








The one-year data from the SAVVE trial represent the “most encouraging clinical data that have ever been produced for a bioprosthetic deep vein valve” in more than half a century of attempts at developing such a device, according to leading venous disease specialist Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK).
The prospective, non-blinded, single-arm, multicentre
Surgical Antireflux Venous Valve Endoprosthesis (SAVVE) pivotal trial enrolled 75 patients with chronic venous insufficiency (CVI) at 21 sites in the USA. The headline findings include 98.4% VenoValve (Envveno Medical) device patency, 85% clinically meaningful benefit and an 80% rate of ulcer size reduction at 12 months.
Fresh from delivering these positive one-year data at the 2024 VEITHsymposium (19–23 November, New York, USA), site principal investigators Matthew Smeds (Saint Louis University, Saint Louis, USA) and Raghu Motaganahalli (Indiana University, Indianapolis, USA) are both ebullient about what the results might mean for the future of the treatment of CVI and venous ulcerations.
The data show that 85% of patients surgically implanted with a bioprosthetic VenoValve reached the one-year milestone having achieved a clinically meaningful benefit of a three or more-point improvement in revised Venous Clinical Severity Score (rVCSS); a 7.91 point average improvement in rVCSS; clinically meaningful benefit across all CEAP (Clinical, Etiological, Anatomical and Pathophysiological) classes of patients enrolled (C4b–C6); 97% target vein patency at one year; and significant resolution in venous ulcerations.
“It is the first device we have had to treat deep venous insufficiency in years,” Smeds tells Vascular News in an interview in the days after presenting at VEITH 2024. “These patients are typically relegated to compression therapy alone. There are no really great surgical options for this issue.” Similarly, Motaganahalli describes the novel device as representing a “game-changer” given how previous attempts at developing a surgical option for the CVI patient population failed to take hold. “The procedure does not have a steep learning curve; technically, this is a procedure that can be accomplished by any trained vascular surgeon. It is not technically demanding,” he says. “Before, these were technically demanding operations: if you really look at the
The procedure does not have a steep learning curve.”
on page 3
New data support role of F/BEVAR for treatment of thoracoabdominal aortic aneurysms
A RECENT PROSPECTIVE, multicentre cohort study provides insights into early and late aorticrelated mortality and rupture after fenestrated and branched endovascular aneurysm repair (F/ BEVAR) of thoracoabdominal aortic aneurysms (TAAAs). Researchers claim these are likely to represent the most comprehensive data on the topic for the foreseeable future, citing the unworkable nature of a randomised study.
Gustavo Oderich, Ying Huang (both University of Texas Health Science Center at Houston–McGovern Medical School, Houston, USA) and colleagues, on behalf of the US Aortic Research Consortium (ARC), write in Circulation that F/ BEVAR has been used as a minimally invasive alternative to open surgical repair to treat patients with TAAAs.
The authors explain that, despite the widespread use of fenestrated and branched aortic devices worldwide, they are not currently commercially approved by the US Food and Drug Administration (FDA). Access to these devices, they continue, is limited to those centres with ongoing physician-sponsored investigational device exemption (PS-IDE) studies. The researchers detail that the US ARC has been collecting prospective data from these studies since 2018.
The aim of the present study, the authors note, was to evaluate aorticrelated mortality and aortic aneurysm rupture after F/BEVAR of TAAAs. In order to do so, the research team analysed patients enrolled in eight prospective, non-randomised, PSIDE studies between 2005 and 2020 who underwent elective F/BEVAR of asymptomatic intact TAAAs.
The primary endpoints of the study, Oderich, Huang et al detail,
Continued on page 4
Iwould like to say a big welcome to you and all of our readers to 2025 and to the 105th issue of Vascular News. I hope that you all enjoyed a good Christmas, New Year and festive season, in general, and are ready to embrace new and current vascular events in 2025.
Vascular News 105 leads with two main stories. The first discusses data from the VenoValve (Envveno Medical) US pivotal trial that were presented by Matt Smeds at the recent 2024 VEITHsymposium in New York, USA. The second story focuses on new data from the US Aortic Research Consortium (ARC), recently published in Circulation, that support the role of fenestrated and branched endovascular aneurysm repair for the treatment of thoracoabdominal aortic aneurysms.
There is an invited commentary from Vascular Society of Great Britain and Ireland (VSGBI) president Ian Chetter, immediate past VSGBI president Andrew Garnham, and Mei Nortley, edited by Anna Pouncey, on the important topic of sexual harassment in surgery that is based on a session at the recent VSGBI annual scientific meeting (VSASM) in Brighton, UK.
This issue’s profile highlights the achievements of Vincent Rowe, professor and chief of vascular surgery at the University of California, Los Angeles (UCLA; Los Angeles, USA).
Regarding vascular access and venous articles, there is coverage of recent data from the WAVE trial by Mahmood Razavi, which indicate benefits for the Wrapsody (Merit Medical) stent graft in arteriovenous grafts at six months; on a novel bioabsorbable perivascular wrap to reduce arteriovenous fistula failure by Ellen Dillavou; and an article on the venous arm of the Vascular Quality Initiative celebrating its decade milestone that was presented by Marc Passman at the 2024 VEITHsymposium, as were the topics by Razavi and Dillavou.
With respect to the aorta, there is an article on a recent CX Aortic Live roundtable discussion with Tilo Kölbel, Mehrdad Ghoreishi, Michael Borger and Davide Pacini on the Endo-Bentall procedure—described by Kölbel as “the last frontier in cardiovascular interventions”.
In the carotid space, an article describes a recent important paper on the safety of shunting strategies during carotid endarterectomy that was published by Xavier Hommery-Boucher and colleagues in the European Journal of Vascular and Endovascular Surgery
Finally, regarding peripheral arterial disease, we look ahead to the upcoming Charing Cross (CX) International
Symposium 2025, which will feature new data from the SWEDEPAD trial among other podium first presentations.
In this section, there is also an update on the LIFE-BTK trial with two-year data presented at last year’s VIVA meeting in Las Vegas, USA.
Elsewhere in the issue there is a summary of interviews conducted at the recent Paris Vascular Insights (PVI) course in Paris, France, including with Stéphan Haulon on the use of artificial intelligence in everyday practice and with Marianne Brodmann and Eric Secemsky.
In concluding, as always please feel free to contact me or the editorial team with any suggestions for improvement or ideas for articles to be included in Vascular News that you may have. I hope that you all enjoy this edition of Vascular News, and I look forward to seeing as many of you as possible in 2025.

We look ahead to the upcoming Charing Cross (CX) International Symposium 2025, which will feature new data from the SWEDEPAD trial among over 50 other podium first presentations.”

ROB MORGAN is professor of interventional radiology at St George’s University Hospitals NHS Foundation Trust (London, UK) and the president of the British Society of Interventional Radiology.
Editorial board: Ian Loftus, Rob Morgan, Stephen Black and Nicholas Inston | Publisher: Stephen Greenhalgh
Content director: Urmila Kerslake
Editor: Jocelyn Hudson Jocelyn@bibamedical.com | Contributing editor: Bryan Kay | Editorial contribution: George Barker, Jamie Bell, Will Date and Éva Malpass | Design: Terry Hawes, Josh Lyon and David Reekie
Advertising: Rav Pankhania Rav@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News and Media, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2025. All rights reserved.
n CX 2025:
Primary results from the SWEDEPAD 1 clinical trial will be be released at the upcoming Charing Cross (CX) International Symposium 2025 (23–25 April, London, UK). The presentation is one of over 50 podium firsts on this year’s programme. The inaugural Roger M Greenhalgh memorial lecture is set to be another highlight, with Gustavo Oderich (Houston, USA) speaking on the challenges of 21st century aortic education, innovation and and evidence. For more on this story go to page 6.
n COMPLEX AORTIC PROCEDURES:

The minimally invasive EndoBentall procedure for the treatment of aortic root pathologies was the focus of a recent roundtable discussion at CX Aortic Live 2024 (7–8 October, Vienna, Austria). After agreeing on a precise definition for this “last frontier” in cardiovascular interventions—as described by discussion chair and course co-director Tilo Kölbel (Hamburg, Germany)—a panel of experts pondered the future of this technique and underscored the importance of collaboration between specialties to ensure the best patient outcomes.
For more on this story go to page 6.
n IVC FILTERS:
A team of researchers in Canada has developed machine learning models that they claim, “can accurately predict oneyear IVC [inferior vena cava] filter complications, performing better than logistic regression”. The research, authored by Ben Li (Toronto, Canada) and colleagues, was recently published in the Journal of Vascular Surgery: Venous and Lymphatic Disorders
"Model performance remained robust across all subgroups"
For more on this story go to page 13.
Scan the QR code to subscribe
If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
SAVVE trial results are ‘most encouraging data ever produced for a bioprosthetic vein valve’
Continued from page 1
historical data from internal valvuloplasty or external valvuloplasty, vein valve reconstruction, vein valve transplant—they were effective in a few select centres and a few select hands, but that result was not reproducible at multiple centres.”
Smeds zeroes in on the topline result of 98.4% device patency and considers how there were nine device occlusions over the course of the one-year study. “However, eight of them recanalised,” he explains. “I had some patients that thrombosed the device. Interestingly, in one of them I sucked out the thrombus via mechanical thrombectomy and then it occluded a few weeks later. I thought that that device was never going to be functional, but, within a month or two, the device recanalised and the patient had decreased reflux below the device and was healing her ulcer.”
Though these thromboses were each marked down as a device “failure,” Smeds continues, the fact eight reopened and many were then functional represented pleasant surprises. “I think you see that in the natural history of people with DVTs [deep vein thromboses] to begin with,” he explains.
Elsewhere among the data, he picks out the trial’s inability to pinpoint a direct relationship between reflux and ulcer healing, and the role of compression therapy compliance as intriguing propositions. “We cannot find a direct one-to-one ratio in terms of if you have a really high decrease in reflux, then you get a really high ulcer healing rate,” Smeds says. “So, there are certainly more complex things going on at a patient-to-patient level with the device as far as who is benefiting from it.
“We also looked at whether there was full
Vascular News is pleased to announce that it is expanding its editorial boards for both Vascular News Europe and Vascular News North America for 2025 onwards. The editorial boards for both newspapers now span several specialties, including vascular surgery, interventional radiology, interventional cardiology, venous surgery, and renal and transplant surgery.
compliance for compression therapy—whether that increased or decreased over the length of the trial—and there was a slight decrease in its use, which demonstrates that the device is doing something to aid in the ulcer healing and the symptom improvement, because it wasn’t necessarily due to an increase in compression.”

Matthew Smeds
For Motaganahalli, ulceration reduction data were stark. In those who had their ulcers for less than three months, the SAVVE trial demonstrated there was 100% resolution—“complete healing,” he says. “Most of the patients who had an ulcer more than a year or so, even then 60% of them showed full resolution of the ulcer. In terms of the average area of the ulcer—especially in those who had a longer duration of ulcer—those patients had an average ulceration of 20.6cm2 at baseline, and that reduced to 12.3cm2.”
Motaganahalli sees a promising future for the device: “The one-year data tell you that it provides a sustained benefit of symptomatic improvement for patients with CVI. The VenoValve is a safe and effective treatment for patients with CVI due to deep valvular
There are a lot of unknown questions that will hopefully begin to be answered once this becomes FDA approved.”
Matthew Smeds
incompetency. It’s not only effective, but effective across the whole spectrum of CEAP classification: for C4b through C5 and C6. The benefits were seen within the first three months and were sustained through the one-year period of observation.”
Motaganahalli also lingered on the significance

Ian Loftus
Consultant and professor in vascular and endovascular surgery at St George's University Hospitals NHS Foundation Trust (London, UK)

Rob Morgan
Professor of interventional radiology and consultant radiologist at St George’s University Hospitals NHS Foundation Trust (London, UK)

Stephen Black
Professor of venous surgery at King's College London; consultant vascular surgeon at Guy's and St Thomas' Hospital (London, UK)

Nicholas Inston
Consultant renal and transplant surgeon at Queen Elizabeth Hospital Birmingham (Birmingham, UK)

Raghu Motaganahalli
of the improvement in average rVCSS among the trial cohort: the 7.91 score recorded at 12 months came amid a US Food and Drug Administration (FDA) mandate for a more than 3-point advance in rVCSS. “Here, more than 85% of the patients had more than a 3-point improvement,” Motaganahalli notes.
On the eve of VEITH 2024, Envveno Medical announced it had submitted an application with the FDA seeking approval to market the VenoValve based on the one-year data. The company is also developing a next-generation, nonsurgical transcatheter-based replacement venous valve called Envve. It is expected to be ready for its own pivotal trial in the middle of 2025.
Smeds is excited by the portent of what might be to come as development of the devices proceeds. The venous system is still “Pandora’s box as far as putting devices and valves in there,” he says. Further, in the SAVVE trial, each patient was implanted with only one replacement valve. Smeds considers the questions of whether more than one device should be fitted in patients to further decrease reflux; whether in patients with duplicate incompetent systems both should receive a VenoValve; and whether the location of the replacement valves should be modified. “There are a lot of unknown questions that will hopefully begin to be answered once this becomes FDA approved,” he adds.
Motaganahalli believes some might query the device cost once it enters the market but sees the potential impact on the heavy cost burden associated with wound care for venous ulcers as a boon. “With appropriate patient selection, the device can do wonders. We have 2.5 million potential patients with CVI in the USA, with close to around 40% missing workdays, US$3 billion in direct medical costs, US$30,000 per patient in terms of the annual cost of ulcer treatment, with the potential for 20–40% of these patients to have a recurrence. This makes the device a very attractive option.”

Ross Milner
Chief of the Section of Vascular Surgery and Endovascular Therapy at University of Chicago Medicine (Chicago, USA)

Erin Murphy
Director of the venous and lymphatic programme at Carolinas HealthCare System's Sanger Heart and Vascular Institute (Charlotte, USA)

Eric Secemsky
Director of vascular intervention and an interventional cardiologist at Beth Israel Deaconess Medical Center; associate professor of medicine at Harvard Medical School (Boston, USA)

Bart Dolmatch
Interventional radiologist at The Palo Alto Medical Foundation (Palo Alto, USA)
New data support role of F/BEVAR for treatment of thoracoabdominal aortic aneurysms
Continued from page 1
were aortic-related mortality—defined as any early mortality (30-day or in-hospital) or late mortality from aortic rupture, dissection, organ or limb malperfusion attributable to aortic disease, complications of reinterventions—or aortic rupture. Secondary endpoints were early major adverse events, TAAA life-altering events— defined as death, permanent spinal cord injury, permanent dialysis, or stroke—all-cause mortality, and secondary interventions.
respectively.
Oderich, Huang et al continue that there were 30 late aortic-related mortalities; the five-year cumulative incidence was 3.8%; and older age and extent I–III TAAAs were independently associated with late aortic-related mortality.
Furthermore, the authors report that 14 late aortic ruptures occurred; the five-year cumulative incidence was 2.7%; extent I–III TAAAs were associated with late aortic rupture; five-year all-cause mortality was 45.7%; and five-year cumulative incidence of secondary intervention was 40.3%.

