17 VAM 2023
‘Early birds’ flock in as registration opens, full spectrum of meeting program comes into focus
SCVS 2023 ENDOVASCULAR-FIRST
By Bryan Kay
MICHAEL S. CONTE, MD, ONE of the foremost experts in the field of peripheral arterial disease (PAD), took attendees of the 2023 Society for Clinical Vascular Surgery (SCVS) Annual Symposium in Miami on a journey through the decades of evidence-based revascularization for limb-threatening ischemia, telling those gathered that the recently published “landmark” BEST-CLI trial evidence should lead to a shift in the current practice landscape, where open bypass surgery is “under-offered and under-utilized.”
Conte, chair, professor and chief of vascular and endovascular surgery at the University of California San Francisco (UCSF), was delivering the SCVS 2023 (March 25–29)

Distinguished Visiting Professor address, devoting a significant portion to the recent publication of the BEST-CLI (Best endovascular versus best surgical therapy in patients with critical limb ischemia) randomized-controlled trial (RCT) data, and what its results means for the treatment of CLI, or chronic limb-threatening ischemia (CLTI) going forward.
Conte posed the ultimate question: What are the take-home messages in the world of CLTI treatment post-publication of the BEST-CLI results? “I think it is really
See page 6
APPROPRIATENESS
A joint effort aimed at ensuring quality vascular care and quality improvement is a universal goal has seen eight pilot programs—four inpatient and four outpatient centers—go through the pilot phase of the verification process.
By Beth Bales
ON OCCASION OF 45TH ANNIVERSARY, CX SYMPOSIUM CHAIRMAN CREDITS CHICAGO VASCULAR SURGICAL TANDEM FOR ENDURING SUCCESS
By Jocelyn Hudson and Bryan Kay
See page 6
THE SOCIETY FOR VASCULAR SURGERY (SVS) and the American College of Surgeons (ACS) have launched the “Vas-cular Verification Program (Vascular-VP),” an ACS Quality Program developed in partnership with the SVS. The newly launched inpatient program reviews not just safety processes against standards created by vascular surgeons, but also emphasizes the importance of using clinical data for tracking outcomes and supporting quality improvement specific to vascular care. It covers the perioperative continuum of care, from pre-hospital to post-discharge care.
“We created this program to ensure quality and quality improvement in vascular care in both the inpatient and outpatient settings,” said SVS President Michael C. Dalsing, MD. “Our own members asked for this program, particularly for outpatient settings, which may have little or no oversight. They understand the importance of standard policies and procedures embedded wherever care is provided to ensure safety and promote quality.”
The verification process ensures that an applicant program:
◆ Has the appropriate infrastructure for the procedures performed
◆ Follows clinical pathways to ensure care is in line with evidence-based clinical guidelines when available
◆ Monitors outcomes, emphasizing the importance of clinically relevant, risk-adjusted, nationally benchmarked data
◆ Submits all needed information to reviewers who will assess all required aspects of the program for verification
◆ Undergoes a verification visit to ensure all standards are complied with and performs case review to ensure that there are internal quality processes in place to ensure safe and appropriate care of patients afflicted with vascular disease is provided
The ACS has long-standing experience in a wide array of quality verification programs to include
See page 3
THE OCCASION OF A SEMINAL MEETING HELD IN Chicago by former Society for Vascular Surgery (SVS) Presidents John J. Bergan, MD, and James S.T. (Jimmy) Yao, MD, played a central role in the formation of the world-renowned Charing Cross (CX) International Symposium, which this year marks its 45th edition.
The year was 1976, and Roger M. Greenhalgh, MD, CX founder and chairman, had not long returned from a tour of the world’s major vascular surgery centers, when he was invited to attend the Bergan and Yao meeting at Northwestern Uni-

THE OFFICIAL NEWSPAPER OF THE Presorted Standard U.S. Postage PAID Permit No. 384 Lebanon Jct. KY ascularV pecialists CHANGE SERVICE REQUESTED 9400 W. Higgins Road, Suite 315 Rosemont, IL 60018 APRIL 2023 Volume 19 Number 4 02 Guest editorial Intentional mentorship— and why it matters 07 Comment & Analysis Corner Stitch looks back at VESS 2023 and open repair volumes among trainees
Diversity
Vascular
bridges
www.vascularspecialistonline.com
12
Society of Black
Surgeons builds
In this issue:
OR -ONLY APPROACH FOR ALL CLTI PATIENTS ‘IS NOT EVIDENCE-BASED CARE’
✓ ✓
Medical Editor Malachi Sheahan III, MD
Associate Medical Editors
Bernadette Aulivola, MD | O. William Brown, MD | Elliot L. Chaikof, MD, PhD
| Carlo Dall’Olmo, MD | Alan M. Dietzek
MD, RPVI, FACS | Professor HansHenning Eckstein, MD | John F. Eidt, MD
| Robert Fitridge, MD | Dennis R. Gable, MD | Linda Harris, MD | Krishna Jain, MD | Larry Kraiss, MD | Joann Lohr, MD
| James McKinsey, MD | Joseph Mills, MD | Erica L. Mitchell, MD, MEd, FACS
| Leila Mureebe, MD | Frank Pomposelli, MD | David Rigberg, MD | Clifford Sales, MD | Bhagwan Satiani, MD | Larry Scher, MD | Marc Schermerhorn, MD | Murray
L. Shames, MD | Niten Singh, MD | Frank
J. Veith, MD | Robert Eugene Zierler, MD
Resident/Fellow Editor
Christopher Audu, MD
Executive Director SVS
Kenneth M. Slaw, PhD
Director of Marketing &
Communications Bill Maloney
Managing Editor SVS Beth Bales
Marketing & Social Media Manager
Kristin Crowe
Communications Specialist
Marlén Gomez
Published by BIBA News, which is a subsidiary of BIBA Medical Ltd.
Publisher Roger Greenhalgh
Content Director Urmila Kerslake
Managing Editor Bryan Kay bryan@bibamedical.com
Editorial contribution
Jocelyn Hudson, Will Date, Jamie Bell, Clare Tierney, Eva Malpass and Benjamin Roche
Design Terry Hawes
Advertising Nicole Schmitz nicole@bibamedical.com
Letters to the editor vascularspecialist@vascularsociety.org
BIBA Medical, Europe
526 Fulham Road, London SW6 5NR, United Kingdom
BIBA Medical, North America
155 North Wacker Drive – Suite 4250, Chicago, IL 60606, USA
Vascular Specialist is the official newspaper of the Society for Vascular Surgery and provides the vascular specialist with timely and relevant news and commentary about clinical developments and about the impact of healthcare policy. Content for Vascular Specialist is provided by BIBA News. Content for the news from SVS is provided by the Society for Vascular Surgery. | The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or the Publisher. The Society for Vascular Surgery and BIBA News will not assume responsibility for damages, loss, or claims of any kind arising from or related to the information contained in this publication, including any claims related to the products, drugs, or services, or the quality or endorsement of advertised products or services, mentioned herein. | The Society for Vascular Surgery headquarters is located at 9400 W. Higgins Road, Suite 315, Rosemont, IL 60018. | POSTMASTER: Send changes of address (with old mailing label) to Vascular Specialist, Subscription Services, 9400 W. Higgins Road, Suite 315, Rosemont, IL 60018. | RECIPIENT: To change your address, e-mail subscriptions@bibamedical.com | For missing issue claims, e-mail subscriptions@bibamedical. com. | Vascular Specialist (ISSN 1558-0148) is published monthly for the Society for Vascular Surgery by BIBA News. | Printed by Vomela
Commercial Group | ©Copyright 2023 by the Society for Vascular Surgery
Intentional mentorship and pushing beyond representation
Chicago medical student Maria Paz, BS, discusses intentional mentorship—and why she believes it matters.

When I started medical school, I never imagined that the most difficult part would be convincing myself that I deserved to be there. I remember at my first interview being separated at lunch to hear a presentation about diversity efforts at the institution. When I rejoined the rest of the students, I remember being asked where I went and one of my fellow interviewees telling me how lucky I was to be a minority. Suddenly, it felt like no one believed I was worthy of being there, least of all myself. I wish I could say this was an isolated experience. From that day forward, I felt as though I had to prove to everyone around me that I deserved the opportunities I had actually earned. Even my advisors at the time would make comments about how much easier it was for me as a Hispanic woman—how my MCAT could be lower, my application less impressive. They even went so far as to say that scholarships would be given to me, and residency spots handed over easily. It didn’t matter that I had worked for five years building a resume worth accepting. My grades, efforts, and dedication were diminished to a singular box I had to check when I applied. While my other classmates were able to start their medical school journey with the joy and excitement of finally achieving their dream, I was left with self-doubt and imposter syndrome that would take me years to unlearn, or at the very least develop effective coping mechanisms for.
The first time I met with my research mentor, she offered me the opportunity to help write a chapter for a textbook about disparities in surgery. I remember immediately feeling like I had to warn her that I would probably underperform. I wanted to set the expectations low for when I inevitably disappointed her. Years of striving to please advisors and admissions committees left me paralyzed with the fear of failure. She stopped me in the middle of yet another self-deprecating comment and said, “Stop worrying, you will be great.” Her singular vote of confidence was a startling contrast to the comments I had received in the past. This was my first experience with what I now consider intentional mentorship. Intentional mentorship goes beyond representation. Instead of simply serving as an example, these mentors use their lived experiences and backgrounds to relate to their students and actively foster a meaningful relationship. The actions that differentiate intentional mentors from the rest often require little effort, like the words of affirmation I received during that first research meeting. As medical students, we crave praise because we are so deprived of it. Words of affirmation are often so sparingly handed out but can have a lasting impact. Luckily, my mentors were not only intentionally telling me when they were impressed, but also did it vocally in front of other physicians and members of the team. Over time, their positive encouragement started to be louder than the negative self-talk to which I was so accustomed to listening. I stopped the self-deprecating comments and my mindset shifted from a fear of disappointment to an excitement about my untapped potential.
Despite the confidence boost from my mentors, the realities of academic surgery continued to reveal themselves, including the significant financial investment required to progress in your training and,
ultimately, your career. I never expected my vascular research mentors to recognize these barriers because they were so far removed from the life and budget of a student. Yet again, they all exceeded my expectations in their understanding and compassion for my circumstances when it came to presenting our research at a national conference. Whenever they could, they allocated funds from their own section budgets to help offset the cost of travel or help with registration fees. When this wasn’t possible, they were intentional about helping me look for travel grants, reminding me about scholarship application deadlines, and writing me recommendation letters for financial aid. They sought out every opportunity they could to help me, even nominating me for awards that not only added to my resume but gave me very real financial stability. I had underestimated the difference it could make to have people looking out for me professionally. All these small acts not only provided me with tangible aid but also helped me believe in myself. Then came my clinical rotations. I was immediately filled with anxiety about performing well on my vascular rotation. It was one of the only opportunities I would get to impress the section during my surgery clerkship. It would also be the first time I would interact with my mentors clinically, and again I was struck with the fear that I would disappoint. After two years of working with them in a research capacity, I was terrified they would regret investing in me if I didn’t “perform” well in the operating room. The night before I started on the service, I was cc’d on the weekly section email that included the faculty and trainees. At the very end of the email, it read “Perhaps everyone is already aware, but we have a medical student joining the team tomorrow, Maria Paz. She is interested in Vascular Surgery so let’s show her a good time.” Those few lines probably took her less than five minutes to write, but it made all the difference in my experience on the rotation. People knew to expect me, and that someone they all admired believed in me. I truly think
this led to opportunities that I wouldn’t have received otherwise. The fellow trusted me to assist with a chart review project he had been too busy to start, the attendings gave me extra learning opportunities during the procedures, and I began to really picture myself as a vascular surgeon. So many of my peers felt like they could never admit to their mentors when they were struggling. Especially given that those same physicians would be the ones reviewing their residency applications or writing their recommendations, it wasn’t an option to openly discuss issues like mental health or burnout. I had so many questions about a career in surgery. How do I balance this dream and all the other things I want out of life? What kind of mother will I be? How do I stay healthy during this journey? The only thing I never had to question was that I could turn to the mentors in my life for help finding answers. Women in medicine who agreed to mentor me truly meant it, and would never judge me for struggling. Maybe without realizing it, these relationcontinued on page 4
2 GUEST EDITORIAL Vascular Specialist | April 2023
“My mentors were not only intentionally telling me when they were impressed, but also did it vocally in front of other physicians and members of the team”
Maria Paz
continued from page 1
cancer, trauma, bariatric surgery, pediatric surgery, to name a few. All these programs use the same structure used in the new Vascular-VP. This structure is based on four pillars:

◆ Standards based on peer-reviewed published data and expert consensus
◆ Appropriate infrastructure based on the standards designed for the program

◆ Data for monitoring outcomes and quality improvement support, using reliable, clinically relevant, benchmark data

◆ External peer-review team to verify compliance with the standards


“It was a win-win to work with the College for the vascular program,” said Anton Sidawy, MD, who, as the vascular regent serving on the Board of Regents of the ACS and past president of the SVS, led the effort to create the Vascular-VP. This is not the first time that the SVS sought such a program to ensure vascular centers are reviewed for quality.
“With the ongoing shift in reimbursement for our services from volume to value, who best to define quality and value than us—vascular surgeons—and the professional organization representing us, the Society for Vascular Surgery?” Sidawy said.
Vascular diseases and issues covered in the program include, but are not limited to, thoracic-aortic, abdominal aortic, carotid disease, peripheral arterial disease (PAD), arteriovenous hemodialysis access, and superficial and deep venous disease.


Those centers that meet the current inpatient standards will be “Verified” at one of two levels: a “comprehensive
inpatient vascular center,” or “verified inpatient vascular center.” Eight pilot programs—four inpatient and four outpatient—have already gone through the pilot phase of the verification process, including both the inpatient and outpatient components of Albany Medical Center in Albany, New York, under the direction of R. Clement Darling III, MD, chief of vascular surgery at the institution, himself an SVS past president. He called the process “invaluable.” That includes ascertaining that
“
Albany Medical Center “had the components we always thought we did. We found out we need to do better in some areas, and actually begin implementing procedures in other areas.”

