Clinical outcome of revision cartilage
tympanoplasty
Persistent local demucosalization after endoscopic sinus surgery: A report of 3 cases
Desmoid tumors of the head and neck: Two decades in a single tertiary care unit and review of the literature
Invasive fungal rhinosinusitis: An Indian perspective
www.entjournal.com A Vendome Publication OCTOBER-NOVEMBER 2018 • VOL. 97, NO. 10-11
Nasal Septal Perforation Prosthesis





The new two-piece magnetically coupled solution for non-surgical closure Round Oval www.inhealth.com We speak ENT ©2017 InHealth Technologies Manufactured by Freudenberg Medical, LLC (161010.06) The voice of experience since 1978
Magnetic4
3Closure
The #1 Most Profitable Ancillary Service for Private Practice ENTs
Y our peers wanted to share an update since adding a FYZICAL Balance Center to their ENT practice:

“This is the single best thing we’ve ever done in our practice. We can’t comprehend why an otolaryngologist would not do this. It’s a no-brainer and it’s taking our profession by storm."

ü We're seeing 40 - 4 5 patients per day for balanc e therapy only.
ü Our hearing aid sales have increased 59%.
ü Our total patient visits are up 15% for new patients and 5% for return patients.
ü Our allergy / immunotherapy population has grown 20% since joining.
"We couldn’t be more
our future.
with FYZICAL.
Discover the systems and procedures behind this opportunity. Call 941-227-4314 to register now or learn more about this event.
*Limited seating and only open to those with open territories*
Discover more at www.BusinessofBalance.com
"We can't stress enough how amazing of an opportunity this really is. You will grow every part of your practice through this vehicle."
excited about
Get in touch
You absolutely, positively, 100% need to see for your own eyes what this can do for your practice before it’s too late.”
Explore FYZICAL Balance at OTO Game Changer
Tuscaloosa ENT Group
EDITORIAL BOARD
EDITORIAL BOARD MEMBERS
Editor-in-Chief
Robert T. Sataloff, MD, DMA, FACS
Professor and Chairman, Department of Otolaryngology–Head and Neck Surgery, and Senior Associate Dean for Clinical Academic Specialties, Drexel University College of Medicine Philadelphia, PA
Jean Abitbol, MD
Jason L. Acevedo, MD, MAJ, MC, USA
Jack B. Anon, MD
Gregorio Babighian, MD
Peter C. Belafsky, MD, PhD
Bruce Benjamin, MD
Gerald S. Berke, MD
Michael J. Brenner, MD
Kenneth H. Brookler, MD
Karen H. Calhoun, MD
Steven B. Cannady, MD
Ricardo Carrau, MD
Swapna Chandran, MD
Chien Chen, MD
Dewey A. Christmas, MD
Nicolle T. Clements, MS
Daniel H. Coelho, MD, FACS
David M. Cognetti, MD
James V. Crawford, MD
David H. Darrow, MD, DDS
Rima Abraham DeFatta, MD
Robert J. DeFatta, MD, PhD
Hamilton Dixon, MD
Paul J. Donald, MD, FRCS
Mainak Dutta, MS, FACS
Russell A. Faust, PhD, MD
Ramón E. Figueroa, MD, FACR
Charles N. Ford, MD
Paul Frake, MD
Marvin P. Fried, MD
Richard R. Gacek, MD
Andrea Gallo, MD
Frank Gannon, MD
Emilio Garcia-Ibanez, MD
Soha Ghossani, MD
William P. R. Gibson, MD
David Goldenberg, MD
Jerome C. Goldstein, MD
Richard L. Goode, MD
Samuel Gubbels, MD
Reena Gupta, MD
Joseph Haddad Jr., MD
Missak Haigentz, MD
Christopher J. Hartnick, MD
Mary Hawkshaw, RN, BSN, CORLN
Garett D. Herzon, MD
Thomas Higgins, MD, MSPH
Jun Steve Hou, MD
John W. House, MD
Glenn Isaacson, MD
Steven F. Isenberg, MD
Stephanie A. Joe, MD
Shruti S. Joglekar, MBBS
Raleigh O. Jones, Jr., MD
Petros D. Karkos, MD, AFRCS, PhD, MPhil
David Kennedy, MD
Seungwon Kim, MD
Robert Koenigsberg, DO
Karen M. Kost, MD, FRCSC
Stilianos E. Kountakis, MD, PhD
John Krouse, MD
Ronald B. Kuppersmith, MD, MBA, FACS
Rande H. Lazar, MD
Robert S. Lebovics, MD, FACS
Keat-Jin Lee, MD
Donald A. Leopold, MD
Steve K. Lewis, BSc, MBBS, MRCS
Daqing Li, MD
Robert R. Lorenz, MD
John M. Luckhurst, MS, CCC-A
Valerie Lund, FRCS
Karen Lyons, MD
A.A.S. Rifat Mannan, MD
Alexander Manteghi, DO
Richard Mattes, PhD
Brian McGovern, ScD
William A. McIntosh, MD
Brian J. McKinnon, MD
Oleg A. Melnikov, MD
Albert L. Merati, MD, FACS
Joseph P. Mirante, MD, MBA, FACS
Ron B. Mitchell, MD
Steven Ross Mobley, MD
Jaime Eaglin Moore, MD
Thomas Murry, PhD
Ashli K. O’Rourke, MD
Ryan F. Osborne, MD, FACS
J. David Osguthorpe, MD
Robert H. Ossoff, DMD, MD
Enrique Palacios, MD, FACR
Michael M. Paparella, MD
Kourosh Parham, MD, PhD
Arthur S. Patchefsky, MD
Meghan Pavlick, AuD
Spencer C. Payne, MD
Kevin D. Pereira, MD, MS (ORL)
Nicolay Popnikolov, MD, PhD
Didier Portmann, MD
Gregory N. Postma, MD
Matthew J. Provenzano, MD
Hassan H. Ramadan, MD, FACS
Richard T. Ramsden, FRCS
Gabor Repassy, MD, PhD
Dale H. Rice, MD
Ernesto Ried, MD
Alessandra Rinaldo, MD, FRSM
Joshua D. Rosenberg, MD
Allan Maier Rubin, MD, PhD, FACS
John S. Rubin, MD, FACS, FRCS
Amy L. Rutt, DO
Anthony Sclafani, MD, FACS
Raja R. Seethala, MD
Jamie Segel, MD
Moncef Sellami, MD
Michael Setzen, MD, FACS, FAAP
Douglas M. Sidle, MD
Herbert Silverstein, MD
Jeffrey P. Simons, MD
Raj Sindwani, MD, FACS, FRCS
Aristides Sismanis, MD, FACS
William H. Slattery III, MD
Libby Smith, DO
Jessica Somerville, MD
Thomas C. Spalla, MD
Matthew Spector, MD
Paul M. Spring, MD
Brendan C. Stack, Jr., MD, FACS
James A. Stankiewicz, MD
Jun-Ichi Suzuki, MD
David Thompson, MD
Lester D.R. Thompson, MD, FASCP
Helga Toriello, PhD, FACMG
Ozlem E. Tulunay-Ugur, MD
Galdino Valvassori, MD
Emre Vural, MD
Donald T. Weed, MD, FACS
Neil Weir, FRCS
Kenneth R. Whittemore, MD
David F. Wilson, MD
Ian M. Windmill, PhD
Ian J. Witterick, MD,MSc, FRCSC
Richard J. Wong, MD
Naoaki Yanagihara, MD
Eiji Yanagisawa, MD, FACS
Ken Yanagisawa, MD, FACS
Anthony Yonkers, MD
Mark Zacharek, MD
Joseph Zenga, MD
Liang Zhou, MD
CLINIC EDITORS
Dysphagia
Peter C. Belafsky, MD, PhD
Gregory N. Postma, MD
Facial Plastic Surgery
Anthony P. Sclafani, MD, FACS
Geriatric Otolaryngology
Kourosh Parham, MD, PhD, FACS
Karen M. Kost, MD, FRCSC
Head and Neck
Ryan F. Osborne, MD, FACS
Paul J. Donald, MD, FRCS
Reena Gupta, MD
Imaging
Enrique Palacios, MD, FACR
Ramón E. Figueroa, MD, FACR
Laryngoscopic
Robert T. Sataloff, MD, DMA, FACS
Otoscopic
John W. House, MD
Brian J. McKinnon, MD
Pathology
Lester D.R. Thompson, MD, FASCP
Pediatric Otolaryngology
Rande H. Lazar, MD
Rhinoscopic
Eiji Yanagisawa, MD, FACS
Dewey A. Christmas, MD
Joseph P. Mirante, MD, MBA, FACS
Ken Yanagisawa, MD, FACS
Special Topics
Robert T. Sataloff, MD, DMA, FACS
Thyroid and Parathyroid
David Goldenberg, MD
330 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
Balloon dilation for Eustachian tube dysfunction
The superior, durable results your patients deserve.
With the safety you can count on.
A randomized, controlled trial comparing balloon dilation with ongoing medical therapy as treatment for persistent Eustachian tube dysfunction reported zero complications and significant symptom improvement through 12 months for patients treated with the XprESS™ ENT Dilation System.1
dilation SUPERIOR to medical management
1 Meyer TA, O’Malley E, Schlosser RJ, et al. A randomized controlled trial of balloon dilation as a treatment for persistent Eustachian tube dysfunction with 1-year follow-up. Otol Neurotol. 2018. DOI: 10.1097/
MAO.0000000000001853
INDICATIONS FOR USE: To access and treat the maxillary ostia/ethmoid infundibula in patients 2 years and older, and frontal ostia/recesses and sphenoid sinus ostia in patients 12 years and older using a transnasal approach. The bony sinus outflow tracts are remodeled by balloon displacement of adjacent bone and paranasal sinus structures. To dilate the cartilaginous portion of the Eustachian tube for treating persistent Eustachian tube dysfunction in patients 18 years and older using a transnasal approach.
Please see Instructions for Use (IFU) for a complete listing of warnings, precautions, and adverse events as well as cleaning, sterilizing and care for surgical instruments.

CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician.
ENTELLUS MEDICAL and XPRESS are trademarks of Entellus Medical, Inc.
DURABLE symptom improvement through 12 months
NEW CLINICAL DATA Read more at: go.ent.stryker.com/ETDRCT1YearData
©2018 Entellus Medical, Inc. 1738-762 rA 07/2018
Balloon Dilation (N=28) Control (N=27) Baseline (N=54) 6 Weeks (N=51) 3 Months (N=52) 6 Months (N=51) 12 Months (N=49) 1 2 3 4 5 6 7 0 1 2 3 4 5 6 7 4.6 2.1 2.12.1 2.1 MILD (NO PROBLEM) MODERAT E SEVERE M EAN OVERALL ETDQ-7 SCORE MEAN OVERALL ETDQ-7 SCORE ∆= -2.9±1.4 ∆= -0.6±1.0 p<0.0001 p<0.0001
follow-up periods BaselineFollow-up Baseline Follow-up ET balloon
Balloon Dilation (N=28) Control (N=27) Baseline (N=54) 6 Weeks (N=51) 3 Months (N=52) 6 Months (N=51) 12 Months (N=49) 1 2 3 4 5 6 7 0 1 2 3 4 5 6 7 4.6 2.1 2.12.1 2.1 MILD (NO PROBLEM) MODERAT E SEVERE M EAN OVERALL ETDQ-7 SCORE MEAN OVERALL ETDQ-7 SCORE ∆= -2.9±1.4 ∆= -0.6±1.0 p<0.0001
periods BaselineFollow-up Baseline Follow-up ET balloon
for change from baseline to all
p<0.0001 for change from baseline to all follow-up
dilation with XprESS is SAFE
0 % COMPLICATION RATE
Editor-in-Chief Robert T. Sataloff, MD, DMA, FACS 219 N. Broad St., 10th Fl., Philadelphia, PA 19107 entjournal@phillyent.com Ph: 215-732-6100
Managing Editor Linda Zinn
Manuscript Editors Martin Stevenson and Wayne Kuznar
Associate Editor, Reader Engagement Megan Combs
Creative Director Eric Collander
National Sales Manager Mark C. Horn mhorn@vendomegrp.com Ph: 480-895-3663
Traffic Manager Eric Collander
Please send IOs to adtraffic@vendomegrp.com
All ad materials should be sent electronically to: https://vendome.sendmyad.com
Customer Service/Subscriptions
www.entjournal.com/subscribe Ph: 888-244-5310 email: VendomeHM@emailpsa.com
Reuse Permissions Copyright Clearance Center info@copyright.com Ph: 978-750-8400 Fax: 978-646-8600
Chief Executive Officer Jane Butler
Chief Marketing Officer Dan Melore
Vice President, Finance Bill Newberry
Vice President, Custom Media Jennifer Turney Director, Circulation Rachel Beneventi
ENT-Ear, Nose & Throat Journal (ISSN: Print 0145-5613, Online 1942-7522) is published 9 times per year in Jan/Feb, Mar, Apr/May, June, July, Aug, Sept, Oct/ Nov and Dec, by Vendome Group, LLC, 237 West 35th Street, 16th Floor, New York, NY 10001-1905.
©2018 by Vendome Group, LLC. All rights reserved. No part of ENT-Ear, Nose & Throat Journal may be reproduced, distributed, transmitted, displayed, published, or broadcast in any form or in any media without prior written permission of the publisher. To request permission to reuse this content in any form, including distribution in education, professional, or promotional contexts or to reproduce material in new works, please contact the Copyright Clearance Center at info@ copyright.com or 978.750.8400.
EDITORIAL: The opinions expressed in the editorial and advertising material in this issue of ENT-Ear, Nose & Throat Journal are those of the authors and advertisers and do not necessarily reflect the opinions or recommendations of the publisher, editors, or the staff of Vendome Group, LLC. ENT-Ear, Nose & Throat Journal is indexed in MEDLINE/PubMed and Current Contents/Clinical Medicine and Science Citation Index Expanded. Editorial offices are located at 812 Huron Rd., Suite 450, Cleveland, OH 44115. Manuscripts should be submitted online at www.editorialmanager.com/entjournal. Instructions for Authors are available at www.entjournal.com

SUBSCRIPTIONS: For questions about a subscription or to subscribe, please contact us by phone: 888-244-5310; or email: VendomeHM@emailpsa.com Individual subscriptions, U.S. and possessions: 1 year $225, 2 years $394; International: 1 year $279, 2 years $488; Single copies $28; outside the U.S., $40.
POSTMASTER: send address changes to Ear, Nose & Throat Journal, PO Box 11404 Newark, NJ 07101-4014.
ENT-Ear,
332 www.entjournal.com
Nose & Throat Journal October-November 2018
ADVERTISER
Pages Arbor Pharmaceuticals, LLC ......... 351, 352 CANT Corp. 337 Carl Zeiss 343 Compulink .................................. 368, CVR3 Entellus Medical ............................ 331, 369 Fyzical ........................................... 329, 370 InHealth Technologies CVR2 Interacoustics 333 Lumenis ................................................. 371 McKeon Products............... 345, 372, CVR4 Medtronic 335, 341 Novartis 355, 356 Optim LLC 373 Reliance Medical ................................... 374 Sinoptim ................................................ 375 Spectrum Audiology 339 Stryker 376
INDEX

