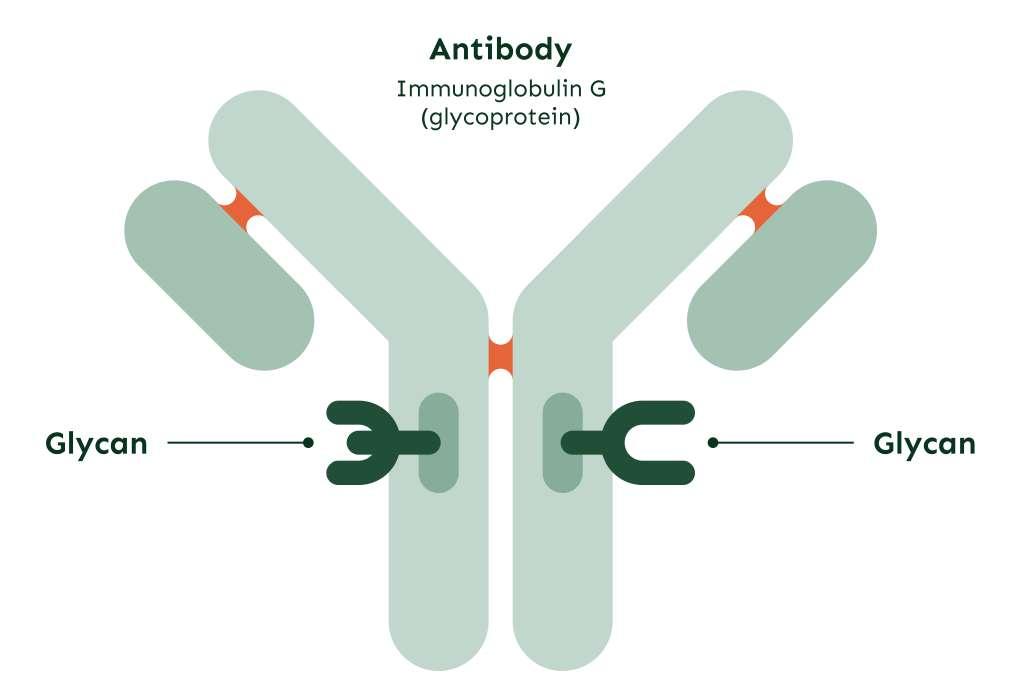
Glycans as biomarkers and functional effectors of ageing and disease
GlycanAge
GlycanAge is a biological age test that analyses glycans. Glycans are complex sugar molecules found in every cell in our bodies.
Glycans are involved in many cellular processes and can be influenced by a variety of factors, such as diet, lifestyle, and genetics. By measuring the levels and patterns of glycans in a person's blood sample, our scientists can calculate an individuals biological age.
The GlycanAge test is based on research that has shown that changes in glycans are associated with aging and age-related diseases. Measuring these changes provides insights into a person's health and potential risk of developing age-related diseases in the future.
Table of contents
What is GlycanAge?
What does it measure?
What is IgG N-Glycosylation?
Who already uses GlycanAge?
How does GlycanAge work?
GlycanAge Partners benefits
Exclusive platform access
What is IgG glycome?
Glycation versus glycosylation
Building blocks of IgG N-glycans
Changes in IgG glycome with age
Changes in IgG glycome with ill-health
Disease-specific changes in IgG glycosylation
Global variability of the human glycome
Heritability and environmental effect on GlycanAge results
Understanding different indexes presented in the GlycanAge report
Glycan Mature
Glycan Median
Glycan Youth
Glycan Shield
This eBook is a free gift for anyone who registered for the Reverse Inflammaging Summit, 2023. Contact for any additional information. partners@glycanage.com
Glycan Lifestyle
How does GlycanAge compare to other tests?
Intro
What is GlycanAge?
GlycanAge is a patented test that analyzes a unique profile of complex carbohydrates (glycans) attached to Immunoglobulin G (IgG), the most abundant antibody in our blood, to determine biological age.
The test was first registered by Genos Ltd, a private research institute founded by Prof. Gordan Lauc, PhD, and later transferred to GlycanAge Ltd, a UK-based biotech start-up. GlycanAge uses capillary electrophoresis to analyze IgG N-glycan profile, which affects its structure and function, as well as interactions with other cells and molecules of the immune system. The ratios of different structures differ depending on age and health.
What does GlycanAge measure?
Biological age tests employ varying biomarkers and aging clocks, yielding distinct values for biological age. The GlycanAge test analyzes age-associated IgG N-glycans to determine biological age.
The GlycanAge test has associations with various chronic diseases and disease risk factors such as BMI, CRP, FEV1, and systolic blood pressure.
Compared to the epigenetic age clocks developed by Horvath and Hannum, the GlycanAge test offers distinct and valuable insights into the aging process based on its analysis of age-associated IgG N-glycans.
What is IgG N-Glycosylation?
IgG N-glycosylation is a process in which complex carbohydrates are attached to IgG, a type of antibody found in human blood. This process affects the structure and function of IgG and its interactions with other cells and molecules of the immune system.
The glycans can be attached to IgG through N-glycosylation, which is linked to the amino acid asparagine. This process occurs within B cells, where IgG is produced, and it is modified by different stimuli during activation and differentiation.
GlycanAge is already used by leading health and longevity experts
We’ve partnered with over 400 providers to:
Take a preventative approach to healthcare •
Measure success of their interventions
•
Optimise health for their clients









