Renal denervation’s rocky road runs on as mixed trial results dent recent optimism
More than eight years after results of the SYMPLICITY HTN-3 trial led the authors of a New England Journal of Medicine (NEJM) editorial to observe that the “renal denervation train” had reached a “grinding halt”, recent months have seen investigators express renewed optimism that the treatment may be back on track as a promising treatment option for hypertension.
This comes amid a flurry of new data from renal denervation trials released at both the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT; 16–19 September, Boston, USA) and the American Heart Association (AHA) annual scientific sessions (5–7 November, Chicago, USA).
On the heels of the latest tranche of data, Medtronic has announced submission of the final module of the premarket approval (PMA) package for its Symplicity Spiral renal denervation system to the US Food and Drug Administration (FDA) for final review and approval.
At AHA 2022, David Kandzari (Piedmont Heart Institute, Atlanta, USA) delivered six-month results from the full cohort of the SPYRAL HTN-ON MED trial. The trial investigated the blood pressure-lowering effect and safety of renal denervation with Medtronic’s radiofrequency (RF)-based Symplicity Spyral system in hypertensive patients prescribed up to three antihypertensive medications. A total of 337 patients were enrolled at 42 sites across the USA, Europe, Japan, Australia, and Canada, and were randomised 2:1 to renal denervation (n=205) versus sham control (n=132).

“The ON MED study demonstrated significant

renal denervation, but did not differ from the sham group, meaning that the trial’s primary endpoint was not met.
Offering some explanation as to why this may have occurred, Kandzari commented that more than 80% of patients in the ON MED expansion group experienced follow-up during the COVID-19 pandemic, and compared with patients enrolled before the pandemic, significant differences in baseline 24-hour ambulatory systolic blood pressure were observed that may reflect changes in patient behaviour and lifestyle during the pandemic. Additionally, patients treated with the sham procedure increased the amount of medication they were taking compared to those treated with renal denervation. “These factors likely contributed to the smaller than expected differences in ambulatory systolic blood pressure”, Kandzari said. The trial met its primary safety endpoint, aligning with previous safety results on the therapy.

In a press release that followed the release of the trial’s results at AHA, Medtronic said that in addition to the consistent absolute drops in blood pressure that have been demonstrated across trials from its SPYRAL HTN global clinical programme, long-term data have “demonstrated the durability and ‘always on’ effect of renal denervation as a treatment strategy”.
Percutaneous coronary intervention (PCI) does not reduce allcause mortality or heart failure hospitalisation in patients with severe left ventricular dysfunction and extensive coronary artery disease, late-breaking research presented at the annual congress of the European Society of Cardiology (ESC 2022; 26–29 August, Barcelona, Spain) has shown.

THIS WAS THE HEADLINE FINDING of the Revascularization for ischemic ventricular dysfunction—REVIVEDBCIS2—trial, a long-anticipated randomised trial of PCI versus optimal medical therapy for severe ischaemic cardiomyopathy. Led by researchers in the UK, results of the trial, which have been published in the New England Journal of Medicine (NEJM), are intended to fill a gap in randomised evidence to determine the benefit of PCI in this patient population.

reductions in office-based blood pressure, the most commonly used measure in clinical practice,” said Kandzari, the trial’s lead principal investigator, adding that researchers also saw reductions in absolute blood pressure that were consistent with earlier renal denervation studies. However, Kandzari’s presentation revealed that 24-hour ambulatory systolic blood pressure declined with
The SPYRAL HTN global clinical programme includes the SPYRAL HTN-OFF MED pivotal trial and the currently-enrolling SPYRAL AFFIRM study, alongside the global SYMPLICITY registry. Long-term results from SMPLICITY HTN-3 were presented at TCT 2022 by Deepak L Bhatt (Brigham and Women's Heart and Vascular Center and Harvard Medical School, Boston, USA). Despite the trial's primary endpoint failure at six months, the long-term data pointed towards significantly greater reductions in office systolic blood pressure, albeit with commentators attaching some caution to the interpretation of the result, due to the crossover of patients later on in the study.
Presentation of the REVIVED-BCIS2 trial results was among the biggest attractions of the four-day ESC congress, and Divaka Perera’s (King’s College London, London, UK) presentation of the primary endpoint— which showed virtually identical rates of allcause death or heart failure hospitalisation for PCI and optimised medical therapy—elicited audible surprise among attendees at a full-tocapacity hot line trial session on day two of the event.
“Coronary artery disease is the most common cause of heart failure and for this reason it has long been assumed that treating the coronary artery disease would be a good treatment for heart failure,” REVIVEDBCIS2 investigator Perera commented in his presentation, adding that despite a lack of randomised trial evidence to support PCI in severe stable left ventricular function, the procedure is “frequently” performed in this context. Furthermore, the use of PCI is supported as a Class 2a recommendation within current European revascularisation guidelines, Perera noted, adding that
“Definitive” data show PCI fails to reduce death, heart failure hospitalisation in patients with severe left ventricular dysfunction
Continued on page 4
in this
Featured
issue:
November 2022 | Issue 67 www.cardiovascularnews.com
Shrilla Bannerjee: Driving better cardiac outcomes for women
page 25
page
The ON MED study demonstrated significant reductions in office-based blood pressure, the most commonly used measure in clinical practice” Cardiovascular News, May 2014
Profile: Timothy D Henry
16
7
Continued on page 3
Joseph Bavaria Low-risk TAVI trends
Edwards
System
outcomes
Edwards Lifesciences strives to set new standards in the treatment of tricuspid regurgitation for the benefit of your patients.
The PASCAL transcatheter valve repair system is proving to be safe and effective, with sustained TR reduction at 1 year in the CLASP TR EFS study.
1. Hahn R. EuroPCR 2022; 2. Baldus S. EuroPCR 2022; TR: tricuspid regurgitation
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, CLASP, PASCAL and PASCAL Ace are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP--EU-4883 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com

CLASP TR EFS 86% TR ≤ 2+ at 1 Year1 Post-market clinical study TriCLASP 90% TR ≤ 2+ at 30 Days2
PASCAL Repair
Unparalleled TR
Early feasibility clinical study
Also pursuing plans to seek US FDA approval for a renal denervation device is Recor Medical. The company is behind the Paradise ultrasound renal denervation system, which has already received the CE mark in Europe. In recent months, the company has shared new data from its RADIANCE global study programme, which it says show “consistent treatment effect and safety across a broad patient population”.
At September’s TCT meeting Ajay Kirtane (Columbia University Irving Medical Center New York-Presbyterian Hospital, New York, USA) presented the primary results from RADIANCE II, looking at the blood pressure-lowering efficacy of the Paradise device at two months. Through the trial, investigators from 60 study centres in eight countries screened 1,038 individuals for trial eligibility, with 224 patients with uncontrolled hypertension ultimately randomised 2:1 to undergo renal denervation with the Paradise system (150) or a sham procedure (74).



Similar reductions in blood pressure were observed in nighttime and 24-hour measures, as well as measurements taken at home and in the physician office. There were no major adverse events in either group through 30 days. The trial’s primary safety endpoint will be measured at six months, and patients will be followed for a total of five years.
“The reason that we did the RADIANCE II trial is to try and build upon prior trials such as RADIANCE HTN SOLO and TRIO which had demonstrated that renal denervation could lower blood pressure in comparison with a sham,” Kirtane told News, referring to earlier studies to have assessed the use of the Paradise system. “I think it pretty clearly showed that renal denervation lowered blood pressure at two months in comparison with the sham, especially on a backdrop of patients with no medication.
“We have also shown that, in a backdrop of a combination therapy with three separate agents, renal denervation can lower blood pressure, and in many ways this is a confirmatory trial, but a larger trial that will hopefully lead to
regulatory approval in the USA,” Kirtane said.
In discussion that followed Kirtane’s presentation at TCT, speakers welcomed the findings of RADIANCE II, and their wider importance for understanding of renal denervation as a treatment for hypertension. “We should not forget that this is the sixth sham-controlled trial that showed and confirmed the efficacy of this approach using different modalities, but also using different patient populations,” said Felix Mahfoud (Saarland University Hospital, Hamburg, Germany), who has himself been heavily involved in renal denervation research, including as an investigator in the RADIANCE-HTN SOLO and TRIO trials.
Naomi Fisher (Brigham and Women’s Hospital and Harvard Medical School, Boston, USA) offered her perspective on the trial as a specialist in hypertension, describing the condition as the “number one global burden of disease risk factor in the world”.
“We need innovation,” she commented. “Our control
rates are poor and they are only falling, if you look at large statistical datasets showing that we are doing a worse and worse job as time goes on. We have had pharmacology around since the 1950s for high blood pressure, our last new drug class was introduced in 2007, that is how long ago that we had an innovation. “Finally we have evidence for an innovative therapy that is really desperately needed to help control blood pressure.”

At AHA 2022, Kirtane also presented a pooled analysis of data from three trials within the RADIANCE programme. These data demonstrated consistency of effect of ultrasound-based renal denervation across each of the three trials, all of which met their primary endpoint for blood pressure reduction. Recor Medical has plans to submit results of its global study programme as part of a PMA to the US FDA for market approval.

News
in brief The latest stories from the world of Cardiology
n PROTECTED TAVR: Hotly-anticipated clinical trial data looking at cerebral embolic protection during transcatheter aortic valve implantation (TAVI) using the Sentinel (Boston Scientific) device were shared at TCT 2022 (16–19 September, Boston, USA). Though the trial failed to meet its primary endpoint, some remain convinced of the benefits of cerebral embolic protection during a TAVI procedure.
For more on this story go to page 5.
n MITRAL
DATA
UPDATE: New data from clinical trials examining transcatether edgeto-edge repair (TEER) for mitral regurgitation offer “reassurance” that the procedure is safe and effective, according to experts. Cardiovascular News rounds up some of the major developments, in both clinical evidence and regulatory approvals, underpinning a renewed sense of optimism in mitral TEER.
For more on this story go to page 9.
n CX AORTIC VIENNA: New technologies and techniques for the treatment of complex aortic disease were on the agenda at the three-day CX Aortic Vienna Digital Edition (24–26 October). The event featured a focus on the collaboration between the cardiac and vascular surgical specialties to improve outcomes.
For more on this story go to page 13.
facebook.com/cardiovascularnews linkedin.com/company/cardiovascular-news @cn_publishing www.cardiovascularnews.com Publisher: Roger Greenhalgh Content Director: Urmila Kerslake Editor-in-chief: Simon Redwood Senior editor: Will Date will@bibamedical.com Editorial contribution: Jamie Bell, Jocelyn Hudson and Clare Tierney Design: Terry Hawes, Wes Mitchell and David Reekie Advertising: sales@bibamedical.com Subscriptions: subscriptions@bibamedical.com Please contact the Cardiovascular News team with news or advertising queries Tel: +44 (0)20 7736 8788 Published by: BIBA Publishing, which is a subsidiary of BIBA Medical Ltd BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323 Printed by: Buxton Press Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2022. All rights reserved. Write to us! If you have comments on this issue or suggestions for upcoming editions write to will@bibamedical.com Make sure you get your copy of Next issue: February
2023
Continued
from page 1
Finally, we have evidence for an innovative therapy that is really desperately needed to help control blood pressure”
Renal denervation's rocky road runs on as mixed trial results dent recent optimism
Samir Kapadia
Global faculty took part in CX Aortic Vienna
Issue67 | November 2022 3 Cover Story
Ajay Kirtane
randomised data from the Surgical treatment for ischemic heart failure (STICH) trial support coronary artery bypass graft (CABG) surgery within this setting.
REVIVED-BCIS2 is the first adequately powered randomised trial to examine the efficacy and safety of PCI in patients with left ventricular systolic dysfunction, enrolling patients with severe left ventricular dysfunction (ejection fraction ≤35%), extensive coronary artery disease and with demonstrable viability in at least four dysfunctional myocardial segments that could be revascularised by PCI. Viability could be assessed by any modality, but cardiac magnetic resonance imaging was used most. Those with a myocardial infarction (MI) within four weeks, decompensated heart failure or sustained ventricular arrhythmias within 72 hours were excluded.
A total of 700 patients from 40 centres in the UK were randomly assigned in a 1:1 ratio to either PCI with optimal medical therapy or optimal medical therapy alone. The median age of participants was 70 years, 88% were men and the mean left ventricular ejection fraction (LVEF) was 27%. The primary outcome was the composite of all-cause death or hospitalisation for heart failure. Secondary outcomes included left ventricular ejection fraction at six and 12 months and quality of life measures.
Despite hypothesising that PCI would improve event-free survival within the patient population, Perera reported that, during a median follow-up of 3.4 years, the primary outcome occurred in 129 (37.2%) patients in the PCI group and 134 (38%) patients in the medical therapy alone group for a hazard ratio (HR) of 0.99 (95% confidence interval 0.78–1.27, p=0.96).
“The first thing I will draw your attention to is that approximately 40% of patients in the control arm either died or had been admitted to hospital during the follow-up period,” said Perera. “This is not a problem that has been addressed already by medical therapy. That is a major event rate. But, PCI failed to reduce that outcome,” the trial investigator commented.
No significant difference was seen between groups in the major secondary outcome of the trial, which Perera described as being a potential “mechanism of benefit of revascularisation” at both six and 12 months. “There was a slight improvement in LV function over time, but that occurred to the same extent in both groups,” Perera noted.
Interestingly, according to Perera, quality of life (the other major secondary outcome) favoured PCI at six and 12 months but there was no difference between groups at 24 months. Asked about this in a press conference, Perera said that more data on this will feed into a health economic analysis of the trial data.
“The key take home message from this where I am concerned is that this is now a definitive result, we finally have RCT [randomised controlled trial] evidence that allow guidelines to be strengthened on the one hand, and allow clinical practice to be rationalised around the world on the other,” Perera commented.
Release of the results has elicited a great deal of discussion and debate within the interventional cardiology and cardiac surgery communities. In an editorial piece accompanying the trial’s results in NEJM , Ajay Kirtane (Columbia University Irving Medical Center, New York–Presbyterian Hospital, New York, USA) writes that “questions remain” over the merits of percutaneous revascularisation within this patient group.
Kirtane suggests that the availability of longterm follow-up data, as was collected through the STICH trial—whereby a mortality benefit was seen at 10 but not five years for CABG—will be important as to the interpretation of the trial’s findings. “Until we have these data, one cannot extrapolate outcomes achieved with surgical revascularisation to the addition of PCI to optimal medical therapy,” he writes.
He also suggests that the improvements in quality of life, as noted among the trial’s secondary endpoints, should not be discounted, before concluding: “While the results of requisite additional analyses and followup data from the REVIVED trial are awaited, the prevailing dictum should be to diagnose the joint conditions of congestive heart failure and coronary artery disease and to provide therapies that are known to be effective for both of these conditions, as the goals of therapies that have not yet met that bar are carefully considered against their risks.”
Asked to reflect on the trial’s findings, Mirvat Alasnag (King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia) told Cardiovascular News that the results were of little surprise, noting that it was unlikely that such patients as those enrolled in the study will gain a survival benefit from revascularisation. However, Alasnag said that the results serve to underscore the need for effective intervention in this population.
“These patients have a very poor prognosis, wherein at 3.4 years of follow-up one in three patients is dead or readmitted with heart failure in the REVIVED-BCIS2 trial,” she told Cardiovascular News . “These are alarming findings suggesting that advanced ischaemic cardiomyopathy has a worse survival than some cancer diagnoses. As such, it is imperative that we intervene sooner at the primary and secondary prevention levels.
“Intensive risk factor modifying therapies should be started sooner along with lifestyle alterations. Guideline-directed medical therapy with the current pillars should be initiated early and optimised. Pathways for heart failure management should be streamlined. In other words, we should avert this late stage of the disease by all means possible.”
Providing a cardiothoracic surgeon’s perspectives on the trial findings, Mario Gaudino (New YorkPresbyterian and Weill Cornell Medicine, New York, USA) told Cardiovascular News that the REVIVED trial adds the idea that revascularisation does not necessarily confer a clinical benefit.

“One trial can never change practice,” he commented. “REVIVED is a great study and adds to the growing evidence that the old paradigm, revascularisation equals survival, is not always true. This is what we have seen in multiple trials comparing PCI versus medical therapy in patients with coronary artery disease and preserved ejection fraction—including ISCHEMIA.
“The question now is if, however, there are patient subsets where revascularisation may be beneficial. In REVIVED there are no data on the efficacy of the revascularisation and also on the presence and amount of ischaemia—most patients were not symptomatic at baseline and may not have ischaemia.
“For now, we can say that probably some of our traditional concepts on myocardial revascularisation may be wrong and more work is needed to understand which patients may—or may not— benefit from it.”
A global initiative will compare coronary artery bypass graft surgery (CABG) and percutaneous coronary intervention (PCI) in patients with ischaemic left ventricular dysfunction, closing the gap for “modern, high-quality evidence” of the optimal treatment strategy in this patient cohort.
Attendees of the 2022 European Association of Cardio-Thoracic Surgery (EACTS) annual meeting (5–8 October, Milan, Italy) were offered a glimpse of the Canadian CABG or PCI trial—STICH3C— by investigator Stephen Fremes (Sunnybrook Research Institute, Toronto, Canada).
STICH3C trial has been granted funding by the Canadian Institutes of Health Research (CIHR) and aims to determine whether CABG, compared to PCI is associated with a reduction in all-cause mortality, stroke, post-procedural myocardial infarction (MI), repeat revascularisation or heart failure readmission over a median follow-up of five years, in patients with multivessel or left main coronary artery disease and ischaemic left ventricular dysfunction.
November 2022 | Issue67 4 Conference Coverage
Continued from page 1
outcome: All-cause death
heart failure hospitalisation 37.2 % PCI 38% Medical therapy
“Definitive” data show PCI fails to reduce death, heart failure hospitalisation in patients with severe left ventricular dysfunction Primary
or
Divaka Perera (centre)
Global studies will fill gap for “high-quality evidence” on invasive treatment of ischaemic cardiomyopathy
Interest in cerebral embolic protection undimmed despite unchanged stroke rates in PROTECTED TAVR
Among patients undergoing transfemoral transcatheter aortic valve implantation (TAVI), the use of a cerebral embolic protection device (Sentinel, Boston Scientific) did not have a significant effect on the incidence of periprocedural stroke. However, a difference in rates of disabling stroke, favouring the use of a cerebral embolic protection device, may yet confer some benefit to its use, researchers claim.
These are the conclusions of the PROTECTED TAVR study, presented during a late-breaking clinical science session at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA) by Samir Kapadia (Cleveland Clinic, Cleveland, USA) and simultaneously published in the New England Journal of Medicine (NEJM)
Despite the trial failing to meet its primary outcome, interest in cerebral embolic protection has not dampened, and all but one of the discussants for the conference session at TCT stated that they would opt to recommend the use of such a device as an adjunct to a TAVI procedure if it were being used in a friend or family member, for example.
PROTECTED TAVR randomised a total of 3,000 aortic stenosis patients from across 51 centres in North America, Europe and Australia to undergo TAVI with or without the use of the Sentinel cerebral embolic protection device, with a primary endpoint of stroke within 72 hours after the procedure, or before discharge. Sentinel is designed to be used during TAVI procedures to reduce the risk of stroke by capturing and removing debris dislodged by such procedures before it reaches the brain.
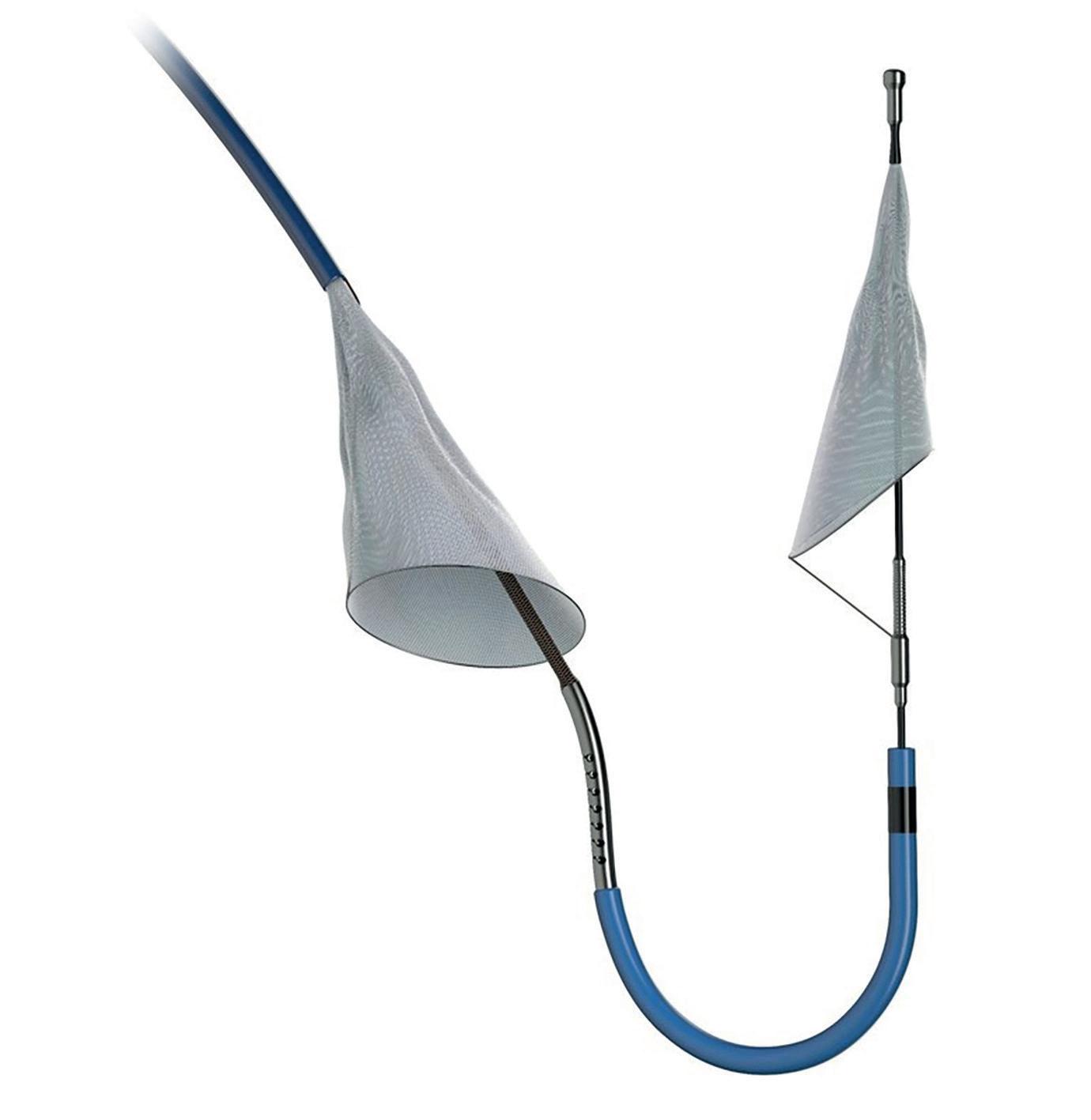
A total of 1,487 patients completed follow-up in the control group and 1,483 in the cerebral embolic protection arm. Use of Sentinel was found to be feasible in 94.4% of patients, and safe, with no major complications. Reporting the study’s findings at TCT, Kapadia reported that the incidence of stroke within 72 hours after TAVI or before discharge did not differ significantly between the cerebral embolic protection group and the control group (2.3% vs. 2.9%; difference, −0.6 percentage points; 95% confidence interval, −1.7 to 0.5; p=0.30). Significantly, according to Kapadia, disabling stroke occurred in 0.5% of the patients in the cerebral embolic protection group and in 1.3% of those in the control group.
There were no substantial differences between the cerebral embolic protection group and the control group in the percentage of patients who died (0.5% vs. 0.3%); had a stroke, a transient
ischaemic attack, or delirium (3.1% vs. 3.7%); or had acute kidney injury (0.5% vs. 0.5%), Kapadia reported.
“If the trial showed that the primary endpoint was positive but all the strokes we prevented were minor strokes, some people would be questioning whether we really need to use the device or not,” Kapadia told Cardiovascular News, digesting the findings of the trial and reiterating his point that though the primary endpoint, the incidence of peri-procedural stroke, was unchanged based upon the use of cerebral embolic protection with the Sentinel device, the rate of disabling strokes did appear to be impacted.
“Even though it is not a primary endpoint, it is a secondary endpoint, I understand that statistically this can be a challenge, but it is a 0.8% difference—
so it is not a little bit of a difference—[it is] a 60% reduction.”
Based upon the trial’s results, the number of patients needed to be treated to prevent disabling stroke is around 125, Kapadia reported, adding that since the safety of the device was established in the trial, cost considerations may be an important determinant of whether uptake of cerebral embolic protection during TAVI is likely to become more widespread.

