









VOL. 32, NO. 2, Summer 2023 8MeettheNewSociety Oficers 41 Emerging Electrochemical ApproachesforChemicalManufacturing 26ECSMournsGordonMoore 47 Ex traterrestrial Electrochemistry Irtsudn i a l E l e c t r ochemistry&Electrochemical En g i neering:Celebrating 80 years
PRIME2024
SWEDEN
October 8-12, 2023
Swedish Exhibition & Congress Centre
May 26-30, 2024
Marriott Marquis San Francisco
TROPIC OF CANCE R
Joint International Meeting
HONOLULU, HI
October 6-11, 2024
Hawaii Convention Center & Hilton Hawaiian Village
CANADA
May 18-22, 2025
Palais des Congrès de Montréal
TROPIC OF CANCER ANTARCTIC CIRCLE
www.electrochem.org/meetings
244th ECS Meeting GOTHENBURG
SWEDEN
FUTURE ECS MEETINGS 244 247 245
PRiME
2024
Joint International Meeting HONOLULU, HI
245th ECS Meeting SAN FRANCISCO, CA
244th ECS Meeting GOTHENBURG
PRiME 2024
CANADA
247th ECS MeetingMONTRÉAL
247th ECS MeetingMONTRÉAL
245th ECS Meeting SAN FRANCISCO, CA


Published by: The Electrochemical Society (ECS) 65 South Main Street Pennington, NJ 08534-2839, USA Tel 609.737.1902, Fax 609.737.2743 www.electrochem.org

School’s Out
The title of this editorial is from the work of the great philosopher Alice Cooper who penned a song of the same name in 1972 (look it up, kids – it was on vinyl!). While for many it is spring that represents new life blooming, for those involved in higher education, spring simply mocks us. As the trees and flowers blossom and birds return from their migration to warmer latitudes, faculty and students are locked in an epic struggle to somehow cover all the material promised in one of the great works of fiction, the syllabus. The faculty hope that some amount of what they have helped students discover sticks through the summer and beyond. Those who teach introductory courses like yours truly live in dread of the future comment from a colleague that the students you taught the semester before say that they have “never heard of” some concept into which you poured your heart and soul to get across its importance, even beauty. Students are trying to figure out if it is possible to learn an entire semester of geology in the weekend before the final (spoiler alert—it is not).
Each year, by some miracle, students get across the finish line of their courses in the spring semester more-or-less intact and stumble out of town, leaving faculty the awful, terrible, nogood job of grading. Worst part of the job, hands down. OK, committee work can be pretty awful, too. But it is in grading that you as an instructor come face-to-face with the reality of your effectiveness, and sometimes it is not a pretty picture. You think of your painstakingly designed activities that focused on critical knowledge and skills so acutely that you were convinced it would require a decision by a student to NOT learn to avoid understanding them. And then you grade the finals. Sure, there are some students who do extremely well (as they had on all the assignments). Those students really didn’t need you, if we are being honest. Your job is about helping those who struggle, and no matter how you much you try, there are some who do so right through the final exam. Some are certainly the classic disaffected college student who just wants the certification of the degree with the minimum effort required. There are others, maybe most, who do want to learn, who come to office hours, and put in all the effort they can muster, but the understanding doesn’t take hold. Those are the ones you get stuck on, trying to figure out what else you should have done.
Of course, the good news is that once the grades are handed in (or the “Submit Grades” button is clicked), the faculty member can look forward to summer. Three months of less demand on their time, or so they think. At the end of the school year at universities throughout the world, you can almost hear the “Things to Get Done This Summer” lists being created. Papers to write, proposals to submit, dissertations to read, conferences to attend, offices to clean … hope springs eternal. Then reality sets in. Before you know it, it is July 1st, and you cannot comprehend what happened to June and a sizable chunk of May, to quote an old daily calendar. Panic begins to set in as all those items you confidently promised others you could “easily” get done in the summer seem to have grown to epic proportions. It is time for triage; all the “would be nice to” items are abandoned without a second thought (probably for the 8th or 9th consecutive summer). Not enough. You cull using the “how bad would it be if I didn’t do this” filter. You still have too many, but you soldier on in a desperate attempt to salvage some shred of reputation. The next thing you know it’s the middle of August, and back the students come like the tide. It is about then that one of your neighbors says, “Must be nice to have the summers off.” Evil thoughts run through your head, but you gather the few wits you have left, and you just smile through gritted teeth. You know from painful experience that thinking you can explain what your summers are like is akin to what they say about second marriages—the triumph of hope over experience. You take a deep breath (but not too obviously) and respond, “Yeah, it beats working.” The look of surprise and disgust makes all the toil worthwhile. Until next time, be safe and happy.
Editor: Rob Kelly
Guest Editor: Maria Inman
Contributing Editors: Christopher L. Alexander, Chris Arges, Scott Cushing, Ahmet Kusolgu, Donald Pile, Alice Suroviec
Director of Publications: Adrian Plummer
Director of Community Engagement: Shannon Reed
Production Editor: Kara McArthur
Graphic Design & Print Production Manager: Dinia Agrawala
Staff Contributors: Frances Chaves, Genevieve Goldy, Mary Hojlo, Christopher J. Jannuzzi, John Lewis, Anna Olsen, Jennifer Ortiz, Beth Schademann, Francesca Spagnuolo
Advisory Board: Brett Lucht (Battery), Dev Chidambaram (Corrosion), Durga Misra (Dielectric Science and Technology), Philippe Vereecken (Electrodeposition), Jennifer Hite (Electronics and Photonics), Mani Manivannan (Energy Technology), Cortney Kreller (High-Temperature Energy, Materials, & Processes), John Weidner (Industrial Electrochemistry and Electrochemical Engineering), Jakoah Brgoch (Luminescence and Display Materials), Hiroshi Imahori (Nanocarbons), James Burgess (Organic and Biological Electrochemistry), Robbyn Anand (Physical and Analytical Electrochemistry), Ajit Khosla (Sensor)
Publications Subcommittee Chair: Colm O'Dwyer
Society Officers: Gerardine Botte, President; Colm O'Dwyer, Senior Vice President; James (Jim) Fenton, 2nd Vice President; Francis D'Souza, 3rd Vice President; Marca Doeff, Secretary; Elizabeth J. Podlaha-Murphy, Treasurer; Christopher J. Jannuzzi, Executive Director & CEO
Statements and opinions given in The Electrochemical Society Interface are those of the contributors, and ECS assumes no responsibility for them.


Authorization to photocopy any article for internal or personal use beyond the fair use provisions of the Copyright Act of 1976 is granted by The Electrochemical Society to libraries and other users registered with the Copyright Clearance Center (CCC). Copying for other than internal or personal use without express permission of ECS is prohibited. The CCC Code for The Electrochemical Society Interface is 1064-8208/92.

ISSN : Print: 1064-8208 Online: 1944-8783

The Electrochemical Society Interface is published quarterly by The Electrochemical Society (ECS), at 65 South Main Street, Pennington, NJ 08534-2839 USA. Subscription to members is part of membership service. © Copyright 2023 by The Electrochemical Society. *“Save as otherwise expressly stated.”
The Electrochemical Society is an educational, nonprofit 501(c)(3) organization with more than 8,500 scientists and engineers in over 75 countries worldwide who hold individual membership. Founded in 1902, the Society has a long tradition in advancing the theory and practice of electrochemical and solid state science by dissemination of information through its publications and international meetings.
FROM THE EDITOR FROM THE EDITOR
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 3
Rob Kelly Editor https://orcid.org/0000-0002-7354-0978
With outstanding resolution and research-grade precision and accuracy, BCS-800 Series battery cyclers stand out for advanced applications including dQ/dV, HPC & native EIS.

www.biologic.net

4 The Electrochemical Society
of Tower Strength
BATTERY CYCLERS
BCS-800 Series
Vol. 32, No. 2
Summer 2023
39 41 47 51
The Future of Industrial Electrochemistry & Electrochemical Engineering
by Maria Inman
Current and Emerging Electrochemical Approaches for Chemical Manufacturing
by Elizabeth J. Biddinger and Paul J. A. Kenis
Extraterrestrial Electrochemistry–Challenges and Opportunities for in-situ Resource Utilization (ISRU) on Mars
by Shrihari Sankarasubramanian,
Bradley
Chambers, and Cheyenne Wilson
Considerations for Industrial Phosphorous Recovery via Electrochemical Processes: A Figures of Merit Approach
3 From the Editor: School's Out
7 From the President: Can You Imagine?
8 Meet the New 2023 Society Officers
11 Society News
24 Websites of Note
26 ECS Mourns Gordon E. Moore
28 People News
29 Reports from the Frontier
37 Tech Highlights
56 Section News
58 Awards Program
60 New Members
64 Student News
Kody D. Wolfe, Ardavan Zanganeh, Richard N. Arthur, Jason P. Trembly, and Damilola A. Daramola
by

This month’s cover is based on a figure from an article in this issue, “Considerations for Electrochemical Phosphorus Precipitation: A Figures of Merit Approach” by Kody D. Wolfe, Ardavan Zanganeh, Richard N . Arthur, Jason P. Trembly, Damilola A. Daramola. The original figure models an electrochemical batch reactor used for phosphorus recovery from storm and treated wastewater and agricultural runoff. The cover image uses rotating arrows to evoke the role of electrochemical recovery and the re-use of essential nutrients in the effort to build a circular economy and to enable the use of renewable energy sources.
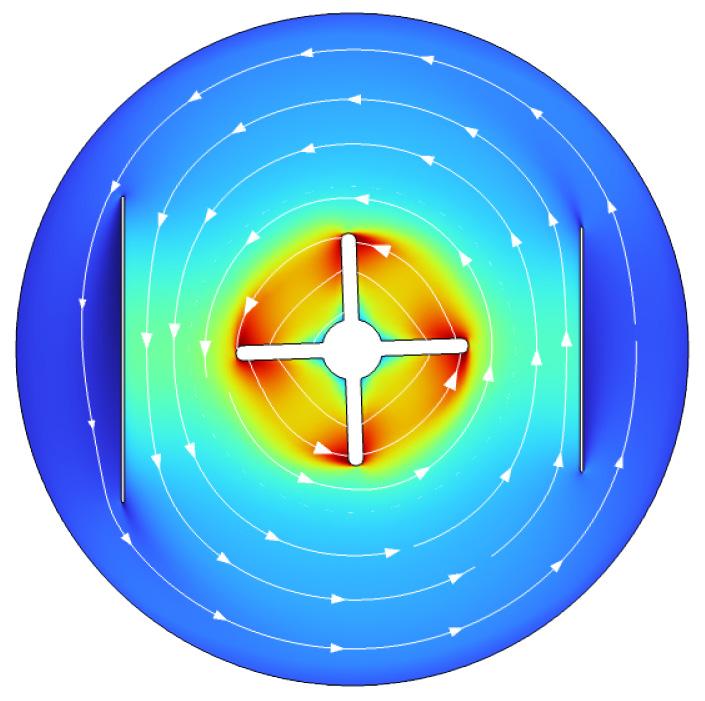

Cover design: Dinia Agrawala

The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 5

6 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Can You Imagine?
Can you imagine a world where fertilizer production is thoroughly sustainable and recycled, thus enabling global food access that meets population growth? Where waste is considered a resource and has value; where buildings monitor air and water to keep us safe; where pathogen-destroying coatings are integrated into fabrics and clothing; where sensors are noninvasive, continuously monitoring our health while personalizing and advancing medicine and treatment; where plastics are upcycled; where the manufacture of chemicals and materials is decarbonized, distributed, circular, modular, with a small footprint and multiple processing steps integrated into a single unit that implements alternative energy sources… Can you imagine?

As members of The Electrochemical Society, we can all imagine these advances because we have seen firsthand how contributions from electrochemical and solid state science contribute to solving the grand challenges facing humanity in the 21st century. Electrochemical and solid state science’s impact on addressing energy sustainability and storage is recognized (since batteries are one of electrochemistry’s most popular applications). However, a significant education gap exists about electrochemistry’s farreaching potential to positively impact society.
Realizing these and additional transformational changes requires convergence research, where intellectually diverse researchers are brought together intentionally to address a major problem. Therefore, it is extremely important to convene industry, policy makers, and public stakeholders to facilitate sciencebased solutions and to anticipate possible unintended consequences of technology adoption.
Although ECS, with its intellectually diverse researchers, already plays a major role in convergence research, we need to do more. As the premier solid state and electrochemical science and technology society in the world, we need to lead a paradigm change that mitigates the educational gap while enabling sustainable education in the field. Our journals are making an impact by disseminating fundamental knowledge, but we need to broadcast this message of our impact on society more broadly, to the media, policy makers, investors, etc.
As President, I would like us to work together to develop programs that explain to different audiences the importance and the fundamental principles of electrochemistry. For example, programs can be targeted to the next generation of STEM scholars, practitioners, policy makers, and investors. There are opportunities for this during our biannual meetings. Forums and sessions discussing the potential commercial aspects of electrochemicalbased technologies can become part of our programs and help us reach out to funding agencies, policy makers, and investors. It is also important to have conversations around how our journals can reach different audiences; for example, we can provide the option to publish special articles in our publications, such as perspectives that include not only technical and scientific analysis but also discussion of policy, economics, and societal impact. I will help facilitate joint collaborations among ECS, federal funding agencies, and industry
to organize and sponsor symposia on topics regarding frontiers and opportunities for electrochemical technologies. I will work with our editorial board and divisions to discuss mechanisms to expand the reach of our publications.

While thinking of the future and the next generation of leaders in our field, it is important to continue to attract young authors, to encourage them to submit their best papers to our journals, and to provide support to rising stars in electrochemical and solid state science. As President, I will help generate resources to increase the number of awards for young authors and to expand the number of travel grants for students and postdocs.
As an international society, we need to grow our global membership and explore programs that benefit everyone. Opportunities exist to organize alternate symposia and sectional meetings, and to cosponsor symposia in different areas of the world. Several regions of the world are underrepresented in our Society, including Central and South America.
ECS Student Chapters have been a tremendous addition to our Society. Currently, we have 123 student chapters in 28 countries (as of April 2023). There is ample room to increase the number of chapters and extend our global reach while increasing diversity of views. As President, I will help catalyze the formation of additional ECS Student Chapters around the world. I would like to take this opportunity to make a call for chapter mentors, who are critical to inspiring and supporting students in this endeavor.
I would also like to expand our traditional journal offerings, enhancing our archival manuscripts by also including relevant data sets and other ancillary information in support of deep learning and knowledge transfer. Finally, we need to continue strengthening diversity and inclusion, which fuels innovation, enhances collaboration, and is required to fully address major societal challenges. As President, I will lead ECS to implement best practices in diversity and inclusion, to seek collaboration with other professional organizations to support these efforts, and to raise resources that reinforce our commitment and impact.
ECS is an integral part of my professional career. It is my family that has welcomed me since I was a student (and I would like many others to have such a wonderful opportunity). Since then, this is where I interact with distinguished scientists around the world, who today are my peers and friends. I am truly humbled and honored to serve as President of this prestigious organization. I look forward to growing the ECS community and serving all of you. I encourage all of us: ECS members, staff, student chapters, and volunteers, to work together to advance electrochemical and solid state science and technology for the benefit of all humanity.
Gerardine G. Botte ECS President https://orcid.org/0000-0002-5678-6669



The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 7 FROM THE PRESIDENT FROM THE PRESIDENT
Graphic: Nathan Caballero, Texas Tech University.
Meet the New 2023 Society Officers O

n June 2, 2023, the newly elected officers of The Electrochemical Society assumed their posts. We are pleased to welcome
Gerardine (Gerri) Botte
as
President
Francis D’Souza as 3rd Vice President.
Gerardine (Gerri) Botte


and
Gerri Botte is Professor and Whitacre Endowed Chair in Sustainable Energy at Texas Tech University (TTU) and the Founding Director of the US National Science Foundation Engineering Research Center for Advancing Sustainable and Distributed Fertilizer Production (CASFER). She also leads a new initiative for sustainability and circular economies under a recently established institute at TTU. She served as the Whitacre Department Chair in Chemical Engineering at TTU for three years before becoming CASFER Director. As department chair, she was instrumental in implementing curricula changes and for significant growth in the department’s research and restrictive research funding.
Gerri has over 25 years of experience in the development of electrochemical processes at the intersection of energy, water, and food sustainability. A visionary and recognized leader in electrochemical science and technology, she has authored 211 publications, including 62 granted patents. Gerri received her PhD in 2000 under the direction of Ralph White and her ME in 1998, both in chemical engineering from the University of South Carolina. She completed her BS in Chemical Engineering at the Universidad de Carabobo in 1994.
Among the awards and honors Gerri has received are the 2015 Science for Solving Society’s Problems Challenge Winner, 2014 Elected Fellow of The Electrochemical Society, 2012 Elected Fellow of the National Academy of Inventors, and 2010 Elected Fellow of the World Technology Network.


An active Society member since 1998, Gerri has served in leadership roles that include Board Member and Chair of the ECS Industrial Electrochemistry and Electrochemical Engineering (IE&EE) Division. What excites her most about electrochemical and solid state science and technology is that they are core platforms with important applications in different aspects of our lives. Though she admits that there is still a lag in education about what electrochemical technologies can do, she believes that ECS, as the premier solid state and electrochemical science and technology society in the world, will lead a paradigm change mitigating this educational gap while enabling sustainable education in the field.
Gerri loves to spend time with her daughters Geri and Andrea, her husband Matt, and her dog Moti, cooking cuisine from her Italian and Venezuelan heritage, and being together with her family outdoors. Whether it is a day at the beach, boating on the high seas, or hitting the links with hot pink golf balls, Gerri brings the same passion and enthusiasm to play and to work!
8 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
2023–2024
Gerardine (Gerri) Botte ECS President, 2023–2024
Gerri and her daughter Andrea celebrate Andrea’s high school graduation.
Photo: Matt Bedell
Gerri with her husband, Matt, and daughters, Geri and Andrea.
Photo: Matt Bedell
Gerri and her dog, Moti.
Photo: Matt Bedell
Gerri and family on the golf course.
• Summer 2023 •
Photo: Matt Bedell
Francis D’Souza
Francis D’Souza is Regents Professor of Chemistry and Materials Science and Engineering at the University of North Texas (UNT) and part of the university’s Applied Materials and Manufacturing Processing Institute. Prior to joining UNT in 2011, he was Professor of Chemistry at Wichita State University. He received his BS and MS from Mysore University and his PhD from the Indian Institute of Science. He held postdoctoral positions at the University of Houston and the Université de Dijon.

Francis’s research covers a wide area of chemistry, nanophotonics, electrochemistry, and materials science. His principal research interests include supra and nanomolecular chemistry of photosensitizercarbon nanomaterials, advanced functional materials for light energy harvesting and photovoltaics, electrochemical and photochemical sensors, and catalysts. Francis has authored or co-authored more than 500 publications, given more than 450 conference talks, and edited 10 Handbooks on Carbon Nanomaterials, resulting in more than 21,500 citations with a cumulative h-index of 75. He has received funding from the NSF, NIH, DOE, and private agencies.
As an active member of The Electrochemical Society since 1993, Francis has served ECS at various levels, including as ECS Nanocarbon Division (NANO) Chair, Vice Chair, Secretary, and Treasurer and now as Member at Large of that division. As NANO Chair, he was instrumental in establishing and securing endowment monies for the division’s Smalley Research Award and Young Investigator Award. He has served on numerous society-level committees and award subcommittees as both chair and member and is currently serving as member of the Meetings Subcommittee and the Vittorio de Nora Award Subcommittee. To date, he has co-organized more than 40 symposia for the Society’s fall and spring biannual meetings. For the last 10 years, Francis has served as Technical and Associate Editor of the ECS Journal of Solid State Science and Technology.


Francis is a Fellow of The Electrochemistry Society and Fellow of the Royal Society of Chemistry. Honors and awards he has received include the Nanocarbons Division Robert C. Haddon Research Award, Fulbright Specialist Scholar, ACS DFW Section Doherty Research Award, Chemical Research Society of India Medal, Global Initiative of Academic Networks Fellow, Japan Society for the Promotion of Science Fellowship, and Wichita State University Excellence in Research Award. UNT has awarded him their Research Leadership Award, Regents Professorship, Toulouse Scholar Award, and Distinguished Teaching Professorship Award.
Francis is married to Mirabilis (Pearl), and they have a daughter and two sons. He enjoys traveling and exploring new places and meeting people and learning about their culture, art, and food.

The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 9
Francis D’Souza
3rd Vice President, 2023–2024
Francis and family visit the Taj Mahal.
Photo: Francis D'Souza
Francis and his wife, Mirabilis (Pearl)
Photo: Francis D'Souza
Francis at the Golden Pavilion Temple in Kyoto, Japan.
• Summer 2023 •
Photo: Francis D'Souza


TESTING ELECTROLYZERS, FUEL CELLS, & BATTERIES Electrochemical Impedance Spectroscopy High Current Rapid Pulsing gamry.com Software development kits make it easy to integrate with process control systems.
SYSTEMS FOR
Publications Update
Opening Doors through Open Access

 by Adrian Plummer, MPA, PMP | Director of Publications
by Adrian Plummer, MPA, PMP | Director of Publications
It’s hard to believe that it has been only 18 months since ECS launched our two new Gold Open Access journals, Sensors Plus Advances
101 articles published in the two journals have garnered the attention of the community with well over 24,000 article downloads and over 1,000 citations. This marks another occasion to celebrate ECS as a champion in the movement to the Science, and the Society’s vision to create “…uninhibited availability of science through open access, and accelerate scientific discovery and innovation, leading the community as the advocate, guardian, and facilitator of our technical domain.”
As we enter the second half of 2023, we continue to pursue growth as a way to fuel the ECS mission and vision. We are expanding our pool of qualified peer reviewers through training and education op portunities and designing new ways to encourage authors to choose ECS as their publication home. Meanwhile, our editorial leadership continues to value the quality of the content published in our journals over the quantity
In collaboration with our publishing partner IOP, in 2023 we have more than doubled the number of our transformative and readand-publish agreements with institutions, funders, and consortiums worldwide. This clears the path for authors to publish open access with ECS without the burden of article processing charges (APCs), welcoming authors from every continent on the globe. We remain focused on opening doors for authors in the pursuit of open, ethical,
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 11 SOCIETY NEWS SOCIETY NEWS
INTERNATIONAL OPEN ACCESS WEEK Experience Unlimited Access to the ECS Digital Library on IOPscience OCTOBER 23 - 29, 2023 iopscience.iop.org/partner/ecs
SOCIETY NEWS SOCIETY NEWS
Editorial Board Updates Summer 2023
The Electrochemical Society Publications Subcommittee, Technical Affairs Committee, and Board of Directors congratulate the newly appointed and reappointed members of our Editorial Board. It is through the unwavering commitment of our Editorial Board Members that The Electrochemical Society family of journals continues to realize great success. Thank you for your service to ECS!
Pratima Solanki


Associate Editor of JSS and JES Sensors topical interest area for the term
March 1, 2023 – February 29, 2024
Amanda Clifford
Associate Editor of ECS Sensors Plus for the term March 1, 2023 –February 29, 2024

Praveen Sekhar


Associate Editor of ECS Sensors Plus for the term
August 6, 2023 –August 5, 2024
Sheng-Joue Young
Associate Editor of JSS and JES Sensors topical interest area for the term




March 1, 2023 –February 29, 2024
Ariel Furst
Associate Editor for ECS Sensors Plus for the term March 1, 2023 –February 29, 2024
Olga Marina

Associate Editor of JES Fuel Cells, Electrolyzers, and Energy Conversion topical interest area for the term May 20, 2023 –May 19, 2025
Trisha Andrews
Associate Editor for ECS Sensors Plus for the term September 5, 2023 –September 4, 2024
ECS Board of Directors Report
The ECS Board of Directors met via video conference on March 17, with board members from around the world putting aside time zone concerns to join the spirited 90-minute online meeting.
ECS President Turgut Gür called the meeting to order and kicked off the 2023 governance year by thanking the Board for their continue leadership, support, and dedication.
ECS Secretary Marca Doeff then presented the minutes from the previous board meeting and had the pleasure of announcing the newly elected board members: Incoming President Gerardine (Gerri) Botte and Incoming 3rd Vice President Francis D’Souza. Their terms begin following the 243rd ECS Meeting in Boston in May. Congratulations to Gerri and Francis!
Marca then asked the Board to approve the appointments of Charuksha Walgama as the ECS Organic and Biological Electrochemistry Division (OBE) representative to the Interdisciplinary Science and Technology Subcommittee and Sabine Kuss as OBE representative to the Honors & Awards Committee. These mid-cycle appointments were necessary to fill vacancies due to the sad passing of our dear colleague and past OBE Chair Diane
Thomas Thundat
Associate Editor of ECS Sensors Plus for the term

April 10, 2023 –April 9, 2024
Smith in late 2022. Please see the spring issue of Interface to read the memorial in Diane’s honor.
Following the Secretary’s report, ECS Treasurer Elizabeth (Lisa) Podlaha-Murphy provided a detailed review of the Society’s financial performance in 2022. Although the value of the Society’s investment portfolio decreased significantly in 2022 in line with last year’s challenging economic climate, strong attendance at the 242nd ECS Meeting in Atlanta and the continued growth of publications revenue helped provide the funds to support the Society’s operations without withdrawing from our portfolio, thus maximizing our potential for recovery and growth when the market rebounds.
Next, ECS Executive Director Chris Jannuzzi reported on the major initiatives for 2023, noting key efforts to expand ECS’s educational offerings (starting a course in Battery Workforce Development) and to significantly grow ECS membership in the coming years. In addition, Chris and Lisa announced a project to be launched after the spring meeting to simplify the divisions’ funding plan. Each year, divisions lead vital efforts on behalf of ECS to advance the Society’s mission. The goal of the simplified funding plan is to provide divisions with the funds required for this critical work in the most streamlined, straightforward, and transparent manner possible. To that end, Chris and Lisa, with ECS Chief Financial Officer Tim Gamberzky, will work with the division treasurers to create a revised funding plan, to be presented for initial review by the Board at the October meeting.
Last, a motion to close the meeting was made, seconded, and unanimously approved. The Board will reconvene in Boston on June 2, 2023, at the 243rd ECS meeting.
12 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
SOCIETY NEWS SOCIETY NEWS
ECS Division Contacts

Battery
Brett Lucht, Chair
University of Rhode Island
Jie Xiao, Vice Chair
Jagjit Nanda, Secretary
Xiaolin Li, Treasurer
Doron Aurbach, Journals Editorial Board Representative
Corrosion
Dev Chidambaram, Chair
University of Nevada Reno
Eiji Tada, Vice Chair
Rebecca Schaller, Secretary/Treasurer
Gerald Frankel, Journals Editorial Board Representative
Dielectric Science and Technology
Uroš Cvelbar, Chair

Jožef Stefan Institute
Sreeran Vaddiraju, Vice Chair
Zhi David Chen, Secretary
Thorsten Lill, Treasurer
Peter Mascher, Journals Editorial Board Representative
Electrodeposition
Natasa Vasiljevic, Chair University of Bristol
Luca Magagnin, Vice Chair
Andreas Bund, Secretary
Antoine Allanore, Treasurer
Takayuki Homma, Journals Editorial Board Representative
Electronics and Photonics
Qiliang Li, Chair
George Mason University
Vidhya Chakrapani, Vice Chair
Zia Karim, Second Vice Chair
Helmut Baumgart, Secretary
Erica Douglas, Treasurer
Fan Ren, Journals Editorial Board Representative
Jennifer Bardwell, Journals Editorial Board Representative
Energy Technology

Katherine Ayers, Chair
Nel Hydrogen
Minhua Shao, Vice Chair
Hui Xu, Secretary
Iryna Zenyuk, Treasurer
Xiao-Dong Zhou, Journals Editorial Board Representative
High-Temperature Energy, Materials, and Processes

Sean R. Bishop, Chair Sandia National Laboratories
Cortney Kreller, Senior Vice Chair
Xingbo Liu, Junior Vice Chair
Teruhisa Horita, Secretary/Treasurer
Xiao-Dong Zhou, Journals Editorial Board Representative
Industrial Electrochemistry and Electrochemical Engineering
Maria Inman, Chair
Faraday Technology, Inc.






