































































26th











In cooperation with:
BPI German Pharmaceutical Industry Association
DECHEMA Society for Chemical Engineering and Biotechnology
European Biotechnology Network
VDGH German Diagnostics Industry Association
ISBN 978-3-928383-93-6

































































26th











In cooperation with:
BPI German Pharmaceutical Industry Association
DECHEMA Society for Chemical Engineering and Biotechnology
European Biotechnology Network
VDGH German Diagnostics Industry Association
ISBN 978-3-928383-93-6



On the one hand, on the other. Is good good enough? These are the thoughts that come to mind when looking at the latest OECD data on the development of the German biotechnology sector. On the one hand, the German economy was in a phase of stagnation last year - even a technology of the future finds it difficult to compete against the leaden mood of late. Significant growth therefore appears to be a particular success. On the other hand, the biotechnology sector is developing solutions to two of today’s megaproblems: climate change and the ageing of societies in industrialised countries. Anyone who jumps on this bandwagon has the chance of exponential growth - something that has not happened again in 2023.
After the growth spurt in the early 2020s due to the novel mRNA vaccines against the coronavirus pandemic, industry figures normalised in 2023 - only revenues continued to fall significantly due to the lack of vaccine sales. By contrast, expenditure on research and development rose to a new record high. Companies invested a total of €3.74 billion, with almost €3.4 billion going into the research of new compounds, technologies and products or the further development of existing healthcare projects.
And the role of biotechnology is also becoming increasingly clear in other areas, such as feeding a growing world population: CRISPR/Cas and precision fermentation are the current growth drivers. In the field of sustainable materials, biotechnology applications offer innovative and climate-friendly solutions, as do new recycling technologies for the circular economy.
Not only has investment in research and development increased significantly, but a large number of new employees have also been hired, despite the prevailing shortage of skilled labour. It is also pleasing to note that a number of new companies have been set up despite the poor general economic climate. All of this testifies to the industry’s strong self-confidence and enthusiasm for innovation.
These assessments are confirmed by the results of BIOCOM’s Biotechnology Company Survey 2024, which analysed the key figures for 2023 in the spring. BIOCOM has been analysing the development of the biotechnology sector in Europe for 38 years. Since 2005, the annual survey of German biotechnology companies has been internationally comparable according to the criteria of the Organisation for Eco -
Key figures of the biotech sector in Germany

Name ›
4base lab AG advanced molecular analysis
Address/P.O. Box › Postal Code/City › State ›
Contact Person › Telephone › Email
Website ›
Founded (year) › Type of Laboratory ›
Areas of Activity ›
Aspenhaustr. 25
72770 Reutlingen
Baden-Wuerttemberg
Dr Johannes Ries
+49-7121-317878-0 pharma@4base-lab.com
https://en.4base-lab.com/pharma/ 1995
GMP, S1/L2
| Identity/quality assessment of mRNA/ DNA vaccines
| Contamination analysis of residual DNA/RNA
| Vaccine quality analysis by Nanopore direct RNA-sequencing
| DNA/cDNA-sequencing by Sanger
| Determination of mRNA poly(A) tail length
| Sequencing the CDS in mRNA
| Direct colony full-length sequencing
| Contract research/outsourcing
| Viral- and microbial detection
For almost three decades, 4base lab has been at the forefront of molecular analysis, maintaining GMP-compliant laboratories that consistently meet the highest industry standards. As a reliable partner of mRNA/DNA vaccine manufacturers including leading pharmaceutical companies, 4base lab offers safety at every stage of the manufacturing process.
Excellence through collaboration is our guiding principle. Our team of experienced scientists and qualified technicians partners closely with you to comprehend your unique objectives. We leverage this understanding to develop customized assays and validation methods that are tailored to your specific requirements and ensure optimal results.
Customized advice and flexible adaptation of our test systems are our strengths.
DNA sequencing: The confirmation of sequence identity is an essential part of the production of therapeutic nucleic acids. 4base lab offers reliable non-GMP and GMP sequencing services according to Sanger, covering all steps necessary for the determination of complete DNA sequences. Even samples with ultra-low DNA concentrations, limited volume or colonies can be sequenced in full-length using validated Rolling Circle Amplification (RCA) methods.
RNA sequencing: The assessment of therapeutic mRNA vaccines directly, without reliance on intermediate molecules, is increasingly in focus of regulatory quality control requirements regarding the sequence coding identity, modifications, and structural motifs. 4base lab offers a qualified analysis pipeline to address these issues directly on the RNA.

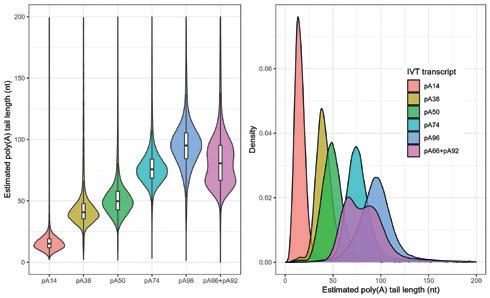


Determination of poly(A) tail length: for determination of poly(A) length of mRNA vaccines, qualified sequencing strategies utilizing Sanger adapter sequencing as well as Oxford Nanopore Technologies (ONT) direct RNA sequencing are available.
Residual nucleic acid analysis: Detection of residual DNA contamination. Safety at every step of your manufacturing process of mRNA vaccines. Various validated qPCR/RT-PCR protocols are established for the detection of residual E. coli genomic DNA, RNA and plasmid DNA contaminant in mRNA vaccines.
Development and Validation: New detection systems can be developed at any time according to customer requirements and validated in accordance with ICH Q2.
Viral and microbial detection: Please inquire for available systems.
4base lab has been maintaining GMP compliant laboratories for molecular analysis for almost three decades. Furthermore, 4base lab is certified according to DIN EN ISO 9001, DIN EN ISO 14001 and accredited according to DIN EN ISO 17025.