Doubters or believers?
Discussing the findings following Kapadia’s presentation, session moderator Michael Mack (Baylor Scott & White The Heart Hospital, Plano, USA) said that the trial’s primary result is unlikely to do much to convince clinicians who are unsure about the merits of using cerebral embolic protection, or to deter those who favour its usage.
“The minority of patients undergoing TAVI in the USA have neuro protection today,” commented Mack. “It seems to me that there are clearly believers and
non-believers. If you are a believer you can say this trial is positive because we prevented disabling strokes. Those that do not want to use it can say that the trial was not positive because all strokes—which is the primary endpoint—were no different.”
Mack’s fellow panellist, the neurologist and stroke specialist Steven Messe (Hospital of the University of Pennsylvania, Philadelphia, USA), responded that the significance of the prevention of disabling stroke should not be downplayed.
“I think it is going to be very interesting to see how this study is received by clinicians in practice,” Messe commented.
“You are right that however you come in with your beliefs might inform how you interpret it, and obviously reducing disabling stroke is the holy grail in neurology, it is clearly the worst kind of stroke and it means something to say that you have reduced it. But, the number needed to treat is somewhat high, I think that is important, and it is a cost consideration given that it is clearly safe.
“As a neurologist I can tell you that disabling stroke is an extremely important outcome for patients, for their family members, [and] for the clinicians that care for patients, you will know that is the worst kind of stroke.”
Asked if the data—based upon a number needed to treat of 125—provide a compelling rationale in his view to use a cerebral embolic protection device, David J Cohen (St Francis Hospital, Roslyn, USA) echoed the view that interpretation of the trial is likely to be coloured by the individual’s existing view of the technology.
“Disabling stroke is clearly a critical endpoint for these patients, there is zero question of that,” said Cohen. “It is costly, it impacts survival, it impacts long-term outcomes in many different ways. But, I do think it is very important to remember that it was a secondary endpoint in a negative trial, there were about five or six secondary endpoints in the trial and the p-value was 0.02, and so our confidence in that 125 is pretty wide. It might even include zero. I think that a lot of the impact on practice is going to depend on people's emotional, Bayesian interpretation of that finding of: 'do you believe it or not?'
“I have said for a very long time, and I think that this trial reinforces it, there are only two right ways to use embolic protection in TAVI: for everybody or for nobody. The in-between does not work and I think this study showed that pretty well. There are two good ways to go depending on your rationale.”
Issue67 | November 2022 5 Cerebral Protection
If you are a believer, you can say this trial is positive because we prevented disabling strokes”
Sentinel Rate of stroke with cerebral embolic protection 2.3% without cerebral embolic protection 2.9% Rate of disabling stroke with cerebral embolic protection 0.5% without cerebral embolic protection 1.3%
Samir Kapadia












*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiovascular field Editorially independent Visit cardiovascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
Latest TVT TAVI data point to positive picture on
progress needed on stroke and pacemaker rates
“Science tells us what we can do, guidelines tell us what we should do, and registries like this one tell us what we are actually doing,” Joseph Bavaria (University of Pennsylvania, Philadelphia, USA) told attendees of the 2022 European Association of Cardio-Thoracic Surgery (EACTS) annual meeting (5–8 October, Milan Italy), where he offered a deep dive into data from The Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) TVT Registry looking at transcatheter aortic valve implantation (TAVI) procedures throughout the USA. The TVT registry is among the world’s largest sources of real-world TAVI data—including data from over 450,000 TAVI patients.
Bavaria’s presentation delivered several important insights into recent trends in TAVI, which includes the growth of the procedure among low surgical risk patients since its approval among this patient cohort in 2019, as well as changes in clinical outcomes over time. The registry now includes data from 813 sites, representing around 81% of the open heart programmes throughout the country, according to Bavaria, indicating that the USA now has one TAVI site for every 60,000 people over the age of 65—more than any other country.
TAVI procedural volume overtook surgical aortic valve replacement (SAVR) in 2019 as the predominant technique for aortic valve replacement throughout the USA, Bavaria recapped, noting that the latest tranche of available data for 2021 show that around 87,020 TAVI procedures took place, as compared to 56,645 SAVR procedures—representing a flattening of the curve for TAVI growth. These figures are likely to be somewhat affected by the pandemic, Bavaria noted, which impacted the volume of elective procedures that were performed in most centres. “TAVI growth has slowed, [and] it is questionable whether total aortic valve replacement rebounded,” he commented in his presentation, reflecting on the data for 2021. Importantly he pointed out that the STS database appears to show that complex aortic valve replacement procedures, as well as Bentall operations, have increased steadily in that time.

Stratified by age, Bavaria revealed that the latest figures show that the average age for high-risk patients undergoing TAVI procedures stood at 81 years, compared to 80 years for intermediate-risk patients, and 75 years for low-risk patients, figures that have remained stable for around the last two years. However, there were changes in the location of where procedures were being performed, showing a growth in the number of procedures being carried out in the cath lab (20%) or the hybrid cath lab (34%), and a decline in the number of procedures being performed in the hybrid operating room (46%).
Turning to the reason for the procedure, as dictated by the heart team, Bavaria commented that there had been a “metamorphosis” in the proportion of procedures being classified as low-, intermediateor high-risk. Since its approval in 2019, Bavaria explained, low-risk TAVI now comprises almost one third (29%) of all procedures carried out, exceeded only marginally by intermediate- (34%) and high-risk (37%) procedures. The volume of low-risk procedures
increased rapidly after the approval of the procedure, but the growth into this risk profile group has been less pronounced since, Bavaria explained. The data also point to the fact that the mean length of stay inhospital is now one day among all risk groups, down from around five days in 2015, something that Bavaria described as “an excellent result for the USA”.
On clinical outcomes, Bavaria noted in his presentation that the rate of inhospital life-threatening bleeding stood at around 2.3% in 2021, while disabling bleeding stands at around 1.5%. This is a trend that has stood relatively flat for the last two to three years, Bavaria commented, but noted there is some indication that 2022 data may show a further decrease. Zooming in on the data for low-risk patients, he noted that major/life-threatening and disabling bleeding stood at 1.5% and 1% respectively, adding that there has been very little change in these rates since 2019.
“Stroke is one of the areas where we have not really made that much progress,” Bavaria added, displaying a slide in which he pointed out that there had been little change in the rates of 30-day stroke (2.3%) over the last eight years. “There has been no real change over eight years, and this is incredibly important as we increase into low-risk so rapidly,” he commented. “This is a problem that needs to be corrected.” The
cerebrovascular event rate for the low-risk cohort specifically was 1.1%, Bavaria added.
Regarding the use of cerebral embolic protection, Bavaria noted that this was a newly added field to the TVT registry, first being used in 2018 upon approval of the Sentinel (Boston Scientific) device. Deployment of Sentinel is “increasing slowly,” Bavaria commented, adding that there is little difference in its usage between different risk groups. Overall, it is used in around 15% of cases added to the registry, he noted.
“TAVI mortality is very good,” Bavaria said of his next slide, in which he showed an overall mortality rate of 11.3% at one year, commenting: “The national results are good and this is probably an effect, to a certain extent, of low risk.” However, one issue he noted was a disparity in the rates of discharge and 30-day mortality, which stood at 1.1% and 2.3% respectively for 2020, the last full year for which figures were available. “The event rates are pretty low, but why do we have 100% increase in 30-day mortality compared to discharge mortality?” he questioned.
“This is something that we have to figure out. Is it because they are going to heart block or having sudden
Highlights from Bavaria’s TVT
data
paravalvular leaks and congestive heart failure and dying? It is a very interesting delta that we have to think about.” Looking at rates of mortality specifically in the low-risk patient group, he noted that this currently stands at about 5% at one year, and suggested that this figure has plateaued since the introduction of this patient cohort into the data. This slide also demonstrated a rate of in-hospital mortality of 0.5%, and 0.8% 30-day mortality for the most recent full year of data.
Issue67 | November 2022 7 TAVI Trends
mortality, but
DATA REVIEW
29% of TAVI procedures performed in lowrisk patients year average age for low-risk TAVI patients pacemaker rate at 30 days in low-risk patients 75 10.1% 87,020 11.3%
Science tells us what we can do, guidelines tell us what we should do, and registries like this one tell us what we are actually doing”
2021 Overall
at one year
TAVI procedures in
mortality
Joseph Bavaria (right)
Transradial access is “gold standard” approach for percutaneous coronary procedures
Transradial access for percutaneous coronary procedures is associated with lower rates of all-cause mortality and major bleeding at 30 days when compared to transfemoral access.
This was the major finding of a metaanalysis of individual patient data from seven multicentre randomised clinical trials, presented during a hot line session at the European Society of Cardiology (ESC) 2022 congress (26–29 August, Barcelona, Spain) by Giuseppe Gargiulo (University Federico II of Naples, Naples, Italy). The findings were simultaneously published in the journal Circulation
The authors of the study—the Radial Trialists’ Collaboration—suggest that the finding of the meta-analysis provides evidence that transradial access should be considered the preferred access-site for percutaneous coronary intervention (PCI) in patients with acute coronary syndrome, supporting recent guideline changes which endorse this approach.
In their paper, the researchers note that there is currently conflicting
evidence on whether the radial approach reduces mortality compared with transfemoral access, with some randomised trials finding an association with lower mortality in coronary patients undergoing invasive management.
In a bid to resolve this question, Gargiulo and colleagues from the Radial Trialists’ Collaboration performed an individual patient data metaanalysis of contemporary randomised trials comparing the two approaches among patients undergoing coronary angiography with or without PCI to assess whether radial access is associated with a lower incidence of mortality and major bleeding. The study team also sought to establish whether these associations are influenced by clinical or procedural characteristics, and if bleeding prevention mediates the mortality reduction.
The meta-analysis included a total of 21,600 patients from across the seven trials, of which 10,775 were randomised to radial access and 10,825 were randomised to femoral access. The median age of participants was 63.9 years, 31.9% were women, 95% presented with acute coronary syndrome, and 75.2% underwent PCI. The primary outcome was all-cause mortality at 30 days and the co-primary outcome was major bleeding at 30 days. The primary analysis was conducted based on the intention-to-treat cohort. The incidence of all-cause death was 1.6% in the radial group and 2.1% in the femoral group, for a hazard ratio of 0.77 (95% confidence interval [CI] 0.63–0.95; p=0.012). Major bleeding was also significantly reduced with radial versus
femoral access, occurring at rates of 1.5% and 2.7%, respectively, for an odds ratio of 0.55 (95% CI 0.45–0.67; p<0.001), the researchers report.
The survival benefit was confirmed in the per-protocol, astreated, PCI, acute coronary syndrome, and myocardial infarction cohorts. The effects of radial access were also consistent across the majority of prespecified subgroups, and the findings indicated that patients with baseline anaemia might have a greater mortality benefit compared to those without anaemia.
In a multivariable model, radial access was independently associated with a significant 24% relative risk reduction of 30-day all-cause mortality and 51% reduction of major bleeding. Mediation analysis showed that the benefit of TRA on mortality was only marginally driven by the prevention of major bleeding.
“Our study provides comprehensive evidence from high-quality multicentre trials that the use of transradial access over transfemoral access is associated with reduced all-cause mortality,” Gargiulo said, summarising the results in a press conference at ESC 2022.
“The meta-analysis provides evidence that transradial access should be considered the gold standard access site for percutaneous coronary procedures, particularly in acute coronary syndromes, supporting [the] most recent recommendations,” Gargiulo concluded.
FRAME-AMI sheds light on best strategy for selecting noninfarct lesions for PCI


