Microcomputed tomography of the stapes: Wide-ranging dimensions An absence of cilia outer microtubules, an etiology not previously recognized in bilateral mucocele
Computational analysis of swallowing mechanics after surgery for obstructive sleep apnea
Vocal tract symptoms: Severity and frequency in patients on statins
www.entjournal.com A Vendome Publication APRIL/MAY 2018 • VOL. 97, NO. 4-5


EDITORIAL BOARD
EDITORIAL BOARD MEMBERS
Editor-in-Chief
Robert T. Sataloff, MD, DMA, FACS
Professor and Chairman, Department of Otolaryngology–Head and Neck Surgery, and Senior Associate Dean for Clinical Academic Specialties, Drexel University College of Medicine Philadelphia, PA
Jean Abitbol, MD
Jason L. Acevedo, MD, MAJ, MC, USA
Jack B. Anon, MD
Gregorio Babighian, MD
Peter C. Belafsky, MD, PhD
Bruce Benjamin, MD
Gerald S. Berke, MD
Michael J. Brenner, MD
Kenneth H. Brookler, MD
Karen H. Calhoun, MD
Steven B. Cannady, MD
Ricardo Carrau, MD
Swapna Chandran, MD
Chien Chen, MD
Dewey A. Christmas, MD
Nicolle T. Clements, MS
Daniel H. Coelho, MD, FACS
David M. Cognetti, MD
Maura Cosetti, MD
James V. Crawford, MD
David H. Darrow, MD, DDS
Rima Abraham DeFatta, MD
Robert J. DeFatta, MD, PhD
Hamilton Dixon, MD
Paul J. Donald, MD, FRCS
Mainak Dutta, MS, FACS
Russell A. Faust, PhD, MD
Ramón E. Figueroa, MD, FACR
Charles N. Ford, MD
Paul Frake, MD
Marvin P. Fried, MD
Richard R. Gacek, MD
Andrea Gallo, MD
Frank Gannon, MD
Emilio Garcia-Ibanez, MD
Soha Ghossani, MD
William P. R. Gibson, MD
David Goldenberg, MD
Jerome C. Goldstein, MD
Richard L. Goode, MD
Samuel Gubbels, MD
Reena Gupta, MD
Joseph Haddad Jr., MD
Missak Haigentz, MD
Christopher J. Hartnick, MD
Mary Hawkshaw, RN, BSN, CORLN
Garett D. Herzon, MD
Thomas Higgins, MD, MSPH
Jun Steve Hou, MD
John W. House, MD
Glenn Isaacson, MD
Steven F. Isenberg, MD
Stephanie A. Joe, MD
Shruti S. Joglekar, MBBS
Raleigh O. Jones, Jr., MD
Petros D. Karkos, MD, AFRCS, PhD, MPhil
David Kennedy, MD
Seungwon Kim, MD
Robert Koenigsberg, DO
Karen M. Kost, MD, FRCSC
Jamie A. Koufman, MD
Stilianos E. Kountakis, MD, PhD
John Krouse, MD
Ronald B. Kuppersmith, MD, MBA, FACS
Rande H. Lazar, MD
Robert S. Lebovics, MD, FACS
Keat-Jin Lee, MD
Donald A. Leopold, MD
Steve K. Lewis, BSc, MBBS, MRCS
Daqing Li, MD
Robert R. Lorenz, MD
John M. Luckhurst, MS, CCC-A
Valerie Lund, FRCS
Karen Lyons, MD
A.A.S. Rifat Mannan, MD
Richard Mattes, PhD
Brian McGovern, ScD
William A. McIntosh, MD
Brian J. McKinnon, MD
Oleg A. Melnikov, MD
Albert L. Merati, MD, FACS
Joseph P. Mirante, MD, MBA, FACS
Ron B. Mitchell, MD
Steven Ross Mobley, MD
Jaime Eaglin Moore, MD
Thomas Murry, PhD
Ashli K. O’Rourke, MD
Ryan F. Osborne, MD, FACS
J. David Osguthorpe, MD
Robert H. Ossoff, DMD, MD
Enrique Palacios, MD, FACR
Michael M. Paparella, MD
Kourosh Parham, MD, PhD
Arthur S. Patchefsky, MD
Meghan Pavlick, AuD
Spencer C. Payne, MD
Kevin D. Pereira, MD, MS (ORL)
Nicolay Popnikolov, MD, PhD
Didier Portmann, MD
Gregory N. Postma, MD
Matthew J. Provenzano, MD
Hassan H. Ramadan, MD, FACS
Richard T. Ramsden, FRCS
Gabor Repassy, MD, PhD
Dale H. Rice, MD
Ernesto Ried, MD
Alessandra Rinaldo, MD, FRSM
Joshua D. Rosenberg, MD
Allan Maier Rubin, MD, PhD, FACS
John S. Rubin, MD, FACS, FRCS
Amy L. Rutt, DO
Anthony Sclafani, MD, FACS
Raja R. Seethala, MD
Jamie Segel, MD
Moncef Sellami, MD
Michael Setzen, MD, FACS, FAAP
Stanley Shapshay, MD
Douglas M. Sidle, MD
Herbert Silverstein, MD
Jeffrey P. Simons, MD
Raj Sindwani, MD, FACS, FRCS
Aristides Sismanis, MD, FACS
William H. Slattery III, MD
Libby Smith, DO
Jessica Somerville, MD
Thomas C. Spalla, MD
Matthew Spector, MD
Paul M. Spring, MD
Brendan C. Stack, Jr., MD, FACS
James A. Stankiewicz, MD
Jun-Ichi Suzuki, MD
David Thompson, MD
Lester D.R. Thompson, MD, FASCP
Helga Toriello, PhD, FACMG
Ozlem E. Tulunay-Ugur, MD
Galdino Valvassori, MD
Emre Vural, MD
Donald T. Weed, MD, FACS
Neil Weir, FRCS
Kenneth R. Whittemore, MD
David F. Wilson, MD
Ian M. Windmill, PhD
Ian J. Witterick, MD,MSc, FRCSC
Richard J. Wong, MD
Naoaki Yanagihara, MD
Eiji Yanagisawa, MD, FACS
Ken Yanagisawa, MD, FACS
Anthony Yonkers, MD
Mark Zacharek, MD
Joseph Zenga, MD
Liang Zhou, MD
CLINIC EDITORS
Dysphagia
Jamie A. Koufman, MD
Peter C. Belafsky, MD, PhD
Gregory N. Postma, MD
Facial Plastic Surgery
Anthony P. Sclafani, MD, FACS
Geriatric Otolaryngology
Kourosh Parham, MD, PhD, FACS
Karen M. Kost, MD, FRCSC
Head and Neck
Ryan F. Osborne, MD, FACS
Paul J. Donald, MD, FRCS
Reena Gupta, MD
Imaging
Enrique Palacios, MD, FACR
Ramón E. Figueroa, MD, FACR
Laryngoscopic
Robert T. Sataloff, MD, DMA, FACS
Otoscopic
John W. House, MD
Brian J. McKinnon, MD
Pathology
Lester D.R. Thompson, MD, FASCP
Pediatric Otolaryngology
Rande H. Lazar, MD
Rhinoscopic
Eiji Yanagisawa, MD, FACS
Dewey A. Christmas, MD
Joseph P. Mirante, MD, MBA, FACS
Ken Yanagisawa, MD, FACS
Special Topics
Robert T. Sataloff, MD, DMA, FACS
Thyroid and Parathyroid
David Goldenberg, MD
98 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018

Editor-in-Chief Robert T. Sataloff, MD, DMA, FACS 219 N. Broad St., 10th Fl., Philadelphia, PA 19107 entjournal@phillyent.com Ph: 215-732-6100
Managing Editor Linda Zinn
Manuscript Editors Martin Stevenson and Wayne Kuznar
Associate Editor, Reader Engagement Megan Combs
Creative Director Eric Collander
National Sales Manager Mark C. Horn mhorn@vendomegrp.com Ph: 480-895-3663
Traffic Manager Eric Collander
Please send IOs to adtraffic@vendomegrp.com
All ad materials should be sent electronically to: https://vendome.sendmyad.com
Customer Service/Subscriptions
www.entjournal.com/subscribe Ph: 888-244-5310 email: VendomeHM@emailpsa.com
Reuse Permissions Copyright Clearance Center info@copyright.com Ph: 978-750-8400 Fax: 978-646-8600
Chief Executive Officer Jane Butler
Chief Marketing Officer Dan Melore
Vice President, Finance Bill Newberry
Vice President, Custom Media Jennifer Turney Director, Circulation Rachel Beneventi
ENT-Ear, Nose & Throat Journal (ISSN: Print 0145-5613, Online 1942-7522) is published 9 times per year in Jan/Feb, Mar, Apr/May, June, July, Aug, Sept, Oct/ Nov and Dec, by Vendome Group, LLC, 237 West 35th Street, 16th Floor, New York, NY 10001-1905.
©2018 by Vendome Group, LLC. All rights reserved. No part of ENT-Ear, Nose & Throat Journal may be reproduced, distributed, transmitted, displayed, published, or broadcast in any form or in any media without prior written permission of the publisher. To request permission to reuse this content in any form, including distribution in education, professional, or promotional contexts or to reproduce material in new works, please contact the Copyright Clearance Center at info@ copyright.com or 978.750.8400.

EDITORIAL: The opinions expressed in the editorial and advertising material in this issue of ENT-Ear, Nose & Throat Journal are those of the authors and advertisers and do not necessarily reflect the opinions or recommendations of the publisher, editors, or the staff of Vendome Group, LLC. ENT-Ear, Nose & Throat Journal is indexed in MEDLINE/PubMed and Current Contents/Clinical Medicine and Science Citation Index Expanded. Editorial offices are located at 812 Huron Rd., Suite 450, Cleveland, OH 44115. Manuscripts should be submitted online at www.editorialmanager.com/entjournal. Instructions for Authors are available at www.entjournal.com.
SUBSCRIPTIONS: For questions about a subscription or to subscribe, please contact us by phone: 888-244-5310; or email: VendomeHM@emailpsa.com. Individual subscriptions, U.S. and possessions: 1 year $225, 2 years $394; International: 1 year $279, 2 years $488; Single copies $28; outside the U.S., $40.
POSTMASTER: send address changes to Ear, Nose & Throat Journal, PO Box 11404 Newark, NJ 07101-4014.
100 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
ADVERTISER INDEX Pages Acclarent, Inc. 103 American Rhinologic Society ............. CVR2 Arbor Pharmaceuticals 123-124 CANT Corporation ................................. 105 Coastal ENT 100 Compulink Business Systems ............ CVR3 Eagle Surgical Products, LLC 111 Entellus .................................................... 99 Fyzical Therapy and Balance 97 InHealth Technologies ........................... 101 McKeon Products, Inc. CVR4 Reliance Medical ................................... 129 Xlear, Inc. 107

ORIGINAL ARTICLES
116 Microcomputed tomography of the stapes: Wide-ranging dimensions
Jason Patrick Calligas, MD; Norman Wendell Todd Jr., MD, MPH
119 An absence of cilia outer microtubules, an etiology not previously recognized in bilateral mucocele
Javier E. Spínola-Hernández, MD; Andrés E. Castell-Rodríguez, MD; Héctor M. Prado-Calleros, MD; Gerardo A. Bravo-Escobar, MD; Andrés Sadek-González, MD
122 Computational analysis of swallowing mechanics after surgery for obstructive sleep apnea
Mark A. Ellis, MD; Mariah B. Pate, MD; Hugh D. Dorris, BA; William G. Pearson Jr., PhD; Jimmy J. Brown, DDS, MD
128 Vocal tract symptoms: Severity and frequency in patients on statins
Abdul-Latif Hamdan, MD, MPH, FACS; Marc Mourad, MD; Ghina Fakhri, BS; Doja Sarieddine, BS; Elie Khalifee, MD; Sami T. Azar, MD
134 Undifferentiated sarcoma presenting as a slowly enlarging facial mass
Alexis Lopez, MD, MPH; Anton M. Kushnaryov, MD; Robert A. Weisman, MD
ONLINE EXCLUSIVES
E1 Anxiety, depression, and hopelessness in patients before and after treatment for peripheral facial paralysis
Fatih Arslan, MD; Mert Cemal Gökgöz, MD; Murat Binar, MD; Emre Aydemir, MD; Abdullah Durmaz, MD
E5 Early experience in endoscopic transoral resection for parapharyngeal space tumors
Ling-zhao Meng, MD; Qi Zhong, MD; Ju-gao Fang, MD; Hong-zhi Ma, MD; Jian-hong Wang, MS; Yong-xiang Wei, MD
E10 Surgical treatment of symptomatic subglottic stenosis during the third trimester of pregnancy
Erin R.S. Hamersley, DO, LT MC USN;
Angel J. Perez, MD, LCDR MC USN;
Michele P. Morrison, DO, CDR MC USN, FACS;
Halton W. Beumer, MD, MAJ MC USAF
E13 A unique manifestation of Langerhans cell histiocytosis: Diagnostic and therapeutic considerations of atypical cases
Judit Kálmán, MD; Tamás Horváth, MD, PhD; Bálint Liktor, MD; Balázs Liktor, MD, PhD
E18 Esthesioneuroblastoma with widespread distant metastasis: Case report and literature review
Manraj Khosla, MD; Cristina Pecci, DO; Annie Do, MD; Lee McGhan, MD; Mahesh Seetharam, MD; Richard Sue, MD
E22 Screening and management of postoperative hypoparathyroidism-induced hypocalcemia in thyroidectomized patients in the endocrine ward compared with the surgical ward
Nina Sauer, MD; Anne Lautenbach, MD; Katharina Pohl, MD; Gerhard Schön, MD; Hans-Peter Brose, MD;
Clarissa Alexandra Schulze zur Wiesch, MD; Jens Carsten Aberle, MD
E27 Powered irrigation with suction evacuation for chronic rhinosinusitis in the office setting: A pilot study
Margherita Bruni, MD; Lindsey E. Ryan, MD; Mark H. Tabor, MD
E31 Horizontal (vs. vertical) closure of the neo-pharynx is associated with superior postoperative swallowing after total laryngectomy
Giannis Thrasyvoulou, MD, PGCert Med, PhD; Petros V. Vlastarakos, MD, MSc, PhD; Michael Thrasyvoulou, BSc(Math); Aristides Sismanis, MD, FACS
E36 Wireless mobile ultrasonography-assisted parotid duct stone removal
Na Rae Oh, MD; Joo Hyun Woo, MD, PhD; Dong Young Kim, MD, PhD; Min Kwan Baek, MD, PhD
102 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018 EDITORIAL OFFICE Robert T. Sataloff, MD, DMA, FACS, Editor-in-Chief • 219 N. Broad St., 10th Fl. • Philadelphia, PA 19107 CONTENTS APRIL/MAY 2018 • VOL. 97, NO. 4-5
DEPARTMENTS 100 Advertiser Index 104 ENT Journal Online 106 Guest Editorial 108 Rhinoscopic Clinic 109 Laryngoscopic Clinic 112 Head and Neck Clinic 114 Dysphagia Clinic E39 Otoscopic Clinic

