

Physicochemical Analysis of Seawater on the East Coast of Doha
Zakarya J. AldeebDoha College, Al Niser St, Doha, Qatar, 7506, Qatar; zakaryajehadaldeeb@gmail.com
ABSTRACT: The quality of seawater is very important for the state of Qatar as it is a peninsula. This paper is focused on the analysis of several physicochemical parameters, such as pH, Conductivity, Total Dissolved Solids (TDS), Turbidity, Alkalinity (M), Total Hardness, Silica, Sulphate, Iron, Nitrate, Phosphate, and Temperature from different sampling sites along major coastal areas on the East Coast of Qatar—namely the Doha Corniche, Al Khor, and Mesaieed. The results revealed that Qatari coastal waters contained typical values of each physicochemical parameter analyzed and were clean and unaffected by the risks of polluted waters or large-scale algal blooming. Across all nine samples, the pH values of the different water samples ranged from 7.7-7.9. Across all nine samples, the conductivity ranged from 57,590 to 64,960 μS/cm. Across all nine samples, the TDS readings ranged from 41110 - 43380 ppm. Across all nine samples, the turbidity ranged from 1 to 2.65 NTU. Across all nine samples, the alkalinity ranged from 110 to 127 ppm as CaCO3. Across all nine samples, the hardness ranged from 6640 to 6515 ppm as CaCO3. Across all nine samples, the Silica levels ranged from 0.15 to 0.23 ppm as Si. Across all nine samples, the sulfate levels ranged from 2140 to 2145 ppm as SO4. Across all nine samples, the iron levels ranged from 0.03 to 0.1 ppm as Fe+3. Across all nine samples, the nitrate levels ranged from 3.2 to 6.6 ppm as NO3. Across all nine samples, the phosphate levels all read 0 ppm as PO4 (below the detection limit). Across all nine samples, the temperature levels ranged from 24 to 28.8°C.
KEYWORDS: Chemistry; Geology; Seawater; Analysis; Physicochemical.
� Introduction
There are three major coastal areas on the east coast of Qatar: Mesaieed, Al Khor, and the Doha Corniche; these areas are exposed directly to the Arabian Gulf. Three samples of seawater were collected from each site and analyzed for each parameter, after appropriate conservation of the samples, in a laboratory for the physicochemical parameters pH, Conductivity, Total Dissolved Solids (TDS), Turbidity, Alkalinity (M), Total Hardness, Silica, Sulphate, Iron, Nitrate, Phosphate, and Temperature. This study aimed to analyze some critical physicochemical characteristics of seawater in these natural bodies of water on the east coast of Qatar to determine whether they were clean and thriving. The State of Qatar has one of the highest per capita water consumption rates in the world,1 as well as being among the countries with the lowest levels of annual rainfall in the world,2 so the state is heavily reliant on seawater and desalination processes to meet this demand. As a result, seawater quality is essential to maintain a healthy living standard. This study analyses seawater from the mentioned major coastal areas and the levels of all the respective physicochemical characteristics tested, comparing them against the sea and natural bodies of water’s quality standards as well as comparison against neighboring countries along the Persian Gulf. The main objective of this study is to evaluate the quality of open seawater on the east coast of Doha and identify any abnormalities that may pose a risk to the marine environment and the State. The individual, location-dependent results of the study are shown in Tables 1, 2, and 3. The mean levels of all parameters across all sample collection areas are shown in Table 4.
� Methods
Samples of surface water were collected from seawater on the east coast of Qatar in the following sites: The Doha Corniche, Al Khor, and Mesaieed. These samples were then analyzed for specific physicochemical characteristics. Three samples were taken from every sample collection site, all collected in sterile, tightly closed glass bottles, leaving nine samples from all sites to be analyzed. Figures 1, 2, and 3 show a map of the sample collection areas. The following physicochemical properties were analyzed: pH, Conductivity, Total Dissolved Solids (TDS), Turbidity, Alkalinity (M), Total Hardness, Silica, Sulphate, Iron, Nitrate, Phosphate, and Temperature. pH was measured through electrometric pH measurement to determine the activity of hydrogen ions by potentiometric measurement using a standard hydrogen electrode and a reference electrode (APHA 4500-H+ B).3 Conductivity was measured through a laboratory method involving conductivity instruments (APHA 2510 B).4 TDS was measured through potentiometric titration. Turbidity was measured through a nephelometric method based on comparing the intensity of light scattered by the sample under defined conditions with the intensity of light scattered by a standard reference suspension under the same conditions (APHA 2130 B).5 Alkalinity (M) was measured through a titration method (APHA 2320 B).6 Total Hardness was measured through an EDTA titrimetric method (APHA 2340 C).7 Silica was measured through a Molybdosilicate method where ammonium molybdate at pH 1.2 is reacted with silica to produce heteropoly acids (APHA 4500-Si D).8 Sulphate was measured through a Turbidimetric
method where sulfate is precipitated in an acetic acid medium with barium chloride to form barium sulfate crystals (APHA 4500-SO4 2- E).9 Iron was measured through a Phenanthroline Method (APHA 3500-Fe B).10 Nitrate was measured through a Cadmium Reduction method where nitrate is reduced in the presence of cadmium to produce NO2, which is determined by diazotizing with sulfanilamide and coupling with n-(1-naphthyl)ethylenediamine dihydrochloride to form a colored azo dye that is measured colorimetrically (APHA 4500-NO3- E).11 Phosphate was measured through a Vanadomolybdophosphoric acid Colorimetric method (APHA 4500-P C).12 The temperature was measured on-site using mercury-filled Celsius thermometers with a scale marked for every 0.1°C, with markings etched on the capillary glass. (APHA 2550 B).13


