Featured in this issue:
WEB 17 device maintains efficacy, safety across ruptured and unruptured aneurysms

The Woven EndoBridge (WEB) 17 device (Microvention/Terumo Corporation) has demonstrated positive efficacy and safety outcomes in the prospective, multicentre CLEVER study, indicating its utility for intrasaccular embolisation across ruptured and unruptured intracranial aneurysms. Twelve-month results from the study were delivered by Laurent Spelle (Bicêtre Hospital, Paris, France) at the LINNC Americas Seminar (16–17 March, Miami, USA).
The CLEVER study was set up to assess whether—despite its lower profile in comparison to prior device iterations— the newer-generation WEB 17 system maintains efficacy and safety in the treatment of bifurcation aneurysms, Spelle reported. It was conducted at 17 clinical sites spanning Finland, France, Germany, Hungary and the UK, with Spelle being one of three trial coordinators alongside Christophe Cognard (Toulouse University Hospital, Toulouse, France) and István Szikora (National Institute of Mental Health, Neurology and Neurosurgery, Budapest, Hungary).

A total of 163 patients (mean age 58.1 years, 68% female) including 103 with unruptured aneurysms and 60 with ruptured aneurysms, none of which had previously been treated, were enrolled from June 2019 to February 2021. CLEVER’s primary safety endpoint was death of non-accidental cause/any major stroke ≤30 days after treatment, or death/major ipsilateral stroke of neurologic cause from day 31 to one year after treatment. Its primary efficacy endpoint—evaluated at James
Measuring success in stroke thrombectomy
David S Liebeskind page 6
www.neuronewsinternational.com
J Mocco page 16 New trial seeks “thorough understanding” of VNS response in depression page 25

Byrne’s (University of Oxford, Oxford, UK) core lab—was adequate occlusion without retreatment at one year. Outlining the study results, Spelle highlighted that there were no treatment failures with WEB 17 across all 163 aneurysms, while adjunctive devices—primarily balloons—were required in just nine unruptured (8.7%) and seven ruptured (11.7%) cases. Based on clinical event adjudication, CLEVER met its key safety endpoint, with three patients (1.8%) suffering a major stroke and zero deaths due to a neurologic cause occurring within one year post-treatment. Touching on the study’s secondary safety endpoints, Spelle also noted a 2% rate of morbidity (defined as modified Rankin scale [mRS] >2) and a 0.6% overall mortality rate at 12 months, as well as one perioperative bleeding event and no rebleeding in patients with ruptured aneurysms, and one haemorrhagic complication in patients with unruptured aneurysms. He further detailed that none of the haemorrhagic complications seen within one year had any clinical consequences, and that no WEB-related morbidity or mortality was observed.
Stryker completes acquisition of Cerus Endovascular
STRYKER HAS ANNOUNCED the completed acquisition of Cerus Endovascular—a medical device company engaged in the design and development of neurointerventional devices for the treatment of intracranial aneurysms.
In an announcement released on 2 May confirming this acquisition, Stryker claimed that technology for the endovascular treatment of aneurysms has been “foundational” to the company’s neurovascular division, and that Cerus’ portfolio will “address the growing need for one-and-done intrasaccular aneurysm therapy”.
Moving on to discuss the study’s primary efficacy endpoint— ultimately assessed in 146 patients—Spelle reported an 82.2% rate of target aneurysm adequate occlusion (complete occlusion or neck remnant) without retreatment at one year. Regarding secondary efficacy outcomes, he stated that four of 152 patients (2.6%) required retreatments at 12 months, and pointed to complete occlusion (62.9%) and neck remnant (21%) rates on the three-grade Raymond-Roy occlusion classification (RROC) scale, resulting in an 83.9% adequate occlusion rate when allowing for retreated aneurysms.
Finally, the speaker relayed 73.1% complete occlusion and 86.6% adequate occlusion rates as per RROC, and one retreatment, in the study’s ruptured aneurysm subgroup. Spelle then drew comparisons between CLEVER and the CLARYS study, which assessed the original, larger WEB device in ruptured aneurysms. He concluded that, “along its evolution, the WEB device remains safe and efficient” in such cases, citing similar maximum aneurysm sac widths in CLARYS and CLEVER’s ruptured subgroup, as well as lower rates of morbidity and mortality observed in the latter patient cohort.
Cerus’ CE-marked products include the intrasaccular Contour neurovascular system and the adjunctive Neqstent coil-assisted flow diverter, both of which gained European clearance for the treatment of intracranial aneurysm in 2020.
The company’s Contour device also received a green light from the US Food and Drug administration (FDA) in 2021—initially in the form of a Breakthrough Device designation in February, and subsequently via an investigational device exemption (IDE) study approval in April.
As per a press release, acquisition of these products will help to expand Stryker’s current haemorrhagic stroke portfolio, which currently includes the Target family of detachable embolisation coils; Surpass Streamline and Surpass Evolve flow diverters; and Neuroform Atlas stent system.

ADEQUATE OCCLUSION RATE (WITH RETREATMENT) RATE OF MAJOR STROKE 83.9% 1.8% { {
Laurent Spelle
June 2023 |
Profile
50
TOWELCOME THE OF5OTHEDITION NEURONEWS MR CLEAN-LATE results published in The Lancet page 5
Fifty issues and counting: Neurointerventional progress shows no sign of slowing
It is my pleasure to welcome readers to the 50th edition of NeuroNews—a hugely gratifying milestone, particularly given the projected ‘death’ of the print industry and the pandemic’s impact on traditional news production. Since our very first issue in September 2010, the neurointerventional space has proliferated in so many ways.
Acute stroke thrombectomy is perhaps the most obvious example. Three negative stroke trials published in 2013 cast doubt on its role for large vessel occlusions (LVOs). However, subsequent publication of the ‘big five’ trials in 2015 proved the positive outcomes achievable in ischaemic stroke.
Thrombectomy continues to expand with iterative advancements in our knowledge, acceptance, and expansion of indications. Its full potential has likely not yet been realised and, as such, ongoing research exploring thrombectomy in more specific patient populations maintains great intrigue and anticipation. Particular efforts to standardise certification of thrombectomy-capable and comprehensive stroke centres globally have set us on a path to optimising how acute stroke patients are transported and triaged— another area of critical importance moving forward.
Technological advancements in the treatment of cerebral aneurysms have also progressed rapidly over the past decade. Many next-generation endovascular devices—both primary and adjunctive—have been developed. Intrasaccular occlusion devices and flow diverters are among notable EU and US approvals here. Now, surface modification, and possibly even reabsorbable technologies, are set to open even more possibilities for the pharmacological management of patients—in part, to limit the risk of haemorrhage.

Augmented by detachable-tip, low-profile microcatheters; even finer guidewires; and miniaturised balloon-occlusion catheters, liquid embolic agents have become more effective and easier to use than ever before, offering hope for improved outcomes in brain arteriovenous malformations (AVMs) and dural arteriovenous fistulas. Even innovations considered almost ‘space-age’ not too long ago like endovascular robotic systems and artificial intelligence (AI) solutions are now being applied to neurointerventional care. With innovations in robotics, there will be the need

to develop entirely new lines of devices to fulfil the promise of access, safety, and remote expertise.
NeuroNews has kept pace with all these topics and the many other advancements made over the past decade, not only across 49 preceding print issues, but also daily via its website and social media channels. The staff at BIBA Medical have done a marvellous job staying abreast of the latest developments—no small feat given the enormous bandwidth of these disciplines and the pace of discovery.
2010
WEB device (Sequent Medical)
2011
Pipeline flow diverter (Medtronic)
US FDA approval
Max reperfusion catheter system (Penumbra)


FDA approval
EU CE marking
2012
Solitaire (Covidien) and Trevo (Stryker) stentrievers
FDA approval
2014
PHIL liquid embolic (Microvention) joins Onyx (Medtronic) and Squid (Balt)
EU approval
As recent history tells us, it is rarely possible to accurately predict the future. When NeuroNews launched 13 years ago, the outlook for stroke thrombectomy was far more uncertain than it is today. However, as mentioned, we seem to have only scratched the surface in terms of what robotics and AI could do to optimise patient outcomes before, during, and even, after stroke treatments.
Sofia catheters (Microvention)

CE marking
Contour device (Cerus Endovascular) CE marking
Medical Specialties (ABMS) approved its Recognition of Focused Practice (RFP) designation for neurointerventionists, recognising the value that physicians who focus most or all their practice within a specific specialty can provide to improving healthcare. For neurointervention, crossing the existing disciplines of neurology, neurosurgery, and neuroradiology, creates synergy to minimally invasive, image-guided therapies. This and similar efforts to standardise training among disciplines will be important for future growth, and maintenance of quality in education and care delivery.
Surpass Streamline flow diverter (Stryker)
FDA approval
As we attempt to improve our understanding of the techniques and devices used in neurointerventional care, comparative effectiveness analyses will have to play an increasingly central role in the future of our speciality. Maintaining relevance and reducing cost cannot be taken for granted. Having established that endovascular therapies can provide benefits in ischaemic stroke, cerebral aneurysms, AVMs, venous diseases, and neoplasia, among others, we now need to optimise them further, and build a clearer picture of specific case types and patient populations in which they are most effective.
In March 2017, the American Board of
EmboTrap II stentriever (Cerenovus) US and EU approval

2019
Siemens Healthineers acquires Corindus’ CorPath
GRX robotic system
P64 flow diverter with hydrophilic coating (Phenox)
CE marking
Stentrode BCI (Synchron)
US breakthrough designation
Viz LVO stroke platform (Viz.ai)
CE marking
Genetics and proteomics, including identification of ischaemic stroke biomarkers that may help prevent the disease prior to clinical manifestation, are another promising endeavour (turn to page 18 for more on this). Progress in the management of subdural haematoma with middle meningeal artery embolisation; communicating hydrocephalus with novel cerebrospinal fluid drainage systems; and neoplasia with intraarterial chemotherapy and targeted drug delivery, will also create growth and development for years to come. Ultimately, the future will host tremendous growth and diversification in minimally invasive procedures and other techniques used to treat neurological diseases. This will be reflected accordingly in NeuroNews—not only with comprehensive, continued coverage of all developments relevant to neurointerventionists, but also through increased focus on neuromodulation, carotid stenosis interventions, and the wider field of stroke medicine.
The timeline (left) features select industry milestones through the period and is not a comprehensive list of device approvals.
Comaneci device (Rapid Medical)

FDA breakthrough designation
PHILIP M MEYERS is a neurointerventionist, endovascular neurosurgeon, and diagnostic and interventional neuroradiologist, based in the USA. He is also former president of the Society of NeuroInterventional Surgery (SNIS).

Editor-in-chief: Prof Philip M Meyers | Publisher: Roger Greenhalgh | Content director: Urmila Kerslake
Editor: Jamie Bell jamie@bibamedical.com | Editorial contribution: Bryan Kay
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: Michael Broughton michael@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
If you have comments on this issue or suggestions for upcoming editions write to jamie@bibamedical.com
2 June 2023 | Issue50 EDITORS LETTER 2015 2017 2018
2020 2022 2021
NeuroNews linkedin.com/company/neuronews/ @NN_publishing
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd | BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
by: Buxton Press Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2023. All rights reserved.
Printed
EDITOR-IN-CHIEF’S LETTER

New WIST guidelines seek to improve access and standardise training in stroke thrombectomy
A new set of guidelines intended to standardise and expand stroke thrombectomy training have been published simultaneously in Europe, in the journal Advances in Interventional Cardiology, and in the USA, in Cardiovascular Revascularization Medicine
THE WORLD FEDERATION FOR Interventional Stroke Treatment (WIST) guidelines for multispecialty endovascular stroke intervention are intended to help develop specific training modules across a wide range of scenarios, including complications, and to facilitate standardised training. A primary goal of the guidelines is to improve patient access to thrombectomy by expanding the range of specialties that can perform these procedures beyond interventional neuroradiologists (INRs) to include clinicians from varying, relevant backgrounds.
A recent University of Dundee (Dundee, UK) press release notes that one of the major constraints to expanding thrombectomy services is the inadequate number of INRs able to perform them.
“When I first experienced the power of endovascular stroke treatment, now nearly 20 years ago, I realised this treatment was here to stay,” said Iris Grunwald (University of Dundee, Dundee, UK), lead author of the guidelines and vice president of WIST.
“On the one hand, we needed more interventionists to cover a worldwide demand; on the other hand, it would not be feasible to ‘produce’ thousands of extra INRs. The number of other neurointerventional cases is just too small. That is when I started advocating cross-specialty training. The manpower of cardiologists and radiologists is much greater, and most hospitals already have angiographic services that would allow incorporating endovascular stroke treatment without much additional infrastructure.”
The new WIST guidelines focus on competency— rather than time-based training—promoting the use of high-fidelity simulation training and unique, perfused cadaveric models to help clinicians acquire both
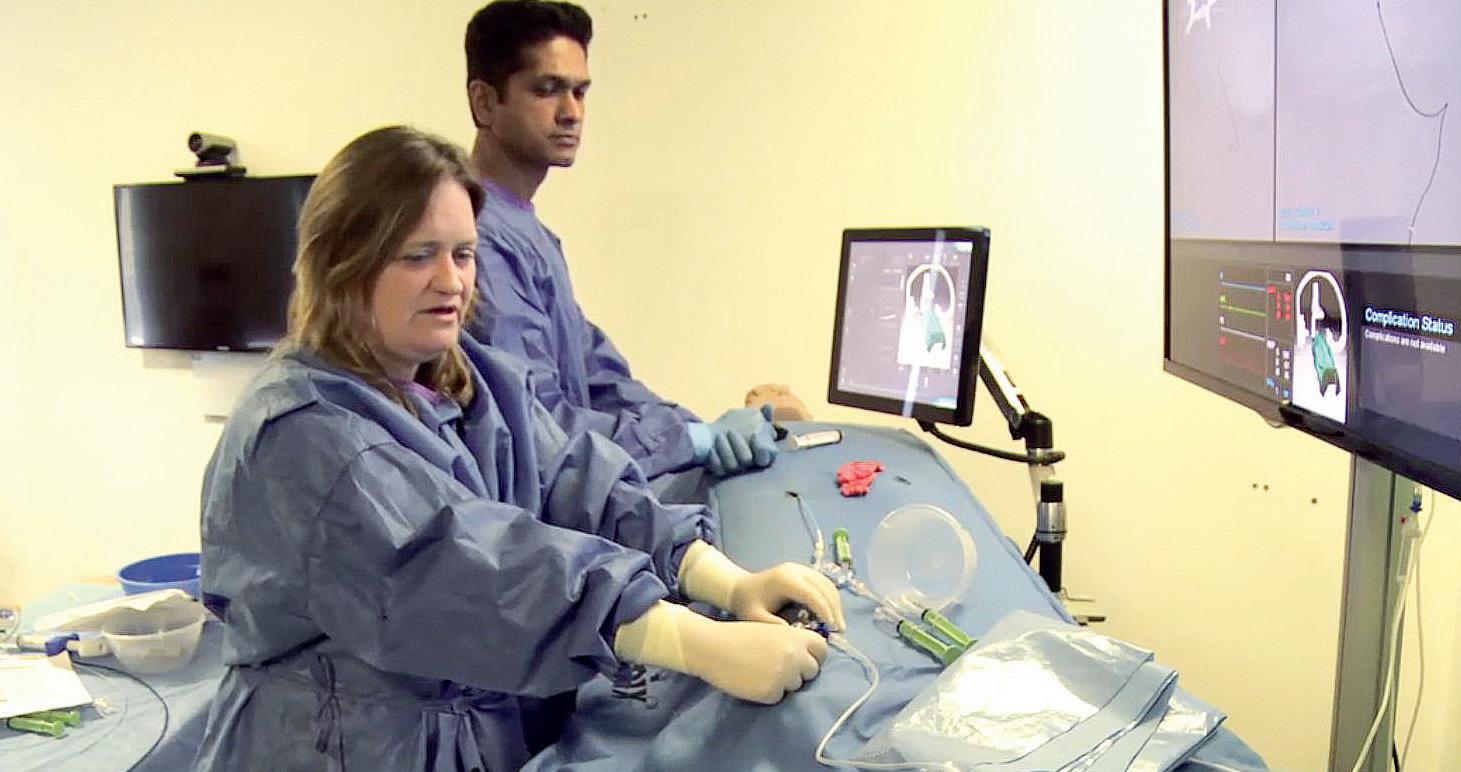
operator as well as team skills.
Training according to WIST guidelines using these innovations in the University of Dundee’s School of Medicine—now accredited as WIST’s official global training centre—has already allowed the setup of successful thrombectomy services in Argentina, Poland, the UK and additional sites. These training protocols are increasingly being adopted worldwide to increase the number of interventionists performing endovascular stroke treatments.
One example of the simulation technologies being deployed here is ANGIO Mentor (Simbionix), which incorporates modern recanalisation techniques, such as carotid revascularisation, intracranial stenting, and mechanical thrombectomy via stent retrieval or aspiration, but also plays a role in complication and team training, as well as decision-making.
“Simulation training is a crucial part of the WIST guidelines, since it is the best way to train and assess physicians and teams in this emergency procedure without risking patients’ lives,” Grunwald stated. “Simbionix’s ANGIO Mentor specifically provides the most realistic proficiency-based training—allowing physicians to prepare for unexpected complications that may arise during the intervention.”
The guidelines, which have been produced in conjunction with other leading figures from the fields of neuroradiology, interventional neurosurgery, stroke neurology and cardiovascular medicine, are designed to map out a training and credentialling pathway for interventionists so that doctors and teams from relevant specialties looking to train in endovascular stroke treatment for WIST accreditation will be able to do so. They offer personalised training to achieve standardised learning outputs, enabling doctors to perform cerebral angiograms, carotid stenting and
Tenecteplase linked to higher haemorrhage rates than alteplase in no-thrombectomy stroke patients
INTRAVENOUS TENECTEPLASE was associated with a higher rate of intracranial haemorrhage (ICH), as compared to alteplase, in a recent analysis of real-world data from acute ischaemic stroke patients who did not undergo a thrombectomy procedure.
Navpreet Bains (University of Missouri, Columbia, USA) and colleagues write in the Journal of Stroke and Cerebrovascular Diseases that, while alteplase has long been considered
the go-to in intravenous thrombolysis (IVT) treatments for acute ischaemic stroke, many US centres are currently switching to tenecteplase. This is in spite of what Bains et al posit is a “paucity of comparative data” between tenecteplase and alteplase in real-world settings.
In an effort to rectify this, the authors analysed the data from 122 healthcare facilities in Cerner real-world data, including patients admitted between February 2016 and April 2022, to
endovascular stroke treatments safely.
“This development is of great significance to healthcare professionals, stroke associations, governments, healthcare services and, ultimately, patients and their families,” Grunwald added. “Despite some important differences in the vessels and organs, many of the techniques involved in endovascular stroke treatment, including thrombectomy, angioplasty and stenting, are familiar to the interventional radiologist and cardiologist. However, while technical nuances can be easily learned by skilled interventionists, WIST emphasises the importance of team training and training in pathophysiology, neuroanatomy, image interpretation, clinical examination and decisionmaking, and the management of complications.”
determine the effect of intravenous tenecteplase versus alteplase. Two outcomes in acute ischaemic stroke patients—non-routine discharge or death, and ICH occurrence—were stratified by use of thrombectomy, and adjusted for potential confounders.
Among the 30,643 acute ischaemic stroke patients who were analysed, 29,480 (96.2%) and 1,163 (3.8%) patients intravenously received alteplase and tenecteplase, respectively. The proportion of patients who received a thrombectomy was “significantly higher” among patients who received tenecteplase (16.7%) compared with those who received alteplase (11%).
“Occurrence of ICH was more common among patients treated with intravenous tenecteplase in acute
ischaemic stroke patients who did not receive thrombectomy (7.9% vs 5.1%) but not in those who received thrombectomy (20.1% vs 16.8%),” Bains et al write.
In the logistic regression analysis, patients treated with tenecteplase who did not receive thrombectomy were found to be at a higher risk of ICH too—after adjusting for age; gender; race/ethnicity; and a number of comorbidities, including hypertension, diabetes mellitus, atrial fibrillation, hyperlipidaemia, and previous ICH.
“There was no difference in the rate of non-routine discharge or death between patients treated with intravenous tenecteplase, and those treated with intravenous alteplase, in the multivariate analyses,” the authors add.
4 June 2023 | Issue50 Mechanical Thrombectomy
Despite some important differences in the vessels and organs, many of the techniques involved in endovascular stroke treatment, including thrombectomy, angioplasty and stenting, are familiar to the interventional radiologist and cardiologist.”
Iris Grunwald (left) demonstrates the use of a surgical simulator
ischaemic stroke
Endovascular therapy (EVT) has been deemed safe and effective in patients with ischaemic stroke caused by an anterior circulation largevessel occlusion (LVO) who present 6–24 hours from onset, or last seen well, who were selected on the basis of the presence of collateral flow on a computed tomography angiography (CTA).
In light of this finding from the MR CLEAN-LATE trial—which is now published in The Lancet and was also presented at last year’s World Stroke Congress (WSC; 26–29 October, Singapore)—study authors Susanne Olthuis (Maastricht University Medical Center, Maastricht, The Netherlands) et al assert that “selection of patients for endovascular treatment in the late window could be primarily based on the presence of collateral flow”.
Initially positing that EVT, via a
mechanical thrombectomy procedure, has been shown previously to be safe and effective in anterior circulation LVO stroke patients who present within an early time window of <6 hours, Olthuis et al set out to assess this approach in the later time window of 6–24 hours from symptom onset, or time last seen well.
MR CLEAN-LATE—a Phase 3, multicentre, open-label, blindedendpoint, randomised controlled trial (RCT)—was set up to this end, and conducted across 18 stroke intervention centres in The Netherlands.
In The Lancet, the authors detail that patients aged 18 years or older with ischaemic stroke, presenting in the late window with an anterior circulation LVO and collateral flow on CTA, and a neurological deficit score on the National Institutes of Health stroke scale (NIHSS) of ≥2, were included. Patients eligible for late-window endovascular treatment were treated according to national guidelines based on criteria derived from the DAWN and DEFUSE-3 trials, and excluded from MR CLEAN-LATE enrolment.
Patients were randomly assigned on a 1:1 basis to receive EVT (treatment group) or no EVT (control group), in addition to best medical treatment, Olthuis et al detail.
The trial’s primary outcome was modified Rankin scale (mRS) score at 90 days after randomisation, while safety outcomes included all-cause mortality at 90 days post-randomisation
and symptomatic intracranial haemorrhage (ICH). All randomly assigned patients who provided deferred consent or died before consent could be obtained comprised the modified intention-to-treat population, in which the primary and safety outcomes were assessed. Analyses were adjusted for predefined confounders, the authors add, and treatment effects were estimated with ordinal logistic regression and reported as an adjusted common odds ratio (OR) with a 95% confidence interval (CI).
Between 2 February 2018 and 27 January 2022, a total of 535 patients were randomly assigned in MR CLEAN-LATE, and 502 (94%) patients provided deferred consent or died before consent was obtained—255 of whom comprised the endovascular treatment group and 247 of whom were included in the control group (52% [261/502] female).
Olthuis et al go on to report that the median mRS score at 90 days was lower in the treatment group (3; interquartile range [IQR] 2–5) than in the control group (4; IQR 2–6), adding that they observed a shift towards better outcomes on mRS for the endovascular treatment group (adjusted common OR 1.67; 95% CI 1.20–2.32). In
Chest, Heart and Stroke Scotland applauds decision to reinstate “vital” thrombectomy funding

