
























Hello, and welcome to the October Edition of Supplyline. In this issue of Supplyline you will find information on the NZSSA AGM, various reports and updates covering a range of topics. A huge thank you to those people who supplied Articles for this edition, it is hugely appreciated.
I had the privilege of attending the NZSSA Conference in Christchurch recently. It was so good to catch up with people, both delegates and Company representatives that I have not seen for ages. The presentations were varied, and all were interesting covering a range of different topics – exactly what you want at a conference. Next year’s conference is set to happen in Wellington, so keep an eye out for updates on the next conference in this and upcoming editions of Supplyline.
If you would like to send in your photos and a wee blurb regarding the photo, I’m more than happy to include it in upcoming editions of Supplyline. It could be about the staff who have completed their studies; any quality improvements projects; or just to introduce your team to the rest of NZ.
It’s not too far away and Christmas will be here – what a thought. We are in my unit, as I’m sure others are, well and truly into the process of planning Christmas rosters, the annual leave for this period and a staff Christmas party. I always think that doing Rosters makes the year go by faster – or at least it feels like it.
I hope that you are all doing well and looking forward to the approaching summer, although the recent cold snap was a shock to the system.
In September we held the NZSSA conference, the first since 2019. It was so wonderful to see everyone again and we had a fantastic turnout from both members and our trade partners.
A few thank you are in order here. First of all to Melanie Pitto of MTANZ who was the behind the scenes organiser of our conference. She liaised with the venues, caterers and the trades to ensure everything came together on the day. I would like to thank our trade partners who made the effort to come to Christchurch and supported us with education re their products that are available to you.
We all enjoyed the array of speakers which covered scientific topics, thought provoking topics around
We care for each other, showing kindness and empathy in all that we do.
We are committed to finding future focused solutions and take personal responsibility to be better every day.
Our diversity is our strength, we back each other and work together in partnership.
We are committed to doing the right thing by ensuring equity and hauora are at the heart of everything we do.
I hope you enjoy this edition and that you learn something you didn’t know before you read through it. If there is anything that you would like to see in Supplyline in the future, please let me know.
Take care everyone, Ngā mihi
Aileen Derby Editor NZSSA Supplyline

culture and equity as well as emotional topics which looked at the human reaction to mass casualty events and then topics which showed to us all that we can expand our horizons by working on the charity hospitals on mercy ships.
The conference dinner was a spectacular event held at the cardboard cathedral. The majority of participants took up the challenge of “Pimping up their Scrubs” and I think everyone had a great time.
A special mention to the team attending from Waikato hospital. These guys could certainly dance. They had all the moves and were a great source of entertainment. Planning for the 2023 conference is now underway. It
shall be held in wellington and we are just confirming venues and dates. This will be announced as soon as contracts are signed.
Prior to the commencement of the conference the executive team had their first face to face meeting since Covid struck. It was great to see everyone, old and new members after such a long time. We were able to plan out a few things for the next year and will start this work this month. We shall put out regular updates on our work on the website throughout the year.
As a profession we are lucky we get to celebrate more than once in a year. In April we have World Sterile Sciences day and for those of you employed by Te Whatu Ora, October the 14th celebrates Allied Health Scientific and Technical day. So if any of your team are celebrating or invited to take part in an AHS&T celebration please send us photos for Supplyline.
In November I am heading of for what will likely be my last WFHSS conference to be held in Spain. It won’t be a holiday as it is quite a quick trip there and back. My role is to represent NZ and to bring back knowledge of the latest research and development that will assist you in your practice as well as information on new products that are available and may be of use in your workplaces. I shall share as much as I can via Supplyline on my return.
For us all the workloads are ramping up again. We have become a bit used to the lighter loads of the last two to three years. Please ensure that you are all taking care of yourselves and seek assistance if you are feeling under pressure at work.
Nga mihi Shelagh ThomasTe Whatu Ora have sterilisation wrap on hand that was central purchased last year when there was a sterile wrap supply issue. It is available to CSSD departments, free of charge. Sterilisation wrap play an essential role in providing protection against contact contamination. Including a microbial barrier that will keep the items sterile, these wraps ensure your surgical instruments are clean. Strong resistance to punctures, tears, and abrasions. Great drapability and, easy to fold. There may also be appropriate uses for this wrap within your hospitals outside of the CSSD department.
Wrap is single-bonded, weighs approximately 40 and 60g/sq., m and is available in the sizes below:
1173346 4501-010
40gsm 4501-010 120x120 100
Blue 60gsm 4501-059 120x120 70 1166468 4501-006
4501-059
Wrap Blue 40gsm 4501-006 75x75 200 1173345 4501-007
Wrap Blue 40gsm 4501-007 90X90 200 1166471 4501-056
Wrap Blue 60gsm 4501-056 90X90 130 1173349 4501-057
Wrap 60gsm 100x100cm 100X100 130 1166516 4501-003
Wrap 40gsm 45x45cm 4501-003 45X45 300 1166490 4501-005
Wrap 40gsm 60x60 4501-005 60X60 300 1173362 4501-011
Wrap Blue 40gsm 130x130cm 130X130 50 1173348 4501-054 Sterilisation Wrap 60gsm 60x60 4501-054 60X60 200
The sterilisation wrap is available in blue and are non-woven SMS polypropylene.
you would like any of the stock, free of charge, please contact COVID.HealthSupplyChain@health.govt.nz with the following information:
Organisation:
name:
address:
number:
number:
of cases required:
If you have any queries or would like to discuss further, please contact Te Whatu Ora’s Supply Chain Team COVID. HealthSupplyChain@health.govt.nz

Sterile reprocessing departments reprocess the used the re-usable medical devices (RMD’s) so that they are fit and safe to be used on patients. Reprocessing consists of three broad steps: decontamination, where RMD’s are received and cleaned either manually or using washer disinfectors, packing/assembly, where RMD’s are inspected for functionality and cleaning and put to together into packs and sterilisation, where RMD’s are sterilised to render free of viable microorganisms and be safe to be used on patients. There has always been an increased focus on producing an effective end product and there are quality indicators to ensure that, but there could be certain steps that can be taken to be efficient while maintaining the effectiveness. Service providers are looking for innovative ways to reduce costs without sacrificing high service quality because of increasing costs of healthcare (Saif and Elhedi, 2019). Efficiency is doing the right thing and characterised by minimising cost, avoiding waste, including waste of equipment supplies, ideas and energy. Efficient management of infrastructure and effective monitoring of disinfection and sterilization procedures is important for quality of patient care (Kumar et al., 2018).
Recently, Covid 19 has impacted the whole world and there were Covid 19 pandemic related slowdowns with decrease in number of surgeries and delays with supply of resources. Leaders should secure resources for improvements, improve efficiency, streamline operations while maintaining, or even improving, quality and safety (Nadeau, 2021).
This research is focused on identifying the factors that affect the efficiency and aim to find some information and explore opportunities that would be helpful to the reprocessing units to increase their efficiency.
Method: The relevant literature was accessed via Toi Ohomai library and Google Scholar, which has further links to databases such as science direct, and Pubmed. . Search terms used were, ‘Sterile reprocessing and efficiency’, ‘work practices and efficiency in sterile processing’, ‘factors that affect efficiency’, ‘How to
increase efficiency in sterilisation’, ‘trends in sterile processing department’. The search was made for literature that was mainly available from since 2018 and then further limiting to academic journals. Articles directly related to reprocessing environment were chosen and key findings related to efficiency were noted.
The findings of the literature review can be summarised into following focal points:
• Staff Training and error reduction
• Workspace design
• Energy, financial costs and carbon foot print
• Preventative maintenance
• Centralised CSSD
• Automation
All staff involved in the reprocessing of RMD’s must be qualified with a plan for continuing education and recertification be obtained at regular intervals (Ling et al., 2018). There is lot of emphasis on training and development in reprocessing environment. This helps in upskilling the staff, make them feel valued and this can help in retaining staff (Jelks, 2019). Experienced staff can organise their work better and can support the inexperienced staff. High staff turnover and reduction in training can be directly correlated to backlog of work and increase in defects of the reprocessed items (Alfred et al., 2021). If the staff are inexperienced, training them and bringing up to speed takes time and the productivity slows down (Nadeau, 2021). Delays in receiving sterilised RMD’s to the operating theatres can cause significant disruptions on surgery schedules (Gokalp and Sanci, 2021).
Reprocessing instruments right the first time saves time and avoids operating room case delays and putting patients at risk. It is important that any non-compliances that occur are recorded and addressed to make continuous improvements (Nadeau, 2021). A study by Kumar et al, 2018, emphasised the need of staff training for implementing the appropriate use of quality checks for efficient sterilisation.
Work system analysis of decontamination by Alfred et al (2020) suggests that insufficient resources such as sinks require technicians to move back and forth thus slowing down the process. Inadequate holding space can result in congestion and poor lighting can affect the bio burden detection. Standardising workstations in the packing area with the same set up can increase efficiency and reduce instrument assembly errors (Nadeau, 2021). Work System Analysis of the assembly step of reprocessing by Alfred et al (2021) proposes that organisational approach to invest in well designed and maintained IT database, work station displays, standardised instrument nomenclature and collaboration between operating rooms and reprocessing units can help in reducing packing tray errors and Operating room delays.



