects and recurrence occurs in almost one-third of cases. Further, patients may still require amputation after extended treatment (Chandler, Bonifaz & Wendy, 2023). Therefore, an affordable and effective treatment is yet to be found.
MycetOS, as an open-source mycetoma research project, is aimed at finding an effective, safe and affordable oral antifungal agent, using a similar strategy as utilised by the Medicines for Malaria Venture through drug repurposing (Lim et al., 2018). The strategy screens drugs already approved for other indications, or drug candidates with a track record of development, screening 800 drug-like molecules from the Pathogen and Stasis boxes. The Pathogen box contains 400 molecules previously shown to be active against pathogens causing tropical and neglected diseases and the Stasis Box contains 400 compounds selected out of 8000 compounds for preclinical or clinical development but have been discontinued for various reasons. Lim’s screening of these drugs identified MMV006357 (Figure 3A) and MMV689244 (Figure 3B) as the most potent exploratory compounds, with MMV006357 resulting in the highest survival rate of 28.6% in G. mellonella larvae infected by M. mycetomatis with IC50 and MIC50 values of 0.40 μM and 0.25 μM, respectively.

Figure 3: Structural formulae of MMV006357 (A) and MMV689244 (B)
In 2018, to assess in vitro activity, the G mellonella grain model was used to effectively predict antifungal activity in mammalian models Such testing was conducted with fenarimol analogues due to difficulty in creating viable grain structures (Lim et al., 2018). Lim found that polarity and charge may play a role in a compound’s ability to access the fungus. However, should be noted that this observation is stated to not be statistically evaluated. Even so, there is a significant possibility of other fenarimol analogues or 2aminothiazole analogues as physicochemical properties of the compounds are proposed to affect drug potency, hence opening up avenues of research into efficable treatments of Eumycetoma through the Open Source Mycetoma project.
Other molecules identified and being tested by the MycetOS consortium include fenarimols, Phenothiazines, antifolates, benzimidazoles and ketoximes. Ketoximes are being explored through the production of optimised analogues of pyrifenox, a horticultural fungicide, for use in humans. Both pyrifenox and fenarimol target the enzyme CYP51F , as do posaconazole and itraconazole, which are currently the best options for treatment (OpenSourceMycetoma, 2021). The inhibition of CYP51 blocks the demethylation of eburicol by interacting with the fungal 14 alpha-demethylase substrate-binding site, disrupting ergosterol synthesis (Figure 4) and compromising cell membrane integrity, thus inhibiting M. mycetomatis (Kurn & Wadhwa 2023).

Figure 4: biosynthetic pathway for ergosterol by CYP51 Source: Lepesheva, Friggeri & Waterman (2018)
MMV 006357 originates from the Stasis box and is a 2-aminothiazole derivative. The mechanism of action (MOA) is still undetermined yet, but the literature shows that 2-aminothiazoles are potent calciumactivated potassium channel inhibitors in humans, more specifically inhibiting the Kca2.3 channel (Gentles et al. 2008). If the channel or a homologue exists in fungi, it would help to explain the efficacy of 2-aminothiazoles in inhibiting M. mycetomatis in humans. MMV006357 as a potential treatment has already been tested extensively in Malaria screenings by GlaxoSmithKline and Novartis, and further screening was conducted in Leishmania Screening by Saint Jude (OpenSourceMycetoma, 2018)
The 2-aminothiazole scaffold was described as having anticancer, antioxidant, antimicrobial and anti-
inflammatory properties (Jakopin, 2020). The structure is therefore a characteristic structure in drug development. The scaffold is featured in medicines such as abafungin, which is used in the treatment of dermatomycoses - a fungal infection of skin – by directly impairing the fungal membrane and inhibiting the enzyme sterol 24C-methyl transferase (Jakopin, 2020). The MycetOS consortium suggest 24C-methyl transferase as a potential target in investigating the mechanism of action of MMV006357 (OpenSourceMycetoma, 2024).
This paper utilises the synthetic pathway for the 2aminothiazole analogue created by the Open Source Mycetoma consortium (Figure 5). Previous analogues of the 2-aminothiazole changed the α-bromoketone as the substituent (Figure 6), only altering the structure of the left aromatic ring. Contrarily, my lead compound changes the right aromatic ring of the 2aminothiazole by the substitution of a trifluomethyl group for a methyl group.

5: Synthetic pathway for 4-(pyridin-2-yl)-N-(4(trifluoromethyl)pyridin-2-yl)thiazole-2-amine (1)

The 2-aminothiazole analogue 4-(pyridin-2-yl)-N-(4(trifluoromethyl)pyridin-2-yl)thiazole-2-amine (1) (Figure 7) has the addition of a trifluoromethyl (CF3) group, substituting for a methyl group (CH3) on the original compound (Figure 8). This addition may increase the drug’s efficacy, and synthesis of the analogue can provide information about the effect of this electron withdrawing substituent on enzyme active site binding efficiency. By promoting electrostatic interactions, the substitution of a trifluoromethyl group instead of a methyl group introduces electron-withdrawing functionality, which may allow the compound to evade the oxidative mechanisms of cytochrome p450 oxidases, which effectively modify the drug to be more readily excreted from the cell. By inhibiting this metabolism of the drug and also increasing cell membrane permeability, the analogue may see increased efficacy in vitro (Nagib & David, 2011).


This research paper uses a method similar to Jeffress’ 2023 synthesis of the same compound (Jeffress, 2023). However, he was unable to perform any testing after successfully synthesising the 2-aminothiazole
analogue, so this research project is aimed at assessing the reliability of this method and maximising the yield and quantity of the desired product so that the analogue can be tested in vitro against M. mycetomatis.
Scientific Research Question
Can a trifluoromethyl analogue of MMV006357 be synthesized in a school lab with higher yield than previous syntheses and tested in vitro against M. mycetomatis?
Scientific Hypothesis
A trifluoromethyl analogue of MMV006357 can be successfully synthesised in a school lab with a yield higher than previous syntheses and sufficient yield to be tested as an anti-fungal agent in vitro against M. mycetomatis.
1H NMR spectra were recorded at 300 K using a Bruker Avance DRX500 NMR spectrometer in deuterated solvents. CDCl3 (δ 7.26) was used as internal reference for 1H NMR spectra. The data is reported as chemical shift (δH ppm), relative integral, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet) and assignment. Atom labels on structures are to illustrate 1H NMR spectral assignments and do not necessarily correspond to the IUPAC names given.
Analytical thin layer chromatography was performed with Merck Kieselgel 60 F254 (0.2 mm) pre-coated aluminium sheets, and visualisation was achieved by inspection under UV light. Throughout the reaction process Thin Layer Chromatography (TLC) was conducted to gauge the progress of the reaction and determine the point of completion. TLC analysis was conducted with 20% EtOAc/hexane. The solvent peak for CDCl3 (δ 77.0) was used as an internal reference for 13C NMR spectra. Mass spectra were recorded by the Mass Spectrometry Unit of the School of Chemistry, The University of Sydney, Sydney. The molecular ion [M+], [M + H+] or [M - H+] is listed.
Step 1: Synthesis of N-((4-trifluoromethyl)pyridin2yl)carbamothioyl)benzamide (2)

Figure 9: N-((4-Trifluoromethyl)pyridin2yl)carbamothioyl)benzamide (2)
In a round bottom flask fitted with a reflux condenser, Benzoyl chloride (4.782g, 34.46mmol, 1.12 equiv.) was added to a suspension of Potassium thiocyanate (3.593g, 36.96mmol, 1.2 equiv,) in acetone (90mL). The reaction was heated to reflux (60°C) and stirred for 30 minutes. The reaction was left to cool slightly, and 4-(trifluoromethyl)pyridin-2-amine (3) (5.001g, 30.85 mmol., 1 equiv) was added. The reaction was returned to reflux (60°C) and stirred for another 30 minutes. The reaction was poured over ice water (100mL) and stirred for another 10 minutes. The precipitate was collected with vacuum filtration, washed with 10mL of ice water and dried in a dessicator overnight to afford N-((4trifluoromethyl)pyridin-2yl)carbamothioyl) benzamide (2) as a brown solid (10.745g, quantitative yield). TLC was conducted with 20% EtOAc/hexane as the eluent.
Step 2: Synthesis of 1-(4(trifluoromethyl)pyridine-2-yl)thiourea (4)

Figure 10: 1-(4-(Trifluoromethyl)pyridine-2-yl)thiourea (4)
In a round bottom flask fixed with a reflux condenser, the benzamide compound from Step 1, N-((4trifluoromethyl)pyridin2yl)carbamothioyl)benzamide (2), (10.745g, 33.23mmol, 1 equiv.) was added to a 2.5M aq. NaOH solution (49.35mL, 105.7mmol, 10 equiv.) and the reaction was heated for 15 mins at 80°C. Using 1M HCl, the pH was adjusted to be within pH 4.0 - 5.0 to quench the remaining NaOH. Then, the pH was adjusted to pH 8.0 using a saturated aqueous Na2CO3 solution to precipitate the product. The precipitate was
collected via vacuum filtration and washed with cold water (25mL), then dried in a desiccator overnight to afford 1-(4-(trifluoromethyl)pyridine-2-yl)thiourea (4) (4.207g, 19.02mmol, 57% yield) as a white crystalline solid.
Step 3: Synthesis of 4-(pyridin-2-yl)-N-(4(trifluoromethyl)pyridin-2-yl)thiazole-2-amine (5)

Figure 11: 4-(Pyridin-2-yl)-N-(4-(trifluoromethyl)pyridin2-yl)thiazole-2-amine (5)
In a round bottom flask fitted with a condenser, 1-(4(trifluoromethyl)pyridine-2-yl)thiourea (4) (4.207g, 19.02mmol, 1 equiv) from step 2 was added to 2bromo-1-(pyridine-2-yl)ethan-1-one (6.130g, 30.60mmol, 1.61 equiv) in ethanol (100mL). The reaction was heated to reflux (80°C) and stirred until completion as determined by TLC (20% EtOAc/hexane). The reaction was cooled by pouring ice water over the mixture (25mL). The pH was adjusted to pH 8.0 using a saturated aqueous Na2CO3 solution to precipitate the product. The precipitate was collected using vacuum filtration and rinsed with cold water (2 x 10mL) to afford 4-(pyridin-2-yl)-N-(4(trifluoromethyl)pyridin-2-yl)thiazole-2-amine (6.558g, 20.30mmol, quantitative yield) (5) as an offwhite solid

12: Mass spectrum after step 3
MS (+ESI): m/z 321.04 (M – H+), 322.03 (M+), 322.96 (M + H+)

Figure 13: 1H NMR spectrum after step 3
1H NMR (500 MHz, CDCl3): δ 11.5525 (1H, br s, NH), 8.5859 (1H, d, J = 4.67 Hz, Ar-H), 8.3891 (1H, d, J = 5.01 Hz, Ar-H), 7.7935 (1H, d, J = 7.89 Hz, Ar-H), 7.5723-7.6064 (1H, m, Ar-H), 7.5916 (1H, s, Ar-H), 7.1383 (1H, dd, J = 5.12, 7.48 Hz, Ar-H), 6.9241 (1H, d, J = 5.23 Hz, Ar-H), 6.7266 (1H, s, Ar-H)

Figure 14: 13C-NMR spectrum after step 3
13C NMR (100 MHz, CDCl3): δ 161.2123 (CH), 152.1775 (C), 151.9498 (CH), 149.5695 (CH), 148.9693 (C), 147.7891 (CH), 136.8058 (CH), 122.4996 (CH), 120.4855 (CH), 111.2560 (C), 111.2310 (C), 110.6251 (CH), 107.0510 (C), 107.0186 (C).



Figure 17: TLC after step 3
Discussion
Step 1: Synthesis of N-((4-trifluoromethyl)pyridin2yl)carbamothioyl)benzamide (2)

Figure 18: Structural equations for the reaction of step 1
In step 1, benzoyl chloride was used to afford benzoyl isothiocyanate in a reaction with potassium thiocyanate suspended in acetone. In this reaction, the
C-Cl bond of the benzoyl chloride is cleaved by the nitrogen atom in potassium thiocyanate to facilitate the substitution reaction in Figure 19 to afford benzoyl isothiocyanate.

Figure 19: Reaction to form benzoyl isothiocyanate
The benzoyl isothiocyanate was then reacted with the starting material, 4-(trifluoromethyl)pyridin-2-amine (3), whereby the nitrogen atom in the starting material reacts with the carbon atom of the isothiocyanate group, and after electron rearrangement affords N-((4trifluoromethyl)pyridin2yl)carbamothioyl)benzamide (2) (Figure 20).
Figure 20: Reaction to form N-((4-trifluoromethyl)pyridin2yl)carbamothioyl)benzamide (2)
The reaction was successful, producing the desired product with a quantitative yield. The clean TLC for step 1 (Figure 15) suggests an absence of any starting material in the product and it is unlikely that any water is increasing the mass as the product was dried for several days.
Jack’s synthesis produced a yield of 60% in the first step, so a significant improvement was made in yield, which is promising for testing and future syntheses. This improvement in yield is likely due to the increased amount of starting material, which likely results in less product loss during filtration. Additionally, in Jack’s methodology, the potassium thiocyanate and benzoyl chloride were used in 1 equivalent to the starting material. Contrarily, the methodology of this paper uses the two reactants in excess, which may also contribute to the increased yield.
Step 2: Synthesis of 1-(4(trifluoromethyl)pyridine-2-yl)thiourea (4)
Figure 21: Structural equation for the reaction of step 2
In Step 2, a hydrolysis reaction facilitates the formation of compound 4 from compound 2 whereby the hydroxide ion hydrolyses the amide bond, detaching the left aromatic ring and allowing for the protonation of the nitrogen atom when acid is added to neutralise the sodium hydroxide, affording 1-(4(trifluoromethyl)pyridine-2-yl)thiourea (4) (Figure 22)

Figure 22: Reaction to form 1-(4(trifluoromethyl)pyridine-2-yl)thiourea (4)
The yield for this step was 57%, which is a significant improvement as Jack’s yield was 43%. This improvement is due to much more optimised reaction times and temperatures and more accurate regulations of pH, as a more precise and reliable pH meter was utilised compared to Jack’s pH meter. Additionally, the increased amount of starting material and higher yield for step 1 may have contributed to this increased yield. The TLC (Figure 16) indicates the product has a high purity as there is little observable starting material in the product of this step, with no observable impurities in the bands
Step 3: Synthesis of 4-(pyridin-2-yl)-N-(4(trifluoromethyl)pyridin-2-yl)thiazole-2-amine (5)

Figure 23: Reaction pathway to form final product, 4(pyridin-2-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)thiazole2-amine (5)
In step 3, 2-bromo-1-(pyridine-2-yl)ethan-1-one was reacted with the thiourea compound (4) to produce the desired final product as shown in the reaction pathway in Figure 23. Due to the weakness of the bromine carbon bond, the sulfur in the thiourea compound (4) reacts with the carbon, resulting in the loss of the bromide ion and the attachment of the sulfur to the carbon as shown above. With this reaction, the sulfur loses its double bond and initiates the deprotonation of the nitrogen. This proton results in the formation of water, enabling a condensation reaction to afford 4-
(pyridin-2-yl)-N-(4-(trifluoromethyl)pyridin-2yl)thiazole-2-amine (5)
The yield for this step was a quantitative yield. The TLC for step 3 (Figure 17) indicates a more polar product than the thiourea compound as it is further down the TLC plate (4), corresponding to the final compound (5) due to the formation of a 2aminothiazole ring. The overall yield for the synthesis was 65.8%, a significant improvement from Jack’s overall yield of 18.8%. With this significantly improved yield and 6.558g of final product, there is certainly sufficient product for any desired testing, providing promising results for future syntheses.
In the 1H-NMR (Figure 13), the downfield singlet at 11.5525ppm was assigned to the 1H NH proton. Doublets at 8.39 ppm, 7.79 ppm, 6.92 ppm, a doublet of doublets at 7.14 ppm, singlets at 7.59 ppm, 6.73 ppm and a multiplet between 7.5723 ppm and 7.6064 ppm were assigned as aromatic protons. The two singlets at 7.59 ppm and 6.73 ppm corresponded with the aromatic hydrogen on the thiazole ring and one of the Hydrogens on the second pyridine ring, respectively. The splitting of the four doublets correlated with the two right-most Hydrogens on the left pyridine ring and the two right-most Hydrogens of the right pyridine ring. The multiplet and doublet of doublets corresponded with the two left-most Hydrogens of the left pyridine ring.
The 13C-NMR (Figure 14) indicated 14 carbon environments, which is representative of the number of carbon environments in the final product (5). The negative ion mass spectrometry (Figure 12) indicated the production of one product with molecular mass 321.03 (M - H+), 322.03 (M) which agrees with the molecular mass 322.31g/mol. From these results, it is clear the desired product (5) was successfully synthesised with high purity.
The synthesis described in this report improves on previous research, with increased yields due to optimised reaction conditions and increased quantity of starting material. However, there is room for further research:
• Due to time constraints, the compound was not tested against M. mycetomatis, so the effect of the substitution of the trifluoromethyl group for the methyl group is still undetermined. A future report could investigate the analogue’s in vitro effects against M. mycetomatis by comparing the product produced in this report with MMV006357. If the analogue does not see improved efficacy against
M. mycetomatis, it may still hold valuable insight into the future development of other analogues which may exhibit a higher inhibition of M. mycetomatis.
• Further analogues of MMV06357 could be investigated in the future, using the results of this research paper and the future in vitro testing to inform decisions about the synthetic pathway and choice of substituents. This will lead to a deeper understanding of the compound’s mechanisms of action, allowing further development of M. mycetomatis treatment.
Therefore, in my research project I successfully synthesised a trifluoromethyl analogue of a 2aminothiazole compound as a potential treatment for eumycetoma - a neglected tropical disease currently with at most 30% treatment success rates. After each step of the synthesis, TLC was performed to confirm the purity of the product, and 1H NMR, mass spectroscopy and 13C NMR were used to confirm the successful synthesis of the final product. The compound was successfully synthesised with 65.8% yield but the analogue could not be tested in vitro against M. mycetomatis due to time constraints. However, the synthesis still provided valuable insight for guiding future syntheses of similar compounds and analogues as I improved upon Jack Jeffress’ yield of the same synthesis in 2023 by 47%.
I would like to thank Dr Katie Terrett for providing me with indispensable guidance throughout this research paper and for ordering and gathering my required chemicals. She has provided invaluable insight into my report and was ready to explain concepts and assist in my understanding of the research. I also extend my gratitude to the Breaking Good project collaborators at University of Sydney who ran the NMR and Mass Spectroscopy analysis of my product.
References
Basma Karrakchou, Ibtissam Boubnane, Senouci, K. and Hassam, B. (2020). Madurella mycetomatis infection of the foot: a case report of a neglected tropical disease in a nonendemic region. BMC Dermatology, [online] 20(1). doi:https://doi.org/10.1186/s12895-019-0097-1.
Chandler, D.J., Bonifaz, A. and Wendy (2023). An update on the development of novel antifungal agents for eumycetoma. Frontiers in Pharmacology, [online] 14. doi:https://doi.org/10.3389/fphar.2023.1165273.
Chen, J., Li, H., Li, R., Bu, D. and Wan, Z. (2005). Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. ˜The œjournal of antimicrobial chemotherapy/Journal of antimicrobial chemotherapy, [online] 55(1), pp.31–37. doi:https://doi.org/10.1093/jac/dkh507.
Das, D., Sikdar, P. and Bairagi, M. (2016). Recent developments of 2-aminothiazoles in medicinal chemistry. European Journal of Medicinal Chemistry, 109, pp.89–98. doi:https://doi.org/10.1016/j.ejmech.2015.12.022. Drugbank.com. (2016). Itraconazole: Uses, Interactions, Mechanism of Action | DrugBank Online. [online] Available at: https://go.drugbank.com/drugs/DB01167 [Accessed 19 May 2024].
Emery, D. and Denning, D.W. (2020). The global distribution of actinomycetoma and eumycetoma. PLoS neglected tropical diseases, [online] 14(9), pp.e0008397–e0008397.
doi:https://doi.org/10.1371/journal.pntd.0008397.
Fahal, A.H. (2021). Mycetoma: the journey from neglect to recognition as a neglected tropical disease. Transactions of the Royal Society of Tropical Medicine and Hygiene, [online] 115(4), pp.292–294.
doi:https://doi.org/10.1093/trstmh/traa195.
Gentles, R.G., Grant-Young, K., Hu, S., Huang, Y., Poss, M.A., Andres, C., Fiedler, T., Knox, R., Lodge, N., C. David Weaver and Harden, D.G. (2008). Initial SAR studies on apamin-displacing 2-aminothiazole blockers of calciumactivated small conductance potassium channels. Bioorganic & Medicinal Chemistry Letters, [online] 18(19), pp.5316–5319.
doi:https://doi.org/10.1016/j.bmcl.2008.08.023.
Jakopin, Ž. (2020). 2-aminothiazoles in drug discovery: Privileged structures or toxicophores? Chemico-Biological Interactions, 330, p.109244.
doi:https://doi.org/10.1016/j.cbi.2020.109244.
Jeffress, J. (2023). Synthesis of a new trifluoromethyl analogue of MMV006357 for the treatment of Mycetoma. Scientific Research in School, [online] 5(1), pp.157–173. Available at:
https://issuu.com/barkercollege/docs/2023_science_journal [Accessed 22 Nov. 2023].
Kemp, C. (2023). Synthesis of a 2-aminothiazole analogue. Scientific Research in School, [online] 5(1), pp.157–173. Available at:
https://issuu.com/barkercollege/docs/2023_science_journal [Accessed 22 Nov. 2023].
Kurn, H. and Wadhwa, R. (2023). Itraconazole. [online] Nih.gov. Available at: https://www.ncbi.nlm.nih.gov/books/NBK557874/ [Accessed 26 Jun. 2024].
Lamis Y M Elkheir, Haroun, R., Magdi Awadalla Mohamed and Ahmed Hassan Fahal (2020). Madurella mycetomatis causing eumycetoma medical treatment: The challenges and prospects. PLoS neglected tropical diseases, [online] 14(8), pp.e0008307–e0008307.
doi:https://doi.org/10.1371/journal.pntd.0008307.
Lepesheva, G.I., Friggeri, L. and Waterman, M.R. (2018). CYP51 as drug targets for fungi and protozoan parasites: past, present and future. Parasitology, [online] 145(14), pp.1820–1836.
doi:https://doi.org/10.1017/s0031182018000562.
Lim, W., Youri Melse, Konings, M., Hung Phat Duong, Eadie, K., Benoît Laleu, Perry, B., Todd, M.H., Jean-Robert Ioset and Wendy (2018). Addressing the most neglected diseases through an open research model: The discovery of fenarimols as novel drug candidates for eumycetoma. PLOS Neglected Tropical Diseases, [online] 12(4), pp.e0006437–e0006437.
doi:https://doi.org/10.1371/journal.pntd.0006437.
Lydie Maresova, Muend, S., Yong Qiang Zhang, Sychrova, H. and Rao, R. (2009). Membrane Hyperpolarization Drives Cation Influx and Fungicidal Activity of Amiodarone. Journal of Biological Chemistry, [online] 284(5), pp.2795–2802. doi:https://doi.org/10.1074/jbc.m806693200.
Nagib, D.A. and David (2011). Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature, [online] 480(7376), pp.224–228. doi:https://doi.org/10.1038/nature10647.
OpenSourceMycetoma (2018). GitHubOpenSourceMycetoma/Series-2-Aminothiazoles: Series 2 [online] GitHub. Available at: https://github.com/OpenSourceMycetoma/Series-2Aminothiazoles/tree/master [Accessed 3 Feb. 2024].
OpenSourceMycetoma (2022). General Synthesis Route [online] GitHub. Available at: https://github.com/OpenSourceMycetoma/Series-2Aminothiazoles/wiki/General-Synthesis-Route [Accessed 23 Jun. 2024].
Shin, J., Kim, J.-E., Lee, Y.-W. and Son, H. (2018). Fungal Cytochrome P450s and the P450 Complement (CYPome) of Fusarium graminearum. Toxins, [online] 10(3), pp.112–112. doi:https://doi.org/10.3390/toxins10030112.
Vineet Relhan, Mahajan, K., Agarwal, P. and Vijay Kumar Garg (2017). Mycetoma: An update. Indian Journal of Dermatology/Indian journal of dermatology, [online] 62(4), pp.332–332. doi:https://doi.org/10.4103/ijd.ijd_476_16.
World (2022). Mycetoma. [online] Who.int. Available at: https://www.who.int/news-room/factsheets/detail/mycetoma#:~:text=Mycetoma%20is%20a%2 0chronic%2C%20progressively,species%20of%20bacteria %20or%20fungi. [Accessed 3 Feb. 2024].
Zhang, J., Li, L., Quanzhen Lv, Yan, L., Wang, Y. and Jiang, Y. (2019). The Fungal CYP51s: Their Functions, Structures, Related Drug Resistance, and Inhibitors. Frontiers in microbiology, [online] 10. doi:https://doi.org/10.3389/fmicb.2019.00691.
Jai Webster Barker College
Vitamin C is an important compound that acts as an antioxidant and is required for the production of collagen within the body which has caused its effects to be extensively researched. However, it is an exogenous compound and therefore must be consumed in food. Oranges contain high concentrations of Vitamin C and so there has been research upon how certain factors affect the concentration of Vitamin C over time. This report will describe an investigation into the effect of storage temperature of oranges on the concentration of Vitamin C. The orange juice was extracted from orange slices that were incubated in the freezer, the fridge and at room temperature over a period of a week. The concentration was then measured through an iodine starch titration to calculate the rate of change in concentration depending on the temperature. The experiment found that there was not a statistically significant difference in the concentration of Vitamin C in the orange slices after storage for a week at room, fridge and freezer temperatures.
What is Vitamin C?
Vitamin C, or otherwise known as Ascorbic acid is a water-soluble vitamin that is highly reactive and unstable due to the two hydroxyl groups in its structure (Figure 1) resulting in ascorbic acid being prone to degradation (Kaleem et al., 2016). There are two main factors that affect the rate of degradation of Vitamin C, temperature and oxygen. This makes conventional preservation methods such as drying and thermal processing to be extremely detrimental for the retention of Vitamin C in the product. This is because the raised temperature increasing the rate of degradation of Vitamin C in oranges (Giannakourou & Taoukis, 2021).

