The head impulse test as a predictor of videonystagmography caloric test lateralization according to the level of examiner experience: A prospective open-label study
Risk of posterior semicircular canal trauma when using a retrosigmoid approach for acoustic neuroma surgery and role of endoscopy: An imaging study
Elastography as a potential modality for screening cervical lymph nodes in patients with papillary thyroid cancer: A review of literature
www.entjournal.com A Vendome Publication JANUARY/FEBRUARY 2018 • VOL. 97, NO. 1-2

EDITORIAL BOARD
EDITORIAL BOARD MEMBERS
Editor-in-Chief
Robert T. Sataloff, MD, DMA, FACS
Professor and Chairman, Department of Otolaryngology–Head and Neck Surgery, and Senior Associate Dean for Clinical Academic Specialties, Drexel University College of Medicine Philadelphia, PA
Jean Abitbol, MD
Jason L. Acevedo, MD, MAJ, MC, USA
Jack B. Anon, MD
Gregorio Babighian, MD
Peter C. Belafsky, MD, PhD
Bruce Benjamin, MD
Gerald S. Berke, MD
Michael J. Brenner, MD
Kenneth H. Brookler, MD
Karen H. Calhoun, MD
Steven B. Cannady, MD
Ricardo Carrau, MD
Swapna Chandran, MD
Chien Chen, MD
Dewey A. Christmas, MD
Nicolle T. Clements, MS
Daniel H. Coelho, MD, FACS
David M. Cognetti, MD
Maura Cosetti, MD
James V. Crawford, MD
David H. Darrow, MD, DDS
Rima Abraham DeFatta, MD
Robert J. DeFatta, MD, PhD
Hamilton Dixon, MD
Paul J. Donald, MD, FRCS
Mainak Dutta, MS, FACS
Russell A. Faust, PhD, MD
Ramón E. Figueroa, MD, FACR
Charles N. Ford, MD
Paul Frake, MD
Marvin P. Fried, MD
Richard R. Gacek, MD
Andrea Gallo, MD
Frank Gannon, MD
Emilio Garcia-Ibanez, MD
Soha Ghossani, MD
William P. R. Gibson, MD
David Goldenberg, MD
Jerome C. Goldstein, MD
Richard L. Goode, MD
Samuel Gubbels, MD
Reena Gupta, MD
Joseph Haddad Jr., MD
Missak Haigentz, MD
Christopher J. Hartnick, MD
Mary Hawkshaw, RN, BSN, CORLN
Garett D. Herzon, MD
Thomas Higgins, MD, MSPH
Jun Steve Hou, MD
John W. House, MD
Amanda Hu, MD, FRCSC
Glenn Isaacson, MD
Steven F. Isenberg, MD
Stephanie A. Joe, MD
Shruti S. Joglekar, MBBS
Raleigh O. Jones, Jr., MD
Petros D. Karkos, MD, AFRCS, PhD, MPhil
David Kennedy, MD
Seungwon Kim, MD
Robert Koenigsberg, DO
Karen M. Kost, MD, FRCSC
Jamie A. Koufman, MD
Stilianos E. Kountakis, MD, PhD
John Krouse, MD
Ronald B. Kuppersmith, MD, MBA, FACS
Rande H. Lazar, MD
Robert S. Lebovics, MD, FACS
Keat-Jin Lee, MD
Donald A. Leopold, MD
Steve K. Lewis, BSc, MBBS, MRCS
Daqing Li, MD
Robert R. Lorenz, MD
John M. Luckhurst, MS, CCC-A
Valerie Lund, FRCS
Karen Lyons, MD
A.A.S. Rifat Mannan, MD
Richard Mattes, PhD
Brian McGovern, ScD
William A. McIntosh, MD
Brian J. McKinnon, MD
Oleg A. Melnikov, MD
Albert L. Merati, MD, FACS
Joseph P. Mirante, MD, MBA, FACS
Ron B. Mitchell, MD
Steven Ross Mobley, MD
Jaime Eaglin Moore, MD
Thomas Murry, PhD
Ashli K. O’Rourke, MD
Ryan F. Osborne, MD, FACS
J. David Osguthorpe, MD
Robert H. Ossoff, DMD, MD
Enrique Palacios, MD, FACR
Michael M. Paparella, MD
Kourosh Parham, MD, PhD
Arthur S. Patchefsky, MD
Meghan Pavlick, AuD
Spencer C. Payne, MD
Kevin D. Pereira, MD, MS (ORL)
Nicolay Popnikolov, MD, PhD
Didier Portmann, MD
Gregory N. Postma, MD
Matthew J. Provenzano, MD
Hassan H. Ramadan, MD, FACS
Richard T. Ramsden, FRCS
Gabor Repassy, MD, PhD
Dale H. Rice, MD
Ernesto Ried, MD
Alessandra Rinaldo, MD, FRSM
Joshua D. Rosenberg, MD
Allan Maier Rubin, MD, PhD, FACS
John S. Rubin, MD, FACS, FRCS
Amy L. Rutt, DO
Anthony Sclafani, MD, FACS
Raja R. Seethala, MD
Jamie Segel, MD
Moncef Sellami, MD
Michael Setzen, MD, FACS, FAAP
Stanley Shapshay, MD
Douglas M. Sidle, MD
Herbert Silverstein, MD
Jeffrey P. Simons, MD
Raj Sindwani, MD, FACS, FRCS
Aristides Sismanis, MD, FACS
William H. Slattery III, MD
Libby Smith, DO
Jessica Somerville, MD
Thomas C. Spalla, MD
Matthew Spector, MD
Paul M. Spring, MD
Brendan C. Stack, Jr., MD, FACS
James A. Stankiewicz, MD
Jun-Ichi Suzuki, MD
David Thompson, MD
Lester D.R. Thompson, MD, FASCP
Helga Toriello, PhD, FACMG
Ozlem E. Tulunay-Ugur, MD
Galdino Valvassori, MD
Emre Vural, MD
Donald T. Weed, MD, FACS
Neil Weir, FRCS
Kenneth R. Whittemore, MD
David F. Wilson, MD
Ian M. Windmill, PhD
Ian J. Witterick, MD,MSc, FRCSC
Richard J. Wong, MD
Naoaki Yanagihara, MD
Eiji Yanagisawa, MD, FACS
Ken Yanagisawa, MD, FACS
Anthony Yonkers, MD
Mark Zacharek, MD
Joseph Zenga, MD
Liang Zhou, MD
CLINIC EDITORS
Dysphagia
Jamie A. Koufman, MD
Peter C. Belafsky, MD, PhD
Gregory N. Postma, MD
Facial Plastic Surgery
Anthony P. Sclafani, MD, FACS
Geriatric Otolaryngology
Kourosh Parham, MD, PhD, FACS
Karen M. Kost, MD, FRCSC
Head and Neck
Ryan F. Osborne, MD, FACS
Paul J. Donald, MD, FRCS
Reena Gupta, MD
Imaging
Enrique Palacios, MD, FACR
Ramón E. Figueroa, MD, FACR
Laryngoscopic
Robert T. Sataloff, MD, DMA, FACS
Otoscopic
John W. House, MD
Brian J. McKinnon, MD
Pathology
Lester D.R. Thompson, MD, FASCP
Pediatric Otolaryngology
Rande H. Lazar, MD
Rhinoscopic
Eiji Yanagisawa, MD, FACS
Dewey A. Christmas, MD
Joseph P. Mirante, MD, MBA, FACS
Ken Yanagisawa, MD, FACS
Special Topics
Robert T. Sataloff, MD, DMA, FACS
Thyroid and Parathyroid
David Goldenberg, MD
2 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
Editor-in-Chief Robert T. Sataloff, MD, DMA, FACS 219 N. Broad St., 10th Fl., Philadelphia, PA 19107 entjournal@phillyent.com Ph: 215-732-6100

Managing Editor Linda Zinn
Manuscript Editors Martin Stevenson and Wayne Kuznar
Associate Editor, Reader Engagement Megan Combs
Creative Director Eric Collander
National Sales Manager Mark C. Horn mhorn@vendomegrp.com Ph: 480-895-3663
Traffic Manager Judi Zeng
Please send IOs to adtraffic@vendomegrp.com
All ad materials should be sent electronically to: https://vendome.sendmyad.com
Customer Service/Subscriptions
www.entjournal.com/subscribe Ph: 888-244-5310 email: VendomeHM@emailpsa.com
Reuse Permissions Copyright Clearance Center info@copyright.com Ph: 978-750-8400 Fax: 978-646-8600
Chief Executive Officer Jane Butler
Chief Marketing Officer Dan Melore
Vice President, Finance Bill Newberry
Vice President, Custom Media Jennifer Turney Director, Circulation Rachel Beneventi
ENT-Ear, Nose & Throat Journal (ISSN: Print 0145-5613, Online 1942-7522) is published 9 times per year in Jan/Feb, Mar, Apr/May, June, July, Aug, Sept, Oct/ Nov and Dec, by Vendome Group, LLC, 237 West 35th Street, 16th Floor, New York, NY 10001-1905.
©2018 by Vendome Group, LLC. All rights reserved. No part of ENT-Ear, Nose & Throat Journal may be reproduced, distributed, transmitted, displayed, published, or broadcast in any form or in any media without prior written permission of the publisher. To request permission to reuse this content in any form, including distribution in education, professional, or promotional contexts or to reproduce material in new works, please contact the Copyright Clearance Center at info@ copyright.com or 978.750.8400.
EDITORIAL: The opinions expressed in the editorial and advertising material in this issue of ENT-Ear, Nose & Throat Journal are those of the authors and advertisers and do not necessarily reflect the opinions or recommendations of the publisher, editors, or the staff of Vendome Group, LLC. ENT-Ear, Nose & Throat Journal is indexed in MEDLINE/PubMed and Current Contents/Clinical Medicine and Science Citation Index Expanded. Editorial offices are located at 812 Huron Rd., Suite 450, Cleveland, OH 44115. Manuscripts should be submitted online at www.editorialmanager.com/entjournal. Instructions for Authors are available at www.entjournal.com
SUBSCRIPTIONS: For questions about a subscription or to subscribe, please contact us by phone: 888-244-5310; or email: VendomeHM@emailpsa.com Individual subscriptions, U.S. and possessions: 1 year $225, 2 years $394; International: 1 year $279, 2 years $488; Single copies $28; outside the U.S., $40.
POSTMASTER: send address changes to Ear, Nose & Throat Journal, PO Box 11404 Newark, NJ 07101-4014.
4 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
ADVERTISER INDEX Pages Acclarent, Inc. 7 American Rhinologic Society .................... 5 Arbor Pharmaceuticals ...................... 33, 34 CANT Corporation 9 Carestream CVR 2 Eosera, Inc......................................... CVR 3 Fyzical Therapy and Balance .................... 1 InHealth Technologies 37 Lannett Company .............................. 17, 18 McKeon Products, Inc. ...................... CVR 4 Optim LLC 3 Optinose 25, 27 Spectrum Audiology ................................ 15 SurgiTel .................................................... 21 Xlear, Inc. 11

ORIGINAL ARTICLES
16 The head impulse test as a predictor of videonystagmography caloric test lateralization according to the level of examiner experience: A prospective open-label study
Ashraf Awadie, MD; Yehuda Holdstein, MD; Margalit Kaminer, MSc; Avi Shupak, MD
24 Risk of posterior semicircular canal trauma when using a retrosigmoid approach for acoustic neuroma surgery and role of endoscopy: An imaging study
Ali Kouhi, MD; Varasteh Vakili Zarch, MD; Ali Pouyan, MD†
31 Elastography as a potential modality for screening cervical lymph nodes in patients with papillary thyroid cancer: A review of literature
Robert Saadi, MD; Salvatore LaRusso, MEd; Kanupriya Vijay, MD; David Goldenberg, MD
ONLINE EXCLUSIVES
E1 The effects of mometasone furoate and strontium chloride in a rat model of allergic rhinitis
Emine Elif Altunta , MD; Ömer Tamer Do an, MD; Bülent Saraç, MD; Nergiz Hacer Turgut, PhD; Kasım Durmu , MD; Melih Akyol, MD
E8 A South African first: Congenital absence of the cartilaginous nasal septum
Yahya Atiya, MBBCh, MMed (ORL), FCORL
E12 Buccal space malignancy in the setting of chronic sialadenitis: A report of 2 cases
Lucas M. Bryant, MD; Patrick Tassone, MD; Joseph Curry, MD; Adam Luginbuhl, MD; David Cognetti, MD
E15 Inferior turbinate reduction: Diode laser or conventional partial turbinectomy?
Venkatesh Doreyawar, MS; Raveendra P. Gadag, MS; Dandi Narasaiah Manjunath, MS; Shivalingappa B. Javali, MPhil, PhD; Nagaraj Maradi, MBBS; Deekshit Shetty, MBBS
E20 Contact allergy to benzalkonium chloride in patients using a steroid nasal spray: A report of 3 cases
Jérôme René Lechien, MD, PhD, MS; Pedro Costa de Araujo, MD; Lisa G. De Marrez, BSc; Jean-Luc Halloy, MD; Mohamad Khalife, MD; Sven Saussez, MD, PhD
E23 Syncope caused by a pleomorphic adenoma: Case report and literature review
Anas Minkara, BHS; Reena Dhanda-Patil MD, MBA; Yash Patil, MD, FACS
E27 Microbial flora and antibiotic resistance in odontogenic abscesses in Upstate New York
Ann W. Plum, MD; Anthony J. Mortelliti, MD; Ronald E. Walsh, NP
E32 The sensitivity and specificity of touch preparation for rapid diagnosis of invasive fungal sinusitis: A pilot study
Theodore A. Schuman, MD; Josephine H. Nguyen, MD; Joshua C. Yelverton, MD; Jorge A. Almenara, PhD; Celeste N. Powers, MD, PhD
E37 A case of squamous cell carcinoma of the nasal cavity in a patient with granulomatosis with polyangiitis (Wegener granulomatosis)
Edward C. Kuan, MD, MBA; Kevin A. Peng, MD; Lyndon O. Gonzalez, MD; Joel A. Sercarz, MD
E42 Use of the microdebrider in the surgical management of rhinophyma
Winsion Chow, MD, MSc; Goran Jeremic, MD, FRCSC; Leigh Sowerby, MD, FRCSC
E46 Granuloma formation secondary to silicone injection for soft-tissue augmentation in facial cosmetics: Mechanisms and literature review
Leo L. Wang, MS; William W. Thomas, MD; Oren Friedman, MD
6 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018 EDITORIAL OFFICE Robert T. Sataloff, MD, DMA, FACS, Editor-in-Chief • 219 N. Broad St., 10th Fl. • Philadelphia, PA 19107 CONTENTS JANUARY/FEBRUARY 2018 • VOL. 97, NO. 1-2
DEPARTMENTS 4 Advertiser Index 8 ENT Journal Online 10 Editorial 13 Rhinoscopic Clinic 14 Pediatric Otolaryngology Clinic E52 Facial Plastic Surgery Clinic E54 Imaging Clinic E56 Laryngoscopic Clinic E57 Otoscopic Clinic E59 Rhinoscopic Clinic

JOURNAL ONLINE
Ear, Nose & Throat Journal's website is easy to navigate and provides readers with more editorial content each month than ever before. Access to everything on the site is free of charge to physicians and allied ENT professionals. To take advantage of all our site has to offer, go to www.entjournal. com and click on the "Registration" link. Once you have filled out the brief registration form, you will have full access. Explore and enjoy!