The charity Chest, Heart and Stroke Scotland (CHSS) has voiced support for the Scottish government’s decision to reinstate “vital” funding of thrombectomy services across the country, stating that a “whirlwind two-week campaign” centred around its Thrombectomy for All petition was a key contributing factor.
“WE WELCOME THAT THE SCOTTISH government has listened to stroke survivors, charities and health professionals, and restored funding for developing a national thrombectomy service,” said CHSS chief executive Jane-Claire Judson. “This cost-effective and life-changing procedure can make a huge difference to stroke survivors, ensuring many more are able to walk again, talk again and live their lives to the full.
“We now need to see work on setting up a 24/7 national service restart as a matter of urgency, and a timetable for delivery, so that every stroke patient who needs it gets the best chance of living life to the full.”
At the beginning of December last year, the Scottish government announced plans to cut £7 million from the funding of a national thrombectomy service—an endeavour it had previously committed to rolling out in an effort to make mechanical thrombectomy treatments accessible to hundreds of stroke patients each year.
Shortly after, an open letter to the UK cabinet secretary for Health and Social Care—penned by several health charities, including CHSS and the UK Stroke Association, and backed by more than 150 stroke clinicians—described this 50% funding cut and an accompanying recruitment freeze as a “mistake”.
“Thrombectomy is the single most effective treatment we have for stroke,” said stroke physician Vera Cvoro (University of Edinburgh, Edinburgh, UK) at the time. “Many patients that come to our hospitals with a stroke could benefit from this treatment that prevents disability. This can mean being able to walk again, talk again and even going back to work. We have the expertise to deliver such treatment and it should be available to all people living in Scotland.” CHSS and the Stroke Association jointly called for the Scottish government’s commitment to continue funding thrombectomy services and recruit the staff necessary for a national rollout, citing the costeffectiveness of these procedures in addition to their proven clinical benefits.
addition, all-cause mortality did not differ significantly between the two groups (24% [62/255] vs 30% [74/247]; adjusted OR 0.72; 95% CI 0.44–1.18). However, more symptomatic ICHs occurred in the treatment group (17 [7%]) compared to the control group (four [2%]; adjusted OR 4.59; 95% CI 1.49–14.10).
“Due to the results of the MR CLEAN-LATE trial, more latepresenting stroke patients are now eligible for endovascular treatment and will overall have better outcomes,” Olthuis said, speaking to NeuroNews. “Hopefully, stroke guidelines will soon recommend the collateral-based selection that we used in our trial because it is more inclusive, but also because it has a few major advantages compared to current selection strategies, which were based on the DAWN and DEFUSE-3 trials.
“For one, collateral-based selection is less complex and therefore more suited for the emergency setting. Another benefit is that advanced imaging is no longer a necessity—consequently, stroke patients presenting in the late window can be selected for treatment even in centres with limited access to these advanced imaging modalities.”
And, after a “tireless campaign” by stroke survivors, healthcare professionals, and CHSS staff and volunteers, the Scottish government announced on 16 December that it would reinstate this £7 million in thrombectomy funding.
“Thanks to you, we gathered over 2,500 signatures for our Thrombectomy for All petition in under two weeks, highlighting the fierce support for this lifechanging surgery that reduces the odds of disability from stroke,” a CHSS press release states.
The announcement came as part of a “record” £19 billion annual budget for health and social care, with the Scottish government pledging more than £1.2 billion for mental health services; over £2 billion to deliver and improve primary healthcare services in the community; and £160 million to address public health emergencies, and reduce the avoidable harms associated with drugs and alcohol.
The other major element of this announcement included “fully restoring the budget for life-saving procedures, such as thrombectomies […] despite the need to make a short-term reduction to tackle the inflationary pressures faced by the whole UK”.
“The stroke community in Scotland was united in alarm over recent cuts to the thrombectomy programme, and we therefore welcome this renewed funding of a lifesaving and cost-saving service,” said John Watson, associate director of the Stroke Association in Scotland. “The cabinet secretary has now given us his assurance that the commitment to a national, round-the-clock thrombectomy service remains.
“We look forward to continuing this positive discussion, with the aim of a clear timetable for delivering one of the most effective and cost-saving procedures available to us.”
5 Issue50 | June 2023 Mechanical Thrombectomy
MR CLEANLATE trial finds endovascular therapy safe and effective in late-presenting
Thrombectomy is the single most effective treatment we have for stroke.”
Vera Cvoro
Susanne Olthuis
First-pass effect or rule of three? Definition of procedural success remains imperfect in stroke thrombectomy
While a number of companies in the neurovascular space have become increasingly focused on the ‘first-pass effect’ in mechanical thrombectomy treatments, many major clinical trials assessing the safety and efficacy of the technique still favour a ‘rule-of-three’ paradigm. And—although it is now broadly accepted that the risk of a poorer clinical outcome grows with every additional clot-retrieval attempt—the exact relationship between the two is still highly debatable. In the view of David S Liebeskind (University of California Los Angeles [UCLA], Los Angeles, USA), this is one of several reasons why the first-pass effect remains an imperfect measure of success in stroke care.
At last year’s Barts Research and Advanced Interventional Neuroradiology (BRAIN) conference (5–8 December 2022, London, UK), in a presentation outlining the import of imaging and angiography core laboratories, Liebeskind stated that success at the first pass is “just a result”—often indicative of a relatively easy case, and little more. He went on to note that variation between individual patients, their specific anatomies, clot types and other features, is one of many factors confounding the use of pass numbers to define success.
“Reperfusion of the downstream territory— measured by a certain threshold of the eTICI [expanded thrombolysis in cerebral infarction] classification system—in itself, is very complicated,” he expounds, speaking to NeuroNews. “That is why I am, not sceptical, but cynical, in saying that, when you get a first-pass effect, it just means things appeared like they went in the right direction.”
According to Liebeskind, discussions around the relevance of the first pass—whereby a physician successfully retrieves a clot from the neurovasculature of an ischaemic stroke patient, restoring sufficient blood flow to the brain, in a single attempt—are a fairly recent emergence. Such debates were “not even on the map” 25 years ago, he notes. This is due to “incremental changes” in neurointerventional technologies that have seen thrombectomies shift from taking up to six hours, in the past, to certain cases now being completed in a matter of minutes.
“There was a lot of focus on time from the start in the stroke field, and [the fact that] we have to treat stroke as fast as possible,” Liebeskind says. “Therefore, conceptually, we were measuring the success of the procedure by how long it took—because we knew that time may be important in terms of [preventing] ischaemic injury.”
However, while the ‘time is brain’ mantra still rings true, the significance of the number of retrieval attempts has evolved and, over time, come to the fore. Liebeskind believes this is an “interesting concept”, not least because it is “truly an interaction between the patient-specific imaging features, operator/ interventional skills, and the device they are using”.
An imperfect metric
There are a number of case- and imaging-specific factors that can contribute to the likelihood of achieving success at the first pass, he continues. The occlusion location; clot size and composition; and extent of downstream ischaemic injury; all contribute to a complex overall picture and cannot be
fully accounted for when measuring success via the number of retrieval attempts alone. The fact it is not yet understood precisely what bearing many of these elements have on the likelihood of an early, successful retrieval only serves to muddy things even further. As Liebeskind also points out, the operator can have a discernible impact on how many passes are needed to restore blood flow—not only because, as prior studies have confirmed, their level of skill and experience can improve the likelihood of a positive outcome, but because how ‘aggressive’ their approach is, and the devices and techniques they opt for, are relevant too. Another current problem with the notion of the first-pass effect stems directly from its nomenclature; how does one define a single pass? Liebeskind queries what constitutes “a go” in mechanical thrombectomy—
instances where a device is not fully deployed, or is used for an off-label indication, or where reperfusion is attempted prior to the device being repositioned slightly for a very quick ‘second pass’, are all examples whereby the definition of a single pass becomes murky.
Aspiration catheters and stent retrievers—the two most prevalent singular devices used in stroke thrombectomy treatments—are both affected by this issue. One ‘pass’ with the former could theoretically involve aspirating for two minutes, or a full 20 minutes, at a time. It is also debatable whether advancing a catheter without turning on the aspiration pump counts as a pass, because such instances fall outside of its intended, on-label use. Even for the more established stent retriever, the definition of a single pass is far from perfect, according to Liebeskind.
“There are interventionists that deploy a stent retriever for 30 seconds, and there are interventionists that deploy for 10 minutes—it is not standardly done,” he says. “And I am excusing myself from being humble here, but I have seen more angiographies of acute ischaemic stroke than anybody in history, and there is extreme variability in what is being done on routine, daily basis. Every case is a little different, and [a thrombectomy] is truly an art in the sense that it is not a technical procedure that you can automate to achieve the same results each time.”
Yet another relevant variable is the way in which technical success is subsequently measured, regardless of how much time or how many passes it takes. Recanalisation refers to the physical reopening of the blood vessel; reperfusion pertains to the actual restoration of downstream blood flow. And, while measuring the latter via eTICI scoring has generally become the gold standard following publication of five landmark stroke trials in 2015, even this system is very broad and leaves the precise definition of ‘success’ open to interpretation.
This is ultimately a “philosophical debate”, as much as anything else, Liebeskind feels, and is one of many reasons why something as ostensibly simple as measuring the number of attempts required to complete a thrombectomy can be “extremely complicated”. He also recalls that, in the wake of the ARISE II study— which saw Osama Zaidat (Mercy Health St Vincent Medical Center, Toledo, USA) et al first coin the firstpass effect—many were quick to jocularly point out that the terminology itself is an imperfect ‘first pass’ at defining the concept.
First pass or three strikes?
“[Another thing] that has come up, which is very important, is the ‘third-pass effect’—which nobody really talks about, [but] is the standard in clinical trials,” Liebeskind says.
The origin of this concept of three passes, or three ‘interventional steps’, can actually be traced back to work done by Liebeskind and his own group at UCLA involving the very first endovascular thrombectomy device: the Merci retriever.
“Decades ago now, once that device [became] available, it was realised there was a certain threshold of passes, or attempts, of device use,” he recalls, “and it seemed like three steps would define a lot of what came after in terms of outcomes—including the final angiographic result, as well as whether you ever achieved success, and then also the clinical outcomes. That is not always the case, but it became a simplistic yet practical approach because, intuitively, three tries at something is a good set of attempts. To use a baseball analogy, you get three strikes before you are out.”
While the mantle of ‘gold standard’ in stent retrieval has since been assumed by the Solitaire (Medtronic) and Trevo (Stryker) devices, the rule-of-three ethos has endured into the present day, according to Liebeskind. Many neurovascular companies currently extol the virtues of their thrombectomy products based on an ability to achieve recanalisation or reperfusion with a single pass, but three attempts is still the more
6 June 2023 | Issue50 Mechanical Thrombectomy
The traditional paradigm for doing clinical trials has to be revisited—they have to be rethought in terms of what we do in practice, and how we declare things as being a success or not.”
CLINICAL STUDIES
David S Liebeskind
commonly used baseline in clinical research.
“Each interventional step is a procedure unto itself,” Liebeskind opines, “and what is involved in clinical trials is this sequential evaluation of success. So, for regulatory purposes, people have employed the practical approach of saying ‘three is enough’—you get three strikes at the plate, and then you are out.”
Clinical versus technical outcomes
Of course, reperfusion, recanalisation, or any other ‘technical’ outcome measure, is only half of the story in stroke thrombectomy—and, fundamentally, also the far less important half. Clinical outcomes, and the eventual impact of the treatment on patient health, are of paramount significance to all other considerations.
“As the family, you would never want to have an interventionist come out of an operating suite and say, ‘the surgery was a tremendous success, but your father died’,” Liebeskind states. “That is not the success you want. And the same concept has been applied to the first-pass effect. No interventionist is going to plant their flag, and beat their chest, and say, ‘we achieved a first-pass effect, but then the rest was a failure’.”
However, while any major clinical trial to date assessing the potential benefits of mechanical thrombectomy—as with the ‘big five’ studies in 2015, as well as more recent investigations involving basilar artery occlusions and larger infarct cores—has used clinical outcomes to measure success, there does appear to be a growing role for what Liebeskind dubs a ‘per-pass methodology’ in analyses of specific devices.


“The PROST trial, which was recently run by Phenox in the USA and Europe using the Preset device, was actually the first randomised controlled trial [RCT] comparing a stent retriever for mechanical thrombectomy to an existing device where the eTICI evaluation was used,” he avers.
In his capacity as professor of Neurology and director of the UCLA Stroke Center, Liebeskind led
the core lab that provided centralised adjudication for PROST—taking on the responsibility of ensuring the procedural results reported by each physician at each centre were consistent, and could be verified on angiographic imaging. Here, Liebeskind also highlights the adoption of a per-pass methodology in the SUMMIT MAX RCT (Route 92 Medical) as another example of technical outcomes gaining more credence in contemporary thrombectomy studies.
Outlining one favourable characteristic of such success measures, he asserts that, when an interventionist is treating a patient—particularly in urgent cases, as all ischaemic strokes are—they are less likely to speculate about modified Rankin scale (mRS) scores and 90-day functional outcomes when deliberating over which specific technique or device to use. This is because there are a myriad of variables that can impact clinical results in the weeks and months following the procedure, making it nye-on impossible to determine the role of the procedure itself in these longer-term developments. The end goal of physically removing a clot and restoring blood flow, on the other hand, can effectively be ascertained in real time.
“Put simply, devices cause a radical change in the pathophysiology—in blood flow, in this case—that is visible, and demonstrable, immediately,” Liebeskind says. “A mechanical thrombectomy is very simplistic in that you can demonstrate, via angiography, exactly what was done and at what timepoint. Angiography tells the story of both the therapeutic intervention as well as the subsequent diagnostic information.”
The role of core labs
“From a careful and thoughtful standpoint, I think the concept of a first-pass effect is useful—it is nice, from a marketing standpoint, to say ‘in a clinical study, […] we achieved a first-pass effect in this number of cases’,” he continues, but asserts that the more objective, standardised adjudication provided by a core
lab is key in substantiating these types of claims.
Liebeskind also highlights the somewhat flawed approach US and European regulators currently take to post-market medical device surveillance. The upshot on both sides of the Atlantic and Pacific is that, “right now, around the world, stroke device usage is selfreported in routine clinical practice,” he adds, “and that is a crazy system to trust and believe in”.
“The traditional paradigm for doing clinical trials has to be revisited—they have to be rethought in terms of what we do in practice, and how we declare things as being a success or not,” Liebeskind argues.
In concluding, he puts forward what he believes is a “very potent, and much more efficient” solution; systematic evaluations delivered by centralised, corelab adjudication—such as in the PROST RCT—being employed more widely in post-market surveillance studies. In Liebeskind’s view, this would create rigorous and objective thrombectomy datasets, against which reperfusion pass rates and other outcomes with novel products could be assessed, alleviating the “extremely high” cost burden and other imperfections carried by today’s comparative device trials.
“You cannot market your device for a treatment indication in the USA—and, probably, anywhere else in the world—if you have not done a randomised, parallel-arm study,” Liebeskind adds. “Will a singlearm vehicle get you through the regulatory approval [processes] in stroke? Probably not, and maybe never, but the way to get around that is to reengineer how you do a randomised or parallel-arm study.

“Your parallel arm does not need to be a treatment randomly assigned to the next prospective patient entered sequentially into a trial; you can use contemporaneous data from patients treated on the same day, at various sites, with approved devices. That becomes your comparator. And that is why post-market surveillance is so important, and why there needs to be a mechanism for doing it.”

7 Issue50 | June 2023 Mechanical Thrombectomy
*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the neuro interventional arena Editorially independent Subscribe today Available in print and digital formats and through our social channels Visit neuronewsinternational.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription**

Stent-assisted coiling fails to show superiority versus coiling alone in unruptured aneurysms
A randomised controlled trial (RCT) involving more than 200 patients has indicated that stent-assisted coiling (SAC) is not superior to coiling alone in unruptured intracranial aneurysm cases with a high risk of recurrence.
Reporting results from the STAT trial in the American Journal of Neuroradiology (AJNR), William Boisseau (Centre Hospitalier de l’Université de Montréal, Montreal, Canada) et al detail that the approach was unable to demonstrate better treatment failure-related outcomes than standard coiling across patients with wide-neck, large or recurrent aneurysms.