Oderich, Huang and colleagues share in Circulation that 1,109 patients were analysed in the study, noting that 589 (53.1%) had extent I–III and 520 (46.9%) had extent IV TAAAs. The patients had a median age of 73.4 years and just under one-third were women.
The investigators report that early mortality was 2.7% and that congestive heart failure was associated with early mortality. Furthermore, they reveal that the incidence of early aortic rupture was 0.4% and that the incidence of early major adverse events and TAAA life-altering events was 20.4% and 7.7%,
not aortic related.
Oderich, Huang et al reiterate that five-year all-cause mortality was high—45.7%— mostly due to non-aortic-related causes, “likely reflecting selection of higher-risk subgroups compared with historical reports of [open surgical repair]”.
patients were analysed in the study
In the conclusion of their findings, Oderich, Huang and colleagues write that aortic-related mortality and aortic rupture are “uncommon” after elective F/BEVAR of asymptomatic intact TAAAs. They add that half of the aortic-related mortalities within the study occurred early, and that most of the late deaths were
These data are likely to be the most reliable and obtainable to validate the role of F/BEVAR for the treatment of TAAA.”
‘Most encouraging data ever produced for a bioprosthetic vein
Vascular News spoke to leading venous disease specialist and Europe-based vascular surgeon Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) to discuss the one-year data from the SAVVE trial (see page 1). While Gohel cautiously welcomed what he described as the “most encouraging clinical data that have ever been produced for a bioprosthetic deep vein valve” in more than half a century of attempts at developing such a device, he also reflected on patient selection, durability and health economics challenges to come.
“THERE HAVE BEEN EFFORTS TO TREAT deep venous reflux for at least 50 years,” says Gohel. “A native valve—a healthy normal valve—is a thing of beauty: it is incredibly flexible, mobile, is not thrombogenic at all, and is also really strong. So that is a tough ask for anything prosthetic.
And, for half a century, people have been trying to do this and have generally failed.”
Case selection will pose the greatest challenge, Gohel explains. “Deep reflux is very common. Of all the patients I see, probably a third, if not a half, of them will have deep venous reflux. The potential population is enormous, but we need to be very selective of the people we put through this procedure because it is still going to be experimental, expensive and invasive.” As such, for Gohel, VenoValve implantation is a third-line treatment option after superficial vein procedures and deep venous stenting “for those people who have still not responded”.
Further, Gohel says he will be closely following the durability of the device as longer-term data emerge. “The one-year data are nice, but one year is nothing in the grand scheme of things when it comes to venous disease. The problem is, if you get device
The valve—which I know they are working on—is going to be a really important next step.”
failure, then the risk is you might get thrombosis of this prosthesis and that is effectively a DVT [deep vein thrombosis].”
Gohel says he will also be following the price at which the VenoValve enters the market in light of health economics considerations.
“Venous ulceration is extremely expensive, but even with the very best treatments, the best they do
The final conclusion the authors make is that secondary interventions were needed in one of four patients. They do stress, however, that three-quarters of these were minor procedures.
The authors recognise several limitations of their study, including possible patient selection bias due to a need to adhere to PS-IDE inclusion and exclusion criteria, the frequency of cause of death being unknown, and the reason for aortic rupture and the aortic diameter at the time of rupture not being available for all patients, among others.
Despite these limitations, the authors underline the significance of the present study.
“Given a perceived lack of clinical equipoise to justify a randomised controlled trial,” they comment, “these data are likely to be the most reliable and obtainable to validate the role of F/BEVAR for the treatment of TAAA.”
On the clinical implications, Oderich, Huang and colleagues remark: “Rigorous surveillance after F/ BEVAR is required because the need for secondary interventions was high among patients undergoing F/BEVAR of TAAAs, and identifying the need for secondary interventions will paradoxically be higher with better surveillance.”
is improve the ulceration rate and reduce recurrence rates by 50%—you don’t cure the problem,” he points out. “Even then, cost effectiveness is only just demonstrated, because the treatments that you are carrying out—dressing the ulcers—cost money but they are still relatively cheap next to a very expensive procedure. So, you’ve got to get a lot of ulcer healing benefit in order to demonstrate cost effectiveness.” Ultimately, says Gohel, reducing the level of invasiveness of the procedure will be key. “At the moment, the procedure is an open surgery and has to be performed by a vascular surgeon,” he adds. “A lot of these patients are quite elderly and frail. You may not want to be putting many of them through relatively major surgery, so the percutaneous valve—which I know they are working on—is going to be a really important next step.”
Recent years have brought other efforts to produce

Late-breaking results from the second year of the LIFEBTK clinical trial demonstrate the long-term effectiveness of the US Food and Drug Administration (FDA)-approved Esprit BTK everolimus-eluting resorbable scaffold system (Abbott Vascular) in patients with the most severe form of below-theknee (BTK) peripheral arterial disease (PAD). The data show that the Esprit BTK offers sustained benefits over balloon angioplasty with fewer repeat procedures at two years.
THE LIFE-BTK TRIAL
evaluated the Esprit BTK in more than 260 patients worldwide with BTK PAD, comparing treatment with the Abbott device to balloon angioplasty—the current standard of care. Following the presentation of one-year results at TCT 2023 (23–26 October, San Francisco, USA), two-year results were revealed during a late-breaking data session at VIVA 2024 (3–6 November, Las Vegas, USA).
“PAD is a dangerous condition that is complex to treat, with limited approved treatment options,” said Brian
DeRubertis (New York Presbyterian–Weill Cornell Medical Center, New York, USA), presenter and one of the principal investigators for the trial, in a press release announcing the results.
“Abbott’s Esprit BTK system offers a new option for treating people with the most severe forms of PAD, helping to heal blood flow and potentially salvage limbs.”
Results after two years of the LIFEBTK clinical trial showed that 90.3% of patients in the Esprit BTK arm did not require a reintervention at 24 months. The trial also showed sustained efficacy
A recent analysis of over 270,000 Medicare fee-for-service beneficiaries has found an increase in adverse outcomes and death after a US Food and Drug Administration (FDA) warning led to decreased use of paclitaxel-coated devices for peripheral revascularisation procedures.
WRITING IN THE JOURNAL OF THE American College of Cardiology (JACC), a team of researchers from the Richard A and Susan F Smith Center for Outcomes Research at the Beth Israel Deaconess Medical Center (Boston, USA) begin by stating that peripheral revascularisation has faced “intense scrutiny” in the past decade.
The authors—Joseph M Kim, Eric Secemsky and colleagues—first cite the impact of a 2018 meta-analysis associating paclitaxel-coated devices with increased mortality when used for peripheral revascularisation. The paper led to a warning from FDA against routine use of such devices that was not reversed until 2023.
Additionally, Kim et al note that COVID-19 “complicated peripheral arterial disease (PAD) management by reducing access to patient care”.
To assess the impact of these events, the researchers conducted a study to evaluate trends in femoropopliteal revascularisation from 2016 to 2023 and to analyse safety outcomes during three key time

at 24 months.
Furthermore, compared to balloon angioplasty, patients treated with Esprit BTK had significantly greater freedom from chronic limb-threatening ischaemia (CLTI), at 61.5% vs. 32.8%.
Additionally, at one year, the trial’s powered secondary endpoints revealed that Esprit BTK had a higher rate of reducing vessel re-narrowing (35.2% improvement) compared to balloon angioplasty.
Alongside the announcement of these results, Abbott also shared that it has launched the Esprit BTK post-approval study (PAS) to assess the continued safety and effectiveness of Esprit BTK in treating CLTI patients in a real-world setting.
periods: 1) before the paclitaxel safety concern; 2) after the onset of the paclitaxel safety concern; and 3) during and after the COVID-19 pandemic.
Kim and colleagues detail that their study included all Medicare fee-for-service beneficiaries aged ≥66 years who underwent femoropopliteal revascularisation by International Classification of Diseases-10th Revision codes between 1 January 2016 and 31 December 2023.
Regarding statistical analysis, the authors state that they examined trends of femoropopliteal artery revascularisation procedures by quarter year across the eight-year study period. Endovascular revascularisation procedures were stratified by percutaneous transluminal angioplasty (PTA) alone, drug-coated balloon (DCB) alone, bare metal stent (BMS) alone, or drug-eluting stent (DES) alone.
The study’s primary outcome was the composite of major amputation and all-cause mortality.
“During the study period, the number of endovascular revascularisation procedures declined 38.2%; the number of surgical revascularisation procedures declined 59.7%,” Kim et al report in JACC
The authors share that PTA was the primary method (27.72%) used for endovascular revascularisation before the onset of the paclitaxel-coated device safety concern, followed closely by DCB (24.91%). After the paclitaxel safety concern, the use of DCBs declined to a low of 17.89% by 2019, with a proportional increase in the use of uncoated PTA (34.52%).
Brian
DeRubertis presenting at VIVA 2024
Abbott’s Esprit BTK system offers a new option for treating people with the most severe forms of PAD, helping to heal blood flow and potentially salvage limbs.”
before the safety concern.
Furthermore, Kim et al reveal that revascularisations performed between the paclitaxel safety concern and the COVID-19 pandemic were associated with a higher rate of the primary outcome compared with procedures performed before the paclitaxel safety concern. They note that this was driven by increased risk of all-cause mortality.
“By contrast,” Kim and colleagues report, “there were lower rates of major amputation after the paclitaxel safety concern.”
“The paclitaxel safety concern sparked a rapid shift away from the use of paclitaxel-coated devices and toward the use of uncoated devices, particularly PTA,” the authors write in their discussion. “The use of DCBs has only recently regained some ground since its nadir in 2019, but their use remains remarkably low relative to PTA despite evidence supporting their superior efficacy and the FDA’s reversal of its warning against their routine use.”
During the study period, the number of endovascular revascularisation procedures declined 38.2%; the number of surgical revascularisation procedures declined 59.7%
In addition, Kim and colleagues write that the proportion of DCB use increased after the onset of COVID-19. “By the time of the FDA’s reversal of its warning against routine use of drug-coated devices in 2023,” the authors continue, “DCB use had reached 23.46%.” They go on to note that the use of uncoated PTA stabilised at a proportion higher than was seen
Regarding limitations to their study, Kim et al recognise that claims data lack detailed anatomical and procedural information, and that the Medicare population “may not represent the US population at large”.
Secemksy, senior author of the study, remarks on the significance of the findings: “This analysis is critical in informing how external events impact patient care. Between device safety concerns and the COVID-19 pandemic, treatment approaches changed dramatically for lower extremity interventions and patient outcomes were negatively impacted. How we approach existential threats to peripheral vascular care will require thought and caution, as informed by these novel data that were generously supported by the SCAI [Society for Cardiovascular Angiography and Interventions] Early Career Research Grant.”
Primary results from the registry-based SWEDEPAD 1 clinical trial are set to be released at the upcoming Charing Cross (CX) International Symposium 2025 (23–25 April, London, UK). The presentation is one of over 50 podium firsts on this year’s programme.
THE SWEDISH DRUG ELUTION trial in peripheral arterial disease (SWEDEPAD) project was designed to assess the clinical efficacy and cost-effectiveness of drug-elution technology versus conventional endovascular techniques in peripheral arterial disease. It consists of two separate parallel studies, SWEDEPAD 1 and SWEDEPAD 2, each defined by the severity of peripheral arterial disease. As noted on clinicaltrials.gov, “patients with critical limb ischaemia are allocated to SWEDEPAD 1 and patients with intermittent claudication are allocated to SWEDEPAD 2”.
Principal investigators Mårten Falkenberg and Joakim Nordanstig (both Gothenburg University, Gothenburg, Sweden) will present the key results from SWEDEPAD 1 during a session on current best practice for the management of chronic limb-threatening ischaemia (CLTI). Following the podium first presentation, Matthew Popplewell (University of Birmingham, Birmingham, UK)—one of the BASIL trial collaborators—will share a response to the data release and, as per the CX style of education, there will be ample time for audience participation and discussion.
CX 2025 will focus on some of the key challenges currently facing the vascular community, continuing the three-year cycle of ‘controversies, challenges and consensus’ the meeting is renowned for. In keeping with this theme, SWEDEPAD has faced significant obstacles over the course of the trial period, all in the pursuit of challenging the available evidence in order to determine best practice for peripheral arterial disease patients.


Mårten Falkenberg Joakim Nordanstig
In January 2019, following the publication of Katsanos et al’s meta-analysis that found a higher risk of death in the long term when paclitaxel devices are used in the leg, SWEDEPAD 1 and 2 stopped enrolment. The BASIL-3 trial—the results from which were presented for the first time at last year’s CX
“We need to collaborate”: CX Aortic Live panel probes future of Endo-Bentall
The minimally invasive Endo-Bentall procedure for the treatment of aortic root pathologies was the focus of a recent roundtable discussion at CX Aortic Live 2024 (7–8 October, Vienna, Austria). After agreeing on a precise definition for this “last frontier” in cardiovascular interventions—as described by discussion chair and course co-director Tilo Kölbel (University of Hamburg, Hamburg, Germany)—a panel of experts pondered the future of this technique and underscored the importance of collaboration between specialties to ensure the best patient outcomes.
KÖLBEL FIRST OUTLINED THAT AN ENDOBentall procedure is, put simply, the combination of a thoracic stent graft in the ascending aorta with an aortic valve. He went on to ask Mehrdad Ghoreishi (Miami Cardiac and Vascular Institute, Miami, USA), who he noted has performed a few cases with fenestrations and connecting stents, whether these can be called Endo-Bentall, or whether the definition must include branches as well.
“If you have connecting stents from a fenestration or branch, then it’s an Endo-Bentall,” Ghoreishi explained. If there are no connecting stents, however, he specified this would be an Endo-Wheat procedure.
CX Aortic Live 2024 executive board member Michael Borger (University of Leipzig, Leipzig, Germany) noted that, while he does not perform endovascular interventions in his role as a conventional cardiac surgeon, his centre in Leipzig has “one of the largest transcatheter valve programmes in the world”—the progress of which he has followed closely over the last decade.
Based on his observations, Borger stressed that the pathologies in question when discussing Endo-Bentall, namely aortic root pathologies such as acute type A aortic dissection, are “hostile”.
“There’s definitely been progress made,” Borger shared. “There is a group of patients that can benefit from this that are too high risk for surgery.” On the other hand, he opined that the treatment of younger patients with aortic root aneurysm disease is “going to stay in the surgical domain for a while yet”, as demonstrated by a presentation shared at CX Aortic
Live 2024.
On this, Kölbel asked Borger whether he was “sceptical” this technique will replace open repair for type A aortic dissection in the foreseeable future.
“Type A would be nice,” Borger responded, noting that these patients have a high perioperative mortality rate. However, he stressed that the vast majority of these patients have an entry site just above the sinotubular junction, rendering a procedure “very difficult” with regard to obtaining a seal without further complications. “Therefore, for type As, I think it would be a very small patient population that would be applicable.”
Here Kölbel asked Davide Pacini (University of Bologna, Bologna, Italy), also a member of the CX Aortic Live 2024 executive board, where he sees the future of the Endo-Bentall procedure.
“We still require more time,” Pacini replied. “Open surgery will remain the gold standard for a long time, even in acute dissection.”
At this point, however, Ghoreishi put forward the question: “what are the other options for inoperable patients?” He posited: “Definitely, in the future, the Endo-Bentall procedure will be for inoperable, highrisk patients.”
Before then, however, Ghoreishi highlighted that there is a need for serious investment from companies. “I think we have a long way ahead of us,” he said. Borger was in agreement, noting that one of the major issues in this regard is that, in order to get US Food and Drug Administration (FDA) or CE-mark approval, the components of a device must be made
gathering—similarly paused recruitment at this time.
It was not until May 2019 when the SWEDEPAD investigators announced the conclusion of a safety committee analysis that recommended the halted SWEDEPAD 1 and 2 trials resume enrolment. The decision to consider resuming enrolment in the SWEDEPAD trials came 12 days after a dedicated session on the paclitaxel controversy took place at CX 2019.
● The largest vascular meeting in the world
● 50+ podium firsts
● Inaugural Roger M Greenhalgh memorial lecture
The following year, the SWEDEPAD investigators published an unplanned interim analysis showing no difference in all-cause mortality for paclitaxel devices.
SWEDEPAD 1 has since completed enrolment and, at CX 2025, primary results from the trial will be shared for the first time.

by one company. “Once you start combining different products from different companies,” he explained, “it complicates the approval process so much that the regulators are not willing to take that risk. And right now, there’s not really a company that has a big enough presence in transcatheter aortic valve technology and vascular graft technology.”
Regarding the possibility of a premarket approval (PMA) study in the meantime, Borger expressed his enthusiasm. “I would definitely be happy to use this in inoperable patients before the CE-mark approval is granted,” he said.
Finally, the panel turned their attention to the importance of collaboration between specialties regarding the success of the Endo-Bentall procedure.
“As cardiac surgeons,” Pacini remarked, “we have to be involved.”
There was agreement that “cross-fertilisation” during training is important. However, Borger was keen to stress that open and endovascular skills should be honed by two different members of a team and not the same person, citing the “exponential” growth in medical knowledge that makes specialisation in multiple areas near impossible.
Pacini was in agreement. “I think that we need to collaborate,” he stated, underlining the involvement of vascular surgery, interventional radiology, and cardiac surgery as key to successful complex aortic procedures. “I think that is the key.”