JOURNAL ONLINE
Ear, Nose & Throat Journal's website is easy to navigate and provides readers with more editorial content each month than ever before. Access to everything on the site is free of charge to physicians and allied ENT professionals. To take advantage of all our site has to offer, go to www.entjournal. com and click on the “Registration” link. Once you have filled out the brief registration form, you will have full access. Explore and enjoy!
ONLINE EXCLUSIVES
Anxiety, depression, and hopelessness in patients before and after treatment for peripheral facial paralysis
Fatih Arslan, MD; Mert Cemal Gökgöz, MD; Murat Binar, MD; Emre Aydemir, MD; Abdullah Durmaz, MD
We conducted a prospective study to investigate the effectiveness of pharmacologic treatment on alleviating facial paralysis, as well as the anxiety and depression that are associated with it. Our study population was made up of 105 patients—59 men and 46 women, aged 18 to 60 years (mean: 38.2)—who had acute idiopathic peripheral facial paralysis. Before treatment, paralysis was classified as House-Brackmann grade II or III in 44 patients (41.9%) and grade IV to VI in the remaining 61 (58.1%). After....
Early experience in endoscopic transoral resection for parapharyngeal
space tumors
Ling-zhao Meng, MD; Qi Zhong, MD;
Ju-gao Fang, MD; Hong-zhi Ma, MD;
Jian-hong Wang, MS; Yong-xiang Wei, MD
The purpose of this study was to investigate the feasibility, safety, and efficacy of the resection of parapharyngeal space (PPS) tumors via an endoscopic transoral approach. We reviewed 9 patients who were diagnosed with PPS tumors and who were treated with an endoscopic transoral approach. PPS tumors ranging from 2.5 to 6 cm were removed completely with no complications and excellent recovery (mean inpatient hospital stay: 6.89 days). Pathology was pleomorphic adenoma (n = 7), schwannoma (n = 1) and malignant pleomorphic adenoma (n = 1)....
Surgical treatment of symptomatic subglottic stenosis during the third trimester of pregnancy
Erin R.S. Hamersley, DO, LT MC USN;
Angel J. Perez, MD, LCDR MC USN;
Michele P. Morrison, DO, CDR MC USN, FACS;
Halton W. Beumer, MD, MAJ MC USAF
Subglottic stenosis is a narrowing of the airway distal to the glottis. Airway narrowing can be severe and, when coupled with pregnancy, can pose a significant threat to the mother and fetus. There is sparse literature describing treatment of these critical patients, posing a challenge for management. We describe our experience with a 31-year-old woman with idiopathic subglottic stenosis who became symptomatic during her pregnancy, requiring surgical intervention early in her third trimester. The....
A unique manifestation of Langerhans cell histiocytosis: Diagnostic and therapeutic considerations of atypical cases
Judit Kálmán, MD; Tamás Horváth, MD, PhD; Bálint Liktor, MD; Balázs Liktor, MD, PhD
Langerhans cell histiocytosis (LCH) is regarded as a clonal disease, usually carrying the activating BRAF mutation V600E. Although LCH theoretically may affect all types of human tissue and typically appears during childhood, temporal bone involvement in adult patients is exceedingly rare. We report an atypical case of a 56-year-old man as one of the oldest patients diagnosed with temporal bone involvement of a BRAF-negative LCH, which caused painless otorrhea and hearing loss. Cutaneous manifestation (multifocal multisystem LCH, also known as Letterer-Siwe....
Esthesioneuroblastoma with widespread distant metastasis: Case report and literature review Manraj Khosla, MD; Cristina Pecci, DO; Annie Do, MD; Lee McGhan, MD; Mahesh Seetharam, MD; Richard Sue, MD
Esthesioneuroblastoma (ENB) is an uncommon sinonasal tract tumor, and it is even more uncommon among all neoplasms. Literature regarding the incidence and spread of the disease is limited. The prognosis of metastatic disease is poor. In this report, we present a case of recurrent ENB in a young woman involving metastasis to the neck, lungs, and ovary. Metastasis to the cervical lymph nodes is relatively common, but metastasis to the lungs is rare. Furthermore, to our knowledge, no cases of ovarian metastases of ENB have been reported. This case highlights....
Screening and management of postoperative hypoparathyroidism-induced hypocalcemia in thyroidectomized patients in the endocrine ward compared with the surgical ward
Nina Sauer, MD; Anne Lautenbach, MD; Katharina Pohl, MD; Gerhard Schön, MD; Hans-Peter Brose, MD;
Clarissa Alexandra Schulze zur Wiesch, MD;
Jens Carsten Aberle, MD
Transient hypoparathyroid-associated hypocalcemia is a common side effect after thyroidectomy. Not only may it be life-threatening, but it also can distinctly affect length of hospital stay and treatment costs. Screening and treatment practices are suspected to differ between clinicians in endocrine and surgical wards. We therefore compared....
104 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018 www.entjournal.com
Powered irrigation with suction evacuation for chronic rhinosinusitis in the office setting: A pilot study
Margherita Bruni, MD; Lindsey E. Ryan, MD; Mark H. Tabor, MD
Bacterial infections in the form of adherent biofilms are frequently implicated in the pathogenesis and recalcitrance of chronic rhinosinusitis. The Hydrodebrider, a disposable powered irrigation and suction device, has been developed specifically to remove biofilm from the paranasal sinuses. We conducted a prospective study to evaluate the tolerability and efficacy of the Hydrodebrider in the office setting with....
Horizontal (vs. vertical) closure of the neo-pharynx is associated with superior postoperative swallowing after total laryngectomy
Giannis Thrasyvoulou, MD, PGCert Med, PhD; Petros V. Vlastarakos, MD, MSc, PhD; Michael Thrasyvoulou, BSc(Math); Aristides Sismanis, MD, FACS
We conducted a cross-sectional study to compare the horizontal and vertical methods used in the surgical closure of the neo-pharynx after total laryngectomy in terms of their effect on swallowing function, swallowing-related quality....
Wireless mobile ultrasonography-assisted parotid duct stone removal
Na Rae Oh, MD; Joo Hyun Woo, MD, PhD;
Dong Young Kim, MD, PhD; Min Kwan Baek, MD, PhD
Ultrasonography is highly sensitive for the diagnosis of sialoliths. Recently, wireless mobile ultrasonography was developed. We describe the case of a 49-year-old man who presented with painful postprandial left cheek swelling. Computed tomography detected a solitary 5-mm parotid duct stone with infection at the anterior portion of the left masseter muscle. Transoral stone removal was planned, although difficulty was expected in view of the surrounding infection. Surgery was performed under the guidance of mobile ultrasonography, and the stone was removed safely.
ONLINE DEPARTMENTS
Otoscopic Clinic: Iatrogenic external auditory canal stenosis induced by silver nitrate
Samantha J. Mikals, MD; Zhen Huang, MD, MBA; Brian K. Reilly, MD, FACS, FAAP; Ashkan Monfared, MD
Volume 97, Number 4-5 www.entjournal.com 105 ENT JOURNAL ONLINE
GUEST EDITORIAL
Rough sailing on a sea of gray
It is not news that our patients are aging. It is not news that older patients pose unique clinical challenges. It is not news that the health insurer for these patients, Medicare, is under tremendous pressure to reduce the cost of providing healthcare for older patients. It is not news that the level of political inaction, which borders on indifference, is resulting in the problems associated with our aging population becoming ever more daunting, and potentially insurmountable. What is not news is that the physician workforce is graying, a process that will increase the burden on those who continue in active practice. What may be news to many of our colleagues is that there is an otolaryngology society, the American Society of Geriatric Otolaryngology, that has made its mission to assist otolaryngologists in navigating the rough sea of gray.
Founded a decade ago, the American Society of Geriatric Otolaryngology (ASGO) has engaged with the American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS), the Triological Society, and other specialty societies, to prepare our specialty for the gray tsunami. ASGO has focused, and will continue to focus, on understanding the unique clinical challenges older patients face, and pose. The AAO–HNS and the Triological Society deserve mention for their support and assistance with arranging meeting space for ASGO’s annual meeting. ASGO, through its annual meeting, has tackled such diverse topics as preoperative management of the older surgical patient, presbylaryngis, presbycusis, presbystasis, swallowing disorders, end of life, head and neck oncology, vestibular evaluation, bone-anchored hearing aid and cochlear implantation in the elderly, the future of Medicare, and when not to operate.
ASGO members not only contribute with lectures and peer-reviewed medical literature, but they also have collaborated to author, with the assistance of Thieme Medical Publishers and AAO–HNS, the textbook Geriatric Otolaryngology (2015, Print ISBN: 9781626239777, EBook ISBN: 9781626239784). Edited by Dr. Robert T. Sataloff, Dr. Michael Johns, and Dr. Karen Kost, the text is an essential resource for otolaryngologists and other health professionals who care for older patients. Why engage with and through ASGO? Where others see only overwhelming challenges, ASGO sees remarkable opportunity for independent thinking and action by a group of like-minded otolaryngologists. While acknowledging the difficult facts of the current reality, ASGO is driven by the belief that by working through those same challenges as concerned and engaged colleagues, we can, in the end, ensure that geriatric otolaryngology patients receive the care they need, and that those who care for geriatric patients are compensated fairly. Annual dues are modest at $100 a year and include the annual meeting. Residents have no annual dues and have an annual opportunity to present their research related to geriatric otolaryngology. For those interested, ASGO’s secretary, Dr. Carl Shipp, would welcome an inquiry: GCShipp@uams.edu.
Brian J. McKinnon, MD, MBA, MPH, FACS
 Associate Professor and Vice Chair
Department of Otolaryngology–Head and Neck Surgery Associate Professor
Department of Neurosurgery
Drexel University College of Medicine
Associate Professor and Vice Chair
Department of Otolaryngology–Head and Neck Surgery Associate Professor
Department of Neurosurgery
Drexel University College of Medicine
Philadelphia
106 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018

Balloon catheter dilation of a septated frontal sinus
A 32-year-old man presented with intermittent recurrent left frontal headaches. He had noticed that the headaches occurred when he had significant nasal congestion. He had a history of allergies for which he had been treated over a 6- year period.
Computed tomography (CT) of the sinuses revealed a large, septated, left frontal sinus with evidence of obstruction of the nasofrontal duct and mucosal thickening (figure, A). Nasal examination was unremarkable other than pale, boggy, allergic appearing
Continued on page 110
From the Department of Otolaryngology, the Halifax Medical Center, Daytona Beach, Fla. (Dr. Mirante and Dr. Christmas); Florida State University School of Medicine, Daytona Beach (Dr. Mirante); and the Section of Otolaryngology, Yale New Haven Hospital–St. Raphael Campus and the Yale University School of Medicine, New Haven, Ct. (Dr. Yanagisawa).
108 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
RHINOSCOPIC CLINIC
Joseph P. Mirante, MD, MBA, FACS; Dewey A. Christmas, MD; Eiji Yanagisawa, MD, FACS
A B C D
Figure. A: CT Scan of the sinus shows septation in the left frontal sinus. B: A cannula is inserted into the left middle meatus and placed into the left frontal recess. C: An inflated balloon is seen in the left frontal recess. D: The left frontal recess is viewed 2 years after the balloon dilation.
LARYNGOSCOPIC CLINIC
An undulating vallecular cyst
Yu-Hsuan Lin, MD; Ming-Yee Lin, MD, PhD
A vallecular cyst is an uncommon and generally benign lesion in the larynx. In adults, it is usually asymptomatic, but it can cause airway distress. Although most lesions are indolent, physicians should maintain full awareness of the potential occurrence of unanticipated catastrophic consequences in those rare cases in which such a cyst might enlarge and produce airway obstruction.
A 47-year-old man presented to our Otolaryngology Department complaining of a decade-long lump-inthe-throat sensation. His medical history was not remarkable. Diagnostic indirect laryngoscopy revealed a well-delineated cystic mass lying on the right vallecular fossa (figure, A). When we asked the patient to phonate, the mass shifted to the left side (figure, B). The tumor
oscillated from side to side along the axis of the median glossoepiglottic fold.
The patient underwent an endoscopic total excision. Histopathology revealed a cystic structure composed of stratified squamous and columnar epithelium, with no lymphatic elements or oncocytic changes, compatible with a diagnosis of a ductal cyst. After surgical excision, the patient’s symptom vanished. He had no recurrence over the next 27 months.
Vallecular cysts (VCs) account for approximately 10.5% of all laryngeal cysts.1-3 VC has a higher occurrence in the fifth and sixth decades of life, with no sex predilection.1-3 There is no consensus on the genesis and development of VCs; a commonly accepted hypothesis

