Featured in this issue:
8
Maarit Venermo Abdominal aortic aneurysm screening


Featured in this issue:
Maarit Venermo Abdominal aortic aneurysm screening

Profile: Jon Boyle
page 16
Surgical training: Change is needed page 25
Data and discussion on revascularisation treatment strategies for patients with chronic limb-threatening ischaemia (CLTI) will take centre stage at the upcoming Charing Cross (CX) Symposium (25–27 April, London, UK), with first results from the BASIL-2 (Bypass versus angioplasty in severe ischaemia of the leg-2) randomised controlled trial set to be a highlight of this year’s programme.
In keeping with CX style, audience participation will be at the heart of the discussion in this headline session. Attendees in London and remote participants will have the opportunity to pose questions to the BASIL-2 investigators, who—along with an expert panel—will interrogate the evidence in a roundtable discussion to reach consensus on this hotly debated topic.

In the peripheral space, revascularisation treatment strategies for patients with CLTI have been firmly in the spotlight following the presentation of first results from BEST-CLI (Best endovascular versus best surgical therapy in patients with critical limb ischaemia) trial in November 2022. CX will provide a platform to move the conversation forward and reach consensus on the topic, with first-topodium results of the BASIL-2 randomised trial forming the centrepiece of a peripheral randomised controlled trial consensus update, taking place on the first day of the CX symposium.
During the session, BASIL-2 chief investigator Andrew Bradbury (University of Birmingham, Birmingham, UK) will deliver the first results from the trial, which Matthew A Popplewell (Heart of England Foundation Trust, Solihull, UK) et al note their 2016 study protocol paper, published in Trials, will “compare, at the point of clinical equipoise, the clinical and costeffectiveness of a ‘vein bypass first’ with a ‘best endovascular treatment first’ revascularisation strategy for severe limb ischaemia due to infrapopliteal (below-the-knee) disease”.
The trial, the authors continue, “is designed to be pragmatic and representative of the ‘real-
world’ [UK] NHS management of severe limb ischaemia due to infrapopliteal disease”.
The original BASIL-1 trial, on which BASIL-2 is based, randomised 452 patients with severe limb ischaemia, mainly due to femoropopliteal disease, to either an angioplasty first or a bypass surgery first strategy, Popplewell and colleagues write. This study, published in The Lancet in 2005, found that, in patients presenting with severe limb ischaemia due to infrainguinal disease and who are suitable for surgery and angioplasty, a bypass-surgeryfirst and a balloon-angioplasty-first strategy are associated with “broadly similar outcomes in terms of amputation-free survival,” and in the short term, “surgery is more expensive than angioplasty”.
BASIL-2 officially began its recruitment phase in July 2014 with its first randomisation from the lead centre— Heart of England NHS Foundation Trust—and, according to the BASIL-2 trial webpage, a total of 41 clinical centres were open to the end of the trial; 31 in England, three in Scotland, two in Wales, three in Denmark, and two in Sweden. On the webpage it is noted that 39 of the 41 clinical centres have cumulatively recruited 345 participants out of a revised sample size of 389 patients.
The headline CX session on revascularisation strategies in CLTI also sees participation from the USA, with BEST-CLI investigators Matthew Menard (Brigham and Women’s Hospital, Boston, USA) and Alik Farber (Boston Medical Center, Boston, USA)
Continued on page 4
A newly published meta-analysis of individual patient data has found that older patients with symptomatic carotid disease are likely to benefit as much from timely intervention as younger patients. Speaking to Vascular News in light of this key finding, senior author Dominic Howard (Oxford University Hospitals NHS Trust, Oxford, UK) stresses that “vascular surgeons must not turn down symptomatic patients just because of their age”.

WRITING IN STROKE, YA YUAN
Rachel Leung (University of Oxford, Oxford, UK), Howard and colleagues note that there is “uncertainty” around whether elderly patients with symptomatic carotid stenosis have higher rates of adverse events following carotid endarterectomy (CEA). “In trials, recurrent stroke risk on medical therapy alone increased with age, whereas operative stroke risk was not related,” they detail, adding, however, that few octogenarians were included in previous trials and that there has been no systematic analysis of all study types. For these reasons, the investigators aimed to evaluate the safety of CEA in symptomatic elderly patients, particularly in octogenarians.
Leung, Howard et al state that they performed a systematic review and metaanalysis of all studies published between 1 Jan 1980 and 1 March 2022 reporting post-CEA risk of stroke, myocardial infarction (MI), and death in patients with symptomatic carotid stenosis. The authors write that they included observational studies and interventional arms of randomised trials if the outcome rates— or the raw data to calculate these—were provided, and that individual patient data from four prospective cohorts enabled multivariate analysis.
The investigators included a total of 47 studies—representing 107,587 patients—in their meta-analysis. Within this cohort, the risk of perioperative stroke was 2.04%
Continued on page 6
The REALITY Study, sponsored by the VIVA physicians,1 demonstrates how the use of the HawkOne™ directional atherectomy system, followed by the IN.PACT™ Admiral™ drug-coated balloon (DCB), can help achieve positive patient outcomes in treating peripheral artery disease.
Outcomes in complex, long, heavily calcified lesions2: Bailout stent rate
92.6% 8.8% 12-month freedom from CD-TLR†
Learn more by visiting medtronic.com/realitystudy





†12-month data reported includes patients beyond the follow-up window.
References
1 The REALITY Study was independently sponsored and conducted by the VIVA Physicians. The study prospectively enrolled 102 participants whose treatment outcomes were independently adjudicated by angiographic and duplex ultrasound core labs and a clinical events committee. The research was funded by Medtronic through the manufacturer’s ERP program.
2 Rocha-Singh KJ, Sachar R, DeRubertis BG, et al. Directional atherectomy before paclitaxel coated balloon angioplasty in complex femoropopliteal disease: The VIVA REALITY Study. Catheter Cardiovasc Interv. September 2021;98(3):549-558.

Brief Statements
















HawkOne Directional Atherectomy System

Important Information: Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device.


Indications for Use: The HawkOne™ directional atherectomy system is intended for use in atherectomy of the peripheral vasculature. The HawkOne catheter is indicated for use in conjunction with the SpiderFX™ embolic protection device in the treatment of severely calcified lesions. The HawkOne catheter is NOT intended for use in the coronary, carotid, iliac, or renal vasculature. Caution: Federal (USA) law restricts this product for sale by or on the order of a physician.
IN.PACT Admiral Paclitaxel-coated PTA Balloon Catheter



















Indications for Use: The IN.PACT Admiral Paclitaxel-coated PTA Balloon Catheter is indicated for percutaneous transluminal angioplasty, after appropriate vessel preparation, of de novo, restenotic, or in-stent restenotic lesions with lengths up to 360 mm in superficial femoral or popliteal arteries with reference vessel diameters of 4–7 mm. Contraindications: The IN.PACT Admiral DCB is contraindicated for use in:
Precautions:
• This product should only be used by physicians trained in percutaneous transluminal angioplasty (PTA).
• Coronary arteries, renal arteries, and supra-aortic/cerebrovascular arteries
• Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy
• Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system • Patients with known allergies or sensitivities to paclitaxel
• Women who are breastfeeding, pregnant, or are intending to become pregnant or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and whether there is a potential for adverse reaction in nursing infants from paclitaxel exposure. Warnings:
• A signal for increased risk of late mortality has been identified following the use of paclitaxelcoated balloons and paclitaxel-eluting stents for femoropopliteal arterial disease beginning approximately 2–3 years post-treatment compared with the use of non-drug coated devices. There is uncertainty regarding the magnitude and mechanism for the increased late mortality risk, including the impact of repeat paclitaxel-coated device exposure. Physicians should discuss this late mortality signal and the benefits and risks of available treatment options with their patients.







• Use the product prior to the Use-by Date specified on the package. • Contents are supplied sterile. Do not use the product if the inner packaging is damaged or opened. • Do not use air or any gaseous medium to inflate the balloon. Use only the recommended inflation medium (equal parts contrast medium and saline solution). • Do not move the guidewire during inflation of the IN.PACT Admiral DCB. • Do not exceed the rated burst pressure (RBP). The RBP is 14 atm (1419 kPa) for all balloons except the 200 and 250 mm balloons. For the 200 and 250 mm balloons the RBP is 11 atm (1115 kPa). The RBP is based on the results of in vitro testing. Use of pressures higher than RBP may result in a ruptured balloon with possible intimal damage and dissection. • The safety and effectiveness of using multiple IN.PACT Admiral DCBs with a total drug dosage exceeding 34,854 μg of paclitaxel in a patient has not been clinically evaluated.
• This product is designed for single patient use only. Do not reuse, reprocess, or resterilize this product. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death. • Assess risks and benefits before treating patients with a history of severe reaction to contrast agents. • The safety and effectiveness of the IN.PACT Admiral DCB used in conjunction with other drug-eluting stents or drug-coated balloons in the same procedure or following treatment failure has not been evaluated. • The extent of the patient’s exposure to the drug coating is directly related to the number of balloons used. Refer to the Instructions for Use (IFU) for details regarding the use of multiple balloons and paclitaxel content. • The use of this product carries the risks associated with percutaneous transluminal angioplasty, including thrombosis, vascular complications, and/or bleeding events. • Vessel preparation using only pre-dilatation was studied in the clinical study. Other methods of vessel preparation, such as atherectomy, have not been studied clinically with IN.PACT Admiral DCB. • This product is not intended for the expansion or delivery of a stent. Potential adverse effects: The potential adverse effects (e.g., complications) associated with the use of the device are: abrupt vessel closure; access site pain; allergic reaction to contrast medium, antiplatelet therapy, or catheter system components (materials, drugs, and excipients); amputation/ loss of limb; arrhythmias; arterial aneurysm; arterial thrombosis; arteriovenous (AV) fistula; death; dissection; embolization; fever; hematoma; hemorrhage; hypotension/hypertension; inflammation; ischemia or infarction of tissue/organ; local infection at access site; local or distal embolic events; perforation or rupture of the artery; pseudoaneurysm; renal insufficiency or failure; restenosis of the dilated artery; sepsis or systemic infection; shock; stroke; systemic embolization; vessel spasms or recoil; vessel trauma which requires surgical repair. Potential complications of peripheral balloon catheterization include, but are not limited to the following: balloon rupture; detachment of a component of the balloon and/or catheter system; failure of the balloon to perform as intended; failure to cross the lesion. Although systemic effects are not anticipated, potential adverse events that may be unique to the paclitaxel drug coating include, but are not limited to: allergic/immunologic reaction; alopecia; anemia; gastrointestinal symptoms; hematologic dyscrasia (including leucopenia, neutropenia, thrombocytopenia); hepatic enzyme changes; histologic changes in vessel wall, including inflammation, cellular damage, or necrosis; myalgia/arthralgia; myelosuppression; peripheral neuropathy. Refer to the Physicians’ Desk Reference for more information on the potential adverse effects observed with paclitaxel. There may be other potential adverse effects that are unforeseen at this time. Please reference appropriate product Instructions for Use for a detailed list of indications, warnings, precautions, and potential adverse effects. This content is available electronically at manuals.medtronic.com. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
providing a podium-first update on the trial and first-topodium quality-of-life data, respectively.
Unanswered questions
The results from BEST-CLI, a major multidisciplinary trial involving investigators from the fields of vascular surgery, interventional cardiology, interventional radiology and vascular medicine, were delivered at the American Heart Association (AHA) scientific sessions (5–7 November, Chicago, USA) and published simultaneously in the New England Journal of Medicine Since their release, the results have ignited debate over which patients are suitable for either an open surgical or endovascular approach.
Results of the trial showed that among a population of 1,830 CLTI patients deemed suitable either for an open or endovascular procedure, surgical bypass with adequate single-segment great saphenous vein (GSV) is a more effective approach. Both strategies, however, were shown to be safe and effective when it came to treating CLTI.
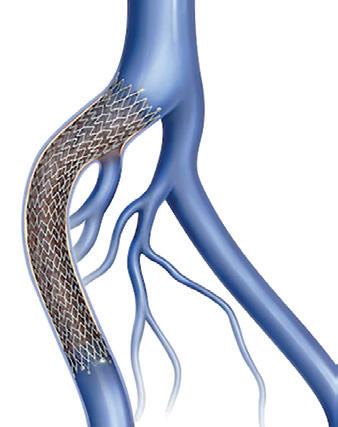
BEST-CLI co-principal investigators—Alik Farber, Matthew Menard, and Kenneth Rosenfield (Massachusetts General Hospital, Boston, USA)— acknowledge that the findings will provide important information regarding the management of CLTI patients. But, recent discussions of the trial’s results suggest that there is still some way to go to settle the question of which strategy should be favoured in patients suitable for either approach. This was brought to the fore in a standalone BEST-CLI session at the recent 2022 VEITHsymposium (15–19 November, New York, USA), which saw commentary being led by interventional cardiologist Eric Secemsky (Beth Israel Deaconess Medical Center, Boston, USA), representing a perspective from the endovascular community, and Michael Conte (University of California, San Francisco, San Francisco, USA), providing a view from the vascular surgery sphere.
Secemsky said BEST-CLI provided robust critical evidence but bore limitations in terms of the inclusion of major reintervention in the primary endpoint, the representativeness of non-surgical specialties in the trial, as well as the “generalisability” of the patients enrolled.
Conte said the trial showed that open surgery and
endovascular intervention “are both safe and have complementary roles in the treatment of CLTI patients”. He said that open bypass with GSV provides more effective revascularisation in suitable candidates, and “is likely under-utilised in current practice,” adding that “an endo-first or endo-only approach to all patients with CLTI is not evidence-based care.” Centres carrying out less than 20% bypass in CLTI “should probably take stock,” he commented.
During the session, Farber delved into potential trial weaknesses. It was a pragmatically designed trial, with the possibility for selection and operator bias in enrolment and intervention, he noted. Farber also acknowledged the trial’s cohort 2 was likely underpowered. “The anatomical complexity is yet to be evaluated,” he added.
Rosenfield, meanwhile, noted the “controversy” the trial will generate: “[Amongst the BEST-CLI investigators] we have differences in the way we think it should be interpreted. My perspective is a little bit more muted than sort of the ‘Okay, this just tells the whole story about how you have to treat CLTI patients.’” His “top line,” he said, is that bypass fundamentally bears an important role in the treatment of CLTI, underscoring how the trial also showed that both procedures are safe. The lesser-discussed cohort 2, Rosenfield said, gets to one of his points of focus—that the study “raises a lot of questions that still need to be answered”.
The trial answers questions “about those patients
n COMPLEX ANEURYSMS:
Data from the UK-COMPASS trial provide new insights into the management of complex aneurysms in England and, according to lead investigator Srinivasa Rao Vallabhaneni (Liverpool, UK), underscore the need for appropriate patient and technique selection to avoid overtreatment.

For more on this story go to page 9.
n PERIPHERAL ARTERIAL DISEASE:
Crossing chronic total occlusion lesions are challenging procedures. The BeBack crossing catheter—Bentley’s first product to be available in both Europe and the USA following the company’s acquisition of Upstream Peripheral Medical Technologies’ GoBack crossing catheter in September 2022— offers a new solution in this space. In a Bentleysponsored advertorial, Andrej Schmidt (Leipzig, Germany) shares his clinical experience with the BeBack, noting how it has been a “gamechanger” in his endovascular peripheral arterial disease practice.
For more on this story go to page 19.
n VENOUS STENTING:

who were randomised in the trial,” he said, continuing, “We need to unpack better what were the characteristics of those patients who were entered into the trial.” That will help specialists determine the degree to which “we can generalise the findings: to which patients does this apply?”
Join the discussion
CX continues its three-year cycle of raising vascular and endovascular controversies in order to challenge the available evidence and to be able to reach a consensus after discussion with a global audience. Register for CX 2023 at www.cxsymposium.com to be one of the first to hear the latest data on revascularisation strategies in CLTI and be part of the data-driven discussion.
Prominent venous disease experts discuss venous stenting, appropriate care, and the quest to refine data and clinical practice in a space where part of the problem, one remarks, involves practitioners moving “freely from being able to do arterial intervention and suddenly assuming they can do a venous intervention”. For more on this story go to page 20.
Editor-in-chief: Roger Greenhalgh

Publisher: Roger Greenhalgh
Content Director: Urmila Kerslake
Editor: Jocelyn Hudson Jocelyn@bibamedical.com
Editorial contribution: Jamie Bell, Will Date, Bryan Kay, Eva Malpass and Clare Tierney
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: Shilpa Suthar shilpa@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Please contact the Vascular News team with news or advertising queries Tel: +44 (0) 20 7736
Make sure you get your copy of Next
The results [of BEST-CLI] have ignited debate over which patients are suitable for either an open surgical or endovascular approach.”