Selection of non-infarct related artery (IRA) lesions for intervention using fractional flow reserve (FFR) is superior to routine angiography-based selection in patients with acute myocardial infarction and multivessel disease, researchers have reported.
THIS WAS THE CONCLUSION OF JOO-YONG Hahn (Samsung Medical Center, Seoul, Republic of Korea) presenting findings of the FAME-AMI trial, an investigator-initiated, open-label trial comparing FFR to angiography-guided percutaneous coronary intervention (PCI) in acute myocardial infarction (AMI) with multivessel disease. Hahn presented the findings at the annual congress of the European Society of Cardiology (ESC 2022; 26–29 August, Barcelona, Spain).
Randomised trials have consistently found that PCI of non-IRA lesions for complete revascularisation in patients with ST-segment elevation myocardial infarction (STEMI) improves clinical outcomes compared with IRA-only PCI, Hahn explained. ESC guidelines recommend that revascularisation of non-IRA lesions should be considered in STEMI patients with multivessel disease during the index procedure or before hospital discharge. But, the optimal strategy to select targets for non-IRA PCI has
not been clarified.
FRAME-AMI was conducted at 14 sites in Korea and randomly assigned patients with AMI and multivessel coronary artery disease who had undergone successful PCI of the IRA to either (FFR-guided PCI of non-IRA with FFR ≤0.80 or (angiography-guided PCI of non-IRA with >50% diameter stenosis. In both groups, complete revascularisation during the index procedure was recommended. Staged procedures were permitted at operators’ discretion. The primary endpoint was a composite of all-cause death, myocardial infarction, or repeat revascularisation.
Between August 2016 and December 2020, a total of 562 patients underwent randomisation. The average age was 63 years and 16% were women. Non-IRA lesions were treated by immediate PCI after successful treatment of IRA in 337 patients (60%) and by staged procedure during the same hospitalisation in 225 patients (40%). During a median follow up of 3.5 years (interquartile range 2.7–4.1 years), the primary endpoint occurred in 18 of 284 patients in the FFR group and 40 of 278 patients in the angiography group (Kaplan–Meier event rates at four years, 7.4% versus 19.7%; hazard ratio [HR] 0.43; 95% confidence interval [CI] 0.25–0.75; p=0.003).
The incidence of death was significantly lower in the FFR group compared with the angiography group, occurring in give patients versus 16 patients, respectively (Kaplan–Meier event rates at four years, 2.1% versus 8.5%; HR 0.30; 95% CI 0.11–0.83; p=0.020). The incidence of myocardial infarction was also significantly lower in the FFR group compared with the angiography group, occurring in seven patients versus 21 patients, respectively (Kaplan–Meier event rates at 4 years, 2.5% versus 8.9%; HR 0.32; 95% CI 0.13–0.75; p=0.009).
Ten patients in the FFR group had an unplanned revascularisation compared with 16 patients in the angiography group, with no significant difference between the two groups (Kaplan–Meier event rates at four years, 4.3% versus 9.0%; HR 0.61; 95% CI 0.28–1.34; p=0.216).
Sharing key messages from the study, Hahn commented that the findings shed light on the efficacy and safety of doing selective PCI of non-IRA lesions using FFR-guided decision making in patients with AMI and multivessel disease. For treatment of non-IRA lesions, FFR-guided PCI reduced the risk of death, MI, or repeat revascularisation with fewer number of stents and less contrast media compared with angiography-guided PCI, Hahn concluded.
November 2022 | Issue67 8 Coronary Interventions
Our study provides comprehensive evidence from highquality multicentre trials that the use of transradial access over transfemoral access is associated with reduced allcause mortality”
Joo-Yong Hahn All-cause death Cardiac death Myocardial infarction Revascularisation Angiography 8.5% 8.2% 8.9% 9% FFR 2.1% 1.4% 2.5% 4.3%
Giuseppe Gargiulo
Promising results for transcatheter mitral valve repair platforms
“expand options” for patients
“THE WHOLE FIELD OF TRANSCATHETER repair is moving forward and these results are getting better and better, such that they are now essentially like the results of a very good mitral surgeon.” This was the assessment of Scott Lim (University of Virginia, Charlottesville, USA) in discussion of results from the CLASP IID trial at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA).
There, Lim co-presented results of the pivotal trial alongside Konstantinos Koulogiannis (Atlantic Health System Morristown Medical Center, Morristown, USA) in a late-breaking clinical science session, with the findings simultaneously published in JACC: Cardiovascular Interventions. CLASP IID is the first randomised trial to compare transcatheter edge-to-edge repair (TEER) using two different devices—Pascal (Edwards Lifesciences) and Mitraclip (Abbott)—in patients with degenerative mitral regurgitation (MR).
Delivery of the trial’s results came just days after the US Food and Drug Administration (FDA) had approved the Pascal Precision TEER system for use in this patient population, the first new device to receive such an approval in the USA since MitraClip in 2013. Design features incorporated in the Pascal implant include a central spacer that is designed to fill the regurgitant orifice and paddles that are intended to maximise coaptation and reduce stress on the native leaflets. As well as its recent FDA approval, the device also received a CE mark for the treatment of both mitral and tricuspid regurgitation in August 2022.
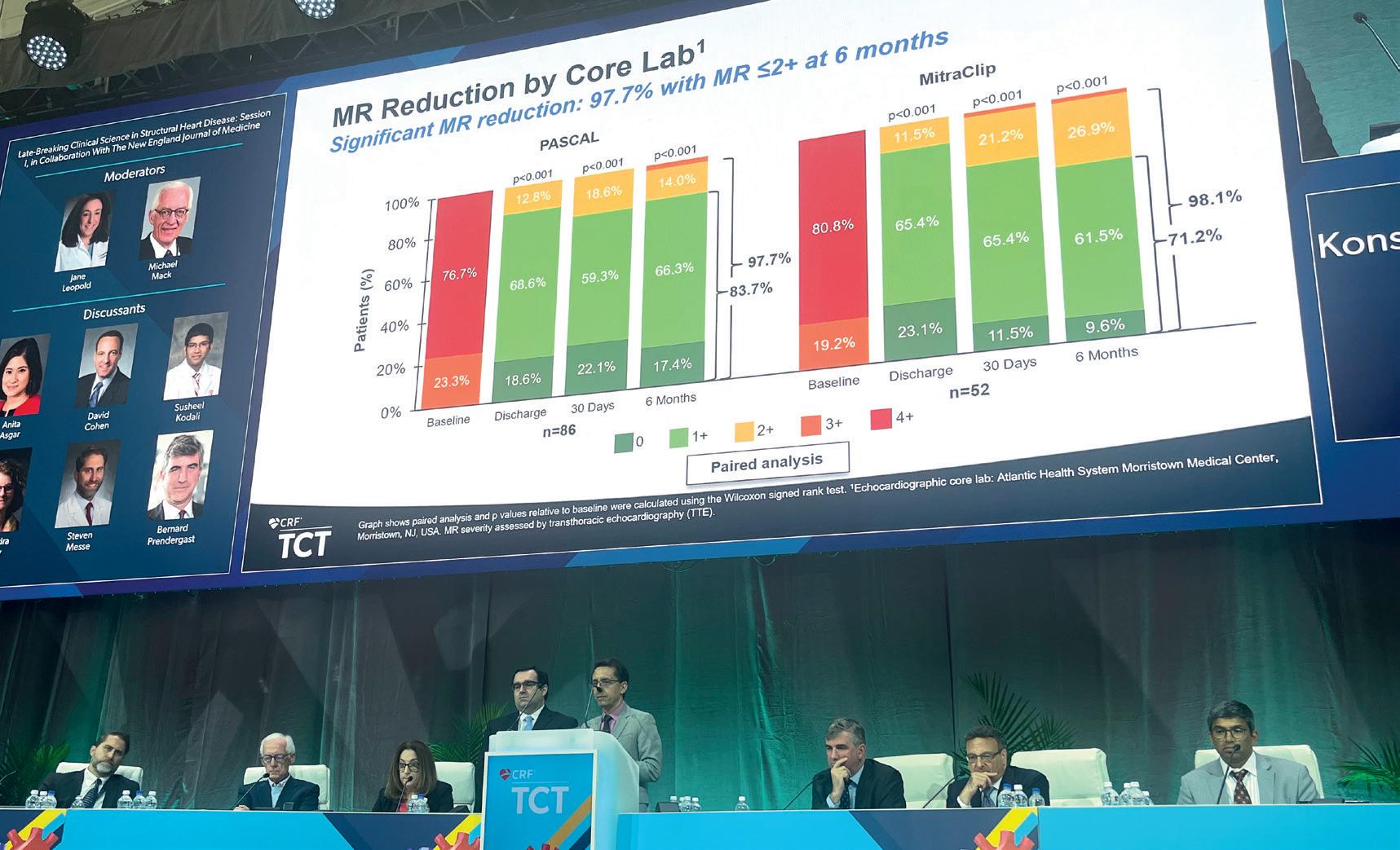
CLASP IID was conducted in 43 sites in the USA, Canada, and Europe, randomising 180 patients at prohibitive surgical with 3+ or 4+ degenerative MR risk 2:1 to receive either the Pascal (n=117) or MitraClip (n=63) device. In their presentation, which was among the most hotly-anticipated of the clinical trials presented across the four-day TCT 2022 programme, Lim and Koulogiannis reported that the trial met its primary safety endpoint, with a 3.4% major adverse event (MAE) rate seen in the Pascal group, compared with 4.8% in the MitraClip arm. Added to this, the trial’s primary effectiveness endpoint was also met, with MR of ≤2+ reported in 96.5% of patients in the Pascal arm, compared with 96.8% of those receiving the MitraClip. Lim and Koulogiannis noted that a “significant and sustained” MR reduction was seen in the trial with 97.7% of patients achieving MR ≤2+ at six months. The investigators noted that the results demonstrated favourable ventricular remodelling, with improved forward stroke volume, while patients also experienced significant improvements in functional capacity and quality of life.
Lim and Koulogiannis concluded that Pascal is a “beneficial therapy for significant symptomatic
degenerative MR, expanding transcatheter options for prohibitive surgical risk patients”.
“This is a really important trial for the field and TEER in general, showing how safe this procedure is and how successful it is for these patients,” panellist Anita Asgar (Montreal Heart Institute, Montreal, Canada) commented in discussion that followed the presentation, which honed in on how clinicians should weigh up the choice between either MitraClip or Pascal. However, Asgar preached caution in a direct comparison of the two devices due to the limited numbers of patients enrolled in the trial. Weighing
of 11.2% at 30 days. He also relayed a “significant and sustained” MR reduction, with 92.4% of patients achieving MR ≤2+ at six months. These findings were allied to significant improvements in symptoms and quality of life, and low post-procedure gradients sustained below 5mmHg, Hausleiter said. The results may expand treatment options for degenerative MR patients with complex valve anatomy who have previously been viewed as unsuitable for TEER.
Though Pascal grabbed many of the headlines at TCT, there were promising signals for MitraClip coming from the EXPAND G4 post-approval study, detailing clinical and echocardiographic outcomes from more than 1,000 patients treated with the Mitraclip G4 system from 60 sites worldwide. G4 is the fourth generation of MitraClip and builds on previous iterations with two wider clip sizes, as well as incorporating an independent grasping feature.
Results of the study, the first complete analysis on core lab assessed 30-day outcomes, were delivered by Ralph Stephan von Bardeleben (Universitätsmedizin Mainz, Mainz, Germany), who reported they “expand the spectrum of TEER suitable patients with data that will redefine complex anatomies and TEER suitability”.
in on this point, Bernard Prendergast (St. Thomas’ Hospital, London, UK) commented that the trial’s findings offered “comfort” that TEER is a safe and effective treatment strategy regardless of the two major devices available, adding: “For individual operators it will come down to cost, ease of use, and operator preference of one device over another.”
In a later session, Jörg Hausleiter (Klinikum der Universität München, Munich, Germany) presented further data on the Pascal device, sharing findings from the PASCAL IID Registry, a prospective registry for TEER in prohibitive risk patients with degenerative MR and complex mitral valve anatomy—patients who were not eligible for randomisation in CLASP IID due to their complex anatomy. Ninety-eight patients from sites in Canada, Germany and the USA were enrolled.
In his presentation, Hausleiter reported that the device achieved favourable safety results with a high rate of survival of 97.9% and a composite MAE rate
Among the headlines from the analysis, which included 1,044 patients with complete follow-up at 30 days, investigators reported significant MR reduction to mild or less (≤ grade 1+ on a four-point scale) achieved in 91% of patients, with a low rate of adverse events of 1.3% all-cause mortality. Clinical improvements including 83% of patients achieving New York Heart Association (NYHA) Functional Class I/II, an improvement of 52% from baseline of 31%; and an 18-point improvement in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score, a 35% improvement from baseline, were also reported.
Von Bardeleben also reported that 65% of patients were treated with one clip, compared to 55% of those studied in EXPAND, using an earlier generation of the device. A wide clip was used in 88% of the patients treated, he added, reporting that wider clips were not associated with increased mitral valve gradients postprocedure.

“The study confirms the safety and effectiveness of this TEER method, [and] it demonstrates it can be tailored to the therapy of individual patient anatomies,”

Von Bardeleben commented in the concluding remarks of his presentation, remarking that the study demonstrates consistent MR reduction with low adverse events with the MitraClip G4 device, as well as improved procedural efficiency. Follow-up will continue out to five years.
Issue67 | November 2022 9 Mitral Update
LIVE FROM TCT
The whole field of transcatheter repair is moving forward and these results are getting better and better, such that they are now essentially like the results of a very good mitral surgeon”
Jörg
Hausleiter Ralph Stephan von Bardeleben
Konstantinos Koulogiannis (left) and Scott Lim



*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the vascular arena Editorially independent Visit vascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
Cardiovascular surgery
call” for trialists over gender balance
CLINICAL TRIALS IN cardiothoracic surgery must ensure that sufficient numbers of women are enrolled to reflect anatomic and symptomatic differences between the sexes, attendees of the 2022 European Association of Cardio-Thoracic Surgery (EACTS) annual meeting (5–8 October, Milan Italy) heard.
This was the message of Jolanda Kluin (University of Amsterdam, Amsterdam, The Netherlands) during a plenary session in which she made the case for a revascularisation trial focused on women.
Kluin detailed that there are numerous differences between men and women relating to cardiovascular disease, including in symptoms, risk factors, and treatment. She also noted that there is growing evidence of differences in outcomes of coronary artery bypass graft (CABG) surgery between the sexes, pointing to a pooled analysis of individual patient data, compiled by Mario Gaudino (Weill Cornell Medicine, New York) et al and published in the European Heart Journal which reached the conclusion that women have worse outcomes than men in the first five years after the procedure.
Speculating on the factors behind this difference, Kluin reflected that there are “multifactorial” underlying causes, suggesting that female patients are more likely to be diagnosed later and therefore face more severe disease. She pointed to anatomical differences—such as smaller coronary arteries—that may make surgery more complicated, and noted differences in hormones, renal function, and coagulation that may also have a bearing.
“There are important differences between men and women in the presentation, symptoms and aetiology in the anatomy and the outcome of cardiovascular disease,” Kluin said in the concluding remarks of her presentation. “Large databases should be exploited to address sex differences and to improve women's health. If you perform clinical study, ensure that sufficient numbers of women and men are included and take a sex-disaggregated approach to collect, analyse and report your data.”
Responding to Kluin’s presentation, EACTS secretary general J Rafael Sádaba (Hospital Universitario de Navarra, Pamplona, Spain), who was moderating the session, said: “It is a wake-up call to take this in mind for future trials.”
Among action that is being taken to address this disparity, Kluin highlighted ROMA Women a global trial a global trial that is randomising female patients undergoing primary isolated nonemergent CABG to either single or ultiple arterial grafts.
Swedish registry data point to survival benefit for younger patients undergoing CABG
An analysis of observational data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) has shown that coronary artery bypass graft (CABG) surgery was associated with a lower rate of mortality than percutaneous coronary intervention (PCI) in patients undergoing revascularisation for left main coronary artery disease.
ACCORDING TO THE AUTHOR OF THE ANALYSIS, Elmir Omerovic (Sahlgrenska University Hospital, Gothenburg, Sweden), the benefit of CABG was greatest in younger patients with a longer life expectancy, while PCI was associated with lower mortality in patients over 80.
Omerovic presented findings of his analysis in a latebreaking clinical trial session at the 2022 European Association of Cardio-Thoracic Surgery (EACTS) annual meeting (5–8 October, Milan Italy), having also presented the same data at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA). Omerovic commented that there are few randomised trials comparing the approaches in left main coronary artery disease, commenting that it remains unclear which—if either—is the superior approach.
The merits of the two revascularisation strategies have been a topic of contention between interventional cardiologists and cardiothoracic surgeons, with Omerovic highlighting controversy relating to the interpretation of results of the EXCEL trial—a randomised trial comparing PCI with CABG for the composite endpoint of death, myocardial infarction (MI), and stroke in patients with left main disease—as an exemplar of this thorny issue.
Aiming to bring some new insight into the debate, Omerovic and colleagues gathered data from SCAAR in
order to compare PCI with CABG for the endpoint of allcause mortality in an unselected left main coronary artery disease population.
For their analysis, investigators analysed data from all patients undergoing coronary angiography in Sweden between 2015 and 2022 who were diagnosed with either stable angina, unstable angina or non-ST-elevation myocardial infarction (NSTEMI). Left main disease was defined as stenosis >50% on angiography. As the study was observational, the investigators used a statistical approach known as an ‘instrumental variable’ analysis, to adjust for known and unknown confounders.
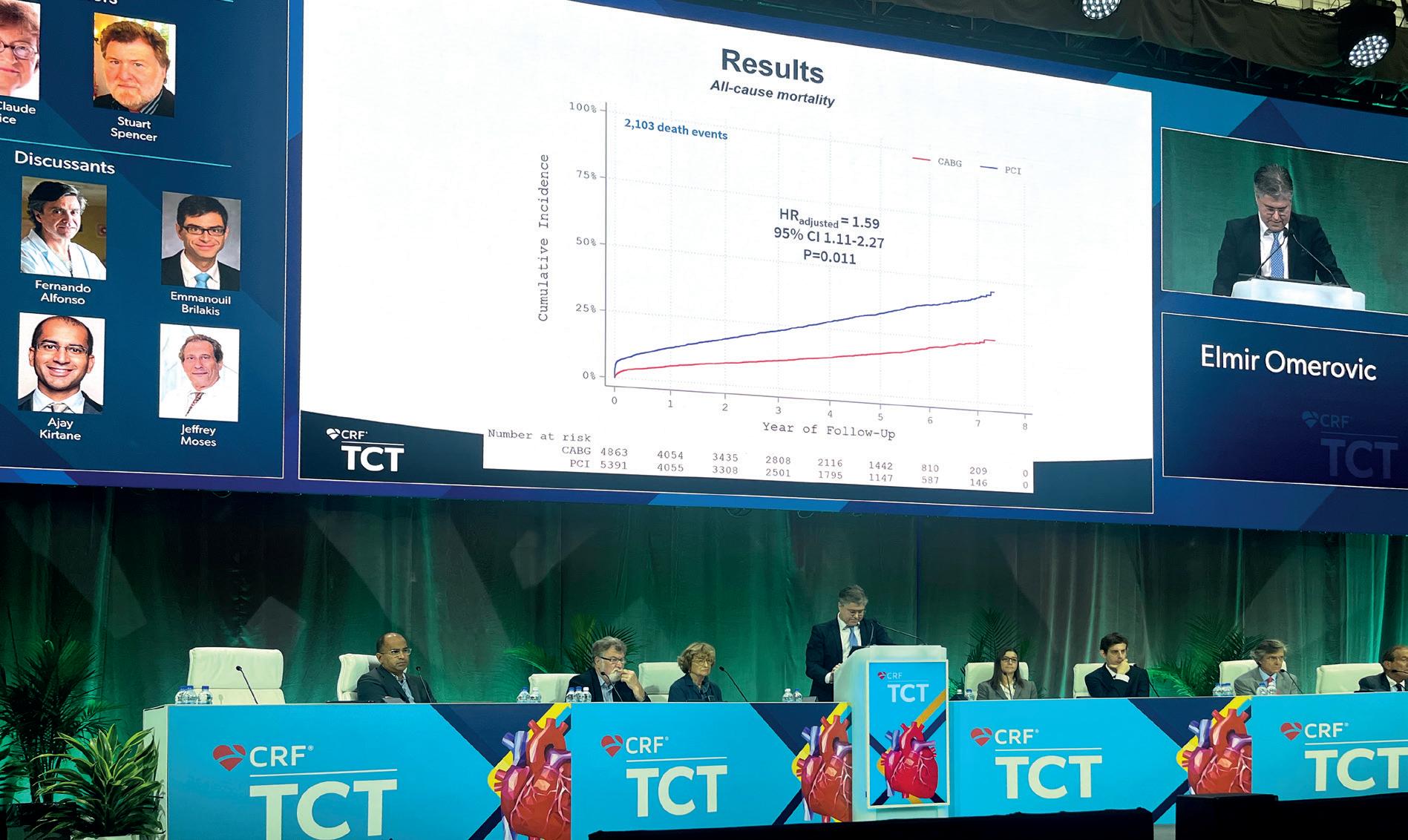
In total, data were captured for 10,254 patients, 5,391 of whom underwent PCI and 4,863 CABG. Omerovic explained that there was a wide variability in the rate at which patients were likely to receive one revascularisation strategy in each hospital from which they analysed data. “We differ so much in our preference for one [approach] or the other, that there is, in other words, an act of randomness of where you happen to live and which hospital of these you belong,” he explained, displaying the data centre by centre. “If a patient is treated in a hospital with a preference for CABG, most of the time they get a treatment for CABG.”
Presenting the primary endpoint data, out of a total of 2,103 death events, Omerovic revealed that the cumulative incidence of all-cause mortality was significantly greater for patients who had undergone PCI than for those who had received CABG, with an adjusted hazard ratio (HR) of 1.59 (95% confidence interval 1.11–2.27, p=0.011). Looking at the differences between the two procedures across various subgroups, Omerovic added that there was very little variability when it came to sex, presence of diabetes, or patients with NSTEMI, but he noted that the investigators had observed a significant interaction of a qualitative nature between those who were <80 and those who were >80, favouring PCI in the older patients, but trending towards CABG in those of lower age.
Issue67 | October 2022 11
The benefit of CABG was highest in younger patients with a longer life expectancy”
“Wake-up
Elmir Omerovic presents at TCT
Cardiovascular surgery
Minimally invasive aortic perfusion cannula receives EACTS
Techno-College innovation award
The developers of a minimally-invasive central aortic perfusion cannula—MIC-Cannula— received the European Association for CardioThoracic Surgery (EACTS) Techno-College Innovation Award. The award, which is chosen by the EACTS New Technology Committee, was presented during the society’s annual meeting (5–8 October, Milan, Italy). Francesco Pollari (Klinikum Nürnberg, Nuremberg, Germany) who developed the device alongside his colleague and wife Michela Cuomo, a paediatric cardiac surgeon, speaks to Cardiovascular News about the device and its potential applications.