ONLINE EXCLUSIVES
The effects of mometasone furoate and strontium chloride in a rat model of allergic rhinitis
Emine Elif Altuntaş, MD; Ömer Tamer Doğan, MD; Bülent Saraç, MD; Nergiz Hacer Turgut, PhD; Kasım Durmuş, MD; Melih Akyol, MD
Neurogenic inflammation plays a role in the pathophysiology of allergic rhinitis. Highly effective in reducing the sensory irritation caused by some substances, strontium salts directly affect C-type nerve fibers. The aim of this study was to compare the efficacy of mometasone furoate and strontium chloride on early-phase symptoms in a rat model of allergic....
A South African first: Congenital absence of the cartilaginous nasal septum
Yahya Atiya, MBBCh, MMed (ORL), FCORL
Congenital absence of the cartilaginous nasal septum has been reported just once in the literature. We present a case of a young child, diagnosed by exclusion, with complete agenesis of the cartilaginous septum. We believe it is only the second case worldwide, and the first in South Africa, to be reported.
Buccal space malignancy in the setting of chronic sialadenitis: A report of 2 cases
Lucas M. Bryant, MD; Patrick Tassone, MD; Joseph Curry, MD; Adam Luginbuhl, MD; David Cognetti, MD
We report the cases of an 89-year-old woman and a 77-yearold woman with long-standing chronic sialadenitis (60 and 10 years, respectively) who were referred to our large academic medical center for sialendoscopy. Both patients presented with symptoms of acute parotid duct obstruction in the background of long-standing parotid duct disease. Imaging demonstrated ductal dilation and periductal....
Inferior turbinate reduction: Diode laser or conventional partial turbinectomy?
Venkatesh Doreyawar, MS; Raveendra P. Gadag, MS; Dandi Narasaiah Manjunath, MS;
Shivalingappa B. Javali, MPhil, PhD; Nagaraj Maradi, MBBS; Deekshit Shetty, MBBS
Hypertrophy of the inferior nasal turbinate is one of the most common causes of nasal obstruction. The diode laser has proven to be as effective as other lasers for this indication. Our objective was to study various outcomes associated with the use of the diode laser, such as improvements in nasal obstruction and postoperative pain, reduction in intraoperative....
Contact allergy to benzalkonium chloride in patients using a steroid nasal spray: A report of 3 cases
Jérôme René Lechien, MD, PhD, MS; Pedro Costa de Araujo, MD; Lisa G. De Marrez, BSc; Jean-Luc Halloy, MD; Mohamad Khalife, MD; Sven Saussez, MD, PhD
Benzalkonium chloride (BAC) is a bactericidal preservative excipient commonly found in steroid nasal sprays used to treat allergic rhinitis and nasal polyposis. In rare cases, BAC can be responsible for type I and type IV hypersensitivity reactions that can manifest as rhinorrhea, which a clinician....
Syncope caused by a pleomorphic adenoma: Case report and literature review
Anas Minkara, BHS; Reena Dhanda-Patil MD, MBA; Yash Patil, MD, FACS
Pleomorphic adenomas are considered the most common salivary gland tumors, although they rarely occur in the parapharyngeal space. To the best of our knowledge, this is the first case report of a parapharyngeal parotid pleomorphic adenoma causing syncope. A 57-year-old man was admitted for left-sided blurred vision, left-sided weakness, dysarthria, lightheadedness, and syncope. Upon his admission....
Microbial flora and antibiotic resistance in odontogenic abscesses in Upstate New York Ann W. Plum, MD; Anthony J. Mortelliti, MD; Ronald E. Walsh, NP
Abscesses in the head and neck frequently have odontogenic sources. As bacterial pathogens and antibiotic resistance patterns may change over time and based on location, we describe the current common bacteria found in odontogenic abscesses, the prevalence of antibiotic resistance, and differences in each between pediatric and adult patients in Upstate New York. This is a retrospective review of patients who....
The sensitivity and specificity of touch preparation for rapid diagnosis of invasive fungal sinusitis: A pilot study
Theodore A. Schuman, MD; Josephine H. Nguyen, MD; Joshua C. Yelverton, MD; Jorge A. Almenara, PhD; Celeste N. Powers, MD, PhD
Invasive fungal sinusitis is a morbid pathology that typically affects immunocompromised patients and may quickly progress to fulminant disease. The purpose of this study was to measure the sensitivity and specificity of touch preparation of nasal debridement specimens as a rapid diagnostic....
8 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018 www.entjournal.com
A case of squamous cell carcinoma of the nasal cavity in a patient with granulomatosis with polyangiitis (Wegener granulomatosis)
Edward C. Kuan, MD, MBA; Kevin A. Peng, MD; Lyndon O. Gonzalez, MD; Joel A. Sercarz, MD
We report a rare case of squamous cell carcinoma (SCC) of the nasal cavity arising in a patient with granulomatosis....
Use of the microdebrider in the surgical management of rhinophyma
Winsion Chow, MD, MSc; Goran Jeremic, MD, FRCSC; Leigh Sowerby, MD, FRCSC
Rhinophyma is a disfiguring end-stage manifestation of acne rosacea. It is characterized by a painless hyperplasia of the sebaceous glands and connective tissues of the nose....
Granuloma formation secondary to silicone injection for soft-tissue augmentation in facial cosmetics: Mechanisms and literature review
Leo L. Wang, MS; William W. Thomas, MD; Oren Friedman, MD
The use of injectable fillers is increasingly popular as an alternative to surgery for facial cosmetic applications. In this....
ONLINE DEPARTMENTS
Facial Plastic Surgery Clinic: Silicone granuloma formation associated with dermal injection
Leo L. Wang, MS; William W. Thomas, MD; Cherie M. Ditre, MD; Oren Friedman, MD
Imaging Clinic: Temporal bone anomalies associated with unbalanced 9;13 chromosome translocation depicted on CT and MRI
Daniel Thomas Ginat, MD, MS
Laryngoscopic Clinic: Transnasal vocal fold augmentation
Michael J. Knabel, BS; Jonathan M. Bock, MD, FACS
Otoscopic Clinic: Distant metastasis to the external auditory canal
Chelsea A. Troiano, MD; Bharat B. Yarlagadda, MD
Rhinoscopic Clinic: Endoscopic finding of two osteomas in the frontal sinus
Jong Seung Kim, MD; Sam Hyun Kwon, MD, PhD
Volume 97, Number 1-2 www.entjournal.com 9 ENT JOURNAL ONLINE
EDITORIAL
Open access: Is there a predator at the door?
Dear Readers:
If your inbox looks like ours, you are barraged daily with requests to send research to a new journal or to join a new editorial board. Many of these “invitations” are from new open access journals, not all of which are legitimate. Open access journals play an increasingly important role in today’s world of medical publication and provide information that would otherwise be difficult or impossible for some to access. Openly sharing peer-reviewed information at no cost to the reader can greatly enhance distribution of legitimate scientific and clinical data. However, there is also an increasing number of journals purporting to serve this mission but acting in a predatory fashion. Here are a few guidelines. Hallmarks of legitimate journals include:
• A well-known editorial board of recognized experts in the field;
• An International Standard Serial Number (ISSN);
• Listing in the Directory of Open Access Journals at https://doaj.org;
• Publisher membership in the Open Access Scholarly Publishers Association;
• Affiliation with recognized societies;
• The journal website provides complete contact information; and
• All publication fees are clearly listed and are not submission fees.
was performed to show the low bar for acceptance of papers that were meaningless, or as they put it, “to maximize amusement.”
In 2014, Van Noorden pointed out in Nature News that several publishers were removing more than 120 papers from their subscription services after it was discovered that “the works were computer-generated nonsense.”3
More subtle forms of non-gibberish, but also non–peer-reviewed publications, seem to be expanding rapidly. Rather than motivation of the publisher to promote the science and practice of medicine, the impetus is clearly financial. They charge large sums of money to publish articles unable to pass a rigorous editorial or peer-review process, which can be quite lucrative. Likewise, authors who might have been rejected by legitimate peer-reviewed journals may find that some open access journals offer an avenue for publication without editorial oversight. Therefore, we suggest the following guidelines when evaluating whether a journal is predatory.
Be cautious if:
• Invitations to submit research or to join editorial boards are overly flattering;
• There is a guarantee of rapid publication;
• The journals’ titles are very similar to those of legitimate journals, but are not established journals.
• The journal website has no address or contact information;
• The mission of the publisher and/or the journal is described in vague terms;
What defines a
predatory journal?
Moher and Moher recently summed up the characteristics neatly by suggesting that such publications can, perhaps, be characterized by their behavior: aggressive recruitment emails, unrealistic promises regarding publication, and ultimately worthless peer review.1
A number of published articles suggest the lack of an editorial review process as a key characteristic of predatory journal publications. One article described predatory journal publications as “gobbledegook.”2 Massachusetts Institute of Technology (MIT) researchers in 2005 invented software called SCIgen, which randomly combined strings of words to produce fake computer-generated science papers that were ultimately published in open access journals. This exercise
• There is no mention of peer-review or basic submission requirements;
• Manuscripts are submitted by email rather than through the publisher’s online manuscript peerreview system.
• There is a requirement to submit a minimum number of articles per year, and there is no clear statement that your open access publication fee will be waived.
We recommend an excellent recent editorial by Roberts that shares our opinion, entitled “Predatory journals: Think before you submit.”4
Our goal is to provide high-quality, rigorously peerreviewed papers and scientific information of value to you and all our readers.
10 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018

EDITORIAL
References
1. Moher D, Moher E. Stop predatory publishers now: Act collaboratively. Ann Intern Med 2016;165(11):826-7
2. Ball P. Computer conference welcomes gobbledegook paper. Nature 2005; 434(7036):946.
3. Van Noorden R. Publishers withdraw more than 120 gibberish papers. Nature News 2014; Feb 24. https://www.nature.com/news/ publishers-withdraw-more-than-120-gibberish-papers-1.14763/ Accessed December 11, 2017.
4. Roberts J. Predatory journals: Think before you submit. Headache 2016;56(4):618-21.
Rakesh Chandra, MD
Co-Editor-in-Chief
American Journal of Rhinology & Allergy
Edward W. Fisher, MA, DM (Oxon), FRCS
Senior Editor
Journal of Laryngology and Otology
Terry M. Jones, MBBS, FRCS(ORL-HNS), MD
Editor-in-Chief
Clinical Otolaryngology
David W. Kennedy, MD
Editor-in-Chief
International Forum of Allergy & Rhinology
Dennis H. Kraus, MD, FACS
Co-Editor-in-Chief
Journal of Neurological Surgery, Part B
John H. Krouse, MD, PhD, MBA
Editor-in-Chief
Otolaryngology–Head and Neck Surgery
Editor-in-Chief
OTO-Open
Michael Link, MD
Co-Editor-in-Chief
Journal of Neurological Surgery. Part B
Lawrence R. Lustig, MD
Editor-in-Chief
Otology & Neurotology
Bert W. O’Malley, Jr., MD
Editor-in-Chief
Journal for Oto-Rhino-Laryngology, Head and Neck Surgery
Jay F. Piccirillo, MD, FACS
Editor-in-Chief
JAMA Otolaryngology–Head & Neck Surgery
Robert Ruben, MD, FAAP, FACS Editor-in-Chief
International Journal of Pediatric Otorhinolaryngology
Robert T. Sataloff, MD, DMA, FACS
Editor-in-Chief
Ear, Nose and Throat Journal
Editor-in-Chief
Journal of Voice
Sandra Schwartz, MS RN CORLN ORL–Head and Neck Nursing
Raj Sindwani, MD
Co-Editor-in-Chief
American Journal of Rhinology & Allergy
Richard J. Smith, MD
Editor-in-Chief
Annals of Otology, Rhinology & Laryngology
Michael G. Stewart, MD, MPH, FACS Editor-in-Chief
The Laryngoscope
Peter C. Weber, MD Editor-in-Chief
American Journal of Otolaryngology
D. Bradley Welling, MD, PhD, FACS Editor-in-Chief
Laryngoscope Investigative Otolaryngology
Robin Youngs, MB, BS, MD, FRCS
Senior Editor
Journal of Laryngology and Otology
12 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
RHINOSCOPIC CLINIC
Endoscopic view of a mucocele obstructing a middle meatal antrostomy
A 38-year old woman presented with a 6-year history of chronic sinus problems. She had undergone bilateral endoscopic anterior ethmoidectomy and bilateral middle meatal maxillary antrostomies 4 years previously. Since she was having recurring sinus symptoms, her primary care physician referred her for evaluation.
Rhinoscopic evaluation revealed a large mucocele in the left middle meatus (figure, A). Computed tomography (CT) of the sinuses revealed a large soft-tissue mass obstructing the left middle meatal antrostomy and extensive mucosal thickening in the right maxillary sinus (figure, B).
The patient elected to undergo revision endoscopic sinus surgery. At the time of surgery, the mucocele obstructing the left middle meatus was identified (figure, C). A large left uncinate remnant was identified (figure, D) and was removed. The left mucocele was removed with a microdebrider (figure, E and F). The right max-
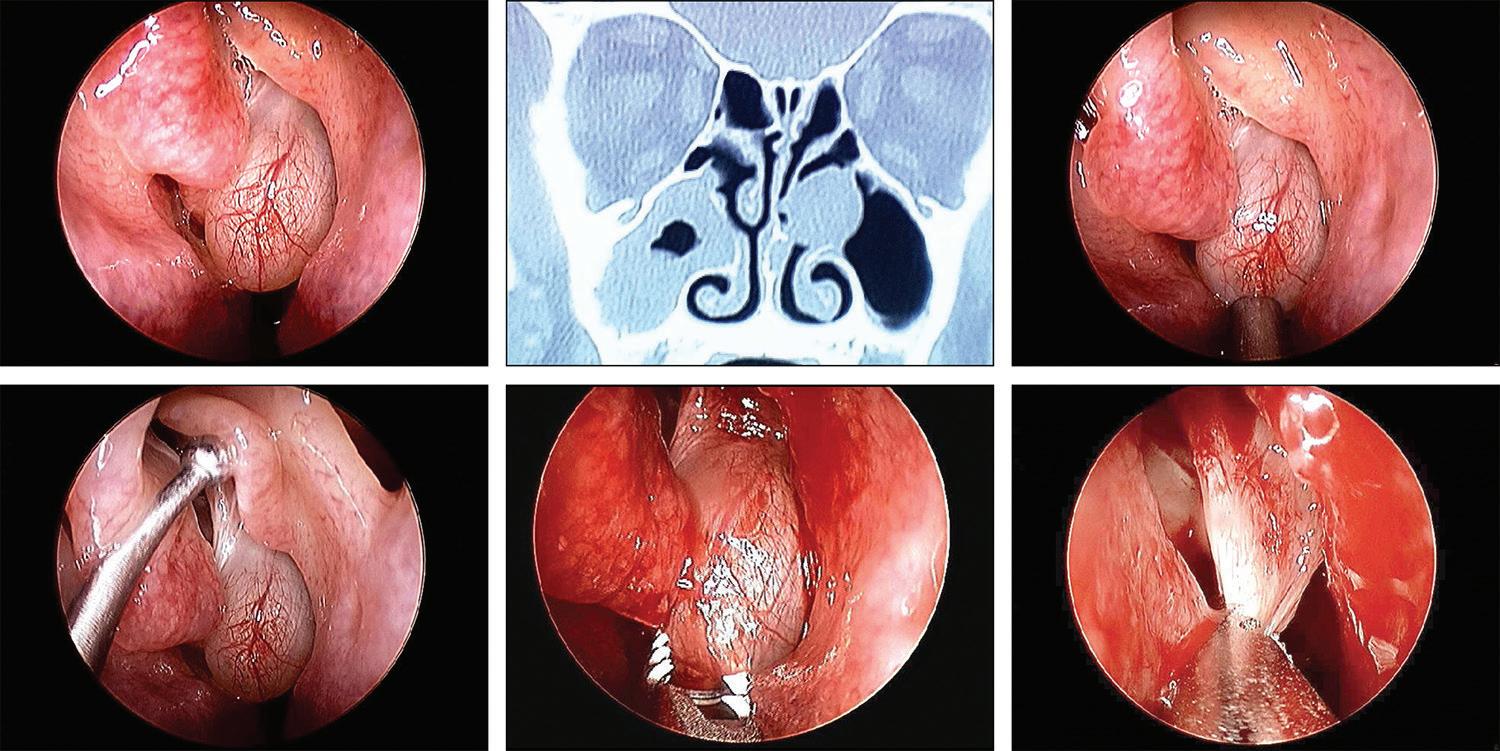
illary sinus was also debrided with the microdebrider. The patient had an uneventful postoperative course. Mucoceles are potentially obstructing and expansile lesions of the sinuses. These can occur naturally as mucus retention cysts or after sinus surgery, by ostial obstruction and formation of a lesion lined by low columnar epithelium.1 Mucoceles can be treated surgically by external or intranasal decompression.2
Acknowledgment
The authors thank Grayson Bertaina for his assistance in preparing this article.