“The use of stents for the treatment of unruptured intracranial aneurysms is an example of the failure of our community to use randomised trials to safely introduce innovations in neurovascular care,” Boisseau et al state. “We must find ways to integrate clinical research into practice to optimise care in real time. Future trials on SAC should probably be integrated into ongoing randomised clinical trials.”
The authors also note that, while their study excluded patients with ruptured aneurysms; patients subjectively adjudged to be “untreatable” without stent placement; and the majority of patients with small unruptured aneurysms, it ultimately showed “no large benefit” to a policy of stent placement plus coiling. Additionally, they feel the trial “raises concerns” regarding potential thromboembolic complications—particularly in patients with small aneurysms at a low risk of rupture, “for whom the crucial question remains: should they be offered preventive treatment at all?”
Backdrop to STAT
While they affirm at the outset of their AJNR paper that SAC may be associated with improved angiographic results in endovascular unruptured aneurysm treatments, as compared to coiling alone, the authors aver “this has never been shown in a randomised trial”. With this in mind, the investigatorled, parallel STAT RCT was set up and conducted across four university hospitals in Canada and France. The trial saw patients with intracranial aneurysms at risk of recurrence (defined as large aneurysms [≥10mm], postcoiling recurrent aneurysms or small aneurysms with a wide neck [≥4mm])
undergo 1:1 randomisation to either SAC or coiling alone.
The trial’s primary efficacy outcome was a composite endpoint of ‘treatment failure’—defined as initial failure to treat the aneurysm; aneurysm rupture or retreatment during follow-up; death or dependency (modified Rankin scale [mRS] >2); or an angiographic residual aneurysm adjudicated by an independent core laboratory at 12 months.
Boisseau et al also detail that their primary hypothesis was that SAC would decrease treatment failure rates from 33% to 15%, with an intention-totreat population of 200 patients being required to sufficiently assess this. This differed from an initial efficacy hypothesis that SAC would decrease angiographic recurrences by 20% at 12 months, as per the trial’s original 2011 protocol, which was modified in July 2021 “before any knowledge of the data”.
New coiling data
Of 205 patients recruited between 2011 and 2021, 94 were allocated to SAC and 111 to coiling alone, the authors
report. The key results from STAT are as follows:
� The primary outcome, ascertainable in a total of 203 patients, was reached in 28/93 patients allocated to SAC (30.1%) versus 30/110 allocated to coiling alone (27.3%; p=0.66).
� Poor clinical outcomes (mRS >2) occurred in 8/94 SAC patients (8.5%) compared with 6/111 coiling-only patients (5.4%; p=0.38).
� There were no incidences of aneurysm rupture during follow-up and three patients—all in the SAC group—were retreated.
� Five deaths were related to treatment complications (three with SAC and two with coiling alone), while adverse events occurred in 25/94 SAC patients (26.6%) and 23/111 coiling-only patients (20.7%; p=0.323).
� Secondary outcomes, including immediate and 12-month angiographic outcomes; days of hospitalisation; discharge disposition; and mRS at discharge and at 12 months; were all similar between groups too.
Boisseau et al’s predefined subgroup analyses of the primary outcome found no significant interactions, with similar results across different subgroups, and “only by redefining a good angiographic outcome as complete occlusion [rather than a combination of complete and near-complete occlusion]—and only by looking at astreated analyses—could SAC be shown superior to coiling alone”.
What are the implications?
“The clinical significance of this finding remains questionable, but it may be a signal in favour of the capacity of stent placement to improve angiographic results of coiling in the long term,” they continue. “This capacity may come at a cost in terms of complications: as-treated analyses also showed complications to be more frequent with SAC, particularly for small aneurysms.
Although in some of these cases complications occurred when stents were being used as a rescue strategy, thromboembolic complications with stent placement remain a concern.”
The authors also posit that STAT was only powered to show a ‘large effect’, claiming that “we cannot exclude that, with the inclusion of a larger number of patients, a more modest but still clinically significant benefit could have been demonstrated”. They further state that a substantial number of crossover patients could have diluted the contrast between treatments in the trial, and that “perhaps, the groups being compared could have been more precisely defined” as SAC (if possible) versus coiling alone plus bailout stent placement (only if necessary).
The overall morbidity and mortality rates in STAT were within the investigators’ initial estimate of 6–12%, as per a secondary hypothesis suggesting stent placement “would not double” the number of dead or dependent (mRS >2) patients at 12 months—a hypothesis that was also dropped in July 2021. However, while safety endpoints were similar between the two groups in intention-to-treat analyses, the authors concede that the trial was “underpowered to draw any conclusions about the safety of SAC over coiling alone”.
STAT is the only RCT to date comparing SAC and coiling alone, according to Boisseau et al. Nevertheless, owing to the many questions it failed to answer conclusively, as well as limitations present—including small numbers of centres and patients stunting the generalisability of the data, and clinical practices having evolved over the 10-year study period—they conclude that more randomised data are needed to determine the role of SAC in the treatment of aneurysms. The authors further note that, as such, the “context of uncertainty” within which STAT was launched in 2011 persists today.
9 Issue50 | June 2023 Coil Embolisation
ANEURYSM CARE
William Boisseau
The use of stents for the treatment of unruptured intracranial aneurysms is an example of the failure of our community to use randomised trials to safely introduce innovations in neurovascular care. We must find ways to integrate clinical research into practice to optimise care in real time.”
Recent addition to European flow diverter market performs well in multicentre study
A recent addition to Europe’s flow diversion market—the Derivo 2 embolisation device (DED2; Acandis)—has demonstrated promise in a small-scale, multicentre study, providing a safe and effective treatment for both ruptured and unruptured intracranial aneurysms.
Writing in Interventional Neuroradiology, study authors Maximilian Thormann (University Hospital Magdeburg, Magdeburg, Germany) et al state that, in addition to demonstrating a safety and efficiency profile that is comparable to other flow diverters, the novel DED2 device showed a high rate of satisfactory aneurysm occlusion, and low rates of permanent major morbidity, at six months of follow-up.
At the outset, the authors describe DED2 as a self-expanding device consisting of up to 64 nitinol wires that promises to provide better X-ray visibility, as well as a more homogeneous distribution of radial force and porosity. They also report that—while a good safety and efficiency profile has been associated with the Derivo 1 (DED1) device in the current literature—there are “no clinical angiographic data on the technologically improved DED2” to date.
As such, Thormann and colleagues performed a multicentre trial across six interventional centres, using prospectively collected data and including all patients treated with the DED2 device. Their primary endpoint was angiographic aneurysm occlusion at six months, as assessed by the O’Kelly Marotta (OKM) grading scale (favourable outcome definition=OKM C [entry remnant] or D [no filling]). Clinical outcomes were evaluated according to the modified Rankin scale (mRS), with favourable outcomes being defined as mRS 0–2, and major morbidity as mRS 3–5 or a deterioration by two points.
In the study, treatment decisions—including systemic heparinisation, and the additional use of coils or balloon angioplasty—were made at the discretion of the treating interventionist, but also confirmed by a
neurovascular board. All procedures were performed under general anaesthesia by a board-certified neuroradiologist, the authors add.
Between August 2020 and July 2021, 37 patients (median age=60 years, 27 females) were treated with DED2 and subsequently included in Thormann et al’s analysis, five of whom presented with ruptured aneurysms and 20 of whom presented asymptomatic. DED2 was successfully implanted and fully opened in all cases, and 21 patients (57%) had an OKM grade of C or above immediately after deployment.
Clinical and angiographic follow-up data were available for 30 patients (81%) and 27 patients (73%), respectively, and the median follow-up time was six months. Twenty-five patients (83%) had an mRS of
Intraoperative adverse events found to impede favourable outcomes in microsurgical aneurysm treatment
A nationwide study drawing on data from Swedish patients has indicated that—while the majority of ruptured intracranial aneurysm patients treated with microsurgery experienced favourable outcomes—intraoperative adverse events were associated with an increased risk of unfavourable outcomes.

WRITING IN THE JOURNAL of Neurology, Neurosurgery and Psychiatry (JNNP), Bryndís Baldvinsdóttir (Lund University, Lund, Sweden) and colleagues put forth that adverse events and complications can arise with microsurgical treatment of aneurysmal subarachnoid haemorrhages (SAH). As such, their study aimed to not only identify adverse events associated with microsurgical occlusion of ruptured brain aneurysms, but also to analyse their risk factors and how they impact functional outcomes. Detailing their methodology,

the authors state that patients with aneurysmal SAH, admitted to neurosurgical centres in Sweden, were prospectively registered across a 3.5year time period—from 2014 to 2018. Adverse events were categorised as being either intraoperative or postoperative. A range of variables relating to patient history and SAH characteristics were explored, Baldvinsdóttir and colleagues also note, as the potential risk factors for these adverse events. Functional outcomes were assessed roughly one year after the occurrence of an SAH using the
0–1. In addition, six of the seven patients with missing mRS data at follow-up had an mRS of 0 at discharge.
Three patients died during the follow-up period—all of whom had ruptured aneurysms initially. No deaths were observed in patients with unruptured aneurysms; no treatment-related major morbidity was observed; and, on follow-up imaging—which was available in 27 patients (90%)—23 patients (85%) showed satisfactory aneurysm occlusion (OKM C or D).
Discussing their findings further, Thormann and colleagues note that the intracranial haemorrhage rate in their study (3%) is comparable with previously published results on the DED1 device, while the rate of in-stent thrombosis in their cohort “did not differ significantly” from rates currently seen in the literature either. They also reiterate that, although the overall mortality seen in their study is “high compared to other studies”, all of the observed deaths occurred in patients with ruptured aneurysm patients, adding that they “intentionally decided to include cases with ruptured aneurysms to demonstrate the usability of the device in both ruptured and unruptured aneurysms”.
“Given our small sample size, the mortality rate within the ruptured aneurysm subgroup may not necessarily be attributed to the device or treatment with flow diverters in general, but rather mirror the severity of specific patient cases,” the authors continue. “While comparisons across studies and devices are difficult due to different inclusion criteria and technical setups, our efficacy and safety results are within the range published for competing flow diverters, and the DED1.”
Thormann et al go on to note improved outcomes in their study compared to a prior trial involving DED1—including lower rates of failure to open, displacement, and use of adjunctive coiling—but concede “our data do not allow us to determine whether this is due to the improved design of DED2”.
Limitations highlighted by the authors in the report include the retrospective nature and small sample size of the analysis, as well as the “relatively short” sixmonth follow-up period, and the fact patient selection, antiplatelet therapy choices and angiographic outcome assessments were all at the interventionists’ discretion.
extended Glasgow outcome scale.
The authors report that a total of 1,037 patients were treated for ruptured aneurysms in their study cohort—with 322 of these undergoing a microsurgical procedure. There were 105 surgical adverse events across 97 of these patients (30%), 94 of which occurred intraoperatively in 79 patients (25%). Homing in on the adverse events they observed, Baldvinsdóttir and colleagues’ JNNP paper details that aneurysm re-rupture occurred in 43 patients (13%); temporary occlusion of the parent artery for more than five minutes occurred in 26 patients (8%); and adjacent vessel injury occurred in 25 patients (8%).
Higher grades on the Fisher scale—a metric used to classify the extent of SAH on medical imaging—as well as brain oedema on computed tomography (CT) scans were related to an increased risk of adverse events. The authors further found that, at followup, 38% of the patients experienced an unfavourable outcome. Those patients
who did suffer adverse events were more likely to experience unfavourable outcomes (odds ratio [OR] 2.3, 95% confidence interval [CI] 1.36 to 4.06). As such, Baldvinsdóttir and colleagues conclude by reiterating that intraoperative adverse events occurred in 25% of ruptured intracranial aneurysms in patients who underwent microsurgery and that these patients were more likely to experience unfavourable outcomes.
“The key take-home message from this study, in my opinion, is the importance of awareness of possible adverse events related to the microsurgical occlusion of a ruptured brain aneurysm,” noted Baldvinsdóttir, in conversation with NeuroNews “In our study, we present results on how temporary occlusion for more than five minutes can have a significant negative impact on patient outcome. We find the results can be relevant for the selection of which treatment modality is most suitable for patients with ruptured brain aneurysms.”
10 June 2023 | Issue50 Interventional Outcomes
ANEURYSM CARE
While comparisons across studies and devices are difficult due to different inclusion criteria and technical setups, our efficacy and safety results are within the range published for competing flow diverters, and the DED1.”
Bryndís Baldvinsdóttir
Systematic review queries how far PPTA aneurysm management is along “the right path”
A SYSTEMATIC REVIEW and analysis published in the journal Interventional Neuroradiology has examined how far along “the right path” management paradigms for persistent primitive trigeminal artery (PPTA) aneurysms currently are—ultimately concluding that, while endovascular techniques in this area are successful, “meticulous” reporting of outcomes and complications remains important.
Gaurav Gupta, Sudipta Roychowdhury (both Rutgers Robert Wood Johnson Medical School and University Hospital, New Brunswick, USA) et al posit from the outset that, since the 1960s, treatment approaches for these types of aneurysms have evolved from ‘conservative’ to
‘surgical’, to ‘endovascular’.
This—in addition to the fact that PPTA aneurysm treatments are “increasingly reported and commonly managed” via endovascular techniques—led Gupta et al to investigate the existing body of research further.
“There are no systematic reviews or meta-analyses which analyse outcomes and complications of treatment modalities for PPTA aneurysms,” they write. “We aim to highlight the change in trend of management of PPTA aneurysms, and to identify clinical and radiological parameters that may influence management paradigms.”
The authors also put forth the following question: how far along the right path are we?
Pipeline Flex embolisation device deemed safe and effective in complex intracranial aneurysms
A retrospective study from China has concluded that the Pipeline Flex embolisation device (Medtronic) is safe and effective in the treatment of complex intracranial aneurysms. Reporting their results in Nature: Scientific Reports, Shun-Qiang Chen (Henan Provincial People’s Hospital, Zhengzhou, China) and colleagues state that the Pipeline Flex—a second-generation flow diverter— was associated with a high complete occlusion rate, decreased complication rate, and good prognosis rate at medium follow-up.
“Although some individual studies have reported similar outcomes, we included [only] complex aneurysms in one study, and reported the treatment outcome as well as follow-up outcomes because of the large volume of patients in our hospital,” the authors write. “Our study contributes to a better understanding of use of the [Pipeline] device in treating complex intracranial aneurysms, in one large medical centre, with the same group of physicians.”
To investigate the safety and short-term effect of the device in the treatment of complex, unruptured intracranial aneurysms, Chen and colleagues conducted a retrospective study between February 2018 and September 2019. Patients with tiny, large, giant, wide-necked, tandem multiple and bloodblister aneurysms, confirmed on imaging, and treated with Pipeline Flex, were included.
A total of 131 patients (67% female, mean age 54.2 years) with 159 complex aneurysms were enrolled. The most common aneurysm location was the internal carotid artery (84.9%), followed by the
Gupta, Roychowdhury and colleagues report that a systematic search of literature was done in PubMed, Embase, Google Scholar, Cochrane library and Medline using keywords including ‘persistent primitive trigeminal artery’, ’aneurysms’, ‘embolisation’, and ‘surgical clipping’. Only cases reporting aneurysms of the PPTA were included, the authors add.
Additionally, they state that three subgroups—conservative, open surgical and endovascular interventional—were studied for outcome evaluation. And, in the endovascular subgroup, relation of clinical and radiological parameters with outcome (complete or partial occlusion) was analysed using the Microsoft Excel Data Analysis ToolPak. Of the 101 articles found to be eligible for assessment, 54 were analysed quantitatively.
Gupta, Roychowdhury et al note that total mortality rates were as follows: 12.5% in the conservative group, 9.09% in the open surgical group, and 8.57% in the endovascular interventional group.
vertebral artery intracranial segment (9.4%), middle cerebral artery (3.8%) and basilar artery trunk (1.9%). Some 24 patients (17.6%) had multiple aneurysms.
Overall, 144 Pipeline Flex devices were all successfully deployed, with a stenting success rate of 100% and a mean operation time of 123.7 minutes. The authors report that Pipeline Flex was deployed alone in 107 aneurysms (67.3%) and combined with coiling to treat the remaining 52 aneurysms (32.7%).
All devices had “good coverage of the aneurysm neck, with good wall adherence and patent parent artery”, Chen et al note, adding that none of the patient population required the ‘massage technique’ or the ‘balloon expansion technique’ to make the Pipeline Flex adherent to the parent arterial wall.
At discharge from the hospital, modified Rankin scale (mRS) scores were as follows: mRS 0 in 101 patients (77.1%), mRS 1 in 25 (19.1%), mRS 2 in four (3.1%), and mRS 4 in one (0.8%). The upshot of this was a good prognosis rate (mRS 0–2) of 99.2%. In addition, periprocedural complications occurred in four patients (3.1%), three of which were ischaemic (2.3%) and one of which was haemorrhagic (0.8%). No deaths occurred, the authors note.
Also, in the endovascular subgroup, complete angiographic occlusion was seen in 88.89% of PPTA aneurysms, and 5.5% warranted retreatment, while location (p=0.17), shape (p=0.69), Saltzman circulation (p=0.26) and status of rupture (p=0.08) did not significantly impact angiographic occlusion outcome.
Multivariate regression analysis did, however, reveal a 6.6% influence of independent variables—those being age; gender; aneurysm location, size and shape (saccular/fusiform); rupture status; and type of Saltzman circulation—on aneurysm occlusion outcome (p=0.27).
“Clinical or radiological parameters do not influence angiographic occlusion outcome,” Gupta, Roychowdhury and colleagues conclude, prior to emphasising the importance of meticulously reporting outcomes and complications associated with endovascular interventional techniques, despite the broadly successful outcomes observed with minimally invasive treatments in their analysis.
found, while asymptomatic in-stent stenosis occurred in four patients (6.6%), Chen et al report.
Discussing their findings further, the authors assert that, while ischaemic complication events were “very low” in their study, prevention is “still needed”, and advocate measures including sensitivity testing of antiplatelet medications, application of tirofiban and insurance of good wall adherence with the Pipeline Flex device.
Six-month complete occlusion rate
Chen et al also posit that the long-term effects of Pipeline Flex in intracranial aneurysms “have not been reported sufficiently”, but may have similar effects to those of the ‘classic’ Pipeline embolisation device—highlighting the fact that three large clinical studies of the latter have demonstrated a complete aneurysm occlusion rate of 75% at six months postembolisation.
85.4% WITH COILING 71.7% WITHOUT COILING
Clinical follow-up was carried out in 87 patients (66.4%) at a median of 28 months after the procedure. No new neurological symptoms were detected, with mRS scores of 0 in 78 patients (89.7%); 1 in five (5.7%); 2 in three (3.4%); and 4 in one (1.1%); being observed. As such, the rate of good prognosis at follow-up was 98.9%. A 1.1% incidence of worsening mRS was calculated—but, overall, no significant difference existed in mRS at discharge versus at clinical follow-up (p=0.16).
Angiographic follow-up was performed in 61 patients (46.7%) with 80 aneurysms (50.3%) at a median of 26 months after embolisation, revealing a complete aneurysm occlusion rate of 71.3% in 57 aneurysms (O’Kelly-Marotta [OKM] grade D) at a median of six months. No aneurysm recurrence was
Touching on the use of coiling alongside Pipeline Flex, the authors reference six-month complete occlusion rates of 85.4% with coiling and 71.7% without in their study, and cite prior research to support their claim that Pipeline-plus-coiling aneurysm treatments are “safe and efficient, with no increased complications when compared to [Pipeline] embolisation alone”.
Chen et al point to the fact that the same group of physicians—with similar levels of procedural proficiency—treated all the aneurysm cases in their study, potentially leading to “greatly reduced” heterogeneity, as well as increased treatment efficiency and decreased complication rates. They also aver that the median clinical and imaging follow-up times of 28 and 26 months, respectively, are “longer than most other studies”.
“Despite this, our study had some limitations, including the retrospective and single-centre study nature; a small cohort of patients; Chinese patients enrolled only; no control; and no randomisation, which may all affect the [generalisability] of the outcomes,” the authors conclude. “Future randomised, controlled, multicentre clinical trials will have to be performed to resolve all these issues for better outcomes.”
11 Issue50 | June 2023 Interventional Outcomes















M4D LLC believes developing innovative endovascular technologies is vital to improving and saving people's lives. We accomplish this by partnering with visionaries to intergrate new ideas into real-world, transformative therapies. 105 North Pointe Dr. Lake Forest, CA 92630 | +1 (949) 491-8327 support@m4dllc.com CELEBRATE CLIENT SUCCESS! UNDERSTAND AND CREATE MANUFACTURE AND TEST DELIVER RESULTS AND PRODUCTS CREATE NEW OPPORTUNITIES OUR PROVEN PROCESS Contact Us Early-stage Concept and Design Rapid Prototyping Design Optimization and Development Regulatory Approvals Quality Management System Commercial Manufacturing Verification and Validation Testing M4D LLC IS A PREMIER DESIGN, DEVELOPER, AND MANUFACTURER OF ENDOVASCULAR TECHNOLOGIES
Early Feasibility Study programme holds potential to advance US neurovascular space

At a time when many of Europe’s medical technology markets are effectively going backwards due to complications created by the EU Medical Device Regulation (MDR), corresponding sectors in the USA are receiving an inevitable boost. Alongside the US Food and Drug Administration (FDA), the country’s leading clinicians and industry figures have seized this opportunity—and, thanks to the Early Feasibility Study (EFS) programme, can now potentially play a leading role in advancing novel devices within the neurovascular space.
ACCORDING TO CHIP HANCE, REGATTA
Medical CEO and a member of the Medical Device Innovation Consortium (MDIC) board of directors, the EFS programme was conceptualised back in 2013 after the FDA released a guidance document on the need to improve domestic innovation in the USA. Hance recalls that the catalyst for this was observed in the structural heart sector.
A decade ago, the USA became the 43rd country, globally, in which the first transcatheter aortic valve implantation (TAVI) device gained regulatory approval; an occurrence Hance states is widely noted, and one that led the FDA to highlight the need for an optimised pathway allowing device developers to conduct early-feasibility research before gaining finalised US approval.