Where did the idea for the device originate?
The idea originated from our experience in minimally invasive cardiac surgery and also from my personal experience in transcatheter aortic valve implantation (TAVI). Typically, these two areas are separate. There are surgeons who exclusively perform minimally invasive surgery and others who may exclusively perform TAVI. In our centre we had the opportunity to perform both, and for this reason I developed the experience in using a wire and to use catheter techniques.
That is why I felt minimally invasive perfusion has some limitations, but also that we could improve those using some techniques that are routinely used in TAVI, for example, the use of a longer wire, and a long cannula that are usually used for advancing the prosthesis in TAVI. I thought that could be the perfect solution to improve perfusion in a minimally invasive setting.
How easy was it to conceptualise the design of the device?
The main problem was to understand if it was only a fantasy! The concept is creating something new. We were worried we were wasting our time or our energy in something that could not become reality. I had the strength in confronting this problem together with my wife. At least two surgeons agreed that it could work, and that encouraged us to write the project and to apply for an international patent. Finally, after three years it was granted from the European Patent Office (EPO) and that was in the same week we received the award from the Techno-College. For a long time, we were not sure it could be a good idea, but finally we have obtained our recognition.
How does the device work?
The device allows antegrade perfusion in a minimally invasive setting. That is the novelty of this device and that was the limitation of the prior state of the art. The present minimally invasive cannulas are not able to perform antegrade perfusion, only retrograde perfusion. This new device overcomes this problem. It is inserted through the
femoral artery, and it is long enough to reach the aortic arch where the oxygenated blood is perfused in the distal part of the cannula.
The whole cannula has a thin diameter and that allows removal without the need for surgery or without using a standard vascular closure device. That is very important because many cardiologists will not have a cardiac surgeon on site. If cardiology has a complication during an elective or an emergency percutaneous coronary intervention (PCI), the only solution they have is to place a perfusion system. With this cannula they could perform a better perfusion, without a cardiac surgeon.
There are also applications in cardiac surgery. As a cardiac surgeon myself I designed this so that it could be used in every minimally invasive elective cardiac surgery. It could also be used in intensive care medicine especially for severe respiratory distress independent from cardiogenic shock. In severe respiratory distress through this new device, it can help to avoid upper body hypoxaemia, known as Harlequin syndrome.
The cannula is also equipped with radiopaque markers and has other characteristics, such as the curved shape of the distal part to respect the anatomy of the aortic arch.
What study has been carried out to date to validate the concept of the device?
There has been an independent study by a group in Aachen who have performed a computer fluid dynamic analysis. They were aware of the problem of Harlequin syndrome and they performed the study simulating how a cannula could improve this problem. That was lucky for us, because they proved with a computer fluid analysis that such a cannula could be the optimal solution to overcome this problem.
That was an independent study that adds value to our idea, but we will go further with our own studies.
What will receipt of the Techno-College innovation award mean for your development of the MIC-Cannula?
The award and the participation at the EACTS annual meeting allows us to get in contact with partners that could help us in developing this idea. To further develop the idea, we will not only need funding but also knowhow. Our knowhow is limited to our area of competence, cardiac surgery, but from bringing the idea from patent to reality there is a long road and the cooperation with partners is indispensable. The award from the Techno-College and the participation at the meeting is the best opportunity to find the right cooperation.
failure compared to aspirin
IN PATIENTS UNDERGOING coronary artery bypass graft (CABG) surgery, the addition of ticagrelor to aspirin was associated with a significantly decreased risk of vein graft failure, but was accompanied by an increased risk of clinically important bleeding, the findings of a systematic review and meta-analysis of four randomised trials has shown.
Findings of the analysis were presented during a late-breaking clinical trials session at the 2022 European Association of Cardio-Thoracic Surgery (EACTS) annual meeting (5–8 October, Milan Italy) by Sigrid Sandner (Medical University of Vienna, Vienna, Austria).
“As we know, early saphenous vein graft failure is mainly due to thrombosis subsequent to endothelial damage, and we also know that inhibition of platelet aggregation with aspirin reduces saphenous vein graft failure,” Sandner said at EACTS 2022. Dual antiplatelet therapy (DAPT) is associated with enhanced platelet inhibitory effects, she noted in the presentation, adding that controversy exists as to the benefit of DAPT for patients after CABG. Studies comparing ticagrelor DAPT with aspirin have yielded conflicting results, Sandner said.
To compare the risks of vein graft failure and bleeding associated with ticagrelor DAPT or ticagrelor monotherapy versus aspirin among patients undergoing CABG, Sandner and colleagues tapped data from four randomised trials comparing the strategies, with individual patient data from each trial synthesised into a combined data set for independent analysis.
The primary endpoint of the analysis was the incidence of saphenous vein graft failure per graft, while secondary outcomes were saphenous vein graft failure per patient and Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding events. A supplementary analysis included randomised trials comparing ticagrelor monotherapy with aspirin.
What is your advice to other surgeons bringing through new innovations?
Brainstorm, speak and think together. I had the luck to have this opportunity in my home because I married my colleague! But I am strongly a believer that the best idea comes from confrontation. Medicine consists of complex problems in every area, it would be very arrogant to think that one doctor could cure a patient alone. Cooperation in medicine is the key, and to develop new ideas it is the same. Through speaking and brainstorming an idea can be born and grow further.
The four trials included 1,316 patients and 1,668 saphenous vein grafts. Of the 871 patients in the primary analysis, 435 received ticagrelor DAPT and 436 received aspirin. The investigators found that ticagrelor DAPT was associated with a significantly lower incidence of saphenous vein graft failure (11.2%) per graft than was aspirin (20%) and was associated with a significantly lower incidence of saphenous vein graft failure per patient (13.2% vs 23%).
12
October 2022 | Issue67
Ticagrelor after CABG associated with a decreased risk of vein graft
To further develop the idea, we will not only need funding but also knowhow.”
Francesco Pollari
Aortic advances: New techniques and technologies in the spotlight
Heinz Jakob (University of Essen, Essen, Germany) and Tilo Kölbel (University of Hamburg, Hamburg, Germany) offer their respective cardiac and vascular surgery perspectives on the potential of some new techniques and technologies at the cutting edge of aortic advances. They detail how novel approaches such as rendezvous access for the Endo-Bentall procedure push the boundaries of treatment and how nascent technologies such as Philips’ Fiber Optic RealShape (FORS) technology might transform current practice.
On day two of the CX Aortic Vienna 2022 Digital Edition (24–26 October), Kölbel presented an edited case on the Endo-Bentall procedure using a rendezvous access technique. Kölbel notes that rendezvous access works by creating a throughand-through guidewire access from the transapical to a transfemoral route, or from a transapical to a transcarotid route. “That is why it is called rendezvous,” he explained, “because the devices meet each other, and then you can do what is otherwise very difficult to do.” He stressed that the access has only been used in three cases so far—one in Hamburg and two in Montpellier, France. “It is not a regular access,” he notes.
According to Kölbel, rendezvous access allows the operating physician to make the Endo-Bentall approach a modular procedure. Prior to the introduction of this technique, he explains that all approaches to combine a transcatheter aortic valve implantation (TAVI) valve with an ascending graft usually aimed to suture or connect those devices outside of the body, and then bring it into the body already connected and deployed. With the rendezvous technique, on the other hand, he details that “these two important parts of the device are connected within the body in a short period of time because of the access from two sides”.
Considering the treatment of Ishimaru zone 0 more generally, Jakob underlines the fact that aortic dissection, for example, is a “dramatic disease,” with a 2% death rate per hour in the first 24 hours. “As cardiac surgeons, this puts us under pressure,” he says. According to Jakob, the rendezvous technique is “an
ingenious approach,” as it enables the treatment of a patient population that are prohibited from classic open repair. “This is the last boundary, the last challenge we are facing in our field,” he remarks.
Both Jakob and Kölbel agreed that there were lessons to be learned about the importance of collaboration between the cardiac and vascular surgery fields, that had been brought to the fore at the meeting.

“I think we should overcome this old thinking that they are taking business from us,” said Jakob. “The technology is there, and the experience is growing, and Tilo and Stéphan Haulon in Paris are excellent protagonists of this approach. The heart is our field and so it makes sense to combine the efforts.”
“I personally think this is an area where vascular surgery cannot take over,” added Kölbel. “This disease is not well understood by vascular surgeons, but vascular surgeons have, in most centres, developed advanced endovascular skills which are needed if you want to treat this. The reason why collaboration is paramount is that you need understanding of the disease and of intracardiac structures and cardiac function and at the same time you need the skills and experience of advanced endovascular repair.”
Imaging advances
“For me, it is always fascinating to see the progress in the endovascular world,” Jakob notes, describing the leap that has been made even over the past year or so with FORS.
Kölbel follows this by noting
that it was “very interesting” to hear experienced speakers discussing the use of carbon dioxide flushing for the de-airing of stent grafts in their own centres.
On this front, things were different a few years ago, he added, as this was not a “regular thing” in stent grafts. “But, watching those presentations, it has become almost the standard—in experienced centres,” he notes.
“We have seen a huge number of small tips and tricks, and they all reduce morbidity and mortality a little bit,” Kölbel continues, “but all of them together suddenly make a procedure possible that has not been [previously].”
Here, Jakob also notes that it will be interesting to see how the community now follows Kölbel’s lead as a pioneer in this field and of many other endovascular treatments.
Kölbel himself adds that, while he has been using FORS for two-and-a-half years in his practice, he learned something new during day two of CX Aortic Vienna—namely that you can ‘zoom out’ on one of the panels while using the technology and view all of the wires. “I learned that from this meeting, here, today— some things you just need to see once to include in your practice,” he says.
And, he remains fascinated by future advances with FORS and is eagerly anticipating what is in the pipeline as well, in the hope these will improve clinical results further. Kölbel concludes that the key is to understand what will actually be of clinical use because, if anything, there is “almost too much information” right now, before complimenting the company (Philips) on their work in this space thus far.
when the situation is more appropriate, or occasionally you might not have to intervene at all. So, I think this is a reminder to all of us that the answer is not always to take the patient quickly to the operating room,” he said.
An important session on day three of CX Aortic Vienna (24–26 October, Digital) reframed the focus from intervention to conservative treatment in highly defined aortic arch patients. Roger Greenhalgh (London, UK) noted that it was “difficult to find a circumstance in the aorta where you can sit tight, with certainty, that the patient will be safe”. Michel Makaroun (University of Pittsburgh, Pittsburgh, USA) remarked, “[this session] really reminds clinicians that the answer is not always an intervention in 100% of patients.”
MAKAROUN SUBMITTED THAT a treatment strategy should never be “a knee-jerk reaction”. “You always have to evaluate what you have to offer and
whether it is going to be for the benefit of the patient. There are many instances where you can wait. You may still have to intervene later, probably electively,
Greenhalgh pointed to the “uphill battle” of trying to define circumstances in which an immediate operation is not required. Makaroun argued that nonoperative management can best serve selected patients with arch injury.
The only published guidelines (Society for Vascular Surgery, 2011) recommend non-operative management for grade I injuries and thoracic endovascular aortic repair (TEVAR) for grades II, III and IV, but more recent data suggests that grade II injuries are also relatively benign, clarified Makaroun.
A review of 192 patients in whom non-operative management was compared with TEVAR showed at three-year follow up that none of the 64 patients treated with non-operative management developed a progression of the lesion and that immediate outcomes were comparable. In the study there were 14 patients with grade III injuries treated conservatively due to location or associated trauma and these did not progress at the three-year mark, either, noted Makaroun.
“Traumatic aortic transection can be managed conservatively in many patients,” he said, commenting that while TEVAR is an excellent treatment for significant blunt thoracic aortic injury, non-operative management is a safe and best treatment for minimal aortic injuries.
Issue67 | October 2022 13
CX Aortic Vienna provides timely reminder that rushing the patient to the operating room is “not always the answer”
Heinz Jakob (left) and Tilo Kölbel
Long-term P2Y12 monotherapy after PCI “may be a promising strategy”
Three-year outcomes from the SMARTCHOICE trial have shown that P2Y12 inhibitor monotherapy was associated with a lower risk of clinically relevant major bleeding than prolonged dual antiplatelet therapy (DAPT) among patients undergoing percutaneous coronary intervention (PCI).
The long-term follow-up from the Korean randomised clinical trial, published in JAMA
Cardiology, suggest that long-term maintenance of P2Y12 inhibitor monotherapy may be a promising antiplatelet strategy after PCI, according to study authors Ki Hong Choi (Sungkyunkwan University School of Medicine, Seoul, Korea) et al
Involving 2,993 patients from 33 hospitals in Korea, 1,495 patients were randomised to receive either P2Y12 monotherapy after three months of DAPT, and 1,498 to receive prolonged DAPT. Investigators assessed the primary endpoint of major adverse cardiac and cerebrovascular events (MACCE), comprising of a composite of all-cause death, myocardial infarction (MI), or stroke, at three years, with secondary endpoints including bleeding, defined as Bleeding Academic Research Consortium (BARC) types 2–5, and major bleeding, BARC types 3–5.
One-year results for the trial were reported at the American College of Cardiology’s (ACC 68th annual scientific session in 2019, and found that 12 months after the index procedure, for the primary endpoint of MACCEs, P2Y12 inhibitor monotherapy was noninferior to standard therapy of 12-month DAPT.
Writing in JAMA Cardiology Choi et al report that at three years, the primary endpoint occurred in 87 patients (6.3%) in the P2Y12 inhibitor monotherapy group and 83 (6.1%) in the prolonged DAPT group (hazard ratio [HR] 1.06, 95% confidence interval [CI]
0.79–1.44, p=0.69).
Furthermore, the authors report that P2Y12 inhibitor monotherapy significantly reduced the risk of bleeding with incidents of bleeding reported in 112 patients (3.2%) when compared to 44 (8.2%) in the prolonged DAPT group (HR 0.39, 95% CI 0.28–0.55, p<0.001). Incidence of major bleeding stood at 17 (1.2%) for the P2Y12 inhibitor monotherapy group compared to 31 (2.4%) in the prolonged DAPT group (HR 0.56, 95% CI 0.31–0.99, p=0.04).
The landmark analyses between three months and three years and per-protocol analyses showed consistent results, the investigators add.
“The current extended follow-up of the SMARTCHOICE study reports three-year ischaemic and bleeding outcomes between patients who received three months of DAPT followed by P2Y12 inhibitor monotherapy and those who received prolonged DAPT,” Choi et al write in JAMA Cardiology “In accordance with the one-year results from the SMART-CHOICE trial, we found that the two treatments had comparable effects in preventing ischaemic events across three years,” the authors add.
Furthermore, they write: “The current results of extended follow-up from the SMART-CHOICE trial support evidence of aspirin dropping strategy with indefinite use of P2Y12 inhibitor after minimum use of DAPT in patients who underwent PCI. Future A-CLOSE and SMART-CHOICE III trials will be helpful to confirm our findings.”
The study team note several limitations of their finding, including that all endpoints in the three-year analysis are exploratory as the completion of followup rate is 92.5%, while also noting that the treatments
were open-label, “reflecting the impossibility of masking for an antiplatelet strategy”. However, all outcomes were adjudicated by an independent committee blinded to the treatment group, they add.
Though the three-year risk of ischaemic cardiovascular events did not show a statistically significant difference between the two groups, this result must be interpreted cautiously because of the limited number of events and sample size, the authors write in their conclusion.
Findings of the SMART-CHOICE are accompanied in JAMA Cardiology by an editorial comment from Ajay Kirtane (Columbia University Irving Medical Center New York-Presbyterian Hospital, New York, USA) and Roxana Mehran (Icahn School of Medicine at Mount Sinai, New York, USA), in which they advocate a “patient-directed” approach to antiplatelet therapy.
“These data should lead clinicians to consider a strategy of monotherapy after a short period of DAPT as a viable one to mitigate bleeding risk, although SMART-CHOICE was underpowered to rigorously assess ischaemic differences, so caution is warranted,” Kirtane and Mehran note. “For patients at greatest risk for recurrent ischaemic events, the role of continued DAPT is always an option, but these data (and other consistent trials) give clinicians more options to pursue individualised treatment decisions. To some, the continually moving field of post-PCI antiplatelet therapy has provided too many choices, which can at times be dizzying. To us, every patient is different, and thoughtful evidence-based consideration is increasingly possible for many of our treatment decisions.”
November 2022 | Issue67 14 Antiplatelet Therapy
The current results of extended followup from the SMARTCHOICE trial support evidence of aspirin dropping strategy with indefinite use of P2Y12 inhibitor after minimum use of DAPT in patients who underwent PCI” Major adverse cardiac and cerebrovascular events: 6.3% 6.1% P2Y12 group DAPT group Bleeding rate: 3.2% 8.2% P2Y12 group DAPT group SMARTCHOICE three-year outcomes
MASTER DAPT continues to show merit for shortened dual antiplatelet therapy out to 15 months post-PCI
Fifteen-month results from the MASTER DAPT trial have shown that the preserved ischaemic benefits and reduced bleeding risk seen with one month of dual antiplatelet therapy (DAPT) among high-bleeding risk patients undergoing percutaneous coronary intervention (PCI) continue beyond one year.
MARCO VALGIMIGLI
(Cardiocentro Ticino Foundation, Lugano, Switzerland) delivered the 15-month data during a hot line session at the annual congress of the European Society of Cardiology (ESC; 26–29 August, Barcelona, Spain), having presented the trial’s 12-month findings a year earlier at the virtual ESC 2021. There, Valgimigli reported that that in high-bleeding risk patients, after intervention using the Ultimaster (Terumo) stent, one month of DAPT was shown to be non-inferior when compared with more than two months of DAPT for the net and major adverse clinical events and was superior for reducing major and clinically relevant non-major bleeding.
At ESC 2022, Valgimigli reported that following the initial 12-month trial period, patients in both the abbreviated and standard DAPT treatment groups received routine clinical care at the discretion of their physician.
The MASTER DAPT researchers recorded medications and adverse events during the following three months, and the results of the prespecified 15-month final analysis were shared for the first time by Valgimigli.

Of 2,295 and 2,284 patients randomised
to abbreviated versus standard DAPT, 2,205 and 2,186 patients started routine care at 12 months, respectively. Complete follow-up at 15 months was available for 99.8% and 99.9% of patients in the abbreviated and standard arms, respectively.
For routine care, physicians tended to continue the treatment assigned in the trial, Valgimigli noted in his presentation. Though guidelines recommend that patients not on oral anticoagulation (OAC) should discontinue DAPT, more than 15% of non-OAC patients were still on DAPT at 15 months in the standard DAPT arm of the MASTER DAPT trial, which was three times higher than the corresponding figure in the abbreviated arm, figures shared by Valgimigli revealed—with the presenter describing this as a “carry-over reflex” from the trial.
Furthermore, though guidelines recommend that OAC patients, if free from recurrent ischaemic events or repeat interventions, should discontinue single antiplatelet therapy (SAPT), more than one quarter were still on SAPT in the standard DAPT arm compared with 14% of the abbreviated treatment group, Valgimigli reported. In multivariate modelling, prior allocation to abbreviated treatment was a strong independent predictor of SAPT versus
Rivaroxaban may be an option for reducing radial artery occlusion in transradial coronary procedures
The use of rivaroxaban for seven days after a transradial coronary procedure reduced the rate of radial artery occlusion, findings of the RIVARAD multicentre randomised trial, presented at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA) have shown.
PRESENTING AUTHOR RANIA HAMMAMI (Hedi Chaker Hospital, Sfax, Tunisia) detailed the findings of the study from five Tunisian centres, which she claimed could impact routine practice by providing a new strategy to reduce radial artery occlusion following transradial access. Radial artery occlusion is the most frequent complication of a transradial procedure, Hammami said, precluding the artery’s future use as an access site for future coronary procedures, coronary artery bypass grafting (CABG) or fistula for haemodialysis.
Through the study, eligible patients aged between 18 and 80 years were randomised to receive either 10mg of rivaroxaban for seven days following a transradial percutaneous coronary intervention (PCI) or coronary angiography procedure, or to receive the current standard of care. Exclusion criteria included the use of thrombolytic therapy or glycoprotein IIb/IIa inhibitors, radial artery complication, high bleeding risk, or haemodynamic instability.
All procedures took place between November 2021 and March 2022, and operators had at least five years of experience with transradial procedures to take part in the investigation.
Investigators tracked the rate of radial artery occlusion seen at 30 days, assessed by ultrasound examination in the wrist, as the study’s primary endpoint, while secondary endpoints included the incidence of haemorrhagic events at the same timepoint, defined according to the Bleeding Academic Research Consortium (BARC) criteria, and local complications on the puncture point including aneurysm, haematoma and arteriovenous fistula.
A total of 538 patients were randomised to the two study groups, 269 patients to the rivaroxaban arm and 269 to the control group. Follow-up data were available for 259 patients in the rivaroxaban group and 262 patients in the control group. Demographic and clinical characteristics of the patient population revealed an average age of 59.7 years among the two patient groups, with 32% female patients, 47% current smokers and 48.1% diabetics.
Reporting the study’s results, Hammami told TCT attendees that the overall incidence of radial artery occlusion seen in the study was 10%, occurring in 6.9% of patients in the rivaroxaban group and 13% in the control group (95% confidence interval [CI] 0.27–0.91, odds ratio [OR]=0.5, p=0.011). The incidence