Paul Kenis, Vice Chair
Elizabeth Biddinger, Secretary/Treasurer
John Harb, Journals Editorial Board Representative
Luminescence and Display Materials

Rong-Jun Xie, Chair
Xiamen University
Eugeniusz Zych, Vice Chair
Dirk Poelman, Secretary/Treasurer
Kailash Mishra, Journals Editorial Board Representative
Nanocarbons


Jeff L. Blackburn, Chair National Renewable Energy Laboratory
Ardemis Boghossian, Vice Chair
Yan Li, Secretary
Hiroshi Imahori, Treasurer
Francis D’Souza, Journals Editorial Board Representative
Organic and Biological Electrochemistry
Shelley Minteer, Chair
University of Utah
Jeffrey Halpern, First Vice Chair
Sabine Kuss, Second Vice Chair
Ariel Furst, Secretary/Treasurer
Janine Mauzeroll, Journals Editorial Board Representative
Physical and Analytical Electrochemistry
Stephen Paddison, Chair
University of Tennessee, Knoxville
Anne Co, Vice Chair
Svitlana Pylypenko, Secretary
Iwona Rutkowska, Treasurer
David Cliffel, Journals Editorial Board Representative
Sensor
Larry Nagahara, Chair Johns Hopkins University
Praveen Kumar Sekhar, Vice Chair
Dong-Joo Kim, Secretary
Leyla Soleymani, Treasurer
Ajit Khosla, Journals Editorial Board Representative
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 13
2023–2024 ECS Committees
Executive Committee of the Board of Directors
Gerardine Botte, Chair President, Spring 2024
Colm O’Dwyer Senior Vice President, Spring 2024
James Fenton 2nd Vice President, Spring 2024
Francis D'Souza 3rd Vice President, Spring 2024
Marca Doeff Secretary, Spring 2024
Elizabeth Podlaha-Murphy Treasurer, Spring 2026
Christopher Jannuzzi Term as Executive Director
Audit Committee
Turgut Gür, Chair
Immediate Past President, Spring 2024
Gerardine Botte President, Spring 2024
Colm O’Dwyer Senior Vice President, Spring 2024
Elizabeth Podlaha-Murphy Treasurer, Spring 2026
Robert Micek Nonprofit Financial Professional, Spring 2025
Education Committee
Alice Suroviec, Chair Spring 2025
Svitlana Pylypenko Spring 2024
Paul Gannon Spring 2024
Stephen Maldonado Spring 2025
David Hall Spring 2025
Wen Shen Spring 2026
Samantha Gateman Spring 2026
Maureen Tang Spring 2027
Damilola Daramola Spring 2027
Mohammad Sabeti Spring 2024
Elif Selin Sahin Spring 2025
Marca Doeff Secretary, Spring 2024
E. Jennings Taylor Chair, Individual Membership Committee, Spring 2026
Ethical Standards Committee
Turgut Gür, Chair
Immediate Past President, Spring 2024
Peter Fedkiw Past Officer, Spring 2026
Esther Takeuchi Past Officer, Spring 2024
Marca Doeff Secretary, Spring 2024
Elizabeth Podlaha-Murphy Treasurer, Spring 2026
Finance Committee
Elizabeth Podlaha-Murphy, Chair Treasurer, Spring 2026
Thorsten Lill Spring 2026
Paul Kenis Spring 2026
Dong-Joo Kim Spring 2025
Robert Micek Spring 2025
Marca Doeff Secretary, Spring 2024
Tim Gamberzky Chief Operating Officer, Term as COO
Honors and Awards Committee
TBD Spring 2027
Vimal Chaitanya Spring 2024
Mikhail Brik Spring 2024
Sabine Kuss Spring 2024
Alanah Fitch Spring 2025
Shigeo Maruyama Spring 2025
Jean St-Pierre Spring 2025
Andrew Hoff Spring 2026
Dev Chidambaram Spring 2026
Shirley Meng Spring 2026
Thomas Thundat Spring 2027
Elizabeth Biddinger Spring 2027
Wilson Chiu Spring 2027
Stanko Brankovic Spring 2027
Gerardine Botte President, Spring 2024
Individual Membership Committee
E. Jennings Taylor, Chair Spring 2026
Kent Jingxu Zheng Spring 2026
Uroš Cvelbar Spring 2026
John Staser Spring 2024
Y. Shirley Meng Spring 2024
Shuthi T. Kumar Raj Spring 2025
Qizhi Liu Spring 2025
Jiaxin Duan Spring 2024
Jedidian Adjetey Adjei Spring 2025
Alex Peroff
Chair, Institutional Engagement Committee, Spring 2025
Marca Doeff Secretary, Spring 2024
Institutional Engagement Committee
Alex Peroff, Chair Spring 2025
Hemanth Jagannathan Spring 2026
Hanping Ding Spring 2026
Vimal Chaitanya Spring 2026
Yuyan Shao Spring 2024
Christopher Beasley Spring 2024
Karen Poe Spring 2024
Yoko Yamakoshi Spring 2025
Santosh Vijapur
Spring 2025
Yaw Obeng Spring 2025
E. Jennings Taylor Chair, Individual Membership Committee, Spring 2026
Elizabeth Podlaha-Murphy Treasurer, Spring 2026
Nominating Committee
Turgut Gür, Chair Immediate Past President, Spring 2024
Jessica Koehne Spring 2024
D. Noel Buckley Spring 2024
John Staser Spring 2024
Francis D'Souza
Christopher Jannuzzi
Technical Affairs Committee
3rd Vice President, Spring 2024
Executive Director, Term as Executive Director
Colm O’Dwyer, Chair Senior Vice President, Spring 2024
Gerardine Botte President, Spring 2024
Turgut Gür
Eric Wachsman
Francis D'Souza
Immediate Past President, Spring 2024
2nd Immediate Past President, Spring 2024
Chair, Meetings Subcommittee, Spring 2024
James Fenton Chair, Publications Subcommittee, Spring 2024
Jennifer Hite Chair, ISTS Subcommittee, Spring 2025
Christopher Jannuzzi Executive Director, Term as Executive Director
Publications Subcommittee of the Technical Affairs Committee
James Fenton, Chair
Francis D'Souza, Vice Chair
2nd Vice President, Spring 2024
3rd Vice President, Spring 2024
Krishnan Rajeshwar JSS Editor, 12/31/2024
Robert Savinell
JES Editor, Spring 2024
Ajit Khosla ECSSP Editor, Fall 2024
Robert Kelly Interface Editor, Spring 2025
Pawel Kulesza
Ahmet Kusoglu
Chunshen Wang
Daniel Schwartz
Meetings Subcommittee of the Technical Affairs Committee
Francis D'Souza, Chair
James Fenton, Vice Chair
Xiaolin Li
Xinfang Jin
Peter Mascher
Spring 2024
Spring 2024
Spring 2025
Spring 2025
3rd Vice President, Spring 2024
2nd Vice President, Spring 2024
Spring 2026
Spring 2024
Spring 2025
Interdisciplinary Science and Technology Subcommittee of the Technical Affairs Committee
Jennifer Hite, Chair Spring 2025
Alanah Fitch Spring 2026
Sreeram Vaddiraju
Vidhya Chakrapani
Spring 2026
Spring 2026
Huyen Dinh Spring 2026
Alok Srivastava
Spring 2024
Charuska Thameera Walgama. Spring 2024
Rangachary Mukundan Spring 2024
Chockkalingam Karuppaiah
Spring 2024
Christopher Johnson Spring 2025
James Noël, Spring 2025
Greg Jackson Spring 2025
Jeff L. Blackburn
Luca Magagnin
Symposium Planning Advisory Board of the Technical Affairs Committee
Francis D'Souza, Chair
Brett Lucht
Dev Chidambaram
Larry Nagahara
Qiliang Li
Katherine Ayers
Shelley Minteer..
Stephen Paddison.
Natasa Vasiljevic
Sean Bishop
Rong-Jun Xie
Uroš Cvelbar
Jeff Blackburn
Spring 2025
Spring 2025
3rd Vice President, Spring 2024
Chair, Battery Division, Fall 2024
Chair, Corrosion Division, Fall 2024
Chair, Sensor Division, Fall 2024
Chair, Electronics and Photonics Division, Spring 2025
Chair, Energy Technology Division, Spring 2025
Chair, Organic and Biological Electrochemistry Division, Spring 2025
Chair, Physical and Analytical Electrochemistry Division, Spring 2025
Chair, Electrodeposition Division, Fall 2023
Chair, High Temperature Materials Division, Fall 2023
Chair, Luminescence and Display Materials Division, Fall 2023
Chair, Dielectric Science and Technology Division, Spring 2024
Chair, Nanocarbons Division, Spring 2024
Maria Inman Chair, Industrial Electrochemistry and Electrochemical Engineering Division, Spring 2024
Jennifer Hite
Chair, Interdisciplinary Science and Technology Subcommittee, Spring 2025 Other Representatives
Society Historian
Roque Calvo
American Association for the Advancement of Science
Christopher Jannuzzi
Science History Institute
TBD
National Inventors Hall of Fame
TBD
Spring 2024
Term as Executive Director
Heritage Councilor, Spring 2024
Chair, Honors & Awards Committee, Spring 2027
14 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org SOCIETY NEWS
SOCIETY NEWS
Slate of Candidates for Division Officers—Fall Elections
These divisions have nominated new officers for the fall 2023 to fall 2025 term. Election results will be reported in the spring 2024 issue of Interface
Electrodeposition
Chair
Luca Magagnin, Politecnico di Milano
Vice Chair
Andreas Bund, Technische Universität Ilmenau
Secretary
Rohan Akolkar, Ernst B. Yeager Center for Electrochemical Sciences at CWRU
Treasurer
Adriana Ispas, Technische Universität Ilmenau
Member at Large
Faisal Alamgir, Georgia Institute of Technology
Trevor Braun, ElectraSteel, Inc.
Amanda Clifford, University of British Columbia
Massimo Innocenti, Università degli Studi di Firenze
Maria Eugenia Toimil-Molares, GSI Helmholtzzentrum für Schwerionenforschung
Toshiyuki Nohira, Kyoto University
High Temperature Energy, Materials, & Processes

Fernando Garzon, University of New Mexico
Srikanth Gopalan, Boston University
Turgut Gür, Stanford University
Liangbing Hu, University of Maryland
Greg S. Jackson, Colorado School of Mines
Tatsuya Kawada, Tohoku University
Hojong Kim, Pennsylvania State University
Jae Jin Kim, Argonne National Laboratory
Kang Taek Lee, Korea Advanced Institute of Science and Technology
Min Hwan Lee, University of California, Merced
Wongyoung Lee, Sungkyunkwan University
Olga Marina, Pacific Northwest National Laboratory
Torsten Markus, Mannheim University of Applied Sciences
Nguyen Minh, University of California, San Diego
Jason Nicholas, Michigan State University
Elizabeth Opila, University of Virginia
Nicola Perry, University of Illinois at Urbana-Champaign
Kannan Ramaiyan, University of New Mexico
Sandrine Ricote, Colorado School of Mines
Jennifer Rupp, Massachusetts Institute of Technology
Yixiang Shi, Tsinghua University
Subhash Singhal, Pacific Northwest National Laboratory
Anna Staerz, Colorado School of Mines
Hitoshi Takamura, Tohoku University
Jianhua Tong, Clemson University
Enrico Traversa, Università di Roma Tor Vergata
Eric Wachsman, University of Maryland
Geoffrey Will, Queensland University of Technology
Chair
Cortney R. Kreller, Los Alamos National Laboratory
Vice Chair
Xingbo Liu, West Virginia University
Junior Vice Chair
Teruhisa Horita, National Institute of Advanced Industrial Science & Technology
Secretary/Treasurer
Dong Ding, Idaho National Laboratory

Jianhua Tong, Clemson University
Member at Large
Stuart Adler, University of Washington
Mark D. Allendorf, Sandia National Laboratories
Jihwan An, Seoul National University of Science and Technology
Di Chen, Tsingua University
Fanglin (Frank) Chen, University of South Carolina
Zhe Cheng, Florida International University
Wilson Chiu, University of Connecticut
Dong Ding, Idaho National Laborator
Chuancheng Duan, Kansas State University
Jan Froitzheim, Chalmers University
Mathias Christian Galetz, DECHEMA-Forschungsinstitut
Paul Gannon, Montana State University Bozeman
Leta Woo, Cummins, Inc.
Bilge Yildiz, Massachusetts Institute of Technology
Luminescence and Display Materials

Chair
Eugeniusz Zych, Uniwersytet Wrocławski
Vice Chair
Dirk Poelman, Universiteit Ghent
Secretary/Treasurer
TBD
Member at Large
Marco Bettinelli, University of Verona
Mikhail Brik, University of Tartu
John Collins, Wheaton College
Won Bin Im, Hanyang University
Tetsuhiko Isobe, Keio University
Luiz Jacobsohn, Clemson University
Ru-Shi Liu, National Taiwan University
Kazuyoshi Ogasawara, Kwansei Gakuin University
Alan Piquette, OSRAM International GmbH
Alok Srivastava, Srivastava Consulting LLC
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 15 SOCIETY NEWS SOCIETY NEWS
Chair
New Division Officers
Mani Manivannan, Global Pragmatic Materials
Electronics and Photonics Division

Qiliang Li, George Mason University
Vice Chair
Vidhya Chakrapani, Rensselaer Polytechnic Institute
2nd Vice Chair
Zia Karim, Yield Engineering Systems
Secretary
Helmut Baumgart, Old Dominion University
Treasurer
Erica Douglas, Sandia National Laboratories
Members at Large
Travis J. Anderson, US Naval Research Laboratory
D. Noel Buckley, University of Limerick
Yu Cao, Fast Power, Inc.
Yu Lun Chueh, National Tsing Hua University
Stefan De Gendt, IMEC
M. Jamal Deen, McMaster University
Jennifer Hite, US Naval Research Laboratory
Andrew M. Hoff, University of South Florida
Hiroshi Iwai, National Yang Ming Chiao Tung University
Hemanth Jagannathan, IBM Corporation Research Center
Soohwan Jang, Dankook University
Daisuko Kiriya, The University of Tokyo
Yue Kuo, Texas A&M University
Qizhi Liu, Global Foundries, Inc.
Robert Lynch, University of Limerick
Junichi Murota, Tohoku University
Colm O’Dwyer, University College Cork
Takahito Ono, Tohoku University
Mark E. Overberg, Sandia National Laboratories
Fred Roozeboom, Universiteit Twente
Tadatomo Suga, Meisei University
Yu-Lin Wang, National Tsing Hua University
Energy Technology Division


Chair
Katherine E. Ayers, Nel Hydrogen
Vice Chair
Minhua Shao, Hong Kong University of Science and Technology
Secretary
Hui Xu, Envision Energy USA
Treasurer
Iryna Zenyuk, University of California, Irvine
Members at Large
Christopher Arges, Pennsylvania State University
Plamen Atanassov, University of California, Irvine
Scott Calabrese Barton, Michigan State University
Rod Borup, Los Alamos National Laboratory
Nemanja Danilovic, Electric Hydrogen
Steven Decaluwe, Colorado School of Mines
Vito Di Noto, Università degli Studi di Padova
Huyen Dinh, National Renewable Energy Laboratory
James Fenton, University of Central Florida
Thomas Fuller, Georgia Institute of Technology
Andrew Herring, Colorado School of Mines
Paul Kenis, University of Illinois
Ahmet Kusoglu, Lawrence Berkeley National Laboratory
Sanjeev Mukerjee, Northeastern University
Sri Narayan, University of Southern California
Peter Pintauro, Vanderbilt University
Bryan Pivovar, National Renewable Energy Laboratory
Krishnan Rajeshwar, University of Texas at Arlington
Cynthia Rice, Plug Power, Inc.
Jacob Spendelow, Los Alamos National Laboratory
Jean St-Pierre, Cummins Technical Center
Vaidynathan Ravi Subramanian, University of Nevada, Reno
Adam Weber, Lawrence Berkeley National Laboratory
John Weidner, University of Cincinnati
Gang Wu, University at Buffalo
Nianqiang Nick Wu, University of Massachusetts Amherst
Thomas Zawodzinski, University of Tennessee, Knoxville
Iryna Zenyuk, University of California, Irvine
Organic and Biological Electrochemistry Division

Chair
Shelley Minteer, University of Utah
Vice Chair
Jeffrey Halpern, University of New Hampshire
2nd Vice Chair
Sabine Kuss, University of Manitoba
Secretary/Treasurer
Ariel Furst, Massachusetts Institute of Technology
Members at Large
Mahito Atobe, Yokohama University
Mekki Bayachou, Cleveland State University
James Burgess, United States Army Research Office
Graham Cheek, United States Naval Academy
Dave Cliffel, Vanderbilt University
Robert Francke, Leibniz-Institut für Katalyse
Carlos Frontana-Vazquez, CIDETEQ
Shinsuke Inagi, Tokyo Institute of Technology
Matt Graaf, Corteva Agriscience
Binbin Huang, Hunan University
Jiri Ludvik, J. Heyrovsky Institute of Physical Chemistry
Flavio Maran, Università degli Studi di Padova
Kevin Moeller, Washington University, St. Louis
Julie Renner, Case Western Reserve University
James Rusling, University of Connecticut
Lior Sepunaru, University of California, Santa Barbara
Charuksha Walgama, University of Houston-Clear Lake
Hai-Chao Xu, Xiamen University
Physical and Analytical Electrochemistry Division
Chair
Stephen J. Paddison, University of Tennessee, Knoxville
Vice Chair
Anne Co, Ohio State University
Secretary
Svitlana Pylypenko, Colorado School of Mines
Treasurer
Iwona Rutkowska, Uniwersytet Warszawski
Members at Large
Robbyn Anand, Iowa State University
Plamen B. Atanassov, University of California, Irvine
16 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
SOCIETY NEWS SOCIETY NEWS
D. Noel Buckley, University of Limerick
Abdoulaye Djire, Texas A&M University
Alanah Fitch, Loyola University
Burcu Gurkan, Case Western Reserve University
David Hickey, Michigan State University
Yasushi Katayama, Keio University
Pawel J. Kulesza, Uniwersytet Warszawski
Johna Leddy, University of Iowa
Robert Mantz, United States Army Research Office
Hang Ren, University of Texas, Austin
Joaquin Rodriguez López, University of Illinois at Urbana Champaign
Alice Suroviec, Berry College
Greg Swain, Michigan State University
Paul Trulove, United States Naval Academy

Petr Vanysek, Northern Illinois University
Valentine Vullev, University of California, Riverside
Yingjie Zhang, University of Illinois Urbana-Champaign
Staff News
Genevieve Goldy Promoted to Awards and Board Relations Manager

Genevieve (Gen) Goldy joined ECS in January 2020 as Board Relations Specialist. This spring she accepted a promotion to Awards and Board Relations Manager, a position which reflects the increased responsibilities she has taken on and the initiative she has shown from the day she joined ECS. In her manager role, she will continue to work with ECS’s diverse, international scientists who are at the forefront of scientific and technological advances in the world. She especially enjoys supporting the ECS volunteers in handling their responsibilities to advance the mission of the Society and finding more efficient ways to make the work of the volunteers easier. She loves travel, organizing, and “working with a great staff at ECS!” In her spare time, Gen also enjoys new and diverse cultural experiences, international cuisine, and fine dining.
ECS Executive Director and CEO Chris Jannuzzi says, “I could not be happier about Gen’s promotion. She brings a wealth of professional experience from her many years working in the legal world, and that experience has helped to bring a new level of engagement with our volunteer leadership. In addition, Gen now manages all aspects of ECS’s robust awards program, which has grown dramatically under her care. This was a major aspect of her promotion and is reflected in the addition of ‘Awards’ to her new title. On a personal level, working with Gen is a joy. I start every week with our Monday morning check-in meeting. Her calm demeanor, insightful guidance, and wonderful sense of humor help set the tone for the week to come and allow me to be at my best. What more could one want in a close colleague? Congratulations and my sincerest thanks to you, Gen!”
Results of the 2023 Election of Officers and Slate of Officers for 2024
The Electrochemical Society announces the results of the 2023 Society election:
Gerardine Botte, Texas Tech University, is President, and Francis D’Souza, University of North Texas, is 3rd Vice President. The terms of Secretary Marca Doeff and Treasurer Elizabeth Podlaha-Murphy were not affected by this election.
At the June 1, 2023 Board of Directors meeting, members voted to approve the slate of candidates recommended by the ECS Nominating Committee.
The next election of ECS Officers takes place from January to March 2024.
The slate of candidates is:
PRESIDENT – Colm O’Dwyer, University College Cork
3RD VICE PRESIDENT – Y. Shirley Meng, University of Chicago/Argonne
National Laboratory, and Robert Savinell, Case Western Reserve University
SECRETARY – Gessie Brisard, Université de Sherbrooke, and Jessica
Koehne, NASA Ames Research Center
Full biographies and candidate statements will appear in the ECS Interface winter 2023 issue.
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 17 SOCIETY NEWS SOCIETY NEWS
Energy Technology Division Successfully Launches New Flagship Electrolysis Symposium
To make green hydrogen affordable, the three leading low-temperature electrolyzer technologies—liquid alkaline (LA), proton exchange membrane water electrolyzers (PEMEL), and anion exchange membrane electrolyzers (AEMEL)—need research to decrease cost, increase power output, and increase durability. The research community in this space needs a home where new ideas can be exchanged. The ECS Energy Technology Division (ETD) responded by creating a new symposium, I01—Low Temperature Water Electrolysis (LT-WE) for H2 Production, offered for the first time at the 243rd ECS Meeting.
The inaugural LT-WE Symposium received more than 170 abstracts that were presented over four-and-a-half days, with more than 30 preeminent invited speakers and panelists. It embraced the international community, with presenters from across the globe. Six topical areas were covered: electrocatalysts and electrocatalysis; membrane and ionomer technologies; electrode and MEA design; stack and system engineering; modeling and diagnostics; and advanced concepts and systems. Different formats were showcased, including electrolysis tutorials, keynote presentations, individual topical sessions, and three rounds of panel discussions covering electrolyzer technology and manufacturing; renewable energy and electrolyzer integration; and green H2 utilization. A symposium reception took place on Tuesday evening.
The past year has been exciting for hydrogen, particularly green hydrogen, which is produced through electrolysis of water using electricity derived from renewable sources such as solar, wind, and hydro. The mass production of green hydrogen is expected to be one of the key solutions to lowering the world’s CO2 emissions and
meeting Net Zero goals—helping to lower or eliminate the carbon footprint of important industrial processes that include transportation, petrochemical processing, and ammonia production, as well as metals and concrete manufacturing. Hydrogen is also an important component of many strategies for long-term (seasonal/annual) energy storage.
Unprecedented investment by governments worldwide is pushing the development and implementation of the three leading lowtemperature electrolyzer technologies: LA, PEMEL, and AEMEL. LA is a well-established technology that uses a KOH electrolyte in conjunction with common metal catalysts. Proton exchange membrane water electrolysis (PEMWE) uses some processes common to hydrogen fuel cells, including an acidic perfluoronated membrane and precious metal catalysts. These systems have higher power than LA and are considered preferable for load-following renewables. They can also produce pressurized hydrogen. Anionexchange membrane water electrolysis (AEMWE) systems are less mature but offer many of the benefits of LA (non-precious metal catalysts) and PEM (a solid separation membrane between the water and hydrogen compartments). In addition to these three, new ideas are emerging for membraneless reactors, and for externally assisted hydrogen production.
The LT-WE Symposium is expected to become a gala event for the researchers, industry leaders, government representatives, and other stakeholders who are passionate about water electrolysis and green H2
Be on the lookout for this symposium at all future ECS spring meetings and reach out to the symposium organizers to get involved!
Editor’s Note
In the spring issue of Interface, the article on the Gordon E. Moore Medal for Outstanding Achievement in Solid State Science and Technology misstated the research focus of the medal winner, Fred Roozeboom. Since 2004, Dr. Roozeboom's
research has focused on selective atomic layer etching (ALE). The article originally stated, “Since 2017, his research has focused on selective atomic layer epitaxy (ALE).”

18 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Be on the lookout for this symposium at all future ECS spring meetings and reach out to the symposium organizers to get involved! SOCIETY NEWS SOCIETY NEWS
HYDROGEN is one of the key solutions to lowering the world’s CO2 emissions and meeting Net Zero goals 170 abstracts 3 rounds of panel discussions >3O preeminent invited speakers and panelists 6 topical areas covered 4 1/2 days ELECTROLYSIS SYMPOSIUM
PRiME 2024
JOINT INTERNATIONAL MEETING of The Electrochemical Society of Japan, The Korean Electrochemical Society, and The Electrochemical Society

HONOLULU, HI

October 6-11, 2024
Hawaii Convention Center & Hilton Hawaiian Village
www.electrochem.org/PRiME2024
The new PAT-Terminal-1, your powerful assistant in the glove box!
The PAT-Terminal-1 is a stand-alone device for sensor adjustment and functionality tests of PAT series battery test cells.

Fully equipped test channel with PStat / GStat / EIS
Ready for use as a stand-alone device directly in the glovebox
For cell functionality checks (e.g. impedance) and sensor adjustments of operando test cells like the PAT-Cell-Force
Integrated display showing live data of inserted test cell
Operable as a regular test channel in conjunction with a PAT-Tester-x-8 potentiostat
SOCIETY NEWS SOCIETY NEWS
sales@el-cell.com +49 40 79012-734 el-cell.com
SAVE THE DATE
Creating Powerful Partnerships
ECS welcomes its newest institutional members. These organizations are great additions to the institutional membership program.
To learn more, visit our Institutional Membership Program Benefits page.
Corteva Agriscience
Corteva, Inc. (NYSE: CTVA) is a publicly traded, global pure-play agriculture company that combines industryleading innovation, high-touch customer engagement, and operational execution to profitably deliver solutions for the world’s most pressing agriculture challenges. Corteva Agriscience generates advantaged market preference through its unique distribution strategy, together with its balanced and globally diverse mix of seed, crop protection, and digital products and services. With some of the most recognized brands in agriculture and a technology pipeline well positioned to drive growth, the company is committed to maximizing productivity for farmers, while working with stakeholders throughout the food system as it fulfills its promise to enrich the lives of those who produce and those who consume, ensuring progress for generations to come.
To learn more, visit Corteva Agriscience at https://www.corteva.com.
Current Chemicals

Current Chemicals is an Ohio specialty materials manufacturer that performs chemical manufacturing, piloting, and development of customers’ formulations in diverse markets, including battery, SOFC, rare earth, and luminescent materials. With 70+ years of experience, Current has the technical knowledge and infrastructure to support small-scale orders or enable rapid scale-up and commercialization.
To learn more, visit Current Chemicals at https://www.currentchemicals.com.
Nel
Nel is a global, dedicated hydrogen company, delivering optimal solutions to produce and distribute hydrogen from renewable energy. We serve industries, energy, and gas companies with leading hydrogen technology. Our roots date back to 1927, and since then, we have had a proud history of development and continuous improvement of hydrogen technologies. Today, our hydrogen solutions cover the entire value chain from hydrogen production technologies to hydrogen fueling stations, enabling industries to transition to green hydrogen, and providing all fuel cell electric vehicles with the same fast fueling and long range as fossil-fueled vehicles—without emissions.
To learn more, visit Nel at www.nelhydrogen.com.
Spectro
Inlets ApS
At Spectro Inlets, we develop real-time and accurate measurement solutions to optimize environmental and chemical processes for a greener, cleaner world. We provide turnkey instruments for effortless coupling of electrochemistry and mass spectrometry, elevating our customers’ Power-to-X and battery research to the next level.
To learn more, visit Spectro Inlets ApS at www.spectroinlets.com NEW
NEXT ISSUE OF IN THE
The fall issue of Interface will be a special issue on commercialization of electrochemistry and material science technologies, guest edited by E. J. Taylor
Featured articles will share insights into the process by which electrochemical research translates into an invention and then into a manufactured product.
Fall 2023 will also include 243rd ECS Meeting highlights, the 2023 Toyota Young Investigator recipients, Pennington Corner, features favorites like EChem Education, Tech Highlights, and Looking at Patent Law, updates on ECS journal impact factors, and the latest news about people, students, and the Society.
20 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org SOCIETY NEWS SOCIETY NEWS
2023 Leadership Circle Awards
Long-term ECS supporters have been recognized with Leadership Circle Awards since fall 2002 to honor and thank our partners in electrochemistry and solid state science. These awards are granted in the anniversary year that an institutional member reaches a milestone level.*
Congratulations and thank you to the following ECS Institutional Members who achieved milestones in 2023. These companies have been tremendous partners and we appreciate all the support they have given the Society over the years.
Legacy Level – 70+ years
General Motors Holdings LLC
General Motors is a global company focused on advancing an all-electric future that is inclusive and accessible to all. At the heart of this strategy is the Ultium battery platform, which powers everything from mass-market to high-performance vehicles. General Motors, its subsidiaries, and its joint venture entities sell vehicles under the Chevrolet, Buick, GMC, Cadillac, Baojun, and Wuling brands.
To learn more, visit General Motors at https://www.gm.com
Gold Level – 25 years
Yeager Center for Electrochemical Sciences
Prof. Ernest B. Yeager founded the Ernest B. Yeager Center for Electrochemical Sciences (YCES) at Case Western Reserve University (CWRU) in Cleveland, OH, in 1976. Although originally its major mission was to promote research and education in electrochemistry across the CWRU campus, its current primary function is to hold the yearly Workshop on Electrochemical Measurements. This unique international event is attended by members of the academic, industry, and National Laboratories communities seeking to gain theoretical and hands-on experimental electrochemistry. These include, among others, interfacial physical chemistry, corrosion, electrocatalysis, energy conversion and energy storage, electrochemical engineering, electrochemical kinetics, and electroanalytical chemistry. The YCES faculty, composed of internationally renowned experts in these fields, contribute greatly to the event’s extraordinary success.
To learn more, visit the Ernest B. Yeager Center for Electrochemical Sciences at https://chemistry.case.edu/research/yces
Bronze Level – 5 years

Cummins Inc.
Cummins Inc., a global power technology leader, is a corporation of complementary business segments that design, manufacture, distribute, and service a broad portfolio of power solutions. The company’s products range from internal combustion, electric, and hybrid integrated power solutions to components including filtration, aftertreatment, turbochargers, fuel systems, controls systems, air handling systems, automated transmissions, electric power generation systems, microgrid controls, batteries, electrolyzers, and fuel cell products. Headquartered in Columbus, IN, since its founding in 1919, Cummins employs approximately 73,600 people committed to powering a more prosperous world through three global corporate responsibility priorities critical to healthy communities: education, environment, and equality of opportunity. Cummins serves its customers online, through a network of company-owned and independent distributor locations, and through thousands of dealer locations worldwide. It earned about $2.2 billion on sales of $28.1 billion in 2022.
To learn more, visit the Cummins Inc. website at https://www.cummins.com.
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 21 SOCIETY NEWS SOCIETY NEWS
Institutional Membership Levels Legacy: 70+ years Medallion: 65 years Diamond: 50 years Gold: 25 years Silver: 10 years Bronze: 5 years Contact Anna Olsen, Senior Manager, Corporate Programs , to learn more about the ECS Institutional Membership Program. ECS Institutional Membership Program LEARN MORE ?
*ECS
SOCIETY NEWS SOCIETY NEWS
2022 Corporate and Institutional Donors
ECS thanks these corporations and institutions. With their support, the Society advances the world’s most cutting-edge research through meetings, publications, and continuing education.
Admiral Instruments
Advance Cell Engineering
Air Liquide Advanced Materials
American Elements
Ametek-Scientific Instruments (PAR/ Solatron)
Applied Materials, Inc.
Arbin Instruments
Army Research Office (ARO)
ASM International N.V.
Attocube Systems AG
BASi
BioLogic USA/BioLogic SAS
Calumix Technologies, Inc.
Case Western Reserve University Alumni Association
Center for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW)
Center for Synthetic Organic Electrochemistry, University of Utah
Central Electrochemical Research Institute (CECRI)
Comet, Inc.
Comsol, Inc.
Corning Incorporated
Cougar Creek Technologies, LLC
Covalent Metrology
Cummins Inc.
Current Chemicals
Cyclikal, LLC
Daicel Corporation
DataPhysics Instruments USA Corporation
De Nora
Department of Homeland Security Science and Technology Directorate
Department of Industrial Engineering of the University of Padova, Italy
Deutsches Zentrum für Luft- und Raumfahrt (DLR)
Duracell US Operations Inc.
easyXAFS, LLC
Electrosynthesis Company, Inc.
EL-CELL GmbH
Energizer Battery
Energy Assurance LLC
E-One Moli Energy (Canada) Limited
Faraday Technology, Inc.
Ford Motor Company
Gamry Instruments
GE Global Research Center
Gelest Inc.
General Motors Holdings LLC
Giner, Inc.
GlobalFoundries
GS Yuasa International Ltd.
Harvard Bioscience
Hiden Analytical Inc.
Honda R&D Co., Ltd.
Hydro-Québec
Ion Power
IOP Publishing
IVIUM Technologies
JSPS Grant-in-Aid for Transformative Research Areas, Dynamic Exciton: Emerging Science and Innovation
Kanto Chemical Co., Inc.
Lam Research Corporation
Lawrence Berkeley National Laboratory
LG Energy Solution
Los Alamos National Laboratory
Maccor, Inc.
Malvern Panalytical
Materials Science and Engineering, National Tsing Hua University (NTHU)
Mattson Technology, Inc.
Medtronic, Inc.
Metrohm USA, Inc.
Microsoft Corporation
Mitsui Metal & Mining Co., Ltd.
MTI Corporation
National Renewable Energy Laboratory
Naura-Akrion, Inc.
Neware Technology Limited
Nissan Group of North America
Nissan Motor Co., Ltd.
Occidental Chemical Corporation, Dallas, Texas
OCI Vacuum Microengineering, Inc.
Pacific Northwest National Laboratory
PalmSens B.V.
Pamarco
Panasonic Energy Corporation
Park Systems
Permascand AB
Physics World
Pine Research Instrumentation
Plug Power
ProSys, Inc.
QuantumScape Corporation
Royal Society of Chemistry
Sandia National Laboratories
Scribner Associates, Inc.
SH Scientific Corporation
Sherwin-Williams Company
Sila Nanotechnologies, Inc.
SK On Co., Ltd.
Spectro Inlets ApS
TA Instruments
Technic, Inc.
Tecnochimica
Teledyne Energy Systems, Inc.
Thermo Fisher Scientific
Tokyo Electron Limited
Toyota Research Institute of North America (TRINA)
UL Research Institutes
United Mineral & Chemical Corporation
US Naval Research Laboratory
VSPARTICLE B.V.
Western Digital GK
Westlake Corporation
Wildcat Discovery Technologies
Wiley
Yeager Center for Electrochemical Sciences at CWRU
Yield Engineering Systems, Inc.
Xenocs, Inc.
Zurich Instruments USA
22 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Member Anniversaries 2023 >>>
It is our great pleasure to recognize the following ECS members who reached their 30, 40, 50, and 60-year anniversaries with the Society in 2023. Congratulations to you all!
60-Year Anniversaries
Charles E. Allman
Vernon A. Bluhm
Maurice I. Hart
Raymond J. Jasinski
Zlata Kovac
William A. McAllister
40-Year Anniversaries
Doron Aurbach
John O. Borland
Edmond F. Bowden
Ernesto Julio Calvo
Dennis H. Evans
Joseph C. Farmer
Heiner J. Gores
Kevin Krist
Uziel Landau
Clovis A. Linkous
Gangadhara S. Mathad
Lubomyr T. Romankiw
Vern D. Shipman
Orlin D. Trapp
John Wagenknecht
Chih Chun Wang
50-Year Anniversaries
Larry R. Faulkner
Turgut M. Gür
Tohru Hara
Adam Heller
Savin Ikonopisov
Harold F. Jones
William J. Kroll
30-Year Anniversaries
Shalini Menes
Toshiaki Murahashi
Zempachi Ogumi
Sandra C. Rondinini
David J. Schiffrin
Kenji Takahashi
Jan B. Talbot
Sing Pin Tay
Jomar Thonstad
Willie J. Yarbrough
Terrill B. Atwater
Helmut
Baltruschat
Jeremy Barker
Joel M. Barnett
John B. Bates
Antonio Martínez
Chaparro
Brian J.
Dougherty
Francis D’Souza
Rasmus Fehrmann
Mark A. Greaney
Geir M. Haarberg
by Frances Chaves
Are you a young researcher just getting started? Do you work in a field that requires frequent job moves? Is your name so common that it leads to confusion about authorship and difficulty following your research contributions?
You need an ORCID iD (Open Researcher and Contributor ID)! ORCID iDs are unique, persistent 16-digit number identifiers which are free of charge and identify an individual author on a global scale across all their published papers, affiliations, peer reviews, grants, and more. Using the iD in systems and platforms ensures that you get credit for your contributions. Readers who want to follow your work can search for you by your ORCID iD and access all your work, unlike searching an individual journal or database.
ORCID iD is integrated across ECS platforms, ScholarOne, and ECSarXiV through ECS’s single-sign-on service. Society members can link their ORCID iD to their membership profile. While accessing ORCID requires an additional sign in, the same credentials as your ECS My Account can be used. ScholarOne—IOPP’s article submission system—uses ORCID as a sign-on option, so including ORCID as part of a member profile eliminates having to remember multiple login names/passwords.