Administrators at Albany Medical Center found it eye-opening to see how much work goes into a successful vascular program, Darling said, including “how comprehensive the infrastructure needs to be to care for these incredibly complex patients. It showed them what infrastructure we need from the hospital to provide the best care for our patients.”
Going through the process “made us better and helped us take better care of our patients,” Darling said, and urged other vascular centers to undertake the verification process. The process also ensures the longitudinal care for which vascular surgeons are known. “Our care doesn’t end in the OR [operating room],” said Darling. “Every move has to be documented and evaluated; this process lets us follow our patients to make sure we’re providing the best care for them.”
“As the program launches, more institutions are waiting in the wings, including my own in Indianapolis, Indiana,” added Dalsing. He believes the new initiative will provide several benefits, including improvement in quality of care for patients, enhanced learning throughout the institution, and external credibility to regulators. “We set the
www.vascularspecialistonline.com 3
SVS, ACS
FROM THE COVER:
LAUNCH NEW QUALITY VERIFICATION PROGRAM
Every
in
Proven to Reduce Amputations
2 Driving Change. TOGETHER.
: 1. American Diabetes Association. https://diabetes.org/newsroom/press-releases/2022/ADA-unveils-amputationprevention-alliance-to-address-diabetes-related-amputation-pandemic 2. Yellin J, et al. Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes Advances in Wound Care, 2021. LEARN MORE
With the ongoing shift in reimbursement for our services from volume to value, who best to define quality and value than us—vascular surgeons—and the professional organization representing us, the Society for Vascular Surgery?” ANTON SIDAWY
3 minutes
the
US a person with diabetes has a limb amputated.1
by 71%
REFERENCES
MB1086-A
Founding Partner
Anton Sidawy, R. Clement Darling III and Michael C. Dalsing
INTENTIONAL MENTORSHIP AND PUSHING BEYOND REPRESENTATION
continued from page 2
ships gave me a framework for how I want to be once I finally achieve all my dreams. I know that when a medical student comes to me one day, I will be intentional about every interaction. I will remember the small things that made all the difference, and strive to show them exactly how to treat their future students. The cycle will continue, and all these small acts will add up to real and significant changes in medicine.
I now realize the power of someone believing in me, which is multiplied when it originates from mentors that you look up to. I hope that sharing more of these experiences will provide physicians with real and actionable examples of ways to be more intentional about mentorship. Improving representation in the surgical workforce must begin with helping students believe they can succeed in this field. I believe that intentional mentorship could make all the difference in recruiting diverse students into a field they historically felt simply wasn’t for them.
MARIA PAZ is a medical student at the University of Chicago Pritzker School of Medicine.
The virtue of a social media presence for surgeons in the internet era
Dear editor,
I RECENTLY READ AN ARTICLE IN VASCULAR SPECIALIST regarding the new age of the “vascular surgery influencer” that discussed how the world of social media has invaded our “once-sane” specialty. While I assume the article was meant to raise awareness of the overuse of social media platforms and how it can violate HIPAA rights, it felt like a personal attack to all who use social media for vascular surgery and neglected all the positive attributes that these platforms have to offer.
I have been in practice for just over two years now. I am by no means an expert vascular surgeon. I am no complex aortic specialist. I am your everyday vascular surgeon. Over the last two years, I have experienced, like all new vascular surgeons, successes—but also failures. While there are many successful revascularizations, the cases that have stuck with me more have been the defeats. All surgeons have these experiences.
We all like to discuss the topic of burnout and our mental health, and we all share our opinions on the matter, but it is not often that we have a good resource to manage these things. For me, social me-
dia is my resource. It is an outlet where I can share my stories, and share my victories. And I have also discovered that I do like a little validation, or a “pat on the back.” They are a reminder that while this specialty is exhausting and filled with tribulation, victories do occur, and they are nice to share with people. Because one thing I have learned as an attending is that I do not always have people with whom to share this.
As a trainee, I was always surrounded by my colleagues in our call room. Our vent sessions—sharing our successful cases and our failures—were a daily occurrence. It was a great place to learn as well: impromptu M&M conference was held every lunchtime. I miss that collegiality as an attending. While I am fortunate to have two great partners who are very supportive, at the same time they have their own lives. We do not often sit in a call room and discuss our cases. We have families to go home to. And at the end of the day, the last thing we want to discuss when we go home is vascular surgery. So, for me, social media is that outlet. It is my “call room” that I visit to find colleagues to share my successes and have them congratulate. It is also the place I can share my failures with those who understand and can benefit from my mistakes, and where I can learn from others. I believe we are fortunate to have a social media presence in the internet era, where surgeons can still stay connected and learn from each other in an ever-evolving field, despite being miles apart. I believe there is a role for validation; there is solace to know that we are not alone when we face those specialty hardships. Of course, there are issues of overuse and HIPAA compliance, which are important matters. As with any tool, these outlets can be misused, and we should know their limitations. However, that does not mean we should abandon social media completely. Instead, should we not embrace the future of our specialty, and help guide its presence in social medial? I doubt I am the only one out there who seeks a little validation from time to time.
Vincent Noori, MD Mercy Medical Center, Baltimore
References
1 Holden A. The IN.PACT AV Access Study: Results through 36 Months. Presented at Charing Cross 2022.
2 Trerotola SO, Saad TF, Roy-Chaudhury P; Lutonix AV Clinical Trial Investigators. The Lutonix AV Randomized Trial of Paclitaxel-Coated Balloons in Arteriovenous Fistula Stenosis: 2-Year Results and Subgroup Analysis. J Vasc Interv Radiol. January 2020;31(1):1-14.e5.
Brief Statement IN.PACT™ AV Drug-coated PTA Balloon Catheter
Indications for Use
The IN.PACT™ AV Paclitaxel-coated PTA Balloon Catheter is indicated for percutaneous transluminal angioplasty, after appropriate vessel preparation, for the treatment of obstructive lesions up to 100 mm in length in the native arteriovenous dialysis fistulae with reference vessel diameters of 4 to 12 mm.
Contraindications
The IN.PACT AV DCB is contraindicated for use in the following anatomy and patient types: Coronary arteries, renal arteries, and supra-aortic/cerebrovascular arteries
• Patients who cannot receive recommended antiplatelet and/or anticoagulant therapy
Patients judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the delivery system
• Patients with known allergies or sensitivities to paclitaxel
Women who are breastfeeding, pregnant, or are intending to become pregnant, or men intending to father children. It is unknown whether paclitaxel will be excreted in human milk and whether there is a potential for adverse reaction in nursing infants from paclitaxel exposure.
Warnings
• A signal for increased risk of late mortality has been identified following the use of paclitaxel-coated balloons and paclitaxel-eluting stents for femoropopliteal arterial disease beginning approximately 2-3 years post-treatment compared with the use of non-drug coated devices. There is uncertainty regarding the magnitude and mechanism for the increased late mortality risk, including the impact of repeat paclitaxel-coated device exposure. Inadequate information is available to evaluate the potential mortality risk associated with the use of paclitaxel-coated devices for the treatment of other diseases/conditions, including this device indicated for use in arteriovenous dialysis fistulae. Physicians should discuss this late mortality signal and the benefits and risks of available treatment options for their specific disease/ condition with their patients. Use the product prior to the Use-by date specified on the package.
• Contents are supplied sterile. Do not use the product if the inner packaging is damaged or opened.
• Do not use air or any gaseous medium to inflate the balloon. Use only the recommended inflation medium (equal parts contrast medium and saline solution).
• Do not move the guidewire during inflation of the IN.PACT AV DCB.
• Do not exceed the rated burst pressure (RBP). The RBP is based on the results of in vitro testing. Use of pressures higher than RBP may result in a ruptured balloon with possible intimal damage and dissection.
• The safety of using multiple IN.PACT AV DCBs with a total drug dosage exceeding 15,105 μgpaclitaxel has not been evaluated clinically.
Precautions
This product should only be used by physicians trained in percutaneous transluminal angioplasty (PTA). Assess risks and benefits before treating patients with a history of severe reaction to contrast agents. Identify allergic reactions to contrast media and antiplatelet therapy before treatment and consider alternatives for appropriate management prior to the procedure.
This product is not intended for the expansion or delivery of a stent.
• Do not use the IN.PACT AV DCB for pre-dilatation or for post-dilatation.
This product is designed for single patient use only. Do not reuse, reprocess, or resterilizethis product. Reuse, reprocessing, or resterilizationmay compromise the structural integrity of the device and/or create a risk of contamination of the device, which could result in patient injury, illness, or death.
The use of this product carries the risks associated with percutaneous transluminal angioplasty, including thrombosis, vascular complications, and/or bleeding events.
• The safety and effectiveness of the IN.PACT AV DCB used in conjunction with other drug-eluting stents or drug-coated balloons in the same procedure has not been evaluated.
The extent of the patient’s exposure to the drug coating is directly related to the number of balloons used. Refer to the Instructions for Use (IFU) for details regarding the use of multiple balloons and paclitaxel content.
Appropriate vessel preparation, as determined by the physician to achieve residual stenosis of ≤ 30%, is required prior to use of the IN.PACT AV DCB. Vessel preparation of the target lesion using high-pressure PTA for pre-dilatation was studied in the IN.PACT AV Access clinical study. Other methods of vessel preparation, such as atherectomy, have not been studied clinically with IN.PACT AV DCB.
Potential Adverse Effects
Potential adverse effects which may be associated with balloon catheterization may include, but are not limited to, the following: abrupt vessel closure, allergic reaction, arrhythmias, arterial or venous aneurysm, arterial or venous thrombosis,death, dissection, embolization, hematoma, hemorrhage, hypotension/hypertension, infection, ischemia or infarction of tissue/organ, loss of permanent access, pain, perforation or rupture of the artery or vein, pseudoaneurysm, restenosis of the dilated vessel, shock, stroke, vessel spasms, or recoil.
Potential complications of peripheral balloon catheterization include, but are not limited to, the following: balloon rupture, detachment of a component of the balloon and/or catheter system, failure of the balloon to perform as intended, failure to cross the lesion. These complications may result in adverse effects.
Although systemic effects are not anticipated, potential adverse effects not captured above that may be unique to the paclitaxel drug coating include, but are not limited to, the following: allergic/immunologic reaction, alopecia, anemia, gastrointestinal symptoms, hematologic dyscrasia (including leucopenia, neutropenia, thrombocytopenia), hepatic enzyme changes, histologic changes in vessel wall, including inflammation, cellular damage, or necrosis, myalgia/ arthralgia, myelosuppression, peripheral neuropathy.
Refer to the Physicians’ Desk Reference for more information on the potential adverse effects observed with paclitaxel. There may be other potential adverse effects that are unforeseen at this time.
Please reference appropriate product Instructions for Usefor a detailed list of indications, warnings, precautions, and potential adverse effects. This content is available electronically at www.manuals.medtronic.com.
CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician.
UC202216301 EN ©2022 Medtronic. All rights reserved. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. For distribution in the USA only. 05/2022 medtronic.com/AVdata
4 Vascular Specialist | April 2023 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Months 0 1 3 6 7 9 12 18 24 30 36 Lutonix DCB 26.9% Log-rank p = 0.087 Probability of target lesion primary patency Probability of target lesion primary patency IN.PACT AV DCB 43.1% Standard PTA 28.6% Months 0 1 3 6 7 9 12 18 24 30 36 Standard PTA 24.4% Log-rank p < 0.001 at 6, 7, 12, 24, and 36 months 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Months 0 1 3 6 7 9 12 18 24 30 36 Lutonix DCB 26.9% Log-rank p = 0.087 Probability of target lesion primary patency Probability of target lesion primary patency IN.PACT AV DCB 43.1% Standard PTA 28.6% Months 0 1 3 6 7 9 12 18 24 30 36 Standard PTA 24.4% Log-rank p < 0.001 at 6, 7, 12, 24, and 36 months
P
GUEST EDITORIAL LETTER TO THE EDITOR
IN.PACT™ AV Drug-Coated Balloon (DCB)
First & only
The first and only DCB with superior, sustained results at 36 months for AV fistula lesions versus PTA.1,2
Separate trials evaluating target lesion primary patency for IN.PACT AV DCB at 36 months and Lutonix™* DCB at 24 months.†
36-month results
vs. PTA at 36 months
vs. PTA at 36 months
26.9%
2.5% vs. PTA at 24 months
months
*Third-party brands are trademarks of their respective owners.
p = 0.087
0.087
24.4%
24.4%
43.1%
43.1% Standard
28.6%
28.6%
†Primary patency endpoints are defined differently; results are from different studies and may vary in a head-to-head comparison; charts are for illustration purposes only.
‡IN.PACT AV Access Trial: Target Lesion Primary Patency Rate was defined as freedom from clinically driven target lesion revascularization (CD-TLR) or access circuit thrombosis measured through 36 months (1,080 days) post-procedure.
§Lutonix AV Clinical Trial: Target Lesion Primary Patency was defined as freedom from clinically driven reintervention of the target lesion or access thrombosis measured through 24 months.
100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Months 0 1 3 6 7 9 12 18 24 30 36 Lutonix DCB
Log-rank p =
Probability of target lesion primary patency Probability of target lesion primary patency IN.PACT AV DCB
Standard PTA
Months 0 1 3 6 7 9 12 18 24 30 36 Standard PTA
Log-rank p < 0.001 at 6, 7, 12, 24, and 36 months 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Months 0 1 3 6 7 9 12 18 24 30 36 Lutonix DCB
Log-rank
Probability of target lesion primary patency Probability of target lesion primary patency IN.PACT AV DCB
PTA
Months 0 1 3 6 7 9 12 18 24 30 36 Standard PTA
Log-rank p < 0.001 at 6, 7, 12, 24, and 36 months
26.9%
IN.PACT
14.5%
Lutonix
14.5%
AV DCB‡1 2.5% vs. PTA at 24
DCB§2
continued from page 1
clear,” said Conte, “that an endo[vascular]-first, or, even more strikingly, endo-only approach, to all patients with CLTI is simply not evidence-based care. And Centers of Excellence must be skilled in both techniques of revascularization.
“Furthermore, informed decision-making with patients in this field should include the results of this evidence, and suitable patients should be offered the option of open bypass surgery, which I suspect is being under-offered and under-utilized in current practice. I think this trial should make that begin to change.
“It’s not surprising—it shouldn’t be surprising to us— that there are trade-offs between effectiveness and invasiveness. This is common in medicine and surgery, and, as I showed you, it is common in coronary artery disease. We need to embrace it and accept it. But I would suggest to you that one of the differences between coronary disease and vascular disease is that, as vascular surgeons doing both of these things, we have the opportunity to make these decisions. That could be either an advantage or burden, because we all have settled into our ways and our workflows, and may be unwilling to change what we currently do in practice.”
Conte then returned to an overarching message he also delivered at a BEST-CLI session hosted at the 2022 VEITHsymposium in New York last November: “I would suggest to you that centers doing less than 20% bypass in CLTI should really probably take stock about whether this is the best treatment for these patients,” he said.
Results from BEST-CLI showed that surgical bypass with adequate single-segment great saphenous vein (GSV) is a more effective revascularization strategy for patients with CLTI who are deemed to be suitable for either an open or
continued from page 1
versity. The brilliance of that educational meeting, said Greenhalgh, inspired him to create something similar back in London, England, where the then-young vascular surgeon, soon-to-be surgery department chair, would birth the CX Symposium at Charing Cross in 1978.