Figure 1: The structure of ascorbic acid as it deteriorates through the aerobic pathway, highlighting the reactive hydroxyl group.
After: Yin et al (2022, p2)
Why is Vitamin C important?
Vitamin C is a “powerful antioxidant essential for the human body”, (Pavlovska et al., 2014) which is important due to its role in binding free radicals. A radical includes any molecule that has one or more unpaired electrons in its outer shell and are classified into two groups, reactive oxygen species (ROS) such as hydroxyl (OH*) and reactive nitrogen species (RNS) such as nitric oxide (NO*). These compounds
are produced by the mitochondria when the cell generates energy using oxygen in ATP production. Free radicals can be beneficial in low concentrations as they are used by phagocytes to destroy pathogenic microbes (Pham-Huay, He & Pham-Huay, 2008). However, due to the highly reactive nature of free radicals, they can damage important biomolecules such as DNA, proteins, carbohydrates and lipids by reacting with them (Yin et al., 2022). Therefore, the build-up of free radicals, also known as oxidative stress, can be very detrimental and is known to play a major role in the development of chronic diseases and cancer. As a result, antioxidants such as Vitamin C are important as they act as free radical scavengers which neutralise the free radicals by acting as an electron donor (Pehlivan, 2017). Vitamin C is also important in the synthesis of collagen as it prevents the autoinactivation of the enzymes, lysyl and prolyl hydroxylase which are necessary in collagen synthesis (Boyera, Galey & Bernard, 1998). This is important as collagen provides structure and support to the skin, muscle bones and ligaments.
Why is the degradation of Vitamin C in food a problem?
Vitamin C cannot be produced in the human body due to the lack of the enzyme, L-gulono-1,4 lactone oxidase in mammals (Yin et al., 2022), meaning it is an exogenous compound that must be supplied in food (Monika, Karolina & Witold, 2021). Due to the susceptibility for Vitamin C to degrade compared to other nutritional compounds such as other vitamins, it is used as an index to measure the overall quality deterioration of a product after storage (Giannakourou
& Taoukis, 2021). It is recommended that people consume at least 80mg of Vitamin C a day to prevent diseases such as scurvy that are caused by a Vitamin C deficiency (Harvard School of Public Health, 2012).
How does Vitamin C degrade?
Vitamin C can degrade through two pathways, the aerobic and anaerobic pathway. The aerobic pathway follows the oxidation of ascorbic acid to dehydroascorbic acid which can degrade through hydrolysis. Vitamin C can also degrade through the anaerobic pathway which causes it to degrade without being oxidised first, forming furfural (Yin et al., 2022).
What are some factors that affect Vitamin C concentration?
Temperature is the major factor attributed by scientists to the degradation of Vitamin C (Yin et al., 2022), (Giannakourou & Taoukis, 2021) as heat can significantly affect many factors such as pH that influence the degradation of vitamin C as well as higher temperatures increasing its reactivity and the rate of degradation (Pavlovska et al., 2014).
The oxidation reaction of ascorbic acid to dehydroascorbic acid is an endothermic reaction (Soceanu et al., 2020). This means that at a higher temperature, the rate of reaction will significantly increase causing the rate of degradation to increase.
Certain enzymes such as ascorbate oxidase can assist the degradation of Vitamin C. (Murao et al., 2014)
Ascorbate oxidase has been found to catalyse the degradation of ascorbic acid most efficiently to dehydroascorbic acid at a temperature of 45°C and at a pH of 4 but was most stable at pH 6-10 and at temperatures of 60°C (Figure 2). A higher temperature not only increases the efficiency of the enzyme, but it also reduces the pH of the solution. This is because the equilibrium reaction, H2O ⇌ H+ + OH- is endothermic, and thus the higher temperature will favour the forward reaction producing more H+ ions and by extension lowering the pH. Therefore, storage at room temperature as opposed to in the fridge will increase the efficiency of the enzyme ascorbate oxidase because it will decrease the pH of the solution due to the endothermic nature of H2O ⇌ H+ + OH- which is the favourable for ascorbate oxidase which prefers warm, acidic environments.

Figure 2: A graph depicting the efficiency of ascorbate oxidase at various temperatures and pH levels.
Source: Murao et al (2014, p6)
How can the Vitamin C concentration be measured?
Vitamin C concentration can be determined through a titration using an iodine titrant and a starch solution. This titration follows the chemical equation in figure 3. As iodine is constantly added to the ascorbic acid, it will continue to react until the iodine becomes in excess compared to the ascorbic acid and so will react with the starch indicator producing a dark blue colouring to the solution.

Figure 3: The chemical equation for the reaction Ascorbic acid + I2 → 2 I- + Dehydroascorbic acid which is used in the titration (Moorthy, 2013).
After: Yin et al (2022, P2)
Project rationale
This report describes experiments that were conducted to investigate the effect of storage temperature of oranges on the BRAIN DRAIN: Investigating concussion-inducing brain accelerations from various baseball pitch speeds BRAIN DRAIN: Investigating concussion-inducing brain accelerations from various baseball pitch speeds Vitamin C concentration after one week. This was chosen because Vitamin C is an essential molecule for the human body due to its role as a powerful antioxidant and its role in collagen synthesis. However, Vitamin C is an exogenous compound so it must be absorbed from food to prevent diseases such as scurvy and so it is extremely important to limit the degradation of Vitamin C in food sources such as oranges which are rich in Vitamin C. Temperature has been proven to have a significant effect on the rate of degradation of Vitamin
C (Yin et al., 2022), (Giannakourou & Taoukis, 2021) and so this experiment sought to emphasise its effects and reveal the importance of maintaining a cool temperature in order to increase the retention of Vitamin C in oranges.
Scientific Research Question
How does the temperature of storage affect the retention of Vitamin C in oranges after a week.
Scientific Hypothesis
With a decrease in the temperature of storage of the orange, there will be an increase in the retention of Vitamin C in the slices of orange after a week of storage.
Methodology
Method A: Storage of Oranges
1. One orange was sliced into 12 equal wedge-shaped parts
2. 3 slices were set apart to be used in the pre-storage titration
3. One slice of orange was placed in a clean sandwich sized Ziploc bag
4. The Ziploc bag was sealed with air still inside
5. Steps 3 and 4 were repeated 8 times so that all orange slices are in a bag
6. 3 Ziploc bags were placed into a fridge set at a temperature of 4 degree Celsius
7. 3 Ziploc bags were placed in a fruit basket at room temperature without sunlight
8. 3 Ziploc bags were placed into a freezer
9. The Ziploc bags were left for one day
10. Each Ziploc bag was opened to replenish the air inside
11. Each Ziploc bag was resealed
12. Steps 9-11 were repeated each day for 5 days
13. The Ziploc bags were removed from the fridge and freezer
14. The oranges were removed from the plastic bags
Method B: Iodine and starch titration (From: Royal Society of Chemistry, 2013)
1. The orange slice was weighed
2. The orange slice was juiced using a citrus reamer
3. The volume of orange juice produced was measured with a measuring cylinder
4. The conical flask was rinsed with distilled water
5. The burette was rinsed with 20ml of 0.04M aqueous iodine solution
6. The burette was filled with 50ml of 0.04M iodine
7. 6g of starch was measured
8. The starch was added to 180ml of water to create the starch solution
9. 20ml of the starch solution was poured into the conical flask
10. The orange juice was poured into the conical flask with the starch solution
11. Iodine was added through the burette until a permanent colour change was observed
12. The volume of iodine used to fully react with the orange juice was recorded
13. Steps 1-12 were repeated for each orange slice
The number of mols of iodine used in the titration could be found by multiplying the volume in litres of iodine used with the concentration. According to Figure 3, ascorbic acid reacts in a 1:1 ratio with iodine, meaning that the number of mols of iodine used is equal to the amount of mols of ascorbic acid in the orange juice. To find the concentration of ascorbic acid in the orange juice, the mols of ascorbic acid was divided by the volume of orange juice produced in litres. To find the mass of Vitamin C per 100g of orange, the mols of ascorbic acid was first multiplied by the molar mass of 176.12g/mol to find the mass of ascorbic acid in the orange juice. The mass of Vitamin C was then divided by the weight of the slice and multiplied by 100 to find the mass of Vitamin C per 100g of orange.
Table 1: Measurements taken of the orange slices before storage. Initial
Table 2: Measurements taken of the orange slices after a week of storage at 18°C to 22°C.
temperature (18 to 22°C)
Table 3: Measurements taken of the orange slices after a week of storage at 4°C to 7°C
Fridge (4 to 7°C)
Table 4: Measurements taken of the orange slices after a week of storage at -5°C to -10°C
Freezer (-5 to -10°C)

4: Graph comparing the average Vitamin C concentration across the different storage conditions

5: Graph comparing the average mass of Vitamin C for every 100g of Orange across the different storage conditions
Table 5: Results of a one-way ANOVA test done on the mass of Vitamin C per 100g of orange across the different storage conditions.
One way ANOVA
of
of Vitamin C
Table 6: Results of a Tukey HSD test on the mass of Vitamin C per 100g of orange across the different storage conditions Treatment
A vs B 3.8157 0.1019070 Insignificant
A vs C 3.6179 0.1240636 Insignificant
A vs D 0.8762 0.8999947 Insignificant
B vs C 0.1979 0.8999947 Insignificant
B vs D 2.9395 0.2383747 Insignificant
C vs D 2.7417 0.2858538 Insignificant
Discussion
The research hypothesis predicts that the temperature of storage will be indicative of the retention of Vitamin C within the orange slices. That is, a lower temperature of storage will lead to an increase in the retention of Vitamin C within the orange slices after being stored at this temperature for a week.
The statistical analysis of the data collected from the orange slices stored at the three temperatures was completed with a one-way ANOVA (analysis of variance) test. In this statistical test, the alpha value was discovered to be 0.061 which is greater than the alpha value of 0.05 as shown in Table 5. Therefore, the alternate hypothesis is rejected, meaning that there is not a significant difference between the Vitamin C concentration after storage at different temperatures. This is further backed up by the data from Table 6 which show there are no significant differences between each of the storage conditions because the Pvalue from the Tukey HSD (honest significant difference) test was greater than 0.5 on each as shown in Table 6. Although there is an observable trend with lower temperatures leading to a higher retention of Vitamin C (Figure 4), there is not a significant difference between the values as shown by the Anova test (Table 5) and the Tukey HSD test (Table 6) which each have P-values greater than 0.05.
Therefore, due to the lack of a significant difference between the Vitamin C concentration across the different storage temperatures, this experiment
indicates that there is a limited effect of storage temperature on the retention of Vitamin C in oranges. However, there is still a correlation between a lower storage temperature and an increase in the retention of Vitamin C in the oranges after one week. Therefore, it is still beneficial to store the oranges at a lower temperature to reduce the rate of degradation of Vitamin C in the oranges although the effect of the storage temperature has been found to have limited impact over the Vitamin C concentration by this experiment.
This experiment was performed from home to mirror the household environment that oranges are stored in causing there to be many limitations that must be taken into consideration when analysing the data produced from this experiment. Firstly, all the orange slices came from one orange. This is because each slice can only be titrated once and therefore the initial must come from the same orange as the other slices so it can be compared to them as they will have the same initial Vitamin C concentration. Despite this, the reliability of this experiment is low as there are outliers in each set of data which cause a change in the average by 5 to 20% (Table 7). Furthermore, the reliability of this data between oranges cannot be measured as only one orange was used, and therefore cannot be generalised to all oranges. Secondly, there was a very large temperature range in the fridge and freezer which would not be observed had the oranges been left in a water bath set at a consistent temperature and therefore decreasing the reliability of this experiment. Furthermore, the temperature range of room temperature is very mild due to it being performed in autumn and so there is a possibility of a large difference in Vitamin C retention being observed in other seasons due to the considerable difference in average temperature across the seasons which limits the validity of this experiment. Thirdly, the oranges left at room temperature became mouldy over the course of the storage period as a result of the high humidity within the sealed plastic bags. This could have impacted the Vitamin C concentration or the mass of Vitamin C per 100g by impacting things such as the orange juice yield. Furthermore, there is a higher surface area as a result of the orange being cut into slices. This increases the area which can be affected by the outside conditions and react with the air and therefore increasing the rate of oxidation of ascorbic acid into dehydroascorbic acid compared to a whole orange. Lastly, each titration only required 0.3 to 0.7ml of titrant before a permanent colour change was observed in the solution. This limits the accuracy of the experiment as it takes very little titrant before it fully reacts and so the point where it has fully reacted
is very unclear and also limits the differentiation in the mols of Vitamin C found in each sample.
Observation of outliers within the data collected for each storage temperature. This can be seen in the mass of Vitamin C per 100g of orange column in Table 1, Table 2, Table 3 and Table 4 with each having an outlier that considerably impacted the averages as seen in Table 7.
This experiment can be improved in many ways as a result of the many limitations associated with performing the experiment in a home environment leading to more uncontrolled variables. This includes things such as, increasing the number of oranges used to assess the reliability of the results and so that it is possible to make a general claim about the effect of storage temperature on Vitamin C in oranges. Alongside this, I will have more repeats in water baths set at the average temperatures of each season to investigate the effect of the different average temperatures. In order to prevent mould growth on the oranges at room temperature, it is necessary to maintain a low humidity which will cause the spores to become dormant until the humidity becomes high enough. Therefore, a desiccant such as silica gel will be added to reduce the humidity within the bag and thus preventing the growth of mould on the oranges. Lastly, I would also further dilute the iodine solution from 0.04M to 0.001M so that it is possible to get more accurate results from the titrations as well as being able to clearly differentiate the exact amount of Vitamin C in the difference slices. Therefore, to increase the reliability of this experiment, increase the accuracy and decrease the effect of outliers by performing my trials with more than one orange, spread those trials across different seasons to observe the effect of the different seasonal temperature ranges and dilute the iodine solution to get more exact titrations and to be able to clearly differentiate between the Vitamin C content in each slice. These improvements will be added next time as there was not enough time to perform a second run of this experiment due to the time-consuming nature of this experiment.
Conclusion
In conclusion, the storage temperature has no significant effect on the retention of Vitamin C in oranges. My research project explored whether the temperature at which oranges were stored at affected the retention of Vitamin C in oranges. I used multiple slices from the same orange to compare the initial concentration of Vitamin C to the concentration after
it was stored for a week at room temperature, the fridge and in the freezer which had temperature ranges 18 to 22°C, 4 to 7°C and -5 to -10°C respectively. The concentration of Vitamin C was measured by conducting an iodine starch titration which is represented in Table 1 through to 4. The data from the titrations were put through a one-way ANOVA test and a Tukey HSD test (Table 5 and Table 6) which found that the difference between the concentration of Vitamin C in the initial as well as the concentration retained after storage at room, fridge and freezer was statistically insignificant due to the P-value of 0.061 being greater than the alpha value of 0.05 although there was a correlation between lower temperatures of storage and higher concentrations of Vitamin C in the oranges after a week of storage.
I would like to acknowledge Dr Katie Terrett for her constant supervision and support throughout the entirety of my experiment by guiding me towards suitable experimental methods and assisting me in analysing the data received from my experiment.
Boyera, N., Galey, I. and Bernard, B.A. (1998). Effect of Vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. International Journal of Cosmetic Science, 20(3), pp.151–158. doi:https://doi.org/10.1046/j.1467-2494.1998.171747.x.
Fadime Eryılmaz Pehlivan (2017). Vitamin C: An Antioxidant Agent. InTech eBooks. [online] doi:https://doi.org/10.5772/intechopen.69660.
Giannakourou, M.C. and Taoukis, P.S. (2021). Effect of Alternative Preservation Steps and Storage on Vitamin C Stability in Fruit and Vegetable Products: Critical Review and Kinetic Modelling Approaches. Foods, 10(11), p.2630. doi:https://doi.org/10.3390/foods10112630.
Harvard University (2012). Vitamin C. [online] The Nutrition Source. Available at: https://nutritionsource.hsph.harvard.edu/vitamin-c/ [Accessed 22 Jun. 2024].
Kaleem, A., Nazir, H. and Pervaiz, S. (2016). Investigation of the effect of temperature on vitamin C in fresh and pack fruit juices. FUUAST Journal of Biology, 6(1), pp.117–120.
Krishna moorthy, R. (2013). Analysis of Effect of Temperature on the Decay of Vitamin – C in Fruit Juices. doi:https://doi.org/10.35543/osf.io/rkgju.
Mieszczakowska-Frąc, M., Celejewska, K. and Płocharski, W. (2021). Impact of Innovative Technologies on the Content of Vitamin C and Its Bioavailability from Processed Fruit and Vegetable Products. Antioxidants, [online] 10(1), p.54. doi:https://doi.org/10.3390/antiox10010054.
Murao, S., Itoh, H., Yajima, T., Ozaki, Y., Fukuyasu, S. and Shin, T. (1992). Isolation and Purification of Ascorbate Oxidase fromAcremoniumsp. HI-25. Bioscience,
Biotechnology, and Biochemistry, 56(6), pp.847–852. doi:https://doi.org/10.1271/bbb.56.847.
Njoku, P.C., Ayuk, A.A. and Okoye, C.V. (2011). Temperature Effects on Vitamin C Content in Citrus Fruits. Pakistan Journal of Nutrition, [online] 10(12), pp.1168–1169. doi:https://doi.org/10.3923/pjn.2011.1168.1169.
Pavlovska, G., Dukovska, E., Knights, V. and Jankuloska, V. (2014). Influence of temperature and time of storage on amount of vitamin C in strawberries. [online] Available at: https://keypublishing.org/jhed/wpcontent/uploads/2020/07/03.-Full-paper-GoricaPavlovska.pdf.
Pham-Huy, L., He, H. and Pham-Huy, C. (2008). Free Radicals, Antioxidants in Disease and Health. InternatIonal journal of BIomedIcal scIence, [online] 4(2). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614697/p df/IJBS-4-89.pdf.
Royal Society of Chemistry (2013). Measuring vitamin C in food. [online] RSC Education. Available at: https://edu.rsc.org/resources/measuring-vitamin-c-infood/1280.article.
Soceanu, A., Matei, N., Dobrinas, S. and Popescu, V. (2020). Degradation Kinetic Modelling of Ascorbic Acid from Orange Juice. Proceedings, 70(1), p.55. doi:https://doi.org/10.3390/foods_2020-07693.
Yin, X., Chen, K., Cheng, H., Chen, X., Feng, S., Song, Y. and Liang, L. (2022). Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants, [online] 11(1), p.153. doi:https://doi.org/10.3390/antiox11010153.
Patrick Liu Barker College
Malaria and Toxoplasmosis are very common infectious diseases, mostly prevalent in Africa, causing significant mortality. Historically, drugs such as Pyrimethamine successfully treated malaria until the most common disease-causing pathogen, Plasmodium falciparum, developed resistance. The research described in this report details efforts towards the synthesis of a novel pyrimethamine analogue that has not yet been synthesised or tested for its efficacy as a treatment for these diseases, in order to understand the mechanism of this established plasmodium resistance. The optimisation and investigation of the first two steps in a three-step synthesis pathway are described, with an increase in yield and purity for the product of Step 1. The optimisation of Step 2 is still ongoing, and investigations are outlined which aim to understand the difficulty in obtaining the second reaction product. In the future, the goal is to test the analogue as an anti-plasmodial agent for treating these diseases
Malaria is an infectious disease in humans caused by five parasite species called Plasmodium and is transmitted by pregnant female anopheles’ mosquitos (WHO, 2023). Plasmodium Falciparum is the major malaria causing species in most endemic continents. The disease begins in the mosquito and spreads to people through its saliva while feeding on blood. Once the disease enters the bloodstream, it affects the liver and red blood cells, potentially causing serious health problems for people and, in some cases, death. Symptoms may be mild, especially for those who have had a malaria infection before (Zekar and Sharman, 2023). Infants, children under 5 years, pregnant women, travellers and people with HIV or AIDS are at higher risk. Severe symptoms include extreme tiredness and fatigue, impaired consciousness, multiple convulsions, and difficulty breathing (Mayo Clinic, 2023).
Over 627,000 fatalities in Africa were caused by the P. falciparum strain in which accounted for 99.6% of malaria cases in the continent 2020 (BylickaSzczepanowska & Korzeniewski 2022). In 2022, there were projected to be 249 million cases of malaria worldwide, with 608,000 malaria-related fatalities. 94% of malaria cases and 95% of malaria deaths in 2022 occurred in the African Region (Figure 1) (WHO, 2022)

Malaria-vulnerable countries often have inadequate healthcare facilities and humid conditions that promote mosquito proliferation (Bardosh et al., 2017). Inadequate availability to affordable antimalarial medications has led to parasite resistance, causing substantial humanitarian and health concerns in affected areas. Malaria incidence is dropping globally, but the rate of reduction in morbidity and mortality in Sub-Saharan Africa has slowed significantly (Oladipo et al., 2022).
Additionally, Toxoplasmosis is another infectious disease which is caused by a parasitic organism, in this case, Toxoplasma gondii (CDC, 2024). It is present in undercooked meats, such as pork, venison, and in polluted water (Food and Drug Administration, 2024). It is often spread when individuals come into contact with infected animal faeces, usually cat faeces. Oocysts grow on raw meats, which if consumed, will experience binary fission and generate tachyzoites, which can transmit through blood or to the foetus if
present (Better Health Channel, 2023). Infected cats, warm-blooded animals, and people are often asymptomatic throughout their lives. A tiny number of infected individuals may have symptoms such as headaches, muscular pains, enlarged lymph nodes, and fever (Cornell University College of Veterinary Medicine, 2018). Individuals with impaired immune systems may experience lasting retinal, brain, and organ damage. In 2023, more than 60% of the global population was infected with toxoplasmosis but did not exhibit symptoms (CDC, 2024). Toxoplasmosis poses a considerable danger to unborn children as it is easily transmitted from mother to child, with 200000 cases reported globally each year (Al-Malki, 2021) Improving access to anti-parasitic drugs to treat malaria and toxoplasmosis in developing countries can reduce prevalence and promote better health outcomes, potentially leading to lower poverty rates, lower infant mortality rates, and longer life expectancy.
Pyrimethamine is sold under the name Daraprim (Figure 2). Pyrimethamine was once widely used as an inhibitor of P. Falciparum plasmodial dihydrofolate reductase (DHFR) enzyme and was effectively used to treat malaria and is still used as a treatment for toxoplasmosis (Sandefur et al., 2007). Due to overuse and subsequent development of P. Falciparum and T. gondii drug resistance, the treatment is no longer as effective. Pyrimethamine is frequently used in combinations with other treatments, including sulfadoxine, to treat malaria (Padberg 2015). It works together to inhibit different enzymes involved in the folic acid synthesis pathway of the malaria parasite. Specifically, DHFR catalyses the transformation of dihydrofolate to tetrahydrofolate, which is part of the thymidylate cycle show (Figure 3) (Bilsland et al., 2018). Folic acid is essential for the parasite's DNA synthesis, and by disrupting its production, these drugs effectively inhibit the growth and reproduction of the Plasmodium parasites that cause malaria. In P. falciparum, this drug resistance in the parasite was caused by rapid mutations in the DHFR enzyme, leading to the formation of a mutant form of DHFR, which has resulted in decreased efficacy of antimalarial agents that inhibit this enzyme. The majority of antifolate-classed antimalarial medicines target DHFR (Hyde 2005)

Figure 2: Pyrimethamine

Research into creating new Pyrimethamine analogues has been a focus over the last 30 years, in order to counteract the alterations in the enzyme and overcome increased resistance (Pandey et al., 2023). Developing novel analogues of synthetic pharmaceuticals is vital for reducing resistance and understanding the structure-activity relationships. The activity of a potential drug molecule can be altered by varying the chemical structure, hopefully leading to improved inhibition of parasite enzymes. It is essential that more research be done to comprehend the distinctions between pyrimethamine's interactions with the mutant DHFR enzyme in order to overcome this medication resistance (Lozovsky et al., 2020). The direct interaction of pyrimethamine with P. falciparum DHFR is a complicated process, and the precise structure of the biofunctional enzyme is currently unknown. As a result, the amount of literature on this topic is still somewhat relatively limited.
One method for creating new drug candidates is to continuously redevelop analogues of medications that have lost their effectiveness. Moreover, the discovery of novel derivatives of pyrimethamine advances our knowledge of how molecular structure influences enzyme interactions with both wild-type and mutant DHFR. The majority of analogues that have been synthesised up to this point feature different halogen replacements at positions R2-R6 on the phenyl ring (Figure 4A) (Kamchonwongpaisan et al., 2017). According to earlier research, binding affinity may be significantly increased by substituting different atoms or functional groups at the R4 position (Figure 4A) (Tarnchompoo et al., 2002). As a result, a lot of work has gone into creating a variety of pyrimethamine analogues and gathering biological information by comparing the activity of these analogues to both mutant and wild-type P. falciparum (Cassera et al., 2011).
To date, analogue development has not focused on the exploration of electron withdrawing substituents on this phenyl ring, such as the effect of a trifluoromethyl substituent. For this reason, the project described in this report outlines our efforts towards the synthesis of a novel trifluoromethyl (CF3) analogue of pyrimethamine (Figure 4B). Molecular models suggest that this analogue is a potentially effective
antimalarial drug; for TbPTR1, the inhibition constant (IC50) is 90 nM, and for TbDHFR, it is 45 nM (Tassone et al., 2021) These modelling also shows its possible that pyrimethamine can bind to DHFR and stabilise the folded enzyme in the endoplasmic reticulum because of its structural resemblance to DHF (Figure 5). Hydrogen bonding at the active site is the primary mechanism via which wild-type DHFR interacts with pyrimethamine. The two amino functional groups on the ring that engage with R211 and D354 and the two amino groups on the heterocyclic pyrimidine component that create three hydrogen bonds with the active site amino acids D290, D240, and E491 combine to form the enzymeinhibitor complex (Figure 5). Most frequently, the non-polar pocket of W405 and W424 contains the ethyl group on the pyrimidine ring (Tropak et al., 2015).
Figure 4: A.) General structure of pyrimethamine analogues, B.) Trifluoromethyl analogue of pyrimethamine, C.) Methylenedioxy analogue of pyrimethamine, D.) Iodo analogue of puramethamine, E.) Structure of pyrimethamine