Linking your member profile to ORCID is easy. First, register for your ORCID iD Then add your iD to your ECS profile. Click “Login” at the top of the electrochem.org screen to get to your ECS My Account page. Here you can access/update your account information. Click “Link my ORCID Account” to go to ORCID and link ORCID to your My Account page. Now you are distinguished and distinguishable!



Arden P. Johnson
Christopher S.
Johnson
Ismail Kashkoush
Carol L.
Korzeniewski
Shawming Ma
Meyya
Meyyappan
John R. Miller
Michael V. Mirkin
Isao Nakatsugawa
Yaw S. Obeng
Elizabeth J. Opila
John J. Michel
Patrick K. Ng
Kemal Nisancioglu
Mark Salomon
Raymond A. Sutula
David J. Young
Yasushi Oura
Martin W. Payne
David R. Peterson
Donald L. Pile
Kenji Sashikata
Chee Burm Shin
Makoto Ue
Palani Velu
Klaus Von Benda
David O. Wipf
Xiao-Qing Yang
Karim Zaghib
Cynthia G. Zoski
ORCID iDs
ORCID Connecting research and researchers www.orcid.org Visit to register. 4 GET YOUR ORCID ID Add your iD to your ECS profile
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 23 SOCIETY NEWS SOCIETY NEWS
Websites of Note

Suggested for you by Alice Suroviec
NASA Technical Reports Server

https://ntrs.nasa.gov
Battery University
Battery University™ is a free educational website that offers battery information for engineers, educators, the media, students, and battery users alike. The tutorials evaluate the advantages and limitations of battery chemistries, advise on the choice of the best battery for different applications, and suggest ways to extend battery life. The information is compiled from specifications and independent test laboratories as well as crowdsourcing.
http://batteryuniversity.com/learn



Modeling of Porous Electrodes
phenomenon in a series of three posts. The problem is very relevant as it appears in a wide variety of systems. His blog is a useful starting point for anyone who is looking to better understand the phenomenon or to model their own systems.

http://www.joshuagallaway.com/?p=215
About the Author
Alice Suroviec is Professor of Bioanalytical Chemistry and Dean of the College of Mathematical and Natural Sciences at Berry College. She earned a BS in Chemistry from Allegheny College in 2000. She received her PhD from Virginia Tech in 2005 under the direction of Dr. Mark R. Anderson. Her research focuses on enzymatically modified electrodes for use as biosensors. She is currently Associate Editor of the PAE Technical Division for the Journal of the Electrochemical Society. She is always looking for new app/podcast/website suggestions, so feel free to contact her.
https://orcid.org/0000-0002-9252-2468

24 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org SOCIETY NEWS SOCIETY NEWS
The NASA STI Repository provides online access to NASA full-text documents, including conference papers, conference presentations, and technical videos. The website is easy to search and can provide data for use both in the classroom and in research.
Prof. Joshua Gallaway has a series of excellent blog posts on modeling porous electrodes on his Northeastern University lab website. He describes the well-known, but not easily taught,
The Electrochemical Society. DOI: 10.1149/2.F02232IF SAVE THE DATE www.electrochem.org/upcoming-meetings 245th ECS Meeting SAN FRANCISCO, CA May 26–30, 2024 Marriott Marquis San Francisco
©
is
244th ECS Meeting
GOTHENBURG l SWEDEN
October 8-12, 2023
UPCOMING ECS SPONSORED MEETINGS
In addition to the ECS biannual meetings and ECS satellite conferences, the Society, its divisions, and its sections sponsor meetings and symposia of interest to the technical audience ECS serves. Here is a partial list of upcoming sponsored meetings. Visit the ECS website for a list of all sponsored meetings.
2023
StorageX International Symposium Series
Ongoing Fridays – Virtual Lectures
Stanford University
2023 International Conference on Green Electrochemical Technologies & the 2023 Annual Meeting of the Electrochemical Society of Taiwan (2023 ICGET-Tw)

October 26–28, 2023
National Taiwan University of Science and Technology
2025
19th International Symposium on Solid Oxide Fuel Cells (SOFC-XIX)
July 13–18, 2025 – Stockholm, Sweden
The Brewery Conference Center
For information on the benefits of ECS meeting sponsorship (including publishing sponsored meetings’ proceedings volumes), or to request ECS sponsorship for your technical event, contact ecs@electrochem.org.

The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 25 SOCIETY NEWS SOCIETY NEWS
Registration
OPEN REGISTER NOW
GORDON E. MOORE, Longtime Society

Moore’s lifelong passion for science was sparked when he was 13 years old and used a neighbor’s chemistry kit to make gun powder, then nitroglycerin and other explosives. He went on to “blow up” the technology world by creating the processes that led to the memory and microprocessors necessary to today’s electronic devices. Along the way, Dr. Moore parlayed a $500 startup investment into a multi-billion-dollar fortune. In keeping with this legacy, the foundation he created with his wife Betty promotes positive outcomes for future generations and fosters path-breaking scientific discovery, environmental conservation, and more.
Dr. Moore joined ECS in 1957. He called the ECS San Francisco Section his “home court ” and described the Society as an asset that makes today’s technological advancements possible. In “The Evolving Technology of the Semiconductor Integrated Circuits,” presented at the San Francisco Section’s 1964 meeting, he outlined the underlying foundation of Moore’s Law and his vision for the future potential of semiconductor electronics. Moore’s Law became the guiding principle for the industry to deliver ever more powerful semiconductor chips at proportionate decreases in cost—one of the most influential technical and business predictions of all time. Dr. Moore went on to deliver “A Perspective on VLSI,” at the 159th ECS Meeting in 1981, and “Fifty Years of Semiconductor Technology” at the 191st ECS Meeting in 1997. He was named ECS Honorary Member in 2007.
Member and Honoree
by Frances N. Chaves
Gordon E. Moore, author of Moore’s Law and co-founder of Intel Corporation, passed away on March 24, 2023. Many at ECS knew him during his 66 years as a Society member. We tracked the extraordinary progress of his research through our meetings and publications. He actively mentored a generation of scientists who followed his lead in the semiconductor revolution. “His seminal work defines the technological world of the 21st Century,” said ECS Past President Turgut Gür.

In a 2016 ECS Masters interview, Dr. Moore describes his progression from a childhood in a small California farming community to co-founding and directing Intel, the world’s largest semiconductor chip manufacturer by revenue.

ECS MOURNS
Fig. 1. Dr. Moore’s 1965 article in Electronics magazine, “Cramming More Components onto Integrated Circuits,” used his own empirical observations to predict that the number of circuits per integrated circuit would double every year for at least the next ten years. © 1965 IEEE. Reprinted, with permission, from Electronics, 38(8), 114 (1965).
Gordon E. Moore
The spring 1997 issue of Interface, a special issue in celebration of the golden anniversary of the invention of the transistor, featured Gordon Moore on the cover.

The ECS Solid State Science and Technology Award was renamed in Dr. Moore’s honor in 2005 through a $150,000 endowment from Intel in honor of its founder. The Gordon E. Moore Medal for Outstanding Achievement in Solid State Science & Technology recognizes scientists and engineers who make distinguished


contributions to solid state science. The 2021 Moore Medal recipient, Hiroshi Iwai (Tokyo Institute of Technology and Vice Dean and Distinguished Chair Professor, National Chiao Tung University) said, “…not only was [Dr. Moore] the father of nanoelectronics and a true titan of industry, he remained active in technical conferences for many years, not only delivering talks himself, but also listening to others’ presentations with sincerity and giving appropriate questions and comments. I was fortunate enough to have met him at conferences in the US from 1980 to 1997. I can still see his gentle gaze and hear his kind voice in my mind.”
In the spring 1997 issue of Interface (featuring his picture on the cover), Dr. Moore predicted, “We have a fair ways to go just to continue to push the technology to smaller and smaller things, higher and higher performance. The people who use that technology to make products will then have billions of transistors on a chip to work with, and that gives them almost open-ended possibilities.” The technological revolution Dr. Moore ignited over 75 years ago continues to generate new innovations. Although he will be sorely missed far into the future by ECS, his family, and the world, his voice continues to urge us on to higher accomplishments.

Fig. 2. Ten years later, in 1975, Moore revised his prediction to doubling every two years. However, this turned out to be a little conservative; for 50 years after 1961, the number of transistors doubled about every 18 months, with consequent reductions in cost. Today, the rate appears to be slowing, but as a goal it continues to drive innovation in the industry. Source: Our World in Data
© The Electrochemical Society. DOI: 10.1149/2.F03232IF

His seminal work defines the technological world of the 21st Century.”
ECS
Outstan d i n g Ahc i etatSdiloSnitnemeve
— ECS Past President Turgut Gür
GordonE
. Moore Medal establishedin1971for
ecneicS & ygolonhceT
In Memoriam ...
Mino Green 1927–2022
Mino Green passed away at age 95, on October 13, 2022. An emeritus professor of electrical device science at Imperial College London, Professor Green joined ECS in 1993 and was a long-standing member of the Electronics and Photonics Division and the Europe Section. His many significant scientific accomplishments include the development of efficient, next-generation lithium-ion batteries, with higher energy densities and longer life cycles.
Prof. Green was born in New York City in 1927, and grew up in the United States, France, and the United Kingdom. He received his BSc in Chemistry from Durham University in 1948, his PhD in Radiochemistry (also at Durham) in 1951, and DSc in 1964. Following his PhD, and marriage in 1951 to his beloved wife Diana, he moved to MIT’s Lincoln Labs in Cambridge, Massachusetts as a Group Leader, followed by stints at University of Pennsylvania and Zenith Radio. He joined Imperial as a Fellow in 1972 and rose steadily there, ultimately retiring as Professor and Head of the Optical and Semiconductor Devices Group in 1992. After retirement, he served Imperial as a Senior Research Investigator for almost 30 years, leading countless research projects.
Throughout his career, Prof. Green’s research took place at the intersection of chemistry and physics, with a focus on nanotechnology. He conducted ground-breaking studies of the surfaces of silicon and germanium and made the seminal investigation of the electrochemistry of the semiconductor-electrolyte interface. His work elucidated many solid-state effects, including electrical transport, thermoelectricity, and photoconductivity. He pioneered the development of electrochromic
Jamie Noël Awarded the 2023 Florence Bucke Science Prize
The Faculty of Science of Western University, Ontario, Canada, has named Professor Jamie Noël the 2023 recipient of the prestigious Florence Bucke Science Prize. The prize is awarded annually to a faculty member based on an assessment of the quality and importance of their recent research achievements. The prize consists of a certificate, a $2000 award, and a public lecture.
Jamie’s recent accomplishments include being named a Western University Faculty Scholar and Fellow of the Electrochemical Society. His research group employs innovative, multidisciplinary approaches to solving problems that straddle the boundaries of chemistry, physics, earth sciences, metallurgy, and materials science, especially those related to materials electrochemistry and corrosion/degradation. This often requires designing and constructing specialized apparatus for novel experiments or extreme environments, performing high resolution surface analyses and precise measurements of fundamental physical chemical quantities by electrochemical and other appropriate means, and detailed data analysis, fitting, and computer modeling. Much of the Noël lab’s work is related to ensuring the safety and longevity of metallic containers for the permanent disposal of nuclear fuel waste.
windows in tungsten bronze thin films. He carried out early work on laser-assisted etching of semiconductors, and on electron-beam modification of glass for optical waveguide formation. He developed a new method of silica-on-silicon waveguide fabrication based on spin-coating and rapid thermal annealing of sol-gel glass. His most recent work was in nanolithography, where he developed methods for fabricating partially ordered nanostructures, using inorganic resists to form closely spaced arrays of nanopillars and nanowells. Nanopillars are now used as highly sensitive substrates for surfaceenhanced Raman spectroscopy in proteomics. Nanowells form the basis of electrochemically shuttered nanoreactors for combinatorial drug discovery, and nanostructured silicon is being used for highperformance anodes in lithium batteries.
Prof. Green was interested in the chemistry of electrical energy storage and consulted for NASA on fuel cells. In 2006, he founded Nexeon to develop nanostructured batteries with improved charge storage capacity. His key contribution was the recognition that damage to conventional planar electrodes caused by repetitive lithium-ion insertion and extraction could be mitigated by using a nanostructured material, with its much larger surface area. He was awarded the Imperial College Medal in 2015 for this work and was a Fellow of the Institute of Electrical Engineers.

He is survived by his son David and daughter Penny, and his four cherished grandchildren.
This notice is compiled from remembrances published by Imperial College and by The Guardian
Dev Chidambaram
Named 2023 Nevada Regents Distinguished Researcher
Dev Chidambaram has been named the 2023 Nevada Regents’ Distinguished Mid-Career Researcher for the State of Nevada. The Nevada Regents’ Researcher Awards are given annually to two permanent full-time Nevada System of Higher Education faculty with distinguished records in research: one Distinguished Career Researcher Award and one Mid-Career Researcher Award. Each institution in the state goes through an internal process to select one faculty member to be the nominee. One recipient is then chosen by the Regents using an external review process. The award carries a $5,000 stipend and use of the title in perpetuity.
Dev Chidambaram is Professor of Chemical & Materials Engineering and the Director of the Nevada Institute for Sustainability at the University of Nevada, Reno. The Regents’ award honors his path-breaking research in sustainable energy, materials characterization, and corrosion science.

28 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org SOCIETY NEWS SOCIETY NEWS PEOPLE NEWS PEOPLE NEWS
Reports from the Frontier
edited by Scott Cushing, Interface Contributing Editor

This feature is intended to let ECS award-winning students and post-docs write primary author perspectives on their field, their work, and where they believe things are going. This month we highlight the work of both Grace Lindquist, the ETD Graduate Student Award Winner, and Matthew J. Liu, the IEEE Student Achievement Award Winner.
Electrifying Chemical Transformations and Separations to Valorize Wastewater Nitrogen
by Matthew J. Liu and William A. Tarpeh

Ammonia (NH3) is an essential compound to modern society, underpinning fertilizer production and chemical manufacturing. Ammonia is also being considered as a hydrogen carrier that can be produced from renewable energy; liquified, stored, and transported readily; and utilized for energy without direct carbon dioxide (CO2) emission.1–3 Global ammonia demand currently exceeds 150 million tons a year (market value 70 billion USD) and is projected to increase over 2% annually.2,4,5 Over 96% of ammonia is currently generated through the Haber-Bosch (HB) process, in which steam-reformed hydrogen (H2) reacts with nitrogen (N2) under reaction conditions (400–500 °C, 100–200 atm) that consume 1–2% of global energy and contribute 1.2–1.4% of anthropogenic CO2 emissions every year.2,5,6
In an environmental context, ammonia is a form of reactive nitrogen. Large amounts of reactive nitrogen, such as HB ammonia, accumulate in the biosphere because 80% of wastewater globally is discharged without treatment.7 The resulting skew in the global nitrogen cycle leads to imbalanced ecosystems and threatens water quality; damages from reactive nitrogen emissions to freshwater alone (including algal blooms and drinking water pollution) cost billions of dollars annually in the United States.8,9 Conventional water treatment removes reactive nitrogen by converting it to N2 (biological nitrification–denitrification); at HB facilities, the N2 is then cycled back to produce ammonia. Directly valorizing reactive nitrogen in waste streams would shortcut the use of N2 as an intermediate in water remediation and ammonia production, allowing savings in energy, emissions, and costs. Indeed, treating nitrogen as a resource to recover rather than simply a pollutant to remove aligns with the US National Academy of Engineering’s call to manage the nitrogen cycle, a challenge central to chemical manufacturing and ecosystem protection.10–12
Two forms of reactive nitrogen dominate aqueous nitrogen emissions: ammonium (NH4+) and nitrate (NO3–). In the context of ammonia recovery, wastewater ammonium can be recovered through processes that selectively separate it from other wastewater constituents. Meanwhile, wastewater nitrate can be recovered as ammonia through a selective reduction reaction followed by a selective separation process. Catalysis has traditionally preceded separations in chemical manufacturing schemes, with the two processes being viewed and developed separately.13,14 Use of impaired feedstocks such as wastewaters has created new opportunities to colocate selective reactions with selective separations.15–20
We define a reactive separation process as an integration of reaction and separation imposed at the system, unit process, or molecular scale, with a particular focus on the unit process scale in this report. Reactive separations can enhance process intensification, process control, and energy consumption in wastewater treatment and valorization. Electricity provides a tunable driving force for reactive separation processes: potential differences control the free energy changes in the electrochemical system while the current controls the electrochemical reaction rate.21 As a result, electrochemical processes enable thermodynamic and kinetic control over multiple length and time scales. In addition, electrochemical processes can be implemented in decentralized settings, which may complement the often-decentralized nature of water treatment and nitrogen pollution. For these reasons, we anticipate that electrochemists and electrochemical engineers can uniquely and meaningfully contribute to nitrogen use practices—both separations and reactions.
Electrochemical Separation Approaches
Selective separations demix solutes using driving forces such as temperature, pressure, and electrochemical gradients.13 Fig. 1 shows electrochemical stripping, a process that uses electrochemical driving forces to recover >93% of wastewater NH3 based on charge and volatility. Ammonium-rich wastewater is fed into the anode chamber (left chamber). The anode and cathode chamber are separated by a cation exchange membrane to allow electromigration of ammonium into the cathode chamber. The catholyte basifies to an alkaline pH (typically 10.5–11.5) that can be tuned through the applied current, thereby avoiding the need for direct chemical input (e.g., of sodium hydroxide to basify the solution). As a result, ammonium deprotonates to ammonia (pKa ~ 9.25), which is a volatile species. Over time, high ammonia vapor pressure builds up in the cathode chamber. The cathode and trap chamber are separated by a hydrophobic gas permeable membrane (e.g., polypropylene) to allow volatilized ammonia to diffuse to the trap chamber for collection. For example, sulfuric acid can be used such that highpurity ammonium sulfate, a commodity fertilizer, is generated.22–25 Traditionally, ammonia stripping has been employed to treat ammonium-rich wastewater. The treatment process is predicated on
(continued on next page)
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 29
Ammonia in the trap chamber protonates to ammonium, maintaining an ammonia concentration gradient between the cathode and trap chambers.

the need for centralized infrastructure that collects wastewater for treatment. However, wastewater is often generated and collected in a distributed fashion, creating a need for self-sufficient processes such as electrochemical stripping that can treat water on-site while generating ammonia in a distributed manner. Electrochemical membrane reactors such as electrochemical stripping can therefore act as reactive separations platforms to recover high-purity ammonia from ammonium-rich wastewaters and/or nitrate-rich wastewaters.
Electrochemical Reaction Approaches
Electrocatalysts mediate the transfer of electrons through a catalyst active site, enabling electrochemical redox reactions to occur with increased reaction rate, energy efficiency, and/or product selectivity.26 Implementation of an ammonia-selective nitrate reduction reaction (NO3RR) catalyst in the cathode chamber of electrochemical stripping could allow simultaneous water treatment and electrified ammonia manufacturing.
NO eH NH H 33 2 89 3O
NO3RR electrocatalysts consist largely of heterogeneous, metallic catalysts such as single metals (e.g., Pt, Pd, Cu, Ti),27,28 alloys (e.g., CuNi, PtRu),29,30 and core–shell nanoparticles (e.g., Ru–oxygendoped-Ru, Cu/CuOx–Co/CoO).31,32 However, the surface structures of these electrocatalysts are difficult to control at an atomic level and they tend to restructure under reaction conditions, making it challenging to isolate the contributions of different surface species to reactivity. For example, titanium, an inexpensive and abundant metal, has been identified as a robust electrocatalytic material for NO3RR.28 The reasons underlying the catalytic performance of titanium remain unclear, especially regarding the role of near-surface titanium hydride (TiHx, 0 < x ≤ 2), a water-stable titanium species that electrochemically forms under protic, reducing conditions.33
We overcame the obstacle of linking catalytic performance with surface structure by combining systematic synchrotron X-ray characterization of Ti electrodes with electrochemical testing (Fig. 2). Through ex situ grazing-incidence X-ray diffraction (GIXRD) and total electron yield X-ray absorption spectroscopy (TEY XAS) measurements, we demonstrated that near-surface TiH2 formation begins at NO3RR potentials ≤ −0.4VRHE and that TiH2 dominates near-surface speciation ≤ −0.8VRHE. For a fixed applied potential, increasing NO3RR duration promotes near-surface TiH2 formation,
though to a lesser extent than varying the potential. These results informed an electrochemical treatment method of Ti to produce TiH2/ Ti electrodes. Controlled potential electrolysis of unamended Ti electrodes vs. TiH2/Ti demonstrated that at all the tested potentials (−0.4, −0.6, −0.8, and −1.0 VRHE), the rate-determining and selectivitydetermining steps of NO3RR were unaffected by the initial nearsurface structure.34 These findings therefore helped decouple hydride formation from NO3RR performance under a variety of reaction durations and applied potentials, which are parameters that may need to be varied to formulate different value-added products on-demand and to remove pollutants to threshold values. In a follow-up study, we found that mass transport effects on interfacial electrolyte pH and solute concentrations played a more impactful role than near-surface structure in regulating NO3RR activity and selectivity.35 The influence of electrolyte properties on NO3RR was especially salient to the context of water treatment, which involves the need to accommodate various wastewater compositions.
Outlook
Ammonia synthesis in the 21st century will be a multifaceted effort that needs to fulfill several goals relating to energy, the environment, and resource equity. First, methods of ammonia synthesis must be increasingly coupled with renewable energy. For example, ammonia could be produced in electrolysis cells powered by electricity from solar or wind energy. According to the US Department of Energy (DOE), electrochemical technologies must operate at current densities >300 mA cm−2 while maintaining energy efficiencies >60% and faradaic efficiencies >90% to be economically viable options for carbon-neutral fuel production (fuel energy cost <$0.3 kWh−1).36 Current state-of-the-art electrochemical ammonia synthesis systems, whether by N2 by NO-3 reduction, can typically achieve one or perhaps two of these metrics,4,37,38 but rarely all three. The technology readiness level (TRL) of electrochemical ammonia synthesis remains at an estimated TRL 1–3,5 meaning that the most mature technologies remain experimental proofs of concept. Substantial work at the intersection of electrocatalyst and electrochemical engineering research, design, and scale-up will help guide electrochemical technologies toward achieving the metrics prescribed by the DOE.
The second goal of 21st century ammonia synthesis is to respond to quality-of-life needs, especially in the context of resource equity. The infrastructure needed for HB production plants requires large capital investment, which favors economies of scale.36 As a result, HB production plants are concentrated in North America and in Western Europe, leading to inequitable distribution and pricing of HB ammonia
Fig. 2. Ex situ synchrotron X-ray characterization of the titanium electrode near-surface. Grazing-incidence X-ray diffraction (GIXRD) characterizes the long-range, crystalline structure of the near-surface while X-ray absorption spectroscopy (XAS) gives insight into the short-range, local Ti coordination environment of the near-surface (e.g., coordination number and interatomic distance). We developed quantitative relationships between near-surface titanium hydride content and various NO3RR durations and applied potentials, allowing us to better decouple the formation of titanium hydride from NO3RR performance. Adapted with permission from M. J. Liu et al., J. Am. Chem. Soc., 144, 5739–5744 (2022). © 2022 American Chemical Society.

30 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org Liu and Tarpeh (continued from previous page)
Fig. 1. Electrochemical stripping reactor. A cation exchange membrane separates the anode and cathode chamber, and a gas permeable membrane separates the cathode and trap chamber. Hydrogen evolution in the cathode chamber produces an alkaline environment, allowing ammonia to diffuse across the gas permeable membrane into the trap chamber.
around the world.39 Electrochemical methods of ammonia synthesis can enable distributed manufacturing of ammonia, where modular process units can leverage distributed feedstocks to deliver products at the source. In this way, technological and economic barriers to accessing ammonia as a resource can be lowered.
The third goal for 21st century ammonia synthesis is to address rather than exacerbate environmental remediation. As we discussed in this report, NO3RR represents an avenue through which water can be simultaneously treated with ammonia generation and recovery. Wastewater can be utilized as a feedstock beyond NO3RR to enable transformations of inorganic nitrogen across the entire oxidation state of nitrogen.40 We envision that fugitive reactive nitrogen emissions of all forms can be converted to high-purity ammonia and recovered, or vice-versa. Such flexibility would allow wastewater to transition from being a waste stream to a valuable feedstock from which tunable and on-demand methods of chemical manufacturing can be utilized to mine the water for maximal value before discharge.13
In his Nobel Prize acceptance speech in 1919, Fritz Haber said of his ammonia synthesis process: “It may be that this solution is not the final one.”41 Indeed, the science, technology, economics, policy, and equity surrounding ammonia synthesis and nitrogen management will continue to evolve in the 21st century. Amidst these multifaceted changes, electrochemists and electrochemical engineers are uniquely positioned to contribute to the opportunities of coupling ammonia synthesis with nitrogen management.
Acknowledgements
This work was supported by the National Aeronautics and Space Administration (NASA) Space Technology Graduate Research Opportunities Fellowship (Award 80NSSC20K1207) and Northern California Chapter of the ARCS Foundation (Rhoda Goldman Memorial Scholarship). The authors thank the Tarpeh Lab for their continued support, feedback, and inspiration.
© The Electrochemical Society. DOI: 10.1149/2.F04232IF
About the Authors
Matthew Liu, Stanford University

Education: BS in chemical engineering (University of California, Berkeley). Currently a 5th year chemical engineering PhD candidate at Stanford University.
Research Interests: Electrochemical reactive separation processes to recover ammonia from nitrogen-rich wastewaters.
Honors & Awards: NASA Space Technology Graduate Researcher Fellowship (2020), Achievement Rewards for College Scientists Award (2022), ECS Industrial Electrochemistry and Electrochemical Engineering Student Achievement Award (2022), American Chemical Society Graduate Student Award in Environmental Chemistry (2023).
William Tarpeh, Assistant Professor of Chemical Engineering, Stanford University

Education: BS in chemical engineering (Stanford University), PhD in environmental engineering (University of California, Berkeley).
Research Interests: Selective separation materials and electrocatalytic processes for refining wastewater into valuable products. The Tarpeh group focuses on wastewater-pollutantproduct combinations that can remediate pollution, establish circular economies, and reduce the environmental impacts of chemical manufacturing.
Work Experience: Assistant professor of chemical engineering, Center Fellow at the Precourt Institute for Energy and the Woods Institute for the Environment, by courtesy, and assistant professor of civil and environmental engineering, by courtesy, all at Stanford.
Honors and Awards: Professor Tarpeh is a recipient of the Forbes 30 Under 30 Award (2019), Chemical & Engineering News Talented 12 Award (2019), Camille Dreyfus Teacher Scholar Award (2022), and Electrochemical Society Toyota Young Investigator Award (2022).
References
1. J. Guo and P. Chen, Chem, 3, 709 (2017).
2. D. R. MacFarlane et al., Joule, 4, 1186 (2020)
3. C. H. Christensen, T. Johannessen, R. Z. Sørensen, and J. K. Nørskov, Catalysis Today, 111, 140 (2006)
4. F.-Y. Chen et al., Nat. Nanotechnol, 17, 759 (2022).
5. C. Smith, A. K. Hill, and L. Torrente-Murciano, Energy Environ. Sci., 13, 331 (2020)
6. V. Kyriakou, I. Garagounis, A. Vourros, E. Vasileiou, and M. Stoukides, Joule, 4, 142 (2020).
7. B. Clark and W. A. Tarpeh, Chemistry – A European Journal, 26, 10099 (2020).
8. X. Zhang, B. B. Ward, and D. M. Sigman, Chem. Rev., 120, 5308 (2020).
9. W. K. Dodds et al., Environ. Sci. Technol., 43, 12 (2009).
10. S. Fields, Environ Health Perspect, 112, A556 (2004)
11. Human Acceleration of the Nitrogen Cycle: Managing Risks and Uncertainty, International Water Association, (2019)
12. http://www.engineeringchallenges.org/challenges/nitrogen. aspx.
13. W. A. Tarpeh and X. Chen, Environmental Science and Ecotechnology, 5, 100078 (2021)
14. D. S. Sholl and R. P. Lively, Nature News, 532, 435 (2016)
15. K. Kim et al., Nat Commun, 14, 823 (2023).
16. V. A. Niemann, P. Benedek, J. Guo, et al., ACS Catal., 13, 6268 (2023)
17. A. Garshasbi et al., Catalysis Today, 331, 18 (2019).
18. K. Kim et al., Advanced Materials, 32, 1906877 (2020).
19. R. Krishna, Chemical Engineering Science, 57, 1491 (2002).
20. A. Stankiewicz, Chemical Engineering and Processing: Process Intensification, 42, 137 (2003).
21. A. Bard and L. Faulkner, Electrochemical Methods. Fundamentals and Applications., 2nd ed., p. page 116, John Wiley & Sons, Inc., (2001).
22. W. A. Tarpeh, J. M. Barazesh, T. Y. Cath, and K. L. Nelson, Environ. Sci. Technol., 52, 1453 (2018)
23. M. J. Liu, B. S. Neo, and W. A. Tarpeh, Water Research, 169, 115226 (2020).
24. M. J. Liu and W. Tarpeh, 50th International Conference on Environmental Systems (2020).
25. H. Dong, C. S. Shepsko, M. German, and A. K. SenGupta, Journal of Environmental Chemical Engineering, 8, 103846 (2020)
26. Z. W. Seh et al., Science, 355, eaad4998 (2017)
27. G. E. Dima, A. C. A. de Vooys, and M. T. M. Koper, Journal of Electroanalytical Chemistry, 554–555, 15 (2003).
28. J. M. McEnaney et al., ACS Sustainable Chem. Eng., 8, 2672 (2020).
29. Y. Wang et al., J. Am. Chem. Soc., 142, 5702 (2020).
30. Z. Wang, S. D. Young, B. R. Goldsmith, and N. Singh, Journal of Catalysis, 395, 143 (2021).
31. J. Li et al., J. Am. Chem. Soc., 142, 7036 (2020).
32. W. He et al., Nat Commun, 13, 1129 (2022).
33. Y. Liu, Z. H. Ren, J. Liu, R. F. Schaller, and E. Asselin, J. Electrochem. Soc., 166, C3096 (2019).
34. M. J. Liu et al., J. Am. Chem. Soc., 144, 5739 (2022).
35. J. Guo, P. Brimley, M. Liu, et al., ACS Sustain. Chem. Eng. (2023).
36. C. A. Fernandez and M. C. Hatzell, J. Electrochem. Soc., 167, 143504 (2020)
37. H.-L. Du et al., Nature, 609, 722 (2022).
38. K. Steinberg et al., Nat Energy, 8, 138 (2022).
39. B. M. Comer et al., Joule, 3, 1578 (2019).
40. D. M. Miller et al., ChemRxiv (2023)
41. F. Haber, (1920).
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 31
Overcoming Limitations for Pure-water
Anion-exchange-membrane Electrolysis


























 by Grace A. Lindquist and Shannon W. Boettcher
by Grace A. Lindquist and Shannon W. Boettcher
Many essential industrial processes rely on the input of high-purity H2, such as fertilizer and steel production.1 However, over 99% of H2 is “gray” (produced using fossil fuels).2, 3 Less than 1% is “green” H2, produced through water electrolysis powered by renewable electricity.2 While gray H2 is currently cheaper to produce, green H2 is expected to become cost-competitive within the next decade as electricity costs decrease and device scale increases.1 Electrolyzer technology also addresses long-duration energy-storage limitations facing scaling renewable electricity sources like wind and solar.4 Pairing these systems with electrolysis stores excess energy as H2, which can be compressed for long-term storage or transport.




