CX 2023: Consensus update marks 45 years of looking forward


New data and global discussion will be the hallmarks of the upcoming Charing Cross (CX) International Symposium, which this year celebrates its 45th anniversary. The 2023 programme offers a consensus update on key topics in the vascular and endovascular space—from revascularisation strategies for patients with chronic limb-threatening ischaemia (CLTI), to treatment options for superficial venous disease. The symposium will bring together world-leading experts and feature three days of in-person education in London, UK, as well as virtual and on-demand viewing options that will be beamed across the globe.
Running from 25th–27th April, the programme will aim to reach consensus on pressing issues in multiple vascular domains, including peripheral arterial, aortic, venous, vascular access, acute stroke, vascular trauma and—for the first time—the wide field of renal interventions.
CX welcomes new co-chairs
This year, CX welcomes three new co-chairs from across the globe to the leadership team. Dittmar Böckler, medical director of the Clinic for Vascular and Endovascular Surgery at University Hospital Heidelberg (Heidelberg, Germany), Andrew Holden, director of Interventional Radiology at Auckland City Hospital (Auckland, New Zealand) and Erin Murphy, director of the Venous and Lymphatic Institute at Sanger Heart and Vascular, Atrium Health (Charlotte, USA) will work alongside CX chair and anchor Roger Greenhalgh (Imperial College London, London, UK) to deliver the CX programme.
Podium First highlight: BASIL-2
The key highlight of this year’s programme is the BASIL-2 (Bypass versus angioplasty in severe ischaemia
of the leg-2) Podium First presentation, which will be published simultaneously in The Lancet. Chief investigator Andrew Bradbury (University of Birmingham, Birmingham, UK) and other members of the trial group will deliver the findings, after which attendees in London and remote participants will have the opportunity to pose questions. A roundtable discussion is planned to include invited commentary from Eleni Whatley (US Food and Drug Administration [FDA], Silver Spring, USA) and UK Secretary of State for Health Steve Barclay (London, UK).
New data
Alongside BASIL-2, there are 12 other Podium First presentations on this year’s CX programme. In the Peripheral Arterial session, stay tuned for new data from the BEST-CLI (Best endovascular versus best surgical therapy for patients with critical limb ischaemia) trial, with Matthew Menard (Brigham and Women’s Hospital, Boston, USA) due to give a clinical trial update and Alik Farber (Boston Medical Center, Boston, USA) an insight into the quality of life data from the study.
The Aortic programme will feature five Podium First presentations, including




CX will run 25th to 27th April
one by Kevin Mani (Uppsala University, Uppsala, Sweden) on statin treatment, and the Vascular Access Masterclass will see Holden, CX co-chair and executive board member, present a three-year data subanalysis from the IN.PACT AV Access study.

Innovation
The CX Innovation Showcase returns for 2023, bringing to the fore an array of cutting-edge technologies and techniques. The CX audience can expect to see presentations on topics ranging from the power of intelligent mapping for complex endovascular aneurysm repair (EVAR), to wearable devices for non-invasive, remote monitoring and classification of access patency and flow characteristics in haemodialysis patients. The session will close with the annual Dragon’s Den-style competition and announcement of the winner of this year’s £1,000 CX Innovation prize.
Hands-on learning
A series of workshops will provide CX attendees with the opportunity to test and improve their practical skills at hands-
on stations showcasing techniques and technologies across all three days of the meeting.
In a dedicated Workshop Wing, attendees will be able visit the Vascular Access Workshop on Tuesday 25th April, the Aortic Workshop on Wednesday 26th April and the Venous and Hurting Leg Workshop on Thursday 27th April.
Global reach
This year’s in-person symposium will be held in the Hilton London Metropole, however there will also be a broadcastquality livestream available to enable participants who cannot be in London to join the global vascular conversation. The CX International Symposium has a global audience and every year sees participation from around the world. There will be a dedicated CX Meets Latin America session at this year’s meeting, with presenters from Brazil and Argentina sharing their research with the global CX audience.
For more information and to register for CX 2023, visit www.cxsymposium.com. Join the global vascular discussion.

Charing Cross Special Edition / April 2023 www.vascularnews.com Featured in this issue: 28 Venous stenting
14 Medical devices MDR updates 20 A year in profiles Interview highlights WELCOME VASCULARTOYOURNEWLOOK NEWSCXSPECIALEDITION 25–27 APRIL 2023 TUESDAY-THURSDAY IN PERSON AND VIRTUAL HILTON LONDON METROPOLE, UNITED KINGDOM Consensus Update Vascular & Endovascular Hurting Leg Consensus C M Y CM MY CY CMY K ai167994811619_CX2023-VNCX2023-COVER-225X36.pdf 1 27/03/2023 21:15:16
Jorinde van Laanen
Reduce the risk of dissections

charge of CLTI
Take
Chocolate™* PTA Balloon Catheter

You can confidently size 1:1 with controlled angioplasty, while reducing
Aortic Consensus
New data take centre stage on CX 2023 Aortic programme
Abdominal and juxtarenal aortic consensus
Wednesday 26th April
Kensington 1
Thoracic aortic consensus and How To Do It (HTDI)
Thursday 27th April
Kensington 1
New data in the aortic field will be a prominent feature of this year’s CX programme, with five Podium Firsts due to be presented on Wednesday 26th April. In the abdominal aortic space, Kevin Mani (Uppsala University, Uppsala, Sweden) is set to deliver a first-to-podium presentation on statin treatment after aortic repair and Hence Verhagen (Erasmus University Medical Center, Rotterdam, The Netherlands) will outline 10-year primary abdominal aortic aneurysm (AAA) outcomes from the ENGAGE OUS registry for the first time.
In the juxtarenal aortic part of the programme, the Podium Firsts include Eric Verhoeven’s (General Hospital Nuremberg, Paracelsus Medical University, Nuremberg, Germany) presentation on eight-year results of a bridging stent for fenestrated and branched endovascular aneurysm repair (F/BEVAR), Luke Terlouw’s (Erasmus University Medical Center) talk on covered stents versus bare-metal stents in chronic atherosclerotic gastrointestinal ischaemia, and a meta-analysis of comparative studies between self- and balloon-expandable bridging covered stents for BEVAR, set to be presented by Konstantinos Spanos (University of Thessaly, Larissa, Greece).
Elsewhere in the programme, Maarit Venermo (Helsinki University Hospital, Helsinki, Finland) will give a presentation on how AAA screening shifts treatment towards elective repairs and decreases rupture repairs and Rao Vallabhaneni (University of Liverpool, Liverpool, UK) will speak on the impact of the ETTAA (Effective treatments for thoracic aortic aneurysms) study and UK-COMPASS (UK complex
aneurysm study) results with reference to the UK National Institute for Health and Care Excellence (NICE) guidelines.
Radiation will also be addressed during the aortic programme, with Gustavo Oderich (University of Texas Health Science Center at Houston, Houston, USA) due to speak on trends of radiation exposure across different imaging systems among other podium presentations.
On Thursday afternoon, stay tuned for the How To Do It (HTDI) session from Roberto Chiesa (Ospedale San Raffaele Clinical Research Institute, Milan, Italy) and his team, who will be presenting on open, hybrid and endovascular management of complex aortic aneurysms post dissection.
Aortic techniques & technologies
Tuesday 25th April
Kensington 2
A number of edited cases will highlight cutting-edge techniques and technologies in the aortic space during a dedicated session on the first day of CX 2023. This part of the programme consists of a series of 10-minute videos, each followed by 10 minutes of discussion.
Wei Guo (Chinese PLA General Hospital, Beijing, China) will open the session with an edited case on aortic arch reconstruction. During this case, Guo will speak on the prospective, multicentre GIANT study on the safety and efficacy of a modular inner branch stent graft system.
Also on the programme is Jean Panneton (East Virginia Medical School, Norfolk, USA), who will give an update on in situ laser fenestration during thoracic endovascular aneurysm repair (TEVAR), describing it as a safe, effective and durable procedure to revascularise arch branches.
In addition, Ross Milner (University of Chicago, Chicago, USA) will speak on transcaval TEVAR with the use of intravascular ultrasound (IVUS), outlining a method for success, and Timothy Resch (University of Copenhagen, Copenhagen, Denmark) will be speaking on the use of laser fenestration to aid in the endovascular treatment of chronic dissection thoracoabdominal aortic aneurysms, alongside other presenters.
The Hurting Leg Consensus
Hurting Leg consensus highlights
CLTI and Hurting Leg consensus 08:00–18:00
Kensington 1
Highlights of this year’s CLTI and Hurting Leg programme include ‘Best of abstracts’ from rising stars in the vascular field, and a roundtable discussion led by Naseer Ahmad (Manchester University NHS Foundation Trust, Manchester, UK) on the possibility of using existing screening programmes like aneurysm and breast screening to opportunistically identify ‘the hurting leg’. Elsewhere on the programme, the winners of a new competition on ‘the hurting leg’ will be announced. The Rouleaux Club—the UK’s national vascular trainee society—in association with CX 2023 and BIBA Medical designed the competition to encourage early detection and treatment of ‘hurting legs’. Students and trainees involved in caring for vascular patients from all over the world were invited to create an infographic and/or infomercial intended to educate members of the public about chronic limb-threatening ischaemia (CLTI) and encourage patients to present to their general practitioner.
Attendees at CX 2023 will be able to view the top five infomercials and infographics and vote for their preferred one. The monetary prizes of £1,000 for the infomercial and £500 for the infographic will be announced during the CLTI and Hurting Leg consensus session.
This year, CX is offering free registration to all fellows who are members of European Vascular Surgeons in Training (EVST), the Rouleaux Club or other equivalent societies worldwide, and 75% off for early career surgeons and physicians (terms apply).

CHARING CROSS SPECIAL EDITION
Editor-in-chief: Roger Greenhalgh | Publisher: Roger Greenhalgh | Content Director: Urmila Kerslake
Editor: Jocelyn Hudson Jocelyn@bibamedical.com | Editorial contribution: Jamie Bell, Will Date, Bryan Kay, Eva Malpass, Benjamin Roche and Clare Tierney
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: Shilpa Suthar shilpa@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
BIBANews April 2023 CHARING CROSS SPECIAL EDITION 4 VascularNews linkedin.com/company/Vascular-news @VascularNews Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd | BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323 Printed by: Buxton Press Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2023. All rights reserved. If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
CX 2023
Peripheral Arterial Consensus
CX 2023 highlight: The BASIL-2 trial
BASIL-2 Podium First presentation
11:10–11:40 Tuesday 25th April
Kensington 1
Roundtable discussion
12:00–13:00 Tuesday 25th April
Kensington 1
Data and discussion on revascularisation treatment strategies for patients with chronic limb-threatening ischaemia (CLTI) will take centre stage at the CX 2023 Consensus update, with results from the BASIL-2 (Bypass versus angioplasty in severe ischaemia of the leg2) randomised controlled trial (RCT) to be presented for the first time. Chief investigator Andrew Bradbury (University of Birmingham, Birmingham, UK) speaks to Vascular News about the background, context and significance of the trial ahead of this year’s meeting.
Bradbury notes that BASIL-2 has its origins in the original BASIL-1 trial, the shortterm results of which were published in The Lancet in 2005. BASIL-1 triallists randomised (1999–2003) patients with severe limb ischaemia, mainly due to femoropopliteal disease, to either a plain balloon angioplasty-first or a bypass surgery-first revascularisation strategy. With “fairly limited follow-up,” Bradbury recalls, there did not seem to be much of a difference in the primary outcome of amputation-free survival. There was, however, a suggestion that the data from both groups were “beginning to diverge,” which prompted the team to follow the patients up for longer. Reporting the key finding from these later outcomes, Bradbury summarises that “people randomised in BASIL-1, and who were likely to live for more than two years, and who had a good vein, were best served by having a vein bypass first rather than a plain balloon angioplasty first”.
Speaking on his motivation for starting the BASIL-2 trial, Bradbury recollects how it became clear there were a number of gaps in peripheral arterial disease (PAD) research during his involvement in the UK National Institute for Health and Care Excellence (NICE) guideline expert group on PAD. One such gap had to do with infrapopliteal, or below-the-knee, disease, and Bradbury notes that NICE made a research recommendation to undertake an RCT to compare a ‘vein bypass-first’ with a ‘best endovascular treatment-first’ strategy for people who required an infrapopliteal procedure. This is what BASIL-2 endeavoured to achieve.
“Endovascular techniques and technologies for lower limb revascularisation are very different now from what they were 15–20 years ago when we did BASIL-1,” Bradbury remarks, noting for example better guidewires, better balloons, more skilful entry and retrograde cannulation to name just a few developments. As a result, the team are keen to see
how the two treatment modalities compare in this new treatment landscape.
In addition, the question at the centre of BASIL-2 represents a “massive global problem” and therefore an important one to solve, Bradbury comments. He elaborates: “I have had the opportunity to visit hospitals in many different countries, and vascular wards wherever you go are essentially full of people with CLTI.” Bradbury explains that BASIL-2 is his and his team’s “attempted contribution” to try and improve the evidence base for the treatment of these patients who are “very challenging” and often “very poorly,” with multiple comorbidities.
BASIL-2 is a superiority trial, Bradbury explains, noting that the hypothesis the investigators started with was that vein bypass would be superior due to the fact that BASIL-1 “seemed to show that vein bypass had advantages over endovascular intervention”. He adds that it is a pragmatic trial, and thus surgeons and interventional radiologists are permitted to use their preferred techniques
are focused in BASIL-2 on below the knee, and [BEST-CLI] did not pre-specify an only below-theknee analysis.” While BEST-CLI is “not exclusively a femoropopliteal trial,” it has similarities to BASIL-1 in that it is more of a femoropopliteal trial with or without infrapopliteal disease, whereas BASIL-2 is specifically looking at infrapopliteal revascularisation, Bradbury explains.
BASIL-2 and BEST-CLI are both RCTs, however Bradbury is keen to stress the limitations of this high level of evidence. “An RCT is not a GPS, it is more like a wobbly compass near the North Pole, and all it can try and do is push you in a certain direction of travel,” he comments. He adds that while both the BASIL-2 and BEST-CLI teams are “huge enthusiasts” for RCTs, this type of research must still be scrutinised.
Looking ahead to CX, Bradbury expresses his excitement at the prospect of presenting the BASIL-2 data for the first time at what he describes as “the big UK vascular and endovascular meeting”. “I am really looking forward to seeing everyone at CX 2023 and it is going to be a great meeting. It is a pleasure and a privilege for us to be part of it and have the opportunity to present our trial data for the first time.”

and equipment, with the primary outcome being amputation-free survival (time to major—abovethe-ankle—amputation, or death from any cause, whichever occurs first).
Considering the BASIL-2 trial in its wider context of randomised data in this space, Bradbury notes that the investigators have been in “friendly dialogue” with the BEST-CLI team “from the getgo”. Results of the BEST-CLI trial were presented late last year, with the headline finding being that surgical bypass with adequate single-segment great saphenous vein is a more effective revascularisation strategy for patients with CLTI who are deemed to be suitable for either an open or endovascular approach.
Bradbury notes that there are a number of differences between the two trials, highlighting for example that BASIL-2 included a different group of patients. “Only about 40% of [BEST-CLI] patients have a below-the-knee intervention,” he says. “We
Bradbury hopes that once the audience hear the results, as well as the limitations, which “every RCT has,” they will “go away and reflect” on the data and think about them “in the context of their own practice, their own healthcare system and come to a decision as to whether these new data are going to influence their practice, or not, as the case may be”.
Join the conversation at CX 2023
During the CX session, Bradbury will deliver the results of the BASIL-2 trial, with co-investigators Catherine Moakes, Gareth Bate and Matthew Popplewell (all University of Birmingham, Birmingham, UK) and Lewis Meecham (University Hospital Cardiff, Wales) set to present on the journey from BASIL-1 to BASIL-2, methodology, study limitations and future work, among other topics.
Attendees in London and remote participants will have the opportunity to pose questions to the BASIL-2 investigators.
A roundtable discussion is planned to include invited commentary from the US Food and Drug Administration (FDA) and UK Secretary of State for Health.
5 BIBANews April 2023 CHARING CROSS SPECIAL EDITION CX 2023
It is a pleasure and a privilege for us to be part of [CX 2023] and have the opportunity to present our trial data for the first time.”

Venous & Lymphatic Consensus
CX 2023 seeks consensus in “rapidly” progressing venous field
Superficial venous & lymphatic consensus
08:00–13:00 Wednesday 26th April
Kensington 2
Deep venous consensus
13:30–18:00 Wednesday 26th April
Kensington 2
The CX 2023 Venous & Lymphatic programme it set to be a “highlight” of this year’s meeting, CX co-chair and executive board member Erin Murphy (Sanger Heart and Vascular Institute, Atrium Health, Charlotte, USA) tells Vascular News

This year’s programme will highlight progress in the space, new data, and areas in which there is a need to “drive movement,” Murphy details, adding that the importance of comprehensive care will be
CX launches dedicated Renal Interventions session alongside Vascular Access Masterclass
Vascular Access CX Masterclass
08:00–13:00 Tuesday 25th April
Admiral
This year, the Vascular Access Masterclass will take place on the morning of day one of CX.

Executive board member Nicholas Inston (University Hospitals
Birmingham NHS Foundation Trust, Birmingham, UK) will anchor the session, with Kate Steiner (East and North Hertfordshire NHS Trust, Stevenage, UK), also an executive board member, Alexandros Mallios (Hôpital Paris Saint-Joseph, Paris, France) and Narayan Karunanithy (Guy’s and St Thomas’ NHS Foundationa Trust, London, UK) set to moderate. The session will feature two Podium First presentations. Executive board member Andrew
a key underlying theme. “We really have to have a full understanding of the venous and lymphatic system and not narrow in too much on one area,” she remarks.
On Wednesday morning, management of superficial venous disease will be the focus. Traditionally, Murphy explains, superficial disease was treated with surgical stripping and phlebectomy. She notes that nowadays, however, treatment is becoming increasingly advanced in this space, with options such as thermal closures, medical adhesive closures, and foam now the standard of care.
Commenting on the use of foam as a closure tool, Murphy believes this is an area in which there is not yet true consensus. “I do not think that [foam] has been maximised yet in its ability,” she remarks. While the technique is used widely in the USA for ulcer-bed sclerotherapy, Murphy states that there may be a higher risk of deep vein thrombosis (DVT) when it is used as a primary closure tool. There is still room for this treatment modality to be “optimised,” she believes.
CX 2023 discussion will focus on which of these treatment options for superficial venous disease— thermal closures, medical adhesive closures, and foam options—might be better suited to certain patient groups and certain clinical scenarios. In this part of the programme, Manj Gohel (Cambridge University Hospitals NHS Healthcare Trust, Cambridge, UK) will deliver a SPECTRUM study programme update on medical adhesive closure versus surgical stripping for patients with chronic venous disease.
Deep venous consensus will be the focus of Wednesday afternoon’s session, with incompetence
Holden (Auckland City Hospital, Auckland, New Zealand) will deliver three-year subanalysis data from the IN.PACT AV Access trial and Frans Moll (University Medical Center Utrecht, Utrecht, The Netherlands) will present on a new kind of graft for vascular access. The Vascular Access Masterclass will also feature edited cases, with Daniel Patel (Daytona Beach, USA) speaking on arteriovenous (AV) revision with a covered stent and Robert Shahverdyan (Asklepios Klinik Barmbek, Hamburg, Germany) on endovascular AV fistula (endoAVF) creation.
Renal Interventions
14:00–18:00 Tuesday 25th April
Admiral
New to CX in 2023, there will be an afternoon of presentations and discussion on renal interventions. Steiner and Inston will be the anchors for the session, with Ounali Jaffer (Barts Health NHS Trust, London, UK), Jeremy Crane (Imperial College Renal and Transplant Centre, London, UK) and Frank Dor (Imperial College London, London, UK) all set to moderate.
Highlights of this new session include a presentation by Charmaine Lok (University of Toronto, Toronto, Canada) on the vascular access treatment algorithm and patient-based choices, and one by Dor on peritoneal dialysis and whether this is an optimal home therapy.
of the deep venous system within the lower extremities and the pelvis set to feature. “We traditionally did open surgery for these patients,” Murphy recalls, noting however that open reconstructions are “very complex surgically” and are associated with a relatively high rate of eventual failure and thrombosis. She notes that there are new technologies available in this space that are “really encouraging”. CX 2023 will look at how these technologies might impact the future of treatment in the space.
Venous stenting will be another topic on the deep venous agenda, with presentations highlighting how to avoid migration and defining patients who should not undergo stenting, among other topics. Murphy is set to look at the data and will outline trends from the ABRE study outcomes.
The venous field is making “huge leaps and bounds”
According to Murphy, the venous field is progressing “very rapidly” and is making “huge leaps and bounds” on a yearly basis. “I think the CX programme has worked very hard to make a comprehensive and up-to-date consensus on what we have available to use now, what we know now, and where we are going”.
The programme is becoming more international every year, Murphy comments, with interest in the programme expanding due to recognition that it “really adds value to the venous space”. “I think [the CX 2023 Venous & Lymphatic programme] is a great update for anybody who is in the venous space and who is interested in what progress we are making.”
Experts debate TCAR versus stenting in Acute Stroke session
08:00–10:10 Wednesday 26th April
Admiral
At this year’s CX Symposium, experts will gather to discuss the most pressing issues in the field of acute stroke. Anchor Maarit Venermo (Helsinki University Hospital, Helsinki, Finland) along with moderators Alexander Zimmermann (University Hospital Zurich, Zurich, Switzerland) and Domenico Valenti (King’s College Hospital NHS Foundation Trust, London, UK) will oversee the session on the second day of CX 2023. The first item on the programme is a CX Debate, during which Peter Schneider (University of California San Francisco, San Francisco, USA) and Valenti will respectively argue for and against the motion that transcarotid artery revascularisation (TCAR) is better than percutaneous carotid stenting.
Among the podium presentations in the session, Venermo is set to speak on aetiology and treatment patterns of ruptured extracranial carotid artery aneurysms, as well as on whether carotid endarterectomy is safe immediately after thrombolysis and the consequences of delay. Zimmermann is due to present on identification of baroreceptors responsible for mechanotransduction in the human aortic arch, and Valenti on simultaneous mechanical thrombectomy and carotid artery stenting for severe internal carotid artery stenosis, outlining indication risks and current evidence. Additionally, Alun Davies (Imperial College Healthcare NHS Trust, London, UK) will argue that NASCET (North America symptomatic carotid endarterectomy trial) and ECST (European carotid surgery trial) need to be reconducted, and Michael Stoner (University of Rochester, Rochester, USA) will speak on how calcified plaque volume predicts haemodynamic restenosis and also on the use of flow-based protection to treat tandem supra-aortic branch stenoses. In keeping with CX style, the session will feature audience participation throughout and close with a panel discussion.
7 BIBANews April 2023 CHARING CROSS SPECIAL EDITION CX 2023
Vascular Access Consensus Renal Interventions Consensus Acute Stroke Consensus
Erin Murphy
Vascular Trauma Consensus