Owing to the successes that followed in interventional cardiology, other spaces soon took an interest. But, as Hance points out, real advancement requires an “enthusiastic clinical champion” to begin with—a mantle he attributes to neurosurgeon Adnan Siddiqui (University of Buffalo/Jacobs Institute, Buffalo, USA), who saw what was happening in structural heart around 2019 and recognised the impact it could have in neurovascular as well.
“Necessity was the mother of invention,” Siddiqui tells NeuroNews, noting that, traditionally, the “vast majority” of endovascular devices created in the USA would be commercialised and enter clinical use across much of the rest of the world while US physicians were still awaiting availability.
“This was routine, and so people like Chip and organisations like the MDIC went to the FDA to try to change that narrative,” he continues. “In the absence of an EFS programme, companies developing innovative therapies do not have a tenable pathway for approval in the USA. Through collaboration of stakeholders working under the MDIC umbrella, the USA has become a competitive environment for early innovation.”
Importance of the EFS programme
Early-feasibility studies are often a “choke point” in making novel technologies available to patients, Hance states, as they are just one aspect of the regulatory process but can take a year or more, in many cases, to navigate.
“We have been on a mission for six or seven years to try and streamline the process, getting sites [on board] that have the experience to actually do these kinds of studies, and what I think it ultimately does is help get novel, game-changing therapies to patients in the USA,” he says. “We can talk about EFS, and contracting, and all of the mechanical aspects, but really it is about getting novel therapies to patients more quickly.”
Here, Siddiqui weighs in, noting that, while the FDA’s original 2013 publication of EFS guidance helped companies get through the agency’s actual review process fairly quickly, other elements— including engagement of clinical study sites and patient enrolment—kept early-stage research moving at a “very slow” pace overall.
Clinical, regulatory and industry leaders turned to MDIC, a public-private partnership seeking to optimise medical technology innovation, to “shepherd this ecosystem forward”—initially in structural heart, but more recently in electrophysiology and, of course, stroke care.
The MDIC does this in numerous different ways, leveraging the expertise and experience of its members to support device developers with contracting, informed consent, legal documentation, best-practices guidance and more via its online toolkit. This, in combination with continuously tracking EFS performance metrics, is ultimately intended to drive overall EFS efficiency and effectiveness for US patients.
According to Hance, a big part of the MDIC’s role here is to identify sites like Siddiqui’s in Buffalo that not only have the clinical knowhow to host a successful, rigorous EFS, but also the structures and processes to conduct these studies efficiently.

While these endeavours have been “slowly but surely” moving forward within the neurovascular sphere over the past 12 months, Siddiqui believes “this has the potential to truly transform how technologies that are being imagined, developed and incorporated in the USA can benefit our patients first”.
Highlighting interest observed thus far, he references dedicated EFS symposia held at the last two Society of NeuroInterventional Surgery (SNIS) meetings, as well as “very wellattended” breakfast seminars involving companies— noting “we have run out of chairs in the room”—and claims an “even bigger footprint” is expected at upcoming neurovascular conferences.
“I think the entire neuro community is really glad to see this initiative, and it is certainly not limited to start-ups—three of our EFS’ are with Medtronic,” Siddiqui adds.
Neurovascular success stories
Having recovered from an initial three-month dead stop in 2021, brought on by the COVID-19 pandemic, early-stage neurovascular research in the USA is now reaping rewards from the years of graft that helped establish the EFS programme.

One example Siddiqui points to is that of Cerevasc’s eShunt device for treating hydrocephalus, which is implanted via an incisionless procedure rather than more traditional open surgery—a “brilliant” idea, he posits, recalling that he wanted the company’s CEO Dan Levangie to conduct first-inhuman studies with the technology in the USA.
Instead, they went with neurosurgical pioneer Pedro Lylyk (Buenos Aires, Argentina). However, while Cerevasc initially planned to remain in Argentina following this, the allure created by the
FDA’s new EFS pathway led Levangie and co to return to the USA for its subsequent feasibility studies. This research is ongoing, with Yale University (New Haven, USA) having performed cases and Siddiqui’s own centre set to conduct a handful in the near future.
“Another example is Synchron, with the Stentrode brain-computer interface,” he goes on. “Same concept; Australian company, but based out of Brooklyn, [and we] wanted them to do first-in-human studies in the USA. And, again, their initial instinct was to start in Australia. They did four cases out there, but then they got really interested in EFS, enquired, and now we have an EFS in the USA with two patients enrolled at Mount Sinai [New York City, USA] and one coming up here [to Buffalo].
“So, I saw these two companies pivot, in real time, from OUS to US, only because we have an EFS pathway, and I am really excited about these two radically different things.”
While Cerevasc and Synchron are relatively small companies at the cutting-edge of current neurovascular innovation, Siddiqui suggests that more established, iterative technologies can also benefit.
For example, next-generation stent retrievers like Medtronic’s Delphi revascularisation device are currently being assessed via EFS’, as are newer flow diverters and detachable coils, including Stryker’s Citadel embolisation device for intracranial aneurysms. Siddiqui feels that—provided companies are willing to engage with the FDA early on—starting with an EFS is becoming an increasingly smooth process whereby device developers can “seamlessly” transition into other regulatory stages: a humanitarian device exemption (HDE) pathway, 510(k) clearance or premarket approval (PMA) application.


Across the Atlantic Hance and Siddiqui are in agreement that the challenges of implementing the EU MDR has drawn new attention to the US EFS pathway—particularly when it comes to the implantables and other Class III devices that constitute many of the novel therapies seen within the structural heart and neurovascular worlds.
According to Hance, European countries are rethinking their clinical strategies due to these regulatory challenges, and have seen less activity lately in medical technology innovation.
When asked if there are any other geographies that can rival the early-feasibility processes established by the FDA and supported by MDIC, Siddiqui notes there is a similar process in Japan, but that it is “not well coordinated” by the country’s regulatory agency, with research endeavours often being “much more ad hoc” than the organised, collective ecosystem the USA now boasts.
Looking to the future, Siddiqui concedes that— while EFS is “unique”, globally, in that the FDA is engaged “from day one”—there are still certain things that need to be ironed out, such as reimbursement policies, and coverage analysis for innovative technologies and investigational devices.
“One of the reasons for our success has been the standardisation of the EFS pathway,” Siddiqui concludes. “It has actually led to a lot of companies, including European companies, coming to the USA for a first clinical site.”
13 Issue50 | June 2023 Neurovascular Trials
INTERVIEW
I think the entire neuro community is really glad to see this initiative.”
Chip Hance Adnan Siddiqui






Programming in conjunction with: European Society of Minimally Invasive Neurological Therapy SNIS 20th Annual Meeting & Fellows Course REGISTER NOW July 31 – August 4, 2023 Marriott Marquis San Diego Marina San Diego, CA http://www.snisannualmeeting.org/
Computing the future: Exploring ChatGPT’s potential role in neurosurgical practice
Brian Fiani & Ryan Jarrah
Point of View
Brian Fiani (Livonia, USA) and Ryan Jarrah (Rochester, USA) take a look at the neurosurgical implications of one of the hottest topics permeating not only the medical industry, but virtually all of society, right now—the natural language processing (NLP) chatbot known as ChatGPT (OpenAI).
Over the last decade, artificial intelligence (AI) and medicine have continuously crossed paths to optimise a physician’s practice and improve patient outcomes. From prognosis prediction calculators to image-based tumour detectors, it is safe to say that AI has revolutionised the current landscape of modern medicine, especially in disciplines like neurosurgery.
One of the most notable AI tools making headlines today is ChatGPT. Also known as GPT 3.5, this NLP chatbot has grown in popularity, especially in the medical field. Among neurosurgeons, ChatGPT’s potential to inform patient decision-making, act as a neurosurgical training tool, and promote neurosurgical research, has seen its stock rise within the community.
Early promise meets sceptic doubt
A recent study from the Huntsman Cancer Institute (HCI; Salt Lake City, USA) assessed if ChatGPT could correctly
answer questions about the National Cancer Institute’s (NCI) common myths and misconceptions. Researchers found that ChatGPT answered the questions with 97% accuracy.
Another article published out of Massachusetts General Hospital (MGH; Boston, USA) found that ChatGPT and other NLP systems, such as GPT 4.0, had achieved passing scores on a mock 500-question neurosurgical written board exam. However, these impressive results are not met without limitations.
The HCI study found that, although ChatGPT had answered with high accuracy, the language of answers was often vague, unclear and indecisive. Moreover, with the MGH study, it was determined that ChatGPT did not perform well with questions with greater word length and higher-order problem-solving.
Where do we stand?
Despite the rapid progressions of AI chatbot systems, the compassion, touch, communication and judgment involved
ChatGPT outperforms medical students and matches residents on neurosurgical boardstyle questions
An analysis published in the Journal of Neurosurgery, which assessed ChatGPT’s (OpenAI) performance on a surrogate for neurosurgical board-style questions, has found that the natural language processing (NLP) algorithm was able to significantly outperform medical students and was only slightly below the performance of residents currently studying for self-assessment neurosurgery (SANS) board exams.
in a physician’s training will remain components that are unlikely to be replaced by AI.
A neurosurgeon’s craft is rejuvenated by the incorporation of humanistic values in medicine, as certain factors— such as safety, ethical compliance, and a holistic investment into a patient’s history and wellbeing—cannot be trained into an AI algorithm.
This would imply that patients should speak to their provider for medical guidance regarding the majority of their health concerns. Hence, it is important to recognise where AI could be used in neurosurgery and where its application will be limited.
Current applications in neurosurgery
Despite the current limitations, physicians should be aware of technologies like ChatGPT, because they do show great potential for augmenting clinical practice—particularly in three key areas.

The first of these is surgical education. Risk identification of patients, surgical planning, explanation of surgical procedures, patient triage and research are all aspects of neurosurgery that ChatGPT may support. ChatGPT could be used in neurosurgical training to help create robust, step-by-step surgical plans; explain complex neuropathological diseases; or inform preoperative or postoperative surgical plans. While it may be premature to consider taking ChatGPT to the operating room, further development of ChatGPT could be very useful for neurosurgical training and enhancing education models.
Patient engagement is also vital to consider. From a patient’s standpoint, ChatGPT could be used to help patients engage with their medical team by answering lingering or last-minute questions rapidly, as well as providing reminders for upcoming appointments, supplying pre-and postoperative instructions, and monitoring patient symptoms. The conversational nature of ChatGPT could help with informing patients about their conditions, while
IN LIGHT OF THE FACT THAT “MACHINE learning in neurosurgery has quickly become a topic of great interest”, authors William Mack (University of Southern California, Los Angeles, USA) et al set out to assess the current performance ability of the artificial intelligence (AI) model ChatGPT on the primary written neurosurgical boards.

A total of 643 Congress of Neurological Surgeons (CNS) SANS questions were copied and pasted into the input section of the model, and used as a surrogate for the overall performance of ChatGPT. Results were calculated using several different methods, including raw overall score; overall score within three regenerations; and raw overall score excluding questions referencing images/diagrams within a stem.
For comparative analyses, the 643 questions were completed by two postgraduate residents and four medical students interested in neurosurgery to establish a “reasonable benchmark for model performance”. Mean performance per category was gathered from the SANS website to provide
also creating opportunities for effective telemedicine consultations.
The third area of note is neurosurgical research. In the realm of research, ChatGPT could assist in creating literature reviews by analysing large amounts of neurosurgical data and extracting relevant information. ChatGPT could find patterns and correlations in data leading to new understandings of neurosurgical trends. For example, one may quickly be able to determine the correlation between selected patient demographics and 30day complications after spine surgery using AI systems. ChatGPT could also be used for the recruitment of clinical trials by filtering patients for eligibility criteria while also quickly answering questions regarding clinical trial rules and regulations.
Plans for the future
It would take ChatGPT several years of training through physician notes, images and operating records before it could be reliably used in a clinical setting. The future may see ChatGPT improve communication between surgeons and operating room staff, provide intraoperative patient monitoring, or quickly detect imaging results.
Current efforts are already experimenting to incorporate ChatGPT into the electronic health record, creating a workflow where physicians can spend more time with the patient. ChatGPT has already shown an impressive number of clinical applications and neurosurgeons should strive to leverage the benefits of AI appropriately.
With further precision and efficiency, we can expect ChatGPT to have an expanded role in neurosurgery, and beyond, for generations to come.
Brian Fiani is a neurosurgeon at Mendelson Kornblum Orthopedics and Spine Specialists in Livonia, USA.

Ryan Jarrah is a clinical trial coordinator at the Mayo Clinic in Rochester, USA.
The authors declared no relevant disclosures.
comparisons with all first-time test takers as well. ChatGPT refused to answer 25 questions (3.9%), all of which included images/diagrams. On the first iteration across all questions responded to, ChatGPT answered 329 (53.2%) correctly. Its performance improved to 54.9% when excluding 166 questions containing images/diagrams; 58.7% when evaluating performance within three input regenerations per question; and 60.2% when allowing for three regenerations and excluding image/diagram questions.
Despite performing better than the four medical students (26.3%) and similarly to the two actively studying residents (61.5%) used as comparators, ChatGPT did worse than the average SANS user (69.3%) in its current state. However, the model did outperform the resident cohort, and performed at an approximately similar level to the average SANS user, in certain categories. “Further research into AI and NLP models is necessary to best understand their place in the future of neurosurgical education and practice,” Mack et al conclude.
15 Issue50 | June 2023 Novel Technologies
J Mocco
As president of the Society of NeuroInterventional Surgery (SNIS), endovascular neurosurgeon J Mocco (Icahn School of Medicine at Mount Sinai, New York, USA) has aspired to take the baton from his predecessors and carry forward their work in improving access to adequate treatments for stroke patients. Here, he discusses the numerous efforts he and his colleagues have undertaken over the past year on this front; his views on the past, present and future of neurointerventional care; a clinical case that he still draws edification from to this day; and much more.
What initially attracted you to medicine, and neurointerventional surgery specifically?
I did not have any doctors in my family growing up, so I really did not understand what it meant to be a doctor in any specific way. The one thing I did know is that I wanted to practise a version of medicine where time was of the essence, and whether I was skilled at my job, made a real and urgent difference. I wanted to be a kind of doctor who pushed themselves to be excellent, and who saw my commitment to my craft as making a tremendous difference for patients. It turns out that those are exactly the qualities required of a neurointerventionist.
Who have your mentors been and how have they impacted your career?
I have been so incredibly fortunate to have many impactful mentors over the course of my life. However, two stand out as particularly impactful: Sander Connolly and Nick Hopkins. Both were individuals who taught me to look at the field with fresh eyes every day, to not be rooted in the dogma of the past, but instead to wonder, “how can this be done better?” Their guidance and inspiration have shaped me more than anyone, outside my family.
What were your goals when you were elected SNIS president—and would you say you have achieved said goals?
I had several goals when I became SNIS president. These included increasing the strength of our relationships with the international community; creating a more data-centric SNIS; solidifying the Neurovascular Quality Initiative (NVQI) registry; initiating new projects to inspire diversity, equity and inclusion in neurointervention; rolling out a neurointerventional match to create a fair playing field for access to quality training; and deepening our relationships with several related societies.
I believe we have been very successful in these endeavours. I want to emphasise that the operative word in that sentence is “we”. The strength of the SNIS is its board of directors and various committees, both of which are made up by amazing individuals, from diverse training backgrounds, who give their time and energy to advancing the society’s goals. Because of these wonderful people, we have created new partnerships with numerous international societies— including shared programming at our respective meetings, new societies choosing the Journal of NeuroInterventional Surgery (JNIS) as their journal, a new guidance document from the World Federation of Interventional and Therapeutic Neuroradiology (WFITN), and other exciting initiatives. On the domestic front, we are working with partner societies to establish the neurointerventional match and to inform the community of the new IR residency pathway to CAST fellowship eligibility.
We also revamped our annual Corporate Advisory Council, our annual meeting, and our webinars to all
be more data-centric; we have committed to funding the roll out of NVQI to all National Institutes of Health (NIH) StrokeNet STEP centres, which will greatly enhance the rigor and volume of data collection; and we initiated a programme to bring neurointerventional doctors and engineers into childrens’ classrooms, to help children realise that doctors/scientists/ engineers are often women or come from traditionally underrepresented minority groups.
The SNIS and Get Ahead of Stroke campaign have contributed a great deal to improving US stroke transport and triage recently. What do you think the priority should be in this space moving forward?
We must continue to bring attention to the importance of patients’ access to lifesaving thrombectomies. If we do not ensure local and state-wide initiatives emphasise prehospital screening and triage, our communities will suffer from continued inadequate access.
What is your proudest achievement to date in the neurointerventional space? I hope that, should anyone ever look back on my career, they will understand that I sought to bring our community together for the betterment of our patients.

