Abdominal and Juxtarenal Aortic
Wednesday, 08:00–18:00 Kensington 1
Superficial Venous & Lymphatic
Wednesday, 08:00–13:00 Kensington 2
A question from Manj Gohel (Cambridge, United Kingdom) on what the CX audience should take back to their multidisciplinary team meetings from the firsttime presentation of BASIL-2 led chief investigator Andrew Bradbury (Birmingham, United Kingdom) to deliver the stark message: a patient who needs a below-the-knee revascularisation with or without a femoropopliteal revascularisation is likely to do better if they are treated with a best endovascular-first strategy rather than a vein bypass-first approach.
In the BASIL-2 (Bypass versus angioplasty for severe ischaemia of the leg) trial of 345 patients with chronic limb-threatening ischaemia (CLTI), a best endovascular treatment-first revascularisation strategy was associated with better amputationfree survival than a vein bypass-first strategy in those who required an infrapopliteal repair—with or without a more proximal infrainguinal procedure. This result was largely driven by fewer deaths in the best endovascular treatment group. Bradbury, of the University of Birmingham, presented this key finding during a Podium First presentation yesterday morning. The results were
simultaneously published in The Lancet
“It all seems to be pointing towards attempting an endovascular procedure first and then if that does not work, doing something else—which could be more endovascular,” Bradbury said in response to Gohel. Alternatively, he added, this could be the point at which the vascular specialist switches over to a bypass approach. BASIL-2, however, “lends quite a lot of weight” to an endovascularfirst revascularisation strategy, “with all the caveats that we have to consider”.
Bradbury, delivering the data for the first time during day one of CX 2023, revealed that 63% of patients randomised to a vein bypassfirst strategy of treatment underwent a major amputation or died during follow-up, compared to just 53% of those allotted to a best endovascular-first approach—BASIL-2’s primary outcome measure (adjusted hazard ratio 1.35, 95% confidence interval [CI] 1.02–1.08, p=0.037).

“Essentially this means that, in this cohort, a vein bypass revascularisation strategy resulted in a 35% increased risk of amputation or death during the follow-up compared with a best

Deep Venous
Wednesday, 13:30–18:00 Kensington 2
Acute Stroke
Wednesday, 08:00–11:00 Admiral
Vascular Trauma
Wednesday, 11:00–13:00 Admiral
CX 2023 OVERVIEW DAY TWO
Deep Venous Consensus Acute Stroke Consensus Vascular Trauma Consensus Superficial Venous & Lymphatic Consensus Aortic Consensus VASCULAR Join our global online vascular community CXVASCULAR.COM Education, News and Insights for the global vascular community SIGN UP AT C M Y CM MY CY CMY K CXVASCULAR-APRIL-2023-225X58.pdf 1 07/04/2023 15:40:00 CXVascular.com Wednesday 26 April 2023 Continued on page 2 Join the community #CX2023 BASIL-2: EXCLUSIVE UPDATE
BASIL-2 points towards endovascular-first revascularisation strategy in CLTI
patients
COVER STORY continued
BASIL-2 points towards endovascular-first revascularisation strategy in CLTI patients

Continued from page 1
endovascular-first revascularisation strategy,” Bradbury told the CX audience.
Median survival for the whole cohort was 3.8 years—3.3 years for the vein bypass group and 4.4 for the endovascular arm, he said. “The significant difference we have observed in favour of best endovascular therapy with amputation-free survival is very largely driven by the fact that there were more deaths in the vein bypass group—53% of vein bypass patients and 45% of best endovascular therapy patients,” Bradbury continued. “There is no significant difference of 30-day mortality but you can see that the median survival of the two groups is quite different.”
CLTI is the “severest manifestation” of peripheral arterial disease (PAD) and presents as ischaemic pain at rest or tissue loss, or both, the authors detail in The Lancet. Against this backdrop, Bradbury and colleagues were comparing effectiveness of a vein bypass-first with a best endovascular treatment-first revascularisation strategy in terms of preventing major amputation and death in patients with CLTI.
“It is important to emphasise that the best way of analysing this trial, which is the way our statistical colleagues have done it, is on the intention-to-treat population; however, for completeness they have done some sensitivity analyses, and this includes a per protocol analysis, which includes only patients who were adherent—that is, they received the allocated intervention they were randomised to,” Bradbury explained at CX. “They also performed an as-treated analysis, which is based upon the first revascularisation that the patient actually received following randomisation, and as you can see here they both trend towards reduced amputation-free survival in the vein bypass-first group.”
The BASIL-2 co-investigators, namely Catherine Moakes, Gareth Bate and Matthew Popplewell (all Birmingham, United Kingdom) and Lewis Meecham (Cardiff, United Kingdom) also presented during the session on the journey from BASIL-1 to BASIL-2, a hypothesis-generating prospective cohort study, methodology, study limitations and future work, among other topics.
BASIL-2 was an open-label, pragmatic, multicentre, phase 3, randomised trial performed at 41 vascular surgery units in three countries: the United Kingdom (n=39), Sweden (n=1) and Denmark (n=1). The central site was the University of Birmingham. “Eligible patients were those who presented to hospital-based vascular surgery units with [CLTI] due to atherosclerotic disease and who required an infrapopliteal, with or without an additional more proximal infrainguinal, revascularisation procedure to restore limb perfusion,” they state in The Lancet Bradbury and colleagues randomly assigned participants 1:1 to receive either vein bypass or best endovascular treatment as their first revascularisation procedure through a secure online randomisation system. The Lancet paper details that participants were excluded if they had ischaemic pain or tissue loss considered not to be primarily due to atherosclerotic PAD. Most vein bypasses used the great saphenous vein and originated from the common or superficial femoral arteries, the authors communicate, while most endovascular interventions comprised plain balloon angioplasty with selective use of plain or drugeluting stents.
Patients were followed up for a minimum of two
years, Bradbury et al write, with data collected locally at participating centres. In England, Wales and Sweden, the authors note, centralised databases were used to collect information on amputations and deaths. They add that data were analysed centrally at the Birmingham Clinical Trials Unit.
The primary outcome of amputation-free survival was defined as time to first major (above the ankle) amputation or death from any cause measured in the intention-to-treat population. Safety was assessed by monitoring serious adverse events up to 30 days after first revascularisation.
Between 22 July 2014 and 30 November 2020, the triallists enrolled and randomised 345 patients with CLTI—65 (19%) women and 280 (81%) men with a median age of 72.5 years (62.7–79.3). The patients were randomly assigned to either the vein bypass group (172 [50%]) or the best endovascular treatment group (173 [50%]).

Bradbury detailed at CX that major amputation or death occurred in 108 (63%) of 172 patients in the vein bypass group and 92 (53%) of 173 patients in the best endovascular treatment group. The relevant mortality numbers were 91 (63%) among the vein bypass group and 77 (53%) in the endovascular arm.
In both groups, the authors write, the most common causes of morbidity and death— including that occurring within 30 days of their first revascularisation—were cardiovascular (61 deaths in the vein bypass group and 49 in the best endovascular treatment group) and respiratory events (25 deaths in the vein bypass group and 23 in the best endovascular treatment group). They add that the number of cardiovascular and respiratory deaths were not mutually exclusive.
In the discussion section of their paper, Bradbury et al consider how their findings compare to those from the BEST-CLI trial, which were presented for the first time last November. “At first glance,” they remark, “our results appear to conflict with the BEST-CLI trial”. However, they note that there were “many differences” between the two trials, including the primary endpoint. “Our clinical experience suggests that few patients with
[CLTI] are deemed suitable and have an optimal vein for infrapopliteal bypass,” Bradbury and colleagues comment, adding that future work is required to determine whether the patients enrolled in BASIL-2 are more like the patients with a non-optimal vein in the BEST-CLI trial.
During the panel discussion following the BASIL-2 presentations at CX, Andres Schanzer (Worcester, United States) asked Bradbury and colleagues to cast the findings against the backdrop of BEST-CLI. Moakes (Birmingham, United Kingdom), who was the statistician for BASIL-2, explained that the team are planning to conduct an individual patient data meta-analysis to answer questions around any relevant differences between the two trials.
“A trial of two strategies”
Also during the discussion, the panel touched on the evolving landscape of endovascular treatment, with moderator Andrew Holden (Auckland, New Zealand) asking Bradbury and colleagues whether they had noted a significant change in endovascular practice in the period between BASIL-2 and its eponymous predecessor.