“John Bergan was a master of education,” remarked Greenhalgh. “I tried to do what he and Jimmy Yao did. Jesse Thompson, from Dallas, came to the first CX meeting in 1978, which was focused on progress in stroke research. Even now, we have an acute stroke session, but the first was entirely about that area.”
Every year since, Greenhalgh recalls, he has been invited to the U.S. “and learned from the giants,” drawing particular attention to Houston, “the mecca of vascular surgery,” and its most famous cardiovascular son, Michael E. DeBakey, MD, with whom he had spent time the year prior to attending the Northwestern meeting. “Nothing after that equalled that amazing experience,” he said. “There was no comparison between the quality of care there seen anywhere else in the world.”
Thus, the CX cornerstone was laid. Over the years, the symposium was the setting for key moments in vascular surgery history. The European Society for Vascular Surgery (ESVS) was convened at the meeting in 1988, with DeBakey involved in prompting vascular societies of Europe to form a pancontinental group, Greenhalgh noted.
In the same decade, Andreas Grüntzig, MD, attended CX to talk about his version of a new balloon angioplasty system, and the symposium was an incubator for the development of key randomized-controlled (RCT) trials, such as the UK Small Aneurysm Trial, EVAR 1 and 2, and IMPROVE.



He references “interesting data” on the subject from Naseer Ahmad, MBChB, from Manchester Royal Infirmary in Manchester, England, which “would seem to suggest that in parts of the population where there is poverty, or inadequate facilities, that these are the areas in which the ‘hurting leg’ does not get the attention it should.” Suggesting what can be done to address this, Greenhalgh stresses that it is important to throw light upon this issue “in order to be able to get more people to get more timely intervention and have their leg saved.”
On the occasion of the 45th anniversary of CX, Greenhalgh ponders what is coming next for the meeting. In particular, he emphasizes the importance of future planning to ensure the concept of CX—education, innovation and evidence—continues.
To that end, he has recently appointed three new cochairs to the CX leadership team who will work alongside him to deliver the CX program going forward: Dittmar Böckler, MD, medical director of the Clinic for Vascular and Endovascular Surgery at University Hospital Heidelberg in Heidelberg, Germany; Andrew Holden, MD, director of interventional radiology at Auckland City Hospital in Auckland, New Zealand; and Erin Murphy, MD, director of the Venous and Lymphatic Institute at Sanger Heart and Vascular, Atrium Health in Charlotte, North Carolina. “We are covering the globe and all vascular subjects,” Greenhalgh said of the new leadership team. “There will be every opportunity for the CX concept to continue if that is considered to be worthwhile doing.”
endovascular approach, the investigators reported. In patients without a suitable single-segment saphenous vein, both surgical and endovascular strategies were found to be effective in treating patients with CLTI, leading the investigators to conclude that there is “a complementary role for both revascularization strategies in these patients.”
BEST-CLI was a “landmark effort by [principal investigators] Alik Farber, Matt Menard and many others,” he said. Their passion and dedication “cannot be overstated in making this a reality over almost a decade, with much more to come from this trial.”
The work exhibited vascular surgeons taking the lead, he continued, “designing and executing science that is practice-changing science,” and “that needs to be recognized,” Conte added. It involved the BEST-CLI researchers taking lessons learned over the 20- to 30-year time span covering the development of evidence-based revascularization that he outlined in his address, he said, rolling them into the design of the trial. Conte, having summarized BEST-CLI’s headline results and delved into characteristics of its two cohorts to underscore his point, also noted the trial’s limitations—a theme of focus among its skeptics. “Any trial can only begin to answer certain questions, and raises many others,” he said. “I think it is currently the standard, landmark trial in our field from which we can launch forward with true evidence-based guidelines and approaches.”
In keeping with this long-standing tradition for being a theater for presentation and discussion of landmark advances in vascular care, this year will see CX play host to delivery of the first results from the much-anticipated BASIL-2 (Bypass versus angioplasty in severe ischemia of the leg-2) trial. They will be revealed by chief investigator Andrew Bradbury, MD, from the University of Birmingham in Birmingham, England, in the presence of representatives of the BEST-CLI (Best endovascular versus best surgical therapy for patients with critical limb ischemia) trial. A roundtable discussion is planned to include invited commentary from Eleni Whatley, from the U.S. Food and Drug Administration (FDA), and the British Secretary of State for Health, Steve Barclay. “At the moment of speaking, publication of the results will take place in The Lancet, which is a huge moment for CX,” he said. “We have had 45 years of a very global CX, and the tradition continues.”

through the years
The CX anchor points to what he sees as one of the central pillars of the CX brand: its multidisciplinary appeal. “If you have an interest in—and are managing patients with—vascular disease,” he says, “there is something for you at CX.” This multidisciplinary approach is particularly important for the education of the next generation, Greenhalgh believes. “It is important to get as much experience alongside as many people as you can, so that you become a compendium of all those people that you have learned from,” he added.
Looking ahead, Greenhalgh believes one of the most pressing issues facing the vascular world is that of patients with a “hurting leg” not being seen by a professional in a timely manner. “My personal interest, not as yet proven, is a suspicion that patients whose legs hurt are at risk of amputation, and it is the responsibility of the vascular profession to do something about it,” he said.
Greenhalgh says he is not talking about patients who are referred to vascular specialists, but instead “the people who are not yet patients, whose legs hurt,” and who might not get advice from a doctor “simply because they think it is the aging process and they do not need to.” He continued: “They live in such an environment where they somehow do not get the advice that would enable them to have something done that would save their legs.”
6
Vascular Specialist | April 2023
CX
“It’s not surprising—it shouldn’t be surprising to us—that there are trade-offs between effectiveness and invasiveness”
MICHAEL S. CONTE
SCVS 2023: ENDOVASCULARFIRST OR -ONLY APPROACH FOR ALL CLTI PATIENTS ‘IS NOT EVIDENCE-BASED CARE’
FROM THE COVER: ON OCCASION OF 45TH ANNIVERSARY, CX SYMPOSIUM CHAIRMAN CREDITS CHICAGO VASCULAR SURGICAL TANDEM FOR ENDURING SUCCESS
2022 1992 1989 1998 1978
This month, Corner Stitch highlights one of the papers recently presented at the Vascular and Endovascular Surgery Society (VESS) 2023 winter meeting in Whistler, British Columbia, Canada (Feb. 23–26). Nallely Saldana-Ruiz, MD, a senior vascular surgery fellow at the University of Washington in Seattle, and colleagues studied the trainee experience in open aortic reconstruction in the modern endovascular era—a topic on the minds of many trainees that sometimes influences how senior medical students rank programs. Here, she tells Christopher Audu, MD, what they found.

CA: Congrats on presenting at VESS 2023! Can you give us a synopsis of the study you presented?
NSR: Thank you. Presenting at VESS 2023 was truly a great experience. We were honored with the opportunity to share our work. Vascular surgery is a rapidly evolving field. While trainees around the country are exposed to many procedures during their years of training, some literature has demonstrated a wide variation in trainee experience and comfort with common procedures, including infrapopliteal revascularizations and in treating abdominal aortic aneurysm (AAA) disease. We noted a paucity of data on the trainee experience with complex aortic surgery, and wanted to understand what the complex thoracoabdominal aortic disease trainee experience was for recent vascular surgery graduates. We collected anonymous survey data from U.S. vascular surgery trainees who graduated in 2020. We wanted to get a better understanding of their experience during training, as well as learn about their current practice and any desire for additional training. Our study adds the unique perspective of early-career vascular surgeons and is strengthened by the anonymous nature of the survey. This allowed participants the opportunity to freely share their experience and how that experience may have shaped their current practice patterns. The limitations of the study include the small number of participants and the overall response rate. While it is certainly possible that the data can be biased by those who chose to answer the survey, we believe the responses provide a valuable insight into the early-career surgeon experience.
CA: What anecdotes or observations prompted this study?
NSR: The impetus for the study came from reading recent data, which demonstrated a wide variation in trainee experience with infrapopliteal bypasses and endovascular procedures. In their 2018 paper “Vascular fellow and resident experience performing infrapopliteal revascularization with endovascular

procedures and vein bypass during training,” McCallum et al demonstrated a significant variation in trainee experience and comfort with treating infrapopliteal arterial disease. They suggest that a quarter of vascular surgery trainees were receiving insufficient exposure to infrapopliteal open and endovascular procedures. Their study found that 27% of vascular surgery trainees performed 10 or less infrapopliteal vein bypasses, while 29% performed 10 or fewer infrapopliteal endovascular procedures. Given these data and the paucity of data on the experience of trainees with treating complex aortic disease, we were compelled to ask the questions.
CA: From your analysis, what does your team think is the “number needed to learn” for trainees to feel comfortable treating complex aortic disease as junior attendings?
NSR: It is important to recognize that we never stop learning, even as we transition out of our trainee roles. It is also essential to acknowledge that the “number needed to learn” will vary from trainee to trainee. Learning is different from mastery, and if you ask five different surgeons the same question you will certainly get five different answers. Still, I think it likely takes anywhere between five to 10 cases before you feel comfortable with attempting to independently manage the pathology. For thoracoabdominal aortic aneurysm (TAAA) disease, learning how to approach and care for patients is challenging on many fronts. As an early-career surgeon, we will be faced with the complexities of decision-making and planning, all while carefully considering our patient’s physiology, anatomy, and fitness. Thus, “learning” is truly a long-term endeavor that is never complete.
CA: What do you propose that trainees who don’t have that sort of TAAA volume do to gain a certain level of comfort with this option—especially the open component?
NSR: The thoracoabdominal aortic disease training at the University of Washington is robust, and as trainees we are very fortunate
to have such opportunities. Still, there is great benefit from cadaveric and simulation courses around the country for all trainees. Learning through simulation and didactics in a controlled environment, such as through courses like “The Big Apple Bootcamp,” the “Moore course,” and the open aortic training course at Houston Methodist Hospital, gives trainees unique exposure to the technical and clinical aspects of managing thoracoabdominal disease. However, I believe that there is no substitute for doing cases with those who manage and treat patients with thoracoabdominal disease often.
learning the intricacies of treating complex pathology through the experience of others. These are skills that cannot be mastered with independent simulation alone. In fact, one of the key findings of our study was that the vascular graduates who continued to treat complex aortic disease in their practice were doing so with the participation of their partners. This highlights that as young surgeons we continue to learn from our mentors and colleagues.
CA: In your estimation, what was the most surprising finding from your study?
NSR: One thing we found most surprising was that while most trainees reported doing a low number of complex open and endovascular aortic cases during their training, many were performing them in practice. When we looked closer at the data, we noted that most of our trainees were doing these cases with partners. Early-career surgeons working closely with senior partners in early practice is not surprising at all, and our data helped us to understand that our current training paradigm is one in which we continue learning from others.
CA: I have a feeling you may have already alluded to it, but what is the biggest takeaway you’d like our readers to gain from this work?
CA: Should the Association of Program Directors in Vascular Surgery, SVS or other vascular surgical societies make this a priority and sponsor open or simulation courses to help address this training gap?
NSR: Simulation and access to additional training should be supported. Additionally, some of the most beneficial aspects of participating in simulations and didactics center on the learning that occurs through interaction. So much of our clinical growth comes from
NSR: Continuing to learn in the years following our formal trainee period is a critical part of our lifelong learning process. Still, because we found that the experience with the management of open and endovascular complex aortic disease treatment varied among trainees in our study, additional training in the form of simulation, dedicated courses, or “super fellowships” can provide effective educational adjuncts. Additionally, regionalization and the high-volume center—which provides dedicated care to patients with specific disease pathologies— may also afford the interested trainee the opportunity to learn a certain skillset in the form of visiting rotations and externships.
www.vascularspecialistonline.com 7
CHRISTOPHER AUDU is the Vascular Specialist resident/fellow editor.
CORNER
Nallely Saldana-Ruiz
STITCH
Spotlight on VESS 2023: ‘While most trainees reported doing a low number of complex open and endovascular aortic cases during training, many were performing them in practice’
“ These are skills that cannot be mastered with independent simulation alone. In fact, one of the key findings of our study was that the vascular graduates who continued to treat complex aortic disease in their practice were doing so with the participation of their partners”
NALLELY SALDANA-RUIZ
YOU’LL























































www.bentley.global







































































































The BeBack crossing catheter: A ‘game-changer’ in endovascular PAD practice
Andrej Schmidt

Crossing chronic total occlusion (CTO) lesions is a challenging procedure. The BeBack catheter—Bentley’s first product to be available in both Europe and the United States following the company’s acquisition of Upstream Peripheral Medical Technologies’ GoBack catheter in September 2022—offers a new solution in this space. In this interview, Andrej Schmidt, MD, a senior interventionalist at University Hospital Leipzig in Leipzig, Germany, who was one of the first to use the catheter, shares his clinical experience with the BeBack, noting how it has been a “game-changer” in his endovascular peripheral arterial disease (PAD) practice.
What does your PAD practice look like, and what do you think are the most difficult aspects to overcome when treating CTO lesions?
The University Hospital Leipzig is one of the larger centers in Germany for the endovascular therapy of peripheral arterial occlusive disease, and we receive a lot of very complex cases—many of them failed in other hospitals. These are very often patients with severely calcified infrainguinal disease, but also complex iliac total occlusions.
Could you talk us through your first experience with the BeBack catheter?
We were struggling with a CTO of the common iliac artery in an abdominal aneurysm patient, and failed to get through the CTO coming from retrograde, cross-over and antegrade using an arm-access. Nothing worked until, eventually, we used the BeBack catheter via the retrograde approach. With this, device passage through the CTO back into the aorta succeeded immediately. This experience was an eye-opener for us.
Can the BeBack also be used as a support catheter?
In addition to its main purpose as a crossing catheter for complex, calcified CTOs, the
BeBack indeed can be used as a support catheter, since it is quite stable and stiff. This feature is very helpful in difficult total occlusions. It also can be used as a re-entry catheter—for example during a recanalization of a CTO of the femoropopliteal segment. In the typical situation of being stuck subintimally, unable to pass the guidewire back into the true lumen distal to the CTO, the BeBack reliably helps to re-enter the distal patent segment of the artery.

