

Introduction A paradigm shift in the management of severe iliac calcification
Charting the history of treatment for severely calcified iliac arteries, Michel Bosiers (University Hospital Bern, Bern, Switzerland) explains how the Shockwave L6—which is now available in selected EU markets—could change the game.

LARGE-VESSEL CALCIFICATION,
particularly in the iliac arteries, poses a significant challenge in vascular interventions. Severely calcified lesions increase the risk of major complications, such as vessel rupture, dissection, and distal embolisation during endovascular treatment. The current approach to managing iliac calcification primarily relies on established techniques like balloon angioplasty and stenting, whether using covered or noncovered stents.
In the past, the debate focused on whether to use self-expandable stents or balloonexpandable stents,1 with a preference for self-expandable stents. However, the advantage of self-expandable stents in calcified lesions seems to have diminished, likely due to the higher radial outward force of balloon-expandable stents. The COBEST trial (not sponsored by Shockwave Medical) compared the use of covered balloonexpandable stents versus bare-metal stents (BMS) and demonstrated the benefit of using covered balloon-expandable stents in treating TASC C and D lesions, outperforming BMS.2 Covered stents, with their polytetrafluoroethylene (PTFE) coating, are not only designed to protect against rupture, but can also mitigate against neointimal
The Shockwave L6 offers consistent, high sonic energy output. Scan the code for an animated illustration.
often under-evaluated. Consequently, the optimal treatment approach for severe iliac calcification remains unclear, highlighting the need for improved methods or alternative strategies.
Although stenting offers proven benefits, successful outcomes require meticulous vessel preparation to ensure procedural safety. In many cases, the complexity of iliac calcification demands careful pre-stenting strategies to minimise complications, or the use of covered stents to mitigate the risk of rupture during excessive dilation in calcified lesions. This raises an important question: can stenting be entirely avoided through better preparation techniques or alternative approaches?
proliferation compared to BMS.3
A recently published systematic review confirmed the efficacy of covered stents over BMS in treating TASC C and D lesions. The primary patency rate for covered stents was 91.4% at 48 months, compared with 83.4% in the BMS group.4
The introduction of the Shockwave L6 catheter offers a promising advancement.”
However, these methods have notable limitations, including the risks mentioned earlier and the potential for under-expansion of deployed stents in heavily calcified lesions. Under-expansion can lead to early failure due to in-stent restenosis or occlusion. While stenting has shown better patency rates compared to standard balloon angioplasty, many randomised controlled trials are underpowered or consist of single-arm studies, with calcification
In recent years, Shockwave introduced its peripheral intravascular lithotripsy (IVL) system, designed for lithotripsy-enhanced, low-pressure balloon dilatation of calcified, stenotic peripheral arteries. The system includes a generator, connector cable, and an IVL catheter equipped with an array of lithotripsy emitters enclosed within an integrated balloon. A cohort analysis from the Disrupt PAD III observational study—a prospective, non-randomised, multicentre, single-arm study evaluating the real-world safety and effectiveness of the Shockwave peripheral IVL system for treating de novo calcified lesions in peripheral arteries—was published in 2020, focusing on its use in the iliac arteries. In total, 118 patients were treated using a 6 or 7mm IVL catheter (larger sizes were not yet available), with a stent placed in 72.9% of cases. No flow-limiting dissections, ruptures, or distal embolisations were observed, supporting the safety of this technology.5 However, it is not uncommon to encounter larger iliac arteries, and IVL therapy is most effective when the balloon is oversized by approximately 10% over the reference vessel diameter.
In this context, the introduction of the Shockwave L6 catheter offers a promising advancement. The Shockwave L6 is designed to overcome the limitations of current practices with its advanced features. The device offers a range of sizes from 8 to 12mm, which is crucial for treating larger iliac vessels, particularly given the need for 10% oversizing with IVL technology to maximise energy transfer into the vessel wall. This 1:1.1 ratio has already been proven safe in numerous trials. Additionally, Shockwave L6 IVL requires ultra-low delivery pressures of 2–4atm, further enhancing the safety and reducing the risk of dissection and rupture in a high-risk vascular bed. The compact
Emitter design