On rati des experum quiam quunt offic testotaturit rem harciis int, simusam quaero beatibus ium eatempos.
surgery:
On rati des experum quiam quunt offic testotaturit rem harciis int, simusam quaero beatibus ium eatempos.
References:
On rati des experum quiam quunt offic testotaturit rem harciis int, simusam quaero beatibus ium eatempos.
“We need to have challenging and awkward conversations”



xxxx rati des experum quiam quunt offic testotaturit rem harciis int, simusam quaero beatibus ium eatempos.
Ian Chetter,
Garnham and Mei Nortley
Point of View
Following a session on sexual misconduct in surgery delivered at the 2024 Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (27–29 November, Brighton, UK), Ian Chetter (Hull, UK), Andrew Garnham (Wolverhampton, UK) and Mei Nortley (Oxford, UK) ask and answer common questions, discuss the pervasiveness of sexual misconduct within professional culture, and highlight what needs to change.
AG: How widespread is the problem?
MN: In September 2023, the Working Party for Sexual Misconduct in Surgery (WPSMS) published their groundbreaking paper demonstrating that sexual misconduct within surgery is rife and pervasive. Within the UK surgical workforce, 81% of men and 91% of women have witnessed sexual harassment. Two-thirds of women have been the target of sexual harassment and one-third reported being sexually assaulted. Over 10% of women had experienced forced physical contact for career opportunities.1 Whilst we struggle to digest the figures, it is automatic to comfort ourselves that this must be happening in other specialities—certainly not in ours. My conversations with vascular trainees across the UK would suggest otherwise, and experiences across Europe are reportedly worse.
Figure 1 represents experiences reported to me. All targets were vascular trainees, and all behaviours carried out by more senior colleagues. As a young surgeon, arriving at work as a highly educated professional— expecting to be trained and treated as a colleague—it is degrading, humiliating, and demoralising to be spoken to like this. These examples represent the ‘mildest’—many more were removed as they are too disgusting to print.
IC: Please explain why misogyny and banter are unacceptable and should not be tolerated.
MN: I used to dismiss comments like those in Figure 1 thinking they “aren’t that bad” or “aren’t really hurting anyone”. Understanding the rape pyramid was pivotal for me.
‘Sexual banter’ involves objectification, sexualisation, misogyny and genderstereotyping and these reinforce
common factors contributing to safety events are human factors, leadership and communication.4 If surgeons are degrading, humiliating, and trying to touch their team members, they will not be focusing on their clinical practice. Moreover, those whom they degrade, humiliate, sexually harass or assault, will also struggle to focus on providing clinical care.
IC: Why is it crucial we address this problem as a matter of urgency?
AG: While it seems intuitive that we should take action, I think the topic can generate considerable discomfort for senior male colleagues who report fear of saying the wrong thing, ‘cultural misappropriation’, or that their contribution would not be valid. It is not unnatural to feel this way; however, female surgeons remain underrepresented in leadership,5 and so I don’t think we can afford to be ‘neutral’ or passive.
MN: Many women avoid this topic too. All of us have normalised these behaviours and many join in due to
me: “In the end it was a boys’ club. The standards you walk past are the standards you set. It takes a village to raise a child, and it takes a village to enable a sexual predator”. Ultimately, this is our “village”, our vascular community, and it has to become our problem.
AG: How do we strike a balance between having a ‘fun’ team culture and maintaining professional boundaries in the work environment?
IC: General Medical Council Good Medical Practice (GMC GMP) guidance states: “You must not act in a sexual way towards colleagues with the effect or purpose of causing offence, embarrassment, humiliation or distress… [which] can include—but isn’t limited to—verbal or written comments, displaying or sharing images, as well as unwelcome physical contact”.
MN: I also find this definition easier and useful in my own interactions: “jokes or behaviour which are at the expense of, or considered offensive by,
the foundation of the rape pyramid (See Figure 2).2 This is a foundation of attitudes and belief that some individuals—often women—are of less value and are present, not as professional colleagues, but instead for sexual or menial purposes. It also contributes to themes of power, control, ownership and entitlement. During a recent tribunal, the panel acknowledged a doctor found guilty of sexual misconduct was “emboldened” by “banter culture” in his department.3 These attitudes and beliefs validate and justify all the behaviours towards the top of the pyramid, including sexual harassment, sexual assault, rape and even murder.
We all know an aspiring, hardworking young person entering a professional sphere—our siblings, children, nieces, nephews or grandchildren. The examples above don’t just represent vascular surgery. They represent a culture that pervades our society. Tolerating, permitting and contributing to comments which sexualise, objectify, gender stereotype or target minority groups, enables harm. Understanding the rape pyramid makes it instantly clear that, as a professional community, we have a choice—we can choose to destablise the beliefs at the base of the pyramid— or maintain them. We can disable damaging attitudes and behaviours—or enable them.
AG: There is so much evidence on the impact of human factors—surely this must be affecting patient safety?
MN: Unlike other professions, instances of sexual harassment in healthcare occur during episodes of patient care—often during live operations. It affects our patients. There is consistent evidence that the most


fear of marginalisation and a pressure to fit in. This culture is so pervasive that many can’t even identify it, and others feel a deep fear of highlighting themselves by speaking out, as it may affect their career progression. Regrettably, inaction leads to inertia and inertia leads to enablement. There was a case within Oxford University Hospitals regarding a surgeon who sexually harassed and intimidated female trainees for over a decade. The words of one of the victims haunt
someone present are inappropriate”. Ensuring colleagues feel respected and psychologically safe at work should not deter a happy team culture; conversely, it generates one and allows humour.
IC: What should someone do if they accidentally touch someone at work?
MN: As a surgical profession, we often work in close quarters. But just as if you were to bump into someone
Continued on page 9
Sexual misconduct in surgery: “We need to have challenging and awkward conversations”
on the street, if you accidentally touch someone, an open, timely acknowledgement and apology shows it was a genuine error and is respectful. “I’m so sorry—I didn’t mean to touch you—my apologies” works well.
IC: What should you do if you are in a consensual relationship with someone you are supervising?
AG: As a head of a school of surgery, I think it would be inappropriate to maintain a romantic as well as supervisory relationship with the same individual. Relationships will happen, but maintaining professional boundaries is crucial. The GMC guidance itself mentions being aware of situations with large differences in power levels between colleagues, or situations where training and career progression opportunities could be impacted.
MN: The Thames Valley Working group is cross-organisational involving NHS trusts, universities and ‘deaneries’ (now Health Education England). We propose any personal relationships should be declared—providing protection for all parties. Addressing
any conflict of interests by removing yourself from educational or clinical supervisory roles linked to personal relationships is advised. It would not be appropriate for a teacher to be in a relationship with a pupil, nor a university professor with a student, nor a surgical trainer with their trainee.
MN: Where can you find relevant resources to learn more on the topic?
AG & IC: We would suggest starting with UK definitions of sexual harassment and sexual assault, GMC GMP guidance and Royal College of Surgeons’ guidance. The WPSMS also provides links to relevant resources. Locally it is important to know what your sexual harassment policies are and what processes are in place.
IC: What can we do to address the issue—both as individuals and leaders?
MN: As individuals, I think we need to appraise ourselves—both men and women. Reading the definitions of sexual harassment and assault was eye opening for me. You may realise that you have experienced sexual harassment or even sexual assault but not acknowledged it to yourself. You may realise that some of the things you have said or done, represent sexual harassment or sexual assault. AG: We need to have challenging and awkward conversations and start educating ourselves on how to handle disclosures of sexual misconduct—and deciding how to act. A good starting point is to think:
At the recent Paris Vascular Insights course (PVI; 12–14 December, Paris, France), Vascular News caught up with some faculty members to get the latest insights on artificial intelligence (AI), peripheral arterial disease (PAD), and intravascular ultrasound (IVUS).
Stéphan Haulon encourages embracement of AI technology

Stéphan Haulon (Hôpital Marie Lannelongue, Paris, France), one of the co-directors of PVI, spoke to Vascular News about AI in vascular surgery now being “a reality” and no longer “a dream” confined to the realms of research alone.
Haulon said: “We’ve seen in the aortic sessions that AI is something that we use in our everyday practice. I follow my patients’ CT [computed tomography] scans with an AI algorithm that tells me if the volume or diameter are decreasing or shrinking. “We’re designing our endografts with AI, with digital twin technology.” In some cases, he noted, AI has shown value in actually predicting some morbidities such as spinal cord ischaemia.
“AI is a game changer. I think that nowadays, if you don’t embrace AI technology, you’re probably not doing a good job. Having said that, AI is here to help you, not to replace you,” Haulon concluded.
“What do I need to do to safeguard the person in front of me?”
“If this was my daughter or son, what would I want to happen for her or him right now?”
We can also choose to challenge behaviours at the bottom of the rape pyramid. That can be anything from just not laughing, staying silent, or vocalising disapproval when comments are not acceptable.
MN: As leaders—if you don’t feel able to advocate, educate or speak up on these issues—facilitate. Get the voices we need to hear a place at the table where meaningful change can happen locally, regionally, nationally and politically. The VSGBI council made this a priority by introducing a code of conduct and putting this topic front and centre stage at the VSGBI annual scientific meeting 2024.
Currently, questions are being asked regarding the lack of institutional safety regarding sexual misconduct at every level. The need is urgent. At every stage of the work being done with my colleagues locally and nationally, we have found we are venturing into unchartered territory, but with that comes opportunity.
References:
1. Begeny C, Arshad H, Cuming T, et al. Sexual harassment, sexual assault and rape by colleagues in the surgical workforce, and how women and men are living different realities: observational study using NHS population-derived weights. British Journal of Surgery 2023;110(11):1518–1526.
2. Alliance, Virginia Sexual and Domestic Violence Action. Virginia Sexual and Domestic Violence Action Alliance [Online]. Available from: http://www. vsdvalliance.org.
3. Doctors.net.uk. Doctor “emboldened” by “banter culture” is suspended for sexual harassment [Online]. Available from: https://www.doctors.net.
Marianne Brodmann reflects on the biggest debates in PAD treatment
Marianne Brodmann (Medical University of Graz, Graz, Austria) shared her opinions on some of the most pressing questions in PAD treatment at present.

On the ongoing competition between sirolimus and paclitaxel, Brodmann remarked that, while paclitaxel is “still the winner above the knee,” limus remains under evaluation. As a result, she stressed that the final conclusion of the debate is “on hold for now”.
Brodmann also weighed in on the discussion surrounding bioresorbable versus non-resorbable scaffolds. After underlining her support for a ‘leave nothing behind’ approach to avoid reinterventions with regard to restenosis, Brodmann shared that she is “very positive” there will be good data for bioresorbable devices in the near future.
Finally, Brodmann considered the treatment of patients with extensive vascular calcification, which she noted is becoming an increasingly prevalent problem. Brodmann commented that there are several new technologies in the works in this space, with Shockwave’s intravascular lithotripsy offering presently representing the “first-line” treatment.
Eric Secemsky drills down on IVUS
In conversation with Vascular News about IVUS, Eric Secemsky (Beth Israel Deaconess Medical Center, Boston, USA), a newly appointed member of the Vascular News North
uk/news/doctor-emboldened-by-banter-culture-issuspended-for-sexual-harassment. 2 August 2023.
4. US Joint Commission. Sentinel Event Statistics Released for 2015 [Online]. Available from: https://info.jcrinc.com/rs/494-MTZ-066/images/ Sentinel39.pdf.
5. Skinner H, Burke J, Young A, et al. Gender representation in leadership roles in UK surgical societies. International Journal of Surgery 2019;67:32–36.
Ian Chetter is chair of surgery at Hull York Medical School, University of Hull (Hull, UK) and current president of the VSGBI.
Andrew Garnham is a consultant vascular surgeon at The Royal Wolverhampton NHS Trust (Wolverhampton, UK), immediate past president of the VSGBI, chair of the Confederation of Postgraduate Schools of Surgery, and head of the School of Surgery in the West Midlands.
Mei Nortley is a consultant vascular surgeon at Oxford University Hospitals NHS Trust (Oxford, UK), deputy director for undergraduate surgical teaching at the University of Oxford (Oxford, UK), co-lead for the Thames Valley Working Group for Sexual Misconduct and member of the Royal College of Surgeons of England (RCSEng) Sexual Misconduct Working Group.
Edited by Anna Pouncey, who is a National Institute for Health and Care Research (NIHR) clinical lecturer, vascular specialist registrar, and member of the Working Group for Sexual Misconduct.
America editorial board, drilled down on the benefits of this technology in the vascular field.
Providing some context, Secemsky noted that IVUS has been available in the USA for several years, with Boston Scientific and Philips representing the two main players. He continued that interest in the technology is now rapidly increasing outside of the USA, highlighting a strong evidence base—including two prospective randomised trials—as one of the main reasons for this.
According to Secemsky, one of the main benefits of IVUS in vascular procedures is that it takes out the guesswork. Previously, he explained, operators were limited in their ability to appropriately size drugcoated devices or determine true lesion lengths due to the incompleteness of angiography alone. IVUS, Secemsky underlined, provides “the ability to make a data-drived decision”.