Figure 5: Three-dimensional modelling of the enzymeinhibitor complex at the pyrimethamine and wild-type DHFR ligand active site.
Source: (Tropak et al., 2015)
The synthetic pathway developed by Sydney Grammar School students in 2016 for the synthesis of pyrimethamine will be the focus of the methodology (Open Source Malaria, 2018). These students have successfully synthesised Pyrimethamine from 4chlorophenylacetontrile in collaboration with the Breaking Good Project and Sydney University. The proposed synthetic pathway is outlined in Figure 6, described herein.
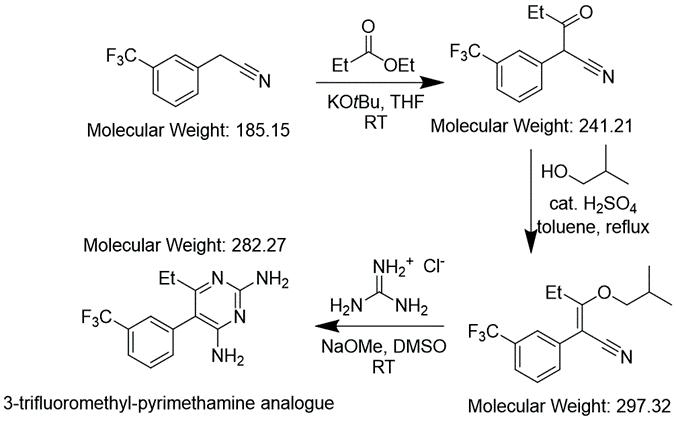
Figure 6: Proposed synthetic pathway to prepare the trifluoromethyl analogue of Pyrimethamine
This paper builds on a series of projects in pyrimethamine synthesis research conducted by a research group at Barker College, particularly the work of Graham (2023). The methylenedioxy analogue (Figure 4C) was created in interest due to the larger size compared to the chlorine atom in pyrimethamine. Since methylenedioxy has a bigger size and is less electronegative than the chlorine atom in pyrimethamine, an analogue of it was developed. The analogue could not, however, be submitted for biological testing against P Falciparum or have its enzyme inhibition data obtained because of a relatively limited yield brought about by impurities and isolation issues presented in steps 2 and 3 of the synthesis (Abbott, 2021). In 2020, Pyrimethamine's iodo analogue was synthesised for the first time by substituting iodine at the R4 position (Figure 4D) (Wong, 2020). Furthermore, with the successful optimisation of the synthesis process through two of the three phases, Wu's (2021) synthesis obtained greater yields and purity. Wu was unable to validate the synthesised chemical, nevertheless, because of problems with solubility that arose in the last stage. Graham (2023), in contrast, attempted the synthesis of a new trifluoromethyl analogue. But in the end, no product was isolated and was extremely impure. The synthesis process depicted in Figure 6 does not alter when considering the trifluoromethyl counterpart. To account for the final molecule's structural modifications, the starting reactant was be modified to 3-triflouromethylphenylacetonitrile. This report is an attempt to synthesize a new trifluoromethyl analogue of pyrimethamine by scaling up the reaction modify the synthesis' starting material.
The research described in this report involves steps towards the synthesis of a new trifluoromethyl analogue of pyrimethamine (Figure 6). The goal of this project was to eventually test this novel pyrimethamine analogue to understand its interaction with the DHFR enzyme of resistant malaria and toxoplasmosis parasites because to the enhanced electronegativity of fluorine atoms and the greater mass of the 3-triflouoromethyl group compared to a
single chlorine atom. The research sought to help this effort to create a new analogue to enhance understanding of how these analogues interact with the enzyme targets, which can provide vital information about the structure-activity relationship, leading to a deeper understanding of the structural features that could lead to increased drug activity.
Scientific Research Question
Can a new trifluoromethyl substituted Pyrimethamine analogue be synthesised in the school laboratory and can its minimum inhibitory concentration (MIC) against Plasmodium Falciparum be measured?
Scientific Hypothesis
That a trifluoromethyl substituted Pyrimethamine analogue can be synthesised in a school laboratory and a minimum inhibitory concentration (MIC) against Plasmodium Falciparum can be determined.
Methodology
General experiment details 1H and 13C NMR spectra were recorded at 300 K using a Bruker Avance DRX400 NMR spectrometer. Residual acetone (δ 2.05) and chloroform (δ 7.26) were used as internal reference for 1H NMR spectra. The data is reported as chemical shift (δH ppm), relative integral, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet) and assignment. Atom labels on structures are to illustrate 1H NMR spectral assignments and do not necessarily correspond to the IUPAC names given. The solvent peak for chloroform (δ 77.0) was used as an internal reference for 13C NMR spectra. Mass spectra were recorded by the Mass Spectrometry Unit of the School of Chemistry, The University of Sydney, Sydney. The molecular ion [M + H+] or [M - H+] is listed.
Analytical thin layer chromatography was performed with Merck Kieselgel 60 F254 (0.2 mm) pre-coated aluminium sheets, and visualisation was achieved by inspection under UV light. Throughout the reaction process Thin Layer Chromatography (TLC) was conducted to gauge the progress of the reaction and determine the point of completion. TLC analysis was conducted with either 50:50 Dichloromethane (DCM): Hexane, 70:30 ethyl acetate: hexane, or pure ethyl acetate.
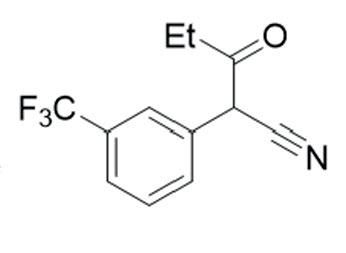
3-triflouromethylphenylacetonitrile (15.00 g, 0.081 mol, 1 equiv.), ethyl propionate (8.69 g, 0.085 mol, 1.05 equiv.) and potassium tert-butoxide (18.1 g, 0.162 mol, 2 equiv.) were combined in tetrahydrofuran (THF) (150 mL) at room temperature, with stirring in a round bottom flask. The mixture changed to a blood red colour, and its temperature increased. After 30 minutes, stirring was turned off as the reaction mixture appeared homogenous. The reaction was sealed and left for 2 hours in a fume hood. The reaction mixture was worked up by the addition of 1.0 M HCl (150 mL) to the reaction vessel. The acidified reaction mixture was moved into a separating funnel and the aqueous layer was extracted with dichloromethane (DCM) (100 mL). The organic layer was washed with brine (100mL) and dried with anhydrous sodium sulfate. This solution was then filtered, and concentrated in vacuo to produce a red oil which was 2-(3-trifluoromethyl)-3-oxopentanenitrile (18.61 g, 0.0772 mol, 95.3%). TLC was conducted with 50:50 DCM: hexane as the eluent. The product: 2-(3-trifluoromethyl)-3-oxopentanenitrile was used in the second step of the synthesis without purification.
Step 2: Synthesis of 2-(3-trifluoromethylphenyl)-3(2-methylpropoxy)-pent-2-enenitrile
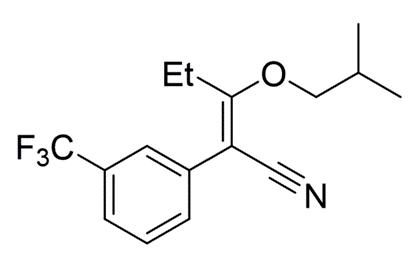
Figure 8: 2-(3-trifluoromethylphenyl)-3-(2methylpropoxy)-pent-2-enenitrile
The product from step 1, 2-(3-trifluoromethyl)-3oxopentanenitrile (18.6 g, 0.0776 mol) was dissolved in a mixture of 2-methylpropan-1-ol (0.101mol, 7.47 mL, 1.3 equiv.) and toluene (175 mL). After the addition of 18 M Sulfuric Acid (1.00 mL), the mixture was refluxed for 10 hours in a Dean Stark apparatus.
This reaction mixture was poured onto saturated sodium hydrogen carbonate (50 ml) in a separating funnel. The aqueous phase was then extracted with DCM (3 x 50 mL), and the combined organic extracts were dried over anhydrous sodium sulfate. 7.0 mL of triethylamine was added to this reaction mixture to convert the unreacted starting material to a triethylammonium enolate salt, which is highly polar. Chromatography silica (25g) was added to the organic mixture, DCM was added to bring the mixture to 200 mL and this was stirred for two hours. This organic phase was decanted and rinsed with 1M HCl (2 x 50 mL) and deionised water (100 mL), to remove all traces of triethylamine. The solvent was removed from the organic solution via evaporation in a fume hood to yield a crude reaction product (9.30g) as a dark red oil.
Results
Step 1: Synthesis of 2-(3-trifluoromethyl)-3oxopentanenitrile

Figure 9: 1H NMR spectra after step 1
1H NMR (500MHz, CDCl3) δ 8.34 (1H, s, CH), 8.29 (H, d, CH), 7.86 (1H, d, CH), 7.60 (2H, m, CH), 4.76 (1H, s, CH), 3.82 (1H, s, CH), 1.07 (3H, t, CH3).
Figure 10: 13C NMR spectra after step 1
13C NMR (100MHz, CDCl3) δ 198.8 (CO), 180.2 (CN), 180.2 (CF3), 125.3 (Ar-CH), 125.1 (Ar-CH),
125.1 (Ar-CH), 125.0 (Ar-CH), 125.1 (Ar-CH), 125.1 (Ar-CH), 33.9 (CH2), 7.7 (CH3).



13:

14: Mass spectra after step 2


Discussion
Step 1: Synthesis of 2-(3-trifluoromethyl)-3oxopentanenitrile
anhydrous solvents to ensure that no water is present to interfere with the base or the reactants. Water can quench the base and reduce its effectiveness, leading to lower yields. The success can also be the use of larger quantity of starting material (15.00 g) compared to prior efforts.
Supporting this high yield, the 1H NMR (Figure 9) confirmed the existence of the desired compound, and only indicated the existence of some slight impurities. The H1 aromatic protons were assigned to the doublet between 8.34-8.26 ppm, whereas the H2 aromatic protons were assigned to the doublet between 7.867.84 ppm. This was expected because the 7-8 ppm region is where hydrogen atoms on a benzene ring typically emerge. The quartet further upfield at 2.70ppm was assigned to the CH2 on the ethyl group. Similarly, the triplet at 1.07ppm was linked to the CH3 group. However, there was an impurity at approximately 3.83 ppm which was caused by unreacted starting material. Impurities continues throughout at 2.40 ppm and 1.06 ppm which is caused by propanoic acid which formed during acid work up from ethyl propionate in excess.
Mass spectroscopy (Figure 11) confirmed the synthesis of the intended molecule, with a peak at m/z 240.03 (M-H+)+. Step 1 of the synthesis had a high yield, evident in the TLC result (Figure 12) and produced a clean crude product that was validated as the desired molecule. This provided a solid foundation for further reactions.
Step 2: Synthesis of 2-(3-trifluoromethylphenyl)-3(2-methylpropoxy)-pent-2-enenitrile
Figure 17: 3-triflouromethylphenylacetonitrile to form 2(3-trifluoromethyl)-3-oxopentanenitrile
The compound was formed in step 1 of the synthetic process via a condensation reaction between ethyl propionate and 2-(3-trifluoromethyl)-3oxopentanenitrile (Figure 17). Initially, the deprotonation of the CH2 group was made possible by the addition of the strong base, potassium tert butoxide. A subsequent series of chemical reactions causes C-C bond to form between 3trifluoromethylphenylacetonitrile and ethyl propionate. and elimination of water afforded the desired product.
This reaction yielded 95.24%, surpassing Sydney Grammar's 2016 Pyrimethamine synthesis yield of 90% and Barker College's 2023 TrifluoromethylPyrimethamine analogue yield of 60% (Graham, 2023). The success of this synthesis can be due to improved reaction conditions by employing

Figure 18: 2-(3-trifluoromethyl)-3-oxopentanenitrile to form (2-(3-trifluoromethylphenyl)-3- (2-methylpropoxy)pent-2-enenitrile)
During Step 2 of the synthesis, which was carried out under reflux, the aim of this step was to form 2-(3trifluoromethylphenyl)-3-(2-methylpropoxy)-pent-2enenitrile by a substitution reaction (Figure 18). In this reaction, water is eliminated and the equilibrium reaction was pushed to the products using a Dean Stark apparatus to remove the water from the reaction mixture. Additionally, this reaction is likely endothermic, which will additionally promote product formation under high temperatures, due to the equilibrium consideration.
Initial TLC analysis revealed the existence of large amount of leftover starting material (Figure 15). The yield for this step was unable to be determined due to the large amount of starting materials remaining. In 2023, Graham’s synthesis of this product was quoted to give a 35% yield, however, no reaction product was indicated by NMR or MS analysis, so this yield is likely a result of starting material and/or impurities. The 1H NMR spectrum was challenging to assign because of its complexity. Analysis of the Mass spectrum indicates a peak at 339.12 (M-H+)+ (Figure 14) which was inconsistent compared to the desired peak of 297.32 (M-H+)+ because there is no obvious side reaction leading to a mass of 339.12. Investigations are ongoing to determine the identity of the major byproduct.
This was to be expected, given Max Graham found leftover starting material following the Dean-Stark reaction (2023). This present research improved Graham’s result by completing the reaction for a second time on the isolated reaction product of this step. In the past, it was hypothesised that the strong electron withdrawing traits of the CF3 group at the R3 location of the ring may have resulted in a more polar molecule, which may have transferred into the THF/water layer during work up. This may explain the loss of some reaction product into the aqueous layer during the extraction step. To remedy this issue, larger volumes of water and dichloromethane were used to promote extraction from the aqueous layer into the dichloromethane layer. This appeared to improve the extraction of the reaction mixture compared to previous attempts.
The TLC of this second attempt (Figure 15) still showed large amounts of starting materials. The product was then purified through column chromatography and TLC was conducted on the collected fractions, and it appeared that fractions 5, 8, 13, and 15 were relatively pure (Figure 16). NMR analysis (Figure 13) was then conducted on those fractions, showing remaining starting materials. The mass spectrum of these fractions were all complex and consisted mainly of starting material, and further purification needs to be done to analyse components of the mixture. Therefore, different reaction conditions or a different sequence of steps all together would need to be taken. However, time constraints and an impure compound for step 2 did not allow for further synthesis or biological testing of any compound as an anti-malarial agent.
The effective synthesis of Pyrimethamine analogues in high school contributes to our growing
understanding of the Sydney Grammar synthesis process, and also offers fresh, intriguing structuralactivity data on the ways in which pyrimethamine interacts with the DHFR enzyme. The trifluoromethyl analogue of pyrimethamine synthesis needs to be updated and then reviewed in light of this findings. Specific areas of interest include:
• In step one of the synthesis, the desired 2-(3trifluoromethyl)-3-oxopentanenitrile is still impure, and hence the reaction needs to be optimised further or the reaction product needs to be purified. This is important so that further 1H NMR and 13C NMR can be conducted, and the spectra for this compound confirmed before being included in the Breaking Good database.
• Step two of the synthesis, which further optimisation is required to get a greater yield, making it possible to conduct further biological testing.
Research must be conducted further in order to stay ahead of the virus's mutations. Generally speaking, this might be accomplished by carrying on with the research and synthesis of novel Pyrimethamine analogues in an effort to create medications that are both accessible and reasonably priced. If not, P. Falciparum mortality and morbidity rates, especially in Sub-Saharan Africa, might show alarming exponential rise.
Using a synthetic process devised by Sydney Grammar School, the trifluromethyl analogue of pyrimethamine was successfully synthesised as a result of the study reported in my article. NMR spectroscopy and mass spectroscopy were used to ensure that the right product had formed at each stage. This synthesis, which is the second counterpart to be produced by this approach, demonstrates that the Sydney Grammar pathway may be used to produce reasonably priced Pyrimethamine analogues, especially those that have different non-halogenic substitutions at the R3-5 locations on the phenyl ring. However, my analogue could not be submitted for biological testing against P Falciparum and to get its enzyme inhibition data because of minor impurities in step 1 and a very modest yield in step 2 due to isolation issues. To enable future biological testing, the reaction pathway should be improved and different reaction conditions or a different sequence of steps all together would need to be taken. By doing this, more structural-activity data about the interactions between
pyrimethamine and the DHFR enzyme may be gathered.
I would like to express my sincere gratitude to Dr. Katie Terrett for all of her tremendous assistance and support throughout the project. The research would not have been made possible without her extensive understanding of organic chemistry and her patient explanations. I would also like to thank the University of Sydney's collaborators on the Breaking Good Project, whose spectroscopy equipment they kindly used to analyse our samples. Finally, I would like to thank the Science Department at Barker College for funding and providing the materials throughout the research.
Bardosh, K.L., Ryan, S.J., Ebi, K., Welburn, S. and Singer, B. (2017). Addressing vulnerability, building resilience: community-based adaptation to vector-borne diseases in the context of global change. Infectious diseases of poverty, [online] 6(1). doi:https://doi.org/10.1186/s40249-017-03752.
Barker College (2020). Science Extension Journal: Volume 2 |Number 1|September 2020. [online] Issuu. Available at: https://issuu.com/barkercollege/docs/2020_science_ext_jou rnal_in_pdf_
Barker College (2021). 2021 Scientific Research in School by Barker College - Issuu. [online] issuu.com. Available at: https://issuu.com/barkercollege/docs/2021_science_journal [Accessed 26 Jun. 2024].
Barker College (2023). Scientific Research in School by Barker College - Issuu. [online] issuu.com. Available at: https://issuu.com/barkercollege/docs/2023_science_journal [Accessed 26 Jun. 2024].
Better Health Channel (2023). Toxoplasmosis. [online] Vic.gov.au. Available at: https://www.betterhealth.vic.gov.au/health/conditionsandtr eatments/toxoplasmosis [Accessed 25 Jun. 2024].
Bilsland, E., Liisa van Vliet, Williams, K., Feltham, J., Carrasco, M.P., Fotoran, W.L., G Cubillos, E.G., Wunderlich, G., Morten Grøtli, Hollfelder, F., Jackson, V., King, R.D. and Oliver, S.G. (2018). Plasmodium dihydrofolate reductase is a second enzyme target for the antimalarial action of triclosan. Scientific reports, [online] 8(1). doi:https://doi.org/10.1038/s41598-018-19549-x
Bunyarataphan, S., Leartsakulpanich, U., Taweechai, S., Tarnchompoo, B., Kamchonwongpaisan, S. and Yuthavong, Y. (2006). Evaluation of the Activities of Pyrimethamine Analogs against Plasmodium vivax and Plasmodium falciparum Dihydrofolate Reductase-Thymidylate Synthase Using In Vitro Enzyme Inhibition and Bacterial Complementation Assays. Antimicrobial agents and chemotherapy, [online] 50(11), pp.3631–3637. doi:https://doi.org/10.1128/aac.00448-06
Bylicka-Szczepanowska, E. and Korzeniewski, K. (2022). Asymptomatic Malaria Infections in the Time of COVID-19 Pandemic: Experience from the Central African Republic.
International journal of environmental research and public health/International journal of environmental research and public health, [online] 19(6), pp.3544–3544. doi:https://doi.org/10.3390/ijerph19063544
Cassera, M.B., Zhang, Y., Hazleton, K.Z. and Schramm, V.L. (2011). Purine and Pyrimidine Pathways as Targets in Plasmodium falciparum. Current topics in medicinal chemistry, [online] 11(16), pp.2103–2115. doi:https://doi.org/10.2174/156802611796575948
CDC (2024). About Toxoplasmosis. [online] Toxoplasmosis. Available at: https://www.cdc.gov/toxoplasmosis/about/index.html [Accessed 25 Jun. 2024].
Cornell University College of Veterinary Medicine (2018). Toxoplasmosis in Cats. [online] Cornell University College of Veterinary Medicine. Available at: https://www.vet.cornell.edu/departments-centers-andinstitutes/cornell-feline-health-center/healthinformation/feline-health-topics/toxoplasmosis-cats [Accessed 25 Jun. 2024].
Food and Drug Administration (2024). Toxoplasma - Food Safety for Moms to Be. [online] U.S. Food and Drug Administration. Available at: https://www.fda.gov/food/people-risk-foodborneillness/toxoplasma-food-safety-momsbe#:~:text=%22What%20is%20Toxoplasma%20gondii%3 F%22,cat%20feces%20can%20be%20found. [Accessed 26 Jun. 2024].
Hyde, J.E. (2005). Exploring the folate pathway in Plasmodium falciparum. Acta tropica, [online] 94(3), pp.191–206. doi:https://doi.org/10.1016/j.actatropica.2005.04.002
Kamchonwongpaisan, S., Quarrell, R., Charoensetakul, N., Ponsinet, R., Vilaivan, T., Vanichtanankul, J., Tarnchompoo, B., Sirawaraporn, W., Lowe, G. and Yuthavong, Y. (2003). Inhibitors of Multiple Mutants of Plasmodium falciparum Dihydrofolate Reductase and Their Antimalarial Activities. Journal of medicinal chemistry, [online] 47(3), pp.673–680. doi:https://doi.org/10.1021/jm030165t
Lozovsky, E.R., Daniels, R.F., Heffernan, G.D., Jacobus, D.P. and Hartl, D.L. (2020). Relevance of Higher-Order Epistasis in Drug Resistance. Molecular biology and evolution, [online] 38(1), pp.142–151. doi:https://doi.org/10.1093/molbev/msaa196
Mayo Clinic (2023). Malaria - Symptoms & causes . [online] Mayo Clinic. Available at: https://www.mayoclinic.org/diseasesconditions/malaria/symptoms-causes/syc-20351184 [Accessed 26 Jun. 2024].
Oladipo, H.J., Tajudeen, Y.A., Oladunjoye, I.O., Yusuff, S.I., Yusuf, R.O., Oluwaseyi, E.M., AbdulBasit, M.O., Adebisi, Y.A. and El-Sherbini, M.S. (2022). Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Annals of Medicine and Surgery, [online] 81(104366). doi:https://doi.org/10.1016/j.amsu.2022.104366.
Open Source Malaria (2018). Daraprim Synthesis. [online] Ourexperiment.org. Available at: https://malaria.ourexperiment.org/daraprim_synthesis/ [Accessed 26 Jun. 2024].
Padberg, S. (2015). Anti-infective Agents. Elsevier eBooks, [online] 2(6), pp.115–176. doi:https://doi.org/10.1016/b978-0-12-408078-2.00007-x.
Pandey, S.K., Anand, U., Siddiqui, W.A. and Tripathi, R. (2023). Drug Development Strategies for Malaria: with the Hope for New Antimalarial Drug Discovery An Update. Advances in Medicine, [online] 2023(1), pp.1–10. doi:https://doi.org/10.1155/2023/5060665
Sandefur, C.I., Wooden, J.M., Quaye, I.K., Worachart Sirawaraporn and Carol Hopkins Sibley (2007). Pyrimethamine-resistant dihydrofolate reductase enzymes of Plasmodium falciparum are not enzymatically compromised in vitro. Molecular and biochemical parasitology, [online] 154(1), pp.1–5. doi:https://doi.org/10.1016/j.molbiopara.2007.03.009
Tarnchompoo, B., Sirichaiwat, C., Phupong, W., Intaraudom, C., Sirawaraporn, W., Kamchonwongpaisan, S., Vanichtanankul, J., Thebtaranonth, Y. and Yuthavong, Y. (2002). Development of 2,4-Diaminopyrimidines as Antimalarials Based on Inhibition of the S108N and C59R+S108N Mutants of Dihydrofolate Reductase from Pyrimethamine-Resistant Plasmodium falciparum. Journal of medicinal chemistry, [online] 45(6), pp.1244–1252. doi:https://doi.org/10.1021/jm010131q
Tassone, G., Landi, G., Linciano, P., Francesconi, V., Tonelli, M., Tagliazucchi, L., Maria Paola Costi, Stefano Mangani and Pozzi, C. (2021). Evidence of Pyrimethamine and Cycloguanil Analogues as Dual Inhibitors of Trypanosoma brucei Pteridine Reductase and Dihydrofolate Reductase. Pharmaceuticals, [online] 14(7), pp.636–636. doi:https://doi.org/10.3390/ph14070636
Tropak, M.B., Zhang, J., Yonekawa, S., Rigat, B.A., Aulakh, V.S., Smith, M.R., Hwang, H.-J., Ciufolini, M.A. and Mahuran, D.J. (2015). Pyrimethamine Derivatives: Insight into Binding Mechanism and Improved Enhancement of Mutant β-N-acetylhexosaminidase Activity. Journal of Medicinal Chemistry, 58(11), pp.4483–4493. doi:https://doi.org/10.1021/jm5017895.
FWorld Health Organization: WHO (2022). World Malaria Report 2022. [online] Who.int. Available at: https://www.who.int/teams/global-malariaprogramme/reports/world-malaria-report-2022 [Accessed 26 Jun. 2024].
World Health Organization: WHO (2023a). Malaria. [online] Who.int. Available at: https://www.who.int/newsroom/fact-sheets/detail/malaria [Accessed 25 Jun. 2024].
Zekar, L. and Sharman, T. (2023). Plasmodium falciparum Malaria. [online] Nih.gov. Available at: https://www.ncbi.nlm.nih.gov/books/NBK555962/ [Accessed 26 Jun. 2024].
Huw Evans Barker College
This paper aims to describe the synthesis of 4-methyl-N-phenylbenzamide (compound 1) using the bio solvent Cyrene ((1R,5S)-7,8-Dioxabicyclo[3.2.1]octan-2-one), in the presence of the coupling agent (Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyBOP). The synthesis was successful, adapting an existing method for HATU mediated amide coupling. Employing Cyrene in PyBOP mediated amide remains an underexamined procedure within the literature. Researching new experimental procedures involving bio solvents allows their commercial viability and use within industry to be expanded. The proposed methodology involves the synthesis of compound 1 by coupling aniline with p-toluic acid in a Cyrene medium. The reaction occurs in the presence of PyBOP and DIPEA. The crude product was then purified with an existing washing procedure within the literature. The product was analysed using Nuclear Magnetic Resonance (NMR) spectroscopy and mass spectroscopy. The analysis revealed that the target molecule (compound 1) had been synthesised. Time limitations prevented the extension of the aforementioned coupling reaction to solid phase peptide synthesis. The practical applications surrounding this project are the possibility of synthesising pharmacologically relevant molecules in a manner that simultaneously reduces the hazard and environmental impact of their synthesis.
Environmental and OHS regulations surrounding both the reagents and solvents used within the pharmaceutical industry are becoming increasingly stringent, increasing the importance of replacing potentially hazardous substances with more environmentally sustainable and non-toxic alternatives (Andrew et al. 2022). On April 30th, 2024, the EPA banned the use of methylene chloride (Rague et al. 2021), a common solvent (created with fossilderived natural gas) used in synthesis reactions (Schlosser et al. 2014). It is toxic to the eyes, skin, liver and heart. It is also a suspected carcinogen (Jaffe et al. 2019). At the forefront of this research is the quest for new suitable bio-solvents. Recently, solvents derived from biomass (cellulose, xylose etc.) have entered the literature as a viable alternative.
An encouraging dipolar aprotic solvent is (1R,5S)-7,8Dioxabicyclo[3.2.1]octan-2-one (Cyrene), which has proven itself to be a “competent replacement in several amide coupling reactions” (Jordan et al. 2022). It is non-toxic and non-mutagenic (Wilson et al. 2018) making it an ideal candidate for the synthesis of pharmacologically desirable bioactive molecules. It is synthesised by pyrolysis of cellulose containing substrate to produce levoglucosenone, followed by catalytic hydration to give dihydrolevoglucosenone (Cyrene) (Wilson et al. 2018). This relatively simple synthesis mechanism means that Cyrene could have
significant commercial use (Yu et al. 2021), if its application was further researched and developed. The two solvents that are most used in amide coupling are dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) (Citarella et al. 2022). These two solvents are conducive to amide coupling because they are polar (owing from the S=O bond in DMSO (Figure 1) and the C=O and C-N bonds in DMF (Figure 2) and aprotic (due to the absence of O-H and N-H bonds) (Brown 1987). Protic solvents lower the reactivity of nucleophiles, causing them to be unsuitable for amide coupling reactions, as this involves an activated carboxylic acid (Ghosh & Shahabi 2021). Additionally, amino acids are insoluble in non-polar solvents (Do et al. 2021


Cyrene (Figure 3) is a bicyclic ketone that has an intermolecular acetal functionality (Kong & Dolzhenko 2022).