Vascular trauma consensus update



11:00–13:00 Wednesday 26th April

Admiral
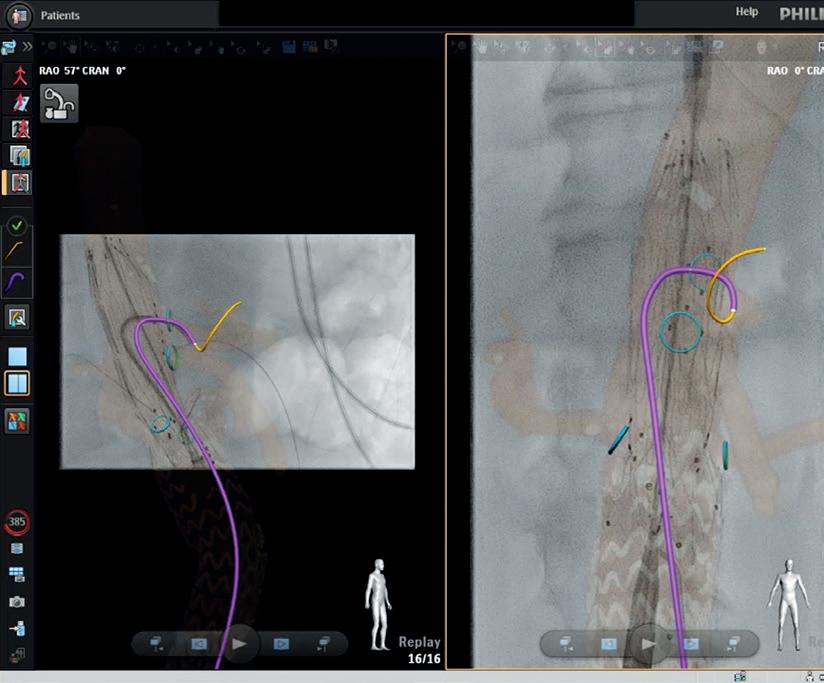
This year’s Vascular Trauma programme at CX will hone in on a number of key topics in the field, namely endovascular trauma, extremity vascular trauma and limb salvage, and cerebrovascular trauma. At the end of the session, the CX audience can expect half an hour of presentations on the best vascular trauma abstracts.

Anchor Ross Davenport (Barts Health NHS Trust, London, UK) and moderator Christopher Aylwin (Imperial College Healthcare NHS Trust, London, UK), both executive board members, will oversee a series of podium presentations, as well as audience participation and discussion, all in the interest of reaching consensus and moving the subject forward.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) will be a central focus of the endovascular trauma section, with presentations including those from Robert Lendrum (Barts Health NHS Trust, London, UK) on refining the use of REBOA for major vessel injury in trauma and Megan Foley (University Hospital, Dublin, Ireland) on limb complications of REBOA, among other presentations.
Moving the focus to extremity vascular trauma and limb salvage, Anna Sharrock (Imperial College London, London, UK) will present on prediction models of limb salvage, and Simon Glasgow (Northwick Park Hospital, Harrow, UK) on decision-making in complex extremity vascular trauma, among other presentations.
The cerebrovascular trauma section will cover blunt cerebrovascular injuries, specifically thresholds for imaging, diagnosis and monitoring, as well as the management of blunt cerebrovascular injuries and penetrating carotid and vertebral injuries.

At the end of the session, the audience can expect to hear about a 10-year experience of vascular trauma in a major trauma centre, pointing to opportunities for training in emergency open approaches, in the ‘Best of Abstracts’ section.


Workshop learning opportunities at CX 2023
08:00–18:00 Tuesday 25th, Wednesday 26th and Thursday 27th April
East Wing (Level -2)
There will be a number of hands-on learning opportunities at CX 2023, with the programme boasting a full complement of workshops for attendees to experience across the three days of the event. The Vascular Access Workshop, Aortic Workshop and Venous & Hurting Leg Workshop will be available to all in-person CX delegates. Attendees will be able to test and improve their practical skills and see new devices up close at hands-on stations in the dedicated Workshop Wing, located in the East Wing of the Hilton London Metropole, which can be accessed via the


staircase entrance by the hotel bar. The Vascular Access Workshop is directed by executive board members Nicholas Inston (University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK) and Kate Steiner (East and North Hertfordshire NHS Trust, Stevenage, UK); the Aortic Workshop and Aortic Course on branched and fenestrated endovascular aneurysm repair (B/FEVAR) physicianmodified procedures by Alexander Zimmermann and Benedikt Reutersberg (both University Hospital
Zurich, Zurich, Switzerland); and the Venous & Hurting Leg Workshop by Hayley Moore (Imperial College
London, London, UK), Tristan Lane (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK), executive board member Manj Gohel (Cambridge University Hospitals NHS Foundation Trust) and CX chair Roger Greenhalgh (Imperial College London, London, UK).

8 BIBANews April 2023 CHARING CROSS SPECIAL EDITION CX 2023
*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiovascular field Editorially independent Available in print and digital formats and through our social channels Visit cardiovascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today
THIS ADVERTORIAL IS SPONSORED BY PHILIPS
Fibre optic technology shows “transformative” potential in endovascular aortic procedures

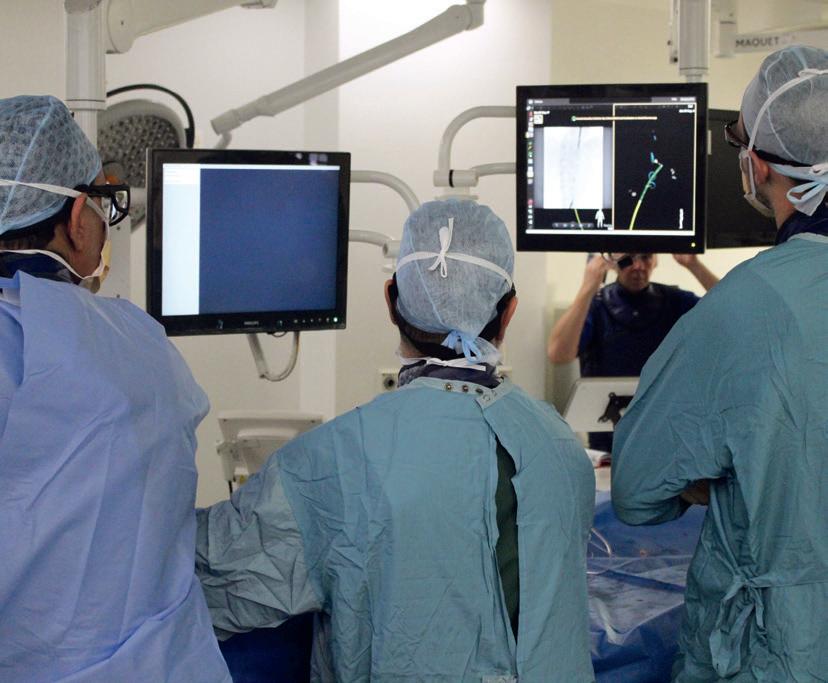
Two early adopters of Fiber Optic RealShape (FORS; Philips) share their expert opinions on the developing technology, highlighting its future potential in reducing radiation and increasing procedural efficiency.
The European Society for Vascular Surgery (ESVS) 2023 clinical practice guidelines on radiation safety1—the first recommendations on the topic to be published in the vascular surgery domain—brought into sharp focus the need to address radiation exposure during endovascular aortic procedures.
Bijan Modarai (Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, London, UK), lead author of the guidelines, notes that X-ray-guided endovascular procedures have become the “mainstay” treatment modality for patients with vascular disease. X-ray is the current standard of care for endovascular procedures, he says. However, it has drawbacks, and the associated radiation poses a threat to patients and operators alike. Based on extensive research into radiation-induced DNA damage, Modarai and colleagues at King’s College London have identified biological evidence that radiation is having a negative effect on both patients’ and operators’ cells.

Adding to the problem are two factors, Modarai
fluoroscopy,” he comments.
Modarai, who recently performed the first fibre optic-guided case at Guy’s and Thomas’ NHS Foundation Trust, concurs, stating that increasing interest in developing technologies to address the radiation issue “has to be high on the list of priorities for all stakeholders”.
“We wanted to disseminate knowledge about what is currently in the pipeline, with the hope that that will stimulate interest in those who read [the guidelines] to come up with new research endeavours,” Modarai explains.
A “novel way” to visualise and navigate the aorta
Van Herwaarden states that he and two other operators have now performed a combined total of around 150 cases with FORS at the University Medical Center Utrecht. “We are getting used to it,” he says, noting that the team have seen improvements with
For Modarai, fibre optic technology represents a positive addition to his aortic practice. “The fact that you can visualise the catheter and the wire relying on this technology rather than X-ray is a conceptual advance,” he says. The benefits of the technology, in Modarai’s view, are multifactorial, pointing to its potential to protect both the patient and the operator from radiation, whilst also providing a “novel way” to visualise and navigate the aorta. “You get a view of the area that you are navigating in multiple planes,” he explains, “and that in itself also facilitates the procedure, allowing the entire team in the hybrid operating theatre to be able to follow what is going on and to be able to visualise what is being done on the screen”.
With regard to the literature on FORS, the evidence base is growing. Most recently, Eric J Finnesgard (University of Massachusetts Chan Medical School, Worcester, USA) and colleagues, under the senior authorship of Andres Schanzer, outlined an initial single-centre experience using FORS guidance in complex endovascular aortic repair. In November 2022, they reported online in the Journal of Vascular Surgery that FORS has shown “promise” in their aortic practice, with “acceptable technical success and reductions in procedural times and radiation usage”.2 Schanzer is set to share these findings, as well as his experience so far using FORS, at the Charing Cross (CX) Symposium 2023 (25–27 April, London, UK).
Van Herwaarden believes that FORS will have positive effects on radiation. He stresses, however, that this still has to be proven, which is why he and his team together with seven other renowned centres in Europe and the USA are conducting a registry study. “We are now busy with the analysis of the more than 600 cases that have been done,” he explains.
While data are being collected, van Herwaarden is keen to stress that FORS is still in its early stages. “It feels like we are still in the beginning of a new technology that is slowly proving itself,” he remarks, and is looking ahead to a “promising” future where FORS could enable less need for radiation, quicker procedures, and fewer complications.
says, noting that practitioners are nowadays being exposed to X-rays throughout their entire career, and that the increasing complexity and thus length of endovascular procedures is resulting in “significant” radiation exposure to both the patient and, “crucially,” the operator.1
The ESVS guidelines aim to raise awareness of the risks of radiation and provide guidance on best practice, closing with a forward-looking chapter on the importance of new and future technologies in reducing and, in the longer term, eradicating the need for radiation. Fibre optic guidance is one such technology, with reference made not only to its radiation-reducing potential, but also to how it might enhance visualisation of catheters and wires and lead to potentially shorter procedure times.1,2
Joost van Herwaarden (University Medical Center Utrecht, Utrecht, The Netherlands), who led the firstin-human clinical feasibility study of endovascular navigation with FORS,3 notes that mention of the technology in the guidelines is important for the purpose of generating awareness and interest among the vascular community. “All of us should be aware that there are other possibilities than just using
the technology in the three years since they started using it. He mentions that with the recent introduction of Philips’ 3D Hub technology, for example, it is possible to use many different commercially available catheters and show them with the FORS technology.
The team now use FORS weekly and in “almost all endovascular aneurysm repairs,” van Herwaarden details. Specifically, he tells Vascular News that FORS “really gives you a better understanding of the position and 3D morphology of the devices in the 3D anatomy or workspace,” noting that the better view the technology provides makes cannulations easier.
According to van Herwaarden, the future of FORS lies also in its combined use with other emerging technologies, for instance robotic tracking, artificial intelligence, and intravascular ultrasound (IVUS), with the combination offering “an even greater promise for simplifying complex procedures and realising radiation dose reduction for patients and for staff”.
Modarai believes the technology will be “transformative” in endovascular aortic procedures, while adding the caveat that there is still some way to go. He looks ahead to iterative improvements with regard to compatible wires and catheters, and the footprint of the ancillary devices, for example, which will make fibre optics “easier and easier” to incorporate into clinical practice. Eventually, he hopes, it will become an “essential” part of the workflow, allowing operators to circumvent the use of X-rays for endovascular procedures. “When that goal is reached, then it is difficult to imagine that one would want to perform these procedures without a technology like this.”
References
1. Modarai B, Haulon S, Ainsbury A, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2023 clinical practice guidelines on radiation safety. European Journal of Vascular and Endovascular Surgery. 2023;62(2):171–222.
2. Finnesgard EJ, Simons JP, Jones DW, et al. Initial single-center experience using Fiber Optic RealShape guidance in complex endovascular aortic repair. Journal of Vascular Surgery. Published online first 12 November 2022.
3. Van Herwaarden J, Jansen MM, Vonken EPA, et al. First in human clinical feasibility study of endovascular navigation with Fiber Optic RealShape (FORS) technology. European Journal of Vascular and Endovascular Surgery. 2021;61(2):317–325.
9 Advertorial BIBANews April 2023 CHARING CROSS SPECIAL EDITION
The fact that you can visualise the catheter and the wire relying on this technology rather than X-ray is a conceptual advance.”
Bijan Modarai
L-R: Bijan Modarai conducting a FORS case (image courtesy of Guy’s and St Thomas’); Cannulation of the coeliac trunk in fenestrated endovascular aneurysm repair (image courtesy of UMC Utrecht).
Bijan Modarai Joost van Herwaarden



10 BIBANews April 2023 CHARING CROSS SPECIAL EDITION CX 2023 LIFT1 LIFT2 LIFT3 LIFT LIFT LIFT LIF RECEPTIONMAIN LOBBYAND MAIN ENTRANCE MAIN ENTRANCE BOWBAR BOWBAR SEMI-PRIVATE WEST WING LOBBY HOXTONROOM 85.3SQM SPEAKER READY ROOM ORGANISERS OFFICE TYBURNMARKET TYBURN BAR TYBURN RESTAURANT HYBRID MEETING ROOMS 1 HYBRID MEETING ROOMS HYBRID MEETING ROOMS HILTON LONDON METROPOLE WEST WING GROUND FLOOR SPEAKER READY ROOM ACCOMMODATION CHECK IN TYBURN RESTAURANT & BAR BOW BAR LIFTS TO LEVELS 1,2, 3 & 4 EVENT REGISTRATION HYBRID MEETING ROOMS ACCESS TO ADMIRAL SUITE GMT SUITE MEZZANINE KENSINGTON 1 AND 2 WEST WING LEVEL 3 KENSINGTON 2 KENSINGTON 1 RICHMOND MEZZANINE WEST WING LEVEL 2 46 SQM CX LIVE STUDIO RICHMONDEXHIBITION WEST WING LEVEL 1 CATERING CATERING A29 A27 A23 A21 B24 A25 D38 B36 B38 C36 C32 B28 B32 C28 D24 E40 D40 E38 D38 E36 D36 E32 D28 D32 F33 F27 F25 F19 D20 B20 A19 A15 F23 G12 G13 G14 F35 G11 G10 A30 MEZZANINE EAST WING LEVEL 1 MEETING ROOMS 26 SQM 56 SQM 66 SQM WORKSHOPS & Abstracts ADMIRAL PLENARY ADMIRAL SUITE EAST WING LEVEL -3 ADMIRAL PLENARY WORKSHOP & ABSTRACTS GMT SUITE EAST WING LEVEL -2 MEETING ROOMS WEST WING KENSINGTON 1 AND 2 RICHMONDEXHIBITION EAST WING ADMIRAL SUITE GMT SUITE MEZZANINE Peripheral Arterial & Acute Limb Ischaemia & Randomised Control Trial Update –P?? Philips Sponsored Education –P?? Nectero Sponsored Education –P?? Terumo Aortic Sponsored Education –P?? Medtronic Sponsored Education –P?? Aortic Techniques & Technologies Consensus Update –P?? Vascular Access CX Masterclass –P?? Vascular Access Workshop –P?? Abdominal Aortic Aneurysm (AAA) Consensus Update –P?? Superfi cial Venous & Lymphatic Consensus Update –P?? Acute Stroke –P?? Aortic Workshop –P?? Thoracic Arch Consensus Update –P?? Thoracic Dissection Consensus Update –P?? Terumo Aortic Sponsored Education –P?? Chronic Limb Threatening Ischaemia (CLTI) Consensus Update –P?? CX Innovation Showcase –P?? Venous & Hurting Leg Workshop –P?? Coffee Tuesday 25 April 08:00 08:10 08:20 08:30 08:40 08:50 09:00 09:10 09:20 09:30 09:40 09:50 10:00 10:10 10:20 Kensington 1 Kensington 2 Admiral Workshop Wing Abstract Gallery Wednesday 26 April 08:00 08:10 08:20 08:30 08:40 08:50 09:00 09:10 09:20 09:30 09:40 09:50 10:00 10:10 10:20 Kensington 1 Kensington 2 Admiral Workshop Wing Abstract Gallery Thursday 27 April 08:00 08:10 08:20 08:30 08:40 08:50 09:00 09:10 09:20 09:30 09:40 09:50 10:00 10:10 10:20 Kensington 1 Kensington 2 Admiral Workshop Wing Abstract Gallery CX 2023 SLIM JIM-COVER-V4.indd 4-10
CX 2023 exhibitors and major sponsors A-Z
PLATINUM SPONSORS
10,000 people worldwide and the products are sold in more than 135 countries.
www.getinge.com/int/insights/ events/exhibitions/2023/cx
■ Inari Medical A15
EXHIBITORS
■ AOTI D32
■
Gore & Associates D24 & Hoxton Lounge
With more than 50 million medical devices implanted over the course of more than 45 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives. Gore is joined in service with clinicians and through this collaboration we are improving lives. www.goremedical.com
■ Medtronic B24
We lead global healthcare technology, boldly attacking the most challenging problems. Our Mission—to alleviate pain, restore health, and extend life—unites a global team of 90,000+ people, and our technologies transform the lives of two people every second, every hour, every day. Expect more from us.
Medtronic. Engineering the extraordinary. www.medtronic.com
GOLD SPONSORS
■ Concept Medical D28
Concept Medical, Inc., headquartered in Tampa, USA, specialises in developing drug-delivery platform technology and products for the treatment of coronary and peripheral arterial disease (PAD). Concept Medical has developed sirolimus-coated balloon with the help of its proprietary Nanolute technology like the Magic Touch percutaneous transluminal angioplasty (PTA) for superficial femoral artery (SFA) and below the knee (BTK) and Magic Touch arteriovenous fistula (AVF) for dysfunctional AVF/ arteriovenous graft (AVG). Concept Medical has recently been granted IDE approval for BTK indication for its Magic Touch PTA.
Visit Concept Medical at booth #D28 and watch the scientific session on 27th April (3:20–3:50pm) to know more about the sirolimus-coated balloon potential in PAD treatment. www. conceptmedical.com
■ Cook Medical C28
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today, we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver
better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: our patients, our employees and our communities. Visit our website to find out more, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
www.cookmedical.com
■ Terumo Aortic B28
At Terumo Aortic, we understand that no two aortas are alike. We are 100% focused on the aorta, from the arch to the iliacs. With our comprehensive portfolio of surgical, endovascular, hybrid and custom solutions, we help you address your patients’ unique challenges—so no patient is left behind.
terumoaortic.com
SILVER SPONSORS
■ Artivion D20
Artivion, Inc. is a medical device company focused on developing simple, elegant solutions that address cardiac and vascular surgeons’ most difficult challenges in treating patients with aortic diseases. Artivion’s four major product groups include: aortic stents, stent grafts, surgical sealants, mechanical heart valves, and implantable cardiac/vascular human tissues.
www.artivion.com
■ Bentley B20
Bentley’s passion is the development, manufacturing and distribution of innovative implants for minimalinvasive treatments of vascular diseases. Since market launch in 2012 we rapidly expanded worldwide. Thanks to our international network of exclusive distribution partners we are represented in more than 80 countries—in some we are already market leader.
www.bentley.global
■ Getinge F23
With a firm belief that every person and community should have access to the best possible care, Getinge provides hospitals and life science institutions with products and solutions aiming to improve clinical results and optimize workflows. The offering includes products and solutions for intensive care, cardiovascular procedures, operating rooms, sterile reprocessing and life science. Getinge employs over
Inari Medical, Inc. is a medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases. Inari has developed two minimally invasive, novel catheterbased mechanical thrombectomy devices—FlowTriever (pulmonary embolism [PE]) and ClotTriever (deep vein thrombosis [DVT]). The company purpose-built its products for the specific characteristics of the venous system and the treatment of the two distinct manifestations of venous thromboembolism (VTE): DVT and PE. www.inarimedical.com/int
■ Endovastec
F19
Lombard Medical Limited is focused solely on the minimally invasive treatment of aortic disease.
In partnership with MicroPort Endovastec, we can provide a broad product portfolio covering endovascular aneurysm repair (EVAR), thoracic endovascular aortic repair (TEVAR) and fenestrated EVAR (FEVAR), adding even more treatment options to the physician’s armamentarium.
To find out more, visit us in the Exhibition Hall. www.lombardmedical.com
■ Penumbra A19
Penumbra, Inc., headquartered in Alameda, USA, is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures and markets novel products and has a broad portfolio that addresses challenging medical conditions in markets with significant unmet need. Penumbra supports healthcare providers, hospitals and clinics in more than 100 countries.
www.penumbrainc.com
■ Shockwave Medical
E32
Shockwave Medical is a company focused on developing and commercialising products intended to transform the way calcified cardiovascular disease is treated. We aim to establish a new standard of care for medical device treatment of atherosclerotic cardiovascular disease through our differentiated and proprietary local delivery of sonic pressure waves for the treatment of calcified plaque, which we refer to as ‘Intravascular Lithotripsy.’
www.shockwavemedical.com
AOTI’s Nexa NPWT and TWO2 topical wound oxygen therapy are designed to increase access and compliance via in-home therapy. TWO2 is clinically proven to heal chronic wounds, reducing hospitalisations and amputations, leading to significant clinical, quality-of-life and cost saving benefits.
www.aotinc.net
■ BD B32
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. BD helps customers enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to healthcare.
eu.bd.com/emea-peripheralinterventions
■ BIBA MedTech Insights
Ground floor
BIBA MedTech Insights is a leading provider of market analysis services. We serve medical professionals and organisations in the medical device industry worldwide. Our research products include quarterly monitors, customised research, and tailored services.
www.bibamedtech.com
■ Boston Scientific F33
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for 40 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of healthcare.
www.bostonscientific.eu
■ Cordis C32
Cordis is a worldwide leader in the development and manufacture of interventional vascular technology, with a reputation for clinical acumen, training and services. For more than 60-years, Cordis has delivered revolutionary products to treat millions of patients.
www.cordis.com/emea/home
■ Cydar D38
Cydar Medical is a global cloud-based software company that provides an integrated solution for planning, navigation and review of surgical procedures using the power of artificial intelligence (AI) to augment a clinician’s decision making.
www.cydarmedical.com
11 BIBANews April 2023 CHARING CROSS SPECIAL EDITION CX 2023
AT-A-GLANCE GUIDE