AI is a game changer. I think that nowadays, if you don’t embrace AI technology, you’re probably not doing a good job. Having said that, AI is here to help you, not to replace you.”
Stéphan Haulon
A rupture prediction-based algorithm is set to enhance patient selection for abdominal aortic aneurysm (AAA) repair. This is according to Randy Moore (University of Calgary, Calgary, Canada), who, at the 2024 VEITHsymposium (19–23 November, New York, USA), reported that the ViTAA system (ViTAA Medical) can predict rupture risk independent of aneurysm size and post-endovascular aneurysm repair (EVAR) neck behaviour.
BEFORE SHARING THE RESULTS OF A retrospective analysis of computed tomography (CT) factors determining AAA wall weakness and strength in AAAs, Moore—who is chief medical officer for ViTAA Medical—began his presentation by outlining an unsuccessful case that highlighted for him an unmet need in vascular surgery. Showing a CT scan, the presenter recalled: “Fifteen years ago, this patient died on my operating table. He wasn’t supposed to die; his aneurysm was small, and I was following guidelines.”
Moore noted that cases such as this are not unique. “In fact,” he said, “a number of large database studies with thousands of patients have demonstrated that up to 12% of patients may have a rupture below size threshold.” Despite this, Moore highlighted that there are investigators who are suggesting the size thresholds for repair can safely be increased due to a low overall risk of rupture.
Moore’s explanation for this “confusion” is the “absolute reliance on AAA size to risk stratify”. In his VEITHsymposium presentation, it was Moore’s aim to suggest to the audience that this firm trust in aneurysm size “remains fundamentally flawed”. “We really need to analyse the aortic wall,” he said. “That’s the key.”
Other investigators have tried to analyse the aortic wall using traditional finite element analyses and stress-based indices, Moore shared, before noting that several publications in the engineering space have underscored the deficits of such strategies. “You’re not taking into account the actual intrinsic patient wall characteristics,” the presenter stated. “In other words, the same stress or force applied to two aortas is going to give you two different clinical outcomes based on the tissue characteristics.”
“We took a different tack,” Moore told the VEITHsymposium audience. Over the past decade, the presenter recounted that he and a team of engineers have processed over 200 aortic specimens. The aim has been “to develop technology that precisely takes into account the integral strength of the aortic wall,” Moore shared.
The presenter explained that, based on a cardiac-
gated scan that allows a clinician to track the motion of the aortic wall, data are uploaded to the cloud where a combination of algorithms assess the intraluminal thrombus thickness, time-averaged wall shear stress with computational fluid dynamics and a peak strain module, all of which are then combined as an output called a regional aortic weakness (RAW) map.
“These maps allow you for the first time to virtually assess the mechanical properties of the aortic wall, not based on population data but based on the individual patient sitting in front of you in the clinic,” Moore elaborated. “We now have an AAA wall analysis that includes wall tissue strength characteristics irrespective of size.”
With a limited number of 38 ruptured patients, we showed a sensitivity of 100% in terms of rupture prediction.”
On use of the technology so far, Moore reported that it has played a role in analysing landing zones for EVAR and has demonstrated that implanting a stent into a weak infrarenal aortic neck is more likely to result in a type 1 endoleak. “That’s not a surprise,” Moore commented, “but now we can actually measure that.”
The technology can also be used for surveillance, Moore continued. He explained that this is due to its ability to analyse peak strain post EVAR and to point out “which endoleaks actually need to be treated, and which can be safely ignored”.
Furthermore, Moore shared that the technology is being used with artificial intelligence (AI) algorithms to predict rapid growth, which he describes as a “surrogate marker for negative outcomes”. These data were shared at the 2023 VEITHsymposium.
During the Sol Cohen prize session at last year’s Vascular Society of Great Britain and Ireland (VSGBI) annual scientific meeting (VSASM; 27–29 November, Brighton, UK), Robert Leatherby (St George’s Vascular Institute, London, UK) shared a presentation on the natural history of splenic artery aneurysms.
BASED ON A DECADE’S experience at St George’s Vascular Institute, Leatherby summarised that splenic artery aneurysms grow slowly
and are prevalent in a female, elderly and comorbid population. The presenter added that heavily calcified aneurysms have particularly slow growth rates.
The “holy grail,” however, is rupture prediction. Moore detailed that he and his team previously published on the ability to identify the site of aortic rupture, but that the more recent aim had been to complete a study looking at the risk of rupture and the ability to predict rupture using the ViTAA technology. “This was a very difficult study to complete,” the presenter remarked, citing specifically the challenge of finding patients with images prior to rupture.
Moore detailed that he and colleagues retrospectively reviewed 38 patients with rupture and matched them in terms of size, age and demographics to 38 non-ruptured patients before conducting a VITAA analysis. “We wanted to make sure we included patients who had a range of aneurysm sizes to reflect real-world practice—roughly 4.6cm all the way up to 12cm,” he said.
The team then completed a traditional analysis using finite element and stress-based indices, finding no statistical difference between ruptured and nonruptured patients. With the ViTAA analysis, however, Moore reported that the team was able to identify a “highly predictive” tool and one that “provides the clinician with an at-a-glance interpretation of aortic wall risk with an accuracy of 92%, a sensitivity for rupture prediction of 100%, specificity of 83% and an area under the curve of 86%”.
“Our AAA wall analysis based on tissue strength and strain allows for patient-specific wall maps that will eventually allow us to more precisely inform patient care,” Moore concluded. “We have a rupture prediction algorithm that will enhance patient selection for repair so that patients like mine 15 years ago will no longer die unnecessarily.”
In Moore’s closing statement, he outlined the longer-term goal of “no longer seeing exclusive reliance on aortic size to determine patient care”.
Data collection “absolutely crucial”
Rao Vallabhaneni (University of Liverpool, Liverpool, UK) commented from the floor on the potential limitations of the 38-patient sample size of the study.
“The difficulty is the great heterogeneity within one person’s aneurysm from different areas of the same aneurysm and between patients,” Vallabhaneni began. “It is such highly heterogenous tissue that I don’t think your sampling is enough to develop a reliable tool.”
While acknowledging Vallabhaneni’s concern, Moore stressed that “the proof is in the pudding”. “With a limited number of 38 ruptured patients, we showed a sensitivity of 100% in terms of rupture prediction,” he reiterated.
However, Moore closed his reply by agreeing with Vallabhaneni that data accumulation is “absolutely critical,” going on to share that he and his team have initiated a series of prospective registries they hope will answer further questions about the technology.
As a result of these findings, Leatherby concluded that it is likely safe to reduce the surveillance for small aneurysms. However, he stressed that this would need to be confirmed in a larger, ideally multicentre, study.
“This study is the first step towards providing a robust and comprehensive surveillance schedule for splenic artery aneurysms,” Leatherby told Vascular News. “Clarity in this area should reduce the burden of scans for patients with small aneurysms and decrease the uncertainty surgeons currently face in managing this condition. A retrospective multicentre study is planned for this year to build upon this research, with an aim to influence future guidelines.”

Recently released six-month results from the single-arm arteriovenous graft (AVG) cohort in the WAVE (Wrapsody arteriovenous access efficacy) trial showed target lesion and access circuit primary patency rates of nearly 82% and 68.8%, respectively.
THE LATEST DATA ON THE Wrapsody cell-permeable endoprosthesis (Merit Medical) for treatment of stenosis or occlusion within the dialysis access outflow circuit were revealed during the final day of the 2024 VEITHsymposium (19–23 November, New York, USA) by co-principal investigator Mahmood K Razavi (St Joseph Heart and Vascular Center, Orange, USA).
“In light of the historically low patency rates for AVG patients, the positive results from the AV graft arm of the WAVE trial are very encouraging
for physicians who manage these patients,” he said.
The WAVE trial was designed to evaluate the efficacy and safety of the Wrapsody endovascular stent graft across two cohorts: up to 244 AV fistula patients randomised 1:1 to either the Wrapsody device or percutaneous transluminal angioplasty, and the AVG cohort. The graft arm enrolled 112 patients across 43 international sites.
Primary efficacy and safety endpoints were assessed by comparing actual rates for the device to performance goals for covered stents (efficacy: 60%;
safety: 89%).
Efficacy of the Wrapsody device was 81.4%—21.4 percentage points higher than the performance goal (p<0.0001).
The proportion of graft patients who were free from an adverse event was higher than the safety performance (95.4% vs. 89%, p=0.0162). “As you well know,” Razavi told VEITH 2024, “these types of numbers are somewhat unusual in this patient population, having this kind of patency.”

between 50–71% at six months.
“The patency results from the WAVE trial are the highest that I have seen to date and are expected to meaningfully improve patients’ quality of life and vascular access survival,” said WAVE trial investigator Leonardo Harduin (Liv Care Centro Clínico, Rio de Janeiro, Brazil). “These results will probably have a positive impact on costs related to the care of these patients.”
The historical controls were based on three prior stent graft studies, he explained, with patency ranging
The patency results from the WAVE trial are the highest that I have seen to date.”
As a part of the ‘Novel technologies in haemodialysis access’ session on the final day of the 2024 VEITHsymposium (19–23 November, New York, USA), Ellen Dillavou (WakeMed Heart Center, Raleigh, USA), primary investigator (PI) for the VenoStent trial, presented new data on the SelfWrap (VenoStent) bioabsorbable perivascular wrap, outlining how the novel device can, in her words, improve arteriovenous fistula (AVF) maturation and patency.
BEGINNING HER PRESENTATION,
Dillavou outlined the problem that the device aimed to solve; according to data published in the Journal of the American Society of Nephrology (JASN) and in the American Journal of Kidney Disease (AJKD), annually, five million patient lives are put at risk by 60% one-year failure rates of AVFs and arteriovenous grafts (AVGs), as well as 20% oneyear failure rates of vein grafts in bypass grafting. She also highlighted that these one-year failure rates were resulting in US$3 billion in direct costs to the Centers for Medicare and Medicaid Services (CMS).
One solution for these issues, Dillavou argued, is the SelfWrap device. Receiving its US Food and Drug Administration (FDA) breakthrough device designation in May 2022, followed by investigational device exemption (IDE) approval in May 2023, this vascular wrap made of bioabsorbable polymers uses an artery-like mechanical support to help veins first “behave” and then “become” like an artery. It also regulates flow—which Dillavou stated will “impart haemodynamic benefits” such as reduced turbulence—and promotes both the outward migration of vascular smooth muscle cells and downstream expansion.
Another benefit of SelfWrap, according to Dillavou, is that it is able to circumvent the primary outcome contributing to a failure rate of over 50% in vascular surgery: neointimal hyperplasia (NH).
“In every large animal model which tested AVFs, AVGs, and bypass grafts over five years and three different centres, the advanced materials approach
that ‘arterialises’ veins significantly reduces NH,” said Dillavou.
The presentation then proceeded to examine the design of the study. The trial’s objective was to demonstrate feasibility and evaluate the safety and performance of the SelfWrap device by enrolling 20 participants in a single-centre, prospective, single-arm study, with follow-up obtained at six months up to 60 months. The primary endpoint was a high patency rate.
For the 20 patients who were enrolled, three SelfWrap sizes were employed, with selection based on vein size. A total of 13 patents (65%) were given brachiocephalic fistulas (BCFs), two received basilic vein transpositions (BVTs), and five were given radiocephalic fistulas (RCFs).
The device is currently being investigated in the USA under a US Food and Drug Administration (FDA) investigational device exemption (IDE).

haemodialysis, and had smaller than average vessel sizes.”
Moving on to the results of the study, Dillavou reported that there were no adverse events probably or definitely related to the device through 36 months, as well as no device deficiencies nor adverse device effects.
Looking at the primary endpoint of the study, the VEITHsymposium audience was told that the SelfWrap device had a high maturation rate; functional maturation (defined as two-needle cannulation for more than 75% of dialysis sessions within four consecutive weeks) was 95%, compared to the <58% expected maturation rate, and unassisted maturation (defined as two-needle cannulation without requiring an intervention [excluding superficialisation and one collateral ligation] to do so) was 90% for SelfWrap, compared to 33% for the benchmark.

The device also had a high catheter removal rate; SelfWrap saw 89.5% of catheters removed at nine months, 94% of which were unassisted, compared to ~27% at nine months for untreated AVFs in a Clarke et al publication.
Five million patient lives are put at risk by 60% one-year failure rates of AVFs and AVGs
One aspect of the study that Dillavou highlighted in particular was that, based on the Lok Criteria—a method for predicting AVF maturation failure based on race, elderly status, peripheral arterial disease (PAD), and carotid artery disease (CAD) status—20 patients were high risk in the trial population, with the criteria predicting that >42% of subjects in the study would fail to mature.
“This doesn’t take into account the presence of more female than male patients”, Dillavou noted, “all of whom had ESRD [end-stage renal disease], were on
The study also saw a low rate of interventions for patients receiving the SelfWrap device. According to Dillavou, there have been four total interventions to date, two of which were on premature AVFs: three were percutaneous transluminal angioplasty (PTA) for juxta/anastomotic stenosis, and one was surgical revision and stent placement for upper-arm cephalic vein stenosis. Through 36-month follow up, the rate of interventions per patient year (PPY) was 0.12, compared to 1.9 interventions PPY for standard of care.
Bringing her presentation to a close, Dillavou noted that, should these results be translated to the ongoing IDE study, “these results would be very impactful for chronic kidney disease/ESRD patients requiring haemodialysis”.
A new Vascular Quality Initiative (VQI) data analysis, recently published in the European Journal of Vascular and Endovascular Surgery (EJVES), has found no statistically significant differences between three carotid endarterectomy (CEA) shunting strategies regarding in-hospital stroke and death rate, including in patients with contralateral carotid occlusion or recent stroke.
IN THEIR EDITOR’S CHOICE PAPER, Xavier Hommery-Boucher (Centre Hospitalier de l’Université de Montréal [CHUM], Montreal, Canada) and colleagues outline that the study aimed to evaluate in-hospital outcomes after CEA according to shunt usage, particularly in high-risk groups of patients such as those with contralateral carotid occlusion or recent stroke.
Hommery-Boucher et al set out to perform a registry-based analysis. Specifically, they analysed data from CEAs registered in the VQI database between 2012 and 2020, excluding surgeons with fewer than 10 CEAs registered in the database, concomitant procedures, reinterventions, and incomplete data.
The authors note that participating surgeons were divided into three groups based on their rate of shunt use: non-shunters (<5%); selective shunters (5–95%), and routine shunters (>95%). They analysed primary outcomes of in-hospital stroke, death, and stroke and death rate in both symptomatic and asymptomatic
patients.
Hommery-Boucher and colleagues share that, in total, 113,202 patients met the study criteria. Of this total, 31,147 were asymptomatic, while a majority of 82,055 were asymptomatic.
Writing in EJVES, the authors report that 12.1% of the 1,645 surgeons included in the study were non-shunters, while 63.6% were selective and the remaining 24.3% were routine shunters. The number of procedures in each group was 10,557, 71,160, and 31,579, respectively.
Hommery-Boucher et al reveal that, in the symptomatic cohort, in-hospital stroke, death, and the combined stroke and death rate were not statistically different among the three groups, based on univariable analysis. Similarly, they note that the asymptomatic group also did not show a statistically significant difference for any of the three primary outcome measures.