Continued from page 1
(1.94–2.14) in octogenarians, or 390 strokes in 19,101 patients, and 1.85% (1.75–1.95) in non-octogenarians, equating to 1.395 strokes in 75,537 patients (p=0.046).
In terms of perioperative death, the investigators report a figure of 1.09% (0.94–1.25) in octogenarians (203 strokes in 18,702 patients) and 0.53% (0.48–0.59) in non-octogenarians (392 strokes in 73,327 patients), with a p value of less than 0.0001.
The authors add that, per five-year age increment, a linear increase in perioperative stroke, MI, and death were observed (p=0.04–0.002). However, during the last three decades, they found that perioperative stroke and/or death has declined “significantly” in octogenarians, from 7.78% (5.58–10.55) before the year 2000 to 2.8% (2.56–3.04) after 2010, with a p value of less than 0.0001.
In the individual patient data multivariate analysis, which included 5,111 patients, age of 85 years or above was independently associated with perioperative stroke (p<0.001) and death (p=0.005). However, the investigators note that survival was similar for octogenarians vs. non-octogenarians at one year (95% [93.2–96.5] vs. 97.5% [96.4–98.6]; p=0.08), as was five-year stroke risk (11.93% [9.98 –14.16] vs. 12.78% [11.65–13.61]; p=0.24).
Leung, Howard and colleagues summarise that they found a “modest” increase in perioperative risk

A systematic review and metaanalysis has demonstrated “convincing evidence” that sex differences exist in carotid atherosclerosis, with all types of plaque features— including those relating to size, composition, and morphology—found to be either larger or more common in men than in women.
“Our results highlight that sex is an important variable to include in both study design and clinical decision-making,” the authors, led by Dianne van Dam-Nolen (Erasmus University Medical Center, Rotterdam, The Netherlands), write in the journal Stroke. “Further investigation of sex-specific stroke risks with regard to plaque composition is warranted.”

Over the past few decades, several individual studies on sex differences
with age in symptomatic patients undergoing CEA. However, they stress that recurrent stroke risk also increases significantly with age when on medical therapy alone, and thus conclude that their findings “support selective urgent intervention in symptomatic elderly patients”.
In the discussion of their findings, Leung, Howard et al claim that theirs is the first meta-analysis of CEA safety by age for patients with symptomatic carotid disease, which includes all study types and individual patient-level data analysis. The study is also novel in its findings, the authors note, writing that “although symptom status is known to be strongly associated with recurrent stroke risk and post-CEA morbidity, this is the first study to confirm the association of age with both short- and long-term outcomes in symptomatic patients”.
The investigators state that their findings have several implications for clinical practice. For example, they highlight the finding that the effect of age on perioperative morbidity was linear without a clear step-change in risk, which “contrasts with the current notion of higher perioperative morbidity in the elderly once they reach a certain age”.
They also address implications around the “major concern” of cardiac risk for elderly patients undergoing intervention. As well as confirming a modest linear increase in perioperative cardiac risk with age, pooled estimates revealed a higher annual stroke or death rate in the elderly compared to younger patients when stratified by prevalence of known coronary artery disease. “This key finding supports clinical caution in selecting elderly patients
in carotid atherosclerosis have been performed, covering a wide range of plaque characteristics and including different populations, the authors state.
In addition to summarising previously reported results on sex differences in this space, the researchers also sought to “present a roadmap explaining next steps needed for implementing this knowledge in clinical practice”.
They began by systematically searching PubMed, Embase, Web of Science, Cochrane Central and Google Scholar for eligible studies, including both male and female participants, and reporting the prevalence of imaging characteristics of carotid atherosclerosis. The eligible studies were then meta-analysed. Van DamNolen et al prespecified which imaging modalities had to be used per plaque characteristic and excluded ultrasonography.

After initially identifying more than 1,000 unique citations, screening of the articles based on the inclusion criteria whittled this number down to a total of 60 articles, with 42 being included in the final meta-analyses.
Six of these studies were included in a meta-analysis on the relationship between sex and atherosclerotic plaque size. All three of the characteristics used to measure plaque
with significant coronary artery disease for CEA. Conversely, it also provides reassurance that for elderly patients without major cardiac comorbidity, outcome following CEA is similar to that of younger patients,” the authors detail.
The authors also acknowledge some limitations of their study. These include the fact that patients in studies published before 2010 may not have been on contemporary intensive medical therapy for control of vascular risk factors, which “may contribute to an overall higher risk of stroke and death than that found in current clinical practice”. To account for this, the investigators relay that their time-trend analyses have confirmed reductions in risk following surgery over the last three decades.
Looking ahead, Leung, Howard et al consider what future research might be of benefit in this space: “Future clinical trials investigating the efficacy of surgery versus best medical therapy alone for very elderly symptomatic patients would be of clinical interest.”
However, they recognise that eliminating selective recruitment bias in such trials “would be very challenging” and thus “interpreting results would require great caution”.
size—maximum wall thickness (1D size), wall area (2D size), and wall volume (3D size)—were more likely to be larger in men than in women, van Dam-Nolen et al report. However, conversely, the normalised wall index, which accounts for the total vessel size, did not statistically significantly differ between male and female participants, which the researchers describe as “surprising”, and possibly indicative of sex differences in plaque size being driven by contrasting vessel sizes.
In addition, analysing three of the studies further regarding the degree of stenosis, the authors found no statistically significant sex difference for stenosis of 50–69%, although highgrade stenosis of 70–99% was more often seen in men than in women.
Meta-analyses relating to plaque composition examined the presence of calcifications, lipid-rich necrotic core (LRNC), and intraplaque haemorrhage (IPH), and found a higher prevalence in men versus women across all three components. Expounding briefly on their calcification findings, van Dam-Nolen et al report statistically significant differences between men and women for the presence and amount of carotid calcifications, but not in terms of calcification percentage i.e. the amount of calcification relative to the total plaque volume.
“Furthermore, we found more pronounced sex differences for LRNC in symptomatic as opposed to asymptomatic participants,” they add. Five studies were also included in the meta-analysis of the relationship between sex and plaque morphology, with the presence of ulceration and the presence of a thin-or-ruptured fibrous cap both being higher in men.
In their report, the authors highlight multiple limitations of their analysis that “deserve comment”, including moderate-to-high heterogeneity among the included studies—especially with regard to plaque size and carotid calcifications—as well as the fact it was not possible to adjust for potential confounders on the relationship between sex and carotid atherosclerosis.
“The found sex differences in carotid atherosclerosis are of clinically significant importance, since the composition of plaque affects the risk of (recurrent) stroke,” van Dam-Nolen et al conclude. “Previous studies have shown that especially IPH contributes to a higher stroke risk. Carotid LRNC, calcifications, total plaque size, and plaque ulceration, have also been reported as important risk factors. With regard to sex-specific risk prediction and treatment, it is essential to investigate the effect of these plaque characteristics per sex separately. We hypothesise that the stroke risk as a result of specific plaque compositions varies among men and women.”
Vascular surgeons must not turn down symptomatic patients just because of their age.”
Dominic HowardYa Yuan Rachel Leung Dominic Howard
Sex found to be “important variable” in studyDianne van Dam-Nolen

Maarit Venermo (University of Helsinki and Helsinki University Hospital, Helsinki, Finland) speaks to Vascular News about population screening for abdominal aortic aneurysm (AAA), sharing key findings from the Nordic AAA study and addressing some of the main challenges associated with implementing a successful national screening programme.

How did the idea for the Nordic AAA study originate?
For some time now, we have been studying how the incidence of ruptured AAA has changed over the last 10–20 years in Finland, particularly in the Helsinki region, and have been following the excellent Swedish publications on aneurysm screening. In addition, we noticed in our own studies that despite not having screening in Finland, the incidence of ruptured AAA has decreased somewhat. Therefore,
we thought that it would be interesting to evaluate how screening has changed the ruptured AAA incidence and also elective AAA repair rates in Sweden, and compare Finland and Sweden. The Nordic countries have similarities in both healthcare and population structure, as well as in geographical distribution of the population, and hence a comparison of AAA epidemiology between these countries offers insights into how organisation of care can affect AAA outcomes.
You shared some key data from the study at CX Aortic Vienna 2022. Could you outline the headline findings?
The main finding I reported was that the incidence of ruptured AAA has decreased in both Finland and Sweden, with the decrease more pronounced in Sweden—the only country out of the four included in the study (Denmark, Finland, Norway and Sweden) with a national screening programme. I also shared that screening has increased elective repair among 65–80-year-old men, and that, among men over the age of 80 years, there has been a significant increase in intact aneurysm repair.
How does the Swedish national screening programme operate, and how has it impacted detection rates?
The Swedish national screening programme was initiated in 2006, and reached national coverage in 2015. It invites men at age 65 to a single ultrasound screening for AAA. The screening programme has been highly successful, reaching >80% coverage in most areas, and with a prevalence of screening-detected AAA of 1.5%. Previous analysis of Swedish data suggests that the screening programme has significantly reduced AAAspecific mortality in Sweden, and health economic evaluation of the programme shows that this was done in a highly costeffective manner. The main challenge for the screening programme going forward is the fact that the prevalence of AAA in the general 65-year-old male population is decreasing, and it is possible that a modification of the programme will be required with a continuous reduction in prevalence of disease. Another challenge that is an area for research is how to manage individuals with detection of subaneurysmal aorta (i.e. aortic diameter of 25–29mm). A large proportion of these individuals are at risk of developing clinically significant AAA over time, but currently there are no clear guidelines on how follow-up should be organised for this cohort.
What are the main costs, and other challenges, associated
The UK’s NHS Abdominal Aortic Aneurysm (AAA) Screening Programme (NAAASP) is fast approaching its 10th anniversary—a milestone that Akhtar Nasim (Sheffield Vascular Institute, Sheffield, UK) and Meryl Davis (Royal Free London NHS Foundation Trust, London, UK) believe provides an opportunity for reflection on progress made, and consideration of future challenges.
IN AN INTERVIEW WITH VASCULAR NEWS —conducted at the 2022 Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (23–25 November, Brighton, UK)—Nasim and Davis looked back on how much has changed with regard to AAA screening in the UK. According to Nasim, clinical lead for the NAAASP in England, a “huge amount of progress” has been made in the years since the MASS (Multicentre aneurysm screening study) trial was published in 2002. In 2008, the UK Government’s Department of Health introduced the AAA screening programme, he noted
in particular, which became fully operational five years later.
In the decade since, Nasim reports that around three million men have been offered screening, over two million have taken up the offer, 8,000 men who reached the threshold size have been referred for AAA surgery, and around 6,000 have been operated on. He relayed “excellent” results in terms of 30day mortality, at around 1.22% overall for both endovascular and open surgery. Davis, who is the AAA screening lead for North London, based at the Royal Free Hospital, highlighted some organisational changes that have occurred over the years. She noted for example that in 2018, the North London aneurysm screening programme was created. This initiative combined services for North Central, North West, and North East London and has seen “huge achievements” in terms of the provision of screening services, she informed Vascular News
Both Nasim and Davis acknowledged that there have been obstacles along the way. Davis noted that that the restoration of services amidst the COVID-19 pandemic has been an ongoing challenge in recent

There are several cost-effectiveness studies on screening and the cost of quality-adjusted life years (QALYs) from Sweden, the UK and also from Denmark. The cost per QALY is different in these studies, being less expensive in Denmark.
Of course the whole set-up is expensive as you have to cover the whole country, train nurses and build a system where all the people who meet the screening criteria will be invited to their appointment. Secondly, the number of elective aneurysm repairs is increasing, causing further expenses. At the same time, however, the incidence of ruptured aneurysms is decreasing, which will have the effect of lowering costs and, more importantly, decreasing the rate of ruptured AAA mortality. Whilst a decreasing prevalence of AAA may challenge the cost-effectiveness of a screening programme, modelling data shows that screening is cost-effective down to a prevalence of 0.5%.
Patient involvement and adequate population information are also crucial factors in a successful screening programme. It is known that individuals who do not attend screening are often socioeconomically disadvantaged, and at higher risk for having AAA. Thus, focused efforts to make screening accessible in such populations are of paramount importance.
years, referencing her involvement in helping the North London screening programme “come back up to speed” since late 2021. “We all think that COVID-19 has disappeared, that it is very much in the background,” she said, going on to warn against overlooking the enduring impact of the pandemic.
This year will mark the 10th anniversary of the NAAASP being fully operational—a milestone which Nasim remarked should be seen as an “opportunity to highlight the programme and particularly encourage men that may have missed screening or those that have not taken up the offer of screening”.
Considering the impact of the programme so far, Nasim pointed to “some signs in the ONS [Office for National Statistics] mortality data” that it has begun to have a beneficial effect. Despite this, Davis emphasised the importance of increasing uptake for AAA screening in general, and especially among men from deprived communities. “We have to work together with those communities to reassure them,” Davis urged, highlighting certain steps being taken on a national level to address this issue, including a “toolkit” to allow local providers to try and work out strategies for how to increase participation in screening among low-uptake communities.
We have got a large cohort of men who have not been invited [for screening], and that is what we are working on.”Akhtar Nasim and Meryl Davis
Maarit Venermo
Data from UK-COMPASS (UK complex aneurysm study) were presented for the first time at the 2022 Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (23–25 November, Brighton, UK).
UK-COMPASS IS A COHORT study of 2,202 patients treated across all hospitals in England over a consecutive two-year period. Speaking to Vascular News, lead investigator Srinivasa Rao Vallabhaneni (University of Liverpool, Liverpool, UK) reports that researchers were able to show “extremely good results” out to three years across three groups of patients based on aneurysm neck length: pararenal (0–4mm), juxtarenal (5–9mm), and those 10mm or more that were deemed unsuitable for an on-label, standard endovascular
aneurysm repair (EVAR). Vallabhaneni notes that, in the pararenal group, both open repair and fenestrated repair are being delivered with “very good safety”.
At the VSGBI annual meeting, Shaneel Patel (Royal Liverpool University Hospital, Liverpool, UK) delivered a presentation on study and corelab methods, after which Michael Jenkins (Imperial College Healthcare NHS Foundation Trust, London, UK) outlined early results. He shared the headline finding that open repair does worst, EVAR does best, and FEVAR is “in between” with regard to perioperative (in-hospital and 30-day) mortality. Jenkins specified that these results were “fairly consistent” across neck lengths, and also noted a high rate of secondary interventions.
Later in the session, Jon Boyle (Cambridge University Hospitals NHS Trust, Cambridge, UK; see page 16 for profile) outlined results out to median follow-up. Reporting unadjusted allcause mortality at three years, he noted a “significant divergence” of results just beyond a year favouring open repair in terms of survival, and at a three-year mortality rate of around 21%.
“In the long term, you have got about twice the risk of dying if you have had an EVAR over open repair, and similarly about twice the risk if you have had FEVAR [fenestrated EVAR] over open repair at long-term follow-up,” the presenter informed attendees.
Looking specifically at the different
treatment groups, Boyle noted that, if a patient with no aortic neck has a standard EVAR, survival in the long term is “significantly worse” than with either open repair and FEVAR—groups in which he noted the outcomes are similar. For longer aortic necks, Boyle stated that open repair in the longer term has better outcomes than both EVAR and FEVAR, and that in patients with aortic necks greater than 10mm in diameter, open repair has “significantly better” outcomes at three years.
In terms of secondary interventions, he said that “not surprisingly,” there were “significantly greater numbers” of reinterventions within the first three years if a patient had EVAR or FEVAR, and that results were “significantly worse” with FEVAR than with EVAR.
“In conclusion,” Boyle relayed, “there is no doubt that the longer-term all-cause mortality is significantly better for open repair,” noting a hazard ratio (HR) for EVAR of around 2.27 and
The UK National Vascular Registry (NVR) recently published its 2022 annual report—the 10th since the registry was launched in 2013—highlighting important outcome data for vascular procedures performed in the period between 2019 and 2021.
The report, which was prepared by the NVR team at the Clinical Effectiveness Unit at the Royal College of Surgeons of England alongside the Vascular Society of Great Britain and Ireland (VSGBI) and the British Society of Interventional Radiology (BSIR), contains comparative information on five major interventions for vascular disease: repair of aortic aneurysms; lower limb bypass; lower limb angioplasty/stenting; major lower limb amputation; and carotid endarterectomy.
Jon Boyle (Cambridge University Hospitals NHS Trust, Cambridge, UK), the 2021–2022 president of the VSGBI, notes in the foreword to the report that it highlights outcome data from a period during which vascular practice “came under unprecedented pressure from the COVID-19 pandemic”. Despite this, he reports that case ascertainment rates in the NVR remain “extremely high,” which is of “great credit” to the vascular community.
The report looks closely at lower-limb interventions for peripheral arterial disease (PAD), with Boyle noting a particular focus on the five-day target for revascularisation for emergency admissions with chronic limb-threatening ischaemia (CLTI). He
relays that, overall, 55% of these patients underwent lower-limb bypass or angioplasty within five days of admission and stresses that the ‘five-day revascularisation target’ has been adopted into clinical practice in many centres.
In addition to the outcome data, the report also includes the findings of an organisational survey of all vascular arterial units that was undertaken in the summer of 2022. “Of particular interest is the wide variation in support for data entry into the NVR in many trusts and limited access to barcode scanning,”