How is the BeBack part of your recanalization strategy?
This depends on the type of lesion and the problem encountered during the intervention. For example, a typical femoropopliteal CTO is usually approached from antegrade. In case of inability to penetrate the guidewire into the CTO, either due to dense fibrosis of the proximal cap or severe calcification, the BeBack catheter is used as a crossing device by pushing the adjustable needle just a little bit out of the tip of the 4F catheter. More frequent, however, is the situation that the guidewire passes the CTO subintimally, and reconnection to the patent lumen, distal to the CTO, fails. As mentioned, the BeBack catheter is then used as a re-entry-device by protruding the curved inner needle further out of the tip of the catheter. Different to other re-entry

devices is that the BeBack is 4F compatible, instead of 6F, and introduction into calcified, tight lesions may be easier. Yet, it can be used over a 0.018” guidewire, which is often the guidewire of choice in difficult CTOs, improving stability and success compared to 0.014” guidewires.
Another situation in which the BeBack is our device of choice is a reocclusion of the femoropopliteal segment with previous spot-stenting. Usually, the guidewire passes subintimally, and entering into the occluded lumen of a stent within a longer CTO is not possible. In this situation, the BeBack—indeed reliably and fast—allows the guidewire to enter into the proximal end of the occluded stent and to finalize the procedure successfully.




Below the knee, we mainly use the 2.9F BeBack device, although the 4F device also is used in the proximal third of the calf for penetrating through calcific CTOs, or for re-entering after subintimal guidewire passage. In some cases of severely calcified infrapopliteal lesions, it can happen that the guidewire passes easily intraluminally through a stenosis, but no balloon would follow due to the tightness and calcification of the lesion. In this situation, the 2.9F BeBack is inserted over the guidewire and, with the needle slightly protruded from the tip of the catheter, it is drilled into the problematic plaque. This technique, mimicking the Japanese technique of transcutaneous plaque-piercing, is very successful in facilitating introduction of balloons into the lesion and finalizing the procedure.
Has the BeBack changed your practice, and, if so, how?
For many years, we and other centers have helped to develop techniques to improve the success rate in difficult peripheral CTOs. The retrograde and bidirectional approach became standard in case of inability to pass a CTO from antegrade. However, in some cases it can be anticipated that establishing a retrograde access will be cumbersome or time consuming. In these cases, we now prefer the BeBack to keep the intervention simple. Furthermore, a bidirectional recanalization can be time consuming and may even fail. In this situation, the BeBack helps to speed up, and may even be the only way to finalize the procedure successfully.
During CTO recanalizations, do you think that sometimes physicians start to switch from one technique to another too late?
Not infrequently, physicians try different guidewires and different catheters many times in order to pass a difficult CTO, or re-enter back into the patent distal lumen. This increases the risk of protruding the dissection distal to the CTO, destroying healthy segments, which sometimes worsens the clinical situation. Furthermore, radiation dose and the amount of contrast medium increase. Complications correlate with the duration of the procedure. The retrograde approach and the BeBack catheter together are our technique and technology of choice in order to shorten the procedure time.
How much time—or how many attempts—would you give yourself with conventional techniques before using the BeBack crossing catheter?
It depends on the complexity of the lesion. If we see a chance to be successful using an antegrade approach, we may proceed for some minutes. In very complex lesions, where it can be anticipated that a conventional approach has a high risk of failure or may take time, we switch to the BeBack catheter within a minute.
If a colleague were to ask you about the BeBack, how would you describe it?
The BeBack is a reliable crossing and re-entry device. It is very slim (2.9F or 4F), yet very stable and can easily be used not only in larger diameter arteries like iliacs, but also in small arteries—even via a retrograde pedal access through a 2.9F sheath. Furthermore, it is possible to use the catheter over 0.014” and stable 0.018” guidewires, the latter of which is usually the wire of choice in more complex lesions. Due to the BeBack’s straightforward design, it is easy to position and reposition the device, and control the depth and direction of the 360-degree adjustable needle from the tip of the catheter. It is helpful in quite a large variety of difficult situations, and handling the BeBack is easy to learn.
1. CTO of the left superficial femoral artery in a male patient suffering from severe claudication in the left calf
2. After subintimal passage, it was impossible to redirect the guidewire into the patent lumen distal to the CTO
3. Positioning of the BeBack catheter to re-enter the guidewire. Arrow indicates an orientation-marker
4. Marker appearing as a “C” indicates the direction of the needle, with the needle protruding maximally out of the BeBack catheter
5. An 0.018” guidewire passing into the patent distal lumen






6. Result after stenting

www.vascularspecialistonline.com 9
ADVERTORIAL | SPONSORED BY BENTLEY
1 2 3 4 5 6
COMMENT& ANALYSIS
Navigating the red tape: A case for hybrid ORs in the VA
By Courtney Morgan, MD
The United States military has access to the most cutting-edge equipment to perform its duties, so we should expect nothing less for its veterans. As vascular surgeons, we all appreciate the importance of high-functioning, cutting-edge equipment. Over the past decade, hybrid operating rooms (ORs) have become more commonplace and are becoming the norm rather than the exception to be able to provide comprehensive vascular care in an efficient manner.1
Any institution that has been involved in the acquisition of a hybrid OR system understands the complex process involved. Multiple stakeholders, including representatives from specialties expecting to utilize the equipment—OR leadership, hospital finance, acquisition committees and biomedical engineering—need to work together to determine the optimal operating system to best serve patients’ needs that will fit within the confines of the physical space and budget.
This can be a particularly overwhelming challenge within the Department of Veterans Affairs (VA) system due to the increased level of bureaucracy involved. Like any institution, the utility of a hybrid OR must first be established locally. This often involves cooperation between services that will utilize the system besides vascular surgeons, such as neurosurgeons, interventional radiologists, cardiologists, pulmonologists and nephrologists, to name a few.
In addition to vascular surgery, the progression of struc-
GRAND ROUNDS EDUCATING THROUGH ADVOCACY
By Anahita Dua, MD
SINCE THE INITIAL LAUNCH OF THE Cancer Moonshot in 2016, the cancer community has made tangible progress towards ambitious goals, including accelerating scientific discovery in cancer, fostering greater collaboration and improving data-sharing.
Just last year, U.S. President Joe Biden announced a re-ignition of the Cancer Moonshot, highlighting new goals, including reducing the cancer death rate by half within 25 years and improving the lives of people with cancer and cancer survivors.
Cancer is a scourge on society, but we as vascular surgeons know that mortality rates in patients with critical limb ischemia (CLI) are higher than all cancers combined.
So how is it that cancer as a disease process has reached the lips of the highest legislators in our society, while peripheral artery disease (PAD) is still essentially an unknown entity?
The answer lies in our need to educate
tural heart programs and transcatheter aortic valve procedures has largely driven this need and may create additional utilization when determining expected volume. Once the need for a hybrid OR system has been established within a specific VA medical center, approval through the Veterans Integrated Service Network (VISN) budget is required.
Even with the support of the VISN, the high cost of hybrid room technology and intricate construction required for installation pushes this acquisition into the classification of High-Cost High-Technical (HCHT), and must go through the VA National Acquisition Center (NAC). The application considers all aspects of the project, including clinical justifications, finance, impact of veterans’ wait time, and how this all fits into strategic planning.
Courtney Morgan
Moreover, in selecting the specific hybrid system, because the purchaser is the federal government, additional layers of scrutiny are required to ensure open and fair competition among vendors. Once the system and construction budget is approved, additional time for construction and installation should be anticipated. Multidisciplinary collaboration for shared use of cardiac catheterization labs or interventional radiology suites may be required to continue to provide clinical services during a prolonged construction phase.
Conquering the alphabet soup of government acquisition approval is well worth it in the end, and many high-com-
plexity VA facilities already have hybrid ORs. Early adopters have been utilizing their hybrid ORs for nearly 20 years, providing equally complex endovascular aortic repairs to their partnering academic institutions.2 The direct impact on care of a hybrid OR for veterans is easily appreciated, but just as important is the impact on training the next generation of vascular surgeons, with more than half of VA vascular surgeons providing direct teaching to vascular surgery trainees.2,3
Unfortunately, the lifetime of a hybrid OR is not indefinite, and several of the early hybrid ORs in the VA system are undergoing—or have already undergone—upgrades and replacement that are equally as involved as installing a new system. Just as the U.S. military is constantly evaluating its technology to ensure access to the optimal equipment, we as vascular surgeons should continue to advocate for the best equipment within our VAs to optimize care for veterans and enhance training. When undertaking such a planning project, start early and anticipate a lengthy, involved process.
References
1. Spenkelink IM, Heidkamp J, Fütterer JJ, Rovers MM (2022) Image-guided procedures in the hybrid operating room: A systematic scoping review. PLOS ONE 17(4): e0266341. https:// doi.org/10.1371/journal.pone.0266341
2. Flannagan CP, Gasper WJ, Caring for the veteran, training the surgeon: The role of the VA in vascular surgery training. Vascular Specialist. 2021 Aug 23
3. Longo WE, Cheadle W, Fink A, Kozol R, DePalma R, Rege R, Neumayer L, Tarpley J, Tarpley M, Joehl R, Miller TA, Rosendale D, Itani K. The role of the Veterans Affairs Medical Centers in patient care, surgical education, research and faculty development. Am J Surg. 2005 Nov;190(5):662-75. doi: 10.1016/j. amjsurg.2005.07.001. PMID: 16226937.
COURTNEY MORGAN is a member of the VA Vascular Surgeons Committee.

through advocacy. In the 1970s, both patients and physicians passionate about eradicating cancer made a concerted effort to lobby government representatives to support passage of legislation that increased National Institutes of Health (NIH) spending, rallied bipartisan support, and ultimately landed successes such as passage of the National Cancer Act of 1971. The latter intended “to amend the Public Health Service Act so as to strengthen the National Cancer Institute in order to more effectively carry out the national effort against cancer.”
Because of these actions, cancer therapies have exploded onto the scene, and the public and our government understand the basics of the disease process and the need to support actions to manage it. This understanding of what is needed and how to achieve it has resulted in significant support through funding and education to ambitiously plan to rid the world of cancer through scientific discovery.
We vascular surgeons now need to turn this tactic towards our patient population. Our patients are dying from cardiovascular
diseases that have undiscovered genetic underpinnings and treatments that need to be studied and financially backed.
We must understand that our legislators cannot be expected to be passionate about supporting bills when they do not fully understand the relevant clinical outcomes and how policies can impact the vascular population.
It is our job to use advocacy to educate our lawmakers about the serious problems in the vascular surgery world so they can get behind the solutions that are best for our patients. For example, in some parts of the country, 50% of the amputations that take place for CLI occur without an angiogram!
Wouldn’t it be nice if our lawmakers understood the relationship between an angiogram and potential limb salvage, and then advocated for a bill that said Medicare won’t pay for an amputation unless an angiogram is performed in a patient without sepsis? And wouldn’t it be nice if our lawmakers understood the labor and skill we put into an angiogram, and the subsequent distal tibial bypass, so we vascular surgeons can be appropriately reimbursed for
Anahita Dua

our work? This is where education through advocacy can truly steer positive change for the next generation of patients and vascular surgeons.
So, I implore you, my fellow vascular surgeons, please support the breadth of SVS’ advocacy programs—including the SVS Political Action Committee (PAC), grassroots advocacy via REACH 535, and whatever other opportunities are available—as these combined efforts are our strongest tools for educating our lawmakers about the diseases that kill our patients and the importance of our specialty.
Without the right legislation in place, we will make little to no progress, as dollars will be funneled to other groups that do make the effort to educate. We have to make our own case; no one will do it for us. We owe this to our patients who need us to advocate for them on the national stage.
For more information on how to help educate with the SVS’ advocacy programs, visit vascular.org/advocacy or contact svsadvocacy@vascularsociety.org
10 Vascular Specialist | April 2023
“The direct impact on care of a hybrid OR for veterans is easily appreciated, but just as important is the impact on training the next generation of vascular surgeons”
ANAHITA DUA is an associate professor of surgery in the division of vascular and endovascular surgery at Massachusetts General Hospital/Harvard Medical School in Boston.
AWARD-WINNING PAPER ESTABLISHED F/BEVAR ‘FEASIBLE AND SAFE’ IN PATIENTS WITH FAILED AORTIC REPAIRS
PRESENTING AWARD-WINNING
new research, Andrea Vacirca, MD, a research fellow at The University of Texas Health Science Center in Houston (UTHealth Houston), revealed how, despite prior records which demonstrate that fenestrated-branched endovascular aortic repair (F/BEVAR) is “feasible and safe” in patients with failed aortic repairs, few studies have outlined the “granular data” to support this claim, which his team aimed to provide through their analysis.
Vacirca was speaking during the opening scientific session at the 2023 Society for Clinical Vascular Surgery (SCVS) Annual Symposium in Miami (March 25–29), where he picked up the Peter B. Samuels Award for work looking at early and midterm outcomes of F/BEVAR in patients with or without a prior history of EVAR or open repair. And he set a course for his team’s main conclusions, he first highlighted the “increasingly utilized” F/BEVAR’s “high” technical success and “low” mortality rates in the study population.