The two dominant commercial low-temperature electrolysis technologies are liquid-alkaline and proton-exchange-membrane (PEM) electrolysis.5 Liquid-alkaline electrolyzers are a mature technology that operate by flowing hot, concentrated aqueous KOH to the anode and cathode separated by a porous diaphragm. They use inexpensive non-platinum-group-metal (non-PGM) catalysts and hardware but historically suffer from high crossover rates, resulting in O2 contamination in the H2 stream, which can be highly combustible. This approach is particularly ill-suited for pairing with renewable technology due to the inherent variability of the power load.6 PEM electrolyzers operate using a cation-selective, solid-polymer membrane which reduces gas crossover, resulting in high-purity H2 output and enabling the variable-load operation for coupling with intermittent electrical sources. The membrane separator also enables the use of pure water rather than supporting electrolyte, improving system safety, simplifying system- and plant-level engineering, and eliminating efficiency losses from shunt currents.7 However, the locally acidic environment of the PEM necessitates more expensive catalysts (Ir, Pt, etc.) and hardware (Ti, etc.).
Anion-exchange-membrane (AEM) electrolysis is an emerging technology that, with further development, can combine the benefits of PEM and liquid-alkaline systems. AEM electrolyzers use an anionselective hydroxide-conducting polymer membrane, which creates a locally alkaline environment that enables the use of non-PGM catalysts (Ni, Co, Fe, etc.) and flow fields (steel) while maintaining the ability to operate in pure water with low gas crossover (Fig. 1).8 However, currently AEM technology is immature, and does not meet the performance and stability needed to compete with PEM. This limitation is largely due to the lack of a high-performing, stable anion-exchange polymer. Further, while many high-performing,
stable non-PGM catalysts for the oxygen-evolution reaction (OER) have been identified in lab-scale testing in KOH, this behavior hasn’t directly translated to industrially relevant device operation in pure water.9, 10
Degradation of Anion Exchange Polymers












For AEM electrolyzers and fuel cells, the stability-limiting component is the anion exchange polymer.5 This polymer is used as a membrane and mixed in the catalyst layer to transport hydroxide to the high-surface-area catalyst and improve contact between the catalyst layer and the membrane. Most polymer development has focused on improving polymer alkaline stability and prevention of OH- nucleophilic attack.11, 12 The oxygen electrode in fuel cells, however, operates at much less-oxidizing potential than in electrolyzers, meaning the ionomers in electrolyzers must have much better oxidative stability. Polymer degradation experiments also often use model ex-situ studies, such as soaking materials in KOH solution or three-electrode studies mimicking oxidative environments.12, 13

When operating in an AEM electrolyzer with pure-water feed, the ionomer in the anode catalyst layer sees substantial oxidative damage. When testing different anion exchange polymers, a similar degradation rate occurs for the initial ~20 h of operation, nearly independent of polymer chemistry across a wide range of materials.14 X-ray photoelectron spectroscopy (XPS) shows an increase in oxidized carbon content and a loss of cation groups (Fig. 2).14 Kim and coworkers isolated phenyl oxidation to phenol in model studies,15 and supporting computational work from Hendon and coworkers suggests the aromatic regions of the polymer are weak sites for oxidation.16 The exact degradation mechanism is complicated, but likely includes both electrochemical reactivity by direct oxidation, perhaps coupled with OH- attack, of the polymer and chemical reactivity with oxygen radical species and other reactive OER intermediates.
Earth-abundant Oxygen-evolving Anode Catalysts










The activity of earth-abundant (particularly Ni, Fe, and Cobased) catalysts for OER has been extensively studied in alkaline conditions.17-19 For all mixed-metal oxyhydroxides, and the oxides/ sulfides/phosphides etc. that serve as pre-catalysts to the oxyhydroxides,20-22 Fe is essential for high activity.23, 24 Fecontaining Ni oxyhydroxides are the most active catalysts in alkaline media,25 with turnover frequencies ten-fold higher than IrOx 26 Co oxides/oxyhydroxides have a lower activity but are more structurally stable under OER conditions. The active catalyst’s phases in both cases are typically molecular-scale metal oxo-/hydroxo species that under OER conditions oxidize from a nominally layered double hydroxide to an oxyhydroxide structure. The transition metal oxidation (i.e., Ni2+ to Ni3+), is generally accompanied by a large increase in electrical conductivity, which contributes to the high performance.19 While structure-compositionperformance relationships have been
















































































32 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Example anion exchange ionomer: OHOHOH - OH - OHOHN CF3 x 100-x
Fig. 1. AEMWE schematic. An anion-selective hydroxide conducting membrane is sandwiched between two gas diffusion electrodes (GDEs) coated with a HER or OER catalyst and anion exchange ionomer. Adapted from ref. 8.
identified in three-electrode studies with a supporting electrolyte, it is unclear how these translate to AEM devices fed with pure water. Despite their high OER activity, Ni-Fe-based catalysts tend to show poor activity and stability in a pure-water membraneelectrode assembly (MEA) environment.10 We found that NiFe2O4 nanoparticles failed rapidly during electrolyzer testing, but Co3O4 nanopowders performed the best out of all non-PGM catalysts tested and showed comparable performance and stability to high-surface area commercial IrOx (Fig 3).9 XPS analysis showed that the leaststable catalysts undergo significant surface transformation during operation (Fig. 3d). The electrolyzer operating voltages trended with the dry powder electrical conductivity (Fig. 3c), indicating that the catalysts were limited by electronic conductivity and not fully restructuring to the conductive oxyhydroxide phase during operation. This observation is likely due to the use of an anion exchange ionomer to supply OH- ions rather than a supporting electrolyte. The hydroxide is confined to near the ionomer cationic backbone and it is not able to transport through the layered sheets, inhibiting the chemical transformation.
We attribute the better performance of Co3O4 to its high electrical conductivity and resistance to structural rearrangement during operation. Notably, others have shown Ni-Fe oxide catalysts perform well in AEM devices when fed with KOH27, 28 or in pure water when using a thin layer of NiFe catalyst on a conductive supporting substrate that compensates for the poor electronic conductivity of the material when not fully converted to the oxyhydroxide form.29, 30
Perspective and Outlook
AEM electrolysis is positioned to play a key role in the predicted exponential growth of green hydrogen technology with essential R&D advances in the coming years. We revealed key design parameters essential to commercialization. First, stable alkaline OER catalysts with high electronic conductivity and minimal surface reconstruction during operation must be designed. Alkaline catalyst layers must also be applied to the MEA with scalable, industrially relevant techniques. Second, ionomer oxidation mitigation strategies must be developed. This approach could also target other creative catalyst layer design, such as phase-separation control to protect oxidation-prone organic components or catalyst engineering to direct selectivity for hydroxide over polymer oxidation. If competitive efficiency and durability can be achieved in pure water, AEM electrolysis has the potential to become a dominant electrolyzer technology.
Acknowledgements
The authors acknowledge support from the US Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) under the Fuel Cell Technologies Office (FCTO) award DE-EE0008841 and the John Keana Research Fellowship from the University of Oregon.


© The Electrochemical Society. DOI: 10.1149/2.F05232IF
About the Authors
Grace Lindquist, Oregon Center for Electrochemistry, Department of Chemistry and Biochemistry, University of Oregon
Education: BA in Chemistry (College of Saint Benedict and Saint John’s University), PhD in Chemistry under Prof. Shannon Boettcher at the University of Oregon.
Research Interests: Water electrolysis, Catalysis, Ion exchange polymer materials
Pubs + Patents: 11 first- and co-author papers, 9 conference and workshop presentations, 1 provisional patent, h-index 6.
Awards: ECS Energy Technology Division Graduate Student Award (2021), Keana Fellow (2020), Dean’s First Year Merit Scholar (2018)
Work with ECS: Energy Technology Division student member since 2020
Website: https://boettcher.uoregon.edu/current-members-boettcherlab/members-v3/ https://orcid.org/0000-0001-5896-2331
Shannon Boettcher, Professor, Oregon Center for Electrochemistry, Department of Chemistry and Biochemistry, University of Oregon

Education: BA Chemistry (physics emphasis) at the University of Oregon, PhD in Inorganic Materials Chemistry under Galen Stucky, NSF Graduate Research Fellow and Chancellor’s Fellow, UC Santa

The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 33
(continued on next page)
Barbara.
C 1s Pristine polymer C=C C-N1 C-N2 O-C=O C-N/C-O C=C C 1s Ir 4d Net fit Net fit b) 294 288 282 Binding energy (eV) C o u n t s / s a) Cell restarted due to power failure 0.7 mV h-1 j = 500 mA cm-2 0 30 60 90 120 150 180 1 8 1 9 2 0 2 1 2 2 2 3 2 4 E ( V ) Time (h) T = 55 ˚C 0 0 0 2 0 4 0 6 0 8 1 0 1 6 1 7 1 8 1 9 2 0 2 1 E ( V ) J (A cm-2) 15 mV h-1 55 ˚C 70 ˚C N 1s 406 402 398 N 1s PiperION anode GDL post-175 hr operation 294 288 282 406 402 398 C 1s Pristine polymer C=C C-N1 C-N2 O-C=O C-N/C-O C=C C 1s Ir 4d Net fit Net fit b) 294 288 282 Binding energy (eV) C o u n t s / s a) Cell restarted due to power failure 0.7 mV h-1 j = 500 mA cm-2 0 30 60 90 120 150 180 1 8 1 9 2 0 2 1 2 2 2 3 2 4 E ( V ) Time (h) T = 55 ˚C 0 0 0 2 0 4 0 6 0 8 1 0 1 6 1 7 1 8 1 9 2 0 2 1 E ( V ) J (A cm-2) 15 mV h-1 55 ˚C 70 ˚C N 1s 406 402 398 N 1s PiperION anode GDL post-175 hr operation 294 288 282 406 402 398
Fig. 2. Electrolyzer degradation. a) Pure-water fed AEM electrolyzer durability. The inset shows temperature-dependent initial performance. b) XPS comparison of polymer in a pristine and operated anode. Adapted from ref. 14.
Lindquist and Boettcher (continued from previous page)
Fig. 3. Non-PGM catalyst operation. a) Polarization curves of anode catalysts tested. All experiments were conducted in pure water at 57 °C. A stainlesssteel woven substrate with the indicated catalyst was used as the anode GDL and Pt black catalyst on Toray carbon paper was used as the cathode GDL. b) Durability testing for each catalyst held at 500 mA·cm-2. c) Electrical conductivity of dry powders measured in a pressed pellet. d) The change in metal ratios of mixed-metal catalysts and changes to Co 2p in Co3O4 and Ni 2p in NiO determined by XPS. All catalysts were operated for 20 h at 500 mA·cm-2. Adapted from ref. 9.
Work Experience: Professor in the Department of Chemistry and Biochemistry at the University of Oregon. His research is at the intersection of materials science and electrochemistry, with a focus on fundamental aspects of energy conversion and storage. In 2019 he founded the Oregon Center for Electrochemistry and the first graduate program in Electrochemical Technology in the USA.
Research Interests: Electrochemistry, Solar energy, Solid state materials chemistry, Catalysis
Pubs + Patents: 143 publications, >150 invited talks and lectures, 8 patents, h-index 62 and ISI Highly Cited Researcher (top ~0.1%) (2019, 2020, 2021, 2022).
Awards: Blavatnik National Award Finalist (top 10 in chemistry, 2021), Camille Dreyfus Teacher Scholar Award (2015), Sloan Fellowship (2015)
Work with ECS: ECS Member for ~10 years; founding member of PNW ECS Chapter (treasurer)

Website: https://boettcher.uoregon.edu/ https://orcid.org/0000-0001-8971-9123
References
1. Hydrogen Council, Path to hydrogen competitiveness: A cost perspecitve (2020). Accessed Dec. 2022 https://hydrogencouncil. com/en/path-to-hydrogen-competitiveness-a-cost-perspective/
2. IEA, The future of hydrogen; IEA: Paris (2019).
3. A. Kusoglu, Electrochem. Soc. Interface, 30, 44 (2021)
4. P. A. Kempler, J. J. Slack, and A. M. Baker, Joule, 6, 280 (2022).
5. K. Ayers, N. Danilovic, R. Ouimet, M. Carmo, B. Pivovar and M. Bornstein, Annu. Rev. Chem. Biomol. Eng., 10, 219 (2019)
6. M. R. Kraglund, M. Carmo, G. Schiller, S. A. Ansar, D. Aili, E. Christensen and J. O. Jensen, Energy & Environmental Science, 12, 3313 (2019).

7. M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, Int. J. Hydrogen Energy, 38, 4901 (2013)
8. G. A. Lindquist, Q. Xu, S. Z. Oener and S. W. Boettcher, Joule, 4, 2549 (2020).
9. R. A. Krivina, G. A. Lindquist, S. R. Beaudoin, et al., Adv. Mater., 34, 2203033 (2022)
10. D. Xu, M. B. Stevens, M. R. Cosby, et al., ACS Catal., 9, 7 (2019).
11. C. G. Arges and L. Zhang, ACS Appl. Energy Mater., 1, 2991 (2018).
34 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
0 0 0 2 0 4 0 6 0 8 1 0 1 4 1 6 1 8 2.0 2 2 2 4 2.6 2 8 E ( V ) j (A•cm-2) 57 °C, pure water a) NiFe2O4 NiO IrO2Co3O4 NiCoO2Ni0.5Co0.5Fe2O4 σ (mS•cm-1) V @ 1 A • c m2 ( V ) *metallic IrO2 1x10-4 0 001 0 01 0 1 1 10 100 2 1 2 2 2 3 2.4 2 5 2 6 2 7 NiFe2O4 NiO Co3O4 NiCoO2 Ni0.5Co0.5Fe2O4 0 2 4 6 8 10 12 14 16 18 20 1 8 2 0 2 2 2.4 2 6 2 8 3.0 3 2 3 4 E ( V ) Time (h) j = 500 mA•cm-2 b) NiFe2O4 Ni0.5Co0.5Fe2O4 NiCoO2 NiO IrO2 Co3O4 c) d) 810 800 790 780 Co 2p in Co3O4 pristine powder after 20-hour testing C o u n t s •s -1 Binding energy (eV) 880 870 860 850 Ni 2p in NiO prisitine powder after 20-hour testing 10 20 30 C o / N i ratio (%) 150 200 250 300 F e / N i r a t i o (%) 60 80 100 120 F e / N i r a t i o (%) NiCoO2 Ni0.5Co0.5Fe2O4 NiFe2O4 pristine 20h pristine 20h pristine 20h
12. K. F. L. Hagesteijn, S. Jiang, and B. P. Ladewig, J. Mater. Sci., 53, 11131 (2018).
13. H. Cao, J. Pan, H. Zhu, Z. Sun, B. Wang, J. Zhao, and F. Yan, Adv. Sci., 8, 2101744 (2021).
14. G. A. Lindquist, S. Z. Oener, R. Krivina, et al., ACS Appl. Mater. Interfaces, 13, 51917 (2021)
15. D. G. Li, I. Matanovic, A. S. Lee, E. J. Park, C. Fujimoto, H. T. Chung, and Y. S. Kim, ACS Appl. Mater. Interfaces, 11, 9696 (2019)
16. R. A. Krivina, G. A. Lindquist, M. C. Yang, et al., ACS Appl. Mater. Interfaces, 14, 18261 (2022)
17. C. C. L. McCrory, S. Jung, J. C. Peters, and T. F. Jaramillo, J. Am. Chem. Soc., 135, 16977 (2013).
18. B. M. Hunter, H. B. Gray, and A. M. Müller, Chem. Rev., 116, 14120 (2016)
19. M. B. Stevens, L. J. Enman, E. H. Korkus, et al., Nano Res., 12, 2288 (2019).
20. E. Fabbri, M. Nachtegaal, T. Binninger, et al., Nat. Mater., 16, 925 (2017).
21. K. Wang, X. Wang, Z. Li, et al., Nano Energy, 77, 105162 (2020).

22. J. Mohammed-Ibrahim, J. Power Sources, 448, 227375 (2020)
23. L. Trotochaud, S. L. Young, J. K. Ranney, and S. W. Boettcher, J. Am. Chem. Soc., 136, 6744 (2014).
24. M. B. Stevens, C. D. M. Trang, L. J. Enman, J. Deng, and S. W. Boettcher, J. Am. Chem. Soc., 139, 11361 (2017).
25. M. S. Burke, S. Zou, L. J. Enman, J. E. Kellon, C. A. Gabor, E. Pledger, and S. W. Boettcher, The J. Phys. Chem. Lett., 6, 3737 (2015).
26. L. Trotochaud, J. K. Ranney, K. N. Williams, and S. W. Boettcher, J. Am. Chem. Soc., 134, 17253 (2012).
27. I. Vincent, E.-C. Lee, and H.-M. Kim, Sci. Rep., 11, 293 (2021).
28. N. Chen, S. Y. Paek, J. Y. Lee, J. H. Park, S. Y. Lee, and Y. M. Lee, Energy Environ. Sci., 14, 6338 (2021).
29. J. Xiao, A. M. Oliveira, L. Wang, et al., ACS Catal., 11, 264 (2021).
30. F. Razmjooei, T. Morawietz, E. Taghizadeh, et al., Joule, 5, 1776 (2021).
About the Editor
Scott Cushing, Assistant Professor of Chemistry, Caltech

Education: BS in Physics, emphasis in Material Science and Chemistry and PhD in Physics, under Nick Wu and Alan Bristow (West Virginia University).
Research Interests: With a multidisciplinary background spanning Chemistry, Materials Science, and Physics, his research focuses on the creation of new scientific instrumentation that can translate quantum phenomena to practical devices and applications. The Cushing lab is currently pioneering the use of attosecond x-ray, time-resolved TEM-EELS, and ultrafast beams of entangled photons for a range of microscopy and spectroscopy applications.
Work Experience: Past appointments include Dept. of Energy EERE Postdoctoral Fellow, Prof. Stephen Leone Group University of California, Berkeley with a Co-Appointment at Lawrence Berkeley National Laboratory. Currently Senior Research Advisor for Pacific Integrated (PI) Energy, San Diego, CA.
Pubs & Patents: >60 publications, 3 patents, h-index >30, cited ~8,000 times
Awards: 2022 Cottrell Scholar, 2022 Shirley Malcom Prize for Excellence in Mentoring, 2019–2021 Young Investigator awards for DOE, AFOSR, ACS, and Rose Hill Foundation.
Work with ECS: ETD Division: assist with organizing and chairing symposium. Member for >15 years.

Website: cushinglab.caltech.edu
https://orcid.org/0000-0003-3538-2259
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 35
See online NOW! MEDIA GUIDE Integrated Advertising Solutions Contact Sponsorship@electrochem.org today!
2023 ECS INSTITUTIONAL MEMBERS
NEWBENEFACTOR PATRON
BioLogic USA/BioLogic SAS (15*)
Duracell US Operations, Inc. (66)
Gamry Instruments (16)
Gelest Inc. (14)
Hydro-Québec (16)
Pine Research Instrumentation (17)
SPONSORING
BASi (8)
Center for Solar Energy and Hydrogen Research BadenWürttemberg (ZSW) (19)
Center for Synthetic Organic Electrochemistry, University of Utah (2)
Central Electrochemical Research Institute (30)
Corteva Agriscience (1)
Deutsches Zentrum für Luft- und Raumfahrt e.V. (DLR) (15)
Electrosynthesis Company, Inc. (27)
EL-CELL GmbH (9)
Ford Motor Company (9)
GS Yuasa International Ltd. (43)
Honda R&D Co. (16)
Medtronic, Inc. (43)
Nissan Motor Co., Ltd. (16)
Pacific Northwest National Laboratory (PNNL) (4)
Panasonic Energy Corporation (28)
Permascand AB (20)
Plug Power, Inc. (2)
Teledyne Energy Systems, Inc. (24)
UL Research Institutes (2)
Energizer Battery (78)
Faraday Technology, Inc. (17)
GE Global Research Center (67)
Lawrence Berkeley National Laboratory (LBNL) (19)
Scribner Associates (27)
Toyota Research Institute of North America (TRINA) (15)
SUSTAINING
Advanced Cell Engineering (2)
Cummins, Inc. (5)
Current Chemicals (1)
General Motors Holdings LLC (71)
Giner, Inc. (37)
Ion Power, Inc. (9)
Kanto Chemical Co., Inc. (11)
Los Alamos National Laboratory (LANL) (15)
Metrohm USA, Inc. (9)
Microsoft Corporation (6)
Occidental Chemical Corporation (81)
Sandia National Labs (47)
Sherwin-Williams (2)
Spectro Inlets ApS (1)
Technic, Inc. (27)
United Mineral & Chemical Corporation (2)
Western Digital GK (9)
Westlake Corporation (28)
Yeager Center for Electrochemical Sciences at CWRU (25)
Please help us continue the vital work of ECS by joining as an institutional member today. To renew, join, or discuss institutional membership options please contact Anna Olsen, Senior Manager, ECS Corporate Programs, anna.olsen@electrochem.org.
36 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
*Years of membership as of 6 January 2023
Methods—Ampero-Coulometry: A New Technique for Understanding Lithium–Sulfur Electrochemistry Lithium–sulfur (Li–S) batteries are one of the most promising beyond–lithium-ion energy storage technologies. However, there are still many challenges to be overcome before widespread commercialization can be possible. The charge storage mechanism for Li–S batteries is complex and there are many open questions about the reduction and oxidation processes which occur during charging and discharging. Consequently, a comprehensive understanding of redox behavior of lithium polysulfides formed during cycling is required to improve the electrochemical performance of Li–S batteries. To this end, researchers from University College Cork, Ireland, have recently presented a new technique they refer to as Ampero-Coulometry, which mathematically transforms galvanostatic charge-discharge curves into a series of curves that reveal the cation diffusional rate inside carbon-sulfur porous electrodes at different states of charge. This novel technique can be used to identify the voltage range where the polysulfide shuttle effect takes place. This will be a useful tool for investigating the influence of several Li–S battery parameters, including sulfur mass loading, the porosity of the sulfur host, and the electrolyte concentration.
Mechanical Failure of Cu Current Collector Films Affecting Li Plating/Stripping Cycles at Cu/LiPON Interfaces
Reversible charging and discharging of Li metal at solid/solid interfaces will be necessary to design the next generation of solid-state Li batteries for microelectronic and automotive applications. In practice, this means Li must plate and de-plate at the interface of a current collector film like Cu and a rigid inorganic solid electrolyte like lithium phosphorous oxynitride (LiPON). To nucleate in these circumstances, there must be Li electrodeposition under high pressure. Consequently, there is significant mechanical work involved in deforming the current collecting film, and this contributes to the overall overpotential. A team led by researchers at Kyushu University has reported work that used finite element simulation to calculate the stresses involved, paired with in situ scanning electron microscopy (SEM) experiments to observe Li plating and stripping at this interface. With a 30-nm current collector, they observed a substantial overpotential caused by mechanical work. However, in subsequent cycles, it was not observed because Li preferentially plated at locations where the current collector fractured. This was confirmed by using a thick 1-μm current
collector that did not fracture, and in this case the mechanical work overpotential was maintained with every cycle.
From: M. Motoyama, M. Ejiri, et al., J. Electrochem. Soc., 170, 012503 (2023)
Baselining Activity and Stability of ORR Catalysts and Electrodes for Proton Exchange Membrane Fuel Cells for Heavy-Duty Applications
Proton-exchange-membrane-fuel-cell (PEMFC) powered heavy-duty vehicles (HDV) show more commercial viability than their light-duty counterparts, thanks to their more predictable routes. This also means that PEMFC system durability for HDV faces more challenges due to longer mileage and lifetime requirements. In this study, Wang et al. performed baseline studies on two state-of-the-art catalysts for HDV applications which contain platinum (a-Pt/C) or platinum-cobalt alloy (d-PtCo/C) with high-surface-area carbon support. Prepared with nearly the same Pt loading and average particle size, both catalysts were tested under accelerated stress test (AST) up to 90,000 cycles. In the first 30,000 AST cycles, d-PtCo/C exhibited twice the mass activity (mA/mgPt) of a-Pt/C, indicating faster kinetics. However, its mass activity loss was higher after 90,000 AST cycles, implying that Co was leaching from the catalyst matrix. Although both catalysts experienced similar electrochemically active surface area (ECSA) loss, the Co leaching issue from d-PtCo/C led to additional kinetics and mass-transport losses. Furthermore, Co poisoned Nafion ionomer in the catalyst layer, resulting in even poorer performance of d-PtCo/C at high current density. All the results prove that a-Pt/C is a better benchmark catalyst to evaluate against the other catalyst systems.
From: X. Wang, L. Hu, et al., J. Electrochem. Soc., 170, 024503 (2023)
Electrochemical Characterization of Biomolecular Electron Transfer at Conductive Polymer Interfaces
Imagine a day when we can use electroactive microorganisms in bio-electrochemical systems to clean up wastewater and generate electricity. One of the key hindrances to realizing this vision is the low electron transfer efficiency between the microorganisms and the electrodes. A recent report by researchers from Massachusetts Institute of Technology and the University of California, Santa Barbara sheds more light on the biotic-abiotic interaction. The model system was a common biological electron shuttle flavin mononucleotide (FMN) and electrodes modified with conductive polymers based on poly{3-[6′(N-methylimidazolium)hexyl]thiophene} (P3HT-Im+). The authors conducted extensive electrochemical impedance studies to model the charge transfer between FMN and different electrodes; and
performed thorough Levich, Cottrell, and Tafel analyses to elucidate the electrode kinetics. The presence of the histidine-like imidazolium groups in the polymers was found to improve the conductivity and charge storage capacity of the polymer layer, yet with negligible faradaic contribution. This finding indicated a non-intuitive way to improve biocatalysis. A concerted twoelectron reduction of FMN was also achieved with P3HT-Im+-modified electrodes.
From: A. Agee, T. Gill, G. Pace, et al., J. Electrochem. Soc., 170, 016509 (2023)
Wide Bandgap Engineering in Power Transistors Using GaN Windows Owing to its wide bandgap, good electron mobility, and high breakdown electric field, Gallium nitride (GaN) has a high potential for application in power electronic devices. In one such power device, the lateral double diffused metal oxide semiconductor (LDMOS), application of GaN poses high on-resistance. Addressing this problem without introducing manufacturing challenges is the core of the work carried out by researchers at Damghan University in Iran. Two innovative features were used to utilize GaN in LDMOS with silicon on insulator (SOI) technology: several GaN windows in the drift region and using interface material silicon nitride (Si3N4) around the GaN windows. Researchers demonstrated modification of the electric field profile horizontally and successfully increased the vertical electric potential. The decrease in on-resistance was demonstrated by the increased drain current at 10V. Optimization of the length and number of GaN windows was carried out to yield the highest breakdown voltage and lowest on-resistance. The team performed the simulation of the proposed device using an ATLAS simulator and showed that GaNbased devices with low on-resistance could be obtained without the introduction of manufacturing complexity.
From: M. Mehrad and M. Zareiee, ECS J. Solid State Sci. Technol., 12, 031004 (2023).
Tech Highlights was prepared by Joshua Gallaway of Northeastern University; Chock Karuppaiah of Vetri Labs; Chao (Gilbert) Liu of Shell; Zenghe Liu of Abbott Diabetes Care; David McNulty of University of Limerick; and Donald Pile of EnPower, Inc. Each article highlighted here is available free online. Go to the online version of Tech Highlights in each issue of Interface, and click on the article summary to take you to the full-text version of the article.
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 37 TECH HIGHLIGHTS TECH HIGHLIGHTS
From: U. Gulzar, A. Lonergan, V. Egorov, et al., J. Electrochem. Soc., 170, 030503 (2023)
WHAT’S ON THE ECS NEWS?
by Jennifer Ortiz
Your connection to the ECS community, career opportunities, and industry-related news and events.


