




























February 2024
Hello, and welcome to the February Edition of Supplyline. This is the first edition for 2024.
We continue in this edition with regular updates relating to Industry Standards, Sterilising Technology Course information, and this year’s conference. Plus, this year it is the NZSSA’s 50th Anniversary. To celebrate this huge achievement, it was decided by the NZSSA Executive to change the NZSSA Logo to reflect this amazing milestone. This new logo will be in circulation for this year only and you can see it on the front cover of this edition of Supplyline.
Planning for this year’s conference is well underway. A conference venue has been chosen, which is the Aotea Centre in Auckland’s CBD. The conference committee along with MTANZ are very keen to put together a very exciting and informative conference for all attendees this year. If there is anything you would like to see included in this year’s conference, please let one of the conference organisers know.
Welcome to the first edition of Supplyline for 2024.
I hope that you have all managed to have some form of leave over the summer period in order to relax and recharge your batteries. I have just completed two weeks annual leave. The first week I decided to clear our cupboards etc. more of a summer clean than a spring clean. For the second week I decided to go off grid in the Wairarapa and just relax and catch up on reading that I wanted to do. If any of you like factual books I recommend that you try Bill Bryson’s “The Body- a Guide for Occupants”.
Anyway the break is over and it is back to the coal face. I hope that by now you or your department have purchased your copy of the new standard AS5369:2003. It has been the official standard since 15 December 2023. There is an official communication from the Ministry of Health regarding the new standard which I put on our NZSSA website. A copy of the communication letter is in this Supplyline for your reference.
Work is about to ramp up again on the requirements that we as a profession need to upgrade to satisfy the Ministry of Health that we are a profession. There is a lot of work and time involved in this.
There are a number of articles in this edition, covering a wide range of topics. Also included are two reports from members who attended the WFHSS World Congress in 2023. It is an exciting event to go to, wherever it is held in the world. I’ve been to two World Congresses over the years, and both were exciting and huge. The first was in London, England; the second in Brisbane, Australia. There are lots more trade displays at the World Congress that what we see at our own conferences and the products on display are usually new and exciting. If you ever get the opportunity to attend a World Congress, then go, you won’t regret it.
Take care everyone and stay safe,
Ngā mihi
Aileen Derby Editor NZSSA Supplyline2024 is a big year for the association. This is the 50th year of our being even though it is only our 48th actual conference thanks to Covid. The executive conference team of Sharon, Aileen and Anthony along with Britta from MTANZ are working very hard to bring you a very exciting conference this year in Auckland to celebrate or 50th Anniversary. Please be sure to register as early as possible as you don’t want to miss this one.
Last October I attended the WFHSS conference in Brussels, Belgium. I was joined there by our international scholarship winner Donna Dador from Southern Cross Hospital Christchurch. You can read my words on the conference in this edition of Supplyline.
As always feel free to contact me.
Shelagh Thomas President/NZSSAWe care for each other, showing kindness and empathy in all that we do.
We are committed to finding future focused solutions and take personal responsibility to be better every day.
Our diversity is our strength, we back each other and work together in partnership.
We are committed to doing the right thing by ensuring equity and hauora are at the heart of everything we do.

Here are some comments made by last year’s delegates.
“It was my first NZSSA Conference and I had a blast over the three days! I learned a lot and got to meet some wonderful colleagues. Te Papa was a great venue, and I really enjoyed the ‘Movie Night’ conference dinner.”
“Loved the venue. Enjoyed the presentations. Good speakers.”
“A well-organised event by all at NZSSA, you all deserve a round of applause!!! Thanks so much for a brilliant conference.”

First, I would like to thank NZSSA for the scholarship that has unlocked a lot of doors and led me to envision a bigger and better SSD environment. I have attended several seminars and conferences in the past and it just keeps getting better each time. This was my first world conference, so that makes it more exciting and something that will stay in my memory for a long time. Everything was an experience, from the time I got the result from the NZSSA secretary Mr. Paul Moody, whom I have to say was amazing, organize and very good when it comes to communication and answering all my queries even before I’ve submitted my application, I have to say, (the best person for the role).The experience continued on, sorting all my documents, itineraries, informing my Southern Cross family about the news, to completing the whole conference with a bonus of visits to other nearby European countries near Belgium.
A little something about me, I was a registered nurse in the Philippines, worked in delivery nursing for 2 years then moved to Singapore and worked as a sterile supplies technician back in 2010, moved up and became SSD Team Leader and hold that position until I left, back in June 2015 to come here in New Zealand to study and work. I have to say it was never easy moving from one place to another, it is hard to leave people, your team has become your family as you tend to work together longer than any other activities that you do. Also because of adapting, learning, and understanding different standards, guidelines, work environment & Working in Health care in 3 different countries with different standards equips me with a lot of lifelong experience and thoughts

about the system and its processes. So, when I have heard about the conference, I was like, “Oh, I want to see, learn and experience how they do it in other parts of the world too” and what better way to do that than to attend a world conference such as WFHSS. I know it is a long shot because I know there are a lot of SSD technicians that are also aspiring to be part of that conference but hey, this just proves there is no dream too big or a goal too impossible if you put your heart and mind in it. So, I packed my bags and got on with my little adventure (This will be my first time travelling solo too) So you see, there are a lot of first times with this trip so, yeah let’s carry on with my little story.
From here to Brussels, it is about 24hrs flight, and I arrived there on a Tuesday Morning, I had met NZSSA president Shelagh Thomas personally for the first time, in the hotel reception. I had heard and read a lot of good things about Shelagh and her work but of course it is always better to meet people live and in action to get to know them better, true enough she is such a wonderful, down-to-earth, and truly knowledgeable person. I felt privileged to attend this conference with her.
The conference did not start until Wednesday 18th October 2023. So that Tuesday as soon as I put my bag down, I went to see some places in Belgium, maximizing my time, I wandered around Brugge and took a quick look at Antwerp the following day before the start of the conference as the registration was not until 3pm on Wednesday.
The conference was held in the Mont des Arts Convention center in Brussels, Belgium. There were 1,648 registered attendees at the conference. The venue was well thought out as it was in central Brussels, within walking distance of most of the tourist attractions, hotels, and train station. The first day was for the registration as well as the start of the set up for most of the stalls. I enjoyed seeing new developments with products and new innovations from different companies. I saw equipment that I have not seen before and probably will not have known the existence of unless I searched for it or saw it from other hospitals, such as the flexible inspection scope from LTA medical that allows you to check
and inspect rigid/lumen/cannulated instruments as small as 1.3mm that will show up on the screen. I also saw in action a wrapping robot which was cool. Another one that stood out to me was the Safeclean box from Bicarmed, it is medical equipment that basically acts like a washer cleaning tool that uses low pressure compressed air and sodium bicarbonate, the intention of this equipment is really good as it revolves around operator/ssd tech safety, good for the environment as it is biodegradable and it helps lengthen the instruments life span and reduce maintenance cost due to its claim of removing biofilm effectively reducing chances of oxidation, my concern is just the amount of time it will take the technician to do all the instruments and possible strain that it may cause on their back, so it might be another story for me. These along with other interesting discoveries and product improvements made me really realize the possibilities are just endless. You just must go out there and explore. Now, enough of that amazing equipment and innovations, moving on to the conference discussion itself, I find it innovative and tech savvy. To start with, the venue was amazing, very conducive for learning. It was my first time attending a conference with a live translator that was translating to 3 different languages which are English, German and French. You are wearing a headset to select the translation that you desire.
Here are the topics that have stood up for me, first is sustainability, this was titled “Hospital mining: The CSSD and OR as goldmine for new raw materials in the circular economy “in which they have presented the ways they are sorting the materials that we use in the operating room, segregating them, recycling them and transforming them giving them new life in different ways. That presentation in sustainability have open my eyes entirely of the things we could have done to do something, nothing is too small or too little if everyone is doing it. I know we already have sustainability initiative across the Southern Cross network and as soon as I got back, I have list down the things that we could do in our department to be involved and be active in the sustainability drive such as printing all our checklist back-to-back as we currently do not do this, it was never too late to start something good.
Another presentation that I liked and totally made sense to me was about the reduction of instruments in circulation. This includes optimization of the sets; it means that instruments in sets should be reviewed from time to time to ensure that they are still fit for their purpose and that no extra instruments sit in the set that ends up not being used all the time. It also consists of modernization and standardization of sets and instruments as well proper calculation of ideal back up / extra sets that we should have. This requires the support of key people in the theatre and the management team. The reduction of instruments in circulation directly impacts SSD efficiency and it also has an impact on cost of reprocessing, maintenance and life span of an instrument too.
It was also good to see the new purposely built facility in the US, where they have use state of the art technology, especially with their washers where instruments are automatically unloaded and delivered to different packing stations, and
it was quite interesting to know how much it has improved their manpower efficiency as evidence by the data that they have provided which shows decrease in staff sickness and promote efficiency.
I have only mentioned three topics here, because I might consume the whole newsletter haha. Kidding aside, the rest of the presentation are equally impressive and relevant, and I promise there was not a time that I doze of ��.I also want to acknowledge one of the best speakers I have ever listened too, whom is now my friend, and she is Belgian, her name is Hannah Siwe, her presentation was an investigation about a UVC LED device for medical devices where she explained one of the reasons for choosing LED over mercury lamp in re processing as LEDs allow for wavelength control. The wavelength control is because LED’s are available in wavelengths so you have a selection to choose from, for example 265, 270, 280 and so forth, whereas mercury lamps are generally either broad spectrum or 254nm peak. And the fact that these lamps contain mercury is also a big disadvantage personally. Her presentation was phenomenal as the delivery was exemplary.
Aside from all the learnings, product samples and amazing food, I am thankful for all the connections and new friends that I’ve made in that conference. I would like to acknowledge Shelagh Thomas NZSSA president and David Bellamy, president of FSRACA (Australia) for guiding me through and ensuring that i am not lost as well as keeping me company most of the time. And oh, I have to say, we did have some party too as there was a conference gala night which was held at Auto world Brussels, the ambiance, the welcome exhibition, the band, everything was perfection! I’m thankful for all the experience.
I really appreciate all the people that have made this journey of mine possible especially for my Southern Cross family and my CSSD team for all the love and support.
You want to know why this is my best trip yet? Aside from this is the first time I have attended a world conference this big, and this was my first time travelling by myself. This was also my first time going to Europe. So, after exploring few places in Belgium such as Brussels, Brugge, Antwerp and Ghent, I continued and went to few other countries just like what I have mention earlier, I took the train and went to Paris, France toured around and saw the magnificent Eiffel tower, eat some macaroons and have a glimpse of that famous Monalisa. Then took the train to Switzerland and be mesmerized by their beautiful landscapes and picture worthy mountains among others and that magical train ride from Chur to Tirano. For my last stop, I took the train from Tirano to Rome wherein I was able to tick one of my bucket lists, which was to come and see and feel the mighty Colosseum, since I was little girl and up to this time, I was always fascinated by its structure and its history, and this trip has made it all possible.
So, from the bottom of my heart. Thank you NZSSA.
Donna Dador Southern Cross Hospital








Reprocessing of medical devices with special reference to CreutzfeldtJakob disease and its variant: A review 20 years after the report of the German vCJD Task Force
M. Beekes1, M. Thanheiser 2, I. Zerr 3, M. Mielke 4
1 Prion and Prionoid Research Unit, Division ZBS 6 - Proteomics and Spektroscopy, Robert Koch-Institute, Nordufer 20 13353 Berlin, Germany; 2 Division 14 – Applied Infection Control and Hospital Hyiene, Robert Koch-Institute, Nordufer 20 13353 Berlin, Germany; 3 Clinical Dementia Center and National Reference Center for CJD Surveillance, University Medical Center, Robert-Koch-Str. 40, 37075 Göttingen, Germany; 4 Department 1 – Infectious Diseases, Robert Koch-Institute, Nordufer 20, 13353 Berlin, Germany
Preliminary remark
Over the past years, the Robert Koch Institute (RKI) has published various original and review articles on the risk and prevention of iatrogenic transmission of classic and, above all, variant CreutzfeldtJakob disease. In particular, two of these review articles, the first of which was published in the immediate context of the vCJD Task Force report (Beekes et al. Der Internist 2002; 43: 738–748) in terms of content and time, and the second as an (interim) review eight years later (Beekes M. Bundesgesundheitsblatt – Gesundheitsforschung – Gesundheitsschutz 2010; 53: 597–605), served as the basis for a new review now 20 years after publication of the vCJD Task Force report. To mark that date, this paper is now intended as a synthesis and update for the readership, which presumably has changed significantly over the course of the recent years. We have incorporated into the present paper from the aforementioned publications several text passages that did not need any changes. General reference will be made here to these text passages from our previous publications since they were not individually marked or referenced.
Abstract
The novel emergence of variant CreutzfeldtJakob disease (vCJD) in the United Kingdom in 1995/96 as a result of the transmission of bovine spongiform encephalopathy (BSE) agents from cattle to humans triggered a previously unprecedented crisis in Europe at the interface of animal health and human public health. This prompted Germany to reconsider, among other things, the practice of reprocessing medical devices with regard to the unconventional BSE/ vCJD pathogens, socalled prions (from “proteinaceous infectious particles”).
Keywords
Creutzfeldt-Jakob disease vCJD Task Force vCJD medical device reprocessing
In 2002, a vCJD task force set up with this objective presented recommendations for minimizing/reducing the risk of vCJD transmission through surgical instruments and other medical devices. According to these recommendations, routine reprocessing should combine at least two procedures that are also (at least partially) suitable for decontamination/inactivation of these pathogens if there is no identifiable risk of contamination with prions. In practice, this includes in particular careful cleaning (decontamination), preferably with alkaline detergents, and subsequent sterilization with moist heat (“steam sterilization”) at 134 °C with a holding time of 5 minutes. The validation of the processes is of great importance. From today’s point of view, the central recommendations of the vCJD Task Force for medical device reprocessing have
Review
Corresponding author:
PD Dr. Michael Beekes
Prion and Prionoid Research Unit
Division ZBS 6 – Proteomics and Spektroscopy
Robert Koch-Institute Nordufer 20 13353 Berlin, Germany
BeekesM@rki.de
Conflict of interest:
All authors confirm that there is no conflict of interest according to the guidelines of the International Committee of Medical Journal editors (ICMJE).
Citation: Beekes M, Thanheiser M, Zerr I, Mielke M. Reprocessing of medical devices with special reference to Creutzfeldt-Jakob disease and its variant: A review 20 years after the report of the German vCJD Task Force. Zentr Steril 2023; 31 (3): 145–158.
Manuscript data:
Submitted: 28 September 2022; revised version accepted: 19 December 2022 (Reprinted from Hyg Med 1-2/2023)
Zentralsterilization | Volume 31 | 3/2023 145
proven to be sustainable with good practicability. The effectiveness of the recommended measures against prions has been confirmed on a broad basis in numerous studies. In line with the paradigmatically high demands that prions place on hygiene in the reprocessing of medical devices, this effectiveness in practice extends simultaneously to conventional pathogens such as bacteria, viruses or fungi. In perspective, this also applies at least in part to potential new challenges, such as those that have been discussed for some time with regard to selfreplicating protein particles of Alzheimer’s or Parkinson’s disease and other protein aggregation diseases.
In 1995/96, the emergence of a novel type of CreutzfeldtJakob disease (CJD) was observed among unusually young patients in the United Kingdom (UK). This human disease, which would later be designated as variant CreutzfeldtJakob disease (vCJD), differed significantly in terms of its aetiology, pathophysiology and clinical manifestations from the classic forms of CJD, constituting a novel independent disease within the group of transmissible spongiform encephalopathies (TSEs) or prion diseases. It was caused by infection with the agent of bovine spongiform encephalopathy (BSE), which was chiefly transmitted through contaminated food. BSE had been observed in cattle in the UK for the first time some 10 years earlier and subsequently assumed epidemic proportions. Against that background, the interspecies transmission of BSE to humans in the form of vCJD made this and other neurodegenerative prion diseases the focus of scientific, political and public health attention, with a severe impact in some cases on agriculture, consumer protection and the healthcare system.
In Germany, the emergence of autochthonous cases of BSE from 2000/2001, coupled with the epidemiological vCJD situation in the UK and other countries at that time as well as the decision in the UK to preferably opt for singleuse devices for those surgical instruments coming into contact with critical (e.g. lymphatic) organs, served as an impetus to review, among other things, the practice of reprocessing medical devices in Germany too. This was done against the backdrop of fears at the
time that the vCJD incubation period was possibly up to 30 years and as a result thousands of vCJD cases could occur in the UK [1]. At the same time, it was expected that vCJD would also emerge in other countries in and outside Europe, while it was still too early to make any sound statements about the further course of vCJD case numbers [2].
An inherent feature of new and crisis events affecting public health is that timely healthpolicy decisions have to be made on the basis of still incomplete data. Furthermore, the long incubation time of prion diseases meant that persons already carrying the causative agent were for a long time not identified with the diagnostic means and methods available, i.e. they could present an “unidentifiable risk” with regard to avoidance of iatrogenic transmission (e.g. through blood, blood products or contaminated surgical instruments). Besides, it was known that the pronounced tenacity of the CJD and vCJD agents called for a special combination of cleaning, disinfection and sterilization measures to minimize or relevantly reduce the risk of iatrogenic or accidental transmission of these diseases in healthcare settings.
At the invitation of the Robert Koch Institute and in collaboration with the scientific board of the German Medical Association (BÄK), a vCJD Task Force was set up to issue specific infection protection recommendations based on incomplete scientific data combined with applied experience. In 2002, this committee then presented recommendations in a report for minimizing the risk of iatrogenic transmission of vCJD through medical devices, especially surgical instruments [3].
At that time, many assumptions on the extent and future evolution of the problem were based on modelling and corresponding projections. Marking the 20 years that have passed since the publication of the vCJD Task Force report, this present article aims to give an overview of the history and the current status of differentiated recommendations for reprocessing medical devices in light of human prion diseases (especially vCJD) in Germany. Special attention will be paid to the recommendations compiled in response to prionrelated issues and which, going beyond the realm of prions, in general lent impetus to the medical device
reprocessing sector, had lasting effects and ideally will also be able to meet new challenges.
Creutzfeldt-Jakob disease and the emergence of its variant as a novel prion disease in humans
The disease that would later be designated as Creutzfeldt-Jakob disease was first described by Hans Creutzfeldt in 1920 and by Alfons Jakob in 1921 – independently of each other – in the scientific literature [4, 5]. Today, a distinction is made between four different types of CJD, which present as neurodegenerative diseases of the central nervous system with varying incidence [6]. These are sporadic, genetically mediated or transmitted types of disease. Most cases, around 85%, occur spontaneously especially in older persons aged between 60 and 70 years (sporadic CJD). Sporadic CJD (sCJD) has a relatively constant global incidence of around 1–2 cases per million inhabitants and year. Some 10–15% of CJD patients develop the disease because of mutations in PRNP, the gene encoding the human prion protein (familial or hereditary CJD), and in a relatively small number of cases the disease is caused by accidental transmission during medical procedures (iatrogenic CJD). In 1995, a hitherto unknown CJD variant (vCJD) which differed from these classic types of CJD was observed in the UK and the following year first described in the literature [7, 8]. vCJD seen predominantly in younger patients (originally observed median age was 29 years [7], current age range 11–74 years [9]) is distinguished from classic CJD by its characteristic aetiology, pathophysiology and clinical manifestations and constitutes a novel independent disease within the group of TSEs.
Classic and variant CreutzfeldtJakob diseases are assigned to the transmissible spongiform encephalopathies because of their transmissibility mediated by a characteristic infectious agent and their specific neuropathological course characterized by the formation of vacuoles in the brain tissue and progressive, partially spongelike degeneration of the brain, as seen under the microscope [10]. Other examples of these diseases in humans are GerstmannSträusslerScheinker (GSS) syndrome and fatal familial insomnia (FFI), both genetically mediated, as well as kuru (acquired).
Kuru is a human TSE transmitted through ritual cannibalism which emerged among the Fore people population group in Papua New Guinea [11]. Today, it is only of historical interest in clinical terms but in a certain sense is paradigmatic for BSE and of considerable information value with regard to vCJD. Since the introduction of epidemiological surveillance for kuru in 1957 around 2,700 cases of the disease have been reported, whereby a total of possibly more than twice as many people were affected because of the kuru epidemic, which had already lasted for a long time beforehand [12]. For a total
population of around 12,000, the mortality rate was as high as 35 deaths per 1000 inhabitants and year [11]. Whereas the mean incubation period in male kuru patients was 3–6 years [11], towards the end of the receding epidemic incubation times thought to exceed 50 years were also observed in isolated cases [13].
In animals, bovine spongiform encephalopathy (BSE), scrapie and chronic wasting disease (CWD) are wellknown examples of TSEs. In all TSEs propagation of the infectious agent occurs predominantly in the central nervous system and following its transmission to a new host the agent
can cause the clinical picture of TSE once again. Neither vaccinations nor effective chemoprophylaxis are available for TSEs. So far, it has not been possible either to treat these always fatal diseases causally.
The unconventional transmissible agent causing TSEs belongs to the pathogen class of infectious prions. Therefore, transmissible spongiform encephalopathies are often also termed prion diseases. According to the widely accepted prion hypothesis [14, 15], prions are infectious protein particles consisting mainly – if not exclusively – of