Vacirca et al had set out to compare these outcomes of EVAR on complex abdominal aortic aneurysm (AAA) and thoracoab-
dominal aortic aneurysms (TAAAs) in a prospective, non-randomized analysis of clinical data from 502 enrollees. They reviewed outcomes in 376 patients with no previous aortic repair (controls), 54 who had prior EVAR (group one), and 72 with prior abdominal open repair (group two).
The researchers reported on 30-day mortality and major adverse events (MAEs), patient survival and freedom from aortic-related mortality (ARM), secondary interventions, any type II endoleak, sac enlargement (≥5mm), and new-onset dialysis. Their results showed that EVAR was performed on average 7±4 and 12±6 years after the prior EVAR and open repair, respectively, with a complex AAA extent in 29% (143) of patients and TAAA in 72% (359) of patients. Breaking this down, Vacirca remarked that patients with prior open repair more frequently experienced TAAA, which were also “more extensive” when compared with the other two groups. However, freedom from type II endoleak and sac enlargement greater than 5mm, was “significantly lower” in patients with prior EVAR, Vacirca told SCVS 2023.
Continuing, he remarked that these ab-
dominal open repair patients were younger at index procedure, and showed “lower” survival and freedom from new-onset hemodialysis which—when prompted on why the latter had occurred during audience discussion—raised a “very important” question for Vacirca and his team. “We evaluated this aspect—if new-onset dialysis was related with lower survival in group two. But we found that only four patients experienced this, and so we associated lower survival with more extensive aortic disease.”
Overall technical success, mortality, and MAE rates were 96%, 1%, and 28%, respectively, Vacirca outlined, though patient survival after 30-month follow-up was “significantly lower” in patients with prior
PAD
Quality Initiative (VQI) registry. The data revealed that 19.2% of patients in the VQI are not discharged on dual antiplatelets after stent placement via TCAR—9% receive a “triple therapy” involving DAPT plus anticoagulation, 5.8% are given single antiplatelet therapy (SAPT) plus anticoagulation, and 4% are directed to take either SAPT or a single anticoagulant.
RECENT
DATA PRESENTATIONS
have revealed reduced risks of stroke and mortality among transcarotid artery revascularisation (TCAR) patients who receive dual antiplatelet therapy (DAPT)—both preoperatively and at discharge—as compared to other drug regimens. Researchers believe these findings underscore the importance of compliance to DAPT regimens before and after a TCAR procedure.
At the 2023 Society for Clinical Vascular Surgery (SCVS) Annual Symposium (March 25–29) in Miami, Hanaa Dakour-Aridi, MD, a vascular surgery resident at Indiana University School of Medicine in Indianapolis, presented the results of a study evaluating post-TCAR discharge regimens among patients in the Vascular
“We demonstrated that patients discharged on a combination of single antiplatelets with anticoagulation witnessed increased [rates of] 30-day stroke, highgrade restenosis, and one-year mortality and stroke/death,” Dakour-Aridi noted. “The use of a single antiplatelet or single anticoagulant after TCAR was associated with increased 30-day and one-year stroke/ death risks. However, there was no significant association between triple therapy and 30-day stroke/death outcomes.”
Nevertheless, Dakour-Aridi concluded that the findings “reinforce our prior study on the importance of compliance to DAPT after TCAR, as well as the need for further follow-up studies to evaluate the appropriateness of TCAR in different patient populations.” Dakour-Aridi et al recently published a study in the Journal of Vascular Surgery (JVS) on the association between preoperative antiplatelet regimens and in-hospital outcomes after TCAR. This research produced similar results.—Jamie Bell
open repair and was reported to be 45% at five years, consistently falling behind the other two groups.
“These procedures carry many technical challenges,” Vacirca stated, their data tentatively weighing up F/BEVAR and open repair for these complex patients. Allowing their research to speak for itself, Vacirca and his team offered an insight into their working practices, describing their hybrid operating room. “Our technique has evolved over the years […] We prefer a total femoral approach, without prophylactic drain whenever possible. We often request a preloaded system, and we now move to use the unibody fenestrated bifurcated devices, and we often double stent vessels coming from the suprarenal fixation devices,” Vacirca explained, making clear that procedural methods must continually be revaluated, and a hybrid approach adopted to provide the best treatment pathway for each individual in this complex patient population.
“We have limited contemporary evidence to support increasing antithrombotic therapies after bypass,” C.Y. Maximilian Png, MD, a vascular surgery resident at Massachusetts General Hospital in Boston told Vascular Specialist on his return from presenting the data at SCVS. The authors set about identifying optimal antithrombotic management of patients after lower-extremity bypass through a restriction analysis of wound, ischemia and foot infection (WIfI) scores. At a single hospital system, Png and colleagues extracted data from infrainguinal bypass procedures completed between January 2018–2021, assigning preoperative WIfI scores to each individual case through the associated documentation. Excluding patients with wound scores of two or three, ischemia scores of zero or one, or foot infection scores of three, Png’s study concerned patients at “[low] risk” of a negative outcome who may “theoretically benefit the most” from increased therapy. “The next challenge is to figure out how to get the most out of this valuable tool, and we thought one use of it could be to help differentiate patients who would benefit from increased anti-thrombotic medication therapy,” Png said.
With 191 procedures in the study, Png et al found 66 (34.6%) patients were discharged on single antiplatelet therapy (SAPT), compared with 125 (65.5%) on either DAPT or AC. The only difference that the authors identified between the two groups was a higher prevalence of atrial fibrillation in the DAPT/AC group. At 30 days, Png et al observed no significant difference in postoperative reintervention or graft occlusion rates, but the DAPT/AC group had a significantly lower rate of mortality (2.2% vs. 9.1%, p<0.05), major amputation (1.6% vs. 7.6%, p<0.05) and major adverse limb events (MALEs). Reflecting on their Kaplan-Meier analysis, the authors determined that MALE-free survival was higher amongst DAPT/AC patients compared with the SAPT group.—Eva Malpass
www.vascularspecialistonline.com 11
“We often request a preloaded system, and we now move to use the unibody fenestrated bifurcated devices”
ANDREA VACIRCA
Andrea Vacirca
DUAL ANTIPLATELET THERAPY LINKED TO BETTER POSTTCAR OUTCOMES VERSUS OTHER DRUG REGIMENS AAA
CAROTID DISEASE
DAPT shows higher survival rate in patients with low WIfI scores, study indicates
A RETROSPECTIVE COHORT STUDY PRESENTED AT THE 2023 Society for Clinical Vascular Surgery (SCVS) Annual Symposium in Miami (March 25–29) has found lower-extremity bypass patients who received dual antiplatelet therapy or anticoagulation (DAPT/AC) postoperatively had a higher 30-day survival rate.
C.Y. Maximilian Png
By Eva Malpass
SOCIETY OF BLACK VASCULAR SURGEONS BUILDS BRIDGES,

CONNECTS
INTERNATIONAL DOTS
A decade ago, if he were asked to name 10 Black vascular surgeons, says one African American vascular chief, he could have come up with six or seven, writes Beth Bales
TODAY AFTER BEING PART OF THE creation and formation of the Society of Black Vascular Surgeons (SBVS), Vincent Rowe, MD, thinks he could list “60, 70, 80” vascular surgeons who are Black. That’s a far cry from 10 years ago, when he thinks could perhaps name just “six or seven.” At a basic level, that’s what the Society has done for him personally, said the chief of vascular surgery at the University of California, Los Angeles (UCLA). “It’s made me feel like I have a lot of colleagues with similar interests, and who have similar challenges to those I have had to face in this profession.”
The SBVS was formed in spring of 2021, after an exploratory meeting in late 2020 discovered enthusiasm and interest in creating such a group, said Edwin Kendrick, MD. It “promotes fellowship amongst members through networking, mentorship and professional development. … [and] has a core mission of education, research, advocacy and provider pipeline development
DIVERSITY DISPARITIES
to advance health equity. The Society seeks to support policies that improve care and overall outcomes by addressing health[care] disparities in underserved patients.”
“Each one of us saw a need,” said Rowe, noting that just 2% of SVS membership is African American. “We felt it necessary to form a collegial networking environment for ourselves, future trainees and colleagues. There’s kind of a dearth of networking systems.”
Kendrick said he has always had “an urgent call to make an impact on improving the pipeline for people entering healthcare.” In 2017 and 2018, he read a report about challenges Black doctors were facing in California. He touched base with Rowe with the idea of trying to create additional opportunities in healthcare for Black physicians in the state.
Rowe began contacting people around the country to gauge interest and discovered “a lot of people who felt they wanted to feel connected,” said Kendrick, who is president of a health equity and technology company. Both men’s visions had a lot of overlap. Kendrick’s long-standing interest was in what he wanted to do for Black physicians, whereas he, Rowe said, was more focused on vascular surgeons specifically.
In late 2020, they met for the first time, and over the next months began forming a potential group. The Society was formed in May 2021, and thanks to the dedication of a diverse board, chaired by Kenneth Simon, MD, the SBVS has continued to flourish and slowly grow. The Society started small, with perhaps 20 to 30 members, but now counts approximately 140 people. “The most revealing and beautiful thing is that the talent pool is so diverse and so strong,” said Kendrick.
“We have a mix of everything in terms of interests—advocacy, entrepreneurship, general surgery, mission work, mentoring, research, a complete coverage of all these different talents.”
It remains a work in progress, both said. They want to be sure not to overreach, but they have ambitions for future projects.
And connections are being formed, to unexpected results. Rowe said he met a surgeon who has been practicing for 20-odd years in West Virginia who regularly travels to Nigeria to train vascular surgeons. A few months ago, Rowe met another doctor who was going to Nigeria. Rowe put the two in contact, and they plan to connect and possibly expand that work.
“We’re doing a lot of this, putting people together because of our network,” Rowe said. “Without it, these two people wouldn’t have connected—and I did not know either one of them a year ago.”
Both Rowe and Kendrick said they feel the SBVS works in parallel with SVS. Their first in-person gathering was at the Vascular Annual Meeting (VAM), and they plan a reception at this year’s meeting outside Washington, D.C. They noted both 2022 vice presidential candidates talked with their members. “We outvoted our percentage,” in terms of demographics, noted Rowe. “Blacks make up 2% of SVS membership and we were 4% of the vote.”
SVS leaders also have made a concerted effort to add diversity to its committees and task forces, which has led to more engagement between SVS and its Black members. One SVS member, in fact, reached out to Rowe to seek a recommendation for an SBVS member to put on a particular task force.
The new SBVS, they noted, is helping build the support required to bring people together, to work to improve healthcare in general and mentor Black surgeons in particular, and to advocate for increased opportunities. It also wants to address the existence of healthcare disparities. Rowe wants SVS members to “remember we are parallel. We’re trying to accomplish some of the same things together. Don’t be afraid to utilize some of our expertise.
“Reach out,” he added. “I know many of our members would say they don’t have a bridge to get involved with SVS. Without that bridge, it’s hard to get places. All of us will help to get people involved where they can and help them get that opportunity.” Interested parties can learn more about the SBVS at blackvascular.org
Tackling health insecurity ‘from the top down and the bottom up’
Texas-based vascular surgeon outlines strategies that can be deployed to tackle problems threatening a patient’s health security, and highlights an example of outreach in San Antonio tackling problems in high-risk population zones head on. By Bryan
The feeling of being “uncertain, anxious and vulnerable” defines a creeping health insecurity problem riddling parts of the U.S. patient population—demographics who are underserved and at higher risk of outcomes such as amputation. And they can be a barrier to obtaining healthcare, according to Rana Afifi, MD, an associate professor of vascular surgery at McGovern Medical School, University of Texas Health Houston. Afifi was speaking during an invited presentation on strategies that can be used to tackle health insecurities during the 2023 Society for Clinical Vascular Surgery (SCVS) Annual Symposium in Miami (March 25–29). “There [are] plenty of data in the literature [showing] that there is a significant increase in the number of publications that show the relationship between social determinants of health and outcomes,”
she told attendees. “However, what is lacking is the data regarding how we do any strategies to improve, implement or actually have any intervention...”
Afifi spoke of health policy reforms that “incentivize healthcare systems to respond to social determinants of health and improve population health as a strategy for reducing excessive costs.” She also focused on strategies already available that can be applied locally. Afifi dealt with health security issues as they relate to such areas of life as housing, finance, transportation and food. “You can work with food banks,” Afifi said. “There have been studies showing their role in care for patients and mitigating some of the health insecurities at risk for this.”
She turned to the example of the SAVE (San Antonio Vascular and Endovascular) Clinic in San Antonio, Texas,
 Kay
Kay
founded and run by vascular surgeon Lyssa Ochoa, MD, as a case in point. Ochoa based her work on identifying the zones where, for example, there are high amputation rates and low socioeconomic status—high-risk populations, observed Afifi. “That’s basically where she based her outreach clinics,” she said. Her network is equipped with vascular care-equipped mobile units that head out into the heart of these communities, “assisting with some of the transportation options.” Ochoa and her team also partner with community healthcare workers to navigate wider needs, such as screening and trust-building. Going forward, the health insecurity issue necessitates a cultural change, and needs to be addressed at multiple levels, Afifi added. “The way to address it is from the top down and the bottom up at the same time—we can’t just wait for policy to change.”
12 Vascular Specialist | April 2023
“I know many of our members would say they don’t have a bridge to get involved with SVS. Without that bridge, it’s hard to get places”
VINCENT ROWE
Vincent Rowe speaks during VAM 2022
Advanced Surgical A llograft Products
AMNIOFIX® and AMNIOEFFECT ® are placental-based sheet allografts providing a protective barrier to support the healing cascade and the development of granulation tissue.
AXIOFILL® is an acellular human placental ECM particulate used in the replacement or supplementation of damaged or inadequate integumental tissue.
RCT Outcomes with DHACM
DFUs: VLUs:
2x to 3.8x faster
defect closure vs. SOC1,2*
MORE