www.shapemem.com LIT1138 Rev A Conforms to the anatomy with unmatched volume Beyond embolization Delivers unmatched volume Generates new healing possibilities INDICATION: The IMPEDE Embolization Plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. INDICATION: The IMPEDE-FX Embolization Plug is indicated for use with the IMPEDE Embolization Plug to obstruct or reduce the rate of blood flow in the peripheral vasculature. CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. The images are illustrative and do not represent the actual size of any products. Shape Memory Medical and IMPEDE are registered trademarks of Shape Memory Medical. IMPEDE® Embolization Plug IMPEDE-FX Embolization Plug
CX 2023 exhibitors and major sponsors A-Z contd.
■ CX Vascular
Ground Floor
CX Vascular is an online community site that delivers curated news and content, year-round education and key insights into the vascular landscape. It will also feature live talk shows and offer access to a state-of-the-art production studio in London, UK. We would welcome you to discuss partnering opportunities to produce roundtables, episodes, podcasts and more. Join the global CX Vascular community at www.cxvascular.com
■ CX Vascular Live Studio
Level 2
The CX Vascular Live Studio makes professional, compelling medical videos with editorial input from experienced medical journalists and a high-end production team in a bespoke studio environment. Contact nathalie@bibamedical.com for more information.
www.cxvascular.com
■ Dendrite Clinical Systems
G11
Vascular surgery databases, registries, MDT, clinical workflow and e-PROMs systems. Clinical data analysis, audit, research, benchmarking. www.e-dendrite.com
■ Frontier bio
G10
We are pushing the boundaries of tissue engineering to combat cardiovascular disease and improve the development pathway for vascular medical devices.
Our innovative technology enables the growth of living blood vessels in vitro, in various shapes and sizes, for applications in surgery and in device testing as alternatives to animals. www.frontierbio.com
■ iVascular
B36
iVascular is a fast-growing company founded in 2010 in Barcelona, Spain, with the aim of developing medical devices and therapies to improve patients’ quality of life. It is empowering the value of technology and innovation in the vascular field. Nowadays, iVascular has fulfilled the quality standards of more than 70 countries. www.ivascular.global
■ Laminate Medical
E38
Laminate Medical Technologies develops solutions for the most significant challenges preventing fistula use in dialysis patients. Laminate’s flagship product, the VasQ external support, is implanted around the arteriovenous anastomosis during


fistula creation and aims to address the high rate of primary failure and repeat procedures experienced by haemodialysis patients.
www.laminatemedical.com
■ NIHR Platform TRIALS TT
endovascular interventions strategies, with different devices designs. Digital twin technique is particularly relevant in planning complex and standard EVAR, where clinical outcomes are uncertain. predisurge.com
■ Scanlan International A25
Celebrating over 100 years and our journey continues...
■
LeMaitre Vascular F25
LeMaitre is a leading global provider of devices for the treatment of cardiovascular and peripheral vascular disease and for arteriovenous (AV) access. We manufacture and market disposable and implantable devices for vascular and cardiac surgeons. Our diversified product portfolio consists of well-known brand name products like the LeMaitre Valvulotomes, XenoSure biologic patches and Omniflow II vascular prosthesis.
www.lemaitre.com
■ LifeTech Scientific F27
Established in 1999, LifeTech Scientific Corporation (Stock Code: 1302.HK) is committed to the R&D, manufacture, and sales of minimally invasive interventional medical devices for cardio-cerebrovascular and peripheral vascular diseases. The company provides patients with innovative device solutions in the treatment of structural heart diseases, peripheral vascular diseases, and bradycardia, and the company also expands its business scope in respiratory interventional business, neurointerventional business and interventional oncology business. The company has over 1,500 highquality patents been filed, and the sales network has penetrated more than 100 countries and regions around the world.
www.lifetechmed.com/en
■ NeoLaser TT
NeoLaser is a world leader in design and manufacturing of surgical laser systems. Specifically, NeoLaser is at the forefront of endovenous laser ablation (EVLA) technology, offering the most advanced platform in the world for EVLA treatments, including the new NeoV1940 system and the revolutionary Infinite Ring fibre family for optimal safety and efficacy.
www.neo-laser.com
■ LSO Medical D40
LSO Medical is a French company specialised in the design and manufacture of Vascular Lasers for over 20 years. Serving patients is a privilege, for which we require the highest standards of quality and ethics. LSO Medical improves the quality of life of patients, by constantly optimising existing treatments.
www.lsomedical.com
The NIHR Health Technology Assessment Programme recently funded two ambitious research projects to develop adaptive platform trials, which will assess new vascular treatments and technologies. The two projects are named PAEDIS and VEIN; they will focus on peripheral arterial disease and venous disease, respectively. The projects are led by the Leicester and Imperial academic vascular units, with the support of three clinical trials units across the UK. We are planning to host a number of surveys, workshops, and trial development focus groups over the next seven months, online and face-toface. Based on this information, we will design two ambitious future trials in the relevant vascular disease areas. If you are a vascular healthcare professional, industry representative, or a patient with a vascular condition, please visit us at the Charing Cross Symposium or email at: as875@le.ac.uk; s.onida@ imperial.ac.uk; f.heatley@imperial. ac.uk. nihr.ac.uk/explore-nihr/fundingprogrammes/health-technologyassessment.htm
■ Philips D36
At Philips, we look beyond technology to the experiences of consumers, patients, providers and caregivers across the health continuum—from healthy living and prevention to diagnosis, treatment and home care. We unlock insights leading to innovative solutions that address the quadruple aim: improved patient experience, better health outcomes, improved staff experience, and lower cost of care. With leading research, design and innovation capabilities, we partner with our customers to transform the delivery of healthcare. www.philips.com
The highest quality surgical instruments designed and manufactured by the Scanlan Family since 1921. Experience the Scanlan difference at our exhibit A25.
www.scanlaninternational.com
■
Scitech C36
Scitech Medical is a minimally invasive medical device company that was founded over 20 years ago and is currently present in more than 45 countries. Its 6950sqm state-of-the-art CE 13485 certified facility is located in Brazil. Currently the company develops a wide portfolio of products for peripheral vascular, interventional cardiology etc.
www.scitechmed.com
■
Shape Memory Medical D38
Shape Memory Medical is reshaping clinical success through the science of smart polymer. Smart polymer upgrades device performance and redefines embolization possibilities. Our conformable smart polymer delivers unparalleled volume, returns imaging clarity, and promotes healing as the material absorbs. We continue to drive a cross-specialty portfolio to meet procedural demands.
www.shapemem.com
■ Starmed E40
The global leader in the thyroid radiofrequency (RF) ablation.
www.starmed4u.com
■
Pie / 3mensio Medical Imaging B38
Pie Medical Imaging is simplifying clinicians’ daily practice with the user friendly 3mensio vascular preoperative planning software; developed specifically for endovascular aneurysm repair (EVAR), thoracic endovascular aortic repair (TEVAR) and fenestrated EVAR (FEVAR) interventions. We cordially invite you to our booth during Charing Cross to test-drive 3mensio yourself.
www.piemedicalimaging.com
■ PrediSurge G12
PrediSurge develops innovative predictive software solutions for cardiovascular interventions. Based on pre-operative imaging, our technology enables the creation of patient-specific digital twins used to simulate different
■ Teleflex E36
Teleflex is a global provider of medical technologies designed to improve the health and quality of people’s lives. We apply purpose driven innovation—a relentless pursuit of identifying unmet clinical needs—to benefit patients and healthcare providers. Our portfolio is diverse, with solutions in the fields of vascular and interventional access, surgical, anaesthesia, cardiac care, urology, emergency medicine and respiratory care. Over 14,000 Teleflex employees worldwide are united in the understanding that what we do every day makes a difference.
www.teleflex.com/emea/en
■
TT
The University of Edinburgh
Study part-time for a Master of Surgery degree in Vascular & Endovascular Surgery completely online and gain extensive knowledge of the specialty.
13 BIBANews April 2023 CHARING CROSS SPECIAL EDITION CX 2023
AT-A-GLANCE GUIDE
CX 2023 exhibitors and major sponsors A-Z contd.

Improve your clinical decision-making skills through learning how to evaluate and apply evidence in your practice. Provides structured learning for those preparing for board exams. Contact chm.info@ed.ac.uk. edin.ac/3kNwa5e
■ Varixio A27
VB Devices develops unique medical devices that resolve practical clinical needs in vascular medicine. It was founded in Barcelona, Spain, in 2016 by vascular surgeon Enric Roche and biomedical entrepreneur Federico Grego. VB Devices’ main product is Varixio, an automated foam-preparation system for use in the treatment of varicose veins with sclerotherapy. The flexibility and versatility Varixio brings to foam sclerotherapy can greatly expand the applicability of this minimally invasive technique, making it possible to treat varicose veins of all sizes. www.varixio.com
■
Veryan Medical Ltd
A23
Veryan Medical, an Otsuka Medical Devices company, is committed to transforming the lives of patients suffering from peripheral vascular disease. The BioMimics 3D stent has a unique three-dimensional helical shape, designed to impart natural curvature to the femoropopliteal artery to promote swirling flow and elevate wall shear which are patency-protective. www.veryanmed.com
■ Wisepress Medical Bookshop
A30
Wisepress.com, Europe’s leading conference bookseller, attends around 200 conferences every year. We have an extensive range of books and journals relevant to the themes of this conference available at our booth. We also have a comprehensive range of STM titles available on our online bookshop. Follow us on Twitter @WisepressBooks. www.wisepress.com
■ Ziehm Imaging
A21
Since 1972, Ziehm Imaging has produced technologies that enhance imaging and streamline clinical workflows. Our devices’ exceptional image quality and flexibility in the operating room serve as an important basis for treatment success.
Ziehm Imaging is specialised in the development and manufacture of mobile C-arms. ziehm.com/en/home.html
Medical devices: Key updates on regulatory changes in Europe
Recent updates from the European Union’s (EU) Council of Ministers and the UK government are intended to mitigate, in part, the impact of the EU Medical Devices Regulation (MDR).

In early March, the EU’s Council of Ministers adopted a resolution to extend the deadline for the certification of medical devices under the MDR.
Producers of medical devices will have until 31 December 2027 for higher risk devices and until 31 December 2028 for medium and lower risk devices to meet the legal requirements.
The extension of the transition period will be granted under certain conditions, ensuring that only devices that are safe and for which manufacturers have already started the certification procedure will benefit from the additional time. The regulation—which changes the way that medical devices are certified for use in the European market—first came into effect in 2021 with an initial three-year transition period, having been delayed by one year in 2020 due to the onset of the COVID-19 pandemic. However, challenges in the implementation of the legislation led to concerns about a potential shortfall in the availability of certain devices, which prompted a rethink in the timetable for the regulation as proposed by the European Commission in December.
The adoption of the resolution by EU Council members, comprising ministers from each of the EU’s member states, means that the decision to extend the implementation period will enter into force on the day of its publication in the Official Journal of the EU
“We have agreed on measures that will allow the industry to continue bringing essential medical devices to the market and ensure that patients have safe access to medical devices,” Acko Ankarberg Johansson, Sweden’s minister for healthcare, was quoted saying.
MedTech Europe, which represents the continent’s device manufacturers, has welcomed the adoption of the amended transitional provisions, which it said will help mitigate the immediate risk that medical devices across all areas of medicine, which are still on the EU market, would no longer be available after May 2024.
“The amendment of the Medical Devices Regulations’ transitional provisions is a needed step forward to help ensure that more medical devices remain available to patients and healthcare systems across Europe. This decision grants Notified Bodies more time to complete certification of more than 500,000 medical devices and accelerates efforts to certify innovative devices in the pipeline,” said Oliver Bisazza, CEO of MedTech Europe.
As soon as the amendment comes into force, MedTech Europe said that alongside its members it will work toward its implementation according to the new provisions and extended deadlines. “In that regard, it is important that all stakeholders have an aligned and clear interpretation of the amendment, including the process for submitting applications to Notified Bodies, and how the extended validity of certificates can be concretely demonstrated,” the organisation’s statement adds.

UK government acts
In February, the UK’s Medical and Healthcare products Regulatory Authority (MHRA) shared in a press release that a CE-marked device can be placed on the UK market until 30 June 2023, but that “there are plans to extend acceptance of the CE-marking in the UK which we will put into law in the coming months. We will publish
guidance on this as soon as possible.”
More recently, His Majesty’s (HM) Treasury in the UK announced that a total of £10 million has been awarded to the Medicines and Healthcare products Regulatory Agency (MHRA)—an executive agency of the UK Department of Health and Social Care (DHSC)—to help bring innovative new medicines and medical technologies to UK patients more quickly.
The funding will be used to accelerate routes for bringing innovative medical products developed in the UK onto the market, as well as those made and approved by other trusted regulatory partners globally.
Over the next two years, the MHRA will use the money to support development of a thorough but shortened process to speed up the approval process for cuttingedge treatments developed in the UK with the greatest opportunity to meet the country’s healthcare priorities, such as cancer vaccines and artificial intelligence (AI)-based therapeutics for mental ill-health.
It will also support the establishment of an international recognition framework, allowing the MHRA to capitalise on the expertise and decision-making of trusted regulatory partners, and provide patients with fast-track access to best-in-class medical products that have been approved in other countries.
The MHRA will still be responsible for the approval of all ‘recognition route’ applications, ensuring that all products are of sufficient quality to be licensed in the UK, and it will operate a robust process promoting patient safety and access to improve the health of the UK population.
Using the agency’s pre-existing international partnerships, developed through the Access Consortium and Project Orbis, the first regulatory partners that the MHRA intends to build new recognition routes with are the Food and Drug Administration (FDA) in the USA and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan.
June Raine, MHRA chief executive, said: “We greatly welcome the £10 million funding announced by HM Treasury today, which will be used to fund our ongoing innovation work and to accelerate the development of ground-breaking global recognition routes, which will give UK patients faster access to the most cutting-edge medical products in the world.
“This cash injection will ensure that we have access to the best resources, talent, and infrastructure to deliver this ambitious vision for patients across the UK.”
Steve Barclay, UK Secretary of State for Health and Social Care, added: “Technology is transforming our care for patients, delivering faster and more accurate diagnoses. This new funding will accelerate the delivery of cuttingedge treatments like cancer vaccines and new artificial intelligence technology that will make therapy more accessible to those who suffer from mental health conditions.
“It will also fast-track access to medical products that have been approved in other countries by trusted regulatory partners, ensuring we continue to provide the best, most innovative and safest treatments in the UK.”
C M Y CM MY CY CMY K
AT-A-GLANCE GUIDE





INNOVATION EDUCATION EVIDENCE CONTROVERSIES CHALLENGES CONSENSUS CXSYMPOSIUM.COM Consensus Update Vascular & Endovascular 25–27 APRIL 2023 TUESDAY-THURSDAY IN PERSON AND VIRTUAL HILTON LONDON METROPOLE, UNITED KINGDOM OF LOOKING FORWARD 3 Full-day hands-on Vascular Access, Venous & Hurting Leg and Aortic workshops 250+ Presentations 170+ Expert Faculty members 3 Parallel streams of world-class Education, Innovation and Evidence 40+ Live and Edited Cases
Deep-dive roundtable discussions Peripheral Arterial Consensus Aortic Consensus Acute Stroke Consensus Vascular Trauma Consensus Venous & Lymphatic Consensus Vascular Access Consensus The Hurting Leg Consensus
2
Getting medical devices to market: The future might not be now, says regulatory expert
The development of medical devices in the vascular space faces increasing challenges in the face of moves afoot at the US Food and Drug Administration (FDA), according to a leading regulatory expert in the field.
AMONG THE DEVELOPMENTAL
vehicles currently caught in the crosshairs are physician-sponsored investigational device exemption (PSIDE) studies, Dorothy Abel, the former FDA official responsible for leading evaluation of vascular and endovascular surgery devices, recently told the 2023 Critical Issues America (CIA) annual meeting (10–11 February, Miami, USA).
She was giving the CIA keynote address, tackling the subject of endovascular grafts across the decades, their evolution and what lies in store for device development down the road.