for FEVAR of about 1.91. Outlining the results of a subgroup analysis, he said that FEVAR “does appear to be equivalent to open repair for shortnecked aneurysms at three years,” again noting “significantly higher” reintervention rates for both EVAR (HR, 2.18) and FEVAR (HR, 2.67).
Considering the wider context of the trial results, Vallabhaneni addressed the question of overtreatment of aortic aneurysms. “Effective clinical decisionmaking in aneurysm repair calls for personalised decision-making,” he stressed. While Vallabhaneni stated that the facets of the “clinical practice ecosystem” in which practitioners are making aneurysm repair decisions— namely evidence, governance structures, and professional attitudes and aspirations—are “all important in their own way,” they provide “in combination, very little scope to provide personalised decision-making in aneurysm practice”.
The presenter posited that “perhaps we are doing too many aneurysm repairs,” adding that there is “relatively good quality evidence to suggest that many a patient who survives the operation may not gain the overall survival benefit we hope for”.
Selection of the appropriate patients and techniques is crucial here, Vallabhaneni reiterated, specifying that clinic-ready tools are needed that would allow practitioners to make personalised decisions regarding aneurysm repair.
Boyle writes. Despite this, he communicates the fact that 60% of aortic devices are being captured for a non-mandatory field, which “reflects the fact that clinicians recognise the importance of collecting this data to drive improvements in the management of aortic aneurysms”.
Speaking to Vascular News, chair of the VSGBI Audit & Quality Improvement Committee and clinical lead for the NVR, Arun Pherwani (University Hospitals of North Midlands NHS Trust, Stoke on Trent, UK) comments on the key takeaway messages from the 2022 report: “We have focused on three particular areas; lower limb interventions for PAD and the ‘five day target for CLTI’, a ‘state of the nation’ report on type B aortic dissection (TBAD) with six-year data and units that treat TBAD in the UK depicted in an interactive map, and the results from the ‘organisational survey’ where all 69 vascular units in the country have participated.” He adds:
“I am confident the VSGBI Quality Improvement Programme for PAD (PAD QIF) helped by the NHS England Commissioning for Quality and Innovation (CQUIN) incentivised payment scheme, will deliver long-term benefits to the care of patients with CLTI.”
Pherwani also highlighted the UK Government’s December 2022 update on the implementation of the recommendations from the 2020 Baroness Cumberlege Independent Medicines and Medical Devices Safety Review. Medical devices safety and information are to be recorded under the new NHS Outcomes and Registries programme and the NVR has been identified as an exemplar registry. Pherwani remarks: “Our work with aortic devices and aortic reintervention data has reinforced our reputation as a ‘world leading vascular registry’.”
In the long term, you have got about twice the risk of dying if you have had an EVAR over open repair.”
Closer ties between cardiologists, cardiac surgeons and vascular surgeons will be a hallmark of the future treatment of diseases of the aorta, a leading figure behind new guidelines for the diagnosis and management of aortic disease tells Vascular News
Jointly published by the American College of Cardiology (ACC) and American Heart Association (AHA) in November 2022, the new guidelines are intended to support decision-making around diagnosis, screening, medical therapy, endovascular and surgical treatment, and long-term surveillance of patients with aortic disease.
Building upon an earlier version of the document, last updated in 2010, the new guideline incorporates latest evidence to reflect advances in care. Central among the latest additions is a focus on multidisciplinary aortic team care to determine appropriate timing and optimal medical, endovascular and surgical therapies.
“The important thing for these guidelines is the multidisciplinary approach that was not evident in prior guidelines,” Ourania Preventza (cardiac surgeon at The Texas Heart Institute and professor of surgery at Baylor College of Medicine, Houston, USA), vice-chair of the guideline writing committee tells Vascular News. “There is a multidisciplinary aortic team that is determining the appropriate type of intervention and a shared decisionmaking approach between the patient

and the providers.”
According to Preventza, this diversity of specialisms was embedded in the writing process from the offset, with the committee chaired by a cardiologist, Eric Isselbacher (Harvard Medical School, Massachusetts General Hospital, Boston, USA), alongside Preventza, a cardiac surgeon, and her fellow vice-chair, James Hamilton Black III (Johns Hopkins Medicine, Baltimore, USA), who is a vascular surgeon. Other spaces on the 26-strong writing committee were also taken, in addition to the cardiologists and to the cardiac and vascular surgeons, by cardiovascular anaesthesiologists, geneticists, and interventional radiologists.
“The thought process when we created this and assembled the committee was really to provide diverse perspectives. The only way to provide these diverse perspectives is when we include all these different specialties with specialist knowledge about therapy and diagnosis of patients with aortic disease,” Preventza adds.
Asked whether it was difficult to balance what
In a “focused update” to their 2019 recommendations, the European Society for Vascular Surgery (ESVS) abdominal aortic aneurysm (AAA) guidelines writing committee has published advice on the surveillance and management of patients treated with the Nellix endovascular aneurysm sealing ( EVAS) system (Endologix).
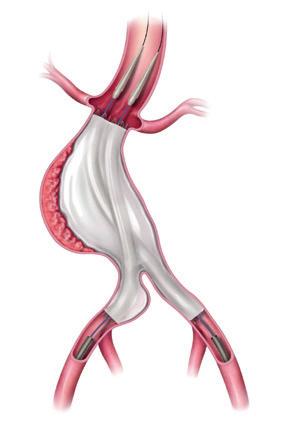
THE COMMITTEE ADVISES THAT ALL patients in whom a Nellix device has been implanted “should be identified, properly informed and enrolled in enhanced surveillance.” If device failure is detected, it states that “early elective device explant should be considered in surgically fit patients”.
The alert, authored by Jon Boyle (Cambridge University Hospitals NHS Trust and University of Cambridge, Cambridge, UK) et al, was published
may sometimes be differing schools of thought, Preventza comments that the writing committee was fundamentally led by evidence available to guide best practice, and says that the proof that they got this right is in the fact that the document has been endorsed by a number of societies in different fields, including the American Association for Thoracic Surgery (AATS), the Society for Thoracic Surgery (STS), the Society for Vascular Surgery (SVS), the Society for Cardiovascular Angiography and Interventions (SCAI), the Society of Cardiovascular Anesthesiologists (SCA) and the Society of Interventional Radiology (SIR).
“The guidelines are there to give the physicians something to base their practice on, guide them, and most importantly, to help patients and make sure that the medical and cardiovascular community have the same or similar approach which is safe and effective.”
Detailing what she sees as the formula for a multidisciplinary aortic team, Preventza says that there is no firm blueprint, rather the approach is guided by shared decision-making in the interest of the patient.
“It is really a collaboration between cardiology, vascular and cardiac [surgeons],” she comments. “For example, an abdominal aneurysm is very well treated by the vascular surgeons. Somebody with an abdominal aneurysm may also have a thoracic aneurysm, involving the ascending aorta, and this is something that has to be taken care of by a cardiac surgeon. Or, perhaps the annuli of the aortic valve or the ascending aorta is not at the size yet that needs intervention, so in this case the patient can be followed by cardiology.”
online ahead of print in the European Journal of Vascular and Endovascular Surgery (EJVES)
Boyle and colleagues note that they performed a scoping review of risk for late serious aortic-related adverse events in patients treated with EVAS for AAA based on a literature search in PubMed up to 7 December 2022. Following synthesis of the available evidence, the writing committee agreed on recommendations graded according to the European Society of Cardiology (ESC) grading system.
“EVAS has a very high incidence of late endograft migration resulting in proximal type 1 endoleak with risk of rupture, requiring open conversion with device implant,” the authors write, detailing their key finding from the review. They add that the reported mortality for elective explantation varies between 0% and 14%, while acute conversion for rupture has a “very dismal prognosis” with 67–75% mortality.
The authors detail that ESVS guidelines are renewed every five years or so, and that ESVS focused updates are issued “to convey important new data that have emerged in between the publication of the full guidelines, that affect patient safety or impact decision-making or management of the patients”.
In their 2019 guidelines on the management of abdominal aortoiliac artery aneurysms, the writing
Patient-specific care is important, she adds, commenting that it is not a onesize-fits-all approach.
Other important takeaways from the revamped document include new thresholds for surgical intervention for sporadic aortic root and ascending aortic aneurysms, an updated definition for rapid aneurysm growth rate, and adjusted recommendations on when to intervene in patients who are smaller or taller than average. “The plan is that the guidelines are a living document, to be updated in the next three years,” adds Preventza. “Nobody has a crystal ball, but given the way that the field is evolving with regards to the treatment options I think it is important for us to re-evaluate and see where we are.”
Important areas to watch include the evolving field of genetics as it relates to aortic disease, further developments in the evidence in the area of endovascular intervention, and research looking at sex and socioeconomic disparities in aortic disease. “These are things that as we evolve we hope to improve, with more inclusive studies, and with advancements in technology; all of which will help us and help our patients and that is why it is important that these guidelines will be updated,” she concludes.
committee recommended that “conceptual new technologies, such as [EVAS], should only be used within studies approved by research ethics committees and with informed consent, until properly evaluated”.
In May of last year, Endologix ended production of the Nellix EVAS system, based on reportedly higher rates of leaks around the device’s implantation, endograft migration and aneurysm sac enlargement. The guideline committee recognised, however, that there was no clear guidance on surveillance and management of patients who have already undergone AAA repair with an EVAS device. It was for this reason that the ESVS AAA guidelines writing committee initiated a literature review on the topic. “The current accumulated knowledge suggests that patients treated with EVAS for AAA may be at high risk for serious aorticrelated adverse events, which justified an updated guidance on the surveillance and management of patients already treated with EVAS,” they write in the new paper.
The committee note that they decided it was important to publish before the fully updated guidelines will be available in 2024, “to highlight the issues with EVAS failure, to promote patient safety and to encourage clinicians to identify all patients in whom a Nellix device has been implanted”.
The plan is that the guidelines are a living document, to be updated in the next three years.”
Nellix EVAS systemOurania Preventza

Athanasios Saratzis (University of Leicester, Leicester, UK) speaks to Vascular News about the EVOCC (Endovascular versus open revascularisation in severe occlusive aortoiliac disease) trial. This randomised study recently received funding from the UK’s National Institute for Health and Care Research (NIHR), marking the first step in what Saratzis states will be “one of the largest peripheral arterial disease [PAD] trials in the UK over the next few years”.
What is the rationale behind this randomised trial?
This was really a patient-initiated study. In Leicester, a lot of our patients feel that there is uncertainty surrounding treatment decisions for those who have severe aortoiliac disease, with regards to how it is decided whether open surgery or endovascular surgery is better. We realised about two or three years ago that there is actually no literature on this. We do not really know whether the results of open surgery are better compared to endovascular treatment, and we do not really know which endovascular surgery or endovascular treatment is the best one in terms of which stents to use and how you use them. Against this backdrop, we performed a meta-analysis that was a joint project between our centre in Leicester and St Thomas’ Hospital in London, UK. We looked at the few studies that have been published in this domain, and found out that actually modern endovascular techniques are probably as good as open surgery, at least for the first few months. However, there is not much data available on what happens after the first three months, especially with regards to reinterventions or late clinical events. When we applied for funding to the NIHR, it was decided that this was an important project, and here we are.
How would you describe the current landscape of care for severe aortoiliac disease across the UK?
In order to design the study, we sent out a national survey to which 144 interventional radiologists and vascular surgeons replied. We also involved 28 patients nationally who had some form of treatment
for this condition in order for us to understand what the treatment landscape is like. About 80% of the surgeons and radiologists who took part in the survey were in agreement that we do not really know whether open surgery is better than modern endovascular treatments in this setting; about 95% felt that we need to do a randomised trial in order to compare modern endovascular treatments and the traditional type of surgery that we have been offering to patients. The patients were all in agreement that they would take part in a study as long as the treatments were safe. Our survey showed that there are still some centres that have got a preference for open surgery and some other centres might have a preference for endovascular surgery, but none of that is actually based on very strong evidence.
How many patients do you hope to enrol and what is the timeline?
This will be a difficult study because of the fact that patients with chronic limb-threatening ischaemia (CLTI) are often frail, which means that it is not very easy to ask them to take part in studies and sometimes decision-making for these patients can be complex. Therefore, I am not expecting that it will be an easy study to complete. In order for us to compare the two treatments in terms of our primary outcome of amputation-free survival, we need to recruit 628 patients. We aim to do this over a period of about 30 months. An internal pilot study, which will start in October 2023, is the first phase of the trial, and that will go on for at least six months. Following that, we will open our main recruitment phase, which will last two years. We are going to follow every patient up
Medtronic has announced the first patient enrolment in the ADVANCE (Endurant stent graft system versus Excluder endoprosthesis) trial, a head-to-head randomised controlled trial of two leading aortic stent graft systems, the Endurant II/IIs stent graft system (Medtronic) and Excluder AAA device family systems (Gore). The ADVANCE trial is a global, post-market, prospective, interventional, multicentre, randomised study that will enrol a minimum of 550 patients at up to 50 centres globally. Patients will be randomised to receive endovascular aneurysm repair (EVAR) with either the Endurant family or Excluder family grafts and will be followed at one month, one year, and annually through five years.
THE FIRST PATIENT IN THE ADVANCE trial was enrolled by the team led by Ray Workman at Novant Health Forsyth Medical Center (Winston-Salem, USA).
“Through the ADVANCE trial, we
are working to deepen our evidence of sac regression as a key indicator of long-term EVAR patient outcomes,” said Hence Verhagen (Erasmus Medical Center, Rotterdam, The Netherlands), co-principal investigator of the trial.
Athanasios Saratzisfor at least two years and for a median of three years. It is our hope that 30 sites (hospitals across the NHS) will take part in the trial. So far, we have been in touch with 35 sites, most of which have confirmed that they would like to participate. It will take a few years before we know the results of the study because it is a big project. I think the internal pilot will give us a lot of interesting information and then hopefully we will be able to recruit the 600 patients we need for the main study.
What do you expect from this trial?
Do you have an idea of how the results might play out?
Not really! If you asked me a few months ago, I would have said that endovascular treatments are definitely seeing major improvements. However, the BEST-CLI trial report from the USA clearly shows that for CLTI patients with disease below the inguinal ligament, surgery seems to do a bit better. So, I am not sure whether we will see the same signal in patients with aortoiliac disease. I think there is just so much uncertainty, and in fact the BEST-CLI results have confirmed even more the fact that we need to do this trial. I am an endovascular enthusiast, but increasingly I am becoming more and more uncertain as to which treatment is the best one. It is a very grey area, let me put it that way.
On the topic of BEST-CLI, how do you think the results might impact the landscape of vascular trials going forward?
I think BEST-CLI is a turning point in vascular surgery, especially in PAD, because we are now funding more effectiveness trials like this one. We really need the support of all vascular clinicians in order to recruit to those studies efficiently in the years to come. It is borderline unacceptable to offer complex endovascular treatments to patients with vascular diseases and not recruit into big effectiveness trials assessing these treatments. There are clearly many questions that have not been answered, and these are not my questions, they are questions that the patients bring up and impact on their care.
“Our hope is that the findings will allow physicians to make evidence-based clinical decisions to improve long-term patient outcomes.”
The ADVANCE trial aims to further the understanding of sac regression by robust evaluation of CT imaging utilising an independent core lab through five years. The trial will provide a comparison of aneurysm sac regression outcomes between the Medtronic Endurant II/IIs stent grafts and the Gore Excluder AAA device family stent grafts with additional evidence to analyse risk factors related to aneurysms that fail to regress. The trial will also compare other key clinical outcomes between the two stent grafts, including endoleaks, migration, secondary interventions, mortality, and renal complications.
“We are pleased to announce the first patient enrolled in the ADVANCE
Enduranttrial,” said Marc Schermerhorn, chief of vascular and endovascular surgery, Beth Israel Deaconess Medical Center (Boston, USA) and co-principal investigator of the trial. “This milestone underscores the commitment to rigorous study of the long-term data around the durability of the Endurant system for patients in need of EVAR. The results of the trial aim to demonstrate contemporary outcomes and our overarching goal to deliver superior aortic patient care through robust and rigorous clinical data.”
The ADVANCE trial draws on clinical data showing that one-year sac regression is an early indicator of improved long-term survival. The outcomes were consistent with the eight-year results from the ENGAGE OUS registry, published in January 2022, which demonstrated the long-term clinical safety and effectiveness of the Endurant stent graft system.










The Sundance (Surmodics) sirolimus drug-coated balloon (DCB) has an “excellent” safety profile in a “challenging, realworld, predominantly CLTI [chronic limb-threatening ischaemia] population,” and has a primary patency rate of 80% at 12 months in a per-protocol analysis population. Ramon Varcoe (Prince of Wales Hospital, Sydney, Australia) first presented these findings from the SWING (Safety and feasibility of Surmodics Sundance DCB) first-in-human study at the 2022 VEITHsymposium (15–19 November, New York, USA).
VARCOE, WHO IS CO-LEAD investigator of the trial, added that the research team observed no major amputations, “very low” rates of major adverse events, and “impressive” luminal gain, which was sustained out to six-month angiogram. In addition, Varcoe reported that Rutherford category and the functional outcome measures were improved.