Laura Capoccia (Frosinone, Italy) writes about vascular surgeons’ role in acute stroke management and the importance of collaboration with other specialties in this setting.
WHILE STROKE TREATMENT has dramatically changed in recent years, still around 795,000 people experience a new or recurrent stroke each year, and of all strokes 87% are ischaemic.1 A high variability in accessing stroke treatments exists across different countries or different regions within the same nation, even if at present guidelines are quite definitive in indicating endovenous thrombolysis and mechanical thrombectomy (MT) in selected cases as key treatment points. However, treatment indications are continuously evolving and so the vascular surgery world should be prepared to evolve accordingly. Looking back, the dawn of stroke treatment started with carotid
revascularisation and, since historia magistra vitae (lessons for the future come from the past; Marcus Tullius Cicero, De Oratore), carotid treatment will continue to play a significant role in ischaemic stroke. Indeed, guidelines dating back to the end of the 1990s indicated carotid endarterectomy (CEA) as a procedure to consider in carotidrelated stroke patients, and some studies have demonstrated the possibility of CEA not only preventing recurrence of embolic neurological events (secondary prevention aim),2 but also of treating brain blood flow impairment when performed in acute patients (therapeutic aim).
It is also to be acknowledged that
The authors go on to state that a multivariable model did not show a statistically significant difference for the primary outcomes between the three shunting cohorts and that, on subgroup analysis, the stroke and death rates were not statistically significantly different for patients with contralateral carotid occlusion and those presenting with a recent stroke.
“This paper adds new data collected from a large registry regarding postoperative outcomes related to shunt use during [CEA],” Hommery-Boucher and colleagues write in EJVES, remarking that the design of the study enabled comparison between three shunting strategies.
The authors summarise that the results of their study “demonstrated that there was a two-fold increase in the percentage of surgeons using the nonshunting strategy between 2012 and 2020, with no significant difference in outcomes compared with the other two strategies”.
In the discussion of their findings, Hommery-Boucher et al recognise some limitations of their research, including those “intrinsic” to the use of a large database like the VQI.
There was a two-fold increase in the percentage of surgeons using the non-shunting strategy between 2012 and 2020
tandem or concurrent intracranial and extracranial (carotid) lesions are responsible for 10–20% of all large vessel occlusion strokes so that the issue of treating them simultaneously or not is at stake. Even if major societies’ guidelines still suggest that urgent symptomatic internal carotid artery (ICA) stenosis should be treated by CEA rather than by carotid artery stenting (CAS),3,4 the European Society for Vascular Surgery (ESVS) and the Society for Vascular Surgery (SVS) still concede that urgent CAS could be performed under certain conditions and the American Heart Association (AHA)/ American Stroke Association (ASA) 2021 guidelines indicate treatment of tandem occlusions when performing MT as reasonable.5
Nowadays, following the publication of new trials’ results on wider time and lesion indications for MT, it is to be expected that the number of endovascular brain procedures will increase over time, and endovascular treatment of carotid lesions by CAS during MT in tandem lesions will increase accordingly, also taking into account the continuous evolution of techniques (different access routes and therapies) and devices (flow-reversal cerebral protection adjuncts, new meshcovered stents).
So, tandem lesions in stroke patients represents the possible meeting point between different specialties (neurology, neuroradiology, and vascular surgery) that should always work together to offer the best solution for patients,
The researchers conclude that, despite its limitations, their study “could not define a preferential shunting strategy,” leading them to advise that the strategy “should be mainly based on a surgeon’s preference and skillset”. They go on to stress that their study has “provided quality data on the impact of a surgeon’s shunting pattern on postoperative stroke and death rate, particularly for the most at-risk groups”.
and, mimicking what happened at the beginning of the endovascular era when vascular surgeons started a close collaboration with interventionalists to acquire endovascular competencies, it could be time to start to involve vascular surgeons in endovascular brain procedures for the benefit of stroke patients. Acquiring specific competencies, certifications, and credentialling in devices and techniques for brain vessel treatment could be the next frontier and challenge for vascular surgery, thus bringing to patients’ care a new experience in managing complications after carotid treatment or in offering a direct carotid access route to MT.
References
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023 Feb 21;147(8):e93–e621.
2. Capoccia L, Sbarigia E, Speziale F, et al. Urgent carotid endarterectomy to prevent recurrence and improve neurologic outcome in mild-to-moderate acute neurologic events. J Vasc Surg 2011 Mar;53(3):622–7; discussion 627–8.
3. Naylor R, Rantner B, Ancetti S, et al. Editor’s choice—European Society for Vascular Surgery (ESVS) 2023 clinical practice guidelines on the management of atherosclerotic carotid and vertebral artery disease. Eur J Vasc Endovasc Surg 2023; 65: 7–111.
4. AbuRahma AF, Avgerinos ED, Chang RW, et al Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J Vasc Surg 2022; 75: 4S–22S.
5. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke 2021; 52: e364–e467.
Laura Capoccia is a vascular surgeon at “F. Spaziani” Hospital in Frosinone, Italy.
A team of researchers in Canada have developed machine learning (ML) models that they claim, “can accurately predict one-year IVC [inferior vena cava] filter complications, performing better than logistic regression”.
WRITING IN THE JOURNAL
of Vascular Surgery: Venous and Lymphatic Disorders (JVS-VL), Ben Li (University of Toronto, Toronto, Canada) and colleagues highlight that IVC filter placement is associated with long-term complications and posit that predictive models for filterrelated issues “may help guide clinical decision-making”. The authors state that it was their objective to develop ML algorithms that predict oneyear IVC filter complications using preoperative data.
Li et al write that they used the Vascular Quality Initiative (VQI) database to identify patients who underwent IVC filter placement
between 2013 and 2024. They identified 77 preoperative demographic and clinical features from the index hospitalisation when the filter was placed.
The authors note that the primary outcome of the study was one-year filter-related complications, specifically a composite of filter thrombosis, migration, angulation, fracture, and embolization or fragmentation, vein perforation, new caval or iliac vein thrombosis, new pulmonary embolism, access site thrombosis, or failed retrieval.
Going into more detail regarding their study methods, Li and colleagues share that the data were divided into
At the recent VEITHsymposium (19–23 November, New York, USA), Marc Passman (University of Alabama at Birmingham, Birmingham, USA) reflected on the achievements of the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) venous arm, in collaboration with the American Venous Forum (AVF), since its inception 10 years ago.
PASSMAN, WHO IS CHAIR OF THE VQI Venous Quality Council (VQC), shared that a total of 1,302,849 procedures have been captured by the VQI as a whole as of 1 November 2024.
Regarding venous interventions, Passman specified that there are currently three venous modules within the VQI, with varicose vein, inferior vena cava (IVC) filter, and venous stent procedures comprising 70,097, 19,793 and 305 of the November 2024 total, respectively.
Passman first highlighted data from the VQI IVC filter registry. Initiated in 2014, the presenter shared that 50 centres were subscribed to the registry as of November 2024, which is the latest participation datapoint available from the registry. He noted that this has decreased by 16 from a peak in July 2017 of 66 centres subscribed.
Participation in the varicose vein registry shows a similar trend, Passman highlighted. Following its inception in 2015, the number of centres subscribed peaked at 45 in May 2021 ahead of falling by 13 to 32 centres as per the latest figures from November 2024.
The venous stent registry was the most recent of the three venous modules to be established. Passman reported that the number of centres subscribed has
training (70%) and test (30%) sets, and that six ML models were trained using preoperative features with 10-fold cross-validation. These were Extreme Gradient Boosting, random forest, Naïve Bayes classifier, support vector machine, artificial neural network, and logistic regression. The primary model evaluation metric was area under the receiver operating characteristic curve (AUROC).
The researchers also assessed model robustness, detailing that they used calibration plot and Brier score to do so, and evaluated performance across subgroups based on age, sex, race, ethnicity, rurality, median Area Deprivation Index, planned duration of filter, landing site of filter, and presence of prior IVC filter placement.
Li et al report in JVS-VL that 14,476 patients underwent IVC filter placement over the course of the 11-year study period, of whom 584 (4%) experienced one-year filter-related complications.
“Patients with a primary outcome were younger and more likely to have thrombotic risk factors including thrombophilia, prior venous thromboembolism (VTE), and family history of VTE,” the authors detail.
been on an upwards trajectory since data capture began in 2020 and currently stands at 10. Providing an update on this newest of the venous VQI registries, Passman noted that there has been a recent revision of the original data fields. Specifically, he shared that less-needed registry variables have been removed and highlighted efforts to decrease registry data entry burden. Passman also detailed that the development of registry reporting measures is now underway, as well as a drive to increase site recruitment.
Moving on to consider how the three venous VQI registries can be used to encourage best practice, Passman shared that a VQI best practices dashboard has been introduced across the board to summarise each individual center’s results, and provide comparison to national VQI benchmarks.
The next topic on the agenda was research, with Passman stating that a venous VQI research advisory council (RAC), chaired by Nicholas Osborne (University of Michigan, Ann Arbor, USA) was set up in 2020. The presenter noted that submission of venous RAC proposals is increasing, and encouraged centres not enrolled in a venous VQI module but are interested in research projects to connect with a venous VQI registry partner. Passman highlighted several publications to have emerged from the venous VQI registries over the past 10 years, including on the impact of COVID-19 on the varicose vein and
The next 10 years offers improvement in data accrual and analytical power thereby expanding the value of the venous VQI quality mission.”
Furthermore, Li and colleagues reveal that the best prediction model was Extreme Gradient Boosting, achieving an AUROC of 0.93. Comparatively, they continue, logistic regression had an AUROC of 0.63. The authors state that calibration plot showed “good agreement” between predicted and observed event probabilities with a Brier score of 0.07.
In addition, Li et al report that that top 10 predictors of one-year filter-related complications were, in order, thrombophilia, prior VTE, antiphospholipid antibodies, factor V Leiden mutation, family history of VTE, planned duration of IVC filter (temporary), unable to maintain therapeutic anticoagulation, malignancy, recent or active bleeding, and age. “Model performance remained robust across all subgroups,” the researchers share.
In their conclusion, Li and colleagues posit that the ML algorithms assessed in the present study “have potential to guide patient selection for filter placement, counselling, perioperative management, and follow-up to mitigate filter-related complications and improve outcomes”.

IVC filter registries and on the use of telemedicine for the management of patients with varicose veins, among various other topics.
In his conclusion, Passman summarised what has been achieved over the past 10 years. He highlighted progress in several regards, notably the improvement of long-term outcomes through standardisation, the availability of evidence-based outcomes analysis, and the use of data to define best clinical practices. In addition, Passman underscored the introduction of site and national benchmarks that have the potential to monitor safety and efficacy, as well as the provision of comparative effectiveness research.
Closing the presentation, Passman gave a glimpse of what the next 10 years of the venous VQI might look like. The presenter alluded to the development of additional venous procedural registries, better integration and coordination between venous modules, the expansion of artificial intelligence (AI) initiatives, electronic medical record (EMR) data extraction and finally cross-module, interactive data analysis for better benchmarking and expanded research opportunities.
Speaking to Vascular News, Passman commented on the significance of the last 10 years of the venous VQI: “After a decade of efforts through multiple participants, administrative resources, clinical sites, and research initiatives, venous VQI modules are now poised to be a valuable source of standardised data for improved quality of venous care, benchmarking, and investigation both at the local sites and nationally. With future expansion plans and technological improvement on the near horizon, the next 10 years offers improvement in data accrual and analytical power thereby expanding the value of the venous VQI quality mission.”
Having initially been geared towards a career in engineering, Vincent Rowe was ultimately drawn to medicine’s dual offering of intellectual stimulation and interpersonal engagement. He overcame a fear of the sight of blood to pursue what has so far been a “deeply rewarding” career in vascular surgery, culminating in his current role as professor of surgery and division chief of vascular and endovascular surgery at the University of California, Los Angeles (UCLA; Los Angeles, USA). Speaking to Vascular News, Rowe reflects on his career to date and shares his thoughts on the state of surgical education, multidisciplinary care, and vascular surgery’s identity.
Why did you choose to pursue a career in medicine and what drew you to vascular surgery?
Medicine has always been an inspiring and fulfilling field, and if I had the chance to start over in college, I would unequivocally choose the same path. My early passion for science initially directed me toward a career in engineering, where I explored the intricacies of problem-solving and innovation. However, I soon realised that while engineering stimulated my intellectual curiosity, it lacked the human connection and interpersonal engagement that I found deeply meaningful. This revelation prompted me to pivot toward medicine, a discipline that seamlessly integrates scientific inquiry with compassionate care.
Before committing to this path, I had to confront a significant obstacle—my fear of the sight of blood. Determined to overcome this challenge, I immersed myself in a trauma centre emergency room, embracing a form of ‘shock therapy’ that ultimately desensitised me to this fear. With the added strength drawn from my faith and reliance on divine support, I was able to transcend this limitation and fully dedicate myself to a career in medicine. This journey not only solidified my resolve, but also deepened my appreciation for the profound impact of this vocation.
Vascular surgery captivated me with its unique combination of specialisation and breadth. I was drawn to the opportunity to work across multiple regions of the body and collaborate with diverse specialists, including nephrologists, neurologists, cardiologists, and podiatrists. Spanning from the neck to the toes, the field offers a dynamic and interdisciplinary approach to patient care.
What continues to fuel my passion for vascular surgery is the constant innovation and technological advancements that keep redefining the specialty. These inspiring developments have made vascular surgery both challenging and deeply rewarding.
Who were your career mentors and what was the best advice that they gave you?
I’ve been asked this question many times, and my answer always comes back to the fact that I had very few mentors early in my career— largely because I was not the best mentee. I’ve worked hard to change that over the years, striving to be the best mentor I can for others now. Upon reflection, I recognise that not reaching out to more people for guidance, early on, was a mistake.
That said, I was fortunate to work closely with Fred Weaver for over two decades. His
guidance played a pivotal role in shaping my development as an academic surgeon, and I am deeply grateful for his unwavering support. One of his many pieces of wisdom that has stayed with me is the simple yet profound reminder that “persistence is a virtue”. I’ve carried those words with me throughout my career and have tried to embody that mindset in all aspects of my work and life.
What have been some of the most important developments in vascular surgery over the course of your career so far?
By far, the most transformative development in vascular surgery has been the advent and evolution of endovascular techniques. I’m old enough to remember a time when endovascular options were entirely absent from our toolkit. Today, they are integral to patient care, seamlessly incorporated into nearly every treatment plan. This paradigm shift has not only expanded our capabilities but also redefined the standard of care for countless conditions.
Another equally impactful advancement has been the growing spirit of collaboration among specialties. The establishment of aortic centres, limb salvage programmes, stroke teams, and similar initiatives reflects our field’s commitment to working together to optimise patient outcomes.
What are the biggest challenges currently facing vascular surgery?
One of the greatest challenges facing our specialty is the issue of identity and branding, which has not yet reached a level that reflects our critical role in patient care. This lack of recognition has, at times, led to vascular surgeons being undervalued, both within the surgical community and by the broader healthcare system. Many complex operations simply wouldn’t happen without the assurance of vascular surgery expertise, whether for direct involvement or for providing essential support. I hope the indispensable role of vascular surgeons in achieving better patient outcomes is fully recognised and appreciated in the future.
What are some of your current research interests?
I have always been deeply interested in preserving limbs through surgical intervention. Early in my career, my focus centred on the technical aspects of limb salvage, including mastering advanced surgical techniques and understanding the intricacies of vascular
CURRENT ACADEMIC APPOINTMENT
December 2022–present: Professor and chief, Division of Vascular and Endovascular Surgery, David Geffen School of Medicine at UCLA
CURRENT HOSPITAL APPOINTMENTS
2023–present: Attending staff, West Los Angeles Veterans Administration Medical Center (Los Angeles, USA)
December 2022–present: Attending staff, UCLA Santa Monica Medical Center (Santa Monica, USA)
December 2022–present: Attending staff, Ronald Reagan UCLA Medical Center (Los Angeles, USA)
EDUCATION
1997–1999: Vascular surgery fellowship, University of Tennessee Medical Center (Knoxville, USA)
1992–1996: General surgery residency, Kaiser Permanente Medical Center (Los Angeles, USA)
1991–1992: General surgery internship, Kaiser Permanente Medical Center
1987–1991: Medical Doctorate, University of Southern California (Los Angeles, USA)
1987: Bachelor of Arts, Biophysics, University of California, Berkeley (Berkeley, USA)
interventions required to save limbs.
Over the past decade, however, my perspective has broadened to include the critical role patient and social factors play in determining outcomes. This includes addressing disparities in access to care, socioeconomic barriers, and the influence of comorbidities on treatment success.
More recently, I have been studying the growing importance of interdisciplinary collaboration, recognising that optimal limb salvage outcomes depend on a team-based approach involving specialists in wound care, infectious disease, endocrinology, and rehabilitation.
What is your experience of working in a multidisciplinary limb salvage team on a day-to-day basis and how has the approach impacted patient care in your centre?
At my previous institution, we developed a strong multidisciplinary team for limb salvage, with daily collaboration among most members of the team. At my current institution, the foundational elements for a highly effective limb salvage programme were already established before my arrival. We are now focused on refining and optimising our integration to enhance the impact and efficiency of the team in delivering the best possible patient outcomes.
You have received numerous teaching awards over the course of your career so far. Could you share some reflection on the state of vascular education and training in 2024? Is there anything that you think needs to change moving forward?
I believe surgical education has never been stronger. For much of my career, teaching was primarily confined to intraoperative instruction or didactic lectures. Today, however, surgical educators have a wealth of tools at their disposal, allowing trainees to engage with simulators, cadavers, and review relevant content or questions via mobile educational platforms.
For surgical training, I believe our integrated training pathway has been revolutionary in enhancing the depth of surgical training. Surgical education is in an excellent place.
You have been active in surgical societies for many years now, including in 2022 as president of the Western Vascular Society. What were your highlights of this presidency?