Throughout, Abel, currently vice president of regulatory strategy at medical device research organisation NAMSA, emphasised the importance of collaboration during the process of shepherding new products toward commercialisation.
Developers should work together across the spectrum of interested parties—manufacturers, testing facilities, regulatory agencies—to establish the best bench-testing and animal study protocols, and what needs to be gleaned from the clinical setting, to get to requirements centered on a riskbased rationale for testing, she said.
This might entail looking at “what does the device need to be able to do; what can go wrong if it does not do that,
what type of testing do we need to help us believe that it is going to do what it is supposed to do,” explained Abel, who cofounded the FDA’s early feasibility studies programme.
She contrasted the difference in standards between what is required in the European Union (EU) and the USA. “In the USA, it is kind of a guidance—it helps FDA understand what should be done,” Abel said. “But in the EU, they take it very seriously,” she added. “If it is written in a standard as something that needs to be done, you are going to have to do it in order to get the device to market.”
Abel posed the overarching question—“Is the future now?”—of the medical device development landscape, answering herself: “I am kind of hoping that it is not.” Her reasoning? Those challenges that potentially limit PS-IDEs.
“The FDA has recently shut down the opportunity for doing some sponsorinvestigator IDEs,” Abel said. “We do not know yet what their thinking is; whether that is going to apply to all IDEs in this space; or if it is going to be focused on particular manufacturers, or types of devices. But, it is a little bit concerning, because we want to make sure people continue to study these devices responsibly.”
Short neck AAAs:
Navigating cases of abdominal aortic aneurysm (AAA) with short neck anatomy pose a serious challenge to vascular surgeons, but the list of potential options available to carry out repairs are numerous—and decision-making can be optimised by using a bespoke algorithm.
THAT WAS THE MESSAGE DELIVERED by Ross Milner (University of Chicago Medicine, Chicago, USA) in a session on endovascular aneurysm repair (EVAR) controversies at the Critical Issues America (CIA) annual meeting (10–11 February, Miami, USA).

Milner outlined the four core pathways open to surgeons when they are confronted with short necks—
Right now, the FDA is focused on devices from a protection standpoint, with the agency often taking a sceptical view of new technology, Abel observed. “What is even more concerning, to go along with that scepticism, is there is a little less communication. They have almost doubled the size of postmarket studies as compared to the premarket studies in the most recent approvals, and it is not clear exactly what their fear is.”
The focus needs to be on patients, she said. “I am afraid that has gone away a little bit. Because there is so much focus on safety, they are not thinking about, ‘We need better things to take care of our patients in general’.”
From another angle, Abel highlighted how blame is apportioned when things go wrong.
“Sometimes we are asking the wrong questions,” she said, referring to the example of ruptures occurring in patients fitted with endovascular devices. “Why did they have the ruptures? Were they not being followed? Is it really the endograft’s fault? Is it your fault? Is it the patient’s fault? But the FDA automatically goes, ‘It is the manufacturers who need to collect more
data to figure out what is going on because we have these ruptures’.”
Investors and developers too are not beyond criticism, Abel continued. “Bad things can happen to potentially ruin good devices, and some of those bad things come from assumptions,” she said.
In order to ensure a successful future in the device development field, Abel returned to the concept of collaboration. And that also includes patients, she said. In the past, Abel recalled, “we kind of fell short. We did not have the patients involved early on. I think we need the patients involved.” Shooting for the stars is laudable, she said, but “be happy to take some baby steps—they keep you moving forward.” Also be realistic, Abel advised. “Often we see timelines driven by investors.” And, whenever possible, be conservative, she added. “If you have a way to test something in a less risky population, why not do that first so you can de-risk the future.
“The goals are to solve problems, improve lives, reduce harm. In order to avoid that, you want to know about the field that you are playing in. You need to know about regulatory, patient safety, study design, technology adoption. This is not for the faint of heart.”
Learning not only from mistakes, but also successes is important, Abel concluded. In the case of the latter, she drew attention to one in particular: “I think one of the big successes in endografts has been the sponsorinvestigator studies. We have learned so much—I know it is over 30 years—but it is a relatively short amount of time when you think about medicine and the complexity of what is being done. And, cooperation is key.”
defined as greater than 4mm and less than 10mm: open repair, fenestrated EVAR (FEVAR) or physicianmodified endografts (PMEGs), off-label chimney EVAR (ChEVAR), and endosuture aneurysm repair (ESAR).
In short, he said, the treatment ultimately elected should be dictated by the individual patient.
“I think in the past you have seen a lot of talks that are designed as debates between one of these techniques, and we commonly joke about different techniques not working well,” he told CIA 2023.
“The reality is, when you are looking at each one of your patients, and you are sitting in the office, and trying to make a decision, I think we incorporate all of these options.”
Milner uses his algorithm, or decision tree, in
order to help establish which approach is going to best serve the patient in front of him. This includes figuring out the patient’s physiologic risk, the level of urgency of a procedure, durability based on risk profile/age, the direction of the renal arteries and the state of the aortic arch anatomy.
“When I look at the anatomy, the things I look at from an anatomic standpoint are: how do the renal arteries come off the aorta?” he said. “At least in my hands, I find that for chimney approaches, downwardgoing renal arteries are easier. For upward-going, fenestrated is easier. But again, in different people’s hands, it is probably different. I also look at the anatomy of the neck and the tortuosity. If you are going to think about ChEVAR, you look at the aortic arch.”
Open repair can be valuable in the right set of
Continued over page
17 BIBANews April 2023 CHARING CROSS SPECIAL EDITION Conference Highlights
You need to know about regulatory, patient safety, study design, technology adoption. This is not for the faint of heart.”
“Choosing what is best for the patient”
AORTIC
Ross Milner
Dorothy Abel
AORTIC
Continued from page 17
patients, Milner continued, but is “highly invasive” and “the risk of renal failure with open surgery is greater than with complex EVAR, and likely to leads to worse outcomes.”
In his practice, Milner and colleagues do not perform procedures with PMEGs but rather on-label FEVARs. “It is FDA approved, it is all transfemoral, it is indicated for 4mm neck, but it can be limited by anatomy, and sometimes the device just cannot be made,” he explained.
ChEVAR, meanwhile, has performed “relatively well” for his team in the treatment of complex anatomy, Milner said. “Its limitations are gutter leaks and stroke risk.”
Then there is short neck ESAR, he added, which incorporates EndoAnchors with an Endurant stent graft (Medtronic).
Overall, said Milner, his decision tree breaks down to, first, physiologic risk. “If it is low, consider open surgery,” he said. “If high, go with an endovascular
approach. For FEVAR, I look at iliac access, the direction of the renals, the neck tortuosity, the urgency of the procedure; for ChEVAR, again, iliac
Study highlights need for “continuous comparative assessments” to guide endograft treatment decisions

A recent study comparing outcomes of endovascular aneurysm repair (EVAR) patients has reported no statistically significant differences in mortality or secondary rupture rates between standard Cook, Medtronic and Gore endografts, suggesting similar safety in a real-world setting.
MICHAEL O FALSTER (CENTRE for Big Data Research in Health, Sydney, Australia), Ramon L Varcoe (Prince of Wales Hospital, Sydney, Australia) and colleagues report this and other outcomes from a large population-based observational study in the European Journal of Vascular and Endovascular Surgery (EJVES). Another key finding was that rates of subsequent aneurysm repair were higher for endografts other than Cook devices, but only statistically significant for Medtronic devices.
In order to compare rates of all cause death, secondary rupture and secondary intervention in their retrospective cohort study, Falster, Varcoe et al used linked clinical registry (Australasian Vascular Audit [AVA]) and all payer administrative data from patients undergoing EVAR for intact abdominal aortic aneurysm (AAA) between 2010 and 2019 in New South Wales, Australia.
The authors identified 2,874 eligible EVAR patients, with a median followup of 4.1 years and a maximum followup of 9.5 years.
Writing in EJVES, Falster, Varcoe and colleagues report similar mortality rates for patients receiving different devices, ranging between 7 and 7.3 per 100 person years. In addition, they reveal that there was no statistically significant difference between devices
in secondary rupture rates, which ranged between 0.4 and 0.5 per 100 person years.
Furthermore, Falster, Varcoe et al note that patients receiving Medtronic and Gore devices tended to have higher crude rates of subsequent aneurysm repair (1.5 per 100 person years) than patients receiving Cook devices (0.8 per 100 person years). This finding, the authors write, remained in the adjusted analysis, but was only statistically significant for Medtronic devices (hazard ratio [HR] 1.57, 95% confidence interval [CI] 1.02–2.47; HR 1.73, 95% CI 0.94–3.18, respectively).
“Major endograft devices have similar overall long-term safety profiles,” the authors summarise in their concluding statement. However, they add, there may be differences in rates of secondary intervention for some devices. “This may reflect endograft durability, or patient selection for different devices based on aneurysm anatomy,” Falster, Varcoe and colleagues posit.
According to the authors, this population-based study is one of the largest to compare outcomes of EVAR patients receiving contemporary endografts from different manufacturers.
In the discussion of their findings, the investigators note that there are no studies that have randomised patients
access, the direction of the renals, neck tortuosity and the aortic arch anatomy; for ESAR, calcification and thrombus, and reverse taper of the neck.”
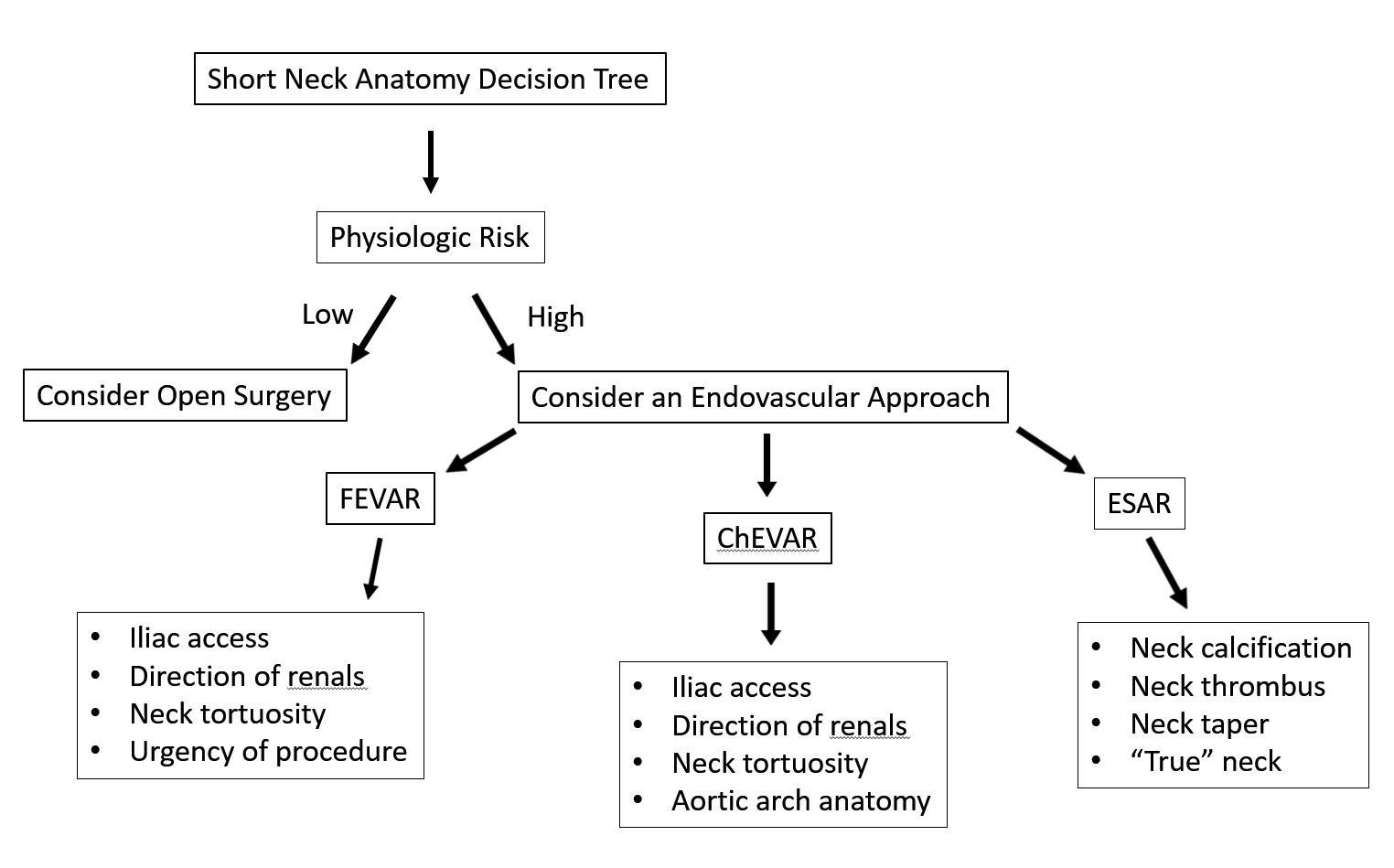
Responding to skepticism over the ESAR procedure, Milner acknowledged controversy centres on a central question: “What is the true neck? And all of us know that a 4mm neck is exceptionally challenging to use EndoAnchors on. I know that this is written as an indication, but that is hard to do,” he said, concluding: “Many options exist; choose what is best for your patient.”
to endograft type to evaluate long-term mortality and secondary intervention rates. Given this absence, Falster, Varcoe et al suggest, observational studies “have a place” in providing “complementary” evidence for postmarket outcomes and are “well positioned to address the evidence gap around emerging technologies”.
The key strength of the present study, the authors claim, was the use of linked clinical registry and population-level administrative data. “This study is one of the largest to have explored outcomes for patients receiving different endografts, and also one of the only studies to have examined a contemporary cohort of patients to directly compare outcomes of endografts in current use,” Falster, Varcoe et al add.
The authors underscore “a need for ongoing surveillance of patient
modification or withdrawal from the commercial market entirely”.
The investigators stress that regulators such as the US Food and Drug Administration are “acutely aware” of the importance of postmarket data to assess the safety of device and encourage surveillance programmes. The present study, Falster, Varcoe et al claim, “demonstrates that linkage of clinical registry and administrative data can strengthen the evidence to help fill the inevitable gaps which occur when [randomised controlled trials] do not exist or are unethical to perform”. They continue: “Increased capacity within clinical registries to identify specific endograft devices, as well as indication for device choice, will further enable this comparative work.”
Considering the limitations of their study, Falster, Varcoe and colleagues note that the drawbacks of administrative data are “well known,” referencing the fact that the present linkage rate of 82.7% between the AVA and administrative datasets means that findings “may not be representative of all EVAR patients in [New South Wales]”.
outcomes to compare and evaluate different devices used in clinical practice” to ensure those grafts are “providing the highest levels of safety and efficacy, with identification of those that are not”. Falster, Varcoe and colleagues opine that the history of endovascular surgery is “littered with examples of underperforming endografts,” which after evaluation were “identified and managed by either temporary pause for device
The investigators stress that their results must be “interpreted with some caution” given the small number of patients receiving some endografts, the relative scarcity of some of the endpoints (e.g. aneurysm rupture) and that the bias which may result from individual surgeons’ subjective device selection methods and the potential for unmeasured confounders has not been eliminated from the study.
“Continuous comparative assessments are needed to guide evidence for treatment decisions across the range of available devices,” Falster, Varcoe et al write in their closing remarks.
18 BIBANews April 2023 CHARING CROSS SPECIAL EDITION Journal Highlight
This study is one of the largest to have explored outcomes for patients receiving different endografts.”
Ramon L Varcoe
Short neck anatomy decision tree




Join the CX Vascular platform today to connect and engage with the global vascular community, share expertise and experiences, and stay up-to-date with the latest education and news in the vascular world. Members will also have access to exclusive content including live discussions on the latest advances in the vascular field and interviews with key thought leaders in the space. Register now Education, News, Insights Join the community at https://cxvascular.com
A Year in Profiles
The last year of Vascular News profiles has seen key leaders in the field share important lessons and advice based on notable careers. This overview of their individual in-depth interviews is an annual look back at their insights and experiences.
Alexander Zimmermann
(University Hospital Zurich, Zurich, Switzerland) spoke to Vascular News last summer about his career to date. A rotation in vascular surgery during a residency opened up “a whole new world of medicine” to his directorship of the Department of Vascular Surgery at the University of Zurich.
Alexander Zimmermann
Issue 94/June 2022
“During my childhood, it was always my wish to become an astronaut,” Zimmermann began, which he said later led to the idea of studying aerospace engineering. However, since he was deployed in the medical service of the German Armed Forces during compulsory military service after school, he got to know the “variety and fulfilment” of the medical profession. “This is how my enthusiasm for medicine developed,” he said, which consequently led to the decision to study medicine instead of aerospace engineering.
From the beginning, Zimmermann told Vascular News, it was clear to him that he wanted to pursue a surgical specialty. “This was originally plastic surgery since here one could immediately see the results of one’s manual work,” he noted. During a residency in plastic surgery, however, a vascular surgery rotation opened up a “whole new world of medicine” for him. He explained: “Endovascular therapy, which was still in its infancy at the time, combined with the possibility of open surgical therapy and the higher meaning of the subject, captivated me from the beginning.” This led to a change in his career aspirations and the continuation of his specialist training in vascular surgery. “A coincidence that I have never regretted to this day,” he remarked.