References
1. Stankiewicz JA, Newell DJ, Park AH. Complications of inflammatory diseases of the sinuses. Otolaryngol Clin North Am 1993; 26(4):643-55.
2. Kennedy DW, Senior BA. Endoscopic sinus surgery. A review. Otolaryngol Clin North Am 1997;30(3):313-30.
Volume 97, Number 1-2 www.entjournal.com 13
From the Section of Otolaryngology, Yale New Haven Hospital–St. Raphael Campus and the Yale University School of Medicine, New Haven, Ct. (Dr. Yanagisawa); the Department of Otolaryngology, the Halifax Medical Center, Daytona Beach, Fla. (Dr. Christmas and Dr. Mirante); and Florida State University School of Medicine, Daytona Beach (Dr. Mirante).
Eiji Yanagisawa, MD, FACS; Dewey A. Christmas, MD; Joseph P. Mirante, MD, MBA, FACS
A B C D E F
Figure. A: Endoscopic view reveals the left middle meatal mucocele. B: CT scan shows the left middle meatal mucocele. C: The large obstructing mucocele is seen in this intraoperative view. D: The probe is retracting the large left uncinate remnant. E and F: The mucocele is removed with a microdebrider from inferiorly to its attachment superiorly.
PEDIATRIC OTOLARYNGOLOGY CLINIC
Langerhans cell histiocytosis presenting as a scalp mass in a child
Omar Hariri, BBA; Seckin Ulualp, MD; Ron B. Mitchell, MD
Langerhans cell histiocytosis (LCH) results from an abnormal accumulation of immature Langerhans cells, causing tumor formation or organ damage. Many parts of the body can be affected, including bone, bone marrow, skin, lymph nodes, liver, lung, spleen, and brain. The course of LCH is variable and can range from a self-limited, asymptomatic, localized lesion to a disseminated multisystem disease with a less favorable prognosis.
A 15-month-old girl presented with a left parietal scalp swelling that had been noted over 3 or 4 days and was increasing in size. There was no history of trauma, fever, or upper respiratory infection. The patient’s birth and medical history were unremarkable. Physical examination of the head and neck showed multiple swellings of the temporoparietal region. These were boggy to touch and nontender, with no overlying skin erythema. The rest of the head and neck and chest examination was unremarkable.
A radiographic survey found multiple lytic lesions involving the left superior orbital rim, left anterior fourth rib, and phalanges of the left hand. The largest lesion, in the left parietal bone, measured 3 cm in its greatest diameter, while the remainder of the lesions measured less than 1 cm.
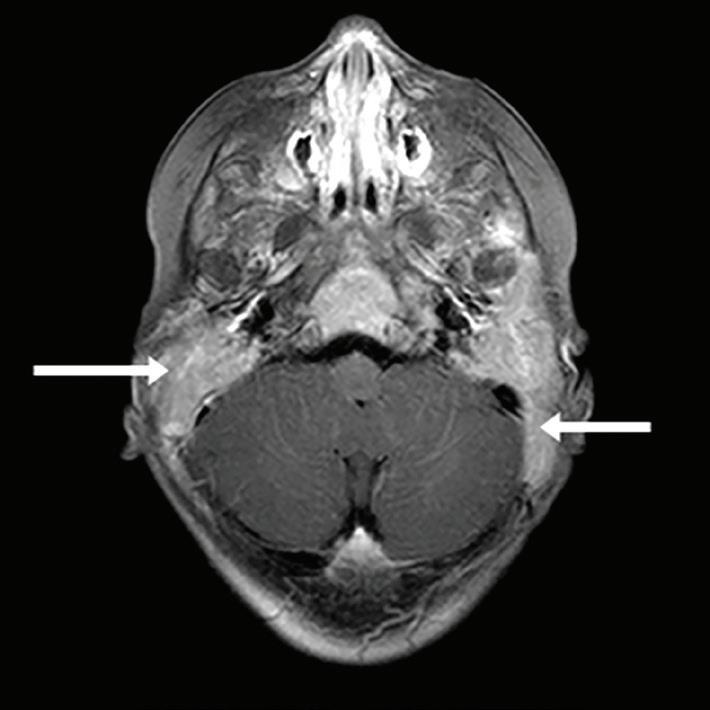
Computed tomography (CT) of the head showed extensive facial, skull base, and calvarial lytic lesions with bony destruction and soft-tissue masses consistent with LCH. There was extensive bilateral lytic destruction involving the temporal bones, with almost complete destructive changes of the mastoid portion and extension up to the external auditory canal (figure 1). There were also bilateral lesions of the petrous temporal bones.
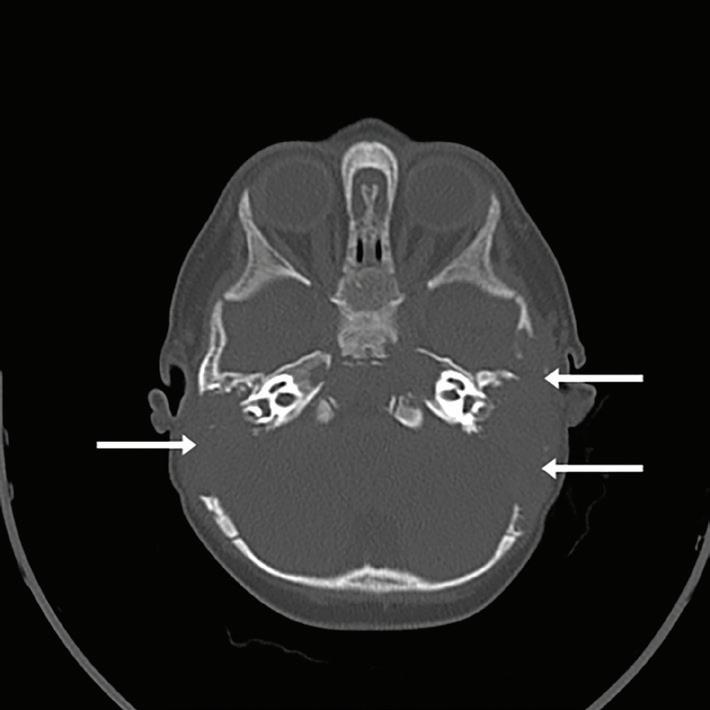
Magnetic resonance imaging (MRI) of the brain showed extensive marrow involvement of the skull base, with the mastoid air cells and middle ear cavities completely replaced by LCH (figure 2). There were also multiple intensely enhancing calvarial lesions. Fine-needle aspiration biopsy of the left parietal mass confirmed the diagnosis of LCH. Blood tests showed a microcytic anemia.
Chemotherapy was started per LCH III protocol, consisting of vinblastine, prednisone, and 6-mercaptopurine for 12 months. A port was placed into the left subclavian vein; this was followed by hoarseness and a left vocal fold paralysis confirmed on endoscopy. MRI at 12 weeks showed improved, but persistent, disease. The child has been healthy and well-appear-
From the Department of Otolaryngology–Head and Neck Surgery, University of Texas Southwestern Medical Center, Dallas.
14 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
Figure 1. CT scan shows extensive bilateral lytic destruction involving the temporal bone with almost complete destruction of the mastoid portion.
Figure 2. MRI shows mastoid air cells and middle ear cavities completely replaced by LCH.
ing; her hoarseness had improved without recovery of movement of the left vocal fold. She continues on the chemotherapy regimen.
LCH most often affects children between the ages of 1 and 3 years but can occur at any age. Its clinical presentation and course are highly variable and can range from a single benign lesion to life-threatening disseminated disease affecting multiple organs. Head and neck involvement occurs in 80% of patients.1 The most common presentation involving the temporal bone is a mass (70%), followed by otitis media and externa (60%).2 Chronic otitis media, recurrent otitis externa, stenosis or polyps in the external auditory canal, mastoiditis, and cholesteatoma can be caused by or misdiagnosed for underlying LCH of the temporal bone. The inner ear is less often affected, as the bony labyrinth is typically resistant to erosion by granulation tissue.3
Imaging modalities such as plain radiography, CT, MRI, positron emission tomography (PET), and bone scan are used to identify, stage, and monitor LCH. CT is useful in delineating bony destruction, whereas MRI is useful in demonstrating soft-tissue and intracranial extension. Therefore, CT and MRI were both used in this child. Bone scan or full skeletal survey is performed to stage LCH. PET has a potential to identify extraskeletal LCH and to evaluate response to treatment.
The natural history of LCH is variable. The factors affecting prognosis are age at presentation (worse in children under 2 years), multisystem involvement, and vital organ dysfunction.4 With high-risk multisystem disease, as in the case reported, a chemotherapy regimen of vinblastine, prednisone, and 6-mercaptopurine for 12 months per LCH III protocol is indicated. This case highlights the need to routinely examine the scalp of a child as part of a head and neck examination. The otolaryngologist should have a high index of suspicion when a child presents with scalp swellings as they may be due to extensive lytic destruction of the temporal bones, even without specific ear symptoms.
References

1. Buchmann L, Emami A, Wei JL. Primary head and neck Langerhans cell histiocytosis in children. Otolaryngol Head Neck Surg 2006;135(2):312-17.
2. Saliba I, Sidani K, El Fata F, et al. Langerhans’ cell histiocytosis of the temporal bone in children. Int J Pediatr Otorhinolaryngol 2008;72(6):775-86.
3. Nanduri VR, Pritchard J, Chong WK, et al. Labyrinthine involvement in Langerhans’ cell histiocytosis. Int J Pediatr Otorhinolaryngol 1998;46(1-2):109—15.
4. Martini A, Aimoni C, Trevisani M, Marangoni P. Langerhans’ cell histiocytosis: Report of a case with temporal localization. Int J Pediatr Otorhinolaryngol 2000;55(1):51—6.
Volume 97, Number 1-2 www.entjournal.com 15 PEDIATRIc OTOLARyNgOLOgy cLINIc
The head impulse test as a predictor of videonystagmography caloric test lateralization according to the level of examiner experience: A prospective open-label study
Ashraf Awadie, MD; Yehuda Holdstein, MD; Margalit Kaminer, MSc; Avi Shupak, MD
Abstract
We conducted a study to compare how well the head impulse test (HIT), without and with eye-movement recordings, would predict videonystagmographic (VNG) caloric test lateralization when performed by a resident and an experienced otoneurologist. This prospective, open-label, blinded study was conducted in an ambulatory tertiary care referral center. Our study population was made up of 60 patients—29 men and 31 women, aged 20 to 82 years (mean: 56.4 ± 11.4)—with peripheral vestibulopathy who underwent HIT and VNG caloric testing. The HIT was conducted in two protocols: HIT0 and HIT1. The HIT0 was performed with passive brisk movements of the patient’s head from the 0° null position to 20° sideways, and the HIT1 was performed toward the center while the null position was a 20° head rotation to the right and to the left. Each protocol was carried out without video eye-movement recordings (HIT0 and HIT1) and with such recordings (rHIT0 and rHIT1). The primary outcome measures were (1) a comparison of the HIT’s sensitivity and specificity when performed by the resident and by the experienced otoneurologist and (2) the ability of video-recorded HIT to predict VNG caloric test lateralization. The sensitivity
From the Otoneurology Unit, Lin Medical Center, Haifa, Israel (all authors); and the Department of Otolaryngology–Head and Neck Surgery, Carmel Medical Center, Haifa, and the Bruce Rappaport Faculty of Medicine, The Technion, Haifa (Dr. Shupak). The study described in this article was conducted at the Lin Medical Center.
Corresponding author: Avi Shupak, MD, Otoneurology Unit, Lin Medical Center, Rotschild Ave. 35, Haifa 3515209, Israel. Email: shupak@012.net.il
Previous presentation: The information in this article has been edited for publication and updated from its original (partial) presentation at the Annual Scientific Meeting of the Israeli Otoneurology Society; Jan. 21-23, 2016; Tiberias, Israel.
and specificity obtained by the resident were 41 and 81%, respectively, for HIT0 and 41 and 90% for HIT1. The sensitivity and specificity obtained by the experienced otoneurologist were 18 and 89% for HIT0 and 32 and 85% for HIT1. Analysis of the recorded eye-movement clips of the HIT0 and HIT1 obtained by a second experienced otoneurologist found a sensitivity and specificity of 32 and 63% for rHIT0 and 33 and 82% for rHIT1. We conclude that the HIT yields high false-negative rates in predicting significant caloric lateralization. Analysis of the eye-movement recordings was no better than normal testing alone for detecting saccades. The experience of the examining physician had no impact on test performance characteristics.
Introduction
The primary laboratory tests used to evaluate vestibular function are electronystagmography (ENG) and videonystagmography (VNG). With ENG, changes in corneoretinal electrical potentials during eye movements are recorded; with VNG, goggle-mounted infrared cameras are used to monitor eye position.1 Standard ENG/VNG testing includes the alternate binaural bithermal caloric test (ABBT) originally described by Fitzgerald and Hallpike.2
The ABBT is of value for detecting impaired horizontal semicircular canal function, which indicates the presence of vestibulopathy on the side of the diminished response. Also, it contributes to the diagnosis of bilateral vestibular loss when all responses to cold and warm stimuli are reduced, and it helps differentiate peripheral from central vestibular involvement by measuring the fixation-induced inhibition of the caloric response.3
16 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018 ORIGINAL ARTICLE


However, the ABBT is the most time-consuming part of the ENG/VNG test battery, and it is often a significant inconvenience for patients due to the repeated administration of the cold- and warm-water ear irrigations needed to elicit significant vestibular stimuli.