sample collection sites within The
The exact GPS coordinates of these sample collection sites are as follows: 25° 41' 9.1284'' N 51° 31' 26.4936'' E, 25° 41' 1.6116'' N 51° 31' 45.0444'' E, and 25° 41' 11.2668'' N 51° 30' 57.9348' E '

The exact GPS coordinates of these sample collection sites are as follows: 24° 52' 10.8768'' N 51° 31' 51.8016'' E, 24° 54' 11.9664'' N 51° 33' 36.9252'' E, and 24° 45' 30.3984'' N 51° 28' 55.3476'' E
� Result and Discussion

pH: The pH values of the different water samples ranged from 7.7-7.9 across all nine samples; Figure 4 shows the mean pH values of the three samples collected in each location. All of these pH values are considered within the recommended range of the WHO (World Health Organization) primary water quality criteria for class SW-I Waters of 6.5 - 8.5 and are weakly alkaline.14 The pH observed from the Corniche samples appeared slightly higher than that of the other two testing sites. pH is a very stable parameter in seawater due to the enormous buffering capacity of seawater. Even during algal blooms with their associated high carbon dioxide consumption, pH fluctuates only slightly.
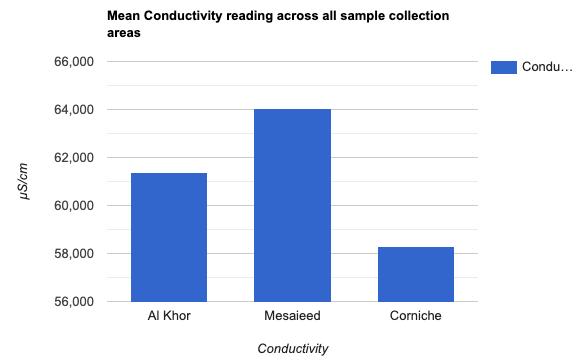
Conductivity: Conductivity is a measure of the ability of water to pass an electric current. These electric currents are conducted by dissolved salts and other inorganic chemicals in water, which play a significant role in determining water quality. More of these impurities lead to higher conductivity. Across all nine samples, the conductivity ranged from 57,590 to 64,960 μS/cm. Figure 5 shows the mean conductivity across the three samples collected in each location (61363, 64043, and 58290 μS/cm, respectively). The typical conductivity for seawater is 5 S/m,15 which is about 50000 μS/cm; we can observe a difference between this specific conductivity and the ones observed in the samples, as it is slightly higher.