Bradbury noted in his interpretation of the data that “there is a much greater willingness now that if you do an endo[vascular], and you are not happy with it, the interventional radiologists will go back and have another go, whereas what we tended to see, I think, in BASIL-1, was that if endo[vascular] did not work, [treatment would] quickly go over to bypass. I think that is the difference.”
Moderator Dittmar Böckler (Heidelberg, Germany) urged the audience to keep in mind the various options that remain open when undertaking an endovascularfirst approach. Greenhalgh underscored the point. “It also came out with BEST-CLI that the quality of the vein is important,” he said. “It is very, very crucial that whatever you do first, it does not have to be the last word.”
UK Secretary of State for Health visits CX 2023

The UK’s Secretary of State for Health and Social Care, Steve Barclay, was in attendance at CX 2023 on Tuesday, where he met Roger Greenhalgh (London, United Kingdom).
The Secretary of State’s visit coincided with the presentation of results from the NIHR-funded BASIL-2 trial, a headline moment on day one of the event.
Böckler said vascular specialists
“need to learn from this trial which patient deserves which treatment”. It is not a case of surgery versus endovascular therapy, but rather a case of learning from the data, he added. Bradbury concurred. “It is a trial of two strategies,” he said.
“That was what BASIL-1 was. It is quite a difficult concept to get across. We are not comparing a vein bypass with an endovascular treatment. We are in a sense—but what we are saying is, ‘What do you do first?’ If you have got equipoise, if you are really on the fence, and you do not know which to do, this trial suggests fairly strongly, I would suggest, that in this subgroup of patients, that you should go endo[vascular] first.”
Wednesday 26 April 2023 2 SECOND EDITION #CX2023 BASIL-2
It all seems to be pointing towards attempting an endovascular procedure first.”
Andrew Bradbury
Innovative solutions for the treatment of thoracic aortic disease
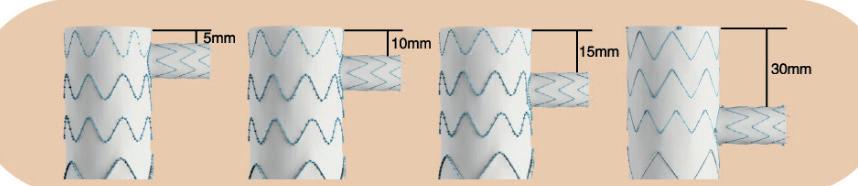



Serve the arch, preserve the branch
• Unibody design with integrated side branch

• High conformability of the branch
• Wide range of sizes*

• Intuitive deployment technique


• 24F Delivery device
Multiple design options for the branch *
* Available as custom-made device
To find out more join our Symposium today at 13.30 in the Admiral Suite or visit us at booth F19



©2023 Lombard Medical. All rights reserved
“Excellent” primary success of limb ischaemia thrombectomy study shared as Podium First
Yesterday morning’s peripheral arterial and acute limb ischaemia programme featured a Podium First presented by Sean Lyden (Cleveland, United States)— primary endpoint results of the STRIDE trial. Designed to report 30-day safety and performance of the Indigo aspiration system (Penumbra), the trial is an international, multicentre, prospective, single-arm, observational study of patients with lower extremity acute limb ischaemia. Lyden informed the audience that the study had successfully met its primary endpoint of target limb salvage at 30 days post-procedure.
STRIDE’s patient cohort numbered 119 patients (of 285 initially screened), across 16 sites in the United States and the European Union. The mean age of patients was 66.3±13.27, and 55/119 were women, the presenter detailed. The highest rates of other medical conditions among patients were hypertension (n=103) and hyperlipidaemia (n=100).
Lyden shared that the inclusion criteria stipulated that patients participating in the trial must have had occlusion of one or more lower limb arteries below the inguinal ligament for up to 14 days, and have a Rutherford category score of I, IIa or IIb. Of the enrolled patients, 10.9% were ‘viable’, with a Rutherford category I score, 54.6% ‘threatened marginally’ classified as IIa, and 34.5% ‘threatened immediately’ with a IIb score.
Patients with a target vessel size of less than 2mm, amputation in the ipsilateral limb, or with target thrombus in a saphenous vein bypass graft were excluded from the trial, the speaker added.
Eight patients of the 119 enrolled exited the study before the 30-day follow-up point—five died, one was lost to follow-up, and two patients withdrew, Lyden supplemented. A further two patients missed 30-day follow-up.
In terms of the primary endpoint, target limb salvage at 30 days was achieved in 109 patients, according to the presenter. Multiple secondary endpoints were also measured— efficacy endpoints comprised patency at 30 days, and modified Society for Vascular Surgery (SVS) runoff score immediately post-procedure versus baseline. Lyden elaborated that the results for these measures were 90.3% success and an improvement of SVS runoff score of 6.3±5.49, respectively. Regarding safetyrelated secondary endpoints, device-related serious
Bioresorbable drugeluting stents swing CX audience vote following peripheral trial update
Following updates from multiple studies assessing bioresorbable drugeluting stents, the majority (69%) of CX audience members in the Peripheral Arterial and Chronic Limb-Threatening Ischaemia (CLTI) session voiced support for these technologies, voting in favour of the statement that ‘bioresorbable wins’.
HOWEVER, QUESTIONS WERE raised over the length of time it takes for these devices to be resorbed following implantation. Responding to session anchor Roger Greenhalgh’s (London, United Kingdom) question regarding their strength, and their longevity, panellist Ramon Varcoe (Sydney, Australia) said one example of these stents—the Esprit below-theknee (BTK) scaffold (Abbott)—is only slightly less strong than a metallic stent, but how long they last is “up for debate”.
“There is a school of thought out there that these stents should actually dissolve more quickly,” Varcoe stated, also touching on the fact that the Esprit device takes roughly 18–24 months to be resorbed, despite the fact drug-eluting stent treatments in BTK patients may need no longer than six months to be effective.
Delivering an update on the LIFE-BTK randomised trial—which he feels has become “the new gold standard” in BTK studies—Varcoe said the “excellent” long-term
adverse events numbered one and major periprocedural bleeding was reported in five patients.
Furthermore, the speaker said, the median time taken to carry out thrombus aspiration with the Indigo system was 22.0 minutes (interquartile range 12.0–47.0).
Lyden concluded that STRIDE study patients treated frontline with the Indigo aspiration system had “excellent” limb salvage rates, a “low” rate of peri-procedural complications, and a “high” technical success rate. “Mechanical aspiration thrombectomy provided a safe and effective alternative therapeutic option for lower extremity acute limb ischaemia patients,” were the presenter’s parting words.
In the discussion following Lyden’s presentation, moderator Gunnar Tepe (Rosenheim, Germany) asked how often during the STRIDE study it was necessary to perform any additional procedures.
The presenter responded that there were a “small proportion of patients” who received postoperative thrombolysis after incomplete mechanical aspiration, the data for which will be included in the paper that principal investigator Thomas Maldonado (New York, United States) is currently working on.
Another question probed what is next for the STRIDE study, with Lyden noting that the first thing the study team are planning to do is “get into the dataset” and look at all the cohorts that were included in terms of type of pathology and the vessels that were treated.
A single first-to-podium is set to be given on the third and final day of CX 2023 (Thursday 27 April), with Andrew Holden delivering first-in-human experiences with the Suture-Tight device (Vesteck)— an innovation intended to eliminate migration in endovascular aneurysm repair (EVAR) procedures.
patency and freedom from targetlesion revascularisation (TLR) Esprit achieved in an earlier proof-of-concept study facilitated this larger, multicentre undertaking. He concluded by noting that LIFE-BTK enrolment has now closed, and results are set to be released later this year.
A similarly positive outlook was then delivered by Michel Bosier (Bern, Switzerland) in his presentation of an update from the MOTIV trial, the results of which showed a 99% rate of technical success in delivery and revascularisation, as well as 88.3% vessel patency after 12 months, in BTK cases. In this prospective, multicentre study assessing the sirolimus-eluting Motiv bioresorbable scaffold (Reva Medical), Bosier emphasised “excellent” tracking and visibility during implantation; low adverse event rates; and a single, clinically-driven TLR across the entire patient cohort.