Additionally, Cyrene has a viscosity that is “an order of magnitude higher than water and methanol” (Abdel et al. 2023), which serves as a limitation when used in aqueous synthesis routes. It has a polarity similar to DMF and DMSO, with a π* of 0.93 (Table 1), compared to 0.88 and 1.00, respectively.
Table 1: Table comparing the density, boiling point, miscibility with water as well as polarity of various solvents.
After: (Jordan et al. 2022, p6755)
Solvent
In the literature, there has been an exploration of amide coupling using Cyrene. Amide coupling is a nucleophilic substitution reaction between a carboxylic acid and an amine, forming an amide, in the presence of a coupling reagent (Table 1). The reaction described within the literature was a coupling reaction between p-toluic acid and aniline in the presence of DIPEA and HATU, forming 4-methyl-Nphenylbenzamide (Figure 5) (Jordan et al. 2022) However, adjustments are required to optimize the procedure for a school setting. The optimal reaction conditions in a professional laboratory were found to be delaying the addition of aniline by 10 minutes, from the addition of the other reagents. Subsequently, the reaction mixture was stirred at room temperature for 1 hour


Building upon the desire to explore medicinal applications, peptides, with particular emphasis on pharmaceutically useful dipeptides serve as a reasonable continuation. Solid phase peptide synthesis (the same reaction pathway as aqueous peptide synthesis, occurring within a resin) (Ferrazzano et al. 2019) must be conducted with solvents that have similar properties to those employed in amide coupling (aapptec, LLC n.d.), making the investigation of Cyrene a reasonable avenue to explore within this field.
How suitable is dihydrolevoglucosenone (Cyrene) for use in amide coupling reactions in PyBOP mediated procedures when conducted in a school laboratory?
Cyrene is a suitable solvent for amide coupling reactions involving PyBOP, within a school setting.
General experiment details
1H spectra were recorded at 300 K using a Bruker Avance DRX500 NMR spectrometer in deuterated solvents. Residual chloroform (δ 7.26) was used as internal reference for 1H NMR spectra. The data is reported as chemical shift (δH ppm), relative integral, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet) and assignment. Atom labels on structures are to illustrate 1H NMR spectral assignments and do not necessarily correspond to the IUPAC names given. Mass spectra were recorded by the Mass Spectrometry Unit of the School of Chemistry, The University of Sydney, Sydney.
Analytical thin layer chromatography was performed with Merck Kieselgel 60 F254 (0.2 mm) pre-coated aluminium sheets, and review was completed by inspection under UV light. Throughout the reaction process Thin Layer Chromatography (TLC) was conducted to determine the progress of the reaction and ascertain the point of completion. TLC analysis was conducted with 20:80 ethyl acetate/hexanes.
Reaction 1: Synthesis of 4-methyl-Nphenylbenzamide (compound 1) using HATU (Citarella et al. 2022)
To a 25 mL round-bottomed flask was added p-toluic acid (0.25 g, 1.8 mmol, 1 equiv), HATU (0.8 g, 2.2 mmol, 1.2 equiv), N,N-diisopropylethylamine (0.712 g, 5.5 mmol, 3 equiv), and Cyrene (9 mL, 0.07 M).
The reaction mixture was stirred at room temperature for 10 mins before the addition of aniline (0.2g, 2 mmol, 1.1 equiv) and subsequently maintained at this temperature for between 1 h and 48 h with stirring.
The solution was then diluted with EtOAc (15 mL), and washed with 1 M HCl (2 x 20 mL), sat. aq. NaHCO3 (15 mL), H2O (10 mL), and brine (20 mL). The organic layer was then dried over Na2SO4 and the solvent was allowed to evaporate in a fume hood to give a residue which was subsequently analysed.
Figure 6: 4-methyl-N-phenylbenzamide
Reaction 2: Synthesis of 4-methyl-Nphenylbenzamide using PyBOP
To a 25 mL round-bottomed flask was added p-toluic acid (0.25 g, 1.8 mmol, 1 equiv), PyBOP (1.14 g, 2.2 mmol, 1.2 equiv), N,N-diisopropylethylamine (0.712 g, 5.5 mmol, 3 equiv), and Cyrene (9 mL, 0.07 M).
The reaction mixture was stirred at room temperature for 10 mins before the addition of aniline (0.2g, 2 mmol, 1.1 equiv) and subsequently maintained at this temperature for between 1 h and 48 h with stirring.
The solution was then diluted with EtOAc (15 mL), and washed with 1 M HCl (2 x 20 mL), sat. aq. NaHCO3 (15 mL), H2O (10 mL), and brine (20 mL). The organic layer was then dried over Na2SO4 and the solvent was allowed to evaporate in a fume hood to give a residue which was subsequently analysed.
Reaction set 3
Reaction 2 was repeated four times, being terminated at 1h, 3h, 8h and 48h, respectively. However, a truncated washing procedure was employed, with no desiccation taking place. The solution was diluted with EtOAc (15 mL), and washed with 1 M HCl (20 mL), sat. aq. NaHCO3 (15 mL), H2O (10 mL), and brine (20 mL). The solvent was allowed to evaporate in a fume hood to give a residue which was subsequently analysed.
Results
Step 1: Synthesis of 4-methyl-N-phenylbenzamide using HATU

Figure 7: 1H NMR spectra after step 1
1H NMR (500 MHz, CDCl3) d 7.85 (1H, br s, NH), d 7.77 (2H, d, Ar-CH), d 7.64 (2H, d, Ar-CH), d 7.36 (2H, dd, Ar-CH), d 7.27 (2H, d, Ar-CH), d 7.14 (1H, dd, Ar-CH), d 2.42 (3H, s, CH3)
Step 2: Synthesis of 4-methyl-N-phenylbenzamide using PyBOP

Figure 8: Mass Spectrometry of PyBOP mediated reaction 1H NMR (500 MHz, CDCl3) d 7.85 (1H, br s, NH), d 7.77 (2H, d, Ar-CH), d 7.64 (2H, d, Ar-CH), d 7.36 (2H, dd, Ar-CH), d 7.27 (2H, d, Ar-CH), d 7.14 (1H, dd, Ar-CH), d 2.42 (3H, s, CH3)

Figure 9: 1H NMR spectra after step 1
1H NMR (500 MHz, CDCl3) d 7.85 (1H, br s, NH), d 7.77 (2H, d, Ar-CH), d 7.64 (2H, d, Ar-CH), d 7.36 (2H, dd, Ar-CH), d 7.27 (2H, d, Ar-CH), d 7.14 (1H, dd, Ar-CH), d 2.42 (3H, s, CH3)

Figure 10: PyBOP TLC
Step 3: series of syntheses of 4-methyl-Nphenylbenzamide using PyBOP

Figure 11: 1 H NMR spectra after 1h
1H NMR (500 MHz, CDCl3) d 7.85 (1H, br s, NH), d 7.77 (2H, d, Ar-CH), d 7.64 (2H, d, Ar-CH), d 7.36 (2H, dd, Ar-CH), d 7.27 (2H, d, Ar-CH), d 7.14 (1H, dd, Ar-CH), d 2.42 (3H, s, CH3)

Figure 12: 1H NMR spectra after 3h
1H NMR (500 MHz, CDCl3) d 7.85 (1H, br s, NH), d 7.77 (2H, d, Ar-CH), d 7.64 (2H, d, Ar-CH), d 7.36 (2H, dd, Ar-CH), d 7.27 (2H, d, Ar-CH), d 7.14 (1H, dd, Ar-CH), d 2.42 (3H, s, CH3)

Figure 13: 1H NMR spectra after 8h
1H NMR (500 MHz, CDCl3) d 7.85 (1H, br s, NH), d 7.77 (2H, d, Ar-CH), d 7.64 (2H, d, Ar-CH), d 7.36 (2H, dd, Ar-CH), d 7.27 (2H, d, Ar-CH), d 7.14 (1H, dd, Ar-CH), d 2.42 (3H, s, CH3)

Figure 14: 1H NMR spectra after 48h
1H NMR (500 MHz, CDCl3) d 7.85 (1H, br s, NH), d 7.77 (2H, d, Ar-CH), d 7.64 (2H, d, Ar-CH), d 7.36 (2H, dd, Ar-CH), d 7.27 (2H, d, Ar-CH), d 7.14 (1H, dd, Ar-CH), d 2.42 (3H, s, CH3)
Reaction 1: Synthesis of 4-methyl-Nphenylbenzamide (compound 1) using HATU
Step 1 was used to confirm the viability of an altered existing method described in the literature in a school setting. It successfully demonstrated that the reaction could be scaled and performed within a school laboratory. It involved the reaction of aniline and ptoluic acid to produce the desired compound 4methyl-N-phenylbenzamide. Specifically, it is a nucleophilic substitution reaction between activated (deprotonated) p-toluic acid and aniline.

Figure 15: Synthesis of compound 1 by amide coupling of aniline and p-toluic acid
In the 1H NMR spectrum (Figure 7) the broad singlet at 7.85ppm was assigned to the 1H NH proton as it was expected to be in this region. The doublets at 7.77ppm and 7.64 were assigned four aromatic protons. The doublet of doublets at 7.36 was assigned to two aromatic protons on the benzene ring to the left of the amino groups (Figure 16), as it experiences more deshielding from the nitrogen atom. The doublet at 7.27ppm was assigned to two aromatic protons to the left of the methyl groups, accounting for comparative upshift. The doublet of doublets at 7.14ppm was assigned to a single aromatic proton to the leftmost side of the molecule. This is the expected value for a proton within an aromatic ring. The singlet at 2.24ppm was assigned to the three protons within the methyl group. This downshift can be attributed to its proximity to an aromatic ring. The 1H NMR of the HATU mediated reaction contained considerably more impurities than the PyBOP mediated reaction pathway, which will be expounded upon in a subsequent section.

Mass spectrometry (Figure 8) was conducted, yet it gave no indication of product or starting material. However, a peak was present with a mass to charge ration of 258.23 that corresponded to a fragment of PyBOP (Figure 17). This is likely due to the acidic workup within the reaction’s parameters.

Step 2 was used to determine whether PyBOP could work as an amide coupling agent in a Cyrene based synthesis. A TLC (Figure 10) conducted 1h after the reaction commenced strongly indicated that the reaction had proceeded successfully. Additionally, the 1H NMR (Figure 10) yielded similar peaks associated with the product that were found in the HATU. In addition to the successful synthesis of the target compound, the 1H NMR (Figure 9) indicated that the final sample contained fewer impurities than the HATU based method.
Step 3: series of syntheses of 4-methyl-Nphenylbenzamide using PyBOP
Step 3 focused on examining the progression of the PyBOP synthesis route by examining its degree of completion at different intervals. A composite image of the 1H NMR (Figure 18) was created, with the 1h reaction at the top of the figure, proceeding to the 48h reaction at the bottom of the figure. The peaks with stars above them correspond to those associated with the product (compound 1). The peaks with the crosses above them correspond to the starting reagents (aniline and p-toluic acid). It is demonstrated that the reaction continues to progress over the duration of the 48h. Further research is needed to determine the optimal reaction time between 8h and 48h, as time constraints did not allow this to occur.
The applications for further research are two-fold, optimising the PyBOP synthesis route and applying Cyrene to pharmacologically relevant dipeptides.

A process of optimisation will involve determining the time at which the reaction reaches completing to a greater degree of accuracy. Additionally, it will involve varying other parameters of the synthesis (such as temperature, pH, purification steps etc.) to determine the most efficient method.
As peptide synthesis is a substantial practical application of amide coupling, it is a logical next step. The success of both a HATU mediated synthesis (Citarella et al. 2022) and a PyBOB synthesis route supports the notion that Cyrene has a broad capacity to replace existing solvents (Jordan et al. 2022). The synthesis of dipeptides involves coupling two amino acids. The example of coupling boc-phenylalanine and phenylalanine tert-butyl ester (Figure 19) is given. This class of reaction involves the same amide linkage and initial conditions (DIPEA and a coupling reagent).

Figure 19: Coupling reaction of boc-phenylalanine and phenylalanine tert-butyl ester
This research concerned developing a successful synthesis pathway of 4-methyl-N-phenylbenzamide using the bio solvent (Cyrene) in the presence of the coupling agent (Benzotriazol-1yloxy)tripyrrolidinophosphonium
hexafluorophosphate (PyBOP). The synthesis was successful, adapting an existing method found within the literature for HATU mediated amide coupling. The PyBOP synthesis pathway afforded less impurities than the HATU mediated pathway, as confirmed by 1H NMR, supporting the need for further research into exact conversion amounts. Additionally, the success of the amide coupling indicates that research into peptide synthesis has a sound foundation.
I would like to thank Dr Katie Terrett for her invaluable input throughout this project, with particular emphasis on her thorough explanation of the theory and nuances surrounding my research. I would also like to thank the University of Sydney chemical faculty for performing the NMR’s and mass spectroscopy used at each step.
aapptec, LLC n.d., Synthesis Notes aapptec, <https://www.peptide.com/custdocs/aapptec%20synthesis %20guide%202-0%20(2).pdf>.
Abdel, N, Steiner-Browne, M, & Rabah Mouras 2023, ‘Cyrene as a greener alternative to harmful solvents used in pharmaceutical cleaning’, Sustainable chemistry and pharmacy, vol. 33, pp. 101149–101149, viewed 26 June 2024, <https://www.sciencedirect.com/science/article/pii/S23525 54123001833>.
Andrew, OB, Sherwood, J, & Hurst, GA 2022, ‘A Greener Synthesis of the Antidepressant Bupropion Hydrochloride’, Journal of Chemical Education, vol. 99, no. 9, pp. 3277–3282, viewed 4 February 2024, <https://pubs.acs.org/doi/epdf/10.1021/acs.jchemed.2c0058 1>.
Brown, ID 1987, ‘Structural chemistry and solvent properties of dimethylsulfoxide’, Journal of Solution Chemistry, vol. 16, no. 3, pp. 205–224.
Citarella, A et al. 2022, ‘Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials’, International Journal of Molecular Sciences, vol. 23, no. 24, p. 15960.
Do, HT et al. 2021, ‘Measurement and modelling solubility of amino acids and peptides in aqueous 2-propanol solutions’, Physical chemistry chemical physics: PCCP, vol. 23, no. 18, pp. 10852–10863, viewed 1 February 2023, <https://pubmed.ncbi.nlm.nih.gov/33908485/>.
Ferrazzano, L et al. 2019, ‘Green Solvent Mixtures for Solid-Phase Peptide Synthesis: A Dimethylformamide-Free Highly Efficient Synthesis of Pharmaceutical-Grade Peptides’, ACS Sustainable Chemistry & Engineering, vol. 7, no. 15, pp. 12867–12877.
Flavio S.P. Cardoso et al. 2023, ‘Practical and Scalable Two-Step Process for 6-(2-Fluoro-4-nitrophenyl)-2-oxa-6azaspiro[3.3]heptane: A Key Intermediate of the Potent Antibiotic Drug Candidate TBI-223’, PubMed Central, vol. 27, no. 7, pp. 1390–1399.
Ghosh, AK & Shahabi, D 2021, ‘Synthesis of amide derivatives for electron deficient amines and functionalized carboxylic acids using EDC and DMAP and a catalytic amount of HOBt as the coupling reagents’, Tetrahedron Letters, vol. 63, p. 152719.
Jaffe, TA et al. 2019, ‘Acute and delayed toxicity from coingestion of methylene chloride and methanol’, Toxicology communications, vol. 3, no. 1, pp. 79–84, viewed 17 June 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6863342/ >.
Jordan, A et al. 2022, ‘Replacement of Less-Preferred Dipolar Aprotic and Ethereal Solvents in Synthetic Organic Chemistry with More Sustainable Alternatives’, Chemical Reviews, vol. 122, no. 6, pp. 6749–6794, viewed 11 February 2024, <https://pubs.acs.org/doi/epdf/10.1021/acs.chemrev.1c006 72>.
Kane, SP 2021, Enalapril - Drug Usage Statistics, ClinCalc DrugStats Database, Clincalc.com, viewed 12 February 2024, <https://clincalc.com/DrugStats/Drugs/Enalapril>.
Kong, D & Dolzhenko, AV 2022, ‘Cyrene: A bio-based sustainable solvent for organic synthesis’, Sustainable chemistry and pharmacy, vol. 25, pp. 100591–100591, viewed 12 May 2024, <https://www.sciencedirect.com/science/article/abs/pii/S23 52554121002187>.
Kostis, JB et al. 2004, ‘Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial’, American Journal of Hypertension, vol. 17, no. 2, pp. 103–111, <https://academic.oup.com/ajh/article/17/2/103/138811>.
Rague, J, Grush, J, & Buchanan, J 2021, Sci-Hub | A Case Series of Chemical Dermal Injury Requiring Operative
Intervention after Prolonged Dermal Methylene Chloride Exposure. Journal of Medical Toxicology, 17(2), 222–226 | 10.1007/s13181-020-00818-z, Sci-hub.se, viewed 13 June 2024, <https://sci-hub.se/10.1007/s13181-020-00818-z>.
Sainsbury, M 2016, Alicyclic Compounds, Elsevier.
Schlosser, P et al. 2014, Sci-Hub | Human Health Effects of Dichloromethane: Key Findings and Scientific Issues. Environmental Health Perspectives | 10.1289/ehp.1308030, Sci-hub.se, viewed 13 June 2024, <https://scihub.se/10.1289/ehp.1308030>.
Sci-Hub | Acute and delayed toxicity from co-ingestion of methylene chloride and methanol. Toxicology Communications, 3(1), 79–84 | 10.1080/24734306.2019.1685222 2019, Sci-hub.se, viewed 17 June 2024, <https://scihub.se/10.1080/24734306.2019.1685222>.
The Consensus Trial Study Group 1987, ‘Effects of Enalapril on Mortality in Severe Congestive Heart Failure’, New England Journal of Medicine, vol. 316, no. 23, pp. 1429–1435.
Wilson, KL et al. 2018, ‘Cyrene as a bio-based solvent for HATU mediated amide coupling’, Organic & Biomolecular Chemistry, vol. 16, no. 16, pp. 2851–2854, <https://pubs.rsc.org/en/content/articlelanding/2018/OB/C8 OB00653A>.
Yu, S et al. 2021, ‘Identifying sustainable alternatives to dimethyl formamide for coating applications using Hansen Solubility Parameters’, Journal of Cleaner Production, vol. 322, p. 129011.

In 2024, five students applied their Physics knowledge to new problems in Science.
Graham built on two previous Barker Science research projects into tensile strength of 3D printed components with a twist. Fascinated by the challenges of filament expiring when exposed to the elements he researched the effect of drying or soaking on tensile strength. Similarly, Evan extended past research into the efficacy of helmets to prevent concussion by extending Clair’s 2022 research with hockey pucks, to the field of baseball pitches and baseball helmets.
Niraj chose a well-known, but surprisingly controversial, historical experiment in Physics – Millikan’s Oil Drop Experiment from 1909 – and sought to investigate why it is difficult for his results to be replicated using similar methods in educational settings. His results and discussion will be particularly fascinating for Year 12 Physics teachers who talk about this experiment each year.
Thomas, fascinated by current energy storage limitations, created his own methodology to investigate a novel method of energy storage. He compressed air into scuba tanks and explored how energy could be obtained by a simple turbine at a later stage. Finally, Rachel explored the principle biomimicry where she applied aerodynamic features of a box fish to computer modelled and 3D printed cargoships and explored the potential benefits. She built her own wind tunnel to validate the results and we look forward to further studies using this apparatus.
Graham Kong Barker College
Commonly used 3D printing polymers, such as Polylactic Acid (PLA), Nylon-66, and High Impact Polystyrene (HIPS), can experience significant material degradation when exposed to moisture. This study investigates the impact of moisture content on the ultimate tensile strength of these 3D printed composites. Understanding how moisture affects these materials is critical for their longterm use and can inform post-processing strategies to enhance durability. PLA, Nylon, and HIPS filaments were subjected to moisture treatments by soaking in deionised water and drying. Standardised ASTM D638 specimens were 3D printed from these filaments for tensile strength testing. The results revealed that moisture content significantly affects Nylon (p= < 0.00001), while it has a less pronounced impact on PLA (p = 0.054749) and HIPS. Nylon showed the strongest correlation (r = -0.8491) due to its hygroscopic nature. PLA exhibited a weaker correlation (r =0.3969), while HIPS showed minimal changes in moisture content due to its hydrophobic properties and therefore, appropriately, minimal changes in tensile strength. These findings highlight the importance of moisture management in improving the performance and reliability of 3D printed parts. By understanding the hygroscopic behaviour of these materials, industries reliant on 3D printing technology can make informed decisions about material selection and post-processing treatments.
3D Printing Introduction
3D printing, otherwise known as additive manufacturing, utilises a layering and an incremental addition of feedstock to craft physical objects (du Plessis et al., 2022). In the realm of 3D printing, its attributes include reduced human intervention, minimised material wastage, simplified manufacturing processes, and enhanced energy efficiency, collectively positioning it as a compelling and sustainable solution for industrial applications (Jandyal et al., 2022) Within the 3D printing industry there are multiple forms of printing, each with their own unique materials and techniques. Fused filament fabrication (FFF), also known as Fused Deposition Modelling (FDM) is the most popular due to its low cost and high usability (Kuhlmann et al., 2021). In addition, 3D printing can be used to create complex geometric parts using materials ranging from glass fibre to titanium (Blok et al. 2018). As a result, the balancing of printability, weight, and strength are important and critical considerations for the overall diverse applications of these parts.
In the 3D printing process, structures are created through the extrusion of molten thermoplastics heated to ~220 °C through a nozzle assembly called a “hotend” (Wickramasinghe et al., 2020). When extruded, the thermoplastic is instantly cooled
retaining its shape and adhering to the adjacent layers. The use of thermoplastics such as polylactic acid (PLA) allows inexperienced home users to produce usable, biodegradable parts without the risk of the potential toxins that may be released from resins in methods such as stereolithography (SLA) where photosensitive resins are used to create layers (Wong & Hernandez, 2012). Increased accessibility, with machines being readily available for prices as low as $200 without requiring prior specialised knowledge, further adds to its use in a wide range of contexts.
FDM does not yet have wider practical application in part due to limitations of strength (Butt and Bhaskar, 2020). Strength is limited because of the 3D printed process. Due to the layered nature, anisotropic strength, specifically the z axis strength, is limited by interlayer adhesion. The second major limitation is the change in mechanical behaviour and material degradation when exposed to moisture or high temperatures for extended periods (Banjo et al., 2022). Prolonged exposure to moisture or high temperatures could lead to a significant decrease in tensile strength (>60%) and failure strain (>50%) (Zaldivar et al., 2018). Additionally, crystallinity has a significant influence on properties of composite material as the degree of crystallinity is highly dependent on the molar mass, thermal history and purity of the polymer.
As a result, it is inversely proportional to the toughness and tensile strength (Södergård and Stolt 2002).
Different types of 3D printers use different materials (such as polymer, ceramic, metal, and composites). For example, the 3D printing method used in this investigation utilises polymers and polymer-based composites with the addition of different types of fillers (Park et al., 2022). The three filament composites used include Polylactic Acid (PLA), High Impact Polystyrene (HIPS) and Polyamide (Nylon). The properties of each are explored in this section.
Polylactic acid is the most extensively researched and utilised biodegradable aliphatic polyester (Farah et al., 2016) in human history being derived from renewable resources such as cornstarch and sugarcane, and therefore, is an environmentally attractive material compared to traditional petroleum-based plastics with a reduced carbon footprint. PLA being an aliphatic polyester is composed of blocks of lactic acid or 2hydroxypropanoic acid (a monomer present in PLA) and can be obtained through fermentation or chemical synthesis (Khouri et al., 2024). Its structure is shown in Figure 1. Moreover, because of its methyl side groups seen in Figure 2, PLA is considered primarily hydrophobic whereby under hydrolytic conditions, the diffusion of water molecules breaks the ester bonds causing chain scission leading to significant reductions in molecular weight and mechanical properties (Banjo et al., 2022).

1: Bifunctionality of lactic acid
Source: (Khouri et al., 2024)

2: Chemical structure of PLA
Source: (Rebelo et al., 2017)
This occurs when water molecules (H₂O) break the ester of PLA, resulting in the degradation of the PLA
material as seen in Figure 3 (Ugaz, 2019; Suharjanto, 2022).

Figure 3: Chemical structure and degradation of PLA
Source: (Suharjanto, 2022)
In research conducted by Banjo et al. (2022), the absence of polar amide groups resulted in a substantial increase in moisture absorption within the first day (Figure 4), but within 7 days, the PLA was degraded to an extent which accurate measurements could not be obtained.

Figure 4: Moisture absorption behaviour of PLA after immersion in DI water
Source: (Banjo et al., 2022)
In addition, PLA is classified as semi-crystalline with an approximate crystallinity of 37% (depending on the molecular weight and polymerisation process), resulting in a tensile strength of 45–70 MPa (Khouri et al., 2024) with an advertised 45-49 MPa (Siddament, n.d.).
1.3.b Nylon
Polyamides, such as Nylon, are semi-crystalline synthetic polymers (Winnacker, 2017). These polymers are composed of monomers linked by amide groups in the structure –CO – NH – and can be derived from both natural and synthetic sources. Due to their unique chemical and structural characteristics, Nylon
is used in a wide range of applications, with a significant focus on biotechnology (Tomasini & LeónSantiesteban, 2015). The Nylon which I will be testing is Nylon-66. Nylon-66 is a polyamide derived from 1,6-hexamethylene diamine and adipic acid (Lin et al., 2006). The structure can be seen below (Figure 5).

Figure 5: Structure of Nylon-66
Source: (Polymer Science Learning Center)
However, Nylon is well known for its extensive hygroscopicity with the more polar the polyamide, the more water it can absorb. Within Nylon, highly polar groups such as amides, amines, and carbonyls are present, which is therefore responsible for the high affinity of moisture (Banjo et al., 2022). When the water molecules break up, hydrogen (H+) and hydroxide (OH-) ions are formed forming both acidic (H+) and alkaline (OH-) solutions. The solutions then react with nylon, breaking up the amide groups in the polymer, creating shorter chains and defects in it, deteriorating the polymer's mechanical properties (Shakiba et al., 2021).
As a result of its extreme hygroscopic nature, Nylon may experience a maximum moisture absorption of 10 wt.%. However, continued immersion results in a decrease in moisture absorbed at around 24 hours, indicating desorption as seen in Figure 6 (Banjo et al., 2022). Moreover, Nylon 66 is expected to yield a tensile strength of ~45 MPa (MatMake, 2024).

Source: (Banjo et al., 2022)
High Impact Polystyrene (HIPS) is one of the oldest styrenic polymers produced through bulk polymerisation of a solution of polybutadiene in styrene (Alfarraj, 2004). The production of HIPS involves a rigorous process in which during polymerisation (Figure 7), the system transitions from a polybutadiene-rich continuous phase to a polystyrene-rich continuous phase with a complex "salami" morphology of dispersed rubber particles, stabilised by poly(butadiene-graft-styrene) copolymers (Leal & Asua, 2009).

Figure 7: Production of hydroxyl-terminated polybutadiene
Source: (Radulović & Milojković, 2017)
Being a non-polar composite, HIPS displays only a slight tendency to absorb water and rarely requires drying prior to use (Martin et al., 2003). Polystyrene is a non-polar polymer that lacks hydroxyl groups (Figure 8), resulting in its inability to dissolve in water (Cook et al., 2003). In addition, polybutadiene, the other polymer HIPS comprises from, is a diene polymer made from a monomer containing two carbon-carbon double bonds. These long, repeating units of non-polar butadiene chains, lack esters and ethers, resulting in the inability to form hydrogen bonds (Liu et al., 2023).
Although HIPS is very hydrophobic, it exhibits moderate tensile strength, typically ranging from 20 to 30 MPa. On the other hand, the addition of polybutadiene in the rubber-toughening process, the polybutadiene is incorporated into the polystyrene matrix, increasing and providing HIPS with its high impact properties (Prasad & Singh, 1997).

Figure 8: Repeating unit of PS polymer chain
Source: Wikipedia
Plastics by nature are hygroscopic and therefore can attract and hold water molecules via either absorption (penetration of water molecules) or adsorption (adhesion of water molecules to the surface) (Hamrol et al., 2023) from the surrounding environment. One of the major issues with moisture in 3D printing is the formation of bubbles while extruding a filament with moisture present, as the water trapped inside the plastic would vaporise and expand upon being heated to temperatures above 100°C resulting in a sequence of bubbles forming (Hadi et al., 2023). This was seen in the surface quality of the printed samples, with high moisture material characterised with high surface roughness (seen in Figure 9).