emitter design of the Shockwave L6, with six emitters condensed into a 30mm integrated balloon length, creates uniform high energy across its entire length, providing efficient remodelling in challenging common iliac artery and external iliac artery cases with no compromise on safety. Moreover, the use of a 0.018” guidewire provides extra support compared to the 0.014” wire currently used with the M5 and M5+, which is particularly beneficial in larger and sometimes tortuous vessels. The amalgamation of these new features suggests a significant shift in how
iliac calcification can be managed, potentially reducing the need for stenting and improving procedural outcomes.
The Shockwave L6 has the potential to revolutionise the management of severe iliac calcification, offering a paradigm shift that may preserve treatment options while enhancing patient safety.5 The cases that follow will illustrate how the Shockwave L6 device could change current practices, address existing limitations, and set a new standard for managing large vessel calcification in the iliac arteries.
Do we need to stent every calcified iliac lesion?
Mark Portou (Royal Free Hospital, London, UK) presents the case of a patient with chronic limb-threatening ischaemia (CLTI) in whom use of the new Shockwave L6 resulted in avoiding the need for a stent, thereby reducing risk to the patient and preserving future access options.
AN 86-YEAR-OLD WOMAN WITH A longstanding history of right-sided, exerciseinduced buttock and thigh pain presented acutely to our clinic with a deteriorating right third toe wound and infection following an injury. At initial presentation, she had an absent right femoral pulse, and a cyanotic right third toe with ulceration and evidence of a soft tissue infection (Figure 1). The patient had a background of bilateral total hip replacements, diabetes mellitus, hypertension and ischaemic heart disease with compensated heart failure.
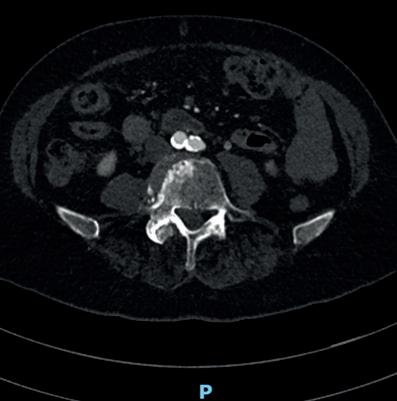
A computed tomography (CT) angiogram revealed a calcified aortic bifurcation (Figure 2) and a focal near-occlusive calcified plaque in the proximal right common iliac artery (CIA; Figure 3). Following urgent outpatient review, she was placed on the next operating list. Her treatment options included open surgical bypass with a very high perioperative risk or, more likely, an endovascular
approach. Before the availability of calciummodification technologies, optimum endovascular treatment would have inevitably required the insertion of at least one stent. The risk of a stent-only approach in this situation given the very tight stenosis would have been under-expansion of the stent and risk of subsequent symptom recurrence or stent thrombosis. Likewise, the insertion of a covered stent risked the patent internal iliac, and the possibility of kissing stents, limiting access options for future interventions.
A retrograde right percutaneous common femoral artery puncture was made under ultrasound guidance using a micropuncture sheath. The iliac lesion was crossed into the aorta and an 8F Brite Tip sheath (Cordis) was placed over an Amplatz wire. Both retrograde and antegrade digital subtraction angiography confirmed a near-occlusive stenosis of the right CIA (Figure 4). A lowcontrast angiography approach was used
References 1. Krankenberg H, Zeller T, Ingwersen M, et al. Self-expanding versus balloon-expandable stents for iliac artery occlusive disease: The randomized ICE trial. JACC Cardiovasc Interv. 2017 Aug 28;10(16):1694–1704. doi: 10.1016/j. jcin.2017.05.015. PMID: 28838480.
2. Mwipatayi BP, Sharma S, Daneshmand A, et al; COBEST coinvestigators. Durability of the balloon-expandable covered versus bare-metal stents in the Covered versus balloon expandable stent trial (COBEST) for the treatment of aortoiliac occlusive disease. J Vasc Surg. 2016 Jul;64(1):83–94.e1. doi: 10.1016/j.jvs.2016.02.064. Epub 2016 Apr 28. PMID: 27131926.B.
3. Dolmatch B, Dong YH, Heeter Z. Evaluation of three polytetrafluoroethylene stent-grafts in a model of neointimal hyperplasia. J Vasc Interv Radiol. 2007 Apr;18(4):527–534.
4. Bontinis V, Bontinis A, Giannopoulos A, et al. Editor's Choice – Covered stents versus bare metal stents in the treatment of aorto-iliac disease: A systematic review and individual participant data meta-analysis. Eur J Vasc Endovasc Surg 2024 Sep;68(3):348–358. doi: 10.1016/j.ejvs.2024.06.008. Epub 2024 Jun 12. PMID: 38876369.
5. Armstrong EJ, Soukas PA, Shammas N, et al. Intravascular lithotripsy for treatment of calcified, stenotic iliac arteries: A cohort analysis from the Disrupt PAD III study. Cardiovasc Revasc Med. 2020 Oct;21(10):1262–1268. doi: 10.1016/j. carrev.2020.02.026. Epub 2020 Mar 2. PMID: 32147133.
Michel Bosiers is a senior consultant at University Hospital Bern in Bern, Switzerland. He is a paid consultant of Shockwave Medical and his views expressed are not necessarily those of Shockwave Medical.
to illustrate flow, augmented with a 0.018” intravascular ultrasound (IVUS) catheter to measure reference vessel diameter (RVD), confirm luminal position, and delineate lesion location, length, composition, and severity of stenosis. A RVD of 10.2mm was measured. The mean luminal area of the pre-occlusive stenosis was measured to be 7.5mm2 (Figure 5). A 3mm pre-dilation with a 3x100mm Sterling balloon (Boston Scientific) over an 0.018” V18 guidewire (Boston Scientific) was performed (Figure 6). For this case, we chose to use the new Shockwave L6, which provides optimal sizing in the iliac arteries. The catheter was passed across the lesion, and inflated to 2 then 4atm. The low inflation pressures provide safe oversized expansion in high-risk vasculature. The device was placed across
Continued on page 4
Preserving future access options is key to the long-term management of patients with CLTI.”
the focal calcific lesion, repositioned across it, and a total of 300 pulses were delivered (Figures 7 and 8). From Figures 7 and 8 you can see, as more energy is delivered to the artery, the vascular compliance of the vessel changes, and the integrated balloon in the Shockwave L6 catheter begins to increase in size. Post intravascular lithotripsy (IVL), IVUS demonstrated significant lumen gain, with an increase in minimal lumen area (MLA) to 68.5mm2, and no evidence of dissection (Figure 9). Following the use of the Shockwave L6 catheter, we had achieved excellent luminal expansion. IVUS and further angiography were performed to assess for vessel recoil and extent of lumen gain. There was sufficient lumen gain and no flowlimiting dissections; therefore, we elected for no further balloon dilatation, and no stent was deployed. The post-procedure IVUS and final antegrade digital subtraction angiography images confirmed a residual stenosis less than 30%, with a 30% or less post-treatment residual stenosis providing a longer-term marker of procedural success (Figure 10).