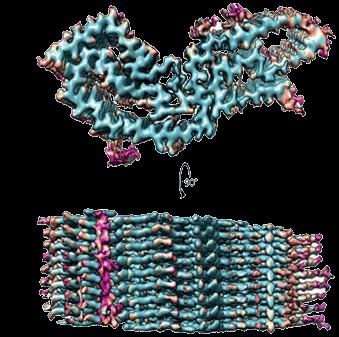
Figure 1: Mechanistic protein folding and aggregation model to explain the occurrence and propagation of infectious nucleation seeds of the prion protein (PrP) in transmissible spongiform encephalopathies (TSE) or prion diseases. Under certain circumstances (usually monomeric) PrP conformers can assemble spontaneously or because of genetic factors into ß-sheet-rich PrP aggregates, giving rise to self-replicating protein particles known as “seeds”. This initial seed formation (primary nucleation) is controlled by a high kinetic barrier. As an example, the primary nucleation of hamster PrP is shown here against a light blue
background, and the aggregation of PrP monomers (1) [123] to a PrPSc seed (2) is schematically illustrated [124, 125]. As soon as PrPSc seeds have been formed endogenously or have entered the body exogenously, they are able to rapidly recruit other PrP monomers and add them to their aggregate structure. During this elongation process the aggregation mass increases by continually adding PrP monomers to the ends of the growing particles. Furthermore, secondary nucleation may occur through the formation of other nucleation sites on the particle surface. When fragmentation of PrP seeds into smaller aggregates occurs,
the progeny seeds enter the replication cycle, expediting propagation of the pathological protein state. This figure was created with modifications based on a template published by Beekes elsewhere ([126]; licensed according to MDPI Open Access Policy. Credits for mounted image components: (1) RCSB Protein Data Bank (PDB) – 4YXL: High-resolution crystal structure of the cellular hamster prion protein (licensed according to PDB Privacy and Usage Policy), and (2) Structure of a hamster scrapie prion ([124]; licensed according to Creative Commons CC0 1.0 Universal (CC0 1.0) Public Domain Dedication).
a misfolded and pathologically aggregated isoform of the host’s own prion protein (PrP) [15]. The cellular prion protein is known as PrPC and its diseaseassociated isoform as PrPSc [15] or PrP TSE [16]. Replication of prions is thought to occur through nucleationdependent polymerization of the prion protein [17 – 19]. According to this concept, prions behave as quasi proteinaceous crystallization seeds that recruit endogenous PrPC and integrate it in misfolded form into their own oligomeric or polymeric aggregate structure. The seeding activity of PrPSc, which can be detected and quantified using sensitive analytical techniques such as protein misfolding cyclic amplification (PMCA) [20] or real-time quaking-induced conversion (RT-QuIC) [21], thus causes autocatalytic propagation of the pathological protein state (Fig. 1). According to that concept, different phenotype characteristics of various prion strains are molecularly encoded by conformational differences in PrPSc [9]. Often, such conformational differences are analysed on Western blot, known as PrPres typing, by studying the molecular weight (Nterminal truncation of the protein) and the glycosylation profile (occupancy of the two asparaginelinked glycosylation sites) of PrPSc after limited proteolytic digestion (generally by proteinase K) [9].
According to the prion concept, onset of familial types of CJD is explained by the fact that inherited mutations in the prion protein gene make the amino acid sequence of PrPC susceptible to structural conversion to the pathogenic isoform PrPSc. Likewise, endogenous misfolded prion protein is thought to be responsible for sporadic CJD. However, other than in familial CJD, the diseaseinducing PrPSc is not caused by a hereditary genetic defect but by a somatic mutation in the PrP gene or by spontaneous structural conversion of PrPC. Finally, acquired forms of CJD are thought to be due to the fact that infectious prion protein enters the body from the outside, for example through the administration of contaminated drugs or during medical procedures conducted with inadequately reprocessed instruments (iatrogenic CJD) or, as was soon suspected in the case of vCJD, through dietary exposure.
According to the current state of research, zoonotic infections with the
BSE agent from cattle are responsible for the vast majority of vCJD cases and the initial emergence of vCJD [8, 22, 23]. According to estimates more than 700,000 infected cattle entered the human food chain [25] in the United Kingdom alone, which was the country first affected by the BSE epidemic. With over 180,000 clinically diseased [24] and around 900,000 infected animals [25], it was also by far the country most severely impacted. Risk materials such as brain and spinal cord as well as separator meat resulting from the processing of spinal cord may have served as a vehicle for transmitting the agent to humans.
As a result, extensive precautionary and protective measures against BSE and its transmission to humans were taken [22, 26]. This has led to a sharp, now almost complete, decline in the number of BSE cases. Therefore, the infection risk presented by BSE to humans appears to be very low or virtually negligible following the effective interruption of infection chains between cattle and from cattle to humans.
However, there are more far-reaching health policy challenges that stem from the possibility of vCJD transmission from person to person (secondary infection) and – as seen already in the past – from iatrogenic and accidental transmission of classic forms of CreutzfeldtJakob disease. But a clear distinction must be made between issues around the potential transmission of, on the one hand, vCJD and, on the other hand, the classic forms of CJD. As regards the latter, various cases of iatrogenic transmission have been reported, albeit only in relatively small numbers [27, 28]. The infection sources and transmission routes implicated in these cases have been identified in the meanwhile and are in principle well under control. Yet, the experiences gleaned from BSE and vCJD, on the other hand, have engendered fears that the hazard potential emanating from the vCJD agent should possibly be deemed to be higher than the largely wellknown risks of transmission of the classic types of CJD.
Before describing in greater detail below the epidemiological situation regarding CJD and vCJD as well as the surveillance and risk assessment of these diseases and their risk management, especially in the context of medical device reprocessing, the clinical and
pathological distinguishing characteristics between CJD and vCJD will be briefly presented.
Variant CJD occurs in unusually young patients, predominantly in the third decade of life. By contrast, classic CJD patients usually develop the disease at a much older age, often in the seventh decade of life or later. The relatively high frequency of vCJD in comparatively young people is thought to be due to agerelated differences in dietary exposure to BSE [29] or to an age-associated increased susceptibility to infection [30]. The median survival time is around 14 months [6], much longer than the 4–7 months observed in the majority of sporadic CJD cases.
The clinical symptoms of vCJD are relatively uniform and are characterized by psychiatric signs of disease such as withdrawal, anxiety, depression and personality changes [31–33]. These symptoms may occur on their own in vCJD or may also be accompanied by various sensory anomalies, such as e.g. joint pain and paresthesia [34]. Additional neurological symptoms appear after around six months. These include visual disorders, progressive ataxia, involuntary movements, such as dystonia, myoclonus and chorea, as well as also progressive deterioration of cognitive abilities [35]. Unlike vCJD, in the classic types of CJD impaired memory and ataxia are often the earliest symptoms of disease [6]. Ultimately, the terminal stage of vCJD is similar to that of patients with sporadic CJD and is often characterized by akinetic mutism.
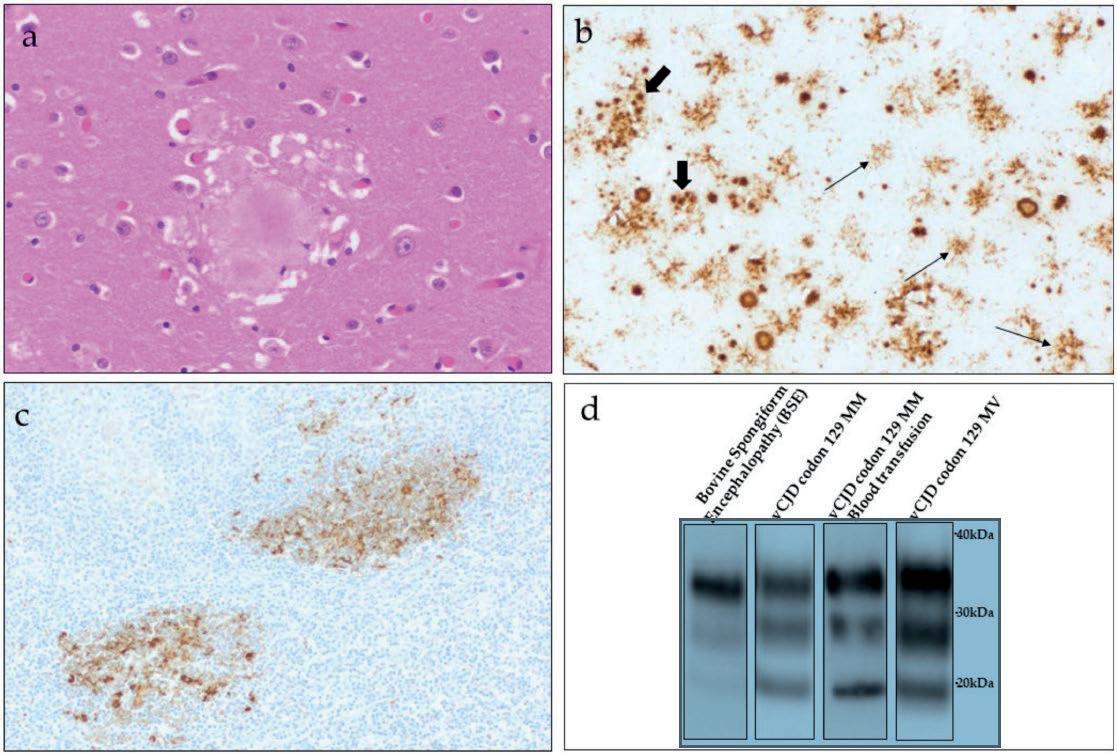
On histology and immunohistochemistry, vCJD exhibits strikingly uniform neuropathological characteristics. This is in marked contrast to the classic forms of CJD, which typically show more pronounced heterogeneity of the pathological phenotype [36, 37]. The most conspicuous characteristic of vCJD is the presence of numerous florid plaques in the cerebral and cerebellar cortex [36] (Fig. 2, a & b). With the exception of type MV2 sporadic CJD, florid plaques are only rarely seen in classic CJD. vCJD differs from classic types of CJD also in that the affected patients have more extensive deposits of PrPSc in peripheral tissues [38–40], particularly in the lymphoreticular system (Fig. 2, c) but, for
example, also in the autonomous and peripheral nervous system. By contrast, the PrPSc deposition pattern in classic CJD is significantly more concentrated in the CNS. However, on using highly sensitive methods in recent times prion infectivity or pathological prion protein and its seeding activity have also been found in the blood plasma [41], olfactory epithelium [42], spleen [43–45], skeletal muscles [44, 46, 47] and skin [47, 48], bone marrow [44, 49], different parts of the eye [50], ganglia of the peripheral nervous system [51] as well as in other peripheral tissues such as e.g. the lungs, liver or kidneys [44, 45, 47] of patients with sporadic types of CJD (sCJD). This should be taken into account in the same way as for vCJD (see below)
when evaluating the potential transmission risks of these forms of disease and formulating corresponding preventive measures, even if the exact correlation between the detection of sCJD prions or their seeding activity and their infectivity to humans has not been conclusively demonstrated so far in high sensitivity bio or in vitro assays.
Finally, the specific PrPres type of vCJD, known as type 2B, serves as a characteristic molecular signature of this disease. PrPres type 2B like PrPres type 2A seen in cases of sporadic CJD is characterized by an unglycosylated fragment size of around 19 kDa, but unlike type 2A has a clear preponderance of the diglycosylated moiety on the electrophoretic band [36, 52] (Fig. 2, d).
Since to date CJD or vCJD can only be definitively diagnosed in autopsy or biopsy CNS tissue samples, in suspected cases of CJD/vCJD a neuropathological examination should be carried out whenever possible to confirm the diagnosis [53].
Risk parameters for person-to-person transmission of vCJD Important risk parameters for personto person transmission of vCJD are the infective power/infectivity and dose of the agent, the incubation time and the time window during which, in particular, unidentified asymptomatic carriers can pass on the infection, the potential modes and routes for transmitting the prion agent as well as the prevalence of pre or subclinically infected carriers (which will be explained in detail at the end of this paper).