Volume 97, Number 4-5 www.entjournal.com 109
From the Department of Otolaryngology, Head and Neck Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainen, Taiwan (Dr. Y.H. Lin); and the Department of Otolaryngology, Head and Neck Surgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan (Dr. M.Y. Lin).
A B
Figure. The laryngoscopic exam reveals a vallecular cyst attached to the median glossoepiglottic fold. The cyst flickers from side to side when the patient is asked to phonate and sniff repeatedly (A and B).
LARyNgOSCOPIC CLINIC
is that the cyst is either an embryologic remnant or a consequence of ductal obstruction.1-3
In contrast to infantile VC—which is recognized as a distinct entity and often occurs with laryngomalacia, leading to choking during feeding, cyanotic spells, failure to thrive, and a feeling of airway obstruction— adult VC is usually asymptomatic.4 Patients sometimes present with relapsing acute epiglottitis or an episode of epiglottic abscess.5 The differential diagnosis includes hemangioma, lymphangioma, teratoma, thyroid ectopia and, thyroglossal duct cyst. Endoscopic excision and transoral laser marsupialization are effective treatments.6 Repeated aspiration of the lesion represents a more conservative strategy, but it is often associated with a higher recurrence rate.4,6
References
1. DeSanto LW, Devine KD, Weiland LH. Cysts of the larynx— classification. Laryngoscope 1970;80(1):145-76.
2. Forte V, Fuoco G, James A. A new classification system for congenital laryngeal cysts. Laryngoscope 2004;114(6):1123-7.
3. Arens C, Glanz H, Kleinsasser O. Clinical and morphological aspects of laryngeal cysts. Eur Arch Otorhinolaryngol 1997; 254(9-10):430-6.
4. Tsai YT, Lee LA, Fang TJ, Li HY. Treatment of vallecular cysts in infants with and without coexisting laryngomalacia using endoscopic laser marsupialization: Fifteen-year experience at a single center. Int J Pediatr Otorhinolaryngol 2013;77(3):424-8.
5. Berger G, Averbuch E, Zilka K, et al. Adult vallecular cyst: Thirteen-year experience. Otolaryngol Head Neck Surg 2008; 138(3):321-7.
6. Su CY, Hsu JL. Transoral laser marsupialization of epiglottic cysts. Laryngoscope 2007;117(7):1153-4.
Continued from page 108
nasal mucosa. Because of the long-standing left frontal headaches and failure of adequate medical therapy, the patient elected to undergo left frontal sinus balloon dilation.
The left frontal sinus balloon catheter dilation was carried out through the left frontal recess (figure, B). The balloon was inflated in the usual manner, and an adequate opening was obtained through the left frontal recess (figure, C).
The patient’s left frontal headache problem resolved after the surgery and has not recurred over a period of 2 years. Endoscopic examination of the nose 2 years postoperatively revealed a widely patent left frontal recess area (figure, D).
Balloon catheter dilation has been described as an excellent tool in the treatment of chronic rhinosinusitis.1 It has been associated with symptomatic relief comparable to that associated with traditional endoscopic sinus surgery, but with less tissue destruction. 2 The case presented shows long-term patency of the frontal recess.
Acknowledgment
The authors thank Grayson Bertaina for his assistance in preparing this article.
References
1. Kuhn FA, Church CA, Goldberg AN, et al. Balloon catheter sinusotomy: One-year follow-up-outcomes and role in functional endoscopic sinus surgery. Otolaryngol Head Neck Surg 2008; 39(3 Suppl 3):S27-37.
2. Bizaki AJ, Taulu R, Numminen J, Rautiainen M. Quality of life after endoscopic sinus surgery or balloon sinuplasty: A randomized clinical study. Rhinology 2014;52(4):300-5.
110 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
CLINIC
RHINOSCOPIC
HEAD AND NECK CLINIC
Aggressive desmoid fibromatosis of the neck after total thyroidectomy
 Alexander Delides, MD, PhD; Ioannis Plioutas, MD; Stephanos Konstantoudakis, MD; Pavlos Maragoudakis, MD, PhD
Alexander Delides, MD, PhD; Ioannis Plioutas, MD; Stephanos Konstantoudakis, MD; Pavlos Maragoudakis, MD, PhD
A 21-year-old woman underwent total thyroidectomy. Three years later, she noticed a neck mass and increasing discomfort in her neck. A computed tomography (CT) scan showed a mass near the site of the previous thyroidectomy, on the right. Fine-needle-aspiration cytology tests were nondiagnostic.
The patient underwent surgery, during which a paralaryngeal mass was found firmly attached to the surrounding structures and impossible to dissect.
Frozen section biopsy was negative for malignancy; a biopsy was taken for permanent section, and the wound was closed. The histologic diagnosis was desmoid tumor.
During the following weeks, the patient’s symptoms rapidly worsened and she was referred to our department, complaining of severe swallowing difficulty. She was not hoarse and had no pain.
Physical examination revealed a firm, palpable neck mass fixated on the right (figure 1, A). Laryngeal endoscopy showed normal vocal fold movement and a projection of the posterior pharyngeal wall. Magnetic resonance imaging (MRI) revealed an 11-cm mass extending from the right to the left sternocleidomastoid (SCM) muscles (figure 1, B). Surgery was scheduled, with the goal to resect as much tumor as possible and to refer the patient for postoperative radiotherapy.

112 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
From the 2nd Otolaryngology Department (Dr. Delides, Dr. Plioutas, and Dr. Maragoudakis) and the 2nd Department of Pathology (Dr. Konstantoudakis), Attikon University Hospital, Athens, Greece.
A B
Figure 1. A: Photo shows the appearance of the neck, with enlargement of the right side and obliteration of the space between the sternocleidomastoid muscle and the larynx. The scar of the previous thyroidectomy is visible. B: T2-weighted MRI of the neck reveals the tumor occupying the space between the larynx and the spine and extending between the large vessels on both sides. The right carotid is enclosed within the tumor.
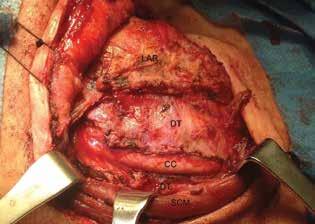
During surgery, a nasogastric tube was placed as a palpable guide to the esophagus. A right hockey-stick incision, including the previous thyroidectomy incision, was made. After elevation of the subplatysmal flaps, the tumor was found completely occupying the space between the right SCM and the larynx. It was dissected away from the SCM, the larynx, the cervical spine, and the esophagus. The right common carotid was surrounded by the tumor but was palpated and used as a landmark (figure 2). The internal jugular vein was saved, but the right inferior laryngeal nerve was sacrificed. An attachment to the right cricothyroid muscle was noticed as a possible point of tumor origin.
Postoperatively, the patient had bilateral vocal fold palsy, but movement of the left vocal fold returned to normal 3 days later. She also had Horner syndrome on the right side, which improved after 3 months. Her swallowing difficulty immediately resolved, and she was discharged on the fifth day. One month later, a follow-up MRI showed no sign of tumor presence, and it was decided to wait before any additional treatment was undertaken.
Two years later, the patient remains disease-free (figure 3), so she is considered cured. Histology showed the lesion to have the morphologic aspects of extraabdominal fibromatosis.
Desmoid tumors are benign musculoaponeurotic tumors with the potential to strangle surrounding structures. They are caused by mutations of fibroblast
cells1 and do not metastasize. Their recurrence rates range between 20 and 77%.2
Treatment is surgical resection.3 External beam radiation or systemic drug treatment may be used when total resection is impossible. A history of prior trauma or surgery at the site of the tumor may be elicited. However, these tumors are considered a neoplastic rather than an inflammatory reactive process.4
This is the third case of a desmoid tumor after thyroidectomy, 5,6 the second after total thyroidectomy, 5 and, to the best of our knowledge, the first with such a large tumor to be completely cured by surgery.
References
1. Ali R, Parthiban N, O’Dwyer T. Desmoid fibromatosis of submandibular region. J Surg Tech Case Rep 2014;6(1):21-5.
2. Hoos A, Lewis JJ, Urist MJ, et al. Desmoid tumors of the head and neck—a clinical study of a rare entity. Head Neck 2000;22(8):814-21.
3. Merchant NB, Lewis JJ, Woodruff JM, et al. Extremity and trunk desmoid tumors: A multifactorial analysis of outcome. Cancer 1999;86(10):2045-52.
4. de Bree E, Zoras O, Hunt JL, et al. Desmoid tumors of the head and neck: A therapeutic challenge. Head Neck 2014;36(10):1517–26.
5. Arena S, Salamone S, Cianci R, et al. Aggressive fibromatosis of the neck initiated after thyroidectomy. J Endocrinol Invest 2006;29(1):78-81.
6. Wang CP, Chang YL, Ko JY, et al. Desmoid tumor of the head and neck. Head Neck 2006;28(11):1008-13.
Volume 97, Number 4-5 www.entjournal.com 113 HEAd ANd NECk CLINIC
Figure 2. Intraoperative view shows the tumor being opened to reveal the common carotid. The sternocleidomastoid muscle is retracted posteriorly and the larynx anteriorly.
Figure 3. T1-weighted MRI of the neck 2 years after surgery reveals no presence of tumor.
DYSPHAGIA CLINIC
A rare cause of dysphagia: Pyriform sinus atypical lipomatous tumor

Atypical lipomatous tumor (ALT) and welldifferentiated liposarcoma (WDL) are rare liposarcoma subtypes that are histologically identical but distinguished by the 2002 WHO classification: ALTs occur in surgically amenable locations, whereas WDLs do not. This distinction exists because these tumors do not tend to recur after complete surgical removal, despite their infiltrative growth pattern and locally aggressive behavior.1
A 60-year-old male nonsmoker presented for evaluation of several months of throat irritation and a sense of something stuck in his throat. Physical examination was normal, except flexible endoscopy revealed a benignappearing, pedunculated mass with normal-appearing mucosa extending from the posterolateral pharyngeal wall onto the arytenoid tower. Computed tomography showed a benign-appearing mass within the left hypopharynx effacing the pyriform sinus.
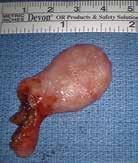
The patient underwent transoral robotic surgical (TORS) excision of the mass. It was 5 cm in size, pedunculated and bilobed (figure 1, A). It was excised, leaving an approximately 1-cm mucosal defect. The specimen was soft and pink (figure 1, B). Microscopic examination
revealed a relatively circumscribed, adipocytic proliferation arranged in lobules of mature-appearing adipose tissue with prominent intervening fibrous septae. Random distribution of enlarged hyperchromatic cells with mild nuclear atypia and occasional multinucleation involved both the adipose and stromal components. Scarce lipoblasts and a rare hibernoma-like nodule were appreciated. Neither necrosis nor mitotic activity was identified. Cells were immunoreactive for MDM2 and p16, and nonreactive for p53, consistent with ALT (figure 2). Three months postoperatively, the patient had no evidence of recurrence.
ALT rarely presents in the laryngopharynx.1-3 In 2009, Davis et al reviewed all head and neck sarcomas at the University of Texas M.D. Anderson Cancer Center over a 60-year period (1945–2005) and found 30 head and neck liposarcomas, of which only 7 were ALT/WDLs.4 Three of these were in the upper aerodigestive tract. In 1999, Mandell et al compiled reports of eight hypopharyngeal liposarcomas from three studies, all of which were ALT/WDLs; they had a strong male predominance (7/8) and a size ranging from 2 to 14.5 cm.3 In 2010, Shi et al reported five ALTs in the laryngopharynx: two
From the Department of Otolaryngology, Tripler Army Medical Center, Hawaii.
Disclaimer: The views expressed in this article are those of the author) and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the United States government.
114 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
Yoseph A. Kram, MD; James M. McCann, DMD, MS; Joseph Golden, MD; Eric Wirtz, MD
A B
Figure 1. A: Intraoperative image shows the pedunculated, bilobed tumor in the left hypopharynx. B: The 5-cm excised tumor is soft and pink with a fatty appearance.
in the hypopharynx and three in the larynx, ranging from 2.0 to 5.0 cm.2 Dysphagia, dysphonia, and globus sensation were typical presenting symptoms.
Immunohistochemistry staining of MDM-2, a negative regulator of the p53 tumor suppressor, and p16 have emerged as markers for ALT/WDL. A 2017 study of 101 ALTs showed that dual p16 and MDM2 testing yielded a 100% positive predictive value and a 100% negative predictive value when MDM2+/p16+ and MDM2–/p16–staining were observed for ALT-WDL, respectively.5

Surgical excision in the hypopharynx can be challenging because of inadequate access and visualization. Classic approaches include lateral pharyngotomy or direct suspension microlaryngoscopy.6 Radiation is not recommended as primary therapy for hypopharyngeal ALTs but has been offered as adjunctive therapy.3,4 If TORS continues to show a low recurrence rate for ALTs, it will further differentiate them from WDLs.
References
1. Dei Tos AP, Pedeutour F. Atypical lipomatous tumour/well differentiated liposarcoma. In: Fletcher C, Unni KK, Mertens F (eds). Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002:35-7.
2. Shi HY, Wei LX, Wang HT, Sun L. Clinicopathological features of atypical lipomatous tumors of the laryngopharynx. J Zhejiang Univ Sci B 2010;11(12):918-22.
3. Mandell DL, Brandwein MS, Woo P, et al. Upper aerodigestive tract liposarcoma: Report on four cases and literature review. Laryngoscope 1999;109(8):1245-52.
4. Davis EC, Ballo MT, Luna MA, et al. Liposarcoma of the head and neck: The University of Texas M.D. Anderson Cancer Center experience. Head Neck 2009;31(1):28-36.
5. Kammerer-Jacquet SF, Thierry S, Cabillic F, et al. Differential diagnosis of atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: Utility of p16 in combination with MDM2 and CDK4 immunohistochemistry. Hum Pathol 2017;59:34-40.
6. Wenig BM, Heffner DK. Liposarcomas of the larynx and hypopharynx: A clinicopathologic study of eight new cases and a review of the literature. Laryngoscope 1995;105(7 Pt 1):747-56.