Screening for vestibular function with shorter tests, such as monothermal caloric testing, has been previously suggested. However, the monothermal test is associated with a false-negative rate of 10%, which could be lowered only by adopting liberal criteria for determining the differences between the two ears.4-7 In addition, monothermal testing is associated with reported specificities of only 22 to 71%, which disqualifies its routine use in the clinical realm.4-7
The horizontal head impulse test (HIT) was developed by Halmagyi and Curthoys as a measure of vestibular semicircular canal function.8 In healthy patients, brisk, passive rotations of the head in the horizontal plane are followed by eye movements in the opposite direction, the amplitude of which is equal to that of the head rotation. Thus, the gaze is stabilized in space. When the vestibulo-ocular reflex (VOR) fails, head movements to the side of the lesion would initiate catch-up saccades to refixate the target on the retina.
The two types of catch-up saccades are early and late:
• Early saccades occur on top of the residual VOR response. With the head still moving, these covert saccades are most likely imperceptible to the clinical observer.9,10
• Late saccades follow head movement. Depending on their amplitude, these overt saccades may be detected by the clinical observer.9,10
The HIT requires only a short time to complete, and it causes only minimal, if any, discomfort. It might prove to be superior to the ABBT if it can yield a high enough sensitivity and specificity for the diagnosis of vestibular impairment. In patients who have a complete unilateral vestibular loss secondary to vestibular neurectomy or labyrinthectomy, the reported sensitivity and specificity of the HIT both reached the area of 100% when the ENG/VNG caloric test results were used as a reference.8,11 However, a partially functioning VOR was associated with a HIT sensitivity of only 34 to 45%, although the specificity remained high at 91 to 100%.12-14 The low sensitivity might have stemmed from a failure to detect the corrective saccades during the HIT. Eye-movement recordings might allow for repeat evaluations and possibly better identification of overt saccades. Also, the accuracy of test interpretations might be dependent on the examiner’s level of training.
In this article, we describe our study to compare the extent to which unrecorded and recorded horizontal HITs performed and interpreted by a resident and an experienced otoneurologist would predict VNG caloric test lateralization results.
Patients and methods
For this prospective, open-label, blinded study, we recruited 78 patients who had been referred to us by their physicians for VNG testing. All patients provided a detailed history and underwent an otoneurologic examination that included otoscopy, testing for spontaneous and gaze-evoked nystagmus with and without Frenzel goggles, the presence of skew-deviation and post-head-shaking nystagmus, the HIT, and the positional, Dix-Hallpike, Romberg, and tandem walking tests.
Our exclusion criteria were (1) age younger than 18 years, (2) the presence of external or middle ear pathology that did not allow for safe and accurate VNG water caloric testing, (3) limitations of neck movements that precluded the complete performance of the HIT and VNG, (4) abnormal ocular motor function, and (5) a diagnosis of central vestibulopathy.
A complete VNG test battery (ICS Chartr 200 VNG System and ICS NCI 480 Water Caloric Stimulator; Otometrics; Taastrup, Denmark) was carried out. This evaluation included tests for oculomotor systems integrity (saccadic, gaze, optokinetic, and pursuit systems); tests for spontaneous, positional, and positioning nystagmus (Dix-Hallpike maneuver); and an ABBT. 3 The Jongkees formula was employed for the quantitative evaluation of the caloric test results.15 Unilateral weakness was defined as a caloric lateralization of more than 25%.
The attending physician reached a diagnosis after reviewing the results of otoneurologic testing, VNG, and additional laboratory tests when indicated (mostly audiometry, computed tomography, and magnetic resonance imaging).
We excluded 18 patients who were diagnosed with central vestibulopathy. This left us with 60 patients—29 men and 31 women, aged 20 to 82 years (mean: 56.4 ± 11.4)—who met our eligibility criteria.
HIT. We performed two versions of the HIT:
• The HIT0 was performed with passive brisk movements of the patient’s head from the 0° null position to 20° sideways.9,10,13,16-18
• HIT1 was performed toward the center while the null position was a 20° head rotation to the right and to the left.8,12
Volume 97, Number 1-2 www.entjournal.com 19 THE HEAD IMPULSE TEST AS A PREDICTOR OF VIDEONYSTAGMOGRAPHY CALORIC TEST LATERALIZATION ACCORDING TO THE LEVEL OF EXAMINER EXPERIENCE: A PROSPECTIVE OPEN-LABEL STUDY
Each of the HIT protocols was conducted without (HIT0 and HIT1) and with eye-movement recordings (rHIT0 and rHIT1).
Two physicians who were blinded to the results of the otoneurologic evaluations separately conducted and interpreted the HIT results. One physician was a postgraduate year 4 resident in otolaryngology–head and neck surgery who was spending a 3-month rotation in our otoneurology unit; he was designated the resident. The second physician was an experienced otolaryngologist who was specializing in otoneurology; he was designated specialist 1. The eye-movement recordings were later evaluated for the presence of corrective saccades by a second experienced otoneurologist designated specialist 2, who was blinded to each patient’s diagnosis and previous HIT results.
The HIT began with the examiner sitting in front of the patient, who was instructed to keep his or her eyes open and fixed on the examiner’s nose. The examiner then performed the test and recorded observations about the presence or absence of corrective saccades. When the patient’s eyes remained fixed on the examiner’s nose, the results were normal. Failure of gaze fixation followed by overt refixation saccades in the opposite direction of the brisk head movement was interpreted as pathologic.16,19
HIT with eye-movement recordings. Recordings of eye movements in response to the same HIT0 and HIT1 protocols were obtained with a 25-Hz infrared camera adapted from our laboratory VNG equipment. Patients were instructed to fixate on a laser-projected dot in dim light while the examiner performed rapid, unpredictable head thrusts. The eye movement recordings were later evaluated for the presence of corrective saccades by specialist 2.
Ethical considerations. The study protocol and procedures were approved by the Committee on Human Experiments of the Meir Medical Center in Kfar Saba, Israel. The study was registered on the ClinicalTrials.gov website (study ID: NCT01426932). All patients signed an informed consent form that explained the purpose of the research and the patient’s role in it.
Results
Videonystagmography. VNG revealed that 22 patients (37%) had significant caloric test lateralization;
15 of them were diagnosed with right-sided canal paresis and 7 with left-sided paresis. The remaining 38 participants (63%) had normal VNG caloric responses.
HIT. A total of 120 HIT examinations were performed on the right and left sides for both the HIT0 and HIT1 protocols (table).
Examination by the resident. According to the resident’s assessments, the sensitivity of the HIT0 as a predictor of significant caloric test lateralization was 41% and the specificity was 81%. For the HIT1 protocol, the sensitivity was also 41%, while the specificity was 90%.
Examination by specialist 1. For the HIT0, specialist 1 found a sensitivity of 18% and specificity 89%. For the HIT1 protocol, the corresponding figures were 32 and 85%.
rHIT. Again, 120 eye-movement recordings were captured for both the rHIT0 and rHIT1 protocols. However, 11 rHIT0 and 11 rHIT1 recordings were excluded from the evaluation due to eye blink, which precluded test interpretation.
Evaluation by specialist 2. Specialist 2’s evaluation of rHIT0 yielded a sensitivity of 32% and a specificity of 63%. For the rHIT1 protocol, the sensitivity was 33% and the specificity was 82% (table).
Discussion
The sensitivities of the HIT0 and HIT1 in predicting significant VNG caloric test lateralization were 18 and 32%, respectively, as determined by specialist 1 and 41% for both protocols as determined by the resident. Such low rates preclude its use as a screening tool for possible vestibular deficits.
Key: CI = confidence interval; HIT0 = head impulse test performed with a 20° head sideways impulse from the 0° null position; HIT1 = test performed with the head impulse directed toward the center with the null position a 20° head rotation to the right and to the left.; rHIT0 = HIT0 with video eye movement recordings; rHIT1 = HIT1 with video eye movement recordings.
20 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
AwADIE, HOLDsTEIN, KAmINER, sHUPAK
Examiner Sensitivity (%) 95%CI (%) Specificity (%) 95%CI (%) Resident HIT0 41 20.1 to 63.6 81 71.3 to 87.9 HIT1 41 20.7 to 63.6 90 82.0 to 94.9 specialist 1 HIT0 18 5.3 to 40.3 89 80.8 to 99.3 HIT1 32 13.9 to 54.8 85 76.0 to 91.1 specialist 2 rHIT0 32 13.9 to 54.8 63 52.2 to 73.3 rHIT1 33 14.6 to 56.9 82 72.1 to 89.2
Table. Sensitivity and specificity of HIT protocols obtained by each examiner
The high HIT0 and HIT1 specificity values of 89 and 85%, respectively, obtained by specialist 1 and 81 and 90% as obtained by the resident suggest that when a positive HIT result is consistent with a patient’s clinical picture, the caloric test might be redundant for the diagnosis of canal paresis. In these patients, the ENG/ VNG test battery could be significantly shortened and patients could avoid caloric-test–induced discomfort.
The discordance between the low sensitivity and the high specificity of the HIT with respect to the ABBT standard might be explained by the different spectra of vestibular function that are measured by the two tests, as well as by the dynamics of VOR recovery after a peripheral vestibular insult. The ABBT water caloric stimuli are equivalent to those produced by sinusoidal head rotations in the frequency range of 0.002 to 0.004 Hz.16,18 However, in everyday life, the head oscillates at a frequency range of 0.5 to 8.0 Hz, and the neural firing of the regular vestibular afferents matches these frequencies. 20
The HIT is performed in velocities and accelerations that correspond to movement frequencies of 2.0 to 3.0 Hz, and thus it represents the stimuli frequencies to which the vestibular system naturally responds better than does the ENG/VNG water caloric stimulation.8,11
In an animal experiment, Angelaki et al reported that after they had plugged the horizontal semicircular canals of rhesus monkeys, recovery of VOR gain and phase in response to yaw rotation was frequency-dependent; recovery was greatest at the highest frequency tested and it progressively diminished at the lower frequencies.21 Also, the residual deficits of the VOR in response to low-frequency stimuli but not to high-frequency stimuli have been previously reported in various peripheral vestibulopathies.22-25 These findings might be explained by the observation that the repair of peripheral vestibular function is highly context-specific.20 For this reason, adaptation of the semicircular canals’ VOR response to the naturally encountered high-frequency stimuli is expected, but the nonphysiologic low-frequency stimulation introduced by the water caloric test remains inadequate.
Restoration of the VOR high-frequency response as described here probably requires at least some partially remaining function of the semicircular canals. Restoration would not take place after complete vestibular ablation where long-term pathologic HIT findings are the rule.8,11
In our study, we found that the post-test evaluation of the recorded eye movements yielded no advantage over the immediate observer interpretation of the HIT.
MacDougall and Curthoys described the importance of identifying covert saccades in making the diagnosis of VOR dysfunction and the dynamics of vestibular compensation.9 In a separate report, MacDougall et al described the successful detection of covert saccades in both the horizontal and vertical planes with a 250-Hz high-speed video camera; their findings were validated by parallel scleral search coil recording.19 This technology was recently implanted in commercial video head impulse test (vHIT) systems that also quantify the VOR gain.10
The 25-Hz infrared camera we used could not support the registration of the short-latency covert saccades that were masked by the VOR-induced eye movements. Weber et al reported that large covert catch-up saccades account for amplitude reduction of the later-appearing overt saccades.10 Thus, the possible presence of covert saccades might have further contributed to the low sensitivity we found for both the HIT and rHIT.
Bartolomeo et al compared the diagnostic value of vHIT (VHIT Ulmer; Synapsys; Marseille, France) with that of caloric testing using the receiver-operating characteristic curve.17 They found a sensitivity of 100% for canal paresis greater than 62.5% and a specificity of 100% for caloric lateralization less than 40%. However, for 30% lateralization, the sensitivity was only 69%.
McCaslin et al reported that a caloric asymmetry of 39.5% was required to optimize discrimination between abnormal and normal vHIT (EyeSeeCam; EyeSeeTec; Munich) results. In their study, optimal prediction of this caloric asymmetry by the vHIT results was defined as a sensitivity of 81% and a specificity of 82%.18 Yet, additional research by Blödow et al with the same recording system found that vHIT had a sensitivity of 55% when using caloric testing results as a reference.26 These results indicate that the sensitivity of the HIT is unsatisfactory, even when using sophisticated and expensive high-tech equipment to capture and evaluate eye movements.
In our study, the sensitivity and specificity of the HIT1 and rHIT1 protocols were mostly better than those of the HIT0 and rHIT0 protocols.
It has been previously suggested that the off-center null position as used in the HIT1 and rHIT1 might allow patients to predict head-thrust direction toward the center, resulting in the production of preprogrammed eye movements that compensate for a failing VOR.27-29 These concerns were not confirmed in our study, as the off-center null position did not affect our HIT and rHIT results.
22 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
AwADIE, HOLDsTEIN, KAmINER, sHUPAK
In our study, the sensitivity scores of the HIT in both protocols when performed by the resident were higher than those obtained by specialist 1. Our findings corroborate those of a study by Jorns-Häderli et al, who found that specialists in otoneurology or neurology with at least 6 months of experience in performing the HIT obtained lower sensitivity scores than did nonexperts in the field.16 In that study, the HIT was performed from a null position of 0° (comparable to that of our HIT0 protocol). Greater specificity was obtained by the trained examiners, a finding similar to ours with the HIT0 protocol.
Even though the point of reference in our study was the ABBT and that of the study by Jorns-Häderli et al was scleral search coil registered eye movements, the results were similar in terms of the examiner’s level of training. This might be explained by the greater confidence level that experienced otoneurologists might require in interpreting a HIT as positive, as well as their tendency to interpret a borderline outcome as negative.
In conclusion, our study found that the HIT was associated with high false-negative rates in terms of predicting significant caloric lateralization. Analysis of eye-movement recordings was no better than the HIT in detecting saccades. Finally, the examining physician’s level of experience had no impact on the test performance characteristics.
References
1. Wuyts FL, Furman J, Vanspauwen R, Van de Heyning P. Vestibular function testing. Curr Opin Neurol 2007;20(1):19-24.
2. Fitzgerald G, Hallpike CS. Studies in human vestibular function. I. Observations on the directional preponderance (“nystagmusbereitschaft”) of caloric nystagmus resulting from cerebral lesions. Brain 1942;65(2):115-37.
3. Barber HO, Stockwell CW. Manual of Electronystagmography. 2nd ed. St Louis: Mosby; 1980.
4. Shupak A, Kaminer M, Gilbey P, Tal D. Monothermal caloric testing in the screening of vestibular function. Aviat Space Environ Med 2010;81(4):369-74.
5. Enticott JC, Dowell RC, O’Leary SJ. A comparison of the monothermal and bithermal caloric tests. J Vestib Res 2003;13(23):113-19.
6. Lightfoot G, Barker F, Belcher K, et al. The derivation of optimum criteria for use in the monothermal caloric screening test. Ear Hear 2009;30(1):54-62.
7. Norré ME. Screening methods for caloric testing. Clin Otolaryngol Allied Sci 1987;12(3):161-6.
8. Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol 1988;45(7):737-9.
9. MacDougall HG, Curthoys IS. Plasticity during vestibular compensation: The role of saccades. Front Neurol 2012;3:21.
10. Weber KP, Aw ST, Todd MJ, et al. Head impulse test in unilateral vestibular loss: Vestibulo-ocular reflex and catch-up saccades. Neurology 2008;70(6):454-63.
11. Halmagyi GM, Curthoys IS, Cremer PD, et al. The human horizontal vestibulo-ocular reflex in response to high-acceleration stimulation before and after unilateral vestibular neurectomy. Exp Brain Res 1990;81(3):479-90.
12. Beynon GJ, Jani P, Baguley DM. A clinical evaluation of head impulse testing. Clin Otolaryngol Allied Sci 1998;23(2):117-22.