How does GlycanAge work?
GlycanAge is a simple and non-invasive test that can be performed in your clinic or lab.
Collect sample
Collect a blood sample from your patient
Send sample
Send the sample to our lab for analysis
insights
Get actionable insights on your patient's biological age and glycan profile

Use this data to tailor personalized treatment plans and monitor the effectiveness of your interventions over time



•
Gain
Use insights 1 2 3 4
Exclusive platform access GlycanAge Partners benefits
We offer premium service and support to all our partners. Our primary goal is to educate and provide meaningful medical value.
Purchase tests at wholesale prices Early access to new products
Additional scientific insights for any qualified partners
Accreditation through our online education course


GlycanAge Partners also get exclusive access to all of our knowledge, experience, technology, as well as early insights into future research.
Experience
We’ve been leading the field of glycobiology for over 20 years now, publishing over 200 papers based on over 200,000 samples.
Technology
We have the laboratory equipment, IP, know-how and technology required to perform high-throughput glycan analysis.
Discoveries
We collaborate with leading universities and process 85% of samples for high-throughput glycomic studies in the entire world!

1 2 3
What is IgG glycome?
The IgG glycome refers to the collection of all the glycans attached to IgG molecules in a person's body.
There are over 30 different N-glycan structures present on IgG molecules in every person, and the ratios of different IgG glycan structures can differ depending on age and health between individuals.