The presenter noted that SWING is a prospective, multicentre, single-arm feasibility study that looked at patients with stenotic or occluded lesions. The researchers enrolled 35 patients over eight sites in Australia, New Zealand, and also in Europe.
Noting some key inclusion criteria,
Varcoe detailed that patients had to be either Rutherford 4 or 5. He added the caveat that Rutherford 3 patients were included, but numbers were capped at 20% of the total cohort. Furthermore, patients had to have de novo or restenotic lesions and at least 50% stenosis by visual estimate of the investigator. They could have up to two distinct lesions in the same or different below-the-knee (BTK) artery, and had to have successfully treated inflow, as well as an unimpaired outflow artery in continuity to the ankle or foot.
The investigators performed both an intention-to-treat and a per-protocol analysis, Varcoe informed the audience.
“The reason for that was because [the
trial] was conducted over the COVID-19 pandemic period, so we lost seven patients to the primary endpoint of angiography,” he noted, adding that there were also three post-protocol deviations. For these reasons, the research team focused on the 25 patients who had the perprotocol analysis.
The presenter stressed that the patients included represent a “realworld” population, including a high proportion of patients who are smokers and have diabetes, as well as a majority of patients with Rutherford 4 or 5 disease. “They also had high proportions of moderate-to-severe calcification in excess of 18%, and around a third of these patients had total occlusions,” he added.
The study had two primary endpoints. The first was a safety primary safety endpoint—freedom from major adverse limb event (MALE) and perioperative death at 30 days following the index procedure. The primary efficacy endpoint was the rate of late lumen loss at six months, as assessed by quantitative vascular angiography.
Both primary endpoints of the SWING trial were achieved, Varcoe revealed. Varcoe reported that, in the per-protocol population, there were no major amputations, no major reinterventions, and complete freedom
from perioperative death, “so it was a safe device”. The team also saw a rate of all-cause death that was 0%, target lesion amputations were 0%, and a “very low” rate of clinically driven target lesion revascularisation at 8%. Varcoe added that the minimal luminal diameter at the end of the procedure was “very high”, which he thinks “represents modern-day angioplasty techniques”, and reported a late lumen loss of 1mm at six months— results that compare “very favourably” to other equivalent DCB trials below the knee, he remarked.
At six months, the researchers observed a primary patency of 88.5%. Varcoe noted that primary patency was retained and consistently retained out to 12 months at 80%—a figure that he said is “very good for this part of the vasculature”.
Varcoe added that, “pleasingly,” the team also saw improvement in Rutherford Becker classification, which was sustained and improved out to 12 months. Regarding quality of life, he noted that patient-reported outcome measures were again consistently improved out to that 12-month endpoint.
In closing, Varcoe shared his belief that the Sundance device has “great promise” and “warrants evaluation in a large-scale pivotal trial” based on these latest findings.
CAGENT VASCULAR HAS ANNOUNCED THE RESULTS OF A comparative subanalysis of the PRELUDE-below-the-knee (BTK) study versus plain balloon angioplasty. The study was led by Marianne Brodmann (Medical University of Graz, Graz, Austria). This subanalysis compared their PRELUDEBTK subset to a consecutive plain balloon angioplasty group.
The Serranator-treated lesions had an average final residual stenosis of 17.2±8.2% vs. 33.7±15.7% in the plain balloon angioplasty group. This represents a 49% average improvement in final residual stenosis. In chronic total occlusions (CTO), there was a 62% improvement in final residual stenosis compared to the plain balloon angioplasty group. The average balloon inflation pressure was 5atm in the Serranator group vs. 9atm in the conventional balloon angioplasty group. Additionally, Serranator-treated arteries demonstrated 2.4 times greater calculated flow improvement versus plain balloon angioplasty These data, analysed by the same independent core lab, were recently published in the Journal of Endovascular Therapy
Brodmann stated: “As an early user of serration angioplasty, it was meaningful to quantify through this subanalysis what we have experienced using the Serranator device. William Tang (UC Irvine, Irvine, USA) developed a novel model, anchored by Poiseuille’s law, from which we were able to derive the volumetric blood flow from lumen gain achieved with the Serranator compared to conventional balloon angioplasty. These results suggest an advantage for serration technology that should allow for superior wound healing and patient outcomes, while minimising the need for stent placement.”
Raman Sharma (Mount Sinai Medical Center, New York, USA) was an early adopter of serration technology, and added: “The results from this subanalysis study support what we have seen in our experience; the Serranator provides greater lumen gain and blood flow versus conventional technology.”

SURMODICS RECENTLY announced it has received a letter from the US Food and Drug Administration (FDA) related to its premarket approval (PMA) application for the SurVeil drugcoated balloon (DCB). In the letter, the FDA indicated that the application is not currently approvable, while providing specific guidance as to a path forward. The letter stated that certain information within two general categories— biocompatibility and labelling—must be added by an amendment to the company’s PMA application to place it in approvable form. Although the information identified by the FDA to put the PMA application in approvable form would require additional testing and analysis, the letter did not question the human clinical data submitted.
“We are disappointed by the FDA’s response to our PMA application and continue to have confidence
in our SurVeil DCB including its compelling performance in the TRANSCEND clinical study,” said Gary Maharaj, CEO of Surmodics. “We are evaluating the issues raised in the FDA’s letter and plan to meet with Agency representatives regarding its contents. Based on our discussion with the Agency, our team and external advisors will determine the appropriate path forward. Concurrently, we will be evaluating options to reduce our use of cash given this development. We expect to address these topics further in connection with our upcoming first quarter fiscal 2023 earnings call.” Surmodics notes in a press release that the SurVeil DCB is a nextgeneration device for the treatment of peripheral arterial disease.
PRELUDE-BTK subanalysis highlights superior lumen gain, greater volumetric blood flow using serration angioplasty
We [...] continue to have confidence in our SurVeil DCB including its compelling performance in the TRANSCEND clinical study.”Ramon Varcoe Serranator
In a recent study of patients with intermittent claudication (IC) caused by isolated superficial femoral artery (SFA) lesions, researchers found that primary stenting conferred benefits in health-related quality of life (HRQoL) at 36 months from treatment compared with best medical therapy (BMT) alone, which were lost at the 60-month mark, where a high crossover rate affected the power of the final analysis.
RESULTS OF THE STUDY
were recently published online in the European Journal of Vascular and Endovascular Surgery (EJVES). The investigators—led by Thordur Gunnarsson (Lund University, Lund, Sweden)— highlight their previous finding that primary stenting of the SFA in IC increased HRQoL after 12 and 24 months in this trial. The current paper, they note, presents an extended followup of HRQoL 36 and 60 months after randomisation.
This was a multicentre randomised controlled trial conducted at seven vascular clinics in Sweden between 2010 and 2020, which included 100 patients randomised to either primary stenting and BMT (n=48) or BMT alone
(n=52) followed for 60 months.
Gunnarsson et al detail that HRQoL—which they assessed using the Short-Form Health Survey (SF-36) and EuroQoL 5 dimensions (EQ5D) 36 and 60 months after randomisation— was the primary outcome. Walking Impairment Questionnaire (WIQ) score, reinterventions, progression to chronic limb-threatening ischaemia (CLTI), amputation, and death were secondary outcomes.
The authors report that, at 36-month follow-up, the stent group (n=32) had “significantly better” scores in the SF36 domain Role Physical (RP, p=0.023) and the Physical Component Summary (PCS, p=0.032) compared to the control group (n=30), however, there was no
significant difference in EQ5D scores (p=0.523).
In addition, they reveal that WIQ was also “significantly better” compared to the control group (p=0.029) at 36 months.
At 60-month follow-up, Gunnarsson and colleagues found no significant difference in HRQoL between the stent (n=31) and the control group (n=32),
months and the 25% crossover from the control group to stent at 60 months “negatively affected the power of the analysis”. In future trials of invasive treatment of IC, they write, crossover and loss to follow-up “need to be taken into account”.
MID-TERM RESULTS FROM A PHASE II STUDY OF SURGICAL bypass using the Human Acellular Vessel (HAV; Humacyte) demonstrated an overall secondary patency rate at 72 months of 60% measured by Kaplan Meier. There was no evidence of graft rejection or infection, the authors reported.
Humacyte said the publication describes the long-term analysis of the clinical trial as it evaluates the bioengineered HAV as a conduit in patients with symptomatic peripheral arterial disease (PAD). The research team, led by firstnamed author Piotr Gutowski (Pomeranian Medical University of Szczecin, Szczecin, Poland) concluded that “the infection-resistant, off-the-shelf human acellular vessel could provide a durable alternative conduit in the arterial circuit setting, to restore lower extremity blood supply in patients with peripheral artery disease.” The results were recently published in the Journal of Vascular Surgery-Vascular Science (JVS-VS).
No patients underwent amputation of the affected limb out to six years, they found, and none reported pain at rest or ischaemic ulcers on the affected legs. Gutowski et al reported that “these data have demonstrated the durability of the HAV and suggest the occurrence of cellular remodelling by the host.”
Gutowski commented: “Synthetic grafts can be limited due to poorly matched mechanical compliance, risk of infection, and variable patency rates. Furthermore, cryopreserved allogenic grafts are limited due to poor durability, thrombosis, and mechanical degradation. The HAV is designed to be consistent in size, durable in high-pressure circulation, show no clinical immunological response, and remodel with the patient’s own cells.”
The HAV has been evaluated in eight clinical studies in the USA, Europe and Israel, including an ongoing phase II/III clinical trial in vascular trauma and an ongoing phase III trial as a haemodialysis access in end-stage kidney disease. The HAV is an investigational product and has not been approved for sale by the US Food and Drug Administration or any international regulatory agency.
and that there was no difference in progression to CLTI, amputation (2.1% vs. 1.9%) or mortality (14.6% vs. 15.4%) between groups. The authors point out that crossover from control to stent group was 25% at this later follow-up point.
The authors recognise that their study has some limitations. They note, for example, that as the study was originally designed to detect differences in the primary outcome at 24 months with an expected 10% loss to followup, both the prolonged follow-up and additional loss of patients at 36 and 60
The Rouleaux Club—the UK’s national vascular trainee society—in association with the Charing Cross (CX) Symposium 2023 and BIBA Medical, has announced a new competition designed to encourage early detection and treatment of “hurting legs”.

‘The Hurting Leg’, involves creating an infographic and/or infomercial intended to educate members of the public about chronic limbthreatening ischaemia (CLTI) and encourage patients to present to their general practitioner.
“We interpret the trial results in favour of stent treatment for at least 36 months for physical HRQoL measures for the [IC] patient,” Gunnarsson et al write in the discussion of their findings. While they acknowledge that the trial does not give robust evidence for a lasting effect of stenting until 60 months, it suggests instead that a “transient period” of improved HRQoL “might be valuable” for an elderly vascular patient.
The trial was conducted at seven vascular clinics in Sweden between 2010 and 2020

They summarise the wider impact their results might have: “This trial provides information relevant when presenting treatment options for patients as well as for health authorities.”
Infographics and short infomercial videos are invited from all students and trainees involved in caring for vascular patients from all over the world. Submission should be made online at www.cxsymposium. com/the-hurting-leg-competitionsubmission-guidelines. The deadline for submission is midnight (GMT) on Tuesday 28 March.
Speaking to Vascular News at the 2022 Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (23–25 November, Brighton, UK), president of the Rouleaux Club Leanna Erete, who is a vascular trainee in the UK’s London deanery, described the competition as “innovative and exciting”.
Attendees at the CX Symposium 2023 (25–27 April, London, UK) will be able to view the top five infomercials and infographics and vote for their preferred one. The monetary prizes of £1,000 for the infomercial and £500 for the infographic will be announced at the symposium.
This year, CX is offering free registration to all fellows who are members of European Vascular Surgeons in Training (EVST), the Rouleaux Club or other equivalent societies worldwide, and 75% off for early career surgeons and physicians (terms apply).
Human-tissue engineered blood vessels remain durable at six years in PAD patients, latest study results show
Demonstrating the “susceptibility” of care delivery to regional market competition, M Libby Weaver (University of Virginia Health System, Charlottesville, USA) recently presented the case that intermittent claudication (IC) is a “novel and underdefined” driver of practice variation in management of patients.
SPEAKING AT THE 2023 SOUTHERN Association for Vascular Surgery (SAVS) annual meeting (18–21 January, Rio Grande, Puerto Rico), Weaver began by outlining guidelines set out by the Society for Vascular Surgery (SVS), who published recent appropriate use criteria for the management of