ORIGINAL ARTICLES
349 Clinical outcome of revision cartilage tympanoplasty
Pei-Yin Wei, MD; Chia-Hui Chu, MD, MPH; Mao-Che Wang, MD, PhD
354 Persistent local demucosalization after endoscopic sinus surgery: A report of 3 cases
Conner J. Massey, MD; Menka M. Sanghvi, MD; Thomas R. Troost, MD, PhD; Vijay K. Anand, MD; Ameet Singh, MD
362 Desmoid tumors of the head and neck: Two decades in a single tertiary care unit and review of the literature
Aleksi Schrey, MD, PhD; Maria Gardberg, MD, PhD; Riitta Parkkola, MD, PhD; Ilpo Kinnunen, MD, PhD
ONLINE EXCLUSIVES
E1 Invasive fungal rhinosinusitis: An Indian perspective
Swati Agrawal, MS, DNB; Sandeep Arora, MS, DNB; Nishi Sharma, MS, DNB
E7 Anxiety and depression in patients with sudden one-sided hearing loss
Fatih Arslan, MD; Emre Aydemir, MD; Yavuz Selim Kaya, MD; Hasan Arslan, MD; Abdullah Durmaz, MD
E11 Serum levels of oxidative stress indicators and antioxidant enzymes in Bell palsy
Nazim Bozan, MD; Ömer Faruk Kocak, MD; Canser Yılmaz Demir, MD;
Mehmet Emre Dinc, MD; Koray Avcı, MD; Halit Demir, PhD; Ahmet Faruk Kıroglu, MD
E15 Paraneoplastic syndrome or metastatic sinonasal neuroendocrine carcinoma?
Clinical conundrum
Panagiotis Kerezoudis, MD;
Patrick R. Maloney, MD; Brandon McCutcheon, MD, MPP; Jeffrey Janus, MD; Mark Jentoft, MD; Timothy Kaufmann, MD; Daniel Honore Lachance, MD;
Jamie J. Van Gompel, MD; Mohamad Bydon, MD
E19 Risk of developing sudden sensorineural hearing loss in patients with hepatitis B virus infection: A population-based study
Yao-Te Tsai, MD; Ku-Hao Fang, MD; Yao-Hsu Yang, MD, MSc; Meng-Hung Lin, PhD; Pau-Chung Chen, MD, PhD; Ming-Shao Tsai, MD; Cheng-Ming Hsu, MD
E28 Primary parapharyngeal leiomyosarcoma: A case report
Luca Giovanni Locatello, MD; Jano Maria De Cesare, MD; Cecilia Taverna, MD; Oreste Gallo, MD
E32 A rare case of coexisting lacrimal sac adenocarcinoma and transitional cell carcinoma
Tsutomu Nomura, MD, DDS, PhD; Daisuke Maki, MD; Fumihiko Matsumoto, MD, PhD; Taisuke Mori, DDS, PhD; Seichi Yoshimoto, MD, PhD
E36 Long-term use of Le Fort I osteotomy for the management of nasopharyngeal rhinosporidiosis: A case series
Vikram Shetty, DNB; Akshaya Kulkarni, MDS; Suman Banerjee, MDS
E44 The effect of zoledronic acid on middle ear osteoporosis: An animal study
Ozgur Surmelioglu, MD; Feyha Kahya Aydogan, MD; Suleyman Ozdemir, MD; Ozgur Tarkan, MD; Aysun Uguz, MD; Ulku Tuncer, MD; Lutfi Barlas Aydogan, MD
334 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018 EDITORIAL OFFICE Robert T. Sataloff, MD, DMA, FACS, Editor-in-Chief • 219 N. Broad St., 10th Fl. • Philadelphia, PA 19107 CONTENTS OCTOBER-NOVEMBER 2018 • VOL. 97, NO. 10-11
DEPARTMENTS 332 Advertiser Index 336 ENT Journal Online 338 Guest Editorial 340 Otoscopic Clinic 342 Imaging Clinic 346 Pathology Clinic 347 Thyroid and Parathyroid Clinic E49 Laryngoscopic Clinic E51 Rhinoscopic Clinic
IRRIGATION. SUCTION. SYNCHRONIZATION.
Now there’s a better way to provide saline washes. The HydroCleanse™ Sinus Wash Delivery System from Medtronic combines pressurized sinus irrigation with builtin suction capability for a more efficient procedure. Disposable and easy to use, it allows you to irrigate the sinuses while limiting pooling and effluent discharge.
§ Disposable and easy to use
§ 360° fan spray
§ Pressurized saline irrigation
§ Built-in suction capability
§ Choice of angled catheter tips
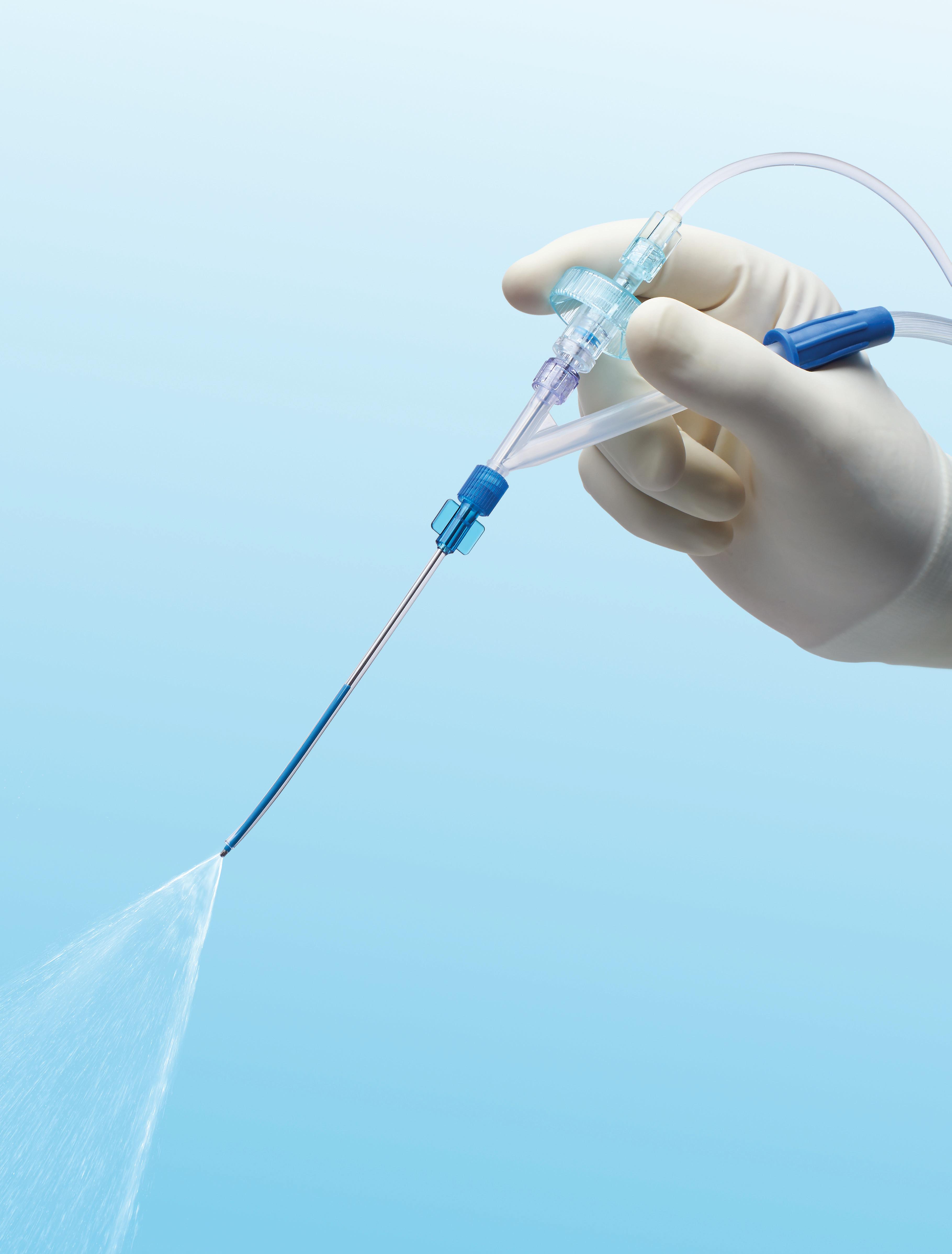
HydroCleanse™ Sinus Wash Delivery System
IMPROVE THE SALINE WASH EXPERIENCE. CHOOSE HYDROCLEANSE.
Rx only. Refer to product instruction manual/package insert for instructions, warnings, precautions and contraindications.
For further information, please call Medtronic ENT at 800.874.5797 or consult Medtronic’s website at www.medtronicent.com
© 2018 Medtronic. All rights reserved. Medtronic, Medtronic logo and Further, Together are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. UC201902765 EN 07.2018
JOURNAL ONLINE
Ear, Nose & Throat Journal's website is easy to navigate and provides readers with more editorial content each month than ever before. Access to everything on the site is free of charge to physicians and allied ENT professionals. To take advantage of all our site has to offer, go to www.entjournal. com and click on the “Registration” link. Once you have filled out the brief registration form, you will have full access. Explore and enjoy!
ONLINE EXCLUSIVES
Invasive fungal rhinosinusitis: An Indian perspective
Swati Agrawal, MS, DNB; Sandeep Arora, MS, DNB; Nishi Sharma, MS, DNB
Invasive fungal rhinosinusitis (IFRS) is a fatal disease of the nose and paranasal sinuses that typically affects immunocompromised patients. Data on this disorder, which is rare and difficult to diagnose, are lacking in the literature. We collected comprehensive data from 9 patients (7 males, 2 females) with a mean age of 34 years (range: 6 to 58) with IFRS who were treated at our center and examined the factors associated with successful treatment. The parameters examined were patient demographics, disease characteristics, clinical course including surgical and medical therapy, treatment, fungal species involved, and long-term survival....
Anxiety and depression in patients with sudden one-sided hearing loss
Fatih Arslan, MD; Emre Aydemir, MD;
Yavuz Selim Kaya, MD; Hasan Arslan, MD; Abdullah Durmaz, MD
Sudden sensorineural hearing loss is a hearing loss of >30 dB in at least three consecutive frequencies that occurs in 3 days. The aim of this study was to investigate anxiety and depression caused by sudden, idiopathic, one-sided hearing loss. The levels of anxiety and depression in patients with this type of hearing loss were determined using the Beck Anxiety Scale (BAS) and the Beck Depression Inventory (BDI) at the time of the patient’s first visit. In total, 56 patients (32 men and 24 women) with a mean age of 32.8 ± 9.9 years (range: 20 to 58 years) were selected as the patient group and 45 individuals without symptoms of anxiety and depression....
Serum levels of oxidative stress indicators and antioxidant enzymes in Bell palsy
Nazim Bozan, MD; Ömer Faruk Kocak, MD;
Canser Yılmaz Demir, MD; Mehmet Emre Dinc, MD; Koray Avcı, MD; Halit Demir, PhD;
Ahmet Faruk Kıroglu, MD
We conducted a prospective study to comparatively evaluate serum levels of malondialdehyde, an oxidative stress indicator, and the antioxidant enzymes glutathione, catalase, and superoxide dismutase in patients with Bell palsy. Our study population was made up of 30 patients with Bell palsy—15 men and 15 women, aged 25 to 68 years (mean: 50.4)—who were seen in the Department of Otorhinolaryngology at a....
Paraneoplastic syndrome or metastatic sinonasal neuroendocrine carcinoma? Clinical conundrum
Panagiotis Kerezoudis, MD; Patrick R. Maloney, MD; Brandon McCutcheon, MD, MPP; Jeffrey Janus, MD; Mark Jentoft, MD; Timothy Kaufmann, MD; Daniel Honore Lachance, MD; Jamie J. Van Gompel, MD; Mohamad Bydon, MD
We report a case of a middle-aged woman with a diffuse, nonenhancing, progressively atrophic T2-hyperintense lesion involving the left frontotemporal lobes and insula found to be synchronous high-grade sinonasal neuroendocrine carcinoma (SNEC) after initial endonasal resection. In 2014, a 47-year old woman underwent resection of a left-sided high-grade ethmoidal neuroendocrine carcinoma after....
Risk of developing sudden sensorineural hearing loss in patients with hepatitis B virus infection: A population-based study
Yao-Te Tsai, MD; Ku-Hao Fang, MD; Yao-Hsu Yang, MD, MSc; Meng-Hung Lin, PhD; Pau-Chung Chen, MD, PhD; Ming-Shao Tsai, MD; Cheng-Ming Hsu, MD
Sudden sensorineural hearing loss (SSNHL) has significant impact on quality of life. It may result from viral infection, but the relationship between hepatitis B virus (HBV) infection and SSNHL remains uncertain. To investigate the risk of developing SSNHL in patients with HBV, we conducted a nationwide, population-based, retrospective cohort study from the Taiwan National Health Insurance Research Database. A total of 33,234 patients diagnosed with HBV infection and 132,936 control subjects without viral hepatitis....
Primary parapharyngeal leiomyosarcoma: A case report
Luca Giovanni Locatello, MD; Jano Maria De Cesare, MD; Cecilia Taverna, MD; Oreste Gallo, MD
Leiomyosarcoma is a rare malignant soft-tissue tumor whose cells resemble smooth-muscle tissue. It has been reported to arise in different areas of the head and neck region. Primary leiomyosarcoma of the parapharyngeal space, however, is extremely rare, as only 4 cases have been previously reported to date. We describe the somewhat urgent case of a primary leiomyosarcoma of the right parapharyngeal space in a 30-year-old man. We also review the diagnostic and therapeutic challenges that clinicians....
336 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018 www.entjournal.com
A rare case of coexisting lacrimal sac adenocarcinoma and transitional cell carcinoma
Tsutomu Nomura, MD, DDS, PhD; Daisuke Maki, MD; Fumihiko Matsumoto, MD, PhD;
Taisuke Mori, DDS, PhD; Seichi Yoshimoto, MD, PhD
Lacrimal sac tumors are rare and difficult to diagnose. We present a case of coexisting lacrimal sac adenocarcinoma and transitional cell carcinoma in a 73-year-old woman who presented with swelling of the inner canthus. Biopsy identified the growth as an adenocarcinoma. After dissection...
Long-term use of Le Fort I osteotomy for the management of nasopharyngeal rhinosporidiosis: A case series
Vikram Shetty, DNB; Akshaya Kulkarni, MDS; Suman Banerjee, MDS
Rhinosporidiosis is a rare, chronic, granulomatous infection of the mucous membranes that mainly involves the nose and nasopharynx; it occasionally involves the pharynx, conjunctiva, larynx, trachea and, rarely, the skin. The characteristic clinical features of this disease include the formation of painless polyps in the nasal mucosa or the nasopharynx that bleed easily on touch. At our center, excision of the lesion with a Le Fort I osteotomy is carried out in patients....
The effect of zoledronic acid on middle ear
osteoporosis: An animal study
Ozgur Surmelioglu, MD; Feyha Kahya Aydogan, MD; Suleyman Ozdemir, MD; Ozgur Tarkan, MD; Aysun Uguz, MD; Ulku Tuncer, MD; Lutfi Barlas Aydogan, MD
Hearing function in older patients may be related to bone structure. We conducted an experiment to evaluate the effect of zoledronic acid on osteoporotic middle ear ossicles in an animal model. Our subjects were 19 female New Zealand....
ONLINE DEPARTMENTS
Laryngoscopic Clinic: Oropharyngeal histoplasmosis in an HIV-negative patient
Mohamedkazim Alwani, MD; Todd J. Wannemuehler, MD; Don-John Summerlin, DMD, MS; Marion E. Couch MD, PhD, FACS
Rhinoscopic Clinic: Endoscopic view of a posterior nasal and nasopharyngeal vascular plexus Dewey A. Christmas, MD; Joseph P. Mirante, MD, MBA, FACS; Eiji Yanagisawa, MD, FACS
Reach More Patients.
People come in different shapes and sizes.The same piece of equipment does not fit them all.Make oropharyngeal surgery easier with a simple extension.The CANT Corporation has created the Dedo Extension that fits between the Mayo Stand and the Crowe-Davis mouth gag so that it can be adjusted to fit larger patients.The DE98-A mounts to the square-sided Mayo Stand,while the DE98-B fits the tubular-sided support. The Dedo Extension - a simple but effective solution.
• All oropharyngeal procedures using a Crowe-Davis mouth gag

Volume 97, Number 10-11 www.entjournal.com 337
ENT JOURNAL ONLINE
The Dedo Extension
The Crowe-Davis mouth gag
The
Mayo Stand
T&A’s
Uvuloplasty
Palatoplasty
Applicable Procedures •
•
•
337 • 233 • 2666, ext.9 • www.jrcant.com • PO Box 3522 • Lafa yette,LA 70502 Extension “DE98-B” also available for tubular support Extension “DE98-A” A B Dedo Ext 9 (ENT1/18/05) 1/19/05 1:51 PM Page 1
GUEST EDITORIAL
Two sides of the same coin
Many years ago, I opened an issue of the U.S. Naval Institute’s Proceedings Magazine. In it I found a column entitled, “Nobody asked me, but….” The intent of the column was to provide a venue for officers, often junior officers, to give commentary on current naval practice or policy. I am certain other publications had similar columns, but what struck me was that the column allowed for constructive, honest, and public critique of an organization, the U.S. Navy, that was not outwardly thought of as being inwardly reflective.
I don’t feel that our profession has the same reputation of not being inwardly reflective, although I can’t recall our profession having a readily available venue for constructive, honest, and public critique. With that in mind, I began to gather my thoughts on the challenges with which otolaryngologists must contend.

As background, I currently practice at Philadelphia ENT Associates, with an academic appointment at the Drexel University College of Medicine. My previous experience has been as a physician in the military, the Veterans Administration, academics, and private practice. My formal education in pursuit of my MBA and MPH also shape what I wish to focus on in this editorial: achieving financial sustainability and achieving economic sustainability
Currently, our practices face tremendous financial stress. Those in the public sector and those in academics are under the greater financial stress. The public sector and academic practices historically have not been prudent managers of their resources; academic practices have been particularly poor in achieving consistent financial sustainability. These practices form the safety net of our healthcare system, as well as the core of the resident and fellow training system.
With increasing enrollment in health exchanges and Medicaid, and facing reimbursement based on outcomes, many of these practices are ill equipped for the business challenges of reduced reimbursement combined with the increased cost of improving care. Academic programs that are financially unsustainable often are left to limp on—an “always sick, never dead” approach. It is intellectually difficult for these programs to institute the most basic revenue and cost-management systems, despite often having very fine business schools affiliated with the parent educational institution. They view themselves as “good stewards” of their patients’ health but fail to realize that stewardship requires the maintenance of a financially sustainable healthcare system. Prudent financial management can markedly improve disease management.
The issue for private practice is somewhat different, in my opinion. The challenge for private practice is to achieve a business that is economically sustainable within the community. Private practices, if not financially viable, will close. Generally, they have fairly good business processes in place, unlike many academic practices. The threat to these practices is the perception that the reporting requirements for MACRA (Medicare Access and CHIP Reauthorization Act of 2015) and MIPS (Merit-Based Incentive Payment System) are insurmountable for smaller groups; or worse, that the cost of reporting successfully is more expensive than the penalties.
If private practices begin to avoid participation in MACRA and MIPS, the lack of quality reporting will ultimately harm all of ENT, not just private
338 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
practice. Reimbursement will be based on the data returned through MACRA and MIPS; poor-quality data, or data based on a very narrow segment of physicians, will adversely impact the “value” assigned to the care otolaryngologists provide.
Further, in their drive for margin, these practices may lose sight of another important fact that is critical to economic sustainability. The otolaryngologist’s charge to the community is a social contract; failing to respect that social contract, with its benefits and obligations, by practicing in a manner no longer economically sustainable within the community, will result in the loss of the economic benefits gained by the social contract. The community is not just those patients who are cared for by the practice, but it also includes fellow otolaryngologists regionally and nationally. Narrowly defined interests usually result in similarly limited achievements.
While our Academy and specialty societies deserve much credit for the resources they have placed in their members’ hands, these resources could make an assertive and concerted effort to educate the public sector and academic practices on their respective fiscal and economic responsibilities and help them develop and use the skills in responsible stewardship of their roles.
Further, our Academy and specialty societies could make an assertive and concerted effort to educate those in private practice on the long-term importance of quality reporting and the need to engage with the Academy, so that the Academy can better provide guidance for successful participation and provide feedback to the public, public officials, and private/public payers on the burden current reporting requirements place on private practice. These actions by the Academy and specialty societies would serve to remind those in academics what their stewardship requires, and those in private practice of the benefits and obligations of their social contract with the community. Such action is essential to the continued high-quality care of our patients, and to the future success of our specialty.
 Brian J. McKinnon, MD, MBA, MPH, FACS Associate Professor and Vice Chair
Department of Otolaryngology–
Head and Neck Surgery Associate Professor
Brian J. McKinnon, MD, MBA, MPH, FACS Associate Professor and Vice Chair
Department of Otolaryngology–
Head and Neck Surgery Associate Professor
Department of Neurosurgery
Drexel University College of Medicine
Philadelphia
Volume 97, Number 10-11 www.entjournal.com 339 GUEST EDITORIAL
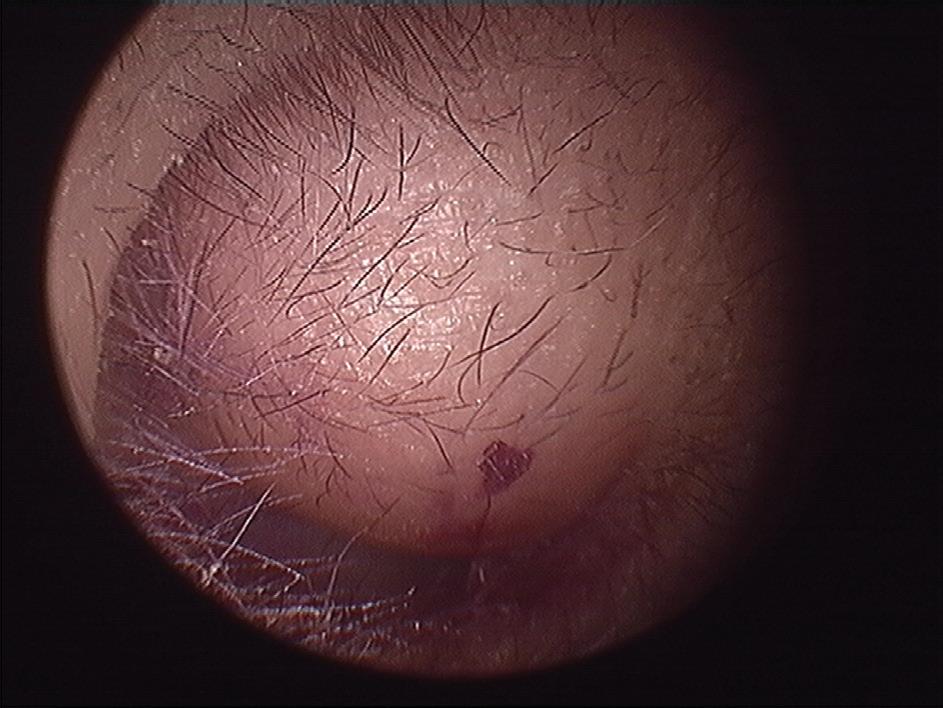
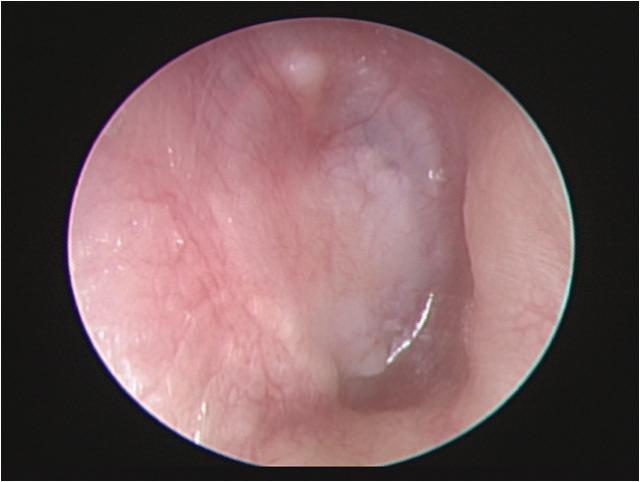
Iatrogenic external auditory canal cholesteatoma with mastoid erosion
A 70-year-old man who had undergone postauricular underlay myringoplasty to treat chronic otitis media 10 years earlier presented with a 6-month history of hearing impairment, aural fullness, and occasional otorrhea of the right ear. Otoscopy revealed a large, bulging mass on the posterosuperior aspect of the external auditory canal (EAC); the tympanic membrane was invisible (figure 1). Computed tomography of the temporal bone revealed a right-sided, 2 × 2-cm soft-tissue mass in the EAC, with erosion of mastoid air cells but a normal eardrum and middle ear cavity.
Considering the postsurgical history and the site of the mass, we diagnosed an iatrogenic EAC cholesteatoma. A whitish, spherical mass was identified on surgical exploration and was completely removed through the prior postauricular incision (figure 2). We subsequently performed canalplasty with EAC
defect reconstruction using fragments of conchal cartilage. The pathologist’s report revealed histopathologic features compatible with cholesteatoma. No evidence of recurrent disease was present during the 5-year follow-up.
Iatrogenic EAC cholesteatoma is a rare complication developing after myringoplasty. The precise incidence of the disease remains unknown. We speculate that inversion or malpositioning of a tympanomeatal flap, or unintentional implantation of epithelium during the prior surgery, causes the subsequent defect.1,2
Small postsurgical EAC inclusion cysts or cholesteatomas are common and can be treated in the office via “unroofing” of the cyst and good ear hygiene practices.3 However, large, complicated iatrogenic EAC cholesteatomas usually require surgical management. The technique chosen is generally based on the extent of the disease and the surgeon’s preference, and it ranges
Continued on page 344
340 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
From the School of Medicine, National Taiwan University, Taiwan (Dr. Lee); the School of Medicine, Chang Gung University, Taiwan (Dr. Hu, Dr. Chuang, and Dr. Chan); the Division of Otology, Department of Otolaryngology–Head and Neck Surgery (Dr. Hu and Dr. Chan); and the Department of Anatomic Pathology (Dr. Chuang), Chang Gung Memorial Hospital, Linkou, Taiwan.
Yen-Hui Lee, MD; Chih-Yu Hu, MD; Wen-Yu Chuang, MD; Kai-Chieh Chan, MD
OTOSCOPIC CLINIC
Figure 1. Otoscopy displays a large, bulging mass in the posterosuperior aspect of the external auditory canal.
WITH YOU, FOR YOU.
Partnership between Medtronic and surgeons like you, working together to advance navigation technology and protect patients — over 20 years strong — has resulted in some of the most advanced ENT technology available.
You can continue to trust navigation surgical solutions from Medtronic, such as the StealthStation™ ENT, to increase your operative efficiency and precision. Let’s advance further, together.
Rx only. Refer to product instruction manual/package insert for instructions, warnings, precautions and contraindications.
For further information, please call Medtronic ENT at 800.874.5797 or consult Medtronic’s website at www.medtronicent.com
© 2018 Medtronic. All rights reserved. Medtronic, Medtronic logo and Further, Together are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. UC201902149a EN 09.2018
IMAGING CLINIC
Eagle syndrome: Transient ischemic attack and subsequent carotid dissection
Thomas Sullivan, MD; Jordan Rosenblum, MD
A 62-year-old man initially presented to our institution 3 months before his final diagnosis with transient right-sided weakness and brief loss of consciousness after reaching across his body in the shower. His medical history was significant for hypertrophic cardiomyopathy and atrial fibrillation. He had an implantable cardiac device and was on lifelong anticoagulation. Head computed tomography (CT) and CT angiography (CTA) were unremarkable, and although a transient ischemic attack (TIA) was thought to be unlikely given the anticoagulation therapy, this was a working diagnosis based on his symptoms, which were otherwise unexplained, and he was sent home.
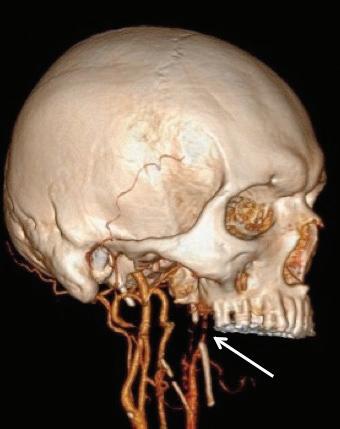
The patient returned 3 months later with sudden-onset behavioral changes, disorganized speech, and right-sided weakness. He reported no specific activity related to symptoms. CTA demonstrated complete left internal carotid occlusion at the level of the styloid tip, 1-cm cranial to the bifurcation, which extended to the carotid terminus (figure 1). Review of imaging obtained at his presentation 3 months earlier demonstrated a normal carotid.
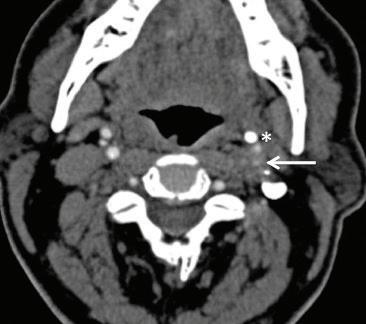
CT of the head obtained at the most recent visit demonstrated new anterior, middle, and posterior circulation infarcts. In addition, we observed not only that his left styloid process was elongated (3.0 cm), but also that his stylohyoid ligament was calcified to the hyoid and possibly fractured (figure 2). We speculate that the fracture might have acted as a lead-point, causing injury
and subsequent dissection of the patient’s carotid. The contralateral styloid process was within normal limits, but the ligament was also calcified.