In his study, McGain F. (2016) found that considerable amount of electricity (about 40%) and water (about 21%) was used by sterilisers on standby. Rotating of idle steam sterilisers and reducing the number of light loads can improve steriliser efficiencies and reduce environmental footprint. Jemmad et al (2020) highlight in their research that induction heating external steam generators are energy saving than ohmic heating steam generators by at least 40% though implementation cost are bit higher. Rizan et al (2022) conducted a study to estimate the carbon and financial costs of reprocessing RMD’s and indicating how that burden can be reduced to drive actions towards net zero carbon surgery. The study shows that the carbon foot print increased two to three fold and financial cost also increased when RMD’s were packed individually as compared to being a part of the set. If four or fewer instruments are required, individually packed items could be preferred because it costs financially less than a pack. The study also emphasises that filling all the machine slots when loading the RMD’s helps to reduce the financial costs and carbon footprint. It further reveals that high temperature incineration of waste (single use packaging) increases carbon footprint by 33-55 percent, whereas recycling reduces it by 6-10 percent.
so that
and the probability
parts are
failure is reduced (Kumar et al., 2018). Proper routine equipment maintenance and timely repair should be done to ensure that equipment is in working order (Alfred et al., 2020).
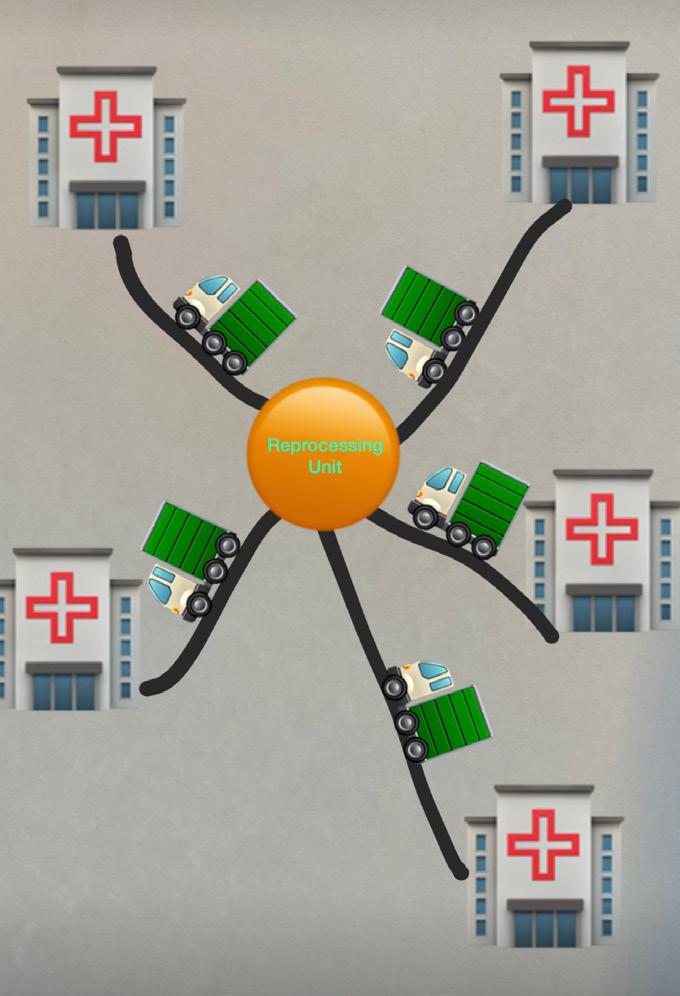
Ling et al., 2018 strongly recommends that the reprocessing be performed in a centralised area and reprocessing requirements related to human resource and physical environment should be complied with. A case study by Saif and Elhedhil (2019) reveal that significant cost savings of about 19.1% can be achieved by centralisation with sterilisation functions being performed in specialised centres as opposed decentralised scheme where sterilisation is performed by each hospital individually. With centralisation, risk pooling effect and better utilisation of resources, cost savings can be made, but the cost of transporting RMD’s to and from hospitals

can go higher and that needs to be weighed against the savings. The study also shows that holding RMD stock in the centralised area and administering to the hospitals also result in small cost savings and that needs to be weighed against operational considerations such as shipping and inconvenience to individual hospitals related to losing control over the RMD stocks. It is the immense variety of instruments than the number of instruments that is challenging for outsourcing processing of sterile services (Fragapane et al., 2021). Infection prevention and control is a concern with centralisation as there is no clear regulatory guidance for transportation of soiled or sterile instruments between organisations (Eisenberg et al., 2021).
There is a rising trend to increase the level of automation in the decontamination process, to increase productivity, and reduce the risk of human error and musculoskeletal injuries (Ghiyasinasab et al., 2021). Automated processing equipment helps in standardising processes, reducing variation and increasing efficiency and staff safety (Nadeau, 2021). For example, staff pushing transportation carts have higher risk for lower back and hand injuries. In a study by Fragapane et al (2021), automated mobile robots (AMRs) have been shown to provide highly flexible and cost efficient transportation of sterile instruments. AMRs are small in size, have intelligent navigation system, and can enter departments to deliver materials closer to point of use, thus increasing efficiency especially in horizontal transportation setting.

training, which can lead to less non-compliance incidents. However, a correlation between minimum staffing levels and work requirements could not be established. Further research as to what could be considered as safe staffing levels would be helpful.
Sufficient resources and ergonomic work design maintains the workflow and increases efficiency. Carbon foot print and financial costs can be reduced by rotating idle sterilisers, loading machines to full capacities, packing RMD’s as part of sets and recycling waste.
With centralisation of CSSD’s, there are significant savings with better utilisation of resources and risk pooling, but
there are also risks associated with infection prevention and control. Further research should be done in relation to centralisation by considering different timings and options of transportation and compare results of busier cities with less busy places. There is increasing trend of automation to improve efficiency in sterile reprocessing areas and reduce health impact on staff. Automated robot systems are fairly new and future research should be done in different hospital settings as each facility’s lay out is different (Fragapane et al, 2021). Further area for research could be optimal surgical tray configuration because of low utilisation rate of instruments in trays (Ahmadi et al., 2019).
Ahmadi E., Masel D. T., Metcalf A.y. and Schuller K. (2019). Inventory management of surgical supplies and sterile instruments in hospital: a literature review. Health Systems, 8(2):134-151. https://doi.org/10.1080/20476965.2018.1496875
Alfred M., Catchpole K., Huffer E., Fredendall, L., & Taaffe, K. M. (2021) Work System Analysis of sterile processing: assembly. BMJ Quality and Safety, 30 (4),271–282 https://qualitysafety.bmj.com/content/qhc/30/4/271.full.pdf
Alfred, M., Catchpole, K., Huffer, E., Fredendall, L., & Taaffe, K. M. (2020). Work systems analysis of sterile processing: decontamination. BMJ Quality & Safety, 29(4), 320. https://doi.org/10.1136/bmjqs-2019-009422
Eisenberg, J., Borland, P., Wilder, J., Oko, W., Marrs, B., and Jagrosse, D. (2021). Driving Innovation: A Transportation Methodology to Support the Transition of Orthopaedic Surgery from Acute to Ambulatory Care. Journal of Orthopaedic Experience & Innovation, 24241 accessed on 09/05/2022
Fragapane G., Hvolby H., Sgarbossa F. & Strandhagen J.O.(2021) Autonomous mobile robots in sterile instrument logistics: an evaluation of the material handling system for a strategic fit framework, Production Planning & Control, 1-15. https://www. tandfonline.com/doi/full/10.1080/09537287.2021.1884914
Ghiyasinasab M., Lahrichi N. & Lehoux N. (2021). A simulation model to analyse automation scenarios in decontamination centers. Health Systems, https://doi.org/10.1080/20476965.2021.2004933
Gokalp E., Sanci E. (2021). Robust capacity planning for sterilisation department of a hospital. International Journal of Production research, https://doi.org/10.1080/00207543.2021.2015807
Jemmad K., Hmidat A., & Saad A. (2020). A novel systematic and comprehensive method for identification of energy-saving measures. EAI Endorsed Transactions on Energy Web, 7(30). https://doi-org.ezproxy.toiohomai.ac.nz/10.4108/eai.13-72018.164827
Jelks, M. (2019). Employee engagement! Managing our best and brightest in sterile processing. Healthcare Purchasing News. 43(12), 34-37
Kumar, N., Lothlikar, V., D’Souza, B., Rani, U., & Swapna, B. V. (2018). Cost analysis of re-sterilization procedure of re-usable devices in a hospital. Journal of Young Pharmacists, 10(1), 109-112. https://www.jyoungpharm.org/sites/default/files/ JYoungPharm_10_1_109.pdf (Accessed on 06/05/2022)
Ling, M.L., Ching, P., Widitaputra, A., Stewart A., Sirijindadirat N., Thu L.T. (2018). APSIC guidelines for disinfection and sterilization of instruments in health care facilities. Antimicrobial Resistance and Infection Control, 7(25). https://doi.org/10.1186/s13756-0180308-2
McGain F., Moore G., Black J. (2016) Steam sterilisation’s energy and water footprint. Australian Health Review 41(1), 26-32. https:// doi.org/10.1071/AH15142
Nadeau, K. (2021). Reevaluating reprocessing workflow: With ORs reopening, SPDs must define their “new normal.” Healthcare Purchasing News, 45(7), 18–22.
Rizan C., Lillywhite R., Reed M., Bhutta M. F. (2022) Minimising carbon and financial costs of steam sterilisation and packaging of reusable surgical instruments. British Journal of Surgery, 109 (2), 200–210, https://doi.org/10.1093/bjs/znab406
Saif, A., Elhedhli, S. (2019) Sterilization network design. EURO Journal on Transortation and Logistics 8(1), 91–115 (2019). https://doi. org/10.1007/s13676-018-0118-y