The presidency of the Western Vascular Society stands as one of the highlights of my career. The camaraderie among members at the meeting and our ‘black and white’-themed banquet, the beautiful venue of Victoria, Canada, and the Empress Hotel of Victoria, and the presence of my wife, close family and friends all contributed to making that meeting truly special for me. I was even able to share some dance moves with past presidents Ben Starnes and Mike Conte.
What do you think is the most important vascular research paper to have been published in 2024?
That’s a hard question. So much effort is put into each publication by the authors, I would hate to place one manuscript over another. I do think the BEST-CLI trial publication, shared in 2022 in the New England Journal of Medicine (NEJM), was one of the most impactful publications I’ve been a part of in my career. Whether the results change the practice patterns of practitioners can be debated but
having the data to help determine the optimal strategy to save limbs is of great importance.
Could you outline one of your most memorable cases?
Last year there was a patient with a previously placed endovascular aneurysm repair (EVAR) who presented with a graft enteric fistula. We took out the graft and reconstructed the patient with homografts. He was lost to follow-up and surprisingly re-presented about six months later with a rupture of the aortic homograft portion of our reconstruction. With balloon control from above, we were able to rereconstruct him.
Thanks to Drs Karen Woo, Megan Brenner, and Jesus Ulloa for their help.
What advice would you give to someone looking to start a career in medicine?
The field of medicine, and surgery in particular, is incredibly rewarding, but it is also exceptionally demanding and requires a significant commitment of your time and
"I believe surgical education has never been stronger.”
energy. It is essential to ensure that medicine is truly the right path for you before embarking on this career.
Despite my own resistance to reaching out to mentors early in my career, I would strongly recommend interacting and connecting with people in the field of medicine early in your careers for guidance.
What are your hobbies and interests outside of medicine?
My original love was always basketball. However, as my schedule became more challenging, I transitioned to cycling. Riding a bike was much easier to do alone or, if my schedule allowed, with a group. Even though my size didn’t make me the ideal cyclist, I still competed and was often just happy to finish with the faster riders. I always heard an important part of wellness was engaging in exercise that would take one to exhaustion. Cycling does that for me. The sport also reinforced the importance of a team, sacrifice, and resilience to me.












First indicated & dedicated platform for bridging stents in FEVAR
Precise & standardised positioning due to a dedicated fenestration marker
2-in-1 design that combines inflation & flaring in one step
In November 2024, vascular surgeon Stéphan Haulon (Hôpital Marie Lannelongue, Paris, France) successfully performed the first-in-human implantation of Bentley’s recently CE-certified BeFlared fenestrated endovascular aneurysm repair (FEVAR) stent graft system. Following this milestone, Haulon spoke to Vascular News about his extensive FEVAR experience, detailing how the BeFlared has been a “gamechanger” in the field.
Haulon has carried out approximately 1,500 FEVARs since first performing the procedure in 2003, following completion of his training at the Cleveland Clinic (Cleveland, USA) with Roy K Greenberg.
Based on his over 20 years of experience, Haulon commented that FEVAR platforms currently show “great” outcomes, as shown in publications evaluating various covered stents as bridging stents. However, he noted that there are certain technical challenges— notably device positioning and flaring—that needed to be addressed. Haulon also highlighted the importance of devices having a “fool-proof” catheter system to avoid going back and forth for flaring, which, in less experienced hands or challenging anatomies, can lead to an “endo-nightmare”.
“Tracking a bridging stent to its target lesion can be a challenge,” Haulon remarked, going into more detail about some issues commonly encountered during FEVAR. “You need to get a sheath all the way from the groin through the fenestration and into the target vessel to protect the tracking of your bridging stent, so you need a bridging stent that can adapt to the anatomy and be flexible enough to get all the way to the target vessel.”
Haulon went on to explain that bridging stents must protrude 3–5mm into the aortic lumen, before flaring to get a perfect seal at the level of the fenestration. “Flaring is a challenging part of the procedure,” he said, “because you need to go back and forth with the sheath into the stent, to exchange balloons.”
It is often at this point during a procedure where difficulties can arise, Haulon continued. “Sometimes you can lose wire access,” he detailed, “or the wire into the target vessel can move and then there’s a risk of dissecting or making a perforation of the target vessel; in addition, it’s sometimes quite difficult to get that flaring balloon stable in the aortic lumen without interfering with the other bridging stents in place.”
visualise, which is positioned at the level of the fenestration, in order to not have too much or too little stent protruding in the aortic lumen,” Haulon continued.
The second feature is the one-step balloon that combines inflation and flaring. “The real challenge was to develop a balloon that would actually have this specific feature without having the stent moving while deploying it,” Haulon said. “And so, what is really unique about [the BeFlared] is when you inflate the balloon, nothing moves—it’s a very stable platform and you have both a perfect seal within the target vessel and a perfect flaring of the stent after a single inflation of the balloon.”
Referring here to his clinical experience with bridging stents, Haulon shared that flaring would often be uneven. Now, postoperative computed tomography (CT) scans of the first patients who have been treated with the device show “perfect” flaring. “The part of the stent that is protruding in the aortic lumen is nicely open, it’s a nice circle so there’s a perfect seal
The BeFlared will make fenestrated endografting much simpler and swifter.”

Haulon moved on to discuss the role of the new BeFlared—which Bentley notes in a recent press release is the world’s first dedicated bridging stent for FEVAR—in addressing some of the aforementioned challenges.
The balloon catheter has two important features, Haulon noted, the first of which is an extra (third) marker for optimal placement. “It’s the first time we have a real dedicated marker to confirm the precise positioning of a bridging stent,” he stated, noting that challenging anatomies can sometimes make this step particularly difficult.
“Now we have a marker that is very easy to
at the level of the fenestration,” he said.
On further benefits of this optimal flaring, Haulon noted: “There are really no concerns about going back through the stent for re-ballooning or implantation of another stent.
It’s actually easier because flaring is achieved spontaneously. Also, later access to the target vessel is very much facilitated.”
Haulon summarised that the BeFlared will make fenestrated endografting “much simpler and swifter”, with the added benefits of reducing endovascular specialists’ exposure to radiation and “resulting in fewer secondary procedures due to better seal at the level of the fenestration”.
Finally, Haulon underlined the “very straightforward” learning curve for this new device, emphasising its potential for widespread use amongst those that are very experienced or less experienced.
“I think that for experts it’s going to make the procedure really fly and avoid those couple of cases where we struggle and have technical issues, and for less experienced colleagues it will enable them to embrace this technology,” he said. “It will be a gamechanger, especially for people who don’t have a high volume of cases.”
Bentley has advised that series production of the BeFlared has started and, after the official market launch at the upcoming Leipzig Interventional Course (LINC 2025; 28–30 January, Leipzig, Germany), it will become commercially available as of February 2025, in line with country-specific market regulations.
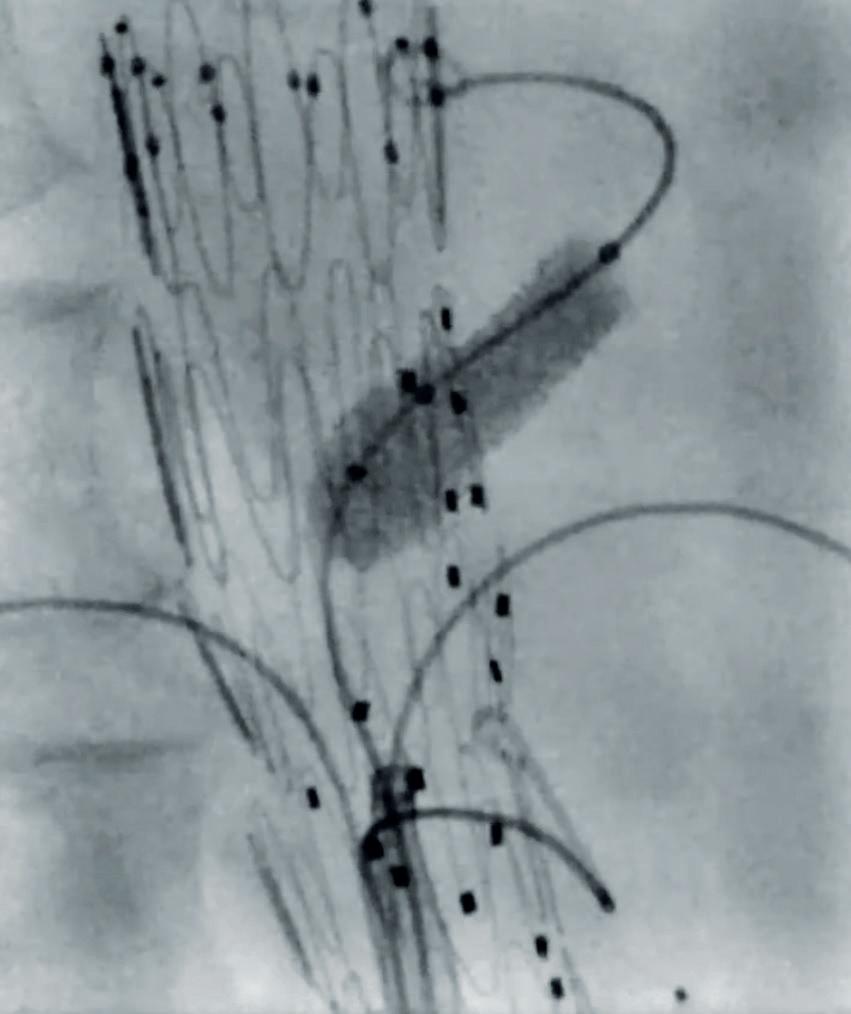


Stéphan Haulon








A wide variety of vessels treated with no device-related adverse events is a key recent finding to emerge from the EMBO postmarket surveillance (EMBO-PMS) registry.
EMBO-PMS is a prospective, multicentre registry of the Impede, Impede-FX, and Impede-FX
RapidFill embolisation plug systems (Shape Memory Medical) when used for peripheral vascular embolisation. The study, which is enrolling up to a combined 125 patients at four centres in the UK and eight in Germany, is evaluating the use of these shape memory polymer-based devices in patients with peripheral vascular disease and their potential to embolise the target area after deployment. Patients are being followed at 90 days and one year.
Robert Morgan (St George’s University Hospitals NHS Foundation Trust, London, UK), co-principal investigator for EMBO-PMS, recently shared one-year outcomes from the trial at the 2024 VEITHsymposium (19–23 November, New York, USA).
During his presentation, Morgan highlighted
Key outcomes of the EMBO-PMS registry are rates of technical success and 30-day serious adverse events (SAEs). Secondary outcomes—through one year—include rates of recurrence of clinical symptoms, rate of treated vessel occlusion, and SAEs.
Regarding demographics, Morgan detailed that, to date, 82 patients have been treated within this study, with 92 vessels embolised. Continuing, Morgan stated that of greatest significance were the variety of vessels treated, which included intercostal arteries, ovarian veins, iliac arteries, and inferior mesenteric arteries. “We see broad utility across multiple applications for standalone use and in combination with other embolic devices,” the presenter added.
Following his presentation of the results to date, Morgan commented that the data illustrate the safety and efficacy of novel shape memory polymer embolic devices for embolisation of peripheral vasculature

and that further experience and longer-term follow-up will demonstrate the utility and durability of the devices.
To date, no device-related SAEs have been reported, and 90-day follow-up has been completed in over half of the 82 patients enrolled with a high rate of sustained occlusion.
Morgan also referenced the initial use of these shape memory polymer devices for peripheral vascular embolisation described by Holden et al, reporting no recurrent clinical symptoms attributable to treated vessel embolisation or recanalisation through up to four-year follow-up.
Shape Memory Medical notes that it continues to study the use of its shape memory polymer devices in other clinical applications outside the scope of their approved indications for use, including the ImpedeFX RapidFill device for active aortic aneurysm sac management in the AAA-SHAPE randomised controlled pivotal trial.
Disclaimers:
Robert Morgan is co-principal investigator of the EMBO-PMS study. For more information about the EMBO-PMS study, please visit https:// clinicaltrials.gov/study/NCT04044443?term=EMBOPMS&rank=1
Figure 1 caption: Shape memory polymer has a porous structure when expanded in a vessel, allowing thrombus formation throughout its structure. (a) Porous, bioabsorbable polyurethane foam; (b) open scaffold enables stable clot formation; (c) Impede-FX embolisation plug; (d) cellular growth with tissue infiltration with no chronic inflammation* *Preclinical data: ACS Biomater Sci Eng 2020 6 2588–2599.
the imaging clarity offered by the Impede devices, emphasising the minimal imaging artifact postimplantation compared to embolisation with coils, which can produce significant artifact on computed tomography (CT) imaging.
Morgan continued that the radiolucent shape memory polymer incorporates a porous polyurethane embolic scaffold that promotes rapid thrombosis followed by collagen ingrowth as shown in preclinical studies.
Elaborating on how the shape memory polymer devices are deployed during a procedure, Morgan explained: “The plugs are crimped for catheter delivery to the target vessel and self expand once exposed to blood for embolisation.”
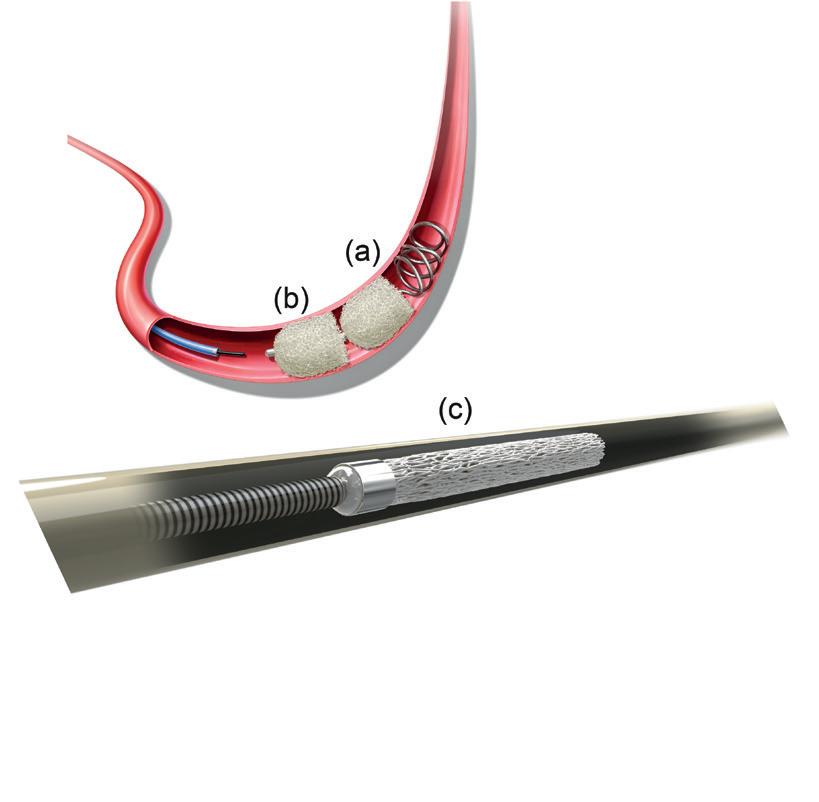
Figure 2 caption: (a) The expanded form of the Impede embolisation plug and (b) Impede-FX embolisation plug with shape memory polymer and a proximal radiopaque marker. (c) Shape memory polymer is a porous polymeric material (polyurethane) that transitions between two ‘memorised’ shapes in different environments (temperature and aqueous)—crimped for catheter delivery and self expands upon contact with blood.
Not all devices discussed in this interview are available in all regions. The content contains information about the AAA-SHAPE pivotal trial, Shape Memory Medical’s prospective, multicentre, randomised, open-label trial of the Impede-FX RapidFill when used for prophylactic abdominal aortic aneurysm (AAA) sac filling during elective endovascular aneurysm repair (EVAR). For more information about the AAA-SHAPE study, please visit https://clinicaltrials.gov/study/NCT06029660 In countries recognising CE marking, the Impede embolisation plug, the Impede-FX embolisation plug, and Impede-FX RapidFill are indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. In the USA, the Impede embolisation plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature and the Impede-FX embolisation plug is indicated for use with the Impede embolisation plug to obstruct or reduce the rate of blood flow in the peripheral vasculature.
The Impede-FX RapidFill device is not approved for sale in the USA or Japan. Data on file at Shape Memory Medical.
The late Professor Roger Greenhalgh, the internationally renowned vascular surgeon who founded the Charing Cross (CX) International Symposium, has been recognised for his commitment to driving forward global education, research and innovation in peripheral arterial disease (PAD) by a worldwide patient-facing support organisation.
GREENHALGH WAS POSTHUMOUSLY conferred with the 2024 Global Legacy Impact Award from the Global PAD Association, also known as The Way to My Heart, the world’s largest PAD patient group, which was accepted on his behalf by his son, Stephen, who continues to operate as managing director of the company behind CX, BIBA Medical.
Presenting the award, the group’s founder Kym McNicholas, said: “This year we celebrate the extraordinary achievements of Professor Roger Greenhalgh, whose pioneering work has left an indelible mark on the field [of PAD care]. His legacy includes significant contributions to PAD treatment through innovative research, the development of new techniques and influential educational initiatives, especially through the most prestigious PAD conference in the world, Charing Cross, held every year in England.
“Professor Greenhalgh’s commitment to improving
patient outcomes and advocating for PAD awareness has been recognised globally, influencing practices and education across regions.”