Medical) to target a “significant” unmet need in peripheral vascular intervention for femoropopliteal disease were presented by Andrew Holden (Auckland, New Zealand). In his update on the trial, Holden disclosed encouraging initial results in short lesions.
Despite “no safe and effective formulation of any [bioresorbable] vascular scaffold” being realised to date in the peripheral vascular bed, according to Holden, development of the EVSS specifically for use in this area has provided high radial strength, “unencumbered” movement of the femoropopliteal axis and prolonged sirolimus elution.
69% AGREE: BIORESORBABLE WINS

During the same session, EFEMORAL I trial results indicating the ability of the Efemoral vascular scaffold system (EVSS; Efemoral
With a look to the future, Varcoe acknowledged the relevance of translating these promising findings from proof-ofconcept to “real-world disease”, before Holden drew the session to a close by stating that inflammation presents an additional challenge moving forward— quipping that “neointimal hyperplasia has been their Achilles’ heel” in the past.
Wednesday 26 April 2023 4 SECOND EDITION #CX2023 Seen First at CX
Peripheral Arterial Consensus
Sean Lyden
Advanta V12 balloon expandable covered stent
Right from the start Still going strong

Advanta V12 is a balloon expandable, fully encapsulated PTFE stent with first to market covered balloon expandable stent technology that has served more than 700,000 patients. Known for its precision and predictability – the versatile Advanta V12 has been meeting the needs of surgeons and patients for 20 years and is the only durable solution backed up by decades of real-world evidence and more than 550 publications.

getinge.com
Getinge, and , are trademarks or registered trademarks of Getinge AB, its subsidiaries, or affiliates in the United States or other countries. Copyright 2023 Atrium Medical Corp. All rights reserved. PN011928 Rev AA 03/2023
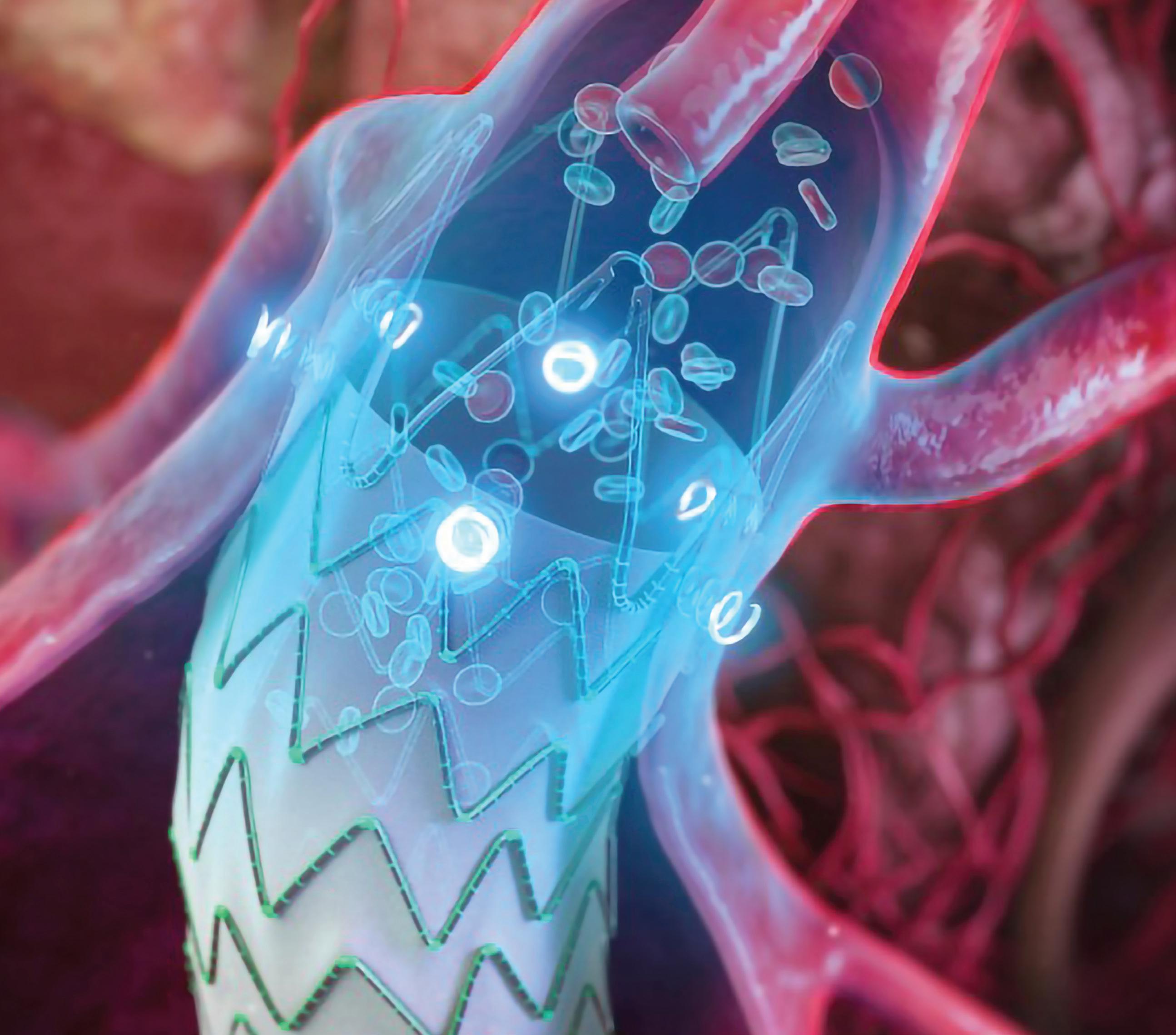
Medtronic.com/aorticpartnerglobal ESAR: Reinforced
redefined outcomes. Risks may include failure to prevent/correct type I endoleaks, migration, sac enlargement. Visit website to learn more and for more complete risk information. See the device manual for detailed information regarding the instructions for use, indications, contraindications, warnings, precautions, and potential adverse events. For further information, contact your local Medtronic representative and/or consult the Medtronic website at medtronic.eu. UC202312111-01 EE ©2023 Medtronic. Medtronic and the Medtronic logo are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. 04/2023
seal,
CX 2023: Day one in focus



The first-to-podium presentation of the BASIL-2 trial were presented to a packed Kensington 1 auditorium, but there was plenty more on offer on day one of CX 2023.






7 Wednesday 26 April 2023 SECOND EDITION #CX2023
“Whatever you do first, it does not have to be the last word.”
ROGER GREENHALGH
First-in-human study update indicates “excellent” patency results with restorative dialysis graft
In a Podium First presentation during this year’s Vascular Access Masterclass, Frans Moll (Utrecht, Netherlands) provided the audience with an update on the novel Axess graft (Xeltis), which is intended to improve haemodialysis access outcomes via endogenous tissue restoration (ETR).