Educational opportunities
Don’t miss upcoming virtual webinars as well as inperson workshops and events for a deep dive into a variety of topics. Home in on the skills you need to master your field!
Meeting news Publication news Awards
With two meetings held per year, there’s an ongoing array of announcements you won’t want to miss, like new topic closeups, important deadlines, networking events, award winner announcements, and more.
Stay “in the know” about the publications world! Find opportunities to publish your work in a focus issue; discover the latest editorial openings; and get the latest announcements on upcoming events like Free the Science Week, educational opportunities, and more.

1 2 3 4 www.electrochem.org/ecsnews
Discover who the latest ECS award winners are; learn about their innovative work; and review upcoming award deadlines to be the next award winner!

38 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
ECS News
The Future of Industrial Electrochemistry & Electrochemical Engineering
by Maria Inman
Since its inception, the work of the Industrial Electrochemistry & Electrochemical Engineering (IE&EE) Division has encompassed a broad range of technologies and applications, including mathematical modeling of electrochemical systems, development and optimization of small- and large-scale industrial processes, environmental remediation, and electrochemical conversion to produce value-added chemicals. A recent focus has been the creation of innovative technologies that will help to alleviate the climate and environmental crises, to improve sustainability, and to decarbonize existing technologies.
In 2023, the ECS Industrial Electrochemistry & Electrochemical Engineering (IE&EE) Division will be Celebrating 80 Years of the Division. The events surrounding the Celebration include a symposium at the 243rd Spring ECS Meeting in Boston, May 28–June 1, 2023, with a presentation on Perspectives on the Past, Current, and Future of IE&EE. The presentation will be followed by a panel discussion, with luminaries such as Dennie Mah, Robert Savinell, and Gerri Botte, who will give their perspectives on the development of major electrochemical industries/processes spanning the years of their careers, as well as on the role of industry in the division.

While we will enjoy looking back on the accomplishments of the IE&EE Division over the past 80 years, the articles in this issue of Interface point to a grand future for the application of electrochemistry and electrochemical engineering in solving societal and industrial problems.
The Future for Industrial Electrochemistry & Electrochemical Engineering
Biddinger and Kenis present exciting developments in Current and Emerging Electrochemical Approaches for Chemical Manufacturing There is strong and growing interest in decarbonization of industrial processes to reduce industrial emissions. Specific to chemical manufacturing, electrification of chemical processes has the potential to improve energy efficiency, expand available reactions and improve catalytic performance, reduce carbon emissions, use renewable feedstocks for power and manufacturing, and increase materials circularity. The opportunity for advances in both fundamental and applied electrochemistry, as well as electrochemical reactor systems, can enable efficient, economical, and sustainable chemical manufacturing, especially when combined with renewable electricity to achieve global goals for decarbonization.
Sankarasubramanian, Chambers, and Wilson take us off-world to look at opportunities, constraints, and solutions for electrochemistry and electrochemical engineering in space. The article Extraterrestrial Electrochemistry: Challenges and Opportunities for In-Situ Resource Utilization (ISRU) on Mars discusses the need for technological solutions that will allow the human race to engage in long-duration missions to explore the solar system. Currently, the duration of space missions is constrained by the need for resupply from Earth.
Electrochemical solutions that can utilize in-situ resources on Mars will enable “living off of the land,” reduce costs and supply chain disruptions, and put into place a sustainable infrastructure that will enable long term development and exploration. This article is supported by well-attended symposia on Electrochemistry in Space at the 236th, 240th, and upcoming 244th ECS meetings that reinforce the interest of the electrochemical community in meeting the needs of the rapidly growing space economy. The challenge is to develop electrochemical technologies that can work under unique extraterrestrial environments such as microgravity and extreme temperatures. The broad interdisciplinary capabilities of the ECS community can meet this challenge and create processes and devices for critical space functions such as energy generation and storage, sensors, and conversion of lunar, Martian, and asteroid materials into propellants, clean water, oxygen, and structural or functional materials/devices.
Wolfe, Zanganeh, Arthur, Trembly, and Daramola bring us back to Earth to understand Considerations for Industrial Phosphorous Recovery via Electrochemical Processes Recovery of phosphorus from treated wastewater, agricultural discharge, and stormwater is a critical component of sustainability. With anticipated increases in global population, and associated food and energy consumption, there is a strong need to reduce dependence on natural resources by recovery of nutrients. Nutrient recovery will also mitigate environmental issues that are a result of fertilizer runoff. This article describes low-cost, modular electrochemical processes that use renewable energy sources to precipitate and recover phosphorus.
The members of the IE&EE Division look forward to the next 80 years, working with The Electrochemical Society to advance the development of electrochemical technologies for both terrestrial and space applications.
© The Electrochemical Society. DOI: 10.1149/2.F07232IF
About the Author
Maria Inman, Vice President, Faraday Technology, Inc.

Education: BE in Metallurgical and Materials Engineering and PhD in Corrosion Engineering, both at the University of Auckland.
Work Experience: Dr. Inman manages Faraday Technology’s pulse and pulse reverse research project portfolio and business development activities.
Pubs + Patents: >108 publications and 7 patents.
Work with ECS: Member for 27 years, Chair of the IE&EE Division.
Website: http://www.faradaytechnology.com/. https://orcid.org/0000-0003-2560-8410

The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 39
“
The articles in this issue of Interface point to a grand future for the application of electrochemistry and electrochemical engineering in solving societal and industrial problems.”
SAVE 20%
The Electrochemical Society book series provides authoritative, detailed accounts on specific topics in electrochemistry and solid state science and technology. These titles are sponsored by ECS and published in cooperation with Wiley.




SECOND EDITION

ATMOSPHERIC CORROSION




LaQue’s Handbook on Marine Corrosion, 2nd Edition

David A. Shifler

ISBN 978-1-119-78883-6 | July 2022

EDITED BY DAVID A. SHIFLER LAQUE’S HANDBOOK OF MARINE CORROSION THE ELECTROCHEMICAL SOCIETY SERIES SECOND EDITION
Shop the Latest ECS Titles at wiley.com

INTERESTED IN PUBLISHING WITH US?
Please contact publications@electrochem.org to discuss your idea.

- CD5827
ON THE MOST TRUSTED RESOURCES IN ELECTROCHEMISTRY New
A practical, single-source reference on the unique nature of seawater as a corrosive environment. Explains practical corrosion control solutions via design, proper materials selection, and implementation of good corrosion control engineering practices.
Christofer Leygraf Inger Odnevall Wallinder Johan Tidblad Thomas Graedel
ECS members receive 20% off all Wiley books.
ECS members use promo code ECS18 at checkout.
Current and Emerging Electrochemical Approaches for Chemical Manufacturing
by Elizabeth J. Biddinger and Paul J. A. Kenis
Society is facing a major challenge: the need to slow and eventually curb climate change induced by excessive anthropogenic emissions of greenhouse gases, in particular carbon dioxide. The scientific community and governments largely agree—as summarized in the Paris Agreement1 that massive cuts in CO2 emissions will be needed over the next two to three decades to avoid the world heating up by more than 2 °C. Already, many of the sectors responsible for the largest fractions of CO2 emissions are making major strides toward that goal. Electric power is increasingly generated using renewable energy sources rather than fossil fuels, transportation applications are increasingly electrified, and commercial and residential buildings have become significantly more energy efficient (e.g., insulation, better HVAC systems).2 Similarly, the industrial sector is searching for ways to achieve the so-called energy transition, implementing changes culminating in becoming (close to) carbon neutral by 2050. In the early 2000s, few people thought that electrified chemical manufacturing approaches would be able to contribute significantly to the reduction of CO2 emissions of this sector. A multitude of developments since then have brought electrified chemical manufacturing approaches much closer to reality:3
(i) Electricity (especially the renewable fraction) is much cheaper now;
(ii) Industry has realized that the CO2 emissions of many conventional chemical manufacturing processes can be cut significantly by electrifying the way in which the process is driven (e.g., switching from burning fossil fuels to generate heat to electrified heating approaches);
(iii) The increased pace by which renewable feeds or waste streams are being identified as potential feeds for electrified chemical conversions; and
(iv) Now that electrochemical synthesis has emerged from a very small field of research to one of the most active fields of study in the chemical sciences, many unprecedented, promising opportunities for electrifying different chemical manufacturing processes are being identified (new chemistry, less harsh, fewer steps, …). Below, after a brief account of historic electrified chemical manufacturing processes being used at scale, we cover some of the emerging developments and opportunities for electrochemical manufacturing, both those that will aid in reducing greenhouse gas emissions, and those that offer new, more efficient synthetic pathways to desired fine chemicals, pharmaceuticals, and other products.
A Bit of History
Despite electrochemical manufacturing methods in the chemical industry often feeling “novel,” “new,” or “not yet implemented,” the foundations of the chemical industry go back to electrochemical manufacturing. The Dow Chemical Company was started in 1897 to electrolyze brines found in Midland, Michigan to recover bromine and chlorine.4 The chlor-alkali process to produce chlorine, hydrogen, and caustic soda (sodium hydroxide) from aqueous sodium chloride is one of the most used chemical processes today and it is an electrochemical process. In fact, this article is part of the 80th anniversary of the founding of what is now the Industrial Electrochemistry & Electrochemical Engineering Division of The Electrochemical Society. This division was originally the “Industrial Electolytics Division” with its membership centered around the chloralkali process. For many years, the division also published a “Report of/on the Electrolytic Industries” as an annual update in The Journal of The Electrochemical Society with a significant focus on the status of the chlor-alkali industry and other updates on electrochemical processes at scale (the last report was on the status of electrochemical manufacturing in 20045).
Electrochemical Manufacturing at Scale Today


While chlor-alkali continues to dominate in terms of electrochemical process production volume and energy input (20 GW installed capacity6), there are other significant electrochemical processes utilized industrially as well. For example, the Monsanto electrohydrodimerization of acrylonitrile to adiponitrile, an intermediate in the production nylon-6,6, went online in 1965 and is used in one-third of all adiponitrile produced today. Additionally, BASF electrochemically dimethoxylates 4-tert-butyltoluene at a scale of tens of thousands of tons per year. A multitude of other chemical products have been manufactured electrochemically over the years, including L-cystine, furan, alkyltoluenes, maleic acid, nitrobenzene, butanone, and many more.7,8 The historical electrochemical processes have been summarized in previous Interface articles9 and handbooks.8, 10
The historical motivation for electrochemical manufacturing of chemicals has largely been to access chemistry otherwise not possible or very difficult, or to eliminate hazardous intermediates, side products, or stoichiometric reagents.9 Significant opportunities exist in the future to pair electrochemical manufacturing with renewable electricity for both decarbonization3,9 and a now-inexpensive oxidizing or reducing reagent (i.e., the electron). Using existing and previous electrochemical manufacturing processes, significant opportunities are emerging for the manufacturing of additional chemicals electrochemically.
Electrochemical Conversion Approaches that Hold Potential for Chemical Manufacturing Hydrogen Production
The production of hydrogen via water electrolysis is already gradually replacing hydrogen generated from fossil fuels (historically steam reforming of natural gas) and is the next big electrochemical process to be realized in the industry. The main driver of water electrolysis today is decarbonization of hydrogen as a chemical feedstock. There are also significant opportunities to use hydrogen for long-term energy storage of variable renewable electricity and for carbon-neutral combustion for industrial heating in the future.3 In 2021, >500 MW of water electrolysis capacity had been installed globally, with rapid growth anticipated to bring capacity to several hundred gigawatts by 2030.6 While water electrolysis can be performed at large scale in an economically feasible manner, research in academia and industry continues to focus on improving the process, in terms of more active and inexpensive catalysts and of improved overall durability for both the cathode (hydrogen evolution) and the anode (oxygen evolution).
CO2 Reduction
As water electrolysis technology has matured, the technological knowledge has expanded to other feedstocks and opportunities for decarbonization. Electrochemical reduction of CO2 has emerged as a possible carbon neutral or even carbon negative approach for the production of intermediates of key interest to the chemical industry, such as CO, formate, methanol, methane, ethanol, and ethylene.11 Over the past 15 years, through an explosion of research activity, (continued on next page)
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 41
Biddinger and Kenis
(continued from previous page)
reasonable active and selective catalysts have been developed for each of these products. Current research efforts focus much more on reactor engineering challenges: achieving high rate and stable performance in membrane-based electrolysis cells. A particularly difficult challenge is the side reaction of CO2 to bicarbonates that tend to precipitate on or in the electrodes, spurring studies on carbonate formation12 as well as the overall carbon balance.13 Indeed, the study and improvement of electrode durability is a major focus of current research.14
Another aspect that electrochemical CO2 reduction and water electrolysis have in common is that both are still energy intensive. A significant fraction of the energy required to drive the process is needed for the anodic process of oxygen evolution. The combination of oxygen evolution thermodynamics (i.e., a very positive theoretical redox potential, 1.23V vs. SHE) and slow kinetics can add several volts to the operating cell potential. Pairing CO2 reduction or hydrogen with less energy intensive half reactions (e.g., oxidation of organics) drastically reduces the cell potential and thus the overall energy demand.15
CO Reduction
The electroreduction of CO, rather than CO2, has also started to garner attention for application at scale. Industry is looking for electrified processes that will replace some of the steps that emit the most CO2. Through decades of research and industrial practice, CO can be produced very efficiently from methane, which continues to be abundantly available and relatively cheap. Furthermore, the CO2 emissions of this process could in principle be reduced to close to zero. Replacing the much more energy intensive and CO2 emitting subsequent conversion of CO to the intermediates needed for the manufacturing of a broad range of chemicals is a much more attractive candidate for electrification. Furthermore, unlike CO2 electroreduction, the CO electroreduction process is not hampered by carbonate formation, simplifying its path toward a process at scale. Indeed, CO reduction has become a very active area of investigation, holding promise for production of intermediates like acetate, ethylene, and acetaldehyde.16,17,18
Electrosynthesis of Organics
As more and more processes are being electrified, interest has returned to utilizing electrochemistry in organic synthesis. Significant research activity in the last decade has occurred in the field of electro-organic syntheses with applications in fine chemicals and pharmaceuticals.19-23 The activity has been driven by progress on multiple fronts: (i) the ability to access chemistries that through traditional synthetic means are difficult, hazardous, or highly waste generating, and (ii) the development of enabling technologies for non-electrochemists such as the IKA Electrasyn systems that provide “plug and play” reactions in standardized vials on a stir plate without the need for a potentiostat paired with recent tutorials geared toward organic chemists.24-26 Electro-organic reactions can be used in coupling reactions such as with C-C and C-N bond formations, functionalization with heteroatoms, selective deprotections of functional groups, reduction of double bonds, hydrogenations, and many more that are of importance for the organic chemist’s toolbox.2 In the most appealing cases, complex multi-step reactions (even 10+ steps) can be performed selectively in single steps using electrochemistry. The electron transfer may be direct at the electrode surface or through a mediator, similar to a homogeneous catalyst that has been activated by electron transfer at the electrode surface. The electron can become a replacement for stoichiometric oxidizing or reducing agents, while also eliminating the stoichiometric generation of salts that would require disposal. While much of the research has been on milligram-gram scales in batch reactors to identify new reactions or to improve yields, efforts are also being made to incorporate flow electrochemistry and scale-up conditions.27,28
New electro-organic reactions in the fine chemical and pharmaceutical industries are likely to be implemented first where substantial process advantages can be obtained. Merely having a
higher yield or selectivity to a desired product is unlikely to meet the activation barrier of implementation of new electrochemical process equipment for fields unseasoned in the use of electrochemical reactors. Processes susceptible to significant minimization of the number of synthesis steps or the elimination of hazardous species are likely to be most sensible for early adoption economically and make the risk of taking on a new transformation method (i.e., electrochemistry) worthwhile. To enable the transformations, scale-up procedures and off-the-shelf electrochemical reactors able to produce 1–1000kg/day need to be widely available. Membranes that are stable and selective in organic solutions need to be developed and economical for when undivided cells are not favorable due to undesired reactions at the counter electrode. Many fine chemical and pharmaceutical chemical processes occur in multi-product facilities—scheduled in short duration runs—rather than in purpose-built single, continuous product facilities. Having electrochemical reactors as part of the inventory available in these multi-product facilities will open up the possibility of performing reactions in which electrochemistry improves yields but does not change the overall production pathway. After the industries have established electrochemical processes and invested in the reactors and infrastructure, electrochemical reactions can become part of the more common “toolbox” of processes to consider.
Biomass-Derived Conversion
Many biomass-derived species have been identified as alternative platform molecules to serve as building blocks for the chemicals and fuels industries27,28 and have led to new and/or re-invigorated investigations into electrochemical upgrading of the building blocks. Biomass-derived species, including furanics, phenolics, glycols, carboxylic acids, and aldehydes, have largely been the focus of electrochemical reactions. Upgrading has included electrochemical hydrogenation (ECH), dehydrogenation (ECD), oxidation (ECO), dimerization, and ring-opening reactions to form intermediates for fuels and chemicals.29–37 The electrochemical depolymerization and upgrading of lignin is also an area of study.37,38 The drive for electrochemical, rather than thermochemical, upgrading of biomassderived species has included the ease of integration of renewable electricity, ability to operate at ambient temperatures and pressures, and reduced infrastructure needs such as steam or hydrogen gas. Electrochemical upgrading of biomass-derived species at Biomass Upgrading Depots (BUDs) has been shown in studies to be economically feasible.39 Additionally, with the advances in hydrogen evolution and CO2 electroreduction, biomass-derived species and wastes have been examined as alternative oxidation reactions for oxygen evolution that would bring both enhanced value in the paired electrolysis and lower overall cell potential compared to oxygen evolution.40
The focus of the research on electrochemical reactions of biomassderived species has largely been from a reaction engineering perspective—improvements in faradaic efficiencies, selectivities, catalyst development, electrolyte influences, and reactor designs. Unlike in fine chemical and pharmaceutical electro-organic syntheses, the value of the biomass-derived species is much closer to that of a commodity/specialty chemicals interface. Electricity costs, while continuing to drop per kilowatt-hour, still make up a significant portion of operating costs for these lower-value chemicals. Efficient, high-surface-area electrocatalysts with minimal precious metals are also becoming an area of significant interest now that the reactions have been demonstrated to be catalytic, not just electron-transfer, in nature. One of the driving factors for electrochemical transformation of biomass-derived species is to improve the stability of the final product. This will entail identifying reaction conditions that enable the handling of reactive species and contaminants so that the desired products are formed, rather than humins or other undesired species. While there are many opportunities for electrochemical conversion of biomass-derived species, the likely first large-scale implementations will be in electrooxidations at the anode paired with hydrogen evolution at the cathode to produce two valuable products and lower the cell potential so that hydrogen can be produced with less energy input.
42 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Ammonia Electrochemistry
Driven by the tremendous global need for ammonia, which is critical for fertilizer manufacturing and other applications, researchers have sought ways to synthesize ammonia directly from nitrogen using a less energy-intense approach than Haber-Bosch. Electrochemists have long recognized this challenge/opportunity too, but finding promising electrocatalysts or an electrochemical approach has proven to be elusive, as has been recently reviewed.41 In recent work, Chorkendorf and colleagues demonstrated that a platinumgold alloy in a 25 cm2 electrolysis cell is able to produce ammonia with a faradaic efficiency of >60%, yet at an energy efficiency that is still only 13%.42 While work likes this represents a major advance, to be feasible for application at scale, energy efficiencies exceeding 50% are needed. An alternative, abundantly available, possible feed for ammonia production is nitrate-containing waste streams such as agricultural runoff. Efficient electrochemical conversion of nitrate to ammonia has been demonstrated43 but the fact that most of the nitrate containing feeds are highly dilute remains a problem.
In addition to interest in electrochemically synthesizing ammonia, ammonia also has emerged as a promising hydrogen carrier since it features a volumetric density of hydrogen that is 1.7 times greater than that of liquid hydrogen. However, present-day approaches like thermal cracking that are used to liberate H2 from ammonia are not energy efficient. Ammonia electrolysis in alkaline media, where ammonia oxidation on the anode to produce N2 is paired with water reduction to produce hydrogen, has the potential to be much less energy intensive. While the reduction reaction to produce H2 is relatively efficient, the ammonia oxidation reaction (AOR) occurring at the anode is not. Present-day catalysts for this reaction exhibit high overpotentials to achieve reasonable rates and are easily poisoned by byproducts of the AOR itself. Research on identifying better AOR catalysts has significantly increased due to the importance ammonia could play in the energy transition, by enabling transfer of green hydrogen over long distances. Recent efforts have started to identify interesting binary and ternary catalysts, in part identified using machine learning approaches, that may address the two challenges of high overpotential and propensity for poisoning.44,45
Process Intensification and Reactor Engineering
Research on the wide range of electrochemical conversions described above has also spurred exploration of a wide range of electrochemical reactor configurations. While typically initial characterization of new catalysts is done in a three-electrode H-cell, evaluation of the performance (activity, selectivity, stability) of those catalysts requires their integration in electrodes for flow cells and/or membrane-electrolyte assembly cells. Several studies have focused on developing such reactors, including multilayer stacks,46 and/or on process intensification by systematic evaluation of a number of parameters, ranging from catalyst loading to operation parameters such as feed and electrolyte flow rates.47 A next level of complexity being studied is so-called tandem approaches, where feeds such as CO2 are converted to desired products in multiple consecutive steps (e.g., CO2 to CO to acetate).48,49 A number of studies also are exploring ways that allow the anode and cathode chemistries to be de-coupled (at times referred to as modular electrochemical synthesis), through the application of bipolar membranes, or heterogeneous redox reservoirs, which for example was used to demonstrate electrochemical hydrogen peroxide synthesis.50 It is these types of approaches that may enable electrochemical manufacturing platform technology capable of coupling large-scale cathodic processes (hydrogen evolution; CO2 or CO reduction) with a number of different smaller scale anodic oxidations of organics.
Remaining Overarching Challenges
The preceding section summarized R&D efforts on some of the many different electrochemical manufacturing approaches being pursued at present. Indeed, many other chemistries are being pursued for electrification. Beyond considering specific electrochemical
conversions, a number of common or overarching challenges still hamper many approaches for electrochemical manufacturing of chemicals from being pursued for scale up.
(i) Lack of suitable electrochemical infrastructure. Unlike other chemical reactors, electrolysis systems are highly specific to the chemical conversion for which they are being used. Going beyond a laboratory scale of benchtop proof-of-concept is thus not trivial. Also, it is not obvious what entity would be most effective in developing and selling these needed durable electrochemical reactors. Furthermore, the end users, here the chemical companies, are hesitant to be the first to invest at a level needed to test new, unproven technology at pilot-plant scale or beyond.
(ii) Lack of familiarity with electrochemical conversion processes across many industries. Implementation of new technology will be slow given the lack of expertise with new reactors etc., leading to hesitancy in their implementation. The field would benefit from facilities where anyone can come in and test electrochemical manufacturing approaches at a larger scale (up to pilot plant) for longer run times (to test durability), to speed up the development of new reactor technology, and to help familiarize designers and implementers with this new technology, thereby removing their hesitancy. The National Renewable Energy Laboratory (NREL) in Colorado may play a major role here.
(iii) Limited availability of (renewable) electrical energy (grid) and electrical hardware to drive the process. It is possible to calculate how much grid power would be necessary to operate electrochemical manufacturing plants at scale. This is quickly followed by the conclusion that the present grid capacity, let alone the present fraction of renewable energy in the grid, is insufficient for even partial electrification of chemical manufacturing. Furthermore, like the electrochemical reactor technology, further development of electrical hardware and controls for operation of electrochemical manufacturing plants at scale will be needed, especially if these electrochemical manufacturing facilities need to become part of regional grid-scale load-leveling efforts to maximize utilization of renewable power while ensuring economic feasibility. Already, sector coupling approaches are being studied, where the variable electrical power supply and demand is connected with hydrogen production (water electrolysis).51
(iv) Variability in feed composition. Many of the approaches for electrochemical manufacturing described above for different applications will rely on feeds that may vary significantly in composition depending on the specific source. Take for example “crude glycerol,” a byproduct of biodiesel production produced by hundreds of plants. Its composition can vary over 30–70% glycerol, 10–40% methanol, 4–25% NaOH, and a few other ingredients. No process would be able to handle such a broad composition range without significant adjustment of the feed and/or the operational parameters. The same is true for captured CO2 streams from different plants or regions. Standardized feed compositions may need to be defined for processes moving to application at scale.52
(v) Need for techno-economic and life cycle analyses and associated models. Due to the much more regional availability of feeds, electrochemical manufacturing facilities will probably be deployed in a more regional fashion compared to the present situation of large-scale chemical production facilities and refineries being deployed only in a limited number of locations across the continents. See Fig. 1. For industry to be willing to invest in the deployment of such regional facilities, high quality techno-economic assessment (TEA) and life cycle assessment (LCA) studies will be needed, coupled with regional models that take into account regional availability of critical resources, such as those developed for biofuel (ethanol)
(continued on next page)
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 43
Biddinger and Kenis (continued from previous page)
Fig. 1: Side-by-side comparison of available biomass resources (left) and oil & natural gas processing and transportation infrastructure (right) in the US. Whereas current fossil fuel processing facilities are located in the vicinity of oil and (shale) gas fields, and along major pipeline networks to/from those, one can foresee that future electrified chemical manufacturing facilities that use biomass resources as the feed will need to be located along the West Coast, the Midwest (utilizing significant amounts of currently stranded wind power?!), along the Mississippi River, along the East Coast, and in the Northeast. Similarly, one can foresee how electrified chemical manufacturing facilities could be co-located with current point sources of CO2 (fossil fuel–based chemical industry and power plants), while those utilizing CO2 from direct air capture could be localized with much fewer constraints, including in vast areas (West/Central) that have limited biomass resources. Sources: Left Map – A. Milbrandt, Technical Report, NREL/TP-560-39181, December (2005) Right Map: Interactive “National Energy and Petrochemical Map,” Fractracker Alliance, accessed April 2023

production and other biomass conversions.53 Almost every electrochemical process will need a dedicated analysis effort to assess its economic feasibility and its remaining carbon footprint. While many TEA and LCA studies on electrifying chemical manufacturing have appeared, most of these lack those detailed, regional deployment considerations, as well as spatiotemporal aspects of variable electricity cost.
Conclusions
We hope that the above summary provides the reader with insight into the promise and remaining challenges associated with electrifying chemical manufacturing across many types of chemistries and applications, ranging from commodity intermediates to specific pharmaceuticals or fine chemicals. We wish to explicitly acknowledge that other approaches and chemistries are being pursued, or maybe have already been implemented in industry. The scenarios and categories we present here are intended as examples.
Many of the directions and/or specific examples covered above have a possible role to play in decarbonizing emissions associated with future chemical manufacturing. In reality, most of these approaches when implemented will not be carbon neutral, let alone be carbon negative, for the simple reason that only a (gradually increasing) fraction of the electrical power needed to drive the process will be derived from renewable energy sources. In a similar vein, it is important to point out that the energy transition, with a goal to arrive at close to carbon neutrality by 2050, is a very gradual process. Industry does not have the resources to abruptly abandon its massive investment in existing plants in lieu of modern carbonneutral processes. Furthermore, the chemical processes needed for a complete electrification of chemical manufacturing are just not available at this point. Beyond the need to reduce CO2 emissions associated with chemical manufacturing, electrochemical processes at times also offer more efficient conversions, avoided steps, elimination of hazardous materials, higher yields, and higher selectivity. Key advances in industrial practice in the production of fine chemicals and pharmaceuticals are evident, underscored by the many chemical and pharma companies that now have dedicated electrosynthesis efforts.
Despite these issues, electrification of chemical manufacturing is already happening, with a focus on the more straightforward opportunities, such as transitioning from heating based on burning fossil fuel to electrified, resistive heating, or the in-situ production of
the hydrogen needed for a process via a water electrolysis approach instead of through thermo-chemical methods. Implementation of new chemical conversion processes will be significantly more challenging. As stated above, centralized facilities where academic and/or corporate teams can try out a process at a larger scale, where they can tackle durability and other challenges, would greatly benefit the progress of the field.
Driven by the need to decarbonize chemical manufacturing, a very healthy number of funding opportunities from the federal agencies and from industry, and the fascination of many researchers with these relatively new processes and the unique capabilities they offer, the field of electrosynthesis indeed is experiencing a sort of renaissance. Indeed, the future of electrochemical manufacturing, in terms of scholarly research opportunities, as well as in terms of potential impact on industrial practice, is bright!
© The Electrochemical Society. DOI: 10.1149/2.F08232IF
About the Authors
Elizabeth J. Biddinger, Associate Professor of Chemical Engineering, The City College of New York

Education: BS in Chemical Engineering (Ohio University), PhD in Chemical Engineering (The Ohio State University), Post-doctoral Fellow (Georgia Institute of Technology).
Research Interests: Electrochemical reaction engineering for green chemistry and energy. In particular, the electrification of chemical processes that transform wastes or renewable resources into valuable materials, chemicals and fuels for decarbonization and sustainability, scale up of electro-organic syntheses, and alternative electrolytes for battery safety and performance.
Pubs & Patents: >45 papers, 1 patent, h-index 23
Awards: US Department of Energy Early Career Award (2018), ECS-Toyota Young Investigator Fellowship (2016–2017)


Work with ECS: IE&EE Secretary/Treasurer (2022–Present) and Student & Early Career Awards Chair (2014–2022); ECS Publications Subcommittee Member (2017–2020); ECS Member since 2004 (19 years).
Website: https://ebiddinger.ccny.cuny.edu
https://orcid.org/0000-0003-3616-1108
44 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Paul J. A. Kenis, Elio Eliakim Tarika
Endowed Chair and Professor of Chemical and Biomolecular Engineering, University of Illinois Urbana-Champaign

Education: BS in Chemistry (Radboud University), PhD Chemical Engineering (Twente University), Post-doctoral Fellow (Harvard University)
Research Interests: Reactor and reaction engineering for applications in energy, sustainability, and health. In particular, reactor technology for (i) Automated continuous synthesis and optimization of nanomaterials such as quantum dots and associated autonomous workflows; (ii) Electrolysis processes for sustainable manufacturing of chemicals and food from renewable resources such as CO2 and bio-derived adducts; (iii) Direct air capture of CO2; (iv) Electro-oxidation of ammonia to enable ammonia as a hydrogen carrier.
Pubs & Patents: >210 publications, 14 patents, h-index 76 Awards: Industry Project Award, Institution of Chemical Engineers (2022), ECS Energy Technology Division Research Award (2020), ECS Fellow (2019).
Work with ECS: ECS Member since 2003 (20 years); ECS Fellow (2019); IE&EE Secretary/Treasurer (2020–2022) and Vice Chair (2022–Present); Energy Technology Division Executive Committee, Member-at-Large (2020–Present), Awards Committee (2020–Present); Mid-America Section Member (2022–Present).