The vCJD agent and its characteristics BSE in cattle and vCJD in humans are caused by similar, hitherto not observed, TSE agents known as BSE and vCJD agents [22, 23]. BSE/vCJD prions can be clearly distinguished biologically and biochemically from the causative agents of the classic types of CJD, i.e. sporadic, familial and iatrogenic CreutzfeldtJakob disease [54–57]. BSE prions have caused in the cattle population of the United Kingdom alone an epidemic involving a total of more than 180,000 cases of clinically diseased animals. This seems all the more remarkable since this agent was probably transmitted predominantly via a very inefficient, i.e. peroral, infection route. The infective potential of this new prion strain has also been underlined by its transmission from cattle to humans, whereby the agent was apparently likewise able to breach perorally a, in principle protective, species barrier; that barrier no longer applies in secondary transmission. Accordingly, there is an obvious infection risk to the recipient from transmission of vCJD agents between humans [23].
In patients with clinically manifest vCJD the highest infectious titres or concentrations of PrPSc, serving as a biochemical marker for the infectious agent, are found in the brain, spinal cord and eye. Tonsil and spleen tissues as well as other components of the lymphatic system (lymph nodes, thymus, gutassociated lymphoid tissue) exhibit infectivity and PrPSc less consistently and in lower quantities [58]. Besides, PrPSc has also been detected in parts of the peripheral nervous system, adrenal glands and rectum [59] as well as in the skeletal muscles [58] and in other organs and tissues (liver, pancreas, kidneys, uterus and skin) [60]. As stated in the following section, blood may also contain critical doses of vCJD prions and be able to transmit the disease.
Within what time window can vCJD prions from infected carriers be passed on? No conclusive answer can be given as such to this question because, among other things, the mean incubation period of vCJD is not known. In principle, the incubation time in prion infections depends on various factors such as the agent strain, host species,
agent transmission route, PrP genotype and infectious dose. In analogy to the shortest incubation time observed for iatrogenic CJD following peripheral infection, an incubation period of at least 4.5 years is assumed for primary BSE infection in humans [8]. Presumably, the incubation period is likely to be longer because primary vCJD infection, unlike iatrogenic CJD, must cross a species barrier. Mathematical modelling estimates came to the conclusion that subject to various assumptions, some of which were necessarily uncertain, the mean incubation time for primary vCJD infection in carriers with the methioninehomozygous MM genotype at PRNP codon 129 was about 15 years [30, 61]. In three cases of secondary transmission via nonleukodepleted erythrocytes the incubation times, i.e. the interval between transfusion and onset of vCJD symptoms, were between six and 8.5 years in the transfusion recipients. These were all homozygous for methionine (MM) at codon 129 of the prion protein gene (a known risk modulator for human prion diseases [9]) [62–64]. This genotype – compared with the heterozygous MV or homozygous VV genotype – is apparently associated with increased susceptibility and/or a shorter incubation time for vCJD infection.
Various findings demonstrate that the infectious agent can in principle apparently be passed on by infected carriers already months to years before the onset of symptoms. For example, PrPSc was detected in archived appendix specimens harvested from two patients eight and 24 months before onset of clinically manifest vCJD [40, 65]. Besides, vCJD was apparently transmitted in four cases through blood and in one other case possibly through plasma withdrawn between 17 months and 3.5 years (blood) [62–64, 66] and six months (plasma) before onset of disease in the donor [67].
There is no evidence that vCJD (or CJD) can be transmitted during normal social or nursing contacts or naturally via the air. Rather, disease transmission requires iatrogenic or accidental introduction of the agent into the recipient. Whether the latter will go on to develop infection and how long that takes to progress to clinical vCJD depends
among other factors, apart from the transmitted infectious dose, also on the exposure route. Intracerebral inoculation is the most effective transmission route for prions. The findings from animal models have revealed that the intravenous, intraperitoneal, subcutaneous or peroral routes are around 101, 102, 104 and 105, respectively, times less effective [22, 23].
Because of the relatively early and widespread distribution of the vCJD agent in the body of clinically asymptomatic carriers, there is a potential risk of transmitting this infectious agent during medical procedures through blood and blood products, transplants/grafts, drugs or other materials of human origin as well as through surgical (or dental) instruments and other medical devices. Furthermore, there is a hypothetical occupational infection risk, for example, in hospitals and medical/dental practices, research laboratories, anatomy or pathology departments, forensic medicine as well as in the undertaker sector. Accordingly, extensive recommendations and guidelines have been drawn up at international and national levels to prevent accidental and iatrogenic transmission of vCJD [68, 69]. Advantageously, these are also effective against transmission risks of sporadic CJD or other human types of TSEs.
It goes without saying that the recommended safety measures are not of a static nature, but must be updated and adapted to the respective new situation with advancing scientific knowledge or a change in basic or operational conditions. Careful consideration must always be given to the extent to which protective measures against vCJD could give rise to problems in other areas of the healthcare sector and to whether the expected benefits justify possible or predictable adverse effects and costs. Serving as a basis for evidencebased decisionmaking, research into TSE makes an important contribution to that end.
The following section will now first give an overview of the prevention of healthcare associated (nosocomial) transmission of vCJD and CJD through surgical instruments and other medical devices before presenting the current state of vCJD surveillance and epidemiology.
Reprocessing of surgical instruments and medical devices with regard to CJD/vCJD
The pronounced tenacity of TSE agents calls for specific prophylactic principles as well as a careful choice of cleaning, disinfection and sterilization measures to minimize the risk of iatrogenic or accidental transmission of classic or variant CJD in healthcare settings.
In order to address this challenge and minimize the transmission risks through possibly inadequately reprocessed instruments, the use of disposable instruments was considered in the UK for frequent surgical procedures, and specifically recommended there for tonsillectomies, because of the developing vCJD situation there at that time. However, due to the associated surgical drawbacks as well as the occurrence of, in some cases, severe bleeding following tonsillectomies performed with single-use instruments [70], this did not appear to be a feasible or sustainable option.
In view of their unusual tolerance against conventional cleaning and inactivation processes, prions, and hence also the CJD and vCJD agents, present a special challenge when it comes to reprocessing invasive, especially surgical, instruments and other medical devices [71, 72]. The preventive measures aiming at risk mitigation include, among other things, risk stratification of patients, tissues/organs and the type of medical intervention. They include differentiated guidelines on risk management and specific instructions for medical device reprocessing tailored to the respective risk. In Germany (which to date has had no vCJD case) the vCJD Task Force in 2002 issued precautionary recommendations to minimize the risk of vCJD transmission from medical devices, especially surgical instruments [3].
Recommendations/guidelines for identifiable CJD/vCJD risks
In the vCJD Task Force report previous guidelines for Disinfection and Sterilization of Surgical Instruments for Suspected Creutzfeldt-Jakob Diseases [73] and for Patient Care and Instrument Sterilization for CJD Patients and Suspected CJD Cases [74] were extended to the effect that instruments and other medical devices used for patients with an identifiable risk of vCJD (clinical suspicion of probable or possible vCJD) are in principle
to be disposed of safely as far as practicable. Where neurological diagnosis is inconclusive, instruments and other medical devices should at first be safely quarantined. If disposal presents a special technical and/or economic problem, such as e.g. with flexible endoscopes, it was recommended that equipment pools should be established at appropriate centres for specific procedures carried out on CJD patients. Endoscopes used to that effect are returned afterwards for specific reprocessing. For endoscopes used on vCJD patients reprocessing has not been considered. Further information and contact details can be found at http://www.cjdaufbereitung.unigoettingen.de/
Procedures in cases where there are no identifiable CJD/vCJD risks
In particular, in view of the problem of possibly unidentified vCJD infection, the vCJD Task Force aimed to recommend a generally applicable reprocessing process that counteracted the theoretic risk of transmission from pre or subclinical CJD/vCJD carriers without essentially changing the usual procedure used to reprocess surgical instruments and other medical devices. Furthermore, the recommended processes should take account of potential crosscontamination of instruments during cleaning and also be suitable for heatsensitive (thermolabile) medical devices. According to the statement issued by the vCJD Task Force, surgical instruments and other medical devices should therefore in principle be reprocessed as per the recommendation jointly compiled by the Commission for Hospital Hygiene and Infection Prevention (KRINKO) and the Federal Institute for Drugs and Medical Devices (BfArM ) in November 2001 [75], and combine at least two procedures that are also (at least partially) suitable for decontamination/inactivation of TSE agents. This includes, in particular, thorough cleaning in an alkaline environment followed by sterilization with moist heat (steam sterilization) at 134 °C with a holding time of 5 minutes (the efficacy of this sterilization process was confirmed only recently in a new study for vCJD and sCJD (VV2) prions [76]).
In terms of sustainable and practical implementation, a combination of a) primary decontamination comprising intensive cleaning and b) validated
standardcompliant sterilization appeared more suitable than a mere extension of the sterilization time to 18 minutes.
Updating the recommendations for reprocessing medical devices pursuant to Section 8 (previously Section 4) of the Medical Device Operator Regulation (MPBetreibV)
In 2012, the Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and of the Federal Institute for Drugs and Medical Devices (BfArM) entitled “Hygiene Requirements for Reprocessing Medical Devices” was fully updated [77]. A key element of this update was that in a new Annex 7 the “Measures for minimization of the transmission risk of (v) CJD through medical devices” were described in specific details. Underlining the statement by the vCJD Task Force, this sets out specific measures that can be applied to an identifiable (or suspected risk) as well as when there is no identifiable risk of (v)CJD. In addition, special measures to be observed when using flexible endoscopes with regard to (v)CJD are presented there separately.
The preventive measures recommended by the KRINKO and BfArM when there is no identifiable risk are met when at least two procedures that are also (at least partially) suitable for decontamination/inactivation of prions are combined [77]. Such processes include e.g. a thorough cleaning process with a demonstrated cleaning efficacy (aimed at reducing the protein load as per the KRINKO and BfArM Recommendation to < 100 µg per medical device) followed by steam sterilization at 134 °C with a holding time of 5 minutes. What is important here is avoidance of thermal or chemical fixation of prion proteins prior to reprocessing. Thorough cleaning is of paramount importance because the efficacy of the subsequent inactivation processes would be jeopardized by prior protein fixation, whereas suitable cleaning processes can greatly reduce the prion contamination load.
A key aspect in light of the KRINKO and BfArM Recommendation relates to appropriate validation of the reprocessing processes. This requirement, which is also legally stipulated in Section 8
MPBetreibV, plays a major role especially with regard to the specific characteristics of prions resulting in their high tolerance against various decontamination/inactivation methods [78, 79]. Apart from the crucial importance of a validated cleaning step targeting prions during reprocessing (see above), sterilization too calls for special care. Based on the current state of knowledge, steam sterilization at 134 °C, as already mentioned above, is an effective prion inactivation method. Other classic steam sterilization processes at 121 °C (and e.g. 15–20 minutes holding time), which are used at times in the healthcare sector, have no or only a slight inactivating effect against the highly temperatureresistant human prions. Even for steam sterilization at a temperature of 134 °C, where already the heat-up phase would suffice to kill or inactivate all bacteria, viruses and fungi, including the spores of the bacterium Geobacillus stearothermophilus which are used at times as bioindicators in steam sterilization, observance of a holding time is prescribed for inactivation of prions [76]. This highlights the practical importance of also providing thermoelectric evidence during validation that throughout the entire holding time of 5 minutes saturated steam at 134 °C can act upon all external and, as applicable, also internal surfaces of the instruments or other medical devices being reprocessed.
In 2004, the RKI published a paper on testing and declaration of suitable processes for the inactivation and removal of prions when reprocessing medical devices [72]. In line with that endeavour, various approaches were taken in recent years aimed at developing new processes and formulations for prionicidal disinfection with improved material compatibility and cleaning performance (to eliminate as far as possible prion contaminations), and also endowed to some extent with activity against bacteria, viruses and fungi, in addition to prions. Recent studies [80, 81] demonstrate that prions are not just a problem but also serve as an excellent paradigm for the reprocessing of surgical instruments and other medical devices. This appears to be all the more significant since good practice stipulates that when reprocessing medical
devices, hygiene requirements be tailored as far as possible to the most resistant pathogens, i.e. the infectious agent most difficult to inactivate or eliminate.
In recent years, the scientific literature has published articles and comments increasingly advocating that, in the interest of preventive patient care, medical device reprocessing processes be designed such that they are also effective against selfreplicating proteinaceous seeds from other protein aggregation diseases, such as e.g. Alzheimer’s or Parkinson’s disease [82–84]. Since the KRINKO and BfArM Recommendation for reprocessing medical devices already ascribes great importance to the removal of protein contamination and at least partial decontamination/ inactivation of prions, it is indeed also addressing this potential new development [85]. The findings of pilot studies suggest various experimental or commercially available detergents and disinfectants, possibly together with subsequent steam sterilization for 5 minutes at 134 °C, are able to reduce by a factor of 100 protein soils consisting of amyloid-β (Aβ), tau and α-synuclein aggregates from Alzheimer’s or Parkinson’s disease from test surfaces of surgical steel [86, 87].
To date, there is no experimental or epidemiological evidence of transmissibility of e.g. Alzheimer’s or Parkinson’s disease by selfreplicating protein particles and, as such, no justification either for any additional specific recommendations for reprocessing medical devices with regard to Aβ, tau or α-synuclein seeds. However, laboratory tests have demonstrated that intracerebrally transmitted inoculates that contained pathological Aβ, tau or α-synuclein aggregates led to stimulation of misfolding and aggregation of endogenous forms of these proteins in the brain of the recipient animals [88–91]. Similar effects have also been detected for Aβ in the meantime in humans who had received dura mater grafts or growth hormone products obtained from deceased donors [83, 92–94]. Whether this could be associated with acceleration of genetically predisposed types of disease or with other adverse health effects below the threshold of full disease transmission is unclear and is being currently under research. Pending further clarification, this speaks in
favour of inactivating or removing as far as possible any potential contamination involving selfreplicating protein seeds also from, what are thought to be, nontransmissible protein aggregation diseases when routinely reprocessing gastroscopes, colonoscopes and other medical instruments.
vCJD today - surveillance and epidemiology
At the time of compiling the Task Force Report, many assumptions on the extent and future development of vCJD were based on modelling and corresponding projections. It is therefore interesting to retrospectively review how the situation actually unfolded over the past 20 years.
Surveillance of human prion diseases
Following the emergence of BSE, human prion diseases have also come into the focus of systematic epidemiological surveillance in the United Kingdom since 1990 in order to be able to detect any changes in their occurrence and presentation as early as possible [95].
In 1993, an international CJD surveillance programme (EUROCJD) was set up with the participation of the following countries: Australia, Germany, Italy, Canada, France, Netherlands, Austria, Switzerland, Slovakia, Spain and United Kingdom); in 1998, this was extended to other countries (Belgium, Denmark, Finland, Greece, Iceland, Israel, Ireland, Norway and Portugal) (NEUROCJD) (http://www.eurocjd. ed.ac.uk). EUROCJD and NEUROCJD, like CJD surveillance in the United Kingdom, were also coordinated by the National CJD Surveillance Unit in Edinburgh. In other countries too, such as the USA and Japan, various forms of systematic surveillance of human TSEs were implemented. In Germany, in compliance with the Infection Protection Act (IfSG), disease and death cases of human spongiform encephalopathy (apart from familial hereditary types) must be reported to the competent Public Health Office. The Robert Koch Institute (RKI) monitors the disease situation on the basis of the reports forwarded from the Public Health Offices. In addition, Germany has a National Reference Centre (NRZ) for the surveillance of CJD (and vCJD). The Prion Research Group at the Department of Neurology of the University Medical Center
of Göttingen (UMG) was designated in 2006 as the National Reference Centre for Transmissible Spongiform Encephalopathies (NRZ-TSE). Its mission is to conduct epidemiological surveillance and research for prion diseases in Germany as well as provide consultation services to German physicians on diagnosis, treatment and infection control/ hygiene measures (http://cjd-goettingen.de/).
Occurrence of vCJD within and outside the United Kingdom
So far, 233 deaths of confirmed or probable vCJD have been reported worldwide [96]: in addition to 178 cases from the United Kingdom, there were 29 other cases from France, five from Spain, four from both Ireland and the USA, three from each the Netherlands and Italy, two from each Portugal and Canada as well as one case each from Saudi Arabia, Japan and Taiwan [97]. In the two cases each from Ireland and
the USA as well as in at least one case from each Canada and Japan it is assumed that the affected patients were exposed to BSE in the United Kingdom in the 1980s or early 1990s. Another patient from the USA is said to have been infected as a child in Saudi Arabia [98].
Figure 3a shows the annual vCJD mortality rate for the UK and France, the countries most affected by the disease. The highest number of deaths per year in the UK was in 2000 with 28 cases and has tended to decrease continuously thereafter. In France, the highest number of cases was observed in 2005 and 2006 (six cases each year). The last vCJD death reported so far from the UK was in 2016 and from France in 2019.
Figure 3b gives the annual number of vCJD cases in the United Kingdom and in the EU (without UK) compared with the course of the number of BSE cases reported there. This highlights how vCJD followed in the wake of BSE.
In Germany, there has so far been no case of vCJD. An overview of the
number of CJD cases in Germany can be found at: http://cjd-goettingen.de/ aktuell/aktuelle-zahlen/
Apart from the vast majority of primary cases of vCJD infections caused by the transmission of BSE from animals to humans, there were in the United Kingdom also four cases of secondary infection through blood as well as one presumed case of secondary transmission through a plasma product. Three of the transfusion recipients developed vCJD [62–64], in both other cases the recipients of transfusion [66] and plasma product (a factor VIII concentrate) [67, 99] died without vCJD symptoms from another cause.
Of the 233 vCJD cases who to date have undergone complete genetic analysis, apart from a single case all were in persons who were homozygous for methionine (MM) at codon 129 of the prion protein gene. The remaining vCJD case was the first pathology-confirmed case of vCJD in the UK in a methionine/valine (MV) heterozygous patient [100]. This rekindled fears of a