When I began my career, many of our constituent physicians were siloed according to their background specialty, and our respective societies were often at odds. I have worked to build bridges across these different training backgrounds, so that we all work together as neurointerventionists to advance the field. The level of coordination and collaboration that exists today is so much greater than we had even 15 years ago. I am proud to have made meaningful contributions towards bringing the field together, and I believe the SNIS—as a truly multidisciplinary society—is an ideal society to help affect such changes.
What do you feel has been the most important development in this field during your career?
The invention, improvement, and eventual widespread adoption of stroke thrombectomy has been, by far, the most important development in this field to date.
The abilities to embolise aneurysms, or open blocked carotid arteries, or cure dangerous fistulas, have all been tremendously important, but none of those developments have so dramatically affected humanity’s health as stroke thrombectomy. We are now moving into a future where neurointervention will possibly facilitate brain-computer interface and, if successful, then that development may be of a magnitude to rival the benefit provided by stroke thrombectomy.
Could you name the one technology or advancement that you think neurointerventional care stands to benefit from the most?
There are so many exciting technologies and potential advancements on the horizon. But, as I mentioned, I believe our field and humanity itself may be most positively affected by the advent of transvascular braincomputer interface technology.
16 Interview PROFILE June 2023 | Issue50
When I began my career, many of our constituent physicians were siloed according to their background specialty, and our respective societies were often at odds.” alisonlang.com
Appointments (selected):
� 2019–present: Kalmon D Post Professor and senior vice chair, Department of Neurological Surgery, Icahn School of Medicine at Mount Sinai (New York, USA)
� 2014–2022: Professor, Department of Neurological Surgery, Icahn School of Medicine at Mount Sinai
� 2012–2014: Associate professor, Departments of Neurological Surgery and Radiology, Vanderbilt University (Nashville, USA)
� 2009–2011: Assistant professor, Departments of Neurological Surgery and Radiology, University of Florida (Gainesville, USA)
Education:
� 2007–2008: Fellow, Endovascular Neurosurgery, University at Buffalo (Buffalo, USA)
� 2004–2007: Master of Science, Biostatistics, Mailman School of Public Health, Columbia University (New York, USA)
� 2002–2007: Resident, Neurological Surgery, The Neurological Institute of New York
With so much focus being placed on thrombectomy, is there a risk that other areas may become slightly neglected? There is always a risk that, when one area dominates in terms of resources and attention, that the other areas might suffer. I believe that it is one of the most important jobs of the SNIS to ensure this does not happen, and that patients still receive their aneurysm care by physicians with appropriate training and expertise, or technological progress continues to occur for patients with carotid disease, brain arteriovenous malformations and other diseases. As the field grows, and new diseases are treated successfully with neurointerventional techniques every year, we must ensure patients are not cheated out of this progress because one area of the field drowns out the rest.
Could you describe one memorable case and what you learned from it?
I had a case once where, during balloon remodelling, the intracranial carotid artery ruptured. I thought for sure it would result in the patient’s death, but I quickly managed things the best I could and took several steps to try and get the patient through this horrible complication. It was very harrowing, and it took us a while, but we got him back to his life. It has been eight years from that surgery and the patient has no neurologic deficits. I am sure much of his good fortune is due to things that were not under my control, but this case reminds me that nihilism has no place in this field. We can be amazed at the outcomes patients can achieve, if we are willing to put in the time and effort—and a little luck, or fate, comes into play.
What are your interests outside of the field of medicine?
My primary interest outside of work is, by far, my family. I have a wonderful wife, Wendy, who is a lawyer by trade and the heart of our family. We have three rambunctious boys—Finn (14), Michael (13), and Conall (10). My mother, Ruth, lives with us too. It is a wonderful, nuclear family, and we also have a rich extended family who visit often. I try to spend all my free time with my family. I serve as a wrestling coach on my kids’ youth teams and driving them around to their activities is one of my favourite ways to spend time. I grew up playing the trumpet and still very much enjoy music. I wrestled in high school and then played rugby a great deal during my late teens through my early 30s, playing the position of inside centre for the South-Eastern US Select Side champions in my senior year in college, and captaining the Columbia Physicians and Surgeons (P&S) ‘Doctor Warriors’ while in medical school. I had a brief flirtation with fame when a photo of me playing rugby was used as the cover image for Delta Sky Magazine. However, it turns out my face is better suited for radio, as that was my first and last magazine cover.
� 1995–2000: Doctor of Medicine, Columbia University College of Physicians and Surgeons
Honours (selected):
� 2022–2023: President, Society of NeuroInterventional Surgery (SNIS)

� 2019: First Place Abstract, Congress of Neurological Surgeons (CNS) annual meeting
� 2011: Exemplary Teacher Award, University of Florida College of Medicine
� 2005: Outstanding Research Award, New York Society of Neurosurgery
17 Interview Issue50 | June 2023
Fact file
Study finds worse post-stroke neurologic outcomes in Spanishspeaking Americans
NEW RESEARCH HAS INDICATED that Mexican Americans have worse outcomes after a stroke than nonHispanic white Americans.
A recent study, which looked at whether the language Mexican American people speak is linked to how well they recover after a stroke, has been published in an online issue of the journal Neurology
“Our study found that Mexican American people who spoke only Spanish had worse neurologic outcomes three months after having a stroke than Mexican American people who spoke only English or were bilingual,” said study author and American Academy of Neurology (AAN) fellow Lewis Morgenstern (University of Michigan, Ann Arbor, USA). “More research is
needed into what factors and barriers may influence these worse outcomes.”
The study involved 1,096 Mexican American people in Corpus Christi, Texas in the USA who had a stroke over a 10-year period.
Researchers looked at results three months after the stroke in three areas: neurologic, functional, and thinking and memory skills. Neurologic results cover areas like muscle strength and coordination, and problems with speech or vision, while functional results include how well people can complete their daily activities, such as showering and preparing meals.
The 170 people who spoke Spanish only were compared to the 926 people who spoke English only or were bilingual. Those who spoke Spanish
only were older, had received less education and had worse neurologic scores at the time of the stroke than those in the other group.
Three months after their stroke, the Spanish-only speakers had average neurologic scores of seven, where scores of five to 14 indicate moderate effects from a stroke. The English-only and bilingual speakers had average scores of four, where scores of one to four indicate mild effects. The results remained after researchers adjusted for the differences between the two groups
Researchers identify novel protein linked to ischaemic stroke risk in older adult patients
By identifying plasma proteomic determinants of incident cardioembolic and noncardioembolic ischaemic stroke, researchers in the USA have found a novel protein believed to be associated with stroke risk in patients with left atrial dysfunction.
Detailing findings from the Cardiovascular Health Study—now published in the journal Neurology—corresponding author Rizwan Kalani (University of Washington, Seattle, USA) and colleagues conclude that N-terminal pro-brain natriuretic peptide (NTproBNP) and macrophage metalloelastase (MMP12) were “independently associated” with ischaemic stroke risk.
“Plasma proteomics may elucidate novel insights into the pathophysiology of ischaemic stroke, identify biomarkers of ischaemic stroke risk, and guide development of nascent prevention strategies,” Kalani et al initially note.
On this basis, they set out to evaluate the relationship between plasma proteome and ischaemic stroke risk in the population-based Cardiovascular Health Study. Eligible study participants were free of prevalent stroke and underwent quantification of 1,298 plasma proteins using the aptamer-based SOMAScan assay platform (SomaLogic) from a 1992–1993 study visit.
For plasma protein concentrations independently associated with incident ischaemic stroke—determined via multivariable Cox proportional hazards regression, and following adjustments for demographics, stroke risk factors and estimated glomerular filtration rate—a secondary stratified analysis evaluated associations
and other factors that could affect stroke risk, such as high blood pressure and diabetes.
The study found no difference between the two groups in how well they recovered their ability to complete daily activities, or in their thinking and memory skills.
“We conducted an earlier study in this same community finding that the language people spoke was not associated with any delay in their getting to the hospital or using emergency medical services after an ischaemic stroke, so we definitely need more information to determine what is driving the differences in outcomes between these two groups,” Morgenstern added.
A study limitation acknowledged by the researchers is that there was a low number of Spanish-only speakers. Also, the majority of Mexican Americans in Corpus Christi are born in the USA, so these results may not be applicable to areas with a larger population of people born outside the country, the researchers note.
in subgroups defined by sex and race. The authors also state that exploratory analyses evaluated plasma proteomic associations with cardioembolic and noncardioembolic ischaemic stroke, as well as proteins associated with stroke risk in participants with left atrial dysfunction but without atrial fibrillation.
Across 2,983 eligible participants, the mean age was 74.3 years, 61.2% were women, and 15.4% were Black. And, over a median follow-up period of 12.6 years, 450 participants experienced an incident ischaemic stroke.
Ultimately, NTproBNP (adjusted hazard ratio [HR] 1.37, 95% confidence interval [CI] 1.23–1.53, p=2.08x10-08) and MMP12 (adjusted HR 1.30, 95% CI 1.16–1.45, p=4.55x10-06) were both found to be independently associated with ischaemic stroke risk in a cohort of older adult patients. Kalani et al further detail that these two associations were similar in men and women, and in Black and non-Black participants.
Additionally, in exploratory analyses, NTproBNP was also independently associated with:
� Incident cardioembolic ischaemic stroke
� E-selectin with incident non-cardioembolic ischaemic stroke
� Secreted frizzled-related protein-1 with ischaemic stroke risk in participants with left atrial dysfunction.
18 June 2023 | Issue50
Stroke Research Plasma proteomics may prove key in preventing stroke before clinical manifestation
More research is needed into what factors and barriers may influence these worse outcomes.”
Early diagnosis and treatment needed in “often underreported” post-stroke cognitive impairment
More than half of all stroke survivors may develop cognitive impairment within a year after their stroke, and one in three are at risk for developing dementia within five years. That is according to a new American Heart Association (AHA) scientific statement published in the journal Stroke, advocating post-stroke screenings and comprehensive interdisciplinary care to support stroke survivors with cognitive impairment.
“COGNITIVE IMPAIRMENT IS AN OFTEN underreported and underdiagnosed yet very common condition that stroke survivors frequently deal with,” said Nada El Husseini (Duke University Medical Center, Durham, USA), chair of the scientific statement writing committee.
“Stroke survivors should be systematically evaluated for cognitive impairment so that treatment may begin as soon as possible after signs appear.”
An AHA scientific statement is an expert analysis of current research and may inform future guidelines. According to the recent statement, entitled “Cognitive impairment after ischemic and hemorrhagic stroke”, cognitive impairment after stroke is common in the first
year after a stroke, occurring in up to 60% of stroke survivors, and is most common within the first two weeks after a stroke. In addition, about 40% of people who survive a stroke have cognitive impairment during the first year that does not meet diagnostic criteria for dementia, yet still impacts their quality of life. Post-stroke cognitive impairment is often associated with other conditions too, including physical disability, sleep disorders, behavioural and personality changes, depression and other neuropsychological changes—each of which may contribute to lower quality of life.
“Cognitive impairment after stroke ranges from mild impairment to dementia and may affect many aspects of life, such as remembering, thinking, planning, language and attention, as well as a person’s ability to work, drive or live independently,” El Husseini said.
There is no gold standard for cognitive screening after a stroke, according to the scientific statement. However, some brief screening tests (30 minutes or less) are widely used to identify cognitive impairment after a stroke: the Mini-Mental State Examination and the Montreal Cognitive Assessment.
While early detection during initial hospitalisation for stroke is important for immediate care planning, it is also important to assess cognitive changes over time, the statement continues. Stroke survivors who experience unexplained difficulties with cognitiverelated activities of daily living, following care instructions or providing a reliable health history may be candidates for additional cognitive screening.
When cognitive impairment is detected, healthcare professionals are encouraged to assess an individual’s daily functioning with neuropsychological screenings, which evaluate areas of brain function that affect behaviour, and may provide a more thorough picture of the individual’s cognitive strengths and weaknesses. Healthcare professionals are also encouraged to offer
Clinical stroke research in Canada receives million-dollar grant boost
Three Canadian charities—the Heart and Stroke Foundation, Brain Canada, and the Canadian Stroke Consortium—have announced the recipients of the 2022 Stroke Clinical Research Catalyst grants. A total of 10 projects were awarded said grants, each worth CAD$100,000, totalling CAD$1 million in funding.
THE PURPOSE OF THIS PROGRAMME, AS per a joint press release, is to increase the capacity for clinical stroke research within Canada, with an aim to reduce the burden of stroke, prevent recurrence, and improve patient outcomes through clinical research that will improve the current understanding of stroke and advance stroke care.
“We are thrilled to be collaborating with two leading organisations in stroke research to ultimately drive discovery,” said Viviane Poupon, president and CEO of Brain Canada. “These 10 investigators are contributing to improved care along the continuum of stroke, which could transform the lives of many people impacted by stroke in Canada.”
“The Canadian Stroke Consortium is committed to reducing the burden of stroke through fostering quality clinical research and translating our learnings into patient care,” added Andrew Demchuk, chair of the Canadian Stroke Consortium. “On behalf of our members, we are delighted to support the important work of these 10 researchers. Their efforts will contribute
to enhancing the lives of Canadians experiencing stroke.”

“This is an exciting opportunity to provide 10 leading stroke researchers with the initial seed funding they need to develop new lines of research and to generate preliminary data,” stated Doug Roth, CEO of the Heart and Stroke Foundation. “The goal is that this initial investment will support successful applications to larger grants to further advance stroke health.”
The release also notes that the Stroke Clinical Research Catalyst programme has been made possible by the Canada Brain Research Fund (CBRF)—an innovative arrangement between the government of Canada, via Health Canada, and the three aforementioned charities.
To date, Health Canada has invested more than CAD$155 million in total through the CBRF, which has been matched by the Brain Canada Foundation, and its donors and partners.
guidance to patients and their caregivers regarding home safety, returning to work and driving after a stroke, and connect caregivers and stroke survivors to community resources for social support.
Interdisciplinary collaboration among healthcare professionals, such as physicians, speech language therapists, occupational therapists, neuropsychologists and nurses, is often needed for optimal monitoring and care for people with cognitive impairment after a stroke. The AHA statement further suggests behavioural cognitive rehabilitation and physical activity may help improve cognition after a stroke.
Preventing another stroke is a key consideration to prevent the worsening of cognitive impairment after a stroke. This includes treatments for stroke risk factors, such as high blood pressure, high cholesterol, Type 2 diabetes and atrial fibrillation. Blood pressure control is associated with reduced risk for recurrent stroke and for mild cognitive impairment too.
There are unanswered questions regarding how cognitive impairment develops after stroke, and the impact of non-brain factors—including infection, frailty and social factors.
Further research is needed to determine best practices for cognitive screening after a stroke, including the development and use of screening instruments that consider demographic, cultural and linguistic factors in determining “normal” function, the statement details.
“Perhaps the most pressing need, however, is the development of effective and culturally relevant treatments for post-stroke cognitive impairment,” El Husseini concluded.
“We hope to see big enough clinical trials that assess various techniques, medications and lifestyle changes in diverse groups of patients that may help improve cognitive function.”
Stroke Clinical Research Catalyst grant recipients:
● Sean Dukelow (University of Calgary, Calgary)—the TeleTaCAS randomised controlled feasibility trial
● Aravind Ganesh (University of Calgary, Calgary)—development and testing of a system for remote ischaemic conditioning in preparation for clinical trials in cerebral small vessel disease and prehospital stroke care
● Raed Joundi (McMaster University, Hamilton)—incidence, trends, determinants and prognosis of post-stroke dementia (INTREPID): a 20-year registry and population-based cohort study
● Aristeidis Katsanos (McMaster University, Hamilton)—blood pressure management in stroke following endovascular treatment (DETECT)

● Ethan MacDonald (University of Calgary, Calgary)—developing a magnetic resonance imaging (MRI)-based pH mapping tool for clinical stroke assessment
● Michelle Ploughman (Memorial University, St John’s)—verifying aerobic training protocols to benefit both heart and brain in subacute stroke
● Alexandre Poppe (Université de Montréal, Montreal)—a multicentre, prospective, randomised, open-label, blinded endpoint (PROBE) controlled trial comparing acute cervical internal carotid artery stenting to no stenting during endovascular thrombectomy for anterior circulation stroke due to acute tandem occlusion: endovascular acute stroke intervention—tandem occlusion trial (EASI-TOC)
● Deborah Siegal (University of Ottawa, Ottawa)—intensive cancer screening for cryptogenic stroke (INCOGNITO) pilot randomised trial
● Nishita Singh (University of Manitoba, Winnipeg)—adaptive platform trial to investigate various therapies in carotidassociated stroke (ACTIVATE-CAS) pilot phase
● Luciano Sposato (Western University, London)—sweet spot for cardiac rhythm monitoring after stroke (STARGATE) pilot trial: a pilotfeasibility randomised controlled trial
19 Issue50 | June 2023 Stroke Research
Image credit: AHA
Nada El Husseini
14 Congress 2022 15thth Congress 2023
13 Congress 2021 th
15 th Congress of the European Society of Minimally Invasive Neurological Therapy

13th Congress of the European Society of Minimally Invasive Neurological Therapy
4 – 6 September 2023
8 – 10 September 2021
Palais du Pharo – Marseille, France & online
Acropolis Convention Centre – Nice, France & online
Congress President
Main Topics:
Prof. Paolo Machi, Switzerland
• Aneurysms
• AVM
Vice Presidents
• Ischemic Stroke
Dr. Mohamed Aggour, United Kingdom
Dr. Anne Christine Januel, France
With thematic focus on:
• Post SAH vasospasm
• Visualization of the diseased vessel wall
• Clinical evidence for new medical devices

Advancing neurointerventional treatment: Between divergent and convergent thinking
Congress President
C. Taschner, Germany
Vice Presidents
W. van Zwam, The Netherlands
Z. Kulcsar, Switzerland
Come and join us for the 15th ESMINT Congress –the fi rst edition to be held in Marseille, France






© katatonia / Fotolia.com
More information at:
More information at www.esmint.eu
www.esmint.eu
In collaboration with EANS, ESO and SNIS
In collaboration with EANS, ESO and SNIS
“The sky has never been the limit”
“Overall, we just need better tools” was one of the salient points that stemmed from a talk focusing on stroke cases with rare pathologies at the LINNC Americas Seminar (16–17 March, Miami, USA).
Jane Khalife (Cooper University Health Care, Camden, USA) presented the case of a 75-yearold woman with a small, subacute infarct in the A3 segment of their left anterior cerebral artery (ACA) who remained symptomatic—experiencing recurrent falls and right-leg weakness—following two spinal operations and despite multiple attempts at medical management via drug regimens.
The patient’s lesion was surmised to have been caused by intracranial atherosclerotic disease (ICAD), and she underwent a stenting procedure. Specifically, an approach involving a sequential angioplasty and self-expanding stent was selected owing to the tortuosity of her vessels. The procedure was a technical success and ultimately produced positive results, with the patient’s symptoms being largely resolved at a seven-month follow-up appointment, as she returned to her premorbid functional status.
In addition to her point on the need for better intracranial stents, Khalife concluded that the case
demonstrated the importance of a multidisciplinary approach to diagnosing and managing patients with rare stroke pathologies. Her other take-home message was that stent selection choices should be made based on lesion aetiology and vessel anatomy.
Following this presentation, there was broad agreement among attendees that improvements to current device options in intracranial stenting are required. LINNC course director Laurent Spelle (Bicêtre Hospital, Paris, France) commented that there are very few stents that can successfully reach locations as distal as the one seen in this case, and it would be “impossible” to track a balloon-mounted stent in such cases as well. He therefore queried why more neurovascular companies are not focusing on these disease types—which are largely absent in Europe but are found more commonly in North America and Asia. “So, it is a big market, [but] we are still missing the proper tools for this,” he noted.
“I think the companies are all here, and they are all well-represented, so they are hearing us—ICAD is coming back,” added course director Vitor Mendes Pereira (St Michael’s Hospital, Toronto, Canada), before highlighting that, owing to positive case results with intracranial stenting like Khalife’s, “we are creating the same movement that we had with [acute] stroke a few years ago”. “We are treating, and seeing good results, and now we need a good trial with good technology [to confirm this],” Pereira averred.
Here, one LINNC Americas attendee noted that previous ICAD trials constitute a similar story to that of the ARUBA study—a randomised trial that suggested medical management alone was noninferior to medical management plus interventional therapy in unruptured brain arteriovenous malformations (AVMs), but was widely criticised for its design and methodology. Subsequent observational studies in ARUBA-eligible patients have indicated favourable safety and efficacy can be achieved with appropriate patient selection and improved treatment
Paediatric case helps highlight benefit of ‘aspiration first’ in young stroke patients
A unique case presentation at this year’s LINNC Americas Seminar (16–17 March, Miami, USA) saw Rafael de Oliveira Sillero (University of Texas Southwestern Medical Center, Dallas, USA) outline how he treated a child with ischaemic stroke at his centre, drawing a consensus from attendees that ‘aspiration first’ is the optimal mechanical thrombectomy approach in such scenarios.
THE CASE DE OLIVEIRA SILLERO delivered was that of an otherwise healthy two-year-old girl who awoke from a nap aphasic and unable to move her right side. He noted that a physical examination found the patient to be wide awake, but unable to follow commands and with a National Institutes of Health stroke scale (NIHSS) score of 14, when she arrived at the intensive care unit (ICU).
“In general, the decision-making process in children is really complicated for a couple of reasons,” De Oliveira Sillero informed the LINNC Americas audience.
“First question: is thrombectomy even indicated, because of the young age—only two years old? As we all know, these children have very good
collaterals. Secondly, is the door open for thrombectomy? We saw that the left carotid [artery] was occluded, or perhaps there was a bad dissection.”
The speaker reported that, ultimately, and in spite of a rare Texan snowstorm making travel to the hospital more challenging, they proceeded with a thrombectomy treatment. Ultrasound— the preferred approach in all paediatric cases at De Oliveira Sillero’s centre— was deployed, and an aspiration-first approach using the Sofia 5Fr catheter (Microvention) was selected.
Through this approach, De Oliveira Sillero and his colleagues were able to achieve first-pass total reperfusion of the occluded left cervical segment of the common carotid artery, with no dissection.

modality choices in multidisciplinary centres.
“This is a great case and it just shows that, in the right hands, and [through] planning things well, the results of angioplasty and stenting intracranially are really good,” another attendee said. “And, just like we need better catheters, we [do] need better stents, but we have to work with what we have available now and I do think that we have some really good options.”
However, they also concluded that what we “really lack” is better studies to “fight against” old data suggesting ICAD patients do worse with angioplasty and stenting—adding that this is key because, in cases like this where stenting is done “appropriately”, the results are “extremely good”.
Providing some balance to the points made previously, Ricardo Hanel (Baptist Neurological Institute, Jacksonville, USA) drew attention to a recent study from China, involving a “super-selective patient population” treated by experienced, top-level physicians from only the highest-enrolling centres, in which stenting still produced negative results in ICAD patients. On this basis, Hanel stated that the disease is “way more complicated than we evaluate”, and posited that it may not be appropriate to blame failures seen in SAMMPRIS and other previous stenting trials on a lack of operator experience alone.
An M1 occlusion in the patient’s intracranial circulation was also treated successfully, with total recanalisation (thrombolysis in cerebral infarction [TICI] 3) being achieved at the second pass.
“Note that there is no spasm in the MCA [middle cerebral artery], which is [one of] the benefits of using aspiration,” he added, presenting postoperative imaging from the case. “And, going back to discussions about the anatomy, I think this is really important in the decisionmaking process.”
De Oliveira Sillero further noted that, had an M2 occlusion been found on imaging, thrombectomy would not have been the recommended approach in this instance.
He went on to report that the patient demonstrated positive outcomes on follow-up magnetic resonance imaging (MRI) and, following rehabilitation, was doing “really well” at one month post-treatment, with “only a mild lefthand weakness”.
Highlighting a couple of discussion
points raised by this case, De Oliveira Sillero noted that “we all love the transradial approach these days— but not for children”, adding that “there is no true reason to do it in a paediatric case”.
He also referred to a 2019 paper in the Journal of Neurosurgery: Pediatrics detailing a total of 16 thrombectomy cases in young children (<5 years), half of which involved MCA occlusions, with the majority favouring a stent retriever-first technique and only one aspiration-first approach being recorded.
Despite this, the speaker reiterated that he favours aspiration in paediatric stroke cases—a point that LINNC course director Vitor Mendes Pereira (St Michael’s Hospital, Toronto, Canada) voiced support for, citing his own experiences of stent retrievers leading to vessel spasm in these patients.
Johanna Fifi (Mount Sinai Health System, New York, USA) also corroborated this view, stating that she employs a “very similar” aspirationfirst approach in her practice. She also noted that a ‘radial-type’ sheath with a smaller outer diameter can be deployed when accessing the femoral artery, enabling a 6Fr sheath to be advanced.