“THIS GRAFT CAN BRING A BRIGHTER future for the increasing number of patients who
depend on dialysis treatments,” Moll told CX Daily News. “And, I think we could see this in the next two-to-three years—the study is running, patients are actively being enrolled, and we are seeing very positive outcomes. We always have to be modest, but we are certainly optimistic about this.”
Initially, the speaker highlighted the goal of this device—to provide an implantable polymer-based technology that harnesses the body’s natural ability to regenerate cells. The Axess graft utilises supramolecular chemistry and precise electrospinning, offering the basis for a tuneable medical device that can act as a scaffold and allow the body to effectively create its own vessel.
Following implantation, ETR enables the graft to gradually be absorbed by the body, resulting in a ‘natural, living’ graft akin to an autologous arteriovenous fistula (AVF). Moll also noted that the proprietary technology behind Axess can be ‘coded’ during the manufacturing process, enabling absorption times to be adjusted.
The goal here—according to Moll—is to combine the early-stage benefits of an arteriovenous graft (AVG), including the possibility for early cannulation and no maturation requirement, with the more long-term advantages associated with AVFs, such as improved patency rates, and a reduced number of interventions and infections over time.
IN.PACT AV three-year subanalysis trial data show “durable” long-term results
Yesterday’s Vascular Access Masterclass session saw a Podium First presentation by Andrew Holden (Auckland, New Zealand) in which he presented new data from the IN.PACT AV Access trial of the IN.PACT AV (Medtronic) drug-coated balloon (DCB). Offering three-year subanalysis data, he said that the study is “the only randomised pivotal trial of a device treating dysfunctional arteriovenous fistulas (AVFs) to demonstrate consistent and sustained clinical benefit” up to three years.
ESTABLISHING SOME
background to the study, Holden noted that there have been eight published peer-reviewed studies with outcomes reported through to three years, but he said that all of these were singlecentre with no adjudication—and that “all except one were single-arm and retrospective”. That means there is, in his words, “a paucity of evidence of long-term outcomes after treatment of stenoses in AV access”.
The IN.PACT AV study was initially planned to extend to 24 months, and a five-year extension meant a shrinking of the pool of participants as a result of death, withdrawal and declined consent to further study. In total, 133 participants completed their threeyear visit out of an original cohort of 330 patients receiving the index
procedure. Of that 330, 170 were in the IN.PACT AV arm, while 160 received percutaneous transluminal angioplasty (PTA). In the IN.PACT AV arm, the mean age was 65.8 years and 112 of 170 were male. The standard PTA arm had a mean age of 65.5 and 101/160 were male.
As presented last year at CX, at 36 months, the DCB arm yielded a target lesion primary patency (TLPP) rate of 43.1% while the PTA arm had a rate of 28.6%, a statistically significant patency advantage for the DCB. This year, Holden set out for the first time the results of subgroup analyses in patients receiving an AVF in the forearm and upper arm at three years.

Those analyses included one in which the results were stratified by AVF type. In that analysis, the
Moving on to outline the latest data with Axess from an EU first-in-human (FIH) study, Moll detailed that enrolment of a total of 20 patients across six sites has now been completed. As per follow-up data, which were available for all 20 patients, primary patency rates of 80% at six months and secondary patency rates of 100% at 12 months were achieved. The speaker also reported that no infections were observed. A full follow-up period of five years is now planned in these patients, and is ongoing.
Moll also provided attendees with insight based on surgeon feedback from the first 20 Axess implants—alluding to the fact it is a similar procedure to standard AVG implantation; the device being easier to suture and softer than traditional expanded polytetrafluoroethylene (ePTFE) products; and its improved adaptation to patient arteriotomy/ venotomy proving “very helpful” in fragile vessels. A prospective, single-arm EU pivotal trial of Axess is now also actively enrolling patients, at up to 25 sites spanning nine countries and with a total followup period of five years. Moll noted that enrolment in this larger-scale study began in Q4 2022. Among the key study endpoints is freedom from devicerelated serious adverse events during the first six months after implantation, and the pivotal trial is also powered to assess both primary and secondary patency rates.
IN.PACT AV arm had a patency rate of 44.5% in forearm (radiocephalic) AVFs and 39.9% in upper arm (brachiocephalic and brachiobasilic) AVFs at 36 months, while the same rates with PTA were 33.8% and 21.3%, respectively. The patency advantage for DCB was significant for both forearm and upper arm fistulas. The authors also stratified results by lesion type, with a DCB rate of TLPP of 50.6% at 36 months for de novo lesions and 40.5% for restenotic lesions. This was statistically superior to TLPP rates for PTA which were 42.2% and 22.7%.
Lesion location
A further subgroup analysis by lesion location focused on peri-anastomotic, cephalic arch and venous outflow lesions. For peri-anastamotic lesions, IN.PACT AV had a TLPP rate of 40.4% while PTA had a rate of 31.1% at 36 months. For cephalic arch lesions, the DCB arm saw a rate of 40.9% and the PTA arm one of 27.9%, while on venous outflow lesions the former had a rate of 45.6% and the latter of 25.5%. While patency was statistically superior at all lesion locations, the most notable effect was seen in perianastomotic and cephalic arch lesions.
For the first time, the 48-month all-cause mortality results were
presented which again showed no evidence of a safety concern using a paclitaxel-eluting device in the AV access circuit. The mortality at 48 months was actually higher in the PTA arm at 41.8% compared to to the DCB arm where mortality was 34.6% although this did not reach statistical significance.
Summarising the findings, Holden stated that they demonstrated “sustained patency benefit for IN.PACT AV DCB compared to PTA in all subgroup analyses”, and he highlighted restenoses, perianastomotic and cephalic arch lesions in particular. Concluding his talk, he said: “Durable long-term data suggest the use of IN.PACT AV DCB as a standard of care for AVF maintenance in patients with end-stage kidney disease.”
Wednesday 26 April 2023 8 SECOND EDITION #CX2023 Seen First at CX
We always have to be modest, but we are certainly optimistic about this.”
Frans Moll
ClotTriever “makes
fast, safe and efficient thrombectomy possible”
“We need to eliminate symptoms as fast as possible—it is not OK just to make things a little better,” says Rick de Graaf (Friedrichshafen, Germany), setting out why intervention in acute deep vein thrombosis (DVT) is important. De Graaf cites quality of life and economic burden as key factors. “That does not allow us to be too risky,” he continues, commenting that avoiding complications, such as bleeding or recurrent DVT are fundamental to any intervention.
According to De Graaf it does not make sense to use a device that takes out, 50, 60 or 70% of thrombus. He argues that full removal should be the ultimate strategy to optimise clinical outcome.
De Graaf cites ClotTriever (Inari Medical) as one such device that makes fast, safe and efficient thrombectomy possible. Outcomes of the device in a real-world DVT population have been demonstrated through the ongoing CLOUT registry, a 500-patient, prospective, multicentre, all-comer study, taking place at 43 centres across the United States, making it the largest mechanical thrombectomy study in the field of DVT. Results have so far been shown out to 30 days, with follow-up planned out to two years. The trial’s primary endpoint is technical success, defined as complete or near-complete (>75%) removal of thrombus by core lab-assessed Marder score.
“That is spectacularly high when you compare that to other devices and benchmarks,” says David Dexter (Norfolk, United States) an investigator in the study, of the primary endpoint goal. However, he offers the view that investigators saw ClotTriever as likely to hit this target, “because of its purely mechanical nature in
the acute phase and maybe in the subacute arm”.
Results out to 30 days, presented at The VEINS 2022 (30–31 October, Las Vegas, United States), demonstrate that the primary endpoint (>75% thrombus removal), was achieved in 91.2% of patients, while nearly two thirds of patients had 100% of clot removed.
The intermediate number of 90% clot removal was seen in around 70% of patients.