13. Harvey SA, Wood DJ, Feroah TR. Relationship of the head impulse test and head-shake nystagmus in reference to caloric testing. Am J Otol 1997;18(2):207-13.
14. Perez N, Rama-Lopez J. Head impulse and caloric tests in patients with dizziness. Otol Neurotol 2003;24(6):913-17.
15. Jongkees LB. Value of the caloric test of the labyrinth. Arch Otolaryngol 1948;48(4):402-17.
16. Jorns-Häderli M, Straumann D, Palla A. Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J Neurol Neurosurg Psychiatry 2007;78(10):1113-18.
17. Bartolomeo M, Biboulet R, Pierre G, et al. Value of the video head impulse test in assessing vestibular deficits following vestibular neuritis. Eur Arch Otorhinolaryngol 2014;271(4):681-8.
18. McCaslin DL, Jacobson GP, Bennett ML, et al. Predictive properties of the video head impulse test: Measures of caloric symmetry and self-report dizziness handicap. Ear Hear 2014; 35(5):e185-91.
19. MacDougall HG, Weber KP, McGarvie LA, et al. The video head impulse test: Diagnostic accuracy in peripheral vestibulopathy. Neurology 2009;73(14):1134-41.
20. Hain TC. Neurophysiology of vestibular rehabilitation. NeuroRehabilitation 2011;29(2):127-41.
21. Angelaki DE, Hess BJ, Arai Y, Suzuki J. Adaptation of primate vestibuloocular reflex to altered peripheral vestibular inputs. I. Frequency-specific recovery of horizontal VOR after inactivation of the lateral semicircular canals. J Neurophysiol 1996;76(5):2941-53.
22. Brantberg K, Magnusson M. The dynamics of the vestibule-ocular reflex in patients with vestibular neuritis. Am J Otolaryngol 1990;11(5):345-51.
23. McGarvie LA, Curthoys IS, MacDougall HG, Halmagyi GM. What does the dissociation between the results of video head impulse versus caloric testing reveal about the vestibular dysfunction in Ménière’s disease? Acta Otolaryngol 2015;135(9):859-65.
24. Park HJ, Migliaccio AA, Della Santina CC, et al. Search-coil headthrust and caloric tests in Ménière’s disease. Acta Otolaryngol 2005;125(8):852-7.
25. Riska KM, Murnane O, Akin FW, Hall C. Video head impulse testing (vHIT) and the assessment of horizontal semicircular canal function. J Am Acad Audiol 2015;26(5):518-23.
26. Blödow A, Heinze M, Bloching MB, et al. Caloric stimulation and video-head impulse testing in Ménière’s disease and vestibular migraine. Acta Otolaryngol 2014;134(12):1239-44.
27. Della Santina CC, Cremer PD, Carey JP, Minor LB. Comparison of head thrust test with head autorotation test reveals that the vestibulo-ocular reflex is enhanced during voluntary head movements. Arch Otolaryngol Head Neck Surg 2002;128(9): 1044-54.
28. Herdman SJ, Hall CD, Schubert MC, et al. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg 2007;133(4):383-9.
29. Sprenger A, Wojak JF, Jandl NM, et al. Predictive mechanisms improve the vestibulo-ocular reflex in patients with bilateral vestibular failure. J Neurol 2014;261(3):628-31.
Volume 97, Number 1-2 www.entjournal.com 23 THE HEAD IMPULSE TEST AS A PREDICTOR OF VIDEONYSTAGMOGRAPHY CALORIC TEST LATERALIZATION ACCORDING TO THE LEVEL OF EXAMINER EXPERIENCE: A PROSPECTIVE OPEN-LABEL STUDY
Risk of posterior semicircular canal trauma when using a retrosigmoid approach for acoustic neuroma surgery and role of endoscopy: An imaging study
Ali Kouhi, MD; Varasteh Vakili Zarch, MD; Ali Pouyan, MD†
Abstract
The rate of hearing preservation after vestibular schwannoma surgery is variable and is not as high as expected, possibly due to injuries to the posterior semicircular canal while exposing the tumor. The aim of this study was to estimate the risk of posterior semicircular canal injuries using temporal bone computed tomography (CT) scan findings. Temporal bone CT scans of 30 patients selected between 2013 and 2015 were studied. The median age of the patients was 40 years. Two planes were studied: (1) the axial plane that shows the common crus of the posterior semicircular canal and (2) the coronal plane that shows the two crura of the posterior semicircular canal. Five lines were drawn and four angles and three distances were measured. In this study, we divided the patients into three groups consisting of 10 patients each: (1) patients with no evidence of inflammatory or neoplastic disease, (2) those with chronic ear disease, and (3) those with vestibular schwannomas. The portion of the internal auditory canal that was exposed by drilling while preserving the posterior semicircular canal was 53 to 64% and 61 ± 9% in whole temporal bones in the three groups. The mean angle of vision with an endoscope was less than 105° in 56% of cases, which means even with a 30° endoscope, the fundus could not be visualized. Therefore, according to our data, it seemed impossible to expose the whole length of the internal audi-
From the Department of Otolaryngology, Head and Neck Surgery, Otorhinolaryngology Research Center, Amir-A’lam Hospital, Tehran University of Medical Sciences, Iran.
†Deceased.
Corresponding author: Varasteh Vakili Zarch, MD, Otorhinolaryngology Research Center, Amir-A’lam Hospital, North Sa’adi Ave., Tehran, Iran. Email: varasteh.vakili@gmail.com
tory canal from the porus to the fundus without causing injury to the posterior semicircular canal. However, the use of endoscopes may help to prevent injury.
Introduction
The three main approaches for acoustic neuroma surgeries are the middle fossa, translabyrinthine, and retrosigmoid. Although the highest hearing preservation rate has been attributed to the middle fossa approach,1,2 the retrosigmoid approach is the most common microsurgical resection method used.2,3 Adequate exposure of the brainstem, access to tumors regardless of size, and hearing preservation are the basic advantages of this technique.3,4
In the retrosigmoid approach, the most common complication after acoustic neuroma surgery is cerebrospinal fluid (CSF) leakage, with a reported rate of 3 to 12%.1,5,6 Opened mastoid air cells, incomplete closure of the dura, and hydrocephaly are probable reasons for the leakage.1 The most common cause of CSF leakage regardless of approach is unrecognized open air cells during drilling.1,3,7
Other morbidities that may arise from the retrosigmoid approach include injury to the cerebellum, cranial nerves, and vessels; headache; and hearing loss despite saving the cochlear nerve anatomy.4 The hearing loss may be the result of an injury to the posterior semicircular canal during the surgeon’s attempt to expose the internal acoustic canal (IAC) posteriorly.1,2,8,9 When exposure of the IAC is required during the retrosigmoid approach, drilling between the posterior semicircular canal, the endolymphatic sac posteriorly, and the canal anteriorly is usually performed 2,8-10 but may result in labyrinthine injury.
24 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018 ORIGINAL ARTICLE

As noted earlier, opened mastoid air cells and labyrinthine injuries are morbidities associated with the retrosigmoid approach during internal acoustic canal exposure. Because the mastoid air cells and the posterior semicircular canal are bony structures, their anatomic locations in relation to the internal acoustic canal are visible in a computed tomography (CT) scan. We estimated the risk of these injuries using temporal bone CT scan findings.
Patients and methods
The temporal bone CT scans of 30 patients selected from 2013 to 2015 were studied. We reviewed the medical records of groups of patients with normal temporal bone, otitis media, or a diagnosis of vestibular schwannoma, with the purpose of comparing pathologies between groups. Images that did not meet the inclusion criteria (i.e., lacking fine sections or not including all structures) were excluded. Measurements were unanimous.
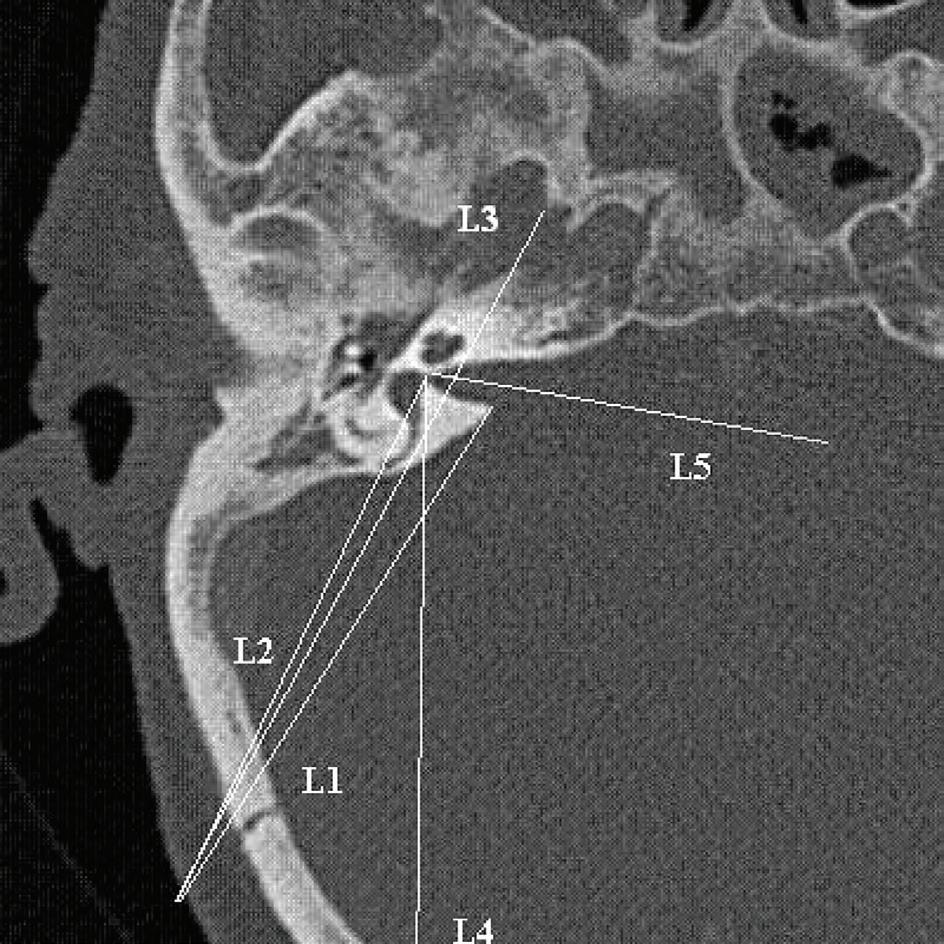
All CT scans were obtained from a 16-slice scanner CT machine (Siemens; Munich, Germany), with 0.6 mm between two slices, in the Amir-Alam University Hospital affiliated with Tehran University of Medical Sciences. The CT scans were not obtained for this study but rather for other indications. In the CT scan, the axial plane that showed the common crus of the posterior semicircular canal (figure) with better IAC visualization was selected.
Five lines were drawn and four angles and three distances were measured. Definitions of angles and distances are provided in table 1. The incision size used in the retrosigmoid approach was assumed to be 4 cm. 3 Pneumatization of bone between the IAC and posterior semicircular canal was presumed positive if air cells were visualized in this area. Pneumatization of different parts of the mastoid was evaluated to indicate extent of air cells and expressed as the percentage of presence of air cells in this compartment of temporal bone.
Statistical Package for the Social Sciences version 18 was used to analyze the data.
Results
Patients. Patients enrolled were divided into three groups of 10 patients each: (1) patients with no evidence of inflammatory or neoplastic disease, (2) patients with chronic ear disease, and (3) patients with vestibular schwannomas. The ratio of men to women was 1.56:1. The median age of the patients was 40 years (mean: 42.17 ± 14.86 years).
Descriptive data regarding the anatomy and the mastoid pneumatization of the groups appear in table 2.
IAC exposure by drilling. P1 indicates the maximum proportion of IAC that can be exposed by drilling while preserving the posterior semicircular canal. Range of the mean P1 was 0.53 to 0.64 in the three groups, and the average was 0.61 ± 0.09 (table 3). This proportion of IAC was not significantly different between the three groups (p > 0.05). Maximum exposure (0.82) was observed in normal temporal bones (with no otitis or vestibular schwannoma). Pneumatization of the posterior or anterior petrous apex or the extent of mastoid pneumatization was not related to this exposure (p > 0.05). In patients with vestibular schwannoma or otitis, no significant difference was observed between the affected and normal side (p > 0.05).
Posterior semicircular canal drilling probability. In all groups of patients, the A1 angle was wider than the A2 angle, indicating that drilling from the porus to the fundus of the IAC is not achievable without removing the posterior semicircular canal (table 3). We also discovered that A1 was always wider than A2, except in one temporal bone. These angles were similar in temporal bones with different pathologies (p > 0.05).
Proper incision site. L4 is drawn from the fundus to the most posterior point of the posterior semicircular canal to achieve proper incision and full exposure of the IAC while preventing canal trauma. The median distance of the posterior incision border was 65.2 ± 11.1 mm (range: 45 to 88.5 mm).
26 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
KOUHI, ZARcH, POUyAN
Figure. This CT scan of the axial plane shows the common crus of the posterior semicircular canal.