Volume 97, Number 4-5 www.entjournal.com 115 dySPHAgIA CLINIC
Figure 2. The tumor is positive for p16 on immunohistochemical staining.
Microcomputed tomography of the stapes: Wide-ranging dimensions
Jason Patrick Calligas, MD; Norman Wendell Todd Jr., MD, MPH
Abstract
Although human stapes are known to have varied dimensions and the footplate is considered to be oval (fitting as it does into the oval window), few studies of high-resolution imaging of these structures have been performed. No study appears to have addressed the bilateral symmetry of stapes dimensions or to have determined if an association exists between the size of the stapes and the size of mastoid pneumatization; a small mastoid pneumatization is an indicator of childhood otitis media. We obtained 41 ear-normal cadaver crania specimens for study in our temporal bone laboratory and isolated 10 for further analysis: the 5 with the largest areas of mastoid pneumatization and the 5 with the smallest. Microcomputed tomography of tissue blocks was performed on the in situ stapes. Using ImageJ software, we created a three-dimensional model of each stapes. The mean height of these stapes was 3.43 mm (range: 3.20 to 3.80), the mean length of the footplates was 2.71 mm (range: 2.52 to 2.97), and the mean width of the footplates was 1.23 mm (range: 1.12 to 1.46). Qualitatively, the footplate was shaped like a human footprint in moist sand, as Eysell described in 1870. The dimensions of the stapes were found to be bilaterally symmetrical in general, but there was no correlation between these dimensions and the size of mastoid pneumatization. The distribution of footplate widths may be bimodal, which is consistent with the observation of Sim et al that men have wider footplates than do women.
Introduction
The stapes, the smallest of all the named bones in the human body, has intrigued clinicians and researchers for many years. More than a century ago, Eysell1 and Urbantschitsch2 published qualitative and quantitative descriptions of the stapes. Dass et al in 1966 reported marked variations in stapedial structure.3 Working with a precision caliper under a microscope, aWengen et al found an impressive range of distances in six measurements between
From the Department of Otolaryngology–Head and Neck Surgery, Emory University School of Medicine, Atlanta.
Corresponding author: Norman Wendell Todd Jr., MD, MPH, Emory Children’s Center, 2015 Uppergate Dr., NE, Atlanta, GA 30329.
Email: ntodd@emory.edu
various points on the stapes.4 Rouset et al, studying normal ears with clinical computed tomography (CT), found that stapes heights ranged from 2.3 to 4.2 mm (mean: 3.7).5
Using microcomputed tomography (microCT), Sim et al reported stapes heights and footplate lengths and widths as 3.28 (±0.21), 2.81 (±0.16), and 1.27 (±0.11) mm, respectively, in predominately right-sided stapes.6 They also reported that men have wider footplates than do women.
In this article, we describe our study to validate the work of Sim et al,6 as well as to assess bilateral symmetry in the quantitative features in pairs of stapes and to determine the qualitative appearance of the footplates. Our hypothesis was that there is symmetry between the stapes pairs—a feature that to the best of our knowledge has not been previously studied. Bilateral symmetry would suggest that genetic influences play a role in stapes development.
A secondary aim of our investigation was to correlate stapes metrics with mastoid pneumatization (minimal temporal bone pneumatization is a correlate of childhood otitis media).7,8 Our hypothesis was that the development of the stapes is independent of the size of mastoid pneumatization. To the best of our knowledge, this issue has not been explored, either.
A third goal was to determine if stapes height, footplate width, and footplate length are correlated. In other words, If a stapes is large in one dimension, is it large in the other dimensions, as well, and vice versa? The question is apropos, since Rodríguez-Vázquez reported that the entire stapes develops from the mesenchyme of the second branchial arch, independent of both the Reichert cartilage and the otic capsule.9
Finally, a fourth objective was to determine if the distribution of footplate widths is bimodal, since Sim et al reported that footplates are wider in men than in women.6
Materials and methods
Specimens. We studied 41 adult skulls (82 temporal bones) that were provided by the anatomy unit at the Emory University School of Medicine. The specimens had been obtained from humans who had bequeathed their bodies to science before they died. No information about the age, sex, and race/ethnicity of the do-
116 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018 ORIGINAL ARTICLE
nors was available. While we knew that none of the donors had died of ear disease, further unambiguous historical ear data were not available.
On plain Law lateral radiography of the 82 temporal bones, the areas of mastoid pneumatization were traced as previously described.10 The 5 crania with the largest areas of mastoid pneumatization and the 5 with the smallest underwent microCT imaging.
MicroCT and 3-D reconstruction. Images of the 20 temporal bones from the 10 selected crania were obtained on a microCT device (Inveon MicroCT; Siemens Healthineers; Erlangen, Germany). Since the gross physical sizes of the temporal bones exceeded the imaging aperture of the scanner, the specimens were physically reduced. The microCT images were then made with a pixel size of 20 × 20 μm, a slice thickness of 21.5 μm, and a resolution of 46.499 pixels/mm.
Segmentation and 3-D volume reconstruction were performed with the ImageJ program and the plug-in BoneJ.11,12

First, each stapes was cropped from the surrounding structures and ambient air by hand. Second, the “background noise” was subtracted. Third, the image stack was processed with the Isosurface algorithm of BoneJ, which converts the stack into a triangular surface model. The surface model was saved into a Standard Tessellation Language (STL) file format for dimensional analysis of the stapes structure. Fourth, the 3-D viewer was used to visualize the STL models. Reference points were marked directly on the 3-D models and recorded as x, y, and z coordinates.
Complete stapes were missing from 2 tissue blocks before microCT imaging, and therefore no data were obtained from them. For 2 other stapes, the crura were
damaged before microCT imaging, making calculation of stapes height impossible.
Stapes measurements. The stapes height was calculated by measuring the distance between a point in the center of the top of the capitulum and a point in the center of the stapes footplate on the medial (vestibular) surface (figure 1). The footplate’s length was calculated by measuring the distance between points on both ends of the long axis of the footplate annulus. The footplate’s width was calculated by measuring points on both ends of the short and long axes of the footplate annulus.
After we created the models in BoneJ, we identified each landmark in 3-D space (x, y, and z) with the point tool. For measurements of the footplate length, we used the landmarks that represented the farthest point on the “big toe” side of the footplate and the farthest point at the “heel.” For footplate width, we determined the widest diameter perpendicular to the footplate length. Footplate dimensions were thus determined in a manner analogous to a shoe salesman measuring a foot (figure 2). Each measurement was performed twice, independently. Calculations and statistics. Distance measurements were calculated according to the Pythagorean theorem in three dimensions. Only if associations were suggested on scatterplot graphs were nonparametric Spearman correlations calculated. No correction for multiple comparisons was done.
Ethical considerations. Our university’s Institutional Review Board determined that IRB approval was not required because this study “does not meet the definition of ‘research involving human subjects’ or the definition of ‘clinical investigation’ under applicable federal regulations.”
Volume 97, Number 4-5 www.entjournal.com 117 MICROCOMPUTEd TOMOgRAPHy Of THE STAPES: WIdE-RANgINg
dIMENSIONS
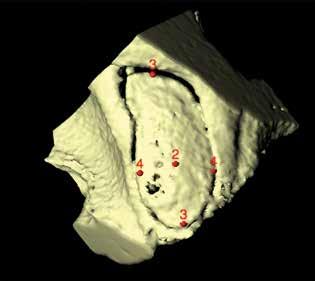
Figure 1. MicroCT shows a right stapes viewed from a surgeon’s perspective, laterally onto the superstructure. Note the footplate’s socked-foot appearance, with the big toe anterorinferior. The red circle (1) indicates the center of the top of the capitulum.
Figure 2. MicroCT demonstrates a right footplate viewed onto the vestibular (medial) surface. The red circles denote the center of the footplate (2), the limits of its length (3), and the limits of its width (4) perpendicular to the length.
Table.
Results
The range of stapes heights was wide (3.20 to 3.80 mm; mean: 3.43), as were the ranges of footplate lengths (2.52 to 2.97 mm; mean: 2.71) and widths (1.12 to 1.46 mm; mean: 1.23). The repeatability of measurements was excellent (table).
Bilateral symmetry of the stapes measurements was suggested, but symmetry was statistically significant only for the footplate width (rs = 0.86; n = 8; 95% confidence interval: 0.39 to 0.97). No stapes feature showed a correlation with the size of mastoid pneumatization.
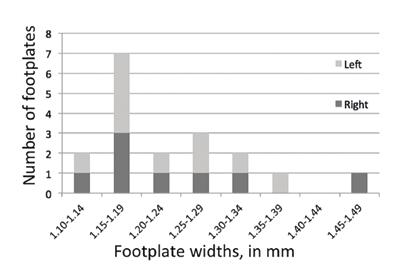
We found no correlation among the linear dimensions themselves—that is, if any one measured dimension of a stapes was small (or large), other measured dimensions of that stapes were not necessarily small (or large). Unlike the distribution of the stapes height and footplate length, the distribution of footplate width may be bimodal (figure 3).
Each footplate qualitatively resembled the appearance of a sock-covered human foot.
Discussion
The stapes measurements acquired in this study are consistent with those of previous reports.2-4,6 The mean height, footplate length, and footplate width for combined right and left stapes in our study was 3.43, 2.71, and 1.23 mm, respectively. Sim et al demonstrated similar findings using microCT analysis.6 They also reported that men have wider footplates than women (mean: 1.32 vs. 1.17 mm, p < 0.001).
Our study aimed to overcome a limitation of previous microCT analyses by looking at pairs of stapes. Bilateral sym-
metry was suggested for all three metrics, but because of the small number of specimens, we found statistical significance only with respect to footplate width.
No correlation of the stapes parameters and the size of mastoid pneumatization was suggested. Thus, these data may indicate that local middle ear environmental factors affecting pneumatization are unrelated to stapes development. The first and second branchial arches and the first pouch contribute to the development of the middle ear. Mastoid bone development occurs at a very different stage of life, but it might nevertheless be influenced by “intrinsic defects.”13 The fact that we did not find a correlation between stapes height and footplate length and width might be surprising, considering the second arch one-anlage origin of the entire stapes.9
* Repeatability is depicted by both the Spearman nonparametric correlation coefficient r s, with 95% confidence interval (CI), and by a clinically relevant value. Each measurement represents the mean of two independent measurements. Continued on page 121
118 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018 CALLIgAS, TOdd
Figure 3. Chart shows that the distribution of footplate widths appears to be bimodal, consistent with two populations (presumably male and female, according to the work of Sim et al6).
Measurement Repeatability, r s, (95% CI) Repeatability, practical Median (range) Mastoid area, right, all crania 0.89, n = 41, (0.80 to 0.94) 31/41, ≤2 cm2 9.6 cm2 (2.4 to 14.2) Mastoid area, left, all crania 0.92, n = 41, (0.85 to 0.95) 34/41 ≤2 cm2 10.0 cm2 (2.0 to 18.0) Height of stapes, right 0.99, n = 7, (0.93 to 0.99) 5/7 ≤0.05 mm 3.30 mm (3.20 to 3.80) Height of stapes, left 0.99, n = 9, (0.95 to 0.99) 7/10 ≤0.05 mm 3.45 mm (3.25 to 3.74) Length of footplate, right 0.80, n = 9, (0.21 to 0.96) 6/8 ≤0.05 mm 2.71 mm (2.62 to 2.97) Length of footplate, left 0.87, n = 10, (0.86 to 0.99) 6/10 ≤0.05 mm 2.68 mm (2.52 to 2.83) Width of footplate, right 0.87, n = 8, (0.42 to 0.98) 5/8 ≤0.05 mm 1.20 mm (1.13 to 1.46) Width of footplate, left 0.96, n = 10, (0.86 to 0.99) 9/10 ≤0.05 mm 1.22 mm (1.12 to 1.33)
Summary of the repeatability and distribution of each measurement*
An absence of cilia outer microtubules, an etiology not previously recognized in bilateral mucocele
Javier E. Spínola-Hernández, MD; Andrés E. Castell-Rodríguez, MD; Héctor M. Prado-Calleros, MD; Gerardo A. Bravo-Escobar, MD; Andrés Sadek-González, MD
Abstract
Most paranasal sinus mucoceles are unilateral and affect one or at most two contiguous sinuses. We describe the case of a 44-year-old woman with bilateral maxillary sinus mucoceles who presented clinically with left malar pain, right-sided swelling, and proptosis of the right eye. The diagnostic workup included computed tomography and magnetic resonance imaging. In addition, because of the atypical bilateral presentation, we analyzed mucosal sinonasal tissue samples by electron microscopy. Microscopic analysis revealed an absence of one of the microtubule doublets in three of the outer doublets of the axoneme, thereby establishing a diagnosis of isolated ciliary dysfunction. To the best of our knowledge, ciliary dysfunction as a cause of bilateral mucoceles has not been previously reported in the literature. The patient underwent successful surgery for removal of the mucoceles, and she exhibited no evidence of recurrence at the 18-month follow-up. When a diagnosis of bilateral mucocele formation is made, we suggest that ciliary dysfunction be considered in the differential diagnosis and that electron microscopy of the sinonasal mucosa be performed in the workup.
Introduction
Most paranasal sinus mucoceles are unilateral and affect one or at most two contiguous sinuses. They produce dilation of the involved sinus, as its interior fills with mucus produced by the mucosa. They usually arise as a
From the Division of Otolaryngology–Head and Neck Surgery, General Hospital “Dr. Manuel Gea González,” Mexico City (Dr. Spínola-Hernández, Dr. Prado-Calleros, Dr. Bravo-Escobar, and Dr. Sadek-González); and the Department of Cell Biology and Tissue, National Autonomous University of Mexico, Mexico City (Dr. Castell-Rodríguez). The case described in this article occurred at General Hospital “Dr. Manuel Gea González.”
Corresponding author: Hector M. Prado-Calleros, MD, Division of Otolaryngology–Head and Neck Surgery, General Hospital “Dr. Manuel Gea González,” Calzada de Tlalpan 4800, Colonia Sección XVI, Delegación Tlalpan, 14080 Mexico City, Mexico. Email: hmpradoc@hotmail.com
result of occlusion of the sinus ostium, which can occur secondary to inflammation, scarring, or a neoplasm.1-3
Bilateral mucoceles are rarely reported. They can occur secondary to surgery, a papillomatous tumor, or chronic inflammation, and they might be related to mucous abnormalities or primary ciliary dyskinesia.1-3
The histologic appearance of mucoceles is remarkable given the intense intralesional pressure. They exhibit strips of flattened ciliated columnar epithelium with bone remodeling and chronic inflammation.4
Primary ciliary dyskinesia is a rare, genetically heterogeneous disorder that results in impairment of mucosal ciliary movement. It usually manifests as recurrent and chronic infections of the upper and lower airways.1 The structure of the ciliary apparatus consists of a microtubule-based axoneme, which is a highly ordered structure made up of nine peripheral microtubule doublets that are arranged around a central core. This core might or might not contain two central microtubules (a 9 + 2 axoneme and a 9 + 0 axoneme, respectively). The cilia of the 9 + 2 axoneme usually contain dynein arms that link the microtubule doublets, and the cilia are motile. Most cilia of the 9 + 0 axoneme lack dynein arms, and they are nonmotile. Mutations of two genes—DNAI1 and DNAH5 —account for about 30 to 50% of all cases of primary ciliary dyskinesia, and they are responsible for the defects in the outer dynein arms.1,5
In this article, we describe a case of bilateral maxillary sinus mucoceles that featured an absence of one of the microtubule doublets in three of the outer doublets of the axoneme, thereby establishing a diagnosis of isolated ciliary dysfunction.
Case report
A 44-year-old woman presented with a 7-month history of left malar pain, right-sided swelling, and proptosis of the right eye. Previously, her dentist had performed a left upper canine extraction. During that procedure,
Volume 97, Number 4-5 www.entjournal.com 119 ORIGINAL ARTICLE
the dentist had found a bulking lesion in the alveolar canine area that was filled with mucinous fluid.
On physical examination at our institution, we observed preserved vision in both eyes, but with a limitation of downward movement of the right eye. Nasal endoscopy detected swelling in the right lateral nasal wall and a white polypoid mass with smooth edges in the left nasal lateral wall. Both middle meatuses were without purulent discharge. Findings on the remainder of the examination were normal.
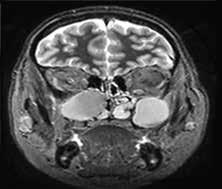
The diagnostic workup included computed tomography (CT) and magnetic resonance imaging (MRI), which clearly demonstrated the bilateral masses in the maxillary sinuses (figure 1). Because of the atypical bilateral presentation of the mucocele, we also performed electron microscopy to examine sinonasal mucosa tissue samples. This analysis revealed an absence of one of the microtubule doublets in three of the outer doublets of the axoneme (figure 2). To rule out etiologies such as cystic fibrosis and Kartagener syndrome, we obtained a chest x-ray and performed a chloride-in-sweat test; findings were normal. Our final diagnosis was isolated ciliary dysfunction.
The patient underwent endoscopic sinus surgery with a medial inferior left endoscopic maxillectomy. The mucoceles were drained via a sublabial approach and endoscopic right mucopyocele drainage.
The surgical outcome was satisfactory. Follow-up CT performed 18 months later detected no sinonasal disease.
Discussion
The presence of mucoceles in both maxillary sinuses is a rare presentation, accounting for only 4% of all reported cases of mucocele.2,3 When bilateral cases do occur, most are bilaterally symmetrical; asymmetrical sinus involvement with or without orbital complications has rarely been reported.2,3 Maxillary sinus involvement is also uncommon, accounting for less than 10% of all mucocele cases.2,3
Many bilateral mucoceles are pseudomucoceles that arise as a result of mucus abnormalities or primary ciliary dysfunction, as is the case with cystic fibrosis or Kartagener syndrome; this presentation is commonly associated with a rhinobronchial syndrome.2,3 Our patient presented without chronic rhinosinusitis symptoms. Because of her atypical presentation, we suspected ciliary dysfunction. Analysis of the sinus mucosa under electron microscopy found alterations of the cilia, which explained the underlying pathophysiology of the mucocele formation.5 To the best of our knowledge, such a cause of bilateral maxillary mucoceles has not been previously described in the literature.
As far as we know, only 1 case of mucocele secondary to primary ciliary dyskinesia has been reported.1 In that case, a 12-month-old boy who presented with such a mucocele and ipsilateral proptosis was found to have an absence of the inner dynein arms linking the microtubule doublets. Our case involved alterations of the outer microtubules. The variations of the ciliary alterations may explain the different clinical presentations in these two cases.
We suggest that in addition to endoscopy and imaging studies, otolaryngologists should consider performing electron microscopy and measuring levels of chloride in sweat in cases of bilateral or atypical mucocele formation (figure 3).