TDS (total dissolved solids) represents the total concentration of substances dissolved in water; this includes inorganic salts and organic matter. Inorganic salts include calcium, magnesium, and potassium salts (cations), carbonates, nitrates, chlorides, and sulfates (anions). A considerable amount of variation was observed in the conductivity across all samples, ranging from 41110 - 43380 ppm. Figure 6 shows the mean levels of TDS across the three samples collected in each location (41210, 43345, and 38030 ppm, respectively). The typical value of TDS in seawater is 35,000 mg/L,16 which is 35,000 ppm. All of the values recorded were found to be above this typical value. However, this is expected as Qatar is located in the Arabian Gulf. The hot and arid climate the Arabian Gulf experiences leads to high evaporation rates, which cause the concentration of dissolved salts and minerals. As a result, a higher TDS is expected. A study conducted at Khafji Beach in the neighboring country Saudi Arabia showed that in the winter (season of sample collection), mean TDS levels could be as high as 45082 ppm, with a minimum value of 43500 and maximum level of 46900 ppm.17 Considering the following, it can be determined that the recorded TDS levels are typical, considering Qatar's geographical location.

Turbidity is a measure of the relative clarity of the water and the degree to which it loses its transparency due to the presence of suspended solids. It is considered a great indicator of water quality as it is a usually obvious optical way to distinguish healthy water. Suspended solids and dissolved colored materials from erosion, runoff, and algal blooms in high quantities have a noticeable effect on the appearance and clarity of the water, as turbid waters will appear murky and colored. Across all nine samples, the turbidity ranged from 1 to 2.65 NTU. Figure 7 shows the mean conductivity across the three samples collected in each location (1.24, 1.14, and 2.35 NTU, respectively). The optimal range for turbidity in seawater is anything below 10 NTU,18 and all recorded values are within this range, all reading very low turbidity. However, higher mean turbidity was observed in samples taken at the Corniche.

Alkalinity is a chemical measurement of water’s ability to neutralize acids. Alkalinity in natural waters like these is primarily because of weak acid salts within the water. Therefore, it is an important indicator as the alkalinity of a body of water informs us about risks, such as how sensitive that body of water will be to acid inputs such as acid rain.19 Across all nine samples, the alkalinity ranged from 110 to 127 ppm as CaCO3. Figure 8 shows the mean alkalinity across the three samples collected in each location (123, 117 and 124, respectively, rounded to the nearest ppm if necessary). According to research conducted by the Global Seafood Alliance,20 seawater alkalinity typically varies from 100-130 mg per L (100-130 ppm) as CaCO3, with an average alkalinity of 116 mg per L (116 ppm) in full-strength seawater. Our recorded values are within this range, with much lower mean alkalinity observed in Mesaieed.

The hardness of water is the measured content of divalent metal cations, mainly the cations Ca2+ and Mg2+ is expressed
as an equivalent of calcium carbonate. Across all nine samples, the hardness ranged from 6640 to 6515 ppm as CaCO3. Figure 9 shows the mean hardness across the three samples collected in each location (6510, 6448, and 6470, respectively, rounded to the nearest ppm if necessary). According to the same research conducted by the Global Seafood Alliance,21 seawater hardness typically varies from 5800-7500 mg per L (58007500 ppm) as CaCO3 in full-strength seawater. Our recorded values are within this range, with a slightly higher mean hardness observed in Al Khor.

Silicon dioxide (Si) or silica is an oxidized form of silicon and the main constituent of sand. It is abundant in nearly all bodies of water. Marine creatures require silica to produce their exoskeletons, such as some phytoplankton, specifically diatoms. Diatoms are among many species that rely on Silica input for life, and they contribute to over 40 percent of photosynthesis in the world’s oceans.22 In addition, silica dissolved in natural bodies of water is considered a good indicator of weathering,23 a necessary natural process contributing to soil formation. Across all nine samples, the Silica levels ranged from 0.15 to 0.23 ppm as Si. Figure 10 shows the mean Silica levels across the three samples collected in each location (0.19, 0.21 and 0.19 ppm, respectively, rounded to two significant figures if necessary). An article by Lenntech detailing Silica in natural bodies of water states that in surface layers of natural water (specifically oceans), silicon concentrations are typically 30 ppb (0.03 ppm) as Si.24 All of our recorded values are close to this typical value.