Prophylactic contralateral styloidectomy was considered, but the patient was deemed a poor surgical candidate given his underlying cardiomyopathy and anticoagulation. After further review of imaging, we felt his right carotid was a safe distance from the right styloid and ligament. As the left carotid was occluded to the skull base, he was not offered revascularization. We speculate that our patient’s initial presentation
From the Department of Radiology, Loyola University Medical Center, Maywood, Ill.
342 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
A B
Figure 1. A: 3-D reconstruction of the patient’s CTA with soft-tissue subtraction demonstrates the elongated and calcified stylohyoid ligament and dissected carotid artery cranial to the ligament (arrow). B: Additional 3-D reconstruction of the CTA with bone and soft tissue removed further demonstrates the dissected left internal carotid artery (arrow).
Boosting Effi ciency.

ZEISS EXTARO 300
INNOVATION
EXTARO® 300 from ZEISS allows you to save valuable time before, during and after each surgical intervention with:












































• Augmented Visualization

• Single-Handed Operation
• Digital Data Management


We understand. Future.
www.zeiss.com/ent/extaro-300
//
MADE BY ZEISS
EN_30_030_0092I SUR.10455
might have been a harbinger of his impending cerebrovascular accident.
Eagle Syndrome is an uncommon but well-described entity with a nonspecific clinical presentation; more benign manifestations include globus sensation, dysphagia, facial neuralgias, throat pain, and cranial neuropathies, for which a differential is extensive.1,2 In the presence of an elongated styloid bone or stylohyoid, diagnostic consideration is often given to Eagle syndrome, but it may have a more insidious presentation. Elongation of the styloid also has been reported as a cause for symptomatic carotid disease, including TIA, Horner syndrome, eye pain, and cluster headache.3 Dissection associated with elongated styloids has been reported in the neurology literature,4 but to the best of our knowledge, no prior reports have demonstrated TIA with a normal carotid and subsequent carotid dissection during two separate clinical presentations.
Surgical and nonsurgical treatments for Eagle syndrome have been reported. Medical treatments, including carbamazepine, local and systemic steroids, and NSAIDS have been used.4 Surgical options include transpharyngeal resection, as originally favored by Eagle, versus an extraoral resection.

References
1. Eagle WW. Elongated styloid process: Report of two cases. Arch Otolaryngol 1937;25(5):584-7.
2. Werhun EL, Weidenhaft MC, Palacios E, Neitzschman H. Stylohyoid syndrome, also known as Eagle syndrome: An uncommon cause of facial pain. Ear Nose Throat J 2014;93(9):384-5.
3. Infante-Cossio P, Garcia-Perla A, Gonzáles-Garcia A, et al. Compression of the internal carotid artery due to elongated styloid process [in Spanish]. Rev Neurol 2004;39(4):339-43.
4. Zuber M, Meder JF, Mas JL. Carotid artery dissection due to elongated styloid process. Neurology 1999;53(8):1886-7.
Continued from page 340
from canalplasty with skin grafting for lesions confined to EAC, to canalplasty or canal-wall-up mastoidectomy with reconstruction of canal defects for lesions involving mastoid cells, to canal-wall-down mastoidectomy for lesions with large wall defects exhibiting mastoid erosion.4 If an EAC defect is to be reconstructed, as in our case, nonretractable materials such as cartilage make ideal grafts.
Myringoplasty is a commonly used and simple type of middle ear surgery associated with a low risk of postoperative, iatrogenic EAC cholesteatoma. However, it is important to ensure high accuracy when repositioning the skin flap. In addition, delicate and meticulous management of every step is essential to avoid trapping or implantation of epithelial debris under the skin flap or graft.
Our case emphasizes the need for long-term follow-up because the average disease latency after otologic surgery is usually more than one decade.1,5
Approval of this case study was obtained from the Institutional Review Board of Chang Gung Memorial Hospital.
References
1. Sweeney AD, Hunter JB, Haynes DS, et al. Iatrogenic cholesteatoma arising from the vascular strip. Laryngoscope 2017;127(3):698-701.
2. Persaud R, Hajioff D, Trinidade A, et al. Evidence-based review of aetiopathogenic theories of congenital and acquired cholesteatoma. J Laryngol Otol 2007;121(11):1013-19.
3. Holt JJ. Ear canal cholesteatoma. Laryngoscope 1992;102(6):608-13.
4. Viswanatha B. External auditory canal cholesteatoma: A rare complication of tympanoplasty. Ear Nose Throat J 2009;88(11): 1206-9.
5. Cronin SJ, El-Kashlan HK, Telian SA. Iatrogenic cholesteatoma arising at the bony-cartilaginous junction of the external auditory canal: A late sequela of intact canal wall mastoidectomy. Otol Neurotol 2014;35(8):e215-21.
344 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018 OTOSCOPIC CLINIC IMAGING CLINIC
Figure 2. A spherical cholesteatoma sac is discovered during surgical exploration.
Figure 2. Axial CT shows the dissected internal carotid artery (arrow) and calcified stylohyoid ligament (asterisk).