Date: Friday, 9th September 2022
Time: 0730
Venue: Christchurch Town Hall and via Teams
Present: Quorum achieved with number of members in person and online via zoom
Prior to proceedings a minute silence was observed to remember Her Majesty Queen Elizabeth II who passed away early on the morning of 9th September 2022.
1. Apologies: 1 apology given
NZSSA AGM Minutes 2021 were circulated to members in the AGM pack prior to the meeting. The motion was put to those present that the minutes be accepted.
Moved by: AD Seconded: CC
Anthony Valvoi and Kelly Swale have have joined the Executive after the resignations of Tracey Kereopa and June Isted. Members were asked to ratify both of these appointments.
Moved by: AD Seconded: JC
The president’s report was circulated with the AGM pack to members prior to the meeting. The meeting was asked if anyone wanted to discuss the content. No discussion requested. It was moved that the report be accepted as written.
Moved by: MS Seconded: JC
The Secretary’s report was circulated with the AGM pack to members prior to the meeting. The meeting was asked if anyone wanted to discuss the content. No discussion requested. It was moved that the report be accepted as written.
Moved by: CS
Seconded: BT
Treasurer’s report was previously circulated in the AGM pack along with the audited annual accounts prior to the meeting. The meeting was asked if anyone wanted to discuss the content. No discussion requested. It was moved that the report be accepted as written.
Moved by: CC Seconded: AV
7. Auditor’s report 2021-2022
Alison briefly covered the auditor’s report completed by Accounting for Charities. There were no issues with the association accounting or use of funds. A motion was raised to retain the audit services of Accounting for Charities for the coming financial year.
Moved by: SM Seconded: AD
8. Conference 2023
Sharon announced next year’s conference is planning to be held in Wellington at the new Takina Wellington Conference and Exhibition Centre in September 2023.
• Shelagh informed the meeting that the association would be offering scholarships in the coming year for members to attend the 2023 NZSSA Conference in Wellington, the 2023 WFHSS Conference in Belgium and the Federal Sterilising Research Advisory Council of Australia (FSRACA) conference in Victoria, Australia. There will also be educational scholarships offered again. Details can be found on the website closer to the time.
• A member asked whether the NZSSA would consider sponsoring someone to work on one of the Mercy Ships. Shelagh responded that a scholarship application would have to be made for consideration by the executive.
No other general business was raised.
Meeting Closed: 0825
Unfortunately getting regional meetings up and running proved to be logistically difficult and to run them by zoom for members even more so as not all members can down tools and attend daytime meetings let alone have access to Zoom. Potentially we will look at recording sessions or podcasts. And putting them on the website for members to listen to in their own time.
The NZSSA are so pleased to be able to bring to its members an annual conference this year.
It has been such a stressful Covid and post Covid period trying to organise a conference with red zones and orange zones and numbers on limitations and travel, yet here we are, we have finally made it.
As an association, and as your executive team, we have continued to meet throughout the pandemic time by regular zoom meetings, which we have all become quite expert at.
Since our last conference we have had a few changes on the executive team. We have farewelled Kevin Green from Whangarei Hospital, Sue Woods from Burwood Hospital, Kerry Nichols from Wakefield Hospital, June Isted from Hawkes Bay Hospital and Tracey Kereopa from Masterton Hospital. Sue, June, and Kevin are still working in sterile services while Kerry has decided to take up retirement and spend more time with grandchildren and Tracey is developing her skills and business in traditional Māori healing/Rongomai. I thank them all for their excellent service to the NZSSA and you the members.
Following those departures, we have some new team members and some previous members who have returned to the executive. We welcome Kelly Swale who is the manager of the SSD for the school of dentistry at Otago University, Anthony Valvoi, SSD manager at Taranaki Base hospital, Sharon Moss, national sterilisation manager for Southern Cross Hospitals, Karen McCormick, SSD manager Capital and Coast hospitals and Paul Moody from Fisher and Paykel Healthcare. We welcome back Jenny Carston, SSD manager from Tauranga hospital and Aileen Derby, SSD manager from Middlemore Hospital. All these new members bring a wealth of sterilising knowledge and experience to the executive. Aileen has graciously agreed to be the Vice president of the NZSSA, and Paul has taken on the role of secretary of the association.
Without having a conference to attend the NZSSA were acutely aware that members would be missing out on educational updates and recent innovations and best practice as well as updates on standards.

To that end we successfully ran leaders meetings via zoom and had attendees from both Australia and NZ.
Education continues to be a major focus of the NZSSA. The courses continue to attract new students from the workforce. For those working in the new environment of Te Whatu Ora and who are paid under a MECA agreement, where you are required to complete the L4 course your employer pays this now rather than you having to pay upfront and try to recover your costs. The NZSSA will be looking to have more regular meetings with Toi Ohomai on our student’s progress and the industry needs going forward.
Tying into education, as president of the NZSSA I was recently invited to be a regular participant on a new committee set up by Sue Waters who is the National Director for Allied health Scientific and technical professions at Te Whatu Ora. Staffing requirements and immigration and local training were on that agenda. Whilst SSD were not the priority profession at this time, it has been minuted that there are difficulties in attracting and employing qualified technicians. One option that is being looked at for the next meeting is better integration with the training and course providers and whether we can open up courses to train technicians without employment and have them in our departments as students or offer a scholarship to study (Te Whatu Ora funded) and then have the technician bonded for a period of time post study. There is a lot going on behind the scenes.
Your executive has been looking at registration, membership, and scope of practice.
Membership is the annual fee paid to belong to an association and is mandated in union contracts to be paid by the employer. Registration is the confusing word for many members. Currently it is the submitting of a Bi-annual professional portfolio for assessment to demonstrate ongoing personal professional development and growth. The NZSSA have discussed changing the portfolio submission to every three (3) years in line with other professions and renaming it to professional development recognition programme (PDRP). We are looking at 2-3 levels of PDRP similar to that of other professions and lining up with career frameworks e.g. competent level for new grads to two year post grad. Expert for those 3-5 years plus post grad and expert for those 5 years plus and with diploma level qualifications. Then there would be a level for those in team leader and managerial roles. As you can gather there is a lot of work to do linking this all into the scope of practice and competencies for sterile technicians. We hope to have the first draft out by June 2023.
The NZSSA have also been looking at the where to from here as a professional group. We have discussed the option of being accepted as a profession under the HPCA, however discussions at a higher level appear to suggest that this would not occur for some years to come and a financial cost. It has been suggested that the NZSSA becomes a self-regulating profession, and we are going to put some effort into developing in that area.
I need to remind you all the standard AS/NZS4187:2014 is still the current standard for best practice that we are required to work to. When we are audited under the Health and Disability Act Clause, clause 5.5.8 refers to our current standard (see below):
5.5.8 - Service providers are able to demonstrate, via evidence of a completed audit across their sterile services in alignment with Standards AS/NZS 4815-2006 and AS/NZS 4187-2014 (or newest version), that equipment has been sterilised prior to use and/or corrective actions have been identified.
When the draft standard AS 5369:2021 goes through final public comment it shall be published as AS5369:202XReprocessing of reusable medical devices and other devices in health and non-health related facilities.
When this occurs, we shall announce it on the website and all facilities shall be expected to obtain a copy of this document and implement it, in the workplace.
2022-23 is expected to be an extremely busy working year for the NZSSA. We are meeting online as a team every six weeks in order to keep the work momentum going, so expect to see more news items on the website.
Already we have booked in next year’s conference for September to be held in Wellington. We are hoping to be able to secure the new convention centre which is across the road from Te Papa.
I would like to thank all of our trades who continue to support us and appreciate that they are also going through trying times. I would like to thank the executive for the dedication and the free time they give due to their passion for the profession, and I would like to thank you the members for supporting your profession.
I would like to end by reminding you all that you are the professionals in Sterilising Technology. You know what we do and why we do it. Your voices and your knowledge count. When something is wrong or you are asked to do something that you know is wrong, speak up and make change for the better.
Shelagh Thomas President NZSSAHow fantastic to be heading to Otautahi, Christchurch where, after over two years of online Teams meetings, we finally get to greet each other in person again. As much as virtual meetings can be efficient and save time, the chance for the executive to sit down and focus on association business for an entire day is so important for planning its future direction. With five brand new members in the executive this year, we are very fortunate to also have the returning experience of Jenny Carston and Aileen Derby and I am looking forward to working with the entire team to ensure the association is adding value to our members and to our industry.
Congratulations to all our members who have bravely taken on further education amongst all the disruptions, shortages, and strains of covid times. Upskilling to achieve level 4 and level 5 diplomas is an immense achievement and a great investment in the future. Please take the time to read the list of this year’s recipients included in the annual report and congratulate them on their success.
Finally, if you have any thoughts or queries regarding the association, things we could do better or things that are working well, please feel free to contact myself or any of the executive team.
Nga mihi.
Paul Moody Secretary, NZSSAThe figures presented in this report are as at 31 March 2022 for the financial year 2021 – 2022. The accounts were prepared by McIntyre Dick & Partners and audited by Accounting for Charities Trust.
The Association remains in a strong financial position. The Executive have managed expenses well during this period of disruption where natural building of equity has been stalled.
The accounts operate on an accrual system in line with the Association reporting as a Tier 3 registered charity.
•
•
•
no conference income for two years there has been two consecutive
years of
Financial year 2020 – 2021
Financial year 2021 – 2022 Deficit
3,894
The solid foundation has meant that the Association has been able to meet its commitments in relation to scholarships without utilizing invested funds. With resumption of the conference and other activities the trend into profit is anticipated.
Each year the Association offers scholarships for conference attendance and education to Registered Members.
Each year the Association allocates funds for up two (2) registered members to study in the NZ Diploma in Sterilising Technology (Level 5). The calibre of appli cant has been such that this scholarship has been awarded to at least one member every year.