21 Issue50 | June 2023 Unique Cases LINNC AMERICAS
‘We need better tools’—new intracranial stent devices required for rare stroke pathologies
I think the companies are all here, and they are all well-represented, so they are hearing us—ICAD is coming back.”
Vitor Mendes Pereira
Rafael de Oliveira Sillero
Initial cases indicate lithotripsy can help TCAR expand into high-risk patients

Lithotripsy may hold the key to enabling more carotid artery disease patients who require calcification treatment to undergo stent placement via a transcarotid artery revascularisation (TCAR) procedure, as per single-centre experiences presented at the recent Society for Clinical Vascular Surgery (SCVS) annual symposium (25–29 March 2023, Miami, USA) by Kathryn DiLosa with principal investigator Misty Humphries (both University of California Davis, Sacramento, USA).
IN CASES OF CIRCUMFERENTIAL OR eccentric calcification, TCAR is precluded and carotid endarterectomy (CEA) often becomes the preferred approach—however, in patients considered ‘high risk’ due to their anatomy or prior surgeries, for example, “another alternative exists”, the speaker averred.
Detailing the use of intravascular lithotripsy (IVL; Shockwave Medical) prior to a TCAR procedure, DiLosa noted that predilation angioplasty may be required to allow passage of a lithotripsy balloon, and the balloon “should be sized to fully oppose the vessel wall, but not extend past the intended coverage area”.
She further stated that lithotripsy technologies have been used in the treatment of kidney stones previously, and are now shifting into the endovascular space.
The speaker also reported a 100% rate of technical success with this approach at her institution, across a
total of seven patients, with comparable procedural and flow-reversal times to standard TCAR, and no observed complications within 30 days of the procedure.
“However, a larger cohort [of patients] is still
Dual antiplatelet therapy linked to better post-TCAR outcomes versus other drug
regimens
Recent data presentations have revealed reduced risks of stroke and mortality among transcarotid artery revascularisation (TCAR) patients who receive dual antiplatelet therapy (DAPT)—both preoperatively and at discharge—as compared to other drug regimens. Researchers believe these findings underscore the importance of compliance to DAPT regimens before and after stent placement via a TCAR procedure.
Astudy published in the Journal of Vascular Surgery (JVS) earlier this year saw Hanaa Dakour-Aridi (Indiana University School of Medicine, Indianapolis, USA), S Keisin Wang (McGovern Medical School at University of Texas Health, Houston, USA) and colleagues examine the association between different preoperative antiplatelet regimens and in-hospital outcomes after TCAR.
This research ultimately found improved clinical outcomes with DAPT compared to other medication regimens in carotid artery disease patients undergoing TCAR.
The authors queried all patients treated with TCAR within the Vascular Quality Initiative (VQI) from September 2016 to June 2022.
They did this against the backdrop of DAPT being the preferred medication regimen in post-TCAR patients, “despite a dearth of quality data”, in an effort to define the risks carried by
different antiplatelet approaches.
Patients on DAPT were compared with those receiving alternative regimens—single antiplatelet therapy (SAPT), anticoagulation, or a combination of the two—with a 1:1 propensity-score match being performed. In-hospital and oneyear outcomes were compared between groups.
The authors report that a total of 29,802 procedures were included in their study population, with 82.7% receiving DAPT and 17.3% receiving an alternative regimen.
Compared with patients on DAPT, in-hospital ipsilateral stroke rates were “significantly higher” among those receiving other types of antiplatelet therapy (1.1% vs 1.7%, respectively).
And, while there was no statistically significant difference between the two groups regarding mortality—0.5% with DAPT and 0.6% without—a composite of stroke/death was more likely in patients receiving an alternative
needed to confirm safety,” DiLosa said.
Responding to an audience query on how lithotripsy is able to successfully break up calcium without leading to embolisation—a concern she admitted to having initially herself—DiLosa added that “all of the available literature has demonstrated that it is able to fracture the calcium, within the wall, without it embolising from the wall”.

Briefly touching on the available literature regarding pre-TCAR lithotripsy, she stated that case studies— but no significant case series—are available at this point, although she and her colleagues are currently compiling a multi-institutional cohort including more than 50 patients.
“This is definitely for a specific patient population— those who cannot tolerate endarterectomy, but who would need the benefit of calcification [treatment],” DiLosa concluded.
regimen (2.4%) rather than DAPT.
The authors also found that immediate stent thrombosis was more common in the ‘alternative’ group of patients, and a non-significant trend towards increased rates of returning to the operating room was observed.
“Conversely, the incidence of perioperative myocardial infarction was lower in the alternative regimen group [than with DAPT],” the authors posit, highlighting rates of 0.4% and 0.7%, respectively. “At one year after the procedure, we observed an increased risk of mortality but not stroke in patients treated with an alternative medication regimen.”
Dakour-Aridi et al ultimately convey that their analysis found an increased risk of both in-hospital stroke and one-year mortality in TCAR recipients who were treated with an alternative approach to DAPT—concluding that “further studies are needed to elucidate the drivers of DAPT failure in patients undergoing TCAR to improve outcomes for carotid stenting patients”.
Further to this JVS publication, Dakour-Aridi also delivered the findings from a similar study at this year’s Society for Clinical Vascular Surgery (SCVS) annual symposium (25–29 March 2023, Miami, USA), with the key distinction being that the

researchers evaluated DAPT regimens at discharge, rather than preoperatively, in patients undergoing TCAR.
They found that 19.2% of patients in the VQI are not discharged on dual antiplatelets after stent placement via TCAR—9% receive a ‘triple therapy’ involving DAPT plus anticoagulation, 5.8% are given SAPT plus anticoagulation, and 4% are directed to take either SAPT or a single anticoagulant.
“We demonstrated that patients discharged on a combination of single antiplatelets with anticoagulation witnessed increased [rates of] 30-day stroke, high-grade restenosis, and one-year mortality and stroke/death,” Dakour-Aridi noted.
“The use of a single antiplatelet or single anticoagulant after TCAR was associated with increased 30-day and one-year stroke/death risks. However, there was no significant association between triple therapy and 30-day stroke/death outcomes [following multivariate analysis adjustments].”
Highlighting study limitations, she touched on the absence of indications for antiplatelet regimens on discharge—increasing the likelihood of selection bias—as well as limited data at follow-up, and the unknown risk of bleeding with triple therapy.
Nevertheless, Dakour-Aridi concluded that “[…] these findings reinforce our prior study on the importance of compliance to DAPT after TCAR, as well as the need for further follow-up studies to evaluate the appropriateness of TCAR in different patient populations”.
22 June 2023 | Issue50 TCAR
CAROTID
This is definitely for a specific patient population—those who cannot tolerate endarterectomy, but who would need the benefit of calcification [treatment].”
Kathryn DiLosa presenting at SCVS 2023
Hanaa Dakour-Aridi S Keisin Wang
Medical therapy improvements mean “the time has come” to
reconduct prior endarterectomy trials
“The time has definitely come to look at the evidence, and redo these studies,” posited Alun Davies (Imperial College London, London, UK), putting forward his argument that the NASCET and ECST clinical trials “need to be reconducted” at this year’s Charing Cross (CX) Symposium (25–27 April, London, UK).
MUCH OF DAVIES’ ARGUMENT
centred around the fact that best medical therapy—the comparator arm against which carotid endarterectomy (CEA) was assessed, and found to produce clinical benefits in carotid artery stenosis patients, in both of these studies—is “significantly better than it was” at the time.
“We are relying on evidence from 1992, and I would say the playing fields have completely changed,” he noted.
After briefly outlining discrepancies between the North American NASCET and European ECST trials regarding how internal carotid artery stenosis was
defined—with ECST having a higher threshold for severity—Davies also reminded the audience that NASCET observed a 3.3% stroke/death rate at one month in its medical therapy arm, compared to roughly 5–6% with CEA.
He then alluded to a 2003 paper by Ross Naylor (University of Leicester, Leicester, UK) et al that, based on primary and secondary analyses of both trials, concluded that not all patients with symptomatic stenosis >70% will benefit from CEA. Davies went on to cite a pooled analysis of prior randomised trials, published in the European Journal of Vascular
and Endovascular Surgery in 2021, that found a lower stroke risk with best medical therapy in earlier studies (1981–1996) versus later ones (2000–2008).
Furthermore, he continued, recent research has shown that targeting and lowering a patient’s cholesterol, and initiating best medical therapy more quickly, can reduce adverse event rates by up to 80% early on. Applying this reduction to the rate of 3.3% seen in NASCET, he theorised that stroke/death incidence could now be as low as 0.7% with today’s best medical therapy.
This, coupled with uncertainties surrounding if—and in which cases— CEA is a cost-effective procedure, led Davies to reiterate that more randomised controlled trial data are now needed to re-evaluate the benefits of surgery versus medical therapy.
“We would certainly all prefer to take a tablet than have an operation or have a metal stent stuck in our neck,” Davies asserted. “I would suggest that, unless we get better evidence, […] we will not be doing CEA much in 2025.”
At the close of his presentation, the speaker highlighted the COMeT
Vascular community split over “death” of traditional carotid stenting
A debate between two prominent carotid interventionists—Peter Schneider (University of California San Francisco, San Francisco, USA) and Domenico Valenti (King’s College London, London, UK)—on the Wednesday of the Charing Cross (CX) symposium (25–27 April 2023, London, UK) revealed that the vascular community is currently divided over the benefits of transcarotid artery revascularisation (TCAR), as compared to percutaneous carotid artery stenting (CAS).
SCHNEIDER KICKED OFF THE DEBATE BY arguing in favour of TCAR, initially outlining some of the limitations of percutaneous CAS that prior studies have demonstrated—for example, unwanted events relating to manipulation of the aortic arch as well as incomplete particulate capture. He went on to query if there is a “better way” to treat carotid artery disease, positing that TCAR may offer a solution that

overcomes these limitations.
Again referring to the current literature, he noted that TCAR can enable greater neuroprotective capabilities; improved safety in symptomatic patients and octogenarians; and carries a more efficient learning curve, versus percutaneous stenting.
After reiterating the “unresolved challenges” CAS faces, including those relating to the aortic arch and
study—a proposed randomised trial comparing carotid intervention and best medical therapy that he and his colleagues are currently preparing to submit to the UK National Institute for Health Research (NIHR) for the second time, and for which they intend to enrol more than 2,500 patients.
Moderator Domenico Valenti (King’s College London, London, UK) said he “completely agreed” with Davies’ views, describing NASCET as an “obsolete” trial and stating that newer best medical therapies are “more than effective”.
Valenti also raised the issue of patient compliance when it comes to taking multiple medications over an extended time period—leading Davies to aver that, “if you actually take the time to educate your patients properly, […] and spend 10–15 minutes talking to them as you might do when consenting them for an intervention, you might find that your compliance is better”.
“I fully accept there are patients who may be intolerant of medications, and could benefit from an intervention, but I think the jury needs to have new data to be able to consider,” Davies concluded.
particulate capture, Schneider concluded that prospective TCAR studies and his own experiences have shown both safety and efficacy with the procedure, demonstrating outcomes that are “competitive” with carotid endarterectomy (CEA) and “substantially better” than percutaneous stenting.
Offering a riposte to these assertions, Valenti stated that TCAR—in essence, at least—has been around for many years, and is therefore not the “disruptive technology” within carotid interventions it is sometimes presented as, also claiming that his opponent and other TCAR advocates have been “mesmerised” by the existing data.
Homing in on said data, Valenti challenged positive conclusions drawn from the results of the ROADSTER 2 trial, noting its non-randomised nature, low proportion of symptomatic patients and, “more importantly”, lack of stratified data on delay to treatment. Additionally, he told attendees that findings from the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative (VQI) are also limited by selection biases, gaps in the data, and the fact they come from a non-randomised trial.
Finally, Valenti noted that CAS has been subject to far more “intense scrutiny” than TCAR to date— for example, in the CREST, ICSS and SPACE randomised controlled trials (RCTs). And, while he commented that TCAR may yet prove useful in certain patients, they are yet to be identified, and the lack of RCT-derived evidence supporting it means the “death” of percutaneous stenting is not imminent.
A subsequent audience poll produced a very close result, but ultimately saw more CX attendees concur with Valenti’s closing gambit that “TCAR is not about to send CAS to oblivion”, as 52% voted against the statement that ‘TCAR is better than percutaneous carotid stenting’.
23 Issue50 | June 2023 CX 2023
Peter Schneider (L) debates with Domenico Valenti
TCAR
stenting 48% 52% For Against
is better than percutaneous carotid







*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the vascular arena Editorially independent Visit vascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
RECOVER trial will provide “thorough understanding” of VNS response in depression patients
A new randomised controlled trial (RCT)—the “largest ever” of its kind in the major depressive disorder (MDD) space—will attempt to provide a “thorough understanding” of how treatmentresistant patients respond to vagus nerve stimulation (VNS) therapy.
treatment group, their VNS devices will be turned on, while sham (placebo) patients’ devices will be implanted but not activated. In both groups, the therapeutic protocol will be followed for 12 months.
Depression ratings are being performed by blinded, offsite parties, Conway and colleagues note. RECOVER’s primary outcome measure is the number of months in ‘response’— defined as a 50% reduction in depression score from baseline score—comparing those receiving active versus sham VNS.
The authors report that a total of 500 unipolar patients (mean age ~54 years) have been enrolled. They relay a mean MADRS score of 34.6 and a mean age at symptom onset of 20.9 years, as well as 20.4 years of lifetime MDE across the patient cohort. In addition, Conway and colleagues note an average failed lifetime of antidepressant medications of 14.1 years, with many patients receiving “aggressive treatments”—including electroconvulsive therapy (ECT [41.9%]), transcranial magnetic stimulation (TMS [46.7%]) and ketamine (21.3%). Finally, they report high numbers of psychiatric hospitalisations (64%) and attempted suicide (43.8%).
“To date, 222 patients—including 40 terminated early— have completed the trial’s first year,” the authors also write.
Office-based removal of sacral neuromodulation devices deemed safe and welltolerated
AS PER A RECENTLY PUBLISHED retrospective chart review, the removal of sacral neuromodulation (SNM) devices in the office setting, under local anaesthesia, is a safe, well-tolerated and effective procedure.
Reporting their findings in the journal Urology, Sarah Martin and Howard Goldman (both Cleveland Clinic, Cleveland, USA) initially state that, in instances whereby SNM devices need to be removed, permanent lead and implantable pulse generator (IPG) explants are “traditionally” performed in the operating room under sedation.
But—due to the fact that officebased removal of SNM devices holds the potential to reduce the length of hospital stays for patients and thus decrease healthcare system costs—the authors relayed experiences of SNM components being removed in the office setting, and under local anaesthesia, by a single surgeon.
Details of the RECOVER trial were presented earlier this year at the 5th International Brain Stimulation Conference (19–22 February, Lisbon, Portugal) and have subsequently been published in the journal Brain Stimulation
Writing in Brain Stimulation, Charles Conway (Washington University School of Medicine, St Louis, USA) and colleagues outline that therapeutic VNS has been approved by the US Food and Drug Administration (FDA) for use in patients with treatment-resistant depression.