IC, which recommends best medical therapy (BMT) and lifestyle modifications as first-line treatments prior to revascularisation.
Atherectomy and tibial-level interventions are specifically “advised against” in the treatment of IC, the presenter noted. Despite guidelines, Weaver continued, high market competition is incentivising physicians to treat patients by aggressive means. She noted how two thirds of interventional procedures for claudication include revascularisation and atherectomy, with an indication of claudication as a “predictor” of independent atherectomy use. Although characteristics such as physician specialty and practice setting have “known” associations with atherectomy and tibial interventions, Weaver made it clear that the causal relationship between IC management and regional competition has “not been explored”.
Examining IC patients undergoing initial endovascular peripheral vascular intervention (PVI) in the SVS Vascular Quality Initiative (VQI) from 2010–2022, the researchers assigned the HerfindahlHirschman Index (HHI)—a measure of regional physician market competition—to each centre based on Census Core-Based Statistical Area, assigning four predefined categories: very high competition (VHC), high competition (HC), moderate competition (MC), and low competition (LC).
The President’s Symposium at the 2022 Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (23–25 November, Brighton, UK) featured Rob Sayers’ (University of Leicester, Leicester, UK) presentation titled ‘CLTI CQUIN has raised the profile of PAD and will lead to fewer amputations’. Sayers’ take-home message for delegates was that the National Health Service’s (NHS) Commissioning for Quality and Innovation (CQUIN) for chronic limb-threatening ischaemia (CLTI) will prove a valuable package of measures when it comes to raising awareness of peripheral arterial disease (PAD) and improving outcomes for patients, but that the question now lies in “how best we measure [these improvements]”.
THE CQUIN, SAYERS BEGAN BY outlining, was started in April 2022, and the premise of it is for the NHS to give “financial rewards for excellence [in the hope] that that translates into better patient outcomes”. The presenter noted, with relevance to the VSGBI audience, that their society had made sure to inform all its members of the CQUIN at its launch, and that the society’s own PAD quality improvement framework (QIF) is complementary in its patient outcome focus.
Then providing background to the CQUIN process, Sayers shared that “you are more likely to be successful if your bid is patient-focused [and it is easy to implement],” adding that it is “competitive”. The resulting financial incentive that a successful bid bestows “depends on the size of your unit,” Sayers proceeded, and in the case of the CLTI CQUIN, “most [units] will receive between £500,000 and £1.5 million”.
Speaking to the merits of the CLTI
CQUIN, Sayers described it as a “wellwritten and clear proposal,” adding that there are benefits for patients. Moreover, the successful application could perhaps be set against the backdrop of “a lot of support from the NHS for various vascular services [… which were] particularly hard-hit by the pandemic.”
Regarding the conditions of the CLTI CQUIN funding, “it requires you to revascularise patients within five days of referral,” Sayers detailed for the symposium audience. “If you revascularise 60% of your patients within five days, you get the full payment and if you revascularise less than 40% you do not get any money at all”. The amount awarded for achieving results in between these benchmark figures is graduated, Sayers expanded.
Data monitoring is key to the CQUIN’s operation, delegates then heard. This is carried out “by local commissioners who compare data such as HES [Hospital Episode
Their inclusion criteria identified 24,669 PVIs, revealing patients undergoing PVI in LC regions were more likely to be younger, white race, self-paid/ uninsured, and active smokers. Continuing, Weaver drew awareness to the “distinct treatment patterns” that became apparent when analysing index peripheral procedures, noting that the odds of patients being on BMT increased with greater market competition, designating a 1.07 increase per class step up in competition quartile.
The probability of undergoing aortoiliac interventions also decreased with increasing competition, but their results showed higher odds of receiving tibial and multi-level interventions in VHC versus LC centres. Primary stenting decreased as competition increased, while likelihood of undergoing atherectomy increased with HC. In subgroup analysis limited to patients undergoing single-artery femoropopliteal intervention with TASC A or B lesions, compliance with BMT remained higher and current smoking status lower in VHC centres. However, odds of undergoing balloon angioplasty and primary stenting only were higher in LC/MC/HC centres, while likelihood of receiving atherectomy remained significantly higher among VHC centres, suggesting disease severity is not a primary driver of these treatment differences, Weaver noted.
Statistics], and NVR [National Vascular Registry…] so if you were tempted to underreport, that would be spotted,” Sayers cautioned.
Moving on to address how the CQUIN can help patients, Sayers underlined that the goal is to “raise awareness of PAD and the PAD QIF, and CLTI, among clinicians and managers”. This then increases the chance of reinvestment to improve services, such as limb salvage clinics.
As of April 2023, the CQUIN will be up for renewal, Sayers conveyed, and the decision will be based on a number of factors, including whether participating units themselves “found [it] worthwhile”. However, there are limitations when it comes to how successful the CQUIN may be at achieving improved outcomes: “It requires very reliable data capture,” Sayers admitted. It “may [also be argued that] the targets are challenging” and “not particularly evidence-based,” he furthered.
Sayers rounded off his presentation by taking a long and wide view of VSGBI’s role in putting patients at the heart of members’ work and calling future presidents to prioritise data gathering to facilitate this. Maintaining relationships with exam boards so that the specialty adheres to “very good” professional standards was also among
Sayers’ pieces of advice. “There is no doubt that the society needs to continue to foster its good relationship with NHS England,” he asserted, before opining that “work with commissioners” can “no doubt” serve as a solution to the problem of the post-COVID-19 vascular services backlog.
Audience questions centred on data monitoring and the quality of datasets,
which reflected Sayers’ message that this is pivotal in the success of measures like those comprising the CLTI CQUIN.
In addition, Sayers was posed the question of whether “in order to ensure the development and appropriate investment in CLTI management, do you think some units will need to take one for the team and fail?” The presenter responded by referring to the “current data” from the CQUIN— “there are 55 units in England that are eligible for the CQUIN scheme [of whom] about 10–12 are currently below the threshold of 40% and [there are] probably another 5% [whose data] we do not [have]”. He reiterated that the CQUIN is “about improving your service to improve outcomes for patients”, and concluded, therefore, that those units that are currently falling short have the choice “whether they want to try to improve things for patients,” rather than it being about ‘taking one for the team’.
You are more likely to be successful if your bid is patient-focused [and it is easy to implement].”Rob Sayers
Following his 2021–2022 presidency of the Vascular Society of Great Britain and Ireland (VSGBI), Jon Boyle (Cambridge University Hospitals NHS Trust, Cambridge, UK) speaks to Vascular News about his career so far. He also shares his thoughts on the future of vascular surgery, anticipating that the management of peripheral arterial disease (PAD) will become a more prominent part of vascular practice due to an ageing population and increased prevalence of diabetes, and details some of his present work in this field. He tells aspiring surgeons that, although COVID-19 and a lack of resources have “cast a shadow” over the last couple of years, he has “no doubt medicine will continue to be a thoroughly rewarding career”. Outside of medicine, Boyle shares his love for sport, and details his involvement in various charity fundraising events.
Why did you decide to pursue a career in medicine and why, in particular, did you choose to specialise in vascular surgery?
I never really had a strong desire to be a doctor, but ended up in medicine because I was good at science at school. I chose vascular surgery after working in Leicester, UK, as a junior doctor for Professor Sir Peter Bell, Professor Rob Sayers and Professor Matt Thompson. I undertook my MD thesis in Leicester from 1995–1997 on the early development of endovascular aneurysm repair (EVAR). I handsewed 45 endografts implanted into abdominal aortic aneurysm (AAA) patients during this time.
Who were your career mentors and what was the best advice that they gave you?
As above to a certain extent. Rob Sayers raised the importance of research in postgraduate surgical training and strengthening this string to my bow helped my rapid career progression. Matt Thompson supervised my higher degree. I am very grateful for a number of my trainers, but in particular would mention Spero Raptis, Rob Fitridge, Michael Berce and Larry Ferguson who supervised me during my senior registrar year at the Royal Adelaide in Australia in 2002, and also Graeme Sutton, Gareth Morris, Mike Phillips, John Cook, Mark Pemberton and Derek Finnis from my Wessex vascular training programme.
What has been the most important development in vascular surgery during your career so far?
The development of endovascular surgery.
What has been the biggest disappointment? Something you hoped would change practice but did not?
From personal experience, the failure of endovascular aneurysm sealing (EVAS). With better medical device surveillance, we would have recognised that these devices were failing much sooner and prevented many patients from being treated with this poorly performing endoprosthesis.
How do you anticipate the field might change in the next decade, and what development would you most like to see realised?
I think management of PAD will become a bigger part of vascular practice due to the ageing population and increased prevalence of diabetes. We now recognise that early treatment of chronic limb-threatening ischaemia (CLTI) prevents amputation and death, and I hope increased focus on the urgent management of this patient group will improve outcomes.
What are the biggest challenges
currently facing vascular surgery?
Increasing workload related to the ageing population and insufficient vascular surgeons to deliver care.
What do you think has been the most important paper published in the last year?
‘Surgery or endovascular therapy for chronic limbthreatening ischaemia’ by Alik Farber (Boston Medical Center, Boston, USA) et al, published in the New England Journal of Medicine. This paper outlined results from the BEST-CLI trial, which showed that surgical bypass with adequate single-segment great saphenous vein is a more effect revascularisation strategy for patients with CLTI.
What have been the highlights of your VSGBI presidency?
I spent 10 years working on the VS council in various different roles. I am most proud of the work I led on PAD quality improvement, aortic device capture within the National Vascular Registry (NVR) and the

achievement of CLTI Commissioning for Quality and Innovation (CQUIN) in England for 2022–2023.
You were the clinical lead for the UK NVR from 2017–2020. Could you give a brief overview of your time in this role?
I felt it was important, following our experience with EVAS, that we started to collect aortic devicespecific information within the NVR. This work was extensive and required collaborating with many stakeholders, and also working with the National Joint Registry team leaders in registry device capture. The aortic device dataset went live in July 2020 and is recognised as an international exemplar. I also led the team responding to the vascular Getting It Right First Time (GIRFT) report in developing the PADQuality Improvement Framework (PAD-QIF) and also the PAD-Quality Improvement Programme (QIP). I obtained funding for two PAD-QIP research fellows
supported by the Circulation Foundation, VS and Royal College of Surgeons of England. I also led the introduction of COVID-19 fields to the NVR in April 2020, shortly after the start of the pandemic. This early addition of vital data fields has enabled a good understanding of the impact of COVID-19 on vascular patients to be gained and a number of reports and research publications. In 2020, the NVR team won the Healthcare Quality Improvement Partnership (HQIP) team of the year report. HQIP stated that “the National Vascular Registry team showed considerable initiative in looking beyond the audit to consider innovations to benefit patients. The nomination demonstrated outstanding work on registry device capture, quality improvement and a rapid response to COVID-19”.
What has been your most memorable case?
I introduced EVAR for ruptured AAAs in Cambridge,
We now recognise that early treatment of CLTI prevents amputation and death, and I hope increased focus on the urgent management of this patient group will improve outcomes.”
alisonlang.com
UK. One of my most memorable cases was being asked to see a ruptured thoracoabdominal aortic aneurysm in a man who was an inpatient at a nonarterial hospital. I arranged his transfer to Cambridge on a Friday afternoon, a thoracic endovascular aortic repair (TEVAR) graft to be delivered from a London hospital and then fixed him on Friday night. This was the first TEVAR rupture treated in Cambridge; the patient survived and lived for many years.
What advice would you give to someone looking to start a career in medicine?
Medicine has been a very enjoyable career for me over the last 31 years. Although the pandemic and lack of resources have cast a shadow over the last couple of years, I have no doubt medicine will continue to be a thoroughly rewarding career.
What are your hobbies and interests outside of medicine?
I still play a lot of sport: hockey in the winter, cricket in the summer, and cycling. In 2013, I organised a 24-hour hockey match (12 hours in Sydney, Australia, 12 hours in Cambridge) for a university friend with pancreatic cancer. We raised nearly £50,000 for Pancreatic Cancer UK and MacMillan. I have done a number of charity cycle rides for Pancreatic Cancer UK, including London to Paris and the Circulation Foundation Ride London twice, and was part of the team that rode across the Outer Hebrides in 2021 for the Circulation Foundation. Overall, I have helped raise over £80,000 for charity.
I also enjoy spending time with my wife, Patricia, and three children, and have been fortunate enough to travel extensively over the last 20 years. I have been one of six owners of my local village pub, The Lidgate Star, for the last 11 years, and I am also active on Twitter—my handle is @jonnyboyle1
Present appointment:
2002–present: Consultant vascular surgeon, Cambridge University Hospitals NHS Trust, Addenbrooke’s Hospital, Cambridge and West Suffolk Hospital
Medical education:
January 2010: MA (Cantab)
University of Cambridge
January 2008: FRCS England ad eundeum
June 2001: FRCS (General Surgery) Edinburgh
September 2000: MD, University of Leicester
January 1996: FRCS Edinburgh
1986–1991: University of Leicester, Faculty of Medicine
Society presidencies:
2021–2022: VSGBI
2013–2015: British Society of Endovascular Therapists

2001–2002: Rouleaux Club
Journal positions
(selected):
2026–2028: Editor-in-chief (recently promoted), European Journal of Vascular and Endovascular Surgery (EJVES)
2023–2025: Senior editor (recently promoted), EJVES
2019–present: Section editor, EJVES
























































































































































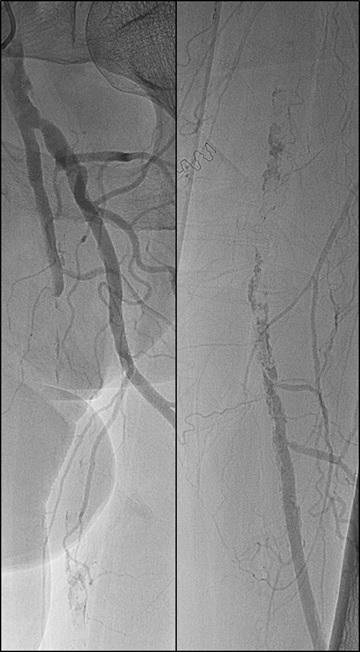
Crossing chronic total occlusion (CTO) lesions are challenging procedures. The BeBack crossing catheter—Bentley’s first product to be available in both Europe and the USA following the company’s acquisition of Upstream Peripheral Medical Technologies’ GoBack crossing catheter in September 2022—offers a new solution in this space.
Andrej Schmidt (University Hospital Leipzig, Leipzig, Germany), one of the first to use the catheter, shares his clinical experience with the BeBack, noting how it has been a “game-changer” in his endovascular peripheral arterial disease (PAD) practice.

What does your PAD practice look like, and what do you think are the most difficult aspects to overcome when treating CTO lesions?
The University Hospital Leipzig is one of the larger centres in Germany for the endovascular therapy of peripheral arterial occlusive disease, and we receive a lot of very complex cases, including many that have failed in other hospitals. These are very often patients with severely calcified infrainguinal disease, but also complex iliac total occlusions.
Could you talk us through your first experience with the BeBack catheter?
We were struggling with a CTO of the common iliac artery in an abdominal aneurysm patient and failed to get through the CTO coming from retrograde, crossover and antegrade using an arm-access. Nothing worked, until eventually we used the BeBack catheter via the retrograde approach. With this, device passage through the CTO back into the aorta succeeded immediately. This experience was an eye-opener for us with regard to the success of the BeBack catheter.
Can the BeBack also be used as a support catheter?
In addition to its main purpose as a crossing catheter for complex, calcified CTOs, the BeBack indeed can be used as a support catheter since it is quite stable and stiff. This feature is very helpful in difficult total occlusions. It also can be used as a re-entry catheter, for example during a recanalisation of a CTO of the femoropopliteal segment. In the typical situation of being stuck subintimally, unable to pass the guidewire back into the true lumen distal to the CTO, the BeBack reliably helps to re-enter the distal patent segment of the artery.
How is the BeBack part of your recanalisation strategy?


This depends on the type of lesion and the problem encountered during the intervention. For example, a typical femoropopliteal CTO is usually approached from antegrade. In case of inability to penetrate the
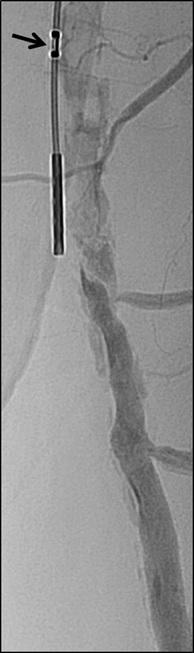
guidewire into the CTO, either due to dense fibrosis of the proximal cap or severe calcification, the BeBack catheter is used as a crossing device by pushing the adjustable needle just a little bit out of the tip of the 4Fr catheter. More frequent however is the situation that the guidewire passes the CTO subintimally and reconnection to the patent lumen distal to the CTO fails. As mentioned above, the BeBack catheter is then used as a re-entry device by protruding the curved inner needle further out of the tip of the catheter. Different to other re-entry devices is that the BeBack is 4Fr compatible, instead of 6Fr, and introduction into calcified, tight lesions may be easier. Yet, it can be used over a 0.018” guidewire, which is often the guidewire of choice in difficult CTOs, improving stability and success compared to 0.014” guidewires.
Another situation, where the BeBack is our device of choice, is a reocclusion of the femoropliteal segment with previous spot-stenting. Usually, the guidewire passes subintimally and entering into the occluded lumen of a stent within a longer CTO is not possible. In this situation, the BeBack indeed reliably and quickly allows the guidewire to enter into the proximal end of the occluded stent and to finalise the procedure successfully.
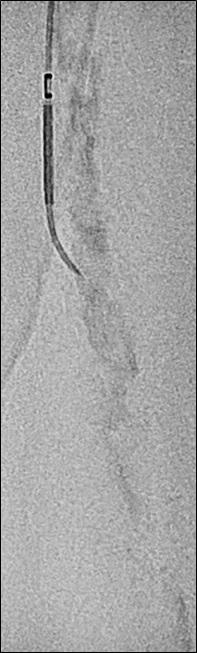
Below the knee, we mainly use the 2.9Fr BeBack device, although also the 4Fr device is used in the proximal third of the calf for penetrating through calcific CTOs or for re-entering after subintimal guidewire passage. In some cases of severely calcified infrapopliteal lesions, it can happen that the guidewire passes easily intraluminally through a stenosis, but no balloon would follow due to the tightness and calcification of the lesion. In this situation, the 2.9Fr BeBack is inserted over the guidewire and, with the needle slightly protruded from the tip of the catheter, it is drilled into the problematic plaque. This technique, mimicking the Japanese technique of transcutaneous plaque-piercing, is very successful in facilitating introduction of balloons into the lesion and finalising the procedure.
Has the BeBack changed your practice and, if so, how?
For many years, we and other centres have helped to develop techniques to improve the success rate in difficult peripheral CTOs. The retrograde and bidirectional approach became standard in case of inability to pass a CTO from antegrade. However, in some cases it can be anticipated that establishing a retrograde access will be cumbersome or time consuming. In these cases, we now prefer the BeBack to keep the intervention simple. Furthermore, a bidirectional recanalisation can be time consuming and may even fail. In this situation, the BeBack helps to speed up and may even be the only way to finalise the procedure successfully.
During CTO recanalisations, do you think that sometimes physicians start to switch from one to another technique too late?
Not infrequently physicians try different guidewires and different catheters many times to pass a difficult CTO or to re-enter back into the patent distal lumen. This increases the risk of protruding the dissection distal to the CTO, destroying healthy segments which sometimes worsens the clinical situation. Furthermore, radiation dose and the amount of contrast medium increases. Complications correlate with the duration of the procedure. The retrograde approach and the BeBack catheter are our technique and technology to shorten the procedure time.
How much time or how many attempts would you give yourself with conventional techniques before using the BeBack crossing catheter?
It depends on the complexity of the lesion. If we see a chance to be successful using an antegrade approach, we may proceed for some minutes. In very complex lesions, where it can be anticipated that a conventional approach has a high risk to fail or may take time, we switch to the BeBack catheter within a minute.
If a colleague were to ask you about the BeBack, how would you describe it?
The BeBack is a reliable crossing and re-entry device. It is very slim (2.9Fr or 4Fr), yet very stable and can easily be used not only in larger diameter arteries like iliacs, but also in small arteries, even via a retrograde pedal access through a 2.9Fr sheath. Furthermore, it is possible to use it over a 0.014” and over a stable 0.018” guidewire, which is usually the wire of choice in more complex lesions. Due to its straightforward design, it is easy to position and reposition the device and control the depth and direction of the 360-degree adjustable needle from the tip of the catheter. It is helpful in quite a large variety of difficult situations and handling is easy to learn.
1. CTO of the left superficial femoral artery in a male patient suffering from severe claudication in the left calf.
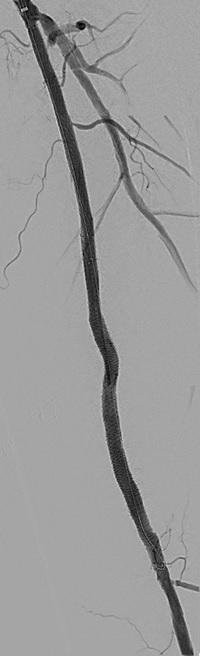
2. After subintimal passage it was impossible to redirect the guidewire into the patent lumen distal to the CTO.
3. Positioning of the BeBack catheter to re-enter the guidewire. Arrow indicates an orientation-marker.
4. Marker appearing as a “C” indicates the direction of the needle, needle protruding maximally out of the BeBack catheter.
5. An 0.018” guidewire passing into the patent distal lumen.
