The IgG N-glycome is also sometimes referred to as the IgG glycome.
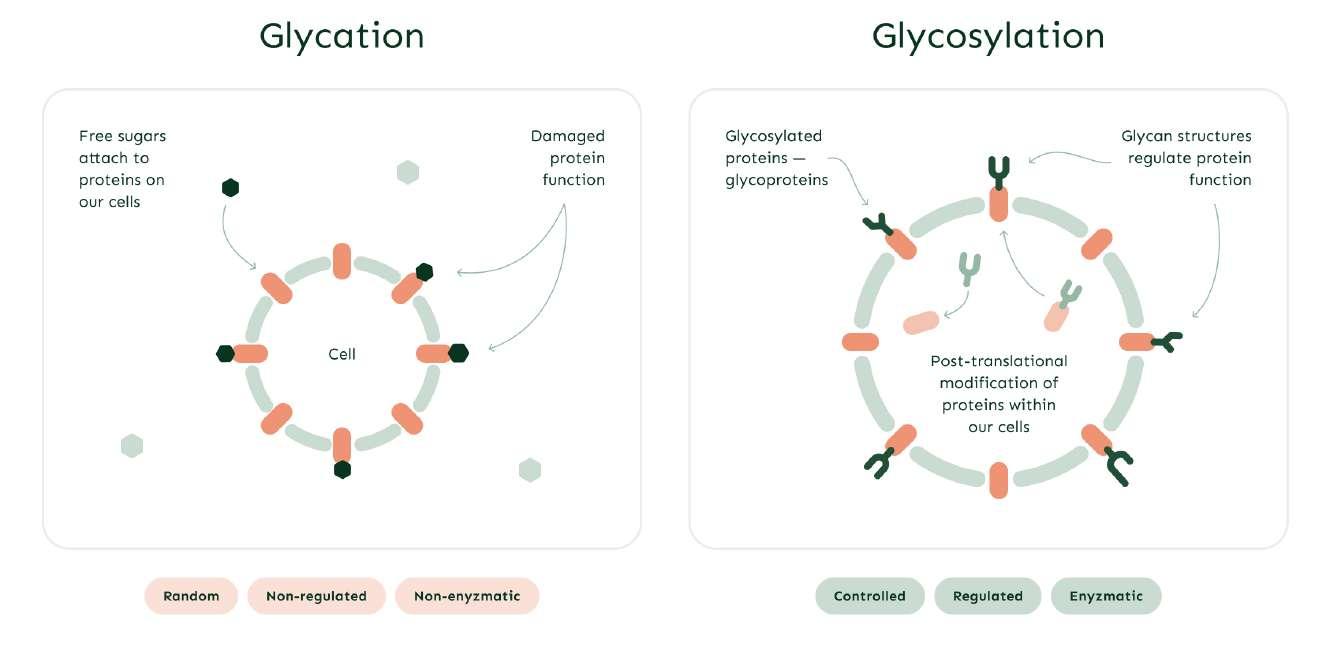
Glycation versus glycosylation
Glycans are complex carbohydrates and one of the four basic building blocks of life. One common misconception is that IgG glycans are formed by the attachment of free blood glucose, which is not correct.
Glycosylation — a controlled process responsible for glycan production by various enzymes; is distinct from glycation — a random and nonenzymatic process.
Within B cells, IgG glycosylation is modulated by various stimuli received during their activation and differentiation.
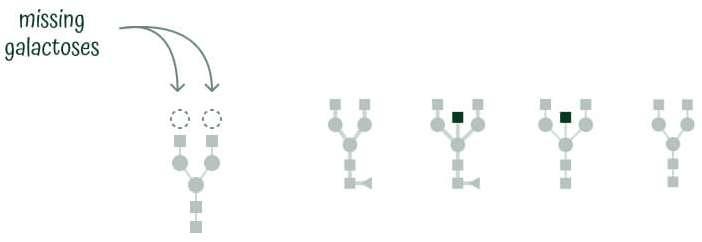
Building blocks of IgG N-glycans
IgG N-glycans are tree-like structures with two branches (or arms) on top of a GlcNAc and mannose base, and sometimes a third branch (arm) – bisecting GlcNAc.
There are five building blocks found in IgG N-glycans in humans: NAcetylglucosamine, core fucose, mannose, galactose, and sialic acid.
All IgG N-glycans share the same backbone of two GlcNAc molecules and three mannose molecules, with additional carbohydrates getting attached to this backbone. To group and classify different glycans, they are separated into groups based on their structural properties.
Glycans with no galactoses are agalactosylated glycans (G0), those with one galactose are monogalactosylated (G1), and those with two galactoses are digalactosylated glycans (G2). Glycans with at least one sialic acid are sialylated glycans (S), those with a core fucose are fucosylated glycans (F), and those with a third middle arm are bisected glycans (B).