They note, however, that in 2007 the US Centers for Medicare and Medicaid Services (CMS) issued a noncoverage decision, “making access difficult for patients”, and in 2019 subsequently requested a coverage with evidence development (CED) trial to determine the potential efficacy of VNS therapies in the US Medicare population of treatmentresistant depression patients.
The RECOVER trial was therefore set up to meet this need. Conway and colleagues state that the study has been cosponsored by CMS and LivaNova—the company that makes the Symmetry device being assessed in the trial. This large, prospective, multicentre, placebo-controlled trial is evaluating VNS’ efficacy over the course of 12 months at a total of 71 US sites.
RECOVER includes patients aged ≥18 years who have been diagnosed with MDD or major depressive episode (MDE) in bipolar disorder—either chronic (two years) or recurrent (≥4 MDEs)—defined as a score ≥22 on the Montgomery-Åsberg depression rating scale (MADRS). The trial’s exclusion criteria include substance use and psychotic disorders; acute suicidal ideation/intention; and severe personality disorder.
Once a patient has been selected as appropriate for the trial, they are being randomised to receive either active or ‘sham’ treatment before being implanted with a VNS device. In the
Concluding their Brain Stimulation report, Conway and colleagues state that RECOVER is “the largest prospective, randomised, device-based, placebo-controlled MDD trial ever undertaken, enrolling severe treatment-resistant depression patients not studied in most MDD clinical trials”. They further claim that the trial will provide a “thorough understanding” of the nature of VNS responses in depression patients, such as the onset of response and potential baseline outcome predictors.
Speaking to NeuroNews, Conway noted that final follow-up for the unipolar 12-month visit is expected in Q2 2024, with publication of the results from RECOVER expected by the end of 2024.
“The results of this trial will likely provide much-needed guidance regarding management of patients with severe treatment-refractory depression,” he added. “As noted, many of the patients in this trial have failed numerous and aggressive treatments, so the results should help guide us to understand the appropriate place for VNS in the treatmentresistant depression armamentarium.”
They conducted a retrospective chart review from 2017–2022 of SNM lead and IPG removals performed in this way, reporting patient characteristics, outcomes, and complications, and found that a total of 41 SNM leads with/ without IPGs were removed via an office-based procedure. Some 88% of the included patients were female, and the mean age was 66 years.
“The most common indications for SNM treatment were urgency incontinence (56%) and non-obstructive urinary retention (24%),” Martin and Goldman write. They further state that 44% (18/41) of SNM device removals were leads removed after failed stage I, while the remaining 56% (23/41) were leads with IPGs removed most commonly for decreased efficacy. In the latter group, median time to removal was 3.1 years, and 52% (12/23) required a separate medial incision for lead retrieval. Additionally, while 9% (2/23) required fluoroscopy, none of these were stage I failures.
Finally, Martin and Goldman relay that 7% (3/41) of leads required cutdown to the sacrum for removal with leads in place between 3.1–3.9 years.
“All leads were removed completely intact and there were no complications,” the authors add, concluding that, as such, the in-office removal of SNM devices under local anaesthesia can be deemed “effective, well-tolerated, and safe” based on their experiences.
25 Issue50 | June 2023
Many of the patients in this trial have failed numerous and aggressive treatments, so the results should help guide us to understand the appropriate place for VNS in the treatment-resistant depression armamentarium.”
Charles Conway
Pope
Point of View
Jason Pope (Santa Rosa, USA) discusses closed-loop spinal cord stimulation (SCS) and its potential to provide sustained, holistic outcomes for chronic pain patients. In addition to describing these theoretical benefits and the mechanisms of action by which they can be achieved, Pope outlines the significance of recent data from the EVOKE study.
SCS has been an established treatment in refractory neuropathic chronic pain for more than 50 years. It works by sending small electrical pulses to electrodes in the epidural space that stimulate the spinal cord to inhibit pain signals to the brain. Randomised controlled trials (RCTs),1,2 systematic reviews3,4 and economic evaluations3,5,6 have demonstrated the clinical effectiveness and cost-effectiveness, and opioidsparing effects, of SCS for the management of chronic pain—although, recently, poorly developed reviews and investigations with significant methodologic errors have been published.
Traditional SCS
SCS requires placement of the electrode within the epidural space for delivery of electricity to the target intradural neural structure (with location typically defined by spinal level). A significant challenge with stimulation of the spinal cord is the ever-changing distance between the stimulating electrode(s) relative to their spinal cord target.
This occurs predictively, because of the anatomic considerations inherent to the epidural and intrathecal space; and is largely contingent to cerebrospinal fluid (CSF) volume and the positional effects of the lead within the epidural space, influenced by body position. This distance, therefore, constantly changes, and is influenced by physiologic activities like breathing and coughing, but also by postural changes and activity.
These small changes in the distance between the electrode and spinal cord
result in large changes in the electricity reaching the spinal cord, impacting the necessary treatment pathway.
Therefore, employing SCS with a fixed-output system inherently defines a system that will be limited by activating the spinal cord with high variability and inaccuracy, not addressing the aforementioned challenges and potentially compromising clinical outcomes.
To date, all commercially available SCS devices—regardless of the stimulation parameters and waveforms—are fixed-output, ‘openloop’ systems. As a consequence, the stimulation paradigm is limited by a lack of identification and maintenance of target neural activation.
Without the ability to both continually measure and adjust stimulation intensity on every stimulation pulse, these turbulent neurophysiological conditions result in frequent and substantial fluctuations in activation of the spinal cord, limiting efficacy and potentially impacting long-term successful outcomes.
As such, these traditional systems are challenged by the absence of a physiologic measurement of neural activation and the inability to adjust stimulation in response to it. To date, open-loop systems are reliant on patientreported validation of neural activation success and, therefore, titration of therapy is often from failure.
Closing the loop
In contrast, a ‘closed-loop’ spinal cord stimulator first measures the neural response elicited by stimulation of the spinal cord through the direct
measurement of Evoked Compound Action Potentials (ECAPs), and then responds to that measurement by adjusting the next stimulation dose in real time to maintain a consistent neural activation target, accommodating the neurophysiologic environment. Therefore, this SCS system is described as a closed-loop, variable-output SCS system.
The theoretical benefit to the patient of a closed-loop physiologic feedback mechanism with consistent neural activation seems simple enough and logically appears to be the next smart focus for innovation.
In an effort to validate this ‘smart loop’, a study was performed, in a double-blinded, multicentre, prospective fashion, with follow-up out to 36 months, comparing the traditional stimulation paradigm (open-fixed) to the smart system (closed-variable).
The EVOKE trial, sponsored by Saluda Medical, was conducted as an investigational device exemption (IDE) study with oversight from the US Food and Drug Administration (FDA), and demonstrated that smart-loop (ECAPcontrolled, closed-loop) SCS delivers superior long-term pain reduction compared to traditional (open-loop, fixed) SCS. The three-year data from EVOKE were presented earlier this year at the North American Neuromodulation Society (NANS) annual meeting (12–15 January 2023, Las Vegas, USA).
Further, the consistent neural activation provided by the smart-loop system leads to improvements in patient outcomes—not only in pain intensity reduction, but also statistical improvements in holistic views of patient outcomes, including quality of life, functional status, mood, and sleep quality and quantity.
Additionally, smart-loop patients voluntarily reduced or eliminated opioids due to pain relief with their SCS device that was much greater than in the traditional stimulation arm.
Based on the cumulative smart-
loop data, aggregating the RCT, the ECAP IDE—a real-world, single-arm, prospective multicentre study—and the Avalon study, all demonstrating replicable, sustained, holistic benefits, this paradigm shift moves to titrating SCS therapy toward success by physiologic measurement and maintenance of neural activation, as compared to titration from failure.
Future directions
The potential utility of physiologic closed-loop technology has applications in the broader field of neuromodulation, including therapies like vagal nerve, sacral nerve and deep brain stimulation.
There are early clinical studies underway in these areas. Further, enhanced strategies to monitor device performance and use, away from the clinic, may also improve outcomes and maintain therapy.
The pathway forward for SCS therapy is to address known challenges with its implementation—with one categorically being the consistency of neural activation. To turn a blind eye, employing open-fixed systems, would ignore the importance of physiologic measurement of neural activation, location, consistency, and accuracy, contributing to less optimal outcomes. Smart-loop systems are creating a new required standard.
References:
1. Slangen R, Schaper N C, Faber C G, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. 2014; 37(11): 3016–24.
2. Mekhail N, Levy R M, Deer T R, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a doubleblind, randomised, controlled trial. The Lancet Neurology. 2020; 19(2): 123–34.
3. Petersen E A, Stauss T G, Scowcroft J A, et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: A Randomized Clinical Trial. JAMA Neurology. 2021; 78(6): 687–98.
4. Kemler M A, Barendse G A, van Kleef M, et al Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. New England Journal of Medicine. 2000; 343(9): 618–24.
5. Mekhail N, Levy R M, Deer T R, et al. Durability of Clinical and Quality-of-Life Outcomes of Closed-Loop Spinal Cord Stimulation for Chronic Back and Leg Pain: A Secondary Analysis of the Evoke Randomized Clinical Trial. JAMA Neurology. 2022; 79(3): 251–60.
6. Russo M, Brooker C, Cousins M J, et al. Sustained Long-Term Outcomes With Closed-Loop Spinal Cord Stimulation: 12-Month Results of the Prospective, Multicenter, Open-Label Avalon Study. Neurosurgery 2020; 87(4): e485–e495.
Jason Pope is the president and CEO of the Evolve Restorative Center in Santa Rosa, USA. A double-boarded pain management specialist and anaesthesiologist by trade, he is also the current president of the American Society of Pain and Neuroscience (ASPN) and chairman of the Pacific Spine and Pain Society (PSPS). Pope previously served two terms on the board of the North American Neuromodulation Society (NANS), and the International Neuromodulation Society (INS).

DISCLOSURES:
The author serves as a consultant for Abbott, Medtronic, Saluda, Flowonix, SpineThera, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific and Thermaquil; has received grant and research support from Abbott, Flowonix, Saluda, Aurora, PainTeq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS and Thermaquil; and is a shareholder of Vertos, SPR Therapeutics, PainTeq, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil and Anesthetic Gas Reclamation.
26 June 2023 | Issue50
The potential utility of physiologic closed-loop technology has applications in the broader field of neuromodulation, including therapies like vagal nerve, sacral nerve and deep brain stimulation. There are early clinical studies underway in these areas.”
The new standard for SCS therapy—neural activation measurement, targeting and maintenance Jason
Review concludes SCS benefits do not outweigh associated risks and costs in low back pain
A recently published Cochrane review has concluded that the clinical benefits of spinal cord stimulation (SCS) do not outweigh the risks and costs associated with the approach in patients with low back pain—leading researchers to claim that “there is clearly a problem here that should be of concern to regulators”.
WRITING IN THE COCHRANE DATABASE OF Systematic Reviews, the researchers involved state that SCS therapy does not provide long-term relief or sustained benefits based on the current evidence, and may cause harm.
“Spinal cord stimulation is invasive and has a great financial cost to people who choose surgery as a last resort to alleviate their pain,” said lead researcher Adrian Traeger (Sydney Musculoskeletal Health, Sydney, Australia). “Our review found that the longterm benefits and harms are essentially unknown.
“Low back pain is one of the leading causes of disability worldwide. Our findings further emphasise the urgent need to review funding arrangements for chronic pain care to help patients in their search for relief. There are evidence-based physical and psychological therapies for back pain; ensuring access
to these is essential.”
The present study reviewed published clinical data on SCS, including randomised controlled trials. The researchers analysed the results of 13 trials, looking at data from a total of 699 participants, comparing SCS treatment with placebo or no treatment for low back pain. They elected to do this via a Cochrane review given these approaches use “robust methodologies to combine evidence from multiple sources, reducing the impact of bias and random error that can make individual studies less reliable”.
The review concluded that SCS is “no better than a placebo” for treating low back pain, with “probably little-to-no benefit” for people with low back pain nor improvement in their quality of life. The researchers add that there were “little-to-no clinical data regarding the long-term effectiveness of SCS”, and that they found data detailing adverse side-effects associated with SCS surgery were “poorly documented overall”, preventing them from conclusively determining the level of risk involved.

Potential sources of harm from SCS could include nerve damage, infection, and the electrical leads moving—all of which may mean repeated surgeries are required, the researchers also detail.
The review team claims to have found multiple gaps in the existing clinical data. For example, there were no studies that investigated the long-term (>12 months) impact of SCS on low back pain, with the longest being a single six-month trial. The majority of clinical trials “only looked at the immediate impact of the device”, which is a time frame of less than one month, the researchers note.


According to a news release from the University of Sydney (Sydney, Australia), these review findings have been submitted to the Federal Department of Health and Aged Care prosthesis list review taskforce.

In Australia, the long-term safety and performance of SCS devices is also currently being re-assessed by the country’s Therapeutic Goods Administration (TGA).


The review team further provided a list of recommendations, including that future clinical trials of SCS should be conducted across at least 12 months; clearly document the number of people who experience adverse events; and make comparisons with other pain treatment options.
“Our review found that the clinical benefit of adding spinal cord stimulation to treat low back pain remains unknown,” said Chris Maher, co-director of Sydney Musculoskeletal Health. “When coupled with the reality that these devices are very expensive and often break down, there is clearly a problem here that should be of concern to regulators.”

A separate Cochrane review led by investigators from the UK and published in late-2021 examined the effect of SCS therapy versus placebo in people with chronic pain and—similarly to the present study— concluded that there was a lack of evidence to suggest long-term benefits.

27 Issue50 | June 2023
A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the spinal arena Editorially independent Subscribe today Visit spinalnewsinternational.com and click ‘Subscriptions’ for e-newsletter subscription Available in digital format and through our social channels
Our findings further emphasise the urgent need to review funding arrangements for chronic pain care to help patients in their search for relief.”
Adrian Traeger
Evidence indicates effectiveness of brain stimulation in upperlimb stroke rehabilitation
Moderate- to high-quality evidence suggests that specific neuromodulation modalities can provide effective treatments in patients undergoing stroke rehabilitation. As per a systematic review and meta-analysis, these therapeutic approaches are able to improve upper-limb motor function in both acute/subacute and chronic stroke.
Writing in Neuromodulation: Technology at the Neural Interface, the authors—Rustem Mustafaoglu (İstanbul ÜniversitesiCerrahpaşa, Istanbul, Turkey) et al—say they analysed the current evidence and potential associated with electric neurostimulation in upper-limb stroke rehabilitation. Treatment modalities assessed included transcranial direct current stimulation (tDCS) and vagus nerve stimulation (VNS).
“We performed a systematic review of randomised controlled trials (RCTs) using network meta-analysis, searching the following databases: PubMed, Web of Science, Cochrane, and Google Scholar,” the authors report, “using specific keywords, from January 2010 to April 2021, and assessing the effects of tDCS or VNS combined with other therapies on upper-limb motor function and activities of daily living (ADL) after stroke.”
The study included 38 RCTs—six involving VNS versus sham and 32 involving tDCS versus sham—with
a total patient population of 1,261. A pairwise network meta-analysis revealed that transcutaneous VNS (mean difference [MD]: 5.5; 95% confidence interval [CI] [0.67–11.67]; p<0.05) and anodal tDCS (MD: 5.23; 95% CI [2.45–8.01]; p<0.05) were effective in improving upper-limb motor function. Mustafaoglu et al further detail that transcutaneous VNS, and both anodal and cathodal tDCS, were effective in improving ADL performance after stroke as well.
In addition, the “best-ranked” treatment in improving upper-limb motor function and performance in
ADL after a stroke was transcutaneous VNS—as revealed by a surface under the cumulative ranking curve (SUCRA) analysis.
Finally, regarding safety outcomes, the authors report that there was “no difference” between VNS and its control interventions. This conclusion was made by measuring reported adverse events with VNS therapy (risk ratio=1.02 [95% CI=0.48–2.17; I2 =0; p=0.96]), which found the technique to be well-tolerated, with no adverse events or discomforts, in the included studies.
“Our network meta-analysis revealed that transcutaneous VNS is an effective method in improving upper-limb motor function after stroke,” Mustafaoglu et al write. “The reason for this might be that VNS may induce motor recovery by increasing the level of brain-derived neurotrophic factors and neurotransmitters, such as noradrenaline, linked to neuroplasticity and recovery after brain lesion.
“Considering the tDCS protocol, anodal tDCS was found to be the most effective treatment option compared with other active tDCS (cathodal and dual tDCS). The relative superiority of cathodal tDCS might be because most studies included in the analysis treated participants with cathodal tDCS. The other reason for this might be
New spectroscopy probe could enhance deep brain stimulation procedures for Parkinson’s
A new probe that performs two types of spectroscopy could make deep brain stimulation (DBS) procedures safer and improve success rates by helping doctors more accurately navigate instruments inside the brain in patients with advanced Parkinson’s disease.
THE RESEARCH TEAM INVOLVED
identified white and grey matter using principal component analysis (PCA), proving that spectroscopic measurements could be suitable for neuronavigation.
Mireille Quémener (CERVO Brain Research Center, Université Laval, Québec City, Canada) recently detailed this new research in a presentation at Optica’s Biophotonics congress (23–27 April, Vancouver, Canada).
“Improving neurosurgical guidance for the DBS electrode insertion would streamline the surgical process, decrease the surgery time, reduce the overall health treatment cost and prevent adverse neuropsychological consequences,” said Quémener.
While DBS therapies are becoming increasingly common and can be “remarkably effective” for patients who no longer benefit from available medications, placement of an electrode in the wrong spot by a surgeon can reduce its effectiveness and lead to psychological disorders, as per a press release from
Optica—a society dedicated to globally promoting the generation, application, archiving and dissemination of knowledge in the fields of optics and photonics. DBS is a two-part procedure, including one surgery to place electrodes in specific parts of the brain and a second surgery to implant a battery pack that delivers electrical current to the electrodes. For the first procedure, doctors typically rely on pre-surgery magnetic resonance imaging (MRI) scans to plan where they will insert electrodes. However, this can sometimes lead to an inaccurate placement, as the brain can shift by up to 2mm during the process of drilling an access hole through the skull.
In the present project, researchers created a DBS electrode that is enhanced with an optical probe to perform coherent anti-Stokes Raman scattering spectroscopy (CARS) and diffuse reflectance spectroscopy (DRS) on brain tissues during the insertion process. The probe fits within the DBS electrode and contains two fibres for CARS and
that cathodal tDCS results in downregulation of overactive, non-lesioned hemisphere, which results in restoring the balance of inter-hemispheric inhibition between two hemispheres, thus promoting motor recovery.”
The authors do, however, highlight some limitations to their work, including a reduced ability to draw conclusions due to the methodological and clinical heterogeneity among the included studies, as well as potential bias of the pooled effect caused by the absence of some unpublished or missing data.
“Finally, although this network metaanalysis suggests that transcutaneous VNS is the best possible treatment for promoting upper-limb motor function and performance in ADL, a network meta-analysis is not adequate to inform treatment, and future, larger multicentre clinical trials need to be conducted to determine the effect of transcutaneous VNS on motor recovery in stroke,” Mustafaoglu et al concede.
While the authors conclude that transcutaneous VNS and anodal tDCS are effective in improving upper-limb motor function in both acute/subacute and chronic stroke, the authors also add that invasive VNS or dual tDCS “can be the second choice of treatment” when the goal is to improve the upper-limb motor function in these patients.
DRS illumination, and a third fibre for collecting the signals. Once the electrode reaches the target position, the optical probe can be deployed while the electrode stays in place.
To test the new probe, a neurosurgeon used it to implant electrodes in six regions of the brain of a human cadaver. CARS and DRS measurements were collected along a total length of 50mm in each of the brain’s two hemispheres. After the procedure, researchers extracted the brain and visually identified the white and grey matter through which the probe had passed. Comparing the readouts from the CARS and DRS measurements to the visual record of brain structures, the researchers found the CARS and DRS methods identified brain tissue with great accuracy— confirming, in their view, that spectroscopy could be a useful tool to help neurosurgeons navigate the brain.
The researchers plan to study whether the approach could be used to collect even more detailed spectroscopic information; for example, to measure neurotransmitters that provide a chemical signature of brain activity.
“Our team is currently working on adapting the optical probe to use it in clinical trials for patients who will receive a DBS surgery,” Quémener stated. “We are convinced that optical methods have enormous potential for surgical guidance, and hope that our technology will emerge in the clinic to assist surgeons in various brain procedures.”
28 June 2023 | Issue50
6 studies of VNS vs sham
studies of tDCS vs sham
participants in total
38
randomised controlled trials
32
1,261
Biotronik announces US FDA approval of Prospera SCS system
Biotronik has announced US Food and Drug Administration (FDA) approval for Prospera—a new spinal cord stimulation (SCS) system—marking the launch of the company’s new business segment, Biotronik Neuro.
The system features RESONANCE, a multiphase stimulation paradigm, paired with Embrace One, a patientcentric care model that makes proactive care possible by offering automatic, objective, daily remote monitoring, and ongoing management and support.
“Biotronik’s remote technologies are moving the industry forward,” said Marc Russo (Hunter Pain Specialists, Newcastle, Australia), principal investigator of the BENEFIT-03 study. “Allowing patient devices to be automatically monitored and remotely programmed seven days a week will redefine therapy in the SCS space. In the coming years, I anticipate a shift in the industry toward increased use of these technologies following the path Biotronik has set, which will provide real benefits to patients and the clinical community.”
BENEFIT-03 is a prospective, multicentre, single-arm study currently underway in Australia to evaluate the safety and effectiveness of Prospera for the treatment of chronic intractable pain of the trunk and limbs. This study is expected to yield important insights regarding the impacts of implementing remote patient management and proactive care to ensure the SCS experience is optimised daily, and over the lifetime of the therapy, as per a Biotronik press release.
SynerFuse announces presentation of IDE proofof-concept study data
SynerFuse has announced that Michael Park (University of Minnesota, Minneapolis, USA)—principal investigator for the company’s investigational device exemption (IDE) proof-of-concept study—presented data from the study’s first eight patients at the 10th annual Minnesota Neuromodulation Symposium (20–21 April, Minneapolis, USA).
According to a press release, this presentation showcased the promising surgical procedure results seen thus far with the world’s first SynerFuse implants, integrating spinal fusion
with neuromodulation of the dorsal root ganglia in patients with chronic neuropathic pain of the low back or leg.
The data showed that, in these eight patients—at an average follow-up of 7.8 months—there were no infections, device failures with loss of stimulation therapy, or lead migrations associated with the novel integrated implant.
Spine surgeons Rohan Lall (M Health Fairview, Minneapolis, USA) and Jonathan Sembrano (University of Minnesota, Minneapolis, USA) performed the implants, with Park and Lall also performing the world’s first solo SynerFuse implant.
Enrolment in the study, which has been set up to evaluate the safety and tolerability of simultaneous implantation of spinal fusion and neuromodulation devices in chronic lower back pain patients, is ongoing.
Theranica publishes positive data on Nerivio in adolescent migraine
Theranica recently had the results from a real-world analysis of Nerivio for acute treatment of migraine in adolescents published in the journal Pediatric Neurology
The study demonstrates the ease of use, safety and efficacy of Nerivio— the company’s remote electrical neuromodulation (REN) device—as an acute treatment for migraine in adolescents, and reflects findings from previous clinical trials in adolescents and similar real-world studies in adults. It analysed prospective, real-world data collected from 1,629 adolescents (aged 12–17) with migraine, treated with Nerivio over a period of one and a half years.
Its results showed that, when Nerivio was used as a standalone therapy, 60.3% of the adolescent users achieved post two-hour pain relief from severe or moderate headache, to mild or no headache, in at least half of their evaluable treatments, and 26.3% of users were completely pain-free after at least half of their treatments. In nearly 65% of treatments, users did not take any medication within two hours of starting Nerivio, while only three device-related adverse events—all of which were minor—were reported out of the 13,682 treatments conducted across 1,629 patients.
“Migraine must be treated early and holistically, with a comprehensive plan tailored for each child’s needs, symptoms, and lifestyle,” said Rashmi Rao (Children’s Hospital New Orleans, New Orleans, USA), a co-author of the study. “For parents and teens, these data add to established clinical efficacy and demonstrate the significant realworld benefits of Nerivio, either as a standalone therapy or as an adjunct to other treatments, as a valuable nondrug tool to treat migraine.”
Reach Neuro gains breakthrough designation in chronic stroke
Reach Neuro has been granted a Breakthrough Device designation by the US Food and Drug Administration (FDA) for its Avantis platform, which uses small electrical impulses delivered to the spinal cord to help restore shoulder, arm and hand movement to individuals with impairment due to chronic stroke.
The Avantis system directly restores a patient’s ability to control movement—not only giving immediate relief, but also making it possible to return to therapy and continue making even further improvements, according to a Reach Neuro press release.
The company recently published a manuscript in the journal Nature Medicine describing results from the first two study participants in whom the technology is currently being tested, showing 40% and 108% improvements in grip strength, and an improvement of up to 124% in joint strength.
These improvements enabled them to perform reaching movements more quickly and smoothly than without stimulation, as well as performing functional tasks like lifting objects, eating with a fork and opening a lock. Reach Neuro claims that early evidence shows Avantis can be effective in chronic stroke patients, even up to nine years after stroke, which is well outside the six-month window in which existing therapies are used.
The company now intends to accelerate the regulatory process for its Avantis platform through close collaboration with the US FDA.
BioWave announces study demonstrating benefit of peripheral nerve stimulation
The journal Pain and Therapy recently published a new study, titled “Reduced pain and improved function following short-term use of noninvasive BioWave high-frequency peripheral nerve stimulation for pain management”, as per a press release.
The release states that the paper was authored by Alaa Abd-Elsayed (University of Wisconsin School of Medicine and Public Health, Madison, USA) and colleagues, and confirmed the findings of smaller prior studies regarding the benefits of BioWave technology to treat chronic pain. The patient population (1,511) is among the largest ever studied for non-invasive peripheral nerve stimulation (PNS), BioWave also claims.
The study looked at the use of the company’s BioWaveHOME device in chronic pain treatment, and found that patients experienced significant reductions in overall pain (46%) and medication needs within two weeks of starting to use this technology. An improvement in quality of life was reported in 87.6% of patients; functional lifestyle metrics—such as mood, sleep, standing and sitting— improved across the board; and no adverse reactions/complications were
reported in 1,511 patient outcomes.
“Although survey studies have inherent limitations, such as duration and compliance biases with such an overwhelming benefit in every category, we believe that noninvasive neuromodulation therapy is a promising, safe, and cost-effective therapy,” the authors state.
Abbott gains US FDA nod to use SCS devices in patients with non-surgical back pain
Abbott has announced receipt of US Food and Drug Administration (FDA) approval of its spinal cord stimulation (SCS) devices in the treatment of chronic pain in patients who have not had or are not eligible for back surgery.
A company press release details that this labelling expansion was supported by results from the DISTINCT study, which demonstrated improvements in pain levels, ability to perform daily activities and emotional wellbeing with BurstDR SCS technology in chronic back pain patients.
DISTINCT data showed “significant” back-pain reductions in the SCS study arm, with an average pain reduction of 69.7% across those who received SCS therapy, the release also notes.
Feinstein Institutes awarded US$3.4 million to study rTMS therapy in schizophrenia
Researchers at the Feinstein Institutes for Medical Research (Manhasset, USA) have received a US$3.4 million grant from London-based charitable foundation Wellcome to study repetitive transcranial magnetic stimulation (rTMS) and its effects— potentially opening the door for a new, non-pharmacological way to treat major mental health disorders like schizophrenia spectrum disorders and psychosis.
Anil Malhotra (Donald and Barbara Zucker School of Medicine at Hofstra/ Northwell, Uniondale, USA), codirector of the Feinstein Institutes’ Institute of Behavioral Science, will lead a new double-blind, randomised clinical trial assessing if rTMS improves social cognitive performance of people living with schizophrenia over the course of five years.
“There is no one silver bullet to treat mental health conditions,” said Malhotra. “The success and effectiveness of rTMS have shown the potential to treat critical symptoms of schizophrenia. With the support of Wellcome, we will get a better understanding of the effectiveness of rTMS to help those with cognitive effects of illness.”