Figure 3: (a) Annual deaths from vCJD in the UK and France, the countries most severely affected by the disease, 1993–2022. Reproduced with modifications from: European Creutzfeldt-Jakob Disease Surveillance Network (EuroCJD; https://www.eurocjd.ed.ac. uk/data). (b) Number of annual vCJD cases in the United Kingdom and EU (without UK) compared with the course of the numbers of BSE cases reported from there, 1988–2013. Reproduced with modifications from: European Centre for Disease Prevention and Control (ECDC; https://www.ecdc.europa.eu/en/vcjd/facts).
potential second wave of vCJD in persons with different PRNP codon 129 genotypes. These concerns were further bolstered by the findings from experimental transmission studies in “humanized” transgenic mice, i.e. with different human PRNP codon 129 genotypes (HuMM, HuMV and HuVV). This indicated that all three PRNP codon 129 genotypes exhibit staggered susceptibility to vCJD infection (MM > MV > VV) and that the genotypes MV and VV are possibly associated with longer incubation times [101].
Based on early estimates, which were, however, still fraught with great uncertainties at that time, it was expected that in total there would be some 100 to 80000 (or more) cases of vCJD in the United Kingdom [102].
Based on later calculations, the United Kingdom was expected to have by 2040 between 150 and 6000 cases of primary vCJD infection [103]. In Germany, based on early estimates, which again were fraught with great uncertainties, it was expected there would be by 2040 several 100 (400–600) primary vCJD cases [104]. That figure compared with around 4000 classic CJD cases expected in Germany for the same period of time. Fortunately, the current situation is much more favourable with so far 178 vCJD cases in the UK and no case to date in Germany. Whether the number of cases will increase again in the future, for example in persons with genotypes other than MM at codon 129 of the prion protein gene, cannot be predicted with certainty at present.
Against that background, various more advanced epidemiological studies were carried out in the UK to estimate the likely maximum number of cases of primary vCJD infections expected there as well as the risk of secondary transmission from sub or preclinical infection carriers.
To estimate the prevalence of unidentified possible vCJD carriers in the British population for a more accurate assessment of the risk of secondary vCJD transmission through blood transfusion and, as applicable, other
medical interventions, in a large scale anonymized survey 11,109 appendix specimens obtained during routine appendectomies were examined for pathological prion protein deposition (Appendix study I) [105, 106]. In the absence of a reliable blood test for vCJD, that approach was based on the observation that all the cases of clinically manifest vCJD examined exhibited pronounced abnormal PrP accumulation in lymphoreticular tissues. Examination of the appendix specimens obtained between 1995 and 2000 identified abnormal prion protein deposition in three donors from the birth cohort 1961–1985. If the abnormal PrP accumulation detected in these cases is a reliable indicator for the presence of a pre or subclinical vCJD infection, based on the study the authors estimated the vCJD prevalence in the United Kingdom’s population between 1995 and 2000 would be 237 infections per 1 million inhabitants (95% confidence interval: 49–692 per 1 million), i.e. 1 case per 4000 inhabitants. If one estimates from the study findings the incidence of pre/subclinical vCJD infection in the age group of 10 to 30 year olds (accounting for around 83% of the group sampled), it would be expected that in that group 3,808 persons (95% confidence interval: 785–11.128) were incubating vCJD. Another investigation of initially 63,007 tonsil samples (obtained from elective tonsillectomies performed in England and Scotland between January 2004 and September 2008) did not, however, yield, any confirmed positive results [107].
Noteworthy is that in all the cases of pre or subclinical vCJD infection reported at that time abnormal PrP deposits were found in the appendix (or spleen) but not always in the tonsils. In February 2008, the Spongiform Encephalopathy Advisory Committee (SEAC) issued a statement on the apparently contrasting results (albeit still formally consistent within the confidence intervals) of the appendix vs tonsil study. Based on that, the SEAC viewed the findings of the appendix study conducted by Hilton et al. [105] as being the most robust indicator for the vCJD prevalence [106, 108].
In a second appendix study the appendectomy samples from a larger birth cohort (1940–1981; n = 32,441) were examined (Appendix study II). Sixteen positive samples were found – again
with a wide confidence interval – pointing to a higher vCJD prevalence of 493 per 1 million, or one case per 2000 inhabitants [109]. The findings of the two appendix studies raised additional concerns due to the fact that the positive appendix samples were from patients with all three PRNP codon 129 genotypes [109, 110].
An additional study (Appendix study III), which after interim analysis in 2016 [111] was finally published in March 2020 [112], ultimately confirmed a similar prevalence to that previously seen in the second appendix study. Admittedly, in that study appendix samples were examined from persons presumed to have not come into contact with the BSE agent (i.e. either had their the appendix removed before 1980 and thus prior to the BSE epidemic or were born after 1996, following the introduction of protective measures to interrupt the infection chain via human food). This led the study authors to believe that the UK population may have been exposed to the BSE agent already for a longer time than originally thought or that there is a low prevalence of abnormal PrP in lymphoreticular tissue that is not associated with the manifestation of vCJD.
Kuru and BSE are impressive examples of the ability of prions to cause outbreaks of TSEs of epidemic proportions under certain conditions among humans or animals. At the same time, BSE has cast light on the considerable zoonotic risks that animal prion diseases can present to humans, whereby epidemiologic data from the kuru epidemic indicate that the incubation time of acquired TSE in humans may be more than 50 years.
In that sense, as the BSE situation unfolded there appeared to be an urgent need at that time to take extensive precautionary and protective measures against the transmission of BSE between cattle (as well as to other animal species) and from cattle to humans.
As a result, the infection chains between cattle and from cattle to humans were effectively interrupted, which meant that the human infection risk from BSE is in the meantime deemed to be extremely low. In retrospect, however, it can also be said that because of the initially unidentified BSE spread the
infection chain between cattle and humans was effectively broken only after at least hundreds of thousands, and possibly millions, of persons had already been exposed to the BSE agent. The fact that to date “only” 178 and 233 deaths have been observed in the UK and worldwide, respectively, is very much due to the presence of an apparently strong species barrier [113, 114] that biologically greatly hampered transmission of the BSE agent to humans. At the time of the emergence of the initial vCJD cases, the strength of that protective species barrier was still of course unknown, something that must be borne in mind when assessing the modelbased risk estimates at that time [e.g. 102], which from today’s perspective make the case numbers projected back then appear to be much too high.
There is, of course, no protective species barrer when it comes to transmission of vCJD and other human TSE between humans. Therefore, back then BSE raised fears that unidentfied infection chains between people could in the worst case lead to extremely widespread transmission as seen in BSE itself or kuru. Due to this potential risk, special attention was therefore also paid to comprehensive protective measures for the prevention of secondary vCJD transmission from person to person e.g. through blood and blood products, organs and tissues or contaminated surgical instruments and other medical devices.
Against that background, the vCJD Task Force issued recommendations for reprocessing medical devices with special reference to CreutzfeldtJakob disease and its variant 20 years ago. In doing so, like many other groups involved in vCJD crisis management, it had to in some cases take action in the interest of infection protection already before reliable data were available. Despite these obstacles, from today’s perspective, these key recommendations of the vCJD Task Force for medical device reprocessing have proven to be sustainable with good practicability. The effectveness of the recommended measures against prions have been confirmed on a broad basis in numerous studies (see, for example, original data and referenced sources in: [76, 79, 115–120]). This effectiveness also extends in practice to conventional pathogens such as bacteria, viruses or fungi because of the paradigmatically
high hygiene demands made by prions on medical device reprocessing [80, 81, 121]. Perspectively, this may also apply at least partially to potential new challenges in the form of selfreplicating protein seeds from protein aggregation diseases such as Alzheimer’s or Parkinson’s disease [85, 86, 122].
Whereas the proposed measures of the vCJD Task Force have been widely evaluated and validated in experimental studies for medical device reprocessing, this has partially been done only to a lesser extent for other areas of BSE/ vCJD risk management. In some cases, therefore, there has been a lack of robust data to provide evidencebased answers to critical questions and objections about the necessity, appropriateness, or duration of certain measures to combat and prevent BSE/vCJD. From today’s perspective, accompanying research concepts for systematic evaluation of the manifold measures applied would therefore have been beneficial. This underlines the usefulness of a critical retrospective review of the infection control measures taken at the outset and subsequently also with regard to new, comparable events, in order to be able to readjust as best as possible where necessary and achieve the greatest possible understanding for and acceptance of the respective health policy crisis management.
Parts of this paper have been taken with the kind permission of Springer Nature from the following previous publications: 1) Der Internist. Aspekte zur Risikoabschätzung und Prävention nosokomialer Übertragungen der klassischen und varianten CJK [Aspects on risk assessment and prevention of healthcareassociated (nosocomial) transmission of classic and variant CJD]. M. Beekes, M. Mielke, R. Kurth; Copyright © 2002 Springer. 2) Bundesgesundheitsblatt – Gesundheitsforschung – Gesundheitsschut z. Die variante CreutzfeldtJakob Krankheit (vCJK). [Variant Creutzfeldt-Jakob disease (vCJD)]. M. Beekes; Copyright © 2010 Springer.
1. Europäische Kommission, CORDIS Forschungsergebnisse der EU. In den nächsten 30 Jahren Tausende von vCJKFällen im VK? https://cord
iseuropaeu/article/id/16496 thousandsofvcjdcasesintheukinthenext30 years/de. 2001.
2. Die bovine spongiforme Enzephalopathie (BSE) des Rindes und deren Übertragbarkeit auf den Menschen. Gemeinsame Information des Robert KochInstitutes (RKI), des Bundesinstitutes für gesundheitlichen Verbraucherschutz und Veterinärmedizin (BgVV), des PaulEhrlichInstitutes (PEI), und des Bundesinstitutes für Arzneimittel und Medizinprodukte (BfArM). Bundesgesundheitsbl – Gesundheitsforsch –Gesundheitsschutz. 2001;44:421–431.
3. Die Variante der CreutzfeldtJakobKrankheit (vCJK). Epidemiologie, Erkennung, Diagnostik und Prävention unter besonderer Berücksichtigung der Risikominimierung einer iatrogenen Übertragung durch Medizinprodukte, insbesondere chirurgische Instrumente – Abschlussbericht der Task Force vCJK zu diesem Thema. Bundesgesundheitsbl – Gesundheitsforsch – Gesundheitsschutz. 2002;45:376–394.
4. Creutzfeldt HG. Über eine eigenartige herdförmige Erkrankung des Zentralnervensystems. Z Ges Neurol Psychiat. 1920;57:1–18.
5. Jakob AM. Über eigenartige Erkrankungen des Zentralnervensystems mit bemerkenswertem anatomischem Befunde (Spastische Pseudosklerose – Enzephalomyelopahie mit disseminierten Degenerationsherden). Deutsch Z Nervenheilk. 1921;70:132–146.
6. Watson N, Brandel JP, Green A, Hermann P, Ladogana A, Lindsay T, et al. The importance of ongoing international surveillance for CreutzfeldtJakob disease. Nat Rev Neurol. 2021;17:362–379.
7. Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, et al. A new variant of CreutzfeldtJakob disease in the UK. Lancet. 1996;347:921–925.
8. Will RG, Ironside JW. Portrait of Variant CreutzfeldtJakob disease. In: Hörnlimann B, Riesner D, Kretzschmar H, editors. Prions in humans and animals. Berlin, New York: De Gruyter; 2006. p. 204–209.
9. Ritchie DL, Peden AH, Barria MA. Variant CJD: Reflections a Quarter of a Century on. Pathogens (Basel, Switzerland). 2021;10:1413.
10. Hörnlimann B, Riesner D, Kretzschmar HA, Will RG, MacDiarmid SC, Wells GAH, et al. Historical Introduction. In: Hörnlimann B, Riesner D, Kretzschmar HA, editors. Prions in Humans and Animals. Berlin, New York: de Gruyter; 2006. p. 3–27.
11. Liberski PP, Gajos A, Sikorska B, Lindenbaum S. Kuru, the First Human Prion Disease. Viruses. 2019;11.
12. Hörnlimann B, Alpers MP. Portrait of Kuru. In: Hörnlimann B, Riesner D, Kretzschmar HA, editors. Pr ions in Hu
mans an Animals. Berlin, New York: de Gruyter; 2006. p. 187–194.
13. Collinge J, Whitfield J, McKintosh E, Frosh A, Mead S, Hill AF, et al. A clinical study of kuru patients with long incubation periods at the end of the epidemic in Papua New Guinea. Philos Trans R Soc Lond B Biol Sci. 2008;363:3725–39.
14. Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144.
15. Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–83.
16. Brown P, Cervenakova L. A prion lexicon (out of control). Lancet. 2005;365:122.
17. Come JH, Fraser PE, Lansbury PT. A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci USA. 1993;90:5959–63.
18. Harper JD, Lansbury PT, Jr. Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the timedependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407.
19. Soto C. Prion hypothesis: the end of the controversy? Trends Biochem Sci. 2011;36:151–158.
20. Saa P, Cervenakova L. Protein misfolding cyclic amplification (PMCA): Current status and future directions. Virus Res. 2015;207:47–61.
21. Candelise N, Schmitz M, Da Silva Correia SM, Arora AS, VillarPiqué A, Zafar S, et al. Applications of the realtime quakinginduced conversion assay in diagnosis, prion straintyping, drug prescreening and other amyloidopathies. Expert Rev Mol Diagn. 2017;17:897–904.
22. Beekes M, Kurth R. BSE und CreutzfeldtJakob Krankheit – Gesundheitspo litische Bedeutung für die Bundesrepublik Deutschland und Europa. Dtsch Med Wochenschr. 2002;127:335–340.
23. Beekes M, Mielke M, Kurth R. Aspekte zur Risikoabschätzung und Prävention nosokomialer Übertragungen der klassischen und varianten CJK. Internist (Berl). 2002;43:738–748.
24. Hörnlimann B, Ryan JB, MacDiarmid SC. The course of the BSE epidemic –Retrospective epidemiological considerations. In: Hörnlimann B, Riesner D, Kretzschmar HA, editors. Prions in Humans and Animals. Berlin, New York: de Gruyter; 2006. p. 449–463.
25. Anderson RM, Donnelly CA, Ferguson NM, Woolhouse ME, Watt CJ, Udy HJ, et al. Transmission dynamics and epidemiology of BSE in British cattle. Nature. 1996;382:779–788.
26. MacDiarmid SC, Infanger P, Hörnlimann B. BSE Control Internationally recommended approaches. In: Hörnlimann B, Riesner D, Kretzschmar H,
editors. Prions in animals and humans. Berlin, New York: De Gryuter; 2006. p. 620–662920.
27. Uttley L, Carroll C, Wong R, Hilton DA, Stevenson M. CreutzfeldtJakob disease: a systematic review of global incidence, prevalence, infectivity, and incubation. Lancet Infect Dis. 2020;20:e2–e10.
28. Brown P, Preece M, Brandel JP, Sato T, McShane L, Zerr I, et al. Iatrogenic CreutzfeldtJakob disease at the millenium. Neurology. 2000;55:1075–81.
29. Cooper JD, Bird SM. UK dietary exposure to BSE in beef mechanically recovered meat: by birth cohort and gender. J Cancer Epidemiol Prev. 2002;7:59–70.
30. Boëlle PY, Cesbron JY, Valleron AJ. Epidemiological evidence of higher susceptibility to vCJD in the young. BMC Infect Dis. 2004;4:26.
31. Heath CA, Cooper SA, Murray K, Lowman A, Henry C, MacLeod MA, et al. Validation of diagnostic criteria for variant CreutzfeldtJakob disease. Ann Neurol. 2010;67:761–770.
32. Zeidler M, Johnstone EC, Bamber RW, Dickens CM, Fisher CJ, Francis AF, et al. New variant CreutzfeldtJakob disease: psychiatric features. Lancet. 1997;350:908–910.
33. Spencer MD, Knight RS, Will RG. First hundred cases of variant CreutzfeldtJakob disease: retrospective case note review of early psychiatric and neurological features. BMJ. 2002;324:1479–82.
34. Macleod MA, Stewart GE, Zeidler M, Will R, Knight R. Sensory features of variant CreutzfeldtJakob disease. J Neurol. 2002;249:706–711.
35. Zeidler M, Stewart GE, Barraclough CR, Bateman DE, Bates D, Burn DJ, et al. New variant CreutzfeldtJakob disease: neurological features and diagnostic tests. Lancet. 1997;350:903–907.
36. Head MW, Ironside JW, Ghetti B, Jeffrey M, Piccardo P, Will RG. Prion diseases. In: Love S, Budka H, Ironside JW, Perry A, editors. Greenfield´s Neuropathology. Volume 2. Boca Raton: CRC Press; 2015. p. 1016–86.
37. Head MW, Bunn TJ, Bishop MT, McLoughlin V, Lowrie S, McKimmie CS, et al. Prion protein heterogeneity in sporadic but not variant CreutzfeldtJakob disease: UK cases 1991–2002. Ann Neurol. 2004;55:851–859.
38. Head MW, Ritchie D, Smith N, McLoughlin V, Nailon W, Samad S, et al. Peripheral tissue involvement in sporadic, iatrogenic, and variant CreutzfeldtJakob disease: an immunohistochemical, quantitative, and biochemical study. Am J Pathol. 2004;164:143–153.
39. Hill AF, Zeidler M, Ironside J, Collinge J. Diagnosis of new variant CreutzfeldtJakob disease by tonsil biopsy. Lancet. 1997;349:99 –100.
40. Hilton DA, Fathers E, Edwards P, Ironside JW, Zajicek J. Prion immunoreactivity in appendix before clinical onset of variant CreutzfeldtJakob disease. Lancet. 1998;352:703–704.
41. Douet JY, Zafar S, PerretLiaudet A, Lacroux C, Lugan S, Aron N, et al. Detection of infectivity in blood of persons with variant and sporadic CreutzfeldtJakob disease. Emerg Infect Dis. 2014;20:114–117.
42. Orrú CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, et al. A test for CreutzfeldtJakob disease using nasal brushings. N Engl J Med. 2014;371:519–529.
43. Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic CreutzfeldtJakob disease. N Engl J Med. 2003;349:1812–20.
44. Douet JY, Huor A, Cassard H, Lugan S, Aron N, Arnold M, et al. Wide distribution of prion infectivity in the peripheral tissues of vCJD and sCJD patients. Acta Neuropathol. 2021;141:383–397.
45. Takatsuki H, Fuse T, Nakagaki T, Mori T, Mihara B, Takao M, et al. Prionseeding activity is widely distributed in tissues of sporadic CreutzfeldtJakob disease patients. EBioMedicine. 2016;12:150–155.
46. Aguzzi A, Heppner FL, Heikenwalder M, Prinz M, Mertz K, Seeger H, et al. Immune system and peripheral nerves in propagation of prions to CNS. Br Med Bull. 2003;66:141–159.
47. Honda H, Mori S, Watanabe A, Sasagasako N, Sadashima S, Dong T, et al. Abnormal prion protein deposits with high seeding activities in the skeletal muscle, femoral nerve, and scalp of an autopsied case of sporadic CreutzfeldtJakob disease. Neuropathology. 2021;41:152–158.
48. Orrú CD, Yuan J, Appleby BS, Li B, Li Y, Winner D, et al. Prion seeding activity and infectivity in skin samples from patients with sporadic CreutzfeldtJakob disease. Sci Transl Med. 2017;9.
49. Huor A, Douet JY, Lacroux C, Lugan S, Tillier C, Aron N, et al. Infectivity in bone marrow from sporadic CJD patients. J Pathol. 2017;243:273–278.
50. Orrù CD, Soldau K, Cordano C, Llibre-Guerra J, Green AJ, Sanchez H, et al. Prion Seeds Distribute throughout the Eyes of Sporadic CreutzfeldtJakob Disease Patients. mBio. 2018;9.
51. Hofmann A, Wrede A, Jürgens-Wemheuer WM, Schulz-Schaeffer WJ. Prion type 2 selection in sporadic CreutzfeldtJakob disease affecting peripheral ganglia. Acta Neuropathol Commun. 2021;9:187.
52. Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, et al. Classification of sporadic CreutzfeldtJakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46:224–233.
53. Zur Situation bei wichtigen Infektionskrankheiten in Deutschland:
CreutzfeldtJakobErkrankung in den Jahren 2010 bis 2011. Analyse und Interpretation der Meldedaten gemäß Infektionsschutzgesetz. Epidemiol Bull. 2013;4:31–37.
54. Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501.
55. Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, et al. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450, 526.
56. Lasmezas CI, Fournier JG, Nouvel V, Boe H, Marce D, Lamoury F, et al. Adaptation of the bovine spongiform encephalopathy agent to primates and comparison with CreutzfeldtJakob disease: implications for human health. Proc Natl Acad Sci USA. 2001;98:4142–7.
57. Scott MR, Will R, Ironside J, Nguyen HO, Tremblay P, DeArmond SJ, et al. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci USA. 1999;96:15137–42.
58. Beekes M, McBride PA. The spread of prions through the body in naturally acquired transmissbile spongiform encephalopathies. FEBS J. 2007;264:588–605.
59. World Health Organization. WHO guidelines on tissue infectivity distribution in transmissible spongiform encephalopathies. 2006.
60. Notari S, Moleres FJ, Hunter SB, Belay ED, Schonberger LB, Cali I, et al. Multiorgan detection and characterization of proteaseresistant prion protein in a case of variant CJD examined in the United States. PLoS One. 2010;5:e8765.
61. Valleron AJ, Boelle PY, Will R, Cesbron JY. Estimation of epidemic size and incubation time based on age characteristics of vCJD in the United Kingdom. Science. 2001;294:1726–8.
62. Health Protection Agency. Health protection report (19 January 2007): Fourth case of transfusionassociated variant CJD infection. 2007.
63. Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, et al. Possible transmission of variant CreutzfeldtJakob disease by blood transfusion. Lancet. 2004;363:417–421.
64. Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, et al. Clinical presentation and premortem diagnosis of variant CreutzfeldtJakob disease associated blood transfusion: a case report. Lancet. 2006;368:2061–7.
65. Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Penney M, et al. Accumulation of prion protein in tonsil and appendix: review of tissue samples. BMJ. 2002;325:633–634.
66. Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD af
ter blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364:527–529.
67. Health Protection Agency. Health protection report (20 February 2009): Post mortem finding of asymptomatic variant CreutzfeldtJakob disease abnormal prion protein in a person with haemophilia. 2009.
68. Hörnlimann B, Pauli G, Lemmer K, Beekes M, Mielke M. Prevention of the transmission of prion diseases in healthcare settings In: Hörnlimann B, Riesner D, Kretzschmar HA, editors. Prions in humans and animals. Berlin, New York: de Gruyter; 2006. p. 546–560.
69. Arbeitskreis Krankenhaus und Praxishygiene der AWMF. Prophylaxe der CreutzfeldtJakob Erkrankung in Krankenhaus und Praxis. Hyg Med. 2007;7/8:301–306.
70. Hopkins C, Geyer M, Topham J. Posttonsillectomy haemorrhage: a 7year retrospective study. Eur Arch Otorhinolaryngol. 2003;260:454–455.
71. Beekes M, Mielke M, Pauli G, Baier M, Kurth R. Aspects of risk assessment and risk management of nosocomial transmission of classical and variant CreutzfeldtJakob disease with special attention to German regulations. Contrib Microbiol. 2004;11:117–135.
72. Bertram J, Mielke M, Beekes M, Lemmer K, Baier M, Pauli G. Inaktivierung und Entfernung von Prionen bei der Aufbereitung von Medizinprodukten. Bundesgesundheitsbl – Gesundheitsforsch – Gesundheitsschutz. 2004;47:36–40.
73. Desinfektion und Sterilisation von chirurgischen Instrumenten bei Verdacht auf CreutzfeldtJakob Erkrankungen. Bundesgesundheitsblatt. 1996;39:282–283.
74. Simon D, Pauli G. Krankenversorgung und Instrumentensterilisation bei CJKPatienten und CJKVerdachtsfällen. Bundesgesundheitsblatt. 1998;41:279–285.
75. Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention beim Robert KochInstitut (RKI) und des Bundesinstitutes für Arzneimittel und Medizinprodukte (BfArM). Bundesgesundheitsbl – Gesundheitsforsch – Gesundheitsschutz. 2001;44:1115–26.
76. Schwenke KA, Wagenführ K, Thanheiser M, Beekes M. Kinetics of the reduction of CJD prion seeding activity by steam sterilization support the use of validated 134 °C programs. J Hosp Infect. 2022;doi: 10.1016/j.jhin.2022.08.014 (Online ahead of print).
77. Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert KochInstitut (RKI) und des Bundesinstitutes für Arzneimittel und Medizinprodukte
(BfArM). Bundesgesundheitsbl – Gesundheitsforsch – Gesundheitsschutz. 2012;55:1244–310.
78. McDonnell G, Burke P. The challenge of prion decontamination. Clin Infect Dis. 2003;36:1152–4.
79. McDonnell G, Dehen C, Perrin A, Thomas V, IgelEgalon A, Burke PA, et al. Cleaning, disinfection and sterilization of surface prion contamination. J Hosp Infect. 2013;85:268–273.
80. Lehmann S, Rauwel GPM, RogezKreuz C, Richard M, Belondrade M, Durand F, et al. New hospital disinfection processes for both conventional and prion infectious agents compatible with thermosensitive equipment. J Hosp Infect. 2009;72:342–350.
81. Beekes M, Lemmer K, Thomzig A, Joncic M, Tintelnot K, Mielke M. Fast, broadrange disinfection of bacteria, fungi, viruses and prions. J Gen Virol. 2010;91:580–589.
82. Abbott A. The redhot debate about transmissible Alzheimer’s. Nature. 2016;531:294–297.
83. Purro SA, Farrow MA, Linehan J, Nazari T, Thomas DX, Chen Z, et al. Transmission of amyloidbeta protein pathology from cadaveric pituitary growth hormone. Nature. 2018;564:415–419.
84. Thomzig A, Wagenführ K, Pinder P, Joncic M, Schulz-Schaeffer WJ, Beekes M. Transmissible α-synuclein seeding activity in brain and stomach of patients with Parkinson’s disease. Acta Neuropathol. 2021;141:861–879.
85. Beekes M, Mielke M, Thomzig A. Avoid brain contamination from surgery. Nature. 2019;565:429.
86. Thomzig A, Wagenführ K, Daus ML, Joncic M, Schulz-Schaeffer WJ, Thanheiser M, et al. Decontamination of medical devices from pathological amyloid-β-, tau- and α-synuclein aggregates. Acta Neuropathol Commun. 2014;2:151.
87. Beekes M, Thomzig A. Sterilization techniques: Counter the risk of Alzheimer’s transfer. Nature. 2016;531:580.
88. Beekes M, Thomzig A, SchulzSchaeffer WJ, Burger R. Is there a risk of prionlike disease transmission by Alzheimer or Parkinsonassociated protein particles? Acta Neuropathol. 2014;128:463–476.
89. Jaunmuktane Z, Brandner S. Invited Review: The role of prionlike mechanisms in neurodegenerative diseases. Neuropathol Appl Neurobiol. 2020:522–545.
90. Melki R. Alphasynuclein and the prion hypothesis in Parkinson’s disease. Rev Neurol (Paris). 2018;174:644–652.
91. Walker LC. Prion-like mechanisms in Alzheimer disease. Handb Clin Neurol. 2018;153:303–319.
92. Banerjee G, Adams ME, Jaunmuktane Z, Alistair Lammie G, Turner B, Wani M, et al. Early onset cerebral amyloid angiopathy following childhood exposure to cadaveric dura. Ann Neurol. 2019;85:284–290.
93. Jaunmuktane Z, Mead S, Ellis M, Wadsworth JD, Nicoll AJ, Kenny J, et al. Evidence for human transmission of amyloidbeta pathology and cerebral amyloid angiopathy. Nature. 2015;525:247–250.
94. Kovacs GG, Lutz MI, Ricken G, Strobel T, Hoftberger R, Preusser M, et al. Dura mater is a potential source of Abeta seeds. Acta Neuropathol. 2016;131:911–923.
95. Will RG. Portrait der neuen Variante der CreutzfeldtJakob Krankheit (nvCJD). In: Hörnlimann B, Riesner D, Kretzschmar H, editors. Prionen und Prionkrankheiten. Berlin, New York: De Gruyter; 2001. p. 152–157.
96. The National CJD Research and Surveillance Unit (NCJDRSU). Current data an vCJD cases worldwide. https:// www.cjd.ed.ac.uk/sites/default/files/ worldfigs.pdf. 2022.
97. CreutzfeldtJakob Disease International Surveillance Network. Variant CJD Cases Worldwide. Available online: https:// www.eurocjd.ed.ac.uk/data_tables.
98. Joint UKBTS/NIBSC professional advisory committee. Postion statement CreutzfeldtJakob disease (12 March 2009). http://www.transfusionguidelines.org.uk/docs/pdfs/dl_ ps_vcjd_2009–03.pdf.
99. Peden A, McCardle L, Head MW, Love S, Ward HU, Cousens SN, et al. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia. 2010;16:296–304.
100. Mok T, Jaunmuktane Z, Joiner S, Campbell T, Morgan C, Wakerley B, et al. Variant CreutzfeldtJakob Disease in a Patient with Heterozygosity at PRNP Codon 129. N Engl J Med. 2017;376:292–294.
101. Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, Thomson V, et al. Predicting susceptibility and incubation time of humanto human transmission of vCJD. Lancet Neurol. 2006;5:393–398.
102. Cousens SN, Vynnycky E, Zeidler M, Will RG, Smith PG. Predicting the CJD epidemic in humans. Nature. 1997;385:197–198.
103. Ghani AC, Ferguson NM, Donnelly CA, Anderson RM. Predicted vCJD mortality in Great Britain. Nature. 2000;406:583–584.
104. Bericht der Arbeitsgruppe Gesamtstrategie Blutversorgung angesichts vCJK. RKI-Hausdruckerei, August 2001.
105. Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Ritchie D, et al. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol. 2004;203:733–739.
106. Spongiform Encephalopathies Advisory Committee (SEAC). Position statement “Prevalence of subclinical variant CreutzfeldtJakob disease infections”. 2008.
107. Clewley JP, Kelly CM, Andrews N, Vogliqi K, Mallinson G, Kaisar M, et al. Prevalence of disease related prion protein in anonymous tonsil specimens in Britain: cross sectional opportunistic survey. BMJ. 2009;338:b1442.
108. Department of Health. Mapping out the consequences of screening blood donations for PrPSc. 2009.
109. Gill ON, Spencer Y, RichardLoendt A, Kelly C, Dabaghian R, Boyes L, et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ. 2013;347:f5675.
110. Ironside JW, Bishop MT, Connolly K, Hegazy D, Lowrie S, Le GM, et al. Variant CreutzfeldtJakob disease: prion protein genotype analysis of positive appendix tissue samples from a retrospective prevalence study. BMJ. 2006;332:1186–8.
111. Public Health England. Summary results of the third national survey of abnormal prion prevalence in archived appendix specimens. Health Protection Report 2016; 10 (26):11–12, 12th August 2016. Available online: https:// www.gov.uk/government/uploads/ system/uploads/attachment_data/ file/546883/hpr2616.pdf.
112. Gill ON, Spencer Y, RichardLoendt A, Kelly C, Brown D, Sinka K, et al. Prevalence in Britain of abnormal prion protein in human appendices before and after exposure to the cattle BSE epizootic. Acta Neuropathol. 2020;139:965–976.
113. Novakofski J, Brewer MS, MateusPinilla N, Killefer J, McCusker RH. Prion biology relevant to bovine spongiform encephalopathy. J Anim Sci. 2005;83:1455–76.
114. Houston F, Andréoletti O. Animal prion diseases: the risks to human health. Brain Pathol. 2019;29:248–262.
115. Lemmer K, Mielke M, Pauli G, Beekes M. Decontamination of surgical instruments from prion proteins: in vitro studies on the detachment, destabilization and degradation of PrPSc bound to steel surfaces. J Gen Virol. 2004;85:3805–16.
116. Lemmer K, Mielke M, Kratzel C, Joncic M, Oezel M, Pauli G, et al. Decontamination of surgical instruments from prions. II. In vivo findings with a model system for testing the removal of scrapie infectivity from steel surfaces. J Gen Virol. 2008;89:348–358.
117. Beekes M. Die variante CreutzfeldtJakob Krankheit (vCJK). Epidemiologie und Schutzmaßnahmen gegen eine Übertragung von Mensch zu Mensch. Bundesgesundheitsbl – Gesundheitsforsch – Gesundheitsschutz. 2010;53:597–606.
118. Belondrade M, Nicot S, Béringue V, Coste J, Lehmann S, Bougard D. Rapid and Highly Sensitive Detection of Variant CreutzfeldtJakob Disease Abnor
mal Prion Protein on Steel Surfaces by Protein Misfolding Cyclic Amplification: Application to Prion Decontamination Studies. PLoS One. 2016;11:e0146833.
119. Belondrade M, JasDuval C, Nicot S, Bruyère-Ostells L, Mayran C, Herzog L, et al. Correlation between Bioassay and Protein Misfolding Cyclic Amplification for Variant Creutzfeldt-Jakob Disease Decontamination Studies. mSphere. 2020;5:e00649–19.
120. McDonnell G. Antiseptics, Disinfection and Sterilization. Washington, DC: ASM Press; 2017.
121. Wagenführ K, Beekes M. Harnessing prions as test agents for the development of broadrange disinfectants. Prion. 2012;6:1–6.
122. Pinder P, Thomzig A, SchulzSchaeffer WJ, Beekes M. Alpha-synuclein seeds of Parkinson´s disease show high prion-exceeding resistance to steam sterilization. J Hosp Infect. 2021;108:25–32.
123. Baral PK, Swayampakula M, Aguzzi A, James MN. Xray structural and molecular dynamical studies of the globular domains of cow, deer, elk and Syrian hamster prion proteins. J Struct Biol. 2015;192:37–47.
124. Kraus A, Hoyt F, Schwartz CL, Hansen B, Hughson AG, Artikis E, et al. Structure of an infectious mammalian prion. bioRxiv. 2021:2021.02.14.431014.
125. Kraus A, Hoyt F, Schwartz CL, Hansen B, Artikis E, Hughson AG, et al. High-resolution structure and strain comparison of infectious mammalian prions. Mol Cell. 2021;81:4540–51.e6.
126. Beekes M. The Neural GutBrain Axis of Pathological Protein Aggregation in Parkinson’s Disease and Its Counterpart in Peroral Prion Infections. Viruses. 2021;13.
127. Jürgens-Wemheuer W, Wrede A, Schulz-Schaeffer W. Defining the Prion Type of Fatal Familial Insomnia. Pathogens (Basel, Switzerland). 2021;10.