Sulphate (as SO4) is one of the most significant anions found in seawater and can be found in almost all natural bodies of water. There is no literature to confirm whether abnormal sulfate levels negatively affect saltwater environments or marine life. However, most sulfate pollution in waters originates from
industrial waste, so abnormally high levels of sulfate pollution may be considered a sign of unclean waters to the extent that they are less suitable for public or industrial supplies. Across all nine samples, the sulfate levels ranged from 2140 to 2145 ppm as SO4. Figure 11 shows the mean Sulphate levels across the three samples collected in each location (2142, 2143, and 2142, respectively, rounded to the nearest ppm if necessary). Research conducted by the WHO on nutrients in drinking water reveals that sulfate concentration in typical seawater is 2649 mg/L.25 All of our recorded values are below this typical value, though not to an extent to which the water would be significantly affected. This is likely because there needs to be more industrial activity/waste disposal within the tested areas.

Iron (as Fe+3) is another naturally occurring ion in seawater. It is an essential dietary requirement for most organisms, and it is necessary for phytoplankton growth. Phytoplankton has a significant effect on the carbon cycle. They play a vital role in maintaining the earth’s climate, as they are responsible for most of the transfer of carbon dioxide from the atmosphere to the ocean. Iron in seawater usually originates from weathering processes and dissolving. So naturally, iron is not very abundant in seawater, typically at 0.01 mg/L.26 The iron levels ranged from 0.03 to 0.1 ppm across all nine samples as Fe+3. Figure 12 shows the mean Iron levels across the three samples collected in each location (0.04, 0.06, and 0.1, respectively, rounded to 2 significant figures if necessary). All of our recorded values are close to this typical value and ideal.

Nitrate (as NO3) is another naturally occurring seawater ion and part of the nitrogen cycle. It is used as a food source by live plants, and nitrate levels from 0 - 40 ppm are generally safe for fish, with levels exceeding 80 at risk of being toxic.27 Nitrates in seawater usually originate from the dissolving of nitrogen gas
from the atmosphere, which is then, through nitrogen fixation, converted into ammonium by cyanobacteria. Most of this ammonium is converted through nitrification by certain nitrifying bacteria into nitrate (and nitrites). It is important to note that ammonia and other nitrogen compounds play an essential role in water, especially in phytoplankton and marine plants unaffected by the risk of possible toxicity. Across all nine samples, the nitrate levels ranged from 3.2 to 6.6 ppm as NO3. Figure 13 shows the mean Nitrate levels across the three samples collected in each location (6.5, 6.6, and 3.3, respectively, rounded to two significant figures if necessary). Literature in the online journal “Reefkeeping” by Randy Holmes Farley revealed that nitrate concentration in water typically ranges between 0.06 - 30 ppm.28 Our recorded values are within this range, with a lower mean nitrate concentration observed in the Corniche. This is likely due to the much more plant-populated nature of the Corniche’s waters observed in the sample collection area. These plants rely on nitrates as their primary nitrogen source, contributing to a lower overall nitrate concentration.

Phosphate (as PO4) is another anion found in seawater. It is scarcely found in high concentrations in water as it is a crucial nutrient for life, including aquatic life, and is actively taken up by plants. As a result, abnormally high phosphate concentrations in waters are primarily responsible for eutrophic conditions 29 and are a great indicator of polluted waters. Across all nine samples, the phosphate levels all read 0 ppm (possibly below the detection limit) as PO4. Figure 14 shows the mean phosphate levels across the three samples collected in each location (All 0 ppm). This means these waters have no phosphate pollution and are very clean. This also indicates that Qatari waters are not vulnerable to massive algal blooming, as phosphorus is a significant factor limiting phytoplankton growth. Therefore, a concentration of 0 ppm phosphorus will not support the outbreak of large-scale algal blooms.

The temperature of water rarely affects clean bodies of water due to the wide range of temperature tolerance in aquatic life and temperature variation within environments worldwide. However, it must be noted that in polluted waters, the temperature can affect BOD (Biological Oxygen Demand) and DO (Dissolved Oxygen) in the water, which can, in turn, harm the water and marine environment. Temperature is also not a reliable indicator of seawater quality. It depends on the time of day, location, and many other factors that could cause drastic differences in the reading. The temperature observed on-site in the sample collection areas is typical of the Arabian Gulf. Along the coast of the Arabian Gulf, summer temperatures may (will not necessarily always) range from 30.6 to 36.8°C, winter temperatures from 11 to 22°C, spring temperatures from 19.7 to 32.8°C and autumn temperatures from 18.8 to 32.8°C. In comparison, the air temperature never drops below 0°C.30 Across all nine samples, the temperature levels ranged from 24 to 28.8°C. Figure 15 shows the mean Temperature across the three samples collected in each location (24.5, 24.8, and 27.9, respectively, rounded to one decimal place if necessary). Our recorded values are typical, considering Qatar’s geographical location and climate.