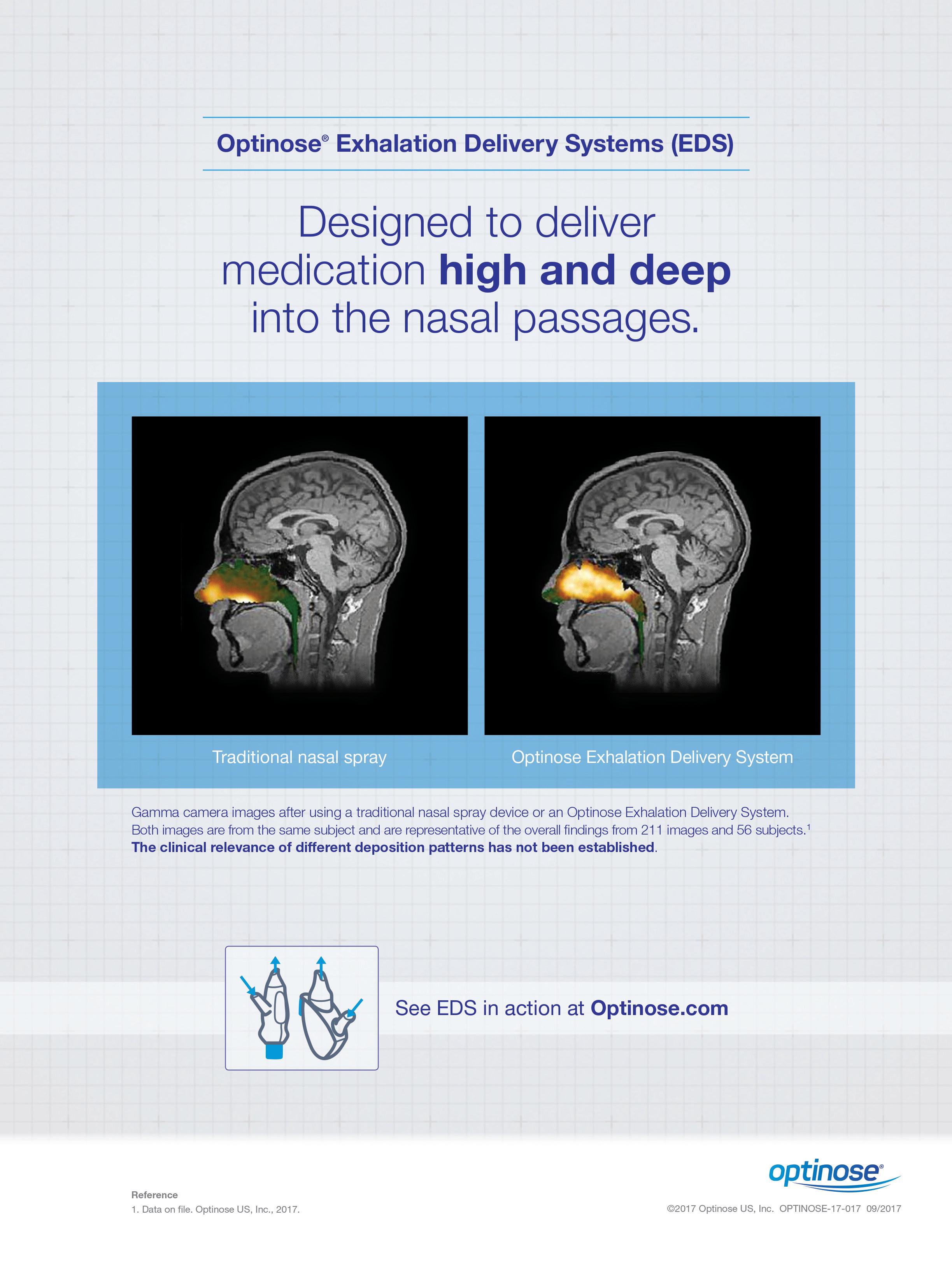
Table 1. Description of parameters used in this study
Parameter
Lines Description
L1 From porus of IAc to most posterior incision site
L2 From fundus of IAc to most posterior incision site
L3 Tangential to posterior semicircular canal from most posterior incision site
L4 From fundus to most posterior point of posterior semicircular canal
L5 IAc vector
Angles
A1 Angle between L1 and L2
A2 Angle between L1 and L3
A3 Angle between direction of posterior semicircular canal and midline in coronal plane
A4 Angle between L5 and L3
Distances
D1 From meet point of L4 to calvaria to retroauricular sulcus
D2 From fondus to porus
D3 From meet point of L3 and IAc to porus
P1 D3/D2
Endoscope vision vector. The mean A4 angle was 103° (standard deviation: 11.96°; range: 76.5 to 128.7°). In 56% of temporal bones, this angle was <105°, which means that even with a 30° endoscope, the fundus cannot be visualized (assuming a 45° visual width and a 30° midline vision vector). However, in >91% of the cases, the fundus was observed using a 45° endoscope.
The possibility of CSF leaks. In 15% of temporal bones, pneumatization of the bone between the IAC and the posterior semicircular canal was evident. This finding meant that by drilling this area, it was possible to create a tract for CSF leakage. This area of pneumatization was not correlated with the extension of mastoid pneumatization (p > 0.05).
Two-sides correlation. Table 3 shows a good correlation between the two sides of the same patient and between the area of pneumatization and pathology.
Discussion
In the 1960s, the development of microscopes revolutionized the treatment of acoustic neuroma. However, recent studies have shown that microsurgical tumor removal is associated with morbidity and a mortality rate reported to be 1% or less.11,12 Although facial nerve injury is always a concern in this surgery, the routine use of neuromonitoring has reduced facial nerve injury to <10% in most cases.13,14
Hearing preservation is currently possible for patients depending on the type of surgical approach, tumor size, patient age, and the use of intraoperative monitoring.15 Hearing loss as a result of a retrosigmoid approach depends on the cochlear nerve position, which is usually anterior to the tumor and cannot be identified early in the surgery before injuring the nerve. Another important etiology of surgery-related hearing loss is posterior semicircular canal injury during the attempt to expose the most lateral aspects of the tumor and IAC.
If the tumor is extended to the lateral parts of the IAC, removing the bone and air cells lateral to the IAC is necessary, and this removal is usually how the labyrinth is injured and hearing is lost.8,16 The posterior semicircular canal is 0.7 cm from the entrance of the posterior wall of the internal auditory canal.17 Our data show that, in most cases, simultaneous removal of the posterior meatal wall to gain full access to the IAC and to preserve the posterior semicircular canal is not feasible. Importantly, this finding is independent of the extent of mastoid pneumatization or other concomitant
* Percentage indicates the percentage of ears. Each side ear is analyzed separately. Some patients had bilateral otitis or bilateral acoustic neuromas.
Key: VS = vestibular schwannoma.
28 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
KOUHI, ZARcH, POUyAN
Pneumatization (%)* Normal Otitis Otitis (contra) VS VS (contra) Total mastoid overall sclerotic 8.3 63.6 0 12.5 0 22 Low 8.3 0 0 0 2.4 High 83 36.4 100 87.5 100 75.6 Zygomatic root 66.7 27.3 66.7 25 28.6 41.5 Anterior apex 0 0 33.3 50 57.1 22 Posterior apex 50 0 66.7 12.5 14.3 24.4
Table 2. Anatomic pneumatization of temporal bone in three patient groups
pathologies. We revealed that if the posterior semicircular canal is to be preserved, approximately 60% of IAC can be visualized.
Lui et al studied the safe zone of drilling with respect to the posterior semicircular canal.18 Their results show that the range of the safe drilling zone is significantly wide, which presents some difficulty in setting a safe zone. Important in the interpretation of their data is that IAC anatomy is not the only parameter that matters; the angle of vision and size of craniotomy can also have great effects on exposure limits. We showed that considering the craniotomy site and angle of vision, finding a safe zone through which the whole length of the IAC may be exposed is difficult.
One complication of the retrosigmoid approach is CSF leakage. This leakage is mainly due to the extensive removal of the posterior meatal wall to more fully expose the IAC and canalicular part of the tumor.19 Our data showed that the chance of finding air cells in this area was considerable. CT scans, however, underestimate the presence of air cells, so their presence may be much more common than CT demonstrates.
In recent years, endoscopes have proven useful in cerebellopontine angle approaches, mainly because they provide increased depth of field, magnification, better illumination and, most important, a wider angle of view.20,21
Our results show that with the use of endoscopes, the far lateral part of the IAC can be visualized easily and the presence of air cell openings can be confirmed. Therefore, the use of endoscopes may potentially reduce both posterior semicircular canal injury and CSF leakage.
Conclusion
In vestibular schwannoma surgery, drilling of the posterior meatal wall of IAC should be conducted with care, as the risk of posterior semicircular canal injury is significant. In addition, exposing the whole length of the IAC from the porus to the fundus without injuring the posterior semicircular canal is not possible. By providing an improved angle of view, endoscopes may help in IAC exposure of the surgery and may eliminate the need for massive drilling.
Acknowledgment
Unfortunately, we lost our dear colleague and coauthor Dr. Ali Pouyan after this work was done.
References
1. Heman-Ackah SE, Golfinos JG, Roland JT Jr. Management of surgical complications and failures in acoustic neuroma surgery. Otolaryngol Clin North Am 2012;45(2):455-70.
2. Sughrue ME, Yang I, Aranda D, et al. Hearing preservation rates after microsurgical resection of vestibular schwannoma. J Clin Neurosci 2010;17(9):1126-9.
3. Elhammady MS, Telischi FF, Morcos JJ. Retrosigmoid approach: Indications, techniques, and results. Otolaryngol Clin North Am 2012;45(2):375-97.
4. Sheth SA, Kwon CS, Barker FG 2nd. The art of management decision making: From intuition to evidence-based medicine. Otolaryngol Clin North Am 2012;45(2):333-51.
5. Harner SG, Beatty CW, Ebersold MJ. Retrosigmoid removal of acoustic neuroma: Experience 1978-1988. Otolaryngol Head Neck Surg 1990;103(1):40-5.
6. Bayazit YA, Celenk F, Duzlu M, Goksu N. Management of cerebrospinal fluid leak following retrosigmoid posterior cranial fossa surgery. ORL J Otorhinolaryngol Relat Spec 2009;71(6):329-33.
Volume 97, Number 1-2 www.entjournal.com 29 RISK OF POSTERIOR SEMICIRCULAR CANAL TRAUMA WHEN USING A RETROSIGMOID APPROACH FOR ACOUSTIC NEUROMA SURGERY AND ROLE OF ENDOSCOPY: AN IMAGING STUDY
Parameter Normal Otitis Otitis (contra) VS VS (contra) Total A1 (degree) 8.46 ± 1.75 10.39 ± 1.92 8.25 ± 3.12 9.19 ± 1.13 9.04 ± 2.01 9.20 ± 1.92 (5.16-11.21) (7.01-12.90) (4.86-11.00) (6.74-10.57) (6.01-11.98) (4.86-12.90) A2 (degree) 5.58 ± 1.70 6.56 ± 1.62 4.58 ± 2.54 6.46 ± 1.42 6.72 ± 1.60 6.13 ± 1.71 (3.13-7.59) (3.00-9.02) (2.49-7.41) (4.47-8.80) (5.00-9.50) (2.49-9.50) A3 (degree) 40.29 ± 8.21 47.51 ± 8.94 47.19 ± 13.00 47.57 ± 8.03 54.53 ± 7.75 46.58 ± 9.52 (26.60-51.25) (26.70-56.20) (32.40-56.77) (29.95-55.30) (38.81-63.43) (26.60-63.43) A4 (degree) 100.30 ± 10.83 101.77 ± 13.77 105.82 ± 17.22 109.03 ± 12.78 103.23 ± 7.97 103.30 ± 11.96 (81.80-117.21) (76.46-122.20) (87.40-121.52) (91.94-128.66) (92.66-117.43) (76.46-128.66) D1 (mm) 65.35 ± 14.13 66.82 ± 10.32 71.53 ± 10.32 70.88 ± 11.06 71.34 ± 7.30 68.30 ± 11.10 (45.00-86.90) (55.20-85.80) (60.2-80.40) (59.60-88.50) (63.00-81.20) (45.0-88.50) P1 0.60 ± 0.12 0.62 ± 0.08 0.53 ± 0.17 0.61 ± 0.08 0.64 ± 0.08 0.61 ± 0.09 (0.40-0.82) (0.52-0.75) (0.38-0.70) (0.50-0.72) (0.55-0.78) (0.38-0.82)
Table 3. Descriptive data of parameters in the three patient groups
7. Chovanec M, Zvĕřina E, Profant O, et al. Impact of videoendoscopy on the results of retrosigmoid-transmeatal microsurgery of vestibular schwannoma: Prospective study. Eur Arch Otorhinolaryngol 2013;270(4):1277-84.
8. Koval J, Molcan M, Bowdler AD, Sterkers JM. Retrosigmoid transmeatal approach: An anatomic study of an approach used for preservation of hearing in acoustic neuroma surgery and vestibular neurotomy. Skull Base Surg 1993;3(1):16-21.
9. Gharabaghi A, Samii A, Koerbel A, et al. Preservation of function in vestibular schwannoma surgery. Neurosurgery 2007; 60(2Suppl1):ONS124-8.
10. Savardekar A, Nagata T, Kiatsoontorn K, et al. Preservation of labyrinthine structures while drilling the posterior wall of the internal auditory canal in surgery of vestibular schwannomas via the retrosigmoid suboccipital approach. World Neurosurg 2014;82(3-4):474-9.
11. Zvĕřina E. Acoustic neuroma—vestibular schwannoma—personal experience of up-to-date management. Cas Lek Cesk 2010; 149(6):269-76.
12. Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: A systematic review of outcome and risk of three therapeutic options. Neurol Res 2003;25(7):682-90.
13. Myrseth E, Pedersen PH, Møller P, Lund-Johansen M. Treatment of vestibular schwannomas. Why, when and how? Acta Neurochir (Wien) 2007;149(7):647-60.
14. Wackym PA. Stereotactic radiosurgery, microsurgery, and expectant management of acoustic neuroma: Basis for informed consent. Otolaryngol Clin North Am 2005;38(4):653-70.

15. Sughrue M, Kane AJ, Kaur R, et al. A prospective study of hearing preservation in untreated vestibular schwannomas. J Neurosurg 2011;114(2):381-5.
16. Göksu N, Yilmaz M, Bayramoglu I, et al. Evaluation of the results of endoscope-assisted acoustic neuroma surgery through posterior fossa approach. ORL J Otorhinolaryngol Relat Spec 2005;67(2):87-91.
17. Ojemann RG. Retrosigmoid approach to acoustic neuroma (vestibular schwannoma). Neurosurgery 2001;48(3):553-8.
18. Liu S, Tong D, Liu M, et al. The safe zone of posterior semicircular canal resection in suboccipital retrosigmoid sinus approach for acoustic neuroma surgery. J Craniofac Surg 2013;24(6):2103-5.
19. Valtonen HJ, Poe DS, Heilman CB, Tarlov EC. Endoscopically assisted prevention of cerebrospinal fluid leak in suboccipital acoustic neuroma surgery. Am J Otol 1997;18(3):381-5.
20. Magnan J, Barbieri M, Mora R, et al. Retrosigmoid approach for small and medium-sized acoustic neuromas. Otol Neurotol 2002;23(2):141-5.
21. Göksu N, Bayazit YA, Bayramoglu I, et al. Surgical exposure in retrosigmoid approach: Do we need cerebellar retractors? Surg Neurol 2006;65(6):631-4.
30 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
KOUHI, ZARcH, POUyAN
Elastography as a potential modality for screening cervical lymph nodes in patients with papillary thyroid cancer: A review of literature
Robert Saadi, MD; Salvatore LaRusso, MEd; Kanupriya Vijay, MD; David Goldenberg, MD
Abstract
Papillary thyroid cancer often presents with cervical lymph node involvement and has a high incidence of recurrence, which requires routine follow-up with ultrasound imaging. Elastography is a novel ultrasound technique that has been demonstrated to be effective clinically in detecting tissue pathology in areas such as the liver and breast. Preliminary data suggest that it may be effective in screening tissues in the neck for malignancy, specifically cervical lymph nodes. However, diagnostic criteria and elastographic techniques vary significantly among the studies we have reviewed, which all tend to focus on populations of patients with many different types of primary malignancies. Further research is required on the feasibility of creating standardized and reproducible clinical criteria in a specific patient population. To study the clinical utility of elastography in cervical lymph nodes, patients with diagnosed papillary thyroid carcinoma may serve as an ideal population because of their need for ultrasound surveillance and the propensity of papillary thyroid cancer to metastasize to and recur in cervical lymph nodes. We will review the limitations, techniques, and reported clinical utility of elastography on cervical lymph nodes and its potential as a screening modality for papillary thyroid cancer.
Introduction
Papillary thyroid carcinoma (PTC) is the most common malignancy affecting the thyroid. Approximately 50% of patients with PTC have lymph node involvement when diagnosed, with up to 20% having an en-
From the Department of Surgery, Division of Otolaryngology–Head and Neck Surgery (Dr. Saadi and Dr. Goldenberg), and the Department of Radiology (Dr. LaRusso and Dr. Vijay), The Pennsylvania State University, College of Medicine, Hershey, Pa.