120 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
SPíNOLA-HERNáNdEz,
CASTELL-ROdRígUEz, PRAdO-CALLEROS, BRAvO-ESCOBAR, SAdEk-gONzáLEz
A B
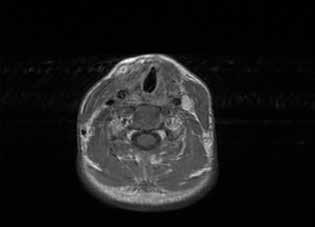
Figure 1. A: Coronal CT shows the homogeneous isodense soft-tissue mass occupying both maxillary sinuses with bone remodeling of their walls, including the orbital floor. B: Axial T2-weighted MRI demonstrates the hyperintense cystic masses in both maxillary sinuses and the expansion of the orbital floor.
Figure 2. Electron microscopy demonstrates the absence of an outer microtubule in three of the nine external doublets of the cilia (one of them is highlighted).
References
1. Berlucchi M, Maroldi R, Aga A, et al. Ethmoid mucocele: A new feature of primary ciliary dyskinesia. Pediatr Pulmonol 2010;45(2):197-201.
2. Varghese L, John M, Kurien M. Bilateral asymmetric mucoceles of the paranasal sinuses: A first case report. Ear Nose Throat J 2004;83(12):834-5.

3. Chong AW, Prepageran N, Rahmat O, et al. Bilateral asymmetrical mucoceles of the paranasal sinuses with unilateral orbital complications. Ear Nose Throat J 2011;90(2):E13.
4. Thompson LD. Paranasal sinus mucocele. Ear Nose Throat J 2012;91(7):276-8.
5. Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development 2006;133(21):4131-43.
MICROCOMPUTEd TOMOgRAPHy Of THE STAPES: WIdE-RANgINg dIMENSIONS
Continued from page 118
The major limitations of our study are that (1) we looked at specimens of only 10 crania (the 5 with the largest areas of mastoid pneumatization and the 5 with the smallest) and (2) we had no specific information about age, sex, or otologic history. Nevertheless, our study does have three distinct positive features: (1) the specimens represented the size extremes of mastoid pneumatization in 41 clinically ear-normal crania, (2) images of the stapes were obtained in situ, and thus any possible damage sustained during harvesting was precluded, and (3) bilateral symmetry of stapes features was checked. In conclusion, stapes heights and footplate lengths and widths in our study were consistent with those reported by Sim et al6 and others.2-4 Studying as few as 7 paired specimens, we found that bilateral symmetry was suggested for superstructure height and footplate length and found to be statistically significant for footplate width. We found no correlation between the stapes measurements and the size of mastoid pneumatization. The distribution of footplate widths may be bimodal, as reported by Sim et al, in that men have wider footplates than do women.6
References
1. Eysell A. Beiträge zur Anatomie des Steigbügels und seiner Verbindungen. Arch Ohrenheilk 1870;5:237-49.
2. Urbantschitsch V. Zur anatomie der Gehörknochelchen des Menschen. Arch Ohrenheilkunde 1876;11:1-10.
3. Dass R, Grewal BS, Thapar SP. Human stapes and its variations. I. General features. J Laryngol Otol 1966;80(1):11-25.
4. aWengen DF, Nishihara S, Kurokawa H, Goode RL. Measurements of the stapes superstructure. Ann Otol Rhinol Laryngol 1995;104(4 Pt 1):311-16.
5. Rousset J, Garetier M, Gentric JC, et al. Biometry of the normal stapes using stapes axial plane, high-resolution computed tomography. J Laryngol Otol 2014;128(5):425-30.
6. Sim JH, Röösli C, Chatzimichalis M, et al. Characterization of stapes anatomy: Investigation of human and guinea pig. J Assoc Res Otolaryngol 2013;14(2):159-73.
7. Swarts JD, Foley S, Alper CM, Doyle WJ. Mastoid geometry in a cross-section of humans from infancy through early adulthood with a confirmed history of otitis media. Int J Pediatr Otorhinolaryngol 2012;76(1):137-41.
8. Chole RA, Sudhoff HH. Chronic otitis media, mastoiditis, and petrositis. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology: Head and Neck Surgery. 6th ed. Philadelphia: Elsevier Saunders; 2014:1965-78.
9. Rodríguez-Vázquez JF. Development of the stapes and associated structures in human embryos. J Anat 2005;207(2):165-73.
10. Todd NW. Orientation of the manubrium mallei: Inexplicably widely variable. Laryngoscope 2005;115(9):1548-52.
11. Doube M, Kłosowski MM, Arganda-Carreras I, et al. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010;47(6):1076-9.
12. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9(7):671-5.
13. Kalter H. Teratology in the Twentieth Century Plus Ten. New York: Springer; 2010.
Volume 97, Number 4-5 www.entjournal.com 121 AN ABSENCE Of CILIA OUTER MICROTUBULES, AN ETIOLOgy NOT PREvIOUSLy RECOgNIzEd IN BILATERAL MUCOCELE
Figure 3. Flow chart shows our suggestion for the diagnosis of ciliary dysfunction.
Computational analysis of swallowing mechanics after surgery for obstructive sleep apnea
Mark A. Ellis, MD; Mariah B. Pate, MD; Hugh D. Dorris, BA; William G. Pearson Jr., PhD; Jimmy J. Brown, DDS, MD
Abstract
Multilevel upper airway surgery for obstructive sleep apnea (OSA) has been shown to cause clinically significant dysphagia in some patients. We describe the cases of 2 adults with OSA who developed persistent dysphagia after multilevel upper airway surgery. Patient-specific computational analysis of swallowing mechanics (CASM) revealed absent pharyngeal shortening and aberrant tongue base retraction in both patients. These findings are consistent with the OSA surgical goal of enlarging the hypopharyngeal airway but likely contributed to our patients’ dysphagia. Patient-specific CASM allows for sensitive identification of swallowing mechanical dysfunction that might otherwise be overlooked, and it may be utilized in future head and neck surgery patients to analyze swallowing dysfunction associated with treatment.
Introduction
Obstructive sleep apnea (OSA) is a common disorder that affects between 5 and 14% of middle-aged adults.1 Continuous positive airway pressure (CPAP) is the first-line therapy for OSA; however, long-term compliance with CPAP is only 50 to 70%.2 Multilevel upper airway surgeries are often employed in OSA patients who do not respond to or who are unable to tolerate
From the Department of Otolaryngology–Head and Neck Surgery, Medical University of South Carolina, Charleston (Dr. Ellis); and the Department of Otolaryngology–Head and Neck Surgery (Dr. Pate and Dr. Brown) and the Department of Cellular Biology and Anatomy (Mr. Dorris and Dr. Pearson), Medical College of Georgia, Augusta University, Augusta.
Corresponding author: Mark A. Ellis, MD, Department of Otolaryngology–Head and Neck Surgery, Medical University of South Carolina, 135 Rutledge Ave., MSC 550, Charleston, SC 29425.
Email: ellismar@musc.edu
CPAP therapy. Uvulopalatopharyngoplasty (UPPP) is the most commonly performed upper airway surgery for OSA and is often used in conjunction with other sleep surgery techniques. Unfortunately, OSA surgical techniques are associated with several untoward outcomes, including clinically significant dysphagia, which has been reported in 13 to 36% of patients.3
The primary sleep surgeon (J.J.B.) at our academic institution employs a variety of sleep surgery techniques. Most patients have evidenced significant improvement in their OSA symptomatology over short- and long-term intervals, but a subset of patients complains of clinically significant dysphagia after surgery. We describe the cases of 2 adults with OSA who developed persistent dysphagia after OSA surgery, and we analyze their postoperative swallowing mechanics using patient-specific computational analysis of swallowing mechanics (CASM).
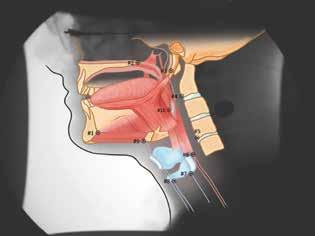
Patient-specific CASM allows for visualization of swallowing mechanics (hyoid movement, laryngeal elevation, tongue base retraction, and pharyngeal shortening) using eigenvectors that characterize the relative contribution of each element of swallowing mechanics. CASM is a multivariate morphometric analysis of coordinates that is used to map muscle groups that underlie oropharyngeal swallow mechanics represented in the lateral-view modified barium swallow (MBS) imaging at 30 frames per second (figure 1).4,5
For patient-specific CASM, these anatomic coordinates are collected for each frame of the oropharyngeal swallow using a semiautomated MATLAB tracker tool.6 Coordinate data are then uploaded into integrated MorphoJ software, and a procrustean superimposition (a mathematical realignment of all coordinates)
122 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018 ORIGINAL ARTICLE


is performed to control for image rotation and patient movement.7 A canonical variate analysis with post hoc discriminant function analysis is performed to statistically evaluate shape change representing the covariate components of swallowing mechanics.