6. Result after stenting.

Prominent venous disease experts discuss venous stenting, appropriate care, and the pursuit of refined data and better education in a space where part of the problem involves practitioners moving “freely from being able to do arterial intervention and suddenly assume they can do a venous intervention”.
Education across the venous care delivery spectrum lies at the heart of efforts to ensure operator judgement is optimal and procedures— like the placement of venous stents—are carried out in the appropriate circumstances.

Or, as Steve Elias (Englewood Health Network, Englewood, USA) says, “the trend is going to be perhaps that too many stents are going to be placed for a while until we educate people about when this is appropriate and when it is not appropriate.”
Elias was speaking ahead of the 2023 American Venous Forum (AVF; 22–25 February, San Antonio, USA) where some of the latest data on venous stent usage trends between 2014 and 2021 were presented by Karem Harth (UH Harrington Heart & Vascular Institute, Cleveland, USA).
Stent migration and overstenting remain recurring themes as new research is presented and surgeons discuss outcomes.
New data from the clinical trial of the Abre dedicated venous stent recently prompted investigator Stephen Black (Guy’s and St Thomas’ Hospital, London, UK)—who led the trial with Erin Murphy (Sanger Heart and Vascular Institute, Atrium Health, Charlotte, USA)—to declare that, given the study showed no stent fractures through three years, and similarly, no delayed stent migrations, “… stent migration really does seem to be an operator issue rather than a stent issue”.
Elias underscores the point: 99.9% of the time the issue is not one of the stent but of the judgment of the operator, or poor training—“or an issue of considering venous stents as similar to arterial stents,” he says.
Elias emphasises the role of device makers in education. “Those of us on the leading edge of this are working with all of industry to set up programmes to better educate their salesforces and then also their physician customers regarding not just stents but also venous disease in general,” he says. “We all realise this is a problem and the only way to solve it is by all of us working together.”
Over the past decade, venous stenting has evolved from a “byword” into a “mainstream and accepted” practice, Black tells Vascular News. Recently, several on-label, venous-specific devices have become
available—four in the USA—along with the first prospective data in the form of investigational device exemption (IDE) trials, and, according to Black, there is now “more enthusiasm for treating patients” among providers in the venous space.
Murphy casts a positive light on how the field has changed over the past five to 10 years, pointing out there has been some “great progress” in technology.
At this juncture, Kush Desai (Northwestern University, Chicago, USA) believes the time to embark on “real-world” studies is now, with the aim of “demonstrating the value of treatment of patients across a variety of disease states from non-thrombotic through post-thrombotic”. He explains that these data will help physicians to “clearly identify which patients benefit and what we can expect for outcomes”.
Black concurs, adding that randomised controlled trials (RCTs) will need to follow, despite the difficulties associated with carrying out such research. The “big problem” here is recruitment—a problem that is affecting various ongoing trials. This recruitment issue is multifactorial, Black notes, specifying that clinicians “feel they do not have equipoise anymore” and among some there is a “financial conflict bias,” while patients “do not want not to be treated.” The solution? According to Black, clinicians need to work as a group to “overcome our own biases,” in order to ensure randomised evidence ensues.
Murphy underlines some specific areas in which data would be beneficial, mentioning the need to determine thresholds for intervention in nonthrombotic disease. She further describes the need for more data supporting good stenting practices, citing intravascular ultrasound as one example. “I think this is something we all agree is essential for good outcomes in this space,” she says, however rigorous data are lacking. She adds that there is also room for “significant improvement” in data encompassing the areas of pre- and postoperative imaging as well as postoperative anticoagulation regimens.
Desai points to the importance of data consolidation, referencing in particular the work of the Deep Venous Academic Research Consortium, which he chairs alongside Black. “The goal of this is to improve the rigour and reproducibility of deep venous
research,” Desai explains, by way of ensuring that studies are all collecting the same trial data, so that they can be compared. He hopes that this will create “more sound data” for the devices that practitioners are placing and will thus have a “downstream effect” on clinical practice.
Rabih Chaer (University of Pittsburgh, Pittsburgh, USA) notes that “longer-term” data might still be lacking for the individual stents and comparative data between the different stents available for venous placement.
“That becomes important because, at least the newer-generation venous dedicated stents have shown us that some have performed better in certain locations based on the design of the stent,” he says. “There may be some variability in the performance of each stent depending on which part of the vein and for
The trend is going to be perhaps that too many stents are going to be placed for a while until we educate people about when this is appropriate and when it is not appropriate.”
Steve Elias
which indication they are used.”



In parallel to the need for more data, Desai posits there is “quite a long way to go” in terms of refining venous stenting practice. “The devices are very good,” he says, noting that, while there is “certainly room for improvement,” outcomes across studies are “very similar, sort of agnostic to the stent device”.
Both Black and Desai highlight the importance of understanding the specifics of venous disease when it comes to best practice. “Part of the problem with venous is people move very freely from being able to do arterial intervention and suddenly assume they can do a venous intervention,” Black remarks. “It is like playing squash and tennis,” he analogises, “they are both racket sports with a ball but the rules are not the same”.
According to Desai, the “biggest issues” in terms of practice are with patient selection and disease state recognition. “Simply put, there are far too many stents placed for non-thrombotic disease in the USA, meaning there is attribution of symptoms that are not likely to significantly improve with placement of a stent.” He states that this can be attributed in part to economic benefits to the operators—which he says “may be a uniquely US thing”—however notes that there are “likely a variety of problems at play”.
Murphy believes the field is “findings its way” in terms of best practice. She highlights that, five years ago, there was a “considerable problem” with undertreatment of venous obstruction.
Considering current practice, she agrees with Desai that overtreatment of non-thrombotic patients is an issue, while also noting that practitioners are “still undertreating” the post-thrombotic group. Many of the latter patients are not referred for treatment.
Black stresses that “inappropriateness of care” is the key issue, and that one of the main challenges facing venous stenting practice is ensuring “the right patient is getting the right treatment by people who know how to do it properly”.
Non-thrombotic iliac vein lesions (NIVLs) are at particular risk of overtreatment, he says, because “the impetus is to treat anybody with any leg problem on the left-hand side” when there are lots of patients who do not need treatment. In the case of chronic occlusions and post-thrombotic disease, and potentially acute iliofemoral deep vein thrombosis (DVT), he notes there are lots of patients who are not getting treatment who would “probably benefit”.
Murphy also highlights the importance of appropriate treatment and is confident that, over the next few years, practitioners will “perfect” their patient selection. “I do not think that people are going awry on purpose,” she stresses, instead noting that this is a “developing” field and that the process of “sorting through who is going to get the most benefit” is ongoing.

Considering how venous stenting practice can be improved, Desai believes education is key. “I think most providers would be open to the discussion that ‘maybe your stent is not helping patients,’ and would correct their behaviour,” he says, while remarking that it is “much more difficult to correct” the practice of providers who are financially driven. “More broadly speaking,” however, he is confident that education remains central “for

providers that are willing to listen”.

Black points out that a number of educational efforts are in place—from company-run symposia and training to workshops at vascular meetings. He stresses however that training is a “twoway thing”. He explains: “You have to engage in training.”

In Murphy’s view, training in the form of “interested” physicians, who are already practicing, shadowing expert practitioners is working well. What is “majorly lacking” in education, she believes, is investment at the fellowship and trainee level, underscoring the need for “dedicated, comprehensive venous training programmes”. She explains that, currently, there are no programmes in the USA that spend a significant amount of time teaching lymphoedema, superficial venous disease, and deep venous obstruction. Programmes that teach all of these aspects in one place, she believes, is “extremely important” for the trainee.
Looking at the wider picture, Black highlights that, while vascular care “continues to suffer from an unreasonable focus on aortic disease,” there is a “huge opportunity in the treatment of venous disease to make a really big difference to a patient’s quality of life”. With this in mind, he encourages “all vascular enthusiasts” to commit to collecting the data, partaking in the training, and delivering the appropriate care that should be the hallmark of venous stenting’s next chapter.
A meta-analysis is first to report the pooled risk of post-thrombotic syndrome (PTS) after isolated distal deep vein thrombosis (DVT). Researchers revealed a one in five risk of long-term PTS after isolated distal DVT, with one in 50 patients progressing to severe PTS, potentially developing to ulceration.
Principal author Benedict R H Turner (Imperial College London, London UK) and colleagues evaluated The Cochrane Library, Embase, Google Scholar and MEDLINE databases following PRISMA guidelines to identify eligible prospective cohort studies analysing the rate of PTS after a first episode of isolated distal DVT. Published in the European Journal of Vascular and Endovascular Surgery the study comprised trials held between 2005 and 2021 that included between 52 and 403 participants, with a total of 1,105 included across all seven studies reporting the development of PTS.
Turner et al note that the baseline characteristics of included articles were varied—follow-up periods ranged between one month and four years after first DVT, and several studies additionally reported which of the distal deep veins were thrombosed and corresponding risk factors. Interventions used to treat PTS included stockings, though duration, adherence and degree of compression were heterogenous. Other patients also received anticoagulation, although duration of treatment was unspecified. Among patients who were anticoagulated, direct oral anticoagulants, low molecular weight heparin and vitamin K antagonists were used.
The authors report that one in five patients are at risk of long-term PTS after isolated distal DVT, with one in 50 experiencing severe symptoms that may potentially include ulceration. Going into detail, the authors observed a post-thrombotic rate of 17% (95% confidence interval [CI] 11–26%, p<0.01) across the seven studies, 217 cases and 1,105 participants. Three of these studies (302 patients) reported on the severity of PTS symptoms, 78% posited as mild (Villalta score 5–9), 11% as moderate (10–14) and 11% were severe
(15 or more).
Even when modulating the follow-up period, the authors did not see a significant change in the risk of developing PTS (p=0.71). This, they write, suggests shorter follow-up periods may be adequate to collect data on symptom development in clinical trials. The authors note that methods of data collection may have been “confounding” due to recall bias, as most included studies only reported rates of PTS at the

option since it demonstrated no additional benefit in the SOX trial. The authors highlight that analysis of stocking use is limited. They detail that trials including CHAPS, SOX and ATTRACT have set about evaluating the treatment for preventing PTS, however they each excluded distal DVT from their eligibility criteria due to assumed lower event rates, although it may constitute up to two thirds of all DVT cases.
Going further, Turner et al comment that the management of isolated distal DVT has been a complex area of consideration in the literature, though there have been no randomised trials to establish the role of therapies preventing PTS in distal DVT. They write that the CACTUS double-blind, randomised trial revealed that outcomes for patients with isolated distal DVT who received six weeks of anticoagulation with Nadroparin were not superior to the placebo group for the composite outcome of venous thromboembolism (VTE) extension, contralateral DVT or pulmonary embolism (PE). Despite a clear risk factor for PTS being poor initial anticoagulation, the author’s meta-analysis shows limited evidence to support its use and benefit when compared with participants who received no anticoagulation.
The investigators note some limitations of their study, including risk of selective reporting due to the inclusion of retrospective studies. Additionally, studies were deemed eligible irrespective of the use of anticoagulation and/or compression stockings, due to little evidence suggesting its effectiveness on reducing rates of PTS, unlike for proximal DVT.
average follow-up duration, rather than pinpointing the exact time at which PTS was diagnosed. Overall, Turner and colleagues state that the risk of PTS after isolated distal DVT appears to be half that in comparison to proximal DVT, although occurring in a similar timeframe. According to the authors, this information is key when considering anticoagulation duration and compression therapy, as “PTS is noted to be the principal moderator of quality of life after VTE [for patients]”.
When considering preventative measures that can be taken by patients, the authors note that the most recent European Society for Vascular Surgery (ESVS) guidance recommends graduated compression stockings in the proximal context of DVT to prevent the onset of PTS, despite the UK National Institute for Health and Care excellence and American College of Chest Physicians withdrawing this treatment
Inari Medical has announced that the first patient has been enrolled in DEFIANCE, a prospective randomised controlled trial (RCT) comparing the clinical outcomes of patients with iliofemoral deep vein thrombosis (DVT) treated with the ClotTriever system versus anticoagulation only.
THE TRIAL IS LED BY principal investigators Xhorlina Marko (Beaumont Health, Dearborn, USA), Steven Abramowitz (MedStar Health, Washington DC/Leonardtown, USA), and Stephen Black (Guy’s and St Thomas’ Hospital, London, UK).
The first DEFIANCE patient was enrolled by Abdullah Shaikh
(Allegheny Health Network, Pittsburgh, USA). DEFIANCE will enrol 300 DVT patients at up to 60 centres worldwide. DEFIANCE is Inari Medical’s second RCT, enrolling in parallel to the PEERLESS pulmonary embolism trial.
“We are thrilled to enrol the first patient and officially kick off this
Although heterogeneity may have been introduced by pooling data in this way, the authors comment on the strengths of the study, noting the “comprehensive inclusion criteria and a thorough statistical analysis that has yielded a credible rate of PTS after distal DVT.” Continuing, they note that future trials of interventional measures to prevent PTS after DVT “should include an isolated distal DVT subgroup considering the established occurrence of PTS in this underserved and clinically significant group […] [and utilise] a survival analysis to delineate the evolution of PTS over time after isolated
The investigators conclude that randomised trials to analyse and support interventions that can effectively prevent PTS are “urgently needed” to improve patient care and subsequent outcomes after isolated distal DVT.
important clinical trial,” said Shaikh. “ClotTriever is fundamentally different to other DVT treatments. This trial is designed to produce definitive evidence to change the
thrombectomy to anticoagulation for DVT.”
“With six major clinical studies, including two ongoing RCTs, Inari is wholly committed to [venous

PTS is noted to be the principal moderator of quality of life after VTE [for patients].”Benedict R H Turner ClotTriever device
84% PRIMARY PATENCY In the VERNACULAR Trial

In the VERNACULAR Trial



In the VERNACULAR Trial
1 The Venovo™ Venous Stent System was studied in the global VERNACULAR clinical trial, which was a prospective, multi-center, non-randomized, single-arm study of 170 patients. The primary effectiveness endpoint of the study was primary patency (PP) at 12 months post-index procedure, defined as: freedom from TVR and freedom from thrombus occlusion and stenosis > 50% as measured by DUS. Patients who received a Venovo Venous Stent had a weighted PP rate of 88.6%, demonstrating a statistically significant difference from a literature-derived performance goal (PG) of 74%. At 36 months, patients who received the Venovo Venous Stent had an unweighted PP of 79.5% (84.0% K-M) (N=141). The primary safety endpoint was freedom from major adverse events (MAE), including stent migration, through 30 days post-index procedure. Freedom from MAE was 93.5%, demonstrating a statistically significant difference from a literature-derived PG of 89%. Stents were evaluated at the 36-month follow-up for fracture analysis. An anteroposterior and lateral x-ray for each evaluated stent were sent to an independent core lab for analysis. 98 subjects’ x-rays were analyzed and no stent fractures were reported. Missing x-ray analyses were recorded as protocol deviations. Dake, Michael D, et al. “Three-Year Results from the Venovo Venous Stent Study for the Treatment of Iliac and Femoral Vein Obstruction.” Cardiovasc Intervent Radiol, vol. 44, no. 12, Dec. 2021, https://doi.org/10.1007/s00270-021-02975-2. Epub 2021 Sep 20.