The Association allocates funds for up to four (4) reg istered members to attend the NZSSA conference. This scholarship has a low take up and it would be interest ing to understand why.
Allocation to international conferences has been made in the past and will be considered in the future when the matter of Covid settles.
“The Strength of the NZSSA comes through its membership.”
It is the opinion of the auditor that the performance report gives a true and fair view, in accordance with Public Benefit Entity Simple Format Reporting –Accrual (Not-For-Profit).
This opinion is based on the belief that the audit evidence obtained was sufficient and appropriate. The audit was conducted in accordance with International Standards on Auditing (ISAs).

Key audit matters are those of most significance in the audit of the performance report for the current period (1/4/2021 – 31/03/2022). Those matters were addressed in the context of the audit as a whole and informing the auditors opinion and a separate opinion is not provided on these matters.
In the opinion of the Executive Committee the Association is a going concern for the foreseeable future.
This summary presents comments on key areas within the financial statement.
The membership continues to rest just below 600 financial members. Reminders are sent out electronically where practical. Some members do not provide emails. Members are held on the books for 12 months after expiry to allow for capture via employer or individual renewal.
Where the employer bulk invoices does mean this income stream is more robust and consistent. The focus in the coming year is to get all members renewed within 3 months of their expiry month.
The companies continue to support the Association through advertising in the journal. Advertising takes the form of regular entry of business cards and full or part page advertising throughout the journal.
The new editor for the journal is going to be proactive in increasing the advertising as this supports the activities of the Association and provides exposure of companies to the membership.
The budget for education scholarships is approximately $13,000 per annum. The actual spend varies per year depending on whether the recipient enrolls in all six courses at one time or in each course individually. This means the $6,500 per recipient may be spread across more than one financial year. The spend in 2021-2022 was $8,360.
Conference scholarships have not been taken up in 2022 but these will be offered again in 2023. They will be for the national conference and international conferences will be considered closer to the time.
Getinge’s manual instrument tracking system that allows you to record every step in the sterile supply workflow.

• Easy-to-use system to help comply with AS/NZS 4187:2014 Standards
• Record and track data on single instruments and instrument trays
• Components available to track the entire sterile supply flow
• Entry level alternative to electronic traceability solutions
more information email sales.nz@getinge.com or call 0800 143 8464

expose them to additional risks over and above what would be procedure?
Patient safety must always be prioritised.
Kaylene Visser RN, RM, PGDip RN Prescriber, CAT NZVS Quality Manager and Consulting Auditor www.nzvalidation.co.nz

Kaylene Visser RN, RM, PGDip RN Presciber, CAT NZVS Quality Manager and Consulting Auditor www.nzvalidation.co.nz
Kaylene champions the health and wellbeing of the people of Aotearoa
Kaylene champions the health and wellbeing of the people of Aotearoa
There is robust discussion around the reprocessing of single use devices in NZ and worldwide. Advocates for environmental sustainability
that this procedure is available to patients with cardiac arrythmias. A proposed solution in a cash-strapped public health care system is to reprocess the EP Catheter used in this procedure despite the fact it is classified by the manufacturer as single use.
AS/NZS 4187: 2014. Reprocessing of reusable medical devices in health service organi Biotronik AlCath Instructions for Use
discussion around the reprocessing of single use devices in NZ and worldwide. environmental sustainability promote a change to how we view the treatment of single that have been classified by the manufact urer as single use. Many surgical category and for the sake of this discussion piece, it is important that we focus on Let’s review Single Use Live Wire Electrophysiology (EP) cardiac catheters and reprocessing of these devices.
a change to how we view the treatment of single use surgical devices that have been classified by the manufacturer as single use. Many surgical devices fit into this category and for the sake of this discussion piece, it is important that we focus on a specific product. Let’s review Single Use Live Wire Electrophysiology (EP) cardiac catheters and thoughts around the reprocessing of these devices.
The Biotronik AlCath and the Livewire Steerable EP Catheter IFUs apply cautions: “DO NOT Reuse this device. Thorough cleaning of biological and foreign material is not possible. Adverse patient reactions may result from reuse of this device.”
DR AS 5369: 2021 Reprocessing of reusable medical devices a nd other devices in health health related facilities
Health & Disability Commissioner (1996). Code of Health and Disability Services Consumers’ Retrieved from Code of Health and Disability Services Consumers' Rights Health and Commissioner (hdc.org.nz)
used to diagnose and treat cardiac arrythmias. EP catheters are devices with are advanced through the venous system, into the heart where electrical activity recorded.
EP Catheters are used to diagnose and treat cardiac arrythmias. EP catheters are devices with insulated wires that are advanced through the venous system, into the heart where electrical activity can be delivered and recorded. Cardiac catheter ablation, a technique where radiofrequency energy is delivered to heart tissue to destroy cells causing arrythmia, is considered amongst physicians as a highly effective intervention for improving symptoms associated with some cardiac arrythmias. A reduction in cardiovascular hospitalisations and an appreciable improvement in quality of life for the patient are cited as beneficial outcomes (Sana et al 2020).
The manufacturer clearly identifies infection control issues associated with the reprocessing of these devices. Further to this, they are used in ablation procedures where metal components conduct energy to cardiac tissue. Could the reprocessing of this kind of single use device lead to device failure? If the physical material and design characteristics associated with the device are thus compromised, could this cause unintended physiological injury to the patient?
Livewire Steerable Electrophysiology Catheter. Instructions for use. Medsafe (2011) Risk Classification of Medical Devices Retrieved from Risk Classification Devices (medsafe.govt.nz)
NZS 8134: 2021 Ngā paerewa Health and disability services standard Wilson, A. L., Timmins, K. A., Allen, R. F., Jones, P. M., Ware, S. L., Boddington, Heald, S. C., & Stiles, M. K. (2020). Initial experience with a novel re -sterilisable
Single use cardiac catheters can cost more than $2000. It seems that we are poised in the centre of a seesaw, considering whether to balance ourselves toward patient benefit or toward withholding of ablation interventions, due to the cost of a single (ablation) procedure. This is the point when we start losing our balance. Physicians who value the benefits of an ablation procedure, are persuasive in their argument to ensure
ablation, a technique where radiofrequency energy is delivered to heart tissue to arrythmia, is considered amongst physicians as a highly effective intervention symptoms associated with some cardiac arrythmias. A reduction in cardiovascular an appreciabl e improvement in quality of life for the patient are cited as (Sana et al 2020).
of interventional cardiac electrophysiology:
of arrhythmias
While Medsafe has a risk classification that categorises a cardiac catheter guide as lla (Medium – Low) risk, this risk has been formulated on the assumption that IFU requirements have been met. Medsafe recognises the cardiac catheter as a single use device. How does the practice of reprocessing affect the patient risk categorisation? In addition, the IFU establishes that Ethylene Oxide (ETO) gas sterilisation method is to be used, to protect the catheter materials. This technology is no longer offered in most NZ Health Service
(2), 177–183. https://doi.org/10.1007/s10840 Sana M. Al-Khatib, MD, MHS, Emelia J. Benjamin, MD, ScM, Alfred E. Buxton, MD, Hugh Calkins, MD, Mina K. Chung, MD, Anne B. Curtis, MD, Patrice Nickens, MD, Pierre Jais, MD, Douglas L. Packer, MD, Jonathan P. Piccini, MD, MHS, Yves Rosenberg, MD, MPH, Andrea M. Russo, MD, Paul J. Wang, MD, Lawton MPH, Alan S. Go, MD, and Workshop Collaborators. Research Needs and Priorities Ablation of Atrial Fibrillation (2020) Circulation Volume 141, Issue 6, 11 February 2020; 492 https://doi.org/10.1161/CIRCULATIONAHA.119.042706
catheters can cost more th at we are poised in see saw, considering ourselves toward toward withholding of interventions, due to the cost of a procedure. This is the losing our bala nce. the benefits of an are persuasive in their that this procedure is patients with cardiac
Organisations, in part related to the risk to personnel. Can we be sure that other available sterilisation methods will not have an adverse effect on the device?
A literary search revealed that there is an international acknowledgment of the expense of single use EP Catheters. Wilson et al (2020) discuss a novel resterilisable decapolar electrophysiology catheter to mitigate the financial barrier to its use. A re-sterilisable handle and inner core was developed with a disposable outer sheath. This technology appears to be still in the testing and review stages.
AS/NZS 4187: 2014, is the guiding standard for sterile service facilities in NZ and Australia. The stance of the standard is that the reprocessing of single use medical devices is a regulated manufacturing-only activity. A review of the draft AS 5369 documents, due to replace AS/NZS 4187 reveals that parameters around the reprocessing of single use devices are prescriptive and the manufacturer’s information for use must guide reprocessing activities.
AS/NZS 4187: 2014 2.4.3.1 requires that reusable medical devices can be tracked to the bedside. Can this be achieved if there are no device-specific serial numbers? Is it feasible to add these devices into a traceability software system?
NZS 8134: 2021 Health and Disabilities Services Standard 5.2.11 advises that a single use medical device shall not be reused or remanufactured unless a formal risk assessment process has been followed, documented, and approved by the governance. To my knowledge there is no evidence that formal rigorous microbial surveillance studies or electrical conduction tests have been performed on these devices. Further to this, there is no evidence of an evaluated system of investigations to determine a precise number of ‘safe’ reprocessing episodes that these devices may be exposed to.
In conclusion, it is important that we benchmark all health service activities against Best Practice Standards. NZS 8134: 2021 standard includes ‘Partners with Choice and Control’. The right of the patient to work with professionals to improve service quality, safety and equity of health and well-being outcomes. This includes Section 5: Infection Prevention and Antimicrobial Stewardship. Health care professionals are required to be transparent in the risk vs benefit assessment process. In addition, the code of rights establishes that services comply with standards and that treatment benefits and risks are fully explained to the patient. Further to this, AS/NZS 4187: 2014 requires that Patient outcomes must be priority.
If the reprocessing of Single Use cardiac catheters was to be considered by a Health Service Organisation, factors for consideration are:
• Have there been rigorous microbial surveillance and electrical conduction / insulation tests performed to support the safety of reprocessing single use EP Catheters?
• Are the available sterilisation methods suitable to preserve the functional integrity of the device?
• Have best practice and innovative practices been investigated to promote patient safety in this area?
• Can a reprocessed single use device be tracked to the bedside to enable the tracing of non-conforming devices?
• Is there a need for open disclosure to the patient if this practice were to occur? Does the patient need to understand that the reprocessing of single use EP catheters may expose them to additional risks over and above what would be expected for the procedure?
Patient safety must always be prioritised.
AS/NZS 4187: 2014. Reprocessing of reusable medical devices in health service organisations.
Biotronik AlCath Instructions for Use
DR AS 5369: 2021 Reprocessing of reusable medical devices and other devices in health and non-health related facilities
Health & Disability Commissioner (1996). Code of Health and Disability Services Consumers’ Rights. Retrieved from Code of Health and Disability Services Consumers’ Rights - Health and Disability Commissioner (hdc.org.nz)
Livewire Steerable Electrophysiology Catheter. Instructions for use. Medsafe (2011) Risk Classification of Medical Devices Retrieved from Risk Classification of Medical Devices (medsafe.govt.nz)
NZS 8134: 2021 Ngā paerewa Health and disability services standard Wilson, A. L., Timmins, K. A., Allen, R. F., Jones, P. M., Ware, S. L., Boddington, D., Swampillai, J., Heald, S. C., & Stiles, M. K. (2020). Initial experience with a novel re-sterilisable decapolar electrophysiology catheter. Journal of interventional cardiac electrophysiology: an international journal of arrhythmias and pacing, 58(2), 177–183. https://doi.org/10.1007/s10840-019-00583-2
Sana M. Al-Khatib, MD, MHS, Emelia J. Benjamin, MD, ScM, Alfred E. Buxton, MD, Hugh Calkins, MD, Mina K. Chung, MD, Anne B. Curtis, MD, Patrice Desvigne-Nickens, MD, Pierre Jais, MD, Douglas L. Packer, MD, Jonathan P. Piccini, MD, MHS, Yves Rosenberg, MD, MPH, Andrea M. Russo, MD, Paul J. Wang, MD, Lawton S. Cooper, MD, MPH, Alan S. Go, MD, and Workshop Collaborators. Research Needs and Priorities for Catheter Ablation of Atrial Fibrillation (2020) Circulation Volume 141, Issue 6, 11 February 2020; Pages 482-492 https://doi.org/10.1161/CIRCULATIONAHA.119.042706
