When considering the topic of mentorship, Zimmermann commented that he only had one mentor, at the beginning of his clinical career in visceral surgery—Ulrich Frank. “[He] took care of me in word and deed,” Zimmermann recalled. In the further course of his training, however,
Zimmermann noted that he was “not granted this good fortune again”. “This experience gave me the deep desire to be able to act as a mentor for the junior staff working in my department. Whether I succeed in this, however, is a matter for other people to judge.”
Despite this, Zimmermann stated that some people have had a lasting influence on his career path. In the field of surgery in general, this was Jörg Rüdiger Siewert. “His visionary thinking and high level of organisation were very impressive,” Zimmermann remarked. In vascular medicine, he added, he views Stéphan Haulon, Tim Resch, and Eric Verhoeven as “inspiring personalities”.
Zimmermann started his clinical career in early 2000, which, he noted, is when endovascular aortic therapy started to become available nationwide in major vascular surgery centres. “The development of this form of therapy still leaves me speechless today,” he said. “Whereas in the early 2000s conventional EVAR [endovascular aneurysm repair] was still considered a complex endovascular aortic procedure, these procedures are now performed percutaneously and on an outpatient basis.”
In this context, Zimmermann has been “particularly fascinated” by two developments: “first, the development of off-the-shelf thoracoabdominal stent grafts, which are immediately available to patients in urgent cases and emergencies, thereby providing access to therapy to a much larger patient population due to the fact they are far less invasive. In this context, I would also like to mention physician-modified stent grafts, as these continue to inspire me with their variability in application.”
Zimmermann also recalled his most memorable case during the interview—the first physicianmodified fenestrated endovascular aneurysm repair (FEVAR) procedure he ever performed.
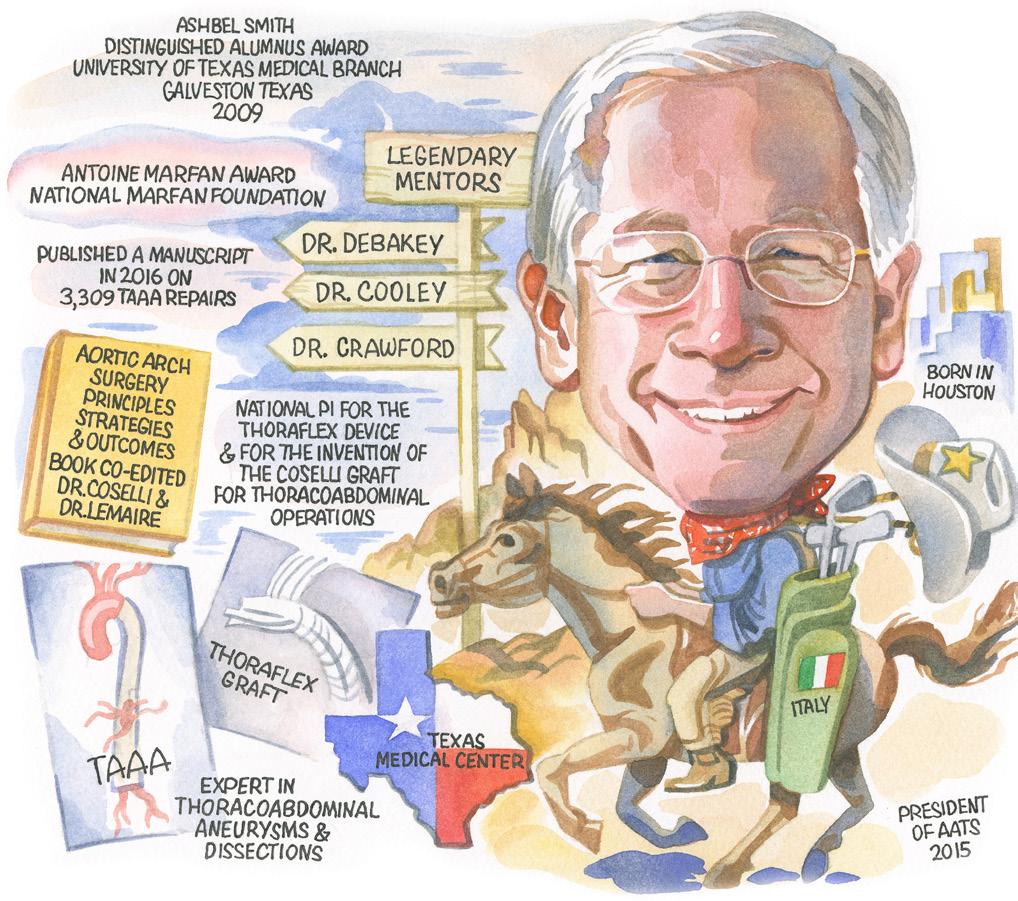
Issue 95/September 2022
Despite initially planning to follow in his family’s footsteps and attend law school, a summer internship at the Texas Heart Institute changed the course of Joseph S Coselli’s (Baylor College of Medicine, Houston, USA) career forever. Speaking to Vascular News last September, he noted that the internship sparked an interest in cardiovascular surgery that would culminate in his current roles as professor of Surgery and executive vice chair of the Michael E DeBakey Department of Surgery at Houston’s Baylor College of Medicine, among others.
Coselli has received widespread recognition for his work in cardiovascular and aortic surgery, and credits his success in large part to the important role of three celebrated mentors—Michael E DeBakey, Denton A Cooley and E Stanley Crawford.
Considering the cardiothoracic field more generally, Coselli commented that the development of numerous minimally invasive and endovascular procedures has been the most significant advancement in cardiothoracic surgery during his career thus far. He listed specifically the impact of abdominal EVAR, descending thoracic endovascular aortic repair (TEVAR), replacement of the aortic valve with a transcatheter aortic valve (TAVR), and, most recently, hybrid open and endovascular aortic devices such as Thoraflex from Terumo Aortic.
While Coselli said that he has not had any major disappointments over the course of his career, he did note one minor defeat in the 1980s. He recalled that, at this time, he and his team pursued the use of laser therapy to open occluded arteries (such as the iliac, superficial femoral and tibial arteries, among others) and it “did not evolve into the spectacular development that we had hoped for at the time”. More recently, however, he noted that similar advancements have been made in opening medium and small vessels.
Cardiothoracic surgery has several “substantial challenges,” Coselli commented. “First, there is an educational challenge.
The complexity of cardiac surgery is increasing, as has its pool of related information. Simply put, the fountain of knowledge has exploded. Organising this into an educational format to prepare future cardiothoracic surgeons remains a daunting task,” he detailed. In addition, he noted that cardiovascular research has been underfunded, for many decades. “There is no question that there are enormous needs in the area of research. Clinically, cardiothoracic surgery is becoming increasingly challenging due to extensive pathology such as infection (i.e. endocarditis), advanced age (i.e. our population is ageing), and increasing patient morbidities,” he said. Against this backdrop, it is Coselli’s belief that partnership with industry will be necessary to address these concerns, particularly from a standpoint of minimally invasive approaches.
20 BIBANews April 2023 CHARING CROSS SPECIAL EDITION Interviews PROFILES
I believe that minimally invasive and endovascular therapies will continue to evolve and advance.”
The development of [endovascular aortic] therapy still leaves me speechless today.”
Joseph Coselli
Steinbauer
Issue 96/November 2022
Following his 2021–2022 presidency of the Vascular Society of Great Britain and Ireland (VSGBI), Jon Boyle (Cambridge University Hospitals NHS Trust, Cambridge, UK) spoke to Vascular News earlier this year about his career so far.

Despite his initial intention to become a transplant surgeon, Markus Steinbauer (Krankenhaus Barmherzige Brüder, Regensburg, Germany) recalled in an interview with Vascular News last November how the rise of endovascular techniques, and the possibilities for procedural advancement they posed in vascular surgery, led him to change career course. Steinbauer is now head of the Department of Surgery at the Krankenhaus Barmherzige Brüder and past president of the German Society of Vascular Surgery (DGG). In the interview, he considered how the field of vascular surgery has changed over the course of his career and anticipated future challenges vascular surgeons might face— including those associated with an ageing population.
Steinbauer spoke on his research interests, noting that he is currently researching vascular graft infections, open surgery in chronic limb-threatening ischaemia and peripheral occlusive disease
Jon Boyle
Issue 97/February 2023
He also shared his thoughts on the future of vascular surgery, anticipating that the management of peripheral arterial disease (PAD) will become a more prominent part of vascular practice due to an ageing population and increased prevalence of diabetes.
Boyle was the clinical lead for the UK National Vascular Registry from 2017–2022, and gave an overview of his time in this role. “I felt it was important, following our experience with EVAS [endovascular aneurysm sealing], that we started to collect aortic device-specific information within the NVR,” he said. “This work was extensive and required collaborating with many stakeholders, and also working with the National Joint Registry team leaders in registry device capture. The aortic device dataset went live in July 2020 and is recognised as an international exemplar.”

Furthermore, Boyle informed Vascular News readers that he led the team responding to the vascular Getting It Right First Time (GIRFT) report in developing the PAD-Quality Improvement Framework (PAD-QIF) and the PAD-Quality Improvement Programme (QIP). He detailed that he obtained funding for two PADQIP research fellows supported by the Circulation Foundation, VS and Royal College of Surgeons of England.
In addition, Boyle led the introduction of
COVID-19 fields to the NVR in April 2020, shortly after the start of the pandemic. “This early addition of vital data fields has enabled a good understanding of the impact of COVID-19 on vascular patients to be gained and a number of reports and research publications,” he commented.
In 2020, the NVR team won the Healthcare Quality Improvement Partnership (HQIP) team of the year report. HQIP stated that ‘the National Vascular Registry team showed considerable initiative in looking beyond the audit to consider innovations to benefit patients. The nomination demonstrated outstanding work on registry device capture, quality improvement and a rapid response to COVID-19,’ Boyle reported.
Boyle detailed that one of his most memorable cases involved being asked to see a ruptured thoracoabdominal aortic aneurysm in a man who was an inpatient at a non-arterial hospital.
“I arranged his transfer to Cambridge [UK] on a Friday afternoon, a TEVAR [thoracic endovascular aortic repair] graft to be delivered from a London hospital and then fixed him on Friday night. This was the first TEVAR rupture treated in Cambridge; the patient survived and lived for many years,” he recounted.
Boyle also had some advice for anyone looking to start a career in medicine. “Medicine has been a very enjoyable career for me over the last 31 years,” he said towards the end of the interview, adding that, although the pandemic and lack of resources have “cast a shadow over the last couple of year,” he as “no doubt medicine will continue to be a thoroughly rewarding career”.
(POD), imaging, and endovascular aortic treatment.
Considering the wider field of vascular surgery research, Steinbauer said that the 2022 paper with “highest relevance for our clinical work in the future” might be ‘Long-term cardiovascular outcomes of COVID-19’ by Yan Xie et al. This was published in February in Nature Medicine. The authors describe an increase in the long-term risk and burden of cardiovascular diseases in COVID-19 patients. We have to elucidate the underlying mechanisms for these late vascular complications and find new solutions for prevention.
However, he added that the paper that interested him the most in 2022 was ‘Neuroimmune cardiovascular interfaces control atherosclerosis,’ by Sarajo Mohanta et al, published last April in Nature. The authors describe a new mechanism of the development and progression of atherosclerosis—a direct interaction of the peripheral nervous system with diseased arteries by neuroimmune cardiovascular interfaces (NICIs). “This might be highly relevant for the POD and diabetic foot patients in the future and might give us the opportunity to develop therapeutic concepts including new surgical approaches,” Steinbauer said.
Looking back at his presidency of the Germany Society of Vascular Surgery, Steinbauer relayed that his personal highlight was the organisation of the “very successful and happy” annual meeting (Jahrestagung) of the German Society of Vascular Surgery in October 2021 under COVID-19 conditions—the first hybrid Jahrestagung of the DGG. “The revival of an onsite congress with cordial meetings and discussions with friends was the best event of my presidency,” he said. Furthermore, he remarked that he “deeply enjoyed the multifaceted and trustworthy conversations/meetings” with DGG members and colleagues and the “tremendous input and devotion” they gave to the development of the society.
Steinbauer also considered how the field of vascular surgery might change in the future. “I would like to see further development in artificial intelligence and imaging,” he said, “for example by mixed virtual reality to improve endovascular treatment modalities and reduce radiation.”
21 BIBANews April 2023 CHARING CROSS SPECIAL EDITION Interviews
I felt it was important, following our experience with EVAS, that we started to collect aortic device-specific information within the National Vascular Registry.”
I would like to see further development in artificial intelligence and imaging.”
Markus
“Urgent action needed” to improve implementation of guideline-directed medical therapy for PVI
Almost one-half of the patients receiving a peripheral vascular intervention (PVI) in the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) registry between 2017 and 2018 were not on guideline-directed medical therapy (GDMT). Kim G Smolderen and Carlos Mena-Hurtado (both Yale University School of Medicine, New Haven, USA) with their Vascular Medicine Outcomes (VAMOS) team report this and other key findings from a recent study in JACC: Cardiovascular Interventions.

The researchers note there has been a “rapid rise” in the volume of PVIs over the last decade, which stands in contrast to what they describe as “clinical inertia” for implementing GDMT in peripheral arterial disease (PAD). The team hypothesised that a lack of GDMT in patients undergoing PVIs may increase mortality and amputation risk and therefore sought to study the association between GDMT and mortality/amputation and to examine GDMT variability among providers and health systems.
Smolderen et al performed an observational study using patients in the VQI registry who underwent PVI during the one-year study period, deriving two-year all-cause mortality and major amputation data from the Medicare claims database. The investigators defined compliance with GDMT as receiving a statin, antiplatelet therapy, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker if hypertensive. They applied propensity 1:1 matching for GDMT versus no GDMT and performed survival analyses to compare outcomes between groups.
The team identified 15,891 patients (mean age, 72) who underwent a PVI in the 2017–18 period, specifying that 48.8% received GDMT and that 6,120 patients in each group were matched. They note that median follow-up was 9.6 months for mortality and 8.4 months for amputation.
Writing in JACC: Cardiovascular Interventions, Smolderen and colleagues report that mortality risk was higher among patients who did not receive GDMT versus those on GDMT (31.2% vs. 24.5%), as was risk of amputation (16% vs. 31.2%). GDMT rates
across sites and provider ranged from 0% to 100%, the authors add, noting that lower performance translated into higher risk. In their conclusion, Smolderen and colleagues summarise that one-half of the patients receiving PVI do not receive optimal GDMT and face an almost 40% increased risk of mortality, and an almost 20% increased risk of major amputation in the two years following their procedure. Speaking to the fact that GDMT rates were “highly variable” across sites and providers, they suggest this highlights “modifiable” rates and comment that “performance can be improved”.

“Offering PVIs without optimal GDMT is a low-value proposition for the patient, their families, health systems, and society at large,” the authors write. In light of this, they stress that “urgent action is needed to ensure the delivery of high-value PAD care as part of the interventional pathway”.

Considering what form this action might take, Smolderen et al state that, as part of the “PVI pathway,” national quality and reporting systems should be developed and tested to improve GDMT rates in patients with PAD.
The authors acknowledge some limitations to their study, including the fact that they limited quality metrics to three medications only, and that there is the “potential for unmeasured residual confounding” in the study due to its observational nature.
In an editorial comment, also published in JACC: Cardiovascular Interventions, Connie N Hess and Marc P Bonaca of the University of Colorado School of Medicine (Aurora, USA) write that Smolderen
New VOYAGER PAD analysis confirms
consistent benefit of low-dose rivaroxaban plus aspirin following lower extremity revascularisation
DATA FROM A NEW
prespecified analysis of the phase III VOYAGER PAD clinical trial show that low-dose rivaroxaban plus aspirin resulted in a 33% reduction in acute limb ischaemia and a 15% reduction in major adverse limb and cardiovascular events, with or without dual antiplatelet therapy (DAPT).
The data were presented at the American College of Cardiology’s 72nd annual scientific
session (ACC.23; 4–6 March, New Orleans, USA).
The new findings reinforce the benefits of the Xarelto (rivaroxaban; Janssen Pharmaceutical Companies of Johnson & Johnson) vascular dose (2.5 mg twice daily plus aspirin 100 mg once daily) over standard of care (aspirin alone), demonstrating consistent benefit at 30 days, 90 days and up to three years following lower extremity revascularisation in patients
and colleagues’ study “adds additional confirmatory evidence of the persistent gap between guidelines and real-world treatment”.
However, they also stress that the observations of the analysis with respect to outcomes should be interpreted with caution. They elaborate that while randomised clinical trials have demonstrated that the individual components of GDMT can reduce major adverse cardiovascular events (MACE) in patients with vascular disease, including PAD, none of these therapies has been proven in randomised trials to reduce all-cause mortality or major adverse limb events (MALE). This, they write, makes it “difficult to infer a causal relationship between GDMT and improved survival and freedom from amputation”.
They deem Smolderen et al’s study an “impressive effort,” although stress that the analysis “illustrates the complementary nature of randomised trials and observational investigations,” labelling the former the “gold standard” for assessing the actual efficacy and safety, and the latter “necessary to understand use in practice and to generate additional hypotheses for further testing in trials”.
The study, Hess and Bonaca conclude, provides a “sobering reminder of the continued gap in PAD care that will continue to persist without better data and the new focus on implementation”. Clinical care “remains paramount,” they say, noting however that there is a need for “accelerated action” to speed up the “glacial pace” of translation of evidence to practice. Newer medical therapies for PAD are already available, they stress, but in terms of implementation, it is “time to step on the gas”.
with peripheral arterial disease (PAD).
“These data demonstrate an evolution in the medical therapy of patients undergoing lower extremity revascularisation for symptomatic [PAD],” said Marc P Bonaca (University of Colorado Anschutz Medical Campus, Aurora, USA), lead study author of the VOYAGER PAD analysis. “We hope these data assist clinicians in understanding how to implement antithrombotic therapy in practice and overall support initiation of rivaroxaban in the first days after revascularisation regardless of whether or not DAPT is utilised.”
Those treated with Xarelto plus aspirin after lower extremity revascularisation saw a 33% reduction in acute limb ischaemia, with a trend toward greater benefit observed early (≤30 days hazard ratio [HR]=0.45;
95% confidence interval [CI] 0.24–0.85) vs. late (>90 days HR=0.75; 95% CI 0.60–0.95).
Xarelto plus aspirin was more effective than antiplatelet therapy alone in preventing acute limb ischaemia after lower extremity revascularisation (Kaplan-Meier estimate from 0 to 90 days 1.02% vs. 2.10%, respectively, and 4.3% and 5.7% from 91 days to three years).
The hazard ratio for the rate of thrombolysis in myocardial infarction (TIMI) major bleeding at 0 to 90 days was HR 2.01 (range 0.9–4.47) and from days 91 up to three years was HR 1.28 (range 0.82–1.99), neither of which were statistically significant.
Janssen Pharmaceutical Companies note in a press release announcing the new results that the Xarelto vascular dose is the first and only approved anticoagulant for PAD.
22 BIBANews April 2023 CHARING CROSS SPECIAL EDITION New Data PERIPHERAL
Offering peripheral vascular interventions without optimal guidelinedirected medical therapy is a low-value proposition for the patient, their families, health systems, and society at large.”
Carlos Mena-Hurtado
Kim Smolderen

© 2023 Shockwave Medical Inc. All rights reserved. SPL- 68027 Rev. A Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events www.shockwavemedical.com Scan for more information UNIQUE MECHANISM OF ACTION is changing the game in PAD treatment Scan QR code to learn more
Top priority: Raising the profile of lower limb amputation research
The UK government’s National Institute for Health and Care Research (NIHR) recently announced it has commissioned a trial in through-knee amputation—a move that has brought into sharp focus the need for research in this area and, it is hoped, will act as a catalyst for change.

According to Robert Hinchliffe, professor of vascular surgery at the University of Bristol in Bristol, UK, and chair of the Vascular Society of Great Britain and Ireland (VSGBI)’s Amputation Special Interest Group (SIG), amputation research has not been a high priority for vascular surgeons in recent years.
Dan Carradice, vascular surgical specialty lead for the Royal College of Surgeons of England based at Hull University Teaching Hospitals NHS Trust in Hull, UK, agrees, stating that, while amputation “does not receive the same attention or investment as revascularisation or limb salvage,” it is “absolutely no less important”. In fact, “in some patients, attempted limb revascularisation is either futile or not associated with salvage of a functional limb and amputation surgery can yield better patient outcomes,” he points out. “I think there is a tendency for surgeons to feel that when they reach the point of an amputation, it is almost a surgical failure,” Carradice explains. “The interest seems to get lost.”
This point of “failure,” however, marks the start of the next significant part of someone’s life following amputation, vascular surgeon George Smith (Hull University Teaching Hospitals) highlights, marking the stage at which a patient’s lifestyle will be “completely turned on its head”. Carradice underlines the crux of the issue: “We struggle to move past the paradigm that success looks like a patient who survives with two legs, and for many patients this is the case. However, we must accept for others
successful treatment looks very different.”
Shigong Guo, a consultant in rehabilitation medicine at the North Bristol NHS Trust in Bristol, UK, notes that this failure “mindset” has changed “significantly” in recent years and is continuing to do so. “Many vascular surgeons I work with today would see (unavoidable) amputation surgery as a pivotal first step in a patient’s successful rehabilitation journey,” he tells Vascular News
have done everything you can, you have used the best evidence base that you possibly can to treat the patient up to the point where, despite your best efforts, they do require an amputation, that is not the point to lose interest. You still have a significant role to play in the future of that patient by getting the amputation right and giving them the best possible future.”
“There shall always be some amputations that are unavoidable,” he précises.
Carradice notes that it is not uncommon for a patient to express their wish that they had agreed to amputation much sooner, pointing out that “different patients require different solutions”. He opines that clinicians are doing themselves and a significant group of patients a “disservice” in not accepting that amputation surgery may lead to the best outcomes in some situations. A focus on research and clinical improvement in this area, he says, “need not detract from our mission to prevent limb loss in other circumstances”.
While Smith believes clinicians’ focus should be on avoiding having to do an amputation where possible, stressing that anything they can do to preserve a functional limb “should be the first choice,” this principle should not get in the way of providing good care for the patients who do require an amputation.
“I think regardless of how good we get, certainly for the foreseeable future there will be a need for amputations,” he believes, highlighting a clinical need to minimise the impact of an amputation, which is going to be a “hugely life-changing event” for the patient. This is a message Smith is keen to pass on to the next generation of vascular surgeons. “If you
Saving limbs is rightly an “essential” focus of research, physiotherapist Hayley Crane (Hull University Teaching Hospitals NHS Trust) adds, noting that in fact it was recently ranked as the top research priority for amputation surgery by the James Lind Alliance priority research setting project. However, she stresses that it is still the case that thousands of people in the UK require major lower limb amputation every year. “The priority for physiotherapists and prosthetists is improving the lives of our patients after amputation,” Crane emphasises, reiterating that while reducing amputations where possible is important, there is a need to stop thinking of amputation as “the end of the line”.
Another issue is that opinion-based care is often the best available, according to Smith. “If [a patient] asks me a question about which of [above-knee or throughknee amputation] is better, at the moment I can give them an opinion, I cannot give them any evidence whatsoever to base that on,” he says. Crane concurs,
24 BIBANews April 2023 CHARING CROSS SPECIAL EDITION Feature
We owe it to our patients and our specialty to leave no stone unturned in understanding how to deliver better care and better outcomes.”
Dan Carradice
Representatives from multiple specialties highlight a pressing need to drive up the quality of research and care for patients who require the “life-changing” intervention that is an amputation of the leg.
PERIPHERAL
noting that clinicians’ opinions, which “vary hugely” across the UK, are the basis of current clinical practice.
“There are many clinical decisions that we have to make without any evidence,” consultant clinical academic physiotherapist Chantel Ostler (Portsmouth Hospitals University NHS Trust, Portsmouth, UK) adds, noting that the evidence base around understanding amputation, as well as prosthetic rehabilitation following amputation, is “really poor”. As a result, she comments, it is “difficult” for clinicians to be able to use that evidence base to help them make decisions.
According to Carradice, the NIHR announcement is “excellent news” for the care of patients in need of lower limb amputation surgery. “We owe it to our patients and our specialty to leave no stone unturned in understanding how to deliver better care and better outcomes.”
she says, may be particularly beneficial for patients who might be at risk of falling or might be at risk of injury when falling, or for those patients who might find that the energy consumption of walking with a traditional prosthesis is too much. “This has had a real impact on how confident our patients feel with their prosthetic limbs, on their fear of falling, and also they tell us on the different activities and the number of activities that they are able to do,” Ostler relays.
The multiple specialist representaives
The hope is that the research will have an international element, with Guo noting that the NIHR are currently considering collaboration and joint funding with the Australian National Health and Medical Research Council (NHMRC). “Having this level of international support elevates the profile of amputation research within the UK and abroad, and hopefully it will spur other trials within this important and challenging field,” he states.
Crane is positive about the impact research will have on the whole care pathway. The greater the evidence, guidance, and consideration given to the amputation at the start of the patient’s journey, she believes, the easier the recovery for the patient.