Figure 9: Influence of material moisture on the quality of the surface of the printed samples. (magnification 240×): (a) FM 0.41%; (b) FM 0.19%.
Source: (Hamrol et al., 2023)
Within the 3D printing industry, all feedstocks (the material that comprises the filament) have their own varying levels of hygroscopicity. However, the amount of water absorbed by the material depends on the duration of exposure and the degree of its moisture (Hamrol et al., 2023). Yet filament can be dried through the process of desorption, where excess water is removed from the polymer. The intensity of the release of particles is heavily reliant on the bonding strength of water with the polymer, resulting in longterm drying for hygroscopic materials.
Table 1: Summary of hygroscopicity and expected tensile strength range
Filament material PLA Nylon HIPS
General tensile strength range (MPa) 45-70 ~45 20-30
Notes on Hygroscopicity
Effect of hygroscopicity
Low hygroscopicity compared to Nylon.
Water molecules diffuse and break ester bonds, leading to chain scission and reduction in molecular weight and mechanical properties.
Extremely hygroscopic. Absorbs up to 10 wt.% moisture, leading to desorption after continued immersion. Not hygroscopic
Water molecules form H+ and OHions, breaking amide groups, creating shorter chains, and causing defects and deterioration in mechanical properties. Not hygroscopic and therefore maintains its structural integrity
How does the moisture content affect the tensile strength of 3D printed composites made from Polylactic Acid (PLA), Nylon (Nylon-66), and High Impact Polystyrene (HIPS)?
The higher the moisture content of all three composites (PLA, Nylon-66, and HIPS) the lower the tensile strength of the 3D printed part.
To test the impact of moisture content in decreasing the interlayer tensile strength, 21 standardised specimens of each of the three different composite filaments consisting of PLA, Nylon and HIPS were produced and tested. The specimens were designed in the Solidworks™ Computer Aided Design software in the standardised ASTM D638 shape (Figure 10). Using Prusa’s PrusaSlicer, the specimens were sliced and prepared for printing with the printing parameters in Table 2
Table 2: Printing parameters and slicing values

To prepare filament for processing, 21 rolls of 2meter-long filament were cut for each composite filament. The filament rolls were then weighed, and their weight recorded. The filament was separated into seven groups, with each group undergoing a different treatment method.
4.1 Treatment 1 – Control
One of the seven groups had no processing done on them to increase or decrease water content and were tested as printed
4.2 Treatment 2 – Soak
Of the 7 groups, 3 of these specimens were immersed in deionised water (Figure 11) for varying time periods ranging from 6 hours to 15 days depending on the known hygroscopicity of the filament (Table 3).
After the filament was soaked for the respective period, the filament was carefully dried and reweighed. The percentage (%) of water absorbed was calculated using: ��������(%) = ��������ₜ ��������ᵢ ��������ᵢ × 100
Where M is percentage moisture absorption, Mt is the mass absorbed after time t (grams), and Mi is the initial mass before immersion (grams).
Table 3: Composite and soaking times
Composite Times Soaked (Days: Hours)
Nylon 5:00 10:00 15:00
HIPS 5:00 10:00 15:00
PLA 0:06 1:00 2:00


The remaining 3 groups of filament samples were dried in the eSun eBox Lite (Figure 12) for varying time periods ranging from 2.5 hours to 36 hours and temperatures depending on the known hygroscopicity of the filament. After the filament was dried for the respective period, the filament was reweighed the above formula and the percentage change calculated.
Table 4: Composite and drying times
Composite Times Dried (Hours)
Nylon 12:00 24:00:00 36:00:00
HIPS 3:00 6:00 9:00
PLA 2.5 5:00 10:00

After the drying and soaking periods, the results were recorded, as seen in Figure 13. Results were as expected, with Nylon increasing/decreasing in mass the most followed by PLA and HIPS. From these results, it is unlikely that further soaking or drying would have impacted the moisture content of PLA or HIPS. Even with extended drying, it can be seen that there was minimal change in mass of HIPS which will be important to note when seeing limited variation in tensile strength in the results. Nylon will be the most informative material for studying the hypothesis that compares moisture content and tensile strength as the changes in mass for Nylon provide a clear correlation between moisture absorption and the resulting impact on tensile strength.
Once the appropriate treatment was applied to the filament, the filament composite was printed into the dogbone shape, and labelled. The specimens were tested using a load-testing machine (Figure 14) where a DC linear actuator was used by the machine to apply a slow, steady load on the specimens, which were clamped securely to the gantry and the base using Gclamps. Elastic
could
specimens before tensile failure due to the steady application of load. The force applied was measured by a load cell (S-Type rated 2450N; model MT501) in the load-testing machine, and the maximum force exerted on the specimen was recorded by an electronic scale set to read peak value. Each specimen was loaded individually, and the scale was tared between each test. The specimens were tested at a load rate of 5 mm/s.


The relationship between water content and tensile strength of each of the three composites can be found using the graph seen in Figure 15. As expected, there was more variation in the water content of Nylon and the least variation in HIPS. The clearest result can be seen for Nylon. The results for each composite have been analysed in turn.
5.1
As can be seen in Figure 15, the PLA samples demonstrated a consistent response to both soaking and drying processes, with all soaked samples increasing in mass and all dried samples losing mass, reflecting water absorption and desorption, respectively. The mass increase for the soaked samples ranged from 0.22% to 0.65%, while the dried samples showed a mass decrease ranging from -0.11% to -0.76%. The tensile strength of PLA samples varied accordingly, with soaked samples generally displaying a slightly lower tensile strength value compared to the control, while dried samples showed higher tensile strength values (Figure 16). A regression analysis was performed on the data from the PLA to measure the relationship between moisture change and tensile strength.
As R2 = 0.158 (p = 0.054749) (after the exclusion of the outlier), there is a weak but not significant correlation between tensile strength and moisture content.

As seen in Figure 15, the Nylon samples exhibited a clear response to both soaking and drying processes, with all soaked samples increasing in mass and all dried samples losing mass, reflecting water absorption and desorption, respectively. The mass increases for the soaked Nylon samples ranged from 0.71% to 1.95%, while the dried samples showed mass decrease ranging from -0.57% to -1.52%. The tensile strength of Nylon samples varied accordingly, with soaked samples generally displaying a lower tensile strength value compared to the control, while dried samples showed higher tensile strength values (Figure 17). `A regression analysis was performed on the data from the Nylon samples to measure the relationship between moisture change and tensile strength. As R2 = 0.721 (p< 0.00001) there is a very strong and
significant correlation between tensile strength and moisture content.

17:
As seen in Figure 15, the HIPS samples exhibited minimal response to both soaking and drying processes, with all soaked samples showing a negligible increase in mass, averaging 0.19%, and many samples showing no change in mass. The dried samples exhibited a mass decrease ranging from0.186% to –0.199%, indicating minor water desorption. The tensile strength of HIPS samples varied slightly with these changes in moisture content, but the variations were minimal. Soaked samples showed a slightly lower tensile strength compared to the control, while dried samples displayed marginally higher tensile strength values (Figure 18). A regression analysis was performed on the data from the HIPS samples to measure the relationship between moisture change and tensile strength.
As R2 = 0.166 there is a very weak correlation between tensile strength and moisture content.

18: Percentage
Discussion
The results, in confirmation with the hypothesis, to an extent, demonstrate the negative effect of moisture
content on the tensile strength of the composites. Among the three composites tested, the impact of moisture content on tensile strength was most pronounced in Nylon, which exhibited a strong negative correlation (R = -0.8491).
In contrast, HIPS, being a hydrophobic composite, showed minimal moisture absorption/desorption, resulting in a weak correlation (R = -0.4073). The correlation test for HIPS is not sufficient to support or refute the hypothesis because the water percentage change was too minimal to significantly impact the tensile strength. This indicates that exposure to moisture had limited effect on both moisture content and tensile strength in HIPS.
PLA, although relatively hygroscopic, showed only a small amount of mass change through soaking and drying. This resulted in a very weak correlation (R =0.2191) between moisture content and tensile strength which is consistent with, but very weak evidence for the hypothesis. The limited mass change suggests that PLA's tensile strength is less affected by moisture content compared to Nylon.
Of the three composites tested to analyse the impact of moisture content on tensile strength, PLA yielded a very weak correlation between moisture content and tensile strength. All PLA samples experienced a tensile strength matching the advertised 45-49 MPa (as identified by the supplier, SIDDAMENT). Moreover, water absorbed by the filament was recorded similar to the advertised 0.3% absorption by SIDDAMENT. Upon the soaking and drying of PLA, a clear trend was observed with mass increasing as immersion time increased, while mass decreased as it was dried. This affirmed the hygroscopic nature of PLA. However, in each soaking process for PLA, one result was on average, higher than the others. This is evident in the one-day soak process, where a mass increase of 0.65% was recorded, which is double the amount that the other two had increased by. This could be a result of the position in which the filament was submerged increasing the surface area in contact with water and thus allowing for higher diffusion rates
However, the impact of water content on the tensile strength was not as evident as hypothesised. During the printing process, the presence of moisture in the PLA filament was noticeable. As the filament was heated to its extrusion temperature, the absorbed moisture boiled and vaporised, creating bubbles within the filament. These bubbles expanded and formed small voids in the printed material, which were audible as popping sounds during printing. The mean
results (excluding outliers) all fell within the advertised MPa range of the filament. However, an outlier was observed in the 2.5-hour dry where a tensile strength of 35.37 MPa was recorded, resulting in a large increase of σ for the process. Thus, the outlier was removed from the regression analysis.
Another interesting observation was the tensile strength recorded for a part immersed for 6 hours. With an increased mass % of 0.33%, a tensile strength of 52.83 MPa was recorded. Although being submersed in water for 6 hours, the MPa yielded was among the higher end of results recorded.
As a result of the weak correlation (r = -0.3969) between the moisture content of PLA and the tensile strength of the part, it may be possible that the soaking times ranging from 2 days to 6 hours were not sufficient to degrade the PLA as stated by Ugaz (2019).
Nylon composite, on the other hand, resulted in a very strong correlation between moisture content and tensile strength. Between the 31 samples printed, an average tensile strength was calculated as 46.15 MPa; slightly higher than the advertised 40 MPa by DuPont™ Zytel®. Upon soaking and drying the filament, a clear trend was established with mass increasing to a maximum of 1.8% when immersed and a minimum of –1.14% when dried.
In comparison to PLA, the impact of water content on tensile strength was significantly more evident: A strong correlation of -0.8491 was found between the moisture content recorded and the tensile strength of the 3D printed part (Pearson’s r = -0.8491, p= < 0.00001). In addition, no significant outliers (α = 0.05) were reported.
When comparing results, it was noted there was a ~35% difference in tensile strength between the highest and lowest moisture content, a result which would be critical for applications requiring precise mechanical performance. This significant variation underscores the importance of understanding and controlling moisture content in 3D printed composites to ensure consistent and reliable performance, particularly in demanding industrial environments.
As the filament was soaked for a relatively longer time compared to PLA, this may have allowed for the highly polar groups to form Hydrogen bonds, deteriorating the polymers mechanical properties as stated by Banjo et al. (2022) and Shakiba et al. (2021).
The results for HIPS indicated that moisture content did not significantly affect the tensile strength (tensile strength) of the material. This observation aligns with the hydrophobic nature of HIPS, which inherently resists moisture absorption. Of the 28 filament samples printed with processes applied, an average tensile strength of 19.69 MPa was recorded, lower than the advertised 27 MPa as advertised by eSUN. Throughout the immersion of HIPS into deionised water, no significant results were found with a surprising 9/12 samples reflecting a 0% increase in mass. This, however, was slightly less evident in the drying process, with only three out of ten samples experiencing a 0% change. Regardless of this, the increases and decreases of mass through the absorption/desorption was insignificant as changes were minor and, in all situations, was a ±1 g change.
In addition, given the minimal moisture absorption, the tensile strength of HIPS samples also did not show significant variation across different soaking and drying treatments. Although a weak correlation of r = -0.4073 was calculated, a correlation test would not be valid as the changes in moisture content were too small to meaningfully affect the tensile strength. This consistency in tensile strength values can be attributed to the hydrophobicity of HIPS, thus confirming the hydrophobic nature of HIPS.
It can also be noted that HIPS is comparatively weaker than Nylon and PLA, regardless of moisture content. When clamping the sample to the BridgeBuster, audible cracking indicated potential fractures in the parts before being pulled apart. Additionally, although printed within a closed, ventilated environment, the lack of an enclosure, as recommended by the producer, might have impacted the printing result and the interlayer bonds of the part, therefore impacting tensile strength.
To draw conclusions, this investigation confirmed that moisture content significantly affects the tensile strength of 3D printed composites, particularly in Nylon-66 and to a lesser extent, in PLA. Through controlled soaking and drying treatments on PLA, Nylon, and HIPS, variations in mass were observed, reflecting changes in moisture content. Nylon specimens exhibited the most significant changes with mass increases in soaked samples up to1.95% and mass decreases in dried samples down to -1.52%. This caused noticeable differences in tensile strength, with soaked samples showing lower and dried samples showing higher tensile strength compared to the
control. The strong negative correlation (r=-0.8491, R2=0.721) highlighted the critical impact of moisture content on Nylon's mechanical performance. PLA samples showed a weaker correlation between moisture content and tensile strength. The mass increase for soaked PLA samples ranged from 0.22% to 0.65%, while the mass decrease for dried samples ranged from -0.11%to -0.76%. The correlation coefficient (r=0.3969, R2=0.158) indicated that the relationship between moisture content and tensile strength in PLA is minimal. HIPS, being hydrophobic, displayed minimal response to both soaking and drying treatments. The mass increase for soaked HIPS samples was negligible at 0.19%, with many samples showing no change in mass. The mass decrease in dried samples ranged from -0.186% to -1.99%. The weak correlation (R2=0.166) confirmed that moisture content had little effect on the tensile strength of HIPS. Future research should focus on extending the soaking and drying times for PLA to provide more definitive insights into its moisture absorption and desorption behaviours and their impact on tensile strength. Additionally, exploring other composite materials with varying hygroscopic properties could broaden the applicability of these findings and offer new insights into material selection for different environmental conditions.
This research has been made possible by the Barker College Science, and Design & Technology departments. I would like to thank Dr Matthew Hill. Throughout this project, he has provided invaluable advice and guidance into my report.
References
Alfarraj, A. and Bruce Nauman, E. (2004). Super HIPS: improved high impact polystyrene with two sources of rubber particles. Polymer, 45(25), pp.8435–8442. doi:https://doi.org/10.1016/j.polymer.2004.10.005.
Banjo, A.D., Agrawal, V., Auad, M.L. and Celestine, A.D.N. (2022). Moisture-induced changes in the mechanical behavior of 3D printed polymers. Composites Part C: Open Access, 7, p.100243. doi:https://doi.org/10.1016/j.jcomc.2022.100243.
Blok, L.G., Longana, M.L., Yu, H. and Woods, B.K.S. (2018). An investigation into 3D printing of fibre reinforced thermoplastic composites. Additive Manufacturing, 22, pp.176–186. doi:https://doi.org/10.1016/j.addma.2018.04.039.
Butt, J. and Bhaskar, R. (2020). Investigating the Effects of Annealing on the Mechanical Properties of FFF-Printed Thermoplastics. Journal of Manufacturing and Materials Processing, 4(2), p.38. doi:https://doi.org/10.3390/jmmp4020038.
Cook, P.A., Hall, S. and Donahue, J. (2003). Pondering Packing Peanut Polymers. Journal of Chemical Education, 80(11), p.1288A. doi:https://doi.org/10.1021/ed080p1288a.
du Plessis, A., Razavi, S.M.J., Benedetti, M., Murchio, S., Leary, M., Watson, M., Bhate, D. and Berto, F. (2022). Properties and applications of additively manufactured metallic cellular materials: A review. Progress in Materials Science, 125(100918), p.100918. doi:https://doi.org/10.1016/j.pmatsci.2021.100918.
Farah, S., Anderson, D.G. and Langer, R. (2016). Physical and mechanical properties of PLA, and their functions in widespread applications A comprehensive review. Advanced Drug Delivery Reviews, [online] 107, pp.367–392. doi:https://doi.org/10.1016/j.addr.2016.06.012.
Hadi, A., Kadauw, A. and Zeidler, H. (2023). The effect of printing temperature and moisture on tensile properties of 3D printed glass fiber reinforced nylon 6. Materials Today: Proceedings, [online] 91, pp.48–55. doi:https://doi.org/10.1016/j.matpr.2023.04.641.
Hamrol, A., Góralski, B., Wichniarek, R., and Kuczko W. (2023). The Natural Moisture of ABS Filament and Its Influence on the Quality of FFF Products. Materials, 16(3), pp.938–938. doi:https://doi.org/10.3390/ma16030938.
Jandyal, A., Chaturvedi, I., Wazir, I., Raina, A. and Ul Haq, M.I. (2022). 3D printing – A review of processes, materials and applications in industry 4.0. Sustainable Operations and Computers, [online] 3, pp.33–42. doi:https://doi.org/10.1016/j.susoc.2021.09.004.
Khouri, N.G., Bahú, J.O., Blanco-Llamero, C., Severino, P., Viktor O.C. Concha and Souto, E.B. (2024). Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications - A Review of the Literature. Journal of molecular structure (Print), 1309, pp.138243–138243. doi:https://doi.org/10.1016/j.molstruc.2024.138243.
Kuhlmann, C., Blum, J.C., Schenck, T.L., Giunta, R.E. and Wiggenhauser, P.S. (2021). Evaluation of the Usability of a Low-Cost 3D Printer in a Tissue Engineering Approach for External Ear Reconstruction. International Journal of Molecular Sciences, 22(21), p.11667. doi:https://doi.org/10.3390/ijms222111667.
Leal, G.P. and Asua, J.M. (2009). Evolution of the morphology of HIPS particles. Polymer, 50(1), pp.68–76. doi:https://doi.org/10.1016/j.polymer.2008.10.035.
Lin, D.-J., Chang, C.-L., Lee, C.-K. and Cheng, L.-P. (2006). Fine structure and crystallinity of porous Nylon 66 membranes prepared by phase inversion in the water/formic acid/Nylon 66 system. European Polymer Journal, 42(2), pp.356–367. doi:https://doi.org/10.1016/j.eurpolymj.2005.07.007.
Liu, J., Yang, D., Li, S., Bai, C., Tu, C., Zhu, F., Xin, W., Li, G. and Luo, Y. (2023). Synthesis and characterization of hydroxyl-terminated polybutadiene modified low temperature adaptive self-matting waterborne polyurethane. RSC advances, 13(10), pp.7020–7029. doi:https://doi.org/10.1039/d3ra00412k.
Martin, M.F., Viola, J.P. and Wuensch, J.R. (2003). Preparation, Properties and Applications of High‐impact Polystyrene. Modern Styrenic Polymers: Polystyrenes and Styrenic Copolymers, pp.247–280. doi:https://doi.org/10.1002/0470867213.ch12.
matmake.com. (n.d.). Nylon 66 (PA-66) - Properties. [online] Available at: https://matmake.com/materialsdata/nylon-66-properties.html [Accessed 6 Jun. 2024].
Park, S., Shou, W., Makatura, L., Matusik, W. and Fu, K.K. (2022). 3D printing of polymer composites: Materials, processes, and applications. Matter, 5, pp.43–76.
Prasad, A.R. and Singh, R.P. (1997). Recent Developments in the Degradation and Stabilization of High-Impact Polystyrene. Journal of Macromolecular Science, Part C, 37(4), pp.581–598.
doi:https://doi.org/10.1080/15321799708009649.
Shakiba, M., Rezvani Ghomi, E., Khosravi, F., Jouybar, S., Bigham, A., Zare, M., Abdouss, M., Moaref, R. and Ramakrishna, S. (2021). Nylon A material introduction and overview for biomedical applications. Polymers for Advanced Technologies, 32(9), pp.3368–3383. doi:https://doi.org/10.1002/pat.5372.
SIDDAMENT. (n.d.). TDS. [online] Available at: https://siddament.com.au/pages/tds [Accessed 13 Jun. 2024].
Södergård, A. and Stolt, M. (2002). Properties of lactic acid based polymers and their correlation with composition. Progress in Polymer Science, [online] 27(6), pp.1123–1163. doi:https://doi.org/10.1016/s0079-6700(02)00012-6.
Suharjanto, G. and Adi, J.P. (2022). Design and manufacture of polylacticacid (PLA) filament storage for 3-dimensional printing with composite material. IOP Conference Series: Earth and Environmental Science, 998(1), p.012028. doi:https://doi.org/10.1088/1755-1315/998/1/012028.
Tomasini, A. and H. Hugo León-Santiesteban (2015). Nylon uses in biotechnology. Elsevier eBooks, pp.319–346. doi:https://doi.org/10.1016/b978-1-78242-373-7.00006-8.
Ugaz, V. (2019). Introduction to Polymers - Lecture 2.4.Polylactic acid (PLA). [online] www.youtube.com. Available at: https://www.youtube.com/watch?v=FSslKK358tM.
Wickramasinghe, S., Do, T. and Tran, P. (2020). FDMBased 3D Printing of Polymer and Associated Composite: A Review on Mechanical Properties, Defects and Treatments. Polymers, 12(7), p.1529. doi:https://doi.org/10.3390/polym12071529.
Winnacker, M. (2017). Polyamides and their functionalization: recent concepts for their applications as biomaterials. Biomaterials Science, 5(7), pp.1230–1235. doi:https://doi.org/10.1039/c7bm00160f.
Wong, K.V. and Hernandez, A. (2012). A Review of Additive Manufacturing. ISRN Mechanical Engineering, [online] 2012, pp.1–10. doi:https://doi.org/10.5402/2012/208760.
Zaldivar, R.J., Mclouth, T.D., Ferrelli, G.L., Patel, D.N., Hopkins, A.R. and Witkin, D. (2018). Effect of initial filament moisture content on the microstructure and mechanical performance of ULTEM® 9085 3D printed parts. Additive Manufacturing, 24, pp.457–466. doi:https://doi.org/10.1016/j.addma.2018.10.022.
Niraj Sesha Barker College
Robert Millikan's seminal 1913 experiment, "On the Elementary Electrical Charge and the Avogadro Constant," provided crucial evidence for the existence of the electron and accurately measured its charge and mass. Despite its acclaim as ingenious and simple, the experiment in modern educational settings, such as high-school laboratories, remains challenging due to the complexity of data analysis and the precision requirements during data collection. This research aimed to address these challenges demonstrate the replicability of Millikan’s experiment. The methodology involved measuring the terminal velocity and balancing the forces acting on the 100 latex droplets, to calculate their respective charge between two charged plates. Statistical analysis and iterative computational methods were employed to identify the elementary charge, though the results highlight inherent difficulties in achieving precise outcomes in a high school setting. Results indicate significant variability in measurements, with many factors influencing accuracy, including environmental conditions and equipment limitations. This study underscores the educational value and practical challenges of performing advanced physics experiments in high school labs, providing insights into improving experimental design and student engagement and posits the question of how beneficial the experiment truly is to teach practically.
Robert Millikan, in his 1913 paper titled "On the Elementary Electrical Charge and the Avogadro Constant”, demonstrates the existence of a previously speculated electron, while, to a relative accuracy determining its charge (Millikan, 1913). His research and discovery brought forth large strides in electrodynamics and quantum mechanics research which was only recently introduced. Although regarded by many to be the “most beautiful experiment of our time” (Klaasen, 2007), its difficult nature posits a question of whether an experiment is truly effective if it is unable to be completed repetitively lower-level settings such as that of a high school laboratory and still produce acceptable results.

Source:
Millikan's meticulous measurements provided a groundbreaking value for the charge of the electron, with remarkable accuracy for its time (Klaasen, 2007). His result, 1.5924 x 10-19 coulombs, is clearly very close to the accepted value of approximately 1.602 x 10-19 coulombs, demonstrating the experiment's enduring accuracy and precision, and suggesting high validity. Milikan used single oil droplets in a controllable charged chamber and analysed their motions to determine the net forces upon each one. He first analysed the motion of each droplet downwards when no opposing voltage was supplied, and concluded the acceleration and mass of each droplets and ultimately the total force downwards. He then adjusted the voltage until it was identified that an oil drop when stationary or moving at constant velocities, had a lack of net acceleration, which, through Newtons second law (F=ma) meant both the force of charge upwards and gravity downwards were balanced using his apparatus seen in Figure 2. Thus, he was able to conclude the Force caused by charge on multiple droplets and therefore the charge on each droplet.

Source: (Millikan, 1913)
“Millikan made repeated measurements of this type on many drops. He observed that the charge changed in steps between one passage of the droplet and the next, and that these steps were multiples of a common elementary charge, now known to be 1.602×10–19C” (University of Oxford, 2022). This development created huge strides in electrodynamics and magnetics as well quantum mechanics through the photoelectric effect which Millikan was also a part of. Although innovative, the final experimental value for e were still questioned by scientists at the time. For example, Sir Arthur Eddington’s paper provides a contrasting perspective on the experiment where he argues that according to only the recently developed theory of quantum mechanics that Millikan’s experimental charge was wrong. He claims that “Since electric charge is only manifested in the interaction between two charges it is useless to consider a solitary electron” (Eddington, 1929, pp.1). While he argues against the purpose of Millikan’s research itself, he also, through calculations claims that Millikan’s calculated charge was wrong, stating that “The fault cannot lie within the theory rather in the error attributed to the experimental value” (Eddington, 1929, pp.1).
Such controversial nature of the initial experiment itself is further drawn on by Niaz (2005). He compares the two prevalent experiments by Millikan and Felix Ehrenhaft, who both formulated an elementary electron charge (see Niaz (2005)). He suggests that “Ehrenhaft’s methodology approximated the traditional scientific method, which did not allow him to discard anomalous data. Ehrenhaft’s method involved using water droplets which may occasionally pick up a stray electron causing it to move towards the positive plate. On the other hand, Millikan’s data selection procedure depended primarily on his commitment to his presuppositions (existence of e) and as such faced some degree of ‘misconduct’. ” (Niaz, 2005, pp.1). This was due to his ability to
disregard outliers, similar to how many may complete the experiment today where they presuppose the accepted value of e and disregard measured charges which don’t approximately represent integer multiples of e. Kapusta (1975) reported on a detailed calculation of the competing effects of reaction time and Brownian motion in performing the Millikan experiment. Kapusta showed that for any apparatus, there is a measuring time producing a minimum relative error. Typically, the optimum measuring time will be of the order of 10 seconds (Kaputsa, 1975).
The paper identifies a central problem, that of appropriate drop selection which Niaz also draws on, while showing why the drop must fall in a particular range of velocities to be selected, thus bringing Millikan’s selection and testing to question.
While Millikan's experiment is lauded for its elegance and significance, reproducing it in a high school laboratory setting presents several challenges. The experiment requires a controlled environment to minimise external factors that could affect the droplets' motion, such as air currents and contamination. (Allen & Raabe, 1982). Additionally, precise measurements of droplet motion and electric field strength are essential for accurate results (Kaputsa, 1975), necessitating sensitive instruments. In the setting of a 21st century high school, sensitivity and variety of instruments is limited, thus accurate measurements of more minor environmental features such as temperature, air density and oil density are either forgone or approximated. Since at least 1933, the Millikan oil drop apparatus has been commercially available for the student physics laboratory (Harnwell & Livingwood, 1933), however it is typically used imprecisely and is simply for students to understand the nature of the experiment rather than complete it. Kruglak concludes that “the experiment (Millikan’s) remains perhaps the most frustrating of all the exercises in the undergraduate laboratory” (Kruglak, 1972, p. 769). Additionally, the ingenious nature of the experiment is further contrasted ‘with the laboratory experience of students and instructors in performing the experiment, for “as a teaching-lab experiment it does not enjoy a good reputation for three principal reasons: eyestrain, tedium, and poor, unconvincing results” questioning whether the experiment, indeed, still has justifiable educational value’ (Heering & Klassen 2011, pp. 1 quoting Jones, 1995).
One of the earliest journal publications discussing the Millikan experiment in the student laboratory was Olsen’s evaluation of the new Millikan oil-drop
apparatus manufactured by Pasco Scientific (Olsen, 1965) (see figure 3).
Figure 3: The Pasco apparatus that Olson used and analysed in his paper
Olson observed that “favourable regard for the pedagogical value of this experiment has not slackened with time” (Olson, 1965, p. 865). In a 1974 paper, Heald (1974) reported on a simplified approach to the student Millikan experiment in which all data from a particular class was pooled and plotted as the class proceeded, so that the aspect of atomicity could be demonstrated in a single period. While this solved the issue of limited data, he argued it did not fix the issues to do with data collection (Heald, 1974).
Some authors suggested the use of a remote-control lab environment (Grober et al., 2007), or simulations (MacIsaac, 2007). Others suggested modifications of the experimental setup such as an improved light source by using a laser to assist in determining droplet motion (Brehmer, 1991; Cheng & Hsu, 2005) or improving the observation of the oil drops using a CCD-camera (Papirio et al 2000, Silva and Mahendra 2005). However, there has been no significant progress in identifying the underlying nature of the problems and solving them. For example, none of the existing studies have analysed the nature of student difficulties from the perspective of the students. Kruglak (1972) does poll students’ attitudes towards the experiment but does not attempt to study the nature of their difficulties. Subsequently, while Klaasen (2009) successfully identifies these issues and discusses the underlying problems of experimentation in school, he fails to provide substantial improvements or viable assistance to teachers and students in fixing such issues. Contrastingly, Sharma & Ahluwalia (2018) tested the feasibility of the experiment through virtual means, using the IOP science application for the Millikan oil drop experiment. Although it was successful it was all virtual and requires far less student engagement.
Hodson affirms the necessity of using such experiments during the study of physics stating that “children learn best by direct experience” (Hodson, 1988, pp.2) thus emphasising the benefits of hands-on activities to gain student engagement and conceptual learning. As the Millikan Oil drop experiment is a well-studied experiment in the Year 12 Physics course in NSW in their study of electro-magnetism and basic quantum mechanics, it, consistent with the views of Hodson (1988), should be replicated in class for maximum engagement and understanding from the experiment.
1. Is it possible to quantify and determine the magnitude of a fundamental unit of charge specifically associated with an electron.
2. Is the Millikan oil drop experiment in its simplistic nature feasible in the setting and constraints of a high-school laboratory to produce accurate results regarding the charge of the electron.
That it is possible to replicate a form of Millikan’s oil drop experiment to determine the fundamental charge of the electron in a high school laboratory with sufficiently simple apparatus and methodology, while achieving sufficiently accurate results.
The research question aimed to quantify the charge of an electron by analysing the motion of a set of 100 oil drops, utilising quantitative data on drop terminal velocity and the voltage applied to the chamber to arrest the motion and balance the downwards force (gravity-buoyancy) with the upwards force (electrostatic). The terminal velocity data (in m/s) were used to determine the mass and thus the total force of gravity acting downward on each drop. Subsequently, the voltage required to keep each drop stationary was recorded.