CONTACT US: Scan the code or visit www.mimedx.com/contact
for the treatment of venous leg ulcers. Int Wound J. 2018;15(1):114-122. 4. Bianchi C, Tettelbach W, Istwan N, et al. Variations in study outcomes relative to intention-to-treat and per-protocol data analysis techniques in the evaluation of efficacy for treatment of venous leg ulcers with dehydrated human amnion/chorion membrane allograft. Int Wound J. 2019;16(3):761-767.
Patents and patents pending see: www.mimedx.com/patents. AMNIOEFFECT, AMNIOFIX, AXIOFILL, PURION, and MIMEDX are trademarks of MIMEDX Group, Inc. ©2023 MIMEDX Group, Inc. All Rights Reserved. www.mimedx.com US-LS-2200045 v1.0

LEARN
WHEN LIMB DEFECTS HAVE DELAYED HEALING, YOUR PATIENT IS AT RISK OF AN AMPUTATION.1
DHACM = Dehydrated Amnion/Chorion Membrane DFU = Diabetic Foot Ulcer VLU = Venous Leg Ulcer SOC = Standard of Care
* 2x = based on mean time and 3.8x = based on median time, ^ Per Protocol group, † Mean percentage in defect size vs. baseline 1. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507. 2. Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12(6):724-732. 3. Bianchi C, Cazzell S, Vayser D, et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EPIFIX®) allograft
THINK ASAP
1.6x the defect closure rate vs. SOC at 16 weeks3,4^
NEW! NEW!
INTERVIEW
 By Bryan Kay
By Bryan Kay
THE FIRST AH-HA MOMENT CAME during a talk at a national meeting being given by one of the foremost vascular surgeons in the U.S. The second came back at home base. The theme common to both? That major vascular operations and complications were being carried out by other specialties engaged in robotics-assisted surgery.


“I was at the Society for Clinical Vascular Surgery one year, and was watching a robotic left renal vein transposition—that’s a very sophisticated operation—by Sam Money, at that time the chief of the Mayo Clinic at Scottsdale,” says Alan Lumsden, MD, the Walter W. Fondren III Presidential Distinguished Chair at Houston Methodist’s DeBakey Heart & Vascular Center in Houston, Texas. “And then he said: ‘I didn’t do this operation, the urologist did it.’”
Then, back home in Houston, Lumsden was called to assist with a bleeding complication during a pelvic procedure being carried out by a gynecologist. When he arrived, the
specialist—performing the procedure robotically—asked Lumsden if he could resolve some bleeding from an iliac artery using the robot. His response was in the negative. “I can’t do that,” he recalls saying. The gynecologist, unperturbed, then told him she would take care of the situation herself. “And I was dismissed,” he says. “Here is a major vascular operation being done by a urologist, and a major complication, that is normally in our bailiwick of repairing, now being done by the gynecologist.”

These experiences led to a realization— and a resolution. “We, in vascular surgery, have missed the boat on this,” says Lumsden. So he set about building a vascular robotics program. Of late, that program has been gaining some interested glances from around the country following dissemination of a video from the popular Houston Methodist DeBakey CV Education YouTube channel via social media. The video features an inferior vena cava (IVC) filter removal procedure performed robotically. The surgery was led by his colleague, Charudatta Bavare, MD, who in a DeBakey Heart & Vascular Center grand rounds from September 2022, proposed robotic vascular surgery as an “un derexplored frontier,” raising the possibility that the open and endovascular era of the specialty may segue “into robotic vascular surgery in the future—that is the hope.”

Lumsden, who recently became president-elect of the Southern Association for Vascular Surgery (SAVS), stresses his role as “enabler” in this quest. For a

long time, one of the challenges that held back vascular surgery was a lack of laparoscopic training, he points out. But these days, even many newer general surgeons “are now bypassing laparoscopic surgery and going straight to robotic,” he notes. “So this need for laparoscopic skills is not an absolute requirement to become a robotic surgeon.”
Lumsden sees his role as one of “pushing this along,” continuing: “You have got to learn the basics before you start taking on the big stuff. Charu[datta] Bavare is one of my mid-level partners, trained as a general surgeon, worked in the community in laparoscopic surgery, vascular surgery, general surgery—he had to go to what we call an underserved community—and he has got all the skills. If he can’t make this work, nobody can make it work.” In Houston, Bavare started out on “relatively trivial cases,” explains Lumsden. Cases that perhaps do not require a robot, he says. “But you have to get up to speed in a safe environment. And you have to get your team
up to speed.” From procedures such as laparoscopic peritoneal dialysis placement and revisions, Bavare moved on to the likes of median arcuate ligament syndrome, and, now, the IVC filter removal. “We have a lot of people here who are sophisticated in robotics, all of whom are interested in helping us do this,” Lumsden relates. “There are these little pieces we need in other specialties that we are actively trying to seek out and grab, and pull this toward the vascular surgery community.”
Lumsden looks back to the man for whom his center is named, Michael E. DeBakey, MD, and his innovation of the Dacron graft, as he looks forward. “To this day, that is probably the single-most durable procedure that has been described for repairing the aorta.” The problem with that was a “not-so-hot” delivery system, which the endovascular revolution sought to remedy. “What we’ve essentially done is given up durability for a delivery system. And that means stent grafts,” he says.
“If you look at other specialties—urology, general surgery—when they went minimally invasive, they went laparoscopic or robotic. They did the same procedures that have been proven for 20, 30 years. They didn’t invent a whole new specialty called endovascular surgery the way we bought into it.”
14 Vascular Specialist | April 2023
surgery: ‘We’ve missed the boat on this,’
CME process changes at the American Board of Surgery (ABS) will impact the way that Diplomates interact with the Board, upload CME credits and meet certification requirements
Society for Vascular Surgery is committed to complying with the new rules and making your ability to claim credit as seamless as possible However, you will need to take steps now to ensure all your CME credits earned with SVS are transferred to the ABS DON'T LOSE EARNED CME CREDITS! The ABS recently made changes to its CME processes. Visit vascular org/NewCreditProcess for more information and to see the steps you can take to ensure your credits are transferred to ABS vascular.org/NewCreditProcess SAVE up to 25% on VESAP5! Take your review to the next level. vascular.org/VESAP5 Robotic surgery for IVC filter removal (top left), the removed filter (right), and Charudatta Bavare (bottom) Alan
Robotic
says Houston vascular chief
The
Lumsden
Creating an artificial intelligence-based tool to help clinicians perform precision AAA analysis
Canadian researcher uses more than a decade’s worth of collected intelligence in abdominal aortic aneurysm (AAA) care to develop algorithms that will allow for forward prediction of disease risk—not just a real-time tool. By
or University of Calgary, Alberta, Canada, vascular surgeon Randy Moore, MD, the route to a world of precision care for individual abdominal aortic aneurysm (AAA) patients—involving aortic wall strength-mapping technology developed over the course of the last 15 years, allied to an artificial intelligence (AI)-powered algorithm—is drawing nearer. Right now, says the associate professor of vascular and endovascular surgery, the decision to treat AAAs is based on a single diameter measure derived from population-based information. “That is flawed,” he implored. “And this hasn’t changed in over half a century.”
Moore was speaking during the 2023 Houston Aortic Symposium (March 16–18), held in Houston, Texas, where he delivered insight into RAW (Regional Areas of Weakness) Maps and its aim to provide a precision medicine solution for AAA patients. “When we look at size alone as a measure for patients at risk of aortic aneurysm disease, we ignore all these other things that are critical to our decision-making,” he told those gathered for a talk on the ViTAA Medical Solutions technology. Moore is the company’s medical director and a co-founder, and it is on his clinical experience that early testing of the tool’s effectiveness is based.

Moore emphasized the core principle behind AI in clinical work: analyzing large amounts of data, recognizing patterns, and predicting outcomes from pattern-recognition algorithms. “In our center, for the past 15 years, we have developed a number of algorithms that allow us to link actual tissue strength to risk in terms of wall strength analysis,” he said. The ViTAA solution he proposes uses a RAW mapping score of aortic wall tissue integrity so that clinicians can talk
Bryan Kay
through with patients the risk associated with their condition. “We knew our mapping technology was very effective at identifying wall strength and weakness,” he said. “But is that map a useful tool to allow for forward prediction—not just a map in real time but the ability to move ahead and provide the clinician with a tool?”
An initial pilot study of 36 patients showed a statistically significant correlation between ViTAA’s RAW Maps and aortic growth over time.
“We could, from a single ViTAA analysis, identify or predict risk in that patient moving forward up to 12 months with a statistically significant outcome,” Moore said. “We knew the mapping tool was good, but we wanted to understand how the addition of artificial intelligence would unlock, not just that mapping technique, but the predictive value moving forward.”
That’s where the ViTAA Aortic Model comes in, he continued. This involves the use of patient imaging—which the system “flattens out,” gathering 3,000 coordinates of the aorta—and comparisons between different aortic scans at “exactly the part of the aortic wall we want to address.” The model enables serial investigations, Moore said, and using explainable AI—unroofing the concept of black-box AI— clinicians can then see what’s being measured and modified for auditable purposes. “Our vision is that with the AI we have combined with the mapping technology, we are going to be able to provide the clinician with a go-forward view of a patient’s down-the-road behavior, which can then inform current real-time decision-making.”
Of course, the AI algorithm requires datasets from which to learn, Moore conceded. As with human facial recognition technology that generates people who do not exist, artificial patients, too, can be created. “Why would we do this?” asked Moore. In order to generate a large enough dataset so that the researchers can get the answers they need in a short period of time, he said. “You can generate human aortic characteristics without actually including any backtraceable, real patient data. How does this work? You take real patient data, feed it in, and a generative adversarial network then creates this synthetic dataset that you can learn to train your AI.”
Moore pointed to a concrete use case his team developed. “In our initial 16-patient pilot study looking at the strength of the aortic neck, we were able to identify a statistically significant relationship to a weak neck and subsequent type 1a endoleak. But if you look at the dataset, it was heavily weighted toward endoseal, with a small number of endoleaks. To make that learning set more applicable, we then created a synthetic database with 5,000 patients, with equipoise between the two groups. That took six minutes on a laptop.” After an analysis probing the synthetic database’s validity, the researchers found that it still produced what is considered a “very excellent” predictive rate.
Moore and colleagues are now focused on validating the model through a North American multi-site registry and a number of research-use only sites accumulating datasets on aortic pathology. “The future of precision aortic care ain’t what it used to be as some of these tools continue to roll out.”
A RECENT STUDY COMPARING OUTcomes of endovascular aneurysm repair (EVAR) patients has reported no statistically significant differences in mortality or secondary rupture rates between standard Cook, Medtronic and Gore endografts, suggesting similar safety in a real-world setting. Michael O. Falster, PhD, a senior research fellow from the Centre for Big Data Research in Health in Sydney, Australia, Ramon L. Varcoe, MBBS, a vascular surgeon from Prince of Wales Hospital, also in Sydney, Australia, and colleagues report this and other outcomes from a large population-based observational study in the European Journal of Vascular and Endovascular Surgery (EJVES).
Another key finding was that rates of subsequent aneurysm repair were higher for endografts other than Cook devices, but only statistically significant for Medtronic devices. In order to compare rates of all-cause death, secondary rupture and secondary intervention in their retrospective cohort study, Falster, Varcoe et al used a linked clinical registry (Australasian Vascular Audit [AVA]) and all-payer administrative data from patients undergoing EVAR for intact abdominal aortic aneurysm (AAA) between 2010 and 2019 in New South Wales, Australia.
The authors identified 2,874 eligible EVAR patients, with a median follow-up of 4.1 years and a maximum of 9.5 years.
Writing in EJVES, Falster, Varcoe and colleagues report similar mortality rates for patients receiving different devices, ranging between 7 and 7.3 per 100 person years. In addition, they reveal that there was no statistically significant difference between devices in secondary rupture rates (between 0.4–0.5 per 100 person years).
Furthermore, Falster, Varcoe et al note that patients receiving Medtronic and Gore devices tended to have higher crude rates of subsequent aneurysm repair (1.5 per 100
person years) than patients receiving Cook devices (0.8 per 100 person years). This finding, the authors write, remained in the adjusted analysis, but was only statistically significant for Medtronic devices.
“Major endograft devices have similar overall long-term safety profiles,” the authors summarize in their concluding statement. However, they add, there may be differences in rates of secondary intervention for some devices. “This may reflect endograft durability, or patient selection for different devices based on aneurysm anatomy,” Falster, Varcoe and colleagues posit.
According to the authors, this population-based study is one of the largest to compare outcomes of EVAR patients receiving contemporary endografts from different manufacturers.
The key strength of the present study, the authors claim, was the use of linked clinical registry and population-level administrative data. “This study is one of the largest to have explored outcomes for patients receiving different endografts, and also one of the only studies to have examined a contemporary cohort of patients to directly compare outcomes of endografts in current use,” Falster, Varcoe et al add.
The authors underscore “a need for ongoing surveillance of patient outcomes to compare and evaluate different devices used in clinical practice” to ensure those grafts are “providing the highest levels of safety and efficacy, with identification of those that are not.” They opine that the history of endovascular surgery is “littered with examples of underperforming endografts,” which, after evaluation, were “identified and managed by either temporary pause for device modification or withdrawal from the commercial market entirely.” The investigators stress that regulators such as the Food and Drug Administration (FDA) are “acutely aware” of the importance of postmarket data to assess the safety of devices and encourage surveillance programs. The present study, Falster, Varcoe et al claim, “demonstrates that linkage of clinical registry and administrative data can strengthen the evidence to help fill the inevitable gaps which occur when [randomized controlled trials] do not exist or are unethical to perform.”
“Continuous comparative assessments” are needed to guide evidence for treatment decisions across devices, they write in their closing remarks.—Jocelyn Hudson
www.vascularspecialistonline.com 15
F
STUDY HIGHLIGHTS NEED FOR ‘CONTINUOUS COMPARATIVE ASSESSMENTS’ BETWEEN DEVICES
EVAR
“For the past 15 years, we have developed a number of algorithms that allow us to link actual tissue strength to risk”
RANDY MOORE
MACHINE LEARNING
Randy Moore
May VRIC program takes shape
By Beth Bales
WITH AN EMPHASIS ON CELLS—STRUCTURAL and immune—the program for the May 10 Vascular Research Initiatives Conference (VRIC) in Boston next month is taking shape.