Safety was also assessed, and the study has shown a serious adverse event (SAE) rate of 0.2%.
Added to this, most patients were treated in a single session (99.4%), with minimal blood loss (40ml) and minimal use of thrombolytic therapy (0.4%). From an improvement standpoint, Dexter says, the first goal is restoration of flow. At baseline only 27.6% of patients in CLOUT had flow, but 30 days post-treatment, 85% of patients had some or all flow restored.
For Steven Abramowitz (Washington DC, United States), putting these data in context with previously
published studies is an important next step to guide future treatment decisions, in line with the universal goal to “function in an evidence-based world”.
To achieve this aim, data from CLOUT have been compared to those from the ATTRACT trial, which assessed the use of pharmacomechanical catheterdirected thrombolysis, in a propensity-matched study.
“ATTRACT is one of the fundamental reference trials in the United States, and I think globally, for guiding intervention for DVT,” says Abramowitz. The analysis looked at 153 pairs of similarly matched patients within the two trials based on 11 baseline covariates. Results show better thrombus extraction with the ClotTriever system, with nearly 88% of the CLOUT patients achieving thrombus clearance ≥75%, compared to 66% who received an intervention in ATTRACT. There was also a significant difference in the number of patients with 100% thrombus clearance, totalling 58% in CLOUT, compared to 30% in ATTRACT. The analysis also compared reduction in Villalta Score—an indicator of the severity of post-thrombotic syndrome in lower-extremity DVT— recorded in the two trials, showing a significant decrease at 30 days in CLOUT with 27% of patients with a Villalta Score ≥5 vs. 40% in ATTRACT.

“Propensity matched analysis shows us that contemporary interventional strategies with ClotTriever do stand their ground and have comparable, if not potentially better results than those patients treated with interventional options available at the time of ATTRACT enrolment,” says Abramowitz.



Charing Cross International Symposium Tuesday 25th - Thursday 27th April 2023 | London | UK Treating the full range of DVT chronicity with the INARI ClotTriever® System 30-minute Symposium | Wednesday 26th April | 15:20 - 15:50 | Room: Kensington 2
Introduction to the DEFIANCE Randomised Clinical Trial Dr
Medicine
Arnsberg ClotTriever Study: Retrospective data of iliofemoral DVT patients treated with mechanical thrombectomy
Expanding the treatment window for the effective management of acute DVT
In-hospital and 30-day outcomes from the fully enrolled CLOUT Registry Inari Medical Europe GmbH, Messeplatz 10, 4058 Basel, Switzerland | www.inarimedical.com 9 Advertorial Wednesday 26 April 2023 SECOND EDITION #CX2023
Prof Stephen Black (Moderator) Vascular Surgery (UK)
Michael Lichtenberg Angiology & Vascular
(Germany)
Dr Andrew Wigham Interventional Radiology (UK)
Dr Steven Abramowitz Vascular Surgery (USA)
THIS ADVERTORIAL IS SPONSORED BY INARI MEDICAL
ClotTriever catheter in anatomy



Wednesday 26 April 2023 10 SECOND EDITION #CX2023 Poll results Floor Plan LIFT1 LIFT2 LIFT LIFT4 FT RECEPTIONMAIN LOBBYAND MAIN ENTRANCE MAIN ENTRANCE BOWBAR BOWBAR SEMI-PRIVATE WEST WING LOBBY HOXTONROOM SPEAKER READY ROOM ORGANISERS OFFICE TYBURNMARKET TYBURN BAR TYBURN RESTAURANT HYBRID MEETING ROOMS 1 HYBRID MEETING ROOMS 2 HYBRID MEETING ROOMS 3 KENSINGTON 2 KENSINGTON 1 CATERING CATERING A27 A29 A23 A21 B24 A25 D38 B36 B38 C36 C32 B28 B32 C28 D24 E40 D40 E38 D38 E36 D36 E32 D28 D32 F33 F27 F25 F19 D20 B20 A19 A15 F23 G12 G13 G14 G11 G10 A30 F35 MEZZANINE BALCONY 26 SQM 26 SQM 56 SQM 66 SQM 45 SQM 45 SQM WORKSHOPS & Abstracts ADMIRAL PLENARY 55 SQM 57 SQM
Key: Aortic Venous Acute Stroke Vascular Trauma Best of CX Abstracts
Day 2: Wednesday 26 April
Kensington 1
Abdominal Aortic
Abdominal Aortic Aneurysm (AAA)
Consensus Update
Time: 08:00–09:40
Anchor: Roger Greenhalgh, London, United Kingdom
Moderators: Tilo Kölbel, Hamburg, Germany Robert Morgan, London, United Kingdom
Sponsored Education
Time: 09:40–10:10
Ruptured Abdominal Aortic Aneurysm
(RAAA) Consensus Update
Time: 10:30–11:10
Anchor: Roger Greenhalgh
Moderators: Janet Powell, London, United Kingdom
Tilo Kölbel
Iliac Consensus Update
Time: 11:10–12:10
Anchor: Roger Greenhalgh
Moderators: Tilo Kölbel
Gustavo Oderich, Houston, United States
Juxtarenal Aortic
Juxtarenal Aortic Consensus Update
Time: 12:10–12:30
Anchor: Andrew Holden, Auckland, New Zealand
Moderators: Gustavo Oderich
Roberto Chiesa, Milan, Italy
Sponsored Education
Time: 12:30–13:00
Juxtarenal Aortic Consensus Update
Continued
Time: 13:30–15:20
Anchor: Roger Greenhalgh
Moderators: Tilo Kölbel, Robert Morgan
Sponsored Education
Time: 15:20–15:50
Juxtarenal Aortic Consensus Update
Continued
Time: 16:10–16:40
Anchor: Andrew Holden
Moderators: Gustavo Oderich, Roberto Chiesa
Kensington 2
Admiral
Superficial Venous & Lymphatic
Superficial Venous & Lymphatic
Consensus Update
Time: 08:00–09:40
Anchor: Erin Murphy, Charlotte, United States
Moderators: Stephen Black, London, United Kingdom
Manj Gohel, Cambridge, United Kingdom
Sponsored Education
Time: 09:40–10:10
Superficial Venous & Lymphatic
Consensus Update Continued
Time: 10:30–12:30
Anchor:Erin Murphy
Moderators: Stephen Black Manj Gohel
Sponsored Education
Time: 12:30–13:00
Deep Venous
Deep Venous Consensus Update
Time: 13:30–15:20
Anchor: Erin Murphy
Moderators: Armando Mansilha, Porto, Portugal Manj Gohel
Sponsored Education
Time: 15:20–15:50
Deep Venous Consensus Update
Continued
Time: 16:10–18:00
Anchor: Erin Murphy
Moderators: Armando Mansilha Manj Gohel
Acute Stroke
Acute Stroke Consensus Update
Time: 08:00–08:30
Anchor: Maarit Venermo, Helsinki, Finland
Moderator: Alexander Zimmermann, Zurich, Switzerland
Acute Stroke Consensus Update Continued
Time: 08:30–10:10
Anchor: Maarit Venermo
Moderator: Domenico Valenti, London, United Kingdom
Acute Stroke: Best of Abstracts
Time: 10:30–11:00
Anchor: Domenico Valenti
Vascular Trauma
Vascular Trauma Consensus Update
Time: 11:00–13:00
Anchor: Ross Davenport, London, United Kingdom
Moderator: Christopher Aylwin, London, United Kingdom
Best of CX Abstracts
Sponsored Education
Time: 13:30–14:00
Best of Aortic Abstracts
Time: 14:00–15:50
Anchor: Afshin Assadian, Vienna, Austria
Moderator: Jan Brunkwall, Cologne, Germany
Best of Aortic Abstracts Continued
Time: 16:10–18:00
Anchor: Afshin Assadian, Vienna, Austria
Moderator: Fiona Rohlffs, Hamburg, Germany
Juxtarenal Aortic Consensus Update: Bridging Stents
Time: 16:40–17:30
Anchor: Andrew Holden
Moderators: Gustavo Oderich, Roberto Chiesa
Radiation Consensus Update
Time: 17:30–18:00
Anchor: Roger Greenhalgh
Moderators: Roberto Chiesa, Tilo Kölbel
11 Wednesday 26 April 2023 SECOND EDITION #CX2023 Programme
All times are London time BST (British Summer Time),
which is GMT+1. The organisers reserve the right to alter timings if necessary.
PROGRAMME AT A GLANCE
Trial updates offer insights on sirolimus in CLTI patients
The peripheral arterial and chronic limbthreatening ischaemia (CLTI) session yesterday afternoon provided delegates with a consensus update and insight into potential advances in this area. The sirolimus drugcoated device section of the session provided updates on three belowthe-knee (BTK) studies, as well as insight into a real-world experience of sirolimus-coated balloon versus conventional balloon angioplasty for CLTI.
Attendees also responded in real time to the statement ‘sirolimus is the answer’—the poll results showed that a plurality of audience members who answered the poll agreed with the statement. These delegates in favour of the motion comprised 56% of respondents, whereas 44% of respondents believed sirolimus was not the answer.
Peter Gaines (Sheffield, United Kingdom) opened the session, offering attendees an update on the SELUTION4BTK trial of a sirolimus drug-coated balloon (DCB) in BTK arteries.