Changes in IgG glycome with age
Research has shown an association between IgG N-glycosylation, aging, and health. A distinct pattern of IgG glycan trends during aging has been identified, with two distinct groups of glycan structures:
Structures associated with aging and pro-inflammatory states (G0 and B)
Structures thought to be mainly antiinflammatory (G2 and S) whose proportion decreases with advancing age and ill-health.
Other glycan structures (G1 and F) are not as strongly associated with aging but show an association with general health. The structures most strongly associated with aging (G0, G2, and S) make up the glycan clock of aging in the GlycanAge test.
Changes in IgG glycome with ill-health
The GlycanAge test is associated with well-known clinical biomarkers of health, including waist circumference, BMI, HbA1C, triglycerides, blood glucose, calcium, D-dimer, cholesterol, LDL C, uric acid, fibrinogen, insulin, and CRP.
Many diseases and conditions, particularly inflammatory, alloimmune, and autoimmune diseases, show abnormal IgG N-glycosylation, characterized by a low level of galactosylation and an increase in agalactosylated glycan structures (G0), and a decrease in digalactosylated (G2) and sialylated (S) glycan structures. Decreased sialylation and increased bisecting GlcNAc also associate with advanced age and obesity.
It is noteworthy that the IgG N-glycosylation profile can turn pro-inflammatory years before the occurrence of any disease symptoms.
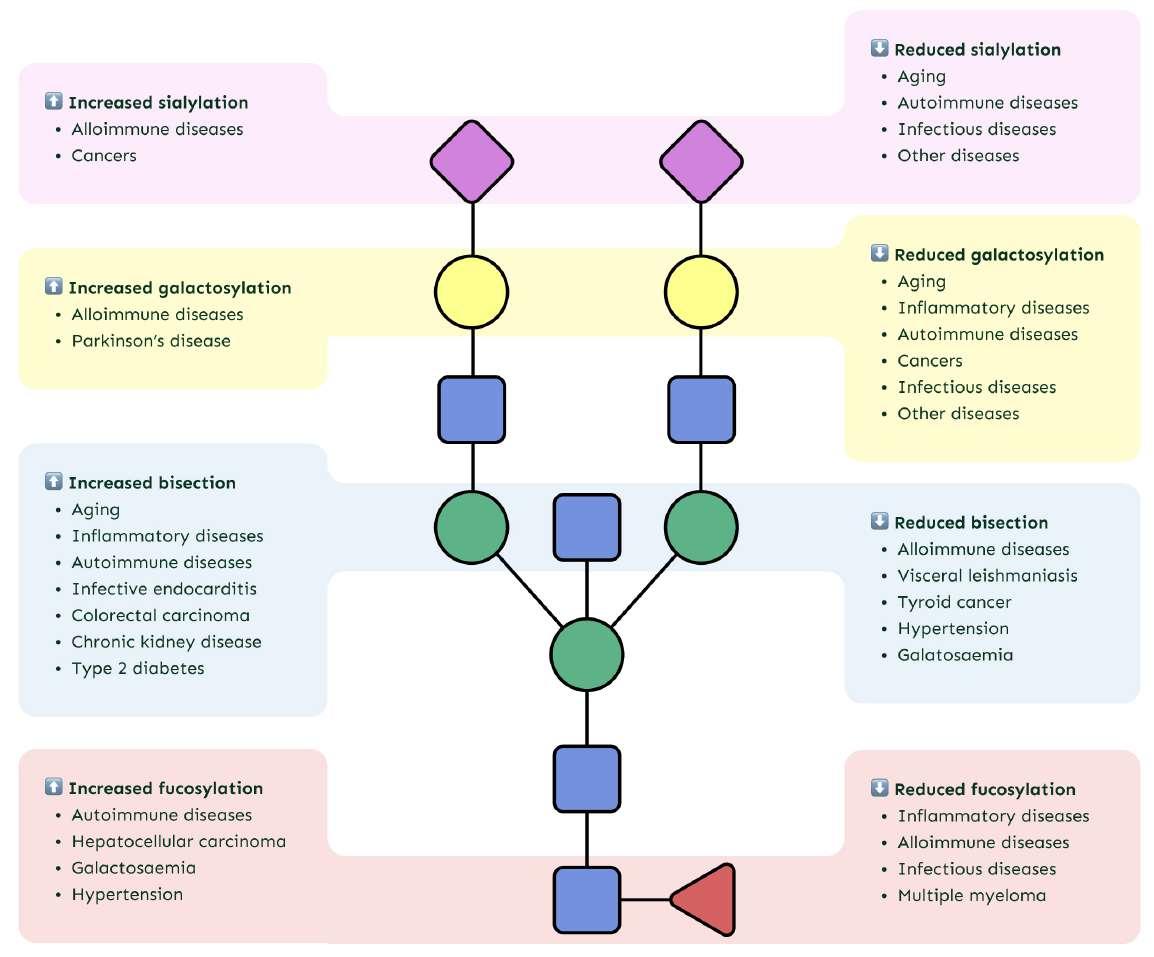
Disease-specific changes in IgG glycosylation
GlycanAge measures biomarkers of inflammaging that can be a trigger or a consequence of many different aging-related conditions, including autoimmune and infectious diseases that generally show low sialylation and galactosylation, while alloimmune diseases show high sialylation and galactosylation.
Malignancies can display either high amounts of highly branched sialylated glycans or low sialylation.
However, it is important to note that changes in IgG N-glycosylation are a reflection of changes in the immune system and not necessarily a consequence of ill health.
Chronic inflammation can appear years before the occurrence of any disease symptoms.
Disclaimer:
GlycanAge is a wellness test and not a medical or diagnostic tool. When considering IgG N-glycosylation in the context of any disease process, other data, including but not limited to an individual's personal medical history, family history, blood test results, and lifestyle assessment should be evaluated to provide holistic care. Table 1 provides a comprehensive list of IgG glycosylation and disease associations reported in published scientific literature to date.