PATHOLOGY CLINIC
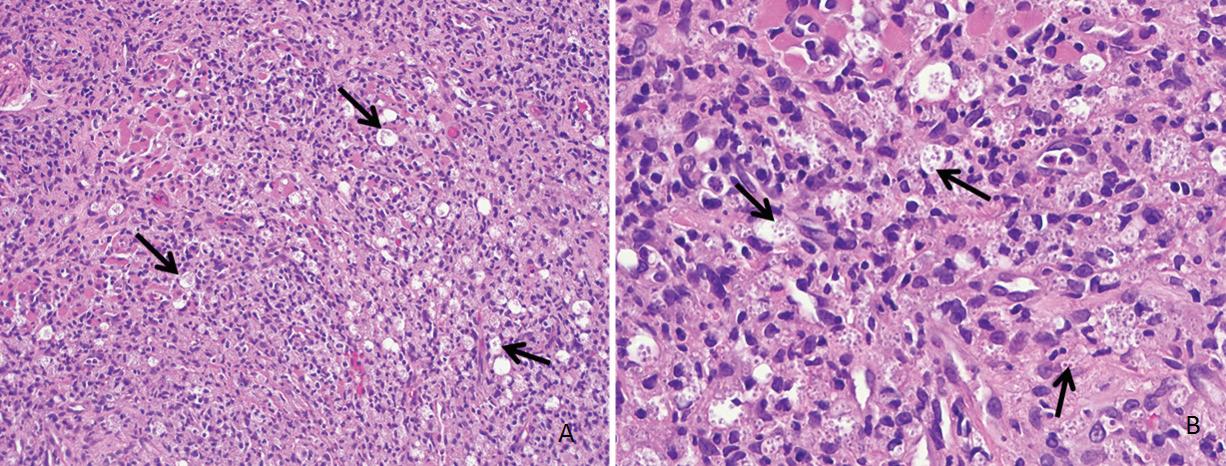
Primary cutaneous histoplasmosis
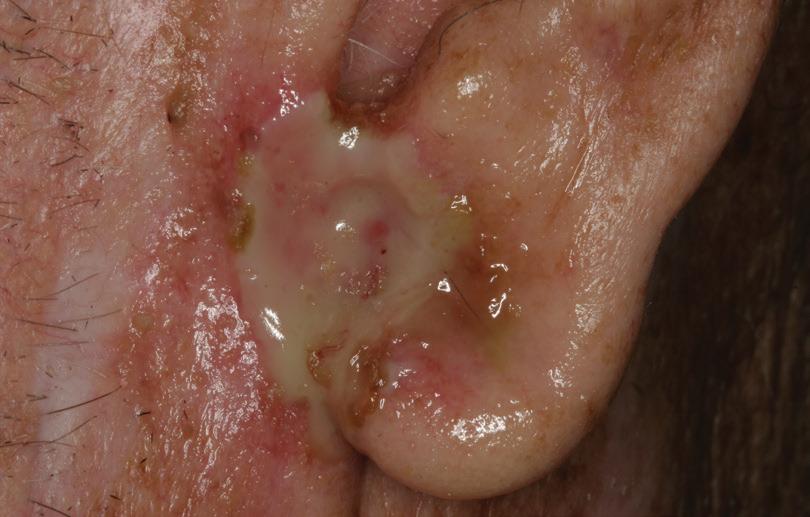
Blake Raggio, MD
An 80-year-old farmer with arthritis, anemia, chronic kidney disease, and a history of shingles presented with a 3-month history of a painful, nonhealing, ulcerative pinna lesion refractory to antibiotics and local wound care (figure 1). He reported a subsequent development of similar ulcerative lesions involving the oral tongue and floor of his mouth.
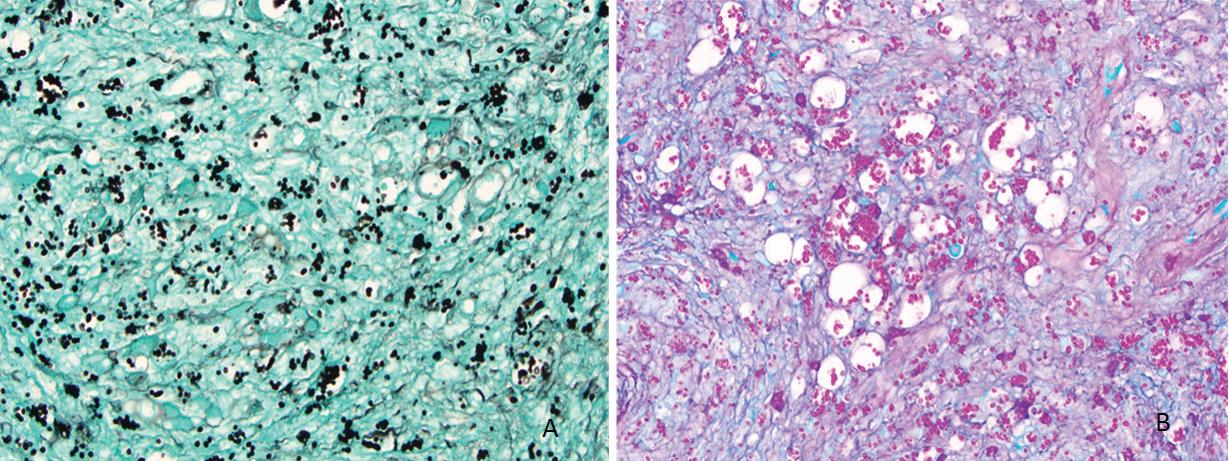
Biopsy of the pinna and oral cavity revealed diffuse, mixed inflammatory infiltrate and numerous epithelioid histiocytes containing small, circular yeast cells compatible with Histoplasma capsulatum (figure 2). Special stains for fungal organisms, including Gomori methenamine silver (GMS) and periodic acid–Schiff (PAS) were strongly positive (figure 3).
The patient had no clinical or laboratory findings to suggest systemic fungal infection, so oral itraconazole was initiated for treatment of primary cutaneous histoplasmosis with dissemination to the oral cavity. Despite a promising initial clinical response, the medication was withdrawn several weeks into therapy because of the patient’s worsening renal function. Hemodialysis was initiated; however, the patient’s health progressively deteriorated, and he passed away a few months later from renal-, heart-, and liver-associated complications.
H capsulatum is a soil saprophyte endemic to the Ohio and Mississippi River valleys, often found in chicken habitats or caves inhabited by bats and birds. Infection by inhalation of aerosolized H capsulatum microconidia primarily affects the lungs of immunosuppressed individuals, although a variety of clinical presentations exist.1 Histoplasmosis of the skin, a less common presentation, occurs either secondarily via systemic infection that disseminates to the skin, or primarily via direct inoculation of H capsulatum during injury to the skin.1,2 The latter disease process, termed primary cutaneous histoplasmosis (PCH), rarely presents in the head and neck and is poorly described in the otolaryngologic literature.3
Patients with PCH present with nonspecific skin lesions including papules, plaques, ulcers, purpura, abscesses, impetigo, or dermatitis.4 Diagnosis hinges on evidence of fungus in the wound and absence of systemic disease. Tissue biopsy of the cutaneous lesion should be performed promptly. Microscopy may reveal fungal elements and granuloma formation, but histopathology showing any distinctive 2- to 4-µm, oval, narrow-based budding yeasts secures the diagnosis (figure 2).
Continued on page 348
346 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
From the Department of Otolaryngology, Tulane University Medical Center; and the Department of Otolaryngology, Ochsner Health System, New Orleans. The case described in this article occurred at the University of Florida Medical Center, Gainesville. This article has been edited and adapted from its presentation as a poster at the American Academy of Otolarayngology–Head and Neck Surgery Annual Meeting; Sept. 10-13, 2017; Chicago.
Figure 1. Photo shows the erosive, ulceronodular lesion of the left pinna and preauricular skin.
THYROID AND PARATHYROID
CLINIC
Huge primary leiomyosarcoma of the thyroid gland
Yong Tae Hong, MD; Ki Hwan Hong, MD
Primary leiomyosarcoma (LMS) of the thyroid gland is a rare thyroid malignancy, accounting for only 0.014% of primary thyroid tumors.1 It has an aggressive clinical course with a low survival rate. Diagnosis of LMS is challenging as it has a pathologic resemblance to other thyroid tumors. Therefore, immunohistochemical staining is essential for diagnosis.
There are 26 previously reported cases of primary thyroid LMS.2,3 Of the 26 patients, 14 died within 6 months due to the disease; only 7 lived >1 year, and only 1 patient survived for >5 years.3
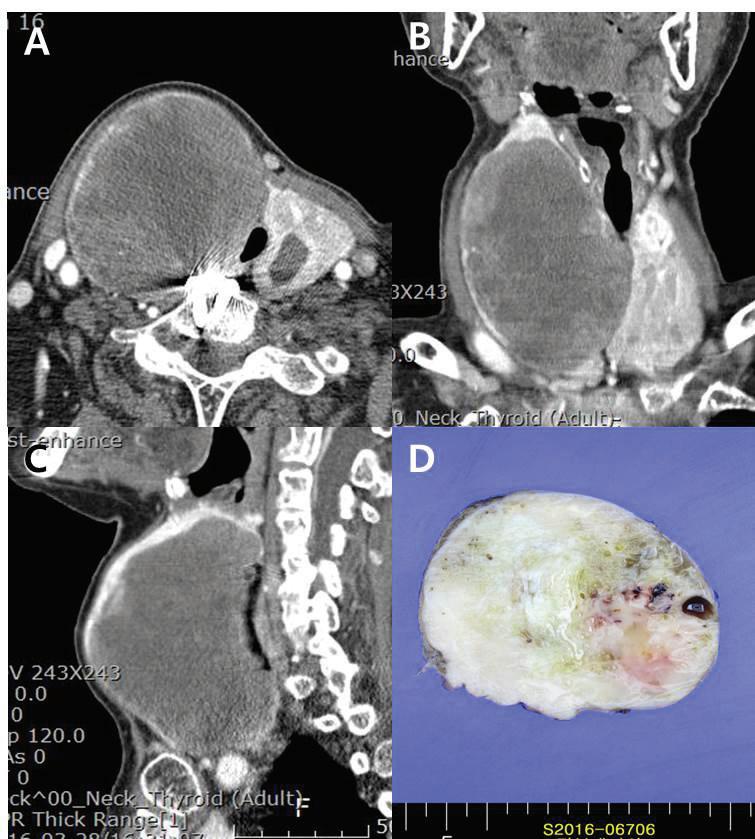
An 83-year-old woman who presented with aggravated dyspnea over a period of 1 month was admitted to our tertiary referral hospital. She had a history of Graves disease with a multinodular goiter involving the thyroid gland and had been receiving medical treatment with methimazole. On physical examination, a hard, protruding mass was noted in the anterior neck over the right thyroid cartilage. The cartilage was shifted toward the left side due to mass effect. Computed tomography (CT) with enhancement of the patient’s neck showed a huge (10 cm) mass in the right thyroid with luminal narrowing of the upper trachea due to mass effect (figure 1).
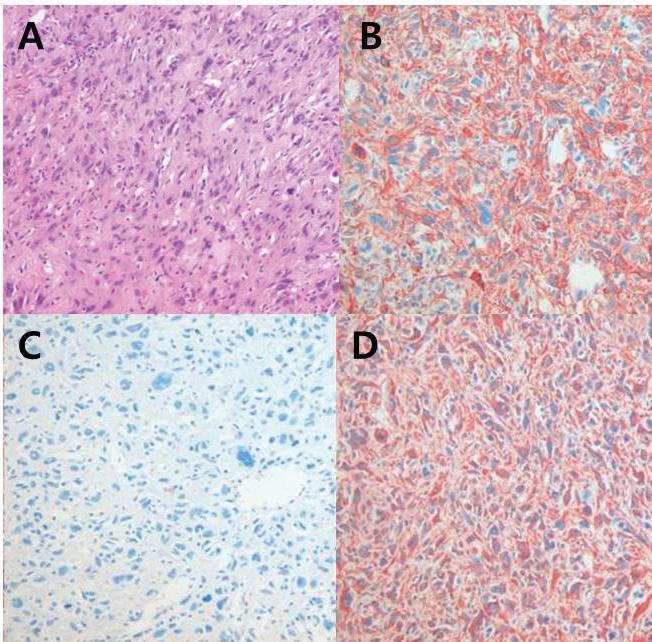
The patient underwent total thyroidectomy. Pathologic examination showed the mass to be positive for vimentin, cytokeratin, and actin, and negative for TTF-1, thyroglobulin, CD34, and EMA (figure 2). Therefore, the diagnosis of primary thyroid LMS was conclusive.
Considering the patient’s age and general condition, no other treatment was planned. At follow-up 12 months after surgery, the patient was still alive with no evidence of recurrence.
The etiology of primary LMS of the thyroid gland is not clear, although some studies have emphasized that the site of origin may be smooth muscles in blood vessels within the capsule of the thyroid.3 It has also been proposed that LMS may originate as a result of smooth-muscle metaplasia from a previously existing anaplastic carcinoma of the thyroid.4,5 There is also a reported case of an LMS of the thyroid associated
Volume 97, Number 10-11 www.entjournal.com 347
From the Department of Otolaryngology–Head and Neck Surgery, Research Institute for Clinical Medicine, Chonbuk National University, Chonbuk, Korea.
Figure 1. A-C: Contrast-enhanced neck CT images show the 10-cm heterogeneous mass with rim calcification on the right thyroid gland. The trachea is deviated to the left. D: An excised surgical specimen shows a homogeneous and partially cystic change.
with Epstein–Barr virus in a child with congenital immunodeficiency.6
There are several treatment options for LMS. In many reported cases, thyroid lobectomy or total thyroidectomy with or without neck dissection has been the first choice for treatment. Although surgery is the most commonly accepted treatment for the disease, no treatment has shown effectiveness. However, it is difficult to make an exact diagnosis of LMS before surgery and to distinguish it from anaplastic thyroid cancer, especially the spindle-cell variant of anaplastic thyroid cancer.1,2
References
1. Tanboon Jantima, Phawin Keskool. Leiomyosarcoma: A rare tumor of the thyroid. Endo Pathol 2013;24:136-43.
2. Chetty R, Clark SP, Dowling JP. Leiomyosarcoma of the thyroid: Immunohistochemical and ultrastructural study. Pathology 1993;25:203-5.
3. Zou ZY. Primary thyroid leiomyosarcoma: A case report and literature review. Oncology Letters 2016;11:3982-6.
4. Tran LM, Mark R, Meier R, et al. Sarcomas of the head and neck: Prognostic factors and treatment strategies. Cancer 1992;70:169–77.
5. Akcam T, Oysul K, Birkent H. Leiomyosarcoma of the head and neck: Report of two cases and review of the literature. Auris Nasus Larynx 2005;32:209–12.
6. Just PA, Guillevin R, Capron F. An unusual clinical presentation of a rare tumor of the thyroid gland: Report on one case of leiomyosarcoma and review of literature. Ann Diag Path 2008;12:50–6.
Continued from page 346
GMS and PAS stains (figure 3) help identify H capsulatum in macrophages and within the tissue. Cultures showing small, oval, budding yeast can be used to confirm the diagnosis, but their results often take several weeks. Antigen detection is a sensitive method used for ruling out disseminated disease.1-3
Treatment of PCH depends on the extent and severity of the disease. Topical amphotericin B and nystatin can be used for limited disease, while systemic amphotericin B and itraconazole often are reserved for more widespread lesions.5 Incision and drainage may be necessary for lesions refractory to medical therapy.6
References
1. Wheat LJ, Azar MM, Bahr NC, et al. Histoplasmosis. Infect Dis Clin North Am 2016;30(1):207-27.
2. Tesh RB, Schneidau JD Jr. Primary cutaneous histoplasmosis. N Engl J Med 1966;275(11):597-9.
3. Kollipara R, Hans A, Hall J, Watson K. A case report of primary cutaneous histoplasmosis requiring deep tissue sampling for diagnosis. Dermatol Online J 2014;20(11). pii: 13030/ qt/11b53041.
4. Chang P, Rodas C. Skin lesions in histoplasmosis. Clin Dermatol 2012;30(6):592-8.
5. Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007;45(7):807-25.
6. Buitrago MJ, Gonzalo-Jimenez N, Navarro M, et al. A case of primary cutaneous histoplasmosis acquired in the laboratory. Mycoses 2011;54(6):e859-61.
348 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
Figure 2. Hematoxylin and eosin (H & E)-stained sections demonstrate numerous vacuolated areas (arrows) surrounded by a diffuse, mixed inflammatory infiltrate (A; original magnification ×20) and numerous epithelioid histiocytes with small, circular yeast cells (arrows), characteristic of H capsulatum (B; original magnification ×40).
Figure 3. GMS (A) and PAS (B) stains highlight numerous aggregates and randomly distributed organisms of H capsulatum (original magnification ×40).
A A B B
Figure 2. Hematoxylin and eosin staining shows a spindle-cell tumor arranged in a fascicular pattern with eosinophilic cytoplasm (A). Immunohistochemical staining of the tumor shows a positive reaction to smooth muscle actin (B), cytokeratin (C), and vimentin (D) (original magnification ×200).
PATHOLOGY CLINIC THYROID AND PARATHYROID CLINIC
Clinical outcome of revision cartilage tympanoplasty
Pei-Yin Wei, MD; Chia-Hui Chu, MD, MPH; Mao-Che Wang, MD, PhD
Abstract
We retrospectively reviewed 32 ears from 30 adult patients with chronic otitis media who underwent revision tympanoplasty using cartilage graft (performed by a single surgeon) from January 10, 2011, to May 10, 2016. All procedures were performed using an endaural incision for both temporalis fascia graft and tragal cartilage graft harvesting. The overall surgical success rate was 93.3%. The average preoperative hearing level was 43.1 ± 17.3 dBHL, and the average postoperative hearing level was 39.2 ± 18.2 dBHL, representing a significant improvement. The average air-bone gap was 19.4 ±7.6 dB preoperatively and 16.9 ± 9.9 dB postoperatively. Also of note, the improvement in air-bone gap reached the level of significance at 500 Hz (p = 0.023). We conclude that using cartilage graft in revision tympanoplasty is a safe and reliable technique with good surgical outcomes. Using one single endaural incision for both fascia and cartilage harvesting is simple while achieving aesthetic wound healing.
Introduction
Tympanoplasty is a commonly performed surgery for the management of chronic otitis media with the aim of preserving the tympanic membrane without infection and restoring the sound-conducting mechanism. Since it was first introduced by Wullstein in 19521 and Zollner in 1955,2 several grafting materials have been described including temporalis fascia, perichondria, periostia, vein, fat, and cartilage.3-5
To date, the temporalis fascia and perichondrium are still the grafting material mainstays in primary tympanoplasty, with a success rate ranging from 35 to 95%.6,7 However, fascia and perichondrium appear to undergo atrophy and subsequently fail in the reconstruction of high-risk perforations,8 such as total or
From the Department of Otorhinolaryngology–Head and Neck Surgery, Taipei Veterans General Hospital, Taipei, Taiwan (Dr. Wei, Dr. Chu, and Dr. Wang); and the School of Medicine, National Yang-Ming University, Taipei (Dr. Chu and Dr. Wang).
Corresponding author: Mao-Che Wang, MD, No. 201, Sec. 2, Shipai Rd., Beitou District, Taipei City, Taiwan 11217, R.O.C. Email: wangmc@vghtpe.gov.tw
subtotal atelectatic perforations, those with cholesteatoma, and revision cases. In these situations, cartilage tympanoplasty is being used increasingly due to its stability, resistance to negative middle ear pressure, and resistance to infections due to the lack of vascularization.9 Compared with fascia graft, similar functional results (hearing improvement) but better morphologic results (intact eardrum) have been reported with the use of cartilage tympanoplasty.10
One of the possible risk factors of failed tympanoplasty is persistent negative middle ear pressure, in which fascia and perichondrium grafting might not be sufficiently robust. Therefore, in revision surgery, cartilage graft may be preferred because of its rigidity, a property that may promote resistance to negative middle ear pressure.
The aim of this study was to analyze the anatomic and audiologic results of revision cartilage tympanoplasty.
Patients and methods
From January 10, 2011, to May 10, 2016, we analyzed 30 adult patients (32 ears) with chronic otitis media who underwent revision cartilage tympanoplasty; all surgeries were performed by a single surgeon. Patients with cholesteatoma or younger than 20 years were excluded.
By retrospective chart review, we recorded each patient’s sex, age at operation, perforation size (small: perforation up to one-third of the tympanic membrane; medium: perforation between one-third and twothirds of the tympanic membrane; large: perforation more than two-thirds of the tympanic membrane), preoperative and postoperative pure-tone audiograms (500, 1,000, 2,000, and 4,000 Hz, respectively), and surgical success.
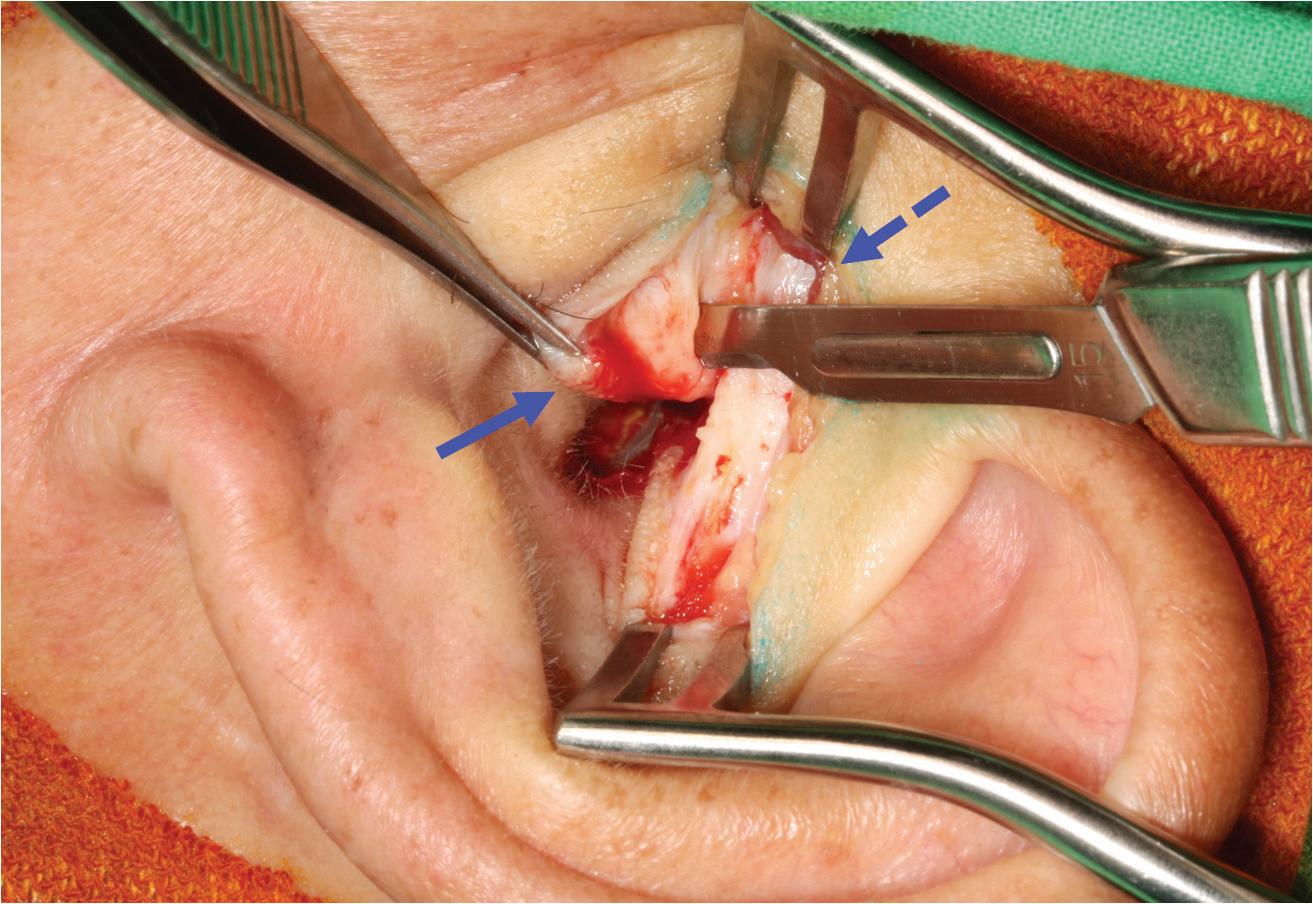
All patients underwent revision cartilage tympanoplasty according to the following protocol. First, an endaural incision was made to facilitate temporalis fascia graft harvesting. Second, the medial part of the tragus was harvested from the same incision and cut into halves to be used as the cartilage graft. Third, after refreshing the perforation edge and elevating the tympanomeatal flap, an underlay grafting technique
Volume 97, Number 10-11 www.entjournal.com 349 ORIGINAL ARTICLE
with a temporalis fascia graft and cartilage reinforcement was performed (figure 1). No patient underwent simultaneous ossiculoplasty.
Surgery was considered successful if no residual perforation of the eardrum was present. Postoperative pure-tone audiometry was measured 3 months after surgery. The average hearing level was defined as the mean air-conduction threshold at 500, 1,000, 2,000, and 4,000 Hz.
A paired t test was performed to determine the difference between preoperative and postoperative pure-tone average and air-bone gaps. Statistical significance was assumed for p < 0.05. Statistical analysis was performed with the Statistical Package for the Social Sciences for Windows, v. 19.0.
This study was approved by the Institutional Review Board of Taipei Veterans General Hospital.
Results
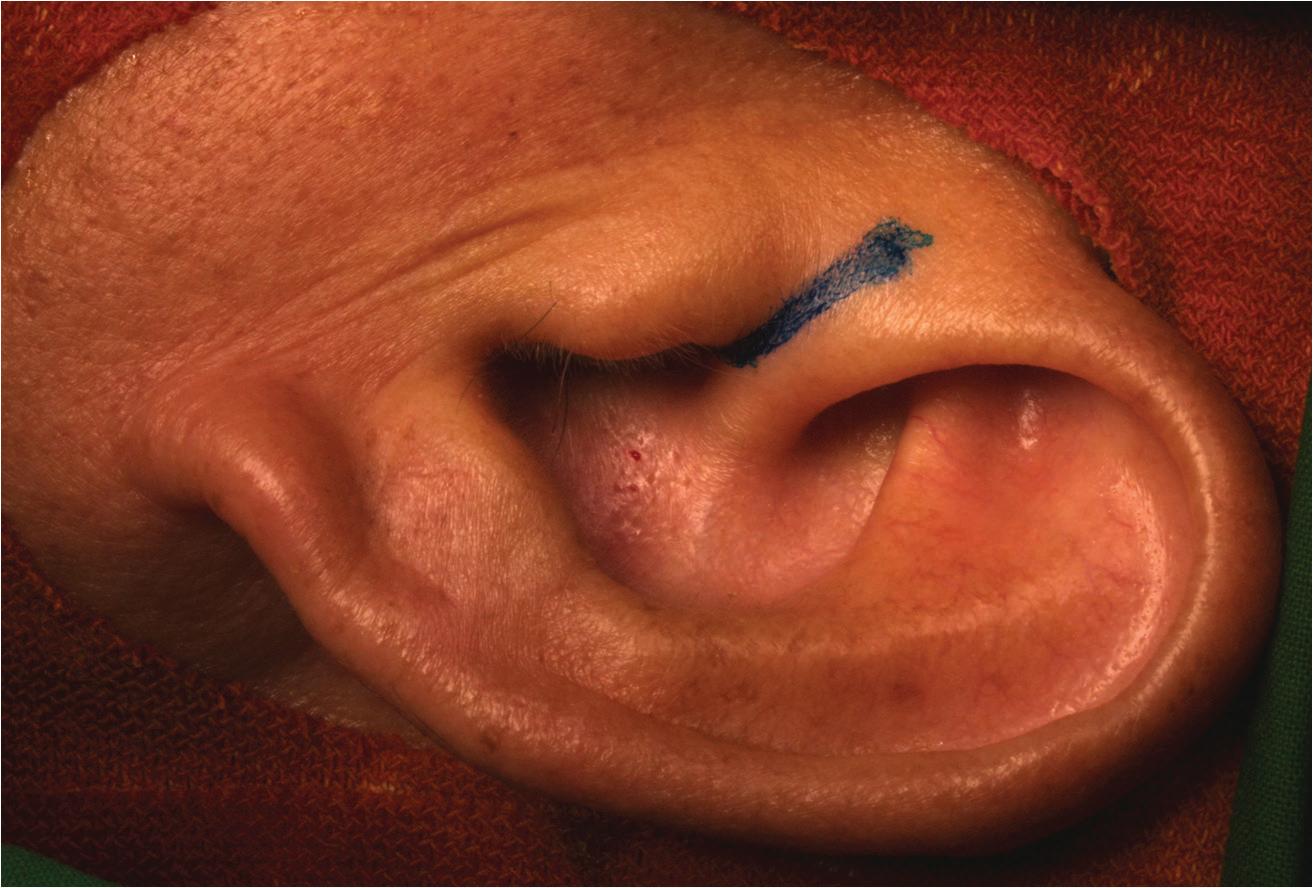
A total of 30 patients (17 men and 13 women) (32 ears) with a mean age of 52 years (range: 20 to 74) were enrolled. The mean number of previous tympanoplasties was 1.18 (range: 1 to 3); perforation size was small in 14 (43.8%) ears, medium in 13 (40.6%) ears, and large in 5 (15.6%) ears, and included 2 total perforations. The average preoperative air-bone gap was 19.4 ± 7.6 dB. The mean follow-up time was 7 months (range: 1 to 45), and the overall surgical success rate was 93.3%. An image obtained 9 months postoperatively is shown in figure 2.
Audiologic outcomes. The average hearing level was 43.1 ± 17.3 dBHL preoperatively and 39.2 ± 18.2 dBHL postoperatively. The change between preoperative and postoperative hearing level was significant (p = 0.029) (table 1). The average air-bone gap declined from 19.4 ± 7.6 dB preoperatively to 16.9 ± 9.9 dB postoperatively, a
trend toward improvement that did not reach statistical significance (p = 0.196) (table 2).
Both the change in hearing level and air-bone gap reached a level of significant improvement at 500 Hz (tables 1 and 2).
Discussion
Use of the cartilage graft was first described in middle ear surgery in 1959,11 and it has been used in the management of high-risk perforations more recently due to its robust and rigid characteristics. In revision tympanoplasty, eustachian tube dysfunction and negative middle ear pressure are the possible causes of previous failure.
Compared with fascia and perichondrium grafts, cartilage graft proved to be a superior choice in revision surgery because of its superior resistance to negative middle ear pressure.
Although primary tympanoplasty has a high success rate, the graft-take rate decreases in revision tympano-
350 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018 WEI, CHU, WANG,
Figure 2. In this image, an intact eardrum without residual perforation can be seen on otoscopy 9 months postoperatively.
Figure 1. These photographs show elements of the single endaural incision approach. A: An endaural incision is made. B: Both the temporalis fascia (dashed arrow) and tragal cartilage (arrow) grafts are harvested with a single incision.
For the treatment of AOMT in pediatric patients due to S. aureus, S. pneumoniae, H. influenzae, M. catarrhalis, and P. aeruginosa.
The difference is in the delivery.
The first and only antibiotic/steroid combination ear drop in single-dose vials
• Manufactured using blow-fill-seal technology— each vial is formed, filled, and sealed in a continuous, automated, sterile operation2,3

• Technology minimizes human intervention in the fill/finish process3

• Single-use vials contain 1 premeasured dose each—dose BID/7 days4












• No drop counting. No mixing or shaking required4



• Demonstrated efficacy and safety in 2 clinical trials1,5 Order
INDICATIONS
OTOVEL® (ciprofloxacin and fluocinolone acetonide) is indicated for the treatment of acute otitis media with tympanostomy tubes (AOMT) in pediatric patients (aged 6 months and older) due to S. aureus, S. pneumoniae, H. influenzae, M. catarrhalis, and P. aeruginosa.
IMPORTANT SAFETY INFORMATION
Contraindications
OTOVEL is contraindicated in:





• Patients with known hypersensitivity to fluocinolone acetonide or other corticosteroids, ciprofloxacin or other quinolones, or to any other component of OTOVEL.
• Viral infections of the external ear canal, including varicella and herpes simplex infections and fungal otic infections.
The following Warnings and Precautions have been associated with OTOVEL: hypersensitivity reactions, potential for microbial overgrowth with prolonged use, and continued or recurrent otorrhea.
The most common adverse reactions are otorrhea, excessive granulation tissue, ear infection, ear pruritis, tympanic membrane disorder, auricular swelling, and balance disorder.
For additional Important Safety Information, please see Brief Summary of Prescribing Information on adjacent page, and full Prescribing Information available at www.otovel.com.
References: 1. Otovel [package insert]. Atlanta, GA: Arbor Pharmaceuticals, LLC. 2016. 2. Data on file. Arbor Pharmaceuticals, LLC. 3. Guidance for industry: sterile drug products produced by aseptic processing—current good manufacturing practice. Food and Drug Administration. https://www.fda.gov/downloads/Drugs/Guidances/ucm070342.pdf.
2016. 5. Spektor Z, Pumarola P, Ismail K, et al. Efficacy and safety of ciprofloxacin plus fluocinolone in otitis media with tympanostomy tubes in pediatric patients: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2017;143(4):341-349.
Otovel is a registered trademark of Laboratorios Salvat, S.A. with the US Patent and Trademark Office and under license by Arbor Pharmaceuticals, LLC. Trademarks are the property of their
September 2004.
March 15, 2018. 4. Orange Book:
products
US Food and Drug Administration. https://www.accessdata.fda.gov/scripts/
May 17, 2013.
July 15,
Published
Accessed
Approved drug
with therapeutic equivalence evaluations.
cder/ob/default.cfm. Updated
Accessed
respective owners. © 2018 Arbor Pharmaceuticals, LLC. All rights reserved. Printed in USA. PP-OTO-US-0268
1
Starter Packs today at otovel.com/hcp/resources
AOMT=acute otitis media with tympanostomy tubes; BID=twice daily.
*Statistically significant.
Key: SD = standard deviation.
plasty. Lesinskas and Stankeviciute reported successful closure rates of primary and revision tympanoplasty of 93.6 and 90.2%, respectively.12 Additionally, the grafttake rates of revision tympanoplasty were 90.9% for temporal fascia, 82.4% for perichondrium, and 93.8% for perichondrium/cartilage as graft material, without a statistically significant difference between them.
Sismanis et al reported a 93.5% graft-take rate with the use of cartilage shield grafts in revision tympanoplasty,13 and Boone et al reported a 94.7% graft-take rate with tragal cartilage–perichondrium island graft or palisaded concha cymba cartilage.14
In our study, the overall success rate was 93.3%, which was comparable to that obtained in previous studies. Residual perforation was found in 2 patients, 1 with a small and 1 with a large preoperative perforation. Neither size of perforation nor number of previous tympanoplasties correlated with surgical success. Possible explanations for surgical failure in these 2 cases were anterior marginal perforation in 1 patient, which was a relative surgical challenge compared with central perforation, and granulation with fibrosis in the middle ear cavity of the other patient, which may imply previous infectious status.
Several techniques for cartilage tympanoplasty have been described, such as cartilage palisade tympanoplasty, cartilage shield graft, perichondrium/cartilage island flap, cartilage butterfly inlay technique, and cartilage reinforcement tympanoplasty.13,15-17
In the present study, we used a single endaural incision for both temporalis fascia and tragal cartilage harvesting. The advantages of this method are that only one incision was needed and there was no deformity of the tragus postoperatively. To our knowledge,
this surgical technique has not been reported previously.
We showed a statistically significant improvement in the mean postoperative hearing level, from 43.1 ± 17.3 dBHL preoperatively to 39.2 ± 18.2 dBHL postoperatively, but not in the mean postoperative air-bone gap, which declined from 19.4 ± 7.6 dB preoperatively to 16.9 ± 9.9 dB postoperatively. However, changes in the hearing level and in the air-bone gap at 500 Hz both improved significantly (42.8 ± 15.5 dBHL to 32.6 ± 18.0 dBHL, and 18.6 ± 11.2 dB to 11.1 ± 14.5 dB, respectively). One possible explanation is that sound transmission was affected because of an increased mass and stiffness of the cartilage graft, especially at high frequency.
Using laser Doppler vibrotomy, Zahnert et al showed that cartilage should not exceed 0.5 mm in thickness to minimize the energy loss in sound transmission.18 Through the use of finite element analysis, Lee et al reported that the optimal thickness of cartilage graft was 0.1 to 0.2 mm for medium (55% perforation) and large perforations (85% perforation), and less than 1.0 mm for small perforations (15% perforation).19 Sound transmission properties at different frequencies require further investigation.
The limitation of the present study was the relatively small sample size.
Conclusion
Revision cartilage tympanoplasty is a safe and reliable technique with both an excellent morphologic result and good audiologic outcome at low frequency. A novel single endaural incision approach was advantageous for both fascia and cartilage harvesting and also achieved aesthetic wound healing.
*Statistically significant. Key: SD = standard deviation.
Volume 97, Number 10-11 www.entjournal.com 353 CLINICAL OUTCOME Of REvISION CARTILAGE TYMPANOPLASTY
Frequency (Hz) Hearing level (dB) ± SD p Value Preoperative Postoperative 500 42.8 ± 15.5 32.6 ± 18.0 0.001* 1,000 41.3 ± 16.2 37.3 ± 18.1 0.103 2,000 40.3 ± 20.0 38.4 ± 19.6 0.387 4,000 47.8 ± 25.8 48.4 ± 22.1 0.786 Average 43.1 ± 17.3 39.2 ± 18.2 0.029*
Table 1. Comparison of preoperative and postoperative hearing level
Frequency (Hz) Air-bone gap (dB) ± SD p Value Preoperative Postoperative 500 18.6 ± 11.2 11.1 ± 14.5 0.023* 1,000 19.7 ± 11.9 18.1 ± 8.6 0.537 2,000 17.7 ± 9.4 18.1 ± 8.6 0.859 4,000 21.8 ± 10.1 19.3 ± 10.4 0.320 Average 19.4 ± 7.6 16.9 ± 9.9 0.196
Table 2. Comparison of preoperative and postoperative air-bone gap
Continued on page 361
Persistent local demucosalization after endoscopic sinus surgery: A report of 3 cases
Conner J. Massey, MD; Menka M. Sanghvi, MD; Thomas R. Troost, MD, PhD; Vijay K. Anand, MD; Ameet Singh, MD
Abstract
Mucosal preservation is paramount to achieving successful outcomes after endoscopic sinus surgery (ESS). Despite best surgical practices and implementation of evidenced-based postoperative care, patients in rare cases might exhibit persistent demucosalization that is recalcitrant to conservative therapies. We retrospectively reviewed the records of 3 patients—a 63-year-old woman, a 67-year-old woman, and a 43-year-old man—who experienced clinically significant local demucosalization after uncomplicated ESS despite routine surgical and postoperative management. We collected data on the characteristics of presentation, wound management strategies, and postoperative care practices. Two patients achieved remucosalization with mechanical debridement, gelatin sponge placement, and intensive moisturization therapy. Our experience suggests that surgical debridement of these chronic, persistent demucosalized wounds may be an effective management strategy for patients who develop this unusual and rare postoperative complication. Biopsy and culture of the persistently demucosalized wound bed may be useful in recognizing the presence of worrisome disease processes and identifying any tenacious infectious agents so that more appropriate therapy can be initiated if necessary.
Introduction
Endoscopic sinus surgery (ESS) is a primary treatment modality for patients with chronic rhinosinusitis (CRS) whose disease is refractory to maximal medical management. ESS functions to aerate the paranasal sinuses and remove polyps, fungus, and debris that can impede mucociliary clearance, all while attempting to preserve normal mucosal tissue as much as possible. In fact, it has been shown repeatedly that mucosal preservation is paramount to achieving successful outcomes after ESS.1,2 Failure to adequately preserve the nasal and sinus mucosa during ESS has been associated with a number of complications that can result in treatment failure, such as adhesions, persistent crusting, osteoneogenesis, osteitis, postoperative chronic sinusitis, and ostial stenosis.3
Mucosal preservation and wound healing in general depend on several surgical and nonsurgical factors. Surgical factors primarily include surgical technique, the type of adjunctive biomaterials used (if any), routine postoperative surgical debridement, and patient compliance with wound care. Nonsurgical factors that can negatively impact wound healing consist of systemic conditions such as diabetes, immunodeficiencies, primary ciliary dyskinesia, and cystic fibrosis. Other factors include exposure to tobacco smoke and cytotoxic medications, and the presence of entrenched bacterial or fungal colonization.4
From the Department of Otolaryngology, University of Colorado School of Medicine, Aurora (Dr. Massey); the Department of Ophthalmology (Dr. Sanghvi) and the Division of Otolaryngology (Dr. Troost and Dr. Singh), George Washington University Hospital, Washington, D.C.; and the Department of Otolaryngology–Head and Neck Surgery, Weill Cornell School of Medicine, New York City (Dr. Anand). The cases described in this article occurred at the George Washington University Hospital and the Weill Cornell School of Medicine.
Corresponding author: Conner J. Massey, MD, Department of Otolaryngology, University of Colorado School of Medicine, 12631 E. 17th Ave., B-205, Aurora, CO 80045. Email: conner.massey@ ucdenver.edu
By and large, patients who undergo ESS with an exacting surgical technique, judicious debridement of the postoperative surgical cavity, proactive wound care, and an absence of deleterious host factors tend to experience excellent wound-healing outcomes. Most such patients experience complete remucosalization by 4 to 6 weeks postoperatively. Histologic animal studies have confirmed this timeline, showing that respiratory mucosa is restored by the 28th day after injury.5 However, there are unusual cases in which patients fail to heal as expected despite a confluence of optimal factors.
354 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018 ORIGINAL ARTICLE
A clear choice for EAR-RESISTIBLE patients
It’s a clear choice ear after ear
CIPRODEX® Otic is the #1 prescribed antibiotic eardrop of ENTs and pediatricians.1



It has been prescribed for AOE and AOMT since 2003, with more than 30 million prescriptions filled.1,2
AOE, acute otitis externa; AOMT, acute otitis media with tympanostomy tubes. Not an actual patient.
INDICATIONS AND USAGE
CIPRODEX® Otic is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in:
• Acute Otitis Media (AOM) in pediatric patients (age ≥ 6 months) with tympanostomy tubes due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa
• Acute Otitis Externa (AOE) in pediatric (age ≥ 6 months), adult and elderly patients due to Staphylococcus aureus and Pseudomonas aeruginosa
DOSAGE
• CIPRODEX® Otic is for otic use only, and not for ophthalmic use, or for injection.
• The recommended dosage is four drops into the affected ear twice daily for seven days.
IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS
• CIPRODEX® Otic is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components in this medication.
• Use of this product is contraindicated in viral infections of the external canal including herpes simplex infections and fungal otic infections.
IMPORTANT SAFETY INFORMATION (CONT) WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions: CIPRODEX® Otic should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones.
Potential for Microbial Overgrowth with Prolonged Use: Prolonged use of CIPRODEX® Otic may result in overgrowth of nonsusceptible bacteria and fungi. If the infection is not improved after one week of treatment, cultures should be obtained to guide further treatment. If such infections occur, discontinue use and institute alternative therapy.
Continued or Recurrent Otorrhea: If otorrhea persists after a full course of therapy, or if two or more episodes occur within six months, further evaluation is recommended to exclude an underlying condition such as cholesteatoma, foreign body, or a tumor.
ADVERSE REACTIONS
The most commonly reported adverse reactions in clinical trials were:
• AOM pediatric patients with tympanostomy tubes: ear discomfort (3.0%), ear pain (2.3%), ear residue (0.5%), irritability (0.5%) and taste perversion (0.5%).
• AOE patients: ear pruritus (1.5%), ear debris (0.6%), superimposed ear infection (0.6%), ear congestion (0.4%), ear pain (0.4%) and erythema (0.4%).
Please see Brief Summary of Prescribing Information on adjacent page.
Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936-1080 © 2018 Novartis 10/18 T-CDX-1364334
References: 1. IQVIA. CIPRODEX® : Annual TRx & NRx 2003-2017. Novartis Pharmaceuticals Corp; February 2018. 2. CIPRODEX® Otic [package insert]. Fort Worth, TX: Alcon Laboratories, Inc; 2015. CIPRODEX® is a registered trademark of Bayer, used with permission.
BRIEF SUMMARY OF PRESCRIBING INFORMATION
For additional information refer to the full prescribing information.
1 INDICATIONS AND USAGE
CIPRODEX® is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed below:
• Acute Otitis Media in pediatric patients (age 6 months and older) with tympanostomy tubes due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa
• Acute Otitis Externa in pediatric (age 6 months and older), adult and elderly patients due to Staphylococcus aureus and Pseudomonas aeruginosa
4 CONTRAINDICATIONS
• CIPRODEX is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components in this medication.
• Use of this product is contraindicated in viral infections of the external canal including herpes simplex infections and fungal otic infections.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
CIPRODEX should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria and itching.
5.2 Potential for Microbial Overgrowth with Prolonged Use
Prolonged use of CIPRODEX may result in overgrowth of non-susceptible, bacteria and fungi. If the infection is not improved after one week of treatment, cultures should be obtained to guide further treatment. If such infections occur, discontinue use and institute alternative therapy.
5.3 Continued or Recurrent Otorrhea
If otorrhea persists after a full course of therapy, or if two or more episodes of otorrhea occur within six months, further evaluation is recommended to exclude an underlying condition such as cholesteatoma, foreign body, or a tumor.
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
• Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
• Potential for Microbial Overgrowth with Prolonged Use [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Phases II and III clinical trials, a total of 937 patients were treated with CIPRODEX. This included 400 patients with acute otitis media with tympanostomy tubes and 537 patients with acute otitis externa. The reported adverse reactions are listed below:
Acute Otitis Media in Pediatric Patients with Tympanostomy Tubes
The following adverse reactions occurred in 0.5% or moreof the patients with non-intact tympanic membranes.
Adverse Reactions Incidence (N=400)
Ear discomfort 3.0%
Ear pain 2.3%
Ear precipitate (residue) 0.5%
Irritability 0.5%
Taste Perversion 0.5%
The following adverse reactions were each reported in a single patient: tympanostomy tube blockage; ear pruritus; tinnitus; oral moniliasis; crying; dizziness; and erythema.
Acute Otitis Externa
The following adverse reactions occurred in 0.4% or more of the patients with intact tympanic membranes.
Adverse Reactions Incidence (N=537)
Ear pruritus 1.5%
Ear debris 0.6%
Superimposed ear infection 0.6%
Ear congestion 0.4%
Ear pain 0.4%
Erythema 0.4%
The following adverse reactions were each reported in a single patient: ear discomfort; decreased hearing; and ear disorder (tingling).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of CIPRODEX®. Because these reactions are reported voluntarily from a population of unknown size it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions include: auricular swelling, headache, hypersensitivity, otorrhea, skin exfoliation, rash erythematous, and vomiting.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects. Pregnancy Category C:
No adequate and well controlled studies with CIPRODEX have been performed in pregnant women. Caution should be exercised when CIPRODEX is used by a pregnant woman.
Animal reproduction studies have not been conducted with CIPRODEX.
Reproduction studies with ciprofloxacin have been performed in rats and mice using oral doses of up to 100 mg/kg and IV doses up to 30 mg/kg and have revealed no evidence of harm to the fetus. In rabbits, ciprofloxacin (30 and 100 mg/kg orally) produced gastrointestinal disturbances resulting in maternal weight loss and an increased incidence of abortion, but no teratogenicity was observed at either dose. After intravenous administration of doses up to 20 mg/kg, no maternal toxicity was produced in the rabbit, and no embryotoxicity or teratogenicity was observed.
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
8.3 Nursing Mothers
Ciprofloxacin and corticosteroids, as a class, appear in milk following oral administration. Dexamethasone in breast milk could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical otic administration of ciprofloxacin or dexamethasone could result in sufficient systemic absorption to produce detectable quantities in human milk. Because of the potential for unwanted effects in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and efficacy of CIPRODEX have been established in pediatric patients 6 months and older (937 patients) in adequate and well-controlled clinical trials. No clinically relevant changes in hearing function were observed in 69 pediatric patients (age 4 to 12 years) treated with CIPRODEX and tested for audiometric parameters.
10 OVERDOSAGE
Due to the characteristics of this preparation, no toxic effects are to be expected with an otic overdose of this product.
Distributed by: Alcon Laboratories, Inc. Fort Worth, TX 76134 USA
U.S. Pat.: www.alconpatents.com
CIPRODEX® is a registered trademark of Bayer Intellectual Property GmbH, licensed to Alcon by Bayer Intellectual Property GmbH.
©2003, 2004, 2008, 2009, 2015 Novartis Revised: December 2015 W9012314-1115
We present a series of 3 otherwise unremarkable patients with CRS and no known comorbidities who experienced clinically significant persistent demucosalization after uncomplicated ESS. We also discuss nasal and sinus mucosal wound healing, possible etiologic factors, and wound management strategies, and we review the current literature.
Case series
Patients were considered for review if they experienced clinically significant and persistent demucosalization after otherwise unremarkable ESS. Patients of any age or sex were eligible provided they had a symptomatic demucosalized lesion that was noted on endoscopic examination that persisted for at least 3 months after uncomplicated sinus surgery despite aggressive medical and topical therapy.
All patients had undergone at least anterior functional ESS (maxillary antrostomy and/or ethmoidectomy) for a diagnosis of CRS. Patients were not considered if they had a medical condition or exposure that predisposed them to poor wound healing (e.g., diabetes,
immunodeficiency, or tobacco use). Patients who were reportedly noncompliant with postoperative care also were excluded.
Medical records and endoscopic images were reviewed retrospectively. Data were collected on several parameters, including baseline demographics, the use of postoperative care, and the time, symptoms, and endoscopic characteristics of wound presentation.
Three patients met our inclusion criteria—a 63-yearold woman, a 67-year-old woman, and a 43-year-old man—and their cases underwent a retrospective chart review (table). All patients had undergone anterior functional ESS with mucosa sparing for CRS. All subsequently developed symptomatic, persistent demucosalization. Patients 1 and 3 were treated with aggressive medical therapies and repeated debridement until full healing was achieved. Patient 2 was lost to follow-up without documentation of resolution.
Discussion
Nasal mucosal wound healing after ESS is a wellorchestrated process that occurs in phases. The first
Key: CRS = chronic rhinosinusitis.
Volume 97, Number 10-11 www.entjournal.com 357 PERSISTENT LOCAL DEMUCOSALIzATION AfTER ENDOSCOPIC SINUS SURGERY: A REPORT Of 3 CASES
Variable Patient 1 Patient 2 Patient 3 Age/sex 63/f 67/f 43/M Medical history CRS, asthma, allergic rhinitis CRS CRS, allergic rhinitis Mucosa-sparing technique Yes Yes Yes Biomaterials used Surgiflo, Gelfoam Merocel floseal, Merocel Postoperative debridement Weeks 1, 3 Weeks 1, 3 Weeks 1, 3, 6 Postoperative antibiotic, saline irrigations, and topical steroid Yes Yes Yes Postoperative acute sinusitis No No No Time of presentation 1 mo postoperatively 2 mo postoperatively 1.5 mo postoperatively Symptoms Dryness, discomfort Discomfort Dryness, discomfort Endoscopic findings Dime-sized mucosal dehiscence on the posterior maxillary wall Denuded mucosa in the posterior ethmoid area Dime-sized mucosal dehiscence on the posterior maxillary wall Topical antibiotic Gentamicin and mupirocin irrigation Gentamicin-soaked Merocel Gentamicin and mupirocin irrigation Aggressive mechanical debridement of wound Yes, with Gelfoam placement 4 mo postoperatively None Yes, with Gelfoam placement 4 mo postoperatively Wound resolution Yes No Yes Time of resolution 18 mo postoperatively Lost to follow-up at 13 mo postoperatively 18 mo postoperatively
Table. Baseline demographics, postoperative care, and wound characteristics in the 3 patients
phase is characterized by coagulation and hemostasis. Injury to blood vessels causes aggregation of platelets and subsequent activation of the coagulation cascade. Deposition of a fibrin matrix leads to formation of a thrombus at the site of injury.
The inflammatory phase then proceeds, with the thrombus serving as a scaffold for migrating neutrophils that are attracted via degranulation of platelets, complement activation, and bacterial degradation.6 Neutrophils stimulate the release of collagenase and elastase, which allows for further infiltration of inflammatory cells. Monocytes begin to migrate to the wound site approximately 2 or 3 days after injury, eventually differentiating into macrophages. As the neutrophil response abates, macrophages become critical for continuation of nasal wound repair, secreting a number of important growth factors and contributing to cellular debridement.7
In the third phase, new tissue formation occurs through fibroplasia, angiogenesis, and re-epithelialization. Epithelial cells migrate into the wound bed within hours of injury.8 Fibroblasts are attracted via cytokines released by macrophages, and they begin to produce and deposit extracellular matrix components such as fibronectin, collagen, and hyaluronic acid. New blood vessels form under the regulation of vascular endothelial growth factor A and fibroblast growth factor 2, two of the most important growth factors guiding angiogenesis.9
Tissue remodeling occurs during the final phase. It generally begins about 2 to 3 weeks after injury and can last as long as 6 months. Cellular maturation and apoptosis occur as the inflammatory and angiogenic responses diminish. The remaining extracellular matrix is actively remodeled with the aid of matrix metalloproteinases, with type III collagen gradually being replaced by type I collagen.
From an endoscopic standpoint, wound healing has been characterized in four phases.10 The first phase, lasting 7 to 12 days postoperatively, is characterized by blood crusts covering the entire wound. In the second phase, the wound consists primarily of granulation tissue, which can persist for 2 to 4 weeks postoperatively. An edematous phase then occurs until finally the wound appears macroscopically normal at 12 to 18 weeks.
Several different management strategies are employed to optimize wound healing after ESS and preserve the normal physiology of the nasal mucosa as much as possible. These include the use of mucosa-sparing surgical techniques, minimizing nasal packing, judicious postoperative debridement, and optimal medical management with either topical or systemic medications.
In ESS, much emphasis has been placed on mucosa-sparing techniques.11 It is well known that the status of the mucosal basement membrane is an important factor in epithelialization. Retention of this layer results in the rapid return to normal epithelial height, while its loss, which occurs during mucosal stripping, may prolong the healing process by weeks or months.4 Damage to the nasal mucosa can also lead to adhesion formation, which occurs when two raw mucosal surfaces are approximated.12
Much has been published regarding the effects of various nasal packs and biomaterials on wound healing post-ESS. Certain products have been shown to promote wound healing in this setting, particularly chitosan gel,13 hyaluronic-acid–containing compounds,14 and fibrin glue.15 Certain biomaterials have exhibited deleterious effects on wound healing. Removable packs may be associated with shearing of the nasal mucosa on removal.
Clinical trials of absorbable biomaterials such as a flowable gelatin-thrombin matrix16 and gelatin film17 have demonstrated a negative impact on wound healing and adhesion formation post-ESS. Collagen, the main component of both of these materials, stimulates fibroblast permeation and allows for nascent deposition of organized collagen fibers.18 Thus, the same mechanism that can lead to adhesion formation after ESS is also responsible for the beneficial effects observed when collagen is used in chronic cutaneous wounds.19
Postoperative in-office debridement of the surgical cavity is commonly performed to remove old blood and secretions, crusts, and absorbable nasal packing. Bugten et al showed that routine debridement post-ESS was associated with reduced crust and adhesion formation at 3 months.20 Alsaffar et al, on the other hand, found no improvement in wound healing in patients who were debrided compared with patients who were not debrided.21
The optimal time and frequency to perform debridement has been somewhat controversial, with some studies demonstrating that early crust debridement may lead to mucosal avulsion.22 The optimal time for initial debridement appears to be 1 week postoperatively.23
The use of saline irrigations post-ESS has also been evaluated. Administration of low volumes of saline (2 ml 3 times daily) has been shown to reduce crusting in the short term, although it has no effect on adhesion formation overall.24 Larger volumes have been shown to be beneficial post-ESS for wound healing in patients with mild CRS, but not in patients with moderate to severe CRS.25
Topical steroid nasal sprays have repeatedly been shown to improve wound-healing–related outcomes when used after ESS. 26,27 When used excessively,
358 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
MASSEY, SANGHvI, TROOST, ANAND, SINGH
however, topical steroids can cause vasoconstriction and local ischemia. Improper use of topical steroids has been shown to cause septal perforations. 28 Systemic steroids in the perioperative period have been shown to improve wound healing in patients with nasal polyposis. 29 However, in an evidence-based review, systemic steroids in the perioperative period did not appear to provide a significant benefit in wound healing. 30 Regardless, systemic steroids tend to be reserved for patients with severe disease given the troublesome side effects often associated with these medications. 31
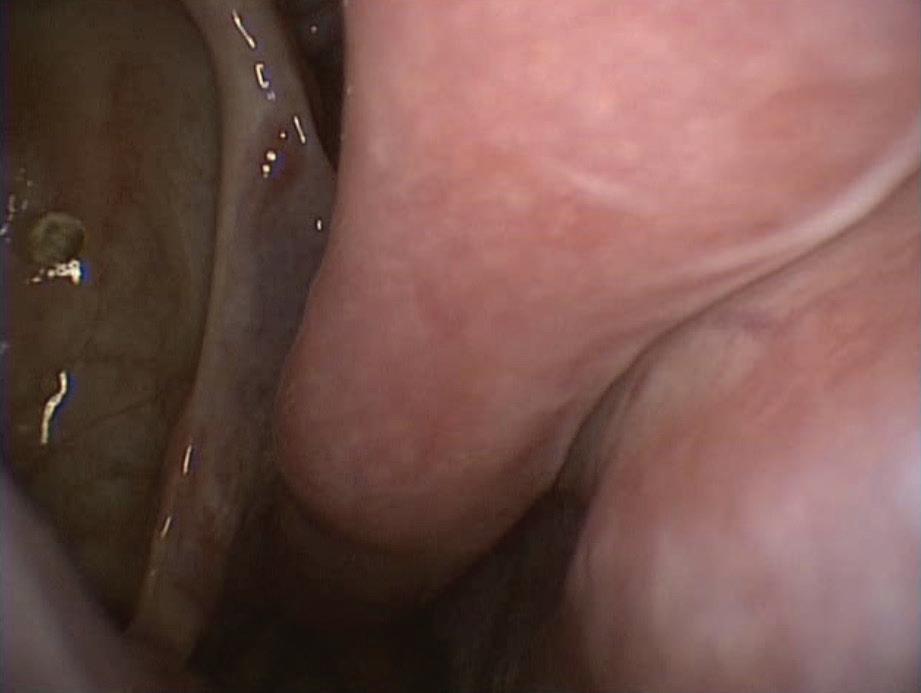
Finally, a 7- to 10-day course of oral antibiotics has been traditionally recommended in the postoperative period, 32 although randomized, controlled trials have demonstrated that the wound-healing benefit is restricted to the early postoperative period.33 Bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa have been known to form biofilms in nasal mucosa, particularly after significant alteration of the normal mucociliary physiology. These biofilms are often difficult to treat, and their persistence may lead to impaired wound healing after ESS.34,35
The 3 cases presented here are particularly unusual because of the presence of a clinically significant demucosalized lesion that persisted after ESS despite the application of the best evidence-based practices. Moreover, these patients had no systemic conditions or exposures that would have had a negative impact on wound healing.
Our patients’ lesions were characterized initially by raw mucosal dehiscence that was first documented within 6 months after ESS. When debrided, these lesions would give way to underlying dry, healthy bone
that was resistant to remucosalization over a protracted period (figure).
Each surgical procedure was performed by a different experienced ESS surgeon using a mucosa-sparing technique. It seems highly unlikely that an inadvertent surgical error such as mucosal stripping could have contributed to the lesions’ formation. Two patients experienced persistent demucosalization centrally on the posterior wall of the maxillary sinus while the immediate medial mucosa was preserved, indicating that a careless surgical maneuver was unlikely. In addition, all affected areas were located at a relatively remote distance from surgical activity.