Two of the important variables in this experiment include velocity (v) and voltage (V). While they use the same letter, it is important to distinguish between the lower-case v for velocity, and upper-case V for voltage.
Part 1: Producing charged oil drops
The product by Industrial Equipment and Control Pty Ltd, titled "Millikan's Apparatus," (See Figure 3) was used for all data collection. Unlike the original method, this apparatus used a latex liquid instead of oil for analysis. A scope was set up to view the drops within the chamber, as the atomised latex drops had micrometer radii. The scope provided a scale ranging from 1 to 5 units, where each unit corresponded to 0.000519047619 meters. A 30-watt light was used to illuminate the inside of the chamber. The atomiser was filled with 3-4 ml of the latex solution and a voltmeter was attached parallel to the voltage source.
In contrast to Millikan, who charged oil droplets using X-rays that ionized the air and caused electrons to attach to the drops, the modern apparatus involved using a latex liquid. After atomization, these drops moved through a rubber tube that interacted with the latex properties of the liquid, imparting a static charge to the drops. This produces an unknown number of electrons on each drop, potentially into the hundreds. After focusing the scope, and starting a stopwatch, multiple latex drops were injected into the chamber using the squeezing/puff mechanism. The particle needed to be sufficiently large such that minute changes in voltage would not drastically alter its motion.
Part 2: Measuring velocity to determine mass, size and net downward force
The stopwatch was used to measure the time taken for the drop to travel four units and was repeated 3 times to calculate the mean fall time. Measuring over four units minimized errors associated with reaction time. This process was repeated 100 times over a span of eight weeks to replicate Millikan’s original method which tested 150 oil drops. The velocity was used to calculate a value of the radius (r) by neglecting the correction to Stokes' law however accounting for buoyancy afterwards.

Equation 1: equation for radius with a given terminal velocity

��������ℎ������������������������ :
=
�������� = 9 8 ��������/�������� 2
�������� = 686 67 ����������������/��������3
≈ 1 82 ∗ (10 5 )
This was then used to calculate the volume of the spherical body, and by multiplying by the density of 686.67 the mass could be calculated. Therefore, from the velocity, the total force downwards could be calculated using Equation 1 (see Figure 6)
Equation 2: Equation for total force downwards
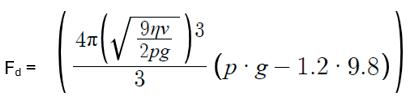

Figure 6: Simplified diagram of the forces acting on the droplet. The diagram does not account for buoyancy, it is solely used to simply represent the net force on each drop
Part 3: Measuring Voltage required to produce an Electric Force to counteract known downward force and hold the drop stationary
The voltage in the chamber was adjusted until the selected drop was stationary (or moving very slowly).
A particle speed was deemed slow enough if it took over 10 seconds to travel one unit (0.000519047619 meters), equivalent to 0.0000519 meters per second. At such low velocities, net acceleration could be approximated to zero, indicating balanced forces, and allowing for further analysis of the drop's motion. The voltage was then recorded from the voltmeter.
Part 4: Calculating an experimental measurement of charge on the droplet
Using the electric force equation Fe =qE where E= V/d, the electric force (Fe) between two charged plates of distance (d) is given by Fe =qV/d. For a given
voltage (V) for each droplet, the net electric force and force downwards are equal due to the stationary nature of the droplet ∴ Fe = Fd ∴ Fd = qV/d ∴ q = Fdd/
Finally, the charge (q) as a function of voltage (V) and velocity (v) is given by
Equation 3: calculation for q using variables v and V

Part 5: Analysis – Finding the highest common factor amongst the identified charges
Identifying the common electron charge from the experimental charge poses a difficulty. While for Millikan this process was complex, but succeeded with his high-quality data, a more realistic replication of the experiment did not do that. The process involved dealing with inherent experimental errors, which can introduce variability in the measurements and required a nuanced approach in analysing and interpreting data. These errors necessitate careful statistical analysis to distinguish between true values and anomalies. Additionally, the data collected might include outliers or inconsistent points that could skew the results if not properly addressed. Furthermore, identifying the elementary charge �������� involves recognizing patterns in the calculated charges, which may not be immediately apparent without sophisticated computational methods.
Iterative computational method of determining common factors
To determine possible candidates for the highest common factor, a program was written that would iteratively test increasing values of a possible common factor for goodness of fit with all the included values for charge. Goodness of fit was measured by calculating the average remainder when each charge was divided by the common factor to be tested. 1000 values were tested from 1.000x10-19 to 2.000x10-19 increasing the potential common factor by 0.001x1019 each time. A scatter plot was produced such that any minimum values of residuals could be further investigated as possible highest common factors.
An alternative method to determine the highest common factor can be done by using the known value of e. Each experimentally calculated charge was divided by e and the remainders can be depicted in a histogram which allowed for analysis of the frequency and amount of charges across drops.
At the end of data collection, a total of 100 random drops had been selected and visually analysed to determine velocity and stationary voltage. The second column of Table (1) titled “velocity (m/s)” records the terminal velocity of all drops’ descent under gravity. All drops were analysed by the same apparatus and had the same chemical makeup.
Table 1: A table showcasing the collected voltage (Column 3) and velocity (Column 2) of all drops and the final calculated charge (Column 4)
The calculated terminal velocities were solely a function of mass which depended on the atomiser. Most drop velocities fell between 5-20 (m/s)-5 meaning that most drops had a net force downwards ranging between: 1.34x10-14 - 1.07x10-13N with 23 other droplets lying outside this range. It is also important to note that the particles that fall within this range have a calculated radius between: 1x10-7 –1.56x10-6 m while the instruction manual for the apparatus states that the atomised particles will have radius approximately = 5x10-7. The average mean velocity 0.0014 m/s and its mean radius 1.32x10-6 m falls outside the expected radius thus identifying certain flaws in the apparatus instructions. This variable was tested independently to determine the size of each droplet.

Determining charge on each drop
After testing all 100 drops by determining velocity and voltage of each one, while assuming other environmental factors (Barometric Pressure, Viscosity of air) were kept consistent, the charge on each drop was calculated using equation 3. In order to visualise the data in a more helpful way, a histogram was produced showing the distribution of charges (Figure 8). The horizontal categories were multiples of the charge of an electron: 1-10, 11-20, etc.

Figure 8: Histogram showcasing the distribution of electrons in the 100 droplets. Shows how many droplets (y axis) have a certain amount of electrons (x axis) using the known charge of the electron (e)). The amount of charges on droplets is shown above with peaks at 10-20 charges with a median score of 41.90059. The three droplets that had greater than 160 electrons were considered and removed as outliers in order to improve the data quality and allow for the highest common factor to be determined more easily.

Figure 9: A plot of the computational results to determine highest common factor. In theory, the average remainder should drop much closer to zero for a common factor. The orange line at HCF=1.602x10-23 represents the known charge of the electron and not a data point. While there is a local minimum at approximately 1.602E-19 this is not considered a valid candidate for the HCF as it is indistinguishable from various other local minimum, it is not the lowest minimum, and it’s not substantially lower than other values as might be expected.

Figure 10: To demonstrate that various local minima appear even for random numbers, the program from Figure 3 was applied to three random data sets with values of the same order of magnitude as the measured charges. Similar patterns emerge. While there is some fluctuation, and a local minimum at approximately 1.602x10-19, there is no obvious common factor between 1.000x10-19 and 2.000x10-19. While it’s possible that it is simply very difficult to identify common factors, it is more probable that the results data could be considered unreliable (due to the similarities to what appears for a set of random numbers).
Each score of q/e is rounded to the nearest integer and the difference between q/e – q/erounded is taken. With a mean value of 0.01017 charges from the nearest integer. The difference is plotted with velocity (v).

Figure 11: A graph of velocity vs Absolute value of remainders when calculated charge q is /e

Figure 12: A graph of Remainder vs test number. The two variables are not correlated as test number is just a unique identifier of each drop. The graph rather showcases the distribution of remainders of q/e. Scores which lie below y=0.1 indicates results closest to a whole number integer when the calculated charge is /e. However, those closer to y=0.5 indicates droplets that are far off a whole number integer when q is /e.
Discussion
Overview of findings
This study aimed to evaluate the feasibility of replicating Millikan’s oil drop experiment in a high school laboratory setting. The experimental data obtained of 100 droplets revealed significant inconsistencies and deviations from the accepted value of the elementary charge (e ≈ 1.602 x 10-19 C). Multiple attempts were made to identify the highest common factor in the data; however, this proved very difficult, introducing another form of difficulty for school environments. These findings challenge the hypothesis and underscore the impracticality of conducting this experiment accurately in a high school environment.
The primary obstacle faced in the completion of the experiment was most likely limitations inherent to the laboratory apparatus. While it is possible the poor experimental practices may have caused errors, the extensive research and preparation involved in setting up and using the apparatus over the 8-week span should have minimised these errors. More
importantly, the literature suggests that there are broader constraints that prevent accurate replication of the historic experiment. Key limitations identified in the literature include: “poor, unconvincing results”
(Heering & Klassen 2011, pp. 1). This is resultant of the lack of precise and sensitive instrumentation essential to accurate results (Kaputsa, 1975). Although written in 1975 Kaputsa’s paper remains relevant today due to the limited enhancements made to technology regarding the Milikan oil drop experiment. Additionally, Kaputsa states that Millikan’s oil drops had to have fallen in a certain range of velocities to be acceptable (Kaputsa, 1975). However, due to the new apparatus, this velocity range cannot be successfully determined and may allow for incorrect data points to be analysed.
The commercially available Millikan apparatus also introduced several significant sources of error:
- The atomiser used to create the latex droplets showed considerable inconsistency, resulting in droplet radii that deviated significantly from the expected values. The manual’s stated radius of 5 x 10-7 meters was rarely achieved. The observed radii varied widely between 7.81x10-7 – 1.56x106, directly affecting the calculated mass and force of each droplet. Possible experimental sources of error for these masses (as determined by the measured velocity) include reaction-time errors in using the stopwatch, or issues with the scale on the scope.
- Using latex instead of oil droplets added variability. The charge distribution on latex droplets likely differs from that on oil droplets, affecting the experiment's replicability. This substitution, necessary due to practical constraints, contributed to the observed discrepancies in charge measurements. The density of the latex solution is a key factor in determining the radius and buoyancy of the drop. Although the density of the latex solution 1030 kg/m3 the company states that the latex liquid used in the actual experiment is “approximately 2 parts latex with 1 part water” which was very ambiguous and may have affected the radius and net Fd calculations which subsequently affected that charge calculations. Using that statement the density of the final solution was measured to be 686.67 kg/m3.
- Multiple instruments were used to measure and adjust each droplet’s motion, including the scope, voltage adjuster, voltmeter and
stopwatch. The microscope's resolution and the voltage source's stability were insufficient for capturing fine details of droplet behaviour, leading to potential measurement errors. The voltmeter had an error of ±3% which resulted in the highly variable voltage readings required to keep drops of similar terminal velocities stationary.
Environmental and experimental constraints also played a large role in determining results. While Millikan and his fellow scientists created a completely controlled environment with a constant temperature, barometric pressure, air currents and humidity levels in his single testing period, limitations inherent to the school setting further proves the lack of feasibility and ability of the Oil Drop experiment is be replicated. Regardless of attempts to mitigate the effect of these factors on the droplets motion, by cleaning the apparatus extensively after each testing period to try and replicate similar environments over the total 8 weeks, the setting inherently lacked the ability to control these environmental variables (see Heering & Klaasen, 2011). These rigorous efforts to standardise the procedure, still provided substantial variability across all measured parameters, consistent with the challenge of replicating such an experiment in the high-school environment (see Kruglak, 1972). Although Milikan’s original experimentation was complete over a 9-hour testing period, limitations of the school timetable rendered me unable to do so.
The outcomes of this study hold significant implications for the teaching of experimental physics at all levels. Millikan’s oil drop experiment, a classic in scientific history, serves not only to demonstrate fundamental concepts of electromagnetism and quantum mechanics but also to impart critical skills in experimental design, data analysis, and scientific reasoning. However, the practical challenges encountered in replicating this experiment within a controlled high school laboratory environment casts doubts on its feasibility as an effective teaching tool, not least raising questions about the abridged and simplified methodology taught in classrooms that may not capture the entire truth about how Millikan completed the historic experiment. In physics education, hands-on experiments have proven to play a pivotal role in fostering students’ understanding of and engagement of theoretical concepts (Hodson, 1988). They allow students to apply their theoretical knowledge to deepen their understanding of scientific principles with direct experience. However, experiments like the oil drop experiment although lauded for its simplicity is most
likely impractical due to its highly difficult nature as demonstrated by this paper. Such experiments, which require meticulous control of environmental variables and precise instrumentation, often exceed the resources and capabilities available in typical educational settings thus reducing its feasibility and purpose as a form of practical physics education method (Harnwell & Livingwood, 1933). The difficulties highlighted in this study underscores the need for physics educators to critically evaluate the suitability of the experiment in achieving physics objectives similar to what (Heald, 1974) argues. While alternative approaches such as virtual simulations have been explored to mitigate these challenges (Sharma & Ahluwalia, 2018), they may not fully substitute for the hands-on learning experience offered by the original experiment. This suggests that while traditional experiments like Millikan’s offer valuable insights into the physical world, their complexity and reliance on precise conditions can hinder rather than enhance learning outcomes.
My research involved replicating the famous Physics Experiment known as “Millikan’s Oil Drop” experiment in a school laboratory to analyse whether the experiment was feasible in a school environment to determine the charge of the electron. Literature suggests this is an exceedingly difficult experiment to replicate. Over the testing period, it has been determined that the experiment is not feasible in a school environment due to the randomness of the data. The research posits that physics educators must critically analyse the feasibility and importance of completing the practical in fostering student’s theoretical knowledge.
I would like to acknowledge Dr Matthew Hill, Director of the Barker Institute and my mentor through this project. His assistance in crafting the paper has been invaluable and is greatly appreciated.
Allen, M.D. and Raabe, O.G. (1982). Re-evaluation of millikan’s oil drop data for the motion of small particles in air. Journal of Aerosol Science, [online] 13(6), pp.537–547. doi:https://doi.org/10.1016/0021-8502(82)90019-2.
Brehmer, S. (1991). Millikan without the eyestrain. ˜The œPhysics teacher/˜The œphysics teacher, 29(5), pp.310–310. doi:https://doi.org/10.1119/1.2343325.
Cheng, D.-L. and Hsu, Y.-C. (2005). A better illumination system for Millikan’s oil-drop experiment. Physics Education, 40(6), pp.498–499.
doi:https://doi.org/10.1088/0031-9120/40/6/f02.
Eddington, A. (1929). The charge of an electron. Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character, 122(789), pp.358–369. doi:https://doi.org/10.1098/rspa.1929.0025.
Gagnon, M. (2012). Millikan’s Oil-Drop Experiment: A Centennial Setup Revisited in Virtual World. The Physics Teacher, 50(2), pp.98–102. doi:https://doi.org/10.1119/1.3677285.
Gröber, S., Vetter, M., Eckert, B. and Jodl, H.-J. (2007). Experimenting from a distance remotely controlled laboratory (RCL). European Journal of Physics, 28(3), pp.S127–S141. doi:https://doi.org/10.1088/01430807/28/3/s12.
Hodson, D. (1988). Experiments in science and science teaching. Educational Philosophy and Theory, 20(2), pp.53–66. doi:https://doi.org/10.1111/j.14695812.1988.tb00144.x.
Jones, R.C. (1995). The Millikan oil‐drop experiment: Making it worthwhile. American Journal of Physics, 63(11), pp.970–977. doi:https://doi.org/10.1119/1.18001.
Kapusta, J.I. (1975). Best measuring time for a Millikan oil drop experiment. American Journal of Physics, 43(9), pp.799–800. doi:https://doi.org/10.1119/1.9710.
Klassen, S. (2007). Identifying and Addressing Student Difficulties with the Millikan Oil Drop Experiment. Science & Education, 18(5), pp.593–607. doi:https://doi.org/10.1007/s11191-007-9126-2.
Lumen Learning (n.d.). 6.4 Drag Force and Terminal Speed | University Physics Volume 1. [online] courses.lumenlearning.com. Available at: https://courses.lumenlearning.com/sunyosuniversityphysics/chapter/6-4-drag-force-and-terminalspeed/.
MacIsaac, D. (2007). Websites for Teaching High School and Introductory College Modern Physics Topics: Teaching about the Millikan Oil Drop Experiment: The Physics Teacher, 45(2), pp.124–124. doi:https://doi.org/10.1119/1.2432096.
Millikan., R.A. (1913). On the Elementary Electrical Charge and the Avogadro Constant. Physical Review, 2(2), pp.109–143. doi:https://doi.org/10.1103/physrev.2.109.
Niaz, M. (2000). The Oil Drop Experiment: A Rational Reconstruction of the Millikan-Ehrenhaft Controversy and Its Implications for Chemistry Textbooks. Journal of Research in Science Teaching, [online] 37(5), pp.480–508. Available at: https://websites.umich.edu/~chemstu/content_weeks/F_06_ Week4/Mullikan_Erenhaft.pdf [Accessed 23 Jun. 2024].
Niaz, M. (2005). An Appraisal of the Controversial Nature of the Oil Drop Experiment: Is Closure Possible? The British Journal for the Philosophy of Science, 56(4), pp.681–702. doi:https://doi.org/10.1093/bjps/axi136.
Rodríguez, M.A. and Niaz, M. (2004). The Oil Drop Experiment: An Illustration of Scientific Research Methodology and its Implications for Physics Textbooks. Instructional Science, 32(5), pp.357–386. doi:https://doi.org/10.1023/b:truc.0000044641.19894.ed.
Rodríguez, M.A., Niaz, M. The Oil Drop Experiment: An Illustration of Scientific Research Methodology and its Implications for Physics Textbooks. Instructional
Science 32, 357–386 (2004). https://doi.org/10.1023/B:TRUC.0000044641.19894.ed
Sharma, S. and Ahluwalia, P.K. (2018). Can virtual labs become a new normal? A case study of Millikan’s oil drop experiment. European Journal of Physics, 39(6), p.065804. doi:https://doi.org/10.1088/1361-6404/aada39. University, O. (2022). Millikan. [online] Ox.ac.uk. Available at: https://web.chem.ox.ac.uk/teaching/Physics%20for%20CH emists/Electricity/Millikan.html.
Evan Wang Barker College
Baseball is a popular sport among children, with millions participating annually. Despite the use of helmets, head injuries, particularly concussions, remain a significant concern due to impacts from high-velocity pitches. This study focuses on the pterion, the weakest part of the skull, to evaluate helmet effectiveness. A 3D-printed head model with a plastic skull and ballistic gel brain was created to simulate human head structure. Using Arduino accelerometers, the model was subjected to baseball impacts at three different velocities. It was found that for the three impact velocities tested (16.3, 23.4, and 29.7 m/s) there was a positive linear relationship between brain acceleration and impact velocity. Even for relatively low velocities, the acceleration of the brain was sufficient to put a person at risk of concussion injury.
Overview
Each year, over six million children worldwide play in organised baseball leagues and up to thirteen million more play non-organised baseball (Cusimano & Zhu, 2017). Injuries in this sport occur because of several mechanisms, the most prevalent come from baseball impacts that causes concussions. (Post et al., 2015). Concussions mostly occur when batters are hit to the head by a pitch at high velocity (which Bartsch (2016) chose to identify as 37 m/s) of a baseball (Bartsch, 2016) and is made especially dangerous as there is no standardised concussion treatment at this stage (Schneider et al., 2017). Ball to player impacts account for 52– 62% of all baseball related injuries, with the most severe resulting from ball impacts to the head (Gessel et al., 2007). At the high school and collegiate/university level, concussive incidents have been reported to occur at 0.08 and 0.23 per 1000 athletes during a game situation (Gessel et al., 2007). Hence baseball helmets are crucial for player safety, providing essential protection against head injuries and their long-term effects, especially due to the lack of true effective treatment.
The pterion, or more commonly known as the temple is a craniometric point near the sphenoid fontanelle of the skull (Gray, 1967). It is a point of convergence of the sutures between the frontal, sphenoid, parietal, and squamous temporal bones (Figure 1). This area is known as the apart of the skull, yet it overlies the course of the anterior division of the middle meningeal artery which supplies blood to the brain (Gray, 1967). The position of the artery close to the pterion makes it
vulnerable to rupture, leading to hematoma in the event of a blunt trauma to the side of the head (Lama & Mottolese, 2023). Brolio et al. (2010) states that linear acceleration of 98“G”’s (that is, 98 times acceleration due to gravity (G=9.8m/s2) so 98Gs = 98 x 9.8m/s2 = 960.4m/s2) and an impact generating a minimum 70–75G’s is necessary to cause injury. Florida (2020) expands on this by stating that the application of 190G’s of force can potentially cause fatal brain damage. The minimal protection from the bone structure and the risk of rupturing the meningeal artery solidifies the pterion as the weakest part of the skull (Gray, 1967), thus the pterion is the most important part to consider for concussion research.

Figure 1: An image depicting the pterion as a weak point convergence of the middle meningeal artery (MMA)
Source: Gough (2019)
Helmet testing and previous investigations
Helmets are the most effective intervention to reduce the incidence and severity of work-related head injury (Gilchrist & Mills., 1987). Despite being the principal preventative measure, today's most frequently used hardhats remain highly similar in design to their predecessors from 70 year ago (Alves et al., 2021).
Type II helmets testing must show impact energy attenuation and penetration resistance for off-centre impacts to the helmet front, back, and sides. Previous research shows that impact energy attenuation is measured by a vertical drop of a helmeted head form onto a hemispherical anvil at 3.5 m/s, corresponding to an 0.6 m free-fall height (Bottlang et al., 2022). Daneshvar et al. (2011) suggested that helmets are only tested at relatively high impact situations and does not test lower impact situations and its correlation between the traumatic brain injury it may cause. Additionally, previous testing has not taken into consideration the anatomical aspect of the pterion, using materials of the same thickness for the frontal bone and the pterion whereas the pterion is much thinner (Kitching, 2022). Furthermore, helmets are predominantly tested using drop tests, where the helmet is fitted to a head form and dropped from a height, with forces measured throughout the impact (Clark et al., 2016). The focus on the top and front of the helmet leaves the area of the pterion insufficiently tested, especially regarding low weight, high velocity objects such as a baseball. Drop tests also often test at a single impact level or through a limited range, meaning that whilst a helmet may meet standards, it will not necessarily be protective across a realistic range of forces nor the realistic mechanisms of injury (Kitching, 2022).
According to the National Operating Committee on Standards for Athletic Equipment 2015, the method used to evaluate the effectiveness of the helmet does not cover the area of the pterion, where it is arguably most crucial for the helmet to function. The lack of testing combined with the presence of ventilation holes that lower structural integrity (as shown in figure 2) presents as a sufficient research gap to investigate further. The purpose of this research is to develop a projectile impact methodology that could be used to evaluate the performance of helmets for head impacts on the pterion– a vulnerable area of concussion in baseball.

What is the effect of increasing the force of a standard baseball hitting a baseball helmet on the acceleration of the ballistic gel brain.
As the velocity of a ball striking a helmet increases, the acceleration that the ballistic gel brain model experiences will increase at an increasing rate.
To test the hypothesis, baseballs at three different launch velocities were collided with a model of a head including both the skull and the brain.
Design and production of the human head
The head was designed to have three different features/layers. Unlike previous research by Kitching (2022) the model in this research had a plastic skull in conjunction with the previous research’s plastic head and ballistic gel. The inclusion of the skull is one of the most important components when attempting to model the acceleration of the brain as it can withstand up to 2300N of force (Matsui 2024), stopping the brain model from breaking but still allowing acceleration to transfer to the ballistic gel brain, allowing concussions to be studied. The accelerometer was embedded in the centre of the ballistic gel brain, as the acceleration of the brain, not acceleration of the head or skull is the most significant factor in concussions (Mo et al., 2022)
The two-piece head and skull structures were made using Fusion 360 (figures 3 and 4) to replicate the standard dimensions of an average sized head 18x21x19 (cm) (Makris et al., 2008). Holes were made in the skull and head template to house the ballistic gel. An Ender - 3 V3 printer was then used to 3D print each of the individual pieces in Cura, the colours blue and orange were chosen for better contrast so signs of damage would be more obvious during testing (figure 5). Six Holes were then drilled into the skull structure to ensure that the liquid ballistic gel would be uniformly layered inside the skull and head.


Ballistic gel
The ballistic gel was made by mixing 2 tablespoons of gelatine powder into 500 ml of 45 oC water and stirred until completely dissolved (Figure 6). The mixture was then slowly poured into the head which contains the skull. The consistency of the mixture was controlled to maintain 1.3kg which is the average weight of the brain (Hartmann, 1994). This step was repeated until the head and the skull were filled with gel resulting in total weight of 5kg with different consistencies for the layer inside and outside the skull to model the brain and other facial tissues respectively. The structure was then left in the fridge at 4oC overnight (figure 7).