Website: https://kenis-group.chbe.illinois.edu
https://orcid.org/0000-0001-7348-0381
References
1. Paris Agreement to the United Nations Framework Convention on Climate Change, Dec. 12, 2015, T.I.A.S. No. 16-1104.
2. Dealing with carbon dioxide at scale Sackler Forum, October 2017 (Published May 2018), National Academy of Sciences, USA; the Royal Society, UK.
3. D. S. Mallapragada, Y. Dvorkin, M. A. Modestino, et al., Joule, 7(1), 23 (2023).
4. Dow Chemical Company: History. Dow Chemical Company, 2023 (accessed 2023 4/16/2023).
5. V. Srinivasan, P. Arora, and P. Ramadass, J. Electrochem. Soc. 153(4), K1 (2006).
6. J. M. Bermudez, S. Evangelopoulou, and F. Pavan, Electrolyzers, Paris, 2022 (accessed 4/21/23).
7. G. G. Botte, Interface, 23(3), 49 (2014)
8. A. J. Fry, Electrochemical processing, organic. In Kirk-Othmer Encyclopedia of Chemical Technology, Vol. 9; John Wiley & Sons, Inc., 652 (2022).
9. E. J. Biddinger and M. A. Modestino, Electrochem. Soc. Interface 29(3), 43 (2020).
10. D. Pletcher and F. C.Walsh, Industrial Electrochemistry, Blackie Academic & Professional (1993).
11. CO2 in general. See for example: S. Nitopi, E. Bertheussen, S. B. Scott, et al., Chem. Rev., 119, 7610 (2019)
12. E. R. Cofell, U. O. Nwabara, S. S. Bhargava, D. E. Henckel, and P. J. A. Kenis, ACS Appl. Mater. Interfaces, 13, 15132 (2020).
13. M. Ma, E. L. Clark, K. T. Therkildsen, et al., Energy Environ. Sci., 13, 977 (2020).
14. U. O. Nwabara, M. P. de Heer, E. R. Cofell, S. Verma, E. Negro, and P. J. A. Kenis, J. Mater. Chem. A, 8, 22557 (2020).
15. S. Verma, S. Lu, and P. J. A. Kenis, Nat. Energy, 4, 466 (2019).
16. M. Jouny, G. S. Hutchings, and F. Jiao, Nat. Cat., 2, 1062 (2019).
17. A. Ozden, Y. Wang, F. Li, M. Luo, et al., Joule, 5, 706 (2021).
18. P. Zhu, C. Xia, C.-Y. et al., Proc. Natl. Acad. Sci. USA 118(2), e2010868118 (2020)
19. C. A. Malapit, M. B. Prater, J. R. Cabrera-Pardo, et al., Chem. Rev., 122(3), 3180 (2022).
20. D. Pollok and S. R. Waldvogel, Chem. Sci, 11(46), 12386 (2020).
21. K. Lam, Synlett, 33(20), 1953 (2022). DOI: 10.1055/a-1890.
22. F. Wang and S. S. Stahl, Acc. Chem. Res., 53(3), 561 (2020).
23. M. Yan, Y. Kawamata, and P. S. Baran, Chem. Rev., 117(21), 13230 (2017).
24. S. B. Beil, D. Pollok, and S. R. Waldvogel, Angew. Chem., Int. Ed., 60(27), 14750 (2021).
25. C. Kingston, M. D. Palkowitz, Y. Takahira, et al., Acc. Chem. Res. 53(1), 72 (2020).
26. C. Schotten, T. P. Nicholls, R. A. Bourne, et al., Green Chem., 22(11), 3358 (2020).
27. T. Noël, Y. Cao, and G. Laudadio, Acc. Chem. Res., 52(10), 2858 (2019).
28. C. Bottecchia, D. Lehnherr, F. Lévesque, et al., Org. Process Res. Dev., 26(8), 2423 (2022).
29. T. Werpy and G. Petersen, Top value added chemicals from biomass: I. Results of screening for potential candidates from sugars and synthesis gas, United States (2004).
30. J. J. Bozell, J. E. Holladay, D. Johnson, and J. F. White, Top value added chemicals from biomass: Volume II: Results of screening for potential candidates from biorefinery lignin, PNNL-16983, Pacific Northwest National Laboratory (2007)
31. S. A. Akhade, N. Singh, O. Y. Gutiérrez, et al., Chem. Rev. 120(20), 11370 (2020).
32. J. Carneiro and E. Nikolla, Ann. Rev. Chemical Biomol. Eng., 10(1), 85 (2019).
33. L. Du, Y. Shao, J. Sun, et al., Catal. Sci. Technol., 8(13), 3216 (2018).
34. F. J. Holzhäuser, B. Mensah, and R. Palkovits, Green Chem., 22(2), 286 (2020).
35. C. H. Lam, W. Deng, L. Lang, et al., Energy Fuels, 34(7), 7915 (2020).
36. J. R. Page, Z. Manfredi, S. Bliznakov, and J. A. Valla, Materials, 16(1), 394 (2023).
37. X. Du, H. Zhang, K. P. Sullivan, et al., Chem. Sus. Chem., 13(17), 4318 (2020).

38. C. Yang, S. Maldonado, and C. R. J. Stephenson, ACS Catal., 11(16), 10104 (2021).
39. C. H. Lam, S. Das, N. C. Erickson, et al., Sustainable Energy & Fuels, 1(2), 258 (2017).
40. M. NaderiNasrabadi, F. Bateni, Z. W. Chen, et al., J. Electrochem. Soc., 166(10), E317 (2019).
41. M. C. Hatzell, ACS Energy Lett., 7(11), 4132 (2022).
42. X. Fu, J. B. Pedersen, Y. Zhou, et al., Science, 379(6633), 707 (2023).
43. K. Kim, A. Zagalskaya, J. L. Ng, et al., Nat. Comm., 14, 823 (2023)
44. H. S. Pillai, Y. Li, S.-H. Wang, et al., Nat. Commun., 14(1), 792 (2023).
45. Y. Tian, Z. Mao, L. Wang, and J. Liang, Small Structures, 2200266 (2023).
46. B. Endrődi, E. Kecsenovity, A. Samu, et al., ACS Energy Lett., 4(7), 1770 (2019).
47. S. S. Bhargava, F. Proietto, D. Azmoodeh, et al., ChemElectroChem, 7(9), 2001 (2020).
48. Y. Liu, H. Qiu, J. Li, et al., ACS Appl. Mater. Interfaces, 13, 40513 (2021).
49. H. Zhang, X. Chang, J. G. Chen, et al., Nat. Commun., 10, 3340 (2019).
50. F. Wang, W. Li, R. Wang, et al., Joule, 5, 149 (2021).
51. G. He, D. S. Mallapragada, A. Bose, et al., Energy & Environ. Sci., 14(9), 4635 (2021)
52. Gaseous carbon waste streams utilization: Status and research needs; National Acadaemies of Sciences, Engineering, and Medicine, Washington, DC, 2019
53. G. Vega, J. Voogt, J. Sohn, et al., Sustainability, 12(9), 3676 (2020).
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 45
ACTIVATE WITH ECS PUBLICATIONS
ECS TOPICAL INTEREST AREAS
Electrochemical
• Batteries and Energy Storage
• Corrosion Science and Technology
• Electrochemical/Electroless Deposition
• Electrochemical Engineering
• Fuel Cells, Electrolyzers, and Energy Conversion
• Organic and Bioelectrochemistry
• Physical and Analytical Electrochemistry, Electrocatalysis, and Photoelectrochemistry
• Sensors (Electrochemical)
Solid State
• Carbon Nanostructures and Devices
• Dielectric Science and Materials
• Electronic Materials and Processing

• Electronic and Photonic Devices and Systems
• Luminescence and Display Materials, Devices, and Processing
• Sensors (Solid State)
46 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
LEARN MORE
Extraterrestrial Electrochemistry –Challenges and Opportunities for Electrolytic in-situ Resource Utilization (ISRU) on Mars
 by Shrihari Sankarasubramanian, Bradley Chambers, and Cheyenne Wilson
by Shrihari Sankarasubramanian, Bradley Chambers, and Cheyenne Wilson
Mars, the most “Earth-like” amongst the other planets in our Solar System, is an exploration priority for several national and private space programs as it may have hosted life at some point in its planetary history. Robotic landers from the US and China and orbiters from the US, China, Europe, Russia, India, and the United Arab Emirates (UAE) are presently active there.1 Furthermore, both China2 and the US3 have laid out concrete plans for future crewed missions to Mars within a decade. Herein, we examine the challenges such missions must overcome, and the key role electrochemical technologies are expected to play in enabling the long-term exploration of Mars and beyond.
Enabling Sustained, Crewed Planetary Expeditions
Relatively longer duration (weeks to months of planetary stay) crewed missions to Mars (or other celestial bodies) need to contend with the challenge of supplying fuel and life-support for the long journey (~350x the distance to the Moon) out-and-back and for the stay on Mars. The traditional route for supplying such missions from Earth runs into the “tyranny of the rocket equation.” The payload ratio (��) of a rocket is defined as4
(1)
Where md is the payload mass, mp is the propellant (fuel and oxidant) mass, and ms is the structural mass of the rocket. Typical values for �� range from ~1% (space shuttle) to ~4% (Saturn V)5 as depicted in Fig. 1(a). Thus, NASA design architecture studies show that completely supplying a Mars mission from Earth, including 35 metric tons of propellant needed for the return journey and the 0.8 to 1.2kg of oxygen a day required by astronauts, would require ~400 metric tons of propellant (fuel and oxidant) transported on 4–5 heavy lift launch vehicles at exorbitant cost.6 A far more cost-effective alternative is the exploitation of resources present on Mars to meet mission needs through in-situ resource utilization (ISRU).
In-situ Resource Utilization on Mars
ISRU requires us to re-examine fundamental design assumptions around terrestrial industrial technologies due to different operating conditions (varying pressure, temperature range, gravity, etc.), resource constraints, and the lack of combustible fuels (and steam) that underpin most of modern industry on Earth. On the other hand, abundant electricity from solar panels or various nuclear sources makes ISRU eminently amenable to electrochemical technologies. Table I summarizes and contrasts the operational conditions encountered on Mars and Earth. The low atmospheric pressure on Mars (0.636 kPa vs. 101.4 kPa
on Earth) and the wide diurnal temperature range suggests pumping and heating energy penalties for any ISRU process carried out there. Furthermore, the low surface gravity can pose challenges for bubble growth and detachment from electrode surfaces. Unlike on Earth, significant seasonal variation (± 20%) in atmospheric pressure and persistent dust storms also need to be accounted for on Mars.
In the Martian context, a key resource is the atmosphere, the predominant constituent ( 95%) of which is CO2. Thus, the ISRU technology farthest in development, NASA’s Mars in-situ resource utilization experiment (MOXIE) utilizes a solid oxide electrolyzer (SOXE) to produce O2 from CO2 17 Hosted onboard the Perseverance rover (Fig. 1(b)), the 10-cell MOXIE stack (assembled stack shown in Fig. 1(c)) has been successfully operated on Mars and shown to produce ~12g/h of O28 (for context, humans need 21g/h when sleeping to ~171g/h when running on a flat surface13). The MOXIE stack (2x 5-cell stacks in series with electrode area = 22.7 cm2) reportedly operates at 50% faradaic efficiency (FE). The 300W power envelope consists of ~115W for operating the stack, with the rest powering the balance of the plant. The stack operation in turn consumes ~35W for electrolysis of CO2 (i = 4A maximum at an 8.7V stack operating voltage) and 80W for maintaining the stack temperature at 1037K. Notably, CO is produced as a side-product of this system; thus, careful downstream processing would be required before the generated O2 could be used in life-support applications.
In addition to abundant CO2 in the Martian atmosphere, relatively recent evidence of water (or brine) on Mars suggests another feedstock for ISRU. Fig. 2(a) depicts the Mars Reconnaissance
(continued on next page)
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 47
m mm
d pS
Fig. 1. (a) Illustration of the payload ratio of a Saturn V rocket (Saturn V image credit: NASA History Office and Kennedy Space Center), (b) Artist’s conception of the Perseverance rover (image credit: NASA/JPL-Caltech), (c) the Mars in-situ resource utilization experiment (MOXIE) solid oxide CO2 electrolyzer stack onboard the Perseverance rover (image credit: NASA/JPL-Caltech).
Sankarasubramanian et al.
(continued from previous page)
Table I. Comparison of Conditions on Earth and on Mars7
CO
Minor: 210 ppm H2O, 100 ppm NO, 2.5 ppm Ne, 0.85 ppm H-D-O, 0.3 ppm Kr, 0.08 ppm Xe
Orbiter’s (MRO) observation of geological features (recurring slope lineae (RSL)) indicating water flows on Mars, at least on geological timescales.9 NASA’s Phoenix Lander discovered water ice under a few inches of regolith and optically observed its sublimation over 4 days (Fig. 2(b)).10 Furthermore, the Martian south pole is expected to be a major water source, as the Mars Advanced Radar for Subsurface and Ionosphere Sounding (MARSIS) instrument onboard the Mars Express spacecraft has detected multiple sub-glacial water bodies underneath the Martian south pole at Ultimi Scopuli.11 The quantity of water present in these bodies is speculated to be on the order of 2x1018 kg. Despite evidence of water, the Martian diurnal temperature range is below the freezing point of water under terrestrial conditions (273K at 101.325 kPa). Furthermore, the low atmospheric pressure also suggests the possibility of sublimation. Fortunately, perchlorates present in the Martian regolith play a key role in solving these issues and enabling the use of this water. The wet chemistry instrument (WCI) on NASA’s Phoenix Lander discovered significant quantities of perchlorate and sulfate salts of sodium and magnesium in the Martian regolith.10 We have shown in our previous work12,13 that perchlorates in the regolith (notably Mg(ClO4)2) depress water’s freezing point to 203K and concurrent boiling point elevation indicates that liquid brines can exist within the temperature and pressure window encountered on Mars. Thus, water is also available as a versatile (albeit geographically constrained) feedstock for ISRU on Mars.

The availability of water led to the proposal of a low-temperature brine electrolyzer (schematic in Fig. 3(a)) to produce H2 (fuel) and O2 (oxidant and for life-support) on Mars in Gayen et al.13 That work sought to address the energy penalty of operating a SOXE at high temperature ( 70% of MOXIE’s stack energy consumption goes toward temperature maintenance) by developing a Mg(ClO4)2 brine electrolyzer that runs at Martian ambient temperatures. While thermodynamic analysis indicates that it should be possible to run this system at Martian atmospheric pressures as well without the brine sublimating, it would be straightforward to throttle the outlet gas flow and achieve terrestrial pressures inside the stack. The electrolyzer was assembled with standard commercial cell hardware of the zero-gap, plate-and-frame type with Ti flow fields and fed with 2.8M Mg(ClO4)2 brine on both sides. The anode consisted of high-performance Pb2Ru2O7-δ pyrochlore oxygen evolution reaction (OER) electrocatalyst deposited onto porous titanium foam, while the cathode consisted of Pt/C hydrogen evolution reaction (HER) electrocatalyst deposited onto porous carbon paper. Both electrodes used anion exchange membrane (AEM) binders and sandwiched a commercially available 50µm Fumasep FAA-3-50 AEM, forming the MEA. Surprisingly, this brine electrolyzer exhibited a peak power density of 1.23 W/cm2 (1.92 V, -36oC) which is comparable to a previously reported solid-state alkaline water electrolyzer operating at 50 oC with a Pb2Ru2O7-δ anode, which achieved a power density of 1.2 W/cm2 at 1.85 V using a DI water feed.15
Building on the demonstrated viability of a Martian ambient temperature water electrolyzer and utilizing both atmospheric CO2
and brine, Shahid et al have recently shown that Martian CO2-brine electrolyzers can serve as a viable route to supplying the upcoming generation of Martian descent vehicles (schematic in Fig. 3(b)). The new generation of Martian descent vehicles plan to use methaneoxygen (methalox) engines due to their superior performance characteristics compared to H2-O2 rocket engines.12 Thus, complete refuelling of these systems requires the production of CH4 and O2. Electrolyzer stack models (validated against experimental electrolyzer data) of stacks with conventional Cu CO2 reduction catalyst, RuO2 OER catalyst, and a cation exchange membrane (CEM) predicted that low-temperature operation of these systems can result in significant energy efficiency gains compared to SOXE systems. A hypothetical 10-cell, 100cm2 electrode-area-per-cell electrolyzer was predicted to produce 0.45gW-1day-1 of CH4 and 3.55gW-1day-1 of O2 at 2V/cell and 50% electrolyzer faradaic efficiency vs. a best-case production of 2.5gW-1day-1 of O2 by MOXIE from NASA’s Perseverance mission
(RSL)) on the slopes of Hale Crater indicating possible water flows on Mars as observed using the Mars Reconnaissance Orbiter (Image credit: NASA/JPL-Caltech/ University of Arizona) (b) sublimation of ice (enclosed in white boxes) in the trench informally called “Dodo-Goldilocks” over the course of four days (June 15 and 19, 2008) as imaged by the Phoenix Mars Lander’s Surface Stereo Imager (Image credit: NASA/JPL-Caltech/University of Arizona/Texas A&M University), (c) Martian south pole as imaged by the Mars Global Surveyor (MGS) Mars Orbiter Camera (MOC) on April 17, 2000 (image credit: NASA).
48 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Fig. 2. (a) Dark, narrow streaks (recurring slope lineae
EARTH MARS Average diurnal surface temperature range (K) 283 to 293 184 to 242 Average surface pressure (kPa) 101.400 0.636 (seasonally variable from 0.400 to 0.870) Surface gravity (m/s2) 9.79 3.71 Escape velocity (km/s) 11.19 5.03 Atmospheric composition Major (vol.%): 78.08% N2, 20.95% O2, >1% H2O (highly variable) Minor: 9340 ppm Ar, 410 ppm CO2, 18.18 ppm Ne, 5.24 ppm He, 1.7 ppm CH4, 1.14 ppm Kr, 0.55 ppm H2 Major (vol.%): 95.1% CO2, 2.59% N2, 1.94% Ar, 0.16% O2, 0.06%
(MOXIE produces no fuel). These counterintuitive results, where brine electrolysis is more performant at -36 oC compared to DI water electrolysis at 50 oC (experimental result) and low-temperature CO2 electrolysis is competitive with SOXE systems on an energy-efficiency basis (model prediction), demonstrate the unusual electrochemistry resulting from the use of highly concentrated perchlorate brine electrolytes and suggest multiple avenues of scientific and technical enquiry.


The Opportunities That Lie Ahead
Typically, low temperature operation of chemical or electrochemical processes is not preferred due to the expected decline in performance (ionic conductivity, diffusivity, electrode kinetics, etc.) predicted by Arrhenius-type relationships. Fortuitously, CO2 reduction on Cu catalysts in perchlorate brine has been found to follow anti-Arrhenius kinetics with increasing kinetic currents and reduced onset potentials at lower temperatures.16 Even more interestingly, the selectivity for CH4 has been found to increase in inverse relationship to the temperature.16 Similarly, belying expectations from Arrhenius theory, brine electrolyzers did not exhibit any dramatic changes in separator resistance when operated at -36 oC. These unexpected results, in conjunction with the lack of kinetic and transport data for a wide variety of electrochemical systems at both sub-zero temperatures
and in highly concentrated electrolytes, points to a rich vein of scientific inquiry under these conditions (especially given increasing technological interest in electrochemical engineering under such conditions) and we speculate that the effect of solvation and its impact on charge transfer and transport will prove to be particularly important in such systems. Some other interesting scientific questions and technical challenges around Martian ISRU are summarized in Fig. 4. Overall, the development of low-temperature electrolytic ISRU promises to enable humanity to reach for the stars and provide insights that will accelerate electrochemical solutions for pressing terrestrial problems.
Acknowledgements
The authors thank the University of Texas at San Antonio for supporting this work. Partial support from NASA grant # 80NSSC22K1766 is also gratefully acknowledged. Washington University in St. Louis and the University of Texas at San Antonio are pursuing patent protections on aspects of the technologies discussed here.
© The Electrochemical Society. DOI: 10.1149/2.F09232IF
About the Authors
Shrihari “Shri” Sankarasubramanian, Assistant Professor, Department of Biomedical Engineering and Chemical Engineering, The University of Texas at San Antonio

Education: BE in Chemical Engineering (Visvesvaraya Technological University, India); PhD in Chemical Engineering (Illinois Institute of Technology).
Research Interests: Shri’s main area of interest is in the development of electrochemical energy conversion and storage devices combining fundamental physical chemistry, materials development, device engineering, and scale-up. His lab is currently working on further developing the Ti-Ce redox flow battery he invented as a postdoc and at realizing the CO2-brine electrolyzer for Martian ISRU. His efforts are supported by the DOE, ARPA-E, and NASA.
Work Experience: 2021 – present, Assistant Professor at UTSA; 2017 – 2021, (successively) Senior Staff Research Scientist, Research Scientist and Postdoctoral Research Associate at Washington University in St. Louis.
Work with ECS: Travel grant reviewer and student award reviewer for the IE&EE division over multiple application cycles.
(continued on next page)
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 49
Fig. 4. Open questions and opportunities in low-temperature electrolytic ISRU on Mars.
Fig. 3. Representations of Martian ISRU electrolyzer developed by the authors: (a) Mg(ClO4)2 brine electrolyzer,13 (b) CO2-brine electrolyzer.12
Sankarasubramanian et al. (continued from previous page)
Awards: 2023 Young Investigator Spotlight Symposium Invitee - American Chemical Society (ACS) Energy and Fuels (ENFL) Division (Spring 2023 meeting); 2021 University of Texas system Rising STARs (Science and Technology Acquisition and Retention) award.

Pubs + Patents: 33 publications, 3 patents issued (6 patent applications pending).
Website: https://ceid.utsa.edu/team/shrihari-sankarasubramanianph-d/ https://orcid.org/0000-0002-0338-4009
Bradley Chambers, Graduate Researcher, Biomedical Engineering, University of Texas at San Antonio

Education: BS in chemical engineering (University of Illinois at Chicago); PhD candidate in Biomedical Engineering (University of Texas at San Antonio)

Research Interests: Space technology, Electrochemistry, Genetic engineering, Biopharmaceuticals

Work Experience: 8/2021– Present: Graduate Research Assistant, Department of Biomedical Engineering and Chemical Engineering, The University of Texas at San Antonio; 8/2021 – 5/2022: Graduate Teaching Assistant, Department of Chemical and Biomedical Engineering, Illinois Institute of Technology; 1/2019 – 4/2021: Lead Night Technician, Ford Chicago Assembly Plant, Ecolab; 6/2017
– 9/2017: Summer Intern, Civil Engineering, Moran Engineering; 10/2016 – 6/2017: Lab Safety Inspector, Environmental Health and Safety Office, University of Illinois at Chicago; 5/2014 – 10/2014: Research Intern, Water Treatment Laboratory, University of Illinois at Chicago.
Awards: 2019 Technician Safety Award – Ecolab; 2018 Best in Category – University of Illinois: Senior Engineering Expo; 2014, 2016 Honors scholarship, University of Illinois at Chicago. Pubs + Patents: M. Shahid, B. Chambers, and S. Sankarasubramanian, AIChE Journal (2022).
Cheyenne Wilson, Undergraduate Research Assistant, University of Texas at San Antonio

References
1. The Planetary Society. https://www.planetary.org/spacemissions/every-mars-mission (2022) accessed May 13, 2022.
2. Reuters, https://www.reuters.com/business/aerospace-defense/ china-plans-its-first-crewed-mission-mars-2033-2021-06-24/ (2021) accessed on April 3, 2023.
3. NASA, https://www.nasa.gov/sites/default/files/atoms/files/ moon-investments-prepare-us-for-mars.pdf (2022) accessed on April 3, 2023.
4. NASA Glenn Research Center, https://www.grc.nasa.gov/ www/k-12/rocket/rktwtp.html accessed on April 4, 2023.
5. D. Petit, https://www.nasa.gov/mission_pages/station/ expeditions/expedition30/tryanny.html#:~:text=The%20 Saturn%20V%20payload%20to,Shuttle%20was%20only%20 about%201%25, (2012) accessed on April 4, 2023.
6. B. G. Drake, https://www.nasa.gov/pdf/373665main_NASASP-2009-566.pdf (2009) accessed May 13, 2022.
7. D. R. Williams, https://nssdc.gsfc.nasa.gov/planetary/factsheet/ marsfact.html, accessed May 10, 2022.
8. M. Hecht, J. Hoffman, D. Rapp, et al., Space Sci Rev, 217, 9 (2021)
9. L. Ojha, M. B. Wilhelm, S. L. Murchie, et al., Nat. Geosci., 8(11), 829 (2015).
10. M. H. Hecht, S. P. Kounaves, R. C. Quinn, et al., Science, 325(5936), 64 (2009)
11. S. E. Lauro, E. Pettinelli, G. Caprarelli, et al., Nat. Astron., 5, 63 (2020)
12. M. Shahid, B. Chambers, and S. Sankarasubramanian, AIChE Journal (2022) DOI: 10.1002/aic.18010
13. P. Gayen, S. Sankarasubramanian, and V. Ramani, PNAS, 117(50), 31685 (2020)