Patients 1 and 3 were treated with a flowable gelatin-thrombin matrix, which has been associated with fibrosis and adhesions but not demucosalization. Patients 2 and 3 had a polyvinyl acetate nasal pack placed postoperatively, but it was unlikely that the packs came into direct contact with the demucosalized area. If the biomaterials or nasal packs were in fact the culprit, one would expect to observe this phenomenon much more often. Another possible etiology would be overly aggressive debridement, although given that every patient receives debridement after ESS, the same argument applies.
All patients were treated with a topical nasal steroid postoperatively, with patient 2 also receiving an oral steroid. All patients underwent topical antibiotic irrigation after the lesions were first identified. Patients 1 and 3 were treated with multiple saline-soaked gelatin foam sponges placed over their lesion in an effort to stimulate re-epithelialization, and patient 2 underwent placement of a polyvinyl acetate sponge that was soaked in gentamicin and triamcinolone. The only intervention
Volume 97, Number 10-11 www.entjournal.com 359 PERSISTENT LOCAL DEMUCOSALIzATION AfTER ENDOSCOPIC SINUS SURGERY: A REPORT Of 3 CASES
Figure. Patient 1. A: At 1 month postoperatively, this endoscopic image demonstrates a roughly 1-cm mucosal dehiscence on the posterior wall of the maxillary sinus with associated crusting. Debridement revealed underlying healthy-appearing bone. B: The same lesion is seen 17 months later, after aggressive surgical debridement, gelatin foam placement, and intensive moisturization therapy, demonstrating near total remucosalization.
that seemed to provide any benefit was surgical debridement and freshening of the wound edge followed by intensive moisturization therapy and stimulation of re-epithelialization with moist gelatin foam.
The exact etiology of these lesions and the reason for their persistence remain unclear. Our literature review failed to find any previous reports of similar cases. In retrospect, a biopsy of the wound edge might have been helpful in ruling out the more concerning agents of aberrant mucosal healing. Such causes include medication-induced ischemia, granulomatous diseases of the head and neck (e.g., sarcoidosis), vasculitides (e.g., Churg-Strauss syndrome and granulomatosis with polyangiitis), and sinonasal malignancies. While these etiologies may be uncommon, their identification would dramatically change management. Cultures also might have been useful in identifying the presence of infectious pathogens that could contribute to biofilm formation.
For patients with idiopathic persistent demucosalization after ESS, mechanical wound debridement with freshening of the wound edge appears to be an effective therapy. We further advocate placement of saline-soaked gelatin foam sponges to stimulate fibroblast permeation and epithelial migration into the wound bed while maintaining an intensive nasal saline irrigation regimen. The patients who underwent this treatment protocol experienced clinical and endoscopic resolution of their chronic wounds.
Our above-stated conclusion is certainly tempered by the small and heterogeneous sample size, as well as the unknown etiology. However, given the fact that to the best of our knowledge this finding has not been previously reported in the literature, we believe it is important to discuss it.
We hope this report will result in accumulation of more examples of this presumably rare and unusual finding among otolaryngologists and possibly point to other etiologies and treatment strategies.
Despite the use of the best evidence-based practices in sinus surgery, patients may still experience events related to adverse wound healing. Our 3 patients all had clinically significant lesions after ESS that were characterized by small, circular areas of demucosalized bone that were recalcitrant to conservative therapy. Based on our experience, we advocate surgical debridement of these chronic, persistent demucosalized wounds with the application of saline-soaked gelatin foam or other collagen-based sponges to stimulate epithelial growth in the wound bed. Furthermore, biopsy and culture of the
wound bed may be useful in recognizing the presence of worrisome disease processes and identifying any tenacious infectious agents so that more appropriate therapy can be initiated if necessary.
References
1. Cohen NA, Kennedy DW. Revision endoscopic sinus surgery. Otolaryngol Clin North Am 2006;39(3):417-35, vii.
2. Javer AR, Alandejani T. Prevention and management of complications in frontal sinus surgery. Otolaryngol Clin North Am 2010;43(4):827-38.
3. Stankiewicz JA, Lal D, Connor M, Welch K. Complications in endoscopic sinus surgery for chronic rhinosinusitis: A 25-year experience. Laryngoscope 2011;121(12):2684-2701.
4. Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr Top Otorhinolaryngol Head Neck Surg 2010;9:Doc07.
5. Khalmuratova R, Kim DW, Jeon SY. Effect of dexamethasone on wound healing of the septal mucosa in the rat. Am J Rhinol Allergy 2011;25(3):112-16.
6. Grose R, Werner S. Wound-healing studies in transgenic and knockout mice. Mol Biotechnol 2004;28(2):147-66.
7. Pierce GF. Macrophages: Important physiologic and pathologic sources of polypeptide growth factors. Am J Respir Cell Mol Biol 1990;2(3):233-4.
8. Wilhelm DL. Regeneration of tracheal epithelium. J Pathol Bacteriol 1953;65(2):543-50.
9. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83(3):835-70.
10. Watelet JB, Bachert C, Gevaert P, Van Cauwenberge P. Wound healing of the nasal and paranasal mucosa: A review. Am J Rhinol 2002;16(2):77-84.
11. Moriyama H. Sinus mucosal wound healing following endoscopic sinus surgery: Mucosal preservation. In: Stramm A, Draf W, eds. Micro-Endoscopic Surgery of the Paranasal Sinuses and Skull Base. Berlin: Springer; 2000.
12. Thornton RS. Middle turbinate stabilization technique in endoscopic sinus surgery. Arch Otolaryngol Head Neck Surg 1996;122(8):869-72.
13. Valentine R, Athanasiadis T, Moratti S, et al. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am J Rhinol Allergy 2010;24(1):70-5.
14. Berlucchi M, Castelnuovo P, Vincenzi A, et al. Endoscopic outcomes of resorbable nasal packing after functional endoscopic sinus surgery: A multicenter prospective randomized controlled study. Eur Arch Otorhinolaryngol 2009;266(6):839-45.
15. Vaiman M, Eviatar E, Shlamkovich N, Segal S. Use of fibrin glue as a hemostatic in endoscopic sinus surgery. Ann Otol Rhinol Laryngol 2005;114(3):237-41.
16. Chandra RK, Conley DB, Haines GK III, Kern RC. Long-term effects of FloSeal packing after endoscopic sinus surgery. Am J Rhinol 2005;19(3):240-3.
17. Catalano PJ, Roffman EJ. Evaluation of middle meatal stenting after minimally invasive sinus techniques (MIST). Otolaryngol Head Neck Surg 2003;128(6):875-81.
18. Doillon CJ. Porous collagen sponge wound dressings: In vivo and in vitro studies. J Biomater Appl 1988;2(4):562-78.
19. Palmieri B. Heterologous collagen in wound healing: A clinical study. Int J Tissue React 1992;14(Suppl):21-5.
360 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
MASSEY, SANGHvI, TROOST, ANAND, SINGH
PERSISTENT LOCAL DEMUCOSALIzATION AfTER ENDOSCOPIC SINUS SURGERY: A REPORT Of 3 CASES
20. Bugten V, Nordgård S, Steinsvåg S. The effects of debridement after endoscopic sinus surgery. Laryngoscope 2006;116(11):2037-43.
21. Alsaffar H, Sowerby L, Rotenberg BW. Postoperative nasal debridement after endoscopic sinus surgery: A randomized controlled trial. Ann Otol Rhinol Laryngol 2013;122(10):642-7.
22. Kühnel T, Hosemann W, Wagner W, Fayad K. How traumatising is mechanical mucous membrane care after interventions on paranasal sinuses? A histological immunohistochemical study [in German]. Laryngorhinootologie 1996;75(10):575-9.
23. Lee JY, Byun JY. Relationship between the frequency of postoperative debridement and patient discomfort, healing period, surgical outcomes, and compliance after endoscopic sinus surgery. Laryngoscope 2008;118(10):1868-72.
24. Freeman SR, Sivayoham ES, Jepson K, de Carpentier J. A preliminary randomised controlled trial evaluating the efficacy of saline douching following endoscopic sinus surgery. Clin Otolaryngol 2008;33(5):462-5.
25. Liang KL, Su MC, Tseng HC, Jiang RS. Impact of pulsatile nasal irrigation on the prognosis of functional endoscopic sinus surgery. J Otolaryngol Head Neck Surg 2008;37(2):148-53.
26. Rowe-Jones JM, Medcalf M, Durham SR, et al. Functional endoscopic sinus surgery: 5 year follow up and results of a prospective, randomised, stratified, double-blind, placebocontrolled study of postoperative fluticasone propionate aqueous nasal spray. Rhinology 2005;43(1):2-10.
27. Jorissen M, Bachert C. Effect of corticosteroids on wound healing after endoscopic sinus surgery. Rhinology 2009;47(3):280-6.
28. Ferguson BJ. Nasal steroid sprays and septal perforations. Ear Nose Throat J 1997;76(2):75-6.
29. Wright ED, Agrawal S. Impact of perioperative systemic steroids on surgical outcomes in patients with chronic rhinosinusitis with polyposis: Evaluation with the novel Perioperative Sinus Endoscopy (POSE) scoring system. Laryngoscope 2007;117(11 Pt 2 Suppl 115):1-28.
30. Rudmik L, Soler ZM, Orlandi RR, et al. Early postoperative care following endoscopic sinus surgery: An evidence-based review with recommendations. Int Forum Allergy Rhinol 2011;1(6):417-30.
31. Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am 2010;43(4):753-68.
32. Stammberger H, Posawetz W. Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolaryngol 1990;247(2):63-76.
33. Albu S, Lucaciu R. Prophylactic antibiotics in endoscopic sinus surgery: A short follow-up study. Am J Rhinol Allergy 2010;24(4):306-9.
34. Zhang Z, Han D, Zhang S, et al. Biofilms and mucosal healing in postsurgical patients with chronic rhinosinusitis. Am J Rhinol Allergy 2009;23(5):506-11.
35. Singhal D, Psaltis AJ, Foreman A, Wormald PJ. The impact of biofilms on outcomes after endoscopic sinus surgery. Am J Rhinol Allergy 2010;24(3):169-74.
CLINICAL OUTCOME Of REvISION CARTILAGE TYMPANOPLASTY
Continued from page 353
References
1. Wullstein HL. Funktionelle operationen im mittelohr mit hilfe des freien spaltlappen-transplantates. Arch Ohren-Nasen-u. Kehlkopfh 1952;161:422-35.
2. Zollner F. The principles of plastic surgery of the soundconducting apparatus. J Laryngol Otol 1955;69(10):637-52.
3. Heermann H. Tympanoplasty with fascial tissue taken from the temporal muscle after straightening the anterior wall of the auditory meatus [in German]. HNO 1961;9:136-7.
4. Shea JJ Jr. Vein graft closure of eardrum perforations. J Laryngol Otol 1960;74:358-62.
5. Nissen AJ, Nissen RL, Yonkers AJ. A historical review of the use of bone and cartilage in otologic surgery. Ear Nose Throat J 1986;65(11):493-6.
6. Caylan R, Titiz A, Falcioni M, et al. Myringoplasty in children: Factors influencing surgical outcome. Otolaryngol Head Neck Surg 1998;118(5):709-13.
7. Vartiainen E. The results of chronic ear surgery in a training programme. Clin Otolaryngol Allied Sci 1998;23(2):177-80.
8. Buckingham RA. Fascia and perichondrium atrophy in tympanoplasty and recurrent middle ear atelectasis. Ann Otol Rhinol Laryngol 1992;101(9):755-8.
9. Beutner D, Huttenbrink KB, Stumpf R, et al. Cartilage plate tympanoplasty. Otol Neurotol 2010;31(1):105-10.
10. Mohamad SH, Khan I, Hussain SS. Is cartilage tympanoplasty more effective than fascia tympanoplasty? A systematic review. Otol Neurotol 2012;33(5):699-705.
11. Utech H. Tympanotomy in disorders of sound conduction; its diagnostic and therapeutic possibilities [in German]. Z Laryngol Rhinol Otol 1959;38(4):212-21.
12. Lesinskas E, Stankeviciute V. Results of revision tympanoplasty for chronic non-cholesteatomatous otitis media. Auris Nasus Larynx 2011;38(2):196-202.
13. Sismanis A, Dodson K, Kyrodimos E. Cartilage "shield" grafts in revision tympanoplasty. Otol Neurotol 2008;29(3):330-3.
14. Boone RT, Gardner EK, Dornhoffer JL. Success of cartilage grafting in revision tympanoplasty without mastoidectomy. Otol Neurotol 2004;25(5):678-81.
15. Dornhoffer J. Cartilage tympanoplasty: Indications, techniques, and outcomes in a 1,000-patient series. Laryngoscope 2003;113(11):1844-56.
16. Eavey RD. Inlay tympanoplasty: Cartilage butterfly technique. Laryngoscope 1998;108(5):657-61.
17. Uslu C, Tek A, Tatlipinar A, et al. Cartilage reinforcement tympanoplasty: Otological and audiological results. Acta Otolaryngologica 2010;130(3):375-83.
18. Zahnert T, Bornitz M, Hüttenbrink KB. Acoustic and mechanical properties of tympanic membrane transplants [in German]. Laryngorhinootologie 1997;76(12):717-23.
19. Lee CF, Chen JH, Chou YF, et al. Optimal graft thickness for different sizes of tympanic membrane perforation in cartilage myringoplasty: A finite element analysis. Laryngoscope 2007;117(4):725-30.
Volume 97, Number 10-11 www.entjournal.com 361
Desmoid tumors of the head and neck: Two decades in a single tertiary care unit and review of the literature
Aleksi Schrey, MD, PhD; Maria Gardberg, MD, PhD; Riitta Parkkola, MD, PhD; Ilpo Kinnunen, MD, PhD
Abstract
Desmoid tumors (DTs) of the head and neck have typically been classified as extra-abdominal, although the anatomic challenges of the head and neck warrant consideration of these DTs as a special entity. We present a review of DTs and describe our series of five patients with DTs of the head and neck treated within 2 decades. Altogether, 53 patients with DTs treated surgically at a tertiary care center over a 20-year period were retrospectively reviewed. Outcomes of the treatment of DTs of the head and neck (n = 5) were analyzed as a case series. DTs are rare, histologically benign, but locally aggressive tumors. In our series of 5 patients with head and neck DTs, no patient experienced a recurrence during a median follow-up of 47.5 months (range 13 to 150), although all had positive histologic margins. The functional integrity of vital structures over meticulous radicality of the tumor resection must be considered, especially in the head and neck.
Introduction
Desmoid tumor (DT), also known as aggressive fibromatosis, is a rare entity, representing only 0.03% of all neoplasms in humans.1 It is classified into three subtypes according to its anatomic position: abdominal, intra-abdominal, and extra-abdominal. Common sites include the extremities, abdominal wall, and mesentery. DTs of the head and neck region comprise approximately 10 to 25% of all cases of extra-abdominal
From the Department of Otorhinolaryngology–Head and Neck Surgery (Dr. Schrey and Dr. Kinnunen), the Department of Pathology (Dr. Gardberg), and the Department of Radiology, Medical Imaging Centre of Southwest Finland (Dr. Parkkola), Turku University Hospital and University of Turku, Turku, Finland.
Corresponding author: Aleksi Schrey, MD, PhD, Department of Otorhinolaryngology–Head and Neck Surgery, Turku University Hospital, FIN-20521, Turku, Finland. Email: aleksi.schrey@utu.fi
DTs, and usually occur in the anterolateral neck or supraclavicular fossa.2-4
Extra-abdominal DT can cause unique clinical problems in relation to its critical anatomic position.2-5 Although surgery is the mainstay of therapy, multimodal treatment including partial or complete excision followed by radiation, chemoradiation, or hormonal therapy can be effective.1 If not completely resected, these tumors have a high likelihood of recurrence.
The rarity of head and neck DTs has precluded prospective, randomized studies to formally assess the importance of positive or negative resection margins or adjuvant therapies. Most studies are retrospective and are composed of patients with DTs from various anatomic locations, including both primary and recurrent tumors, and those with recurrence after surgery have received diverse adjuvant therapies.
Patients and methods
Institutional approval (T225/2016; Nr TO6/035/16) for the study was granted. A search for the patients with DTs was carried out from patient charts in the Hospital District of Southwest Finland (HDSWF), and included the words “desmoid tumor” and “agressive fibromatosis,” as well as the ICD-10 diagnostic codes M72.9 and D21.0. The search covered the period from January 1997 until December 31, 2016. The initial search resulted in a total of 53 patients with DTs, 5 of whom (9.4%) had head and neck DTs (table 1), who were treated at HDSWF and followed in the outpatient department. The Auria Biobank of Turku University implemented the search from the electronic patient database.
The charts of these 5 patients with primary head and neck DTs were further analyzed for tumor location, tumor size, and treatment data, as well as for findings
362 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018 ORIGINAL ARTICLE
on immunohistochemistry (table 2). Each of these patients is reported separately with selected representative images obtained by magnetic resonance imaging (MRI).
Of the 5 patients with head and neck DTs, 3 were women and 2 were men. The median age at diagnosis was 48 years (range: 26 to 60). Four of the 5 patients were <50 years old at presentation.
Patient 1. A previously healthy, 46-year-old Caucasian nonsmoking man, presented with complaints of swelling in the temporal region, especially during forceful biting. During the few months of symptoms, the swelling had become larger. On examination, a painless tumor approximately 2 cm in size was easily palpable when the patient flexed the temporal muscle. An ortopantomogram was normal.
An MRI performed with contrast enhancement revealed an 18 × 15 × 22-mm clearly visible tumor under the zygomatic arch closely attached to the processus coronoideus and the temporal muscle without infiltration of the muscle (figure 1). There was no clinical evidence of cervical lymphadenopathy. Preoperative cytology was not conclusive of a specific disease but was suspicious for DT and was diagnosed as benign. A mesenchymal tumor, such as a dermatofibroma, leiomyoma, or dense fibroma was a possible diagnosis.
The patient underwent en bloc resection via coronal incision. The tumor was surrounded by the temporal muscle tendons, some of which approached the tumor. Meticulous dissection preserved the facial nerve with clinically complete excision of the tumor. The posterior deep fascia of the temporal muscle remained intact. An intraoperative frozen section from a tumor specimen was analyzed as benign. Postoperatively, the histology from the formalin-fixed sample showed a spindle cell tumor with collagenous stroma. The elongated tumor cells were uniform, and no mitoses were found. The tumor vasculature was prominent. There were no complications during outpatient
follow-up. The incision scar healed nicely, and function of the facial nerve and mouth opening was optimal. No extra bulging, even during forceful biting, could be identified. After 11 years, evidence of the tumor was absent according to outpatient clinical control and images generated by MRI (figure 2). The patient is without neurologic deficit.
Patient 2. A 60-year-old nonsmoking Caucasian man with a history of arterial hypertension and rheumatoid arthritis noticed a lump in the left clavicular fossa within the previous year. The lump had been asymptomatic, although during the previous 3 months the patient had experienced pain in the shoulder and neck regions ipsilaterally. Fine-needle aspiration (FNA) biopsy was not diagnostic although fibromatotic tissue was suspected.
The patient was operated on by a team consisting of a plastic surgeon, hand surgeon, and a cardiothoracic surgeon working in close cooperation. An axial T2-weighted fat-saturated MRI and a coronal T1-weighted gadolinium-enhanced MRI of the tumor of the left supraclavicular area were obtained. The images demonstrated a complex 65 × 65 × 55-mm left neck mass in the clavicular fossa extending and fixating to the ipsilateral brachial plexus, as well as extending laterally to the common carotid artery, between the subclavian artery and vein, as well as to the surface of the first thoracic rib (figure 3).
The patient was notified of the possibility that the tumor was not totally resectable without sacrificing the (brachial) nerves. He accepted the possibility of a
Volume 97, Number 10-11 www.entjournal.com 363 DESMOID TUMORS Of THE HEAD AND NECk: TWO DECADES IN A SINGLE TERTIARY CARE UNIT AND REvIEW Of THE LITERATURE
Location Extremity Abdominal wall Chest wall Mesentery Pelvic Head/neck n (%) 15 (28.3) 13 (24.5) 9 (17.0) 7 (13.2) 4 (7.5) 5 (9.4) Median age, yr (range) 38 (1-79) 53 (23-81) 28 (24-38) 53 (23-81) 38 (36-66) 48 (26-60) Male:female 10:27 3:4 1:4 3:4 1:4 2:3
Table 1. Desmoid tumors characterized in patients treated in the Hospital District of Southwest Finland, Turku, between 1997 and 2016 (n = 53)
Patient no. a-SMA Desmin S-100 Beta-catenin CD34 Ki-67 (%) 1 + – – + – 2 2 + – – + – 3 3 + – – + – 4 4 – – – + – 5 5 + – – + – <1
Table 2. Immunohistochemical findings in the 5 patients with head and neck desmoid tumors in Turku University Hospital, Turku, Finland
Key: SMA = smooth muscle actin.
possible nonradical operation, preferring to save the brachial plexus nerves.
The patient underwent en bloc resection of the mass, with preservation of the brachial plexus, subclavian vessels, and spinal accessory nerve. The clavicula required an osteotomy, which was fixated with a titanium plate and lock screws. The phrenic nerve was injured/ sacrificed in the operation, and a postoperative consultation with a physiotherapist for breathing support was needed. The deep margins of resection were clinically and microscopically positive.
The patient received postoperative external beam radiation (EBR) therapy at a dosage of 54 Gy with 1.8 Gy fractions. The tumor did not disappear but diminished to the size of less than 2 cm. It has not grown during 120 months of follow-up after the resection and radiotherapy. The patient is without neurologic deficit other than that caused by the cranial position of the left diaphragm due to the injury of the phrenic nerve.
Patient 3. A 48-year-old Caucasian nonsmoking woman with a history of arterial hypertension, diabetes mellitus, and asthma noticed a lump laterally in the right side of the neck, with occasional pain in the neck and in the ipsilateral arm. A core needle biopsy suggested a DT, although this 56 × 42 × 36-mm mass was suspected to be a sarcoma according to findings on MRI. The mass infiltrated to the levator scapulae muscle, as well the scalenus muscles ipsilaterally.
The patient underwent en bloc resection of the mass performed by a plastic surgeon, with preservation of the brachial plexus. The spinal accessory and the phrenic nerves were sacrificed during
the operation due to the tumor’s invaginating these nerves.
Sacrifice of the phrenic nerve necessitated a postoperative consultation with a physiotherapist for breathing assistance. The margins of resection were clinically and microscopically positive.
The patient received postoperative EBR therapy at a dosage of 57 Gy with 1.8 Gy fractions. She suffered from pain in the ipsilateral arm. The neurologic deficit caused by the cranial positioning of the right diaphragm due to the injury of the phrenic nerve, as well as from the spinal accessory nerve injury, reduced this patient’s quality of life. She is currently disease-free 13 months after the resection and EBR therapy.
Patient 4. A previously healthy 26-year-old nonsmoking Caucasian woman developed a mass on the left side of the neck. FNA biopsy was not diagnostic, and ultrasound investigation found the 12 × 30-mm mass to be homogenous.
The patient underwent en bloc resection of the mass by a head and neck surgeon, with preservation of the spinal accessory nerve.
An MRI performed 6 months postoperatively showed no signs of tumor residue. The patient is currently free of disease 32 months after the tumor resection and is without neurologic deficit.
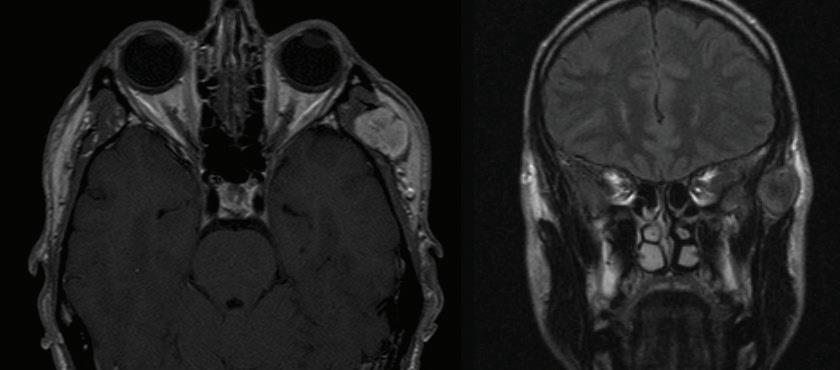
Patient 5. A previously healthy 49-year-old, smoking Caucasian woman developed hoarseness 1 month after a bout of influenza. A firm, solid, fixated tumor was identified on the right side of her neck, measuring 50 × 40 mm on MRI. The tumor, which extended between the transverse processes of the lower cervical vertebrae, was barely visible on contrast-enhanced computed tomography (CT).
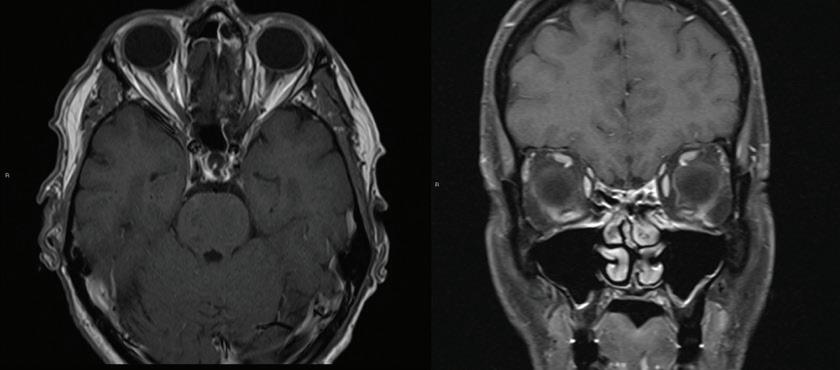
364 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
SCHREY, GARDBERG, PARkkOLA, kINNUNEN
Figure 1. These preoperative MRI (axial and coronal) scans from a 46-year-old nonsmoking Caucasian man who had complaints of swelling in the temporal region (patient 1) show an 18 × 15 × 22-mm tumor involving left temporal fossa.
Figure 2. These postoperative MRI (axial and coronal) scans taken 11 years after surgery performed on patient 1 show no evidence of residual tumor and no remarkable visible postoperative changes.
The patient underwent en bloc resection of the mass by a head and neck surgeon, with preservation of the spinal accessory and phrenic nerves. The tumor located lateral to the brachial plexus and partly fixated to the plexus was sharply dissected from the superior part of the tumor. No additional therapies were given. The patient is currently disease-free 150 months after the resection and is without neurologic deficit.
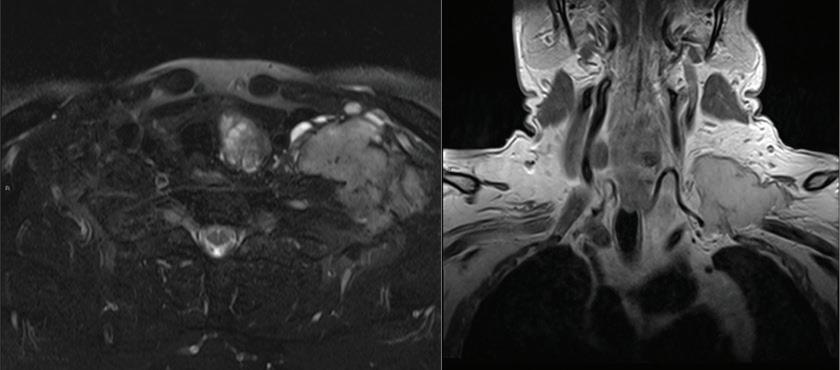
Tumor histology and immunohistochemistry
Tumor samples were formalin-fixed and paraffin-embedded according to standard procedure. Histologic findings on hematoxylin and eosin and van Gieson staining were typical for DTs.6 To rule out differential diagnostic possibilities and to verify the diagnosis, immunohistochemical stainings were performed using a Ventana Benchmark XT Autostainer Device (Ventana Medical Systems; Strasbourg, France). All five tumor samples were stained for S-100 protein, CD34 smooth muscle actin (SMA), desmin, pancytokeratin, vimentin, Ki-67, and beta-catenin. The immunohistochemical findings were compatible with a diagnosis of DT in all patients (table 2).
Staining was positive for vimentin and SMA in all samples with the exception of patient 4, and nuclear accumulation of beta-catenin was observed (figure 4). Tumor cells were negative for CD34, SMA, desmin, and pancytokeratin. Four tumors were negative for S-100 protein; in patient 2, a small fraction (2%) of S-100-positive cells was found. Ki-67 staining revealed low proliferation indexes (5% at the maximum).
Results
Symptoms at first presentation. As described above, the most common presentations were either an asymptomatic/painless mass (n = 3) (patients 1, 2, and 4) or
neurologic symptoms, including pain or radiculopathy (n = 2) (patients 3 and 5). None of the patients reported a history of surgery or trauma at the site of the head and neck tumor. Diagnostic modalities. The diagnosis was established in 2 patients by performing FNA biopsy (patients 1 and 2). When FNA was nondiagnostic (patients 2 and 4), a core needle (patient 3), open biopsy, or tumor surgery was performed according to the findings on MRI (patients 4 and 5). Preoperative extent of tumor growth and involvement of vital anatomic structures were assessed by MRI (patients 1 to 3 and 5), CT (patient 5), ultrasound (patient 4), or a mixture of these modalities. Examples of preoperative radiologic appearances of DTs in the temporal region and in the supraclavicular fossa are shown in figure 1 and figure 3, respectively.
Tumor characteristics. The anatomic distribution of tumors revealed 2 patients (40%) with primary DTs of the supraclavicular fossa or anterior lower neck, 2 patients with tumors arising within other muscles of the neck (40%), and 1 patient (20%) with a tumor of the craniofacial area. Three tumors ranged in size from 5 to 10 cm and two tumors were smaller than 5 cm.
Resection margins. All 5 patients underwent resection of their tumors as attempted curative treatment. Three patients had a grossly complete surgical resection performed. Of these, 1 patient was found to have microscopically positive margins. Two patients had grossly positive margins and incomplete resection because of the proximity of the tumor to the brachial plexus and subclavian veins.
Extent of resection. Resection of the tumor alone was possible in 3 patients (60%). Anatomic structures such as the accessory nerve, the phrenic nerve, parts of the brachial plexus, the jugular vein, or the clavicle were resected in the other 2 (40%) patients.
Clinical course. The median follow-up of the 5 patients was 47.5 months (range: 13 to 150). The 2 patients who had incomplete resection because of the proximity of the tumor to the brachial plexus and subclavian veins received adjuvant EBR therapy.
All 5 patients have remained free of symptoms. No recurrent tumor could be documented, although
Volume 97, Number 10-11 www.entjournal.com 365 DESMOID TUMORS Of THE HEAD AND NECk: TWO DECADES IN A SINGLE TERTIARY CARE UNIT AND REvIEW Of THE LITERATURE
Figure 3. Shown here are the preoperative MRI (axial and coronal) scans from a 60-year-old nonsmoking Caucasian man (patient 2) who presented with a slow-growing lump in the left supraclavicular fossa that was noticed by the patient approximately 1 year earlier.
patient 2 had residual tumor around the subclavian vessels and brachial plexus after radiotherapy, with no signs of growth according to postoperative MRI scans over follow-up of 120 months. Overall, no local recurrences have developed after resection of the primary tumor, although 2 patients (patients 2 and 5) had a residual but asymptomatic tumor. No patient had metastatic disease, and there were no tumor-related deaths.
Functional outcome. Three patients (60%) had good functional outcome after surgery. One patient (20%) displayed persistent functional problems caused by iatrogenic accessory nerve injury during resection. Physical therapy was helpful for this patient to improve the range of motion of that extremity. Two patients (40%) suffered iatrogenic phrenic nerve injuries due to DT surgery, causing a paralytic diaphragm ipsilaterally.
Discussion
DT is a rare, slow-growing, nonmalignant neoplasm arising from fibroblasts, with the potential for locally aggressive growth. DTs arise as uncontrolled fibroblastic proliferations of musculoaponeurotic structures. The pathogenesis of DTs is multifactorial, involving genetic alterations, physiologic factors (i.e., surgical trauma), and endocrine factors.1,2 DTs can arise in virtually any part of the body. These tumors can occur in anyone at any age but often occur in women older than 30 years.
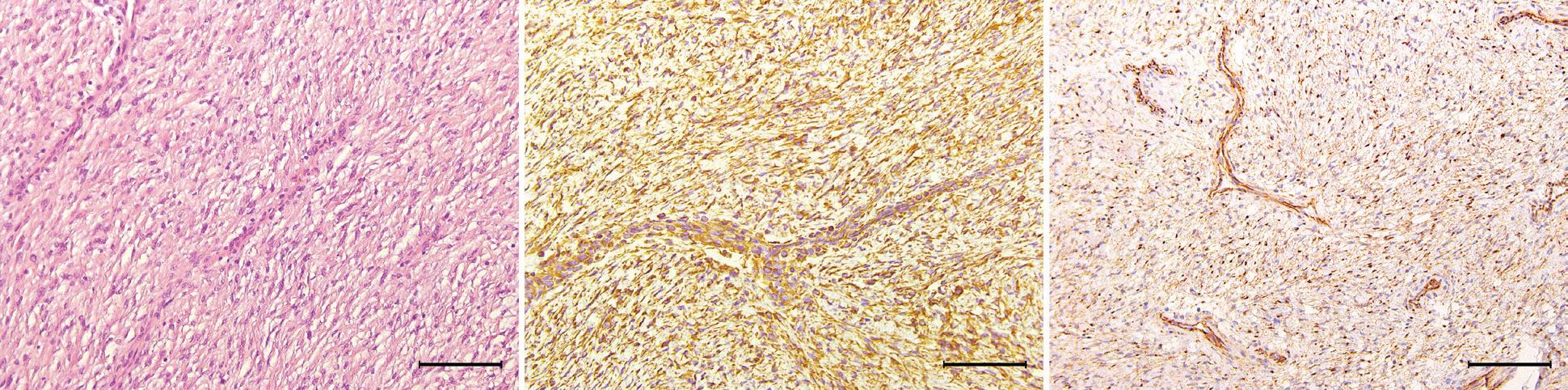
Histologically, DTs are typically poorly circumscribed. They are composed of spindle cells in a dense collagenous stroma with prominent blood vessels. In contrast to fibrosarcomas, their cellularity is low, necrosis is not present, and mitoses are rare.
Importantly, DTs do not have metastatic potential or malignant transformation unless related to radiation. However, despite having a benign histology, these growing tumors themselves or their treatment
can cause significant morbidity. 3 DT of the head and neck usually presents with a painless mass or, less commonly, with pain, obstructive features, or neurologic symptoms.7
As with fibromatosis arising elsewhere in the body, surgical resection is the treatment of choice. 3,6-8 The primary goal is to achieve a clear resection with wide margins, but preservation of function and major vessels is of equal importance. Due to the complex anatomy and frequent entrapment of neurovascular structures in the head and neck region, these aims are often difficult to achieve. Resection often leads to injury of important surrounding tissues such as the carotid artery, internal jugular vein, or brachial plexus, and is associated with a high rate of recurrence (46 to 62%) that requires repeated excisions.8 The timing of most recurrences is within 2 years of resection, which is the minimum recommended follow-up time. The recurrence risk is higher for younger patients.4,9
Several series in the literature have confirmed that margin status after resection does not necessarily correlate with local disease recurrence. Therefore, the sacrifice of major neurovascular structures in an attempt to obtain negative margins is not recommended.10 Two patients (patients 2 and 3) in our series suffered iatrogenic nerve injuries during resection of the tumor; however, the resection was not radical and the patients received EBR therapy. The possibility of phrenic nerve injury must be considered carefully, and preoperative discussion with the patient is mandatory concerning the radicality of the resection. Multidisciplinary cooperation is essential, and referring patients to centers with the most experience with management of DTs of the head and neck is recommended.
The precise role of adjuvant therapy and the optimal therapeutic agent have not been established, probably due to the rarity of the disease in the head and neck. The occasional observation of spontaneous regression
366 www.entjournal.com ENT-Ear, Nose & Throat Journal October-November 2018
SCHREY, GARDBERG, PARkkOLA, kINNUNEN
Figure 4. These slides depict the histologic and immunohistochemical findings from the resected tumor of patient 1. A: Hematoxylin and eosin staining demonstrates a spindle cell tumor with prominent blood vessels. B: Immunohistochemical staining for vimentin is positive in tumor cells. C: Beta-catenin staining is positive in most tumor cell nuclei, a finding typical for desmoid tumors (scale bars: 100 µm).
DESMOID TUMORS Of THE HEAD AND NECk: TWO DECADES IN A SINGLE TERTIARY CARE UNIT AND REvIEW Of THE LITERATURE
and arrested growth after incomplete excision, and the finding that the resection margin correlates poorly with the likelihood of local recurrence, also may be partly responsible.3 Our study supports the poor correlation with the positive resection margins and the likelihood of local recurrence.
Although the efficacy of radiotherapy is controversial, good local control rates using radiotherapy as either adjuvant or primary therapy have been reported.10-12 Radiotherapy should thus be employed if primary excision is not feasible, with postoperative residual tumor, or if the tumor recurs.9,11,12
Although rare, DT should be considered in the differential diagnosis of patients with neck or facial swelling. Detailed attention should be paid to all relevant clinical information. Any unusual presentation or disease course should raise suspicion for further investigation and reassessment.
DT is best managed by a multidisciplinary approach. The disease is usually partially resectable when presented in the neck below the deep cervical fascia. Postoperative radiation along with tamoxifen therapy should be considered when complete excision is not possible due to involvement of neurovascular structures. Follow-up has to be maintained in all cases of DTs due to their high recurrence rate, especially during the first 2 years of follow-up.
References
1. Nuyttens JJ, Rust PF, Thomas CR Jr, Turrisi AT 3rd. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer 2000;88(7):1517–23.