VRIC will be held at the Boston Marriott Copley Place in Boston, in conjunction with the American Heart Association’s “Vascular Discovery 2023: From Genes to Medicine.” The AHA sessions will be held May 10–13 at the venue.
The conference emphasizes discussions of research of basic and translational vascular science, in a thoughtful atmosphere intended to motivate participants to discover solutions to important problems affecting vascular patients. VRIC brings together vascular surgeon-scientists, vascular biologists and other cardiovascular physicians researching vascular disease, and trainees working in the cardiovascular disease space. The 2023 theme is “Structural and Immune Cells in Vascular Disease.”
Four abstract sessions will focus on venous disease and novel devices; vascular regeneration, stem cells and wound-healing; atherosclerosis and the role of the immune system; and aortopathies.
Esther Lutgens, MD, will deliver the Alexander W. Clowes Distinguished Lecture, established in 2017 to honor the late SVS member and surgeon-scientist who had a big hand in shaping the conference over the years. Her topic is “Co stimulatory immune checkpoints in atherosclerosis: Novel immunotherapeutic targets to combat atherosclerotic cardiovascular disease.”
Lutgens is a renowned molecular biologist and professor
of cardiovascular medicine at the Mayo Clinic in Rochester, Minnesota. The day’s agenda also includes the Translational Panel. Chiara Giannarelli, MD, will focus on deep phenotyping of human atherosclerosis, and Bhama Ramkhelawon, PhD, will discuss structural triggers of macrophages in aortic aneurysms.
“This is going to be an outstanding conference with a focus on vascular discovery. We had a near record number of abstracts submitted this year, many of which were by trainees performing exceptional research that will one day advance our understanding and treatment of cardiovascular disease,” said Katherine Gallagher, MD, chair of the SVS Basic and Translational Research Committee, which plans VRIC.
“I don’t think anyone will leave VRIC 2023 anything but inspired, and hopefully the knowledge gained here will lead investigators to other pathways of inquiry.”
Areck Ucuzian, MD, will present his research into “The benefits of moderate aerobic exercise on maintaining vessel structure in mouse models of aortopathy,” as part of his SVS Foundation Mentored Clinical Scientist Research Career Development Award.
Four vascular trainees were selected as recipients of the SVS Foundation VRIC Trainee Award; their abstracts were scored among the highest of those submitted. They will present their work at the conference:

● Sharika Bamezai, BA, a Sarnoff Research Fellow at Stanford University School of Medicine and a medical student at University of Michigan Medical School, “Safety and efficacy of pro-efferocytic nanoparticles to treat atherosclerosis in a porcine model.” Her mentor is Nick Leeper, MD
● Tyler Bauer, MD, a general surgery resident at University of Michigan Medical School, “Inflammatory macrophages dictate fibroblast function via epigenetic reprogramming in diabetic wounds.” Mentor: Katherine Gallagher, MD
● Rodrigo Meade, BS, a research fellow at Washington University in St. Louis, “Fatty acid synthase targeting reduces aortic atherosclerosis and inflammation.” Mentor: Mohamed Zayed, MD
● Humraaz Samra, MBBCh, an integrated vascular surgery resident at Indiana University School of Medicine, “Allogeneic mesenchymal stromal cells significantly increase type 1 regulatory T cells, decrease effector Th17 cells and decrease aneurysm volume in a dose-dependent fashion in patients with small abdominal aortic aneurysms—Results of the phase I Aortic aneurysm repression with mesenchymal stem cells (ARREST) trial.” Mentor: Michael P. Murphy, MD
A poster competition and cocktail reception also are included. For more information on the conference and to register, visit vascular.org/VRIC23
SVS Foundation Vascular Innovation Partners (VIP)


SVS Foundation VIP Program Member: Texas Vascular Associates

Why is Texas Vascular Associates supporting the SVS Foundation?
oundation by becoming a VIP essential to advancing the field to provide to our patients atients with vascular disease ic awareness.
at interest
agnosis and treatment of Foundation to advance the as Vascular Associates is elp support the Foundation’s
on and public awareness of n and treatment, with dation’s commitment to nals need to deliver the best VIP program helps raise tients have the information
The Vascular Innovation Partners (VIP) program, a new initiative of the SVS Foundation, consists of institutional partners whose values align with the mission of the Foundation.
Learn more at
ntial to improving the care gram member, Texas work of the Foundation and it of patients everywhere.
16 Vascular Specialist | April 2023 VASCULAR SCIENCE
vascular.org/VIP
“We had a near record number of abstracts submitted this year, many of which were by trainees performing exceptional research that will one day advance our understanding and treatment of cardiovascular disease”
KATHERINE GALLAGHER
Depiction of fibroblast cells
EDUCATIONAL
WITH SESSIONS COVERING A WIDE swath of topics important to vascular surgeons, including many proposed by Society for Vascular Surgery (SVS) members themselves, participants at the 2023 SVS Vascular Annual Meeting (VAM) are sure to find valuable information on both clinical and non-clinical topics important to the professional lives of vascular surgeons.
That’s the word from William Robinson, MD, chair of the SVS Postgraduate Education Committee (PGEC), which plans the educational sessions at VAM. The meeting takes place June 14–17 at the Gaylord National Resort and Convention Center in National Harbor, Maryland, just outside Washington, D.C.
“Our committee has striven to achieve a really good mix of topics,” he said of the 17 clinical and eight non-clinical sessions that comprise the educational sessions. “We have something for everybody, irrespective of where they are in their careers. We also try to mix clinical information with sessions related to education, practice management, advocacy for our specialty and professional well-being. In all of them, we strive for programs which are innovative and current, and try to use inventive and interactive formats to increase engagement.”
The PGEC evaluated—blindly—63 proposals submitted by members in the fall of 2022. Committee members ultimately selected 18 proposals, and then worked with the submitters to develop the educational sessions. Those represent a large portion of PGEC programming. The remainder of the sessions cover clinical topics highly desired based upon feedback from SVS members, but which have not been addressed in three to four years.
Throughout the meeting, sessions will cover what Robinson calls “updates and innovations in the meat and potatoes of vascular surgery,” such as hemodialysis, acute limb ischemia, venous disease and arterial intervention, while mixing in specialized and more focused topics.
For example, the schedule includes a session on the “onco-vascular surgeon,” on vascular surgeons’ assistance in cancer operations. “This highlights the role vascular surgeons play in assisting multiple other specialties, of which cancer surgery is just one area,” said Robinson.
SHOWCASING A ‘REWARDING FIELD’: STUDENT-TRAINEE PROGRAM AT VAM DEALS WITH FULL SPECTRUM OF VASCULAR SURGERY
BESIDES BEING ABLE TO ATTEND the scientific and educational sessions at the Vascular Annual Meeting (VAM), medical students and general surgery residents have an entire program devoted specifically to their career stage.

The VAM Resident and Student Program includes:
Students and residents also enjoy highly discounted rates to attend VAM, ranging from $50–$100 for SVS member medical students to $375–$525 for SVS member residents. The exact fee depends on the registration date, which includes three zones.
“Every year, we consider what topics need particular emphasis based on current practice trends, important data and member feedback,” he said. This year, such sessions will cover venous disease, two of them offered in collaboration with the American Venous Forum, one on iliocaval stenting, and a second on “what’s new in deep venous thrombosis treatments.”
In addition, a session is planned that takes a deeper dive into the BEST-CLI and BASIL-2 trials.
The PGEC also works with the four SVS sections—Young Surgeons, Community Practice, Women’s, and Sub-Section on Outpatient & Office Vascular Care (SOOVC)—to develop educational programs sponsored by each. Topics include financial literacy (Women’s Section), the business side of running an outpatient facility (SOOVC), tips for surgeons in their first five years of practice (Young Surgeons Section), and a session on recognizing, enhancing and promoting the vascular surgeon’s value (Community Practice Section). Physician assistants will have separate educational sessions, including a luncheon and “Cases Over Cocktails” with the Society for Vascular Nursing.
The educational sessions organized by the sections proved popular last year, said Robinson.
“They allow a particular membership group to identify what large portions of SVS members think are important. It therefore gives all of our members an opportunity for a voice in the meeting content, and helps ensure this content is applicable and important to a diverse group of members,” he said.
View the VAM Online Planner for detailed information on all presentations at vascular.org/OnlinePlanner23
◆ An introduction to vascular surgery, for pre-medical and medical students interested in learning more about the field of vascular surgery, 6:45 to 8 a.m. Thursday, June 15
◆ Succeeding as a vascular surgery residency applicant, for medical students who are planning to apply or who have already matched into vascular surgery residency training, 6:45 to 8 a.m. Thursday, June 15
◆ Succeeding as a vascular surgery fellowship applicant, for general surgery residents who are considering or plan to pursue vascular fellowship training, 6:45 to 8 a.m. Thursday, June 15
◆ A mock interview session, to prepare students and general surgery residents for vascular surgery training program interviews, 6:30 to 8 a.m.
Friday, June 16
◆ A Residency and Fellowship Fair, from 1:30 to 3 p.m. Friday, June 16
◆ A welcome reception specifically for medical students and general surgery residents, from 5:15 to 6:15 p.m. Wednesday, June 14
◆ Mentor Mentee match, pairing students and general surgery residents with a vascular surgeon for on-site mentorship that often continues on in subsequent months and years
In addition, with the support of a faculty member, trainees are encouraged to submit cases for an education session set for Friday, June 16, called “Endovation II.”
“The Resident and Student Outreach Committee (RSOC) carefully plans this program to showcase our specialty and the Society. VAM serves as an introduction to vascular surgery to those considering vascular surgery as a career choice; they can get an in-depth look at the research, technology and clinical cases that make vascular surgery a rewarding field of practice. Also, students and residents have the opportunity to meet and network with peers, vascular trainees and faculty already practicing in this rewarding specialty,” said Bernadette Aulivola, MD, committee chair. “Over the years, it’s proved highly successful. Some of our most active SVS members became committed to vascular surgery after attending VAM as a student or resident.”
It’s not just the programming, she said. Former attendees specifically mention the welcoming atmosphere and camaraderie amongst vascular surgeons at all levels, from newly minted surgeons to those with decades of experience. Aulivola also pointed to the depth and breadth of knowledge that is showcased at VAM, including cutting-edge research presented at the plenaries.
Learn more at vascular.org/VAM.
REGISTRATION FOR THE SOCIETY FOR Vascular Surgery (SVS) 2023 Vascular Annual Meeting (VAM) is off to a brisk start, with more than 620 attendees taking advantage of this year’s new early-bird pricing to lock in discounts.
VAM 2023 will be June 14–17 at the Gaylord National Resort & Convention Center on the Maryland coast, close to Washington, D.C. New this year is a requirement that attendees must register for the meeting itself before booking hotel rooms at the venue. The deadline to book rooms at the SVS group rate is May 17.
For a detailed look at this year’s schedule, sessions, abstracts, receptions and more, people can take a look at the VAM Online Planner at vascular.org/OnlinePlanner23 Certain events require separate payments, including:
1. The Connect@VAM Building Community welcome party from 7 to 9 p.m. Wednesday, June 14
2. The Women’s Leadership Dinner, from 7:45 to 9:30 p.m. Thursday, June 15
3. The SVS Foundation “Great Gatsby Gala,” which in March had very limited tables available
4. VQI@VAM, the Vascular Quality Initiative’s annual meeting
5. The Society for Vascular Nursing’s Annual Conference
6. The Registered Physician Vascular Interpretation (RPVI) course, moved to Wednesday this year
Sign up for all such events via the registration site, or for those who have already registered, modify existing registrations at vascular.org/vam23reg
The Connect@VAM party will be free to those 16 and younger, and will be open to non-VAM guests, such as family members. In addition, with permission of SVS staff, children 16 and younger can be in the Exhibit Hall.
VAM 2023
“This highlights the role vascular surgeons play in assisting multiple other specialties, of which cancer surgery is just one area”
WILLIAM ROBINSON
www.vascularspecialistonline.com 17
‘EARLY BIRDS’ FLOCK IN AS MEETING REGISTRATION DISCOUNT PERIOD OPENS
William Robinson
VAM
SESSIONS SET TO COVER GAMUT OF CLINICAL AND VASCULAR PRACTICE MATTERS
Compiled by Beth Bales
SOCIETY BRIEFS
Compiled by Beth Bales and Marlén Gomez
Gala organizers seek auction donations