On Saturday 36 CSSD staff members from Rotorua, Whakatane, and Tauranga attended the CSSD Regional Training Day held at Hauora A Bay of Plenty at the hospital Education Centre.
The morning was a chance for all CSSD staff to learn and even have a go at the equipment that they constantly reprocess for theatre. Unless they are given an inservice on the equipment, they often don’t know how an instrument works or how it is used in theatre.
The morning started with a couple of presentations: Ginene Scott from 3M spoke about Indicators, the different types and the importance of them being placed in every pack etc.
Kaylene Visser from NZ Validation Services had the CSSD staff interacting with a bit of jazz-ercise and then answering questions with either a touch of the ear or shoulder for your answer. This was designed to promote right and left hemisphere crossover brain function and was a lot of fun!
Then Audrey Williams from Multigate had an early start to zoom in from Australia to talk about the Sterile storeroom and what should be considered when setting one up.
After a lovely Brunch supplied by our reps, we carried on with the workshops.
J&J DePuy Synthes – Lag Screws (hands on with drills, plates and screws)
Medtronic: Signia and laparoscopic training (Stapler and the laparoscopic simulator, moving elastic bands and tying knots in string using lap graspers)
Dental: Dental instruments and extraction sets (the how and why of extractions)
Multigate: Pro-Zone sterilizing wraps (blindfold speed wrapping)
These went down a treat especially when the staff were able to get hands-on and have a go. All in all, it was a great day had by all, and with the reports I am getting back everyone had a great time.
Jenny CarstonSterilising Technician Coordinator
Hauora a Toi Bay of Plenty





During October 2023 I had the opportunity to attend the WFHSS conference which was held in Brussels Belgium. To be able to attend these conferences is a great privilege. While I am in attendance I make it my mission to tell as many people as possible about New Zealand, our profession and our qualifications. I believe it is so important to tell the world how great we are and how far we have come as a profession. For this conference I also had a special person with me, to experience first-hand for themselves the extravaganza. With me was our 2023 scholarship recipient Donna Dador from Southern Cross Hospital Christchurch. This was Donna’s first major adventure on her own and I am certain she enjoyed every minute of her time in Belgium.
I arrived a couple of days early into Brussels to overcome the jet lag and to get my bearings in this city. Fortunately I have enough school level French to get around.
I also needed to arrive early in order to attend a forum hosted by the WFHSS. It is similar to our AGM, however occurs before the opening of the conference. Here I get to meet national association leaders from all around the globe. Additionally, this is where each country gets to vote on any major changes being made by the WFHSS. It is important that NZ votes at this meeting and that the world knows who we are and what we have to contribute.
The conference officially opened on the Wednesday evening. There were the obligatory speeches then we were entertained by a fantastic female choir who were all between 18 and 20 years. This choir enter competitions and sing all around the world. They have a conductor who was entertainment in himself by the way he threw himself around enthusiastically waving his hands with directions. Post the entertainment it was time for the opening of the trade exhibition, where we were treated to drinks and finger food and of course CHOCOLATE. Belgium is the home of amazing chocolate and each company who was presenting at the conference was giving it away in spade loads. It was at this point we also discovered there were several others from NZ attending the conference, so we had a chat about what we were expecting to see and hear at the conference.
Thursday was the first full day of conference with presentations in the main auditorium and smaller presentations at lunchtime by sponsors e.g., Steris or ASP in the smaller meeting rooms. Talking of lunch at this conference lunch was presented in individual lunch boxes. The good thing about these lunch boxes was that they were reusable and made from recycled material. I managed to bring mine all the way back to NZ to use as my work lunch boxes.
Friday was a repeat of Thursday with sessions beginning at 8.30am and going on until 4.30pm and a final session on the Saturday morning post the gala dinner, for the few of us who actually made it after the previous night.
At the end of the hectic first day Donna and myself were invited to attend a chocolate making class hosted by Belimed. There were around 40 delegates who went along to learn the art of making and eating chocolate. We shared a work table with the delegates from Latvia who immediately adopted us two from NZ and quickly became great friends for the rest of the conference. Following on from being covered in chocolate and realising we would never get jobs as chocolatiers we headed on to a hosted dinner in the centre of Brussels. The conference sessions were full on and at break times there was a rush to get to meet all the trades and eat there chocolate offerings and free coffee.
A major theme throughout the conference was around the circular economy and the impact that theatres and CSSD have on this. One presentation was entitled”Hospital MiningCSSD and OR a goldmine for new raw materials in the circular economy.” A Dutch company conducted research into the amount of waste generated by CSSD and OR and a value was attached to this. They looked at what they could reuse and for what types of products. For example sterilising wrap was collected along with other plastics e.g. bowls and this was put through a process where it was cleaned and almost blended down to fine pellets which could then be melted and redeveloped into new products. One such product from this company we already have here in NZ for holding open hinges on instruments. The whole presentation was thought provoking and made one think why we are not doing this

in NZ on a large scale, particularly when it is getting more difficult to get supplies from overseas.
Continuing on this theme, during the lunch break two UK doctors sponsored by Steris talked on the UK-NHS initiative , which is now mandatory for using reusable RMD where applicable thus cutting down on the use of single use items which go direct to the landfill.
An interesting development was discussed in the presentation on an “Automated Endoscope Channel Cleaner-AECC ” It is still in production testing , however the US FDA have accepted the machine into their safer technologies programme so hopefully clearance is not far off. This is recognition that the AECC has the potential to improve the safety in endoscope cleaning.
The machine eliminates brushing and flushing through automation and significantly reduces the number of steps in the process. Tests undertaking on 1,4mm lumens with biofilm growth compared manual cleaning with the AECC. The lumens were stained with crystal violet. Post cleaning results demonstrated that the lumens manually cleaned still contained biofilm and the lumens cleaned with AECC were clear. This is a machine of interest. Not only has it so far demonstrated excellent cleaning efficacy, however as the presenter stated it is also good for the technician who has to manually brush and clean as there is less potential for shoulder and back injury from vigorous brushing. Hopefully it will not be too long before it is on the market.
Another major topic was the “Access to Reliable Product Data” The benefit of this, is in as having a uniform dataset for RMD. This would include:
• Complete and standardised data set for all medical devices
• Fully integrated in GDSN- one single source of truth
• Up to date
• Less error prone
• More efficient
• Real time available
The benefits of this would be that Suppliers and Hospitals worldwide are using the same data via the global data synchronisation network.
There was a lengthy presentation on the “Alignment of Global Medical Device Standards and their Acceptability for Regulatory Purposes.” Basically there are numerous standards out there in different countries all aiming for the same goal but not all quite on the same page. I do have slides of the presentation if anyone wants more information and I am sure this will be published shortly in Zentral Sterilisation
A topic of great interest to me was that of the use of UVC for Disinfection. A lot of research has been undertaken in Europe around the use of UVC for disinfection of non lumened endoscopes and rigid scopes particularly in ENT settings. The results of the research have shown that UVC is effective on scopes and in reducing the bacterial load.
There is so much interest in UVC that there were 3 companies demonstrating there UVC disinfectors.
The speaker from Healthmark Industries presented on the need for examining and testing of laparoscopic instruments. His presentation came from a personal perspective as his young daughter had received burns internally due to the insulation on the laparoscopic sheath being faulty. He conducted a study aimed at identifying how common insulation failures were on RMD. For the study he selected insulated laparoscopic instruments from random trays and bipolar insulated forceps and cables. For the testing he used a cable continuity teste, an insulation tester and an enhanced inspection microscope. All of the testing equipment is readily available and can be found in most CSSD but often not used. The results of his audits were dire. Of 416 instruments tested, 233 showed failures on insulation testing or inspection. Of all the facilities in the study, 16 showed a failure rate of 75% - 100% of all devices tested. On average insulated cables showed a 6% failure rate across 32 facilities .Bipolar Forceps had the highest failure rate with 27 facilities having 75%100% failure rate.
I can see that there is potential to replicate this study in NZ. It would be interesting to see how we measure up against this study.
There were so many interesting topics that I could possibly fill the whole Supplyline, but have picked my favourites.




Friday evening the conference gala dinner was held at the Brussels car museum. If you love cars as much as I do this is the place to go, I think every car ever made is housed here. This dinner had a dress code theme which was”the roaring 20’s”. The venue is huge and everyone or almost everyone (you know who you are Kiwis) turned up in costume. I have added a few photos of the event here including me with my new found Latvian friends.
On the final morning of the conference there were definitely reduced numbers attending the lectures. I can only presume there were a few tired people post the gala dinner, however Donna and I stayed until the very end.