In this article, the eigenvectors of this analysis are reported to demonstrate the patient-specific mechanics associated with surgical intervention.8
Case reports
Patient 1. The first patient was a 49-year-old woman with diagnosed OSA who demonstrated CPAP intolerance and desired surgical intervention. Preoperative polysomnography revealed a baseline apnea-hypopnea index (AHI) of 41 and an Epworth sleepiness scale (ESS) score of 22. Physical examination revealed a body-mass index (BMI) of 44.2 and a Friedman class III tongue position. The fiberoptic nasal endoscopy with modified Müller maneuver (FNMM) scores were 4/4, 4/4, and 3/4 for retropalatal, retrolingual, and lateral pharyngeal wall collapse, respectively.
The patient underwent UPPP, relocation pharyngoplasty, transoral robotic surgery tongue base resection, and tracheotomy. She was decannulated on postoperative day 17. After surgery, she complained of persistent dysphagia with nasal regurgitation and globus sensation.
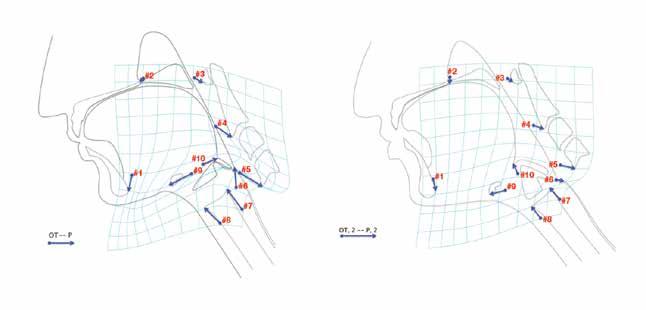
An MBS study performed 8 months after surgery revealed a penetration aspiration scale (PAS) score of 2 (penetration without aspiration). Findings were otherwise unremarkable, including complete velopharyngeal valve closure. Patient-specific CASM was performed on the MBS imaging. When visually compared with normative values for CASM in nonsurgical controls (figure 2, A), our patient’s analysis (figure 2, B) demonstrated an absent pharyngeal shortening (vector #6)
Volume 97, Number 4-5 www.entjournal.com 125 COMPUTATIONAL ANALySIS Of SWALLOWINg MECHANICS AfTER SURgERy fOR OBSTRUCTIvE SLEEP APNEA
Figure 1. Coordinates are used to map the swallowing mechanics in the suprahyoid muscle group (#9 to the mandible [vectors #1 to #3]) and in the thyrohyoid (#8 to #9), the stylopharyngeus (#7 to #3), the palatopharyngeus (#6 to #2), and the styloglossus and hyoglossus (#10 to #3) muscles. It also shows the movement of three skeletal levers, including the skull base (#2 to #3), the mandible (#1 to #3), and vertebrae (#3, #4, and #5). Illustrations courtesy of Mark A. Ellis, MD, and William G. Pearson Jr., PhD.
A B
Figure 2. Patient 1. A: Vectors in nonsurgical controls show normal swallowing mechanics. B: Vectors in patient 1 demonstrate an absent pharyngeal shortening (vector #6) and an aberrant (superior vs. posterior) tongue base retraction (#10). (OT = oral transport; P = pharyngeal swallowing; 1 = patient 1).
and an aberrant (superior vs. posterior) tongue base movement (vector #10).
Patient 2. The second patient was a 23-year-old man with OSA who could not tolerate CPAP due to claustrophobia. Preoperative polysomnography revealed a baseline AHI of 7.3 and an ESS score of 17. Physical examination revealed a BMI of 31.6 and a Friedman class III tongue position. The FNMM scores were 4/4, 4/4, and 3/4 for retropalatal, retrolingual, and lateral pharyngeal wall collapse, respectively.
The patient underwent UPPP, relocation pharyngoplasty, transoral robotic surgery tongue base resection, and tracheotomy. He was decannulated on postoperative day 4. After surgery, he complained of nasal regurgitation with liquids and clinically significant dysphagia.
An MBS study performed 2 months after surgery revealed a PAS score of 2. Findings were otherwise unremarkable, including complete velopharyngeal valve closure. Patient-specific CASM was performed on the MBS imaging. When visually compared with normative values for CASM in nonsurgical controls (figure 3, A), our patient’s analysis (figure 3, B) demonstrated an absent pharyngeal shortening (vector #6) and an aberrant (superior vs. posterior) tongue base retraction (vector #10).
Discussion
We used computational analysis to examine swallowing mechanics and structural changes in postoperative OSA surgery patients. It is interesting that our 2 patients demonstrated similar changes in swallowing mechanics—namely the absence of pharyngeal shortening and the presence of aberrant movement of the tongue base in the superior vs. posterior direction compared with swallowing mechanics of nonsurgical patients. There were no significant differences in laryngeal elevation and hyoid excursion between our 2 patients and normal controls.
Relocation pharyngoplasty is a modified UPPP technique that includes tonsillectomy, removal of supratonsillar mucosa and adipose tissue, suturing of the superior pharyngeal constrictor muscle to the anterior tonsil pillar, and suturing of the posterior pillar to the anterior pillar.9 The palatopharyngeus muscle resides within the posterior pillar mucosa and aids in pharyngeal shortening.10
Our preliminary data suggest that relocation pharyngoplasty alters the function of the palatopharyngeus muscle by tethering it to the anterior tonsil pillar. In normal swallowing, the pharynx shortens, allowing the pharyngeal esophageal segment to engulf the bolus head. In these patients, the middle pharyngeal constrictor
126 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
ELLIS, PATE, dORRIS, PEARSON, BROWN
A B
Figure 3. Patient 2. A: Vectors in nonsurgical controls show normal swallowing mechanics. B: Vectors in patient 2 demonstrate an absent pharyngeal shortening (vector #6) and an aberrant (superior vs. posterior) tongue base retraction (#10). (OT = oral transport; P = pharyngeal swallowing; 2 = patient 2).
muscle appears to compensate by collapsing toward the tongue base to help drive the bolus through the pharyngeal esophageal segment.
Preoperatively, both patients demonstrated 4/4 retrolingual collapse on FNMM and were deemed candidates for transoral robotic surgery tongue base reduction. CASM results indicated a reduction in tongue base retraction, which is consistent with the surgery’s goal of stabilizing the hypopharyngeal airspace and relieving tongue base obstruction. Reduced tongue base retraction has been associated with incomplete bolus clearance in head and neck cancer patients, thus compromising airway safety during swallowing.11
The PAS score for both patients was 2, indicating that the bolus penetrated the larynx but was ejected without aspiration. Findings of a recent study suggested that reduced tongue base retraction impairs epiglottic inversion, which acts as an element of airway protection.4 Since our patients had reduced tongue base retraction, they likely had associated reduced epiglottic inversion and thus poorer protection of their airway and a greater likelihood of penetrating (PAS score of 2, penetration).
Li and Lee reported “no serious dysphagia,” including no nasal regurgitation, 3 months postoperatively in their initial cohort of 10 patients who underwent relocation pharyngoplasty.9 They did not report how dysphagia was measured or determined. By objective measures in our study, the procedure did not result in serious dysphagia; a PAS score of 2, while not normal, may be considered to be within functional limits. However, in view of patients’ complaints of postoperative swallowing difficulty, our concern is whether relocation pharyngoplasty impairs swallowing mechanics.
The current standard of care does not include a preoperative MBS swallowing study for OSA patients. In the absence of preoperative MBS swallowing studies, impaired swallowing mechanics before treatment cannot be ruled out. However, since dysphagia symptoms are newly onset and the altered mechanics of swallowing are consistent with the anatomic changes of the procedure, it is likely that the OSA surgery resulted in impaired swallowing mechanics. Future directions for research include preoperative and postoperative MBS imaging to determine if these alterations in swallowing mechanics are consistent with this procedure.
In conclusion, patient-specific CASM revealed impaired swallowing mechanics, including absent pharyngeal shortening and aberrant tongue base retraction, in 2 postsurgical OSA patients. These findings are consistent with the OSA surgical goal of enlarging and/or stabilizing the hypopharyngeal airway, and they may also have contributed to our patients’ dysphagia symptoms. Patient-specific CASM allows for sensitive identification of swallowing mechanical dysfunction that might otherwise be overlooked, and it may be utilized in future head and neck surgery patients to analyze swallowing dysfunction associated with treatment.
References
1. Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006-14.
2. Richard W, Venker J, den Herder C, et al. Acceptance and longterm compliance of nCPAP in obstructive sleep apnea. Eur Arch Otorhinolaryngol 2007;264(9):1081-6.
3. Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea—a systematic review. Sleep 2009;32(1):27-36.
4. Pearson WG Jr., Taylor BK, Blair J, Martin-Harris B. Computational analysis of swallowing mechanics underlying impaired epiglottic inversion. Laryngoscope 2016;126(8):1854-8.
5. Thompson TZ, Obeidin F, Davidoff AA, et al. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp 2014;(87).
6. Natarajan R, Stavness I, Pearson W Jr. Semi-automatic tracking of hyolaryngeal coordinates in videofluoroscopic swallowing studies. Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization 2017;5(6):379-89.
7. Klingenberg CP. MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources 2011; 11(2):353-7.
8. Tran TT, Martin-Harris B, Pearson WG Jr. Improvements resulting from respiratory-swallow phase training visualized in patient-specific computational analysis of swallowing mechanics. Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization 2016 March:1-7.
9. Li HY, Lee LA. Relocation pharyngoplasty for obstructive sleep apnea. Laryngoscope 2009;119(12):2472-7.
10. Choi DY, Bae JH, Youn KH, et al. Anatomical considerations of the longitudinal pharyngeal muscles in relation to their function on the internal surface of pharynx. Dysphagia 2014;29(6):722-30.
11. Pauloski BR, Logemann JA. Impact of tongue base and posterior pharyngeal wall biomechanics on pharyngeal clearance in irradiated postsurgical oral and oropharyngeal cancer patients. Head Neck 2000;22(2):120-31.
Volume 97, Number 4-5 www.entjournal.com 127 COMPUTATIONAL ANALySIS Of SWALLOWINg MECHANICS AfTER SURgERy fOR OBSTRUCTIvE SLEEP APNEA
Vocal tract symptoms: Severity and frequency in patients on statins
Abdul-Latif Hamdan, MD, MPH, FACS; Marc Mourad, MD; Ghina Fakhri, BS; Doja Sarieddine, BS; Elie Khalifee, MD; Sami T. Azar, MD
Abstract
The objective of the study was to analyze the frequency and severity of vocal tract symptoms in patients on statins. A total of 73 patients were enrolled in this study, 44 patients who were taking statins and 29 controls not taking statins. The severity and frequency of vocal tract discomfort was assessed using the Vocal Tract Discomfort scale. The most frequent vocal tract symptom in patients on statins was dryness followed by tightness and lump sensation. The difference in the mean of the total score and in the mean frequency of any vocal tract symptom was not significant between patients taking statins and controls. The most severe (highest mean values) vocal tract symptom in patients taking statins also was dryness followed by tightness and lump sensation. The difference in the mean of the total score and in the mean severity of any vocal tract symptom between patients taking statins and controls was not significant. This study failed to demonstrate a higher prevalence or severity of vocal tract symptoms in patients receiving statins. Despite the lack of a significant difference in the means of vocal tract discomfort symptom frequency and severity, this study carries clinical significance when considering that a higher prevalence and severity of vocal tract discomfort symptoms should alert physicians to the possible development of statin-induced myotoxicity in the laryngopharyngeal complex.
Introduction
The statin class of cholesterol-lowering medications has truly revolutionized preventive cardiology. Since they were first introduced in 1987, the use of statins has grown remarkably, reaching more than 100 million prescriptions per year.1 Statins, like other medica-
From the Department of Otolaryngology–Head and Neck Surgery (Dr. Hamdan, Dr. Mourad, Mrs. Sarieddine, and Dr. Khalifee), the Department of Medicine (Ms. Fakhri), and the Department of Internal Medicine (Dr. Azar), American University of Beirut Medical Center, Lebanon.
Corresponding author: Sami T. Azar, MD, Internal Medicine Department, American University of Beirut, PO Box 11-0236, Beirut, Lebanon. Email: sazar@aub.edu.lb
tions, have side effects, and myopathy seems to be the most common one.2 Myotoxicity usually occurs within the first month of treatment and is more common in women than in men and in patients older than 75 compared with their younger counterparts.3-6
The clinical spectrum of myopathy ranges from an asymptomatic increase in the concentration of creatine kinase to myalgia, myositis, and rhabdomyolysis. The incidence of myalgia is reported to be 1.5 to 3% in randomized, controlled trials and up to 10 to 13% in prospective clinical studies. 2,4,7,8 Patients taking statins may complain of muscle weakness, cramps, or nonspecific muscle aches. These side effects, although subclinical, must not be taken lightly as they may limit physical activity, affect quality of life, and reduce adherence to statin treatment, thus depriving patients of the clinical benefits.9,10
Given that the laryngopharyngeal complex is a musculoskeletal structure prone to the same statin-induced myotoxicity as the remaining musculoskeletal structures in the body, the purpose of this investigation is to analyze the frequency and severity of throat symptoms in patients on statins using a validated questionnaire, the Vocal Tract Discomfort scale. We hypothesize that the frequency and severity of throat symptoms are greater in patients receiving statins compared with subjects not taking statins.
Patients and methods
A total of 73 patients (44 statin recipients and 29 controls not taking statins) agreed to participate in this study after having read and signed the informed consent approved by the Institutional Review Board. Patients underwent fiberoptic or telescopic laryngeal examination before their enrollment in this study. Exclusion criteria included recent history of laryngeal manipulation, upper respiratory tract infection, and/ or abnormal laryngeal findings.
Demographic data included age, sex, Reflux Symptom Index (RSI) as established by Belafsky et al, and the dosage and duration of statin therapy.11 The severity
128 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018 ORIGINAL ARTICLE