Arduino sensor
The Arduino LIS2DH three axis accelerometers were used in conjunction with an Arduino uno board and wired (figure 8). The accelerometer was made to measure all forces and acceleration both inside the middle of the modelled brain.

The head and skull structure were placed inside a baseball batting cage 60 feet 6 inches (18.44 meters) from the pitching machine (figure 9). The ballistic gel head was placed on the ground with a cardboard box 5 cm away from the back of the head to allow the head movement consistent with an impact with a baseball but still supported so that it doesn’t roll over after impact. The location of each piece of equipment was traced over with a black marker. The first Arduino accelerometer was inserted into the ballistic gel of the brain, covered by a ziploc bag to prevent damaging the sensor. An MLB-H baseball helmet was then fitted snugly onto the head model, making sure that the padding covered its corresponding area. This particular helmet was chosen was as its common for children and adults to use in the amateur baseball leagues local to the researcher. The Arduino Uno “motherboard” was finally connected to a laptop via extension cord so that the laptop and the scientist can be stood outside the batting cage for safety. A full diagram of the setup can be seen in figure 12.




Standard 150g baseballs were loaded into the pitching machine for each trial. The baseline trial was initiated with the lowest level of external force, set to 20 meters per second to establish foundational measurements and to calibrate the accelerometer to a negligible value of 0+- 0.5m/s2
Baseballs were ejected at speeds of 20.1168, 26.8224, and 33.528 meters per second with each speed repeated five times. In between each test the head and helmet were repositioned against the black marker outlines to ensure validity, repositioning of the head
into its testing position is shown in figure 10 and 11. The head and skull were also checked to ensure there were no damage on the 3D printed plastic or the ballistic gel. If damage was present, an identical backup head model was used.
Recording the results
During testing, two assumptions were made, one was that the ball came out of the IHASPM at the same speeds for each of the different speeds tested, and that it was consistent with the speed setting indicator on the control panel. To determine the impact velocity, 0.44m/s is lost per 2.1m of travel for a standard baseball pitch (Seroyer et al., 2010). Hence, by assuming that this relationship applies to all the tested speeds, the baseball loses 19-11% of its speed, and the impact velocity was calculated for starting velocities of 20 to 33.5 meters per second. The inaccuracy of this assumption will be explored later. Furthermore, it was assumed that the acceleration that the LIS2DH sensor measured was the acceleration experienced throughout the gel model brain. Therefore, by doing a vector addition of the x, y, and z-axis acceleration of the LIS2DH sensor (measured in Gs), the acceleration of the modelled brain could be recorded.
The maximum acceleration vector over the duration of each impact was recorded in a table and the average acceleration of the Arduino sensor in the brain across the five tests for each velocity was calculated and plotted against launch velocity. An ANOVA was used to confirm if there were significant differences in acceleration for the three baseball velocities. The graph allowed for determination of whether there was a linear or non-linear relationship, to test the hypothesis.
For each of the 15 tests conducted, raw data from the Arduino was recorded (acceleration in G’s), representing the acceleration of the brain. The summary results are presented in Table 1.
Table 1: Simplified recorded results of experiment
An ANOVA was completed to confirm that the mean acceleration was different between the three launch velocities. Unsurprisingly, there was a significant difference between the three means (p<0.00001, F=11355.43).
Table 2: ANOVA test results for experiment
The f-ratio value is 11355.4350. The p-value Is < .00001. the result is significant at p < .05.

Figure 12: Acceleration experienced by the brain vs impact speed of baseball on helmet (error bars and standard deviations excluded as they are too small to be noticeable)
The change in the resultant vector acceleration of the modelled brain against increasing baseball velocities were very consistent with only an average standard deviation of 1.8G’s across the 3 speeds tested. A standard error was also calculated to ensure the reliability of the experiment that averaged 0.83. The extremely small standard error and standard deviation indicates that the three speeds tested were reliable. As a result, a line graph with the average of the results were plotted (as seen in figure 11) that suggests a linear increase in the acceleration experienced by the brain as the impact speed of the baseball increased. This result indicating a linear relationship which was contradictory to the hypothesis that proposed a nonlinear, increasing acceleration at an increasing rate. However, the trend of the curve between each data point, and also for lower speeds, remains unexplored due to the nature of the IHASPM only having three
speeds, the limitations of which will be explored more in the discussion.
The results show a clear positive relationship between the force applied externally to the helmet and the magnitude and direction of the impact in the centre of the ballistic gel, by an LIS2DH sensor. The higher the resultant acceleration vector (calculated by the vector sum of acceleration in the x, y and z direction), the more force experienced by the gel. Despite all impacts not being in identical places on the side of the head form, the slight variation only influenced the direction of the forces rather than the magnitude (See Appendix). As stated by Kitching (2022), the slight variation is influenced by the direction of the forces rather than the magnitude. Because a resultant vector was calculated, the direction of the force was not of utmost importance, and the resultant vector had the same magnitude regardless of the combination of impact directions, meaning the slight variation in the point of impact did not affect results. Thus, 5 consistently recorded results on 3 data points is enough to suggest a strong linear relationship but further testing of intermediate or external values will continue to validate this conclusion. Logically, the linear relationship present for the graph cannot be present for all speeds, which calls the conclusion of a linear relationship into question. Specifically, the linear relationship cannot be extrapolated for lower speeds, as this would suggest that an impact speed of 20km/h would produce 0G’s of acceleration. It’s possible that the relationship is non-linear for lower speeds (0-35m/s) then appears linear for the impact speeds tested.
Broglio et al. (2010) states that linear acceleration is most likely to cause a concussion with a mean threshold for injury to be 98G’s and an impact generating a minimum 70–75G’s necessary to cause injury. However, as seen above in the data in figure 11, all impacts recorded are above the threshold of 75G’s of acceleration, indicating that a baseball fired at a beginner/youth setting at 33.528 meters per second on the I-Hack Attack Softball Pitching Machine (IHASPM) is likely to cause a concussion which is a concern as it’s the setting amateur baseball
players use as well as the mean speed that 10 year olds are capable of pitching (Axe et al., 2014). As explored earlier in the literature review, the threshold for potentially fatal brain acceleration is 120-122G’s in a single plane of movement or 190G’s combined (Florida 2020). The results indicate that this acceleration occurs at baseball velocities of 26.8 and 33.5 meters per second as seen in figure 11, which is of great concern as children who are 14 and play baseball usually have an average pitching speed of 26.8m/s (Axe et al., 2014), suggesting that potential life-threatening concussions can be common. Thankfully not all collisions of over 190G’s of acceleration result in serious injury or death, however the risk is still worrying.
The literature shows that a concussion rate of 0.08 per 1000 athletic exposures has been calculated for high school baseball players (Gessel et al., 2007). Therefore, it is surprising that there are only 8 concussions per 100,000 matches of baseball given that (if the research is correct) acceleration of over 98G’s will result in a likely concussion.
More testing should be done on a range of different brands of baseball helmets with different helmet technologies to determine what can make the helmet more effective at preventing traumatic brain injuries. The lack of imitation of the neck during testing limits the realistic features of the experiment, meaning that potentially more or less G’s of force should have been transferred to the brain ultimately reducing validity. A greater number of speeds should also be used in future research as pitchers are obviously not limited to 3 speeds. This would allow a plot of more data points to better determine the trend of the graph in figure 11 between, and most importantly, at the extremes of the tested speeds (e.g. the 0-35m/s range). Additionally, the study was limited to the area of the pterion, further research should also focus on other vulnerable areas such as the temporal region. The head model was also very simple and could not mimic the neck movement or the height of an actual human given the time constrains of this research, thus the head had to be placed on the ground, decreasing the validity of the experiment.
The results of this investigation raise significant concern, especially regarding junior baseball players who are most vulnerable to concussions as youth have incomplete myelination, limited neck muscle development, and a higher head-body ratio (Campolettano et al., 2019). One way to ensure safety of the players is to change the rules of the game but
that changes the very nature of baseball itself. Another option is to redesign the baseball to lower the amount of G’s that’s transferred to the brain such as adopting a softer or lighter ball. Again, that would entail major changes in the rules of the game, potentially discouraging players. Therefore, this experiment guides further research into the different forces experienced by the brain in various types of impacts at different speeds. Ultimately, however, this research should be used to design a better helmet that is more suited for junior baseball players that can effectively protect the player from traumatic brain injuries.
As discussed above in the literature review, helmets are predominantly tested using drop tests, where the helmet is fitted to a head form and dropped from a height, with forces measured throughout the impact (Clark et al., 2016). Sensors are also predominantly placed on the outside of the helmet despite the fact that its the brain that helmets need to protect the most (Daneshvar et al., 2011). This research demonstrated that helmets do not eliminate concussions despite companies branding helmets as laterally or rotationally safe. This is consistent with previous sources that emphasise the importance of a sensor not only placed outside the helmet but inside the model head in order to represent accurately how effective baseball helmets are (Bottlang et al., 2022). This research can be used in the future to highlight the importance to test across a realistic range of forces, targeting the vulnerable areas of the head such as the pterion, ensuring that data is read from both inside and outside the helmet.
The research examined how varying baseball velocities affect brain acceleration in a ballistic gel model. ANOVA revealed a significant difference, suggesting a linear relationship between impact force and brain acceleration. Additional testing at lower speeds is required to better understand the trend, particularly to avoid unrealistic implications at high velocities.
I would like to acknowledge Dr Matthew Hill for his continued guidance throughout the experiment, providing crucial feedback that advanced my project, Mrs Claire Kitching for giving invaluable advice for the design of my experiment. I also want to thank Mr Phil Barden for helping with the design of the 3D printed skull, and instructions on the Arduinos.
Alves, A, Sorce, J, Schonning, A & Bevill, G 2021, ‘The Influence of Hard Hat Design Features on Head Acceleration Attenuation’, Journal of applied biomechanics, vol. 37, International Society of Biomechanics, no. 2, pp. 80–86, viewed 8 May 2024, <https://pubmed.ncbi.nlm.nih.gov/33373975/>.
Axe, MJ, Strube, M, Osinski, D, Andrews, JR & SnyderMackler, L 2014, ‘A speed distance-based classification system for injury prevention and research in international and domestic youth baseball players’, International journal of sports physical therapy, vol. 9, United States, no. 3, pp. 346–55, viewed 26 April 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4060312/ >.
Bartsch, A 2016, Analysis of Baseball-to-Helmet Impacts in Major League Baseball - Aravind Athiviraham, Adam Bartsch, Prasath Mageswaran, Edward C. Benzel, Brian Perse, Morgan H. Jones, Mark Schickendantz, 2012, The American Journal of Sports Medicine, viewed 26 January 2024, <https://journals.sagepub.com/doi/10.1177/036354651246 1754>.
Bottlang, M, DiGiacomo, G, Tsai, S & Madey, S 2022, ‘Effect of helmet design on impact performance of industrial safety helmets’, Heliyon, vol. 8, Elsevier BV, no. 8, pp. e09962–e09962, viewed 8 May 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9379520/ >.
Broglio, SP, Schnebel, B, Sosnoff, JJ, Shin, S, Feng, X, He, X & Zimmerman, J 2010, ‘Biomechanical Properties of Concussions in High School Football’, Medicine and science in sports and exercise, vol. 42, Lippincott Williams & Wilkins, no. 11, pp. 2064–2071, viewed 26 April 2024,<https://www.ncbi.nlm.nih.gov/pmc/articles/PMC294 3536/#:~:text=The%20findings%20indicated%20linear%2 0acceleration,75g%20necessary%20to%20cause%20injury .>.
Campolettano, ET, Gellner, RA, Smith, EP, Srinidhi Bellamkonda, Tierney, CT, Crisco, JJ, Jones, DA, Kelley, ME, Urban, JE, Stitzel, JD, Amaris Genemaras, Beckwith, JG, Greenwald, RM, Maerlender, AC, Per Gunnar Brolinson, Duma, SM & Rowson, S 2019, ‘Development of a Concussion Risk Function for a Youth Population Using Head Linear and Rotational Acceleration’, Annals of biomedical engineering, vol. 48, Springer Science+Business Media, no. 1, pp. 92–103, viewed 11 June 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6928097/ #:~:text=Physical%20differences%20between%20youth% 20and,susceptibility%20of%20youth%20to%20concussion .>.
Cusimano, MD & Zhu, A 2017, ‘Systematic Review of Traumatic Brain Injuries in Baseball and Softball: A Framework for Prevention’, Frontiers in Neurology, vol. 8, Frontiers Media, viewed 26 January 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5670156/ >.
Daneshvar, DH, Baugh, CM, Nowinski, CJ, McKee, AC, Stern, RA & Cantu, RC 2011, ‘Helmets and Mouth Guards: The Role of Personal Equipment in Preventing Sport-Related Concussions’, Clinics in Sports Medicine, vol. 30, Elsevier BV, no. 1, pp. 145–163, viewed 26 January 2024,
<https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2987604/ >.
Easton Z5 2.0 Matte Batting Helmet - Senior 2024, RBI Australia, viewed 13 February 2024, <https://rbiaustralia.com.au/product/easton-z5-2-0-mattebatting-helmet-senior/>.
Florida, Lori. "Brain Concussion: How Easily Does It Occur?" Critical Analysis RN Consulting, 17 Jan. 2020, criticalanalysisrn.com/what-does-ear-wax-have-to-do-with drug-testing-2/. Accessed 26 April 2024.
Gessel 2017, ‘Concussions among United States high school and collegiate athletes’, Journal of athletic training, vol. 42, J Athl Train, no. 4, viewed 26 January 2024, <https://pubmed.ncbi.nlm.nih.gov/18174937/>.
Gilchrist, A & Mills, NJ 1987, ‘Construction site workers helmets’, Journal of occupational accidents, vol. 9, Elsevier BV, no. 3, pp. 199–211, viewed 8 May 2024, <https://www.sciencedirect.om/science/article/abs/pii/0376 634987900125>.
Gough, M 2019, Cranial Foramina | Skull Anatomy | Foramen | Geeky Medics, Geeky Medics, viewed 7 February 2024, <https://geekymedics.com/cranialforamina/>.
Gray, H. (1967). Gray’s Anatomy: Anatomy of the Human Body. Basic Human Anatomy. Annals of Internal Medicine, 66(2), p.462. doi:https://doi.org/10.7326/00034819-66-2-462_1.
Hartmann, P., Ramseier, A., Gudat, F., Mihatsch, M.J. and Polasek, W. (1994). [Normal weight of the brain in adults in relation to age, sex, body height and weight]. Der Pathologe, [online] 15(3), pp.165–170. doi:https://doi.org/10.1007/s002920050040.
Makris, N, Angelone, L, Tulloch, S, Sorg, S, Kaiser, J, Kennedy, D & Giorgio Bonmassar 2008, ‘MRI-based anatomical model of the human head for specific absorption rate mapping’, Medical & biological engineering & computing, vol. 46, Springer Science+Business Media, no. 12, pp. 1239–1251, viewed 26 June 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2828153/ >.
Matsui 2024, ‘[Experimental studies of skull fracture in the temporal region (author’s transl)]’, No shinkei geka. Neurological surgery, vol. 3, No Shinkei Geka, no. 2, viewed 8 May 2024, <https://pubmed.ncbi.nlm.nih.gov/1238919/>.
Mo, Gen-Lin, et al. “Influence of Impact Velocity and Impact Attack Angle of Bullets on Damage of Human Tissue Surrogate Ballistic Gelatin.” Chinese Journal of Traumatology, Mar. 2022, www.sciencedirect.com/science/article/pii/S100812752200 0256, 10.1016/j.cjtee.2022.03.004. Accessed 21 May 2022.
National Operating Committee on Standards for Athletic Equipment Commissioning research and establishing standards for athletic equipment, where feasible, and encouraging dissemination of research findings on athletic equipment and sports injuries NOCSAE Advances Development in Athletic Equipment Standards n.d., viewed 26 June 2024, <https://nocsae.org/wpcontent/uploads/2015/06/NOCSAE-2015-June-StandardsMeeting-Release-6-18-15.pdf>.
Kitching, C. (2022). Grey matter matters: An investigation into the role of helmets in preventing force on the brain. Scientific Research in Sch ool, [online] 4(1), pp.79–88. Available at: https://barker.institute/publications/studentresearch-science/ [Accessed 10 Feb. 2024].
Lama M;Mottolese C 2023, ‘Middle meningeal artery aneurysm associated with meningioma’, Journal of neurosurgical sciences, vol. 44, J Neurosurg Sci, no. 1, viewed 26 January 2024, <https://pubmed.ncbi.nlm.nih.gov/10961495/>.
Post, A, Karton, C, T. Blaine Hoshizaki, Gilchrist, MD & Bailes, JE 2015, ‘Evaluation of the protective capacity of baseball helmets for concussive impacts’, Computer Methods in
Schneider, K, Leddy, JJ, Guskiewicz, KM, Seifert, T, McCrea, M, Silverberg, ND, Feddermann-Demont, N, Iverson, GL, Hayden, A & Makdissi, M 2017, ‘Rest and treatment/rehabilitation following sport-related concussion: a systematic review’, British Journal of Sports Medicine, vol. 51, BMJ, no. 12, pp. 930–934, viewed 26 January 2024, <https://pubmed.ncbi.nlm.nih.gov/28341726/>.
Seroyer, ST, Nho, SJ, Bach, BR, Bush-Joseph, CA, Nicholson, GP & Romeo, AA 2010, ‘The Kinetic Chain in Overhand Pitching: Its Potential Role for Performance Enhancement and Injury Prevention’, Sports health, vol. 2, SAGE Publishing, no. 2, pp. 135–146, viewed 19 June 2024, <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3445080/ >.
Thomas Wunderlich Barker College
This study examines the relationship between fill times (relating to stored air pressure) and the output energy of a compressed air energy storage (CAES) battery. The efficiency and power dynamics of the system were also explored. The investigation hypothesized that higher fill times would yield greater output energy. Results indicated that increased fill times indeed resulted in higher output energy, although the system exhibited low efficiency due to significant energy losses, likely from heat dissipation and mechanical inefficiencies. Power curves revealed a maximum power limit that the system could sustain for varying durations based on fill time. These findings underscore the potential of CAES while highlighting the need for efficiency improvements for viable large-scale application.
The Need for Alternative Energy Storage Solutions
The escalating global environmental and climate change challenges have driven most countries and regions to prioritize sustainable development and the efficient utilization of renewable energy sources (Erdiwansyah et al., 2021). There is a growing consensus on the need to achieve a high proportion of renewable energy in the power supply (Yang et al., 2021). EU, the USA, and China have set ambitious targets to reach 100%, 80%, and 60% renewable energy in their power supply by 2050, respectively (Zhou et al., 2022).
However, the intermittent and unpredictable nature of new energy sources of solar and wind where there is a chance that the sun does not shine and the wind does not blow there should be way to store the supply glut of energy (Xuezhao et al., 2023).
Current Approaches to Energy Storage
Currently, the most common energy storage option is battery technology, particularly lithium-ion batteries, which are widely used due to their high energy density and efficiency. However, their limitations, including potential supply constraints of lithium and cobalt, high costs, and environmental concerns related to mining and disposal, necessitate exploring alternative storage methods (Yang et al., 2021). Compressed air energy storage (CAES) emerges as a promising candidate, offering several advantages such as the use of abundant resources (air) and potential for large-scale storage (Barbour & Pottie, 2022).
CAES involves compressing air during periods of low energy demand and storing it in large underground caverns or high-pressure tanks (Kim et al., 2023). When energy demand increases, the compressed air is released, expanded, and used to drive turbines that generate electricity (Burian & Dančová, 2023). This method leverages established technology and infrastructure, making it a viable option for grid-scale energy storage (Lee, 2021).
The operation of reciprocating piston compressors, which are essential in CAES systems, involves a piston moving within a cylinder to compress air. During the intake stroke, the piston creates a vacuum that draws in air. As the piston ascends, the air is compressed, increasing its pressure and reducing its volume (Venkataramani, Ramalingam & Viswanathan, 2018). This compressed air is then stored and can be released to drive a generator, converting the stored potential energy back into electrical energy (Hollingsworth et al., 2019).
The process of generating electricity from compressed air involves utilizing a generator that operates on the principle of electromagnetic induction. When the compressed air is released, it drives the turbine connected to a generator, converting the kinetic energy of the moving air into electrical energy This conversion process is critical for the efficiency and feasibility of CAES systems (Chen et al., 2016).
Despite its potential, CAES faces several challenges, including energy loss during compression and
expansion, the need for suitable geological formations for large-scale storage, and safety concerns related to high-pressure air storage (Yu, Seiji Engelkemier & Emre Gençer, 2022). Additionally, while the basic principles of compressors and generators are wellunderstood, integrating these components into efficient systems, reducing the amount of heat loss in the system, and making it cost-effective requires further research and development (Han et al., 2018).
Lithium-ion batteries, while popular for their high energy density, face significant supply constraints and environmental concerns (Zakeri and Syri, 2015).
Lithium-ion batteries typically have an energy density ranging from 150 to 250 watt-hours per kilogram (Wh/kg) and an efficiency of around 85-90%. However, the extraction and processing of lithium and cobalt, which are critical for these batteries, pose environmental and ethical challenges. Whereas CAES is significantly less efficient compared to battery storage. The entire process of compressing air, storing it, and then converting it back into electricity has an efficiency rate of only 60 to 65 percent (Clemens, 2023).
Advanced adiabatic CAES systems capture and store heat generated during compression, significantly improving overall efficiency (Budt et al., 2016). Traditional CAES systems release the heat generated during air compression into the environment, leading to significant energy losses and reducing overall efficiency. In contrast, advanced adiabatic CAES systems utilize a thermal energy storage (TES) unit to capture this heat (Demir & Dincer, 2023).
During the compression phase, the air heats up as it is pressurized. Instead of dissipating this heat, advanced adiabatic CAES systems store it in a thermal energy storage unit. When the stored compressed air is later expanded to generate electricity, the stored heat is returned to the air, increasing its pressure. This process enhances the efficiency of the expansion phase, as the air can produce more work, thereby generating more electricity.
New materials like advanced composites and highstrength alloys are being developed to enhance the performance and safety of CAES systems (Raju et al., 2018).
Environmental Impact CAES has a lower environmental footprint compared to battery storage systems, primarily due to the use of air and minimal
reliance on mining (Parker, Clifford and Cohen, 2024).
How does efficiency and output energy of a compressed energy battery compare across various fill times (pressures)? What was the relationship between fill times (which related to pressures) and the output energy of a compressed air energy battery was? Were the efficiency of the system and power curves also examined to understand the dynamics of energy release?
That the output energy of a compressed air energy battery increases with greater fill times (pressures).
The valve of the LPG bottle was opened fully and the bottle inverted to ensure that it was free of any residual gas. It was then connected to the air compressor. the air compressor was connected to mains power through a Powermeter to measure the power used by the compressor. A separate hose system was prepared to be attached to the LPG bottle to receive the output air. This included the turbine generator which was placed in a circuit with a 5 Ohm resistor. The current through the resistor, and voltage drop across the resistor was measured with Pasco data loggers set to take measurements at 50 Hz.
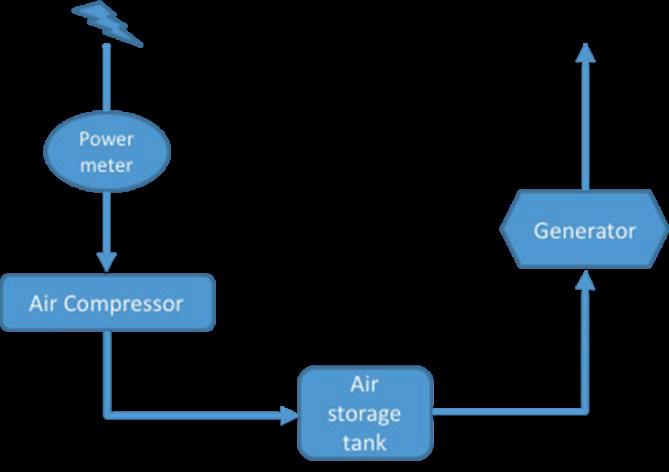
Figure 1: Flow chart of energy through the apparatus
The air compressor was started and a stopwatch was used to time the fill durations. The air storage tank was disconnected from the air compressor and connected to the generator using the air hose. The generator was set up to convert the expanding air into electricity
efficiently. The compressed air was gradually released from the storage tank and the energy produced by the generator was measured. The air compressor was started again and a stopwatch was used to time the fill durations. Tests were performed at fill times of 1, 1.5, 2, and 3 minutes. The power consumption of the air compressor was recorded every 20 seconds using the wattmeter, and the energy input was calculated using the trapezoidal rule.
After each fill, the compressed air was released through the generator. An energy data logger was used to record the voltage output and the Pasco data energy logger was used to log the energy data. The output energy was measured in joules.
Each fill time test was conducted twice to ensure consistency and reliability of data, resulting in a total of eight tests. The total output energy for each test was recorded. Observations were noted regarding the noise level, temperature of the air compressor, and any audible changes during discharge.
The goal was to perform tests at fill times of 1, 1.5, 2, and 3 minutes. Initially, tests were conducted at 1, 2, and 3 minutes. Observations of the output power curves revealed significant differences between the 1 and 2-minute fills, prompting additional tests at 1.5 minutes. Each fill time test was conducted twice, leading to a total of eight tests although the first 1.5 minute test was found to be a outlier. The generator was connected to the amp resistor and the Pasco data energy logger. A resistor is necessary to measure current because it allows the current to flow through a known resistance, enabling the measurement of the current's magnitude. Without a resistor, the current would not be able to flow through the measuring device, making it impossible to determine the current's value. The data tracker recorded the measurements at 50 Hz therefore it was decided that using the trapezoidal rule would be unnecessary.
Although it was desired to have more than two data points for each fill time in total 50 tests were conducted for preliminary testing and to achieve completion within the time frame given the amount of tests needed to be reduced.
When filling the LPG tank with air, the air compressor made a low amount of noise. The decibels could not be measured as there was no access to the equipment. It was also noted that the air compressor itself
increased in temperature. This was observed by touching the air compressor before and after. This all indicates a “loss” of energy in the battery system as it is not being turned into potential energy. For the first test that was conducted, the LPG tank was filled for 2 minutes. A 16-volt generator with an airflow regulator was used. When the battery was discharged, the output power was increasing but did not drop off when there was an audible reduction of the amount of air being released. It was decided to remove the regulator because it was believed that the regulator restricted the airflow so much that it was unable to pass the generator's coefficient of static friction.
1: Fill time vs output energy
*test number 4 was determined to be a outlier
Correlation analysis
A Pearson’s R correlation test was performed on the 7 values. The P-Value is 0.003612. The result is significant at p < .05. With a r value of 0.9173. Test of the experiment was conducted one after another
number 4 was determined to be a outlier Average efficiency excluding test 4 is 0.00151% efficient
Each test was conducted one after another. The efficiency generally decreased as the test progressively went on. This can be explained as when the experiment goes on the air compressor gets hot and it being hotter makes it more efficient.
The data shows a clear trend where higher fill times result in greater output energy (see table 1 and figure 2). Despite some variation and outliers, the overall trend supports the hypothesis that more pressure leads to more energy output. This is supported by the correlation test with a r value of 0.9173.
Efficiency
The efficiency of the system is low, as shown in table 2, ranging in values from 0.000354% to 0.00234% This low efficiency suggests significant energy losses, likely due to heat dissipation and mechanical inefficiencies in the system. It was observed that there was a jet of air coming out of the generator thus suggesting the generator is source of inefficiency as it is not transforming all the kinetic energy. The air compressor was hot to the touch therefore underscores the significant energy losses associated with heat
dissipation and mechanical inefficiencies. These findings highlight the need for further research and development to improve the method, particularly in capturing and reusing waste heat to enhance overall system efficiency. As observed from the power curve the graphs when flat and was unable to go past 0.24 joules/sec therefore could suggest that the data tracker or the generator maxed it to its specifications and therefore finding that source of systematic error and eliminating it could improve the efficiency of the system.
The power curves from the tests show distinct trends that align with the fill times. Notably, the 1-minute fill time did not reach maximum power output from the generator. Higher fill times not only reached maximum power but also sustained it for longer periods. This indicates a more stable and prolonged energy release, which is critical for practical energy storage applications where a consistent power output is desirable.
A key question arising from these observations is how the system would perform with different resistors or a different generator. The current setup, with a 5 Ohm resistor, limits the current flow, and thus the power output. If a resistor with a lower resistance were used, it could potentially allow more current to flow, increasing the power output. Similarly, using a generator with a higher power rating could also result in higher power outputs, assuming the turbine could handle the increased load. This could lead to a higher overall efficiency and more usable energy from the system.
Potential for Higher Power and Usable Energy