Table 3: The table shows the levels and mean levels of the tested physicochemical characteristics across all sample collection sites in The Corniche.

Table 4: Table 4 shows the mean levels of the tested physicochemical characteristics across all sample collection areas

� Conclusion
The analysis results revealed that (as shown in Table 4) the mean values of parameters were as follows. The mean pH level was 7.7; the mean Conductivity level was 61232 μS/cm; TDS levels were 40861.667 ppm; turbidity levels were 1.577 NTU, Alkalinity (M) levels were 121.333 ppm as CaCO3, Total Hardness levels were 6476 ppm as CaCO3, Silica concentrations were 0.197 ppm as Si, Sulphate concentrations were 2142.333 ppm as SO4, Iron concentrations were 0.067 ppm as Fe+3, Nitrate levels were 5.467 ppm as NO₃, Phosphate levels were 0 ppm as PO4. Temperature levels were 25.733 degrees Celsius. The study confirmed that Qatari waters contain typical amounts of each parameter analyzed and that the waters are clean and unaffected by risks of polluted waters or large-scale algal blooming.
� References
1. Water Use Statistics: Worldometer Water Use Statistics, Published by Worldometer https://www.worldometers.info/water/
2. Average precipitation in depth (mm per year) | Data: Average precipitation in depth (mm per year), Published by World Bank https://data.worldbank.org/indicator/AG.LND.PRCP.MM?most_recent_value_desc=true
3. Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017. https://books.google.com/books/about/Standard_Methods_for_ the_Examination_of.html?id=V2LhtAEACAAJ&source=kp_ book_description&redir_esc=y
4. Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
5. Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
6. Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
7. Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
8. Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
9. Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
10.Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
11.Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
12.Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
13. Standard Methods for the Examination of Water and Wastewater: Baird, Rodger. and Laura Bridgewater. Standard Methods for the Examination of Water and Wastewater. 23rd edition. Washington, D.C., American Public Health Association, 2017.
14.64.0 WATER QUALITY STANDARDS FOR COASTAL WATERS MARINE OUTFALLS: “64.0 WATER QUALITY STANDARDS FOR COASTAL WATERS MARINE OUTFALLS” Published by the Central Pollution Control Board (CPCB) https://cpcb.nic.in/wqm/coasteal_water_standards.pdf
15.Water conductivity - Lenntech: “Water Conductivity” Published by Lenntech https://www.lenntech.com/applications/ultrapure/conductivity/ water-conductivity.htm
16.WATER QUALITY PARAMETERS & INDICATORS: “WATER QUALITY PARAMETERS & INDICATORS” Published by the Sustainable Sanitation and Water Management Toolbox https://sswm.info/sites/default/files/reference_attachments/MCCAFFREY%20ny%20Water%20Quality%20Parameters%20 &%20Indicators.pdf
17.Evaluating suitability of source water for a proposed SWRO plant location: Mohamed O. Saeed, Mohammed A. Al-Nomazi, Ahmed S. Al-Amoudi. Evaluating suitability of source water for a proposed SWRO plant location. Heliyon 5 (2019) e01119. doi: 10.1016/j. heliyon.2019. e01119 https://www.swcc.gov.sa/uploads/Evaluating%20suitability%20 of%20source%20water%20for%20a%20proposed%20SWRO%20 plant%20location.pdf
18.WATER QUALITY PARAMETERS & INDICATORS: “WATER QUALITY PARAMETERS & INDICATORS” Published by the Sustainable Sanitation and Water Management Toolbox https://sswm.info/sites/default/files/reference_attachments/MCCAFFREY%20ny%20Water%20Quality%20Parameters%20 &%20Indicators.pdf