I have attended world conferences previously where scholarship winners have joined me on the trip. I can honestly say that we receive such positive feedback about the experience and knowledge that they gain from attending. Next year the WFHSS conference is to be held in Santiago Chile only one flight away from Auckland. We look forward to receiving your applications to attend.
Shelagh Thomas President NZSSAdioxide has chlorine in its name and has a similar smell to chlorine, it differs chlorine in its chemical structure and behaviour. Discovered in 1814 by Sir hlorine dioxide is a molecule consisting of two oxygen atoms and one whereas chlorine exists as a diatomic molecule, meaning it consists of two bonded together (Ganiev et al., 2016) (see Figure 1).
While chlorine dioxide has chlorine in its name and has a similar smell to chlorine, it differs from elemental chlorine in its chemical structure and behaviour. Discovered in 1814 by Sir Humphrey Davey, chlorine dioxide is a molecule consisting of two oxygen atoms and one chlorine atom, whereas chlorine exists as a diatomic molecule, meaning it consists of two chlorine atoms bonded together (Ganiev et al., 2016) (see Figure 1).
Literature indicates there is no single encapsulating mode of action, and chlorine dioxide has different biocidal effects depending on the type of organism. Chlorine dioxide causes the chemical disruption of cell walls and the inhibition of protein synthesis for bacteria, fungi and yeasts (Benarde et al., Wei et al., Wen et al.). The proteins are denatured for viruses, amino acids are modified, and genetic material is impaired (Miura and Shibata, Noszticzius et al., Alvarez and O’Brien). For bacterial spores, research suggests that chlorine dioxide causes severe bacterial cell inner membrane damage (Young and Setlow). For biofilms (the aggregation of microbial species, such as bacteria and fungi), chlorine dioxide can penetrate the slime layers and oxidise the polysaccharide matrix that holds the biofilm together (Kim et al., 2022).
Antimicrobial Resistance (AMR) happens when microorganisms develop the ability to overcome the drugs designed to kill them. The more resistant the microorganisms become to these drugs, the harder they are to kill. A similar interaction may occur when an insufficient disinfectant is used. This has led to a significant impact on the healthcare environment. Chlorine dioxide’s mode of action is not affected by these resistance pathways, as it is unaffected by defensive molecular features, such as cell walls. It steals electrons from within the microorganism, making it unstable and inevitably destroying it. This mode of action means that microorganisms cannot build resistance (Andrés et al., 2022; Noszticzius et al., 2013).
. The structure of chlorine and chlorine dioxide molecules
Oxidising agents accept electrons from another substance, causing the other substance to lose electrons and become oxidised. Both chlorine dioxide and chlorine are oxidising agents. However, they have different oxidation capacities and strengths. Oxidation capacity refers to the number of electrons one molecule can obtain from another molecule. Chlorine dioxide can steal five electrons, whereas chlorine can take only two, making chlorine dioxide more effective than chlorine at lower concentrations (Ran et al., 2019).
accept electrons from another substance, causing the other substance to become oxidised. Both chlorine dioxide and chlorine are oxidising they have different oxidation capacities and strengths. Oxidation capacity number of electrons one molecule can obtain from another molecule. Chlorine five electrons, whereas chlorine can take only two, making chlorine dioxide chlorine at lower concentrations (Ran et al., 2019).
Chlorine dioxide has been used as a globally approved chemistry for the disinfection of many medical devices and surfaces by generating the active biocidal concentration at the point of use. The generation is typically done through a chemical reaction between sodium chlorite and an acid to create the active concentration.
A disinfectant manufacturer has pioneered the inherent ease of use product designs that combine the precursor solutions of sodium chlorite and citric acid with the pull of a trigger, the bursting of a sachet, or the press of a pump at the point of use.
potential describes how strongly an oxidiser reacts with an oxidisable
The oxidation potential describes how strongly an oxidiser reacts with an oxidisable substance. Chlorine dioxide has a lower potential than chlorine. It is not as reactive as chlorine and has been shown to produce little to no by-products compared to chlorine (Ran et al., 2019) due to the different reaction mechanisms. The first step in the reaction of chlorine dioxide with many organic compounds is a single-electron oxidation. This is different from electrophilic addition, which is what is observed with chlorine. This mechanism prevents the formation of harmful chlorinated organic compounds such as Haloacetic acid (HAAs) and Trihalomethanes (THMS), which are proven carcinogens (Hrudey et al., 2009).
Chlorine dioxide is a more selective oxidiser than chlorine; this contributes to the effective microbicidal efficacy of chlorine dioxide at concentrations far lower than typically required for chlorine, which also results in a lower environmental impact (Wu et al., 2010) (Hrudey et al., 2009).
How Chlorine Dioxide works to Destroy Microorganisms
Chlorine dioxide has a lower potential than chlorine. It is not as reactive as been shown to produce little to no by-products compared to chlorine (Ran the different reaction mechanisms. The first step in the reaction of with many organic compounds is a single-electron oxidation. This is electrophilic addition, which is what is observed with chlorine . This revents the formation of harmful chlorinated organic compounds such as (HAAs) and Trihalomethanes (THMS), which are proven carcinogens 2009).
The Use of Chlorine Dioxide as a Disinfectant in Healthcare Chlorine dioxide has been utilised as a chemical disinfectant to offer point-of-care disinfection (performed near or at the site of a patient) in various human medicine sectors, such as Ophthalmology, Otorhinolaryngology (ENT), Ultrasound, Endoscopy, Radiology, Urology, Emergency Services, Gynaecology and Obstetrics to name a few. These products are intended to disinfect non-invasive and invasive semicritical medical equipment and devices, including endoscopes that cannot be sterilised and general surface disinfection, including floors, walls, countertops, and equipment.
Chlorine dioxide is currently used in the following formats on the market:
• Decontamination wipes.
A three-wipe system for the decontamination of non-
a more selective oxidiser than chlorine; this contributes to the effective
lumened invasive and non-invasive medical devices such as nasendoscopes, laryngoscopes, transoesophageal echocardiographic probes, transvaginal and transrectal ultrasound probes. It comprises a cleaning wipe for the removal of soil and organic matter before high-level disinfection, a pre-impregnated wipe with the base solution, and when combined with an activator foam, generates chlorine dioxide for the high-level disinfection of medical devices in 30 seconds, and a sterile water rinsing wipe to remove chemical residues from the medical device after disinfection.
• Disinfectant foams.
The foam products comprise a dual-sided bottle; one chamber contains base solution, and the other has activator solution, which, when dosed or sprayed combine to generate the active chlorine dioxide for:
- The high-level disinfection of ophthalmic medical devices in two minutes, such as diagnostic contact lenses, reusable tonometers, pachymeters, and ophthalmic ultrasound probes (A-scan and B-scan probes).
- The intermediate-level disinfectant of non-invasive ultrasound probes, cables, plugs, probe holders, monitors, and control panels at the intermediate level in 30 seconds.
- The sporicidal disinfection of hard, non-porous surfaces of medical equipment in two minutes such as IV poles, tourniquets, surfaces of transfusion pumps, dialysis machines, patient monitoring equipment, mattresses, bed rails, dressing trolleys, commodes, instrument tables, bench tops, general building, and fitting surfaces.
• Immersion disinfectant.
The chlorine dioxide immersion disinfectant comprises a dual-sided laminated sachet, each side containing base and activator solution. Folding the sachet in half and applying pressure to one side separates the seal, leading to the two compartments mixing to generate chlorine dioxide ready to be added to five litres of water. This disinfectant was specifically designed for a 5-minute high-level disinfection cycle in a semi-automated washer disinfector for the disinfection of invasive and non-invasive, heat sensitive, non-lumened and single-lumened endoscopes, laparoscopes, or other suitable medical devices which are invasive either by body orifice or surgical procedure.
The Importance of Water Quality in EWDs.
Water impurities can adversely affect a medical device, (re-) processing procedures, and the patient. Tap water is often of insufficient quality for specific reprocessing activities and, therefore, generally needs water treatment to remove impurities or inactivate microorganisms that may be present.
The Association for the Advancement of Medical Instrumentation (AAMI) published AAMI ST108:2023 - Water for the processing of medical devices, standard in 2023. This standard establishes the minimum requirements for water quality necessary to process medical devices intended for patient use effectively.
AAMI ST108:2023 outlines three types of water used in
healthcare facilities:
1. Utility Water: water as it comes from the tap, predominantly used for medical device processing except for the final rinse, where critical Water is recommended.
2. Critical Water: extensively treated water that removes microorganisms and inorganic and organic materials, usually by a multistep treatment process. It is mainly used as the final rinse after high-level disinfection, for the final rinse for critical devices before sterilisation, and feedwater for process steam production.
3. Steam: vaporised water produced by a centralised boiler or a generator/heat exchanger near the steriliser.
Utility water is suitable for all stages of the manual preclean until the stage of disinfectant dilution and final rinse. Here, the emphasis is on the Endoscope Washer Disinfector (EWD) manufacturer to decide on the type of water required, stipulated in the device’s instructions for use (AAMI, 2023). Therefore, with the introduction of this guidance, it is speculated that there will be a shift in the use of critical water in EWDs.
Other guidance on water quality used in EWDs, such as the Australian Standards AS 5369: 2023 – Reprocessing of reusable medical devices and other devices in health and non-health related facilities, the United Kingdom Health Technical Memorandum (HTM) 01-06 – Management and decontamination of flexible endoscopes, EN ISO 15883Washer-disinfectors - Part 4: Requirements and tests for washer-disinfectors employing chemical disinfection for thermolabile endoscopes also recognises that rinsing with microbial-free water is as significant in the reprocessing cycle as adequate high-level disinfection.
Biofilms and bacteria in the pipelines can supply contaminated water to EWDs. The EWDs themselves are challenging to disinfect due to their complex design. This leads to moist surfaces within them, leading to further bacterial proliferation, favouring biofilm formation. Contaminated water can compromise medical devices, which, if inadequately dried and stored, can provide favourable conditions for biofilm formation on the device and any ridges, indentations and lumen(s) present (de Bruijn A. & van Drongelen A., 2010, Roberts, 2013)). This poses a risk to the patient and staff handling the device. To prevent contamination of the water flowing through the EWDs and subsequent re-contamination of devices, it is paramount to for the water supplied to EWDs to be microbial-free (de Bruijn A & van Drongelen A., 2010).
Reverse Osmosis (RO) plays an integral part in producing quality water. It can remove microbial and ionic contaminants, lowering the outgoing water’s conductivity levels by passing them through a semi-permeable membrane and a final 0.2μm filter. However, the significant concern of RO systems is biofouling (the growth and deposition of biofilms) of pipelines to the EWDs, the membranes, and the filters that purify the water passing through, which can lead to the presence of biofilm in the treated water (Flemming 2002). Endotoxins in rinse water are another significant concern. While RO systems are usually effective in removing
endotoxins, if biofouling occurs, this can inhibit RO’s ability to remove endotoxins, resulting in contaminated rinse water.
Chlorine dioxide is utilised in a Class I Medical Device water purification system (Medical Device Regulation (EU) 2017/745) that offers optional RO, chemical dosing in low concentrations, and filtration. The water purification system is listed in the Australian Register of Therapeutic Goods as a Class I medical device. It meets the requirements set by the AAMI ST108 and AS 5369 2023 for the acceptable levels of endotoxins in the rinse water, and also complies with EN ISO 15883 and HTM 01-06 guidance.
The chlorine dioxide is dosed before the final 0.2μm filter, preventing bacterial proliferation and biofilm formation while simultaneously treating the 0.2μm filter used during an EWD’s decontamination cycle. This saves the user time and money as a separate manual disinfection process is not required to decontaminate the filter.
Since its discovery, chlorine dioxide has been integral to many industrial and healthcare applications owing to its versatility. Over the past 30 years a UK manufacturer has utilised a proprietary method for generating chlorine dioxide at the point of care.
Chlorine dioxide products have been successfully utilised as a part of standard infection prevention and control procedures in healthcare. They destroy a broad spectrum of microorganisms in a short, uniform contact time. This allows quick turnaround of patient rooms.
A water purification system that utilises chlorine dioxide for chemical dosing at low concentrations can also eliminate bacteria, preventing biofilm formation, and significantly lowering endotoxin levels, ensuring microbial-free water.
Alvarez, ME & O’Brien, RT 1982, ‘Mechanisms of Inactivation of Poliovirus by Chlorine Dioxide and iodine.’, Applied and Environmental Microbiology, vol. 44, no. 5, pp. 1064–1071.
Andrés, CMC, Lastra, JMP de la, Andrés Juan, C, Plou, FJ & Pérez-Lebeña, E 2022, ‘Chlorine Dioxide: Friend or Foe for Cell Biomolecules? a Chemical Approach’, International Journal of Molecular Sciences, vol. 23, no. 24, p. 15660.
Association for the Advancement of Medical Instrumentation (AAMI) 2023, ANSI/AAMI ST108:2023 Water for the Processing of Medical Devices, American National Standards Institute, Inc. Benarde, MA, Snow, WB, Olivieri, VP & Davidson, B 1967, ‘Kinetics and Mechanism of Bacterial Disinfection by Chlorine Dioxide’, Applied Microbiology, vol. 15, no. 2, pp. 257–265.
de Bruijn A & van Drongelen A 2010, ‘Quality of the Final Rinse Water for Endoscope Washer disinfectors. a Literature Review. Report 360050019/2009’, National Institute for Public Health and the Environment.
Flemming, H-C. 2002, ‘Biofouling in Water Systems – Cases, Causes
and Countermeasures’, Applied Microbiology and Biotechnology, vol. 59, no. 6, pp. 629–640.
Ganiev, IM, Timergazin, QK, Kabalnova, NN, Shereshovets, VV & Tolstikov, GA 2016, ‘Reactions of Chlorine Dioxide with Organic Compounds’, Eurasian Chemico-Technological Journal, vol. 7, no. 1, p. 1.
Hrudey, SE 2009, ‘Chlorination disinfection by-products, public health risk tradeoffs and me’, Water Research, vol. 43, no. 8, pp. 2057–2092.
International Organization for Standardization (ISO) 2018, ISO 15883
- Washer-disinfectors - Part 4: Requirements and Tests for washerdisinfectors Employing Chemical Disinfection for Thermolabile Endoscopes, European Committee for Standardisation (CEN), Brussels.
Kim, S & Park, SH 2022, ‘Chlorine dioxide gas mediated inactivation of the biofilm cells of’, Journal of Food Science and Technology, vol. 59, Springer Science+Business Media, no. 12, pp. 4863–4869.
Miura, T & Shibata, T 2010, ‘Antiviral Effect of Chlorine Dioxide against Influenza Virus and Its Application for Infection Control’, The Open Antimicrobial Agents Journal, vol. 2, no. 2, pp. 71–78.
Noszticzius, Z, Wittmann, M, Kály-Kullai, K, Beregvári, Z, Kiss, I, Rosivall, L & Szegedi, J 2013, ‘Chlorine Dioxide Is a Size-Selective Antimicrobial Agent’, in P Schlievert (ed.), PLoS ONE, vol. 8, no. 11, p. e79157.
Ran, Y, Chen Qing-min & Fu Maorun 2019, ‘Chlorine Dioxide Generation Method and Its Action Mechanism for Removing Harmful Substances and Maintaining Quality Attributes of Agricultural Products’, Food and Bioprocess Technology, vol. 12, Springer Science+Business Media, no. 7, pp. 1110–1122.
Roberts, CG 2013, ‘The Role of Biofilms in Reprocessing Medical Devices’, American Journal of Infection Control, vol. 41, no. 5, pp. S77–S80.
Standards Australia LTD. 2023, AS 5369 Reprocessing of Reusable Medical Devices and Other Devices in Health and Non-health Related Facilities, Joint Standards Australia/Standards New Zealand Committee HE-023.
United Kingdom Department of Health 2016, Health Technical Memorandum (HTM) 01-06: Decontamination of flexible endoscopes.
Wei, M, Wu, Q, Huang, Q, Wu, J-M & Zhang, J-M 2008, ‘Plasma Membrane Damage to Candida albicans caused by Chlorine Dioxide (ClO2)’, Letters in Applied Microbiology, vol. 47, Oxford University Press, no. 2, pp. 67–73.
Wen, G, Xu, X, Huang, T, Zhu, H & Ma, J 2017, ‘Inactivation of Three Genera of Dominant Fungal Spores in Groundwater Using Chlorine dioxide: Effectiveness, Influencing factors, and Mechanisms’, Water Research, vol. 15, Elsevier BV, no. 125, pp. 132–140.
Wu, VCH & Rioux, A 2010, ‘A simple instrument-free gaseous chlorine dioxide method for microbial decontamination of potatoes during storage’, Food Microbiology, vol. 27, no. 1, pp. 179–184.
Young, SB & Setlow, P 2003, ‘Mechanisms of Killing of Bacillus Subtilis Spores by Hypochlorite and Chlorine Dioxide’, Journal of Applied Microbiology, vol. 95, no. 1, pp. 54–67.

The New Zealand Sterile Sciences Association awarded me a full scholarship to complete the Level 5 Diploma in Sterilising Technology through the Toi Ohomai Institute of Technology. This editorial reflects how the learning has furthered my knowledge and skills in sterilising sciences and the value the experience gave me.
Firstly, this was an incredible opportunity, with the outcome being completing my Sterilising Technology Level 5 Diploma. As an adult learner, it has been meaningful to me. As an adult, I had to balance work, family life and the demands of studying, which sometimes felt overwhelming. Studying as an adult has also rekindled my interest in learning. It has allowed me to gain new knowledge, skills, and perspectives that have positively impacted my personal and professional life.
The experience of having someone believe in me and my capability to succeed also played a significant role in this, and it has increased my confidence in my ability to pursue future opportunities and challenges.
During my Sterilising Level 5 Diploma, I completed:
• Quality Management Systems for Reprocessing Facilities
• Continuous Improvement of Reprocessing Services
• Validation for the Sterilisation Reprocessing Cycle
• Monitoring for the Reprocessing Cycle and
• Leadership Skills for Sterilisation Management
• Evidence-based Practice for the Sterile Sciences
Quality Management Systems for Reprocessing Facilities taught me the essential components to establish and maintain a robust quality management system. This included understanding the principles of quality management, developing standard operating procedures, implementing quality control measures, and conducting regular audits. The key area covered in quality management systems was the importance of ensuring safe and effective sterilisation processes following the guidelines outlined in AS/NZS 4187:2014 Reprocessing of reusable medical devices in healthcare service organisations (This was one of the governing standards throughout my study).
Continuous Improvement of Reprocessing Services: I learned the importance of continually assessing and improving reprocessing services and ensuring optimal sterilisation outcomes. This included implementing performance improvement initiatives, analysing data, and engaging in regular evaluation and feedback processes.
From the Validation for the Sterilisation Reprocessing Cycle module, I gained insight into the various methods and techniques used to ensure the sterilisation cycles are effective and capable of consistently achieving the proper outcomes. This involved understanding the principles of validation and ensuring compliance with regulatory requirements. Through this process, I gained knowledge of the three stages of validation: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Monitoring for the Reprocessing Cycle, I learned the importance of monitoring key indicators during sterilisation and implementing measures to control and prevent potential issues. This included monitoring sterilisation equipment, performing biological testing, using chemical indicators, and ensuring proper documentation and record-keeping.
Leadership Skills for Sterilisation Management were another vital aspect covered in the diploma. I learned the qualities and abilities required to lead and manage a sterilisation department effectively. This included developing effective communication skills, fostering teamwork and collaboration, and promoting a culture of continuous improvement.
Evidence-based Practice for the Sterile Sciences was a topic that further reinforced the importance of basing decisions and practices on reliable evidence and research findings. I learned about the various sources of evidence and how to critically appraise and apply them in the context of sterilisation processes. This included understanding research methodologies, conducting a literature review, and interpreting and implementing evidence-based guidelines. In conclusion, completing the Sterilising Level 5 Diploma has been a transformative personal growth experience. This study has not only been about acquiring knowledge, but also the positive impact on me. This diploma has enhanced my ability to contribute to the field of sterilisation management and has prepared me for future challenges and advancements in this area. Having someone believe in me and believing in myself has demonstrated the importance of support, feedback, and encouragement in achieving personal and professional goals. “If you have just one person believe in you, you’ll always find your way”- Sarah Dessen
Thank you, New Zealand Sterile Sciences Association, for the fantastic opportunity to further my studies in sterilising and to those who believed in me.
Nga Mihi Shelley MorrisonDear Central Service Readers,
The Quality Committee published a total of 126 recommendations between 1998 and 2022. While some of these recommendations continue to be valid, others are no longer fully applicable due to new developments in science, regulations or standards.
So far, all recommendations are available on the website of the German Society of Sterile Supply (DGSV) and perhaps it is not always easy for the reader to evaluate the content of older recommendations.
The Quality Task Group has therefore begun revising the recommendations. Topics that are no longer of relevance will gradually be placed in an archive, so that they can still be consulted for research purposes but will be clearly separated from currently valid recommendations.
If you have any suggestions about the hitherto recommendations or about new topics, you can send them to us at any time at qualitaet@dgsv-ev.de
Ulrike Zimmermann and Dr. Gerhard Kirmse Coordinators for the Quality Task Group
use of test objects to verify the cleaning performance at the time of validation of cleaning and
Authors: T. Appel, S. Bungardt, C. Diekmann, M. Fažon, A. Forster, A. Hartwig, M. Hunold, M. Igla, A. Jones, G. Kirmse, K. Mann, J. Metzing, M. Pohl, G. Regnieth, D. Schricker, A. van Waveren, U. Zimmermann
The standard DIN EN ISO 15883 Part 5 stipulates that a defined test soil and soiling method as well as instruments harbouring routinely encountered soils be used for verification of the cleaning performance. The Guideline Compiled by the DGKH (German Society of Hospital Hygiene), DGSV (German Society of Sterile Supply) and AKI (Instrument Preparation Working Group) for Validation and Routine Monitoring of Automated Cleaning and Thermal Disinfection Processes for Medical Devices specifies a defined test object (process challenge device) to be used to verify the cleaning performance at the time of validation. This test object meets the requirements for specification of the cleaning performance and with blood as a test soil it simulates the contaminants routinely found on surgical instruments. Using defined test objects, the reproducibility of the cleaning process can be assessed in addition to the minimum cleaning performance and if necessary optimized, for example by modifying the influencing variables or the load specifications. To that end, the test object must be appropriately standardized and manufactured subject to the pertinent quality assurance requirements. In-depth QUALITY ASSURANCE understandably sets high standards for the manufacture of the test objects.
The material and surface composition must be standardized. That applies especially if the clamps are repeatedly used. The factors that play a role include e.g.: alloy quality, passivated surface, rust, residues, care and sterilization.
As TEST SOIL , heparinised sheep blood is used while adding the appropriate amount of protamine sulphate to counteract the anticoagulant effects of heparin. 0.1 ml of this test soil is pipetted into the clamp joint (Fig. 1) and dried under standardized conditions. Neither human nor ovine blood has an absolutely unchanging composition or unchanging coagulation properties. This applies for this test soil too. When manufacturing test objects each blood batch must therefore be checked to ensure it is within the specified tolerance limits. In all cases the REPRODUCABILITY of the test results must be assured. Important preconditions for blood of an unchanging quality include the maintenance of the cold chain during blood transportation and observance of the blood shelf life.
TEST SOIL
Before releasing the test objects, the manufacturer must check the quality, including the cleaning performance in a defined process. Batch assignment must be assured by the manufacturer’s internal quality management system. The test object packaging must be accordingly labelled. To pre-empt occurrence of adverse changes during transportation the shelf life – in relation to the time, temperature and vacuum packaging – must be investigated and defined.
TEST OBJECT
INSTRUCTIIONS BY THE MANUFACTURER
TESTING
LABORATORY
TESTING REAL-LIFE INSTRUMENTS
Handling test objects at the time of validation
Before using the test objects, the user/validation engineer must check the expiry date and other INSTRUCTIIONS BY THE MANUFACTURER. The positions of the test objects in the washer-disinfector reference load must be documented. The test process is started and the test objects are withdrawn for inspection before the disinfection step. TESTING comprises visual inspection for cleanliness and investigation for protein residues using an eluted sample and a quantitative detection method, e.g. biuret, BCA (bicinchoninic acid) or OPA (ortho-phthalaldehyde) method. Immediately after inspection, clean, disinfect and dry the test objects in the washer-disinfector. This helps to avoid rusting of the joint region.
If the test objects are sent for evaluation to a LABORATORY, they must be dried at room air temperature immediately after withdrawal from the washer-disinfector and visual inspection, then placed in the return packaging provided, labelled according to positions and dispatched.
Blood residues are the most common type of contamination found on surgical instruments. But in addition to blood, instruments may also harbour other substances, e.g. fat, mucus, bone meal, drugs, antiseptics, etc. The test object described here serves as a model for testing the cleaning performance in relation to blood soils. Testing is done on site with the specified routine reference loads. These tests may also reveal application errors, e.g. loading errors or process deficits.
During validation the effectiveness of the cleaning process must be verified additionally by TESTING REAL-LIFE INSTRUMENTS, i.e. instruments harbouring routine soils; these are also visually inspected for cleanliness and investigated with a quantitative protein detection method. Especially in view of their specific design and the various operational influences as well as the drying times, additional shortcomings in the reprocessing process may come to light. The validation engineer must take account of these preconditions when carrying out the tests. Here, cooperation with the reprocessing management personnel is absolutely necessary.