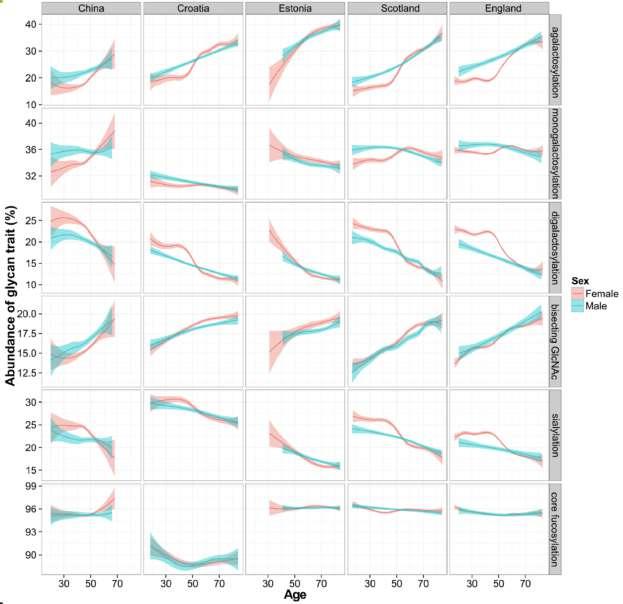
Global variability of the human glycome
We all have unique profiles of IgG N-glycosylation. A 2020 study found that IgG N-glycosylation is associated with the country of residence and the development index of a country. Individuals living in developing countries had higher levels of chronic low-grade inflammation.
Monogalactosylation had the strongest association with the country of residence/development index among all analyzed glycan traits. A 2022 study found that the heritability of the glycan clock of age was 39% on average, and that shared environmental factors (such as chronological age and molecular aging) contributed to the majority of environmental contributions, while 14-19% were attributed to unique environmental effects that may be attributed to lifestyle.