and frequency of vocal tract discomfort symptoms were assessed subjectively using the Vocal Tract Discomfort scale first described by Woźnicka et al.12 The Vocal Tract Discomfort scale is a questionnaire that assesses both the severity and frequency of eight commonly encountered vocal tract symptoms (lump sensation, burning, itching, tightness, dryness, aching, soreness, and irritability) using a 0 to 6 Likert scale for each. The total score ranges from 0 to 48 for each of the severity and frequency subscales. Higher scores mean more severe and more frequent symptoms.12
Descriptive statistics were used to compute the means and the standard deviation of the continuous variables and the frequencies of the categorical variable. The Mann-Whitney U test was used to compare the means of the continuous variables between patients and controls. As for the categorical variables, the Pearson chi-square test was used to compute the p value, and the Fisher exact test was reported when more than 20% of the cells had a cell count <5. Statistical analysis was performed using the IBM Statistical Package for the Social Sciences software for Windows, v. 22.13
Results
Demographic data. The mean age of patients on statins was 54.68 ± 8.12 years, and the mean age of controls was 45.24 ± 13.69 years. The ratio
of men to women was 1:1. The duration of therapy ranged from 3 to 288 months (mean: 66). Mean scores on the RSI in patients taking statins and in controls were 6 and 7, respectively. Eight of 44 (18.2%) patients taking statins had an RSI score >13 compared with 7 of 29 (24.1%) controls.
Frequency of vocal tract discomfort symptoms. The most frequent vocal tract symptom in patients taking statins was dryness (mean of frequency: 0.59) followed by tightness (mean of frequency: 0.34) and lump sensation (mean of frequency: 0.32). The most frequent vocal tract symptom in the controls was dryness (mean frequency: 0.38) followed by burning (mean frequency: 0.34) and lump sensation (mean frequency: 0.31). The observed difference in the mean of the total score and the mean frequency of any vocal tract symptom between patients
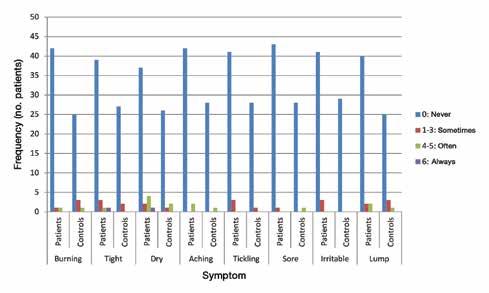
130 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
HAMdAN, MOURAd, fAkHRI, SARIEddINE, kHALIfEE, AzAR
Patients Controls p Value Mean Range Mean Range Burning 0.14 ± 0.668 0-4 0.34 ± 0.974 0-4 0.170 Tight 0.34 ± 1.140 0-6 0.17 ± 0.658 0-3 0.529 dry 0.59 ± 1.515 0-6 0.38 ± 1.208 0-5 0.503 Aching 0.23 ± 1.054 0-5 0.14 ± 0.743 0-4 0.793 Tickling 0.14 ± 0.554 0-3 0.07 ± 0.371 0-2 0.539 Sore 0.07 ± 0.452 0-3 0.14 ± 0.743 0-4 0.750 Irritable 0.14 ± 0.554 0-3 0.00 ± 0.00 0-0 0.154 Lump 0.32 ± 1.073 0-5 0.31 ± 0.891 0-4 0.596 Total 1.95 ± 4.979 0-26 1.55 ± 3.813 0-20 0.518
Table 1. Comparison of the means of vocal tract discomfort frequency of symptoms between statin recipients (n = 44) and controls (n = 29)
Figure 1. Bar chart compares the Vocal Tract Discomfort scale frequency per symptom between patients and controls.