Roger Greenhalgh
Asked to speak about his father’s legacy, Stephen highlighted Professor Greenhalgh’s relentless commitment to patient-centred care. “He really cared about the patient; he wanted the patient to get better and everything that he did, whether it was education, research or particularly the management of his patients, was to do the right thing,” he commented.
Stephen also reflected on Professor Greenhalgh’s open mind to emerging technologies and techniques and how he placed innovation at the heart of the CX Symposium, and how he stressed the importance of level-one evidence to demonstrate that they worked.
“He led about a dozen randomised controlled trials, and that’s evidence,” he said.
“It’s no good having stuff that’s new if it doesn’t work, so my father was relentless in wanting to show things worked and that was his passion—someone who wanted studies that showed or didn’t show that this was something that would be the best way of treating the patient.
“He not only conducted those studies, but he created the platform by which the evidence that he helped create and others created, that would define future care of PAD, through Charing Cross.”
He really cared about the patient.”
Stephen Greenhalgh
A new photographic initiative reveals the scale of preventable amputations in the UK and the impact on both patients and the UK National Health Service (NHS).
AMPUNATION, A PROJECT LED by Abbott, launches with a series of confronting portraits of real people living with amputation. Shot by iconic photographer Rankin, the images accompany a health economics report published in the British Journal of Surgery and aims to help people see and acknowledge the problem behind the statistics.
The Abbott-funded BJS report, written by Athanasios Saratzis (University of Leicester, Leicester, UK) and colleagues, found that the NHS could save more than £8 million a year if the rate of major lower-limb amputations among chronic limb-threatening ischaemia (CLTI) index procedures is reduced from 10% to 3% across England and Wales.
An Abbott press release notes that the AmpuNATION initiative calls for increased awareness of PAD and the threat of amputation in key at-risk groups, faster diagnosis and referrals, consistent standards of care and a clear pathway for people living with PAD.
“Our clinical and health economics assessment shows that the NHS could save millions of pounds a year, and improve outcomes for patients, by increasing revascularisation rates and reducing the number of preventable amputations in people with PAD. As a treating clinician, I see the devastating impact that amputation has on people. They don’t just lose a limb, they lose their confidence, their freedom, autonomy, and many lose the motivation to keep fighting their condition. We must increase awareness, provide timely diagnosis and treatment, and embrace the innovations that can reduce the number of amputations in the NHS,” Saratzis commented in the press release.
“Every preventable amputation is a tragedy, not just for the patient but the loved ones around them. At Abbott, we are proud to support the AmpuNATION initiative to raise awareness of the signs of PAD to reduce the number of amputations,” Jonathan Wood, regional director, Abbott Vascular, North Europe, remarked.
THE WORLD FEDERATION OF Vascular Societies (WFVS) staged its inaugural Roger M Greenhalgh Lecture in honour of his work as a leading pioneer in the field of vascular surgery.
The memorial lecture took place during the 31st annual meeting of the Vascular Society of India (17–19 October, Jaipur, India) and was presented by Alan Lumsden (Houston Methodist DeBakey Heart and Vascular Center, Houston, USA).
Fittingly, the talk focused on another frontierpushing topic: robotic vascular surgery and artificial intelligence.
Like Greenhalgh, Lumsden is British and known for pushing the envelope in the field of vascular surgery. His team at Houston Methodist DeBakey Heart and Vascular Center is at the vanguard of efforts to bring robotics into the vascular surgical space.
In the lecture, entitled ‘Robotics and AI: Next revolution in vascular surgery’, the chair of cardiovascular surgery outlined a roadmap for bringing robotic vascular surgery into the fold of clinical practice.
Lumsden referenced his moment of clarity around the concept, how he realised major vascular operations were being handled robotically by other specialties, and how vascular surgery must not miss the boat.

We must increase awareness, provide timely diagnosis and treatment, and embrace the innovations that can reduce the number of amputations in the NHS.”
On 3 October 2024, a significant milestone was achieved as Terumo Aortic announced the first implant in the world using the TREO FIT system. The procedure was performed by Benjamin W Starnes (Harborview Medical Center and the University of Washington, Seattle, USA). In the USA, TREO FIT is currently only available in the physiciansponsored investigational device exemption (PSIDE) study under Starnes’ guidance.
TREO FIT IS USED TO FACILITATE physician-modified endovascular grafts (PMEGs). It guides surgeons in locating and marking fenestrations on the partially deployed TREO abdominal stent graft system to align precisely with the patient’s anatomy, reducing the need for manual measurements of fenestration locations. TREO FIT is indicated solely for use with the TREO abdominal stent graft system.
The TREO FIT system in combination with the TREO abdominal stent graft system is used in complex abdominal aortic aneurysms (AAAs), namely juxtarenal aortic aneurysms presenting with a high risk (e.g. large aneurysm size), symptomatic aneurysms and/or ruptured aneurysms. According to Terumo Aortic, this device provides an innovative solution for patients who require urgent complex AAA treatment and are unable to wait for a companymanufactured device before having surgery.
Starnes, who is professor of vascular surgery at Harborview Medical Center and the University of Washington and performed the first implant of TREO FIT, commented: “As recommended through our PSIDE, I used TREO FIT for these patients due to their unique, complex anatomy and urgency. This device, currently available in our IDE, significantly simplifies the steps involved in identifying the location of fenestrations. The features of the TREO abdominal stent graft system, along with TREO FIT, optimise the performance due to the deliverability, conformability and graft design, which is suitable for creating fenestrations. The TREO stent graft has ample fabric available, ensuring that the renal fenestrations were always strut free and could be easily located on the endograft.”
A further milestone was achieved on 23 October 2024 when the first implant in Europe using the TREO FIT device took place in Bordeaux, France. The procedure was performed by Éric Ducasse (CHU Bordeaux Hospital, Bordeaux, France). In Europe,


TREO FIT is currently only available through the custom-made programme.
Following the implant, Ducasse, who is professor of vascular surgery at CHU Bordeaux Hospital, stated: “The first use of the TREO FIT platform in Europe marks a major landmark in endovascular treatment for complex abdominal aortic aneurysms. The procedure utilised a three-fenestration device to treat a fast-growing AAA. Visibility of fenestrations, target arteries, cannulation and endograft delivery were optimal. The procedure was performed in 139 minutes, including the device modification and reloading. The patient was discharged at day two with full target-vessel patency and no endoleaks. This first case validates the relevance of the TREO FIT concept
This device significantly simplifies the steps involved in identifying the location of fenestrations.”
Benjamin W Starnes
and its applicability to treat emergent and semiemergent complex AAA pathologies.”
Terumo Aortic states that the importance of the first implants in the USA and Europe demonstrates a significant step forward in innovation and confirms how the TREO FIT platform, in combination with the TREO abdominal stent graft system, provides a pioneering solution for treating complex abdominal aortic aneurysms.
PM-08870
Product name: TREO Fenestration Into Template (FIT)
Product availability status:
• USA: under PSIDE study
• Europe: under custom-made programme
Overview:
The first case highlights the TREO FIT platform’s potential in managing both emergent and semiemergent complex AAA cases, particularly when swift intervention is critical. Terumo Aortic notes that the successful deployment of the fenestrated device in this context validates the platform’s design and functionality, setting a precedent for the treatment of similar complex aneurysmal pathologies in Europe and potentially worldwide.
■ Urgent treatment
■ Patient specific
■ Physician modified
■ AortaFit software to determine location of target ostia
■ Tooling equipment provided
■ Future IFU for on-label use
This first case validates the relevance of the TREO FIT concept and its applicability to treat emergent and semiemergent complex AAA pathologies.” Éric Ducasse

The TREO FIT system is available in the USA under PSIDE study and in Europe under a custom-made programme
Biotronik enrols first patients in BIO-OSCAR FIRST trial
Biotronik recently announced that it has enrolled the first patients in its BIO-OSCAR FIRST trial, a study designed to confirm the safety and clinical performance of the Oscar peripheral multifunctional catheter for dilation of lesions in the femoral, popliteal and infrapopliteal arteries, including both above-the-knee (ATK) and below-the-knee (BTK) lesions.
The BIO-OSCAR FIRST trial is a prospective, multicentre, single-arm observational study involving 16 European sites within CE mark-accepting territories.
Over the next 12 months, the trial aims to enrol 200 patients—100 for ATK and 100 for BTK lesions. This study is designed to evaluate Oscar’s performance in a practical clinical setting.
two studies showing clot-sensing guidewire successfully identifies fresh clot to support decisionmaking in PAD treatment
Sensome has announced positive results from two studies of its Clotild smart guidewire system demonstrating its ability to successfully identify fresh clot—thrombus rich in red blood cells (RBCs)—in peripheral arterial disease (PAD) and differentiate it from other tissue encountered during PAD procedures. Results from the SEPARATE and E-SEPARATE studies were presented at the Paris Vascular Insights (PVI) course 2024 (12–14 December, Paris, France).

“The Oscar catheter design holds exciting possibilities for peripheral arterial disease (PAD) care, particularly in the more challenging cases,” says Koen Deloose (AZ Sint Blasius Hospital, Dendermonde, Belgium). “This trial will provide key data on how we can better streamline procedures, assess the algorithm of treatment and reduce the need for reinterventions.”
The first patients were included in the trial by Deloose and Marianne Brodmann (Medical University of Graz, Graz, Austria).
The primary endpoint of the study is the Oscar device procedural success rate, which is defined by successful lesion crossing and a residual stenosis of ≤30%, without significant Oscarrelated procedural complications such as distal embolisation, vessel rupture, perforation, or acute occlusion.
“With the BIO-OSCAR FIRST study, we’re looking to validate Oscar’s potential to simplify complex interventions while confirming safety and efficacy,” says Stuart Perks, vice president at Biotronik Vascular Intervention. “Many physicians have reported excellent procedural outcomes with Oscar. Our focus now is to generate the hard clinical evidence to support clinicians’ decision-making and the care they deliver to their patients.”
A company press release details that the Oscar device is an all-inone solution indicated for femoral, popliteal and infrainguinal lesions. The Oscar support catheter and dilator are used in tandem to enable lesion access and crossing with a compatible guidewire, offering a range of support strength dependent on the extension of the dilator.
Sensome announces data from
A press release details that the Clotild clot-sensing guidewire integrates the world’s smallest electrical impedance sensor with machine learning. For PAD, the clot-sensing technology is being developed to instantly identify fresh clot and differentiate it from organised clot as well as plaque, calcium and other tissue in real-time in order to inform individualised PAD treatment.
Sensome notes that the technology can be integrated into devices commonly used during PAD procedures, such as guidewires and catheters, and has the potential to enable the first device capable of objectively identifying fresh clot during a procedure without changing current workflow.
The prospective, single-arm SEPARATE study encompassed 17 patients treated by Koen Deloose (AZ Sint Blasius Hospital, Dendermonde, Belgium). The study showed in a post-procedure analysis that there was a high level of agreement between the technology’s identification of fresh clot, the expert’s assessment of fresh clot and the treatment decisions appropriate for fresh clot.
“Differentiating among tissues in an obstructed vessel in order to achieve successful peripheral revascularisation is often limited by indistinct angiographic imaging, inaccurate patient medical history and a lack of tactile guidewire feedback. The SEPARATE study shows us that Sensome’s clot-sensing technology could become a novel, real-time modality to reliably identify fresh clot at an expert level in interventional procedures, with the potential to improve vessel preparation and treatment decision-making for physicians of all experience levels treating PAD,” said Deloose.
A second study of the Sensome technology—E-SEPARATE—was conducted with 15 PAD patients (scheduled for amputation or bypass) at a single centre in France and had two interesting findings, according to a press release. The study showed the technology’s ability to differentiate
fresh clot from other tissue collected from these PAD patients and examined ex vivo. It also demonstrated an excellent correlation between the technology’s ability to determine the RBC content of clots collected from PAD patients with subacute and chronic lesions, and a histological analysis of the same clot by an outside core lab.
“The E-SEPARATE study findings clearly demonstrate that symptom onset is an unreliable way to judge clot composition prior to treating a patient with PAD. The clots retrieved from both chronic and subacute lesions contained both RBC-rich fresh clot and organised RBC-poor clots. In light of this, it’s important for us to know the clot type to decide how to treat these patients…do we aspirate, dissolve the clot, or use another method?
The Sensome technology has the potential to provide us with important information we are missing today to more effectively guide treatment and achieve better patient outcomes,” said Yann Gouëffic (Groupe Hospitalier Paris Saint-Joseph, Paris, France).
In two previous peer-reviewed publications, the company’s microsensor technology was shown to reliably predict the composition of RBCs in retrieved clot with good sensitivity and specificity consistent with histologic findings.
First patient enrolled in InspireMD’s CGUARDIANS II pivotal study
InspireMD recently announced that the first patient has been enrolled in the company’s CGUARDIANS II clinical trial evaluating its CGuard Prime carotid stent system in patients undergoing carotid artery stenting (CAS) via the transcarotid artery revascularisation (TCAR) approach. The patient was enrolled by Patrick Muck at Good Samaritan Hospital, part of the TriHealth System in Cincinnati, USA. Muck serves as both the site principal investigator as well as a co-lead investigator of the CGUARDIANS II study.
USA this year. I would like to thank Dr Muck for helping us achieve this initial and critical enrolment milestone, and I look forward to the efficient execution of this important study as we work to enable the use of CGuard Prime in the broadest application, offering patients and physicians this nextgeneration stenting platform, which has demonstrated best-in-class clinical outcomes in rigorous clinical studies and with over 60,000 devices sold to date.”
Muck, who is programme director and chief of vascular surgery at Good Samaritan Hospital, stated: “As we begin this study of CGuard Prime in a TCAR setting, we value tremendously the prior data from the C-GUARDIANS PMA [premarket approval], the real-world results of this implant and its potential to advance patient care through these unmatched clinical results. The protective qualities of the MicroNet mesh offer patients the sustainable protection which is so important in both short- and long-term outcomes of this procedure. We look forward to the efficient enrolment of this study, contribution from the team of investigators and working with InspireMD on this important programme.”
Xeltis completes enrolment in EU pivotal trial Xeltis has announced the completion of enrolment in its EU pivotal trial for aXess, its restorative vascular access conduit, in adults with end-stage renal disease. The trial is taking place in 22 centres across Europe.