The Venovo Venous Stent System is indicated for the treatment of symptomatic iliofemoral venous outflow obstruction. The Venovo Venous Stent System is contraindicated for use in patients with a known hypersensitivity to nitinol (nickel-titanium) and tantalum and patients who cannot receive intraprocedural anti-coagulation therapy. The stent is not designed for repositioning or recapturing.
Hematoma, puncture site ·Hypotension/hypertension · Incorrect positioning of the stent requiring further stenting or surgery
Potential Adverse Events include, but are not limited to: Allergic/anaphylactic reaction
Malposition (failure to deliver the stent to the intended site) · Open surgical repair
Pulmonary embolism · Stent Fracture
Stent Migration
Venous occlusion/thrombosis/restenosis Please consult product labels and instructions for the use of indications, contraindications, hazards, warnings, and precautions BD, the BD logo, and Venovo are property of Becton, Dickinson and Company or






Q1: Endovascular arch repair with a 2- or 3-branched device, what is left for the open approach?
Cook Medical Symposium: A short neck is a diseased neck and deserves a durable dedicated solution
Q2: What is a short aortic neck for you?
Q3: What is the most important thing for you during deployment?
10mm: 56%
5mm: 23%
<5mm: 14%
15mm 7%
Many procedures:75% Very little: 25%
CX AORTIC VIENNA RETURNED LAST OCTOBER FOR ITS third edition (24–26 October, Digital), bringing together world-leading specialists from the cardiac and vascular fields to discuss all facets of aortic care from selection to investigation, diagnosis, techniques and technologies. The meeting showcased the latest approaches—open and endovascular—for the treatment of complex aortic problems spanning the aortic valve to the iliac arteries. The 2022 edition featured three days of high-quality digital programming, delivering a total of 15 hours of aortic education, which is currently available on-demand to registered attendees.
The full programme was curated by a Vascular, Endovascular and Cardiothoracic Executive board comprised of leaders in the field of aortic care including CX Aortic Vienna founding chair Roger Greenhalgh (London, UK), alongside Tilo Kölbel (Hamburg, Germany), Afshin Assadian (Vienna, Austria), Roberto Chiesa (Milan, Italy), Martin Grabenwöger (Vienna, Austria), Stéphan Haulon (Paris, France), Gustavo Oderich (Houston, USA), Markus Steinbauer (Regensburg, Germany) and Alexander Zimmermann (Zürich, Switzerland).
The format for the digital edition followed the CX style of short talks, debates, and audience interaction, presented live via broadcast. Podium presentations focusing on open and endovascular aortic data, techniques and technologies were punctuated by a series of edited aortic cases. The programme encompassed discussion and debate spanning key talking points in the aortic space including aortic arch interventions, thoracic dissection, thoracic imaging, thoracoabdominal techniques, juxtarenal, abdominal aortic and iliac artery therapies.
The importance of multidisciplinary aortic approaches was a key strand running through CX Aortic Vienna’s content and discussion, and the 2022 event continued its mission to bring together specialists of all skills to provide a comprehensive overview of cutting-edge aortic treatment.
Audience polling is central to the CX model, with the charts on this page offering a visual representation of the outcomes of key debates and discussions. Stay tuned for more information about CX Aortic Vienna 2023.
Dreiländertagung Global Discussion –Abdominal Aortic Pathologies
Q8: Does the CX Aortic Vienna 2022 audience consider that sac diameter increase is an indicator of endoleak somewhere?
Dreiländertagung Global Discussion –Thoracic Aortic Pathologies
Q9: The location of the primary entry tear and the presence or absence of malperfusion is the be all and end all
Q4: What is most useful when dealing with challenging renals?
Precision: 68%
Control: 17%
Ease of use: 11%
Speed: 4%
Q5: What is your preferred option for short necks in elective cases?
Steerable sheaths: 67%
Ability to adjust the position of the endograft: 28%
Try another catheter: 5%
Open: 28%
Endostaples: 28%
FEVAR: 22%
Pararenal bypass: 22%
Q6: When would the use of Fiber Optic RealShape technology and seeing the full shape have the highest value?
Navigation of target vessels in complex aortic repair: 73%
Catheterization of tortuous access vessels: 23%
Peripheral interventions for PAD: 4%
Q7: When would unrestricted viewing angles be most useful?
Catheterization of supra-aortic arteries: 59%
Catheterization of visceral arteries: 35%
Catheterization of the internal iliac artery: 6%
7 93
Abdominal Aortic
Q10: Debate: The key risk of adverse event after standard EVAR relates to original aortic aneurysm diameter
Agree: % Disagree: %
Yes: % No: % 4 96
Q11: Sac regression is a predictor of positive EVAR outcome
Q12: Debate: TEVAR for acute uncomplicated Type B aortic dissection patients is the gold standard 31 69 Against: % For: %
Q13: Dynamic CT imaging is the new gold standard for diagnosis and interventional planning
Agreed: %
Not agreed: %


Vascular trainee Claire Dawkins argues that vascular surgical training needs to “move with the times” in order to avoid “alienating” the next generation.


SURGICAL TRAINING HAS ALWAYS BEEN thought of as a particularly gruelling time, with a demanding rota, long on-calls, little sleep and difficulties fitting a family life around it. But vascular training is something that needs to move with the times, particularly as we are seeing an increase in the number of primary caregivers within our ranks.
At present, doctors looking to commence speciality training are between a rock and a hard place. Applying for a National Training Number (NTN) is competitive and can be stressful, only to find that you have met the requirements for a training number but, due to your ranking nationally, you will be dispatched to a distant region. You move there, disrupting any family you may have, only to be told that over the following six years you
will be rotating through several distant hospitals. Do you find somewhere to settle in the region and face significant commutes throughout your training, or accept that you may move yearly (even up to six monthly for some)?
At work you may find that some of your colleagues in the same role did not apply for or get an NTN. They have a fixed job, in one hospital, where they can settle their family without the disruption of moving every year. They know the team who understand how they work due to the consistency they have had. Unfortunately, it is not all rosy as a non-NTN trainee. A recent UK survey suggests that non-NTN trainees do not have comparable access to educational and clinical supervisors, they do not have priority when there is competition for surgical cases or projects (audit, quality improvement work or research) and can struggle to gain leadership and management experience. The Certificate of Eligibility for Specialist Registration (CESR) process, which technically expects the same level of evidence as is required of an NTN trainee to complete training, is a laborious and expensive process without the structure of NTN training.
The junior doctor workforce is fed up and downtrodden. They are balloting for potential strike action. The intensity and demands of medical care continues to increase. Their working conditions and quality of life need to change. But this is unlikely to improve without a significant change in the structure of training. In vascular surgery,
we are already facing a workforce planning deficit and need to increase our inclusivity rather than alienating both NTN and non-NTN trainees. We need to manage the pathway better, with better locality and less disruption for NTN trainees and more structure and opportunity for non-NTN trainees. I accidentally managed to achieve a relatively undisrupted pathway through my training—only rotating every two years. This gave me the continuity to work with a team for a longer amount of time, to better develop professional relationships and to gain a better understanding of different approaches, techniques and pathways, while not having to frequently move house or disrupt my family to meet my training requirements. However, there are individuals for whom this would still pose difficulties, particularly single parents with school age children, or those with non-traditional care-giving responsibilities. Unfortunately, I do not have the answers. I do not think it is a problem that one person can easily fix. It needs a complete overhaul of both NTN and non-NTN pathways. The potential gains of better recruitment and retention of the best individuals to vascular surgery (or any UK training pathway) are huge, but depend on being able to provide a stable, supportive working environment for medical professionals, usually in their thirties, to develop their skills and attain specialist registration.
VentureMed Group announces “impressive” 12-month Flex vessel prep data
VentureMed Group, a privately held medical device innovator in access management for arteriovenous (AV) fistulas and grafts and vessel preparation for interventional treatment of peripheral arterial disease (PAD) announced data presented at the VEITHsymposium (15–19 November, New York, USA). Overall, the data presented demonstrated that Flex vessel prep used prior to balloon angioplasty improves 12-month outcomes both in PAD and AV fistulas and grafts.
“AV Access management is a critical component of successfully treating AV patients over time,” said John Aruny (Dialysis Access Institute, Orangeburg, USA), primary investigator of the Flex Vessel Prep AV registry clinical study, the 12-month outcomes of which he presented at VEITHsymposium. “The FLEX AV Registry 12-month outcomes shows that utilising Flex vessel prep provides more time between interventions and continues to excel in the very difficult cephalic arch lesions.”
The study was a single-arm, prospective study conducted in eight centres in the USA with 114 real-world patients.
The Flex AV Registry 12-month outcomes demonstrate sustained patency across most patients and particularly good results specifically in the cephalic arch.
49% patency for all AV fistula patients (comparable historical data 26%)
59.7% patency for cephalic arch lesions (comparable historical data 0–33.9%)
AV grafts had average time to target lesion revascularisation of 228 days (41.2% patency at nine months)
No observed serious adverse events.
“Vessel preparation has become a necessary step for better patient outcomes,” said Eric Secemsky, director of vascular intervention, Beth Israel Deaconess Medical Center (Boston, USA), who presented the 12-month results of the Belong study with Flex vessel prep system prior to drug-coated balloon (DCB). “Vessel preparation in PAD with Flex creating longitudinal microincisions prior to DCB therapy had impressive freedom from clinically driven target lesion revascularisation and looks to improve outcomes by lowering balloon inflation pressures and potentially enhancing drug delivery.”
The Belong study was a singlecentre, single-arm, prospective study conducted with 41 patients in Fribourg, Switzerland.
12-month efficacy results
97.5% (39/40) freedom from
clinically driven target lesion revascularisation
84.2% (32/38) freedom from target lesion restenosis via duplex
14/32 patients had stents
100% freedom from major amputation.
Viz.ai announces positive new data from large aortic dissection AI real-world study
Viz.ai recently announced new data from a large aortic dissection artificial intelligence (AI) real-world study that supports the use of its AI technology for the detection of suspected aortic dissection. Data from the new study, which were presented at VEITHsymposium 2022 (15–19 November, New York, USA), validate the company’s industry-leading dissection detection algorithm, part of the Viz Aortic solution.
An abstract by Viz.ai, in collaboration with Avicenna.ai, entitled “Real-world validation of a deep learning AI-based detection algorithm for suspected aortic dissection,” reported the performance of the Viz Aortic Dissection Algorithm on 1,303 computed tomography angiography scans collected from over 200 US cities. The algorithm demonstrated a sensitivity of 94.2%, specificity of 97.3%, as well as a positive predictive value of 80.1% and negative predictive value of 99.3%. The authors concluded: “These findings provide significant real-world validation of a deeplearning AI-based detection algorithm for suspected aortic dissection. Automated detection may have a positive downstream effect on patient triage leading to accelerated care coordination, earlier diagnosis, timely initiation of life-saving interventions, and better patient outcomes.”
“My experience as a clinician and AI developer has shown me that care teams need solutions that are not only accurate but that will enable them to be more efficient,” said Peter Chang (UC Irvine, Irvine, USA). “The application of this aortic algorithm into the workflow has the potential to move the needle for care teams treating this deadly disease.”
According to a company press release, Viz Aortic accelerates time-tonotification to specialists giving them access to clinically-relevant imaging and patient information for appropriate patient treatment plans. The solution includes AI-powered alerts, highfidelity mobile image viewing, relevant clinical information, and HIPAAcompliant communication to facilitate workflow and improve patient care for all aortic conditions.
“Acute aortic dissection is a deadly disease, and mortality for an untreated dissection is about 50% by 24 hours. In 2011, the IRAD [International
Registry of Acute Aortic Dissections] investigators showed us that there is often a delayed recognition and treatment of acute aortic dissection, leaving many patients at risk for another cardiovascular event,” said Jayme Strauss, chief clinical officer at Viz.ai. “These new data show that Viz Aortic has the power to help care teams coordinate and improve care for these patients in a real-world setting with a diverse patient population.”
Contego Medical has announced that enrolment of the PERFORMANCE II clinical trial has been completed.
PERFORMANCE II is intended to evaluate the safety and effectiveness of the Neuroguard integrated embolic protection (IEP) three-in-one carotid stent and post-dilation balloon system with IEP. The Neuroguard IEP system is designed to treat clinically significant carotid artery stenosis while improving procedural safety through the integration of the stent, balloon and an embolic filter, all in a single device.
“The Neuroguard IEP system combines a truly innovative stent design with a multi-function delivery platform for advanced protection from stroke, while reducing the number of catheter exchanges needed to complete the procedure,” said Christopher Metzger (Holston Valley Medical Center, Kingsport, USA), lead enroller in the trial. “Additionally, it has outstanding conformability for a closed-cell stent, allowing the advantages of this stent system in a wide variety of carotid anatomies.”
PERFORMANCE II is a prospective, multicentre, single-arm, open-label study for the treatment of carotid artery stenosis in subjects at high risk for carotid endarterectomy (CEA). The trial enrolled 305 patients at 32 sites in the USA and Europe. The principal investigators of the study are William Gray (Main Line Health, Wynnewood, USA) and Ralf Langhoff (Sankt Gertrauden Hospital, Berlin, Germany). Results of the study will be used to support a US Food and Drug Administration (FDA) premarket approval application.
“The Neuroguard IEP technology may offer significant benefits to patients suffering from severe carotid stenosis, specifically with the potential to lower the embolic burden and overall stroke rates,” said Gray.
Neuroguard IEP system is both safe and effective,” added Stacy Enxing Seng, chairman of the board of Contego. “We believe that, once available, the Neuroguard IEP system has the potential to be the standard of care for the treatment of extracranial carotid disease.”
The Neuroguard IEP system is designed to safeguard against stroke by integrating a novel, next-generation stent, a pre-positioned post-dilation balloon, and a micro-embolic filter.
The stent has a closed-cell design with FlexRing technology for optimal balance of plaque coverage, radial strength, and flexibility, according to a Contego press release. The integrated 40μm filter is designed to capture more micro-embolic debris than traditional filters.

SPYRAL HTN-ON MED study demonstrates meaningful clinical benefits consistent with other SPYRAL HTN renal denervation trials
Medtronic has announced the sixmonth results from the full cohort of the SPYRAL HTN-ON MED clinical trial. The late-breaking data were presented at the American Heart Association (AHA) Scientific Sessions 2022 (5–7 November, Chicago, USA). With this news, Medtronic has submitted the final module of the Symplicity Spyral premarket approval (PMA) package to the US Food and Drug Administration (FDA) for review and approval.
Patients who were prescribed antihypertensive medications and were treated with the Medtronic Symplicity Spyral renal denervation (RDN) system had a statistically significant and clinically meaningful reduction in office-based systolic blood pressure (OSBP), a key secondary endpoint, compared to patients in the sham control group. However, in the primary endpoint, RDN did not demonstrate a statistically significant reduction in 24-hour ambulatory systolic blood pressure (ABPM) due to increased medications in the sham control group and the potential impacts of the COVID-19 pandemic on the clinical trial environment. The study also included win ratio, a pre-specified secondary endpoint that combines reduction in blood pressure with reduction in medication burden, which enables assessment of the overall beneficial effect of RDN. The win ratio demonstrated significance in favour of RDN versus a sham procedure. Finally, the study met its primary safety endpoint, with a low incidence of procedure-related and clinical adverse events.
“The ON MED study demonstrated significant reductions in office-based blood pressure, the most commonly used measure in clinical practice. Additionally, we saw reductions in absolute blood pressure that were consistent with earlier RDN studies,” said David Kandzari (Piedmont Heart Institute and Cardiovascular Services, Atlanta, USA), SPYRAL HTN-ON MED principal investigator.
Xeltis starts pivotal clinical trial of “first-ever” restorative synthetic haemodialysis access graft
Xeltis announced today the initiation of a pivotal trial with the “first-ever” restorative synthetic haemodialysis access graft, dubbed ‘Axess’.
The first two patients have been successfully implanted as part of the AXESS European pivotal trial at AZ Sint-Jan Brugge-Oostende AV (Bruges, Belgium) by vascular surgeon Jan de Letter, and discharged from hospital.
The AXESS EU pivotal trial is a prospective, single-arm study to evaluate the safety and performance of Axess in patients with end-stage kidney disease who need haemodialysis. The study will enrol 110 patients at up to 25 centres in Europe and will follow them for five years.
The Axess graft has been previously successfully implanted in 20 patients, as part of the AXESS first-in-human (FIH) trial, which completed enrolment in September 2022. AXESS FIH full cohort data are expected in 2023.

“A device that enables immediate use, as seen with the existing synthetic ePTFE [expanded polytetrafluoroethylene] grafts, and turns into a living blood vessel that
recovers promptly after puncturing from each dialysis session may become the safer and longer-lasting solution that patients on haemodialysis need,” explained De Letter, who also has previous experience with Axess, having implanted it during the FIH trial.
“We are encouraged by the promising preliminary experience with this device from the FIH trial, and confirmation in a larger trial involving more patients and implanting sites is an important next step.”
The Axess graft is a restorative, synthetic, electrospun blood vessel for arteriovenous haemodialysis access. Once implanted, its porous microstructure gets colonised by the patient’s own tissue cells through the body’s natural healing process, turning into a living vessel made of their own tissue over time.
“Life for patients on haemodialysis means multiple hospital visits each week, involving puncturing, bleeding, waiting, healing and risk of infections from all of the above, in addition to poor renal function,” added An de Vriese, head of Nephrology and Infectious Diseases at AZ Sint-Jan Brugge-Oostende AV, and one of the co-ordinating investigators of the AXESS EU pivotal trial. “If a novel device can spare part of this burden through reduced bleeding, prompt coagulation and healing, lower infection risks and longer durability, it would be a life-changing experience for most patients.”
ReCor Medical announces consistent reduction of blood pressure in pooled analysis of three clinical trials
ReCor Medical and its parent company, Otsuka Medical Devices, announced consistent and significant blood pressure-lowering results across a range of patients with uncontrolled hypertension, including across differences in age, sex, baseline blood pressure, medication level and ethnicity. The results come from analysis of the pooled data from ReCor’s RADIANCE global clinical trial programme: three prospectively powered, randomised and sham-controlled clinical trials which evaluated the endovascular Paradise ultrasound renal denervation (uRDN) system in patients with uncontrolled hypertension.
The RADIANCE pooled analysis includes data from more than 500 patients randomised in the three studies from ReCor’s RADIANCE global programme: RADIANCE-HTN TRIO, which studied patients with resistant hypertension, and RADIANCE-HTN SOLO and RADIANCE II, which studied patients with mild-to-moderate hypertension. The combined dataset showed an overall reduction in daytime ambulatory systolic blood pressure in the uRDN group of -8.5mmHg (p<0.0001) with a difference between treatment and sham at two months of -5.9mmHg (p<0.0001), favouring uRDN. Blood pressure results were similarly positive in the 24-hour, nighttime, home, and office measures. A favourable safety profile was consistently observed following uRDN treatment across the studies.
“Pooling the data from the RADIANCE programme demonstrates that treatment with the Paradise uRDN System results in a consistent reduction in blood pressure across differing severities of hypertension.
The consistent and clinically meaningful blood pressure reduction across multiple patient groups increases our interest in the use of uRDN as a potential therapeutic option, when added to lifestyle modification and medications for our patients with uncontrolled blood pressure,” said Ajay Kirtane (Columbia University Irving Medical Center, New York USA), study co-principal investigator.