Level 4
Technology Course Certificate
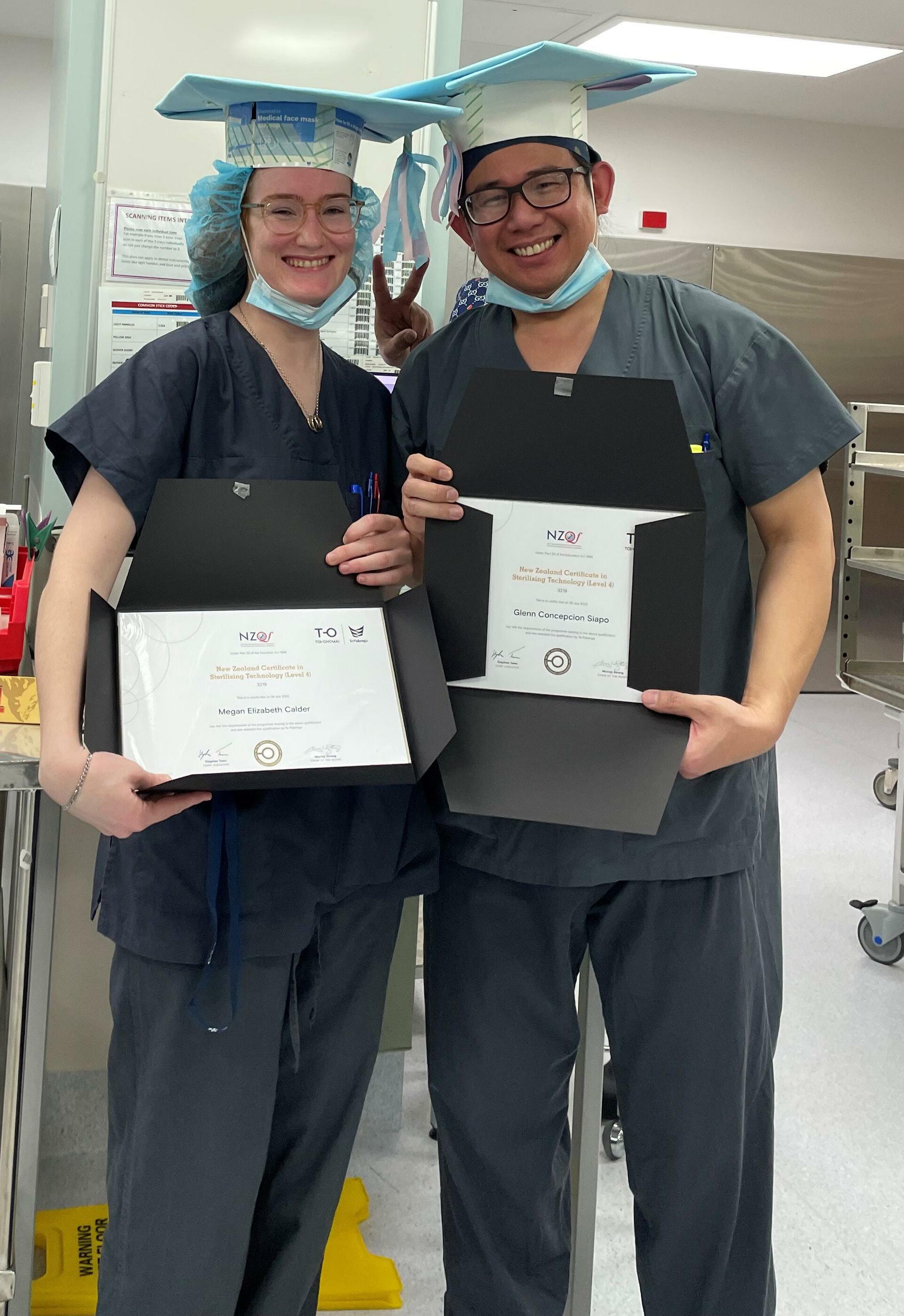
Congratulations to three staff from the Sterile Services Department at Wellington Hospital. Megan Calder, Glenn Siapo and Louie Pingol have all graduated and completed their studies for the Level 4 Sterilising Technology Course Certificate.