CGUARDIANS II is a prospective, multicentre, single-arm pivotal study that aims to enrol a minimum of 50 evaluable patients. The objective of this study is to evaluate acute device success and technical success of the CGuard Prime when used in conjunction with a US Food and Drug Administration (FDA)-cleared TCAR neuroprotection system in patients at high risk for adverse events from carotid endarterectomy.
Marvin Slosman, chief executive officer of InspireMD, commented: “As we approach potential FDA approval of CGuard Prime with a CAS indication in the first half of next year, we are thrilled to have initiated the CGUARDIANS II study that, if successful, will address an everexpanding TCAR market of roughly 30,000 procedures performed in the

The aXess EU pivotal trial is a prospective study investigating the patency, safety, and performance of aXess in adult patients with end-stage renal disease requiring vascular access to start or maintain haemodialysis therapy. A total of 120 patients have been recruited across 22 centres in nine countries across Europe. This trial follows outstanding 12-month data from the first-in-human EU trial, which demonstrated a significant improvement in performance compared to current haemodialysis vascular access solutions and showed 0% (zero) infection rate.
Xeltis is now enrolling up to 140 patients in the aXess US pivotal trial.
Loreto Gesualdo (University of Bari, Bari, Italy), principal investigator, commented: “I am very proud to be contributing to this EU pivotal trial, which is a vital step towards demonstrating the potential of aXess to transform patient outcomes for those with end-stage renal disease. Xeltis’ technology allows for the natural creation of living and long-lasting vessels, effectively addressing the issue of fistula non maturation, without the risk of reverting to catheter use. This results in an improved dialysis patient experience, a clear unmet medical need.”



SYMPOSIUM FEATURES
• Comprehensive and Practical Programming
• Practice Management Essentials
• Clinical Case Reviews
• New Methods, Early Results, Clinical Trials and Innovation
• Daily Physician-in-training sessions (Scholarships Available)
• Allied Health Session
• International Call for Abstracts and E-Poster Presentations
• Latin Session in Collaboration with CADECI
• Extensive Exhibition Hall
• Social Networking Events
• Live Patient Consultations, Examinations
• Ultrasound Protocols
• Interactive Discussions on The Evaluation, Diagnosis, Treatment and Follow Up of The Patients



US FDA approves Humacyte’s Symvess for vascular trauma Humacyte recently shared that the US Food and Drug Administration (FDA) has granted a full approval for the Symvess acellular tissueengineered vessel.
A press release notes that Symvess is indicated for use in adults as a vascular conduit for extremity arterial injury when urgent revascularisation is needed to avoid imminent limb loss, and when autologous vein graft is not feasible.
“We are very excited and proud to provide patients suffering from arterial injury with a novel treatment option. Symvess has been made possible by our innovative bioengineering science along with the contributions of many patients, healthcare providers and Humacyte team members,” said Laura Niklason, founder and chief executive officer of Humacyte. “Symvess approval in this first indication for arterial injury repair is a milestone for regenerative medicine overall, as well as for Humacyte. The FDA’s full approval of Symvess is a transformational event for the company and our bioengineering technology platform. Even more importantly, we believe Symvess provides a new means of treating patients with devastating arterial injuries, which is a population that has not benefited from substantial innovation in decades. We look forward with great excitement to our upcoming commercial launch of Symvess, and we have recruited and trained a terrific team to execute on our sales and marketing missions.”
“The approval of a vascular conduit that resists infection and remodels into native arteries is an extraordinary technological advancement that will have a huge impact on the quality of trauma care around the world,” said Charles J Fox (University of Maryland Capital Region, Lake Arbor, USA), a clinical investigator in the V005 clinical trial. “Symvess is perfectly sized to treat most injuries, has excellent handling properties, and reduces time necessary to save both life and limbs. The Humacyte team has responsibly and scientifically solved a major clinical problem that I believe will reduce the amputation rate for traumatic vascular injury. They should be congratulated on an accomplishment that will undoubtedly advance our specialty to the next level.”
“I believe that Symvess will revolutionise vascular trauma care and be profoundly beneficial to our patients,” said Rishi Kundi
(University of Maryland Medical System, Baltimore, USA). “From my experience so far, Symvess will allow reconstructions that are currently impracticable because of contamination and infection. It will make reconstructions that we now perform with prosthetic or even biologic grafts more successful. I am most excited about the promise that Symvess holds for the long-term experience of our patients. I hope that, with Symvess, the 19-year-old patient with vascular reconstruction after trauma will no longer spend the six decades after their surgery anticipating disaster, but that their chances for reintervention will be no different than if they had autologous conduit.”
A press release details that Symvess is a first-in-class bioengineered human tissue that is designed to be a universally implantable vascular conduit for use in arterial replacement and repair. “While harvesting vein from a trauma patient takes valuable surgical time, Symvess is available offthe-shelf, and does not require further injuring the patient to obtain vascular repair material,” the release notes.
Humacyte’s biologics license application (BLA) included positive results from the V005 pivotal Phase 2/3 clinical study, as well as realworld evidence from the treatment of wartime injuries in Ukraine under a humanitarian aid programme. Symvess was used to repair many types of traumatic injuries including car accidents, gunshot wounds, blast wounds, and industrial accidents. It was utilised by vascular and trauma surgeons in level-one trauma centres throughout the USA and Israel to repair severe limb-threatening and lifethreatening injuries, and in frontline hospitals in Ukraine to treat wartime injuries. Results from these studies were published in JAMA Surgery in November 2024. In the civilian and military clinical studies, Symvess was observed to have high rates of patency, or blood flow, and low rates of amputation and infection.
“Finally, we have an innovative technology for battlefield vascular injuries using a tissue-engineered human arterial replacement that can resist infections that are so prevalent in modern combat zones,” added Fox. “Symvess shows promise to reduce amputation rates since an alternative conduit for war injuries is often needed but up until now has not been a good option.”
“The FDA approval of Symvess will make it the preferred conduit for complex vascular injuries, and particularly those at risk for infection,” said Ernest E Moore (Denver Health, Denver, USA), a clinical investigator in the V005 trial. “I look forward to using Symvess in my practice.”
Humacyte shares that the Symvess trauma programme was granted
regenerative medicine advanced therapy (RMAT) designation by the FDA in May 2023, a BLA was submitted to the FDA in December 2023, and in February 2024 the FDA granted a priority review. On 9 August 2024, the FDA informed Humacyte that it needed additional time to complete its review of the BLA, although there were no outstanding pre-approval requirements for Symvess as of that date. The FDA completed its review on 19 December, granting full approval.
First patients treated with Contego Medical’s Neuroguard IEP system
Neuroguard IEP system Contego Medical has announced the successful completion of the first commercial cases with its newly US Food and Drug Administration (FDA)-approved Neuroguard IEP system.
A press release notes that John Gaughen Jr, an interventional neuroradiologist at Centra Medical Group (Lynchburg, USA), and Srinivas Attanti, an interventional cardiologist at the University of Florida Health Leesburg Hospital (Leesburg, USA), treated the first patients.
A press release details that Liberty is the world’s first single-use, fully disposable robotic system for endovascular procedures. The 510(k) submission follows the successful completion of Microbot Medical’s multicentre, single-arm trial to evaluate the performance and safety of Liberty in human subjects undergoing peripheral vascular interventions.
The company anticipates FDA marketing clearance during the second quarter of 2025, with US commercialisation activities expected to commence after the clearance.
“This is a pivotal milestone for our company, as the 510(k) submission reflects the commencement of our transition to a commercially focused company,” commented Harel Gadot, chairman, chief executive officer and president. “We are excited to transition our focus towards preparing for our expected US launch in the second quarter of 2025 and targeting the more than two million peripheral vascular procedures performed in the USA each year. We believe, based on feedback from physicians and the medical community, that Liberty is positioned to redefine the peripheral endovascular space with the introduction of the world’s first commercially available single-use robotic system.”

“The Neuroguard system is very easy to use and streamlines the steps for treating our patients with carotid artery disease. I believe this innovation will reduce the risk of stroke during and after carotid artery stenting procedures,” said Gaughen. Attanti added: “The pores on the integrated filter are much smaller than those on traditional technology, improving stroke protection for our patients.”
Contego Medical highlights clinical studies of the Neuroguard IEP system, including the PERFORMANCE I trial and PERFORMANCE II investigational device exemption (IDE) trial, that have consistently reported “unprecedented” low event rates—zero major strokes, zero neurologic deaths, and zero stent thromboses—at up to two-year follow-up.
“When we started Contego, we always had our sights set on this day. The introduction of the Neuroguard IEP system ushers in a new era for treating patients with carotid artery disease, providing a level of safety and confidence that was previously unmatched,” said Ravish Sachar, Contego Medical’s chief executive officer and founder.
Microbot Medical announces US FDA submission for commercialisation of Liberty endovascular robotic system Microbot Medical recently announced that it has submitted a 510(k) premarket notification to the US Food and Drug Administration (FDA) for its Liberty endovascular robotic system.
Microbot Medical states in the press release that Liberty eliminates the need for large and expensive capital equipment and streamlines customers’ access to robotics. With its remote control, Liberty is designed to significantly reduce radiation exposure to physicians and staff, and improve ergonomics, which has the potential to reduce the physical strain on healthcare providers. The company also believes that Liberty has the potential to lower procedure costs, increase procedure efficiency and improve the overall quality of care.
Merit Medical announces US FDA approval of the Wrapsody cellimpermeable endoprosthesis
Merit Medical Systems recently announced that the Wrapsody cellimpermeable endoprosthesis has received premarket approval from the US Food and Drug Administration (FDA).
With this approval, a press release notes, Merit can begin commercialisation of the device in the USA in 2025.
The company details that Wrapsody is designed to extend long-term vessel patency in dialysis patients. “Preserving vascular access for dialysis patients is critical for them to maintain lifesaving treatment,” said Bart Dolmatch (Palo Alto Medical Foundation, Palo Alto, USA), who is credited as co-inventor of the Wrapsody device, in a Merit press release.
“I believe the advancements that the Wrapsody device offers will translate to better outcomes for haemodialysis patients.”



Flow Medical secures US$5 million seed funding to advance pulmonary embolism therapy
Flow Medical has announced the closing of an oversubscribed US$5 million seed funding round, which includes US$2 million previously secured from friends and family. A press release notes that this investment positions Flow Medical to accelerate the development and launch of its pulmonary embolism (PE) device.
The company shares that the seed round was supported by a strategic group of investors with an interest in healthcare innovation, including the University of Chicago Medical Center (Chicago, USA) as part of its UCM Ventures initiative. The funding represents UCM Ventures’ first direct investment in a med-tech startup. All three founders of Flow Medical are faculty members at the University of Chicago.
“This is the second company I have built out of UChicago, and I am thrilled to have such strong support from the
28–30 January
Leipzig Interventional Course (LINC) Leipzig, Germany leipzig-interventional-course.com
20–22 February
International Multidisciplinary Endovascular Forum – IM Endo Forum Florence, Italy imendoforum.com

investment community,” said Jennifer Fried, chief executive officer of Flow Medical. “We are at the forefront of a new era in PE treatment and are poised to translate our innovative ideas into meaningful clinical advancements that can save lives.”
Boston Scientific to acquire IVL developer Bolt Medical Boston Scientific has announced it has entered into a definitive agreement to acquire Bolt Medical, the developer of an intravascular lithotripsy (IVL) advanced laser-based platform for the treatment of coronary and peripheral artery disease.
“Representing one of the fastest growing medical device segments, intravascular lithotripsy therapy addresses a significant unmet need for patients with complex calcified arterial disease through a minimally invasive approach,” said Lance Bates, senior vice president and president, Interventional Cardiology Therapies, Boston Scientific. “Bolt Medical is developing a nextgeneration technology that is highly complementary to our existing portfolio. The addition of this system to our offerings can help us better serve physicians and their patients and provides a platform for future innovation.”
Lithotripsy is a procedure in which a
9–11 March European Vascular Course (EVC) Maastricht, The Netherlands vascular-course.com
29 March–1 April Society of Interventional Radiology (SIR) annual scientific meeting Nashville, USA sirmeeting.org
3–4 April CLI-C GLOBAL Venice, Italy cli-courses.com
physician breaks up hardened masses such as calcium to help restore blood flow.
The Bolt IVL system is designed with a novel application of lithotripsy to fracture calcium by creating acoustic pressure waves inside of a balloon catheter. The system also includes visible, directional emitters for consistent energy delivery in the treatment of the calcified lesions.
Boston Scientific initially developed the concept for the Bolt IVL system which helped establish Bolt Medical in 2019. As a strategic investor in Bolt Medical, Boston Scientific has an equity stake of approximately 26%. As a result, the transaction consists of an upfront payment of approximately US$443 million for the 74% stake not yet owned and up to US$221 million upon achievement of certain regulatory milestones.
Boston Scientific anticipates the transaction to be completed in the first half of 2025, subject to customary closing conditions.
Stryker announces definitive agreement to acquire Inari Medical
Stryker has announced a definitive agreement to acquire all of the issued and outstanding shares of common stock of Inari Medical for US$80 per share in cash, representing a total fully diluted equity value of approximately US$4.9 billion.
A Stryker press release notes that Inari, which was founded in 2011, will
23–25 April Charing Cross (CX) Symposium London, UK cxsymposium.com
8–10 May Venous Symposium New York, USA venous-symposium.com
22–23 May 17th Pacific Northwest Endovascular Conference (PNEC) Seattle, USA pnec-seattle.org
bring a leading peripheral vascular position in the fast-growing segment of venous thromboembolism (VTE) to Stryker. “Inari’s innovative product portfolio is highly complementary to Stryker’s Neurovascular business and includes mechanical thrombectomy solutions for peripheral vascular diseases such as deep vein thrombosis and pulmonary embolism,” the release reads.
“The acquisition of Inari expands Stryker’s portfolio to provide lifesaving solutions to patients who suffer from peripheral vascular diseases,” said Kevin Lobo, chair and chief executive officer (CEO), Stryker.
“These innovations elevate the standard of care for venous thromboembolism patients and will accelerate Stryker’s impact in endovascular procedures.”
“Inari has positively impacted the lives of hundreds of thousands of patients through the development of purpose-built tools that address unmet patient needs,” said Drew Hykes, CEO, Inari. “With Stryker’s capabilities and global infrastructure, we will be even better positioned to accelerate the development of innovative new solutions and expand our footprint.”
Stryker advises that the transaction is anticipated to close by the end of the first quarter of 2025, subject to customary closing conditions.
Expected impacts to 2025 financial results will be discussed on Stryker’s upcoming fourth quarter 2024 earnings call scheduled for 28 January 2025.
4–7 June
Vascular Annual Meeting (VAM) New Orleans, USA vascular.org/vam-2025
26–27 June
British Society of Endovascular Therapy (BSET) annual meeting Wotton-under-Edge, UK bset.co.uk/meetings/bset-annualmeeting-2025





















When you hold someone’s
in your hands, you need to trust your aortic technology. So choose endovascular solutions that have helped over 600,000 patients and are substantiated in peer-reviewed journals. We’re your aortic ally.
Learn more, visit gmd.cm/aortic-outcomes