133 Molesworth Street
PO Box 5013
Wellington 6140
New Zealand
T+64 4 496 2000
7 February 2024
Sue Waters - Interim National Chief Allied Health, Scientific and Technical Officer , Te Whatu Ora
Via email
Shelagh Thomas - President, New Zealand Sterile Supply Association
Via email
Carlton Irving - Clinical Chief Officer, Allied Health, Te Aka Whai Ora
Via email
Aaron Watson - General Manager, SC Bio Ltd
Via email
Private Surgical Hospital Network Members
Via email
Designated Auditing Agencies
Via email
Tēnā koutou katoa,
This letter provides clarification regarding standards for the reprocessing of medical and surgical equipment. As of 15 December 2023, both AS/NZS 4187:2014 and AS/NZ 4815-2006 were replaced by AS 5369:2023. New Zealand representatives were involved in the development of AS 5369:2023 and it reflects current best practice. While this is an Australian standard, it is the most up-to-date version of the previous standards and should be utilised in the absence of a New Zealand-specific standard
AS 5369:2023 is the standard which HealthCERT will expect sterile services to demonstrate compliance against through their evidence of internal audit processes The Ministry of Health will liaise with relevant agencies to reinstate a shared Australian/New Zealand standard so that we continue to have appropriate standards which are fit for purpose in our environment.
Please circulate this letter across your networks.
Ngā manaakitanga,
 Lauren Hancock Acting Chief Allied Health Professions Officer
Lauren Hancock Acting Chief Allied Health Professions Officer
 Kirsten Lassey Manager, HealthCERT
Kirsten Lassey Manager, HealthCERT
Original article
Brian Kirk
Corresponding author: Dr Brian Kirk
Brian Kirk Sterilization Consultancy Group Ltd 10 Harcourt Place, Castle Donington, Derby UK
bkirk0256@outlook.com
Conflict of interest:
The work was carried out by Brian Kirk Sterilization Consultancy Group Ltd, supported by a research grant from 3M St Paul, USA.
Citation:
Kirk B. Evaluation of a number of chemical indicators for monitoring vaporized hydrogen peroxide (VH2O2) sterilization processes. Zentr Steril 2020; 28 (4): 214–223
Manuscript data:
Submitted: 9 April 2020
Revised version accepted: 17 June 2020
Zentralsterilization | Volume 28 | 4/2020
Abstract
Background: The study evaluated the characteristics and performance of eight chemical indicators (CIs) for monitoring VH2O2 sterilization processes of type 1 and 4 according to EN ISO 11140-1.
Method: CIs were exposed to processes in which VH2O2 concentration ([c]), temperature (T) and time (t) were changed to give type 1 and 4 pass and fail conditions as described in EN ISO 11140-1. Changes to individual variables then explored the performance of the CIs similar to the approach taken for steam and ethylene oxide type 5 CIs. Colour change was evaluated visually, according to manufacturer’s instructions, by comparison to a colour reference when supplied and by reflectance colourimetry. Results were also expressed as L*, a*, b* colour coordinates and plotted as a*, b* graphs (International Commission on Illumination, CIE).
Results: Of the type 1 CIs tested, the ASP Sterrad® (A) and the Steris Verify® (D) gave appropriate pass and fail results.
The Shinva® (B) gave all pass results and the Steris Celerity® all fail results (C). Of the type 4 CIs tested, the 3M Tri-Metric® (E) and SPS® (G) gave appropriate pass and fail results. The gke® CI (F) gave appropriate pass and fail results but colour differentiation was slight. The Terragene Chemdye® (H) gave a colour change interpreted as a pass, when tested in pass or fail conditions.
W hilst EN ISO 11140-1 does not require such tests when T, t and VH2O2[c] were individually reduced, CI A, B, D, F and H gave similar colour changes in all three test conditions. CI G showed a different colour change in T and t and gave different colorimetric measurements in all the different exposure conditions. CI C had not reached its reference colour (orange) but it was possible by colorimetric measurements to detect the reduction in VH2O2[c] and the combined reduction in T and t but the colours ap-
peared similar (red). It was possible by visual observation and colorimetric measurement to observe differences in response of CI E to each of the reduced exposure conditions T, t and VH2O2[c].
Conclusion: Eight different CIs were tested. Two of the type 1 CIs showed appropriate pass and fail results. Another CI gave all passes and one all fails. Two of the type 4 CIs gave appropriate pass and fail results. Another showed slight differences in colour in pass or fail tests and another gave the same pass colour.
Keywords
Chemical Indicators
EN ISO 11140-1
Multivariable (Type 4) Indicators
Process Indicators (Type 1)
Sterilization Assurance
VH2O2
Users should ensure purchased CIs are suitable for use in their own situation and that clear instructional material is available for end users.
Introduction
Sterile medical devices must be sterilized by a validated sterilization process [1, 2]. There are international standards describing the validation methods for sterilization processes employing ethylene oxide (EO) [3] and low temperature steam with formaldehyde (FORM) [4] but no such standards exist for vaporised hydrogen peroxide sterilization (VH2O2) processes; these are in development [5]. However VH2O2 has become one of the most popular low temperature sterilization processes employed in health care facilities.
Along with validation sterilization processes should also be routinely monitored using systems which ensure sterilizing conditions are met. Monitors for sterilization processes usually take one of three forms. Those which
Table 1: A list of the CIs tested including their stated values (SVs), specified colour change, including the International Commission on Illumination CIE L*,a*,b* colour coordinates, presence or absence of a colour reference on the CI or in the Instructions for Use (IfU) and a description of the manner in which the colour change should be interpreted as printed on the CI or contained in the IfU.
CI colour change (L*,a*,b* values)
Colour Reference (L*,a*,b*)
Interpretation instructions
A: ASP STERRAD® Chemical Indicator Strip (14100)
B: Shinva® VH2O2 label (010104)
12.3 / 50 / 360
1a N/A
C: Steris Celerity® HP Chemical Indicator (PCC074)
D: Steris Verify® HPU Chemical Indicator (PCC061)
E: 3M Attest VH2O2 Tri-Metric® Indicator (1348)
F: gke SteriRecord® CI for VH2O2 (C-V-P-6)
G: SPS Medical®
VH2O2 Indicator Strip (GPS-250R)
H: Terragene Chemdye® VH2O2 CI (CD40)
12.3 / 50 / 360 b
Dark Red to Orange/Yellow (42,61,10 to 91,-2,8)
Red to Yellow (58,51,41 to 90,1,105)
Red to Orange/ Yellow (64,33,3 to 89,6,38)
12.3 / 50 / 360
45.1 / 50 / 60
42.3 / 50 / 360
Magenta to Yellow (40,61,-29 to 93,4,29)
Orange printed on CI (67,32,70)
Six reference colours from Red to Yellow in IfU (58,48,65)
Orange printed on CI (76,22,34)
Yellow printed on CI (87,7,60)
Blue to Pink (40,0,-58 to 74,24,-1) None
Light Blue to Light Green (60,-7,-36 to 81,-7,-7)
42.3 / 50 / 360
blue to light green in IfU (81,-6,-7)
“Bar changes from red to colour indicated by comparator bar [orange] or lighter” printed on CI.
Orange or lighter (towards yellow) is a Pass (ref chart)
An arrow pointing from the indicator strip to the reference colour printed on the indicator.
“If the indicator colour matches the orange reference colour or is lighter…” in the IfU.
“Accept only if magenta circle turns yellow” printed on CI
“Any pink in accept zone is a pass” printed on the CI
Blue to green. “If the indicator dot remains purple/ blue or has not turned to the final colour completely” in IfU.
Dark Pink to Blue (73,31,2 to 82,-6,-20)
42.3 / 50 / 360
Purple to dark green / blue to greenc (28,43,-42 to 78,-48,8)
none
Green printed on CI. Five ref colours from purple to light green in IfU (not the same green) (71,-48,8)
“Indicator turns BLUE when exposed to VH2O2 during the sterilization process” printed on CI
“The indicator must turn to the reference colour for considering that the indicated conditions were met” in IfU.
a: The Shinva packaging and accompanying technical sheets did not specify which type therefore it was assumed that because it was an adhesive label for attachment to the outside of packs it would be a process indicator.
b: The Celerity indicator is claimed to be a type 1 which according to EN ISO 11140-1 has defined SVs as shown.
c: The Terragene Chemdye tested showed two types of unexposed colour. In the first set of samples supplied CIs were in a cardboard box and had a blue starting colour. A second set of samples were provided in a foil pouch and had a purple starting colour.
measure the physical characteristics of the process (physical indicators). Those which consist of a preparation of bacterial spores, presenting a known resistance to the process but inactivated by an efficacious process (biological indicators) [6]. Those which consist of a mixture of reactive chemicals printed on a substrate which respond to defined characteristics of the processes so that a visible change is observed after suitable exposure (chemical indicators; CIs) [7].
The standard EN ISO 11140-1 [7] specifies the performance requirements for six different types of CI. Type 1 are process indicators for placement
on the outside of instrument sets to indicate processing. Type 2 are used for special tests. Type 3 are single variable indicators having limited utility, whilst type 4, 5 and 6 are multivariable, integrating and emulating CIs, respectively, having specific performance characteristics for placement inside sterile packs. The exposure conditions under which a type 1 CI should show an endpoint (pass) result and a fail result are specified in EN ISO 11140-1 (see Table 2). The exposure conditions for a type 4 indicator are however related to the manufacturers claimed stated values (SVs) for VH2O2 concentration, tem-
perature and time (pass conditions).
The fail exposure conditions are then related to specified reductions in each of the SVs according to EN ISO 11140-1 [7] (see Table 2).
Purpose of the study
The purpose of the study was to evaluate the characteristics and performance of eight commercially produced type 1 and 4 VH2O2 CIs (table 1) in pass and fail test conditions according to EN ISO 11140-1.
Whilst not a requirement of EN ISO 11140-1, the sensitivity of each indi-
a: The stated value for the 3M Tri-Metric was 5.1 mg/L, 50 °C, 60s which, if using a square wave VH2O2 exposure condition would equal an integrated exposure dose (AuC) of 306 mg-s/L. In order to deliver an integrated exposure dose of approximately 306 mg-s/L a target peak exposure concentration of 4.3 mg/L was employed for the operating cycle of the exposure apparatus (see Figure 1).
b: The exposure apparatus exposure time could be programmed in 5 second increments therefore 288s was rounded up to 290s
c: Type 1 Pass and Fail exposure conditions are specified in EN ISO 11140-1 and are shown in the table.
d: Type 4 Pass exposure conditions are the SVs specified by the CI manufacturer. The Fail exposure conditions are specified in EN ISO 11140-1 as SV[c] – 20%, SV(T) – 3 °C, SV(t) – 25%
cator to changes in individual process variables was examined by exposure to conditions of reduced VH2O2 concentration, then reduced time and finally a combination of reduced temperature and time (see table 2).
Either type 1 or 4 CIs were purchased from commercial sources (Table 1). All chemical indicators were stored in their original packaging and in a laboratory environment of 20 to 25 oC and 40 to 70% rh.
With the exception of one product, the CIs consisted of a printed ink on
a suitable substrate. The 3M Trimetric product had indicator ink printed on a substrate covered with a polymer sheet with gaps cut along its length. Beyond the “accept” line printed on top of the CI, the overlying sheet enclosed the ink creating a small gap down which VH2O2 penetrated to effect colour change.
The colour characteristics of the unchanged and exposed indicators and any reference colour printed on the indicator or in the accompanying instructions for use were measured us-
ing a hand held reflectance colourimeter (X-Rite eXact Standard spectrophotometer, https://www.xrite.com/ categories/portable-spectrophotometers/exact) with an aperture diameter of 1.5mm and white light illumination. Readings were expressed in the International Commission on Illumination (CIE) L*,a*,b* notation [8] as described previously [9]. The L*a*b* notation describes a three-dimensional colour space with L* representing the brightness of the colour (0=black, 100=white) this being perpendicular to the colour axes, a* representing red
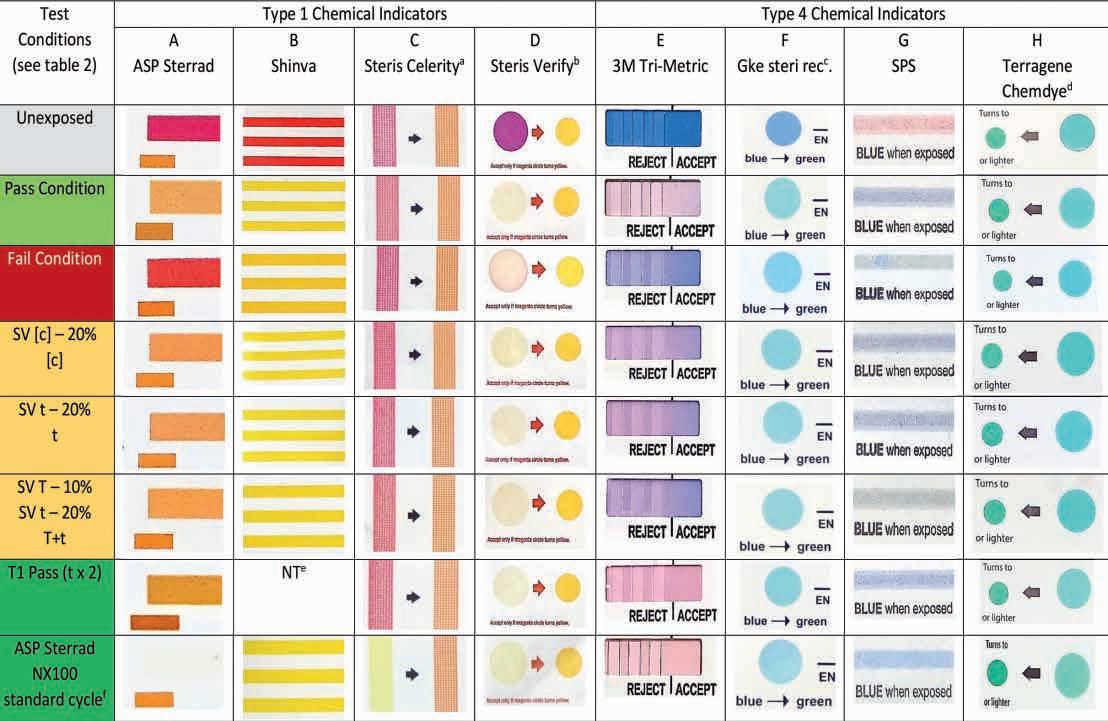
a: When the Steris Celerity CI was exposed to 3.7mg/L VH2O2 peak concentration, (2.8 mg average, range 3.7 to 2.1 see Figure 1) at 50 °C for 720 seconds the indicator ink matched the reference orange colour.
b: The Verify indicator appeared yellow by visual observation however the pictures shown in the table show this as a light pink colour which can only be assumed an anomaly of reproduction.
c: The gke IfU shows pale green endpoint and the CI is printed with green as an endpoint. The observed colours which are annotated Pass appeared aquamarine (light blue/ green) rather than a pure green.
d: The Terragene Chemdye IfU indicates an aquamarine (light blue/green) endpoint colour. The indicator itself has a green reference dot. All samples tested changed to aquamarine not green.
e: The CI was not tested (NT) in this condition having turned Yellow (endpoint) at the type 1 Pass condition.
f: The ASP Sterrad CI turned a very pale yellow, almost white colour when exposed to this test condition resulting in poor photographic reproduction.
(positive values) to green (negative values) and b* representing yellow (positive values) to blue (negative values).
For coloured areas greater than 20 mm2 three readings were taken from different positions within the printed surface. For coloured areas less than 20 mm2 a single measurement was taken. Colour change was also visually interpreted by the author in bright daylight next to a west facing window and categorised as pass or fail according to the manufacturer’s instructions, and, if present, by comparison with the colour reference provided. Photographic records of colour change were taken under similar illumination (see Table 3). Colour measurements were taken within 48 hours after exposure to the operating cycle.
The variability of the measurements taken by the reflectance spectrophotometer were determined and found to have a standard deviation of less than 0.1 units of L*, a* and b*.
Vaporised hydrogen peroxide exposure apparatus
CI samples were exposed to VH2O2 using a Sterilucent® PSD 85LS programmable sterilizer (http://www.sterilucent.com/ pages/products_psd85.php) having a parallel piped configuration with the approximate dimensions 660 mm length, 430 mm width and 300 mm height giving a chamber volume of ca 85 L All internal surfaces were heated to the exposure temperature prior to commencement of an operating cycle. VH2O2 was introduced into the chamber through a baffled port mounted in the geometric centre of the base of the chamber. All elements of the operating cycle were programmable through a user interface panel connected to the controller.
Process monitoring
VH2O2 concentration, temperature, pressure and time, along with electromechanical operations were monitored throughout the operating cycle by the monitoring system incorporated into the controller. VH2O2 concentration was measured within the chamber using an ultraviolet light spectrophotometric method. The sensor was mounted in the upper front part of the chamber and measured across its width giving a pathlength of approximately 430 mm Process data was transferred from the controller to a PC based logging system for
further analysis. An example of the operating cycle profile is shown in Figure 1.
Sample preparation
Samples of CIs were mounted on a polyester film sample holder, ca 1 m m thick
and cut to a shape to allow mounting of various sizes and shapes of CIs. CIs were orientated so that the indicator ink was in close proximity to the central cut out of the sample holder (see
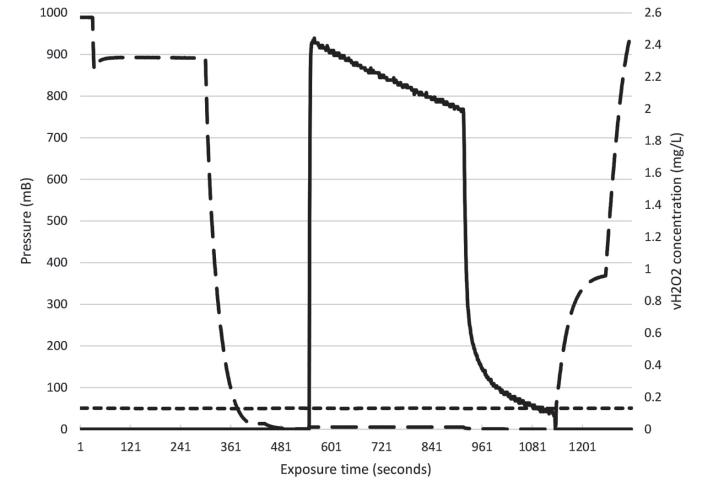
was 2.2 with a range 2.0 to 2.4 mg/L).
conditions in this case were 2.3 +/- 0.4 mg/L, 50 +/- 0.5 °C and 360 +/- 1 seconds.
Five CIs of each type were exposed to operating cycles in groups of 3 (Figure 2). A total of 30 indicators of each type were exposed to each of the different exposure conditions employed (Table 2).
A series of tests were carried out in which the exposure conditions shown in Table 2 were employed. The exposure conditions consisted of a target VH2O2 peak concentration, which occurred at the beginning of the exposure time, (see Figure 1), at a defined temperature for a defined period of time.
Table 2 also shows a variable, not considered in EN ISO 11140-1, which is the product of the VH2O2 concentration x time of exposure, in seconds, and represents the area under the VH2O2 concentration curve (AuC or dose; mg-s/L). The values given in EN ISO 11140-1 assume “square wave” exposure conditions ie a rapid rise in VH2O2 which remains stable followed by a rapid fall after the exposure time [10]. The AuC variable is useful when the exposure apparatus has a profile similar to that shown in Figure 1 where a single injection of VH2O2 is followed by an exposure period during which the concentration slowly decreases ending in a chamber vent where a rapid fall in concentration takes place but a residual tail remains as concentration falls to zero. This is typical of sterilization processes used in production equipment which often use multiple vapour exposure periods.
A series of tests were also carried out in which 5 samples of each CI type were exposed to an ASP Sterrad® NX100 standard cycle (https:// www.asp.com/product/terminal-sterilization/sterrad-100nx). Thirty cycles were carried out and the CIs exposed to the cycle which exhibited the greatest AuC for VH2O2 dose were used as examples of the maximum colour change observed during the study (final row in Table 2).
Bespoke PC based computer software supplied with the exposure apparatus was used to analyse operating cycle data including the calculation of