DAPT in non-OAC patients and no antiplatelet versus SAPT in OAC patients.
The researchers reported 15-month results according to original treatment assignment for the three co-primary outcomes of the main trial: net adverse clinical events (NACE; composite of all-cause death, myocardial infarction, stroke, and major or clinically relevant nonmajor bleeding); major adverse cardiac and cerebral events (MACCE; composite of all-cause death, myocardial infarction, and stroke); and major or clinically relevant non-major bleeding (Bleeding Academic Research Consortium type 2, 3 or 5 bleeding).
NACE occurred in 199 (8.7%) and 214 (9.5%) patients in the abbreviated and standard DAPT groups, respectively, for a hazard ratio (HR) of 0.92 (95% confidence interval [CI] 0.76–1.12; p=0.399) and a risk difference of −0.75 percentage points (95% CI −2.42 to 0.93). MACCE occurred in 158 (6.9%) and 167 (7.4%) patients in the abbreviated and standard DAPT arms, respectively (HR 0.94; 95% CI 0.76– 1.17; p=0.579), for a risk difference of −0.51 percentage points (95% CI −2.01 to 1.00). Major or clinically relevant non-major bleeding remained lower in the abbreviated compared with the standard therapy arm (167 [7.4%] versus 239 [10.7%]; HR 0.68; 95% CI 0.56–0.83; p=0.0001), for a risk difference of −3.25 percentage points (95% CI −4.93 to -1.58).
of haemorrhagic complications seen at 30 days was slightly higher in the rivaroxaban group (2.7% vs. 1.9%, 95% CI 0.44–4.5, OR=1.4, p=0.54), though this was not statistically significant.
Limitations of the study, according to Hammami, included that best practice for the radial approach— such as compression of the ipsilateral ulnar artery or the use of patent haemostasis—were not always observed, however she acknowledged that this reflects the hectic nature of real-world clinical practice.
The findings prompted Hammami to conclude that use of rivaroxaban could be a good option to prevent radial artery occlusion, particularly where ambulatory procedures are increasing, though she cautioned that interventional cardiologists must take more care to implement preventive measures by using best practices for transradial access.
Issue67 | November 2022 15 Antiplatelet Therapy
Marco Valgimigli
Rania Hammami
Timothy D Henry
A pioneer of one of the first regional ST-segment elevation myocardial infarction (STEMI) systems in the USA, Timothy D Henry boasts an illustrious career, which most recently has seen him lead the interventional cardiology field on COVID-19 research, and serve as president of the Society for Cardiovascular Angiography and Interventions (SCAI). He discusses how the field has developed during his 30 years in practice.
Why did you become a doctor?
I grew up in rural North Dakota. When I was in high school, my grandfather Howard Henry—who had run for state governor—died from a myocardial infarction (MI). That stimulated my interest in medicine, as nobody in my family had ever been a doctor.
When I was graduating from medical school, interventional cardiology did not really exist. I knew that I wanted to do internal medicine, rather than surgery, and it was clear at that time that cardiology was changing dramatically. You could do interventions that made people’s lives better, and almost everything we did was for the first time—you really had the opportunity to blaze a trail, which was great.
Who were your biggest influences?
This is a hard question because there are a lot of people to mention. Robert Wilson is an interventional cardiologist who was among the first to do coronary flow reserve. When I did my fellowship at the University of Minnesota, Bob was very involved in clinical research, and he developed the Doppler flow catheter, for example.
In my first job at Hennepin County Medical Center, I worked with Bob van Tassel who was the founder of the Minneapolis Heart Institute and was involved in the first angioplasty in the state of Minnesota. Later, I had the opportunity to work with Bob as the director of research at the Institute. One of the important things that Bob taught me was that when it comes to making plans, you cannot think big enough.
The TIMI Study Group have also been really important to me. It was through working with them that I learned how to do clinical trials, alongside the likes of Eugene Braunwald and Mike Gibson.
My father too was a big influence. He was an outstanding athlete and innovator, having trained as an architect, but then later working as a farmer. He taught me to be positive and innovative.
What has been the most important development during your career?
The most important development to me personally, and something I have been directly involved in, has been primary percutaneous coronary intervention (PCI). The development of regional STEMI systems—or even regional systems for care of all acute cardiovascular emergencies—have also been important.
Despite not being a structural heart specialist myself, you cannot talk about anything in interventional cardiology without mentioning transcatheter aortic valve implantation (TAVI). Looking back to the PARTNER Trial, which included people who had been turned down for surgery, TAVI has cut mortality among those patients in half.
Obviously, angioplasty itself has made a clearcut difference in people’s lives, but there have been so many innovations. When I look back at my career it is remarkable to think about how far things have come.

What has been the biggest disappointment?
One of the main things I do is to take care of patients with refractory angina, those who are not amenable to
further revascularisation—including both advanced coronary artery disease and microvascular angina. I would say something that has not got across the goal line has been stem cell therapy. There has perhaps been too much hype, and this happens when we get away from treating it like medicine. I think some people saw it as a magic bullet that cures everything, which it was never going to be. When you look critically at some of the data, there have been some positive signals from double blind, placebo-controlled trials.
What are your research priorities?
Refractory angina and microvascular dysfunction are important areas of interest of mine right now. Those patients are still out there, and I probably do more microvasculature function tests than anyone else in the world! Microvasculature is the big unmet need. This includes young people who have angina, including more women than men. These could be postPCI or post-chronic total occlusion (CTO) patients who still have chest pain. Heart attack patients who do the worst are those who have microvascular obstruction, and 75% of heart failure with preserved ejection fraction (HFpEF) patients have abnormal coronary flow reserve—the point being that microvasculature is involved with everything. I think that in the next 10 years there is going to be a big focus on how we improve the microvasculature.
In Minneapolis, you developed the Level I heart attack network. How has this changed STEMI care?
When you start anywhere new, you have to recognise the strengths and weaknesses, and look at the unmet needs to fix them. The best example I have is from when I went to the Minneapolis Heart Institute—a big hospital that does outreach among some 30 hospitals. It was perfectly designed to do the first STEMI system, which we called the Level I MI system. I could not have done that anywhere else.
We knew it was the right thing to do. People look at mortality and major adverse cardiovascular events (MACE), but the impact of primary PCI is even far beyond that. It is not just the procedure but having a standardised protocol before and after so that you get comprehensive STEMI care in a guideline-based way. The outcome was shocking to me, we cut MI mortality in the state of Minnesota by 50% within two years.
You were at the forefront of research on COVID-19 through the North American COVID-19 STEMI (NACMI) registry. What is the impact of the pandemic on STEMI care?
Three years ago, I would never have foreseen myself specialising in COVID-19, but now I have over 30 papers on the subject published. In March 2020 we recognised many reports on social media that COVID-19 patients were coming to hospitals and were having MIs, but they appeared to have normal coronary arteries. The second thing that we noticed was that STEMI volumes were down, and we were wondering where they had all gone.
We have a STEMI registry called the Midwest
STEMI consortium, made up of four large centres. Right now, that consists of more than 20,000 STEMI activations and it is a goldmine of information. I called all those sites and asked if they had seen their volumes drop, and they all had. I then called five other hospitals around the country, and we got their data from 2018 and 2019. We submitted a paper to the Journal of the American College of Cardiology (JACC) on 3 April and it was published on 5 April, and it showed a 40% reduction in STEMI activations. What we had seen on social media was true.
16 Interview November 2022 | Issue67
alisonlang.com
PROFILE
I probably do more microvascular function tests than anyone else in the world!”
We put the NACMI registry together within two weeks, through a partnership between SCAI, the American College of Cardiology, and the Canadian Association of Interventional Cardiology. We had 70 sites, and we now have more than 800 COVID-19positive patients with STEMI in our database. We had the idea in March of 2020 and we were up and running by April 2020, with late-breaking data shared at TCT and published in October 2020. I think that has really impacted our treatment of COVID-19-positive patients. It has shown that it is safe to go to the cath lab, and that you still need to do primary PCI. COVID-19 is very bad, but our public health response was to scare people, and that meant that they did not come to the hospital, which was also bad.
What were you proudest to achieve as president of SCAI?

Being president of SCAI was one of the most satisfying years of my life. It has been really fantastic. During that time we started JSCAI The Journal of the Soceity for Cardiovascular Angiography and Interventions and started a Scientific Oversight Committee, pushing SCAI to be more scholarly and to increase research output from registries, surveys, and clinical trials. NACMI is a great example of how important SCAI is.
What is your most memorable case?
On my very last weekend on call in Minneapolis, I was involved in 21 cases, all involving STEMI, or out-of-hospital cardiac arrest (OHCA). Case number 21 was at 2am on the Monday morning. It was a STEMI from a town around 100 miles away. The patient had been working in a factory and had a tearing in her chest. She came by helicopter—a part of our Level I programme—arrested twice on the way, was in cardiogenic shock and had a spontaneous coronary dissection on her left main. Despite being two hours away from a PCI centre, within 10 minutes of her reaching us we had a wire into the true lumen, and had stented the left main. It was a nasty left main dissection, God’s final exam! It is now eight years later and she has written a book and her ejection fraction (EF) is 60%. I think in most places in the world she may have died, especially being so far away from a PCI centre and if we had not had the Level I system.
Outside of medicine, what are your hobbies and interests?
I was involved in athletics in high school and college, and I still enjoy all sports—both watching and participating—I run, I play golf, I cycle, I hike. I have climbed Mount Kilimanjaro with my son. I also like music and the theatre, and I love to travel. My family is really important—I have three great kids and my family are a key part of my life.
17 Interview Issue67 | November 2022
SCAI President JSCAI Editorial Board Research interests Acute myocardial infarction Refractory angina Education University of California at San Francisco University of Colorado, internal medicine residency University of
Medical Center, interventional cardiology fellowship programme Awards and accolades Best Doctors in America 2007‒2017 American Heart Association Heart and Stroke Hero Award in Research 2013 LUMEN Global Lifetime Achievement Award in MI 2012 Master Fellow, SCAI Fact file
Current and past appointments
Lindner Family Distinguished Chair in Clinical Research, The Christ Hospital
Medical Director, The Carl and Edith Lindner Center for Research, The Christ Hospital
Chief of Cardiology, Cedars Sinai Medical Center
Minnesota
AORTIC VIENNA











WATCH ON-DEMAND
Catch up on 3 days’ worth of content from CX Aortic Vienna 2022.

Watch individual talks, discussions, edited cases and abstracts from the CX Aortic Vienna 2022 programme via our on-demand library.

Visit our partners channels for selected content from our sponsors.

VISIT CXAORTIC.COM
cxaortic.com
Structural Heart Interventions
TEER


failure hospitalisation
Analysis of data from the Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) TVT Registry found that most patients with severe mitral regurgitation (MR) and cardiogenic shock (CS) undergoing mitral transcatheter edge-to-edge repair (TEER) achieved successful MR reduction, and that successful repair was associated with lower mortality and heart failure (HF) hospitalisations at one year.
Pioneering Mitraclip procedure performed using 4D imaging

Physicians at UC Davis Health (Davis, USA) are among the first to utilise a 4D holographic guidance system to support a Mitraclip (Abbott) procedure.

“The procedure went smoothly thanks to this new technology,” said Gagan Singh, who performed the procedure aided by the EchoPixel holographic therapy guidance (HTG) system. “As a group, we feel HTG has the potential to increase procedural efficiency and simplify many complex structural heart procedures in addition to Mitraclip.”

Amulet occluder keeps pace with Watchman at three years
LONG-TERM FOLLOW-UP of patients with non-valvular atrial fibrillation (AF) receiving the Amplatzer Amulet left atrial appendage occlusion (LAAO) device have shown continued safety and efficacy through three years, results from the Amulet investigational device exemption (IDE) trial shared at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA) indicate.
The results, presented by Dhanunjaya Lakkireddy (Kansas City Heart Rhythm Institute at HCA Midwest Health, Kansas City, USA) suggest that the device offers “immediate and durable closure to reduce the risk of stroke,” Lakkireddy commented.
The randomised controlled trial pitted Amulet head-to-head with the Watchman (Boston Scientific) device, with primary
results presented at the European Society of Cardiology’s 2021 congress (ESC 2021, 27–30 August, virtual), subsequently published in Circulation, having shown that Amulet was superior with respect to LAAO at 45 days and noninferior with respect to safety and effectiveness through 18 months compared to the Watchman device.
Abbott’s Amulet features Dual-Seal technology—a lobe to fill the body of the LAA and a disc to close off the opening into the LAA—which, according to the device manufacturer, offers immediate closure of the LAA, reducing the risk of stroke and immediately eliminating the need for anticoagulation post-implant.
The trial enrolled more than 1,800 patients at sites internationally, 934 randomised to receive the Amulet, and 944 Watchman. Three-year follow-up data were available for
92% of patients in the Amulet arm, and 86.7% for Watchman.
Detailing the key findings from the trial, Lakkireddy reported that more patients in the Watchman arm (7%) were placed on oral anticoagulation, than those receiving Amulet (4%). Furthermore, cardiovascular death (6.6% vs. 8.5%) and all-cause death (14.6% vs. 17.9%) trended lower with Amulet than Watchman, while stroke and major bleeding were comparable between the two groups.
Device factors, including devicerelated thrombus or peri-device leak preceded strokes in more Watchman patients (17) than Amulet patients (4) after six months, Lakkireddy noted. Offering up a final takeaway message from the trial, Lakkireddy commented that the results provide “confidence in the Amulet occluder to provide immediate and prolonged closure so patients can safely remove oral anticoagulant medications from their everyday life”.
FINDINGS WERE PRESENTED DURING a late-breaking clinical science session at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA) by Mohamad Alkhouli (Mayo Clinic, Rochester, USA) and published simultaneously in the Journal of the American College of Cardiology (JACC)
Alkhouli’s JACC paper acknowledges that there is a growing recognition of the high prevalence of concomitant valvular heart disease in patients with CS. Several studies have shown that moderate-severe MR may be present in as many as one in five patients admitted with CS, the paper notes, and is associated with mortality rates of up to 60%.
Mitral TEER is an established therapy for patients with significant MR, the JACC paper notes, however, it adds that data addressing the role of TEER in CS patients with significant MR has been limited to smaller cohorts, albeit with promising outcomes.
Using data from the STS/ACC TVT Registry from 22 November 2013 to 31 December 2021, researchers assessed outcomes among a total of 3,797 patients undergoing TEER in the USA who met at least one of the prespecified inclusion criteria for CS due to the presence of cardiogenic shock, inotrope use, or mechanical circulatory support prior to TEER. Among this group, 3,249 (85.6%) patients achieved device success defined as MR reduction of ≥1 grade and a final MR ≤ moderate (2+).
The primary endpoint was the impact of device success on clinical outcomes—specifically, mortality and HF admissions at one year. At one-year post procedure, device success was associated with significantly lower all-cause mortality (34.6% vs. 55.5%, adjusted HR 0.49, 95% CI 0.41–0.59, p<0.001), and a composite of mortality and HF admissions (29.6% vs. 45.2%, adjusted HR 0.51, 95% CI 0.42–0.62, p<0.001).
“This analysis examined the characteristics and outcomes of TEER in patients with cardiogenic shock,” said Alkhouli. “Our findings not only show that TEER in patients with CS can achieve successful MR reduction the majority of the time, but also that device success in this high-risk population is associated with a better one-year survival rate as well as fewer heart failure hospitalisations.”
19 Structural Heart Interventions Issue67 | November 2022
in cardiogenic shock patients shows promise for lowering mortality and heart
TAVI costs comparable to surgery in low-risk patients, Canadian researchers conclude
A budget impact analysis comparing transcatheter aortic valve implantation (TAVI) with surgical aortic valve replacement (SAVR) has concluded that the incremental annual cost of implementing TAVI in low-risk aortic stenosis patients is small, “making TAVI likely an affordable strategy”.
THE RESEARCH, PUBLISHED IN THE Canadian Journal of Cardiology, used a one-year time horizon to quantify the total cost of healthcare resource utilisation to initially treat low-risk AS patients and manage subsequent adverse events.
Derrick Y Tam (University of Toronto, Toronto, Canada) and colleagues estimated the total cost of care for various uptake rates of TAVI for a hypothetical centre with 100 low-risk AS patients. This showed that increasing the use of TAVI from baseline (10% of patients) to 50% and 70% had a small impact on the hospital budget (increases of 3% and 4.5% respectively).
In the first year of managing these patients, the upfront cost of the procedure was partially offset by the reduced cost of adverse events. The main contributors to the difference were intensive care unit (ICU) length of stay, new pacemaker implantation rate, and total hospital length of stay. The average total cost of managing a low-risk patient for one year with TAVI (CAN$45,897) represents a nominal increase compared to SAVR (CAN$42,659).
“Although the cost-effectiveness of most transcatheter valve interventions is well studied, given the incremental costs associated with TAVR, the affordability from the hospital payer’s perspective was unknown,” said Tam. “As low-risk aortic stenosis patients likely represent the majority of