Objectives: To assess the efficacy of a standard operational procedure to clean flexible intramedullary bone reamers, as well as the sterilization level, and to show the cytotoxicity of the residual dirtiness of a flexible reamer used in care practice. Methods: Flexible intramedullary bone reamers were weighed before processing, after challenge contamination and after cleaning. They were contaminated with the Soil Test™, Geobacillus stearothermophilus suspension, in the concentration of 106 cfu/ml, and bovine bone flour. After processing, the samples were inoculated into a culture medium and incubated for 21 days. Residual dirtiness of a flexible intramedullary bone reamer used in practice was submitted to in vitro cytotoxicity test.
Results: Despite being sterilized, the samples indicate to accumulated dirtiness and the processing was inefficient. Residual dirtiness presented a cytotoxic effect.
Conclusion: It is recommended that the flexible design of reamers is discontinued by the lack of safety of reprocessing.
Keywords: Nursing. Orthopedics. Sterilization.
Flexible intramedullary bone reamers are medical devices that can be reprocessed, besides being thermoresistant, with complex conformation. They present extreme cleaning diffi-culties. These products are constituted of stainless steel and are characterized by a shaft whose flexibility is provided by two stainless steel ribbons overlapped in a spiral shape, one spiralled
clockwise and another one spiralled counter-clock-wise, forming a flexible structure similar to a coil. This shaft is connected to a tip developed to ream the surface of the intramedullary canal of long bones.
The difficulty in cleaning is attributed not only to its conformation, but also to the dirtiness resulting from the surgi-cal procedure itself, which includes blood, bone, and bone marrow. This dirtiness is spread over the shaft, and most of it is retained in the space between the two steel ribbons, especially in the extremities (with lower flexibility), making it difficult to remove it, since this space is inaccessible to the artifacts and technologies available for cleaning.
The literature about flexible intramedullary bone reamers is concentrated on functional aspects and is scarce as to the safety in cleaning and sterilization. This is a matter of concern, once the scientific literature reports the survival of microorganisms in its vegetative form in the instruments used for orthopedic surgeries after steam sterilization, due to flaws in cleaning.
In 1999, a report was published on three cases of Staphylococcus epidermidis septic arthritis. The authors iden-tified dry organic matter in cannulas that would be used in orthopedic procedures1. In another study, published in 2009, the authors found organic matter in lumens, with positive culture for coagulase-negative Staphylococcus, S. epidermidis, and Streptococcus mitis2. In 2011, an outbreak of Pseudomonas aeruginosa was also associated with flaws in the reprocessing of orthopedic surgery instruments, which had residues of organic matter3.
Besides the risks related to the infection, associated with the difficulty or the impossibility of cleaning, the toxicity
1 Nurse. PhD in Sciences, Nursing School at Universidade de São Paulo – São Paulo (SP), Brazil. E-mail: rafaelqsouza@hotmail.com
2 Nurse. PhD student at the Nursing School at Universidade de São Paulo – São Paulo (SP), Brasil. E-mail: jeanebronzatti@usp.br
3 Electrical engineer. PhD student at the Nursing School at Universidade de São Paulo –São Paulo (SP), Brazil. E-mail: prlaranjeira@usp.br
4 Doctor. Professor, Associated Professor of Microbiology. Department of Pathological Sciences, School of Medical Sciences at Santa-Casa de São Paulo – São Paulo (SP), Brazil. E-mail: lmimica@uol.com.br
5 Pharmacist Biochemist, Master’s degree in Sciences, Service of Hospital Infection Control – Irmandade Santa-Casa de Misericórdia de São Paulo – São Paulo (SP), Brazil. E-mail: cely.silva@santacasasp.org.br
6 Biologist. Scientific Research VI, PhD at Universidade de Ciências Farmacêuticas, in Universidade de São Paulo, Instituto Adolfo Lutz – São Paulo (SP), Brazil. E-mail: aurcruz@ial.sp.gov.br
7 Nurse. Senior Professor at the Department of Medical-Surgical Nursing in the Nursing School of Universidade de São Paulo, and Pedagogical Coordinator of the MBA Course of Management of a Central Sterile Supply Department, Instituto Nacional de Ensino e Pesquisa – São Paulo (SP), Brazil. E-mail: kugrazia@usp.br
Rafael Queiroz
of residual dirtiness contained inside the reamers has not been shown yet, and the impact of these residues in the occurrence of local and systemic inflammatory processes is not known.
On the basis of these reports, this article aims at asses-sing the efficiency of a standard operating procedure (SOP) for cleaning flexible intramedullary bone reamers, as well as the sterilization level, besides showing the cytotoxicity of residual dirtiness in a flexible intramedullary bone reamer used in care practice.
This is a laboratory experimental study conducted in two stages, between 2015 and 2016, and carried out in the following laboratories: Laboratory of Microbiological Trials, in the Nursing School of Universidade de São Paulo, Laboratory of Microbiology in the Pathology Department of the School of Medical Sciences at SantaCasa de São Paulo, and the Group of Cell Culture in Instituto Adolfo Lutz (São Paulo).
Samples were flexible intramedullary bone reamers for the humerus, 27.5 mm long and with internal diameter of 0.4 mm (Tech Tools®, Brazil) (Figure 1). In this evaluation, three newly manufactured reamers were used, which had not been used in care practice. They were identified by the colors, green, blue, and red.
To evaluate the cleaning, each reamer was weighted in three moments: before each reprocessing (basal weight), after the challenge contamination, and after cleaning. So, the accu-mulated value was calculated, given by the difference between weight after cleaning and basal weight, using a weigh digital scale (0.01g sensitivity) (Shimadzu Corp., Japan). To simu-late its care use, each sample was submitted to a challenge contamination, internally and externally, with Soil Test™, and Geobacillus stearothermophilus suspension in the concen-tration of 106 cfu/ml, containing spores.