taking statins and controls was not significant, with a p value ≥0.05 (table 1).
The distribution of vocal tract discomfort symptom frequencies as never, sometimes, often, and always in both groups is shown in figure 1.
Severity of vocal tract discomfort symptoms. The highest means for vocal tract symptom severity in patients taking statins were for dryness (mean severity: 0.64), tightness (mean severity: 0.35), and lump sensation (mean severity: 0.32). The highest means for vocal tract symptom severity in controls were for lump sensation (mean severity: 0.52), burning (mean severity: 0.48), and dryness (mean severity: 0.41). The difference in the mean of the total score and the mean severity of any vocal tract symptom between patients taking statins and controls was not significant (table 2).
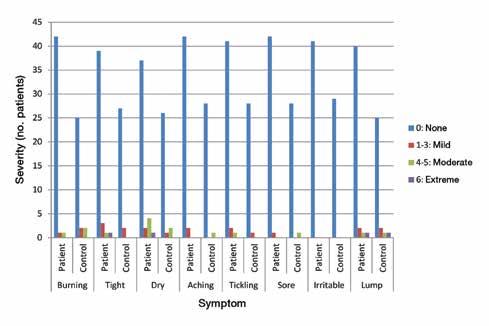
The distribution of vocal tract discomfort symptom severity as none, mild, moderate, and extreme in both groups is shown in figure 2.
Discussion
The mechanism of statin-induced myopathy is controversial. Several theories that revolve around the following known actions of statins exist: reduction of metabolic intermediates such as mevalonic acid, farnesol, geranylgeraniol, and ubiquinone; upregulation of
apoptotic pathways; modification of chloride channel conductance; alteration of muscle membrane function; impairment of Na+/K+-ATPase and Na+/Ca+-ATPase pump function; and reduction in selenoprotein production.13-15
Several studies have documented that the inhibition of protein prenylation is behind the muscle injury and results in the induction of pro-apoptotic pathways. In contrast, various reports of statin-induced polymyositis, dermatomyositis, and other autoimmune diseases are in favor of immunologic and toxic mechanisms.16 To that end, the role of statins as immune modulators is very controversial, with few reports describing statins as agents capable of inducing autoimmune disorders.17-19
132 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
HAMdAN, MOURAd, fAkHRI, SARIEddINE, kHALIfEE, AzAR
Patients Controls p Value Mean Range Mean Range Burning 0.16 ± 0.805 0-5 0.48 ± 1.299 0-5 0.163 Tight 0.36 ± 1.222 0-6 0.17 ± 0 .658 0-3 0.529 dry 0.64 ± 1.586 0-6 0.41 ± 1.268 0-5 0.497 Aching 0.11 ± 0.538 0-3 0.14 ± 0 .743 0-4 0.844 Tickling 0.23 ± 0.922 0-5 0.07 ± 0.371 0-2 0.506 Sore 0.07 ± 0.457 0-3 0.14 ± 0.743 0-4 0.762 Irritable 0.14 ± 0.554 0-3 0.00 ± .000 0-0 0.154 Lump 0.32 ± 1.137 0-6 0.52 ± 1.430 0-6 0.519 Total 2.02 ± 4.967 0-25 1.93 ± 3.954 0-20 0.420
Table 2. Comparison of the means of vocal tract discomfort severity of symptoms between stain recipients (n = 44) and controls (n = 29)
Figure 2. This bar chart compares the Vocal Tract Discomfort scale severity per symptom between patients and controls.
With the mechanism of myopathy still under investigation, the number of reports on the effect of statins on muscle strength and exercise tolerance is increasing.1 The results of our investigation failed to show a significant difference in the means of the frequency and severity of vocal tract discomfort symptoms between patients taking statins compared with those not taking statins. These findings can be attributed to two factors.
First, the association between statin intake and muscle pain and weakness is controversial despite the many reports stating that adult patients taking statins are significantly more likely than the general population to report musculoskeletal pain.20 Based on Buettner et al, patients taking statins have 50% greater odds of any musculoskeletal pain and 50 to 60% greater odds of back and lower extremity pain.20 These results corroborate the findings of several previous observational and postmarketing surveillance studies.4,21-23 Still, numerous randomized clinical trials showed no significant association between myalgia and statins.24,25
Similarly, findings regarding statin-induced muscle weakness are contradictory. Some studies showed a decline in muscle strength with statin treatment whereas others found no change or even an increase in strength.26-31 For instance, Loenneke and Loprinzi found that statin treatment indirectly decreases muscle force by decreasing the engagement in muscle strengthening activities due to the muscle pain induced by statin therapy.32 In contrast, Bruckert et al demonstrated that muscular symptoms are exacerbated by exercise and are more apparent in those who are physically active compared with those who are sedentary.4
Second, studies have shown that myopathy is related to the duration of therapy. Worth noting is that statin-induced myotoxicity is more likely to occur during the first 6 months of therapy, whereas the patients enrolled in this study were on therapy for an average of 66 months.33
Our study has two main limitations. First, it lacks objective measurements on a confounding disease, namely laryngopharyngeal reflux disease that may present with symptoms similar to those listed in the Vocal Tract Discomfort scale. Although the demographic data shed information on the frequency of laryngopharyngeal symptoms in both groups of patients, this investigation lacks objective measurements such as double-probe pH metry or pharyngeal impedance to either refute or confirm the presence of laryngopharyngeal reflux disease.
Another limitation is the duration of the investigation and the enrollment of patients who were on statins for an extended period. Nevertheless, this study is the first to investigate the prevalence of vocal tract discomfort symptoms in patients taking statins compared with a control group.
Despite the lack of a significant difference in the means of vocal tract discomfort symptom frequency and
severity between patients taking statins and controls, this study carries clinical significance if we consider that a higher prevalence and severity of vocal tract discomfort symptoms in patients taking statins should alert the treating physician to the possible development of statin-induced myopathy.
Based on the Prediction of Muscular Risk in Observational conditions study, among the several predictors for the development of statin-induced myotoxicity are hypothyroidism, history of muscle symptoms, and/or unexplained muscle cramps.4,5 The list has increased recently to include aging, female sex, body mass index, and intake of several medications and/or alcohol.
Conclusion
This study failed to demonstrate a higher prevalence or severity of vocal tract symptoms in patients taking statins despite the known statin-induced myotoxicity. Three possible reasons for the negative results are the small sample size, the late time of investigation that could have missed the early phase of toxicity, and the possible presence or lack of laryngopharyngeal reflux disease, an important confounding variable that should be excluded in the future using objective measures.
References
1. Sathasivam S, Lecky B. Statin induced myopathy. BMJ 2008;337: a2286.
2. Scott RS, Lintott CJ, Wilson MJ. Simvastatin and side effects. N Z Med J 1991;104(924):493–5.
3. Skilving I, Eriksson M, Rane A, Ovesjö ML. Statin-induced myopathy in a usual care setting—a prospective observational study of gender differences. Eur J Clin Pharmacol 2016;72(10): 1171-6.
4. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients —the PRIMO Study. Cardiovasc Drugs Ther 2005;19(6):403-14.
5. Pasternak RC, Smith SC Jr., Bairey-Merz CN, et al. ACC/AHA/ NHLBI clinical advisory on the use and safety of statins. Stroke 2002;33(9):2337-41.
6. Walsh JE, Pignone M. Drug treatment of hyperlipidemia in women. JAMA 2004;291(18):2243-52.
7. Law M, Rudnicka AR. Statin safety: A systematic review. Am J Cardiol 2006;97(8A):52C-60C.
8. Bays H. Statin safety: An overview and assessment of the data— 2005. Am J Cardiol 2006;97(8A):6C-26C.
9. Chapman MJ, Carrie A. Mechanisms of statin-induced myopathy: A role for the ubiquitin-proteasome pathway? Arterioscler Thromb Vasc Biol 2005;25(12):2441-4.
10. Ballantyne CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med 2003;163(5):553-64.
11. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice 2002;16(2):274-7.
12. Woźnicka E, Niebudek-Bogusz E, Kwiecień J, et al. Applicability of the vocal tract discomfort (VTD) scale in evaluating the effects of voice therapy of occupational voice disorders. Med Pr 2012;63(2):141-52.
Continued on page 136
Volume 97, Number 4-5 www.entjournal.com 133 vOCAL TRACT SyMPTOMS: SEvERITy ANd fREqUENCy IN PATIENTS ON STATINS
Undifferentiated sarcoma presenting as a slowly enlarging facial mass
Alexis Lopez, MD, MPH; Anton M. Kushnaryov, MD; Robert A. Weisman, MD
Abstract
Head and neck sarcomas are rare and consist of a variety of histologic subtypes. We present a case of undifferentiated/unclassified sarcoma (UUS) of the maxillary sinus, a tumor subtype historically known as malignant fibrous histiocytoma (MFH) or undifferentiated pleomorphic sarcoma (UPS). A 50-year-old female patient presented with worsening facial pain and dysphagia. Physical examination demonstrated a large, ulcerated mass protruding from the oral cavity. Computed tomography demonstrated a large, enhancing mass centered in the right maxillary sinus with local invasion. The initial biopsy was read as “central giant cell granuloma.” Conservative management yielded no improvement, and the tumor grew steadily. The patient underwent a total maxillectomy with resection of the orbital floor and an anterior ethmoidectomy, followed by radiation and chemotherapy. In addition to treatment of this patient, we discuss a review of the literature and the clinical presentation, radiologic, and histologic findings of this disease.
Introduction
Sinonasal tumors are uncommon and represent approximately 5% of head and neck neoplasms; more than 80% of these tumors are of epithelial origin, arising predominantly from squamous cells.1 Risk factors for these tumors include occupational exposure to toxins and wood dust, smoking tobacco, and infection with human papilloma virus (HPV) subtypes 16 and 18. A wide range of less common, nonepithelial tumor types can originate from the sinuses, including lymphomas, melanomas, neuroblastomas, and sarcomas.1
Head and neck sarcomas account for up to 2 to 15% of all head and neck malignancies and consist of a broad range of histologic subtypes, including those originating from adipocytes, smooth muscle, and nerve sheath.2 A small, yet significant subgroup of sarcomas remains classified as undifferentiated/unclassified sarcoma (UUS), also associated with the terms malignant fibrous histiocytoma (MFH) and undifferentiated pleomorphic sarcoma (UPS).3
Historically considered the most common type of soft-tissue sarcoma in the head and neck region, these tumors remain rare at 3 to 10% of all sarcoma cases. 2 Common sites are the craniofacial bones, larynx, and soft tissue of the neck. Within the sinonasal tract, the most common location is the maxillary sinus, followed by the ethmoid sinus and nasal cavity.4 Presentation is more common in men and in patients aged 50 to 60 years. 2 A risk factor for these tumors is a history of exposure to ionizing radiation. In a series of 25 patients with sinonasal MFH, 65% were diagnosed after radiation therapy to the head and neck. 5
Case report
From the Department of Surgery, Otolaryngology Division, University of New Mexico School of Medicine, Albuquerque (Dr. Lopez); and the Head and Neck Division, Department of Surgery, University of California–San Diego (Dr. Kushnaryov and Dr. Weisman). The case described in this article occurred at the University of California–San Diego.
Corresponding address: Alexis Lopez, MD, MPH, Department of Surgery, Division of Otolaryngology, UNM School of Medicine, MSC 10 5610, 1 University of New Mexico, Albuquerque, NM 87131.
Email: alexislf@gmail.com
A 50-year-old woman presented after several months of significant enlargement of a long-standing facial mass with worsening facial pain and dysphagia. She had no history of smoking, occupational exposure, or radiation therapy. She had undergone a prior endoscopic biopsy of a maxillary sinus mass, and initial pathology had demonstrated a giant cell-rich neoplasm most consistent with central giant cell granuloma. On physical examination, she had an immobile, 8-cm, firm, ulcerated, submucosal mass protruding from the oral cavity situated over the right maxillary alveolus.
Computed tomography (CT) demonstrated a large, enhancing mass centered in the right maxillary sinus measuring 5.6 × 6.7 × 10.8 cm (figure). The mass extended through the alveolus and ethmoid sinuses, with erosion of the walls of the maxillary sinus and the hard palate. Mass effect was noted on the inferior wall of the right orbit and nasopharynx, with proptosis.
134 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018 ORIGINAL ARTICLE
Treatment with corticosteroids resulted in a stable size of the mass for a few months. However, the mass subsequently continued to enlarge, and a repeat debulking was performed in the office and tissue was sent for pathologic examination. The final pathology demonstrated undifferentiated pleomorphic sarcoma.
The patient underwent a right total maxillectomy with anterior ethmoidectomy with preservation of the orbital contents. After complete gross tumor resection, the inferior orbital floor was reconstructed with a 3-D printed, customized orbital implant and temporalis myofascial flap. The patient underwent adjuvant radiation and chemotherapy and was disease-free at the 6-month follow-up.
Discussion
Classification of this tumor type has been debated since it was first described as MFH in the 1960s, characterized as histiocytes and fibroblasts arranged in a storiform or cartwheel-like pattern. In the early 1990s, improvements in immunohistochemistry and electron microscopy led to classification into five subtypes: storiform-pleomorphic, myxoid, giant cell, inflammatory, and angiomatoid.6 In 2002, the World Health Organization (WHO) adjusted this classification with more stringent criteria and replacement of MFH with UPS.6 The WHO classification was revisited in 2013 and includes a subset of radiation-associated sarcomas and undifferentiated tumors with round cell, spindle cell, epithelioid, and pleomorphic morphology.3 Developments in genetics have allowed further characterization of these tumor types with round cell morphology.3
Patients often present at advanced stages once tumor size is significant enough to cause obstruction, mass effect, or erosion of adjacent structures. Symptoms commonly include nasal obstruction, facial pain, rhinorrhea, and/or epistaxis. Advanced presentations may include proptosis, diplopia, and neurologic symptoms. Although reported to have primarily nonspecific findings, CT and magnetic resonance imaging (MRI) can provide valuable information regarding tumor size, extension, and composition. 2
On CT, UUS may appear as a large, lobulated soft-tissue mass that occasionally can demonstrate calcification or ossification. On MRI, UUS can appear isointense to muscle on T1-weighted images and heterogeneously hyperintense on T2-weighted images.4
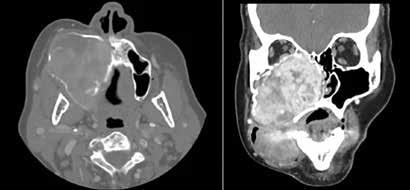
Reasonable attempts should be made to classify such tumors to guide therapy and to provide prognostic significance. Positivity for vimentin, α1-antichymotrypsin, and Ki-67, along with negativity for S-100 protein and cytokeratins have demonstrated diagnostic importance.2 Initial treatment is wide local excision which, in the paranasal sinuses, remains a challenge because of the proximity of numerous critical structures. Tumors are often high-grade and recur locally; treatment generally includes adjuvant chemotherapy and radiation therapy.7
Because of their rarity, few case series have focused solely on tumors arising from the maxillary sinus and have encompassed all of those found in the head and neck. In earlier case series most, if not all, cases were treated with wide excision as the primary form of treatment. 8-10 Radiotherapy was reserved for those with regional or distant metastases for palliation. Most of the tumors were classified as high-grade and metastatic, with 5-year survival varying from 50 to 74%. 8-10
Sabesan et al reviewed 54 patients with MFH, all of whom were treated with radical excision without radiation or chemotherapy.11 Of these, 78% were highgrade, and recurrence occurred in 57% of patients. The overall 5-year survival rate was 48%.
Wang et al reported 25 patients with sinonasal MFH tumors, 8 of which were considered primary and 17 of which were diagnosed after irradiation to the head and neck.5 All except 2 of the patients underwent excision, with one-third receiving adjuvant radiotherapy. A significant difference in 5-year, disease-free survival between those with primary MFH (72.9%) and those with post-irradiated MFH (0%) was found.
Salcedo-Hernández et al reported a case series of maxillary sinus sarcomas including 4 (20%) diagnosed as MFH.12 Among these, all were considered high-grade although none exhibited metastases. Two-thirds of these patients underwent excision, and of these, all but one underwent postoperative radiotherapy.12
Volume 97, Number 4-5 www.entjournal.com 135
UNdIffERENTIATEd SARCOMA PRESENTINg AS A SLOWLy ENLARgINg fACIAL MASS
A B
Figure. Axial (A) and coronal (B) views show the large, enhancing mass centered in the right maxillary sinus with local invasion and protrusion into the oral cavity.
In summary, sinonasal MFH/UPSs are a group of rare, locally aggressive sarcomas of the head and neck. Historically, these have been treated with primary excision. Radiation therapy is commonly implemented after surgery because of local spread. Distant metastases appear in one-third of cases and are more common to the lung, bone, and liver. The overall 5-year survival has been reported to be 58 to 77%.4 Prognosis and survival are worse for radiation-induced sarcomas, ranging from 10 to 30% for 5-year survival. 2
Despite improvements in treatment, survivors have a high incidence of morbidity related to complications after surgery and adjuvant therapy, and quality of life issues may make treatment decisions challenging.13
References
1. Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: Clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol 2014;11(8):460-72.
2. O’Neill JP, Bilsky MH, Kraus D. Head and neck sarcomas: Epidemiology, pathology, and management. Neurosurg Clin N Am 2013;24(1):67-78.
3. Fletcher CD. The evolving classification of soft tissue tumours— an update based on the new 2013 WHO classification. Histopathology 2014;64(1):2-11.
4. Park SW, Kim HJ, Lee JH, Ko YH. Malignant fibrous histiocytoma of the head and neck: CT and MR imaging findings. AJNR Am J Neuroradiol 2009;30(1):71-6.
5. Wang CP, Chang YL, Ting LL, et al. Malignant fibrous histiocytoma of the sinonasal tract. Head Neck 2009;31(1):85-93.
6. Matushansky I, Charytonowicz E, Mills J, et al. MFH classification: Differentiating undifferentiated pleomorphic sarcoma in the 21st Century. Expert Rev Anticancer Ther 2009;9(8):1135-44.
7. Clark DW, Moore BA, Patel SR, et al. Malignant fibrous histiocytoma of the head and neck region. Head Neck 2011;33(3):303-8.
8. Blitzer A, Lawson W, Zak FG, et al. Clinical-pathological determinants in prognosis of fibrous histiocytomas of head and neck. Laryngoscope 1981;91(12):2053-70.
9. Freedman AM, Reiman HM, Woods JE. Soft-tissue sarcomas of the head and neck. Am J Surg 1989;158(4):367-72.
10. Tran LM, Mark R, Meier R, et al. Sarcomas of the head and neck. Prognostic factors and treatment strategies. Cancer 1992; 70(1):169-77.
11. Sabesan T, Xuexi W, Yongfa Q, et al. Malignant fibrous histiocytoma: Outcome of tumours in the head and neck compared with those in the trunk and extremities. Br J Oral Maxillofac Surg 2006;44(3):209-12.
12. Salcedo-Hernández RA, Lino-Silva LS, Luna-Ortiz K. Maxillary sinus sarcomas: Epidemiological and clinicopathological experience of 25 Years in a National Reference Cancer Center. Indian J Otolaryngol Head Neck Surg 2014;66(4):359-64.
13. Robbins KT, Ferlito A, Silver CE, et al. Contemporary management of sinonasal cancer. Head Neck 2011;33(9):1352-65.
Continued from page 133
13. Owczarek J, Jasiñska M, Orszulak-Michalak D. Drug-induced myopathies. An overview of the possible mechanisms. Pharmacol Rep 2005;57(1):23-34.
14. Jamal SM, Eisenberg MJ, Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. Am Heart J 2004;147(6):956-65.
15. Moosmann B, Behl C. Selenoprotein synthesis and side-effects of statins. Lancet 2004;363(9412):892–4.
16. Noël B. Autoimmune disease and other potential side-effects of statins. Lancet 2004;363(9425):2000.
17. Ulivieri C, Baldari CT. Statins: From cholesterol-lowering drugs to novel immunomodulators for the treatment of Th17-mediated autoimmune diseases. Pharmacol Res 2014;88:41–52.
18. Kobashigawa JA. Statins and cardiac allograft vasculopathy after heart transplantation. Semin Vasc Med 2004;4(4):401–6.
19. Kwak B, Mulhaupt F, Veillard N, et al. The HMG-CoA reductase inhibitor simvastatin inhibits IFN-gamma induced MHC class II expression in human vascular endothelial cells. Swiss Med Wkly 2001;131(3-4):41–6.
20. Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008;23(8):1182–6.
21. de Sauvage Nolting PR, Buirma RJ, Hutten BA, et al. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the examination of probands and relatives in statin studies with familial hypercholesterolemia [ExPRESS fh]). Am J Cardiol 2002;90(2):181–4.
22. Franc S, Dejager S, Bruckert E, et al. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther 2003;17(5-6):459–65.
23. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: A systematic overview of randomized clinical trials. Circulation 2006;114(25):2788–97.
24. Jukema JW, Bruschke AV, van Boven AJ, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels: The Regression Growth Evaluation Statin Study (REGRESS). Circulation 1995;91(10):2528–40.
25. Kerzner B, Corbelli J, Sharp S, et al. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol 2003;91(4):418–24.
26. Dobkin BH. Underappreciated statin-induced myopathic weakness causes disability. Neurorehabil Neural Repair 2005;19(3): 259–63.
27. Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002;137(7):581-5.
28. Scott D, Blizzard L, Fell J, Jones G. Statin therapy, muscle function and falls risk in community-dwelling older adults. QJM 2009;102(9):625–33.
29. Ashfield TA, Syddall HE, Martin HJ, et al. Grip strength and cardiovascular drug use in older people: Findings from the Hertfordshire cohort study. Age Ageing 2010;39(2):185–91.
30. Traustadóttir T, Stock AA, Harman SM. High-dose statin use does not impair aerobic capacity or skeletal muscle function in older adults. Age (Dordr) 2008;30(4):283–91.
31. Agostini JV, Tinetti ME, Han L, et al. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc 2007;55(3):420–5.
32. Loenneke JP, Loprinzi PD. Statin use may reduce lower extremity peak force via reduced engagement in muscle-strengthening activities. Clin Physiol Funct Imaging 2018;38(1):151-4.
33. Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med 2005;165(22):2671-6.
136 www.entjournal.com ENT-Ear, Nose & Throat Journal April/May 2018
HAMdAN, MOURAd, fAkHRI, SARIEddINE, kHALIfEE, AzAR
LOPEz, kUSHNARyOv, WEISMAN









 Associate Professor and Vice Chair
Department of Otolaryngology–Head and Neck Surgery Associate Professor
Department of Neurosurgery
Drexel University College of Medicine
Associate Professor and Vice Chair
Department of Otolaryngology–Head and Neck Surgery Associate Professor
Department of Neurosurgery
Drexel University College of Medicine




 Alexander Delides, MD, PhD; Ioannis Plioutas, MD; Stephanos Konstantoudakis, MD; Pavlos Maragoudakis, MD, PhD
Alexander Delides, MD, PhD; Ioannis Plioutas, MD; Stephanos Konstantoudakis, MD; Pavlos Maragoudakis, MD, PhD

