severe aortic stenosis patients requiring intervention, understanding the cost impact of treating more patients with TAVI becomes critically important for health policy and resource planning.”
The researchers concluded that while survival rates do not vary, studies have shown that TAVI is associated with more rapid improvement in quality of life compared to SAVR, and that these findings persist to one year.
“As such, it is very reasonable that TAVI may be preferential to patients after a discussion of the risks and benefits. Similarly, the nominal incremental in hospital cost, can be offset by improved efficiencies in the system. Reduced overall length of hospital stay and ICU stay can relieve pressure on hospital bed capacity for optimising resource use,” noted Tam.
In an accompanying editorial Fiona Clement and Derek Chew (University of Calgary, Calgary, Canada), welcome the study as an important contribution to support evidence-informed policy decisions about the adoption and balance between TAVI and SAVR. They point out that the true value of the work is making the model underlying the analysis openly available to decision-makers and researchers globally.
“This model represents a significant infrastructure. By making the model open access, the required investment of time and human resources need not be duplicated as decision-makers face similar questions about shifting from SAVR to TAVI. Further, the model can be responsive as science continues to develop,” they write. However, Clement and Chew warn that costs alone should not drive the discussion of technology adoption and implementation. Recognition of other important factors should be taken into consideration, such as if an intervention is immediately lifesaving; impact on quality of life; number of people eligible; vulnerable patient populations; underlying baseline health; likelihood of the treatment being successful; and its impact on equality of access to therapy.
“Looking through this lens with respect to TAVI funding underscores the complexity of the decisionmaking process in the context of finite resources and the associated opportunity costs. These decisions will only become more challenging as innovation continues, often coming at increased cost,” Clement and Chew concluded.
Faster progression of aortic stenosis linked to poorer outcomes
A fast progression from moderate to severe aortic stenosis is associated with worse outcomes, research published in the American Journal of Cardiology indicates.
MOHAMMED RIZWAN AMANULLAH (National Heart Centre Singapore, Singapore) and colleagues investigated risk factors of progression of aortic stenosis and their impact on clinical outcomes. The researchers studied a total of 954 patients with moderate aortic stenosis—defined as a valve area of between >1cm² and ≤1.5cm². The degree of aortic stenosis progressed to severe in 589 patients (61.7%) over a median follow-up of 2.46 (interquartile range [IQR] 1.29–3.91) years.
Of those who progressed to severe aortic stenosis, patients were then divided into several subgroups, based upon whether their disease progress was deemed to be ‘slow’ (n=294, over 3.91 years [IQR 3.11–5.10]) or ‘fast’ (n=295, over 1.29 years [IQR 0.85–1.85]), according to the median time between the two echocardiograms. The correlates of fast aortic stenosis progression and its impact on cumulative survival and freedom from valve intervention were evaluated.
On multivariate analysis, age, thickened left ventricle posterior wall, severe renal impairment, and aortic valve area were significantly associated with fast aortic stenosis progression, Amanullah et al found.
Over a median follow-up of 6.34 years, 228 patients (38.7%) died. Despite similar aortic valve intervention rates, fast progressors had worse five-year survival (61.2% vs. 81.9%, p<0.001) and event-free survival rates (16.2% vs. 55.9%, p <0.001). Amanullah and colleagues report that, upon multivariable Cox analysis, shorter progression to severe aortic stenosis was independently associated with increased risk of all-cause mortality (hazard ratio [HR] 1.26, 95% confidence interval [CI] 1.16–1.37, p<0.001), or combined aortic valve intervention and death (HR 1.46, 95% CI 1.38–1.55, p<0.001).
Risk score predicts mortality for AF patients after TAVI
Researchers have developed a risk score to predict mortality for patients with atrial fibrillation (AF) who have undergone a successful transcatheter aortic valve implantation (TAVI) and have been discharged home.
The risk score is a product of the
ENVISAGE-TAVI AF trial, which compared the safety and efficacy of the direct oral anticoagulant (DOAC) edoxaban with vitamin K antagonists (VKAs) in AF patients with an indication for oral anticoagulation after TAVI. George Dangas (The Zena and Michael A Wiener Cardiovascular Institute at the Icahn School of Medicine at Mount Sinai, New York, USA) presented findings of the investigation as a late-breaking clinical trial at the European Society of Cardiology (ESC) 2022 congress
(26–29 August, Barcelona, Spain).
“Our study focuses solely on high-risk TAVI patients with atrial fibrillation, which is a wellrecognised surrogate of unfavourable prognosis,” said Dangas. “Although past research has been mostly focused on procedure risks, this new risk assessment tool focuses on how to stratify patients after completion of successful TAVI when they are ready for discharge, to
improve outcomes.”
Approximately 33% of patients post-TAVI have prevalent or incident AF, Dangas noted in his presentation, commenting that AF is a wellestablished risk factor for mortality after the procedure. Surgeons often rely on the Society of Thoracic Surgeons (STS) risk score that was developed for open heart surgery, or other similar risk scores for this procedure, he added, pointing out that there is no wellestablished risk score for prognosis after successful completion of TAVI.
ENVISAGE-TAVI, which took place in 173 centres in 14 countries, compared the safety and efficacy of different therapies in AF/ TAVI patients requiring oral anticoagulation. Researchers analysed 1,426 patients starting five to 12 days after TAVI and followed for up to one year to evaluate predictors of mortality.
Of the 178 patients (12.5%) who died within that timeframe, most were over age 64; had kidney disease and/ or heart failure; higher weight; had non-paroxysmal AF; consumed more than three alcoholic drinks per day; and had a history of major bleeding or predisposition to bleeding.
Investigators assigned a risk level to each of those predictors. Once they calculated total risk, they classified patients into three categories: low risk (between 0–10), moderate risk (between 11–15), and high risk (above 16). They validated the risk score and found that the mortality rate was more than double in the moderaterisk patients (10.1%) and triple in the high-risk group (17%) compared to the low-risk group (4.8%).
“We will continue to perform focused analyses on high-risk TAVI patients based on combinations of different/other clinical risks to enhance our understanding of patient risks after TAVI so we can then plan clinical investigations on improving prognosis,” said Dangas.
20 Structural Heart Interventions November 2022 | Issue67
George Dangas
The PREVUE-Valve study: A novel approach to understanding valvular heart disease in the community
Michael I Brener & David J Cohen
Comment & Analysis
Michael I Brener (Columbia Presbyterian Medical Center, New York, USA) and David J Cohen (Cardiovascular Research Foundation, New York, USA) offer details of the PREVUE-Valve study, a groundbreaking research project that seeks to shed new light on valvular heart disease using
Over the past two decades, transcatheter aortic valve intervention (TAVI) has revolutionised the treatment landscape for patients with valvular heart disease. The remarkable success of this disruptive technology has also inspired efforts to treat other forms of valvular heart disease using catheter-based approaches. While the field is advancing rapidly, areas of uncertainty still remain for patients, the clinicians who care for them, and the policy stakeholders that influence the delivery of valvular heart disease-related healthcare.
One of these fundamental questions surrounds the prevalence of valvular heart disease in the USA and the developed world. The relevance of this question stems from several considerations. First, it is important to understand precisely how common
valvular heart disease is in order to meet the growing demand for valvular heart disease-related care that will continue to increase as the population ages. A number of studies have been conducted over the years aimed at answering this question, but they have been drawn almost exclusively from “convenience” samples where prevalence was estimated among a cohort of patients who may have already self-selected or been selected by virtue of the study design to undergo screening tests that identify forms of valvular heart disease. Given these issues, we still do not have clear, unbiased insights into the present-day frequency of valvular heart disease, and whether or not it is similarly distributed across different segments of the population. A second key consideration is understanding how
AI picks up “missed” high-risk aortic stenosis
Late-breaking research presented at the European Society of Cardiology (ESC) annual congress (26–29 September, Barcelona, Spain) has demonstrated the use of an artificial intelligence (AI) diagnostic tool—ECHO IQ—in identifying moderate-to-severe aortic stenosis.
INVESTIGATOR GEOFFREY STRANGE (University of Notre Dame, Fremantle, Australia) the principal investigator in the AI-ENHANCED AS trial told ESC attendees that the AI algorithm could be used in clinical practice to identify patients who may be at high risk of aortic stenosis and require further assessment for treatments such as transcatheter aortic valve implantation (TAVI) or surgical valve replacement.
best to screen for valvular heart disease in large populations. Echocardiography is currently the standard approach for diagnosis of valvular heart disease. However, widespread application of echocardiography as a screening test may be highly impractical owing to the cost and time required for testing.
To address this critical knowledge gap, we designed the PREVUE-VALVE trial. The goals of PREVUE-Valve are: (1) to determine the true community prevalence of moderate to severe valvular heart disease in a representative US population; (2) to examine the relationship between age, sex, and race and the prevalence of various forms of valvular heart disease; and (3) to test several novel, artificial intelligencebased approaches to valvular heart disease screening in a real-world, community-based setting. To do this, PREVUE-Valve aims to recruit ~5,000 diverse individuals between the ages of 65‒85 from across the USA in the span of 24 months. All patients will undergo a detailed echocardiogram to identify the presence or absence of aortic, mitral, and tricuspid valvular heart disease. Patients will also undergo several potential screening tests including an electrocardiogram (ECG), digital phonocardiogram and a blood sample will be obtained for future studies of biomarkers and genetic determinants of valvular heart disease.
One of the unique aspects of the PREVUE-Valve trial is that it is designed as a completely “virtual” clinical trial— bypassing both the medical clinic and
hospital system. Instead of asking older individuals (in whom the prevalence of valvular heart disease is naturally higher than in younger individuals) to come to medical centers for their study procedures, PREVUE-VALVE is designed to engage participants in their homes. Although the study is being led by investigators at the Cardiovascular Research Foundation and Columbia Presbyterian Medical Center, it involves a number of collaborating institutions. These include CVS Health (who are assisting with patient recruitment) and Hawthorne Effect, a company aimed conducting clinical trials in the home. Additional collaborators include the University of Michigan’s Survey Research Center (who have expertise in community-based sampling) and the Vanderbilt University Biobank (for storage and analysis of blood samples).
The study is currently in its pilot phase, during which we have been experimenting with different approaches to recruitment and screening. We anticipate transitioning to Phase 2, during which the majority of participants will be recruited and hope to present the primary results in early 2025. All told, PREVUE-Valve has the potential to provide important new information about how common valvular heart disease is among older Americans and how we can screen for it using alternative diagnostic tests that complement traditional echocardiography. The revolutionary study design may also influence how future epidemiological studies are conducted, and specifically, how clinicians and researchers can reach patients with valvular heart disease.
David J Cohen is director of Clinical and Outcomes Research, Cardiovascular Research Foundation, New York, USA and a practicing interventional cardiologist at St Francis Hospital and Heart Center, Roslyn, USA.


Michael I Brener is a cardiology fellow at Columbia Presbyterian Medical Center, New York, USA.
The proprietary AI-decision support algorithm (AIDSA) used in the study was trained using data from the National Echo Database of Australia (NEDA), which contains more than 1,000,000 echocardiograms from over 630,000 patients and is linked to mortality information, Strange detailed. The algorithm was also trained to ensure all guideline-defined severe aortic stenosis was detected. Training was performed using 70% of the NEDA data, which were randomly selected.
Using the remaining 30% of NEDA data, the researchers compared five-year death rates in patients with the moderate-to-severe and severe aortic stenosis phenotypes with five-year death rates in patients without significant risk of severe aortic stenosis. Out of 179,054 individuals, the AI-DSA identified 2,606 (1.4%) with a moderate-to-severe phenotype and 4,622 (2.5%) with a severe phenotype. Of those with a severe phenotype, 3,566 (77.2%) met guideline criteria for severe aortic stenosis.
The five-year mortality rate was 56.2% in patients with the moderate-to-severe phenotype and 67.9% in those with the severe phenotype. Those without either phenotype had a 22.9% five-year mortality rate. Compared with the reference group, the age- and sex-
adjusted odds ratio (OR) for all-cause mortality was 1.82 (95% confidence interval [CI] 1.63–2.02) and 2.80 (95% CI 2.57–3.06) for patients with the moderate-tosevere and severe phenotypes, respectively.
Within the severe aortic stenosis phenotype identified by the AI-DSA (4,622; 2.5%), those that met current guidelines (77%) had a five-year mortality of 69.1%. The additional population identified by the AI-DSA with a severe phenotype, but who do not meet current guidelines, had a mortality rate of 64.4%.
Commenting on the findings, Strange said: “This proprietary AI algorithm picks up patients with a high risk (and all patients within current guidelines) of dying within five years that may be missed by conventional definitions.”
Strange concluded: “Given the rising prevalence of aortic stenosis and its impact on mortality, it is time to revisit the practice of watchful waiting and consider more proactive attempts to identify those at risk.
More research is needed to determine if aortic valve replacement improves survival and quality of life in patients identified by the AI-DSA as having a high risk of mortality, but who do not meet current guideline definitions.”
21 Structural Heart Interventions Issue67 | November 2022
an innovative study model.
The study design may influence how future epidemiological studies are conducted”
TAVI valve durability and reliability: How patient lifetime management is evolving
Durability, reliability, accuracy. These are just some of the characteristics that interventionalists look for in a transcatheter aortic valve implantation (TAVI) device to achieve the predictable results that their patients need in the long run. With TAVI procedures now being performed across a younger, lowerrisk patient population, reliable haemodynamic performance, predictable rates of permanent pacemaker implant and proven durability come ever more to the fore.
According to Nicolas Dumonteil (Clinique Pasteur, Toulouse, France), the importance of valve durability is one of the components that is now front and centre in the interventionalist’s mind when planning a TAVI procedure. “It has become more and more important since we have had the extension of indications to patients having a longer life expectancy,” Dumonteil tells Cardiovascular News “Fifteen years ago, we used to treat patients who had maybe three, four, or five years of life expectancy and therefore valve durability was less of a concern. As we are now treating patients who can have ten or more years of life expectancy, and, for the majority, that are still good surgical candidates, it has become a crucial concern at the time of the first valve selection process.”
Perhaps the most important recent development regarding valve durability is the pooled analysis of five-year data from the CoreValve US Pivotal and SURTAVI trials, delivered by Michael J Reardon (Houston Methodist DeBakey Heart and Vascular Center, Houston, USA) at the American College of Cardiology (ACC) Scientific Sessions (2–4 April, Washington, DC, USA). The study evaluated the five-year incidence and predictors of haemodynamic valve deterioration (using standardised definitions) and clinical outcomes among patients who had been randomised to undergo TAVI—using either the CoreValve or Evolut (Medtronic) platforms—or surgical aortic valve replacement (SAVR).
Researchers looked at 1,484 intermediate-risk (792 TAVI, 692 SAVR) and 615 high-risk (336 TAVI, 279 SAVR) patients for structural valve deterioration (SVD), finding that in patients with symptomatic severe aortic stenosis at intermediate or high surgical risk, the five-year rate of SVD was 4.38% in patients undergoing surgery, versus 2.57% in patients undergoing TAVI using the Evolut and CoreValve
platforms (p=0.0095).
“Those data are crucial for our community because we started with TAVI thinking that it was a treatment for patients who were inoperable,” says Dumonteil. “Now, for the first time, we have robust, large-scale data to inform our patients that so far, at five years’ follow-up, with such a platform we do not have to express any concerns regarding valve durability as compared to surgical implants. That is crucial, especially at the time of preoperative discussion with the patients and explanation of the pros and cons of TAVI and SAVR.”
An important concept to consider is the design of the CoreValve and Evolut platforms—both are self-expanding, supra-annular valves—which potentially explains some of the mechanism behind its performance at five years. Dumonteil says that the findings from the pooled analysis gives weight to the hypothesis that the supraannular positioning of the leaflets in the CoreValve and Evolut platforms, and the design of thoses leaflets contributes to its promising five-year results.
The unique design of the Evolut platform may also confer benefits among patients with small aortic annuli, with the data suggesting that the difference in SVD was observed to be more profound in patients with smaller (≤23mm) annuli (SVD: 5.84% surgery vs. 1.32% TAVI; p=0.02). “The valve has been developed to have a decoupling of the constrained portion of the frame in the small orifice of the aortic annulus. This hypothesis has been mentioned since the early ages of CoreValve implantations, but now we have some data that suggest it could be true,” Dumonteil explains, discussing the theoretical mechanism behind this finding.

This year marks 20 years since the first TAVI procedure was performed by Alain Cribier—in Dumonteil’s native France—and structural heart specialists now benefit from two
decades’ worth of experience in planning and executing the procedure. This helps to determine the right strategy, and ensure that the patient is receiving the right valve to match their anatomy. As valve technologies have developed, so have the implant techniques, and an experienced operator will now have knowledge of many technical variations that they can deploy to optimise outcomes in complex cases.
One such new approach is the cusp overlap technique (COT). Cusp overlap is intended to lower the risk of interference with the conduction system, by providing a more accurate assessment of valve depth upon implant, thereby lessening the risk that the TAVI patient may need a pacemaker implantation—which has been a common feature of the procedure. “We need to thank those who proposed to shift to this practice,” says Dumonteil. “They [have] helped us understand that in this projection we have a better understanding of the real and natural depth of implantation in the left ventricular outflow tract (LVOT), and therefore we must be more accurate in terms of implant depth or positioning of
pacemaker implantation rate (9.2%), and upon discharge, no cases of moderate or severe paravalvular leak (PVL; 0%). The lowest pacemaker implantation rates were observed when all steps of the cusp overlap technique were followed, investigators found.

“It was necessary to have this kind of large-scale registry data to validate these theoretical advantages,” says Dumonteil, of the study. “The most interesting outcome coming from that is to validate, in real-world patients, through a multicentre observational study, that if we use this simple, but rigorous, technique to implant the supra-annular Evolut platform, then we can dramatically increase our procedural outcomes, regarding those two potential complications, that are pacemakers and PVL.”
Taking all of these data together, Dumonteil believes that perception of the TAVI procedure is changing within the interventional cardiology community.
“What has changed, and what is still changing, is that now, at the time of a first transcatheter valve implant, related to the life expectancy of a patient with 10 years of life expectancy after aortic valve treatment, then we must take into
the valve.”
The merits of the technique, when used in conjunction with the Evolut valve, has been among the key learnings from the Optimize PRO study, which was conducted among 400 patients treated in the USA and Canada. Recent findings from the study have shown that cusp overlap led to more “predictability and control,” according to investigators, resulting in a single-digit
account some factors related to this,” he says.
“To end with Evolut—the good thing with the recent data we have at five years, and of course we need the 10year data—we initially implanted those valves thinking about maybe needing after six, seven or eight years to implant a second one. But, the hope we have now, is that these five-year data will be confirmed at 10 years and finally we will have the proof that the first implant we are doing is the only one the patient will have until the end of their life, and this would be the ideal scenario.”
22 Advertorial November 2022 | Issue67
THIS ADVERTORIAL IS SPONSORED BY MEDTRONIC
The findings from the pooled analysis give weight to the hypothesis that the supra-annular positioning of the leaflets in the CoreValve and Evolut platforms, and the design of thoses leaflets contributes to its promising five-year results”
Medtronic's Evolut Pro+ TAVI device
Nicolas Dumonteil






*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiac rhythm field Editorially independent Visit cardiacrhythmnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
Study highlights need for “gender specific” strategy for percutaneous revascularisation in STEMI patients
An analysis of ten-year follow-up data from the EXAMINATION-EXTEND trial, assessing gender-specific outcomes following ST-segment elevation myocardial infarction (STEMI), has shown no differences in patient-oriented endpoints including myocardial infarction (MI) or revascularisation between women and men, but a trend towards higher all-cause death in women not driven by cardiac death.
THE FINDINGS, WHICH WERE PRESENTED at the European Society of Cardiology (ESC 2022; 26–29 August, Barcelona, Spain) by Rami Gabani (Hospital Clinic of Barcelona, Barcelona, Spain) and published in JACC: Cardiovascular Interventions, point to the need for focused, personalised medicine in women after percutaneous revascularisation aimed at both cardiovascular and gender-specific risk factor control and targeted treatment, according to investigators.
Short-term outcomes of women following STEMI are worse than men, with specifically a higher mortality rate, Gabani and colleagues noted, acknowledging that it is unknown if gender may play a role in long-term outcomes. The study’s aim was to assess if very long-term outcomes following STEMI may be influenced by gender.
Investigators used data from the EXAMINATION trial, an allcomers, multicentre, prospective 1:1 randomised, two-arm, singleblind, controlled trial, conducted at 12 centres in three countries, assessing the superiority of the Xience (Abbott) everolimuseluting stent compared to the Multilink Vision (Abbott) bare metal stent in STEMI patients for the primary endpoint of all-cause death, myocardial infarction, and revascularisation at one year.
Outcomes of the study have been reported out to five years, demonstrating the superior efficacy and safety of the second-generation, Xience, drug-eluting stent as compared with the bare metal stent, with a significant 28% reduction of all-cause mortality. The trial was then reinitiated as EXAMINATIONEXTEND to evaluate patient-oriented composite endpoint (POCE) and device-oriented composite endpoints at 10 years.
Of the 1,498 patients randomised, 254 patients (17%) were women, and 1,244 (83%) were men. Overall, women enrolled in the trial were found to be older by an average of eight years, fewer were smokers and had a higher prevalence of hypertension than men, Gabani et al report.
At 10-year follow-up, the POCE had occurred in 103 women (40.6%) and in 426 men (34.2%) (adjusted hazard ratio [HR]: 1.14; 95% confidence interval [CI]: 0.91‒1.42; p=0.259), Gabani and colleagues note. A trend toward higher all-cause death was observed in women, occurring in 70 women (27.6%) and 249 men (19.4%) (adjusted HR: 1.30; 95% CI: 0.99‒1.71; p=0.063). The rate of cardiac death did not differ between groups (adjusted HR: 1.19; 95% CI: 0.821.72; p=0.356). No differences were found in terms of
other secondary endpoints between the two groups.
Discussing the findings, the authors write in JACC: Cardiovascular Interventions that their study demonstrates that women with STEMI did not have worse long-term outcomes in terms of POCE compared with men, but note that they did exhibit a numerically higher incidence of all-cause death, which was not driven by cardiac death. Gabani and
a trend toward higher all-cause death at the 10-year follow-up in women compared with men (27.6% vs. 19.4%; p=0.063), which was not driven by cardiac deaths (15.7% vs. 10.1%; p=0.356).
Life expectancy of patients after STEMI is closely related to their survival in the first 30 days, with consistent data suggesting gender as an independent predictor of in-hospital and up to 30-day mortality, implying that heart failure and cardiogenic shock may lead to worse outcomes in women, the authors add.
“At long-term follow-up, the available evidence is contradictory, with some studies showing higher longterm all-cause mortality in women versus men and others showing no differences” the study team writes. “Thus, the debate on whether gender plays a role in long-term mortality is still ongoing,” they note.
Gabani et al state that the findings are in line with previous studies, showing an unadjusted higher rate of all-cause death in women versus men after PCI, likely related to age and comorbidities, specifically due to the older age at the time of STEMI presentation, at which point more non-cardiac comorbidities would have been accumulated, increasing the risk for non-cardiac death in the subsequent years.
Although the authors state that further investigation is needed to confirm this finding, they suggest that the focus should “go beyond cardiac death, by managing comorbidities that may be gender associated, in order to prevent non-cardiac death.
“The higher POCE rate in women versus men, related mainly to age and hypertension as the only independent predictors of events, also underlines the need for focused personalised medicine in women after percutaneous revascularisation aimed at both cardiovascular and gender-specific risk factor control and targeted treatment,” the authors continue. “This genderbased attention should be promoted and become the standard of care.”
Investigators note that their findings are subject to some limitations, including that they were unable to account for some potential variables that may be associated with outcomes or patient management, such as long-term patient adherence to drug treatments.
Further to this, the study included a smaller proportion of women than and men, and thus the authors note that their power to assess for differences in outcomes was limited. Association between gender and outcomes may be driven by confounders, including over-time lifestyle and behavioural factors which have not been recorded on the day.
In an editorial piece accompanying the article in JACC: Cardiovascular Interventions, Stefano Savonitto (Manzoni Hospital, Lecco, Italy) and Nuccia Morici (IRCCS Fondazione Don Carlo Gnocchi, Milan, Italy) note that awareness of genderrelated differences in the risk factors, development, treatment, and long-term care of acute coronary syndromes should be increasingly integrated in the cardiology curriculum, “as it involves, particularly at a younger age, female-specific factors and strategies for prevention”. They write: “Social, cultural, and economic factors are being increasingly recognised to increase the risk of ischemic heart disease progression in women across their lifecycle.”
colleagues note that women had a lower risk for target vessel revascularisation after STEMI than men in the first five years of follow-up, but not thereafter, while also reporting that age was as an independent predictor of POCE in both men and women.
Looking specifically at the individual components of POCE, Gabani and colleagues note that there was
Furthermore, Savonitto and Morici add: “inequalities and opportunities do exist to improve quality of care, especially regarding an increased awareness of cardiovascular risk among women: as underlined in recent consensus documents, most of these opportunities lie in improvements in dietary habits, weight, and physical activity, rather than in drugs and the catheterisation laboratory.”
November 2022 | Issue67 24 Gender Outcomes
Inequalities and opportunities do exist to improve quality of care, especially regarding an increased awareness of cardiovascular risk among women” INDIVIDUAL CLINICAL ENDPOINTS AT 10 YEARS All-cause death WOMEN 28% MEN 19% Myocardial infarction WOMEN 6% MEN 6% Any revascularisation WOMEN 14% MEN 19%
Driving better cardiac care outcomes for women through technology and increased representation
Shrilla Banerjee
Comment & Analysis
In its many guises, coronary artery disease (CAD) can impact different genders and ethnicities to widely varying degrees, impacting no one gender or group with the same symptoms, frequency or severity. As cardiologists, we must ensure that we understand these nuances and appreciate what they might mean for different patients.
One of the starkest examples of this is coronary microvascular dysfunction (CMD). CMD is a form of CAD that is undetectable with a traditional angiogram and is accompanied by unexplained chest pains that cannot be attributed to other cardiac dysfunctions. CMD is a significant diagnosis, accounting for up to 50% of patients with ischaemia and non-obstructive coronary artery disease (INOCA)1
CMD can occur secondary to changes within the microcirculation or vasomotor dysfunction. This may result in an inability to increase flow when demand increases, an impaired coronary flow reserve (CFR), and is often associated with an adverse prognosis2
Its presence can leave patients feeling frustrated, worried, and anxious about their lack of diagnosis. CMD disproportionately impacts women because of years of under-representation in clinical data studies and a lack of understanding of inherent differences in how they report or cope with chest pain—those differences themselves being rooted in under-recognised gender-specific pathophysiologies.
When women’s presentations are unrecognised or misdiagnosed—which has long been the case with CMD, previously dubbed as the ambiguous “Syndrome X”—their frustration with the healthcare system grows. Inherent biases, over-confidence in diagnosis and an unintended lack of empathy for
female patients can see cardiologists avoid ordering further tests and discharging patients without adequate investigation.
According to Abbott’s Beyond Intervention 2021 research, female patients can face significantly more challenges whilst on the pathway to diagnosis and treatment than male patients. This appears to be at odds with physicians’ and healthcare leaders’ opinions of the patient experience for cardiology patients, where physicians (52%) and healthcare Leaders (64%) said that the patient experience was ideal—compared to just 44% of patients3. Such a disconnect is at best concerning or at worst reflective of healthcare professionals’ inherent biases and a significant gap between patients’ and providers’ viewpoints, which will only serve to leave women enduring less desirable experiences and outcomes.
Addressing structural shortcomings
Forcing movement on the gender imbalance that exists in interventional cardiology is crucial to improving the patient journey going forward. Many organisations, including Women As One and the British Cardiovascular Intervention Society (BCIS), have highlighted that there is a significant gender imbalance amongst cardiologists, with women only making up 6% of the workforce4. Support for training and recruiting more female interventional cardiologists may help to build a workforce more conscious of women’s perspectives; more aware of conditions that disproportionately impact women and more sympathetic of the anxieties and suffering of female patients.
On this front, progress is being made—albeit slowly. The pipeline is growing and in 2021, 27% of interventional cardiology trainees in the UK were women4. It is vital that this
EMPOWER CAD trial will answer questions on coronary lithotripsy in an all-female population
Investigators have initiated a prospective coronary intervention study consisting of all female patients with coronary artery disease (CAD)—EMPOWER CAD—seeking to gather evidence on the safety of coronary intravascular lithotripsy (IVL) in a real-world population of female patients.
THE STUDY WILL SEEK TO DETERMINE whether results from earlier coronary IVL studies, which have used the Shockwave C2 (Shockwave Medical) coronary IVL catheter, showing similar safety outcomes across both sexes, can be replicated in an expanded, real-world population of female patients with severely calcified coronary lesions.
The prospective, multicentre registry will enrol up to 400 female patients with symptomatic ischaemic heart disease in up to 50 investigational centres in the USA and Europe and will include a three-year follow-up.
Co-principal investigators Margaret McEntegart (Columbia University Medical Center/New YorkPresbyterian Hospital, New York, USA) and Alexandra Lansky (Yale University School of Medicine, New Haven, USA) along with the study’s European lead, Nieves Gonzalo (Hospital Clinico
San Carlos, Madrid, Spain), announced the study at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA).
“When it comes to CAD, females are often underinvestigated, under-treated and have less favourable outcomes than males due to a variety of different factors,” said McEntegart. “Previous reports with atherectomy have shown that females with calcified CAD are more susceptible to adverse procedural outcomes compared to males. Despite often being more challenging to treat, female patients are underrepresented in published data, and there have been no dedicated prospective studies performed on this population. EMPOWER CAD will be an extremely valuable study to better inform interventional cardiologists on the optimal treatment strategy for these complex patients.”
progress is continued and accelerated recognising equality as a virtue in and of itself, but also for the sake of creating a more empathetic system that recognises unique and diverse patient perspectives.
Tech will also play a pivotal role in helping us to overcome structural gender inequalities in the vascular patient experience. New imaging tech that is more precise, more accurate, artificial intelligence-enabled and that takes into account all-important differences in gender pathophysiologies will help us to improve outcomes for women and begin to address the challenges of gender equality in cardiac care. While some cardiology centres have invested in this technology, a wider implementation rollout is needed across all centres.
Failure to address these structural challenges contributes to less desirable cardiac outcomes for women. When the solutions are this clear, it is simply not an option to sit back and do nothing. As both a moral and medical imperative, we must better support a group of people who make up half of our patients.
Shrilla Banerjee is a consultant interventional cardiologist at Surrey and Sussex Healthcare NHS Trust, Redhill, UK. She has a particular interest in coronary artery disease (prevention and complex intervention) in women, and also in aviation cardiology.