Strong association High = high amounts of corresponding glycan structures Weak association Low = low amounts of corresponding glycan structures
Table: Disease-specific changes in IgG glycosylation
Heritability and environmental effect on GlycanAge results
As mentioned earlier, a 2022 study looked at the heritability and environmental factors that contribute to the glycan clock of biological age. The study found that the glycan clock has an average heritability of 39%.
The majority of environmental contributions came from shared environmental factors such as chronological age and molecular aging. About 14-19% of environmental effects were due to unique environmental factors that could be attributed to lifestyle. This suggests that lifestyle factors such as diet, exercise, and environmental exposure can play a role in the variability of an individual's glycan clock.
Understanding different indexes presented in the GlycanAge report
The GlycanAge test calculates one's biological age based on the glycan clock, a set of glycan structures on IgG that have been scientifically proven to be associated with age and age-related health. The test uses a multivariable polynomial regression model and has multiple baselines based on sex, geography, and ethnicity.
In addition to the GlycanAge result, clients receive five GlycanAge indexes in their personal report that group similar glycans together and weigh them against a baseline of adults aged 20-80. These indexes provide additional information about analyzed IgG N-glycan groups, but they are not components of the final biological age result.
The GlycanAge biological age result is a separate measure that takes into consideration only the structures that are most relevant for the glycan clock.
Glycan Mature
Glycan Mature is an index that measures IgG N-glycan structures that are agalactosylated or G0. This means they do not contain any galactose units, which is a hallmark of aging and many diseases, including autoimmune and inflammatory diseases, cancers, and cardiometabolic syndrome.
Low galactosylation may precede the onset of disease, as demonstrated in individuals with high agalactosylation who have a higher risk of developing rheumatoid arthritis.
G0 glycans are often characterized as pro-inflammatory and cannot be sialylated (S) due to the absence of galactose, but may have bisecting GlcNAc (B) structural features.
Insight into the relevance of G0 glycans for the immune system and disease pathogenesis
It is important to note that the exact mechanisms of how agalactosylated (G0) IgG N-glycans contribute to disease pathogenesis are still being studied and understood. While there are some proposed mechanisms, such as complement activation, their exact role and contribution may vary depending on the specific disease and context.
It is also important to consider that the association between G0 and disease does not necessarily imply causation, and other factors may be involved in disease pathogenesis as well.
Further research is needed to better understand the role of IgG Nglycosylation in disease and to identify potential therapeutic targets.
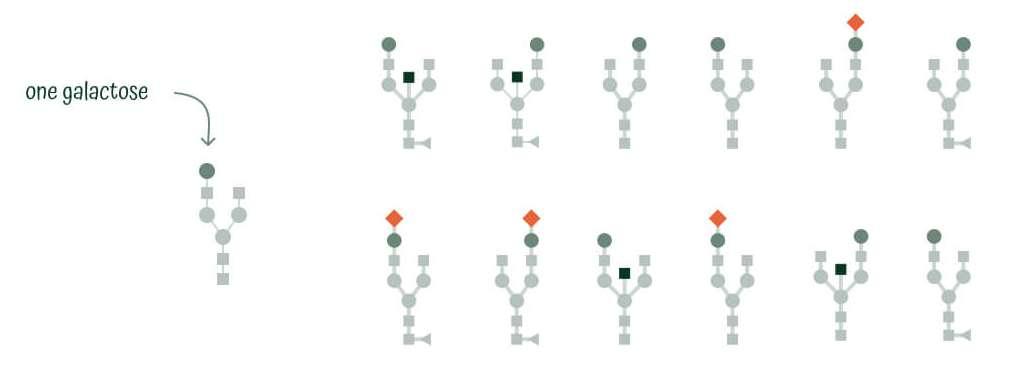
Glycan Median
Glycan Median consists of monogalactosylated structures (G1), which do not contribute to GlycanAge result but have a shared genetic causal variant with various diseases including cardiovascular diseases.
A study found that 38% of G1 glycans variability is explained by the socio-economic development level of a country.
A decreased Glycan Median index in women might be used to investigate their Glyco Cardio index.
Insight into the relevance of G1 glycans for the immune system and disease pathogenesis
A recent study found that the cytokine IFN-γ increases the proportion of mono-galactosylated (G1) N-glycans in vitro. However, it is important to note that in-vivo and in-vitro IgG glycosylation differ, and the clinical significance of this finding requires further investigation.
Glycan Youth
Glycan Youth includes a subset of IgG N-glycan structures that are digalactosylated (G typically found in high amounts in young and healthy individuals and are




However, it's worth noting that the end effect of these structures on the immune system can be modified by other structural features, such as the presence or absence of sialic acid or bisecting GlcNAc.
Insight into the relevance of G2 glycans for the immune system and disease pathogenesis:
Galactosylation of IgG molecules has been reported to have both antiinflammatory and pro-inflammatory effects. In mice, IgG1 Fc galactosylation was found to be necessary for inhibition of proinflammatory signalling cascade by binding to the inhibitory receptor FcγRIIB, which is involved in the maintenance of immune tolerance and autoimmune disease susceptibility.
Highly galactosylated IgG molecules have also been reported to inhibit the pro-inflammatory activity of complement component C5a, a fragment released upon cleavage of C5 complement component.
However, several other studies report a pro-inflammatory effect of IgG galactosylation, especially terminal galactosylation. This occurs through enhancement of C1q complement component and pro-inflammatory Fcγ receptors, which boost pro-inflammatory processes such as complementdependent cytotoxicity (CDC) and antibody-dependent cytotoxicity (ADCC).
There are some diseases in which an increase of galactosylation in comparison to healthy controls has been reported, such as fetal neonatal alloimmune thrombocytopenia (FNAIT) and Parkinson's disease. Most papers reporting on associations between disease and IgG N-glycosylation observe a decrease in digalactosylation (G2) in disease states.
Glycan Shield
Glycan Shield is a term used to describe complex structures of sialylated IgG N-glycans that contain at least one galactose and one or more sialic acids. These structures are highly present in healthy and young individuals and are generally considered to be anti-inflammatory.