14. S. Sankarasubramanian, P. Gayen, and V. Ramani, The Chemical Engineer (IChemE), 957 (2021)
15. J. Parrondo, M. George, C. Capuano, et al., J Mater Chem A, 3(20), 10819 (2015)
16. E. Sargeant, A. Kolodziej, C.S. LeDuff, and P. Rodriguez, ACS Catal., 10, 7464 (2020)
17. E. J. Taylor and G. S. Jackson, ECS Interface, 30(2), 79 (2021)
www.electrochem.org
Considerations for Electrochemical Phosphorus Precipitation: A Figures of Merit Approach
 by Kody D. Wolfe, Ardavan Zanganeh, Richard N. Arthur, Jason P. Trembly, and Damilola A. Daramola
by Kody D. Wolfe, Ardavan Zanganeh, Richard N. Arthur, Jason P. Trembly, and Damilola A. Daramola
Phosphorus (P) recovery from treated wastewater, agricultural discharge, and stormwater is a critical challenge in reducing our dependency on first-use natural resources as the population grows and per-capita energy consumption continues to rise.1,2 Significant reserves of naturally occurring P are sequestered in geographically isolated areas and the utilization of P varies extensively across the globe, resulting in myriad P recovery needs and scenarios.1,3,4 Applying chemical fertilizer to meet growing food supply demands exacerbates P demand due to over-application and slow kinetics of natural P fixation, resulting in poor nutrient use efficiency. Nutrient recovery is also necessary to mitigate environmental issues such as the eutrophication of freshwater ecosystems due to fertilizer runoff.4 For these reasons, recovery of P and other nutrients from waste streams is of significant international interest due to a combination of localized concentration, the growing difference between the rate of natural P fixation and anthropogenic P mining/extraction, and the continued accumulation of mined/extracted P in waterbodies.5
Existing methods for P recovery from aqueous phases are based upon either chemical precipitation or P adsorption, with integrated biotechnology for improved processing.6–8 These methods have seen the most significant development in Japan and the European Union as indicated by databases of commercially deployed technologies and technologies under development curated by the European Sustainable Phosphorus Platform.9 Electrochemical P precipitation (EPP) targets the same goal as the above-mentioned recovery processes, which is to build a circular economy around using P and other major nutrients; however, the electrochemical methods have specific advantages.
First, utilizing electricity as a reactant expands the sustainability of P recovery by enabling the use of renewable energy sources, further reducing the total carbon footprint. Secondly, due to their modular configuration, electrochemical systems can be applied to distributed waste discharges (e.g., rural water treatment facilities or small-to-midsize farms), negating the high capital costs associated with economies of scale in traditional chemical processes. Operational costs are also expected to be lower. EPP is typically pH-driven; therefore the operational costs can be based on the cost of hydroxide delivery, which is $0.025/OH- when supplied via NaOH dosing (at $625/t) versus $0.0044/OH- when generated from electrons (at $0.083/kWh and 2.0V).10–12 Lastly, adopting integrated process controls common to energy conversion systems can lower the barrier to the required domain knowledge for the implementation of EPP.
Currently, EPP processes reported in the literature span a wide range of P recovery efficiencies (40–90%) and associated energy use (3–2238 kWh.kg -1 P).1,6 Fig. 1 below shows the energetic performance of reported EPP processes (blue ellipses) in comparison to operating chemical P recovery processes (red ellipses).6,9 Notice that Fig. 1 includes both the a) specific energy and the b) volumetric energy versus the P conversion. The importance and implications of such reporting metrics will be further discussed. The widely varying EPP values highlight the need for carefully considered benchmark metrics and the diversity of EPP technologies. Fig. 1 shows that a gap exists both among the current EPP technologies and between these technologies and industry because only a small minority of EPP technologies fall within the operational energetic region of (continued on next page)
6,9
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 51
Fig. 1. Literature values for a) Specific energy and b) Volumetric energy plotted against P conversion (equivalent to P recovery) for P recovery technologies. Blue ellipses indicate electrochemical processes found in the literature and red ellipses are reported values from operating chemical P recovery systems. Ellipse vertices were defined as the low and high bounds of reported values for each study or report.
Daramola et al.
(continued from previous page)
the currently implemented chemical processes. To address this deficiency and to expedite the development of EPP, figures of merit (FOM) specific to electrochemical engineering may be used while also considering the diverse P recovery conditions and challenges of operating at scale.
Figures of Merit in Electrochemical Engineering
FOM are quantitative metrics used to express specific characteristics of a process or instrumentation.13 Further, FOM are simple and effective means of reporting experimental results relevant to specific applications. FOM are essential when comparing competing technologies and considering their viability for scaleup to industrial application.13–16 Unsurprisingly, the use of FOM in industrial electrochemistry and electrochemical engineering (IE&EE) has been widely discussed. Fig. 2 highlights FOM collated from various sources used for electrochemical reactors and systems.2,13–16 The FOM in Fig. 2 are organized such that quantities easily measured at a lab or benchtop scale begin at the left and progress in complexity to the right, where FOM typically used for large-scale or continuous reactors are shown. As an electrochemical system is scaled, the relevant FOM change and generally increase in number and complexity due to an increase in design variables.15 A handful of FOM are ubiquitous in electrochemical systems, such as charge efficiency (ϕ), while others are specific to reactor designs or applications.16 Indeed, not all of the FOM presented in Fig. 2 apply
to EPP studies, primarily because these technologies are currently at low technology readiness levels (TRLs).1 Therefore, investigators must carefully consider, discuss, and choose the relevant FOM. This article aims to outline FOM for EPP systems, consider how they can be used to compare EPP with other P recovery technologies, and give recommendations on relevant reactor conditions.
FOM Considerations in Reporting Electrochemical P Recovery
Expressing the viability and performance of EPP technologies requires defining potential competitive advantages with respect to the product being delivered. Foremost, the product must be specifically chosen and well-defined. For example, when the focus of implementing EPP is on P resource recovery or waste conversion, a gravimetric figure of merit based upon the P product is most relevant. However, when the performance is based upon the volume of the waste stream being treated, a volumetric figure of merit should be chosen. Additionally, careful consideration is necessary when reporting FOM for emerging technologies such as EPP systems because subtle differences in calculations and assumptions can skew the perceived success, especially in early studies. The following sections discuss the choice and adaptation of select FOM from Fig. 2 for use when reporting on EPP technologies.
Recovery Efficiency
The recovery efficiency of P from an input stream is a necessary and often-reported metric, and, as previously mentioned, the range of P recovery for EPP technologies is vast. Likely causes for the wide-ranging values include differing conditions and experimental methods; however, another important consideration is the means of calculation. The primary goal of reporting P recovery is to describe the extent of reaction; however, multiple FOM exist for describing this quantity. For example, P recovery may be determined via dividing P in the outlet stream by that of the P in the inlet stream (conversion of P). Reporting the conversion of P is one essential figure of merit; however, the selectivity or yield of a particular product, such as struvite, may be more important in other scenarios. Considering the conversion (X), selectivity (S), and yield (Y) of a P recovery reaction is critical regardless of the method used for P precipitation and these FOM can be readily used for comparison between purely chemical, electrochemical, or other processes for recovery. Care should be taken when reporting speciesspecific values. Specifically, the molecular weight of the species or the chemical formula should be included in the discussion so the values can be converted to compare to other work (e.g., converting from P2O5 yield to P yield).1 The ease of measuring X, S, and Y makes them excellent FOM for technologies in the early stages of development, and careful calculation, reporting, and discussion of these FOM allow for clear and concise descriptions of the achieved reaction.
52 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Variables & Constants Reactor Design Normalized Electrode Area (Reactor/Electrode) Mass Transport Coeff. & Area Normalized Mass Transport Coeff Reynolds, Sherwood, & Schmidt Numbers Space Time & Space Velocity Space Time Yield Energetics & Efficiency Current/Charge Efficiency Cell Potential Specific Energy Consumption Volumetric Energy Consumption Energy Efficiency Notes Generic Equation Assumptions Mass transfer limited conditions Normalized to a specified conversion (typically 90%) Extent of Reaction Conversion Yield Selectivity
Fig. 2. FOM used in electrochemical engineering. The FOM are organized to show the transition from benchtop/ lab scale (low TRLs) on the left to pilot scale on the right. This is not an exhaustive list and the relevance of each FOM, as well as possible adaptations for specific applications, should be considered when reporting results.2,13–16
Specific and Volumetric Energy Consumption
Perhaps the most industrially relevant figure of merit for reporting on EPP is the energetic cost of the product.1 The energetic cost is then used to evaluate EPP technologies in comparison to traditional P recovery methods. There are many variables to consider when determining a process’s energy consumption, and many may not be accurately accounted for in the case of laboratory or benchscale processes. In the case of EPP aimed at producing a particular product, one recommended approach is to begin with the specific energy calculation with respect to electrical energy consumption (see Fig. 2) and then to adjust this value to account for unavoidable costs related to the processing scenarios. For example, the cost of a reactant may be included as an embodied energy value to better compare electrochemical methods with chemical precipitation, whose cost is primarily based upon the required feed chemicals.9,17,18 Any consumables necessary to achieve conversion of P to the desired product may be included as embodied energy values. Equation (1) shows a formula that can be used to calculate an adjusted specific energy that includes the embodied energy of any consumed reactants (EEreact.) in addition to the consumed electrical energy during the reaction. A specific example used in this article includes the EE of magnesium chloride (MgCl2) as the magnesium source for EPP via struvite precipitation (Fig. 3b). Equation (1) is leveraged in Fig. 3 as an example of how adjusted FOM can be used to evaluate EPP technologies. For more developed EPP technologies, the environmental cost of reactants and waste products may be included to provide a holistic comparison with current wastewater treatment practices.19
As previously mentioned, another important consideration when reporting specific energy values is the choice of reporting with respect to the product generated (kWh.kgP−1) or with respect to volumetric throughput (kWh.m-3). The choice is likely dependent on the target of the proposed technology and comparison to state-of-the-art processes. Reporting the kWh.kgP−1 for a process facilitates comparison to the process energy required to produce or the embodied energy within a commercial P-based fertilizer.1,18 Delivering solid phosphorus fertilizers such as phosphate rock, triple superphosphate, and diammonium phosphate requires process energetics between 0.21 and 4.5 kWh.kgP−1 , 20 and embodied energetics between 2.5 and 16 kWh. kgP−1 . 21 Therefore, the competitive advantage of the final product from an emergent P recovery technology can be expressed with respect to these commercial costs and sustainability metrics. On the other hand, reporting the kWh.m-3 treated indicates energy consumption related to the process throughput and facilitates comparison to alternative unit operations on the same stream. The energy consumed in the treatment of sludge-related streams, typically where P recovery technologies are located, is approximately 0.04 kWh.m-3 19,22
Current Efficiency
The current or charge efficiency (ϕ) is used to report electron transfer efficiency to the target product in electrochemical engineering. However, in the case of pH-driven precipitation, the basis of most EPP technologies, determining ϕ is complicated by the intermediate steps between electrochemical hydroxide (OH-) generation and the product precipitation. Therefore, assumptions based upon the expected solution chemistry must be made to approximate the stoichiometry between OH- and the product. For example, equation (2) can be used to determine ϕ based on the actual charge passed (q) and the moles of struvite (MgNH4PO4•6H2O) collected, assuming all OH- produced electrochemically results in formation. However, a more thorough approach includes the effects of phosphate ion speciation, based on a Bjerrum plot, to determine the stoichiometric OH- required first to produce the necessary phosphate ions and then to precipitate the product. For example, at 25°C and an initial pH of 6.9, orthophosphate ions speciate as 32% HPO42− and 68% H2PO4− on a molar basis. Under these conditions it can be assumed, by ignoring intermediate phosphate species, that only 32% of the currently
available orthophosphate can be immediately precipitated. The remaining 68% will require OH- to stoichiometrically convert H2PO4 to HPO42− prior to precipitation of MgNH4PO4•6H2O. To summarize, both the pH and the assumptions made will greatly affect ϕ and the effects of solution chemistry should be given extra attention when considering the efficiency of an EPP reactor system.
Regardless of the feasibility of determining ϕ, the implications of imperfect charge transfer must be considered when scaling an electrochemical process. Fig. 3 shows a comparison of the effects of ϕ on the specific energy of P recovery as struvite when a) only the electrical energy used during the electrochemical reaction is considered and b) when the EE of the magnesium source (MgCl2) is included, via equation (1), to determine the adjusted specific energy. The data shown in Fig. 3 is based on a thermodynamic model of P precipitation as struvite at a P conversion of 90% and an EE of MgCl2 of 2.06 kWh.kgMgCl2 or 0.80 kWh.kgstruvite at various solution phase magnesium to phosphorus ratios (Mg:P).21 Several observations can be drawn from the effects of the hypothetical ϕ in Fig. 3. First, the effect of ϕ on the specific energy decreases as ϕ approaches unity in either case, highlighting the importance of ϕ, yet showing that incremental optimization of ϕ may not be a leading factor in improving the energy efficiency of EPP. Second, including the EE of MgCl2 (Fig. 3b) increases the specific energy of P recovery. This effect is important in comparing to the currently implemented chemical processes shown in Fig. 1 (red ellipses), because the energetic costs of reactants are the primary cost for chemical precipitation. Lastly, the behavior of specific energy for P recovery across the Mg:P ratios invert between the two cases. That is, when only electrical energy is considered, high Mg:P ratios have lower specific energetic costs, while when the energetic cost of the MgCl2 is considered, high Mg:P ratios have higher specific energy costs of P recovery. The observations from Fig. 3 show that although ϕ may be difficult to elucidate from experimental results, the efficiency of the electrochemical charge transfer is an insightful consideration in optimizing EPP technologies.
Mass Transport Considerations
Scaling electrochemical reactions from benchtop to pilot scale is a resource-intensive endeavor. A primary topic in scale-up is the mass transfer behavior within the reactor because scaling inevitably demands reactor design changes to optimize efficiency and throughput.15 Therefore, knowledge of and reporting of FOM that are useful in determining mass transport scenarios is critical for technologies at low TRLs. As seen in Fig. 2, the mass transfer coefficient, normalized mass transfer coefficient, and the Reynolds, Sherwood, and Schmidt numbers are useful FOM for electrochemical technologies currently at the benchtop scale (such as a stirred batch reactor). Determining and studying these FOM will allow researchers to better understand the limitations of the technology and the key areas for improvement or optimization before scaling to large, continuous flow reactors.
Belarbi et al. showed the relevance of using dimensionless fluid mechanics numbers and the mass transfer coefficient to compare a rotating cylinder electrode geometry to a stirred reactor for EPP. Their analysis showed the ability to compare results from a smallscale electrode geometry to that of a more complex system quickly and easily. Building upon this concept, we have modeled momentum and charge transport in COMSOL to visualize fluid dynamics and electroactive species concentrations in a stirred batch reactor (Fig. 4). The use of modeling tools can be coupled with FOM to enable faster technology optimization at the bench scale and to elucidate areas for further research efforts. For example, in Fig. 4b a depletion of ferricyanide is seen on the left side of the working electrode (dark blue region), highlighting a unique region for mass transfer within this simplified system. Electrochemical reactor modeling enables testing of conditions ranging from active species concentrations to electrode geometries, and even temporal behavior.
(continued on next page)
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 53
Adjusted EE kWhkgEEkWh kg P S P S Preact P (. )( 11.) (1)
(2)
–1 –1
Daramola
(continued from previous page)
Fig. 3. The effects of charge efficiency (ϕ) on the a) electrical (or electrochemical) specific energy of recovery of P (EP S)and b) the adjusted specific energy of recovery of P (Adjusted EPS) using MgCl2 as a magnesium source across a range of solution phase Mg:P ratios. The data shown herein is a result of a thermodynamic model of struvite precipitation from synthetic wastewater via electrochemical hydroxide production using OLI as the modeling platform and includes the electricity used to drive the electrochemical precipitation and the embodied energy of MgCl2 (2.06 kWh.kgMgCl2), according to Equation (1).21
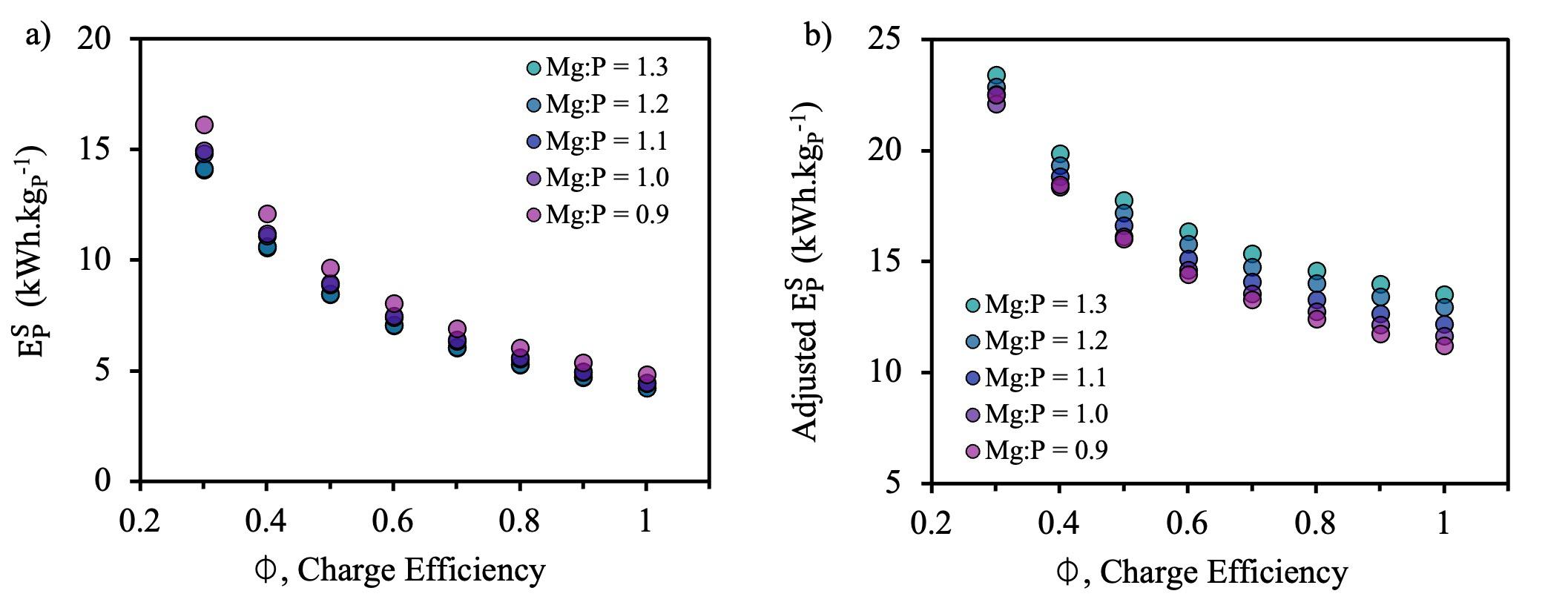
Concluding Remarks
The use of established FOM and the adaptation of FOM for EPP can improve comparisons between P recovery technologies, expedite scale-up, and yield valuable insight into reactor design and optimization. The FOM approach also provides a useful template for integrating fundamental electrochemical engineering principles into other innovative and emerging electrochemical technologies such as energy storage,23 CO2 utilization,24 and decarbonized manufacturing.25 Lastly, we emphasize that leveraging FOM can help accelerate the adoption of electrochemical technologies by providing benchmarks for comparison between techniques and across TRLs.
Acknowledgements
This material is based upon work supported by the US Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) under the Advanced Manufacturing Office, Research and Development for Advanced Water Resource Recovery Systems award number DE-EE0009502. The views expressed herein do not necessarily represent the views of the US Department of Energy or the United States Government.
© The Electrochemical Society. DOI: 10.1149/2.F10232IF

54 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Fig. 4. Modeling an electrochemical batch reactor using COMSOL Multiphysics (Version 6.0, CFD and Fuel Cell & Electrolyzer Modules) to visualize the qualitative relationships between impeller rotational speed, mass transport conditions, and distribution of active species. a) Electrolyte velocity at steady state conditions and b) ferricyanide concentration at steady state conditions. Initial concentration of both ferri/ferrocyanide was 10 mol.m-3. The reactor conditions were 400 rpm impeller speed, 40 ℃, and the applied potential at the working electrode is -1.3 vs Ag/AgCl. The working electrode is at left and the counter (grounded) is at right. et al.
About the Authors
Kody D. Wolfe, Research Engineer, Chemical & Biomolecular Engineering, Institute for Sustainable Energy and the Environment, Ohio University

Education: BS in Chemical Engineering (Ohio University); PhD in Materials Science & Engineering (Vanderbilt University).
Research Interests: Energy, Electrode material design, Electron transfer Work Experience: Electrochemical Engineer (Prieto Battery, Inc.)
Awards: ECS H. H. Uhlig Summer Fellow (2020)
Pubs: 5+ peer-reviewed articles. https://orcid.org/0000-0002-6452-0107
Ardavan Zanganeh, PhD Student, Chemical & Biomolecular Engineering, Institute for Sustainable Energy and the Environment, Ohio University

Education: BS in Chemical Engineering (Amirkabir University of Technology); MS in Chemical Engineering (University of Tehran)
Research Interests: Mathematical modeling, Transport phenomena, Electrochemical engineering
Pubs: 2 peer-reviewed articles
Richard N. Arthur, PhD Student, Chemical & Biomolecular Engineering, Institute for Sustainable Energy and the Environment, Ohio University

Education: BS in Chemical Engineering (Kwame Nkrumah University of Science and Technology); Master of Business Administration (Accra Business School).
Research Interests: Modeling and simulation, Electrochemical engineering, Reaction engineering, Energy and environmental sustainability, Chemical products development, Water and wastewater treatment
Work Experience: Electrochemical Process Engineer (Volta Aluminum Company Ltd., Ghana)
Jason P. Trembly, Russ Professor of Mechanical Engineering and Director, Institute for Sustainable Energy and the Environment, Ohio University
Education: BS in Chemical Engineering (Ohio University); PhD in Chemical Engineering (Ohio University).
Research Interests: Solid oxide fuel cells, Electrochemical nutrient recovery, Sustainable building materials
Work Experience: Team Leader and Research Chemical Engineering (RTI International) and ORISE Fellow (U.S. Department of Energy-National Energy Technology Laboratory).
Awards: ORISE Fellowship
Pubs + Patents: 40+ publications, 10+ patents.
Website: https://www.ohio.edu/engineering/isee https://orcid.org/0000-0002-9851-2914
Damilola A. Daramola, Assistant Professor, Chemical Engineering and Assistant Professor, Chemistry and Chemical Biology, Northeastern University


Education: BS in Chemical Engineering (Ohio University); PhD in Chemical Engineering (Ohio University).
Research Interests: Energy conversion, Resource recovery, and Phenolic composites
Work Experience: Assistant Professor, Chemical and Biomolecular Engineering and Assistant Director, Institute for Sustainable Energy and the Environment (Ohio University); Technical Business Development (Ohio University); Research Chemist Intern (Ashland Chemical Inc.)
Work with ECS: ECS Member since 2007, Webmaster for Ohio University ECS Student Chapter (2011), Education Committee (2023)
Awards: Ralph E. Powe Junior Faculty Enhancement Award (2022), NextProf Nexus Participant (2019), Carl Storm Underrepresented Minority Fellow (2018), DOW Best Symposium Participant (2013), and ECS Dokiya Fund Travel Grant (2007, 2009).
Pubs: 10+ peer-reviewed articles, 1 book chapter, 40+ presentations. https://orcid.org/0000-0002-6737-415X



References
1. D. A. Daramola and M. C. Hatzell, ACS Energy Lett., 8, 1493 (2023)
2. Z. Belarbi, D. A. Daramola, and J. P. Trembly, J. Electrochem. Soc., 167, 155524 (2020).
3. D. J. Batstone, T. Hülsen, C. M. Mehta, and J. Keller, Chemosphere, 140, 2 (2015)
4. C. Langhans, A. H. W. Beusen, J. M. Mogollón, and A. F. Bouwman, Nat. Sustain., 5, 57 (2022)
5. P. J. A. Withers, J. J. Elser, J. Hilton, H. Ohtake, W. J. Schipper, and K. C. van Dijk, Green Chem., 17, 2087 (2015)
6. Y. Wang, P. Kuntke, M. Saakes, R. D. van der Weijden, C. J. N. Buisman, and Y. Lei, Water Res., 209, 117891 (2022)
7. R. Hirota, K. Motomura, and A. Kuroda, in Phosphorus Recovery and Recycling, H. Ohtake and S. Tsuneda, Editors, p. 499, Springer, Singapore (2019)
8. W. Fan, L. Bryant, M. Srisupan, and J. Trembly, Clean Technol. Environ. Policy, 20, 1467 (2018).
9. European Sustainable Phosphorus Platform, Last Accessed: March 11th, 2023 (2023)
10. Monthly Electric Power Industry Report, U.S. Energy Information Administration, Last Accessed: April 1st, 2023



11. K. Hays, SP Glob. Commod. Insights (2023).
12. R. M. Navarro, R. Guil, and J. L. G. Fierro, in Compendium of Hydrogen Energy, Woodhead Publishing Series in Energy. V. Subramani, A. Basile, and T. N. Veziroğlu, Editors, p. 21, Woodhead Publishing, Oxford (2015).
13. C. M. Sánchez-Sánchez, E. Expósito, J. Solla-Gullón, V. GarcíaGarcía, V. Montiel, and A. Aldaz, J. Chem. Educ., 80, 529 (2003)
14. S. C. Perry, C. Ponce de León, and F. C. Walsh, J. Electrochem. Soc., 167, 155525 (2020)
15. D. Pletcher and F. C. Walsh, Industrial Electrochemistry, Springer Dordrecht, (1993).
16. F. Walsh and G. Reade, Analyst, 119, 791 (1994)
17. C. Kabbe, Sustainable sewage sludge management fostering phosphorus recovery and energy efficiency, Last Accessed: March 11th, 2023 (2015).
18. B. Butler, Struvite or Traditional Chemical Phosphorus Precipitation—What Option Rocks, Australian Meat Processor Corporation, (2018).
19. S. Çapa, A. Özdemir, Z. Günkaya, A. Özkan, and M. Banar, J. Water Process Eng., 49, 103002 (2022)
20. T. K. Jenssen and G. Kongshaug, Energy Consumption and Greenhouse Gas Emissions in Fertiliser Production, International Fertiliser Society (IFS), (2003).
21. M. G. Bhat, B. C. English, A. F. Turhollow, and H. O. Nyangito, Energy in synthetic fertilizers and pesticides: Revisited, Oak Ridge National Lab., TN (United States); Tennessee Univ., Knoxville, TN (United States). Dept. of Agricultural Economics and Rural Sociology, (1994)
22. J. C. Pasqualino, M. Meneses, and F. Castells, J. Ind. Ecol., 15, 49 (2011)
23. E. E. Michaelides, Energies, 14 (2021).
24. R. Chauvy and G. De Weireld, Energy Technol., 8, 2000627 (2020)
25. J. Peng, D. Schwalbe-Koda, K. Akkiraju, et al., Nat. Rev. Mater., 7, 991 (2022)
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 55
ECS, a prestigious nonprofit professional society, has led the world in electrochemistry, solid state science and technology and allied subjects since 1902, providing a rigorous and high-quality home for the whole community.
ECS is dedicated to moving science forward by empowering researchers globally to leave their mark on science. The Society connects a diverse and representative constituency of members and nonmembers to accelerate scientific discovery, facilitate the engagement of an inclusive network, and champion the dissemination of research to support a sustainable future.
For more information on becoming a member, or publishing in ECS publications, visit electrochem.org
56 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
SECTION NEWS SECTION NEWS
Section Name
Section Leadership

Section Chair
Arizona Section Candace Kay Chan, Chair
Brazil Section
Raphael Nagao, Chair
Canada Section Heather Andreas, Chair
Chile Section Jose H. Zagal, Chair
China Section Yongyao Xia, Chair
Detroit Section Erik Anderson, Chair
Europe Section Philippe Marcus, Chair
Georgia Section Seung Woo Lee, Chair
India Section Sinthai A. Ilangovan, Chair
Israel Section Daniel Mandler, Chair
Japan Section Yasushi Idemoto, Chair
Korea Section Won-Sub Yoon, Chair
Mexico Section Carlos E. Frontana-Vázquez, Chair
Mid-America Section Nosang Vincent Myung, Chair
National Capital Section Chunsheng Wang, Chair
New England Section Sanjeev Mukerjee, Chair
Pacific Northwest Section Corie Cobb, Chair
Pittsburgh Section Open
San Francisco Section Gao Liu, Chair
Singapore Section Zichuan J. Xu, Chair
Taiwan Section Chi-Chang Hu, Chair
Texas Section Jeremy P. Meyers, Chair
Twin Cities Section Victoria (Vicki) Gelling, Chair
BioLogic ........................................................................... 4 ECS Transactions 243rd ECS Meeting inside back cover El-Cell............................................................................. 19 Gamry 10 Ion Power 6 IOP back cover Pine Research Instrumentation 2 Scribner Associates .......................................................... 1 Wiley 40 Advertisers Index The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 57 SECTION NEWS
NEWS
SECTION
more about ECS sections at www.electrochem.org/sections
Learn
AWARDS PROGRAM AWARDS PROGRAM
Awards, Fellowships, Grants
ECS distinguishes outstanding technical achievements in electrochemistry, solid state science, and technology, and recognizes exceptional service to the Society through the Honors & Awards Program. Recognition opportunities exist in the following categories: Society Awards, Division Awards, Student Awards, and Section Awards. ECS recognizes that today’s emerging scientists are the next generation of leaders in our field, and offers competitive Fellowships and Grants to allow students and young professionals to make discoveries and shape our science long into the future.
See
Society Awards
Charles W. Tobias Young Investigator Award: Established in 2003 to recognize outstanding scientific and/or engineering work in fundamental or applied electrochemistry or solid-state science and technology by a young scientist or engineer, the award consists of a framed certificate, $5,000 prize, Society Life Membership, complimentary meeting registration, and travel support. Materials deadline: October 1, 2023
ECS Toyota Young Investigator Fellowship: Established in 2015 in partnership with the Toyota Research Institute of North America, the award encourages young professionals and scholars to pursue research into batteries, fuel cells and hydrogen, and future sustainable technologies. Each year, at least one candidate receives the fellowship restricted grant of no less than $50,000 to conduct the proposed research within one year, and a one-year complimentary ECS membership. Recipients must present at a Society biannual meeting and publish their research in a relevant ECS journal within 24 months of receiving the award. Materials deadline: January 31 annually
Edward Goodrich Acheson Award: Established in 1928 for distinguished contributions to the advancement of any of the objects, purposes, or activities of The Electrochemical Society, the award consists of a gold medal; a plaque with a bronze replica of the medal; $10,000 prize; Society Life Membership; and complimentary meeting registration. Materials deadline: October 1, 2023.

Fellow of The Electrochemical Society: Established in 1989 for advanced individual technological contributions in the field of electrochemical and solid state science and technology, and active membership and involvement in The Electrochemical Society, the award consists of a framed certificate and lapel pin. Materials deadline: February 1, 2024
Leadership Circle Awards: Established in 2002 to honor and thank our partners in electrochemistry and solid state science, these awards are granted in the year that an institutional member reaches a





milestone level. Awardees receive a commemorative plaque and recognition on the ECS website and in Interface magazine. Nominations not accepted

Division Awards
Corrosion Division H. H. Uhlig Award: Established in 1972 to recognize excellence in corrosion research and outstanding technical contributions to the field of corrosion science and technology, the award consists of a framed certificate; $1,500 prize; and travel assistance. Materials deadline: December 15, 2023
Corrosion Division Rusty Award for Mid-Career Excellence: Established in 2021 to recognize a scientist or engineer’s mid-career achievements and contributions to the field of corrosion science and technology, the award consists of a framed certificate; $1,000; and possibly travel expenses and meeting registration. Materials deadline: December 15, 2023
Electronics and Photonics Division Award: Established in 1969 to encourage excellence in electronics research and outstanding technical contribution to the field of electronics science, the award recognizes authors who have made noteworthy scientific contributions and enhanced the scientific stature of the Society in the areas of physics, chemistry, and metallurgy of electronic materials and devices by presenting well received papers in Society journals and meetings. The award consists of a scroll; $1,500 prize; and ECS Life Membership or up to $1,000 in meeting travel expenses. Materials deadline: August 1, 2023
Energy Technology Division Research Award: Established in 1992 to encourage excellence in energyrelated research, the award consists of a framed certificate; $2,000 prize; and ECS Energy Technology Division membership for the duration of the recipient’s ECS membership. Materials deadline: September 1, 2023
Energy Technology Division Supramaniam Srinivasan Young Investigator Award: Established in 2011 to recognize and reward an outstanding young researcher in the energy technology field, the award consists of a framed certificate; $1,000 prize; and complimentary meeting registration. Materials deadline: September 1, 2023
58 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
www.electrochem.org/awards for more information.
highlights below and visit
AWARDS AWARDSAWARDSPROGRAM AWARDS PROGRAM
Nanocarbons Division Richard E. Smalley Research Award: Established in 2006 to encourage excellence in fullerenes, nanotubes, and carbon nanostructures research, the award recognizes researchers who have made outstanding contributions to the understanding and applications of fullerenes. The award consists of a framed certificate; $1,000 prize; and up to $1,500 in travel assistance. Materials deadline: September 1, 2023
Nanocarbons Division SES Research Young Investigator Award: Established in 2007 to recognize one outstanding young researcher in the field of fullerenes, carbon nanotubes, and carbon nanostructures, the award consists of a framed certificate, $500 prize, and complimentary meeting registration. Materials deadline: September 1, 2023


Physical and Analytical Electrochemistry Division

David C. Grahame Award: Created in 1981 to encourage excellence in physical electrochemistry research and to stimulate publication of high-quality research papers in the Journal of The Electrochemical Society, the award consists of a framed certificate and $1,500 prize. Materials deadline: October 1, 2023
Student Awards
Corrosion Division Morris Cohen Graduate Student Award: Established in 1991 to recognize and reward outstanding graduate research in the field of corrosion science and/or engineering and to encourage especially promising researchers to remain active in the field after their graduate research is completed, the award consists of a framed certificate and $1,000 prize. The recipient may receive up to $1,000 toward travel expenses to facilitate attendance at a designated Corrosion Division symposium where the recipient is expected to present a lecture on his or her research work. Materials deadline: December 15, 2023
Energy Technology Division Graduate Student Award sponsored by BioLogic: Established in 2012 to recognize and reward promising young engineers and scientists in fields pertaining to the division, the award consists of a framed certificate, $1,000 prize, complimentary student meeting registration and admission to the division’s business meeting. Materials deadline: September 1, 2023
Georgia Section Outstanding Student Achievement Award: Established in 2011 to recognize academic accomplishments in any area of science or engineering in which electrochemical and/ or solid state science and technology is the central consideration, the award consists of a $500 prize. Materials deadline: August 15, 2023



Industrial Electrochemistry and Electrochemical Engineering Division H. H. Dow Memorial Student Achievement Award: Established in 1990 to recognize promising young engineers and scientists in the field of electrochemical engineering and applied electrochemistry, the award consists of a framed certificate and $1,000 for expenses associated with the recipient’s education or research project. Materials deadline: September 1, 2023
Industrial Electrochemistry and Electrochemical Engineering Division Student Achievement Award: Established in 1989 to recognize promising young engineers and scientists in the field of electrochemical
engineering, the award consists of a framed certificate and $1,000 prize. Materials deadline: September 1, 2023
Korea Section Student Award: Established in 2005 to recognize academic accomplishments in any area of science or engineering in which electrochemical and/or solid state science and technology is the central consideration, the award consists of a $500 prize. Materials deadline: December 31, 2023