References 1. Marinescu MA, Loffler, AI, Ouellette M, et al. Coronary Microvascular Dysfunction, Microvascular Angina, and Treatment Strategies. JACC: Cardiovascular Imaging Vol 8, Issue 2, February 2015, 210‒220.
2. Perera D, Berry C, Hoole SP, et al. Invasive coronary physiology in patients with angina and non-obstructive coronary artery disease: a consensus document from the coronary microvascular dysfunction workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart Published Online First: 22 March 2022. doi: 10.1136/ heartjnl-2021-320718
3. Abbott, 2021, Beyond Intervention 2021 Report: Improving Patient Experience By Addressing Unmet Needs In Vascular Disease. Available here: https:// abbo.tt/3QgVH0R
4. BCIS Audit Data 2021. Available here: https://www. bcis.org.uk/audit-results/
“Early retrospective analyses have suggested that coronary IVL can potentially bridge the disparity in clinical outcomes between sexes, however the studies only included a limited number of females with strict inclusion criteria,” said Lansky. “Information that will be gathered in EMPOWER CAD will be immensely valuable, as it will provide more robust data with longer-term outcomes in a larger, all-comers patient cohort to determine whether coronary IVL should be considered the front-line calcium modification approach in female patients.”
Issue67 | November 2022 25 Gender Outcomes
When it comes to coronary artery disease, females are often under-investigated, under-treated and have less favourable outcomes than males due to a variety of different factors”











*Available for US and EU readers only **Available worldwide Visit renalinterventions.net and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels October 2021 www.renalinterventions.net In this issue: Profile: Alexandros Twelve-month Wrapsody results at CIRSE 2021 Dialysis: Latest debates in dialysis care Transplantation: Healthcare disparities in the spotlight page 20 kidney care The National Kidney Foundation (NKF) and the American Society of Nephrology (ASN) have jointly released a report outlining new race-free approach to diagnosing kidney disease. In its report, the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease recommends the adoption of the new estimated glomerular ltration rate (eGFR) 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation that estimates kidney T to eliminate disparities in the diagnosis and treatment of kidney disease.” “As the largest organisations representing kidney patients and health professionals, NKF and ASN are committed to eliminating health disparities that harm kidney patients, and ensuring that racial bias does not ect the diagnosis and subsequent treatment of kidney diseases,” said ASN president Susan Quaggin. “By recommending the CKD-EPI creatinine equation re without the race variable, the task force has taken action and demonstrated how nephrology continues to lead the way in promoting healthcare justice. time for other medical specialties to follow our lead, and NKF and ASN stand ready to help however we can.” In the USA, more than 37 million adults have kidney diseases and 90% are not aware they have diminished kidney function, the NKF-ASN statement adds, with disproportionate number of these patients being Black or Bioarti cial device receives KidneyX award after reaching preclinical testing AN IMPLANTABLE BIOARTIFICIAL kidney device (iBAK) has moved closer to becoming reality after being awarded US$650,000 prize from KidneyX. The device’s creator, the Kidney Project, received this award following the rst ever demonstration of its functional prototype. The Kidney Project US-wide collaboration led by Shuvo Roy (University of California San Francisco [UCSF], San Francisco, USA) and William Fissell (Vanderbilt University Medical Center, Nashville, USA). In the past few years, has successfully tested the two essential components that make up its arti cial kidney technology—a haemofilter, ent technologies included wearable, lightweight, dialysate-free arti cial kidney (US Kidney Research Corporation) and genetically engineered pig kidneys designed to increase supplies of transplantable organs (Makana Therapeutics). “The NKF and ASN urge all laboratories and healthcare systems nationwide to adopt this new approach as rapidly as possible so that we can move towards a consistent method of diagnosing kidney diseases that is independent of race.” Paul Palevsky
removal
kidney
A trusted provider
latest news,
A specialised news source
the
renal disease management
independent
Renal community reckons with
of race variable in
disease diagnosis
of
review of cutting-edge research, congress coverage and opinion from thought leaders
in
field of
Editorially
Integrated imaging technologies will support complex cases in the cath lab
Supporting physicians to tackle more complex cases with integrated imaging systems and devices in the cath lab will be an important future area of focus for Philips’ healthcare technology business, Chris Landon, the general manager for image guided therapy at Philips tells Cardiovascular News

LANDON WAS SPEAKING AT THE 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA), where Philips showcased several diagnostic and interventional technologies from across its portfolio. These included Philips’ intracardiac echocardiography [ICE] catheter for 2D and 3D live imaging in structural heart procedures and new a software package for the Azurion ultrasound system integrating transoesophageal echocardiography (TEE) and X-ray fluoroscopy.
“Our mission within the imageguided therapy organisation is to disrupt clinical practice through the application of imaging technologies, data-oriented technologies and interventional technologies, and find better ways to
Haemodynamic support system for heart failure patients wins TCT innovation prize
deliver therapy,” Landon comments to Cardiovascular News
One such example of this philosophy in action, cited by Landon, is the addition of co-registration functionality to its flagship Azurion platform, combining intravascular ultrasound (IVUS) and angiogram usability to aid percutaneous coronary intervention (PCI) procedure planning.
“That allows physicians to get a better view and a more precise idea of where to direct therapy during complex PCI procedures,” he says. “It is a disruption that we think improves
outcomes and potentially drives economics by preventing the need for delivery of stents where maybe they are not going to have an impact on the patient outcome.”
Commenting on the trends that are shaping innovations in image guidance within Philips, Landon said that facilitating more complex interventions will be an important driver for future advances in technology. “What we are seeing more and more in cath labs are physicians willing to take on more complex cases and raising the bar on the outcomes that they achieve,” he says. “To help support our physicians going after those more complex cases, a lot of things have to happen from an innovation perspective.”
This includes facilitating the capture and interpretation of data across multiple imaging platforms, and integrating new technologies that will enhance visualisation capabilities, he comments. “In the future, we hope to be able to deliver better ways to visualise the patient anatomy using technologies like augmented reality or even virtual reality, potentially even doing things with new types of sensor platforms,” says Landon. “For instance, we have some innovation in place right now using fibre optic sensors in order to create images of the heart in
Chris Landon
three dimensions.”
Turning to the immediate priorities for the company, Landon says that the strategy remains bringing alternative interventional imaging and diagnostic capabilities, allied to therapeutic interventions, into the market. He pointed to the 2020 acquisition by Philips of Intact Vascular, developer of the Tack endovascular system, a dissection repair device for patients with peripheral arterial disease [PAD] as an exemplar of this approach.
“There is no other device on the market approved for dissection treatment,” he comments. “It is a niche market but one that is very relevant to our customers who are already interacting with the devices that we put on to the market. That is the kind of growth we are going to move into over time within the idea of image guided therapy.”
Puzzle Medical Devices was announced as the winner of the Shark Tank innovation competition at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA).
THE COMPANY IS DEVELOPING A NOVEL circulatory support device that is implanted percutaneously in the abdominal aorta—known as ModulHeart. The device provides haemodynamic support through three endovascular pumps inserted in series and assembled in parallel into a selfexpandable anchor implanted in the descending aorta. Puzzle Medical designed the ModulHeart device for patients with advanced heart failure (HF) who are not candidates for heart transplant or surgical mechanical circulatory support. The device is intended to enable safe percutaneous implantation, with low risk of bleeding, stroke, and pump thrombosis, while providing sustained symptom relief, reduced rehospitalisation and improved overall quality of life.
Results from the device’s first-in-human study showed 100% procedural success in four patients who received circulatory support whilst undergoing high-risk percutaneous coronary intervention (PCI). In addition, cardiac output among these patients
increased by 25%, urine output increased nine-fold, and left ventricular end-diastolic pressure decreased by 78%, Puzzle Medical has reported.
“I am extremely pleased with the first series of cases performed. Device implantation and explantation were easy and technically successful. The pumps functioned as expected and provided patients with significant improvement in haemodynamic parameters. I am excited about Puzzle Medical’s progress and the potential for this device to disrupt HF treatment,” said Philippe Genereux (Morristown Medical Center, Morristown, USA) of the first-in-human procedures. Genereux also serves as the company’s chief medical officer.
ModulHeart has received US Food and Drug Administration (FDA) breakthrough device designation, and an initial indication is being pursued for in-hospital therapy of acute decompensated heart failure (ADHF).
Puzzle Medical saw off competition from a number of early stage companies to scoop the US$200,000 Shark Tank prize, including Magneto, developer of a pulmonary embolism solution, Solopace, with a purpose-built pacing system for transcatheter aortic valve implantation (TAVI), Cardionomic and its cardiac autonomic modulation therapy, Restore Medical, developing pulmonary banding for HF treatment, and NXT Biomedical, which entered with a pulmonary to venous shunt for pulmonary hypertension in patients with heart failure and preserved ejection fraction (PH-HFpEF).
“We are excited to name Puzzle Medical as this year’s TCT Shark Tank Innovation Competition winner,” said Juan F Granada, president and chief executive officer of the Cardiovascular Research Foundation (CRF), the sponsor of TCT. “Their
ModulHeart device has the potential to change the course of treatment for heart failure patients in a minimally invasive way.”
Granada presented Genereux and the Puzzle Medical team with the award at TCT 2022, alongside Robert Schwartz, president of the Jon DeHaan Foundation, which has supported the last five editions of the competition.

“It is an honour to be recognised by CRF and the Jon DeHaan Foundation,” said Jade DoucetMartineau, co-founder and CEO of Puzzle Medical Devices. “Our team is grateful to have been chosen from the other innovative companies that presented at this year’s TCT Shark Tank innovation competition. With the successful completion of our first-in-human study, further investigations are underway using the ModulHeart device.”
“CRF is truly grateful to the Jon DeHaan Foundation for their continued support of pioneers in the field dedicated to developing novel technologies for the diagnosis and treatment of cardiovascular diseases,” added Granada.
Issue67 | November 2022 27 Market Watch
Puzzle Medical is awarded the Shark Tank prize
To help support our physicians going after those more complex cases, a lot of things have to happen from an innovation perspective”
MEDTECH INSIGHT
Latest Sapien TAVI valve features anti-calcification technology
Edwards Lifesciences has announced the launch of the Sapien 3 Ultra Resilia valve—combining Resilia tissue technology with the Sapien 3 Ultra transcatheter aortic valve implantation (TAVI) system. The launch follows recent US Food and Drug Administration (FDA) approval.
Resilia tissue is a bovine pericardial tissue treated with advanced anticalcification technology and serves as the platform for Edwards’ new class of valves, including the next generation Sapien X4 valve, which is currently undergoing clinical trials.
Resilia tissue delivers important advances to the Sapien 3 Ultra platform including enhanced calcium blocking properties and dry tissue packaging conditions that facilitate ease of use, Edwards Lifesciences said in a press release. Resilia tissue has demonstrated freedom from structural valve deterioration at five years and provides the potential to extend the durability of the Sapien 3 Ultra Resilia valve. It is used in the Edwards’ surgical aortic valve, the Inspiris Resilia valve.
The Sapien 3 Ultra Resilia valve will be available in the USA in limited release in the fourth quarter of 2022.
First US TAVI procedures performed using SavvyWire guidewire
OpSens has announced that Philippe Genereux (Morristown Medical Center, Morristown, USA) has performed the first use of SavvyWire in a transcatheter aortic valve implantation (TAVI) procedure in the USA, following recent 510(k) approval by the US Food and Drug Administration.
balloon-expandable and self-expandable valves, and balloon valvuloplasty. There is no doubt the SavvyWire allowed us to optimise our efficiency and workflow, while enhancing accuracy and patient safety. I am excited to collaborate with OpSens to bring this disruptive technology to patients.”
OpSens plans a controlled release to a limited number of hospitals in the USA through the end of 2022, and will then initiate full launch in early 2023.
The company has also recently announced the successful completion of the first cases in a clinical study— SAFE-TAVI—studying SavvyWire left ventricular rapid pacing in TAVI procedures in Europe. Ander Regueiro (Hospital Clínic de Barcelona, Barcelona, Spain) conducted the procedures as one of the primary investigators of the study. This study is part of the OpSens pre-CE mark clinical strategy that will lead to the commercialisation of SavvyWire in Europe.
Medtronic launches Onyx Frontier coronary stent following CE mark approval Medtronic has announced the launch of its latest generation drug-eluting coronary stent—Onyx Frontier— following recent CE mark approval.
In a press release, Medtronic details that Onyx Frontier leverages the same stent platform as the Resolute Onyx drug-eluting stent (DES), with an enhanced delivery system “designed to improve deliverability and increase acute performance in the most challenging cases”.
Design changes incorporated in the latest generation device include a duallayer balloon, lower crossing profile, and increased catheter flexibility leading to a 16% improvement in deliverability versus the previous generation Resolute Onyx DES without compromising on radial strength, Medtronic’s press release states.
approval to expand the instructions for use labelling for the current-generation Watchman FLX left atrial appendage closure (LAAC) device to include a 45day dual anti-platelet therapy (DAPT) option as an alternative to 45-day oral anticoagulation (OAC) plus aspirin for post-procedural treatment of patients with non-valvular atrial fibrillation (NVAF).
causing a significant number of patients to screen fail,” said Denti. “I believe that ShortCut Mitral provides a solution that may almost double the number of patients eligible for TMVR.”
Abbott’s Amplatzer
Talisman PFO occlusion system launched in Europe
Abbott has announced the European launch of its Amplatzer Talisman patent foramen ovale (PFO) occlusion system.
The new Talisman PFO occluders come pre-attached to the delivery cable, designed to reduce preparation time for doctors and making the system easier to use.
Clinical evidence submitted to the FDA to support the labelling update included analyses spanning approximately 8,300 patients from the Left Atrial Appendage Occlusion Registry (LAAO Registry) within the American College of Cardiology Foundation’s (ACCF) National Cardiovascular Data Registry (NCDR).
The data were submitted to the FDA to support the safety and efficacy of DAPT as a post-procedural antithrombotic regimen in patients with NVAF who may have a reason for seeking an alternative to OAC. The labelling in Europe has included the choice of either OAC or a DAPT postprocedural drug regimen for Watchman technology since 2017.
First-in-human procedure completed using ShortCut Mitral device
Pi-Cardia has announced a successful first-in-human treatment of the mitral valve with the ShortCut Mitral device in Europe.