Sialylated IgG N-glycans are the most complex structures found on ed by other structural features such
Insight into the relevance of G2 glycans for the immune system and disease pathogenesis:
Sialylated IgG N-glycans are the most complex structures present on IgG, and are characterized by at least one galactose and one or more sialic acid units. They are generally thought to have an antiinflammatory effect on the immune system by lowering the affinity for the FcyRIIIa receptor on natural killer cells, which can cause unwanted cellular damage.
However, there are conflicting results regarding the possible proinflammatory function of the sialylated N-glycan IgG Fc region that regards its binding to the C1q component of the complement and complement-dependent cytotoxicity.
Most publications report a decrease in sialylation (S) in disease states when compared to healthy controls. However, in some alloimmune diseases, such as fetal neonatal alloimmune thrombocytopenia (FNAIT), and some malignancies, increased sialylation has been observed.
Increased Glycan Lifestyle is associated with aging and unhealthy lifestyle/ environmental factors such as obesity and COPD in smokers. Conversely, decreased agalactosylated glycans with bisecting GlcNAc are an early feature associated with familial longevity.
Insight into the relevance of G2 glycans for the immune system and disease pathogenesis:
One study in mice found that low sialylation was enough to cause hypertension, while supplementation with a sialic acid precursor broke the link between hypertension and obesity.
Glycan Lifestyle
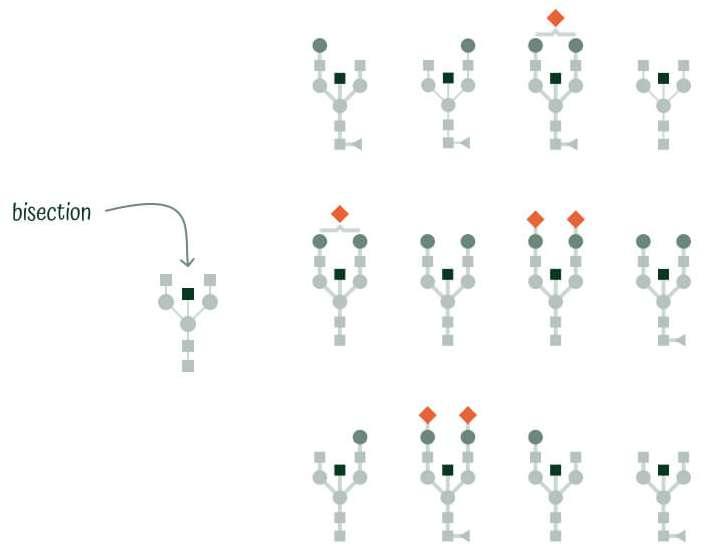

The presence of bisecting GlcNAc on IgG Fc domain has been shown to enhance the binding of IgG to FcγRIIIa, leading to increased ADCC activity. While ADCC is an important mechanism for clearing cells infected with viruses or tumor cells, it can also contribute to unwanted cellular damage in some autoimmune diseases.
Glycan Lifestyle indexes the structures containing bisecting GlcNAc (B


Though categorized as a wellness rather than a diagnostic test, the GlycanAge test and indexes can still provide useful insights into their client's overall health if users are familiar with the limitations of the test. For instance, while Glycan Youth, Glycan Mature, Glycan Shield and Glycan Lifestyle indexes change with age (1), genetics and environment explain the inter-individual differences in monogalactosylated glycans (G1) which are presented in form of the Glycan Median index.
How does GlycanAge compare to other tests?
GlycanAge measures glycans, which change rapidly in response to lifestyle factors and as we age. Periodic measurements can help track changes and adjust interventions such as diet and exercise accordingly. It captures both rapid and slow changes for a comprehensive picture of biological age, offering actionable feedback on interventions.
Biomarker Glycans
Measures biological age
Stable with repeat testing
Correlates with autoimmune and chronic conditions
Has prognostic relevance
Gives insight into current health
Responds to interventions with statistical significance
Measures inflammation
Unproven Unproven
Unproven
Partly
DNA DNA
Partly
Telomeres Methylation GlycanAge

GlycanAge is great! It allows us to measure success of our methods within months, not years.
— Jim LaValle, Metabolic Code
Join world’s leading clinics and partner with GlycanAge today!
Ready to take your practice to the next level with GlycanAge?
Contact us today to learn more about how we can help you optimize
your patients' health and well-being.
Sign up for free or book a call to learn more.
Book a call Sign up for free
No credit card required.