3:
to different test conditions identified in Table 2. The figure shows the a* and b* International Commission on Illumination CIE coordinates. Each point represents a different exposure condition for VH2O2 concentration, temperature and time. The legend shows the exposure condition for each data point marker. Markers labelled Pass and Fail represent the Pass and Fail conditions specified in EN ISO 11140-1 (see also Table 2) L* values for exposed indicators were within the range 53 to 91, ASP Sterrad (A), 83 to 90, Shinva (B), 66 to 88 Steris Celerity (C), 87 to 93 Steris Verify (D), 57 to 74, 3M TriMetric (E), 70 to 76, gke (F), 74 to 81 SPS (G), 75 to 78, Terragene Chemdye (H).
the time and temperature of exposure to VH2O2 and a determination of the AuC.
Colour measurements from the reflectance spectrophotometer were automatically uploaded into a PC spreadsheet (Excel, Microsoft Corporation, https://www.office.com/) for further analysis. Mean reflectance values and variation around the mean for each of the three CIE notations, L*,a*,b*, were calculated for replicate samples. The mean values are shown in the form of two-dimensional plots of a* and b* data. L* values are reported in the graph legends (see Figure 3).
Results
Exposure apparatus reproducibility
Table 2 shows the CI type and test condition for the target peak exposure concentration for VH2O2, temperature and time and their tolerances, the average and range of the measured AuC for VH2O2 concentration and the products exposed to the test condition. For each group type 1 and 4 pass test conditions had significantly higher AuCs (at p=0.05) compared with their respective fail conditions.
The AuC for test conditions in which VH2O2 concentration and time were reduced by 20% and temperature and time by 10% and 20% respectively did not differ significantly.
Observed colour change
Table 1 shows the manufacturers description of the colour change including a description of any reference colour provided. The table also shows the L*, a*, b* values for each colour.
The colour change exhibited by CIs exposed to a standard Sterrad NX100 sterilization process delivering a total VH2O2 AuC of 7036 mg-s/L (sum of the two VH2O2 exposures delivered by the process with peak concentrations of 7 and 13 mg/L respectively). This represented the maximum VH2O2 exposure employed during the study and therefore the maximum colour change observed.
Figure 3 shows a series of charts in which the colour change of each CI exposed to the different test conditions (Table 2) is plotted as a*, b* coordinates. L* values are shown in the legend. As the differences in a*, b* values increased visible differences became more apparent.
Type 1 CIs
ASP Sterrad CI
The CI reached its endpoint colour when exposed to the pass condition, the ink being a lighter orange than the reference. The CI did not reach its endpoint when exposed to a fail condition showing a darker orange colour (Table 3 column A). Colorimetric measurements indicated clear differences between the Type 1 pass and fail (Δa* 24, Δb*15; Figure 3A).
The CI did not differentiate, by visual observation, between the test conditions employing individually reduced SVs for concentration, time and temperature and time ([c],t,T+t) and the pass all being a uniform orange colour slightly lighter than the reference (Table 3 column A). Colorimetric measurements indicated slight differences in colour change (Δa*2, Δb*2).
Shinva CI
The Shinva CI was a self-adhesive label for attachment to the outside of packs. Although no apparent claim was made Type 1 appeared appropriate.
The CI reached its endpoint colour when exposed to a pass condition, the indicator ink being yellow, compared to the orange/red on the reference chart. When exposed to a fail condition a slightly darker yellow was observed but this was still much lighter than the reference and was therefore also interpreted as a pass result ( Table 3 column B). Colorimetric measurements indicated clear differences between the pass and fail (Δa*10, Δb*1) (Figure 3B) but both were nevertheless yellow in colour.
The CI did not differentiate, by visual observation, between the conditions employing individually reduced SVs ([c],t,T+t) and the pass with all of the changed colours being yellow compared to the orange/red reference therefore indicating a pass. Only slight differences in colour (Δa*1, Δb*3) were observed by colorimetric measurement.
Steris Celerity CI
The CI did not reach its endpoint colour when exposed to the pass condition, being dark red compared to the orange reference printed on the indicator. When exposed to a fail condition a slightly darker red was observed (Table 3 column C). Colorimetric measurements indicated a difference between pass and fail (Δa*7, Δb*8) however these
colours were red rather than the orange of the reference and therefore interpreted as fails (Figure 3C). The CI was also exposed to a higher concentration of VH2O2 for a longer time (2.8 mg average, range 3.7–2.1 at 50 °C for 720s.). Under these conditions, the ink matched the reference orange colour.
The CI did not differentiate, by visual observation, between the pass and the reduced concentration and reduced time conditions ([c],t). When exposed to the T+t reduced test conditions the indicator had a very slightly more red tinge. Colorimetric measurements showed similar differences (Δa*6, Δb*6 spanning the range of colours observed).
Steris Verify CI
The CI reached its endpoint colour when exposed to the pass condition, having a much lighter yellow colour compared to the deep yellow reference printed on the indicator. When exposed to a fail condition the indicator ink showed a central light yellow area fringed by a magenta colour around the circumference indicating a fail (Table 3 column D). Colorimetric measurements indicated clear differences between the pass and fail (Δa*9, Δb*9) (Figure 3D).
The CI did not differentiate between the test conditions employing individually reduced SVs ([c],t,T+t) and the pass by visual observation or colorimetric measurement with there being only slight differences in colour change measured (Δa*1, Δb*1). None of the changed colours matched the a* b* values for the reference colour all being a much lighter yellow (see Table 3 column D).
Type 4 CIs
gke CI
The CI colour change was difficult to interpret since the instruction printed on the CI stated a change from blue to green. The colour printed within the IfU was an aquamarine colour. The ink on the CI reached a colour similar to, but not the same as, that shown in the IfU and was therefore assumed to be at its endpoint when exposed to the pass condition. When exposed to the fail condition the ink showed a barely perceptible darker bluish colour (Table 3 column F) making visual interpretation difficult. Colorimetric measurements indicated a slight difference (Δa*2, Δb*2; Figure 3F) between pass and fail.
The CI did not differentiate, by visual observation, between the pass and those test conditions employing individually reduced SVs ([c],t,T+t). Colorimetric measurements showed slight differences in colour (Δa*1, Δb*3).
SPS CI
The CI reached its endpoint colour when exposed to the pass condition. When exposed to a fail condition the indicator ink showed a green colour indicating a fail (Table 3 column G). Colorimetric measurements indicated a difference between the pass and fail (Δa*0, Δb*8).
The CI did not differentiate, by visual observation, between the pass and the test conditions employing reduced [c] and reduced t. When exposed to the combined reduced T+t test condition the indicator had a green/blue colour indicating a fail (Table 3, column G). Colorimetric measurements showed similar differences (Δa*1.5, Δb*10 spanning the range of colours observed) (Figure 3G).
Terragene CI
The batch of Terragene Chemdye CIs tested were supplied in a cardboard box. Unexposed indicators had a blue colour (Table 3 column H). A subsequent batch was supplied in a foil pouch. Unexposed indicators had a purple colour. The reasons for these differences are unknown.
The CI colour change was difficult to interpret since there was a green reference colour printed on the indicator but the colour printed within the IfU was closer to aquamarine. The ink on the CI reached a colour similar to, but not the same as, that shown in the IfU which was assumed to be its endpoint colour when exposed to the pass conditions but it did not match the green colour printed on the CI. When exposed to the fail conditions the indicator ink showed a colour indistinguishable from the pass (Table 3 column H). Colorimetric measurements indicated minor differences between pass and fail (Δa*1, Δb*1; Figure 3H).
The CI did not differentiate, by visual observation, between the pass and the reduced SV test conditions ([c],t,T+t) all showing the same colour. Colorimetric measurements showed barely detectable differences in colour (Δa*1, Δb*1 spanning the range of colours observed).
The 3M Tri-Metric CI is a type 4 CI with SVs of 5.1mg/L, 50 oC, 60s (306 mg-s/L AuC) and is the only product which is claimed to be able to detect individual changes in each critical process variable of a VH2O2 sterilization process. The stated values for the CI assume a square wave exposure profile (see EN ISO 18472; 10). However since the exposure apparatus, whilst working within the tolerances required, did not provide such a profile (see Figure 1) it was decided to employ a target peak concentration of 4.3 mg/L so that the AuC was close to the integrated exposure of 306 mg-s/L (a target peak concentration of 5.1 mg/L would have delivered an AuC of ca 370 mg-s/L). The fail condition was therefore 3.4 mg/L (peak concentration), 47 oC, 45 s (153 mg-s/L AuC).
The CI showed an endpoint colour when exposed to the pass condition having a pink colour immediately after the accept line according to the instructions printed on the CI. When exposed to a fail condition the indicator ink immediately after the accept line was a dark mauve/blue colour indicating a fail (Table 3 column E). Colorimetric measurements indicated a clear difference between the pass and fail (Δa*12, Δb*18, Figure 3E).
The CI showed a slight observable difference between the pass and the individual reduced SVs [c] and t, having a darker mauve colour immediately after the accept line. The indicator showed a more obvious observable difference between the pass and indicators exposed to the combined reduced SVs, T+t, having a darker mauve colour. Colorimetric measurements showed similar differences (Figure 3E) with the pass to reduced [c] and reduced t difference being Δa*3, Δb*2 and for the combined reduced SVs T+t, Δa*5, Δb*4.
Response to pass and fail conditions according to EN ISO 11140-1
Previous reports have discussed the performance of type 6 steam CIs and type 2 Bowie and Dick test CIs [9, 11]. The indicators evaluated in this report were subjected to the test conditions described in EN ISO 11140-1 which should create a pass or fail response in type 1 and 4 chemical indicators for VH2O2 sterilization. Of the type 1 CIs tested,
the ASP Sterrad and the Steris Verify gave appropriate pass and fail results. The Shinva gave all pass results and the Steris Celerity all fails. Of the type 4 CIs tested, the 3M Tri-Metric and SPS gave appropriate pass and fail results. The gke CI gave appropriate pass and fail results but colour differentiation was slight making interpretation difficult. The Terragene Chemdye gave a similar colour change when tested in pass or fail conditions which were interpreted as a pass.
Can the tested samples detect slight variations in process?
EN ISO 11140-1 describes 6 types of CI. Whilst the standard describes requirements for type 5 indicators for steam and EO, no such requirements are specified for FORM or VH2O2 CIs. The requirements for type 5 CIs are different to those for the other types in that they require multiple SVs to be specified. A type 5 steam CI must have 3 stated values for time at 121 and 135 °C and these should be greater than 16.5 and 1.2 minutes, respectively. At least one additional test point is required. After testing at the SVs (pass) the CI is tested at the fail condition (t –15%, T –1 °C) for each of the temperature SVs. For EO two SVs for time are required at 37 and 54 °C which should be ≥ 75 and 30 minutes respectively at a gas concentration of 600mg/L and 60% rh. The fail condition is represented by a 20% reduction in time at the same gas and rh conditions. Whilst no requirements are specified for type 5 VH2O2 CIs in the standard [7| it was thought interesting to establish if the CIs could detect differences in the operating cycle when the [c], t and T+t were reduced as shown in Table 2, these having similarities to the requirements for type 5 steam and EO CIs.
When the [c], t and T+t exposures were changed the ASP Sterrad , gke, Shinva, Steris Verify and Terragene CIs, did not detect differences in the operating cycles by both visual observation and colorimetric measurement. The SPS CI detected the combined reduction in t+T by visual observation (green) and each of the exposure conditions by colorimetric measurement compared to the pass condition. Whilst the Steris Celerity CI had not reached its reference colour it was possible by colorimetric measurements to detect the reduction
in [c] and t+T but not by visual observation. It was possible by both visual observation and colorimetric measurement to observe differences in response between the pass condition and each of the reduced exposure conditions with the 3M Tri-Metric CI.
The value of type 4 versus type 1 Type 1 chemical indicators are placed on the outside of sterilization packs and are designed to show exposure to a sterilization process. Bearing in mind that the criteria for a pass or fail condition depends on a 353 second difference in exposure time (360 s vs 7 s) they will offer limited value for detecting process failures when used as internal pack indicators. Type 4 indicators are for placement within sterilization packs and show that sterilization conditions have been attained at the point of placement.
Bearing in mind that the difference between a pass and fail response relates to the SVs for the CI, which should have some relationship to the sterilization process, and a defined reduction in the SVs for each critical process variable ([c]-20%, T-3 °C, t-25%) they will prove more useful for providing evidence that sterilization conditions have been met but more importantly if inadequate conditions have been encountered and this will be described in a subsequent publication (in preparation).
How useful is a printed reference colour; does it cause confusion?
The perception of colour is subjective [12] depending on several factors e.g. the intensity and spectrum of the incident light and the age, health and visual acuity of the observer. This is why the colour change of CIs should be clear and unambiguous and from one distinct colour to another [7].
The use of a reference colour printed in the instructions for use or on the CI is helpful in interpreting the colour change correctly but is only useful if the colour bears a strong likeness to the indicator ink endpoint otherwise uncertainty and misinterpretation can arise. The description of an endpoint colour is a useful alternative and avoids a direct comparison of the ink colour with that of a reference, however such information should be a precise description of the endpoint colour in order to avoid similar confusion and misinterpretation.
Internal sterile pack CIs are interpreted by the end user working remotely from the sterile processing department and are employed to assure “the sterility of every pack” [13]. Use of reference colours covering the span of those likely to be observed during use are essential and should be readily accessible printed reference charts and on-line material. The use of mobile telephone Apps to provide colour interpretation may also be of value.
Should VH2O2 dose (AuC) be used rather than fixed test conditions?
EN ISO 11140-1 describes test conditions expressed in the form of fixed concentrations of VH2O2(mg/L), temperature and time w ith appropriate tolerances. This approach is appropriate for exposure apparatus designs which are able to introduce the sterilizing agent to a fixed value and then modulate around that value as might be observed in a steam resistometer. In VH2O2 exposure apparatus designs, it may not be possible to continuously introduce vapour into the chamber as the concentration declines, therefore under these conditions (Figure 1), it may be more appropriate to describe a target VH2O2 concentration which is initially introduced, along with a tolerance, followed by a VH2O2 dose expressed as the area under the VH2O2 concentration curve (AuC, mg-s/L). This approach would then take into account various designs of exposure apparatus. This approach will be further discussed in a further publication (in preparation) and which could inform discussion within ISO TC 198 wg 6 for future revisions of EN ISO 11140-1.
Conclusions
Monitoring the ongoing efficacy of validated sterilization processes is of vital importance in maintaining an effective quality system but more importantly protecting patients from harm. CIs are useful for establishing penetration of sterilant and the presence of sterilizing conditions at the point of placement within surgical sets. Many regulatory authorities and national guidelines require or recommend such use [14]. The reliability and performance of CIs is vital if quality is to be maintained. This study examined the performance of eight type 1 and 4 CIs for VH2O2 sterilization processes. A mixture of respons-
es was observed. Some indicators gave appropriate pass and fail results, others gave all passes and some all fails.
Performance was also examined when exposure to process variables was individually reduced ([c], t -20%) and where a combination of T+t were both reduced together (10% and 20%). Some indicators were unable to detect these decreases, some were able to detect some of the changes and one was able to detect all three of the changes made.
The inclusion of a reference colour printed on the indicator or within IfUs is of great concern since in most cases the colour did not match the endpoint of the indicator ink leading to confusion in interpretation.
The author would like to thank Dr Marco Bommarito, Mr Lawrence Talapa and Mr Sandy Reilly of 3M who helped in the preparation of this manuscript.
1. Regulation (EU) 2017/745 of the European Parliament and of the council of 5 April 2017 on medical devices , amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC, Official Journal of the European Union, 5th May 2017
2. Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labelled as Sterile. Guidance for Industry and Food and Drug Administration Staff, January 21 2016, Food and Drug Administration, Washington, USA
3. EN ISO 11135, Sterilization of healthcare products – Ethylene Oxide – Requirements for the development, validation and routine control of a sterilization process for medical devices, 2014, CEN-CENELEC Management Centre: Avenue Marnix 17, B-1000 Brussels
4. EN ISO 25424, Sterilization of healthcare products – Low temperature steam and formaldehyde – Requirements for the development, validation and routine control of a sterilization process for medical devices, 2014, CEN-CENELEC Management Centre: Avenue Marnix 17, B-1000 Brussels
5. ISO/CD 22441 Sterilization of healthcare products – Low temperature vaporized hydrogen peroxide – Requirements for the development, validation and routine
control of a sterilization process for medical devices, 2019, International Standards Organisation, Geneva.
6. EN ISO 11138 Sterilization of health care products – Biological indicators –Part 1: General requirements, 2017, CEN-CENELEC Management Centre:
Avenue Marnix 17, B-1000 Brussels
7. EN ISO 11140-1, Sterilization of health care products – Chemical indicators –Part 1: General requirements, 2014, CEN-CENELEC Management Centre: Avenue Marnix 17, B-1000 Brussels
8. The International Commission on Illumination (Commission internationale de l’éclairage), CIE L*,a*,b* colour standard, 1976, CIE Central Bureau, Babenbergerstraße 9/9A, 1010 Vienna, Austria
9. Van Doornmalen J.P.C.M, Hermsen R.J., Kopinga K. Six commercially available class 6 chemical indicators tested against their stated values. Central Service 2012; 6, 395–404.
10. EN ISO 18472 Sterilization of health care products – Biological and Chemical indicators – Test equipment, 2018, CEN-CENELEC Management Centre: Avenue Marnix 17, B-1000 Brussels
11. Kirk B. An evaluation of nine Bowie and Dick test products available in the United Kingdom, Medical Device Decontamination 2012; 17(1): 01.
12. Hurlbert A., Pearce B., Aston S. All illuminations are not created equal: The limits of colour constancy. In: 38th European Conference on Visual Perception
(ECVP). 2015, Liverpool, UK: Sage Publications Ltd.
13. World Health Organisation, WHO surgical safety checklist, 2009. WHO, Avenue Appia 20, 1211 Geneva
14. ANSI/AAMI ST 79 Comprehensive guide to steam sterilization and sterility assurance in health care facilities, 2017, Association for the Advancement of Medical Instrumentation, 901 N. Glebe Road, Suite 300, Arlington, VA 22203 USA.

Join us in celebrating this significant milestone at the Aotea Centre, Auckland. 25th – 27th September 2024.
Conference Dinner: Thursday 26th September
Dinner theme: Fashion, Style, Music, History, and Events of 1974.
This year is your chance to join the NZSSA Executive team and help drive the future direction of our industry. If you’re interested in building the profile of sterile sciences, and contributing to how the association is run, then step forth and consider being nominated for the 2024-2027 executive term.
You can apply to be a member of the executive team or one of the elected offices of the executive (President or Secretary), or both. The association members will vote on the candidates in July and successful members will join the executive in September as we celebrate 50 years of the NZSSA!
For further information read the Executive Nomination form on our website at https://nzssa.org/membership/, contact one of the current executive members or email secretary@nzssa.org .
March Nomination forms will be emailed out to NZSSA members.
April 30th Last day for candidates to submit completed nomination forms.
June Successful candidate biographies will be published in Supplyline
Voting forms sent to eligible association members.
July 12th Last day for acceptance of voting forms.
September Executive committee ratified at Association AGM
President: Shelagh Thomas
CSSD
Hutt Valley
Capital, Coast and Hutt Valley
Phone: 04 566 6999 ext 2745
Mobile: 027 589 6473
Email: shelagh.thomas@huttvalleydhb.org.nz
Maureen Scott
Sterile Services
Hamilton
Waikato District Health Board
Email: Maureen.Scott@waikatodhb.health.nz
Sharon Moss
National Sterile Services Manager
Southern Cross Health
Christchurch
Email:
sharon.moss@southerncrosshospitals.co.nz
Jenny Carston
CSSD Manager
Tauranga
Hauora a Toi Bay of Plenty
Email: Jenny.Carston@bopdhb.govt.nz
Secretary:
Paul Moody
Senior Product Development Manager
Fisher & Paykel
Auckland
Email: paul.moody@fphcare.co.nz
Anthony Valvoi
Sterile Services
New Plymouth
Taranaki
Email: anthony.valvoi@tdhb.org.nz
Martin Bird
Sterile Services
Dunedin
Southern
Email: martin.bird@southerndhb.govt.nz
Aileen Derby
CSSD Manager
Manukau
Counties Manukau
Email:
Aileen.Derby@middlemore.co.nz
Treasurer: Alison Stewart
NZSSA Treasurer
28 Brighton Street
Island Bay
Wellington 6023
Mobile: 021 209 8127
Email: nzsterilescienceassoc@gmail.com
Kelly Swale
Sterile Services Manager
Faculty of Dentistry, University of Otago
Dunedin
Email: kelly.swale@otago.ac.nz