ShortCut is a dedicated device designed to split the leaflets prior to transcatheter valve treatment in patients at risk for coronary obstruction after transcatheter aortic valve implantation (TAVI) or left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR).
“Guidelines from industry organisations and clinical trial data continue to reinforce the benefits of PFO closure for people who have had a PFO-associated stroke,” said Matthew Daniels (The University of Manchester, Manchester, UK), who completed the first European procedure with the Talisman system. “We are excited that physicians around Europe now have access to the Talisman device to help reduce patients’ risk and worry of experiencing another stroke.”
The Amplatzer Talisman system builds on the company’s Amplatzer PFO occluder. While physicians had to attach the Amplatzer PFO Occluder to the delivery cable before performing the implant procedure, the Talisman occluder now comes pre-assembled to the Amplatzer Trevisio delivery cable, reducing prep time ahead of the procedure and making it easier to use, according to Abbott. The Talisman system accommodates a wide range of patient anatomies and an updated 30mm occluder size optimises how it fits in the heart, the company adds in a press release. The Talisman device is fully recapturable and repositionable so doctors can easily make adjustments for accurate placement.
Medtronic expands US rollout of Evolut FX TAVI system
SavvyWire is a sensor-guided TAVI solution which, according to OpSens, provides a three-in-one solution for stable aortic valve delivery and positioning, continuous accurate haemodynamic measurement during the procedure, and reliable left ventricular pacing without the need for adjunct devices or venous access.

“I am extremely pleased with the performance of the SavvyWire,” said Genereux. “We successfully treated 10 consecutive patients with a variety of anatomies and levels of complexity including bicuspid valve, severe vessel tortuosity, horizontal aorta, failed prior surgical valve (valve-in-valve) using both
Onyx Frontier offers a broad size matrix to treat patients ranging from 2mm to 5mm diameters and its 4.505mm sizes can be expanded to 6mm— specifically designed to support extralarge vessels including the left main. Onyx Frontier inherits the same clinical data and indications of Resolute Onyx, including approval for bifurcation lesions, left main percutaneous coronary intervention (PCI), and one month of dual antiplatelet therapy (DAPT) in high bleeding risk patients.
The Onyx Frontier DES received US Food and Drug Administration (FDA) approval in the USA in May and recently received CE mark in Europe.
New DAPT labelling approved for Watchman FLX device
Boston Scientific has received US Food and Drug Administration (FDA)
The ShortCut Mitral compassionate case was performed at the University Hamburg Eppendorf, Eppendorf, Germany by Lenard Conradi and Niklas Schofer, in collaboration with Paolo Denti (San Raffaele Hospital, Milan, Italy). “We were able to successfully treat a patient who was at risk of LVOT obstruction by effectively splitting the anterior leaflet in a simple and controlled manner prior to a TMVR procedure,” said Conradi. “With the ShortCut Mitral device, we will now be able to treat more patients with mitral valve disease who otherwise have no other option.”
The TMVR market is growing exponentially and is predicated to reach several billion dollars in the next decade. LVOT obstruction often occurs when the anterior mitral leaflet is displaced following TMVR and is associated with morbidity and mortality.
“LVOT obstruction has been a major limitation for TMVR procedures,
Medtronic has announced the expanded US market release of its newestgeneration, self-expanding transcatheter aortic valve implant (TAVI) system, Evolut FX.
Evolut FX adds new features to the existing Evolut platform to enhance ease of use and predictable valve deployment for physicians, Medtronic said in a press release.
The full commercial release of the Evolut FX system follows US Food and Drug Administration (FDA) approval and a limited rollout earlier this year. With this latest expansion, the Evolut FX system will be available to all commercial TAVI sites across the USA.
Updates in the new platform include gold markers built into the frame to provide implanters with direct visualisation of commissure alignment; a redesigned catheter tip for a smoother insertion profile; and a more flexible delivery system that features an optimised stability layer for more stable deployment.
28 Market Watch November 2022 | Issue67
Watchman FLX
SavvyWire
Product News
Product News
Edwards gains US and European regulatory approvals for its Pascal Precision TEER system
Edwards Lifesciences has announced that its Pascal Precision transcatheter valve repair system for transcatheter edge-toedge repair (TEER) has received US Food and Drug Administration (FDA) approval for the treatment of patients with degenerative mitral regurgitation (DMR). The news comes shortly after the device was granted CE mark for the treatment of mitral and tricuspid regurgitation (MR and TR).

The Pascal Precision system is utilised in the treatment of patients with mitral or tricuspid regurgitation, through a single delivery system. The new system is designed to enable precise navigation and implant delivery.
“The Pascal Precision system provides significant advancements in operator experience with implant delivery,” said Jörg Hausleiter (Ludwig-Maximilians University in Munich, Munich, Germany). “Having successfully treated my first patient with the Pascal Precision system, I found it easy to operate and the improved catheter response and stability gave me greater control to place the implant exactly where I needed to.”
The Pascal Precision system includes Pascal and Pascal Ace implants, which feature independent grasping, atraumatic clasp and closure, and implant versatility including the ability to elongate and navigate complex anatomy.
The Pascal Precision system is one of multiple transcatheter repair or replacement therapies in development by Edwards that are designed to address mitral and tricuspid valve diseases, the company said in a press release.
Breakthrough device designation granted for SirPlux Duo coronary DCB in small vessels
Advanced NanoTherapies has been granted US Food and Drug Administration (FDA) breakthrough device designation for its SirPlux Duo drug-coated balloon (DCB) for coronary artery disease in vessels less than 3mm.
This comes shortly after the company received two other critical breakthrough device designations for its SirPlux Duo DCB in coronary instent restenosis (ISR) and peripheral below-the-knee (BTK) lesions.
“Despite decades of advances in drug-eluting stents, treating small
coronary vessels continues to be challenging due to the unacceptable incidence of restenosis and target lesion failure,” commented Rishi Puri (Cleveland Clinic, Cleveland, USA), co-principal investigator of the company’s ADVANCE-DCB clinical trial. “Advanced NanoTherapies is championing a novel stent-free option to address this clinical gap. I look forward to seeing how this technology can advance the field of coronary interventions by improving long-term clinical outcomes and preventing the need for repeat revascularisations.”
The clinical profile of small vessel disease (SVD) patients translates into poor long-term outcomes, including a higher rate of target lesion failure when using drug-eluting stents (DES). DCBs are becoming an attractive therapeutic strategy for de novo lesions, with potential advantages of lower risk of acute thrombosis, favourable vascular remodelling, and shortened dual antiplatelet therapy (DAPT), Advanced NanoTherapies said in a press release.
SirPlux Duo DCB delivers lowdose, long-term release of sirolimus and paclitaxel to inhibit cell growth. Advanced NanoTherapies’ proprietary nanoparticle drug-encapsulation and delivery platform provides safe, reliable, and sustained bioavailability of the two synergistic drugs in tissue for long-term outcomes, the company adds.

Haemonetics’ Vascade vascular closure devices gain CE mark
Haemonetics Corporation has received CE mark certification for its Vascade vascular closure and Vascade MVP venous vascular closure systems.
The CE marking will allow Haemonetics to engage in the next steps of country-specific entrance of both products into the European Union (EU) and forms the basis for entry into other geographies that recognise the CE mark, the company said in a press release.
The Vascade system is designed for “small-bore” femoral arterial and venous closure, generally used in interventional cardiology and peripheral vascular procedures. The Vascade MVP system is designed for “mid-bore” multi-access femoral venous closure—generally used in electrophysiology procedures— and is the only US Food and Drug Administration (FDA)-approved closure device for use following cardiac ablation procedures requiring two or more access sites within the same limb.
Both devices include proprietary collapsible disc technology and a resorbable collagen patch to achieve haemostasis. Vascade and Vascade MVP are designed to save time for hospital staff, while helping patients
reach haemostasis faster with fewer complications on average.
In 2021, Vascade MVP received an FDA indication for same-day discharge following atrial fibrillation (AF) ablation. While not all countries in the EU are able to offer same-day discharge to patients, all patients can benefit from the quicker time to ambulation and reduced discomfort that this product provides.
CathWorks gains Japanese regulatory approval for FFRangio system
CathWorks has announced approval of the fourth generation CathWorks FFRangio system by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA). The FFRangio system is also commercially available in the USA and Europe.
“The FFRangio System enables us to quickly perform a comprehensive physiologic assessment of coronary artery disease without the need for invasive pressure wires or hyperaemic agents, providing significant benefits to clinicians and patients,” said Yutaka Hikichi (Saga-Ken Medical Center Koseikan, Saga, Japan). “The approval of the fourth-generation application offers significant automation and enhancements, further simplifying the utilisation of the platform.”
“Coronary artery disease is a lifethreating disease, and it is critical that we can quickly, effectively, and cost-effectively diagnose the disease and determine the appropriate treatment plan,” said Mamoru Nanasato (Sakakibara Heart Institute, Tokyo, Japan). “We have been able to experience first-hand how FFRangio can transform the diagnosis and treatment of our patients with coronary artery disease.”
Abiomed gains FDA approval for Impella low profile sheath
The US Food and Drug Administration (FDA) has granted 510(k) clearance to Abiomed to market a new low profile sheath for delivery of its Impella device.
Compared to the existing 14Fr sheath used for placement of Impella CP, the new sheath maintains the same inner diameter, but reduces the outer diameter by nearly 2Fr.
As a result of its smaller size and other technological advancements, the low profile sheath will facilitate easier Impella insertion and removal, reduce procedural steps and help improve outcomes, Abiomed said in a press release.
The low profile sheath is the first
sheath specifically engineered to be compatible with the Impella singleaccess technique, which removes the need for an additional access site, the press release goes on to add. Additionally, it is designed to simplify access and ease-of-use by eliminating the peel-away sheath and the need for the re-access sheath for patients who are sent to the intensive care unit.
These features are also intended to further minimise vascular complications and bleeding, facilitate easier delivery into the vasculature with a hydrophilic coated long-taper dilator, reducing the need for multiple steps of serial dilation, and enable easier management of the patient during heart pump removal and vascular closure as Impella can be removed directly from the sheath without re-wiring.
HeartFlow receives US FDA approval for Plaque and RoadMap analysis AI products
HeartFlow has received US Food and Drug Administration (FDA) 510(k) clearance on two new, AI-powered products: Plaque and RoadMap analysis.
With its expanded product portfolio, HeartFlow is the first and only company to provide noninvasive coronary artery anatomy (RoadMap analysis), physiology (HeartFlow FFRCT), and plaque information (Plaque analysis) based on cardiac computed tomography angiography (CCTA), the company said in a press release. These products enable physicians to gain a more comprehensive understanding of a patient’s coronary artery disease and are the most accurate approach to predict risk of a heart attack.
HeartFlow’s Plaque analysis provides physicians with comprehensive and actionable data showing plaque characteristics and volume in all major coronary arteries. It enables critical information regarding coronary plaque to be delivered conveniently to physicians along with anatomy and physiology.
RoadMap analysis enables CT readers to improve coronary artery disease diagnosis by providing visualisation and quantification of the location and severity of anatomic narrowings in the coronary arteries on every CCTA.
“It is exciting to note the work HeartFlow is doing to bring forward the innovative technologies to help us advance our understanding and care for patients with coronary artery disease. Combining anatomy, physiology, and plaque morphology would be essential for personalised patient care,” said Jagat Narula (Mount Sinai Morningside Hospital, New York, USA).
With this FDA 510(k) clearance, HeartFlow will begin real-world clinical use of the Plaque and RoadMap analyses with select hospitals and health systems. HeartFlow’s latest technologies will play a vital role in improving cardiovascular patient care.
29 Market Watch Issue67 | November 2022
SirPlux Duo DCB
Vascade system
Selution SLR receives US FDA investigational device exemption approval for coronary clinical trial
Selution SLR, MedAlliance’s novel sirolimus-eluting balloon, has received US Food and Drug Administration (FDA) investigational device exemption (IDE) approval to initiate its coronary pivotal clinical trial.
This is the first sustained limus release coronary drug eluting balloon (DEB) to receive FDA IDE approval for in-stent restenosis (ISR) indication. The study has already begun enrolment in Europe.

“We are pleased with the speed of European enrolment and look forward to enrolling the first US patient before the year end; US site selections are underway and will be finalised over the next several weeks. This study has the potential to address the important unmet need of a non-stent treatment for the ongoing problem of in-stent restenosis,” commented Don Cutlip, principal investigator of the IDE SELUTION4ISR Study and the chief medical officer at Baim Institute for Clinical Research (Boston, USA).
Selution SLR was awarded CE mark approval for the treatment of peripheral artery disease in February 2020 and for the treatment of coronary artery disease in May 2020.
Selution SLR is commercially available in Europe, Asia, the Middle East and the Americas (outside the USA) and most other countries where the CE mark is recognised.
Last month (October) it was announced that MedAlliance is to be acquired by Cordis, after the two companies reached agreement for an acquisition which includes an initial investment of US$35 million and upfront closing payment of US$200 million.
Clinical trial of TAVI in severe aortic regurgitation completes enrolment
JenaValve Technology has announced the successful completion of patient enrolment in the ALIGN-AR Pivotal trial, a landmark prospective, singlearm investigation device exemption (IDE) study designed to assess the safety and efficacy of the Trilogy transcatheter aortic valve implantation (TAVI) system in high-risk patients with symptomatic, severe aortic regurgitation.
Results from the study are intended to support a future premarket approval (PMA) submission to the US Food and Drug Administration (FDA).
“Completing the ALIGN-AR trial is a significant step forward for the cardiology community in addressing an unmet need for patients suffering from significant AR,” said Martin B Leon (Columbia University Irving Medical Center, New York, USA) global programme chair for the trial. “The unique design of the Trilogy system has the potential to address the shortcomings of existing TAVI devices when treating high surgical risk AR patients, enabling physicians to address a critical and long-overdue need for a minimally invasive solution that can meaningfully improve a patient’s quality of life.”
“This is a potentially game-changing clinical trial for the treatment of symptomatic, severe aortic regurgitation in a significant patient population which has not been addressed by other solutions,” said Raj Makkar (CedarsSinai Medical Center, Los Angeles, USA) leading enroller for the trial. “If approved, the Trilogy System will be the only transcatheter device indicated for the treatment of symptomatic, severe aortic regurgitation patients, with a potential to become the standard of care for these patients.”
Abiomed given green light for RECOVER IV randomised trial
Abiomed has announced two approvals from the US Food and Drug Administration (FDA) related to clinical research of Impella heart
pumps in acute myocardial infarction (AMI) cardiogenic shock patients.
The FDA has approved the on-label RECOVER IV randomised controlled trial (RCT) for AMI cardiogenic shock patients. RECOVER IV is a two-arm trial that will assess whether percutaneous coronary intervention (PCI), with Impella support initiated prior to the PCI, is superior to PCI without Impella support.
“This landmark trial will be the culmination of over 20 years of research in the interventional therapy of AMI and will apply all the clinical advancements we have made to improve survival and heart recovery for AMI patients with cardiogenic shock as demonstrated in multiple prospective studies,” said William O’Neill (Henry Ford Health, Detroit, USA), a RECOVER IV national co-principal investigator.
The primary endpoint of RECOVER IV is all-cause mortality at 30 days. Secondary endpoints include major adverse cardiovascular and cerebrovascular events (MACCE) at 30 days, days alive out of the hospital at six months, recovery of left ventricular (LV) function, need for durable ventricular assist device (VAD) or heart transplant, and health-related quality of life as measured by responses to the Kansas City Cardiomyopathy Questionnaire (KCCQ) at one year. Abiomed’s goal in conducting the trial is to achieve a global AMI cardiogenic shock Class I guideline recommendation for Impella and related best practice protocols, including Impella implantation prePCI.
“I am optimistic that RECOVER IV will further demonstrate the benefits of haemodynamic support and best practice protocols. These benefits include ventricular unloading using Impella pre-PCI, reduced LV wall stress, reduced pulmonary congestion, increased collateral coronary blood flow, and enhanced cardio protection so that more AMI cardiogenic shock
patients can survive and achieve native heart recovery. The heart team and field have evolved and understand how important myocardial recovery is for both AMI and AMI cardiogenic shock to reduce the growing epidemic of heart failure,” said Navin K Kapur (Tufts Medical Center, Boston, USA), a national co-principal investigator for RECOVER IV.
First patient enrolled in Hyper II study evaluating hybrid approach in long diffuse coronary artery disease
Cardionovum has announced the enrolment of the first patient in the international Hyper II clinical study designed to evaluate the safety and efficacy at 12 months of the hybrid treatment approach combining the Restore drug-coated balloon (DCB) with new a generation drug-eluting stent (DES) in long diffuse coronary artery disease (CAD).
The first procedure of the Hyper II clinical study was performed on 27 July at Sant’Ambrogio Cardio-Thoracic Center in Milan, Italy by Alfonso Ielasi, principal investigator of the study.
The Restore paclitaxel-coated balloon utilises a unique combination of a rapid absorption drug— paclitaxel—and patented Safepax, paclitaxel coating matrix.
The Hyper II is a prospective, non-randomised, multicentre clinical trial that will enrol up to 500 patients across 15 international sites and is built upon the findings of the Hyper Study presented at the EuroPCR 2022 (17–20 May, Paris, France), showing encouraging clinical results in terms of device-oriented composite endpoint (DoCE) of 3.7% at 12 months with no thrombosis reported in DES and DCB treated segments. The primary endpoint of the Hyper II study has been defined as the target lesion failure (TLF) incidence and will be assessed within 12 months after the index procedure.
“De novo diffuse coronary artery disease still represents an open issue in the field of interventional cardiology,” said Ielasi. “Overlapping DES and DCB in order to limit the total stent length may reduce the risks associated with permanent rigid metallic cages within the coronary wall. This concept could be particularly important in case of very long lesions involving small vessels and in diabetic patients.”
Calendar of events
27–29 November
PCR London Valves London, UK pcronline.com
14–15 December
GulfPCR-GIM Dubai, UAE pcronline.com
2023
18–20 January
British Cardiovascular Intervention Society (BCIS) Advanced Cardiovascular Intervention (ACI) London, UK millbrook-medical-conferences. co.uk
21–23 January
Society of Thoracic Surgeons (STS) Annual Meeting San Diego, USA sts.org/meetings
9–11 February
Joint Interviontal Meeting (JIM) Milan, Italy jim-vascular.org
25–28 February
Cardiovascular Research Technologies (CRT) 23 Washington DC, USA crtmeeting.org 4–6 March
American College of Cardiology (ACC) 2023 Scientific Sessions New Orleans, USA expo.acc.org
16–19 May
EuroPCR Paris, France pcronline.com
18–20 May
Society for Cardiovascular Angiography and Interventions (SCAI) 2023 Phoenix, USA scai.org/education-and-events
30 Market Watch November 2022 | Issue67
Clinical News
Selution SLR
Impella CP
C M Y CM MY CY CMY K

Consensus Update Vascular & Endovascular SEE YOU IN 2023 CXSYMPOSIUM.COM Peripheral Arterial Consensus Aortic Consensus Acute Stroke Consensus Venous & Lymphatic Consensus Vascular Access Consensus The Hurting Leg Consensus INNOVATION EDUCATION EVIDENCE 25–27 APRIL 2023 TUESDAY-THURSDAY HILTON LONDON METROPOLE, UNITED KINGDOM CONTROVERSIES CHALLENGES CONSENSUS
Onyx Frontier™ DES
Great just got better
Onyx Frontier DES inherits the same stent platform, clinical data, and indications you’ve come to rely on with Resolute Onyx™ DES. What makes it even better is its delivery system resulting in best-in-class with a lower crossing profile.2
deliverable
†Stent delivery system updates were implemented on the 2.0–4.0 mm Onyx Frontier DES diameters.
See more at medtronic.com/ OnyxFrontierGlobal
1 Based on bench test data on file at Medtronic. May not be indicative of clinical performance. N = 7 DES of each tested: Onyx Frontier DES, Resolute Onyx DES, Orsiro®* Mission DES, XIENCE Sierra™* DES, XIENCE Skypoint™* DES, SYNERGY™* DES, SYNERGY™* XD DES, Ultimaster™* Tansei™* DES.
2 Based on bench test data on file at Medtronic. May not be indicative of clinical performance. N = 5 DES of each tested: Onyx Frontier DES, Orsiro Mission DES, Resolute Onyx DES, XIENCE Skypoint DES, SYNERGY DES, Ultimaster Tansei DES.
Europe
Medtronic Intl. Trading SARL Tel: 41.21.802.7000 Asia Pacific Medtronic Intl. Ltd. Tel: 65.6436.5000 Latin America Medtronic USA, Inc. Tel: 786.709.4200
medtronic.com/OnyxFrontierGlobal
UC202211208 ML ©2022 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. ™*Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. For distribution only in markets where the Onyx Frontier coronary stent has been approved. Not for distribution in the USA, Canada, Japan, or France. 08/2022


































































