Future research
Looking to the future of amputation surgery considering the NIHR news, Smith notes that he and his team are applying for funding to conduct a trial that will compare through-knee with above-knee amputation for people who are not suitable for a below-knee amputation, examining quality of life in particular. “The trial is about the fact that there is a perception that through-knee amputations may not heal as well as an above-knee amputation, and that is why a lot of people have traditionally shied away from them,” he explains. In registry data, however, he notes that there is no real signal towards this negative outcome. “If anything,” he continues, “the healing is equivalent.” In addition, Smith notes that the researchers want to look further into some positive benefits of through-knee amputation signalled to in the registry data, including fewer medical complications, shorter length of hospital stay, and a shorter rehabilitation period.
The trial, dubbed HAMLET (Through knee or not through knee, that is the question), involves multiple stakeholders and multiple centres, Carradice continues, specifying for example that the Amputation SIG and UK Vascular Clinical Trials Network are all behind the work. “To date in the UK through-knee amputation has made up a very small proportion of procedures and it is therefore important to establish whether these potential benefits are seen in a prospective randomised study and can be translated into a wide-scale change in practice and clinical benefits for this important group of patients.”
Prosthetic advancements
With regard to life after amputation surgery, Ostler notes that clinicians are now able to provide some high-tech prosthetic equipment. In 2016, she reveals, NHS England allowed for the prescription of microprocessor knees on the NHS. These prosthetics,

Guo also refers to several advancements in prosthetics in recent years, while pointing out that some further research is still needed. One of these developments is osseointegration (OI) prosthetic limbs, also known as direct skeletal fixation. He explains how they work: “In transfemoral amputees for example, instead of having a conventional socket attached to a prosthetic limb, OI involves surgically inserting an intramedullary nail-like stem into the medullary canal of the femur. The stem protrudes through the skin and attaches to a prosthetic limb.”
Guo reports that initial studies focusing on younger, fitter patients with trauma or combat injury amputations have been “promising” in terms of potentially avoiding the problems of a traditional socket interface—such as skin issues, sweating, socket discomfort and fitting issues, as well as reduced control of the prosthetic limb. However, he also notes that complications have been reported such as peri-prosthetic fractures, implant loosening and breakage, as well as infection. “Currently, the NHS in England does not recommend OI as a treatment for transfemoral amputation, especially for vascular patients. As time progresses, it will be interesting to see the long-term outcomes of OI.”
Carradice acknowledges that while advanced prosthetics “come at a cost,” if they do improve function, this could have a “dramatic” impact on patient outcomes and the social care budget.

“A team sport”
There is consensus that, in conjunction with research, multidisciplinary collaboration will be key to improving amputation care going forward. Carradice stresses that the multidisciplinary approach, while important across medicine, is especially crucial when it comes to amputation care. “Resources for highly visible and well-promoted areas within surgery are in short supply, much less is often available for less visible and less celebrated treatment such as amputation,” he says. “We must ensure that our patients receive the correct balance of evidencebased clinical, operative, psychological and pain management, as well as physical therapy, prosthetics, physical adaptations and social care so that they can realise the best possible outcomes from their and from societal perspectives.” This, he believes, will require “passionate multidisciplinary academic and
clinical leaders working nationally and locally to drive positive change”.

Ostler underscores the importance of including all members of a multidisciplinary team in considering what level of amputation might allow a patient to have the best outcome. She mentions, for example, the significance of thinking about the different types of residual limbs that are created and the impact that they might have on how comfortable the team can get patients in their prosthetic socket, but also about the processes that happen during the inpatient stay that can speed up patient recovery.
While multidisciplinary care is the goal, it is not always the reality, Guo tells Vascular News. A discussion between the operating surgeon and the amputee rehabilitation team with regards to the level of amputation, and even whether to amputate or not, can be a “pivotal step” in managing the patient’s clinical sequelae, he says. Often, however, Guo notes that amputee rehabilitation centres are located off-site, away from the acute (or non-acute) hospital where the amputation surgery is being performed, and that hospitals’ surgical departments may not have access to an amputee rehabilitation centre within close proximity.
According to Guo, he and colleagues are “very fortunate” in Bristol to have an “ingrained and embedded” interdisciplinary and inter-specialty framework, between the rehabilitation team and surgical colleagues. “Despite the Bristol Centre for Enablement (BCE) being off-site to Southmead Hospital, there is a regular weekly in-reach review of selected pre-, peri- and post-amputation inpatients by the amputation rehabilitation team (including the Consultation in Rehabilitation Medicine [i.e. myself],

Hayley Crane
and Amputee Psychological Counsellor),” he explains. “We are continuing to build up our links with major trauma, vascular and plastic surgery and the limb reconstruction team in order to provide a more holistic approach to this complex and lifechanging condition.”
Ostler agrees, noting that “better links” with prosthetic rehabilitation are needed. In parallel to this, she believes it is important to link data. For example, she asks how data collected through the National Vascular Registry could be integrated with data that could be collected in prosthetic settings. “This could give a good idea about the longer-term outcome of amputation, particularly for those who may be prosthetic users, and whether that information then might help us to make better decisions about patients who have an amputation, the information that we provide to them and how we manage their expectations and their adjustment following amputation.”
Amputee care is “very much a team sport,” Smith summarises. “There is no single individual that can make this pathway work.”
25 BIBANews April 2023 CHARING CROSS SPECIAL EDITION Feature
As physiotherapists, our role is to rehabilitate the patient to the best of our ability after amputation, but we are ultimately bound by the limitations of the amputation surgery the patient has had.”
For more information on the HAMLET trial, please contact George Smith at george. smith16@nhs.net
Clockwise from top right: Dan Carradice, Chantel Ostler, Hayley Crane, Shigong Guo, Robert Hinchliffe and George Smith












































Modifying Beveled Tip 1 Continuous Active Aspiration 3 Rotating Abrading Vortex 2 Refining Atherectomy Please consult package insert for more detailed safety information and instructions for use. BD, the BD logo, and Rotarex are trademarks of Becton, Dickinson and Company or its a liates. © 2023 BD. All Rights Reserved. © 2023 Illustrations by Mike Austin. BD-19798 bd.com BD, Tempe, AZ, USA, 1 800 321 4254
New study demonstrates IVC filters “safe and effective” in treating venous thromboembolism
Few adverse events are connected to the use of inferior vena cava (IVC) filters to help prevent deep vein blood clots from developing into pulmonary embolisms (PEs), according to the findings of the Predicting the safety and effectiveness of inferior vena cava filters (PRESERVE) trial, published jointly in the Journal of Vascular and Interventional Radiology (JVIR) and the Journal of Vascular Surgery: Venous and Lymphatic Disorders (JVS-VL)
PRESERVE is a US Food and Drug Administration (FDA)-directed multicentre, prospective, openlabel, non-randomised trial that studied the safety and efficacy of IVC filters from six manufacturers. It was a joint effort of the Society of Interventional Radiology (SIR) and the Society for Vascular Surgery (SVS). The study was conducted at 54 sites in the USA between 10 October 2015 and 31 March 2019. During that time, filters were implanted in 1,421 patients, of whom 1,019 patients had an existing deep vein thrombosis (DVT) or PE.
Researchers found that IVC filters were effective in helping to prevent PEs in patients experiencing a DVT where anticoagulation medicines failed or were not an option for the patient. Approximately half of the patients in the study had their filters removed within three months of placement without complication or recurrence of DVT or PE, according to study authors.
“The question should not be only ‘should we place a filter?’ but ‘how should we offer comprehensive filterinclusive care of patients with venous blood clots, comprised of a detailed patient evaluation, a plan for retrieval after placement, and frequent followup with evaluation for filter removal or replacement,’” said Matthew S Johnson, an interventional radiologist
and professor of radiology and surgery at Indiana University School of Medicine (Indianapolis, USA) and co-principal investigator on PRESERVE.

“PRESERVE showed what questions we should ask as clinicians: ‘does this person continue to require protection against PE, and, in light of changing clinical status and available therapies, is the current filter needed?’ and then make an informed decision on how to continue care.”
“DVTs and PEs are a significant cause of death worldwide and understanding fully how tools like IVC filters can be used to prevent the progression of a DVT into a PE allow physicians to safely treat patients at risk of death from venous thromboembolism [VTE]” said David L Gillespie, a vascular surgeon at Beth Israel Deaconess Medical Center (Brockton, USA), and co-principal investigator on PRESERVE. “Now that the study is complete, we now have a roadmap for better filter utilisation.
We need to solidify a clearer set of practice guidelines for venous thromboembolic disease, based on its symptoms, location and complications. Further studies will focus on how the different manifestations of venous thromboembolic disease may benefit from filter-inclusive care.”
Speaking at VEITHsymposium 2022 (15–19 November, New York, USA), Gillespie outlined that “approximately ten years ago or so, the clinical management of patients with VTE and the prophylaxis of potential VTE in trauma patients was not well studied with regard to the use of IVC filters”. The result of the PRESERVE trial has been that “essentially, these filters are safe and effective for therapeutic [and prophylactic] use in the patient population [in question],”
Gillespie continued, and this, despite the “challenges” of the COVID-19 pandemic, the “high dropout rate”, and the fact that “a large number of patients” had their filters removed.
To date, PRESERVE is the largest prospective study investigating the realworld patient outcomes of IVC filter use. “This trial represents an important step in collaborating across specialties to benefit the health and safety of our patients,” said SIR President Parag J Patel (Medical College of Wisconsin, Milwaukee, USA). “Thanks to the work of Drs Johnson and Gillespie and all the investigators and patients
Study indicates reflux time “best utilised as a cut-off” for diagnosis of venous insufficiency
In a prospective study of patients undergoing great saphenous vein (GSV) ablation, researchers found no correlation between maximum reflux time and symptom severity as measured by Venous clinical severity score (VCSS).
AUTHORS DAMIANOS G KOKKINIDIS (Yale University, New Haven, USA) and colleagues write in the February edition of Phlebology that it is unclear whether reflux time independently correlates with severity of symptoms in patients with GSV reflux. It was their aim, therefore, to compare venous reflux time and VCSS in this patient group.
The investigators prospectively enrolled 80 patients (mean age 64 years, 56% female) presenting for treatment of symptomatic venous reflux to one teaching hospital between August 2020 and January
2022. They note that VCSS was conducted by the operating physicians as part of the initial screening visit or immediately before the ablation.
Of the total number of patients included in the study, the authors specify that 57 underwent ablation with radiofrequency, while 23 were treated with cyanoacrylate adhesive.
Kokkinidis et al report in Phlebology that VCSS values ranged from two to 20, with a median value of seven, and that the mean reflux time was 5.3 seconds. Additionally, they relay that the Spearman
involved in the trial, we now have higher quality evidence to support appropriate utilisation and management of IVC filters in patients with venous thromboembolic disease.”
“Congratulations to Dr Gillespie, Dr Johnson and their many colleagues for shepherding this large collaborative multispecialty, multicentre clinical trial to completion,” said Michael C Dalsing (Indiana University Health, Indianapolis, USA), president of the SVS. “This highly impactful study provides the real-world evidence needed when recommending IVC filter placement to protect our patients from a potentially lethal disease and when to remove that filter after it has accomplished the desired effect. It is a stellar example of collaboration across specialties for the betterment of patient care.”

“Very valuable” results
At the Society of Interventional Radiology 2023 annual scientific meeting (4–9 March, Phoenix, USA), Johnson gave a presentation on PRESERVE. Rounding off his time on the podium, Johnson asserted his stance on the efficacy of the IVC filter for VTE—“filters do prevent PE; I have no question that this study demonstrated this very well”.
He then ventured that where clots do occur in patients with filters placed, it could be that, rather than the filter having caused the clot, that its formation is linked to patients’ “thromboembolic tendencies” that therefore preclude anticoagulation. “I do not believe that they cause clot”, rather, asserting the conviction, as supported by the PRESERVE results, that they are “very valuable” in ensuring patients for whom they are suitable are “well cared for”.
rank correlation yielded a value of rs=-0.123, which they say was not significant. The patients with concomitant deep vein reflux had higher VCSS, Kokkinidis and colleagues add, noting finally that analysis of patients with only superficial vein reflux (n=45) also demonstrated a poor correlation between VCSS and reflux time.
Based on their conclusion that this prospective study did not demonstrate a correlation between reflux time and VCSS, the authors suggest that reflux time is “best utilised as a cut-off” for the diagnosis of the presence of venous insufficiency.
In the discussion of their findings, Kokkinidis et al recognise that their study has some limitations, including its small sample size, which they write “may have led to an inability to detect correlation between reflux time and VCSS”. They also note that the number and size of affected tributaries, as well as the duration and presence of symptoms, were not evaluated.
27 BIBANews April 2023 CHARING CROSS SPECIAL EDITION New Data
VENOUS
Further studies will focus on how the different manifestations of venous thromboembolic disease may benefit from filter-inclusive care.”
David L Gillespie
STEVECO trial calls into question randomisation in the venous stenting field
Recruitment proved to be a major challenge for the STEVECO (Stent versus conservative treatment in patients with deep venous obstruction) randomised controlled trial (RCT), prompting discussion on how best to randomise patients in future trials, the ethics of doing so, and possible alternatives to RCTs.
JORINDE VAN LAANEN (MAASTRICHT
University Medical Center, Maastricht, The Netherlands) presented results of the STEVECO trial at this year’s European Vascular Course (5–7 March, Maastricht, The Netherlands).

While venous stenting is “basically common practice” and supported by various guidelines, the presenter noted, the problem is that the evidence available on venous stenting is limited. Most reports focus on stent safety and patency, with only three studies—according to a literature review van Laanen referenced—reporting on quality of life. “Are we really helping the patients? Are the clinical symptoms improving?” she questioned.
The STEVECO trial, sponsored by Optimed, was designed as a multicentre, randomised study. Van Laanen detailed that patients had to be treated conservatively for one year before they could be included in the trial, which had
a primary outcome of VEINES-QoL/Sym score at 12 months and secondary outcomes of general quality of life, clinical improvement and stent patency.
“The trial had a lot of challenges,” van Laanen remarked, with randomisation of patients being the most notable. She explained that patients present to the Maastricht centre after tertiary referral because they have been struggling with symptoms for a long time. “These patients have already been treated conservatively and are sent to expert centres for treatment, so they want a treatment, an intervention,” the presenter continued. When the investigators tried to randomise patients—with the possibility that they would be assigned to the conservative arm for at least one more year—she noted that patients often refused to participate. As a result of this and other challenges, van Laanen detailed that the investigators stopped recruitment at 63 patients out of a target of 130 (21 in the control group and 42 in the intervention group).
The presenter reported an eight-point VEINESQoL score advantage for the stent group at one year, as well as a six-point VEINES-Sym benefit. In terms of secondary outcomes, she revealed no significant difference in general quality of life—as assessed by the EQ5D questionnaire—and a significantly diminished pain score (11 points) in the stented group. Finally, she detailed a significant reduction in venous clinical severity score (VCSS) but not in Villalta score.

The patency results, van Laanen added, were comparable to other studies, with a primary patency of 91% and secondary patency of 97%.
The presenter stressed that there are some key questions that must be asked going forward. “How should we do this in the future?” van Laanen asked the audience at EVC. “If we really want to prove stenting is better than conservative treatment, how are we
Large bore mechanical thrombectomy reduces adverse outcomes in high-risk PE patients
LARGE BORE MECHANICAL THROMBECTOMY WITH THE FlowTriever system (Inari Medical) in patients with high-risk pulmonary embolism (PE) was associated with a significantly lower occurrence of meaningful in-hospital adverse clinical outcomes compared to other contemporary treatments, data presented at the American College of Cardiology (ACC) 2023 Scientific Sessions (4–6 March, New Orleans, USA) indicate.
These were among the results of the FLAME study, a prospective, nonrandomised study of interventional treatment in high-risk PE, a patient population with a historical mortality rate of 25–50%, which were presented at ACC 2023 by Mitchell Silver (OhioHealth Heart and Vascular, Columbus, USA).
The study collected data on patients treated with the FlowTriever and on those treated with other therapies in a context arm.
The primary endpoint measured a composite of meaningful in-hospital clinical outcomes, including mortality, major bleeding, clinical deterioration, and escalation to an alternate therapy.
Silver reported that FLAME was stopped early after meeting the pre-specified interim analysis criterion at 50 FlowTriever patients. Sharing the results of the study, Silver noted that the endpoint was met in the FlowTriever arm, in which a mortality rate of 1.9% was recorded, compared to 29.5% in the context arm. In all, the composite primary endpoint occurred in 17% of patients in the FlowTriever arm, compared to 63.9% in the context arm.
“The remarkably low mortality seen with FlowTriever demonstrates the benefit of rapidly identifying PE patients and getting them to an interventionalist for assessment,” Silver was quoted as saying in a press release issued shortly after the presentation of the results at ACC 2023.
“It is time for our hospital systems to develop standardised care pathways for PE, similar to what has been done in other major cardiovascular diseases such as heart attack and stroke.”
going to include patients in an RCT when we are all doing the treatment all of the time?”
“You need to be applauded for trying this,” panellist Stephen Black (Guy’s and St Thomas’ Hospital, London, UK) remarked in the discussion following van Laanen’s presentation. He stressed that recruiting for a venous RCT is not an uncommon problem, noting BEST-PTS is “struggling” and C-TRACT is “at risk of failing to recruit”. He also referred to past trials, highlighting that ATTRACT and CAVA both took 10 years to recruit.
Considering what investigators need to do differently in the future, van Laanen suggested that “maybe the only option” would be to offer stenting exclusively to patients who participate in an RCT. Alun Davies (Imperial College London, London, UK) expressed his support for this idea, telling the audience it is “exactly what happened in the UK” with the EVAR 1 and EVAR 2 trials. “The only way you could get a device was by actually going into the trial,” he said.
Black remarked that the ethics of randomised trials are “quite difficult,” expressing the opinion that “we need to find alternatives to RCTs”. These trials are “exceptionally difficult to run” and “exceptionally expensive,” he said, adding that he is not sure the narrative that an RCT is the only level of acceptable evidence is completely correct.
Looking ahead, he underlined the potential of artificial intelligence (AI) and other new technologies in looking at large datasets. “There are opportunities there to run studies that provide you with exactly as robust a level of evidence, without having to randomise patients,” he explained. “I suspect the only way we are going to get around ethics is really looking at study design in a more robust fashion.”
New York, USA). The subanalysis was drawn from the 800-patient prospective, multicentre, real-world registry in light of a paucity of data regarding the use of the percutaneous system among highrisk patients, Horowitz noted.
PE patients
AMONG 61 HIGH-RISK pulmonary embolism (PE) patients followed through to the 30-day visit in the US cohort of the FLASH registry, no mortalities were recorded, while at 48 hours post-treatment with the FlowTriever mechanical embolectomy system (Inari Medical), likewise, there were no major adverse events (MAEs), nor serious adverse events (SAEs) reported. The outcomes emerged at the 2023 American Venous Forum (22–25 February, San Antonio, USA) in a paper delivered by James Horowitz (NYU Grossman School of Medicine,
Horowitz reported that, postembolectomy using the FlowTriever system, haemodynamic improvements were observed, with mean pulmonary artery pressure decreasing from 31.5mmHg to 24.3mmHg, and cardiac index (CI) increasing from 1.5L/ min/m2 to 1.9L/min/m2. “Patients demonstrated immediate haemodynamic and vital-sign improvements following the procedure,” he added.
Horowitz and colleagues highlighted that the large-bore mechanical embolectomy system “has a favourable safety and effectiveness profile” in the high-risk group. They concluded:
“In high-risk PE patients, there were no mortalities through 30 days or MAEs after mechanical embolectomy with the FlowTriever system. Highrisk patients demonstrated significant improvement of acute haemodynamics and functional outcomes. Results from the FLASH registry suggest mechanical embolectomy is safe and effective for high-risk PE, leading to markedly lower acute mortality compared to previously reported mortality rates for this population.”
28 Conference Highlights
FLASH registry suggests
“favourable safety and effectiveness profile” in high-risk
How should we do this in the future?”
ACC 2023 AVF 2023
Jorinde van Laanen at EVC 2023