After being contaminated with the solution, the samples were contaminated, internally and externally, with
bovine bone flour (~3,5 g), simulating the bone residue at the end of a surgical procedure4. Contact with contaminants was main-tained for 3 hours, which is the estimated time of a surgical procedure. After this period, the samples were processed according to SOP: prehumectation in tap water for 5 minu-tes; brushing of the external shaft surfaces (Mack Medical®, Brazil); brushing of the external surfaces of the tip (Mack Medical®, Brazil); clearance of the lumen with a guidewire (when necessary); lumen washing with a pressure water gun (RFQ®, Germany), for 5 seconds, or until it is no longer obstructed; brushing the lumen five times (Mack Medical®, Brazil); rinsing in tap water; washing with enzymatic deter-gent Endozime™ Xtreme Power (Ruhof®, United States of America) in ultrasonic cleaner (Medisafe® SI Digital, United Kingdom) with cannulated instrument connections at 50ºC for 5 minutes; rinsing in tap water; complementary rinsing with purified water; drying the internal surfaces with filtered compressed air; inspection; individually wrapped in surgical grade papers/ film (Amcor®, Australia); and sterilization in autoclave at 135ºC for 4 minutes (Tuttnauer®, Israel).
After sterilization, the samples were inoculated in 250-ml test tubes, with sterile tryptic soy broth medium (BD®, United States of America). This procedure was carried out with an aseptic technique, inside a biological safety cabinet. Then, the samples were incubated at 56°C for 21 days, with daily reading of the recovery of Geobacillus stearothermophilus.
These procedures were repeated three times to simulate three reuses of each sample. To test the validity of the results, the simulated reuses were followed-up with a negative con-trol, that is, a new reamer without a challenge contamination, submitted to reprocessing and incubated in a sterile tryptic soy broth medium for 21 days. We also used a positive control, that is, a reamer submitted to the challenge contamination and incubated in a tryptic soy broth medium right after – both at 56ºC.
In vitro cytotoxicity assays are methods aiming at determining the biological response of mammal in vitro cells, through defi-ned biological parameters, constituting an attempt to simu-late or exaggerate conditions of clinical use, in order to show toxic risk5. In this stage, a flexible intramedullary reamer for femur was used after eight reuses in care practice. The external steel ribbon was removed, and the dirtiness adhered to the internal ribbon was collected, aseptically, in a test tube. After this procedure, the in vitro cytotoxicity test of the resi-dual was conducted, using the agar diffusion test. Triplicate analyses were carried out.
This assay used the cell line National Collection of Type Cultures (NCTC) clone 929 (L cell, L-929, derivative of Strain L) (American Type Culture Collection® CCL 1™),
elaborated SOP, based on updated scientific references and pertinent regulation7. This study used manual and automatic cleaning methods, with demonstrated efficacy, but still, the confor-mation of the reamers did not allow the complete removal of dirtiness.
Residual dirtiness obtained in the flexible intramedul lary reamer for femur pointed out to a cytotoxic effect with degree 3 of biological reactivity, average of 0.31 cm of halo in the triplicates, that is, moderate cytotoxic effect.
registered in the collection of the Group of Cell Culture in Instituto Adolfo Lutz, number CCIAL020. This test used the following procedures: NCTC clone 929 cells were seeded in Petri dishes, treated for cellular cultures, measuring 60x15 mm (TPP®, Switzerland), in a concentration of 3×105 cells/mL and volume of 5 mL. The cultures were incubated for 48 hours at 37ºC±1ºC, in an atmosphere containing 5% of CO2. After this period, cellular monolayers were assessed as to confluence, and the culture medium was replaced by an overlay medium composed of a twice concentrated Eagle’s medium and agar (BD®, United States of America) at 1.8% with 0.01% of neutral red vital stain. In preparation6, agar was proportionally mixed (1:1) to the Eagle’s medium, both at 44°C. Cellular toxicity was observed in a microscope by the alteration of morphology or the death of cells around or under the sample; and, macroscopically, for the formation of the colorless halo around the cytotoxic material6. After the measurement of the extension of the colorless halo taken from the sample, the cytotoxicity was classified according to the levels of reactivity for the agar diffusion test described in ISO 10.993-5:2009 standard5.
Brazilian legislation demands that each stage of surgical instrument reprocessing be conducted using an elaborated SOP, based on updated scientific references and pertinent regulation7. This study used manual and automatic cleaning methods, with demonstrated efficacy, but still, the confor mation of the reamers did not allow the complete removal of dirtiness.
The cleaning SOP used was inefficient, as it was not able to completely remove the dirtiness of the samples. The mean difference in the weight of the samples after the reuses was of 0.30 g (Table 1).
In complex conformation instruments, the impossibility to totally remove the dirt was also observed in a study con ducted with instruments used for minimally invasive surge ries, which used the contaminant ATS® (Artificial Test Soil), composed of 85.2 mg/mL of protein, 12.3 mg/mL of car bohydrates, and 4.12 mg/mL of hemoglobin. In this case, the authors obtained a reduction of 99% in contaminants after the ultrasonic cleaning of the samples8.
Even though the SOP had failed, the required sterilization level was observed in all samples; therefore, no turbi-dity was observed in the culture medium at the end of the 21-day-incubation period. The results of the negative and positive control were in accordance with expectancies for the three simulated reuses.
Residual dirtiness obtained in the flexible intramedul-lary reamer for femur pointed out to a cytotoxic effect with degree 3 of biological reactivity, average of 0.31 cm of halo in the triplicates, that is, moderate cytotoxic effect.
The quantification of insoluble residues is a method that has been described in the literature to assess the dirtiness in the orthopedic instrument. An investigation from 2012 used the membrane filtration technique and the weighing of inso luble organic matter (clotted blood and bovine bone flour) in products with lumen for orthopedic surgeries9. In this study, this technique was not feasible due to the internal space bet ween the metallic ribbons constituting the body of the rea mers, which did not allow the elution of insoluble dirt; the refore, to perform residues control using the total weight of the samples was necessary.
Brazilian legislation demands that each stage of surgical instrument reprocessing be conducted using an
In complex conformation instruments, the impossibility to totally remove the dirt was also observed in a study con-ducted with instruments used for minimally invasive surge-ries, which used the contaminant ATS® (Artificial Test Soil), composed of 85.2 mg/mL of protein, 12.3 mg/ mL of car-bohydrates, and 4.12 mg/mL of hemoglobin. In this case, the authors obtained a reduction of 99% in contaminants after the ultrasonic cleaning of the samples8.
The same study analyzed residues in orthopedic devi ces with lumen, which were artificially contaminated with bone cement, and observed the retention of residues after ten cycles of contamination and cleaning 9 . The authors concluded that the complexity of the design influences the retention of dirt, similarly to what was observed for flexible intramedullary reamers.
Because of the design of the flexible reamer, the SOP included several manual steps during the cleaning stage, which would require special attention from the cleaning staff. During the experiments, only the application of the cleaning stages, including drying, required approximately 15 minutes for each sample.
The quantification of insoluble residues is a method that has been described in the literature to assess the dirtiness in the orthopedic instrument. An investigation from 2012 used the membrane filtration technique and the weighing of inso-luble organic matter (clotted blood and bovine bone flour) in products with lumen for orthopedic surgeries9. In this study, this technique was not feasible due to the internal space bet-ween the metallic ribbons constituting the body of the rea-mers, which did not allow the elution of insoluble dirt; therefore, to perform residues control using the total weight of the samples was necessary.
In the daily routine of a central sterile service depart ment (CSSD), especially in major general hospitals, with great diversity in instruments, it would be impossible for an employee to spend 45 minutes of his or her six-hour shift in only three products. Besides cleaning, other activities are also required, like receiving, checking, separating, disassembling, and referring the products.
The same study analyzed residues in orthopedic devi-ces with lumen, which were artificially contaminated with bone cement, and observed the retention of residues after ten cycles of contamination and cleaning9. The authors concluded that the complexity of the design influences the retention of dirt, similarly to what was observed for flexible intramedullary reamers.
In most cases, SOPs are validated in laboratory condi tions, without considering the dynamics of work processes or the dimension of human resources and infrastructure. Even though the products of complex conformation require more elaborated SOPs, it is necessary to observe that a very long SOP, with many manual cleaning steps, may lead to the non-adherence to all of the stages, especially at times with higher demand in the cleaning area of the CSSD.
Because of the design of the flexible reamer, the SOP included several manual steps during the cleaning stage, which would require special attention from the cleaning staff. During the experiments, only the application of the cleaning stages, including drying, required approximately 15 minutes for each sample.
Non-systematic observations show that the services tend to sacrifice the efficacy and favor the efficiency of processes. In other words, the high demand for production, added to the deficit in infrastructure and human resources, may lead to the suppression of important steps of product cleaning. Therefore, it is urgent that health product manufacturers prio ritize not only functionality, but also the effective processing, investing in more accessible design for manual or automatic cleaning stages, such as semi-rigid reamers.
In the daily routine of a central sterile service department (CSSD), especially in major general hospitals, with great diversity in instruments, it would be impossible for an employee to spend 45 minutes of his or her sixhour shift in only three products. Besides cleaning, other activities are also required, like receiving, checking,
separating, disassembling, and referring the products.
In most cases, SOPs are validated in laboratory conditions, without considering the dynamics of work processes or the dimension of human resources and infrastructure. Even though the products of complex conformation require more elaborated SOPs, it is necessary to observe that a very long SOP, with many manual cleaning steps, may lead to the non-adherence to all of the stages, especially at times with higher demand in the cleaning area of the CSSD.
Non-systematic observations show that the services tend to sacrifice the efficacy and favor the efficiency of processes. In other words, the high demand for production, added to the deficit in infrastructure and human resources, may lead to the suppression of important steps of product cleaning. Therefore, it is urgent that health product manufacturers prio-ritize not only functionality, but also the effective processing, investing in more accessible design for manual or automatic cleaning stages, such as semi-rigid reamers.
The main facilitator for the management of SOPs in the CSSD is the accessible design for cleaning. The management of SOPs by brand and type of product is required; however, in a major general hospital that works with several surgical specialties, there would be an excessive number of SOPs in the cleaning area, which could prevent its application because of reasons related to the efficiency of processes and the ratio-nalization of work. An alternative that should be discussed in the scientific field is to advance from a SOP addressed to each type of product to the categorization of products by design, which would have a standard SOP. As an example, SOP for <5 mm lumen products and SOP for conventional surgical tweezers would make it easier to separate and direct the workflow in the cleaning area, whereas specific SOPs would be addressed to exotic or delicate products, such as products with electronic components and hydrodissection cannulas for ophthalmological surgery.
Unlike the expected, the samples, even with residual dirti-ness, did not allow the recovery of microorganisms. A similar study contaminated the cannulas for meniscus repair with 0.5 mL of blood containing 200–500 cfu of coagulase-negative Staphylococcus, which were tested in three SOPs:
1. Manual cleaning and rinsing in the operating room, sterilization in the unwrapped flash cycle (132ºC for 10 minutes);
2. Cleaning and rinsing with enzymatic detergent, pressure water jet, sterilization (132ºC for 45 minutes);
3. Cleaning in ultrasonic cleaner, sterilization (132ºC for 45 minutes).
No microorganism was recovered after these procedu-
res; however, the authors still observed blood in the cannulas processed in SOPs 1 and 21. These results reinforce the pos-sibility of a product being without viable microorganisms, even with the adhered organic matter, although it cannot be considered safe for use due to the biological response, such as systemic inflammatory response syndromes and toxic anterior segment syndrome.
Another study showed dirtiness in cannulated products from the DePuy Mitek® Intrafix system, with positive culture for coagulase-negative Staphylococcus, S. epidermidis and S. mitis. In this study, the authors identified that the CSSD did not have brushes with the proper diameter to remove the dirt of the instrument2, and this fact indicates a major deviation in good practices of health product processing. There was no mention to the monitoring of the steam sterilization process, so it was not possible to make other interpretations of the results obtained. In 2011, during an outbreak of infections associated with flaws in the processing of orthopedic surgery instruments, the authors mentioned residues of organic matter and brush bristles in the products, besides the non-adherence to good practices, as the arthroscopy cannulas were only washed with tap water. Another important aspect is that, even with the observance of the SOPs provided by the manufacturer, some products still had residues of organic matter3. In this study, the fact that the residues were found in places whose visua-lization was only possible through a borescope was remar-kable. That means that the visual inspection of the external surfaces of the products with internal spaces was not effec-tive, reinforcing the need to invest in technologies of visua-lization in the preparation area, cleaning monitors, and qua-lification of automatic cleaning equipment, according to the Brazilian legislation7. Besides, the accessible design for clea-ning and the validation of the practical application of SOPs are important to mention, because, in the aforementioned study, the instructions of the manufacturer were not efficient.
Sterilization is a critical safety aspect of medical devices; howe-ver, it is not the only one, because, even if sterile, a product can be toxic to the body. In the processes of cleaning valida-tion, the possibility of reaching the “absolute zero” in organic residues is ruled out, even though reductions of about 99% have been reported8. Therefore, it is essential that the bio-logical response to that residual dirtiness, even if minor, be demonstrated, so that a processed product can be considered safe, as proposed by laboratory studies of SOP validation10,11.
In the obtained data, the toxicity of residual dirtiness obtained degree 3, which is therefore an unacceptable risk for use in surgical procedures, emphasizing the thesis that if a product cannot be cleaned, it cannot be safely reused.
The SOP used, elaborated in the best sequence of steps, in accordance with the practicable, was ineffective; flexible intra-medullary reamers did not show the recovery of Geobacillus stearothermophilus; however, residual dirtiness had a cytoto-xic effect. Therefore, the results sustain the discontinuity of the flexible design due to the lack of safety in the reproces-sing. It is important to mention that this fact should be con-sidering by the committees of health products reprocessing, and also by all surgical teams, product manufacturers, and regulating institutions.

To the company Tech Tools®, which provided the samples used in the trials.