Biannual Meeting Travel Grants: Many ECS divisions and sections offer travel grants to undergraduates, graduate students, postdoctoral researchers, and young professionals and faculty presenting papers at ECS biannual meetings. The awards consist of financial support ranging from complimentary meeting registration to luncheon/reception tickets, travel support, and more. Each division and section has its own application requirements. 245th ECS Meeting Travel Grant applications accepted from December 1, 2023 to February 26, 2024.
ECS General Student Poster Session Awards: This poster session provides a forum for graduate and undergraduate students to present research results of general interest to ECS. The session was established in 1993 to foster and promote work in electrochemical and solid state science and technology and to stimulate active student interest and participation in ECS. Posters accepted for presentation in the session are eligible for these General Student Poster Awards: $1500 for first place, $1000 for second place, and $500 for third place. To be considered for awards, student poster authors must (1) submit an abstract to the Z01 General Student Poster Session; (2) be accepted for inclusion in the poster session; (3) upload a digital poster; and (4) be present during the in-person judging session. 245th ECS Meeting abstract deadline: December 1, 2023.
ECS Outstanding Student Chapter Award: Established in 2012, the award recognizes distinguished student chapters that demonstrate active participation in the Society’s technical activities; establish community and outreach activities in the areas of electrochemical and solid state science and engineering education; and create and maintain a robust membership base. Up to three winners are selected, with one named the Outstanding Student Chapter and up to two named Chapters of Excellence. The Outstanding Student Chapter receives a recognition plaque; $1,000; and award recognition and a chapter group photo in Interface or other electronic communications. Chapters of Excellence members receive mailed recognition certificates and acknowledgement in Interface Materials deadline: April 15 annually
ECS Summer Fellowships: Established in 1928, the award assists students pursue work in a field of interest to ECS. Each year, The Society awards the Edward G. Weston Fellowship, Joseph W. Richards Fellowship, F. M. Becket Fellowship, and the H. H. Uhlig Fellowship. Each fellowship consists of $5,000 in funding to support the recipient’s research from June through August and publication of a summary report in Interface Materials deadline: January 15 annually.
Colin Garfield Fink Fellowship: First awarded in 1962, the fellowship assists a postdoctoral scientist/ researcher during the months of June through September to pursue research work in a field of interest to the Society. The award consists of $5,000 in funding and publication of a summary report in Interface. Materials deadline: January 15 annually
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 59
NEW MEMBERS NEW MEMBERS
ECS is proud to announce the following new members for January, February, and March 2023 (Members are listed alphabetically by family/last name.)
Members
A
Kiran Adepalli, Lewis Center, OH, USA
Chi-Yeong Ahn, Daejeon, Chungcheongnamdo, South Korea
Saeed Alhassan, Abu Dhabi, Abu Dhabi, UAE
Isao Amemiya, Soka, Saitama, Japan
Arava Aravaz, Goleta, CA, USA
B
Natalia Bannyk, Dnipro, Dnipropetrovsk, Ukraine
Christian Beauger, Sophia Antipolis, Provence-Alpes-Côte d'Azur, France
John Buttles, Los Angeles, CA, USA
C
R. Kramer Campen, Duisburg, NRW, Germany
Daren Caruana, London, England, UK
Guy Cassuto, Brooklyn Center, MN, USA
Mark Clapp, Sonning Common, Reading, Oxfordshire, UK
D
Pilar de la Cruz, Toledo, Castilla-La Mancha, Spain
Ernest Delmo, Sai Kung, Hong Kong, China
Gabriel Dera, Castelfranco Veneto, Treviso, Italy
Kyle Diederichsen, Seattle, WA, USA
G
Andrew (Bean) Getsoian, Canton, MI, USA
Ebru Gungor, Burdur City, Burdur, Turkey
H
Umer Hassan, Piscataway, NJ, USA
Seiichiro Higashi, Higashi, Hiroshima, Japan
Yi-Ting Hsieh, Taipei, Taipei, Taiwan
I
Abubakr Ibrahim, Cambridge, Cambridgeshire, UK
John Ilkka, Royal Oak, MI, USA
Ian Jakupca, Cleveland, OH, USA
Nathan Kempema, Grosse Pointe Farms, MI, USA
Jung Kyu Kim, Suwon, Gyeonggi-do, South Korea
Myeongjin Kim, Daegu, Yeongnam, South Korea
Wooyul Kim, Naju, South Jeolla, South Korea
Valentina Knysh, Dnipro, Dnipropetrovsk, Ukraine
Genki Kobayashi, Wako, Saitama, Japan
Vadym Kovalenko, Dnipro, Dnipropetrovsk, Ukraine
Tim Kucharski, Natick, MA, USA
Raghunathan Kuppuswamy, Bangalore, KA, India
Adolfo La Rosa-Toro Gómez, Lima, Lima, Peru
Jong-Won Lee, Seoul, Gyeonggi-do, South Korea
Sanghan Lee, Gwangju, Gyeonggi-do, South Korea
Yoon Koo Lee, Daejeon, South Chungcheong, South Korea
Luxi Li, Lemont, IL, USA
Shu-Ping Lin, Taichung City, Taichung, Taiwan
Sateesh Madhi, Bellaire, TX, USA
Filipe Marques Mota, Lincoln, East Midlands, UK
Vishal Metri, Bangalore, KA, India
Soraya Muzayanha, Jakarta Timur, DKI, Indonesia
Maria Myroniak, Dnipro, Dnipropetrovsk, Ukraine
Nanda Nagarajan, Burlington, ON, Canada
Kyung-Wan Nam, Seoul, Gyeonggi-do, South Korea
Konstantin Nikolaev, Singapore, Singapore, Singapore
Liv Oftedal, Kristiansand Vest, Agder, Norway
Rachel O’Malley, Bristol, Gloucestershire, UK
Bin Ouyang, Tallahassee, FL, USA
Georgios Polizos, Oak Ridge, TN, USA
Vyacheslav Protsenko, Dnipro, Dnipropetrovsk, Ukraine
Yahya Rabbani, Lausanne, VD, Switzerland
Julienne Regele, Jefferson, MA, USA
Iryna Reshetnyak, Dnipro, Dnipropetrovsk, Ukraine
Debra Rolison, Washington, DC, USA
Jacek Ryl, Gdańsk, Pomerania, Poland
S
Niloufar Sharif, Lausanne, VD, Switzerland
Jaswinder Sharma, Oak Ridge, TN, USA
Takatoshi Shimada, San Jose, CA, USA
Vasyl Shvalya, Ljubljana, Central, Slovenia
Uk Sim Naju-si, South Jeolla, South Korea
Karen Smith, Bordentown, NJ, USA
Grigorii Soloveichik, Reston, VA, USA
LKostiantyn Sukhyi, Dnipro, Dnipropetrovsk, Ukraine
Ravishankar Sundararaman, Troy, NY, USA
T
Thomas Turek, Clausthal-Zellerfeld, Lower Saxony, Germany
V
Roel van de Krol, Berlin, Berlin, Germany
Barbara Ventura, Bologna, EmiliaRomagna, Italy
Al Rey Villagracia, Malate, Metro Manila, Philippines
W
Colleen Wallace, Lafayette, CO, USA
Wim Wenseleers, Antwerp, Flanders, Belgium
Y
Hyoungsik Yim, Melrose, MA, USA
Xiaoguang Yin, Shenzhen, Guangdong, China
Li Yunchao, Troy, MI, USA
Z
Yanwen Zhang, Idaho Falls, ID, USA
Student Members A
Archana, Delhi, DL, India
Dharshini A, Namakkal, TN, India
Ehsan Abbasi, Lubbock, TX, USA
Nooruddin Abdel Rahman, Golden, CO, USA
Davoud Adinehloo, Tonawanda, NY, USA
Jedidian Adjei, Lubbock, TX, USA
Nitisha Ahuja, State College, PA, USA
Fei Ai Shatin, Hong Kong, Hong Kong, China
Belinda Akabueze, Philadelphia, PA, USA
Toshihiro Akashige, Brooklyn, NY, USA
Ali A. Alizadehmojarad, Houston, TX, USA
Shatha Alrasheed, West Lafayette, IN, USA
Lennart Alsheimer, Münster, NRW, Germany
Aniqa Anjum, College Park, MD, USA
Ashly Antony, Oxford, MS, USA
Karla Aranda, Austin, TX, USA
J
K
M
N
O
P
R
60 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Jorge Arredondo Espanola, Guanajuato, San Luis Potosí, México
Raad Asif, Fukuoka, Fukuoka, Japan
Sethi Asis, Chennai, TN, India
Yahreli Audeves, Santiago de Querétaro, Querétaro, México
Sachinthani Ayabadda Devage, Stillwater, OK, USA
B
Farzaneh Bahmani, Rapid City, SD, USA
Avinash Bairwa, West Lafayette, IN, USA
Yuna Bang, Gwanju, Gyeonggi-do, South Korea
Amira Ben Abderrahmane, Montpellier, Occitanie, France
Jay Bender, Austin, TX, USA
Matthew Bergschneider, Plano, TX, USA
Elizabeth Bernhardt, Cambridge, MA, USA
Muhammad Bilal, Bethlehem, PA, USA
Tianyu Bo, New York, NY, USA
María Fernanda Bósquez Cáceres, Riobamba, Tungurahua, Ecuador
Jan Bosse, Villigen, Aargau, Switzerland
Deaglan Bowman, Limerick, Munster, Ireland
Ana Brandão, Porto, Porto, Portugal
Taylor Brandt, Ann Arbor, MI, USA
Jeremy Brinker, Austin, TX, USA
Daniel Broadhurst, Glasgow, Scotland, UK
Lia Bu, Cambridge, MA, USA
C
Jessica Cárdenas, Santiago de Querétaro, Querétaro, México
Uriel Cárdenas, Santiago de Querétaro, Querétaro, México
Emily Carroll, Salt Lake City, UT, USA
Isabella Caruso, Cambridge, MA, USA
Jorge Castro Fernández, Querétaro, Querétaro, México
Joshua Chamberland, Sudbury, ON, Canada
Nai Jen Chang, Hsinchu, Hsinchu County, Taiwan
Sarmistha Chatterjee, Kuala Lumpur, Selangor, Malaysia
Nan Chen, Baltimore, MD, USA
Moses Chilunda, New York, NY, USA
Tae Cho, Ann Arbor, MI, USA
Hyun Won Chu, Cambridge, MA, USA
Chikaodili Chukwuneke, Austin, TX, USA
Mireia Cifre Herrando, València, VAL, Spain
César Coello Mauleón, Querétaro, Querétaro, México
Zachary Cohen, New York, NY, USA
Thomas Colburn, Oak Ridge, TN, USA
Anthony Concepcion, Austin, TX, USA
Oğuz Kağan Coşkun, Cleveland, OH, USA
Yingdan Cui, Columbia, SC, USA
Zhihao Cui, Columbus, OH, USA
NEW MEMBERS NEW MEMBERS
B. Dass, Madurai, TN, India
Samuel Degnan-Morgenstern, Cambridge, MA, USA
Jean-Nicolas Deraspe, Montréal, QC, Canada
Sathya Dewarathna Samaraweerage, Stillwater, OK, USA
Rachel DeWees, Louisville, KY, USA
Maria Carmenza Diaz Lacharme, Milan, Lombardia, Italy
Ana Díaz Patiño, Irapuato, Guanajuato, México
Hannah Dickinson, Glasgow, Scotland, UK
Tanye Dornelles, Porto Alegre, Rio Grande do Sul, Brazil
Changyue Du, Montréal, QC, Canada
Hisham Elaqapa, New Cairo, Cairo, Egypt
Ahmed El-Harairy, Lincoln, NE, USA
Hajar El-Ouahabi, Temara, Rabat-SaleKenitra, Morocco
Yaritza Enríquez Izazaga, Querétaro, Ciudad de México, México
Marius Enstrup, Clausthal-Zellerfeld, Lower Saxony, Germany
Md Ershad, Oxford, MS, USA
Daniel Esau, Karlsruhe, BW, Germany
Silvia Escayola Gordils, Tossa de Mar, CAT, Spain
F
Chien-Wei Fan, Zhubei City, Hsinchu County, Taiwan
Hsu Fang, Hsinchu, Hsinchu County, Taiwan
Hafiz Muhammad Umar Farooq, New York, NY, USA
Jin Feng, Trondheim, Sør-Trøndelag, Norway
Jannatul Ferdous, Oxford, MS, USA
Gus Floerchinger, Golden, CO, USA
Emily Foley, Santa Barbara, CA, USA
Maurice Friedrichs-Schucht, Braunschweig, Lower Saxony, Germany
G
Pooja Gaikwad, New York, NY, USA
Jessica Gallawa, Loveland, CO, USA
Veka Sri Ganesan, Detroit, MI, USA
Saahir Ganti-Agrawal, New York, NY, USA
Nagalakshmi Gayathri M, Chennai, TN, India
Candeniz Gercek, Münster, NRW, Germany
Michael Geserer, Munich, Bavaria, Germany
Gregor Glanz, Vienna, Bundesland, Austria
Megan Gober, Huntsville, AL, USA
Dana Goerzen, New York, NY, USA
Sadhana Golani, Karlsruhe, BW, Germany
Luma Gomes, Porto Alegre, Rio Grande do Sul, Brazil
Patricia Gonzales, College Park, MD, USA
Abigail Gonzalez, La Plata, Buenos Aires, Argentina
Corbinian Groen, Munich, Bavaria, Germany
Cedric Grosselindemann, Karlsruhe, BW, Germany
Alyssa Grube, Lincoln, NE, USA
Liqun Guo, Houston, TX, USA
HMajid Haji Bagheri, Waterloo, ON, Canada
Alan Halverson, West Lafayette, IN, USA
Md Wahidul Hasan, Rapid City, SD, USA
Joshua Hazelnis, Ann Arbor, MI, USA
Marcel Heidbuechel, Münster, NRW, Germany
Marie Heidler, Münster, NRW, Germany
Ángel Hernández, Santiago de Querétaro, Querétaro, México
EEvelyn Hernández Rodríguez, Querétaro, Querétaro, México
Jan Hettig, Ulm, BW, Germany
Laura Hoagland, Fort Worth, TX, USA
Gustavo Hobold, Cambridge, MA, USA
Tara Hoffman, Austin, TX, USA
Wei-Lun Hsiao, Tucheng, New Taipei, Taiwan
Fang-Rong Hsu, Hsinchu, Hsinchu County, Taiwan
Genzhi Hu, East Lansing, MI, USA
Kedi Hu, New York, NY, USA
Po-Wei Huang, Atlanta, GA, USA
Julia Huddy, Hanover, NH, USA
Matthew Humbert, Footscray, Victoria, Australia
I
Mairis Iesalnieks, Riga, Riga, Latvia
Sarang Ismail, Lincoln, NE, USA
Atara Israel, Hewlett, NY, USA
J
Tyng Woei Jang, Hsinchu, Hsinchu County, Taiwan
Mayuresh Janpandit, Buffalo, NY, USA
Marek Janssen, Braunschweig, Lower Saxony, Germany
Theresa Jaster, Oberhausen, NRW, Germany
Kyle Jiang, Cambridge, MA, USA
Diana Jimenez Acambay de Ruez, Castañeda, Estado de México, México
Xiaojia Jin, Cambridge, MA, USA
Gan Jing En, Hsinchu, Hsinchu County, Taiwan
K
Sachithra K, Thrissur, KL, India
Iwona Kaczmarzyk, Gdańsk, Pomerania, Poland
Vishal Mani Kalaimani, Hsinchu City, Hsinchu County, Taiwan
Tanunya Kamthong, Mueang, Nakhon Ratchasima, Thailand
Matheus Antonius Da Costa, Porto Alegre, Rio Grande do Sul, Brazil
Ishaku Dagare, Dutse, Jigawa, Nigeria
Sumanta Das, Chennai, TN, India
DVictor Gonzalez, Querétaro, Ciudad de México, México
Joachim Grieg, Trondheim, Sør-Trøndelag, Norway
Jerren Grimes, Evanston, IL, USA
Riham Kanaan, Antibes, Provence-AlpesCôte d'Azur, France
Hassan Karaki, Braunschweig, Lower Saxony, Germany
(continued on next page)
The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 61
(continued from previous page)
Sandun Bogahawaththa Kasthuri Dias, Stillwater, OK, USA
Mark Kathol, Lincoln, NE, USA
Erin Keblish, New York, NY, USA
Chan Gi Kim, Cambridge, MA, USA
Moonseong Kim, West Lafayette, IN, USA
Tae Jeong Kim, Baltimore, MD, USA
Kyler Krupp, Austin, TX, USA
Samuel Sandesh Kuchu, Williamsville, NY, USA
Felix Kullmann, Karlsruhe, BW, Germany
Navneet Kumar, Edmonton, AB, Canada
Tzu Kuo, Hsinchu City, Hsinchu County, Taiwan
Sameera Kurumbara Arachchige, Buffalo, NY, USA
Sz-Nian Lai, Hsinchu, Hsinchu County, Taiwan
Christian Landry, College Station, TX, USA
Rebeca Leal Avila, Querétaro, Querétaro, México
Yen-Che Lee, Hsinchu, Hsinchu County, Taiwan
Jeremy Leger, Broussard, LA, USA
Jiafeng Lei, Sha Tin, Hong Kong, China
Julia Lenef, Ann Arbor, MI, USA
Karla Leon, Zuiga, Pedro Escobedo, México
Karen Li, Seattle, WA, USA
Chia Kai Lin, Taipei, Taipei, Taiwan
Chia-Ju Lin, Hsinchu, Hsinchu County, Taiwan
Mingo Lin, Hsinchu, Hsinchu County, Taiwan
Adrian Lindner, Karlsruhe, BW, Germany
Alex Liu, San Diego, CA, USA
Fan Liu, Manhattan, KS, USA
Yanting Liu, Karlsruhe, BW, Germany
Marco Loeffelholz, Clausthal-Zellerfeld, Lower Saxony, Germany
Alejandro Lopez, Santiago de Querétaro, Querétaro, México
Rosa Lopez, Pedro Escobedo, Querétaro, México
Josua Lopez Montelongo, Querétaro, Querétaro, México
Alexander Lucas, Recklinghausen, NRW, Germany
Carlos Lucecki, Detroit, MI, USA
Marco Luether, Münster, NRW, Germany
Karen Ly, Rapid City, SD, USA
Varsha M V, Trivandrum, KL, India
Peiyuan Ma, Chicago, IL, USA
Yi-Chun Ma, Hsinchu City, Hsinchu County, Taiwan
Krystian Machaj, Warsaw, Mazovia, Poland
Moksh Madan, Sonipat, HR, India
Mostafa Mahmoudi, Fayetteville, AR, USA
Katharina Mairhofer, Vienna, Bundesland, Austria
Likhith Manjunatha, Fukuoka, Fukuoka, Japan
NEW MEMBERS NEW MEMBERS
Vasanthapandiyan Mari, Gautam Buddha Nagar, UP, India
Maria Martin Martinez, Querétaro, Ciudad de México, México
Laura Lupita Martinez Rodriguez, Pedro Escobedo, Querétaro, México
Ricardo Mathison, Brooklyn, NY, USA
Derrick Maxwell, Maynard, MA, USA
Charles McCabe, Cohoes, NY, USA
Mitchell McGowan, South Bend, IN, USA
Vivian Meier, Grafing, Bavaria, Germany
Samuel Merk, Garching bei München, Bavaria, Germany
Christopher Merkel, Omaha, NE, USA
Alvaro Miguel, West Lafayette, IN, USA
Pretty Mitra, West Lafayette, IN, USA
Marvin Mohrhardt, Münster, NRW, Germany
Archer Montgomery, Lubbock, TX, USA
Xiaowei Mu, Amherst, MA, USA
Debashis Mura, Raghunathganj, WB, India
Erlind Mysliu, Trondheim, Sør Trøndelag, Norway
N
Jungheyn Nam, Buk-gu, Busan, South Korea
Anurupa Naskar, Bloomington, IN, USA
Huy Qui Vinh Nguyen, Tartu, Tartu County, Estonia
Viet Phuong Nguyen, Daejeon, South Chungcheong, South Korea
Ningyuan Nie, Singapore, Singapore, Singapore
O
Oghenetega Obewhere, Lincoln, NE, USA
Everardo Olide, Ann Arbor, MI, USA
Margarita Orlova, Nashville, TN, USA
Jens Osiewacz, Clausthal-Zellerfeld, Lower Saxony, Germany
Christopher Owen, New York, NY, USA
Kyra Owensby, Knoxville, TN, USA
P
Sri Krishna Murthy Padavala, Corvallis, OR, USA
Jesús Pérez García, San Juan del Río, Querétaro, México
Julia Petersen, Boston, MA, USA
Charles Phu, Boston, MA, USA
Pattiya Pibulchinda, Evanston, IL, USA
Thomas Porter, Cambridge, MA, USA
Hossein Pourrahmani, Sion, VS, Switzerland
Divyansh Prakash, Oxford, MS, USA
Shwetha Prakash, Buffalo, NY, USA
Nicholas Price, Cambridge, MA, USA
Arvind Pujari, Cambridge, Cambridgeshire, UK
R
Darshana Rajput, Santiago de Querétaro, Querétaro, México
Sachin Ramdin, Ozone Park, NY, USA
Gayatri Ranade, Buffalo, NY, USA
Pooja Ranganathan, West Lafayette, IN, USA
Haresh Raygor, Chennai, TN, India
Jakob Reinke, Chicago, IL, USA
Anton Resing, Brookline, MA, USA
Collin Rodmyre, Blaine, MN, USA
Julymar Rodríguez, Besancon, BourgogneFranche-Comté, France
Jyoti Rohilla, Hsinchu, Hsinchu County, Taiwan
Abraham Rojas Zuniga, Bentley, Western Australia, Australia
Anthony Romero, Seattle, WA, USA
Charlotte Roullier, Lausanne, VD, Switzerland
Andrea Russo, Copenhagen, Hovedstaden, Denmark
Amelia Ryan, New York, NY, USA
Changhyun Ryu, Austin, TX, USA
S
Sisheed S, Palakkad, KL, India
Syama S, Chennai, TN, India
Sepehr Saadat, Ulm, BW, Germany
Shashika Sabaragamuwe J. K. M. R., East Lansing, MI, USA
Aleksei Sadykov, Münster, NRW, Germany
Mohammed Sahal, Mesa, AZ, USA
Elif Selin Sahin, Lubbock, TX, USA
Diego Salazar, Lincoln, NE, USA
Alexander Sananes, Cambridge, MA, USA
Monserrat Santos, Corregidora, Querétaro, México
Giuseppe Sassone, Grenoble, AuvergneRhône-Alpes, France
Witchayatip Satianram, Nakhon Ratchasima, District Nakhon Phanom, Thailand
William Schubert, Arlington, VA, USA
Bret Schumacher, New York, NY, USA
Armin Sedighi, Detroit, MI, USA
Sahand Serajian, Lincoln, NE, USA
Alejandro Sevilla, Cambridge, MA, USA
Yang Shi Shatin, Hong Kong, Hong Kong, China
Her-Yih Shieh, Hsinchu, Hsinchu County, Taiwan
Shubhendra Shukla, Oxford, MS, USA
Rachel Silcox, Ypsilanti, MI, USA
Amarjeet Singh, Chandigarh, CH, India
Dhyllan Skiba, Boston, MA, USA
Lettie Smith, Austin, TX, USA
Devan Solanki, Lodi, NJ, USA
Katharina Steier, Manchester, Lancashire, UK
Blake Stringer, Ypsilanti, MI, USA
Javier Suarez, Barajas, Querétaro, México
Majharul Islam Sujan, Oxford, MS, USA
Anish Sukumar, Cambridge, MA, USA
Kerry Sun, New York, NY, USA
TKaito Takeuchi, Sendai, Miyagi, Japan
Sarvarjon Talipov, New York, NY, USA
Christopher Tapia, Troy, MI, USA
Modeste Tegomoh, Columbus, OH, USA
Anusha Thampi V V, Thiruvananthapuram, KL, India
Micah Thorpe, Ann Arbor, MI, USA
L
M
62 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org
Gunnar Thorsteinsson, New York, NY, USA
Ugur Topkiran, Fort Worth, TX, USA
Heron Torres, Porto Alegre, Rio Grande do Sul, Brazil
David Tran, Kgs. Lyngby, Hovedstaden, Denmark
Hoang Tran, Potsdam, NY, USA
Andrew Tuokkola, Ann Arbor, MI, USA
Zane Turner, Oxford, MS, USA
Kenneth Tuul, Tartu, Tartu County, Estonia
Nathalie Tuya, New York, NY, USA
Truong Tuyen, Raleigh, NC, USA
Marco Uscanga Olea, Pedro Escobedo, Querétaro, México
Lonneke van Eijk, Golden, CO, USA
Travis van Leeuwen, Bozeman, MT, USA
Theadora Vessella, Smithfield, RI, USA
Molly Vitale-Sullivan, Corvallis, OR, USA
Heng Wang, Cambridge, Cambridgeshire, UK
Jiacheng Wang, Amherst, MA, USA
NEW MEMBERS NEW MEMBERS
Wenlu Wang, Boston, MA, USA
Yian Wang, Guangzhou, Guangdong, China
Zeyi Wang, Hyattsville, MD, USA
Brian Washington, Knoxville, TN, USA
Nicholas Watkins, Thousand Oaks, CA, USA
Bryce Watson, Rapid City, SD, USA
Nicholas Wayman, Blair, NE, USA
Courtney Weber, Columbia, SC, USA
Miaomiao Wen, Sophia Antipolis, ProvenceAlpes-Côte d'Azur, France
Lydia Weseler, Clausthal-Zellerfeld, Lower Saxony, Germany
Tyler Williams, Provo, UT, USA
Corbin Witt, Columbia, SC, USA
Cindy Wong, Cambridge, MA, USA
Henry Woolley, Münster, NRW, Germany






You-cheng Wu, Hsinchu City, Hsinchu County, Taiwan
X





Xiao Xiao, Singapore, Singapore, Singapore
Zhengxi Xuan, Buffalo, NY, USA
Y






















Moises Yanez, Quertero, Ciudad de México, México
Fei Yang, Shenzhen, Guangdong Sheng, China
Jixiang Yang, Hong Kong, Hong Kong, China
Kevin Yang, Cambridge, MA, USA
Mengya Yang, London, England, UK
Ruoyu Yang, Atlanta, GA, USA
Masahiro Yasutake, Fukuoka, Fukuoka, Japan
Julio Cesar Yepez, El Marquez, Querétaro, México
David Kumar Yesudoss, College Station, TX, USA
Chen Yi, Hsinchu, Hsinchu County, Taiwan
Ho Yi-Jui, Hsinchu, Hsinchu County, Taiwan
Kevin Yu, Pasadena, CA, USA
Baba Yussif, Lincoln, NE, USA
Z



Alexis Zabalegui, Querétaro, Querétaro, México
Guan-Cheng Zeng, XinZhu, Hsinchu County, Taiwan
Bingzhang Zhang, Getzville, NY, USA
Shulin Zhang, London, England, UK

Zhen Zhang, Cambridge, MA, USA
Zhaoyi Zheng, Malden, MA, USA
Kosovare Zullufi, Ilmenau, Thuringia, Germany
U
V
W
Arg en�na Australia Austria Belgium Brazil Canada China Denmark Ecuador Egypt Estonia France Germany Hong Kong India Indonesia Ireland Italy Japan Latvia Malaysia Mexico Morocco Nigeria Norway Peru Philippines Poland Portugal Sing apore Slov enia South Korea Spain Switzerland Taiwan Thailand Turkey UAE UK Ukraine USA Argentina 1 Australia 2 Austria . 2 Belgium 1 Brazil 4 Canada. 6 China 4 Denmark 2 Ecuador . 1 Egypt 1 Estonia 2 France. 6 Germany 33 Hong Kong 4 India 18 Indonesia 1 Ireland . 1 Italy 3 Japan 7 Latvia . 1 Malaysia 1 Mexico 27 Morocco. 1 Nigeria 1 Norway 4 Peru. 1 Philippines 1 Poland 3 Portugal 1 Singapore 3 Slovenia. 1 South Korea 12 Spain 3 Switzerland. 5 Taiwan 23 Thailand 2 Turkey. 1 UAE 1 UK 12 Ukraine. 7 USA 201 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org 63
New Members by Country Look who joined ECS in the First Quarter of 2023.
Chapter News
ECS University of the Philippines Student Chapter Student



The new ECS University of the Philippines Student Chapter is the Society’s first student chapter in the Philippines. The Executive Committee officers are Chair DJ Donn C. Matienzo; Vice-Chair Daniel Loresca; Secretary Maricor F. Divinagracia-Luzadas; Treasurer Darlene T. Pudolin; and Director of Communications and Publicity Rhoda Lyn H. Ramos. The chapter, which is based at the University of the Philippines Diliman (UPD), recognizes faculty advisors Assistant Professor Joey D. Ocon, Assistant Professor Michael T. Castro, and Laboratory Manager Goran T. Tomacruz for their unwavering support of chapter members and their activities.
The chapter’s February 13–18 Battery Week celebration, “Powering the Future: A Celebration of Battery Innovation,” commemorated National Battery Day. It was held in partnership with the Advanced Batteries Center, a collaborative R&D program between the Technological Institute of the Philippines and the University of the Philippines Diliman (UPD). A seminar at the UPD Department of Chemical Engineering on February 17, “Charging Ahead: Pioneering New Frontiers in Battery Technology,” highlighted the event. Guest speakers—Julie Anne D. del Rosario-Paraggua, Associate Professor and Department Chair, UPD; Drandreb Earl Juanico, Principal Researcher, CATALYST TechnoCore, Technological Institute of the Philippines; Lawrence Limjuco, Senior Lecturer, UPD and Supervising Science Research Specialist, Advanced Batteries Center Philippines; and Prince Elmer A. Reyes, Assistant Vice President and Head of Technical R&D, Philippines Batteries Inc.—shared their expertise and experiences in industry and academia. The talks were informative and insightful, with discussions ranging from the latest battery technologies to emerging trends in energy storage. Around 60 undergraduate and graduate students attended and were treated to free snacks and game prizes, making the event both educational and enjoyable.
A poster exhibit throughout the week provided viewers—mostly undergraduate and graduate students—with an overview of recent developments and ongoing research projects in batteries and battery technology. Some of the most significant research conducted by members of the UPD Laboratory of Electrochemical Engineering (LEE) was showcased, providing students with opportunities to interact with researchers. The poster exhibit was valuable for researchers to share their findings with a wider audience, to promote knowledge sharing, and to inspire students to pursue further research and exploration in battery technology. Moreover, the exhibit was an excellent way to introduce the chapter and encourage students to join.
The chapter’s first major event of the year shared and highlighted
the importance of battery technology and its role in sustainable development. It was a great success with a wonderful turnout and attendee engagement. The chapter is committed to organizing similar events in the future. Interface readers are invited to keep abreast of chapter activities and events on the chapter’s Facebook page.
64 The Electrochemical Society Interface • Summer 2023 • www.electrochem.org STUDENT NEWS STUDENT NEWS
ECS University of the Philippines Student Chapter members attend the first General Assembly and induction of the executive committee at the University of the Philippines Diliman (UPD) Department of Chemical Engineering.
ECS University of the Philippines Student Chapter Battery Seminar participants and organizers.
Chapter members gather for their Battery Week poster exhibit launch in the lobby of the UPD Department of Chemical Engineering.

Full issues now available for purchase and download from the ECS Online Store: www.electrochem.org/online-store Volume 111: 243rd Meeting of The Electrochemical Society with SOFC-XVIII 20% ECS Members’ Discount ENHANCE YOUR MEETING EXPERIENCE





































 by Adrian Plummer, MPA, PMP | Director of Publications
by Adrian Plummer, MPA, PMP | Director of Publications








































































































 by Shrihari Sankarasubramanian, Bradley Chambers, and Cheyenne Wilson
by Shrihari Sankarasubramanian, Bradley Chambers, and Cheyenne Wilson








 by Kody D. Wolfe, Ardavan Zanganeh, Richard N. Arthur, Jason P. Trembly, and Damilola A. Daramola
by Kody D. Wolfe, Ardavan Zanganeh, Richard N. Arthur, Jason P. Trembly, and Damilola A. Daramola






















