Corresponding author: David Goldenberg, MD, FACS, Division of Otolaryngology–Head and Neck Surgery, The Pennsylvania State University, College of Medicine, 500 University Dr., H091, Hershey, PA 17033-0850. Email: dgoldenberg@hmc.psu.edu
larged lymph node as the presenting symptom. Metastatic spread of PTC most often occurs in ipsilateral cervical lymph nodes, detection of which is important in planning selective lymph node dissection.1,2
When thyroid carcinoma is suspected, each nodal station in the neck is examined by B-mode and color or power Doppler ultrasound (US) for the presence of abnormal features, which include calcification, increased or irregular peripheral vascularity, round shape, size, loss of fatty hilum, hyperechogenicity relative to adjacent muscle, thickened cortex, or cystic change (figures 1 and 2). The diagnostic yield of these findings varies when considered individually, with wide ranges for sensitivity (20 to 93%), specificity (70 to 100%) and accuracy (55 to 90%).2,3
Loss of fatty hilum (LOFH) is the most common finding in metastatic lymph nodes, but the sole finding of LOFH has a low yield in differentiating benign from malignant lymph nodes. LOFH has even been found to decrease accuracy when included in the diagnostic criteria.2,4
Approximately 40% of all PTC lymph node metastases undergo cystic degeneration, which is associated with the common finding of dark/black nodes during surgery.1,5,6 This finding on US is highly specific for metastatic disease, approaching 100%, but it is often found to have a low sensitivity (20 to 30%).2,3,7 Some US findings require a degree of clinical judgment for accurate interpretation, but most studies agree that the overall utility of preoperative US of cervical lymph nodes in patients with diagnosed thyroid cancer is strong. Stulak et al reported 84% sensitivity, 98% specificity, and 95% accuracy with B-mode US in these patients.8
Metastasis in abnormal-appearing lymph nodes on US can be subsequently confirmed with a fine-needle aspiration biopsy (FNAB). Central as well as lateral lymph node neck dissection is recommended at the time of thyroidectomy in cases of confirmed metastasis.9
Volume 97, Number 1-2 www.entjournal.com 31 ORIGINAL ARTICLE
Additionally, diagnosis of lymph node invasion on presentation also serves as an important prognostic indicator for locoregional recurrence after the initial surgery.10,11
Approximately 30% of patients may develop recurrence following resection of primary disease, which is often associated with increased morbidity and mortality.12-14 PTC most often recurs or persists in cervical lymph nodes; therefore post-thyroidectomy surveillance with sonography of the neck and measurement of serum thyrogobulin level is required in the first 6 to 15 months and at regular intervals thereafter. The frequency with which patients are evaluated depends on serum thyroglobulin level and other risk factors.9
In evaluating recurrent disease, US findings have a reported sensitivity of 90%, specificity of 79%, and accuracy of 87.9%.8 The propensity of PTC for lymphatic spread and its effect on management of the disease underscores the need for accurate evaluation of cervical lymph nodes before and after thyroidectomy.
Elastography is a novel imaging modality that can be used clinically to assess elasticity of soft tissues. Elasticity is a parameter based on the strain or deformation of tissue in response to a physical force. Most malignancies reliably exhibit reduced elasticity and increased stiffness due to the nature of neoplastic growth, making this procedure a promising new development in the diagnosis of cancerous and metastatic foci.
The elastrography technique involves applying a force on the tissue and subsequently imaging the resultant deformation. This technique initially required the operator to physically push the transducer into the tissue to be examined, now termed quasi-static imaging. The unintended variable with
this technique was the differences in the degree of pressure applied by each observer. In efforts to increase objectivity and reproducibility, the acoustic radiation force impulse (ARFI) technique was developed, which involves emitting acoustic pulses inside tissue to generate micron-scale displacement.15
The distortion of tissues can be observed by ultrasonographic imaging, and areas of decreased strain can be recorded, creating a map of elasticity, known as an elastogram (figures 3 and 4). Additionally, whereas deformation can provide qualitative indications of tissue stiffness, measuring the speed of the shear wave, which is the speed with which the tissue wave travels perpendicularly from the pulse, can provide quantitative measurements of higher clinical value.16
Newer ARFI models can be used to measure shear wave velocity, which is inversely proportional to tissue elasticity, providing a means of numerical data collection.17 Supersonic shear imaging (SSI) or shear wave elastography (SWE) combines the technology of an applied ultrasonographic beam with ultrafast US imaging to create real-time, quantitative maps of tissue stiffness.18 Many newer models of US machines now have ARFI and/or SSI/SWE-based capabilities.
Review of literature
We searched PubMed and Ovid using key words elastography, cervical, lymph node, and malignancy from December 2014 to February 2015 for articles that were published over the past 5 years. Excluded from our review were studies that focused on other neck tissue, such as the thyroid gland; studies of lymph nodes in
32 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
sAADI, LARUssO, VIJAy, gOLDENbERg
Figure 1. The ultrasound appearance of a normal lymph node shows the oval shape and preserved central fatty hilum (left). Note the normal vascularity within the central hilum by Doppler ultrasound (right).
Figure 2. An abnormal lateral cervical lymph node with loss of fatty hilum and microcalcifications is shown (left). Another morphologically abnormal cervical lymph node is shown (right), demonstrating round shape, loss of fatty hilum, and microcalcifications. Both images are highly suspicious for papillary thyroid cancer metastasis.

other areas of the body, such as the axilla; and studies on patients without cervical node malignancies. Here, we review the current literature on the use of elastography in diagnosing metastatic invasion of cervical lymph nodes.
Quasi-static elastography. To collect numerical data with the quasi-static method, Lyshchik et al used the lymph node strain index, or the muscle-to-lymph node strain ratio, and found that a cutoff value of 1.5 was associated with 98% specificity, 85% sensitivity, and 92% specificity.19 This was a prospective study, and the inclusion criterion was preoperative suspicion of thyroid cancer (74% with PTC) or hypopharyngeal cancer. The Sonoline Elegra US scanner (Siemens; Malvern, Pa.) was used for elastographic and US imaging. Diagnosis was confirmed by histopathologic examination after surgical excision. The authors encountered issues in imaging in the neck due to noise from nonaxial motion of lesions and pulses from the carotid artery.19
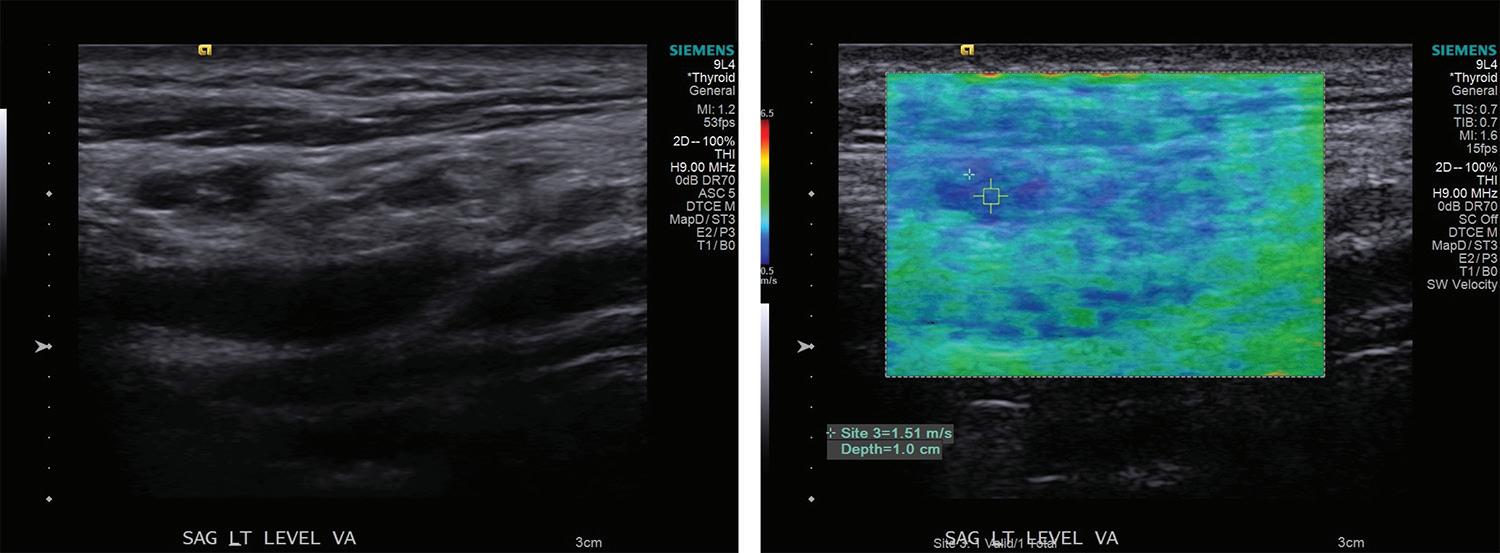
Alam et al created a scoring system associated with five qualitative elastographic patterns. 20 This study compared elastography to B-mode US in distinguishing reactive vs. metastatic processes in enlarged cervical nodes. Sensitivity, specificity, and accuracy of B-mode US were 98, 59, and 84%, respectively, compared with 83, 100, and 89%, respectively, with free-hand elastography. They also found that when the two methods were used together, overall diagnostic accuracy was 93%, which was superior to that with either strategy used alone. Lymph nodes were diagnosed by histopathology, and metastatic nodes represented a variety of primary malignancies, including squamous cell, thyroid, and breast carcinoma.20
ARFI elastography. Fujiwara et al showed that ARFI-induced shear wave velocities could be useful in differentiating reactive from malignant lymph nodes. 21 Shear wave velocities of reactive lymph nodes were significantly lower at 1.52 ± 0.48 m/s than that of malignant nodes, which were 2.46 ± 0.75 m/s. In
the study, the best cutoff value was 1.9 m/s, which provided a 95% sensitivity, 81.8% specificity, and 88% accuracy. The receiver operating characteristic (ROC) area under the curve (AUC) was 0.923 (95% confidence interval [CI]: 0.842 to 1.000). Final diagnosis was based solely on histopathologic results. Imaging was performed with a Siemens S2000 US system, and measurements were taken three times and averaged.
Fujiwara et al also reported that in 40% of the patients with confirmed malignant disease, the shear wave velocity could not be recorded by the machine (value X.XX recorded) because of the necrotic or liquid component of the tissue.21 Immeasurable shear wave velocity in lymph nodes by elastography was found to always harbor metastasis in this study. The study was small and retrospective and included patients with a variety of primary cancer sites including squamous cell carcinoma, PTC, and lymphoma. Only patients with lymph node swelling were included in the study, and measurements were made preoperatively.21
Meng et al reported similar findings with ARFI-based imaging. This study showed shear wave speed for benign lymphadenopathy to be 2.01 ± 0.95 m/s and shear wave speed of malignant lesions to be 4.61 ± 2.56 m/s.22 They found the most accurate cutoff value to be 2.595 m/s, and with the ROC curve, this value predicted malignancy with 82.9% sensitivity and 93.1% specificity and gave an AUC of 0.906 (95% CI: 0.857-0.954). Also, 93.6% of malignant nodes were noted to be substantially darker than surrounding tissue on elastogram, whereas only 28.4% of benign nodes were qualitatively defined in this way.
Imaging in the Meng study was performed with the Siemens S2000 US machine, and measurements were taken five times and averaged.22 The machine recorded X.XX m/s in a few subjects, with the suspected reason being interference by micro- and macrocalcification in the region of interest (ROI). This was a prospective study and cervical lymph nodes were included only if at least one abnormality was found on conventional US, such as increased size, roundness, calcifications, or disordered vasculature. As a result, final diagnoses were quite varied, including metastatic nodes from a wide range of malignancies and reactive nodes, tuberculous nodes, and necrotizing granulomatous lymphadenitis.22
Volume 97, Number 1-2 www.entjournal.com 35 ELASTOGRAPHY AS A POTENTIAL MODALITY FOR SCREENING CERVICAL LYMPH NODES IN PATIENTS WITH PAPILLARY THYROID CANCER: A REVIEW OF LITERATURE
Figure 3. An ultrasonographic image of a normal cervical lymph node is shown (left). The same node is displayed with a color mapping elastogram using Virtual Touch Imaging (Siemens), acoustic radiation force impulse-based elastography with a shear wave velocity of 1.51 m/s in the region of interest (right).
Supersonic shear impulse/SWE. Bhatia et al found that with real-time SWE, malignant cervical lymph nodes were significantly stiffer (median: 25.0 kPa, range: 6.9 to 278.9 kPa) than benign nodes (median: 21.3kPa, range: 8.9 to 30.2 kPa).23 A cutoff value of 30.2 yielded the highest accuracy of 61.8%, with 41.9% sensitivity and 100% specificity. They also found that malignant lymph nodes were more likely to have heterogeneous elasticity, even when the nodes appeared uniform on US.
Nodes with cystic spaces had foci with no elasticity signals due to failure of shear waves to travel through liquids; the vast majority of these nodes were malignant. This was a prospective study conducted on patients undergoing US evaluation, and results were confirmed by FNAB for cytology. A variety of primary malignancies was found in malignant nodes, and no subgrouping of elastography results based on histology was performed.23
Choi et al found higher values for sensitivity, specificity, and accuracy (91%, 97%, and 94%, respectively) than did Bhatia et al, with a cutoff of 19.44 kPa.24 The ROI of the SWE measurements was placed in the stiffest-appearing areas of the lymph nodes to obtain the maximum shear wave elasticity modulus, which was significantly higher in metastatic (41.06 ± 36.34kPa) than in benign (14.226 ± 4.19 kPa) nodes. They compared these values with those of traditional gray-scale US, which had sensitivity of 88%, specificity of 94%, and accuracy of 91%.
Lymph nodes were considered malignant in the Choi study if they had one of the following criteria by US: LOFH, heterogeneity in cortex, calcification, cystic or necrotic area, or a long-to-short axis diameter ratio greater than 2.0.24 Multivariate regression analyses for US (r = 0.837) and elastography (r = 0.882) were not significantly different but were independently associated with metastatic nodes. This was a prospective study, and patients were included if they had confirmed head and neck malignancies (67 lymph nodes from 15 patients) and if the final diagnosis was based on histopathologic examination of surgical specimens. Most patients and metastatic lymph nodes that were evaluated had PTC.24
A retrospective study by Jung et al examined cervical lymph nodes using SWE preoperatively in patients with PTC, exclusively.25 This study showed that elasticity indices of the region of interest of Emean, Emin, Emax, and the calculated ratio with the surrounding muscle of Emean-m
were all significantly higher in metastatic lymph nodes, with highest accuracy (72.6%) using Emean with a cutoff value of 29 kPa. B-mode US accuracy was 78.57%, but they found that Emean significantly improved diagnostic performance of B-mode US when used adjunctively (AUC increased from 0.738 to 0.811). Additionally, quantitative SWE values were significantly higher when extranodal extension of metastasis was present, and correlated with the number of positive lymph nodes per lymph node dissected, thereby predicting prognostic factors of lymph node metastasis. The values were measured at the stiffest portion of the lymph nodes and correlated to histopathologic findings. Suspicious lymph nodes based on US were included, and final diagnosis was by FNAB or surgical specimen depending on indication.25
Discussion
The articles reviewed in the present study are quite varied in terms of techniques used, methods of data collection, and types of primary malignancies. However, malignant nodes were shown consistently to be significantly stiffer and more heterogeneous, even when measured by different forms of elastography.
Most studies agree that the diagnostic value of elastography is at least comparable, if not in some ways superior, to B-mode US, the current standard of care for papillary thyroid carcinoma. When compared directly, it appears that the technology trends towards higher specificity and lower sensitivity than US. More importantly, elastography seems to increase AUC values when used in conjunction with B-mode US.