Haemostasis was achieved with an 8F Angioseal (Terumo Interventional Systems), with an immediate return of the right femoral pulse noted. One week post procedure the patient reported complete resolution of pain and a significant improvement in the third toe wound (Figure 11). They were completely healed by six weeks (Figure 12) and are now walking unlimited distances pain free.
Prior to the availability of Shockwave L6, this lesion would have required placement of a stent, possibly kissing stents. As mentioned earlier in this report, this would have led to certain challenges for this patient by limiting access options as the likelihood of a patient with CLTI requiring further treatment in the future is high. In our practice, patients require surveillance arterial Duplex scans at regular intervals post stent insertion.
IVL technology to the larger diameter vessels. The low-pressure inflation technique and ability to increase vessel wall compliance through medial wall and intimal plaque calcium modification present a genuine opportunity to change the paradigm for calcified iliac and common femoral disease. In the situations where stents are required, adequate vessel preparation will result in better stent expansion and reduce the need to stent above the bifurcation into the aorta. This case demonstrates, however, that lumen gain can be achieved through the actions of IVL alone, without the need for balloon post dilatation or stent insertion, reducing risk and preserving options.
Mark Portou is a consultant vascular surgeon at the Royal Free Hospital in London, UK. He is a paid consultant of Shockwave Medical and his views expressed are not necessarily those of Shockwave Medical.
Patients with CLTI must be considered in the same manner as cancer patients, with a progressive disease that, post treatment, at best, should be considered as entering a state of remission. Preserving future access options is key to the long-term management of these patients. The Shockwave L6 IVL catheter now brings the safe and effective Scan code to link to corresponding video interview