9 CME HOURS
• Lower Extremity
• Venous
• Physician Practice
• Technical Pearls, Tips and Debate
MAY 25, 2023
SIMULATION SUMMIT FOR VASCULAR TRAINEES
Scholarship includes:
• Registration to Summit & PNEC

• Lodging at the Hyatt Regency Seattle
• Travel reimbursement (U.S.-based)
CONCURRENT SESSIONS
• Updates for Advanced Practice
Providers
• Limb Preservation & Wound Care
• The Business of Medicine
clinical outcomes,” said Chris Mansi, CEO of Viz.ai. “The feedback from our early adopters has been highly positive, and we look forward to expanding access to this solution to our over 1,300 hospital customer base.”
New data presented at VEITHsymposium
a lower profile and a soft, atraumatic tip designed to help navigate the complex and delicate anatomy of the body. When used together, the device is intended to help remove blood clots quickly while minimising potential blood loss.
and provides more consistency in the procedure. We believe that the Vesseal will support enhanced outcomes in microsurgical procedures, as well as improved patient care.”
Viz.ai has announced the launch of Viz Vascular Suite—artificial intelligence (AI)-powered software enabling vascular care teams to automatically detect and triage care for suspected pulmonary embolism, right heart strain, aortic dissection, and abdominal aortic aneurysm (AAA). The company submitted a new 510(k) application to the US Food and Drug Administration (FDA) for the AAA algorithm, a press release notes.
“Recognising the symptoms of serious vascular conditions, such as aortic dissections, is hugely important because any delay in treatment can have a direct impact on patient outcomes,” said Philip Batista (Cooper University Health Care, Camden, USA). “When Viz identifies an abnormal scan, it quickly notifies the appropriate specialists regardless of their location, facilitating seamless communication via mobile application. We have been using the Viz software for the last several months and have seen improvements in patient care across our institution.”
Viz.ai claims that the Viz Vascular Suite uses AI to automatically analyse an array of imaging modalities, including computerised tomography (CT), electrocardiogram (ECG), and more for suspected vascular diseases. If a suspected pathology is found, the app automatically alerts and displays high-fidelity patient scans on providers’ mobile devices. Clinicians can use Viz Vascular Suite to remotely co-ordinate vascular pathology care within a hospital system’s hub and spoke network and enable synchronous, HIPAA-compliant communication among specialists. The platform is enhanced by AI for immediate team activation and facilitates informed, efficient treatment decisions.
“We set out to build a solution where any patient with life threatening diseases can benefit from AI-powered care coordination with triage and real-time mobile communication.
The Viz Vascular Suite will bring the advantages of intelligent care coordination to even more patients and help clinical teams achieve better
The launch follows Viz.ai’s announcement late last year of new data from a large aortic dissection artificial intelligence (AI) real-world study that support the use of the company’s AI technology for the detection of suspected aortic dissection. Data from the new study were presented at VEITHsymposium 2022 (15–19 November, New York, USA).
Penumbra recently announced the US Food and Drug Administration (FDA) clearance and launch of its Lightning Flash mechanical thrombectomy system.
“Lightning Flash features Penumbra’s novel Lightning intelligent aspiration technology, now with dual clot detection algorithms,” the company notes in a press release, adding that the system is designed to quickly remove large blood clots in the body, including venous thrombus and pulmonary emboli (PE).
“Penumbra’s Lightning Flash gives physicians a highly torqueable, larger catheter that is designed to remove a large clot burden in the pulmonary arteries or deep venous system more efficiently while maintaining an excellent safety profile because of Lightning’s computer-aided algorithms,” said James F Benenati, chief medical officer at Penumbra. “We believe that patients can have improved outcomes with this new technology because of the exceptional trackability and unique ability to distinguish flowing blood from clot.”
The company details that Lightning Flash, powered by the Penumbra Engine, uses both pressure and flowbased algorithms to detect blood clot and blood flow. The catheter is made with MaxID hypotube technology, allowing an inner diameter similar to large-bore catheters while maintaining
“Lightning Flash will fundamentally change how blood clots are removed from the body,” said Adam Elsesser, president and chief executive officer of Penumbra. “With this latest advancement, physicians are more likely to adopt mechanical thrombectomy because a broad spectrum of blood clots can be removed much quicker and less invasively than current interventional or surgical methods. This means that we are able to help even more patients with our technology, which is core to who we are as a company.”
Lightning Flash is part of Penumbra’s Indigo system with Lightning portfolio. The company claims that Lightning products are the only computer-aided mechanical thrombectomy systems currently available in the USA and early data have shown improvement in clinical outcomes and quality of life.
Lydus Medical has announced that the Vesseal has received US Food and Drug Administration (FDA) 510(k) clearance. The Vesseal is a microvascular anastomosis suture deployment system, for standardised omnivessel anastomoses, enabling simple, fast, safe, and effective procedures.
An anastomosis is one of the most complicated steps of microvascular surgery and fundamental to the success of these demanding surgical interventions. Varied clinical fields require microvascular anastomosis, including breast reconstruction; head and neck reconstruction; surgical lymphoedema treatment; and vascular access for haemodialysis.
Until now, microvascular anastomoses have been performed manually. Manual microanastomoses are time- and labour-intensive, require a long learning curve, a unique skillset and great surgical dexterity. The Vesseal is designed to mimic the skillset and dexterity needed to deliver quality patient care in microsurgical anastomoses. It provides simple, accurate, dependable and consistent results through symmetrical placement of eight microsutures at the anastomosis site.
“FDA clearance of the Vesseal is a significant milestone both for innovation in microvascular anastomoses, and for Lydus Medical. It is common knowledge that there are large clinical unmet needs around microvascular anastomosis,” said Jessica Weiss, CEO of Lydus Medical. “Clinicians who used the Vesseal stated that it shows a significant advantage over the manual anastomosis
AVS has announced that it raised US$20 million in Series B financing, which the company says will accelerate clinical trial timelines for its device for peripheral application in pulsatile intravascular lithotripsy (PIVL) cases and advance development and preclinical work on a PIVL device for coronary cases.
BioStar Capital, the lead investor in the company’s Series A round, also led the Series B round. BioStar Capital is focused on transformational investments in medical technologies with an emphasis on cardiovascular and orthopaedics.
“AVS is one step closer to offering a new treatment solution for patients with severely calcified peripheral arterial disease and progressing toward preclinical studies for coronary cases,” said Mark Toland, managing director for BioStar Capital and executive chairman/CEO of AVS. “Patients in this disease state too often face the prospect of limb amputation due to a lack of treatment options. We see a significant opportunity to address that need and advance the intravascular lithotripsy space through minimally invasive technology in both peripheral and coronary therapy.”
AVS’s novel balloon-based platform, the Pulse IVL system, shatters calcium with pressure waves in frequent bursts and expands calcified arteries, all with a single device.

“We are proud to support AVS in both its successful Series A and Series B funding rounds,” said Louis Cannon, founder and senior managing partner of BioStar Capital. “The preclinical results of the Pulse IVL System have shown the potential to raise the standard of care and significantly impact the wellbeing of patients with calcified arterial disease. We are excited to partner with AVS as it looks to future clinical trials and development.”
In September 2022, AVS announced enrolment, successful treatment, and positive 30-day follow-up data of the first patients in its POWER PAD I clinical trial, a first-in-human study. Jon George (University of Pennsylvania Health System, Philadelphia, USA) an interventional cardiologist and medical advisor to AVS, assisted in trial cases in the Dominican Republic.
“Our early trial results showed that we can successfully treat patients with multiple lesions using a single device,” said George. “We saw patients report a reduction in leg pain, increase in blood flow to the leg, and improvement in their ability to walk in our initial study.
This is a patient population that needs easier access to advanced therapies and this platform has the potential to provide that access.”






Cardiovascular Systems (CSI), recently acquired by Abbott, announced that Innova Vascular (Innova) has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) for its thrombectomy devices intended to treat peripheral vascular disease.

CSI intends to acquire and commercialise each of the novel thrombectomy devices from Innova targeting peripheral vascular disease. Commercialisation of the thrombectomy devices will be complementary to CSI’s broader portfolio of advanced technologies used in the treatment of cardiovascular disease.
Sanjay Shrivastava, CEO of Innova, said: “The FDA submission of the thrombectomy devices for use in the peripheral vasculature marks an important milestone in our commitment to develop innovative technologies targeting large, underserved markets. We are excited to partner with CSI, which has been serving the interventional cardiology, interventional
radiology, and vascular surgery communities that will be the primary users of this thrombectomy system.”
Pending regulatory clearance in the USA and completion of the acquisition of the first Innova system, CSI could begin to commercialise a portfolio of aspiration catheters and clot retrieval devices for use in peripheral vasculature in approximately six months. The portfolio and corresponding indications for use will be expanded to include the treatment of deep vein thrombosis and pulmonary embolism following completion of the respective clinical trials and subsequent 510(k) clearances. These trials are expected to begin enrolling later in 2023.
Endologix recently announced that it has received US Food and Drug Administration (FDA) approval for a premarket approval (PMA) supplement relating to the AFX2 system.
According to a company press release, Endologix received approval to include an updated warning and the most contemporary clinical information in the labelling for the AFX2 system.
Clinical data added to the instructions for use (IFU) includes: final results from the five-year LEOPARD randomised controlled trial, and results from the recently published, independently performed, VQIVISION analysis on abdominal aortic aneurysm (AAA) endografts.



Endologix notes that in both reported studies, out to three years, the rates shown for rupture and reintervention are comparable between the AFX/AFX2 device cohort and endovascular aneurysm repair (EVAR) comparator devices.
Additionally, results from the LEOPARD study demonstrated no significant difference in aneurysmrelated mortality, all-cause mortality, rupture, secondary interventions, and Type I and Type III endoleaks between the AFX/AFX2 device cohort and the EVAR comparator devices.
balloon for treatment of PAD receives FDA approval
Genesis MedTech Group announced that the US Food and Drug Administration (FDA) has approved
the Chocolate Touch drug-coated balloon (DCB) percutaneous transluminal angioplasty (PTA) catheter, developed by TriReme Medical, for the treatment of patients with peripheral arterial disease (PAD) in the superficial femoral artery (SFA) and the popliteal artery.
The Chocolate Touch DCB was shown to have statistically superior patency and non-inferior safety at 12 months as compared with Lutonix DCB (BD) based on a head-tohead, randomised trial of patients with symptomatic femoropopliteal disease.
The Chocolate Touch showed statistically superiority in its primary efficacy endpoint of 12-month true DCB success—a measure of the target vessel remaining patent without the need for bail-out stenting. Primary patency by Kaplan-Meier (KM) estimate was 83.3% for the Chocolate Touch and 73.0% for Lutonix DCB at 12 months.
The primary safety endpoint of 12-month freedom from major adverse events (MAEs) was 88.9% for those treated with the Chocolate Touch versus 84.6% for Lutonix DCB.
AFX2
Additionally, Chocolate Touch results reported the lowest all-cause KM mortality value as compared to reported mortality for FDA-approved DCBs in pivotal studies. Mortality rates for Chocolate Touch using KM estimates at three years were 6.7% for the Chocolate Touch and 11.1% for the Lutonix DCB.

Corindus has been rebranded to Siemens Healthineers Endovascular Robotics and will sit as a dedicated business within the Advanced Therapies area of Siemens Healthineers, the company has announced.

This brand unification is the final step of the company integration process that commenced in 2019 with the acquisition of Corindus by Siemens Healthineers, the company says in a press release. The aim of the Endovascular Robotics business is to advance interventions with robotics and change the way that care is delivered through innovations that enhance physician technique and bring precision to interventional procedures, the press release adds.
Siemens Healthineers Endovascular Robotics says it will continue to offer the CorPath GRX system to support endovascular procedures while working to revolutionise treatment of emergent conditions by developing technologies to provide specialised and timely medical care to more patients around the world.
Wayne Markowitz, worldwide executive vice president of Siemens Healthineers and head of endovascular
22–25 February
35th American Venous Forum (AVF) Annual Meeting San Antonio, USA venousforum.org/education/ venous2023
5–7 March

26th European Vascular Course (EVC) Maastricht, The Netherlands vascular-course.com
robotics, said: “The completion of our brand evolution marks the final step to becoming a united company and we remain focused on pioneering breakthroughs for everyone, everywhere. Our flagship product, the CorPath GRX system, is designed to support endovascular interventions, and we look forward to working with our customers as Siemens Healthineers Endovascular Robotics while fuelling our vision to connect more patients to advanced care.”
Boston Scientific announces investment to acquire majority stake of Acotec Scientific
Boston Scientific has announced that it will make a partial offer to acquire a majority stake, up to a maximum of 65%, of shares of Acotec Scientific, a Chinese medical technology company that offers solutions designed for a variety of interventional procedures.
The proposed price is HK$20 per share, which represents a total upfront cash payment consideration of approximately US$523 million for the 65% stake at current exchange rates.
Acotec is a leader in medical solutions, including drug-coated balloons (DCBs), which are used in the treatment of vascular and other diseases.
In 2016, the company launched the

23–25 March IM Endo Forum –International Multidisciplinary Endovascular Forum Florence, Italy imendoforum.com
25–27 April
Charing Cross (CX) Symposium 2023 London, UK cxsymposium.com
first peripheral DCB in China after receiving approval from the National Medical Products Administration. The Acotec portfolio also includes radiofrequency ablation technologies and thrombus aspiration catheters, as well as more than 20 other products in various stages of development across a range of specialties. In the 12-month period ending June 30, 2022, Acotec generated sales of RMB339 million (approximately US$53 million), growing 25% year-over-year in the first six months of 2022 with strong double-digit growth in each of the two years prior.
“Acotec is a profitable, fast-growing company with a strong portfolio and innovative pipeline of medical technologies, and we believe this investment will generate growth opportunities for both companies,” said Art Butcher, executive vice president and group president, MedSurg and Asia Pacific, Boston Scientific. “We expect completion of the partial offer to further strengthen our presence in China and create the potential for commercialisation of Acotec products globally, providing an increased number of physicians and patients access to our robust and complementary product portfolios.”
Boston Scientific expects the impact to adjusted earnings per share to be immaterial in 2023 and the impact to generally accepted accounting principles (GAAP) earnings per share to be less accretive, or dilutive, as the case may be, due to amortisation expense and acquisition-related
net charges.

The completion of the transaction, which is anticipated in the first half of 2023, is subject to acceptance and approval by Acotec shareholders and other conditions set forth in related filings.
Abbott and Cardiovascular Systems (CSI) have announced a definitive agreement for Abbott to acquire CSI. Under terms of the agreement, CSI stockholders will receive US$20 per common share at a total expected equity value of approximately US$890 million. CSI claims to be a leader in devices for atherectomy, the procedural use of which can help maximise the benefits of standard balloon angioplasty or stent treatments in restoring blood flow in complex arterial disease. CSI also has an early-stage pipeline of complementary vascular intervention devices in development.
“The acquisition of CSI will add new, complementary technologies to Abbott’s leading vascular device offerings,” said Lisa Earnhardt, executive vice president, Medical Devices, Abbott.
“We are pleased to have reached an agreement with a leading global company that shares our passion for the development and commercialisation of innovative solutions for treating complex peripheral vascular disease and coronary artery disease,” said Scott Ward, CSI’s chairman, president, and chief executive officer.

26–29 April
13th International Congress of the Vascular Access Society (VAS) Porto, Portugal vas2023.com
27–29 April
International Vein Congress (IVC) Miami Beach, USA ivcmiami.com/index.php
10–13 May Venous Symposium 2023 New York, USA venous-symposium.com
25–26 May Pacific Northwest Endovascular Conference (PNEC) 2023 Seattle, USA pnec-seattle.org

6–9 June
Leipzig Interventional Course (LINC) 2023 Leipzig, Germany leipzig-interventional-course.com
14–17 June
Society for Vascular Surgery (SVS) Vascular Annual Meeting 2023 National Harbor, USA vascular.org/vam-2023