2. Wang CP, Chang YL, Ko JY, et al. Desmoid tumor of the head and neck. Head Neck 2006;28(11):1008–13.
3. Hoos A, Lewis JJ, Urist MJ, et al. Desmoid tumors of the head and neck—a clinical study of a rare entity. Head Neck 2000;22(8):814–21.
4. Fasching MC, Saleh J, Woods JE. Desmoid tumors of the head and neck. Am J Surg 1988;156(4):327–31.
5. Weiss SW, Goldblum JR. Cartilaginous soft tissue tumors. In: Weiss SW, Goldblum JR, Enzinger FM (eds). Enzinger and Weiss’s Soft Tissue Tumors. 4th ed. St. Louis: Mosby; 2001:1361-8.
6 Heim-Hall J, Yohe SL. Application of immunohistochemistry to soft tissue neoplasms. Arch Pathol Lab Med 2008;132(3):476-89.
7 Shields CJ, Winter DC, Kirwan WO, Redmond HP. Desmoid tumours. Eur J Surg Oncol. 2001;27(8):701-6.
8 Lazar AJ, Tuvin D, Hajibashi S, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 2008;173(5):1518-27.
9. Lessow AS, Song P, Komisar A. Unusual fibromatosis of the head and neck. Otolaryngol Head Neck Surg 2004;130(3):366–9.
10. Collins BJ, Fischer AC, Tufaro AP. Desmoid tumors of the head and neck: A review. Ann Plast Surg 2005;54(1):103–8.
11. Siegel NS, Bradford CR. Fibromatosis of the head and neck: A challenging lesion. Otolaryngol Head Neck Surg 2000;123(3):269–75.
12. Tse GM, Chan KF, Ahuja AT, et al. Fibromatosis of the head and neck region. Otolaryngol Head Neck Surg 2001;125(5):516–9.
Volume 97, Number 10-11 www.entjournal.com 367
How an ENT-specific EHR can benefit your practice
By Romeo C. Agbayani, Jr, MD, CEO Marin ENT, Greenbrae, CA