Changing the treatment mindset for calcified external iliac artery disease
In this case report, Stefano Fazzini (University of Rome Tor Vergata, Rome, Italy) illustrates how the new Shockwave L6 offers an “ideal solution” for severely calcified iliac lesions as part of an optimised approach to imaging and functional assessment.
THE EXTERNAL ILIAC ARTERY
(EIA) is a very tortuous segment with a high flexion point at the level of the inguinal ligament and usually presents with a smaller diameter compared with the proximal (common iliac artery [CIA]) and distal (common femoral artery [CFA]) segments. Therefore, considering a tapered diameter at the level of these locations, a stenting procedure can be complicated by anatomical mismatch of the stent where over/undersizing of the stent selection occurs. Occlusion of the collateral vessels, such as the internal iliac artery (IIA), is also a consideration when selecting a covered stent.
This is a case example of a 71-year-old man with lifestyle-limiting claudication of the left leg (Rutherford III, walking distance <50 meters). He was an active smoker affected by arterial hypertension, dyslipidaemia and obesity. The preoperative Duplex scan showed monophasic flow at the level of the left CFA with a significant stenosis at the origin of the left EIA. The ankle-brachial index (ABI) was 0.6; two further lesions (common femoral bifurcation and distal superficial femoral artery) were identified in the ipsilateral femoropopliteal segment.
Computed tomography (CT) angiography (Figure 1) showed diffuse aortoiliac calcification on the left side, with a severe calcified eccentric stenosis at the origin of the EIA (with an ectatic CIA) and confirmed stenoses at the origins of the profunda and superficial femoral artery as moderate and severe, respectively.
In cases of severe claudication, our strategy is treating the main inflow, focusing the procedure to treat the external iliac lesion. Because of the calcific morphology of the plaque and the large vessel diameter, we believed that the patient could benefit from an endovascular treatment with the new Shockwave L6.
As per protocol in our institution, our plan was to stent the lesion only in case of complications (flow-limiting dissection or rupture) and/or suboptimal result of intravascular lithotripsy (IVL) treatment.
The reference vessel diameter (measured media-to-media) at the level of the EIA stenosis was between 8 and 8.5mm so we selected a 9mm Shockwave L6 catheter in order to have an ideal oversizing to facilitate optimised energy transfer to the vessel wall. Under local anaesthesia, a percutaneous left femoral access was performed positioning an 8F sheath. In this case, the 8F size has been used to ensure readiness in case of vessel rupture and subsequent use of a covered stent. The initial angiogram confirmed the presence of a tight and focal stenosis at the origin of the EIA and a chronically occluded IIA. The CIA diameter was between 14mm (proximally) and 20mm (distally); therefore, in case of stenting, a bare-metal stent was contraindicated for the risk of turbulence in this tapered segment (20mm>8mm) and two different covered stents or a single iliac limb of an aortic endograft would be required,
Shockwave L6 builds on the safety and efficacy of the heritage peripheral IVL catheter portfolio.”
due to the large proximal landing zone. The preoperative pressure gradient across the lesion was 41mmHg.
Pre-dilatation was performed with a 3.5x40mm plain angioplasty balloon and, thanks to the new 0.018” platform, the trackability of the Shockwave L6 made it easy to deliver into position. We proceeded to deliver all 10 cycles of therapy (five cycles at 2atm, five at 3atm; Figure 2). The completion angiogram (at least two different projections) showed an optimal result, without evidence of rupture or dissections, with a low residual stenosis <15%. Furthermore, the pressure gradient across the lesion after the treatment was <5mmHg and extravascular
ultrasound (EVUS) at the level of the left CFA showed triphasic flow (Figure 3). The distal angiogram showed direct flow to the below-the-knee vessels and the left pedal pulse was present at the end of the procedure. We were satisfied with the post-IVL result and therefore we decided to avoid any stent and any further local or distal treatment.
The patient was discharged on the first postoperative day with an ABI of 0.9 (+50%) and single antiplatelet therapy, without any symptoms. Two months after the procedure a Duplex scan confirmed the success of the procedure with triphasic flow at the left CFA, left pedal pulse and complete resolution of the claudication.
This case is an example of three different methods to assess an ideal result, in term of imaging and functional assessment. In our daily practice this new approach (multiple angiograms + gradient pressure + EVUS) is becoming the standard to treat iliac lesions and allows us to leave nothing behind after optimal IVL treatment. This ‘no-stent option’ could be of benefit in different locations and for different reasons. Some of the benefits could be avoiding unnecessary kissing stents, maintaining the patency of collaterals, treating a tapered iliac segment, and avoiding stents that could be prone to fractures in the distal zone.
Shockwave L6 builds on the safety and efficacy of the heritage peripheral IVL catheter portfolio and provides an ideal solution for severely calcified lesions in the iliac arteries. This new concept of ‘leave nothing behind’ in the iliac territory is reshaping this kind of treatment and presents the opportunity to maintain future treatment options for our patients.
Stefano Fazzini is an associate professor of vascular surgery at the University of Rome Tor Vergata in Rome, Italy. He is a paid consultant of Shockwave Medical and his views expressed are not necessarily those of Shockwave Medical.
Continued on page 6
Continued from page 5