A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the venous arena Editorially independent Visit venousnews.com and click ‘Subscriptions’ for e-newsletter subscription Subscribe today Available in print and digital formats and through our social channels







*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the interventional field Editorially independent Visit interventionalnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
ESC and EAPCI publish renal denervation consensus statement
Renal denervation represents another treatment option in patients with uncontrolled resistant hypertension and may be used in selected patients deemed intolerant to antihypertensive drugs.
These are among the messages of a new consensus statement published in EuroIntervention following a review of evidence by the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI).
Initial excitement over the potential for renal denervation as a treatment for hypertension was dampened following the release of initial results of the SYMPLICITY HTN-3 trial in 2014 in which it was shown that at six months, renal denervation did not elicit significant incremental blood pressure lowering benefit compared with a sham procedure.
Subsequently, joint ESC and European Society of Hypertension (ESH) guidelines on the management of arterial hypertension, published in 2018, advocated against the routine use of device-based therapies for hypertension, until further evidence of their safety and efficacy came to light. However, newer
sham-controlled trials have revitalised hope in the potential of renal denervation, with a number showing statistically significant and clinically meaningful reductions in blood pressure.
In producing the latest consensus statement, the ESC and EAPCI expert panel have reviewed evidence from several “second-generation” randomised, shamcontrolled trials, which, they say, demonstrate the safety and the blood-pressure lowering efficacy of radiofrequency and ultrasound renal denervation. The second generation of trials have involved either the Symplicity Spyral (Medtronic) multielectrode radiofrequency device or the Paradise (ReCor Medical) ultrasound system.
“Since the publication of the 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension, several high-quality, randomised, sham-controlled trials have been published, demonstrating a bloodpressure-lowering efficacy over 24 hours for both radiofrequency and ultrasound renal denervation in a broad spectrum of patients whose hypertension ranges from mild-to-moderate to severe and resistant,” Emanuele Barbato (University of Rome, Rome, Italy) and colleagues write in EuroIntervention
“This expert group proposes that renal denervation is an adjunct treatment option in uncontrolled resistant hypertension, confirmed by ambulatory blood pressure measurements, despite best efforts at lifestyle and pharmacological interventions,” the statement notes. Renal denervation may also be used in patients who are unable to tolerate antihypertensive medications in the long term, the writing committee has concluded.
The authors of the paper suggest that a shared decision-making process, taking into account the patient’s global cardiovascular risk and the presence
Results on ReCor Medical’s Paradise ultrasound renal denervation system published in two JAMA Network publications
ReCor Medical and its parent company, Otsuka Medical Devices, recently announced that primary endpoint results from the RADIANCE II pivotal trial were published in the Journal of the American Medical Association (JAMA).

RADIANCE II IS A RANDOMISED, sham-controlled US Food and Drug Administration (FDA) investigational device exemption (IDE) pivotal trial of the Paradise ultrasound renal denervation (uRDN) system in the treatment of patients with uncontrolled hypertension. Conducted as an international multicentre study at more than 60 study centres in eight countries, 224 patients with uncontrolled hypertension were randomised 2:1 to uRDN or a sham.
Patients were to remain off antihypertensive medications throughout the two months of followup unless specified blood pressure criteria were exceeded. At the twomonth primary efficacy endpoint, patients treated with the Paradise uRDN system had a mean reduction in daytime ambulatory systolic blood pressure of -7.9mmHg, compared to a reduction of -1.8mmHg in the sham
arm, corresponding to a statistically significant and clinically relevant between-group difference of -6.3mmHg (p<0.0001). The study also achieved its primary safety composite outcome with no major adverse events observed.
Concurrently published in JAMA Cardiology, a RADIANCE pooled analysis includes data from more than 500 patients randomised in the three studies from ReCor’s RADIANCE global programme: RADIANCEHTN TRIO, which studied patients with resistant hypertension, and RADIANCE-HTN SOLO and RADIANCE II, which studied patients with mild-moderate hypertension.
The combined dataset showed an overall reduction in daytime ambulatory systolic blood pressure in the uRDN group of -8.5mmHg with a difference between treatment and sham at two months of -5.9mmHg (p<0.0001), favouring uRDN. Blood
of hypertension-mediated organ damage, should be followed when considering renal denervation as a treatment option.
Furthermore, they state that interventionalists require expertise in renal interventions and specific training in renal denervation procedures. “Centres performing these procedures require the skills and resources to deal with potential complications,” Barbato et al state.
Presently both the Symplicity Spyral and Paradise system carry a CE mark, and Medtronic and ReCor both filed premarket approval applications to the US Food and Drug Administration (FDA) in late 2022 for their respective devices.

“To date, there are at least 18 societal and/or expert consensus documents published, and the increasing number of citations seems to parallel the mounting evidence for renal denervation therapy,” David Kandzari (Piedmont Heart Institute and Cardiovascular Services, Atlanta, USA), a member of the writing committee for the ESC/EAPCI consensus statement and prinicipal investigator in the SPYRAL HTN-ON MED trial, told Vascular News. “In all, these documents are important for providing clinicians with guidance regarding the evidence basis for renal denervation safety and effectiveness, patient selection, and procedural technique. Many of the documents also underscore the need for shared-decision making and accounting for patient preference.
“The ESC/EAPCI document offers the most contemporary evidence and informed clinical considerations for renal denervation, including recommendations for not only patient selection but also for institutions related to renal denervation programme development, patient selection, and operator proficiency.”
pressure results were similarly positive in the 24-hour, night-time, home, and office measures. A favourable safety profile was consistently observed following uRDN treatment across the studies.
Results in line with new consensus statement

“The results of the RADIANCE clinical trials are meaningful in that they solidify the role of the Paradise uRDN system as an adjunctive therapy for hypertension treatment, in addition to medications and lifestyle modification. Having three consistent shamcontrolled clinical trials demonstrating that the Paradise uRDN system can safely lower blood pressure across a range of patients is a very high bar to have met,” said co-principal investigator Ajay Kirtane
(New York-Presbyterian Hospital, New York, USA).
Co-principal investigator Michel Azizi (Université Paris Cité, Hôpital Européen Georges Pompidou, Paris, France) added: “The pooled analysis of RADIANCE SOLO, TRIO, and RADIANCE II shows a remarkable consistency of effect in patients with mild to moderate hypertension and those with resistant hypertension. These results are in line with the new 2023 consensus statement of the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). The publication of these results in JAMA and JAMA Cardiology will bring the evidence of the performance of uRDN in the treatment of hypertension to a broad audience of physicians.”
31 BIBANews April 2023 CHARING CROSS SPECIAL EDITION Renal Denervation
These results are in line with the new 2023 consensus statement of the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI).”
Paradise system
Kidney model

Stepping up: The transition to consultant
Claire Dawkins (Newcastle, UK) writes about the career milestone for any trainee that is becoming a consultant. “I just need to remember, it is a marathon, not a sprint,” she says of the road ahead.


I STEPPED UP TO ‘ACTING CONSULTANT’ three months ago and, although I knew it was going to be a big change from my time as a registrar, nothing could have prepared me for the difference I was going to experience. I heard from senior colleagues that when they first made that transition they were bored. One told me how he would sit in his office reading a book. In contrast, I have a growing stack of books gathering dust and a continuous stream of paperwork, phone calls and emails. This is partly a reflection of how the demands on the vascular department have changed over the last 10 years, and partly my attempts to run before I can walk!
When I first took on the role I was faced with an initial job plan that had not yet been altered to accommodate me, with the only elective work being a single half-day clinic a week of legacy patients from a retired senior colleague, and a
CAROTID
1-in-10 on-call rota. I set myself a task of finding other ways to entertain myself and before I knew it I had easily filled my time. I over-reached.
Training had not really prepared me for consultant life. All the extras I did in my own time during my registrar years had better prepared me than the training programme itself: rota coordination, teaching, event organisation, being a Rouleaux Committee member and representing trainees on the Vascular Society council. But nothing quite prepared me for the mental drain of doing a week on call as the ‘vascular consultant of the week’, juggling the needs of over 60 inpatients and various outpatients, particularly regarding patients who have had complications or whose families have questions and concerns regarding their care. It is different when the buck stops with you.
As a registrar at the end of training I was used to being the person that a lot of people turned to, to answer questions and to get things done. But I could not do that in the same way anymore. My mental capacity was stretched as was my resilience and I found myself getting frustrated when things I had planned had not worked out. One day I felt angry because my colleagues had kindly arranged for our specialty and specialist (SAS) colleague to do my operating list as I had been called in for an emergency overnight. That is when I realised I was trying to do too much too soon.
I am not special nor different from the majority of trainees stepping up to consultant level.
Distal embolic protection linked to significantly better outcomes in carotid stenting
Distal embolic protection using a filter has been associated with improved transfemoral carotid artery stenting (tfCAS) outcomes in terms of in-hospital stroke and death risks—underpinning current Society for Vascular Surgery (SVS) guidelines recommending routine use of distal embolic protection during carotid stenting, and supporting the notion that, “if a filter cannot be placed safely, an alternative approach to carotid revascularisation should be considered”.
SOPHIE WANG, PATRIC LIANG
(both Harvard Medical School, Boston, USA) and colleagues deliver this message in a recent Journal of Vascular Surgery (JVS) publication detailing a retrospective, propensity score-matched cohort analysis of tfCAS patients in the Vascular Quality Initiative (VQI) across a 16-year period.

“Transfemoral carotid stenting without use of embolic protection is associated with a significant increase in in-hospital stroke/death compared with tfCAS performed with distal filter placement,” the authors write. “Failed attempted filter placement is associated with worse outcomes after tfCAS, but stroke/death rates are no different to those seen in patients in whom a filter was never attempted.
“These findings support the current guideline recommendation for routine use of distal embolic filters during tfCAS. However, the proportion of non-protected tfCAS performed annually is increasing, and there is significant variability in routine filter usage across physicians and centres in the VQI.”
In an effort to assess in-hospital outcomes in patients undergoing tfCAS with and without embolic protection using a distal filter, Wang, Liang and colleagues identified all patients undergoing this procedure in the VQI from March 2005 to December 2021— excluding those who received proximal
We are generally high-fliers with big ambitions who are used to excelling clinically as registrars. But even though I was warned to start slowly and to say no to extra responsibilities, I thought I could do everything straight away and it took time and unnecessary stress to realise that I needed to start small and build up slowly.
So how do we make this transition easier? Now I am at the end of my acting-up period I look back and see how useful it has been. I have learnt a lot about how the NHS, my hospital and the department work. More importantly, I have learnt a lot about myself and about the team that I hope to join as a full-time consultant. I certainly recommend giving all trainees this opportunity. But understanding what consultant life is like needs to start earlier than the final three months of training. Including trainees in different aspects of nonclinical consultant life, such as consultant business meetings, may give a small insight.
Unfortunately, until you do the job itself and feel the pressures and responsibility of consultancy I do not think you can have a full grasp of what it involves. After three months I am only scratching the surface. I am starting to realise how much I do not know. But if these last few months are anything to go by, I am going to enjoy growing into consultancy even more than I enjoyed getting here, I just need to remember, it is a marathon, not a sprint.
CLAIRE DAWKINS is a vascular trainee at the Newcastle Upon Tyne Hospitals NHS Foundation Trust in Newcastle, UK and a committee member for the Rouleaux Club, the UK national vascular trainee society.
embolic balloon protection. As well as creating propensity score-matched cohorts of patients who underwent tfCAS with and without attempted filter placement, they performed subgroup analyses of patients with ‘failed’ versus ‘successful’ filter placement, and ‘failed’ versus ‘no attempt’ at filter placement.
In-hospital outcomes were assessed using log binomial regression, adjusted for protamine use, the authors detail. Their outcomes of interest were composite stroke/death, stroke, death, myocardial infarction (MI), transient ischaemic attack (TIA), and hyperperfusion syndrome.
Among 29,853 patients who underwent tfCAS, 28,213 (95%) had a filter attempted for distal embolic protection and 1,640 (5%) did not. After propensity-score matching, 6,859 patients were identified.
In comparison to cases in which a filter placement was attempted, no attempted filter was associated with a significantly higher risk of in-hospital stroke/death (6.4% vs 3.8%)— constituting a more than two-fold increase in stroke/death risk, as per the analysis’ key outcome of interest. Similar trends were observed regarding
stroke (3.7% vs 2.5%) and mortality (3.5% vs 1.7%) too.
In a secondary analysis of patients who underwent a failed attempt at filter placement—versus successful filter placement—failure was associated with worse outcomes (stroke/death: 5.8% vs 2.7%; stroke: 5.3% vs 1.8%, respectively). However, Wang, Liang and colleagues observed no statistical difference in outcomes in patients with failed versus no attempted filter placement (stroke/death: 5.4% vs 6.2%; stroke: 4.7% vs 3.7%; death: 0.9% vs 3.4%) after adjusted analysis.
“Distal embolic protection confers a benefit in stroke/death rates following tfCAS compared to unprotected stenting,” the authors conclude, putting forward their ‘take-home message’.

33 BIBANews April 2023 CHARING
SPECIAL EDITION Trainee Perspective
CROSS
Launch Pad
These findings support the current guideline recommendation for routine use of distal embolic filters during tfCAS.”
Sophie
Wang Patric Liang
Recruitment complete in BEVAR study for on-label use of the Bentley BeGraft peripheral Plus as a bridging stent
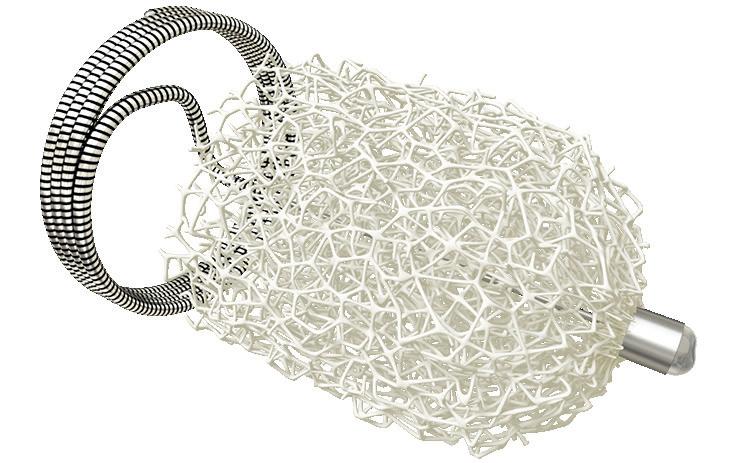
Recruitment is now complete with more than 100 patients as planned per protocol in the branched endovascular aneurysm repair (BEVAR) study that uses Bentley’s BeGraft peripheral Plus balloon expandable covered stent as a bridging stent in complex aortic aneurysms repairs.
Sebastian Büchert (CEO, Bentley) mentioned, “At Bentley we became aware that a significant share of the BeGraft peripheral Plus is being used as a bridging stent for the treatment of thoracoabdominal aneurysms in BEVAR procedures. Since none of the available covered stents are holding this indication, Bentley aims to step up and bring this off-label application on-label.”
The study, which is led by Martin Austermann (St, Franziskus Hospital, Münster, Germany) is evaluating the safety and performance of the BeGraft peripheral Plus as a bridging stent in BEVAR procedures. When successful, this would bring the on-label use in this indication a step closer.
Austermann explained: “In BEVAR interventions, bridging stents are not supported by the native vessel wall, so the risk of failure is high. Although we have not yet analysed study data, the BeGraft peripheral Plus with its special design met the requirements of high radial force combined with flexibility quite well. At first glance the data are promising, and with the potential indication for on-label usage as a bridging stent in BEVAR procedures, it will change the approach from the physicians’ perspective.”
The study endpoints include technical success of the procedure and patency of the bridging stent after one year. Safety will be evaluated for the one-year time point and will include the absence of procedure-related complications and endoleaks associated with the bridging stents. Results are expected in the second half of 2024.

The study is a collaboration between the Foundation for Cardiovascular Research and Education (FCRE, Belgium) and Bentley. It has been
approved by the German competent authority BfArM. Participating German centres are the St Franziskus Hospital Münster, University Hospitals Aachen, University Clinic HamburgEppendorf, LMU Munich, University Clinic Regensburg, Stuttgart Hospital, University Hospital Leipzig, University Hospital Heidelberg, University Hospital Schleswig-Holstein-Kiel and the Nuremberg Hospital.
Humacyte publishes six-year outcomes in study of HAV for peripheral arterial bypass Humacyte, a clinical-stage biotechnology platform company developing universally implantable bioengineered human tissues and advanced tissue constructs and organ systems at commercial scale, announced the publication of “Sixyear outcomes of a phase two study of human-tissue engineered blood vessels for peripheral arterial bypass,” in the Journal of Vascular SurgeryVascular Science
The publication describes the long-term analysis of the company’s phase two clinical trial evaluating the bioengineered human acellular vessel (HAV) as a conduit in patients with symptomatic peripheral artery disease (PAD). The researchers concluded that “the infection-resistant, off-theshelf HAV could provide a durable alternative conduit in the arterial circuit setting, to restore lower extremity blood supply in patients with peripheral artery disease”.
This paper reports a 60% overall secondary patency rate for the phase two study at 72 months, including all patients originally enrolled, and was estimated by Kaplan Meier analysis. There was no evidence of graft rejection or infection. Additionally, no patients underwent amputation of the affected limb out to six years—a meaningful clinical and quality-of-life result, as amputation is a common outcome in many severe PAD patients. Furthermore, no patients reported pain at rest or ischaemic ulcers on the affected legs. Researchers reported that “these data have demonstrated the durability of the HAV and suggest the occurrence of cellular remodelling by the host.”
Piotr Gutowski (Pomeranian Medical University, Szczecin, Poland), lead manuscript author, commented: “Synthetic grafts can be limited due to poorly matched mechanical compliance, risk of infection, and variable patency rates. Furthermore, cryopreserved allogenic grafts are limited due to poor durability, thrombosis, and mechanical degradation. The HAV is designed to be consistent in size, durable in highpressure circulation, show no clinical immunological response, and remodel
with the patient’s own cells.”
“With an increasing global prevalence of PAD and more than 200 million people living with the disease, there still remains a major unmet need for long-term solutions,” said Laura Niklason, chief executive officer of Humacyte. “Key findings of this publication show that the HAV was durable and performed well in a medically complex patient cohort for long-term treatment of PAD.
The HAV is designed to be available off-the-shelf, has the potential for a regenerative capacity and low infection risk, all of which are particularly important in this patient group.”
The HAV has been evaluated in eight clinical studies in the USA, Europe, and Israel, including an ongoing phase two/three clinical trial in vascular trauma and an ongoing phase three trial as a haemodialysis access in end-stage kidney disease. The HAV is an investigational product and has not been approved for sale by the US Food and Drug Administration (FDA) or any international regulatory agency.
affected limb, where they encourage the generation of new, healthy blood vessels. By regenerating the blood flow to the limb, the therapy “potentially prevents limb amputation, or even death, and allows the patients to resume normal activity,” they write in the press release.
Yael Porat, founder and CEO of BioGenCell, commented on the company update: ”This is great news for patients with ischaemic vascular diseases who are at risk of limb amputation.”
Shlomo Baytner of Laniado Hospital in Netanya, Israel, remarked: “BioGenCell’s innovative treatment is intended for patients whom we can longer offer the option of catheterisation or bypass. These patients suffer constant pain over a long period of time, and I am optimistic and hope that we will finally have a treatment available that can help these patients.”
Shape Memory Medical announces first patient treated in EMBO postmarket surveillance registry in Germany
Shape Memory Medical has announced the first patient treated in Germany as part of the EMBO postmarket surveillance registry (EMBO-PMS), the company’s prospective, multicentre registry study of its Impede and Impede-FX embolisation plugs when used for peripheral vascular embolisation.
BioGenCell begins recruitment for clinical trial of stem cell-based treatment for preventing limb amputation
BioGenCell has announced that it is recruiting for an international clinical trial to assess the company’s stem cell-based therapy for chronic limbthreatening ischaemia patients who are at risk for limb amputation.
The clinical trial is currently taking place in medical centres in Israel, Europe, and the USA, a press release details.
The company notes that the news follows a recent investment round that brought in US$16 million, led by the entrepreneur Marius Nacht, as well as US Food and Drug Administration (FDA) and Health Authorities approvals in Israel and Europe to carry out the study.
BioGenCell states that the company previously demonstrated the efficacy of their treatment in the prevention of limb amputation based on cells from patients’ blood in lab settings and in animal models, and reported promising results in a phase one clinical trial.