Following Gaines came Edward Choke (Bukit Merah, Singapore), who spoke about his centre’s experience comparing sirolimus DCB and conventional balloon angioplasty in patients with CLTI.
Ramon Varcoe (Sydney, Australia) took over from Gaines at the podium, to present the 12-month results from the SWING BTK multicentre study, evaluating a sirolimus DCB.
Rounding off this section of the afternoon’s programme was Marianne Brodmann (Graz, Austria), with an update on the RESOLV study, which is evaluating a bioabsorbable sirolimus-coated scaffold for BTK treatment.
Latin America comes to CX 2023

LEADERS IN VASCULAR disease from Argentina, Brazil and further afield came together for the inaugural CX Meets Latin America session, taking place on Tuesday morning in Mezzanine 10. Session chair Bruno Freitas (Leipzig, Germany) was joined by an international panel of moderators including Luiz Furuya (Sao Paulo, Brazil), Frans Moll (Utrecht, Netherlands) and Sophie Renton (London, United Kingdom), who oversaw a vibrant session taking in the latest perspectives from Latin America on topics spanning aortic and peripheral procedures.
Freitas opened the session with a presentation covering complex multilevel chronic limb-threatening ischaemia, in which he opened the toolbox to offer technical tips to overcome challenging scenarios. Results of an in-situ fenestration technique for preservation of the left subclavian artery in the endovascular repair of acute aortic syndromes were delivered by Andre Pinheiro (Bahia, Brazil), followed by a case report from Tony Furuie (Sao Paulo, Brazil) concerning ipsilateral iliac branch repair
Aortic device conformability: Adapting to the anatomy for better clinical outcomes
During one of yesterday’s satellite symposia at CX, speakers addressed the “key factor” of conformability in the endovascular repair of multiple vascular pathologies.
First to address the audience, Michele Antonello (Padua, Italy) argued that true conformability can reduce the risk of distal sine in the endovascular repair of type B aortic dissection (TBAD). Speaking to CX Daily News ahead of CX, Antonello remarked that conformability is a “key factor” in device selection, and especially so in relation to TBAD as the aorta is more “fragile” in this pathology. “Theoretically,” the presenter explained, “the less you try to modify the anatomy of the aorta, the less the endograft will shock the adventitia.”
Antonello explained that the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System “keeps the curvature of the arch of the aorta” and stays in the position to which it is moved. This has the effect of reducing the possibility of trauma to the aortic wall, Antonello noted. In addition, Antonello detailed that there is a theoretically lower risk of a new dissection created by the TAG Conformable Device than with a standard device.
Data from the GREAT Registry of a real-world population have shown that the TAG Conformable Device has a low distal sine rate of 4% in chronic TBAD aortic dissection patients, Antonello communicated with the audience at CX. The results from this study are “particularly good,” he shared with this newspaper, citing low incidence of three events: neurological complications, new endoleaks and new dissections created by the endograft. “I think [the TAG
Conformable Device] is the only endograft that has such robust data coming from a real-life registry.” Later in the symposium, Jan Heyligers (Tilburg, Netherlands) addressed the question ‘Does conformability have an impact on the outcomes of endovascular repair of iliac limb performance?’ In an interview with CX Daily News, Heyligers remarked that conformability is important in treating iliac pathology since the flow and curvature of the iliacs are challenging. “We are aiming to have a device that is conformable to the anatomy.”
According to Heyligers, there are differences in conformability in the iliac branch devices (IBDs) available on the market. “There are some devices that are stiffer than others,” he explained, noting that the GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE) seems to be the most conformable to the actual anatomy, based on his clinical experience with the device.
Heyligers detailed that he and his team have researched the Gore endograft in tortuous anatomy. He shared, for example, that in one study they implanted 17 IBEs and conducted electrocardiography (ECG)-gated computed tomography (CT) analysis preand postoperatively. “We have learned
using a looped wire—what he described as a precannulated gate technique.
Later in the session, Mariano Castelli (Buenos Aires, Argentina) spoke on predictors of type IIIa endoleak after endovascular aneurysm repair (EVAR) with an anatomic fixation endograft, after which Luiz Furuya (Sao Paulo, Brazil) delivered a presentation on a bridged stent for fenestrated EVAR (FEVAR) and branched EVAR (BEVAR), addressing the question ‘Is there really a difference between them?’ Castelli delivered the penultimate presentation, which focused on FEVAR with a physician-modified endograft after previous failed EVAR in an emergent setting. Finally, Freitas considered current evidence and technical insights on intramural haematoma and penetrating aortic ulcer. All presentations were punctuated with audience participation and discussion, and the session closed with a roundtable
that after implantation of an IBE there is a significant increase in motion,” he reported.


“In general, the more straightforward your anatomy is, the more probable it is that your device will endure,” Heyligers remarked. Then again, he noted that the more adapted the system is to the anatomy, the more likely it is to translate into good outcomes, irrespective of the anatomy. The IBE is a “proven solution” in this regard, he said in summary.
Giovanni Pratesi (Genoa, Italy) also spoke during the session, sharing his insights on the impact of conformability on the outcomes of endovascular repair of abdominal aortic aneurysm (AAA) with angulated infrarenal necks.
In endovascular aneurysm repair (EVAR), Pratesi told CX Daily News, conformability is “intrinsic” to how a stent graft adapts to a patient’s anatomy and can improve procedural outcomes.

During the symposium, Pratesi discussed three-year follow-up data from the European multicentre EXCeL registry on the GORE® EXCLUDER® Conformable AAA Endoprosthesis with ACTIVE CONTROL System, noting how it adds to the long-term evidence from numerous trials and registries worldwide that support the efficacy of all Gore conformable devices.

Postoperative angio-CT showing true lumen expansion and optimal visceral perfusion after thoracic endovascular aortic repair with the GORE TAG.
The indication to treat challenging anatomies within the Instructions for Use (IFU) is “undoubtedly” an advantage of the GORE EXCLUDER Conformable Device, Pratesi remarked. In particular, he explained, the device allows for the treatment of short as well as severely angulated necks, offering the capability to treat anatomy previously considered unsuitable for standard EVAR.