Figure 2. Shockwave L6 (9x30mm) at first (A), fifth (B) and tenth (C) cycle showing an increasing balloon profile by cracking a tight calcific stenosis at 2–3atm of the left proximal EIA. Multiple angiograms showing imaging preoperatively (D), and after five (E) and 10 (F) cycles.
Figure 1. Preoperative CT angiography showing severe iliac calcification with concentric severe stenosis at the origin of left EIA (A) and wide mismatch in diameter of left iliac with ectatic CIA (B).

Treatment of the common iliac arteries with Shockwave L6: Preserving future options
Ashish Patel (Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, London, UK) outlines a case report demonstrating how the new Shockwave L6 catheter represents a “significant addition” to clinicians’ armamentarium for the endovascular treatment of aortoiliac occlusive disease.
A 75-YEAR-OLD FEMALE PATIENT who was previously being treated for bilateral intermittent claudication with best medical therapy and ongoing watchful waiting was referred to our institution with rest pain in her right foot. She reported worsening of her symptoms bilaterally over the preceding six months. Her symptoms suggested shortdistance claudication, with onset of pain in both of her calves after walking less than 25 metres. As a result of the new onset rest pain, she was not able to sleep, with her symptoms waking her up most nights during
the course of a week. She was an ex-smoker, and her medical history included treated hypertension and hyperlipidaemia as well as stage 3A chronic kidney disease. She had previously undergone a left common femoral artery (CFA) to above-knee popliteal bypass using reversed great saphenous vein in 2008, which was confirmed to be patent on a recent Duplex scan with a moderate (20–49%) stenosis of the distal graft anastomosis. On clinical examination, she had cool feet bilaterally, with ischaemic rubor of her right toes. Her femoral pulses were weak
Scan code to link to corresponding video interview
Figure 3. Comparison of preoperative (A-C-E) and postoperative (B-D-F) imaging and functional assessment. Severe angiographic stenosis (A), 41mmHg gradient pressure (B) and monophasic duplex waveform at the level of left CFA (E). Complete resolution of the stenosis (residual stenosis <15%, no flow-limiting dissection; B), 4mmHg gradient pressure (D) and triphasic duplex waveform at the level of left CFA (F).
bilaterally, and she had an ankle brachial pressure index (ABPI) of 0.4 on the left and 0.5 on the right.
Computed tomography (CT) angiography revealed evidence of severe bilateral common iliac artery (CIA) stenoses from their origins, with diffuse heavy vascular calcification. She also had focal calcified stenoses of her external iliac arteries (EIAs) bilaterally and an occluded left internal iliac artery. During our multidisciplinary team discussion, it was noted that her distal aorta was very small (10.4mm) and more reasonably sized approximately 3cm above the bifurcation where it measured 14.7mm. In addition, her CIAs only measured 4.8–4.9mm on both sides (Figure 1). The technical options were discussed, including a covered endovascular reconstruction of the aortic bifurcation (CERAB) or high kissing stents into the larger segment of her aorta. However, given her previous left leg bypass, it was also noted that it was important to consider technical options that would preserve the aortic bifurcation to ensure she would have an ‘up and over’ access option, should the bypass (or her other outflow/run-off native vessels)
require further endovascular intervention in the future. The planned recommended strategy was, therefore, to treat both CIAs and EIAs with intravascular lithotripsy (IVL) prior to covered stenting (with concomitant bare-metal stenting of the EIAs), in order to forego the need for either high kissing stents or a CERAB.
Due to her rest pain the procedure was performed under general anaesthesia. Bilateral CFA retrograde access was obtained, heparinisation commenced with target activated clotting time >200 seconds, and 7F sheaths advanced into both EIAs. Intravascular ultrasound (IVUS; Visions PV 0.014P, Philips) was used to accurately measure the distal aorta and both CIAs, which measured just 5.4mm and 4.1mm at their origins (Figure 2), but near 6–6.5mm distally. Intra-arterial digital subtraction angiography was used to confirm the bilateral CIA and EIA disease, accurately mark the aortic bifurcation and to carry out the remainder of the procedure using ‘image overlay’.
An 8mm Shockwave L6 IVL catheter was easily advanced into the right CIA and four cycles of IVL were delivered to this segment whilst supporting the contralateral CIA with a 6x40mm plain angioplasty ballon (Sterling balloon, Boston Scientific) that was inflated to nominal pressure (Figure 3). As a result of the need for minimal emitter overlap using the new Shockwave L6 device, we were able to treat a total of 5cm of diseased proximal iliac artery, with a further cycle of IVL delivered to the focal right EIA stenosis. In addition, we know that despite oversizing the Shockwave L6 catheter well beyond 50% for the proximal CIA, the semi-compliant balloon is never inflated above the lowest required pressure to achieve wall apposition, so the risk of vessel perforation is very low; this flexibility in sizing allows cost saving by enabling use of the same Shockwave catheter in the EIA segment. This process was repeated for the left CIA and EIA for the remaining five cycles. Repeat IVUS and angiography confirmed significant luminal gain in the treated segments (right CIA area increased from 17mm2 to 36mm2, Figure 4). The procedure was completed by deploying an 8x39mm covered Gore Viabahn VBX stent graft (Gore Medial) very accurately into each CIA, below the aortic bifurcation (Figure 4). Below these, two bare-metal Zilver 635 self-expanding 7x40mm and 7x100mm stents (Cook Medical) were deployed to treat the right and left diseased EIA segments respectively, with IVUS used to mark distal healthy landing zones and to assess for adequate stent expansion. Final angiography showed brisk flow through the aortoiliac segments (Figure 4) with no evidence of
distal embolisation and preservation of flow through the bypass graft and its run-off. A ProStyle (Abbott) was used for closure of the CFA access on both sides and check ultrasonography confirmed that both CFAs remained widely patent.
Duplex ultrasound follow-up at six weeks confirmed patency of all treated segments. The patient’s rest pain has fully resolved, she is no longer describing symptoms of claudication and has an ABPI of 0.9 bilaterally. This case demonstrates how IVL, when judiciously applied to the iliac segment, especially in cases of anatomical size mismatch between the aorta and iliac vessels, can be used to prevent the need for stenting into the aorta. The use of Shockwave L6 to lower the ‘stent zone’ by preparing the CIA origin also preserves future