Compulink is the ideal electronic health record system for my otolaryngology private practice!
In response to the requirements imposed on physicians to implement an electronic health record system, I researched and contacted numerous companies. Obviously, the larger systems seemed ideal for integration and HIPAA-compliant sharing of patient information with other providers and their systems. However, larger systems do not cater to the needs of the smaller private practices. Some would not separate financial confidentiality of patient’s ledgers between different providers and staff.
In addition, many electronic health record systems did not focus on the specific ENT templates required to streamline an otolaryngology examination.
Compulink, however, was receptive to all my needs. When problems arose, Compulink had an IT team available to quickly address my practice needs without significant downtime. Their training team was very polite and helpful.
Please contact us for more information.
I had several patients comment that Compulink’s pre-registration link to be very helpful and pleasantly unique. My billing staff find the billing tools easy to use and supportive. My front office likes how easy it is to navigate within Compulink to make appointments.
Finally, I found the cost of their system extremely competitive. I implemented Compulink into my three surgeon otolaryngology practice over a year ago, and am highly satisfied with my decision.
I strongly recommend Compulink’s EHR and Practice Management system to any otolaryngology practice for its ENT specificity, ease of use, cost, confidentiality, and friendly personal customer support
Compulink
www.compulinkadvantage.com/ENTEHR | 800.456.4522
SPONSORED CONTENT
In our field, we are constantly striving to provide excellent patient care. To innovate new treatments. To reduce pain and minimize downtime. Studies have proven we have a tool that can help us accomplish all those things for patients with chronic rhinosinusitis: the XprESS™ ENT Dilation System.
Not only does this technology enable the flexibility to perform a procedure in the OR or office, studies show that for the right patients it can deliver results that are as successful and as long-lasting as a traditional FESS.1 XprESS is uniquely designed for maximum patient comfort and ease of use, so it can be integrated seamlessly into your office or OR. Its malleable tip can be customized to fit a patient’s unique anatomy for efficient, effective and comfortable dilation of the frontal, sphenoid and maxillary sinuses, all with the same device. It’s sleek profile is designed to improve your visualization and access, while enabling navigation through tight nasal anatomy—even in pediatric patients.
In fact, XprESS is the only balloon dilation device to have received FDA clearance to treat frontal and sphenoid sinuses in adolescents (12+), as well as maxillary sinuses in children (2+).† And the results speak for themselves, with studies producing a 100 percent technical success rate and 92 percent of children showing improvement after six months—all with zero complications or revisions needed.2

One reason for the success of XprESS treatments is the ability to confirm correct device replacement through five different methods, including the tactile feel similar to traditional surgical seekers, depth markings on the device itself, an LED light that provides transillumination through the skin, and more. And in situations where natural anatomical landmarks aren’t present, the XprESS system is compatible with the Fiagon™ Image Guidance System.*
Less pain. A quicker recovery. Improved quality of life. The XprESS ENT Dilation System delivers it all, while providing you with an easy-to-adopt, easy-to-use tool that’s sure to become one of the most valuable in your practice.
Dive deep into balloon dilation with XprESS clinical data at: entellusmedical.com/clinicaldata
1 Chandra RK, Kern RC, Cutler JL, Welch KC, Russell PT. REMODEL larger cohort with long-term outcomes and meta-analysis of standalone balloon dilation studies. Laryngoscope. 2016:126:44-50.
2 Soler ZM, Rosenbloom JS, Skarada D, Gutman M, Hoy MJ, Nguyen SA. Prospective, multicenter evaluation of balloon sinus dilation for treatment of pediatric chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016 Nov 26. [Epub ahead of print]
* Fiagon™ Image Guidance System is compatible with the XprESS LoProfile device only.
† As of August 1, 2018.
©2018 Entellus Medical, Inc.
Enhanced patient experience
1
Average days to return to normal activity:
Average days prescription pain medication needed:
Average follow-up care visits per patient:
Safe and effective for pediatrics
100 percent technical success rate in children, with 92 percent showing improvement after six months— all with zero complications or revisions needed.
By the numbers1 6 846 97.5% 1.4-3.2%
2
Sinuses treated
Studies Technical success rate Revision rates
I NDICATIONS FOR USE: To access and treat the maxillary ostia/ethmoid infundibula in patients 2 years and older, and frontal ostia/recesses and sphenoid sinus ostia in patients 12 years and older using a transnasal approach. The bony sinus out flow tracts are remodeled by balloon displacement of adjacent bone and paranasal sinus structures. To dilate the cartilaginous portion of the Eustachian tube for treating persistent Eustachian tube dysfunction in patients 18 years and older using a transnasal approach. Please see Instructions for Use (IFU) for a complete listing of warnings, precautions, and adverse events. CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician.
ENTELLUS MEDICAL and XPRESS are trademarks of Entellus Medical, Inc.
FIAGON is a trademark of Fiagon GmbH. Fiagon is distributed by Entellus Medical. Manufactured by Fiagon GmbH
SPONSORED CONTENT
1738-769 rA 08/2018 vs vs vs vs Balloon patients 1.7 1.0 0.2 5.0 2.8 1.0
FESS patients
XprESS™ ENT Dilation System
Improved outcomes. Supported by evidence. Proven in clinical practice.
“…because FYZICAL gave me the financial cushion to step back from treating, grow my practice and plan for the future. With the help of FYZICAL, I’ve been able to hire additional physician employees that generate revenue in the practice, even when I’m not there. I’ve now built a business that is actually worth something, and when the time comes, I can retire with wealth.”
- Dr. Vien Phommachanh
“…because FYZICAL showed us how to increase our hearing aid sales by 59%! And that’s with half the number of audiologists in our practice! We were missing so many opportunities before, and FYZICAL has helped us to supercharge our ancillary services.”
- Dr. Carl Stephenson
“…because I was doing my patients a disservice by not providing balance services. Since joining FYZICAL, my therapists have received the most up-to-date, world-class balance and vestibular training available and we’re now able to provide substantially better patient care across the board.”
- Dr. Jason Boole
www.BusinessofBalance.com

“…because before opening our center, when dizzy patients would come to me, I’d end up sending them to a therapist down the road and cross my fingers they were getting the care they needed. Now, I’m able to refer them to the balance center inside our practice and see their treatment the whole way through… and with FYZICAL’s proprietary balance program, I know they are getting top notch care.”
-
Dr.
Bill McFeely
“…because we never realized that our practice was worth next to nothing— unless we stayed in the practice and became an employee for someone else down the road. Now, we have significantly increased our ancillary revenue and the success and growth of our practice is no longer dependent on our presence, thus, creating an extremely valuable, attractive business.”
- Dr. Seth Palmer & Dr. Rich Strabbing

“…because it’s completely rejuvenated my passion as a mature practitioner again. I’m at the tail end of my career. I stopped performing surgeries a few years ago and have been treating common colds and runny noses. My passion had dwindled over the past few years, but I still wanted to help my patients. I am now able to have meaningful interactions with patients I was unable to help for so long. We’re also preventing falls, and making a true difference in these patients’ lives.
- Dr. Ed Shotts
“…because of the business skills I’ve learned. Like so many of my physician peers I didn’t receive education or experience in business while I was in school. FYZICAL has helped us implement daily management reports, pillars of success vital stats and so much more. I know all of our performance metrics, and it makes a huge difference managing our business. It isn’t just with our balance center either, we are actually improving quite a few things on the ENT side using what FYZICAL has taught us as well.”
- Dr. Sarah Powell
“…because it has been the biggest revenue generator in our practice, and FYZICAL made it easy. The FYZICAL team helped me design my balance center and trained my entire team with the most advanced balance protocols my therapists have ever seen. My team is so excited to be able to help our patients more than ever!”
- Dr. Chip
Hebert
“…because I’m now able to move on to the next stage of my career! FYZICAL has shown me how to operate a profitable, systems-driven practice. I have an exit strategy and a secure financial future I am now focused on helping physicians across the country add balance and help these patients too.”
- Dr. Daniel Deems
SPONSORED CONTENT
I Wish I Added a BALANCE & DIZZINESS CENTER Sooner… Private practice otolaryngologists from across the country share their thoughts on why they wish they added a balance center to their practice sooner.

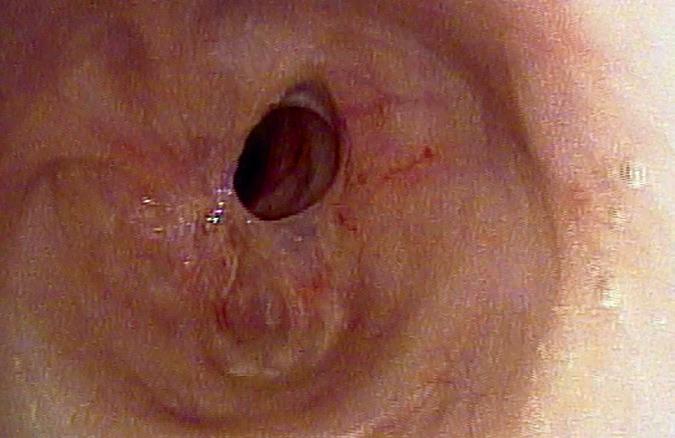

SPONSORED CONTENT
#1 DOCTOR RECOMMENDED EARPLUG BRAND IN THE U.S.*


#1-SELLING EARPLUG BRAND IN THE U.S.**
#1-SELLING & ORIGINAL FORMULA MACK’S® PILLOW SOFT® SILICONE EARPLUGS**


#1-SELLING MACK’S® SNORE BLOCKERS® SOFT FOAM EARPLUGS**

THE OFFICIAL EAR CARE OF USA SWIMMING®
Company Overview
Since 1962, McKeon Products, Inc. has led the hearing protection and ear care industry with its Mack’s® brand line of products. Today, McKeon Products, Inc. manufactures a full range of health care products for consumers. The Mack’s® line of products includes hearing protection (earplugs and earmuffs), ear drying aids, earwax removal aids, sleep masks, breathing improvement products, dental guards, and screen and lens wipes. McKeon, an ISO 9001:2015 quality certified company, is committed to supplying the highest quality, innovative consumer products.
macksearplugs.com
*Business Research Group, February 2014 | **Numbers based on Total FDM plus Walmart Nielsen Data 52 weeks ending 07/14/18
SPONSORED CONTENT
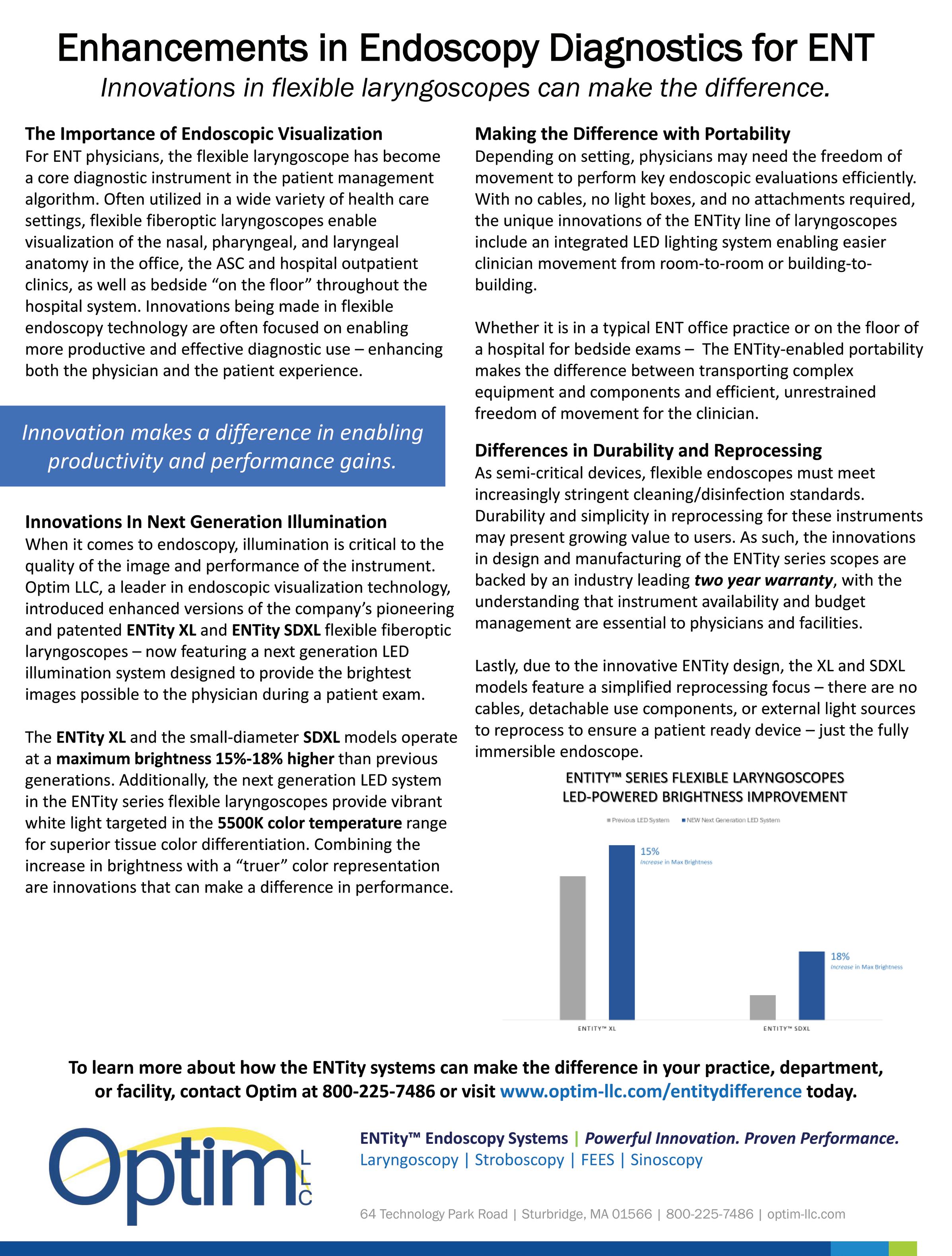
SPONSORED CONTENT
By Dr. John McElveen, Jr. –Carolina Ear and Hearing Clinic, Raleigh, NC
1. FIRST IMPRESSIONS MATTER – Like it or not, the way your office looks and is designed is a reflection of your practice. Get cabinets and chairs that help your practice stand out, are comfortable for patients, and that still function efficiently for you.
2. USE YOUR SPACE EFFECTIVELY – It’s critical to use your space wisely, because you’re paying rent by the square foot. My attention was drawn to Reliance Medical Products exam chairs. They were comfortable, but not bulky, have efficient control panels, and move smoothly and easily.

3. MAKE YOUR EQUIPMENT ACCESSIBLE TO ALL – Be sure that your chairs and cabinets can meet everyone’s needs. I’ve found success with Reliance chairs, which give you the ability to raise both arm and foot rests. This makes them accessible for both handicapped patients, and patients of all sizes.
4. RELIABILITY & DURABILITY ARE EVERYTHING – The most important features of any exam chair are reliability and endurance, because every chair that’s down means there is a room that’s down. Make sure you are not swayed by a low price point, but instead, make an investment in equipment that is built and designed to last.
“REMEMBER: THE CEO GETS PAID LAST.”
Four tips to equip your office with reliable chairs and cabinets.
For all the training we receive, many doctors get little training in the business aspects of medicine. I was no exception. After completing my ENT residency, neurotology fellowship and six years as an attending at Duke, I decided to set up my own private practice. Suddenly, I went from just taking care of patients to taking care of patients and my employees. I had to equip my office and temporal bone lab using my own money, and was met with the reality that the CEO gets paid last.
A major expense you’ll make as a private practitioner is for your chairs and cabinets, and the last thing you want is to continue spending money every couple of years to replace them. To help you find the right chairs and cabinets for your practice, and to protect your investment longer, here are four things to keep in mind as you search.
THE EQUIPMENT I USE
I made the right choice with chairs and cabinets from Reliance Medical Products. I opened the Carolina Ear & Hearing Clinic in 1993, and not only am I still using Reliance chairs, I am still using the SAME Reliance chairs I purchased back in 1993. With Reliance I don’t have to worry. Their chairs and cabinets have always been reliable. I know they can be for you as well.
Dr. John T. McElveen, Jr.* carolinaear.com
*Per Dr. McElveen’s request, he received no compensation for his endorsement.
To learn more about Reliance ENT Cabinets and Chairs, call 1-800-735-0357, or visit BuiltToSupport.com.
Reliance 7000 Procedure Chair
SPONSORED CONTENT
NOT ONLY AM I STILL USING RELIANCE CHAIRS, I AM STILL USING THE SAME CHAIRS I PURCHASED BACK IN 1993.
Samantha was a 45-year old married mother of three, working full-time and battling chronic sinusitis that seemed to have worsened despite the best efforts of her primary care providers.
Additionally, according to a 2012 study published in JAMA, the lack of efficacy of oral antibiotics for acute rhinosinusitis is documented (“antibiotics provide little if any benefit for patients with clinically diagnosed acute rhinosinusitis”), and alternative therapies such as nasal irrigation are encouraged. (JAMA. 2012;307(7):685-692. doi:10.1001/jama.2012.138)
Not only is saline nasal irrigation (SNI) as effective as antibiotics, but, when done regularly, the health benefits include improved resistance to infection, as cited by the National Center for Biotechnology:

After years of observing patients suffer, and knowing there must be a better way, Dr. Chandler invented a new design for the old nasal irrigation bottle - he calls it “ResQRinse®.”

She was referred to ENT specialist Dr. Stephen Chandler by her primary GP after multiple rounds of oral antibiotics, steroidal nasal sprays, and OTC decongestants had failed to provide sustained symptom relief.
Dr. Chandler recalls her predicament, saying Samantha was a fairly typical case of the ‘revolving-door’ approach to symptom management. Rather than searching for an underlying anatomic or medical predisposing symptom cause, patients are often treated based on symptom profile. She was prone to sinus infections, for which she was repeatedly treated with oral antibiotics and steroids.
So what if the cycle were broken and the emphasis was on prevention and early natural treatment for common sinus maladies?
Dr Chandler points out:
“Nasal irrigation (also known as nasal rinsing) is an inexpensive, simple, self-administered treatment, and has been shown to help relieve the symptoms of various sinus conditions, such as acute, sub-acute and chronic sinusitis, congestion, common colds and allergies.“
Nasal rinsing has been cited as being an appropriate adjunctive therapy for the symptoms of chronic rhinosinusitis, viral upper respiratory infections and allergic rhinitis. (Am Fam Physician. 2009 Nov 15; 80(10): 1117–1119.)
SNI may enhance the nasal mucosa’s ability to resist the effects of infectious agents, inflammatory mediators and irritants... Physiological effects (include) the direct cleansing of irrigation, the removal of inflammatory mediators, and improved mucociliary function, demonstrated by increased ciliary beat frequency. (Am Fam Physician. 2009 Nov 15; 80(10): 1117–1119)
Many medical professionals are optimistic at this proposal. However, the greatest obstacle to realizing the benefits of sino-nasal irrigation is patient compliance.
Dr. Chandler notes:
“Nasal rinsing is a centuries-old practice. While there are many nasal rinse systems on the market today, most have drawbacks that deter regular use, therefore limiting their efficacy (with nasal rinsing, regular compliance is key for best results). Common negative side effects of many of these systems include choking, gagging and burning—even nausea—as a result of saline solution running from the nasal passages down the back of the throat. For kids in particular—the largest user group of nasal rinse systems—that pain and irritation is an obvious obstacle.“
ResQRinse® can eliminate these typical negative, uncomfortable side effects through a physiological process called choanal occlusion. The process creates a barrier between the nasal passages and the back of the throat, and is triggered automatically when using ResQRinse®, due to the system’s patent-pending design. This creates a more pleasant user experience by preventing the saline solution from reaching the throat. The ResQRinse® system provides highly effective delivery of saline solution throughout the sinus cavity. Also, ResQRinse® is designed to work with the user in an upright position, further enhancing comfort (whereas many other nasal rinse systems require the user to tilt the head at an awkward angle).
ResQRinse® is easy to use for medical or surgical adjunctive treatment, as well as comprehensive sinonasal wellness and preventative care. The system is affordable, all-natural, drug-free, and travel-ready, making it easy for patients to follow prescribed treatment protocols.
Now available at Amazon, Walmart. com, independent neighborhood pharmacies, and www.ResQRinse.com.
ResQRinse® is a registered trademark of SinOptum, LLC, 2018 205 Ken Pratt Blvd. Ste 120 #77, Longmont, CO 80501 1.800.707.1414 ResQRinse.com NASAL RINSING
NON-COMPLIANT
&
PATIENTS
SPONSORED CONTENT
Nasal Lock™ Design Breath action activates natural palatial-choanal closure which prevents choking.
ENT solutions
Success is you in control
As an ENT, your role is complex. Your patients and staff look to you to lead. At Stryker, we deliver technology that puts control in your hands, from the XprESS ENT dilation system, to the LATERA absorbable implant, to our full product offering. Together, we are making success the standard. Explore Stryker’s ENT solutions at ent.stryker.com.

Stryker or its affiliated entities own, use, or have applied for the following trademarks or service marks: LATERA, XprESS and Stryker.












































SMART is Better TRANSFORM YOUR PRACTICE WITH ARTIFICIAL INTELLIGENCE Our Advantage SMART Practice™ suite of All-in-One Solutions use real-time data from your clinic to optimize efficiency, improve outcomes, and increase revenue. EHR | PM | RCM | ASC ANALYTICS | PATIENT ENGAGEMENT TELEHEALTH | 2015 ONC CERTIFIED ENT SPECIFIC EHR & PM Leader for 33 Years Request a demo TODAY www.compulinkadvantage.com/ENTEHR | 800.456.4522
Bath time should be fun, not painful.

Mack’s® is the #1 Doctor Recommended earplug brand to help prevent otitis externa.*

Available at Albertson’s, Big 5, Discount Drug Mart, Hy-Vee, Meijer, Publix and Wegman’s
*Business Research Group, February 2014.
















 Brian J. McKinnon, MD, MBA, MPH, FACS Associate Professor and Vice Chair
Department of Otolaryngology–
Head and Neck Surgery Associate Professor
Brian J. McKinnon, MD, MBA, MPH, FACS Associate Professor and Vice Chair
Department of Otolaryngology–
Head and Neck Surgery Associate Professor



































































































































