Wednesday 26 April 2023 12 SECOND EDITION #CX2023 CX Round-up
THIS ADVERTORIAL IS SPONSORED BY GORE
Image courtesy of Michele Antonello
Bruno Freitas


















Renal Interventions Consensus
Thrombosis focus and dialysis discussion at first CX renal interventions session
Yesterday’s renal interventions afternoon session yielded a rich vein of education and audience participation on everything from stent positioning to interventional training. With speakers including Matthew Gibson (Reading, United Kingdom) and Robert Shahverdyan (Hamburg, Germany), its talks provided a wide snapshot of the renal space.
SHAHVERDYAN OPENED THE SESSION BY presenting on a new endovascular arteriovenous fistula (endoAVF) device, the Velocity system (Venova Medical). Setting out a few of the challenges in dialysis access, he said that endoAVFs can pose particular challenges—including the fact that “not all patients have appropriate anatomy” and, centrally, the risks of juxta-anastomotic stenoses, partial deep venous flow and associated cannulation difficulties. The Velocity system, Shahverdyan averred, is the only endoAVF system “that would replicate surgical AVF (sAVF) anatomy”, offering as it does an end-to-side connection from the proximal radial artery to the perforator vein “without losing any flow into the deep venous system”. Citing the first-in-human VENOS trial results on the technology, he said that early use of the system “demonstrates clear proof” of its ability to improve vascular access care.
One early presentation by Matthew Gibson (Reading, United Kingdom) set out “a real-world
approach” to managing thrombosed haemodialysis access. Clotted access is usually preventable, Gibson said, and can be due to a “failure”. This failure could be the surgical or radiological formation technique, monitoring needling technique or homeostasis (dehydration/hypotension), Gibson added. He also established the particular problems raised by clotted access. Compared to a preventative fistulopasty, he said, a declotting procedure results in a worse patient experience, worse success and complications rates, and was more time- and resourceconsuming. It also potentially poses the need for temporary access if delayed or unsuccessful.
But, he asked, what should you do when presented with access thrombosis? First, he urged listeners to “act quickly and effectively” to remove a thrombus

PAVE 2 trial to meet need for improved evidence on drug-coated balloons
Speaking at yesterday’s CX vascular access masterclass session, Narayan Karunanithy (London, United Kingdom) set out “the need for more evidence” as part of a presentation on the upcoming, National Institute for Health and Care Research (NIHR)-funded, investigator-initiated PAVE 2 trial providing a comparison between multiple drug-coated balloons (DCBs) and plain-balloon angioplasty for the treatment of dysfunctional arteriovenous fistulas (AVFs) in dialysis patients.
THE VASCULAR ACCESS
programme—directed by CX executive board members Nicholas Inston (Birmingham, United Kingdom) and Kate Steiner (Stevenage, United Kingdom)—also hosted a number of workshops intended to provide delegates with hands-on education, and a chance to hone their practical skills while getting acquainted with many novel technologies. Demonstrators including Gavin Corrigan (Dublin, Ireland), John McCafferty and Daniela Romero (both Edinburgh, United Kingdom) showcased the Ellipsys (Medtronic)
and WavelinQ (BD) endovascular AVF (endoAVF) systems along with several other innovations.
In his presentation, Karunanithy noted that, while plain-balloon fistuloplasty continues to be the “mainstay” in dysfunctional AVF treatments, its benefits may only be short-term— with reported six-month primary patency rates of 60–70% decreasing to 40–50% at 12 months. Reviewing the published literature on paclitaxel-coated balloon use, the speaker reflected that discrepancies between the findings of multiple studies in this space may have
and to treat the culprit lesion. Though there are a range of techniques available, there are no randomised controlled trials to guide choice of technique. While there have been meta-analyses performed, Gibson argued that there is “no clear winner” on the issue. This is where real-world considerations could help to expedite a decision.
In the real world, he said, clinical decision-making during the COVID-19 pandemic changed to favour methods that were both the least invasive and most likely to be successful—as well as less resourceintensive. He noted that this had led to a fall in declotting procedures, especially surgical declotting, per a report on access management during the pandemic published in the Journal of Vascular Access by Christopher Seet (London, United Kingdom) et al in 2021. Gibson’s concluding message was to “treat [thrombosis] quickly and effectively, but be realistic and think of the future”. He added that technique is “less important than availability, skill and enthusiasm”.
Also presenting was Georgia Georgopoulou (Patras, Greece), who detailed the Nephrology Partnership for Advancing Technology in Healthcare (N-PATH) interventional nephrology training programme. She described it as “the first European advanced training course in diagnostic and interventional nephrology”, explaining that it comprises component modules in molecular pathology, vascular access, medical ultrasound and peritoneal dialysis, each with handson training sessions in cities across Europe including Milan and Prague, while also including online educational resources.
been caused by differences in the choice of balloon device and drug dosage; ethnicity of participants; and proportion of prior-revascularisation patients, in each.
Next, Karunanithy discussed emerging evidence evaluating the role of sirolimus-coated balloons, such as the MATILDA study of the MagicTouch (Concept Medical) device, which has reported target-lesion primary patency (TLPP) rates of 83% at six months and 58% at 12 months, and the ISABELLA study of the Selution DCB (MedAlliance), which found TLPP rates of 72% at six months and 44% at 12 months. Further evidence on the MagicTouch balloon is awaited from the ongoing IMPRESSION randomised controlled trial, he added.
Finally, the speaker announced plans for the upcoming PAVE 2 trial—the primary objective of which will be to evaluate and compare the efficacy of the paclitaxel-based IN.PACT AV DCB (Medtronic), the aforementioned MagicTouch DCB, and plainballoon angioplasty treatments.

According to Karunanithy, this three-arm study aims to recruit 642 patients across approximately 20 high-volume haemodialysis centres in the United Kingdom, and is scheduled to commence recruitment in Autumn 2023.

Karunanithy’s presentation was
preceded by one from Ounali Jaffer (London, United Kingdom), who took the opposing stance of querying: Is there “too much evidence” on DCBs? He called into question the impact patient factors may have had in contrasting trial results to date; emphasised the difference between “efficacy” and “costeffectiveness”, and the need for greater uniformity of trial designs; and noted that artificial intelligence may have a key role to play if global database analyses are embraced in the future.
Audience polling during the vascular access masterclass revealed differences of opinion on another hot topic in dialysis access: endoAVFs. Following presentations on this subject from Alexandros Mallios (Paris, France) and Tobias Steinke (Düsseldorf, Germany), among others, 59% of attendees voted against the motion that “endovascular AVFs are the way forward”, while 41% voted in favour. The topic of endoAVFs was also the focus of an edited case delivered by Robert Shahverdyan (Hamburg, Germany) later in the
Wednesday 26 April 2023 14 SECOND EDITION #CX2023 Renal Interventions
Consensus
Vascular Access
Narayan Karunanithy
Robert Shahverdyan

Wednesday 26 April: Satellite symposia
Kensington 2 — 09:40–10:10
Decision-making in superficial venous disease treatment: The roles of data, device, and patient voice
09:40–09:45
Introduction
Speaker: Manj Gohel, Cambridge, United Kingdom
09:45–09:55
How is the VenaSeal Spectrum programme designed?
Speaker: Kathleen Gibson, Bellevue, United States
09:55–10:05
How is patient voice measured in the VenaSeal Spectrum programme?
Speaker: Manj Gohel, Cambridge, United Kingdom
10:05–10:10
Discussion & questions
Speaker: Manj Gohel, Cambridge, United Kingdom
Admiral — 13:30–14:00