1: Preoperative planning shows a 14.7mm mid-aorta (A) which tapers to just 10.2mm before the bifurcation (B). The calcified CIAs are both smaller than 5mm in diameter (C) with further calcified disease of both EIAs (D).

3: Treatment of the right (A) and left (B) CIAs with Shockwave L6 IVL with support of the contralateral iliac artery. Treatment of both focal EIA lesions with IVL (C and D).
Scan code to link to corresponding video interview
endovascular options by preserving the aortic bifurcation. In addition, the flexibility of the device with regards to sizing also allows for the treatment of calcium affecting the EIA in order to ensure maximum expansion of self-expanding stents deployed in this segment. In conclusion, the new Shockwave L6 IVL catheter is a significant addition to our armamentarium for the endovascular treatment of aortoiliac occlusive disease.
Ashish Patel is a consultant vascular and endovascular surgeon at Guy’s and St Thomas’ NHS Foundation Trust and a clinical senior lecturer in vascular surgery at King’s College London in London, UK. He is a paid consultant of Shockwave Medical and his views expressed are not necessarily those of Shockwave Medical.

Figure 2: Intra-arterial digital subtraction angiography showing a large median sacral artery, small iliac artery origins and stenoses of the EIAs (A). The aorta (B) and diameters/luminal areas of the right and left CIAs (C and D) were measured using IVUS.

Figure 4: (A) IVUS after IVL of the right CIA showing an increase in the vessel diameter (without the use of plain balloon angioplasty) from 4.1mm to 7.2mm as a result of a change in compliance and, as a result, an increase in luminal area from 12mm2 to 36mm2. (B) Deployment of 8x39mm covered VBX stent grafts into each CIA without the need for protrusion into the distal aorta (C). (D) Treatment of the EIAs with bare-metal self-expanding stents. (E) The final angiogram confirming successful treatment of the lesions.
Figure
Figure