1. Blevins FT, Salgado J, Wascher DC, Koster F. Septic arthritis following arthroscopic meniscus repair: a cluster of three cases. arthroscopy. 1999;15(1):35-40. doi: 10.1053/ar.1999.v15.015003
2. Parada Sa, Grassbaugh Ja, Devine JG, arrington eD. Instrumentation-specific infection after anterior cruciate ligament reconstruction. Sports Health. 2009;1(6):481-5. doi: 10.1177/1941738109347975
3. Tosh PK, Disbot M, Duffy JM, Boom Ml, Heseltine G, Srinivasan a, et al. Outbreak of Pseudomonas aeruginosa surgical site infections after arthroscopic procedures: Texas, 2009. Infect Control Hosp epidemiol. 2011;32(12):1179-86. doi: 10.1086/662712.
4. Haugen SP, Duraiswamy N, Hitchins vM. Quantification by mass of residual debris in reusable medical devices. Biomed Instrum Technol. 2012;Suppl:61-7. doi: 10.2345/0899-8205-12.1.61.
5. International Standard Organization. ISO 10993-5. 2009. Biological evaluation of medical devices part 5: tests for cytotoxicity: in vitro methods.
6. Rogero SO, lugão aB, Ikeda TI, Cruz aS. Teste in vitro de citotoxicidade: estudo comparativo entre duas metodologias. Materials Research. 2003;6(3):317-20. doi: 10.1590/S151614392003000300003
7. Brasil. Ministério da Saúde. Resolução da diretoria colegiada n.º 15, de 15 de março de 2012. Dispõe sobre requisitos de boas práticas para o processamento de produtos para saúde e dá outras providências. Brasília; 2012.
8. alfa MJ, Nemes R. Manual versus automated methods for cleaning reusable accessory devices used for minimally invasive surgical procedures. J Hosp Infect. 200458(1):50-8. doi: 10.1016/j. jhin.2004.04.025
9. lucas aD, Nagaraja S, Gordon ea, Hitchins vM. evaluating device design and cleanability of orthopedic device models contaminated with a clinically relevant bone test soil. Biomed Instrum Technol. 2015;49(5):354-62. doi: 10.2345/0899-8205-49.5.354.
10. Tamashiro NS, Souza RQ, Gonçalves CR, Ikeda TI, luz Ra, Cruz aS, et al. Cytotoxicity of cannulas for ophthalmic surgery after cleaning and sterilization: evaluation of the use of enzymatic detergent to remove residual ophthalmic viscosurgical device material. J Cataract Refract Surg. 2013;39(6):937-41. doi: 10.1016/j. jcrs.2012.12.039.
11. Souza RQ, Gonçalves CR, Ikeda TI, Cruz aS, Graziano Ku. The impact of the final rinse on the cytoxicity of critical products submitted for processing. Rev esc enferm uSP. 2015;49:87-92. doi: 10.1590/S0080-623420150000700013.
AS 5369 –
Reprocessing of reusable medical devices and other devices in health and non-health related facil ities
Combining AS/NZS 4187 & AS/ NZS 4815 standards. Commit tee currently reviewing public comments
Publication date to be con firmed
Until AS 5369 is published con tinue to refer to AS/NZS
4187 and AS/NZS 4815 as they are the current best practice standards.
NZS 8134:2021 Ngā paerewa New Zealand health & disability service standards
Infection Prevention & Control in Endoscopy 2021
This standard supersedes the previous 2008 suite of stan dards and came into effect on 28 February 2022.
The new standard is now avail able via Standards NZ: https:// www.standards.govt.nz/ search/doSearch?Search=8134
Services should be complet ing audit to identify the gaps in how they comply with this standard.
EN 16442, Controlled en vironment storage cabinet for processed thermola bile endoscopes
ISO 15883.5 Washer -dis infectors – Part 5 Perfor mance requirements and test method criteria for demonstrating cleaning efficacy
ISO 17664.1 Processing of health care prod ucts – information to be provided by the medical device manufacturer for the processing of medical devices – Part 1: Critical and semi-critical medical devices
ISO 17664.2 Processing of health care prod ucts – information to be provided by the medical device manufacturer for the processing of medical devices – Part 2: Non-crit ical medical devices
These guidelines supercede the previous editions of GENCA Infection prevention guidelines pertaining to endoscopy.
Can be purchased through Standards NZ or direct through EN standards
The guidelines have been made available by GESA: https:// www.gesa.org.au/education/ clinical-information/
Standard published in 2015
The document is available for download and services should audit how well they align with the guidelines.
To align with the revised IP&C Endoscopy guidelines this standard should be referred to when procurring controlled environment storage cabinets
Supercedes the 2005 docu ment
Can be purchased through Standards NZ or direct through ISO standards
Supercedes ISO 17664:2004 and 2017
There are now two parts to this standard
Standard published July 2021
Published July 2021
Supercedes ISO 17664:2004 and 2017
This is the second part
Published July 2021
In our National Standards AS/NZS 4187-2014 section A 9.5 f and gives us the ideal ranges of temperature and humidity supplied for the handling, transport and storage of released reprocessed RMD’s.
For temperature the range is 18°C to 25°C
For humidity the range is 35% to 70% relative humidity. These are generally comfortable ranges of temperature and humidity and allow you to have comfortable temperature depending on whether it is summer, and you want a cooler work area or winter and you want to have a slightly warmer work area or gives us a range that hopefully air conditioners of differing ages and performances can supply.
However while it seems simple there is always a potential impact over some part of the process depending on what you experience as your real temperature and humidity depending on how hot summer gets or how cold winter is and if your hospital air conditioning can cope with the wide range of conditions.
For example in the packing area if you have the temperature set at 18°C then the items you are packing are cool and will create more condensate inside the pack or container in the steam sterilizing process and make drying harder to achieve. I have found by practical experience and validation that items of large mass wrapped at 21 or 22°C are easier to dry than the same items at 18°C.
From a human point of view 18°C in summer seems a comfortable temperature when working hard but in winter it can feel cold. 22°C in winter can be very comfortable but in summer is oppressive.
But temperature alone is not the full picture. We always need to consider humidity and its possible effects on people, electronic equipment, testing consumables, loads.
First, we need to understand humidityRelative humidity is the ratio of the current absolute humidity to the highest possible absolute humidity (which depends on the current air temperature). A reading of 100 percent relative humidity means that the air is totally saturated with water vapor and cannot hold any more, creating the possibility of rain or inside a room environment a very wet feeling atmosphere and wet clothes.
People are very sensitive to humidity, as the skin relies on the air to get rid of body moisture. The process of sweating is our body’s attempt to keep cool and maintain its current temperature. If the air is at 100 percent relative humidity, sweat will not evaporate into the air. As a result, we feel much hotter than the actual temperature when the relative humidity is high. Your shirt may become saturated with perspiration that doesn’t evaporate, leaving you feeling fatigued, exhausted, dehydrated.
If the relative humidity is low, we can feel much cooler than the actual temperature because our sweat evaporates easily, cooling us off. For example, if the air temperature is 24°C and the relative humidity is zero percent, the
air temperature feels like 21 °C to our bodies. If the air temperature is 24 °C and the relative humidity is 100 percent, we feel like it’s 27 °C.
In New Zealand inside buildings our average range of humidity is 30% to 50% in a dry building and 50% to 70% in a damp building. An interesting side fact in a damp house at night time in winter the range of humidity is between 80% to 90% humidity.
Our design standard for ventilation in hospitals is AS/ NZS 1668 and while we don’t need to go into too much detail there are some basic rules for decontamination area, packing area, sterile stores.
• Dirty Utility & Infectious Rooms area air, under a negative air pressure (sucked to outside) not allowed to recirculate to other enclosures, 10 air changes per hour.
• Packing area and sterile stores supply air, not allowed to be re-circulated from other enclosure types, must be under a positive air pressure so dirty air can’t come in, air shall be filtered through a hepa filter of 99.9% filtering and there must be 10 litres or air per person and 2 litres of air per square metre every second.
In Europe it is considered best practice that the air in the packing area should be a higher pressure than in the stores area. The rational is that you need the cleanest possible air while the instruments are unwrapped and have the greatest risk of expose to contaminates.
It means that the hospital air conditioning system has the responsibility to control the temperature and humidity between a set range (that is generous) and it should be capable of doing this all year round in extreme summers and extreme winters and everywhere in between. Or in simple terms, every day that the department is open and used it must be correct. If it fails to control the temperature and humidity in the correct range for even one day then the system is faulty and needs to be improved. If not already recorded daily, departments should record the temperatures and humidity readings daily in these areas as part of the quality management system required in standards to monitor and prove that the hospital systems are correctly functioning.
In high humidity areas electronics will have a higher failure rate due to corrosion.
Consumables have an ideal range of temperature and humidity for storage and use and if you are outside that range they may become ineffective or faulty before the use by date.
Staff may require more frequent breaks, more hydration breaks, and in extreme examples suffer for hyperthermia symptoms- Dehydration, Fatigue, Muscle cramps, Heat exhaustion, Fainting, Heat stroke.
Sterilizer loads during cooling on racks may cause sweating of the load giving false wet load symptoms.
President: Shelagh Thomas CSSD
Hutt Valley Capital, Coast and Hutt Valley
Phone: 04 566 6999 ext 2745
Mobile: 027 589 6473
Email: shelagh.thomas@huttvalleydhb.org.nz
Maureen Scott
Sterile Services Hamilton Waikato District Health Board
Email: Maureen.Scott@waikatodhb.health.nz
Karen McCormick
CSSD Manager
Wellington Capital, Coast and Hutt Valley
Email: Karen.mccormick@ccdhb.org.nz
Sharon Moss
National Sterile Services Manager Southern Cross Health Christchurch
Email: sharon.moss@southerncrosshospitals.co.nz
Jenny Carston CSSD Manager Tauranga Hauora a Toi Bay of Plenty
Email: Jenny.Carston@bopdhb.govt.nz
Treasurer: Alison Stewart
NZSSA Treasurer 28 Brighton Street Island Bay Wellington 6023
Mobile: 021 209 8127 Email: nzsterilescienceassoc@gmail.com
Secretary: Paul Moody
Senior Product Development Manager Fisher & Paykel Auckland Email: paul.moody@fphcare.co.nz
Anthony Valvoi Sterile Services New Plymouth Taranaki Email: anthony.valvoi@tdhb.org.nz
Martin Bird Sterile Services Dunedin Southern Email: martin.bird@southerndhb.govt.nz
Aileen Derby CSSD Manager Manukau Counties Manukau Email: Aileen.Derby@middlemore.co.nz
Kelly Swale
Sterile Services Manager
Faculty of Dentistry, University of Otago Dunedin Email: kelly.swale@otago.ac.nz