Preserve the LSA: A simple and safe solution with Castor



13:30–13:42
Castor benefits, implantation and applicability
Speaker: Christoph Nienaber, London, United Kingdom
13:42–13:54
The University Hospital of Zurich clinical experience with Castor
Speaker: Alexander Zimmermann, Zurich, Switzerland
13:54–14:00
Q&A
Kensington 1 — 12:30–13:00
EnChEVAR: Elevating ChEVAR
therapy
12:30–12:35
What we mean with EnChevar and why it is different from what we know
Speaker: Stefano Fazzini, Rome, Italy
12:35–12:40
Audience participation & discussion
12:40–12:45
Juxtarenal aneurysms can be successfully treated with EnChevar
Speaker: Theodoros Kratimenos, Athens, Greece
12:45–12:50
Audience Participation & Discussion
12:50–12:55
Proper treatment indications and procedural planning are crucial to succeed with EnChevar
Speaker: Philipp Geisbusch, Stuttgart, Germany
12:55–13:00
Audience participation & discussion
Poll results

























Kensington 1 — 15:20–15:50
When to consider inner branches in your treatment algorithm for the visceral aorta
15:20–15:24
Introduction
Speaker: Eric Verhoeven, Nuremberg, Germany
15:24–15:32
Customising the device to patient´s anatomy - Patient selection
Speaker: Nuno Dias, Lund, Sweden
15:32–15:40
Customising the device to patient´s anatomy - Case presentations

Speaker: Fiona Rohlffs, Hamburg, Germany
15:40–15:50
Q&A from the audience
Kensington 1 — 09:40–10:10
The importance of staying on label with off-the-shelf devices
09:40–09:45


Confidence to address the entire aortic arch whilst being on label
Speaker: Bartosz Rylski, Freiburg, Germany
09:45–09:50
Clinical reassurance that Treo provides

Speaker: David Murray, Manchester, United Kingdom
09:50–09:55
Gold standard Thoraflex Hybrid continues to serve global clinical community






















Speaker: Aung Ye Oo, London, United Kingdom
09:55–10:10
Audience participation & panel discussion
Kensington 2 — 15:20–15:50
Treating the full range of DVT chronicity with the Inari ClotTriever system
15:20–15:21
Welcome
Speaker: Stephen Black, London, United Kingdom
15:21–15:28
Expanding the treatment window for the effective management of acute DVT

Speaker: Andrew Wigham, Oxford, United Kingdom
15:35–15:42
Arnsberg ClotTriever study: Retrospective data of iliofemoral DVT patients treated with mechanical thrombectomy











Speaker: Michael Lichtenberg, Arnsberg, Germany
15:42–15:47
Audience participation & discussion / Q&A
15:47–15:50
Closing
Speaker: Stephen Black, London, United Kingdom
16 #CX2023
Sponsored Education
SATELLITE PREVIEW All times are London time BST (British Summer Time), which is GMT+1. The organisers reserve the right to alter timings if necessary.

























































































































































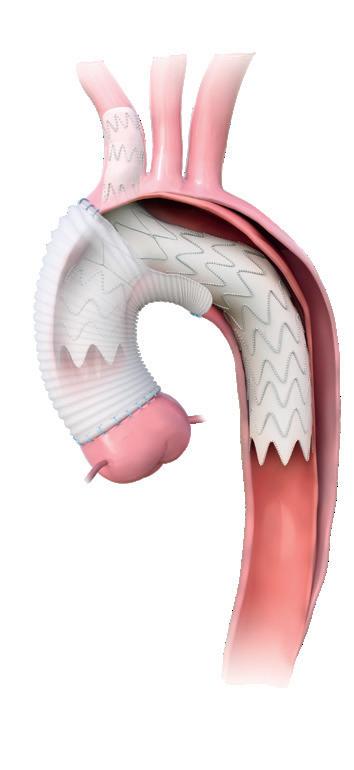



Aortic Arch Solutions AAA Solutions TAAA Solutions All trademarks are owned by Artivion, Inc. or its subsidiaries. Note: All products and indications are not available/approved NEXUS® is manufactured by Endospan Ltd. NEXUS® product illustration is shown in combination with surgical graft. Global Headquarters: 1655 Roberts Blvd., NW, Kennesaw, GA 30144 USA Telephone: +1.888.427.9654 Domestic Fax: +770.590.3753 For information on additional Artivion locations, please visit www.artivion.com/contact Trusted together E-liac™ Stent Graft System Artivion, CryoLife, and Jotec are trademarks owned by Artivion, Inc. or its subsidiaries. Note: All products and indications are not available/approved in all markets. © 2023 Artivion, Inc. All rights reserved. JR-CROL-0930200-EN V01 01/2023. NEXUS® Aortic Arch Stent Graft System E-vita® Open Neo Hybrid Stent Graft System AMDS Hybrid Prosthesis E-xtra Design Multibranch Stent Graft System E-tegra Stent Graft System E-liac Stent Graft System E-nside TAAA Multibranch Stent Graft System TM TM TM TM TM
When the Need is Aortic, the Solution is Artivion
Edited case showcases new technique for aortic arch lesions
The emerging WeFlow modular inner branch stent graft system is suitable for the aortic arch and partial ascending lesions, yesterday’s aortic techniques and technologies session heard.

Wei Guo (Beijing, China) opened the session, reporting on the ongoing, prospective, multicentre GIANT study, evaluating the WeFlow device for the treatment of patients with aortic arch disease. Guo presented an edited case, informing CX attendees that further clinical data are expected to confirm its safety and efficacy.
“Segmental release [of the system] does not affect the cerebral blood flow,” Guo said in his presentation. The system has “perfect stability” and “simple localisation and release.” It has an adjustable delivery system, “an easy bridging technique, no gutter endoleak, and good inner branch morphology,” he added.
TERUMO AORTIC SYMPOSIUM
The Importance Of Staying On Label With Off-the Shelf Devices
Wednesday 26th April 2023 • 09:40 - 10:10 • Kensington 1
Moderator: Hence Verhagen, Netherlands
Confidence to Address the Entire Aortic Arch Whilst Being on Label
Bartosz Rylski, Germany
Clinical Reassurance that TREO Provides
David Murray, United Kingdom
Gold Standard Thoraflex Hybrid Continues to Serve Global Clinical Community

Aung Oo, United Kingdom
Please visit us at booth 28 & 30 to discuss our wide range of Surgical, Endovascular and Hybrid solutions.
Discover Aortic Solutions
terumoarotic.com/products

Wednesday 26 April 2023 18 SECOND EDITION #CX2023 Aortic Techniques & Technologies
Poll results
Peripheral arterial consensus Potential advances consensus
Atherectomy is overused? The CX community has its say on atherectomy in peripheral arterial treatment.
27
Technical advance is the future? Attendees vote on whether technique development holds the key to staving off adverse outcomes.
Agree % Disagree % 88 12
Sirolimus is the answer? CX asks the audience to chime in on the role of sirolimus-coated devices in CLTI treatment.
Agree % Disagree % 56 44
73
Agree % Disagree %
Randomised control trial consensus
Vein bypass beats endovascular? The audience reacts in the wake of the landmark BASIL-2 trial results.
54 46
Agree % Disagree %
Bioresorbable wins? Attendees ponder the key to tackling PAD both above and below the knee.
69% 31% Agree Disagree Vascular access consensus
Agree % Disagree %
59
59 Endovascular AVF are the way forward?
41
41
19 Wednesday 26 April 2023 SECOND EDITION #CX2023
POLL
RESULTS

EXCLUDER™*/EXCLUDER™* Conformable AAA Endoprosthesis
is designed to empower your decisions — advancing sac regression evidence to improve patient outcomes. ADVANCE
EndurAnt stent graft system versus ExcluDer endoprosthesis — a global, prospectiVe, rANdomized Clinical trial in sac rEgression UC202313018 IE ©2023 Medtronic. All rights reserved. Medtronic and the Medtronic logo are trademarks of Medtronic. ™*Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. 03/2023 Endurant™ II/IIs AAA Stent Graft System Scan QR code to find out more about this trial
You’re invested. So are we. Strength in evidence ADVANCE
Trial
















































































































































































