Also, specific types of histology likely produce different degrees of elasticity, although all malignant tissues are generally stiffer than benign tissues. A recent study documented that even SWE measurements of malignancies limited to the thyroid revealed disparity in stiffness based on histopathologic properties.26 This highlights the need for prospective studies in a specific patient population to develop definitive elastographic criteria. Limiting
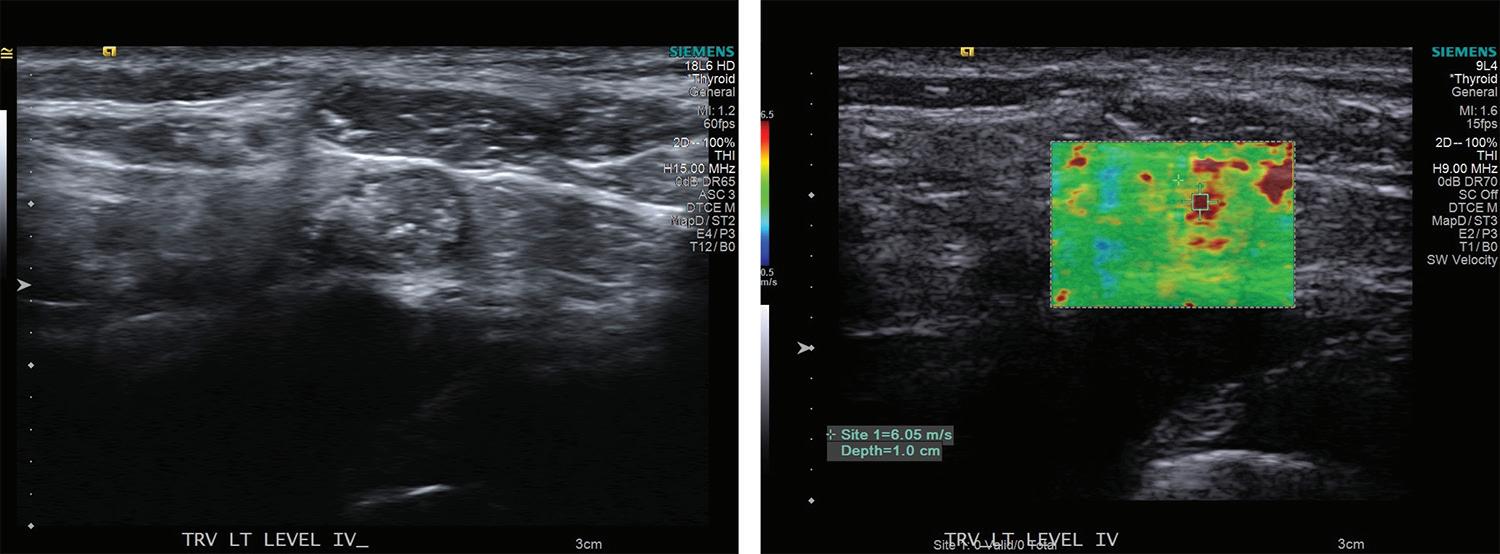
36 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
sAADI, LARUssO, VIJAy, gOLDENbERg
Figure 4. This ultrasonographic image demonstrates an abnormal lymph node with invasion by papillary thyroid carcinoma (left). The same node is shown to have increased tissue stiffness on elastogram with a shear wave velocity of 6.05 m/s (right).
the variation of primary malignancies or subgrouping based on histologic type may help to achieve this goal.
PTC has a significant probability of cervical lymph node invasion and recurrence. As such, PTC may be an excellent model for developing diagnostic elastographic criteria. Jung et al show that in this population of patients, elastography can reliably detect metastasis, predict prognostic factors such as extranodal spread, and increase accuracy when used in combination with B-mode US.25
Many lymph nodes with PTC tend to undergo cystic degeneration. As noted by Fujiwara et al and Meng et al, any fluid component present in a lymph node cannot be read by elastography (figure 5).21,22 However, if elastography can detect cystic change reliably, it may represent a potential advantage in diagnosis because US has a low sensitivity for cystic change (20%) and cystic change can be a cause of false negatives and false positives in FNAB.3,5 Unreadable elastography results may therefore serve to aid in diagnosis and raise suspicion of a subsequent, hypocellular FNAB. Furthermore, cystic necrosis has been found to predict the presence of BRAF V600E mutation, advanced disease, and decreased survival.27-30 Therefore, presence of cystic change by elastography may serve as an independent prognostic and diagnostic criterion for malignancy. Whether fluid collections smaller than those visualized by US may be detected by values of X.XX on elastography remains to be determined.
Currently, screening by US could benefit from more definitive numerical criteria, as it relies heavily on morphologic findings and, specifically, the type of morphologic abnormalities present. Size criteria can be difficult to quantify as the size of lymph nodes can vary in different levels of the neck.31 US findings of calcification and cystic necrosis are specific (100%), but sensitivity for these features is only about 50% and 20%, respectively.3 LOFH, the most common finding in metastatic lymph nodes, has been shown in some cases to decrease overall accuracy when included in criteria.2 FNAB is a minimally invasive procedure that has high diagnostic performance but may be avoided if elastographic data are used in cases with solitarily lowyield ultrasonographic findings, such as LOFH. The quantifiable data gained by elastography may serve as a specific cutoff for disease, and numbers may be trended over time to uncover changes in tissue consistency early during the close follow-up required after surgical resection in patients with
PTC. Ultrasonography may have less accuracy in diagnosing recurrence than in diagnosing initial metastatic spread, so elastographic trending may be particularly helpful during this period of patient management.8
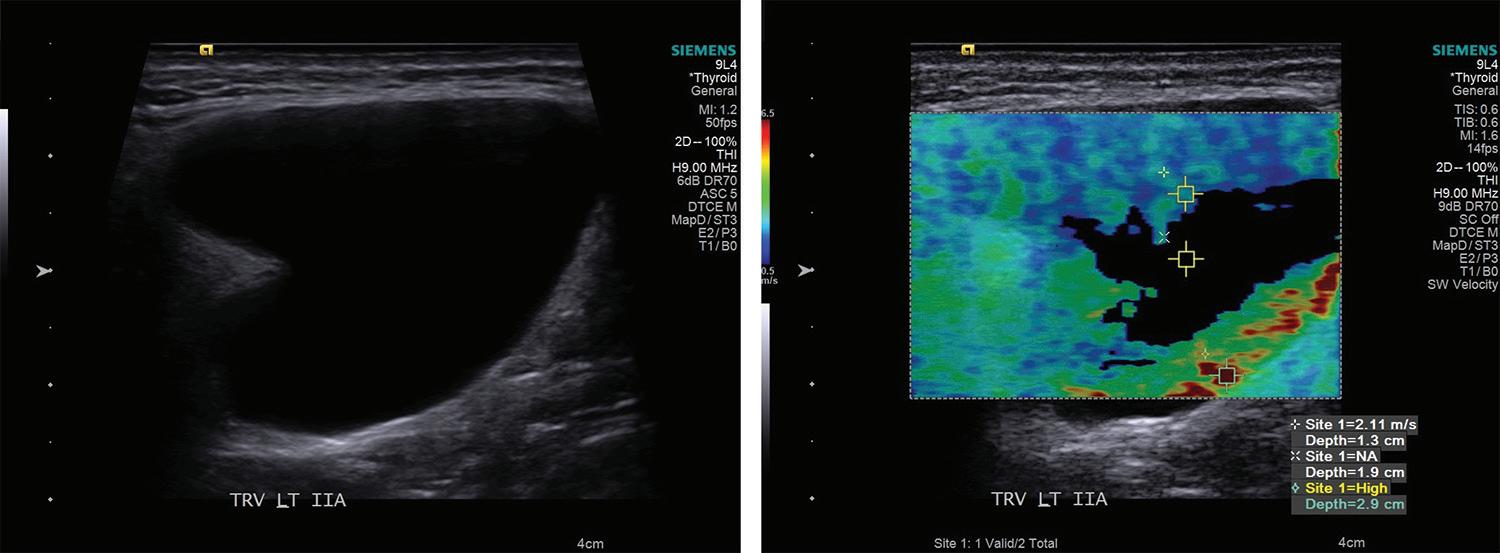
As mentioned above, typical advanced findings of disease such as necrosis with calcification and fluid components cannot be quantified by elastography. However, it can be argued that quantification is no longer necessary in the presence of such specific and concerning findings. Although it has not yet been studied, it stands to reason that abnormalities may be apparent on elastography before US, considering B-mode sonography requires visualization of structural changes, whereas elastography measures changes in cellular composition. Because the technology has now become standard in many US machines, the use of elastography in combination with required US screening in PTC patients is a cost- and time-effective strategy that may improve overall accuracy.
Many studies mentioned possible flaws in evaluation of malignancy by elastography. Calcifications and necrotic or cystic change have been noted to interfere with elastographic readings; however, this is less of a problem in cervical lymph nodes in the setting of PTC, where these findings would be highly specific for malignancy.
Some other concerns for elastography in the neck are the curvature and mobility of the neck, selection of representative regions of interest in heterogeneous tissue, and interference by carotid pulses.19,21,22,32 Methods that have been used in the presently reviewed studies for managing these issues are: applying minimal pressure to the neck with the probe, measuring maximum values of elasticity in heterogeneous tissue, and orienting the probe longitudinally to the plane of the carotid.
Conclusion
PTC has a high rate of spread to cervical lymph nodes and locoregional recurrence with relatively characteristic histology. Elastography has been shown to detect metastatic change reliably and would likely be useful in improving
38 www.entjournal.com ENT-Ear, Nose & Throat Journal January/February 2018
sAADI, LARUssO, VIJAy, gOLDENbERg
Figure 5. An ultrasonographic image of cervical cyst is shown (left). Virtual Touch Imaging of this cyst demonstrates “null” information (right).
screening, determination of prognosis, and management. Currently, elastographic imaging of cervical lymph nodes requires further prospective studies in patient populations with the same primary cancer, preferably one with a propensity for spread to cervical lymph nodes, such as PTC. Success of the technology can be determined after consistent, reproducible elastographic criteria of malignant node invasion are created. Given the research so far, elastography is extremely promising as a diagnostic imaging modality in the neck, especially the techniques that limit user variability, such as ARFI and SWE.
References
1. Wunderbaldinger P, Harisinghani MG, Hahn PF, et al. Cystic lymph node metastases in papillary thyroid carcinoma. AJR Am J Roentgenol 2002;178(3):693-7.
2. Sohn YM, Kwak JY, Kim EK, et al. Diagnostic approach for evaluation of lymph node metastasis from thyroid cancer using ultrasound and fine-needle aspiration biopsy. AJR Am J Roentgenol 2010;194(1):38-43.
3. Rosário PW, de Faria S, Bicalho L, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med 2005; 24(10):1385-9.
4. Miah CF, Zaman JA, Simon M, et al. The utility of lymph node mapping sonogram and thyroglobulin surveillance in post thyroidectomy papillary thyroid cancer patients. Surgery 2014; 156(6):1491-6; discussion 1496-7.
5. Ustün M, Risberg B, Davidson B, Berner A. Cystic change in metastatic lymph nodes: A common diagnostic pitfall in fineneedle aspiration cytology. Diagn Cytopathol 2002;27(6):387-92.
6. Crist HS, Evans JJ, Mani H, et al. Do black (dark) lymphnode metastases in papillary thyroid carcinoma suggest more advanced or aggressive disease? Thyroid 2013;23(8):977-81.
7. Langer JE, Mandel SJ. Sonographic imaging of cervical lymph nodes in patients with thyroid cancer. Neuroimaging Clin N Am 2008;18(3):479-89.
8. Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg 2006;141(5):48994; discussion 494-6.
9. Coquia SF, Chu LC, Hamper UM. The role of sonography in thyroid cancer. Radiol Clin North Am 2014;52(6):1283-94.
10. Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22(11):1144-52.
11. Joo JY, Jin J, Seo ST, et al. Recurrence in regional lymph nodes after total thyroidectomy and neck dissection in patients with papillary thyroid cancer. Oral Oncol 2015;51(2):164-9.
12. Hundahl SA, Cady B, Cunningham MP, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer 2000;89(1):202-17.
13. Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): Temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 2002;26(8):879-85.
14. Simon D, Goretzki PE, Witte J, Röher HD. Incidence of regional recurrence guiding radicality in differentiated thyroid carcinoma. World J Surg 1996;20(7):860-6; discussion 866.
15. Zaleska-Dorobisz U, Kaczorowski K, Pawluś A, et al. Ultrasound elastography - review of techniques and its clinical applications. Adv Clin Exp Med 2014;23(4):645-55.
16. Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: Principles and techniques. Diagn Interv Imaging 2013;94(5):487-95.
17. Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: In vivo and ex vivo results. Ultrasound Med Biol 2003;29(12):1715-23.
18. Bercoff J, Tanter M, Fink M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51(4):396-409.
19. Lyshchik A, Higashi T, Asato R, et al. Cervical lymph node metastases: Diagnosis at sonoelastography—initial experience. Radiology 2007;243(1):258-67.
20. Alam F, Naito K, Horiguchi J, et al. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparison with conventional B-mode sonography. AJR Am J Roentgenol 2008;191(2):604-10.
21. Fujiwara T, Tomokuni J, Iwanaga K, et al. Acoustic radiation force impulse imaging for reactive and malignant/metastatic cervical lymph nodes. Ultrasound Med Biol 2013;39(7):1178-83.
22. Meng W, Xing P, Chen Q, Wu C. Initial experience of acoustic radiation force impulse ultrasound imaging of cervical lymph nodes. Eur J Radiol 2013;82(10):1788-92.
23. Bhatia KS, Cho CC, Tong CS, et al. Shear wave elasticity imaging of cervical lymph nodes. Ultrasound Med Biol 2012;38(2):195-201.
24. Choi YJ, Lee JH, Lim HK, et al. Quantitative shear wave elastography in the evaluation of metastatic cervical lymph nodes. Ultrasound Med Biol 2013;39(6):935-40.
25. Jung WS, Kim JA, Son EJ, et al. Shear wave elastography in evaluation of cervical lymph node metastasis of papillary thyroid carcinoma: Elasticity index as a prognostic implication. Ann Surg Oncol 2015;22(1):111-6.
26. Sebag F, Vaillant-Lombard J, Berbis J, et al. Shear wave elastography: A new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab 2010;95(12):5281-8.
27. Kabaker AS, Tublin ME, Nikiforov YE, et al. Suspicious ultrasound characteristics predict BRAF V600E-positive papillary thyroid carcinoma. Thyroid 2012;22(6):585-9.
28. Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 2005;90(12):6373-9.
29. Kim SJ, Lee KE, Myong JP, et al. BRAF V600E mutation is associated with tumor aggressiveness in papillary thyroid cancer. World J Surg 2012;36(2):310-7.
30. Ricarte-Filho J, Ganly I, Rivera M, et al. Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic BRAF, the number of nodal metastases, and extra-nodal extension. Thyroid 2012;22(6):575-84.
31. van den Brekel MW, Castelijns JA, Snow GB. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: How reliable is it? AJNR Am J Neuroradiol 1998; 19(4):695-700.
32. Gallotti A, D’Onofrio M, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med 2010;115(6):889-97.
Volume 97, Number 1-2 www.entjournal.com 39
ELASTOGRAPHY AS A POTENTIAL MODALITY FOR SCREENING CERVICAL LYMPH NODES IN PATIENTS WITH PAPILLARY THYROID CANCER: A REVIEW OF LITERATURE

