WITH TABLES NEARLY SOLD OUT, THE “GREAT Gatsby Gala” Committee is turning its attention to gathering auction items to entice bidders in both the Live and Silent auctions.
The sky’s the limit, organizers say—anyone up for donating rides in a hot-air balloon, for example? In the past, members have donated weekend or even week-long stays at vacation homes across the country, in the mountains, and along the water. There even have been trips offered on other continents. In 2022, multiple people donated stays of five to seven days at their vacation homes or timeshares, raising more than $30,000 in donations.
All proceeds benefit the Society for Vascular Surgery (SVS) Foundation. The Gala, with its Roaring Twenties-inspired theme, harkening the days of F. Scott Fitzgerald’s 1925 classic, The Great Gatsby, will take place Friday, June 16, during VAM 2023.
Though the Gala’s Live Auction will be available only to ticket-holders, anyone, anywhere, can bid on the Silent Auction prizes, via computer. Those items will be available in early June, ahead of the Gala itself, with bidding closing during the event. Artwork from Chris Robb and a gift card to Enrico Cuini already have been donated.
To donate items for the auctions, email CLampi@vascularsociety.org
BEST-CLI takes center stage in inaugural ‘SVS Presents’ webcast
What do the results of the much-debated BEST-CLI trial mean for the vascular surgery community?
SVS members Alik Farber, MD, and Matthew Menard, MD, co-principal investigators of the trial, presented data, discussed the trial with panelists and took questions from participants during the inaugural “SVS Presents” webcast on April 12.
The ongoing series will take place on the first or second Wednesday evening monthly.
SPOT LIGHT
Contribute to PAC in April: Be honored at VAM in June
This year’s VAM will include an “enhanced” Society for Vascular Surgery Political Action Committee (SVS PAC) contributor display, which will recognize SVS PAC donors from both 2022 and 2023. Deadlines for recognition for 2023 are April 15 for groups and April 30 for individuals.
The SVS will recognize practices that achieved 100% SVS PAC contributor participation within their groups in 2022 and those that achieve 100% 2023 participation in advance of the April deadline.
Members who believe their practices qualify for either 2022 or 2023 should email SVSAdvocacy@vascularsociety.org to share the name of their practice and their colleagues’ names. Members who plan to make an SVS PAC contribution for 2023, and who do so by April 30, will have their names included on the 2023 SVS PAC contributor list at VAM.
Member WILLIAM D. JORDAN JR., MD, a former president of the Association of Program Directors in Vascular Surgery (APDVS), has been named chair of the Department of Surgery at the Medical College of Georgia at August University. He will join the institution July 1. He currently is chief of the division of vascular surgery and endovascular therapy at Emory University in Atlanta.
Member BRUCE L. GEWERTZ, MD, has been named the CedarsSinai healthcare organization of Los Angeles surgeon-in-chief, to be the vice dean of clinical system development and faculty affairs. He is the former chair of the Cedars-Sinai Department of Surgery, and also is vice president of interventional services and the H & S Nichols Distinguished Chair in Surgery.
In memoriam
ROBERT HUGH JOHNSTON JR., MD, Feb. 22, Victoria, Texas, at age 92. Johnston, who trained with surgical titans Michael DeBakey, MD, and Denton A. Cooley, MD, was a cardiovascular surgeon who started the first heart program in Victoria, located in the south of the state. He also was a colonel in the United States Army, a pilot and, finally, an Episcopal priest.
Read more at vascular.org/RobertJohnstonObit
FDA
The development of medical devices in the vascular space faces increasing challenges amid moves afoot at the Food and Drug Administration (FDA), according to a leading regulatory expert in the field.
Among the developmental vehicles currently caught in the crosshairs are physician-sponsored investigational device exemption (PS-IDE) studies, Dorothy Abel, the former FDA official responsible for leading evaluation of vascular and endovascular surgery devices, recently told the 2023 Critical Issues America (CIA) annual meeting (Feb. 10–11) in Miami.
She was giving the CIA keynote address, tackling the subject of endovascular grafts across the decades, their evolution, and what lies in store for device development down the road.
Throughout, Abel, currently vice president of regulatory strategy at medical device research organization NAMSA, emphasized the importance of collaboration during the process of shepherding new products toward commercialization.

Developers should work together across the spectrum of interested parties—manufacturers, testing facilities, regulatory agencies—to establish the best bench-testing and animal study protocols, and what needs to be gleaned from the clinical setting, to get to requirements centered on
a risk-based rationale for testing, she said. This might entail looking at “what does the device need to be able to do; what can go wrong if it does not do that, what type of testing do we need to help us believe that it is going to do what it is supposed to do,” explained Abel, who co-founded the FDA’s early feasibility studies program.
She contrasted the difference in standards between what is required in the European Union (EU) and the U.S. “In the U.S., it is kind of a guidance—it helps FDA understand what should be done,” Abel said. “But in the EU, they take it very seriously,” she added. “If it is written in a standard as something that needs to be done, you are going to have to do it in order to get the device to market.”
Abel posed the overarching question—“Is the future now?”—of the medical device development landscape, answering herself: “I am kind of hoping that it is not.” Her reasoning? Those challenges that potentially limit PS-IDEs.
“The FDA has recently shut down the opportunity for doing some sponsor-investigator IDEs,” Abel said. “We do not know yet what their thinking is; whether that is going to apply to all IDEs in this space; or if it is going to be focused on particular manufacturers, or types of devices. But, it is a little bit concerning, because we want to
Short neck AAAs: ‘Choosing what is best for the patient’
NAVIGATING CASES OF ABDOMINAL aortic aneurysm (AAA) with short neck anatomy poses a serious challenge to vascular surgeons, but the list of potential options available to carry out repairs are numerous— and decision-making can be optimized by using a bespoke algorithm.
That was the message delivered by Ross Milner, MD, chief of vascular surgery and co-director of the Center for Aortic Diseases at the University of Chicago Medicine, in a session on endovascular aneurysm repair (EVAR) controversies at the 2023 Critical Issues America (CIA) annual meeting in Miami (Feb. 10–11).
Milner outlined the four core pathways open to surgeons when they are confronted with short necks—defined as greater than 4mm and less than 10mm: open repair,
fenestrated EVAR (FEVAR) or physicianmodified endografts (PMEGs), off-label chimney EVAR (ChEVAR), and endosuture aneurysm repair (ESAR).
In short, he said, the treatment ultimately elected should be dictated by the individual patient.
Milner
“I think in the past you’ve seen a lot of talks that are designed as debates between one of these techniques, and we commonly joke about different techniques not working well,” he told CIA 2023. “The reality is, when you’re looking at each one of your patients, and you’re sitting in the office, and trying to make a decision, I think we incorporate all of these options.”
Milner uses his algorithm, or decision tree, in order to help establish which approach is going to best serve the patient in front of
18 Vascular Specialist | April 2023
Getting medical devices to market: The future might not be now, says regulatory expert
NEWS
CLINICAL&DEVICE
Ross
Gala 2022
Compiled by Bryan Kay and Jocelyn Hudson
make sure people continue to study these devices responsibly.”
Right now, the FDA is focused on devices from a protection standpoint, with the agency often taking a skeptical view of new technology, Abel observed. “What is even more concerning, to go along with that skepticism, is there is a little less communication. They have almost doubled the size of postmarket studies as compared to the premarket studies in the most recent approvals, and it is not clear exactly what their fear is.”
The focus needs to be on patients, she said. “I am afraid that has gone away a little bit. Because there is so much focus on safety, they are not thinking about, ‘We need better things to take care of our patients in general.’”

From another angle, Abel highlighted how blame is apportioned when things go wrong. “Sometimes we are asking the wrong questions,” she said, referring to the example of ruptures occurring in patients fitted with endovascular devices. “Why did they have the ruptures? Were they not being followed? Is it really the endograft’s fault? Is it your fault? Is it the patient’s fault? But the FDA automatically goes, ‘It is the manufacturers who need to collect more data to figure out what is going on because we have these ruptures.’”
him. This includes figuring out the patient’s physiologic risk, the level of urgency of a procedure, durability based on risk profile/ age, the direction of the renal arteries, and the state of the aortic arch anatomy.
“When I look at the anatomy, the things I look at from an anatomic standpoint are: how do the renal arteries come off the aorta?” he said. “At least in my hands, I find that for chimney approaches, downwardgoing renal arteries are easier. For upwardgoing, fenestrated is easier. But again, in different people’s hands, it’s probably different. I also look at the anatomy of the neck and the tortuosity. ”
Open repair can be valuable in the right set of patients, Milner continued, but is “highly invasive,” and “the risk of renal failure with open surgery is greater than with complex EVAR, and likely leads to worse outcomes.”
In his practice, Milner and colleagues do not perform procedures with PMEGs but rather on-label FEVARs. “It’s FDA approved, it’s all transfemoral, it’s indicated for 4mm neck, but it can be limited by anatomy, and sometimes the device just can’t be made,” he explained.
Investors and developers too are not beyond criticism, Abel continued. “Bad things can happen to potentially ruin good devices, and some of those bad things come from assumptions,” she said.
In order to ensure a successful future in the device development field, Abel returned to the concept of collaboration. And that also includes patients, she said. In the past, Abel recalled, “we kind of fell short. We did not have the patients involved early on. I think we need the patients involved.”
Shooting for the stars is laudable, she said, but “be happy to take some baby steps—they keep you moving forward.” Also be realistic, Abel advised. “Often we see timelines driven by investors.” And, whenever possible, be conservative, she added. “If you have a way to test something in a less risky population, why not do that first so you can de-risk the future? The goals are to solve problems, improve lives, reduce harm.” Learning not only from mistakes, but also successes is important, Abel concluded. “I think one of the big successes in endografts has been the sponsor-investigator studies. We have learned so much—I know it is over 30 years—but it is a relatively short amount of time when you think about medicine and the complexity of what is being done. And, cooperation is key.”
ChEVAR, meanwhile, has performed “relatively well” for his team in the treatment of complex anatomy. “Its limitations are gutter leaks and stroke risk,” Milner said.
Then there is short neck ESAR, he added, which incorporates EndoAnchors with an Endurant stent-graft. Overall, said Milner, his decision tree breaks down to, first, physiologic risk. “If it’s low, consider open surgery,” he said. “If high, go with an endovascular approach. For FEVAR, I look at iliac access, the direction of the renals, the neck tortuosity, the urgency of the procedure; for ChEVAR, again, iliac access, the direction of the renals, neck tortuosity and the aortic arch anatomy; for ESAR, calcification and thrombus, and reverse taper of the neck.”
Responding to skepticism over the ESAR procedure, Milner acknowledged controversy centers on a central question: ”What is the true neck? And all of us know that a 4mm neck is exceptionally challenging to use EndoAnchors on. I know that this is written as an indication, but that is hard to do,” he said, concluding: “Many options exist; choose what is best for your patient.”
Getinge receives FDA premarket approval for the iCast covered stent system
GETINGE‘S iCAST covered stent system has received premarket approval from the Food and Drug Administration (FDA) for the treatment of patients with iliac arterial occlusive disease.
The iCast covered stent system, sold outside the U.S. under the brand name Advanta V12, has been used by clinicians for 20 years and is the most clinically evaluated balloon-expandable polytetrafluoroethylene (ePTFE)-covered stent in the world, with data published in over 550 articles, Getinge says.

VIZ.AI RECEIVES FDA 510(K) CLEARANCE FOR ABDOMINAL AORTIC ANEURYSM AI ALGORITHM
Viz.ai recently announced it has received Food and Drug Administration (FDA) 510(k) clearance for its algorithm intended to detect suspected abdominal aortic aneurysm (AAA). Viz AAA is the first FDA-cleared AI-powered solution for the detection and triage of suspected AAA, the company claims.
“There is an estimated 1.1 million Americans living with an AAA. The majority of these patients are asymptomatic and many are unaware of their disease until a rupture occurs. This is a catastrophic medical emergency, resulting in over 10,000 deaths each year,” said Philip Batista, MD, from Cooper University Health Care in Camden, New Jersey. “This algorithm is a powerful new tool for healthcare professionals to more readily identify and capture individuals with AAA and, importantly, automatically refer those at imminent risk for rupture.”
Viz AAA uses artificial intelligence (AI) to automatically search for the presence of a AAA from computed tomography angiography (CTA) from any scanner in a hospital network, a press release details. The new AI algorithm and clinical workflow solution will be a part of the Viz Aortic Module, an AI solution designed to accelerate treatment decisions for all aortic pathology.
GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts. CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available.
used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS Heparin Surface. Products listed may
life, VIABAHN and designs are trademarks of W. L. Gore & Associates.
2021 W. L. Gore & Associates, Inc. 21373436-EN DECEMBER
www.vascularspecialistonline.com 19
Consult Instructions for Use eifu.goremedical.com
GORE,
2021 01: 4.5” x 5.625” 21373436-EN-VSX-Indications-Ad.indd 1 12/8/21 11:10 AM
*As
not be available in all markets.
Together, improving
©
“It’s a little bit concerning because we want to make sure people continue to study these devices responsibly”
DOROTHY ABEL
SAFE AND EFFECTIVE TREATMENT OF SFA ISR
The Gore RELINE MAX Clinical Study demonstrated safe and effective treatment of real-world superficial femoral artery (SFA) in-stent restenosis (ISR) through three years with the VIABAHN® Device.1







65% 100% freedom from major amputations and VIABAHN® Device stent fractures

freedom from target lesion revascularization

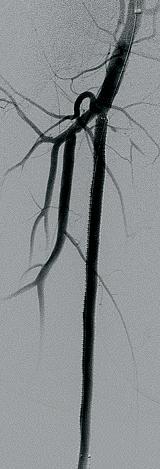
See

* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS ® Heparin Surface.





Occluded bare metal stent Post VIABAHN® Device placement Images courtesy of Peter Soukas, M.D. Used with permission.

Products
GORE, Together, improving life, VIABAHN and designs
W. L. Gore & Associates. © 2023 W. L. Gore & Associates, Inc. 22820057-EN JANUARY 2023
GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface* W. L. Gore & Associates, Inc. Flagstaff, Arizona 86004 goremedical.com Please see accompanying prescribing information in this journal.
listed may not be available in all markets.
are trademarks of
1. Soukas P. Three-year results of the GORE® VIABAHN® stent-graft in the superficial femoral artery for in-stent restenosis. Presented at VEINS (Venous Endovascular INterventional Strategies) and VIVA (Vascular InterVentional Advances) Annual Conference; October 31, 2022- November 2, 2022; Las Vegas, NV. the data










































































































 Kay
Kay

 By Bryan Kay
By Bryan Kay

