29 Issue50 | June 2023
Prospera SCS system
Anil Malhotra
Industry News
RapidAI receives US FDA 510(k) clearance for new imaging product to accelerate acute
stroke triage
RapidAI announced recently that it has received US Food and Drug Administration (FDA) 510(k) clearance for Rapid non-contrast computed tomography (NCCT) Stroke, which is intended to support faster treatment and transfer decisions in stroke care.
A company press release describes this as a “major addition” to the RapidAI suite of non-contrast-based solutions for stroke and trauma care, and the “first and only” FDA-cleared medical device for detecting suspected intracranial haemorrhage (ICH) and large vessel occlusion (LVO) from value-based CT imaging.
Rapid NCCT Stroke uses artificial intelligence (AI) to analyse NCCT images to determine suspicion of ICH and LVO of the distal internal carotid artery (ICA) and middle cerebral artery (MCA-M1). The fully automated system then delivers triage and prioritisation notifications through picture archiving and communication system (PACS), email, and the Rapid mobile app.
Because NCCT imaging is readily available and is the initial imaging modality used for stroke and trauma patients, care teams can make timesensitive workflow and transfer decisions faster.
For hospitals that do advanced imaging, Rapid NCCT Stroke may significantly reduce the time between CT and CT angiography (CTA) scans, the release also states.
Penumbra launches new RED 43 reperfusion catheter for mechanical thrombectomy
Penumbra has announced the launch of the latest addition to its RED family of catheter devices for mechanical thrombectomy, the RED 43 reperfusion catheter.
According to the company, this device is intended to take distal aspiration “to the next level” with optimised lumen occupation as well as a soft distal design that allows for enhanced and compliant delivery in tortuosity.
With a 0.043-inch lumen, RED 43 provides powerful distal aspiration alongside the Penumbra ENGINE and features REDglide technology for advanced hydrophilic coating for smooth trackability.
As per the company’s website, additional features of this newly launched device include:
� A 1.52mm (0.060-inch) outer dimension: low profile and atraumatic design for distal deliverability
� Precision tip: articulating marker band with short polymer tip design for optimal visualisation and placement
� Full-length polytetrafluoroethylene (PTFE) liner: designed for durability and lumen integrity under powerful aspiration
� Proximal hybrid stainless steel and nitinol coil wind: engineered to provide optimal support and softer profile atraumatic navigation
The RED 43 reperfusion catheter gained approval from the US Food and Drug Administration (FDA) on 20 December 2022, as per a 510(k) premarket notification.
NeuroOne announces US commercial launch of Evo sEEG electrodes

NeuroOne Medical Technologies has announced the commercial launch of the Evo stereoelectroencephalography (sEEG) electrode product line in the USA having received US Food and Drug Administration (FDA) clearance in October 2022.
Zimmer Biomet holds exclusive worldwide distribution rights to NeuroOne’s Evo Cortical and sEEG product lines, and the Evo product line is expected to utilise Zimmer Biomet’sROSA One Brain—a robotic platform that assists surgeons in planning and performing complex minimally invasive neurosurgical procedures.
NeuroOne has described the Evo sEEG platform launch as “one of the most impactful milestones” in its history, also stating that it expects to complete initial cases within 30 days of this announcement on 2 May.
In a press release, the company notes that there is a “significant opportunity” to expand market adoption of sEEG technology with broad US distribution of its Evo product line.
NeuroOne’s Evo sEEG electrode technology offers sEEG recording, brain stimulation and future capabilities for spinal cord stimulation, and ablation solutions targeted for patients suffering from epilepsy, chronic back pain, and Parkinson’s disease. The release adds that it is the company’s most advanced diagnostic electrode, designed to be secure, precise, and less invasive, with “proven placement accuracy and signal quality”, and made in a mostly automated manufacturing process.
Cerevasc appoints Adel Malek as its new chief medical officer
Cerevasc has named neurosurgeon Adel Malek as its new chief medical officer. This appointment will allow Cerevasc to further accelerate the development and clinical strategy of the eShunt system—a minimally invasive device designed to treat communicating hydrocephalus—according to a press release from the company.
Malek is chief of Neurovascular Surgery, and director of the Cerebrovascular and Endovascular Division, in the Department of Neurosurgery at Tufts Medical Center—and professor of Neurosurgery at Tufts University School of Medicine—in Boston, USA.
The Cerevasc press release notes that he is also a co-inventor of the endovascular eShunt device.
“I am excited about the opportunity to contribute to the great pioneering innovations at Cerevasc as we embark on expanded clinical studies and product development initiatives,” Malek said.
“Having worked with the team for the past eight years, I have been impressed with the passion for improving patient care and dedication to transformative innovation, and am looking forward to supporting this important mission.”
Route 92 gains US FDA clearance for complete stroke treatment system
Route 92 Medical has announced receipt of 510(k) clearance from the US Food and Drug Administration (FDA) for its FreeClimb 70 reperfusion system, which includes the FreeClimb 70 aspiration catheter along with the Tenzing 7 delivery catheter.
The FreeClimb 70 reperfusion system enables physicians to treat patients experiencing an acute ischaemic stroke by removing clots rapidly and safely, a company press release notes.
This “innovative, first-to-market system” is designed to work together harmoniously for superior deliverability and high procedural efficiency, making it a more refined bi-axial approach for restoring blood flow to the brain during endovascular thrombectomy procedures, the release adds.
“In our initial experience with FreeClimb 70 and Tenzing 7, the system easily delivered to the target occlusion allowing rapid, effective and safe reperfusion,” said James Caldwell (Auckland City Hospital, Auckland, New Zealand), an early user of the system.
“Tenzing-based delivery can reduce procedure time and may reduce complications. This comprehensive solution will help clinicians overcome challenging neurovascular anatomy with greater ease and efficiency, providing better care for their patients.”
According to Route 92, the FreeClimb 70 reperfusion system is the first fully integrated solution designed around the Tenzing 7 delivery catheter, which offers superior navigation and predictable access to occluded distal vessels without the need for a guidewire.
The system’s advanced design is also intended to eliminate the ‘ledge effect’ commonly found in largebore catheters.
Cerenovus announces commercial availability of Cerepak detachable coils and first patient cases in the USA
Cerenovus has announced that its new Cerepak detachable coils are now commercially available in the USA. In a press release, the company details that the first patient cases with these devices have also been performed.
Cerepak is the latest innovation to join the company’s haemorrhagic stroke portfolio.
The Cerepak detachable coils offer three shapes and multiple coil sizes, providing physicians with comprehensive options to embolise brain aneurysms—including coils shaped specifically to achieve concentric aneurysm filling and contribute large volumetric filling.
As per a company press release, Cerepak provides physicians with a state-of-the-art delivery system designed for ease of use and reliable detachment; next-generation microcatheter stability; effortless tracking through tortuous anatomy; and reduction of radiation (fluoroscopy) exposure with fluoro-saver markers.
“I am privileged to be one of the first users of the new Cerepak detachable coils,” said Osama Zaidat (Mercy Hospital, Toledo, USA), study co-principal investigator of the STERLING registry.
“The advancement of greater tools in neurology may improve patient outcomes and my team looks forward to continuing the utilisation of Cerepak coils in our treatment plans.”
This latest addition to the Cerenovus portfolio will also be included in the STERLING registry, which is currently collecting real-world data on ruptured/ unruptured aneurysms treated with Micrusframe and Galaxy coils, the release further details.
“The Cerepak detachable coils provide important advancements for embolising brain aneurysms and we are pleased to expand the STERLING registry to include this latest Cerenovus offering,” said Reade De Leacy (Mount Sinai Health System, New York, USA), study co-principal investigator of the STERLING registry.
30 Market Watch June 2023 | Issue50
RED 43 Tenzing 7 and FreeClimb 70
Clinical
MIVI completes enrolment for study of Q revascularisation system in acute ischaemic stroke
MIVI Neuroscience has announced that it has completed enrolment in the EVAQ clinical trial to assess the safety and effectiveness of the Q revascularisation system for the treatment of acute ischaemic stroke.
The EVAQ trial has enrolled 121 patients at 17 centres globally. Data from the study will be used as part of an application to the US Food and Drug Administration (FDA) for market clearance of the Q device in the USA.
“While aspiration catheters are used in nearly every stroke treatment, their design has not significantly changed over the last decade,” said Lucas Elijovich (Semmes-Murphey Neurologic Institute, Memphis, USA), EVAQ US principal investigator.

“The Q aspiration catheter is a novel design that delivers greater aspiration power without increasing the size of the catheter tip. This may be especially advantageous in smaller vessels, where
Conference calendar
5–7 June
LINNC Paris 2023 Paris, France www.linnc.com/Courseinformation/LINNC-Paris-2023
19–23 June

The European Course in Minimally Invasive Neurological Therapy (ECMINT) 5.1 Oxford, UK www.esmint.eu/ecmint
large-bore catheters may not safely access and single-lumen catheters offer limited efficacy.”
“It is only through clinical research like EVAQ and innovative technologies like the Q aspiration catheter that stroke treatment will continue to advance,” added Christophe Cognard (Hôpital de Purpan, Toulouse, France), French principal investigator.
“We are proud to contribute to the study with our US colleagues and look forward to the results of the trial.”
Carthera publishes “promising” results with SonoCloud-9 in glioblastoma patients
Carthera has announced the publication in The Lancet Oncology of results from a Phase 1 clinical trial carried out by scientists at Northwestern University Feinberg School of Medicine (Chicago, USA). In the trial, they used the SonoCloud-9 implantable ultrasound device to repeatedly open the bloodbrain barrier (BBB) and deliver
1–4 July 9th Congress of the European Academy of Neurology (EAN) Budapest, Hungary www.ean.org/congress2023
31 July–4 August Society of NeuroInterventional Surgery (SNIS) 20th Annual Meeting and Fellows Course San Diego, USA www.snisannualmeeting.org
chemotherapy in patients with recurrent glioblastoma.
A Carthera press release notes that the results show the treatment was safe and well-tolerated, with some patients receiving up to six cycles of treatment.
In the study, opening the BBB led to an approximately four- to six-fold increase in drug concentrations in the brain— an effect observed with two different chemotherapy drugs, paclitaxel and carboplatin.
“This is potentially a huge advance for glioblastoma patients,” said lead investigator Adam Sonabend (Northwestern Medicine, Chicago, USA).
“Temozolomide, the current chemotherapy used for glioblastoma, does cross the BBB, but is a weak drug. In the past, studies that injected paclitaxel or carboplatin directly into the brain of patients with these tumours observed promising signs of efficacy, but the direct injection was associated with toxicity. In our study, the use of the SonoCloud-9 system has demonstrated promise for safe and tolerable delivery to patients.”
NICO announces initial positive results from ENRICH trial
NICO Corporation has announced initial positive results from the completed ENRICH trial at the 2023 American Association of Neurological Surgeons (AANS) annual
4–6 September 15th Congress of the European Society of Minimally Invasive Neurological Therapy (ESMINT) Marseille, France www.esmint.eu/esmint-congress
9–13 September Congress of Neurological Surgeons (CNS) Annual Meeting Washington DC, USA www.cns.org/annualmeeting
scientific meeting (21–24 April, Los Angeles, USA).
ENRICH was a randomised, multicentre trial designed to evaluate safety, efficacy and economic outcomes for intracranial haemorrhage (ICH), or haemorrhagic stroke, comparing early minimally invasive parafascicular surgery (MIPS) using NICO’s complete technology solution versus standard of care across 300 patients.

At six months, functional outcomes in ENRICH were assessed using the utility-weighted modified Rankin scale (UWmRS), with results meeting the primary endpoint, and the MIPS group achieving a statistically significant and clinically meaningful improvement in UWmRS versus medical management. In addition, ENRICH found MIPS to be safe, demonstrating an overall mortality at six months of 20%, compared to 23.3% in the medical management group.
“ENRICH is the first randomised clinical trial to meet its primary endpoint demonstrating early MIPS with BrainPath and Myriad improves outcomes for these deadly strokes,” said Gustavo Pradilla (Emory University School of Medicine, Atlanta, USA), co-lead investigator for the study.
“I believe this trial will change how we treat haemorrhagic stroke moving forward and I look forward to sharing the further details of the ENRICH trial, which will be published soon.”
20–24 September
European Society of Neuroradiology (ESNR)


Annual Meeting Vienna, Austria www.esnr.org/en/46th-esnrannual-meeting-6456
10–12 October 15th World Stroke Congress (WSC) Toronto, Canada www.worldstrokecongress.org
18–19 October Stroke Live Course (SLICE) Worldwide TBA www.masterandfellow.com/ slice/ww
16–18 November
Society of Vascular and Interventional Neurology (SVIN) Annual Meeting Miami, USA www.svin.org/i4a/pages/index. cfm?pageid=3601
News 31 Issue50 | June 2023 Sign up for a free print subscription* and e-newsletter subscription** www.neuronewsinternational.com NeuroNews is a trusted, independent source of news and opinion in the neurointerventional and neurosurgical world. *Available for US and EU readers only ** Available worldwide Market Watch
Q revascularisation system







a. Devices shown at same scale. Tests performed and data on file at Penumbra, Inc. All testing was completed by the same operator during the same testing session under the same 29.2 inHg of power aspiration. Bench test results may not be indicative of clinical performance. Clinical results may vary. Data on file at Penumbra, Inc. b. Data on file at Penumbra, Inc. Photographs taken by and on file at Penumbra, Inc. Product availability varies by country. Prior to use, please refer to the Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events, and detailed instructions for use. For the complete Risk Statements, please visit: http://bit.ly/2BYj7Yj. Please contact your local Penumbra representative for more information. Copyright ©2023 Penumbra, Inc. All rights reserved. The Penumbra P logo, RED, and Penumbra System are registered trademarks or trademarks of Penumbra, Inc. in the USA and other countries. 26882, Rev. A 04/23 OUS Penumbra System ™ RED Reperfusion Catheters COMPROMISED lumen resulting in reduced tip area b TRUE LUMEN When It Matters Lumen Integrity Under Full Vacuum a Competitive Catheter Penumbra’s RED™ Reperfusion Catheters feature a full-length PTFE liner which is designed to maintain their true lumen under powerful vacuum MAINTAINED lumen integrity maximises aspiration force b RED Reperfusion Catheters SCAN HERE to learn more (Including Risk Statements) 68 72 62







































































