19.Alkalinity and Water | U.S. Geological Survey: “Alkalinity and Water” Published by the United States Geological Survey (USGS), Written by “Water Science School” https://www.usgs.gov/special-topics/water-science-school/science/ alkalinity-and-water#:~:text=Alkalinity%20is%20the%20most%20 readily,and%20streams%20to%20acidic%20deposition
20.Typical chemical characteristics of full-strength seawater - Responsible seafood Advocate: Claude E. Boyd “Typical chemical characteristics of full-strength seawater” https://www.globalseafood.org/advocate/typical-chemical-characteristics-of-full-strength-seawater/?headlessPrint=AAAAAPIA9c
21.Typical chemical characteristics of full-strength seawater - Responsible seafood Advocate: Claude E. Boyd “Typical chemical characteristics of full-strength seawater”
22.The evolution of diatoms and their biogeochemical functionsPMC: Benoiston AS, Ibarbalz FM, Bittner L, Guidi L, Jahn O, Dutkiewicz S, Bowler C. The evolution of diatoms and their biogeochemical functions. Philos Trans R Soc Lond B Biol Sci. 2017 Sep 5;372(1728):20160397. Doi: 10.1098/rstb.2016.0397. PMID: 28717023; PMCID: PMC5516106. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5516106/
23.Silica origin and solubility in groundwater from the weathered zone of sedimentary rocks of the Intra-Sudetic Basin, SW Poland: Dobrzyński, Dariusz. “Silica origin and solubility in groundwater from the weathered zone of sedimentary rocks of the Intra-Sudetic Basin, SW Poland.” Acta Geologica Polonica 55 (2005): 445-462. https://www.researchgate.net/publication/235889942_Silica_origin_and_solubility_in_groundwater_from_the_weathered_zone_ of_sedimentary_rocks_of_the_Intra-Sudetic_Basin_SW_Poland
24.Silicon (Si) and water: “Silicon (Si) and water” Published by Lenntech (No DOI) https://www.lenntech.com/periodic/water/silicon/silicon-and-water.htm
25.Nutrients in Drinking Water: J.A. Cotruvo, Desalination Guidelines Development for Drinking Water: Background, Joseph Cotruvo & Associates LLC, Washington D.C., USA, 2016. https://www.barrier.ru/upload/%D0%94%D0%BE%D0%BA%D0%BB%D0%B0%D0%B4 _%D0%92%D0%9E%D0%97.pdf#page=22
26. Nutrients in Drinking Water: J.A. Cotruvo, Desalination Guidelines Development for Drinking Water: Background, Joseph Cotruvo & Associates LLC, Washington D.C., USA, 2016.
https://www.barrier.ru/upload/%D0%94%D0%BE%D0%BA%D0%BB%D0%B0%D0%B4 _%D0%92%D0%9E%D0%97.pdf#page=22
27.nitrate and nitrite: “Nitrate and Nitrite” Published by Lenntech https://www.lenntech.com/hazardous-substances/nitrate-and-nitrite.htm
28.What is Seawater? by Randy Holmes-Farley - Reefkeeping.com: Randy Holmes-Fairley, What is Seawater? Reefkeeping: an Online Magazine, 2005. Issue 2005-11, 18 pp. https://reefkeeping.com/issues/2005-11/rhf/index.php#19
29.Phosphorus and Freshwater Eutrophication Pressure Narrative Contents: UK Technical Advisory Group for the Water Framework Directive “Freshwater Eutrophication” https://consult.environment-agency.gov.uk/++preview++/environment-and-business/challenges-and-choices/user_uploads/ phosphorus-pressure-rbmp-2021.pdf
30.https://books.google.com.qa/books?hl=en&lr=&id=y4h9CAAAQ BAJ&oi=fnd&pg=PT18&ots=2x9cUTM2F-&sig=C7kaefSys8oDmXqbGlHPenhUWM4&redir_esc=y#v=onepage&q&f=false Al-Sayari, Saad S., and Josef G. Zötl, eds. Quaternary period in Saudi Arabia: 1: sedimentological, hydrogeological, hydrochemical, geomorphological, and climatological investigations in central and eastern Saudi Arabia. Springer Science & Business Media, 2012.
�
Authors Zakarya Jehad Aldeeb: 12-year-old secondary student at Doha College (Year 8).