The company claims that their technology “transforms the patient’s self-blood cells to cells with rehabilitative functions”. These personal cells are then injected back into the patient, directly into the
The patient was treated by Götz Martin Richter, chairman of the Clinics for Diagnostic and Interventional Radiology at the Center for Minimally Invasive Medicine, Klinikum Stuttgart (Stuttgart, Germany) in cooperation with the Clinic for Vascular Surgery, Endovascular Medicine and Transplant Surgery in the Klinikum Stuttgart.
The EMBO-PMS has been initiated at two centres in the UK and has now expanded to include up to ten centres in Germany, with the goal of treating up to 125 patients. Results thus far in the UK reflect treatment for conditions including iliac artery aneurysm, type II endoleak after thoracic endovascular aneurysm repair (TEVAR), type 1b endoleak after endovascular aneurysm repair (EVAR), gluteal artery aneurysm, and splenomegaly. “We are pleased to participate in this important study and look forward to the follow-up from our treated patients,” said Richter, who is also the principal investigator for the EMBO-PMS.
Both the Impede and Impede-FX embolisation plugs incorporate Shape Memory Medical’s shape memory
34 Market Watch BIBANews April 2023 CHARING CROSS SPECIAL EDITION
Clinical News
Martin Austermann
The BioGenCell lab grows educated cells from the patient’s blood within a day
Impede embolisation plug, expanded
Clinical News
polymer, which is a porous, radiolucent, embolic scaffold that is crimped for catheter delivery and self-expands upon contact with blood, enabling conversion to organised thrombus followed by gradual healthy tissue formation. Preclinical and clinical studies have shown that shape memory polymer offers effective and predictable space-filling, stable clot formation for embolisation, and progressive healing as the material is absorbed.
“We look forward to collaborating with colleagues from Germany to add to the experience within EMBOPMS,” said Robert Morgan, consultant vascular and interventional radiologist, St George’s NHS Trust (London, UK) and principal investigator, EMBO-PMS UK registry. “We are continuing to obtain data to support the safety and effectiveness of the Impede devices across a variety of peripheral vascular anatomies with technical success and target vessel thrombosis achieved in all cases,” continued Morgan.
Gore initiates EMBRACE registry to evaluate VBX stent graft as bridging stent

W L Gore & Associates announced that it is initiating the EMBRACE registry to capture real-world data about the
Gore Viabahn VBX balloon-expandable endoprosthesis used as a bridging stent in conjunction with a branched/ fenestrated stent graft.
This multicentre, retrospective and prospective registry is being conducted to evaluate the clinical performance and safety of the VBX stent graft as a bridging stent. Up to 15 sites in Europe will be required to enrol a minimum of 220 patients that have had treatment with the VBX stent graft. These patients will have prospective follow-up visits up to five years from the initial procedure.
The primary efficacy endpoint will be the target vessel patency at 12 months. Other secondary endpoints will address technical success, reinterventions and target vessel instability from a performance perspective, and aneurysm-related mortality and major adverse events from a safety perspective. One-year results of the registry are expected
mid-2023 and results are intended to be published and presented at major congresses thereafter.
“Currently, all stents are used offlabel during fenestrated and branched endovascular procedures. This study will evaluate the VBX stent graft clinical performance and eventually support device indication expansion for on-label-use,” said Luca Bertoglio (Spedali Civili Brescia, Brescia, Italy), coordinating investigator of the registry. “The EMBRACE registry will track the real-world effectiveness and safety of the device, with minimal inclusion and exclusion criteria, and I look forward to the results and seeing the long-term value of this device.”
The VBX stent graft offers precise delivery and supports positive outcomes in complex aortoiliac applications, Gore claims in a press release. The device was developed utilising the small diameter, ePTFE stent graft technology from the Gore Viabahn endoprosthesis. The VBX stent graft is available in a range of diameters from 5 to 11mm and lengths of 15, 19, 29, 39, 59 and also 79mm. It also offers a range of diameter adjustability in a single device, with a maximum post-dilated diameter up to 16mm with 8L or 11mm devices.

Theraclion announces results of first US trial
Theraclion has announced the results of the first Sonovein trial in the USA,
which the company describes as a “major milestone” towards receiving US Food and Drug Administration (FDA) approval and accessing the US varicose veins market.
Theraclion reports a 100% feasibility result—the primary endpoint of the trial. It notes also that patients did not experience any serious adverse event, and that abolition of venous reflux, as measured by duplex ultrasound, was achieved in 95% of cases.
A total of 20 patients were enrolled in this clinical trial, which was approved by the FDA in early 2022. The study compliance was 100%, meaning all 80 visits were completed and timely and none lost to follow-up or missed visits, a press release details.
Steve Elias (Englewood Health Network, Englewood, USA), Antonios Gasparis and Nicos Labropoulos (both Stony Brook Medicine, Stony Brook, USA) conducted the trial.
Elias commented: “I am happy to have finished the first FDA-approved trial with Sonovein in the USA. More importantly, the results are as good— if not a little bit better—than some of the results with the more traditional technologies. Ninety-five per cent of patients had a successful healing of the abnormal vein. We treat people who have abnormal veins, and not just the veins. Most important, all patients felt better. Sonovein really is the next evolution of a completely noninvasive, transcutaneous way of treating vein diseases.”
Reduced registration fee until May 6 CET!
LINC 2023, the Leipzig Interventional Course, will feature numerous live cases performed from leading interventional centers worldwide, news on the latest developments, KOL in-depth discussions on hot topics, company symposia, and poster sessions.
LINC 2023 will take place from Tuesday, 6 June through Friday, 9 June 2023 in Leipzig, Germany.
more information visit our website at www.leipzig-interventional-course.com
35 Market Watch BIBANews April 2023 CHARING CROSS SPECIAL EDITION
Viabahn VBX balloon-expandable endoprosthesis
Leipzig Interventional Course | 6 – 9 June 2023
For
LINC 2023 Bringing the Global Vascular Community Together Insight. Innovation. In Person. In One Place. Leipzig, Germany Don’t miss reduced registration fee! LI23_vascular_news225x153plus3_march.indd 1 14.03.23 12:19
JOIN ENDOVASCULAR EXPERTS
















Explore
Learn
Loews Miami Beach
Miami Beach, Florida
2024
22–25 JANUARY
More and Register at iset.org INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
USE CODE JOURNAL10 BY 31 MAY FOR 10% OFF*
groundbreaking techniques, technologies, and procedures while engaging in discussion and debate with the experts in your field to drive patient care forward.
*Valid for new, clinician registrations only.
Product News
Shockwave Medical announces US launch of new peripheral IVL catheter
Shockwave Medical announced the full US commercial availability of the Shockwave L6 peripheral intravascular lithotripsy (IVL) catheter following clearance by the US Food and Drug Administration (FDA).
A press release details that the Shockwave L6 catheter is purpose-built to modify calcification in otherwise difficult-to-treat lesions in large peripheral vessels, including the iliac and the common femoral arteries.

“The Shockwave L6 catheter pushes the boundaries of what IVL can help physicians achieve for patients with severe peripheral arterial disease,” said Frank Arko (Sanger Heart and Vascular Institute, Charlotte, USA). “The characteristics of the new catheter allow physicians to optimise IVL therapy in large peripheral vessels, which represent over 20% of peripheral interventions performed in the USA each year. The Shockwave L6 catheter may also be beneficial when IVL is utilised to facilitate transfemoral access for large bore procedures like TAVR [transcatheter aortic valve replacement], TEVAR [thoracic endovascular aortic repair] and EVAR [endovascular aortic repair] to minimise the risks of rupture and dissection.”
According to Shockwave Medical, the L6 IVL catheter’s compact emitter configuration, in conjunction with four new device sizes (8-, 9-, 10- and 12-mm diameters), enables effective delivery of sonic pressure waves in larger vessels.
“Shockwave L6 is another example of our team’s commitment to developing technologies to address specific market needs while still maintaining the safety, effectiveness and ease of use of our core IVL technology,” said Doug Godshall, chief executive officer of Shockwave Medical. “We are pleased with the success of our limited release and the great feedback we have received from our customers. We now look forward to offering Shockwave L6 as an additional IVL option for the most complex and high-risk large vessel cases.”
VeinWay crossing tool sees first-in-human compassionate use for venous recanalisation
In a first-in-human, compassionate-use case approved by the US Food and Drug Administration (FDA), University of Michigan Health (Ann Arbor, USA) interventional radiologists David M Williams and Minhaj S Khaja successfully used VeinWay’s Traversa for venous recanalisation to save a patient’s leg that was close to being amputated.
Traversa, a newly developed tool for crossing tough occlusions in veins, enabled the physicians to recanalise a previously uncrossable vascular pathway, which included a 20cm-long blocked vein with four occluded stents.
Williams made the following comment on the procedure—“I had crossed the segment in this patient three years ago, but today it would have been impossible with existing devices. The patient’s leg was in a desperate condition. Traversa not only made this procedure possible, but also much easier and impressively faster.”
Khaja said that “the device allowed us to take the path that we wanted rather than being forced to troubleshoot the path chosen by the sharp recanalisation devices available today. With Traversa, we were able to choose the angle and the length of the throw of the needle. This enabled us to pick the pathway and if we did not like it, we were able to readjust. I think that allowed us to safely get through the occlusions and the stents.”
VeinWay CEO Jordan Pollack stated that his company’s “mission is to give surgeons the control they need to recanalise the veins of their patients safely, in a timely manner, and successfully. In doing so, we hope to inspire physicians to perform more venous recanalisations for the patients that need it to relieve pain and improve mobility.
“The procedure at University of Michigan Health was a huge step in changing the medical landscape of venous recanalisation. We would like to thank the US FDA for approving the use of Traversa in this first compassionate use case as well as Drs Williams and Khaja for their agreement to try something new. We were honoured to be of service.”
Biotronik launches Oscar multifunctional peripheral catheter
Biotronik has announced the US Food and Drug Administration (FDA) 510(k) clearance and CE mark of its Oscar (One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter.
The company notes in a press release
that Oscar is intended for percutaneous transluminal interventions in the peripheral vasculature. The device was developed to provide support during access into and to dilate stenoses in femoral, popliteal and infrapopliteal arteries.
The Oscar peripheral multifunctional catheter is comprised of three useradjustable components:
Oscar support catheter with integrated Lock Grip
Oscar extendable dilator
Oscar length-adjustable percutaneous transluminal angioplasty (PTA) balloon
The Oscar system will be available in 11 total size configurations with either 0.014″/4Fr or 0.018″/6Fr guidewire/ introducer sheath compatibility, Biotronik states. Additional standalone Oscar PTA balloons are also available separately to be used with the Oscar support catheter.
The company details that the Oscar support catheter and dilator are used in tandem to enable lesion access and crossing with a compatible guidewire, offering a wide range of support strength, adjusted with the extension of the dilator. At its strongest support level, Biotronik claims, the combination provides up to 55% more pushability than the leading 0.035″ support catheter.
The first procedure with the Oscar system in the USA was performed by Jihad A Mustapha (Advanced Cardiac & Vascular Centers for Amputation Prevention, Grand Rapids, USA).

“Oscar is a game-changing product. In cases where we had previously failed with multiple treatment approaches, the Oscar catheter made it possible to succeed, while also saving time and reducing how long the patient needs to spend on the table. This is one of the best support systems I have ever used, and the length-adjustable balloon functionality is exceptional,” stated Mustapha.
More than 70 cases have been performed in US hospitals with the Oscar device as part of Biotronik’s
Touch sirolimus DCB below the knee
The Magic Touch percutaneous transluminal angioplasty (PTA) sirolimus drug-coated balloon catheter (DCB) has received investigational device exemption (IDE) for the treatment of below-the-knee (BTK) atherosclerotic lesions in peripheral arterial disease (PAD). Concept Medical received its first IDE approval for the Magic Touch for coronary in-stent restenosis (ISR) indication in September 2022. The Magic Touch has also been granted with a breakthrough device designation in BTK by the US Food and Drug Administration (FDA).
Currently, plain balloon angioplasty (POBA) is the standard of care for the treatment of the BTK arterial occlusion disease, along with paclitaxel-coated balloons, as well as stents. The IDE approval will allow Concept Medical to gather safety and effectiveness data for the Magic Touch PTA sirolimuscoated balloon to support a future premarket approval (PMA) in the USA, providing patients and physicians with an alternative product for the treatment of BTK arterial disease.
The CE-marked Magic Touch has already been widely studied in multiple clinical trials outside the USA and has shown promising safety and efficacy results. The product is currently being investigated in Europe in two randomised controlled trials (RCTs) for the BTK indication. The LIMES RCT is a study designed to compare the Magic Touch with POBA, while the Debate BTK Duell compares the Concept Medical catheter with a paclitaxel-coated balloon catheter.
Sahil Parikh (Columbia University Irving Medical Center, New York, USA) stated that, “Concept Medical’s proposed clinical trial studying the Magic Touch PTA in BTK indication will collect significant data on safety and efficacy of the device, thus paving its way to treating patients in USA. With the sirolimus-coated balloon having already received an IDE approval in coronary arteries, and breakthrough device designations for multiple indications, along with the vast clinical data [outside the USA], it will surely be looked up to by the US physicians and patients with PAD.”
pre-launch evaluations. Despite highly complex disease, the Oscar catheter demonstrated a 90% crossing success rate and a PTA technical success rate of 95%, the company reveals. In some cases, physicians had previously tried and failed with traditional crossing devices and strategies.
Biotronik advises that the product will be commercially available in the USA starting in the Spring of 2023 and in CE mark-accepting regions in the second half of 2023.
Concept Medical granted IDE approval for Magic
Edward Choke (Sengkang General Hospital, Singapore), one of the early investigators of the Magic Touch PTA balloon, who is also conducting a RCT against POBA (FUTURE BTK) adds, “the field of BTK angioplasty needs effective solutions to its problem of poor patency rates. This is an exciting phase three trial that will determine whether the novel Magic Touch PTA can maintain the patency of BTK arteries for a longer period of time, compared with our current gold standard of POBA. This is a key goal in our efforts to reduce the number of repeated interventions and to save the legs of our patients with the severest form of PAD. If successful, this has the potential to be a game-changer.”
37 Market Watch BIBANews April 2023 CHARING CROSS SPECIAL EDITION
Jordan Pollack, CEO, VeinWay (left) and Minhaj S Khaja (right) on the day of the first-in-human, compassionate use-case
Oscar multifunctional peripheral catheter
Xeltis secures funding to progress clinical trials
Xeltis has raised €32 million in a Series D2 equity fundraise, backed by a syndicate of current and new investors, which the company says will enable it to progress its clinical programmes into pivotal trials.

Investors include Grand Pharma, DaVita Venture Group, EQT Life Sciences, Invest-NL and others.
Xeltis’ proprietary endogenous tissue restoration (ETR) platform utilises an advanced polymer-based material which triggers the body’s natural healing response to regenerate the patient’s own tissue around it, forming new, living and long-lasting vessels and valves.
The company’s most advanced program, aXess, is a vascular access graft for patients with chronic kidney disease (CKD) requiring haemodialysis. Xeltis is also pursuing clinical programmes in pulmonary valve replacement and coronary artery bypass grafts.
Alongside the equity fundraise,
Conference calendar
25–27 April
Charing Cross (CX) Symposium 2023 London, UK cxsymposium.com
27–29 April
International Vein Congress (IVC) Miami Beach, USA ivcmiami.com/index.php
10–13 May
Venous Symposium 2023 New York, USA venous-symposium.com
Xeltis and Grand Pharma have completed a license deal, covering Greater China, for aXess and other potential haemodialysis products developed under the same technology platform. Under the agreement, Grand Pharma will have exclusive rights to develop, produce and commercialise these products in Greater China.

“The strategic support of Grand Pharma and DaVita alongside our existing investor base represents a strong validation of our technology and potential to transform the landscape of cardiovascular surgery. We remain focused on progressing our aXess clinical trials, as well as exploring our next steps in other indications,” commented Eliane Schutte, CEO of Xeltis. “Securing this financing is an important milestone for Xeltis. We are proud to have attracted such high-profile investors to our company and look forward to leveraging their respective insights—from clinical expertise to the product development lifecycle—as we continue on the next phase of growth.”
25–26 May
Pacific Northwest Endovascular Conference (PNEC) 2023
Seattle, USA pnec-seattle.org
6–9 June
Leipzig Interventional Course (LINC) 2023
Leipzig, Germany leipzig-interventional-course.com
14–17 June Society for Vascular Surgery (SVS) Vascular Annual Meeting 2023


National Harbor, USA vascular.org/vam-2023
Cydar Medical raises US$11.5m in Series A funding round, appoints new chairman





Cydar Medical recently announced it has successfully completed a US$11.5 million (£9.3 million) Series A funding round including a US$3.7 million (£3 million) cornerstone investment by Pembroke Venture Capital Trust (VCT). The company also revealed that Ken Hitchner has been appointed as chairman of the board with immediate effect. A press release notes that proceeds from the financing round will enable the company to advance its pioneering artificial intelligence (AI) surgical maps platform and also bolster the company’s ongoing commercial expansion.
This Series A funding round will support the expansion of the company’s Operations, Science & Development, Business Development and Customer Onboarding & Service teams, the release continues. These
expanded capabilities will support the successful execution of the key strategic collaborations announced by the company last year with leading industry players, including Medtronic Vascular and BrainLab.

The proceeds will also enable the ongoing development of Cydar EV Maps. That includes the planned release of the first version of Cydar EV Intelligent Maps in 2023, which will enable clinicians planning their minimally invasive surgery cases to compare patient anatomy with prior cases undertaken globally and will, over time, provide predictive procedure-planning capabilities. The company also plans to release a generally applicable Cydar Maps product offering a solution for minimally-invasive procedures beyond the current endovascular indication.
The Series A financing was led by Pembroke VCT, managed by Pembroke Investment Managers, who focus on growth stage companies across a range of sectors. Pembroke VCT were joined in the round by existing shareholders, as well as a number of new investors which included Downing, through its Downing Healthcare Ventures fund, which focuses on investment in healthcare and life science companies based in the UK.
22–24 June

23rd European Venous Forum (EVF) Annual Meeting Berlin, Germany europeanvenousforum.org/index. php/meetings
29–30 June
British Society of Endovascular Therapy (BSET) Annual Meeting 2023 Wotton-under-Edge, UK bset.co.uk/meetings/bset-annualmeeting-2023
9–13 September
Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Annual Congress 2023 Copenhagen, Denmark cirsecongress.cirse.org/about/ theannualcongress
17–21 September
UIP 2023 World Congress Miami Beach, USA myavls.org/annual-congree-2023. html
26–29 September
European Society for Vascular Surgery (ESVS) Annual Meeting 2023 Belfast, UK esvs.org/events/annual-meeting/ annual-meeting-2023
30 October–2 November
Vascular Interventional Advances (VIVA) 2023 Las Vegas, USA viva-foundation.org/future-meetings
Sign up for a free print subscription* and e-newsletter subscription** www.vascularnews.com Vascular News is a trusted, independent source of news and opinion in the vascular and endovascular world. *Available for US and EU readers only ** Available worldwide
News 38 Market Watch BIBANews April 2023 CHARING CROSS SPECIAL EDITION Next generation of vascular surgeons need open and endovascular skills E Head-to-head trial for peripheral interventions “There was total support favour the multidisciplinary philosophy, with 100% vote for the motion”
Industry
CLASSICAL OPEN AND ENDOVASCULAR SOLUTIONS

CARDIAC, VASCULAR AND ENDOVASCULAR AORTIC ADVANCES

HOLD THE DATES
MONDAY–WEDNESDAY

2–4 OCTOBER 2023
IN PERSON AND VIRTUAL ANDAZ VIENNA AM BELVEDERE, VIENNA, AUSTRIA









AORTIC
cxaortic.com
VIENNA



Visit our website for more information on use, indications, contraindications, warnings/precautions and availability within your market. Custom made devices are specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications; which gives (1) specific design characteristics provided under that person’s responsibility and (2) is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs. Custom made devices are not available in the US and availability is subject to local regulatory approval. Manufactured by: Bolton Medical Inc, 799 International Parkway, Sunrise, Florida 33325, United States OUS_0247-A Feb23 • PM-06701 Advancing Fenestrated Horizons Fenestrated TREO To find out more, visit: terumoaortic.com/fenestrated-treo












































































































































































