To explore this further, future experiments could involve varying the resistance in the circuit and testing with generators of different power ratings. This would help determine the optimal configuration for maximizing both power output and system efficiency. It would also be valuable to investigate how different fill pressures (beyond the current limits) impact the overall performance and energy output of the system.
A table of the duration at max power for different fill times shows that longer fill times allow the system to maintain maximum power for longer periods, which is consistent with the hypothesis.
The research confirms that higher fill times (which is related to pressures) result in increased output energy from a compressed air energy battery. However, the system is not very efficient, with a lot of energy lost during the compression and generation process. The generator’s power curve reveals a maximum power limit that constrains the system’s performance. These findings suggest that while CAES can store and release energy, its efficiency needs improvement to be considered a viable large-scale energy storage solution.
To significantly enhance the efficiency of compressed air energy storage (CAES) systems, focusing on optimizing turbine and generator design is crucial. As a water turbine was use for this experiment. Research into high-efficiency turbine designs can lead to substantial improvements in the conversion of compressed air energy into mechanical energy. This involves exploring turbine geometries and materials that maximize aerodynamic performance and minimize energy losses due to friction and turbulence. Innovations in blade design, such as the use of advanced composites or coatings that reduce wear and improve airflow, can further enhance turbine efficiency.
Conclusion
In this study, I examined the relationship between fill times (related to stored air pressure) and the output energy of a compressed air energy storage (CAES) battery. I also explored the efficiency and power dynamics of the system. I hypothesized that higher fill times would yield greater output energy. The results indicated that increased fill times did indeed result in higher output energy, although the system exhibited low efficiency due to significant energy losses, likely from heat dissipation and mechanical inefficiencies.
The power curves revealed a maximum power limit that the system could sustain for varying durations based on fill time. These findings underscore the potential of CAES while highlighting the need for efficiency improvements for viable large-scale application.
Acknowledgements
I would like to thank Ian Wunderlich and Mike Wunderlich for support and help with the experimentation process. I would like to thank Dr Matthew Hill for supervision and guidance throughout.
Worku, M.Y. (2022). Recent Advances in Energy Storage Systems for Renewable Source Grid Integration: A Comprehensive Review. Sustainability, [online] 14(10), pp.5985–5985. doi:https://doi.org/10.3390/su14105985.
Barbour, E.R. and Daniel L.F. Pottie (2022). Adiabatic Compressed Air Energy Storage Systems. Elsevier eBooks, [online] pp.188–203. doi:https://doi.org/10.1016/b978-012-819723-3.00061-5.
Yang, Y., Okonkwo, E.G., Huang, G., Xu, S., Sun, W. and He, Y. (2021). On the sustainability of lithium ion battery industry – A review and perspective. Energy storage materials, [online] 36, pp.186–212. doi:https://doi.org/10.1016/j.ensm.2020.12.019.
Hollingsworth, J., Phillippi, G., Hinchliff, M., Kulhanek, C., Rimpel, A.M. and Maywald, F. (2019). Reciprocating Compressors. Elsevier eBooks, [online] pp.167–252. doi:https://doi.org/10.1016/b978-0-12-814683-5.00005-5.
Erdiwansyah, Mahidin, H. Husin, Nasaruddin, Zaki, M. and Muhibbuddin (2021). A critical review of the integration of renewable energy sources with various technologies. Protection and control of modern power systems, [online] 6(1). doi:https://doi.org/10.1186/s41601021-00181-3.
Yang, Y., Qin, C., Zeng, Y. and Wang, C. (2022). Optimal Coordinated Bidding Strategy of Wind and Solar System with Energy Storage in Day-ahead Market. Journal of modern power systems and clean energy, [online] 10(1), pp.192–203.
doi:https://doi.org/10.35833/mpce.2020.000037.
Zhou, Q., Sun, Y., Lu, H. and Wang, K. (2022). Learningbased Green Workload Placement for Energy Internet in Smart Cities. Journal of modern power systems and clean energy, [online] 10(1), pp.91–99.
doi:https://doi.org/10.35833/mpce.2020.000271.
Olusola Joshua Olujobi (2020). The legal sustainability of energy substitution in Nigeria’s electric power sector: renewable energy as alternative. Protection and control of modern power systems, [online] 5(1).
doi:https://doi.org/10.1186/s41601-020-00179-3.
Zheng, X., Cai, G., Guo, J., Gao, W., Huang, Y. and Tong, X. (2023). Combustion characteristics and thermal decomposition mechanism of the flame-retardant cable in urban utility tunnel. Case studies in thermal engineering,
[online] 44, pp.102887–102887. doi:https://doi.org/10.1016/j.csite.2023.102887.
Burian, O. and Dančová, P. (2023). Compressed Air Energy Storage (CAES) and Liquid Air Energy Storage (LAES) Technologies A Comparison Review of Technology Possibilities. Processes, [online] 11(11), pp.3061–3061. doi:https://doi.org/10.3390/pr11113061.
Gayathri Venkataramani, Ramalingam, V. and Viswanathan, K. (2018). Harnessing Free Energy From Nature For Efficient Operation of Compressed Air Energy Storage System and Unlocking the Potential of Renewable Power Generation. Scientific reports, [online] 8(1). doi:https://doi.org/10.1038/s41598-018-28025-5.
Chen, L., Zheng, T., Mei, S., Xue, X., Liu, B. and Lu, Q. (2016). Review and prospect of compressed air energy storage system. Journal of modern power systems and clean energy, [online] 4(4), pp.529–541. doi:https://doi.org/10.1007/s40565-016-0240-5.
Feng, B. and Yu, B. (2022). Application research of compressed-air energy storage under high proportion of renewable energy. Clean energy, [online] 6(2), pp.305–312. doi:https://doi.org/10.1093/ce/zkac017.
Borri, E., Alessio Tafone, Comodi, G., Romagnoli, A. and Cabeza, L.F. (2022). Compressed Air Energy Storage An Overview of Research Trends and Gaps through a Bibliometric Analysis. Energies, [online] 15(20), pp.7692–7692. doi:https://doi.org/10.3390/en15207692.
Barbour, E. and Pottie, D.L. (2021). Adiabatic compressed air energy storage technology. Joule, [online] 5(8), pp.1914–1920. doi:https://doi.org/10.1016/j.joule.2021.07.009.
Yu, H., Seiji Engelkemier and Emre Gençer (2022). Process improvements and multi-objective optimization of compressed air energy storage (CAES) system. Journal of cleaner production, [online] 335, pp.130081–130081. doi:https://doi.org/10.1016/j.jclepro.2021.130081.
Han, Z., Guo, S., Wang, S. and Li, W. (2018). Thermodynamic analyses and multi-objective optimization of operation mode of advanced adiabatic compressed air energy storage system. Energy conversion and management, [online] 174, pp.45–53. doi:https://doi.org/10.1016/j.enconman.2018.08.030.
Parker, S.S., Clifford, M.J. and Cohen, B.S. (2024). Potential impacts of proposed lithium extraction on biodiversity and conservation in the contiguous United States. Science of the total environment, [online] 911, pp.168639–168639.
doi:https://doi.org/10.1016/j.scitotenv.2023.168639.
Guo, H., Kang, H., Xu, Y., Zhao, M., Zhu, Y., Zhang, H. and Chen, H. (2023). Review of Coupling Methods of Compressed Air Energy Storage Systems and Renewable Energy Resources. Energies, [online] 16(12), pp.4667–4667. doi:https://doi.org/10.3390/en16124667.
Arabkoohsar, A. (2021). Compressed air energy storage system. Elsevier eBooks, [online] pp.45–71. doi:https://doi.org/10.1016/b978-0-12-820023-0.00003-1.
Kim, S., Dusseault, M.B., Oladipupo Babarinde and Wickens, J. (2023). Compressed Air Energy Storage (CAES): Current Status, Geomechanical Aspects, and Future Opportunities. [online] ResearchGate. Available at: https://www.researchgate.net/publication/367477786_Com pressed_Air_Energy_Storage_CAES_Current_Status_Geo
mechanical_Aspects_and_Future_Opportunities [Accessed 25 Jun. 2024].
Clemens, K. (2023). The Ins and Outs of Compressed Air Energy Storage. [online] Eepower.com. Available at: https://eepower.com/tech-insights/the-ins-and-outs-ofcompressed-air-energystorage/#:~:text=Another%20problem%20with%20CAES %20is,high%2080%20percent%20efficiency%20range. [Accessed 25 Jun. 2024].
Demir & Dincer (2023). Performance assessment of compressed air energy storage systems with and without phase change materials. International journal of thermofluids, [online] 20, pp.100489–100489. doi:https://doi.org/10.1016/j.ijft.2023.100489.
Social Science Statistics. (2024). Pearson Correlation Coefficient Calculator. [online] Available at: https://www.socscistatistics.com/tests/pearson/default2.asp x [Accessed 25 Jun. 2024].
Rachel Mathews Barker College
With the 2023 International Maritime Organization’s 40% reduction target of greenhouse gas emissions by 2030, maritime cargo ships require innovative designs to improve aerodynamic efficiency and minimise energy consumption. The biomimicry of a box-fish frontal carapace was adapted to the superstructure of a cargo ship to reduce aerodynamic drag and consequent energy consumption. Analysis of 3D models through Computational Fluid Dynamics and wind tunnel testing revealed that despite the boxfish’s low drag coefficient, its application to maritime transport ships was ineffective due to the production of a large wake region
Literature Review
Minimising Maritime Transport Emissions
In 2018, globalized maritime transport systems contributed to 1076 million tonnes of CO2 emissions and an overall 2.9% of worldwide greenhouse gas (GHG) emissions, representing a critical point for fuel consumption minimization (Psaraftis and Kontovas, 2020). There are over 93,000 container ships that transport goods across the world, majority of which use diesel combustion engines to burn low grade bunker fuel that is environmentally inefficient (Gallucci, 2018). In response, the International Maritime Organization (IMO) introduced a revised strategy in July 2023 to minimise GHG emissions in international shipping, highlighting the centrality of technological innovation to find energy efficient designs and alternative fuel solutions (IMO, 2023). These new commitments aim to work towards the 13th UN Sustainable Development Goal calling for urgent action to cut emissions that worsen global warming (United Nations, 2023). Progress towards the IMO’s new target to reduce shipping CO2 emissions by at least 40% for 2030 will be monitored through data collection systems and enforced through upcoming local regulations on shipping fuel consumption (IMO, 2023).
Aerodynamic Drag
To reduce the aerodynamic drag, the resistive force that occurs when the superstructure of maritime transports ships is travelling at high speeds through air needs to be minimized (Srinivas, 2023). The drag force is determined by a multitude of variables including the density of air, the square of the velocity travelling opposite to the direction of movement, the
air’s viscosity and compressibility and the body’s shape and size (Hall, 2022). Complex dependencies are represented by a single variable, the drag coefficient ‘Cd’ which is often calculated experimentally.
Equation 1: Drag coefficient

Equation 2: Drag force

As seen in Equation 1, the drag coefficient is proportional to twice the drag force ( N ), and inversely proportional to the density of air (1.293 kg/m^3 at room temperature ), flow speed of the object relative to the fluid ( v^2 in m/s ) and the reference area ( A ). From this a simplified drag equation is derived as seen in Equation 2.
Previous friction-reducing design upgrades to the propeller, hull and bulbous bow design have successfully contributed to reducing the carbon consumption of container ships by over 30% since 2008 (Faber, et al., 2020). These adaptations have successfully minimized the container ships hull resistance which was measured to have a wind drag coefficient to 0.1 for a 1200 TEU container ship, however the above water drag coefficient remains inefficient at 0.7 with the same ship (Ngô Văn Hệ et
al., 2020). Especially for container ships that have a large windward area because of its cubiform shape, wind drag accounts for a large percentage of total resistance.
A study conducted by Ngo Van He et al. (2020) analyses the effectiveness of a curved frontal nose to replace the ships accommodation in combination with the flat alignment shipping containers to reduce above water wind drag. The accommodation encompasses living quarters and the bridge at the bow of a ship and is a popular option for aerodynamic improvement. Through CAD models and CFD imaging, the study recorded that up to 30% of the total wind drag due to head winds could be reduced through streamlined accommodation.

Another research investigation conducted by Van Nguyen, Shimizu and Kinugawa (2016) tested the addition of a various winglet formations at the bow of container ships to aid pressure distribution and achieve a 4-6% reduction in the drag coefficient. The winglet configuration forms a stationary vortex, preventing the separation of airflow and hence reducing wind induced drag. Computational Fluid Dynamics helped prove computational predictions and visualize the prevalence of vortex’s and highpressure areas to continually reiterate the winglet design.
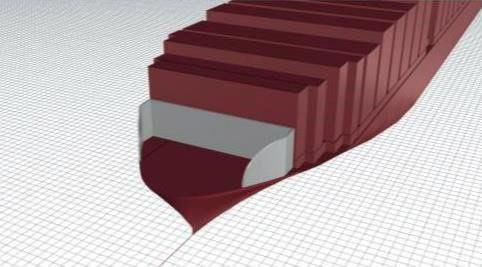


Biomimicry and the Yellow Box Fish
Nature-inspired design or ‘biomimicry’ draws upon six hundred million years of nature’s problem solving to draw upon invaluable insights into energy efficient design (Kozlov, 2015). Existing natural designs are pre-purposed to optimise energy and materials tailored for specific physical environments and are hence being increasingly drawn upon for scientific invention such as Velcro inspired by the burdock plant and termite mound cooling systems (Chayaamor-Heil and
HannachiBelkadi, 2017). Overall, the integration of nature into scientific developments promotes an appreciation for natural knowledge and ethical innovation rather than past exploitative uses.
Despite its cube-like morphology and large crosssectional area, the Yellow Boxfish (Ostracion cubicus) has a remarkably low flow resistance for aerodynamic optimization with a drag coefficient of 0.1 (Chowdury and Loganathan, 2022). Residing in shallow tropical waters, the boxfish’s unique carapace structure allows it to automatically direct water flow and control its movement in coral reef habitats (Kozlov et al., 2015).

In 2005, Mercedes-Benz collaborated with biologists to harness the unique characteristics of the boxfish and introduced its Bionic Concept Car. This successfully united practical features of a two-door, four-seater sedan while achieving a calculated drag coefficient of 0.095 which is 35% less than the drag of standard passenger cars (Daimler Chrysler, 2005) and a predicted improvement in fuel efficiency to 70 miles per gallon compared to the industry standard of 35 miles per gallon (Conceptcarz, 2006). Contrary to computational predictions, the bioinspired car faced significant slowing in all attempts make turning movements. This miscalculation is the result of an oversight on the box fish’s use of shallow water currents to float around rather than its own fins resulting in the aerodynamic design being completely inefficient for the purpose of sharp turns (Van Wassenbergh et al., 2015).

4: Adaptation of boxfish CAD to passenger car
Source: Chowdury et al. (2022, pg.15)
Despite previous limitations of a boxfish inspired car, further research was conducted by Srinivas (2023) to investigate the application of boxfish biomimicry to reduce aerodynamic drag of Class 8 Heavy Vehicle Trailers for the purpose of meeting emission targets of the US Environmental Protection Agency’s Clean
Truck Plan. The study used processes of CFD Analysis and a small-scale high precision wind tunnel experiment to find a drag coefficient reduction from 0.65 (standard trailer) to 0.56.
Scientific Research Question
To what extent can the integration of box fish biomimicry mitigate wind induced drag in the abovewater superstructure of maritime transport vessels to achieve consequential effects on fuel efficiency optimization and environmental sustainability.
Scientific Hypothesis
Integrating box fish carapace features in the superstructure of maritime freight carriers will reduce wind induced drag force.
Methodology
The overall approach to analyse the effects of box-fish biomimicry for cargo ships can be separated into three primary steps including the creation of the geometric model, computational simulations, and physical wind tunnel testing of a 3D-printed model.
Part 1: Creation of a boxfish inspired cargo ship model
A geometric model was created using ComputerAided-Design (CAD) on Autodesk Fusion 360. The base ship reciprocates the dimensions and proportionality of a 2000 TEU Fully Cellular container ship model. Two final models were created, one maintaining the original superstructure design with a vertically flat front surface, and one with the added bio-inspired frontal accommodation. The depth of the models include a portion of the below water level hull shape to aid load cell installation at a later stage, but is kept consistent between both models. These CAD models help visualize the simplicity in a biomimicry adaptation to the external design of any vehicle.

Secondly, computational simulations were conducted with the Autodesk CFD software with incompressible, laminar equivalent air flow at a speed of 20 m/s. The ship’s material was set to be steel. A virtual wind tunnel was built around each ship using the external volume and auto size mesh features of the CFD software.

6: Mesh Autosize feature on CAD
The virtual wind tunnel was made significantly larger in both cross section and length relative to the truck so that the boundaries of the wind-tunnel don’t interfere with the air flow around the container ship. Two boundary conditions were set including the inlet velocity speed of air (wind speed) and an unknown condition at the end of the wind tunnel in order to measure the resultant wind speed and drag. The simulations were run through for 50 iterations to allow for stabilized flow around the models, stabilizing around the 40th iteration.
Table 1: Size & Velocity Boundary Conditions of domain geometry

Figure 7: Convergence plot with stabilization
Part 3: Wind tunnel testing with 3D printed models
In order to validate the results drawn from CFD Analysis, a physical wind tunnel was constructed and used to test 3D printed models of both original and bio-inspired ships.
Design & build of wind tunnel
A bell mouth inlet was created using CAD and 3D printed to be attached to the body of the tunnel with a metal latch for easy access to internal features. The internal width of the inlet holds a 3D printed honeycomb mesh for ideal laminar flow, minimizing systematic errors that may arise from uneven air
pressure. The opposing outlet was fitted with a VEVOR 300mm Industrial Ventilation/ Suction Fan controlled by a 0-240V Variable Speed Rheostat to suction a desired amount of air through the wind tunnel. Furthermore, an Anemometer was glued to the roof of the wind tunnel’s interior chamber and connected to an external digital reader to measure wind speed (km/h) in real time.

A vertically aligned 1kg load cell attached to a 3D printed cylinder (15cm in length) in a way which allows the cylinder to enter the wind chamber and hold the cargo ship models stationary and transfer the drag force to the load cell calibrator. This allows the load cell to measure force applied horizontally as a result of aerodynamic drag, which is then recorded using an Arduino Nano. These values are also displayed at intervals of 1 second on a Liquid Crystal Display screen and can be tared to zero with the Arduino Push Button.
Testing
Both 10km/h and 20km/h wind speeds were tested for both the original and bioinspired model. Prior to initiating the fan, the model was inserted on top of the load cell extension and pushed towards the inlet allowing the load cell to re-calibrate to a near-zero value (± 0.01). Closing the glass screen, the Rheostat was increased until the Anemometer steadied on a 10km/h wind speed value. Load cell measurement was recorded after 60 seconds of wind allowing the airflow and highly sensitive load cell readings to stabilize, promoting consistency across readings. This process was repeated three (Tuesday = five) times at the same wind speed, then repeated with a wind speed of 20km/h before repeating the entire process with the bio-inspired cargo ship model.


Table 2: Wind-induced drag force of base ship versus bio-inspired ship

Three t-tests were performed to evaluate whether the mean drag for the Base Ship was significantly different from the mean drag for the Bio-Inspired Ship.
→ At 10km/h wind speed (t=0.75, p=0.48>0.05)
→ At 15km/h wind speed (t=0.17, p=0.86>0.05)
→ At 20km/h wind speed (t=0.39, p=0.70>0.05)
Discussion
In a combination of wind tunnel testing and CFD Analysis, differences in the wind speed are evident despite not being statistically significant. The lowest wind speed saw an average 27% increase of windinduced drag while the highest wind speed recorded an average 36% increase represented in Figure 11. The increasing growth of the drag force in relation to wind speed evident in the line graph is comparable to the relationship F ∝ v2 established in the drag formula (Equation 2 ). However, since the p-value in the t-test is above the acceptable alpha value, these results are not significant enough to disprove the hypothesis that the application of a boxfish-adapted will produce a notable decrease in drag-reduction properties when analysed with wind tunnel testing when analysed alone.
Despite not being statistically significant, the numerical data and graphical representation aligns with observations made in CFD Imaging (Figure 9&10) in which the boxfish adapted cargo ship model produces a larger wake region than the unaltered base
model. As the airflow enters through the inlet and separates around the cargo ship, a region of low pressure and wind speed or the ‘wake’ region forms (Srinivas,2023). Indicated by the significantly larger blue region in the bioinspired CFD Image in Figure 9 and 10, this creates a vacuum against surrounding high-speed wind and hence increases the backwards drag force on the vessel. The wake region increases with increasing wind speed, validating the larger percentage difference between both models at the 20km/h wind speed.
Further analysis of CFD reveals the existence of high wind speed above the base ship model and the formation of air vortex’s that contribute to the resistance of separation and reduction of drag. The boxfish aerodynamic front encourages flow separation as expected from its biological uses for selfstabilization in high current streams (Van Wassenbergh et al., 2015). This feature works against the drag reduction method measured in a physical wind tunnel.
A statistic t-test reveals the lack of statistical significance in the difference between model drag averages. The high variability of results measured evident in the p-value makes it significantly higher than the standard alpha value that is required to surpass for the null hypothesis to be rejected. This statistical test reveals the imprecise nature of measurements because of fluctuating load cell calibration difficulties.
Despite the wind tunnel’s necessity to validating computational data; cost, material, precision, calibration limitations could contribute to minor data inaccuracies. A wood-built wind tunnel has the potential to leak airflow, vibrate and resonate which can influence load cell measurements. Arduinocompatible sensors often lack high precision required for aerodynamic calculations despite affordability and ease-of-use. Increasing the power/voltage maximum of fans could contribute to data accuracy with larger and more measurable load cell readings.
In regards to 3D design, the application of a back guide may have potential to reduce the wake region behind the ship and make a boxfish inspired frontal nose a more viable design. A back guide involves a simple curved structure at the rear side of the ship’s superstructure that encourages the reconnection of airflow (Srinivas, 2023). Calculated results of velocity distributions for a cargo ship model with the back guide have presented reduction in overall drag by 18.18% according to the research study by Van Nguyen, Shimizu and Kinugawa (2016) aimed at testing a wide variety of aerodynamic add-ons to improve efficiency.
Despite ineffectiveness for cargo ships, boxfish biomimicry features retain potential applications in combination with standard aerodynamic features or in static objects. A study of boxfish bionic drag reduction for Box Girders was found to be effective to reduce wind loads and hence reduce the vibration and shock of the structure in high airflow fields (Wang et al., 2020).
This experiment disproved the application of a boxfish biomimicry for an aerodynamic front that encourages drag reduction and hence environmental efficiency. Modifying the front section of a maritime container ship led to a larger wake region at the end of the model and reduced speed of airflow around the model as evident through CFD imaging. This was further confirmed by a physical wind tunnel test which produced an average difference but failed to evidentiate a statistical dfference due to the high variability of readings because of a self-built low speed and low-cost wind tunnel. These results represent previous aerodynamic issues found in the Mercedes Benz Bionic Car highlighted in the study by Wang et al. (2020) in which the low drag coefficient
of the boxfish carapace was incompatible with moving vehicles.
I would like to thank Dr Matthew Hill for his guidance in this scientific study. The physical wind tunnel set up was made possible by Mr Phil Barden and the resources of the Design and Technology Department.
Andersen, I.M.V. (2013). Wind loads on post-panamax container ship. Ocean Engineering, [online] 58, pp.115–134. doi:https://doi.org/10.1016/j.oceaneng.2012.10.008.
Chowdhury, H. and Loganathan, B. (2022). Chapter EightBiomimetics of boxfish: Designing an aerodynamically efficient passenger car. [online] ScienceDirect. Available at: https://www.sciencedirect.com/science/article/abs/pii/B978 0128210741000074
Elkafas, A.G., Elgohary, M.M. and Zeid, A.E. (2019). Numerical study on the hydrodynamic drag force of a container ship model. Alexandria Engineering Journal, 58(3), pp.849–859. doi:https://doi.org/10.1016/j.aej.2019.07.004.
Hall, N. (2022). Drag Equation. [online] Glenn Research Center | NASA. Available at: https://www1.grc.nasa.gov/beginners-guide-toaeronautics/drag-equation/ Imo.org. (2015). IMO’s work to cut GHG emissions from ships. [online] Available at: https://www.imo.org/en/MediaCentre/HotTopics/Pages/Cut tingGHGemissions.aspx#:~:text=2023%20IMO%20Strategy% 20on%20Reduction%20of%20GHG%20Emissions%20fro m%20Ships&text=In%20particular%2C%20the%202023% 20IMO [Accessed 13 Feb. 2024].
Kozlov, A., Chowdhury, H., Mustary, I., Loganathan, B. and Alam, F. (2015). Bio-Inspired Design: Aerodynamics of Boxfish. Procedia Engineering, 105, pp.323–328. doi:https://doi.org/10.1016/j.proeng.2015.05.007.
Ngô Văn Hệ, Ngo Van Hien, Van Thuan Truong and Lam Thu Bui (2020). Interaction Effect between Hull and Accommodation on Wind Drag Acting on a Container Ship. Journal of Marine Science and Engineering, 8(11), pp.930–930. doi:https://doi.org/10.3390/jmse8110930.
Srinivas, V. (2023). Biomimicry as a tool for the Aerodynamic Drag Reduction of Class 8 Heavy Vehicle Trailers: A Computational Analysis and Wind Tunnel Study. Intersect: The Stanford Journal of Science, Technology, and Society, 16(3).
Van Nguyen, T., Shimizu, N. and Kinugawa, A. (2016). Studies on Air Resistance Reduction Methods for a Large Container Ship (Part 1).
Van Wassenbergh, S., van Manen, K., Marcroft, T.A., Alfaro, M.E. and Stamhuis, E.J. (2015). Boxfish swimming paradox resolved: forces by the flow of water around the body promote manoeuvrability. Journal of The Royal Society Interface, 12(103), p.20141146. doi:https://doi.org/10.1098/rsif.2014.1146.
Wang, Y., Cheng, W., Du, R. and Wang, S. (2020). Bionic Drag Reduction for Box Girders Based on Ostracion cubicus. Energies, 13(17), p.4392. doi:https://doi.org/10.3390/en13174392.
Yang, C.-M., Hung, J.-Y., Wang, Y.-L. and Lien, Y.-H. (2019). Analysis of Mercedes-Benz Concept Car Using Biomimicry Design Spiral and Template Analysis -An Exploratory Study. International Journal of Innovation in Management, 7(2), pp.49–56.